Attached files
| file | filename |
|---|---|
| EX-5.1 - OPINION OF ANSLOW & JACLIN, LLP - Tanke Biosciences Corp | fs12012a7ex5i_tankebio.htm |
| EX-23.1 - AUDITOR'S CONSENT - Tanke Biosciences Corp | fs12012a7ex23i_tankebio.htm |
| EX-23.2 - CONSENT OF PARKER RANDALL CF (H.K.) CPA LIMITED * - Tanke Biosciences Corp | fs12012a7ex23ii_tankebio.htm |
| EX-5.3 - OPINION OF MARTIN HU & PARTNERS, DATED JANUARY 24, 2011 * - Tanke Biosciences Corp | fs12012a7ex5iii_tankebio.htm |
| EX-23.5 - CONSENT OF MARTIN HU & PARTNERS * - Tanke Biosciences Corp | fs12012a7ex23v_tankebio.htm |
As filed with the Securities and Exchange Commission on September 28, 2012
Registration No. 333-172240
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
TO
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
(Exact Name of Registrant as Specified in its Charter)
|
Nevada
|
0200
|
26-3853855
|
||
|
(State or Other Jurisdiction of
Incorporation or Organization)
|
(Primary Standard Industrial
Classification Code Number)
|
(I.R.S. Employer
Identification Number)
|
Room 2801, East Tower of Hui Hao Building, No. 519 Machang Road
Pearl River New City, Guangzhou, People’s Republic of China 510627
(Address, Including Zip Code, and Telephone Number, Including Area Code, of Registrant’s Principal Executive Offices)
Chief Executive Officer
c/o Guangzhou Tanke Industry Co., Ltd.
Room 2801, East Tower of Hui Hao Building, No. 519 Machang Road
Pearl River New City, Guangzhou, People’s Republic of China 510627
+86-20-3885-9025
CT Corporation
111 Eighth Avenue, 13th Floor
New York, New York 10011
(Name, Address Including Zip Code and Telephone Number, Including Area Code, of Agent For Service)
Richard I. Anslow, Esq.
Thomas Slusarczyk, Esq.
Anslow & Jaclin, LLP
195 Route 9 South
Manalapan, NJ 07726
Telephone: (732) 409-1212
Facsimile: (732) 577-1188
Approximate date of Commencement of Proposed Sale to the Public: As soon as practicable after this Registration Statement becomes effective.
If any of the securities being registered on this form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box. x
If this form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
If this form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ¨
|
Accelerated filer ¨
|
|
Non-accelerated filer (Do not check if a smaller reporting company) ¨
|
Smaller reporting company x
|
CALCULATION OF REGISTRATION FEE
|
Title of each class of securities
to be registered
|
Amount to be
registered(1)
|
Proposed
maximum
offering
price per
unit (2)
|
Proposed
maximum
aggregate
offering
price
|
Amount of
registration fee
|
|||||||||||
|
common stock, par value $0.001 per share, offered by selling stockholders
|
2,237,455 shares
|
$
|
1.15
|
$
|
2,573,073.25
|
$
|
294.87
|
||||||||
|
common stock, par value $0.001 per share, underlying the principal of convertible notes held by selling stockholders (1)
|
6,669,627 shares
|
(3)
|
$
|
1.15
|
$
|
7,670,071.05
|
$
|
878.99
|
|||||||
|
common stock, par value $0.001 per share, underlying warrants held by selling stockholders (1)
|
6,669,627 shares
|
(4)
|
1.40
|
$
|
9,337,477.80
|
$
|
1,070.07
|
||||||||
|
common stock, par value $0.001 per share, underlying agent warrants held by selling stockholders (1)
|
666,963 shares
|
(5)
|
1.15
|
$
|
767,007.45
|
$
|
87.90
|
||||||||
|
TOTAL
|
16,243,672 shares
|
$
|
$
|
20,347,629.55
|
$
|
2,331.84
|
|||||||||
|
(1)
|
Pursuant to Rule 416 of the Securities Act of 1933, also registered hereby are such additional and indeterminable number of shares as may be issuable due to adjustments for changes resulting from stock dividends, stock splits and similar changes.
|
|
(2)
|
Estimated solely for the purpose of calculating the amount of the registration fee pursuant to Rule 457(g) of the Securities Act of 1933.
|
|
(3)
|
The 6,669,627 shares of common stock are being registered for resale by certain selling stockholders named in this registration statement, which shares are issuable by the registrant upon the conversion of the registrant’s 8% Convertible Notes due February 9, 2013.
|
|
(4)
|
The 6,669,627 shares of common stock are being registered for resale by certain selling stockholders named in this registration statement, which shares are issuable by the registrant upon the exercise of the registrant’s common stock purchase warrants issued on February 9, 2011.
|
|
(5)
|
The 666,963 shares of common stock are being registered for resale by a certain selling stockholder named in this registration statement, which shares are issuable by the registrant upon the exercise of the registrant’s placement agent warrants issued on February 9, 2011.
|
The registrant hereby amends this registration statement on such date or dates as may be necessary to delay its effective date until the registrant shall file a further amendment which specifically states that this registration statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933 or until the Registration Statement shall become effective on such date as the Commission, acting pursuant to said Section 8(a), may determine.
The information in this preliminary prospectus is not complete and may be changed. These securities may not be sold until the registration statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell, nor does it seek an offer to buy these securities, in any jurisdiction where the offer or sale is not permitted.
|
Preliminary Prospectus
|
Subject to Completion, Dated September 28, 2012
|
Tanke Biosciences Corporation
16,243,672 Shares of
Common Stock
This prospectus relates to the sale of up to a total of 16,243,672 shares of common stock of Tanke Biosciences Corporation, a Nevada corporation, that may be sold from time to time by the selling stockholders named in this prospectus and their successors and assigns. The shares of common stock subject to this prospectus include: (i) 2,166,913 shares issued to certain selling stockholders pursuant to a contractual arrangement with us, (ii) 6,669,627 shares issuable upon conversion by certain selling stockholders of the principal underlying our 8% Convertible Notes due February 9, 2013, which we refer to herein as the Notes; (iii) 6,669,627 shares issuable upon the exercise by certain selling stockholders of our common stock purchase warrants issued on February 9, 2011; (iv) 666,963 shares issuable upon exercise by certain selling stockholders of our placement agent warrants issued on February 9, 2011, which we refer to herein collectively as the Warrants; and (v) 70,542 shares issued to a selling stockholder. The securities offered for resale hereby were issued to the applicable selling stockholders in a private placement transaction completed prior to the filing of the registration statement of which this prospectus is a part.
The selling stockholders may sell all or a portion of their shares through public or private transactions at prevailing market prices or at privately negotiated prices. Information regarding the selling stockholders and the times and manner in which they may offer and sell the shares under this prospectus is provided under “Selling Stockholders” and “Plan of Distribution” in this prospectus. We have agreed to pay all the costs and expenses of this registration.
We will not receive any of the proceeds from the sale of shares by the selling stockholders. We may receive proceeds upon conversion of the Notes and exercise of the Warrants, and any proceeds we receive will be used for general corporate purposes and for working capital.
Our common stock is listed for quotation on the Over-the-Counter Bulletin Board, or OTCBB, under the symbol “TNBI” (formerly “GHND”). There is very limited trading in our common stock. On September 21, 2012, the most recent day that our stock traded, the last reported price per share of our common stock was $0.40. You are urged to obtain current market quotations of our common stock before purchasing any of the shares being offered for sale pursuant to this prospectus.
We are an emerging growth company as that term is used in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”).
An investment in our securities is highly speculative, involves a high degree of risk and should be considered only by persons who can afford the loss of their entire investment. See “Risk Factors” beginning on page 7 of this prospectus.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
The date of this prospectus is , 2012.
|
PROSPECTUS SUMMARY
|
1
|
|
RISK FACTORS
|
8
|
|
CAUTIONARY NOTE ON FORWARD LOOKING STATEMENTS
|
25
|
|
USE OF PROCEEDS
|
26
|
|
DETERMINATION OF OFFERING PRICE
|
26
|
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
|
27
|
|
DESCRIPTION OF BUSINESS
|
42
|
|
DESCRIPTION OF PROPERTY
|
60
|
|
LEGAL PROCEEDINGS
|
61
|
|
MANAGEMENT
|
61
|
|
EXECUTIVE COMPENSATION
|
63
|
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
|
64
|
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
|
65
|
|
SELLING STOCKHOLDERS
|
67
|
|
PLAN OF DISTRIBUTION
|
77
|
|
DESCRIPTION OF OUR COMMON STOCK
|
78
|
|
MARKET PRICE OF AND DIVIDENDS ON THE COMPANY’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
|
79
|
|
DISCLOSURE OF COMMISSION POSITION ON INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
|
80
|
|
LEGAL MATTERS
|
81
|
|
EXPERTS
|
81
|
|
WHERE YOU CAN FIND ADDITIONAL INFORMATION
|
81
|
|
FINANCIAL INFORMATION
|
F-1
|
This prospectus is part of a registration statement we filed with the Securities and Exchange Commission (the “SEC”). You should rely only on the information provided in this prospectus. We have not authorized anyone to provide you with information different from that contained in this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities other than the common stock offered by this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any common stock in any circumstances in which such offer or solicitation is unlawful. The selling stockholders are offering to sell, and seeking offers to buy, shares of common stock only in jurisdictions where offers and sales are permitted.
Neither the delivery of this prospectus nor any sale made in connection with this prospectus shall, under any circumstances, create any implication that there has been no change in our affairs since the date of this prospectus or that the information contained by reference to this prospectus is correct as of any time after its date. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or of any sale of common stock. The rules of the SEC may require us to update this prospectus in the future.
PROSPECTUS SUMMARY
The following summary is qualified in its entirety by the more detailed information appearing elsewhere in this prospectus. In this prospectus, unless the context requires otherwise, the terms “we”, “our”, “us” and the “Company” refer to Tanke Biosciences Corporation, a Nevada corporation formerly known as Greyhound Commissary, Inc. (“Greyhound”), as well as our direct and indirect subsidiaries, and our principal operating business, Guangzhou Tanke Industry Co., Ltd. (“Guangzhou Tanke”), a company organized under the laws of the People’s Republic of China (“China” or the “PRC”), which we control via a series of variable interest entity contractual agreements (the “VIE Agreements”) more fully described below.
We conduct our business through our subsidiaries, principally our wholly-owned subsidiary China Flying Development Limited (“China Flying”), a Hong Kong incorporated company, and its wholly-owned subsidiary Guangzhou Kanghui Agricultural Technology Co., Ltd. (“Kanghui Agricultural” or the “WFOE”), a wholly foreign owned enterprise incorporated as a limited liability company under the laws of the PRC. The Company operates and controls Guangzhou Tanke through Kanghui Agricultural and China Flying and in connection with the VIE Agreements.
“RMB” and “Renminbi” refer to the legal currency of China and “$”, “US dollar” and “US$” refer to the legal currency of the United States.
Overview of Our Business
Through Guangzhou Tanke, our principal operating business, we are one of the leading animal nutrition and innovative feed additive providers in China. Our products are distinguished from traditional artificial feed additives in that they are environmentally-friendly and are designed to optimize the growth and health of livestock such as pigs and cattle, as well as farmed fish. One of our most popular products is an organic trace mineral additive that we believe is one of the few Chinese-developed organic products in the trace mineral market.
Our target customers are mid-to-large sized feed product factories and large scale producers. The market for our products is significant and growing, as China seeks to meet the demand of its over 1.3 billion citizens for safe and reasonably priced food. With feed additives used in China at less than half the rate of that of the United States and Europe, we are seeking to capitalize on a significant market opportunity as Chinese feed producers modernize and expand the use of modern and more effective additives.
The Company currently produces 22 branded feed additives, with each brand available in seven different mixes that correspond to different stages of an animal’s life cycle. Our major products address most key market categories within China’s animal feed additive industry including: Organic Trace Mineral Additives, which account for approximately 79% of our revenue, Feed Acidifiers, Seasonings and Flavor Enhancers, which account for approximately 17% of our revenue, and Herbal Medicinal Additives, which account for approximately 1% of our revenue. Our extensive distribution network reaches China’s top 10 feed producers and the 500 largest animal farming operations. While the majority of our sales are domestic, international sales, mainly in Southeast Asia, Latin American and other developing countries, currently account for approximately 1.64% of our total sales.
Background and Key Events
Greyhound, our predecessor corporation, was organized on May 24, 1989 under the laws of the State of Idaho and was re-incorporated under the laws of the State of Nevada on November 1, 2007. Greyhound was initially created to provide a variety of services related to the operation of a nearby greyhound dog-racing track. Following inception, Greyhound raised funds to assist it in providing food, shelter, healthcare and other services to animals used in the greyhound racing. Subsequently the track was closed and the business was curtailed. Beginning in 1995, Greyhound engaged in an ongoing search for suitable business opportunities, including a potential merger.
On February 9, 2011, Greyhound closed (i) a share exchange transaction (the “Share Exchange”), pursuant to which (among other things) we became the sole stockholder of China Flying and changed our name to “Tanke Biosciences Corporation”, and (ii) a private placement (the “Private Placement”) of $7,670,071.50 for 6,669,627 units (the “Units”), with each Unit consisting of a $1.15 principal amount 8% Senior Convertible Note (each, a “Note”) and a Common Stock Purchase Warrant (each, a “Warrant”) to purchase one share of our common stock, with an exercise price of $1.40 per share. The Share Exchange and the Private Placement are more fully described below.
Our principal offices are located at East Tower of Hui Hao Building, No. 519 Machang Road, Pearl River New City, Guangzhou, China. Our telephone number is +86-20-3885-9025.
VIE Agreements and Call Option Agreement
On January 3, 2011, Kanghui Agricultural entered into the VIE Agreements, which included a Consulting Services Agreement, Operating Agreement, Voting Rights Proxy Agreement, Equity Pledge Agreement and an Option Agreement, with Guangzhou Tanke and the shareholders of Guangzhou Tanke, namely Mr. Guixiong Qiu (“Mr. Qiu”), Mr. Bi Gao (“Mr. Gao”), Ms. Xiuzhen Liang (“Ms. Liang”) and Mr. Bing Teng (“Mr. Teng”) (collectively referred to as the “Tanke Shareholders”), who are all PRC citizens. Pursuant to the VIE Agreements, Kanghui Agricultural effectively assumed management of the business activities of Guangzhou Tanke and has the right to appoint all executives and senior management and the members of the board of directors of Guangzhou Tanke.
In addition, on January 3, 2011, the Tanke Shareholders each entered into a call option agreement (the “Call Option Agreement”) with Golden Genesis Limited (“Golden Genesis”), a British Virgin Islands company, and Wong Kwai Ho (“Ms. Wong”), a Hong Kong resident owning 100% of the issued and outstanding shares of Golden Genesis. Pursuant to the Call Option Agreement, the Tanke Shareholders each received three year options exercisable for shares of common stock in the Company and options for 34% of the shares shall vest and become exercisable on December 31, 2011, options for 33% of the shares shall vest and become exercisable on December 31, 2012 and options for 33% of the shares shall vest and become exercisable on December 31, 2013. Upon exercise of the options, the Tanke Shareholders may purchase each share for $0.01 and if the Tanke Shareholders exercise all of their options they will own a majority of our outstanding shares of Common Stock and Golden Genesis will no longer be a stockholder of the Company. The Call Option Agreement provides that Golden Genesis shall not dispose of the respective portion of the shares of common stock without the Tanke Shareholders’ prior written consent.
Pursuant to the VIE Agreements, Kanghui Agricultural has the right to advise, consult, manage and operate Guangzhou Tanke for an annual fee in the amount of Guangzhou Tanke’s yearly net profits after tax. Additionally, the Tanke Shareholders pledged their rights, titles and equity interest in Guangzhou Tanke as security for Kanghui Agricultural to collect consulting and services fees provided to Guangzhou Tanke through an Equity Pledge Agreement. For an equity pledge agreement to be legally established, the agreement must be filed with the local Administration for Industry and Commerce (“AIC”) which issues the business license to the pledgor. The pledge agreement was filed with the local AIC in July 2012. Under Chinese law, the pledgee, Kanghui Agricultural, will not automatically receive the equity and become the shareholder of the operating company if the pledgor, the Tanke Shareholders, do not pay off the debt secured by the equity. Without an agreement between pledgee and pledgor, the pledged equity cannot be transferred to any third party. The pledgee has priority in receiving the proceeds from the auction or sale of the equity, or can further negotiate with the pledgor to acquire the equity pledged.
In order to further reinforce Kanghui Agricultural’s rights to control and operate Guangzhou Tanke, the Tanke Shareholders granted Kanghui Agricultural an exclusive right and option to acquire all of their equity interests in Guangzhou Tanke through the Option Agreement.
Pursuant to the Call Option Agreement, Golden Genesis shall transfer up to 100% of the shares of the Company’s common stock to the Tanke Shareholders over the next three years. Upon the completion of such transfers the Tanke Shareholders will be stockholders of the Company and Golden Genesis will no longer be a stockholder of the Company.
Details regarding how restrictions on foreign ownership and other regulations in China may impact the VIE agreements and call option agreement are set out in “Description of Business - Government Regulation” on page 50.
We entered into the VIE Agreements and Call Option in order to comply with applicable Chinese law and because such structure was a tax-efficient alternative, along with other reasons as described below, for the Tanke Shareholders.
VIE Compared to Direct Equity Structure
Currently, PRC laws do not restrict foreign investment in our industry and we could function with a direct equity structure. As our business is an encouraged industry under the Catalogue of Industries for Guiding Foreign Investment (the “Catalogue”), there are no restrictions or limitations on the shareholding percentage for the registrant or its Chinese subsidiaries. The other laws of China also do not impose shareholding limitations or restrictions on such direct acquisition. Under the current laws of China, both the VIE structure and direct equity structure are allowable.
We chose a VIE structure over a direct equity structure because for a direct equity structure (i) regulatory approval would be required, (ii) we would have been required to pay the Tanke Shareholders cash for the stock of Guangzhou Tanke (the operating company), and (iii) tax efficiency reasons. Other benefits for a VIE structure as opposed to a direct equity structure generally also include a shorter transaction time and lower transaction costs.
Regulatory Approval
In China, a direct equity ownership of the Chinese operating company can be accomplished in two ways:
(1) A foreign company, the registrant or China Flying, could acquire the operating company. In this instance, Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the “New M&A Rules”, apply and the foreign company will need to submit relevant documents to the Chinese authorities which are determined by Chinese laws and regulations related to the establishment of a foreign invested company, with the consideration of formation, the industry in which the Chinese company operates, and total investment scale of the acquired company (Article 21 of the New M&A Rules). The New M&A Rules were jointly issued by six Chinese government agencies, namely, the Ministry of Commerce, or MOFCOM, the State Administration for Industry and Commerce, or SAIC, the China Securities Regulatory Commission, or CSRC, the State Administration of Foreign Exchange, or SAFE, the State Assets Supervision and Administration Commission, or SASAC, and the State Administration for Taxation, or SAT, and became effective on September 8, 2006; or
2
(2) A Chinese subsidiary of the registrant, Kanghui Agricultural, as the WFOE, could acquire the operating company. Generally, Chinese law allows for a WFOE to acquire a Chinese operating company, which is not conducting business in a limited or prohibited industry under the Catalogue of Industries for Guiding Foreign Investment (the “Catalogue”), without the approval from the foreign investment authority in China. However, if the acquisition of a Chinese domestic company (even though the Chinese domestic company is not in a limited or prohibited industry under the Catalogue) by a WFOE is immediately after the establishment of the WOFE, the relevant foreign investment authority may challenge such acquisition for its possible intention of bypassing the New M&A Rules and may request that the WFOE go through the regulatory approval procedure. Generally, no further approval from the foreign investment authority is required for a WOFE that has been in operation for more than two years and the acquisition involves a Chinese domestic company which is not in a limited or prohibited industry under the Catalogue. In this instance, Kanghui Agricultural did not exist for two years prior to the transaction.
Payment of Cash for Capital Stock of Guangzhou Tanke
Under the direct equity structure, we would be required to pay the Tanke Shareholders cash for the capital stock of Guangzhou Tanke because stock-for-stock acquisitions are not permitted under Chinese law. It is stipulated in Article 29 of the New M&A Rules that if the equity of a foreign company is intended to be used as consideration in a stock-for-stock acquisition, such foreign company shall have been listed in an overseas market (excluding over-the-counter market) with a stable stock price for one year. If such foreign company is not a listed company, it has to be a “special purpose vehicle.” Under the New M&A Rules, the Company does not fall within definition of a “special purpose vehicle” because it is not directly or indirectly controlled by a Chinese company or natural person (Article 39 of the New M&A Rules). Further, the foreign investor which intends to adopt the stock-for-stock acquisition must also apply to the Ministry of Commerce , or MOFCOM. Currently, there is no successful precedent regarding MOFCOM approval of such stock-for-stock acquisition.
Tax Reasons
The structure provided by the VIE Agreements is tax-free to the Tanke Shareholders under applicable PRC law. Under the direct equity structure, the price for the equity of the operating company is based on the valuation by a qualified asset appraisal institution (Article 14 of the New M&A Rules). The income tax is based on the difference between the valued price and the actual selling price. A VIE structure will not trigger the shareholders of the operating company to pay income tax which will be otherwise applied in case of direct equity structure. We are unable to calculate the actual tax savings benefit because we did not have an appraisal of the operating company completed for the transaction.
Share Exchange
On February 9, 2011, pursuant to a Share Exchange Agreement, dated January 3, 2011 (the “Share Exchange Agreement”), between Greyhound, Golden Genesis and China Flying, the Company closed the Share Exchange and acquired all of the outstanding equity securities of China Flying from Golden Genesis, which was the sole shareholder of China Flying immediately prior to the closing of the Share Exchange. In exchange, we issued to Golden Genesis 10,758,000 newly issued shares of our common stock. In addition, pursuant to the terms of the Share Exchange Agreement, the Company effected a 1 for 8.512 reverse stock split to modify the Company’s capital structure to accommodate the transactions contemplated by the Share Exchange and the Private Placement and to put in place an appropriate capital structure for the Company following the closing of the Share Exchange and the Private Placement. Such securities were not registered under the Securities Act of 1933, as amended (the “Securities Act”). These securities qualified for exemption under Section 4(2) of the Securities Act since the issuance of securities by us did not involve a public offering. All references to number of shares and per share amounts included in this prospectus give effect to the 1 for 8.512 reverse stock split.
We consummated the Share Exchange in order to acquire China Flying and Kanghui Agricultural. Pursuant to the terms of the VIE Agreements, the Share Exchange also resulted in us acquiring control of the business and operations of Guangzhou Tanke.
Private Placement
On February 9, 2011, we entered into a Securities Purchase Agreement (the “Securities Purchase Agreement”) with certain accredited investors who are selling stockholders in this offering (each, including their respective successors and assigns, an “Investor” and collectively, the “Investors”) and, with respect to certain sections of the Securities Purchaser Agreement, Euro Pacific Capital, Inc. (“Euro Pacific”) and Newbridge Securities Corporation, relating to the Private Placement of 6,669,627 Units at a purchase price of $1.15 per Unit. Each Unit consisted of a $1.15 principal amount 8% Senior Convertible Note and a Common Stock Purchase Warrant to purchase one share of the Company’s common stock, with an exercise price of $1.40 per share. We sold 6,669,627 Units in the Private Placement, for gross proceeds of $7,670,071.50. In addition, in connection with the Private Placement, we also issued to certain affiliates of Euro Pacific, our lead placement agent in the Private Placement, three-year warrants (the “Agent Warrants”) to purchase an aggregate of 666,963 shares of our common stock at an exercise price of $1.15 per share. The Private Placement met the requirements to qualify for exemption under Regulation D promulgated under the Securities Act.
The Notes are convertible into shares of our common stock at a price of $1.15 per share (subject to customary weighted average and stock based anti-dilution protection) and are payable 24 months from the date of their issuance with an interest rate of 8% per annum payable semiannually in arrears. Each three-year Warrant entitles the holder to purchase one share of our common stock, with an exercise price of $1.40 per share (subject to customary weighted average and stock based anti-dilution protection). We also issued to certain affiliates of Euro Pacific, our lead placement agent in the Private Placement, three-year Agent Warrants to purchase an aggregate of 666,963 shares of common stock at an exercise price of $1.15 per share. The Agent Warrants also contain a cashless exercise option. The issuance of the Notes and the Warrants was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D. The issuance of the Agent Warrants was exempt from registration under Section 4(2) of the Securities Act.
In connection with the closing of the Private Placement, we also entered into the following additional agreements:
|
●
|
Registration Rights Agreement. We entered into a Registration Rights Agreement (the “Registration Rights Agreement”) with the Investors which sets forth the rights of the Investors to have the shares of common stock underlying the Notes and Warrants registered with the SEC for public resale. The filing of the registration statement of which this prospectus is a part is intended to satisfy certain of our obligations under the terms of the Registration Rights Agreement. We also entered into a Registration Rights Agreement with the Investors.
|
|
●
|
Pursuant to the Registration Rights Agreement, we agreed to file, no later than April 11, 2011, a registration statement to register the shares underlying the Notes and the Warrants and to have such registration statement effective no later than September 18, 2011. If the registration statement was not filed by April 11, 2011 (the “Filing Failure”), is not effective by September 18, 2011 (the “Effectiveness Failure”) or if, after the effective date, sales of securities included in the registration statement cannot be made (including, without limitation, because of a failure to keep the registration statement effective, to disclose such information as is necessary for sales to be made pursuant to the registration statement, to register a sufficient number of shares of Common Stock or to maintain the listing of the Common Stock) (a “Maintenance Failure”) then, as liquidated damages (and in complete satisfaction and to the exclusion of any claims or remedies inuring to any holder of the securities) the Company is required to pay an amount in cash equal to 1% of the aggregate purchase price paid by the Investors on each of the following dates: (i) 20 days following the date of a Filing Failure; (ii) 30 days following the initial day of a Maintenance Failure; (iii) on every thirtieth day thereafter (pro-rated for periods totaling less than thirty days) until such failure is cured; (iv) on every thirtieth day after the day of an Effectiveness Failure and thereafter (pro rated for periods totaling less than thirty days) until such Effectiveness Failure is cured; (v) on every thirtieth day after the initial day of a Maintenance Failure and thereafter (pro rated for periods totaling less than thirty days) until such Maintenance Failure is cured. The payments to be made by the Company are limited to a maximum of 6% of the aggregate amount paid by the Investors ($460,204.29). As of March 30, 2012, we accrued the maximum amount of $460,206 for registration delay penalty.
|
|
●
|
Interest Escrow Agreement. We entered into an Escrow Agreement (the “Interest Escrow Agreement”) with Euro Pacific and Escrow, LLC (the “Escrow Agent”), as escrow agent, pursuant to which the Company deposited into escrow an amount of proceeds of the Private Placement equal to one semi-annual interest payment on the Notes to secure prompt interest payments under the Notes. Until such time as 75% of the Notes are converted into shares of common stock, if such escrow is depleted in order to make interest payments, the Company has agreed to promptly replenish such escrow amount.
|
|
●
|
Securities Escrow Agreement. We entered into a Securities Escrow Agreement (the “Securities Escrow Agreement”) with Euro Pacific, as representative of the Investors, Golden Genesis and the Escrow Agent, as escrow agent, pursuant to which Golden Genesis placed in escrow 2,000,000 shares of common stock, to be disbursed to either the Investors on a pro rata basis or to Golden Genesis based on the financial performance of Guangzhou Tanke, our principal operating business, for the fiscal years ending December 31, 2011 and 2012.
We did not achieve the Adjusted Income of $4,652,410 for the year ended December 31, 2011, therefore 1,000,000 shares of common stock placed in escrow have been distributed to the Investors on a pro rata basis, pursuant to the terms of the Securities Escrow Agreement.
|
Corporate Name Change
On February 8, 2011, in connection with the closing of the Share Exchange and Private Placement, we filed a Certificate of Amendment to our Articles of Incorporation with the Secretary of State of the State of Nevada to change our corporate name from “Greyhound Commissary, Inc.” to “Tanke Biosciences Corporation”, a name that more accurately reflects the business operations of the Company following the closing of the Share Exchange. The name change was effective as of February 10, 2011.
Our Organizational Structure
Our organization structure is illustrated below:
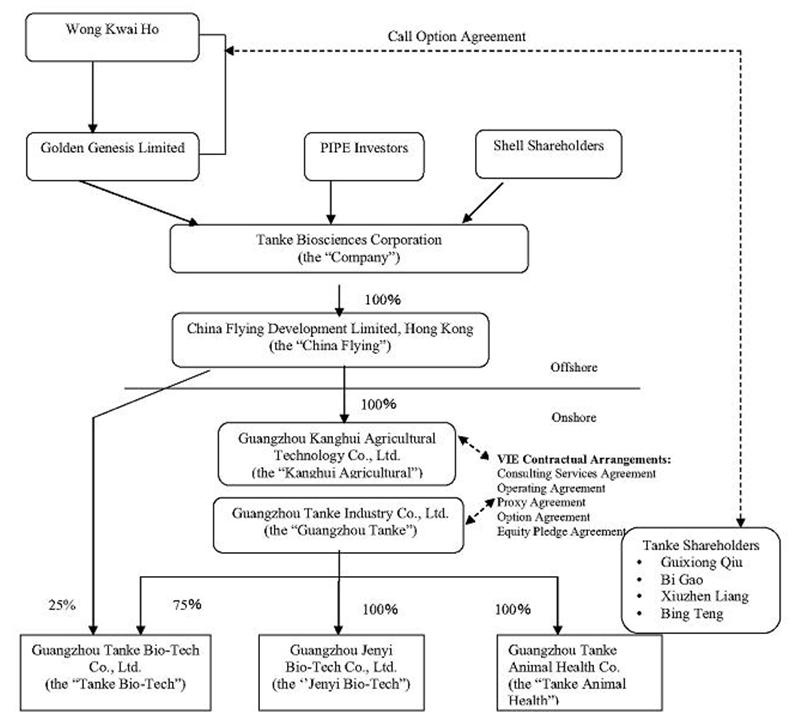
Mr. Guixiong Qiu is the sole director of the Company and Golden Genesis, the Company’s largest stockholder. Other than Mr. Qiu, Golden Genesis does not have other directors or officers. Ms. Wong Kwai Ho is the sole director of China Flying. Ms. Wu Chun Rui is the sole director of Kanghui Agricultural and the legal representative who is authorized to sign corporate documents on behalf of Kanghui Agricultural. Mr. Guixiong Qiu, Mr. Bi Gao, Mr. Xugang Shu, and Ms. Xiuzhen Liang are the directors and officers of Guangzhou Tanke and its subsidiaries, Guangzhou Tanke Bio-Tech Co., Ltd., Guangzhou Jenyi Bio-Tech Co., Ltd., and Guangzhou Tanke Animal Health Co.
Implications of Being an Emerging Growth Company
We qualify as an emerging growth company as that term is used in the JOBS Act. An emerging growth company may take advantage of specified reduced reporting and other burdens that are otherwise applicable generally to public companies. These provisions include:
|
•
|
A requirement to have only two years of audited financial statements and only two years of related MD&A;
|
|
•
|
Exemption from the auditor attestation requirement in the assessment of the emerging growth company’s internal control over financial reporting under Section 404 of the Sarbanes-Oxley Act of 2002;
|
|
•
|
Reduced disclosure about the emerging growth company’s executive compensation arrangements; and
|
|
•
|
No non-binding advisory votes on executive compensation or golden parachute arrangements.
|
We have already taken advantage of these reduced reporting burdens in this prospectus, which are also available to us as a smaller reporting company as defined under Rule 12b-2 of the Exchange Act.
In addition, Section 107 of the JOBS Act also provides that an emerging growth company can take advantage of the extended transition period provided in Section 7(a)(2)(B) of the Securities Act for complying with new or revised accounting standards. We have elected to use the extended transition period provided above and therefore our financial statements may not be comparable to companies that comply with public company effective dates.
We could remain an emerging growth company for up to five years, or until the earliest of (i) the last day of the first fiscal year in which our annual gross revenues exceed $1 billion, (ii) the date that we become a “large accelerated filer” as defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, which would occur if the market value of our common stock that is held by non-affiliates exceeds $700 million as of the last business day of our most recently completed second fiscal quarter, or (iii) the date on which we have issued more than $1 billion in non-convertible debt during the preceding three year period.
The Offering
|
Common stock outstanding immediately before this offering:
|
13,324,083 shares
|
|
Common stock offered by the selling stockholders:
|
Up to 16,243,672 shares underlying securities held by the selling stockholders
|
|
Common stock outstanding immediately after this offering:
|
Up to 27,330,300 shares, assuming full conversion of the principal on the Notes and exercise of the Warrants, but no conversion of any interest accrued on the Notes.
|
|
OTCBB Symbol:
|
TNBI (formerly GHND). No active market for our common stock presently exists.
|
|
Use of proceeds:
|
We will not receive any proceeds from the sale of the common stock offered hereby. However, we may receive up to a maximum of $17,007,548.85 of proceeds from the conversion of Notes and exercise of Warrants by certain selling stockholders, which proceeds we would expect to use for general working capital. No assurances can be given, however, that all or any portion of such warrants will ever be exercised.
|
|
Risk Factors:
|
Investing in our securities involves a high degree of risk. As an investor you should be able to bear a complete loss of your investment. You should carefully consider the information set forth in the section of this prospectus entitled “Risk Factors.”
|
RISK FACTORS
Our business, as well as our common stock, are highly speculative in nature and involve a high degree of risk. Our securities should be purchased only by persons who can afford to lose their entire investment. Accordingly, prospective investors should carefully consider, along with other matters referred to herein, the following risk factors in evaluating our business before purchasing any of our common stock.
Risks Related to Our Business
We may not possess all the licenses required to operate our business, or may fail to maintain the licenses we currently hold. This could subject us to fines and other penalties, which could have a material adverse effect on our results of operations.
We are required to hold a variety of permits, licenses and certificates to conduct our business in China and we may not possess all of the permits, licenses and certificates required for our business. We entered into a Land Lease Agreement valid from May 20, 2006 to May 20, 2021 with the government of Huaqiao Town, Huadu District, Guangzhou under which the Government of Huaqiao Town granted us a land use right covering the land where our manufacturing facility currently stands. This lease will be superseded by a Land Use Right Certificate and will be automatically terminated once we obtain such certificate. Due to changes in the relevant PRC regulations, we have not been granted such certificate for this land. Therefore, pursuant to applicable PRC law, we are not permitted to operate our manufacturing facility without such certificate and as a result, there is a risk that the PRC government may declare our Land Lease Agreement invalid.
We are currently negotiating with the local government to obtain a Land Use Right Certificate, which would permit us to operate our business as it is currently conducted. Without the Land Use Right Certificate, we are unable to apply for a Property Ownership Certificate for our manufacturing facilities. Until we obtain the Land Use Right Certificate, the PRC government may evict our personnel from the premises and remove our manufacturing facilities that we built on the premises. Such action would have a very significant and negative impact on our operations and business.
In addition, there may be other circumstances under which the approvals, permits, licenses or certificates granted by the governmental agencies are subject to change without substantial advance notice, and it is possible that we could fail to obtain the approvals, permits, licenses or certificates that are required to expand our business as we intend. If we fail to obtain or to maintain such permits, licenses or certificates, or renewals are granted with onerous conditions, we could be subject to fines and other penalties and be limited in the number or the quality of the products that we would be able to offer. As a result, our business, result of operations and financial condition could be materially and adversely affected.
Concerns with the safety and quality of agricultural feed additive products could cause customers to avoid our products.
We could be adversely affected if our customers and the ultimate consumers of our feed additive products lose confidence in the safety and quality of various feed additive products. Adverse publicity about these types of concerns may discourage our customers from buying our products or cause production and delivery disruptions. Any negative change in customer perceptions about the safety and quality of our feed additive products could adversely affect our business and financial condition.
If our feed additive products become adulterated or misbranded, we would need to recall those items and may experience product liability claims if consumers are injured as a result.
Animal feed products occasionally contain contaminants due to inherent defects in those products or improper storage or handling. Under adverse circumstances, animal feed manufacturers may need to recall some of their products if they become adulterated or misbranded, and may also be liable if the consumption of any of their products causes injury. A widespread product recall could result in changes to one or more of our business processes, product shortages, loss of customer confidence in our products or other adverse effects on our business. If we are required to defend against a product liability claim, whether or not we are found liable under the claim, we could incur substantial costs, our reputation could suffer and our customers might substantially reduce their existing or future orders from us.
We face significant competition in the sales of our agricultural feed additive products.
Competition in the feed additive industry, especially with companies with greater resources, may make us unable to compete successfully, which could adversely affect our business.
In general, the competitive factors in the feed additive industry in China include:
|
●
|
price;
|
|
●
|
product quality;
|
|
●
|
brand identification;
|
|
●
|
breadth of product line; and
|
|
●
|
customer service.
|
To the extent that our products and services do not exhibit these qualities, our ability to compete will be hindered.
The markets in which we operate are highly competitive and fragmented and we may not be able to maintain market share.
We operate in highly competitive markets and compete with numerous local Chinese feed additive manufacturers. We expect competition to persist and intensify in the future. Our domestic competitors are mainly leaders in the feed additive markets in China. Our small local competitors may have better access in certain local markets to customers and prospects, an enhanced ability to customize products to a particular region or locality and more established local distribution channels within a small region. We also compete with large Chinese national and multi-national competitors who may have competitive advantages over us in certain areas such as access to capital, technology, product quality, economies of scale and brand recognition and may also be better positioned than us to develop superior product features and technological innovations and to exploit and adapt to market trends. Due to the lack of publicly available information about our competitors and industry, we may not be able to conduct in-depth research and analysis on our current or new markets. Therefore, we may not be able to determine our direct competitors, such competitors' revenues or market share.
In addition, China’s entry into the World Trade Organization may lead to increased foreign competition for us. International producers and traders import products into China that generally are of higher quality than those produced in the local Chinese market. We may face additional competition if these products are considered to be better than the type of feed additives we produce. If we are not successful in our marketing and advertising efforts to increase awareness of our brands, our revenues could decline, which could have a material adverse effect on our business, financial condition, results of operations and share price. We may not be able to compete successfully against existing or new competition in our markets.
We may not be able to fully implement our current business strategy if we are unable to develop a second manufacturing facility.
As part of our current business strategy, we intend to continue to increase our production volume in order to gain additional market share. In connection with that strategy, we plan to build a second manufacturing facility that would double our organic trace mineral production capacity. There is a risk that we may be unable to finish construction of the new facility, or operate it at a profit. If we are unable to achieve any or all of the foregoing it could have a material adverse effect on our business and results of operations.
We cannot be certain that our feed additive product innovations and marketing achievements to date will continue.
We believe that our past performance has been based on, and our future prospects will depend upon, in large part, our ability to continue to improve our existing feed additive products or develop new feed products. We may not be successful in introducing, marketing and producing any new feed products or feed additive product innovations, or that we will develop and introduce, in a timely manner, innovations to our existing products which satisfy customer needs or achieve market acceptance. Our failure to develop new feed additive products and introduce them successfully and in a timely manner could harm our ability to grow our business and could have a material adverse effect on our business, results of operations and financial condition.
We purchase many commodities that we use for raw materials and packaging; price changes for such commodities may adversely affect our profitability.
The raw materials used in our feed additive business are largely commodities that experience price fluctuations caused by external conditions and changes in governmental agricultural programs that we cannot control. As a result, we try to recover our commodity cost increases by increasing prices, promoting a higher-margin product mix and creating additional operating efficiencies. Substantial increases in the prices of packaging materials, such as corrugated cardboard, aluminum products, films and plastics, or higher prices of our raw materials could adversely affect our operating performance and financial results. Any substantial fluctuation in the prices of raw materials, if not offset by increases in our sales prices, could adversely affect our profitability.
Outbreaks of livestock disease can adversely affect sales of our products.
Outbreaks of livestock diseases can significantly affect demand for our feed additive products and could result in governmental restrictions on the sale of livestock products to or from customers, or require our customers to destroy their feeds. This could result in the cancellation of orders of feed additive products by our customers and create adverse publicity that may have a material adverse effect on the agricultural products industry and our ability to market our products successfully.
We do not typically have long-term sales contracts with our customers and our customers could at any time reduce purchases of, or entirely cease purchasing, our products, harming our operating results and business.
We typically do not have long-term volume sales contracts with our customers. Accordingly, our customers could reduce their purchases from us or cease purchasing our products altogether when a particular contract expires. A variety of factors, including economic, health, regulatory, political and social instability, could contribute to a slowdown in the demand or a reduction in the market price for our products because poultry demand and pricing is highly correlated with general economic activities. If any of our customers experience serious financial difficulties, it may lead to a decline in sales and write-offs of accounts receivable, which could harm our results of operations.
The cessation of tax exemptions and deductions by the Chinese government may affect our profitability.
On March 16, 2007, the National People’s Congress of China enacted a new tax law, or the New Tax Law, whereby both foreign investment enterprises, or FIEs, and domestic companies will be subject to a uniform income tax rate of 25%. On November 28 2007, the State Council of China promulgated the Implementation Rules of the New Tax Law, the “Implementation Rules”. Both the New Tax Law and the Implementation Rules have become effective on January 1, 2008. Both the New Tax Law and the Implementation Rules provide tax exemption treatment for enterprises engaged in agricultural industries, such as farming, foresting, fishing and animal husbandry. We have been informed that certain of our Chinese subsidiaries are eligible for relevant preferential tax treatment, including tax reduction and exemption, and certain of our products are exempted from value added tax. In the future, if the relevant tax authorities determine that Guangzhou Tanke, our principal operating business, is not eligible for tax exemption treatment it may materially and adversely affect our profits, business and financial performance.
Potential environmental liability could have a material adverse effect on our operations and financial condition.
As a manufacturer, we are subject to various Chinese environmental laws and regulations on air emission, waste water discharge, solid wastes and noise. The Environmental Protection Bureau of Huadu District, Guangzhou issued the Opinion on the Environmental Impact Statement regarding the Construction Project of Tanke Group on February 16, 2004. To date, we have not obtained any documentation of environment appraisal and acceptance inspection with respect to the completion of projects of the factory buildings and the production lines, because we have not obtained a Land Use Right Certificate for such site nor have we been granted Property Ownership Certificate. We are in the process of applying for a Land Use Right Certificate, which will enable us to obtain an environmental appraisal and acceptance inspection with respect to our facilities. Following our receipt of a Land Use Right Certificate, we anticipate that we will be able to obtain the necessary environmental approvals, but there can be no assurance in this regard. We may not be able to comply with environmental regulations at all times as the Chinese environmental legal regime is evolving and becoming more stringent. Therefore, if the Chinese government imposes more stringent regulations in future, we may have to incur additional and potentially substantial costs and expenses in order to comply with new regulations, which may negatively affect our results of operations. Furthermore, no assurance can be given that all potential environmental liabilities have been identified or properly quantified or that any prior owner, operator, or tenant has not created an environmental condition unknown to us. If we fail to obtain our Land Use Right Certificate or to comply with any of the present or future environmental regulations in any material aspects, we may suffer from negative publicity and be subject to claims for damages that may require us to pay substantial fines or have our operations suspended or even be forced to cease operations.
We do not presently maintain business disruption insurance and any disruption of the operations in our production facility would damage our business.
All of our feed additive products are currently manufactured in our production facility in the Huadu Economic Development Zone near the capital city of Guangzhou in the province of Guangdong, China. Our operations could be interrupted by fire, flood, earthquake and other events beyond our control. Any disruption of the operations in our production facility would have a significant negative impact on our ability to manufacture and deliver products as we would likely be unable to outsource our production on terms favorable to us, if at all. Failure to replace any lost production capability would cause a potential reduction in sales, the cancellation of orders, loss of valuable employees, damage to our reputation and potential lawsuits.
Our products and processes can expose us to product liability claims.
Product liability claims or product recalls can adversely affect our business reputation and expose us to increased scrutiny by local, provincial, and central governmental regulators. The packaging, marketing and distribution of agricultural feed additive products entail an inherent risk of product liability and product recall and the resultant adverse publicity. We may be subject to significant liability if the consumption of any of our products causes injury, illness or death of livestock, other animals or humans. We could be required to recall certain of our feed additive products in the event of contamination or damage to the products. In addition to the risks of product liability or product recall due to deficiencies caused by our production or processing operations, we may encounter the same risks if any third party tampers with our feed additive products. We may be required to perform product recalls, or that product liability claims will be asserted against us in the future. Any claims that may be made may create adverse publicity that would have a material adverse effect on our ability to market our feed additive products successfully or on our business, reputation, prospects, financial condition and results of operations. A successful product liability claim in excess of our insurance coverage could have a material adverse effect on us and could prevent us from obtaining adequate product liability insurance in the future on commercially reasonable terms.
Our current and future operations substantially depend on our management team and other key personnel, the loss of any of whom could disrupt our business operations.
Our business does and will depend in substantial part on the continued service of our senior management and founder, including but not limited to Guixiong Qiu, Xugang Shu and Bo Jun. The loss of the services of one or more of our key personnel could impede implementation and execution of our business strategy and result in the failure to reach our goals. We do not carry key person life insurance for any of our officers or employees. Our future success will also depend on the continued ability to attract, retain and motivate highly qualified personnel in the diverse areas required for continuing our operations. The rapid growth of the economy in China has caused intense competition for qualified personnel. We may not be able to retain our key personnel or that we will be able to attract, train or retain qualified personnel in the future.
Volatile energy prices could adversely affect our operating results.
In the last few years, energy prices have risen dramatically and are now volatile, which has resulted in increased and unpredictable increases for our raw materials costs. Continued or volatile increases in energy prices could adversely affect demand for our feed products and increase our operating costs, both of which would reduce our operating income.
If we need additional financing, we may not be available to find such financing on satisfactory terms or at all. The cost of obtaining additional financing may adversely affect our stockholders or the financial interests of the Company.
Our capital requirements may be accelerated as a result of many factors, including the growth and timing of our business development activities, budget shortfalls, unanticipated expenses or capital expenditures, future product opportunities, future licensing opportunities and future business combinations. Consequently, we may need to seek additional debt or equity financing, which may not be available on favorable terms, if at all, and which may be dilutive to our stockholders.
We may seek to raise additional capital through public or private equity offerings, debt financings or additional corporate collaboration and licensing arrangements. To the extent we raise additional capital by issuing equity securities, our stockholders may experience dilution. To the extent that we raise additional capital by issuing debt securities, we may incur substantial interest obligations, may be required to pledge assets as security for the debt and may be constrained by restrictive financial and/or operational covenants. Any provider of debt financing would also be superior position to our stockholders’ interest in the event of a bankruptcy or liquidation. To the extent we raise additional funds through collaboration and licensing arrangements, it may be necessary to relinquish some rights to our technologies or product candidates, or grant licenses on unfavorable terms.
We face risks associated with currency exchange rate fluctuations; any adverse fluctuation may adversely affect our operating margins
Almost all of our revenues are denominated in Renminbi. Conducting business in currencies other than US dollars subjects us to fluctuations in currency exchange rates that could have a negative impact on our reported operating results. Fluctuations in the value of the US dollar relative to other currencies impact our revenues, cost of revenues and operating margins and result in foreign currency translation gains and losses. If the exchange rate of the Renminbi is affected by lowering its value as against the US dollar, our reported profitability when stated in US dollars will decrease. Historically, we have not engaged in exchange rate hedging activities and have no current intention of doing so.
We may not be able to adequately protect and maintain our intellectual property, trademark, and brand names.
Our business has and will depend on our ability to continue to develop and market feed additive products. We currently own two patents and have entered into an exclusive licensing agreement with respect to two other patents covering aspects of the manufacturing and production of feed additives. We have filed applications for five additional patents. Our inability to protect our rights to this intellectual property may adversely affect our ability to prevent competitors from using our products and developments.
Intellectual property rights in China are still developing, and there are uncertainties involved in the protection and the enforcement of such rights. We will need to pay special attention to protecting our intellectual property and trade secrets. Failure to do so could lead to the loss of a competitive advantage that could not be compensated by our damages award.
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(2)(b) of the JOBS Act and therefore our financial statements may not be comparable to companies that comply with public company effective dates.
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(2)(b) of the JOBS Act allowing us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies and therefore our financial statements may not be comparable to companies that comply with public company effective dates.
Risks Related to Our Corporate Structure
The Chinese government may determine that the VIE Agreements which we utilize to operate Guangzhou Tanke are not in compliance with applicable Chinese laws, rules and regulations and that they are therefore unenforceable.
In China it is widely understood that foreign investment enterprises, or FIEs, are forbidden or restricted from engaging in certain businesses or industries which are sensitive to the economy. As we intend to centralize our management and operation in China without being restricted to conduct certain business activities which are important for our current or future business but are restricted or might be restricted in the future, we believe our VIE Agreements will be essential for our business operation.
A WFOE that operates for certain period of time is allowed to further acquire or invest in a Chinese domestic company, which is not in a limited or prohibited industry under the Catalogue, without approval from the Chinese foreign investment authority. Assuming that the WFOE, Kanghui Agricultural, has been operating for a certain period of time, the acquisition of the operating company (as being in the encouraged industry) by the WFOE will need no approval from foreign investment authority. However, after acquisition by WFOE, if the operating company intends to expand or invest in another Chinese domestic company (which is a limited or prohibited industry under the Catalogue) in the future, such acquisition or investment may need further approval from the foreign investment authority or may be rejected in case of prohibited industry because of its direct foreign investment background, such further acquisition or investment by the operating company may raise the concern of the foreign investment authority for its possible intention of bypassing the restrictions imposed under the Catalogue regarding the limited and prohibited industry for foreign investment.
Under the VIE structure, the operating company has no foreign investment background. Its future business expansion or investment will not be subject to the restrictions imposed under the Catalogue for foreign investment.
Pursuant to the terms of the VIE Agreement, almost all of our business activities in China are managed and operated by China Flying though Kanghui Agricultural, and almost all economic benefits and risks arising from the business of Guangzhou Tanke are transferred to China Flying and Kanghui Agricultural.
