Attached files
| file | filename |
|---|---|
| 10-Q/A - 10-Q/A - AUXILIUM PHARMACEUTICALS INC | a12-25893_110qa.htm |
Exhibit 10.4
Confidential treatment requested under 17 C.F.R. §§ 200.80(b)(4) and 240.24b-2. The confidential portions of this exhibit have been omitted and are marked accordingly. The confidential portions have been filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request.
CO-PROMOTION AGREEMENT
This Co-promotion Agreement (hereafter, the “Agreement”) is entered into as of May 18, 2012, by and between Auxilium Pharmaceuticals, Inc., a Delaware corporation (“Auxilium”), and GlaxoSmithKline LLC, a Delaware limited liability company (“GSK”).
Recitals
A. Auxilium is the owner of all right, title and interest in and to the Product.
B. Auxilium believes additional resources are necessary to adequately meet the demands for information about, and access to, the Product in the Territory and, therefore, desires to co-promote the Product with GSK in the Territory.
C. GSK desires to co-promote the Product with Auxilium in the Territory, and Auxilium is willing to grant GSK the right to co-promote the Product in the Territory, all on the terms and subject to the conditions set forth in this Agreement.
1. Definitions
1.1 AAA shall have the meaning set forth in Section 17.2.
1.2 AAA Rules shall have the meaning set forth in Section 17.3.
1.3 Act shall mean the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 301 et seq, as it may be amended from time to time, and the regulations promulgated thereunder.
1.4 Adverse Event(s) shall mean any of: an “adverse drug experience,” a “life-threatening adverse drug experience,” a “serious adverse drug experience,” or an “unexpected adverse drug experience,” as those terms are defined in Title 21 of the U. S. Code of Federal Regulations, §314.80, as amended from time to time and published in the Federal Register.
1.5 Affiliate shall mean, in relation to a Party, any person, corporation, firm, partnership or other entity, whether de jure or de facto, which directly or indirectly through one or more intermediaries, controls, is controlled by or is under common control with such Party. An entity shall be deemed to control another entity if it: (i) owns, directly or indirectly, at least fifty percent (50%) of the outstanding voting securities or capital stock (or such lesser percentage which is the maximum allowed to be owned by a foreign corporation in a particular jurisdiction) of such other entity, or has other comparable ownership interest with respect to any entity other than a corporation; or (ii) has the power, whether pursuant to contract, ownership of securities or otherwise, to direct the management and policies of the entity.
1.6 Agreement shall have the meaning set forth in the preamble.
1.7 ANDA shall mean an Abbreviated New Drug Application as described in Section 505(j) of the Act, 21 U.S.C. § 355(j).
1.8 Androgel shall mean the [**] testosterone gel products marketed under the Androgel brand.
1.9 Androgel or Other Generic Entry shall mean the date of the first [**] in the Territory of an FDA-approved (a) Androgel Generic, or (b) another Generic [**] testosterone [**] with the same or substantially similar indications for use to those of the Product (other than a Generic version of the Product).
1.10 Androgel or Other Generic Erosion Date shall mean the date on which there is a loss of [**] percent ([**]%) or more of the market share of the Product, measured in [**] as reported by [**], for any [**] period following an Androgel or Other Generic Entry when compared to such market share measured in [**], as reported by [**], for the [**] period immediately prior to such Androgel or Other Generic Entry. For the purposes of this Section 1.10, the market for the Product shall include any [**] testosterone [**] with the same or substantially similar indications for use to those of the Product.
1.11 Annual Baseline Net Sales Amount shall mean the amount of Net Sales per Year as set forth in the Overall Marketing Plan. The Annual Baseline Net Sales Amount (a) may be adjusted by mutual agreement of the Parties upon an [**] or [**], to become effective upon the [**] or the [**], and (b) for the 2012 calendar year shall be the sum of the Quarterly Baseline Net Sales Amounts for each of the calendar quarters in 2012 commencing with the GSK First Promotion Date.
1.12 Annual Marketing Plan shall mean, subject to Section 2.2, a document prepared annually by Auxilium, and submitted to the Joint Steering Committee for review and approval. The Annual Marketing Plan will set forth the responsibilities of both Auxilium and GSK with respect to promoting and marketing the Product in the Territory for the applicable Year, including the minimum number (which with respect to GSK, shall be the GSK Minimum Details unless otherwise agreed to by the Parties, and which with respect to Auxilium, shall be the Auxilium Minimum Details unless otherwise agreed to by the Parties), a marketing budget for the Product for the applicable Year and all aspects of commercialization of the Product. The Annual Marketing Plan will supplement the Overall Marketing Plan and in the event of a conflict between the Overall Marketing Plan and the Annual Marketing Plan, the terms of the Overall Marketing Plan shall control unless the Parties otherwise agree in writing. The Annual Marketing Plan for the 2012 Year will be agreed to by the Parties within thirty (30) days after the Effective Date.
1.13 Annual Scientific Engagement Plan shall mean, subject to Section 2.2, a document prepared annually by Auxilium and submitted to the Joint Steering Committee for review and approval and which provides a comprehensive summary of the planned activities of Auxilium, in the Territory, with external physicians and communities about the Product and conditions associated with a deficiency or absence of endogenous testosterone for the applicable Year, including medical affairs activities, clinical studies, publication strategies, scientific conferences, patient advocacy group activities, advisory boards and consultancies, and grants and sponsorships.
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
1.14 Applicable Health Care Laws shall mean the Anti-Kickback Statute (42 U.S.C. § 1320a-7b et seq.), the Health Insurance Portability and Accountability Act of 1996 (Pub. L. 104-191) as amended by the Health Information Technology for Economic and Clinical Health Act (Subtitle D of the Health Information Technology for Economic and Clinical Health Act of 2009 and any regulations or other guidance promulgated thereunder), the Sunshine Act, the Federal False Claims Act (31 U.S.C. § 3729-3733), the Civil Monetary Penalties Statute (42 U.S.C. § 1320a-8), Health Care Fraud (18 U.S.C. § 1347), False Statements Relating to Health Care Matters (18 U.S.C. § 1035), and Laws and Regulations related to Government Price Reporting.
1.15 Arbitration Request shall have the meaning set forth in Section 17.3.
1.16 Authorized Testim Generic Entry shall mean the date of the first [**] in the Territory by (a) an Auxilium authorized Third Party, or (b) Auxilium or an Affiliate of Auxilium, of a Generic of the Product.
1.17 Auxilium shall have the meaning set forth in the preamble.
1.18 Auxilium Minimum Details shall have the meaning set forth in Section 5.5(c).
1.19 Auxilium Parties shall have the meaning set forth in Section 13.4.
1.20 Auxilium Patents shall mean all patents and patent applications in the Territory that are or become owned by or licensed to Auxilium, or to which Auxilium otherwise has, now or in the future, the right to grant licenses and license rights, that generically or specifically cover the Product or a use or formulation of the Product. Included within the definition of Auxilium Patents are all continuations, continuations-in-part, divisionals, patents of addition, reissues, reexaminations, renewals or extensions thereof. The current list of patent applications and patents encompassed within Auxilium Patents is set forth in Appendix B attached hereto.
1.21 Auxilium Sales Support Expenses shall mean at least $[**] to be spent by Auxilium [**] in support of sales of the Product.
1.22 Auxilium Trademarks shall mean the Testim® trademark owned by or licensed to Auxilium in the Territory, and any other trademark, service mark or logo developed, applied for, registered, or to be applied for or registered for use in connection with the sale of the Product in the Territory. The current list of Auxilium Trademark registrations and applications is set forth in Appendix A attached hereto.
1.23 Base Auxilium Marketing Expenses shall have the meaning set forth in Section 5.8(a).
1.24 Baseline Net Sales Amount means the Annual Baseline Net Sales Amount or the Quarterly Net Sales Amount, as the context may require.
1.25 Benefit Plans shall have the meaning set forth in Section 5.6(e).
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
1.26 Business Day shall mean a day on which commercial banks are open for business in New York City. References in this Agreement to “days” other than Business Days shall mean calendar days.
1.27 cGMP shall mean the then-current Good Manufacturing Practices as defined in the U.S. Code of Federal Regulations that are applicable to the Product and the manufacturing thereof under this Agreement, as amended from time to time, including the current good manufacturing practices promulgated by the FDA under the Act, found in Title 21 of the U.S. Code of Federal Regulations, Parts 210 and 211.
1.28 Change of Control shall mean, with respect to Auxilium: (a) (i) any Third Party acquires, directly or indirectly, beneficial ownership of any voting security of Auxilium representing fifty percent (50%) or more of the total voting power of the then-outstanding voting securities of Auxilium; (ii) the consummation of a merger, consolidation, recapitalization, or reorganization of Auxilium with or by a Third Party which would result in fifty percent (50%) or more of the total voting power of the capital stock being transferred to a Third Party; (iii) the stockholders of Auxilium approve a plan of complete liquidation of Auxilium; or (iv) an agreement for the sale or disposition by Auxilium of all or a substantial portion of Auxilium’s assets, other than to an Affiliate, and (b) after consummation of any of the foregoing events, the acquiring Third Party or surviving, consolidated, recapitalized or reorganized entity then owning the Product also sells, promotes or markets [**].
1.29 CIAs shall mean any corporate integrity agreement.
1.30 Claims shall have the meaning set forth in Section 13.3.
1.31 Codes shall mean the Code on Interactions with Healthcare Professionals promulgated by the Pharmaceutical Research and Manufacturers of America (PhRMA), the American Medical Association Opinion 8.061, Gifts to Physicians from Industry, and the Compliance Program Guidance for Pharmaceutical Manufacturers by the Department of Health and Human Services - Officer of Inspector General, as any of the foregoing may be amended.
1.32 Commercially Reasonable Efforts shall mean, with respect to a Party’s obligation to perform or achieve a specified obligation for the Product or generally under this Agreement, the efforts, expertise, degree of skill, and resources that are comparable in quality and scope to those efforts, expertise, degree of skill and resources that are generally used by such Party to perform or achieve a comparable obligation for a pharmaceutical product owned or controlled by such Party, which has the same regulatory requirements or status (for example, requires a prescription or is available over-the-counter), is at a comparable stage of development or product life as the Product, and that has similar market potential as the Product, taking into account issues of patent coverage, relative safety and efficacy, product profile, the competitiveness of the marketplace, the proprietary position of the compound or product, relevant regulatory circumstances, the profitability of the product (including pricing and reimbursement status achieved) and other relevant risk factors, including technical, legal, scientific, commercial and/or medical risk factors.
1.33 Competing Product shall have the meaning set forth in Section 11.1.
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
1.34 Compliance Committee shall have the meaning set forth in Section 5.11(a).
1.35 Compliance Dispute shall have the meaning set forth in Section 5.11(a).
1.36 Compliance Records shall mean records created or generated by or for each Party during the Term and related to the sale, promotion or marketing of the Product, any disease state or scientific engagement activities related to hypogonadism in males or any activities conducted under this Agreement, including applicable (a) Compliance Committee meeting minutes, (b) compliance hotline complaints, inquiries, and responses thereto, (c) Promotion Materials, (d) Scientific Materials, (e) sales representative call notes and call logs; (f) documents related to interactions with healthcare professionals or Product customers; (g) customer contracts; (h) service arrangements; (i) contracts and payment terms related to relationships with healthcare professionals; or (j) results of compliance audits or investigations, whether internal or external.
1.37 Core Sales Aid shall mean that certain core piece of Promotional Material that is agreed upon by the Parties prior to the Effective Date.
1.38 Dispute shall have the meaning set forth in Section 17.1.
1.39 Early Termination Date shall have the meaning set forth in Section 4.2(b).
1.40 Effective Date shall mean the date upon which this Agreement becomes effective and shall be the date first written above.
1.41 Intentionally Omitted.
1.42 Existing Confidentiality Agreement shall mean that certain Confidentiality Agreement between the Parties dated as of December 7, 2010.
1.43 Expiration Date shall have the meaning set forth in Section 3.1.
1.44 FDA shall mean the United States Food and Drug Administration and any successor agency thereto.
1.45 Force Majeure Event shall have the meaning set forth in Section 16.2.
1.46 GAAP shall mean Generally Accepted Accounting Principles in the United States.
1.47 Generic or “generic” shall mean a product approved pursuant to either [**] or [**].
1.48 Good Clinical Practice shall mean standards for the design, conduct, monitoring, auditing, recording, analysis, reporting of studies and clinical trials that provide assurance that the data and reported results are credible and accurate, and that the rights, integrity, safety, and confidentiality of subjects are protected. Good Clinical Practice includes the requirements of Title 21 of the U.S. Code of Federal Regulations, Parts 50, 54, 56, and 312, applicable FDA
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
guidance, and the International Conference on Harmonisation (ICH) E6 Good Clinical Practice: Consolidated Guidance.
1.49 Government Price Reporting shall have the meaning set forth in Section 7.5.
1.50 GSK shall have the meaning set forth in the preamble.
1.51 GSK First Promotion Date shall mean, subject to Section 2.2, the first date on which a GSK field sales representative first conducts a Product Detail after having received the training agreed in the Training Plan.
1.52 GSK Marketing Expenses shall have the meaning set forth in Section 5.8(a).
1.53 GSK Minimum Details shall have the meaning set forth in Section 5.5(c).
1.54 GSK Parties shall have the meaning set forth in Section 13.3.
1.55 GSK Policies shall mean the product promotional, compliance, scientific, anti-corruption and other related policies and procedures generally applicable to GSK that have been provided in writing to Auxilium and listed on Schedule 1.55 and such additional or modified written policies and procedures as are provided by GSK to Auxilium from time to time.
1.56 Incremental Marketing Spend shall have the meaning set forth in Section 5.8(b).
1.57 Incremental Net Sales shall mean the amount by which the Net Sales of the Product for a particular period exceeds the Baseline Net Sales Amount for such period.
1.58 Indemnitee shall have the meaning set forth in Section 13.5.
1.59 Indemnitor shall have the meaning set forth in Section 13.5.
1.60 Ineligible or Debarred Person shall mean an individual who (a) is currently excluded, debarred, suspended or otherwise ineligible to participate in a federal health care program or in federal procurement or nonprocurement programs, (b) to the knowledge of Auxilium or GSK, as applicable, has been convicted of a criminal offense that falls within the ambit of 42 U.S.C. § 1320a-7(a), but has not yet been excluded, debarred, suspended or otherwise declared ineligible, (c) is currently debarred under Subsection (a) or (b) of Section 306 of the Act, or (d) to the knowledge of Auxilium or GSK, as applicable, has been convicted of a criminal offense that makes the individual eligible for debarment under Subsection (a) or (b) of Section 306 of the Act, but has not yet been debarred.
1.61 Intellectual Property shall have the meaning set forth in Section 13.1(i).
1.62 Inventions shall have the meaning set forth in Section 9.1.
1.63 Joint Co-Promotion Activities shall have the meaning set forth in Section 5.5.
1.64 Joint Defense Agreement shall mean that certain Joint Defense Agreement by and between the Parties dated as of April 5, 2012.
1.65 Joint Steering Committee shall have the meaning set forth in Section 5.1.
1.66 Know-How shall mean all present and future information developed by Auxilium, whether or not in written form, that is not in the public domain or already rightfully known to GSK and that relates to the Product, including all biological, chemical, pharmacological, toxicological, medical or clinical, analytical, quality, manufacturing, research, or sales and marketing information as well as all processes, methods, procedures, techniques, plans, programs, and data and any other information relating to the Product or useful for the development or commercialization of the Product in the Territory.
