Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AIkido Pharma Inc. | a12-14872_18k.htm |
Exhibit 99.1: Spherix Incorporated Overview Presentation, June 18, 2012
Exhibit 99.1
|
|
Spherix Incorporated (NASDAQ: SPEX) June 18, 2012 |
|
|
Forward-Looking Statements This presentation contains forward-looking statements made pursuant to provisions of Section 21E of the Securities Exchange Act of 1934. Investors are cautioned that such statements, including statements relating to planned clinical study design, regulatory and business strategies, plans and objectives of management and growth opportunities for existing or proposed products, constitute forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those anticipated by the forward-looking statements. The risks and uncertainties include, without limitation, risks that product candidates may fail in the clinic or may not be successfully marketed or manufactured, we may lack financial resources to complete development of our products, the FDA may interpret the results of studies differently than us, competing products may be more successful, demand for new pharmaceutical products may decrease, the biopharmaceutical industry may experience negative market trends, our continuing efforts to develop products may be unsuccessful, our common stock could be delisted from the Nasdaq Capital Market, and other risks and challenges detailed in our filings with the U.S. Securities and Exchange Commission. You are cautioned not to place undue reliance on any forward-looking statements that speak only as of the date of this presentation. We undertake no obligation to publicly release the results of any revisions to these forward-looking statements that may be made to reflect events or circumstances that occur after the date of this presentation or to reflect the occurrence of unanticipated events. |
|
|
Overview Spherix operates two subsidiaries: Biospherics – Pharmaceutical development with pipeline products for: Lowering triglycerides Lowering cholesterol Metabolic syndrome Aortic aneurysm Hunner’s ulcers and other painful bladder syndrome sub-populations |
|
|
