Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a12-17844_18k.htm |
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a12-17844_1ex99d1.htm |
Exhibit 99.2
|
|
At the Forefront of Therapies for Rare and Orphan DiseasesTM August 7, 2012 |
|
|
Safe Harbor This presentation contains “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995 relating to business, operations and financial conditions of Amicus including but not limited to preclinical and clinical development of Amicus’ candidate drug products, the timing and reporting of results from preclinical studies and clinical trials evaluating Amicus’ candidate drug products, the projected cash position for the Company, and business development and other transactional activities. Words such as, but not limited to, “look forward to,” “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “would,” “should” and “could,” and similar expressions or words, identify forward-looking statements. Although Amicus believes the expectations reflected in such forward-looking statements are based upon reasonable assumptions, there can be no assurance that its expectations will be realized. Actual results could differ materially from those projected in Amicus’ forward-looking statements due to numerous known and unknown risks and uncertainties, including the “Risk Factors” described in our Annual Report on Form 10-K for the year ended December 31, 2011. All forward-looking statements are qualified in their entirety by this cautionary statement, and Amicus undertakes no obligation to revise or update this news release to reflect events or circumstances after the date hereof. Slide 2 |
|
|
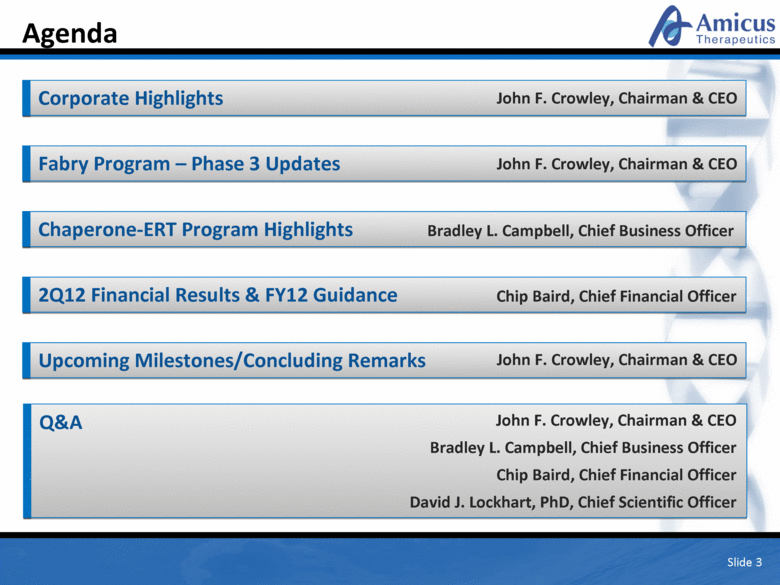
Slide 3 Agenda Corporate Highlights Fabry Program – Phase 3 Updates 2Q12 Financial Results & FY12 Guidance Upcoming Milestones/Concluding Remarks Chaperone-ERT Program Highlights Q&A John F. Crowley, Chairman & CEO Bradley L. Campbell, Chief Business Officer Chip Baird, Chief Financial Officer David J. Lockhart, PhD, Chief Scientific Officer Chip Baird, Chief Financial Officer John F. Crowley, Chairman & CEO John F. Crowley, Chairman & CEO John F. Crowley, Chairman & CEO Bradley L. Campbell, Chief Business Officer |
|
|
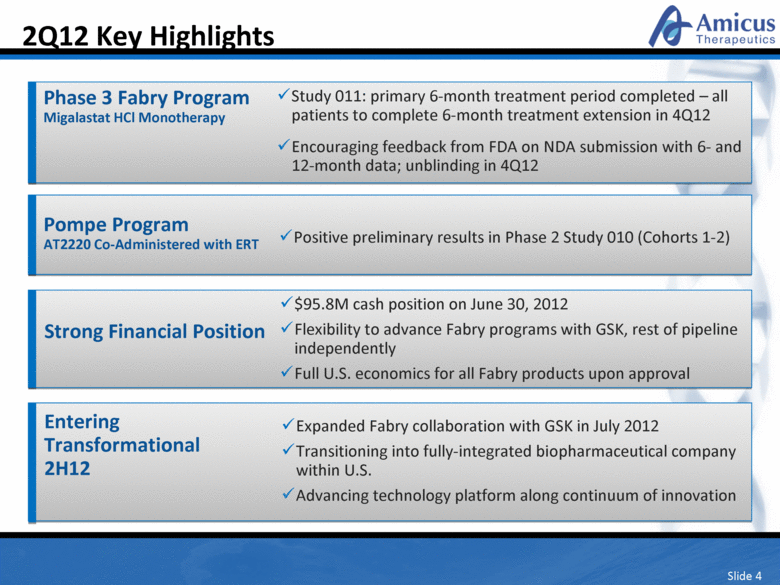
Slide 4 2Q12 Key Highlights Phase 3 Fabry Program Migalastat HCl Monotherapy Study 011: primary 6-month treatment period completed – all patients to complete 6-month treatment extension in 4Q12 Encouraging feedback from FDA on NDA submission with 6- and 12-month data; unblinding in 4Q12 Strong Financial Position $95.8M cash position on June 30, 2012 Flexibility to advance Fabry programs with GSK, rest of pipeline independently Full U.S. economics for all Fabry products upon approval Entering Transformational 2H12 Expanded Fabry collaboration with GSK in July 2012 Transitioning into fully-integrated biopharmaceutical company within U.S. Advancing technology platform along continuum of innovation Pompe Program AT2220 Co-Administered with ERT Positive preliminary results in Phase 2 Study 010 (Cohorts 1-2) |
|
|
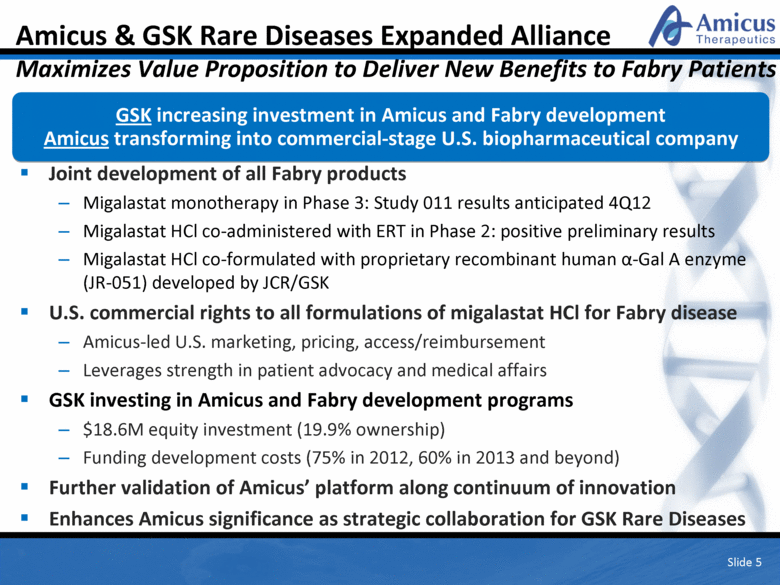
