Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a12-19541_28k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a12-19541_2ex99d1.htm |
Exhibit 99.2
|
|
A dual – acting muscarinic antagonist, beta 2 – agonist (MABA) molecule (GSK961081) improves lung function in COPD: A randomised trial Pascal Wielders*, Andrea Ludwig-Sengpiel, Nicholas Locantore, Suus Baggen, Robert Chan and John H. Riley * Catharina Hospital Eindhoven, The Netherlands, on behalf of The Investigators The study was registered on the Clinical Trials Register NCT01319019, used the study code MAB115032 and was funded by GlaxoSmithKline. My institution has received grants from GSK, AstraZeneca and Novartis Speakers fee from GSK, AstraZeneca, Boehringer Ingelheim, Novartis |
|
|
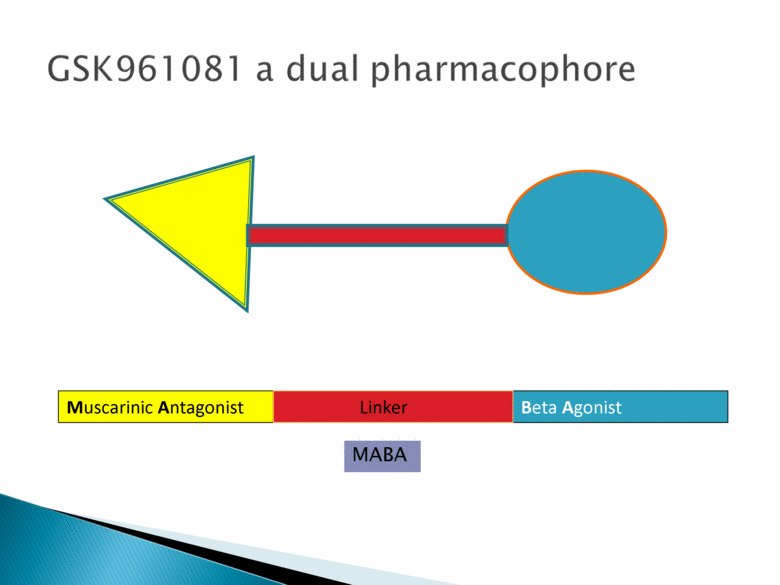
GSK961081 a dual pharmacophore Beta Agonist Muscarinic Antagonist Linker MABA |
|
|
Study aims To assess the safety and efficacy of GSK961081 in moderate and severe COPD subjects To assess the dose and dosing regimen MAB115032 – Design. A phase IIb multicentre, randomised, double-blind, double-dummy, placebo- and active-controlled, parallel-group, dose-ranging and dose-interval study. |
