Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Born, Inc. | Financial_Report.xls |
| EX-32 - EXHIBIT 32.1 - Born, Inc. | tcln0810form10kex321.htm |
| EX-31 - EXHIBIT 31.2 - Born, Inc. | tcln0810form10kex312.htm |
| EX-32 - EXHIBIT 32.2 - Born, Inc. | tcln0810form10kex322.htm |
| EX-31 - EXHIBIT 31.1 - Born, Inc. | tcln0810form10kex311.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, DC 20549
FORM 10-K
☑ ANNUAL REPORT UNDER SECTION 13 OR 15 (D) OF THE SECURITIES EXCHANGE ACT OF 1934
FOR THE FISCAL YEAR ENDED APRIL 30, 2012
☐ TRANSITION REPORT UNDER SECTION 13 OR 15 (d) OF THE EXCHANGE ACT OF 1934
For the transition period from _______________ through _______________
TECHS
LOANSTAR, INC.
_______________________________________
(Name of small business in its charter)
| Nevada | 0-4682058 |
| (State or other jurisdiction of incorporation) | (IRS employer ID Number) |
| 319 Clematis Street, Suite 400 | |
| West Palm Beach, FL. 33401 | |
| 561-514-9042 | |
| (Address and Telephone Number including area code of registrant's principal executive offices) | |
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act: Yes ☐ No ☑
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☑
Check whether the issuer: (1) filed all reports required to be filed by Section 13 or 15(d) of the Exchange Act during the past 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☑ No ☐
Indicate by checkmark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§229.405 of this chapter) during the preceding 12 months ( or for such shorter period that the registrant was required to submit and post such files.
Yes ☐ No ☑ (Not required by smaller reporting companies)
Check if there is no disclosure of delinquent filers in response to Item 405 of Regulation S-K is not contained in this form, and no disclosure will be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ☑
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company filer. See definition of "accelerated filer" and "large accelerated filer" in Rule 12b-2 of the Exchange Act (Check one):
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☐ | Smaller reporting company ☑ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☑
State issuer's revenues for its most recent fiscal year. $40,900
As of October 31, 2011, the aggregate market value of the shares of common stock held by non-affiliates (computed by reference to the most recent offering price of such shares) was $5,014,823
As of August 8, 2012, there were 2,214,556,789 shares of common stock issued and outstanding.
| TABLE OF CONTENTS | ||
| PART I | Page | |
| Item 1. | Description of Business | 4 |
| Item 1A. | Risk Factors | 16 |
| Item 1B. | Unresolved Staff Comments | 26 |
| Item 2. | Description of Property | 26 |
| Item 3. | Legal Proceedings | 26 |
| Item 4. | Mine Safety Disclosures | 26 |
| PART II | ||
| Item 5. | Market for Common Stock and Related Stockholder Matters | 26 |
| Item 6. | Selected Financial Data | 28 |
| Item 7. | Management's Discussion and Analysis of Plan of Operations | 28 |
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk | 30 |
| Item 8. | Financial Statements and Supplementary Data | 30 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 31 |
| Item 9A(T). | Controls and Procedures | 31 |
| Item 9B. | Other Information | 32 |
| PART III | ||
| Item 10. | Directors, Executive Officers, Promoters and Control Persons; Compliance with Section 16(a) of the Exchange Act | 33 |
| Item 11. | Executive Compensation | 34 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 37 |
| Item 13. | Certain Relationships and Related Transactions | 37 |
| Item14. | Principal Accountant Fees and Services | 38 |
| PART IV | ||
| Item 15. | Exhibits, Financial Statement Schedules | 38 |
| Signatures | 40 | |
| Certifications |
PART I
SPECIAL CAUTIONARY NOTICE REGARDING FORWARD-LOOKING STATEMENTS
This Report contains statements that constitute forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, (the "Securities Act) and the Securities Exchange Act of 1934, as amended, (the "Exchange Act"). Various matters discussed in this document and in documents incorporated by reference herein, including matters discussed under the caption "Plan of Operation," may constitute forward-looking statements for purposes of the Securities Act and the Exchange Act. These statements are based on many assumptions and estimates and are not guarantees of future performance and may involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of Techs Loanstar, Inc. (the "Company" or "Techs Loanstar") to be materially different from future results, performance or achievements expressed or implied by such forward-looking statements. The words "expect," "anticipate," "intend," "plan," "believe," "seek," "estimate," and similar expressions are intended to identify such forward-looking statements. The Company's actual results may differ materially from the results anticipated in these forward-looking statements.
ITEM 1. DESCRIPTION OF BUSINESS
OVERVIEW
References in this Report to "Techs Loanstar," "Registrant," "we," "our," "us," and the "Company" refer to Techs Loanstar, Inc. Techs Loanstar was incorporated in the State of Nevada as a for-profit company on April 7, 2006. On March 14, 2012 the Company changed its name with the Secretary of State of Nevada to Quture International, Inc. The Company plans to file with the appropriate regulatory bodies the name change as well as a new ticker symbol to better reflect the company's core business.
SHARE EXCHANGE TRANSACTION WITH QUTURE, INC.
On July 25, 2011, the Company entered into a securities exchange agreement with Quture, Inc., a Nevada corporation (“Quture”). Pursuant to the Agreement, the Company agreed to acquire all of the outstanding capital stock of Quture in exchange for a number of shares of the Registrant’s common stock, par value $0.001 per share (the “Common Stock”) equal to eighty-five percent (85%) of the issued and outstanding common stock of the Company following the Exchange.
On August 9, 2011, the Registrant entered into and consummated the First Amendment to the Agreement Concerning that Exchange of Securities (the “Share Exchange Agreement”) with Quture and the shareholders of Quture. Upon consummation of the transactions set forth in the Share Exchange Agreement (the “Closing”), the Registrant adopted the business plan of Quture.
Pursuant to the Share Exchange Agreement, the Registrant acquired all of the outstanding capital stock of Quture in exchange (the “Exchange”) for the original issuance of an aggregate of 1,938,543,110 shares (the “Exchange Shares”) of the Registrant’s common stock, par value $0.001 per share (the “Common Stock”). The Registrant initially issued 400,000,000 Exchange Shares to the shareholders of Quture and, following an amendment of the Registrant’s Articles of Incorporation, the remaining Exchange Shares were available to be issued to Quture shareholders. The Exchange Shares were issued on a pro rata basis, on the basis of the shares held by such security holders of Quture at the time of the Exchange. As a result of the Exchange, Quture became a wholly-owned subsidiary of the Registrant and the shareholders of Quture beneficially at Closing, owned approximately eighty-five percent (85%) of the issued and outstanding Common Stock of the Registrant. The parties have taken the actions necessary to provide that the Exchange is treated as a “tax free exchange” under Section 368 of the Internal Revenue Code of 1986, as amended. The Agreement contains customary representations, warranties and covenants of the Registrant and Quture for like transactions. The foregoing descriptions of the above referenced agreements do not purport to be complete. For an understanding of their terms and provisions, reference should be made to the Agreement attached as Exhibit 10.1 to the Current Report on Form 8-K filed August 12, 2011 with the Securities and Exchange Commission (the “SEC”) and the Current Report on Form 8K/A filed on September 22, 2011 with the SEC.
On August 10, 2011, as a covenant to the Agreement, holders of a majority of the Registrant’s outstanding Common Stock voted to amend the Registrant’s Articles of Incorporation to increase the number of its authorized shares of capital stock from 900,000,000 shares to 2,510,000,000 par value $0.001 shares (the “Amendment”) of which (a) 2,500,000,000 shares were designated as Common Stock and (b) 10,000,000 shares were designated as blank check preferred stock.
At the effective time of the Exchange, our board of directors and officers was reconstituted by the resignation of Henry Fong as Director, President and Chief Executive Officer of the Registrant and the appointment of G. Landon Feazell as a member of the Registrant’s Board of Directors, Chief Executive Officer and President, and Geoffrey L. Feazell as Treasurer and Secretary of the Registrant.
For SEC reporting purposes, Quture is treated as the continuing reporting entity that acquired Techs (the historic registrant). The reports filed after the transaction have been prepared as if Quture (accounting acquirer) were the legal successor to Techs’ reporting obligation as of the date of the acquisition. Therefore, all financial statements filed subsequent to the transaction reflect the historical financial condition, results of operations and cash flows of Quture, for all periods prior to the share exchange and consolidated with Techs from the date of the share Exchange. Quture previously had a December 31 fiscal year end, but has now assumed the fiscal year end of Techs Loanstar, Inc., the legal acquirer. Accordingly, the financial statements presented herein are the audited financial statements for the years ended April 30, 2012 and 2011 are of Quture, Inc., and from August 9, 2011 are consolidated with Techs Loanstar, Inc. All share and per share amounts of Quture have been retroactively adjusted to reflect the legal capital structure of Techs pursuant to FASB ASC 805-40-45-1.
Quture was incorporated in the state of Nevada on April 21, 2011. The Company develops medical software with tools and analytics to reduce costs while improving clinical performance, outcomes, predictive insight, and evidence-based best clinical processes. Effective July 1, 2011, Quture merged with Q3, LLC (“Q3”), a Florida Limited Liability Company, whereby Quture was the legal acquirer and Q3 is the accounting acquirer. Accordingly, Quture’s historical financial results, includes Q3 prior to July 1, 2011 and consolidated with Quture from July 1, 2011.
Quture is an emerging healthcare knowledge solution company created to transform health and healthcare by developing the standard in measuring clinical performance and outcomes. The Company’s products focus on using actual clinical data from existing electronic databases to measure performance and outcomes applying analytics. The Quture technology leverages its Application Partnership with InterSystems Corporation. InterSystems’ technology is already used in over 66% of the hospitals in America. Quture, using this fully developed technology platform converts manual processes to electronic processes to integrate clinical data from existing disparate electronic data sources in healthcare organizations. The Company’s product uses InterSystems’ data integration product Ensemble already programmed for every electronic medical record (EMR) and database. Quture is immediately able to collect and integrate performance metrics into the InterSystems Cache database customized for the Quture application through this partnership. By licensing the InterSystems technology and implementing the company’s clinical content and performance measures, Quture has the potential to develop the most powerful clinical knowledge database in the world. Quture will deliver to customers the clinical data for value-based purchasing. Performance-based and value calculated clinical data will ultimately determine what payers do and do not want to pay for and the clinical knowledge to support new mandated payment systems while improving care.
Quture, Inc. (“Quture”), a Nevada corporation, was formed in April 2011 and on July 25, 2011 entered into a Share Exchange Agreement (the “Agreement) with Techs Loanstar (“TCLN”). Pursuant to the Agreement, Quture became a subsidiary of TCLN. Corporate strategy includes filing for a name change of Techs Loanstar to Quture International, Inc., in Nevada and completing negotiations with another subsidiary to leverage its technology for Quture’s planned expansion. The initial focus of Quture’s products is for hospital customers and influencers. Quture then intends to market its other product to physicians and physician groups, especially invasive physicians. Quture’s fully developed and existing products, discussed below, are being enhanced and migrated to the InterSystems technology platform. Performance and outcomes measurement functionality is the core intellectual property, technology and clinical content of both products, one primarily for hospitals and one primarily for surgery centers and physicians performing invasive procedures. Quture then plans to rapidly expand its products and business to patients and payers, leveraging its clinical performance knowledge and database. Product strategy inevitably first focuses on health care and then expands to personalized medicine, health and wellness.
We believe the Company’s products and services will become an essential technology and tool to improve care and health, while reducing costs. The demand to measure performance is coming from all stakeholders involved in both payment and delivery. Measuring performance is now mandatory and is the new foundation of how providers are reimbursed, granted privileges to practice in hospitals, and, in the near future, selected by patients for their care.
The core competence of Quture pivots on its product with clinical content to measure performance. Quture’s clinical content is evidence-based optimal clinical processes and clinical performance measures developed for over 35 years as the leading the hospital performance measurement company. This content has been developed through external peer review and peer review products and services. We know what to measure, where to find it, and how to use it to continuously improve clinical processes. Quture intends to license its transformative and disruptive technology and, through relationships with its customers, continuously develop its clinical database and expand upon its clinical content to know what works, which best provides these specific clinical services, and the relative cost to outcome ratio determining value.
Quture’s mission is to become the international standard in healthcare performance and outcomes measurement. Quture provides healthcare organizations, insurers, government and private sector payers, and others in the healthcare community with performance and outcomes measurement tools and data sets. Industry experts agree that these performance measures are the “transformative tool” that reduce medical cost and improve quality of care. Quture’s management team has 35 years of experience and a long history of working with many of the nation’s leading healthcare institutions as a leader and innovator in measuring clinical performance. The Company intends to potentially accelerate its growth and revenues through mergers and acquisitions. Existing relationships provide the Company with unique business opportunities for such mergers and acquisitions.
Quture is conceived on the premise that optimal health and health care can be empowered by its performance and outcomes tools and the clinical data that will be derived from that technology. Quture is dedicated to the proposition that its quality-value equation is an unparalleled business opportunity and within the existing core competence of the Company.
TARGET MARKETS AND MARKETING STRATEGY
Our target markets encompass the total array of customers of health and healthcare products, data, and services. Marketing strategy sequences this market beginning with hospitals, then physicians and physician groups, and then patients and payers.
We intend to introduce the Company’s QualOptima performance measurement product into the market after completion of a formal “clinical trial” at the University of Miami, Miller School of Medicine and Jackson Memorial Hospital. The proof of concept Phase I portion of that demonstration project has been successfully completed based on sample data and to the satisfaction of Quture and InterSystems The QualOptima product is further demonstrated at Niagara Falls Memorial Hospital, Niagara Falls, New York, for performance measurement of nursing-sensitive measures to become “interdisciplinary” performance measurement. An existing customer of Quture, Springhill Medical Center, Springhill, LA, is evaluating the QualOptima product to replace its existing license with Quture for peer review. The QualOptima product will then have been demonstrated, if successful, to transition from strictly peer review to performance measurement on the InterSystems platform for interdisciplinary performance and outcomes measurement. Quture then intends to evaluate data from these hospital projects for return on investment (ROI) potential of the QualOptima product for commercial marketing and sales. After successful demonstration in cooperation with InterSystems, where the InterSystems products are being demonstrated to meet the requirements and specifications of Quture, the QualOptima product will be formally launched and then commercialized. QualOptima is now being expanded from the demonstration in anesthesiology to the more than 40 medical specialties. The Company plans to negotiate contracts and licenses to install QualOptima in reference hospitals during the fall months of 2012 as part of the go to market strategy of the Company.
The “go to market strategy” will focus on existing strong relationships with major hospital corporations, including their self-insured trusts, at several flagship hospitals, designed to mature into sales to large hospital systems. We anticipate other reference hospital installations will focus on specific product components, including the relationship between performance and outcomes measures, one focused on the natural language processing software component and aggregated medical specialties and specific federal initiatives, such as re-hospitalizations and hospital acquired conditions. Significantly, the Niagara Falls Memorial Hospital project is focused on a hospital acquired condition, eliminating or at least reducing cather-acquired urinary tract infections.
Initial sales are being pursued through marketing and sales for installation. Initial customers will be carefully coordinated with InterSystems. InterSystems will accompany Quture sales engineers with their own sales engineers for sales presentations. The Company is also partnering with prominent national healthcare law firms and accounting firms, jointly conducting several summit meetings for their clients and inviting potential customers – stressing compliance and the threat of negligent credentialing as motivators and leveraging their representation and influence with hospital boards and senior executives. The Quture product database and clinical process, quality and patient safety management, and medical staff credentialing processes will provide new and unique consulting opportunities for these firms in concert with Quture.
The Company anticipates marketing and sales of the QSurg product will follow introduction of the QualOptima product, projected by Quture to begin six (6) months after commencement of marketing and sales of QualOptima.
PRODUCTS
Quture owns two (2) fully developed and tested products; both are being migrated from a Microsoft technology platform onto the InterSystems technology platform, with the Cache object-oriented database serving as the foundation for each and providing a unified Quture database to be called the “Qualytx” database. Real-time clinical data is captured electronically from multiple vendor disparate databases using the Ensemble interface engine and aggregated into the Qualytx database. The DeepSee dashboard and Zen report writer technology are functionalities existing in the InterSystems Ensemble Enterprise suite of products. The Quture products on the InterSystems platform are (QualOptima) or are planned to be (QSurg) as follows:
QualOptima – clinical performance measurement with analytics,
And
QSurg – surgery center electronic medical record (EMR) with a simplified QualOptima (“QuOp”) performance analytics application embedded.
