Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Orexigen Therapeutics, Inc. | d406133d8k.htm |
 Exhibit 99.1
September 2012 |
 2
Forward-Looking Statements
Forward-Looking Statements
This presentation contains forward-looking statements about Orexigen Therapeutics, Inc.
Words such as "believes," "anticipates," "plans,"
"expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed,“ “probability” and
similar expressions are intended to identify forward-looking statements. These statements
are based on the Company's current beliefs and expectations. These forward-looking
statements include statements regarding the timing of the enrollment of the
Contrave® Light Study, the timing of a resubmission of the Contrave NDA, the Special
Protocol Assessment, or SPA, the cost of conducting the Light Study, the timing expected
for the interim analysis of the Light Study, the timing of FDA input for Empatic™,
the potential for, and timing of, approval for Contrave or Empatic, and the potential for the therapeutic or
commercial value of Contrave or Empatic. The inclusion of forward-looking statements should
not be regarded as a representation by Orexigen that any of its plans will be achieved.
Actual results may differ from those set forth in this presentation due to the risk and
uncertainties inherent in Orexigen’s business, including, without limitation: the SPA is not
binding on the FDA if public health concerns unrecognized at the time the SPA agreement was
entered into become evident, other new scientific concerns regarding product safety or
efficacy arise, or if Orexigen fails to comply with the agreed upon trial protocol; the
progress and timing of the Light Study; Orexigen's ability to demonstrate in the Contrave outcomes trial that the
risk of major adverse cardiovascular events in overweight and obese subjects treated with
Contrave does not adversely affect the product candidate's benefit-risk profile; the
potential that earlier clinical trials may not be predictive of future results in the
Light Study or in a Phase 3 trial for Empatic; the potential for adverse safety findings
relating to Empatic or Contrave to delay or prevent regulatory approval or
commercialization, or result in product liability claims; the potential for early termination of the
collaboration agreement between Orexigen and Takeda; the costs and time required to complete
additional clinical, non-clinical or other requirements prior to any resubmission of
an NDA for Contrave and the Phase 3 trials for Empatic; the therapeutic and commercial
value of Contrave or Empatic; Orexigen's ability to attract and retain key personnel; Orexigen's ability to maintain
sufficient capital; and other risks described in the Company's filings with the Securities and
Exchange Commission (SEC). You are cautioned not to place undue reliance on these
forward-looking statements, which speak only as of the date hereof, and Orexigen
undertakes no obligation to revise or update this presentation to reflect events or circumstances after the date hereof.
Further information regarding these and other risks is included under the heading "Risk
Factors" in Orexigen’s Quarterly Report on Form 10-Q, which was filed with
the SEC on August 9, 2012 and is available from the SEC's website (www.sec.gov) and on
Orexigen’s website (www.orexigen.com) under the heading "Investor Relations”. All
forward-looking statements are qualified in their entirety by this cautionary
statement. This caution is made under the safe harbor provisions of Section 21E of the Private