|
|
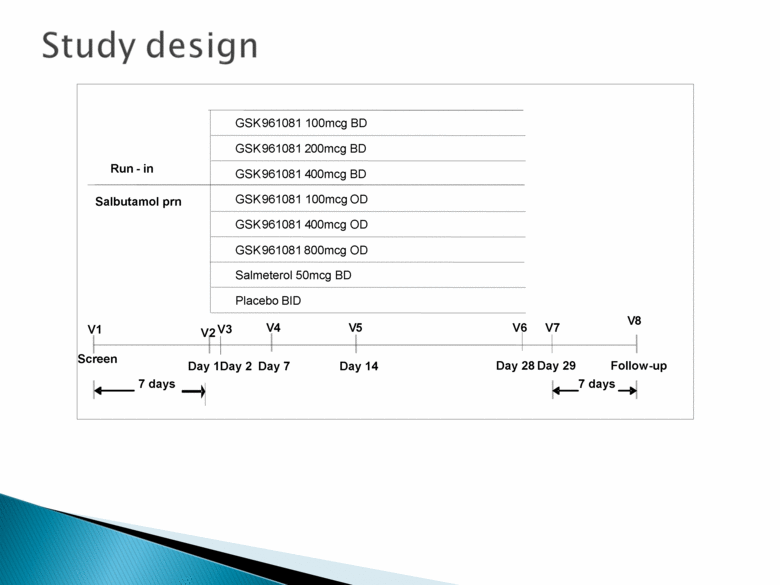
Study design Placebo BID Salmeterol 50mcg BD GSK961081 100mcg BD GSK961081 200mcg BD GSK961081 400mcg BD GSK961081 100mcg OD GSK961081 400mcg OD GSK961081 800mcg OD V1 Day 29 Day 14 Day 7 Day 1 Screen Run - in 7 days Follow-up 7 days V4 Salbutamol prn Day 28 V2 V6 V7 V8 V3 Day 2 V5 |
|
|
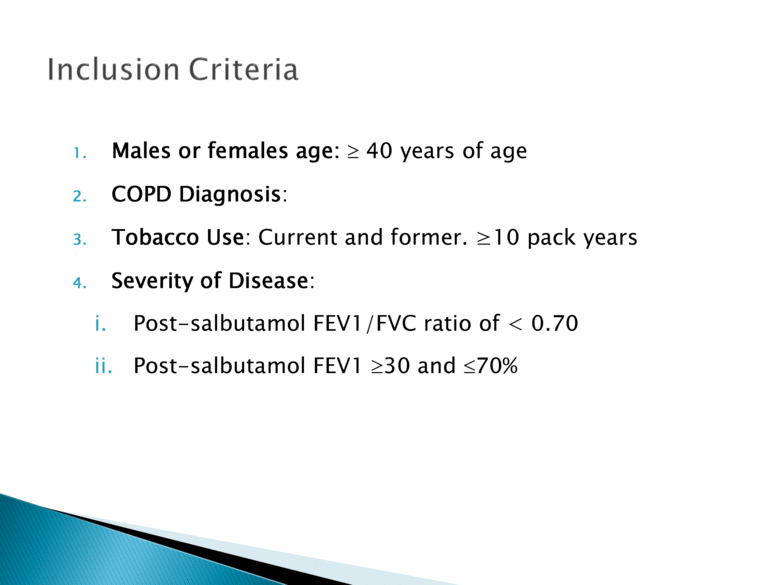
Males or females age: 40 years of age COPD Diagnosis: Tobacco Use: Current and former. >10 pack years Severity of Disease: Post-salbutamol FEV1/FVC ratio of < 0.70 Post-salbutamol FEV1 30 and 70% |
|
|
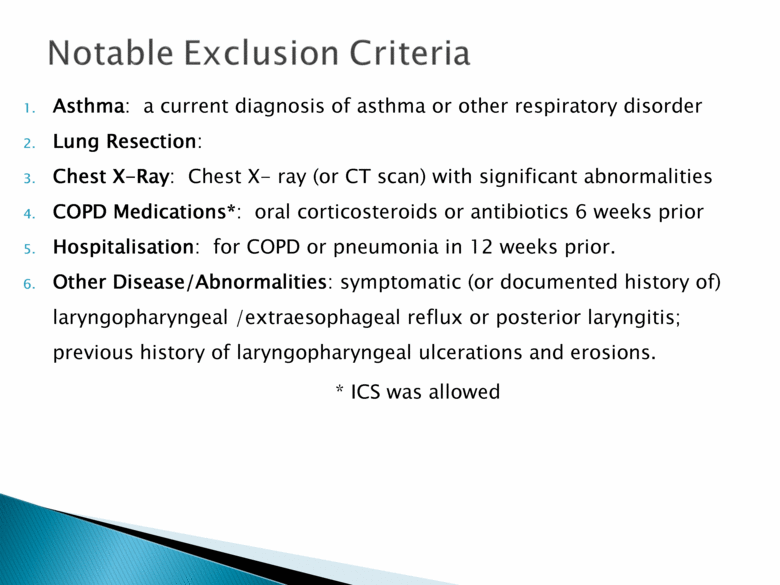
Asthma: a current diagnosis of asthma or other respiratory disorder Lung Resection: Chest X-Ray: Chest X- ray (or CT scan) with significant abnormalities COPD Medications*: oral corticosteroids or antibiotics 6 weeks prior Hospitalisation: for COPD or pneumonia in 12 weeks prior. Other Disease/Abnormalities: symptomatic (or documented history of) laryngopharyngeal /extraesophageal reflux or posterior laryngitis; previous history of laryngopharyngeal ulcerations and erosions. * ICS was allowed |