QUALOPTIMA
QualOptima – electronically captures, integrates, and analyzes performance and outcomes data from Quture’s performance metrics from multiple vendor product disparate hospital databases. QualOptima contains the proprietary library of performance measures, with precisely defined numerator and denominator specifications, including all performance measures published by the Physician Consortium for Performance Improvement (PCPI) of the American Medical Association. The performance measures are mapped at key interventions or data “nodes” in an evidence-based optimal clinical process, as follows:

The clinical data structure includes individual patient risk factors, performance measures based on evidence-based optimal clinical processes, and outcomes measures. The product demonstration underway with Keith Candiotti, M.D, is based on his design for the product in anesthesiology, as follows:
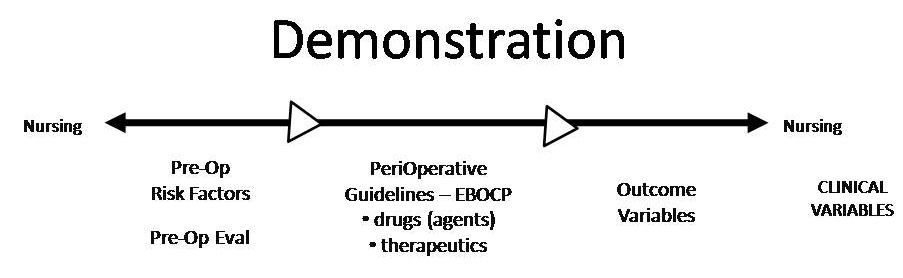
QualOptima is a single product (not a “suite” of products) with components designed to satisfy the mandates imposed on hospitals through governmental regulation and accreditation standards (see Governmental Regulations). These components are:
Performance measurement (the fundamental component discussed above)
Proctoring
Triggers
Peer review
Cost analysis for value calculations
The performance measurement component implements the InterSystems data capture and integration functionality (“interface engine”) of the Ensemble product. Ensemble uses data connections known as application programming interfaces (“API’s”) which are already programmed for every vendor’s EMR and database. The driver for this component is the accreditation standard of the Joint Commission requiring measurement of performance of each individual physician for each clinical privilege the hospital must grant to practice every two years but measured at least every six (6) months. Manual data abstraction to satisfy this requirement is no longer practical after recent imposition of this standard. This requirement and publication of performance measures as the minimum threshold requirement to avoid negligent credentialing litigation presents a compelling need for the Quture product.
Quture’s product provides the clinical “failure mode and effects analysis” (FMEA) used in other industries to proactively design optimal processes. Improving processes of care and reducing costs is not a marketing mantra for Quture, but it is the compelling business case for hospital customers to license QualOptima.
“Proctoring” is the required process for hospitals to evaluate the current clinical competence of practitioners to perform specific patient interventions or treat certain conditions. Proctoring is required of all physicians new to a hospital or on the medical staff and applying to perform a new clinical “privilege”. Quture’s existing product QReview is ideally suited to perform proctoring, and the new QualOptima product will use this software methodology to electronically conduct proctoring.
“Triggers” are clinical indicators and are required as part of Ongoing Professional Practice Evaluation to monitor safety concerns. QualOptima implements the published triggers, but electronically conducts this required process instead of traditional manual data abstraction. The Company further employs its license to electronically “machine read” free text in crucial EMR documents to identify triggers and to “learn” from electronic pattern and trend analytics.
Peer review is the traditional business of the Founder of Quture, with over 35 years of performance measurement using this methodology and with the best software product for conducting peer review formally called QReview. When this product is completely migrated onto the InterSystems platform, peer review will be integrated with the three (3) components above for performance measurement, proctoring, and triggers to dramatically enhance the functionality of the former product. For example, we expect peer review will be capable of being performed remotely by physicians in Quture’s QualPRO expert panel of physicians in every medical specialty. The former strength of the product to impose quality of care question sets to standardize peer review for “structured” review will be dramatically improved to use performance measures electronically for “structured explicit review”.
QualOptima also introduces another new functionality to integrate quality and patient safety data into the Qualytx database using the Ensemble interface engine from the multitude of data “silos” of all the quality and risk functions in hospitals, each with its own software product. Overcoming the impediment of disparate databases has been a product design now included in the QualOptima product.
The QualOptima product has two different configurations to accommodate both large hospitals, some with systems and system-wide programs, and small and rural hospitals. Small and rural hospitals will be able to contract for monthly access to cloud computing the QualOptima solution that would otherwise not be affordable. The InterSystems product platform will be installed on secure servers and operated remotely at a reduced operating cost using InterSystems’ Ensemble Enterprise pricing model. For larger hospitals, who want their servers on their side of the “firewall” for data security and control, the pricing model and product server licensing from InterSystems provides the other product configuration.
QSURG
QSurg - is a fully developed and tested electronic medical record product for surgery centers. This product is the only EMR with a fully functional performance analytics application embedded in the workflow and data capture. The QSurg EMR introduced capturing patient data at point of care in the operative and patient care areas. The product was built for tablet PC’s several years ago, anticipating this hardware opportunity and in an Application Partnership with Motion Computing, the best tablet for operative areas since it can be antiseptically cleaned and is a closed system with a special Intel processor.
QSurg is the electronic medical record (EMR) for surgery centers and hospitals, eliminating storing paper and having patient data readily available at point of care, measures performance focused on efficiency and effectiveness (such as OR turnover times) and absence of complications (such as infection rates), enhances revenue, and improves patient safety and outcomes, and complies with federal and state regulations.
Dr. Candiotti has been the consulting physician for the anesthesiology application of the QSurg product. The demonstration project at the University of Miami, Jackson Memorial Hospital, has already resulted in a unique anesthesiology application embedded in the QSurg product.
CUSTOMERS
Quture has begun marketing its products to the array of heath care providers, primarily focused on hospitals, hospital systems, and their insured trusts, as well as health maintenance organizations, physician groups, and provider groups (collaboratives). Initial operations and sales are structured in traditional licensing models, most favorably subscription based sales. Operationally, the Company provides performance measures for customer selection from the QuOp Performance Measures Library. The metrics of numerator and denominator integration using the Ensemble interface engine will be installed in coordination with InterSystems engineers. Support for the product will be first and primarily with InterSystems, supported in an advisory capacity by Quture. Regional Health Information Exchanges are a target customer for the InterSystems HealthShare product and should be considered as a potential customer by Quture.
New federal incentives have created new health care organizational models that may be targets for Quture products. First and most important among these are Accountable Care Organizations (ACO’s). New regulations creating Health Homes, where payments are bundled for those organizations to divide between providers and can be divided based on performance and results for value are important as customers. State cooperative insurance exchanges are another example of new structures as potential customers. Purchasers ranging from the federal government to employers to health savings accounts are target customers, as well.
Customers of the proctoring product are unique and include device manufacturers, pharmaceutical and proteonomics companies, and medical equipment companies.
The QSurg product is designed for physician office surgical/invasive procedure facilities, ambulatory surgery centers, and surgical hospitals.
Products, data sets and clinical knowledge, including predictive clinical and personalized knowledge, are planned as discussed for patients as customers of Quture. As performance results become transparent and medicine becomes personalized, participatory, and predictive, patients will become customers of Quture.
Performance measurement is intended to evolve into customers for measuring Good Clinical Practice (GCP) applications of the Quture’s products and data sales to pharmaceutical and stem cell companies, medical device, equipment and supply manufacturers.
COMPETITION
Quture competitors are profiled for the performance measurement and analytics product. There is no identical competitor for this product, as demonstrated by the matrix graphic below. However, there are three (3) categories of companies and products that provide some elements similar to the Quture product: performance measurement but based on billing/administrative data rather than Quture’s clinical data, clinical content products that attempt to connect with a software product to deliver extensive content (primarily publishing companies), and niche products primarily for peer review (where the peer review product of Quture now enhanced for performance and outcomes measurement).
Performance Measurement Based on Billing/Administrative Data:
Premier (www.premierinc.com) - Products such as QualityConnect and the Premier Integrated Performance Platform provide users with performance and quality data. The legacy products remain primarily reliant on billing/administrative data rather than point of care clinical data. The Quest program, for example, connects healthcare providers to share knowledge about quality, performance, patient safety and group contracting for hospital products and services. These programs collect data from participating hospitals to share clinical knowledge so that hospitals can learn from one another..
3M Health Information Systems (http://solutions.3m.com) – Software analyzing billing/administrative data and claiming significance as performance measurement and analysis. Reliance on administrative data correlates co-morbidity data and uses algorithms and formulas to claim calculation of quality of care information. Software and consulting services solutions across the health care continuum for transcription, speech recognition, clinical documentation improvement, document management, computer-assisted coding, quality, and revenue cycle management.
Xerox (www.midasplus.com) – ACS Healthcare Solutions was acquired including the Midas+ product solutions, operated as a separate business unit of Affiliated Computer Services, Inc., a Xerox Company, The product array relies primarily upon billing/administrative data to collect quality, patient safety, and financial information. Midas+ became an integral component of the transformational outsourcing, consulting, revenue cycle and analytics solution that HCS provides to its hospital clients. Midas+ continues to be responsible for its direction, development, sales, implementation, and support with respect to the products and services it offers its clients. As part of the ACS and HCS family, Midas+ has the financial, technical, and administrative support of ACS, a premier provider of business process and information technology solutions.
Thompson-Reuters (www.thomsonreuters.com) – Thomson Reuters announced they were exiting the health care business segment, then introduced their new suite of software called “Payment Reform Solutions” suite. Quture believes that this portfolio of products has now been sold but is without details as to that sale. The new product suite claims to include risk analytics to use real time clinical data to better coordinate care, manage costs and maintain quality for value-based purchasing. It is unclear how this new product relates to their treatment pathways (see Clinical Content Companies) and the performance product called “Advantage Suite” (including the CareDiscovery module) and Physician Performance Assessment. However, these products are clearly based on their “Medical Episode Grouper” (MEG) and, therefore, use billing and administrative data, not clinical data, as the basis for these products.
Quantros (www.quantros.com) - The Quantros suite of products includes safety and risk management, with its “Safety Events Manager” the core application, for analysis of events and near misses entered into the system, regulatory and compliance reporting, and infection control, quality and performance reporting and analysis off the events application. The suite of products includes a patient safety organization system and a dashboard graphics display for multiple constituencies.
Clinical Content Companies
Elsevier – Elsevier is a major publisher of prominent medical books and journals. In an effort to leverage the clinical content in these publications, as “the world’s leading provider of science and health information,” the European company has acquired software tools such as MEDai. MEDai Pinpoint claims to provide analytics for population management, clinical surveillance, predictive analytics, physician profiling and performance, and outcomes analysis. Data collection requires developing API connections for customers, however.
Hearst – Hearst Corporation acquired First DataBank to enter the patient safety and quality healthcare quality focused primarily on drug databases. Hearst acquired Zynx Health to expand clinical content to evidence-based resources of physician order sets, interdisciplinary plans of care, alerts and reminders. The Zynx customizable templates are intended to provide clinical support and include content to reduce care disparities, minimize errors, and save staff time through goals and expected outcomes, clinical interventions, and embedded links to relevant research and quality measures.
Thomson Reuters – Micromedix (www.micromedix.com) – The clinical decision support system of Thomson Reuters and aggregated products that run off the clinical content acquired in their acquisition of Micromedix includes products running on their evidence-based reference information for drug, disease, and toxicology management and for patient education. These can interface with products like MarketScan® Treatment Pathways. Thomson Reuters’ modules for real-time clinical decision support and then performance, quality, and financial analytics (see Performance Measurement products above).
Niche Products:
Medkinetics (www.medkinetics.com) – The Medkinetics product is primarily designed as medical staff credentialing for Ongoing and Focused Professional Practice Evaluation through performance measurement and peer review. The Electronic Physician Record® (EPR®) quality & performance improvement product is a physician peer review product. The system relies on user development of individual performance improvement plans.
Acesis (www.acesis.com) – The Acesis Healthcare Improvement Platform provides templates for performance improvement projects, process control/checklists, patient experience/satisfaction, peer review, root cause analysis, adverse event reporting, incident reporting, and morbidity and mortality review. The system combines core measures with other custom criteria for peer review in the product template. The system combines core measures and other custom criteria to determine reasons for peer review in the template. The product relies upon each organization developing safety event classification definitions, taxonomy and algorithms in order to determine the level of harm and event type. .
HCPRO – HCPRO is essentially a publishing company focused on compliance in health care. The Greeley Company is primarily a services company that provides educational and templates for credentialing, quality and risk management processes including external peer review. The Core Privilege Plus web-based software product with core privileging data designed to implement criteria-based privileging.
The Quture performance and outcomes analytics product does not compete with electronic medical record (EMR) companies. The product integrates clinical data from these EMR products but integrates data from multiple vendor products for each customer.
There are competitors to Quture’s surgery center QSurg electronic medical record producte, primarily ZChart and source Medical (www.sourcemed.net), but this is primarily a company whose products are focused on scheduling, billing, and inventory. Neither of these products competed with the performance measuremenet and quality improvement component of the Quture product. There are two significant outcomes measurement companies, including Outcome Sciences (www.outcome.com) and Surgical Outcomes (SOIX).
| COMPANY | CLINICAL DATA BASED | BILLING CODE BASED | PERFORMANCE MEASURES* | DISPARATE DATA** | PEER REVIEW | TJC / MU*** COMPLIANT | FINANCIAL |
| QutureX | X | X | X | X | X | X | |
| Medkinetics | X | X | |||||
| Premier | X | X | X | ||||
| Acesis | X | X | X | X | |||
| HCPRO | X | X | |||||
| 3M | X | X | X | ||||
| XEROX | X | X |
* QualOptima – is the only product integrating nationally published performance measures and electronically capturing numerator/denominator data.
** Disparate data in competitor products based solely on billing codes.
*** TJC (The Joint Commission) and MU (Meaningful Use) relevant to the HITECH Act.
There can be no assurance that the Company will compete successfully with existing or new competitors, or that the competition will not have a material adverse effect on the business, operating results or financial condition of the Company (See “RISK FACTORS”).
LIQUIDITY AND CAPITAL RESOURCES
Quture now has significant opportunities to capitalize the Company. The traditional potential sources of capital are possible proceeds from private placements, issuance of notes payable, loans from its officers, and cash from future revenues after the Company commences sales as well as funding from accounts receivable. The Company may require additional financing to continue operations, and there is no assurance that such additional financing will be available. However, the Company is now engaged in exploring funding opportunities based on funding other subsidiary companies of Quture, specifically the established subsidiaries of Quture Euro, LLC and the Qx Health Exchange, as well as potential additional subsidiaries including but not limited to a finance company subsidiary and/or a personalized medicine subsidiary.
POTENTIAL FUTURE PROJECTS AND CONFLICTS OF INTEREST
Members of the Company’s Management may serve in the future as an officer, director or investor in other entities. Neither the Company nor any shareholder would have any interest in these projects. Management believes that they have sufficient resources to fully discharge their responsibilities to all projects they have organized or will organize in the future, if any.
GOVERNMENT REGULATION
Statutes unique to health care that could potentially impact the Company include but are not limited to the following federal statutes:
Health Insurance Portability and Accountability Act of 1996 (HIPPA) Privacy and Security Rules – protects the privacy of individually identifiable health information. The HIPPA Privacy and Security Rules set national standards for the security of electronically protected health information. The confidentiality provisions of the Patient Safety Rule protect identifiable information being used to analyze patient safety events and improve patient safety. There are severe monetary sanctions for violations of these rules and statutory provisions.
Patient Protection and Affordable Care Act (2010) – provides that all Americans have access to quality, affordable health care and will create the transformation within the health care system necessary to contain costs. The Congressional Budget Office (CBO) has determined that the Patient Protection and Affordable Care Act is fully paid for, will provide coverage to more than 94% of Americans while staying under the $900 billion limit that President Obama established, bending the health care cost curve, and reducing the deficit over the next ten years and beyond. The Patient Protection and Affordable Care Act contains nine titles, each addressing an essential component of reform:
| • | Quality, affordable health care for all Americans |
| • | The role of public programs |
| • | Improving the quality and efficiency of health care |
| • | Prevention of chronic disease and improving public health |
| • | Health care workforce |
| • | Transparency and program integrity |
| • | Improving access to innovative medical therapies |
| • | Community living assistance services and supports |
Revenue provisions
Some of the significant provisions to the Company include development of new patient care and payment models. A new Center for Medicare & Medicaid Innovation will be established within the Centers for Medicare and Medicaid Services to research, develop, test, and expand innovative payment and delivery arrangements. Accountable Care Organizations (ACOs) that take responsibility for cost and quality received by patients will receive a share of savings they achieve for Medicare. The HHS Secretary will develop a national, voluntary pilot program encouraging hospitals, doctors, and post-acute providers to improve patient care and achieve savings through bundled payments. A new demonstration program for chronically ill Medicare beneficiaries will test payment incentives and service delivery using physician and nurse practitioner-directed home-based primary care teams. Beginning in 2012, hospital payments will be adjusted based on the dollar value of each hospital’s percentage of potentially preventable Medicare readmissions.
Title III. Improving the Quality and Efficiency of Health Care provides for improvement of quality and efficiency of U.S. medical care services for everyone, and especially for those enrolled in Medicare and Medicaid. Payment for services will be linked to better quality outcomes. The Patient Protection and Affordable Care Act will make substantial investments to improve the quality and delivery of care and support research to inform consumers about patient outcomes resulting from different approaches to treatment and care delivery. New patient care models will be created and disseminated. Rural patients and providers will see meaningful improvements. Payment accuracy will improve. The Medicare Part D prescription drug benefit will be enhanced and the coverage gap, or donut hole, will be reduced. An Independent Medicare Advisory Board will develop recommendations to ensure long-term fiscal stability.
