Attached files
| file | filename |
|---|---|
| EX-16.3 - EXHIBIT 16.3 - CHINA-BIOTICS, INC | v315639_ex16-3.htm |
| EX-31.2 - EXHIBIT 31.2 - CHINA-BIOTICS, INC | v315639_ex31-2.htm |
| EX-32.2 - EXHIBIT 32.2 - CHINA-BIOTICS, INC | v315639_ex32-2.htm |
| EX-21.1 - EXHIBIT 21.1 - CHINA-BIOTICS, INC | v315639_ex21-1.htm |
| EX-31.1 - EXHIBIT 31.1 - CHINA-BIOTICS, INC | v315639_ex31-1.htm |
| EX-32.1 - EXHIBIT 32.1 - CHINA-BIOTICS, INC | v315639_ex32-1.htm |
| EXCEL - IDEA: XBRL DOCUMENT - CHINA-BIOTICS, INC | Financial_Report.xls |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended March 31, 2012
¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Transition Period from _______ to _________
333-110733
(Commission File Number)
CHINA-BIOTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 98-0393071 |
(State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) |
No. 26 Orient Global Headquarter
Lane 118, Yonghe Road
Zhabei District, Shanghai 200072
People’s Republic of China
(Address of principal executive offices)
Telephone number: (86 21) 5834 9748
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class | Name of each exchange on which registered |
Securities registered pursuant to Section 12(g) of the Act: Common stock, par value $0.0001 per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ¨ No þ
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No þ
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer | ¨ | Accelerated filer | ¨ | |
| Non-accelerated filer | ¨ (Do not check if a smaller reporting company) | Smaller reporting company | þ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No þ
The aggregate market value of voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold was approximately $6,558,489 as of the last business day of the registrant’s most recently completed second fiscal quarter.
As of September 11, 2012, 22,150,200 shares of the registrant’s common stock were outstanding.
TABLE OF CONTENTS
| Page | ||
| Part I | ||
| ITEM 1. | BUSINESS | 3 |
| ITEM 1A. | RISK FACTORS | 17 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS | 30 |
| ITEM 2. | PROPERTIES | 31 |
| ITEM 3. | LEGAL PROCEEDINGS | 31 |
| Part II | ||
| ITEM 4. | [REMOVED AND RESERVED] | 32 |
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES | 32 |
| ITEM 6. | SELECTED FINANCIAL DATA | 33 |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS | 33 |
| ITEM 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK | 47 |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA | 48 |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE | 72 |
| ITEM 9A. | CONTROLS AND PROCEDURES | 73 |
| ITEM 9B. | OTHER INFORMATION | 75 |
| Part III | ||
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE | 76 |
| ITEM 11. | EXECUTIVE COMPENSATION | 79 |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS | 82 |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE | 84 |
| ITEM 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES | 84 |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES | 85 |
| EX-31.1 (Certifications required under Section 302 of the Sarbanes-Oxley Act of 2002) | ||
| EX-31.2 (Certifications required under Section 302 of the Sarbanes-Oxley Act of 2002) | ||
| EX-32.1 (Certifications required under Section 906 of the Sarbanes-Oxley Act of 2002) | ||
| EX-32.2 (Certifications required under Section 906 of the Sarbanes-Oxley Act of 2002) | ||
| 2 |
PART I
The information in this document contains forward-looking statements which involve risks and uncertainties, including statements regarding our capital needs, business strategy and expectations. Any statements contained herein that are not statements of historical fact may be deemed to be forward-looking statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “should,” “will,” “expect,” “plan,” “intend,” “anticipate,” “believe,” “estimate,” “predict,” “potential,” “forecast,” “project,” or “continue,” the negative of such terms or other comparable terminology. You should not rely on forward-looking statements as predictions of future events or results. Any or all of our forward-looking statements may turn out to be wrong. They can be affected by inaccurate assumptions, risks and uncertainties and other factors which could cause actual events or results to be materially different from those expressed or implied in the forward-looking statements.
In evaluating these statements, you should consider various factors, including the risks described in “Item 1A. Risk Factors” beginning on page 17 and elsewhere in this Form 10-K. These factors may cause our actual results to differ materially from any forward-looking statement. In addition, new factors emerge from time to time and it is not possible for us to predict all factors that may cause actual results to differ materially from those contained in any forward-looking statements. We disclaim any obligation to publicly update any forward-looking statements to reflect events or circumstances after the date of this document, except as required by applicable law.
ITEM 1. BUSINESS
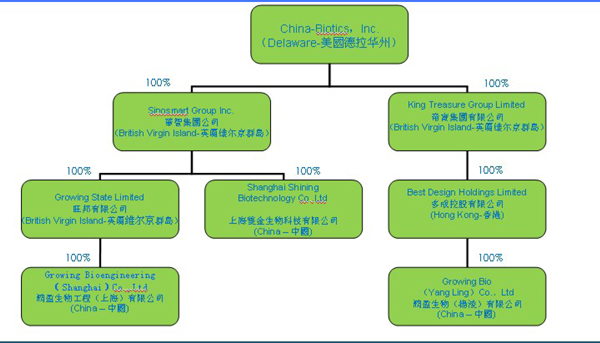
Except as otherwise indicated by the context, references in this Annual Report on Form 10-K to “we,” “us,” and “our” are to the combined business of China-Biotics, Inc. (the “Company”) and to the Company’s wholly owned direct subsidiaries, Sinosmart Group Inc. (“SGI”), Growing State Limited (“GSL”) and King Treasure Group Limited (“KTG”); SGI’s wholly owned subsidiary, Shanghai Shining Biotechnology Co. Ltd. (“Shining”); GSL’s wholly owned subsidiary, Growing Bioengineering (Shanghai) Co. Ltd. (“Growing”); KTG’s wholly owned subsidiary, Best Design Holdings Limited (“BDH”); and BDH’s wholly owned subsidiary, Growing Bio (Yangling) Co. Ltd (“Growing Yangling”). References to “China” or to the “PRC” are references to the People’s Republic of China. All references to “dollars” or “$” refers to United States dollars.
History
We were incorporated under the name of Otish Resources, Inc. in Delaware in February 2003. Prior to March 2006, we were a mineral exploration stage company specializing in acquiring and consolidating mineral properties with potential for commercial ore bodies. Although we conducted some preliminary exploration work with respect to our mineral properties, we never achieved full operations with respect to our mineral properties. We never generated any revenue from our mineral exploration operations.
On March 22, 2006, we entered into and completed a Securities Exchange Agreement with SGI and SGI’s shareholders pursuant to which the SGI shareholders transferred all of the equity securities of SGI to us in exchange for the issuance of shares of our common stock. We refer to this transaction in this document as the “Share Exchange.” At the closing of the Share Exchange, we issued to the SGI shareholders an aggregate of 15,980,000 shares of our common stock in exchange for their shares of SGI, and SGI became our wholly owned subsidiary. SGI owns all of the equity securities of Shining. As a result of the Share Exchange, we are no longer a mineral exploration stage company, and SGI’s business operations became our primary operations. We are currently engaged in the research, development, production, marketing, and distribution of probiotics products. These products contain live microbial food supplements that beneficially affect the host by improving its intestinal microbial balance.
SGI was incorporated in the British Virgin Islands on February 13, 2004. On December 9, 2005, SGI incorporated a wholly owned subsidiary, GSL, in accordance with the laws of the British Virgin Islands. On September 22, 2006, GSL established a wholly foreign-owned enterprise, Growing, in China.
On December 11, 2007, we sold a 4% Senior Convertible Promissory Note in the amount of $25,000,000 (the “Note”) with a maturity date of December 11, 2010, to Pope Investments II LLC, an affiliate of Pope Investments, LLC, in a private placement. In connection with the sale, we entered into an Investment Agreement (the “Investment Agreement”) and a Registration Rights Agreement. In addition, Mr. Jinan Song, the company’s Chief Executive Officer, Chairman and largest shareholder, entered into a Guaranty Agreement (the “Guaranty Agreement”) and a Pledge Agreement (the “Pledge Agreement”), pursuant to which Mr. Song agreed to guaranty the company’s obligations under the Note and to secure such guaranty with a pledge of 4,000,000 shares of China-Biotics’ common stock owned by Mr. Song. The principal amount of the Note was convertible into shares of our common stock at an exercise price of $12.00 per share at any time until the maturity date. Pursuant to the Investment Agreement, payment of our obligations under the Note was secured with a pledge of 100% of the stock of our subsidiary, SGI, to Pope Investments II LLC. Net proceeds of the Note were used to fund the construction of a 150 metric ton per-year manufacturing facility (now expanded to 300 metric tons per year) in Shanghai Qingpu Industrial Park District in Shanghai (“Qingpu”) and other capital expenditures.
| 3 |
On December 9, 2010, the Company repaid in full its obligations under the Note in the original aggregate principal amount of $25,000,000. The payoff amount of $29,684,932, consisting of $25,000,000 of outstanding principal and $4,684,932 of accrued interest, was paid to Pope Investments II LLC, and all security interests and liens held by Pope Investments II LLC were terminated and released, including (1) the Guaranty Agreement; and (2) the Pledge Agreement.
On October 5, 2009, the Company closed an underwritten public offering of 4,600,000 shares of its common stock at a price of $15.00 per share. On October 26, 2009, an additional 690,000 shares were sold pursuant to the exercise of an over-allotment option at the same price. Net proceeds of the offering, including the over-allotment, after deducting underwriting discounts and offering expenses, were approximately $74.9 million. Net proceeds from the offering were used for general corporate purposes, including expanding our retail operations, expanding our products, funding Phase 2 of our bulk manufacturing facility in Qingpu, funding our new project in the Yangling Agricultural High-Tech Industries Demonstration Zone in Shaanxi Province of China (the “Yangling Zone”), and for general working capital purposes.
On May 4, 2010, the Company incorporated a wholly owned subsidiary, KTG, in accordance with the laws of the British Virgin Islands. On June 25, 2010, KTG incorporated a wholly owned subsidiary, BDH, in accordance with the laws of the Special Administrative Region of Hong Kong (“Hong Kong”). On July 12, 2010, BDH incorporated a wholly owned subsidiary, Growing Yangling, in accordance with the laws of China. Growing Yangling commenced construction of a manufacturing plant in the Yangling Zone in April 2012 and expects to complete its construction by June 2014 and commence trial production by 2015.
Set forth below is a list of the Company’s direct and indirect subsidiaries and their respective jurisdiction of incorporation or registration:
| Name of Subsidiary | Jurisdiction | |
| Sinosmart Group Inc. (SGI) | British Virgin Islands | |
| Growing State Limited (GSL) | British Virgin Islands | |
| King Treasure Group Limited (KTG) | British Virgin Islands | |
| Shanghai Shining Biotechnology Co. Ltd. (Shining) | PRC | |
| Growing Bioengineering (Shanghai) Co. Ltd. (Growing) | PRC | |
| Best Design Holdings Limited (BDH) | Hong Kong | |
| Growing Bio (Yangling) Co. Ltd (Growing Yangling) | PRC |
| 4 |
Current Operations
Overview
We are engaged in the research, development, production, marketing, and distribution of probiotics products (which we sometimes refer to simply as “probiotics”), which are products that contain live microbial food supplements that beneficially affect the host by improving its intestinal microbial balance.
Our first product, Shining Essence, was approved by the Chinese Ministry of Health for production and sale as a health product in August 2000. We launched Shining Essence in Shanghai in April 2001, and Shining Essence remains one of our best-selling retail products. The Health Food Association of China named Shining Essence as the best-selling liver health product in 2001.
As of March 31, 2012, we had 12 patents for a number of processes and products as described in “—Government Regulation—Intellectual Property” on Page 14. We have applied the technologies for which we hold patents in the manufacturing process of retail products under the “Shining” and “Growing” brands. We have a total of 22 patents in different stages of the application, review or grant process, including, in addition to the 12 patents referenced above, ten patents currently under review.
We obtained the four certifications below from the TÜV Rheinland/Berlin-Brandenburg Group of Companies for our production plant in Pudong.
| · | ISO 9001. We obtained ISO 9001:2008 certification from TÜV Anlagentechnik GmbH in respect of our production process for our leading retail product, Shining Essence. This certification is valid through January 2015. According to the American National Standards Institute, ISO 9001:2008 specifies requirements for a quality management system where an organization needs to demonstrate its ability to consistently provide products that meet customer and applicable regulatory requirements, and aims to enhance customer satisfaction through effective application of the system, including processes for continual improvement of the system and assurance of conformity to customer and applicable regulatory requirements. All requirements of this international standard are generic and are intended to be applicable to all organizations, regardless of type, size, and product provided. |
| · | ISO 14001. We obtained ISO 14001:2004 certification from TÜV Anlagentechnik GmbH in respect of our production process for our Shining Essence product. This certification is valid through February 2013. According to the American National Standards Institute, ISO 14001:2004 specifies requirements for an environmental management system to enable an organization to develop and implement a policy and objectives which take into account legal requirements and other requirements to which the organization subscribes, and information about significant environmental aspects. It applies to those environmental aspects that the organization identifies as those which it can control and influence. It does not itself state specific environmental performance criteria. |
| · | OHSAS 18001. We obtained OHSAS 18001:1999 certification from TÜV Hong Kong Ltd in respect of our production process for our Shining Essence product. This certification was valid through June 2012, and we are currently following the relevant procedures for renewal of the certificate. According to BSI Management Systems - Asia, Occupational Health and Safety Assessment Series specification relates to an entity’s occupational health and safety management systems that enable organizations to control its occupational health and safety risks and improve its performance. It does not state specific occupational health and safety performance criteria, nor does it give detailed specifications as to the design of a management system. OHSAS 18001 is an assessment specification developed in response to the need for companies to meet their health and safety obligations in an efficient manner. |
| · | HACCP DS 3027 E. We obtained HACCP DS 3027 E: 1997 certification from TÜV Anlagentechnik GmbH in respect of our production process for our Shining Essence product. This certification was valid through July 2012, and we are currently following the relevant procedures for renewal of the certificate. The term “HACCP” stands for Hazard Analysis Critical Control Point. The HACCP DS 3027 E:1997 standard was developed to ensure food safety among food manufacturers and their suppliers in Denmark. |
In addition to the foregoing, we hold two certificates from the Shanghai Food and Drugs Administration for our two production plants in Pudong and Qingpu, Shanghai: we obtained the Good Manufacturing Process (“GMP”) certification, which is valid through March 28, 2013, for our new bulk production facility in Qingpu, and on July 1, 2010, Shining’s GMP certificate was also renewed and is valid through June 30, 2013. The GMP certification program is a global quality assurance system that covers the testing and manufacturing of food, pharmaceutical products and medical devices. GMP stipulates stringent approval guidelines on various aspects of production based on evaluations of factory and equipment, materials, hygiene certificates, waste and recycling and after sales, among other things.
| 5 |
We are currently applying for the Current Good Manufacturing Practices (cGMP) certification for our production plant in Qingpu, Shanghai, from the National Safety Foundation (NSF). cGMP refers to the Current Good Manufacturing Practice regulations enforced by the US Food and Drug Administration (FDA). cGMPs provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the cGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations. This includes establishing strong quality management systems, obtaining appropriate quality raw materials, establishing robust operating procedures, detecting and investigating product quality deviations, and maintaining reliable testing laboratories. This formal system of controls at a pharmaceutical company, if adequately put into practice, helps to prevent instances of contamination, mix-ups, deviations, failures and errors. This assures that drug products meet their quality standards.
The cGMP requirements were established to be flexible in order to allow each manufacturer to decide individually how to best implement the necessary controls by using scientifically sound design, processing methods, and testing procedures. The flexibility in these regulations allows companies to use modern technologies and innovative approaches to achieve higher quality through continual improvement. Accordingly, the “c” in cGMP stands for “current,” requiring companies to use technologies and systems that are up-to-date in order to comply with the regulations. Systems and equipment that may have been “top-of-the-line” to prevent contamination, mix-ups and errors ten or 20 years ago may be less than adequate by today’s standards.
Products
We manufacture and sell several health supplements under the “Shining” or “双金 (Chinese)” brand in China as set forth below. All of these products have been approved by the Ministry of Health in China (“MOH”) and their content has been tested by the Shanghai Preventative Medicine Research Institute, which validated that our products contain the specified quantities of bacteria. While management believes these products to be effective, their effectiveness has not been conclusively established.
Our major retail products are:
| · | Shining Essence - Composed of lactobacillus acidophilus and bifidobacterium bifidum, aimed at balancing the microecology of the human digestive system, enhancing intestinal health, and protecting and strengthening liver function; |
| · | Shining Signal - Composed of monascus rice and lactobacillus acidophilus, focused on reducing high blood pressure, high blood sugar level, and hyperlipidemia; |
| · | Shining Golden Shield - Composed of bifidobacterium adolescentis and lentinusedodes, focused on enhancing the human immune system; |
| · | Shining Energy - Composed of Vitamin C, L. Arginine, and other amino acids, aimed at promoting the development of the human brain cells and enhancing alertness and energy; |
| · | Shining Beauty Essence - Composed of soy bean isoflavones and pueraria lobata p extracts, aimed at increasing bone mineral density of elderly people and reducing negative effects associated with the aging process; |
| · | Shining Companion Bifidus Factor Granule - Composed of bifidus, focused on enhancing the growth of bifidus in the human body and enhancing intestinal health; |
| · | Shining Stomach Protection Capsules - Composed of lactobacillus acidophilus, aimed at protecting stomach walls and improving the digestive system; |
| · | Shining Sicanel Capsules - Composed of lactobacillus acidophilus, focused on reducing hyperlipidemia, or excess fat in the blood; |
| · | Shining Golden Shield (for children) - Composed of bifido bacterium adolescentis and lentinusedodes, focused on enhancing human body’s immune system; |
| · | Starter Culture - Composed of streptococcus thermophilus and others which improve immunity and gastrointestinal function and promote digestion and prevention of enteritis and other gastrointestinal diseases; and |
| · | Yogurt Powder - Composed of streptococcus thermophilus and safe quality milk powder, which improve immunity and gastrointestinal function and promote digestion and prevention of enteritis and other gastrointestinal diseases. |
In February 2010, we commenced production at our new facility in Qingpu and began producing bulk additive probiotics products, which are sold to institutional customers, such as dairy manufacturers, animal feed manufacturers, pharmaceutical companies, and food companies. We intend to continue to develop new retail and bulk products to strengthen our product pipeline so that we may offer a wider array of products for sale in the market. Two years after commencing production in Qingpu, our customer base for bulk additive probiotics products has grown from 60 customers as of March 31, 2011 to 71 customers as of March 31, 2012. With respect to retail products, in keeping with the Company’s focus on bulk products and owing to increasing rent and labor costs, the Company has transitioned since January 2011 from focusing on point-of-sales to internet sales. Although the Company’s retail products sales during this transition period were negatively affected, the Company has succeeded in improving its business model for selling retail products via the internet.
| 6 |
Recent Changes in our Business
Leveraging on what our management believes to be our technical competence, cost efficiencies, and highly recognized brand name, our management expects to achieve significant growth through:
| · | Introduction of bulk additives probiotics products through our Qingpu Facility - We have expanded into the bulk additives business for institutional customers through the completion of our project to build a 300-metric ton capacity plant in Qingpu, which commenced commercial production in February 2010. See –Facilities” for further information about our Qingpu facility. |
| · | We are currently focused on two fast-growing industries in China: the dairy and animal feed sectors. As of March 31, 2012, we had entered into contracts with 71 customers for the bulk additives business. In this regard, we have created a number of formulations for testing by many potential customers. We have established business relationships with a variety of commercial customers located in major cities, including Beijing, Tianjin, Chongqing, and Shanghai, and 16 provinces, including Guangdong, Jiangsu, and Jiangxi, among others. These growing companies are among the leaders in the dairy, animal feed, baked food, and pharmaceuticals industries. |
| · | Construction of a new facility in the Yangling Zone - Encouraged by the growing demand for animal feed additives in China, we plan to leverage our technology and R&D capability in probiotics-based animal feed applications to build a new facility in the Yangling Zone in Shaanxi Province, China. The cost for constructing the Yangling Zone facility is expected to be over $58 million invested over two years. The facility will produce probiotics and probiotics-related biological additives for the animal feed industry. Currently, the facility is in its construction stage, and the plan is subject to government approval prior to implementation. During the year ended March 31, 2011, the Company made total payments of approximately $3.25 million (RMB20,608,112) to acquire the land use right of approximately 122,600 square meters (183.94 mu). We received the land use right certificate on January 28, 2012 and commenced construction in June 2012. We expect to complete construction by June 2014 and trial production is expected to commence in 2015. In the existing market environment, the Company will utilize its available cash and bank facilities to fund such construction. See “–Land use rights” in Note 2 of the Notes to the Consolidated Financial Statements for the Years Ended March 31, 2012 and 2011 for further information about land use rights in the PRC. |
| · | Closure of retail outlets and expansion to wholesale and e-commerce businesses - In our continuing effort to transition from a retail, business-to-consumer model to a wholesale, business-to-business model, and to improve operating efficiency, we have completed the consolidation of our retail outlets. We closed all of our retail outlets by the end of fiscal year 2011, as we believe our distribution network is more efficient for our retail products sales. Comparatively, direct selling through retail outlets involves increasing leasing expenses and staffing costs. By selecting six new distributors, we have recently expanded our distribution network into the greater Beijing area to sell the Company’s retail products. The local distributors sell the Company’s retail probiotics products through established distribution networks, including malls, supermarkets, and functional food stores. During the quarter ended March 31, 2012, we added five more distributors. We now have a total of 39 distributors for retail products as of March 31, 2012. In light of increasing online sales of health food and supplements in China and to maintain our existing retail customer base, “Community Network,” we established an in-house e-commerce department during the quarter ended March 31, 2011, which is dedicated to promoting and selling our retail products online through the Company’s website at http://www.shiningbt.com/Product/. Since its establishment, the in-house e-commerce department is working to better access the market and existing and potential customers. We also have been working with two online selling companies to sell our retail products, including www.ule.com.cn and www.yihaodian.com. In addition, we have established a customer service center to reply to inquiries from our end users. |
| · | Improvement in research and development of new products and services - We continue to develop new retail products aimed at improving general human health conditions, enhancing the immune system, and reducing health problems. To further improve our competency in the bulk additives market, we continue to improve and provide our value-added service to institutional customers by assisting their lab and production testing and providing customized technical support, among other things. In addition, we are also working with certain universities, including Northeast Agricultural University, to carry out research and development projects in order to seek to increase the probiotics industry’s capability. |
| 7 |
Industry overview and market conditions
Probiotics
We manufacture and sell probiotics. Most probiotics are bacteria-based and naturally exist in the human body in the lower intestinal tract. The introduction of “helpful” bacteria and other organisms may aid in preventative fights against infection and improve digestion, especially with respect to dairy products.
Probiotics generally have a very short lifespan. Water, acid, and oxygen are critically harmful to probiotics subsistence. Most probiotics die or cease to function after a short period of time after extraction from their source. A reduction of these naturally-occurring organisms due to poor eating habits, stress, use of antibiotic drugs, or other factors may disrupt the natural equilibrium of the human body and could lead to a variety of abdominal ailments and the weakening of the immune system. Based on information available on the website www.usprobiotics.org, a non-profit research and education website sponsored by the California Dairy Research Foundation and Dairy & Food Culture Technologies, researchers are also studying the potential links between probiotics and their beneficial effects on a variety of ailments such as hypertension, certain types of cancer, high cholesterol, and allergies (to access this information, click on the Section “Probiotics Basics,” and then click on the Section “Health Effects of Probiotics.” The subsections of “Health Effects of Probiotics” include the following topics: hypertension, cancer, elevated blood cholesterol, and allergy, among others.).
Chinese market
China has limited capacity to produce probiotics. We believe that demand for probiotics and functional foods in China will continue to increase in the foreseeable future. We believe that China lacks manufacturing capabilities of bulk additives in a scale necessary to support the domestic functional foods industry. This has forced processed food producers to either import most of their probiotics or produce finished products abroad and re-import the final products. We believe this creates significant inefficiencies in both cost and probiotics efficacy, as some bacteria cease to function during transportation.
Demand for functional food products is expected to grow significantly. As the discretionary income and health-consciousness of the average Chinese consumers increase, we expect the demand for functional foods and dietary supplements to increase in tandem. We believe that the demand for functional foods and dietary supplements will be bolstered by the stated commitment of the Chinese government to reduce the use of antibiotics and promote the use of probiotics and other preventative measures.
Curtailment of the use of antibiotics may stimulate demand for probiotics. According to a Chinese newspaper article entitled “80,000 people in China die from inappropriate use of antibiotics each year, children suffer the most,” published in Xin Kuai Bao, dated December 12, 2003, available in Chinese at http://info.china.alibaba.com/news/detail/v8-d5779326.html,” China has the highest per capita consumption of antibiotics in the world. In 2000, the World Health Organization cautioned that “Super Diseases” are being created by the overuse of antibiotics. In order to stem the tide of these drug resistant strains, many nations have taken steps to limit the use of antibiotic drugs. In July 2004, the Chinese government took an active role in the fight against the overuse of antibiotics by requiring prescriptions for these drugs. To further reduce the use of antibiotics, the Chinese government has slashed the retail price of antibiotics by 60%, so that it is no longer profitable for a large number of antibiotics manufacturers to continue to manufacture such products. This resulted in a marked increase in the use of other products to not only treat existing infections, but to prevent infections from occurring as well. In addition, on May 20, 2005 (effective July 1, 2005) the State Food and Drug Administration (reference no. Guo Shi Yao Jian Zhu (2005) no. 202) issued a notice acknowledging that probiotics are beneficial to human health and also introduced guidelines for regulating manufacturers of probiotics products and registration of probiotics products with the State Food and Drug Administration.
Demand for dairy product additives is expected to increase significantly. The demand for functional foods and foods that use probiotics supplements is growing at a significant rate. According to AC Nielsen (article available at http://cn.en.acnielsen.com/news/20050916.shtml), yogurt and yogurt drinks are the fastest-growing products in the food products segment in China, with sales increasing by 38% in 2004 alone. Sales of infant formula grew 23% in the same year. Moreover, according to statements made by the Nutrition Development Centre of National Development and Reform Commission in China, effective April 1, 2007, probiotics were to be added to baby milk powders produced in China, although it is unclear when this rule will come into effect. On October 24, 2011, the MOH published a list of probiotics which could be used in baby food for babies aged one year or older, all of which are currently produced and sold by the Company. These factors could translate into significant growth in demands in China for live bacteria as food additives.
More use of probiotics in animal feed industry. Antibiotics have been widely used in animal feed by Chinese farmers. Human health has been negatively impacted, albeit indirectly, as a result of consuming dairy and meat produced by farms using antibiotics in animal feed. With the increasing public awareness of those issues, the government is expected to set up stricter regulations to monitor the non-therapeutic use of antibiotics in livestock feed to ensure food safety. At the same time, organic food is attracting more attention among Chinese consumers. This creates a favorable trend of using more probiotics in animal feed.
| 8 |
Business strategies
Leveraging on what we believe is our technical competence, cost efficiencies, and highly recognized brand name, our management expects to achieve significant growth through the:
| · | introduction of bulk additives products, with a focus on fast-growing industries, including dairy and animal feed; |
| · | geographic expansion of our retail sales through the use of third party distributors, who distribute to traditional retail outlets and networks, and online sales; and |
| · | enhancement in R&D to develop new products and provide value-added services. |
Bulk market
In China, most probiotics used for the manufacturing of yogurt, milk powder products, and food preservatives are currently imported. However, we believe imported probiotics are generally more expensive and can be of lower quality, as some bacteria may not survive long distance transportation and the bacteria that do survive could lose some of their efficacy. We believe our bulk probiotics are of higher quality than most imported probiotics, and our lower costs provide an attractive alternative to domestic manufacturers of dairy, animal feed, and other food products that utilize probiotics as a raw material. In addition, as noted above, the government of the PRC is considering requiring that probiotics be added to baby milk powders and other products produced in China, with the relevant regulations and laws to be implemented at a future date.
Also as noted above, we have expanded into the bulk additives business for functional foods through completion of Phases 1 and 2 of our new Qingpu facility.
Geographic expansion
Our retail products are sold to distributors, who then distribute them to various retail channels such as drug stores and supermarkets. Over the past decade, we believe we have firmly established ourselves in Shanghai as the leading supplier and manufacturer of probiotics products. We are now expanding the sale of our retail products to other metropolitan cities in China through our distribution network and via internet sales, which in recent years have been the most efficient and effective way to increase sales in China due to the increasing number of Chinese internet users.
In the past, we have operated our own retail outlets for direct sales. With the successful commercial production at the Qingpu facility since February 2010, the Company began a strategic move from retail operations (a business-to-consumer model) to wholesale and bulk product sales (a business-to-business model). Therefore, we also consolidated our retail outlets starting around the beginning of fiscal year 2011 and, by the end of fiscal year 2011, the Company had closed all of its retail outlets.
As of March 31, 2012, we had a distribution network of 39 distributors for our retail products in China, and we have a fast-growing customer base of 71 institutional customers for our bulk products in dairy, animal feed, pharmaceutical, and other food industries. In addition, we have a website and two active online selling partners for selling our retail products online.
Introduction of new products
During fiscal year 2012, we launched several new retail products under the Shining brand. We currently have regulatory approval to produce 46 retail products that can be marketed under the Shining brand. We are continuing to develop new products to strengthen our retail product pipeline so that we may offer an array of retail products for sale in the market.
Under the “Growing” or “润盈(Chinese)” brand name, we have launched over ten new bulk additive products since May 2010, including one for animal feed, one for a medical and health supplement, one for a food product, and several others for yogurt products. In order to meet the demand of our institutional customers, we continue to develop new products to enrich our bulk products series. As of March 31, 2012, we had a total of 62 bulk additive products available for sale, of which 25 are actively sold.
Our Business Prospects
Growth potential from geographic expansion leveraging on the Shining brand.
We have experienced continued, long-term sales growth of our retail products that are sold through retail channels, mainly in the greater Shanghai area under our Shining brand. Management believes that the Shining brand is one of the most recognized health supplement brands in Shanghai. We are expanding the distribution network of our retail products to another major metropolitan area, Beijing. Given the high level of disposable income in the Beijing area, management anticipates that distribution in the greater Beijing area could be profitable. Expansion of retail sales is also a key component in helping to grow our food additives products. We intend to co-brand with food producers allowing consumers to identify food products that use our additives as high quality and beneficial. As of March 31, 2012, we had entered into agreements with 71 institutional customers for use of our bulk products.
| 9 |
Significant potential from the new bulk business (yogurt).
Live bacteria are essential to the formulation of yogurt and yogurt-based drinks. Yogurt and yogurt drinks were the fastest-growing food product segments in China in 2004 according to AC Nielsen. However, yogurt producers in China currently import most of their probiotics additives. We believe that importing probiotics is costly and that a portion of the effective ingredient (bacteria) ceases to function during transportation. Our new Qingpu plant is being used for local production and supply of bulk manufacturing of probiotics for use as food additives for foods such as yogurt and yogurt drinks.
Significant potential from the new bulk business (milk powder).
Manufacturers have begun to add probiotics to infant formula and milk powders to improve digestion and absorption, as well as to strengthen the immune systems of infants. Currently, infant formula made in China by some multinational companies such as Nestle and Mead Johnson uses imported probiotics produced by Rodia SA, Chr. Hansen and other producers. As noted above, according to statements made by the Nutrition Development Centre of National Development and Reform Commission in China, effective April 1, 2007, probiotics are required to be added to baby milk powders produced in China, and products made by the Company were included on a list published on October 24, 2011 by the MOH of probiotics applicable for baby food for babies aged one year and older. Besides, owning to the continuing monitoring of food safety in China, updated relevant rules and regulations are expected to be announced at a later date and from time to time. Currently, the Company’s management believes there is no other manufacturing facility in China that can meet the demand for probiotics if the aforementioned requirement is imposed. We believe we are well positioned to capture this significant new demand for probiotics.
Advanced technology provides product quality advantages.
We believe our proprietary production technologies give us the following competitive advantages:
| · | Product shelf life - Our proprietary technology helps to protect the live bacteria in probiotics and allows a survival rate of 70% two years after manufacture. |
| · | Concentration - The concentration of active ingredients we produce is over 100 times that of the minimum governmental standards in China. |
| · | Human compatibility - The probiotics we produce originate from organisms cultured from human sources, reducing the risk that the active ingredients will be rejected by the human body. |
Revenue and Cash Flow
Our probiotics products may or may not be able to generate sufficient cash flow to finance our operations. Sales of our probiotics products decreased 45.9% to $58.9 million in fiscal year 2012 from $108.8 million in fiscal year 2011. Income before taxes decreased from $45.9 million in fiscal year 2011 to $5.6 million in fiscal year 2012. Net cash provided by operating activities was $1.3 million and $26.1 million for the years ended March 31, 2012 and 2011, respectively.
Production
We use microecology technologies to produce live bacteria, which is the active ingredients of our probiotics. We use a multi-stage fermentation process under a strictly controlled environment utilizing our proprietary technology. Solid bacteria are then extracted and stored using controlled freeze drying method. Prior to sales to our customers, we transform the solid bacteria into capsule form and place it in sealed double aluminum packaging using our patented equipment.
We have registered the following patents in China:
| · | Nutrition Gas Injection Capability and Double Aluminum Packaging Machine (patent registration number ZL 01 2 04515.2): enables the probiotics bacterium to retain their vitality for up to two years and to better resist gastric acid; |
| · | Packaging for Shining Essence (patent registration number ZL 01 3 01526.5); |
| 10 |
| · | High Quality Microecologics and Microencapsulation Technology (patent registration number ZL 01 1 09063.4): increases the vitality rate, maintaining large quantities of active bacterium; |
| · | Blood Cholesterol Reduction Agent (patent registration number ZL 2004 1 0025139.8); |
| · | Home-made Direct Flow Style Yogurt Leaven and Preparation Method (patent registration number ZL 2008 1 0202071.4); |
| · | Yogurt Powder (patent registration number ZL 2008 1 0202072.9); |
| · | A disk centrifuge for probiotics separation (patent registration number ZL 2011 2 0516219.9); |
| · | An airlift fermenter for lactic acid bacteria production (patent registration number ZL 2011 2 0516395.2); |
| · | A fermenter with dentate stirring blade (patent registration number ZL 2011 2 0516398.6); and |
| · | Equipment of lactic acid bacteria granulation and quantitative package (patent registration number ZL 2011 2 0516215.0). |
From fiscal years 2008 through 2012, we submitted to the Intellectual Property Bureau of China 22 applications for registration of patents regarding the production of our products, which applications are in different stages: patents for ten such applications have been granted and have not yet expired, two patents have been granted but have expired and applications for ten patents are currently under review.
Our management believes that we enjoy the following competitive advantages in utilizing such microecologics technology in our production process:
| · | With advanced fermentation, bacteria extraction and micro-encapsulation technology, we can easily increase our production output and reduce costs. |
| · | Since probiotics are extremely sensitive to water, acid and oxygen, their life span is very short. We use technology that significantly extends the survival rate of the bacteria and, as a result, our products have a survival rate of up to two years from manufacturing at room temperature. |
| · | According to rules governing live bacteria products in China which took effect in 2001, such products need to maintain concentrations of live bacteria at a level of 106/g within their stated effective period. Our products maintain a 108/g concentration of live bacteria during their stated effective period. This concentration level is also over 200 times higher than the current commonly accepted international standard. |
| · | Most probiotics producers extract their bacteria base from animals. The probiotics we produce originate from organisms cultured from human sources to reduce the risk that the active ingredients will be rejected by the human body. |
Distribution
We sell our retail products primarily in the greater Shanghai area to large distributors who then sell them through their networks to supermarkets, hypermarkets, and drug stores. As of March 31, 2012, we had 39 distributors located in Beijing, Shanghai, Jiangsu, Zhejiang, Harbin, Anhui and Hong Kong. As of March 31, 2011, we closed all of our retail outlets, as we believe the distribution network for, and internet sales of, our retail products sales are more efficient. Comparatively, direct selling through retail outlets involves increasing leasing expenses and staffing costs, while internet sales are the most cost-effective and direct way of reaching customers across China.