Overview Spherix operates two subsidiaries: Spherix Consulting – Scientific and regulatory consulting on food and drug approvals for clients worldwide: Expertise in scientific and technical aspects of drug safety and efficacy Innovative approach provides capital efficiency and institutional memory that benefits Biospherics R&D With consulting expertise, Spherix validates its methods and models more effectively than similar companies through the increased science flow |
|
|
Transition from an asset centric strategy to a product development engine. Employed by Spherix to model complex metabolic disease pathways, testing potential binary therapies in simulations at various combinations of two points in the pathways, dynamic data-driven application simulation (DDDAS) chooses the most effective pair-wise combinations. Implemented a combination-drug discovery platform based on a DDDAS approach. Business Strategy |
|
|
Develop combination therapies for complex diseases: Currently in metabolic syndrome, a multifactorial disease with no good therapies Looking to expand into a new therapeutic area, another multifactorial disease with no good therapies In addition to developing its own combination drugs, Spherix is looking to in-license Phase 1 and Phase 2 assets. Business Strategy |
|
|
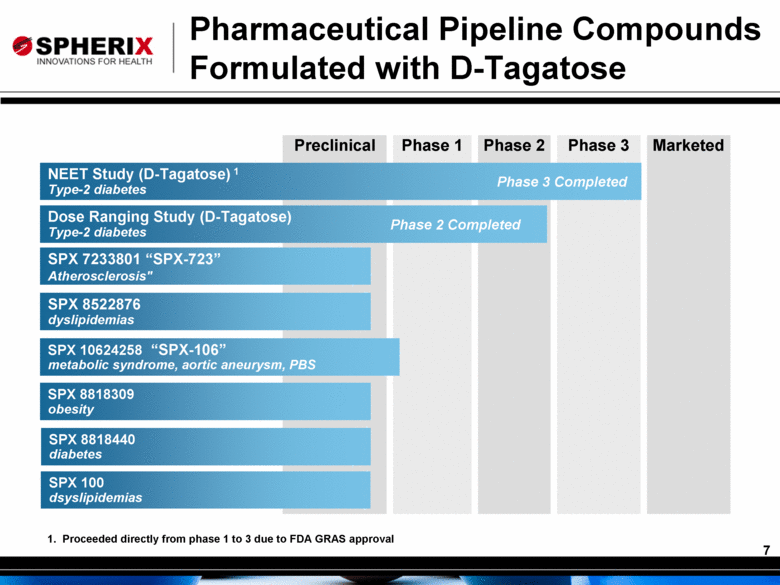
Preclinical Phase 1 Phase 2 Phase 3 Marketed Pharmaceutical Pipeline Compounds Formulated with D-Tagatose SPX 8522876 dyslipidemias Dose Ranging Study (D-Tagatose) Type-2 diabetes Phase 2 Completed SPX 7233801 “SPX-723” Atherosclerosis" SPX 10624258 metabolic syndrome, aortic aneurysm, PBS SPX 8818309 obesity SPX 8818440 diabetes NEET Study (D-Tagatose) 1 Type-2 diabetes Phase 3 Completed 1. Proceeded directly from phase 1 to 3 due to FDA GRAS approval “SPX-106” SPX 100 dsyslipidemias |
|
|
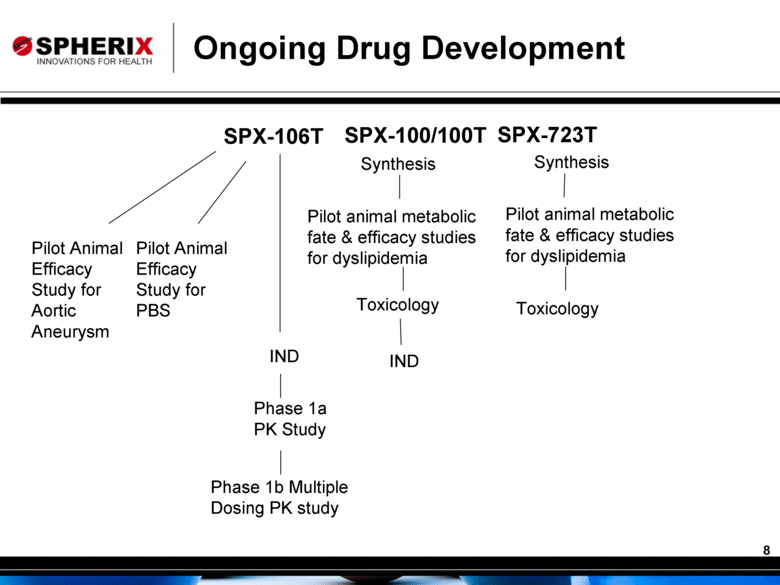
Ongoing Drug Development SPX-106T SPX-100/100T IND IND Phase 1a PK Study Phase 1b Multiple Dosing PK study Synthesis Pilot animal metabolic fate & efficacy studies for dyslipidemia Toxicology SPX-723T Pilot animal metabolic fate & efficacy studies for dyslipidemia Synthesis Toxicology Pilot Animal Efficacy Study for Aortic Aneurysm Pilot Animal Efficacy Study for PBS |
|
|