The Health Care Quality Improvement provisions create a new program to develop community health teams supporting medical homes to increase access to community-based, coordinated care. It supports a health delivery system research center to conduct research on health delivery system improvement and best practices that improve the quality, safety, and efficiency of health care delivery. And, it support medication management services by local health providers to help patients better manage chronic disease. The Act provides for the establishment of a private, nonprofit entity (the Patient-Centered Outcomes Research Institute) governed by a public- private board appointed by the Comptroller General to provide for the conduct of comparative clinical outcomes research. The Act provides Support for Prevention and Public Health Innovation, which empowers the HHS Secretary to provide funding for research in public health services and systems to examine best prevention practices.
Title IV: Prevention of Chronic Disease and Improving Public Health provides for better orienting the nation’s health care system toward health promotion and disease prevention, a set of initiatives will provide the impetus and the infrastructure. A new interagency prevention council will be supported by a new Prevention and Public Health Investment Fund. Barriers to accessing clinical preventive services will be removed. Developing healthy communities will be a priority, and a 21st century public health infrastructure will support this goal.
The Act provides for the creation of American Health Benefit Exchanges. By 2014, each state will establish an Exchange to help individuals and small employers obtain coverage. Plans participating in the Exchanges will be accredited for quality, will present their benefit options in a standardized manner for easy comparison, and will use one, simple enrollment form.
American Recovery Act and Reinvestment Act of 2009 (ARRA) – HITECH Act “Meaningful Use” provisions and financial incentives - The Medicare and Medicaid EHR Incentive Programs provide a financial incentive for the "meaningful use" of certified EHR technology to achieve health and efficiency goals. These incentives are designed to reduce medical errors, makes patient records and data available to providers, create reminders and alerts, clinical decision support, and e-prescribing/refill automation. The criteria for meaningful use will be staged in three steps over the course of the next five years. Stage 1 (2011 and 2012) sets the baseline for electronic data capture and information sharing. Stage 2 (expected to be implemented in 2013) and Stage 3 (expected to be implemented in 2015) will continue to expand on this baseline and be developed through future rule making.
To demonstrate meaningful use successfully, eligible professionals, eligible hospitals and CAHs are required also to report clinical quality measures specific to eligible professionals or eligible hospitals and CAHs. Eligible professionals must report on 6 total clinical quality measures: 3 required core measures (substituting alternate core measures where necessary) and 3 additional measures (selected from a set of 38 clinical quality measures). Eligible hospitals and CAHs must report on all 15 of their clinical quality measures.
Health Information Technology for Economic and Clinical Act of ARRA (see above) – basically establishes four (4) major goals that advance the use of health information technology, such as electronic medical records by:
Requiring the government to take a leadership role to develop standards by 2010 that allow for the nationwide electronic exchange and use of health information technology to improve quality and coordination of care;
Investing $20 billion in health information technology infrastructure and Medicare and Medicaid incentives to encourage doctors and hospitals to use HIT (health information technology) to electronically exchange patients’ health information;
Saving the government $10 billion, and generating additional savings throughout the health sector, through improvements in quality of care and care coordination, and reductions in medical errors and duplicative care; and strengthening federal privacy and security law to protect identifiable health information from misuse as the health care sector increases use of Health IT.
The Act provides immediate funding for health information technology infrastructure, training, dissemination of best practices, telemedicine, inclusion of health information technology for clinical education, and State grants to promote health information technology.
The legislation establishes a voluntary certification process for health information technology products. The National Institute of Standards and Technology will provide for the testing of such products to determine if they meet national standards that allow for the secure electronic exchange and use of health information.
The Act further provides significant financial incentives through Medicare and Medicaid programs to encourage doctors and hospitals to adopt and use certified electronic health records. Physicians will be eligible for $40,000 to $65,000 for showing that they are meaningfully using health information technology, such as through the reporting of quality measures. Hospitals are eligible for many millions of dollars in the Medicare and Medicaid programs to similarly use health information technology. Federally qualified health centers, rural health clinics, children’s hospitals and others will be eligible for funding through the Medicaid program.
Incentive payments for both physicians and hospitals to continue for several years, but are phased out over time. Eventually, Medicare payments are reduced for physicians and hospitals that do not use a certified electronic health record that allows them to communicate with others.
ITEM 1A - RISK FACTORS
RISK FACTORS
OUR SECURITIES ARE HIGHLY SPECULATIVE, AND PROSPECTIVE PURCHASERS SHOULD BE AWARE THAT AN INVESTMENT IN THE SECURITIES INVOLVES A HIGH DEGREE OF RISK. ACCORDINGLY, PROSPECTIVE PURCHASERS SHOULD CAREFULLY CONSIDER THE FOLLOWING RISK FACTORS IN ADDITION TO THE OTHER INFORMATION IN THIS CURRENT REPORT AND RELATED EXHIBITS, INCLUDING OUR FINANCIAL STATEMENTS.
RISK FACTORS ASSOCIATED WITH OUR BUSINESS
We have a limited operating history on which to base an investment decision.
We are a newly created startup company that has very limited operating history. As a startup company, we are subject to unforeseen costs, expenses, problems and difficulties inherent in new business ventures. There is no history upon which to base any assumption as to the likelihood that the company will prove successful. We cannot provide investors with any assurance that our services will attract customers; generate any operating revenue or ever achieve profitable operations. If we are unable to address these risks, there is a high probability that our business can fail.
We have inadequate capital and need for additional financing to accomplish our business and strategic plans.
We have very limited funds, and such funds are not adequate to develop our current business plan. Our ultimate success may depend on our ability to raise additional capital. In the absence of additional financing or significant revenues and profits, the company will have to approach its business plan from a much different and much more restricted direction, attempting to secure additional funding sources to fund its growth, borrowing money from lenders or elsewhere or to take other actions to attempt to provide funding. We cannot guarantee that we will be able to obtain sufficient additional funds when needed, or that such funds, if available, will be obtainable on terms satisfactory to us.
We have a limited history of success and profitability.
We have limited current business operations and may incur significant losses and negative operating cash flow for the foreseeable future, and we may never achieve or maintain profitability. We may incur losses for the foreseeable future and may never become profitable.
Our management team may not be able to successfully implement our business strategies.
If our management team is unable to execute on its business strategies, then our development, including the establishment of revenues and our sales and marketing activities would be materially and adversely affected. In addition, we may encounter difficulties in effectively managing the budgeting, forecasting and other process control issues presented by any future growth. We may seek to augment or replace members of our management team or we may lose key members of our management team, and we may not be able to attract new management talent with sufficient skill and experience.
Our products are new and innovative and have not been established in the healthcare marketplace.
The Company’s initial products are new to the hospital and physician market and may take time or even fail to become accepted as having value or sufficient return on investment for potential users. We could encounter significant delays in adoption of these innovations and new tools, which would harm or at least delay our revenue projections. The Company’s business plan and revenue projections are based upon rapid market penetration and achieving critical mass to become the international standard for performance measurement. The marketing and sales of our products depends upon our “blue ocean” strategy to introduce “disruptive” unique technology and define our market niche with a new series of products. Health care has traditionally resisted clinical quality data and information, and the Company’s strategy is directly related to federal initiatives and accreditation standards that are now favorable, but are subject to change and to enforcement variables that could significantly affect the Company and adversely affect its gross revenue, margins and projections.
Our products and services may suffer from uncertainty of market acceptance, and we may have difficulty commercializing them.
The Company is currently engaged in migrating developed products to a new technology platform and is in various stages of developing new software and technology products. Continued product and service development and commercialization efforts are subject to all of the risks inherent in the development of new products and services, including unanticipated development problems, lack of acceptance in the market, as well as the possible insufficiency of funds to undertake development and commercialization, any of which could result in abandonment or substantial change in the development of a specific product or service. In addition, demand and market acceptance for newly developed products and services are subject to a high level of uncertainty and the Company does not have any assurance that its concepts and ideas will achieve market acceptance. Achieving market acceptance of the Company’s new products and services will require substantial marketing efforts and the expenditure of significant funds. If the Company’s business concepts and device concepts and devices do not achieve market acceptance in a fairly cost-effective and quick time frame, the Company and its prospects will be materially adversely affected and it could well cause the Company to fail.
We may not be able to compete initially or maintain market share even if originally successful.
The sale of the Company’s proposed products and services involve competitive businesses, and the Company faces or may face significant competition. The Company will compete with many other companies, most of which have far greater experience, product penetration, financial and personnel resources, marketing and distribution organizations and overall market power than the Company. There can be no assurance that the Company will be able to compete effectively or respond successfully to any future changes in the competition or that additional competitors will not enter the market. There is also no assurance that other parties will not try to copy ideas developed by the Company. The failure to meet these challenges could have a material adverse effect on the Company and its business and could cause the Company to fail. There can also be no assurance that the Company’s products will remain viable and attractive alternatives for customers.
We may not be able to protect our intellectual property, which would potentially harm or jeopardize the Company.
The Company is currently attempting to secure provisional patents and convert provisional patents to permanent patents on its products and on the products being developed or marketed by the Company. There is no assurance that the Company will receive the patents that may be necessary to protect our intellect property; that the patents would not be significantly narrowed in their scope; that the patents would turn out to be a material advantage to the Company; that other parties will not find ways to circumvent the benefits of any such patents; that other ideas on which the Company’s business plan is based will not be utilized by competitors of the business or that competitors will not find improved methods of achieving what the Company plans to achieve, leaving the Company without the ability to effectively compete.
We are significantly dependent on key software and technology partnerships.
The Company’s products and development strategy rely upon an application partnership with InterSystems and a strategic vendor relationship with a development company that are not essential but are vital to our products and success.
If we are unable to achieve our milestones and strategic challenges our success could be delayed or jeopardized.
The product plan during capitalization focuses on software products for hospitals, which are the primary customer for the Company’s initial product offering. There are other potential customer and influencers, such as health and liability insurance companies, healthcare law firms, financial healthcare consulting and accounting firms, and governmental and regulatory entities. There are numerous newly formulated entities, as well, such as accountable care organizations and state insurance exchanges (“co-ops”) that appear to be logical customers and influencers in the Company’s strategic product plan. Initially, our product plan relies upon the successful migration of our products to a new technology platform and the success of that technology to achieve the technical specifications and requirements of the product design. Despite initial assessments of product performance, there could be any series of recognized or unrecognized performance challenges and/or impediments that could affect the Company’s product development and performance milestones and strategic challenges with customers and influencers. Our other existing product, an electronic medical record for surgery centers with integrated performance measurement integrated, has similar risks.
If we fail to manage growth effectively or prepare for product scalability, it could have an adverse affect on our employee efficiency, product quality, working capital levels and results of operations.
Any significant growth in the market for our products or our entry into new markets may require an expansion of our employee base for managerial, operational, financial, and other purposes. As of July 31, 2011, we had zero full time employees. During any period of growth, we may face problems related to our operational and financial systems and controls, including quality control and delivery and service capacities. We would also need to continue to expand, train and manage our employee base. Continued future growth will impose significant added responsibilities upon the members of management to identify, recruit, maintain, integrate, and motivate new employees.
Aside from increased difficulties in the management of human resources, we may also encounter working capital issues, as we will need increased liquidity to finance the development of new products, and the hiring of additional employees. For effective growth management, we will be required to continue improving our operations, management, and financial systems and controls. Our failure to manage growth effectively may lead to operational and financial inefficiencies that will have a negative effect on our profitability. We cannot assure investors that we will be able to timely and effectively meet that demand and maintain the quality standards required by our existing and potential customers.
If we are unable to retain key executives and other key affiliates, particularly our founder and medical/clinical experts, our growth could be significantly inhibited and our business harmed with a material adverse affect on our business, financial condition and results of operations.
Our success is, to a certain extent, attributable to the management, sales and marketing, and operational and technical expertise of certain key personnel. Mr. G. Landon Feazell, our Chief Executive Officer and President, performs key functions in the operation of our business. The loss of Mr. Feazell could have a material adverse effect upon our business, financial condition, and results of operations. We do not maintain key-person insurance for members of our management team because it is cost prohibitive at this point. If we lose the services of any senior management and medical experts, including our QualPRO panels, we may not be able to locate suitable or qualified replacements, and may incur additional expenses to recruit and train new personnel, which could severely disrupt our business and prospects.
Our management team may not be able to successfully implement our business strategies.
If our management team is unable to execute on its business strategies, then our development, including the establishment of revenues and our sales and marketing activities would be materially and adversely affected. In addition, we may encounter difficulties in effectively managing the budgeting, forecasting and other process control issues presented by any future growth. We may seek to augment or replace members of our management team or we may lose key members of our management team, and we may not be able to attract new management talent with sufficient skill and experience.
Our success in the future may depend on our ability to establish and maintain strategic alliances, and any failure on our part to establish and maintain such relationships would adversely affect our market penetration and revenue growth.
We may be required to establish strategic relationships with third parties. Our ability to establish strategic relationships will depend on a number of factors, many of which are outside our control, such as the competitive position of our product and marketing plan relative to our competitors. We may not be able to establish other strategic relationships in the future. In addition, any strategic alliances that we establish may subject us to a number of risks, including risks associated with sharing proprietary information, loss of control of operations that are material to developed business and profit-sharing arrangements. Moreover, strategic alliances may be expensive to implement and subject us to the risk that the third party will not perform its obligations under the relationship, which may subject us to losses over which we have no control or expensive termination arrangements. As a result, even if our strategic alliances with third parties are successful, our business may be adversely affected by a number of factors that are outside of our control.
Our financial results may not meet the expectations of investors and may fluctuate because of many factors and, as a result, investors should not rely on our revenue and/or financial projections as indicative of future results.
Fluctuations in operating results or the failure of operating results to meet the expectations investors may negatively impact the value of our securities. Operating results may fluctuate due to a variety of factors that could affect revenues or expenses in any particular quarter. Fluctuations in operating results could cause the value of our securities to decline. Investors should not rely on revenue or financial projections or comparisons of results of operations as an indication of future performance. As a result of the factors listed below, it is possible that in future periods results of operations may be below the expectations of investors. This could cause the market price of our securities to decline. Factors that may affect our quarterly results include:
| • | delays in sales resulting from potential customer sales cycles | |
| • | variations or inconsistencies in return on investment models and results | |
| • | delays in demonstrating product performance or installations | |
| • | resistance from medical and/or clinical professionals to the products | |
| • | changes in competition | |
| • | Changes or threats of significant changes in legislation or rules or standards that would change the drivers for product adoption. |
Our strategy may include acquiring companies which may result in unsuitable acquisitions or failure to successfully integrate acquired companies, which could lead to reduced profitability.
We may embark on a growth strategy through acquisitions of companies or operations that complement existing product lines, customers or other capabilities. We may be unsuccessful in identifying suitable acquisition candidates, or may be unable to consummate desired acquisitions. To the extent any future acquisitions are completed, we may be unsuccessful in integrating acquired companies or their operations, or if integration is more difficult than anticipated, we may experience disruptions that could have a material adverse impact on future profitability. Some of the risks that may affect our ability to integrate, or realize any anticipated benefits from, acquisitions include:
| • | unexpected losses of key employees or customer of the acquired company; | |
| • | difficulties integrating the acquired company’s standards, processes, procedures and controls; | |
| • | difficulties coordinating new product and process development; | |
| • | difficulties hiring additional management and other critical personnel; | |
| • | difficulties increasing the scope, geographic diversity and complexity of our operations; | |
| • | difficulties consolidating facilities, transferring processes and know-how; | |
| • | difficulties reducing costs of the acquired company’s business; | |
| • | diversion of management’s attention from our management; and | |
| • | adverse impacts on retaining existing business relationships with customers. |
Our Chief Executive Officer and certain stockholders possess the majority of our voting power, and through this ownership, control our Company and our corporate actions.
Our current CEO and President, G. Landon Feazell, holds approximately 70% of the voting power of the outstanding shares immediately after this Share Exchange Agreement and shares distribution. Mr. Feazell has a controlling influence in determining the outcome of any corporate transaction or other matters submitted to our stockholders for approval, including mergers, consolidations and the sale of all or substantially all of our assets, election of directors, and other significant corporate actions. As such Mr. Feazell also has the power to prevent or cause a change in control; therefore, without his consent we could be prevented from entering into transactions that could be beneficial to us. The interests of Mr. Feazell may give rise to a conflict of interest with the Company and the Company’s shareholders. For additional details concerning voting power please refer to the section below entitled “Description of Securities.”
There is a substantial lack of liquidity of our common stock and volatility risks.
Our common stock is quoted on the OTCQB under the symbol “TCLN.” The liquidity of our common stock may be very limited and affected by our limited trading market. The OTCQB market is an inter-dealer market much less regulated than the major exchanges, and is subject to abuses, volatilities and shorting. There is currently no broadly followed and established trading market for our common stock. An established trading market may never develop or be maintained. Active trading markets generally result in lower price volatility and more efficient execution of buy and sell orders. Absence of an active trading market reduces the liquidity of the shares traded.