Securities Litigation Reform Act of 1995. |
 Large, growing
market Unmet need, limited
competition
Differentiated product
profile
Skilled partner with heavy
resourcing
Path to Commercial Success
Path to Commercial Success
Orexigen: Contrave
®
Poised to Deliver
Orexigen: Contrave
®
Poised to Deliver
Regulatory clarity on
the Light Study design
and potential for
approvability at interim
analysis
High probability of
technical success
Path to approval
Path to approval
3 |
 Path to
Approval Path to Approval
4 |
 5
The Light Study was designed to satisfy the single
approval deficiency identified in the CRL
CRL identified a single approval deficiency of theoretical CV risk
Complete NDA already reviewed by FDA
–
Efficacy threshold has been met
–
General safety & tolerability profile established
–
Positive Ad-Com
World-class advisors assembled to steer the study |
 6
The Light Study began enrolling patients June 2012
Protocol based on guidance from OND and conducted under a SPA
Contrave could be approved with a positive interim analysis at 87
MACE events
–
Must exclude a doubling of MACE at the interim analysis to be approved
–
Must exclude a 40% increase at the final analysis (371 events)
Enrollment update: Orexigen expects to complete enrollment of
patients
required
for
the
interim
analysis
in
the
fourth
quarter
of
2012 and to accrue 87 MACE in 2013 |
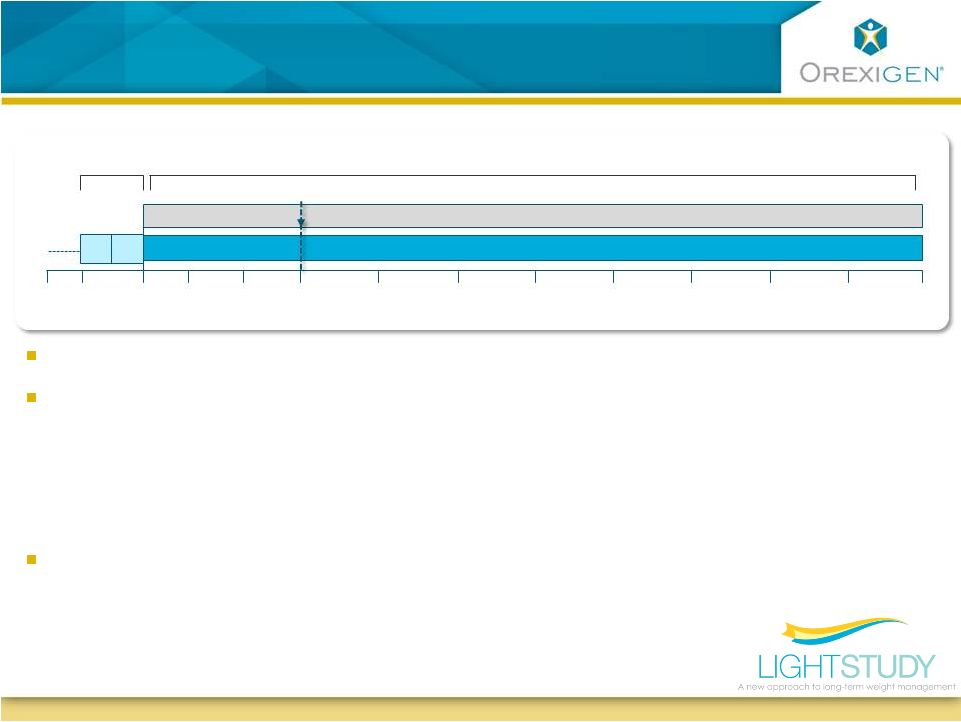 7
Light Study Design
Primary endpoint is ITT analysis of MACE (MI, stroke, CV death)
Targeting MACE event rate of 1.5% per year
Streamlined study -
large sample size, but limited information per subject
Contrave32 + WeightMate
Placebo + WeightMate
Wk 208
Wk 182
Wk 130
Wk 104
Wk 78
Wk 52
Wk 26
Wk 16
Wk 156
Wk 8
Wk 2
Day1
Wk -2
Evaluation of appropriateness of treatment
Lead-in
Treatment Period
–
Real-world
test
-
long term exposure to study drug only in patients demonstrating
early potential to benefit from therapy
–
Those with limited weight loss or increased blood pressure drop study drug at Week
16 but remain in ITT analysis
–
No ongoing labs, infrequent visits, minimal data collection
–
Total cost to interim analysis and approval is estimated at ~$100M
|
 8
Probability of Technical Success of the
Light Study is High
Hurdle for approval requires excluding a doubling of CV risk in non-
inferiority design, not demonstrating CV benefit
Bupropion well characterized in >50M patients over >25 years
–
Large patient registries and AERS have not identified evidence of
increased CV events
Risk Engine modeling of 1-year Contrave Phase 3 data predicts
reduced
10-year CV risk compared to placebo
Trial focus on responders to drug therapy allows real-world test
–
Long term exposure to study drug is only in those demonstrating early
potential to benefit from therapy |
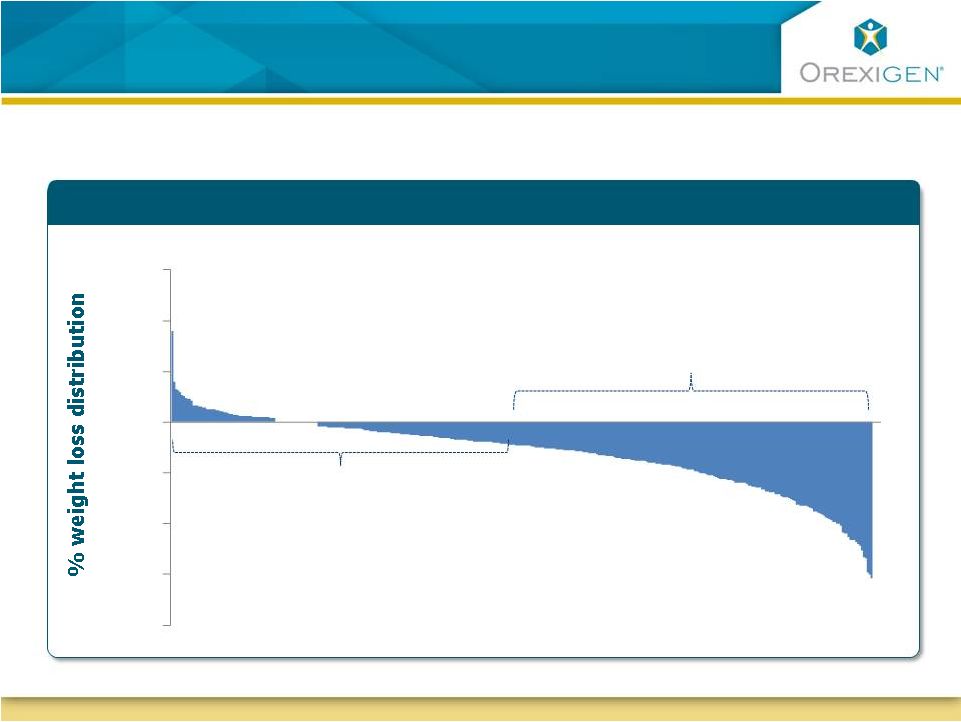 9
Our Focus is on Responders to Contrave Therapy
Our Focus:
Responders
Non-responders discontinue
drug therapy
0
10
20
30
Illustrative : Typical distribution of weight loss response to obesity therapy
-10
-20
-30
-40 |
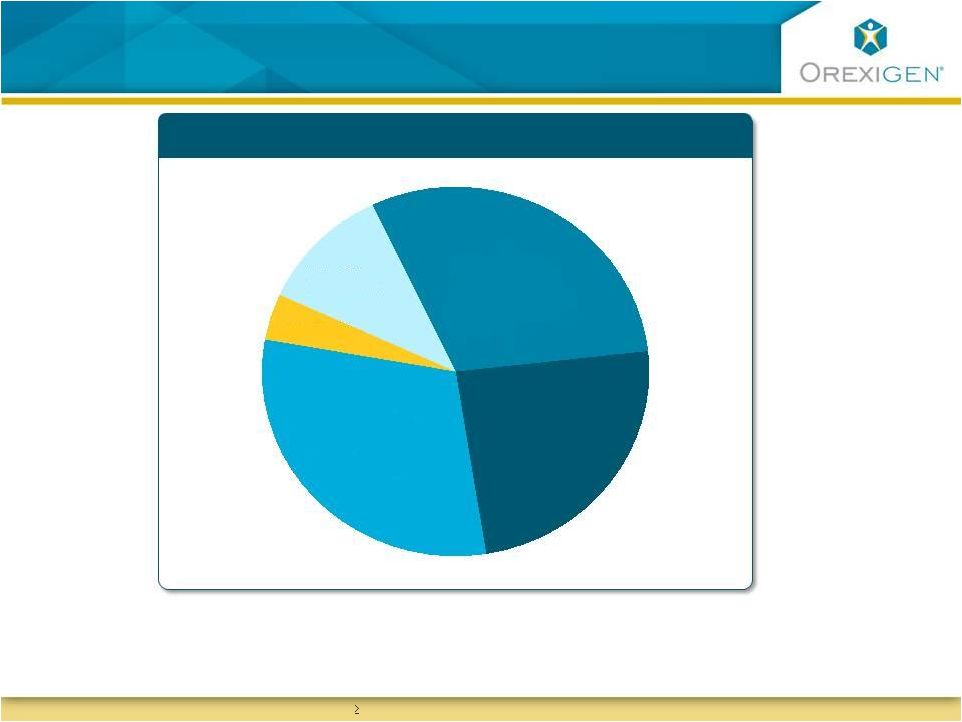 10
Responders Maintained Meaningful Weight Loss
Contrave Responders Achieved Significant Weight
Loss
Represents
one
year
data
for
responders
(patients
with
5%
weight
loss
at
week
16
per
recommended
treatment
protocol)
In the Contrave
®
clinical development program the most frequent treatment-emergent
adverse
events
on
Contrave
®
were
nausea,
constipation,
headache,
vomiting
and
dizziness. These were mostly mild to moderate in severity, transient, and typically
occurred during the first weeks of treatment.