1.67 Laws and Regulations shall mean all applicable statutes, ordinances, regulations, codes, rules or orders of any kind whatsoever of any governmental authority in the Territory, including the Act, the Generic Drug Enforcement Act of 1992 (21 U.S.C. § 335a et seq.), the Anti-Kickback Statute (42 U.S.C. § 1320a-7b et seq.), the Health Insurance Portability and Accountability Act of 1996 as amended by the Health Information Technology for Economic and Clinical Health Act, the Sunshine Act, the Federal False Claims Act (31 U.S.C. § 3729-3733), the Codes, the PDMA, the Department of Health and Human Services Office of Inspector General Compliance Program Guidance for Pharmaceutical Manufacturers, released April 2003, the Antifraud and Abuse Amendment to the Social Security Act (P.L. 95-142 amending the Social Security Act § 1128), the Social Security Act section regarding reporting of information relating to drug samples (the Social Security Act § 1128H), the Department of Veteran Affairs (“VA”) Final Rule regarding Drug and Drug-Related Supply Promotion by Pharmaceutical Company Representatives at VA Facilities (77 Fed Reg. 12997), the Public Health Service Act (42 U.S.C. § 254b et seq.), the Civil Monetary Penalties Statute (42 U.S.C. § 1320a-8), Health Care Fraud (18 U.S.C. § 1347), and False Statements Relating to Health Care Matters (18 U.S.C. § 1035), and any state or local laws impacting the promotion of pharmaceutical products, all as amended from time to time.
1.68 Minimum Details shall mean the GSK Minimum Details or the Auxilium Minimum Details, as applicable.
1.69 NDA shall mean a New Drug Application, as described in Section 505(a) of the Act, 21 U.S.C. § 355(a).
1.70 Net Sales shall mean the gross amount billed or invoiced on sales of the Product in the Territory to Third Parties, less only the following deductions (without any duplication), which are actually incurred, allowed, paid, accrued or specifically allocated to the extent that such amounts are deducted from gross invoiced sales amounts as reported by Auxilium, its Affiliates, licensees or agents, in its financial statements in accordance with GAAP, applied on a consistent basis:
(i) customary trade, quantity, prompt pay, and cash discounts;
(ii) fees to wholesalers based on shipment activity under the terms of wholesaler service agreements;
(iii) Product rebates and Product charge-backs and other price reduction programs granted to managed care entities, pharmaceutical benefit management service entities and government health plans;
(iv) redemption of Product coupons and co-pay assistance programs; and
(v) the allowances or credits to customers, in each case on account of rejection or return of the Product or on account of price reductions affecting the Product.
In the event that non-monetary consideration is received for the Product, Net Sales shall be calculated based on the average price charged for the Product during the preceding calendar quarter. In addition, Net Sales shall be determined on, and only on, the first sale by a Party or its Affiliates, licensees or agents to a Third Party.
If Auxilium chooses to sell the Product together with another Auxilium product with composite pricing, Net Sales for the affected Product will be recalculated using the prorated portion of the combined net selling price of the bundled products based on the relative net selling price for each of the individual products in such bundle (a) during the last quarter in which each such product was not sold as part of a bundle, or (b) if any such product continues to be individually sold, at each such product’s then-current net selling price. Where any increase or reduction in the invoice price or deduction therefrom is based on sales of a bundle of products in which the Product for use in the Territory is included, the increase or reduction in price or deduction therefrom would be allocated to such Product on a pro rata basis based on the sales value (i.e., the unit average selling price multiplied by the number of units) of such Product relative to the sales value contributed by the other products in the bundle with respect to such sale based on the relative net selling prices of such products (y) during the last quarter in which each such product was not sold as part of a bundle, or (z) if any such product continues to be individually sold, at such product’s then-current net selling price.
1.71 OIG shall mean the Office of the Inspector General of the U.S. Department of Health and Human Services and any successor office thereto.
1.72 Overall Marketing Plan shall mean the marketing plan attached hereto as Schedule 1.72. For the avoidance of doubt, the Overall Marketing Plan may only be amended or modified by written agreement of the Parties.
1.73 Party(ies) shall mean each of Auxilium and GSK.
1.74 PDMA shall mean the Prescription Drug Marketing Act of 1987, as incorporated in the Act, and the regulations promulgated thereunder, found in Title 21 of the U.S. Code of Federal Regulations Part 203.
1.75 Permitted Shortfall shall mean that (i) with respect to each of the [**] after [**], a shortfall in the number of Product Details required to be performed by a Party during such [**], not to exceed [**] of the Minimum Details to be performed by such Party during such [**], and (ii) for any [**] thereafter during the Term, a shortfall in the number of Product Details required
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
to be performed by a Party during such [**], not to exceed [**] of the Minimum Details to be performed by such Party during such [**].
1.76 PM Working Group shall have the meaning set forth in Section 5.5(d).
1.77 Prescriber shall mean a practitioner licensed under Laws and Regulations to administer or prescribe the Product.
1.78 Prime Rate means the US Prime Rate as published from time to time in The Wall Street Journal.
1.79 Product shall mean Auxilium’s Testim® 1% testosterone gel product for all indications approved by the FDA.
1.80 Product Detail(s) shall mean a face-to-face meeting, between (i) a professional representative of the applicable Party, who is not an Ineligible or Debarred Person and who meets minimum professional standards of [**], and (ii) a Prescriber, during which a promotional presentation of at least one of the Product’s attributes is orally presented in a manner consistent with the quality of such presentations made by a Party’s professional representatives for such Party’s other products. When used as a verb, “Product Detail” or “Product Detailing” shall mean to engage in the Product Detail. For purposes of clarity, a Product Detail shall not include a Sample drop or coupon drop by such a professional representative.
1.81 Product Manager shall have the meaning set forth in Section 5.5(h).
1.82 Promotion Conditions shall have the meaning set forth in Section 2.2.
1.83 Promotional Materials shall mean all written, printed, digital, electronic, audio-visual or other graphic advertising, promotional, educational and communication materials for marketing, advertising, promotion, sale and Product Detailing for use in the Territory, including disease awareness materials, coupons, materials related to patient assistance/reimbursement programs, public relation campaign materials, sales training and speaker training materials. Promotional Materials do not include: (a) public announcements or press releases made in accordance with Section 10.3, (b) periodic presentations to the investment community that include factual information regarding the Product that is not promotional in nature, or (c) Scientific Materials.
1.84 Promotional Payment shall have the meaning set forth in Section 4.1.
1.85 Quarterly Baseline Net Sales Amount shall mean the amount of Net Sales per calendar quarter for each Year as set forth in the Overall Marketing Plan. The Quarterly Baseline Net Sales Amount (a) may be adjusted upon mutual agreement of the Parties upon an [**] or [**] to become effective within thirty (30) days after the [**] or [**], and (b) for the first quarter after the Effective Date shall be adjusted ratably for the period from the GSK First Promotion Date to the last day of the first calendar quarter thereafter.
1.86 Reserved Matter shall have the meaning set forth in Section 5.3(b).
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
1.87 Samples shall mean Product that is provided free of charge as defined in section 503 of the Act.
1.88 Scientific Materials shall mean publications, papers, scientific posters and presentations, advisory board materials, written prepared responses to unsolicited requests for information related to indications for use of the Product that are not included in FDA-approved Product labeling, and similar materials used for specific educational purposes or in connection with medical affairs functions.
1.89 Specifications shall mean specifications for or concerning the manufacturing and testing of the Product which are utilized by Auxilium (or a Third Party on behalf of Auxilium) in the manufacture of the Product and as approved by the FDA.
1.90 Sunshine Act shall mean the Transparency Reports and Reporting of Physician Ownership or Investment Interests (42 U.S.C. § 1320a-7h) and all relevant regulations.
1.91 Tail Payment(s) shall have the meaning set forth in Section 4.2.
1.92 Term shall have the meaning set forth in Section 3.1.
1.93 Territory shall mean the United States of America and its territories and possessions, including Puerto Rico, and the District of Columbia.
1.94 Third Party(ies) shall mean any person or entity other than Auxilium and GSK or their Affiliates.
1.95 Training Plan shall mean a plan, in reasonable detail, for training the Auxilium and GSK sales representatives that shall be agreed to by the Parties within thirty (30) days after the Effective Date.
1.96 Unauthorized B-Rated Testim Generic Entry shall mean the date of the first [**] in the Territory by a Third Party (other than a person or entity related to, licensed or otherwise authorized by Auxilium) of a Generic version of the Product that is not A-rated by the FDA as listed in the publication Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book.
1.97 Unauthorized B-Rated Testim Generic Erosion Date shall mean the date on which there is a loss of [**] percent ([**]%) or more of the market share of the Product measured in [**] as reported by [**] for any [**] period following an Unauthorized B-Rated Testim Generic Entry, when compared to such market share measured in [**], as reported by [**], for the [**] period immediately prior to such Unauthorized B-Rated Testim Generic Entry. For the purposes of this Section 1.97, the market for the Product shall include any [**] testosterone [**] with the same or substantially similar indications for use to those of the Product.
1.98 Unauthorized A-Rated Testim Generic Entry shall mean the date of the first [**] by a Third Party (other than a person or entity related to, licensed or otherwise authorized by Auxilium) of a Generic version of the Product in the Territory that is A-rated by the FDA as
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
listed in the publication Approved Drug Products with Therapeutic Equivalence Evaluations, commonly known as the Orange Book.
1.99 Year shall mean January 1st to December 31st of each calendar year, except for 2012 which shall be the Effective Date to December 31, 2012 and for 2015 which shall be January 1, 2015 to September 30, 2015.
2. Co-Promotion
2.1 Grant of Rights. Auxilium grants to GSK the exclusive right to co-promote the sale of the Product in the Territory, subject to (i) Auxilium’s right to co-promote the Product in the Territory and (ii) all the other terms and conditions of this Agreement. Auxilium shall retain exclusively all of its rights with respect to the Product outside the Territory, and GSK shall have no rights with respect to the Product outside the Territory as a result of the execution or performance of this Agreement or otherwise. Auxilium shall not grant any rights to, permit or authorize any Third Party to promote, sell or Product Detail the Product in the Territory during the Term without GSK’s prior written consent. GSK shall have no other rights relating to the Product except as specified in this Agreement.
2.2 Promotion Conditions. GSK’s obligations to promote the Product and conduct Product Details hereunder shall be subject to the satisfaction of each of the following conditions precedent (the “Promotion Conditions”), in a manner acceptable to each Party in each Party’s sole and absolute discretion:
(a) Completion of the 2012 Annual Marketing Plan;
(b) Completion of the Training Plan and completion of all training activities contemplated by the Training Plan;
(c) Completion of the other significant Promotional Materials set forth on Schedule 2.2, which support the Core Sales Aid;
(d) Completion of the initial Annual Scientific Engagement Plan;
(e) Execution of a Pharmocovigilance Agreement in accordance with Section 7.2; and
(f) Ensure [**] that Auxilium maintains written policies and procedures describing Auxilium’s administrative systems with respect to drug sampling, as required by 21 CFR 203.34.
The Parties shall cooperate in good faith to complete each of the foregoing actions as promptly as practicable after the Effective Date.
2.3 Efforts. From and after the date that all of the Promotion Conditions are first satisfied, each of Auxilium and GSK shall deploy such of their respective sales forces, which, in the case of GSK, shall be GSK’s [**] field sales force consisting of approximately [**] sales representative positions as of the Effective Date, and, in the case of Auxilium, shall be
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
Auxilium’s Testim field sales force consisting of approximately 148 sales representative positions as of the Effective Date, and expend Commercially Reasonable Efforts to conduct the Product Details and promote the Product in accordance with the terms of this Agreement, as provided in the Overall Marketing Plan and each Annual Marketing Plan. Subject to Section 2.2, GSK shall be obligated to conduct the GSK Minimum Details and Auxilium shall be obligated to conduct the Auxilium Minimum Details. The Parties agree that each Party has the right to reconstitute their respective sales forces to meet their business needs. Subject to the preceding sentence, each Party agrees to use Commercially Reasonable Efforts to maintain their field sales force in such a manner and with as much consistency to enable them to fulfill their obligations under this Agreement.
2.4 Compliance with Law. Notwithstanding anything to the contrary contained herein, each of Auxilium and GSK agree that in conducting their activities hereunder, including promoting the Product and performing Product Details, such Party shall comply with (a) all Laws and Regulations, (b) the terms of this Agreement, (c) the [**] (assuming Auxilium does not exercise its termination rights under Section 3.2(b)(ii)), and (d) relevant provisions of the CIAs to which GSK is a party and that are attached hereto as Exhibit 2.4 or are otherwise provided in a timely manner and in writing to Auxilium (assuming Auxilium does not exercise its termination rights under Section 3.2(b)(ii)); provided that with respect to any CIA provisions [**] provided to Auxilium after the Effective Date, Auxilium’s obligations with respect to such CIA provisions [**] under this Section 2.4 shall apply only after Auxilium’s receipt of such CIA provisions [**]. Auxilium agrees that in conducting its activities hereunder, Auxilium shall comply with all Laws and Regulations related to Government Price Reporting obligations for the Product. No Party shall be required to undertake any obligation, participate in, or incur any cost or reimbursement obligation, in connection with any activity under this Agreement (i) that such Party believes, in good faith with the advice of counsel, may violate or conflict with any Laws and Regulations, (ii) that such Party believes, in good faith may violate or conflict with the GSK Policies (in the case of GSK) or any Auxilium product promotional, compliance, or other related policy or procedure (in the case of Auxilium), (iii) that such Party believes, in good faith may violate or conflict with any CIA, or (iv) with respect to the Product or any use thereof which such Party believes, in good faith, poses a material safety concern. Each Party agrees to maintain a compliance program consistent with Laws and Regulations with respect to its activities pursuant to this Agreement, including, as applicable, but subject to Section 7.1(f), reporting to the appropriate governmental entity all amounts spent on Product Detailing. Each Party acknowledges and agrees that it will, from time to time during the Term and at the request of the other Party, (y) participate in discussions with the other Party regarding changes in Laws and Regulations or in the business or regulatory environment that may impact Product Detailing, and (z) if necessary, enter into good faith negotiations to amend this Agreement as appropriate in order to comply with such changes.
3. Term, Termination and Renegotiation
3.1 Term. This Agreement shall commence on the Effective Date, and this Agreement and any licenses granted hereunder shall terminate at 11:59 p.m. Eastern Standard Time on September 30, 2015 (the “Expiration Date”), unless earlier terminated pursuant to Section 3.2 (the “Term”).
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
3.2 Early Termination.
(a) Each Party shall have the right to terminate this Agreement and any licenses granted hereunder before the end of the Term:
(i) immediately upon a material breach of this Agreement (excluding a breach of Section 5.5(c)) by the other Party where such breach is not cured within [**] ([**]) days following the other Party’s receipt of written notice of such breach;
(ii) upon [**] ([**]) days written notice in the event the FDA or any other governmental regulatory authority takes any action that [**] the Product, or the Parties’ rights to promote, and Auxilium’s right to sell, the Product, as evidenced by [**], in each case, in the Territory;
(iii) upon [**] ([**]) days written notice in the event there is any [**] for the Product in the Territory as evidenced by [**] in the Territory;
(iv) immediately upon the bankruptcy or insolvency, or the making or seeking to make or arrange an assignment for the benefit of creditors of the other Party, or the initiation of proceedings in voluntary or involuntary bankruptcy, or the appointment of a receiver or trustee of such Party’s property that is not discharged within [**] ([**]) days;
(v) immediately upon written notice delivered to the other Party following an [**];
(vi) immediately upon written notice if the Parties fail to agree on a new Annual Baseline Net Sales Amount, Base Auxilium Marketing Expenses, GSK Marketing Expenses, and Auxilium Sales Support Expenses to be effective within [**] ([**]) days after [**] as required by Section 5.10(a);
(vii) immediately upon written notice if the Parties fail to agree on a new Annual Baseline Net Sales Amount, Base Auxilium Marketing Expenses, GSK Marketing Expenses, and Auxilium Sales Support Expense to be effective within [**] ([**]) days after the [**], as required by Section 5.10(b).
(viii) upon [**] ([**]) days written notice in the event of (1) the filing of federal or state criminal charges against the other Party or one of the other Party’s directors, officers or senior management employees relating to activities taken in connection with this Agreement, the promotion or marketing of such Party’s products, or an alleged violation of the Act and/or Applicable Health Care Laws or (2) the felony conviction of the other Party or one of the other Party’s directors, officers or senior management employees relating to activities taken in connection with this Agreement, the promotion or marketing of such Party’s products, or an alleged violation of the Act and/or Applicable Health Care Laws;
(ix) upon [**] ([**]) days written notice delivered to the other Party upon the inability of a Party to perform its obligations under this Agreement due to a Force Majeure Event exceeding [**] days in [**];
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
(x) immediately upon written notice if the [**] of the Product for any [**] periods following the [**] fails to [**] a [**] for such [**] periods [**];
(xi) immediately upon written notice if all of the Promotion Conditions have not been satisified by December 31, 2012; or
(xii) immediately upon written notice of a breach of Section 5.5(c).
