Attached files
| file | filename |
|---|---|
| EX-21.1 - EXHIBIT 21.1 - Tongli Pharmaceuticals (USA), Inc. | v317170_ex21-1.htm |
| EX-31.2 - EXHIBIT 31.2 - Tongli Pharmaceuticals (USA), Inc. | v317170_ex31-2.htm |
| EX-10.2 - EXHIBIT 10.2 - Tongli Pharmaceuticals (USA), Inc. | v317170_ex10-2.htm |
| EX-10.3 - EXHIBIT 10.3 - Tongli Pharmaceuticals (USA), Inc. | v317170_ex10-3.htm |
| EX-31.1 - EXHIBIT 31.1 - Tongli Pharmaceuticals (USA), Inc. | v317170_ex31-1.htm |
| EXCEL - IDEA: XBRL DOCUMENT - Tongli Pharmaceuticals (USA), Inc. | Financial_Report.xls |
| EX-32.1 - EXHIBIT 32.1 - Tongli Pharmaceuticals (USA), Inc. | v317170_ex32-1.htm |
| EX-32.2 - EXHIBIT 32.2 - Tongli Pharmaceuticals (USA), Inc. | v317170_ex32-2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| ¨ | Annual report pursuant to section 13 or 15(d) of the Securities Exchange Act of 1934. |
For the fiscal year ending March 31, 2012
OR
| ¨ | Transition report pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934. |
For the transition period from ________ to ________.
Commission file number 000-52954
Tongli Pharmaceuticals (USA), Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 84-1090791 | |
| (State or other jurisdiction | (IRS Employer | |
| of incorporation or organization) | Identification number) | |
| 136-17 Maple Ave, 11F | ||
| Flushing, NY | 11355 | |
| (Address of Principal Executive Offices) | (Zip Code) |
212-842-8837
(Registrant’s Telephone Number, Including Area Code)
Securities registered pursuant to Section 12(b) of the Act:
None
(Title of Class)
Name of each exchange on which registered
None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $.001 par value per share
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x .
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x .
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨ .
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer ¨ |
| Non-accelerated filer ¨ | Smaller reporting company x. |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common stock, other than shares held by persons who may be deemed affiliates of the registrant, computed by reference to the closing sales price for the registrant’s Common Stock on September 30, 2011, as reported on the OTC Bulletin Board, was approximately $2.9 million.
As of July 6, 2012, there were 13,548,122 outstanding shares of common stock of the registrant, par value $.001 per share.
DOCUMENTS INCORPORATED BY REFERENCE
None
TABLE OF CONTENTS
| Page | ||
| Cautionary Note On Forward Looking Statements | -i- | |
| Part I | ||
| Item 1. | Business | 1 |
| Item 1A. | Risk Factors | 8 |
| Item 1B. | Unresolved Staff Comments | 20 |
| Item 2. | Description of Properties | 20 |
| Item 3. | Legal Proceedings | 21 |
| Item 4. | Mine Safety Disclosure | 21 |
| Part II | ||
| Item 5. | Market For Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 21 |
| Item 6. | Selected Financial Data | 22 |
| Item 7. | Management’s Discussion and Analysis or Plan of Operation | 22 |
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk | 27 |
| Item 8. | Financial Statements and Supplementary Data | 27 |
| Item 9. | Changes In and Disagreements With Accountants On Accounting and Financial Disclosure | 27 |
| Item 9A. | Controls and Procedures | 27 |
| Item 9B. | Other Information | 29 |
| Part III | ||
| Item 10. | Directors, Executive Officers, Promoters and Control Persons; Compliance With Section 16(A) of the Exchange Act | 30 |
| Item 11. | Executive Compensation. | 32 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 34 |
| Item 13. | Certain Relationships and Related Party Transactions | 35 |
| Item 14. | Principal Accountant Fees and Services | 36 |
| Part IV | ||
| Item 15. | Exhibits | 36 |
| Index to Financial Statements | F-1 | |
Unless otherwise provided in this Annual Report on Form 10-K, references to “Tongli,” “we,” “us,” “our” and similar terminology refer to Tongli Pharmaceuticals (USA), Inc. and its subsidiaries.
CAUTIONARY NOTE ON FORWARD LOOKING STATEMENTS
In addition to historical information, this Annual Report on Form 10-K contains forward looking statements which are subject to certain risks and uncertainties that could cause actual results to differ materially from those reflected in such forward-looking statements. Factors that might cause such a difference include, but are not limited to, those discussed in the sections entitled “Business”, “Risk Factors”, and “Management’s Discussion and Analysis or Plan of Operation.” Readers are cautioned not to place undue reliance on these forward-looking statements, which reflect management’s opinions only as of the date thereof. We undertake no obligation to revise or publicly release the results of any revision of these forward-looking statements. Readers should carefully review the risk factors described in this Report and in other documents that we file from time to time with the Securities and Exchange Commission.
In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “projects,” “potential,” “proposed,” “intended,” or “continue” or the negative of these terms or other comparable terminology. You should read statements that contain these words carefully, because they discuss our expectations about our future operating results or our future financial condition or state other “forward-looking” information. There may be events in the future that we are not able to accurately predict or control. You should be aware that the occurrence of any of the events described in our risk factors and other disclosures included in this Report could substantially harm our business, results of operations and financial condition, and that upon the occurrence of any of these events, the trading price of our securities could decline. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, growth rates, and levels of activity, performance or achievements. Factors that may cause actual results, our performance or achievements, or industry results, to differ materially from those contemplated by such forward-looking statements include without limitation:
| · | obtain sufficient working capital to support our business plans; |
| · | maintain or protect our intellectual property; |
| · | maintain our proprietary technology; |
| · | expand our product offerings and maintain the quality of our products; |
| · | manage our expanding operations and continue to fill customers’ orders on time; |
| · | maintain adequate control of our expenses allowing us to realize anticipated revenue growth; |
| · | the impact of government regulation in China and elsewhere; |
| · | implement our product development, marketing, sales and acquisition strategies and adapt and modify them as needed; |
| · | integrate any future acquisitions; |
| · | our implementation of required financial, accounting and disclosure controls and procedures and related corporate governance policies; and |
| · | anticipate and adapt to changing conditions in the Chinese herbal medicines industry resulting from changes in government regulations, mergers and acquisitions involving our competitors, technological developments and other significant competitive and market dynamics. |
Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results. The following discussion should be read in conjunction with our financial statements and the related notes that appear elsewhere in this report.
We cannot give any guarantee that these plans, intentions or expectations will be achieved. All forward-looking statements involve risks and uncertainties, and actual results may differ materially from those discussed in the forward-looking statements as a result of various factors, including those factors listed above and described in the “Risk Factors” section of this Report.
| i |
PART I
| Item 1. | Business |
Organization and Business Description
Tongli Pharmaceuticals (USA), Inc., through its indirectly wholly-owned subsidiaries, Harbin Tianmu Pharmaceuticals Co., Ltd. (“HTP” or “Tianmu Pharmaceuticals”) and Harbin Lvnong Plant Ltd.(“Lvnong”), develops, produces and sells a wide variety of pharmaceuticals and healthcare products in the People’s Republic of China (“PRC” or “China”) that are based on traditional Chinese medicine, or TCM. We were formerly known as American Tony Pharmaceutical, Inc. (“American Tony”), which was a holding company incorporated in the state of Delaware on November 17, 2006 and had no significant operations since its inception. The name change became effective on October 30, 2008 and was done to better represent the origin and ongoing business of our company.
We became a U.S. Public Company on August 12, 2008, American Tony completed a reverse merger with Aim Smart Corporation (“Aim Smart”), a dormant public shell, which was originally incorporated on April 27, 1988 in the State of Colorado under the name “Gatwick, Ltd” for the purpose of seeking out and completing a merger or acquisition with one or more companies or businesses, and was reorganized as a Delaware corporation in September 2007. The acquisition was effected by the merger of American Tony into a wholly-owned subsidiary of Aim Smart.
Under the terms of the merger agreement, the former American Tony stockholders exchanged their shares for Aim Smart shares so that, upon the closing of the merger, the former American Tony stockholders owned 96.7% of the outstanding shares of Aim Smart. America Tony acquired its controlling interest in Aim Smart for a cost of $525,000. This interest was acquired solely to effectuate the reverse merger and was paid for with $276,000 of its own funds and a $249,000 loan from our Chairman, Mingli Yao. Aim Smart changed its name to American Tony upon the closing of the reverse merger.
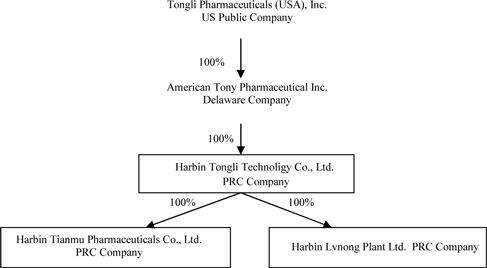
Tianmu Pharmaceuticals was formed under laws of the PRC on November 26, 1999. In February 2007, American Tony acquired Tianmu Pharmaceuticals through a recapitalization transaction which was accomplished through the exchange of shares with Heilongjiang Tongli Technology Co., Ltd. (“TT” or “Tongli Technology”), a wholly-owned subsidiary of American Tony formed in 2007 in the PRC. TT owns 100% of Tianmu Pharmaceuticals and doesn’t have any other operations since its inception. In August 2011, we formed Lvnong to grow chinese herbs in China. The primary purpose of Lvnong is to provide required chinese herbs to Tianmu Pharmaceuticals . Lvnong is 100% owned by our indirect wholly owned subsidiary, Tongli Technology.
Our corporate structure as of the date of this Report is as follows:
Industry Background
Overview of Traditional Chinese Medicine
In China, TCM is not an alternative form of therapy but is used in the state-run hospitals alongside modern medicine. For its practitioners and advocates, TCM is a complete medical system that is used to treat disease in all its forms. TCM is also believed to promote long term wellness and vigor. Many modern-day drugs have been developed from herbal sources. These include drugs designed to treat asthma and hay fever such as ephedrine; hepatitis remedies from fruits and licorice roots and a number of anticancer agents from trees and shrubs.
| 1 |
The roots of TCM date back thousands of years and include a number of therapeutic approaches. These include herbal medications, acupuncture, dietary manipulation, massage and others. Very early works of Chinese medical literature date back as much as 2,500 years while other classics appeared approximately 2,000 years ago during the Han Dynasty. Medicine in China continued to develop throughout the Middle Ages when emperors commissioned the creation of various scholarly works that compiled and documented hundreds of medicines derived from herbs, animal sources and minerals. In addition, these works described their therapeutic uses. In the 1950s, TCM was further modernized and reformed by the PRC government.
The emphasis on wellness and the avoidance of disease is considered by some to be a key distinction between TCM and western medical practice which has been seen as more heavily oriented toward the treatment of disease and less toward prevention. While TCM has remained a substantial part of medical treatment in China and throughout East Asia, recent decades have seen increasing acceptance throughout the United States, Europe and elsewhere. This growth is, in part, driven by increasingly educated and empowered consumers of medical care who seek organic, natural and alternative approaches to western medical treatments and prescription drugs. Medical doctors are also accelerating the process of acceptance, as doctors trained in the western tradition in Europe, the United States and elsewhere are integrating TCM and alternative treatments in their everyday practice. Additionally, a growing number of physicians specifically trained in TCM, acupuncture and other modalities are opening offices in communities in the U.S. and around the world.
We believe that the sales of TCM in China reflect the central and still growing role these therapies play in medical care in that nation. According to IMS Health, a market intelligence firm, the Chinese pharmaceutical market was predicted to grow to more than $50 billion by the end of 2011, which would make China the world’s third-largest pharmaceutical market.
Overview of the Chinese Market
The People’s Republic of China is undergoing the world’s most important and powerful economic transformation. This transformation includes the confluence of its ancient culture with modern trends in business, technology and finance. As a result, Chinese operating companies are capitalizing on unmatched growth opportunities in this evolving and growing marketplace. Although average income is approximately one-tenth that of developed western nations, business growth and market reform-driven policies have given the country’s 1.3 billion citizens more purchasing power than ever.
TCM Industry Drivers
We believe that demographic, governmental and related factors in the China will be favorable to growth and expansion of our business.
Growing Prosperity of the Chinese People. The increased spending power of China’s population continues to be reflected in the increased consumption of health products and medical services between 2007 and 2011. According to Euromonitor data, spending by Chinese people on these goods and services was projected to increase from $100 billion in 2007 to $160 billion in 2011.
Population and Aging
| · | The total population of China was 1.37 billion at the end of 2011 according to official government estimates. |
| · | Due to improved healthcare, the elderly population of China is growing. |
| · | The health/medical costs associated with care for elderly in China are approximately five (5x) times that of younger people. |
| · | China had 170 million elderly people in 2007 but will have an expected 230 million elderly by 2015 according to “Consumer Lifestyles in China: Consumer Trends, China’s Grey Population,” by Euromonitor, 2009. |
| · | The proportion of the China’s population aged 65 and over will rise from just 10% of the overall population in 1995 to 22% by 2030, according to the World Bank. |
| · | From 1995 to 2030 it is estimated that the ratio of working-age people to pensioners will decrease from 9.7:1 to 4.2:1. China’s national estimates vary slightly from World Bank figures, but still show in increase in the proportion of the population over 65 years from 7% in 2000 to 9.4% in 2007, according to China Country Profile 2009, The Economist Intelligence Unit Ltd. |
Government Policies in Health Care and TCM. In April of 2009 the PRC government implemented a new national medical and health plan. Among other features, this new plan extended national medical insurance coverage to China’s rural areas, where the bulk of the population resides. This expanded coverage will eventually encompass virtually all of China’s 1.3 billion citizens, greatly expanding the market for TCM pharmaceuticals, as well as other health care products and services. This has led to massive potential for increased sales growth for Tongli and other providers of TCM pharmaceutical products.
| 2 |
Government Support of Traditional Chinese Medicine. Among its public health initiatives, the Chinese government officially supports use of TCM to enhance wellness and to treat chronic and acute diseases. The government has also commenced a program to evaluate TCM and herbal-based pharmaceuticals for coverage and reimbursement under national medical insurance. In 2002, TCM was declared a “national strategic industry” in the government’s “Development Outline of Traditional Chinese Medicine Modernization (2002 – 2010).”
Decreased Competition. According to the Information Office of the State Council of the PRC, prior to 2009, there were approximately 6,000 Chinese pharmaceutical manufacturers. That number is being significantly reduced through both marketplace attrition and direct government involvement, decreasing competition and increasing potential sales opportunities for the surviving companies. Other companies are expected to fail through lack of size and innovative and aggressive management. According to a 2009 report published by KMPG, of the approximately 4,500 pharmaceutical companies in China, the majority are small players with limited local market reach, and rapid consolidation between medium and large players in the sector is anticipated since the Chinese government has been encouraging industry consolidation with an effort to improve the Good Manufacturing Practice (GMP) standard, enforce GMP certification and to better control the pricing of drugs.
Our Products
Our Chinese operating subsidiaries have been developing, manufacturing and distributing pharmaceuticals and health care products that incorporate elements of Chinese traditional medicine with elements of western medicine since 1999. In August 2011, we formed Lvnong to grow chinese herbs in PRC, which herbs will be provided to Tianmu Pharmaceuticals .Tianmu Pharmaceuticals currently offers drugs and health care products in several distinct categories, including:
| · | Antihyperlipidemics. These tablets, based on principles of Chinese traditional medicine, are used to reduce cholesterol levels and soften blood vessels in order to improve circulation. Our antihyperlipidemics are offered as an affordable alternative to the statins commonly used for this purpose in western medicine. For the year ended March 31, 2012, sales of Antihyperlipidemics accounted for approximately 20% of our total sales revenue during the year. |
| · | Panax and Radix Polygoni Capsule. We began to market this new product in October 2010. This product is designed to reinforce the liver and kidney, replenish Qi (life energy) and blood, with major application on Qi and blood infirmity, beard and hair early whiteness, neurastheria, morbid forgetfulness and insomnia, lack of appetite and fatigue. For the year ended March 31, 2012, sales of Panax and Radix Polygoni Capsule accounted for approximately 45% of our total sales revenue during the year. |
| · | Yuxiang Anti-Bacterial Mouthwash. Comprised of a mixture of medicinal ingredients that counter disease and odor in the oral cavity and throat, Yuxiang Mouthwash is designed to purge bad breath caused by gum disease, abnormal sleep, nervousness, food, alcohol and smoking. We package Yuxiang Mouthwash in bottles that are small enough to be carried conveniently, and we target customers who are travelling or away from home. Our primary points of distribution for Yuxiang Mouthwash are restaurants and transport carriers such us trains. For the year ended March 31, 2012, sales of Yufang Anti-Bacterial Mouthwash accounted for approximately 15% of our total sales revenue during the year. |
| 3 |
| · | Calcium Gluconate Oral Liquid. This is a calcium supplement used for the prevention and treatment of diseases caused by calcium deficiency, such as osteoporosis, bone hypoplasis, and rickets. The liquid is particularly recommended for women during menopause or lactation. We believe that our product has a competitive advantage over other similar products provided by our competitors, because we have obtained a pharmaceutical license for this product, which is considerably more authoritative than the health license under which most of our competitors market their calcium supplements. For the year ended March 31, 2012, sales of Calcium Gluconate Oral Liquid accounted for approximately 14% of our total sales revenue during the year. |
| · | Fuke Zhidai Tablets. This product is used to treat abnormal leucorrhea which caused by chronic cervicitis, endometritis and endocolpitis. For the year ended March 31, 2012, sales of Fuke Zhidai Tablets accounted for approximately 6 % of our total sales revenue during the year. |
We have obtained Drug Register License and Drug Production Certificate for each of the products listed above from China State Food & Drug Administration (“SFDA”). For further information, please refer to “Government Regulation” below.
Lanhai Agreement
In September 2008, we entered into a Patent Assignment Agreement (the “Lanhai Agreement”) with Harbin Lanhai Biochemical Company Limited (“Lanhai”) under which we agreed to acquire a patent related to a product called Lanhai Spirulina Calcium Tablet. Pursuant to the Lanhai Agreement, Lanhai agreed to provide us with the product formula as well as the technical support and assistance necessary for us to obtain SFDA approval for the product. Under the Lanhai Agreement, we made an aggregate payment of RMB7,030,000 (approximately $1.03 million) in October 2008, representing the entire purchase price for the subject patent. We are not obligated to make any subsequent payment. The patent application was accepted by the State Intellectual Property Office of China (“SIPO”) on March 14, 2008 and, if granted, the patent will expire on March 13, 2028 pursuant to PRC patent laws. The term of the Lanhai Agreement (meaning the term under which Lanhai is to provide support and assistance to us) is from November 1, 2008 to October 30, 2013. Title to this patent will not be assigned to us until the patent assignment is properly recorded with SIPO. The recording of this assignment is yet to be confirmed by SIPO. Based on ASC 805-50-30 “Initial Measurement of Acquisition of Assets Rather Than a Business” and ASC350-30 “General Intangibles Other Than Goodwill”, the payment of RMB7,030,000 for acquiring the patent was recorded as an asset at cost. The payment was originally recorded as a deposit which would be recorded as an intangible asset once we obtain the title to this patent, which asset will be subsequently amortized over the life of the patent. During 2010, we reached an unwritten mutual understanding with Lanhai that if we cannot obtain title to the patent before the end of the contract period (i.e., October 30, 2013), the entire payment will be refunded to us. Although we believe this understanding to be binding on the parties, it was not explicitly stipulated in the Lanhai Agreement or otherwise memorialized.
Subsequently, on June 17, 2011, we entered into a Termination Agreement with Lanhai pursuant to which the Lanhai Agreement was terminated. The parties agreed that, because the patent assignment recording process took too long, we shall return the Lanhai Spirulina Calcium Tablet product and related intellectual property to Lanhai. Pursuant to the Termination Agreement, Lanhai agreed to refund the total purchase price of RMB7,030,000 in three installments. The first installment of RMB2,000,000 shall be refunded prior to June 30, 2011, the second installment of RMB2,000,000 shall be refunded prior to July 30, 2011 and the last installment of RMB3,030,000 shall be refunded prior to August 30, 2011. The Termination Agreement also provides that we are entitled to initiate litigation against Lanhai if Lanhai fails to fully refund the total purchase price before August 30, 2011. In June 2011, Lanhai refunded the first installment of RMB 2,000,000. The recording of the patent assignment was terminated in June 2011 as well. The second installment of the refund of RMB 2,000,000 was received on July 18, 2011. The third installment of the refund of RMB 3,030,000 was received on August 2, 2011.
Sanmu Agreement
In August 2009, we signed a contract with a third party Harbin Sanmu Pharmaceuticals to purchase the exclusive rights to manufacture and sell a new product named Yan Li Xiao Capsule nationwide for the next seven years. We paid Harbin Sanmu Pharmaceuticals RMB 1,200,000 (approximate to USD 0.18 million) for this new product namely Yan Li Xiao Capsule in October 2009 and started to manufacture and distribute it in late 2009. However, in July 2010, Harbin Sanmu Pharmaceuticals decided to withdraw the exclusive sales right and the cooperative relationship between us and Harbin Sanmu Pharmaceuticals was terminated. Accordingly, management has written off the intangible asset related to this exclusive sales right.
