Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Fortress Biotech, Inc. | d405471d8k.htm |
 Exhibit 99.1
September 2012
Bobby W. Sandage, Jr., PhD
President & Chief Executive Officer
Developing Unique Products for Autoimmune
Diseases and Cancer (NASDAQ: CNDO) |
 Statements in this
presentation that are not descriptions of historical facts are forward-looking statements
within the meaning of the “safe harbor” provisions of the Private Securities
Litigation Reform Act of 1995. We have attempted to identify forward-looking statements by
terminology including “anticipates,”
“believes,”
“can,”
“continue,”
“could,”
“estimates,”
“expects,”
“intends,”
“may,”
“plans,”
“potential,”
“predicts,”
“should,”
or “will”
or the negative of these terms or other
comparable terminology. Forward-looking statements are based on management’s current
expectations and are subject to risks and uncertainties that could negatively affect our business,
operating results, financial condition and stock price. Factors that could cause actual results
to differ
materially
from
those
currently
anticipated
risks
include
those
set
forth
in
our
SEC
filings
including, in particular, risks relating to: the results of research and development activities;
uncertainties relating to preclinical and clinical testing, financing and strategic agreements
and relationships; the early stage of products under development; our need for substantial
additional funds; government regulation; patent and intellectual property matters;
dependence on third party manufacturers; and competition. We expressly disclaim any obligation
or undertaking to update or revise any statements contained herein to reflect any change in our
expectations or any changes in events, conditions or circumstances after the date of this