There are risks involved with the operation of Guangzhou Tanke under the VIE Agreements. We received an opinion, dated January 4, 2011, from Martin Hu & Partners, our PRC legal counsel, that if the Chinese government determines the VIE Agreement used to control the operating company to be unenforceable as they circumvent the Chinese restrictions relating to foreign investment restrictions, the relevant regulatory authorities would have broad discretion in dealing with such breach, including:
|
●
|
imposing economic penalties;
|
|
|
●
|
discontinuing or restricting the operations of China Flying, Kanghui Agricultural or Guangzhou Tanke;
|
|
|
●
|
imposing conditions or requirements in respect of the VIE Agreements with which Kanghui Agricultural or Guangzhou Tanke may not be able to comply;
|
|
|
●
|
requiring us to restructure the relevant ownership structure or operations;
|
|
|
●
|
taking other regulatory or enforcement actions that could adversely affect our business; and
|
|
|
●
|
revoking the business license and/or the licenses or certificates of China Flying or Kanghui Agricultural, Guangzhou Tanke, and/or voiding the VIE Agreements.
|
Any of these actions could have a material adverse impact on our business, financial condition and results of operations. Under PRC law the feed additive industry is not currently prohibited from receiving direct foreign investments. We implemented the VIE Agreements in order to ensure that if PRC law changes in the future that it will not adversely affect us.
We depend upon the VIE Agreements in conducting our operations in China, which may not be as effective as direct ownership.
We conduct our business through our Chinese operating subsidiaries and generate the revenues through the VIE Agreements. The VIE Agreements may not be as effective as direct ownership in providing us with control over Guangzhou Tanke. Under the VIE structure, if the parties breach or terminate any one of the VIE agreements, the WFOE (namely, Kanghui Agricultural) may not be able to receive the agreed benefits or profits from the operating company, which will in turn have an impact on distributing funds to the registrant.
The VIE Agreements are governed by Chinese laws and provide for the resolution of disputes through arbitration proceedings pursuant to Chinese laws. Accordingly, the VIE Agreements will be interpreted in accordance with Chinese laws. If Guangzhou Tanke or its shareholders fail to perform the obligations under the VIE Agreements, we may have to rely on legal remedies under Chinese laws, including seeking specific performance or injunctive relief, and claiming damages, and there is a risk that we may be unable to obtain these remedies. The legal environment in China is not as developed as in other jurisdictions. As a result, uncertainties in the Chinese legal system could limit our ability to enforce the VIE Agreements.
The pricing arrangement under the VIE Agreements may be challenged by Chinese tax authorities.
We could face adverse tax consequences if Chinese tax authorities determine that the VIE Agreements were not entered into based on arm’s length negotiations. If the Chinese tax authorities determine that the VIE Agreements were not entered into on an arm’s length basis, they may adjust the income and expenses of our company for Chinese tax purposes which could result in higher tax liability.
Any deterioration of the relationship between Kanghui Agricultur al and Guangzhou Tanke could materially and adversely affect the overall business operation of our company.
Our relationship with Guangzhou Tanke is governed by the VIE Agreements, which are intended to provide us, through our ownership of China Flying and indirect ownership of Kanghui Agricultural, with effective control over the business operations of Guangzhou Tanke. Guangzhou Tanke could violate the VIE Agreements, go bankrupt, suffer from difficulties in its business, fail to renew necessary permits and certifications or otherwise become unable to perform its obligations under the VIE Agreements and, as a result, our operations, reputation, business and stock price could be severely harmed.
If Kanghui Agricultural exercises the purchase options over Guangzhou Tanke’s equity pursuant to the VIE Agreements, the payment of purchase prices could materially and adversely affect our financial position.
Under the VIE Agreements, China Flying, through its ownership of Kanghui Agricultural, holds an option to purchase all or a portion of the equity of Guangzhou Tanke at a price, pro rata in case of not all, based on the capital paid in by the Tanke Shareholders ($1,147,704 or 9.5 million RMB). If applicable Chinese laws and regulations require an appraisal of the equity interest or provide other restrictions on the purchase price, the purchase price shall be the lowest price permitted under the applicable Chinese laws and regulations. As Guangzhou Tanke is already a contractually controlled affiliate to our company, Kanghui Agricultural’s purchase of Guangzhou Tanke’s equity would not bring immediate benefits to us. Due to the existence of the VIE Agreements, we currently receive all of the revenue of Guangzhou Tanke, after the payment of taxes. Therefore, even if we own Guangzhou Tanke, we will not have a stronger claim to its revenue. In addition, payment of the option price would significantly decrease our cash and this reduction of cash would have a material adverse impact on our financial position.
If the consulting service agreement between Kanghui Agricultural and Guangzhou Tanke is terminated, it could materially and adversely affect the business operation of our company.
Either Guangzhou Tanke or Kanghui Agricultural may terminate the consulting service agreement entered into by and between them as part of the VIE agreements if circumstances arise that could materially and adversely affect the performance of the objectives of this agreement. We may close our control over the operations of Guangzhou Tanke if the consulting service agreement is terminated.
Risks Associated With Doing Business in China
If Guangzhou Tanke’s land use rights are revoked, we would have no operational capabilities.
Under Chinese law, land is owned by the state or rural collective economic organizations. The rural collective economic organizations issue to tenants the rights to use rural land. Use rights can be revoked and the tenants forced to vacate at any time when redevelopment of the land is in the public interest. The public interest rationale is interpreted quite broadly and the process of land appropriation may be less than transparent. We rely heavily on Guangzhou Tanke’s land use rights for our operations, and the loss of such rights would have a material adverse effect on our business.
We entered into a Land Use Right Grant Agreement with the government of Huaqiao Town, Huadu District, Guangzhou under which the Government of Huaqiao Town granted us certain land use rights for an area of approximately 60 mu where our manufacturing facility is built. The land use right of such parcel of land is owned collectively by local farmers. However, we currently do not maintain a Land Use Right Certificate for such parcel of land. Having no use right certificate of our land would have a material adverse effect on us as we would be required to relocate our facilities and obtain new land use rights, and there is a risk that we would not be able to accomplish such a relocation with reasonable cost or at all.
In addition, we currently do not maintain a building ownership certificate for our manufacturing facility. Because Guangzhou Tanke does not have land use right certificate on this parcel of land, it neither applied for nor will be granted a building ownership certificate for the manufacturing facility it built on this parcel of land. We may not be able to eventually obtain the building ownership certificate for the foresaid land with reasonable cost.
Economic, political and social conditions in China are subject to significant uncertainty and could affect our business.
All of our operations are located in China and our business is subject to political and economic uncertainties in China. The economy of China differs from the economies of most developed countries in many respects, including the level of government involvement, the level of development, control of foreign exchange, and methods for allocating resources. A substantial portion of productive assets in China are owned by the Chinese government. Changes in Chinese policies, laws and regulations, or in their interpretation or the imposition of confiscatory taxation, restrictions on currency conversion, restrictions or prohibitions on imports, restrictions or prohibitions on dividend payments to stockholders, devaluations of currency or the nationalization or other expropriation of private enterprises may occur from time to time without notice and could have a material adverse effect on our business. Nationalization or expropriation could result in the total loss of our investment in China. We have no control over most of these risks and may be unable to anticipate changes in Chinese economic and political conditions.
The Chinese government exerts substantial influence over the manner in which we must conduct our business activities.
We are dependent on our relationship with the local government in the province in which we operate our business. Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its laws and regulations, including those relating to taxation, environmental regulations, land use rights, property and other matters. The Chinese central or local governments may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties.
Future inflation in China may inhibit our ability to conduct business in China. In recent years, the Chinese economy has experienced periods of rapid expansion and high rates of inflation. Rapid economic growth can lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may have an adverse effect on profitability. These factors have led to the adoption by Chinese government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. High inflation may in the future cause Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in China, and thereby harm the market for our products.
If relations between the United States and China worsen, our share price may decrease and we may have difficulty accessing U.S. capital markets.
At various times during recent years, the United States and China have had disagreements over political and economic issues. Any political or trade controversies between the United States and China in the future could adversely affect the market price of our common stock and our ability to access U.S. capital markets.
In the fiscal year ended December 31, 2011, we derived approximately 99% of our sales in China. A slowdown or other adverse developments in China’s economy may materially and adversely affect our customers, demand for our products and our business.
During the fiscal year ended December 31, 2011, we generated 99% of our sales in China. We continue to focus our growth of business primarily in China in the next few years simply due to the market growth potential and the cost of doing business is still relatively low comparing with selling overseas. However, the significant growth of China’s economy in recent years may not continue. The industry which we are involved in China is relatively new and growing, but we do not know how sensitive we are to a slowdown in economic growth or other adverse changes in the Chinese economy which may affect demand for our products. In addition, the Chinese government also exercises significant control over Chinese economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Although we are in one of the industries the Chinese government highly supports – agriculture, and receive certain preferential treatment, we are uncertain how significant an overall economic slowdown or recession in China will reduce the demand of our products and adversely affect our business.
We may have limited legal recourse under Chinese laws if disputes arise under our contracts with third parties.
The Chinese government has enacted some laws and regulations dealing with matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. However, their experience in implementing, interpreting and enforcing these laws and regulations is limited, and our ability to enforce commercial claims or to resolve commercial disputes is unpredictable. If our new business ventures are unsuccessful, or other adverse circumstances arise from these transactions, we face the risk that the parties to these ventures may seek ways to terminate the transactions, or, may hinder or prevent us from accessing important information regarding the financial and business operations of these acquired companies. The resolution of these matters may be subject to the exercise of considerable discretion by agencies of the Chinese government, and forces unrelated to the legal merits of a particular matter or dispute may influence their determination. Any rights we may have to specific performance, or to seek an injunction under Chinese laws, in either of these cases, are severely limited, and without a means of recourse by virtue of the Chinese legal system, we may be unable to prevent these situations from occurring. In addition, the inability to enforce or obtain a remedy under any of our future agreements could result in a significant loss of business, business opportunities or capital and could have a material adverse impact on our operations business and financial condition.
Because our principal assets are located outside of the United States and our sole director and officer and all of our key employees reside in China, outside of the United States, it may be difficult for you to enforce your rights based on the United States federal securities laws against us and our officers and directors in the United States or to enforce judgments of United States courts against us or them in China.
Our sole director and officer and all of our key employees reside in China, outside of the United States. In addition, our principal operating business is located in China and all of our assets are located outside of the United States. China does not have a treaty with United States providing for the reciprocal recognition and enforcement of judgments of courts. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the United States federal securities laws against us in the courts of either the United States or China and, even if civil judgments are obtained in courts of the United States, to enforce such judgments in Chinese courts. Further, it is unclear if extradition treaties now in effect between the United States and Chinese would permit effective enforcement against us or our officers and directors of criminal penalties, under the United States federal securities laws or otherwise.
We may have difficulty establishing adequate management, legal and financial controls in China, which could impair our planning processes and make it difficult to provide accurate reports of our operating results.
China historically has been deficient in Western style management and financial reporting concepts and practices, as well as in modern banking, and other control systems. We may have difficulty in hiring and retaining a sufficient number of qualified employees familiar with these concepts, practices and systems to work in China. As a result of these factors, and especially given that we expect to be a publicly listed company in the U.S. and subject to regulation as such, we may experience difficulty in establishing management, legal and financial controls, collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices that meet Western standards. We may have difficulty establishing adequate management, legal and financial controls in China.
Currency fluctuations and restrictions on currency exchange may adversely affect our business, including limiting our ability to convert Chinese Renminbi into foreign currencies and, if Chinese Renminbi were to decline in value, reducing our revenue in U.S. dollar terms.
Our reporting currency is the U.S. dollar and our operations in China use their local currency as their functional currency. Substantially all of our revenue and expenses are in Chinese Renminbi. We are subject to the effects of exchange rate fluctuations with respect to any of these currencies. For example, the value of the Renminbi depends to a large extent on Chinese government policies and China’s domestic and international economic and political developments, as well as supply and demand in the local market. Since 1994, the official exchange rate for the conversion of Renminbi to the U.S. dollar had generally been stable and the Renminbi had appreciated slightly against the U.S. dollar. However, on July 21, 2005, the Chinese government changed its policy of pegging the value of Chinese Renminbi to the U.S. dollar. Under the new policy, Chinese Renminbi may fluctuate within a narrow and managed band against a basket of certain foreign currencies. It is possible that the Chinese government could adopt a more flexible currency policy, which could result in more significant fluctuation of Chinese Renminbi against the U.S. dollar. We can offer no assurance that Chinese Renminbi will be stable against the U.S. dollar or any other foreign currency.
Our financial statements are translated into U.S. dollars at the average exchange rates in each applicable period. To the extent the U.S. dollar strengthens against foreign currencies, the translation of these foreign currencies denominated transactions results in reduced revenue, operating expenses and net income for our international operations. Similarly, to the extent the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions results in increased revenue, operating expenses and net income for our international operations. We are also exposed to foreign exchange rate fluctuations as we convert the financial statements of our foreign consolidated subsidiaries into U.S. dollars in consolidation. If there is a change in foreign currency exchange rates, the conversion of the foreign consolidated subsidiaries’ financial statements into U.S. dollars will lead to a translation gain or loss which is recorded as a component of other comprehensive income. In addition, we have certain assets and liabilities that are denominated in currencies other than the relevant entity’s functional currency. Changes in the functional currency value of these assets and liabilities create fluctuations that will lead to a transaction gain or loss. We have not entered into agreements or purchased instruments to hedge our exchange rate risks. The availability and effectiveness of any hedging transaction may be limited and we may not be able to hedge our exchange rate risks.
The application of Chinese regulations relating to the overseas listing of Chinese domestic companies is uncertain, we may be subject to penalties for failing to request approval of the Chinese authorities prior to listing our shares in the U.S. and we may be subject to additional approval requirements for Kanghui Agricultural’s acquisition of Guangzhou Tanke.
On August 8, 2006, six Chinese government agencies, namely, the Ministry of Commerce, or MOFCOM, the State Administration for Industry and Commerce, or SAIC, the China Securities Regulatory Commission, or CSRC, the State Administration of Foreign Exchange, or SAFE, the State Assets Supervision and Administration Commission, or SASAC, and the State Administration for Taxation, or SAT, jointly issued the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, which we refer to as the “New M&A Rules”, which became effective on September 8, 2006. The New M&A Rules purport, among other things, to require offshore “special purpose vehicles,” that are (1) formed for the purpose of overseas listing of the equity interests of Chinese companies via acquisition and (2) are controlled directly or indirectly by Chinese companies and/or Chinese individuals, to obtain the approval of the CSRC prior to the listing and trading of their securities on overseas stock exchanges.
There are substantial uncertainties regarding the interpretation, application and enforcement of the New M&A Rules and CSRC has yet to promulgate any written provisions or formally declare or state whether the overseas listing of a China-related company structured similar to ours is subject to the approval of CSRC. Any violation of these rules could result in fines and other penalties on our operations in China, restrictions or limitations on remitting dividends outside of China, and other forms of sanctions that may cause a material and adverse effect to our business, operations and financial conditions.
The new mergers and acquisitions regulations also established additional procedures and requirements that are expected to make merger and acquisition activities by foreign investors more time-consuming and complex, including requirements in some instances that the Ministry of Commerce be notified in advance of any change-of-control transaction in which a foreign investor takes control of a Chinese domestic enterprise that owns well-known trademarks or China’s traditional brands. If within two years, the relevant PRC authority determines that Kanghui Agricultural lacks a business in the ordinary course, Kanghui Agricultural may be considered as a wholly foreign owned holding company and therefore Kanghui Agricultural’s acquisition of Guangzhou Tanke may be subject to additional approvals that are required under the new M&A Rule. Complying with the requirements of the new mergers and acquisitions regulations in completing this type of transactions could be time-consuming, and any required approval processes, including CSRC approval, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
Recent Chinese regulations relating to the establishment of offshore special purpose companies by Chinese residents may subject our Chinese resident shareholders to penalties in certain circumstance. After exercising their rights under the Call Option Agreement, the Tanke Shareholders will need to register with SAFE according to Circular 75. Circular 75 affects the Tanke Shareholders and does not apply to us, our subsidiaries including Guangzhou Tanke, or any shareholders who are not PRC residents.
The SAFE issued a public notice in October 2005, or the SAFE Circular No. 75 (“Circular 75”), requiring Chinese residents to register with the local SAFE branch before establishing or controlling any company outside of China for the purpose of capital financing with assets or equities of Chinese companies, referred to in the Circular 75 as special purpose vehicles, or SPVs. Chinese residents who are shareholders of SPVs established before November 1, 2005 were required to register with the local SAFE branch before June 30, 2006. Further, Chinese residents are required to file amendments to their registrations with the local SAFE branch if their SPVs undergo a material event involving changes in capital, such as changes in share capital, mergers and acquisitions, share transfers or exchanges, spin-off transactions or long-term equity or debt investments.
To date, the Chinese residents who are shareholders of Guangzhou Tanke do not own any equity in the Company, and for such reason they do not need to file registrations with the foreign exchange authority pursuant to Circular 75. The ground for this statement is Article 2 of Circular 75, according to which a Chinese resident is required to file the registration under Circular 75 only when it is to directly establish or indirectly control an offshore special purpose vehicle. Strictly speaking, by executing the Call Option Agreement alone, Tanke Shareholders shall not be deemed to own any shares in the Company and therefore will not trigger the registration of Circular 75. Unless and until Tanke Shareholders intend to exercise the call option to own the shares of the Company, they will not be required to complete the registration under Circular 75.
Pursuant to the vesting schedule set forth in the Call Option Agreement, the Tanke Shareholders are permitted to exercise their options for shares of common stock in the Company. When exercising their right to acquire Option Shares, the Tanke Shareholders will need to file registrations with SAFE. Any failure by the Tanke Shareholders to file their registration with SAFE will restrict and prohibit such individuals from transferring the proceeds from a sale of common stock to China. If such sales proceeds are transferred to China without SAFE registration, any individual in violation thereof could be subject to a fine imposed by the PRC foreign exchange control agency in an amount of approximately 30% of the sales price as well as criminal liabilities. See “Business—Government Regulation – Foreign Exchange Regulation”.
Under applicable PRC laws, a Chinese domestic resident legal person or natural person (collectively, the “Resident Person”) shall register with SAFE if such Resident Person “directly establishes or indirectly controls” a Special Purpose Vehicle in another country. The aforementioned “directly establish or indirectly controls” could be by an individual Resident Person or by several Resident Persons collectively.
The Special Purpose Vehicle means a foreign enterprise established for the purpose of an equity financing (including convertible bond financing) outside of China by using the assets or interests held by such individual or several Resident Persons in the Chinese enterprise. In this regard, if the Tanke Shareholders exercise their right under the Call Option Agreement and, therefore, individually or collectively control us, under applicable PRC law, we will be deemed to be a Special Purpose Vehicle because we are controlled by a Resident Person and our interests in Guangzhou Tanke, through the VIE Agreements, was used in connection with our equity financing in the US.
Before exercising their rights under the Call Option Agreement, none of the Tanke Shareholders may obtain shares in us and, therefore, shall not be deemed to be controlling us. In this regard, Circular 75 will not apply. After exercising their rights under the Call Option Agreement, the Tanke Shareholders (all of them are Chinese residents and, therefore, fall into the definition of Resident Person) will be deemed as individually or collectively controlling us. Therefore, the Tanke Shareholders will need to register with SAFE according to Circular 75. Circular 75 affects the Tanke Shareholders and does not apply to any shareholders who are not PRC residents.
It is expressly stated in the Article 1 of Circular 75 that Circular 75 only applies to Chinese residents (including the natural person holding Chinese identity card or passport; the natural person habitually residing in China for economic or other benefit ties without holding Chinese identity card or passport; and the company, institution or other economic entity incorporated in China) who directly establish or indirectly control the special purpose vehicle. Therefore, under Circular 75, only a Chinese resident who directly establishes or indirectly controls the special purpose vehicle is liable for registration with local foreign exchange authority. Circular 75 does not extend its application to any non-Chinese resident, such as the Company, or any non-Chinese shareholders of the Company. As to Kanghui Agricultural, although it is a Chinese resident, it does not engage in any activity within the scope of “directly establishing or indirectly controlling the special purpose vehicle”, and therefore is not within the application of Circular 75
For such reason, the Company, the non-Chinese shareholders of the Company, and the subsidiaries of the Company (including Kanghui Agricultural) will not be subject to any administrative or criminal liabilities due to Tanke Shareholders failure of complying with Circular 75.
The uncertainty regarding the interpretation and implementation of Circular 75 that may have impact on the Company is the definition of "Control" stipulated in Article 1 of Circular 75. Circular 75 applies only to Chinese residents who intend to directly establish or INDIRECTLY CONTROL an offshore purpose vehicle for the purpose of raising capital overseas. It is stipulated in Circular 75 that "Control" shall refer to "the behavior that a Chinese resident obtains the rights to carry out business operation of, to gain proceeds from or to make decisions for a special purpose vehicle by means of acquisition, trusteeship, holding shares on behalf of others, voting rights, repurchase, convertible bonds, etc."
Mr. Qiu Guixiong, one of the Tanke shareholders, is the sole director of the Company and therefore has the decisive power to the operation of the Company. However, he obtains the control over the Company by means of directorship in the Company rather than any of the means expressly set out in Circular 75 (i.e., acquisition, trusteeship, holding shares on behalf of others, voting rights, repurchase, convertible bonds).
Strictly speaking, the Call Option Agreement placed a condition on exercising of the voting power by Golden Genesis (the “Grantor”). It is expressly stated in Article 2.8 of the Call Option Agreement that the Grantor “agrees not to exercise any of its voting rights with respect to the Option Shares……on behalf of the Grantees without the prior written consent of the Grantees,……before all of the Grantor’s shares are transferred to the Grantee,…”. This arrangement does not directly grant the voting power to the Grantees (which includes Mr. Qiu), rather limiting the voting power of the Grantor. Such condition placed cannot be strictly equivalent to the “voting power” mentioned in Circular 75 because that, under the Chinese law perspective, the voting power is attached to the equity and Tanke Shareholders do not have the title of the Option Shares before exercising the option right. To date, there is no further interpretation regarding the “voting power” under the Circular 75.
Circular 75 remains unclear on whether the control gained through the directorship should be deemed “Control” within its application. To date, there is no further amendment or detailed interpretation regarding the definition of "Control" of Circular 75 . Our informal consultation with some officials of foreign exchange authorities confirmed that the control over a special purpose vehicle through the position of directorship does not belong to the scope of the “Control” under Circular 75 . We are therefore of the opinion that, in absence of further amendments or detailed regulations regarding the definition of "Control" in Circular 75 , the chance that Mr. Qiu Guixiong's position will be definitely determined as "control" over the Company is rare.
However, if the Chinese local foreign exchange authority determines under its own discretion that Mr. Qiu Guixiong is a de facto controller of the Company and his control over the Company should be deemed to be within the scope of Notice 75, Mr. Qiu Guixiong may be required to finish the registration under Circular 75 , even before he exercises the call option right on the shares of the Company. Under such circumstance, Mr. Qiu’s non-compliance under Circular 75 will not affect Kanghui Agricultural to distribute funds to us because that, as being mentioned before, Tanke Shareholders’ failure of complying with the Circular 75 will not trigger Kanghui Agricultural or us to be subject to any administrative or criminal liabilities.
Under Chinese law, Mr. Qiu’s failure of complying with Circular 75 will not affect his ability to serve as the registrant’s chief executive officer and sole director because the Circular 75 only regulates the matters relating to the administration of foreign exchange.
We face uncertainty from the Circular on Strengthening the Administration of Enterprise Income Tax on Non-resident Enterprises' Share Transfer, or Circular 698, released in December 2009 by China's State Administration of Taxation, or the SAT, effective as of January 1, 2010.
Pursuant to the Circular 698 effective on January 1, 2010, where a foreign investor transfers the equity interests of a Chinese resident enterprise indirectly via disposing of the equity interests of an overseas holding company, which we refer to as an Indirect Transfer, and such overseas holding company is located in a tax jurisdiction that: (i) has an effective tax rate less than 12.5% or (ii) does not tax foreign income of its residents, the foreign investor shall report such Indirect Transfer to the competent tax authority of the Chinese resident enterprise. The Chinese tax authority will examine the true nature of the Indirect Transfer, and if the tax authority considers that the foreign investor has adopted an abusive arrangement in order to avoid Chinese tax, they will disregard the existence of the overseas holding company and re-characterize the Indirect Transfer and as a result, gains derived from such Indirect Transfer may be subject to Chinese withholding tax at the rate of up to 10%. Circular 698 also provides that, where a non-Chinese resident enterprise transfers its equity interests in a Chinese resident enterprise to its related parties at a price lower than the fair market value, the competent tax authority has the power to make a reasonable adjustment to the taxable income of the transaction.
Wong Kwai Ho’s transfer of her 100% shareholding interest in China Flying to Golden Genesis may be deemed an indirect disposal of equity interest of Guangzhou Tanke, a Chinese resident enterprise, through China Flying. This transfer may be subject to examination by Chinese tax authorities and could be subject to Wong Kwai Ho been required to pay a withholding tax of 10%.
SAFE rules and regulations may limit our ability to transfer the net proceeds from future offerings to Guangzhou Tanke, which may adversely affect the business expansion of Guangzhou Tanke.
On August 29, 2008, SAFE promulgated Circular 142, a notice regulating the conversion by a foreign-invested company of foreign currency into Renminbi by restricting how the converted Renminbi may be used. The notice requires that the registered capital of a foreign-invested company settled in Renminbi converted from foreign currencies may only be used for purposes within the business scope approved by the applicable governmental authority and may not be used for equity investments within the PRC. In addition, SAFE strengthened its oversight of the flow and use of the registered capital of a foreign-invested company settled in Renminbi converted from foreign currencies. Violations of Circular 142 will result in severe penalties, such as heavy fines. As a result, Circular 142 may significantly limit our ability to transfer the net proceeds from future offerings.
We may be considered a resident enterprise of the PRC, and if so, the Enterprise Income Tax Law of PRC relating to PRC taxation on resident enterprise may subject us or our Shareholders to penalties, limit our ability to distribute dividend to our shareholders or otherwise adversely affect us.
The Enterprise Income Tax Law (the “EIT Law”) of the PRC and its implementing rules provide that enterprises established outside China whose “de facto management bodies” are located in China are considered “resident enterprises” and subject to the uniform 25% enterprise income tax rate on global income. Our management is substantially based in the PRC and expects to be based in the PRC in the future. It is unclear and uncertain how the PRC tax authorities would interpret and implement the EIT Law and its implementing rules and it remains uncertain whether the PRC tax authorities would determine that we are a “resident enterprise” or a “non-resident enterprise”. If we are deemed as resident enterprise, we will be subject to a 25% enterprise income tax rate. If we are deemed as resident enterprise, any failure to pay the enterprise income tax may result in a fine imposed by relevant PRC tax authorities in an amount from RMB2,000 to RMB10,000, and any failure to pay the income tax within a limited period of time as requested by the PRC tax authorities may result in a fine in amount from 50% to 500% of the taxes unpaid or underpaid.
Due to various restrictions under Chinese laws on the distribution of dividends by our Chinese operating companies, we may not be able to pay dividends to our stockholders.
The Wholly-Foreign Owned Enterprise Law (1986), as amended, the Wholly-Foreign Owned Enterprise Law Implementing Rules (1990), as amended and the Company Law of China (2006) contain the principal regulations governing dividend distributions by wholly foreign-owned enterprises. Under these regulations, wholly foreign-owned enterprises may pay dividends only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. Additionally, prior to the distribution of the dividend, wholly foreign-owned enterprises are required to set aside 10% of their annual net income as certain company statutory reserves, until the total amount of such reserves reach to 50% of the registered capital of the foreign-owned enterprises. These reserves can only be utilized to make up the loss or be converted to the registered capital of the foreign-owned enterprises and are not distributable as cash dividends except in the event of liquidation.
In accordance with these regulations, Kanghui Agriculture is required to remain 10% of its net income annually, as determined in accordance with the PRC’s accounting rules and regulations, as the statutory reserve fund until the amount of such statutory reserve reaches 50% of its registered capital. Such statutory reserve cannot be distributed in cash to China Flying and in turn to our common stock shareholders, until and unless Kanghui Agriculture is liquidated. To date, we have segregated $373,406 related to the statutory reserve requirements.
Additionally, the Circular 75 requires Chinese residents to register with the local SAFE branch before establishing or controlling any company outside of China for the purpose of capital financing with assets or equities of Chinese companies, referred to in the Circular 75 as special purpose vehicles, or SPVs. Chinese residents who are shareholders of SPVs established before November 1, 2005 were required to register with the local SAFE branch before June 30, 2006. Further, Chinese residents are required to file amendments to their registrations with the local SAFE branch if their SPVs undergo a material event involving changes in capital, such as changes in share capital, mergers and acquisitions, share transfers or exchanges, spin-off transactions or long-term equity or debt investments.
To date, the Chinese residents who are shareholders of Guangzhou Tanke do not own any equity in the Company, and for such reason such Chinese residents currently do not need to file registrations with SAFE pursuant to Circular 75. When the Chinese residents exercise their options in the future to receive any share of the Company pursuant to the Call Option Agreement, they will need to file registrations with SAFE. In that case, before the Chinese residents complete the registration with SAFE, Kanghui Agriculture cannot remit out of China its dividend and, as a result, we may experience delay or difficulty in paying the dividends on our common stock.
The Chinese government also imposes controls on the conversion of RMB into foreign currencies and the remittance of currencies out of China. We may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from the profits of Guangzhou Tanke. Furthermore, if our subsidiaries in China incur debt on their own in the future, the instruments governing the debt may restrict their ability to pay dividends or make other payments. If we unable to receive all of the revenues from our operations through these contractual or dividend arrangements, we may be unable to pay dividends on our common stock.
The State Administration of Foreign Exchange restrictions or changes in foreign exchange regulations in China may affect our ability to pay dividends in foreign currency or conduct other foreign exchange business.
We receive substantially all of our revenues in RMB, which is currently not a freely convertible currency. The restrictions on currency exchanges may limit our ability to use revenues generated in RMB to make dividends or other payments in United States dollars. The Chinese government strictly regulates conversion of RMB into foreign currencies. Over the years, foreign exchange regulations in China have significantly reduced the government’s control over routine foreign exchange transactions under current accounts. In China, SAFE regulates the conversion of the RMB into foreign currencies. Pursuant to applicable Chinese laws and regulations, foreign invested enterprises incorporated in China are required to apply for “Foreign Exchange Registration Certificates.” Currently, conversion within the scope of the “current account” (e.g. remittance of foreign currencies for payment of dividends, trade and service-related foreign exchange transactions, etc.) can be effected without requiring the approval of SAFE, instead, need to be registered with the SAFE. However, conversion of currency in the “capital account” (e.g. for capital items such as direct investments, loans, securities, etc.) still requires the approval of SAFE. In addition, failure to obtain approval from SAFE for currency conversion on the capital account may adversely impact our capital expenditure plans and our ability to expand in accordance with our desired objectives.
All of our income is derived from the consulting fees we receive from Guangzhou Tanke through the VIE Agreements. SAFE restrictions may delay the payment of dividends, since we have to comply with certain procedural requirement and we may experience difficulties in completing the administrative procedures necessary to obtain and remit foreign currency for the payment of dividends from the profits of Kanghui Agricultural, and it thus may delay our payment of dividend to the equity holders.
Foreign exchange transactions by Chinese operating subsidiaries under the capital account continue to be subject to significant foreign exchange controls and require the approval of and need to register with Chinese government authorities, including SAFE. In particular, if Guangzhou Tanke, which we control via the VIE Agreement, borrows foreign currency through loans from us or other foreign lenders, these loans must be registered with SAFE, and if we finance Guangzhou Tanke by means of additional capital contributions, these capital contributions must be approved by certain government authorities, including the Ministry of Commerce, or their respective local counterparts. These limitations could affect Guangzhou Tanke’s ability to obtain foreign exchange through debt or equity financing.
The Chinese government may also at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining foreign currency, we may be unable to pay dividends or meet obligations that may be incurred in the future that require payment in foreign currency.
We are subject to Chinese environmental laws that adversely affect our results of operations.
We are subject to multiple Chinese environmental laws, including the Law on Environmental Protection of China and the Law of Prevention of Effluent Pollution of China and other environmental regulation governing the classification and disposal of waste. We expect that national, provincial and local governmental agencies will adopt stricter pollution controls in the future. Our production process may produce waste which may be harmful to the environment. There can be no assurance that future changes in environmental laws and regulations will not impose costly compliance requirements on us or otherwise subject us to future liabilities. Our profitability may be adversely affected if additional or modified environmental control regulations are imposed upon us.
Our failure to fully comply with Chinese labor laws, including laws relating to social insurance, may expose us to potential liability and increased costs.
Companies operating in China must comply with a variety of labor laws, including the Labor Contract Law of China enacted in June 2007, or the New Labor Contract Law, and laws requiring us to make social insurance (including unemployment insurance, medical insurance, and pension) and other staff welfare-oriented payments (such as housing funds). Our failure to comply with these laws could have a material adverse effect on our business. The New Labor Contract Law, which became effective on January 1, 2008, imposes stricter obligations on employers including a requirement that employers execute written labor contracts with all of their employees. Among our 165 employees, we have paid social insurance for 151 of our employees and 14 employees participated in commercial insurance due to the fact that they are transferred to the Company from other companies who have paid their social insurance. Our failure to remain in compliance with Chinese labor laws including social insurance requirements in the future could adversely impact our results of operations.
Furthermore, the New Labor Contract Law governs standard terms and conditions for employment, including termination and lay-off rights, contract requirements, compensation levels and consultation with labor unions, among other topics. In addition, the law limits non-competition agreements with senior management and other employees who have access to confidential information to two years and imposes restrictions or geographical limits. This new labor contract law will increase our labor costs, which could adversely impact our results of operations.
We must comply with the Foreign Corrupt Practices Act.
We are required to comply with the United States Foreign Corrupt Practices Act, which prohibits U.S. companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some of our competitors, are not subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in mainland China. If our competitors engage in these practices, they may receive preferential treatment from personnel of some companies, giving our competitors an advantage in securing business or from government officials who might give them priority in obtaining new licenses, which would put us at a disadvantage. We have no formal policies in place to prevent our employees or other agents from engaging in such conduct. If such conduct is undertaken, we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties, which could adversely impact our business and results of operations.
Risks Related to Our Common Stock
An active and visible trading market for our common stock may not develop.
We cannot predict whether an active market for shares of our common stock will develop in the future. In the absence of an active trading market:
|
●
|
Investors may have difficulty buying and selling or obtaining market quotations;
|
|
●
|
Market visibility for shares of our common stock may be limited; and
|
|
●
|
A lack of visibility for shares of our common stock may have a depressive effect on the market price for shares of our common stock.
|
The OTCBB is an unorganized, inter-dealer, over-the-counter market that provides significantly less liquidity than NASDAQ or the NYSE AMEX. The trading price of our common stock is expected to be subject to significant fluctuations in response to variations in quarterly operating results, changes in analysts’ earnings estimates, announcements of innovations by us or our competitors, general conditions in the industry in which we operate and other factors. These fluctuations, as well as general economic and market conditions, may have a material or adverse effect on the market price of shares of our common stock.
The market price for shares of our common stock may be volatile.
The market price for shares of our common stock may be volatile and subject to wide fluctuations in response to factors including the following:
|
●
|
actual or anticipated fluctuations in our quarterly operating results;
|
|
●
|
changes in financial estimates by securities research analysts;
|
|
●
|
conditions in commodities markets;
|
|
●
|
changes in the economic performance or market valuations of other feed additive technology companies;
|
|
●
|
announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments;
|
|
●
|
addition or departure of key personnel;
|
|
●
|
fluctuations of exchange rates between RMB and the U.S. dollar;
|
|
●
|
intellectual property litigation; and
|
|
●
|
general economic or political conditions in China.
|
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of shares of our common stock.
We do not anticipate paying cash dividends in the foreseeable future and, as a result, our investors’ sole source of gain, if any, will depend on capital appreciation, if any.
We do not plan to declare or pay any cash dividends on our shares of common stock in the foreseeable future and currently intend to retain any future earnings for funding growth. As a result, investors should not rely on an investment in our securities if they require the investment to produce dividend income. Capital appreciation, if any, of our shares may be investors’ sole source of gain for the foreseeable future. Moreover, investors may not be able to resell their shares of common stock at or above the price they paid for them.
We have considerable discretion in the use of proceeds from the selling stockholders’ conversion of the Notes and exercise of the Warrants, and we may use these proceeds in ways with which you may not agree.
Our Board of Directors and our management will have considerable discretion in the application of the net proceeds received by us from the selling stockholders’ conversion of the Notes and exercise of the Warrants. You will not have the opportunity, as part of your investment decision, to assess whether proceeds are being used appropriately. You must rely on the judgment of our Board of Directors and our management regarding the application of the net proceeds of this offering. The net proceeds may be used for corporate purposes that do not improve our efforts to maintain profitability or increase the price of our securities. The net proceeds from this offering may be placed in investments that do not produce income or that lose value. See “Use of Proceeds.”
Shares eligible for future sale may adversely affect the market price of our common stock, as the future sale of a substantial amount of outstanding stock in the public marketplace could reduce the price of our common stock.
The former shareholders of Guangzhou Tanke are eligible to sell all or some of their shares of our common stock by means of ordinary brokerage transactions in the open market pursuant to Rule 144 promulgated under the Securities Act (“Rule 144”), subject to certain limitations. In general, pursuant to Rule 144, a stockholder (or stockholders whose shares are aggregated) who has satisfied an one-year holding period may, under certain circumstances, sell within any three-month period a number of securities which does not exceed the greater of 1% of the then outstanding shares of common stock or the average weekly trading volume of the class during the four calendar weeks prior to such sale. As of the closing of the Private Placement, 1% of our issued and outstanding shares of common stock equals approximately 133,241 shares (without giving effect to any conversion of Notes or exercise of Warrants or Agent Warrants). Rule 144 also permits, under certain circumstances, the sale of securities, without any limitations, by a non-affiliate that has satisfied a one-year holding period. Any substantial sale of common stock pursuant to any resale prospectus or Rule 144 may have an adverse effect on the market price of our common stock by creating an excessive supply. A stockholder that is not an affiliate of the Company, and that acquired shares of common stock pursuant to its conversion of the Notes or its exercise of the Warrants, shall be permitted to sell its shares of common stock pursuant to Rule 144(i) on or after February 10, 2012.
We will incur increased costs as a result of being a public company.
As a public company, we will incur significant legal, accounting and other expenses, in excess of $100,000, that we did not incur as a private company. In addition, the Sarbanes-Oxley Act of 2002, as well as new rules subsequently implemented by the SEC, has required changes in corporate governance practices and internal control and procedures of public companies. We expect these new rules and regulations to increase our legal, accounting and financial compliance costs and to make certain corporate activities more time-consuming and costly. In addition, we will incur additional costs associated with our public company reporting requirements. We are currently evaluating and monitoring developments with respect to these new rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
We have determined that our internal control over financial reporting is currently not effective. The lack of effective internal controls could materially adversely affect our financial condition and ability to carry out our business plan.
Our management team for financial reporting, under the supervision and with the participation of our Chief Executive Officer and our Chief Financial Officer, conducted an evaluation of the effectiveness of the design and operation of our internal controls. At December 31, 2011, we concluded that our internal control over financial reporting was not effective because we (1) lack of controls designed or implemented over financial reporting process as well as lack of qualified staff to effectively carry out the activities over financial reporting process, (2) lack of policies over comingling Company funds with personal bank accounts and policies over cash transactions, (3) Incomplete and inappropriate accounting records keeping, and (4) lack of U.S. GAAP expertise to identify and assess unusual or complex accounting transactions. Until we have been able to test the operating effectiveness of remediated internal controls, any material weaknesses may materially adversely affect our ability to report accurately our financial condition and results of operations in the future in a timely and reliable manner. In addition, although we continually review and evaluate internal control systems to allow management to report on the sufficiency of our internal controls, we cannot assure you that we will not discover additional weaknesses in our internal control over financial reporting. Any such additional weakness or failure to remediate the existing weakness could materially adversely affect our financial condition or ability to comply with applicable financial reporting requirements and the requirements of the Company’s various financing agreements.
Our common stock may be considered a “penny stock,” and thereby be subject to additional sale and trading regulations that may make it more difficult to sell.
Our common stock, which is currently and will be quoted for trading on OTCBB, may be considered to be a “penny stock” if it does not qualify for one of the exemptions from the definition of “penny stock” under Section 3a51-1 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). Our common stock may be a “penny stock” if it meets one or more of the following conditions: (i) the stock trades at a price less than $5.00 per share; (ii) it is not traded on a “recognized” national exchange; (iii) it is not quoted on the Nasdaq Capital Market, or even if so, has a price less than $5.00 per share; or (iv) is issued by a company that has been in business less than three years with net tangible assets less than $5 million. The principal result or effect of being designated a “penny stock” is that securities broker-dealers participating in sales of our common stock will be subject to the “penny stock” regulations set forth in Rules 15-2 through 15g-9 promulgated under the Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to: (i) obtain from the investor information concerning his or her financial situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives. Compliance with these requirements may make it more difficult and time consuming for holders of our common stock to resell their shares to third parties or to otherwise dispose of them in the market or otherwise.
Our majority stockholder and its affiliates will control the outcome of matters requiring stockholder approval.
Golden Genesis is our majority stockholder and beneficially owns 10,011,469 shares of common stock, or approximately 75.14% of the issued and outstanding shares of common stock (without giving effect to any conversion of Notes or exercise of Warrants or Agent Warrants). Golden Genesis is controlled (and will be majority owned, upon consummation of the transactions contemplated in the Call Option Agreement described herein) by our Chairman and Chief Executive Officer, Mr. Qiu. Consequently, Golden Genesis and Mr. Qiu will have the ability, when acting alone or with others, to control the election of our directors and the outcome of corporate actions requiring stockholder approval, such as changes to our Articles of Incorporation and Bylaws and a merger or a sale of our company or a sale of all or substantially all of our assets. This concentration of voting power and control could have a significant effect in delaying, deferring or preventing an action that might otherwise be beneficial to our other stockholders and be disadvantageous to our stockholders with interests different from those of our officers, directors and affiliates. Golden Genesis and Mr. Qiu also have significant control over our business, policies and affairs. Additionally, this significant concentration of share ownership may adversely affect the trading price for our common stock because investors often perceive disadvantages in owning stock in companies with controlling stockholders.
Provisions in our Bylaws could discourage, delay or prevent a change of control of our company and may result in an entrenchment of management and diminish the value of our common stock.
Our Bylaws provide that, unless otherwise prescribed by statute, special meetings of the stockholders can only be called by the Chairman of our Board of Directors, our President, or by the Board of Directors, or in their absence or disability, by any Vice President; and can be called by the President or, in his or her absence or disability, by a Vice President or by the Secretary upon the written request of the holders of not less than 15% of all the shares entitled to vote at the meeting. These provisions may discourage, delay or prevent a merger, acquisition or other change of control that our stockholders may consider favorable. Such provisions could impede the ability of our common stockholders to benefit from a change of control and, as a result, could materially adversely affect the market price of our common stock and your ability to realize any potential change-in-control premium.
Provisions in our Bylaws limit the rights of dissenting stockholders in transactions involving the purchase by a third party of a controlling equity interest in the Company and may result in dissenting stockholders in such transactions not obtaining payment of the fair market value of its shares.
Pursuant to our By-Laws, we have elected to limit the rights, under Chapter 78 of the Nevada Revised Statutes, of dissenting stockholders in transactions involving the purchase by a third party of a controlling equity interest in the Company. Under Nevada law, following the acquisition of a controlling interest of a company by an acquiring person, a stockholder whose shares are not voted in favor of authorizing voting rights for the control shares may dissent, and obtain payment of the fair value of his or her shares. The Company has elected not to be governed by this provision. As a result of not being able to dissent in such transactions under Nevada law, the dissenting stockholder may not be able to obtain payment of the fair market value of its shares.
Our employees and management do not have significant experience in the preparation or supervising the preparation of our financial statements in accordance with US GAAP.
Prior to the completion of this offering, Guangzhou Tanke operated as a private company located in China. In connection with the completion of the offering the Company acquired control of Guangzhou Tanke by virtue of VIE agreements. In the process of taking these steps to prepare our company for this offering, Guangzhou Tanke’s senior management became the senior management of the Company. Guangzhou Tanke’s senior management has limited experience managing a public company in the U.S. or complying with such U.S. laws, regulations and obligations. These obligations can be burdensome and complicated, and failure to comply with such obligations could have a material adverse effect on the Company. In addition, we expect that the process of learning about such new obligations as a public company in the United States will require senior management to devote time and resources to such efforts that might otherwise be spent on the operation of the business of the Company.
Historically our operations have been in China, and we have prepared our financial statements and maintained our books and records in accordance with China GAAP and have not previously been required to comply with U.S. GAAP. We recently hired a new Chief Financial Officer that is familiar with U.S. GAAP. In addition, we have engaged outside consultants who have significant experience with U.S. GAAP to prepare the conversion from China GAAP into U.S. GAAP. Furthermore, our financial statements are audited by our independent registered public accounting firm for compliance with U.S. GAAP standards. Yet, we cannot assure that our management has identified or fully remedied all material weaknesses in our internal controls of financial reporting pursuant to U.S. GAAP. If deficiencies go undetected or unremedied, our financial statements may not accurately reflect our financial condition as required by U.S. GAAP. We will evaluate these risk factors that impact our ability to prepare financial statements and maintain our books and records in accordance with U.S. GAAP in the future in concluding on the effectiveness of disclosure controls and procedures under Item 307 of Regulation S-K and internal control over financial reporting under Item 308 of Regulation S-K.
CAUTIONARY NOTE ON FORWARD LOOKING STATEMENTS
The information contained in this prospectus, includes some statements that are not purely historical and that are forward-looking statements. Such forward-looking statements herein which are not historical reflect our current expectations and projections about the Company’s future results, performance, liquidity, financial condition, prospects and opportunities and are based upon information currently available to the Company and our management and their interpretation of what is believed to be significant factors affecting the businesses, including many assumptions regarding future events. Such forward-looking statements include statements regarding, among other things:
|
●
|
our ability to produce, market and generate sales of our products;
|
|
●
|
our ability to develop, acquire and/or introduce new products;
|
|
●
|
our projected future sales, profitability and other financial metrics;
|
|
●
|
our future financing plans;
|
|
●
|
our plans for expansion of our facilities;
|
|
●
|
our anticipated needs for working capital;
|
|
●
|
the anticipated trends in our industry;
|
|
●
|
our ability to expand our sales and marketing capability;
|
|
●
|
acquisitions of other companies or assets that we might undertake in the future;
|
|
●
|
our operations in China and the regulatory, economic and political conditions in China;
|
|
●
|
our ability as a U.S. company to operate our business in China through an indirect wholly-owned subsidiary; and
|
|
●
|
competition existing today or that will likely arise in the future.
|
Forward-looking statements, which involve assumptions and describe our future plans, strategies and expectations, are generally identifiable by use of the words “may,” “should,” “expect,” “anticipate,” “estimate,” “believe,” “intend,” “seek,” or “project” or the negative of these words or other variations on these words or comparable terminology. Actual results, performance, liquidity, financial condition and results of operations, prospects and opportunities could differ materially from those expressed in, or implied by, these forward-looking statements as a result of various risks, uncertainties and other factors, including the ability to raise sufficient capital to continue the Company’s operations. Actual events or results may differ materially from those discussed in forward-looking statements as a result of various factors, including, without limitation, the risks outlined under “Risk Factors” and matters described in this prospectus generally. In light of these risks and uncertainties, there can be no assurance that the forward-looking statements contained in this prospectus will in fact occur. Potential investors should not place undue reliance on any forward-looking statements. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
Potential investors should not place undue reliance on any forward-looking statements. Except as expressly required by the federal securities laws, there is no undertaking to publicly update or revise any forward-looking statements, whether as a result of new information, future events, changed circumstances or any other reason.
The specific discussions in this prospectus about the Company include financial projections and future estimates and expectations about the Company’s business. The projections, estimates and expectations are presented in this prospectus only as a guide about future possibilities and do not represent actual amounts or assured events. All the projections and estimates are based exclusively on the Company management’s own assessment of our business, the industry in which it works and the economy at large and other operational factors, including capital resources and liquidity, financial condition, fulfillment of contracts and opportunities. The actual results may differ significantly from the projections.
Potential investors should not make an investment decision based solely on the Company’s projections, estimates or expectations.
USE OF PROCEEDS
The selling security holders will receive all of the proceeds from the sale of shares of common stock pursuant to this prospectus. We will not receive any proceeds from the sale of the shares of common stock offered by the selling security holders to the public. However, we will receive proceeds from the conversion of the Notes and exercise of the Warrants. If all of the Notes are converted and Warrants are exercised, then we will receive gross proceeds of $17,007,548.85. Any such proceeds will be used for working capital and general corporate purposes of the Company. No assurance can be given, however, that all or any portion of such Notes will be converted or all or any portion of such Warrants will be exercised.
DETERMINATION OF OFFERING PRICE
The selling stockholders may sell these shares in the over-the-counter market or otherwise, at market prices prevailing at the time of sale, at prices related to the prevailing market price, or at negotiated prices. We will not receive any proceeds from the sale of shares by the selling stockholders but we may receive proceeds upon the conversion or exercise, if any, of the Notes and the Warrants.
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION
AND RESULTS OF OPERATIONS
The following discussion and analysis should be read in conjunction with our financial statements and the related notes. This discussion contains forward-looking statements based upon current expectations that involve risks and uncertainties, such as its plans, objectives, expectations and intentions. Its actual results and the timing of certain events could differ materially from those anticipated in these forward-looking statements. As a result of the Share Exchange and the VIE Agreements, the Company, through Kanghui Agricultural, its indirect wholly owned subsidiary, assumed management of the business activities of Guangzhou Tanke and has the right to appoint all executives and senior management and the members of the board of directors of Guangzhou Tanke. As used in this section, the terms “we”, “our”, “us” and the “Company” refer to the Company, our direct and indirect subsidiaries and Guangzhou Tanke, our principal operating business.
Overview
We are one of the leading animal nutrition and feed additive providers in China and in 2001 were designated a certified high-tech company by the Guangzhou City Commission of Science and Technology as recognition for new technology developed by us in the agriculture industry. Our products optimize the growth and health of livestock such as pigs and cattle, as well as farmed fish, and seek to capitalize on China’s growing demand for safe and reasonably priced food. Feed additives are utilized in China at less than half the rate of the United States and Europe, and we have a significant growth opportunity as Chinese farmers and ranchers include a greater amount of increasingly sophisticated additive to their feed.