Tonghua Agreement
On March 21, 2010, we entered a New Drug Assignment Agreement (“Tonghua Agreement”) with a third party TonghuaYisheng Pharmaceuticals Company Limited (“Tonghua”) to purchase a new drug candidate, called Nafarelin, which we believe has great market potential. Total consideration for this patent transaction amounted to RMB 33,000,000 (approximate to $4.85 million) payable in three installments. We paid the first installment of RMB 11,000,000 (approximate to $1.6 million) to Tonghua in March 2010 upon execution of the patent purchase agreement. The second installment of RMB 11,000,000 has been paid to Tonghua during the fourth quarter of 2011. As of March 31, 2012, we have paid total of RMB 22,000,000 to Tonghua on this patent agreement.
| 4 |
On June 6, 2012, we entered into a Termination Agreement with Tonghua pursuant to which the Tonghua Agreement was terminated. Pursuant to the Termination Agreement, Tonghua will refund prepayment of RMB 22,000,000 (approximately $3.4 million) in four installments. The first installment of RMB 5,000,000 (approximately $783,000) is expected to be refunded prior to August 30, 2012, the second installment of RMB 6,000,000 (approximately $939,000) will be refunded prior to November 30, 2012, the third installment of RMB 8,000,000 (approximately $1.3 million) will be refunded prior to February 28, 2013 and the last installment of RMB 3,000,000 (approximately $469,000) will be refunded prior to May 30, 2013.
Xinyu Breath Spray
On April 30, 2012, we entered into Xinyu Breath Spray Purchase Agreement (“Xinyu Purchase Agreement”) with Harbin Junde Healthcare Product Company (“Harbin Junde”). Pursuant to the Xinyu Purchase Agreement, we will acquire the right to manufacture the Xinyu Breath Spray at the total purchase price of RMB 8,500,000 (approximately $1.33 million). We will pay 50% of the purchase price at time of closing of the Xinyu Purchase Agreement and pay the rest of the purchase price after we manufacture three batches of Xinyu Breath Spray that meet the quality standards of product. We paid RMB 1,370,000 (approximately $214,000) on March 29, 2012 and RMB 3,630,000 (approximately $568,000) on May 30, 201, which includes the first installment payment of RMB 4,250,000 and RMB 750,000 for advance payment of testing fees and materials.
Xinyu Breath Spray was marketed primarily through adware. The major markets include supermarkets, hotels and internet. We have two medium-size packages with two flavors for the product, one for man and another for woman. We are targeting to high-end consumers.
We anticipate we will start production of Xinyu Breath Spray in July 2012.
Manufacturing
Our manufacturing and warehouse facilities are located in the Limin Pharmaceutical Technology Park in the City of Harbin. Our entire site was constructed in compliance with Chinese State Drug Administration GMP (Good Manufacturing Practices) standards at a total construction cost of 50 million RMB (approximately $7.3 million), with a goal of achieving world class standards. In recognition of our accomplishment, our manufacturing facility has received the National Drug GMP (Good Manufacturing Practices) Certificate, which is required by laws in order to carry on pharmaceutical manufacturing in the PRC. We have also received certificates from the International Organization for Standardization: specifically, ISO9001:2000 International Quality Management System Certificate and ISO14001 Environmental Management System Certificate.
At the present time, our manufacturing facility has the capacity to produce an annual output of products with a sales value over 100 million RMB (approximately $14,650,000). We believe that our current capacity is adequate for at least the next two years. In the meantime, we have budgeted $5 million for capital investment to expand our capacity, and we will need to raise capital or obtain other funding to finance such expansion.
Marketing
We currently market exclusively within the PRC. Our distribution network is comprised of our own direct sales personnel as well as a network of authorized distribution agents. Currently our sales network includes:
| · | 15 regional distribution agents; |
| · | over 200 city and county level distribution agents; and |
| · | four national distributors, each of whom has the exclusive right to market one or more of our products if certain designated sales targets are achieved. For example, we have given Jilin Province Wan Min Medical Ltd. the exclusive right to market our Calcium Gluconate Oral Solution and our Clindamycin Hydrochloride Capsule nationwide through March 2011 if it purchases certain designated minimum quantities of each product. |
We have entered into agreements with four distributors to provide agreed upon amounts of products at pre-agreed price. In the event a distributor does not purchase a fixed percentage of the agreed upon amounts for three consecutive months, we may terminate the agreement. In addition to that, one agreement provides, among other things, that the distributor can become the exclusive distributor for a geographical area if certain sales targets are met.
| 5 |
We also market online through the “China Flagship Medicine Net”, a consortium website that offers subscribers medical information services and an online purchasing platform.
Major Customers
During the year ended March 31, 2012, approximately 26% of sales were generated from two major distributors. In addition, our four major products (Antihyperlipidemics, Yuxiang Anti-Bacterial Mouthwash, Calcium Gluconate Oral Liquid and Panax and Radix Polygoni Capsule represented approximately 94% of the total sales for the year ended March 31, 2012.
Research and Development
We currently have limited resources to devote to and limited capabilities to conduct the development of new products, and as such research and development activities are not presently material to our business. We, like other TCM manufacturers, enjoy relatively low research and development expenses as most TCM medicines are based on standardized formula. In 2008, SFDA promulgated a notice of registration of Chinese traditional medicine providing that TCM composed of classic prescriptions shall be exempted from pharmacological and toxicological tests and studies. The notice defined classic prescription and classic TCM formulas as those herbal remedies recorded in ancient Chinese medicine books from Qing Dynasty or earlier which are currently widely used. According to such notice, the production and manufacturing of TCM products are subject to non-clinical safety studies only and exempted from pharmacological and toxicological tests and studies. Thus, TCM products are entitled to obtain faster SFDA approval. As such, we enjoy relatively low research and development expenses because most of our products are based on classic TCM formulas that are covered by this notice.
We have decreased research and development expenses incurred in the fiscal year ended March 31, 2012 because our current products are still under normal product cycle and our patents have provided us sufficient capabilities to meet our current production and marketing demand, accordingly, we cut off our investment in our own research and development activities. In addition, we have switched our research and development strategy to acquiring new products with significant market potential from third parties instead of relying on our own efforts which we believe will be more efficient.
Raw Materials
We have developed purchasing relationships with a considerable number of suppliers, and have multiple sources for most of the raw materials that we require. Our business would not be significantly affected by the loss of any one supplier.
A considerable portion of the raw materials that we require are volatile herbs, which have a brief shelf life. This situation imposes a risk on our suppliers, who will often grow the herbs to order in order to insure an immediate market for their herbs. The situation also necessitates that we assure ourselves that our raw material requirements are available precisely when needed. To satisfy these conditions, it is our practice to make substantial cash advances to our suppliers in order to lock-in our raw material requirements. As of March 31, 2012, we have $1,016,294 of advances paid to suppliers.
Competition
We compete with other companies, many of whom are developing, or can be expected to develop, products similar to ours. Some of our competitors are better established than we are, have better brand recognition of products that compete with ours, and have more financial, technical, marketing and other resources than we presently possess and a larger customer base. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers or adopt more aggressive pricing policies. We cannot assure you that we will be able to compete effectively with current or future competitors or that the competitive pressures we face will not harm our business.
We intend to establish a significant market share by advertising the demonstrated efficacy of Tianmu Pharmaceutical’s products. We have extensively tested our products and can cite studies that demonstrate the efficacy of many of them. This contrasts with a large portion of the over-the-counter pharmaceutical market in China, which is characterized by unproven products.
Growth Strategies
In our fiscal year ended March 31, 2012, we continued the execution of our product channel expansion strategy that resulted in increased market penetration of our products and expanded revenue growth. Management plans to continue the emphasis on expanded and enhanced marketing and sales in our 2013 fiscal year and beyond. Part of this strategy involves increasing and improving our marketing and sales activities to enhance the market leadership of our key leading products and to increase the sales of other products by expanding our sales force, solidifying our distribution network and expanding our market segment coverage, and increasing our marketing and promotional activities.
| 6 |
Management also plans to pursue strategic acquisitions of new products with significant market potential as part of our growth strategy in 2013 and beyond. We plan to selectively pursue strategic acquisition opportunities to further consolidate our resources and expand our market coverage. We believe that such an initiative will provide efficient means to broaden our product lines, increase our market coverage and complement our research and development capabilities.
Management believes that our emphasis on further commercializing and broadening our product lines, enhanced sales and marketing efforts has the potential to yield significant increases in revenue in 2013 and beyond.
Government Regulation
The pharmaceutical industry in China, including the TCM sector, is highly regulated. The primary regulatory authority is the SFDA, including its provincial and local branches. As a developer, producer and distributor of medicinal products, we are subject to regulation and oversight by the SFDA and its provincial and local branches. The Law of the PRC on the Administration of Pharmaceuticals provides the basic legal framework for the administration of the production and sale of pharmaceuticals in China and covers the manufacturing, distributing, packaging, pricing and advertising of pharmaceutical products. Its implementing regulations set forth detailed rules with respect to the administration of pharmaceuticals in China. We are also subject to other PRC laws and regulations that are applicable to business operators, manufacturers and distributors in general.
Under the SFDA guidelines for licensing of pharmaceutical products, all pharmaceutical manufacturers must obtain and maintain Good Manufacturing Practices (“GMP”) Certificate. In [year] the SFDA has implemented new GMP policy requiring new standards for all drug manufacturers, which will become fully effective between 2013 and 2015.
Because our manufacturing facility has obtained the National GMP Certificate, we are authorized to produce products in four modes: tablets, capsules, granules, and oral suspensions. In addition to that, in order to market our products as pharmaceuticals, we are required to obtain Drug Register License and Drug Production Certificate specific to each product from the provincial branch of China SFDA. The process of application for such licenses is rigorous, requiring considerable testing. On average, it costs us approximately 1 million RMB (approximately $150,000) to get the approval for each product by the SFDA. To date we have obtained Drug Register License and Drug Production Certificate for our products listed under “Our Products” above.
The more readily available license is for “health care products”, which are governed by the Heilongjiang Province Public Health Bureau. Tianmu Pharmaceuticals has registered its Yuxiang Anti-Bacterial Mouthwash with this Bureau.
Currently we have not developed a market in U.S. so we believe we are not subject to any of regulations by the U.S. Food and Drug Administration.
Environmental Matters
Our manufacturing and warehouse facilities are located in the Limin Pharmaceutical Technology Park in the City of Harbin. We believe that the industrial zone where we have located our manufacturing facilities is equipped with all necessary equipment that will enable us to comply with the applicable national, provincial and local environmental laws related to our operation. We maintain all the permits and licenses required by the PRC environment regulations through Limin Pharmaceutical Technology Park, to whom we pay certain amount of management fees every year.
Intellectual Property
We have one registered trademark for the Chinese character of “Tianmu” approved by the China Trademark Office covering drug capsule, medicinal capsule and active pharmaceutical ingredients. This registered trademark relates to our drug products. The trademark registration will expire in August 2012 and we plan to renew the registration for another ten-year period.
We have one design patent registered in China that covers the method of applying blue polyethylene packaging to the bottles of our Calcium Gluconate Oral Solution. This design patent relates to our healthcare products and will expire on May 10, 2015.
We purchased a patent related to a healthcare product named Lanhai Spirulina Calcium Tablet from Harbin Lanhai Biochemical Company Limited in September 2008. This patent will expire in August 2027. Subsequently, on June 17, 2011, we entered into a Termination Agreement with Lanhai pursuant to which the Lanhai Agreement was terminated. The parties agreed that, because the patent assignment recording process took too long, we returned the Lanhai Spirulina Calcium Tablet product and related intellectual property to Lanhai.
All of our employees are bound by our company policy of non-disclosure our proprietary information, although we have no written confidentiality agreements with individual employee.
| 7 |
Employees
We currently have approximately 120 employees, all of whom are employed on a full-time basis. 20 employees are in executive management and human resource and administration, 5 in accounting, 15 in technical and 80 in manufacturing.
Executive Offices in China
Our executive offices in China are located at 1 Beijing Road, Limin Development Zone, Harbin, China. We maintain a website at www.tmyy.com.cn. Information contained on or accessed through our website is not intended to constitute and shall not be deemed to constitute part of this Report.
Item 1A. Risk Factors
Our business, operations and financial condition are subject to various risks. Some of these risks are described below and you should take these risks into account in making a decision to invest in our common stock. If any of the following risks actually occurs, we may not be able to conduct our business as currently planned and our financial condition and operating results could be seriously harmed. In that case, the market price of our common stock could decline and you could lose all or part of your investment in our common stock.
Risks Related to Our Business
We may need additional financing, which may not be available on satisfactory terms or at all.
The revenues from the production and sale may not be adequate to support our expansion plans or our business generally. We may need substantial additional funds to build new production facilities, acquire new products, obtain regulatory approvals, market our products, and file, prosecute, defend and enforce our intellectual property rights.
At present we have no commitment from any source for those funds. We cannot determine, therefore, the terms on which we will be able to raise the necessary funds. To the extent we raise additional capital by issuing equity securities, our stockholders may experience dilution. To the extent that we raise additional capital by issuing debt securities, we may incur substantial interest obligations, may be required to pledge assets as security for the debt and may be constrained by restrictive financial and/or operational covenants. Debt financing would also be superior to our stockholders’ interest in bankruptcy or liquidation.
There are no assurances that future funding will be available to us on favorable terms or at all. If additional funding is not obtained, we will need to reduce, defer or cancel development programs, planned initiatives or overhead expenditures, to the extent necessary. The failure to fund our capital requirements would have a material adverse effect on our business, financial condition and results of operations.
We have been heavily dependent on key products.
Our four major products (Antihyperlipidemics, Yuxiang Anti-Bacterial Mouthwash, Calcium Gluconate Oral Liquid and Panax and Radix Polygoni Capsule) represented approximately 86.6% of the total sales for the year ended March 31, 2011. We expect that a significant portion of our future revenue will continue to be derived from sales of these four products. If any of these four products were to become subject to a problem such as loss of patent protection, unexpected side effects, regulatory proceedings, publicity adversely affecting user confidence or pressure from competing products, or if a new, more effective treatment should be introduced, the impact on our revenues could be significant.
We face competition in the pharmaceutical market in the PRC and such competition could cause our sales revenue and profits to decline.
The certificates, permits, and licenses required for pharmaceutical operation in the PRC create a potentially significant barrier for new competitors seeking entrance into the market. Despite these obstacles, we face competitors that will attempt to create, or are already marketing, products in the PRC that are similar to ours. Many of our current and potential competitors have significantly longer operating histories and significantly greater managerial, financial, marketing, technical and other competitive resources, as well as greater name recognition, than we do. These competitors may be able to respond more quickly to new or changing opportunities and customer requirements and may be able to undertake more extensive promotional activities, offer more attractive terms to customers or adopt more aggressive pricing policies. We cannot assure you that we will be able to compete effectively with current or future competitors or that the competitive pressures we face will not harm our business.
| 8 |
Our business and growth will suffer if we are unable to hire and retain key personnel that are in high demand.
Our future success depends on our ability to attract and retain highly skilled chemists, pharmaceutical engineers, technical, and marketingl, especially qualified personnel for our operations in China. Qualified individuals are in high demand in China, and there are insufficient experienced personnel to fill the demand. Therefore we may not be able to successfully attract or retain the personnel we need to succeed.
Our business development would be hindered if we lost the services of some key personnel. Yao Mingli is the Chief Executive Officer of our company and of its operating subsidiary, Tianmu Pharmaceuticals. Mr. Yao is responsible for strategizing not only our business plan but also the means of financing it. If Mr. Yao were to leave Tianmu Pharmaceuticals or become unable to fulfill his responsibilities, our business would be imperiled. At the very least, there would be a delay in the development of Tianmu Pharmaceuticals until a suitable replacement for Mr. Yao could be retained.
Our results of operations are dependent on continually developing or acquiring new and advanced products, technologies and processes, and failure to do so may cause us to lose our competitiveness in the pharmaceutical industry and may cause our profits to decline.
To remain competitive in the pharmaceutical industry, it is important to continually develop new and advanced products, technologies and processes. There is no assurance that our competitors’ new products, technologies and processes will not render our company’s existing products obsolete or non-competitive. Our company’s competitiveness in the pharmaceutical market therefore relies upon our ability to enhance our current products, introduce new products, and develop and implement new technologies and processes. Our company’s failure to technologically evolve and/or develop new or enhanced products may cause us to lose our competitiveness in the pharmaceutical industry and may cause our profits to decline. It is likely that our efforts to grow our products lines will be focused on acquisitions of such products from third parties. There are many risks attendant to the acquisition of assets or companies, including availability, pricing, competition and, if acquisitions are consummate, integration. If we are unable to so acquire and integrate new products, our revenue and profitability may suffer.
The commercial success of our products depends upon the degree of market acceptance among the medical community and failure to attain market acceptance among the medical community may have an adverse impact on our operations and profitability.
The commercial performance of our products depends upon the degree of market acceptance among the medical community, such as hospitals and physicians. Even if our products are approved by SFDA, and even if our products are authorized to be eligible for reimbursement under Chinese national medical insurance programs, there is no assurance that physicians will prescribe or recommend our products to patients. Furthermore, a product’s prevalence and use at hospitals may be contingent upon our relationship with the medical community. The acceptance of our products among the medical community may depend upon several factors, including but not limited to, the product’s acceptance by physicians and patients as a safe and effective treatment, cost effectiveness, potential advantages over alternative treatments, and the prevalence and severity of side effects. Failure to attain market acceptance among the medical community may have an adverse impact on our operations and profitability.
We may not be able to obtain the regulatory approvals or clearances that are necessary to manufacture and commercialize our products.
The PRC and other countries impose significant statutory and regulatory obligations upon the manufacture and sale of pharmaceutical products. Each regulatory authority typically has a lengthy approval process in which it examines pre-clinical and clinical data and the facilities in which the product is manufactured. Regulatory submissions must meet complex criteria to demonstrate the safety and efficacy of the ultimate products. Addressing these criteria requires considerable data collection, verification and analysis. We may spend time and money preparing regulatory submissions or applications without assurances as to whether they will be approved on a timely basis or at all.
Our product candidates, some of which are currently in the early stages of development, will require significant additional development and pre-clinical and clinical testing prior to their commercialization. These steps and the process of obtaining required approvals and clearances can be costly and time-consuming. If our potential products are not successfully developed, cannot be proven to be safe and effective through clinical trials, or do not receive applicable regulatory approvals and clearances, or if there are delays in the process:
| · | the commercialization of our products could be adversely affected; |
| · | any competitive advantages of the products could be diminished; and |
| · | revenues or collaborative milestones from the products could be reduced or delayed. |
| 9 |
Governmental and regulatory authorities may approve a product candidate for fewer indications or narrower circumstances than requested or may condition approval on the performance of post-marketing studies for a product candidate. Even if a product receives regulatory approval and clearance, it may later exhibit adverse side effects that limit or prevent its widespread use or that would force us to withdraw the product from the market.
Any marketed product and its manufacturer will continue to be subject to strict regulation after approval. Results of post-marketing programs may limit or expand the further marketing of products. Unforeseen problems with an approved product or any violation of regulations could result in restrictions on the product, including its withdrawal from the market and possible civil actions.
In manufacturing our products we will be required to comply with applicable good manufacturing practices regulations, which include requirements relating to quality control and quality assurance, as well as the maintenance of records and documentation and utilization of qualified raw materials. If we cannot comply with regulatory requirements, including applicable good manufacturing practice requirements, we may not be allowed to manufacture, develop or market the product candidates. If we or our manufacturers fail to comply with applicable regulatory requirements at any stage during the regulatory process, we may be subject to sanctions, including fines, product recalls or seizures, mandatory disposal of unqualified raw materials, injunctions, refusal of regulatory agencies to review pending market approval applications or supplements to approve applications, total or partial suspension of production, civil penalties, withdrawals of previously approved marketing applications and criminal prosecution.
Our current and future products may have inadvertent and/or harmful side effects which would expose us to the risks of litigation and a loss of revenue.
All medicines have certain side effects. Although all of our medicines sold on market have passed proper testing and are approved by SFDA, the products may still inadvertently adverse effects on the health of the consumers. If such side effect is identified after marketing and sale of the products, the products may be required to be withdrawn from the market, or have a change in labeling. If a product liability claim is brought against us, it may, regardless of merit or eventual outcome, result in damage to our reputation, breach of contracts with consumers, decreased demand for our products, costly litigation and loss of revenue.
Natural disasters, weather conditions and other environmental factors affect our raw material supply, and a reduction in the quality or quantity of our herb supplies may have material adverse consequences on our financial results.
Our business may be adversely affected by weather and environmental factors beyond our control, such as natural disasters and adverse weather conditions. The production of our products depends on the availability of raw materials, a significant portion of which are herbs. These herbs tend to be very sensitive crops, which can be readily damaged by harsh weather, by disease, and by pests. If our suppliers’ crops are destroyed by drought, flood, storm, blight, or the other woes of farming, we will not be able to meet the demands of our customers, which will have a material adverse effect on our business and financial condition and results.
If we lost control of our distribution network, our business would fail.
We depend on our distribution network for the success of our business. Competitors may seek to pull our distribution network away from us. In addition, if dominant members of our distribution network become dissatisfied with their relationship with Tianmu Pharmaceuticals, a concerted effort by the distribution network could force us to accept less favorable financial terms from the distribution network. Either of these possibilities, if realized, would have an adverse effect on our business.
We may not be able to adequately protect our intellectual property, which could cause us to be less competitive.
We regard our trademarks, trade secrets, patents and similar intellectual property as material to our success. We rely on trademark, patent and trade secret law, as well as confidentiality and license agreements with certain of our customers and others to protect our proprietary rights. We have received trademark and patent protection for certain of our products in the PRC. No assurance can be given that our patents and licenses will not be challenged, invalidated, infringed or circumvented, or that our intellectual property rights will provide competitive advantages to us. There can be no assurance that we will be able to obtain a license from a third-party technology that we may need to conduct our business or that such technology can be licensed at a reasonable cost.