presentation. 2
Forward-Looking Statements |
 Two biologic product candidates in clinical stage development
-
Focused on autoimmune diseases and cancer immunotherapy
-
Strong proprietary position
Novel treatments with broad therapeutic applications
addressing multi-billion dollar markets
Four efficacy clinical trials completed and multiple additional trials
ongoing
-
TSO:
Trichuris
suis
ova
(CNDO-201)
in
Crohn’s
Disease,
Ulcerative
Colitis (UC) and Multiple Sclerosis (MS)
-
CNDO-109
Tumor
Activated
NK
Cells
in
relapsed
Acute
Myeloid
Leukemia (AML)
Experienced management team and board of directors
3
Value Proposition
Value Proposition
: |
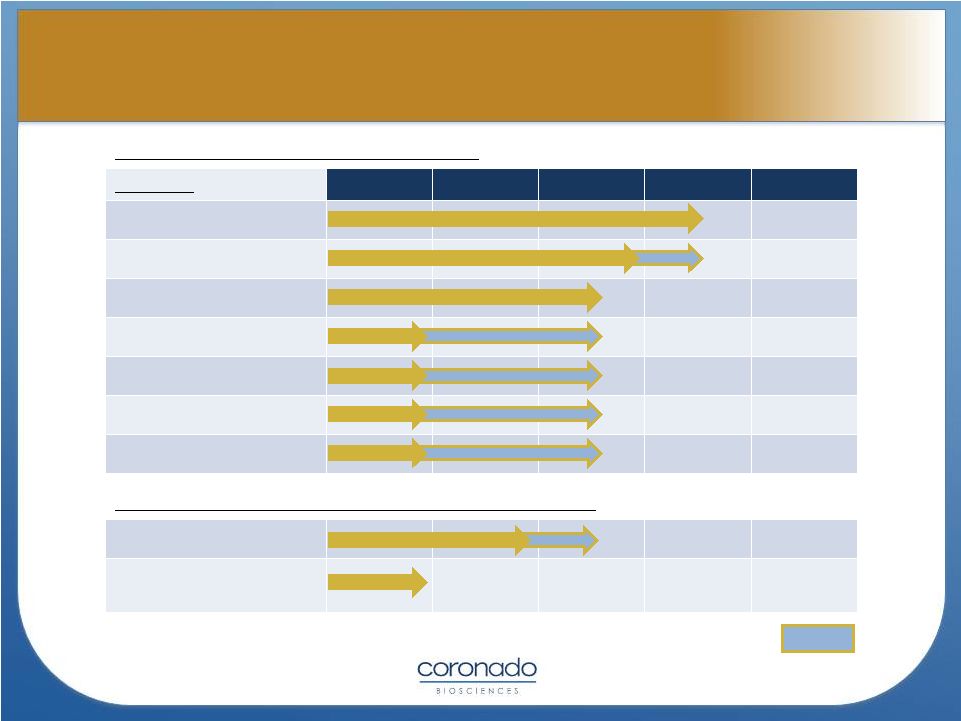 TSO
(Trichuris suis ova or CNDO-201) Indication
Pre-Clinical
Phase 1
Phase 2a*
Phase 2
Phase 3
Crohn’s Disease
Ulcerative Colitis
Multiple Sclerosis
Autism
Psoriasis
Type-1 Diabetes
Other indications**
CNDO-109 (Tumor-Activated Natural Killer Cells)
Acute Myeloid Leukemia
Multiple Myeloma, Solid
Tumors
4
2H2012
4Q2012
* TSO Phase 2a studies being conducted as Investigator-Initiated Studies
** Other indications include Rheumatoid Arthritis and Psoriatic Arthritis
Planned
4Q2012
1Q2013
2013
1Q2013
Coronado Pipeline Overview
Coronado Pipeline Overview |
 Porcine whipworm
ova Represents a novel approach to treating autoimmune diseases –
the
“Hygiene Hypothesis”
Natural immunomodulator -
regulates T-Reg cells and inflammatory
cytokines
Clinical proof of principle established in Inflammatory Bowel
Disease and Multiple Sclerosis
Ongoing Phase 2 studies ongoing in Crohn’s disease
Planned studies in multiple additional autoimmune indications
Natural properties suggest strong potential for a safe profile
North and South America and Japanese rights for all indications