Customers
We have two different types of customers: individual retail products consumers and institutional bulk additive products customers. Institutional customers include dairy manufacturers, animal feed manufacturers, pharmaceutical companies, and food companies. Individual consumers are primarily located in major metropolitan areas and they are middle-aged or above and have middle to higher income levels. We believe that these individuals are becoming increasingly health-conscious and, as their income levels increase, they will likely spend more on health-related products such as probiotics products. We sell our retail products through large distributors, who then sell them through their networks to supermarkets, hypermarkets, and drug stores, where they are purchased by consumers. For the fiscal year ended March 31, 2012, we maintained active business relationship with 39 distributors for retail products and 71 institutional customers for bulk products, and no customer accounts for more than 10% of our sales revenue.
| 11 |
Backlog
We do not have any material backlog. Due to market fluctuation and the high profile on food safety supervision by the PRC Government in recently years, we sell our products on a spot-basis as orders are made.
Seasonality
Regarding our retail products, many of our customers purchase our products as gifts during the Chinese festivals and holidays, and the seasonal effect is correlated to these days during the year. For the first two fiscal quarters ending in September, there are no major Chinese festivals or holidays, except for the Dragon Boat Festival and mid-Autumn Festival. However, in the last two fiscal quarters ending in March, there are some major Chinese and Western festivals and national holidays, including National Day, Christmas, New Year, and Chinese New Year.
With respect to our bulk additive products, while it is still too early to tell, we expect that our bulk additives sales will not be seasonal in nature because the bulk products are purchased by food manufacturers consistently over the year. Except for the possibility of the PRC government implementing from time to time new or different rules and regulations for the food industry, including with respect to additives and related products, we are not aware of and do not foresee any seasonal effects on our bulk additive products business.
Marketing and Advertising
We promote our products through the media by placing advertisements in newspapers and magazines and on television and radio mainly in Shanghai, China. From time to time, we also sponsor local and territorial charitable events mainly in Shanghai to increase public awareness of the benefits of our health products.
Competition
We believe that we are well-positioned to compete in the Chinese pharmaceutical and nutra-ceutical market with our proprietary technology, strong brand, diverse product portfolio, research and development capabilities, established sales and service network and favorable cost structure. Other factors affecting competitive conditions in the Chinese pharmaceutical and nutra-ceutical market are our managerial and technological expertise, the ability to identify and capitalize on commercially viable products, time to market, patent position, product efficacy, safety, convenience, reliability, availability and pricing.
For retail products, our primary competitors in the Chinese domestic probiotics market are Biostime Inc., Shanghai Jiao Da Onlly Co., Ltd. and Shanghai Pharmaceutical Group Co. Ltd. – SINE Pharmaceutical Co. Ltd. These competitors produce similar retail probiotics products in the Chinese domestic market. In addition to these primary competitors, there are other secondary domestic competitors that compete with us in the Chinese market.
With respect to the bulk additives market, we believe that our competition comes mainly from large overseas producers and food importers, including Danisco A/S and Chr Hansen A/S, which produce their own supplements in facilities located outside China. In addition, a few foreign companies, like DSM, also entered the Chinese domestic market for probiotics products in recent years. However, our management believes that we are well-positioned to compete in the bulk additives market based on the high quality of our products, our favorable cost structure, our advantages in local production and transportation, our time-to-market in the domestic market and our established sales and service network. We are directing efforts toward encouraging customers to switch from imported bacteria to our products as additives for the production of yogurt, sour milk and other food products.
Research and Development
We have a strong research and development team supported by a technical advisory board of experts. As of March 31, 2012, we had 41 staff members with Masters Degrees or PhDs. In addition to having advanced technology in bacteria culturing and protection, we also conduct research and development into complimentary technology, including genetically engineered drugs, drug delivery solutions and Chinese medicine, in an effort to formulate solutions to address specific health problems and expand our product line. We incurred research and development costs of approximately $5,084,949 and $6,730,514 for the fiscal years ended March 31, 2012 and 2011, respectively. Such research and development costs are mainly comprised of raw material costs, laboratory expenses and staff salaries in the research and development division, which were included as part of the production costs in our financial statements for such periods.
| 12 |
Government Regulation
The Food Business
Laws and regulations governing our business include the following: the Law on the Food Conditions of the PRC promulgated and effective as of October 30, 1995; the Administrative Rules for Healthy Food promulgated by the Ministry of Health, or MOH, on March 15, 1996 and effective as of June 1, 1996; the Notice of Circulating the Appraisal Standard of Fungal and Good-Live-Bacterial Healthy Food and its appendixes: the Appraisal Standard of Fungal and Good-Live-Bacterial Healthy Food and the List of Good-Live-Bacteria Applicable for Healthy Food, both of which are promulgated by the MOH and effective as of March 23, 2001; and the Administration Rules for the Registration of Healthy Food (experimental) promulgated by the State Food and Drug Administration, or SFDA, on April 30, 2005 and effective as of July 1, 2005.
The previous governing authority of healthy food was the MOH. Since the General Office of the State Council of the PRC promulgated the Regulations on the Internal Organizations and Staff Schedule of the State Food and Drug Administration on April 25, 2003, the responsibility of approving healthy food of MOH has been assigned to the SFDA. The SFDA is a direct subordinate authority under the State Council and its responsibilities are generally supervising the safety control of food, healthy food and cosmetics, and supervising drugs.
Pursuant to the Law on the Food Conditions of the PRC, a food manufacturing or other food-related enterprise may not engage in any food manufacturing or other food-related business until it obtains a Health License issued by the competent health administration. While using a new resource in manufacturing food, before the formal production, the company must apply for an approval in accordance with applicable standard food condition application procedures, and obtain a New Food and Food-used Products Health Approval.
Pursuant to the Administrative Rules for Healthy Food, the MOH applied an approval system for healthy food. Any food claiming to have health care functions was required to be inspected and confirmed by the MOH, which would issue a Certificate of Healthy Food upon a successful inspection. After the Administration Rules for the Registration of Healthy Food were enacted, SFDA will make an integral appraisal and inspection of the safety, effectiveness, quality control and the label and introduction of the healthy food. If permitted, the SFDA will issue an Approval Certificate of Native Healthy Food, which is valid for five years.
Pursuant to the Appraisal Standard of Fungal and Good-Live-Bacterial Healthy Food, an enterprise using good-live-bacteria in manufacturing healthy food must satisfy the following requirements: form a Good Manufacturing Practice (GMP) and a step-by-step Hazard Analysis Critical Control Point (HACCP) quality control system; possess a pilot scale experiment manufacturing scale (at least 500 cubic liters), and submit its pilot scale experiment products for approval; have special plants or workshop, specific manufacturing equipment and devices, a good-live-bacteria lab, special staffs looking after the bacteria under the supervision of experts with at least middle level expert title, and specific technical rules and procedures. In addition, these Rules require that good-live-bacterial healthy food must maintain its active bacteria population at more than 106 cfu/ml during its storage term.
Pursuant to the List of Good-Live-Bacteria Applicable for Healthy Food issued by MOH on April 22, 2010 in accordance with the Food Safety Law of the PRC, 21 good-live-bacteria can be used in healthy foods, including
| A | Bifidobacterium |
| 1 | Bifidobacterium adolescentis |
| 2 | Bifidobacterium animalis (Bifidobacterium lactis) |
| 3 | Bifidobacterium bifidum |
| 4 | Bifidobacterium breve |
| 5 | Bifidobacterium infantis |
| 6 | Bifidobacterium longum |
| B | Lactobacillus |
| 1 | Lactobacillus acidophilus |
| 2 | Lactobacillus casei |
| 3 | Lactobacillus crispatus |
| 4 | Lactobacillus delbrueckii subsp. Bulgaricus (Lactobacillus bulgaricus) |
| 5 | Lactobacillus delbrueckii subsp. lactis |
| 6 | Lactobacillus fermentium |
| 7 | Lactobacillus gasseri |
| 8 | Lactobacillus helveticus |
| 9 | Lactobacillus johnsonii |
| 10 | Lactobacillus paracasei |
| 11 | Lactobacillus plantarum |
| 12 | Lactobacillus reuteri |
| 13 | Lactobacillus rhamnosus |
| 14 | Lactobacillus salivarius |
| C | Streptococcus |
| 1 | Streptococcus thermophilus |
| 13 |
Intellectual Property
The tables below set forth the trademarks and patents that we have registered in China. The trademarks were granted by the Trademark Office of State Administration of Industry and Commerce of the People’s Republic of China and the patents were granted by the State Intellectual Property Administration Office of the People’s Republic of China. Each of these trademarks and patents is enforceable only within China.
Trademarks
| Description | Registration No. |
Class | Term | |||
| Logo of Shanghai Shining Biotechnology Co. Ltd. and device | 1610780 | 30 | July 28, 2001 to July 21, 2021 | |||
| “Shining” | 1554223 | 30 | Up to April 13, 2021 | |||
| Two Chinese characters “双金”(translation: “Shining”) | 1675162 | 30 | November 28, 2001 to November 27, 2021 | |||
| “Shining Essence” | 1675163 | 30 | November 28, 2001 to November 27, 2021 | |||
| Device containing 2 cartoon figures | 3304485 | 30 | January 21, 2004 to January 20, 2014 | |||
Four Chinese characters “双金金盾 “ (translation: “Shining Golden Shield”) |
3304374 | 30 | Up to November 6, 2013 | |||
Four Chinese characters “医生有益 “ (translation: “Probiotics are beneficial”) |
5227367 | 30 | March 28, 2009 to March 27, 2019 | |||
Five Chinese characters “ 双金有益菌 “ (translation: “Shining beneficial bacteria”) |
4095567 | 30 | October 14, 2006 to October 13, 2016 | |||
Logo of Five Chinese characters “ 双金有益菌 “ (translation: “Shining beneficial bacteria”) |
6974213 | 30 | Up to September 27, 2020 | |||
| Two Chinese characters “ 润盈” (translation: “Growing”) | 7269694 | 1 | January 7, 2011 to January 6, 2021 | |||
| Two Chinese characters “ 润盈” (translation: “Growing”) | 7269746 | 31 | March 14, 2012 to March 3, 2022 | |||
| “Growing” | 7269680 | 1 | January 14, 2011 to January 13, 2021 | |||
| “Growing” | 7269757 | 31 | May 7, 2011 to May 6, 2021 | |||
| Logo of Growing Bioengineering (Shanghai) Co. Ltd. and device | 8701433 | 5 | October 7, 2011 to October 6, 2021 | |||
| Logo of Growing Bioengineering (Shanghai) Co. Ltd. and device | 8701393 | 1 | October 7, 2011 to October 6, 2021 | |||
| Logo of Growing Bioengineering (Shanghai) Co. Ltd. and device | 8701413 | 31 | October 21, 2011 to October 20, 2021 | |||
| “Biogrowing” | 8701389 | 1 | October 7, 2011 to October 6, 2021 | |||
| “Biogrowing” | 8701419 | 31 | October 21, 2011 to October 20, 2021 |
Pursuant to the Trademark Law of the PRC and its implementation rules amended on October 27, 2001, a registered capital may refer to a trademark registered with the Trademark Bureau, including products, service trademarks, collective trademarks, and attest trademarks; a trademark owner shall have exclusive rights to use the trademark under the protection of applicable law. The exclusive rights of using a trademark is limited within a registered trademark and the registered products on which a trademark can be used. The Trademark Bureau of the State Administration for Industry and Commerce is responsible for managing the trademark registration and administration throughout the PRC. The protection period of a registered trademark is ten years from the registration date.
Patents
| Type | Patent No | Term | ||
| High Quality Microecologics and Microencapsulation Technology | ZL 01 1 09063.4 | February 28, 2001 to February 27, 2021 | ||
| Blood Cholesterol Reduction Agent | ZL 2004 1 0025139.8 | June 11, 2004 to June 10, 2014 | ||
| Home-made Direct Flow Style Yogurt Leaven and Preparation Method | ZL 2008 1 0202071.4 | October 31, 2008 to October 30, 2028 | ||
| Yogurt Powder | ZL 2008 1 0202072.9 | October 31, 2008 to October 30, 2028 | ||
| Probiotics biscuit materials, biscuit and its production method | ZL 2008 1 0035001.4 | March 12, 2008 to March 20, 2028 | ||
| Piggy Compound Microorganism Feeding Additive | ZL 2009 1 0045386.7 | January 15, 2009 to January 14 ,2029 | ||
| A disk centrifuge for probiotics separation | ZL 2011 2 0516219.9 | December 12, 2011 to December 11, 2021 | ||
| An airlift fermenter for lactic acid bacteria production | ZL 2011 2 0516395.2 | December 12, 2011 to December 11, 2021 | ||
| A fermenter with dentate stirring blade | ZL 2011 2 0516398.6 | December 12, 2011 to December 11, 2021 | ||
| Equipment of lactic acid bacteria granulation and quantitative package | ZL 2011 2 0516215.0 | December 12, 2011 to December 11, 2021 |
| 14 |
Retired Patents
| Type | Patent No | Term | ||
| Nutrition Gas Injection Capability and Double Aluminum Packaging Machine | ZL 01 2 04515.2 | February 28, 2001 to February 27, 2011 | ||
| Packaging for Shining Essence | ZL 01 3 01526.5 | February 28, 2001 to February 27, 2011 |
We have a total of 22 patents in different stages of the application, review or grant process, including ten patents that have been granted and have not yet expired, two patents that have been granted but have expired, and ten patents currently under review.
Pursuant to the Patent Law of the PRC and its implementation rules amended on August 25, 2000, Chinese laws protect the following three kinds of patents: patents for inventions, patents for utility models, and patents for designs. The State Bureau of Intellectual Property is responsible for the management of patents in China, accepting and reviewing patent applications and granting patents pursuant to applicable laws and regulations. Any invention or utility model for which a patent right may be granted must possess novelty, inventiveness, and practical applicability. Any design for which a patent right may be granted must neither be identical with, or similar to, any design which, before the date of filing, has been publicly disclosed in publications in the country or abroad or has been publicly used in the country, nor conflict with legal rights of any third party obtained before. The protection period of a patent for invention is 20 years, and the protection period of a patent for utility model or design is ten years, both calculated from the application date.
Pursuant to the Patent Law of the PRC, a retired patent is not subject to patent protection. However, the loss of protection of the retired patents has had no adverse effect on the production and sales of our products.
Under the Patent Law of the PRC, we may enforce our rights attached to the registered patents against an infringer by applying to the relevant governing authorities for an injunction. We may also apply to the Supreme People’s Court of the PRC for an order of specific performance, which prohibits any third parties from using the registered patents. The relevant governing authorities may also impose a fine up to three times the profits made by the infringer from the unauthorized use of the registered patents or a fixed fine up to RMB50,000 for cases in which the infringer has not earned any profits from such unauthorized uses.
Taxation and Local Governmental Support
Income tax of a foreign-invested enterprise in China is principally governed by the Law on the Income Tax of Foreign-Invested Enterprises and Foreign Enterprises of the PRC and its implementation rules promulgated and effective as of July 1, 1991. Pursuant to those law and regulations, the corporate income tax rate of a foreign-invested enterprise is 25%, and the local income tax rate is 3%. However, foreign-invested enterprises which are located in certain areas or satisfy certain qualifications are entitled to a corporate income tax exemption or deduction. For instance, a manufacturing foreign-invested enterprise established in Pudong District, Shanghai, is entitled to pay its corporate income tax at a reduced tax rate of 15%. In addition, a manufacturing foreign-invested enterprise, with a business term in excess of ten years, is entitled to a two-year corporate income tax exemption calculating from its first profitable year, and for the following three years, such foreign-invested enterprise is entitled to a half deduction of its applicable corporate income tax rate. From January 1, 2008, the income tax rate has gradually increased to the standard rate of 25% over a five-year transition period. The five-year transitional rates for former tax rate that was 15% was 18% in 2008, 20% in 2009, 22% in 2010, 24% in 2011, and 25% in 2012. Pursuant to PRC laws and regulations, if an enterprise is qualified as “high-tech” enterprise, it would be entitled to the 15% preferential rate under the new CIT regime.
Foreign Exchange
Foreign exchange in China is principally governed by the PRC Foreign Exchange Control Regulations promulgated by the State Council and effective as of April 1, 1996, and the Regulations on the Administration of Foreign Exchange Settlement, Sale and Payment promulgated by the State Council and effective as of July 1, 1996. Under these regulations, upon payment of the applicable taxes, foreign-invested enterprises may convert the dividends they received in Renminbi into foreign currencies and remit such amounts outside China through their foreign exchange bank accounts.
| 15 |
If a foreign-invested enterprise needs foreign exchange transaction services in relation to the current account item, it may make such payment through its foreign exchange account or make an exchange and payment at one of the designated foreign exchange banks by providing applicable receipts and certificates, and with an approval from the State Administration of Foreign Exchange, or SAFE. If a foreign-invested enterprise distributes dividends to its shareholders, it will be deemed as foreign exchange transaction services in relation to the current account item, therefore, as long as it provides the board resolutions and other documents authorizing the distribution of dividends, it may make such payment through its foreign exchange account or make an exchange and payment at one of the designated foreign exchange banks.
Notwithstanding the above, foreign exchange conversion matters under the capital account item are still subject to regulatory restrictions, and prior approval from SAFE or its relevant branches is required before conversion between Renminbi and other foreign currencies.
Facilities
On July 22, 2010, we acquired a property located at No. 26 Orient Global Headquarter Lane 118, Yonghe Road, Zhabei District, Shanghai 200072, People’s Republic of China as our principal executive office.
We conduct our retail probiotics products production from a leased facility in Pudong, Shanghai. Pursuant to our lease for this facility, which expires on December 31, 2013, we pay annual rent of $124,894 (RMB798,919), payable in quarterly installments of $31,223 (RMB199,730). This facility, which includes a level 100,000 clean room and a level 10,000 clean room, houses our office space, manufacturing facilities and warehouse. The maximum current production capacity at this location is approximately 3.5 million capsules per month. We have received GMP, ISO 9001, ISO 14001, OHSAS 18001 and HACCP certifications for this facility. See “Business—Current Operations—Overview” for further information with respect to these certifications.
We have expanded into the bulk additives business for functional foods through the completion of our 300-metric ton capacity plant in Qingpu, which commenced production in February 2010. Phase 1 of the project involved constructing a facility with a production capacity of 150 metric tons of probiotics per year that cost $36 million in total, the sum of which was invested in calendar year 2010. Phase 2 of this project commenced in September 2010 and cost $18 million, $11 million of which was paid in calendar year 2010 and the remaining $7 million of which was paid off by the end of 2011, the date when construction of Phase 2 was completed. Phase 2 added an additional 150 metric tons of annual capacity. The construction of Phase 1 of the plant was funded by cash received from the sale of a convertible promissory note in December 2007 and the proceeds of the public offering of our common stock in October 2009. The construction cost of Phase 2 of the plant was funded by cash received from the public offering of our common stock in October 2009.
On March 21, 2006, GSL, our subsidiary, entered into an agreement, as amended, with Shanghai Qingpu Industrial Park District Development (Group) Company Limited for the lease of 36,075 square meters of land in the Qingpu on which we constructed the 300-metric ton capacity production plant described above for a term of 50 years beginning January 15, 2008. The agreement provides for the payment of leasing fees of approximately $1.8 million. In February 2009, the formal land use right certificate was issued. There are no future lease payments under this land lease.
The Company has commenced construction of a new facility in the Yangling Zone in the Shaanxi Province of China. The cost for constructing the Yangling Zone facility is expected to be over $58 million invested over two years. The facility will produce probiotics and probiotics-related biological additives for the animal feed industry. Currently, the facility is in the construction stage, and the plan is subject to government approval prior to implementation. During the year ended March 31, 2011, the Company made total payments of approximately $3.25 million (RMB20,608,112) to acquire the land use right of approximately 122,600 square meters (183.94 mu). We received the land use right certificate on January 28, 2012 and commenced construction in June 2012. We expect to complete construction by June 2014 and trial production is expected to commence in 2015. In the existing market environment, the Company will utilize its available cash and bank facilities to fund such construction.
Employees
As of March 31, 2012, we had 387 staff and employees. The following table summarizes the functional distribution of our employees:
| Department | Headcount | |
| Management and Administrative | 46 | |
| Sales and Marketing | 100 | |
| Research and Development | 44 | |
| Production | 169 | |
| Finance and Accounting | 18 | |
| Engineering | 10 | |
| Total | 387 |
| 16 |
All of these employees were full-time. As of March 31, 2012, we did not have any payment obligations for any retirees and were not retaining any contractors.
According to Article 10 of the Trade Union Law of the People’s Republic of China, an enterprise, public institution, or government organ with 25 or more members must establish a basic-level trade union committee. However, a union is established only if it is voluntarily formed by the employees. As of March 31, 2012, we did not have a trade union.
Available Information
We file periodic reports with the Securities and Exchange Commission (the “SEC”), including Annual Reports, on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports. All of our periodic reports may be inspected without charge at the Public Reference Room maintained by the SEC at 100 F Street, NE, Washington, D.C. 20549. You may obtain copies of any registration statements, including the exhibits thereto, and all of our periodic reports, after payment of the fees prescribed by the SEC. For additional information regarding the operation of the Public Reference Room, you may call the SEC at 1-800-SEC-0330. The SEC also maintains a website which provides on-line access to reports and other information regarding registrants that file electronically with the SEC at the following web address: http://www.sec.gov.
On June 15, 2011, the Company timely filed a Form 12b-25 informing the SEC of its inability to file timely its Annual Report on Form 10-K for the year ended March 31, 2011. Since that filing, the Company has not filed Quarterly Reports on Form 10-Q for the quarters ended June 30, 2011, September 30, 2011 or December 31, 2011, although it has timely filed Forms 12b-25 disclosing its inability to file timely such Quarterly Reports. On June 29, 2012, the Company filed a Form 12b-25 disclosing its inability to file timely this Form 10-K for the year ended March 31, 2012. Since June 15, 2011, the Company has continued to comply with its obligations to file Forms 8-K when required.
On September 15, 2011, the staff of the SEC informed the Company that it intended to recommend that the SEC institute a public administrative proceeding against the Company for alleged violations of Section 13(a) of the Securities Exchange Act of 1934 and Rules 13a-1 and 13a-13 or 13a-16 promulgated thereunder. On October 7, 2011, the SEC issued an Order Instituting Proceedings to determine whether it was necessary and appropriate to suspend or revoke the registration of the Company’s securities. On February 22, 2012, the administrative law judge overseeing the proceedings issued a decision ordering that the registration of the Company’s securities be revoked. On March 26, 2012, the SEC granted the Company’s petition for review of this decision. The results of the SEC’s review are pending as of September 13, 2012. If the registration of the Company’s securities is revoked, no U.S. registered broker-dealer may execute trades in the Company’s shares and the trading market for our common stock may cease to exist. In such event, investors may not be able to liquidate their investment.
We maintain an internet website at http://www.chn-biotics.com. Our website contains a link to the SEC’s website at http://www.sec.gov, which provides free online access to our periodic reports. We will also provide electronic or paper copies of our filings free of charge upon request.
ITEM 1A. RISK FACTORS.
If the SEC revokes the registration of our common stock, the trading market for our common stock may cease to exist.
On September 15, 2011, the staff of the SEC informed the Company that it intended to recommend that the SEC institute a public administrative proceeding against the Company for alleged violations of Section 13(a) of the Securities Exchange Act of 1934 and Rules 13a-1 and 13a-13 or 13a-16 promulgated thereunder. On October 7, 2011, the SEC issued an Order Instituting Proceedings to determine whether it was necessary and appropriate to suspend or revoke the registration of the Company’s securities. On February 22, 2012, the administrative law judge overseeing the proceedings issued a decision order that the registration of the Company’s securities be revoked. On March 26, 2012, the SEC granted the Company’s petition for review of this decision. The results of the SEC’s review are pending as of September 13, 2012. If the registration of the Company’s securities is revoked, no U.S. registered broker-dealer may execute trades in the Company’s shares and the trading market for our common stock may cease to exist. In such event, investors may not be able to liquidate their investment.
Risks Related to Our Business
We have identified a lack of U.S. GAAP experience among our senior management personnel, a weakness in efficiency and controls related to the financial statement closing process and inconsistent corporate governance and financial controls as material weaknesses in our disclosure controls and procedures and our internal control over financial reporting, which have affected and could continue to affect our ability to ensure timely and reliable financial reports, have affected and could continue to affect the ability of our auditors to attest to the effectiveness of our internal controls should we once again become an accelerated filer in the future, and weaken investor confidence in our financial reporting.
| 17 |
As directed by Section 404 of the Sarbanes-Oxley Act of 2002, the SEC adopted rules requiring public companies in their annual reports to include a report of management on the reporting company’s disclosure controls and procedures and internal controls over financial reporting. As set forth in the report of our management included under Item 9A. “Controls and Procedures” of this Annual Report on Form 10-K, after reviewing our Annual Report on Form 10-K for the year ended March 31, 2010 and our Quarterly Report on Form 10-Q for the period ended December 31, 2010, the SEC concluded in a letter to the Company dated as of January 9, 2012, that the Company’s lack of U.S. GAAP experience is a material weakness in our disclosure controls and procedures and in our internal control over financial reporting. In its letter, the SEC also required the Company to file an amended Annual Report on Form 10-K for the year ended March 31, 2010 disclosing such material weakness, which the Company intends to file as promptly as is reasonably practicable. Also, as further set forth in the report of our management included under Item 9A. “Controls and Procedures” of this Annual Report on Form 10-K, our management has concluded that our disclosure controls and procedures were not effective as of March 31, 2012, and there existed material weaknesses in our internal control over financial reporting as of March 31, 2012. The Company’s Audit Committee and our management are still engaged in discussions about how best to rectify fully such weakness. The Audit Committee has recommended that the Company hire, and management has retained the services of, additional qualified personnel to prepare the Company’s books and records and financial statements in accordance with U.S. GAAP.
We depend on the services of our directors and key employees, the loss of which could harm our business.
We believe our success relies on the strategies, vision, efforts and technical expertise of our directors and key management personnel, including Mr. Song Jinan. The resignation or departure of any of these key people could have a material adverse impact on our operations and future prospects. In addition, if any of these key people join a competitor or form a competing company, we could lose customers and incur additional expenses to recruit replacements and train personnel. We have entered into standard form confidentiality agreements with our technical employees with the exception of our directors and our key executives, which contain non-competition clauses. We do not maintain key-man life insurance for any of our key executives.
Failure to attract and retain qualified employees may adversely affect our business.
Our continued success depends largely on our ability to attract and retain highly skilled executive, managerial and technical employees. We may face difficulties in recruiting skilled personnel in our industry due to its specialized nature. If we are unable to attract and retain a sufficient number of suitably skilled and qualified personnel, our business would be materially and adversely affected. We may also have to pay substantial wages to attract sufficient numbers of skilled employees and professionals, which may adversely affect our operating margins.
We are not insured against potential losses and could be seriously harmed by natural disasters, catastrophes or acts of war.
Our facilities and inventories could be materially damaged by hurricanes, floods and other natural disasters, catastrophes, acts of war or other catastrophic circumstances. We do not maintain insurance covering such events. If any of these events occur, we could incur material losses and liabilities, which could negatively affect our operating results.
We may incur material product liability claims, which could increase our costs and adversely affect our reputation, revenues and operating income.
As a manufacturer of products designed for human consumption, we are subject to product liability claims that the use of our products has resulted in injury. Our products contain three types of live bacteria, lactobacillus acidophilus, bifidobacterium bifidum and bifidobacterium adolescentis, which fall within the nine types of “good” live bacteria that are approved for direct sale to the public in China as health food. We obtain our bacteria from human sources. Although we believe this reduces the risk that it will be rejected by the human body, there can be no assurance that consumption of such bacteria could not result in adverse health effects. We do not maintain any product liability insurance. A product liability claim against us could result in costly litigation and could adversely affect our reputation with our customers, which in turn could adversely affect our revenues and operating income.
Our revenues primarily depend on sales of one product, and a decline in sales of this product could cause our revenues to decrease.
We receive a large portion of our revenue from the sale of our Shining Essence product. Sales of this product represented approximately 21% and 19% of our total sales of retail products for the years ended March 31, 2012 and 2011, respectively. We expect that Shining Essence will continue to account for a sizable portion of our revenues for the foreseeable future. In addition to Shining Essence, our research and development team has successfully developed other new retail products, such as Shining Probiotics Protein Powder, which represented approximately 38% and 24% of our total sales of retail products for the year ended March 31, 2012 and 2011, respectively. Any factors adversely affecting the pricing of, demand for, or market acceptance of, Shining Essence, including increased competition, could cause our revenues to decline and our business and future operating results to suffer.
| 18 |
Consumer concerns regarding the safety and quality of food products or health concerns could adversely affect sales of our products.
Our sales performance could be adversely affected if consumers lose confidence in the safety and quality of our products. Consumers in China are increasingly conscious of food safety and nutrition. Consumer concerns about, for example, the safety of pork products, or the safety of food additives used in processed meat products, could discourage them from buying certain products and cause our results of operations to suffer. Specifically, in 2011, there was some negative publicity regarding the quality and safety of certain meat products which had a broad effect on Chinese consumer habits with respect to many food products. While we believe that we maintain an advanced system for quality assurance and control, our operations may be impacted by the deteriorating reputation of the food industry in China due to recent food safety scandals. In addition, the product return program we commenced at the end of calendar year 2011 in response to certain of our products which were below the Company’s quality standards, and the special concession credits we made to certain of our distributors to address complaints from their customers in respect of such products, could have a negative effect on our customers’ perception of the safety and reliability of our probiotics products.
We are subject to concentrations of credit risk that could adversely affect our operations.
Our principal operations are in China and all of our sales during fiscal years 2012 and 2011 arose in China. A significant number of our financial instruments, principally cash and accounts receivable, are located in China. These financial instruments include:
| · | cash deposits in China, which includes the Special Administrative Region of Hong Kong, where there is currently no rule or regulation in place for obligatory insurance of bank accounts; |
| · | accounts receivable; and |
| · | a loan receivable. |
The concentration of these financial instruments in China subjects us to concentrations of credit risk that could adversely affect our operating results.
If our products fail to keep pace with advances in the industry, they may be displaced by competitors’ newly developed products.
Other companies in our industry may gain significant competitive advantages by introducing new products to the market, delivering constant innovation in products and techniques and offering competitive prices. Our future growth partially depends on our ability to develop products that are more effective in meeting consumer needs. In addition, we must be able to manufacture and effectively market those products. The sales of our existing products may decline if a competing product is introduced by other companies.
We may have difficulty competing with larger and better-financed companies in our industry, which could require us, among other things, to lower our prices and could result in the loss of our customers.
Some of our existing and future competitors may have greater technical and financial resources than we do and may use these resources to pursue a competitive position that threatens our products. Our products could be rendered obsolete or uneconomical by the development of new products to treat conditions addressed by our products, as a result of technological advances affecting the cost of production, or as a result of marketing or pricing action by one or more of our competitors.
Additionally, with China’s accession to the World Trade Organization, the Chinese government has undertaken to open up the Chinese market to foreign companies. China reduced its average import tariff rate overall to 11.50% in 2003 and has further reduced it to 9.90% in 2005. As a result, foreign competitors may form alliances with or acquire companies in our industry in China. Intensified competition from these foreign competitors may lead to lower profit margins due to price competition, loss of customers and slower than anticipated growth.
Unfavorable publicity or research reports casting a negative light on our industry or our products could change consumer perceptions and have an adverse affect on our ability to market and sell our products.
We believe that our industry is affected by media attention. Future research reports or publicity about the quality of products in our industry generally, or our products in particular, could have a material adverse effect on our business. Scientific research to date is preliminary and there can be no assurance that future scientific research or publicity will be favorable to our industry or any particular product or consistent with earlier favorable research or publicity. Adverse publicity could arise even if the adverse effects associated with such products resulted from consumers’ failure to consume such products appropriately. Given our dependence upon consumer perceptions, adverse publicity, whether or not accurate, associated with illness or other adverse effects resulting from the consumption of our products or any similar products distributed by other companies could have a material adverse effect on our business.
| 19 |
Our expansion into the bulk additives business may not generate sufficient revenues, and the construction of our new facilities to accommodate this business may result in increased costs and losses.
We are expanding our operations into the bulk additives business through the supply of high quality probiotics to be used as additives in, among other things, dairy products to manufacturers in China. We have completed construction of Phase I of our new Qingpu production plant, which has a 150-metric tons annual capacity that can accommodate our new bulk additives business. We began commercial production at this plant in February 2010. In addition, we commenced construction in June 2012 on a new facility in Yangling related to the production of probiotics and probiotics-related biological additives for the animal feed industries. This will expose us to many risks, including the following:
| · | there may not be sufficient market demand for bulk probiotics additives or our products in particular; |
| · | we may experience delays and cost overruns during construction of our new facilities, which may result in losses; and |
| · | we may experience substantial start up losses when the plants are first commissioned. |
Our shift in focus from retail to the bulk additives business, and the shift from direct selling to selling through a distribution network, may fail.
In our continuing effort to shift our focus from retail to bulk additives business and to improve operating efficiency, we have consolidated our retail outlets, as we believe the distribution network for our retail products is more efficient. Comparatively, directly selling through retail outlets involves increased leasing expenses and large staffing cost. By selecting six new distributors, we have expanded our distribution network into the Pan-Beijing area to sell the Company’s retail products. The local distributors sell the Company’s retail probiotics products through established distribution networks, including malls, hypermarkets, supermarkets, and functional food stores, adding approximately 30 new points of sale. As of March 31, 2012, we had a total of 39 distributors for retail products. In light of the increasing online sales of health food in China, we have started to work with two on-line selling companies, www.ule.com.cn and www.yihaodian.com, to sell our retail products. Furthermore, the Company has established and launched an in-house e-commerce department dedicated to promoting and selling our retail products online through the Company’s website at http://www.shiningbt.com/Product/.
With respect to our bulk additives products business, we had 71 customers for bulk additives probiotics products as of March 31, 2012. Among the 12 small to medium-sized new customers, one is an animal feed manufacturer, five are functional food, nutritional products and pharmaceutical producers, and the remaining six are dairy companies.
As we shift our focus from retail to bulk additives and implement a distribution network selling model, there is a risk that our current systems may not be able to accommodate the increased volume or the complexity of the future business. Our short term operating results may be adversely affected as additional capital investments will have to be made for system upgrades, replacements, or improvements.
We face potential tax exposure.
In addition to the Enterprise Income Tax (“EIT”), companies in the PRC that are engaged in the sale of goods are generally required to pay value added taxes (“VAT”) at a rate of 17% of the gross sales proceeds received, less any deductible VAT already paid or borne by the taxpayer.
Our management believes that our operations in China were exempted from EIT and VAT for all years prior to 2005 because we had been recognized by the local government as an advanced technology enterprise. However, the Company never received a written confirmation from the appropriate tax authorities for the tax exemption status of our operations in China prior to 2005. As a result, there is no way to ascertain the ultimate position which may be taken by the relevant PRC tax authorities in the future and accordingly, full provisions for tax liabilities in the amount of $12,734,640, for all years prior to 2005 have been recorded by the Company. Beginning in January 2006, we made tax payments to the PRC tax authorities for 2005 and we have made regular tax payments to the PRC tax authorities for all subsequent periods.
| 20 |
In addition, in connection with dividends paid to the Shining shareholders between April 2003 to June 2005, Shining did not deduct a withholding tax at the rate of 20% as required by applicable Chinese laws and regulations. The Company has accrued the dividend withholding tax and interest on the dividend withholding tax.
According to PRC tax regulations, the overdue tax liabilities in the PRC for the calendar years prior to 2005 may be subject to interest at the 0.05% per day, and potential penalties for the late payment of taxes which is calculated on the basis of 0.5 times to five times the amount of taxes payable. Through March 31, 2011, the Company accrued the interest that may be potentially payable upon these taxes incurred prior to 2005 and the unpaid dividend withholding tax. Interest related to the unpaid taxes was recognized in the Company’s consolidated statements of operations as a component of its income tax provision.
We may not be able to protect our intellectual property against claims by other parties or enforce our rights with respect to our intellectual property.
Without patents, we may have no legal recourse in the event that our processes and technologies are replicated by other parties. If our competitors are able to replicate our processes, technologies, and systems at lower costs, we may lose our competitive advantage and our profitability will be adversely affected.
In addition, we believe that over the last five years our “Shining” brand has become a highly recognizable brand in our industry in Shanghai. To protect this brand, which we consider important to our continued success, we have registered eight trademarks in China. If our competitors introduce products of inferior qualities to the market using trademarks that are confusingly similar to the “Shining” or “Growing” trademarks, our reputation and operating results will be adversely affected.
From time to time, we may have to resort to litigation to enforce our rights with respect to our intellectual property. This type of litigation could result in substantial costs and diversion of our resources, which would adversely affect our results of operations.