5%
-
1
0%
Weight
Loss
Avg.
Wt
.
Loss
=
17
.0
lbs
10
%
-
1
5%
Weight
Loss
Avg.
Wt
.
Loss
=
2
6.7
lbs
>
1
5
%
Weight
Loss
Avg.
Wt
.
Loss
=
43
.9
lbs
Weight
Gain
0%
-
5%
Weight
Loss |
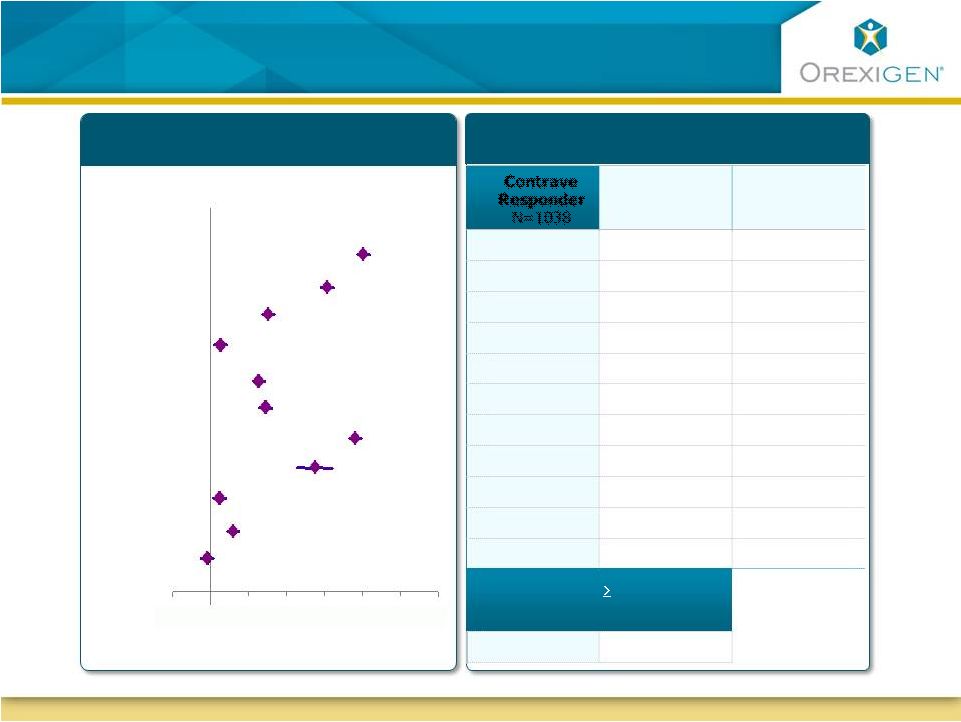 11
Contrave Responders Achieve Improvements Across
Multiple Cardiometabolic Parameters
Contrave Responders Achieve Improvements Across
Multiple Cardiometabolic Parameters
Responders Maintained Meaningful
Weight Loss
Contrave32 Standardized
Mean Change from Baseline at 1 Year
Change from Baseline at 1 Year
Body Weight
Waist Circ.
HDL
LDL
Triglycerides
hsCRP
IWQOL
HbA1c
SBP
DBP
Heart Rate
Improvement
(Standardized Mean Change from Baseline)
Contrave
Responder
N=1038
Placebo
Responder
N=254
All Placebo
N=1319
-11.3%
-8.6%
-2.4%
-9.5 cm
-7.2 cm
-3.5 cm
5.2 mg/dL
2.9 mg/dL
0.0 mg/dL
-2.5 mg/dL
1.7 mg/dL
0.0 mg/dL
-16.0%
-9.2%
-2.8%
-38.9%
-36.2%
-14.5%
14.7
12.3
8.3
-1.0%
-0.6%
-0.1%
-0.9 mm Hg
-2.8 mm Hg
-1.6 mm Hg
-1.6 mm Hg
-2.6 mm Hg
-1.3 mm Hg
0.2 bpm
-2.3 bpm
-0.2 bpm
Proportion w/
5% Wt Loss
Wk 16
51%
19%
-0.4
0
0.4
0.8
1.2
1.6
2
2.4 |
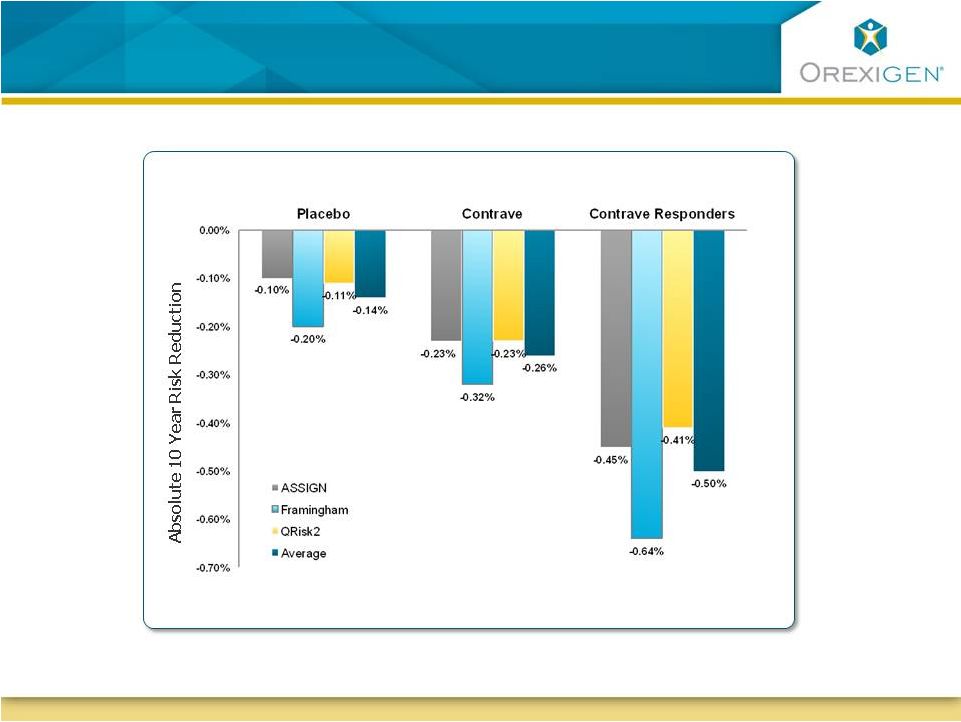 12
Risk Engine Modeling Suggests Long-term CVD Risk
Reduction on Contrave
Risk Engine Modeling Suggests Long-term CVD Risk
Reduction on Contrave |
 13
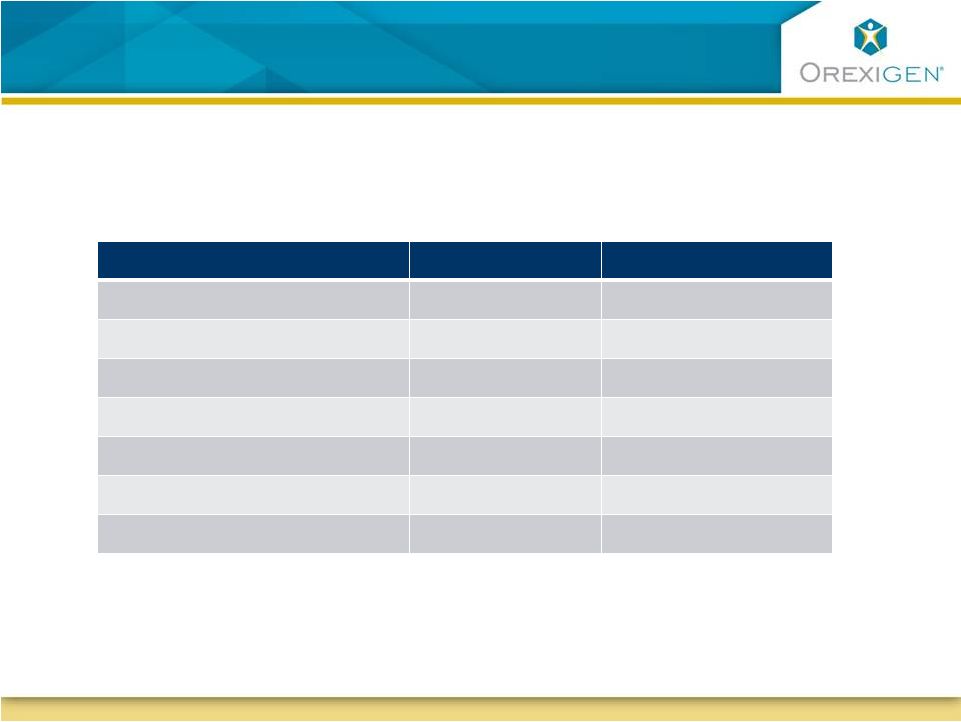
Patient demographic targets are based on
