Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - LA JOLLA PHARMACEUTICAL CO | d380455d8k.htm |
 Corporate Presentation
July 2012
Exhibit 99.1 |
 Mission
La Jolla Pharmaceutical is Committed to
Discovering and Developing Novel Therapies
Designed to Modulate Immune Function
to Treat Organ Failure and Cancer
2 |
 Corporate Highlights
3
Technology
•
Immune therapy platform targeting, Galectin-3, an emerging key with a
demonstrated role in organ failure and cancer via immune regulation
•
Clear path to proof-of-concept
Pipeline
•
Product
candidate:
GCS-100
-
Leading,
clinical
stage
galectin-3
antagonist
May prevent or reverse organ failure by mediating fibrosis via galectin-3
sequestration Binds galectin-3 and reverses T-cell suppression
Extensive clinical data with clear single agent activity and favorable safety
profile Management and Investors
•
Experienced and driven
Milestones
•
Near-term, cost-efficient clinical and preclinical milestones to drive
value |
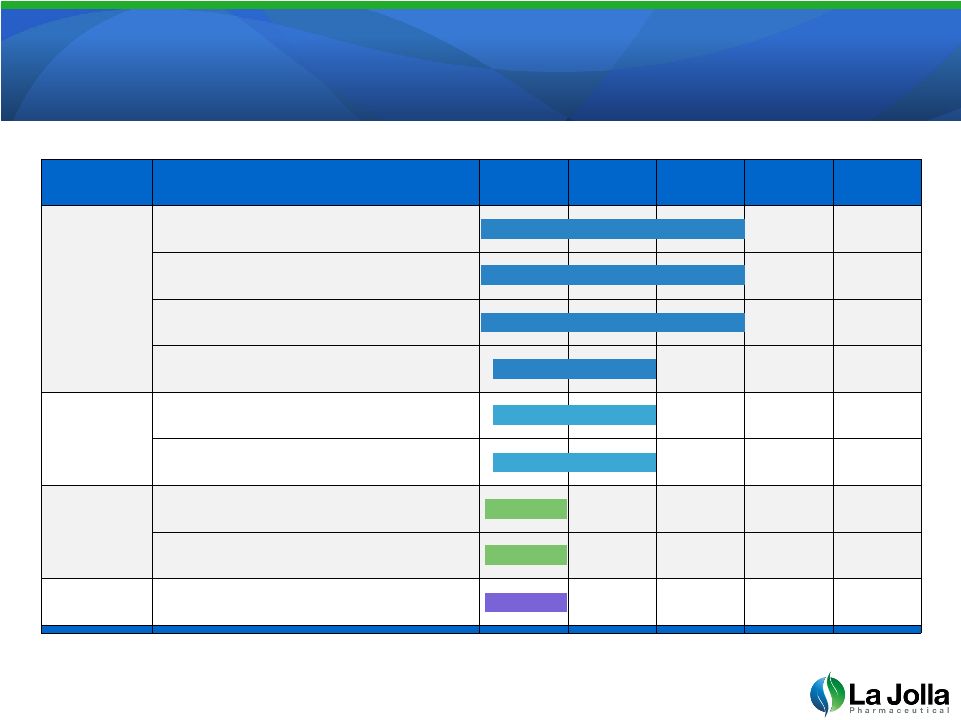 Robust Product Pipeline
Robust Product Pipeline
4
Products
Program/Indication
R&D
IND
Phase 1
Phase 2
Phase 3
GCS-100
Cancer with Renal Insufficiency
Cancer Immunotherapy
Prevention of Chemotherapy Toxicity
End-Stage Renal Disease
LJPC-101
Renal Transplantation
End-Stage Renal Disease