We have more than 165 employees, with 40 engaged in sale or sales-related activities. Our headquarters and manufacturing facilities are in a state-of-the-art 34,000 square-meter facility in the capital city of Guangzhou, in Guangdong province. We currently produce 22 branded feed additives, with each brand available in seven different mixes that correspond to different states of an animal’s life cycle.
Our major products address most key market categories within China’s animal feed additive industry including: Organic Trace Mineral Additives, which account for approximately 83% of our revenue, Functional regulation, which account for approximately 15% of our revenue, and Herbal Medicinal Additives, which account for approximately 1% of our revenue. Our extensive distribution network reaches China’s top 10 feed producers and the 500 largest animal farming operations. We currently market 22 different brands of feed additives at various price points to meet the demands of existing and prospective customers. Within each brand there are seven different mixes that correspond to the different growth stages of an animal’s life cycle. While the majority of our sales are domestic, international sales, mainly in Southeast Asia, Latin American and other developing countries, currently account for approximately 1% of our total sales.
Critical Accounting Policies
While our significant accounting policies are more fully described in Note 2 to our consolidated financial statements, we believe that the following accounting policies are the most critical to aid you in fully understanding and evaluating this “Management’s Discussion and Analysis of Financial Condition and Results of Operations.”
Basis of Preparation
The Company’s consolidated financial statements have been stated in US dollars and prepared in accordance with generally accepted accounting principles in the United States of America ("US GAAP") and have been consistently applied.
Basis of consolidation
These consolidated financial statements include the financial statements of the Company, its subsidiaries and its VIE-Guangzhou Tanke (the “Group''). All significant inter-company balances and transactions within the Group have been eliminated.
Use of Estimates
In preparing consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of the amount due from related parties, the net realizable value of inventories, the estimation of useful lives of property and equipment and intangible assets, allowance of bad debt and the value of warrants. Actual results could differ from those estimates.
Concentrations of Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash and cash equivalents, restricted cash, accounts receivable and amounts due from related parties. The Company places its cash with financial institutions with high-credit ratings and quality. The Company maintains bank accounts in the PRC only. In addition, the Company conducts periodic reviews of the related party financial conditions and payment practices.
Approximately 99% of the Company’s revenue is generated from buyers in mainland China.
Concentrations of Suppliers
|
All the Company’s suppliers are located in mainland China.
|
|
Cash and Cash Equivalents
|
|
The Company considers all highly liquid investments with initial maturities of three months or less to be cash equivalents.
|
|
Restricted Cash
|
|
Deposits that are restricted in use are classified as restricted cash.
|
|
Trade Receivables
|
|
The Company periodically assesses its accounts receivable for collectability on a specific identification basis. If collectability of an account becomes unlikely, an allowance is recorded for that doubtful account. Once collection efforts have been exhausted, the account receivable is written off against the allowance. The Company does not require collateral for trade or other accounts receivable.
|
|
Inventories
|
|
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the weighted average method. The cost of inventories includes the purchase cost and other costs incurred in bringing the inventories to their present location and condition. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and the estimated costs necessary to make the sale.
|
|
Property and Equipment
|
|
Property and equipment are stated at cost less accumulated depreciation. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use.
|
|
Depreciation of property and equipment is calculated using the straight-line method over their estimated useful lives. The estimated useful lives are as follows:
|
|
Buildings
|
15-20 years
|
|
Plant and machinery
|
3-20 years
|
|
Motor vehicle
|
10 years
|
|
Office equipment
|
3-10 years
|
|
Expenditures for additions, major renewals and betterments are capitalized and expenditures for maintenance and repairs are charged to expense as incurred.
|
|
Intangible Asset
|
|
The intangible asset primarily represented two land use rights and purchased developed technology, and they are recorded at cost less accumulated amortization.
According to the laws of China, land in the PRC is owned by the government and cannot be sold to an individual or company. However, the government grants the users a land use right to use the land. The land use rights granted to the Company are being amortized using the straight-line method over the lease term of fifty years.
During the second quarter of 2012, we purchased from an agricultural research institute the right of commercializing and applying for a patent for a new product technology developed by that research institute.
|
|
Impairment of Long-lived Assets
|
|
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book values of such assets exceed the future undiscounted cash flows attributable to such assets.
|
Statutory Reserves
In accordance with the relevant laws and regulations of the PRC and the articles of associations of the Company, Guangzhou Tanke is required to allocate 10% of their net income reported in the PRC statutory accounts, after offsetting any prior years’ losses, to statutory reserve, on an annual basis. When the balance of such reserve reaches 50% of the respective registered capital of the subsidiaries, any further allocation is optional.
As of December 31, 2011 and 2010, the statutory reserves of the subsidiary already reached 50% of the registered capital of the subsidiary and the Company did not have any further allocation on it.
The statutory surplus reserves can be used to offset prior years’ losses, if any, and may be converted into registered capital, provided that the remaining balances of the reserve after such conversion is not less than 25% of registered capital. The statutory surplus reserve is non-distributable.
|
Revenue Recognition
|
|
The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) 605-10, Revenue Recognition, and SEC Staff Accounting Bulletin No. 104. Pursuant to these pronouncements, revenue is recognized when all of the following criteria are met:
|
|
- Persuasive evidence of an arrangement exists;
|
|
- Delivery has occurred or services have been rendered;
|
|
- The seller's price to the buyer is fixed or determinable; and
|
|
- Collectability is reasonably assured.
|
|
The Company’s revenue is generated through the wholesale and retail sale of livestock feed additives including organic trace mineral additives, functional regulation additives, herbal medicinal additives and raw materials. Before the Company recognizes revenue on these product sales, written purchase orders and contracts are received in advance of all shipments of goods to customers. For sales within the Company’s own province, delivery is made by Company employees. Such delivery occurs on the same day as shipment. For delivery outside the province, shipment is made through a separate logistics company that assumes the risk of loss. Revenue is recognized upon shipment of goods to the customers. The Company typically does not incur bad debt losses because this type of loss is deducted from the salesperson’s compensation, thereby mitigating the loss to the Company. Therefore, collectability is reasonably assured.
|
|
Revenue is presented net of sales returns, which are not significant. However, the Company continually performs analyses of returns and records a provision at the time of sale if necessary. As of December 31, 2011 and 2010, it was determined that potential returns and allowances were not material so the Company did not record a provision for returns. The Company revisits this estimate regularly and adjusts it if conditions change.
|
|
Cost of Goods Sold
|
|
Cost of revenue consists primarily of material cost, labor cost, overhead associated with the manufacturing process and directly related expenses.
|
|
Research and Development Costs
|
Research and development costs are charged to expense as incurred and are included in operating expenses. As of December 31, 2011 and 2010, the Company incurred research and development costs amounted to $246,038 and $91,397, respectively.
|
Value Added Tax
|
|
In accordance with the relevant tax laws in the PRC, VAT is levied on the invoiced value of sales and is payable by the purchaser. The Company is required to remit the VAT it collects to the tax authority, but may deduct the VAT it has paid on eligible purchases. The difference between the amounts collected and paid is presented as VAT recoverable or payable balance on the balance sheet.
Income Taxes
|
|
The Company uses the asset and liability method of accounting for income taxes pursuant to ASC 740, ”Income Tax”. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
|
|
Comprehensive Income
|
|
Comprehensive income is defined to include all changes in equity except those resulting from net income or loss, investments by owners and distributions to owners. The Company’s only component of other comprehensive income is the foreign currency translation adjustment.
|
Earnings per share (EPS)
Earnings per share is calculated in accordance with ASC 260-10 which requires the Company to calculate net income (loss) per share based on basic and diluted net income (loss) per share, as defined. Basic EPS excludes dilution and is computed by dividing net income (loss) by the weighted average number of shares outstanding for the period. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock.
Commitments and Contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, penalties and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
Foreign Currency Translation
The Company, its subsidiaries and VIE maintain financial statements in the functional currency of each entity. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
The financial statements of each entity are prepared using the functional currency, and have been translated into United States dollars (“US$” or “$”). Assets and liabilities are translated at the exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates for the period. Stockholders’ equity is translated at historical exchange rates. Any translation adjustments are included as a foreign exchange adjustment in other comprehensive income, a component of stockholders’ equity.
RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US$ at the rates used in translation.
Financial Instruments
The carrying amounts of cash and cash equivalents, restricted cash, accounts receivable, due to/from related parties, notes payable, other payable and accrued liabilities and income tax payable approximate their fair values due to the short-term nature of these items. The carrying amounts of long-term borrowings approximate the fair value based on the Company’s expected borrowing rate for debt with similar remaining maturities and comparable risk.
Convertible notes are not carried at fair value due to the discounts for warrants and the beneficial conversion feature. As the interest on these notes approximates market interest, the fair value is their face value of $7,670,071.
It is management’s opinion that the Company is not exposed to significant interest, price or credit risks arising from these financial instruments.
Consolidation of Variable Interest Entities
According to the requirements of Statement of Financial Accounting Standards No. 810-10, “Variable interest Entities”, the Company has evaluated the economic relationships of its wholly owned subsidiary, China Flying and its wholly-owned subsidiary Kanghui Agricultural with Guangzhou Tanke and has determined that it is required to consolidate China Flying, Kanghui Agricultural and Guangzhou Tanke pursuant to the rules of FASB ASC Topic 810-10. Therefore Guangzhou Tanke is considered to be a VIE, as defined by FASB ASC Topic 810-10 , of which China Flying is the primary beneficiary as a result of its wholly owned subsidiary Kanghui Agricultural. China Flying, as mentioned above, will absorb a majority of the economic risks and rewards of all of these VIE that are being consolidated in the accompanying financial statements.
The carrying amount of the VIEs’ assets and liabilities are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Current assets and Long term notes receivables
|
$
|
13,929,777
|
$
|
8,647,731
|
||||
|
Property, plant and equipment
|
4,801,723
|
4,332,006
|
||||||
|
Intangible assets
|
837,525
|
286,892
|
||||||
|
Total assets
|
19,569,025
|
13,266,629
|
||||||
|
Total liabilities
|
(1,572,020
|
)
|
(2,932,483
|
)
|
||||
|
Net assets
|
$
|
17,997,005
|
$
|
10,334,146
|
||||
Results of Operations for the Three Months Ended June 30, 2012 as Compared to the Three Months Ended June 30, 2011
The following is a comparison of our revenue, costs of sales and gross profit by segment for the three months ended June 30, 2012 and 2011.
Revenue and Costs of Sales
The Company operates in four segments: (1) Organic Trace Mineral Additives, (2) Functional Regulation Additives, (3) Herbal Medicinal Additives and (4) Other. Management tracks each of these operations separately. The Company evaluates the performance of its operating segments based on segment revenue and gross profit, and management uses aggregate segment gross profit as a measure of the overall performance of the business. The Company believes that information about aggregate segment gross profit assists investors by allowing them to evaluate changes in the gross profit of the Company’s various offerings separate from factors other than product offerings that affect net income.
|
2012
|
2011
|
$ Change
|
% Change
|
|||||||||||||
|
Segment revenues
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$
|
6,512,375
|
$
|
3,939,129
|
$
|
2,573,246
|
65.3
|
%
|
||||||||
|
Functional Regulation Additives
|
844,638
|
948,663
|
(104,025
|
)
|
-11.0
|
%
|
||||||||||
|
Herbal Medicinal Additives
|
54,254
|
83,016
|
(28,762
|
)
|
-34.6
|
%
|
||||||||||
|
Other
|
20,219
|
19,035
|
1,184
|
6.2
|
%
|
|||||||||||
|
$
|
7,431,486
|
$
|
4,989,843
|
$
|
2,441,643
|
48.9
|
%
|
|||||||||
|
Segment costs of sales
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$
|
4,030,699
|
$
|
2,520,275
|
$
|
1,510,424
|
59.9
|
%
|
||||||||
|
Functional Regulation Additives
|
576,489
|
601,392
|
(24,903
|
)
|
-4.1
|
%
|
||||||||||
|
Herbal Medicinal Additives
|
64,179
|
74,878
|
(10,699
|
)
|
-14.3
|
%
|
||||||||||
|
Other
|
19,596
|
21,256
|
(1,660
|
)
|
-7.8
|
%
|
||||||||||
|
$
|
4,690,963
|
$
|
3,217,801
|
$
|
1,473,162
|
45.8
|
%
|
|||||||||
|
Segment gross profit
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$
|
2,481,676
|
$
|
1,418,854
|
$
|
1,062,822
|
74.9
|
%
|
||||||||
|
Functional Regulation Additives
|
268,149
|
347,271
|
(79,122
|
)
|
-22.8
|
%
|
||||||||||
|
Herbal Medicinal Additives
|
(9,925
|
)
|
8,138
|
(18,063
|
)
|
-222.0
|
%
|
|||||||||
|
Other
|
623
|
(2,221
|
)
|
2,844
|
-128.1
|
%
|
||||||||||
|
$
|
2,740,523
|
$
|
1,772,042
|
$
|
968,481
|
54.7
|
%
|
|||||||||
Organic Trace Mineral Additives
Organic trace mineral additives constitute the largest and fastest growing area of our business. We are one of China’s largest domestic providers of organic trace mineral additives, specializing in the development and production of chelated organic trace mineral additives. Our current trace mineral manufacturing facility is the largest chelating facilities in China and has the capacity to produce approximately 350 metric tons of organic trace minerals per week.
Revenue from organic trace mineral additives in the second quarter of 2012 increased by $2,573,246, or 65.3%, as compared to 2011. During the second quarter of 2012, demand for organic trace mineral additives rebounded after a slower first quarter due to the Chinese New Year holiday and soft overall economic growth. Our revenue in the second quarter of 2012 increased due in part to strict food safety rules and regulations enforced by the State Council of China regarding the animal feed and feed additive industries. We have benefited from these new industry standards, as smaller companies who produce sub-standard feed additives will not be able to operate. In addition, pig diseases began to seriously impact the pig farming industry in the second quarter of 2011, lowering demand for feed and feed additives, and driving pork prices to a record level. This did not occur in 2012. In 2012, this revenue accounted for approximately 88% of our revenues for the three months ended June 30, 2012 as compared to 79% for the three months ended June 30, 2011. Our gross profit percentage for the organic trace mineral additive sales amounted to 38.1% and 36.0% for the quarter ended June 30, 2012 and 2011, respectively, as our prices have increased in 2012 as compared to 2011.
Functional Regulation Additives
Functional feed additives are widely used to enhance the properties of other products, improve feed efficiency and stimulate the rapid maturation of the immune system. We currently produce two types of functional regulation additives: feed acidifiers and flavor enhancers. Feed acidifiers are used to prevent microbial degradation of raw materials or finished feeds and to maintain the quality of feed. Flavor enhancers are used to improve feed palatability, enhance animal appetite and stimulate saliva, gastric and pancreatic juices and other digestive juice secretion and gastrointestinal motility and ultimately feed consumption and yield from production animals.
Revenue from functional regulation additives for the quarter ended June 30, 2012 decreased by $104,025, or 11.0%, as compared to the second quarter of 2011. The decrease in sales is primarily due to the segment being less emphasized during the second quarter of 2012, as more attention was placed pushing the growth of the organic trace mineral additive market.
Herbal Medicinal Additives
Chinese herbal feed additives utilize traditional Chinese medicine theory to improve an animal’s digestion and appetite and to regulate the yin and yang balance of an animal’s health. Herbal medicines come from plants, plant extracts, fungal and bee products, minerals, shells and certain animal parts. Compared to synthetic antibiotics or inorganic chemicals, these naturally-derived products are less toxic, residue free and thought to be ideal feed additives in food animal production.
Revenue from herbal medicinal additives for the quarter ended June 30, 2012 decreased by $28,762, or 34.6%, as compared to the quarter ended June 30, 2011.
Herbal Medicinal Additives are still in a development and introductory stage. Material cost has been rising and customers are reluctant to pay higher prices. We have been developing new products and production processes to lower costs and offer more affordable products in this category, as well as new marketing strategy to grow sales. While customers are adapting to the usage, Tanke is making minor modifications to products. Prices and costs will continue to fluctuate in the near future until the right balance is reached.
Other
Other revenue mainly consists of buying and then reselling raw materials. Revenue from raw material sales accounted for 0.3% of our revenue for the three months ended June 30, 2012 and remained consistent with the second quarter of 2011. Going forward, we do not expect this to be a significant source of revenue.
Operating Expenses and Other Income / Expenses
The following table reconciles aggregate segment gross profit to net income for the three months ended June 30, 2012 and 2011.
|
Three Months Ended
|
||||||||||||||||
|
June 30,
|
||||||||||||||||
|
2012
|
2011
|
$ Change
|
% Change
|
|||||||||||||
|
Gross profit
|
$
|
2,740,523
|
$
|
1,772,042
|
$
|
968,481
|
54.7
|
%
|
||||||||
|
Selling expenses
|
(548,179
|
)
|
(618,806
|
)
|
(70,627
|
)
|
-11.4
|
%
|
||||||||
|
Administrative expenses
|
(680,232
|
)
|
(780,518
|
)
|
(100,286
|
)
|
-12.8
|
%
|
||||||||
|
Other operating expenses
|
-
|
(91,689
|
)
|
(91,689
|
)
|
-100.0
|
%
|
|||||||||
|
Depreciation and amortization
|
(14,763
|
)
|
(51,460
|
)
|
(36,697
|
)
|
-71.3
|
%
|
||||||||
|
Income from operations
|
1,497,349
|
229,569
|
1,267,780
|
-552.2
|
%
|
|||||||||||
|
Other income/expense
|
||||||||||||||||
|
Interest income
|
148,252
|
2,220
|
146,032
|
6578.0
|
%
|
|||||||||||
|
Interest expense
|
(368,782
|
)
|
(423,495
|
)
|
(54,713
|
)
|
-12.9
|
%
|
||||||||
|
Amortization of discount on notes
|
(690,903
|
)
|
(1,381,806
|
)
|
(690,903
|
)
|
-50.0
|
%
|
||||||||
|
Foreign exchange losses, net
|
-
|
26,646
|
26,646
|
-100.0
|
%
|
|||||||||||
|
Income (loss) before income taxes
|
585,916
|
(1,546,866
|
)
|
2,132,782
|
137.9
|
%
|
||||||||||
|
Income taxexpense
|
(313,502
|
)
|
(106,953
|
)
|
206,549
|
193.1
|
%
|
|||||||||
|
Net income (loss)
|
$
|
272,414
|
$
|
(1,653,819
|
)
|
$
|
1,926,233
|
116.5
|
%
|
|||||||
Selling, General and Administrative Expenses
Selling expenses for the three months ended June 30, 2012 decreased by $70,627 and 11.4% as compared to 2011. The selling expenses decreased despite the increase in sales for the quarter, as the number of sales people did not increase and have been closely monitored due to the lower sales results experienced in the first quarter of 2012.
General and administrative expenses for the three months ended June 30, 2012 decreased by $100,286 and 12.8% as compared to 2011. This was due to higher accounting and legal expenses associated with our public filings in 2011, which did not occur in 2012.
Other Income/Expenses
Interest income for the three months ended June 30, 2012 increased by $146,032 as compared with the three months ended June 30, 2011. This increase is due primarily to interest income received during the second quarter of 2012 resulting from loans we made to a customer and a supplier in the fourth quarter of 2011.
Interest expense for the three months ended June 30, 2012 decreased by $54,713 as compared to 2011. The primary reason for this decrease was due to lower amortization of capitalized offering costs recorded in 2012 resulting from a change in estimate for the life of the offering costs. The offering costs were being amortized over a one year period in the first half of 2011, and a 2 year period in 2012.
The expense associated with the amortization of discounts on our convertible notes payable for the three months ended June 30, 2012 amounted to $690,903 as compared to $1,381,806 in 2011. As discussed above with the amortization of the capitalized offering costs, the convertible notes payable were issued in February 2011 and were being amortized over a period of one year in the first half of 2011. In 2012 we revised our estimate to amortize the amounts over a 2 year period. These amounts are expected to be amortized through February 2013.
Income Tax Expense
Our income tax expense increased by $206,549 for the three month ended June 30, 2012 as compared to 2011. The increase was attributable to an increase in taxable income during the quarter.
Pursuant to Section 26 of the Inland Revenue Ordinance (“IRO”), the governing statute of Hong Kong taxation, any dividend income received by any entity subject to IRO would not be taxable in Hong Kong. Furthermore, foreign (non-Hong Kong) investment income that is repatriated to Hong Kong is not subject to Hong Kong profits (income) tax.
Results of Operations for the Six Months Ended June 30, 2012 as Compared to the Six Months Ended June 30, 2011
The following is a comparison of our revenue, costs of sales and gross profit by segment for the six months ended June 30, 2012 and 2011.
Revenue and Costs of Sales
The Company operates in four segments: (1) Organic Trace Mineral Additives, (2) Functional Regulation Additives, (3) Herbal Medicinal Additives and (4) Other. Management tracks each of these operations separately. The Company evaluates the performance of its operating segments based on segment revenue and gross profit, and management uses aggregate segment gross profit as a measure of the overall performance of the business. The Company believes that information about aggregate segment gross profit assists investors by allowing them to evaluate changes in the gross profit of the Company’s various offerings separate from factors other than product offerings that affect net income.
|
Six Months Ended
|
||||||||||||||||
|
June 30,
|
||||||||||||||||
|
Segment revenues
|
2012
|
2011
|
$ Change
|
% Change
|
||||||||||||
|
Organic Trace Mineral Additives
|
$
|
9,898,283
|
$
|
8,801,243
|
$
|
1,097,040
|
12.5
|
%
|
||||||||
|
Functional Regulation Additives
|
1,746,539
|
1,658,775
|
87,764
|
5.3
|
%
|
|||||||||||
|
Herbal Medicinal Additives
|
96,839
|
84,268
|
12,571
|
14.9
|
%
|
|||||||||||
|
Other
|
230,267
|
387,027
|
(156,760
|
)
|
-40.5
|
%
|
||||||||||
|
$
|
11,971,928
|
$
|
10,931,313
|
$
|
1,040,615
|
9.5
|
%
|
|||||||||
|
Segment costs of sales
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$
|
6,151,155
|
$
|
5,399,720
|
$
|
751,435
|
13.9
|
%
|
||||||||
|
Functional Regulation Additives
|
1,159,312
|
1,025,072
|
134,240
|
13.1
|
%
|
|||||||||||
|
Herbal Medicinal Additives
|
115,244
|
75,674
|
39,570
|
52.3
|
%
|
|||||||||||
|
Other
|
218,463
|
232,401
|
(13,938
|
)
|
-6.0
|
%
|
||||||||||
|
$
|
7,644,174
|
$
|
6,732,867
|
$
|
911,307
|
13.5
|
%
|
|||||||||
|
Segment gross profit
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$
|
3,747,128
|
$
|
3,401,523
|
$
|
345,605
|
10.2
|
%
|
||||||||
|
Functional Regulation Additives
|
587,227
|
633,703
|
(46,476
|
)
|
-7.3
|
%
|
||||||||||
|
Herbal Medicinal Additives
|
(18,405
|
)
|
8,594
|
(26,999
|
)
|
-314.2
|
%
|
|||||||||
|
Other
|
11,804
|
154,626
|
(142,822
|
)
|
-92.4
|
%
|
||||||||||
|
$
|
4,327,754
|
$
|
4,198,446
|
$
|
129,308
|
3.1
|
%
|
|||||||||
Organic Trace Mineral Additives
Organic trace mineral additives constitute the largest and fastest growing area of our business. We are one of China’s largest domestic providers of organic trace mineral additives, specializing in the development and production of chelated organic trace mineral additives. Our current trace mineral manufacturing facility is the largest chelating facilities in China and has the capacity to produce approximately 350 metric tons of organic trace minerals per week.
Revenue from organic trace mineral additives for the six months ended June 30, 2012 increased by $1,097,040, or 12.5%, as compared to 2011. During the second quarter of 2012, demand for organic trace mineral additives rebounded after a slower first quarter due to the Chinese New Year holiday and soft overall economic growth. Our revenue in the second quarter of 2012 increased due in part to strict food safety rules and regulations enforced by the State Council of China regarding the animal feed and feed additive industries. We have benefited from these new industry standards, as smaller companies who produce sub-standard feed additives will not be able to operate. In 2012, this revenue accounted for approximately 83% of our revenues for the six months ended June 30, 2012 as compared to 81% for the six months ended June 30, 2011. Our gross profit percentage for the organic trace mineral additive sales amounted to 37.9% and 38.6% for the six months ended June 30, 2012 and 2011, respectively.
Functional Regulation Additives
Functional feed additives are widely used to enhance the properties of other products, improve feed efficiency and stimulate the rapid maturation of the immune system. We currently produce two types of functional regulation additives: feed acidifiers and flavor enhancers. Feed acidifiers are used to prevent microbial degradation of raw materials or finished feeds and to maintain the quality of feed. Flavor enhancers are used to improve feed palatability, enhance animal appetite and stimulate saliva, gastric and pancreatic juices and other digestive juice secretion and gastrointestinal motility and ultimately feed consumption and yield from production animals.
Revenue from functional regulation additives for the six months ended June 30, 2012 increased by $87,764, or 5.3%, as compared to the six months ended June 30, 2011. The increase in sales is primarily due to increased sales from the successful introduction of two new products in the 2nd half of 2011. However, the gross profit percentage for the six months ended June 30, 2012 decreased to 33.6%, as compared to 38.2% in the six months ended June 30, 2011 due to higher material and labor costs.
Herbal Medicinal Additives
Chinese herbal feed additives utilize traditional Chinese medicine theory to improve an animal’s digestion and appetite and to regulate the yin and yang balance of an animal’s health. Herbal medicines come from plants, plant extracts, fungal and bee products, minerals, shells and certain animal parts. Compared to synthetic antibiotics or inorganic chemicals, these naturally-derived products are less toxic, residue free and thought to be ideal feed additives in food animal production.
Revenue from herbal medicinal additives for the six months ended June 30, 2012 increased by $12,571, or 14.9%, as compared to the six months ended June 30, 2011. However, gross profit was negative due to significant increase in material cost.
Herbal Medicinal Additives are still in a development and introductory stage when customers are adapting to the usage and we have been developing new products and production processes to lower costs and offer more affordable products in this category, as well as new marketing strategy to grow sales. Prices and costs will continue to fluctuate in the near future until the right balance is reached.
Other
Other revenue mainly consists of buying and then reselling raw materials. Revenue from raw material sales accounted for 2% of our revenue for the six months ended June 30, 2012 and decreased by $156,760 as compared to 2011. This revenue is very price sensitive, and since our costs increased to the point that selling raw materials actually lost money, we plan on curtailing this activity. Going forward, we do not expect this to be a significant source of revenue.
Operating Expenses and Other Income / Expenses
The following table reconciles aggregate segment gross profit to net income for the six months ended June 30, 2012 and 2011.
|
Six Months Ended
|
||||||||||||||||
|
June 30,
|
||||||||||||||||
|
2012
|
2011
|
$ Change
|
% Change
|
|||||||||||||
|
Gross profit
|
$
|
4,327,754
|
$
|
4,198,446
|
$
|
129,308
|
3.1
|
%
|
||||||||
|
Selling expenses
|
(1,094,232
|
)
|
(1,204,056
|
)
|
(109,824
|
)
|
-9.1
|
%
|
||||||||
|
Administrative expenses
|
(1,221,236
|
)
|
(3,324,905
|
)
|
(2,103,669
|
)
|
-63.3
|
%
|
||||||||
|
Other operating expenses
|
-
|
(91,689
|
)
|
(91,689
|
)
|
-100.0
|
%
|
|||||||||
|
Depreciation and amortization
|
(26,429
|
)
|
(63,179
|
)
|
(36,750
|
)
|
-58.2
|
%
|
||||||||
|
Income (loss) from operations
|
1,985,857
|
(485,383
|
)
|
2,471,240
|
509.1
|
%
|
||||||||||
|
Other income/expense
|
||||||||||||||||
|
Interest income
|
169,453
|
3,610
|
165,843
|
4594.0
|
%
|
|||||||||||
|
Interest expense
|
(741,869
|
)
|
(682,949
|
)
|
58,920
|
8.6
|
%
|
|||||||||
|
Amortization of discount on notes
|
(1,381,806
|
)
|
(2,141,040
|
)
|
(759,234
|
)
|
-35.5
|
%
|
||||||||
|
Foreign exchange losses, net
|
-
|
(52,400
|
)
|
(52,400
|
)
|
-100.0
|
%
|
|||||||||
|
Income (loss) before income taxes
|
31,635
|
(3,358,162
|
)
|
3,389,797
|
100.9
|
%
|
||||||||||
|
Income tax expense
|
(430,198
|
)
|
(320,308
|
)
|
109,890
|
34.3
|
%
|
|||||||||
|
Net loss
|
$
|
(398,563
|
)
|
$
|
(3,678,470
|
)
|
$
|
3,279,907
|
89.2
|
%
|
||||||
Selling, General and Administrative Expenses
Selling expenses for the six months ended June 30, 2012 decreased by $109,824, or 9.1%, as compared to 2011. The amount represents primarily travel and meeting expenses for sales department staff to develop new customers and expand the market. The amounts for the six months ended June 30, 2012 decreased as compared to 2011 despite the increase in sales for the same period of 9.5%, as we have more closely controlled our expenses due to the reduced amount of sales experienced in the first quarter of 2012.
General and administrative expenses for six months ended June 30, 2012 decreased by $2,103,669 as compared to 2011. This was due to higher accounting and legal expenses associated with our public filings in 2011, which did not occur in 2012. In particular, we issued shares of common stock to a consultant as compensation in the first quarter of 2011, which accounted for $2,116,000 of the total administrative expenses. There were no such expenses in 2012.
Other Income/Expenses
Interest income for the six months ended June 30, 2012 increased by $165,843 as compared with the three months ended June 30, 2011. This increase is due primarily to interest income received during the second quarter of 2012 resulting from loans we made to a customer and a supplier in the fourth quarter of 2011.
Interest expense for the six months ended June 30, 2012 increased by $58,920 as compared to 2011. The primary reason for the increase in interest expense was due to new borrowings during the second quarter of 2012.
The expense associated with the amortization of discounts on our convertible notes payable for the six months ended June 30, 2012 amounted to $1,381,806 as compared to $2,141,040 in 2011. The convertible notes payable were issued in February 2011 and were being amortized over a period of one year in the first half of 2011. In 2012 we revised our estimate to amortize the amounts over a 2 year period. These amounts are expected to be amortized through February 2013.
Income Tax Expense
Our income tax expense increased by $109,890 for the six month ended June 30, 2012 as compared to 2011. The increase was attributable to an increase in taxable income during the quarter.
Pursuant to Section 26 of the Inland Revenue Ordinance (“IRO”), the governing statute of Hong Kong taxation, any dividend income received by any entity subject to IRO would not be taxable in Hong Kong. Furthermore, foreign (non-Hong Kong) investment income that is repatriated to Hong Kong is not subject to Hong Kong profits (income) tax.
Results of Operations of the Year Ended December 31, 2011 as Compared to the Year Ended December 31, 2010
The following is a comparison of our revenue, costs of sales and gross profit by segment for the year ended December 31, 2011 and 2010.
Revenue and Costs of Sales
The following is comparison of our revenue, costs of sales and gross profit by segment for the years ended December 31, 2011 and 2010.
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2011
|
2010
|
$ Change
|
% Change
|
|||||||||||||
|
Segment revenues
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$
|
18,855,958
|
$
|
15,197,415
|
$
|
3,658,543
|
24.1
|
%
|
||||||||
|
Functional Regulation Additives
|
4,163,823
|
3,388,111
|
775,712
|
22.9
|
%
|
|||||||||||
|
Herbal Medicinal Additives
|
281,036
|
419,450
|
(138,414
|
)
|
-33.0
|
%
|
||||||||||
|
Other
|
531,910
|
1,092,808
|
(560,898
|
)
|
-51.3
|
%
|
||||||||||
|
$
|
23,832,727
|
$
|
20,097,784
|
$
|
3,734,943
|
18.6
|
%
|
|||||||||
|
Segment costs of sales
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$
|
11,907,294
|
$
|
9,291,424
|
$
|
2,615,870
|
28.2
|
%
|
||||||||
|
Functional Regulation Additives
|
2,571,920
|
2,098,021
|
473,899
|
22.6
|
%
|
|||||||||||
|
Herbal Medicinal Additives
|
219,856
|
381,205
|
(161,349
|
)
|
-42.3
|
%
|
||||||||||
|
Other
|
363,449
|
926,676
|
(563,227
|
)
|
-60.8
|
%
|
||||||||||
|
$
|
15,062,519
|
$
|
12,697,326
|
$
|
2,365,193
|
18.6
|
%
|
|||||||||
|
Segment gross profit
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$
|
6,948,664
|
$
|
5,905,991
|
$
|
1,042,673
|
17.7
|
%
|
||||||||
|
Functional Regulation Additives
|
1,591,903
|
1,290,090
|
301,813
|
23.4
|
%
|
|||||||||||
|
Herbal Medicinal Additives
|
61,180
|
38,245
|
22,935
|
60.0
|
%
|
|||||||||||
|
Other
|
168,461
|
166,132
|
2,329
|
1.4
|
%
|
|||||||||||
|
$
|
8,770,208
|
$
|
7,400,458
|
$
|
1,369,750
|
18.5
|
%
|
|||||||||
Organic Trace Mineral Additives
Organic trace mineral additives constitute the largest and fastest growing area of our business. We are one of China’s largest domestic providers of organic trace mineral additives, specializing in the development and production of chelated organic trace mineral additives. Our current trace mineral manufacturing facility is the one of the largest chelating facility in China and has the capacity to produce approximately 350 metric tons of organic trace minerals per week.
Revenue from organic trace mineral additives for the year ended December 31, 2011 increased by $3,658,543, or 24.1%, as compared to 2010 due to a volume variance. In 2011, this revenue accounted for approximately 79% of our revenues for 2011 as compared to 76% for 2010. Our gross profit margin for the organic trace mineral additive sales amounted to 36.9% and 38.9% for the year ended December 31, 2011 and 2010, respectively. The decrease in our gross profit margin was primarily due to higher material and labor costs compared with last year while we needed to keep our prices steady to increase our volume.
Functional Regulation Additives
Functional feed additives are widely used to enhance the properties of other products, improve feed efficiency and stimulate the rapid maturation of the immune system. We currently produce two types of functional regulation additives: feed acidifiers and flavor enhancers. Feed acidifiers are used to prevent microbial degradation of raw materials or finished feeds and to maintain the quality of feed. Flavor enhancers are used to improve feed palatability, enhance animal appetite and stimulate saliva, gastric and pancreatic juices and other digestive juice secretion and gastrointestinal motility and ultimately feed consumption and yield from production animals.
Revenue from functional regulation additives for the year ended December 31, 2011 increased by $775,712, or 22.9%, as compared to the same period in 2010. The increase in sales is primarily due to increased volume sales to existing customers due to adopting a lower pricing strategy. The gross profit percentage for functional regulation additives for 2011 amounted to 38.2% as compared to 38.1% in the same period of 2010. The gross profit percentage for functional regulation additives had been trending down in 2011 due to increase in cost but the introduction of higher margin new products in the third quarter reversed the trend. As a result, the gross profit margin for the year 2011 remained consistent overall with 2010.
Herbal Medicinal Additives
Chinese herbal feed additives utilize traditional Chinese medicine theory to improve an animal’s digestion and appetite and to regulate the yin and yang balance of an animal’s health. Herbal medicines come from plants, plant extracts, fungal and bee products, minerals, shells and certain animal parts. Compared to synthetic antibiotics or inorganic chemicals, these naturally-derived products are less toxic, residue free and thought to be ideal feed additives in food animal production.
Revenue from herbal medicinal additives for the year ended December 31, 2011 decreased by $138,414, or 33.0%, as compared to the same period in 2010. The decrease is primarily due to unstable market demand for the products.
Other
Other revenue decreased by $560,898, or 51.3%, during the year ended December 31, 2011 as compared to the same period in 2010. Other revenue mainly consists of buying and then reselling raw materials. This revenue is very price sensitive, and since our costs increased in the second quarter of 2011 to the point that selling raw materials actually lost money, we have curtailed this activity. Going forward, we do not expect this to be a significant source of revenue.
Operating Expenses and Other Income / Expenses
The following table reconciles aggregate segment gross profit to net income for the year ended December 31, 2011 and 2010.
|
Year Ended
|
||||||||||||||||
|
December 31,
|
||||||||||||||||
|
2011
|
2010
|
$ Change
|
% Change
|
|||||||||||||
|
Gross profit
|
$
|
8,770,208
|
$
|
7,400,458
|
$
|
1,369,750
|
18.5
|
%
|
||||||||
|
Selling expenses
|
(2,463,901
|
)
|
(1,885,845
|
)
|
(578,056
|
)
|
30.7
|
%
|
||||||||
|
Administrative expenses
|
(4,486,490
|
)
|
(834,761
|
)
|
(3,651,729
|
)
|
437.5
|
%
|
||||||||
|
Depreciation and amortization
|
(81,004
|
)
|
(47,159
|
)
|
(33,845
|
)
|
71.8
|
%
|
||||||||
|
Other operating expenses
|
-
|
(258,584
|
)
|
258,584
|
-100.0
|
%
|
||||||||||
|
Income from operations
|
1,738,813
|
4,374,109
|
(2,635,296
|
)
|
-60.2
|
%
|
||||||||||
|
Other income/expense
|
||||||||||||||||
|
Interest income
|
95,834
|
4,828
|
91,006
|
1885.0
|
%
|
|||||||||||
|
Interest expense
|
(1,361,703
|
)
|
(100,265
|
)
|
(1,261,438
|
)
|
1258.1
|
%
|
||||||||
|
Amortization of discount on notes
|
(2,467,511
|
)
|
-
|
(2,467,511
|
)
|
100.0
|
%
|
|||||||||
|
Registration rights agreement expense
|
(460,206
|
)
|
-
|
(460,206
|
)
|
100.0
|
%
|
|||||||||
|
Foreign exchange losses, net
|
-
|
(1,899
|
)
|
1,899
|
-100.0
|
%
|
||||||||||
|
Income (loss) before income taxes
|
(2,454,773
|
)
|
4,276,773
|
(6,731,546
|
)
|
-157.4
|
%
|
|||||||||
|
Income tax expense
|
(681,321
|
)
|
(582,493
|
)
|
(98,828
|
)
|
17.0
|
%
|
||||||||
|
Net income (loss)
|
$
|
(3,136,094
|
)
|
$
|
3,694,280
|
$
|
(6,830,374
|
)
|
-184.9
|
%
|
||||||
Selling, General and Administrative Expenses
Selling expenses for the year ended December 31, 2011 increased by $578,056 as compared to 2010. The amount increased in 2011 due to the increase in our volume of sales, along with an increase in travel and meeting expenses for the sales department staff to develop new customers and expand the market.
Our selling, general and administrative expenses may fluctuate in the future due to a variety of factors, including, but not limited to:
|
●
|
Additional expenses as a result of becoming a reporting company including, but not limited to, director and officer insurance, compensation for the director, SEC reporting and compliance, transfer agent fees, additional staffing, professional fees and similar expenses;
|
|
●
|
Expenses resulting from developing new products or expansion of new markets, including travel and entertainment and advertising expenses.
|
General and administrative expenses for the year ended December 31, 2011 increased by $3,651,729 as compared to 2010. The increase was largely due to $2,491,938 of expenses associated with the issuance of shares of common stock to service providers in 2011. Additionally, we had higher professional service expenses associated with our public filings in 2011 and higher bank charges for capital transfer to offshore subsidiary and VIE, which did not occur in 2010.
Other Income/Expenses
Interest income for the year ended December 31, 2011 increased by $91,006 as compared to 2010 primarily due to higher cash balances on hand during 2011 relating to the private placement transaction which provided net proceeds of $6,522,563 in February 2011.
Interest expense for the year ended December 31, 2011 amounted to $1,361,703, which consisted primarily of amortization of capitalized offering costs of $709,409 relating to the convertible notes payable issued in February 2011. Interest expense also includes the interest on our convertible notes of $545,445 and interest expense from other debt outstanding of $106,849.
Additionally, we recorded amortization associated with the discounts on our convertible notes payable of $2,467,511 for the year ended December 31, 2011. These expenses did not occur in 2010.
The registration rights agreement expense relates to the amount we owe investors for failing to have our registration statement effective as of September 18, 2011. As a result, we estimated the total amount we will need to pay and have recorded it in this account. We did not have this agreement in 2010 and therefore there was no corresponding expense.
Income Tax Expense
Approximately 99% of our income was generated from mainland China and was generally subject to tax at a statutory rate of 25% on income reported in the statutory financial statements after appropriate tax adjustments, except for one subsidiary, Guangzhou Tanke Bio-Tech Co. Ltd, which obtained tax exemption on April 2007 from the State Tax Bureau of Huadu District, Guangzhou. Guangzhou Tanke Bio-Tech was a joint venture enterprise and was eligible for tax reduction and exemption, which enjoys the “two year exempt, three years half” benefits. These benefits are the result of a new tax law passed by the National People’s Congress of China in 2007. It means Tanke was exempt from tax for the first two years, which ended in 2008, and now pays one half the 25% tax rate for the next three years, through 2011.
Taking into consideration the new tax law, the effective statutory tax rate for Guangzhou Tanke Bio-Tech was 12.5% both for years ended December 31, 2011 and 2010. Beginning in 2012, Guangzhou Tanke Bio-Tech will be required to pay tax under tax rate of 15% as it is designated as a certified high-tech company since 2009, which is valid for 3 years.
The income tax expense amounted to $681,321 for the year ended December 31, 2011, an increase of $98,828 and 16.9% compared to 2010, which was attributed to the increase in taxable income for the year ended December 31, 2011. Without the tax rate benefit afforded to Guangzhou Tanke Bio-Tech, the Company’s income tax expense would have been $1,299,035 and $1,139,595 for 2011 and 2010, respectively.
Liquidity and Capital Resources
Comparison of June 30, 2012 and 2011
As of June 30, 2012 and 2011 we had cash balances of $10,032,346 and $10,519,392, respectively. The following table provides detailed information about our net cash flow for all financial statement periods presented in this report. To date, we have financed our operations primarily by cash from operations, issuance of convertible notes and capital contribution by our stockholders.
The following table sets forth a summary of our cash flows for the periods indicated.
|
Six Months Ended
|
||||||||||||||||
|
June 30,
|
$
|
%
|
||||||||||||||
|
2012
|
2011
|
Change
|
Change
|
|||||||||||||
|
Net cash provided by operating activities
|
$
|
2,380,298
|
$
|
2,896,022
|
$
|
(515,724
|
)
|
-17.81
|
%
|
|||||||
|
Net cash used in investing activities
|
(1,350,282
|
)
|
(764,537
|
)
|
(585,745
|
)
|
76.61
|
%
|
||||||||
|
Net cash provided by financing activities
|
1,091,217
|
6,282,132
|
(5,190,915
|
)
|
-82.63
|
%
|
||||||||||
|
Effect of foreign currency conversion on cash
|
210,957
|
(116,250
|
)
|
327,207
|
-281.47
|
%
|
||||||||||
|
Net increase in cash
|
$
|
2,332,190
|
$
|
8,297,367
|
$
|
(5,965,177
|
)
|
-71.89
|
%
|
|||||||
Operating Activities
Net cash provided by operating activities was $2,380,298 for the six months ended June 30, 2012 compared to cash provided of $2,896,022 for the six months ended June 30, 2011. Although we had a net loss of $398,563 during the six months ended June 30, 2012, a significant amount of our expenses were non-cash related such as $1,381,805 for the amortization of the discounts recorded on our convertible notes payable, as well as $397,271 of offering cost amortization. These non-cash expenses offset the loss and allowed us to continue generating positive cash from operations of $2,380,298. During the same period in 2011, our net loss of $3,678,470 included non-cash expenses of $2,141,040 for the amortization of the discounts on the convertible notes payable, $2,491,938 for common stock issued for services, as well as $615,549 in capitalized offering cost amortization. As a result of these non-cash expenses, and a scheduled payment from the shareholders of $1,288,062 from an outstanding receivable, we generated positive cash flows from operations of $2,896,022 during the six months ended June 30, 2011.
Investing Activities
Net cash used in investing activities was $1,350,282 for the six months ended June 30, 2012, which was the result of an increase in loans to customers and suppliers and other receivables of $479,712, spending for the acquisition of property and equipment of $388,346 and the purchase of intangible assets of $482,224. During the six months ended June 30, 2011, we had net cash used in investing activities of $764,537 due to an increase in other receivables of $740,433 and spending for the acquisition of property and equipment of $100,179, offset by cash of $76,075 acquired as a result of the VIE agreement with China Flying in the first quarter of 2011.
Financing Activities
Net cash provided by financing activities was $1,091,217 for the six months ended June 30, 2012 due to an additional loan of approximately $1.6 million received in the second quarter of 2012, offset by paydown of bank borrowings of approximately $631,000. During the six months ended June 30, 2011, we had net cash provided by financing activities of $6,282,132, resulting primarily from the net proceeds from the issuance of convertible notes of $6,522,563, and net proceeds from bank borrowings of $458,215, offset by an increase of restricted cash of $698,646.
We have historically funded our operation primarily through cash generated from operations. Over the next twelve months, we intend to pursue organic and acquisitive growth and increase our market share in mainland China. We believe that our cash on hand and cash flow from operations will meet our present operating cash needs for the next 12 months. However, we will require additional cash resources to meet the cash requirements of our planned growth.
Additionally, we may require additional cash resources due to changed business conditions, implementation of our strategy to ramp up our marketing efforts and increase brand awareness, or acquisitions we may decide to pursue. If our own financial resources are insufficient to satisfy our capital requirements, we may seek to sell additional equity, securities, convertible notes or warrants in the future.
Comparison of December 31, 2011 and 2010
As of December 31, 2011, and 2010 we had cash balances of $7,700,156 and $2,222,025, respectively. The following table provides detailed information about our net cash flow for all financial statement periods presented in this report. To date, we have financed our operations primarily by cash from operations, issuance of convertible notes and capital contribution by our stockholders.
The following table sets forth a summary of our cash flows for the periods indicated.
|
Year Ended
|
||||||||||||||||
|
December 31,
|
$
|
%
|
||||||||||||||
|
2011
|
2010
|
Change
|
Change
|
|||||||||||||
|
Net cash provided by operating activities
|
$
|
3,544,279
|
$
|
4,384,988
|
$
|
(840,709
|
)
|
19.2
|
%
|
|||||||
|
Net cash used in investing activities
|
(3,559,483
|
)
|
(2,821,822
|
)
|
(737,661
|
)
|
26.1
|
%
|
||||||||
|
Net cash provided by financing activities
|
5,815,761
|
(1,220,739
|
)
|
7,036,500
|
-576.4
|
%
|
||||||||||
|
Effect of foreign currency conversion on cash
|
(322,427
|
)
|
61,723
|
(384,150
|
)
|
-622.4
|
%
|
|||||||||
|
Net increase in cash
|
$
|
5,478,130
|
$
|
404,150
|
$
|
5,073,980
|
1255.4
|
%
|
||||||||
Operating Activities
Net cash provided by operating activities was $3,544,279 for the year ended December 31, 2011 compared to cash provided of $4,384,988 for the year ended December 31, 2010. Although we had a net loss of $3,136,094 during the year ended December 31, 2011, a significant amount of our expenses were non-cash related such as $2,491,938 in expenses associated with the issuance of common stock for services, $2,467,511 for the amortization of the discounts recorded on our convertible notes payable, as well as $709,409 of offering cost amortization. These non-cash expenses offset the loss and allowed us to continue generating cash from operations. In addition, during 2011 we prepaid some of our suppliers in order to get favorable pricing on raw materials. The prepayment of $3,633,674 was partially offset by receipt of cash of $2,584,892 from related parties who were repaying their notes. The related party notes originated in 2010 when certain payments on customers’ accounts receivable were paid to individuals instead of the Company. The intent was for the individuals to repay the Company for these collections.
During the 4 th quarter of 2011, as material prices were on the verge of increasing substantially, the Company negotiated with key raw material suppliers to secure continuous supply and lower cost in the coming year. Many of these arrangements require a prepayment or deposit of up to a year’s projection of purchases. As we continue purchasing from these suppliers throughout the year, some prepayments are being used up but the majority of them require replenishments in order to lock in lower prices. The Company is constantly monitoring the raw material pricing trends and in contact with the suppliers to make adjustments to the prepayment arrangements as necessary.
Investing Activities
Net cash used in investing activities was $3,559,483 for the year ended December 31, 2011, which was the result of an increase in other receivables of $2,404,598, spending for the acquisition of property and equipment of $701,022, spending for the purchase of intangible assets of $529,938, offset by cash of $76,075 acquired as a result of the acquisition of China Flying in the first quarter of 2011. The other receivable balance reflects loans made during 2011 to unrelated parties. We expect these loans to be repaid during 2012. During 2010, our net cash used in investing activities was $2,821,822 due primarily to spending for the acquisition of property and equipment of and construction in progress $2,970,511.
Financing Activities
Net cash provided by financing activities was $5,815,761 for the year ended December 31, 2011. The proceeds from financing activities were the result of net proceeds from the issuance of convertible notes of $6,522,563, offset by an increase in restricted cash of $706,802. During 2010, our cash used in financing activities was $1,220,739 resulting from payments made on bank borrowings of $892,134, payments made to related parties of $328,605.
Our bank borrowings stem from an original note with the bank in the amount of RMB15,000,000. The interest rate is divided between different borrowings, and ranges between 5.4% and 5.85%. If we fail to use the funds in accordance with the agreement, specifically for technical innovation, there is a penalty equal to 100% of upward fluctuation in the interest rate. To date we have not violated this provision of the note. Furthermore, if we are late on any payments, the interest rate increases by 50%. At December 31, 2011, the outstanding balance on the note was $1,413,821, of which $471,274 is due May 21, 2012, $314,182 is due October 30, 2012 and the remainder is due January 30, 2013.