epidemiologic modeling and clinical trial experience
Patient demographic targets are based on
epidemiologic modeling and clinical trial experience
Target
Current (August)
Age
64
61
Younger Age Group
<25%
14%
Gender
50:50 (M:F)
44:56
Minority
15%
>20%
CV disease
30%
35%
Diabetes
>70%
87%
Smoking status
>5%
X
Orexigen is targeting to enroll a patient population with a modeled
background
annual
MACE
rate
of
>2%
expecting
to
observe
a
MACE
rate of ~1.5% |
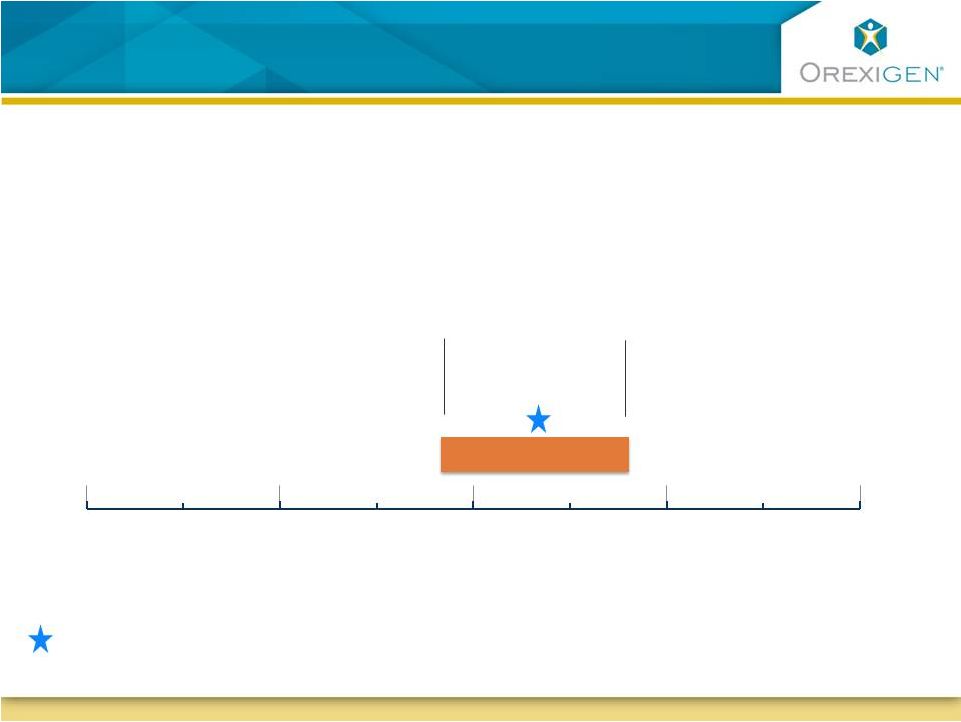 14
Precise timing of the 87
th
event dependent on
the actual observed event rate
Precise timing of the 87
th
event dependent on
the actual observed event rate
87
th
event
3Q 12 4Q 12 1Q 13
2Q 13
3Q 13
4Q 13
1Q 14
2Q 14
Timing of 87
th
event based on 1.5% event rate
2%
1.25%
Interim analysis triggered by official MACE adjudication
Assumed event rate (ER) |
 15
Path to Commercial
Path to Commercial
Success
Success |
 16
Contrave Poised for Commercial Success
Contrave Poised for Commercial Success
Blockbuster Building Blocks
Blockbuster Building Blocks
Market Size / Market Growth
Unmet Need / Limited Competition
Differentiated Product Profile
Heavy Resourcing |
 17
Obesity: A Large Untapped Market in Need of Solutions
Obesity: A Large Untapped Market in Need of Solutions
US Population
Source: CDC (2009)
~
1
in