LJPC-201
Heart Failure
Hepatic Fibrosis
LJPC-301
Cancer –
Solid Tumors |
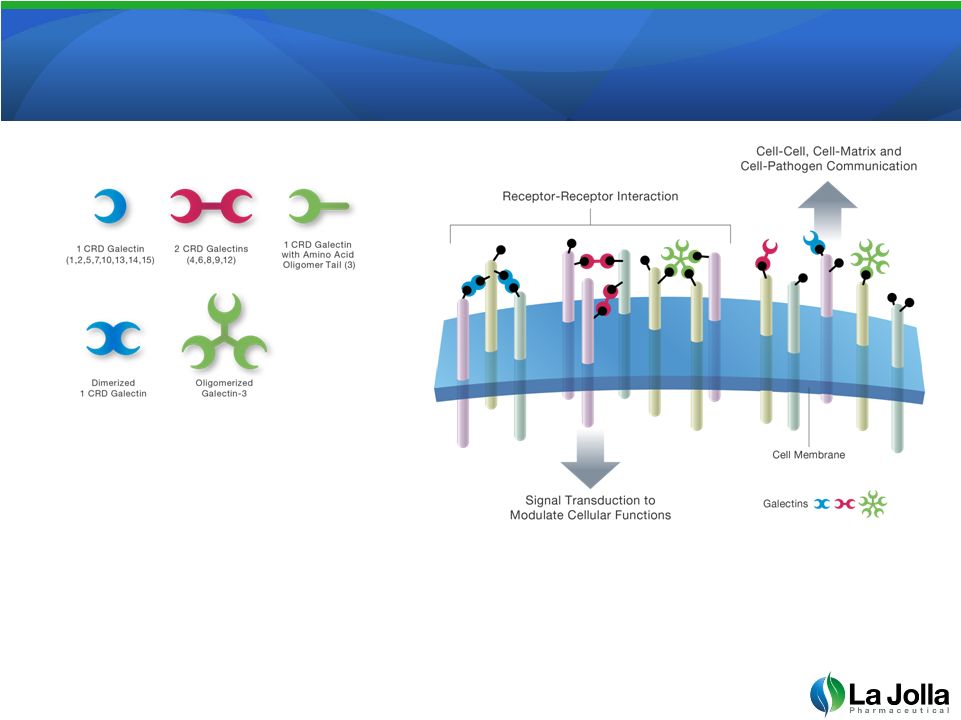 Galectins and
Galectin-3 •
Galectins are proteins that can
bind to sugars proteins to modulate
function and communication
•
Normally present at low concentration, but up-regulated in cancer and organ
failure
5 |
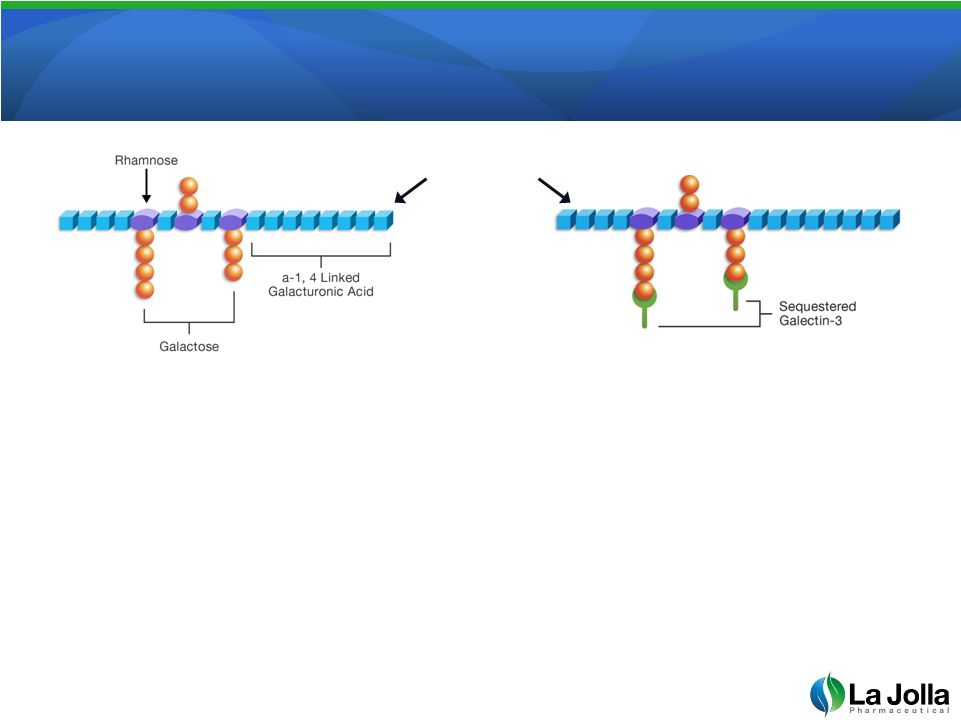 GCS-100: The Lead Galectin-3 Antagonist
GCS-100: The Lead Galectin-3 Antagonist
•
GCS-100 is a well-characterized, complex sugar
•
GCS-100 binds to and neutralizes galectin-3. Binding activity
is localized to the galactose containing side-branches
•
Patented manufacturing process required for biologic activity;
unmodified pectin has reduced biologic activity
6
GCS-100 |
 Galectin-3 and Organ
Failure Galectin-3 and Organ Failure
•
Galectin-3 knockout mice develop significantly less kidney
fibrosis
and
failure
after
damage
compared
to
normal
mice
•
Galectin-3 knockout mice do not develop liver fibrosis when
exposed
to
toxin
•
Galectin-3 serum assay is FDA approved to identify patients at
risk
for
death
due
to
heart
failure
•
Serum galectin-3 levels identify patients with end-stage renal
disease
who
are
at
highest
risk
for
death
7
4
5
3
1,2
1
The American Journal of Pathology, 2008; Vol. 172, No. 2: 288-298.