If our products fail to receive regulatory approval or are severely limited in the products scope of use, then we may be unable to recoup our research and development expenditures and we may not be able to adequately sell such products.
Our products that are approved to be manufactured as of March 31, 2012 include four medicines. The production of our pharmaceutical products is subject to the regulatory approval of the SFDA. The regulatory approval procedure for pharmaceuticals can be quite lengthy, costly, and uncertain. Depending upon the discretion of the SFDA, the approval process may be significantly delayed by additional clinical testing and require the expenditure of currently unavailable resources; in such an event, it may be necessary for us to abandon our application. Even where approval of the product is granted, it may contain significant limitations in the form of narrow indications, warnings, precautions, or contra-indications with respect to conditions of use. If approval of our product is denied, abandoned, or severely limited in terms of the scope of products use, it may result in the inability to recoup considerable research and development expenditures already incurred.
| 10 |
Our certificates, permits, and license are subject to governmental control and renewal, and the failure to obtain renewal would cause all or part of our operation to be suspended and have a material adverse effect on our financial condition.
We are subject to various PRC laws and regulations pertaining to the pharmaceutical industry. We have has attained certain certificates, permits, and licenses required for the operation of a pharmaceutical enterprise and the manufacturing of pharmaceutical products in the PRC. We obtained the Medicine Production Permit in 2003 and 2004, which are subject to annual checks by the SFDA. We also have GMP certificates which are subject to annual checks by the SFDA. The pharmaceutical production permits and GMP certificates are each valid for a term of five years and must be renewed before their expiration. During the renewal process, we will be re-evaluated by the appropriate governmental authorities and must comply with the prevailing standards and regulations, which may change from time to time. In the event that we are not able to renew the certificates, permits and licenses, all or part of our operations may be suspended by the government, which would have a material adverse effect on our financial condition. Furthermore, if escalating compliance costs associated with governmental standards and regulations restrict or prohibit any part of our operations, it may adversely affect our results of operations and profitability.
We may be subject to the People’s Republic of China’s price control of drugs which may limit our profitability and even cause us to stop manufacturing certain products .
The State Development and Reform Commission of the PRC (“SDRC”) and the price administration bureaus of the relevant provinces of the PRC in which the pharmaceutical products are manufactured are responsible for the retail price control over our pharmaceutical products. The SDRC sets the price ceilings for pharmaceutical products that are listed in an “Essential Drug List” (“EDL”) prescribed by the Chinese government. Products (other than under the new drug protection periods) are subject to such price controls. Previously, two of the ten products which we produced (Metformin Hydrochloride Tablets and Clindamycin Hydrochloride Capsules) were included in EDL. The new drug protection periods for both products have expired and thus these two products are under price control. We stopped production of these two products for the fiscal years ended March 31, 2011 or March 31, 2010 because there was no profit margin to support the production. There is no assurance as to whether the Chinese government will impose pricing control on products outside of the EDL. Where our products are subject to a price ceiling, we will need to adjust the product price to meet the requirement and to accommodate for the pricing of competitors in the competition for market shares. The price ceilings set by the SDRC may limit our profitability, and in some instances, such as where the price ceiling is below production costs, may cause us to stop manufacturing certain products which may adversely affect our results of operations.
Because we may not be able to obtain business insurance in the PRC, we may not be protected from risks that are customarily covered by insurance in the United States.
Business insurance is not readily available in the PRC. To the extent that we suffer a loss of a type which would normally be covered by insurance in the United States, such as product liability and general liability insurance, we would incur significant expenses in both defending any action and in paying any claims that result from a settlement or judgment. We have not obtained fire, casualty and theft insurance, and there is no insurance coverage for our raw materials, goods and merchandise, furniture and buildings in China. Any losses incurred by us will have to be borne by us without any assistance, and we may not have sufficient capital to cover material damage to, or the loss of, our production facility due to fire, severe weather, flood or other cause, and such damage or loss would have a material adverse effect on our financial condition, business and prospects.
We may be subject to product liability claims, for which we have no insurance.
We may produce products which inadvertently have an adverse pharmaceutical effect on the health of individuals. Existing laws and regulations in China do not require us to maintain third party liability insurance to cover product liability claims. However, if a product liability claim is brought against us, it may, regardless of merit or eventual outcome, result in damage to our reputation, breach of contracts with our customers, decreased demand for our products, costly litigations, product recalls, loss of revenue, and our inability to commercialize some products.
Our indemnification obligations could adversely affect our business, financial condition and results of operations.
Our governing documents require us to indemnify our current and former directors, officers, employees and agents against most actions of a civil, criminal, administrative or investigative nature. Generally, we are required to advance indemnification expenses prior to any final adjudication of an individual’s culpability. The expense of indemnifying our current and former directors, officers and employees and agents in their defense or related expenses as a result of any actions related to the internal investigation and financial restatement may be significant and in excess of any insurance coverage we may have. As such, there is a risk that our indemnification obligations could divert needed financial resources and may adversely affect our business, financial condition and results of operations.
| 11 |
A large portion of our common stock is controlled by a small number of stockholders and as a result, these stockholders are able to influence and ultimately control the outcome of stockholder votes on various matters.
Mr. Mingli Yao, our Chairman and CEO, together with his wife and daughter owns 3,761,337, or 27.8% of our outstanding shares as of the date of this Form 10-K. As a result, these stockholders are able to influence and potentially control the outcome of stockholder votes on various matters, including the election of directors and other corporate transactions including business combinations. In addition, the occurrence of sales of a large number of shares of our common stock, or the perception that these sales could occur, may affect our stock price and could impair our ability to obtain capital through an offering of equity securities. Furthermore, the current ratios of ownership of our common stock reduce the public float and liquidity of our common stock which can in turn affect the market price of our common stock.
If we are unable to maintain appropriate internal financial reporting controls and procedures, it could cause us to fail to meet our reporting obligations, result in the restatement of our financial statements, harm our operating results, subject us to regulatory scrutiny and sanction, and cause investors to lose confidence in our reported financial information.
Effective internal controls are necessary for us to provide reliable financial reports and effectively prevent fraud. As a public company, we have significant additional requirements for enhanced financial reporting and internal controls. We will be required to document and test our internal control procedures in order to satisfy the requirements of Section 404 of the Sarbanes-Oxley Act of 2002, which requires annual management assessments of the effectiveness of our internal controls over financial reporting and a report by our independent registered public accounting firm addressing these assessments. The process of designing and implementing effective internal controls is a continuous effort that requires us to anticipate and react to changes in our business and the economic and regulatory environments and to expend significant resources to maintain a system of internal controls that is adequate to satisfy our reporting obligations as a public company.
We cannot assure you that we will not, in the future, identify areas requiring improvement in our internal control over financial reporting. We cannot assure you that the measures we will take to remediate any areas in need of improvement will be successful or that we will implement and maintain adequate controls over our financial processes and reporting in the future as we continue our growth. If we are unable to establish appropriate internal financial reporting controls and procedures, it could cause us to fail to comply with Sarbanes-Oxley and meet our reporting obligations, result in the restatement of our financial statements, harm our operating results, subject us to regulatory scrutiny and sanction, and cause investors to lose confidence in our reported financial information.
Moreover, because our business is in China, our management there lacks experience in Western accounting practices and related U.S. public company requirements. This lack of experience may result in the production of financial statements that are inaccurate or do not fully comply with U.S. generally accepted accounting principles. If inaccurate financial statements are prepared, we may later be required to restate them, which could cause great harm to our stock price and our company generally.
We have not developed independent corporate governance.
Although we have one director that is “independent” (as defined under Nasdaq Marketplace Rules), we have no audit, compensation, or nominating committees of our board of directors. Our independent director does not speak English and resides in China. We are inexperienced in formal U.S. corporate governance procedures. A lack of functioning independent controls over our corporate affairs may result in potential or actual conflicts of interest between our management and our stockholders. We presently have no policy to resolve such conflicts. The absence of such standards of corporate governance may leave our stockholders without protections against interested director or executive transactions, conflicts of interest and similar matters, which could negatively impact an investment in our company.
We incur significant costs as a result of being a public company.
As a public company, we incur significant legal, accounting and other expenses that we did not incur as a private company. In addition, the Sarbanes-Oxley Act of 2002, as well as new rules subsequently implemented by the SEC, have required changes in corporate governance practices of public companies. We expect these new rules and regulations to increase our legal, accounting and financial compliance costs and to make certain corporate activities more time-consuming and costly. In addition, we will incur additional costs associated with our public company reporting requirements. We are currently evaluating and monitoring developments with respect to these new rules, and we cannot predict or estimate the amount of additional costs we may incur or the timing of such costs.
Our directors and officers liability insurance may lapse or be invalid or may fail to cover any expenses and losses due to lawsuits related to financial reporting errors, and our indemnification obligations could adversely affect our business, financial condition and results of operations.
Our director and officer liability insurance may lapse or otherwise be unable to cover lawsuit expenses and losses related to financial reporting errors. Our bylaws require us to indemnify our current and former directors, officers, employees and agents against most actions of a civil, criminal, administrative or investigative nature. Generally, we are required to advance indemnification expenses prior to any final adjudication of an individual’s culpability. The expense of indemnifying our current and former directors, officers and employees and agents in their defense or related expenses as a result of any actions related to the internal investigation and financial restatement may be significant. Therefore, our indemnification obligations could result in the diversion of our financial resources and may adversely affect our business, financial condition and results of operations.
| 12 |
We are not likely to hold annual stockholder meetings in the next few years.
Management does not expect to hold annual meetings of stockholders in the next few years, due to the expense involved. The current members of the Board of Directors were appointed to that position by the previous directors. If other directors are added to the Board in the future, it is likely that the current directors will appoint them. As a result, our stockholders will have no effective means of exercising control over the operations of our company.
Potential environmental liability could have a material adverse effect on our operations and financial condition.
As a manufacturer, we are subject to various Chinese environmental laws and regulations on air emission, waste water discharge, solid wastes and noise. Although we believe that our operations are in substantial compliance with current environmental laws and regulations, we may not be able to comply with these regulations at all times as the Chinese environmental legal regime is evolving and becoming more stringent. Therefore, if the Chinese government imposes more stringent regulations in the future, we may have to incur additional and potentially substantial costs and expenses in order to comply with new regulations, which may negatively affect our results of operations. Further, no assurance can be given that all potential environmental liabilities have been identified or properly quantified or that any prior owner, operator, or tenant has not created an environmental condition unknown to us. If we fail to comply with any of the present or future environmental regulations in any material aspects, we may suffer from negative publicity and be subject to claims for damages that may require us to pay substantial fines or have our operations suspended or even be forced to cease operations.
Risks Associated With Doing Business In China
There are substantial risks associated with doing business in China, as set forth in the following risk factors.
The Chinese government exerts substantial influence over the manner in which we must conduct our business activities.
We are dependent on our relationship with the local government in the Chinese province in which we operate our business. The Chinese government has exercised and continues to exercise substantial control over virtually every sector of the Chinese economy through regulation and state ownership. Our ability to operate in China may be harmed by changes in its laws and regulations, including those relating to taxation, environmental regulations, land use rights, property and other matters. The central or local governments of in the PRC jurisdictions may impose new, stricter regulations or interpretations of existing regulations that would require additional expenditures and efforts on our part to ensure our compliance with such regulations or interpretations. Accordingly, government actions in the future, including any decision not to continue to support recent economic reforms and to return to a more centrally planned economy or regional or local variations in the implementation of economic policies, could have a significant effect on economic conditions in China or particular regions thereof, and could require us to divest ourselves of any interest we then hold in Chinese properties.
Future inflation in China may inhibit our ability to conduct business in China. In recent years, the Chinese economy has experienced periods of rapid expansion and high rates of inflation. Rapid economic growth can lead to growth in the money supply and rising inflation. If prices for our products rise at a rate that is insufficient to compensate for the rise in the costs of supplies, it may have an adverse effect on profitability. These factors have led to the adoption by Chinese government, from time to time, of various corrective measures designed to restrict the availability of credit or regulate growth and contain inflation. High inflation may in the future cause Chinese government to impose controls on credit and/or prices, or to take other action, which could inhibit economic activity in China, and thereby harm the market for our products.
If our land use rights are revoked, we would have no operational capabilities.
Under Chinese law land is owned by the state or rural collective economic organizations. The state issues to tenants the rights to use property. Land use rights can be revoked and the tenants forced to vacate at any time when redevelopment of the land is in the public interest. The public interest rationale is interpreted quite broadly and the process of land appropriation may be less than transparent. Through our operating subsidiaries, we rely on these land use rights as the cornerstone of our operations for both our manufacturing facility and our corporate headquarters. The loss of such rights would have a material adverse effect on our company as we would be required to relocate our facilities and obtain new land use rights, and there is no assurance that we would be able to accomplish such a relocation with reasonable cost or at all. Even if we could relocate our facilities to an appropriate piece of land, we would be required to go through the inspection and approval procedure to obtain the Good Manufacture Practice (“GMP”) certificate, which may take up to three years. Such disruption may cause us to outsource manufacture and as a result have material adverse effect on our costs of goods sold and our result of operations.
| 13 |
Our operations and assets in China are subject to significant political and economic uncertainties and the company may lose all of its assets and operations if the Chinese government alters its policies to further restrict foreign participation in business operating in the PRC.
Changes in PRC laws and regulations, or their interpretation, or the imposition of confiscatory taxation, restrictions on currency conversion, imports and sources of supply, devaluations of currency or the nationalization or other expropriation of private enterprises could have a material adverse effect on our business, results of operations and financial condition. Under its current leadership, the Chinese government has been pursuing economic reform policies that encourage private economic activities and greater economic decentralization. There is no assurance, however, that the Chinese government will continue to pursue these policies, or that it will not significantly alter these policies from time to time without notice. We may lose all of our assets and operations if the Chinese government alters its policies to further restrict foreign participation in business operating in the PRC.
We derive all of our sales in China and a slowdown or other adverse developments in the PRC economy may materially and adversely affect our customers, demand for our products and our business.
All of our sales are generated in China. We anticipate that sales of our products in China will continue to represent all of our total sales in the near future. Although the PRC economy has grown significantly in recent years, we cannot assure you that such growth will continue. The industry which we are involved in the PRC is relatively new and growing, but we do not know how sensitive we are to a slowdown in economic growth or other adverse changes in the PRC economy which may affect demand for our products. In addition, the Chinese government also exercises significant control over Chinese economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Efforts by the Chinese government to slow the pace of growth of the Chinese economy could result in reduced demand for our products. A slowdown in overall economic growth, an economic downturn or recession or other adverse economic developments in the PRC may materially reduce the demand for our products and materially and adversely affect our business.
Currency fluctuations and restrictions on currency exchange may adversely affect our business, including limiting our ability to convert Chinese Renminbi into foreign currencies and, if Chinese Renminbi were to decline in value, reducing our revenue in U.S. dollar terms.
Our reporting currency is the U.S. dollar and our operations in China use their local currency as their functional currencies. Substantially all of our revenue and expenses are in the Chinese currency, the Renminbi. We are subject to the effects of exchange rate fluctuations with respect to any of these currencies. For example, the value of the Renminbi depends to a large extent on Chinese government policies and China’s domestic and international economic and political developments, as well as supply and demand in the local market. Since 1994, the official exchange rate for the conversion of the Renminbi to the U.S. dollar had generally been stable and the Renminbi had appreciated slightly against the U.S. dollar. In July 2005, the Chinese government changed its policy of pegging the value of the Renminbi to the U.S. dollar. Under this policy, which was halted in 2008 due to the worldwide financial crisis, the Renminbi was permitted to fluctuate within a narrow and managed band against a basket of certain foreign currencies. In June 2010, the Chinese government announced its intention to again allow the Renminbi to fluctuate within the 2005 parameters. It is possible that the Chinese government could adopt an even more flexible currency policy, which could result in more significant fluctuation of Renminbi against the U.S. dollar, or it could adopt a more restrictive policy. We can offer no assurance that the Renminbi will be stable against the U.S. dollar or any other foreign currency.
Our financial statements are translated into U.S. dollars at the average exchange rates in each applicable period. To the extent the U.S. dollar strengthens against foreign currencies, the translation of these foreign currencies denominated transactions results in reduced revenue, operating expenses and net income for our international operations. Similarly, to the extent the U.S. dollar weakens against foreign currencies, the translation of these foreign currency denominated transactions results in increased revenue, operating expenses and net income for our international operations. We are also exposed to foreign exchange rate fluctuations as we convert the financial statements of our foreign consolidated subsidiaries into U.S. dollars in consolidation. If there is a change in foreign currency exchange rates, the conversion of the foreign consolidated subsidiaries’ financial statements into U.S. dollars will lead to a translation gain or loss which is recorded as a component of other comprehensive income. In addition, we have certain assets and liabilities that are denominated in currencies other than the relevant entity’s functional currency. Changes in the functional currency value of these assets and liabilities create fluctuations that will lead to a transaction gain or loss. We have not entered into agreements or purchased instruments to hedge our exchange rate risks, although we may do so in the future. The availability and effectiveness of any hedging transaction may be limited and we may not be able to hedge our exchange rate risks.
| 14 |
Changes in foreign exchange regulations in the PRC may affect our ability to pay dividends in foreign currency or conduct other foreign exchange business.
We receive substantially all of our revenues in Renminbi, the Chinese currency, which is currently not a freely convertible currency. The restrictions on currency exchanges may limit our ability to use revenues generated in RMB to make dividends or other payments in United States dollars. The PRC government strictly regulates conversion of RMB into foreign currencies. Over the years, foreign exchange regulations in the PRC have significantly reduced the government’s control over routine foreign exchange transactions under current accounts. In the PRC, SAFE regulates the conversion of the RMB into foreign currencies. Pursuant to applicable PRC laws and regulations, foreign invested enterprises incorporated in the PRC are required to apply for “Foreign Exchange Registration Certificates.” Currently, conversion within the scope of the “current account” (e.g. remittance of foreign currencies for payment of dividends, etc.) can be effected without requiring the approval of SAFE. However, conversion of currency in the “capital account” (e.g. for capital items such as direct investments, loans, securities, etc.) still requires the approval of SAFE. In addition, failure to obtain approval from SAFE for currency conversion on the capital account may adversely impact our capital expenditure plans and our ability to expand in accordance with our desired objectives.
The PRC government also may at its discretion restrict access in the future to foreign currencies for current account transactions. If the foreign exchange control system prevents us from obtaining foreign currency, we may be unable to pay dividends or meet obligations that may be incurred in the future that require payment in foreign currency.
The application of PRC regulations relating to the overseas listing of PRC domestic companies is uncertain, and we may be subject to penalties for failing to request approval of the PRC authorities prior to listing our shares in the U.S.
On August 8, 2006, six PRC government agencies, namely, the Ministry of Commerce, the State Administration for Industry and Commerce, the China Securities Regulatory Commission (the “ CSRC ”), the State Administration of Foreign Exchange, the State Assets Supervision and Administration Commission, and the State Administration for Taxation, jointly issued the “Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors” (the “New M&A Rules”), which became effective on September 8, 2006. The New M&A Rules purport, among other things, to require offshore “special purpose vehicles,” that are (1) formed for the purpose of overseas listing of the equity interests of Chinese companies via acquisition and (2) are controlled directly or indirectly by Chinese companies and/or Chinese individuals, to obtain the approval of the CSRC prior to the listing and trading of their securities on overseas stock exchanges.
The reverse merger that took place between Aim Smart and American Tony in July 2008 may be subject to the New M&A Rules and thus may be required to obtain CSRC approval. Our management believes that, to date, there remain substantial uncertainties regarding the interpretation, application and enforcement of the New M&A Rules. CSRC has yet to promulgate any written guidance with regard to the application procedure to obtain CSRC approval for the overseas listing of a company structured similar to ours. As a result, our management was unable to determine if we should (and, therefore, we did not) apply for CSRC approval prior to the consummation of its reverse merger in July 2008. It is management’s intention to apply for CSRC approval when more specific and explicit interpretations are issued by the PRC government and when it therefore become clear that such approval is required. Should the PRC government take the position that such rules were or are applicable to us, there are substantial risks associated with our failure to apply the CSRC approval including but not limited to the following:
| · | fines and other penalties on our operations in China, |
| · | suspension or limitation on our operating privileges in China, |
| · | restriction or limitation on remittance of dividends outside of China, |
| · | restriction or delay the repatriation of the proceeds from future financings into China; and |
| · | other forms of sanctions that may cause a material and adverse effect to our business, operations and financial conditions. |
If any of these risks should come to reality, it could have a material adverse affect on our business.
| 15 |
We may face regulatory uncertainties that could restrict our ability to issue equity compensation to our directors and employees and other parties who are PRC citizens or residents under PRC law.
On April 6, 2007, SAFE issued the “Operating Procedures for Administration of Domestic Individuals Participating in the Employee Stock Ownership Plan or Stock Option Plan of An Overseas Listed Company, also know as “Circular 78.” It is not clear whether Circular 78 covers all forms of equity compensation plans or only those which provide for the granting of stock options. For any equity compensation plan which is so covered and is adopted by a non-PRC listed company after April 6, 2007, Circular 78 requires all participants who are PRC citizens to register with and obtain approvals from SAFE prior to their participation in the plan. In addition, Circular 78 also requires PRC citizens to register with SAFE and make the necessary applications and filings if they participated in an overseas listed company’s covered equity compensation plan prior to April 6, 2007. We have begun to make option and stock grants to some of our directors and employees, who are PRC citizens and have adopted an equity compensation plan of 2 million shares. Circular 78 may require PRC citizens who receive option grants to register with SAFE. We believe that the registration and approval requirements contemplated in Circular 78 will be burdensome and time consuming. Failure to comply with such provisions may subject us and recipients of such options to fines and legal sanctions and prevent us from being able to grant equity compensation to our PRC employees. In that case, our ability to compensate our employees and directors through equity compensation would be hindered and our business operations may be adversely affected.