Slide 5 Joint development of all Fabry products Migalastat monotherapy in Phase 3: Study 011 results anticipated 4Q12 Migalastat HCl co-administered with ERT in Phase 2: positive preliminary results Migalastat HCl co-formulated with proprietary recombinant human a-Gal A enzyme (JR-051) developed by JCR/GSK U.S. commercial rights to all formulations of migalastat HCl for Fabry disease Amicus-led U.S. marketing, pricing, access/reimbursement Leverages strength in patient advocacy and medical affairs GSK investing in Amicus and Fabry development programs $18.6M equity investment (19.9% ownership) Funding development costs (75% in 2012, 60% in 2013 and beyond) Further validation of Amicus’ platform along continuum of innovation Enhances Amicus significance as strategic collaboration for GSK Rare Diseases Amicus & GSK Rare Diseases Expanded Alliance Maximizes Value Proposition to Deliver New Benefits to Fabry Patients GSK increasing investment in Amicus and Fabry development Amicus transforming into commercial-stage U.S. biopharmaceutical company Slide 5 |
|
|
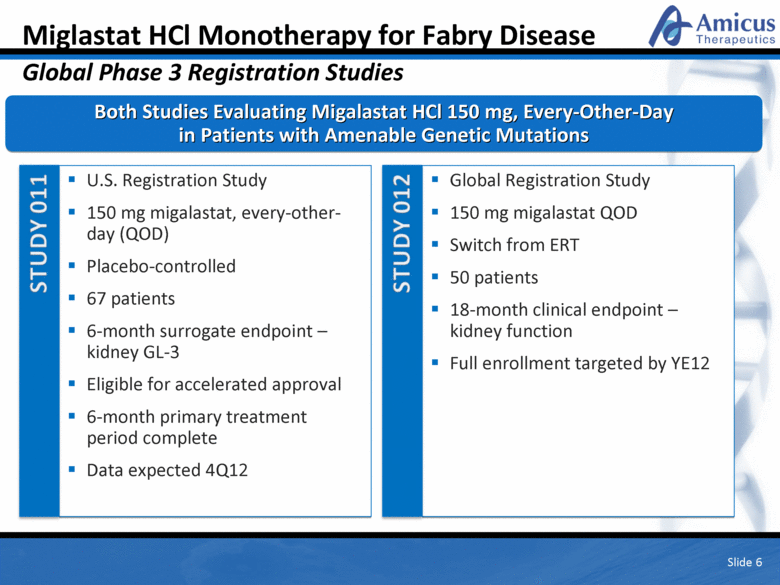
Slide 6 Miglastat HCl Monotherapy for Fabry Disease Global Phase 3 Registration Studies Both Studies Evaluating Migalastat HCl 150 mg, Every-Other-Day in Patients with Amenable Genetic Mutations U.S. Registration Study 150 mg migalastat, every-other-day (QOD) Placebo-controlled 67 patients 6-month surrogate endpoint – kidney GL-3 Eligible for accelerated approval 6-month primary treatment period complete Data expected 4Q12 Global Registration Study 150 mg migalastat QOD Switch from ERT 50 patients 18-month clinical endpoint – kidney function Full enrollment targeted by YE12 |
