Attached files
| file | filename |
|---|---|
| 8-K - MAINBODY - Amarantus Bioscience Holdings, Inc. | mainbody.htm |
| EX-99.1 - EXHIBIT 99.1 - Amarantus Bioscience Holdings, Inc. | ex99_1.htm |

Banyan Biomarkers Inc.
Evaluation of MANF effectiveness as neuroprotector of necrotic and apoptotic cell death in rat primary cortical cultures. Reproducibility of observed neuroprotective effects observed on 3-5-15
Requested by Gerald Commissioning, President and CEO
Amarantus Biosciences Inc.
Prepared and executed by Juan A. Martinez, Ph.D.
Senior Scientist, Banyan Biomarkers Inc.
Alachua, FL
| 12076 Technology Avenue | www.banyanbio.com | Ph: (386) 518-6741 |
| Alachua, FL 32615 | Fax: (386) 518-67513 |
Introduction:
Mesencephalic Astrocyte-derived Neurotrophic Factor (MANF) is a novel 18 kDa astrocyte-secreted protein that has been shown to have anti-apoptotic/neuroporotective/neurorestorative properties. Amaranthus Biosciences Inc owns the intellectual property rights of this protein.
Objective:
Reproduce results observed during previous determination of dose-response effect of MANF on cytotoxicity induced in primary cortical cultures (CTX) by NMDA (necrosis-induction) and STS (apoptosis-induction).
Materials and Methods:
Seven 12-well clusters of primary cortical neuronal cultures (from P1 pups) were provided by Banyan staff according to our standard protocol. Visual inspection indicated cultures were in good health with few astrocytes present (estimated >60% neurons based on single randomly selected field for each well; estimated plate was >70% confluent). Our method selects for neurons by inducing death of replicating cells (5 µM Ara-c added for 24 hours after cultures are seeded and attached). Cells are grown at 37° C in DMEM + 10% horse serum + penicillin/streptomycin + glutamine in 9% CO2 humidified incubator. Seeding and selection process takes about 10 days and cultures remain viable for experimentation for 7-10 days.
Each of the seven clusters received a different dose of MANF (in citrate buffer-provided). These were 0, 2.5, 5, 10, 25, 50 and 100 ng/ml of MANF. Necrotic cell death was induced by N-methyl D-aspartate (NMDA). Apoptotic cell death was induced by kinase inhibitor staurosporine. Set conditions on each cluster provided four wells for each experimental condition (i.e. Control, 0.5 µM STS and 300 µM NMDA). STS and NMDA were prepared in serum-free, low antibiotic (1/3 normal amount) DMEM (1 ml per well). Different concentrations of MANF were added immediately after and cultures were incubated for 24 hours.
After 24 hours cells were visually evaluated under microscope and then the medium was removed from each cluster and centrifuged at 800 x G for 5 min in microcentrifuge to pellet any floating cells and debris. Supernatant was transferred and aliquot tested for cytotoxicity by lactate dehydrogenase (LDH) release assay (Promega).
Cells were lysed in-well by triton-based lysis buffer with protease inhibitor cocktail (Roche) for 90 min at 4°C in orbital shaker (300 µl per well). The tubes spun at 800 x G containing any cells/debris were also lysed with 10 µl of solution and pooled with corresponding sample. After lysis genomic DNA was removed from each well and samples were centrifuged for 20 minutes at 4°C. Protein in supernatant was determined by Pierce 660 assay and equal total protein was loaded onto 7.5% polyacrylamide gels for SDS-PAGE and semi-quantitative western blot analysis. Β-actin served as normalizing control for quantitative analysis of spectrin breakdown products (SBDP-150, SBDP-145 and SBDP-120). These spectrin breakdown products are generated by the proteolytic cleavage of calpain-1 (SBDP-145) and caspase-3 (SBDP-120). SBDP-150 is produced primarily, but not exclusively by the action of calpain-1, thus we consider this SBDP a “mixed” biomarker. The fact that these are all breakdown products of brain spectrin indicates degradation of neuronal cytoskeletal components that are associated with specific modalities of cell death (i.e. necrosis and apoptosis).
Semi-quantitative analysis of protein expression levels were determined by densitometric analysis of western blots with high resolution flatbed scanner (1200 dpi) and Image J software.
Statistical analysis and graphical representation for each category was performed in Prism v 5. Significant difference between different concentrations in each group was performed by student’s t-test (two-tailed) relative to corresponding control.
Results:
LDH assay of cytotoxicity: Neuroprotective properties of MANF were tested in vitro by determining the dose-response in cultures challenged by serum deprivation (control), necrosis induced by NMDA and apoptosis induced by staurosporine. Figure 1 summarizes results obtained by lactate dehydrogenase release assay. Serum deprivation (growth factor withdrawal) is our control condition because of the incompatibility of serum with LDH enzyme assay. Results indicate that MANF concentrations of 50 ng/ml and above in serum-deprived conditions (green bar) increased the amount of LDH released, suggesting an increase in cytotoxicity. MANF concentrations between 2.5-10 ng/ml showed no significant difference in LDH release compared to citrate-vehicle in control condition (serum deprived). Staurosporine treatment (red bar) increased LDH release more than 2-fold over control (green bar). This kinase inhibitor is commonly used to induce apoptosis in vitro. MANF concentrations between 2.5-10 ng/ml significantly decreased cytotoxicity induced by STS. MANF intermediate concentration (25 ng/ml) did not provide significant neuroprotection against STS-induced neurotoxicity, and concentrations of 50 ng/ml and above significantly increased LDH release, suggesting cytotoxicity at high dosage in vitro. Necrotic cell death challenge induced by NMDA increased LDH release more than 3-fold over control (blue bar-NMDA vs green bar-control). MANF concentrations between 2.5 and 25 ng/ml provided significant neuroprotection from NMDA-induced necrotic cell death. Concentrations of 50 ng/ml and 100 ng/ml did not show significant neuroprotection, but instead the tendency shifted towards enhanced cytotoxicity. This high dose cytotoxic effect (> 50 ng/ml) was consistent in all conditions tested. These results clearly indicate an effective neuroprotective dose of MANF to both necrotic and apoptotic cell death in vitro.