The trading volume of our common stock may be limited and sporadic. This situation is attributable to a number of factors, including the fact that we are a small company which is relatively unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that current trading levels will be sustained. As a result of such trading activity, the quoted price for our common stock on the OTCQB may not necessarily be a reliable indicator of our fair market value. In addition, if our shares of common stock cease to be quoted, holders would find it more difficult to dispose of or to obtain accurate quotation as to the market value of, our common stock and as a result, the market value of our common stock likely would decline.
The market price for our stock may be volatile and subject to fluctuations in response to factors, including the following:
•
|
the increased concentration of the ownership of our shares by a limited number of affiliated stockholders following the Share Exchange may limit interest in our securities; |
| • | variations in quarterly operating results from the expectations of securities analysts or investors; |
| • | revisions in securities analysts’ estimates or reductions in security analysts’ coverage; |
•
|
announcements of new attractions or services by us or our competitors; |
•
|
reductions in the market share of our services; |
•
|
announcements by us or our competitors of significant acquisitions, strategic partnerships, joint ventures or capital commitments; |
•
|
general technological, market or economic trends; |
•
|
investor perception of our industry or prospects; |
•
|
insider selling or buying; |
•
|
investors entering into short sale contracts; |
•
|
regulatory developments affecting our industry; and |
•
|
additions or departures of key personnel. |
Many of these factors are beyond our control and may decrease the market price of our common stock, regardless of our operating performance. We cannot make any predictions or projections as to what the prevailing market price for our common stock will be at any time, including as to whether our common stock will sustain current market prices, or as to what effect that the sale of shares or the availability of common stock for sale at any time will have on the prevailing market price.
Our common stock may never be listed on a major stock exchange.
We anticipate seeking the listing of our common stock on a national or other securities exchange at some time in the future, assuming that we can satisfy the initial listing standards for such exchange. We currently do not satisfy the initial listing standards and cannot ensure that we will be able to satisfy such listing standards or that our common stock will be accepted for listing on any such exchange. Should we fail to satisfy the initial listing standards of such exchanges, or our common stock is otherwise rejected for listing, the trading price of our common stock could suffer, the trading market for our common stock may be less liquid, and our common stock price may be subject to increased volatility.
A decline in the price of our common stock could affect our ability to raise working capital and adversely impact our ability to continue operations.
A prolonged decline in the price of our common stock could result in a reduction in the liquidity of our common stock and a reduction in our ability to raise capital. A decline in the price of our common stock could be especially detrimental to our liquidity and our operations. Such reductions may force us to reallocate funds from other planned uses and may have a significant negative effect on our business plan and operations, including our ability to develop new services and continue our current operations. If our common stock price declines, we can offer no assurance that we will be able to raise additional capital or generate funds from operations sufficient to meet our obligations. If we are unable to raise sufficient capital in the future, we may not be able to have the resources to continue our normal operations.
Concentrated ownership of our common stock creates a risk of sudden changes in our common stock price.
The sale by any shareholder of a significant portion of their holdings could have a material adverse effect on the market price of our common stock.
Sales of our currently issued and outstanding stock may become freely tradable pursuant to Rule 144 and may dilute the market for your shares and have a depressive effect on the price of the shares of our common stock.
A substantial majority of the outstanding shares of common stock are “restricted securities” within the meaning of Rule 144 under the Securities Act of 1933, as amended (the “Securities Act”) (“Rule 144”). As restricted shares, these shares may be resold only pursuant to an effective registration statement or under the requirements of Rule 144 or other applicable exemptions from registration under the Securities Act and as required under applicable state securities laws. Rule 144 provides in essence that a non-affiliate who has held restricted securities for a period of at least six months may sell their shares of common stock. Under Rule 144, affiliates who have held restricted securities for a period of at least six months may, under certain conditions, sell every three months, in brokerage transactions, a number of shares that does not exceed the greater of 1% of a company’s outstanding shares of common stock or the average weekly trading volume during the four calendar weeks prior to the sale (the four calendar week rule does not apply to companies quoted on the OTCQB). A sale under Rule 144 or under any other exemption from the Securities Act, if available, or pursuant to subsequent registrations of our shares of common stock, may have a depressive effect upon the price of our shares of common stock in any active market that may develop.
The securities issued in connection with the Share Exchange are restricted securities and may not be transferred in the absence of registration or the availability of a resale exemption.
The shares of common stock being issued in connection with the Share Exchange are being issued in reliance on an exemption from the registration requirements under Section 4(2) of the Securities Act and Regulation D promulgated thereunder or Regulation S. Consequently, these securities will be subject to restrictions on transfer under the Securities Act and may not be transferred in the absence of registration or the availability of a resale exemption. In particular, in the absence of registration, such securities cannot be resold to the public until certain requirements under Rule 144 promulgated under the Securities Act have been satisfied, including certain holding period requirements. As a result, a purchaser who receives any such securities issued in connection with the Share Exchange may be unable to sell such securities at the time or at the price or upon such other terms and conditions as the purchaser desires, and the terms of such sale may be less favorable to the purchaser than might be obtainable in the absence of such limitations and restrictions.
If we issue additional shares or derivative securities in the future, it will result in the dilution of our existing stockholders.
Our Certificate of Incorporation authorizes the issuance of up to 2,500,000,000 shares of common stock, $0.001 par value per share, and 10,000,000 shares of preferred stock, $0.001 par value per share. Our board of directors may choose to issue some or all of such shares, or derivative securities to purchase some or all of such shares, to provide additional financing in the future.
We do not plan to declare or pay any dividends to our stockholders in the near future.
We have not declared any dividends in the past, and we do not intend to distribute dividends in the near future. The declaration, payment and amount of any future dividends will be made at the discretion of the board of directors and will depend upon, among other things, the results of operations, cash flows and financial condition, operating and capital requirements, and other factors as the board of directors considers relevant. There is no assurance that future dividends will be paid, and if dividends are paid, there is no assurance with respect to the amount of any such dividend.
The requirements of being a public company may strain our resources and distract management.
As a public company, we are subject to the reporting requirements of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), and the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”). These requirements are extensive. The Exchange Act requires that we file annual, quarterly and current reports with respect to our business and financial condition. The Sarbanes-Oxley Act requires that we maintain effective disclosure controls and procedures and internal controls over financial reporting.
We may incur significant costs associated with our public company reporting requirements and costs associated with applicable corporate governance requirements. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. This may divert management’s attention from other business concerns, which could have a material adverse effect on our business, financial condition and results of operations. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers. We are currently evaluating and monitoring developments with respect to these rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
If we do not file our quarterly or annual reports with the SEC, we may be de-listed from the OTCQB.
OTCQB is the middle tier of the OTC market. OTCQB companies report to the SEC or a U.S. banking regulator, making it easy for investors to identify companies that are current in their reporting obligations. There are no financial or qualitative standards to be in this tier. OTCQB securities may also be quoted on the FINRA BB. The OTCQB allows investors to easily identify reporting companies traded in the OTC market regardless of where they are quoted.
Under OTCQB rules relating to the timely filing of periodic reports with the SEC, any OTCQB issuer who fails to file a periodic report (Forms 10-Q or 10-K) by the due date of such report, period we may be removed from the OTCQB and our common stock may only be able to be traded on the OTC Pink. The OTC Pink is the bottom tier of the OTC market – a speculative trading marketplace that helps broker-dealers get the best prices for investors. Accordingly, our securities may become worthless and we may be forced to curtail or abandon our business plan.
If we become subject to product liability claims, personal injury claims or defective products, our business may be harmed.
The testing, marketing and sale of the Company’s products and services entails risk of product liability and there can be no assurance that product liability claims will not be asserted against the Company. While the Company intends to obtain some business liability insurance, insurance designed to cover product liability is expensive, difficult to obtain in some cases and may not be available now or in the future on acceptable terms, if at all. Furthermore, there can be no assurance that such insurance coverage will be adequate, or that a product liability claim, even one without merit, would not have a material adverse effect on the business or financial condition of the Company. As a result, any imposition of product liability could materially harm our business, financial condition and results of operations. In addition, we do not have any business interruption insurance due to the limited coverage of any such business interruption insurance, and as a result, any business disruption or natural disaster could severely disrupt our business and operations and significantly decrease our revenue and profitability.
Persons associated with securities offerings, including consultants, may be deemed to be broker dealers.
In the event that any of our securities are offered without engaging a registered broker-dealer, we may face claims for rescission and other remedies. If any claims or actions were to be brought against us relating to our lack of compliance with the broker-dealer requirements, we could be subject to penalties, required to pay fines, make damages payments or settlement payments, or repurchase such securities. In addition, any claims or actions could force us to expend significant financial resources to defend our company, could divert the attention of our management from our core business and could harm our reputation.
Future changes in financial accounting standards or practices may cause adverse unexpected financial reporting fluctuations and affect reported results of operations.
A change in accounting standards or practices can have a significant effect on our reported results and may even affect our reporting of transactions completed before the change is effective. New accounting pronouncements and varying interpretations of accounting pronouncements have occurred and may occur in the future. Changes to existing rules or the questioning of current practices may adversely affect our reported financial results or the way we conduct business.
“Penny Stock” rules may make buying or selling our common stock difficult.
Trading in our common stock is subject to the “penny stock” rules. The SEC has adopted regulations that generally define a penny stock to be any equity security that has a market price of less than $5.00 per share, subject to certain exceptions. These rules require that any broker-dealer that recommends our common stock to persons other than prior customers and accredited investors, must, prior to the sale, make a special written suitability determination for the purchaser and receive the purchaser’s written agreement to execute the transaction. Unless an exception is available, the regulations require the delivery, prior to any transaction involving a penny stock, of a disclosure schedule explaining the penny stock market and the risks associated with trading in the penny stock market. In addition, broker-dealers must disclose commissions payable to both the broker-dealer and the registered representative and current quotations for the securities they offer. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers from effecting transactions in our common stock, which could severely limit the market price and liquidity of our common stock.
We have the right to issue up to 10,000,000 shares of preferred stock, which may adversely affect the voting power of the holders of our other securities and may deter hostile takeovers or delay changes in management control.
We may issue up to 10,000,000 shares of preferred stock from time to time in one or more series, and with such rights, preferences and designations as our board of directors may determine from time to time. To date, our board of directors have authorized Series A and B of preferred stock. Additionally, our board of directors, without further approval of our common stockholders, is authorized to fix the dividend rights and terms, conversion rights, voting rights, redemption rights, liquidation preferences and other rights and restrictions relating to any series of our preferred stock. Issuances of shares of preferred stock, while providing flexibility in connection with possible financings, acquisitions and other corporate purposes, could, among other things, adversely affect the voting power of the holders of our other securities and may, under certain circumstances, have the effect of deterring hostile takeovers or delay changes in management control.
The Company currently has outstanding convertible debt on its balance sheet and the issuance of common stock in satisfaction of the convertible debt will cause significant dilution to our shareholders.
We currently have $315,000 of convertible debt on our balance sheet that has terms that include issuing our common stock at up to a fifty percent (50%) discount to the market price of our common stock upon its conversion. This will cause significant dilution to our existing shareholders. Additionally other current obligations may be settled by the issuance of common stock which will cause dilution to our shareholders.
An investment in the Company may be diluted in the future as a result of the issuance of additional securities, the exercise of options or warrants or the conversion of aged debt and convertible debt.
In order to raise additional capital to fund its strategic plan, we expect to issue additional shares of common stock or securities convertible, exchangeable or exercisable into common stock from time to time, which could result in substantial dilution to investors. If we are successful in raising capital, we expect to issue securities convertible into common stock which would result in substantial dilution to investors. The current condition of the credit markets make it probable that if we are able to raise any working capital, it will be through the issuance of convertible debt, or the sale of restricted securities at a significant discount to the market price of our common stock. We may not be successful in raising additional capital.
Sales of additional equity securities may adversely affect the market price of our common stock and your rights in the Company may be reduced.
We expect to continue to incur research and development and selling, general and administrative costs, and in order to satisfy our funding requirements, we may need to sell additional equity securities. Our stockholders may experience substantial dilution and a reduction in the price that they are able to obtain upon sale of their shares. Also, any new securities issued may have greater rights, preferences or privileges than our existing common stock which may adversely affect the market price of our common stock and our stock price may decline substantially.
ITEM 1B. UNRESOLVED STAFF COMMENTS
None.
ITEM 2 - DESCRIPTION OF PROPERTY
FACILITIES
The Company’s corporate offices are located at:
121 South Orange Avenue
Suite 1500, North Tower
Orlando, FL 32801
The Company’s primarily operations offices are located at:
200 Magnolia Avenue
Daytona Beach, FL 32114
The Company’s primary financial and reporting offices are located at:
319 Clematis Street
West Palm Beach, FL 33401
We do not own any real estate or other properties.
ITEM 3 - LEGAL PROCEEDINGS
There are no pending legal proceedings to which the Company is a party or in which any director, officer or affiliate of the Company, any owner of record or beneficially of more than 5% of any class of voting securities of the Company, or security holder is a party adverse to the Company or has a material interest adverse to the Company.
ITEM 4. MINE SAFETY DISCLOSURES
None.
ITEM 5 - MARKET FOR REGISTRANTS COMMON STOCK, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASE OF EQUITY SECURITIES
Our authorized capital stock consists of 2,500,000,000 common shares, par value $0.001 per share (“Common Stock”). Holders of our common stock are entitled to one vote for each share held of record on all matters submitted to a vote of stockholders. As of August 9, 2012, the Company has 2,214,556,789 shares of Common Stock issued and outstanding and had 187 active shareholders of record. We have not paid cash dividends and as of April 30, 2012 we have outstanding options to purchase 10,500,000 shares of common stock at an exercise price of $0.03667 per share and 87,000,000 outstanding warrants to purchase 87,000,000 shares of common stock at an average exercise price of $0.008 per share. Additionally, the Company has a remaining reserve pursuant to the Exchange of 120,061,687 shares of Common Stock.
On July 19, 2010, we paid a stock dividend of two shares for each share of Common Stock owned as of the record date of July 16, 2010.
Techs Loanstar's ticker symbol for its shares of common stock quoted on the Over-the-Counter Quotation Board is "TCLN". For the period indicated, the following table sets forth the high and low closi prices per share of common stock. These prices represent inter-dealer quotations without retail markup, markdown, or commission and may not necessarily represent actual transactions.
| Fiscal Year 2012 | High ($) | Low ($) |
| Fourth Quarter | 0.0175 | 0.0082 |
| Third Quarter | 0.0325 | 0.0135 |
| Second Quarter | 0.02 | 0.009 |
| First Quarter | 0.0194 | 0.0016 |
| Fiscal Year 2012 | High ($) | Low ($) |
| Fourth Quarter | 0.0069 | 0.0011 |
| Third Quarter | 0.0104 | 0.0033 |
| Second Quarter | 0.045 | 0.0099 |
| First Quarter | 0.1633 | 0.0283 |
Dividend Policy
We have not paid cash dividends on our common stock and do not plan to pay such dividends in the foreseeable future. Our Board of Directors (“Board”) will determine our future dividend policy on the basis of many factors, including results of operations, capital requirements, and general business conditions.
Securities authorized for issuance under equity compensation plans
We have the following securities authorized for issuance under our equity compensation plans as of April 30, 2012, including options available for future issuance under our 2010 Equity Incentive Plan approved by our security holders effective January 29, 2010.
Equity Compensation Plan Information
| Plan category | Number of securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) | ||
| (a) | (b) | (c) | |||
| Equity compensation plans approved by security holders | 10,500,000 | $0.0367 | -0- | ||
| Equity compensation plans not approved by security holders | -0- | $-0- | -0- | ||
| Total | 10,500,000 | $0.0367 | -0- |
Recent Sales of Unregistered Equity Securities
During the year ended April 30, 2012 the Company issued 23,008,388 shares of common stock upon the conversion of $147,500 of debentures and $5,900 of accrued and unpaid interest. The shares were issued at an average price of approximately $0.0067 per share.
During the year ended April 30, 2012, we issued 55,000,000 shares of our common stock to unaffiliated accredited investors pursuant to a private placement. The shares were sold for $275,000 or $0.005 per share.
During the year ended April 30, 2012 the Company issued 70,000,000 shares of common stock for shares that were to be issued in connection with the Share Exchange transaction with Quture.
On December 12, 2011 the Company issued 2,000,000 shares of common stock to an unaffiliated Company in connection with the purchase of computer equipment. The Company valued the stock at $10,000 and recorded the computer equipment as of February 1, 2012, the date the Company received the equipment.
On December 5, 2011, the Company issued 9,043,164 shares of common stock pursuant to Debt Settlement and Release Agreements in exchange for the cancellation of $60,589 of notes and interest payable. The shares were issued at approximately $0.0067 per share.