|
|
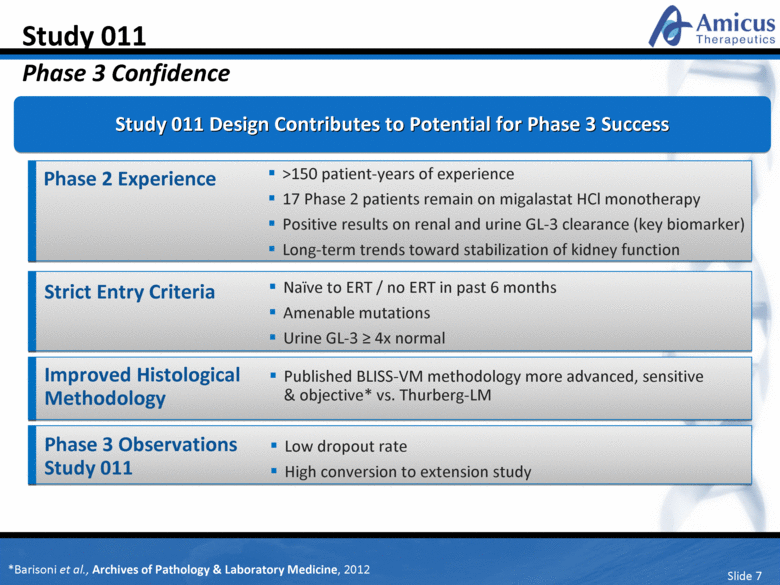
Slide 7 Study 011 Phase 3 Confidence Phase 2 Experience Study 011 Design Contributes to Potential for Phase 3 Success >150 patient-years of experience 17 Phase 2 patients remain on migalastat HCl monotherapy Positive results on renal and urine GL-3 clearance (key biomarker) Long-term trends toward stabilization of kidney function Improved Histological Methodology Published BLISS-VM methodology more advanced, sensitive & objective* vs. Thurberg-LM Phase 3 Observations Study 011 Low dropout rate High conversion to extension study Strict Entry Criteria Naïve to ERT / no ERT in past 6 months Amenable mutations Urine GL-3 > 4x normal *Barisoni et al., Archives of Pathology & Laboratory Medicine, 2012 |
|
|
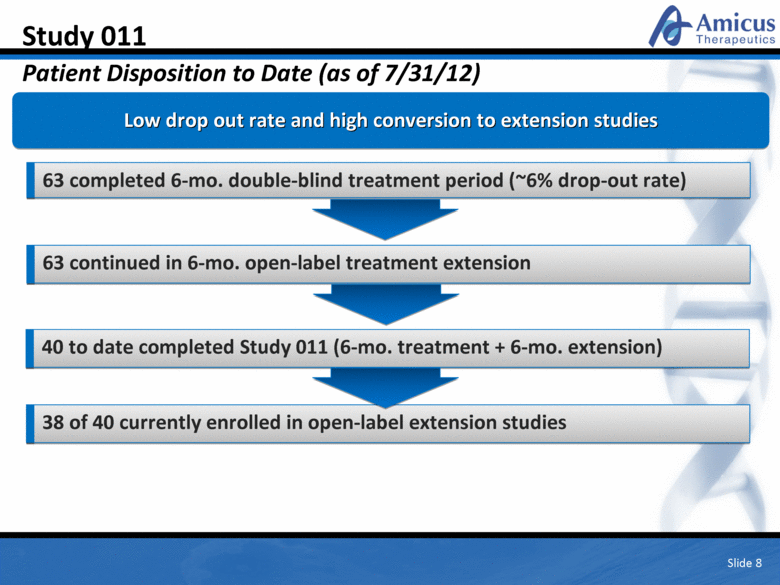
Slide 8 Study 011 Patient Disposition to Date (as of 7/31/12) Low drop out rate and high conversion to extension studies 63 completed 6-mo. double-blind treatment period (~6% drop-out rate) 63 continued in 6-mo. open-label treatment extension 40 to date completed Study 011 (6-mo. treatment + 6-mo. extension) 38 of 40 currently enrolled in open-label extension studies |
|
|
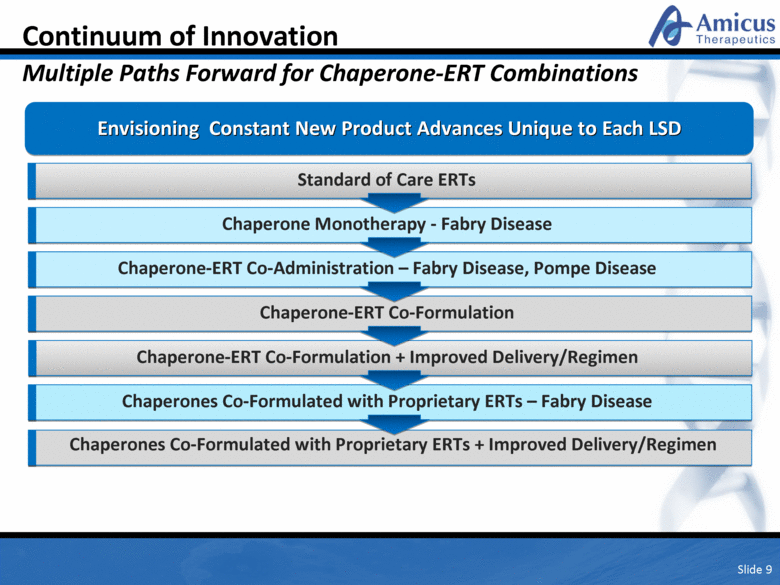
Continuum of Innovation Multiple Paths Forward for Chaperone-ERT Combinations Slide 9 Envisioning Constant New Product Advances Unique to Each LSD Chaperone Monotherapy - Fabry Disease Chaperone-ERT Co-Administration – Fabry Disease, Pompe Disease Chaperone-ERT Co-Formulation Chaperones Co-Formulated with Proprietary ERTs – Fabry Disease Chaperones Co-Formulated with Next Generation ERTs + Improved Delivery/Regimen Standard of Care ERTs Chaperone-ERT Co-Formulation + Improved Delivery/Regimen Chaperones Co-Formulated with Proprietary ERTs + Improved Delivery/Regimen |