Management by a small team of officers may create conflicts of interests and impede the successful implementation of our growth plans.
Mr. Song and Dr. Chang, our only executive officers, are responsible for all managerial functions of the Company. We have been hiring additional employees to complete our management team, but we cannot assure you that we can assemble a management team that can tackle the expansion plans that we have. The concentration of management could be disadvantageous to stockholders with interests different from those of Mr. Song or Dr. Chang.
Risks Related to Government Regulations
We are subject to government regulation in China, and changes in Chinese regulations may substantially increase the cost of manufacturing and selling our products.
The manufacturing and marketing of our products are subject to various governmental regulations in China. Government regulation includes inspection of and controls over manufacturing, safety and environmental controls, efficacy, labeling and the sale and distribution of wellness products.
As a company which produces probiotics supplements, we are subject to the Law on the Food Conditions of the PRC which became effective on October 30, 1995, the Administrative Rules for Healthy Food promulgated by the Ministry of Health on March 15, 1996 which became effective on June 1, 1996, the Notice of Circulating the Appraisal Standard of Fungal and Good-Live-Bacterial Healthy Food and its appendixes-Appraisal Standard of Fungal and Good-Live-Bacterial Healthy Food, and List of Good-Live-Bacteria Applicable for Healthy Food, promulgated by the Ministry of Health which became effective on March 23, 2001, the Administration Rules for the Registration of Healthy Food (experimental) promulgated by the State Food and Drug Administration on April 30, 2005, which became effective on July 1, 2005, and other relevant rules and regulations issued by the Ministry of Health and the State Food and Drug Administration. In addition, Shining is a Chinese corporation and therefore is subject to the Company Law of China and more specifically to the Foreign Company provisions of the Company Law and the Law on Foreign Capital Enterprises of China.
| 21 |
Our industry is relatively new in China, and the manner and extent to which it is regulated is evolving. Changes in existing laws or new interpretations of such laws may have a significant impact on our methods and costs of doing business. For example, new legislative proposals that affect our product pricing, reimbursement levels, approval criteria and manufacturing requirements may be proposed and adopted.
The costs of compliance with current or future legislation or regulatory requirements may be significant, and could force us to curtail our operations or otherwise have a material adverse effect on our financial condition, results of operations or cash flows. For example, we have obtained three licenses and permits which are required for us to operate our business in China. If the regulations regarding these licenses and permits are changed, it may be materially burdensome for us to obtain or renew these licenses and permits or they may be otherwise unavailable.
Government regulation of our retail prices and advertising methods may adversely affect our results of operations.
We are subject to government regulations with respect to the prices we charge, the rebates we may offer to customers and our marketing methods. In addition, we are required to obtain approval from Chinese government authorities regarding the contents of advertisements related to our products before they can be published. If the Chinese government requires that we set our retail prices at undesirable prices or significantly limits our ability to advertise our products, it could have a material adverse effect on our results of operations.
We may not be able to obtain regulatory approvals for our products or reimbursement from the sale of our products.
The manufacture and sale of our products in China is highly regulated by a number of state, regional and local authorities. These regulations significantly increase the difficulty and costs involved in obtaining and maintaining regulatory approval for marketing new and existing products. In addition, our future growth and profitability are, to a significant extent, dependent upon our ability to obtain timely regulatory approvals from the relevant authorities.
We may not be able to obtain manufacturing or marketing approvals or pass on-site inspections for our current and future products, including re-registration certification and re-evaluation of our products or our production facilities, and failure to obtain the necessary approvals or pass as referenced above could materially harm our business prospects.
Certain capsules that are used by the Company as delivery methods for some of its probiotics products are considered medicines and so are regulated as such. All medicines must be approved by the State Food and Drug Administration (SFDA) before they can be manufactured, marketed or sold in the PRC. The SFDA requires a pharmaceutical manufacturer to successfully complete clinical trials of a new medicine and demonstrate its manufacturing capability before approval to manufacture that new medicine is granted. Clinical trials are expensive and their results are uncertain. It usually takes two to five years for a manufacturer to obtain approval of a typical SFDA production application. However, the SFDA may not strictly adhere to such general timeline and may alter the review procedure. We have no ability to foresee or control any such changes in procedure, which could delay our launch of new products. Furthermore, the SFDA and other regulatory authorities may apply new standards for safety, manufacturing, labeling, marketing and distribution of future products. Complying with these standards may be time-consuming and expensive. In addition, our future products may not be efficacious or may have undesirable or unintended side effects, toxicities or other characteristics that may preclude us from obtaining approval or may prevent or limit their commercial use. As a result, we may not be able to obtain SFDA or other governmental approvals for our future products on a timely basis or at all.
Furthermore, even after we obtain such approvals for a proposed product, we may not be able to pass the on-site inspections required prior to the launch of such proposed product. If we fail to pass the on-site inspection in connection with a production permit application, we will not be able to obtain the production permit and commence production. Failure to obtain or renew approvals or pass on-site inspections for our existing or future products could materially harm our business prospects. In addition, in connection with our manufacture of any new products that will require us to add to or expand our existing production lines or to construct new production lines, we will be required to obtain production permits. Failure to obtain such permits could render us unable to produce any new products.
Failure to comply with applicable GMP standards could have a material adverse effect on our business, financial condition and results of operations.
We are required to comply with applicable GMP regulations, which include requirements relating to personnel, premises and equipment, raw materials and products, qualification and validation, document management, production management, quality control and quality assurance and products distribution and recall. Manufacturing facilities must be approved by governmental authorities before we can use them to commercially manufacture our products and are subject to inspection by regulatory agencies. The SFDA has implemented more stringent GMP standards which are aimed at improving drug production management and controlling risks in the production process and introduce internationally-recognized quality control mechanisms. The latest update to the GMP standards requires increased quality control and documentation and improved overall manufacturing processes, thus causing an increase of cost in manufacturing and decrease of profit margins.
| 22 |
A pharmaceutical manufacturer must meet the new GMP standards, which became effective on March 1, 2011, for each of its production facilities in China with respect to each form of pharmaceutical products it produces within a five-year grace period. Manufacturers of injectables, blood products or vaccines have a three-year grace period to bring existing facilities in line with the revisions.
Although each of our eight production lines meets GMP guidelines promulgated in 1998 and we are in the process of upgrading our production facilities to bring them in line with the new GMP standards, we may not obtain clearance from the SFDA in the event that we are inspected. Any failure to comply with the new GMP standards may subject us to fines or other penalties, which may have a material and adverse impact on our business, financial condition and results of operations.
The PRC Government has focused more on food safety in recent years and we may be subject to substantial liabilities should the consumption of any of our products cause personal injury or illness, and unlike most food processing companies in the United States, we do not maintain product liability insurance to cover our potential liabilities.
The sale of food products for human consumption involves an inherent risk of injury to consumers. Such injuries may result from tampering by unauthorized third parties, product contamination or degeneration, including the presence of foreign contaminants, chemical substances or other agents or residues during the various stages of the procurement and production process. The PRC’s Food Safety Law, which became effective on June 1, 2009, enhances the supervision and examination of PRC governmental authorities over food production and provides that no exemption from such inspections and examinations shall be permitted. While we are subject to governmental inspections and regulations, we cannot assure you that consumption of our products will not cause a health-related illness in the future, or that we will not be subject to claims or lawsuits relating to such matters.
Even if a product liability claim is unsuccessful or is not fully pursued, the negative publicity surrounding any assertions that our products caused personal injury or illness could adversely affect our reputation with customers and our corporate and brand image. In addition, the product return program we commenced at the end of calendar year 2011 in response to certain of our products which were below the Company’s customary quality standards, and the special credits that were given to certain of our distributors to address complaints from their customers in respect of such products, could have a negative effect on our customers’ perception of the safety and reliability of our probiotics products. Unlike most food processing companies in the United States, but in line with industry practice in China, we do not maintain product liability insurance. Furthermore, our products could potentially suffer from product tampering, contamination or degeneration, or they could be mislabeled or otherwise damaged. Under certain circumstances, we may be required to recall products. Even if a situation does not necessitate a product recall, we cannot assure you that product liability claims will not be asserted against us as a result. A product liability judgment against us or a product recall could have a material adverse effect on our revenues, profitability and business reputation.
Risks Related to Doing Business in China
Adverse changes in China’s economic, political and social conditions and government policies could have a material adverse effect on the overall economic growth of China, which could adversely affect our results of operations and financial condition.
We currently conduct our business solely in China. Changes in the economic and political situation in China and the economic, financial, fiscal and other policies adopted by the Chinese government may affect our operations, performance and profitability. The economy of China differs from the economies of most developed countries in many respects, including:
| · | structure; |
| · | extent of government involvement; |
| · | level of development; |
| · | growth rate; |
| · | control of foreign exchange; and |
| · | allocation of resources. |
China’s economy has traditionally been subject to central planning, with a series of economic plans promulgated and implemented by the Chinese government. Over the past 30 years, the Chinese government has been reforming the economic and political systems in China in an attempt to achieve economic and social advancements. Many of these reforms were unprecedented and are expected to continue while political, economic and social factors may also lead to further adjustments to China’s reform measures. These reforms and adjustments may not always have a positive effect on our operations. Accordingly, we cannot assure you that our performance and profitability will not be adversely affected from these measures. In addition, there is no assurance that the Chinese government will continue to pursue economic liberalization and other reforms.
| 23 |
Macroeconomic measures taken by the Chinese government may cause the Chinese economy to slow down.
In response to concerns relating to China’s high rate of growth in industrial production, bank credit, fixed investment and money supply and growing inflationary pressures, the Chinese government has taken measures to slow economic growth to a more manageable level. Among the measures that the Chinese government has taken are restrictions on bank loans in certain sectors and the increase of interest rates. We cannot assure you that those measures will not result in a slowdown in economic growth and hence a reduction in demand for consumer products in China. These measures and any additional measures could contribute to a slowdown in the Chinese economy and could potentially cause the economy to enter a recession, which could have an adverse impact on demand for a wide range of products in China, including our products.
There are risks inherent in doing business in China.
The PRC is a developing country with a young market economic system overshadowed by the state under heavy regulation and scrutiny. Its political and economic systems are very different from the more developed countries. China also faces many social, economic and political challenges that may produce major shocks and instabilities and even crises, in both its domestic arena and in its relationship with other countries, including but not limited to the United States. Such shocks, instabilities and crises may in turn significantly and adversely affect our performance.
Substantially all of our assets are located in the PRC and all of our revenues are derived from our operations in the PRC. Accordingly, our results of operations and prospects are subject, to a significant extent, to the political and legal developments in the PRC.
Substantially all of our assets are located in the PRC and all of our revenues are derived from our operations in the PRC. Accordingly, our results of operations and prospects are subject, to a significant extent, on the economic, political and legal developments in the PRC. The PRC economy differs from the economies of most developed countries in many respects.
The PRC economy has been transitioning from a planned economy to a more market-oriented economy. Although in recent years the PRC government has implemented measures emphasizing the use of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of productive assets in the PRC is still owned by the PRC government. In addition, the PRC government continues to play a significant role in regulating industry development by imposing industrial policies. It also exercises significant control over PRC economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. We cannot assure you that changes in the PRC’s economic, political or legal systems will not detrimentally affect our business, prospects, financial conditions and results of operations.
There are uncertainties regarding interpretation and enforcement of Chinese laws and regulations.
China’s legal system is a civil law system based on statutory law. Prior legal decisions and judgments have little precedential value. China is still in the process of developing a comprehensive statutory framework and its legal system is still considered to be underdeveloped in comparison with the legal systems in some western countries. Since 1979, the Chinese government has formulated and enacted a large number of laws and regulations governing economic matters, securities activities and foreign investments.
Despite significant development in its legal system, China does not have a comprehensive system of laws. The interpretation of Chinese law by courts and tribunals may be inconsistent and influenced by government policies and other considerations. In addition, the enforcement of existing laws and regulations can be uncertain and unpredictable. Judgments and arbitration rulings may be unenforceable. The promulgation of new laws, changes to existing laws and inconsistent interpretation of laws could have a negative impact on our business.
Our business may be affected by unexpected changes in regulatory requirements in the jurisdictions in which we operate.
Our company, and its subsidiaries, is subject to many general regulations governing business entities and their behavior in China and in other jurisdictions in which we and our subsidiaries have, or plan to have, operations and market products. In particular, we are subject to laws and regulations covering food, dietary supplements and pharmaceutical products. Such regulations typically deal with licensing, approvals and permits. Any change in product licensing may make our products more or less available on the market. Such changes may have a positive or negative impact on the sale of our products and may directly impact the associated costs in compliance and our operational and financial viability. Such regulatory environment also covers any existing or potential trade barriers in the form of import tariff and taxes that may make it difficult for us to import our products to certain countries and regions, such as Hong Kong, which would limit its international expansion.
| 24 |
All of our officers and directors, and substantially all of our assets, are located in China, thus it may be extremely difficult to acquire jurisdiction and enforce liabilities against our management and our assets.
Because our executive officers and directors are Chinese citizens, it may be difficult, if not impossible, to acquire jurisdiction over them in the event a lawsuit is initiated against us or our management by a stockholder or group of stockholders in the United States. We anticipate that future members of our management will also be Chinese citizens. Because the majority of our assets are located in China, it would also be extremely difficult to access those assets to satisfy an award entered against us in U.S. court.
Restrictions on currency exchange may limit our ability to receive and use our revenues effectively.
Since almost all of our future revenues may be in the form of Renminbi, any future restrictions on currency exchanges may limit our ability to use revenue generated in Renminbi to fund any future business activities outside China or to make dividend or other payments in U.S. dollars. There are significant restrictions on convertibility of the Renminbi for current account transactions, including primarily the restriction that foreign invested enterprises may only buy, sell or remit foreign currencies, after providing valid commercial documents, at those banks authorized to conduct foreign exchange business. In addition, conversion of Renminbi for capital account items, including direct investment and loans, is subject to governmental approval in China, and companies are required to open and maintain separate foreign exchange accounts for capital account items. We cannot be certain that the Chinese regulatory authorities will not impose more stringent restrictions on the convertibility of the Renminbi, especially with respect to foreign exchange transactions.
Inflation in the PRC could negatively affect our profitability and growth.
While the PRC economy has experienced rapid growth, it has been uneven among various sectors of the economy and in different geographical areas of the country. Rapid economic growth can lead to growth in the money supply and rising inflation. If prices for our products do not rise at a rate that is sufficient to fully absorb inflation-driven increases in our costs of supplies, our profitability can be adversely affected.
In addition, in order to control inflation, the PRC government might impose controls on bank credits, limits on loans for fixed assets and restrictions on state bank lending. The implementation of these and other similar policies may impede economic growth and thereby harm the market for our products in the future.
Deterioration of the PRC’s political relations with the U.S., Europe, or other nations could make Chinese businesses less attractive to Western investors.
The relationship between the U.S. and the PRC is subject to sudden fluctuation and periodic tension. Changes in political conditions in the PRC and changes in the state of Sino-foreign relations are difficult to predict and could cause potential target businesses or services to become less attractive. This could lead to a decline in our profitability. Any weakening of relations between the U.S., Europe, or other nations and the PRC could also have a material adverse effect on our ability to raise additional capital.
The enforcement of new labor contract law and its implementation rules and increase in labor costs in the PRC may adversely affect our business and our profitability.
China adopted the PRC Employment Contract Law, or the new Labor Contract Law, effective January 1, 2008 and the implementation rules effective September 18, 2008. The new Labor Contract Law and its implementation rules impose more stringent obligations on employers for, among others, entering into written employment contracts, hiring temporary employees, dismissing employees, setting compensations for dismissal and protecting certain sick or disabled employees from dismissal and setting forth detailed requirements relating to the contents of the employment contracts. The implementation of the new Labor Contract Law may increase our operating expenses, in particular our personnel expenses, as the continued success of our business depends significantly on our ability to attract and retain qualified personnel. In the event that we decide to terminate some of our employees or otherwise change our employment or labor practices, the new Labor Contract Law may also limit our ability to effect those changes in a manner that we believe to be cost-effective or desirable, which could adversely affect our business and results of operations.
| 25 |
Failure to comply with PRC regulations regarding the registration requirements for employee equity incentive plans may subject our PRC citizen employees or us to fines and other legal or administrative sanctions.
On March 28, 2007, SAFE promulgated the Application Procedure of Foreign Exchange Administration for Domestic Individuals Participating in Employee Stock Holding Plan or Share Option Plan of Overseas-Listed Company, or the Share Option Rule. Under the Share Option Rule, PRC citizens who are granted share options or other employee equity incentive awards by an overseas publicly-listed company are required, through a PRC agent who may be a PRC subsidiary of such overseas publicly-listed company, to register with the SAFE and complete certain other procedures related to the share options or other employee equity incentive plans. We and our PRC citizen employees who are granted share options or other equity incentive awards under our 2010 Equity Incentive Plan, or PRC optionees, are subject to the Share Option Rule. If we or our PRC optionees fail to comply with these regulations, we or our PRC optionees may be subject to fines and legal sanctions.
We are subject to environmental regulations and may be exposed to liability and potential costs for environmental compliance.
We are subject to PRC laws and regulations concerning the discharge of waste water, gaseous waste and solid waste during our manufacturing processes. We are required to establish and maintain facilities to dispose of waste and report the volume of waste to the relevant government authorities, which conduct scheduled or unscheduled inspections of our facilities and treatment of such discharge. We may not at all times comply fully with environmental regulations. Any violation of these regulations may result in substantial fines, criminal sanctions, revocations of operating permits, shutdown of our facilities and obligation to take corrective measures. Our cost of complying with current and future environmental protection laws and regulations and our liabilities which may potentially arise from the discharge of effluent water and solid waste may materially adversely affect our business, financial condition and results of operations. The government may take steps towards the adoption of more stringent environmental regulations. Due to the possibility of unanticipated regulatory or other developments, the amount and timing of future environmental expenditures may vary substantially from those currently anticipated. If there is any unanticipated change in the environmental regulations, we may need to incur substantial capital expenditures to install, replace, upgrade or supplement our pollution control equipment or make operational changes to limit any adverse impact or potential adverse impact on the environment in order to comply with new environmental protection laws and regulations. If such costs become prohibitively expensive, we may be forced to cease certain aspects of our business operations.
The 2006 M&A Rule establishes more complex procedures for some acquisitions of Chinese companies by foreign investors, which could make it more difficult for us to pursue growth through acquisitions in China.
On August 8, 2006, six PRC regulatory agencies (the Ministry of Commerce, the State Assets Supervision and Administration Commission, or SASAC, the State Administration for Taxation, the State Administration for Industry and Commerce, the CSRC and SAFE) jointly adopted the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or the 2006 M&A Rule, which became effective on September 8, 2006. The 2006 M&A Rule establishes additional procedures and requirements that could make some acquisitions of PRC companies by foreign entities, such as our company, more time-consuming and complex, including requirements in some instances that the approval of the Ministry of Commerce be obtained for transactions involving the shares of an offshore-listed company being used as the acquisition consideration by foreign entities, including Sino-foreign joint ventures. In the future, we may grow our business in part by acquiring complementary businesses. Complying with the requirements of the 2006 M&A Rule to complete such transactions could be time-consuming, and any required approval processes, including obtaining approval from the Ministry of Commerce, may delay or inhibit our ability to complete such transactions, which could affect our ability to expand our business or maintain our market share.
The increased scrutiny of acquisition transactions by the PRC tax authorities may have a negative impact on our acquisition strategy.
In connection with the new EIT law, the Ministry of Finance and State Administration of Taxation jointly issued the Notice on Issues Concerning Process of Enterprise Income Tax in Enterprise Restructuring Business, or Circular 59, on April 30, 2009. On December 10, 2009, the State Administration of Taxation issued the Notice on Strengthening the Management on Enterprise Income Tax for Non-resident Enterprises Equity Transfer, or Circular 698. Both Circular 59 and Circular 698 became effective retroactively to January 1, 2008. By promulgating and implementing these circulars, the PRC tax authorities have strengthened their scrutiny over the direct or indirect transfer of equity interest in a PRC resident enterprise by a non-resident enterprise. For example, Circular 698 specifies that the PRC State Administration of Taxation is entitled to redefine the nature of an equity transfer where offshore vehicles are interposed as abusing corporate structures for tax-avoidance purposes and without reasonable commercial intention. We may pursue acquisitions as one of our growth strategies, and may conduct acquisitions involving complex corporate structures. We cannot be assured that the PRC tax authorities will not, at their discretion, adjust the applicable capital gains, tax thus causing us to incur additional acquisition costs.
| 26 |
Our China-sourced income is subject to PRC withholding tax under the new Enterprise Income Tax Law of the PRC, and we may be subject to PRC enterprise income tax at the rate of 25% when more detailed rules or precedents are promulgated.
We are a Delaware holding company with substantially all of our operations conducted through our operating subsidiary in China. Under the new PRC Enterprise Income Tax Law, or the new EIT law, and its implementation rules, both of which became effective on January 1, 2008, China-sourced income of foreign enterprises, such as dividends paid by a PRC subsidiary to its overseas parent, is generally subject to a 10% withholding tax. The new EIT law, however, also provides that enterprises established outside China whose “de facto management bodies” are located in China are considered “tax resident enterprises” and will generally be subject to the uniform 25% enterprise income tax rate as to their global income. Under the implementation rules, “de facto management bodies” are defined as the bodies that have, in substance, overall management control over such aspects as the production and business, personnel, accounts and properties of an enterprise. In April 2009, the PRC tax authority promulgated the Notice on Determination of Tax Resident Enterprises of Chinese-controlled Offshore Incorporated Enterprises in accordance with Their De Facto Management Bodies, or Circular 82, to clarify the criteria for determining whether the “de facto management bodies” are located within the PRC for enterprises incorporated overseas with controlling shareholders being PRC enterprises. As all of our operational management is currently based in the PRC, and we expect them to continue to be located in China, our company may be deemed a PRC resident enterprise and therefore be subject to the PRC enterprise income tax at a rate of 25% on our worldwide income, which excludes the dividends received directly from another PRC resident enterprise. Due to the lack of clear guidance on the criteria pursuant to which the PRC tax authorities will determine our tax residency under the new EIT law, it remains unclear whether the PRC tax authorities will treat us as a PRC resident enterprise. Therefore, we are unable to confirm whether we are subject to the tax applicable to resident enterprises or non-resident enterprises under the new EIT law. Furthermore, in connection with the new EIT law and Tax Implementation Regulations, the Ministry of Finance and State Administration of Taxation jointly issued, on April 30, 2009, the Notice on Issues Concerning Process of Enterprise Income Tax in Enterprise Restructuring Business, or Circular 59, which became effective retroactively to January 1, 2008. As Circular 59 has only recently been promulgated, it is uncertain to us as to how it will be implemented and the respective tax base and the tax exposure cannot be determined reliably at this stage. In case we are required to pay the income tax on capital gains by the relevant PRC tax authorities, our financial conditions and results of operations could be adversely affected.
Dividends payable by us to our foreign investors and gain on the sale of our shares may become subject to taxes under PRC tax laws.
Under the new EIT law, dividends, interests, rent, royalties and gains on transfers of property payable by a foreign-invested enterprise in China to any of its foreign investors who is a non-resident enterprise will be subject to a 10% withholding tax, unless such non-resident enterprise’s jurisdiction of incorporation has a tax treaty with China that provides for a reduced rate of withholding tax. Under the arrangement for avoidance of double taxation between mainland China and Hong Kong, the effective withholding tax applicable to a Hong Kong non-resident company is currently 5% if it directly owns no less than a 25% stake in the Chinese foreign-invested enterprise and meets the definition of “beneficial owner.”
Under the new EIT law and its implementation rules, to the extent that we are considered a “resident enterprise” which is “domiciled” in China, PRC income tax at the rate of 10% (which must be withheld by the Company on all such distributions) is applicable to dividends payable by us to investors that are “non-resident enterprises” so long as such “non-resident enterprise” investors do not have an establishment or place of business in China or, despite the existence of such establishment or place of business in China, the relevant income is not effectively connected with such establishment or place of business in China. Similarly, any gain realized on the transfer of our shares by such investors is also subject to a 10% PRC income tax if such gain is regarded as income derived from sources within China and we are considered a “resident enterprise” which is domiciled in China for tax purposes. Additionally, there is a possibility that the relevant PRC tax authorities may take the view that our purpose is that of a holding company, and the capital gain derived by our overseas stockholders would be deemed China-sourced income, in which case such capital gain may be subject to PRC withholding tax at the rate of up to 10%. It is unclear how this tax would be applied to or enforced against non-PRC citizens or residents. If we are required under the new EIT law to withhold PRC income tax on our dividends payable to our foreign stockholders who are “non-resident enterprises”, or if you are required to pay PRC income tax on the transfer of our shares under the circumstances mentioned above, the value of your investment in our shares may be materially and adversely affected. It is unclear whether, if we are considered a PRC “resident enterprise,” holders of our shares would be able to claim the benefit of income tax treaties or agreements entered into between China and other countries or areas.
Failure to comply with the United States Foreign Corrupt Practices Act could subject us to penalties and other adverse consequences.
As our ultimate holding company is a Delaware corporation, we are subject to the United States Foreign Corrupt Practices Act, which generally prohibits United States companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some that may compete with our company, are not subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices may occur from time-to-time in the PRC. We can make no assurance, however, that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties and other consequences that may have a material adverse effect on our business, financial condition and results of operations.
| 27 |
Because significant portion of our funds are held in banks in the PRC that do not provide insurance, the failure of any bank in which we deposit our funds could affect our ability to continue in business.
Banks and other financial institutions in the PRC do not provide insurance for funds held on deposit. A significant portion of our assets are in the form of cash deposited with banks in the PRC, and in the event of a bank failure, we may not have access to our funds on deposit. Depending upon the amount of money we maintain in a bank that fails, our inability to have access to our cash could impair our operations, and, if we are not able to access funds to pay our suppliers, employees and other creditors, we may be unable to continue in business.
Any outbreak of the Swine Flu (H1N1), severe acute respiratory syndrome, or SARS, the Avian Flu, or another widespread public health problem in the PRC could adversely affect our operations.
There have been recent outbreaks of the highly pathogenic Swine Flu, caused by the H1N1 virus, in certain regions of the world, including parts of China, where all of our manufacturing facilities are located and where all of our sales occur. Our business is dependent upon our ability to continue to manufacture and distribute our products, and an outbreak of the Swine Flu, or a renewed outbreak of SARS, the Avian Flu, or another widespread public health problem in China, could have a negative effect on our operations. Any such outbreak could have an impact on our operations as a result of:
| · | quarantines or closures of our manufacturing or distribution facilities or the retail outlets, which would severely disrupt our operations; |
| · | the sickness or death of our key officers and employees; and |
| · | a general slowdown in the Chinese economy. |
Any of the foregoing events or other unforeseen consequences of public health problems could adversely affect our operations.
Any outbreak of earthquake, tsunami, adverse weather or oceanic conditions or other calamities may result in disruption in our operations and could adversely affect our sales.
We are based in Shanghai, which is situated in southeast China on the coast of the East China Sea. Shanghai is a vital navigation hub between the East China Sea and South China Sea, and is also rich in agricultural and marine resources.
In 2004, an undersea earthquake occurred off the west coast of Sumatra, Indonesia. This earthquake triggered a series of devastating tsunamis along the costs of most landmasses boarding the Indian Ocean. More than 225,000 people in 11 countries were killed, and coastal communities were inundated with waves up to 100 feet.
On May 12, 2008, there was an 8.0 magnitude scale earthquake centered on Sichuan Province of China. It was also known the Wenchuan earthquake, which by any name killed at least 69,000 people and injured more than 374,000 people, with 18,000 listed as missing. The earthquake left about 4.8 million people homeless, thought the number could be as high as 11 million. It was the deadliest earthquake to hit China since the 1976 Tangshan earthquake.
Due to the location of our business, we may be at risk of experiencing another tsunami, earthquake or other adverse weather or oceanic conditions. This may result in the breakdown of our facilities, such as our cold storage facilities, which will in turn lead to deterioration of our products with the potential for spoilage. This could adversely affect our ability to fulfill our sales orders and adversely affect our profitability.
Adverse weather conditions affecting the normal operation order of Shanghai such as storms, heavy rainfall, cyclones and typhoons or cataclysmic events such as heavy rainfall may affect our transportation of products outside Shanghai area.
We may be affected by global climate change or by legal, regulatory or market responses to such changes. The growing political and scientific sentiment is that increased concentrations of carbon dioxide and other greenhouse gases in the atmosphere are influencing global weather patterns. Changing weather patterns, along with the increased frequency or duration of extreme weather conditions, could impact the availability or increase the cost of key raw materials that we use to produce our products.
Concern over climate change, including global warming, has led to legislative and regulatory initiatives directed at limiting greenhouse gas emissions. For example, proposals that would impose mandatory requirements on greenhouse gas emissions may be considered by policy makers in the territories that we operate. Laws enacted that directly or indirectly affect our production, distribution, packaging, cost of raw materials, fuel, ingredients, and water could all impact our business and financial results.
| 28 |
Risks Related to our Common Stock
Shares of our common stock which are eligible for immediate sale by our stockholders may decrease the market price of our common stock.
We had 22,150,200 shares outstanding as of March 31, 2012, including approximately 12,523,745 shares which are free trading and may be sold immediately by our stockholders. If our stockholders sell substantial amounts of our common stock, or there is a perception in the market that such sales may occur, then the market price of our common stock could decrease.
Concentration of our ownership by Mr. Song, our Chairman and Chief Executive Officer and a Director, and his family may dissuade new investors from purchasing our securities, which could result in a lower trading price for our securities than if our ownership was less concentrated.
As of March 31, 2012, Mr. Song, our Chairman and Chief Executive Officer and a Director, owned approximately 23.1% of our issued and outstanding common stock on a fully diluted basis. As a result, Mr. Song has the ability to exert substantial influence over all matters requiring approval by our stockholders, including the election and removal of directors, any proposed merger, consolidation or sale of all or substantially all of our assets and other corporate transactions. This concentration of control could be disadvantageous to other stockholders with interests different from those of Mr. Song. In addition, this concentration of share ownership may adversely affect the trading price for our common stock because investors often perceive disadvantages in owning stock in companies with a significant concentration of ownership among a limited number of stockholders.
Our preferred stock may make a third-party acquisition of our company more difficult, which in turn would make a purchase of our shares less desirable, thereby potentially reducing our stock price or the liquidity of our shares.
Our Certificate of Incorporation authorizes our Board of Directors to issue up to 10,000,000 shares of preferred stock having such rights as may be designated by our Board of Directors, without stockholder approval. The issuance of preferred stock could inhibit a change in our control by making it more difficult to acquire the majority of our voting stock and thereby making the purchase of our shares by new investors less likely. A lesser interest in the purchase of our shares could reduce our market price or make it more difficult for stockholders to sell their shares. No shares of preferred stock are currently outstanding.
“Penny Stock” rules may make buying or selling our common stock difficult.
Trading in our common stock is subject to the “penny stock” rules. The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. These rules require that any broker-dealer that recommends our common stock to persons other than prior customers and accredited investors, must, prior to the sale, make a special written suitability determination for the purchaser and receive the purchaser’s written agreement to execute the transaction. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated with trading in the penny stock market. In addition, broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for the securities they offer. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our common stock, which could severely limit the market price and liquidity of our common stock.
There is currently a limited trading market for our common stock and a more liquid trading market may never develop or be sustained and stockholders may not be able to liquidate their investment at all, or may only be able to liquidate the investment at a price less than the Company’s value.
There is currently a limited trading market for our common stock and a more liquid trading market may never develop. As a result, the price of our shares may not reflect the value of the Company. Consequently, investors may not be able to liquidate their investment at all, or if they are able to liquidate it may only be at a price that does not reflect the value of our business. Because the price for our stock is low, many brokerage firms may not be willing to effect transactions in the securities. Even if an investor finds a broker willing to effect a transaction in our stock, the combination of brokerage commissions, transfer fees, taxes, if any, and any other selling costs may exceed the selling price. Further, many lending institutions will not permit the use of common stock like ours as collateral for any loans. Even if a more active market should develop, the price may be highly volatile.
Our common stock is currently quoted in the Pink Sheets and under “caveat emptor”. We do not satisfy the listing standards of the New York Stock Exchange, The Nasdaq Stock Market or any other U.S. securities exchange. If we never are able to satisfy any of those listing standards our common stock will never be listed on an exchange, or we may choose not to apply for any such listing. As a result, the trading price of our stock may be lower than if we were listed on an exchange. Our stock may be subject to increased volatility. When a stock is thinly traded, a trade of a large block of shares can lead to a dramatic fluctuation in the share price. These factors may make it more difficult for our shareholders to sell their shares.
| 29 |
Restrictions on the use of Rule 144 by shell companies or former shell companies could affect your ability to resale our shares.
Historically, the SEC has taken the position that Rule 144 under the Securities Act, as amended, is not available for the resale of securities initially issued by companies that are, or previously were, blank check companies like us, to their promoters or affiliates despite technical compliance with the requirements of Rule 144. The SEC has codified and expanded this position in its amendments to Rule 144, effective on February 15, 2008, and which apply to securities acquired both before and after that date by prohibiting the use of Rule 144 for resale of securities issued by shell companies (other than business transaction related shell companies) or issuers that have been at any time previously a shell company. The SEC has provided an exception to this prohibition if the following conditions are met: the issuer of the securities that was formerly a shell company has ceased to be a shell company; the issuer of the securities is subject to the reporting requirements of Section 13 or 15(d) of the Exchange Act; the issuer of the securities has filed all Exchange Act reports and material required to be filed, as applicable, during the preceding 12 months (or such shorter period that the issuer was required to file such reports and materials), other than Form 8-K reports; and at least one year has elapsed from the time that the issuer filed current Form 10-type information with the SEC reflecting its status as an entity that is not a shell company. As such, due to the fact that we had been a shell company prior to October 2007, holders of “restricted securities” within the meaning of Rule 144, when they resell their shares pursuant to Rule 144, shall be subject to the conditions set forth in Rule 144.
We do not anticipate paying dividends.
We do not anticipate paying dividends in the foreseeable future. Any dividends which we may pay in the future will be at the discretion of our board of directors and will depend on our future earnings, any applicable regulatory considerations, our financial requirements and other similarly unpredictable factors. For the foreseeable future, we anticipate that we will retain any earnings which we may generate from our operations to finance and develop our growth.
The market price for our common stock may be volatile and subject to wide fluctuations, which may adversely affect the price at which you can sell our shares.
The market price for our common stock may be volatile and subject to wide fluctuations in response to factors including the following:
| · | actual or anticipated fluctuations in our quarterly operating results; |
| · | changes in financial estimates by securities research analysts; |
| · | conditions in foreign or domestic food processing or agricultural markets; |
| · | changes in the economic performance or market valuations of other food processing companies; |
| · | announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| · | addition or departure of key personnel; |
| · | fluctuations of exchange rates between the RMB and the U.S. dollar; |
| · | intellectual property litigation; and |
| · | general economic or political conditions in China. |
Furthermore, certain short sellers and other funds have in the past, and may again in the future, take a short position or positions in our shares for the specific purpose of driving down our share price. Short sellers may also publish articles or other allegations in conjunction with such attacks. Even when there is no truth to their claims and we rebut their allegations, such attacks can have a material impact on our share price and divert management resources and attention. The securities market has also experienced significant price and volume fluctuations from time to time that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our stock.
ITEM 1B. UNRESOLVED STAFF COMMENTS.
As discussed further in the report of our management included under Item 9A. “Controls and Procedures” of this Annual Report on Form 10-K, after reviewing our Annual Report on Form 10-K for the year ended March 31, 2010 and our Quarterly Report on Form 10-Q for the period ended December 31, 2010, the SEC concluded in a letter to the Company dated as of January 9, 2012, that the Company’s lack of U.S. GAAP experience is a material weakness in our disclosure controls and procedures and in our internal control over financial reporting. In its letter, the SEC also directed the Company to file an amended Form 10-K for the year ended March 31, 2010 identifying the Company’s lack of U.S. GAAP experience as a material weakness in our disclosure controls and procedures and in our internal control over financial reporting, which the Company intends to file as promptly as is reasonably practicable.
| 30 |
ITEM 2. PROPERTIES.
On July 22, 2010, we acquired a property located at No. 26 Orient Global Headquarter Lane 118, Yonghe Road, Zhabei District, Shanghai 200072, People’s Republic of China as our principal executive office.