The securities described above were offered and sold in reliance upon exemptions from registration pursuant to Section 4(2) under the Securities Act and Rule 506 of Regulation D promulgated there under. The agreements executed in connection with this sale contain representations to support the Registrant’s reasonable belief that the Investor had access to information concerning the Registrant’s operations and financial condition, the Investor acquired the securities for their own account and not with a view to the distribution thereof in the absence of an effective registration statement or an applicable exemption from registration, and that the Investor are sophisticated within the meaning of Section 4(2) of the Securities Act and are “accredited investors” (as defined by Rule 501 under the Securities Act). In addition, the issuances did not involve any public offering; the Registrant made no solicitation in connection with the sale other than communications with the Investor; the Registrant obtained representations from the Investor regarding their investment intent, experience and sophistication; and the Investor either received or had access to adequate information about the Registrant in order to make an informed investment decision.
ITEM 6 - SELECTED FINANCIAL DATA
We are a smaller reporting company as defined in Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
ITEM 7 - MANAGEMENT'S DISCUSSION AND ANALYSIS OF PLAN OF OPERATION
The following discussion and analysis of our financial condition and results of operations should be read in conjunction with our financial statements and related notes included elsewhere in this report.
For SEC reporting purposes, Quture is treated as the continuing reporting entity that acquired Techs (the historic registrant). The reports filed after the transaction have been prepared as if Quture (accounting acquirer) were the legal successor to Techs’ reporting obligation as of the date of the acquisition. Therefore, all financial statements filed subsequent to the transaction reflect the historical financial condition, results of operations and cash flows of Quture, for all periods prior to the share exchange and consolidated with Techs from the date of the share Exchange. Quture previously had a December 31 fiscal year end, but has now assumed the fiscal year end of Techs Loanstar, Inc., the legal acquirer. Accordingly, the financial statements presented herein are the audited financial statements for the years ended April 30, 2012 and 2011 are of Quture, Inc., and from August 9, 2011 are consolidated with Techs Loanstar, Inc.
This annual report contains forward looking statements relating to our Company's future economic performance, plans and objectives of management for future operations, projections of revenue mix and other financial items that are based on the beliefs of, as well as assumptions made by and information currently known to, our management. The words "expects", "intends", "believes", "anticipates", "may", "could", "should" and similar expressions and variations thereof are intended to identify forward-looking statements. The cautionary statements set forth in this section are intended to emphasize that actual results may differ materially from those contained in any forward looking statement.
Our auditor's report on our April 30, 2012 financial statements expresses an opinion that substantial doubt exists as to whether we can continue as an ongoing business. This opinion is limited to the period ending April 30, 2012 and does not consider potential changes in the condition of the Company if successful commercial sales occur in the transition to a revenue-based ongoing business or if additional sources of revenue are obtained after this date. Following the merger of Techs Loanstar with Quture, the Company has continued to raise sufficient capital to achieve its objectives and meet its development milestones to transition to revenue generating financial status. See "April 30, 2012 Audited Financial Statements - Auditors Report."
As of April 30, 2012, we had $51,521 cash on hand and in the bank. Management believes this amount will not satisfy our cash requirements for the next twelve months or until such time that additional proceeds are raised. We plan to satisfy our future cash requirements - primarily the working capital required for operations and the costs of being a public Company as set forth in the Liquidity and Capital Resources section above.
If we are unsuccessful in raising such additional proceeds , we will then have to seek capital from other sources, which may not even be available to the Company. However, if such financing were available, we would likely have to pay additional costs associated with high risk loans and be subject to an above market interest rate. At such time these funds are required, management will evaluate the terms of such debt financing and determine whether the business could sustain operations and growth and manage the debt load. If we cannot raise additional proceeds via a private placement of our common stock or secure debt financing we would be required to cease business operations. As a result, investors in Techs Loanstar's common stock could lose all of their investment.
The Company has generated $40,900 of revenue during the year ended April 30, 2012, compared to $19,900 for the year ended April 30, 2011. Total operating expenses for the year ending April 30, 2012 and 2011 were $2,326,080 and $139,169. Total other expenses for the year ended April 30, 2012 were $391,077 resulting in a net loss for the year ended April 30, 2012 of $2,676,257 compared to $119,269 for the year ended April 30, 2011.
Operating expenses for the year ended April 30, 2012 and 2011 are comprised of:
| 2012 | 2011 | |
| Salaries and benefits | $ 227,000 | $ 40,000 |
| Warrant costs | 1,307,000 | - |
| Software development | 460,055 | 66,514 |
| Rent | 64,158 | 16,000 |
| General and administrative costs | 160,900 | 8,755 |
| Professional fees and consulting | 106,967 | 7,900 |
| Total | $ 2,326,080 | $ 139,169 |
Salaries and benefits
Prior to the Share Exchange Agreement, the Company had agreed to pay Landon Feazell, the President and Chief Executive Officer $10,000 a month (cash flow permitting) for his management services, and effective with the Share Exchange Agreement (August 9, 2011) the Company agreed to increase Mr. Feazell’s compensation to $13,600 per month. Beginning August 9, 2011, the Company has agreed to compensate Mr. Barry Hollander $5,000 monthly for his services as Chief Financial Officer. The amounts are paid when the Company has available funds, and in the absence of such funds the Company accrues the monthly fee. For the year ended April 30, 2012 Mr. Feazell has been paid $132,122 and as of April 30, 2012 is owed $60,278 of accrued and unpaid fees. For the year ended April 30, 2012 Mr. Hollander has been paid $31,191 for his services and as of April 30, 2012 is owed $13,809 of accrued and unpaid fees.
Software development
The Company has an agreement with Q’Zure, LLC (“Q’Zure”) to provide software and technology services that Quture requires. Geoffrey Feazell, an officer of our Company is the managing partner of Q’Zure. For the years ended April 30, 2012 and 2011 we incurred $460,055 and $66,514 respectively, of software development costs. These costs were primarily related to the design, development and migration of our software product to our licensor’s platform, technology support and outsourced programming services. As of April 30, 2012 we owe $377,122 to Q’Zure which is included in accounts payable, related parties on the balance sheet presented herein.
Rent and office expenses
Through April 25, 2012, the Company leased office space in Port Orange, Fl. from Sunset Quay Outfitters, LLC (“Sunset Quay”). Sunset Quay is a Florida limited liability Company that is controlled by Geoffrey Feazell, an Officer of our Company. Under the terms of the lease agreement, the Company, the rent was $4,000 per month on a net lease. For the years ended April 30, 2012 and 2011 the Company has expensed $48,000 and $16,000 respectively in rent expense, and as of April 30, 2012 owes $23,000 to Sunset Quay for accrued and unpaid rent which is included in accounts payable and accrued expenses, related parties on the balance sheet as of April 30, 2012.
General and administrative
For the year ended April 30, 2012 the Company incurred $160,067 compared to$139,169 for the year ended April 30, 2011of general and administrative expenses. Included in these costs are office administration, trade shows, travel and promotion.
Professional fees and consulting
Professional fees and consulting expenses for the years ended April 30, 2012 and 2011 were $106,967 and $7,900 respectively. Included in this amount was $40,000 for the year ended April 30, 2012 related to our product demonstration costs at the University of Miami. Legal and accounting costs were $15,774 and $20,194, respectively for the year ended April 30, 2012. Lastly, we amortized the remaining balance of $28,499 during the year ended April 30, 2012 relating to a marketing consultant.
We anticipate that our current cash and cash equivalents and cash generated from financing activities will be sufficient to satisfy our liquidity requirements for the next 12 months. We expect to incur development, marketing, professional and administrative expenses as well expenses associated with maintaining our SEC filings. We will require additional funds during this time and will seek to raise the necessary additional capital. If we are unable to obtain additional financing, we may be required to reduce the scope of our business activities, which could harm our business plans, financial condition and operating results. Additional funding may not be available on favorable terms, if at all.
OFF BALANCE SHEET ARRANGEMENTS.
As of the date of this Annual Report, the funds currently available to the Company will not be sufficient to continue operations. The cost to continue operations is estimated to be approximately $480,000 over the next twelve months and the cost of maintaining our reporting status is estimated to be $48,000 over this same period. These amounts do not include the $18,600 monthly compensation due our officers. These anticipated funds essentially correspond to the amounts of operating capital the Company has obtained and expended since the merger with Quture..
Other than the above described situation the Company does not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on the Company's financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors.
ITEM 7A - QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are a smaller reporting company as defined in Rule 12b-2 of the Exchange Act and are not required to provide the information required under this item.
ITEM 8 - FINANCIAL STATEMENTS
The required Financial Statements and the notes thereto are contained in a separate section of this report beginning with the page following the signature page.
ITEM 9 - CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
On July, 14, 2010, the Board of Directors of the Registrant dismissed Seale and Beers, CPA’s as its independent accountant. On the same date the accounting firm of R.R. Hawkins and Associates International, a PC (“RR Hawkins”) was engaged as the Registrant's new independent registered public accounting firm. The Board of Directors of the Registrant approved the dismissal of Seale and Beers, CPAs and the engagement of RR Hawkins as its independent auditor.
During the Registrant's two most recent fiscal years and the subsequent interim periods thereto, there were no disagreements with RR Hawkins whether or not resolved, on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which, if not resolved to RR Hawkins’ satisfaction, would have caused it to make reference to the subject matter of the disagreement in connection with its report on the registrant's financial statements.
ITEM 9A(T) - CONTROLS AND PROCEDURES.
Evaluation of Disclosure Controls and Procedures
A review and evaluation was performed by the Company's management, including the Company's Chief Executive Officer (the "CEO") and the Chief Financial Officer (the “CFO”), of the effectiveness of the design and operation of the Company's disclosure controls and procedures as of the end of the period covered by this annual report. Based on that review and evaluation, the CEO and CFO has concluded that as of April 30, 2012, disclosure controls and procedures, were not effective at ensuring that the material information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported as required in the application of SEC rules and forms.
Management’s Report on Internal Controls over Financial Reporting
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting. Internal Control over financial reporting is defined in rule 13a-15(f) or 15d-15(f) promulgated under the Securities Exchange Act of 1934 as a process designed by, or under the supervision of the Company's principal executive and principal financial officers and effected by the Company's board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America and includes those policies that:
| · | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the tractions and dispositions of the assets of the Company; |
| · | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with accounting principles generally accepted in the United States of America and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| · | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company's assets that could have a material effect on the financial statements. Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Because of the inherent limitations of internal control, there is a risk that material misstatements may not be prevented or detected on a timely basis by internal control over financial reporting. However, these inherent limitations are known features of the financial reporting process. Therefore, it is possible to design into the process safeguards to reduce, though not eliminate, this risk. |
As of April 30, 2012, management assessed the effectiveness of our internal control over financial reporting based on the criteria for effective internal control over financial reporting established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO") and SEC guidance on conducting such assessments. Based on that evaluation, they concluded that, during the period covered by this report, such internal controls and procedures were not effective to detect the inappropriate application of US GAAP rules as more fully described below. This was due to deficiencies that existed in the design or operation of our internal control over financial reporting that adversely affected our internal controls and that may be considered to be material weaknesses.
The matters involving internal controls and procedures that the Company's management considered to be material weaknesses under the standards of the Public Company Accounting Oversight Board were: (1) lack of a functioning audit committee and lack of a majority of outside directors on the Company's board of directors, resulting in ineffective oversight in the establishment and monitoring of required internal controls and procedures; (2) inadequate segregation of duties consistent with control objectives; and (3)ineffective controls over period end financial disclosure and reporting processes. The aforementioned material weaknesses were identified by the Company's Chief Financial Officer in connection with the review of our financial statements as of April 30, 2012.
Management believes that the material weaknesses set forth in items (2) and (3) above did not have an effect on the Company's financial results. However, management believes that the lack of a functioning audit committee and lack of a majority of outside directors on the Company's board of directors, results in ineffective oversight in the establishment and monitoring of required internal controls and procedures could result in material misstatement in our financial statements in future periods.
This Annual Report does not include an attestation report of our independent registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our independent registered public accounting firm pursuant to the rules of the SEC that permit us to provide only management’s report in this annual report.
We are committed to improving the internal controls and will (1) consider to use third party specialists to address shortfalls in staffing and to assist us with accounting and finance responsibilities, (2) increase the frequency of independent reconciliations of significant accounts which will mitigate the lack of segregation of duties until there are sufficient personnel and (3) may consider appointing additional outside directors and audit committee members in the future.
We will continue to monitor and evaluate the effectiveness of our internal controls and procedures and our internal controls over financial reporting on an ongoing basis and are committed to taking further action and implementing additional enhancements or improvements, as necessary and as funds allow.
Changes in Internal Control over Financial Reporting
There have been no changes in the Company’s internal controls over financial reporting that have materially affected, or are reasonably likely to materially affect, the Company’s internal controls over financial reporting.
ITEM 9B - OTHER INFORMATION
None.
ITEM 10 - DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
The name, address, age, and position of our present officers and directors are set forth below:
| Name | Age | Position |
| G. Landon Feazell | 68 | Chief Executive Officer, President and Chairman of the Board of Directors |
| Geoffrey Feazell | 43 | Treasurer and Secretary |
| Barry Hollander | 55 | Chief Financial Officer and Director |
G. Landon Feazell, 68, President, Chief Executive Officer, Director.
Landon Feazell has served as Chairman and Chief Executive Officer of Quture, Inc. since April 2011. Prior thereto and from 1975 to 2011, Mr. Feazell served as the President and Chief Executive Officer of Medico-Legal Management, then becoming QualVal Systems and Q3 LLC. Mr. Feazell is nationally recognized for his leadership, innovation, and expertise in measuring clinical performance, pioneering proactive clinical risk management and patient safety and physician peer review processes. His companies have developed and implemented systems and processes in hundreds of hospitals, hospital corporations and trusts, university health systems, health maintenance organizations, large and small physician practices, federal and state governmental agencies, and health and liability insurance companies throughout the United States.
Mr. Feazell received a Bachelor of Science degree in zoology from Duke University, followed by extensive graduate studies in medical sciences at the West Virginia University Medical Center, where he was a teaching fellow, conducting research in mycology and its relationship to leukemia and preventive medicine, then law at the University of Denver, College of Law, where he was student assistant to the deans. He served as Chairman of the National Conference of Law Reviews (1968-69). His community service includes chairing many public education initiatives for Jefferson County Board of Education, mental health chairmanships and board education grants, and successful political campaign cabinets for Governor and United States Senator in Colorado. He is widely published in professional articles, and he lectures, teaches, and participates on national expert panels at universities, professional organizations, private corporations, and forums and seminars and has taught at the undergraduate and graduate level at several universities.
Geoffrey L. Feazell, 43, Treasurer, Secretary.
Geoffrey Feazell is the Managing Member and Chief Executive Officer of Q’Zure LLC, a Florida limited liability company responsible for design, programming, testing, and implementation of all Quture, Inc.’s technology products and has served in this capacity since April 2004. Prior thereto and from August 1999 to April 2004, Geoffrey served as the Manager of Technology of QualVal Systems, Inc. For over 10 years his contributions have been developing IT solutions and tools for measuring quality, clinical performance, and productivity. Geoffrey has served as team leader for enhancements and the report writer of QReview and QSurg. These products integrate performance measurement and peer review with electronic medical records and connect disparate databases. He has managed implementation of these products and client projects for healthcare organizations throughout the United States. His recent focus has been quality software and technology analytics to develop clinical knowledge solutions based on optimal evidence-based clinical processes. He has further become proficient in the application of Natural Language Processing to integrate unstructured data into object-oriented databases to migrate former products onto the new Quture technology platform. Geoffrey’s educational background includes undergraduate studies from the University of North Carolina at Wilmington, multiple certificates from the University of Texas, Quality Software Institute, multiple certifications from the Diver’s Institute of Technology, and Medical Billing Specialist certification from Pittsburg Technology Institute.
Barry S. Hollander, 55, Chief Financial Officer and Director
Mr. Hollander has been the Chief Financial Officer of the Registrant since February 2010. Mr. Hollander has been the Acting Chief Executive Officer of FastFunds Financial Corporation, a publicly traded company since January 2007. Prior to becoming the Acting CEO of FastFunds, Mr. Hollander had been a financial consultant to FastFunds. Mr. Hollander has been the Chief Financial Officer of SurgLine International, Inc. (formerly China Nuvo Solar Energy, Inc.) a publicly traded company since May 2002. Mr. Hollander is the sole Director, president and chief financial officer of Mint Capital, Inc. (“Mint”), a blank check shell company. Mint is seeking to merge with a target company. Mint filed a Form 10 registration statement which went effective in January 2010. Mr. Hollander is an affiliate (greater than 10% shareholder) of PB Capital International, Inc. (“PBCI”). PBCI is a blank check shell company, seeking to merge with a target company. PBCI filed a Form 10 registration statement which went effective in October 2009. Mr. Hollander has been the Chief Financial Officer of VP Sports since March 1999. From 1994 to 1999, Mr. Hollander was the Chief Financial Officer of California Pro Sports, Inc., an in-line skate importer, marketer and distributor. In 1999 California Pro merged with Imaginon, Inc. Mr. Hollander has been since 1980 in various accounting, senior management and executive positions. Mr. Hollander has a BS degree from Fairleigh Dickinson University.