| [MANF] (ng/ml) | Control | STS (0.5 µM) | NMDA (300 µM) | |||||||||
| 0.0 | 0.2890 | 0.1733 | 0.1958 | 0.1808 | 0.6190 | 0.5574 | 0.6522 | 0.6306 | 0.9404 | 0.8720 | 0.9322 | 0.8447 |
| 2.5 | 0.3941 | 0.1482 | 0.1468 | 0.1537 | 0.4785 | 0.3868 | 0.2714 | 0.2350 | 0.5069 | 0.3027 | 0.2631 | 0.1256 |
| 5.0 | 0.1763 | 0.1631 | 0.1500 | 0.2403 | 0.2918 | 0.3320 | 0.3273 | 0.4552 | 0.6210 | 0.5395 | 0.6573 | 0.4519 |
| 10.0 | 0.1595 | 0.1593 | 0.1482 | 0.2252 | 0.3590 | 0.4035 | 0.2410 | 0.3191 | 0.4323 | 0.3654 | 0.3175 | 0.4320 |
| 25.0 | 0.3849 | 0.3178 | 0.4391 | 0.4667 | 0.4904 | 0.4108 | 0.4086 | 0.7374 | 0.5867 | 0.2849 | 0.2544 | 0.5943 |
| 50.0 | 0.7682 | 0.7432 | 0.6953 | 0.5117 | 1.0132 | 0.8813 | 1.1202 | 0.8320 | 1.2435 | 1.1194 | 0.7443 | 0.5818 |
| 100.0 | 0.5706 | 0.5586 | 0.4742 | 0.5913 | 1.3131 | 1.4694 | 0.9755 | 0.7925 | 1.7594 | 1.2493 | 0.6678 | 0.9122 |
| [MANF] (ng/ml) | Control | STS (0.5 µM) | NMDA (300 µM) | ||||||
| Mean | SEM | n | Mean | SEM | n | Mean | SEM | n | |
| 0.000 | 0.209725 | 0.02683572 | 4 | 0.6148 | 0.02033224 | 4 | 0.897325 | 0.02324233 | 4 |
| 2.500 | 0.2107 | 0.06115147 | 4 | 0.342925 | 0.05557969 | 4 | 0.299575 | 0.07884116 | 4 |
| 5.000 | 0.182425 | 0.02002471 | 4 | 0.351575 | 0.03568805 | 4 | 0.567425 | 0.04571071 | 4 |
| 10.000 | 0.17305 | 0.01758268 | 4 | 0.33065 | 0.03449803 | 4 | 0.3868 | 0.02794897 | 4 |
| 25.000 | 0.402125 | 0.03284368 | 4 | 0.5118 | 0.07756962 | 4 | 0.430075 | 0.09284338 | 4 |
| 50.000 | 0.6796 | 0.05797401 | 4 | 0.961675 | 0.06523085 | 4 | 0.92225 | 0.1553604 | 4 |
| 100.000 | 0.548675 | 0.02572714 | 4 | 1.137625 | 0.1544512 | 4 | 1.147175 | 0.2363371 | 4 |
Figure 1. Dose-response effect of MANF on cytotoxicity in CTX cultures induced by serum deprivation (control), staurosporine (STS) and N-methyl D-aspartate (NMDA) determined by LDH assay. LDH released into medium was measured by enzymatic reaction according to Promega CytoTox assay. Each point represents the average and standard error of mean, n=4. Tables with raw data and statistical analyses shown below.
Semi-quantitative western blot analysis of SBDP’s: The previous results showed very similar profiles in the protein levels of SBDP-145 and SBDP-150. Resolution of these two bands in a 7.5% gel was not good enough to allow independent quantitative analysis and thus we measured the combination of this biomarker which is increased by calpain activity. To better illustrate details of biomarker expression in each category we analyzed each individually (by challenge) and indicate statistically significant differences from corresponding control.