2
Transplantation International, 2008; Vol. 21, No. 10: 999-1007.
3
Proceedings of the National Academy of Sciences, 2006; Vol. 103, No. 13: 5060-5065.
4
Annals of Medicine, 2011; 43: 60–68.
5
Galectin-3 and Outcomes in Patients with End-Stage Renal Disease: Data from the German
Diabetes and Dialysis Study, presented by Rudolf de Boer, MD, PhD, Associate Professor of
Cardiology at the University of Groningen, the Netherlands; American Heart Association Scientific Presentation,
November 2011.
|
 Galectin-3 in ESRD:
2011 AHA Presentation Galectin-3 in ESRD: 2011 AHA Presentation
8
1
Department of Cardiology, University Medical Center Groningen, The Netherlands
2
Department of Medicine, Division of Nephrology, University Hospital Würzburg, Germany
Galectin-3 and Cardiovascular Outcomes in Patients
with End-Stage Renal Disease
Data from the German Diabetes and Dialysis Study
Rudolf
A.
de
Boer
MD
¹
Christoph
Wanner
MD
²
Katja
Blouin
MSc
²
Christiane
Drechsler
MD
² |
 Galectin-3 in ESRD:
Galectin-3 in ESRD:
2011 AHA Presentation Conclusions
2011 AHA Presentation Conclusions
•
Galectin-3 levels are extremely elevated in patients with ESRD
on hemodialysis
•
High galectin-3 in independently associated with stroke, CV
events, all-cause and infectious mortality in dialysis patients
9 |
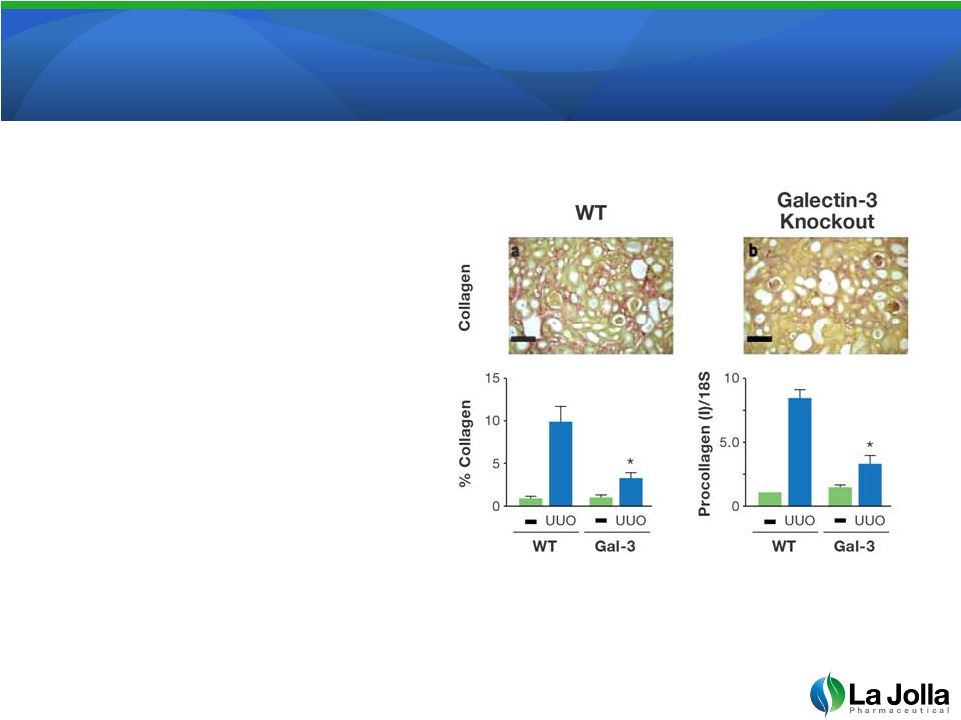 Galectin-3:
Promotes Organ Failure Galectin-3: Promotes Organ Failure
via Scar Formation
via Scar Formation
•
Mice genetically altered to lack
galectin-3 produce much less
scar tissue in the kidney after
injury. Normal (wild-type, WT)
mice or galectin-3 knockout mice
were either left alone (-) or
surgically injured by obstructing
the outflow of urine from the
kidney (UUO). The amount of
collagen and procollagen
produced is a measure of scar
formation. As indicated, galectin-
3 knockout mice produced much
less scaring.