The persons named above have held their offices/positions since August 9, 2011, the date of the Exchange Agreement and are expected to hold their offices/positions at least until the next annual meeting of our stockholders. Directors receive no compensation for serving on the Board of Directors other than the reimbursement of reasonable expenses incurred.
SIGNIFICANT EMPLOYEES
The Company does not, at present, have any employees other than the current officers and directors. We have not entered into any employment agreements, as we currently do not have any employees other than the current officer and director.
FAMILY RELATIONS
Geoffrey Feazell is the son of G. Landon Feazell.
INVOLVEMENT IN LEGAL PROCEEDINGS
No executive Officer or Director of the Company has been convicted in any criminal proceeding (excluding traffic violations) or is the subject of a criminal proceeding that is currently pending.
No Executive Officer or Director of the Company is involved in any bankruptcy petition by or against any business in which they are a general partner or executive officer at this time or within two years of any involvement as a general partner, executive officer, or Director of any business.
AUDIT COMMITTEE
We do not have a separately-designated standing audit committee. The entire Board of Directors performs the functions of an audit committee, but no written charter governs the actions of the Board when performing the functions of what would generally be performed by an audit committee. The Board approves the selection of our independent accountants.
CODE OF ETHICS
As of April 30, 2012, we have not adopted a Code of Ethics for Financial Executives, which would include our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions.
ITEM 11 - EXECUTIVE COMPENSATION
The Company has non written arrangements with G. Landon Feazell and Barry Hollander for their management services, whereby it pays Mr. Feazell $13,600 in consideration of his services to the Company as President and Mr. Hollander $5,000 a month in consideration of his services as Chief Financial Officer. The amounts are paid when the Company has available funds, and in the absence of such funds the Company accrues the monthly fee.
The following table sets forth all of the compensation awarded to, earned by or paid to (i) each individual serving as the Company’s principal executive officer during the last three completed fiscal years ending April 30, 2012; (ii) each other individual that served as an executive officer of the Company at the conclusion of the fiscal year ended April 30, 2012 and who received in excess of $100,000 in the form of salary and bonus during such fiscal year.
| Name and Principal Position | Year | Salary (a) | Bonus | Restricted Stock Awards | Option Awards (b) | Nonequity incentive plan | All other compensation | Total |
G. Landon Feazell Chief Executive Officer & President |
2012 2011 2010 |
$152,400 $40,000 - |
- - - |
- - - |
- - - |
- - - |
- - - |
$152,400 $40,000 - |
| Barry S. Hollander, Chief Financial Officer | 2012 2011 2010 |
$45,000 - - |
- - - |
- - - |
- |
- - - |
- - - |
$45,000 - - |
Geoffrey Feazell Secretary and Treasurer |
2012 2011 2010 |
-
-
- |
-
-
- |
-
-
- |
-
-
- |
-
-
- |
-
-
- |
-
-
- |
The Company has month-to-month arrangements with G. Landon Feazell and Barry Hollander for their management services, whereby it pays Mr. Feazell $13,600 in consideration of his services to the Company as President and Mr. Hollander $5,000 a month in consideration of his services as Chief Financial Officer. The amounts are paid when the Company has available funds, and in the absence of such funds the Company accrues the monthly fee. For the year ended April 30, 2012 Mr. Feazell has been paid $132,122 and as of April 30, 2012 is owed $60,278 of accrued and unpaid fees. For the year ended April 30, 2012 Mr. Hollander has been paid $31,191 for his services and as of April 30, 2012 is owed $13,809 of accrued and unpaid fees.
Outstanding Equity Awards at Fiscal Year-End
Pursuant to the Exchange Agreement, the Company acquired the outstanding balances of the 2010 Equity Incentive Plan (“EIP”). The following table sets forth information regarding each unexercised option and non-vested stock award held by each of the named executive officers as of April 30, 2012.
| Name | Number of Securities Underlying Unexercised Options Exercisable |
Number of Securities Underlying Unexercised Options Unexercisable | Option Exercise Price ($) |
Option Expiration Date | ||||
| Barry S. Hollander | 2,258,064 | - | 0.0367 | 6/25/2020 |
Compensation of Directors
The Company has not paid fees to their respective directors for attendance at meetings of the board; however, the Company may adopt a policy of making such payments in the future. The Company will reimburse out-of-pocket expenses incurred by directors in attending board and committee meetings.
There are no annuity, pension or retirement benefits proposed to be paid to officers, directors or employees of the corporation in the event of retirement at normal retirement date pursuant to any presently existing plan provided or contributed to by Company.
ITEM 12 - SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT RELATED STOCKHOLDER MATTERS
The following table sets forth, as of August 9, 2012, certain information as to shares of our common stock owned by (i) each person known by us to beneficially own more than 5% of our outstanding common stock, (ii) each of our directors, and (iii) all of our executive officers and directors as a group:
| Name of Beneficial Owner | Number of Shares of Common Stock Beneficially Owned | Percent of Common Stock (1) |
G. Landon Feazell 200 Magnolia Avenue Daytona Beach, FL. 32114 |
1,575,935,342(2) | 68.1% |
Barry Hollander 319 Clematis Street, Suite 400 West Palm Beach, Fl. 33401 |
30,166,071 (3) | 1.3% |
Geoffrey Feazell 200 Magnolia Avenue Daytona Beach, FL. 32114 |
- | -% |
Starslide Global Holdings Company, LLC 200 Magnolia Avenue Daytona Beach, FL. 32114 |
1,575,935,432 (2) | 68.1% |
| All Executive Officers and Directors as a Group (3 persons) | 1,606,101,413 (4) | 69.4% |
| (1) | Based on 2,312,690,505 shares of our common stock outstanding. |
| (2) | This includes 1,575,935,432 shares of common stock held by Starslide Global Holdings, LLC. (“Starslide”), also shown separately in this table. Mr. Feazell is the Managing Member of Starslide. |
| (3) | This includes: (i) 27,908,007 shares of restricted common stock owned by a limited liability company that Mr. Hollander is the managing partner and (ii) and an option to purchase 2,258,064 shares of common stock at an exercise price of $0.03667 granted under our 2010 Stock Option Plan. |
| (4) | Includes an option to purchase 2,258,064 shares of common stock at an exercise price of $0.03667 granted under our 2010 Stock Option Plan. |
ITEM 13 - CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
As of April 30, 2012, the President and Chief Financial Officer or their affiliates are owed from the Company $163,068 and $30,790, respectively, for loans and advances made to the Company. These amounts are unsecured, non-interest bearing, without specific terms of repayment and are recorded on the balance sheet as Notes payable, related parties. Additionally, the following amounts comprise accounts payable and accrued expenses related parties as of April 30, 2012 and 2011:
| 2012 | 2011 | |
| Accrued salaries | $ 132,575 | $ 40,000 |
| Accrued expenses | 26,817 | 16,000 |
| Accrued payable | 377,122 | 66,514 |
| Total | $ 536,515 | $ 122,514 |
The Company has agreed to compensate Mr. Feazell (the President) $13,600 per month and Mr. Hollander (the Chief Financial Officer) $5,000 per month for the services they provide the Company as cash flow permits. In months where the cash flow does not permit the monthly fee to be paid, the amount is accrued. There is no formal written employment agreement or other contracts with our current officers and there is no assurance that the services to be provided by them will be available for any specific length of time in the future.
ITEM 14 - PRINCIPAL ACCOUNTING FEES AND SERVICES
During the fiscal year ended April 30, 2012 and 2011, we incurred approximately $8,000 and $7,500 respectively in fees to our principal independent accountants for professional services rendered in connection with the audit of financial statements for the fiscal years ended April 30, 2012 and 2011. For review of our financial statements for the quarters ended in fiscal year April 30, 2012 and 2011 we incurred approximately $7,500 and $4,500 respectively in fees to our principal independent accountants for professional services.
During the fiscal years ended April 30, 2012 and 2011, we did not incur any other fees for professional services rendered by our principal independent accountants for all other non-audit services which may include, but not limited to, tax related services, actuarial services or valuation services.
ITEM 15 - EXHIBITS
(a) Exhibits:
EXHIBITS
2.1 Agreement Concerning the Exchange of Securities by and among Techs Loanstar, Inc. and ZenZuu USA, Inc. dated February 10, 2010 (Incorporated by reference to Exhibit 2.1 of Registrant’s Current Report on Form 8-K filed on February 17, 2010).
2.2 Articles of Exchange relating to the share exchange by and between Techs Loanstar, Inc. and ZenZuu USA, Inc. as filed with the Nevada Secretary of State on February 17, 2010 (Incorporated by reference to Exhibit 2.2 of Registrant’s Current Report on Form 8-K filed on February 17, 2010).
3.1 Amendment to Articles of Incorporation (Incorporated by reference to Exhibit 3.1 of Registrant’s Current Report on Form 8-K filed on August 12, 2011).
10.1 Website Hosting and License Agreement dated May 20, 2008 by and between ZZPartners, Inc. and ZenZuu, Inc. (Incorporated by reference to Exhibit 10.1 of Registrant’s Current Report on Form 8-K filed on February 17, 2010).
10.2 First Amendment to the Agreement Concerning that Exchange of Securities by and among Techs Loanstar, Inc., Quture, Inc. and the Security Holders of Quture, Inc. (Incorporated by reference to Exhibit 10.1 of Registrant’s Current Report on Form 8-K filed on August 12, 2011).
99.1 Techs Loanstar, Inc. 2010 Equity Incentive Plan. (Incorporated by reference to Exhibit 99.1 of Registrant’s Form S-8 filed on June 28, 2010).
99.2 Techs Loanstar, Inc 2010 Stock Incentive Plan. (Incorporated by reference to Exhibit 99.1 of Registrant’s Form S-8 filed on June 28, 2010).
99.3 Audited financial statements as of July 31, 2011 Quture, Inc. (incorporated by reference to Exhibit 99.1 of Registrant’s Form 8-K/A filed on September 22, 2011).
99.4 Audited financial statements of Q3, LLC as of December 31, 2010 and 2009 (incorporated by reference to Exhibit 99.2 of Registrant’s Form 8-K/A filed on September 22, 2011).
99.5 Unaudited interim financial statements of Q3, LLC. as of June 30, 2011 (incorporated by reference to Exhibit 99.3 of Registrant’s Form 8-K/A filed on September 22, 2011).
31.1 Chief Executive Officer Certification of Periodic Financial Report Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002*
31.2 Chief Financial Officer Certification of Periodic Financial Report Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002*
32.1 Chief Executive Officer Certification Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to section 906 of the Sarbanes-Oxley Act of 2002*
32.2 Chief Financial Officer Certification Pursuant to 18 U.S.C. Section 1350, as adopted pursuant to section 906 of the Sarbanes-Oxley Act of 2002*
101.INS XBRL Instance Document
101.SCH XBRL Taxonomy Extension Schema Document
101.CAL XBRL Taxonomy Extension Calculation Linkbase Document
101.DEF XBRL Taxonomy Extension Definition Linkbase Document
101.LAB XBRL Taxonomy Extension Label Linkbase Document
101.PRE XBRL Taxonomy Extension Presentation Linkbase Document
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Exchange Act, Techs Loanstar has duly caused this report to be signed on its behalf by the undersigned persons, and in the capacities so indicated on August 13, 2012.
TECHS LOANSTAR, INC.
By: /s/ G. Landon Feazell
Name: G. Landon Feazell
Title: President, Secretary and Chairman of the Board
(Principal Executive Officer)
By: /s/ Barry Hollander
Name: Barry Hollander
Title: Chief Financial Officer, Secretary
and Director
(Principal Financial Officer)
TECHS LOANSTAR, INC.
AUDITED FINANCIAL STATEMENTS
TABLE OF CONTENTS
| Page(s) | |
| REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM | 2 |
| BALANCE SHEETS | 3 |
| STATEMENTS OF OPERATIONS | 4 |
| STATEMENTS OF STOCKHOLDERS’ EQUITY (DEFICIT) | 5 |
| STATEMENTS OF CASH FLOWS | 6 |
| NOTES TO FINANCIAL STATEMENTS | 7 - 18 |

Board of Directors
Techs Loanstar, Inc. and subsidiary
West Palm Beach, Florida
Report of Independent Registered Public Accounting Firm
We have audited the accompanying balance sheets of Techs Loanstar, Inc. as of April 30, 2012 and 2011, and the related statements of operations, stockholders’ deficit, and cash flows for each of the two years in the period ended April 30, 2012 and 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Techs Loanstar, Inc. as of April 30, 2012 and 2011, and the results of its operations, and its cash flows for each of the two years in the period ended April 30, 2012 and 2011 in conformity with U.S. generally accepted accounting principles.
The accompanying financial statements have been prepared assuming the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred net losses since inception, which raise substantial doubt about its ability to continue as a going concern. The financial statements do not include any adjustment that might result from the outcome of this uncertainty.