Figure 2. Dose-response effect of MANF on SBDP-145/150 expression in primary neuronal cultures (rat cortex-CTX) exposed to apoptosis and necrosis inducers. CTX cultures were maintained in serum-free conditions during experimental protocol of 24 hours; (A) control, (B) 0.5 µM STS and (C) 300 µM NMDA. Each bar represents the mean and SEM for n=4. Asterisk indicates statistically significant difference by two-tailed t-test (p<0.05).
The effects of MANF on SBDP-145/150 expression resulting from STS and NMDA challenge in vitro are summarized in figure 2. In control condition (A) we observed a significant decrease in SBDP-145/150 expression between 5-10 ng/ml. We know that growth factor deprivation does not affect overall cell viability in these short course experiments but we have observed that there is an increase of about two fold in cellular levels of SBDP-145 and SBDP-150 resulting from subtle intracellular events related perhaps to cytoskeletal rearrangement in response to growth factor deprivation. The fact that MANF was able to effectively reduce generation of SBDP-145/150 in this condition might suggest a modulatory role in calpain-mediated cytoskeletal rearrangement. Staurosporine is a kinase inhibitor that is commonly used as apoptosis inducer in vitro, although the precise molecular mechanisms have not been clearly elucidated. In our assay paradigm it consistently generates increased levels of SBDP-120, a caspase-mediated proteolytic product of αII-spectrin that indicates apoptosis. But we also know that in this in vitro assay STS also increases what we call SBDP-150i, a caspase-3-generated fragment that is indistinguishable by the current method from SBDP-150 (a calpain product). Thus, this additive effect observed in the level of SBDP-145/150 measured in STS-treated cells could be attributed to increased SBDP-150i levels. Relative to control, STS increased SBDP-145/150 by about 2-fold. MANF concentrations between 5-25 ng/ml were effective in decreasing the level of SBDP-145/150 induced by STS. Further evidence that this reduction is likely associated with caspase-3 inhibition during apoptosis induction is provided by analysis of SBDP-120 (figure 3) where the same concentration range significantly decreased the level of this apoptosis biomarker. NMDA-induced expression of SBDP-145/150 is associated with increased calpain activation resulting from increased intracellular calcium levels upon NMDA receptor activation in neurons. NMDA caused a 2.3-fold increase in SBDP-145/150 level relative to control. In this challenge, MANF was able to significantly decrease level of SBDP-145/150 induced at all doses tested, but most significantly in concentrations of 5 ng/ml and above. This suggests that MANF could be a mediator of calpain-mediated cytoskeletal rearrangement during NMDA-induced necrosis/oncosis. It must be clarified that intracellular molecular mechanisms that take place during this challenge are complex and cannot be dissected into components, but must be analyzed in the context of a living cell. From this perspective it is understandable that cytoskeletal rearrangement is a property of a living organism. The fact that high doses of MANF showed deleterious effect in cell viability during NMDA challenge is not necessarily inconsistent with the decreased SBDP-145/150 levels observed at their corresponding concentrations. We have observed that proposed neuroprotection based on calpain inhibition depends not only on the challenge but on intrinsic cell-specific properties. Interfering with cytoskeletal rearrangement during specific cellular events could favor either cell survival or cell death.
Analysis of the caspase-3 proteolytic product of αII-spectrin SBDP-120 is summarized in figure 3. Serum starvation (Figure 3A) did not cause significant changes in the level of SBDP-120 measured by quantitative western blotting (notice the scale range). As expected, STS increased level of SBDP-120 (by about 10-fold), indicating effective activation of caspase-3. MANF concentrations between 5-25 ng/ml significantly decreased SBDP-120 levels induced by STS (Figure 3B). This is consistent with effective concentration range that caused significant decrease in SBDP-145/150 in STS-treated cells (Figure 2B). In that figure we explained that the observed decrease could have been attributed to caspase-3 generated SBDP-150i (see above). Thus, analysis of apoptosis biomarker SBDP-120 indicates that MANF has anti-apoptotic properties. Figure 3C showed that NMDA challenge did not enhance SBDP-120 and that MANF had no significant effect in SBDP-120 generation.