(b) Auxilium shall have the right to terminate this Agreement and any licenses granted hereunder:
(i) immediately upon written notice to GSK of GSK’s material breach of Section 2.4; or
(ii) upon [**] ([**]) days written notice delivered to GSK following (1) Auxilium’s receipt of any new CIA provisions or GSK Policies delivered by GSK to Auxilium under this Agreement that [**] under this Agreement, or (2) GSK’s entry into any other settlement agreement with any governmental authority that relates in any way to an alleged breach of Laws and Regulations by GSK with respect to the Product, in each case during the Term.
(c) GSK shall have the right to terminate this Agreement and any licenses granted hereunder:
(i) after the [**] anniversary of the Effective Date, for any reason upon [**] days prior written notice to Auxilium (which notice may not be given prior to the [**] anniversary of the Effective Date);
(ii) immediately upon prior written notice to Auxilium in the event [**];
(iii) upon [**] ([**]) days written notice delivered to Auxilium following Auxilium’s inability to supply the Product for [**] ([**]) days [**] during any [**] period;
(iv) immediately upon written notice to Auxilium of Auxilium’s material breach of Section 2.4;
(v) upon [**] ([**]) days written notice delivered to Auxilium following (1) Auxilium’s entry into any CIA that [**] under this Agreement or (2) Auxilium’s entry into any other settlement agreement with any governmental authority that relates in any way to an alleged breach of Laws and Regulations by Auxilium with respect to the Product, in each case during the Term;
(vi) immediately upon written notice to Auxilium upon the occurrence of [**];
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
(vii) upon [**] ([**]) days notice to Auxilium in the event of a Change of Control.
4. Fees and Payments
4.1 Payment on Net Sales. Subject to Section 5.8(a), Auxilium shall pay GSK, in consideration for the performance of its obligations hereunder, sixty-five percent (65%) of Incremental Net Sales (the “Promotional Payment”). The amount of such Promotional Payment shall be determined and paid on a quarterly basis, and shall be calculated following each of the first three (3) calendar quarters of a Year by (A) subtracting the Quarterly Baseline Net Sales Amount from the Net Sales for such calendar quarter, and (B), if such amount is a positive number, multiplying such amount by sixty-five percent (65%). No Promotional Payment for a calendar quarter shall be made if the Quarterly Baseline Net Sales Amount exceeds the Net Sales for such quarter. The Promotional Payment to be paid by Auxilium following the fourth calendar quarter of each Year (or upon any termination of this Agreement that is effective other than at the end of a Year) shall be calculated by (X) subtracting the Annual Baseline Net Sales Amount from the Net Sales for such Year (or partial Year), and then (Y) multiplying such amount by sixty-five percent (65%), and then (Z) subtracting from such amount the aggregate amount of all Promotional Payments paid by Auxilium following the first three calendar quarters during such Year (or partial Year) (the “Reconciliation Amount”). In the event the Reconciliation Amount is a negative number, upon submission by Auxilium of a written invoice to GSK, GSK shall pay to Auxilium the Reconciliation Amount within sixty (60) days following receipt of such invoice, unless GSK disputes the calculation of the Reconciliation Amount, and in such case, GSK shall pay to Auxilium all undisputed amounts within such sixty (60) day period. For clarity, in no event shall the Reconciliation Amount to Auxilium be greater than the previous three (3) Promotional Payments paid by Auxilium to GSK resulting in a net payment to Auxilium for such Year. The calculation of all Promotional Payments and all Reconciliation Amounts shall include reductions for GSK Marketing Expenses and any Incremental Marketing Spend as set forth in Sections 5.8(a) and 5.8(b). Subject to Auxilium’s receipt of a written invoice from GSK, all Promotional Payments required to be paid by Auxilium shall be paid by Auxilium by the later of (a) sixty (60) days following the end of each calendar quarter and (b) fifteen (15) days after GSK has submitted a written invoice to Auxilium. In the event that this Agreement is terminated other that at a Year end, the amount of any Promotional Payment owed to GSK will be calculated by subtracting the pro-rated Quarterly Baseline Net Sales Amount from the pro-rated Net Sales for such quarter, based on the number of days that have elapsed during such quarter immediately prior to the effective date of such termination.
4.2 Tail Payments. Except as otherwise provided in this Section 4.2, GSK shall be entitled to receive the following additional payments from Auxilium (each a “Tail Payment,” and together the “Tail Payments”):
(a) in the event there is [**] of this Agreement, (i) for each of the first four (4) three-calendar-month periods following the Expiration Date, the lesser of (1) ten percent (10%) of Net Sales for such three-month period and (2) thirty seven and one half (37.5%) of the Promotional Payment paid by Auxilium with respect to the corresponding three-month period in the 12-month period immediately preceding the Expiration Date, and (ii) for each of the fifth
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
through eighth three-calendar-month periods following the Expiration Date, the lesser of (1) eight percent (8%) of the Net Sales for such three-month period and (2) twenty seven and one half (27.5%) of the Promotional Payments paid by Auxilium with respect to the corresponding three-month period in the 12-month period immediately preceding the Expiration Date; or
(b) in the event this Agreement is terminated (1) [**] pursuant to Section [**] at any time, (2) by [**] pursuant to [**] after the [**] or (3) by [**] after the [**] due to [**] (the “Early Termination Date”), then for each of the first four (4) three-calendar-month periods following the Early Termination Date, the lesser of (x) five percent (5%) of the Net Sales for such three-month period and (y) twenty percent (20%) of the Promotional Payment made by Auxilium with respect to the corresponding three-month period in the 12-month period immediately preceding the Early Termination Date; provided, however that, no Tail Payment shall be owed to GSK if [**] terminates this Agreement at any time pursuant to Section [**] as the result of [**].
Unless specifically required under Section 4.2(a) or 4.2(b), no Tail Payment shall be owed by Auxilium under this Agreement.
Notwithstanding any other provision of this Section 4.2, Auxilium shall not be obligated to pay any Tail Payment with respect to any period after the date, if any, on which GSK first promotes, markets, or sells [**].
The amount of such Tail Payment shall be determined and paid on a quarterly basis. Subject to Auxilium’s receipt of a written invoice from GSK, all Tail Payments required to be paid by Auxilium shall be paid by Auxilium by the later of (a) sixty (60) days following the end of each calendar quarter and (b) fifteen (15) days after GSK has submitted a written invoice to Auxilium.
4.3 Past Due Amounts. Either Party’s failure to make any required and undisputed payment to the other Party under this Agreement will constitute a material breach of this Agreement by such Party if such amount remains unpaid [**] after such Party receives written notice from the other Party that such amount has not been paid when due. All amounts owing by a Party to the other Party pursuant to this Agreement that are not timely paid by such Party will bear interest from the due date at an annual interest rate equal to the lesser of (i) the Prime Rate plus [**] percent ([**]%) or (ii) the highest rate permissible by Laws and Regulations, with such interest accruing from the date the payment was originally due, and any late payment pursuant to this Section shall be credited first to interest and then to any outstanding amounts owing hereunder. Such interest will not prejudice any rights and remedies of the other Party hereunder or under Laws and Regulations.
4.4 Effect of Termination on Payments. The termination or expiration of this Agreement shall not affect either Party’s obligation to reimburse or pay the other Party any amount due and owing under this Agreement through the effective date of such termination or expiration.
4.5 Payments. All payments under this Agreement shall be in U.S. dollars in immediately available funds, and, unless instructed otherwise by the receiving Party, shall be made via wire transfer to the account designated from time to time by the receiving Party.
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
4.6 Taxes. All payments required to be paid to a Party pursuant to this Agreement shall be paid without deduction therefrom for withholding for, or on account of, any sales tax, use tax, or other similar tax (other than taxes imposed on, measured by, or credited against net income). Unless otherwise required by Laws and Regulations, each Party shall be responsible for paying and reporting all of its own taxes and fees, including income taxes, payroll taxes, franchise taxes and all taxes and fees in connection with the Party conducting business in any jurisdiction.
4.7 Reports.
(a) Within forty-five (45) days following the end of each of the first three (3) calendar quarters of each Year during the Term, Auxilium shall provide GSK with a written statement containing a calculation of the Net Sales for such quarter and for the year-to-date period through the end of such quarter and a calculation of the Promotional Payment for such quarter, with reasonable supporting detail.
(b) Within fifty (50) days following the end of each calendar Year of the Term and within fifty (50) days following the end of each partial calendar Year of the Term upon expiration or earlier termination of the Agreement, Auxilium shall provide GSK with a written statement containing a calculation of the Net Sales for such Year and a calculation of the Promotional Payment and the Reconciliation Amount for such Year pursuant to Section 4.1, with reasonable supporting detail.
(c) Within forty-five (45) days following the end of each quarter for which a Tail Payment is owed, Auxilium shall provide GSK with a written statement of the Net Sales for the calendar quarter and the amount of the Promotional Payment for the corresponding calendar quarter during the 12-month period immediately preceding the Expiration Date or Early Termination Date, as applicable, and a calculation of the Tail Payment for such quarter pursuant to Section 4.2.
5. Co-Promotion Management
5.1 Joint Steering Committee. Immediately following the Effective Date, Auxilium and GSK shall assemble a team of appropriate personnel from both Auxilium and GSK (the “Joint Steering Committee”) to plan and oversee the commercialization, promotional and scientific engagement practices relating to the Product in accordance with this Agreement and to periodically discuss any Compliance Disputes that may be referred to the Joint Steering Committee by the Compliance Committee, subject to Section 5.3(b). The Parties will have the opportunity, through the Joint Steering Committee, to confer with each other on such sales, marketing, and promotional matters, and the Joint Steering Committee will have the ultimate decision making power with respect to the matters set forth in Section 5.2. The Joint Steering Committee shall consist of an equal number of representatives from each of Auxilium and GSK, not to be less than four (4) representatives and not to exceed a total of eight (8) representatives. A Party may change any of its representatives on the Joint Steering Committee at any time by giving written notice to the other Party; provided, however, that, without limiting the foregoing, the Parties acknowledge that a key objective with respect to membership in the Joint Steering Committee shall be preserving continuity. Auxilium shall designate one (1) of its representatives
on the Joint Steering Committee to serve as the chairperson of the Joint Steering Committee. The Joint Steering Committee may, from time to time, include additional non-voting ad-hoc representatives from either Party on specific issues as the need arises. As appropriate, the Joint Steering Committee may establish one or more sub-committees to consider matters that may arise between meetings of the Joint Steering Committee or matters that would be more effectively considered by a smaller committee. Such sub-committees, however, shall have no authority to bind the Parties.
5.2 Responsibilities of the Joint Steering Committee. In addition to the other functions and responsibilities of the Joint Steering Committee provided for in this Agreement, the Joint Steering Committee shall perform the following functions, subject to compliance with Section 2.4 in all respects:
(a) Reviewing available quarterly gross sales, quarterly Net Sales, and other Product performance data as determined by the Joint Steering Committee;
(b) Reviewing and approving new Promotional Materials or changes to existing Promotional Materials, subject to Section 5.5(d);
(c) Reviewing coupon demand forecast and data on coupon usage / redemption;
(d) Reviewing the efforts of sales representatives employed by, or on behalf of, GSK and Auxilium who engage in Product Details and other promotional efforts with respect to the Product to maintain desired frequency of Product Details and to avoid unintended duplication of Product Details;
(e) Subject to Section 2.2, reviewing and approving the Annual Marketing Plan for the Product, including appropriate budgets for the provision of Samples;
(f) Subject to Section 2.2, reviewing and approving the Annual Scientific Engagement Plan;
(g) Reviewing the Parties’ respective training needs and training schedule;
(h) Discussing Compliance Disputes referred to the Joint Steering Committee by the Compliance Committee;
(i) Establishing monthly reporting forms related to each Party’s Product Detailing;
(j) Discussing any changes to Product pricing;
(k) Discussing the progress and results of any studies relating to the Product;
(l) Reviewing medical inquiry procedures related to the Product that have been established by the Compliance Committee;
(m) Discussing any events affecting the integrity or reputation of the Parties, in accordance with Section 7.8;
(n) Discussing and approving any Auxilium Trademarks for use with the Product pursuant to Section 9.7; and
(o) Performing such other responsibilities as may be mutually agreed upon by the Parties in writing from time to time; provided, however, that, notwithstanding anything to the contrary in this Agreement, the Joint Steering Committee shall have no authority to (1) amend any provision of this Agreement; (2) determine any dispute related to the interpretation or performance of this Agreement; or (3) increase (with respect to GSK) or decrease (with respect to Auxilium) any obligation of the Parties, financial or otherwise.
5.3 Voting Procedures of Joint Steering Committee.
(a) Each Party shall have one (1) vote on all matters. In the event that the members of the Joint Steering Committee are unable to agree on any matter, such matter shall be submitted to the Chief Executive Officer of Auxilium and the GSK President of North America Pharmaceuticals, or their designees, who shall meet in person, by videoconference or by telephone within five (5) Business Days to review and resolve such matter. If such executives of the Parties, using good faith efforts, are not able to resolve such matter, the chairperson of the Joint Steering Committee shall make a final decision with respect to such matter that shall be binding on the Parties, subject to GSK’s applicable termination rights set forth in Section 5.3(b). For the avoidance of doubt, the chairperson of the Joint Steering Committee shall not have the authority to make a final decision related to those matters listed in Section 2.2.
(b) Notwithstanding anything to the contrary contained in this Agreement, GSK shall have the [**] rights set forth in Section [**] if a decision by the Compliance Committee or the Joint Steering Committee is made without the consent of GSK and such decision results in: (i) [**], (ii) [**], or (iii) [**] (each a “Reserved Matter”).
5.4 Meetings of the Joint Steering Committee. Meetings of the Joint Steering Committee shall be held monthly for the first three months after the Effective Date, with the first meeting to occur within thirty (30) days following the Effective Date, and quarterly thereafter or more often as determined by the Joint Steering Committee. All meetings may be held by teleconference or videoconference so long as all representatives participating in the meeting can hear one another. The Parties will use commercially reasonable efforts to conduct at least one (1) face-to-face meeting of the Joint Steering Committee annually. Minutes of each Joint Steering Committee meeting shall be transcribed and issued by a designee of the Joint Steering Committee within ten (10) Business Days after each meeting and shall be approved by the chairperson not later than the first order of business at the immediately succeeding Joint Steering Committee meeting. Each Party shall bear its own expenses in connection with attending meetings of the Joint Steering Committee. The Joint Steering Committee shall also establish a procedure for either (a) calling special interim meetings of the Joint Steering Committee in the event a need for Joint Steering Committee decisions arises between regularly scheduled meetings or (b) establishing a process for making such interim decisions.
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
5.5 Joint Co-Promotion Activities. Subject to satisfaction of the Promotion Conditions in Section 2.2, Section 2.4 and the Overall Marketing Plan, each of Auxilium and GSK shall be responsible for performing the co-promotion activities described below (the “Joint Co-Promotion Activities”), in each case in accordance with the minimum activity levels established in this Agreement and in each Annual Marketing Plan. Accordingly, except as otherwise provided in this Agreement, each Party shall fully bear all of the costs incurred by such Party in connection with such Party’s performance of the Joint Co-Promotion Activities.
(a) Annual Marketing Plan. Not less than ninety (90) days prior to the commencement of each Year, Auxilium shall share a draft of the Annual Marketing Plan with GSK. Thereafter, in consultation with GSK through the Joint Steering Committee and subject to the terms of this Agreement, Auxilium will prepare the final Annual Marketing Plan for each Year, which will be presented to the Joint Steering Committee for its review and approval not less than thirty (30) days prior to the commencement of such Year. Although the Parties intend that the Annual Marketing Plan will be more detailed in scope than the Overall Marketing Plan, each Annual Marketing Plan shall be consistent with the financial and other metrics set forth in the Overall Marketing Plan unless otherwise agreed by the Parties. In the event there is any inconsistency between any Annual Marketing Plan and the Overall Marketing Plan that is not agreed to by the Parties, the Overall Marketing Plan shall control. Each Party shall have the sole and exclusive right to determine how to carry out its responsibilities under each Annual Marketing Plan, subject to Section 5.5(b) and 5.8(a).