|
|
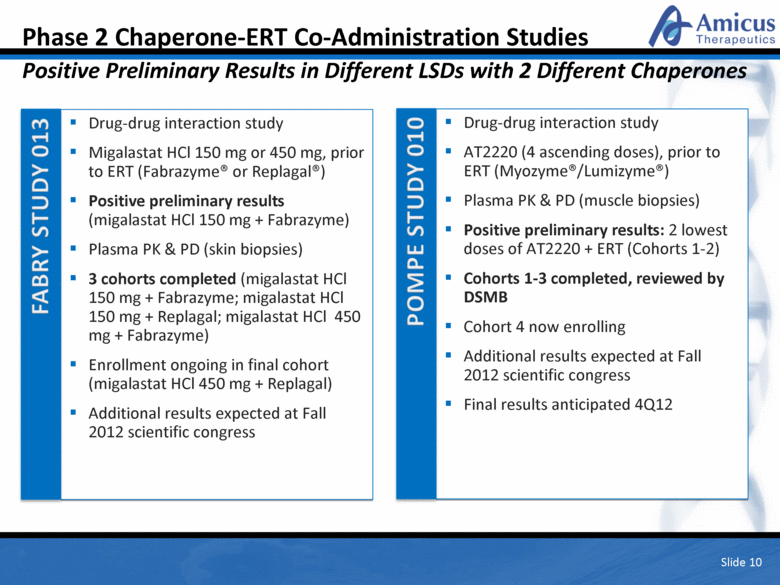
Slide 10 Phase 2 Chaperone-ERT Co-Administration Studies Positive Preliminary Results in Different LSDs with 2 Different Chaperones Drug-drug interaction study Migalastat HCl 150 mg or 450 mg, prior to ERT (Fabrazyme® or Replagal®) Positive preliminary results (migalastat HCl 150 mg + Fabrazyme) Plasma PK & PD (skin biopsies) 3 cohorts completed (migalastat HCl 150 mg + Fabrazyme; migalastat HCl 150 mg + Replagal; migalastat HCl 450 mg + Fabrazyme) Enrollment ongoing in final cohort (migalastat HCl 450 mg + Replagal) Additional results expected at Fall 2012 scientific congress Drug-drug interaction study AT2220 (4 ascending doses), prior to ERT (Myozyme®/Lumizyme®) Plasma PK & PD (muscle biopsies) Positive preliminary results: 2 lowest doses of AT2220 + ERT (Cohorts 1-2) Cohorts 1-3 completed, reviewed by DSMB Cohort 4 now enrolling Additional results expected at Fall 2012 scientific congress Final results anticipated 4Q12 |
|
|
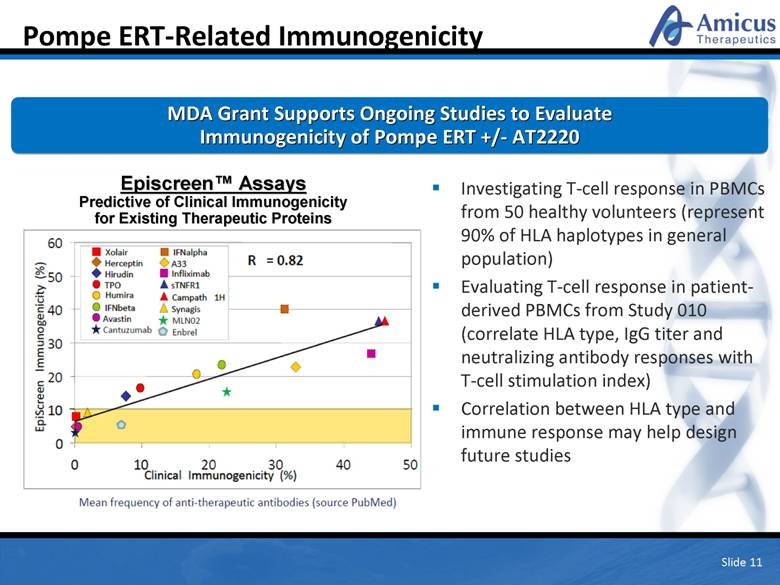
Pompe ERT-Related Immunogenicity Slide 11 MDA Grant Supports Ongoing Studies to Evaluate Immunogenicity of Pompe ERT +/- AT2220 Investigating T-cell response in PBMCs from 50 healthy volunteers (represent 90% of HLA haplotypes in general population) Evaluating T-cell response in patient-derived PBMCs from Study 010 (correlate HLA type, IgG