Investment Highlights SPX-106 and D-tagatose show promise in multiple clinical applications Hypertriglyceridemia (Dyslipidemia) ($26 billion treatment market) Preclinical and Phase 2 data show significant lowering of triglycerides Clinical development program initiated Cash position sufficient to fund current development plan Phase 3 study demonstrated statistically significant reductions in HbA1c in patients with mild Type 2 diabetes Company expertise well positioned to pursue specific new drug opportunities. |
|
|
In-Licensing for the Pipeline Biospherics’ goal to diversify pipeline by licensing in new drug candidates The Company has preclinical cardiovascular and metabolic drug candidates licensed from UKRF The Company is seeking opportunities for: Orphan drugs: Developed specifically to treat a rare medical condition 7 years of data exclusivity in addition to patent life in US In the US and EU, may be easier to gain marketing approval For indications such as familial hypertriglyceridemia, Hunner’s ulcers and other PBS sub-populations |
|
|
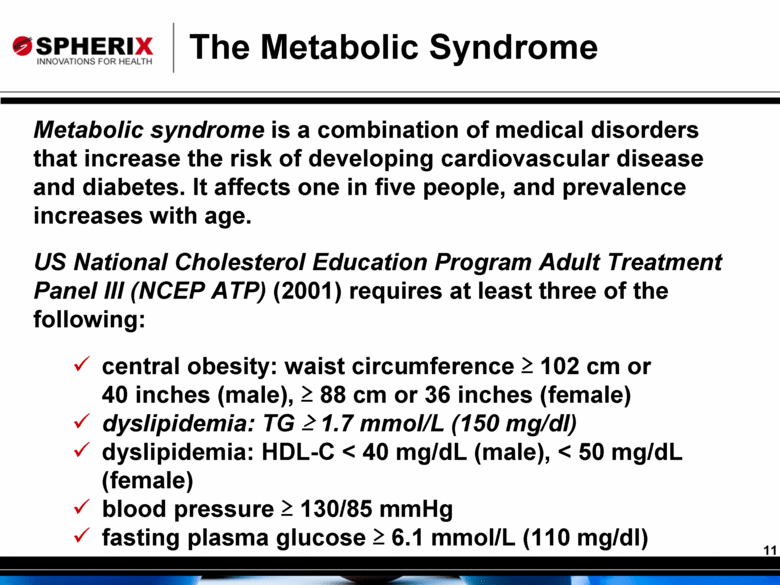
The Metabolic Syndrome Metabolic syndrome is a combination of medical disorders that increase the risk of developing cardiovascular disease and diabetes. It affects one in five people, and prevalence increases with age. US National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP) (2001) requires at least three of the following: central obesity: waist circumference > 102 cm or 40 inches (male), > 88 cm or 36 inches (female) dyslipidemia: TG > 1.7 mmol/L (150 mg/dl) dyslipidemia: HDL-C < 40 mg/dL (male), < 50 mg/dL (female) blood pressure > 130/85 mmHg fasting plasma glucose > 6.1 mmol/L (110 mg/dl) |
|
|
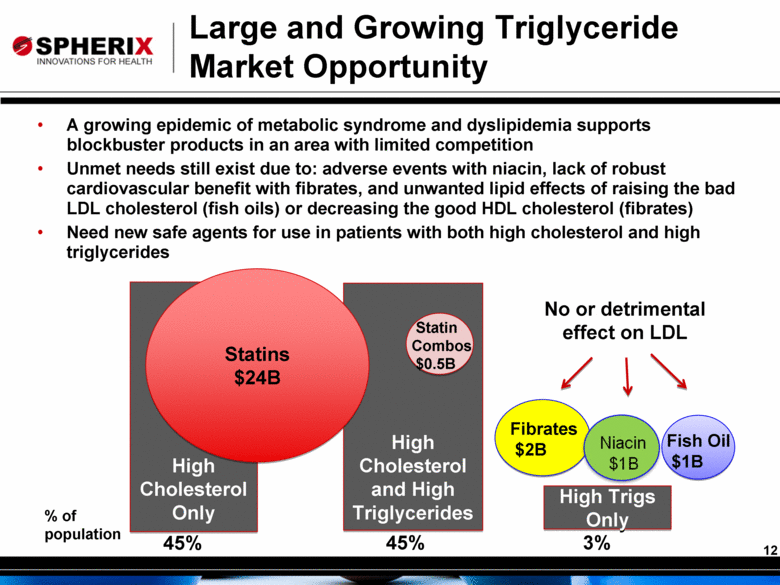
Large and Growing Triglyceride Market Opportunity A growing epidemic of metabolic syndrome and dyslipidemia supports blockbuster products in an area with limited competition Unmet needs still exist due to: adverse events with niacin, lack of robust cardiovascular benefit with fibrates, and unwanted lipid effects of raising the bad LDL cholesterol (fish oils) or decreasing the good HDL cholesterol (fibrates) Need new safe agents for use in patients with both high cholesterol and high triglycerides \ High Cholesterol Only High Cholesterol and High Triglycerides High Trigs Only Statins $24B Fibrates $2B Fish Oil $1B 45% 45% 3% Statin Combos $0.5B No or detrimental effect on LDL % of population |
|
|
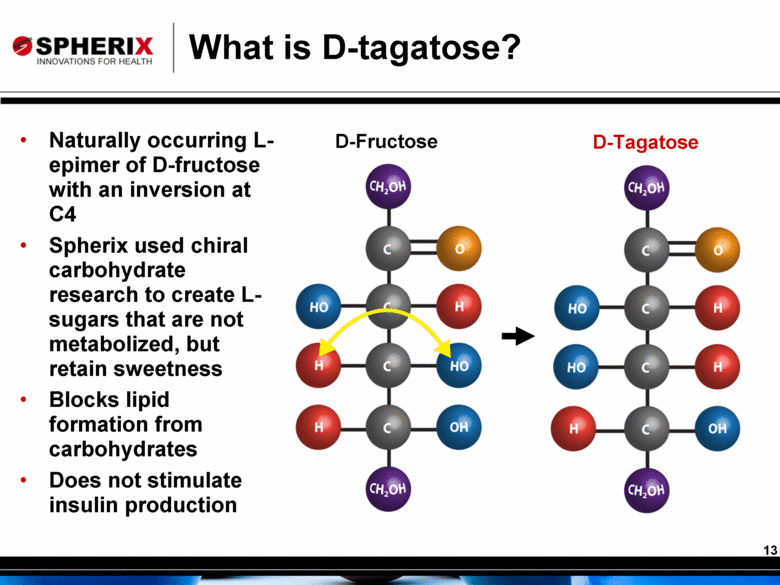