We conduct our retail probiotics products production from a leased facility in Pudong, Shanghai. Pursuant to our lease for this facility, which expires on December 31, 2013, we pay annual rent of $124,894 (RMB798,919), payable in quarterly installments of $31,223 (RMB199,730). This facility, which includes a level 100,000 clean room and a level 10,000 clean room, houses our office space, manufacturing facilities and warehouse. The maximum current production capacity at this location is approximately 3.5 million capsules per month. We have received GMP, ISO 9001, ISO 14001, OHSAS 18001 and HACCP certifications for this facility. See “Business—Current Operations—Overview” for further information with respect to these certifications.
We have expanded into the bulk additives business for functional foods through the completion of our 300-metric ton capacity plant in Qingpu, which commenced production in February 2010. Phase 1 of the project involved constructing a facility with a production capacity of 150 metric tons of probiotics per year that cost $36 million in total, the sum of which was invested in calendar year 2010. Phase 2 of this project commenced in September 2010 and cost $18 million, $11 million of which was paid in calendar year 2010 and the remaining $7 million of which was paid off by the end of 2011, the date when construction of Phase 2 was completed. Phase 2 added an additional 150 metric tons of annual capacity.
On March 21, 2006, GSL, our subsidiary, entered into an agreement, as amended, with Shanghai Qingpu Industrial Park District Development (Group) Company Limited for the lease of 36,075 square meters of land in Qingpu on which we constructed our 300-metric ton capacity production plant described above for a term of 50 years beginning January 15, 2008. The agreement provides for the payment of leasing fees of approximately $1.8 million. In February 2009, the formal land use right certificate was issued. There are no future lease payments under this land lease.
The Company has commenced construction of a new facility in Yangling in the Shaanxi Province of China. The cost for constructing the Yangling facility is expected to be over $58 million invested over two years. The facility will produce probiotics and probiotics-related biological additives for the animal feed industry. Currently, the facility is in the construction stage, and the plan is subject to government approval prior to implementation. During the year ended March 31, 2011, the Company made total payments of approximately $3.25 million (RMB20,608,112) to acquire the land use right of approximately 122,600 square meters (183.94 mu). We received the land use right certificate on January 28, 2012 and commenced construction in June 2012. We expect to complete construction by June 2014 and trial production is expected to commence in 2015. In the existing market environment, the Company will utilize its available cash and bank facilities to fund such construction.
ITEM 3. LEGAL PROCEEDINGS
The Company and certain of its current and former officers and directors have been named as defendants in two putative shareholder class action lawsuits, one in the United States District Court for the Central District of California (Mohapatra v. China-Biotics, Inc., et al., No. 10-cv-6954 (C.D. Cal.), the “California Action”) and the other in the United States District Court for the Southern District of New York (Hill v. China-Biotics, Inc., et al., No. 10-cv-7838 (S.D.N.Y.), the “New York Action”). After certain shareholders filed motions for appointment as lead plaintiff in both lawsuits, the plaintiff in the California Action voluntarily dismissed its case and the plaintiff in the New York Action, together with another shareholder, were appointed as lead plaintiffs. The lead plaintiffs filed an amended complaint in which they allege that the defendants violated Sections 11, 12(a)(2), and 15 of the Securities Act of 1933, and Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 by making material misstatements or failing to disclose certain material information regarding, among other things, the Company’s financial condition, operations, and future business prospects, and the quality, nature, and quantity of the Company’s retail outlets. The lead plaintiffs seek to represent a class of shareholders who bought the Company’s securities between July 10, 2008 and August 27, 2010.
On August 18, 2011, the Company filed a motion to dismiss the lead plaintiffs’ amended complaint. The court dismissed the lead plaintiffs’ Section 11 claim, but gave them leave to replead. The court did not rule on the motion to dismiss the Section 10(b) claim. On January 9, 2012, the lead plaintiffs filed a second amended complaint that included a new named plaintiff and new allegations for the Section 11 claim. On February 27, 2012, the Company filed a motion to dismiss the amended Section 11 claim. Both that motion and the original motion to dismiss the Section 10(b) and Section 20(a) claims are currently pending before the court. The Company intends to defend this action vigorously.
| 31 |
The Company and certain of its current and former officers and directors have been named as defendants in a putative stockholder class action in the United States District Court for the Southern District of New York (Casper v. Jinan, et al., No. 12-cv-4202 (S.D.N.Y.). The plaintiff alleges that the defendants violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 by making material misstatements about the Company’s projected revenue growth for 2011. The plaintiff seeks to represent a class of stockholders who bought the Company’s securities between February 9, 2011 and July 1, 2011. On September 5, 2012, the court appointed the Blanck Investor Group as lead plaintiff. The Company intends to defend this action vigorously.
The Company and its directors have been named as defendants in a derivative lawsuit filed in the United States District Court for the District of Columbia (Marteney v. Song Jinan, et al., No. 10-cv-1983 (D.D.C.)). The complaint alleges that the directors breached their fiduciary duties by disseminating false and misleading financial statements and seeks unspecified damages. On March 26, 2012, the plaintiff filed an amended complaint in which he added Roth Capital Partners LLC and Maxim Group LLC as defendants. On September 7, 2012, Roth Capital Partners LLC and Maxim Group LLC filed a motion to dismiss. The defendants intend to defend this action vigorously.
On September 15, 2011, the staff of the SEC informed the Company that it intended to recommend that the SEC institute a public administrative proceeding against the Company for alleged violations of Section 13(a) of the Securities Exchange Act of 1934 and Rules 13a-1 and 13a-13 or 13a-16 promulgated thereunder. On October 7, 2011, the SEC issued an Order Instituting Proceedings to determine whether it was necessary and appropriate to suspend or revoke the registration of the Company’s securities. On February 22, 2012, the administrative law judge overseeing the proceedings issued a decision ordering that the registration of the Company’s securities be revoked. On March 26, 2012, the SEC granted the Company’s petition for review of this decision. The results of the SEC’s review are pending as of September 13, 2012.
ITEM 4. [REMOVED AND RESERVED]
PART II
ITEM 5. MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock was traded on the Nasdaq Global Stock Market between October 23, 2008 and July 20, 2011, under the symbol “CHBT.” It was formally de-listed from the Nasdaq Global Stock Market on July 21, 2011. Since July 1, 2011, our common stock has been quoted in the Pink Sheets and under “caveat emptor”.
Prior to listing on the Nasdaq Global Stock Market, our common stock was quoted on the OTC Bulletin Board.
Set forth below is information with respect to the intraday high and intraday low sales prices of our common stock for the periods indicated on the OTC Bulletin Board, the Nasdaq Global Stock Market and via the Pink Sheets.
| Period | High | Low | ||||||
| Quarter Ended June 30, 2010 | $ | 18.95 | $ | 12.10 | ||||
| Quarter Ended September 30, 2010 | $ | 15.65 | $ | 8.75 | ||||
| Quarter Ended December 31, 2010 | $ | 15.70 | $ | 10.00 | ||||
| Quarter Ended March 31, 2011 | $ | 17.90 | $ | 7.35 | ||||
| Quarter Ended June 30, 2011 | $ | 12.21 | $ | 3.46 | ||||
| ON PINK SHEETS: | ||||||||
| Quarter Ended September 30, 2011 | $ | 3.72 | $ | 0.05 | ||||
| Quarter Ended December 31, 2011 | $ | 0.75 | $ | 0.40 | ||||
| Quarter Ended March 31, 2012 | $ | 2.10 | $ | 0.57 | ||||
| Quarter Ended June 30, 2012 | $ | 1.67 | $ | 0.70 | ||||
| Quarter Ended September 30, 2012 (through September 11, 2012) | $ | 1.70 | $ | 0.94 | ||||
Security Holders
As of September 11, 2012, there were 22,150,200 shares of our common stock outstanding held by approximately 13 stockholders of record.
| 32 |
Dividend Policy
We have not historically paid any cash dividends and do not intend to pay any dividends in the foreseeable future. We plan to use retained earnings, if any, to finance our growth. The declaration and payment of dividends in the future will be determined by our Board of Directors in light of conditions then existing, including, but not limited to, our financial condition, capital requirements, and restrictions in our financing agreements.
Equity Compensation Plans (As of March 31, 2012)
On January 16, 2011, the Board adopted the 2010 Equity Incentive Plan (the “2010 Plan”) and reserved 1,500,000 shares of common stock for issuance under the 2010 Plan. On March 9, 2011, the 2010 Plan was approved by the Company’s stockholders at the 2010 Annual Meeting of Stockholders. As of March 31, 2012, options to purchase 660,000 shares were outstanding under the 2010 Plan.
| Plan Category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities
remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | 660,000 | $ | 14.81 | 840,000 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 660,000 | $ | 14.81 | 840,000 | ||||||||
Recent Sales of Unregistered Securities
There were no sales of unregistered securities during the year ended March 31, 2012.
Issuer Purchases of Equity Securities
During the Company’s fiscal year ended March 31, 2012, no purchases of the Company’s common stock were made by, or on behalf of, the Company or any “affiliated purchaser,” as defined in Rule 10b-18(a)(3) under the Securities Exchange Act of 1934, as amended.
ITEM 6. SELECTED FINANCIAL DATA
Not applicable to smaller reporting companies.
ITEM 7. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION
The following discussion should be read in conjunction with the financial statements and the notes thereto appearing elsewhere in this Form 10-K. The following discussion contains forward-looking statements reflecting our plans, estimates, and beliefs. Our actual results could differ materially from those discussed in the forward-looking statements. You are also urged to carefully review and consider our discussions regarding the various factors which affect our business, including the information provided in the risk factors discussion beginning on page 17 of this Form 10-K. See the cautionary note regarding forward-looking statements at the beginning of Part I of this Form 10-K.
General
We were incorporated under the name of Otish Resources, Inc. in Delaware in February 2003. Until March 2006, we were a mineral exploration stage company specializing in acquiring and consolidating mineral properties with potential for commercial ore bodies. Although we conducted some preliminary exploration work with respect to our mineral properties, we never achieved full operations with respect to our mineral properties. We had never generated any revenue from our mineral exploration operations. We incurred a total of expenses of $257,914 from inception to February 28, 2006.
| 33 |
On March 22, 2006, we entered into and completed a securities exchange transaction with SGI and the shareholders of SGI, pursuant to which the SGI shareholders transferred all of the equity securities of SGI to us in exchange for an aggregate of 15,980,000 shares of our common stock. At the closing of the Share Exchange, SGI became our wholly owned subsidiary. Immediately after the Share Exchange and related transactions described elsewhere in this document, the former SGI shareholders and their designees collectively owned 98.7% of our common stock. As a result of the Share Exchange, we are no longer a mineral exploration stage company, and SGI’s business operations became our primary operations. We are currently engaged in the research, development, production, marketing and distribution of probiotics products. These products contain live microbial food supplements which beneficially affect the host by improving its intestinal microbial balance. See “Business – History.”
We accounted for the Share Exchange as a recapitalization, whereby the historical financial statements and operations of SGI became our historical financial statements, with no adjustment to the carrying value of the assets and liabilities. Our issued and outstanding common stock immediate prior to the Share Exchange is accounted for at the net book value at the time of the transaction.
Upon consummation of the Share Exchange, we changed our fiscal year end from August 31 to March 31 to conform to the year-end date of SGI. We filed a quarterly report on Form 10-QSB on April 14, 2006, for the quarter ended February 28, 2006. That quarterly report was our last filing under our previous fiscal year end date of August 31 and as a mineral exploration stage company. In our subsequent filings with the SEC, we have reported our business activities as a manufacturer and distributor of probiotics products based on our new fiscal year end date of March 31. SGI’s historical financial statements have become our historical financial statements.
The results of operations related to Otish Resources, Inc. as a mineral exploration stage company are not material and are therefore not included in the discussion below. Unless otherwise noted, all references to the “Company,” “we,” “us” and “our” hereafter in this section refer to the current business of China-Biotics, Inc., SGI and its subsidiaries, Shining, Growing, GSL, KTG, BDH and Growing Yangling, as applicable.
The accompanying consolidated financial statements are presented in United States dollars. The functional currency of the Company is the Renminbi (“RMB”). Capital accounts of the consolidated financial statements are translated into United States dollars from RMB at their historical exchange rates when the capital transactions occurred. Assets and liabilities are translated at the exchange rate as of the balance sheet date. Income and expenditures are translated at the average exchange rate for the period presented.
The RMB is the functional currency of Shining, Growing, and Yangling Growing (the “Operating Subsidiaries”) as it is the currency of the People’s Republic of China, which is the primary economic environment the Operating Subsidiaries operate in and the environment in which the Company primarily generates and expends cash. RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US dollars at the rates used in translation.
Overview
We manufacture and sell probiotics products. Probiotics comprise mainly live bacteria, which we produce using advanced proprietary fermentation technology. Currently, our products are sold primarily in the Chinese domestic market.
Our retail products are mainly sold to distributors, who then distribute them to various retail outlets such as drug stores and supermarkets or sell directly to enterprise accounts. Typically, 60 to 90 days’ credit is given to the distributors. Our bulk additives products are primarily sold to institutional customers, such as dairy manufacturers, animal feed manufacturers, pharmaceutical companies, and food companies. Typically, 60 to 90 days’ credit is given to the bulk additives products customers.
Because of the increasing cost of rents and sales personnel in recent years, since January 2011 we have expanded our retail product sales in China through our growing distribution network and via internet sales. Our management believes that as China becomes more affluent and the number of Chinese internet users increases, Chinese citizens are becoming more health conscious, which has led to higher demand for healthy and functional foods such as probiotics and yogurt.
Our first retail product, Shining Essence, was launched in April 2001 and remains one of our best-selling products. Sales of Shining Essence represented approximately 21%, and 19% of our total sales of our retail products for the years ended March 31, 2012 and 2011, respectively. In addition to Shining Essence, our research and development team has successfully developed other new retail products, such as Shining Probiotics Protein Powder, which represented approximately 38% and 24% of our total sales of our retail products for the years ended March 31, 2012 and 2011, respectively. As of March 31, 2012, we had a retail product portfolio of 46 products, and we are currently selling 14 of them in the market. As we have released new products, the percentage contribution of Shining Essence to our total retail sales has decreased.
| 34 |
As our retail products comprise mainly live bacteria, which are reproduced by fermentation, we have historically had a low cost of production of which packaging costs represent the largest cost item. Based on sales invoiced value, our bulk products have a revenue contribution of 53% in fiscal year 2012, increased from 42% in fiscal year 2011. The significant increase reflects the stability of the additive market as compared to the retail products market in the current adverse economic environment. In February 2010, our new bulk production facility in Qingpu commenced commercial production. The state of art bulk additives production facility has a full capacity of 150 metric tons. Management believes our new facility will help us to continue to meet the increasing market demand for high quality and low cost products.
Our management believes that the following trends in China will have an important impact on, and present significant opportunities for, our business:
| · | Increasing demand for functional food and health supplement products. As the discretionary income and health-consciousness of the average Chinese consumer increase, we expect the demand for functional foods and health supplements to increase. |
| · | Curtailment of the use of antibiotics and preservatives and government support for probiotics. China has the highest per capita consumption of antibiotics in the world. To curtail the overuse of antibiotics, the Chinese government has taken steps to limit the use of antibiotic drugs and preservatives for both humans and animals. Moreover, the Chinese State Food and Drug Administration has also acknowledged that probiotics are beneficial for human health. Recently, the Ministry of Health in China announced an expanded list of probiotics strains allowed to be used in the food industry. The number of probiotics strains on the list has doubled. Management believes that this reflects that the Chinese government is encouraging wider uses of probiotics products in the food industry. Management also believes that it also demonstrates the rapidly expanding probiotics market in China. |
| · | Increasing demand for dairy product additives. The demand for functional foods and foods that use probiotics supplements is growing at a significant rate, and our management believes that it will continue to do so. According to statements made by the Nutrition Development Centre of National Development and Reform Commission in China, effective April 1, 2007, probiotics will be required to be added to baby milk powders produced in China. On October 24, 2011, the MOH published a list of probiotics applicable for baby food for babies aged one year or older. |
Our management expects to capitalize on the opportunities created by these trends to achieve significant growth through:
| · | Introduction of bulk additives probiotics products through our Qingpu Facility - We have expanded into the bulk additives business for institutional customers through the completion of our project to build a 300-metric ton capacity plant in Qingpu, which commenced commercial production in February 2010. Phase 1 of the project involved constructing a facility with a production capacity of 150 metric tons of probiotics per year that cost $36 million in total, the sum of which was invested in calendar year 2010. Phase 2 of this project commenced in September 2010 and cost $18 million, $11 million of which was paid in calendar year 2010 and the remaining $7 million of which was paid off by the end of 2011, when the construction of Phase 2 was completed. Phase 2 added an additional 150 metric tons of annual capacity. The construction of Phase 1 of the plant was funded by cash received from the sale of a convertible promissory note in December 2007 and the proceeds of the public offering of our common stock in October 2009. The construction cost of Phase 2 of the plant was funded by cash received from the public offering of our common stock in October 2009. On March 21, 2006, GSL, our subsidiary, entered into an agreement, as amended, with Shanghai Qingpu Industrial Park District Development (Group) Company Limited for the lease of 36,075 square meters of land in the Qingpu on which we constructed the 300-metric ton capacity production plant described above for a term of 50 years beginning January 15, 2008. The agreement provides for the payment of leasing fees of approximately $1.8 million. In February 2009, the formal land use right certificate was issued. There are no future lease payments under this land lease. |
| · | We are currently focused on two fast-growing industries in China: the dairy and animal feed sectors. As of March 31, 2012, we had entered into contracts with 71 customers for the bulk additives business. In this regard, we have created a number of formulations for testing by many potential customers. We have established business relationships with a variety of commercial customers located in major cities, including Beijing, Tianjin, Chongqing, and Shanghai, and 16 provinces, including Guangdong, Jiangsu and Jiangxi, among others. These growing companies are among the leaders in the dairy, animal feed, baked foods, and pharmaceutical industries. |
| · | Construction of a new facility in Yangling - Encouraged by the growing demand for the animal feed market in China, the Company has commenced construction of a new facility in the Yangling Zone in the Shaanxi Province of China. The cost for constructing the Yangling facility is expected to be over $58 million invested over two years. The facility will produce probiotics and probiotics-related biological additives for the animal feed industry. Currently, the facility is in the construction stage, and the plan is subject to government approval prior to implementation. During the year ended March 31, 2011, the Company made total payments of approximately $3.25 million (RMB 20,608,112) to acquire the land use right of approximately 122,600 square meters (183.94 mu). We received the land use right certificate on January 28, 2012 and commenced construction in June 2012. We expect to complete construction by June 2014 and trial production is expected to commence in 2015. In the existing market environment, the Company will utilize its available cash and bank facilities to fund such construction. |
| 35 |
| · | Closure of retail outlets and expansion to wholesale and e-commerce businesses - In our continuing effort to transition from a retail, business-to-consumer model to a wholesale, business-to-business model, and to improve operating efficiency, we have completed the consolidation of our retail outlets. We closed all of our retail outlets by the end of fiscal year 2011, as we believe our distribution network is more efficient for our retail products sales. Comparatively, direct selling through retail outlets involves increasing leasing expenses and staffing costs. By selecting six new distributors, we have recently expanded our distribution network into the greater Beijing area to sell the Company’s retail products. The local distributors sell the Company’s retail probiotics products through established distribution networks, including malls, supermarkets, and functional food stores. During the quarter ended March 31, 2012, we added five more distributors. We now have a total of 39 distributors for retail products as of March 31, 2012. In light of increasing online sales of health food and supplements in China and to maintain our existing retail customer base, “Community Network,” we established an in-house e-commerce department during the quarter ended March 31, 2011, which is dedicated to promoting and selling our retail products online through the Company’s website at http://www.shiningbt.com/Product/. Since its establishment, the in-house e-commerce department is working to better access the market and existing and potential customers. We have also been working with two online selling companies to sell our retail products, including www.ule.com.cn and www.yihaodian.com. In addition, we have established a customer service center to reply to inquiries from our end users. |
| · | Improvement in research and development of new products and services - We continue to develop new retail products aimed at improving general human health conditions, enhancing the immune system, and reducing health problems. To further improve our competency in the bulk additives market, we continue to improve and provide our value-added service to institutional customers by assisting their lab and production testing and providing customized technical support, among other things. In addition, we are also working with certain universities, including Northeast Agricultural University, to carry out research and development projects in order to seek to increase the probiotics industry’s capability. |
Our operations are generally not labor-intensive. We employed 387 people as of March 31, 2012. With production ramp up in the Phase 1 facility and the construction of Phase 2 in Qingpu, we expect significant increases in our number of employees over the next two years. We have been recruiting senior executives to strengthen our management team. However, as wages in China are relatively inexpensive, we expect that labor costs will remain insignificant.
Results of Operations for the Year Ended March 31, 2012 Compared with the Year Ended March 31, 2011
Our results for 2012 and 2011 are summarized below:
| Year ended March 31, 2012 | Year ended March 31, 2011 | |||||||||||||||
| Amount | % of Net sales | Amount | % of Net sales | |||||||||||||
| Net sales | $ | 58,873,629 | 100.00 | % | $ | 108,794,932 | 100.00 | % | ||||||||
| Cost of sales | 26,163,629 | 44.44 | % | 38,926,924 | 35.78 | % | ||||||||||
| Cost of product returns | 7,162,229 | 12.17 | % | - | - | |||||||||||
| Gross profit | 25,547,771 | 43.39 | % | 69,868,008 | 64.22 | % | ||||||||||
| Operating expenses: | ||||||||||||||||
| Selling expenses | 5,628,820 | 9.56 | % | 13,904,849 | 12.78 | % | ||||||||||
| General and administrative expenses | 16,320,988 | 27.72 | % | 20,229,024 | 18.59 | % | ||||||||||
| Total operating expenses | 21,949,808 | 37.28 | % | 34,133,873 | 31.37 | % | ||||||||||
| Income from operations | 3,597,963 | 6.11 | % | 35,734,135 | 32.85 | % | ||||||||||
| Other income and expense: | ||||||||||||||||
| Gain on extinguishment of derivative liability | - | - | 14,797,000 | 13.60 | % | |||||||||||
| Interest expense | (344,957 | ) | (0.59 | )% | (4,930,896 | ) | (4.53 | )% | ||||||||
| Interest income | 2,571,306 | 4.37 | % | 512,578 | 0.47 | % | ||||||||||
| Other income | 4,885 | 0.01 | % | 1,665 | 0.01 | % | ||||||||||
| Other expenses | (126,149 | ) | (0.21 | )% | (55,049 | ) | (0.05 | )% | ||||||||
| Exchange losses, net | (94,070 | ) | (0.16 | )% | (141,607 | ) | (0.13 | )% | ||||||||
| Total other income | 2,011,015 | 3.42 | % | 10,183,691 | 9.36 | % | ||||||||||
| Income before taxes | 5,608,978 | 9.53 | % | 45,917,826 | 42.21 | % | ||||||||||
| Provision for income taxes | 2,833,168 | 4.81 | % | 8,982,349 | 8.26 | % | ||||||||||
| Net income | $ | 2,775,810 | 4.71 | % | $ | 36,935,477 | 33.95 | % | ||||||||
| 36 |
Net sales
Net sales in our financial statements are stated at invoiced value less sales discount and sales tax. Our net sales for the fiscal years 2012 and 2011 comprised the following:
| Year ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Invoiced value of sales | $ | 61,663,280 | $ | 113,007,667 | ||||
| Less: Sales discount | (2,350,314 | ) | (3,779,603 | ) | ||||
| Less: Sales tax | (439,337 | ) | (433,132 | ) | ||||
| $ | 58,873,629 | $ | 108,794,932 | |||||
Net sales decreased by $49,921,303 or 45.89% to $58,873,629 for the year ended March 31, 2012 from $108,794,932 for the year ended March 31, 2011. The decrease was mainly attributable to the implementation by the PRC government of new rules and regulations on health care products, including our probiotics products, during 2011. Certain health care products, including our probiotics products, have been repositioned as food and so are now governed by the PRC’s Food Safety Law, resulting in the Company and its competitors having to adapt to the new rules and regulations. In addition, due to the overestimation of market demand by the Company’s distributors for the first calendar quarter of 2011, we agreed to accept the return of probiotics products worth $2,590,841 from certain distributors during May 2011, in part to avoid the Company suffering a negative image in the probiotics market. All such returned goods were held in the Company’s warehouse until they were fully destroyed in September 2011. During June 2011, we also found a batch of probiotics products worth $474,505 that were below the Company’s quality standards due to production problems; the Company tracked and destroyed all such products after obtaining them from its distributors.
The contributions of our products as a percentage of invoiced value on sales for the years ended March 31, 2012 and 2011, respectively, are summarized below.
| Year ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Retail products | 49.41 | % | 58.88 | % | ||||
| Bulk additives | 50.59 | % | 41.12 | % | ||||
| 100.00 | % | 100.00 | % | |||||
Unit volume and unit prices comparatives (on the invoiced value of sales) for 2012 and 2011 are summarized below.
| Percentage increase (decrease) from the prior year | ||||||||||||||||||||||||
| Year ended March 31, | ||||||||||||||||||||||||
| 2012 | 2011 | |||||||||||||||||||||||
| Unit volume | Selling prices | Overall increase / (decrease) | Unit Volume | Selling prices | Overall increase / (decrease) | |||||||||||||||||||
| Retail products | (67 | )% | 21 | % | (60 | )% | (7 | )% | 11 | % | 3 | % | ||||||||||||
| Bulk additives | (39 | )% | 8 | % | (35 | )% | 70 | % | 13 | % | 92 | % | ||||||||||||
Based on the invoiced value of sales, the retail products invoiced sales contributions decreased to 49.41% in the year ended March 31, 2012 from 58.88% in the year ended March 31, 2011. The decrease was mainly attributable to the implementation by the government of the PRC of new rules and regulations on health care products during calendar year 2011, as noted above. Many Chinese customers, including many of our customers, have developed in recent years a lack of confidence in the safety of locally produced food and healthcare products, which has had a noticeable effect on our sales volume.
| 37 |
Cost of sales
Cost of sales for the year ended March 31, 2012 was $26,163,629 compared with $38,926,924 for the year ended March 31, 2011. The decrease in cost of sales was primarily caused by decrease in sales for the year ended March 31, 2012.
Unit volume and unit costs comparatives for the year ended March 31, 2012 and 2011 are summarized below.
| Percentage increase (decrease) from the prior year | ||||||||||||||||||||||||
| Year ended March 31, | ||||||||||||||||||||||||
| 2012 | 2011 | |||||||||||||||||||||||
| Unit volume | Unit Costs | Overall increase / (decrease) | Unit Volume | Unit Costs | Overall increase / (decrease) | |||||||||||||||||||
| Retail products | (67 | )% | 10 | % | 52 | % | (7 | )% | 30 | % | 21 | % | ||||||||||||
| Bulk additives | (39 | )% | 29 | % | (22 | )% | 70 | % | 38 | % | 134 | % | ||||||||||||
Cost of product returns
For the year ended March 31, 2012, product returns of $474,505 were deducted from sales due to a batch of probiotics products produced in June 2011 that were below the Company’s quality standards due to production problems. For the year ended March 31, 2011, product returns of $2,590,841 were deducted from sales due to the overestimation of market demand by the Company’s distributors for the first calendar quarter of 2011. We agreed to accept the return of probiotics products from certain distributors during May 2011, in part to avoid the Company suffering a negative image in the probiotics market. All such returned goods were held in the Company’s warehouse until they were fully destroyed by September 2011.
As a result of a product returns after certain of our products did not meet our quality standards, and in order to improve the confidence of the Company’s distributors and end-user customers in our probiotics products, we provided special concession credits to our distributors during August 2011 to address the complaints and concerns of customers worried about food and healthcare products in the PRC. Such credits were made available for our distributors to reimburse end-user customers for products which were recalled. We treated these credits as being comparable to a warranty on our probiotics products, and we incurred a cost of $7,162,229 for these credits for the year ended March 31, 2012 that has been reflected as an additional cost of product returns in the accompanying consolidated financial statements.
Gross profit
Gross profit decreased by $44,320,237 or 63.43% to $25,547,771 for the year ended March 31, 2012, from $69,868,008 for the year ended March 31, 2011. The decrease was mainly attributable to the decrease in sales volume and also the write-off of the special payments to distributors, which we consider a cost of the product returns described above, for the year ended March 31, 2012.
Selling expenses
Selling expenses were $5,628,820 or 9.56% of net sales for the year ended March 31, 2012, compared with $13,904,849 or 12.78% of net sales for the year ended March 31, 2011. The decrease was mainly attributable to the reduction in operating costs of the retail outlets, all of which were closed by December 31, 2010.
General and administrative expenses
General and administrative expenses decreased by $3,908,036 or 19.32% to $16,320,988 or 27.72% of net sales for the year ended March 31, 2012, compared with $20,229,024 or 18.59% of net sales for the year ended March 31, 2011. The decrease in general and administrative expenses was mainly attributable to the reduction of legal and professional fees for legal actions filed against the Company by third parties, including stockholders and the SEC, by $1,308,286 to $731,841 for the year ended March 31, 2012 from $2,040,127 for the year ended March 31, 2011. General and administrative expenses for the year ended March 31, 2012, were also composed of audit fees of $600,000, salaries and allowance of $2,765,532, research and development expenses of $5,084,949 and depreciation of $975,911, and were offset by the impairment of accounts receivable of $1,665,188.
Income from operations
Income from operations decreased by $32,136,172 or 89.93% to $3,597,963 for the year ended March 31, 2012, from $35,734,135 for the year ended March 31, 2011. The decrease is mainly attributable to the decrease in sales and decrease of gross profit margin for the year ended March 31, 2012.
| 38 |
Total other income (expenses)
Total other income decreased by $8,172,676 or 80.25% to $2,011,015 for the year ended March 31, 2012, from $10,183,691 for the year ended March 31, 2011. The decrease is mainly attributable to the gain on extinguishment of derivative liability of $0 and $14,797,000 for the years ended March 31, 2012 and 2011, respectively.
Provision for income taxes
Provision for income taxes was $2,833,168 and $8,982,349 for the years ended March 31, 2012 and 2011, respectively. The decrease in the provision for income taxes is primarily attributable to decrease in operating profit.
Net income
Net income decreased by $34,159,667 or 92.48% to $2,775,810 for the year ended March 31, 2012 from $36,935,477 for the year ended March 31, 2011. The decrease of net income is mainly attributable to the decrease of income from operations of $32,136,172 and decrease of total other income of $8,172,676.
Segment reporting
We have adopted the “products and services” approach for segment reporting. For fiscal years 2012 and 2011, the management considers there to be two reporting segments: retail products and bulk additive products, as the new bulk production plant commenced production since February 2010 with a separate production and distribution channel. As the separate entity of production, there are also separate operational management teams and operations and marketing strategies. Therefore, we have adopted the segment reporting since fiscal year 2011.
Retail products
The results of retail products segment for the years ended March 31, 2012 and 2011 are summarized as below:
| Year ended March 31, 2012 | Year ended March 31, 2011 | |||||||||||||||
| Amount | % of Net sales | Amount | % of Net sales | |||||||||||||
| Net sales, retail products | $ | 27,890,558 | 100.00 | % | $ | 62,564,210 | 100.00 | % | ||||||||
| Cost of sales | 13,720,712 | 49.19 | % | 22,844,520 | 36.51 | % | ||||||||||
| Cost of product returns | 7,162,229 | 25.68 | % | - | - | % | ||||||||||
| Gross profit, retail products | 7,007,617 | 25.13 | % | 39,719,690 | 63.49 | % | ||||||||||
| Operating expenses: | ||||||||||||||||
| Selling expenses | 3,114,752 | 11.17 | % | 10,357,414 | 16.55 | % | ||||||||||
| General and administrative expenses | 4,922,325 | 17.65 | % | 6,203,158 | 9.91 | % | ||||||||||
| Total operating expenses | 8,037,077 | 28.82 | % | 16,560,572 | 26.47 | % | ||||||||||
| Income from operations, retail products | $ | (1,029,460 | ) | (3.69 | )% | $ | 23,159,118 | 37.02 | % | |||||||
Net sales
Net sales in our financial statements are stated at invoiced value less sales discount and sales tax. Our net sales for the fiscal years 2012 and 2011 comprised the following:
| Year ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Invoiced value on sales | $ | 30,470,340 | $ | 66,536,259 | ||||
| Less: Sales discount | (2,350,314 | ) | (3,779,603 | ) | ||||
| Less: Sales tax | (229,468 | ) | (192,446 | ) | ||||
| $ | 27,890,558 | $ | 62,564,210 | |||||
Net sales decreased by $34,673,652 or 55.42% to $27,890,558 for the year ended March 31, 2012 from $62,564,210 for the year ended March 31, 2011. The decrease in sales of retails products is preliminary attributable to the implementation by the government of the PRC of new rules and regulations on health care products during 2011, as noted above.
| 39 |
Cost of sales
Cost of sales decreased by $9,123,808 or 39.94% to $13,720,712 for the year ended March 31, 2012 from $22,844,520 for the year ended March 31, 2011. The decrease in cost of sales was primarily caused by the decrease of products sold during the fiscal year 2012.
Cost of product returns
For the year ended March 31, 2012, product returns of $474,505 were deducted from sales due to a batch of probiotics products produced in June 2011 that were below the Company’s quality standards due to production problems. For the year ended March 31, 2011, product returns of $2,590,841 were deducted from sales due to the overestimation of market demand by the Company’s distributors for the first calendar quarter of 2011. We agreed to accept the return of probiotics products from certain distributors during May 2011, in part to avoid the Company suffering a negative image in the probiotics market. All such returned goods were held in the Company’s warehouse until they were fully destroyed by September 2011.
As a result of a product returns after certain of our products did not meet our quality standards, and in order to improve the confidence of the Company’s distributors and end-user customers in our probiotics products, we provided special concession credits to our distributors during August 2011 to address the complaints and concerns of customers worried about food and healthcare products in the PRC. Such credits were made available for our distributors to reimburse end-user customers for products which were recalled. We treated these credits as being comparable to a warranty on our probiotics products, and we incurred a cost of $7,162,229 for these credits for the year ended March 31, 2012 that has been reflected as an additional cost of product returns in the accompanying consolidated financial statements.
Gross profit
Gross profit decreased by $32,712,073 or 82.36% to $7,007,617 for the year ended March 31, 2012 from $39,719,690 for the year ended March 31, 2011. The decrease in gross profit was primarily caused by the decrease of products sold and also the write-off of a special payment to distributors, which we consider a cost of the product returns described above for the year ended March 31, 2012.
Selling expenses
Selling expenses were $3,114,752 or 11.17% of net sales for the year ended March 31, 2012, compared with $10,357,414 or 16.55% of net sales for the year ended March 31, 2011. The selling expenses mainly included salary and advertising expenses. This decrease in selling expenses was primarily attributable to the closing of all retail outlets on or before December 31, 2010, which directly reduced the operating expenses of those outlets, including salary and rental payments.
General and administrative expenses
General and administrative expenses decreased by $1,280,833 or 20.65% to $4,922,325 or 17.65% of net sales for the year ended March 31, 2012 compared with $6,203,158 or 9.91% of net sales for the year ended March 31, 2011. The decrease in general and administrative expenses was mainly attributable to a decrease of research and development costs of $1,400,610 to $2,487,074 for the year ended March 31, 2012 from $3,887,684 for the year ended March 31, 2011.
Income from operations
Income from operations decreased by $24,188,578 or 104.45% to become a loss from operations of $1,029,460 for the year ended March 31, 2012 from income from operations of $23,159,118 for the year ended March 31, 2011. The decrease is mainly attributable to decrease of products sold for the year ended March 31, 2012.
| 40 |
Bulk products
The results of bulk additives products segment for the years ended March 31, 2012 and 2011 are summarized as below:
| Year ended March 31, 2012 | Year ended March 31, 2011 | |||||||||||||||
| Amount | % of Net sales | Amount | % of Net sales | |||||||||||||
| Net sales, bulk additives products | $ | 30,983,071 | 100.00 | % | $ | 46,230,722 | 100.00 | % | ||||||||
| Cost of sales | 12,442,917 | 40.16 | % | 16,082,404 | 34.79 | % | ||||||||||
| Gross profit, bulk additives products | 18,540,154 | 59.84 | % | 30,148,318 | 65.21 | % | ||||||||||
| Operating expenses: | ||||||||||||||||
| Selling expenses | 2,514,068 | 8.11 | % | 3,547,435 | 7.67 | % | ||||||||||
| General and administrative expenses | 6,049,185 | 19.52 | % | 6,172,289 | 13.35 | % | ||||||||||
| Total operating expenses | 8,563,253 | 27.64 | % | 9,719,724 | 21.02 | % | ||||||||||
| Income from operations, bulk additives products | $ | 9,976,901 | 32.20 | % | $ | 20,428,594 | 44.19 | % | ||||||||
Net sales
Net sales in our financial statements are stated at invoiced value less sales discount and sales tax. Our net sales for the fiscal years 2012 and 2011 comprised the following:
| Year ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Invoiced value on sales | $ | 31,192,940 | $ | 46,471,408 | ||||
| Less: Sales tax | (209,869 | ) | (240,686 | ) | ||||
| $ | 30,983,071 | $ | 46,230,722 | |||||
Net sales decreased by $15,247,651 or 32.98% to $30,983,071 for the year ended March 31, 2012, from $46,230,722 for the year ended March 31, 2011. The decrease in sales of bulk additive products is primarily attributable to the implementation by the government of the PRC of new rules and regulations on health care products during 2011, as noted above.