titer and neutralizing antibody responses with T-cell stimulation index) Correlation between HLA type and immune response may help design future studies Episcreen™ Assays Predictive of Clinical Immunogenicity for Existing Therapeutic Proteins |
|
|
Formulation and Preclinical Studies Conducted Over 16+ Months Chaperone-ERT Co-Formulation: Strategic Relationship Leverages JCR’s Biological Expertise Slide 12 |
|
|
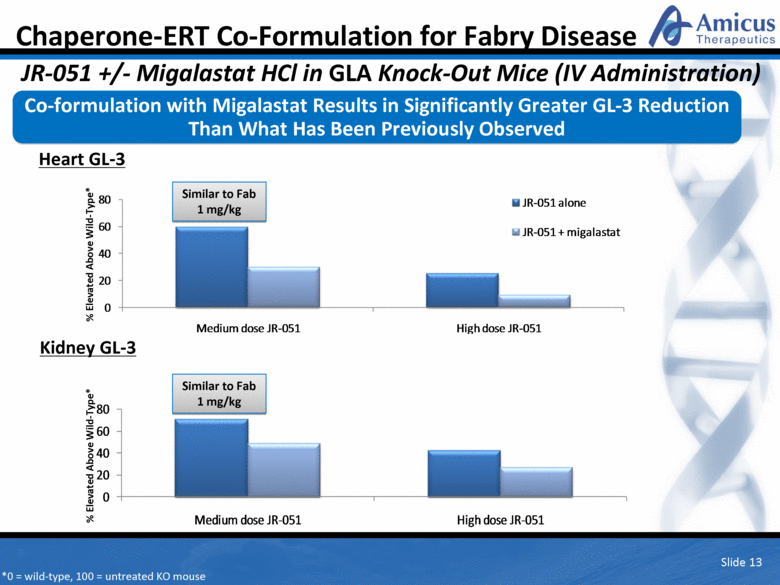
Slide 13 Heart GL-3 Kidney GL-3 *0 = wild-type, 100 = untreated KO mouse Similar to Fab 1 mg/kg Similar to Fab 1 mg/kg Co-formulation with Migalastat Results in Significantly Greater GL-3 Reduction Than What Has Been Previously Observed Chaperone-ERT Co-Formulation for Fabry Disease JR-051 +/- Migalastat HCl in GLA Knock-Out Mice (IV Administration) % Elevated Above Wild-Type* % Elevated Above Wild-Type* 0 20 40 60 80 Medium dose JR - 051 High dose JR - 051 JR - 051 alone JR - 051 + migalastat 0 20 40 60 80 Medium dose JR - 051 High dose JR - 051 |
|
|
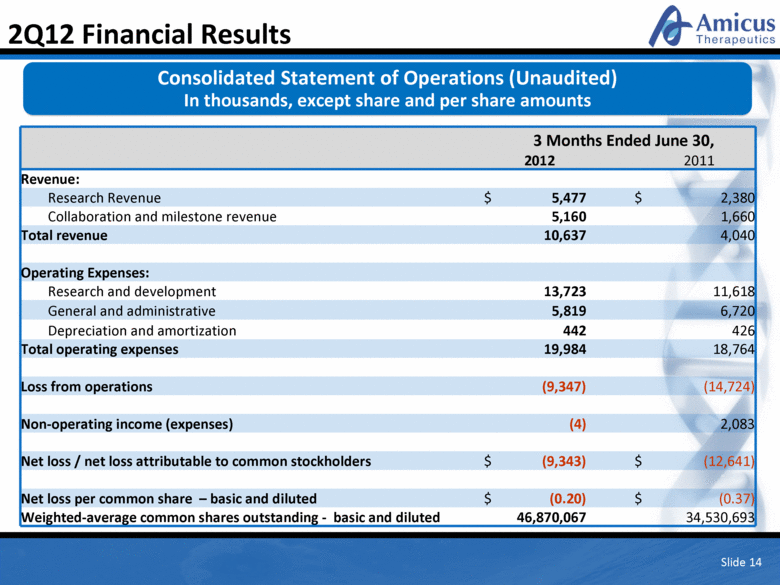
Slide 14 2Q12 Financial Results 3 Months Ended June 30, 2012 2011 Revenue: Research Revenue $ 5,477 $ 2,380 Collaboration and milestone revenue 5,160 1,660 Total revenue 10,637 4,040 Operating Expenses: Research and development 13,723 11,618 General and administrative 5,819 6,720 Depreciation and amortization 442 426 Total operating expenses 19,984 18,764 Loss from operations (9,347) (14,724) Non-operating income (expenses) (4) 2,083 Net loss / net loss attributable to common stockholders $ (9,343) $ (12,641) Net loss per common share – basic and diluted $ (0.20) $ (0.37) Weighted-average common shares outstanding - basic and diluted 46,870,067 34,530,693 Consolidated Statement of Operations (Unaudited) In thousands, except share and per share amounts |