|
|
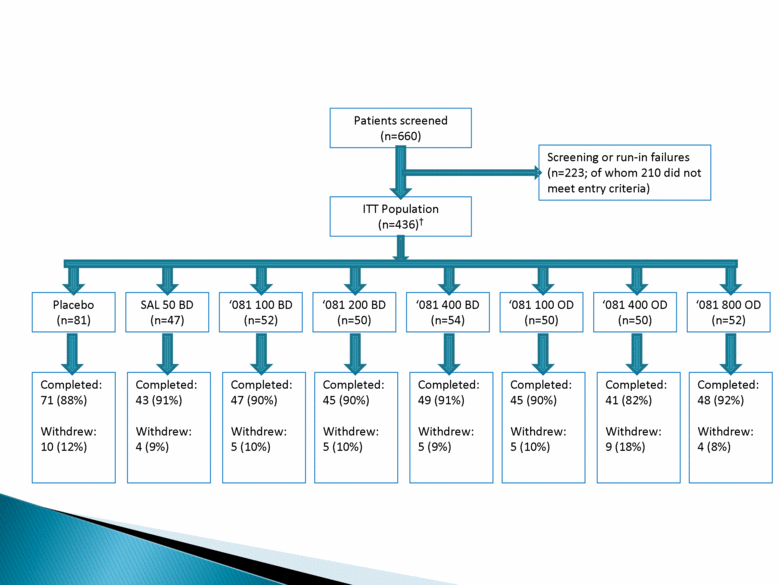
Patients screened (n=660) Screening or run-in failures (n=223; of whom 210 did not meet entry criteria) ITT Population (n=436)† Placebo (n=81) SAL 50 BD (n=47) ‘081 100 BD (n=52) ‘081 200 BD (n=50) ‘081 400 BD (n=54) ‘081 100 OD (n=50) ‘081 400 OD (n=50) ‘081 800 OD (n=52) Completed: 71 (88%) Withdrew: 10 (12%) Completed: 43 (91%) Withdrew: 4 (9%) Completed: 47 (90%) Withdrew: 5 (10%) Completed: 45 (90%) Withdrew: 5 (10%) Completed: 49 (91%) Withdrew: 5 (9%) Completed: 45 (90%) Withdrew: 5 (10%) Completed: 41 (82%) Withdrew: 9 (18%) Completed: 48 (92%) Withdrew: 4 (8%) |
|
|
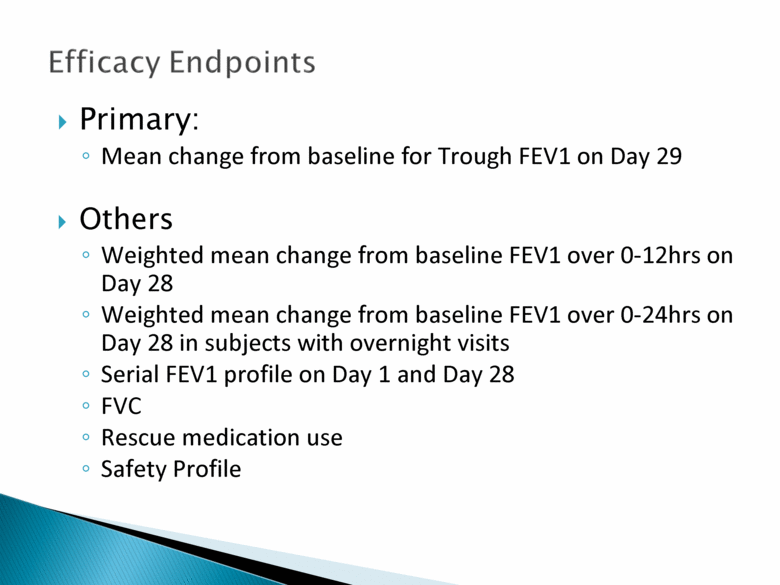
Efficacy Endpoints Primary: Mean change from baseline for Trough FEV1 on Day 29 Others Weighted mean change from baseline FEV1 over 0-12hrs on Day 28 Weighted mean change from baseline FEV1 over 0-24hrs on Day 28 in subjects with overnight visits Serial FEV1 profile on Day 1 and Day 28 FVC Rescue medication use Safety Profile |
|
|
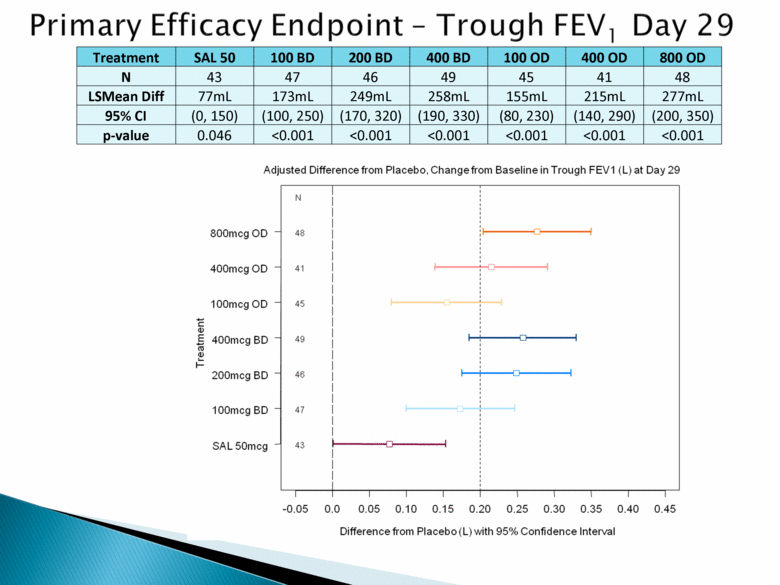