Because our principal assets are located outside of the United States and with the exception of one director, our directors and all our officers reside outside of the United States, it may be difficult for you to enforce your rights based on the United States Federal securities laws against us and our officers and directors in the United States or to enforce judgments of United States courts against us or them in the PRC.
All of our officers and directors reside outside of the United States. In addition, our operating subsidiaries are located in the PRC and all of their assets are located outside of the United States. China does not have a treaty with United States providing for the reciprocal recognition and enforcement of judgments of courts. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the United States Federal securities laws against us in the courts of either the United States or the PRC and, even if civil judgments are obtained in courts of the United States, to enforce such judgments in PRC courts. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement against us or our officers and directors of criminal penalties, under the United States Federal securities laws or otherwise.
We may have limited legal recourse under PRC law if disputes arise under our contracts with third parties.
The Chinese government has enacted some laws and regulations dealing with matters such as corporate organization and governance, foreign investment, commerce, taxation and trade. However, their experience in implementing, interpreting and enforcing these laws and regulations is limited, and our ability to enforce commercial claims or to resolve commercial disputes is unpredictable. If our new business ventures are unsuccessful, or other adverse circumstances arise from these transactions, we face the risk that the parties to these ventures may seek ways to terminate the transactions, or, may hinder or prevent us from accessing important information regarding the financial and business operations of these acquired companies. The resolution of these matters may be subject to the exercise of considerable discretion by agencies of the Chinese government, and forces unrelated to the legal merits of a particular matter or dispute may influence their determination. Any rights we may have to specific performance, or to seek an injunction under PRC laws, in either of these cases, are severely limited, and without a means of recourse by virtue of the Chinese legal system, we may be unable to prevent these situations from occurring. The occurrence of any such events could have a material adverse effect on our business, financial condition and results of operations. Although legislation in China over the past 30 years has significantly improved the protection afforded to various forms of foreign investment and contractual arrangements in China, these laws, regulations and legal requirements are relatively new and their interpretation and enforcement involve uncertainties, which could limit the legal protection available to us, and foreign investors, including you. The inability to enforce or obtain a remedy under any of our future agreements could result in a significant loss of business, business opportunities or capital and could have a material adverse impact on our operations.
We must comply with the Foreign Corrupt Practices Act.
We are required to comply with the United States Foreign Corrupt Practices Act, which prohibits U.S. companies from engaging in bribery or other prohibited payments to foreign officials for the purpose of obtaining or retaining business. Foreign companies, including some of our competitors, are not subject to these prohibitions. Corruption, extortion, bribery, pay-offs, theft and other fraudulent practices occur from time-to-time in mainland China. If our competitors engage in these practices, they may receive preferential treatment from personnel of some companies, giving our competitors an advantage in securing business or from government officials who might give them priority in obtaining new licenses, which would put us at a disadvantage. Although we inform our personnel that such practices are illegal, we have not established formal policies or procedures for prohibiting or monitoring this conduct, and we can not assure you that our employees or other agents will not engage in such conduct for which we might be held responsible. If our employees or other agents are found to have engaged in such practices, we could suffer severe penalties.
| 16 |
Due to various restrictions under PRC laws on the distribution of dividends by our PRC operating companies, we may not be able to pay dividends to our stockholders.
The Wholly-Foreign Owned Enterprise Law (1986), as amended and the Wholly-Foreign Owned Enterprise Law Implementing Rules (1990), as amended and the Company Law of the PRC (2006) contain the principal regulations governing dividend distributions by wholly foreign owned enterprises. Under these regulations, wholly foreign owned enterprises, such as WFOE, may pay dividends only out of their accumulated profits, if any, determined in accordance with PRC accounting standards and regulations. Additionally, WFOE is required to set aside a certain amount of their accumulated profits each year, if any, to fund certain reserve funds. These reserves are not distributable as cash dividends except in the event of liquidation and cannot be used for working capital purposes.
Furthermore, if our consolidated subsidiaries in China incur debt on their own in the future, the instruments governing the debt may restrict its ability to pay dividends or make other payments. If we or our consolidated subsidiaries are unable to receive all of the revenues from our operations due to these contractual or dividend arrangements, we may be unable to pay dividends on our common stock.
We may have difficulty establishing adequate management, governance, legal and financial controls in the PRC.
The PRC historically has been deficient in western style management, governance and financial reporting concepts and practices, as well as in modern banking, and other control systems. Our current management has little experience with western style management, governance and financial reporting concepts and practices, and we may have difficulty in hiring and retaining a sufficient number of qualified employees to work in the PRC. As a result of these factors, we may experience difficulty in establishing management, governance legal and financial controls, collecting financial data and preparing financial statements, books of account and corporate records and instituting business practices that meet western standards. We may have difficulty establishing adequate management, governance, legal and financial controls in the PRC. Therefore, we may, in turn, experience difficulties in implementing and maintaining adequate internal controls as required under Section 404 of the Sarbanes-Oxley Act of 2002 and other applicable laws, rules and regulations. This may result in significant deficiencies or material weaknesses in our internal controls which could impact the reliability of our financial statements and prevent us from complying with SEC rules and regulations and the requirements of the Sarbanes-Oxley Act of 2002. Any such deficiencies, weaknesses or lack of compliance could have a materially adverse effect on our business and the public announcement of such deficiencies could adversely impact our stock price.
It may be difficult to protect and enforce our intellectual property rights under PRC laws.
Intellectual property rights in China are still developing, and there are uncertainties involved in the protection and the enforcement of such rights. We will need to pay special attention to protecting our intellectual property and trade secrets. Failure to do so could lead to the loss of a competitive advantage that could not be compensated by our damages award.
Our failure to fully comply with PRC labor laws, including laws relating to social insurance, may expose us to potential liability and increased costs.
Companies operating in China must comply with a variety of labor laws, including certain pension, health insurance, unemployment insurance and other welfare-oriented payment obligations. Our failure to comply with these laws, including those relating to the payment of social insurance, could have a material adverse effect on our business in the form of payment obligations as well as paying administrative fines.
In addition, the new PRC Labor Contract Law took effect January 1, 2008 and governs standard terms and conditions for employment, including termination and lay-off rights, contract requirements, compensation levels and consultation with labor unions, among other topics. In addition, the law limits non-competition agreements with senior management and other employees who have access to confidential information to two years and imposes restrictions or geographical limits. This new labor contract law could impact our labor costs, which could adversely impact our results of operations.
Any recurrence of severe acute respiratory syndrome, or SARS, or another widespread public health problem, could adversely affect our operations.
A renewed outbreak of SARS or another widespread public health problem in the PRC, where all of our revenue is derived, could have an adverse effect on our operations. Our operations may be impacted by a number of health-related factors, including quarantines or closures of some of our offices that could leave us without many employees to conduct our business which would materially and adversely affect our operations and financial condition.
| 17 |
If we become directly subject to the recent scrutiny, criticism and negative publicity involving U.S.-listed Chinese companies, we may have to expend significant resources to investigate and resolve the matter which could harm our business operations, stock price and reputation and could result in a loss of your investment in our stock, especially if such matter cannot be addressed and resolved favorably.
Recently, U.S. public companies that have substantially all of their operations in China, particularly companies like us which have completed so-called reverse merger transactions, have been the subject of intense scrutiny, criticism and negative publicity by investors, financial commentators and regulatory agencies, such as the SEC. Much of the scrutiny, criticism and negative publicity has centered around financial and accounting irregularities, a lack of effective internal controls over financial accounting, inadequate corporate governance policies or a lack of adherence thereto and, in many cases, allegations of fraud. As a result of the scrutiny, criticism and negative publicity, the publicly traded stock of many U.S. listed Chinese companies has sharply decreased in value and, in some cases, has become virtually worthless. Many of these companies are now subject to shareholder lawsuits and SEC enforcement actions and are conducting internal and external investigations into the allegations. It is not clear what effect this sector-wide scrutiny, criticism and negative publicity will have on our company, our business and our stock price. If we become the subject of any unfavorable allegations, whether such allegations are proven to be true or untrue, we will have to expend significant resources to investigate such allegations and/or defend our company. This situation may distract our management from growing our company. If such allegations are not proven to be groundless, our company and business operations will be severely hampered and your investment in our stock could be rendered worthless.
Risks Related to Our Common Stock
There is currently an extremely limited trading market for our common stock, and the limited public trading market that may develop in the future may cause extreme volatility in our stock price.
Although our common stock is listed for quotation on the OTCBB and the OTCQB, there has been extremely limited trading in our stock. Trading in our common stock may not fully evolve for a variety of reasons, and even if a market for our stock develops, it may continue to be limited. Even if a market for our common stock does develop, there is a risk that a meaningful, consistent and liquid trading market may not develop. Moreover, stocks with limited trading markets have historically experienced extreme price and volume fluctuations and have particularly affected the market prices of many smaller companies like us. Our common stock is thus expected to be subject to significant volatility when and if meaningful trading commences. In addition, sales of substantial amounts of our common stock, or the perception that such sales might occur, could adversely affect prevailing market prices of our common stock.
An active and visible trading market for our common stock may not develop.
We cannot predict whether an active market for our common stock will develop in the future. In the absence of an active trading market:
| · | Investors may have difficulty buying and selling or obtaining market quotations; |
| · | Market visibility for our common stock may be limited; and |
| · | A lack of visibility for our common stock may have a depressive effect on the market price for our common stock. |
The OTCBB and the OTCQB are unorganized, inter-dealer, over-the-counter markets that provides significantly less liquidity than NASDAQ or the NYSE AMEX. No assurances can be given that we will ever obtain a listing for our securities on a senior exchange. The trading price of our common stock is therefore expected to be subject to significant fluctuations in response to variations in quarterly operating results, changes in analysts’ earnings estimates, announcements of innovations by us or our competitors, general conditions in the industry in which we operate and other factors. These fluctuations, as well as general economic and market conditions, may have a material or adverse effect on the market price of our common stock.
The market price for our stock may be volatile.
The market price for our stock may be volatile and subject to wide fluctuations due to factors such as:
| · | the perception of US investors and regulators of US-listed Chinese companies; |
| · | actual or anticipated fluctuations in our quarterly operating results; |
| · | changes in financial estimates by securities research analysts; |
| 18 |
| · | conditions in pharmaceutical markets; |
| · | changes in the economic performance or market valuations of other pharmaceutical companies; |
| · | announcements by us or our competitors of new products, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| · | addition or departure of key personnel; |
| · | fluctuations of exchange rates between RMB and the U.S. dollar; |
| · | intellectual property or other litigation; |
| · | general economic or political conditions in China; |
| · | period-to-period fluctuations in our financial results; |
| · | common stock sales in the public market by one or more of our larger stockholders, officers or directors; |
| · | a negative outcome in any litigation or potential legal proceedings; or |
| · | other potentially negative financial announcements including (i) reviews of any of our public filings by the SEC, (ii) changes in accounting treatment or restatement of previously reported financial results or (iii) delays in our filings with the SEC. |
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our stock.
In the past, following periods of volatility in the market price of a company’s securities, stockholders have often instituted class action securities litigation against those companies. Such litigation, if instituted, could result in substantial costs and diversion of management attention and resources, which could significantly harm our profitability and reputation.
In addition, the securities market has from time to time experienced significant price and volume fluctuations that are not related to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price of our stock.
We are likely to remain subject to “penny stock” regulation and as a consequence there are additional sales practice requirements and additional warning issued by the SEC.
As long as the trading price of our common stock is below $5.00 per share, the open-market trading of our common stock will be subject to the “penny stock” rules of the SEC. The “penny stock” rules impose additional sales practice requirements on broker-dealers who sell securities to persons other than established customers and accredited investors (generally those with assets in excess of $1,000,000 or annual income exceeding $200,000 or $300,000 together with their spouse). For transactions covered by these rules, the broker-dealer must make a special suitability determination for the purchase of securities and have received the purchaser’s written consent to the transaction before the purchase. Additionally, for any transaction involving a penny stock, unless exempt, the broker-dealer must deliver, before the transaction, a disclosure schedule prescribed by the SEC relating to the penny stock market. The broker-dealer also must disclose the commissions payable to both the broker-dealer and the registered representative and current quotations for the securities. Finally, monthly statements must be sent disclosing recent price information on the limited market in penny stocks. These additional burdens imposed on broker-dealers may restrict the ability of broker-dealers to sell the common stock and may affect a stockholder’s ability to resell the common stock.
| 19 |
There can be no assurance that our common stock will qualify for exemption from the “penny stock” rules. In any event, even if our common stock is exempt from such rules, we would remain subject to Section 15(b)(6) of the Exchange Act, which gives the SEC the authority to restrict any person from participating in a distribution of a “penny stock” if the SEC finds that such a restriction would be in the public interest.
Stockholders should be aware that, according to SEC Release No. 34-29093, the market for penny stocks has suffered in recent years from patterns of fraud and abuse. Such patterns include (i) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (ii) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (iii) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (iv) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (v) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market.
We do not foresee paying cash dividends in the foreseeable future.
We currently intend to retain any future earnings for funding growth. We do not anticipate paying any dividends in the foreseeable future. As a result, you should not rely on an investment in our securities if you require dividend income. Capital appreciation, if any, of our shares may be your sole source of gain for the foreseeable future. Moreover, you may not be able to resell your shares in our company at or above the price you paid for them.
Our Articles of Incorporation authorize the issuance of shares of preferred stock which, if issued, the rights, preference, designations and limitations of such preferred stock could operate to the disadvantage of the shares of our outstanding common stock.
Our articles of incorporation authorize the issuance of shares of preferred stock, the rights, preferences, designations and limitations of which may be set by the board of directors. While no preferred stock is currently outstanding or subject to be issued, the articles of incorporation have authorized issuance of up to 1,000,000 shares of preferred stock (“Preferred Stock”) in the discretion of the board of directors. Such Preferred Stock may be issued upon filing of amended Articles of Incorporation and the payment of required fees; no further shareholder action is required. If issued, the rights, preferences, designations and limitations of such Preferred Stock would be set by our board of directors and could operate to the disadvantage of the shares of our outstanding Common Stock. Such terms could include, among others, preferences as to dividends and distributions on liquidation.
Item 1B Unresolved Staff Comments
None
Item 2. Description of Properties
The executive offices and manufacturing facilities of our wholly-owned operating subsidiary, Tianmu Pharmaceuticals, are located in the Limin Pharmaceutical Technology Park in the City of Harbin, which is the capital of Heilongjiang Province.
Under the PRC law, land is owned by the state or rural collective economic organizations. The state issues to tenants the rights to use land. Tianmu Pharmaceuticals has been granted by the government of China, a 50-year land use right certificate, which will expire in April 2046, to use 50,000 square meters of land for manufacturing purposes. Currently our offices and manufacturing facility occupy 15,000 square meters, and our warehouse occupies 1,800 square meters. There is a risk that the Chinese government could revoke our land use rights, which would have a very material adverse effect on our company and our results of operation.
On December 20, 2010, we entered into a contract with third party to acquire the right to use a parcel of land for twenty years (from December 20, 2010 to December 30, 2030) with a total contract price of RMB 20 million (approximately to $3 million). Through our Lynong subsidiary we started to use this parcel of land to plant the Chinese herbs in 2011. We paid the contract price in two installments. The first installment of RMB 12 million (equivalent to $1,817,541) was paid in December 2010 and the second installment payment of RMB 8 million (equivalent to $1,211,694) was paid to the third party upon closing of the transaction on February 21, 2011.
In addition, in March 2011, we entered into a supplemental contract with the same third-party to acquire the right to use a nearby fish pond for twenty years with total contract price of RMB 5.5 million (approximate to $0.85 million). RMB5,270,665 (approximately $825,000) was paid as of March 31, 2012. We use this fish pond to provide water supply to the traditional Chinese medicine field.
| 20 |
On October 26, 2010, we entered into an Office Service Agreement for office space located at room 317A, No 8 Aijian Road, Harbin city, Heilongjiang Province, China. The term of the lease is from November 5, 2010 to November 4, 2011. Annual rent under the lease was RMB 32,000(Approximate $4,700) and was paid upon execution of the Office Service Agreement.
Item 3. Legal Proceedings
We are not a party to any current or pending legal proceedings that, if decided adversely to us, would have a material adverse effect upon our business, results of operations, or financial condition, and we are not aware of any threatened or contemplated proceeding by any governmental authority against us. To our knowledge, we are not a party to any threatened civil or criminal action or investigation. However, from time to time, we may become involved in various lawsuits and legal proceedings that arise in the ordinary course of business. Litigation is subject to inherent uncertainties, and an adverse result in these or other matters may arise from time to time that may harm our business.
Item 4. MINE SAFETY DISCLOSURE
Not applicable
PART II
Item 5. Market For Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities Market Information
Our Common Stock is traded on the OTC Bulletin Board under the symbols “TGLPE.OB”. The following tables set forth, for the calendar quarter indicated, the quarterly high and low sales price for our common stock as reported on the OTC Bulletin Board. Trading in the common stock in the over-the-counter market has been limited and sporadic and the quotations set forth below are not necessarily indicative of actual market conditions. Further, the quotations merely reflect the prices at which transactions were proposed, and do not necessarily represent actual transactions.
| High | Low | |||||||
| 2012 by Quarter | ||||||||
| April 1, 2011 - June 30 , 2011 | $ | 0.70 | $ | 0.30 | ||||
| July 1, 2011 - September 30, 2011 | $ | 0.60 | $ | 0.30 | ||||
| October 1, 2011 - December 31, 2011 | $ | 0.05 | $ | 0.30 | ||||
| January 1, 2012 - March 31 , 2012 | $ | 0.90 | $ | 0.02 | ||||
| 2011 by Quarter | ||||||||
| April 1, 2010 - June 30 , 2010 | $ | 1.18 | $ | 0.05 | ||||
| July 1, 2010 - September 30, 2010 | $ | 0.55 | $ | 0.35 | ||||
| October 1, 2010 - December 31, 2010 | $ | 1.00 | $ | 0.40 | ||||
| January 1, 2011 - March 31 , 2011 | $ | 0.89 | $ | 0.39 | ||||
On June 20, 2012, the closing price for shares of our common stock, as reported by the Over-the-Counter Bulletin Board, was $0.15.
Record Holders.
As of March 31, 2012, there were 468 registered holders of our common stock and 13,294,778 shares of common stock issued and outstanding.
Dividends
We have not paid dividends on our common stock in the past and do not anticipate doing so in the foreseeable future. We currently intend to retain future earnings, if any, to fund the development and growth of our business. The decision whether to pay cash dividends on our common stock will be made by our board of directors, at their discretion, and will depend on our financial condition, operating results, capital requirements and other factors that the board of directors considers significant.
| 21 |
Recent Sales of Unregistered Securities
On April 14, 2011, we issued 100,000 shares of common stock to Stephen Sax, an independent director of our company, as compensation for his services. On April 18, 2011, we issued 28,571 shares of common stock to a law firm as consideration for services. The issuance of shares to these holders was exempt from registration pursuant to Section 4(2) of the Securities Act of 1933, as amended.
Securities Authorized for Issuance Under Equity Compensation Plans
The following table indicates shares of common stock authorized for issuance under our 2009 Incentive Plan as of March 31, 2012:
Plan category | Number of securities to
be issued upon exercise of outstanding options, warrants and rights (a) |
Number of securities remaining available for future issuance | ||||||
| Equity compensation plans approved by security holders | — | 580,907 | ||||||
| Equity compensation plans not approved by security holders | — | — | ||||||
| Total | — | 580,907 | ||||||
Repurchases of Equity Securities.
None.
Item 6. Selected Financial Data
We are a “smaller reporting company” as defined by Regulation S-K and as such, are not required to provide the information contained in this item pursuant to Regulation S-K.
Item 7. Management’s Discussion and Analysis of Financial Conditions and Results of Operations.
The following discussion and analysis of financial condition and results of operations relates to the operations and financial condition reported in the financial statements of Tongli Pharmaceuticals (USA) Inc. for the years ended March 31 2012 and 2011, and should be read in conjunction with such financial statements and related notes included in this report. Those statements in the following discussion that are not historical in nature should be considered to be forward looking statements that are inherently uncertain. Actual results and the timing of the events may differ materially from those contained in these forward looking statements due to a number of factors, including those discussed in the “Cautionary Note on Forward Looking Statements” set forth above.
Company Overview
Tongli Pharmaceuticals (USA), Inc., a Delaware corporstion (the “Company”, “we,” “us” or “our”), owns all of the outstanding capital stock of American Tony Pharmaceutical, Inc., a Delaware corporation (“American Tony”).
American Tony is a holding company, incorporated in Delaware. In February 2007, American Tony acquired, through its wholly owned subsidiary, Heilongjiang Tongli Technology Co., Ltd., a PRC company (“Tongli Technology”), all of the registered capital of Harbin Tianmu Pharmaceuticals Co., Ltd. (“Tianmu Pharmaceuticals”), a corporation organized under the laws of the PRC on November 26, 1999. In August 2011, we formed Harbin Lvnong Plant Ltd. (“Lvnong”) to grow herbs in China. Lvnong is a PRC company and is 100% owned by Tongli Technology. Tianmu Pharmaceuticals and Lvnong are our principal operating subsidiaries. Tianmu Pharmaceutical is engaged in developing, manufacturing and marketing pharmaceutical and health care products that incorporate elements of Chinese Traditional Medicine with elements of western medicine. Our research and development activities have been carried out at relatively low cost because they have been carried out by our in house research and development team and, in the past, in concert with a number of research institutes and universities, including the Jilin Research Institute of Chinese Traditional Medicine, the Sichuan Research Institute of Chinese Medicine, the Heilongjiang Institute of Chinese Traditional Medicine, the Chemistry Department of Tsinghua University, and the R&D Center of Harbin Medical University.