Cost of sales
Cost of sales decreased by $3,639,487 or 22.63% to $12,442,917 for the year ended March 31, 2012, from $16,082,404 for the year ended March 31, 2011. The decrease in cost of sales was primarily caused by the decrease in products sold for the year ended March 31, 2012.
Gross profit
Gross profit decreased by $11,608,164 or 38.50% to $18,540,154 for the year ended March 31, 2012, from $30,148,318 for the year ended March 31, 2011. The decrease of gross profit was primarily caused by the decrease in products sold for the year ended March 31, 2012.
Selling expenses
Selling expenses were $2,514,068 or 8.11% of net sales for the year ended March 31, 2012, compared with $3,547,435 or 7.67% of net sales for the year ended March 31, 2011. The selling expenses mainly included advertising expenses, promotion expenses and salary. This decrease in selling expenses was primarily attributable to decrease of the advertising and promotion expenses of $219,004 and $760,286, respectively, for the year ended March 31, 2012.
General and administrative expenses
General and administrative expenses were $6,049,185 or 19.52% of net sales for the year ended March 31, 2012, compared with $6,172,289 or 13.35% of net sales for the year ended March 31, 2011. The decrease in general and administrative expenses was mainly attributable to a decrease of depreciation charges and research and development costs by $244,955 to $2,597,875 for the year ended March 31, 2012 from $2,842,830 for the year ended March 31, 2011.
Income from operations
Income from operations decreased by $10,451,693 or 51.16% to $9,976,901 for the year ended March 31, 2012, from $20,428,594 for the year ended March 31, 2011. The decrease is mainly attributable to the decrease of gross profit of $11,608,164 from a decrease in sales and net of the decrease in selling expenses, and general and administrative expenses for the year ended March 31, 2012 stated above.
| 41 |
Liquidity and Capital Resources
Liquidity
We had cash of $70.0 million and working capital of $57.2 million as of March 31, 2012 and cash of $144.0 million and working capital of $132.3 million as of March 31, 2011.
Our statements of cash flow for the years ended March 31, 2012 and 2011 are summarized as below:
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Net cash provided by operating activities | $ | 1,294,041 | $ | 26,090,309 | ||||
| Net cash used in investing activities | (83,930,458 | ) | (15,223,029 | ) | ||||
| Net cash provided by (used in) financing activities | 12,192,030 | (25,000,119 | ) | |||||
| Effect of exchange rate changes on cash | (3,457,887 | ) | 2,541,816 | |||||
| Net decrease in cash and cash equivalents balances | $ | (73,902,274 | ) | $ | (11,591,023 | ) | ||
Operating activities
Cash generated from operations was $1.3 million and $26.1 million for the years ended March 31, 2012 and 2011, respectively.
During the year ended March 31, 2012, we had positive cash flow from operating activities of $1.3 million, primarily attributable to income from operations of $3.6 million. During the year ended March 31, 2011, we had positive cash flow from operating activities of $26.1 million, primarily attributable to income from operations of $35.7 million. The primary reason for the decrease in cash flow from the year ended March 31, 2011 to the year ended March 31, 2012, was a decrease in income from operations.
Our business is not capital or labor intensive. Typically, 60% of our sales take place in the second half of the fiscal year. Since our customers have historically been large distributors with whom we have done business for a number of years, our cash flows from our existing business have been, and we expect them to continue to be, fairly reliable.
Investing activities
We had capital expenditures totaling $83.9 million for the year ended March 31, 2012, primarily related to: a $20.8 million loan receivable to a trust fund; deposits of $28.9 million for certain capital expenditures and acquisitions; $13.9 million for the construction, machinery and equipment for completion of the Phase 2 construction at our Qingpu facility discussed above; and $18.0 million in the prepayment of capital expenditures with respect to our Yangling facility, also discussed above.
On December 19, 2011, the Company entered into an agreement with Jiangxi International Trust Co., Ltd. (“Jiangxi”) to loan $20,753,343 (RMB 131,100,000) to the Jiangxi International Trust of Yinhe #7 Property, an investment fund sponsored by Jiangxi. The loan principal is due on December 26, 2013, is unsecured, and according to the loan agreement, the Company is to earn 20% interest per annum, less a 5% investment management fee per annum. Interest income for the year ended March 31, 2012 includes $1,078,320 of interest accrued on the outstanding balance. Jiangxi is a major trust and investment company that is carrying different investment projects in the PRC, including real estate projects, listed company restructuring, and other financial services.
On March 27, 2012, the Company paid a deposit of $23.7 million (RMB150 million) to the government of Yangling for a bid to acquire a local probiotics company located in Yangling, Shaanxi Province that is in receivership. According to the relevant regulations, the deposit will be treated as part of the purchase price of the acquisition if the transaction is approved. As of September 13, 2012, the government of Yangling is reviewing bids received for the probiotics company and expects to complete this process by the end of calendar 2012. If the Company does not win the auction to acquire the probiotics company, its deposit will be returned.
On February 1, 2012, our subsidiary, Growing, entered into an agreement to acquire a patent regarding a production technology for $6,647,147 (RMB 42,000,000) from an unrelated PRC company. Growing made a deposit of $6,330,616 (RMB 40,000,000) to the seller for the transfer of the patent within 210 days, or by August 30, 2012. The original terms of the agreement were that if the patent cannot be transferred to Growing within 210 days, Growing will be refunded the deposit paid and also be compensated an additional $159,000 (RMB 1,000,000) for damages. The transfer was not completed by August 30, 2012, and on September 3, 2012, Growing and the seller mutually agreed to extend the completion date to December 30, 2012. As of September 13, 2012, the transfer had not yet been completed because the Company is waiting for approval of the transfer from the State Intellectual Property Office of the PRC. The Company has no information to allow it to estimate when such confirmation will be obtained, but expects the transaction to close in 2013, after which the patent is planned to be used in the Company’s operations.
| 42 |
Financing activities
Cash provided from financing activities was $12.2 million for the year ended March 31, 2012, and we used net cash of $25.5 million in financing activities in the year ended March 31, 2011. Details of our financing activities for such periods are as follows:
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Repayment of convertible note | $ | - | $ | (25,000,000 | ) | |||
| Purchase of treasury stock | - | (2,739,634 | ) | |||||
| Proceeds from loans | 7,609,308 | - | ||||||
| Advance from director | 4,582,722 | 2,739,515 | ||||||
| NET CASH PROVIDED (USED IN) BY FINANCING ACTIVITIES | $ | 12,192,030 | $ | (25,000,119 | ) | |||
The net cash provided by financing activities for the year ended March 31, 2012 was primarily from the proceeds of a bank loans and the repayment of an advance from the Company to a director amounting to $7.6 million and $4.6 million, respectively.
The net cash used in financing activities for the year ended March 31, 2011 was used primarily on the repayment of a convertible note of $25.0 million (detail discussed below under “Capital Resources”) and the purchase of stock (now held as treasury stock) for $2.7 million.
During the years ended March 31, 2012 and 2011, the advance from a director, Mr. Song Jinan, was entered into because of the difficulty the Company faced in paying expenses incurred outside China in non-PRC currency due to the difficulties the Company had in converting currency because of foreign exchange controls.
Capital Resources
Issuance of Common Stock
On October 5, 2009, the Company closed an underwritten public offering of 4,600,000 shares of its common stock at a price of $15.00 per share. On October 26, 2009, an additional 690,000 shares were sold pursuant to the exercise of an over-allotment option at the same price. Net proceeds of the offering, including the over-allotment, after deducting underwriting discounts, and offering expenses, were approximately $74.9 million. The Company expects to use the net proceeds from the offering for general corporate purposes, including expanding its retail operations, expanding its products, funding Phase 2 of our bulk manufacturing facility, funding our newly announced Yangling project, and for general working capital purposes.
The offering was made pursuant to an Underwriting Agreement, dated September 29, 2009, by and between the Company and Roth Capital Partners, LLC, as sole manager and representative of the underwriters named therein. The offering of the shares was registered under the Securities Act of 1933, as amended, pursuant to the Company’s shelf registration statement on Form S-3, as amended by Amendment No. 1 and Amendment No. 2 to Form S-3 (File No. 333-160519).
Issuance and Repayment of Convertible Note
On December 11, 2007, we issued the Note to Pope Investments II LLC, an affiliate of Pope Investments, LLC, with a maturity date of December 11, 2010. The principal amount of the Note was convertible into shares of our common stock at an exercise price of $12.00 per share at any time until the maturity date. Net proceeds of the Note were used to fund the construction of the 150-metric-ton-per-year manufacturing facility in Qingpu and other capital expenditures.
On December 9, 2010, the Company repaid in full its obligations under the Note in the original aggregate principal amount of $25,000,000. The payoff amount of $29,684,932, consisting of $25,000,000 of outstanding principal and $4,684,932 of accrued interest, was paid to Pope Investments II LLC, and all security interests and liens held by Pope Investments II LLC were terminated and released, including (1) a guaranty by Mr. Song Jinan of the Company’s obligations under the Note backed by a pledge of 4,000,000 shares of China-Biotics’ common stock owned by Mr. Song; and (2) a pledge by the Company of 100% of the stock of SGI to Pope Investments II LLC.
| 43 |
Taking into account our current cash position and our anticipated cash flows from operations, we expect that we will be able to meet all our funding needs in the next twelve months, including payments required to settle our contractual obligations and for the construction of our Yangling plant. No assurance, however, can be given that our business plan will succeed. In the event that our business plan does not materialize as predicted, we may need to seek external financing to fund our expansion plan. There can be no assurance that we will be able to raise needed capital on favorable terms, if at all. In addition, there is no assurance that our estimate of our liquidity needs is accurate or that new business development or other unforeseen events will not occur, resulting in the need to raise additional funds.
Commitments
Operating Leases
As of March 31, 2012, future minimum lease payments under non-cancellable operating leases for office, warehouse and factory were as follows:
| 2012 | ||||
| For the year ended March 31, | ||||
| 2013 | $ | 622,396 | ||
| 2014 | 94,832 | |||
| Thereafter | - | |||
| $ | 717,228 | |||
Rental expense, which was charged to expense, amounted to $275,424 and $1,689,680 for the years ended March 31, 2012 and 2011, respectively.
Capital commitments
On August 12, 2010, BDH, a subsidiary of the Company, entered into agreements with a government agency to establish manufacturing facilities for animal probiotics products in the Yangling Agricultural High-tech Industries Demonstration Zone in Shaanxi Province of China. In furtherance of such agreements, BDH incorporated a foreign, wholly owned subsidiary, Growing Yangling, with a registered capital of $50 million. As of March 31, 2012, the Company had injected into Growing Yangling $7.5 million as registered capital. According to the approval from the government agency dated October 12, 2010, the remaining balance of Growing Yangling’s registered capital of $42.5 million must be injected before July 13, 2013
The cost for constructing the Yangling facility is expected to be over $58 million invested over two years, which would be mainly facilitated by the capital injection from BDH. The facility will produce probiotics and probiotics-related biological additives for the animal feed industry. Currently, the facility is in its construction stage. During the year ended March 31, 2011, the Company made total payment of approximately $3.25 million (RMB 20,608,112) to acquire the land use right of approximately 122,600 square meters (183.94 mu). We received the land certificate on January 28, 2012 and commenced construction in June 2012. We expect to complete construction by June 2014. As of March 31, 2012, Growing Yangling entered into agreements with contractors related to the construction of the plant and manufacturing facilities for future payment of $2,548,749 and $2,458,630, respectively. Subsequent to March 31, 2012, Growing Yangling entered into agreements with contractors for approximately $15,750,000 (RMB 99,400,000) for the purchase of production equipment.
On February 1, 2012, our subsidiary, Growing entered into an agreement to acquire a patent regarding a production technology for $6,647,147 (RMB 42,000,000) from an unrelated PRC company. Growing made a deposit of $6,330,616 (RMB 40,000,000) to the seller for the transfer of the patent within 210 days, or by August 30, 2012. The original terms of the agreement were that if the patent cannot be transferred to Growing within 210 days, Growing will be refunded the deposit paid and also be compensated an additional $159,000 (RMB 1,000,000) for damages. The transfer was not completed by August 30, 2012, and on September 3, 2012, Growing and the seller mutually agreed to extend the completion date to December 30, 2012. As of September 13, 2012, the transfer had not yet been completed because the Company is waiting for approval of the transfer from the State Intellectual Property Office of the PRC. The Company has no information to allow it to estimate when such confirmation will be obtained, but expects the transaction to close in 2013, after which the patent is planned to be used in the Company’s operations. Subject to the completion of the transfer of the patent, the Company will have to pay the balance, the future payment of which amounts to $316,531 (RMB2 million), which amount was contracted but not provided for as of March 31, 2012.
| 44 |
Purchase obligations
Shining and Growing entered into agreements with suppliers to purchase raw materials and packing materials, with respect to which the amount of future payments is $8,019,273.
Other Obligations
Growing entered into an agreement with a university in the PRC to perform research and development. The amount of future payments is $1,424,389 (RMB9 million).
Inflation
We believe that inflation has not had a material impact on our results of operations for the years ended March 31, 2012 and 2011.
Seasonality
Regarding our retail products, many of our customers purchase our products as gifts during the Chinese festivals and holidays, and the seasonal effect is correlated to these holidays during the year. For the first two quarters, there are no major Chinese festivals or holidays, except for the Dragon Boat Festival (June) and mid-Autumn Festival (September). However, in the last two quarters, there are some major Chinese festivals and holidays, including National Day (October), Christmas (December), New Year (January), and Chinese New Year (January to February).
With respect to our bulk additive products, while it is still too early to tell, we expect that our bulk additives sales will not be seasonal in nature because the bulk products are purchased by food manufacturers consistently over the year. Except for the possibility of the PRC government implementing from time to time new or different rules and regulations for the food industry, including with respect to additives and related products, we are not aware of and do not foresee any seasonal effects on our bulk additive products business.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements.
Critical Accounting Policies
This MD&A discusses our consolidated financial statements for the years ended March 31, 2012 and 2011. These financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. In preparing these financial statements, we are required to make estimates and assumptions affecting the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. On an ongoing basis, we evaluate our estimates and judgments. We base our estimates and judgments on historical experience and on various other factors we believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
Those estimates and assumptions include estimates for allowance for doubtful accounts, inventory valuation, impairment consideration, assumptions used in the valuation of derivative liabilities, and estimates for potential penalties for late payment of taxes.
Revenue Recognition
Revenues of the Company are from the sale of our probiotics products. We recognize revenue from the sale of goods when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred; (3) the sales price is fixed or determinable; and (4) collectability is reasonably assured. Revenues are presented net of value added tax (“VAT”). In our revenue arrangements, physical delivery is the point in time when customer acceptance occurs since title and risk of loss are transferred to the customer.
Allowance for doubtful accounts
We maintain an allowance for doubtful accounts for estimated losses that may result from the inability of our customers to make required payments. Such allowances are based upon several factors including, but not limited to, historical experience and the current and projected financial condition of specific customers. Since our inception of business, we have never experienced any unrecoverable receivables. We also have never experienced situations causing us to cast doubt on the ability of our customers to make required payments. We had trade receivables totaling $35,057,662 and $26,194,313 as of March 31, 2012 and 2011, respectively, and an allowance for doubtful accounts of $1,665,188 and $0 for the years ended March 31, 2012 and 2011, respectively. We have considered all relevant factors, including the financial conditions, affecting the payment abilities of customers comprising these receivables up to the date of this 10-K, and we believe these customers are able to make required payments. We, however, cannot give assurance that these factors, including the financial conditions of these customers, will not change adversely in the future. We will continue to evaluate the ability of all our customers to make required payments. Were the financial condition of a customer to deteriorate, resulting in an impairment of its ability to make payments, allowances may be required.
| 45 |
Stock based compensation
The Company periodically issues stock options and warrants to employees and non-employees in non-capital raising transactions for services and for financing costs. The Company accounts for stock option and warrant grants issued and vesting to employees based on the authoritative guidance provided by the Financial Accounting Standards Board, whereas the value of the award is measured on the date of grant and recognized over the vesting period. The Company accounts for stock option and warrant grants issued and vesting to non-employees in accordance with the authoritative guidance of the Financial Accounting Standards Board, whereas the value of the stock compensation is based upon the measurement date as determined at either a) the date at which a performance commitment is reached, or b) at the date at which the necessary performance to earn the equity instruments is complete. Non-employee stock-based compensation charges generally are amortized over the vesting period on a straight-line basis. In certain circumstances where there are no future performance requirements by the non-employee, option grants are immediately vested and the total stock-based compensation charge is recorded in the period of the measurement date.
The fair value of the Company’s common stock option grant is estimated using the Black-Scholes-Merton option pricing model, which uses certain assumptions related to risk-free interest rates, expected volatility, expected life of the common stock options, and future dividends. Compensation expense is recorded based upon the value derived from the Black-Scholes-Merton option pricing model, and based on actual experience. The assumptions used in the Black-Scholes-Merton option pricing model could materially affect compensation expense recorded in future periods.
Use of estimates as applied to potential penalties for the late payment of taxes
In addition to the Enterprise Income Tax (“EIT”), companies in the PRC that are engaged in the sale of goods are generally required to pay value added taxes (“VAT”) at a rate of 17% of the gross sales proceeds received, less any deductible VAT already paid or borne by the taxpayer.
Our management believes that our operations in China were exempted from EIT and VAT for all years prior to 2005 because we had been recognized by the local government as an advanced technology enterprise. However, the Company never received a written confirmation from the appropriate tax authorities for the tax exemption status of our operations in China prior to 2005. As a result, there is no way to ascertain the ultimate position which may be taken by the relevant PRC tax authorities in the future and accordingly, full provisions for tax liabilities in the amount of $12,734,640, for all years prior to 2005 have been recorded by the Company. Beginning in January 2006, we made tax payments to the PRC tax authorities for 2005 and we have made regular tax payments to the PRC tax authorities for all subsequent periods.
In addition, in connection with dividends paid to the Shining shareholders between April 2003 to June 2005, Shining did not deduct a withholding tax at the rate of 20% as required by applicable Chinese laws and regulations. The Company has accrued the dividend withholding tax and interest on the dividend withholding tax.
According to PRC tax regulations, the overdue tax liabilities in the PRC for the calendar years prior to 2005 may be subject to interest at the 0.05% per day, and potential penalties for the late payment of taxes which is calculated on the basis of 0.5 times to five times the amount of taxes payable. Through March 31, 2011, the Company accrued the interest that may be potentially payable upon these taxes incurred prior to 2005 and the unpaid dividend withholding tax. Interest related to the unpaid taxes was recognized in the Company’s consolidated statements of operations as a component of its income tax provision.
For the year ended March 31, 2012, management made an assessment of whether it was necessary to provide a further provision for interest and penalties on these unpaid amounts. Management determined that no further provision for interest and penalties was necessary given the unlikelihood of payment of the amount already accrued and provided in the financial statements. This assessment was based upon receipt by the company of a report by an independent tax expert that the amount of taxes prior to 2005 may not be payable, the length of time the amount has been outstanding, and that there have been no requests from PRC tax authorities for payment of the unpaid taxes outstanding amounts. As such, management believes that the previously recorded amounts are sufficient to cover any settlement of this liability.
| 46 |
For further information about our accounting policies, see Item 9. “Changes in and Disagreements with Accountants on Accounting and Financial Disclosure” and Item 9A. “Controls and Procedures” below.
Recent Accounting Pronouncements
See Note 2 to the Notes to the Consolidated Financial Statements for the Years Ended March 31, 2012 and 2011.
Other recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force), the AICPA, and the SEC did not or are not believed by management to have a material impact on the Company's present or future consolidated financial statements.
ITEM 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable to smaller reporting companies.
| 47 |
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
CHINA-BIOTICS, INC. AND SUBSIDIARIES
CONSOLIDATED FINANCIAL STATEMENTS
YEARS ENDED MARCH 31, 2012 AND 2011
TABLE OF CONTENTS
| Report of Independent Registered Public Accounting Firm | 49 | |
| Audited Consolidated Financial Statements | ||
| Consolidated Balance Sheets as of March 31, 2012 and 2011 | 50 | |
| Consolidated Statements of Operations and Other Comprehensive Income for the years ended March 31, 2012 and 2011 | 51 | |
| Consolidated Statements of Changes in Stockholders' Equity for the years ended March 31, 2012 and 2011 | 52 | |
| Consolidated Statements of Cash Flow for the years ended March 31, 2012 and 2011 | 53 | |
| Notes to the Consolidated Financial Statements | 54 | |
| Schedule I - Condensed Parent Company Financial Statements as of and for the years ended March 31, 2012 and 2011 | 69 |
| 48 |
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders of
China-Biotics Inc.
We have audited the accompanying consolidated balance sheets of China-Biotics, Inc. and Subsidiaries (the “Company”) as of March 31, 2012 and 2011, and the related consolidated statements of operations and comprehensive income, changes in stockholders’ equity, and cash flows for each of the years then ended. Our audits also included the financial statement schedules listed in the accompanying index. These consolidated financial statements and schedules are the responsibility of the Company’s management. Our responsibility is to express an opinion on these consolidated financial statements and schedules based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement. We were not engaged to perform an audit of the Company’s internal control over financial reporting. Our audits included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the consolidated financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the consolidated financial position of China-Biotics, Inc. and Subsidiaries at March 31, 2012 and 2011, and the consolidated results of its operations and its cash flows for each of the years then ended, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, the related financial statement schedules, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
On September 15, 2011, the staff of the Securities and Exchange Commission (“SEC”) informed the Company it intended to recommend that the SEC institute a public administrative proceeding against the Company for alleged violations of Section 13(a) of the Securities Exchange Act of 1934 and Rules 13a-1 and 13a-13 or 13a-16 promulgated thereunder. On October 7, 2011, the SEC issued an Order Instituting Proceedings to determine whether it was necessary and appropriate to suspend or revoke the registration of the Company’s securities. On February 22, 2012, the administrative law judge overseeing the proceedings issued a decision ordering that the registration of the Company’s securities be revoked. On March 26, 2012, the SEC granted the Company’s petition for the Commission’s review of this decision. The results of the review are pending as of September 13, 2012. If the registration of the Company’s securities is revoked, no U.S. registered broker-dealer may execute trades in the Company’s shares.
/s/ Weinberg and Company, P.A.
Los Angeles, California
September 14, 2012
| 49 |
CHINA-BIOTICS, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(Amounts expressed in US Dollars)
| March 31, 2012 | March 31, 2011 | |||||||
| ASSETS | ||||||||
| Current assets | ||||||||
| Cash and cash equivalents | $ | 70,086,074 | $ | 143,988,348 | ||||
| Accounts receivable, net | 33,392,474 | 26,194,313 | ||||||
| Inventories | 1,942,549 | 1,772,321 | ||||||
| Deposits - short term | 1,114,367 | 85,546 | ||||||
| Prepayments | 356,157 | 1,245,565 | ||||||
| Other receivables | 102,530 | 276,047 | ||||||
| Total current assets | 106,994,151 | 173,562,140 | ||||||
| Loan receivable | 20,753,343 | - | ||||||
| Deposits - long term | 30,070,428 | - | ||||||
| Prepayments - long term | 22,007,427 | 3,146,229 | ||||||
| Property, plant and equipment, net | 72,080,332 | 59,142,556 | ||||||
| Land use rights | 5,056,883 | 1,780,354 | ||||||
| Total assets | $ | 256,962,564 | $ | 237,631,279 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Accounts payable | $ | 2,873,904 | $ | 5,532,443 | ||||
| Other payables and accruals | 5,425,821 | 4,439,986 | ||||||
| Tax payables | 33,364,611 | 30,942,752 | ||||||
| Short term loan | 3,165,308 | - | ||||||
| Amount due to director | 4,954,345 | 371,623 | ||||||
| Total current liabilities | 49,783,989 | 41,286,804 | ||||||
| Long term loan | 4,747,963 | - | ||||||
| Total liabilities | 54,531,952 | 41,286,804 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders' equity: | ||||||||
| Preferred stock, par value of $0.01, 10,000,000 shares authorized, none issued | - | - | ||||||
| Common stock, par value of $0.0001, 100,000,000 shares authorized,42,370,000 shares issued and 22,150,200 outstanding as of March 31, 2012 and 2011, respectively | 4,237 | 4,237 | ||||||
| Additional paid-in capital | 84,727,616 | 83,242,926 | ||||||
| Retained earnings | 105,153,281 | 102,377,471 | ||||||
| Treasury stock at cost, 20,219,800 shares as of March 31, 2012 and 2011, respectively | (2,741,634 | ) | (2,741,634 | ) | ||||
| Accumulated other comprehensive income | 12,261,318 | 10,435,681 | ||||||
| Capital and statutory reserves | 3,025,794 | 3,025,794 | ||||||
| Total stockholders’ equity | 202,430,612 | 196,344,475 | ||||||
| Total liabilities and stockholders' equity | $ | 256,962,564 | $ | 237,631,279 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| 50 |
CHINA-BIOTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS AND OTHER COMPREHENSIVE INCOME
(Amounts expressed in US Dollars)
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Net sales | $ | 58,873,629 | $ | 108,794,932 | ||||
| Cost of sales | 26,163,629 | 38,926,924 | ||||||
| Cost of product returns | 7,162,229 | - | ||||||
| Gross profit | 25,547,771 | 69,868,008 | ||||||
| Operating expenses: | ||||||||
| Selling expenses | 5,628,820 | 13,904,849 | ||||||
| General and administrative expenses | 16,320,988 | 20,229,024 | ||||||
| Total operating expenses | 21,949,808 | 34,133,873 | ||||||
| Income from operations | 3,597,963 | 35,734,135 | ||||||
| Other income and expenses: | ||||||||
| Gain on extinguishment of derivative liability | - | 14,797,000 | ||||||
| Interest expense | (344,957 | ) | (4,930,896 | ) | ||||
| Interest income | 2,571,306 | 512,578 | ||||||
| Other income | 4,885 | 1,665 | ||||||
| Other expenses | (126,149 | ) | (55,049 | ) | ||||
| Exchange losses, net | (94,070 | ) | (141,607 | ) | ||||
| Total other income | 2,011,015 | 10,183,691 | ||||||
| Income before taxes | 5,608,978 | 45,917,826 | ||||||
| Income taxes | 2,833,168 | 8,982,349 | ||||||
| Net income | 2,775,810 | 36,935,477 | ||||||
| Other comprehensive income | ||||||||
| Foreign currency translation adjustment | 1,825,637 | 5,495,851 | ||||||
| Comprehensive income | $ | 4,601,447 | $ | 42,431,328 | ||||
| Weighted average number of shares | ||||||||
| Basic | 22,150,200 | 22,241,076 | ||||||
| Diluted | 22,266,993 | 23,689,004 | ||||||
| Income per common stock | ||||||||
| Basic | $ | 0.13 | $ | 1.66 | ||||
| Diluted | $ | 0.12 | $ | 0.93 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| 51 |
CHINA-BIOTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY
FOR THE YEARS ENDED MARCH 31, 2012 AND 2011
(Amounts expressed in US Dollars)
| Common Stock | Accumulated | |||||||||||||||||||||||||||||||
| Shares | Par value $0.0001 | Additional Paid-in Capital | Retained Earnings | Treasury Stock | Other Comprehensive Income | Capital & Statutory Reserves | Total | |||||||||||||||||||||||||
| Balance- April 1, 2010 | 42,370,000 | $ | 4,237 | $ | 82,769,074 | $ | 65,441,994 | $ | (2,000 | ) | $ | 4,939,830 | $ | 3,025,794 | $ | 156,178,929 | ||||||||||||||||
| Purchase of treasury stock | (2,739,634 | ) | (2,739,634 | ) | ||||||||||||||||||||||||||||
| Fair value of vested options | 473,852 | 473,852 | ||||||||||||||||||||||||||||||
| Net income | 36,935,477 | 36,935,477 | ||||||||||||||||||||||||||||||
| Foreign currency translation adjustments | 5,495,851 | 5,495,851 | ||||||||||||||||||||||||||||||
| Balance- March 31, 2011 | 42,370,000 | 4,237 | 83,242,926 | 102,377,471 | (2,741,634 | ) | 10,435,681 | 3,025,794 | 196,344,475 | |||||||||||||||||||||||
| Fair value of vested options | 1,484,690 | 1,484,690 | ||||||||||||||||||||||||||||||
| Net income | 2,775,810 | 2,775,810 | ||||||||||||||||||||||||||||||
| Foreign currency translation adjustments | 1,825,637 | 1,825,637 | ||||||||||||||||||||||||||||||
| Balance- March 31, 2012 | 42,370,000 | $ | 4,237 | $ | 84,727,616 | $ | 105,153,281 | $ | (2,741,634 | ) | $ | 12,261,318 | $ | 3,025,794 | $ | 202,430,612 | ||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| 52 |
CHINA-BIOTICS, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Amounts expressed in US Dollars)
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net income | $ | 2,775,810 | $ | 36,935,477 | ||||
| Adjustments to reconcile net income to cash provided by operating activities: | ||||||||
| Gain on extinguishment of derivative liability | - | (14,797,000 | ) | |||||
| Loss on disposal of property, plant and equipment | - | 296,568 | ||||||
| Amortization | 49,664 | 97,925 | ||||||
| Depreciation | 3,672,224 | 4,591,402 | ||||||
| Allowance for doubtful accounts | 1,644,804 | - | ||||||
| Fair value of vested options | 1,484,690 | 473,852 | ||||||
| Change in deferred tax | - | 310,007 | ||||||
| Change in operating assets and liabilities : | ||||||||
| - Accounts receivable | (7,599,639 | ) | (4,317,173 | ) | ||||
| - Inventories | (101,221 | ) | (624,846 | ) | ||||
| - Deposits | (986,287 | ) | (85,275 | ) | ||||
| - Prepayments | 899,146 | (96,187 | ) | |||||
| - Other receivables | 176,581 | 546,345 | ||||||
| - Accounts payable | (2,751,418 | ) | (554,839 | ) | ||||
| - Other payables and accruals | 791,474 | 2,542,559 | ||||||
| - Taxes payable | 1,238,213 | 771,494 | ||||||
| NET CASH PROVIDED BY OPERATING ACTIVITIES | 1,294,041 | 26,090,309 | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||
| Issuance of loan receivable | (19,956,170 | ) | - | |||||
| Deposits for long-term capital expenditures | (28,915,369 | ) | - | |||||
| Prepayments for long-term capital expenditures | (18,025,813 | ) | (3,136,270 | ) | ||||
| Acquisition of land use right | (3,137,586 | ) | (8,365 | ) | ||||
| Purchase of property, plant and equipment | (13,895,520 | ) | (12,078,394 | ) | ||||
| NET CASH USED IN INVESTING ACTIVITIES | (83,930,458 | ) | (15,223,029 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Repayment of convertible note | - | (25,000,000 | ) | |||||
| Purchase of treasury stock | - | (2,739,634 | ) | |||||
| Proceeds from loans | 7,609,308 | - | ||||||
| Cash advance from director | 4,582,722 | 2,739,515 | ||||||
| NET CASH (USED IN) PROVIDED BY FINANCING ACTIVITIES | 12,192,030 | (25,000,119 | ) | |||||
| Effect of exchange rate changes on cash and cash equivalents | (3,457,887 | ) | 2,541,816 | |||||
| NET CHANGES IN CASH AND CASH EQUIVALENTS BALANCES | (73,902,274 | ) | (11,591,023 | ) | ||||
| CASH AND CASH EQUIVALENTS BALANCES, beginning of period | 143,988,348 | 155,579,371 | ||||||
| CASH AND CASH EQUIVALENTS BALANCES, end of period | $ | 70,086,074 | $ | 143,988,348 | ||||
| Supplemental disclosure of cash flow information: | ||||||||
| Interest paid | $ | 74,752 | $ | 5,434,983 | ||||
| Income taxes paid | $ | 2,430,857 | $ | 6,742,742 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| 53 |
CHINA-BIOTICS, INC. AND SUBSIDIARIES
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEARS ENDED MARCH 31, 2012 AND 2011
| 1. | ORGANIZATION AND DESCRIPTION OF BUSINESS |
China-Biotics, Inc. (sometimes referred to as “China-Biotics” or the “Company”) is engaged in the research, development, production, marketing, and distribution of probiotics products (which we sometimes refer to simply as “probiotics”), which are products that contain live microbial food supplements that beneficially affect the host by improving its intestinal microbial balance.
Corporate Organization and History
The Company was incorporated under the name Otish Resources, Inc. in Delaware in February 2003. On March 22, 2006, the Company completed a securities exchange transaction (the “share exchange”) with Sinosmart Group Inc. (“SGI”) and the shareholders of SGI and issued to the SGI shareholders an aggregate of 15,980,000 shares of newly issued common stock in exchange for all of SGI’s ordinary shares issued and outstanding. The share exchange was treated as a recapitalization with SGI treated as the accounting acquirer, and as a result, the Company became engaged in SGI’s business operations of probiotics products.
SGI was incorporated in the British Virgin Islands on February 13, 2004. SGI’s original shareholders were Mr. Song Jinan, Ms. Yan Li, Mr. Huang Weida, and Ms. Yan Yihong (the “Original SGI Shareholders”). Until August 2005, the Original SGI Shareholders owned 99.5% of the outstanding stock of Shanghai Shining Biotechnology Co. Ltd. (“Shining”), with Shanghai Shengyuan Property Co., Ltd. (“Shengyuan”) owning the remaining 0.5% of the Shining equity.
On August 11, 2005, SGI entered into an agreement to acquire 100% of the outstanding Shining shares from the Original SGI Shareholders and Shengyuan in exchange for a total cash consideration of $2.3 million dollars (RMB 18,350,000). On August 19, 2005, the transaction was approved by the Economic and Trade Bureau of the Pudong New District, Shanghai, PRC, and in October 2005, SGI made full payment of $2.3 million dollars to the Original SGI Shareholders and Shengyuan. In December 2005, a revised business license was issued to Shining as a Wholly Owned Foreign Corporation, signifying the formal recognition of SGI as Shining’s sole shareholder by the Chinese government authorities.
Also on August 11, 2005, SGI granted the Original SGI Shareholders the option to purchase an aggregate of 9,000 shares of SGI for $1.00 per share. On October 25, 2005, the Original SGI Shareholders exercised the option and purchased 9,000 shares of SGI (representing 90% of the ownership of SGI) for an aggregate of $9,000. In the share exchange, the Original SGI Shareholders exchanged 9,000 shares of SGI for 10,067,400 shares of the Company’s common stock, which represented 63% of the total of 15,980,000 shares received by all SGI shareholders in this transaction.
On December 9, 2005, SGI incorporated a wholly owned subsidiary, Growing State Limited (“GSL”), in accordance with the laws of the British Virgin Islands. On September 22, 2006, GSL established a wholly foreign-owned enterprise, Growing Bioengineering (Shanghai) Company Limited (“Growing”) in the PRC.
Both Shining and Growing are manufacturers and distributors of probiotics products in the PRC.