Treatment SAL 50 100 BD 200 BD 400 BD 100 OD 400 OD 800 OD N 43 47 46 49 45 41 48 LSMean Diff 77mL 173mL 249mL 258mL 155mL 215mL 277mL 95% CI (0, 150) (100, 250) (170, 320) (190, 330) (80, 230) (140, 290) (200, 350) p-value 0.046 <0.001 <0.001 <0.001 <0.001 <0.001 <0.001 |
|
|
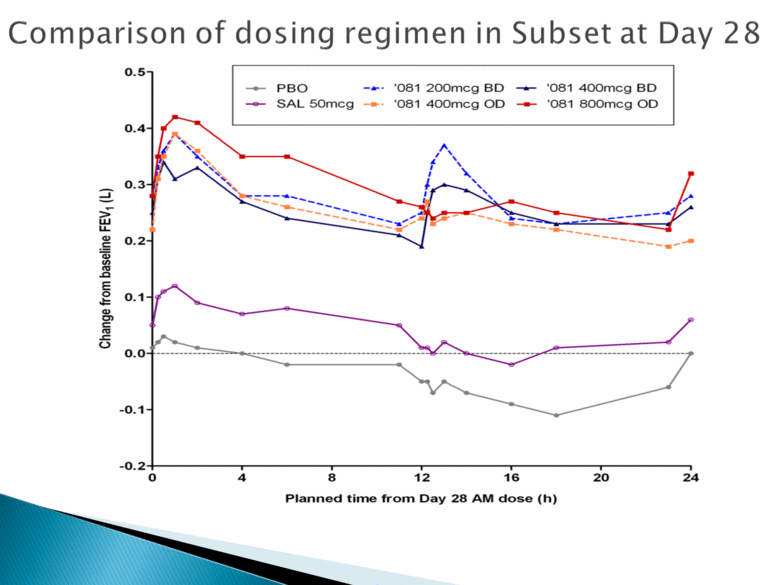
Comparison of dosing regimen in Subset at Day 28 PBO ‘081 200mcg BD ‘081 400mcg BD SAL 50mcg ‘081 400mcg OD ‘081 800mcg OD Change from baseline FEV1 (L) 0.5 0.4 0.3 0.2 0.1 0.0 -0.1 -0.2 0 4 8 12 16 20 24 Planned time from day 28 AM dose (h) |
|
|
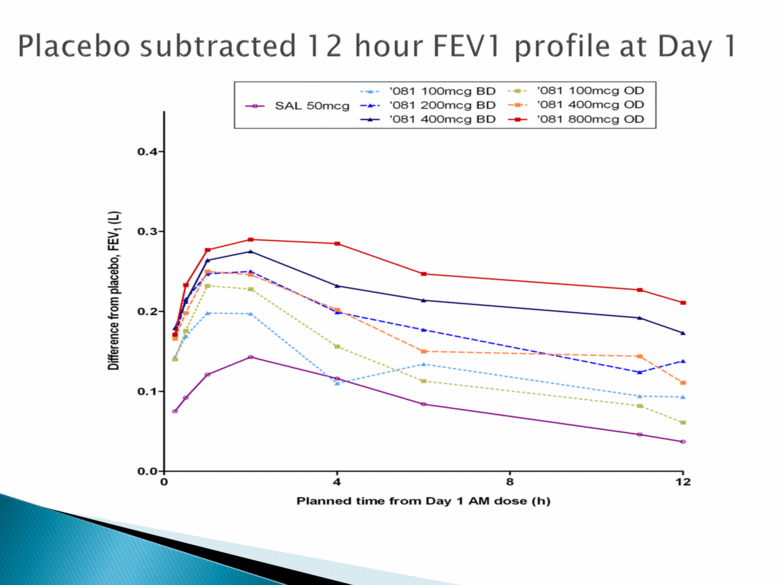
Placebo subtracted 12 hour FEVI profile at day 1 ‘081 100mcg BD ‘081 100mcg OD SAL 50mcg ‘081 200mcg BD ‘081 400mcg OD ‘081 400mcg BD ‘081 800mcg OD Difference from place placebo, FEV1 (L) 0.0 0.1 0.2 0.3 0.4 Planned time from Day 1 AM dose (h) 0 4 8 12 |
|
|
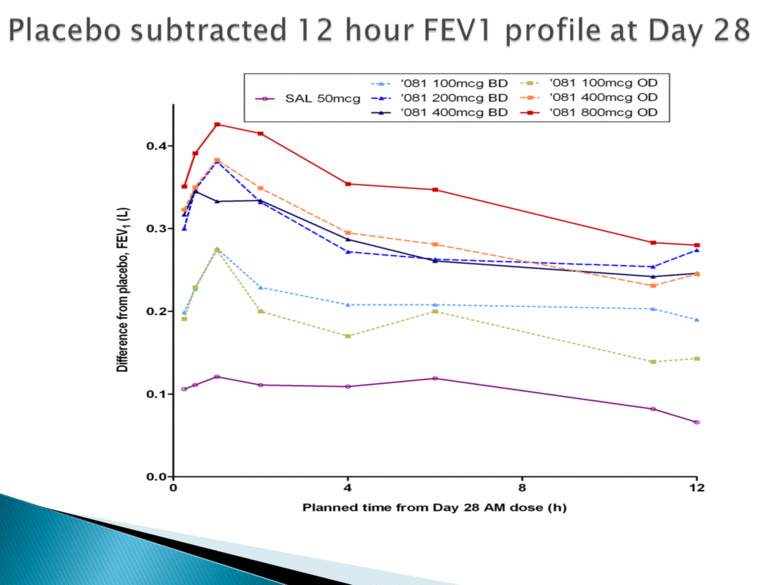
Placebo subtracted 12 hour FEVI profile at day 28 ‘081 100mcg BD ‘081 100mcg OD SAL 50mcg ‘081 200mcg BD ‘081 400mcg OD ‘081 400mcg BD ‘081 800mcg OD Difference from placebo, FEV1 (L) 0.0 0.1 0.2 0.3 0.4 Planned time from day AM dose (h) 0 4 8 12 |
|
|
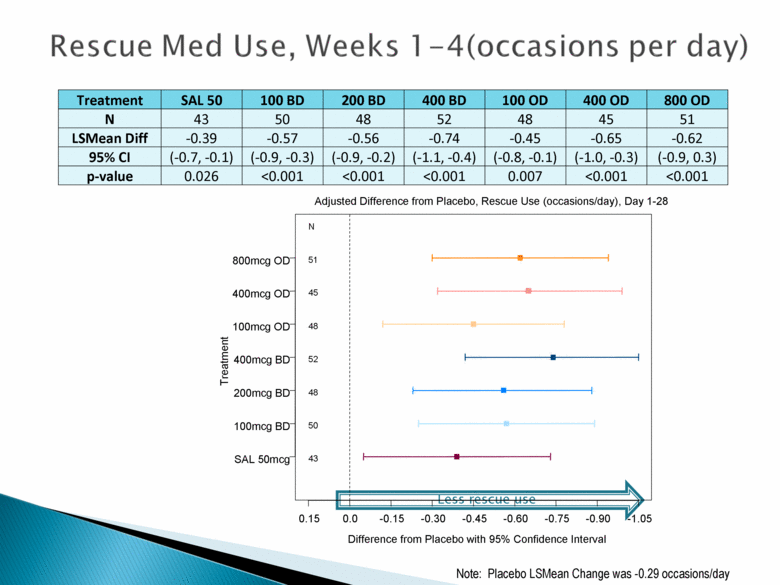