3
People
in the US are
Obese
US Obesity Rate Growth
Source: CDC (2010)
The obesity epidemic is also expected to grow globally
n= ~685M people
n= ~484M people
Blockbuster Market Size
Blockbuster Market Growth |
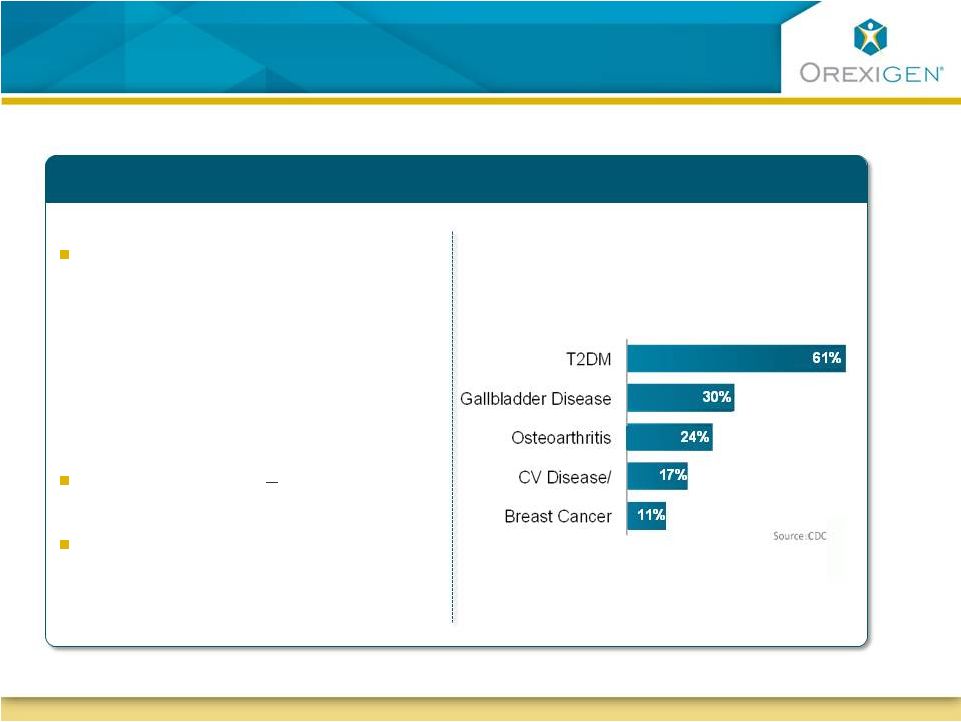 Significant
Unmet Need in the Market Significant Unmet Need in the Market
T2DM, 61% of which is
attributable to obesity, is an
epidemic
1 in 3 adults in the U.S. may
have diabetes by 2050 (CDC)
Associated with premature
death & physical/psychosocial
consequences
Weight loss of 5
10% confers
significant benefits
Large gaps in current treatment
paradigm
Serious Disease Attributable to Obesity
–
–
Treatment Options Needed
18 |
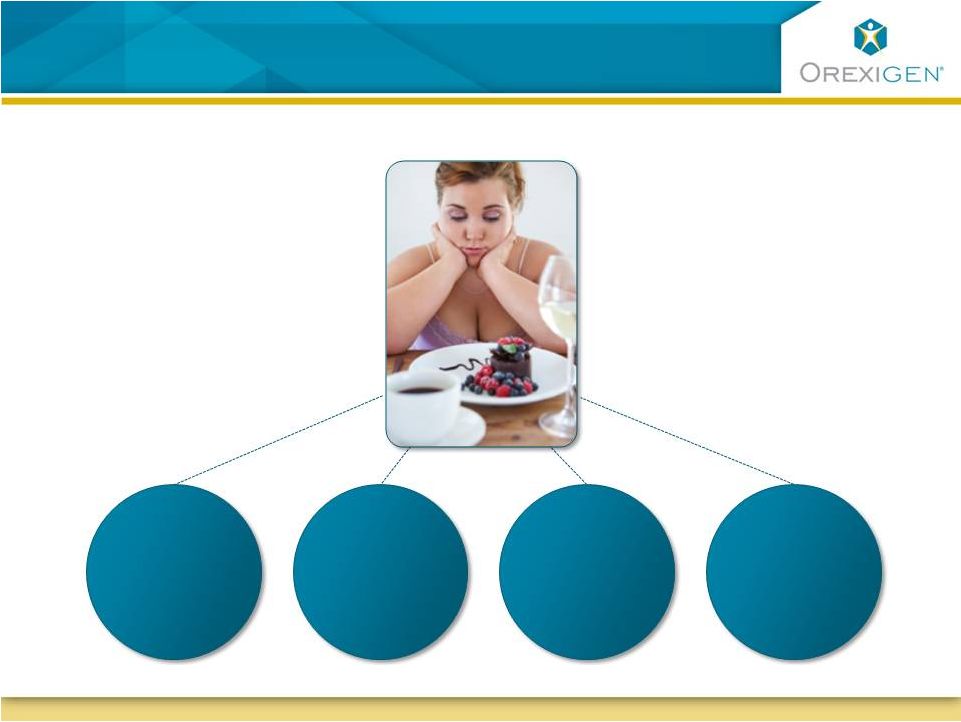 19
Contrave is Uniquely Positioned for Significant
Segments of the Obese Population
Contrave is Uniquely Positioned for Significant
Segments of the Obese Population
A Typical Obese Patient
Has diabetes
and/or
dyslipidemia
62
%
Has difficulty
controlling eating
in response to
food cravings
50
%
Is depressed or
has depressive
symptoms
63
%
Is female,
many of child
bearing age
72
% |
 20
Patients With Diabetes On Contrave Achieve
Improvements In Glycemic Control with Weight Loss
Patients With Diabetes On Contrave Achieve
Improvements In Glycemic Control with Weight Loss
As little as 5 -
10% weight loss