5
TSO:
Trichuris
suis
ova
(CNDO-201) |
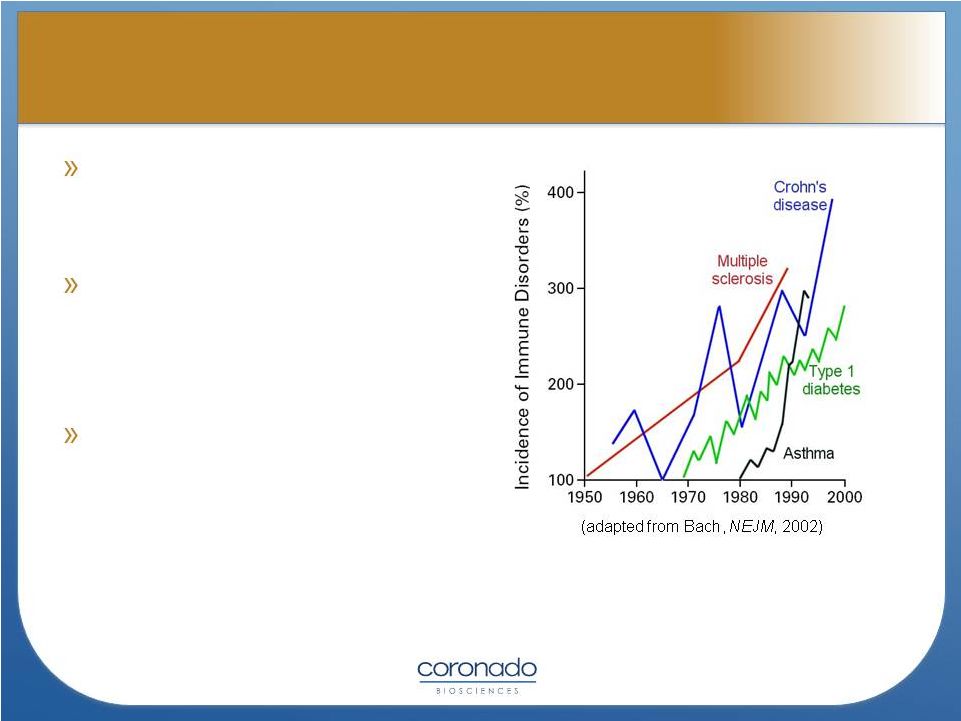 6
There are >100 immune-
mediated diseases affecting
50 million Americans
Second highest cause of
chronic disease in United
States and number one
cause of morbidity in women
In contrast, most of these
diseases are rare in less
developed countries
Walsh SJ, Rau LM. Am J Public Health 2000
Faustman, D. Institute of Medicine Report, “Women’s Health Research: Progress,
Pitfalls, and Promise, 2010
Rapid
Emergence
of
Autoimmune
and
Immune
Mediated
Diseases |
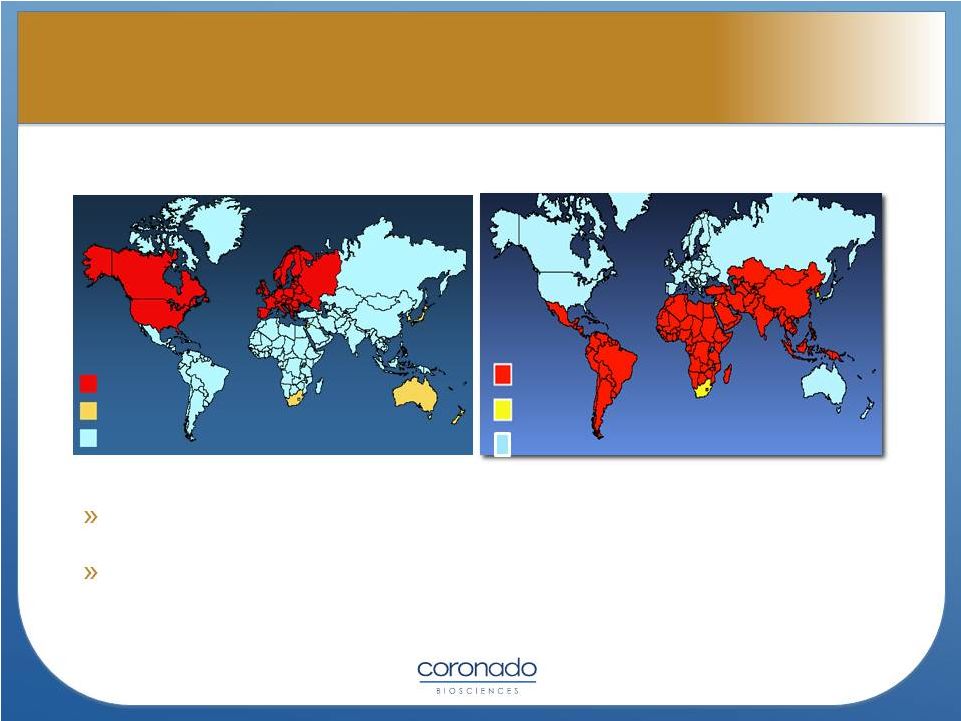 7
Autoimmune disorders incidence
Helminths infestation incidence
High
High
High
Moderate
Moderate
Moderate
Low
Low
Low
High
High
Moderate
Moderate
Low
Low
Various immunological and autoimmune diseases are much less common in the
developing world than the industrialized world
Immigrants
to
the
industrialized
world
from
the
developing
world
increasingly
develop immunological disorders in relation to the length of time since arrival in
the industrialized world
Epidemiological data demonstrate:
Distribution of Autoimmune Disorders
Distribution of Autoimmune Disorders
and Helminths
and Helminths |
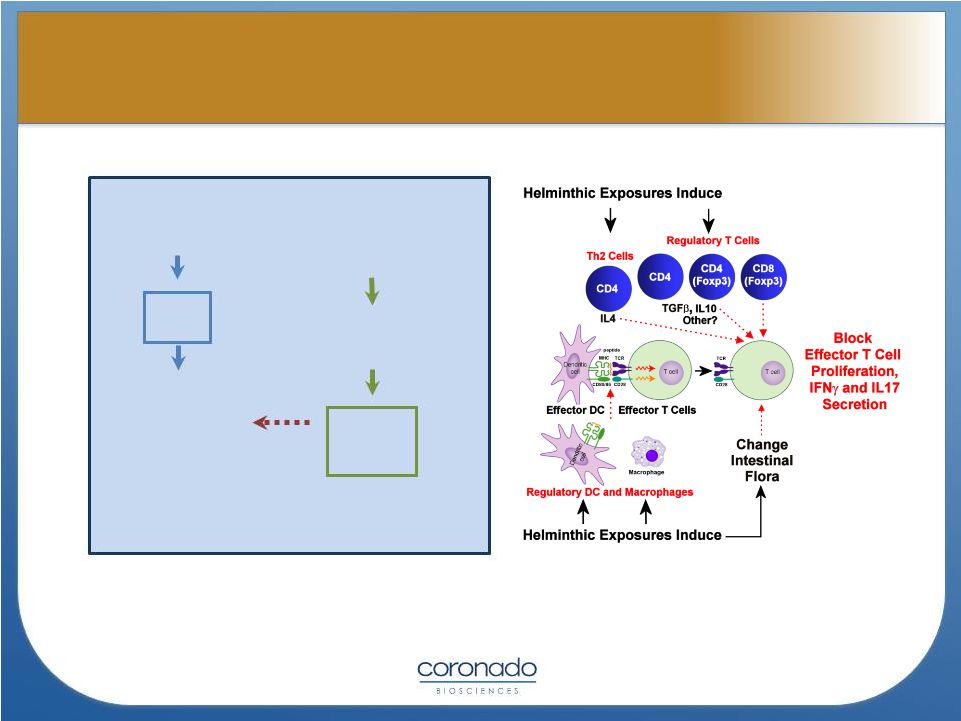 8
Weinstock and Elliott, Inflamm Bowel Dis, Jan 2009
POOR SANITATION,
IMPURE FOOD
AND CROWDED
LIVING CONDITIONS
Helminthic
and bacterial
exposures
Inhibits
Excess
Reactivity
Viral, bacterial and
protozoan
infections
Crohn’s Disease,
Ulcerative Colitis,
Multiple Sclerosis
&
other
autoimmune
diseases
Elliott & Weinstock, Ann NY Acad Sci, 2012
(Prevents)
The Biology Supporting the Hygiene
The Biology Supporting the Hygiene
Hypothesis
Hypothesis
Excess
Th1 |
 Does not multiply in
human host Colonization is self-limited in
humans
No systemic phase