Rescue Med Use, Weeks 1 – 4 (occasion per day) Treatment SAL 50 100 BD 200 BD 400 BD 100 OD 400 OD 800 OD N 43 50 48 52 48 45 51 LSMean Diff -0.39 -0.57 -0.56 -0.74 -0.45 -0.65 -0.62 95% CI (-0.7, -0.1) (-0.9, -0.3) (-0.9, -0.2) (-1.1, -0.4) (-0.8, -0.1) (-1.0, -0.3) (-0.9, 0.3) p-value 0.026 <0.001 <0.001 <0.001 0.007 <0.001 <0.001 Less rescue use Difference from Placebo with 95% Confidence Interval Adjusted Difference from Placebo, Rescue Use (occasions/day), Day 1-28 Treatment 0.15 0.0 -0.15 -0.30 -0.45 -0.60 -0.75 -0.90 -1.05 43 50 48 52 48 45 51 N SAL 50mcg 100mcg BD 200mcg BD 400mcg BD 100mcg OD 400mcg OD 800mcg OD Note: Placebo LSMean Change was -0.29 occasions/day |
|
|
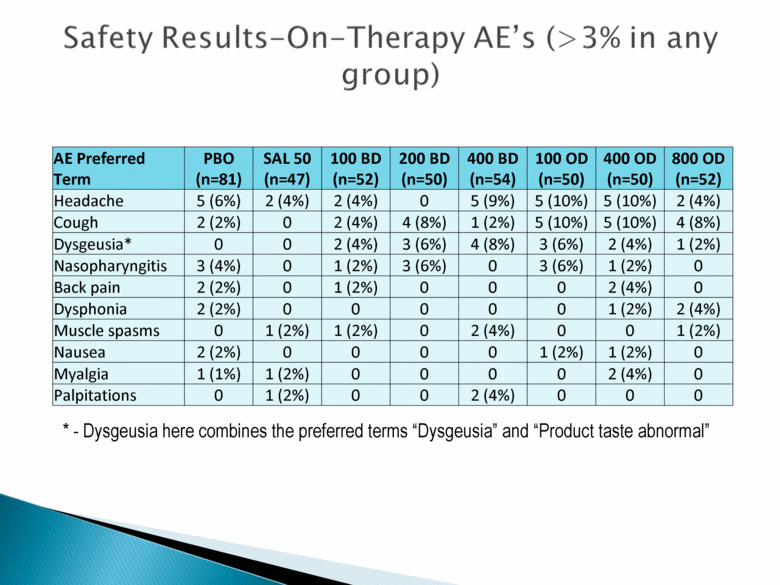
Safety Results – On – Therapy AE’s (> 3% in any group) AE Preferred Term PBO (n=81) SAL 50 (n=47) 100 BD (n=52) 200 BD (n=50) 400 BD (n=54) 100 OD (n=50) 400 OD (n=50) 800 OD (n=52) Headache 5 (6%) 2 (4%) 2 (4%) 0 5 (9%) 5 (10%) 5 (10%) 2 (4%) Cough 2 (2%) 0 2 (4%) 4 (8%) 1 (2%) 5 (10%) 5 (10%) 4 (8%) Dysgeusia* 0 0 2 (4%) 3 (6%) 4 (8%) 3 (6%) 2 (4%) 1 (2%) Nasopharyngitis 3 (4%) 0 1 (2%) 3 (6%) 0 3 (6%) 1 (2%) 0 Back pain 2 (2%) 0 1 (2%) 