(b) Field Sales. The Parties shall mutually agree upon the target audience and the monthly call reach and frequency objectives for each of the Parties’ sales organizations, and will agree upon which physicians each Party’s sales representatives will call upon, and the Parties will use their Commercially Reasonable Efforts to implement such agreements. All healthcare providers in the target audience must be reasonably expected to write prescriptions for an approved use of the Product as it is indicated in the FDA-approved labeling.
(c) Product Details. Each Annual Marketing Plan will provide that (i) GSK will be obligated to perform no fewer than [**] Product Details per [**], which shall be distributed evenly throughout [**] of a [**] subject to the provisions of this Section 5.5(c) and prorated for any partial [**] in the Term (“GSK Minimum Details”), and (ii) Auxilium will be obligated to perform no fewer than [**] Product Details per [**], which shall be distributed evenly throughout [**] of a [**] subject to the provisions of this Section 5.5(c) and prorated for any partial [**] in the Term (“Auxilium Minimum Details”). Each Party shall have [**] to cure any Permitted Shortfall that occurs in any [**], but at least [**] ([**]) of any Permitted Shortfall must be made up in the [**] immediately following the [**] in which the Permitted Shortfall occurs. Product Details performed by a Party in a given [**] shall first be applied to the Minimum Details required for such [**]. Notwithstanding the foregoing, by the Expiration Date, each Party shall have performed the Minimum Details without any right to cure a Permitted Shortfall that exists as of the Expiration Date. Each Party shall maintain records of each Product Detail by its sales force representatives using a call document that records the name and address of the member of the target audience and the date and position of the Product Detail, and shall supply a monthly record of the number of Product Details performed to the other Party within sixty (60) days of the end of each month in a form to be established by the Joint Steering Committee.
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
(d) Promotional Materials. From and after the date that all of the Promotion Conditions are first satisfied and during the Term, each Party shall promote the Product using only the Core Sales Aid, those Promotional Materials that are pre-approved and listed on Schedule 2.2, or other Promotional Materials approved pursuant to this Section 5.5(d), and, in each case, provided by Auxilium, at Auxilium’s expense in accordance with Section 5.8(a). Immediately following the Effective Date, the Parties shall form a joint Promotional Materials working group (the “PM Working Group”), comprised of representatives of Auxilium and of GSK, whose responsibility shall be to oversee the creation of any new, or changes to, Promotion Materials. Any compliance matters brought before the PM Working Group shall be unanimously approved by the Parties prior to submitting such matter to the Joint Steering Committee and all other matters shall ultimately be decided by Auxilium. The Promotional Materials must comply with (i) all Laws and Regulations, (ii) the [**], and (iii) relevant provisions of the CIAs that are attached hereto as Exhibit 2.4 or are otherwise provided in a timely manner to Auxilium in accordance with Section 2.4. The Promotional Materials used by field sales force representatives may not include any pricing information (i.e., wholesale acquisition costs or any discounts, rebates, or other price concessions) regarding the Product.
(e) Samples. The Joint Steering Committee shall determine the appropriate budget for the provision of Samples annually. All Sample distribution and reporting shall be conducted by Auxilium in accordance with Laws and Regulations. GSK shall comply with Auxilium’s procedures for requesting shipment of Samples of Product to physicians, which written procedures are attached hereto as Exhibit 5.5(e). Auxilium shall use Commercially Reasonable Efforts to fulfill requests for shipments of Samples in accordance with each Annual Marketing Plan. Auxilium will maintain written procedures to ensure compliance with all Laws and Regulations relating to the distribution of, and accountability for, Samples. This compliance includes obtaining written requests, obtaining the licensed healthcare professional’s signature for all Samples delivered, ensuring validity of the practitioner’s state license and storage of all Samples under Auxilium’s control at label conditions.
(f) Labeling. The Parties shall promote the Product in compliance with the FDA-approved product labeling and all written, printed or graphic material affixed to the container or physically accompanying the Product. Auxilium shall promptly notify GSK of all Product labeling modifications, but in any event within one (1) Business Day of any such modification.
(g) Sales Force Training. Each Party shall train (or have trained) its own sales force and provide periodic on-going training on the Product to its sales force in accordance with the Training Plan. Such training will address, at a minimum, compliance with Laws and Regulations and [**]. No sales representative employed by either Party will Product Detail without having undergone such training. Each Party shall bear its own expenses in connection with all training efforts and meetings for its sales force. Auxilium shall (1) provide to GSK copies of training materials, (2) up to [**] per [**], at GSK’s reasonable request, train a reasonable number of GSK sales force trainers, in each case to the extent commensurate with Auxilium’s internal training efforts, and (3) reasonably in advance thereof, notify GSK of any Auxilium internal Product or compliance training efforts so as to permit GSK, at GSK’s expense, to attend and participate in any such internal training efforts. GSK shall provide to Auxilium copies of its training materials relating to compliance matters, and shall up to [**] per [**] train a
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
reasonable number of Auxilium sales force trainers in compliance policies and procedures, in each case to the extent commensurate with GSK’s internal training efforts with respect to such policies and procedures. Following this initial training, each Party shall periodically make available its trainers to train the other Party’s trainers upon reasonable request by such Party, and solely to the extent commensurate with their internal training efforts.
(h) Product Manager. Auxilium and GSK will each identify an individual with appropriate authority to serve as the primary contact with the other Party about the Product and the Parties’ relationship under this Agreement (each a “Product Manager”). Each Party’s Product Manager will generally be responsible for overseeing the day-to-day activities of each of the respective Parties with respect to the commercialization of the Product and coordinating each respective Party’s internal resources in order to facilitate collaboration between the Parties under this Agreement.
(i) Communications with Field Sales Representatives. Each Party shall only communicate with designated representatives of the other Party (including the members of the Joint Steering Committee and the other Party’s Product Manager). Notwithstanding the foregoing, nothing herein shall restrict the field sales teams from communicating directly with one another.
5.6 Other Co-Promotion Matters.
(a) Booking of Sales Revenue. Auxilium shall record on its books all revenue from gross and Net Sales of the Product, which shall be recorded in accordance with GAAP.
(b) Packaging. Auxilium shall have the responsibility for selecting and approving all packaging materials, subject to Laws and Regulations and [**].
(c) Trademarks. Auxilium shall approve the names, design, and usage in the Territory of all Auxilium Trademarks associated with the Product.
(d) Field Sales Representatives. Except with the prior written consent of the other Party, all sales representatives who are responsible for Product Details shall be employees of GSK or Auxilium, as applicable. All sales representatives employed by each Party who are responsible for Product Details shall not be Ineligible or Debarred Persons and shall have any and all licenses, permits, and insurance or other coverage as may be required to enable the sales representatives to perform under this Agreement. All sales representatives shall be hired and recruited by each Party in accordance with Laws and Regulations. Prior to any GSK or Auxilium sales representative performing a Product Detail, each Party will ensure that such individuals (a) have not been listed (1) as an excluded Person on the Office of Inspector General’s List of Excluded Individuals/Entities (2) on the General Services Administration Excluded Parties List, (3) FDA Clinical Investigator enforcement lists, including the Disqualified/Totally Restricted List, Restricted List, or Adequate Assurances List, or (4) FDA Debarment List (b) have passed a criminal background check conducted by a firm reasonably selected by a Party, (c) have not been debarred, nor be currently under investigation by the FDA for debarment action or pursuant to the Generic Drug Enforcement Act of 1992 (21 U.S.C. § 335a et seq.), (d) have passed a drug screening test administered by a firm reasonably selected
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
by a Party, (e) have successfully passed a driving record screening conducted by a firm reasonably selected by a Party, and (f) have been evaluated under all other procedures required by [**] background screening process, a copy of which is attached to this Agreement as Exhibit 5.6(d).
(e) Status of Field Sales Representatives. Each Party acknowledges and agrees that its sales representatives are not employees of the other Party or any of the other Party’s Affiliates, and that such individuals are not, and are not intended to be, eligible to participate in any benefits programs or in any “employee benefit plans” (as such term is defined in section 3(3) of ERISA) that are sponsored by the other Party or the other Party’s Affiliates or that are offered from time to time by the other Party or the other Party’s Affiliates to its own employees (the “Benefit Plans”). All matters of compensation, benefits and other terms of employment for each Party’s sales representatives shall be solely a matter between GSK and GSK’s sales representatives and Auxilium and Auxilium’s sales representatives. Each Party shall not be responsible to the other Party (or the other Party’s sales representatives) for any compensation, expense reimbursements or benefits (including vacation and holiday remuneration, healthcare coverage or insurance, life insurance, severance or termination of employment benefits, pension or profit-sharing benefits and disability benefits), payroll-related taxes or withholdings, or any governmental charges or benefits (including unemployment and disability insurance contributions or benefits and workmen’s compensation contributions or benefits) that may be imposed upon or be related to the performance by the other Party (or the other Party’s sales representatives), all of which shall be the sole responsibility of GSK with respect to GSK’s sales representatives or Auxilium with respect to Auxilium’s sales representatives, as applicable, even if it is subsequently determined by any court or governmental agency that any such individual may be an employee or a common law employee of the other Party or any of the other Party’s Affiliates or is otherwise entitled to such payments and benefits.
(f) Sales Incentive Plan. Each Party may use its own method of compensating its sales representatives throughout the Term of this Agreement, subject to such Party’s compliance with all Laws and Regulations.
5.7 Annual Scientific Engagement Plan. In consultation with GSK through the Compliance Committee and subject to the terms of this Agreement, Auxilium will prepare the Annual Scientific Engagement Plan for each Year, which will be presented to the Joint Steering Committee for its approval not less than thirty (30) days prior to the commencement of such Year.
5.8 Expenses.
(a) Each party shall bear the full cost of its respective sales force. Auxilium shall solely incur and be responsible for paying the costs (but not GSK’s sales force costs) for the marketing of the Product, subject to and in accordance with the budgets provided in each Annual Marketing Plan. In addition to those marketing expenses that will be incurred by Auxilium in Auxilium’s continued marketing of the Product in accordance with the Overall Marketing Plan (the “Base Auxilium Marketing Expenses”), additional amounts related to marketing expenses shall be paid by Auxilium and allocated to support GSK’s promotion of the Product (the “GSK Marketing Expenses”) and shall be agreed each year by the Parties in the Annual Marketing
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
Plan; provided, however, that (i) expenses will not constitute GSK Marketing Expenses in any applicable period unless and until Auxilium has incurred all of the Base Auxilium Marketing Expenses for such period, and (ii) in the absence of a written agreement by the Parties, the GSK Marketing Expenses shall be set at $[**] per [**] pro rated according to [**] (or such pro rata amount of $[**] for a [**] that includes less than [**]). The GSK Marketing Expenses, determined quarterly, but not the Base Auxilium Marketing Expenses, shall be subtracted from the Incremental Net Sales amount before calculating each quarterly Promotional Payment due to GSK.
(b) In the event that GSK requests, including through its field sales force representatives and including requests for Samples, that Auxilium spend more (or otherwise causes Auxilium to incur more) than the budgeted amount of the Base Auxilium Marketing Expenses and the budgeted amount of the GSK Marketing Expenses for any period, and the Parties do not mutually agree to increase the GSK Marketing Expenses as provided in the Annual Marketing Plan (such additional amount that is not agreed to, the “Incremental Marketing Spend”), the Promotional Payment for such period shall be reduced by the amount of the Incremental Marketing Spend incurred during such period.
(c) Within twenty (20) Business Days following the end of each calendar month during the Term, Auxilium will provide GSK with a report setting forth the amounts, if any, of Base Auxilium Marketing Expenses, GSK Marketing Expenses and Incremental Marketing Spend incurred during such month and for the Year to date.
(d) From and after the Effective Date and for so long as there is no [**], with respect to each [**] during the Term Auxilium shall incur and spend, and be solely responsible for, a minimum amount equal to the Auxilium Sales Support Expenses in support of the sale of the Product by the Parties. Such amount shall be prorated for any partial calendar Year in the Term.
5.9 Pricing. Auxilium shall determine pricing for the Product. Auxilium shall notify GSK of all changes in the price of the Product within three (3) Business Days of any change. Notwithstanding the foregoing, Auxilium shall not make any material changes to the pricing assumptions of the Product as set forth in the Overall Marketing Plan without prior discussion at the Joint Steering Committee.
5.10 Amendment to Annual Baseline Net Sales Amount.
(a) Promptly following [**], the Parties shall negotiate in good faith a revised Annual Baseline Net Sales Amount (and the applicable Quarterly Baseline Net Sales Amounts), Base Auxilium Marketing Expenses, GSK Marketing Expenses, and Auxilium Sales Support Expenses to become effective no later than thirty (30) days after the [**].
(b) Promptly following [**], the Parties shall negotiate in good faith a revised Annual Baseline Net Sales Amount (and the applicable Quarterly Baseline Net Sales Amounts), Base Auxilium Marketing Expenses, GSK Marketing Expenses, and Auxilium Sales Support Expenses to become effective no later than thirty (30) days after the [**].
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
5.11 Compliance Committee.
(a) Immediately following the Effective Date, the Parties shall form a Compliance Committee (the “Compliance Committee”) whose responsibility shall be to, subject to Section 2.4 in all respects: (i) oversee compliance activities relating to (1) the Parties’ commercialization efforts under this Agreement and (2) the Annual Scientific Engagement Plan, (ii) advise the Joint Steering Committee on compliance-related issues and compliance monitoring activities, (iii) oversee and monitor the Parties’ compliance training efforts as they relate to the Product, and (iv) subject to Section 5.3(b), consider and attempt to resolve any dispute concerning issues related to compliance with Laws and Regulations, GSK Policies, Auxilium policies and procedures or any CIA and arising out of promotional and Product Detailing activities, including sales, marketing, Product Samples, medical affairs and training practices, sales training materials, the contents of coupons, patient assistance/reimbursement programs, Promotional Materials, Scientific Materials and training materials, and any related FDA or other governmental reporting in the Territory (each such dispute, a “Compliance Dispute”).
(b) The Compliance Committee shall consist of two (2) representatives from each of Auxilium and GSK. Auxilium shall designate one (1) of its representatives on the Compliance Committee to serve as its chairperson. The Compliance Committee may from time to time include additional non-voting ad-hoc representatives from either Party on specific issues as the need arises. Each Party shall have one (1) vote on all matters. In the event that the members of the Compliance Committee are unable to agree on any matter set forth in Section 5.11(a) above, such matter shall be submitted to the Joint Steering Committee for resolution, subject to Section 5.3(b).
(c) Meetings of the Compliance Committee shall be held at least once per quarter commencing with the first meeting to occur within thirty (30) days following the Effective Date or at such other frequency as is determined by the Compliance Committee. The location of Compliance Committee meetings and whether to hold them via teleconference and/or videoconference or in person shall be determined by the Compliance Committee, provided that one Party does not bear an undue travel burden. Each Party shall bear its own expenses in connection with attending meetings of the Compliance Committee. The Compliance Committee shall also establish a procedure for either (i) calling special interim meetings of the Compliance Committee in the event a need for Compliance Committee decisions arises between regularly scheduled meetings or (ii) establishing a process for making such interim decisions.
6. Manufacturing and Supply
6.1 Auxilium Responsibilities.
(a) Auxilium shall have, at its sole cost and expense, the sole responsibility for the manufacture, fill, finish, supply, testing, labeling, packaging, release, storage and delivery of the Product. Auxilium shall use its Commercially Reasonable Efforts to manufacture or have manufactured and to supply or have supplied the quantities of the Product required to meet market demand in the Territory during the Term.
(b) Auxilium warrants that Product for sale or distribution in the Territory shall be manufactured, filled, finished, packaged, labeled, tested, released, stored, distributed, sold and offered for sale in compliance with the Product NDA, Specifications, cGMP, all Laws and Regulations, and the requirements of this Agreement. Auxilium further warrants that Product has not been, is not and shall not be adulterated or misbranded within the meaning of the Act.