No direct transmission
Ova stable
Oral dosing; 1 tbsp solution
taken once every 2 weeks
-
Clear, odorless, tasteless
9
Benefits of Trichuris suis ova (TSO)
Benefits of Trichuris suis ova (TSO) |
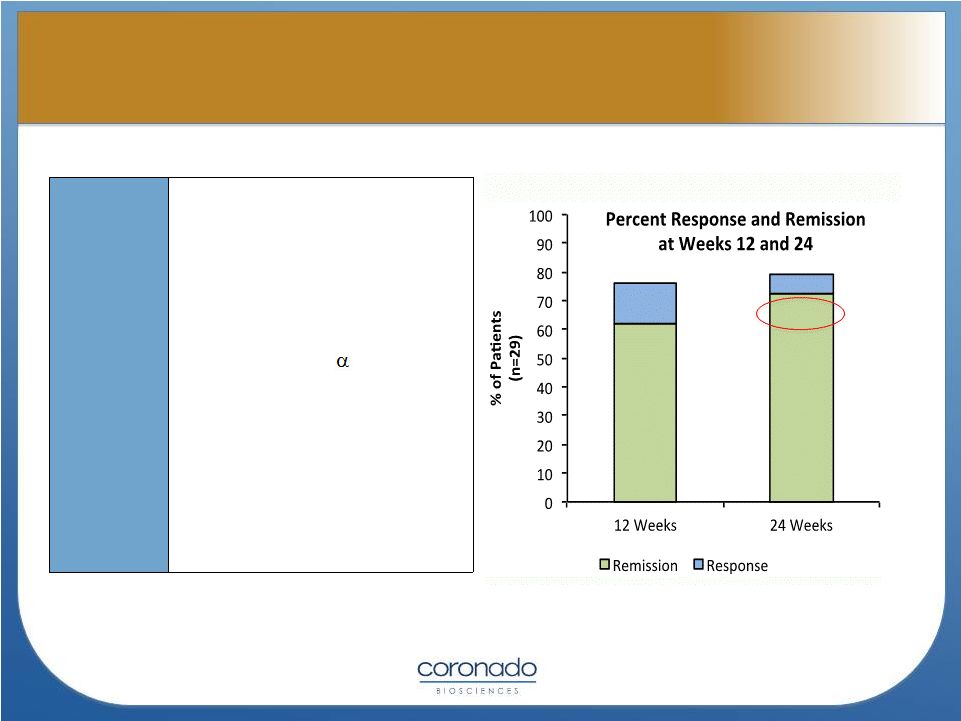 10
75.9
62.1
79.3
72.4
Patients
and
Methods
•
29 CD patients with
CDAI>220 (mean=296)
•
Median duration of the
disease : 4 yrs
•
Baseline meds –
5ASA, low
dose steroids, 6-MP or Aza,
washout of TNF
inhibitors
•
2500 TSO every 3 weeks for
24 weeks
•
Remission defined as a
CDAI of < 150 points
•
Response defined as a
CDAI > 100 point drop from
baseline
Summers, et.al., GUT
2005
Effect of TSO in Crohn’s Disease
Effect of TSO in Crohn’s Disease |
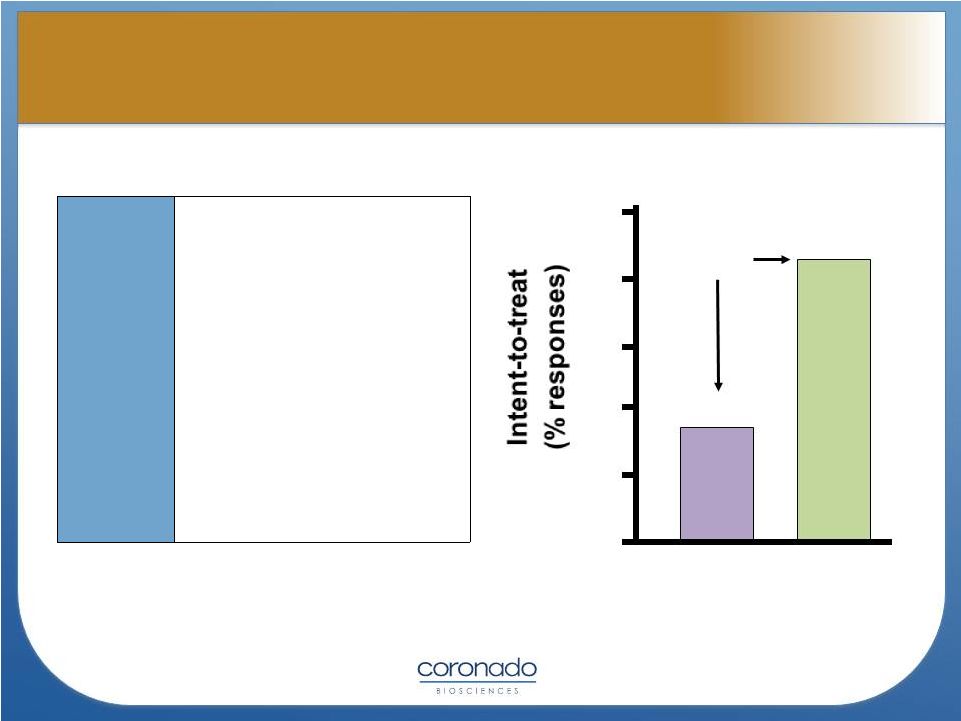 11
50
40
30
20
10
0
p=0.04
16.7%
43.3%
Placebo
T. suis
Summers, et.al., Gastroenterology 2005
Patients
and
Methods
•
n = 54 UC patients with a
UCDAI score > 4 points
•
Average score 8.7-8.8
•
Duration of disease
averaged 8 years
•
2500 TSO every 2 weeks
for 3 months
•
Most patients refractory to
previous therapy
•
Response was defined as
> 4 point drop
Effect of TSO in Ulcerative Colitis
Effect of TSO in Ulcerative Colitis |
 12 week, dose
ranging study -
Double-blind, randomized,
placebo controlled
-
TSO 250, 2500, 7500 or placebo
-
N=250 (2
nd
interim)
-
Crohn’s patients
•
CDAI = 220-350
•
CRP 2X ULN or Calprotectin 1X
ULN
Outcome –
Remission rates
2
nd
Interim –
Mid-2013
12
TRUST -
I
TRUST -
II
12 week study
-
Double-blind, randomized,
placebo controlled
-
TSO 7500 or placebo
-
N=220
-
Crohn’s patients
•
CDAI = 220-450
•
Endoscopic evidence of
inflammation
Outcome –
Response rates
Topline data –
2H 2013
TSO Phase 2 Crohn’s Disease Studies
TSO Phase 2 Crohn’s Disease Studies |
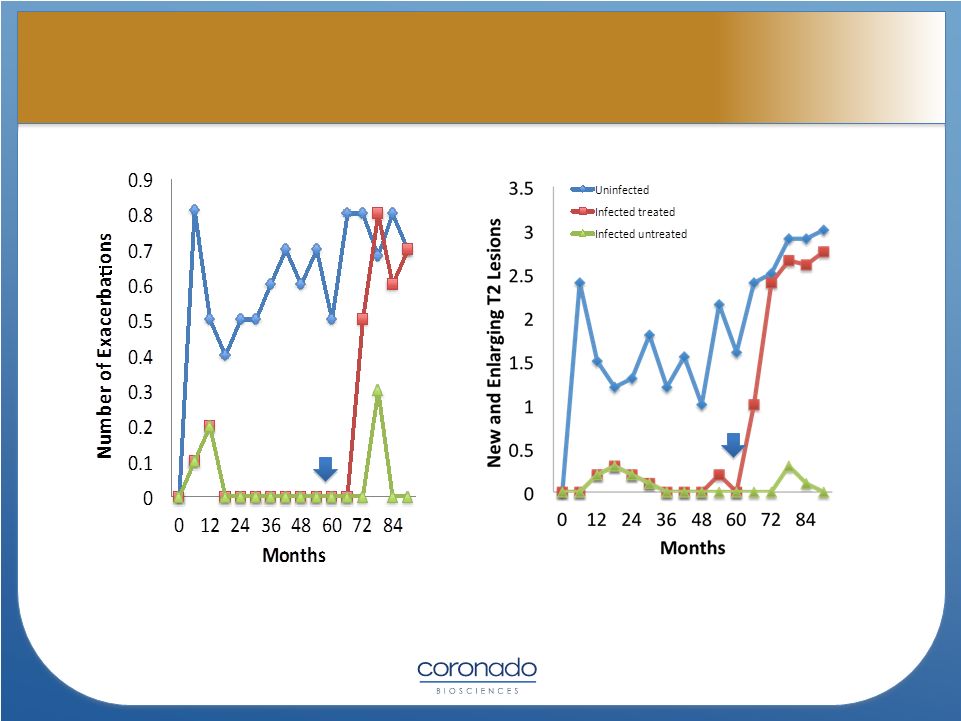 Correale
J,
Farez
MF.