The Company’s main products include Panax and Radix Polygoni Capsule, cholesterol reduction pill, mouthwash, anti-inflammatory tablet and calcium supplement. These products are sold through distributors or directly to customers; no service is required of the Company after sales are made. The Company’s primary customers are drug stores and hospitals located in China.
Development and Strategy
For the year ended March 31, 2012, we continued the execution of our product channel expansion strategy that resulted in increased market penetration of our products and expanded revenue growth.
Panax and Radix Polygoni Capsule
In October, 2010, we began to manufacture and sell our new product Panax and Radix Polygoni Capsule. This new product has helped us to generate about 44% and 27% of our total sales for the year ended March 31, 2012 and 2011, respectively. We believe that this new product have great market potential and will continue to generate sustainable income for our company in the foreseeable future.
| 22 |
Xinyu Breath Spray
On April 30, 2012, we entered into Xinyu Breath Spray Purchase Agreement (“Xinyu Purchase Agreement”) with Harbin Junde Healthcare Product Company (“Harbin Junde”). Pursuant to the Xinyu Purchase Agreement, we will acquire the right to manufacture the Xinyu Breath Spray at the total purchase price of RMB 8,500,000 (approximately $1.33 million). We will pay 50% of the purchase price at time of closing of the Xinyu Purchase Agreement and pay the rest of the purchase price after we manufacture three batches of Xinyu Breath Spray that meet the quality standards of product. We paid RMB 1,370,000 (approximately $214,508) on March 29, 2012 and RMB 3,630,000 (approximately $568,369) on May 30, 201, which includes the first installment payment of RMB 4,250,000 and RMB 750,000 for other payment orally agreed by the parties. On July 2, 2012, the Company entered into a supplementary agreement to record the oral agreement the parties reached in May with regard to our payment obligation in respect to inspection fees, improvement fees and raw material fees.
Xinyu Breath Spray was marketed primarily through adware. The major markets include supermarkets, hotels and internet. We have two medium-size packages with two flavors for the product, one for man and another for woman. We are targeting to high-end consumers.
We anticipate we will start production of Xinyu Breath Spray in July 2012.
New Land Parcel
On December 20, 2010, we entered into a contract with third party to acquire the right to use a parcel of land for twenty years (from December 20, 2010 to December 30, 2030) with a total contract price of RMB 20 million (approximate to $3 million). We started to use this parcel of land to plant the traditional Chinese medicine in 2011. We paid RMB 12 million (equivalent to $1,817,541) in December 2010 and RMB 8 million (equivalent to $1,211,694) in February 21, 2011.
In addition, in March 2011, we entered into a supplemental contract with the same third-party to acquire the right to use a nearby fish pond for twenty years with total contract price of RMB 5.5 million (approximate to $0.85 million). RMB5,270,665 (approximately $825,000) was paid as of March 31, 2012. We used this fish pond to provide water supply to the traditional Chinese medicine field.
Marketing and sales
Management plans to continue to emphasize on expanding and enhancing marketing and sales in the fiscal year 2013 and beyond. Part of this strategy involves increasing and improving marketing and sales activities to enhance the market position of our key products and to increase the sales of other products by expanding our sales force, solidifying our distribution network and expanding market segment coverage, and increasing marketing and promotional activities. Management also plans to selectively pursue strategic acquisition opportunities to further consolidate our resources and expand our market coverage. We believe that such initiatives will provide effective means to broaden our product lines, expand our market coverage and complement our research and development capabilities. As of the date of this report, we are not engaged in any material discussions regarding any potential acquisition.
Management believes that our emphasis on further commercializing and broadening our product lines, enhanced sales and marketing efforts shall continue to yield increases in revenue in fiscal 2013 and beyond.
Discussion of Operating Results
Operating results for the years ended March 31, 2012 and 2011
Revenues, cost of sales and gross profit
Revenue in U.S. dollar (“USD”) increased 6% for the year ended March 31, 2012 compared to revenue in the year ended March 31, 2011, but revenue in Chinese Renminbi (“RMB”) decreased 2% compared to revenue in RMB for the year ended March 31, 2011. The manufacturing process was interrupted by the breakdown of a boiler and related pipeline in January, 2012 and didn’t get fully resumed until July 2012.
Our reporting currency is USD, but our functional currency is RMB. The average translation rates applied to the income statements accounts fluctuated from USD$1: RMB 6.3122 for the year ended March 31, 2012 to USD$1: RMB 6.8289 for the year ended March 31, 2011, representing a 8% decrease. Our sales revenue increased by approximately $1.2 million as a result of the foreign currency translation rate fluctuation.
| 23 |
The Company had distribution contracts with various distributors during the years ended March 31, 2012 and 2011 to sell its products in China. The Company manufactures its products and ships the products to distributors based on the sales order from distributors each month. Revenue is recognized at the time of the shipment. No return is allowed, unless there is a quality problem. During the years ended March 31, 2012 and 2011, there were no returns from distributors or customers. Distributors normally make payments upon receiving the products; however, in some cases, the Company may extend credit to certain creditable distributors for certain period until the next shipment.
Cost of sales was approximately $9.7 million for the year ended March 31, 2012 compared to $7.7 million for the year ended March 31, 2011. Approximately $0.9 million increase in cost of sales was related to expenses increased due to disposals of materials and approximately $1.0 million increase was related to RMB appreciation against USD. Cost of sales as a percentage of revenue increased to 62.6% from 52.8% as compared to the prior comparative period.
Gross profit was approximately $5.8 million for the year ended March 31, 2012 compared to $6.9 million for the year ended March 31, 2011, a decrease of $1,1 million or 16.4% primarily due to decreased sales of our products and increased expenses due to disposals of materials. The gross profit as a percent of revenues for the year ended March 31, 2012 decreased to 37.4% compared to 47.2% in the prior comparative period. The decrease was mainly related to the decrease in sales and the increase in cost of sales for the year ended March 31, 2012 described above.
Operating expenses
Total operating expenses increased by approximately $1.45 million from $1.04 million for the year ended March 31 2011 to $2.49 million for the year ended March 31, 2012, representing a 139% increase. The increase in our total operating expense for the year ended March 31, 2012 as compared to the prior comparative period was primarily related to increase in stock based compensation expenses, our marketing and advertisement expenses, additional amortization expenses of land use right and repairing for broken boiler in February 2012. The increase in stock-based compensation expenses accounted for about 50% of the increase in our total operating expense. During the year ended March 31, 2012, the Company issued 1,605,571 shares of Company Common Stock to several officers, directors, an independent director, employees and consultants for their services and recorded $771,533 of stock-based compensation expenses in general and administration expenses. During the year ended March 31, 2011, we reported an impairment loss of $159,732 associated with the written-off Yan Li Xiao when the agreement with third party was terminated.
Interest expense, net
Interest expense, net was $24,133 for the year ended March 31 2012 compared to interest expense of $58,225 for the year ended March 31, 2011. Interest expense for the period included approximately $27,000 interest accrued on the loans from related parties and shareholders, net of interest income of approximately $2,000. Decrease in interest expense was mainly attributed to the decrease of net amount of outstanding of related party advances.
Income taxes
All of our net income for the year ended March 31, 2012 and 2011 were from Tianmu Pharmaceuticals, which conducts substantial part of our operation in the PRC. Our Chinese subsidiaries are governed by the Income Tax Law of the PRC concerning the private-run enterprises, which are generally subject to tax at a statutory rate of 25% on income reported in the statutory financial statements after appropriate tax adjustments.
Income tax expense of $1.12 million for the year ended March 31, 2012, a decrease of $0.44 million or 28% from $1.56 million for the year ended March 31 2011, was due to the decrease in income before income taxes.
Net income
As a result of the above factors, net income was $2.15 million for the year ended March 31, 2012, a decrease of $2.11 million, or 49.5%, as compared to the net income of $4.26 million for the year ended March 31, 2011.
| 24 |
Other comprehensive income
We operate primarily in the PRC and the functional currency of our operating subsidiary is RMB. RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is intended to imply that the RMB amounts could have been, or could be, converted, realized or settled into USD at the rate on March 31 2012 or at any other rate.
The value of RMB against USD may fluctuate and is affected by changes in political and economic conditions. Our revenues, costs and financial assets are mostly dominated in RMB while our reporting currency is the U.S. dollar. Accordingly, this may result in gains or losses from currency translation on our financial statements.
Translation adjustments resulting from this process amounted to a gain of $371,822 and $562,020 as of March 31, 2012 and 2011, respectively. The balance sheet amounts with the exception of equity at March 31, 2012 were translated at 6.3867 RMB to 1.00 USD as compared to 6.5468 RMB to 1.00 USD at March 31, 2011. The equity accounts were stated at their historical rate. The average translation rates applied to the income statements accounts for the years ended March 31, 2012 and 2011 were 6.3122 RMB and 6.7077 RMB, respectively.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis. We have financed our operation and capital expenditures through cash from operations as well as loans from shareholders and an entity owned by our Chairman. As of the date of this report, we do not have any outstanding bank loans..
As of March 31, 2012 and 2011, due to (from) related parties consists the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Due to Harbin Tianmu Real Estate Development Co. Ltd | $ | (599,151 | ) | $ | 344,899 | |||
| Due to Yao, Mingli, Chairman of the Company | 570,324 | 541,304 | ||||||
| Due to US Hua Sky International Investment LLC | 27,727 | 27,727 | ||||||
| Due to Tianmu Investment Co. Ltd | (7,829 | ) | - | |||||
| Due to Yao, Yuan, a shareholder of the Company | (78,609 | ) | 158,141 | |||||
| Total | $ | (87,538 | ) | $ | 1,072,071 | |||
As of March 31, 2012, the balance of due from Harbin Tianmu Real Estate Development Co. Ltd. was 599,151. Harbin Tianmu Real Estate Development Co., Ltd. is an entity owned by our Chairman, Mr. Mingli Yao. Due to US Hua Sky International Investment LLC was $27,727. US Hua Sky International Investment LLC is an entity owned by our Chairman, Mr. Mingli Yao. Due from Tianmu Investment Co. Ltd was $7,829. Tianmu Investment Co., Ltd. is an entity partially owned by our Chairman, Mr. Mingli Yao.
Mr. Mingli Yao has personally guaranteed the loan receivable from Harbin Tianmu Real Estate Development Co. Ltd and Tianmu Investment Co. Ltd. If the loan could not been repaid during the year ended March 31, 2013, Mr. Yao has agreed to offset the balance due to him referenced above.
We plan to fund operations and capital expenditures with cash from operations, as well as loans from major shareholders and management members and their affiliates. We might also pursue sources additional financing in the form of debt, equity or convertible security offerings. There can be no assurance that we will be able to obtain such additional financing at acceptable terms to us, or at all.
Total current assets increased to approximately $9.1 million as of March 31, 2012 as compared to $3.98 million at March 31, 2011. The primary changes in our current assets during this period were from changes in accounts receivable, advance to suppliers and a reclassification of part of contract deposit to current. The increase of accounts receivable from approximately $2.0 million at March 31, 2011 to approximately $4.2 million at March 31, 2012 was due to increased credit sales. The increase in advance to suppliers from $0 at March 31, 2011 to approximately $1.0 million at March 31, 2012 was due to advances paid to suppliers to lock-in our raw material requirements when needed. The increase in contract deposits from approximately $1.1 million at March 31, 2011 to $3.2 million was due to payments to Harbin Junde for our Xinyu Breath Spray product.
Our current liabilities as of March 31, 2012 was approximately $1.31 million compared to $1.79 million as of March 31, 2011.The decrease in our current liabilities during this period were mainly from the decrease in accounts payable.
| 25 |
The growth of our company will require additional debt and/or equity financing. Currently we have budgeted $3.5 million for capital improvements. We intend to pursue additional debt financing which could be secured by our property and equipment and approach international equity markets for additional debt and/or equity financing. To date we have no commitment from any source for the funds we require.
Discussion of Cash Flow
Comparison of cash flows results for the year ended March 31, 2012 and 2011 is summarized as follows:
| 2012 | 2011 | |||||||
| Net cash provided by (used in) operating activities | $ | (285,025 | ) | $ | 5,781,232 | |||
| Net cash provided by (used in) investing activities | 895,805 | (5,441,527 | ) | |||||
| Net cash providedy by (used in) financing activities | (1,205,604 | ) | 210,479 | |||||
| Effect of exchange rate changes on cash | 25,661 | 12,421 | ||||||
| Net increase (decrease) in cash | (569,163 | ) | 562,605 | |||||
| Cash, beginning of year | 592,671 | 30,066 | ||||||
| Cash, end of year | $ | 23,508 | $ | 592,671 | ||||
Operating activities
Cash flows used in operating activities for the year ended March 31, 2012 amounted to $285,025, which consists of our net income of $2,151,772 adds back noncash adjustments of $1,049,166 (including depreciation and amortization of $568,319, accrued interest expense on related party loan of $26,862, stock issued for services of $805,500 and deferred tax asset of $351,515) and offset by net changes in operating assets and liabilities, primarily including increase of accounts receivable of $2,077,397 which represented the temporarily uncollected amount from our increased credit sales during the year.
Cash flows provided by operating activities for the year ended March 31, 2011 amounted to $5,781,232, which consist of our net income of $4,260,069, adds back noncash adjustments of $681,088 (including depreciation of property and equipment of $336,249, amortization of land use right of $49,431, amortization of intangible assets of $6,389, accrued interest expense on related party loan of $58,225, stock issued for services of $117,500, impairment of intangible asset of $159,732 and deferred tax asset of $46,438 and offset by net changes in operating assets and liabilities, primarily including increase of accounts receivable of $1,653,704 which represented the temporarily uncollected amount from our increased credit sales during the year, a decrease of advance to vendors of $1,491,546 because we collected back the same amount from these vendors when the contracts expired.
Investing activities
Net cash provided by investing activities for the year ended March 31 2012 amounted to $895,805 which consists of $1,113,716 of contract deposit refunded from the termination agreement with Lanhai and $217,040 of payment made to Harbin Junde for Xinyu Breath Spray as deposit. .
Net cash used in investing activities for the year ended March 31, 2011 amounted to $5,441,527. $3,801,615 was paid to acquire a land use right and a use right of fish pond for twenty years. In addition, we paid RMB 11 million (equivalent to $1.6 million) to Tonghua under our Tonghua Agreement. On June 6, 2012, the Tonghua Agreement was terminated.
| 26 |
Financing activities
Cash flows used in financing activities amounted to $1,205,604 for the year ended March 31, 2012, which consists of $1,213,404 of payments to related party loans and advances to related parties.
Cash flows provided by financing activities amounted to $210,479 for the year ended March 31, 2011, which consists of proceeds from related party loans to support our working capital needs.
Off-Balance Sheet Arrangements
As of the date of this report, we do not have any off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to investors. The term "off-balance sheet arrangement" generally means any transaction, agreement or other contractual arrangement to which an entity unconsolidated with us is a party, under which we have: (i) any obligation arising under a guarantee contract, derivative instrument or variable interest; or (ii) a retained or contingent interest in assets transferred to such entity or similar arrangement that serves as credit, liquidity or market risk support for such assets.
Inflation
Inflation has not had a material impact on our business and we do not expect inflation to have a material impact on our business in the near future.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
We are a “smaller reporting company” as defined by Regulation S-K and as such, are not required to provide the information contained in this item pursuant to Regulation S-K.
Item 8. Financial Statements and Supplementary Data
Our Consolidated Financial Statements and Notes thereto and the report of Paritz & Company, P.A., our independent registered public accounting firm, are set forth on pages F-1 through 16 of this Report.
Item 9. Changes In and Disagreements With Accountants On Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Disclosure Controls and Procedures.
Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of the design and operation of our disclosure controls and procedures, as defined in Rules 13a-15(e) and 15d-15(e) under the Securities Exchange Act of 1934, as amended, as of the end of the period covered by this Annual Report on Form 10- K (the “Evaluation Date”). The evaluation of our disclosure controls and procedures included a review of our processes and the effect on the information generated for use in this Annul Report on Form 10-K. In the course of this evaluation, we sought to identify any material weaknesses in our disclosure controls and procedures and to confirm that any necessary corrective action, including process improvements, was taken. The purpose of this evaluation is to determine if, as of the Evaluation Date, our disclosure controls and procedures were operating effectively such that the information, required to be disclosed in our SEC reports (i) was recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and (ii) was accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate to allow timely decisions regarding required disclosure. Based on that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures were not effective as of March 31, 2012 based on the significant deficiencies described below.
| 27 |
Management’s Report on Internal Control over Financial Reporting
Management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting (ICFR) as defined in Rule 13a-15(f) under the Securities Exchange Act of 1934. Our internal controls over financial reporting are designed by, or under the supervision of, our Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, and effected by our board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles.
Our internal control over financial reporting, and includes those policies and procedures that:
| 1. | Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets; |
| 2. | Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and directors; and |
| 3. | Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
Management has evaluated the effectiveness of our internal control over financial reporting as of March 31, 2012 based on the control criteria established in a report entitled Internal Control—Integrated Framework , issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our assessment and those criteria, our management has concluded that our internal control over financial reporting was not effective due to the following significant deficiencies (as defined in Public Company Accounting Oversight Board Standard No. 2) in the Company’s internal controls over financial reporting. This is due to the fact that the Company lacks sufficient personnel with the appropriate level of knowledge, experience and training in the application of US generally accepted accounting principles (“GAAP”), especially related to complicated accounting issues. This could cause the Company to be unable to fully identify and resolve certain accounting and disclosure issues that could lead to a failure to maintain effective controls over preparation, review and approval of certain significant account reconciliation from Chinese GAAP to US GAAP and necessary journal entries. The Company has relatively small number of professionals employed by the Company in bookkeeping and accounting functions, which prevents the Company from appropriately segregating duties within its internal control systems. The inadequate segregation of duties is a weakness because it could lead to the untimely identification and resolution of accounting and disclosure matters or could lead to a failure to perform timely and effective reviews.
Remediation of Significant Deficiencies
We attempted to carry out certain remedial measures in the fiscal year ended March 31, 2012 to address the identified weaknesses. However, we have not accomplished all the remedial measures listed in Item 9 in the Annual Report for fiscal year ended March 31, 2012 and we believe we should continue to implement the specific remediation initiatives described below:
| · | We are evaluating the roles of our existing accounting personnel in an effort to realign the reporting structure of our internal auditing staff in China that will test and monitor the implementation of our accounting and internal control procedures. |
| · | We will evaluate and revise the documentation of the Company’s internal control procedures and policies. |
| · | We will provide training to our staff in China to ensure the importance of internal controls and compliance with established policies and procedures are fully understood throughout the organization and will provide additional U.S. GAAP training to all employees involved with the performance of or compliance with those procedures and policies. |
| · | We will implement a formal financial reporting process that includes review by our Chief Executive Officer and the full Board of Directors of financial statements prior to filing with the SEC. |
| 28 |
| · | We will increase our accounting and financing personnel resources, by retaining more U.S. GAAP knowledgeable financial professionals. |
The remedial measures being undertaken may not be fully effectuated or may be insufficient to address the significant deficiencies we identified, and there can be no assurance that significant deficiencies or material weaknesses in our internal control over financial reporting will not be identified or occur in the future. If additional significant deficiencies (or if material weaknesses) in our internal controls are discovered or occur in the future, among other similar or related effects: (i) the Company may fail to meet future reporting obligations on a timely basis, (ii) the Company’s consolidated financial statements may contain material misstatements, (iii) the Company’s business and operating results may be harmed.
Based on this evaluation, our Chief Executive Officer and Chief Financial Officer concluded that, as of the Evaluation Date, our disclosure controls and procedures were not operating effectively.
Inherent Limitations Over Internal Controls
Because of its inherent limitations, internal control over financial reporting may not prevent or detect all errors or misstatements and all fraud. Therefore, even those systems determined to be effective can provide only reasonable, not absolute, assurance that the objectives of the policies and procedures are met. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
This annual report does not include an attestation report of the company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by the company’s registered public accounting firm pursuant to temporary rules of the SEC that permit the company to provide only management’s report in this annual report.
Changes in Internal Control over Financial Reporting
There were no changes in our internal controls over financial reporting identified in connection with the evaluation that occurred during the quarter ended March 31, 2012 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting. Our management has initiated the steps outlined above under “Remediation of Significant Deficiencies.”
Item 9B. Other Information
Not applicable.
| 29 |
PART III
Item 10. Directors, Executive Officers, Promoters and Control Persons; Compliance With Section 16(A) of the Exchange Act.
The following table sets forth the names and ages of our directors and executive as of the date of this report.
| Name | Age | Title | ||
| Mingli Yao | 62 | Chief Executive Officer, Chairman | ||
| Ailing Zhao | 55 | Corporate Secretary, Director | ||
| Yuan Yao | 32 | Director | ||
| Li Li | 45 | Chief Financial Officer | ||
| Zhijian Hao | 62 | Independent Director |
The term of office of each director other than Mr. Shao and Mr. Sax expires at our annual meeting of stockholders or until their successors are duly elected and qualified. Our officers serve at the discretion of our Board of Directors.