(c) Auxilium shall ship Samples of the Product directly to physicians in accordance with the annual budget for the provision of Samples in each Annual Marketing Plan and in accordance with Auxilium’s current practices and procedures and Laws and Regulations. The Parties will maintain all records required by Laws and Regulations in respect to Samples and, subject to receipt of necessary information from GSK, Auxilium shall be solely responsible for the filing of any necessary reports to the FDA, as required by Laws and Regulations.
7. Regulatory, Safety and Surveillance
7.1 Regulatory Matters.
(a) Responsibility. All regulatory matters in the Territory regarding the Product shall remain under the exclusive control of Auxilium, including responsibility for all communications with the FDA related to the Product, and Auxilium shall have sole responsibility to seek and/or obtain any necessary approvals of any label, labeling (including changes thereto), package inserts, and Promotional Materials used in connection with the Product in the Territory, and for determining whether the same requires approval. Auxilium shall have sole responsibility for the submission of Promotional Materials to the FDA’s Office of Prescription Drug Promotion. Auxilium shall ensure that the incidence, severity and/or nature of Adverse Events are accurately reflected in the package inserts for the Product to the extent required under, and in accordance with, Laws and Regulations.
(b) Permits. Auxilium shall be responsible for and maintain all regulatory and governmental permits, licenses and approvals that may be necessary to manufacture, ship, sell, store and market the Product in the Territory.
(c) Reporting. Auxilium shall be responsible for any reporting of matters, or other communications with the FDA, regarding the Product, including Adverse Events, to the FDA and other relevant regulatory authorities, in accordance with Laws and Regulations. Auxilium shall provide GSK with copies of: [**] submitted to the FDA regarding the Product, within [**] ([**]) [**] submitted by Auxilium to the FDA, within [**] ([**]) [**], with redactions of any Auxilium proprietary information unrelated to this Agreement, including [**] and related to the Product in the Territory, within [**] ([**]) [**] and related to the Product within [**] ([**]) [**] and related to the Product within [**] ([**]) [**]. Auxilium shall otherwise provide GSK with copies of its reports to the FDA relating to the Product promptly upon request of GSK. GSK shall promptly, but in no event later than one (1) Business Day, notify Auxilium of any Adverse Event of which it should become aware.
(d) Actions by Governmental Authorities. Auxilium shall promptly, but in no event later than [**], notify GSK of any FDA inspections, proposed regulatory actions,
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
investigations or material requests, and any corrective actions initiated by Auxilium with the FDA, in each case, relating to the Product, and Auxilium shall provide GSK with [**].
(e) Compliance with Risk Evaluation and Mitigation Strategy. Auxilium shall be responsible for complying with the requirements of the Risk Evaluation and Mitigation Strategy (the “REMS”) for the Product as updated from time to time and shall, through the Compliance Committee, discuss the REMS, and any changes thereto, with GSK. GSK shall comply with any requirements of the REMS that are applicable to its activities hereunder.
(f) GSK Involvement. Subject to Section 7.4, GSK shall not, without the prior written consent of Auxilium, unless so required by Laws and Regulations, correspond or communicate with the FDA or with any other governmental authority, concerning the Product, or otherwise take any action concerning any authorization or permission under which the Product is sold. GSK shall provide to Auxilium, promptly upon receipt, copies of any communication from the FDA or other governmental authority related to the Product. If GSK is advised by its outside counsel that it must communicate with the FDA or other governmental authority, then GSK shall so advise Auxilium and GSK shall, if the Laws and Regulations permit, comply with any and all reasonable direction of Auxilium concerning any meeting or communication with the FDA or other governmental authority.
7.2 Pharmacovigilance Agreement. The Parties shall enter into a separate and mutually agreeable Pharmacovigilance Agreement prior to the GSK First Promotion Date.
7.3 Studies. Auxilium shall retain all rights and responsibilities with respect to, and shall bear all costs of, all matters relating to the support for, or conduct of, investigator-initiated studies or any clinical studies, including post-approval studies, or other studies, including health economics and outcomes research and Phase IV studies, with respect to the Product. Any such studies will be performed in compliance with Good Clinical Practice. Auxilium shall keep GSK informed of the conduct and results of such studies through regular reports at meetings of the Joint Steering Committee.
7.4 Sunshine Act Reporting. Auxilium shall be responsible for the reporting of matters required under the Sunshine Act and related state laws, if any, relating to the commercialization and promotion of the Product by Auxilium and GSK. GSK shall promptly provide to Auxilium all information that Auxilium notifies GSK in writing is required to be reported by Auxilium pursuant to the Sunshine Act or related state laws in connection with GSK’s commercialization and promotion of the Product, in a commercially reasonable electronic format designated by Auxilium. Notwithstanding the foregoing, GSK reserves the right to report matters as required under the Sunshine Act, related state laws or in accordance with GSK’s voluntary reporting policies. Auxilium shall promptly provide to GSK all reasonable information reasonably requested by GSK to enable GSK to fulfill its reporting obligations under the Sunshine Act, related state laws or GSK’s voluntary reporting policies; provided that GSK will provide Auxilium with excerpted copies of any such reports as they relate to the Product prior to filing thereof.
7.5 Government Price Reporting. With respect to the Product in the Territory, Auxilium shall be responsible for any and all price reporting, payment and rebate obligations
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
including average manufacturer price, best price, average sales price, non-federal average manufacturer price, and federal ceiling prices, and best price, under government healthcare programs including Medicare, Medicaid, 340B, Tricare, or VA, and any successor government program, including the accuracy and completeness of all reports relating thereto (“Government Price Reporting”).
7.6 Medical Inquiries. Auxilium shall respond to all reasonable requests for medical information. Such responses shall be made in accordance with Laws and Regulations, as well as the FDA’s Draft Guidance for Industry: Responding to Unsolicited Requests for Off-Label Information About Prescription Drugs and Medical Devices, issued in December 2011. Promptly after the Effective Date, the Parties, through the Compliance Committee, shall establish procedures in accordance with the applicable portions of Auxilium’s processes and standard operating procedures, to enable GSK to send such requests to specific parties.
7.7 Recalls and Market Withdrawals. In the event Auxilium or GSK should become aware of information that may require a recall, field alert, Product withdrawal or field correction arising from any defect in the Product (collectively, an “Market Event”), it shall immediately notify the other Party in writing. To the extent reasonably practicable prior to initiating a Market Event in the Territory and in any event within [**] thereafter, Auxilium shall notify GSK of its intent to initiate a Market Event and, to the extent reasonably practicable, provide GSK a reasonable opportunity to comment on the merits or manner of such Market Event; provided, however, that Auxilium will have the sole right to determine the response to any Market Event and shall have sole responsibility for carrying out any such response. Auxilium will bear all costs of any action taken with respect to any Market Event, including costs associated with any recall, withdrawal or stock recovery (including mailings to health care professionals). The Parties acknowledge and agree that GSK shall have no liability for any Market Event and Auxilium shall indemnify GSK for any costs incurred under this Section 7.7.
7.8 Events Affecting Integrity or Reputation. During the Term, the Parties shall notify each other immediately of any circumstances of which they are aware and which could impair the integrity and reputation of the Product or if a Party is threatened by the unlawful activity of any Third Party in relation to the Product, which circumstances shall include, by way of illustration, deliberate tampering with or contamination of the Product by any Third Party as a means of extorting payment from the Parties or another Third Party. In any such circumstances, the Parties shall use Commercially Reasonable Efforts to limit any damage to the Parties and/or to the Product with the understanding that the health and welfare of patients is of foremost importance. The Joint Steering Committee shall promptly call a meeting to discuss and resolve such circumstances.
8. Audit Rights; Access to Records
8.1 Product Detailing and Samples Audits. Each of Auxilium and GSK shall keep complete and accurate records of its respective Product Details for the Product by sales representatives, and, in the case of Auxilium, records related to Product Samples. Each Party shall have the right, at such Party’s expense, through an independent certified public accountant or like person reasonably acceptable to the other Party, upon execution of a confidentiality agreement, to examine such records and documentation during regular business hours upon
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
reasonable written notice during the Term of this Agreement and for two (2) Years thereafter; provided, however, that (a) such examination shall not take place more often than [**] per [**] and shall not cover such records for more than that portion of the year in which the audit takes place and the two (2) preceding years (but not for any period prior to the Effective Date, for any period after the Term, or with respect to any period that has previously been audited) and (b) such accountant shall report to the Party conducting the audit only such information as is required to be disclosed under this Agreement and in a summary fashion as is necessary to report such Third Party’s conclusions. All such information disclosed or generated by the auditor shall be deemed Confidential Information of the Party being audited. All costs and expenses incurred in connection with performing audits under this Section 8.1 shall be paid by the Party requesting the audit; provided, however, that the Party being audited shall reimburse the Party conducting the audit for such reasonable out-of-pocket costs and expenses in the event the audit reveals material noncompliance with this Agreement.
8.2 Compliance Audits. Each Party shall have the right, at such Party’s expense, through an independent Third Party, upon execution of a confidentiality agreement, to examine, at [**] during the Term of the Agreement and for two (2) Years thereafter and upon reasonable written notice, any and all Compliance Records of the other Party. All such information disclosed under this Section 8.2 shall be deemed Confidential Information of the Party being audited; provided, however, that the Party being audited shall reimburse the Party conducting the audit for such reasonable out-of-pocket costs and expenses in the event the audit reveals material noncompliance with this Agreement.
8.3 Financial Audits. Auxilium shall keep complete and accurate records of the Net Sales of the Product, and any other reimbursable expenses. GSK shall have the right, at GSK’s expense, through an independent certified public accountant or like person reasonably acceptable to Auxilium, upon execution of a confidentiality agreement, to examine such records during regular business hours upon reasonable written notice during the Term of this Agreement and any period during which a Tail Payment is due to GSK and for two (2) years thereafter; provided, however, that (a) such examination shall not take place more often than [**] per [**] and shall not cover such records for more than that portion of the year in which the audit takes place and the two (2) preceding years (but not for any period prior to the Effective Date or with respect to any period that has previously been audited), and (b) such accountant shall report to GSK only such information as is required to be disclosed under this Agreement and in a summary fashion as is necessary to report such Third Party’s conclusions. All such information disclosed or generated by the auditor shall be deemed Confidential Information of Auxilium. Subject to Section 4.3, any adjustments required as a result of overpayments or underpayments identified through GSK’s exercise of audit rights shall be made by subtracting or adding, as appropriate, amounts from or to the next Promotional Payment or Tail Payment in accordance with Section 4.1 and Section 4.2 or, if no further Promotional Payments or Tail Payments are due, by payment to GSK after identification of such adjustment within sixty (60) days of Auxilium’s receipt of a written invoice from GSK. All costs and expenses incurred in connection with performing audits under this Section 8.3 shall be paid by GSK, provided, however, Auxilium shall reimburse GSK for reasonable out-of-pocket costs and expenses in the event the audit reveals an error of overstatement or understatement equal to or exceeding [**] in the numbers reported in any Year. In the event that a Party disputes an invoice or other payment obligation under this Agreement, such Party shall timely pay the amount of the invoice or other
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
payment obligation that is not in dispute, and the Parties shall resolve such dispute in accordance with Article 17 and neither Party shall be deemed to be in breach of this Agreement during the pendency of such dispute.
8.4 Access to Records. During the Term of this Agreement and for six (6) Years thereafter and with respect to the records maintained by each Party under Sections 8.1, 8.2 and 8.3, each Party shall have the right, at such Party’s expense, through an independent Third Party, upon execution of a confidentiality agreement, during regular business hours and upon reasonable written notice, to reasonably request, access and copy such records of the other Party solely to comply with any governmental authority inquiry, investigation, or proceeding.
9. Intellectual Property
9.1 Ownership of Intellectual Property. Each Party shall have and retain sole and exclusive right, title and interest in and to all inventions, discoveries, writings, trade secrets, know-how, methods, practices, procedures, engineering information, designs, devices, improvements, manufacturing information and other technology, whether or not patentable or copyrightable, and any patent applications, patents, or copyrights based thereon (“Inventions”) that are made, discovered, conceived, reduced to practice or generated by such Party (or its employees or representatives). The Parties shall jointly own, without any duty to account, the right, title and interest in and to any Inventions made, discovered, conceived, reduced to practice or generated jointly by the Parties (or their employees or representatives); provided that such joint Inventions do not relate to the Product or the active ingredients in the Product. Auxilium shall solely own all right, title and interest in and to all joint Inventions relating to the Product or the active ingredients in the Product. GSK shall not represent to any Third Party that it has any proprietary or property right or interest in the Product, or in any patent relating thereto, or in any trademark used in connection therewith.
9.2 Patent Prosecution. Auxilium, at its expense, shall have responsibility for filing, prosecution and maintenance of all Auxilium Patents in the Territory and for all joint Inventions relating to the Product or the active ingredient in the Product. Each Party shall hold all non-public information disclosed to it under this Section as confidential subject to the provisions of Article 10. With respect to any jointly-owned Inventions that do not relate to the Product or the active ingredients of the Product referred to in Section 9.1, both Parties shall cooperate in the patent prosecution and maintenance of such Inventions and shall share the costs for such prosecution and maintenance; provided, however, that if either Party decides not to prosecute or maintain any jointly-owned Inventions in any country, such Party shall assign its interest in such specific Inventions to the other Party in such country free of charge.
9.3 Notification of Patent Litigation. In the event of the institution of any suit by a Third Party against Auxilium or GSK for patent infringement involving the manufacture, use, sale, license, marketing or promotion of the Product anywhere in the Territory during the Term, the Party sued shall promptly notify the other Party in writing.
9.4 Patent Infringement. In the event that after the Effective Date Auxilium or GSK becomes aware of actual or threatened infringement of an Auxilium Patent as such infringement relates to the Product anywhere in the Territory, that Party shall promptly notify the other Party
in writing. Auxilium shall investigate and/or bring an infringement action against any Third Party as it reasonably determines to be appropriate. Auxilium shall have full control over the conduct of such investigations and litigation, including the settlement thereof. The cost of such investigation and litigation shall be borne entirely by Auxilium, with Auxilium being entitled to the entire proceeds, if any, of such litigation. GSK shall reasonably assist Auxilium and reasonably cooperate in any such investigation and litigation at Auxilium’s request and expense.
9.5 Title to Trademarks. The ownership and all goodwill from the use of any Auxilium Trademarks shall at all times vest in and inure to the benefit of Auxilium. Except as expressly provided in this Agreement or as mutually agreed by the Parties, neither Party shall use the Trademarks of the other Party for any purpose.
9.6 Trademark License. Auxilium grants to GSK, during the Term, an irrevocable (except as expressly provided in this Agreement), royalty-free, nonexclusive, non-transferable (except as set forth in Section 14), non-sublicensable license to use the Auxilium Trademarks in the Territory during the Term in connection only with the marketing and promotion of the Product as contemplated in this Agreement, subject to Auxilium retaining full rights to use the Auxilium Trademarks in the Territory, and without limiting in any way Auxilium’s rights with respect to the Auxilium Trademarks outside the Territory.
9.7 Maintenance of Trademarks. Auxilium agrees to search, file, register and maintain a registration for the Auxilium Trademarks in the Territory for the Term of this Agreement and for the period of time during which GSK is entitled to receive any Tail Payments. Such expenses incurred in connection with the Auxilium Trademarks shall be paid solely by Auxilium. In the event that the Auxilium Trademarks are not available for use and registration in connection with the Product in the Territory due to a rejection of the trademark by a government agency, actual or threatened opposition, cancellation or litigation as to use and/or registration of the Auxilium Trademarks by a Third Party, and/or a decision by the Joint Steering Committee that use of the Auxilium Trademarks is likely to cause confusion with another’s trademark, Auxilium shall provide an alternate Auxilium Trademark and shall develop, search, file, register and maintain such alternate Auxilium Trademark at Auxilium’s sole expense, provided that the selection of such alternate Auxilium Trademark shall be made by the Joint Steering Committee in accordance with Section 5.2.