J
Neuroimmunol.
2011;233:6
Antihelminth
Treatment
(n=4)
Antihelminth
Treatment
(n=4)
12 patients/group
13
Impact of Parasitic Infections on the
Impact of Parasitic Infections on the
Course of Multiple Sclerosis
Course of Multiple Sclerosis |
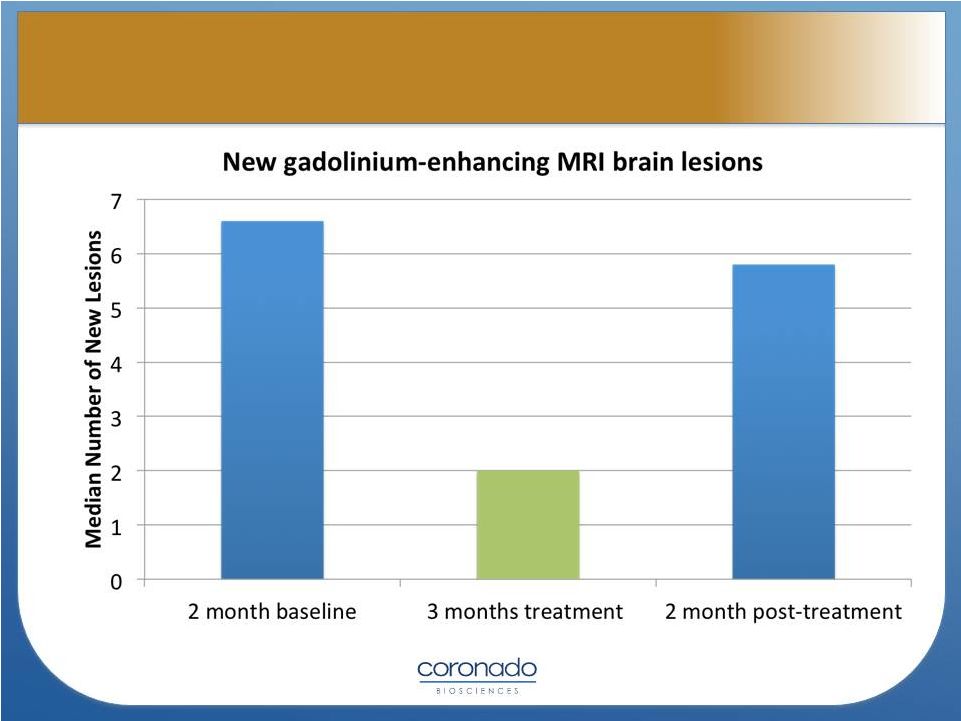 Fleming, et.al.,
Multiple Sclerosis Journal 2011 14
Effect of TSO in Multiple Sclerosis |
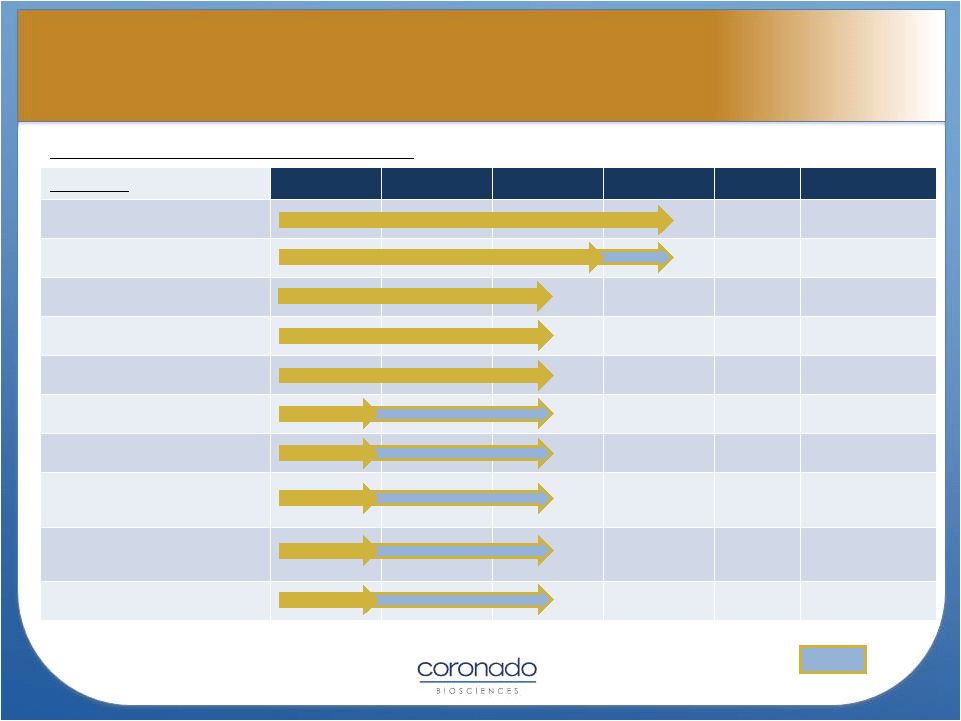 TSO
(Trichuris suis ova or CNDO-201) Indication
Pre-Clinical
Phase 1
Phase 2a*
Phase 2
Phase 3
Design
Crohn’s Disease
~500pts, DB, PC
Ulcerative Colitis
120 pt, DB, PC
Ulcerative Colitis (MOA)
18 pt, OL
Multiple Sclerosis (US)
16 pt, SB
Multiple Sclerosis (EU)
50 pt, DB, PC
Autism
10 pt, DB, Cross
Psoriasis
20 pt, OL
Type-1 Diabetes (early
intervention
60 pt, DB, PC
Type-1 Diabetes
(prevention)
150 pt, DB, PC
Psoriatic Arth/RA
20-30 pt, DB, PC
15
* TSO Phase 2a studies being conducted
as Investigator-Initiated Studies
Planned
1Q2013
4Q2012
Two phase 2 studies
4Q2012
1Q2013
2013
1Q2013
OL = Open-Label DB = Double-Blind, SB= Single-Blind PC =
Placebo-Controlled TSO Pipeline
TSO Pipeline |
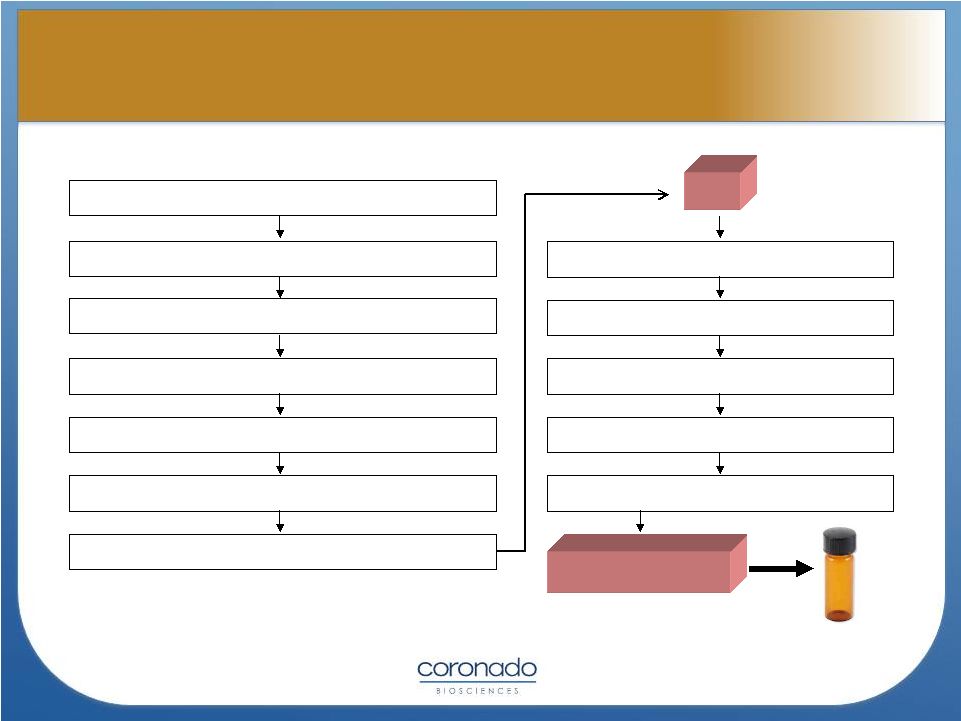 Certified specific
pathogen-free minipigs Observation and pathogen testing
Oral Inoculation with OVA from master bank