|
|
FY12 Financial Guidance Cash position $95.8M at June 30, 2012 vs. $56.0M at December 31, 2011 > $90M projected at December 31, 2012, expected to fund current operating plan beyond 2013 Strengthening balance sheet in 3Q12 $18.6M GSK equity investment $3.5M development milestone received from GSK FY12 OpEx guidance: Upper end of previous guidance range of $37M - $43M Net of anticipated Fabry cost-sharing |
|
|
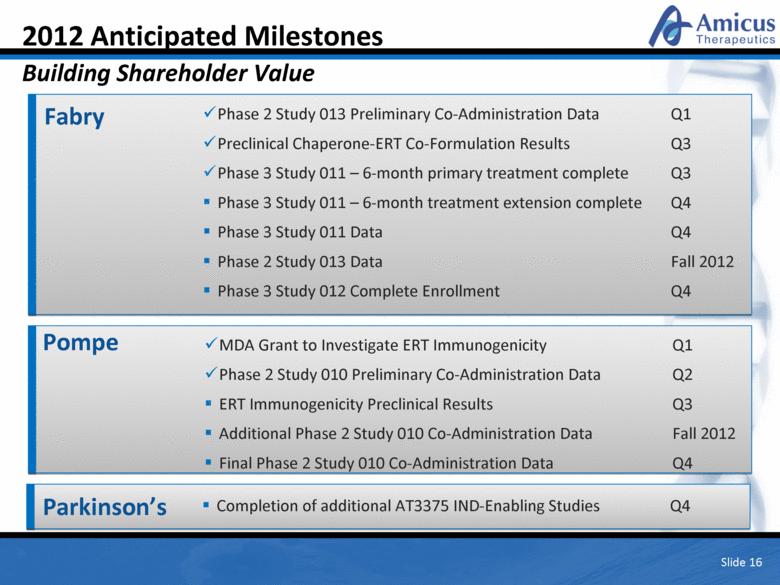
Slide 16 2012 Anticipated Milestones Building Shareholder Value Fabry Phase 2 Study 013 Preliminary Co-Administration Data Q1 Preclinical Chaperone-ERT Co-Formulation Results Q3 Phase 3 Study 011 – 6-month primary treatment complete Q3 Phase 3 Study 011 – 6-month treatment extension complete Q4 Phase 3 Study 011 Data Q4 Phase 2 Study 013 Data Fall 2012 Phase 3 Study 012 Complete Enrollment Q4 Pompe MDA Grant to Investigate ERT Immunogenicity Q1 Phase 2 Study 010 Preliminary Co-Administration Data Q2 ERT Immunogenicity Preclinical Results Q3 Additional Phase 2 Study 010 Co-Administration Data Fall 2012 Final Phase 2 Study 010 Co-Administration Data Q4 Parkinson’s Completion of additional AT3375 IND-Enabling Studies Q4 |
|
|
Q&A Slide 17 John F. Crowley, Chairman & CEO Bradley L. Campbell, Chief Business Officer Chip Baird, Chief Financial Officer David J. Lockhart, PhD, Chief Scientific Officer |