We have historically funded our operation primarily through cash generated from operations. Over the next twelve months, we intend to pursue organic and acquisitive growth and increase our market share in mainland China. We believe that our cash on hand and cash flow from operations will meet our present operating cash needs for the next 12 months. However, we will require additional cash resources to meet the cash requirements of our planned growth.
Additionally, we may require additional cash resources due to changed business conditions, implementation of our strategy to ramp up our marketing efforts and increase brand awareness, or acquisitions we may decide to pursue. If our own financial resources are insufficient to satisfy our capital requirements, we may seek to sell additional equity, securities, convertible notes or warrants in the future.
Transfer of Offshore Funds into China
The most commonly accepted way to transfer offshore funds to our subsidiaries is through the increase of a subsidiary’s registered capital. Specifically, overseas funds such as proceeds of an offering can be contributed to our wholly foreign owned subsidiaries in China, Kanghui Agricultural, as a result of an increase in the registered capital of Kanghui Agricultural. China Flying contributed $3,999,900 into Kanghui Agricultural and Kanghui Agricultural has filed its increase of registered capital certificate from $75,000 to $4,074,990 with Guangzhou Administration for Industry and Commerce on May 5th, 2011.
The easiest way to transfer funds from Kanghui Agricultural to Guangzhou Tanke is via a loan agreement from Kanghui Agricultural to Guangzhou Tanke for Guangzhou Tanke’s operation. In connection with this loan, Guangzhou Tanke may be required by Kanghui Agricultural to pledge all or part of its assets as collateral to secure such loans.
Kanghui Agricultural may also buy certain Guangzhou Tanke’s assets or businesses. Upon the completion of such acquisition, such business or assets will be utilized by Kanghui Agricultural in providing Guangzhou Tanke with the specified consulting and technical services under the Consulting Services Agreement between Guangzhou Tanke and Kanghui Agricultural.
Alternatively, Kanghui Agricultural may make a capital contribution to Guangzhou Tanke, subject to the restriction below and provided that there is not a change in the current PRC law that prohibits such equity investment. Due to the restrictions under the Circular 142, RMB converted from registered capital of our WFOE, Kanghui Agricultural, cannot be used to make an equity investment in Guangzhou Tanke to increase the registered capital of Guangzhou Tanke. The converted RMB can only be used for activities falling under the respective business scope as provided in the respective business licenses of Guangzhou Tanke. PRC law does not prohibit a WFOE from making an equity investment, however Circular 142 restricts a WFOE from using RMB converted from its registered capital to making such equity interest.
Kanghui Agricultural may invest cash in Guangzhou Tanke pursuant to methods that are not restricted by Circular 142:
1. Kanghui Agricultural can utilize the RMB generated by its operations in PRC to increase the registered capital of Guangzhou Tanke, which is not subject to regulation of Circular 142;
2. We can expand our investment in Guangzhou Tanke through asset investments rather than through equity investment in the future, which is not restricted under the Circular 142; and
3. We can restructure our WFOE, Kanghui Agricultural, to foreign-invested investment companies because the Circular 142 does not regulate the equity investment activities of a foreign-invested investment company. However, the threshold to set up a foreign-invested investment company is fairly high, with a minimum registered capital of $30,000,000 and other strict conditions which cannot be fulfilled by us at this point in time. Incorporation of a foreign-invested investment company is subject to approval of the PRC Ministry of Commerce or its local counterparts, and each equity investment made by such foreign-invested investment company is also be subject to SAFE’s verification.
Interest Transfer from Guangzhou Tanke to Kanghui Agricultural
Kanghui Agricultural and Guangzhou Tanke have entered into a Technical Services Agreement, under which Guangzhou Tanke will transfer substantially all of its profit to Kanghui Agricultural as the technical service fee.
Cash Transfer out of China
The PRC government imposes currency exchange controls on all cash transfers out of China; A China domiciled subsidiary can instruct one of a number of banks authorized by the government to handle outbound foreign exchange transactions in China to make a cash transfer out of China, and the bank will make such transfer after its receipt and satisfactory review of documents evidencing compliance with the regulations and procedures mandated by the Chinese government;
Cash transfers related to product or service purchases, or payments for royalties and related charges do not require pre-approval, but require the China domiciled subsidiary to furnish to the bank the following supporting documents:
|
•
|
a form which describes the amount and type of the proposed transfer in accordance with China’s currency exchange control regulations;
|
|
|
•
|
documentation that all required approvals or filings for the product, service or license to be purchased (where applicable)
|
|
|
•
|
have been obtained (for example, if a China domiciled entity desires to purchase imported technology, the purchase requires approval from the Ministry of Commerce);
|
|
|
•
|
relevant agreements or contracts (e.g., sales contracts, service agreements or license agreements, etc.);
|
|
|
•
|
corresponding invoices or payment notices; and
|
|
•
|
tax payment receipts.
|
Cash transfers out of China to a foreign parent for purposes of profits repatriation and dividends do not require pre-approval. Pursuant to applicable regulations, foreign-invested enterprises in China, like Kanghui Agricultural, may pay dividends only out of their accumulated profits, if any, determined in accordance with Chinese accounting standards and regulations. In calculating accumulated profits, foreign investment enterprises in China are required to allocate at least 10% of their accumulated profits each year, if any, to fund certain reserve funds unless these reserves have reached 50% of the registered capital of the enterprises. The regulations require the China domiciled subsidiary to furnish to the bank the following supporting documents:
|
•
|
a form which describes the amount and type of the proposed transfer in accordance with China’s currency exchange control regulations;
|
|
|
•
|
a foreign-invested enterprise foreign exchange registration certificate;
|
|
|
•
|
a board resolution or shareholder’s resolution approving the repatriation of profits or dividends;
|
|
|
•
|
a copy of its statutory accounts for the most recently completed fiscal year (financial statements prepared in accordance with Chinese governmental regulations and audited by an approved independent auditor); and
|
|
•
|
corresponding tax payment receipts.
|
The cash transfer mechanisms described above are examples of current account transactions under China’s currency exchange controls. Other types of transactions, such as repatriations of investment by or loans to foreign owners, or direct equity investments in a foreign entity by a China domiciled entity are examples of capital account transactions under China’s currency exchange controls. Capital account transactions require prior approval from China’s State Administration of Foreign Exchange (SAFE) or its provincial branch to convert a remittance into a foreign currency, such as U.S. dollars, and transmit the foreign currency outside of China.
It is possible that the requirements for a China domiciled subsidiary to transfer cash out of China may be changed without prior notice or any due process. It is also possible that the Company may experience delays in accomplishing transfers of cash from its China domiciled subsidiary.
Effect of Changes in the Foreign Exchange Rate
Upon translation of the Company’s financial statements into US Dollars for the purpose of financial reporting in the United States, the exchange rate between the Chinese Renminbi and the US Dollar can have an impact on the amount of reported cash on hand.
However all of the Company’s revenue is generated in China, and currently over 90% of its cost is within China. As a result, from an operational standpoint, a change in the exchange rate has relatively little impact on the Company. Such change is not expected to affect the Company’s liquidity in any significant way.
Economy and Inflation
Inflation and changing prices have not had a material effect on our business and we do not expect that inflation or changing prices will materially affect our business in the foreseeable future.
We have not experienced any significant cancellation in orders due to the downturn in the economy. Furthermore, we have also had only a small number of customer-requested delays in delivery or production.
Off Balance Sheet Arrangements
We do not have any off balance sheet arrangements that have, or are reasonably likely to have a current or future effect on our financial statements.
Seasonality
Our operating results and operating cash flows historically have not been subject to significant seasonal variations. However, sales around Chinese New Year are typically comparatively lower than other months. This pattern may change as a result of new market opportunities or new product introduction.
Recent Accounting Pronouncements
See Note 2 of the accompanying consolidated financial statements for a description of recent accounting pronouncements. We do not anticipate that the adoption of these recent accounting pronouncements will have a material impact on our financial statements.
DESCRIPTION OF BUSINESS
Those statements in the following discussion that are not historical in nature should be considered to be forward looking statements that are inherently uncertain. Actual results and the timing of the events may differ materially from those contained in these forward looking statements due to a number of factors, including those discussed in the “Cautionary Note on Forward Looking Statements” and “Risk Factors” set forth elsewhere in this prospectus. As a result of the Share Exchange and the VIE Agreements, the Company, through Kanghui Agricultural, its indirect wholly owned subsidiary, assumed management of the business activities of Guangzhou Tanke and has the right to appoint all executives and senior management and the members of the board of directors of Guangzhou Tanke. As used in this section, the terms “we”, “our”, “us” and the “Company” refer to the Company, our direct and indirect subsidiaries and Guangzhou Tanke, our principal operating business.
Overview
Through Guangzhou Tanke, our principal operating business, we are one of the leading animal nutrition and innovative feed additive providers in China. Our products are distinguished from traditional artificial feed additives in that they are environmental-friendly and are designed to optimize the growth and health of livestock such as pigs and cattle, as well as farmed fish. One of our most popular products is an organic trace mineral additive that we believe is one of the few Chinese-developed organic products in the trace mineral market.
In 2001, we were designated a certified hi-tech company by the Guangzhou City Commission of Science and Technology as recognition of new technology that we developed in the agricultural industry, and in addition to our headquarters, we operate in a modern 34,000 square-meter manufacturing facility in the Huadu Economic District, also in Guangdong province. As the Chinese economy continues to evolve and prosper, the opportunity for technology companies like the Company should increase dramatically.
Our feed additive products are distinguished from traditional artificial feed additives in that they are non-hazardous, environmentally friendly and safe for livestock and their human consumers, making them compatible with China's efforts to develop a safer food supply. Such feed additive products are environmentally friendly because animals that consume them produce fewer waste products than other animals and a decrease in the amount of waste produced is beneficial to the environment.
As a growing player in providing advanced, environmentally-friendly and innovative feed additives, we believe that sales will rapidly increase as more large scale farms and feed processing and production companies in China seek “Pollution-Free” certifications from the Chinese government. These certifications indicate that the farm or food production facility has taken material steps to make its manufacturing process as environmentally friendly as possible and the food provided as safe as possible. Large scale farms and feed processing and productions companies in China that seek "Pollution-Free" certifications may be more likely to receive such certifications if they use our Organic Trace Mineral Additives. Pursuant to a May 3, 2005, published by the MU Extension of the University of Missouri-Columbia, the main advantage of feeding organic trace minerals to animals is to increase the bioavailability to the animal, thereby decreasing the amount of waste and, correspondingly, the amount of pollution. In addition, feed additives are utilized in China at less than half the rate of the United States and Europe, and we have a significant growth opportunity as Chinese farmers and ranchers include a greater amount of increasingly sophisticated additives in their feeds.
Our major products address most key market categories within China’s animal feed additive industry, including:
|
●
|
Organic Trace Mineral Additives, which accounted for approximately 76% of our revenue in 2010 and 79% of our revenue in 2011;
|
|
●
|
Feed Acidifiers, Seasonings and Flavor Enhancers, which accounted for approximately 17% of our revenue in 2010; and 17% of our revenue in 2011; and
|
|
●
|
Herbal Medicinal Additives, which account for approximately 2% of our revenue in 2010 and 1% of our revenue in 2011.
|
Our extensive distribution network reaches China’s top 10 feed producers and the 500 largest animal farming operations. As a result of our diversified products and extensive distribution network, we believe that we are ideally positioned to help meet China’s growing demand for safe and reasonably priced food.
From Guangzhou Tanke’s incorporation in April 1997, when it manufactured one product utilizing a small rented facility in Gaotang in the Guangdong province, Guangzhou Tanke has grown into one of China’s largest feed additive producers. As of December 31, 2011, we marketed 22 different brands of feed additives and had an aggregate production capacity of approximately 560 metric tons per week of feed additives, of which 350 metric tons are organic trace minerals. We estimate that we are currently operating at a blended average across our product lines of approximately 53% of our manufacturing capacity at our current facility. To satisfy increasing demand of our products and to provide better services, we have purchased land and started construction of our second manufacturing facility in Qingyuan, Guangdong. The new facility will utilize the most advanced technology and automated systems which greatly reduce per unit cost and improve product quality. It will be fully operational at the beginning of 2013.
We launched two new products in the second half of 2011. One is “Shibujian” used in helping improve the health and appetite of piglets. The other is “Shufukang”, an animal vitamin product that enhances the effectiveness of the Company’s trace minerals. In 2012, we have about 5 – 7 new product introductions in the pipeline, mainly focus on improving health and enhancing growth in pigs, cattle, chicken, and seafood.
Development of Business
We believe our rapid growth in recent years has been supported by the continuing expansion of the market for feed additive products in China as well as our introduction of new products to meet increased demand by our customers for more diversified and efficient products.
In 2000, we introduced our first organic trace mineral additive and this segment of our business has become our fastest growing. In 2008, sales of organic trace minerals accounted for the majority of our revenue, generating approximately $4.8 million, or 56% of our total sales. The growth continued in 2009 as sales increased to approximately $8.5 million, or 70% of our total sales for that year. As of December 31, 2010, sales of organic trace minerals rose to $15.2 million, or 76% of our total sales. In 2011, organic trace mineral products continued to rise 24% to $18.9 million, or 79% of total sales.
In 2003, we became a member of the National Feed Industry Standardization Technical Committee, a prestigious appointment that added significantly to our credibility. Over the years, we have helped the government established three national standards and one industry standard for animal feed additives. These are: (1) GB/T 21694-2008 product standard for feed additive of zinc methionine; (2) GB/T 20802-2006 product standard for feed additive of copper methionine; (3) NY/T 1498-2008 product standard for feed additive of methionine iron and (4) GB/T 22489-2008 product standard for feed additive of methionine manganese.
In 2006, we were recognized as an “Excellence Enterprise” by the provincial government for our technology innovation in the feed additive industry. During the same year, we formed two fully owned subsidiaries, Guangzhou Jenyi Bio-Tech and Guangzhou Tanke Animal Health Product to further expand and diversify our business into herbal feed additives and veterinary drugs.
In 2007, our production of organic trace minerals was granted “National Torch Project” by the Chinese Ministry of Science and Technology. Launched in 1988 by the Chinese government, the Torch Program is China’s most important program to encourage and recognize high technology projects and achievements based on certain technological standards and national economic benefits.
During the period of 2006 to 2011, we received three patents granted by the State Intellectual Property Office and have five patents pending in the areas of (1) the methodology of feed additive production; (2) the procedure associated with testing products, among others.
Much effort and resources were put into building our technology innovation center at the existing manufacturing facility in Huadu. Once the new facility in Qingyuan is complete, the production of high volume products will be moved there and the existing facility will focus on R&D and developing and testing new products. Some low volume, high impact, specialty products will still be manufactured at the Huadu facility.
We launched two new products in the second half of 2011. One is “Shibujian” used in helping improve the health and appetite of piglets. The other is “Shufukang”, an animal vitamin product that enhances the effectiveness of the Company’s trace minerals. In 2012, we have about 5 – 7 new product introductions in the pipeline, mainly focus on improving health and enhancing growth in pigs, cattle, chicken, and seafood.
Overview of the Chinese Feed Additive Market
Over the past decade, as a result of a series of market-based reforms, China’s economy has experienced unprecedented growth, with an average annual GDP growth rate of over 10%. As China has become more prosperous, the rapid growth in per capita income and consumer choices has led to a dramatic improvement in living standards and dietary patterns. Chinese consumers have significantly increased their consumption of high-protein food such as meat and other livestock and in the place of traditional staple grain-based foods. This growing demand for high-protein foods has had a material impact on the growth of the feed additive market.
Beginning in the mid-1970s, in response to China’s increasing and diversifying food consumption, its domestic feed industry began to experience rapid development and transitioned into the world’s second largest feed producer behind the United States. According to a recent research report issued by IBISWorld in March 2011, (originated in 1971, IBISWorld is the market research organization that specializes in the long range forecasting of industries and business environments in Australia, the US and China), revenue of the animal feed manufacturing industry (including pet food) increased from $39.8 billion in 2007 to a forecast $97.62 billion in 2011. This represents an annualized growth rate of 27.8%. This growth momentum is expected to continue in future years with projected total revenue of $158.63 billion in 2017, or an average growth rate of 14.8% per year.
In the past decade, feed producers have become more efficient, with new, high production mills replacing older, smaller mills. As part of their effort to improve the quality of agricultural output and the efficiency of animal production, commercial feed producers have increased their use of feed additives. According to the Chinese Ministry of Agriculture, the Chinese market for all feed additives in 2010 was $5.5 billion, compared to $4.6 billion in 2009.
According to Chinese Feed Industry Information, there has been rapid growth in the animal feed industry in China, however, development and research of feed additives in China has not had similar growth. Many of the highly efficient, low toxic and less residual feed additive products are heavily dependent on importation thereby creating a significant opportunity for domestic manufacturers, like the Company, to increase its market share in China. Additionally, while, according to the European Union Register of Feed Additives published June 1, 2011, the European Union has hundreds of feed additive formulas approved for use, to date China has only approved approximately 220 feed additive formulas, of which most are imported. As China permits more feed additive formula approvals in an attempt to close the gap with the western developed countries, the Company will benefit since it has the technology and manufacturing capability to produce these new and more effective products.
One of the primary components of the Company’s business is Feed Acidifiers and it has gained significant momentum in recent years as a substitute for growth promoters that rely on antibiotics as the primary ingredient. The desirability of Feed Acidifiers is a result of growing concerns about drug-resistant “superbugs” in humans and animals resulting from the indiscriminate use of antibiotics. The global feed industry has been under scrutiny for years for its use of antibiotics as growth promoters in the rearing process of livestock, prompting the European Union to ban the use of Antibiotic Growth Promoters (AGPs) in January 2006. The Chinese government is currently tightening the industry standard and may follow the EU’s lead to restrict or ban the use of AGPs. Our Feed Acidifiers differ from other growth promoters because they contain alkaloids to stimulate acid production in an animal’s stomach, lowering the pH levels to improve overall animal health. Such Feed Acidifiers do not rely on antibiotics as a primary ingredient. Rather, the ingredients in our Feed Acidifiers comply with the more restrictive requirements of the EU and the increased regulation that we anticipate in the future from China’s regulatory authorities.
In response to quality control breakdowns from isolated Chinese manufacturers in 2007, in June, 2009, the Chinese Ministry of Agriculture announced Bulletin No. 1224 (the Safe Use of Feed Additive Specification), which tightened feed additive quality control standards by specifying “norms” of trace elements and other feed additives usage. The maximum normative amounts set by the Chinese government are required to be strictly followed and implemented. As a result of these regulations, the Company expects this new regulation to drive the market to shift from high dosage, low absorption rate inorganic products to low dosage, high absorption rate organic products. Our products meet the requirements described in the new regulations in that they require a smaller amount of additive (a lower dosage) for the same nutritional effect as inorganic products. In addition, our products increase the bioavailability of the animal resulting in less excretion to the environment. As one of the pioneers in the organic trace minerals additive segment, Guangzhou Tanke, as a member of the Chinese Feed Association, participated in setting the national standards on the usage of organic trace minerals additives. As a result, we are well positioned to further expand our market position.
Competition in the Chinese Market
The Chinese feed additive market is highly fragmented, with approximately 2,500 feed additive companies nationwide and no participant having a greater than 1.2% market share. While many of the Company’s domestic competitors are smaller businesses that operate in relatively specialized niche product areas, the industry is in the process of transforming itself from small, family-based operations into large, enterprise based businesses.
Foreign firms are also attempting to gain a foothold in the Chinese feed additive market, but generally charge higher prices than those of domestic manufacturers.
Our largest Chinese competitors include:
|
●
|
Changsha Xingjia Bio Tech Co., Ltd., which is engaged in developing, marketing and producing safe, environmental friendly trace mineral feed additives. Changsha offers compound acidifier, amino acid chelated trace elements, copper chloride and other products. Changsha has sales office nationwide and subsidiaries in Thailand and Singapore.
|
|
●
|
Debon Bio Tech Co., Ltd., which was established in 2004 and is a Sino-German joint venture engaged in feed additive development and raw material trading. Debon has a long term partnership with its German partner and imports piglet nutrition and feed additives from overseas.
|
We also compete with the following large international manufacturers:
|
●
|
Zinpro Corporation, a manufacturer of trace minerals. Zinpro offers iron, copper, manganese, zinc and cobalt products used in the dairy, beef, poultry, swine, and equine industries. Headquartered in Eden Prairie, Minnesota, Zinpro has sales offices in the United States, Canada, Mexico, the Netherlands, China, Japan, Thailand, Brazil, Australia and New Zealand.
|
|
●
|
Alltech Inc., an animal health and nutrition company. Alltech manufactures nutritional products and solutions for the feed industry. It provides natural feed ingredients and Sel-Plex organic selenium for use in animal species with selenium deficiencies for feed and food manufactures in North America, Latin America, the Asia-Pacific, Europe, the Middle East, and Africa. Alltech is headquartered in Nicholasville, Kentucky and has bioscience centers in the United States, Ireland, and Thailand.
|
Growth Strategy
Our goal is to become the leading provider of feed additives in China. Our primary growth strategy is as follows:
Strengthen our leading position in the organic trace mineral market and substantially increase our Chinese market share within the next three years.
In recent years, Chinese inorganic minerals have been linked to contamination by heavy metals and dioxins. As a result, farmers and feed producers are increasingly switching to organic trace minerals based on research that indicates that quality organic minerals are superior to inorganic minerals in bioavailability, health and performance.
As the largest organic trace minerals producer in China, accounting for approximately 6.6% of total production on an annual basis, the Company is ideally positioned to benefit from the substantial growth it anticipates in the organic trace mineral market. To meet the expected demand, we are building a second manufacturing facility that would double our organic trace mineral production capacity.
Expand sales of the Company’s products to more regions within China.
As of the end of 2011, we had sales representatives in six of the major agriculture centers in China, including China’s northeastern and southern regions. To expand our reach into China’s other regions, we intend to establish sales offices in the northern and central regions of China and hire additional sales personnel in 2012.
Increase the Company’s production capacity.
In 2011, we added a new production line at our current manufacturing facility in Huadu city, increasing total production capacity by 40% to 560 metric tons per week. In addition, we have begun remodeling and upgrading the old production line in 2012, investing over $760,000 to further improve efficiency and capacity of the current facility. Due to a longer process of getting all the various government approvals and permits, the Qingyuan new facility project was delayed but it has no impact to our ability to produce and service our customers because of the improvements at the current facility. We anticipate the new facility will be fully operational by early 2013 and at that time, all the production of our core products in Organic Trace Minerals will be moved to Qingyuan to take advantage of the most advanced technology and highly automated processes. Our Huadu facility will focus on producing other product categories and developing new and high impact products.
Increase the Company’s investment in research and development.
To maintain a competitive advantage in the marketplace, we plan to devote greater resources to our in-house research and development team and to enhance and expand our collaborations with institutions and universities. Our in-house research team typically requires one to two years to develop a new product and bring it to market. We intend to enhance our trial testing program by acquiring a farm operation to streamline our testing.
Additionally, we plan to strengthen our collaboration with institutions and universities as part of our effort to stay on the cutting edge of the feed additive business. We believe that as the Chinese market continues to grow and mature, companies will face increasing competitive pressure both in the area of technology and talented personnel. Our partnership with institutions and universities will not only provide us with skilled human resources, but also will assist us in staying on the cutting edge of the industry. Currently, the majority of our research partners are located in Guangzhou, and we intend to establish similar cooperative relationships with schools in other regions.
Strengthen international sales.
We plan to increase our attendance at industry exhibitions worldwide to market our products to a broader market of potential customers. In our experience, participating in these exhibitions is an effective way to introduce our products overseas and develop new customers.
Competitive Strengths
We believe that the following competitive strengths have contributed to our current market position and enable us to capitalize on the growth opportunities in the feed additive market in China:
We have a leading market position in the organic trace mineral market.
We are the largest provider and producer of organic trace minerals in China with production capacity of 350 metric tons per week. We acquired land use rights in Qingyuan, Guangdong province, to build a second manufacturing facility, a project we expect to take one year to be finished by early 2013. Upon the completion of this facility, we expect to double our production capacity of organic trace minerals.
We offer a diversified product portfolio.
Following the introduction of Guangzhou Tanke’s first flavor enhancer into the market in 1997, we have introduced a broad product portfolio to the market, including organic trace mineral, functional regulation additives and herbal medicinal additives. Within each of these segments, we produce a diverse array of products.
We have strong research and development capabilities.
We have made significant investments in research and development. We focus our research and development efforts on creating new products with large potential markets and on improving existing technologies, both with a view towards increasing market share and growing the business.
We are in compliance with standard manufacturing guidelines for our industry.
We have an ISO 9001/2000 International Quality Management System Certificate for our operations management system and Good Manufacturing Practice (GMP) for our manufacturer compliance for animal drugs, certifying our commitment to the integrity of our products. The World Health Organization (WHO) initiated the GMP system in the 1960s, and China adopted it in the early 1980s. GMP guidelines define standards for the pharmaceutical manufacturing process to reduce the possibility of contamination errors. Companies that fail to meet GMP specifications will be restricted or banned from production.
Our management team has extensive knowledge of, and experience in, the feed additive industry.
Our management team, led by Guixiong Qiu, Guangzhou Tanke’s founder and the Company’s Chief Executive Officer, match their academic backgrounds in agriculture and chemistry with extensive knowledge of the feed additive industry in China and a proven track record of developing and marketing quality feed additive products.
Products and Services
We currently market 22 different brands of feed additives at various price points to meet the demands of existing and prospective customers. Within each brand there are seven different mixes that correspond to the different growth stages of an animal’s life cycle. Our business focuses on four key business areas: organic trace minerals additives, functional regulation additives, herbal medicinal additives and other. The following chart shows each segment’s contributions to fiscal 2011 net sales and gross profit.
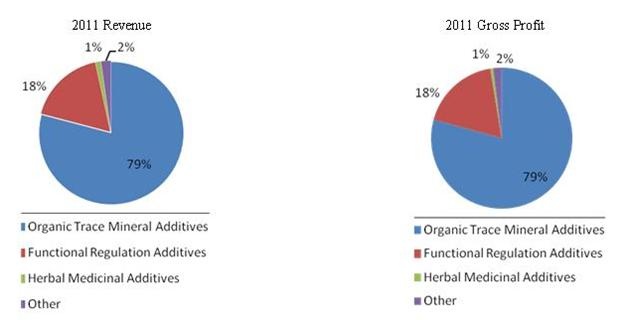
Organic Trace Mineral Additives
We are China’s largest domestic provider of organic trace mineral additives, specializing in the development and production of chelated organic trace minerals additives. Our current trace mineral manufacturing facility is the largest chelating facility in China and has the capacity to produce approximately 350 metric tons of organic trace mineral per week.
Our total revenue for organic trace minerals in 2011 was approximately $18.9 million. Such revenue accounted for approximately 79% of our 2011 revenues and net sales and carried a gross profit margin of approximately 37%.
Minerals play an important role in the growth and development of fish, livestock, pigs and cattle and are routinely used by breeders to supplement their animals’ diets. Animals require two classes of minerals: major minerals, which include sodium, potassium, chloride, calcium, magnesium, phosphorus, and sulfur, and trace minerals, which include copper, iron, manganese, molybdenum, zinc, chromium, fluorine, selenium and silicon. Major and trace minerals are differentiated primarily by the amount of a particular mineral that an animal requires. Animals require a minimum of 100 milligrams per day of the major minerals to carry out normal bodily functions and less than 100 milligrams per day of trace minerals.
While major minerals are typically present in most feed products provided to animals, Chinese farmers and ranchers are placing an increasing emphasis on the consumption of trace minerals, which help the animal’s body perform its daily routines more efficiently.
Trace minerals are widely available as feed additives in two main forms: organic and inorganic. Although both forms are commonly used, important differences exist in their bioavailability and environmental impact. Bioavailability means the degree or rate at which a nutrient of medication is absorbed and becomes available to the body. Organic trace minerals increase bioavailability, reducing feed costs and minimizing nutrient buildup in the soil. Environmentally, new restrictions are likely to be imposed on producers to reduce nutrient excretion, making the Company’s organic feed additives more appealing to breeders. The Company’s products are more appealing because they achieve similar production results to inorganic feed additives while requiring smaller amounts of trace materials.
Our organic trace minerals are marketed primarily in, but also outside of, China to large scale feed producers and farmers under the brand name “Qili”. Our principle organic trace minerals products are:
|
●
|
Iron glycine chelate (G/Fe-140);
|
|
|
●
|
Iron glycine chelate (G/Fe-185);
|
|
|
●
|
Zinc glycine chelate (G/Zn-220);
|
|
|
●
|
Manganese glycine chelate (G/Mn-220);
|
|
|
●
|
Copper glycine chelate (G/Cu-210);
|
|
|
●
|
Chromium glycine chelate (G/Cr-001);
|
|
|
●
|
Iron methionine chelate (M/Fe-155);
|
|
|
●
|
Zinc methionline chelate (M/Zn-190);
|
|
●
|
Manganese methionine chelate (M/Mn-155);
|
|
|
●
|
Copper methionine chelate (M/Cr-001);
|
|
|
●
|
Zinc lysine chelate (L/Zn-105);
|
|
|
●
|
Zinc lysine chelate (L/Zn-145); and
|
|
|
●
|
Copper lysine chelates (L/Cu-100).
|
According to a certificate report issued by Guangdong Feed Industry Association dated April 28, 2011, Qili products provide the essential minerals (zinc, copper, manganese and chromium) and lysine, which is an amino acid essential to a nutritious livestock feed program. Such products provide nutritional balance for the animals in order for them to have a healthier life. Qili products also help animals absorb these essential minerals and lysine in order to slow the process by which nutrients pass through the animal.
We also produce and market multiple trace mineral premix products for livestock and poultry under the trademark “Qilimix,” which has been particularly successful in foreign markets. These products contain highly bio-available minerals and result in the lowest excretion of minerals into the environment, especially for high content copper and zinc. Qilimix products are used to improve the reproductive performance of sows and breeder poultry, the growth and reproductive performance of pigs and the quality and color of animal carcasses.
Functional Regulation Additives
We are one of the leading developers and providers of functional regulation additives in China. According to the Chinese Ministry of Agriculture, the Chinese market for functional feed additives in 2009, including feed acidifiers and flavor enhancers, was $328 million. Our total revenues and net sales of functional feed additives in 2010 and 2011 were approximately $3.4 million and approximately $4.2 million, respectively, accounting for approximately 1% of China’s total production.
Sales from functional regulation additives represent approximately 18% of our 2011 revenues and net sales and carry a gross profit margin of approximately 38%.
Functional feed additives are widely used to enhance the properties of other products, improve feed efficiency and stimulate the rapid maturation of the immune system. We currently produce two types of functional regulation additives: feed acidifiers and flavor enhancers.
Feed Acidifiers
Feed acidifiers are used to prevent microbial degradation of raw materials or finished feeds and to maintain the quality of feed. We produce and market feed acidifiers under the trademark “Qilicid.” Qilicid products consist of alkaloids that stimulate acid production and lower pH levels, inhibiting the development of pathogenic bacteria in the stomach and stimulating endogenous pepsin activities in the stomach and enzyme production in the intestine.
According to a certificate report issued by Guangdong Feed Industry Association dated April 28, 2011, Qilicid products slow the passage of the feed through the animal’s intestine, allowing ample time for digestion, increasing feed intake and nutritional efficiency, reducing undesired gut microorganisms, supporting endogenous digestive enzymes and improving animal growth performance.
Flavor Enhancers
Flavor enhancers are widely used throughout the world as an important agent in the production of blended and high-grade feed to ensure animals obtain the required nutrients and to improve feed efficiency. There are two types of flavor agents: aroma agents, which impart a pleasant scent to feed and come from the roots, stems, leaves, and fruits of natural plants and from artificial compounds, and taste agents, which include sweeteners, which improve the feed’s taste and promotes continuous eating and come from flavor agents, salty agents and other flavoring materials.
All flavor enhancers are used to improve feed palatability, enhance animal appetite and stimulate saliva, gastric and pancreatic juices and other digestive juice secretion and gastrointestinal motility and ultimately feed consumption and yield from production animals.
As animals grow, their nutritional needs change, requiring corresponding changes to feed. Such feed changes often result in reduced intake by animals accustomed to the flavor of prior feeds. Flavor agents can be mixed with different feeds to result in the same or similar flavor as previous feeds, which help animals maintain their food intake and successfully switch to a new formula. Additionally, during periods of weaning and transportation, animals and fish normally reduce feed intake. Adding a flavor agent to blended feeds can help alleviate stress and unease, increasing feed consumption and ensuring that an animal obtains the nutrients it requires.
We produce and sell the following (non-sugar) natural sweeteners, feed flavor enhancers, and attractants for use with feed for pigs, piglets, fish and other aquatic animals under the trademark “Tankeball™” Functional Flavoring Series:
|
●
|
Tanksweet ST (a mixed sweetener designed to improve the palatability and acceptability of all pig feed);
|
|
●
|
Tankarom ST (a feed flavor enhancer and functional physiological regulator that assists animals in overcoming the negative effects of weaning, stress, disease, medications or mal-flavored feedstuffs);
|
|
●
|
Tankmix SA (a co-mixed product with sweetener and flavoring that makes feed more attractive);
|
|
●
|
Tankebaal sweet (a mixed sweetener to improve the palatability and acceptability of all pig feed);
|
|
●
|
Tankarom (a functional physiological regulator);
|
|
●
|
Fishy Spicy (an aroma agent added to fishmeal to enhance fishy taste, cover-up mal-flavors in feeds and improve the palatability of feed products);
|
|
●
|
Aquatic Lives’ Attractants (consisting of concentrated extracts from natural seafood and high efficient attractants rich in amino acids that improve the feed intake of fish); and
|
|
●
|
Kimyso™ (a micro-granulated solid dispersion Kitasamycin premix).
|
Herbal Medicinal Additives
We have placed an emphasis on developing and promoting herbal medicine additives blended with feed products in China. Chinese herbal feed additives utilize traditional Chinese medicine theory to improve an animal’s digestion and appetite and to regulate the yin and yang balance of an animal’s health.
Herbal medicines come from plants, plant extracts, fungal and bee products, minerals, shells and certain animal parts. Compared to synthetic antibiotics or inorganic chemicals, these naturally-derived products are less toxic, residue free and thought to be ideal feed additives for animal consumption. Herbal Medicinal Additives represent approximately 1% of our 2011 revenues and net sales and carry a gross profit margin of approximately 22%.
We produce and sell the following trademarked products in the Herbal Medicinal Additives sector, all of which are derived from Chinese natural plants, herbs and minerals:
|
●
|
Extra-Health™ (improves animal immune system and functions);
|
|
●
|
Qilimix™ (a natural feed additive for livestock and poultry designed to improve the reproductive and growth performance of farm animals); and
|
|
●
|
Recoccider™ (a highly efficient anticoccidial premix containing Ethopabate and Diclazuril designed to inhibit DHSS and DHRS).
|
Our total revenue for Herbal Medicinal Additives in 2011 was approximately $281,036. Such revenue accounted for approximately 1.2% of our total 2011 revenue, and carried a gross profit margin of approximately 22%.
In 2010, we successfully developed and introduced a new antioxidant with the active element extracted from an Asian tree vine, which can be used to maintain the quality of feed products. During 2011, we produced and delivered approximately 320 tons of this new antioxidant product from sales to approximately 90 customers, generating revenues and net sales of RMB 4.08 million or approximately $630,934.
Other Revenues
The Company and its subsidiaries have received numerous awards from industry associations, including the National Innovative and Outstanding Enterprises of Feed Additive Industry award and a Certificate of Honor, from the China Feed Industry Association, and various local and national government agencies, including the National Award Certificate of Advanced Science and Technology, from the Chinese State Department and a Certificate of National Torch Program from the Torch High Technology Industry Development Center of Ministry of Sciences and Technology, in recognition for the products and processes it has developed over the years and, as a result, is considered a well respected company in its field.
We also engage in various businesses including the domestic distribution of raw materials and providing technical support and know-how to our customers. As one of the leading producers and distributors of feed additives in China, we are able to engage in certain distribution activities to other Chinese companies. In connection with this, we purchase raw materials from certain manufacturers and sell such materials domestically to other feed additive manufacturers. We are able to purchase raw material at a relatively lower price. Subsequently, we sell those raw materials to other customers at a premium.
Our total revenue for Other Revenue in 2011 was approximately $531,910. Such revenue accounted for approximately 2% of our total 2011 revenue, and carried a gross profit margin of approximately 32%. Revenue from raw material trading accounted for about 50% of Other Revenues.
Marketing
The majority of our marketing in China is conducted through sales visits to feed producers, farmers and other potential customers. During these sales meetings, the Company’s sales team distributes marketing materials and shares its extensive knowledge on husbandry and cultivation of farm animals.
As part of our marketing effort, every two years, we co-sponsor the Chinese Academy’s international seminar on Animal Health Products and Feed Additives, inviting speakers and participants who are academic professionals, industry experts or key managers in the agriculture business. The seminar is designed to introduce recent developments and trends in the feed additive business and to provide a platform for increasing awareness of our products. Since beginning the seminar in 2002, it has become one of the important events in the industry and typically attracts more than five hundred professionals.
Outside of China, we market our products mainly through participation in industry exhibitions.
Sales and Distribution
Our target customers are mid-to-large sized feed product factories and large scale producers. These customers have substantial bargaining power and require the feed additive products that they use to meet the highest standards of quality, productivity and efficiency, which we believe gives us a competitive advantage over our smaller competitors. In total, we employ 43 sales or sales-related employees, including 21 in regional sales, 5 in distribution, 4 in the aquatic group, 4 in international sales, 3 in marketing and 6 in technical support.
As of December 31, 2011, we had two customers that accounted for more than ten percent of our consolidated revenues. For the 2011 fiscal year, sales to our top 10 customers accounted for approximately 59% of our revenue and for the 2010 fiscal year, sales to our top 10 customers accounted for approximately 75% of our revenue. The following table identifies customers that purchased more than 10% of our products during the years ended December 31, 2011 and 2010:
|
Customers
|
% of Consolidated
Revenue in 2011
|
Customers
|
% of Consolidated
Revenue in 2010
|
|||
|
Guangzhou Tienhe Lianhua Agriculture Technology Co., Ltd.
|
14%
|
Guangdong Huanong Wenshi Animal Husbandry Co.
|
22%
|
|||
|
Jin Yin Ke Bio-Tech Co., Ltd.
|
12%
|
Nanbao Group
|
14%
|
|||
|
Wenshi Food Group
|
12%
|
|||||
|
Guangzhou Zhan Da Lan Ke Feed Company
|
12%
|
We sell our products throughout China with a combination of our internal sales teams and a network of agents in 28 regions, including Guangdong, Sichuan, Shandong and Liaoning provinces, which have significant animal breeding industries. Presently, about 80% of our sales are generated through our internal sales force, with the remaining 20% via our agents. The Company currently has sales offices in the cities of Shengyang, Chengdu, Xiameng, Xi’an, Nanning and Zhengjiang.
Our products are sold in Xinjiang, Gansu, Neimeriggu, Sichuan, Yunnan, Guanxi, Haihan, Guizhou, Chongqing, Shanxi, Henan, Hubei, Hunan, Guangdong, Fujian, Jiangxi, Anhui, Jiangsu, Shanghai, Shandong, Tianjin, Beijing, Liaoning, Jilin and Heilongjiang provinces.
While the vast majority of our sales are domestic, an aggregate of approximately 1% of our sales are to customers in Thailand, Vietnam, Cambodia, South Korea, The Philippines, Uruguay, Malaysia, Russia, and India.
Customer Service
Our service and support infrastructure quickly and efficiently provides clients with customized products, technical support and advice. We assign our technicians to prospective customers to conduct a thorough analysis of the customer’s needs, followed by a detailed customized product manual. While the manuals vary according to the specific product, they typically include a product introduction that includes nutrition facts, a user guide, expiration dates and storage and packaging information, among other things. For the large-scale farmer or feed producer, our team also provides training courses to help our customers understand our products and how to use them most effectively.
Upon the delivery of our products to new customers, we provide after-sales support, which not only serves to resolve any technical issues, but also helps identify other opportunities for increasing business with current customers. We utilize a multi-tiered product strategy pursuant to which we tailor our products to the needs and preferences of the feed market.
Raw Materials and Suppliers
The raw materials for our products include agricultural commodities and fine powders like amino acid, organic trace minerals and organic acid. Although most of our principal raw materials are widely available in China, the price for certain raw materials can fluctuate. We have adopted measures to reduce our risks in both raw material supply costs and availability, including establishing long-term relationships with suppliers and diversifying supply sources.
To assure the consistency of our raw material supplies, we source most of our materials from mid-to large size companies. Before making any purchase with a new vendor, we evaluate the vendor’s products and attempt to select the most reliable and reputable vendor. We regularly conduct similar evaluations throughout our purchasing process to ensure that we are purchasing high quality raw materials at competitive prices. Because we source our raw materials from several vendors, we are not dependent on any particular vendor or merchant as a sole provider for our raw materials.
Our top ten suppliers provide approximately 73% of our total raw material purchases in 2011 and 50% in 2010. The following table identifies suppliers that provide more than 10% of our raw materials during the years ended December 31, 2011 and 2010:
|
Suppliers
|
% Supplied in 2011
|
Suppliers
|
% Supplied in 2010
|
|||
|
Guangzhou Tienhe Lianhua Agriculture Technology Co., Ltd.
|
23%
|
Zhejiang Shenghua Bai Ke Bio Co., Ltd.
|
19%
|
|||
|
Shijiazhuang Guqiao Chemistry Industry Co., Ltd.
|
10%
|
Guangzhou Nan Hua Run Material Co., Ltd.
|
16%
|
|||
|
Jinzhou City Fu Li Chemical Co., Ltd.
|
12%
|
|||||
|
Guangzhou Guanqiu Chemical Co., Ltd.
|
11%
|
Research and Development
We are strongly committed to the development of new products and processes and the enhancement of our existing products and technology. We conduct research and development and acquire new technologies through our in-house research team in collaboration with various universities and research institutions and through technology acquisitions from third parties. We plan to achieve the status of National Level Technology and Engineering Center. This is a status awarded by the national government to enterprises after they possess certain minimum requirements of assets, sales, credit rating, R&D structure and spending, scientific and technical resources, plus having developed a number of industry standards, published research papers, obtained a number of famous trademarks and patents, and commercialized a number of valuable new products. Achieving this honor will allow us to attract more technical talents, be more competitive in the industry, and receive more projects and grants from the government.
Our in-house development team consists of seven PhD’s and forty-eight researchers of which 80% have college degrees and nineteen hold graduate degrees. This team is responsible for developing new products and responding to customer needs. Spending on research and development was $246,038 and $91,397 in 2011 and 2010, respectively. Our in-house team has contributed to the establishment of four national standards for feed additives, has applications pending for five Chinese patents covering synthetic methods for manufacturing additives and holds three Chinese patents covering new products, methodologies and machines used to mix and dry feed additives. We believe that our participation in the development of national standards provides us with an insight into Chinese regulators’ focus and a competitive advantage versus our competitors. We do not receive any compensation or sponsorship from customers for our research and development activities.
Our most recent in-house development is CA-130 (Shibujian), a new type of transitional feed for early-wean piglets. CA-130 is an antibiotic-free product that improves a piglet’s immune system while reducing the days needed for the production of finished pigs. CA-130 was officially launched in the third quarter of 2011.
To further expand our development platform, we have entered into development agreements with several universities and research institutions, including the Institute of Subtropical Agro-ecology of the Chinese Academy of Sciences, Guangdong University of Technology, Zhongkai Institute of Agriculture Engineering, Northeast Agriculture University and Southeast Agriculture University. These cooperative ventures are conducted under agreements that grant us the exclusive right to commercially exploit these new processes and procedures in exchange for licensing fees to the research partner. Besides partnering with Chinese universities and research institutes on developing new technology, we are actively seeking to develop relationship with top agricultural researchers in the U.S. and other countries. Our goal is to find the best and most advanced technology and apply it into developing new products.
Manufacturing
We operate from a modern 34,000 square-meter manufacturing facility in the Huadu Economic District, in Guangdong province. As of December 31, 2011, we had an aggregate production capacity of approximately 560 metric tons per week of feed additive, of which 350 metric tons are organic trace minerals. We estimate that we are currently operating at a blended average across our product lines of approximately 53% of our manufacturing capacity at our current facility. We expect our manufacturing output to increase in 2012 and beyond, and are constructing a second facility, which is currently scheduled to be operational in the first quarter of 2013. This second facility is being built at the economic and industrial development zone in Qingyuan where the Company has acquired a Land Use Agreement from the local government. This particular area is designated specifically for the purpose of building manufacturing factories of this kind and we are only one of the new occupants among many other similar manufacturers. There is no land use right issue at this facility.
In 2003, Guangzhou Tanke entered into a Land Use Agreement valid from May 20, 2006 until May 20, 2021 with the government of Huaqiao Town, Huadu District, Guangzhou under which the Government of Huaqiao town granted Guangzhou Tanke a land use right covering the land where Guangzhou Tanke’s manufacturing facility currently stands. This agreement will be superseded by a Land Use Right Certificate and will be automatically terminated once the Company obtains such certificate. We are currently negotiating with the local government to obtain such certificate, which would permit us to operate our business as it is currently conducted. If the Company is not able to obtain a Land Use Right Certificate, the PRC government may declare the Land Lease Agreement invalid, evict the Company’s personnel from the premises and remove the Company’s manufacturing facilities.
Intellectual Property
As a result of our extensive research and development, we own valuable patents, trademarks and licenses and regard our intellectual property as a major component of our competitive strategy. Our granted patents include:
|
●
|
A Chinese utility model patent (ZL200620154038.5) the Company owns for a mixed drier of feed additives. The patent was issued in November 2007 and will expire on March 2017.
|
|
●
|
A Chinese patent (ZL200710030121.0), that the Company jointly owns with the Guangdong University of Technology for the methodology of preparation of copper and zinc glycine complexes by ball milling and solid-static doping. The patent was issued in July 2010 and will expire in July 2030.
|
|
●
|
A Chinese patent (ZL200810198628.1), that the Company owns for a method of detecting and determining the rate of chelation in amino acid trace mineral chelation. The patent was issued in 2011.
|
We have five pending Chinese patent applications that were developed either through our in-house research team or in collaboration with various Chinese research institutions or universities. Two of our patent applications were submitted in 2008 and the other three were submitted in 2010. Based on our experience and the advice we received from Chinese counsel, there is a two to three year period prior to the receipt of a patent approval. We expect that these five applications, without any delay due to any dispute and/or other causes, will be granted by 2012 or 2013 respectively.
On June 17, 2008, Tanke Bio-Tech entered into an exclusive Licensing Agreement with the Institute of Subtropical Agro-ecology of the Chinese Academy of Sciences (the “Institute”), whereby the Institute granted Tanke Bio-Tech an exclusively license for the use of (a) Chinese Patent ZL200410013212.X which governs the methodology to extract metal sulfur protein from pork liver and (b) Chinese Patent ZL200510120505.2 which is a formula and methodology to enhance the immune system of piglets. Tanke Bio-Tech agreed to pay the Institute an aggregate amount of RMB 6.6 million for the exclusive use of the two patents. The term of the exclusive license for the patents is from January 1, 2008 to December 30, 2012 unless earlier terminated by Tanke Bio-Tech pursuant to the terms of the agreement.
In 2011, the trademark “Tanke” was evaluated and recognized by the City of Guangzhou as “Famous Trademark”. We have also registered seven trademarks with the Trademark Office of the State Administration for Industry and Commerce of China, including “Tanke”, “Qilimix,” “Qili” and “CTanke”.
Seasonality
Although our business is not significantly affected by seasonality, demand for our products tends to be lower from January to April because large amount of animals raised by husbandry farms are sold during the Chinese New Year in February. The demand for our products tends to be higher from May to December, because many feed manufactures will complete stocking by December before the Chinese New Year.
Quality Control
Following quality control breakdowns from isolated Chinese manufacturers in the past several years and the resultant negative publicity, we have placed a strong emphasis on maintaining the quality and integrity of our products. To that end, the Company’s internal quality controls are implemented in accordance with the requirements of ISO 9001/2000. The Company has also received the ISO 9001/2000 International Quality Management System and HACCP management system certificates and the GMP certification for an animal drug production line.
Our quality control center reviews the quality of the factors involved in production of our products, including the examination of raw material, product testing and sampling. Our laboratory maintains samples of each of our delivered products in the event of customer issues or a regulatory review.
Government Regulation
Our domestic business activities are regulated by various governmental agencies in China and Guangdong province and our foreign sales are subject to similar requirements in the countries in which the Company does business. These laws govern current operations and product safety and may require remediation of environmental incidents.
Regulations of PRC Feed Additive Industry
The animal feed additive market in China is managed under a legal system that includes registration, permits, supervision and inspection. The major regulations applying to feed additive industry in China include (i) the Regulation on Administration of Feed and Feed Additive; (ii) the Administrative Measure on Approval Reference Number of Feed Additive and Additive Premixed Feed; (iii) the Administrative Measure on Production License of Feed Additive and Additive Premixed Feed; and (iv) the Administrative Measure on New Feed and New Feed Additive. The Ministry of Agriculture of China also promulgates various notices and rules regulating the feed and feed additive industry from time to time.
Tanke Bio-Tech, a subsidiary of Guangzhou Tanke that we control via the VIE agreements, currently holds the Inspection and Quarantine Registration Certificate for Enterprise of Production, Processing and Storage of Feed for Export, Feed Additive Production License, Premixed Additive Feed Production Certificate and Food Safety Management System Certification. Jenyi Bio-Tech, a subsidiary of Guangzhou Tanke that we control via the VIE agreements, currently holds Certificate for Compliance for Company of Feed Production, the Feed Additive Production License and Additive Premixed Feed Production License. Tanke Animal Health, a subsidiary of Guangzhou Tanke that we control via the VIE agreements, currently holds Veterinary Medicine Production Certificate and Veterinary Medicine GMP Certificate. We believe that we are in compliance with PRC environmental and agricultural laws and regulations.
Feed Additive Industry
The feed additive industry in China falls within the encouraged category for foreign investment in accordance with the Catalogue of Industries for Guiding Foreign Investment. As a result of this designation companies in the feed additive industry, including Guangzhou Tanke its subsidiaries, qualify for certain tax incentives and VAT exemptions.