Development of infection
Harvest OVA from pigs
Isolation and purification
Processing and pathogen inactivation
API
Quality and pathogen testing
Incubation and sterilization
Formulation process
Fill/finish
DRUG Product
Quality and microbiological testing
16
TSO Manufacturing Process
TSO Manufacturing Process |
 Three issued US
patents entitled ‘Use of Parasitic Biological Agents for Prevention and Control of
Autoimmune Disease’ directed to compositions,
methods of producing compositions, and methods of autoimmune disease with
helminths –
exp. 12/2018
Five additional pending patents
-
‘Use
of
Parasitic
Biological
Agents
for
Disease
Prevention
and
Control’
-
directed
to
the
treatment
of
animals/man
with
a
Th1
or
Th2
mediated
autoimmune
disease
–
exp.
11/2023
-
‘Production
of
a
Viable,
Storable
Worm
Egg
Suspension’
-
directed
to
a
process
for
preparation
of
TSO
using
an
acid
wash
-
exp.
3/2028
-
‘Method for Characterizing the Biological Activity of Helminth Eggs, in particular
Trichuris
Eggs’
–
exp. 5/2029
-
‘Treatment
with
Helminths’
directed
to
methods
of
treating
obesity
and
IBS
-
exp.
10/2029
-
‘Compositions
and
Methods
for
Treating
IBD’
-
directed
to
a
method
of
treating
IBD
by
contacting
an
isolated
dendritic/macrophage
cell
with
a
helminth
–
exp.