has been shown to reduce
mortality risk by ~25%
Half the Contrave patients who
completed therapy lost
5%
body weight
Half the Contrave patients who
completed therapy achieved
ADA HbA1c guideline of <7%
Patients on Contrave
experienced a reduction in
rescue medications
Source: Williamson, Diabetes Care, Vol 23, No. 10 Oct (2000)
HbA1c, change from baseline
ITT-LOCF (Mean baseline A1C = 8.0%) |
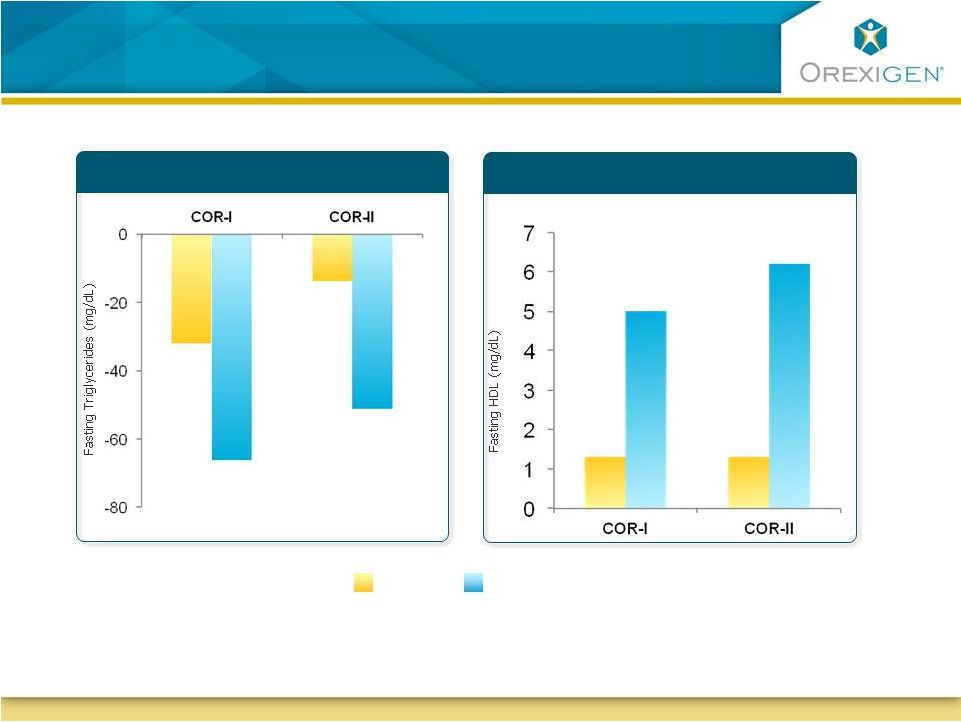 21
Patients with Dyslipidemia Experienced Clinically
Significant Improvement in Lipid Profiles
Patients with Dyslipidemia Experienced Clinically
Significant Improvement in Lipid Profiles
Placebo
P < 0.05
P < 0.05
P < 0.05
P < 0.05
Data is for completers in high-risk subgroups. High risk is defined by ATPIII Guidelines;
Baseline 200-222mg/dl for TGs, 40-41 mg/dl for HDL
N=82
N=87
N=69
N=135
N=122
N=121
N=117
N=184
HDL Cholesterol
Triglycerides
Contrave32 |
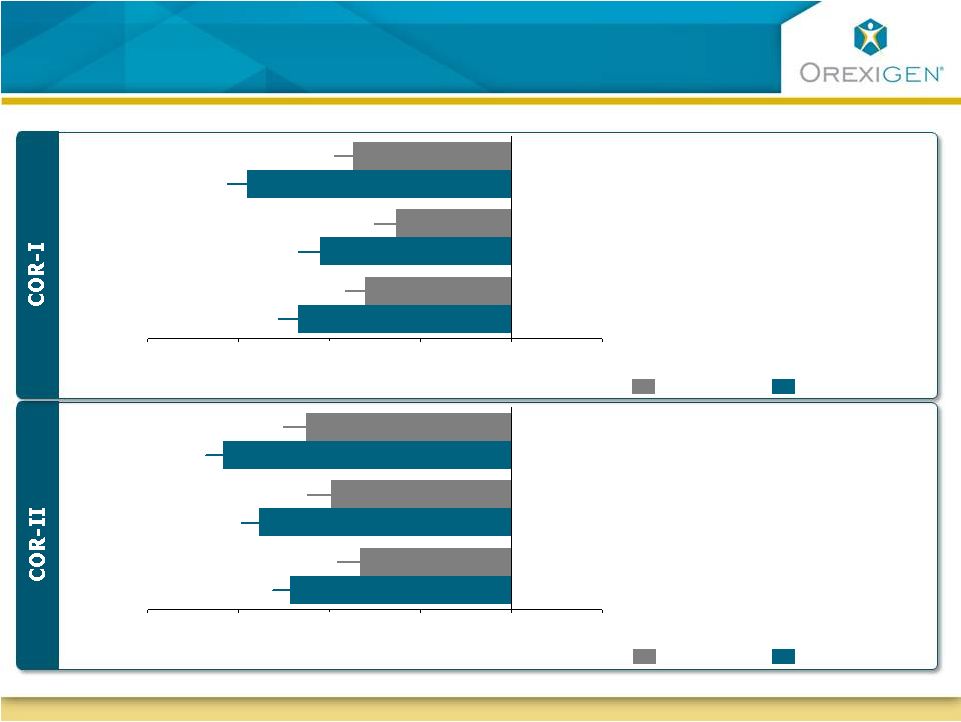  22
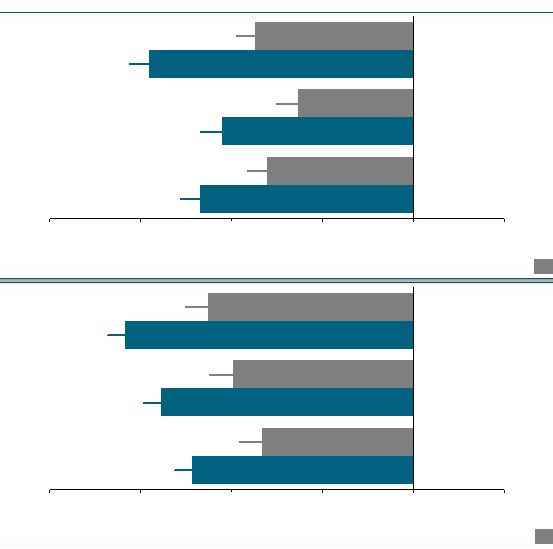
Patients Experienced Statistically Significant
Improvements in Eating Control
Patients Experienced Statistically Significant
Improvements in Eating Control
-20
-15
-10
-5
0
5
*
*
*
Change from baseline (mm)
-20
-15
-10
-5
0
5
*
*
*
Change from baseline (mm)
Less
More
Placebo (N=511)
Contrave32 (N=471)
How difficult has it been to control your eating?