On May 4, 2010, the Company incorporated a wholly owned subsidiary, King Treasure Group Limited (“KTG”), in accordance with the laws of the British Virgin Islands. On June 25, 2010, KTG incorporated a wholly owned subsidiary, Best Design Holdings Limited (“BDH”), in Hong Kong and BDH established a wholly foreign-owned enterprise, Growing Bio (Yangling) Company Limited (“Growing Yangling”) in the PRC. Growing Yangling commenced construction a manufacturing plant in Yangling Agricultural High-tech Industries Demonstration Zone in June 2012.
| 2. | SUMMARY OF PRINCIPAL ACCOUNTING POLICIES |
Basis of presentation and consolidation
The consolidated financial statements for China-Biotics, Inc. and its subsidiaries for the years ended March 31, 2012 and 2011 are prepared in accordance with accounting principles generally accepted in the United States of America and include the accounts of SGI, Shining, Growing, GSL, KTG, BDH and Growing Yangling. Intercompany accounts and transactions have been eliminated in consolidation.
| 54 |
Use of Estimates
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Because of the use of estimates inherent in the financial reporting process, actual results could differ from those estimates. Those estimates and assumptions include estimates for allowance for doubtful accounts, inventory valuation, impairment consideration, and assumptions used in the valuation of derivative liabilities.
Revenue Recognition
Revenues of the Company are from the sale of our probiotics products. We recognize revenue from the sale of goods when all of the following criteria are met: (1) persuasive evidence of an arrangement exists; (2) delivery has occurred; (3) the sales price is fixed or determinable; and (4) collectability is reasonably assured. Revenues are presented net of value added tax (VAT”). In our revenue arrangements, physical delivery is the point in time when customer acceptance occurs since title and risk of loss are transferred to the customer.
Cash and cash equivalents
Cash and cash equivalents include all highly liquid investments with an original maturity of three months or less.
Accounts receivable
Accounts receivable are recognized and carried at the original invoiced amount less an allowance for any uncollectible accounts. The Company uses the aging method to estimate the valuation allowance for anticipated uncollectible receivable balances. Under the aging method, bad debts determined by management are based on historical experience as well as the current economic climate and are applied to customers’ balances categorized by the number of months the underlying invoices have remained outstanding. The valuation allowance balance is adjusted to the amount computed as a result of the aging method. When facts subsequently become available to indicate that an adjustment to the bad debt allowance should be made, this is recorded as a change in estimate in the current year. As of March 31, 2012, allowance for doubtful accounts was $1,665,188. As of March 31, 2011, no allowance for doubtful accounts was provided.
Inventories
Inventories are stated at the lower of cost or market. Cost, which is calculated using the weighted average method, comprises all costs of purchases, costs of conversion, and other costs incurred in bringing the inventories to their present location and condition. Market value is determined by reference to the sales proceeds of items sold in the ordinary course of business after the balance sheet date.
Property, plant and equipment
Property, plant and equipment, and land use rights are recorded at cost and are stated net of accumulated depreciation. Gains or losses on disposals are reflected as gain or loss in the year of disposal. The cost of improvements that extend the life of plant and equipment are capitalized. These capitalized costs may include structural improvements, equipment, and fixtures. All ordinary repair and maintenance costs are expensed as incurred.
Plant and equipment are depreciated at rates sufficient to write off their cost over their estimated useful lives on a straight-line basis. Leasehold improvements are depreciated over the lease term of the related leased properties. Depreciation relating to property, plant, and equipment used in production is used in our determination of gross profit. The estimated useful lives of the assets are as follows:
| Land use right | Terms defined in related legal documents | |
| Building | 20 years | |
| Plant and machinery | 10 years | |
| Office equipment | 5 years | |
| Motor vehicles | 5 years | |
| Leasehold improvements | The shorter of 5 years or lease term of related leased properties |
| 55 |
Construction in progress includes project costs paid to third parties that are clearly associated with the acquisition, development, and construction of an asset and are capitalized as a cost of that project prior to the use of the assets. Such costs include the costs of construction, equipment, interest and direct labor costs. These capitalized project costs are not subject to depreciation until the assets to which they are related are placed into service.
Land use rights
According to the law of China, the government owns all the land in China. Companies or individuals are authorized to possess and use the land only through land use rights granted by the Chinese government. Land use rights are amortized using the straight-line method over the related lease terms.
Impairment of Long-Lived Assets
The Company's policy is to record an impairment loss against the balance of a long-lived asset in the period when it is determined that the carrying amount of the asset may not be recoverable. This determination is based on an evaluation of such factors as the occurrence of a significant event, a significant change in the environment in which the business assets operate or if the expected future non-discounted cash flows of the business is determined to be less than the carrying value of the assets. If impairment is deemed to exist, the assets will be written down to fair value. Management also evaluates events and circumstances to determine whether revised estimates of useful lives are warranted. Based upon management’s assessment, there were no indicators of impairment of the Company’s long lived assets as of March 31, 2012 or 2011.
Foreign Currency Translations and Transactions
The accompanying consolidated financial statements are presented in United States dollars. The functional currency of the Company is the Renminbi (“RMB”). Capital accounts of the consolidated financial statements are translated into United States dollars from RMB at their historical exchange rates when the capital transactions occurred. Assets and liabilities are translated at the exchange rate as of the balance sheet date. Income and expenditures are translated at the average exchange rate for the period presented.
The RMB is the functional currency of Shining, Growing, and Yangling Growing (the “Operating Subsidiaries”) as it is the currency of the People’s Republic of China, which is the primary economic environment the Operating Subsidiaries operate in and the environment in which the Company primarily generates and expends cash. RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into US dollars at the rates used in translation.
Comprehensive Income
Under authoritative guidance of the FASB on reporting comprehensive income, disclosure of all components of comprehensive income and loss on an annual and interim basis is required. Comprehensive income is defined to include all changes in equity which for the Company principally compose foreign currency translations.
Fair value of financial instruments
Fair value measurements are determined using authoritative guidance issued by the FASB, with the exception of the application of the guidance to non-recurring, non-financial assets and liabilities as permitted. Fair value is defined in the authoritative guidance as the price that would be received to sell an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants at the measurement date. A fair value hierarchy was established, which prioritizes the inputs used in measuring fair value into three broad levels as follows:
Level 1-Quoted prices in active markets for identical assets or liabilities.
Level 2-Inputs, other than the quoted prices in active markets, are observable either directly or indirectly.
Level 3-Unobservable inputs based on the Company’s assumptions
The Company is required to use observable market data if available without undue cost and effort. The following table presents certain investments and liabilities of the Company’s financial assets measured and recorded at fair value on the Company’s consolidated balance sheets on a recurring basis and their level within the fair value hierarchy as of March 31, 2012 and 2011:
| 56 |
| Fair Value Measurements as at March 31, 2012 | ||||||||||||||||
| Balance at March 31, 2012 | Quoted Prices in Active Markets (Level 1) | Significant Other Observable Inputs (Level 2) | Significant Unobservable Inputs (Level 3) | |||||||||||||
| Loan receivable | $ | 20,753,343 | $ | - | $ | 20,753,343 | $ | - | ||||||||
The changes in level 3 liabilities measured at fair value on a recurring basis are summarized as follows:
| Embedded derivatives – conversion right | ||||
| Balance at April 1, 2010 | $ | 14,797,000 | ||
| Gain on extinguishment of derivative liability related to of convertible note payable | (14,797,000 | ) | ||
| Balance at March 31, 2011 and 2012 | $ | - | ||
Derivative financial instruments
On December 11, 2007, the Company issued a 4% Senior Convertible Promissory Note in an amount of $25,000,000 (the “Note”), which was due on December 11, 2010. Pursuant to ASC Topic 815, “Derivatives and Hedging” (Formerly, SFAS No. 133 “Accounting For Derivatives Instruments And Hedging Activities” and EITF Issue No. 00-19 “Accounting For Derivatives Financial Instruments Indexed To And Potentially Settled In A Company’s Own Stock”), the Company bifurcated the conversion options with a mandatory conversion feature (“embedded derivatives”) from the Note as the embedded derivatives were determined to be not clearly and closely related to the host contract. The embedded derivatives were recorded at fair value, mark-to-market at each reporting period, and were reflected on a separate line in the balance sheet. On December 9, 2010, the Note was repaid and the derivative liability was extinguished resulting in a gain of $14,797,000 during the year ended March 31, 2011.
Stock based compensation
The Company periodically issues stock options and warrants to employees and non-employees in non-capital raising transactions for services and for financing costs. The Company accounts for stock option and warrant grants issued and vesting to employees based on the authoritative guidance provided by the Financial Accounting Standards Board, whereas the value of the award is measured on the date of grant and recognized over the vesting period. The Company accounts for stock option and warrant grants issued and vesting to non-employees in accordance with the authoritative guidance of the Financial Accounting Standards Board, whereas the value of the stock compensation is based upon the measurement date as determined at either a) the date at which a performance commitment is reached, or b) at the date at which the necessary performance to earn the equity instruments is complete. Non-employee stock-based compensation charges generally are amortized over the vesting period on a straight-line basis. In certain circumstances where there are no future performance requirements by the non-employee, option grants are immediately vested and the total stock-based compensation charge is recorded in the period of the measurement date.
The fair value of the Company’s common stock option grant is estimated using the Black-Scholes-Merton option pricing model, which uses certain assumptions related to risk-free interest rates, expected volatility, expected life of the common stock options, and future dividends. Compensation expense is recorded based upon vesting schedule of these options using the value derived from the Black-Scholes-Merton option pricing model, and based on actual experience. The assumptions used in the Black-Scholes-Merton option pricing model could materially affect compensation expense recorded in future periods.
Treasury Stock
The Company treats common stock repurchased, but not yet canceled, as treasury stock. Treasury stock is accounted for by the cost method, with par value charged to common stock, and any excess charged against additional paid-in capital or retained earnings.
Advertising costs
All advertising costs incurred in the promotion of the Company’s products are expensed as incurred. Advertising costs, which are included in selling expenses in the accompanying consolidated statements of operations and other comprehensive income, amounted to $582,028 and $2,973,683 for the years ended March 31, 2012 and 2011, respectively.
| 57 |
Income Taxes
The Company uses an asset and liability approach for financial accounting and reporting for income taxes that allows recognition and measurement of deferred tax assets based upon the likelihood of realization of tax benefits in future years. Under the asset and liability approach, deferred taxes are provided for the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes. A valuation allowance is provided for deferred tax assets if it is more likely than not these items will either expire before the Company is able to realize their benefits, or that future deductibility is uncertain. The Company’s policy is to recognize interest and/or penalties related to income tax matters in income tax expense.
Research and Development Costs
Research and development costs are expensed as incurred. For the years ended March 31, 2012 and 2011, there were $5,084,949 and $6,730,514 of research and development expenses recorded and are included in general and administrative expenses in the accompanying consolidated statements of income and other comprehensive income.
Earnings Per Share
Basic earnings per share is computed in accordance with ASC Topic 260 “Earnings Per Share” (Formerly, SFAS No.128, “Earnings Per Share”), by dividing the net income by the weighted average number of shares of outstanding common stock during the period. The diluted earnings per share calculation includes the impact of dilutive convertible securities, if applicable. The weighted average number of shares of outstanding common stock is determined by relating the portion of time within a reporting period that a particular number of shares of common stock has been outstanding to the total time in that period (see Note 3).
Segment Reporting
ASC Topic 280 “Segment Reporting” (Formerly, SFAS No. 131, “Disclosure about Segments of an Enterprise and Related Information”), requires use of the “management approach” model for segment reporting. The management approach model is based on the way a company’s management organizes segments within the Company for making operating decisions and assessing performance. Reportable segments are based on one of the followings: (a) products and services, (b) geographical areas, (c) legal structure, (d) management structure, or (e) any other manner in which management disaggregates a company. The Company’s management has adopted the “products and services” approach for segment reporting.
The Company operates its business on the basis of two reportable segments — retail products and bulk additive products.
Recent Accounting Pronouncements
In May 2011, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update (“ASU”) No. 2011-04, “Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs”. ASU No. 2011-4 does not require additional fair value measurements and is not intended to establish valuation standards or affect valuation practices outside of financial reporting. The ASU is effective for interim and annual periods beginning after December 15, 2011. The Company adopted ASU No. 2011-04 effective January 1, 2012 and it did not affect the Company’s results of operations, financial condition or liquidity.
In June 2011, the FASB issued ASU No. 2011-05, “Presentation of Comprehensive Income”. The ASU eliminates the option to present the components of other comprehensive income as part of the statement of changes in shareholders’ equity, and instead requires consecutive presentation of the statement of net income and other comprehensive income either in a continuous statement of comprehensive income or in two separate but consecutive statements. ASU No. 2011-5 is effective for interim and annual periods beginning after December 15, 2011. The Company adopted ASU 2011-05 effective January 1, 2012 and it did not affect the Company’s results of operations, financial condition or liquidity.
In September 2011, the FASB issued ASU 2011-08, “Testing Goodwill for Impairment”, an update to existing guidance on the assessment of goodwill impairment. This update simplifies the assessment of goodwill for impairment by allowing companies to consider qualitative factors to determine whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount before performing the two step impairment review process. It also amends the examples of events or circumstances that would be considered in a goodwill impairment evaluation. The amendments are effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. The Company adopted ASU 2011-08 effective January 1, 2012. We do not believe that the adoption of this new accounting guidance will have a significant effect on our goodwill impairment assessments in the future.
| 58 |
In December 2011, the FASB issued ASU No. 2011-11, “Balance Sheet (Topic 210): Disclosures about Offsetting Assets and Liabilities.” This ASU requires an entity to disclose information about offsetting and related arrangements to enable users of its financial statements to understand the effect of those arrangements on its financial position. ASU No. 2011-11 will be applied retrospectively and is effective for annual and interim reporting periods beginning on or after January 1, 2013. The Company does not expect adoption of this standard to have a material impact on its consolidated results of operations, financial condition, or liquidity.
Other recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force), the AICPA, and the Securities and Exchange Commission (the “SEC”) did not or are not believed by management to have a material impact on the Company's present or future consolidated financial statements.
| 3. | EARNINGS PER SHARE |
The following table sets forth the computation of basic and diluted earnings per share:
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Earnings per share – Basic | ||||||||
| Income for the year | $ | 2,775,810 | $ | 36,935,477 | ||||
| Basic average common stock outstanding | 22,150,200 | 22,241,076 | ||||||
| Net earnings per share | $ | 0.13 | $ | 1.66 | ||||
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Earnings per share – Diluted | ||||||||
| Income for the year | $ | 2,775,810 | $ | 36,935,477 | ||||
| Gain on extinguishment of derivative liability | - | (14,797,000 | ) | |||||
| $ | 2,775,810 | $ | 22,138,477 | |||||
| Basic average common stock outstanding | 22,150,200 | 22,241,076 | ||||||
| Diluted effect from derivative liability | - | 1,444,064 | ||||||
| Diluted effect from vested stock options | 116,793 | 3,864 | ||||||
| Diluted average common stock outstanding | 22,266,993 | 23,689,004 | ||||||
| Net earnings per share | $ | 0.12 | $ | 0.93 | ||||
Basic earnings per share is computed by dividing the net income by the weighted average number of outstanding common stock during the period. The diluted earnings per share calculation includes the impact of dilutive convertible securities, if applicable. The weighted average number of outstanding common stock is determined by relating the portion of time within a reporting period that a particular number of shares of common stock has been outstanding to the total time in that period.
| 4. | RISKS, UNCERTAINTIES, AND CONCENTRATIONS |
Economic and political risks
The Company’s operations are conducted in the PRC and are subject to various political, economic, and other risks and uncertainties inherent in this country. Among other risks, the Company’s operations are subject to the risks of restrictions on transfer of funds; export duties, quotas and embargoes; domestic and international customs and tariffs; changing taxation policies; foreign exchange restrictions; and political conditions and governmental regulations.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist principally of cash and accounts receivable. As of March 31, 2012 and 2011, the Company had cash deposits of $70.09 million and $143.99 million placed with several banks in the PRC, which includes the Special Administrative Region of Hong Kong, where there is currently no rule or regulation in place for obligatory insurance of bank accounts. For the years ended March 31, 2012 and 2011, all of the Company’s sales arose in the PRC. In addition, all accounts receivable as at March 31, 2012 and 2011 are from customers in the PRC.
| 59 |
Concentration of Customers
The Company had no sales to a single customer that accounted for more than 10% of total gross sales. For the year ended March 31, 2012, there is no customer that accounted over 5% of our sales revenue. For the year ended March 31, 2011, there is one customer that accounted for 5.2% of our sales revenue. As of March 31, 2012, there is one customer that accounted for 4.8% of our accounts receivable. As of March 31, 2011, there is one customer that accounted for 2.9% of our accounts receivable.
| 5. | ACCOUNTS RECEIVABLE |
Accounts receivable consisted of the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Accounts receivable | $ | 35,057,662 | $ | 26,194,313 | ||||
| Less: Allowance for doubtful accounts | (1,665,188 | ) | - | |||||
| $ | 33,392,474 | $ | 26,194,313 | |||||
| 6. | INVENTORIES |
Inventories consisted of the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Raw materials | $ | 801,288 | $ | 744,667 | ||||
| Work-in-progress | 99,763 | 94,756 | ||||||
| Finished goods | 1,041,498 | 932,898 | ||||||
| $ | 1,942,549 | $ | 1,772,321 | |||||
| 7. | LOAN RECEIVABLE |
On December 19, 2011, the Company entered into an agreement with Jiangxi International Trust Co., Ltd. (“Jiangxi”) to loan $20,753,343 (RMB 131,100,000) to the Jiangxi International Trust of Yinhe #7 Property, an investment fund sponsored by Jiangxi. The loan principal is due on December 26, 2013, is unsecured, and according to the loan agreement, the Company is to earn 20% interest per annum, less a 5% investment management fee per annum. Interest income for the year ended March 31, 2012 includes $1,078,320 of interest accrued on the outstanding balance. Jiangxi is a major trust and investment company that is executing different investment projects in the PRC, including real estate projects, listed company restructuring, and other financial services.
| 8. | DEPOSITS |
Deposits for the acquisition of long-term assets and operating trading deposits consisted of the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Long term | ||||||||
| Deposit for acquisition of a company | $ | 23,739,812 | $ | - | ||||
| Deposit for acquisition of patent | 6,330,616 | - | ||||||
| 30,070,428 | - | |||||||
| Short term | - | |||||||
| Other operating deposits | 1,114,367 | 85,546 | ||||||
| $ | 31,184,795 | $ | 85,546 | |||||
| 60 |
On March 27, 2012, the Company made a deposit of $23,739,811(RMB 150,000,000) to the government of Yangling for a bid to acquire a probiotics company located in Yangling, Shaanxi Province that is in receivership. According to the relevant regulations, the deposit will be treated as part of the purchase price of the acquisition if the transaction is approved. As of September 13, 2012, the government of Yangling is reviewing bids received for the probiotics company and expects to complete this process by the end of calendar 2012. If the Company does not win the auction to acquire the probiotics company, its deposit will be returned.
On February 1, 2012, our subsidiary, Growing, entered into an agreement to acquire a patent regarding a production technology for $6,647,147 (RMB 42,000,000) from an unrelated PRC company. Growing made a deposit of $6,330,616 (RMB 40,000,000) to the seller for the transfer of the patent within 210 days, or by August 30, 2012. The original terms of the agreement were that if the patent cannot be transferred to Growing within 210 days, Growing will be refunded the deposit paid and also be compensated an additional $159,000 (RMB 1,000,000) for damages. The transfer was not completed by August 30, 2012, and on September 3, 2012, Growing and the seller mutually agreed to extend the completion date to December 30, 2012. As of September 13, 2012, the transfer had not yet been completed because the Company is waiting for approval of the transfer from the State Intellectual Property Office of the PRC. The Company has no information to allow it to estimate when such confirmation will be obtained, but expects the transaction to close in 2013, after which the patent is planned to be used in the Company’s operations.
| 9. | LAND USE RIGHTS |
The land use rights consisted of the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Land use rights | $ | 5,231,955 | $ | 1,900,734 | ||||
| Less: Accumulated amortization | (175,072 | ) | (120,380 | ) | ||||
| $ | 5,056,883 | $ | 1,780,354 | |||||
On March 21, 2006, GSL, our subsidiary, entered into an agreement, as amended, with Shanghai Qingpu Industrial Park District Development (Group) Company Limited for the lease of 36,075 square meters of land in the Shanghai Qingpu Industrial Park District on which we constructed our 300-metric ton capacity production plant for a term of 50 years beginning January 15, 2008. The agreement provides for the payment of leasing fees of approximately $1.8 million. In February 2009, the formal land use right certificate was issued. There are no future lease payments under this land lease. The Qingpu land use right was pledged to a bank as a credit guarantee for a loan of $4,747,963 (RMB 30,000,000 million, see Note 12). At March 31, 2012, and 2011, the net book value of such land use right was $1,806,203 and $1,780,354, respectively.
In 2012, our Growing Yangling, subsidiary of the Company that operates in Yangling, the PRC, has acquired a land use right for a period of 50 years, beginning January 18, 2012. At March 31, 2012, the net book value of the land use right was $3,250,680.
Amortization expense amounted to $49,664 and $97,925 for the years ended March 31, 2012, and 2011, respectively.
The estimated amortization expense for the land use rights over each of the five years and thereafter is summarized as below:
| March 31, 2012 | ||||
| For the year ended March 31: | ||||
| 2013 | $ | 104,639 | ||
| 2014 | 104,639 | |||
| 2015 | 104,639 | |||
| 2016 | 104,639 | |||
| 2017 | 104,639 | |||
| Thereafter | 4,533,688 | |||
| $ | 5,056,883 | |||
| 61 |
| 10. | PROPERTY, PLANT AND EQUIPMENT |
Property, plant and equipment, net, consisted of the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Building | $ | 44,754,344 | $ | 12,047,068 | ||||
| Plant and machinery | 23,963,145 | 51,430,868 | ||||||
| Leasehold improvements | 1,814,783 | 1,750,615 | ||||||
| Office equipment | 4,220,655 | 4,007,571 | ||||||
| Motor vehicles | 543,569 | 445,632 | ||||||
| Construction in progress | 13,217,574 | 1,762,970 | ||||||
| 88,514,070 | 71,444,724 | |||||||
| Less: Accumulated depreciation | (16,433,738 | ) | (12,302,168 | ) | ||||
| $ | 72,080,332 | $ | 59,142,556 | |||||
For the years ended March 31, 2012 and 2011, depreciation expense amounted to $3,672,224 and $4,591,402, respectively.
At March 31, 2012, construction in progress consisted primarily of Phase 2 construction of a research and development center and other ancillary facilities in Qingpu. At March 31, 2011, construction in progress consisted of Phase 1 construction of a research and development center and other ancillary facilities in Qingpu, and an office building, which is used as our corporate headquarters.
At March 31, 2012, the corporate headquarters office building was pledged to banks as a guarantee for a total of loans of $3,165,308 (RMB 20,000,000, see Note 12). The net book value of the building was $8,919,818.
| 11. | AMOUNT DUE TO DIRECTOR |
As of March 31, 2012 and 2011, the amount due to a director, Mr. Song Jinan, represented advances to the Company which are unsecured, interest-free and repayable on demand. The advances from such director were entered into because of the difficulty the Company faced in paying expenses incurred outside China in non-PRC currency due to the difficulties the Company had in converting currency because of foreign exchange controls.
| 12. | LOANS PAYABLE |
Short term loan
On March 19, 2012, the Company received a loan from the Bank of China for $3,165,308 (RMB 20,000,000), secured by the corporate headquarters office building of Growing (see Note10), due September 19, 2012. Interest is 7.2% per annum, based on 118% of the six months’ of RMB borrowing prime rate established by the People’s Bank of China at the time of funding. Total principal and interest are due September 19, 2012. The loan is renewable with the bank upon maturity.
Long term loan
On December 7, 2011, the Company received a loan from Shanghai Rural Commercial Bank for $4,747,963 (RMB 30,000,000), secured by the land use right in Qingpu of Growing (see Note 9), due May 27, 2014. Interest is 7.65% per annum, based on 115% of the 1-to-3 years’ borrowing of RMB prime rate set by the People’s Bank of China at the time of funding, and is due at the end of each calendar quarter. On May 25, 2012, the Company repaid $791,327 (RMB 5,000,000) of the loan.
| 13. | CONVERTIBLE NOTES PAYABLE |
On December 11, 2007, the Company issued a senior convertible promissory note for $25,000,000, due December 11, 2010 in a private placement. Mr. Song Jinan, the Company’s Chief Executive Officer and largest shareholder, agreed to guarantee the Company’s obligations under the Note with a pledge of 4,000,000 shares of China-Biotics common stock owned by Mr. Song. The note was convertible at an exercise price of $12 per share into shares of the Company’s common stock at any time until maturity. If the Note was not converted before maturity, the Company agreed to redeem the Note with a total yield of 10% per annum. The Note also included a mandatory conversion into the Company’s common stock if the Company achieved net income of $60 million in fiscal year 2010. For the year ended March 31, 2010, the Company had net income of $15.6 million. Since the Company did not attain net income of $60 million, the Note was not converted. On December 9, 2010, the Note was paid off in full and the guarantee by Mr. Song was released. The Company had been paying interest at 4% on a quarterly basis, and had accrued an additional 6% for a total of 10% since the Note was not converted at maturity. For the years ended March 31, 2012 and 2011, interest expense was $0 and $4,684,932, respectively.
| 62 |
The Company accounted for the net proceeds from the issuance of the Note as two separate components: an embedded conversion feature and a debt component. The Company determined that the fair value of the conversion feature of the notes resulted in a derivative liability of $9,118,000 being recorded when the notes were issued. The $9,118,000 amount was allocated to debt discount and amortized over the term of the note using the effective interest method. The embedded derivatives were recorded at fair value, mark-to-market at each reporting period, and were reflected on a separate line in the balance sheet. On December 9, 2010, the Note was repaid and the derivative liability was extinguished resulting in a gain of $14,797,000 during the year ended March 31, 2011.
| 14. | INCOME TAXES PAYABLE |
For the years ended March 31, 2012 and 2011, the components of earnings before income taxes were:
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Income before income taxes generated in the PRC | $ | 10,573,208 | $ | 43,374,799 | ||||
| (Loss) income before income taxes generated in the United States of America | (4,564,027 | ) | 2,972,262 | |||||
| Loss before income taxes generated in the British Virgin Islands | (399,402 | ) | (394,616 | ) | ||||
| Loss before income taxes generated in Hong Kong | (801 | ) | (34,629 | ) | ||||
| $ | 5,608,978 | $ | 45,917,816 | |||||
For the years ended March 31, 2012 and March 31, 2011, there was no tax provision related to income (loss) generated in the United States of America, the British Virgin Islands, and Hong Kong. For the years ended March 31, 2012 and March 31, 2011, the provision for income taxes relating to income generated in the PRC consists of the following:
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Current-PRC | $ | 3,140,086 | $ | 8,675,431 | ||||
| Deferred-PRC | (306,918 | ) | 306,918 | |||||
| $ | 2,833,168 | $ | 8,982,349 | |||||
For the years ended March 31, 2012 and 2011, the reconciliation between the PRC statutory tax rate and effective tax rate are as follows:
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Statutory rate | 25.0 | % | 25.0 | % | ||||
| Preferential tax rate | - | % | (5.0 | )% | ||||
| Effect of different tax rates in other jurisdictions | 5.5 | % | (1.0 | )% | ||||
| Expenses not deductible for tax purpose | 6.5 | % | - | % | ||||
| Effect of taxable temporary difference | 8.5 | % | (5.1 | )% | ||||
| Interest on accrued taxes | - | % | 5.0 | % | ||||
| Tax losses not recognized | 5.0 | % | (0.4 | )% | ||||
| Deferred tax | - | % | 1.1 | % | ||||
| Effective tax rate | 50.5 | % | 19.6 | % | ||||
As of March 31, 2012 and 2011, the Company had incurred tax losses of approximately $4.2 million and $2.2 million, respectively which can be carried forward in various jurisdictions from five to 25 years.
| Year ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Deferred tax assets | ||||||||
| Net operating loss carryforwards | ||||||||
| - PRC | $ | 320,292 | $ | 63,970 | ||||
| - United States of America | 334,633 | - | ||||||
| - Hong Kong | 132 | 5,714 | ||||||
| Less: Valuation allowance | (655,057 | ) | (69,684 | ) | ||||
| Net deferred tax assets | $ | - | $ | - | ||||
The Company is incorporated in Delaware and is subject to U.S. tax law. The daily operations of the Company in the United States principally relate to payment of legal and professional fees. The income generated from the United States in 2011 primarily related to the change in the fair value of derivative liability, which is a permanent difference between book and tax reporting. The Company has no taxable income in the United States and does not expect it will be able to realize the U.S. tax losses.
There is no income tax for companies not carrying out business activities in the British Virgin Islands or Hong Kong. Accordingly, the Company's financial statements do not present any income tax provisions related to the British Virgin Islands or Hong Kong tax jurisdictions.
The Company has its principal operations in the PRC and is subject to a PRC Enterprise Income Tax (“EIT”) rate of 25% in calendar years 2012 and 2011, subject to certain rate reductions. The Company’s subsidiary Shining is located in the Shanghai Jinqiao special economic zone and was awarded the status of “high technology” enterprise for the calendar year 2007 until 2011. Therefore, Shining enjoyed a preferential income tax rate of 15% in 2007 to 2011. Beginning January 1, 2012, Shining’s EIT rate is 25%.
The Company’s subsidiary Growing is located in Qingpu and has similar business operations as Shining, but with a larger production scale. Growing was exempted from PRC Enterprise Income Tax in calendar 2008 and 2009, followed by 50% tax exemption for calendar 2010 to 2012.
The Company’s subsidiary, Growing Yangling, is located in Yangling and as of March 31, 2012, its manufacturing plant was under construction. According to the investment agreement with the local government, Growing Yangling is entitled to have a tax incentive that exempts the local portion of EIT for three calendar years from the commencement of production, followed by 50% tax exemption of local portion for the next year. There is no financial effect from the tax holiday as Growing Yangling did not generate any assessable profit in fiscal years 2012 or 2011.
| 63 |
At March 31, 2012 and 2012, taxes payable consisted of the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Taxes arising prior to 2005: | ||||||||
| Value added tax and other taxes | $ | 5,707,746 | $ | 5,505,930 | ||||
| Income taxes | 3,112,441 | 3,002,391 | ||||||
| Dividends withholding tax | 3,914,453 | 4,095,745 | ||||||
| Total taxes prior to 2005 | 12,734,640 | 12,604,066 | ||||||
| Value added tax and other taxes | 1,366,106 | 515,637 | ||||||
| Income taxes | 2,100,059 | 1,317,961 | ||||||
| Accrued interest on taxes | 17,163,806 | 16,505,088 | ||||||
| $ | 33,364,611 | $ | 30,942,752 | |||||
In addition to the EIT, companies in the PRC that are engaged in the sale of goods are generally required to pay value added taxes (“VAT”) at a rate of 17% of the gross sales proceeds received, less any deductible VAT already paid or borne by the taxpayer.
Our management believes that our operations in China were exempted from EIT and VAT for all years prior to 2005 because we had been recognized by the local government as an advanced technology enterprise. However, the Company never received a written confirmation from the appropriate tax authorities for the tax exemption status of our operations in China prior to 2005. As a result, there is no way to ascertain the ultimate position which may be taken by the relevant PRC tax authorities in the future and accordingly, full provisions for tax liabilities in the amount of $12,734,640, for all years prior to 2005 have been recorded by the Company. Beginning in January 2006, we made tax payments to the PRC tax authorities for 2005 and we have made regular tax payments to the PRC tax authorities for all subsequent periods.
In addition, in connection with dividends paid to the Shining shareholders between April 2003 to June 2005, Shining did not deduct a withholding tax at the rate of 20% as required by applicable Chinese laws and regulations. The Company has accrued the dividend withholding tax and interest on the dividend withholding tax.
According to PRC tax regulations, the overdue tax liabilities in the PRC for the calendar years prior to 2005 may be subject to interest at the 0.05% per day, and potential penalties for the late payment of taxes which is calculated on the basis of 0.5 times to five times the amount of taxes payable. Through March 31, 2011, the Company accrued the interest that may be potentially payable upon these taxes incurred prior to 2005 and the unpaid dividend withholding tax. Interest related to the unpaid taxes was recognized in the Company’s consolidated statements of operations as a component of its income tax provision.
For the year ended March 31, 2012, management made an assessment of whether it was necessary to provide a further provision for interest and penalties on these unpaid amounts. Management determined that no further provision for interest and penalties was necessary given the unlikelihood of payment of the amount already accrued and provided in the financial statements. This assessment was based upon receipt by the company of a report by an independent tax expert that the amount of taxes prior to 2005 may not be payable, the length of time the amount has been outstanding, and that there have been no requests from PRC tax authorities for payment of the unpaid taxes outstanding amounts. As such, management believes that the previously recorded amounts are sufficient to cover any settlement of this liability.
The Company’s PRC subsidiaries are deemed “high technology” enterprises subject to preferred tax rates (tax holiday). The table below shows the effect of using the higher rates and earnings per share.
| Year ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Income per common share-basic | $ | 0.13 | $ | 1.66 | ||||
| Effect of tax holiday | 0.01 | 0.11 | ||||||
| Pro forma income per common share-basic | $ | 0.14 | $ | 1.77 | ||||
| 15. | TREASURY STOCK |
On March 22, 2006, the Company repurchased 24,381,004 shares of common stock of the Company with a total cost of $2,438 under the approval of the Board of Directors. Before year ended March 31, 2010, 4,381,004 shares of common stock of the Company were cancelled and the balance of 20,000,000 shares of common stock of the Company with a total cost of $2,000 remained in treasury. The Company recorded the entire purchase price of the remaining treasury stock as a reduction of equity.
During the year ended March 31, 2011, the Company repurchased 219,800 shares of its common stock for an aggregate cost of $2,739,634. The Company recorded the entire purchase price of the treasury stock as a reduction of equity.
| 16. | CAPITAL AND STATUTORY RESERVES |
The Company’s PRC subsidiary, Shining, is required to make appropriations to reserve funds, comprising the statutory surplus reserve, statutory public welfare fund and discretionary surplus reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (the “PRC GAAP”). Appropriation to the statutory surplus reserve should be at least 10% of the after tax net income determined in accordance with the PRC GAAP until the reserve is equal to 50% of the subsidiary’s registered capital. Appropriations to the statutory public welfare fund are at 5% to 10% of the after tax net income determined in accordance with the PRC GAAP. Appropriations to the discretionary surplus reserve are made at the discretion of the Board of Directors. The statutory public welfare fund is established for the purpose of providing employee facilities and other collective benefits to the employees and is non-distributable other than in liquidation. The statutory surplus reserve and discretionary surplus reserve can be used to make good losses or to increase the capital of the relevant company.
| 17. | OPTION INCENTIVE PLAN |
On January 16, 2011, the Board adopted the 2010 Equity Incentive Plan (the “2010 Plan”) and reserved 1,500,000 shares of common stock for issuance under the 2010 Plan. On March 9, 2011, the 2010 Plan was approved by the Company’s stockholders at the 2010 Annual Meeting of Stockholders.
| 64 |
On January 16, 2011, the Company granted to the directors and officers an option to purchase 910,000 shares of common stock under the 2010 Plan. The options have exercise price of $14.81 per share, an expiration date of five to ten years from the date of grant, and vest over 48 consecutive months: 20% in the first 12-month period; 20% in the second 12-month period; 30% in the third 12-month period; and 30% in the forth 12-month period. The Company determined the fair value of the options on the date of grant was $9,477,024 using the Black-Scholes-Merton option pricing model with the following assumptions: expected volatility, 76%; risk-free interest rate, 1.95%; expected weighted average life, five to ten years; and expected dividend yield, 0%. As of March 31, 2012 and 2011, the total vested options were 165,000 shares and 45,500 shares, respectively.
During June 2011, two candidates resigned from their position and terminated their services to the Company. They also did not exercise their vesting options within three months from the date of their termination. According to the option agreement, their options were forfeited and cancelled; the total number of forfeited and cancelled options was 250,000.
During the years ended March 31, 2012 and 2011, no vested options were exercised.
At March 31, 2012 and 2011, outstanding options were as follows:
| Number of Shares under Options | Weighted Average Exercise Price | |||||||
| Options outstanding at April 1, 2010 | - | $ | - | |||||
| Options granted | 910,000 | 14.81 | ||||||
| Options expired or forfeited | - | - | ||||||
| Options exercised | - | - | ||||||
| Options outstanding at March 31, 2011 | 910,000 | 14.81 | ||||||
| Options granted | - | - | ||||||
| Options expired or forfeited | (250,000 | ) | 14.81 | |||||
| Options exercised | - | - | ||||||
| Options outstanding at March 31, 2012 | 660,000 | $ | 14.81 | |||||
The following table summarizes information about options outstanding at March 31, 2012:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||
| Exercise price | Number of shares under Option | Weighted average remaining contractual life (years) | Weighted Average Exercise Price | Number of shares under Option | Weighted Average Exercise Price | |||||||||||||||||
| $ | 14.81 | 660,000 | 7.15 | $ | 14.81 | 165,000 | $ | 14.81 | ||||||||||||||
At March 31, 2012 and 2011, the options outstanding and exercisable had no intrinsic value.