0 0 0 2 (4%) 0 Dysphonia 2 (2%) 0 0 0 0 0 1 (2%) 2 (4%) Muscle spasms 0 1 (2%) 1 (2%) 0 2 (4%) 0 0 1 (2%) Nausea 2 (2%) 0 0 0 0 1 (2%) 1 (2%) 0 Myalgia 1 (1%) 1 (2%) 0 0 0 0 2 (4%) 0 Palpitations 0 1 (2%) 0 0 2 (4%) 0 0 0 * - Dysgeusia here combines the preferred terms “Dysgeusia” and “Product taste abnormal” |
|
|
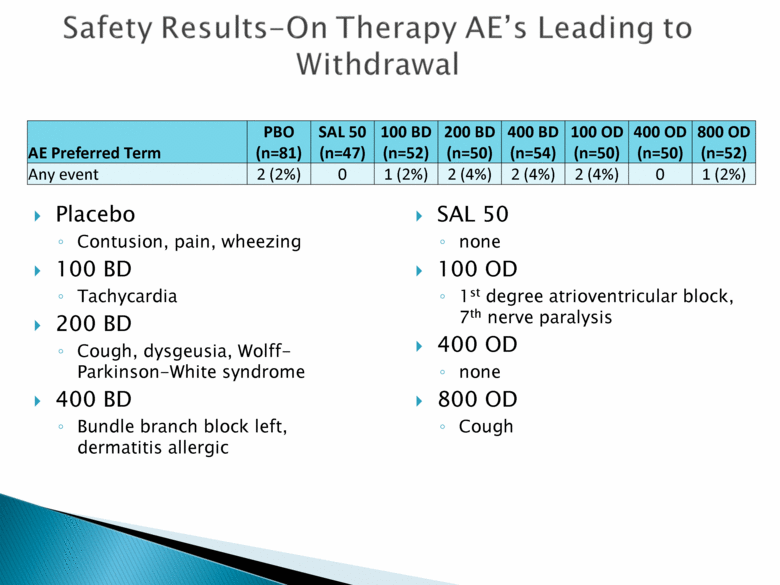
Safety Results – On – Therapy AE’s Leading to Withdrawal AE Preferred Term PBO (n=81) SAL 50 (n=47) 100 BD (n=52) 200 BD (n=50) 400 BD (n=54) 100 OD (n=50) 400 OD (n=50) 800 OD (n=52) Any event 2 (2%) 0 1 (2%) 2 (4%) 2 (4%) 2 (4%) 0 1 (2%) Placebo Contusion, pain, wheezing 100 BD Tachycardia 200 BD Cough, dysgeusia, Wolff-Parkinson-White syndrome 400 BD Bundle branch block left, dermatitis allergic SAL 50 none 100 OD 1st degree atrioventricular block, 7th nerve paralysis 400 OD none 800 OD Cough |
|
|
Conclusions GSK961081 is a dual pharmacophore having both muscarinic antagonist and beta 2-agonist activities GSK961081 appears safe and well tolerated GSK961081 provides rapid symptom relief in moderate and severe COPD subjects GSK961081 is a potent bronchodilator in moderate and severe COPD subjects |