9.8 Notification of Trademark Litigation. In the event of the institution of any suit by a Third Party against Auxilium or GSK for trademark infringement involving the marketing, promotion or sale of the Product in accordance with the Annual Marketing Plan in the Territory, the Party sued shall promptly notify the other Party in writing.
9.9 Trademark Infringement. In the event that Auxilium or GSK becomes aware of actual or threatened infringement of an Auxilium Trademark anywhere in the Territory, that Party shall promptly notify the other Party in writing. Auxilium shall investigate and shall have the sole right to bring an infringement and/or opposition or cancellation action against a Third Party. Auxilium shall have full control over the conduct of such investigations and litigation, including the settlement thereof, and the cost of such investigation and litigation shall be borne entirely by Auxilium, with Auxilium being entitled to the entire award or judgment, if any,
arising from such litigation. GSK shall reasonably assist Auxilium and cooperate in any such investigation and litigation at Auxilium’s request and expense.
9.10 Information and Settlements. Auxilium shall keep GSK informed of the status of any patent or trademark infringement litigation or settlement thereof concerning the Product, Auxilium Patents or the Auxilium Trademarks to which GSK is entitled to be notified pursuant to this Article 9; provided however, that no settlement or consent judgment or other voluntary final disposition of any suit defended or action brought pursuant to this Article 9 shall be entered into without the consent of GSK if such settlement shall require GSK to make a monetary payment.
10. Confidentiality
10.1 Disclosure of Know-How. To the extent that Auxilium has disclosed, or in the future discloses, to GSK any Know-How, GSK shall not acquire any ownership rights in such Know-How by virtue of this Agreement or otherwise.
10.2 Confidential Information.
(a) Confidentiality. Each Party (the “Recipient”) may receive or have access to certain Confidential Information of the other Party (the “Discloser”). For purposes of this Agreement, “Confidential Information” means any information disclosed by the Discloser to the Recipient, whether technology-related or business-related, furnished after the Effective Date and irrespective of the form of communication, that is reasonably considered by a Party to be competitive, confidential or proprietary in nature. The mere existence of this Agreement (including its title and the Parties’ identities) is not confidential. The specific terms of this Agreement, however, are Confidential Information of both Parties and will not be disclosed by GSK or Auxilium except: (x) to any Third Party proposing to enter into a business transaction with a Party, but only to the extent reasonably necessary for carrying out the proposed transaction and only under terms of a written confidentiality agreement that limits the disclosure of this Agreement’s terms to personnel necessary to carry out the proposed transaction or (y) each Party shall have the right to disclose the terms and conditions of this Agreement to its attorneys and to its bankers, auditors, and significant investors on an “as-needed” basis so long as any such parties are subject to written obligations of confidentiality at least as stringent as those set forth in this Section 10.
(b) Protection of Confidential Information. During the Term and for a period of five (5) years thereafter, Auxilium and GSK shall not use or disclose to Third Parties any Confidential Information received from the other Party or otherwise developed or attained (including during the Term or during any period in which the Parties have audit rights pursuant to Article 8) by either Party in the performance of activities in furtherance of this Agreement without first obtaining the written consent of the Discloser, except as may be otherwise provided in, or required in order for a Party to fulfill its obligations under, this Agreement. The Recipient will be responsible for any breach of this Agreement by it or its employees. Without limiting the foregoing, the Recipient will protect the Discloser’s Confidential Information by using efforts at least as great as the precautions the Recipient takes to protect its own Confidential Information
from unauthorized use or disclosure, and in any event such efforts will not be less than are reasonably necessary to maintain the confidentiality of the Discloser’s Confidential Information.
(c) Exclusions. The foregoing confidentiality obligations will not apply to any Confidential Information that the Recipient demonstrates:
(i) is or becomes a matter of public knowledge (other than by breach of this Agreement by the Recipient or its Affiliates or its or their Affiliates, agents or advisors);
(ii) is disclosed in response to a valid order of a court of competent jurisdiction or other governmental authority of competent jurisdiction; provided, however, that the Recipient shall, if legally permissible, first have given notice to the Discloser and given the Discloser a reasonable opportunity to quash such order or to obtain a protective order requiring that the Confidential Information or documents that are the subject of such order be held in confidence by such court or governmental authority or, if disclosed, be used only for the purposes for which the order was issued; and provided further that if a disclosure order is not quashed or a protective order is not obtained, the Confidential Information disclosed in response to such court or governmental order shall be limited to that information which is legally required to be disclosed in such response to such court or governmental order;
(iii) is required by Laws and Regulations to be disclosed; provided, however, that the Recipient shall, if legally permissible, first have given notice to the Discloser and given the Discloser a reasonable opportunity to obtain a protective order requiring that the Confidential Information or documents that are required by Laws and Regulations to be disclosed be held in confidence by the applicable governmental authority or, if disclosed, be used only for the purposes for which Laws and Regulations requires; and provided further that if a protective order is not obtained, the Confidential Information disclosed shall be limited to that information which is legally required to be disclosed by such governmental authority;
(iv) was already known to it or was in its possession at the time of disclosure;
(v) was independently developed by persons in its employ without the use of such information; or
(vi) is disclosed to the Recipient by a Third Party having the right to do so and who was not known to the Recipient to be bound by any confidentiality obligation with respect to such information.
The Parties shall take reasonable measures to assure that no unauthorized use or disclosure is made by others to whom access to such information is granted.
(d) Confidential Treatment Request. If either Party is required to file or disclose this Agreement or any portion thereof with the United States Securities and Exchange Commission (the “SEC”) or any other governmental authority, such Party shall notify the other Party in writing and shall provide the other Party with at least five (5) Business Days to request redactions thereof prior to making such filing or disclosure. Each Party shall use its commercially reasonable efforts to procure confidential treatment of the Agreement or relevant
provisions thereof pursuant to the Securities Act of 1933 or the Securities Exchange Act of 1934, in each case as amended, and the rules, regulations and guidelines promulgated thereunder, or any Laws and Regulations. Each Party will use its commercially reasonable efforts to procure confidential treatment for such portions of the Agreement as may be reasonably requested in a timely manner by the other Party.
(e) Disclosure to Affiliates. Nothing in this Agreement shall be construed as preventing either Party from disclosing any information received from the other to an Affiliate of the receiving Party who is necessary for the purposes of enabling the receiving Party to fulfill its obligations under this Agreement, provided, the receiving Party shall be responsible for breaches of the confidentiality obligations by such Affiliate.
(f) Certification of Destruction of Confidential Information. Within fourteen (14) days following the receipt of a written request from the other Party, which shall only be given after the termination or expiration of this Agreement, each Party will certify the destruction of all Confidential Information of the other Party and all copies thereof (in any media), except to the extent a Party is required to retain such material under Laws and Regulations or entitled to retain such material under any other written agreement between the Parties.
(g) Retention of Confidential Information. Notwithstanding Section 10.2(f), upon expiration or earlier termination of this Agreement, a Party may retain one (1) archival copy of the other Party’s Confidential Information, for the sole purpose of establishing compliance with the terms of this Agreement. All other copies of Confidential Information will be destroyed.
(h) Joint Defense Agreement. The Parties reaffirm their rights and obligations under the Joint Defense Agreement.
10.3 Public Announcements. No public announcement or other disclosure to Third Parties concerning the existence of or terms of this Agreement shall be made, either directly or indirectly, by either Party, without first obtaining the written approval of the other Party and agreement upon the nature, text and timing of such announcement or disclosure; provided, however, either Party shall have the right to make any such public announcement or other disclosure required by Laws and Regulations after such Party has provided to the other Party a copy of such announcement or disclosure and an opportunity to comment thereon. Notwithstanding anything in this Section 10.3 to the contrary, neither Party shall be required to provide the other Party with any advance notice of any public announcements or other disclosures related to periodic, routine financial reporting or periodic presentations to the investment community that include factual information regarding the Product that is not promotional in nature. Each Party acknowledges the other Party’s responsibilities under the federal and state securities laws in the United States with respect to trading in the securities of GSK and Auxilium, as applicable, while in possession of material non-public information relating to GSK and Auxilium, as applicable, and agrees to maintain and enforce appropriate policies to prevent the violations of such Laws and Regulations by its employees and representatives.
11. Non-Competition and Non-Solicitation
11.1 Non-Competition. During the Term, GSK agrees that it will not promote, market, or sell any Competing Product [**]. For purposes of this Section 11.1, a “Competing Product” shall mean any [**] with the same or substantially similar indications for use to those of the Product.
11.2 Non-Solicitation. During the Term of this Agreement, neither Party shall solicit, induce, encourage or attempt to induce or encourage any employee of the other Party to terminate his or her employment or relationship with such other Party or to breach any other obligation to such other Party. The Parties agree that general solicitation, such as through advertisements or recruiting services, not targeted at the other Party’s employees shall not be a violation of the foregoing sentence.
12. Rights And Duties Upon Termination
12.1 Continuing Obligations. The following provisions shall survive the termination of this Agreement for any reason: Sections 1, 5.5 (solely the last sentence of the opening paragraph), 5.5(g) (solely the fourth sentence), 5.6(a), 5.6(e), 5.8(a) (solely the first sentence), 6.1(a), 6.1(b), 6.1(c) (solely the last sentence), 7.1(c) (solely the first sentence), 7.1(f) (solely the first and last sentence), 7.4, 7.5, 7.7 (solely the last two sentences), 9.1, 9.2, 9.5 (solely the first sentence), 9.7 (solely the first and second sentence), 9.10 (solely the proviso), and Articles 4, 8, 10, 12, 13, 15, 16, and 17. In addition, any other provision required to interpret and enforce the Parties’ rights and obligations under this Agreement shall also survive, but only to the extent required for the full observation and performance of this Agreement.
12.2 Remedies. Termination of this Agreement in accordance with its provisions shall not limit the remedies that may be otherwise available to either Party in law or equity; provided that neither Auxilium nor GSK shall be liable under this Agreement for any indirect, incidental, punitive, exemplary, special or consequential damages of any kind whatsoever sustained as a result of a breach of this Agreement, whether based on breach of contract, tort (including negligence), warranty or otherwise arising out of or that relate in any way to this Agreement or its performance. This exclusion will apply regardless of the legal theory upon which any claim for such damages is based, whether such Party had been advised of the possibility of such damages, whether such damages were reasonably foreseeable, or whether application of the exclusion causes any remedy to fail of its essential purpose. The foregoing sentence shall not limit the obligations of either Party to indemnify, defend and hold harmless the other Party from and against Claims (as defined in Section 13.3) under Section 13.3 or Section 13.4. Any payments due hereunder by a Party to the other Party shall not be deemed to be indirect, incidental, punitive, exemplary, special or consequential damages.
12.3 Returns. Promptly upon termination of this Agreement, GSK shall return to Auxilium all Promotional Materials and other sales and communication materials, marketing plans and reports and other tangible items provided by Auxilium to GSK pursuant to the terms and intent of this Agreement.
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
13. Representations, Warranties, Covenants and Indemnification
13.1 Representations, Warranties and Covenants of Auxilium. Except as disclosed on Schedule 13.1, Auxilium represents and warrants that, as of the Effective Date:
(a) Auxilium (i) is a company duly incorporated, validly existing, and in good standing under the Laws and Regulations of the jurisdiction of its incorporation; (ii) is duly qualified and in good standing under the Laws and Regulations of each jurisdiction where its ownership or lease of property or the conduct of its business requires such qualification, where the failure to be so qualified would have a material adverse effect on its financial condition or its ability to perform its obligations hereunder; (iii) has the requisite corporate power and authority and the legal right to conduct its business as now conducted and hereafter contemplated to be conducted; and (iv) is in compliance with its charter documents.
(b) The execution, delivery and performance of this Agreement by Auxilium and all instruments and documents to be delivered by Auxilium hereunder (i) are within the corporate power of Auxilium; (ii) have been duly authorized by all necessary or proper action; (iii) do not conflict with any provision of the charter documents of Auxilium; (iv) will not, to the best of Auxilium’s knowledge, violate any Laws and Regulations or any order or decree of any court of governmental instrumentality; and (v) will not violate or conflict with any terms of any indenture, mortgage, deed of trust, lease, license, agreement, or other instrument to which Auxilium is a party, or by which Auxilium or any of its property is bound, which violation would have a material adverse effect on its financial condition or on its ability to perform its obligations hereunder.
(c) This Agreement has been duly executed and delivered by Auxilium and upon execution by Auxilium constitutes a legal, valid and binding obligation of Auxilium, enforceable against Auxilium in accordance with its terms, except as such enforceability may be limited by applicable insolvency and other Laws and Regulations affecting creditors’ rights generally, or by the availability of equitable remedies.
(d) Neither the execution and delivery of this Agreement nor the performance hereof by Auxilium requires Auxilium or any of its Affiliates to obtain any permits, authorizations or consents from any governmental authority or from any other person, not previously obtained.
(e) No Third Party agreement to which Auxilium is a party or by which Auxilium is bound in any way limits Auxilium’s ability to perform all of the obligations undertaken by Auxilium under this Agreement.
(f) None of the representations and warranties of Auxilium contained herein omit to state a fact necessary to make the statement herein not misleading in any material respect.
(g) Auxilium shall use Commercially Reasonable Efforts to ship and distribute in a timely manner the Product in response to purchase orders;
(h) Appendix A contains a correct and complete list of all trademarks and Appendix B contains a correct and complete list of all patents which (x) are pending, applied for, granted or registered in the Territory, (y) are owned by or licensed to Auxilium or any of its Affiliates, and (z) relate to the Product in the Territory.
(i) Auxilium or its Affiliates own or possess adequate licenses or other valid rights to use all patents, patent rights, trademarks, applications and Know-How (collectively, “Intellectual Property”) as necessary to manufacture and package the Product for sale in the Territory, and to distribute, use, promote and sell the Product in the Territory for their FDA-approved indications, all free of any material lien, encumbrance, liability or other restriction. Except as set forth in Schedule 13.1(i), to the knowledge of Auxilium, there are no actions, suits, proceedings or claims pending against Auxilium or any of its Affiliates, or, to the knowledge of Auxilium, threatened against Auxilium or any of its Affiliates, at law or in equity, or before or by any governmental authority relating to the ownership, enforceability, use or validity of any of the Intellectual Property. There are no investigations of public record pending or, to Auxilium’s knowledge, any investigations ongoing or threatened against Auxilium or any of its Affiliates, at law or in equity, or before or by any governmental authority relating to the Product or the manufacture, marketing, distribution, use, promotion, offer for sale or sale thereof contemplated under this Agreement. To Auxilium’s knowledge, there have not at any time anywhere in the world been any material Third Party Claims (as defined in Section 13.3) involving death or bodily injury resulting from the use of the Product.
(j) To the best of Auxilium’s knowledge, the manufacture, marketing, distribution, use, promotion, offer for sale or sale of the Product, and the creation, use and dissemination of Promotional Materials or other materials as contemplated hereunder do not infringe, and will not infringe, any Third Party intellectual property right in the Territory.
(k) Auxilium is in compliance in all material respects with all Laws and Regulations applicable to the subject matter of this Agreement.
(l) The NDAs for the Product filed with the FDA and any amendments and supplements thereto (i) have been prepared in all material respects in accordance with all applicable requirements of the Act and any other Laws and Regulations, and (ii) have been approved by FDA, and none of Auxilium or any of its Affiliates has received any written notice which has, or reasonably should have, led Auxilium or any of its Affiliates to believe that such NDAs and any amendments thereto are not currently in good standing in all respects with FDA. Auxilium (or its designated agent) has filed, or caused to be filed, with the FDA all required notices, supplemental applications and reports, including Adverse Event reports, required to be filed by the NDA holder for the Product. None of Auxilium or any of its Affiliates has received any written notice which has led Auxilium or any of its Affiliates to believe that any governmental authority (including the FDA) has commenced, or threatened to initiate, any action anywhere in the world to withdraw any approval for the Product or to limit the ability of Auxilium or any of its Affiliates to manufacture (or to have manufactured for it by a Third Party) the Product or to request the recall of the Product or commenced, or threatened to Auxilium’s knowledge, to initiate any action to enjoin production of the Product at any facility.