Mingli Yao. Mr. Yao has been the Chief Executive Officer and a director of our company since March 2008. Since 1999, Mr. Yao has been employed as Chairman of Tianmu Pharmaceuticals, our PRC operating subsidiary, and he is the founder of Tianmu Pharmaceuticals. From 1998 to 1999, Mr. Yao was employed as a manager for a construction company owned by the People’s Government of Heilongjiang Province. In 1983, Mr. Yao graduated with an Associate Degree in Electronic Engineering from the China Harbin College of Light Industry. Mr. Yao has over 20 years management experience in variety industries and has over 10 years experience in the medical and pharmaceuticals industry in China. Mr. Yao is the husband of Ailing Zhao and the father of Yuan Yao.
Yuan Yao. Ms. Yao has been a director of our company since March 2008. Since 2005, Ms. Yao has been appointed as General Manager in charge of business operations of Tianmu Pharmaceuticals. In 2005, Ms. Yao graduated with a Bachelor of Arts Degree with a concentration in Law from the China University of Political Science and Law. Ms. Yao is the daughter of Mingli Yao and Ailing Zhao. Ms. Yao has in-depth knowledge of our business accrued in the years of serving as a General Manager of Tianmu Pharmaceutical.
Ailing Zhao. Mrs. Zhao has been a director of our company since March 2008. Since 1999, Ms. Zhao has been employed as Deputy General Manager in charge of administration of Tianmu Pharmaceuticals. Ms. Zhao has over 30 years management and administration experience in variety industries including Pharmaceutical industry. In 1980 Ms. Zhao graduated with a Vocational Degree in Business from the Harbin Business Vocational School. Ms. Zhao is the wife of Mingli Yao and the mother of Yuan Yao.
Li Li. Mr. Li was appointed as Chief Financial Officer on March 16, 2009. Mr. Li joined Harbin Tianmu Pharmaceutical Inc., a wholly owned operating subsidiary of our company, as Chief Financial Officer in 2009. Prior to that, he had served as Chief Financial Officer of Sanpu Pharmaceutical Co., Ltd. from 2006 to 2008, and Chief Financial Officer of Harbin Qiulin Co., Ltd. from 1999 to 2005. Mr. Li earned an M.B.A. in Finance from Peking University.
Zhijian Hao. On January 21, 2010, Mr. Hao was appointed as an independent director. Mr. Hao had more than three decades of experience in P.R.C. tax regulation and tax planning. From 1979 to 1994, Mr. Yao was the Deputy Chief and the Section Chief of Taiping Local Taxation Bureau of Harbin city, Heilongjiang Province. Since 1994 to present, he has been the Deputy Director of Daoli Local Taxation Bureau and DaoWai Local Taxation Bureau of Harbin city, Heilongjiang Province. In 1985, Mr. Hao graduated with an Associate Degree in Finance and Taxation from Dongbei University of Finance and Economics.
Family Relationships
Mr. Mingli Yao and Ms. Ailing Zhao are husband and wife. Ms. Yuan Yao is their daughter. No other family relationships exist among our directors or executive officers.
Involvement in Certain Legal Proceedings
Our directors, executive officers and control persons have not been involved in any of the following events during the past ten years:
| 1. | any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer either at the time of the bankruptcy or within two years prior to that time; |
| 30 |
| 2. | any conviction in a criminal proceeding or being subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| 3. | being subject to any order, judgment, or decree, not subsequently reversed, suspended or vacated, of any court of competent jurisdiction, permanently or temporarily enjoining, barring, suspending or otherwise limiting his involvement in any type of business, securities or banking activities; or |
| 4. | being found by a court of competent jurisdiction (in a civil action), the SEC or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law, and the judgment has not been reversed, suspended, or vacated. |
Code of Conduct and Ethics
We have not adopted a code of ethics or a code of conduct that applies to its principal executive officer, principal financial officer, principal accounting officer, controller, or to persons performing similar functions.
Director Independence
Our board has determined that Mr. Zhijian Hao is an independent director within the meaning of applicable NASDAQ Stock Market and SEC rules.
Board Meetings and Committees; Annual Meeting Attendance
Our board of directors did not hold any formal meeting during the most recently completed fiscal year and all proceedings of the board of directors were conducted by resolutions consented to in writing by all the directors and filed with the minutes of the proceedings of the directors.
We do not have a formal policy regarding attendance by directors at shareholders’ annual meetings. However, if any board members do attend the annual meeting of security holders, their expenses will be reimbursed.
Committees of the Board of Directors
We currently do not have an audit committee, compensation committee, nominating committee, executive committee, or any other committees.
Functions of the Audit Committee
We do not presently have a separately-designated standing audit committee. The entire Board of Directors performs the functions of an audit committee, but no written charter governs the actions of the Board when performing the functions of what would generally be performed by an audit committee.
The primary functions of an audit committee carried out by the entire Board of Directors include:
| · | appointing, approving the compensation of, and assessing the independence of our independent auditors; |
| · | assisting in the oversight of the integrity of our financial statements, our company’s compliance with legal and regulatory requirements, its independent auditors’ qualifications, and independence and the performance of the independent auditors; |
| · | overseeing the work of our independent auditors, including through the receipt and consideration of certain reports from the independent auditors; |
| · | reviewing and discussing with management and the independent auditors our annual and quarterly financial statements and related disclosures; |
| 31 |
| · | coordinating the oversight of our internal control over financial reporting, disclosure controls and procedures and code of conduct and ethics; and |
| · | establishing procedures for the receipt and retention of accounting related complaints and concerns; meeting independently with our internal accounting staff, independent auditors and management. |
The Board of Directors has determined that no one on our Board qualified as an “audit committee financial expert” as defined in Item 401(e) of Regulation S-B. We believe that our directors are collectively capable of analyzing and evaluating our financial statements and understanding internal controls and procedures for financial reporting.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Securities Exchange Act of 1934, as amended, requires our officers, directors and persons who beneficially own more than 10% of a registered class of our equity securities (“ten percent stockholders”) to file reports of ownership and changes in ownership with the SEC. Officers, directors and ten percent stockholders are charged by the SEC regulations to furnish us with copies of all Section 16(a) forms they file.
Based solely upon a review of copies of Section 16(a) reports and representations received by us from reporting persons, and without conducting any independent investigation of our own, in fiscal year ended on March 31, 2012, our officers, directors and ten percent stockholders are in compliance with Section 16(a).
Stockholder Communications with the Board of Directors
Stockholders may communicate with our directors by writing to us as follows: 136-17 Maple Ave., Apt. 11F, Flushing, New York, NY Attn: Corporate Secretary. Stockholders who would like their submission directed to a particular member of the board may so specify and the communication will be forwarded as appropriate.
Item 11. Executive Compensation.
The following table sets forth all compensation received during the last two fiscal years by our Chief Executive Officer, Chief Financial Officer and each of the other most highly compensated executive officers whose total compensation exceeds $ 100,000 in such fiscal years. These officers are referred to as the Named Executive Officers in this Report.
All the executive officers were paid in RMB and the amounts reported in this table have been converted from Renminbi to U.S. dollars based on the March 31, 2012 conversion rate of RMB6.3122 to $1.00
| SUMMARY COMPENSATION TABLE | ||||||||||||||||||||||||||||||||
| Name and principal position | Fiscal year ($) | Salary ($) | Bonues ($) | Stock award ($) | Non-equity
Incentive plan compensation ($) | Change in
Pension Value and Nonqualified Deferred Compensation Earnings ($) | All Other
Compensation ($) | Total ($) | ||||||||||||||||||||||||
| Mingli Yao | 2012 | $ | 24,000 | - | 100,000 | - | - | - | $ | 124,000 | ||||||||||||||||||||||
| Chief Executive officer | 2011 | $ | 9,162 | - | - | - | - | - | $ | 9,162 | ||||||||||||||||||||||
| Ailing Zhao | 2012 | $ | 12,000 | - | 50,000 | - | - | - | $ | 62,000 | ||||||||||||||||||||||
| Corporate Secretary | 2011 | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| Li Li | 2012 | $ | 9,505 | - | - | - | - | - | $ | 9,505 | ||||||||||||||||||||||
| Chief Financial officer | 2011 | $ | 9,162 | - | - | - | - | - | $ | 9,162 | ||||||||||||||||||||||
| 32 |
We do not have any written employment agreements with our executive officers, aside from those entered into between our subsidiaries in China and all employees in China.
DIRECTOR COMPENSATION
| Name (a) | Fees Earned or Paid in Cash ($)(1) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Non-Qualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||||
| Mingli Yao | $ | 24,000 | 100,000 | - | - | - | - | 124,000 | ||||||||||||||||||||
| AilingZhao | $ | 12,000 | 50,000 | - | - | - | - | 62,000 | ||||||||||||||||||||
| Yuan Yao | $ | 5,703 | 75,000 | - | - | - | - | 80,703 | ||||||||||||||||||||
| Hui Shao (2) | $ | 8,000 | 40,000 | - | - | - | - | 48,000 | ||||||||||||||||||||
| Zhijian Hao | $ | 4,753 | 25,000 | - | - | - | - | 29,753 | ||||||||||||||||||||
| Stephen Sax(3) | $ | 30,000 | 65,000 | - | - | - | - | 95,000 | ||||||||||||||||||||
(1) All the directors other than Mr. Hui Shao and Mr. Stephen Sax were paid in RMB and the amounts reported in this table have been converted from Renminbi to U.S. dollars based on the March 31, 2012 conversion rate of RMB6.3122 to $1.00.
(2) On December 12, 2011, Mr. Hui Shao resigned from the Board. The compensation reported in this table reflects all compensation Mr. Shao received during the period of his service on the Board.
(3) On April 9, 2012, Mr. Stephen J. Sax, resigned from the Board. The compensation reported in this table reflects all compensation Mr. Sax received during the period of his service on the Board.
Mr. Mingli Yao, Ms. Ailing Zhao and Mr. Yuan Yao are paid in their capacity as executive officers of our company and they do not receive any additional compensation for their service as directors.
We have historically entered into written agreements with our non-employee directors.
Mr. Hui Shao was appointed as a director in August 2008 and was entitled to receive an annual base salary of $12,000 and an annual award of 40,000 shares of our restricted stock pursuant to certain director agreement. As of the date of this Annual Report, an aggregate of 120,000 shares for his services during the periods from August 2009 to August 2010 and from August 2010 to August 2011 have been issued as of the date of this Report.
Pursuant to a Director Agreement we entered into with Mr. Hao, we will provide for an annual base salary of RMB30,000 (approximately $4,753 based upon the March 31, 2012 annual average exchange rate of $1.00 = RMB6.3122) and an annual award of 50,000 shares of our restricted stock starting from January 1, 2011. The Director Agreement has a term of three years, subject to election by our stockholders. As of the date of this Annual Report, the 50,000 shares have been issued.
In April 2011, we entered into a Director Agreement with Mr. Steven Sax where we shall provide for an annual cash compensation of $30,000 and grant Mr. Sax 100,000 shares of the Company’s restricted stock on the date of appointment. As of the date of this Annual Report, the 100,000 shares have been issued.
All directors entitled to reimbursement for reasonable and substantiated expenses incurred by them in furtherance of their performance of duties to our company.
Outstanding Equity Awards Narrative Disclosure
The following sets forth certain information about the securities authorized for issuance under our 2009 Incentive Plan as of March 31, 2012.
As of March 31, 2012, 1,605,571 shares have been issued to employees and consultants of the Company..
| 33 |
2009 Incentive Plan
On August 13, 2009, our Board of Directors and the stockholders holding a majority of the Company’s outstanding voting stock approved the 2009 Incentive Plan (the “Plan”). The purpose of the Plan is to provide additional incentive to selected employees, officers, directors and consultants of our company or its affiliates, to attract and retain the best available personnel for positions of substantial responsibility, and to promote the long-term success of our business.
The Plan currently provides that a maximum of 2,000,000 shares of Common Stock may be issued under the Plan (subject to adjustment in the event of stock split or other changes in the Common Stock as provided in the Plan).
Administration
The Plan is administered by our Board of Directors. The Board has, by resolution, delegated authority to our executive officers to issue up to 200,000 shares or options under the Plan to any single recipient without prior Board of Directors approval (the “Issuance Cap”), with the proviso that: (i) any single or series of related issuances in excess of the Issuance Cap shall require the approval of the Board of Directors or a designated committee thereof and (ii) such officers are required to review with the Board of Directors or a designated committee thereof no less than quarterly all issuances made under the Plan.
Type of Awards
We may grant the following types of awards under the Plan:
| · | Incentive Stock Option |
| · | Non-Qualified Stock Option |
| · | Restricted Shares |
Option Terms
The options granted under the Plan will be evidenced by an award agreement. The Administrator shall specify the grant date, exercise price, terms, conditions, and restriction for the exercise of the options. No option shall in any event be exercisable after ten years from the date of grant, and no Incentive Stock Option granted to a ten percent shareholder shall become exercisable five years from the date of grant.
The exercise price shall be no less than the Fair Market Value per Share on the date of grant and the exercise price of an Incentive Stock Option granted to an employee who owns more than 10% of the our stock shall be no less than one hundred and ten percent of the fair market value per Share on the date of grant.
Eligibility
Employees, directors, officers and consultants in the service of our company or its affiliated are eligible to participate in the Plan. Determinations as to eligibility shall be made by the Administrator. Options issued in the form of Incentive Stock Options may only be granted to our employees.
Transferability
No unexercised or restricted award shall be assignable or transferable by a recipient other than by will or the laws of descent and distribution; provided, unless that the Administrator determines otherwise.
Restrictions on Resale
Certain of our officers and directors may be deemed to be “affiliates” of the Company as that term is defined under the Securities Act. The Common Stock acquired under the Plan by an affiliate may be reoffered or resold only pursuant to an effective registration statement or pursuant to Rule 144 under the Securities Act or other exemption from the registration requirements of the Securities Act.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Security Ownership of Certain Beneficial Owners and Management [please update the table below and footnotes]
The table sets forth below certain information regarding the beneficial ownership of our common stock as of July 6, 2012, based on 13,548,122 aggregate shares of common stock outstanding as of such date, by: (i) each person who is known by us to own beneficially more than 5% of our outstanding common stock with the address of each such person, (ii) each of our present directors and officers, and (iii) all officers and directors as a group.
| 34 |
Beneficial ownership is determined in accordance with SEC rules and generally includes voting or investment power with respect to securities. Unless otherwise indicated, the stockholders listed in the table have sole voting and investment power with respect to the shares indicated. Unless otherwise noted, the principal address of each of the stockholders, directors and officers listed below is c/o 1 Beijing Road, Limin Development Zone, Harbin, China.
| Name of Beneficial Owner | Amount and Nature of Beneficial Ownership | Percentage of Class | ||||||
| Mingli Yao Chief Executive Officer and Chairman | 3,421,667 | (1) | 25.2 | % | ||||
| Yuan Yao Director | 3,371,667 | (2) | 24.8 | % | ||||
| Ailing Zhao Secretary and Director | 3,321,667 | (3) | 24.5 | % | ||||
| Li Li Director | 0 | 0 | % | |||||
| Zhijian Hao Director | 0 | 0 | % | |||||
| All officers and directors as a group (7 persons) | 3,761,667 | 27.8 | % | |||||
(1) Includes 700,000 shares owned by Yuan Yao who is Mingli Yao’s daughter and 786,667 shares owned by Ailing Zhao who is Mingli Yao’s wife.
(2) Includes 1,735,000 shares owned by Mingli Yao who is Yuan Yao’s father and 786,667 shares owned by Ailing Zhao who is Yuan Yao’s mother.
(3) Includes 700,000 shares owned by Yuan Yao who is Ailing Zhao’s daughter and 1,735,000 shares owned by Mingli Yao who is Ailing Zhao’s husband.
Item 13. Certain Relationships and Related Party Transactions
As of March 31, 2012 and March 31, 2011, we had a balance of $87,538 and $1,072,071 respectively due to(from) related parties. This includes amounts payable to (receivable from) shareholders, members of our management and their affiliates.
| March 31, 2012 | March 31, 2011 | |||||||
| Due to Harbin Tianmu Real Estate Development Co. Ltd | $ | (599,151 | ) | $ | 344,899 | |||
| Due to Yao, Mingli, Chairman of the Company | 570,324 | 541,304 | ||||||
| Due to US Hua Sky International Investment LLC | 27,727 | 27,727 | ||||||
| Due to Tianmu Investment Co. Ltd | (7,829 | ) | - | |||||
| Due to Yao, Yuan, a shareholder of the Company | (78,609 | ) | 158,141 | |||||
| Total | $ | (87,538 | ) | $ | 1,072,071 | |||
As of March 31, 2012, the balance of due from harbin Tianmu Real Estate Development Co. Ltd was 599,151. Harbin Tianmu Real Estate Development Co., Ltd. is an entity owned by our Chairman, Mr. Mingli Yao. Due to US Hua Sky International Investment LLC was $27,727. US Hua Sky International Investment LLC is an entity owned by our Chairman, Mr. Mingli Yao. Due from Tianmu Investment Co. Ltd was $7,829. Tianmu Investment Co., Ltd. is an entity partially owned by our Chairman, Mr. Mingli Yao.
Mr. Mingli Yao has personally guaranteed the loan receivable from Harbin Tianmu Real Estate Development Co. Ltd and Tianmu Investment Co. Ltd. If the loan has not been repaid in the year ended March 31, 2013, Mr Yao has agreed to offset the balance due to him referenced above.
Review, Approval or Ratification of Related Party Transactions
Our Board of Director is responsible for reviewing all “Related Person Transactions” as defined by Item 404 of Regulation S-K of the rules promulgated by the SEC. Directors and executive officers are responsible for bringing a potential Related Person Transaction to the attention of our Board.
| 35 |
In reviewing a related person transaction, the Board will, after reviewing all material information regarding the transaction, take into account, among other factors it deems appropriate, whether the transaction is on terms no less favorable than terms generally available to an unaffiliated third party under the same or similar circumstances and the extent of the related person’s interest in the transaction.
Item 14. Principal Accountant Fees and Services.
The following table sets forth fees billed to us by our independent registered public accounting firms Paritz & Company, P.A. during the fiscal years ended March 31, 2012 and 2011 for: (i) services rendered for the audit of our annual financial statements and the review of our quarterly financial statements; (ii) services by our independent registered public accounting firms that are reasonably related to the performance of the audit or review of our financial statements and that are not reported as audit fees; (iii) services rendered in connection with tax compliance, tax advice and tax planning; and (iv) all other fees for services rendered.
| March 31, 2012 | March 31, 2011 | |||||||
| Audit Fees | $ | 50,000 | $ | 50,000 | ||||
| Audit Related Fees | $ | - | $ | 0 | ||||
| Tax Fees | $ | 1,350 | $ | 1,250 | ||||
| All Other Fees | $ | - | $ | 0 | ||||
| TOTAL | $ | 51,350 | $ | 51,250 | ||||
PART IV
Item 15. Exhibits, Financial Statement Schedules.
(a)(1)(2) Financial Statements and Financial Statement Schedule.
The financial statements listed in the Index to Financial Statements beginning on the next page are filed as part of this annual report.
(a)(3) Exhibits.