How often have you eaten in response to food cravings?
How difficult has it been to resist any food cravings?
How difficult has it been to control your eating?
How often have you had food cravings?
How difficult has it been to resist any food cravings?
*P<0.05 vs. Placebo; ITT-LOCF; results were measured based on
measurements using 100mm VAS scale Less
More
Placebo (N=456)
Contrave32 (N=702) |
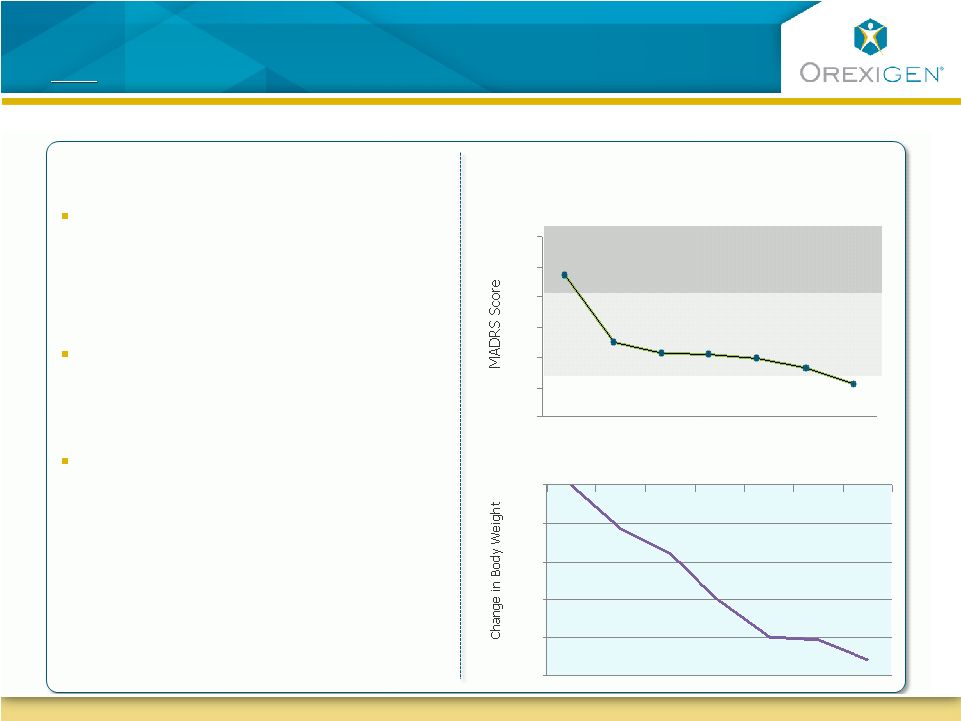 Depressed
Patients Experienced Significant Weight Loss and
Improvements in Depressive Symptoms
Depressed Patients Experienced Significant Weight Loss
and
Improvements in Depressive Symptoms
In a 24-week Phase 2 open-label trial
of obese depressed patients,
Contrave significantly improved
measures of depression
At the same time,
patients who
completed the trial lost over 9%
of their body weight
In addition, in a Control of Eating
Questionnaire, patients reported
decreases in hunger and cravings,
and less difficulty resisting cravings
Week 0
(N=23)
Week 12
(N=14)
Week 24
(N=13)
Not depressed
Mildly depressed
Moderately depressed
Montgomery Asberg Depression Rating Scale
(Observed Case)
Week 0
Week 12
Week 24
Change in Body Weight
(Observed Case)
Trial included 25 obese patients that met DSM-IV criteria for major depression.
0
5
10
15
20
25
30
-10%
-8%
-6%
-4%
-2%
0%
23 |
 24
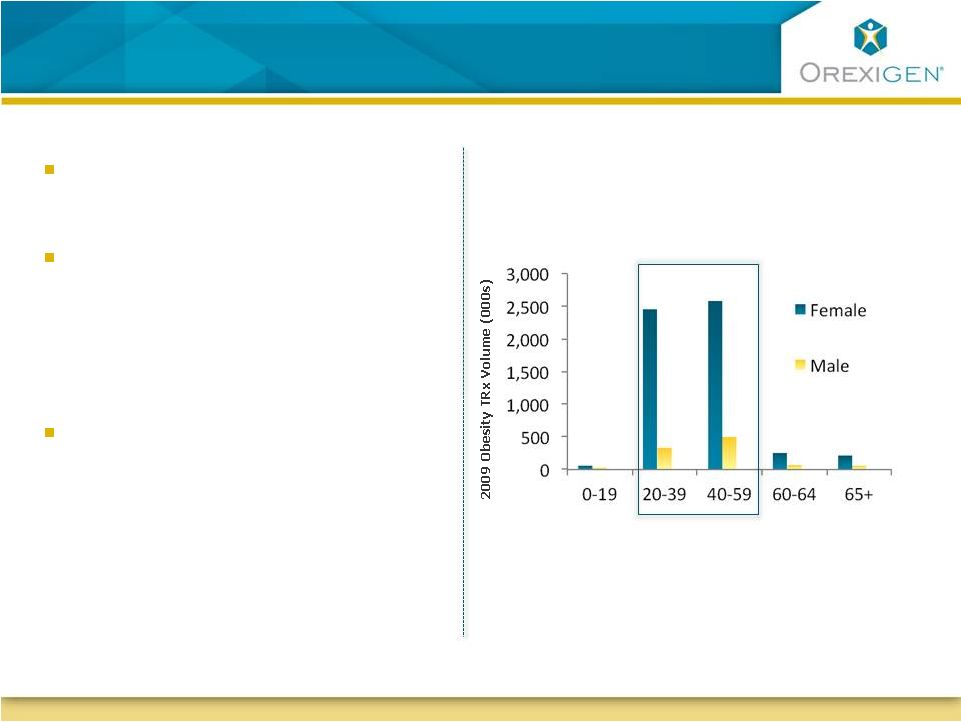
Contrave May Be The Logical Treatment Choice for
Women
Contrave May Be The Logical Treatment Choice for
Women
Source: Orexigen market research; IMS Health, National Disease and
Therapeutic Index, Obesity (2010) 18: 347-353 Women seek obesity therapy
more than men
Women tend to crave food more
than men, and younger women
tend to crave more than older
women
(Pelchat, 1997)
Female gender is one of the few
factors that has been consistently
associated with an increased risk
of depression among obese
individuals
(Ma, 2010)
Majority of the obesity Rxs today go to
women of child-bearing age
Age (years) |
 25
Empatic™
is Following Behind Contrave Providing a
Different Tool for the Treatment of Obesity
Empatic™
is Following Behind Contrave Providing a
Different Tool for the Treatment of Obesity
Empatic™
(zonisamide SR / bupropion SR) works by stimulating POMC
and inhibiting AGRP/NPY
–
Enhances 5HT and DA release leading to synergistic effects on POMC
firing
Associated with robust effects on weight loss in Phase 2
Different profile of constituent drugs provides the potential for
alternative, complementary positioning vs. Contrave |
 26
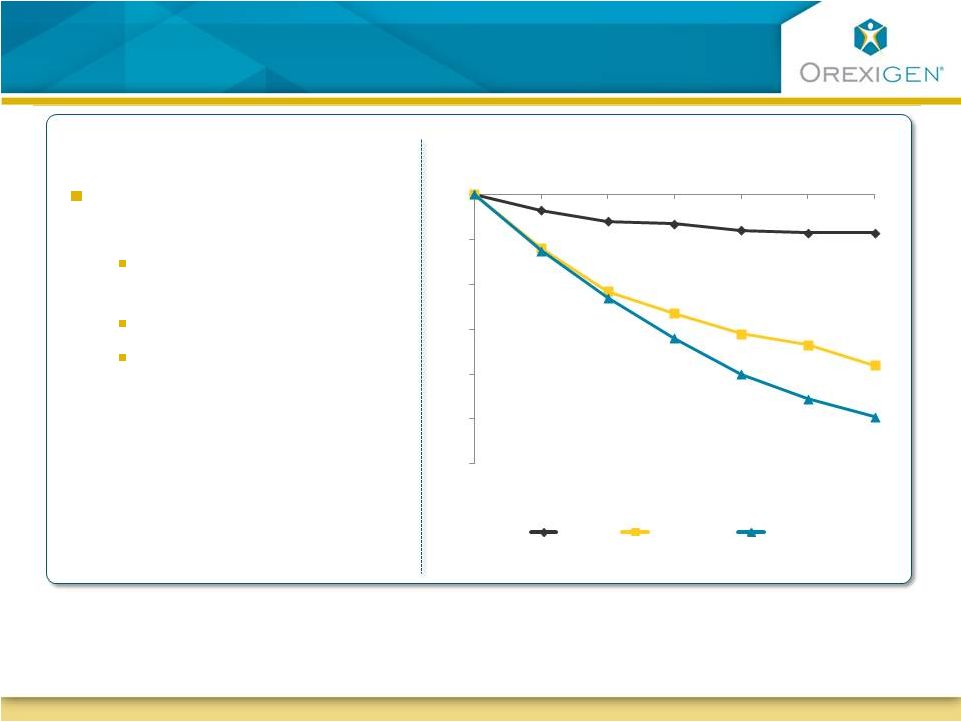