(m) Auxilium is in compliance in all material respects with all Laws and Regulations related to the Government Price Reporting of the Product.
(n) All regulatory filings related to the NDA for the Product, and the data and information in Auxilium’s submissions related to the Product are and shall be free from fraud or any false statements. The data and information in Auxilium’s submissions are and shall be accurate and reliable for purposes of supporting approval of the submissions, and the NDA for the Product has been obtained without illegal or unethical behavior of any kind.
(o) All manufacturing operations conducted by Auxilium and its Affiliates (or by Third Parties on their behalf) relating to the manufacturing of the Product are being conducted in material compliance with cGMP and other Laws and Regulations.
(p) Auxilium and its officers, and directors, and employees, as applicable, have not been and are not under consideration to be (i) excluded, disqualified, banned, debarred or suspended from, or otherwise ineligible to provide services pursuant to Section 306 of the Act; (ii) excluded, disqualified, banned, debarred or suspended from, or otherwise ineligible to participate in, any federal or state health care program, including Medicare, Medicaid, TRICARE, the U.S. Department of Defense, or the U.S. Department of Veterans Affairs pursuant to the U.S. Department of Health and Human Services Office of Inspector General’s exclusion authority under 42 U.S.C. § 1320a-7a, as implemented at 42 C.F.R. §§ 1001.101; (iii) excluded, disqualified, banned, debarred or suspended from, or otherwise ineligible to participate in federal procurement or non-procurement programs (as that term is defined in 42 U.S.C. §1320a-7b(f)); (iv) disqualified by any government or regulatory agencies from performing the hereunder, and are not subject to a pending disqualification proceeding; or (v) convicted of a criminal offense related to the provision of health care items or services, or under investigation or subject to any such action that is pending.
(q) Within [**], Auxilium has not engaged in the practice of “channel stuffing” or other program (including any rebate, discount, chargeback or refund policy or practice) with respect to the Product that would reasonably be expected to result, directly or indirectly, in a trade buy-in of the Product that is significantly in excess of normal customer purchasing patterns consistent in all material respects with the past practices of Auxilium’s business during [**].
(r) Auxilium acknowledges that it has received and read GSK’s ‘Prevention of Corruption — Third Party Guidelines’ (either in hard copy or at http://www.gsk.com/policies/Prevention-of-Corruption-Third-Party-Guidelines.pdf).
13.2 Representations, Warranties and Covenants of GSK. GSK represents and warrants that, as of the Effective Date:
(a) GSK (i) is a company duly formed, validly existing, and in good standing under the Laws and Regulations of the jurisdiction of its formation; (ii) is duly qualified and in good standing under the Laws and Regulations of each jurisdiction where its ownership or lease of property or the conduct of its business requires such qualification, where the failure to be so qualified would have a material adverse effect on its financial condition or its ability to perform
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
its obligations hereunder; (iii) has the requisite limited liability company power and authority and the legal right to conduct its business as now conducted and hereafter contemplated to be conducted; and (d) is in compliance with its charter documents;
(b) The execution, delivery and performance of this Agreement by GSK and all instruments and documents to be delivered by GSK hereunder (i) are within the limited liability company power of GSK; (ii) have been duly authorized by all necessary or proper action; (iii) do not conflict with any provision of the charter documents of GSK; (iv) will not, to the best of GSK’s knowledge, violate any Laws and Regulations or any order or decree of any court of governmental instrumentality; and (v) will not violate or conflict with any terms of any indenture, mortgage, deed of trust, lease, license, agreement, or other instrument to which GSK is a party, or by which GSK or any of its property is bound, which violation would have a material adverse effect on its financial condition or on its ability to perform its obligations hereunder.
(c) This Agreement has been duly executed and delivered by GSK and upon execution by GSK constitutes a legal, valid and binding obligation of GSK, enforceable against GSK in accordance with its terms, except as such enforceability may be limited by applicable insolvency and other Laws and Regulations affecting creditors’ rights generally, or by the availability of equitable remedies.
(d) Neither the execution and delivery of this Agreement nor the performance hereof by GSK requires GSK or any of its Affiliates to obtain any permits, authorizations or consents from any governmental authority or from any other person.
(e) No Third Party agreement to which GSK is a party or by which GSK is bound in any way limits GSK’s ability to perform all of the obligations undertaken by GSK under this Agreement.
(f) GSK is in compliance in all material respects with all Laws and Regulations applicable to the subject matter of this Agreement.
(g) GSK and its officers, managers and employees, as applicable, have not been and are not under consideration to be (i) excluded, disqualified, banned, debarred or suspended from, or otherwise ineligible to provide services pursuant to Section 306 of the Act; (ii) excluded, disqualified, banned, debarred or suspended from, or otherwise ineligible to participate in, any federal or state health care program, including Medicare, Medicaid, TRICARE, the U.S. Department of Defense, or the U.S. Department of Veterans Affairs pursuant to the U.S. Department of Health and Human Services Office of Inspector General’s exclusion authority under 42 U.S.C. § 1320a-7a, as implemented at 42 C.F.R. §§ 1001.101; (iii) excluded, disqualified, banned, debarred or suspended from, or otherwise ineligible to participate in federal procurement or non-procurement programs (as that term is defined in 42 U.S.C. §1320a-7b(f)); (iv) disqualified by any government or regulatory agencies from performing the hereunder, and are not subject to a pending disqualification proceeding; or (e) convicted of a criminal offense related to the provision of health care items or services, or under investigation or subject to any such action that is pending.
(h) None of the representations and warranties of GSK contained herein omit to state a fact necessary to make the statement herein not misleading in any material respect.
13.3 Indemnification by Auxilium.
(a) Auxilium shall defend, indemnify and hold harmless GSK and its Affiliates and their respective officers, directors, managers, shareholders, employees, agents, representatives, successors and assigns (the “GSK Parties”) from and against all Third Party claims, complaints, or lawsuits for damages (collectively referred to as “Claims”) arising out of (i) any act or omission prior to the GSK First Promotion Date (or if the GSK First Promotion Date shall not occur, the date on which this Agreement is terminated), (ii) the failure by Auxilium or any of its Affiliates to comply with Laws and Regulations, (iii) any assertion of the infringement or misappropriation of any Third Party patent, copyright, trademark, service mark, trade secret, or other intellectual property as a result of the development, registration, marketing, promotion, labeling, use, sale or distribution of the Product, (iv) any breach of any covenant, representation or warranty of Auxilium or any of its Affiliates under this Agreement, and (v) promotional activities that are outside the scope of, or are contrary to, the Overall Marketing Plan, the applicable Annual Marketing Plan or the Promotional Materials. Auxilium shall not be obligated to indemnify for any such Claim under this Section 13.3(a) to the extent that GSK is obligated to indemnify Auxilium for such Claim pursuant to Section 13.4.
(b) In addition to the indemnity set forth in Section 13.3(a), Auxilium shall defend, indemnify and hold harmless the GSK Parties from and against all product liability and consumer fraud Claims arising out of the development, registration, manufacture, design, promotion, labeling, sale or distribution of the Product by Auxilium or GSK, or any of their respective Affiliates, or any use of the Product; provided, however, that Auxilium shall not be obligated to indemnify for any such Claim under this Section 13.3(b) to the extent that such Claim arises from promotional activities of the GSK Parties that are outside the scope of, or are contrary to, the Overall Marketing Plan, the applicable Annual Marketing Plan or the Promotional Materials.
13.4 Indemnification by GSK. GSK shall defend, indemnify and hold harmless Auxilium and its Affiliates and their respective officers, directors, managers, shareholders, employees, agents, representatives, successors and assigns (the “Auxilium Parties”) from and against all Claims arising out of (i) any assertion of the infringement or misappropriation by GSK or any of its Affiliates of any Third Party patent, copyright, trademark, service mark, trade secret or other intellectual property, as a result of GSK’s or such Affiliate’s marketing or promotion of the Product which is not pursuant to the terms of this Agreement or consistent with the direction of the Joint Steering Committee or the Compliance Committee, (ii) the failure by GSK or any of its Affiliates to comply with Laws and Regulations, (iii) any breach of any covenant, representation or warranty of GSK or any of its Affiliates under this Agreement, and (iv) promotional activities that are outside the scope of, or are contrary to, the Overall Marketing Plan, the applicable Annual Marketing Plan or the Promotional Materials. GSK shall not be obligated to indemnify for any such Claim under this Section 13.4 to the extent that Auxilium is obligated to indemnify GSK for such Claim pursuant to Section 13.3(a) or for the Claims Auxilium is obligated to indemnify GSK pursuant to Section 13.3(b).
13.5 Indemnification Procedures. Promptly after receipt by any indemnified party (“Indemnitee”) of any Claim or the commencement of any action, such Indemnitee shall (a) notify the indemnifying party (“Indemnitor”) in writing of any such claim (including a copy of any related complaint, summons, notice or other instrument); (b) provide the Indemnitor with reasonable assistance to settle or defend such claim, at the Indemnitor’s own expense; and (c) grant to the Indemnitor the right to control the defense and/or settlement of such Claim, at the Indemnitor’s expense; provided however that (i) the failure to so notify, provide assistance and grant authority and control shall not relieve the Indemnitor of any of its obligations hereunder except to the extent the Indemnitor is prejudiced by such failure, and (ii) the Indemnitor shall not, without the Indemnitee’s consent (such consent not to be unreasonably withheld or delayed), agree to any settlement which (1) makes any admission on behalf of the Indemnitee; or (2) consents to any injunction against the Indemnitee (except an injunction relating solely to the Indemnitee’s continued use of any infringing intellectual property), and (iii) the Indemnitee shall have the right, at its expense, to participate in any legal proceeding to contest and defend a Claim and be represented by legal counsel of its choosing, but shall have no right to settle a claim without the Indemnitor’s written consent.
13.6 Disclaimer of Warranties. EXCEPT FOR THE EXPRESS WARRANTIES SET FORTH IN THIS AGREEMENT, AUXILIUM AND GSK MAKE NO REPRESENTATIONS AND GRANT NO WARRANTIES, EXPRESS OR IMPLIED, EITHER IN FACT OR BY OPERATION OF LAW, BY STATUTE OR OTHERWISE, AND AUXILIUM AND GSK EACH SPECIFICALLY DISCLAIM ANY OTHER REPRESENTATIONS AND WARRANTIES, WHETHER WRITTEN OR ORAL, EXPRESS, STATUTORY OR IMPLIED, INCLUDING ANY WARRANTY OF QUALITY, MERCHANTABILITY OR FITNESS FOR A PARTICULAR USE OR PURPOSE.
13.7 Insurance.
(a) During the Term and continuing for a period of [**] after the expiration of this Agreement or the earlier termination thereof, Auxilium shall obtain and/or maintain, at its sole cost and expense, product liability insurance in an amount not less than $[**] in the aggregate (with a $[**] deductible). The Product liability insurance shall insure against all liability, including personal injury, physical injury, or property damage arising out of the manufacture, sale, storage, distribution, marketing and/or promotion of the Product in the Territory.
(b) During the Term and continuing for a period of [**] after the expiration of this Agreement or the earlier termination thereof, Auxilium shall carry insurance in amounts not less than the following for each type specified or as otherwise might be required by Laws and Regulations:
(i) commercial general liability insurance, including coverage for bodily injury and property damage, with limits of not less than $[**] per occurrence and $[**] in the aggregate;
(ii) automobile liability insurance with coverage of not less than $[**] per occurrence; and
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
(iii) worker’s compensation with limits in accordance with statutory requirements, including employer’s liability coverage of not less than $[**].
(c) GSK hereby represents and warrants to Auxilium that it is self-insured against liability and other risks associated with its activities and obligations under this Agreement in such amounts and on such terms as are customary for prudent practices for similarly situated companies in the pharmaceutical industry for the activities to be conducted by GSK under this Agreement.
(d) Each Party shall provide written proof of the existence of such insurance or self-insurance, as applicable, to the other Party upon reasonable request. If requested by GSK, Auxilium shall cooperate with GSK in purchasing and maintaining, at GSK’s expense, insurance regarding GSK’s activities under this Agreement.
14. Assignment
Neither Party shall assign or transfer its rights or obligations, or any other right or obligation, under this Agreement (including in connection with the insolvency or bankruptcy of either Party) to any Third Party without the prior written consent of the other Party; provided however, that either Party may make such an assignment or transfer without such consent (a) to an Affiliate of such Party, or (b) subject to Section 3.2(c)(vii), in connection with a merger, or consolidation with, or the sale to a Third Party of, the entire pharmaceutical business of such Party. Any attempted assignment in derogation of the foregoing shall be null and void. In the event of any such permitted assignment or transfer with such consent, as a condition to such assignment or transfer, the assigning or transferring Party must confirm to the other Party in writing that it will remain fully liable for all obligations under this Agreement as if such assignment or transfer had not occurred.
15. Notices
Any notice, request, approval or other document required or permitted to be given under this Agreement shall be in writing and shall be deemed to have been given when delivered in person, or sent by overnight courier service, postage prepaid, or sent by certified or registered mail, return receipt requested, or by facsimile transmission, to the following addresses of the Parties and to the attention of the persons identified below (or to such other address, addresses or persons as may be specified from time to time in a written notice). Any notices given pursuant to this Agreement shall be deemed to have been given and delivered upon the earlier of (i) if sent by overnight courier service, on the date when received at the address set forth below as proven by a written receipt from the delivery service verifying delivery, or (ii) if sent by certified or registered mail, three (3) Business Days after mailed by certified or registered mail postage prepaid and properly addressed, with return receipt requested, or (iii) if sent by facsimile transmission, on the day when sent by facsimile as confirmed by automatic transmission report coupled with certified or registered mail or overnight courier service receipt proving delivery, or (iv) if delivered in person, on the date of delivery to the address set forth below as proven by written signature of the recipient.
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
Auxilium Pharmaceuticals, Inc:
40 Valley Stream Parkway
Malvern, PA
Facsimile: (484) 321-5996
Attention: Adrian Adams, Chief Executive Officer and President
Copy to:
Auxilium Pharmaceuticals, Inc.
40 Valley Stream Parkway
Malvern, PA
Facsimile: (484) 321-5996
Attention: Andrew I. Koven, Chief Administrative Officer & General Counsel
Copy to (which shall not constitute notice):
Holland & Knight LLP
701 Brickell Avenue
Suite 3000
Miami, FL 33131
Facsimile: (305) 789-7799
Attention: Rodney H. Bell
GlaxoSmithKline LLC:
200 N. 16th Street
Philadelphia, PA 19102
Facsimile: (678) 802-2868
Attention: President, North America Pharmaceuticals
Copy to (which shall not constitute notice):
GlaxoSmithKline
2301 Renaissance Boulevard
RN 0220
King of Prussia, PA 19406
Facsimile: (610) 787-7084
Attention: Vice President, Legal Operations, Business Development Transactions
Copy to (which shall not constitute notice):
Sidley Austin LLP
One South Dearborn
Chicago, Illinois 60603
Facsimile: (312) 853-7036
Attention: Jeffrey S. Rothstein
16. Miscellaneous
16.1 Cooperation. The Parties shall take commercially reasonable steps to cooperate, including taking such actions as are reasonably requested by the other Party, in performing their obligations hereunder.
16.2 Force Majeure. If the performance of any part of this Agreement by either Party, or of any obligation under this Agreement, is prevented, restricted, interfered with or delayed by reason of any cause beyond the reasonable control of the Party liable to perform (a “Force Majeure Event”), unless conclusive evidence to the contrary is provided, the Party so affected shall, upon giving written notice to the other Party, be excused from such performance to the extent of such prevention, restriction, interference or delay, provided that the affected Party shall use its reasonable best efforts to avoid or remove such causes of nonperformance and shall continue performance with the utmost dispatch whenever such causes are removed.
16.3 No Partnership or Joint Venture. It is expressly agreed that Auxilium and GSK shall be independent contractors and that the relationship between the two Parties shall not constitute a partnership, joint venture or agency. Neither Auxilium nor GSK shall have the authority to make any statements, representations or commitments of any kind, or to take any action, which shall be binding on the other, without the prior written consent of the other Party to do so.