~9/2032
* Expiration dates do not include any patent term extension
17
TSO Intellectual Property
TSO Intellectual Property |
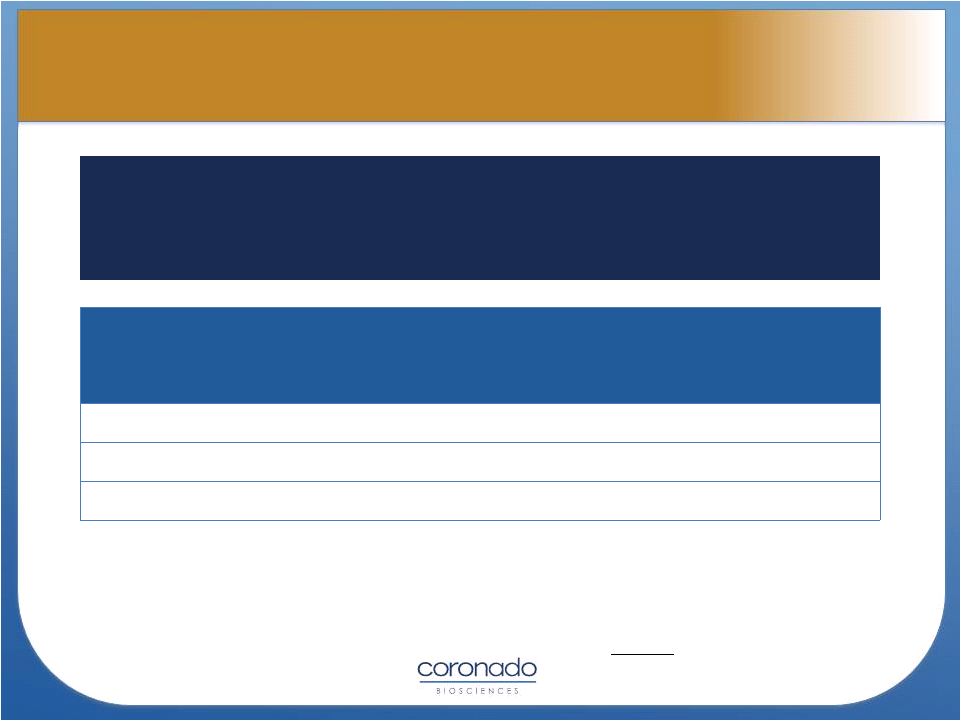 Potential TSO Target
Indications
U.S./Japan
Prevalence
U.S./Japan Annual
Market Sales
(USD Mil)
Ulcerative Colitis
669,000
$1,300
Crohn’s Disease
534,000
$2,600
Multiple Sclerosis
485,000
$6,400
Sources:
Decision
Resources
2012
The mechanism of action of TSO should, if approved, allow it to be
positioned in a variety of autoimmune disorders, including inflammatory
bowel
diseases
and
multiple
sclerosis
as
well
as
other
potential
disorders
such as rheumatoid arthritis and psoriasis.
18
TSO Market Opportunity
TSO Market Opportunity |
 NK cells represent
the key component of the body’s innate immune surveillance system
Proof of principle established in patients with high-risk refractory or
relapsed acute myeloid leukemia (AML)
Activation with CNDO-109 does not require toxic cytokines or long-
term culture/expansion, and does not change NK cell phenotypes
Preclinical activity demonstrated in multiple myeloma, breast
cancer, prostate cancer and ovarian cancer
19
CNDO-109: Activated Natural Killer Cells
CNDO-109: Activated Natural Killer Cells |
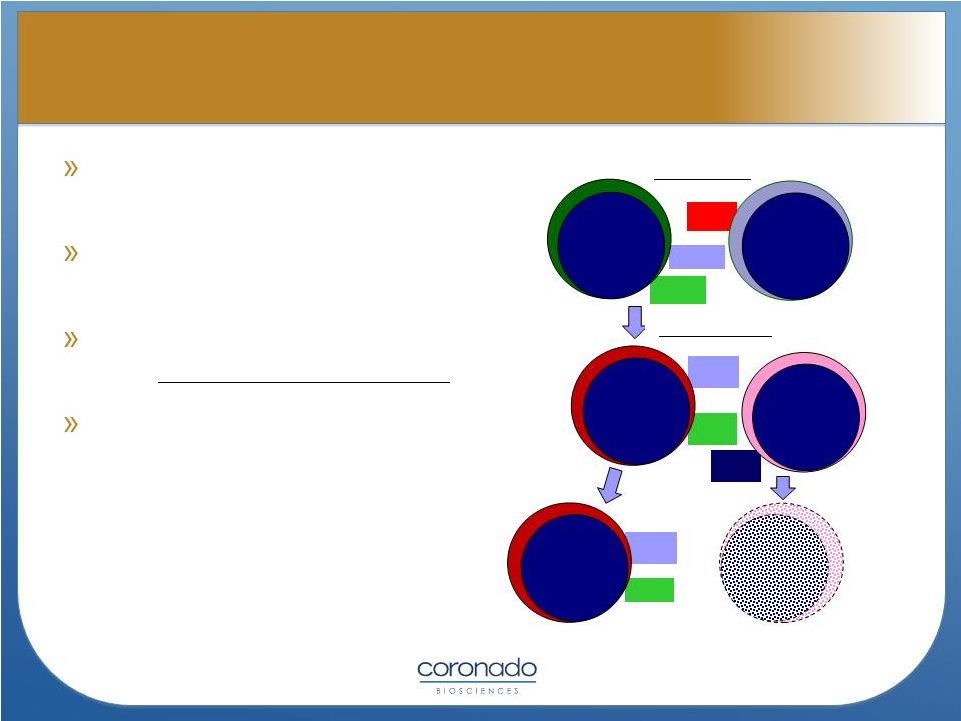 Activated ex vivo
by tumor cell lysate (CNDO-109)
Effective from autologous or
allogeneic NK cell source
Uniquely positioned in patients
with “minimal residual disease”
Remains active after
freeze/thaw
20
Resting
Donor NK
Primed
Donor NK
CNDO-109
Patient’s
tumor
Dead
Tumor
cell
Priming –
Signal 1
Triggering –
Signal 2
Primed
Donor NK
Tumor lysis
Priming
receptor
Trigger
receptor
Priming
receptor
Trigger
receptor
Trigger
receptor
Priming
signal
Priming
receptor
Trigger
ligand
“Serial Killer”
CNDO-109 Mechanism of Action
CNDO-109 Mechanism of Action |
 »
Phase 1 investigator sponsored open-label trial
»
To determine the safety of infusion of allogeneic Tumor-activated
NK (TaNK) cells after low dose radiotherapy plus chemotherapy
in high-risk relapse or refractory AML patients
»
Enrolled 8 AML patients
-
5 in Complete Remission 2 or 3 (CR2 or CR3)
-
1 patient in partial relapse (PR)
»
3/5 experienced a longer CR than their previous CR, in addition
PR patient achieved CR
21
Kottaridis, et al., ASH 2011
CNDO-109 Phase 1 Study in AML
CNDO-109 Phase 1 Study in AML |
 1Q 2012
Filed IND
2H 2012
Initiate Phase 1/2 allogeneic clinical trial for the treatment of
relapsed AML
Once the dose is selected, initiate a randomized Phase 2 trial
-
Potential for regulatory approval with single randomized, controlled
clinical trial if data are clinically meaningful and statistically persuasive
Future autologous studies planned in other tumor types (including
multiple myeloma, breast, ovarian and prostate)
22
CNDO-109 Clinical Development
CNDO-109 Clinical Development |
 Core patent
- “Method for activating natural killer cells by tumor
cell preparation in vitro”
-
Issued in U.S. -
exp. 1/2029
-
Issued
in
Australia
–
exp.