For the years ended March 31, 2012 and 2011, the Company has recorded $1,484,690 and $473,852, respectively, as the fair value of the vested options.
As of March 31, 2012, total compensation cost related to non-vested stock options and restricted stock not yet recognized was approximately $5,054,193, which is expected to be recognized over the next 33 months.
| 18. | PRODUCT RETURN CONCESSIONS |
For the year ended March 31, 2012, product returns of $474,505 were deducted from sales due to a batch of probiotics products produced in June 2011 that were below the Company’s quality standards due to production problems. For the year ended March 31, 2011, product returns of $2,590,841 were deducted from sales due to the overestimation of market demand by the Company’s distributors for the first calendar quarter of 2011. We agreed to accept the return of probiotics products from certain distributors during May 2011, in part to avoid the Company suffering a negative image in the probiotics market. All such returned goods were held in the Company’s warehouse until they were fully destroyed by September 2011.
As a result of a product returns after certain of our products did not meet our quality standards, and in order to improve the confidence of the Company’s distributors and end-user customers in our probiotics products, we provided special concession credits to our distributors during August 2011 to address the complaints and concerns of customers worried about food and healthcare products in the PRC. Such credits were made available for our distributors to reimburse end-user customers for products which were recalled. We treated these special credits as being comparable to a warranty on our probiotics products, and we incurred a cost of $7,162,229 for these credits for the year ended March 31, 2012 that has been reflected as an additional cost of product returns in the accompanying consolidated financial statements.
| 65 |
| 19. | RETIREMENT COSTS |
The Company’s employees are required to participate in a central pension scheme operated by the local municipal government and the Company is required to contribute a certain percentage of their payroll costs to the central pension. In accordance with the rules of the central pension, contributions are recorded when due and included in general and administrative expenses in the accompanying consolidated statements of operations and comprehensive income. Forfeited contributions are available to reduce contributions payable in future years. For the years ended March 31 2012 and 2011, the Company’s retirement costs are summarized as follows:
| Years ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Contributions to central pension operated by local municipal government | $ | 1,790,857 | $ | 2,210,103 | ||||
| 20. | COMMITMENTS AND CONTINGENCIES |
Operating Leases
As of March 31, 2012, future minimum lease payments under non-cancellable operating leases for office, warehouse and factory were as follows:
| 2012 | ||||
| For the year ended March 31: | ||||
| 2013 | $ | 622,396 | ||
| 2014 | 94,832 | |||
| Thereafter | - | |||
| $ | 717,228 | |||
Rental expense, which was charged to expense, amounted to $275,424 and $1,689,680 for the years ended March 31, 2012 and 2011, respectively.
Capital commitments
On August 12, 2010, BDH, a subsidiary of the Company, entered into the agreements with a government agency to establish manufacturing facilities for animal probiotics products in the Yangling Agricultural High-tech Industries Demonstration Zone in Shaanxi Province of China. In furtherance of such agreements, BDH incorporated a foreign, wholly owned subsidiary, Growing Yangling, with a registered capital of $50 million. As of March 31, 2012, the Company had injected into Growing Yangling $7.5 million as registered capital. According to the approval from the government agency dated October 12, 2010, the remaining balance of Growing Yangling’s registered capital of $42.5 million must be injected before July 13, 2013.
The cost for constructing the Yangling facility is expected to be over $58 million invested over two years, which would be mainly facilitated by the capital injection from BDH. The facility will produce probiotics and probiotics-related biological additives for the animal feed industry. Currently, the facility is in its construction stage. During the year ended March 31, 2011, the Company made total payment of approximately $3.25 million (RMB 20,608,112) to acquire the land use right of approximately 122,600 square meters (183.94 mu). We received the land certificate on January 28, 2012 and commenced construction in June 2012. We expect to complete construction by June 2014. As of March 31, 2012, Growing Yangling entered into agreements with contractors related to the construction of the plant and manufacturing facilities for future payment of $2,548,749 and $2,458,630, respectively. Subsequent to March 31, 2012, Growing Yangling entered into agreements with contractors for approximately $15,750,000 (RMB 99,400,000) for the purchase of production equipment. Shining also entered into agreements with contractors for initial work on the project in the amount of $125,726, which had not been paid as of March 31, 2012.
| 66 |
On February 1, 2012, our subsidiary, Growing, entered into an agreement to acquire a patent regarding a production technology for $6,647,147 (RMB 42,000,000) from an unrelated PRC company. Growing made a deposit of $6,330,616 (RMB 40,000,000) to the seller for the transfer of the patent within 210 days, or by August 30, 2012. The original terms of the agreement were that if the patent cannot be transferred to Growing within 210 days, Growing will be refunded the deposit paid and also be compensated an additional $159,000 (RMB 1,000,000) for damages. The transfer was not completed by August 30, 2012, and on September 3, 2012, Growing and the seller mutually agreed to extend the completion date to December 30, 2012. As of September 13, 2012, the transfer had not yet been completed because the Company is waiting for approval of the transfer from the State Intellectual Property Office of the PRC. The Company has no information to allow it to estimate when such confirmation will be obtained, but expects the transaction to close in 2013, after which the patent is planned to be used in the Company’s operations.
Purchase obligations
The Company entered into the agreements with the suppliers to purchase raw materials and packing materials. The amount of future payments is $8,019,273.
Other obligations
The Company entered into an agreement with a university to perform research and development. The amount of future payments is $1,424,389 (RMB 9,000,000).
Pending litigation
The Company and certain of its current and former officers and directors have been named as defendants in two putative shareholder class action lawsuits, one in the United States District Court for the Central District of California (Mohapatra v. China-Biotics, Inc., et al., No. 10-cv-6954 (C.D. Cal.), the “California Action”) and the other in the United States District Court for the Southern District of New York (Hill v. China-Biotics, Inc., et al., No. 10-cv-7838 (S.D.N.Y.), the “New York Action”). After certain shareholders filed motions for appointment as lead plaintiff in both lawsuits, the plaintiff in the California Action voluntarily dismissed its case and the plaintiff in the New York Action, together with another shareholder, were appointed as lead plaintiffs. The lead plaintiffs filed an amended complaint in which they allege that the defendants violated Sections 11, 12(a)(2), and 15 of the Securities Act of 1933, and Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 by making material misstatements or failing to disclose certain material information regarding, among other things, the Company’s financial condition, operations, and future business prospects, and the quality, nature, and quantity of the Company’s retail outlets. The lead plaintiffs seek to represent a class of shareholders who bought the Company’s securities between July 10, 2008 and August 27, 2010.
On August 18, 2011, the Company filed a motion to dismiss the lead plaintiffs’ amended complaint. The court dismissed the lead plaintiffs’ Section 11 claim, but gave them leave to replead. The court did not rule on the motion to dismiss the Section 10(b) claim. On January 9, 2012, the lead plaintiffs filed a second amended complaint that included a new named plaintiff and new allegations for the Section 11 claim. On February 27, 2012, the Company filed a motion to dismiss the amended Section 11 claim. Both that motion and the original motion to dismiss the Section 10(b) and Section 20(a) claims are currently pending before the court. The Company intends to defend this action vigorously.
The Company and certain of its current and former officers and directors have been named as defendants in a putative stockholder class action in the United States District Court for the Southern District of New York (Casper v. Jinan, et al., No. 12-cv-4202 (S.D.N.Y.). The plaintiff alleges that the defendants violated Sections 10(b) and 20(a) of the Securities Exchange Act of 1934 by making material misstatements about the Company’s projected revenue growth for 2011. The plaintiff seeks to represent a class of stockholders who bought the Company’s securities between February 9, 2011 and July 1, 2011. On September 5, 2012, the court appointed the Blanck Investor Group as lead plaintiff. The Company intends to defend this action vigorously.
The Company and its directors have been named as defendants in a derivative lawsuit filed in the United States District Court for the District of Columbia (Marteney v. Song Jinan, et al., No. 10-cv-1983 (D.D.C.)). The complaint alleges that the directors breached their fiduciary duties by disseminating false and misleading financial statements and seeks unspecified damages. On March 26, 2012, the plaintiff filed an amended complaint in which he added Roth Capital Partners LLC and Maxim Group LLC as defendants. On September 7, 2012, Roth Capital Partners LLC and Maxim Group LLC filed a motion to dismiss. The defendants intend to defend this action vigorously.
With respect to the above-referenced litigation matters, the Company is unable at this time to estimate possible losses, if any, or any other impact of the outcome of the litigation matters on the consolidated financial statements.
| 67 |
Review of decision ordering that the registration of the Company’s securities be revoked
On September 15, 2011, the staff of the SEC informed the Company that it intended to recommend that the SEC institute a public administrative proceeding against the Company for alleged violations of Section 13(a) of the Securities Exchange Act of 1934 and Rules 13a-1 and 13a-13 or 13a-16. On October 7, 2011, the SEC issued an Order Instituting Proceedings to determine whether it was necessary and appropriate to suspend or revoke the registration of the Company’s securities. On February 22, 2012, the administrative law judge overseeing the proceedings issued a decision ordering that the registration of the Company’s securities be revoked. On March 26, 2012, the SEC granted the Company’s petition for review of this decision. The results of the SEC review are pending as of September 13, 2012. If the registration of the Company’s securities is revoked, no U.S. registered broker-dealer may execute trades in the Company’s shares and the trading market for our common stock may cease to exist. In such event, investors may not be able to liquidate their investment.
| 21. | BUSINESS SEGMENTS |
The Company operates two business segments for the years ended March 31, 2012 and 2011, which are retail probiotics products as a health supplement and bulk additives for institutional customers in the PRC. The following is the summary information by segment as of March 31, 2012 and 2011, and for the years ended March 31, 2012 and 2011:
| Year Ended March 31, 2012 | Retail products | Bulk additives | Segment Total | Corporate | Total | |||||||||||||||
| Net revenue | $ | 27,890,558 | $ | 30,983,071 | $ | 58,873,629 | $ | - | $ | 58,873,629 | ||||||||||
| Income (loss) from operations | (1,029,460 | ) | 9,976,901 | 8,947,441 | (5,349,478 | ) | 3,597,963 | |||||||||||||
| Income taxes | 1,278,070 | 1,555,098 | 2,833,168 | - | 2,833,168 | |||||||||||||||
| Total assets | 115,981,279 | 102,438,295 | 218,419,574 | 38,542,990 | 256,962,564 | |||||||||||||||
| Depreciation and amortization | 1,091,171 | 2,599,604 | 3,690,775 | 31,113 | 3,721,888 | |||||||||||||||
| Year Ended March 31, 2011 | Retail products | Bulk additives | Segment Total | Corporate | Total | |||||||||||||||
| Net revenue | $ | 62,564,210 | $ | 46,230,722 | $ | 108,794,932 | $ | - | $ | 108,794,932 | ||||||||||
| Income (loss) from operations | 23,159,118 | 20,428,594 | 43,587,712 | (7,853,577 | ) | 35,734,135 | ||||||||||||||
| Income taxes | 6,225,158 | 2,757,191 | 8,982,349 | - | 8,982,349 | |||||||||||||||
| Total assets | 114,292,840 | 78,356,139 | 192,648,979 | 44,982,300 | 237,631,279 | |||||||||||||||
| Depreciation and amortization | 2,001,845 | 2,675,588 | 4,677,433 | 11,894 | 4,689,327 | |||||||||||||||
Reconciliation is provided for unallocated amounts relating to corporate operations, which is not included in the segment information.
| Years ended March 31, | ||||||||
| Reconciliation | 2012 | 2011 | ||||||
| Total segment operating income | $ | 8,947,441 | $ | 43,587,712 | ||||
| Corporate overhead expenses | (5,349,478 | ) | (7,853,577 | ) | ||||
| Other income, net | 2,011,015 | 10,183,691 | ||||||
| Income tax expense | (2,833,168 | ) | (8,982,349 | ) | ||||
| Total consolidated net income | $ | 2,775,810 | $ | 36,935,477 | ||||
| 68 |
CHINA-BIOTICS, INC.
SCHEDULE I – CONDENSED PARENT COMPANY FINANCIAL STATEMENTS
BALANCE SHEETS
(Amounts expressed in US Dollars)
| March 31, 2012 | March 31, 2011 | |||||||
| ASSETS | ||||||||
| Cash and cash equivalents | $ | 5,974 | $ | 63,513 | ||||
| Investments in and advances to subsidiaries | 207,407,664 | 200,636,597 | ||||||
| Total assets | $ | 207,413,638 | $ | 200,700,110 | ||||
| LIABILITIES AND SHAREHOLDERS’ EQUITY | ||||||||
| Current liabilities | ||||||||
| Accounts payable and accrued expenses | $ | 2,971,694 | $ | 2,764,303 | ||||
| Amount due to director | 2,011,332 | 1,591,332 | ||||||
| Total current liabilities | 4,983,026 | 4,355,635 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders’ equity: | ||||||||
| Preferred stock | - | - | ||||||
| Common stock | 4,237 | 4,237 | ||||||
| Additional paid-in capital | 84,727,616 | 83,242,926 | ||||||
| Retained earnings | 108,179,075 | 105,403,265 | ||||||
| Treasury stock | (2,741,634 | ) | (2,741,634 | ) | ||||
| Accumulated other comprehensive income | 12,261,318 | 10,435,681 | ||||||
| Total stockholders’ equity | 202,430,612 | 196,344,475 | ||||||
| Total liabilities and stockholders’ equity | $ | 207,413,638 | $ | 200,700,110 | ||||
The accompanying note is an integral part of these condensed financial statements.
| 69 |
CHINA-BIOTICS, INC.
SCHEDULE I - CONDENSED PARENT COMPANY FINANCIAL STATEMENTS
STATEMENTS OF OPERATIONS AND OTHER COMPREHENSIVE INCOME
(Amounts expressed in US Dollars)
| Year ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| General and administrative expenses | $ | (4,564,076 | ) | $ | (7,159,455 | ) | ||
| Exchange loss, net | (23 | ) | (32,185 | ) | ||||
| Interest income | 73 | 483 | ||||||
| Interest expenses | - | (4,885,468 | ) | |||||
| Gain on extinguishment of derivative liability | - | 14,797,000 | ||||||
| Income (loss) before equity in earnings of subsidiaries | (4,564,026 | ) | 2,720,375 | |||||
| Equity in earnings of subsidiaries | 7,339,836 | 34,215,102 | ||||||
| Net income | 2,775,810 | 36,935,477 | ||||||
| Other comprehensive income | ||||||||
| Foreign currency translation adjustment | 1,825,637 | 5,495,851 | ||||||
| Comprehensive income | $ | 4,601,447 | $ | 42,431,328 | ||||
| Weighted average shares outstanding | ||||||||
| Basic | 22,150,200 | 22,241,076 | ||||||
| Diluted | 22,266,993 | 23,689,004 | ||||||
| Earnings per share: | ||||||||
| Basic | $ | 0.13 | $ | 1.66 | ||||
| Diluted | $ | 0.12 | $ | 0.93 | ||||
The accompanying note is an integral part of these condensed financial statements.
| 70 |
CHINA-BIOTICS, INC.
SCHEDULE I - CONDENSED PARENT COMPANY FINANCIAL STATEMENTS
STATEMENTS OF CASH FLOWS
(Amounts expressed in US Dollars)
| Year ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES | ||||||||
| Net income | $ | 2,775,810 | $ | 36,935,477 | ||||
| Adjustments for: | ||||||||
| Investments in and advances to subsidiaries | (4,945,430 | ) | 4,777,278 | |||||
| Gain on extinguishment of derivative liability | - | (14,797,000 | ) | |||||
| Fair value of vested options | 1,484,690 | 473,852 | ||||||
| - Accounts payable and accrued expenses | 207,391 | (1,177,792 | ) | |||||
| NET CASH USED PROVIDED BY (USED IN) IN OPERATING ACTIVITIES | (477,539 | ) | 26,211,815 | |||||
| CASH FLOWS FROM FINANCING ACTIVITIES | ||||||||
| Repayment of convertible note | - | (25,000,000 | ) | |||||
| Purchase of treasury stocks | - | (2,739,634 | ) | |||||
| Cash advance from a director | 420,000 | 1,591,332 | ||||||
| NET CASH PROVIDED BY (USED IN) FINANCING ACTIVITIES | 420,000 | (26,148,302 | ) | |||||
| NET CHANGES IN CASH AND CASH EQUIVALENTS BALANCES | (57,539 | ) | 63,513 | |||||
| CASH AND CASH EQUIVALENTS BALANCES, beginning of period | 63,513 | - | ||||||
| CASH AND CASH EQUIVALENTS BALANCES, end of period | $ | 5,974 | $ | 63,513 | ||||
| Supplemental disclosure of non-cash information: | ||||||||
| Taxes paid | $ | - | $ | - | ||||
| Interest paid | $ | - | $ | 5,434,983 | ||||
The accompanying note is an integral part of these condensed financial statements.
1 These condensed parent company only financial statements should be read in connection with the consolidated financial statements and notes thereto.
| 71 |
ITEM 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
As previously reported by the Company in a Current Report on Form 8-K filed with the SEC on June 23, 2011, on June 22, 2011, the Company received a letter of resignation from BDO Limited, or BDO, which through that date had been the Company’s independent auditor, stating that it had resigned as such, effective immediately. The reasons for BDO’s resignation were set forth in its letter to the Company and are summarized in the Company’s Current Report on Form 8-K filed with the SEC on June 23, 2011, which is incorporated herein by reference. A copy of BDO’s resignation letter is incorporated herein by reference.
In a Form 12b-25 filing with the SEC dated as of June 15, 2011, the Company announced that its Audit Committee was investigating the issues raised in BDO’s letter, as well as other matters. On February 26, 2012, the Company engaged Weinberg & Company, P.A. (“Weinberg”) as the Company’s principal registered independent public accounting firm to audit the Company’s financial statements. Weinberg’s engagement is currently effective for the audit of the Company’s annual financial statements for the Company’s fiscal years ending from and after March 31, 2011. The engagement of Weinberg was approved by the Audit Committee of the Board of Directors of the Company. The Audit Committee also conducted an independent investigation of the issues raised in BDO’s letter and engaged Thornhill Capital LLC (“Thornhill”) on June 4, 2012 to perform limited financial due diligence on the Company and review the alleged irregularities identified by BDO that were the reasons BDO cited for its resignation as the Company’s independent auditor.
On July 27, 2012, the Audit Committee reviewed and adopted the results of Thornhill’s due diligence review, as summarized in a Due Diligence Report prepared by Thornhill for the Audit Committee of the Company, and concluded that the alleged irregularities identified by BDO Limited were not valid or material. Thornhill analyzed each of the alleged irregularities identified by BDO and, subject to certain assumptions and qualifications set forth in its report, concluded that:
| · | with respect to a Company sales contract in which the purchaser’s chop (i.e., the official signature or seal) affixed on the signature page of the sales contract belonged to a different company than the one named in the sales contract, it was likely that an isolated clerical error caused the discrepancy in the chop; |
| · | with respect to an incident in which, in connection with BDO’s review of the Company’s bank account through the Company’s e-banking system using the Company’s computer, BDO was directed by the Company to access a suspected fake website for the bank, (i) it was unlikely that the online banking system at the Bank of Communications that is used by the Company is fake, or that the balances or activity are misstated, and (ii) the online system is an accurate reflection of the Bank of Communication’s online system and the balances and activity reflected therein are properly recorded in the Company’s financial records; |
| · | with respect to a bank advice dated March 21, 2011 documenting a portion of the Company’s interest income that contained mathematical errors that the Company’s management dismissed as clerical mistakes made by the bank, and which the Company later replaced with a “corrected” advice from the bank, the reprinted bank advice is very likely valid and represents the bank transaction that occurred and was recorded in the Company’s financial statements (and Thornhill also confirmed that the demand deposit interest rates reflected on such bank advice agreed with the rates published by the People’s Bank of China); and |
| · | with respect to the use by the aforementioned bank advice of a deposit interest rate to calculate the interest income earned by the Company, which differed from the interest rate announced by the People’s Bank of China for the relevant deposit period as referred to in an undated deposit agreement that was presented to BDO to corroborate the Company’s interest income, the contract given to BDO was the actual contract signed with the bank, despite the fact that it was not dated by the Company (this was confirmed directly by the bank). |
In compliance with Items 304(a)(2) and (a)(3) of Regulation S-K, the Company has provided each of Thornhill and BDO with a copy of the disclosures made in this Annual Report on Form 10-K and has (i) notified Thornhill of its right to review such disclosures and furnish to the Company a letter addressed to the SEC containing any new information, clarification of the Company’s expression of its views or the respects in which it does not agree with the statements made by the Company and (ii) requested that BDO furnish to the Company a letter addressed to the Securities and Exchange Commission stating whether BDO agrees with the statements made by the Company in this Form 10-K in response to Item 304(a) of Regulation S-K and, if not, stating the respects in which it does not agree. Such letter from Thornhill is attached hereto as Exhibit 23.2.
| 72 |
ITEM 9A. CONTROLS AND PROCEDURES
Evaluation of Disclosure Controls and Procedures
In a letter to the Company on January 9, 2012, the SEC concluded that the Company’s lack of U.S. GAAP experience is a material weakness in our disclosure controls and procedures. The SEC required the Company to include disclosure about this material weakness in subsequent filings. For information on the Company’s correspondence with the SEC with respect to the Company’s internal controls over financial reporting, see “– Management’s Annual Report on Internal Control over Financial Reporting” below.
Since the SEC’s letter of January 9, 2012, the Company has endeavored to address the concerns raised by the SEC. The Company’s Audit Committee and our management are still engaged in discussions about how best to rectify fully the material weakness in our disclosure controls and procedures. The Audit Committee has recommended that the Company hire additional qualified personnel to prepare the Company’s books and records and financial statements in accordance with U.S. GAAP. The Company has retained the services of an accounting professional to perform our internal audit and assist with SEC compliance for purposes of all future reporting. Management believes that these remediation measures have improved and will materially improve the Company’s disclosure controls and procedures for periods subsequent to March 31, 2012, and that the corresponding material weaknesses are being addressed with respect to periods subsequent to March 31, 2012.
An evaluation was performed under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, of the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934) as of the end of the period covered by this Annual Report on Form 10-K. Based on that evaluation, our management, including our principal executive officer and principal financial officer, concluded that, as of such date, our disclosure controls and procedures were not effective and suffered from a material weakness because of the Company’s lack of training and/or experience with preparing financial statements in accordance with U.S. GAAP.
Management’s Annual Report on Internal Control over Financial Reporting
The Company’s management is responsible for establishing and maintaining adequate internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act). The Company’s internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of its financial reporting and the preparation of published financial statements in accordance with generally accepted accounting principles. However, because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or the degree of compliance with policies may deteriorate.
In a letter dated January 7, 2011, the SEC commented on the Company’s Annual Report on Form 10-K for Fiscal Year ended March 31, 2010 and Quarterly Report for the Period Ended September 30, 2010. The SEC asked the Company a number of questions about how we prepare financial statements and how we assess our internal controls over financial reporting. The Company replied to the SEC’s comment letter on February 16, 2011, and the SEC and the Company subsequently exchanged numerous comment and reply letters, respectively, through January 9, 2012. In a letter to the Company on that date, the SEC concluded that the Company’s lack of U.S. GAAP experience is a material weakness in our internal control over financial reporting. For information on the Company’s correspondence with the SEC with respect to the Company’s disclosure controls and procedures, see “–Evaluation of Disclosure Controls and Procedures” above.
In reaching such decision, the SEC concluded that those primarily responsible for the preparation of the Company’s books and records and financial statements do not have the requisite U.S. GAAP experience to prepare financial statements in accordance with U.S. GAAP. The SEC noted that the Company’s former and interim Chief Financial Officers do not hold a license such as that of a Certified Public Accountant in the U.S. and have not attended U.S. institutions or extended educational programs that would provide sufficient relevant education relating to U.S. GAAP; the SEC also noted that most of the U.S. GAAP audit experience, if any, of the Company’s former and interim Chief Financial Officers consists of audits of subsidiary financial information that supports or is included in the U.S. GAAP financial statements of parent registrants. The SEC also noted that the Company’s retention of a consultant to assist with the preparation and fulfillment of all SEC and U.S. GAAP reporting requirements was further evidence that our accounting department does not possess the requisite U.S. GAAP knowledge to prepare financial statements in accordance with U.S. GAAP. The SEC required the Company to file an amended Annual Report on Form 10-K for the year ended March 31, 2010 disclosing such material weakness, which the Company intends to file as promptly as is reasonably practicable, and also required the Company to include disclosure about this material weakness in subsequent filings.
| 73 |
Since the SEC’s letter of January 9, 2012, the Company has endeavored to address the concerns raised by the SEC. The Company’s Audit Committee and our management are still engaged in discussions about how best to rectify fully the material weakness in our internal control over financial reporting. The Audit Committee has recommended that the Company hire additional qualified personnel to prepare the Company’s books and records and financial statements in accordance with U.S. GAAP. The Company has retained the services of an accounting professional to perform our internal audit and assist with SEC compliance for purposes of all future reporting. Management believes that these remediation measures have improved and will materially improve the Company’s internal control over financial reporting for periods subsequent to March 31, 2012, and that the corresponding material weaknesses are being addressed with respect to periods subsequent to March 31, 2012.
Management is continuing its review of the Company’s internal control over financial reporting as it believes the following material weaknesses still exist: (i) a lack of senior management personnel who have the requisite U.S. GAAP experience to prepare financial statements in accordance with U.S. GAAP; (ii) the Company did not maintain an adequate financial reporting organizational structure (in part due to the turnover of the executives in the CFO and Interim CFO positions) to support the complexity and operating activities of the Company resulting in a weakness in efficiency and controls related to the financial statement closing process; and (iii) the Company may not have maintained sufficiently consistent corporate governance (including proper recording of minutes of meetings and decisions of the Company’s Board of Directors) and financial controls to ensure proper delegation of authority limits and timeliness of Board approval for significant transactions. However, the Company has taken steps described above to remediate these deficiencies.
Inherent Limitations on the Effectiveness of Controls
Management does not expect that the Company’s disclosure controls and procedures or its internal controls over financial reporting will prevent or detect all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control systems are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in a cost-effective control system, no evaluation of internal control over financial reporting can provide absolute assurance that misstatements due to error or fraud will not occur or that all control issues and instances of fraud, if any, have been or will be detected.
These inherent limitations include the realities that judgments in decision-making can be faulty and that breakdowns can occur because of a simple error or mistake. Controls can also be circumvented by the individual acts of some persons, by collusion of two or more people, or by management override of the controls. The design of any system of controls is based in part on certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions. Projections of any evaluation of controls effectiveness to future periods are subject to risks. Over time, controls may become inadequate because of changes in conditions or deterioration in the degree of compliance with policies or procedures.
Changes in Internal Controls over Financial Reporting
On February 22, 2012, the Company retained the services of Mr. Yun Hon Man as part of management’s effort to use additional qualified personnel to prepare the Company’s books and records and financial statements in accordance with U.S. GAAP. Mr. Yun has served as:
| · | an independent director and the Chair of the Audit Committee of the Chisen Electric Corporation (OTCBB: CIEC) since November 24, 2008; |
| · | a Corporate Consultant with Smart Pine Investment Limited, a consulting firm organized under the laws of Hong Kong, since September 2007; |
| · | an independent director and the Chair of the Audit Committee of CH Lighting International Corporation (OTCBB: CHHN) since July 28, 2008; and |
| · | an independent director and the Chair of the Audit Committee of Xinde Technology Company (OTCBB: WTFS) since January 2010. |
Mr. Yun also served as Chief Operating Officer of China INSOnline Corp. (NASDAQ: CHIO) from January 2008 through April 2010. Prior to that, Mr. Yun served as Corporate Controller of Hi-Tech Wealth Inc. (n/k/a China Mobile Media Technology, Inc.) (OTCBB: CHMO) from January 2007 through August 2007. From January 2003 through December 2006, Mr Yun served as Corporate Controller of General Components, Inc. (n/k/a China Mobile Media Technology, Inc.) (OTCBB: CHMO). Mr. Yun is a Chartered Accountant, having memberships with the Institute of Chartered Accountants in England and Wales. He is also a Fellow Member of the Chartered Association of Certified Accountants and a member of the Hong Kong Institute of Certified Public Accountants. He was a member of Association of International Accountants, the Society of Registered Financial Planners, the Institute of Financial Accountants and the Institute of Crisis and Risk Management. Mr. Yun received his MBA at the University of Western Sydney in 2007.
| 74 |
ITEM 9B. OTHER INFORMATION
On March 6, 2012, Ms. Marie Yang, the interim Chief Financial Officer, submitted her resignation to the Company, effective immediately. Ms. Yan Yihong became the Company’s interim Chief Financial Officer as of the same date. Ms. Yan is also the Company’s Chief Administration Officer (assistant to the general manager). Ms. Yan has served as a director of Shining since 1999. She was appointed as the Company’s Chief Administration Officer in 2004. She will serve as the interim Chief Financial Officer of the Company until a successor can be appointed.
On January 6, 2012, Dr. Hui S. Chang, the Chief Operating Officer, submitted his resignation to the Company, effective immediately. The Company did not appoint a replacement Chief Operating Officer.
| 75 |
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Directors, Executive Officers, and Key Employees and Advisors
We seek directors with established strong professional reputations and experience in areas relevant to the strategy and operations of our businesses. We also seek directors who possess the qualities of integrity and candor, personal and professional ethics, commitment to understanding the Company’s business, industry knowledge and contacts, leadership, strong analytical skills, and the willingness to engage management and each other in a constructive and collaborative fashion. We also seek directors who have the ability and commitment to devote significant time and energy to service on the board of directors and its committees. We believe that all of our directors meet the foregoing qualifications.
The criteria described above are used as guidelines to evaluate the experience, qualifications, skills and diversity of our current and potential board members. With respect to diversity, certain of our directors have strong scientific backgrounds that are relevant to our industry; another of our directors has a background in accounting, finance, and management. We believe that the backgrounds and skills of our directors bring a diverse range of experience, opinion and perspectives to the board.
The following is a summary of the business experience of our executive officers and directors:
Mr. Song Jinan, age 50, has been Chief Executive Officer, President, Treasurer and Secretary since March 2006, when he was also appointed to the board of directors. His current term as director expires with the 2012 Annual Meeting of Stockholders. Mr. Song was one of the founders of Shining in 1999, and has been the principal executive officer of Shining since inception. Prior to founding Shining, Mr. Song served as the chief engineer of Sai Bao Bio-Chemical Manufacturing Corporation. Mr. Song received his Bachelor’s Degree in Polymers from the University of Hei Long Jiang and his Master’s degree in Politics and Economics from Habin Industrial University.
Mr. Song’s day-to-day leadership as our Chief Executive Officer provides him with detailed knowledge of our business and operations. Among other professional experiences, qualifications and skills, Mr. Song brings in-depth knowledge and understanding of the probiotics industry, scientific expertise and management skills that have been critical to formulating the Company’s short and long-term strategies and to establishing the Company as a leading probiotics developer and producer in China. As the Company’s largest shareholder, Mr. Song’s interest is aligned with other China-Biotics shareholders interest in increasing the long-term value of the Company.
Dr. Chin Ji Wei, age 55, has been a director since January 2007 and his current term expires with the 2012 Annual Meeting of Stockholders. Dr. Chin has over 20 years of academic experience as a lecturer and researcher in the field of horticulture, where he has been focused on the areas of efficient agriculture industry and food safety. Dr. Chin has served as a Vice Principal, professor, and lecturer at Northeast Agricultural University in China since 1999. From 1985 to 1995, Dr. Chin served as a Researcher at the Northeast Agricultural University and the Northeast Agricultural Institute. Dr. Chin has Bachelors, Masters, and Doctorate degrees, all from Northeast Agricultural University. Among other professional experiences, qualifications and skills, Dr. Chin’s expertise in the horticulture and agriculture industries, and in particular his focus on food safety, provide scientific knowledge and expertise in the regulatory aspects of food safety that are critical to the development and distribution of the Company’s products.
Dr. Du Wen Min, age 44, has been a director since January 2007 and his current term expires with the 2012 Annual Meeting of Stockholders. Dr. Du has served as the Deputy Director in charge of the Centre for Adverse Drug Reactions in Shanghai since 2001. The centre was established in June 2001 as a technology unit governed by The Shanghai Food and Drug Authority. Dr. Du has also served as the Vice Chairman of Evaluation of Pharmacology & Clinical Pharmacy in Shanghai, China, and the Vice Chairman at the Centre for the Study of Liver Disease in Shanghai, China since 2006. Dr. Du has Bachelors and Masters Degrees from Shaanxi Medical University and a Doctorate in Medicine from Fudan University. Among other professional experiences, qualifications and skills, Dr. Du’s experience in the pharmaceutical industry and knowledge of food and drug safety issues are critical to the development and distribution of the Company’s products.
Mr. Ivan Chu, Siu-Lun, age 31, has been a director since February 2012. Mr. Chu brings over eight years of professional experience in governmental, executive and corporate finance with various public companies and investment institutions. Since September 2011, Mr. Chu has been the project director for Suncorp Technology Limited (HKG: 1063), a company listed on the Hong Kong Exchange. Since July 2011, he also has served as an independent director and a member of the audit committee of CNC Holding Limited (HKG: 8356), a Hong Kong Exchange-listed company. Since September 2007, Mr. Chu also has served as the founder and executive director of HUDA Asia Investments Limited, a corporate financing company that provides services in IPO consultation, advisory services in mergers and acquisition of listed companies, management of investment funds and other securities-related services. Through his professional experience, Mr. Chu has developed expertise in financial management, account reporting, mergers and acquisitions, risk control, fund valuation, SOX 404 compliance and operational management of project development. Mr. Chu holds a Bachelor of Art (Honors) degree in Business Studies from the Bolton Institute of the University of Bolton, UK. He is certified by the Institute of Financial Accountants, UK as a Financial Accountant and by Institute of Financial Planners of Hong Kong as a Financial Planner (AFP TM). Mr. Chu is currently studying for his Master of Science degree in Finance at the National University of Ireland.
| 76 |
Ms. Yan Yihong, age 49, became the Company’s interim Chief Financial Officer as of March 6, 2012 and is also the Chief Administration Officer (assistant to the general manager). Ms. Yan has served as a director of Shining since 1999. She was appointed as the Company’s Chief Administration Officer in 2004. During the past five years, Ms. Yan has been an employee of Shining in various capacities and has, among other things, participated in formulating the company’s development plans, implemented the company’s internal control procedures and represented the company in business negotiations with relevant government authorities and other external parties.
Board Structure and Composition; Committees
Our board of directors currently consists of four members: Mr. Song Jinan, Dr. Chin Ji Wei, Dr. Du Wen Min and Mr. Ivan Chu. Dr. Chin Ji Wei, Dr. Du Wen Min and Mr. Ivan Chu are independent directors under the independence definitions established by the SEC, the American Stock Exchange and NASDAQ.
Our board of directors believes that Mr. Song’s service as both Chairman of the Board and Chief Executive Officer of the Company is in the best interest of the Company and its stockholders. Mr. Song possesses detailed and in-depth knowledge of the issues, opportunities and challenges facing the Company, its business and the probiotics industry, and is thus best positioned to develop agendas that ensure that our board’s time and attention are focused on the most critical matters. His combined role enables decisive leadership, ensures clear accountability, and enhances the Company’s ability to communicate its message and strategy clearly and consistently to the Company’s shareholders, employees, customers and suppliers.
Audit Committee. On May 28, 2008, we established an Audit Committee of the Board of Directors. The members of the Audit Committee are Dr. Chin Ji Wei, Dr. Du Wen Min and Mr. Ivan Chu. Mr. Chu serves as the chairperson of the Audit Committee. Our Board of Directors has determined that Mr. Chu qualifies as an Audit Committee financial expert and is an independent director under the independence definitions established by the SEC, the American Stock Exchange and NASDAQ.