10
The American Journal of Pathology, 2008; Vol. 172, No. 2: 288-298. |
 Galectin-3 and
Cancer Galectin-3 and Cancer
•
Over-expression of galectin-3 correlates with aggressiveness
and relapse in multiple human cancers
1,2
•
Galectin-3 expression via transfection increases metastasis in
animal models
1,2
•
Galectin-3
suppresses
anti-tumor
T-cell
activity
3
•
Lung tumorigenesis is reduced in galectin-3-/-
vs. wild-type
mice
4
•
Galectin-3
knockdown
reduces
proliferation
and
tumor
growth
5
11
1
Reviewed in: Liu, F., et al. 2005. Galectins as Modulators of Tumour Progression. Nature Reviews
Cancer. 5:29-41proliferation ane
2
Reviewed in: Takenaka, Y., et al. 2004. Galectin-3 and Metastasis. Glycoconj J.
19:543-549
3
Demotte, N. et al. 2008. Restoring the Association of the T Cell Receptor with CD8 Reverses Anergy in
Human Tumor-Infiltrating Lymphocytes. Immunity 28, 414-424 4
Abdel-Aziz, H., et al. 2008. Targeted Disruption of the Galectin-3 Gene Results in Decreased
Susceptibility to NNK-Induced Lung Tumorigenesis: An Oligonucleotide Microarray
Study. J Cancer Res Clin Oncol. 134:777-788
5
Peng W, et al. 2008. Tumor Associated Galectin-3 Modulates the Function of Tumor Reactive
T-cells. Cancer Research 68:7228-7236. |
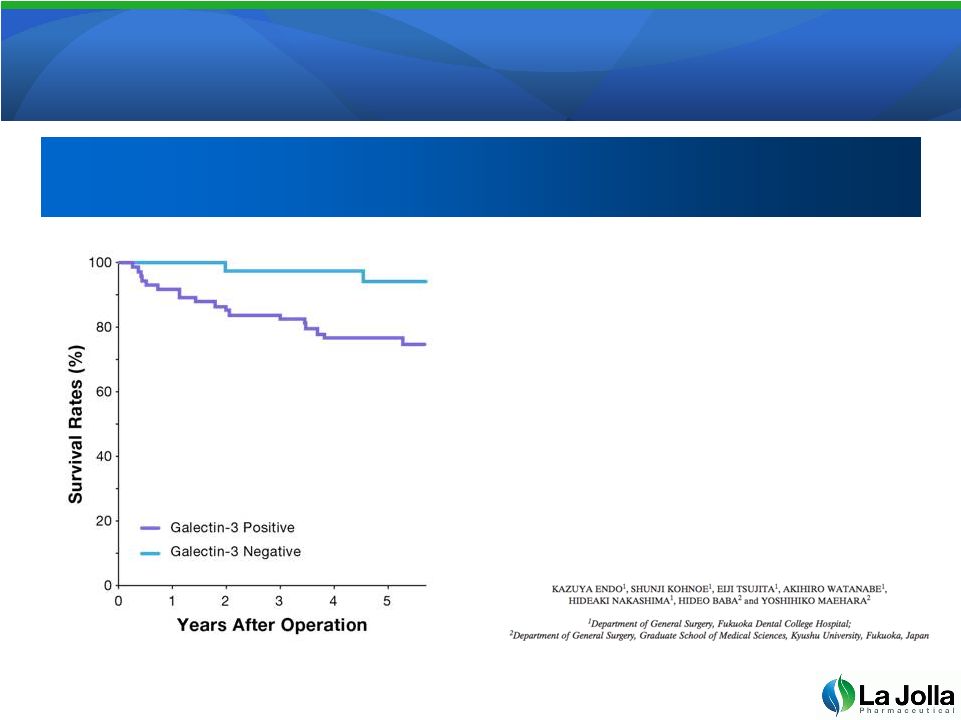 Galectin-3
Expression Correlates with Galectin-3 Expression Correlates with
Reduced Patient Survival
Reduced Patient Survival
•
Survival curve of colorectal cancer
patients with positive and negative
galectin-3 expression. The
prognosis of patients was
significantly worse in patients with
galectin-3-positive expression
(purple line) than in galectin-3-
negative patients (blue line)
(p=0.0027).
