Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a12-14721_18k.htm |
Exhibit 99.1
POSTER NO. 1188
Fluticasone furoate and vilanterol suppress allergen-induced bronchial hyper-responsiveness to methacholine
Oliver A(1), Quinn D(2), Saggu P(1), Thomas P(3), Lötvall J(4), Bjermer L(5)*
(1)GlaxoSmithKline Respiratory and Immuno-Inflammation Medicines Development Centre, Uxbridge, UK; (2)P3 Research, Wellington, NZ; (3)Faculty of Medicine, The University of New South Wales, Sydney NSW 2052, Australia; (4)Krefting Research Centre, University of Gothenburg, Gothenburg, Sweden; (5)Department of Respiratory Medicine and Allergology, Institute for Clinical Science, Lund, Sweden, *Presenting Author
INTRODUCTION
· A high proportion of people with asthma are affected by airborne allergens.(1)
· Allergen exposure may lead to a biphasic decline in lung function consisting of the early asthmatic response (EAR) and the late asthmatic response (LAR); the latter is associated with the development of airway hyper-responsiveness (AHR).(2)
· Fluticasone furoate (FF)(3) and vilanterol trifenatate (VI)(4) are promising agents for a combined, long-acting, once-daily treatment of asthma.
OBJECTIVES
· Primary: to compare the effect of FF/VI combination on EAR (vs FF or VI monotherapy) and LAR (vs placebo).
· Secondary: to compare the effects of treatments on AHR.
METHODS
· Randomised, double-blind, 4-way crossover study
· 21 days treatment administered in the morning via a novel dry powder inhaler (Figure 1).
Figure 1. Study design
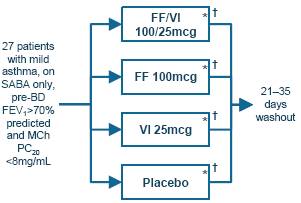
* Allergen challenge on Day 21, 1h post-final dose
† Assessment of AHR on Day 22, 24h post-allergen challenge (25h post-dose) using doubling concentrations of methacholine to induce a 20% fall in forced expiratory volume in 1s (FEV1) (PC20)
RESULTS
Study population and demographics
· Baseline characteristics of study participants are outlined in Table 1.
· Of the 27 patients randomised, one withdrew consent and four protocol deviations during treatment period 1 led to those data being excluded from the analysis for that treatment period.
Pre-challenge lung function
· FEV1 improved from Day 1 to Day 21 with FF/VI, FF and VI by 230mL (95% CI: 145, 315), 116mL (30, 202) and 183mL (95, 272) respectively. With placebo, FEV1 declined by 61mL (-147, 24).
Figure 2. Methacholine challenge treatment differences (PC20) performed 25h post-dose and 24h following an inhaled allergen challenge
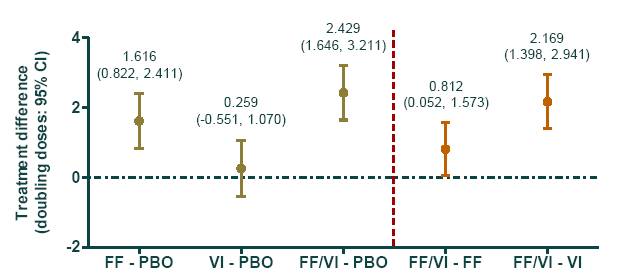
Table 1. Baseline characteristics
Demographics
|
Mean age, |
|
30.8 |
|
years (range) |
|
(18-49) |
|
Female, % |
|
30 |
|
Mean BMI, |
|
25.5 |
|
kg/m2 (range) |
|
(19.2-35.0) |
|
White race, % |
|
93 |
Lung function
|
Mean pre-bronchodilator FEV1, L |
|
3.7 |
|
(range) |
|
(2.7-5.0) |
|
Mean pre-bronchodilator FEV1 |
|
92.3 |
|
% pred. (range) |
|
(71.3-119.8) |
|
Methacholine PC20, mg/mL |
|
<8 |
Allergen, n (%)
|
House dust mite |
|
15 (56) |
|
Cat hair/dander |
|
10 (37) |
|
Birch tree |
|
1 (4) |
|
Grass pollen |
|
1 (4) |
BMI = body mass index
Allergen challenge (EAR/LAR)
· At all time points assessed, FF/VI exhibited the greatest attenuation of the allergen-induced response; the LAR to allergen challenge was significantly reduced with all active treatments, while the EAR was significantly reduced by FF/VI and FF, relative to placebo.
AHR
· 25h post-dose FF alone and combined with VI significantly reduced AHR vs placebo (Figure 2).
· Combination therapy with FF/VI was superior to monotherapy with FF or VI alone (Figure 2).
Safety
· No serious adverse events or withdrawals were reported.
Safety cont’d.
· On-treatment, treatment-related adverse events occurring in >2 patients are listed in Table 2.
Table 2. Treatment-related adverse events
|
|
|
|
|
|
|
|
|
FF/VI |
|
|
|
|
PBO |
|
FF 100 |
|
VI 25 |
|
100/25 |
|
|
n (%) |
|
(n=27) |
|
(n=27) |
|
(n=27) |
|
(n=27) |
|
|
Any AE |
|
7 (26) |
|
5 (19) |
|
4 (15) |
|
6 (22) |
|
|
Headache |
|
4 (15) |
|
1 (4) |
|
2 (8) |
|
4 (15) |
|
|
Oral candidiasis |
|
2 (7) |
|
0 |
|
0 |
|
0 |
|
|
Oropharyngeal pain |
|
1 (4) |
|
1 (4) |
|
0 |
|
2 (7) |
|
|
Throat irritation |
|
0 |
|
1 (4) |
|
1 (4) |
|
0 |
|
CONCLUSION
· FF/VI provides significant protection from allergen-induced airway hyper-responsiveness shown by an increase in PC20 methacholine at 25h post-dose, compared with placebo and FF and VI alone.
REFERENCES
(1) Lötvall J, et al. J Allergy Clin Immunol 2011;127:355–360.
(2) O’Byrne PM. Allergy Asthma Immunol Rev 2009;1:3–9.
(3) Woodcock A, et al. Respir Res 2011;12:160.
(4) Lötvall J, et al. Eur Respir J 2012 [Epub ahead of print].
ACKNOWLEDGEMENTS
· The presenting author, Dr L Bjermer, declares the following real or perceived conflicts of interest during the last 3 years in relation to this presentation: received honoraria for speaking and consulting and/or financial support for attending meetings from Almirall, AstraZeneca, Airsonette, Andre Pharma, Boehringer Ingelheim, GlaxoSmithKline, Merck, Mundipharma, Nigaard, Novartis, Nycomed/Takeda and Orion Pharma.
· This study was funded by GlaxoSmithKline; GSK Study Code HZA113126, Clinicaltrials.gov NCT01128595.
· Editorial support was provided by Geoff Weller, PhD, at Gardiner-Caldwell Communications; this support was funded by GlaxoSmithKline.

Presented at the European Academy of Allergy and Clinical Immunology Congress 2012 Geneva, Switzerland, 16–20 June, 2012