Environmental Laws and Regulations
As required under the PRC Environmental Laws and Regulations, we have obtained an Opinion on the Environmental Impact Statement issued by the Environmental Protection Bureau of Huadu District on February 16th, 2004, approving the construction of our manufacturing site. Since we have neither obtained the Land Use Right Certificate of the manufacturing site nor the Property Ownership Certificate, we are unable to apply for the examination and acceptance of environmental appraisal. Since this delay to obtain environmental approval was caused by the change of zoning regulations, the environmental bureau of Guangdong Province issued us the waste disposal permit on March 2, 2011 valid from March 2011 to March 30, 2014. The relevant procedures for environmental protection will be completed along with the settlement of the land issue. As long as we obtain the environmental permit, our business will continue. However, if we lose such permit, the applicable PRC government may require us to discontinue our operating business. The actual cost for environmental law compliance will vary depending upon the nature and quantity of the waste generated along with the daily production of the products. The Company estimates its expenditures for waste disposal is approximately $7,692/year.
Foreign Exchange Regulations
We receive substantially all of our revenues in Chinese Renminbi, which is currently not a freely convertible currency. In China, SAFE regulates the conversion of Chinese Renminbi into foreign currencies. Pursuant to applicable Chinese laws and regulations, foreign invested enterprises incorporated in China are required to apply for “Foreign Exchange Registration Certificates.” Currently, conversion within the scope of the “current account” (e.g. remittance of foreign currencies for payment of dividends, trade and service-related foreign exchange transactions, etc.) can be effected without requiring the approval of SAFE, instead, need to be registered with the SAFE. However, conversion of currency in the “capital account” (e.g. for capital items such as direct investments, loans, securities, etc.) still requires the approval of SAFE. On July 21, 2005, the Chinese government changed its policy of pegging the value of Chinese Renminbi to the U.S. dollar. Under the new policy, Chinese Renminbi may fluctuate within a narrow and managed band against a basket of certain foreign currencies. It is possible that the Chinese government could adopt a more flexible currency policy, which could result in more significant fluctuation of Chinese Renminbi against the U.S. dollar.
PRC M&A Rule, Circular 75 and Circular 638
On August 8, 2006, six Chinese government agencies, namely, the Ministry of Commerce, or MOFCOM, the State Administration for Industry and Commerce, or SAIC, the China Securities Regulatory Commission, or CSRC, the State Administration of Foreign Exchange, or SAFE, the State Assets Supervision and Administration Commission, or SASAC, and the State Administration for Taxation, or SAT, jointly issued the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, referred to as the “New M&A Rules”, which became effective on September 8, 2006. The New M&A Rules purport, among other things, to require offshore “special purpose vehicles,” that are (1) formed for the purpose of overseas listing of the equity interests of Chinese companies via acquisition and (2) are controlled directly or indirectly by Chinese companies and/or Chinese individuals, to obtain the approval of the CSRC prior to the listing and trading of their securities on overseas stock exchanges. Based on our understanding of current Chinese Laws and pursuant to a legal opinion, dated January 4th, 2011, that we received from Martin Hu and Partners, (i) Kanghui Agricultural was incorporated by a foreign investor and was not deemed to be an acquisition of the equity or assets of a “Chinese domestic company” as such term is defined under the New M&A Rules and (ii) no provision in the New M&A Rules clearly classifies the contractual arrangements between Kanghui Agricultural and Guangzhou Tanke as a type of transaction falling within the New M&A Rules.
The SAFE issued a public notice in October 2005, or the Circular 75, requiring Chinese residents to register with the local SAFE branch before establishing or controlling any company outside of China for the purpose of capital financing with assets or equities of Chinese companies, referred to in the Circular 75 as special purpose vehicles, or SPVs. Chinese residents who are shareholders of SPVs established before November 1, 2005 were required to register with the local SAFE branch before June 30, 2006. Further, Chinese residents are required to file amendments to their registrations with the local SAFE branch if their SPVs undergo a material event involving changes in capital, such as changes in share capital, mergers and acquisitions, share transfers or exchanges, spin-off transactions or long-term equity or debt investments. To date, the Chinese residents who are shareholders of Guangzhou Tanke do not own any equity in the Company, currently such Chinese residents do not need to file registrations with SAFE pursuant to Circular 75. When the Chinese residents exercise their options in the future to receive any share of the Company pursuant to the Call Option Agreement, they will need to file registrations with SAFE.
If within two years of formation the applicable PRC authorities determine that Kanghui Agricultural lacks a business in the ordinary course, Kanghui Agricultural may be deemed to be a wholly foreign owned holding company and therefore the relevant PRC authority may determine that Kanghui Agricultural’s purchase of shares of Guangzhou Tanke pursuant to the Option Agreement may be subject to approvals required under the new M&A Rule. The relevant PRC authorities may further determine Tanke Shareholders’ exercise of their options to acquire shares of Common Stock of the Company pursuant to the Call Option Agreement may be subject to approvals required under the new M&A Rule. Tanke Shareholders who are Chinese residents and acquire shares of common stock may be subject to registration requirements with SAFE pursuant to Circular 75.
Pursuant to the Circular 698, where a foreign investor transfers the equity interests of a Chinese resident enterprise indirectly via disposing of the equity interests of an overseas holding company, which we refer to as an Indirect Transfer, and such overseas holding company is located in a tax jurisdiction that: (i) has an effective tax rate less than 12.5% or (ii) does not tax foreign income of its residents, the foreign investor shall report such Indirect Transfer to the competent tax authority of the Chinese resident enterprise. The Chinese tax authority will examine the true nature of the Indirect Transfer, and if the tax authority considers that the foreign investor has adopted an abusive arrangement in order to avoid Chinese tax, they will disregard the existence of the overseas holding company and re-characterize the Indirect Transfer and as a result, gains derived from such Indirect Transfer may be subject to Chinese withholding tax at the rate of up to 10%. Circular 698 also provides that, where a non-Chinese resident enterprise transfers its equity interests in a Chinese resident enterprise to its related parties at a price lower than the fair market value, the competent tax authority has the power to make a reasonable adjustment to the taxable income of the transaction.
Employees
As of September 2012, we employed 165 full-time personnel. We believe that we maintain a satisfactory and safe working environment that it has a history of low turnover. We believe that we comply in all material respects with applicable Chinese labor laws.
Recent Events
VIE Agreements
On January 3, 2011, Kanghui Agricultural, a wholly-owned subsidiary of China Flying, entered into the VIE Agreements with Guangzhou Tanke and the Tanke Shareholders. Pursuant to the VIE Agreements, Kanghui Agricultural effectively assumed management of the business activities of Guangzhou Tanke and has the right to appoint all executives and senior management and the members of the board of directors of Guangzhou Tanke. The VIE Agreements are comprised of a series of agreements, including a Consulting Services Agreement, Operating Agreement, Voting Rights Proxy Agreement, Equity Pledge Agreement and Option Agreement, through which Kanghui Agricultural has the right to advise, consult, manage and operate Guangzhou Tanke for an annual fee in the amount of Guangzhou Tanke’s yearly net profits after tax. The Tanke Shareholders have pledged their rights, titles and equity interest in Tanke as security for Kanghui Agricultural to collect consulting and services fees provided to Guangzhou Tanke through an Equity Pledge Agreement. In order to further reinforce Kanghui Agricultural’s rights to control and operate Guangzhou Tanke, the Tanke Shareholders have granted Kanghui Agricultural an exclusive right and option to acquire all of their equity interests in Tanke through an Option Agreement.
|
●
|
Equity Interest Pledge Agreement. The WFOE and the Tanke Shareholders have entered into Equity Interest Pledge Agreements, pursuant to which each shareholder pledges all of his shares of Guangzhou Tanke to the WFOE in order to guarantee cash-flow payments under the applicable Consulting Services Agreement. The Equity Pledge Agreement further entitles the WFOE to collect dividends from Guangzhou Tanke during the term of the pledge.
|
|
●
|
Consulting Service Agreement. Guangzhou Tanke and the WFOE has entered into a Consulting Services Agreement, which provides that the WFOE will be the exclusive provider of consulting and management services to Guangzhou Tanke and Guangzhou Tanke will pay all of its net income based on the quarterly financial statements to the WFOE for such services. Any such payment from the WFOE to the Company would need to comply with applicable Chinese laws affecting payments from Chinese companies to non-Chinese companies. See “Risk Factors – Risks Associated With Doing Business in China – ‘Due to various restrictions under Chinese laws on the distribution of dividends by our Chinese operating companies, we may not be able to pay dividends to our stockholders’ and ‘The State Administration of Foreign Exchange restrictions or changes in foreign exchange regulations in China may affect our ability to pay dividends in foreign currency or conduct other foreign exchange business’.”
|
|
●
|
Operating Agreement. Pursuant to the operating agreement among the WFOE, Guangzhou Tanke and each of Tanke Shareholder, the WFOE provides guidance and instructions on Guangzhou Tanke’s daily operations and financial affairs. The Tanke Shareholders must designate the candidates recommended by the WFOE as their representatives on their respective boards of directors. The WFOE has the right to appoint senior executives of Guangzhou Tanke. In addition, the WFOE agrees to guarantee Guangzhou Tanke’s performance under any agreements or arrangements relating to Guangzhou Tanke’s business arrangements with any third party. Guangzhou Tanke, in return, agrees to pledge its accounts receivable and all of its assets to the WFOE. Moreover, Guangzhou Tanke agrees that without the prior consent of the WFOE, Guangzhou Tanke will not engage in any transactions that could materially affect its assets, liabilities, rights or operations, including, without limitation, incurrence or assumption of any indebtedness, sale or purchase of any assets or rights, incurrence of any encumbrance on any of its assets or intellectual property rights in favor of a third party or transfer of any agreements relating to its business operation to any third party.
|
|
●
|
Option Agreement. Pursuant to the option agreement among the WFOE, Guangzhou Tanke and each of Tanke Shareholder, the Tanke Shareholders have granted Kanghui Agricultural an exclusive right and option to acquire all of their equity interests in Guangzhou Tanke upon an event of default.
|
|
●
|
Voting Right Proxy Agreement. Pursuant to the voting right proxy agreement among the WFOE, Guangzhou Tanke and its shareholders, the Tanke Shareholders have granted the WFOE a voting and proxy right to vote their equity interest on Guangzhou Tanke.
|
We have been advised by our PRC legal counsel, Martin Hu & Partners, in an opinion dated January 4th, 2011, that: (1) our inner-PRC shareholding structure complies with PRC laws and regulations; (2) the contractual arrangements between the WFOE, China Flying, Guangzhou Tanke and its shareholders are valid and binding on all parties to these arrangements and do not violate relevant PRC laws or regulations; (3) the each of the WFOE and Guangzhou Tanke has the requisite corporate power to own, lease and operate its properties, to enter into contracts and to conduct its business and (4) each of the WFOE and Guangzhou Tanke is qualified to do business in the respective jurisdiction of its establishment.
We entered into the VIE Agreements and Call Option in order to comply with applicable Chinese law and because such structure was a tax-efficient alternative for the Tanke Shareholders. First, under an alternative structure, we would be required to obtain the approval of the PRC government because the WOFE did not exist for two years prior to the transaction. Second, under an alternative structure, we would be required to pay the Tanke Shareholders cash for the capital stock of Guangzhou Tanke because stock-for-stock acquisitions are not permitted. Lastly, the structure provided by the VIE Agreements is tax-free to the Tanke Shareholders under applicable PRC law.
Call Option Agreement
In addition, on January 3, 2011, the Tanke Shareholders each entered into the Call Option Agreement with Golden Genesis and Ms. Wong. Under the terms of the Call Option Agreement, the Tanke Shareholders each received three year options for their Shares. Pursuant to the Call Option Agreement, options for 34% of the shares shall vest and become exercisable on December 31, 2011, options for 33% of the shares shall vest and become exercisable on December 31, 2012 and options for 33% of the shares shall vest and become exercisable on December 31, 2013. The Tanke Shareholders may purchase each share for $0.01 and if the Tanke Shareholders exercise all of their options they will own a majority of our outstanding shares of Common Stock and Golden Genesis will no longer be a stockholder of the Company. The Call Option Agreement provides that Golden Genesis shall not dispose of the respective portion of the shares of common stock without the Tanke Shareholders’ prior written consent.
Share Exchange
On February 9, 2011, the Company closed the Share Exchange and acquired all of the outstanding equity securities of China Flying from Golden Genesis, which was the sole shareholder of China Flying immediately prior to the closing of the Share Exchange. In exchange, the Company issued to Golden Genesis 10,758,000 newly issued shares of our common stock. In addition, pursuant to the terms of the Share Exchange Agreement, the Company effected a 1 for 8.512 reverse stock split to modify the Company’s capital structure to accommodate the transactions contemplated by the Share Exchange and the Private Placement and to put in place an appropriate capital structure for the Company following the closing of the Share Exchange and the Private Placement. Such securities were not registered under the Securities Act. These securities qualified for exemption under Section 4(2) of the Securities Act since the issuance of securities by us did not involve a public offering. As a result of the VIE Agreements, since the closing of the Share Exchange, we have operated and controlled Guangzhou Tanke through Kanghui Agricultural and China Flying. We acquired Guangzhou Tanke through the VIE Agreements, rather than an acquisition of Guangzhou Tanke’s assets or equity, because: (i) the tax and other consequences of a share exchange with a foreign entity that result in the acquisition of a Chinese company are uncertain due to PRC laws that were effective on September 8, 2006 and (ii) if Guangzhou Tanke is not acquired via a share exchange transactions, PRC authorities may require it to be acquired for cash, however the Company was not able to raise a sufficient amount of cash to purchase Guangzhou Tanke.
Private Placement
On February 9, 2011, in connection with the closing of the Share Exchange, we closed the Private Placement of 6,669,627 Units, at a purchase price of $1.15 per Unit, pursuant to the Securities Purchase Agreement. Each Unit consisted of a $1.15 principal amount 8% Senior Convertible Note and a Common Stock Purchase Warrant to purchase one share of the Company’s common stock, with an exercise price of $1.40 per share. We sold 6,669,627 Units in the Private Placement, for gross proceeds of $7,670,071.50. In addition, in connection with the Private Placement, the Company also issued to certain affiliates of Euro Pacific, the lead placement agent in the Private Placement, three-year warrants Agent Warrants to purchase an aggregate of 666,963 shares of our common stock at an exercise price of $1.15 per share. The issuances of the Notes, the Warrants and the Agent Warrants were exempt from registration under Section 4(2) of the Securities Act, and the Private Placement met the requirements to qualify for exemption under Regulation D promulgated under the Securities Act. The first $1,474,006 of proceeds from the Private Placement were used primarily to pay expenses of the Share Exchange and the Private Placement, including $400,000 reserved for financial marketing of the Company for the U.S. capital markets. Proceeds above these amounts will be used primarily to develop new products, acquire patents, add manufacturing capacity, advertising, promotion, increasing and training sales personnel and for general working capital needs of the Company. The net proceeds from the Private Placement shall be not be used for the satisfaction of any portion of the Company’s bank debt or debt due to related parties (other than payment of accounts payable in the ordinary course of the Company’s business and consistent with prior practices), or to redeem any common stock or common stock equivalents of the Company. In connection with the closing of the Private Placement, we also entered into the following additional agreements:
Notes. We offered and sold $7,670,071.50 worth of Notes convertible into up to 6,669,627 shares of our common stock, in conjunction with a purchase of the Units. The Notes are payable 24 months from February 9, 2011 with an interest rate of 8% per annum payable semiannually in arrears. At the option of the holder, the Notes may be converted into common stock at a price of $1.15 per share, which is subject to customary weighted average and stock based anti-dilution protection. The issuance of the Notes was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D.
Warrants. We offered and sold Warrants to purchase up to 6,669,627 shares of common stock in conjunction with a purchase of the Units. Each Warrant entitles the holder to purchase one share of our common stock. The Warrants will be exercisable in whole or in part, at an initial exercise price per share of $1.40, which is subject to customary weighted average and stock based anti-dilution protection. The Warrants may be exercised at any time upon the election of the holder, beginning on the date of issuance and ending of the third anniversary of the closing of the Private Placement. The issuance of the Warrants was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D.
Agent Warrants. We issued to certain affiliates of Euro Pacific, our lead placement agent in the Private Placement, three-year Agent Warrants to purchase an aggregate of 666,963 shares of common stock at an exercise price of $1.15 per share. The Agent Warrants also contain a cashless exercise option. The issuance of the Agent Warrants was not registered under the Securities Act. The issuance of the Agent Warrants was exempt from registration under Section 4(2) of the Securities Act.
Registration Rights Agreement. Under the terms of the Registration Rights Agreement, we agreed to file a Registration Statement on Form S-1 with the SEC within 60 days of the closing of the Private Placement registering the number of shares of common stock underlying the Units sold in the Private Placement, and to use our best efforts to have the registration statement declared effective prior to September 15, 2011. As of March 30, 2012, we accrued the maximum amount of $460,206 for registration delay penalty.
Interest Escrow Agreement. Pursuant to the Interest Escrow Agreement, the Company deposited into escrow an amount of proceeds of the Private Placement equal to one semi-annual interest payment on the Notes to secure prompt interest payments under the Notes. Until such time as 75% of the Notes are converted into shares of common stock, if such escrow is depleted in order to make interest payments, the Company has agreed to promptly replenish such escrow amount.
Securities Escrow Agreement. Pursuant to the Securities Escrow Agreement, Golden Genesis placed in escrow 2,000,000 shares of common stock (the “Escrow Shares”), to be disbursed to either the Investors on a pro rata basis or to Golden Genesis based on the financial performance of Guangzhou Tanke, our principal operating business. If our “Adjusted Income” (as defined below) for the year ending December 31, 2011 is (i) at least $4,652,410, then Golden Genesis shall receive an aggregate of one million (1,000,000) Escrow Shares or (ii) less than $4,652,410, then the Investors shall receive an aggregate of one million (1,000,000) Escrow Shares. If our Adjusted Income for the year ending December 31, 2012 is (i) at least $7,571,111, then Golden Genesis shall receive an aggregate of one million (1,000,000) Escrow Shares or (ii) less than $7,571,111, then the Investors shall receive an aggregate of one million (1,000,000) Escrow Shares. For the purposes of the Securities Escrow Agreement, “Adjusted Income” means the sum of: (A) the Company’s net income; plus (B) any expense incurred in connection with the transactions contemplated by the Securities Purchase Agreement in connection with the Private Placement, including, without limitation, expenses related to the filing of a registration statement; plus (C) any depreciation and amortization expenses related to the expenses described in (B) above for the fiscal year ending December 31, 2011 or December 31, 2012 (as applicable), in each case as determined in accordance with GAAP, as reported in the Company’s Annual Report on Form 10-K as filed with the SEC.
We did not achieve the Adjusted Income of $4,652,410 for the year ended December 31, 2011, therefore 1,000,000 shares of common stock placed in escrow have been distributed to the Investors on a pro rata basis, pursuant to the terms of the Securities Escrow Agreement.
Organization and Consolidated Subsidiaries
The Company’s organizational structure, which is illustrated under “Prospectus Summary – Our Organizational Structure”, was carefully developed to abide by Chinese laws and to maintain Guangzhou Tanke’s tax benefits as well as internal organizational efficiencies.
DESCRIPTION OF PROPERTY
Our corporate office is located at East Tower of Hui Hao Building, No. 519 Machang Road, Pearl River New City, Guangzhou, China. Our manufacturing facility is in a modern 34,000 square-meter facility in the Huadu Economic District, in Guangdong province.
Guangzhou Tanke entered into a Land Lease Agreement valid from May 20, 2006 to May 20, 2021 with the government of Huaqiao Town, Huadu District, Guangzhou, under which the Government of Huaqiao Town granted Guangzhou Tanke a land use right covering the land where our manufacturing facility currently stands. This lease will be superseded by a Land Use Right Certificate and will be automatically terminated once Tanke obtains such certificate. Due to changes in the relevant PRC regulations, we have not been granted such certificate for this land. Therefore, pursuant to applicable PRC law, we are not permitted to operate our manufacturing facility without such certificate and as a result, there is a risk that the PRC government may declare our Land Lease Agreement invalid.
We are currently negotiating with the local government to obtain a Land Use Right Certificate, which would permit us to operate our business as it is currently conducted. Without the Land Use Right Certificate, we are unable to apply for a Property Ownership Certificate for our manufacturing facilities. Until we obtain the Land Use Right Certificate, the PRC government may evict our personnel from the premises and remove our manufacturing facilities that we built on the premises. Such action would have a very significant and negative impact on our operations and business.
A second manufacturing facility is being built at the economic and industrial development zone in Qingyuan where the Company has acquired a Land Use Agreement from the local government. This particular area is designated specifically for the purpose of building manufacturing factories of this kind and we are only one of the new occupants among many other similar manufacturers. There is no land use right issue for this facility. This facility is projected to be fully operational in early 2013.
60
LEGAL PROCEEDINGS
We have no material proceedings pending nor are we aware of any pending investigation or threatened litigation by any third party.
MANAGEMENT
The following table sets forth the name, age, and position of our directors and officers as of the date of this prospectus. Executive officers are elected annually by our board of directors. Each executive officer holds his office until he resigns, is removed by the board, or his successor is elected and qualified. Directors are elected annually by our stockholders at the annual meeting. Each director holds his office until his successor is elected and qualified or his earlier resignation or removal. Each person listed below was appointed to his respective office on February 9, 2010. Mr. Qiu was appointed as a the sole director or the Company on or about February 13, 2011, effective as of the tenth (10th) day following the later of the date of the filing of the Information Statement pursuant to Section 14(f) of the Securities Exchange Act of 1934, as amended, and Rule 14f-1 promulgated thereunder.
|
Name
|
Age
|
Position
|
|
Guixiong Qiu
|
45
|
Founder, CEO and Chairman of the Board of Directors
|
|
Xugang Shu
|
34
|
Vice President of Research and Development
|
|
Bo Jun
|
32
|
Marketing Director
|
|
Gilbert Lee
|
53
|
Chief Financial Officer
|
Guixiong Qiu. Mr. Qiu has more than 13 years experience in the feed additives industry. Mr. Qiu earned an associate degree from South China Agricultural University with a major in Animal Inspection in 1987 and completed an Advanced Program in Agriculture Industrial and Business Management from Tsinghua University in 2005. After his graduation from college, Mr. Qiu worked for Zhengda Kangdi, a large feed producer, for three years. In 1991, he formed a logistic company trading animal drugs. From 1993 to 1996, Mr. Qiu assisted an associate in managing a flavor enhancer feed additive company. Mr. Qiu founded Guangzhou Tanke in 1997 and has been its CEO and Chairman of the Board since its inception.
Mr. Qiu served as a Vice President of the China Feed Industry Association and is a trustee of the Guangdong Province & Guangzhou City Feed Industry Association, Vice President of China Animal Health Association and trustee of China Green Industries Union Association. Mr. Qiu is also the President and a Director of Guangzhou Hai Hong Chuang Ltd.
Having worked for over 13 years in the feed additive industry, Mr. Qiu brings specialized knowledge of Guangzhou Tanke’s business to our board of directors.
Xugang Shu. Dr. Shu joined Guangzhou Tanke in 2002 after receiving his Masters Degree in Chemistry from Guangdong University of Technology. From December 2002 until December 2004, he was in charge of Guangzhou Tanke’s product quality meeting ISO 9001 and 2000 standards and compliance with GMP. In 2005, Dr. Shu became Guangzhou Tanke’s Vice President for Research & Development and organized the technology research center that has been responsible for 5 patent applications. He has received numerous awards and received a PhD in Chemistry from Guangdong University of Technology.
Bo Jun. Prior to joining Guangzhou Tanke in 2003, Mr. Jun was a sales manager responsible for the sale of feed products at Hunan Haihong Group. From 2002 to 2003, Mr. Jun was a business development officer of technology for Shunde Zhongtian Feed Industrial Co. He has a BA in veterinary from Chongqing Southwest University.
Gilbert Kwong-Yiu Lee. Mr. Lee joined the Company as Chief Financial Officer on August 1, 2011 after a finance executive role at Dimensional Merchandising Inc., a family-owned beauty aid and pharmaceutical contract manufacturer based in Wharton, New Jersey, from 2010 to 2011. From 2008 to 2010, Mr. Lee served as Director of Finance of Two’s Company, a wholesale distributor that imports giftware and fashion accessories from China and India and is based in Elmsford, New York. From 1998 to 2008, Mr. Lee held director level positions in finance, operations, and marketing for Essilor of America, a U.S. subsidiary of Essilor International which is the world’s largest eyeglass lens producer based in France. Mr. Lee received his MBA degree from the University of Texas at Austin after earning a master degree in accounting and a bachelor degree in marketing. Mr. Lee is also a CPA and CMA.
62
Committees and Meetings
We do not have a standing audit committee of the Board of Directors. Management has determined not to establish an audit committee at present because of our limited resources and limited operating activities do not warrant the formation of an audit committee or the expense of doing so. We do not have a financial expert serving on the Board of Directors or employed as an officer based on management’s belief that the cost of obtaining the services of a person who meets the criteria for a financial expert under Item 407(d) of Regulation S-K is beyond its limited financial resources and the financial skills of such an expert are simply not required or necessary for us to maintain effective internal controls and procedures for financial reporting in light of the limited scope and simplicity of accounting issues raised in its financial statements at this stage of its development.
Family Relationships
There are no family relationships between our director and executive officers.
Involvement in Certain Legal Proceedings
Our sole director and officer has not been convicted in a criminal proceeding, excluding traffic violations or similar misdemeanors, nor has been a party to any judicial or administrative proceeding during the past ten years that resulted in a judgment, decree or final order enjoining the person from future violations of, or prohibiting activities subject to, federal or state securities laws, or a finding of any violation of federal or state securities laws, except for matters that were dismissed without sanction or settlement. Except as set forth in our discussion below in “Certain Relationships and Related Transactions,” our sole director and officer has not been involved in any transactions with us or any of our affiliates or associates which are required to be disclosed pursuant to the rules and regulations of the SEC.
Code of Ethics
We currently do not have a code of ethics that applies to our officers, employees and directors.
EXECUTIVE COMPENSATION
The following table sets forth all cash compensation paid by the Company for the fiscal years of 2010 and 2011. The table below sets forth the positions and compensation for each officer and director of the Company. All the officers (except Gilbert Lee) were paid in RMB and the amounts reported in this table have been converted from Renminbi to U.S. dollars based on the 2011 year average conversion rate of RMB 6.4725 to $1.00, as certified for customs purposes by the Federal Reserve Bank of New York.
Summary Compensation Table
|
Name and Principal
Position
|
Fiscal Year
|
Salary
($)
|
Bonus
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
All Other
Compensation
($)
|
Total
($)
|
|||||||||||||||||||||||||
|
Guixiong Qiu (1)
|
2011
|
$
|
46,350.00
|
46,350.00
|
-
|
-
|
-
|
-
|
-
|
$
|
92,700.00
|
|||||||||||||||||||||||
|
Founder, CEO and Chairman
|
2010
|
$
|
31,384.61
|
38,461.53
|
-
|
-
|
-
|
-
|
-
|
$
|
69,846.14
|
|||||||||||||||||||||||
|
Gilbert Lee (2)
|
||||||||||||||||||||||||||||||||||
|
Chief Financial Officer
|
2011
|
$
|
52,083.30
|
-
|
-
|
-
|
-
|
-
|
-
|
$
|
52,083.30
|
|||||||||||||||||||||||
|
Xugang Shu (1)
|
||||||||||||||||||||||||||||||||||
|
Vice President of Research
|
2011
|
$
|
23,175.00
|
23,175.00
|
-
|
-
|
-
|
-
|
-
|
$
|
46350.00
|
|||||||||||||||||||||||
|
And Development
|
2010
|
$
|
20,307.69
|
15,384.61
|
-
|
-
|
-
|
-
|
-
|
$
|
35,692.30
|
|||||||||||||||||||||||
|
Bo Jun (1)
|
2011
|
$
|
7,725.00
|
30,900.00
|
-
|
-
|
-
|
-
|
-
|
$
|
38,625.00
|
|||||||||||||||||||||||
|
Marketing Director
|
2010
|
$
|
30,769.23
|
-
|
-
|
-
|
-
|
-
|
-
|
$
|
30,769.23
|
|||||||||||||||||||||||
|
Geoff Williams (3)
|
||||||||||||||||||||||||||||||||||
|
President, CEO and Director
|
2010
|
$
|
0
|
0
|
0
|
0
|
0
|
0
|
0
|
$
|
0
|
|||||||||||||||||||||||
(1) The compensation data for Guiziong Qiu, Xugang Shu and Bo Jun prior to the Share Exchange dated February 9, 2010 reflects compensation paid by Guangzhou Tanke.
(2) Mr. Lee was appointed as chief financial officer of the Company effective August 1, 2011.
(3) Prior to the Share Exchange, Geoff Williams served as a principal executive officer and director of Greyhound. Since its inception, Greyhound had not paid its officers any salary or consulting fees.
Employment Agreements
Since our inception and prior to the closing of the Share Exchange, we did not pay any salary or consulting fees to our officers.
On July 26, 2011, we have signed an engagement agreement with Mr. Gilbert Kwong-Yiu Lee (“Mr. Lee”) as the Chief Financial Officer of the Company. Mr. Lee’s employment agreement provides for an initial term of two (2) years and an annual base compensation of $125,000, subject to a 10% increase on January 1, 2012. Pursuant to Mr. Lee’s employment agreement, he will be entitled to (i) an annual cash bonus, as may be determined by the Board of Directors of the Company; (ii) 30,000 options of common stock of the Company at an exercise price equal to the market price of the Company’s Common Stock on the issuance date, which shall vest on a yearly basis at a rate of 15,000 options each year for two years provided that he is employed by the Company, (iii) four weeks of vacation; and (iv) two months of severance if (A) he terminates the agreement with good reason; (B) is terminated by the Company without cause , or (C) is terminated by the Company due to the CFO’s permanent disability or death as defined in his employment agreement. For a period of twenty-four months following termination, Mr. Lee shall not compete with, or solicit any current or prospective clients, customers, vendors, business or strategic partners or accounts of the Company.
Guangzhou Tanke has signed standard employment agreements in compliance with PRC labor laws and regulations with our current directors and senior executives. Each of these employment agreements provides for a five-year term valid until 2015. Under Chinese law, Guangzhou Tanke is obligated to pay an employee compensation equal to one month’s salary for each year Guangzhou Tanke has employed such employee, up to twelve years, upon termination (or upon expiration of any applicable employment agreement), except where (1) the employee has committed a crime or the employee’s actions or inactions have resulted in a material adverse effect to Guangzhou Tanke; (2) the employee is then-receiving a pension; (3) the employee dies or is declared missing; and (4) Guangzhou Tanke offers to renew the employment agreement at equal or higher consideration and the employee does not accept it. Except as described above, we currently have no compensatory plans or arrangements resulting from the resignation, retirement or any other termination of any of our executive officers, from a change-in-control, or from a change in any executive officer’s responsibilities following a change-in-control.
We do not have nonqualified deferred compensation plan under the U.S. laws and regulations. Guangzhou Tanke has established its own non-qualified employee welfare plan in accordance with Chinese law and regulations, including unemployment insurance, medical insurance, pension (collectively referred to as the “social insurance”) and housing funds, etc. Guangzhou Tanke makes contribution to our employees’ social insurance according to the minimum standard permitted by the PRC labor laws and regulations. Guangzhou Tanke makes contributions 5% of the employee’s base salary to his employee housing fund and the employees contribute 5%-12% of his base salary to his housing fund.
Furthermore, the New Labor Contract Law governs standard terms and conditions for employment, including termination and lay-off rights, contract requirements, compensation levels and consultation with labor unions, among other topics. In addition, the law limits non-competition agreements with senior management and other employees who have access to confidential information to two years and imposes restrictions or geographical limits. This new labor contract law will increase our labor costs, which could adversely impact our results of operations.
Option/SAR Grants in Last Fiscal Year
We did not grant any stock options to our executive officers or directors from inception through December 31, 2011.
Director Compensation
We do not pay our directors any fees or other compensation for acting as directors. We have not paid any fees or other compensation to any of our directors for acting as directors to date.
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS
Notes Receivables
Notes receivables – related parties consisted of the following:
|
Current portion
|
December 31,
|
December 31,
|
||||||
|
2011
|
2010
|
|||||||
|
Advance to director
|
$
|
239,149
|
$
|
2,033,622
|
||||
|
Others
|
327
|
-
|
||||||
|
$
|
239,476
|
$
|
2,033,622
|
|||||
|
Non-current portion
|
December 31,
|
December 31,
|
||||||
|
2011
|
2010
|
|||||||
|
Advance to director
|
$
|
-
|
$
|
974,532
|
||||
|
$
|
-
|
$
|
974,532
|
|||||
Advance to directors represents advance payment made to directors for business development activities and will normally be repaid within one year.
Loans to Affiliates
On December 24, 2010, Mr. Guixiong Qiu, Mr. Bi Gao, and Ms. Xiuzhen Liang, affiliates of the Company and stockholders of Guangzhou Tanke, executed a Promissory Note with Guangzhou Tanke Industry Co., Ltd., under the law of the PRC in amount of $3,443,198.67 for a loan that the Company previously made to them. The loan is unsecured, bears interest at a rate of 4% per annum and will mature on June 30, 2012, at which point the outstanding amount, plus accrued but unpaid interest, is due. If the principal or interest is not paid timely, or the borrower files for bankruptcy or liquidation, or an order or judgment is entered approving the borrower's bankruptcy, the note is in default and the interest will increase to 5% per annual.
Reorganization-Related Transactions
On February 9, 2011, in connection with the Private Placement, the Company entered into the Securities Escrow Agreement with Euro Pacific, as agent, Golden Genesis and the Escrow Agent, as escrow agent. Pursuant to the Securities Escrow Agreement, Golden Genesis placed in escrow 2,000,000 Escrow Shares, to be disbursed to either the Investors on a pro rata basis or to Golden Genesis based on the financial performance of Guangzhou Tanke, the Company’s principal operating business through the VIE Agreements. If the Company’s “Adjusted Income” (as defined below) for the year ending December 31, 2011 is (i) at least $4,652,410, then Golden Genesis shall receive an aggregate of one million (1,000,000) Escrow Shares or (ii) less than $4,652,410, then the Investors shall receive an aggregate of one million (1,000,000) Escrow Shares. If the Company’s Adjusted Income for the year ending December 31, 2012 is (i) at least $7,571,111, then Golden Genesis shall receive an aggregate of one million (1,000,000) Escrow Shares or (ii) less than $7,571,111, then the Investors shall receive an aggregate of one million (1,000,000) Escrow Shares. For the purposes of the Securities Escrow Agreement, “Adjusted Income” means the sum of: (A) the Company’s net income; plus (B) any expense incurred in connection with the transactions contemplated by the Securities Purchase Agreement in connection with the Private Placement, including, without limitation, expenses related to the filing of a registration statement; plus (C) any depreciation and amortization expenses related to the expenses described in (B) above for the fiscal year ending December 31, 2011 or December 31, 2012 (as applicable), in each case as determined in accordance with generally accepted accounting principles in the United States of America, as reported in the Company’s Annual Report on Form 10-K as filed with the SEC.
We did not achieve the Adjusted Income of $4,652,410 for the year ended December 31, 2011, therefore 1,000,000 shares of common stock placed in escrow have been distributed to the Investors on a pro rata basis, pursuant to the terms of the Securities Escrow Agreement.
See “Description of Business – Recent Events – VIE Agreements and Call Option Agreement” for disclosure of additional reorganization-related transactions.
Other
Other than employment and the foregoing arrangements, none of the following persons has any direct or indirect material interest in any transaction to which we are a party since our incorporation or in any proposed transaction to which we are proposed to be a party: (i) any of the Company’s directors or officers; (ii) any person who beneficially owns, directly or indirectly, shares carrying more than 10% of the voting rights attached to our common stock; or (iii) any relative or spouse of any of the foregoing persons, or any relative of such spouse, who has the same house as such person or who is a director or officer of any parent or subsidiary of the Company.
Director Independence
As part of our obligations under the Securities Purchase Agreement entered into in connection with the Private Placement, we are required to appoint a Board of Directors consisting of a majority of “independent” directors (as defined under the Nasdaq Marketplace Rules) and one director designated by Euro Pacific, the lead placement agent in the Private Placement, with at least two of such directors being fluent in English, within six months of the closing of the Private Placement. Upon such appointment, we will seek to form audit and other board committees in a manner consistent with Nasdaq listed companies.
Conflict of Interest Policy
Our Board of Directors is reviewing our Conflicts of Interest Policy and such Policy may be approved following the effectiveness of the Registration Statement. Once approved, we will file it in compliance with our obligations under the rules and regulations of the Securities and Exchange Commission.
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth certain information regarding the beneficial ownership of our common stock as of September 28, 2012, by (i) each person known by us to be the beneficial owner of 5% or more of the outstanding common stock, (ii) each executive officer and director of the Company, and (iii) all of our executive officers and directors as a group.
Unless otherwise indicated, the Company believes that all persons named in the table have sole voting and investment power with respect to all shares of common stock beneficially owned by them.
|
Name and Address
of Beneficial Owner (1)
|
Title
|
Shares of
Common Stock
Beneficially Owned
|
Percent of Class
Beneficially Owned (2)
|
|||||||
|
Directors and Executive Officers
|
||||||||||
|
Guixiong Qiu (3)
Room 401, No. 5 Building, Chashan Street
South China Agricultural University
No. 483 Wushan Road, Tianhe District
Guangzhou City, Guangdong
P.R. China
|
Chief Executive Officer and
Chairman of the Board of Directors
|
4,055,161
|
30.43
|
%
|
||||||
|
Xugang Shu
Room 401, No. 5 Building, Chashan Street
South China Agricultural University
No. 483 Wushan Road, Tianhe District
Guangzhou City, Guangdong
P.R. China
|
Vice President of Research and Development
|
0
|
0
|
%
|
||||||
|
Bo Jun
Room 401, No. 5 Building, Chashan Street
South China Agricultural University
No. 483 Wushan Road, Tianhe District
Guangzhou City, Guangdong
P.R. China
|
Director of Marketing
|
0
|
0
|
%
|
||||||
|
Gilbert Lee
23 Langdale Road
Wayne, New Jersey 07470
|
Chief Financial Officer
|
0
|
0
|
%
|
||||||
|
Officers and Directors as a Group (a total of four persons beneficially owning stock)
|
4 ,055,161
|
30.43
|
%
|
|||||||
|
5% Shareholders
|
||||||||||
|
Bi Gao (4)
Room 704 , No. 10 Tianhe Guanghe Road
Guangzhou City, Guangdong
P.R. China
|
2,883,670
|
21.64
|
%
|
|||||||
|
Xiuzhen Liang (5)
Room 404, No. 10 Quan Fu Li
Fangcun District
Guangzhou City, Guangdong
P.R. China
|
1,802,294
|
13.53
|
%
|
|||||||
|
Golden Genesis Limited (6)(7)
Suite 2108, Nan Fung Tower
173 Des Voex Road
Hong Kong
|
9,011,469
|
67.63
|
%
|
|||||||
|
(1)
|
Under Rule 13d-3 of the Exchange Act, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights.
|
|
(2)
|
Based on 13,324,083 shares of common stock issued and outstanding as of September 28, 2012.
|
|
(3)
|
Includes (i) 3,605,161 shares of common stock issued in the name of Golden Genesis that shall be transferred within the next 3 years to Mr. Qiu pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Mr. Qiu without the prior written consent of Mr. Qiu, before all of such shares are transferred to Mr. Qiu; and (ii) 450,000 shares of common stock over which Mr. Qiu has voting power that are issued in the name of Golden Genesis, but held in escrow, subject to the terms of the Securities Escrow Agreement, which provides that 450,000 shares will be disbursed in 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Mr. Qiu), based on whether the Company achieves certain financial benchmarks for the fiscal year ending December 31, 2012. Mr. Qiu is the CEO, President and sole director of the Company.
|
|
(4)
|
Includes (i) 2,563,670 shares of common stock issued in the name of Golden Genesis that shall be transferred within the next 3 years to Mr. Gao pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Mr. Gao without the prior written consent of Mr. Gao, before all of such shares are transferred to Mr. Gao; and (ii) 320,000 shares of common stock over which Mr. Gao has voting power that are issued in the name of Golden Genesis, but held in escrow, subject to the terms of the Securities Escrow Agreement, which provides that 320,000 shares will be disbursed in 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Mr. Gao), based on whether the Company achieves certain financial benchmarks for the fiscal year ending December 31, 2012.
|
|
(5)
|
Includes (i) 1,602,294 shares of common stock issued in the name of Golden Genesis that shall be transferred within the next 3 years to Ms. Liang pursuant to the Call Option Agreement, under the terms of which Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of Ms. Liang without the prior written consent of Ms. Liang, before all of such shares are transferred to Ms. Liang; and (ii) 200,000 shares of common stock over which Ms. Liang has voting power that are issued in the name of Golden Genesis, but held in escrow, subject to the terms of the Securities Escrow Agreement, which provides that 200,000 shares will be disbursed in 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, Ms. Liang), based on whether the Company achieves certain financial benchmarks for the fiscal year ending December 31, 2012.
|
|
(6)
|
Includes an aggregate of 8,011,469 shares of common stock issued in the name of Golden Genesis that shall be transferred within the next 3 years to the Tanke Shareholders in accordance with the Call Option Agreement. Pursuant to the Call Option Agreement, Golden Genesis has agreed not to exercise any of its voting rights with respect to such shares on behalf of the Tanke Shareholders without the prior written consent of the Tanke Shareholders.
|
|
(7)
|
Includes an aggregate of 1,000,000 shares of common stock over which the Tanke Shareholders have voting power that are issued in the name of Golden Genesis, but held in escrow, subject to the terms of the Securities Escrow Agreement, which provides that an aggregate of 1,000,000 shares will be disbursed in 2013 to either the Investors, on a pro rata basis, or to Golden Genesis (and, subsequently, to the Tanke Shareholders, on a pro rata basis), based on whether the Company achieves certain financial benchmarks for the fiscal years ending December 31, 2012.
We did not achieve the Adjusted Income of $4,652,410 for the year ended December 31, 2011, therefore 1,000,000 shares of common stock placed in escrow have been distributed to the Investors on a pro rata basis, pursuant to the terms of the Securities Escrow Agreement.
|
SELLING STOCKHOLDERS
On February 9, 2011, we closed the Private Placement, in which we sold 6,669,627 Units, with each Unit consisting of a Note and a Warrant. The Notes are immediately convertible into shares of our common stock at a conversion price of $1.15 per share. The Warrants are immediately exercisable, expire on the third anniversary of their issuance and entitle their holders to purchase up to including (i) 6,669,627 shares of our common stock at $1.40 per share (subject to customary weighted average and stock based anti-dilution protection) and (ii) 666,963 shares of our common stock at $1.15 per share.
The selling stockholders may sell all, some or none of their shares in this offering. The following table sets forth, as of the date of this prospectus:
|
●
|
the name of the selling stockholders;
|
|
●
|
the number of shares of our common stock that may be offered for resale for the account of the selling stockholder under this prospectus;
|
|
●
|
the number and percentage of shares of our common stock that the selling stockholder beneficially owned prior to the offering for resale of the shares under this prospectus; and
|
|
●
|
the number and percentage of shares of our common stock to be beneficially owned by the selling stockholder after the offering of the resale shares (assuming all of the offered resale shares are sold by the selling stockholders).
|
The 16,243,672 shares of our common stock registered for public resale pursuant to this prospectus consists of (i) shares issued to Regeneration Capital Group, LLC, and its investors, pursuant to a consulting agreement with the Company which provided that Regeneration would provide certain consulting and due diligence services to the Company and Guangzhou Tanke prior to the Private Placement in exchange for such shares, (ii) shares issuable upon conversion or exercise of Notes and Warrants that were issued in the Private Placement (not including shares issuable upon conversion of interest accrued on the Notes) and (iii) shares issued to a selling stockholder.