12
Anticancer Research 25: 3117-3122 (2005)
Galectin-3 Expression is a Potential
Galectin-3 Expression is a Potential
Prognostic Marker in Colorectal Cancer
Prognostic Marker in Colorectal Cancer |
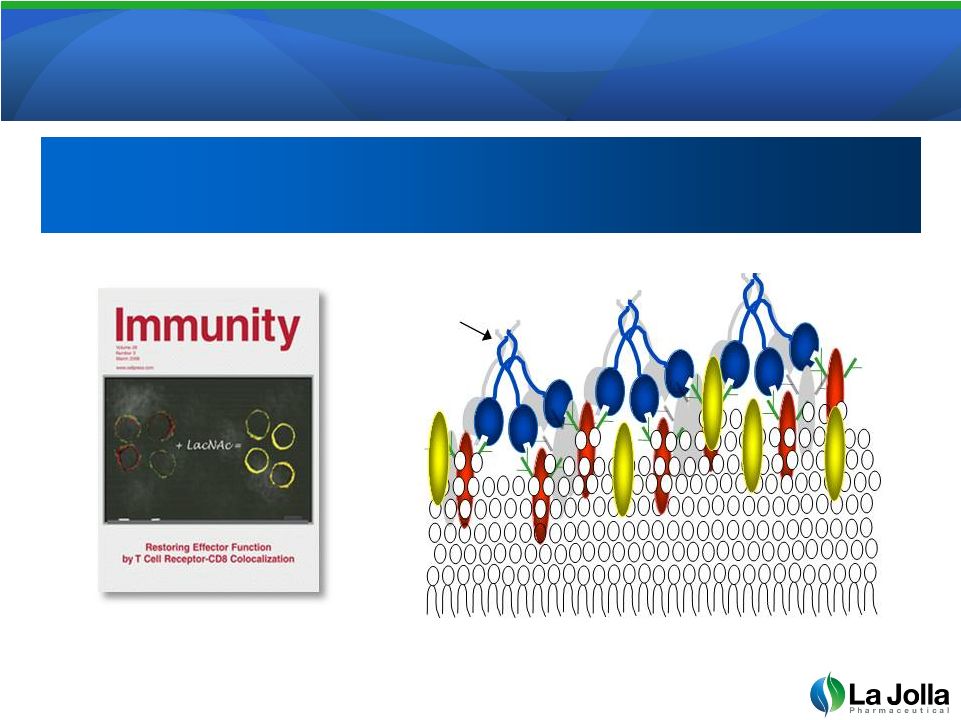 Galectin-3
Suppresses T-Cell Activation Galectin-3 Suppresses T-Cell Activation
13
Source: Pierre van der Bruggen, Ludwig Institute for Cancer Research
Galectin-3
Galectin-3 binding to cell surface glycoproteins blocks
Galectin-3 binding to cell surface glycoproteins blocks
co-receptor association and cell signal transmission
co-receptor association and cell signal transmission |
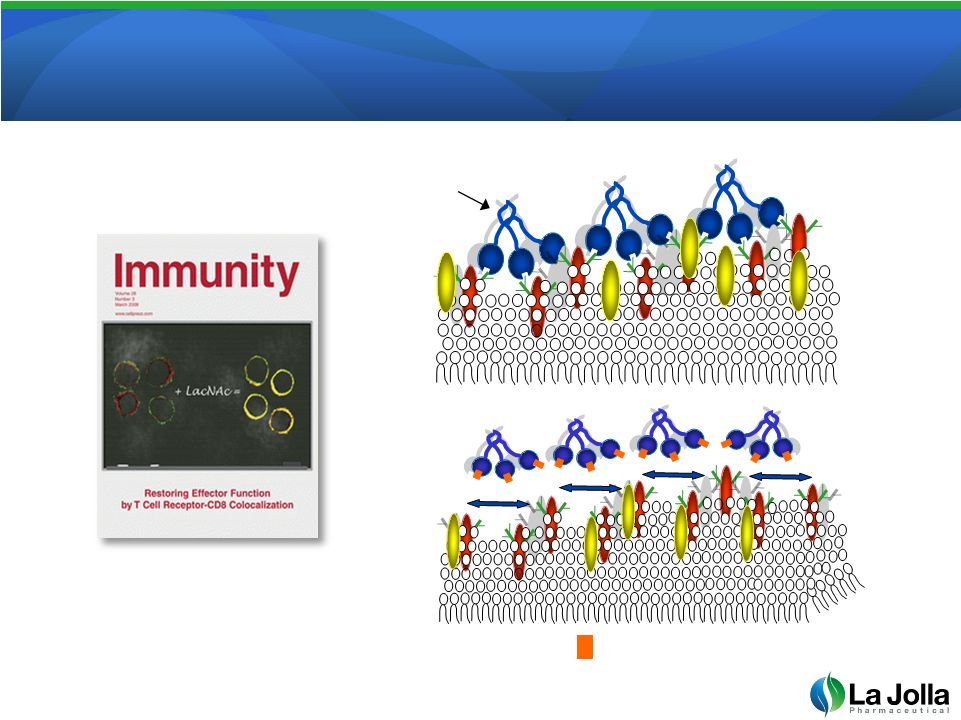 GCS-100 Reverses Galectin-3
T-Cell Suppression
14
Source: Pierre van der Bruggen, Ludwig Institute for Cancer Research
Galectin-3
GCS-100 |
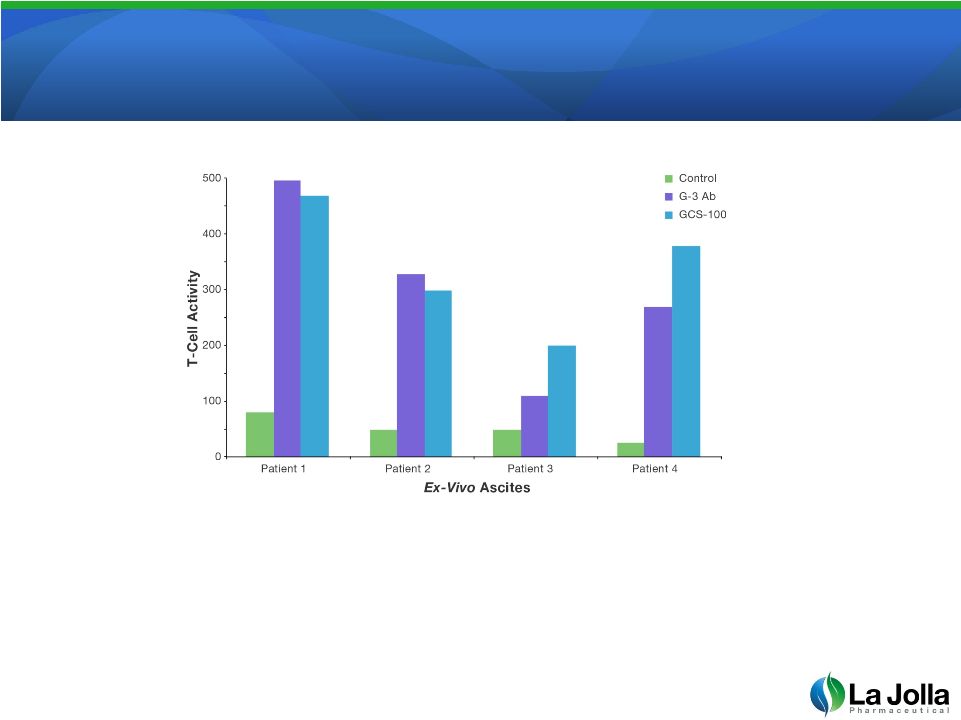 GCS-100 Reverses
Galectin-3 T-Cell Suppression
•
T-cells were collected from 4 ovarian cancer patients and tested for their
ability to kill the tumor (T-cell Activity). Untreated cells have little activity.
After treating the T-cells with either an antibody to galectin-3 (purple) or with
GCS-100 (blue), the T-cells regain their activity and ability to kill the tumor.
15
Source: Dermotte et al. A Galectin-3 Ligand Corrects the Impaired Function of Human
CD4 and CD8 Tumor-Infiltrating Lymphocytes and Favors Tumor Rejection in Mice, Cancer
Research, Oct 1, 2010 70 (19), pgs. 7476-7488 |
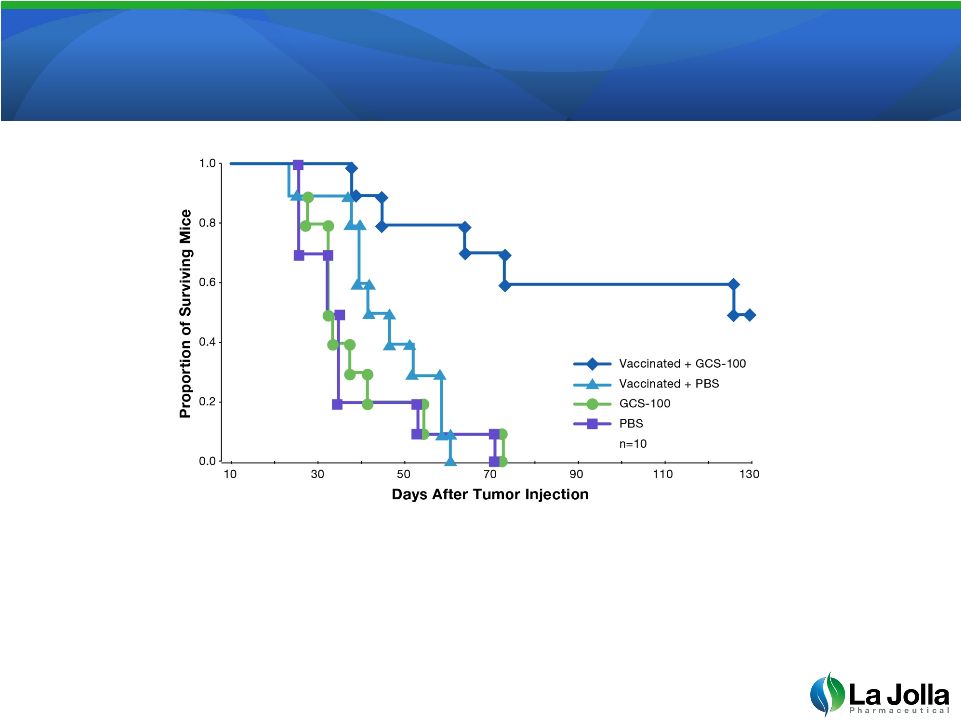 GCS-100 Improves Melanoma
GCS-100 Improves Melanoma
Cancer Vaccine
Cancer Vaccine
•
Animals treated with GCS-100 in addition to a vaccine against the tumor
live much longer than untreated animals or animals treated with vaccination
or GCS-100 alone
16
Source: Dermotte et al. A Galectin-3 Ligand Corrects the Impaired
Function of Human CD4 and CD8 Tumor-Infiltrating Lymphocytes and Favors
Tumor Rejection in Mice, Cancer Research, Oct 1, 2010 70 (19), pgs. 7476-7488 |
 GCS-100 Clinical Summary