/s/ R.R. Hawkins & Associates International, a PC
Los Angeles, CA
August 10, 2012
11301 W. Olympic Blvd. # 714
Los Angeles, CA 90064
T: 310.553.5707 F: 310.553.5337
www.rrhawkins.com
| TECHS LOANSTAR, INC | |||||||
| CONSOLIDATED BALANCE SHEETS | |||||||
| April 30, 2012 | April 30, 2011 | ||||||
| ASSETS | |||||||
| Current Assets | |||||||
| Cash | $ | 51,521 | $ | 87 | |||
| Prepaid assets | 8,561 | — | |||||
| Deferred financing costs | 7,692 | — | |||||
| Total current assets | 67,774 | 87 | |||||
| Property, plant and equipment, net of accumulated] | |||||||
| depreciation of $1,207 (2012) | 16,871 | ||||||
| Security deposit | 1,000 | ||||||
| Total assets | $ | 85,645 | $ | 87 | |||
| LIABILITIES AND SHAREHOLDERS' DEFICIT | |||||||
| Current liabilities: | |||||||
| Accounts payable and accrued expenses | $ | 833,790 | $ | — | |||
| Accounts payable and accrued expenses, related parties | 536,515 | 122,514 | |||||
| Notes payable | 198,850 | — | |||||
| Notes payable, related parties | 193,858 | 168,658 | |||||
| Convertible notes, net of discount | 184,606 | — | |||||
| Derivative Liability | 341,380 | — | |||||
| Total Liabilities | 2,288,999 | 291,172 | |||||
| STOCKHOLDERS' DEFICIT | |||||||
| Common stock, par value $0.001, 2,500,000,000 | |||||||
| shares authorized and 2,192,681,818 (2012) and | |||||||
| 1,693,481,423 (2011) outstanding | 2,192,628 | 1,693,481 | |||||
| Common stock to be issued | 120,062 | 245,062 | |||||
| Preferred stock, par value $0.001, 10,000,000 shares | |||||||
| authorized, none issued | — | — | |||||
| Additional paid in capital | 1,459,298 | 1,069,457 | |||||
| Deficit | (5,975,342) | (3,299,085) | |||||
| Total Stockholders' Deficit | (2,203,354) | (291,085) | |||||
| Total Liabilities and Stockholders' Deficit | $ | 85,645 | $ | 87 | |||
| TECHS LOANSTAR, INC | |||||
| CONSOLIDATED STATEMENTS OF OPERATIONS | |||||
| For the year ended | |||||
| April 30, 2012 | April 30, 2011 | ||||
| REVENUE | $ | 40,900 | $ | 19,900 | |
| OPERATING EXPENSES: | |||||
| Salaries and management fees | 227,000 | 40,000 | |||
| Warrants and stock based compensation | 1,307,000 | — | |||
| General and administrative expenses | 160,900 | 8,755 | |||
| Professional fees & consultants | 106,967 | 7,900 | |||
| Rent and office expenses | 64,158 | 16,000 | |||
| Software development | 460,055 | 66,514 | |||
| Total Operating Expenses | 2,326,080 | 139,169 | |||
| LOSS FROM OPERATIONS | (2,285,180) | (119,269) | |||
| OTHER INCOME (EXPENSES): | |||||
| Interest expense | (289,976) | — | |||
| Interest expense, related parties | (4,152) | — | |||
| Change in derivative liability | (96,949) | — | |||
| Total Other Income (Expenses) | (391,077) | — | |||
| LOSS BEFORE INCOME TAXES | (2,676,257) | (119,269) | |||
| PROVISION FOR INCOME TAX | — | — | |||
| NET LOSS | $ | (2,676,257) | $ | (119,269) | |
| Basic and diluted net loss | |||||
| per common share | $ | (0.01) | $ | (0.01) | |
| Basic and diluted weighted average | |||||
| common shares outstanding | 2,078,584,752 | 2,045,187,155 | |||
| TECHS LOANSTAR, INC | |||||||||||||||
| CONSOLIDATED STATEMENTS OF CHANGES IN STOCKHOLDERS' EQUITY (DEFICIT) | |||||||||||||||
| FOR THE YEAR ENDED APRIL 30, 2012 | |||||||||||||||
| Additional | Total | ||||||||||||||
| Common stock | Common stock to be issued | Paid- in | stockholders' | ||||||||||||
| Shares | Amount | Shares | Amount | Capital | Deficit | deficit | |||||||||
| Balances April 30, 2011 | 1,693,481,423 | $ | 1,693,481 | 245,061,687 | $ | 245,062 | $ | 1,069,457 | $ | (3,299,085) | $ | (291,085) | |||
| Reverse merger with Quture, Inc. | 342,095,843 | 342,096 | (1,648,833) | - | (1,306,737) | ||||||||||
| Shares of common stock issued for conversions of debenture | |||||||||||||||
| and related accrued interest of $5,900 | 23,008,388 | 23,008 | 130,392 | 153,400 | |||||||||||
| Shares of common stock issued pursuant to private placement | 55,000,000 | 55,000 | (55,000,000) | (55,000) | 275,000 | 275,000 | |||||||||
| Warrants issued in connection with private placement | 1,307,000 | 1,307,000 | |||||||||||||
| Redemption of subordinated debentures | 254,736 | 254,736 | |||||||||||||
| Shares of common stock issued pursuant to cancellation of | |||||||||||||||
| notes payable | 9,043,164 | 9,043 | 51,546 | 60,589 | |||||||||||
| Shares issued pursuant to consulting agreements | 19,500,000 | 19,500 | (19,500,000) | (19,500) | - | ||||||||||
| Shares issued from common stock to be issued | 47,500,000 | 47,500 | (47,500,000) | (47,500) | - | ||||||||||
| Shares of common stock issued for financings | 3,000,000 | 3,000 | (3,000,000) | (3,000) | 20,000 | 20,000 | |||||||||
| Net loss for the period | (2,676,257) | (2,676,257) | |||||||||||||
| Balances April 30, 2012 | 2,192,628,818 | $ | 2,192,628 | 120,061,687 | $ | 120,062 | $ | 1,459,298 | $ | (5,975,342) | $ | (2,203,354) | |||
| TECHS LOANSTAR, INC | |||||
| CONSOLIDATED STATEMENTS OF CASH FLOWS | |||||
| For the year ended | |||||
| April 30, 2012 | April 30, 2011 | ||||
| Operating activities: | |||||
| Net loss | $ | (2,676,257) | $ | (119,269) | |
| Adjustments to reconcile net loss to net cash used in operating | |||||
| activities: | |||||
| Initial derivative liability expense on convertible notes | 164,515 | ||||
| Non cash interest expense | 10,000 | ||||
| Amortization of debt discount | 211,809 | - | |||
| Stock and warrant compensation expense | 1,307,000 | - | |||
| Amortization of consulting agreement and prepaid expenses | 53,700 | - | |||
| Cash acquired in merger | 3,064 | - | |||
| Amortization of debt issuance costs | 13.564 | - | |||
| Change in deravitive liability | (67,566) | - | |||
| Depreciation | 1,180 | - | |||
| Change in operating assets and liabilities: | |||||
| Other assets | (8,561) | - | |||
| Increase (decrease) in accounts payable and accrued liabilities | 59,998 | - | |||
| Increase in accounts payable and accrued liabilities, related parties | 334,979 | 122,514 | |||
| Net cash used in operating activities | (592,575) | 3,245 | |||
| Investing activities: | |||||
| Purchases of equipment | (8,051) | 0 | |||
| Net cash used in investing activities | (8,051) | - | |||
| Financing activities: | |||||
| Proceeds from sale of common stock | 275,500 | 0 | |||
| Proceeds from issuance of notes payable, related parties | 4,770 | 36,487 | |||
| Proceeds from issuance of convertible notes | 282,500 | 0 | |||
| Proceeds from issuance of notes payable | 178,650 | ||||
| Payments of convertible notes | (35,000) | ||||
| Prepayment of services | (25,200) | ||||
| Payments of notes payable, related parties | (9,160) | (39,677) | |||
| Payment of deferred financing costs | (18,500) | 0 | |||
| Payment of security deposits | (1,000) | ||||
| Net cash provided by financing activities | 652,060 | (3,190) | |||
| Net increase in cash and cash equivalents | 51,434 | 55 | |||
| Cash and cash equivalents, beginning of period | 87 | 32 | |||
| Cash and cash equivalents, end of period | $ | 51,521 | $ | 87 | |
| Supplemental disclosures of cash flow information: | |||||
| Cash paid during the year for interest | $ | 0 | $ | 0 | |
| Cash paid during the year for taxes | $ | 0 | $ | 0 | |
| Non-cash investing and financing activities: | |||||
| Common stock issued for notes payable | $ | 60,589 | $ | 0 | |
| Common stock issued for debentures payable and accrued interest | $ | 153,400 | $ | 0 | |
| Common stock issued for assets | $ | 20,000 | $ | 0 | |
TECHS LOANSTAR, INC.
NOTES TO THE CONDENSED CONSOLIDATED
FINANCIAL STATEMENTS
Audited
April 30, 2012
NOTE 1 – NATURE OF OPERATIONS AND BASIS OF PRESENTATION
Techs Loanstar, Inc. (“Techs” or the “Registrant”) was incorporated on April 7, 2006 in the State of Nevada. The fiscal year end of the Registrant is April 30. On March 14, 2012 the Company changed its name with the Secretary of State of Nevada to Quture International, Inc. The Company plans to file with the appropriate regulatory bodies the name change to better reflect the company's core business.
SHARE EXCHANGE TRANSACTION WITH QUTURE, INC.
On July 25, 2011, the Company entered into a securities exchange agreement with Quture, Inc., a Nevada corporation (“Quture”). Pursuant to the Agreement, the Company agreed to acquire all of the outstanding capital stock of Quture in exchange for a number of shares of the Registrant’s common stock, par value $0.001 per share (the “Common Stock”) equal to eighty-five percent (85%) of the issued and outstanding common stock of the Company following the Exchange.
On August 9, 2011, the Registrant entered into and consummated the First Amendment to the Agreement Concerning that Exchange of Securities (the “Share Exchange Agreement”) with Quture and the shareholders of Quture. Upon consummation of the transactions set forth in the Share Exchange Agreement (the “Closing”), the Registrant adopted the business plan of Quture.
Pursuant to the Agreement, the Registrant acquired all of the outstanding capital stock of Quture in exchange (the “Exchange”) for the original issuance of an aggregate of 1,938,543,110 shares (the “Exchange Shares”) of the Registrant’s common stock, par value $0.001 per share (the “Common Stock”). The Registrant initially issued 400,000,000 Exchange Shares to the shareholders of Quture and, following an amendment of the Registrant’s Articles of Incorporation, the remaining Exchange Shares were issued to Quture shareholders. The Exchange Shares were issued on a pro rata basis, on the basis of the shares held by such security holders of Quture at the time of the Exchange. As a result of the Exchange, Quture became a wholly-owned subsidiary of the Registrant and the shareholders of Quture beneficially at Closing, owned approximately eighty-five percent (85%) of the issued and outstanding Common Stock of the Registrant.
On August 10, 2011, as a covenant to the Agreement, holders of a majority of the Registrant’s outstanding Common Stock voted to amend the Registrant’s Articles of Incorporation to increase the number of its authorized shares of capital stock from 900,000,000 shares to 2,510,000,000 par value $0.001 shares (the “Amendment”) of which (a) 2,500,000,000 shares were designated as Common Stock and (b) 10,000,000 shares were designated as blank check preferred stock.
At the effective time of the Exchange, our board of directors and officers was reconstituted by the resignation of Henry Fong as Director, President and Chief Executive Officer of the Registrant and the appointment of G. Landon Feazell as a member of the Registrant’s Board of Directors, Chief Executive Officer and President, and Geoffrey L. Feazell as Treasurer and Secretary of the Registrant.
For SEC reporting purposes, Quture is treated as the continuing reporting entity that acquired Techs (the historic registrant). The reports filed after the transaction have been prepared as if Quture (accounting acquirer) were the legal successor to Techs’ reporting obligation as of the date of the acquisition. Therefore, all financial statements filed subsequent to the transaction reflect the historical financial condition, results of operations and cash flows of Quture, for all periods prior to the share exchange and consolidated with Techs from the date of the share Exchange. Quture previously had a December 31 fiscal year end, but has now assumed the fiscal year end of Techs Loanstar, Inc., the legal acquirer. Accordingly, the financial statements presented herein are the audited financial statements for the years ended April 30, 2012 and 2011 are of Quture, Inc., and from August 9, 2011 are consolidated with Techs Loanstar, Inc. All share and per share amounts of Quture have been retroactively adjusted to reflect the legal capital structure of Techs pursuant to FASB ASC 805-40-45-1.
Quture was incorporated in the state of Nevada on April 21, 2011. The Company develops medical software with tools and analytics to reduce costs while improving clinical performance, outcomes, predictive insight, and evidence-based best clinical processes. Effective July 1, 2011, Quture merged with Q3, LLC (“Q3”), a Florida Limited Liability Company, whereby Quture was the legal acquirer and Q3 is the accounting acquirer. Accordingly, Quture’s historical financial results, includes Q3 prior to July 1, 2011 and consolidated with Quture from July 1, 2011.
NOTE 2 – GOING CONCERN AND MANAGEMENTS PLANS
We have incurred losses from operations of $2,676,257 and have limited revenues from operations from inception on April 26, 2011 through the period ended April 30, 2012. Further, the Company has a working capital deficit of approximately $2,203,354 and to maintain or develop its operations is dependent upon funds from private investors and the support of certain stockholders.
These factors raise substantial doubt about the ability of the Company to continue as a going concern. Management may raise necessary additional funds through loans and additional sales of its common stock. There is no assurance that the Company will be successful in raising additional capital or in further developing its operations.
Managements’ Plans
Quture is an emerging healthcare knowledge solution company created to transform health and healthcare by developing the standard in measuring clinical performance and outcomes. The Company’s products focus on using actual clinical data from existing electronic databases to measure performance and outcomes applying analytics. The Quture technology leverages its Application Partnership with InterSystems Corporation. More than two-thirds of the U.S. population is served by InterSystems-based healthcare technology. Quture, using this fully developed technology platform converts manual processes to electronic processes to integrate clinical data from existing disparate electronic data sources in healthcare organizations. The Company’s product uses InterSystems’ data integration product Ensemble already programmed for every electronic medical record (EMR) and database. Quture is immediately able to collect and integrate performance metrics into the InterSystems Cache database customized for the Quture application through this partnership. By licensing the InterSystems technology and implementing the company’s clinical content and performance measures, Quture has the potential to develop the most powerful clinical knowledge database in the world. Quture will deliver to customers the clinical data for value-based purchasing. Performance-based and value calculated clinical data will ultimately determine what payers do and do not want to pay for and the clinical knowledge to support new mandated payment systems while improving care.
Quture owns two (2) fully developed and tested products; both are being migrated from a Microsoft technology platform onto the InterSystems technology platform, with the Cache object-oriented database serving as the foundation for each and providing a unified Quture database to be called the “Qualytx” database. Real-time clinical data is captured electronically from multiple vendor disparate databases using the Ensemble interface engine and aggregated into the Qualytx database. The DeepSee dashboard and Zen report writer technology are functionalities existing in the InterSystems Ensemble Enterprise suite of products. The Quture products on the InterSystems platform are QualOptima (clinical performance measurement with analytics) and QSurg (surgery center electronic medical record (EMR) with a simplified QualOptima (“QuOp”) performance analytics application embedded).
We intend to introduce the Company’s QualOptima performance measurement product into the market after completion of a formal “clinical trial” at the University of Miami, Miller School of Medicine and Jackson Memorial Hospital. The proof of concept Phase I portion of that demonstration project has been successfully completed based on sample data and to the satisfaction of Quture and InterSystems. The QualOptima product is further demonstrated at Niagara Falls Memorial Hospital, Niagara Falls, New York, for performance measurement of nursing-sensitive measures to become “interdisciplinary” performance measurement. An existing customer of Quture, Springhill Medical Center, Springhill, LA, is evaluating the QualOptima product to replace its existing license with Quture for peer review. The QualOptima product will then have been demonstrated, if successful, to transition from strictly peer review to performance measurement on the InterSystems platform for interdisciplinary performance and outcomes measurement. Quture then intends to evaluate data from these hospital projects for return on investment (ROI) potential of the QualOptima product for commercial marketing and sales. After successful demonstration in cooperation with InterSystems, where the InterSystems products are being demonstrated to meet the requirements and specifications of Quture, the QualOptima product will be formally launched and then commercialized. QualOptima is now being expanded from the demonstration in anesthesiology to the more than 40 medical specialties. The Company plans to negotiate contracts and licenses to install QualOptima in reference hospitals during the fall months of 2012 as part of the go to market strategy of the Company.
The “go to market strategy” will focus on existing strong relationships with major hospital corporations, including their self-insured trusts, at several flagship hospitals, designed to mature into sales to large hospital systems. We anticipate other reference hospital installations will focus on specific product components, including the relationship between performance and outcomes measures, one focused on the natural language processing software component and aggregated medical specialties and specific federal initiatives, such as re-hospitalizations and hospital acquired conditions. Significantly, the Niagara Falls Memorial Hospital project is focused on a hospital acquired condition, eliminating or at least reducing cather-acquired urinary tract infections.
Initial sales are being pursued through marketing and sales for installation. Initial customers will be carefully coordinated with InterSystems. InterSystems will accompany Quture sales engineers with their own sales engineers for sales presentations. The Company is also partnering with prominent national healthcare law firms and accounting firms, jointly conducting several summit meetings for their clients and inviting potential customers – stressing compliance and the threat of negligent credentialing as motivators and leveraging their representation and influence with hospital boards and senior executives. The Quture product database and clinical process, quality and patient safety management, and medical staff credentialing processes will provide new and unique consulting opportunities for these firms in concert with Quture.
The Company anticipates marketing and sales of the QSurg product will follow introduction of the QualOptima product, projected by Quture to begin six (6) months after commencement of marketing and sales of QualOptima.
NOTE 3 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
These financial statements are presented in United States dollars and have been prepared in accordance with accounting principles generally accepted in the United States.
Use of Estimates and Assumptions
Preparation of the financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect certain reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the period. Accordingly, actual results could differ from those estimates.
Financial Instruments
All significant financial assets, financial liabilities and equity instruments of the Company are either recognized or disclosed in the financial statements together with other information relevant for making a reasonable assessment of future cash flows, interest rate risk and credit risk. Where practical the fair values of financial assets and financial liabilities have been determined and disclosed; otherwise only available information pertinent to fair value has been disclosed.
Loss per Common Share
Basic earnings (loss) per share includes no dilution and is computed by dividing income (loss) available to common stockholders by the weighted average number of common shares outstanding for the period. Stock options, warrants and common stock underlying convertible promissory notes are not considered in the calculations for the years ending April 30, 2012 and 2011, as the impact of the potential common shares would be antidilutive and decrease loss per share. Therefore, diluted loss per share presented for the periods ended April 30, 2012 and 2011 is equal to basic loss per share.
Advertising expense
Advertising is expensed when incurred. Advertising expense was $4,548 and $0 for the years ended April 30, 2012 and 2011 respectively.
Software Development Costs
The Company expenses all software development costs when incurred. Software development costs was $460,055 and $66,514 for the years ended April 30, 2012 and 2011.
Income Taxes
The Company follows the asset and liability method of accounting for income taxes under ASC 740, Income Taxes. Under this method, deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax balances and tax loss carry-forwards. Deferred tax assets and liabilities are measured using enacted or substantially enacted tax rates expected to apply to the taxable income in the years in which those differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the date of enactment or substantive enactment.
Stock-based Compensation
The Company accounts for stock-based compensation issued to employees based on ASC Topic 718- Compensation- Stock Compensation, which establishes standards for the accounting for transactions in which an entity exchanges its equity instruments for goods or services. It also addresses transactions in which an entity incurs liabilities in exchange for goods or services that are based on the fair value of the entity’s equity instruments or that may be settled by the issuance of those equity instruments. ASC Topic 718 primarily focuses on accounting for transactions in which an entity obtains employee services in share-based payment transactions. This statement requires a public entity to expense the cost of employee services received in exchange for an award of equity instruments. These statements also provide guidance n valuing and expensing these awards, as well as disclosure requirements of these equity arrangements.
Intangible Assets
The Company evaluates the recoverability of intangible assets that are amortized whenever events indicate the carrying amount of any such asset may not be fully recoverable. Our evaluation is based upon, among other things, our assumptions about the estimated future cash flows that the asset are reasonably expected to generate. When that amount exceeds the carrying value of the asset, we will recognize an impairment loss to the extent the carrying value exceeds the fair value. We apply our best judgment in our determination.
Recent Accounting Pronouncements
In June 2011, the FASB issued ASU 2011-05 to require an entity to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income, or in two separate but consecutive statements. ASU 2011-05 eliminates the option to present components of other comprehensive income as part of the statement of equity. ASU 2011-05 will be effective for the Company beginning February 1, 2012, and the Company will be required to apply it retrospectively. The adoption of this standard may only impact the presentation of our financial statements and will have no impact on the reported results.
In May 2011, the FASB issued new authoritative guidance to provide a consistent definition of fair value and ensure that fair value measurements and disclosure requirements are similar between GAAP and International Financial Reporting Standards. This guidance changes certain fair value measurement principles and enhances the disclosure requirements for fair value measurements. This guidance is effective for interim and annual periods beginning after December 15, 2011 and is applied prospectively. The Company does not expect that the adoption of this guidance will have a material impact on its financial statements.