The below table assumes that all of the currently outstanding Notes and Warrants will be exercised and converted for common stock and all of the securities will be sold in this offering. However, any or all of the securities listed below may be retained by any of the selling stockholders, and therefore, no accurate forecast can be made as to the number of securities that will be held by the selling stockholders upon termination of this offering. The selling stockholders are not making any representation that any shares covered by this prospectus will be offered for sale. We believe that, based on information provided to us by each of the selling stockholders, the selling stockholders listed in the table have sole voting and investment powers with respect to the securities indicated. As indicated below, certain selling stockholders are broker-dealers or affiliates of broker-dealers.
|
Beneficial Ownership Before
Offering (1)
|
Beneficial Ownership After
Offering (1)
|
|||||||||||||||||
|
Name
|
Shares of Common Stock
|
Shares
|
Percentage* (2)
|
Shares (3)
|
Percentage* (3)
|
|||||||||||||
|
Barry Cervantes (4)
|
21,739
|
21,739
|
0
|
|||||||||||||||
|
Steven Straub (4)
|
21,739
|
21,739
|
0
|
|||||||||||||||
|
Fei Fang (4)
|
34,782
|
34,782
|
0
|
|||||||||||||||
|
Crown World Service PSP/Trust fbo Brigitte Durand (4)
|
17,391
|
17,391
|
0
|
|||||||||||||||
|
Island Capital Management, LLC (4)
|
10,000
|
10,000
|
0
|
|||||||||||||||
|
Crown World Service PSP/Trust fbo Perry Lerner (4)
|
86,957
|
86,957
|
0
|
|||||||||||||||
|
John H van Merkensteijn III (4)
|
275,968
|
275,968
|
2.07
|
%
|
0
|
|||||||||||||
|
IRA f/b/o Barry Fox (4)
|
156,403
|
156,403
|
1.17
|
%
|
0
|
|||||||||||||
|
Crown World Management LLC (4)
|
69,453
|
69,453
|
0
|
|||||||||||||||
|
Emanuel Addington Living Trust Dated 10/11/96 (4)
|
107,894
|
107,894
|
0
|
|||||||||||||||
|
Joseph Douek (4)
|
107,894
|
107,894
|
0
|
|||||||||||||||
|
SAI LLC (4)
|
107,894
|
107,894
|
0
|
|||||||||||||||
|
Richard Kaufman (4)(5)
|
170,991
|
170,991
|
1.28
|
%
|
0
|
|||||||||||||
|
Altbachco LLC (4)(6)
|
170,991
|
170,991
|
1.28
|
%
|
0
|
|||||||||||||
|
La Mancha Capital LLC (4)
|
170,991
|
170,991
|
1.28
|
%
|
0
|
|||||||||||||
|
Regeneration Capital Group LLC (4)(7)
|
563,326
|
563,326
|
3.68
|
%
|
0
|
|||||||||||||
|
Jason Landver
|
70,000
|
75,248
|
5,248
|
|||||||||||||||
|
NFS/FMTC Rollover IRA FBO Susan M Carder
|
80,000
|
85,997
|
5,997
|
|||||||||||||||
|
Timothy Carroll
|
80,000
|
85,997
|
5,997
|
|||||||||||||||
|
Marc A Pierre Patricia Barragan P/Adm Center for Phys Hlth 401k PSP (9)
|
80,000
|
85,997
|
5,997
|
|||||||||||||||
|
Kevin Cornelius Janet L Cornelius
|
80,000
|
85,997
|
5,997
|
|||||||||||||||
|
NFS/FMTC Sep IRA FBO Helen Mary Erskine
|
80,000
|
85,997
|
5,997
|
|||||||||||||||
|
Alexander & Yana Galuz TTEE The Alexander Galuz and Yana Galuz Jt Living Tst, U/A 8/24/05 (10)
|
80,000
|
85,997
|
5,997
|
|||||||||||||||
|
Beneficial Ownership Before
Offering (1)
|
Beneficial Ownership After
Offering (1)
|
|||||||||||||
|
Name
|
Shares of Common Stock
|
Shares
|
Percentage* (2)
|
Shares (3)
|
Percentage* (3)
|
|||||||||
|
Lawrence Allen Glenn Linda Karen Sorensen TTEE Lawrence Allen Glenn Trust U/A 6/18/02 (11)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Linda Krug Leslie Beiers
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC Rollover IRA FBO Jerrold Allen Lester
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Jerry F McWilliams
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Devon Miller
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
O’Connor Investments LP (12)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Skee Goedhart TTEE The Paracelsus Revocable Trust U/A 7/25/97 (13)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
James Pugh
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Lynn Rollins Stull TTEE Lynn Rollins Stull Trust U/A 8/1/08 (14)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
John D Smead
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC IRA FBO Jeffrey p baker
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC Rollover IRA FBO Myra Margaret Diamond
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Trisha L Freres Theodore Kyle Freres TTEE Trisha L Freres Living Trust U/A 12/20/04 (15)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
David W Larson Jennifer L Larson
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
David J Schilder Lee Ann Schilder
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC IRA FBO Steven Styers
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Harnek Thiara Talwinder Thiara TTEE The Thiara Family Trust U/A 10/29/04 (16)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Yellowstone Holdings (17)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Louis A Larson Pamela J Larson TTEE Larson Family 2006 Trust U/A 7/13/06 (18)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC Rollover IRA FBO Curtis Ellsworth
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Beneficial Ownership Before
Offering (1)
|
Beneficial Ownership After
Offering (1)
|
|||||||||||||
|
Name
|
Shares of Common Stock
|
Shares
|
Percentage* (2)
|
Shares (3)
|
Percentage* (3)
|
|||||||||
|
Sherry Morse Maccabee John Greenberger Maccabee TTEE Maccabee Trust U/A 1/24/97 (19)
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC IRA FBO George Madaraz
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC Roth IRA FBO Mark Miller
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC Rollover IRA FBO Brent W Anderson
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Leah Berson
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Mary Heinking
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
John Russell Riedmueller Nicole Cameron Riedmueller
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
NFS/FMTC Rollover IRA FBO Timothy P Gandy
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Steffan Imhoff Tod Abby Margalith
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Andrew Craig
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Gregor Said Jackson
|
80,000
|
85,997
|
5,997
|
|||||||||||
|
Curtis Andrew Beeson Brenda Batenburg
|
86,800
|
93,307
|
6,507
|
|||||||||||
|
Jorge Fernando Echeverria Olga Clemencia Echeverria Tod Olga Clemencia Echeverria
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Paul Fosse
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Kathryn Hacker Richard Hacker
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Ulrich Honighausen Amanda Honighausen P/Adm Hausenware 401K PSP (20)
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
NFS/FMTC Sep IRA FBO Gerald E Manwill
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Barbara S Meister TTEE The Meister Non-Exempt Marital Tr, U/A 11/17/83 (21)
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Russell Parker
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Brent Paulger Sharissa Paulger
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Miriam Schwartz
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
NFS/FMTC Roth IRA FBO Howard W Wahl
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Frank Cardile Sr
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Beneficial Ownership Before
Offering (1)
|
Beneficial Ownership After
Offering (1)
|
|||||||||||||
|
Name
|
Shares of Common Stock
|
Shares
|
Percentage* (2)
|
Shares (3)
|
Percentage* (3)
|
|||||||||
|
Ralph Michael Defay
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Edward S Garcia Jr
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Jim Griffin Cust Daniel J Griffin Utma Oh
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Jim Griffin Cust Michelle E Griffin Utma Oh
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Julia L Griffin
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Scott R Griffin
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Deepak Munjal
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
NFS/FMTC Rollover IRA FBO Richard Wayne Ruess
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
NFS/FMTC Rollover IRA FBO James R Barnes
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Royce V Jackson Patricia Jackson TTEE The Royce V Jackson Sr Rev Tr U/A 12/3/94 (22)
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
QMI Fertilizer & Grain Inc (23)
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Edgar Simons
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
NFS/FMTC IRA FBO Robert Stephen Adams
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Michael Baldwin
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Balfour Hollow LLC (24)
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Robert L Bishop Tod Francine Bishop
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Vince Bacolini
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Herschel Hunter TTEE Herschel Hunter Trust U/A 11/30/88 (25)
|
86,956
|
93,475
|
6,519
|
|||||||||||
|
Bill Meurer TTEE William Henry Meurer 1997 Trust U/A 8/5/97 (26)
|
87,826
|
94,410
|
6,584
|
|||||||||||
|
NFS/FMTC IRA FBO Scott Grosskreutz
|
87,826
|
94,410
|
6,584
|
|||||||||||
|
Patrick Robert Durocher Amy Lynne Durocher
|
90,260
|
97,026
|
6,766
|
|||||||||||
|
Hemant Kathuria P/Adm H Kathuria Investments II P Plan
|
90,416
|
97,194
|
6,778
|
|||||||||||
|
Gerald L. Mars (8)
|
48,993
|
48,993
|
0
|
|||||||||||
|
William G. McBean (8)
|
205,990
|
205,990
|
1.55%
|
0
|
||||||||||
|
Peter D. Schiff (8)
|
205,990
|
205,990
|
1.55%
|
0
|
||||||||||
|
Beneficial Ownership Before
Offering (1)
|
Beneficial Ownership After
Offering (1)
|
||||||||||||||||
|
Name
|
Shares of Common Stock
|
Shares
|
Percentage* (2)
|
Shares (3)
|
Percentage* (3)
|
||||||||||||
|
Thomas Tan (8)
|
205,990
|
205,990
|
1.55
|
%
|
0
|
||||||||||||
|
KK Swogger Asset Management (27)
|
90,434
|
97,214
|
6,780
|
||||||||||||||
|
Buckthorn LLC (28)
|
94,000
|
101,047
|
7,047
|
||||||||||||||
|
Dr George K Ching P/Adm Val Eye Care Med Grp FBO Staff
|
94,260
|
101,326
|
7,066
|
||||||||||||||
|
Karl K Spielman Melinda G Elkin TTEE The Spielman and Elkin Rev Tr U/A 6/14/99 (29)
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
Spongbob Ventures II LLC (30)
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
Sarah J Basler Donald S Basler TTEE The Sarah J Basler Living Trust U/A 7/2/98 (31)
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
William Bradley P/Adm Bradley Anesthesiology PSP
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
Ronald A Griff Marilyn Jane Griff
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
Henry Louis Schairer Jr
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
Robert Neal Spady Linda Spady
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
Richard L Stone Rita L Tuzon TTEE Stone Tuzon Family Trust U/A 2/25/04 (32)
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
Carter Laren
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
Carolyn R Long
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
NFS/FMTC Rollover IRA FBO Gerald Mona
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
NFS/FMTC Rollover IRA FBO Paul Harper Zink
|
100,000
|
107,497
|
7,497
|
||||||||||||||
|
William Wiley Marianne Wiley TTEE Wiley Family Living Trust U/A 7/19/95 (33)
|
100,868
|
108,430
|
7,562
|
||||||||||||||
|
Sandeep Garg P/Adm Pacific Heart Assoc PC PS Plan (34)
|
102,904
|
110,618
|
7,714
|
||||||||||||||
|
James A Sheahan Melody K Sheahan
|
104,000
|
111,797
|
7,797
|
||||||||||||||
|
Bruce & Vanessa Franklin Greg Sad P/Adm Bafbiz Inc 401k FBO Vanessa Franklin
|
104,000
|
111,797
|
7,797
|
||||||||||||||
|
Beneficial Ownership Before
Offering (1)
|
Beneficial Ownership After
Offering (1)
|
||||||||||||||||
|
Name
|
Shares of Common Stock
|
Shares
|
Percentage* (2)
|
Shares (3)
|
Percentage* (3)
|
||||||||||||
|
Dr Uzzi Reiss Mrs Yael Reiss TTEE The Reiss Family Trust U/A 12/29/88 (35)
|
104,346
|
112,168
|
7,822
|
||||||||||||||
|
William Ten Brink TTEE Ten Brink Trust U/A 10/2/86 (36)
|
104,346
|
112,168
|
7,822
|
||||||||||||||
|
Maureen K Nass Kenneth H Nass TTEE Maureen K Nass Living Trust U/A 5/16/05 (37)
|
125,216
|
134,603
|
1.00
|
%
|
9,387
|
||||||||||||
|
Stephen Ben Son Sang Rim Son Tod Soo Sum Son
|
128,694
|
138,342
|
1.03
|
%
|
9,648
|
||||||||||||
|
Damon Suter Malinda Suter TTEE The Suter Family Trust U/A 4/12/02 (38)
|
129,000
|
138,671
|
1.03
|
%
|
9,671
|
||||||||||||
|
Francis Brent May P/Adm F Brent May DMD MSD PC PS Plan (39)
|
129,738
|
139,464
|
1.04
|
%
|
9,726
|
||||||||||||
|
Proud Fndtn
|
130,000
|
139,746
|
1.04
|
%
|
9,746
|
||||||||||||
|
Tina C Peterson P/Adm Hendrikus Grp Inc 401k FBO H Schraven
|
140,000
|
150,495
|
1.12
|
%
|
10,495
|
||||||||||||
|
Tina C Peterson P/Adm Hendrikus Grp Inc 401k FBO Tina C Peterson, Dtd 10/1/00
|
140,000
|
150,495
|
1.12
|
%
|
10,495
|
||||||||||||
|
J Mark McPherson
|
160,000
|
171,995
|
1.28
|
%
|
11,995
|
||||||||||||
|
Bruce & Vanessa Franklin Greg Sad P/Adm Bafbiz Inc 401k FBO Bruce Franklin
|
160,000
|
171,995
|
1.28
|
%
|
11,995
|
||||||||||||
|
Matula Family LP Class 2 a Partnership (40)
|
165,210
|
177,595
|
1.32
|
%
|
12,385
|
||||||||||||
|
James Bacon TTEE James V Bacon Trust U/A 9/14/95 UAD 03/26/09 (41)
|
170,000
|
182,744
|
1.35
|
%
|
12,744
|
||||||||||||
|
Blake Everett Lisa Everett
|
173,912
|
186,950
|
1.39
|
%
|
13,038
|
||||||||||||
|
NFS/FMTC IRA FBO Diane D Spolum
|
173,912
|
186,950
|
1.39
|
%
|
13,038
|
||||||||||||
|
Dennis Fried Joyce Fried TTEE Fried Family Trust U/A 7/25/91 (42)
|
173,912
|
186,950
|
1.39
|
%
|
13,038
|
||||||||||||
|
Eugene Dupont
|
173,914
|
186,952
|
1.39
|
%
|
13,038
|
||||||||||||
|
NFS/FMTC Rollover IRA FBO James A Tamborello
|
174,000
|
187,044
|
1.39
|
%
|
13,044
|
||||||||||||
|
R Steven Smith
|
174,000
|
187,044
|
1.39
|
%
|
13,044
|
||||||||||||
|
Dr John Arguelles P/Adm John C Arguelles DDS Inc DBPP (43)
|
174,000
|
187,044
|
1.39
|
%
|
13,044
|
||||||||||||
|
Beneficial Ownership Before
Offering (1)
|
Beneficial Ownership After
Offering (1)
|
||||||||||||||||
|
Name
|
Shares of Common Stock
|
Shares
|
Percentage* (2)
|
Shares (3)
|
Percentage* (3)
|
||||||||||||
|
Stephen Raskin P/Adm Drew & Raskin P/S Plan FBO Stephen Raskin
|
176,000
|
189,194
|
1.40
|
%
|
13,194
|
||||||||||||
|
Mark R Mitchell P/Adm Mark R Mitchell MD a Medical Corp DBPP (44)
|
176,000
|
189,194
|
1.40
|
%
|
13,194
|
||||||||||||
|
David Brisbin TTEE Innes Brisbin Living Trust U/A 6/8/04 (45)
|
208,694
|
224,339
|
1.66
|
%
|
15,645
|
||||||||||||
|
Richard Griff Jackie Griff
|
220,000
|
236,493
|
1.75
|
%
|
16,493
|
||||||||||||
|
Robert C Sayson Alice K Sayson
|
220,000
|
236,493
|
1.75
|
%
|
16,493
|
||||||||||||
|
Gary J May Beth C May TTEE Gary J May DMD MSD PC PS Plan (46)
|
221,216
|
237,800
|
1.76
|
%
|
16,584
|
||||||||||||
|
PETER D SCHIFF
|
347,826
|
373,901
|
2.73
|
%
|
26,075
|
||||||||||||
|
ALLEN GWYNN CHEROVLET
|
360,000
|
386,988
|
2.83
|
%
|
26,988
|
||||||||||||
|
RICHARD POTAPCHUK
|
520,000
|
558,983
|
4.04
|
%
|
38,983
|
||||||||||||
|
PRICE TARGET MEDIA, INC.
|
45,000
|
45,000
|
0
|
||||||||||||||
|
JAMES CAPLAN
|
10,000
|
10,000
|
0
|
||||||||||||||
|
VICTOR NOSTAS
|
10,000
|
10,000
|
0
|
||||||||||||||
|
JOHN LOWY
|
70,542
|
70,542
|
0
|
||||||||||||||
|
MICHAEL DRAZNIN
|
7,500
|
7,500
|
0
|
||||||||||||||
|
TOTAL
|
16,243,672
|
17,243,670
|
1,000,000
|
||||||||||||||
* Less than 1%, unless otherwise specified.
(1) Under Rule 13d-3 of the Exchange Act, a beneficial owner of a security includes any person who, directly or indirectly, through any contract, arrangement, understanding, relationship, or otherwise has or shares: (i) voting power, which includes the power to vote, or to direct the voting of shares; and (ii) investment power, which includes the power to dispose or direct the disposition of shares. Certain shares may be deemed to be beneficially owned by more than one person (if, for example, persons share the power to vote or the power to dispose of the shares). In addition, shares are deemed to be beneficially owned by a person if the person has the right to acquire the shares (for example, upon exercise of an option) within 60 days of the date as of which the information is provided. In computing the percentage ownership of any person, the amount of shares outstanding is deemed to include the amount of shares beneficially owned by such person (and only such person) by reason of these acquisition rights. Includes 1,000,000 share distributed to the Investors on a pro rata basis pursuant to the terms of the Securities Escrow Agreement.
(2) Based on 13,324,083 shares of common stock issued and outstanding as of the date of the registration statement of which this prospectus is a part.
(3) Assumes that all securities registered will be sold.
(4) Such shares were issued to Regeneration Capital Group, LLC, and its investors, pursuant to a consulting agreement with the Company which provided that Regeneration would provide certain consulting and due diligence services to the Company and Guangzhou Tanke prior to the Private Placement in exchange for such shares.
(5) Richard Kaufman is a Principal of Regeneration Capital Group LLC, the sole advisor to the Company.
(6) Altbachco LLC is owned by Ronald S. Altbach. Mr. Altbach is a Principal of Regeneration Capital Group LLC, the sole advisor to the Company.
(7) The shares held by the selling stockholder include 101,413 shares of common stock issued in the name of Regeneration Capital Group LLC, but held in escrow to pay the Company’s investor relations-related expenses. Regeneration Capital Group, LLC is the sole advisor to the Company.
(8) Selling stockholder is an affiliate of Euro Pacific, a FINRA member and SEC registered broker-dealer that acted as the lead placement agent in our February 9, 2011 private placement. Securities represent shares of common stock underlying the Placement Agent Warrant issued to the selling stockholder. The selling stockholder acquired the shares in the ordinary course of business and at the time of his receipt of the securities, such individual did not have any agreement or understanding, directly or indirectly, with any person to distribute the securities.
(9) Marc A. Pierre and Patricia Barragan are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(10) Alexander Galuz and Yona Galuz are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(11) Lawrence Allen Glenn and Linda Karen Sorensen are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(12) Timothy O’Connor is the natural person with voting or investment power over the shares of common stock owned by this entity.
(13) Skee Goedhart is the natural person with voting or investment power over the shares of common stock owned by this entity.
(14) Lynn Rollins Stull is the natural person with voting or investment power over the shares of common stock owned by this entity.
(15) Trisha L Freres and Theodore Kyle Freres are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(16) Harnek Thiara and Talwinder Thiara are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(17) James Pire is the natural person with voting or investment power over the shares of common stock owned by this entity.
(18) Luis A. Larson and Pamaca J. Larson are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(19) Sherry Morse Maccabee and John Greenberger Maccabee are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(20) Ulrich Honighausen and Amanda Honighausen are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(21) Barbara S. Meister is the natural person with voting or investment power over the shares of common stock owned by this entity.
(22) Royce Jackson is the natural person with voting or investment power over the shares of common stock owned by this entity.
(23) Quincy Murphy is the natural person with voting or investment power over the shares of common stock owned by this entity.
(24) Hope Bash is the natural person with voting or investment power over the shares of common stock owned by this entity.
(25) Herschel Hunter is the natural person with voting or investment power over the shares of common stock owned by this entity.
(26) William Meurer is the natural person with voting or investment power over the shares of common stock owned by this entity.
(27) Kurt Swogger is the natural person with voting or investment power over the shares of common stock owned by this entity.
(28) Llord E Hendrix is the natural person with voting or investment power over the shares of common stock owned by this entity.
(29) Karl K. Spielman and Melinda G. Elkin are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(30) Steven B. Linker and Carey S. Linker are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(31) Sarah J. Basler and Donald S Basler are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(32) Richard L Stone and Rita L. Stones are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(33) William Wiley and Marianne Wiley are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(34) Sandeep Garg is the natural person with voting or investment power over the shares of common stock owned by this entity.
(35) Dr. Uzzi Reiss and Mrs. Yael Reiss are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(36) William Ten Brink is the natural person with voting or investment power over the shares of common stock owned by this entity.
(37) Maureen K. Nass andKenneth H. Nass are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(38) Damon Suter and Malinda Suter are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(39) Francis Brent May is the natural person with voting or investment power over the shares of common stock owned by this entity.
(40) Michael Matula is the natural person with voting or investment power over the shares of common stock owned by this entity.
(41) James Bacon is the natural person with voting or investment power over the shares of common stock owned by this entity.
(42) Dennis Fried and Joyce Fried are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(43) Dr. John Arguelles is the natural person with voting or investment power over the shares of common stock owned by this entity.
(44) Mark R. Mitchell is the natural person with voting or investment power over the shares of common stock owned by this entity.
(45) David Brisbin is the natural person with voting or investment power over the shares of common stock owned by this entity.
(46) Gary May and Beth May are the natural persons with voting or investment power over the shares of common stock owned by this entity.
(47) Does not include 800,413 shares potentially issuable upon conversion by certain selling stockholders of interest accrued under the notes.
PLAN OF DISTRIBUTION
The selling stockholders identified in this prospectus may offer and sell up to 16,243,672 shares of our common stock, of which (i) 2,166,913 shares were issued to certain selling stockholders pursuant to a contractual arrangement with the Company, (ii) 13,339,254 shares shall be issued to certain selling stockholders upon the conversion of the Notes or exercise of the common stock purchase warrants held by them, as applicable; (iv) 666,963 shares issuable upon exercise by certain selling stockholders of our placement agent warrants; and (v) 70,542 shares issued to a selling stockholder. The selling stockholders may sell all or a portion of their shares of common stock through public or private transactions at prevailing market prices or at privately negotiated prices.
All of the Notes and Warrants were issued on February 9, 2011, in or in connection with the Private Placement.
The selling stockholders may sell all or a portion of the shares of common stock beneficially owned by them and offered hereby from time to time directly or through one or more underwriters, broker-dealers or agents. If the shares of common stock are sold through underwriters or broker-dealers, the selling stockholders will be responsible for underwriting discounts or commissions or agent’s commissions. The shares of common stock may be sold in one or more transactions at fixed prices, at prevailing market prices at the time of the sale, at varying prices determined at the time of sale, or at negotiated prices. These sales may be effected in transactions, which may involve crosses or block transactions:
|
●
|
On any national securities exchange or quotation service on which the securities may be listed or quoted at the time of sale;
|
|
●
|
In the over-the-counter market;
|
| ● |
In transactions otherwise than on these exchanges or systems or in the over-the-counter market;
|
|
●
|
Through the writing of options, whether such options are listed on an options exchange or otherwise;
|
|
●
|
Ordinary brokerage transactions and transactions in which the broker-dealer solicits purchasers;
|
|
●
|
Block trades in which the broker-dealer will attempt to sell the shares as agent but may position and resell a portion of the block as principal to facilitate the transaction;
|
|
●
|
Purchases by a broker-dealer as principal and resale by the broker-dealer for its account;
|
|
●
|
An exchange distribution in accordance with the rules of the applicable exchange;
|
|
●
|
Privately negotiated transactions;
|
|
●
|
Short sales;
|
|
●
|
Sales pursuant to Rule 144;
|
|
●
|
Broker-dealers may agree with the selling stockholders to sell a specified number of such shares at a stipulated price per share;
|
|
●
|
A combination of any such methods of sale; and
|
| ● |
Any other method permitted pursuant to applicable law.
|
If the selling stockholders effect such transactions by selling shares of common stock to or through underwriters, broker-dealers or agents, such underwriters, broker-dealers or agents may receive commissions in the form of discounts, concessions or commissions from the selling stockholders or commissions from purchasers of the shares of common stock for whom they may act as agent or to whom they may sell as principal (which discounts, concessions or commissions as to particular underwriters, broker-dealers or agents may be in excess of those customary in the types of transactions involved). In connection with sales of the shares of common stock or otherwise, the selling stockholders may enter into hedging transactions with broker-dealers, which may in turn engage in short sales of the shares of common stock in the course of hedging in positions they assume. The selling stockholders may also sell shares of common stock short and deliver shares of common stock covered by this prospectus to close out short positions and to return borrowed shares in connection with such short sales. The selling stockholders may also loan or pledge shares of common stock to broker-dealers that in turn may sell such shares.
The selling stockholders and any broker-dealer participating in the distribution of the shares of common stock may be deemed to be “underwriters” within the meaning of the Securities Act, and any commission paid, or any discounts or concessions allowed to, any such broker-dealer may be deemed to be underwriting commissions or discounts under the Securities Act. At the time a particular offering of the shares of common stock is made, a prospectus supplement, if required, will be distributed which will set forth the aggregate amount of shares of common stock being offered and the terms of the offering, including the name or names of any broker-dealers or agents, any discounts, commissions and other terms constituting compensation from the selling stockholders and any discounts, commissions or concessions allowed or re-allowed or paid to broker-dealers.
Under the securities laws of some states, the shares of common stock may be sold in such states only through registered or licensed brokers or dealers. In addition, in some states the shares of common stock may not be sold unless such shares have been registered or qualified for sale in such state or an exemption from registration or qualification is available and is complied with.
There can be no assurance that any selling stockholder will sell any or all of the shares of common stock registered pursuant to the registration statement of which this prospectus is a part.
The selling stockholders and any other person participating in such distribution will be subject to applicable provisions of the Exchange Act and the rules and regulations thereunder, including, without limitation, Regulation M promulgated under the Exchange Act (“Regulation M”), which may limit the timing of purchases and sales of any of the shares of common stock by the selling stockholders and any other participating person. Regulation M may also restrict the ability of any person engaged in the distribution of the shares of common stock to engage in market-making activities with respect to the shares of common stock. All of the foregoing may affect the marketability of the shares of common stock and the ability of any person or entity to engage in market-making activities with respect to the shares of common stock.
We have agreed to pay all expenses of the registration of the shares of common stock including, without limitation, SEC filing fees and expenses of compliance with state securities or “Blue Sky” laws; provided, however, that a selling stockholder will pay all underwriting discounts and selling commissions, if any. We will indemnify the selling stockholders against liabilities, including some liabilities under the Securities Act, in accordance with our agreement to register the shares, or the selling stockholders will be entitled to contribution. We may be indemnified by the selling stockholders against civil liabilities, including liabilities under the Securities Act, that may arise from any written information furnished to us by the selling stockholder specifically for use in this prospectus, in accordance with the related registration rights agreements, or we may be entitled to contribution.
Once sold under the registration statement of which this prospectus is a part, the shares of common stock will be freely tradable in the hands of persons other than our affiliates.
DESCRIPTION OF OUR COMMON STOCK
Our common stock was registered under the Exchange Act pursuant to a Form 10 (File Number 000-53529) that was filed with the SEC on December 16, 2008.
As of the date of this prospectus, the Company is authorized to issue 50,000,000 shares of common stock, par value $0.001 per share. As of September 28, 2012, there were 13,324,083 shares of our common stock outstanding held by approximately 58 stockholders of record.
Our common stock is entitled to one vote per share on all matters submitted to a vote of the stockholders, including the election of directors. Except as otherwise required by law, the holders of our common stock will possess all voting power. Generally, all matters to be voted on by stockholders must be approved by a majority (or, in the case of election of directors, by a plurality) of the votes entitled to be cast by all shares of our common stock that are present in person or represented by proxy. Holders representing 50 percent (50%) of our common stock issued, outstanding and entitled to vote, represented in person or by proxy, are necessary to constitute a quorum at any meeting of our stockholders. A vote by the holders of a majority of our outstanding shares is required to effectuate certain fundamental corporate changes such as liquidation, merger or an amendment to our Articles of Incorporation. Our Articles of Incorporation do not provide for cumulative voting in the election of directors. Holders of our common stock have no pre-emptive rights, no conversion rights and there are no redemption provisions applicable to our common stock.
In addition, pursuant to our By-Laws, we have elected to limit the rights, under Chapter 78 of the Nevada Revised Statutes, of dissenting stockholders in transactions involving the purchase by a third party of a controlling equity interest in the Company. Under Nevada law, following the acquisition of a controlling interest of a company by an acquiring person, a stockholder whose shares are not voted in favor of authorizing voting rights for the control shares may dissent, and obtain payment of the fair value of his or her shares. The Company has elected not to be governed by this provision.
MARKET PRICE OF AND DIVIDENDS ON THE COMPANY’S COMMON EQUITY AND RELATED STOCKHOLDER MATTERS
We are a reporting company under the Exchange Act, and our public filings can be accessed at www.sec.gov. Effective February 10, 2011, our common stock is listed for quotation on the Over-The-Counter Bulletin Board under the trading symbol “TNBI” (formerly “GHND”). There has been limited trading in our shares since they became eligible for trading on the OTCBB during the third quarter of 2009.
The following table sets forth the reported high and low bid prices for our common stock for the periods indicated as reported by the OTCBB during the last two fiscal years (N/A indicates no trading during such period):
|
TNBI
(formerly GHND)
|
||||||||
|
High
|
Low
|
|||||||
|
Fiscal Year 2010
|
||||||||
|
First Quarter
|
N/A | N/A | ||||||
|
Second Quarter
|
N/A | N/A | ||||||
|
Third Quarter
|
$ | 0.10 | $ | 0.10 | ||||
|
Fourth Quarter
|
N/A | N/A | ||||||
|
Fiscal Year 2011
|
||||||||
|
First Quarter
|
N/A | N/A | ||||||
|
Second Quarter
|
N/A | N/A | ||||||
|
Third Quarter
|
N/A | N/A | ||||||
|
Fourth Quarter
|
N/A | N/A | ||||||
|
Fiscal Year 2012
|
||||||||
|
First Quarter
|
$ | 0.35 | $ | 0.15 | ||||
|
Second Quarter
|
$ | 0.40 | $ | 0.30 | ||||
|
Third Quarter
|
$ | 0.60 | $ | 0.33 | ||||
As of September 28, 2012, there were 13,324,083 shares of our common stock outstanding held by approximately 58 stockholders of record. The number of our stockholders of record excludes any estimate by us of the number of beneficial owners of shares held in street name, the accuracy of which cannot be guaranteed.
Since inception we have not paid any dividends on our common stock. We currently do not anticipate paying any cash dividends in the foreseeable future on our common stock. The Notes we issued in the private placement contain restrictive covenants that restrict us from paying dividends or make any other distribution on shares of the capital stock of the Company without the consent of Euro Pacific, as representative of the Investors.
Although we intend to retain our earnings, if any, to finance the exploration and growth of our business, our Board of Directors will have the discretion to declare and pay dividends in the future provided that such decision is in compliance with the restrictive covenants that we made in connection with the Private Placement. Payment of dividends in the future will depend upon our earnings, capital requirements, and other factors, which our Board of Directors may deem relevant.
DISCLOSURE OF COMMISSION POSITION ON
INDEMNIFICATION FOR SECURITIES ACT LIABILITIES
The current Bylaws of the Company provides that the Board of Directors shall cause the Company to indemnify a current or former director, officer and Secretary of the Company, or a current or former director, officer and Company of a corporation of which the Company is or was a stockholder and the heirs and personal representative of any such person against all costs, charges and expenses to settle an action, judgment or proceeding to which they are made a party by reason of their position of director or officer of the Company.
The Company is permitted by the Bylaws to purchase and maintain insurance for any director, officer, employee or agent of the Company or as a director, officer, employee or agent of the Company of which the Company is or was a stockholder and his or her heirs or personal representatives against a liability incurred by him as a Director, officer, employee or agent.
Our company is incorporated under the laws of the State of Nevada. Section 78.7502 of the Nevada Revised Statutes (the “NRS”) provides that a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with the action, suit or proceeding if the person acted in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction or upon a plea of nolo contendere or its equivalent, does not, of itself, create a presumption that the person did not act in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and that, with respect to any criminal action or proceeding, the person had reasonable cause to believe that his conduct was unlawful.
Section 78.7502 of the NRS further provides a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys’ fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if the person acted in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation. Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
Section 78.751 of the NRS provides that any discretionary indemnification under Section 78.7502 of the NRS, unless ordered by a court or advanced pursuant to subsection 2 of Section 78.751 of the NRS, may be made by the corporation only as authorized in the specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances. The determination must be made by:
|
●
|
By the stockholders;
|
|
●
|
By the board of directors by majority vote of a quorum consisting of directors – who were not parties to the action, suit or proceeding;
|
|
●
|
If a majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding so orders, by independent legal counsel in a written opinion; or
|
|
●
|
If a quorum consisting of directors who were not parties to the action, suit or proceeding cannot be obtained, by independent legal counsel in a written opinion.
|
The Company’s Articles of Incorporation and Bylaws or an agreement made by the Company may provide that the expenses of officers and directors incurred in defending a civil or criminal action, suit or proceeding must be paid by the corporation as they are incurred and in advance of the final disposition of the action, suit or proceeding, upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it is ultimately determined by a court of competent jurisdiction that the person is not entitled to be indemnified by the corporation. The provisions of this subsection do not affect any rights to advancement of expenses to which corporate personnel other than directors or officers may be entitled under any contract or otherwise by law.
The indemnification and advancement of expenses authorized in or ordered by a court pursuant to Section 78.751 of the NRS:
|
●
|
does not exclude any other rights to which a person seeking indemnification or advancement of expenses may be entitled under the articles of incorporation or any bylaw, agreement, vote of stockholders or disinterested directors or otherwise, for either an action in his official capacity or an action in another capacity while holding his office, except that indemnification, unless ordered by a court pursuant to section 78.7502 of the NRS or for the advancement of expenses made pursuant to subsection 2 of section 78.751 of the NRS, may not be made to or on behalf of any director or officer if a final adjudication establishes that his acts or omissions involved intentional misconduct, fraud or a knowing violation of the law and was material to the cause of action; and
|
|
●
|
continues for a person who has ceased to be a director, officer, employee or agent and inures to the benefit of the heirs, executors and administrators of such a person.
|
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of our Company under Nevada law or otherwise, we have been advised the opinion of the SEC is that such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event a claim for indemnification against such liabilities (other than payment by us for expenses incurred or paid by a director, officer or controlling person of our company in successful defense of any action, suit, or proceeding) is asserted by a director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction, the question of whether such indemnification by it is against public policy in the Securities Act and will be governed by the final adjudication of such issue.
LEGAL MATTERS
The validity of our common stock offered hereby has been passed upon by Anslow & Jaclin, LLP, Manalapan, New Jersey.
EXPERTS
The audited consolidated financial statements of the Company and our subsidiaries as of and for the year ended December 31, 2010 as well as related footnotes appearing in this registration statement have been so included in reliance on the Report of Parker Randall CF (H.K.) CPA Limited, an independent registered public accounting firm, appearing elsewhere in this prospectus, given on the authority of such firm as experts in accounting and auditing.
The audited consolidated financial statements of the Company and our subsidiaries as of and for the year ended December 31, 2011 as well as related footnotes appearing in this registration statement have been so included in reliance on the Report of EFP Rotenberg LLP, an independent registered public accounting firm, appearing elsewhere in this prospectus, given on the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND ADDITIONAL INFORMATION
We have filed with the Commission a registration statement on Form S-1 (File No. 333-172240) under the Securities Act, as amended, with respect to the shares of Common Stock being offered by this prospectus. This prospectus does not contain all of the information included in the registration statement. For further information pertaining to us and our Common Stock, you should refer to the registration statement and the exhibits and schedules filed with the registration statement. Whenever we make reference in this prospectus to any of our contracts, agreements or other documents, and you should refer to the exhibits attached to the registration statement for copies of the actual contract, agreement or other document.
The registration statement and any other material we may file with the Commission may be read and copied at the Commission’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549, on official business days during the hours of 10:00 am to 3:00 pm. The public may obtain information on the operation of the Public Reference Room by calling the Commission at 1-800-SEC-0330. The Commission maintains a web site (www.sec.gov) that contains the registration statements, reports, proxy and information statements and other information regarding registrants that file electronically with the Commission.
We file periodic reports (Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K) with the Commission. You may read and copy any reports, statements or other information that we have filed with the Commission at the addresses indicated above and you may also access them electronically at the web site set forth above. These Commission filings are also available to the public from commercial document retrieval services.
TANKE BIOSCIENCES CORPORATION
See accompanying notes to the condensed consolidated financial statements
CONDENSED CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$ | 10,032,346 | $ | 7,700,156 | ||||
|
Restricted cash
|
583,214 | 706,802 | ||||||
|
Accounts receivable, net
|
1,815,485 | 1,917,699 | ||||||
|
Inventory
|
1,257,031 | 1,187,895 | ||||||
|
Note receivable-related parties, current portion
|
- | 239,476 | ||||||
|
Loans to customers and suppliers
|
2,889,492 | 2,513,460 | ||||||
|
Other receivables
|
157,616 | 53,936 | ||||||
|
Prepayment
|
3,325,391 | 3,633,674 | ||||||
|
Other current assets
|
542,953 | 914,594 | ||||||
|
Deferred tax assets
|
46,532 | 46,042 | ||||||
|
Total current assets
|
20,650,060 | 18,913,734 | ||||||
|
Property, plant and equipment, net
|
4,871,577 | 4,771,299 | ||||||
|
Construction in progress
|
79,382 | 35,878 | ||||||
|
Intangible asset, net
|
1,320,313 | 838,089 | ||||||
|
Other non-current assets
|
368,258 | 328,006 | ||||||
|
Total assets
|
$ | 27,289,590 | $ | 24,887,006 | ||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$ | 582,052 | $ | 784,777 | ||||
|
Other payable and accrued liabilities
|
1,048,362 | 758,907 | ||||||
|
Income tax payable
|
1,476,642 | 1,216,841 | ||||||
|
Convertible notes, net
|
5,870,686 | - | ||||||
|
Current portion of long-term borrowing
|
793,817 | 785,456 | ||||||
|
Total current liabilities
|
9,771,559 | 3,545,981 | ||||||
|
Convertible notes, net
|
- | 4,488,881 | ||||||
|
Note payable - related party
|
13,722 | 13,722 | ||||||
|
Advance from government grant
|
249,979 | 355,754 | ||||||
|
Long term borrowing
|
1,587,633 | 628,365 | ||||||
|
Total liabilities
|
11,622,893 | 9,032,703 | ||||||
|
Commitments and contingencies
|
||||||||
|
Stockholders' equity:
|
||||||||
|
Common stock, $0.001 par value, 50,000,000 shares authorized, 13,324,083 issued and outstanding as of June 30, 2012 (unaudited) and December 31, 2011, respectively
|
13,324 | 13,324 | ||||||
|
Additional paid-in capital
|
12,220,181 | 12,220,181 | ||||||
|
Retained earnings
|
2,297,420 | 2,695,983 | ||||||
|
Statutory reserve
|
373,406 | 373,406 | ||||||
|
Accumulated other comprehensive income
|
762,366 | 551,409 | ||||||
|
Total stockholders' equity
|
15,666,697 | 15,854,303 | ||||||
|
Total liabilities and stockholders' equity
|
$ | 27,289,590 | $ | 24,887,006 | ||||
See accompanying notes to the condensed consolidated financial statements
TANKE BIOSCIENCES CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME (LOSS)
(UNAUDITED)
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||
|
June 30,
|
June 30,
|
|||||||||||||||
|
2012
|
2011
|
2012
|
2011
|
|||||||||||||
|
Net sales
|
$ | 7,431,486 | $ | 4,989,843 | $ | 11,971,928 | $ | 10,931,313 | ||||||||
|
Costs of sales
|
(4,690,963 | ) | (3,217,801 | ) | (7,644,174 | ) | (6,732,867 | ) | ||||||||
|
Gross profit
|
2,740,523 | 1,772,042 | 4,327,754 | 4,198,446 | ||||||||||||
|
Selling expenses
|
(548,179 | ) | (618,806 | ) | (1,094,232 | ) | (1,204,056 | ) | ||||||||
|
Administrative expenses
|
(680,232 | ) | (780,518 | ) | (1,221,236 | ) | (3,324,905 | ) | ||||||||
|
Other operating expenses
|
- | (91,689 | ) | - | (91,689 | ) | ||||||||||
|
Depreciation and amortization
|
(14,763 | ) | (51,460 | ) | (26,429 | ) | (63,179 | ) | ||||||||
|
Income (loss) from operations
|
1,497,349 | 229,569 | 1,985,857 | (485,383 | ) | |||||||||||
|
Other income/expense
|
||||||||||||||||
|
Interest income
|
148,252 | 2,220 | 169,453 | 3,610 | ||||||||||||
|
Interest expense
|
(368,782 | ) | (423,495 | ) | (741,869 | ) | (682,949 | ) | ||||||||
|
Amortization of discount on notes
|
(690,903 | ) | (1,381,806 | ) | (1,381,806 | ) | (2,141,040 | ) | ||||||||
|
Foreign exchange losses, net
|
- | 26,646 | - | (52,400 | ) | |||||||||||
|
Income (loss) before income taxes
|
585,916 | (1,546,866 | ) | 31,635 | (3,358,162 | ) | ||||||||||
|
Income tax expense
|
(313,502 | ) | (106,953 | ) | (430,198 | ) | (320,308 | ) | ||||||||
|
Net income (loss)
|
$ | 272,414 | $ | (1,653,819 | ) | (398,563 | ) | $ | (3,678,470 | ) | ||||||
|
Other comprehensive income, net of tax:
|
||||||||||||||||
|
Effects of foreign currency conversion
|
98,471 | 649,101 | 210,957 | 240,768 | ||||||||||||
|
Translation attributable to non-controlling interest
|
- | - | - | - | ||||||||||||
|
Comprehensive income (loss)
|
$ | 370,885 | $ | (1,004,718 | ) | (187,606 | ) | $ | (3,437,702 | ) | ||||||
|
Net income (loss) available to common shareholders per share:
|
||||||||||||||||
|
Basic
|
$ | 0.02 | $ | (0.12 | ) | (0.03 | ) | $ | (0.29 | ) | ||||||
|
Diluted
|
$ | 0.02 | $ | (0.12 | ) | (0.03 | ) | $ | (0.29 | ) | ||||||
|
Weighted average shares outstanding:
|
||||||||||||||||
|
Basic
|
13,324,083 | 13,324,083 | 13,324,083 | 12,756,993 | ||||||||||||
|
Diluted
|
13,324,083 | 13,324,083 | 13,324,083 | 12,756,993 | ||||||||||||
See accompanying notes to the condensed consolidated financial statements
TANKE BIOSCIENCES CORPORATION
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
|
Six Months Ended
|
||||||||
|
June 30,
|
||||||||
|
2012
|
2011
|
|||||||
|
Operating activities:
|
||||||||
|
Net loss
|
$ | (398,563 | ) | $ | (3,678,470 | ) | ||
|
Adjustments to reconcile net income to net cash
|
||||||||
|
used in operating activities:
|
||||||||
|
Change in allowance for doubtful accounts
|
3,341 | |||||||
|
Depreciation and amortization
|
244,564 | 63,179 | ||||||
|
Common stock issued for services
|
- | 2,491,938 | ||||||
|
Amortization of discount on convertible notes payable
|
1,381,805 | 2,141,040 | ||||||
|
Amortization of capitalized offering costs
|
397,271 | 615,549 | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
102,214 | 98,710 | ||||||
|
Inventories
|
(69,136 | ) | 172,608 | |||||
|
Note receivables - related parties
|
239,476 | 1,288,062 | ||||||
|
Deferred tax asset
|
(490 | ) | 15,822 | |||||
|
Prepayment
|
308,283 | - | ||||||
|
Other current assets
|
(25,630 | ) | (565,838 | ) | ||||
|
Other non-current assets
|
(40,252 | ) | - | |||||
|
Government grant
|
(105,775 | ) | (63,425 | ) | ||||
|
Accounts payable
|
(202,725 | ) | (3,282 | ) | ||||
|
Other payables and accrued liabilities
|
289,455 | 22,023 | ||||||
|
Income tax payable
|
259,801 | 297,978 | ||||||
|
Advance from customer
|
- | (3,213 | ) | |||||
|
Net cash provided by operating activities
|
2,380,298 | 2,896,022 | ||||||
|
|
||||||||
|
Investing activities:
|
||||||||
|
Increase in loans to customers and suppliers
|
(376,032 | ) | - | |||||
|
Increase in other receivables
|
(103,680 | ) | (740,433 | ) | ||||
|
Purchase of property and equipment
|
(388,346 | ) | (100,179 | ) | ||||
|
Purchase of intangible assets
|
(482,224 | ) | - | |||||
|
Increase in cash due to acquisition of China Flying
|
- | 76,075 | ||||||
|
Net cash used in investing activities
|
(1,350,282 | ) | (764,537 | ) | ||||
|
Financing activities:
|
||||||||
|
Change in restricted cash
|
123,588 | (698,646 | ) | |||||
|
Net proceeds from issue of convertible notes
|
- | 6,522,563 | ||||||
|
Increase (decrease) in bank borrowings
|
967,629 | 458,215 | ||||||
|
Net cash provided by (used in) financing activities
|
1,091,217 | 6,282,132 | ||||||
|
Effect of foreign currency translation
|
210,957 | (116,250 | ) | |||||
|
Net increase (decrease) in cash
|
2,332,190 | 8,297,367 | ||||||
|
Cash and cash equivalents, beginning of period
|
7,700,156 | 2,222,025 | ||||||
|
Cash and cash equivalents, end of period
|
$ | 10,032,346 | $ | 10,519,392 | ||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Cash paid for interest
|
$ | 37,797 | $ | 67,405 | ||||
|
Cash paid for income taxes
|
$ | 184,227 | $ | 31,562 | ||||
See accompanying notes to the condensed consolidated financial statements
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
1. ORGANIZATION AND PRINCIPAL ACTIVITIES
In these condensed consolidated financial statements, unless the context requires otherwise, the terms “we”, “our”, “us” and the “Company” refer to Tanke Biosciences Corporation, a Nevada corporation formerly known as Greyhound Commissary, Inc. (“Greyhound”), as well as our direct and indirect subsidiaries, and our principal operating business, Guangzhou Tanke Industry Co., Ltd. (“Guangzhou Tanke”), a company organized under the laws of the People’s Republic of China (“China” or the “PRC”), which we control via a series of variable interest entity contractual agreements (the “VIE Agreements”) more fully described below.
We conduct our business through our subsidiaries, principally our wholly-owned subsidiary China Flying Development Limited (“China Flying”), a Hong Kong incorporated company, and its wholly-owned subsidiary Guangzhou Kanghui Agricultural Technology Co., Ltd. (“Kanghui Agricultural” or the “WFOE”), a wholly foreign owned enterprise incorporated as a limited liability company under the laws of the PRC. The Company operates and controls Guangzhou Tanke through Kanghui Agricultural and China Flying and in connection with the VIE Agreements.
On January 3, 2011, Guangzhou Tanke entered into a series of agreements with Kanghui Agricultural, pursuant to which Kanghui Agricultural effectively assumed management of the business activities of Guangzhou Tanke. Kanghui Agricultural is entitled to 100% of the net income of Guangzhou Tanke and is able to direct Guangzhou Tanke’s actions.
Also on January 3, 2011, our board of directors unanimously approved a resolution to enter into a Share Exchange Agreement with China Flying, and Golden Genesis Limited, a British Virgin Islands company ("Golden Genesis"), the sole stockholder of China Flying. Under the terms of the Share Exchange, Golden Genesis exchanged 100% of its capital stock in China Flying for 10,758,000 shares of authorized, but previously unissued Greyhound common stock, post-split as described below. Also, at the closing, we issued an aggregate of 2,166,903 shares (post split) of our authorized, but previously unissued common stock to a U.S. advisor. Following the closing of the agreement on February 9, 2011, China Flying became our wholly owned subsidiary.
Our board of directors further approved unanimously on January 3, 2011, a one share for 8.512 shares reverse split of our issued and outstanding common stock. The effective date of the split was established by our board on a date prior to the closing of the acquisition of China Flying.
The acquisition of China Flying was contingent upon the completion of our planned private placement in which we sold 6,669,627 units (the “Units”), with net proceeds of $6,522,563. Each Unit consisted of a $1.15 principal amount convertible note and a three year warrant to purchase one share of Greyhound common stock. On February 9, 2011, the Company entered into a Securities Purchase Agreement with individual investors relating to the private placement and completed the private placement transaction (see Note 9 below). The proceeds from such sale will be used to finance the operations and growth of Guangzhou Tanke.
At the time of the Share Exchange Agreement, Greyhound had 3,397,787 shares of common stock issued and outstanding. Following the reverse split, but prior to the issuance of shares pursuant to the acquisition of China Flying, the outstanding shares were reduced to 399,180 shares. Split shares issued in connection with the reverse stock split were fully paid and non-assessable. The number of stockholders will remain unchanged as a result of the reverse split. The par value of our common stock remained unchanged.
As management of Guangzhou Tanke obtained control of the Company, the Share Exchange was treated as a reverse merger. Accordingly, for accounting purposes Guangzhou Tanke was the acquirer so historical financial information presented herewith is that of Guangzhou Tanke. Consequently, there was no step-up in the basis of the assets of Guangzhou Tanke, as Guangzhou Tanke was the acquirer for accounting purposes.
Pursuant to the VIE Agreements, Kanghui Agricultural has the right to advise, consult, manage and operate Guangzhou Tanke for a quarterly fee equal to Guangzhou Tanke’s net income. Additionally, the Tanke Shareholders pledged their rights, titles and equity interest in Guangzhou Tanke as security for Kanghui Agricultural to collect consulting and services fees provided to Guangzhou Tanke through an Equity Pledge Agreement. In order to further reinforce Kanghui Agricultural’s rights to control and operate Guangzhou Tanke, the Tanke Shareholders granted Kanghui Agricultural an exclusive right and option to acquire all of their equity interests in Guangzhou Tanke through an Option Agreement. Neither Tanke Biosciences nor Kangui Agricultural own the assets or are responsible for the liabilities of Guangzhou Tanke.
The VIE Agreements were necessary because without them, the shareholders of Tanke Biosciences would not have control of Guangzhou Tanke. With these in place, however, Guangzhou Tanke is contractually equivalent to a subsidiary of Tanke Biosciences.
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
Guangzhou Tanke has historically self financed, and has been a profitable enterprise. However, on February 9, 2011, Tanke Biosciences sold convertible notes payable (see Note 9 below) with net proceeds of $6,522,563. Such proceeds will be used to finance the operations and growth of Guangzhou Tanke.
As Tanke Biosciences has complete control over Guangzhou Tanke, all assets presented on the balance sheet of Tanke Biosciences are available to settle obligations of Tanke Biosciences. Furthermore, there are no liabilities on the balance sheets of Guangzhou Tanke that do not have recourse against the assets of Tanke Biosciences. As Guangzhou Tanke is our sole operating entity, nearly all operating assets and liabilities are those of Guangzhou Tanke.
“RMB” and “Renminbi” refer to the legal currency of China and “$”, “US dollar” and “US$” refer to the legal currency of the United States.
Overview of Our Business
Through Guangzhou Tanke, our principal operating business, we are one of the leading animal nutrition and innovative feed additive providers in China. Our products are distinguished from traditional artificial feed additives in that they are environmentally-friendly and are designed to optimize the growth and health of livestock such as pigs and cattle, as well as farmed fish.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Basis of Preparation
The Company’s consolidated financial statements as of June 30, 2012 and for the three and six months ended June 30, 2012 and 2011 have been stated in US dollars and prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) for interim financial statements and with instructions to Form 10-Q pursuant to the rules and regulations of Securities and Exchange Act of 1934, as amended (the “Exchange Act”) and Article 8-03 of Regulation S-X under the Exchange Act. In the opinion of our management, we have included all adjustments (consisting only of normal recurring adjustments) considered necessary in order to make the financial statements not misleading. Operating results for the three and six months ended June 30, 2012 are not indicative of the results that may be expected for the fiscal year ending December 31, 2012. The condensed consolidated balance sheet information as of December 31, 2011 was derived from the audited consolidated financial statements included in the Form 10-K. These unaudited financial statements and related notes should be read in conjunction with our audited annual financial statements for the year ended December 31, 2011 included in our Form 10-K filed with the Securities and Exchange Commission on April 16, 2012.
(b) Basis of consolidation
These consolidated financial statements include the financial statements of the Company and its subsidiaries (the “Group''). All inter-company balances and transactions within the Group have been eliminated.
(c) Use of Estimates
In preparing consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of the amount due from related parties, the net realizable value of inventories, the estimation of useful lives of property and equipment and intangible assets, and the value of warrants. Actual results could differ from those estimates.
(d) Concentrations of Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash and cash equivalents, restricted cash, accounts receivable and amounts due from related parties. The Company places its cash with financial institutions with high-credit ratings and quality. The Company maintains bank accounts in the PRC only. In addition, the Company conducts periodic reviews of the related party financial conditions and payment practices. The Company has not experienced losses related to these concentrations in the past.
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
Approximately 99% of the Company’s revenue is generated from buyers in mainland China.
(e) Concentrations of Suppliers
All the Company’s suppliers are located in mainland China.
|
(f) Cash and Cash Equivalents
|
|
The Company considers all highly liquid investments with initial maturities of three months or less to be cash equivalents.
|
|
(g) Restricted Cash
|
|
Deposits that are restricted in use are classified as restricted cash. The Company has restricted cash in an escrow account and represents one year of interest (approximately $300,000) on the convertible notes payable. When the notes mature or are converted into stock, the cash in this account will be released from restriction. Another escrow account holds the fund set aside for use on Investor Relations activities (approximately $280,000.)
|
|
(h) Trade and Other Receivables
|
|
The Company periodically assesses its accounts receivable for collectability on a specific identification basis. If collectability of an account becomes unlikely, an allowance is recorded for that doubtful account. Once collection efforts have been exhausted, the account receivable is written off against the allowance. The Company does not require collateral for trade or other accounts receivable.
|
|
(i) Inventories
|
|
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the weighted average method. The cost of inventories includes the purchase cost and other costs incurred in bringing the inventories to their present location and condition. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and the estimated costs necessary to make the sale.
|
|
As of June 30, 2012 and December 31, 2011, the Company’s provision for slow-moving or defective inventories amounted to $42,028 and $41,585, respectively.
|
(j) Prepayments
Prepayments represent cash paid in advance to suppliers for purchases of raw materials.
|
(k) Property, Plant and Equipment
|
|
Property and equipment are stated at cost less accumulated depreciation. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use.
|
|
Depreciation of property and equipment is calculated using the straight-line method over their estimated useful lives. The estimated useful lives are as follows:
|
|
Buildings
|
15-20 years
|
|
Plant and machinery
|
3-20 years
|
|
Motor vehicle
|
10 years
|
|
Office equipment
|
3-10 years
|
|
Expenditures for additions, major renewals and betterments are capitalized and expenditures for maintenance and repairs are charged to expense as incurred.
|
|
Upon sale or disposition, the applicable amounts of asset cost and accumulated depreciation are removed from the accounts and the net amount less the proceeds from disposal is charged or credited to income.
|
|
(l) Intangible Asset
|
|
The intangible asset primarily represented two land use rights and purchased developed technology, and they are recorded at cost less accumulated amortization.
According to the laws of China, land in the PRC is owned by the government and cannot be sold to an individual or company. However, the government grants the users a land use right to use the land. The land use rights granted to the Company are being amortized using the straight-line method over the lease term of fifty years.