GCS-100 Clinical Summary
•
>140 patients dosed in 9 Phase 1 and Phase 2 clinical trials
Significant clinical activity in multiple cancers, including chronic
lymphocytic leukemia (CLL), multiple myeloma (MM), renal cell
carcinoma
•
Pharmacokinetic parameters established
Single-
and
multiple-dose
administration
of
30-160
mg/m
²
–
Once daily for 5 consecutive days (21-day cycle)
–
Effective half-life of 36 hours
•
Demonstrated single-agent activity
•
Broad-based synergy in vitro with both chemotherapy and
other targeted agents
17 |
 GCS-100 Side
Effects Consistent with GCS-100 Side Effects Consistent with
Immune Modulation
Immune Modulation
•
Doses
up
to
160
mg/m
²
well
tolerated
in
24-patient
solid
tumor
study
No hematological toxicity
Only drug-related grade 3 AEs = rash (8%), arthralgia (8%) and myalgia
(4%), all consistent with immune effects
•
Dose-Limiting Toxicity
Grade 3 rash
•
Rash
Immune-mediated leucoclastic vasculitis
Responds to systemic steroid therapy
Not associated with additional organ involvement
Develops during, or within 1-2 days after completion of, dosing during a
treatment cycle
No clinical reports of skin desquamation, necrosis, or ulceration
Did not preclude additional treatment cycles with GCS-100
18 |
 Phase 2a
Single-Agent CLL Study: Design Phase 2a Single-Agent CLL Study: Design
•
Enrolled 24 patients with one or two prior therapies
Median age 67 years old; 15 patients > 67 years, 4 > 80 years
Prior therapies include Fludara-, Rituxan-
and chlorambucil-
containing regimens
•
Dosing regimen
160
mg/m
²
i.v.
daily
for
5
days
on
21-day
cycle
Regimen from Phase 1 solid tumor study
Short (~1 hour) infusion
No steroid prophylaxis
•
Primary objectives
Evaluate the short-term effect of GCS-100 on markers of cell death
Evaluate decreases in peripheral leukocyte count
19 |
 Phase 2a
Single-Agent CLL Study: Results Phase 2a Single-Agent CLL Study: Results
•
GCS-100 was well tolerated
Minimal hematologic toxicity
1 patient discontinued due
to grade 3 rash
No drug-related SAEs
•
In vivo evidence of caspase activation and apoptotic cell death
observed
1
•
Preliminary data indicate 6/24 patients (25%) achieved PR and
12/24
(50%)
achieved
SD,
for
a
75%
disease
control
rate
2
3 Patients >50% decrease in LN size
1 Patient >50% decrease in WBC with LN shrinkage
2 Patients >50% decrease in WBC
20
1
Cotter F. et al. 2008. 10th International Conference on Malignant Lymphoma
2
Cotter F. et al. 2009. 45th American Society of Clinical Oncology Annual Meeting
|
 GCS-100 Clinical Summary
GCS-100 Clinical Summary
•
Clinical activity in multiple cancers, including chronic
lymphocytic leukemia (CLL), multiple myeloma (MM), and
renal cell
•
Well tolerated and has low toxicity based on >140 patients
treated
•
Broad-based synergy in vitro with both chemotherapy and
other targeted agents
•
Single agent activity observed
•
Rash, arthralgias, myalgias and increase in neutrophil count
consistent with immune effects
21 |
 Next
Steps: Clinical Trial Rational Next Steps: Clinical Trial Rational
•
Galectin-3 is is increased in patients with ESRD and the level
correlates to overall survival
•
Galectin-3 knockout mice develop less kidney scar after injury
•
Patients with ESRD have no existing therapeutic options
outside of renal transplantation
•
ESRD patients have a high mortality rate and biomarkers
correlate with survival
Albumin
Galectin-3
Preservation of urine output
22 |
 Next
Steps: Clinical Trial Rational Next Steps: Clinical Trial Rational
•
GCS-100 has shown single-agent activity in cancer
•
Galectin-3 is is increased in patients with renal failure
•
Galectin-3 knockout mice develop less kidney scar after injury
•
Cancer therapies often cause kidney injury and scar formation
with reduced renal function
•
There are no approved therapies for treating renal insufficiency
in cancer patients
23 |
 Next
Steps: Phase 1/2 Study in Cancer Next Steps: Phase 1/2 Study in Cancer