As more fully described in its charter, the Audit Committee has responsibility for, among other things:
| · | appointing, determining the funding for, and overseeing the independent registered public accounting firm; |
| · | assisting our board in monitoring the integrity of our financial statements and other SEC filings; |
| · | discussing with our management and our independent registered public accounting firm significant financial reporting issues and judgments and any major issues as to the adequacy of our internal controls; |
| · | reviewing our annual and quarterly financial statements prior to their filing with the SEC and prior to the release of our results of operations; |
| · | reviewing the independence, performance and qualifications of our independent registered public accounting firm and presenting its conclusions to our board and approving, subject to permitted exceptions, any non-audit services proposed to be performed by the independent registered public accounting firm; and |
| · | assisting the Board of Directors in its oversight responsibilities regarding the performance of the Company’s internal audit function. |
Nominating Committee. On May 28, 2008, we also established a Nominating Committee of the Board of Directors. The members of the Nominating Committee are Mr. Song Jinan, Dr. Chin Ji Wei, and Dr. Du Wen Min. Mr. Song serves as the chairperson of the Nominating Committee.
As more fully described in its charter, the Nominating Committee has the responsibility for, among other things:
| · | recommending persons to be selected by the board as director nominees for the Annual Meeting of the Stockholders and from time to time to fill vacancies on the board; |
| · | assessing our directors’ and our board’s performance; and |
| 77 |
| · | making recommendations to the board regarding membership and chairs of the board’s committees. |
Our Bylaws provide that a vote of the majority of our independent directors is required for the selection of director nominees for election at each annual meeting of stockholders or at any special meeting of stockholders called for the purpose of electing directors.
Compensation Committee. On May 28, 2008, we also established a Compensation Committee of the Board of Directors. The members of the Compensation Committee are Dr. Chin Ji Wei, Dr. Du Wen Min and Mr. Ivan Chu. Dr. Du Wen Min serves as the chairperson of the Compensation Committee.
The Compensation Committee has responsibility for, among other things:
| · | reviewing and approving our Chief Executive Officer’s compensation and making recommendations to the board with respect to compensation of other executive employees; |
| · | evaluating the Chief Executive Officer’s performance in light of corporate goals and objectives and setting the Chief Executive Officer’s compensation level based on that evaluation, as well as the short-term and long-term performance of the Company; |
| · | reviewing and recommending to the board for approval by a majority of the independent members of the board, the compensation of all executive officers other than the CEO, based on such factors as the disinterested members of the Compensation Committee may deem relevant; |
| · | administering our incentive compensation plans and equity-based plans and making recommendations to the board with respect to those plans; and |
| · | producing an annual report on executive compensation for inclusion in the Company’s Proxy Statement in accordance with applicable rules and regulations. |
The charters of the three committees are posted on the Company’s website and can be accessed free of charge at www.chn-biotics.com. They are also available in print to anyone who requests them.
Risk Oversight
Our board of directors takes an active role, as a whole and also at the committee level, in overseeing the material risks facing the Company, including operational, financial, legal and regulatory, and strategic and reputational risks. Risks are considered in virtually every business decision and as part of the Company’s overall business strategy. Our board committees also regularly engage in risk assessment as a part of their regular function. The Audit Committee discusses with management the Company’s major financial risk exposures and the steps management has taken to monitor and control such exposures. The Compensation Committee is responsible for overseeing the management of risks relating to the Company’s executive compensation plans and arrangements. The Nominating Committee manages risks associated with corporate governance, including risks associated with the independence of the board and reviews risks associated with potential conflicts of interest affecting directors and executive officers of the Company. While each committee is responsible for evaluating certain risks and overseeing the management of such risks, our entire board of directors is regularly informed through committee reports about such risks. The board of directors regularly engages in discussion of financial, legal, technology, economic and other risks. Because overseeing risk is an ongoing process that is inherent in the Company’s strategic decisions, our board of directors discusses risk throughout the year at other meetings in relation to specific proposed actions. Additionally, our board of directors exercises its risk oversight function in approving the annual budget and quarterly forecasts and in reviewing the Company’s long-range strategic and financial plans with management.
Section 16(a) Beneficial Ownership Reporting Compliance
On October 23, 2008, our common stock became registered under the Securities Exchange Act of 1934, as amended, at which time the Company’s officers, directors and any 10% shareholders became subject to the reporting requirements of Section 16(a). To our knowledge, based solely on our review of the copies of such reports furnished to us during the fiscal year ended March 31, 2012, all applicable Section 16(a) filing requirements were met, and all such filings were timely except that a Form 3 was not filed by Mr. Ivan Chu upon his appointment as a director of the Company.
Code of Business Conduct and Ethics
Our Board of Directors has adopted a Code of Conduct and Ethics applicable to our directors and executive officers, including our chief executive officer, chief financial officer, and other of our senior financial officers and employees in accordance with applicable rules and regulations of the SEC and NASDAQ. Our Code of Conduct and Ethics is posted on our website at www.chn-biotics.com.
| 78 |
ITEM 11. EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
Our compensation program is designed to attract and retain employees and reward them for their efforts toward helping us achieve both long-term and short-term goals. Currently, compensation for our executive officers consists of base salary and the granting of equity incentive awards, which are based on relevant factors, such as:
| · | the short-term and long-term performance of the Company; |
| · | the performance of the executive officers in light of relevant corporate goals and objectives; |
| · | executive compensation levels at comparable companies; and |
| · | the recommendations of our Chief Executive Officer. |
Base salaries are reviewed annually and adjustments are made to reflect performance-based factors, such as the individual performance of the executive officer and the financial performance of the Company, as well as competitive conditions in the industry. The Compensation Committee uses the Company’s financial performance for fiscal year 2010 as a baseline to determine its executive officers’ future compensation, including any adjustments to their current salaries in the annual review process. Other specific performance goals and specific corporate goals and objectives that may be used to set or adjust executive compensation are currently under discussion by the Compensation Committee but have not yet been formally approved.
On January 16, 2011, the board approved the 2010 Equity Incentive Plan (the “2010 Plan”), which was subsequently approved by the stockholders of the Company at the 2010 Annual Meeting of Stockholders held on March 9, 2011. The Company believes that equity awards will (i) promote long-term performance by its executives; (ii) create an ownership culture among our executive officers; and (iii) will foster beneficial long-term performance by the Company. The Company further believes that an equity compensation program will provide our employees with incentives to help align their interests with the interests of stockholders. The Compensation Committee believes that the use of stock-based awards promotes our overall executive compensation objectives and expects that stock options will become a significant source of compensation for our executives.
On January 16, 2011 (the “Grant Date”), upon recommendation by the Compensation Committee and pursuant to the 2010 Plan, the Company granted to Mr. Song options to purchase 300,000 shares of the Company’s common stock. The exercise price of the options is $14.81 per share, which is equal to 110% of the closing price per share of the Company’s common stock as quoted on the NASDAQ Stock Exchange on the last trading day immediately prior to the Grant Date. The options will vest according to the Vesting Schedule (as defined below) and are exercisable as of March 9, 2011.
With respect to the options granted to Mr. Song, the “Vesting Schedule” means a series of 48 successive monthly installments on the last day of each month (beginning with the calendar month including the Grant Date) such that (1) during the first 12-month period, the options will vest in equal monthly installments such that on the one-year anniversary of the Grant Date, 20% of the options shall be fully vested; (2) during the second 12-month period, the options will vest in equal monthly installments such that on the two-year anniversary of the Grant Date, an additional 20% of the options shall be fully vested; (3) during the third 12-month period, the options will vest in equal monthly installments such that on the three-year anniversary of the Grant Date, an additional 30% of the options shall be fully vested; and (4) during the fourth 12-month period, the options will vest in equal monthly installments such that on the four-year anniversary of the Grant Date, an additional 30% of the options shall be fully vested.
Dr. Chang, our former Chief Operating Officer, received a base salary of $80,347 for his service during the period from April 1, 2011 to January 6, 2012.
Ms. Yan Yihong, our interim Chief Financial Officer and the Chief Administrative Officer, receives a base salary of $192,027 per year in her capacity as Chief Administrative Officer. Ms. Yan does not receive any additional compensation in her capacity as the interim Chief Financial Officer.
The compensation of our executive officers was unanimously approved by our Compensation Committee based on their respective salary histories, their respective professional experience, their personal attributes and experience, an analysis of the compensation levels of executive officers of comparable companies, and on the recommendation of our Chief Executive Officer. The three companies that were identified as comparable for purposes of setting our executive officer compensation were American Oriental Bioengineering, Inc., Shanghai Bright Dairy & Food, and Tiens Biotech Group (USA), Inc. American Oriental Bioengineering is a New York Stock Exchange listed, U.S.-based company focused on producing pharmaceutical and neutra-ceutical products for the Chinese market. Shanghai Bright Dairy & Food is a Shanghai Stock Exchange listed company that produces dairy products for the Chinese market. Tiens Biotech Group is U.S.-based, NYSE Alternext US-listed company focused on developing and producing nutrition supplement products for the Chinese and international market.
| 79 |
In the future, the Compensation Committee will seek to identify additional companies that are considered comparable for the purposes of setting executive compensation. Any future adjustments to the compensation of our executive officers will take into account the factors described above, their future performance, the Company’s future financial performance, and any additional factors the Compensation Committee considers appropriate at that time.
Our Compensation Committee is responsible for advising and assisting the Board in its responsibilities related to compensation of the Company’s executive officers, and ensuring that compensation plans are appropriate and competitive and properly reflect the objectives and performance of our management and the Company.
Director Compensation
The table below lists the compensation received by the non-independent director of China-Biotics for the years ended March 31, 2012 and 2011.
| Annual Compensation | |||||||||||||||||||||||
| Name of non-independent | Option | Other Annual | |||||||||||||||||||||
| director | Year | Fee | Bonus | Awards(5) | Compensation | Total | |||||||||||||||||
| Song Jinan(1) | 2012 | $ | 60,000 | — | — | — | $ | 60,000 | |||||||||||||||
| 2011 | $ | 60,000 | — | 2,796,060 | — | $ | 2,850,060 | ||||||||||||||||
(1) Reflects only the compensation paid to Mr. Song in his capacity as a director. Does not include the compensation paid to Mr. Song as Chief Executive Officer.
The table below lists the compensation received by the independent directors of China-Biotics for the years ended March 31, 2012 and 2011.
| Annual Compensation | ||||||||||||||||||||||||
| Option | Other Annual | |||||||||||||||||||||||
| Name of independent directors | Year | Fee(1) | Bonus | Awards(6) | Compensation | Total | ||||||||||||||||||
| Dr. Chin Ji Wei(2) | 2012 | $ | 18,238 | — | — | — | $ | 18,238 | ||||||||||||||||
| 2011 | $ | 15,267 | — | 876,192 | — | $ | 891,459 | |||||||||||||||||
| Dr. Du Wen Min(3) | 2012 | $ | 18,238 | — | — | — | $ | 18,238 | ||||||||||||||||
| 2011 | $ | 15,267 | — | 876,192 | — | $ | 891,459 | |||||||||||||||||
| Mr. Simon Yick(4) (5) | 2012 | $ | 11,598 | — | — | — | $ | 11,598 | ||||||||||||||||
| 2011 | $ | 58,465 | — | 1,095,240 | — | $ | 1,153,705 | |||||||||||||||||
| Mr. Jonathan Chan(7) | 2012 | $ | 20,619 | — | — | — | $ | 20,619 | ||||||||||||||||
| 2011 | $ | — | — | — | — | $ | — | |||||||||||||||||
| Mr. Ivan Chu(8) | 2012 | $ | 3,866 | — | — | — | $ | 3,866 | ||||||||||||||||
| 2011 | $ | — | — | — | — | $ | — | |||||||||||||||||
(1) Chin Ji Wei and Dr. Du Wen Min were paid in RMB; Mr. Simon Yick, Mr. Jonathan Chan and Mr. Ivan Chu were paid in Hong Kong dollars. The US dollar amounts set forth above were calculated using an average exchange rate for the relevant period.
(2) On January 16, 2011, the Company granted Dr. Chin options to purchase 80,000 shares of the Company’s common stock at an exercise price of $14.81 per share. The options are subject to graded vesting over a four-year period. Vested options became exercisable on March 9, 2011, the day on which the 2010 Plan was approved by the Company’s stockholders. As of March 31, 2012, 20,000 options held by Dr. Chin were vested.
| 80 |
(3) On January 16, 2011, the Company granted Dr. Du options to purchase 80,000 shares of the Company’s common stock at an exercise price of $14.81 per share. The options are subject to graded vesting over a four-year period. Vested options became exercisable on March 9, 2011, the day on which the 2010 Plan was approved by the Company’s stockholders. As of March 31, 2012, 20,000 options held by Dr. Du were vested.
(4) On January 16, 2011, the Company granted Mr. Yick options to purchase 100,000 shares of the Company’s common stock at an exercise price of $14.81 per share. The options are subject to graded vesting over a four-year period. Vested options became exercisable on March 9, 2011, the day on which the 2010 Plan was approved by the Company’s stockholders.
(5) On June 23, 2011, Mr. Simon Yick resigned his position as director and Chairman of the Audit Committee of the Company. As of the date of such resignation, Mr. Yick lost any right or claim to unvested options. Because Mr. Yick did not exercise his options within three months of his resignation, all vested options held by Mr. Yick were forfeited and cancelled in accordance with the 2010 Plan.
(6) Option awards amounts shown in this table represent the grant date fair value computed in accordance with FASB ASC 718.
(7) On October 31, 2011, Mr. Jonathan Chan was appointed as director and Chairman of the Audit Committee of the Company. On February 25, 2012, Mr. Chan communicated his resignation orally to the Company and the board of directors of the Company accepted his resignation. Mr. Chan received compensation of HK$40,000 per month for his services as director and Chairman of the Audit Committee.
(8) On February 25, 2012, Mr. Ivan Chu was appointed as director and Chairman of the Audit Committee of the Company. Mr. Chu will receive compensation of HK$30,000 per month for his services as director and Chairman of the Audit Committee.
Executive Officer Compensation
The following table sets forth information concerning compensation awarded to, earned by, or paid to Mr. Song Jinan, the Chief Executive Officer of China-Biotics, Dr. Hui S. Chang, the former Chief Operating Officer of China-Biotics, Ms. Yan Yihong, the interim Chief Financial Officer and the Chief Administrative Officer of the Company and Mr. Travis Cai, former Chief Financial Officer of China-Biotics. Mr. Song and Ms. Yan are currently the only executive officers of the Company. No other officer of China-Biotics or SGI received compensation in excess of $100,000 during the years ended 2012 and 2011.
SUMMARY COMPENSATION TABLE
| Option | ||||||||||||||||||||
| Name | Year | Salary | Bonus | Awards(7) | Total | |||||||||||||||
| Song Jinan | 2012 | $ | 718,194 | $ | — | $ | — | $ | 718,194 | |||||||||||
| CEO, Treasurer, Secretary, and | 2011 | $ | 450,310 | (2) | $ | 1,150,000 | $ | — | (2) | $ | 1,600,310 | |||||||||
| Principal Executive Officer of SGI (1) | ||||||||||||||||||||
| Travis Cai | 2012 | $ | 26,751 | $ | — | $ | — | $ | 26,751 | |||||||||||
| Former Chief Financial Officer (3) | 2011 | $ | 150,000 | $ | — | $ | 1,642,860 | $ | 1,792,860 | |||||||||||
| Dr. Hui S. Chang | 2012 | $ | 80,347 | $ | — | $ | — | $ | 80,347 | |||||||||||
| Former Chief Operating Officer(4) | 2011 | $ | 45,195 | $ | — | $ | — | $ | 45,195 | |||||||||||
| Maria Yang | 2012 | $ | 32,829 | $ | — | $ | — | $ | 32,829 | |||||||||||
| Former interim Chief Financial Officer(5) | 2011 | $ | — | $ | — | $ | — | $ | — | |||||||||||
| Ms. Yan Yihong | 2012 | $ | 192,027 | $ | — | $ | — | $ | 192,027 | |||||||||||
| Interim Chief Financial Officer and Chief Administrative Officer(6) | 2011 | $ | 244,634 | $ | — | $ | 2,190,480 | $ | 2,435,114 | |||||||||||
(1) Reflects only the compensation paid to Mr. Song in his capacity as Chief Executive Officer. Does not include the compensation paid to Mr. Song as director. Mr. Song became our Chief Executive Officer, Chief Financial Officer, Treasurer, and Secretary as of March 22, 2006. He was the sole executive officer of China-Biotics prior to November 2006 and the principal executive officer of SGI for the periods indicated. On November 13, 2006, Mr. Song resigned from the office of Chief Financial Officer, and appointed Mr. Raymond Li to serve as the Chief Financial Officer. Mr. Lewis Fan replaced Mr. Li as the Chief Financial Officer as of March 6, 2009. Mr. Travis Cai became the Chief Financial Officer on January 22, 2010 and resigned on June 23, 2011. On June 23, 2011, Ms. Maria Yang was appointed to serve as the interim Chief Financial Officer; she resigned on March 6, 2012. On March 6, 2012, Ms. Yan Yihong was appointed to serve as the interim Chief Financial Officer until a successor can be appointed.
(2) Mr. Song was paid in RMB. The US dollar amounts were calculated using an average exchange rate for each of fiscal years 2012 and 2011. The amounts include social insurance contributions of $9,595 (RMB60,883) and $8,082 (RMB56,496) for Mr. Song for the years ended March 31, 2012 and 2011, respectively. On January 16, 2011, Mr. Song was granted options to purchase 300,000 shares of the Company’s common stock at an exercise price of $14.81 per share. The options are subject to graded vesting over a four-year period. Vested options became exercisable on March 9, 2011, the day on which the 2010 Plan was approved by the Company’s stockholders.
| 81 |
(3) Mr. Travis Cai’s base salary was $150,000 per year. In addition to his base salary, on January 16, 2011, Mr. Cai was granted options to purchase 150,000 shares of the Company’s common stock at an exercise price of $14.81 per share. The options are subject to graded vesting over a four-year period. Vested options became exercisable on March 9, 2011, the day on which the 2010 Plan was approved by the Company’s stockholders. On June 24, 2011, Mr. Cai resigned his position as Chief Financial Officer of the Company. As of the date of such resignation, Mr. Cai lost any right or claim to unvested options.
(4) Dr. Hui S. Chang joined the Company on, and worked as our Chief Operating Officer since, November 11, 2010. Dr. Chang received an annual salary of $80,347 for the period from April 1, 2011 to January 6, 2012. On January 6, 2012, Dr. Chang resigned his position as Chief Operating Officer of the Company.
(5) Ms. Yang received no salary for serving in the position of interim Chief Financial Officer.
(6) Ms. Yan Yihong, our interim Chief Financial Officer and Chief Administrative Officer, received an annual salary of $192,027 for serving in the position of Chief Administrative Officer only. On January 16, 2011, Ms. Yan was granted options to purchase 200,000 shares of the Company’s common stock at an exercise price of $14.81 per share. The options are subject to graded vesting over a four-year period. Vested options became exercisable on March 9, 2011, the day on which the 2010 Plan was approved by the Company’s stockholders.
(7) Option awards amounts shown in this table represent the grant date fair value computed in accordance with FASB ASC 718.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
| Equity | |||||||||||||||
| Incentive | |||||||||||||||
| Number of | Number of | Plan Awards: | |||||||||||||
| Securities | Securities | Number of | |||||||||||||
| Underlying | Underlying | Securities | |||||||||||||
| Unexercised | Unexercised | Underlying | Option | ||||||||||||
| Options | Options | Unexercised | Exercise | Option | |||||||||||
| (#) | (#) | Unearned Options | Price | Expiration | |||||||||||
| Name | Exercisable | Unexercisable | (#) | ($) | Date | ||||||||||
| Song Jinan, CEO, Treasurer, Secretary, and Principal Executive Officer of SGI |
75,000 | 225,000 | 300,000 | 14.81 | January 16, 2016 |
||||||||||
| Travis Cai, former Chief Financial Officer(1) | - | - | - | 14.81 | January 16, 2021 |
||||||||||
| Yan Yihong, interim Chief Financial Officer and Chief Administrative Officer | 50,000 | 150,000 | 200,000 | 14.81 | January 16, 2021 |
||||||||||
| (1) | On June 24, 2011, Mr. Cai resigned his position as Chief Financial Officer of the Company. As of the date of such resignation, Mr. Cai lost any right or claim to unvested options. Because Mr. Cai did not exercise his vested options within three months of his resignation, all vested options held by Mr. Cai were forfeited and cancelled in accordance with the 2010 Plan. As of March 31, 2012, Mr. Cai held no options. |
ITEM 12. SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
The following table sets forth information with respect to the beneficial ownership of our common stock as of September 11, 2012, including shares which the listed beneficial owner has the right to acquire within 60 days from such date from options, warrants, rights, conversion privileges or similar obligations, by:
| · | each holder of more than 5% of our common stock; |
| · | each of our executive officers and directors; and |
| · | our executive officers and directors as a group. |
| 82 |
Unless otherwise noted below, the addresses of each beneficial owner set forth below is No. 26, Orient Global Headquarter, Lane 118, Yonghe Road, Zhabei District, Shanghai 20072, People’s Republic of China. The numbers and percentages are based on 22,150,200 shares of our common stock outstanding as of September 11, 2012.
| Number of | ||||||||
| Shares of | Percent of | |||||||
| Common Stock | Common | |||||||
| Name and Address of Beneficial Owner | Owned | Stock Owned | ||||||
| Song Jinan | 9,139,030 | (1) | 40.95 | % | ||||
| Chin Ji Wei | 20,000 | (2) | 0.09 | % | ||||
| Du Wen Min | 20,000 | (3) | 0.09 | % | ||||
| Simon Yick(4) | 221,000 | (5) | 0.99 | % | ||||
| Travis Cai | - | (6) | - | % | ||||
| Yan Yihong | 540,662 | (7) | 2.42 | % | ||||
| Yan Li | 9,139,030 | (8) | 40.95 | % | ||||
| Richard E. Azar | 4,050,000 | (9) | 18.15 | % | ||||
| Executive officers and directors (4 persons) | 9,719,692 | 43.56 | % | |||||
(1) Includes 75,000 shares that are exercisable pursuant to options and 3,979,993 shares held by Ms. Yan Li, Mr. Song’s spouse.
(2) Includes 20,000 shares that are exercisable pursuant to options.
(3) Includes 20,000 shares that are exercisable pursuant to options.
(4) Each of Mr. Yick and his spouse owns 50% of Master Talent Group Limited, which owns 221,000 shares of our common stock. On June 23, 2011, Mr. Simon Yick resigned his position as director and Chairman of the Audit Committee of the Company.
(5) On June 23, 2011, Mr. Simon Yick resigned his position as director and Chairman of the Audit Committee of the Company. As of the date of such resignation, Mr. Yick lost any right or claim to unvested options. Because Mr. Yick did not exercise his options within three months of his resignation, all vested options held by Mr. Yick were forfeited and cancelled in accordance with the 2010 Plan. As of March 31, 2012, Mr. Yick held no options.
(6) On June 24, 2011, Mr. Cai resigned his position as Chief Financial Officer of the Company. As of the date of such resignation, Mr. Cai lost any right or claim to unvested options. Because Mr. Cai did not exercise his options within three months of his resignation, all vested options held by Mr. Cai were forfeited and cancelled in accordance with the 2010 Plan. As of March 31, 2012, Mr. Cai held no options.
(7) Includes 50,000 shares that are exercisable pursuant to options.
(8) Includes 5,159,037 shares (including 75,000 shares that are exercisable pursuant to options) held by Mr. Song Jinan, Ms. Yan’s spouse.
(9) Based on Form 4 filed with the SEC on May 26, 2011. Richard E. Azar is the sole director and owner of Value Holdings Capital, S.A. (“Value Holdings”) and Value Assets International, S.A. (“Value Assets”), each of which owns 1,536,336 shares and 2,463,664 shares, respectively. In addition, based on Amendment No. 1 to Schedule 13G filed with the SEC on May 23, 2011, Mr. Azar is a director of GBWU Investment Holdings, S.A. (“GBWU”), which owns 50,000 shares. As such, Mr. Azar may be deemed to beneficially own 4,050,000 shares as collectively held by Value Holdings, Value Assets, and GBWU. The address of Value Holdings, Value Assets, and GBWU is as follows: c/o Harney’s Corporate Services, Ltd., Craigmuir Chambers, P.O. Box 71, Road Town, Tortola, British Virgin Islands. The address of Mr. Azar is 7-9 St. Claire Place, 2nd Fl., St. Clair Ave., Port of Spain, Trinidad Tobago.
Equity Compensation Plan Information
The following table sets forth information regarding the Company’s equity compensation plans as of March 31, 2012 and 2011.
| 83 |
| Number of | Number of securities | |||||||||||
| securities to be | Weighted-average | remaining available | ||||||||||
| issued upon | exercise | for future issuance | ||||||||||
| exercise of | price of | under equity | ||||||||||
| outstanding | outstanding | compensation plans | ||||||||||
| options, | options, | (excluding securities | ||||||||||
| warrants and | warrants and | reflected in column | ||||||||||
| Plan Category | rights | rights | (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | 660,000 | $ | 14.81 | 840,000 | ||||||||
| Equity compensation plans not approved by security holders | - | - | - | |||||||||
| Total | 660,000 | $ | 14.81 | 840,000 | ||||||||
At March 31, 2012 and 2011, outstanding options were as follows:
| Number of | Weighted | |||||||
| Shares under | Average | |||||||
| Options | Exercise Price | |||||||
| Options outstanding at April 1, 2010 | - | $ | - | |||||
| Options granted | 910,000 | 14.81 | ||||||
| Options expired or forfeited | - | - | ||||||
| Options exercised | - | - | ||||||
| Options outstanding at March 31, 2011 | 910,000 | 14.81 | ||||||
| Options granted | - | - | ||||||
| Options expired or forfeited | (250,000 | ) | 14.81 | |||||
| Options exercised | - | - | ||||||
| Options outstanding at March 31, 2012 | 660,000 | $ | 14.81 | |||||
The following table summarizes information about options outstanding at March 31, 2012:
| Options Outstanding | Options Exercisable | |||||||||||||||||||||
| Number of | Weighted average | Weighted | Number of | Weighted | ||||||||||||||||||
| Exercise | shares | remaining contractual life | Average | shares | Average | |||||||||||||||||
| price | under Option | (years) | Exercise Price | under Option | Exercise Price | |||||||||||||||||
| $ | 14.81 | 660,000 | 7.15 | $ | 14.81 | 165,000 | $ | 14.81 | ||||||||||||||
At March 31, 2012 and 2011, the options outstanding and exercisable had no intrinsic value.
ITEM 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS AND DIRECTOR INDEPENDENCE
As of March 31, 2012 and 2011, the amount due to a director, Mr. Song Jinan, represented advances to the Company which are unsecured, interest-free and repayable on demand. The advances from such director were entered into because of the difficulty the Company faced in paying expenses incurred outside China in non-PRC currency due to the difficulties the Company had in converting currency because of foreign exchange controls.
Any transaction we enter into with any related party is required to be made on terms no less favorable to us than could have been obtained from unaffiliated third parties and the terms of any such transaction are reviewed by our Audit Committee and approved by a majority of our independent directors.
Dr. Chin Ji Wei, Dr. Du Wen Min and Mr. Ivan Chu are independent directors under the independence definitions established by the SEC, the American Stock Exchange and NASDAQ.
ITEM 14. PRINCIPAL ACCOUNTANT FEES AND SERVICES
Set forth below is a summary of the fees we paid our principal auditor for professional services rendered for the years ended March 31, 2012 and 2011. All of the audit fees were approved by the board of directors acting as the Company’s Audit Committee.
Audit Fees
The aggregate fees billed for professional services rendered by Weinberg & Company, P.A. for the audit of our annual financial statements and SEC filings for the fiscal years ended March 31, 2012 and 2011 were $600,000.
| 84 |
The aggregate fees billed for professional services rendered by BDO Limited for the audit of our annual financial statements and review of financial statements and SEC filings for the fiscal year ended March 31, 2011 was $356,000.
Audit-Related Fees
During the year ended March 31, 2012, our current principal accountant did not render assurance and related services reasonably related to the performance of the audit or review of financial statements.
Tax Fees
During the year ended March 31, 2012, our current principal accountant did not render assurance and related services reasonably related to the performance of the audit or review of financial statements.
All Other Fees
During the year ended March 31, 2012, there were no fees billed for products and services provided by the current principal accountant other than those set forth above.
Policy on Audit Committee Pre-Approval of Audit and Audit-Related Services of Independent Auditors
Our Audit Committee pre-approves all audit and audit-related services provided by the independent auditors. On an ongoing basis, management communicates specific projects and categories of services for which advance approval of the board of directors is requested. The Audit Committee reviews these requests and makes a recommendation to the board of directors. The board of directors then advises management whether it has approved the engagement of the independent auditors for specific projects.
ITEM 15. EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)(1) Financial Statements.
| Report of Independent Registered Public Accounting Firm | 49 |
| Audited Consolidated Financial Statements | |
| Consolidated Balance Sheets as of March 31, 2012 and 2011 | 50 |
| Consolidated Statements of Operations and other comprehensive income for the years ended March 31, 2012 and 2011 | 51 |
| Consolidated Statements of Changes in Stockholders’ Equity for the years ended March 31, 2012 and 2011 | 52 |
| Consolidated Statements of Cash Flow for the years ended March 31, 2012 and 2011 | 53 |
| Notes to the Consolidated Financial Statements | 54 |
| Schedule I - Condensed Parent Company Financial Statements as of and for the years ended March 31, 2012 and 2011 | 69 |
| 85 |
(b) Exhibits.
| Number | Exhibit | |
| 3.1 | Amended and Restated Certificate of Incorporation (incorporated by reference to Exhibit 3.1 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006). | |
| 3.2 | Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006) as amended by the Amendment to the Amended and Restated Bylaws (incorporated by reference to Exhibit 3.2 to China-Biotics, Inc.’s Form 10-Q filed on November 10, 2008). | |
| 10.1 | Securities Exchange Agreement dated March 22, 2006 (incorporated by reference to Exhibit 10.1 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006). | |
| 10.2 | Form of Lockup Agreement dated March 22, 2006 (incorporated by reference to Exhibit 10.2 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006). | |
| 10.3 | Put Agreement dated March 22, 2006 (incorporated by reference to Exhibit 10.3 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006). | |
| 10.4 | Registration Rights Agreement dated March 22, 2006 (incorporated by reference to Exhibit 10.4 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006). | |
| 10.5 | Investors’ Rights Agreement dated March 22, 2006 (incorporated by reference to Exhibit 10.5 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006). | |
| 10.6 | Stan Ford Agreement dated March 22, 2006 (incorporated by reference to Exhibit 10.6 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006). | |
| 10.7 | Summary of English translation of Investment Agreement for lease of land dated March 21, 2006 (incorporated by reference to Exhibit 10.7 to China-Biotics, Inc.’s Form 8-K filed on March 23, 2006). | |
| 10.8 | Escrow Agreement dated March 22, 2006 (incorporated by reference to Exhibit 10.8 to China-Biotics, Inc.’s Form 10-KSB filed on June 30, 2006). | |
| 10.9 | Stock Purchase Agreement with Fred Cooper dated February 6, 2006 (incorporated by reference to Exhibit 10.9 to China-Biotics, Inc.’s Form 10-KSB filed on June 30, 2006). | |
| 10.10 | Loan agreement dated as of September 22, 2005 (incorporated by reference to Exhibit 10.10 to China-Biotics, Inc.’s Form 10-KSB filed on June 30, 2006). | |
| 10.11 | Convertible Bond dated as of September 22, 2005 (incorporated by reference to Exhibit 10.11 to China-Biotics, Inc.’s Form 10-KSB filed on June 30, 2006). | |
| 10.12 | Subscription Agreement dated as of September 22, 2005 (incorporated by reference to Exhibit 10.12 to China-Biotics, Inc.’s Form 10-KSB filed on June 30, 2006). | |
| 10.13 | English Translation of Equity Transfer Agreement dated August 11, 2005 (incorporated by reference to Exhibit 10.13 to China-Biotics, Inc.’s Form SB-2/A filed on April 27, 2007). | |
| 10.14 | English Translation of Subscription Agreement dated August 11, 2005 (incorporated by reference to Exhibit 10.13 to China-Biotics, Inc.’s Form SB-2/A filed on April 27, 2007). | |
| 10.15 | Investment Agreement dated December 11, 2007 (incorporated by reference to Exhibit 10.1 to China-Biotics, Inc’s Form 8-K filed on December 12, 2007). | |
| 10.16 | Registration Rights Agreement dated December 11, 2007 (incorporated by reference to Exhibit 10.2 to China-Biotics, Inc.’s Form 8-K on December 12, 2007). | |
| 10.17 | 4% Senior Convertible Promissory Note dated December 11, 2007 (incorporated by reference to Exhibit 10.3 to China-Biotics, Inc.’s Form 8-K filed on December 12, 2007). |
| 86 |
| Number | Exhibit | |
| 10.18 | Guaranty by Song Jinan in favor of Pope Investments II LLC dated December 11, 2007 (incorporated by reference to Exhibit 10.5 to China-Biotics, Inc.’s Form 8-K filed on December 12, 2007). | |
| 10.19 | Pledge Agreement between Song Jinan and Pope Investments II LLC dated December 11, 2007 (incorporated by reference to Exhibit 10.5 to China-Biotics, Inc.’s Form 8-K filed on December 12, 2007). | |
| 10.20 | Form of Purchase Agreement dated January 21, 2009 (incorporated by reference to Exhibit 10.15 to China-Biotics, Inc.’s Form 10-Q filed on February 13, 2009). | |
| 10.21 | Form of Purchase Agreement dated May 19, 2009 (incorporated by reference to Exhibit 10.1 to China-Biotics, Inc.’s Form 8-K filed on May 20, 2009). | |
| 10.22 | Share Charge dated September 21, 2009 (effective as of January 24, 2008) (incorporated by reference to Exhibit 10.22 to China-Biotics, Inc.’s Form 10-Q filed on November 16, 2009). | |
| 10.23 | Underwriting Agreement dated September 29, 2009 (incorporated by reference to Exhibit 1.1 to China-Biotics, Inc.’s Form 8-K filed on September 30, 2009). | |
| 10.24 | District Entrance Project Agreement dated August 12, 2010 (incorporated by reference to Exhibit 10.19 to China-Biotics, Inc.’s Form 10-Q filed on February 14, 2011). | |
| 10.25 | 2010 Equity Incentive Plan (incorporated by reference to Exhibit 4.1 to China-Biotics, Inc.’s Registration Statement on Form S-8 filed on March 11, 2011). | |
| 14.1 | Code of Ethics (incorporated by reference to Exhibit 14.1 to China-Biotics, Inc.’s Form 10-KSB for the year ended March 31, 2006). | |
| 16.1 | Letter dated April 13, 2006 from Malone & Bailey PC to the United States Securities and Exchange Commission (incorporated by reference to Exhibit 16.1 to China-Biotics, Inc.’s Form 8-K filed on May 31, 2006). | |
| 16.2 | Resignation Letter of BDO Limited, dated June 22, 2011 (incorporated by reference to Exhibit 16.1 to China-Biotics, Inc.’s Form 8-K filed on June 23, 2011). | |
| 21.1 | List of subsidiaries | |
| 23.1 | Letter from BDO Limited, dated June 23, 2011, to the United States Securities and Exchange Commission (incorporated by reference to Exhibit 16.2 to China-Biotics, Inc.’s Form 8-K filed on June 23, 2011). | |
| 23.2 | Letter from Thornhill Capital LLC to the United States Securities and Exchange Commission, dated September 10, 2012. | |
| 31.1 | Certification of CEO pursuant to Rule 13a-14(a)/15(d)-14(a). | |
| 31.2 | Certification of CFO pursuant to Rule 13a-14(a)/15(d)-14(a). | |
| 32.1 | Certification of CEO pursuant to Section 1350. | |
| 32.2 | Certification of CFO pursuant to Section 1350. |
| 87 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on September 14, 2012.
| CHINA-BIOTICS, INC. | ||
| By: | /s/ Song Jinan | |
| Mr. Song Jinan | ||
| Chairman of the Board, Chief Executive Officer, | ||
| Treasurer and Secretary (Principal Executive | ||
| Officer) | ||
Pursuant to the requirements of Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities indicated on September 14, 2012.
| /s/ Song Jinan | Chairman of the Board and Chief Executive Officer | |
| Song Jinan | (Principal Executive Officer) | |
| /s/ Yan Yihong | Chief Financial Officer | |
| Yan Yihong | (Principal Financial and Accounting Officer) | |
| /s/ Chin Ji Wei | Director | |
| Chin Ji Wei | ||
| /s/ Du Wen Min | Director | |
| Du Wen Min | ||
| /s/ Ivan Chu | Director | |
| Ivan Chu |
| 88 |