Empatic Demonstrates Early and Progressive
Weight Loss
Empatic Demonstrates Early and Progressive
Weight Loss
The most frequently reported adverse events in patients treated with Empatic were
headache, insomnia, nausea and constipation. The most common reasons for
discontinuation from treatment were insomnia, headache, irritability and nausea.
At 24 weeks, Empatic
demonstrates:
Clinically meaningful
weight loss
No evidence of plateau
Both Empatic doses
appear efficacious and
could provide dosing
flexibility
Observed Case Analysis
-12%
-10%
-8%
-6%
-4%
-2%
0%
4
8
12
16
20
24
Placebo
Empatic120
Empatic360
-9.9%
-7.6%
-1.7%
Study Week
Note :
All differences between Empatic and placebo statistically significant at all time points |
 27
In a Phase IIa Empatic clinical trial, completers
demonstrated 15% weight loss at one year
In a Phase IIa Empatic clinical trial, completers
demonstrated 15% weight loss at one year
Completers
Modified
ITT-LOCF
Week
LS mean ±SE
ZB-201 through Week 48
Placebo (N=72 through Week 24)
ZB360 (N=66 through Week 24; N=39 from Week 28 through
48 )
18
15
12
9
6
3
0
0
4
8
12
16
20
24
28
32
36
40
44
48
52
-
-
-
-
-
- |
 28
Commercialization plans
Commercialization plans
North American Contrave rights
$50mm upfront to Orexigen
$1 billion milestones including
$100mm before first sale
Tiered royalties on net sales
from 20 –
35%
Takeda has multi-year contractual
promotional requirements
Orexigen retains a right to co-
promote, paid for by Takeda
under certain circumstances
Heavy Resourcing for Contrave:
Takeda is an ideal partner
Licensing terms:
SCALE
Top 15 Pharma
Significant U.S.
Presence
Primary Care Focus
COMMITMENT TO
OBESITY
Partnership
with Amylin
Stated
Strategy
DOMAIN
EXPERIENCE
Leader in
Cardiometabolic
Care
Excellence in
Life Cycle
Management
SUCCESSFUL
BRAND BUILDER
Actos ~$3B in U.S.
Sales in 2009
Prevacid ~$3B in
U.S. Sales in 2009
•
ROW Strategy: Partner Contrave and Empatic
•
Orexigen plans to keep U.S. Empatic rights |
 29
Attractive Value Proposition for Orexigen
Attractive Value Proposition for Orexigen
Significant revenue potential projected from lead product, Contrave
Retained ROW rights for future catalysts and upside
Sufficient cash (~$126.2m as of June 30, 2012) to fund operations
through Contrave NDA resubmission
World-class clinical advisory board to steer the Light Study
Large capable partner in Takeda responsible for North American
commercialization
Maintain global rights to second product candidate, Empatic, which
has completed Phase II
Focused, experienced and capable team |
 30 |