3/2026
-
Pending in Europe, Canada, Japan and India
Patent pending -
“Preserved Compositions of Activated NK Cells
and
Methods
of
Using
the
Same”
–
exp.
7/2030
Provisional
application
pending
in
the
U.S.
-
“Compositions
and
Methods for Treating Viral Infections”
* Expiration dates do not include any patent term extension
23
CNDO-109 Intellectual Property
CNDO-109 Intellectual Property |
 Potential
CNDO-109 Target Indications
G7 Drug
Treatable
Population
G7 Market
Sales
(USD Mil)
Mortality
AML
43,500
$165
5 year mortality rate is 85-90% but varies by age
Multiple Myeloma
44,000
$2,870
Stage III: median survival of 29 months
Breast Cancer
494,000
$10,100
Stage IV: 5 year mortality rate is 76%
Ovarian
57,110
$424
Overall 5 year survival rate of 45%
Prostate
500,000
$4,000
Overall 5-year survival rate of +99%, but
2 leading cause of cancer deaths in men
G7 = U.S., U.K., Germany, France, Italy, Spain, Japan
Sources:
Decision
Resources
2011/2012
If Coronado establishes the efficacy of CNDO-109 activated NK cells in the treatment of
AML, Coronado believes the market opportunity for CNDO-109 activated NK cell therapy
is
large
due
to
the
fact
that
many
types
of
tumors
are
sensitive
to
killing
by
activated
NK
cells.
24
CNDO-109 Market Opportunity
CNDO-109 Market Opportunity
nd |
 25
Key Management and Board Members
Key Management and Board Members
Bobby W. Sandage, Jr., PhD
President & Chief Executive Officer
»
Served as EVP & CSO of Indevus Pharmaceuticals, Inc.
»
Over 30 years of pharmaceutical/biotechnology experience
Noah D. Beerman
EVP & Chief Operating Officer
»
Served as President & CEO of RXi Pharmaceuticals
»
Over 25 years of pharmaceutical/biotechnology experience
Karin Hehenberger, MD, PhD
EVP & Chief Medical Officer
»
Served in senior management positions at Juvenile Diabetes
»
Over 12 years of pharmaceutical/biotechnology experience
Lucy Lu, MD
EVP & Chief Financial Officer
»
Served Senior Analyst Citi, Lazard and First Albany
»
Over 10 years of equity research experience
Glenn L. Cooper, MD
Executive Chairman
»
Served as Chairman & CEO of Indevus Pharmaceuticals, Inc.
»
Over 25 years of pharmaceutical/biotechnology experience
Eric K. Rowinsky, MD
Vice Chairman
»
World renown oncologist, former CMO at ImClone, board of
»
Over 25 years of healthcare experience
Lindsay Rosenwald, MD
Director and Founder
»
A prolific and successful investor in the life sciences industry
for
Research Foundation and at JNJ Diabetes
Biogen/Idec
over 20 years |
 26
Listed on NASDAQ: CNDO
12/19/2011
Market
Cap
as
of
8/29/2012
$140M
Shares Outstanding
24.4M
-
Additional 3.4M options and warrants
Cash
Position
as
of
6/30/2012
$38.2M
-
Additional $15M loan secured in August
Financials |
 27
Principal
$15M
Interest
9.25%*
Payment Terms
42 Months
-First 12 months, interest only
-Next 30 months, principal and interest
Warrants
73,009
-Exercise price $5.65
*The loan bears interest at a rate per annum equal to the greater of
(i) 9.25% or (ii) 9.25% plus the sum of the prevailing
prime rate minus 3.25% $15M Hercules Loan |
 TSO
Initiate Phase 2 Crohn’s study
Completed
Initiate Multiple Investigator Initiated Studies
2H 2012
TRUST-II (Falk) Crohn’s study 2nd Interim Results
Mid-2013
TRUST-I (CNDO) Crohn’s study Topline Results
2H 2013
CNDO-109
Initiate US Phase 1/2 study
2H 2012
28
Upcoming Milestones
Upcoming Milestones |
 Two biologic product candidates in clinical stage development
-
Focused on autoimmune diseases and cancer immunotherapy
-
Strong proprietary position
Novel treatments with broad therapeutic applications
addressing multi-billion dollar markets
Four efficacy clinical trials completed and multiple additional trials
ongoing
-
TSO:
Trichuris
suis
ova
(CNDO-201)
in
Crohn’s
Disease,
Ulcerative
Colitis (UC) and Multiple Sclerosis (MS)
-
CNDO-109:
Tumor
Activated
NK
Cells
in
relapsed
Acute
Myeloid
Leukemia (AML)
Experienced management team and board of directors
29
Investment Highlights
Investment Highlights |