What is D-tagatose? D-Tagatose D-Fructose Naturally occurring L-epimer of D-fructose with an inversion at C4 Spherix used chiral carbohydrate research to create L-sugars that are not metabolized, but retain sweetness Blocks lipid formation from carbohydrates Does not stimulate insulin production |
|
|
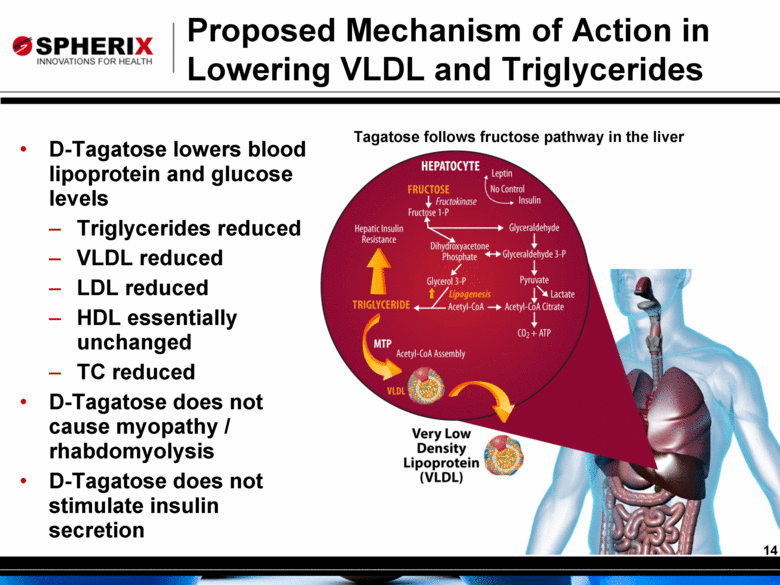
Proposed Mechanism of Action in Lowering VLDL and Triglycerides D-Tagatose lowers blood lipoprotein and glucose levels Triglycerides reduced VLDL reduced LDL reduced HDL essentially unchanged TC reduced D-Tagatose does not cause myopathy / rhabdomyolysis D-Tagatose does not stimulate insulin secretion Tagatose follows fructose pathway in the liver 14 |
|
|
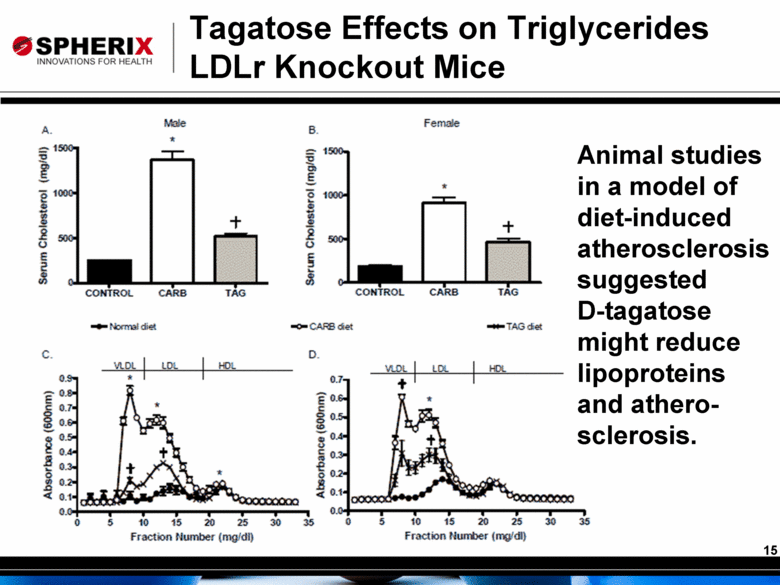
Tagatose Effects on Triglycerides LDLr Knockout Mice Animal studies in a model of diet-induced atherosclerosis suggested D-tagatose might reduce lipoproteins and athero-sclerosis. |
|
|
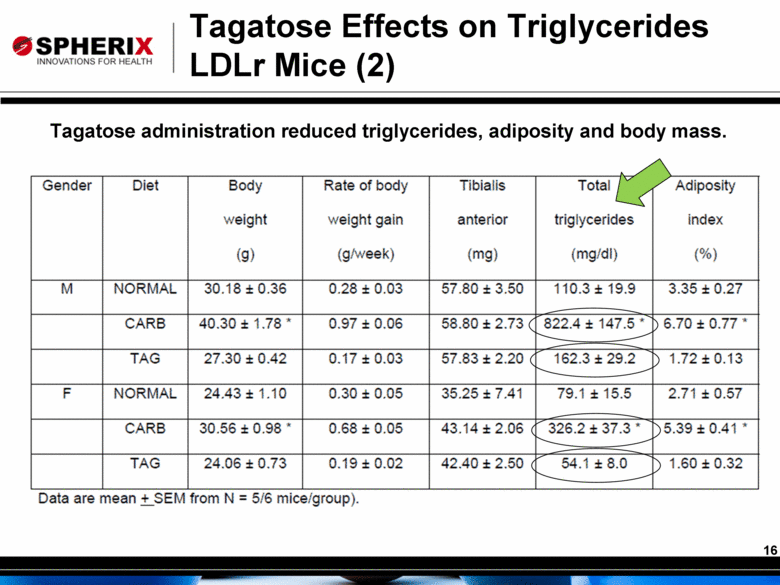
Tagatose Effects on Triglycerides LDLr Mice (2) Tagatose administration reduced triglycerides, adiposity and body mass. |
|
|
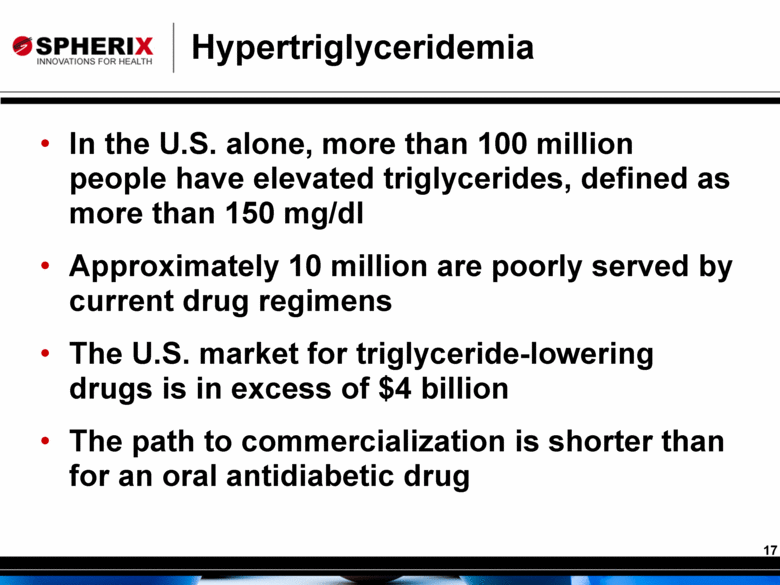
Hypertriglyceridemia In the U.S. alone, more than 100 million people have elevated triglycerides, defined as more than 150 mg/dl Approximately 10 million are poorly served by current drug regimens The U.S. market for triglyceride-lowering drugs is in excess of $4 billion The path to commercialization is shorter than for an oral antidiabetic drug |
|
|
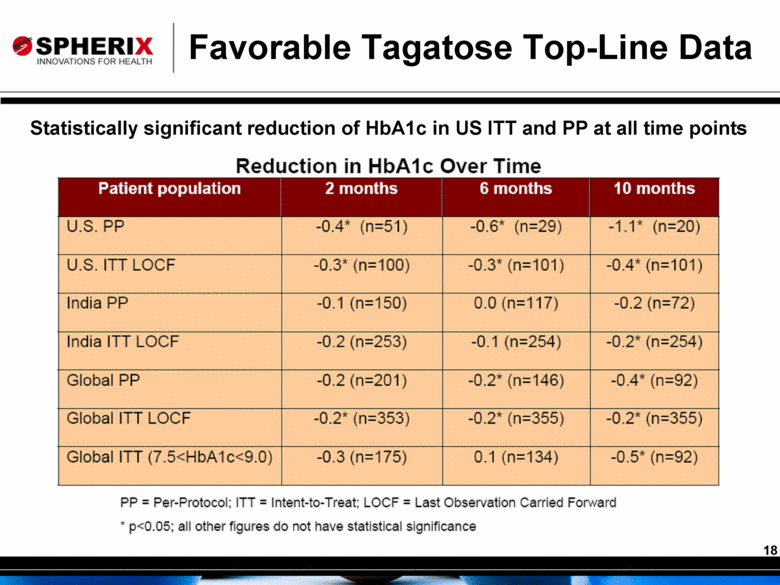
Favorable Tagatose Top-Line Data Statistically significant reduction of HbA1c in US ITT and PP at all time points |
|
|
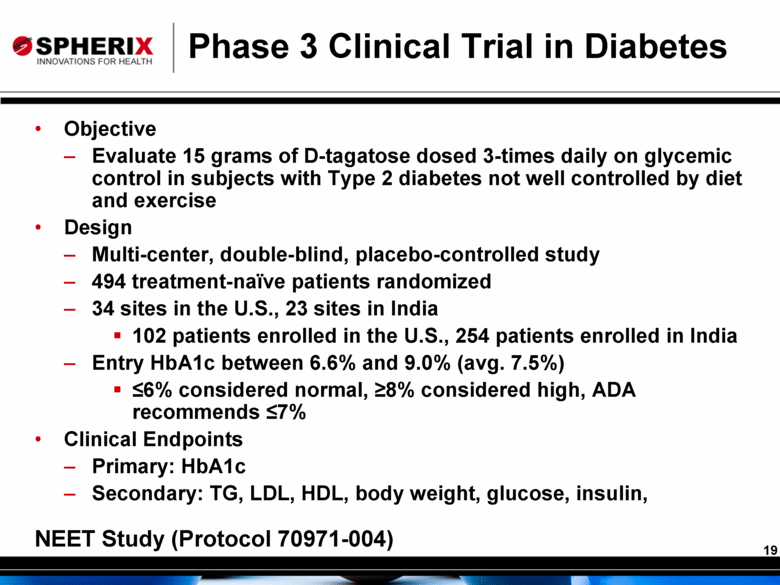
Phase 3 Clinical Trial in Diabetes Objective Evaluate 15 grams of D-tagatose dosed 3-times daily on glycemic control in subjects with Type 2 diabetes not well controlled by diet and exercise Design Multi-center, double-blind, placebo-controlled study 494 treatment-naïve patients randomized 34 sites in the U.S., 23 sites in India 102 patients enrolled in the U.S., 254 patients enrolled in India Entry HbA1c between 6.6% and 9.0% (avg. 7.5%) <6% considered normal, >8% considered high, ADA recommends <7% Clinical Endpoints Primary: HbA1c Secondary: TG, LDL, HDL, body weight, glucose, insulin, NEET Study (Protocol 70971-004) |
|
|
