Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIOVEST INTERNATIONAL INC | d361471d8k.htm |
| EX-99.1 - PRESS RELEASE - BIOVEST INTERNATIONAL INC | d361471dex991.htm |
 Biovest (OTCQB: BVTI) Interview with
OneMedRadio
Brett Johnson
President & Executive Editor
OneMedPlace
Samuel S. Duffey, Esq.
President & CEO
Biovest International, Inc.
Carlos F. Santos, Ph.D.
SVP, Product Development &
Regulatory Affairs
Biovest International, Inc.
May 31, 2012
Exhibit 99.2 |
 Statements in this presentation/interview that are not strictly historical in
nature constitute "forward- looking statements.“
Such statements include, but are not limited to, statements about Biovest and
its
product
candidate,
BiovaxID®
and
any
other
statements
relating
to
products,
product
candidates, product development programs, the FDA or clinical study process
including the commencement, process, or completion of clinical trials or the
regulatory process. Such statements may include, without limitation,
statements with respect to the Company's plans, objectives, expectations and
intentions, and other statements identified by words such as "may,"
"could," "would," "should," "believes,"
"expects," "anticipates," "estimates," "intends," "plans," or
similar expressions. In particular (and without limitation), statements regarding
the timing of anticipated filing of marketing applications for BiovaxID with
Health Canada and the EMA, pre-filing meetings with the FDA or other
jurisdictions an/or commercial plans reflect current expectations but are
subject to inherent risks of delay in compilation and finalization of all components of the
licensing application. Such forward-looking statements involve known and
unknown risks, uncertainties, and other factors that may cause the actual
results of Biovest to be materially different from historical results or
from any results expressed or implied by such forward-looking
statements. These factors include, but are not limited to, risks and
uncertainties related to the progress, timing, cost, and results of clinical
trials and product development programs; difficulties or delays in obtaining
regulatory approval for product candidates; competition from other
pharmaceutical or biotechnology companies; and the additional risks discussed in
filings with the Securities and Exchange Commission. All
forward-looking statements are qualified in their entirety by this
cautionary statement, and Biovest undertakes no obligation to revise or update this news
release to reflect events or circumstances after the date hereof. The product
names used in this statement are for identification purposes only. All
trademarks and registered trademarks are the property of their respective
owners. Safe Harbor Statement |
 2
Late-Stage Personalized Cancer Vaccine
Poised to Seek Approval(s) for Follicular non-
Hodgkin’s Lymphoma in 2012/2013 |
 Regulatory Strategy
If Approved, BiovaxID Represents the First
Cancer Vaccine Available for Lymphoma Patients
Canada:
Based on guidance from pre-filing clinical meeting
conducted with Health Canada, Biovest plans to file a New Drug
Submission (NDS) later this year seeking marketing approval in
Canada.
European
Union:
Based
on
guidance
from
pre-filing
clinical
meeting
conducted with EU-member drug agencies, Biovest plans to file a
Marketing Authorization Application (MAA) with the EMA seeking
marketing approval in the EU. An Intent-to-File Letter is planned to
be soon submitted to the EMA.
U.S.A.:
Biovest is preparing to conduct and report on a pre-filing
clinical meeting with the FDA and expects to announce the outcome
and expected U.S. regulatory pathway in Summer 2012.