Figure 3. Dose-response effect of MANF on SBDP-120 expression in primary neuronal cultures (rat cortex-CTX) exposed to apoptosis and necrosis inducers. CTX cultures were maintained in serum-free conditions during experimental protocol of 24 hours; (A) control, (B) 0.5 µM STS and (C) 300 µM NMDA. Each bar represents the mean and SEM for n=4. Asterisk indicates statistically significant difference by two-tailed t-test (p<0.05).
Concluding remarks:
Although experimental reproducibility did not show 100% fidelity relative to previous experiment, the tendency was clearly in the same direction. It seems clear that MANF has neuroprotective properties on primary cortical neurons when these are challenged with apoptotic and necrotic inducers. Dose between 5-10 ng/ml appeared to be effective in both apoptosis and necrosis in vitro models, although the 2.5 ng/ml dose significantly reduced overall cytotoxicity induced by both STS and NMDA. Thus, although analysis of biomarkers indicated that MANF could be associated with specific calpain and caspase-mediated proteolytic events, this is unlikely to be either the only or principal mechanism by which MANF is conferring neuroprotection in this in vitro model. Results here also clearly suggest that high dose/exposure could be neurotoxic. These results were clearly reproduced in both experiments, where concentrations of 50 ng/ml and above appeared to decrease cell viability. Another discrepancy between the two studies was observed in the serum starved control, where the percentage of viable cells was better preserved in the latest experiment and no significant neuroprotection was observed at any MANF dose. This could be a result of variability in the neuron:glia ratio in the culture. The presence of more glia in the more recent experiment could have provided a more supportive environment that ameliorated cell death previously observed in serum-starved control. It is noteworthy that the previously observed protection under these conditions was statistically significant but small in magnitude.
In conclusion, we believe that results from this in vitro study should be carefully reviewed and interpreted, but one conclusion is clear, MANF appears to be neuroprotective against both NMDA-mediated necrosis induction and STS-mediated apoptosis induction in vitro. These results are consistent with previously executed experiment and support further testing in an in vivo model to confirm whether these events translate into a more complex organismal system/pre-clinical model. This is certainly an exciting compound that deserves further investigation to identify not only molecular mechanisms of action but also potential clinical applications.
Raw data files:


Table above is values obtained by image signal processing, background and actin normalization.
Images in sequence 0-2.5-5-10-25-50 and 100 ng/ml
Replicates n=4 in blocks, left is control, middle is STS and right is NMDA




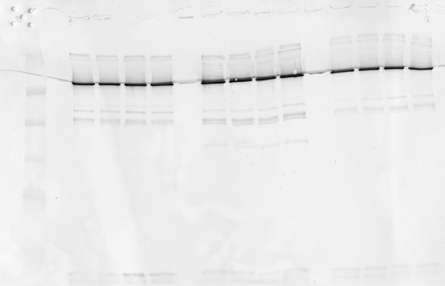

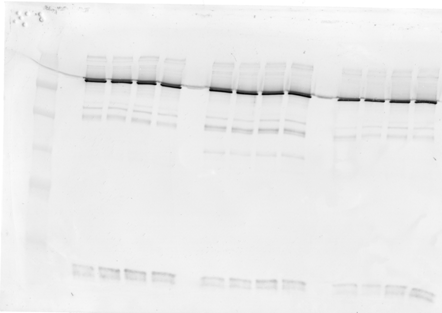
Extensive image analysis was performed to enhance contrast/S/N ratio, background normalization, minimize interference from artefacts via individual ROI selection and image processing. Method had to be developed following procedures established by imaging consultant using Kodak Molecular Imaging software v. 5.0.
Statistical analyses performed in Prism v5.0:
Data 1 corresponds to SBDP-120-STS (only significant data recorded)

SBDP-145/150 control statistics:

SBDP-145/150 STS and NMDA stats: