Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ZIOPHARM ONCOLOGY INC | v316370_8k.htm |
Exhibit 99.1

Jonathan Lewis, MD, PhD Chief Executive Officer 2012 Research & Development Day June 19, 2012

Forward - Looking Statements This presentation contains certain forward - looking information about ZIOPHARM Oncology that is intended to be covered by the safe harbor for “forward - looking statements” provided by the Private Securities Litigation Reform Act of 1995 , as amended . Forward - looking statements are statements that are not historical facts . Words such as “expect(s),” “feel(s),” “believe(s),” “will,” “may,” “anticipate(s)” and similar expressions are intended to identify forward - looking statements . These statements include, but are not limited to, statements regarding our ability to successfully develop and commercialize our therapeutic products ; our ability to expand our long - term business opportunities ; financial projections and estimates and their underlying assumptions ; and future performance . All of such statements are subject to certain risks and uncertainties, many of which are difficult to predict and generally beyond the control of the Company, that could cause actual results to differ materially from those expressed in, or implied or projected by, the forward - looking information and statements . These risks and uncertainties include, but are not limited to : whether any of our therapeutic candidates will advance further in the clinical trials process and whether and when, if at all, they will receive final approval from the U . S . Food and Drug Administration or equivalent foreign regulatory agencies and for which indications ; whether any of our therapeutic candidates will be successfully marketed if approved ; whether our DNA - based biotherapeutics discovery and development efforts will be successful ; our ability to achieve the results contemplated by our collaboration agreements ; the strength and enforceability of our intellectual property rights ; competition from pharmaceutical and biotechnology companies ; the development of and our ability to take advantage of the market for DNA - based biotherapeutics ; our ability to raise additional capital to fund our operations on terms acceptable to us ; general economic conditions ; and the other risk factors contained in our periodic and interim reports filed with the SEC including, but not limited to, our Annual Report on Form 10 - K for the fiscal year ended December 31 , 2011 , and our Quarterly Report on Form 10 - Q for the fiscal quarter ended March 31 , 2012 . Our audience is cautioned not to place undue reliance on these forward - looking statements that speak only as of the date hereof, and we do not undertake any obligation to revise and disseminate forward - looking statements to reflect events or circumstances after the date hereof, or to reflect the occurrence of or non - occurrence of any events .

Compound Preclinical Phase 1 Phase 2 Phase 3 Palifosfamide Soft Tissue Sarcoma Small Cell Lung Cancer Other Phase 2 Study Oral DNA Therapeutics Ad - IL12 DC - IL12 Other Candidates Indibulin Breast Cancer Darinaparsin Oral Data - Driven Oncology Portfolio

Our Guests – Wael Harb, MD, Chief Community Officer of the Hoosier Oncology Group, Director of Cancer Services and Clinical Research at Indiana University Health Arnett – Ramaswamy Govindan, MD, Head of the Thoracic Oncology Program, Co - Director of Medical Oncology, and Professor of Medicine, Oncology Division at Washington University School of Medicine – Robert Maki, MD, PhD, Chief of the Pediatric Hematology/Oncology Division, Medical Director of the Sarcoma Cancer Program, Tisch Cancer Institute at Mount Sinai Medical Center – Larry Norton, MD, Deputy Physician - in - Chief for Breast Cancer Programs, Memorial Sloan - Kettering Cancer Center, Medical Director, Breast Cancer Programs From ZIOPHARM – Hagop Youssoufian, MSc, MD, President of Research and Development and Chief Medical Officer – Mark Thornton, MD, PhD, Executive Vice President, Government Affairs, Health Policy and Advocacy and Chief Quality Compliance Officer Many Thanks

Palifosfamide Small Cell Lung Cancer Sarcoma DNA Therapeutics Rational Drug Development Program – Rational Design and Preclinical Activity – Phase 1 Bridging Study in SCLC – MATISSE: A Phase 3 Study – FDA, PFS & the Shifting Landscape in Sarcoma – The PICASSO 3 Study – AD - IL12 and DNA Therapeutics – Cancer Biology in the 21st Century: Translation in the Clinic

Palifosfamide

Biosynthesis & Metabolism 2 - and 3 - dechloroethylifosfamide + chloroacetaldehyde ALDH1A1 c hloroacetic acid m ainly CYP3A4/5 and CYP2B6 ifosfamide m ainly CYP3A4/5 4 - ketoifosfamide 4 - hydroxyifosfamide ADH 4 - thiofosfamide carboxyifosfamide alcoifosfamide 1,3, - oxazolidine - 2 - one aldofosfamide chloroethylamine IPM acrolein AKR1 GST GST ALDH1A1 ALDH3A1 HEMORRHAGIC CYSTITIS NEUROTOXICITY NEPHROTOXICITY IPM conjugate +

C H 2 N H P - O H O H N - C H 2 - C H 2 5 ' - X - G - X - C - X - 3 ' 3' - X - C - X - G - X - 5' C H 2 + +++++ ++ 5 ' - X - G - C - X - X - 3 ' 3 ' - X - C - G - X - X - 5 ' 5 ' - X - G - X - X - C - 3 ' 3 ' - X - C - X - X - G - 5 ' 5 ' - X - G - X - C - 3 ' 3 ' - X - C - X - G - 5 ' Palifosfamide Safety ~ Bypass Drug Resistance ~ Target Stem Cells Struck RF et al. Cancer Chemother Pharmacol (2000) 45: 59 - 62 MW 221.02 • Crosslinks DNA: – Preferentially targets 5 ’ - G - X - C - 3’ – Generates 7 - atom crosslinks • Impedes DNA replication

Rational Drug Development: Extensive Early Clinical Study Informed Late - Stage Programs Phase 1 Dose Phase 2 Proof - of - Concept in Advanced Disease Phase 1 with Doxorubicin Phase 2 Randomized Phase 3 Randomized, Double Blind, Placebo - Controlled (prior HOG Phase 3 positive + Ifosfamide) Phase 1 with Carboplatin/ Etoposide Adaptive Phase 3 Breast Pancreatic Prostate

Wael Harb, MD Small Cell Lung Cancer Rational Design and Preclinical Activity Phase 1 Bridging Study
Broad Application Effect in solid tumors and hematological malignancies Safety, Efficacy and Accessibility Active in ALDH hi cells, rapid on - off kinetics, less toxic and ease of administration Addressing Unmet Needs Orphan Drug status for STS in U.S. and Europe Long Commercial and Development Runway U.S. pharmaceutical composition patent rights extending to 2029; other pending applications WW A Novel DNA - Targeted Drug
Low Toxicity No neurotoxicity or bladder toxicity Minimal Patient - to Patient Variability On/off rapid kinetics minimize interpatient variability; target cancer Broad Activity Active in multiple tumor cell lines and human cancer xenografts, including sarcoma Activity in Resistant Tumors Bypasses several known mechanisms of drug resistance Active in ifosfamide - and cyclophosphamide - resistant tumor cell lines and xenografts Activity in ALDH (Cancer Stem Cells) Active in ALDH3A1 - overexpressing xenografts Preclinical Activity & Safety

ALDH is a validated marker of cancer stem cells ALDH+ cells are generally resistant to treatment and chemotherapy ALDH+ cells are readily detectable in multiple subtypes of epithelial cancer, bone marrow derived cancer and sarcoma SCLC direct patient xenografts have an abundant population of tumor cells that are ASCL1+/CD133+/ALDH1+ ALDH: Potential Impact on Cancer Stem Cells Hingorani P et al. Cancer Chemother Pharmacol (2009) 64: 733 - 40 Jiang T et al. Cancer Res (2009) 69: 845 - 54 Minna J. 12 th Annual Targeted Therapies of Lung Cancer Meeting (2012), IASLC, Santa Monica
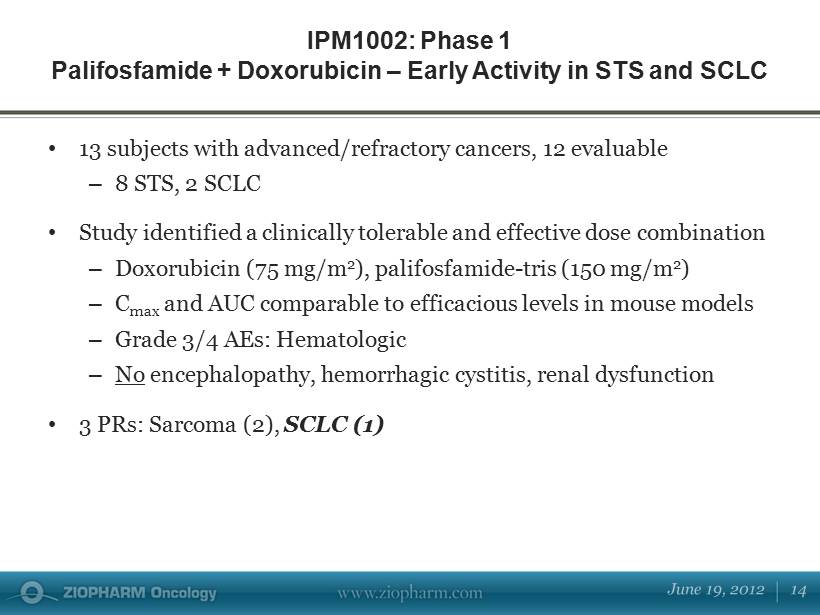
IPM1002 : Phase 1 Palifosfamide + Doxorubicin – Early Activity in STS and SCLC • 13 subjects with advanced/refractory cancers, 12 evaluable – 8 STS, 2 SCLC • Study identified a clinically tolerable and effective dose combination – D oxorubicin (75 mg/m 2 ), palifosfamide - tris (150 mg/m 2 ) – C max and AUC comparable to efficacious levels in mouse models – Grade 3/4 AEs: Hematologic – No encephalopathy, hemorrhagic cystitis, renal dysfunction • 3 PRs: Sarcoma (2), SCLC (1)
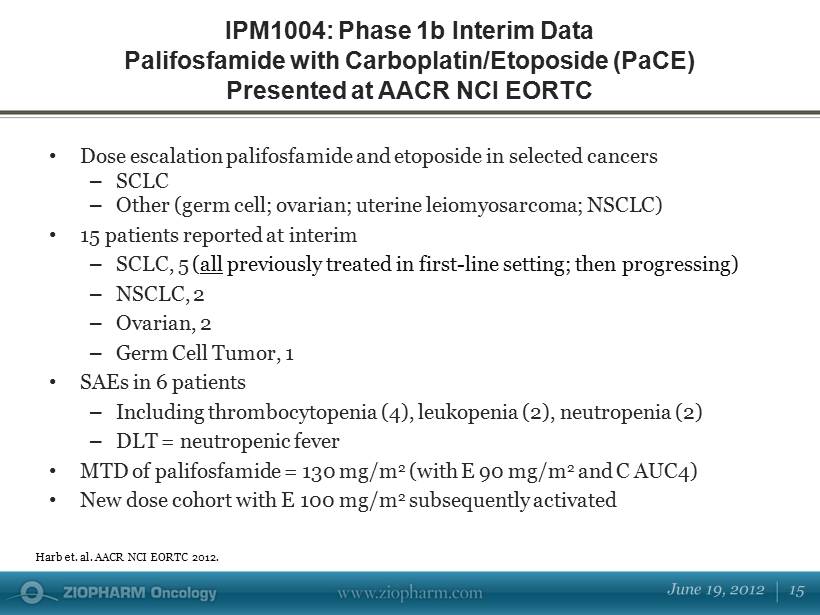
• Dose escalation palifosfamide and etoposide in selected cancers – SCLC – Other (germ cell; ovarian; uterine leiomyosarcoma; NSCLC) • 15 patients reported at interim – SCLC, 5 ( all previously treated in first - line setting; then progressing) – NSCLC, 2 – Ovarian, 2 – Germ Cell Tumor, 1 • SAEs in 6 patients – Including thrombocytopenia (4), leukopenia (2), neutropenia (2) – DLT = neutropenic fever • MTD of palifosfamide = 130 mg/m 2 (with E 90 mg/m 2 and C AUC4) • New dose cohort with E 100 mg/m 2 subsequently activated IPM1004: Phase 1b Interim Data Palifosfamide with Carboplatin/Etoposide (PaCE) Presented at AACR NCI EORTC Harb et. al. AACR NCI EORTC 2012.

• Normalization of tumor markers: ─ Previously treated ( including ifosfamide containing regimen – developed encephalopathy) ─ β HCG reduction: 2358 to 12 mU/Ml ─ Completed 4 cycles of therapy Rapid Clinical Activity: Primary Mediastinal Nonseminomatous Germ Cell Tumor 0 500 1000 1500 2000 2500 β HCG mU/Ml 1 mo. 2 mo. 3 mo. 4 mo. Harb et. al. AACR NCI EORTC 2012.

Updated Phase 1b Data Bridge to Adaptive Phase 3 (MATISSE) Clinical Benefit Rate = 67% Range of cycles 2 – 17+ Response Assessment 1 Patients N=18 Cancer Types PR 6 Germ Cell Tumor, NSCLC, Ovarian, SCLC (2), Uterine Leiomyosarcoma SD 7 Ovarian (2), NSCLC, SCLC (2), Squamous Cell, DFSP PD 5 Adenoc arcinoma , Carcinoid, Pancreatic, SCLC (2) 1 Response Assessment per RECIST 1.1 definitions and/or assessed per Biomarkers. Tumor Response

Ramaswamy Govindan, MD MATISSE: A Phase 3 Study in Extensive - Stage Small Cell Lung Cancer

• Significant unmet medical need in cancer with no new front - line therapies in decades – Approximately 15% of lung cancers – 30,000 - 35,000 U.S. incidence/200,000 worldwide 1 (China annual incidence alone growing to >150,000 2 ) • Platinum plus etoposide SOC front - line; concurrent w/radiation in limited stage; only topotecan approved second - line Small Cell Lung Cancer (SCLC) 1 SEER, Globocon. 2 Derived from: Liu et. al. Emerging tobacco hazards in China: 1. Retrospective proportional mortality study of one million deaths. BMJ. 1998;317:1411.

Contemporary Phase 3 Trials in 1 st Line ES - SCLC: Ifosfamide – Only Positive Study Study Regimen Results Irinotecan Lara PN et al. J Clin Oncol 2009;27:2530 Cisplatin + etoposide vs cisplatin + IRINOTECAN No difference in PFS or OS Topotecan Eckardt JR et al. J Clin Oncol 2006;24:2044 TOPOTECAN + cisplatin vs cisplatin + etoposide No difference in RR or OS Bevacizumab Intergroupe Francophone de Cancerologie Thoracique Chemotherapy (including cisplatin + etoposide ) ± BEVACIZUMAB Suspended Paclitaxel Niell HB et al. J Clin Oncol 2005;23:3752 Cisplatin + e toposide ± PACLITAXEL No difference in OS ; tx - related mortality higher w/paclitaxel Pemetrexed Socinski MA et al. J Clin Oncol 2009;27:4787 PEMETREXED + carboplatin vs carboplatin + etoposide No difference in PFS or OS Ifosfamide Loehrer PJ et al. J Clin Oncol 1995;13:2594. (Hoosier Oncology Group) Cisplatin + etoposide ± IFOSFAMIDE Significant survival advantage for ifosfamide

Cisplatin plus etoposide with or without ifosfamide in extensive - stage small - cell lung cancer : Hoosier Oncology Group - Einhorn et. al. VIP regimen: etoposide (75 mg/m 2 ), ifosfamide (1.2 g/m 2 ), cisplatin (20 mg/m 2 ) - days 1 to 4 x four cycles. » Statistically significant difference in median survival 7.3 months vs 9.0 months (P=.045) » 2 - year survival 5% vs 13 % » Difference in time to progression statistically different (P = .039) • Excess toxicity: grade 3/4 myelotoxicity , neurotoxicity, pneumonitis • In - patient therapy, inconvenience despite efficacy, NEVER pursued as Rx Efficacy Rationale: Positive Phase 3 Randomized Ifosfamide Study in SCLC

The MATISSE Study: M ulticenter A daptive T rial I nvestigating S mall Cell Lung Cancer S urvival E ndpoints • A Multi - center, Open - Label, Adaptive, Randomized Study of Palifosfamide, a Novel DNA Cross - linker , in Combination with Carboplatin and Etoposide (PaCE) Chemotherapy versus Carboplatin and Etoposide (CE) Alone in Patients with Extensive - Stage Small Cell Lung Cancer who have not received initial chemotherapy Phase 3 in SCLC (“MATISSE”)

Design Multinational, multi - center, randomized controlled, open - label, adaptive trial N Up to 548 subjects Population Males and females, age ≥18 years, with extensive - stage small cell lung cancer Primary Endpoint Overall survival (OS) Secondary Endpoints Progression - free survival (PFS) Objective response rate (ORR) Quality of Life (QOL) Disease related symptoms MATISSE Study Design

Adaptive Clinical Trial Design: • Prospectively planned opportunity to modify the study by adjusting specified aspects of the study design and/or hypotheses • Adjustments based on analysis of real time data (usually interim data) from subjects in the study • Analyses of accumulating study data performed at prospectively planned time points clearly defined in the study protocol • C an occur with or without formal statistical hypothesis testing F DA Guidance on Adaptive Clinical Trial Design Conventional Trial Design: • Fixed sample size • Does not have any adaptive elements or characteristics

MATISSE Adaptive Trial Design Single pre - planned evaluation by the IDMC • Optimize chances of success • Stop trial for efficacy/futility • Complete trial as planned or resize study 125 Events Continue trial with decreased sample size Complete trial as planned Continue trial with increased event size

• Rationale: – Ifosfamide has been only front - line therapy to show survival benefit added to SOC… but with excessive toxicity , never adopted – High mutant variability in SCLC necessitates a treatment with broad DNA, multi - genomic, cancer stem cell activity – Palifosfamide added to SOC demonstrating activity in refractory, progressing patients… combination well tolerated Palifosfamide: Rational Development in High Unmet Medical Need

Mark Thornton, MD, PhD Sarcoma FDA, PFS & the Shifting Landscape
• A PFS primary endpoint in Phase 3 registration trials has undergone pendulum swings over time, and is now in favor: – In 2011, four products were approved based on PFS as primary trial endpoint – In 2012, pazopanib (Votrient ® ) became the 5 th precedent, and the first for STS • Rajeshwari Sridhara, PhD, Deputy Director, DBV, at FDA in 2012: PFS is considered clinical benefit in certain circumstances, and therefore meets requirement to grant full approval under FDA flexibility standards and practices The Changing Landscape of PFS as a Primary Endpoint

• Criteria for PFS (Sridhara, May 18, 2012): – Rare cancer – Large magnitude of benefit (“months not weeks”) – High quality data – No negative impact/trend on OS – Favorable risk/benefit • Note that setting (1 st line, 2 nd line) is not a factor in applying the policy. Rare is rare. – However, for a lead indication, sufficient numbers in safety database will be necessary “PFS as Primary Endpoint Is Here to Stay” Criteria for Full Approval Based on PFS
• PDUFA reauthorization cycle coincides with intensive reform lobbying by advocacy community – PDUFA will mandate FDA: • Listen to patient input regarding acceptable risk/benefit • Show evidence of flexibility in rare disease settings • Enhance use of accelerated approval beyond HIV/oncology • Relevance to PFS – FDA’s OHOP is leading the way on demonstrating increased flexibility on endpoints for rare cancers – Full approval of pazopanib for STS is type of evidence of flexibility that Congress is looking for – United sarcoma patient community showing FDA support Impact of Rare Disease Advocates on PDUFA

• Current policy is clear by both precedent, as well as by recent public comments from senior FDA officials • Pazopanib full approval for STS confirms sound strategy of PFS as primary endpoint for palifosfamide to attain FDA approval as lead indication in front - line STS • PFS is appropriate primary endpoint for STS (rare cancer) • Encouraging data to date (safety/efficacy), as well as favorable regulatory landscape, indicates palifosfamide optimized to meet other key criteria of PFS - based approval Relevance to Regulatory Strategy for Palifosfamide in STS

Robert Maki, MD, PhD The PICASSO 3 Study

Soft Tissue Sarcoma $ in Millions • Currently over 100,000 people diagnosed with STS worldwide; estimated metastatic front - line of 23,000 people for U.S. and Europe* – Estimated 9,000 treated for front - line metastatic disease in U.S., target indication – Estimated 14,000 being treated in Europe • Doxorubicin alone, doxorubicin - based therapy currently SOC in front - line setting; NCCN recommends clinical trial participation whenever possible • March 2012 ODAC; April 2012 FDA full approval of Votrient ® validate PFS endpoint; May 2012 positive opinion from European CHMP * Source: U.S.: IntrinsiQ Data, © Copyright 2012, IntrinsiQ, LLC an AmerisourceBergen Specialty Group company. All rights reser ved; remainder of world Company estimated from epidemiology (SEER, NCCN).

IPM2001: Phase 1/2 (Palifosfamide Monotherapy) Effect on Tumor Mass Presented at the Liddy Schriver Sarcoma Initiative, 2009 Refractory L iposarcoma Baseline 12 Weeks 13.9 x 11.2 cm 9.0 x 7.0 cm
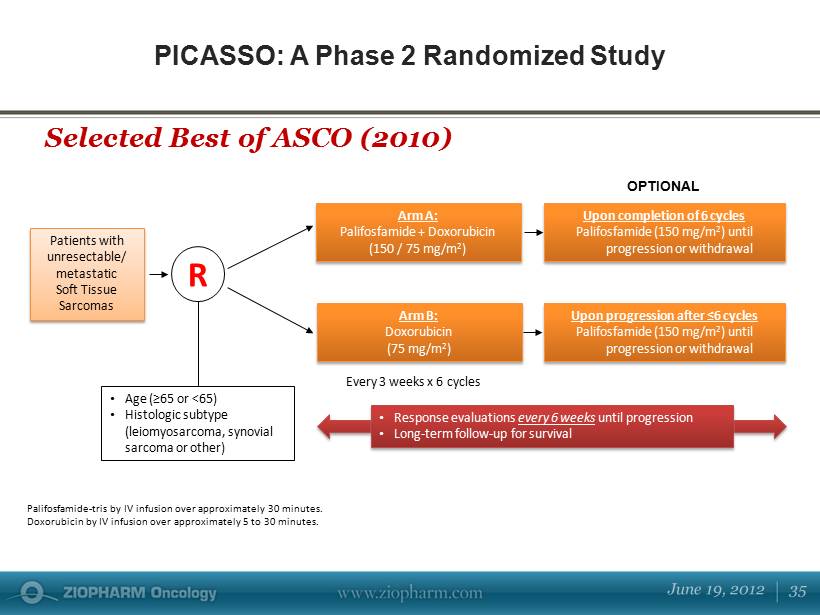
PICASSO: A Phase 2 Randomized Study Selected Best of ASCO (2010) Patients with unresectable/ metastatic Soft Tissue Sarcomas Arm B: Doxorubicin (75 mg/m 2 ) • Age (≥65 or <65) • Histologic subtype (leiomyosarcoma, synovial sarcoma or other) E very 3 weeks x 6 cycles U pon completion of 6 cycles Palifosfamide (150 mg/m 2 ) until progression or withdrawal R U pon progression after ≤6 cycles Palifosfamide (150 mg/m 2 ) until progression or withdrawal Arm A: Palifosfamide + Doxorubicin (150 / 75 mg/m 2 ) Palifosfamide - tris by IV infusion over approximately 30 minutes. Doxorubicin by IV infusion over approximately 5 to 30 minutes. OPTIONAL • Response evaluations every 6 weeks until progression • Long - term follow - up for survival
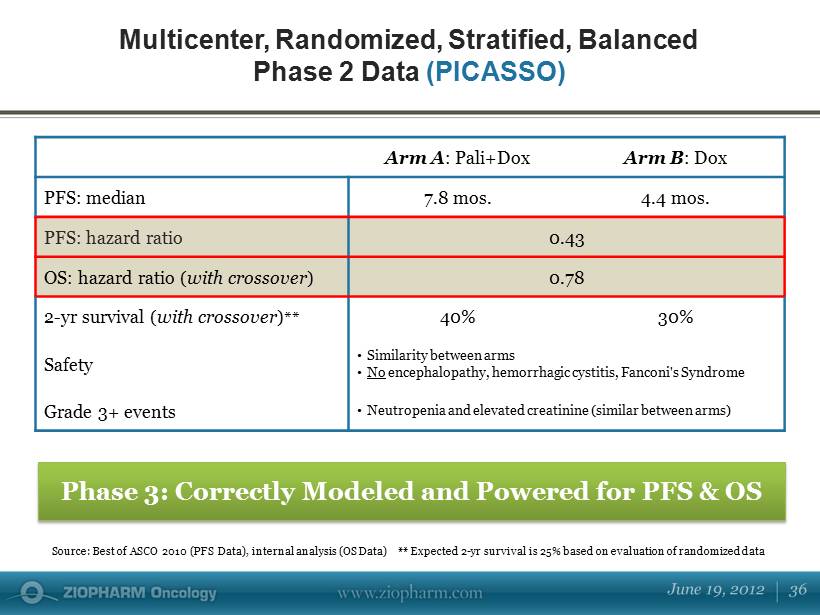
Arm A : Pali+Dox Arm B : Dox PFS: median 7.8 mos. 4.4 mos. PFS: hazard ratio 0.43 OS: hazard ratio ( with crossover ) 0.78 2 - yr survival ( with crossover ) ** 40% 30% Safety • Similarity between arms • No encephalopathy, hemorrhagic cystitis, Fanconi's Syndrome Grade 3+ events • Neutropenia and elevated creatinine (similar between arms) Multicenter, Randomized, Stratified, Balanced Phase 2 Data (PICASSO) Phase 3: Correctly Modeled and Powered for PFS & OS Source: Best of ASCO 2010 (PFS Data), internal analysis (OS Data) ** Expected 2 - yr survival is 25% based on evaluation of ran domized data

Phase 3 Structure ( PICASSO 3) N : Approximately 424 patients; front - line metastatic STS Regimen: Palifosfamide + doxorubicin vs doxorubicin + placebo Primary Endpoint: PFS for accelerated approval; OS for full approval Powered for PFS & OS PFS Power: 85% power to detect 0.60 HR, 3 month median Δ (p=0.0005, one - tailed) PFS Analysis: Evaluation of PFS by IDMC following a pre - determined number of PFS events Study sites: > 150 centers worldwide Results: Pivotal PFS data expected 2H 2012 Design Based on Successful Randomized Phase 2 Study with Similar Design

PICASSO 3 Study Schema R A N D O M I Z E 1:1 Palifosfamide 150 mg/m 2 d1,2,3 + Doxorubicin 75 mg/m 2 d1 q 21 d x 6 cycles Histology (LMS, SS, other) A ge (<65 vs >=65) Placebo d1,2,3 + Doxorubicin 75 mg/m 2 d1 • Frontline metastatic STS (central review of histology) • Neo/adjuvant Gemzar ® and Taxotere ® allowed for non - metastatic • Measurable disease • Not eligible: alveolar soft - part sarcoma, chondrosarcoma, dermatofibrosarcoma, EWS, GIST, KS, mixed mesodermal tumor/ carcinosarcoma, osteo, radiation - induced sarcoma, purely low grade • LVEF ≥50%

• High unmet medical need with no new approved treatments in over 30 years in the front - line setting • April 2012 FDA full approval of Votrient ® validate PFS endpoint • Objective of palifosfamide in combination with doxorubicin as standard of care for front - line metastatic STS • PICASSO 3 results highly anticipated Palifosfamide for Front - Line Soft Tissue Sarcoma

DNA Therapeutics

• A revolutionary technology for the precise, controlled delivery of therapeutic proteins in vivo • Lead therapeutic / early validation of the platform – IL - 12 is a prime target for a controlled release approach – IL - 12 is an increasingly compelling target to big pharma • “Next - wave” of therapeutic approaches in the research pipeline (antibody technology, protein - protein decoys, immunotoxins, etc.) • Focused, disciplined approach to development will minimize expense while driving value through near - term proof - of - concept studies DNA Therapeutics

Hagop Youssoufian, MD AD - IL12 and DNA Therapeutics
Leverage the power of DNA technology to build novel therapeutic products • Precision: control over biologic drug distribution (local/systemic) • Controlled: in vivo control of dosing through first in - human biological on/off switch (RheoSwitch Therapeutic System ® ) • Adaptable: multiple therapeutic protein approaches DNA Therapeutics: Capabilities
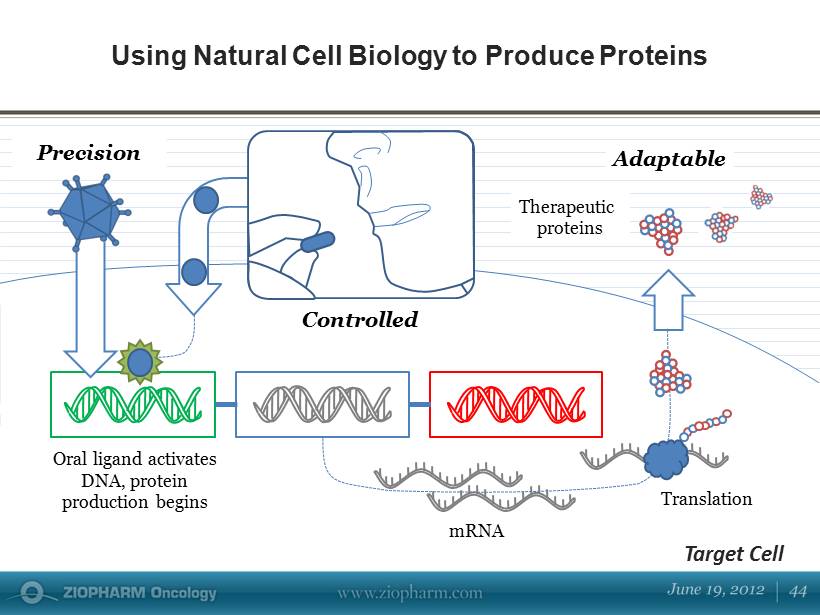
Using Natural Cell Biology to Produce Proteins Controlled mRNA Oral ligand activates DNA, protein production begins Therapeutic proteins Target Cell Precision Translation Adaptable

• IL12 – A renewed interest in an well characterized, naturally occurring cytokine – Activity alone and in combination • Indications of interest – Melanoma – Breast cancer – Head and neck cancer IL - 12 DNA

IL12: Foundation for Immunotherapy • Preserve anti - tumor potency • Deliver safely IL12 Remodel Tumor Environment Antiangiogenic Enhance Tumor Immunity

Two Clinical Stage Product Candidates • DC - IL12 Phase 1b in metastatic melanoma – Safety profile predictable – 2 PRs, 2 SDs, biomarker effects • Ad - IL12 Phase 1b in metastatic melanoma – S afe to date – Preliminary activity – Significant data expected 2H 2012 P ost - treatment Pre - treatment CT PET Baseline Necrosis prior to cycle 2

Next Phase of Development: • Remains a significant unmet need in metastatic melanoma despite evolving landscape • Phase 2 to begin at confirmation of biologically effective dose • Phase 2 modifications to include additional patient - selection criteria to maximize signal detection • Planned to be completed 1H 2013 Phase 1/2 of Ad - IL12 + AL in Melanoma

Additional Clinical and Preclinical Directions: Breast Cancer • Significant unmet medical need • Potential for multiple approaches to treatment: - Ad - IL12 monotherapy - Ad - IL12 in combination with palifosfamide (novel - novel strategy) • Study design under evaluation

Additional Clinical and Preclinical Directions: Head and Neck Cancer + Erbitux ® • Head and neck cancer ─ Significant unmet medical need ─ Ad - IL12 in combination with Erbitux ® ─ May lead to a pivotal study ─ Various paths forward

Conclusions • IL12 - based intratumoral therapy has yielded initial proof - of - concept and appears to be well tolerated • Melanoma to be pursued as lead indication - Protocol discussions with FDA to establish and maintain alignment and clarity toward an approval path • Head - and - neck and breast cancer opportunities being considered

Larry Norton, MD Cancer Biology in the 21 st Century: Translation in the Clinic

Tycho Brahe 1546 - 1601 Johannes Kepler 1571 - 1630

But Ki67 (Mitotic Fraction) is Not Greater than in Non - Metastatic Lines 0 100 200 300 400 500 600 700 800 0 10 20 30 40 50 4175 (n=9) 1834 (n=10) 1833 (n=5) Parental (n=5) Tumor volume (mm 3 ) Days post - injection Growth in Mammary Fat Pad Minn , Gupta et al., Nature 2005 4175 1833

The Self - Seeding Model of Epithelial Cancer Norton, Massagué: Nature Med 2006

0.00000001 0.000001 0.0001 0.01 1 100 0 20 40 60 80 100 dN t / dt = k* N t a /c N t b /c Arbitrary (Arithmetic) Time Scale Cubic Centimeters Self - Seeding Explains Gompertzian Growth Bigger Tumor = Lower Surface to Volume Ratio = Slower Growth at Surface Smaller Tumor = Greater Surface to Volume Ratio = Faster Growth at Surface Growth from Outside In Creates a Stellate Pattern Norton L et al. , Nature, 1976

dN t / dt = k* N t a /c N t b /c

Jonathan Lewis, MD, PhD In Conclusion…

Compound Preclinical Phase 1 Phase 2 Phase 3 Palifosfamide Soft Tissue Sarcoma Small Cell Lung Cancer Other Phase 2 Study Oral DNA Therapeutics Ad - IL12 DC - IL12 Other Candidates Indibulin Breast Cancer Darinaparsin Oral Data - Driven Oncology Portfolio

Two Pivotal Programs Representing Significant, Global Market Potential Blended EU/US Price Per Patient STS Patients Treated $ 30,000 $ 40,000 $ 50,000 Soft Tissue Sarcoma 9,000 $ 270 $ 360 $ 450 10,000 $ 300 $ 400 $ 500 11,000 $ 330 $ 440 $ 550 Small Cell Lung Cancer 14,000 $ 420 $ 560 $ 700 16,000 $ 480 $ 640 $ 800 18,000 $ 540 $ 720 $ 900 Asia/Latin America Opportunity: +$600 – $900 M (sales in millions) * Source: Based on Company projections. >$1 Bn total market potential

IL - 12 DNA Program Phase 1 Data 2012 Phase 2 Melanoma 2H 2012 New DNA Candidates • Preclinical Data 2012 Palifosfamide and DNA Therapeutics: 2012 Anticipated Key Clinical Milestones Palifosfamide DNA Therapeutics PICASSO 3 STS Pivotal PFS Data 2H 2012 MATISSE SCLC Phase 3 Initiation 2Q 2012

• Primary shares outstanding: approximately 79.5MM • Cash: approximately $ 128MM @ 3/31/12 Balance Sheet Support Through Key Milestones

An intelligent portfolio of therapeutics addressing unmet medical needs in cancer • Palifosfamide – Wholly - owned asset – Near - term, Phase 3 study results in STS – Phase 3 for SCLC now enrolling – Potential in multiple solid and hematological tumors – >$1 Bn total market potential in two indications alone • DNA therapeutics targeting established treatment pathway into Phase 2 study – Exploring multiple avenues for revolutionary treatment modality • Balance sheet support through multiple key milestones ZIOPHARM Highlights

Better Cancer Medicine Questions