|
 Body of Clinical Evidence
4
Follicular Lymphoma Phase 2
demonstrates nearly universal immune
response to BiovaxID, durable molecular remission in FL with highly
encouraging Overall Survival signal (15-year follow-up)
Follicular Lymphoma Phase 3:
randomized, multicenter, controlled
clinical trial improved DFS by 13.6 months in patients in first remission
who received at least one dose of BiovaxID
This study was conducted in 12 major cancer centers across the US with a
median follow-up of 4.7 years
Mantle Cell Lymphoma Phase 2
demonstrates strong association
between vaccine-induced GM-CSF cytokine response and Overall
Survival (10-year follow-up) following a rituximab containing induction
therapy
No significant safety risks have been identified for BiovaxID
|
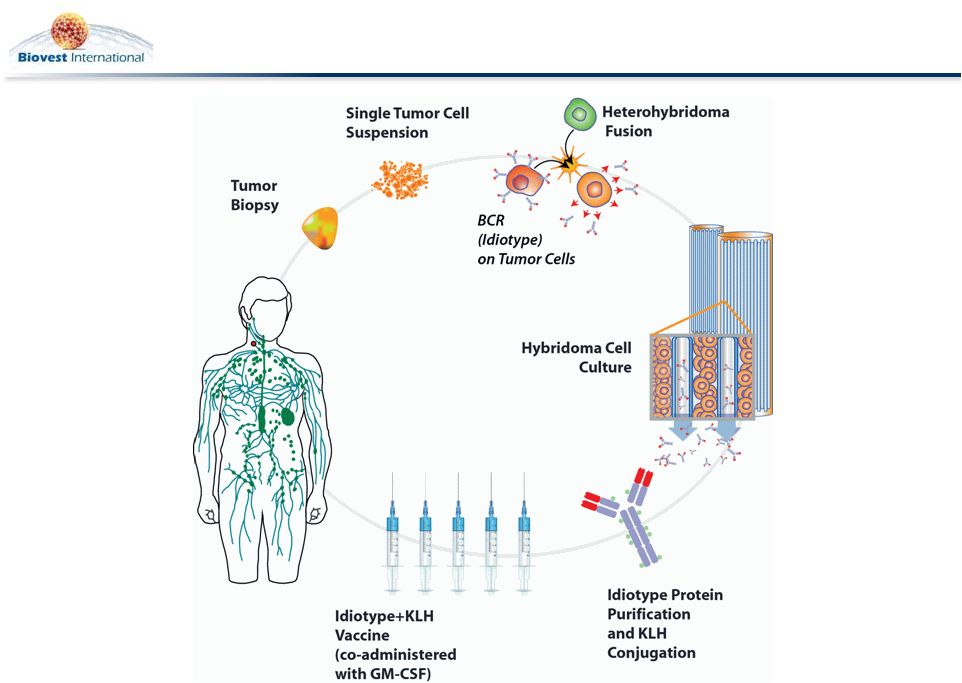 BiovaxID Personalized Manufacturing Process
5 |
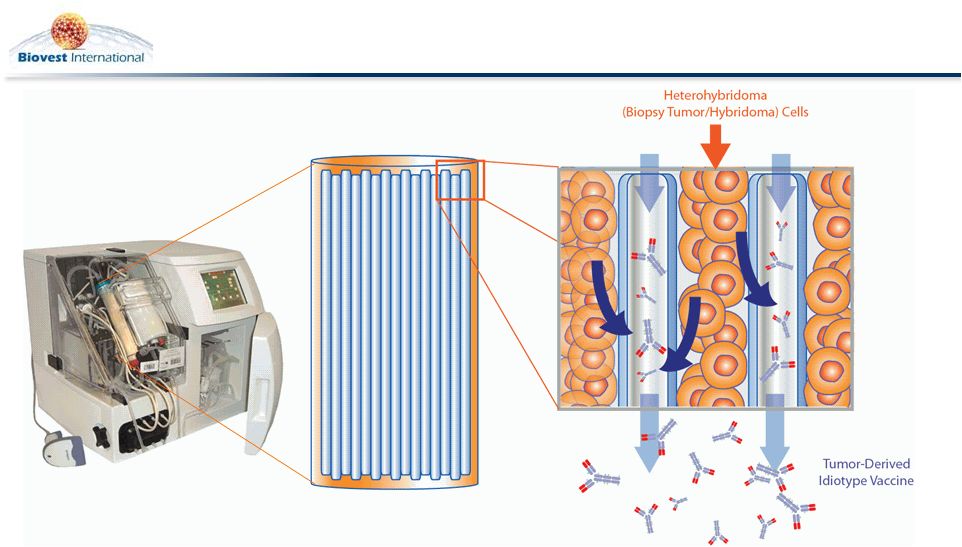 AutovaxID Automated Manufacturing System
6
Hollow-Fiber Bioreactor
Cultures Tumor Derived Cells
(Heterohybridoma)
for Id Protein Production
Isotype-Matched Id Protein
Secreted and
Purified |
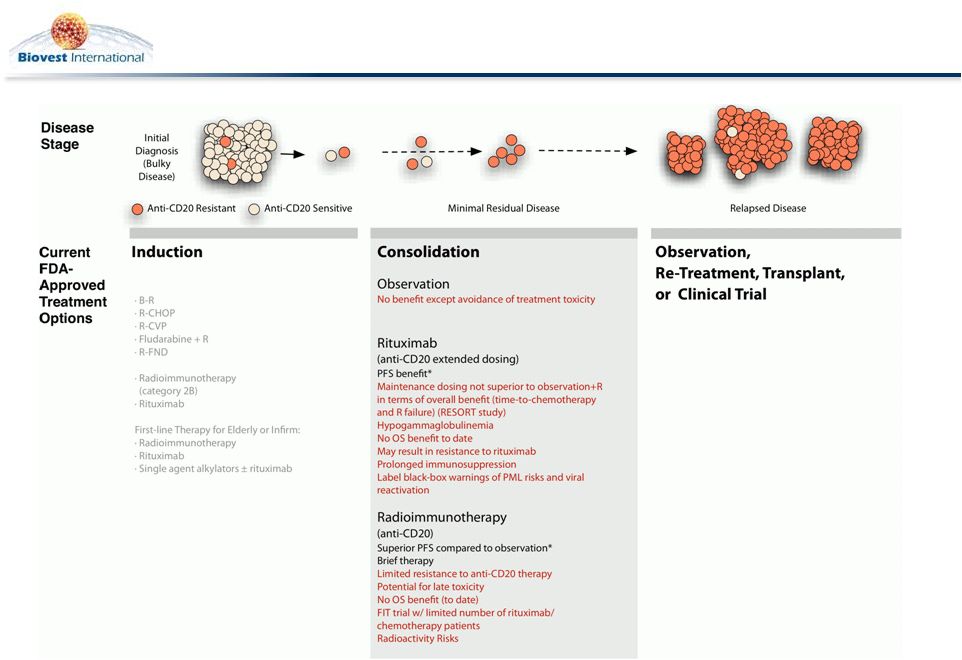 Post-Induction Consolidation Therapy: An Unmet Need in
Follicular Lymphoma
7 |
 BiovaxID Consolidation Therapy
BiovaxID acts as a novel, non-cross-resistant, consolidation therapy for
patients with Follicular Lymphoma (FL) that achieve a Complete Response
following induction treatment.
Why do we need another consolidation therapy in FL?
Maintenance rituximab appears equivalent to rituximab retreatment and is being
challenged as a consolidation option (Kahl, et al. ASH 2011 RESORT study
outcomes)
1
Practicing oncologists reconsidering the role of maintenance rituximab based on
the RESORT trial
1
Safety profile of vaccine compares favorably to maintenance rituximab
Vaccination, unlike all other consolidation therapies, generates
long-term anti-
lymphoma immunity
Vaccination does not preclude future retreatment with rituximab and may even
enhance the benefit of subsequent therapies
Vaccine mechanism of action complements rituximab’s mechanism of action
Not all patients are eligible or can tolerate prolonged immunosuppression
associated with currently available consolidation therapies.
1
Kahl, B. et al. Results of Eastern Cooperative Oncology Group Protocol E4402
(RESORT): A Randomized Phase III Study Comparing Two Different Rituximab
Dosing Strategies for Low Tumor Burden Follicular Lymphoma. Abstract LBA-6, ASH
2011
8 |
 Corporate Contact:
Douglas W.
Calder
Vice President, Strategic Planning
& Capital Markets
324 South Hyde Park Ave.
#350 Tampa, FL
33606 Phone:
813-864-2558
Email:
dwcalder@Biovest.com
OTCQB: BVTI
www.Biovest.com |