|
|
Acknowledgements The GSK study team including Helen Griffiths, Francis Arkhurst, the medical monitor Dmitriy Galkin and Ginny Norris for Scientific advice. The Subjects for taking part in the trial. The investigators for their collection of subjects and running of the trial: Abdullah I (SA), Arpasova K (RS), Aisanov Z (RU), Bantje T (NL) Bateman E (SA), Bjermer L (SW), Blazhko V (UE), Bruning A (SA), De Munck D (NL), Dzurilla M (RS), Feshchenko Y (UE), Foerster K (GE), Gavrysiuk V (UE), Goossens M (NL), Hajkova M (RS), Hukelova H (RS), Iashyna L (UE), Irusen E (SA), Jogi R (ES), Joubert J (SA), Kornmann O (GE), Kuulpak EM (ES), Leshchenko I (RU), Lindberg A (SW), Linnhoff A (GE), Löfdahl M (SW), Lundback B (SW), Ludwig-Sengpiel A (GE), Mihaescu T (RO), Mihaicuta S (RO), Mostovoy Y (UE), Nemes R (RO), Ogorodova L (RU), Ostrovskyy M (UE), Pertseva T (UE), Pribulova E (RO), Rascu A (RO), Richter (SA), Samaruutel P (ES), Schenkenberger I (GE), Schroeder-Babo W (GE), Sinninghe Damste H(NL), Sooru E (ES), Stallaert R (NL), Sushko V (UE), Trofimov V (RU), Wielders P(NL), Wuerziger J (GE), Zorin V (UE) |