The exhibits required by this item are set forth on the Exhibit Index immediately following the signature page of this annual report.
| 36 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| March 31, 2012 | March 31,2011 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash | $ | 23,508 | $ | 592,671 | ||||
| Accounts receivable | 4,166,178 | 2,060,773 | ||||||
| Inventories | 13,613 | 194,052 | ||||||
| Advance to suppliers | 1,016,294 | - | ||||||
| Deferred tax assets | 399,081 | 47,566 | ||||||
| Contract deposit-current portion | 3,189,441 | 1,073,512 | ||||||
| Prepaid expenses and other current assets | 241,738 | 10,072 | ||||||
| Due from related parties | 87,538 | - | ||||||
| Total current assets | 9,137,391 | 3,978,646 | ||||||
| Long-term assets: | ||||||||
| Property and equipment, net | 6,193,526 | 6,383,717 | ||||||
| Land use right, net | 4,041,032 | 4,144,912 | ||||||
| Contract deposits-long term | 469,726 | 3,359,497 | ||||||
| Total long-term assets | 10,704,284 | 13,888,126 | ||||||
| Total assets | $ | 19,841,675 | $ | 17,866,772 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current liabilties: | ||||||||
| Accounts payable | $ | 429,117 | $ | 756,355 | ||||
| Taxes payables | 879,386 | 834,934 | ||||||
| Accrued expenses | 192,340 | 199,474 | ||||||
| Total current liabilities | 1,500,843 | 1,790,763 | ||||||
| Long-term liabilities: | ||||||||
| Due to related parties - long term | - | 1,072,071 | ||||||
| Stockholders' Equity | ||||||||
| Preferred stock, $0.001 par value,authorized 1,000,000 shares; none issued and outstanding | - | - | ||||||
| Common stock, $0.001 par value,authorized 200,000,000 shares issued and outstanding 13,294,778 and 11,663,207 shares, as of March 31, 2012 and 2011, respectively | 13,295 | 11,663 | ||||||
| Additional paid-in-capital | 8,842,699 | 8,031,031 | ||||||
| Retained earnings | 7,408,863 | 5,257,091 | ||||||
| Accumulated other comprehensive income | 2,075,975 | 1,704,153 | ||||||
| Total Stockholders' equity | 18,340,832 | 15,003,938 | ||||||
| Total liablities and Stockholders' equity | $ | 19,841,675 | $ | 17,866,772 | ||||
The accompanying notes are an intergral part to the consolidated financial statements
| F-1 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF INCOME AND OTHER COMPREHENSIVE INCOME
| For the Years Ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Revenue | $ | 15,487,083 | $ | 14,655,239 | ||||
| Cost of sales | 9,699,648 | 7,733,684 | ||||||
| Gross Profit | 5,787,435 | 6,921,555 | ||||||
| Operating expenses: | ||||||||
| General and administrative | 1,724,711 | 613,611 | ||||||
| Impairment | - | 159,732 | ||||||
| Depreciation and amortization | 362,553 | 208,985 | ||||||
| Selling expenses | 402,666 | 58,998 | ||||||
| Total operating expenses | 2,489,930 | 1,041,326 | ||||||
| Operating income | 3,297,505 | 5,880,229 | ||||||
| Other expenses: | ||||||||
| Interest expense, net | 24,133 | 58,225 | ||||||
| Total other expenses | 24,133 | 58,225 | ||||||
| Income before income taxes | 3,273,372 | 5,822,004 | ||||||
| Income Taxes | 1,121,600 | 1,561,935 | ||||||
| Net Income | 2,151,772 | 4,260,069 | ||||||
| Other Comprehensive income | ||||||||
| Foreign Currency Translation adjustment | 371,822 | 562,020 | ||||||
| Comprehensive income | $ | 2,523,594 | $ | 4,822,089 | ||||
| Basic and diluted income per share | $ | 0.17 | $ | 0.37 | ||||
| Basic and diluted weighted average shares outstanding | 12,565,139 | 11,541,526 | ||||||
The accompanying notes are an integral part to the consolidated financial statements
| F-2 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Cash flows from operating activities | ||||||||
| Net income | $ | 2,151,772 | $ | 4,260,069 | ||||
| Adjustments to reconcile net income to net cash provided by operating activities: | ||||||||
| Depreciation and amortization | 568,319 | 392,069 | ||||||
| Accrued interest- related party | 26,862 | 58,225 | ||||||
| Stock issued for services | 805,500 | 117,500 | ||||||
| Deferred Tax | (351,515 | ) | (46,438 | ) | ||||
| Impairment of intangible asset | - | 159,732 | ||||||
| Changes in operating assets and liabilities: | - | |||||||
| Accounts receivable | (2,077,397 | ) | (1,653,704 | ) | ||||
| Inventory | 187,546 | (78,134 | ) | |||||
| Advance to suppliers | (1,028,288 | ) | 1,491,546 | |||||
| Prepaid expenses and other current assets | (234,101 | ) | (6,102 | ) | ||||
| Accounts payable | (350,493 | ) | 635,009 | |||||
| Accrued expenses and tax payables | 16,770 | 451,460 | ||||||
| Net cash provided by (used in) operating activities | (285,025 | ) | 5,781,232 | |||||
| Cash flows from investing activities | ||||||||
| Acquisition of land use right | - | (3,801,615 | ) | |||||
| Acquistion of property and equipment | (871 | ) | - | |||||
| Refund(Payment) of contract deposit | 896,676 | (1,639,912 | ) | |||||
| Net cash provided by (used in) investing activities | 895,805 | (5,441,527 | ) | |||||
| Cash flows from financing activities | ||||||||
| Proceeds from sale of common stock | 7,800 | - | ||||||
| Proceeds from related party loans | - | 210,479 | ||||||
| Repayment of related party loans | (1,213,404 | ) | - | |||||
| Net cash provided by (used in) financing activities | (1,205,604 | ) | 210,479 | |||||
| Effect of exchange rate changes on cash | 25,661 | 12,421 | ||||||
| Net increase (decrease) in cash | (569,163 | ) | 562,605 | |||||
| Cash, beginning of the year | 592,671 | 30,066 | ||||||
| Cash , end of the year | $ | 23,508 | $ | 592,671 | ||||
| Supplemental cash flow information | ||||||||
| During the period, cash was paid for the following: | ||||||||
| Income taxes | $ | 2,050,471 | $ | 1,348,552 | ||||
The accompanying notes are an integral part to the cosolidated financial statements
| F-3 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENT OF CHANGES IN STOCKHOLDERS' EQUITY
| Accumulated | ||||||||||||||||||||||||||||||||
| Other | ||||||||||||||||||||||||||||||||
| Preferred Stock | Common Stock | Additional | Retained | Comrehensive | ||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Paid in capital | Earnings | Income | Total | |||||||||||||||||||||||||
| Balance - March 31, 2010 | - | $ | - | 11,395,025 | $ | 11,395 | $ | 7,913,799 | $ | 997,022 | $ | 1,142,133 | $ | 10,064,349 | ||||||||||||||||||
| Stock issued to employees under 2009 stock incentive plan | 250,000 | 250 | 97,250 | 97,500 | ||||||||||||||||||||||||||||
| Common stock isssued for service rendered | 18,182 | 18 | 19,982 | 20,000 | ||||||||||||||||||||||||||||
| Net Income | 4,260,069 | 4,260,069 | ||||||||||||||||||||||||||||||
| Foreign currency translation adjustment | 562,020 | 562,020 | ||||||||||||||||||||||||||||||
| Balance - March 31, 2011 | - | - | 11,663,207 | 11,663 | 8,031,031 | 5,257,091 | 1,704,153 | 15,003,938 | ||||||||||||||||||||||||
| Common stock issued for service | 1,605,571 | 1,606 | 803,894 | 805,500 | ||||||||||||||||||||||||||||
| Proceeds from the sale of common stock | 26,000 | 26 | 7,774 | 7,800 | ||||||||||||||||||||||||||||
| Net Income | 2,151,772 | 2,151,772 | ||||||||||||||||||||||||||||||
| Foreign currency translation adjustment | 371,822 | 371,822 | ||||||||||||||||||||||||||||||
| Balance - March 31, 2012 | - | $ | - | 13,294,778 | $ | 13,295 | $ | 8,842,699 | $ | 7,408,863 | $ | 2,075,975 | $ | 18,340,832 | ||||||||||||||||||
The accompanying notes are an integral part to the consolidated financial statements
| F-4 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
| 1 | ORGANIZATION |
Tongli Pharmaceuticals (USA), Inc. (the “Company”), through its indirect wholly-owned subsidiaries, Harbin Tianmu Pharmaceuticals Co., Ltd. (“Tianmu Pharmaceuticals”) and Harbin Lvnong Plant Ltd. (“Lvnong”), develops, produces and sells a wide variety of pharmaceuticals and healthcare products in the People’s Republic of China (“PRC” or “ China”) that are based on traditional Chinese medicine (“TCM”). The Company’s products include a cholesterol reduction pill, mouthwash, an anti-inflammatory tablet and a calcium supplement. These products are sold through distributors and directly to customers; no service is provided after sales are made. The Company’s primary customers are drug stores and hospitals located in China.
In August 2011, the Company formed Lvnong to grow Chinese herbs in China. The primary purpose of this business is to provide Chinese herbs to Tianmu Pharmaceuticals. Lvnong is 100% owned by the Company’s indirect wholly owned subsidiary, Heilongjiang Tongli Technology Co., Ltd .
| 2 | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Principles of consolidation
The consolidated financial statements include the accounts of: (i) the Company (ii) the Company’s wholly owned subsidiary American Tony Pharmaceuticals, Inc., a Delaware corporation (“American Tony”), (iii) American Tony’s wholly owned subsidiary, Heilongjiang Tongli Technology Co., Ltd., a PRC company (“Tongli Tech”) and (iv) Tongli Tech’s wholly owned PRC subsidiaries, Tianmu Pharmaceuticals and Lvnong. All significant inter-company accounts and transactions have been eliminated upon consolidation.
Basis of presentation
The financial statements have been prepared in conformity with accounting principles generally accepted in the United States of America. The functional currency of Tongli Technology, Tianmu Pharmaceuticals and Lvnong is the Chinese Renminbi (“RMB”). The accompanying financial statements include the financial statements of foreign subsidiaries that have been translated and presented in United States dollars (“USD”).
Uses of estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of net revenue and expenses during each reporting period. The significant areas requiring the use of management estimates include, but not limited to, the allowance for doubtful accounts receivable, estimated useful life and residual value of property, plant and equipment, recognition and measurement of deferred income taxes and valuation allowance for deferred tax assets. Although these estimates are based on management’s knowledge of current events and actions management may undertake in the future, actual results may ultimately differ from those estimates
Foreign currency translation
Since the Company operates primarily in the PRC, the Company’s functional currency is RMB. For financial reporting purposes, RMB has been translated into USD as the reporting currency. Equity accounts are translated at historical rates. Assets and liabilities are translated at the exchange rate in effect at the balance sheet date. Equity accounts are translated at historical rates. Revenues and expenses are translated at the average rate of exchange prevailing during the reporting period. Translation adjustments arising from the use of different exchange rates from period to period are included as a component of stockholders' equity as "Accumulated other comprehensive income". Gains and losses resulting from foreign currency translations are included in accumulated other comprehensive income.
Revenue Recognition
Revenue is recognized at the date of shipment to customers when a formal arrangement exists, the price is fixed or determinable, the delivery is completed, and no other significant obligations of the Company exist and collectability is reasonably assured. Payments received before all of the relevant criteria for revenue recognition are satisfied are recorded as advances from customers. The Company sells primarily to distributors who subsequently sell the merchandise. The agreements with distributors do not provide for the right of return. Return from distributors has historically been immaterial and accordingly no allowance for returns is deemed necessary as of March 31, 2012 and 2011.
| F-5 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Shipping and Handling Costs
Shipping and handling amounts billed to customers in related sales transactions are included in sales revenues and shipping expenses incurred by the Company are reported as a component of selling expenses. The shipping and handling expenses of $0 and $0 for the year ended March 31, 2012 and 2011, respectively.
Advertising and Promotional Expense
Advertising and promotional costs are expensed as incurred and are included in selling expenses. The Company incurred $258,280 and $1,695 in advertising and promotional costs for the years ended March 31, 2012 and 2011, respectively.
Cash
Cash includes cash on hand and cash in time deposits, certificates of deposit and all highly liquid debt instruments with original maturities of three months or less.
The Company maintains cash with financial institutions in the PRC, which are not insured or otherwise protected. Should any of these institutions holding the Company’s cash become insolvent, or if the Company is unable to withdraw funds for any reason, the Company could lose the cash on deposit with that institution.
Accounts Receivable
Accounts receivables consist of receivables resulting from sales of products, and are stated at net realizable value. This value includes an appropriate allowance for estimated uncollectible accounts. The Company maintains a policy of reserving for uncollectible accounts based on our best estimate of the amount of probable credit losses in existing accounts receivable. The Company extends credit to our customers based on an evaluation of their financial condition and other factors. The Company generally does not require collateral or other security to support accounts receivable. The Company performs ongoing credit evaluations of customers and maintains an allowance for potential bad debts if required.
The Company determines whether an allowance for doubtful accounts is required by evaluating specific accounts where information indicates the customers may have an inability to meet financial obligations. In these cases, the Company uses assumptions and judgment, based on the best available facts and circumstances, to record a specific allowance for those customers against amounts due to reduce the receivable to the amount expected to be collected. These specific allowances are re-evaluated and adjusted as additional information is received. The amounts calculated are analyzed to determine the total amount of the allowance. The Comapny may also record a general allowance as necessary. Direct write-offs are taken in the period when the Company has exhausted efforts to collect overdue and unpaid receivables or otherwise evaluate other circumstances that indicate that the Comapny should abandon such efforts. Management believes that an allowance for doubtful accounts is not required for any of the years presented.
Inventory
Inventory is stated at the lower of cost, determined using the weighted average cost method, or net realizable value. Costs include materials, labor and manufacturing overhead. Net realizable value is the estimated selling price, in the ordinary course of business, less estimated costs to complete and dispose. Management periodically compares the cost of inventory with the market value and an allowance is made to write down the inventory to its net realizable value, if lower than cost. No allowance for inventory markdown is required for the years ended March 31, 2012 and 2011.
| F-6 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Costs associated with purchasing, planting and growing of Chinese herbs by Lvnong aggregating $121,359 during the year ended March 31, 2012 were charged to product development expense as the Company has no experience in the area of growing these products and cannot reasonably estimate the future revenue stream resulting from this activity.
Advances to Suppliers
Advance to suppliers represents the payments made in advance for goods and services to be received. The Company’s management has developed purchasing relationships with a considerable number of suppliers to ensure multiple sources for most of the raw materials required. In the opinion of management, the Company’s business would not be significantly damaged by the loss of any one supplier.
A considerable portion of the raw materials that production requires are volatile herbs, which have a brief shelf life. This situation imposes a risk on suppliers, who often grow such herbs to order to insure an immediate market for the herbs. The situation also necessitates that management is assured that raw material requirements are available precisely when needed. To satisfy these conditions, it is the practice to make substantial cash advances to suppliers in order to lock-in raw material requirements.
Property, equipment and land use right
Property, equipment and land use rights are recorded at cost. The cost of an asset comprises its purchase price and any directly attributable costs of bringing the asset to its present working condition and locations for its intended use. Depreciation is provided in amounts sufficient to amortize the cost of the related assets over their useful lives using the straight line method for financial reporting purposes.
Depreciation is calculated using the straight-line method over the following useful lives:
| Buildings and improvements | 40 years |
| Right to use land | Life of lease |
| Machinery and equipment | 10 years |
| Office equipment | 5 years |
| Vehicle | 5 years |
Impairment of Long Lived Assets
Long-lived assets, which include property, plant and equipment and intangible assets, are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of long-lived assets to be held and used is measured by a comparison of the carrying amount of an asset to the estimated undiscounted future cash flows expected to be generated by the assets.
The Company accounts for the impairment of long-lived assets in accordance with the guidance of FASB ASC 360-10-20. Long-lived assets are reviewed for impairment when circumstances indicate the carrying value of an asset may not be recoverable. For assets that are to be held and used, impairment is recognized when the estimated undiscounted cash flows associated with the asset or group of assets is less than their carrying value. If impairment exists, an adjustment is made to write the asset down to its fair value, and a loss is recorded as the difference between the carrying value and fair value. Fair values are determined based on quoted market values, discounted cash flows or internal and external appraisals, as applicable. Assets to be disposed of are carried at the lower of carrying value or estimated net realizable value. Based on its review, the Company believes that, as of March 31, 2012, there were no impairment of its long-lived assets.
| F-7 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Acquisition of Intangible assets
During the normal course of business the Company acquires patents, formulation, and other intangible assets from third parties. The Company accounts for these acquisitions according to ACS 350-30 Acquisition of intangibles other than goodwill. According to ACS 350-30, intangibles acquired are initially recognized and measured based on their fair values. The contracts to acquire these intangibles frequently call for advance payments to be made. These advances are recorded as deposits until the final allocation of the purchase price is made according to ACS 350-30, which is consistent with the accounting guidance in accordance with ASC 820 Fair Value Measurement.
Fair Value of Financial Instruments
The Company adopted ASC 820, Fair Value Measurements and Disclosures. ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The carrying amounts reported in the balance sheets for cash, accounts receivable, inventory, prepaid expense, accounts payable and other accrued expenses approximate their fair market value based on the short-term maturity of these instruments. The Company did not identify any assets or liabilities that are required to be presented on the consolidated balance sheets at fair value in accordance with ASC 820.
Stock Based Compensation
The Company recognizes compensation expense for stock-based compensation in accordance with ASC Topic 718. For employee stock-based awards, the Company calculates the fair value of the award on the date of grant using the Black-Scholes method for stock options and the quoted price of its common stock for unrestricted shares. The expense is recognized over the service period for awards expected to vest. For non-employee stock-based awards, the fair value of the award is calculated on the date of grant in the same manner as employee awards, however, the awards are revalued at the end of each reporting period and the pro rata compensation expense is adjusted accordingly until such time the nonemployee award is fully vested, at which time the total compensation recognized to date equals the fair value of the stock-based award as calculated on the measurement date, which is the date at which the award recipient’s performance is complete. The estimation of stock-based awards that will ultimately vest requires judgment, and to the extent actual results or updated estimates differ from original estimates, such amounts are recorded as a cumulative adjustment in the period estimates are revised. The Company considers many factors when estimating expected forfeitures, including types of awards, employee class, and historical experience.
Deferred income taxes
The Company accounts for income taxes in accordance with FASB ASC 740 “Income Taxes”which requires that deferred tax assets and liabilities be recognized for future tax consequences attributable to differences between financial statement carrying amounts of existing assets and liabilities and their respective tax bases. In addition, ASC 740 requires recognition of future tax benefits, such as carry-forwards, to the extent that realization of such benefits is more likely than not and that a valuation allowance be provided when it is more likely than not that some portion of the deferred tax asset will not be realized. Management reviews this valuation allowance periodically and makes adjustments as warranted.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on derecognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Company has no material uncertain tax positions for any of the reporting periods presented.
| F-8 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Company has filed tax returns in China and in the U.S. that are subject to audit by tax authorities beginning with the year ended December 31, 2009.The Company’s policy is to classify assessments, if any, for tax and related interest and penalties as tax expense.
Earnings per share
The Company computes earnings per share (“EPS’) in accordance with FASB ASC 260 “Earnings per Share” which requires companies with complex capital structures to present basic and diluted EPS. Basic EPS is measured as net income divided by the weighted average common shares outstanding for the period. Diluted EPS is similar to basic EPS but presents the dilutive effect on a per share basis of potential common shares (e.g., convertible securities, options and warrants) as if they had been converted at the beginning of the periods presented, or issuance date, if later. Potential common shares that have an anti-dilutive effect (i.e., those that increase income per share or decrease loss per share) are excluded from the calculation of diluted EPS.
Comprehensive income
In accordance with FASB ASC 220, comprehensive income is defined to include all changes in equity except those resulting from investments by shareholders and distributions to shareholders. Among other disclosures, all items that are required to be recognized under current accounting standards as components of comprehensive income are required to be reported in a financial statement that is presented with the same prominence as other financial statements. Comprehensive income includes net income and the foreign currency translation gain, net of tax.
Statement of Cash Flows
In accordance with FASB ASC 230 “Statement of Cash Flows,” cash flows from the Company’s operations are calculated based upon the local currencies. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheet.
Segment reporting
FASB ASC 280-10-50 “Segment Reporting" requires the use of the “management approach” model for segment reporting. The management approach model is based on the way a company’s management organized segments within the company for making operating decisions and assessing performance. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company. ASC 280-10-50 has an immaterial effect on the Company’s financial statements, as the Company consists of only one reportable business segment. All revenues are from sales to customers in the PRC. Substantially all of the Company’s assets are located in the PRC.
New Accounting Pronouncements
In June 2011, the FASB issued guidance which eliminates the option to report other comprehensive income and its components in the statement of changes in stockholders' equity and requires an entity to present the total of comprehensive income, the components of net income and the components of other comprehensive income either in a single continuous statement or in two separate but consecutive statements. This pronouncement is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. The adoption of this guidance is not expected to have a material impact on the Company’s consolidated financial statements.
In May 2011, the FASB amended ASC 820, “Fair Value Measurements.” This amendment is intended to result in convergence between U.S. GAAP and International Financial Reporting Standards (“IFRS”) requirements for measurement of and disclosures about fair value. This guidance clarifies the application of existing fair value measurements and disclosures, and changes certain principles or requirements for fair value measurements and disclosures. The updated guidance is effective on a prospective basis for financial statements issued for interim and annual periods beginning after December 15, 2011. The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
| F-9 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
In September 2011, the FASB issued ASU No. 2011-08, “Testing Goodwill for Impairment.” ASU 2011-08 simplifies the goodwill impairment assessment by permitting a company to make a qualitative assessment of whether it is more likely than not that a reporting unit's fair value is less than its carrying amount before applying the two-step goodwill impairment test. If the conclusion is that it is more likely than not that the fair value of a reporting unit is less than its carrying amount, the company would be required to conduct the current two-step goodwill impairment test. Otherwise, it would not need to apply the two-step test. The adoption of ASU 2011-08 did not have any impact on the Company’s financial position, results of operations or cash flows.
| 3 | INVENTORY |
As of March 31, 2012 and 2011, inventory consists the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Raw materials | $ | 13,553 | $ | 61,478 | ||||
| Finished goods | 60 | 132,574 | ||||||
| Total | $ | 13,613 | $ | 194,052 | ||||
| 4 | CONTRACT DEPOSIT |
Contract deposit represents payments under material contracts by which the Company intends to purchase drug formula to be used in the manufacturing and increasing its product lines.
As of March 31, 2012 and 2011, contract deposit consists the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Contract deposit-Lanhai (1) | $ | - | $ | 1,073,512 | ||||
| Contract deposit-Tonghua (2) | 3,444,658 | 3,359,497 | ||||||
| Contract deposit-Xinyu (3) | 214,509 | - | ||||||
| 3,659,167 | 4,433,009 | |||||||
| Less: current portion | (3,189,441 | ) | (1,073,512 | ) | ||||
| Total | $ | 469,726 | $ | 3,359,497 | ||||
| (1) | In September 2008, the Company entered into a Patent Assignment Agreement (the “Lanhai Agreement”) with Harbin Lanhai Biochemical Company Limited (“Lanhai”) under which the Company agreed to acquire a patent related to a product called LanhaiSpirulina Calcium Tablet. Pursuant to the Lanhai Agreement, Lanhai agreed to provide the Company with the product formula as well as the technical support and assistance necessary for the Company to obtain approval for the product by the China State Food & Drug Administration (“SFDA”). Under the Lanhai Agreement, the Company made a payment of RMB7,030,000 (approximately $1.03 million) in October 2008, representing the entire purchase price for the subject patent. The Company is not obligated to make any subsequent payment. The patent application was accepted by the State Intellectual Property Office of China (“SIPO”) on March 14, 2008 and, if granted, the patent will expire on March 13, 2028 pursuant to PRC patent laws. |
The term of the Lanhai Agreement is from November 1, 2008 to October 30, 2013. Title to this patent will not be assigned to the Company until the patent assignment is properly recorded with SIPO. The recording of this assignment was yet to be confirmed by SIPO.