During the second quarter of 2012, we purchased from an agricultural research institute the right of commercializing and applying for a patent for a new product technology developed by that research institute.
|
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
|
(m) Impairment of Long-lived Assets
|
|
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book values of such assets exceed the future undiscounted cash flows attributable to such assets.
|
(n) Statutory Reserves
In accordance with the relevant laws and regulations of the PRC and the articles of associations of the Company, Guangzhou Tanke is required to allocate 10% of their net income reported in the PRC statutory accounts, after offsetting any prior years’ losses, to statutory reserve, on an annual basis. When the balance of such reserve reaches 50% of the respective registered capital of the subsidiaries, any further allocation is optional.
As of June 30, 2012 and December 31, 2011, the statutory reserves of the subsidiary already reached 50% of the registered capital of the subsidiary and the Company did not have any further allocation on it.
The statutory surplus reserves can be used to offset prior years’ losses, if any, and may be converted into registered capital, provided that the remaining balances of the reserve after such conversion is not less than 25% of registered capital. The statutory surplus reserve is non-distributable.
|
(o) Revenue Recognition
|
The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) 605-10, Revenue Recognition, and SEC Staff Accounting Bulletin No.104. Pursuant to these pronouncements, revenue is recognized when all of the following criteria are met:
|
- Persuasive evidence of an arrangement exists;
|
|
- Delivery has occurred or services have been rendered;
|
|
- The seller's price to the buyer is fixed or determinable; and
|
|
- Collectability is reasonably assured.
|
|
The Company’s revenue is generated through the wholesale and retail sale of livestock feed including organic trace mineral additives, functional regulation additives, herbal medicinal additives and raw materials. Before the Company recognizes revenue on these product sales, written purchase orders and contracts are received in advance of all shipments of goods to customers. For sales within the Company’s own province, delivery is made by Company employees. Such delivery occurs on the same day as shipment. For delivery outside the province, shipment is made through a separate logistics company that assumes the risk of loss. Revenue is recognized upon shipment of goods to the customers. The Company typically does not incur bad debt losses because this type of loss is deducted from the salesperson’s compensation, thereby mitigating the loss to the Company. Therefore, collectability is reasonably assured.
|
|
Revenue is presented net of sales returns, which are not significant. However, the Company continually performs analyses of returns and records a provision at the time of sale if necessary. As of June 30, 2012 and December 31, 2011, it was determined that potential returns and allowances were not material so the Company did not record a provision for returns. The Company revisits this estimate regularly and adjusts it if conditions change.
|
|
(p) Cost of Goods Sold
|
|
Cost of revenue consists primarily of material cost, labor cost, rent of land allocated to production, overhead associated with the manufacturing process and directly related expenses.
|
|
(q) Research and Development Costs
|
Research and development costs are charged to expense as incurred and are included in operating expenses.
|
(r) Value Added Tax
|
|
In accordance with the relevant tax laws in the PRC, VAT is levied on the invoiced value of sales and is payable by the purchaser. The Company is required to remit the VAT it collects to the tax authority, but may deduct the VAT it has paid on eligible purchases. The difference between the amounts collected and paid is presented as VAT recoverable or payable balance on the balance sheet.
|
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
(s) Income Taxes
|
The Company uses the asset and liability method of accounting for income taxes pursuant to ASC 740, ”Income Tax”. Under the asset and liability method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
The Company is not subject to United States income tax. Furthermore, the Company is audited every year by an agency of the Chinese tax authority. Consequently, there are no uncertain tax positions requiring accrual or disclosure in accordance with ASC 740-10, Income Taxes.
|
|
(t) Comprehensive Income
|
|
Comprehensive income is defined to include all changes in equity except those resulting from net income or loss, investments by owners and distributions to owners. The Company’s only component of other comprehensive income is the foreign currency translation adjustment.
|
(u) Earnings per share (EPS)
Earnings per share is calculated in accordance with ASC 260-10 which requires the Company to calculate net income (loss) per share based on basic and diluted net income (loss) per share, as defined. Basic EPS excludes dilution and is computed by dividing net income (loss) by the weighted average number of shares outstanding for the period. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock.
(v) Commitments and Contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, penalties and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
(w) Foreign Currency Translation
The Company, its subsidiaries and VIE maintain financial statements in the functional currency of each entity. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
The financial statements of each entity are prepared using the functional currency, and have been translated into United States dollars (“US$” or “$”). Assets and liabilities are translated at the exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates for the period. Stockholders’ equity is translated at historical exchange rates. Any translation adjustments are included as a foreign exchange adjustment in other comprehensive income, a component of stockholders’ equity.
|
Exchange rates used (RMB to USD)
|
2011
|
2012
|
|||||||
|
Assets and liabilities
|
Balance sheet date (6/30)
|
0.1547 | 0.1588 | ||||||
|
Balance sheet date (12/31)
|
0.1571 | ||||||||
|
Revenue and expenses
|
Period average (1/1to 3/31)
|
0.1518 | 0.1588 | ||||||
|
Period average (1/1to 6/30)
|
0.1527 | 0.1584 | |||||||
RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US$ at the rates used in translation.
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
(x) Financial Instruments
The carrying amounts of cash and cash equivalents, restricted cash, accounts receivable, due to/from related parties, notes payable, other payable and accrued liabilities and income tax payable approximate their fair values due to the short-term nature of these items. The carrying amounts of long-term borrowings approximate the fair value based on the Company’s expected borrowing rate for debt with similar remaining maturities and comparable risk.
Convertible notes are not carried at fair value due to the discounts for warrants and the beneficial conversion feature. As the interest on these notes approximates market interest, the fair value is their face value of $7,670,071.
It is management’s opinion that the Company is not exposed to significant interest, price or credit risks arising from these financial instruments.
(y) Recent Accounting Updates
In June 2011, the FASB issued ASC Topic 220 “Comprehensive Income” that amends the presentation of comprehensive income in the financial statements by requiring an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. The update also eliminates the option to present the components of other comprehensive income as part of the statement of equity. The guidance is effective for interim and annual reporting periods beginning on or after December 15, 2011, with early adoption permitted. The adoption of this ASU did not have a material impact on the Company’s financial statements.
In May 2011, the FASB issued ASU 2011-04, Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS. ASU 2011-04 amends ASC Topic 820 to require additional disclosures regarding fair value measurements. One of the areas concerned is to add disclosures about the sensitivity of fair value measurements categorized within Level 3 of the fair value hierarchy, which require the most judgment in determining fair value. The provisions of ASU 2011-04 will be effective for years beginning after December 15, 2011 for both public and nonpublic entities. Public entities will begin adoption in the first interim period beginning after December 15, 2011. Early adoption is not permitted. The adoption of this ASU did not have a material impact on the Company’s financial statements.
3. INVENTORIES
Inventories as of June 30, 2012 and December 31, 2011 consisted of the following.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Raw materials
|
$ | 720,274 | $ | 538,465 | ||||
|
Finished goods
|
337,425 | 402,458 | ||||||
|
Work in pogress
|
199,983 | 242,748 | ||||||
|
Packaging material
|
41,377 | 45,809 | ||||||
|
Inventory allowance
|
(42,028 | ) | (41,585 | ) | ||||
| $ | 1,257,031 | $ | 1,187,895 | |||||
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
4. ACCOUNTS RECEIVABLE
Accounts receivable as of June 30, 2012 and December 31, 2011 consisted of the following.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Account receivables
|
$ | 2,051,250 | $ | 2,150,981 | ||||
|
Less: Allowance for doubtful accounts
|
(235,765 | ) | (233,282 | ) | ||||
| $ | 1,815,485 | $ | 1,917,699 | |||||
5. DUE FROM RELATED PARTIES
Due from related parties consisted of the following.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Advance to director
|
$ | - | $ | 239,149 | ||||
|
Others
|
- | 327 | ||||||
| $ | - | $ | 239,476 | |||||
Advance to directors represents advance payment made to directors for business development activities and will normally be repaid within one year.
6. OTHER RECEIVABLES
Other receivables consisted of the following.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Deposit and others
|
$ | 11,557 | $ | 13,425 | ||||
|
Advance to staff
|
146,059 | 40,511 | ||||||
| $ | 157,616 | $ | 53,936 | |||||
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
Loans to customers and suppliers represent 8% interest bearing advances to one of the Company’s customers and one supplier, both of which are effective in December 2011 and expected to be repaid within one year.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Loan to customers and suppliers
|
2,889,492 | 2,513,460 | ||||||
| $ | 2,889,492 | $ | 2,513,460 | |||||
7. PREPAYMENT AND OTHER CURRENT ASSETS
In order to secure key raw materials and supplies and to lock in rising purchase prices, the Company paid substantial amounts of cash in advance on purchase orders. In addition, a few major R&D projects were paid in advance for developers’ work.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Prepayment to suppliers
|
$ | 3,325,391 | $ | 3,633,674 | ||||
Other current assets as of June 30, 2012 and December 31, 2011 consisted of the following.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Deferred expenses
|
25,630 | - | ||||||
|
Offering costs, net
|
517,323 | 914,594 | ||||||
| $ | 542,953 | $ | 914,594 | |||||
In connection with the private placement, the Company incurred $1,624,002 of offering costs. These costs have been reflected as other current assets and are being amortized using the interest method over the expected life of the related convertible notes payable. Amortization of these costs is recorded as interest expense. As of June 30, 2012, the remaining book value of these offering costs amounted to $517,323.
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
8. PROPERTY, PLANT AND EQUIPMENT, NET
Property, plant and equipment as of June 30, 2012 and December 31, 2011 consisted of the following.
|
June 30,
2012
|
December 31,
2011
|
|||||||
|
|
||||||||
|
Buildings and leasehold improvements
|
$ | 4,568,872 | $ | 4,518,397 | ||||
|
Plant and equipment
|
1,154,445 | 1,024,245 | ||||||
|
Motor vehicles
|
169,250 | 166,884 | ||||||
|
Office equipment
|
326,708 | 164,907 | ||||||
|
Total property, plant and equipment
|
6,219,275 | 5,874,433 | ||||||
|
Accumulated depreciation
|
1,347,698 | 1,103,134 | ||||||
|
Property, plant and equipment, net
|
$ | 4,871,577 | $ | 4,771,299 | ||||
|
Construction in progress
|
79,382 | 35,878 | ||||||
| $ | 4,950,959 | $ | 4,807,177 | |||||
The Company has buildings on the site it occupies, including factory buildings. Due to the lack of a Land Use Right Certificate, the Company is unable to apply for the Property Ownership Certificate for the buildings. However, as the buildings are in use, the Company depreciates them over their expected useful lives. During the quarter ended June 30, 2011, the Company’s factory campus construction project was completed and the costs were moved from construction in process to the buildings account. Upon placement in service, the Company began depreciating them.
9. INTANGIBLE ASSET, NET
The intangible asset as of June 30, 2012 and December 31, 2011 consisted of the following.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Deposit for land use right
|
$ | 842,659 | $ | 835,985 | ||||
|
New product technology
|
476,290 | |||||||
|
Other
|
1,364 | 2,104 | ||||||
| $ | 1,320,313 | $ | 838,089 | |||||
On November 21, 2003, the Company applied to the Government of Huaqiao Town, Huadu District, Guangzhou, for the land use right of No. 2 Industry Area of Huaqiao Town (i.e., Laohutou Lot, Wangongtang) covering an area of around 430,000 square feet.
On October 22, 2010, the Company applied to the Administration Committee of Qingyuan Huaqiao Industrial District for the land use right covering an area of around 60 acres. The Company has paid $576,211 as consideration for the land use right and started the construction project during the year.
The Company plans to start amortizing the land use right as soon as the official certificate is issued on Huadu facility. The amortization of the land use right for the Qingyuan facility should begin in the 3rd quarter of 2012.
The amount included in new product technology represents the right to commercialize and apply for a patent a new product developed by an agricultural research institute, that was sold to the Company during the quarter ended June 30, 2012. Patent protection applied will last for five years and amortization of this intangible asset will start when production of the new product begins.
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
10. OTHER NON-CURRENT ASSETS
Other non-current assets consisted of the following.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Staff loan
|
$ | 325,465 | $ | 265,167 | ||||
|
Others
|
42,793 | 62,839 | ||||||
| $ | 368,258 | $ | 328,006 | |||||
Staff loans are made to employees under terms that call for repayment of the amounts within two years.
11. OTHER PAYABLE AND ACCRUED LIABILITIES
Other payable and accrued liabilities as of June 30, 2012 and December 31, 2011 consisted of the following.
|
June 30,
2012
|
December 31,
2011
|
|||||||
|
|
||||||||
|
Other payables
|
$ | 110,388 | $ | 67,751 | ||||
|
Staff welfare payable
|
68,686 | 68,057 | ||||||
|
Accrued payroll
|
112 | 46,294 | ||||||
|
Value added tax payable
|
72,435 | 93,140 | ||||||
|
Registration rights penalties
|
460,206 | 460,206 | ||||||
|
Accrued Interest
|
315,691 | - | ||||||
|
Other tax payable
|
20,844 | 23,459 | ||||||
| $ | 1,048,362 | $ | 758,907 | |||||
Other payables represent loans from third parties, which are interest free, unsecured and repayable on demand. Registration rights penalties associated with the registration rights agreement. Accrued interest for the 8% convertible note payable, paid semi-annually.
12. CONVERTIBLE NOTES PAYABLE
On February 9, 2011, the Company entered into a Securities Purchase Agreement with individual investors relating to a private placement transaction by the Company (the “Private Placement”) of 6,669,627 units. Each unit consisted of a $1.15 principal amount 8% Senior Convertible Note (the “Notes”) and a Common Stock Purchase Warrant (the “Warrants”) to purchase one share of the Company’s common stock at an exercise price of $1.40 per share.
As a result of the Private Placement, the Company offered and sold $7,670,071 worth of Notes convertible into 6,669,627 shares of common stock. The Notes are payable 24 months from February 9, 2011 with an interest rate of 8% per annum payable semiannually in arrears. The Company placed in escrow an amount of the proceeds of the Private Placement equal to one semi-annual interest payment on the Notes to secure prompt interest payments. Until such time as 75% of the Notes are converted into shares of Common Stock, if such escrow is depleted in order to make interest payments, the Company will replenish such escrow amount. At the option of the holder, the Notes may be converted into Common Stock at a price of $1.15 per share, which is subject to customary weighted average and stock based anti-dilution protection. The issuance of the Notes was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D.
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
The Notes contain customary events of default and affirmative and negative covenants of the Company, including negative covenants which restrict the Company’s ability to do the following (among other things) without the consent of the investors: (i) incur, or permit to exist, any indebtedness for borrowed money in excess of (A) US$3,000,000 during the twelve (12) month period beginning on February 9, 2011, or (B) US$5,000,000 during the two-year period beginning on February 9, 2011 and ending on February 9, 2013 (the maturity date of the Notes), except in the ordinary course of the Company’s business; (ii) lend or advance money, credit or property to or invest in (by capital contribution, loan, purchase or otherwise) any person or entity in excess of US$1,000,000 except: (A) investments in United States Government obligations, certificates of deposit of any banking institution with combined capital and surplus of at least $200,000,000; (B) accounts receivable arising out of sales in the ordinary course of business; and (C) inter-company loans between and among the Company and its subsidiaries; (iii) pay dividends or make any other distribution on shares of the capital stock of the Company; (iv) create, assume or permit to exist, any lien on any of the Company’s property or assets now owned or hereafter acquired, subject to existing liens and certain exceptions; (v) assume guarantees, subject to certain exceptions; (vi) engage in “sale-leaseback” transactions, subject to certain exceptions; (vii) make capital expenditures in excess of US$5,000,000 in any fiscal year, subject to certain exceptions; and (viii) materially alter the Company’s business.
In connection with the issuance of the Notes, we entered into a Registration Rights Agreement (the “Registration Rights Agreement”) with the investors which sets forth the rights of the investors to have the shares of common stock underlying the Notes and Warrants registered with the SEC for public resale. Pursuant to the Registration Rights Agreement, we agreed to file, no later than April 11, 2011, a registration statement to register the shares underlying the Notes and the Warrants and to have such registration statement effective no later than September 18, 2011. If the registration statement was not filed by April 11, 2011 (the “Filing Failure”), was not effective by September 18, 2011 (the “Effectiveness Failure”) or if, after the effective date, sales of securities included in the registration statement cannot be made (including, without limitation, because of a failure to keep the registration statement effective, to disclose such information as is necessary for sales to be made pursuant to the registration statement, to register a sufficient number of shares of Common Stock or to maintain the listing of the Common Stock) (a “Maintenance Failure”) then, as liquidated damages (and in complete satisfaction and to the exclusion of any claims or remedies inuring to any holder of the securities) the Company is required to pay an amount in cash equal to 1% of the aggregate purchase price paid by the Investors on each of the following dates: (i) 20 days following the date of a Filing Failure; (ii) 30 days following the initial day of a Maintenance Failure; (iii) on every thirtieth day thereafter (pro-rated for periods totaling less than thirty days) until such failure is cured; (iv) on every thirtieth day after the day of an Effectiveness Failure and thereafter (pro rated for periods totaling less than thirty days) until such Effectiveness Failure is cured; (v) on every thirtieth day after the initial day of a Maintenance Failure and thereafter (pro rated for periods totaling less than thirty days) until such Maintenance Failure is cured. The payments to be made by the Company are limited to a maximum of 6% of the aggregate amount paid by the Investors ($460,204.29). The registration statement was not declared effective by December 31, 2011, as such an Effectiveness Failure occurred and the Company accrued the full $460,204. The Company will continue to assess the likelihood of payments under this arrangement.
The warrants issued as a component of the units were valued using the Black-Scholes method using the following assumptions: (1) life of warrants of 3 years, (2) annualized volatility of 100%, (3) fair value of stock as of grant date of $1.15, (4) exercise price of $1.40, (5) annual dividend rate of 0%, and (6) discount rate of 1.34%. Such calculation resulted in a warrant value of $4,470,536.
The proceeds of the unit offering were allocated to the Notes and warrants based on their relative fair values on a weighted average basis, with the resulting allocated value of the warrants of $2,824,350 being classified to additional paid in capital. Such discount to the Notes is being amortized over their expected life.
The beneficial conversion feature associated with the issuance of the above Notes, amounted to $2,824,350, which has also been recorded as a discount to the convertible notes payable and is being amortized over the life of the Notes. The Notes do not contain any embedded derivatives which require liability classification.
As of June 30, 2012, the book value of the Notes amounted to $5,870,686, which consisted of the aggregate face value of $7,670,071, less the remaining discount of $1,799,385.
13. INCOME TAX
The Company’s operating subsidiary, Guangzhou Tanke, is a “domestic enterprise” that is registered and operated in Guangzhou, the PRC. Income tax expense for the six months ended June 30, 2012 and 2011 of $430,198 and $320,308 respectively, represents the provision for current income tax expenses in the PRC. The statutory income tax rate in PRC is 25%.
Tanke Bio-Tech, a subsidiary of Guangzhou Tanke, is a joint venture with a foreign entity that received a full exemption from income taxes in 2007 and 2008 and half rate reduction (12.5%) for years 2009, 2010 and 2011 in accordance with the Law of the People's Republic of China on Income Tax of Enterprises with Foreign Investment and Foreign Enterprises and Notification of the State Council on Carrying out the Transitional Preferential Policies concerning Enterprise Income Tax (Guofa (2007) No. 39). Beginning in 2012, Tanke Bio-Tech starts benefiting from a different reduced tax rate of 15% due to its status of an official new, high-tech company.
As of June 30, 2012 and December 31, 2011, the income tax payable for the Company amounted to $1,476,642 and $1,216,841, respectively. All of the Company’s U.S. net operating loss carry forward was fully reserved.
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
Significant components of the Company’s deferred tax asset are as follows.
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Deferred tax asset
|
||||||||
|
Allowance for doubtful accounts
|
$ | 46,532 | $ | 46,042 | ||||
14. LONG-TERM LOANS
The details of the Company’s long-term borrowings are as follows:
|
June 30,
|
December 31,
|
|||||||
|
2012
|
2011
|
|||||||
|
|
||||||||
|
Bank loans bearing interest at 5.85% and 7.36% per annum, maturing on January 30, 2013 and April 30, 2015.
|
||||||||
|
The loans are uncollateralized other than restricted cash deposited at the bank.
|
$ | 2,381,450 | $ | 1,413,821 | ||||
|
Less: Current portion
|
(1,270,107 | ) | (785,456 | ) | ||||
| $ | 1,111,343 | $ | 628,365 | |||||
The bank loans consist of $793,817 (RMB 5,000,000) and $1,587,633 (RMB 10,000,000), bearing interest at 5.85% and 7.36% per annum, maturing on January 30, 2013 and April 30, 2015, respectively. Interest is calculated and paid on the 20th of each month. Principle payments are made on a quarterly basis.
| Payment Schedule | |||||||||||||||||||
| 2012 |
2013
|
2014
|
2015
|
2016
|
|||||||||||||||
|
$
|
529,211
|
$ | 617,413 | $ | 617,413 | $ | 617,413 | $ | - | ||||||||||
15. GOVERNMENT GRANT
The government grant liability represents an advance from the Chinese government for research and development projects. The Company has recorded the grants received as a government grant liability, and ratably recognizes the amount as a reduction of research and development expense when the related research and development activities are performed. As of June 30, 2012 and December 31, 2011, government grant balances are $249,979 and $355,754, respectively.
16. SEGMENT INFORMATION
The Company operates in four segments: organic trace mineral additives, functional regulation additives, herbal medicinal additives and other revenues. Management oversees each of these operations separately.
Property, equipment and other assets are shared and not tracked separately by segment. Administrative expenses are also not tracked by segment. Following is a breakdown of revenue, costs of sales and gross profit by segment.
TANKE BIOSCIENCES CORPORATION
NOTES TO FINANCIAL STATEMENTS
June 30, 2012
(Unaudited)
|
Three Months Ended
|
Six Months Ended
|
|||||||||||||||
|
June 30,
|
June 30, | |||||||||||||||
|
2012
|
2011
|
2012
|
2011
|
|||||||||||||
|
Segment revenues
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
$ | 6,512,375 | $ | 3,939,129 | $ | 9,898,283 | $ | 8,801,243 | ||||||||
|
Functional Regulation Additives
|
844,638 | 948,663 | 1,746,539 | 1,658,775 | ||||||||||||
|
Herbal Medicinal Additives
|
54,254 | 83,016 | 96,839 | 84,268 | ||||||||||||
|
Other
|
20,219 | 19,035 | 230,267 | 387,027 | ||||||||||||
| 7,431,486 | 4,989,843 | $ | 11,971,928 | $ | 10,931,313 | |||||||||||
|
Segment costs of sales
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
4,030,699 | 2,520,275 | $ | 6,151,155 | $ | 5,399,720 | ||||||||||
|
Functional Regulation Additives
|
576,489 | 601,392 | 1,159,312 | 1,025,072 | ||||||||||||
|
Herbal Medicinal Additives
|
64,179 | 74,878 | 115,244 | 75,674 | ||||||||||||
|
Other
|
19,596 | 21,256 | 218,463 | 232,401 | ||||||||||||
| 4,690,963 | 3,217,801 | $ | 7,644,174 | $ | 6,732,867 | |||||||||||
|
Segment gross profit
|
||||||||||||||||
|
Organic Trace Mineral Additives
|
2,481,676 | 1,418,854 | $ | 3,747,128 | $ | 3,401,523 | ||||||||||
|
Functional Regulation Additives
|
268,149 | 347,271 | 587,227 | 633,703 | ||||||||||||
|
Herbal Medicinal Additives
|
(9,925 | ) | 8,138 | (18,405 | ) | 8,594 | ||||||||||
|
Other
|
623 | (2,221 | ) | 11,804 | 154,626 | |||||||||||
| 2,740,523 | 1,772,042 | $ | 4,327,754 | $ | 4,198,446 | |||||||||||
TANKE BIOSCIENCES CORPORATION
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
CONTENTS
|
Report of Independent Registered Public Accounting Firm - EFP Rotenberg LLP
|
F-2
|
|||
|
Report of Independent Registered Public Accounting Firm - Parker Randall CF (H.K.) CPA Limited
|
F-3
|
|||
|
Consolidated Balance Sheets – As of December 31, 2011 and 2010
|
F-4
|
|||
|
Consolidated Statements of Income and Comprehensive Income – For the Years ended December 31, 2011 and 2010
|
F-5
|
|||
|
Consolidated Statements of Shareholders’ Equity – For the Years Ended December 31, 2011 and 2010
|
F-6
|
|||
|
Consolidated Statements of Cash Flows – For the Years ended December 31, 2011 and 2010
|
F-7
|
|||
|
Notes to Consolidated Financial Statements
|
F-8 to F-20
|
|
||
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of Tanke Biosciences Corporation
We have audited the accompanying consolidated balance sheet of Tanke Biosciences Corporation as of December 31, 2011 and the related consolidated statements of operations, stockholders’ equity, and cash flows for the year ended December 31, 2011. Tanke Biosciences Corporation’s management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Tanke Biosciences Corporation as of December 31, 2011 and the results of its operations and its cash flows for the year ended December 31, 2011 in conformity with accounting principles generally accepted in the United States of America.
/s/ EFP Rotenberg, LLP
EFP Rotenberg, LLP
Rochester, New York
April 16, 2012
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of
TANKE BIOSCIENCES CORPORATION
We have audited the accompanying consolidated balance sheets of Tanke Biosciences Corporation fka Guangzhou Tanke Industry Co. Ltd. (the “Company”) as of December 31, 2010 and the related consolidated statements of income and comprehensive income, shareholders’ equity and cash flows for the year ended December 31, 2010. These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2010, and the results of its operations and its cash flows for the year ended December 31, 2010 in conformity with accounting principles generally accepted in the United States of America.
Parker Randall CF (H.K.) CPA Limited
Certified Public Accountants
Hong Kong
May 12, 2011, except as to note 2(a), which is as of April 16, 2012
TANKE BIOSCIENCES CORPORATION
CONSOLIDATED BALANCE SHEETS
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Current assets:
|
||||||||
|
Cash and cash equivalents
|
$
|
7,700,156
|
$
|
2,222,025
|
||||
|
Restricted cash
|
706,802
|
-
|
||||||
|
Accounts receivable, net
|
1,917,699
|
1,767,968
|
||||||
|
Inventory
|
1,187,895
|
1,354,282
|
||||||
|
Note receivable-related parties, current portion
|
239,476
|
2,033,622
|
||||||
|
Other receivables
|
2,567,396
|
112,569
|
||||||
|
Other current assets
|
4,548,268
|
164,846
|
||||||
|
Deferred tax assets
|
46,042
|
17,887
|
||||||
|
Total current assets
|
18,913,734
|
7,673,199
|
||||||
|
Property, plant and equipment, net
|
4,771,299
|
1,554,589
|
||||||
|
Construction in progress
|
35,878
|
2,777,417
|
||||||
|
Intangible asset, net
|
838,089
|
286,892
|
||||||
|
Notes receivable-related parties, long-term portion
|
-
|
974,532
|
||||||
|
Other non-current assets
|
328,006
|
-
|
||||||
|
Total assets
|
$
|
24,887,006
|
$
|
13,266,629
|
||||
|
LIABILITIES AND SHAREHOLDERS' EQUITY
|
||||||||
|
Current liabilities:
|
||||||||
|
Accounts payable
|
$
|
784,777
|
$
|
604,913
|
||||
|
Other payable and accrued liabilities
|
758,907
|
192,298
|
||||||
|
Income tax payable
|
1,216,841
|
699,637
|
||||||
|
Current portion of long-term borrowing
|
785,456
|
905,975
|
||||||
|
Advance from customers
|
-
|
3,176
|
||||||
|
Total current liabilities
|
3,545,981
|
2,405,999
|
||||||
|
Convertible notes payable
|
4,488,881
|
-
|
||||||
|
Note payable - related party
|
13,722
|
-
|
||||||
|
Advance from government grant
|
355,754
|
73,497
|
||||||
|
Long term borrowing
|
628,365
|
452,987
|
||||||
|
Total liabilities
|
9,032,703
|
2,932,483
|
||||||
|
Commitments and contingencies
|
||||||||
|
Stockholders' equity:
|
||||||||
|
Common stock, $0.001 par value, 50,000,000 shares
|
||||||||
|
authorized, 13,324,083 and 10,758,000 issued and
|
||||||||
|
outstanding as of December 31, 2011 and 2010,
|
||||||||
|
respectively
|
13,324
|
10,758
|
||||||
|
Additional paid-in capital
|
12,220,181
|
1,417,098
|
||||||
|
Retained earnings
|
2,695,983
|
5,832,077
|
||||||
|
Statutory reserve
|
373,406
|
373,406
|
||||||
|
Accumulated other comprehensive income
|
551,409
|
530,070
|
||||||
|
Total stockholders' equity
|
15,854,303
|
8,163,409
|
||||||
|
Non-controlling interest in subsidiary
|
-
|
2,170,737
|
||||||
|
Total equity
|
15,854,303
|
10,334,146
|
||||||
|
Total liabilities and stockholders' equity
|
$
|
24,887,006
|
$
|
13,266,629
|
||||
The accompanying notes are an integral part to these consolidated financial statements
TANKE BIOSCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF OPERATIONS AND COMPREHENSIVE INCOME
|
Year Ended
|
||||||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Net sales
|
$
|
23,832,727
|
$
|
20,097,784
|
||||
|
Costs of sales
|
(15,062,519
|
)
|
(12,697,326
|
)
|
||||
|
Gross profit
|
8,770,208
|
7,400,458
|
||||||
|
Selling expenses
|
(2,463,901
|
)
|
(1,885,845
|
)
|
||||
|
Administrative expenses
|
(4,486,490
|
)
|
(834,761
|
)
|
||||
|
Depreciation and amortization
|
(81,004
|
)
|
(47,159
|
)
|
||||
|
Other operating expenses
|
-
|
(258,584
|
)
|
|||||
|
Income from operations
|
1,738,813
|
4,374,109
|
||||||
|
Other income/expense
|
||||||||
|
Interest income
|
95,834
|
4,828
|
||||||
|
Interest expense
|
(1,361,703
|
)
|
(100,265
|
)
|
||||
|
Amortization of discount on notes
|
(2,467,511
|
)
|
-
|
|||||
|
Registration rights agreement expense
|
(460,206
|
)
|
-
|
|||||
|
Foreign exchange losses, net
|
-
|
(1,899
|
)
|
|||||
|
Income (loss) before income taxes
|
(2,454,773
|
)
|
4,276,773
|
|||||
|
Income tax expense
|
(681,321
|
)
|
(582,493
|
)
|
||||
|
Net income (loss)
|
$
|
(3,136,094
|
)
|
$
|
3,694,280
|
|||
|
Non-controlling interest in earning of subsidiaries
|
-
|
(956,025
|
)
|
|||||
|
Net (loss) income available to shareholders
|
$
|
(3,136,094
|
)
|
$
|
2,738,255
|
|||
|
Other comprehensive income, net of tax:
|
||||||||
|
Effects of foreign currency conversion
|
56,629
|
203,605
|
||||||
|
Translation attributable to non-controlling interest
|
-
|
(39,125
|
)
|
|||||
|
Comprehensive income (loss)
|
$
|
(3,079,465
|
)
|
$
|
2,941,860
|
|||
|
Net income (loss) available to common shareholders per share:
|
||||||||
|
Basic
|
$
|
(0.24
|
)
|
$
|
0.34
|
|||
|
Diluted
|
$
|
(0.24
|
)
|
$
|
0.34
|
|||
|
Weighted average shares outstanding:
|
||||||||
|
Basic
|
13,083,333
|
10,758,000
|
||||||
|
Diluted
|
13,083,333
|
10,758,000
|
||||||
The accompanying notes are an integral part of these consolidated financial statements
TANKE BIOSCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
|
Additional
|
Accumulated Other
|
Non-
|
||||||||||||||||||||||||||||||
|
Common Stock
|
Paid-in |
Retained
|
Statuory
|
Comprehensive
|
Controlling
|
Total
|
||||||||||||||||||||||||||
|
Shares
|
Amount
|
capital
|
Earnings
|
Reserve
|
Income
|
Interest
|
Equity
|
|||||||||||||||||||||||||
|
Balance at December 31, 2008, restated in terms of the Share
|
||||||||||||||||||||||||||||||||
|
Exchange Agreement
|
10,758,000
|
$
|
10,758
|
$
|
1,343,868
|
$
|
1,101,492
|
$
|
373,406
|
$
|
361,595
|
$
|
583,165
|
$
|
3,774,284
|
|||||||||||||||||
|
Increase in paid-in capital
|
73,230
|
73,230
|
||||||||||||||||||||||||||||||
|
Net income
|
1,992,330
|
591,671
|
2,584,001
|
|||||||||||||||||||||||||||||
|
Foreign currency translation
|
3,993
|
751
|
4,744
|
|||||||||||||||||||||||||||||
|
Balance at December 31, 2009, restated in terms of the Share
|
||||||||||||||||||||||||||||||||
|
Exchange Agreement
|
10,758,000
|
10,758
|
1,417,098
|
3,093,822
|
373,406
|
365,588
|
1,175,587
|
6,436,259
|
||||||||||||||||||||||||
|
Net income
|
2,738,255
|
956,025
|
3,694,280
|
|||||||||||||||||||||||||||||
|
Foreign currency translation
|
164,482
|
39,125
|
203,607
|
|||||||||||||||||||||||||||||
|
Balance at December 31, 2010, restated in terms of the Share
|
||||||||||||||||||||||||||||||||
|
Exchange Agreement
|
10,758,000
|
10,758
|
1,417,098
|
5,832,077
|
373,406
|
530,070
|
2,170,737
|
10,334,146
|
||||||||||||||||||||||||
|
Effect of VIE Agreement with China Flying
|
2,133,917
|
(35,290
|
)
|
(2,170,737
|
)
|
(72,110
|
)
|
|||||||||||||||||||||||||
|
Effect of Share Exchange Agreement
|
399,180
|
399
|
54,200
|
54,599
|
||||||||||||||||||||||||||||
|
Effect of Private Placement
|
6,125,195
|
6,125,195
|
||||||||||||||||||||||||||||||
|
Shares issued for consulting services
|
2,166,903
|
2,167
|
2,489,771
|
2,491,938
|
||||||||||||||||||||||||||||
|
Net loss
|
(3,136,094
|
)
|
(3,136,094
|
)
|
||||||||||||||||||||||||||||
|
Foreign currency translation
|
56,629
|
56,629
|
||||||||||||||||||||||||||||||
|
Balance at December 31, 2011
|
13,324,083
|
$
|
13,324
|
$
|
12,220,181
|
$
|
2,695,983
|
$
|
373,406
|
$
|
551,409
|
-
|
$
|
15,854,303
|
||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
TANKE BIOSCIENCES CORPORATION
CONSOLIDATED STATEMENTS OF CASH FLOWS
|
Year Ended
|
||||||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Operating activities:
|
||||||||
|
Net income (loss)
|
$
|
(3,136,094
|
)
|
$
|
3,694,280
|
|||
|
Adjustments to reconcile net income to net cash
|
||||||||
|
used in operating activities:
|
||||||||
|
Depreciation and amortization
|
400,013
|
136,140
|
||||||
|
Common stock issued for services
|
2,491,938
|
-
|
||||||
|
Amortization of discount on convertible notes payable
|
2,467,511
|
-
|
||||||
|
Amortization of offering asset
|
709,409
|
-
|
||||||
|
Provision for bad debt
|
90,185
|
-
|
||||||
|
Inventory provision
|
41,585
|
-
|
||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Accounts receivable
|
(169,664
|
)
|
(239,613
|
)
|
||||
|
Inventories
|
173,356
|
(333,943
|
)
|
|||||
|
Other current assets
|
(3,380,518
|
)
|
139,307
|
|||||
|
Other assets
|
(61,660
|
)
|
-
|
|||||
|
Government grant
|
274,171
|
(81,032
|
)
|
|||||
|
Due from (to) related parties
|
2,584,892
|
-
|
||||||
|
Accounts payable
|
153,420
|
555,220
|
||||||
|
Other payables and accrued liabilities
|
457,109
|
(161,118
|
)
|
|||||
|
Income tax payable
|
478,810
|
401,961
|
||||||
|
Deferred tax asset
|
(26,946
|
)
|
17,887
|
|||||
|
Investment in unconsolidated entities
|
-
|
252,772
|
||||||
|
Advance from customer
|
(3,238
|
)
|
3,127
|
|||||
|
Net cash provided by operating activities
|
3,544,279
|
4,384,988
|
||||||
|
Investing activities:
|
||||||||
|
Increase in other receivables
|
(2,404,598
|
)
|
-
|
|||||
|
Change in restricted cash
|
-
|
148,689
|
||||||
|
Purchase of property and equipment
|
(701,022
|
)
|
(76,071
|
)
|
||||
|
Increase in construction in progress
|
-
|
(2,894,440
|
)
|
|||||
|
Purchase of intangible assets
|
(529,938
|
)
|
-
|
|||||
|
Increase in cash due to acquisition of China Flying
|
76,075
|
-
|
||||||
|
Net cash provided by investing activities
|
(3,559,483
|
)
|
(2,821,822
|
)
|
||||
|
Financing activities:
|
||||||||
|
Due from (to) realted parties
|
-
|
(328,605
|
)
|
|||||
|
Change in restricted cash
|
(706,802
|
)
|
-
|
|||||
|
Net proceeds from issue of convertible notes
|
6,522,563
|
-
|
||||||
|
Decrease in bank borrowings
|
-
|
(892,134
|
)
|
|||||
|
Net cash provided by financing activities
|
5,815,761
|
(1,220,739
|
)
|
|||||
|
Effect of foreign currency translation
|
(322,427
|
)
|
61,723
|
|||||
|
Net increase in cash
|
5,478,130
|
404,150
|
||||||
|
Cash, beginning of period
|
2,222,025
|
1,817,875
|
||||||
|
Cash, end of period
|
$
|
7,700,155
|
$
|
2,222,025
|
||||
|
Supplemental disclosures of cash flow information:
|
||||||||
|
Cash paid for interest
|
$
|
652,294
|
$
|
100,265
|
||||
|
Cash paid for income taxes
|
$
|
249,916
|
$
|
184,989
|
||||
The accompaning notes are an integral part of these consolidated financial statements
TANKE BIOSCIENCES CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
|
1.
|
ORGANIZATION AND PRINCIPAL ACTIVITIES
|
In these consolidated financial statements, unless the context requires otherwise, the terms “we”, “our”, “us” and the “Company” refer to Tanke Biosciences Corporation, a Nevada corporation formerly known as Greyhound Commissary, Inc. (“Greyhound”), as well as our direct and indirect subsidiaries, and our principal operating business, Guangzhou Tanke Industry Co., Ltd. (“Guangzhou Tanke”), a company organized under the laws of the People’s Republic of China (“China” or the “PRC”), which we control via a series of variable interest entity contractual agreements (the “VIE Agreements”) more fully described below.
We conduct our business through our subsidiaries, principally our wholly-owned subsidiary China Flying Development Limited (“China Flying”), a Hong Kong incorporated company, and its wholly-owned subsidiary Guangzhou Kanghui Agricultural Technology Co., Ltd. (“Kanghui Agricultural” or the “WFOE”), a wholly foreign owned enterprise incorporated as a limited liability company under the laws of the PRC. The Company operates and controls Guangzhou Tanke through Kanghui Agricultural and China Flying and in connection with the VIE Agreements.
On January 3, 2011, Guangzhou Tanke entered into a series of agreements with Kanghui Agricultural, pursuant to which Kanghui Agricultural effectively assumed management of the business activities of Guangzhou Tanke. Kanghui Agricultural is entitled to 100% of the net income of Guangzhou Tanke and is able to direct Guangzhou Tanke’s actions.
Also on January 3, 2011, our board of directors unanimously approved a resolution to enter into a Share Exchange Agreement with China Flying, and Golden Genesis Limited, a British Virgin Islands company ("Golden Genesis"), the sole stockholder of China Flying. Under the terms of the Share Exchange, Golden Genesis exchanged 100% of its capital stock in China Flying for 10,758,000 shares of authorized, but previously unissued Greyhound common stock, post-split as described below. Also, at the closing, we issued an aggregate of 2,166,903 shares (post split) of our authorized, but previously unissued common stock to a U.S. advisor. Following the closing of the agreement on February 9, 2011, China Flying became our wholly owned subsidiary.
Our board of directors further approved unanimously on January 3, 2011, a one share for 8.512 shares reverse split of our issued and outstanding common stock. The effective date of the split was established by our board on a date prior to the closing of the acquisition of China Flying.
The acquisition of China Flying was contingent upon the completion of our planned private placement in which we sold 6,669,627 units (the “Units”), with net proceeds of $6,522,563. Each Unit consisted of a $1.15 principal amount convertible note and a three year warrant to purchase one share of Greyhound common stock. On February 9, 2011, the Company entered into a Securities Purchase Agreement with individual investors relating to the private placement and completed the private placement transaction (see Note 9 below). The proceeds from such sale will be used to finance the operations and growth of Guangzhou Tanke.
At the time of the Share Exchange Agreement, Greyhound had 3,397,787 shares of common stock issued and outstanding. Following the reverse split, but prior to the issuance of shares pursuant to the acquisition of China Flying, the outstanding shares were reduced to 399,180 shares. Split shares issued in connection with the reverse stock split were fully paid and non-assessable. The number of stockholders will remain unchanged as a result of the reverse split. The par value of our common stock remained unchanged.
As management of Guangzhou Tanke obtained control of the Company, the Share Exchange was treated as a reverse merger. Accordingly, for accounting purposes Guangzhou Tanke was the acquirer so historical financial information presented herewith is that of Guangzhou Tanke. Consequently, there was no step-up in the basis of the assets of Guangzhou Tanke, as Guangzhou Tanke was the acquirer for accounting purposes.
Pursuant to the VIE Agreements, Kanghui Agricultural has the right to advise, consult, manage and operate Guangzhou Tanke for a quarterly fee equal to Guangzhou Tanke’s net income. Additionally, the Tanke Shareholders pledged their rights, titles and equity interest in Guangzhou Tanke as security for Kanghui Agricultural to collect consulting and services fees provided to Guangzhou Tanke through an Equity Pledge Agreement. In order to further reinforce Kanghui Agricultural’s rights to control and operate Guangzhou Tanke, the Tanke Shareholders granted Kanghui Agricultural an exclusive right and option to acquire all of their equity interests in Guangzhou Tanke through an Option Agreement. Neither Tanke Biosciences nor Kangui Agricultural own the assets or are responsible for the liabilities of Guangzhou Tanke.
The VIE Agreements were necessary because without them, the shareholders of Tanke Biosciences would not have control of Guangzhou Tanke. With these in place, however, Guangzhou Tanke is contractually equivalent to a subsidiary of Tanke Biosciences.
Guangzhou Tanke has historically self financed, and has been a profitable enterprise. However, on February 9, 2011, Tanke Biosciences sold convertible notes payable (see Note 9 below) with net proceeds of $6,522,563. Such proceeds will be used to finance the operations and growth of Guangzhou Tanke.
As Tanke Biosciences has complete control over Guangzhou Tanke, all assets presented on the balance sheet of Tanke Biosciences are available to settle obligations of Tanke Biosciences. Furthermore, there are no liabilities on the balance sheets of Guangzhou Tanke that do not have recourse against the assets of Tanke Biosciences. As Guangzhou Tanke is our sole operating entity, nearly all operating assets and liabilities are those of Guangzhou Tanke.
“RMB” and “Renminbi” refer to the legal currency of China and “$”, “US dollar” and “US$” refer to the legal currency of the United States.
Overview of Our Business
Through Guangzhou Tanke, our principal operating business, we are one of the leading animal nutrition and innovative feed additive providers in China. Our products are distinguished from traditional artificial feed additives in that they are environmentally-friendly and are designed to optimize the growth and health of livestock such as pigs and cattle, as well as farmed fish.
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
(a) Basis of Preparation
The Company’s consolidated financial statements have been stated in US dollars and prepared in accordance with generally accepted accounting principles in the United States of America ("US GAAP") and have been consistently applied.
Restatement of 2010 financial statements
As stated in Note1, as management of Guangzhou Tanke Co. obtained control of Tanke Biosciences Corporation, the Share Exchange was treated as a reverse merger, and accordingly, for accounting purposes Guangzhou Tanke was the accounting acquirer therefore. Consequently, per ASC 805-40-45-2, retroactive adjustments have to be made to reflect the legal capital of the legal acquirer( accounting acquiree). As a result, the Company’s contributed capital of $ 1,427,856 has been restated as common stock of $10,758 and additional paid-in capital $1,417,098. The restatements have been reflected in the balance sheet and statement of stockholders’ equity.
Previously the Company included $373,406 related to statutory reserves in retained earnings, the Company now separately presents the statutory reserves from the retained earnings. Such restatement is reflected in the balance sheet and statement of stockholders’ equity.
(b) Basis of consolidation
These consolidated financial statements include the financial statements of the Company, its subsidiaries and its VIE-Guangzhou Tanke (the “Group''). All significant inter-company balances and transactions within the Group have been eliminated.
(c) Use of Estimates
In preparing consolidated financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of the amount due from related parties, the net realizable value of inventories, the estimation of useful lives of property and equipment and intangible assets, allowance of bad debt and the value of warrants. Actual results could differ from those estimates.
(d) Concentrations of Credit Risk
Financial instruments that potentially expose the Company to concentrations of credit risk consist primarily of cash and cash equivalents, restricted cash, accounts receivable and amounts due from related parties. The Company places its cash with financial institutions with high-credit ratings and quality. The Company maintains bank accounts in the PRC only. In addition, the Company conducts periodic reviews of the related party financial conditions and payment practices.
Approximately 99% of the Company’s revenue is generated from buyers in mainland China.
(e) Concentrations of Suppliers
|
All the Company’s suppliers are located in mainland China.
|
|
(f) Cash and Cash Equivalents
|
|
The Company considers all highly liquid investments with initial maturities of three months or less to be cash equivalents. The Company maintains certain cash accounts in individuals’ names. The accounts are used solely and exclusively to facilitate certain corporate transactions that cannot be consummated through the use of corporate accounts, due to PRC banking regulations.
|
|
(g) Restricted Cash
|
|
Deposits that are restricted in use are classified as restricted cash.
|
|
(h) Trade Receivables
|
|
The Company periodically assesses its accounts receivable for collectability on a specific identification basis. If collectability of an account becomes unlikely, an allowance is recorded for that doubtful account. Once collection efforts have been exhausted, the account receivable is written off against the allowance. The Company does not require collateral for trade or other accounts receivable.
|
|
(i) Inventories
|
|
Inventories are stated at the lower of cost or net realizable value. Cost is determined using the weighted average method. The cost of inventories includes the purchase cost and other costs incurred in bringing the inventories to their present location and condition. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and the estimated costs necessary to make the sale.
|
|
(j) Property and Equipment
|
|
Property and equipment are stated at cost less accumulated depreciation. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use.
|
|
Depreciation of property and equipment is calculated using the straight-line method over their estimated useful lives. The estimated useful lives are as follows:
|
|
Buildings
|
15-20 years
|
|
Plant and machinery
|
3-20 years
|
|
Motor vehicle
|
10 years
|
|
Office equipment
|
3-10 years
|
|
Expenditures for additions, major renewals and betterments are capitalized and expenditures for maintenance and repairs are charged to expense as incurred.
|
|
(k) Intangible Asset
|
|
The intangible asset consists of two land use rights, which are recorded at cost less accumulated amortization. Amortization is provided over the term of the land use right agreements on a straight-line basis.
|
|
(l) Impairment of Long-lived Assets
|
|
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be recoverable. The Company recognizes impairment of long-lived assets in the event that the net book values of such assets exceed the future undiscounted cash flows attributable to such assets.
|
(m) Statutory Reserves
In accordance with the relevant laws and regulations of the PRC and the articles of associations of the Company, Guangzhou Tanke is required to allocate 10% of their net income reported in the PRC statutory accounts, after offsetting any prior years’ losses, to statutory reserve, on an annual basis. When the balance of such reserve reaches 50% of the respective registered capital of the subsidiaries, any further allocation is optional.
As of December 31, 2011 and 2010, the statutory reserves of the subsidiary already reached 50% of the registered capital of the subsidiary and the Company did not have any further allocation on it.
The statutory surplus reserves can be used to offset prior years’ losses, if any, and may be converted into registered capital, provided that the remaining balances of the reserve after such conversion is not less than 25% of registered capital. The statutory surplus reserve is non-distributable.
|
(n) Revenue Recognition
|
|
The Company recognizes revenue in accordance with Accounting Standards Codification (“ASC”) 605-10, Revenue Recognition, and SEC Staff Accounting Bulletin No. 104. Pursuant to these pronouncements, revenue is recognized when all of the following criteria are met:
|
|
- Persuasive evidence of an arrangement exists;
|
|
- Delivery has occurred or services have been rendered;
|
|
- The seller's price to the buyer is fixed or determinable; and
|
|
- Collectability is reasonably assured.
|
|
The Company’s revenue is generated through the wholesale and retail sale of livestock feed additives including organic trace mineral additives, functional regulation additives, herbal medicinal additives and raw materials. Before the Company recognizes revenue on these product sales, written purchase orders and contracts are received in advance of all shipments of goods to customers. For sales within the Company’s own province, delivery is made by Company employees. Such delivery occurs on the same day as shipment. For delivery outside the province, shipment is made through a separate logistics company that assumes the risk of loss. Revenue is recognized upon shipment of goods to the customers. The Company typically does not incur bad debt losses because this type of loss is deducted from the salesperson’s compensation, thereby mitigating the loss to the Company. Therefore, collectability is reasonably assured.
|
|
Revenue is presented net of sales returns, which are not significant. However, the Company continually performs analyses of returns and records a provision at the time of sale if necessary. As of December 31, 2011 and 2010, it was determined that potential returns and allowances were not material so the Company did not record a provision for returns. The Company revisits this estimate regularly and adjusts it if conditions change.
|
|
(o) Cost of Goods Sold
|
|
Cost of revenue consists primarily of material cost, labor cost, overhead associated with the manufacturing process and directly related expenses.
|
|
(p) Research and Development Costs
|
Research and development costs are charged to expense as incurred and are included in operating expenses. As of December 31, 2011 and 2010, the Company incurred research and development costs amounted to $246,038 and $91,397, respectively.
|
(q) Value Added Tax
|
|
In accordance with the relevant tax laws in the PRC, VAT is levied on the invoiced value of sales and is payable by the purchaser. The Company is required to remit the VAT it collects to the tax authority, but may deduct the VAT it has paid on eligible purchases. The difference between the amounts collected and paid is presented as VAT recoverable or payable balance on the balance sheet.