Other Diabetes Clinical Findings Patients with >1 treatment-emergent adverse events in the active group (163) was comparable to the placebo group (166) No serious adverse event deemed treatment related No episodes of hypoglycemia or pancreatitis were reported among any trial subjects The study was not powered for significance in secondary endpoints (e.g., triglycerides) |
|
|
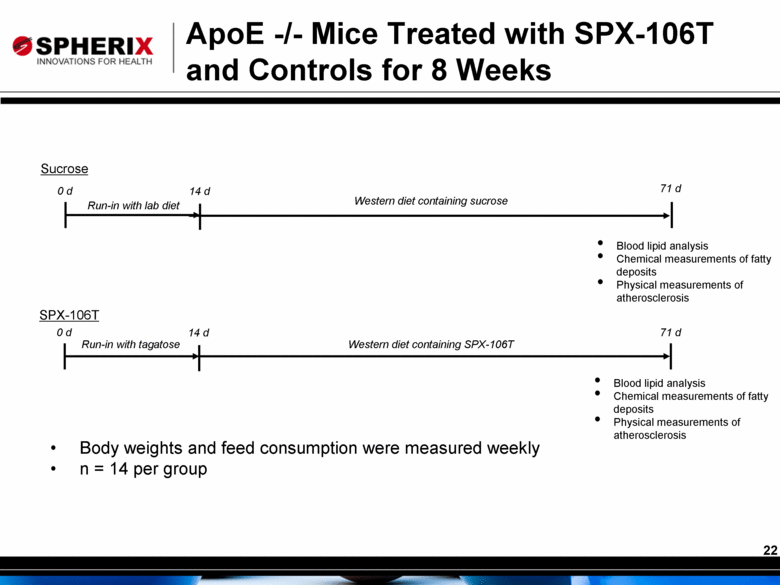
ApoE -/- Mice Treated with SPX-106T and Controls for 8 Weeks 26 Male Apolipoprotein E knockout (ApoE -/-) mice were fed high fat/high carbohydrate diet for 8 weeks Treated mice received D-tagatose in their carbohydrate source and control mice received sucrose Body weights monitored throughout the experiment Mice were exsanguinated via cardiac puncture and the serum analyzed for cholesterol and triglycerides Tissue samples were also taken: aorta, aortic sinus, liver, spleen, and fat pads Aorta and aortic sinus were both analyzed for atherosclerosis |
|
|
0 d 14 d 71 d 0 d 14 d Sucrose SPX-106T Western diet containing sucrose Body weights and feed consumption were measured weekly n = 14 per group Western diet containing SPX-106T 71 d Run-in with tagatose Blood lipid analysis Chemical measurements of fatty deposits Physical measurements of atherosclerosis ApoE -/- Mice Treated with SPX-106T and Controls for 8 Weeks Run-in with lab diet Blood lipid analysis Chemical measurements of fatty deposits Physical measurements of atherosclerosis |
|
|
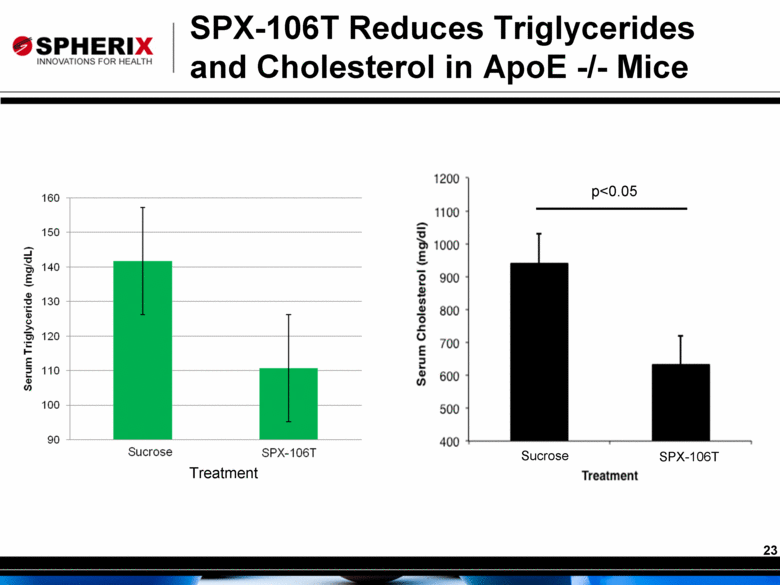
SPX-106T Reduces Triglycerides and Cholesterol in ApoE -/- Mice p<0.05 Sucrose SPX-106T Treatment |
|
|
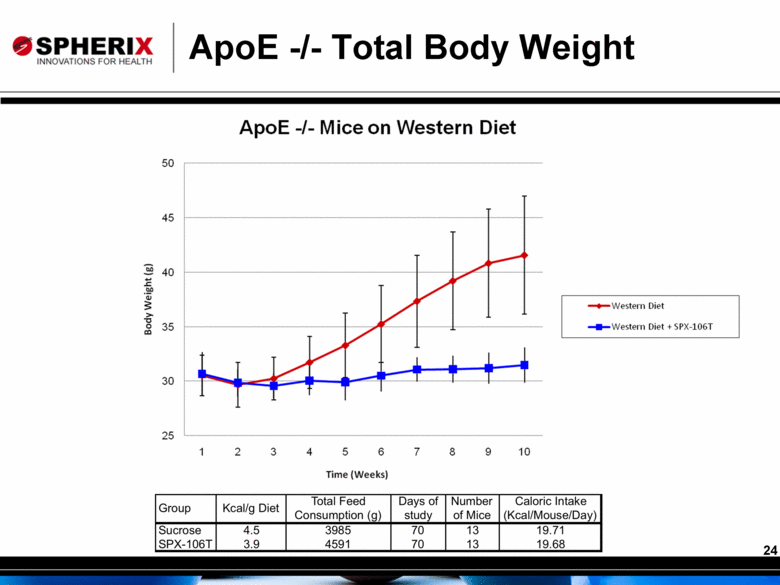
ApoE -/- Total Body Weight |
|
|
ApoE -/- Mouse Organ Weights Black = Western diet White = Western with SPX-106T Mass reduction comes from fat pads in the body * p<0.01 * * |
|
|
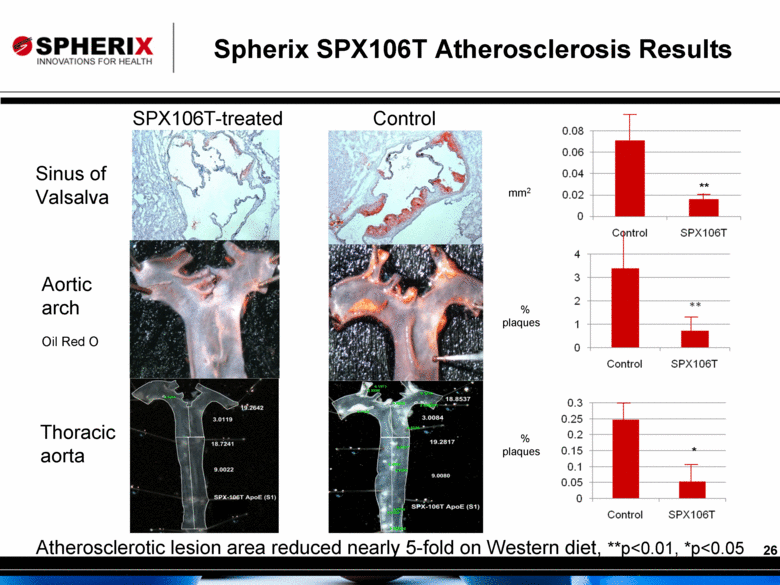
Spherix SPX106T Atherosclerosis Results Atherosclerotic lesion area reduced nearly 5-fold on Western diet, **p<0.01, *p<0.05 SPX106T-treated Control Sinus of Valsalva Aortic arch Thoracic aorta Oil Red O mm2 % plaques % plaques ** * |
|
|
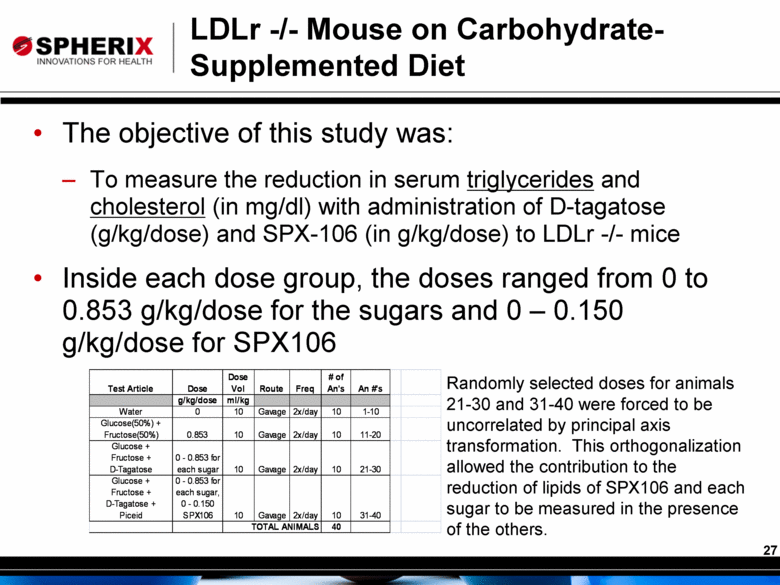
LDLr -/- Mouse on Carbohydrate-Supplemented Diet The objective of this study was: To measure the reduction in serum triglycerides and cholesterol (in mg/dl) with administration of D-tagatose (g/kg/dose) and SPX-106 (in g/kg/dose) to LDLr -/- mice Inside each dose group, the doses ranged from 0 to 0.853 g/kg/dose for the sugars and 0 – 0.150 g/kg/dose for SPX106 Randomly selected doses for animals 21-30 and 31-40 were forced to be uncorrelated by principal axis transformation. This orthogonalization allowed the contribution to the reduction of lipids of SPX106 and each sugar to be measured in the presence of the others. 0 - 0.853 for each sugar, 0 - 0.150 SPX106 |
|
|
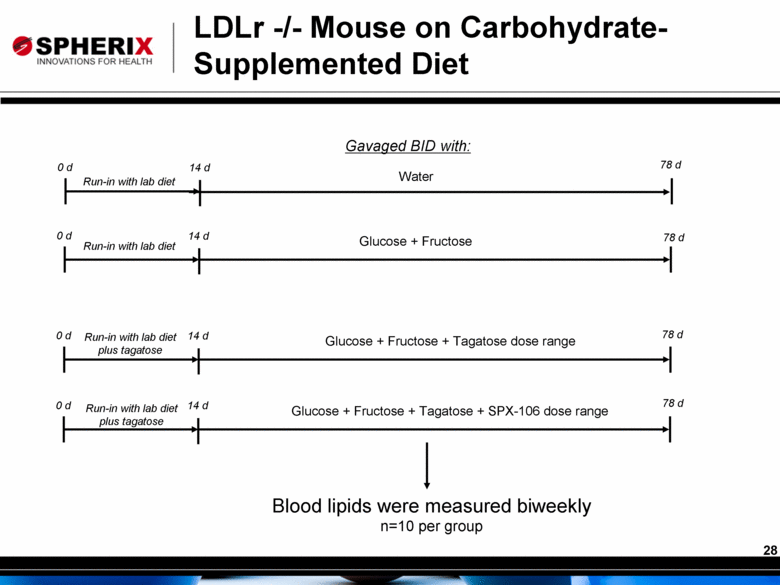
0 d 14 d 0 d 14 d Water Glucose + Fructose 78 d 0 d 14 d Run-in with lab diet plus tagatose 0 d 14 d Glucose + Fructose + Tagatose dose range Glucose + Fructose + Tagatose + SPX-106 dose range Gavaged BID with: Blood lipids were measured biweekly n=10 per group LDLr -/- Mouse on Carbohydrate-Supplemented Diet Run-in with lab diet Run-in with lab diet Run-in with lab diet plus tagatose 78 d 78 d 78 d |
|
|
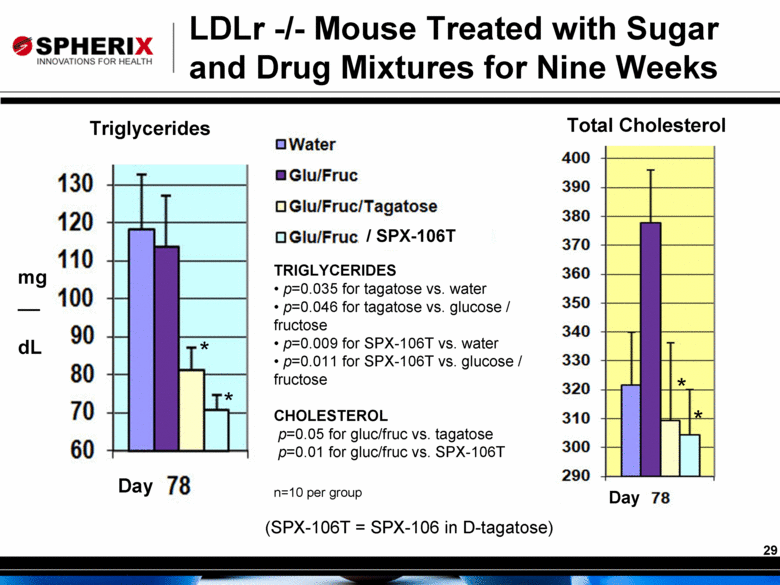
/ SPX-106T Triglycerides Total Cholesterol LDLr -/- Mouse Treated with Sugar and Drug Mixtures for Nine Weeks mg__ dL Day Day TRIGLYCERIDES p=0.035 for tagatose vs. water p=0.046 for tagatose vs. glucose / fructose p=0.009 for SPX-106T vs. water p=0.011 for SPX-106T vs. glucose / fructose CHOLESTEROL p=0.05 for gluc/fruc vs. tagatose p=0.01 for gluc/fruc vs. SPX-106T n=10 per group * * * * (SPX-106T = SPX-106 in D-tagatose) |
|
|
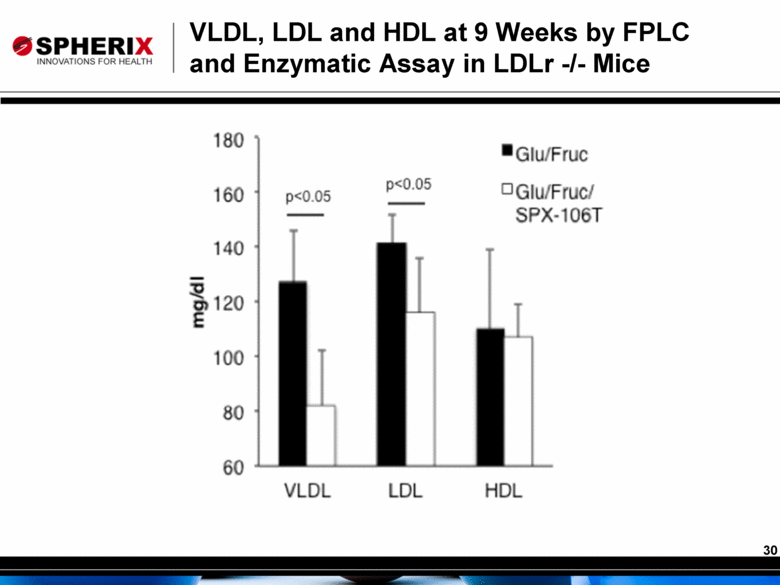
VLDL, LDL and HDL at 9 Weeks by FPLC and Enzymatic Assay in LDLr -/- Mice |
|
|
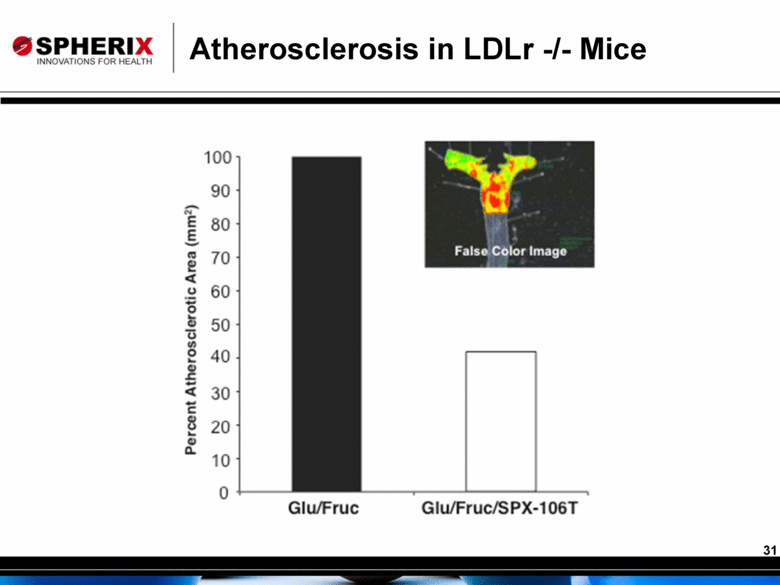
Atherosclerosis in LDLr -/- Mice |
|
|
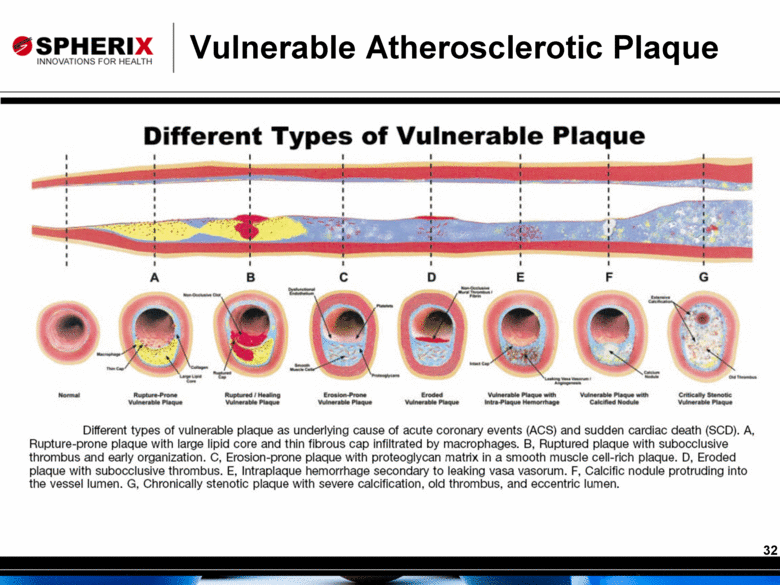
Vulnerable Atherosclerotic Plaque |
|
|
Future Development Additional testing of drug therapies to combat aortic aneurysms SPX106T reduces the hyperlipidemic background required in order for AAA to develop reduces MMP-9, a key enzyme for degrading collagen 33 |
|
|
Control Mouse, Western diet B-mode ultrasonography Visible light 34 |
|
|
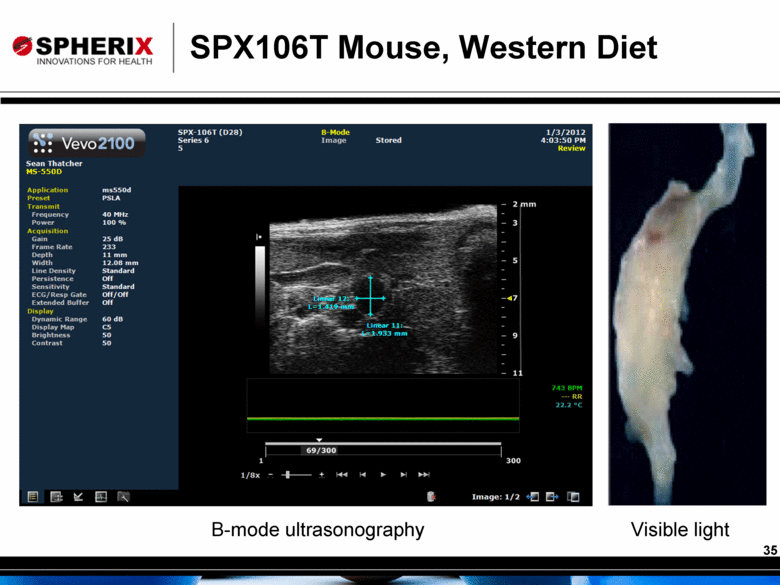
SPX106T Mouse, Western Diet B-mode ultrasonography Visible light 35 |
|
|
Intellectual Property Protection Patents include: Ten national filings for SPX-106T and SPX723T based on PCT publication # 2010/054001 as anti-metabolic, atherosclerosis, obesity & diabetes methods and composition Filing for a tagatose-metformin formulation for diabetes Filings for AAA and IC/PBS New Chemical Entity Exclusivity (Hatch-Waxman) – U.S. 5-year exclusivity (no aNDA’s accepted) usually granted to new drug products containing chemical entities never previously approved by FDA either alone or in combination Essentially ~6 years exclusivity (base case) Pediatric Exclusivity – U.S. 6 months exclusivity for conducting studies in pediatric population Added to end of all existing marketing exclusivity and patent periods 36 |
|
|
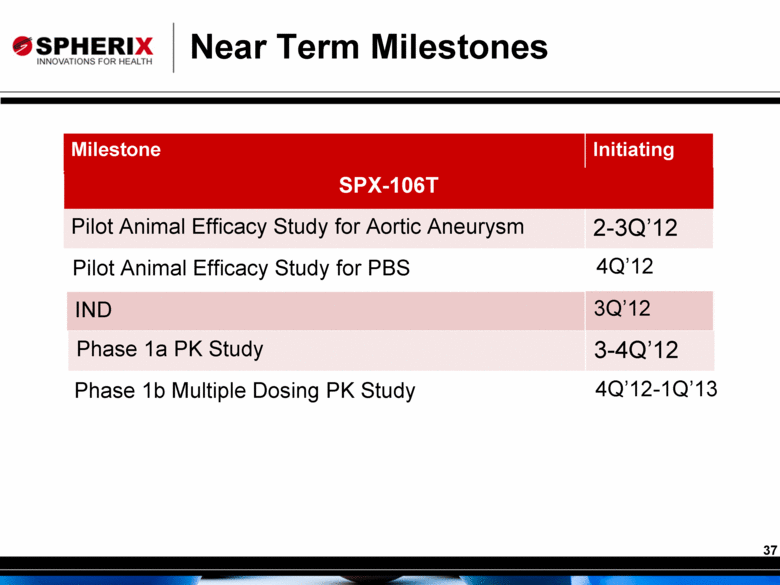
Near Term Milestones Milestone Initiating Pilot Animal Efficacy Study for Aortic Aneurysm 2-3Q’12 IND 3Q’12 Pilot Animal Efficacy Study for PBS 4Q’12 Phase 1a PK Study 3-4Q’12 Phase 1b Multiple Dosing PK Study 4Q’12-1Q’13 SPX-106T 37 |
|
|
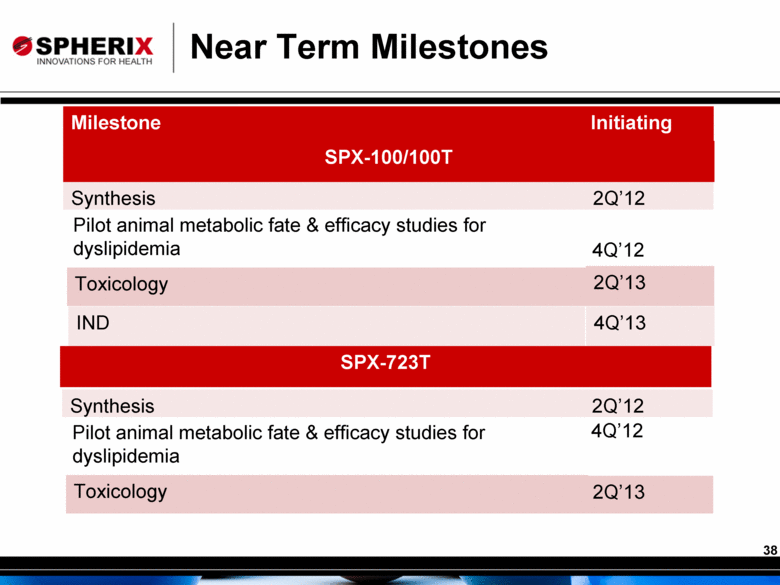
Near Term Milestones Milestone Initiating Synthesis 2Q’12 Toxicology 2Q’13 Pilot animal metabolic fate & efficacy studies for dyslipidemia 4Q’12 IND 4Q’13 SPX-100/100T SPX-723T Synthesis 2Q’12 Toxicology 2Q’13 Pilot animal metabolic fate & efficacy studies for dyslipidemia 4Q’12 38 |
|
|
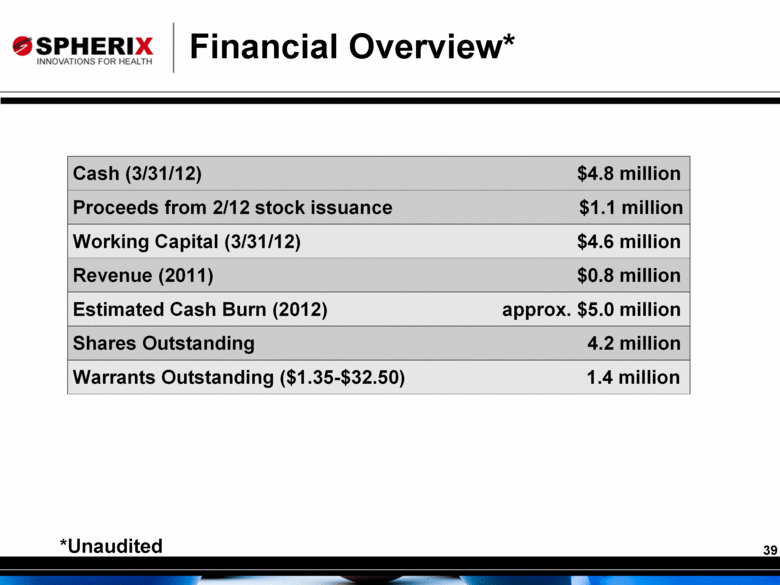
Financial Overview* *Unaudited Cash (3/31/12) $4.8 million Proceeds from 2/12 stock issuance $1.1 million Working Capital (3/31/12) $4.6 million Revenue (2011) $0.8 million Estimated Cash Burn (2012) approx. $5.0 million Shares Outstanding 4.2 million Warrants Outstanding ($1.35-$32.50) 1.4 million 39 |
|
|
Experienced Leadership Claire L. Kruger, Ph.D., Chief Executive Officer Toxicologist with 25 years of consulting experience; primary area of expertise is in pharmaceuticals, consumer products and foods, where she provides scientific, regulatory, and strategic support to clients in both the US and international regulatory arenas Robert A. Lodder, Ph.D., President Founder of InfraReDx, Inc., Prescient Medical, Inc., and Escent Technologies, Inc. Professor of Pharmaceutical Sciences at the College of Pharmacy, University of Kentucky Medical Center. Joint appointments in Electrical & Computer Engineering and in Chemistry. Inventor with over 100 publications and 17 patents. Robert L. Clayton, Chief Financial Officer 16 years of experience in finance and accounting, including 5 years in public accounting; previously served as Director of Finance and Controller for Spherix Katherine M. Brailer, Corporate Secretary and Director of Administrative Services 25 years of experience in all aspects of administrative services, including 10 years as an Officer of Spherix 40 |
|
|
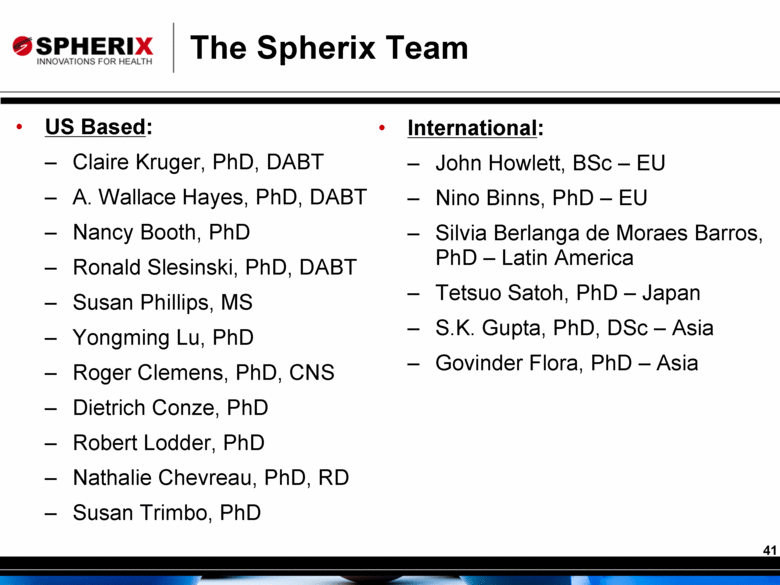
The Spherix Team US Based: Claire Kruger, PhD, DABT A. Wallace Hayes, PhD, DABT Nancy Booth, PhD Ronald Slesinski, PhD, DABT Susan Phillips, MS Yongming Lu, PhD Roger Clemens, PhD, CNS Dietrich Conze, PhD Robert Lodder, PhD Nathalie Chevreau, PhD, RD Susan Trimbo, PhD International: John Howlett, BSc – EU Nino Binns, PhD – EU Silvia Berlanga de Moraes Barros, PhD – Latin America Tetsuo Satoh, PhD – Japan S.K. Gupta, PhD, DSc – Asia Govinder Flora, PhD – Asia 41 |
|
|
UK Scientific Investigative Team Lisa A. Cassis, Ph.D. – adipocytes and aneurysm Chair of the Department of Nutritional Sciences in the College of Medicine. A primary research interest is the study of production of components of the renin-angiotensin system by adipocytes, and the impact of an adipose renin-angiotensin system on the development of obesity and obesity-associated diseases (diabetes, hypertension, atherosclerosis). Alan Daugherty, Ph.D. – lipids and atherosclerosis Editor-in-Chief of the AHA Journal Arteriosclerosis, Thrombosis, and Vascular Biology (ATVB). College of Medicine Senior Associate Dean for Research, Director, Saha Cardiovascular Research. Studies macrophages, endothelial cells and smooth muscle cells in the development of dyslipidemias, atherosclerosis, and abdominal aortic aneurysm. Phillip Kern, M.D. – metabolic syndrome Professor, Division of Endocrinology and Molecular Medicine, Director, Barnstable Brown Kentucky Diabetes and Obesity Center, Director, Center for Clinical and Translational Sciences. Basic and clinical research related to obesity, metabolic syndrome, and diabetes. Clinical studies have examined adipose tissue and muscle gene expression in relation to insulin resistance, tissue lipotoxicity and inflammation in obesity related insulin resistance. Other research involves the gene expression of lipoprotein lipase (LPL), an important enzyme in adipose tissue that is involved in triglyceride metabolism, diabetes, and obesity. 42 |