16.4 No Third Party Beneficiaries. Except as may be specifically provided in any applicable provisions of this Agreement: (a) this Agreement is for the benefit of, and will be enforceable by, only the Parties hereto; and (b) this Agreement is not intended to, and does not, confer any right or benefit on any Third Party, other than the GSK Parties and the Auxilium Parties. No action may be commenced or prosecuted against a Party by any Third Party claiming as a Third Party beneficiary of this Agreement.
16.5 Execution In Counterparts. This Agreement may be executed in any number of counterparts, each of which shall be deemed an original but all of which together shall constitute one and the same instrument. This Agreement may be executed and delivered by facsimile, email (in portable document format (.pdf) or any other image format in common use) or similar electronic means.
16.6 Governing Law. This Agreement shall be deemed to have been made in the State of Delaware, without giving effect to its conflicts of laws principles, and its validity, construction and effect shall be determined in accordance with the laws of the State of Delaware.
16.7 Waiver Of Breach. The failure of either Party at any time or times to require performance of any provision hereof shall in no manner affect its rights at a later time to enforce the same. No waiver by either Party of any condition or term in any one or more instances shall be construed as a further or continuing waiver of such condition or term or of another condition or term.
16.8 Severability. In the event any portion of this Agreement were to be held illegal, void or ineffective, the remaining portions of this Agreement shall remain in full force and effect. If any of the terms or provisions of this Agreement are in conflict with any Laws and
Regulations, then such terms or provisions shall be deemed inoperative to the extent that they may conflict therewith and shall be deemed to be modified to conform with such Laws and Regulations. In the event that the terms and conditions of this Agreement are materially altered as a result of this Section 16.8, the Parties shall renegotiate the terms and conditions of this Agreement to resolve any inequities.
16.9 Entire Agreement; Modifications. This Agreement, together with all Exhibits, Appendices and Schedules hereto, shall constitute the entire agreement between the Parties relating to the subject matter thereof and shall supersede all previous writings and understandings other than the Joint Defense Agreement and the Existing Confidentiality Agreement. The Parties hereby agree that the Existing Confidentiality Agreement shall not be applicable with respect to Confidential Information disclosed under this Agreement. No terms or provisions of this Agreement shall be varied or modified by any prior or subsequent statement, conduct or act of either of the Parties, except that the Parties may amend this Agreement by written instruments specifically referring to and executed in the same manner as this Agreement. In the event of any conflict between the Agreement and its Exhibits, Schedules or Appendices, the terms of the Agreement shall control unless the Parties otherwise expressly agree in writing.
16.10 Interpretation. The Parties have been represented by counsel in connection with the negotiation and execution of this Agreement. Accordingly, any rule of law or any legal decision that would require interpretation of any claimed ambiguities in this Agreement against the drafting Party has no application and is expressly waived. Captions are included for convenience of reference only and shall be ignored in the construction or interpretation of this Agreement. The terms “this Agreement,” “herein,” “hereof,” “hereunder” and similar expressions refer to this Agreement and not to any particular section or other portion of this Agreement. Lists of examples following “including”, “e.g.”, “such as”, or “for example” are interpreted to include “without limitation”, unless qualified by words such as “only” or “solely.” Unless stated or context requires otherwise: (i) all internal references are to this Agreement, its parties, its Exhibits and Schedules; (ii) “days” means calendar days; (iii) “may” means that the applicable Party has a right, but not a concomitant duty; (iv) “partner,” if used in this Agreement or related documents, is used in its common, marketing sense and does not imply a partnership; (v) “current” or “currently” means “as of the Effective Date” but “then-current” means the present time when the applicable right is exercised or performance rendered or measured; (vi) “notify” means to give notice as provided in Section 15; (vii) a Party’s choices under this Agreement are in its sole discretion; and (viii) any reference to any agreement defined herein shall include such agreement as amended, modified or supplemented in accordance with its terms.
17. Dispute Resolution
17.1 Internal Resolution. Any dispute, controversy or claim arising out of or relating to the interpretation of this Agreement other than a Reserved Matter (collectively referred to as “Dispute”) shall be attempted to be settled by the Parties, in good faith, by submitting each such Dispute to the Joint Steering Committee, which shall meet in person, by videoconference or by telephone within five (5) Business Days of submission of such dispute; provided that, any Dispute submitted to the Joint Steering Committee may only be resolved by unanimous decision. If the Dispute is not resolved by the Joint Steering Committee within fifteen (15) Business Days
after the Dispute has been submitted to the Joint Steering Committee, either Party may at any time thereafter provide the other written notice specifying the terms of such Dispute in reasonable detail. Within five (5) Business Days of receipt of such notice, the Chief Executive Officer of Auxilium and the President of North America Pharmaceuticals of GSK, or a member of management designated by the respective officers, shall meet in person (at a mutually agreed upon time and location), by videoconference or by telephone for the purpose of resolving such Dispute. They will discuss the problems and/or negotiate for a period of up to fifteen (15) Business Days in an effort to resolve the Dispute. Notwithstanding the foregoing, the Parties agree that any dispute, controversy or claim that falls within the responsibility of the Joint Steering Committee (which, for the avoidance of doubt, does not include any dispute regarding the interpretation of this Agreement) shall not constitute a Dispute and shall be resolved by the Joint Steering Committee in accordance with the provisions of Section 5.3. For the avoidance of doubt, this Section 17 shall not be applicable to, or have any effect on, any Reserved Matter.
17.2 Mediation. The Parties agree that they shall try in good faith to resolve such Dispute by confidential, non-binding mediation under the Commercial Mediation Procedures of the American Arbitration Association (“AAA”), in effect as of date of the Agreement, before resorting to arbitration. If the Parties have not agreed on a mediator within fourteen (14) days after the Dispute was referred for mediation, the mediator shall, upon request of either Party, be appointed pursuant to the AAA Mediation Procedures. The cost of mediation shall be borne equally by the Parties. No statements made by any Party during the mediation may be used by another Party or referred to in any subsequent proceedings. Any Dispute not resolved within forty-five (45) days (or within such other time period as may be agreed to by Parties in writing) after appointment of a mediator shall be finally resolved by binding arbitration by providing a notice of arbitration (“Arbitration Request”) to the other Party.
17.3 Arbitration. From the date of the Arbitration Request and until such time as the Dispute has become finally settled, the running of the time periods as to which a Party must cure a breach of this Agreement shall become suspended as to any breach that is the subject matter of the Dispute. Unless otherwise agreed by the Parties, disputes relating to patents shall not be subject to arbitration, and shall be submitted to a court of competent jurisdiction. The arbitration shall be held in Philadelphia, Pennsylvania under the Commercial Arbitration Rules of AAA (the “AAA Rules”). The arbitration shall be conducted by one arbitrator, who shall be chosen in accordance with the AAA Rules. If the total amount in controversy exceeds $15 million, the arbitration shall be conducted by three (3) arbitrators. One (1) arbitrator will be selected by Auxilium, one (1) arbitrator will be selected by GSK, and the third arbitrator will be selected by mutual agreement of the two (2) arbitrators selected by the Parties. If the arbitrators selected by the Parties are unable or fail to agree upon the third arbitrator, the third arbitrator shall be appointed by AAA. The procedures for the taking of evidence shall be governed by the IBA Rules on the Taking of Evidence in International Arbitration. The arbitration panel shall collectively resolve any discovery disputes or, if the panel unanimously decides, they may designate the neutrally-selected arbitrator as the chair capable of resolving such disputes without the need to convene the entire arbitration panel. The Parties agree that the arbitration panel and counsel of record in any arbitration hereunder shall have the power to subpoena witnesses to appear to provide their testimony at a hearing or deposition. As set forth in the AAA Rules, the arbitrators may proceed to an award, notwithstanding the failure of either Party to participate in the proceedings. The arbitrators shall promptly, after the conclusion of the arbitration hearing,
issue a written award and statement of decision describing the essential findings of fact and conclusions of law on which the award is based, including the calculation of any damages awarded, and designating one (1) Party or the other as the prevailing party in the arbitration, as appropriate. The arbitrators shall be authorized to award compensatory damages, but shall not be authorized to award non-economic damages or punitive, special, consequential, or any other similar form of damages, or to reform, modify or materially change this Agreement. The arbitrators also shall be authorized to grant any temporary, preliminary or permanent equitable remedy or relief the arbitrators deem just and equitable and within the scope of this Agreement, including an injunction or order for specific performance. Any decision which requires a monetary payment shall require such payment to be payable in United States dollars. The arbitration panel shall make reasonable efforts to conduct and complete such proceeding within six (6) months from submission of the Arbitration Request. The award of the arbitrators shall be the sole and exclusive remedy of the Parties (except for those remedies set forth in this Agreement). The enforceability of this Section 17.3 and, subject to the terms of this Section 17.3, the enforcement of any award hereunder, shall be governed by the Federal Arbitration Act (Title 9, U.S. Code). Judgment on the award rendered by the arbitrators may be enforced in any court having competent jurisdiction thereof. Each Party shall bear its own costs and expenses and attorneys’ fees; but the arbitration panel shall be authorized to require the Party that does not prevail in the arbitration proceeding to pay the arbitrators’ and any administrative fees of arbitration. Except to the extent necessary to confirm an award or as may be required by Laws and Regulations, neither a Party nor an arbitrator may disclose the existence, content, or results of an arbitration without the prior written consent of both Parties. EACH PARTY HERETO WAIVES ITS RIGHT TO TRIAL OF ANY ISSUE BY JUDGE OR JURY.
[Remainder of Page Intentionally Left Blank.]
NOW THEREFORE, the Parties, through their authorized officers, have executed this Agreement as of the date first written above.
AUXILIUM PHARMACEUTICALS, INC
|
By: |
/s/ Adrian Adams |
|
|
Name: |
Adrian Adams |
|
|
Title: |
CEO & President |
|
GLAXOSMITHKLINE LLC
|
By: |
/s/ Deirdre P. Connelly |
|
|
Name: |
Deirdre P. Connelly |
|
|
Title: |
President |
|
SCHEDULES, EXHIBITS AND APPENDICES
The following are schedules, exhibits and appendices to the Co-Promotion Agreement, dated as of May 18, 2012 (the “Agreement”), by and between Auxilium Pharmaceuticals, Inc., a Delaware corporation (“Auxilium”), and GlaxoSmithKline LLC, a Delaware limited liability company (“GSK”). Unless otherwise defined, capitalized terms have the meanings set forth in the Agreement.
INDEX TO SCHEDULES, EXHIBITS AND APPENDICES
|
SCHEDULE 1.55 |
GSK Policies |
|
SCHEDULE 1.72 |
Overall Marketing Plan |
|
SCHEDULE 2.2 |
Promotional Materials |
|
SCHEDULE 13.1 |
Auxilium Disclosure Schedule |
|
SCHEDULE 13.1(i) |
Auxilium IP Claims |
|
|
|
|
EXHIBIT 2.4 |
GSK CIAs |
|
EXHIBIT 5.5(e) |
Samples Procedures |
|
EXHIBIT 5.6(d) |
Background Screening Process |
|
|
|
|
APPENDIX A |
Auxilium Trademarks |
|
APPENDIX B |
Auxilium Patents |
Schedule 1.55
GSK Policies
[**]
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
Schedule 1.72
Overall Marking Plan
[**]
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
Schedule 2.2
Promotional Materials
See attached.
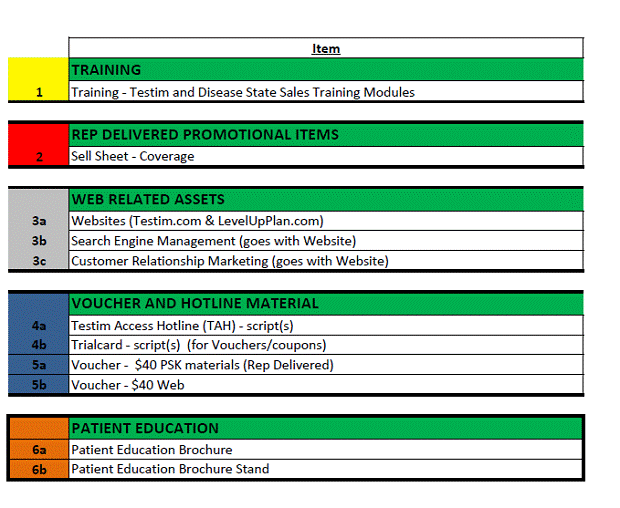
Schedule 13.1
Auxilium Disclosure Schedule
[**]
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
Schedule 13.1(i)
Auxilium IP Claims
Auxilium Pharmaceuticals, Inc., et al. v. Upsher-Smith Laboratories, Inc., C.A. No. 08-908-SLR, D.Del. Auxilium and CPEX Pharmaceuticals, Inc. (predecessor in interest to FCB I Holdings Inc. (“FCB”)) filed on December 4, 2008 a lawsuit against Upsher-Smith Laboratories, Inc. (“Upsher-Smith”) for infringement of the ‘968 Patent, which covers Testim (the “Upsher-Smith Litigation”). The lawsuit was filed in the United States District Court for the District of Delaware. Auxilium and FCB are seeking a judgment (1) declaring that Upsher-Smith’s act of filing the Abbreviated New Drug Application seeking approval from the FDA to market a generic version of Testim prior to the January 2025 expiration of the ‘968 Patent is an act of infringement, (2) declaring that the making, using or selling of the product for which Upsher-Smith is seeking approval infringes the ‘968 Patent, (3) ordering that the effective date of the FDA’s approval of the product not be until the expiration of the ‘968 Patent, (4) enjoining Upsher-Smith from making, using or selling the product for which it seeks approval until the expiration of the ‘968 Patent, and (5) awarding Auxilium and FCB their costs and expenses in the action. Auxilium and CPEX filed this lawsuit under the Hatch-Waxman Act in response to the notice from Upsher-Smith of its filing of an ANDA with the FDA containing a Paragraph IV certification under 21 U.S.C. Section 355(j) for testosterone gel. Upsher-Smith has alleged that its proposed generic version of Testim would not infringe the ‘968 Patent, and that the ‘968 Patent is invalid. The Paragraph IV certification notice states that Upsher-Smith does not believe that the testosterone gel product for which it is seeking approval infringes the ‘968 Patent and that it seeks to market its generic product before the expiration of the ‘968 Patent. The ‘968 Patent is listed in the Orange Book, published by the FDA, and will expire in January 2025. The district court has issued a protective order prohibiting disclosure of confidential information relating to the litigation. On December 13, 2011, the district court issued an order administratively closing the case. This administrative closure has effectively stayed the case, but has not dismissed it. In April 2012, Auxilium and FCB received a notice from Upsher-Smith in connection with its ANDA advising Auxilium and FCB of Upsher-Smith’s Paragraph IV certification relating to the eight additional patents listed in the Orange Book in addition to the ‘968 patent-in-suit, and asserting that Upsher-Smith does not believe that the product for which it is seeking approval infringes any of the Orange Book listed Testim patents and that those patents are invalid.
On April 13, 2012, Auxilium and FCB I LLC (“FCB LLC”) received a notice from Watson Laboratories, Inc. (“Watson”) that advises them of Watson’s filing of an Abbreviated New Drug Application (ANDA) containing a Paragraph IV certification under 21 U.S.C. Section 355(j) for testosterone gel. This Paragraph IV certification notice refers to FCB LLC’s nine U.S. patents, covering Testim®, 1% testosterone gel, that are listed in Approved Drug Products with Therapeutic Equivalence Evaluations (commonly known as the Orange Book), published by the U.S. Food and Drug Administration. The referenced patents will expire between 2023 and 2025.
EXHIBIT 2.4
GSK CIAs
None.
EXHIBIT 5.5(e)
Sample Procedures
[**]
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
EXHIBIT 5.6(d)
Background Screening Process
[**]
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
Appendix A
Auxilium Trademarks
TESTIM [Reg. No. 2767622]
Testim 1% (testosterone gel) [Reg. No. 3190743]
THE POWER OF T. [Reg. No. 3281302]
Appendix B
Auxilium Patents
[**]
** CERTAIN INFORMATION IN THIS EXHIBIT HAS BEEN OMITTED AND FILED SEPARATELY WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO A CONFIDENTIAL TREATMENT REQUEST.