(r) Income Taxes
|
|
The Company uses the asset and liability method of accounting for income taxes pursuant to ASC 740, “IIncome Tax”. Under the asset and liability method of ASC 740, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The Company is subject to value added tax (“VAT”) and corporate income taxes. In connection with these taxes, the Company is audited every year by the various taxing authorities. The Company is subject to the normal annual audit for the year ended December 31, 2011. In the event that fraud or impropriety is found, the taxing authorities can also go back five years to audit the Company’s tax compliance.
|
|
(s) Comprehensive Income
|
|
Comprehensive income is defined to include all changes in equity except those resulting from net income or loss, investments by owners and distributions to owners. The Company’s only component of other comprehensive income is the foreign currency translation adjustment.
|
(t) Earnings per share (EPS)
Earnings per share is calculated in accordance with ASC 260-10 which requires the Company to calculate net income (loss) per share based on basic and diluted net income (loss) per share, as defined. Basic EPS excludes dilution and is computed by dividing net income (loss) by the weighted average number of shares outstanding for the period. Diluted EPS reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock.
(u) Commitments and Contingencies
Liabilities for loss contingencies arising from claims, assessments, litigation, fines, penalties and other sources are recorded when it is probable that a liability has been incurred and the amount of the assessment can be reasonably estimated.
(v) Foreign Currency Translation
The Company, its subsidiaries and VIE maintain financial statements in the functional currency of each entity. Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchange rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
The financial statements of each entity are prepared using the functional currency, and have been translated into United States dollars (“US$” or “$”). Assets and liabilities are translated at the exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates for the period. Stockholders’ equity is translated at historical exchange rates. Any translation adjustments are included as a foreign exchange adjustment in other comprehensive income, a component of stockholders’ equity.
RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US$ at the rates used in translation.
(w) Financial Instruments
The carrying amounts of cash and cash equivalents, restricted cash, accounts receivable, due to/from related parties, notes payable, other payable and accrued liabilities and income tax payable approximate their fair values due to the short-term nature of these items. The carrying amounts of long-term borrowings approximate the fair value based on the Company’s expected borrowing rate for debt with similar remaining maturities and comparable risk.
Convertible notes are not carried at fair value due to the discounts for warrants and the beneficial conversion feature. As the interest on these notes approximates market interest, the fair value is their face value of $7,670,071.
It is management’s opinion that the Company is not exposed to significant interest, price or credit risks arising from these financial instruments.
(x) Consolidation of Variable Interest Entities
According to the requirements of Statement of Financial Accounting Standards No. 810-10, “Variable interest Entities”, the Company has evaluated the economic relationships of its wholly owned subsidiary, China Flying and its wholly-owned subsidiary Kanghui Agricultural with Guangzhou Tanke and has determined that it is required to consolidate China Flying, Kanghui Agricultural and Guangzhou Tanke pursuant to the rules of FASB ASC Topic 810-10. Therefore Guangzhou Tanke is considered to be a VIE, as defined by FASB ASC Topic 810-10 , of which China Flying is the primary beneficiary as a result of its wholly owned subsidiary Kanghui Agricultural. China Flying, as mentioned above, will absorb a majority of the economic risks and rewards of all of these VIE that are being consolidated in the accompanying financial statements.
The carrying amount of the VIEs’ assets and liabilities are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Current assets and Long term notes receivables
|
$
|
13,929,777
|
$
|
8,647,731
|
||||
|
Property, plant and equipment
|
4,801,723
|
4,332,006
|
||||||
|
Intangible assets
|
837,525
|
286,892
|
||||||
|
Total assets
|
19,569,025
|
13,266,629
|
||||||
|
Total liabilities
|
(1,572,020
|
)
|
(2,932,483
|
)
|
||||
|
Net assets
|
$
|
17,997,005
|
$
|
10,334,146
|
||||
(y) Recent Accounting Updates
In June 2011, the FASB issued ASC Topic 220 “Comprehensive Income” that amends the presentation of comprehensive income in the financial statements by requiring an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. The update also eliminates the option to present the components of other comprehensive income as part of the statement of equity. The guidance is effective for interim and annual reporting periods beginning on or after December 15, 2011, with early adoption permitted. The adoption of this guidance will not have a material effect on the Company’s financial condition, results of operations or cash flows.
In May 2011, the FASB issued ASU 2011-04, Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS. ASU 2011-04 amends ASC Topic 820 to require additional disclosures regarding fair value measurements. One of the areas concerned is to add disclosures about the sensitivity of fair value measurements categorized within Level 3 of the fair value hierarchy, which require the most judgment in determining fair value. The provisions of ASU 2011-04 will be effective for years beginning after December 15, 2011 for both public and nonpublic entities. Public entities will begin adoption in the first interim period beginning after December 15, 2011. Early adoption is not permitted, The Company is currently evaluating the impact of this standard upon its adoption on the Company’s consolidated financial statements.
3. RESTRICTED CASH
As of December 31, 2011 and 2010, the Company has restricted cash amounts to $706, 802 and $0, respectively.
On February 9, 2011, in connection with the Private Placement, the Company entered into an Escrow Agreement (the “Interest Escrow Agreement”) with the Lead Placement Agent and the Escrow Agent, as escrow agent. Pursuant to the terms of the Interest Escrow Agreement, the Company deposited into escrow an amount of proceeds of the Private Placement equal to one semi-annual interest payment on the convertible notes to secure prompt interest payments under the notes amounted to $306,802. When the notes mature or are converted into stock, the cash in this account will be released from restriction.
The Company also sets aside restricted fund amounted to $400,000 for investor relations expenditure in another escrow account.
4. INVENTORIES
Inventories consisted of the following:
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Raw materials
|
$
|
538,465
|
$
|
653,212
|
||||
|
Finished goods
|
360,873
|
182,631
|
||||||
|
Work in pogress
|
242,748
|
472,060
|
||||||
|
Packaging material
|
45,810
|
46,379
|
||||||
|
$
|
1,187,895
|
$
|
1,354,282
|
|||||
As of December 31, 2011 and 2010, the Company’s provision for slow-moving and obsolete inventories amounted to $41,585 and $0, respectively.
5. ACCOUNTS RECEIVABLE
Accounts receivable consisted of the following:
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Account receivables
|
$
|
2,150,981
|
$
|
1,911,065
|
||||
|
Less: Allowance for doubtful accounts
|
(233,282
|
)
|
(143,097
|
)
|
||||
|
$
|
1,917,699
|
$
|
1,767,968
|
|||||
As of December 31, 2011 and 2010, the Company’s allowance for doubtful accounts amounted to $233,282 and $143,097, respectively.
6. NOTES RECEIVABLES – RELATED PARTIES
Notes receivables – related parties consisted of the following.
|
Current portion
|
December 31,
|
December 31,
|
||||||
|
2011
|
2010
|
|||||||
|
Advance to director
|
$
|
239,149
|
$
|
2,033,622
|
||||
|
Others
|
327
|
-
|
||||||
|
$
|
239,476
|
$
|
2,033,622
|
|||||
|
Non-current portion
|
December 31,
|
December 31,
|
||||||
|
2011
|
2010
|
|||||||
|
Advance to director
|
$
|
-
|
$
|
974,532
|
||||
|
$
|
-
|
$
|
974,532
|
|||||
Advance to directors represents advance payment made to directors for business development activities and will normally be repaid within one year.
7. OTHER RECEIVABLES
Other receivables consisted of the following.
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Deposit and others
|
$
|
13,425
|
$
|
13,724
|
||||
|
Advance to staff
|
40,511
|
98,845
|
||||||
|
Loan to customers and suppliers
|
2,513,460
|
-
|
||||||
|
$
|
2,567,396
|
$
|
112,569
|
|||||
Loans to customers and suppliers represent 8% interest bearing advances to one of the Company’s customers and one supplier, both of which are effective in December 2011 and expected to be repaid within one year.
8. OTHER CURRENT ASSETS
Other current assets consisted of the following:
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Prepayment to suppliers
|
$
|
3,633,674
|
$
|
115,078
|
||||
|
Deferred expenses
|
-
|
49,768
|
||||||
|
Offering costs, net
|
914,594
|
-
|
||||||
|
$
|
4,548,268
|
$
|
164,846
|
|||||
Prepayment primarily represents advance payment to suppliers for purchase of raw material.
In connection with the private placement, the Company incurred $1,624,002 of closing costs. These costs have been reflected as other current assets and are being amortized using the interest method over the expected life of the related convertible notes payable. Amortization of these costs is recorded as interest expense. As of December 31, 2011, the remaining book value of these closing costs amounted to $914,594.
9. PROPERTY, PLANT AND EQUIPMENT AND CONSTRUCTION IN PROGRESS
Property, plant and equipment consisted of the following.
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Buildings and leasehold improvements
|
$
|
4,518,397
|
$
|
1,507,916
|
||||
|
Plant and equipment
|
1,024,245
|
613,287
|
||||||
|
Motor vehicles
|
166,884
|
52,908
|
||||||
|
Office equipment
|
164,907
|
83,599
|
||||||
|
Total property, plant and equipment
|
5,874,433
|
2,257,710
|
||||||
|
Accumulated depreciation
|
1,103,134
|
703,121
|
||||||
|
Property, plant and equipment, net
|
$
|
4,771,299
|
$
|
1,554,589
|
||||
|
Construction in progress
|
35,878
|
2,777,417
|
||||||
|
$
|
4,807,177
|
$
|
4,332,006
|
|||||
The Company has buildings on the site it occupies, including factory buildings. Due to the lack of a Land Use Right Certificate, the Company is unable to apply for the Property Ownership Certificate for the buildings. However, as the buildings are in use, the Company depreciates them over their expected useful lives. During the quarter ended June 30, 2011, the Company’s factory campus construction project was completed and the costs were moved from construction in process to the buildings account. Upon placement in service, the Company began depreciating them.
As of December 31, 2011 and 2010, the Company recorded depreciation expense amounted to $365.996 and $136,140, respectively.
10. INTANGIBLE ASSET, NET
The intangible asset primarily represents two land use rights and consisted of the following.
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Deposit for land use right
|
$
|
835,985
|
$
|
286,892
|
||||
|
Other
|
2,104
|
-
|
||||||
|
$
|
838,089
|
$
|
286,892
|
|||||
On November 21, 2003, the Company applied to the Government of Huaqiao Town, Huadu District, Guangzhou, for the land use right of No. 2 Industry Area of Huaqiao Town (i.e., Laohutou Lot, Wangongtang) covering an area of around 430,000 square feet. The Company has paid $259,774 as consideration for the land use right.
On October 22, 2010, the Company applied to the Administration Committee of Qingyuan Huaqiao Industrial District for the land use right covering an area of around 60 acres. The Company has paid $576,211 as consideration for the land use right and started the construction project during the year.
As of December 31, 2011 and 2010, the Company recorded amortization expense amounted to $34,017 and $0, respectively.
11. OTHER NON-CURRENT ASSETS
Other non-current assets consisted of the following.
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Staff loans
|
$
|
265,167
|
$
|
-
|
||||
|
Others
|
62,836
|
-
|
||||||
|
$
|
328,003
|
$
|
-
|
|||||
Staff loans are made to employees under terms that call for repayment of the amounts within two years.
12. OTHER PAYABLE AND ACCRUED LIABILITIES
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Other payables
|
$
|
67,751
|
$
|
911
|
||||
|
Staff welfare payable
|
68,057
|
96,417
|
||||||
|
Accrued payroll
|
46,294
|
|||||||
|
Value added tax payable
|
93,140
|
50,230
|
||||||
|
Registration rights penalties
|
460,206
|
-
|
||||||
|
Other tax payable
|
23,459
|
44,740
|
||||||
|
$
|
758,907
|
$
|
192,298
|
|||||
Other payables represent registration rights penalties associated with the registration right agreement, loans from third parties, which are interest free, unsecured and repayable on demand.
13. CONVERTIBLE NOTES PAYABLE
On February 9, 2011, the Company entered into a Securities Purchase Agreement with individual investors relating to a private placement transaction by the Company (the “Private Placement”) of 6,669,627 units. Each unit consisted of a $1.15 principal amount 8% Senior Convertible Note (the “Notes”) and a Common Stock Purchase Warrant (the “Warrants”) to purchase one share of the Company’s common stock at an exercise price of $1.40 per share.
As a result of the Private Placement, the Company offered and sold $7,670,071 worth of Notes convertible into 6,669,627 shares of common stock. The Notes are payable 24 months from February 9, 2011 with an interest rate of 8% per annum payable semiannually in arrears. The Company placed in escrow an amount of the proceeds of the Private Placement equal to one semi-annual interest payment on the Notes to secure prompt interest payments. Until such time as 75% of the Notes are converted into shares of Common Stock, if such escrow is depleted in order to make interest payments, the Company will replenish such escrow amount. At the option of the holder, the Notes may be converted into Common Stock at a price of $1.15 per share, which is subject to customary weighted average and stock based anti-dilution protection. The issuance of the Notes was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D.
The Notes contain customary events of default and affirmative and negative covenants of the Company, including negative covenants which restrict the Company’s ability to do the following (among other things) without the consent of the investors: (i) incur, or permit to exist, any indebtedness for borrowed money in excess of (A) US$3,000,000 during the twelve (12) month period beginning on February 9, 2011, or (B) US$5,000,000 during the two-year period beginning on February 9, 2011 and ending on February 9, 2013 (the maturity date of the Notes), except in the ordinary course of the Company’s business; (ii) lend or advance money, credit or property to or invest in (by capital contribution, loan, purchase or otherwise) any person or entity in excess of US$1,000,000 except: (A) investments in United States Government obligations, certificates of deposit of any banking institution with combined capital and surplus of at least $200,000,000; (B) accounts receivable arising out of sales in the ordinary course of business; and (C) inter-company loans between and among the Company and its subsidiaries; (iii) pay dividends or make any other distribution on shares of the capital stock of the Company; (iv) create, assume or permit to exist, any lien on any of the Company’s property or assets now owned or hereafter acquired, subject to existing liens and certain exceptions; (v) assume guarantees, subject to certain exceptions; (vi) engage in “sale-leaseback” transactions, subject to certain exceptions; (vii) make capital expenditures in excess of US$5,000,000 in any fiscal year, subject to certain exceptions; and (viii) materially alter the Company’s business.
In connection with the issuance of the Notes, we entered into a Registration Rights Agreement (the “Registration Rights Agreement”) with the investors which sets forth the rights of the investors to have the shares of common stock underlying the Notes and Warrants registered with the SEC for public resale. Pursuant to the Registration Rights Agreement, we agreed to file, no later than April 11, 2011, a registration statement to register the shares underlying the Notes and the Warrants and to have such registration statement effective no later than September 18, 2011. If the registration statement is not effective by September 18, 2011 (the “Effectiveness Failure”) or if, after the effective date, sales of securities included in the registration statement cannot be made (including, without limitation, because of a failure to keep the registration statement effective, to disclose such information as is necessary for sales to be made pursuant to the registration statement, to register a sufficient number of shares of Common Stock or to maintain the listing of the Common Stock) (a “Maintenance Failure”) then, as liquidated damages (and in complete satisfaction and to the exclusion of any claims or remedies inuring to any holder of the securities) the Company is required to pay an amount in cash equal to 1% of the aggregate purchase price paid by the Investors on each of the following dates: (i) 20 days following the date of a Filing Failure; (ii) 30 days following the initial day of a Maintenance Failure; (iii) on every thirtieth day thereafter (pro-rated for periods totaling less than thirty days) until such failure is cured; (iv) on every thirtieth day after the day of an Effectiveness Failure and thereafter (pro rated for periods totaling less than thirty days) until such Effectiveness Failure is cured; (v) on every thirtieth day after the initial day of a Maintenance Failure and thereafter (pro rated for periods totaling less than thirty days) until such Maintenance Failure is cured. The payments to be made by the Company are limited to a maximum of 6% of the aggregate amount paid by the Investors ($460,204.29). As of June 30, 2011, the Company did not expect to incur any registration delay payments and has not accrued any such payments. The Company will continue to assess the likelihood of payments under this arrangement, and will recognize these estimates into earnings in the period in which they become likely in accordance with ASC 825-20, Registration Payment Arrangements.
As required by FASB ASC 820, assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to their fair value measurement. Our financial instruments that are measured at fair value on a recurring basis under FASB ASC 815 are all measured at fair value using Level 3 inputs. Level 3 inputs are unobservable inputs that are supported by little or no market activity and that are significant to the fair value of the assets or liabilities.
The warrants issued as a component of the units were valued using the Black-Scholes method using the following assumptions: (1) estimated life of warrants of 3 years, (2) annualized volatility of 100%, (3) fair value of stock as of grant date of $1.15, (4) exercise price of $1.40, (5) annual dividend rate of 0%, and (6) discount rate of 1.34%. Such calculation resulted in a warrant value of $4,470,536.
In accordance with ASC 470-20-25, the proceeds of the unit offering were allocated to the Notes and warrants based on their relative fair values on a weighted average basis, with the resulting allocated value of the warrants of $2,824,350 being classified to additional paid in capital. Such discount to the Notes is being amortized over their expected life.
The beneficial conversion feature associated with the issuance of the above Notes, amounted to $2,824,350, which has also been recorded as a discount to the convertible notes payable and is being amortized over the life of the Notes.
As of December 31, 2011, the book value of the Notes amounted to $4,488,881, which consisted of the aggregate face value of $7,670,071, less the remaining discount of $3,181,190.
14. INCOME TAX
The Company’s VIE, Guangzhou Tanke, is a “domestic enterprise” that is registered and operated in Guangzhou, the PRC.
The PRC's legislative body, the National People's Congress, adopted the unified Enterprise Income Tax ("EIT") Law on March 16, 2007. This new tax law replaces the existing separate income tax laws for domestic enterprises and foreign-invested enterprises and became effective on January 1, 2008. Under the new tax law, a unified income tax rate is set at 25% for both domestic enterprises and foreign-invested enterprises. However, there is a transition period for enterprises, whether foreign-invested or domestic, that are currently receiving preferential tax treatments granted by relevant tax authorities. Enterprises that are subject to an enterprise income tax rate lower than 25% may continue to enjoy the lower rate and will transit into the new rate over a five year period beginning on the effective date of the EIT Law. Enterprises that are currently entitled to exemptions for a fixed term may continue to enjoy such treatment until the exemption term expires.
Tanke Bio-Tech, a subsidiary of Guangzhou Tanke, is a joint venture with a foreign entity that received a full exemption from income taxes in 2007 and 2008 and half rate reduction for years 2009, 2010 and 2011 in accordance with the Law of the People's Republic of China on Income Tax of Enterprises with Foreign Investment and Foreign Enterprises and Notification of the State Council on Carrying out the Transitional Preferential Policies concerning Enterprise Income Tax (Guofa (2007) No. 39).
As of December 31, 2011 and 2010, the income tax payable for the Company amounted to $1,216,841 and $699,637, respectively.
A reconciliation of the provision for income taxes with the expected income tax computed by applying the US Federal statutory income tax rate to the income before the provision for income taxes is as follows.
|
Years Ended
|
||||||||
|
December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Income tax at US Federal statutory rate
|
$
|
(834,624
|
)
|
$
|
1,256,055
|
|||
|
Difference in Chinese rate versus US rate
|
(441,937
|
)
|
(116,460
|
)
|
||||
|
Tax holiday for Chinese subsidiary
|
(617,714
|
)
|
(557,102
|
)
|
||||
|
Difference in Hong Kong tax rate
|
204,827
|
-
|
||||||
|
Other
|
4,008
|
-
|
||||||
|
Change in valuation allowance
|
2,366,760
|
-
|
||||||
|
Total
|
$
|
681,321
|
$
|
582,493
|
||||
The Company uses the liability method, where deferred tax assets and liabilities are determined based on the expected future tax consequences of temporary differences between the carrying amounts of assets and liabilities for financial and income tax reporting purposes. As approved by the relevant tax authority in the PRC, income tax rates will be 25% for 2012 and thereafter.
The income tax provision consists of the following.
|
Year Ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Current provision
|
||||||||
|
PRC
|
$
|
709,476
|
$
|
582,493
|
||||
|
Deferred provision
|
(28,155
|
)
|
-
|
|||||
|
$
|
681,321
|
$
|
582,493
|
|||||
Significant components of the Company’s deferred tax asset are as follows.
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Allowance for doubtful accounts in China
|
$
|
46,042
|
$
|
17,887
|
||||
|
Net operating loss carryforward
|
1,873,030
|
-
|
||||||
|
Share based payment
|
493,730
|
-
|
||||||
|
Valuation allowance
|
(2,366,760
|
)
|
-
|
|||||
|
Net deferred tax asset
|
$
|
46,042
|
$
|
17,887
|
||||
The Company’s uncertain tax positions are related to tax years that remain subject to examination by relevant tax authorities and the major one is the China Tax Authority. The Company is audited every year by an agency of the Chinese tax authority and remains subject to the normal annual audit for the year ended December 31, 2011. In the event that fraud or impropriety is found, the taxing authorities can also go back five years to audit the Company’s tax compliance. As the Company is audited every year, there are no uncertain tax positions requiring accrual or disclosure in accordance with ASC 740-10, Income Taxes.
15. EARNINGS (LOSS) PER SHARE
Basic earnings (loss) per share is computed by dividing net income (loss) attributable to common shareholders by the weighted average number of common shares outstanding during the period. Diluted earnings (loss) per share reflects the potential dilution of securities by including other potential common stock, including convertible preferred stock, stock options and warrants, in the weighted average number of common shares outstanding for the period, if dilutive. There is no dilution factor occurred during 2011 and 2011, the basic EPS equals diluted EPS.
The numerators and denominators used in the computations of basic and dilutive earnings (loss) per share are presented in the following table:
|
For The Years Ended Dec. 31
|
||||||||
|
BASIC/DILUTED
|
2011
|
2010
|
||||||
|
Numerator for basic/diluted earnings (loss) per share attributable to the Company’s common stockholders
|
||||||||
|
Net income (loss) used in computing basic/diluted earnings per share
|
$
|
(3,136,094
|
)
|
$
|
3,694,280
|
|||
|
Basic/diluted weighted average shares outstanding
|
13,083,333
|
10,758,000
|
||||||
|
Basic/diluted earnings (loss) per share
|
$
|
(0.24
|
)
|
$
|
0.34
|
|||
16. LONG-TERM BORROWINGS
The details of the Company’s long-term borrowings are as follows:
|
December 31,
|
December 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
Bank loans bearing interest at 5.4% and 5.85% per annum, maturing on May 21, 2012 and January 30, 2013.
|
||||||||
|
The loans are uncollateralized other than restricted cash deposited at the bank.
|
$
|
1,413,821
|
$
|
1,358,962
|
||||
|
Less: Current portion
|
(785,456
|
)
|
(905,975
|
)
|
||||
|
$
|
628,365
|
$
|
452,987
|
|||||
The bank loans consist of $785,456 (RMB 5,000,000) and $628,365 (RMB 4,000,000), bearing interest at 5.4% and 5.85% per annum, maturing on May 21, 2012 and January 30, 2013, respectively.
17. GOVERNMENT GRANT
The government grant liability represents an advance from the Chinese government for research and development projects. The Company has recorded the grants received as a government grant liability, and ratably recognizes the amount as a reduction of research and development expense when the related research and development activities are performed. Government grant balances are $355,754 and $73,497 as at December 31, 2011 and 2010, respectively.
18. SEGMENT INFORMATION
Tanke operates in four segments: organic trace mineral additives, functional regulation additives, herbal medicinal additives and other revenues. Management oversees each of these operations separately.
Organic trace mineral additives constitute the largest and fastest growing area of our business. These are various minerals added to animal feed to provide a balanced diet. Functional feed additives are widely used to enhance the properties of other products, improve feed efficiency and stimulate the rapid maturation of the immune system. Chinese herbal feed additives utilize traditional Chinese medicine theory to improve an animal’s digestion and appetite and to regulate the yin and yang balance of an animal’s health. Other revenue consists of the reselling of raw materials.
Property, equipment and other assets are shared and not tracked separately by segment. Administrative expenses are also not tracked by segment. There are also no intersegment sales or purchases made by the Company. The following is a breakdown of revenue and costs of sales by segment.
|
Year Ended December 31,
|
||||||||
|
2011
|
2010
|
|||||||
|
Segment revenues
|
||||||||
|
Organic Trace Mineral Additives
|
$
|
18,855,958
|
$
|
15,197,415
|
||||
|
Functional Regulation Additives
|
4,163,823
|
3,388,111
|
||||||
|
Herbal Medicinal Additives
|
281,036
|
419,450
|
||||||
|
Other
|
531,910
|
1,092,808
|
||||||
|
$
|
23,832,727
|
$
|
20,097,784
|
|||||
|
Segment costs of sales
|
||||||||
|
Organic Trace Mineral Additives
|
$
|
11,907,294
|
$
|
9,291,424
|
||||
|
Functional Regulation Additives
|
2,571,920
|
2,098,021
|
||||||
|
Herbal Medicinal Additives
|
219,856
|
381,205
|
||||||
|
Other
|
363,449
|
926,676
|
||||||
|
$
|
15,062,519
|
$
|
12,697,326
|
|||||
|
Segment gross profit
|
||||||||
|
Organic Trace Mineral Additives
|
$
|
6,948,664
|
$
|
5,905,991
|
||||
|
Functional Regulation Additives
|
1,591,903
|
1,290,090
|
||||||
|
Herbal Medicinal Additives
|
61,180
|
38,245
|
||||||
|
Other
|
168,461
|
166,132
|
||||||
|
$
|
8,770,208
|
$
|
7,400,458
|
|||||
You should rely only on the information contained in this document. We have not authorized anyone to provide you with information that is different. This document may only be used where it is legal to sell these securities. The information in this document may only be accurate on the date of this document.
Additional risks and uncertainties not presently known or those are currently deemed immaterial may also impair our business operations. The risks and uncertainties described in this document and other risks and uncertainties which we may face in the future will have a greater impact on those who purchase our common stock. These purchasers will purchase our common stock at the market price or at a privately negotiated price and will run the risk of losing their entire investment
TANKE BIOSCIENCES CORPORATION
16,243,672 Shares
Common Stock____________________
PROSPECTUS
____________________
____, 2012
PART II
INFORMATION NOT REQUIRED IN THE PROSPECTUS
Other Expenses of Issuance and Distribution
The following table sets forth the costs and expenses payable by the Company in connection with the offer and sale of the securities being registered. All amounts are estimates except the SEC registration fee.
|
Nature of Expense
|
||||
|
SEC registration fee
|
$
|
2,331.84
|
||
|
Legal fees and expenses
|
$
|
60,000.00
|
||
|
Accounting fees and expenses
|
$
|
2,000.00
|
||
|
Total
|
$
|
64,331.84
|
||
Indemnification of Directors and Officers
The current Bylaws of the Company provides that the Board of Directors shall cause the Company to indemnify a current or former director, officer and Secretary of the Company, or a current or former director, officer and Company of a corporation of which the Company is or was a stockholder and the heirs and personal representative of any such person against all costs, charges and expenses to settle an action, judgment or proceeding to which they are made a party by reason of their position of director or officer of the Company.
The Company is permitted by the Bylaws to purchase and maintain insurance for any director, officer, employee or agent of the Company or as a director, officer, employee or agent of the Company of which the Company is or was a stockholder and his or her heirs or personal representatives against a liability incurred by him as a Director, officer, employee or agent.
Our company is incorporated under the laws of the State of Nevada. Section 78.7502 of the Nevada Revised Statutes (the “NRS”) provides that a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative, except an action by or in the right of the corporation, by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise, against expenses, including attorneys’ fees, judgments, fines and amounts paid in settlement actually and reasonably incurred by the person in connection with the action, suit or proceeding if the person acted in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and, with respect to any criminal action or proceeding, had no reasonable cause to believe his conduct was unlawful. The termination of any action, suit or proceeding by judgment, order, settlement, conviction or upon a plea of nolo contendere or its equivalent, does not, of itself, create a presumption that the person did not act in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation, and that, with respect to any criminal action or proceeding, the person had reasonable cause to believe that his conduct was unlawful.
Section 78.7502 of the NRS further provides a Nevada corporation may indemnify any person who was or is a party or is threatened to be made a party to any threatened, pending or completed action or suit by or in the right of the corporation to procure a judgment in its favor by reason of the fact that the person is or was a director, officer, employee or agent of the corporation, or is or was serving at the request of the corporation as a director, officer, employee or agent of another corporation, partnership, joint venture, trust or other enterprise against expenses, including amounts paid in settlement and attorneys' fees actually and reasonably incurred by him in connection with the defense or settlement of the action or suit if the person acted in good faith and in a manner which the person reasonably believed to be in or not opposed to the best interests of the corporation. Indemnification may not be made for any claim, issue or matter as to which such a person has been adjudged by a court of competent jurisdiction, after exhaustion of all appeals therefrom, to be liable to the corporation or for amounts paid in settlement to the corporation, unless and only to the extent that the court in which the action or suit was brought or other court of competent jurisdiction determines upon application that in view of all the circumstances of the case, the person is fairly and reasonably entitled to indemnity for such expenses as the court deems proper.
Section 78.751 of the NRS provides that any discretionary indemnification under Section 78.7502 of the NRS, unless ordered by a court or advanced pursuant to subsection 2 of Section 78.751 of the NRS, ay be made by the corporation only as authorized in the specific case upon a determination that indemnification of the director, officer, employee or agent is proper in the circumstances. The determination must be made by:
| ● |
By the stockholders;
|
|
●
|
By the board of directors by majority vote of a quorum consisting of directors - who were not parties to the action, suit or proceeding;
|
|
●
|
If a majority vote of a quorum consisting of directors who were not parties to the action, suit or proceeding so orders, by independent legal counsel in a written opinion; or
|
|
●
|
If a quorum consisting of directors who were not parties to the action, suit or proceeding cannot be obtained, by independent legal counsel in a written opinion.
|
The Company’s Articles of Incorporation and Bylaws or an agreement made by the Company may provide that the expenses of officers and directors incurred in defending a civil or criminal action, suit or proceeding must be paid by the corporation as they are incurred and in advance of the final disposition of the action, suit or proceeding, upon receipt of an undertaking by or on behalf of the director or officer to repay the amount if it is ultimately determined by a court of competent jurisdiction that the person is not entitled to be indemnified by the corporation. The provisions of this subsection do not affect any rights to advancement of expenses to which corporate personnel other than directors or officers may be entitled under any contract or otherwise by law.
The indemnification and advancement of expenses authorized in or ordered by a court pursuant to Section 78.751 of the NRS:
|
●
|
does not exclude any other rights to which a person seeking indemnification or advancement of expenses may be entitled under the articles of incorporation or any bylaw, agreement, vote of stockholders or disinterested directors or otherwise, for either an action in his official capacity or an action in another capacity while holding his office, except that indemnification, unless ordered by a court pursuant to section 78.7502 of the NRS or for the advancement of expenses made pursuant to subsection 2 of section 78.751 of the NRS, may not be made to or on behalf of any director or officer if a final adjudication establishes that his acts or omissions involved intentional misconduct, fraud or a knowing violation of the law and was material to the cause of action; and
|
|
●
|
continues for a person who has ceased to be a director, officer, employee or agent and inures to the benefit of the heirs, executors and administrators of such a person.
|
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of our Company under Nevada law or otherwise, we have been advised the opinion of the SEC is that such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable. In the event a claim for indemnification against such liabilities (other than payment by us for expenses incurred or paid by a director, officer or controlling person of our company in successful defense of any action, suit, or proceeding) is asserted by a director, officer or controlling person in connection with the securities being registered, we will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction, the question of whether such indemnification by it is against public policy in the Securities Act and will be governed by the final adjudication of such issue.
Recent Sales of Unregistered Securities
Share Exchange
On February 9, 2011, we closed the Share Exchange and acquired all of the outstanding equity securities of China Flying from Golden Genesis, which was the sole stockholder of China Flying immediately prior to the closing of the Share Exchange. In exchange, we issued to Golden Genesis 10,758,000 newly issued shares of our common stock. In addition, pursuant to the terms of the Share Exchange Agreement, the Company effected a 1 for 8.512 reverse stock split to modify our capital structure to accommodate the transactions contemplated by the Share Exchange and the Private Placement and to put in place an appropriate capital structure for the Company following the closing of the Share Exchange and the Private Placement. Such securities were not registered under the Securities Act. These securities qualified for exemption under Section 4(2) of the Securities Act since the issuance of securities by us did not involve a public offering.
Private Placement
On February 9, 2011, in connection with the closing of the Share Exchange, we closed the Private Placement of 6,669,627 Units at a purchase price of $1.15 per Unit. Each Unit consisted of a Note convertible into shares of our common stock at a conversion price of $1.15 per share, and a Warrant to purchase one share of the Company’s common stock with an exercise price of $1.40 per share. We sold 6,669,627 Units in the Private Placement, for gross proceeds of $7,670,071.50. In addition, in connection with the Private Placement, the Company also issued to certain affiliates of Euro Pacific, the Company’s lead placement agent in the Private Placement, three-year Agent Warrants to purchase an aggregate of 666,963 shares of our common stock at an exercise price of $1.15 per share. The Agent Warrants also contain a cashless option. The issuances of the Notes, the Warrants and the Agent Warrants were exempt from registration under Section 4(2) of the Securities Act, and the Private Placement met the requirements to qualify for exemption under Regulation D promulgated under the Securities Act.
Notes
On February 9, 2011, in connection with the Private Placement, we offered and sold $7,670,071.50 worth of Notes convertible into up to 6,669,627 shares of our Common Stock, in conjunction with a purchase of the Units. The Notes are payable 24 months from February 9, 2011 with an interest rate of 8% per annum payable semiannually in arrears. The Company shall place in escrow with the Escrow Agent an amount of the proceeds of the Private Placement equal to one semi-annual interest payment on the Notes to secure prompt interest payments. Until such time as 75% of the Notes are converted into shares of Common Stock, if such escrow is depleted in order to make interest payments, we will replenish such escrow amount. At the option of the holder, the Notes may be converted into Common Stock at a price of $1.15 per share, which is subject to customary weighted average and stock based anti-dilution protection. The issuance of the Notes was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D.
The Notes contain customary events of default and affirmative and negative covenants of the Company, including negative covenants which restrict the Company’s ability to do the following (among other things) without the consent of Euro Pacific, as representative of the Investors: (i) incur, or permit to exist, any indebtedness for borrowed money in excess of (A) US$3,000,000 during the twelve (12) month period beginning on February 9, 2011, or (B) US$5,000,000 during the two-year period beginning on February 9, 2011 and ending on February 9, 2013 (the maturity date of the Notes), except in the ordinary course of the Company’s business; (ii) lend or advance money, credit or property to or invest in (by capital contribution, loan, purchase or otherwise) any person or entity in excess of US$1,000,000 except: (A) investments in United States Government obligations, certificates of deposit of any banking institution with combined capital and surplus of at least $200,000,000; (B) accounts receivable arising out of sales in the ordinary course of business; (C) inter-company loans between and among the Company and our subsidiaries; and (D) the loan described under “Certain Relationships and Related Transactions – Loans to Affiliates”; (iii) pay dividends or make any other distribution on shares of the capital stock of the Company; (iv) create, assume or permit to exist, any lien on any of the Company’s property or assets now owned or hereafter acquired, subject to existing liens and certain exceptions; (v) assume guarantees, subject to certain exceptions; (vi) engage in “sale-leaseback” transactions, subject to certain exceptions; (vii) make capital expenditures in excess of US$5,000,000 in any fiscal year, subject to certain exceptions; and (viii) materially alter the Company’s business.
Warrants
On February 9, 2011, in connection with the Private Placement, we offered and sold Warrants to purchase 6,669,627 shares of Common Stock in conjunction with a purchase of the Units. Each Warrant entitles the holder to purchase one share of our Common Stock. The Warrants will be exercisable in whole or in part, at an initial exercise price per share of $1.40, which is subject to customary weighted average and stock based anti-dilution protection. The Warrants may be exercised at any time upon the election of the holder, beginning on the date of issuance and ending of the third anniversary of the closing of the Private Placement. The issuance of the Warrants was not registered under the Securities Act as such issuance was exempt from registration under Section 4(2) of the Securities Act and Regulation D.
Upon the expiration of the Warrant exercise period, the Warrants will expire and become void. In order to exercise the Warrants, the Warrant must be surrendered at the office of the Warrant Agent (as defined in the Warrants) prior to the expiration of the Warrant exercise period, with the form of exercise appearing with the Warrant completed and executed as indicated, accompanied by payment of the full exercise price for the number of Warrants being exercised. Payment shall be by wire transfer or certified check payable to the Company. In the case of partial exercise, the Company will issue a new warrant to the exercising warrant holder, or assigns, evidencing the Warrants which remain unexercised.
In the event of our liquidation, dissolution or winding up, the holders of Warrants will not be entitled to participate in the distribution of our assets.
Holders of Warrants do not have voting, pre-emptive, subscription or other rights of shareholders in respect of the Warrants, nor shall the holders of the Warrants be entitled to receive dividends.
Agent Warrants
In connection with the Private Placement, on February 9, 2011, we issued to certain affiliates of Euro Pacific, the lead placement agent in the Private Placement, three-year Agent Warrants to purchase an aggregate of 666,963 shares of common stock at an exercise price of $1.15 per share. The Agent Warrants also contain a cashless exercise option. The issuance of the Agent Warrants was not registered under the Securities Act. The issuance of the Agent Warrants was exempt from registration under Section 4(2) of the Securities Act.
Exhibits and Financial Statement Schedules
|
Exhibit No.
|
Description
|
|
|
2.1
|
Share Exchange Agreement, dated January 3, 2011, by and among Greyhound Commissary, Inc. (as now known as Tanke Biosciences Corporation, the “Company”) Golden Genesis Limited (“Golden Genesis”) and China Flying Development Limited (“China Flying”) (1)
|
|
|
3.1
|
Articles of Incorporation of the Company (2)
|
|
|
3.2
|
Certificate of Amendment to Articles of Incorporation for reverse stock split (3)
|
|
|
3.3
|
Certificate of Amendment to Articles of Incorporation for change of corporate name (4)
|
|
|
3.4
|
Bylaws of the Company (5)
|
|
|
4.1
|
Form of Note issued to the investors (the “Investors”) in the private placement of 6,669,627 units (the “Private Placement”), dated February 9, 2011 (6)
|
|
|
4.2
|
Form of Warrant issued to the Investors in the Private Placement, dated February 9, 2011 (7)
|
|
|
4.3
|
Form of Agent Warrant issued to Euro Pacific Capital, Inc. and to Newbridge Securities Corporation, dated February 9, 2011 (8)
|
|
|
5.1
|
Opinion of Anslow & Jaclin, LLP *
|
|
|
5.2
|
Opinion of Martin Hu & Partners, dated August 3, 2012 †
|
|
|
5.3
|
Opinion of Martin Hu & Partners, dated January 24, 2011 *
|
|
|
10.1
|
Securities Purchase Agreement, dated February 9, 2011, by and among the Company, the Investors and, with respect to certain sections thereof, Euro Pacific Capital, Inc. and Newbridge Securities Corporation †
|
|
|
10.2
|
Registration Rights Agreement, dated February 9, 2011, by and among the Company and the Investors (10)
|
|
|
10.3
|
Securities Escrow Agreement, dated February 9, 2011, by and among the Company, Euro Pacific Capital, Inc., as representative of the Investors, Golden Genesis and Escrow, LLC, as escrow agent (11)
|
|
|
10.4
|
Interest Escrow Agreement, dated February 9, 2011, by and among the Company, Euro Pacific Capital, Inc., as representative of the Investors, and Escrow, LLC, as escrow agent (12)
|
|
|
10.5
|
Consulting Services Agreement, dated January 3, 2011, between Guangzhou Tanke Industry Co., Ltd. (“Guangzhou Tanke”) and Guangzhou Kanghui Agricultural Technology Co., Ltd. (the “WFOE”) (13)
|
|
|
10.6
|
Operating Agreement, dated January 3, 2011, by and among Guixiong Qiu, Bi Gao, Xiuzhen Liang and Bing Teng (the “Tanke Shareholders”), Guangzhou Tanke and the WFOE (14)
|
|
|
10.7
|
Voting Rights Proxy Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (15)
|
|
|
10.8
|
Equity Pledge Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (16)
|
|
|
10.9
|
Option Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (17)
|
|
|
10.10
|
Call Option Agreement, dated January 3, 2011, between Golden Genesis, Wong Kwai Ho and the Tanke Shareholders (18)
|
|
|
10.11
|
Employment Agreement, dated July 26, 2011, by and between the Company and Gilbert Lee (19)
|
|
|
99.1
|
Unofficial English translation of land lease agreement dated April 15, 2006, between Guangzhou Baoyuhua Industry Co., Ltd. and Guangzhou Tanke †
|
|
|
99.2
|
Unofficial English translation of form of employment agreement †
|
|
|
99.3
|
Unofficial English translation of promissory note dated December 24, 2010 signed by Guixiong Qiu, Bi Gao and Xiuzhen Liang †
|
|
|
99.4
|
Unofficial English translation of loan agreement dated May 2009 †
|
|
|
21.1
|
List of the Company’s Subsidiaries †
|
|
|
23.1
|
Consent of EFP Rotenberg LLP *
|
|
|
23.2
|
Consent of Parker Randall CF (H.K.) CPA Limited *
|
|
|
23.3
|
Consent of Anslow & Jaclin, LLP, as in Exhibit 5.1.
|
|
|
23.4
|
Consent of Martin Hu & Partners, as in Exhibit 5.2.
|
|
|
23.5
|
Consent of Martin Hu & Partners *
|
|
|
101
|
Interactive Data File [incorporated by reference to Exhibit 101 filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 2011 filed on April16, 2012 and the Quarterly Report on Form 10-Q/A for the six months ended June 30, 2012 filed on August 27, 2012]
|
|
*
|
Filed herewith
|
|
†
|
Previously filed
|
|
(1)
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 6, 2011.
|
|
(2)
|
Incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form 10 filed with the SEC on December 16, 2008.
|
|
(3)
|
Incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(4)
|
Incorporated by reference to Exhibit 3.3 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(5)
|
Incorporated by reference to Exhibit 3.2 to the Company’s Registration Statement Form 10 filed with the SEC on December 16, 2008.
|
|
(6)
|
Incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(7)
|
Incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(8)
|
Incorporated by reference to Exhibit 4.3 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(9)
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(10)
|
Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(11)
|
Incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(12)
|
Incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(13)
|
Incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(14)
|
Incorporated by reference to Exhibit 10.6 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(15)
|
Incorporated by reference to Exhibit 10.7 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(16)
|
Incorporated by reference to Exhibit 10.8 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(17)
|
Incorporated by reference to Exhibit 10.9 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(18)
|
Incorporated by reference to Exhibit 10.10 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(19)
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on August 1, 2011.
|
Undertakings
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i) To include any prospectus required by section 10(a)(3) of the Securities Act of 1933;
(ii) To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of prospectus filed with the SEC pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement;
(iii) To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or any material change to such information in the registration statement;
(2) That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser, if the registrant is subject to Rule 430C, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A, shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness. Provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Guangzhou, Province of Guangdong, People’s Republic of China, on September 28, 2012.
|
TANKE BIOSCIENCES CORPORATION
|
|||
|
By:
|
/s/ Guixiong Qiu
|
||
|
Guixiong Qiu
|
|||
|
Chief Executive Officer
|
|||
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following person in the capacity and on the date indicated.
|
Signature
|
|
Title
|
Date
|
|
|
/s/ Guixiong Qiu
|
||||
|
Guixiong Qiu
|
|
Chief Executive Officer and
Chairman of the Board of Directors
(principal executive officer)
|
September 28, 2012
|
|
|
/s/ Gilbert Lee
|
||||
|
Gilbert Lee
|
Chief Financial Officer (principal
financial officer and principal
accounting officer)
|
September 28, 2012
|
||
EXHIBIT INDEX
|
Exhibit No.
|
Description
|
|
|
2.1
|
Share Exchange Agreement, dated January 3, 2011, by and among Greyhound Commissary, Inc. (as now known as Tanke Biosciences Corporation, the “Company”) Golden Genesis Limited (“Golden Genesis”) and China Flying Development Limited (“China Flying”) (1)
|
|
|
3.1
|
Articles of Incorporation of the Company (2)
|
|
|
3.2
|
Certificate of Amendment to Articles of Incorporation for reverse stock split (3)
|
|
|
3.3
|
Certificate of Amendment to Articles of Incorporation for change of corporate name (4)
|
|
|
3.4
|
Bylaws of the Company (5)
|
|
|
4.1
|
Form of Note issued to the investors (the “Investors”) in the private placement of 6,669,627 units (the “Private Placement”), dated February 9, 2011 (6)
|
|
|
4.2
|
Form of Warrant issued to the Investors in the Private Placement, dated February 9, 2011 (7)
|
|
|
4.3
|
Form of Agent Warrant issued to Euro Pacific Capital, Inc. and to Newbridge Securities Corporation, dated February 9, 2011 (8)
|
|
|
5.1
|
Opinion of Anslow & Jaclin, LLP *
|
|
|
5.2
|
Opinion of Martin Hu & Partners, dated August 3, 2012 †
|
|
|
5.3
|
Opinion of Martin Hu & Partners, dated January 24, 2011 *
|
|
|
10.1
|
Securities Purchase Agreement, dated February 9, 2011, by and among the Company, the Investors and, with respect to certain sections thereof, Euro Pacific Capital, Inc. and Newbridge Securities Corporation †
|
|
|
10.2
|
Registration Rights Agreement, dated February 9, 2011, by and among the Company and the Investors (10)
|
|
|
10.3
|
Securities Escrow Agreement, dated February 9, 2011, by and among the Company, Euro Pacific Capital, Inc., as representative of the Investors, Golden Genesis and Escrow, LLC, as escrow agent (11)
|
|
|
10.4
|
Interest Escrow Agreement, dated February 9, 2011, by and among the Company, Euro Pacific Capital, Inc., as representative of the Investors, and Escrow, LLC, as escrow agent (12)
|
|
|
10.5
|
Consulting Services Agreement, dated January 3, 2011, between Guangzhou Tanke Industry Co., Ltd. (“Guangzhou Tanke”) and Guangzhou Kanghui Agricultural Technology Co., Ltd. (the “WFOE”) (13)
|
|
|
10.6
|
Operating Agreement, dated January 3, 2011, by and among Guixiong Qiu, Bi Gao, Xiuzhen Liang and Bing Teng (the “Tanke Shareholders”), Guangzhou Tanke and the WFOE (14)
|
|
|
10.7
|
Voting Rights Proxy Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (15)
|
|
|
10.8
|
Equity Pledge Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (16)
|
|
|
10.9
|
Option Agreement, dated January 3, 2011, by and among Guangzhou Tanke, the Tanke Shareholders and the WFOE (17)
|
|
|
10.10
|
Call Option Agreement, dated January 3, 2011, between Golden Genesis, Wong Kwai Ho and the Tanke Shareholders (18)
|
|
|
10.11
|
Employment Agreement, dated July 26, 2011, by and between the Company and Gilbert Lee (19)
|
|
|
99.1
|
Unofficial English translation of land lease agreement dated April 15, 2006, between Guangzhou Baoyuhua Industry Co., Ltd. and Guangzhou Tanke †
|
|
|
99.2
|
Unofficial English translation of form of employment agreement †
|
|
|
99.3
|
Unofficial English translation of promissory note dated December 24, 2010 signed by Guixiong Qiu, Bi Gao and Xiuzhen Liang †
|
|
|
99.4
|
Unofficial English translation of loan agreement dated May 2009 †
|
|
|
21.1
|
List of the Company’s Subsidiaries †
|
|
|
23.1
|
Consent of EFP Rotenberg LLP *
|
|
|
23.2
|
Consent of Parker Randall CF (H.K.) CPA Limited *
|
|
|
23.3
|
Consent of Anslow & Jaclin, LLP, as in Exhibit 5.1.
|
|
|
23.4
|
Consent of Martin Hu & Partners, as in Exhibit 5.2.
|
|
|
23.5
|
Consent of Martin Hu & Partners *
|
|
|
101
|
Interactive Data File [incorporated by reference to Exhibit 101 filed with the Company’s Annual Report on Form 10-K for the year ended December 31, 2011 filed on April16, 2012 and the Quarterly Report on Form 10-Q/A for the six months ended June 30, 2012 filed on August 27, 2012]
|
|
*
|
Filed herewith
|
|
†
|
Previously filed
|
|
(1)
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 6, 2011.
|
|
(2)
|
Incorporated by reference to Exhibit 3.1 to the Company’s Registration Statement on Form 10 filed with the SEC on December 16, 2008.
|
|
(3)
|
Incorporated by reference to Exhibit 3.2 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(4)
|
Incorporated by reference to Exhibit 3.3 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(5)
|
Incorporated by reference to Exhibit 3.2 to the Company’s Registration Statement Form 10 filed with the SEC on December 16, 2008.
|
|
(6)
|
Incorporated by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(7)
|
Incorporated by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(8)
|
Incorporated by reference to Exhibit 4.3 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(9)
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(10)
|
Incorporated by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(11)
|
Incorporated by reference to Exhibit 10.3 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(12)
|
Incorporated by reference to Exhibit 10.4 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(13)
|
Incorporated by reference to Exhibit 10.5 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(14)
|
Incorporated by reference to Exhibit 10.6 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(15)
|
Incorporated by reference to Exhibit 10.7 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(16)
|
Incorporated by reference to Exhibit 10.8 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(17)
|
Incorporated by reference to Exhibit 10.9 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(18)
|
Incorporated by reference to Exhibit 10.10 to the Company’s Current Report on Form 8-K filed with the SEC on February 10, 2011.
|
|
(19)
|
Incorporated by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on August 1, 2011.
|
II-10