Patients with Renal Insufficiency
Patients with Renal Insufficiency
•
Advanced-stage cancer patients (n=18)
•
GCS-100
alone
160
mg/m
2
weekly
•
Compare baseline to post-treatment:
Serum galectin-3
Renal function
Tumor response
Immune parameters
24 |
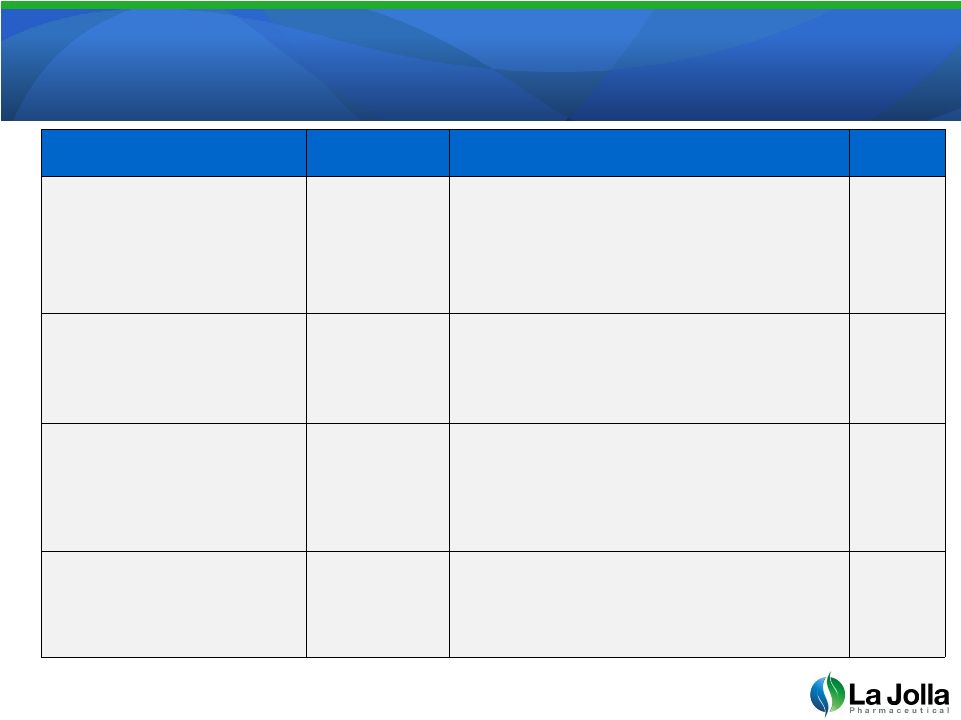 Intellectual Property
Position Intellectual Property Position
25
Title
Status
Coverage
Expiration
Modified Pectins,
Compositions and
Methods Related Thereto
Issued
US 8,128,966
Methods for making biologically active
modified or unmodified pectin using tangential
flow filtration having a molecular weight
between 10 and 250 kD.
2028
Modified Pectins,
Compositions and
Methods Related Thereto
Alllowed
US 13/357,325
Biologically active pectin (MW 10-250 kD)
from modified or unmodified pectin using
tangential flow filtration.
2025
Modified Pectins,
Compositions and
Methods Related Thereto
Pending
US 13/400,007
Highly bioactive forms of modified pectin and
methods of making modified pectin of high
molecular weights and/or lacking low
molecular weight materials
2025
Compositions and Uses
of Galectin Antagonists
Pending
US 11/803,150
Methods
for
reducing
the
rate
of
cancer
growth
by treating with a galectin inhibitor, such as
modified pectin, and a topoisomerase inhibitor
2027 |
 Corporate Highlights
Corporate Highlights
Technology
•
•
Pipeline
•
May prevent or reverse organ failure by mediating fibrosis via galectin-3
sequestration Binds galectin-3 and reverses T-cell suppression
Extensive clinical data with clear single agent activity and favorable safety
profile Management and Investors
•
Milestones
•
26
Immune therapy platform targeting, Galectin-3, an emerging key with a
demonstrated role in organ failure and cancer via immune regulation
Clear path to proof-of-concept
Product
candidate:
GCS-100
-
Leading,
clinical
stage
galectin-3
antagonist
Experienced and driven
Near-term, cost-efficient clinical and preclinical milestones to drive
value |
 Thank You |