NOTE 4 - LICENSING AGREEMENT
Quture has a non-exclusive five year license as an Application Partner with InterSystems Corporation (“InterSystems”). The license allows the Company to use InterSystems proprietary software in conjunction with the Company’s software. More than two-thirds of the U.S. population is served by InterSystems-based healthcare technology. InterSystems data integration connections, known as application programming interfaces (“API’s”) are already programmed for every EMR and database. Utilizing the world’s number 1 database in clinical healthcare applications, Quture is able to collect and integrate performance metrics immediately through this partnership, creating a powerful clinical knowledge database. The initial five year license agreement expires in September 2012, and the Company is currently in discussions regarding its renewal.
NOTE 5 - FAIR VALUE OF FINANCIAL INSTRUMENTS
Determination of Fair Value
At April 30, 2012 and 2011, the Company calculated the fair value of its assets and liabilities per ASC 820 for disclosure purposes as described below.
The carrying value of cash and cash equivalents, employee advances, prepaid expenses, accounts payable and accrued liabilities approximate their fair value due to the short period to maturity of these instruments pursuant to ASC 825.
Valuation Hierarchy
ASC 820 establishes a three-level valuation hierarchy for the use of fair value measurements based upon the transparency of inputs to the valuation of an asset or liability as of the measurement date:
Level 1 Inputs to the valuation methodology are quoted prices (unadjusted) for identical assets or liabilities in active markets. Level 1 assets and liabilities include debt and equity securities and derivative financial instruments actively traded on exchanges, as well as U.S. Treasury securities and U.S. Government and agency mortgage-backed securities that are actively traded in highly liquid over the counter markets.
Level 2. Observable inputs other than Level 1 prices such as quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets or liabilities in markets that are not active, and inputs that are observable or can be corroborated, either directly or indirectly, for substantially the full term of the financial instrument.
Level 3. Inputs to the valuation methodology are unobservable but significant to the fair value measurement. Examples in this category include interests in certain securitized financial assets, certain private equity investments, and derivative contracts that are highly structured or long-dated.
Application of Valuation Hierarchy
A financial instrument's categorization within the valuation hierarchy is based upon the lowest level of input that is significant to the fair value measurement. The following is a description of the valuation methodology used to measure fair value, as well as the general classification of such instruments pursuant to the valuation hierarchy.
Convertible notes payable, net of debt discount. Market prices are not available for the Company's convertible notes payable, nor are market prices of similar convertible notes available. The Company assessed that the fair value of this liability approximates its carrying value due to its nature, the stated interest rate of the notes and the embedded conversion features as calculated.
The method described above may produce a current fair value calculation that may not be indicative of net realizable value or reflective of future fair values. If readily determined market values became available or if actual performance were to vary appreciably from assumptions used, assumptions may need to be adjusted, which could result in material differences from the recorded carrying amounts. The Company believes its method of determining fair value is appropriate and consistent with other market participants. However, the use of different methodologies or different assumptions to value certain financial instruments could result in a different estimate of fair value.
The Company has determined the estimated fair value of financial instruments using available market information and appropriate valuation methodologies. The fair value of financial instruments classified as current assets or liabilities approximate their carrying value due to the short-term maturity of the instruments.
NOTE 6 – CONVERTIBLE AND PROMISSORY NOTES PAYABLE
During the year ended April 30, 2012, the Company entered into seven separate note agreements with an unaffiliated investor for the issuance of seven convertible promissory notes, which in the aggregate were a total of $282,500 in principal (the “CY Convertible Notes”). Among other terms, each of the CY Convertible Notes are due nine months from their issuance dates, bears interest at 8% per annum, payable in cash or shares at the Conversion Price as defined herewith, and are convertible at a conversion price (the “Conversion Price”) for each share of common stock equal to 50% of the average of the lowest three trading prices (as defined in the note agreements) per share of the Company’s common stock for the ten trading days immediately preceding the date of conversion. Upon the occurrence of an event of default, as defined in the CY Convertible Notes, the Company is required to pay interest at 22% per annum and the holders may at their option declare the CY Convertible Notes, together with accrued and unpaid interest, to be immediately due and payable. In addition, the CY Convertible Notes provide for adjustments for dividends payable other than in shares of common stock, for reclassification, exchange or substitution of the common stock for another security or securities of the Company or pursuant to a reorganization, merger, consolidation, or sale of assets, where there is a change in control of the Company. The Company may at its own option prepay the CY Convertible Notes and must maintain sufficient authorized shares reserved for issuance under the CY Convertible Notes. As of April 30, 2012 the Company has reserved 140,000,000 shares of common stock pursuant to this provision.
We received net proceeds of $264,000 after debt issuance costs of $18,500 paid for lender legal fees. These debt issuance costs are amortized over the terms of the CY Convertible Notes, and accordingly $13,564 has been expensed as debt issuance costs (included in interest expense) for the year ended April 30, 2012. Pursuant to the Share Exchange Agreement, the Company acquired a balance of $2,755 of deferred debt issuance costs, which has been amortized and has been expensed as debt issuance costs (included in interest expense) for the year ended April 30, 2012.
We determined that the conversion feature of the CY Convertible Notes represents an embedded derivative since the CY Convertible Notes are convertible into a variable number of shares upon conversion. Accordingly, the CY Convertible Notes are not considered to be conventional debt and the embedded conversion feature must be bifurcated from the debt host and accounted for as a derivative liability. Accordingly, the fair value of these derivative instruments have been recorded as a liability on the consolidated balance sheet with the corresponding amount recorded as a discount to the CY Convertible Notes. Such discount will be accreted from the date of issuance to the maturity dates of the CY Convertible Notes. The change in the fair value of the liability for derivative contracts will be recorded to other income or expenses in the consolidated statement of operations at the end of each quarter, with the offset to the derivative liability on the balance sheet. The beneficial conversion feature included in the CY Convertible Notes resulted in an initial debt discount of $282,500 and an initial loss on the valuation of derivative liabilities of $164,515 for a derivative liability initial balance of $447,015 on the CY Convertible Notes. At April 30, 2012, the Company revalued the derivative liability based on the face value of the balance of $165,000 of the CY Convertible Notes. At April 30, 2012 the derivative liability for the remaining $165,000 for the CY Convertible Notes is $341,380.
Pursuant to the Exchange Agreement, the Company assumed a derivative liability balance of $216,667 (the “Assumed Convertible Note”) to the same investor under terms identical to the CY Convertible Notes. For the period from August 9, 2011 (the Closing of the Share Exchange Agreement) to April 30, 2012, the Assumed Convertible Note was redeemed (January 2012), and accordingly there is no remaining derivative liability.
The fair value of the CY Convertible Notes was calculated at issue date utilizing the following assumptions:
| Issuance Date |
Fair Value |
Term |
Assumed Conversion Price |
Market Price on Grant Date | Volatility Percentage |
Interest Rate |
| 8/17/11 | $85,227 | 9 months | $0.0088 | $0.018 | 259% | 1.4% |
| 9/16/11 | $52,000 | 9 months | $0.0075 | $0.0149 | 259% | 1.4% |
| 10/19/11 | $70,000 | 9 months | $0.006 | $0.014 | 258% | 1.4% |
| 12/1/11 | $41,250 | 9 months | $0.01 | $0.02 | 250% | 0.05% |
| 1/18/12 | $59,770 | 9 months | $0.0087 | $0.0185 | 222% | 0.05% |
| 3/23/12 | $56,038 | 9 months | $0.00593 | $0.012 | 149% | 0.08% |
| 4/27/12 | $82,730 | 9 months | $0.00484 | $0.013 | 140% | 0.09% |
The fair value of the CY Convertible Notes and the Assumed Convertible Note was calculated at April 30, 2012 utilizing the following assumptions:
|
Fair Value |
Term |
Assumed Conversion Price |
Volatility Percentage |
Interest Rate |
| $343,280 | 9 months | $0.004833 | 140% | 0.1% |
Additionally, pursuant to the Share Exchange Agreement, the Company assumed $150,000 of outstanding Notes due from ZZUSA. As of April 30, 2012 a summary of convertible notes payable is as follows:
| CY Convertible Notes Payable | $ | 165,000 |
| Assumed Note | 150,000 | |
| Total face value | 315,000 | |
| Less discount on above | 130,394 | |
| Convertible notes, net of discount | $ | 184,606 |
NOTE 7 – CONTINGENCIES AND COMMITMENTS
The Company is not aware of any legal proceedings against it as of April 30, 2012. No contingencies have been provided in the financial statements.
Lease Agreements
Through April 25, 2012, the Company leased office space in Port Orange, Fl. from Sunset Quay Outfitters, LLC (“Sunset Quay”). Sunset Quay is a Florida limited liability Company that is controlled by Geoffrey Feazell, an Officer of our Company. Under the terms of the lease agreement, the monthly rent was $4,000 on a net lease. For the year ended April 30, 2012 the Company has included $48,000 in rent expense and as of April 30, 2012 owes $23,000 to Sunset Quay for accrued and unpaid rent which is included in accounts payable and accrued expenses, related parties on the balance sheet as of April 30, 2012.
Effective on May 1, 2012 Quture entered into a one year agreement to rent approximately 4,100 square feet of office space in Daytona Beach, Florida. The monthly rent is $4,014 plus applicable taxes. Our fiscal year annual payment obligation under the lease is as follows:
| 2013 | $48,168 |
NOTE 8 – STOCKHOLDERS EQUITY
Common Stock
During the year ended April 30, 2012 the Company issued 23,008,388 shares of common stock upon the conversion of $147,500 of debentures and $5,900 of accrued and unpaid interest. The shares were issued at an average price of approximately $0.0067 per share.
During the year ended April 30, 2012, we issued 55,000,000 shares of our common stock to unaffiliated accredited investors pursuant to a private placement. The shares were sold for $275,000 or $0.005 per share.
During the year ended April 30, 2012 the Company issued 70,000,000 shares of common stock for shares that were to be issued in connection with the Share Exchange transaction with Quture.
On December 12, 2011 the Company issued 2,000,000 shares of common stock to an unaffiliated Company in connection with the purchase of computer equipment. The Company valued the stock at $10,000 and recorded the computer equipment as of February 1, 2012, the date the Company received the equipment.
On December 5, 2011, the Company issued 9,043,164 shares of common stock pursuant to Debt Settlement and Release Agreements in exchange for the cancellation of $60,589 of notes and interest payable. The shares were issued at approximately $0.0067 per share.
Options
Pursuant to the Exchange Agreement, the Company acquired the outstanding balances of the 2010 Equity Incentive Plan (“EIP”). A summary of outstanding option balances under the EIP at May 1, 2011 and April 30, 2012 are as follows:
|
2010 EIP |
Options | Weighted-average exercise price | Weighted-average remaining contractual life (years) | |||||
| Outstanding and exercisable at May 1, 2011 | 10,500,000 |
$0.03667 |
9.2 | |||||
| Granted | ||||||||
| Expired | - | - | - | |||||
| Exercised | - | - | - | |||||
| Outstanding and exercisable at April 30, 2012 | 10,500,000 | $ 0.03667 | 8.2 |
Warrants
In connection with the sale of 55,000,000 shares of common stock, the Company issued warrants to purchase 55,000,000 shares of common stock. The warrants expire on the three year anniversary and have an exercise price of $0.01 per share. The Company valued the warrants at $735,000 based on the Black Scholes formula.
In conjunction with the issuance of 2,000,000 shares of common stock in exchange for the purchase of equipment, the Company issued a warrant to purchase 2,000,000 shares of common stock. The warrant expires on the three year anniversary and has an exercise price of $0.01 per share. The Company valued the warrant at $32,000 based on the Black Scholes formula.
Additionally, in February 2012, the Company issued to a consultant, a warrant to purchase 30,000,000 shares of common stock at an exercise price of $0.005, the warrant expires January 31, 2015. The Company valued these warrants at $540,000 based on the Black Scholes formula.
A summary of the activity of the Company’s outstanding warrants at May 1, 2011 and April 30, 2012 is as follows:
| Warrants | Weighted-average exercise price | Weighted-average grant date fair value | ||||
| Outstanding and exercisable at May 1, 2011 | 7,722,102 | $ 0.0782 | $ 0.037 | |||
| Granted | 87,000,000 | 0.015 | 0.015 | |||
| Expired | (7,722,102) | 0.0782 | 0.037 | |||
| Exercised | - | - | - | |||
| Outstanding and exercisable at April 30, 2012 | 87,000,000 | $ 0.008 | $ 0.015 |
The following table sets forth the exercise price range, number of shares, weighted average exercise price and remaining contractual lives of the warrants by groups as of April 30, 2012:
| Exercise price range | Number of options outstanding | Weighted-average exercise price | Weighted-average remaining life | |||
| $0.005 | 30,000,000 | $ 0.005 | 0.95 | |||
| $0.01 | 57,000,000 | $ 0.01 | 1.77 years | |||
| 87,000,000 | $ 0.008 | 2.72 years |
NOTE 9 – RELATED PARTY TRANSACTIONS
The Company has an agreement with Q’Zure, LLC to provide software and technology services that Quture requires. Pursuant to the terms of the agreement, Quture will provide Task Orders to Q’Zure on process and other terms to be negotiated on an order by order basis. For the year ended April 30, 2012 the Company incurred $460,055 of software development costs and as of April 30, 2012 the Company owes Q’Zure $353,982, which is included in accounts payable and accrued expenses, related parties on the balance sheet as of April 30, 2012, included herein.
Effective January 1, 2011 through the date of the Share Exchange Agreement, the Company has agreed to compensate Landon Feazell $10,000 per month for his services to the Company as President and Chief Executive Officer, to be paid as cash flow permits. Effective with the Share Exchange Agreement the Company has agreed to increase Mr. Feazell’s compensation to $13,600 per month. Accordingly, for the year ended April 30, 2012 the Company has included $152,400 in salaries and benefits, and as of April 30, 2012, the Company owes Mr. Feazell $60,379 for accrued and unpaid fees. Effective with the Share Exchange agreement, the Company has agreed to compensate Barry Hollander $5,000 per month for his services as Chief Financial Officer. The Company has included $45,000 in salaries for the year ended April 30, 2012, and as of April 30, 2012 the Company owes Mr. Hollander $13,809 for accrued and unpaid fees which is included in accounts payable and accrued expenses, related parties on the balance sheet as of April 30, 2012.
As of April 30, 2012 the Company owed Mr. Feazell, our Chief Executive Officer $163,068 for advances received, which was assumed by the Company pursuant to the Q3 merger. The advances are due on demand and bear no interest. Additionally, the Company owes $30,790 to Mr. Hollander, for advances he has previously made to the Company.
NOTE 10 – INCOME TAXES
As of April 30, 2012, the Company had net operating loss carry forwards of approximately $1,138,000 that may be available to reduce future years’ taxable income and will expire commencing in 2027. Availability of loss usage is subject to change of ownership limitations under Internal Revenue Code 382. Future tax benefits which may arise as a result of these losses have not been recognized in these financial statements, as their realization is determined not likely to occur and, accordingly, the Company has recorded a full valuation allowance for the deferred tax asset relating to these tax loss carryforwards.
The difference between income tax expense computed by applying the federal statutory corporate tax rate and actual income tax expense is as follows:
April 30 2012 |
|||
| Statutory federal income tax rate | 34.0% | ||
| State income taxes and other | 5.4% | ||
Effective tax rate |
39.4% |
Deferred income taxes result from temporary differences in the recognition of income and expenses for the financial reporting purposes and for tax purposes. The tax effect of these temporary differences representing deferred tax asset and liabilities result principally from the following:
April 30 2012 |
|||
| Loss provision | 448,372 | ||
| Valuation allowance | (448,372) | ||
| Deferred income tax asset | $ | - |
NOTE 11 – SUBSEQUENT EVENTS
On May 7, 2012 the Company formed Quture Euro LLC., (“Quture Euro”) a Florida Limited Liability Company. Quture Euro, a wholly owned subsidiary of the Company was formed to take advantage of the strength of our partner InterSystems in Europe. A subsidiary focused on Europe provides the Company with a significant array of business strategies and opportunities including the potential to raise capital within the subsidiary to support European operations.
Effective June 4, 2012, Qx Health Exchange, LLC. (“Qx Health Exchange) was formed as a Florida Limited Liability Corporation. The Company owns fifty (50%) of Qx Health Exchange. Qx Health Exchange intends to bring quality and competitively priced products and services to the global medical and healthcare marketplace. The QX Health Exchange intends to establish a B2B and Health Provider to Patient (HP2P) Quality Health Exchange that will serve to connect hospitals/medical facilities; physicians; health workers; insurance providers, medical tourism and patients by providing quality health through valued products, services and technologies at competitive prices. The Qx Health Exchange also intends to sell its own branded health programs and products.
Management performed an evaluation of the Company’s activity through the date these financials were issued to determine if they must be reported. The Management of the Company determined that there were no other reportable subsequent events to be disclosed.