Based on ASC 805-50-30 “Initial Measurement of Acquisition of Assets Rather Than a Business” and ASC350-30 “General Intangibles Other Than Goodwill”, the payment of RMB7,030,000 for acquiring the patent was recorded as an asset at cost. The payment was originally recorded as a deposit which would be recorded as an intangible asset once the Company obtains title to this patent, which asset will be subsequently amortized over the life of the patent.
| F-10 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
During 2010, the Company reached an unwritten mutual understanding with Lanhai that if the Company cannot obtain title to the patent before the end of the contract period (i.e., October 30, 2013), the entire payment will be refunded to the Company. Although the Company believes this understanding to be binding on the parties, it was not explicitly stipulated in the Lanhai Agreement or otherwise memorialized.
On June 17, 2011, the Company entered into a Termination Agreement with Lanhai pursuant to which the Lanhai Agreement was terminated. The parties agreed that, because the patent assignment recording process took too long, the Company will return the LanhaiSpirulina Calcium Tablet product and related intellectual property to Lanhai. Pursuant to the Termination Agreement, Lanhai agreed to refund the total purchase price of RMB7,030,000 in three installments.The recording of the patent assignment was terminated in June 2011 as well. On June 27, 2011, Lanhai refunded the first installment of RMB2,000,000. The second portion of refund of RMB 2,000,000 was received on July 18, 2011. The third portion of refund of RMB 3,030,000 was received on August 2, 2011.
| (2) | On March 21, 2010, the Company entered into a New Drug Assignment Agreement (the “Tonghua Agreement”) with TonghuaYisheng Pharmaceuticals Company Limited (“Tonghua”) pursuant to which the Company agreed to purchase a new drug candidate, called Nafarelin, from Tonghua for a purchase price of RMB33,000,000 (approximately $4.85 million). The total purchase price is payable in three installments: 33% of the total purchase price (approximately $1.6 million) was paid upon execution of the Tonghua Agreement in March 2010; another 33% (approximately $1.6 million) was paid in March 2011 (although it is required to be paid upon conclusion of third clinical trial for the product pursuant to the terms of the Tonghua Agreement) and the balance is payable upon conclusion of the transfer. |
Nafarelin is a proposed treatment for endometriosis and prostate cancer. At a minimum, completion of the third phase clinical trail is a pre-condition to receipt of the New Drug Approval Certificate from the SFDA. The SFDA new drug approval procedure is separate and distinct from the patent prosecution procedure. To date, neither Tonghua nor the Company has applied or intend to apply for patent protection for Nafarelin or the formula or process related to Nafarelin. Once Tonghua is granted the New Drug Approval Certificate for Nafarelin, according to pharmaceutical laws of PRC, the Company, as the assignee of the New Drug Approved Certificate, shall receive 4 years of exclusive protection. As described below, the Company regards such protection period as the appropriate amortization period. The patent protection is irrelevant for purposes of amortization in determining the life of the drug as the Company will not apply for patent protection for Nafarelin.
The Company is not be responsible for conducting or paying for any clinical trial and/or research and development in connection with the new drug application for Nafarelin. Pursuant to the Tonghua Agreement, Tonghua shall continue the research, development and clinical trial work for Nafarelin until the SFDA grants the New Drug Approval Certificate and the product is ready to be marketed. Tonghua is listed as the applicant for the SFDA new drug approval process for this product. Once SFDA approval is obtained, the Company will be able to consummate the assignment and enjoy the protection afforded by the New Drug Approval Certificate. In addition, the Company’s ability to commercialize this new product also requires assistance and cooperation from Tonghua, which Tonghua is obligated to provide to us under the Tonghua Agreement.
Under PRC law, the SFDA’s New Drug Approval Certificate grants certain exclusive protections to approved new drugs, meaning that the subject formula may not be manufactured by other parties in China. The exclusive protection periods for the new drugs vary from 4 to 12 years depending on the category of the new drug. Because the Company shall be the beneficiary of the exclusive protection during the applicable period (the so-called “new drug approval period”), upon consummation of the transaction contemplated by the Tonghua Agreement, the Company will regard the protection period as the appropriate amortization period. Based on ASC 805-50-30 “Initial Measurement on Acquisition of Assets Rather Than a Business” and ASC350-30 “General Intangibles Other Than Goodwill”, the total payment pursuant to the Tonghua Agreement will be recorded as an intangible asset once the Company obtains the SFDA New Drug Approval Certificate and will be amortized over the term of the new drug protection period.
Based on a written mutual understanding between the Company and Tonghua, any payments the Company made to Tonghua under the Tonghua Agreement will be refunded if SFDA approval is not abtained for Nafarelin. Such mutual understanding, although the Company believes to be binding, was not explicitly stipulated in the Tonghua Agreement or otherwise memorialized. As such, the first two installment under the Tonghua Agreement was accounted as a deposit and the remaining last installment payment will be also accounted as deposits until the New Drug Approval Certificate is obtained, at which point the total payment will be recorded as intangible asset and amortized over the term of the new drug protection period.
| F-11 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The Tonghua Agreement also provides that the Company and Tonghua will compensate one another for all losses incurred by the other party if the purchase is not concluded due to reasons attributable to the offending party.
On June 6, 2012, the Company entered into a Termination Agreement with Tonghua pursuant to which the Tonghua Agreement was terminated. Pursuant to the Termination Agreement, Tonghua will refund prepayment of RMB 22,000,000 (approximate to $3.5 million) in four installments. The first installment of RMB5, 000,000 (approximately $794,000) will be refunded prior to August 30, 2012, the second installment of RM6, 000,000 (approximately $950,000) will be refunded prior to November 30, 2012, the third installment of RMB8,000,000 (approximately $1.3 million) will be refunded prior to February 28, 2013 and the last installment of RMB3,000,000 (approximately $476,000) will be refunded prior to May 30, 2013.
| (3) | On April 30, 2012, the Company entered into Xinyu Breath Spray purchase agreement with Harbin Junde Healthcare product Company. Pursuant to the agreement, the total purchase price is RMB8,500,000(approximately $1.33 million). The Company will pay 50% of the purchase price after commencement of the Xinyu Breath Spray purchase agreement and pay the rest of the purchase price after the Company manufactured three batches of Xinyu Breath Spray that meet the quality standards of the product. The Company paid RMB1,370,000 (approximately $214,509) on March 29, 2012 and RMB3,630,000 (approximately $568,369) on May 30, 2012 as deposits. |
| 5. | PROPERTY AND EQUIPMENT |
A summary of property and equipment is as follows:
| As of March 31, 2012 | As of March 31, 2011 | |||||||
| Building | $ | 7,602,491 | $ | 7,414,535 | ||||
| Machinery and equipment | 1,093,276 | 1,066,246 | ||||||
| Office equipment | 17,431 | 16,377 | ||||||
| Vehicle | 106,254 | 103,627 | ||||||
| $ | 8,819,452 | $ | 8,600,785 | |||||
| Less: accumulated depreciation | (2,625,926 | ) | (2,217,068 | ) | ||||
| Property and equipment, net | $ | 6,193,526 | $ | 6,383,717 | ||||
Depreciation expense was $356,901 and $336,249 for the years ended March 31, 2012 and 2011.
| 6. | LAND USE RIGHTS |
The executive offices and manufacturing facilities of the Company’s subsidiary Tianmu Pharmaceuticals are located in the Limin Pharmaceutical Technology Park in the City of Harbin, which is the capital of Heilongjiang Province in The People’s Republic of China. In 2001, Tianmu Pharmaceuticals from the local government obtained a land use right of 50,000 square meters for 50 years for manufacturing purposes for RMB 2,700,000(approximately $400,000). The Company amortizes this land use right on a straight-line basis over 50 years.
On December 20, 2010, the Company entered into a contract with a non-affiliated individual to acquire rights to use a parcel of land and fixtures thereon for twenty years (from December 20, 2010 to December 30, 2030) for RMB20 million (approximately $3 million). Total payment of contract price was recorded as long-term assets and is amortized over the twenty years use right period.
In March 2011, the Company acquired the lease of fish pond for twenty years with total contract price of RMB 5.5 million (approximate to $0.85 million). The Company will use this fish pond to provide water supply to the planted traditional Chinese medicine. Total amount of this contract was amortized on a straight-line basis over twenty years.
| F-12 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Land use rights consist of the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Land use rights | $ | 4,415,426 | $ | 4,306,264 | ||||
| Less: Accumulated amortization | (374,394 | ) | (161,352 | ) | ||||
| $ | 4,041,032 | $ | 4,144,912 | |||||
The amortization expense for the years ended March 31, 2012 and 2011 was $211,418 and $49,431 respectively.
| 7. | DUE TO/(FROM) RELATED PARTIES |
Due to (from) related parties consist of the following:
| March 31, 2012 | March 31, 2011 | |||||||
| Due to Harbin Tianmu Real Estate Development Co. Ltd (a) | $ | (599,151 | ) | $ | 344,899 | |||
| Due to Yao, Mingli, Chairman of the Company | 570,324 | 541,304 | ||||||
| Due to US Hua Sky International Investment LLC (b) | 27,727 | 27,727 | ||||||
| Due to Tianmu Investment Co. Ltd (c) | (7,829 | ) | - | |||||
| Due to Yao, Yuan, a shareholder of the Company | (78,609 | ) | 158,141 | |||||
| Total | $ | (87,538 | ) | $ | 1,072,071 | |||
(a) Harbin Tianmu Real Estate Development Co., Ltd. is an entity owned by our Chairman, Mr. Mingli Yao.
(b) US Hua Sky International Investment LLC is an entity owned by our Chairman, Mr. Mingli Yao
(c)Tianmu Investment Co., Ltd. is an entity partially owned by our Chairman, Mr. Mingli Yao.
Mr. Mingli Yao has personally guaranteed the loan receivable from Harbin Tianmu Real Estate Development Co. Ltd and Tianmu Investment Co. Ltd. If theloan has not been repaid in the year ended March 31, 2013, Mr. Yao has agreed to offset the balance due to him referenced above.
| 8. | STOCKHOLDERS’ EQUITY |
In December 2009, the Company established its 2009 incentive plan (“the plan”) to provide additional incentive to selected employees, officers, directors and consultants of the Company and to retain the best available personnel for positions of substantial responsibility and to promote the long-term success of the Company. 2,000,000 shares of Company common stock were initially reserved for issuance pursuant to awards granted under the plan. Awards may be in the form of incentive stock options, non-qualified stock options, restricted shares or other arrangements or programs. On September 8, 2010, 250,000 shares of common stock were issued from the plan to several employees and consultants for services rendered. The Company recorded the fair value of $97,500 for these issued shares. As of March 31, 2012, total 1,419,093 shares were issued from the plan.
On November 15, 2010, the Company issued 18,182 common shares to its legal consul for services rendered. The Company recorded the fair value of $20,000 for these issued shares, which approximated the fair value of the shares on the date of issuance.
Pursuant to an Independent Director Agreement the Company entered into with Mr. Stephen J. Sax on April 14, 2011, the Company granted an award of 100,000 shares of restricted stock of the Company on the date of appointment. The Company recorded the fair value of $65,000 for these issued shares.
On April 18, 2011, the Company issued 28,571 common shares to its legal counsel Ellenoff Grossman & Schole LLP for services rendered. The Company recorded the fair value of $20,000 for these issued shares.
On September 21, 2011, the Company issued 1,377,000 common shares to several officers, directors, employees and consultants for their services. 580,000 shares were issued to the officers and directors. The Company recorded the fair value of $688,500 for these issued shares.
| F-13 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
On November 1, 2011, the Company issued 100,000 common shares to its consultants in China for services rendered. The Company recorded the fair value of $32,000 for these issued shares.
On November 1, 2011, the Company sold 26,000 common shares to an individual at the price of $0.3 per share.
| 9. | TAXES |
| (a) | Corporation income tax (“CIT”) |
The Company’s Chinese subsidiaries are governed by the Income Tax Law of the People’s Republic of China concerning the private-run enterprises, which are generally subject to tax at a new statutory rate of 25%. The Company’s PRC entities remain open for statutory examination by PRC tax authorities for the tax year ended December 31, 2011.
The Company is incorporated in the United States. It had net operating loss carry forwards for United States income tax purposes amounted to $1,871,117 and $1,359,147 for the years ended March 31, 2012 and 2011 respectively, which may be available to reduce future years' taxable income in the United States. These carry forwards will expire between 2028 and 2031. Management doesn't expect to remit any of its net income back to the United States in the foreseeable future. Accordingly, the Company recorded a full valuation allowance as of March 31, 2012 and 2011.
The reconciliation of income tax expense at the U.S. statutory rate at 35% in 2012 and 2011, to the Company’s effective tax is as follows:
| For the Years Ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| U.S. Statutory income tax rate | $ | 1,145,680 | $ | 2,037,701 | ||||
| Taxable difference between U.S. and China | (378,534 | ) | (615,249 | ) | ||||
| Expenses not deductable for tax purpose in China | 165,459 | 23,762 | ||||||
| Change in valuation allowance | 188,995 | 115,721 | ||||||
| Effective income tax | $ | 1,121,600 | $ | 1,561,935 | ||||
The provisions for income taxes for the years ended March 31, 2012 and 2011 are summarized as follows:
| For the Years Ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| Current | $ | 1,473,115 | $ | 1,608,374 | ||||
| Deferred-United States | (179,189 | ) | (115,671 | ) | ||||
| Deferred-China | (361,321 | ) | (46,489 | ) | ||||
| Change in Valuation Allowance | 188,995 | 115,721 | ||||||
| Total | $ | 1,121,600 | $ | 1,561,935 | ||||
The components of income (loss) before income taxes from China and U.S. for the years ended March 31, 2012 and 2011 were as follows:
| For the Years Ended March 31, | ||||||||
| 2012 | 2011 | |||||||
| China | $ | 3,785,342 | $ | 6,152,493 | ||||
| United States | (511,970 | ) | (330,489 | ) | ||||
| Income before income tax | $ | 3,273,372 | $ | 5,822,004 | ||||
| F-14 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The tax effects of temporary differences that give rise to the Company’s net deferred tax assets as of March 31, 2012 and 2011 are as follows:
| As of March 31, 2012 | As of March 31, 2011 | |||||||
| Deferred tax assets resulted from temporary difference in PRC | ||||||||
| Impairment of intangible assets | $ | 35,995 | $ | 35,995 | ||||
| Depreciation difference | 15,776 | 10,564 | ||||||
| Accrued expenses | 96,830 | - | ||||||
| Inventory write-off | 44,794 | - | ||||||
| Other temporary difference | 11,115 | 1,007 | ||||||
| Net operationg loss carry-forward - China | 204,697 | 320 | ||||||
| Net Operationg loss carry-forward - United States | 654,891 | 475,702 | ||||||
| 1,064,098 | $ | 523,588 | ||||||
| Less: valuation allowance | (665,017 | ) | (476,022 | ) | ||||
| Deferred tas assets | $ | 399,081 | $ | 47,566 | ||||
| (b) | Value added tax (“VAT”) |
Enterprises or individuals who sell commodities, engage in repair and maintenance or import or export goods in the PRC are subject to a value added tax in accordance with the PRC laws. The value added tax standard rate is 17% of the gross sales price. A credit is available whereby VAT paid on the purchases of semi-finished products or raw materials used in the production of the Company’s finished products can be used to offset the VAT due on the sales of the finished products.
| (c) | Other taxes |
The Company is also subject to 5% of business tax, 7% of City Construction Tax and 5% of Education Fees based on VAT.
| 10 | CONCENTRATION |
For the year ended March 31, 2012, four products manufactured by the Company, including Panax and Radix Polygoni Capsule, Antihyperlipidemics, Calcium Gluconate Oral Liquid and Anti-bacterial Mouthwash, represented 45%, 20%, 14% and 15% respectively of the total sales.
For the year ended March 31, 2011, four products manufactured by the Company, including Panax and Radix Polygoni Capsule, Antihyperlipidemics, Calcium Gluconate Oral Liquid and Anti-bacterial Mouthwash, represented 27%, 24%, 18% and 18% respectively of the total sales.
| 11 | VULNERABILITY DUE TO OPERATIONS IN PRC |
The Company’s operations may be adversely affected by significant political, economic and social uncertainties in the PRC. Although the PRC government has been pursuing economic reform policies for more than thirty years, no assurance can be given that the PRC government will continue to pursue such policies or that such policies may not be significantly altered, especially in the event of a change in leadership, social or political disruption or unforeseen circumstances affecting the PRC’s political, economic and social conditions. There is also no guarantee that the PRC government’s pursuit of economic reforms will be consistent or effective.
Substantially all of the Company’s businesses are transacted in RMB, which is not freely convertible. The Peoples Bank of China or other banks are authorized to buy and sell foreign currencies at the exchange rates quoted by the Peoples Bank of China. Approval of foreign currency payments by the Peoples Bank of China or other institutions requires submitting a payment application form together with suppliers’ invoices, shipping documents and signed contracts.
| F-15 |
TONGLI PHARMACEUTICALS (USA), INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Since the Company has its primary operations in the PRC, the majority of its revenues will be settled in RMB, not U.S. Dollars. Due to certain restrictions on currency exchanges that exist in the PRC, the Company’s ability to use revenue generated in RMB to pay any dividend payments to its shareholders outside of China may be limited.
The Company’s business depends on maintaining licenses of its current products from SFDA. Obtaining licenses for additional products can be expensive and is usually time consuming. Failure to obtain the required licenses can cause the Company’s business plan to be delayed. If the delays prevent the Company from generating positive cash flows or introducing a significant number of products, there will be a material adverse effect on the Company.
| 12 | SUBSEQUENT EVENT |
The Company has evaluated events after the date of these financial statements through the date that these financial statements were issued and no subsequent event is required to be disclosed.
| F-16 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
Dated: July 16, 2012
| /s/ Mingli Yao | |
| Mingli Yao | |
| Chief Executive Officer and Chairman |
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Mingli Yao | Chief Executive Officer and Chairman | July 16, 2012 | ||
| Mingli Yao | (principal executive officer) | |||
| /s/Li Li | Chief Financial Officer | July 16, 2012 | ||
| Li Li | (principal financial and accounting officer) | |||
| /s/ Ailing Zhao | Corporate Secretary and Director | July 16, 2012 | ||
| Ailing Zhao | ||||
| /s/ Yuan Yao | Director | July 16, 2012 | ||
| Yuan Yao | ||||
| /s/ Zhijian Hao | Director | July 16, 2012 | ||
| Zhijian Hao |
| 37 |
Exhibit Index
| Exhibit No. | Description | |
| 3.1 | Amended and Restated Bylaws - filed as an exhibit to the Current Report on Form 8-K filed on April 14, 2011 and incorporated herein by reference. | |
| 10.1 |
Unofficial English translation of Director Agreement with Mr. Zhijiang Hao - filed as an exhibit to the Current Report on Form 8-K filed on January 24, 2011 and incorporated herein by reference
| |
| 10.2* |
Unofficial English translation of Termination Agreement with Tonghua Yisheng Pharmaceuticals Company Limited
| |
| 10.3* |
Unofficial English translation of Xinyu Breath Spray Purchase Agreement with Harbin Junde Healthcare Product Company (“Xinyu Purchase Agreement”) and Supplementary Agreement to the Purchase Agreement.
| |
| 10.4 |
Unofficial English translation of Termination Agreement with Harbin Lanhai Biochemical Company Limited, filed as an exhibit to Annual Report on Form 10-K for year ended March 31, 2011 filed on July 14, 2011
| |
| 10.5 | Unofficial English translation of Loan Agreement with Harbin Tianmu Real Estate Development Co., Ltd. filed as an exhibit to Annual Report on Form 10-K for year ended March 31, 2011 filed on July 14, 2011 | |
| 10.6 |
Unofficial English translation of Loan Agreement with Mingli Yao, filed as an exhibit to Annual Report on Form 10-K for year ended March 31, 2011 filed on July 14, 2011
| |
| 10.7 |
Loan Agreement with US Hua Sky International Investment LLC, filed as an exhibit to Annual Report on Form 10-K for year ended March 31, 2011 filed on July 14, 2011
| |
| 10.8 | Unofficial English translation of the Land Use Right Transfer Agreement and Supplementary Agreement with Shuai Li, filed as an exhibit to Annual Report on Form 10-K for year ended March 31, 2011 filed on July 14, 2011 | |
| 21.1* |
Subsidiaries of Registrant.
| |
| 31.1* |
Certification of our principal executive officer pursuant to Rules 13a-14 and 15d-14 under the Securities Exchange Act of 1934, as amended.
| |
| 31.2* |
Certification of our chief financial officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
| |
| 32.1* |
Certification of our principal executive officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
| |
| 32.2* | Certification of our chief financial officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
| 101.INS ** | XBRL Instance Document | |
| 101.SCH ** | XBRL Taxonomy Extension Schema Document | |
| 101.CAL ** | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF ** | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB ** | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE ** | XBRL Taxonomy Extension Presentation Linkbase Document |
* Filed herewith.
** XBRL (Extensible Business Reporting Language) information is furnished and not filed or a part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, is deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise is not subject to liability under these sections.
| 38 |
