Attached files
| file | filename |
|---|---|
| 8-K - SYNERGETICS USA, INC. 8-K - SYNERGETICS USA INC | synergetics8k.htm |

Investor Presentation
MAY 2012
OPHTHALMOLOGY
NEUROSURGERY
QUALITY. PERFORMANCE. INNOVATION.
OPHTHALMOLOGY
NEUROSURGERY
QUALITY. PERFORMANCE. INNOVATION.

Safe Harbor Statement
Certain statements made in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. Among other, statements concerning managements expectations of future financial results, potential business, acquisitions, government agency approvals, additional indications and therapeutic applications for medical devices, as well as their outcomes, clinical efficacy and potential markets are forward looking. Forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from predicted results. For a discussion of such risks and uncertainties, please refer to the information set forth under Risk Factors included in Synergetics USA, Inc.s Annual Report on Form 10-K for the year ended July 31, 2011, and information contained in subsequent filings with the Securities and Exchange Commission. These forward looking statements are made based upon our current expectations and we undertake no duty to update information provided in this presentation.
Certain statements made in this presentation are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. Among other, statements concerning managements expectations of future financial results, potential business, acquisitions, government agency approvals, additional indications and therapeutic applications for medical devices, as well as their outcomes, clinical efficacy and potential markets are forward looking. Forward-looking statements involve risks and uncertainties that could cause actual results to differ materially from predicted results. For a discussion of such risks and uncertainties, please refer to the information set forth under Risk Factors included in Synergetics USA, Inc.s Annual Report on Form 10-K for the year ended July 31, 2011, and information contained in subsequent filings with the Securities and Exchange Commission. These forward looking statements are made based upon our current expectations and we undertake no duty to update information provided in this presentation.

Overview
Corporate Information
Synergetics USA, Inc. is a medical device company focused in the fast-growing ophthalmology and neurosurgery markets
Formed through a reverse merger of Synergetics, Inc. and Valley Forge Scientific in 2005
Synergetics was founded in 1991 and Valley Forge was founded in 1980
Corporate Headquarters: OFallon, MO
Manufacturing Facilities: OFallon, MO and King of Prussia, PA
Market Information*
NASDAQ: SURG
Market Cap: $132mm
52 Week Range: $4.61 $7.55
Shares Outstanding: 25mm
Institutional Ownership: 44%
Russell 2000 & 3000 Indexes
*Source: Google Finance, as of 5/24/12.
Corporate Information
Synergetics USA, Inc. is a medical device company focused in the fast-growing ophthalmology and neurosurgery markets
Formed through a reverse merger of Synergetics, Inc. and Valley Forge Scientific in 2005
Synergetics was founded in 1991 and Valley Forge was founded in 1980
Corporate Headquarters: OFallon, MO
Manufacturing Facilities: OFallon, MO and King of Prussia, PA
Market Information*
NASDAQ: SURG
Market Cap: $132mm
52 Week Range: $4.61 $7.55
Shares Outstanding: 25mm
Institutional Ownership: 44%
Russell 2000 & 3000 Indexes
*Source: Google Finance, as of 5/24/12.
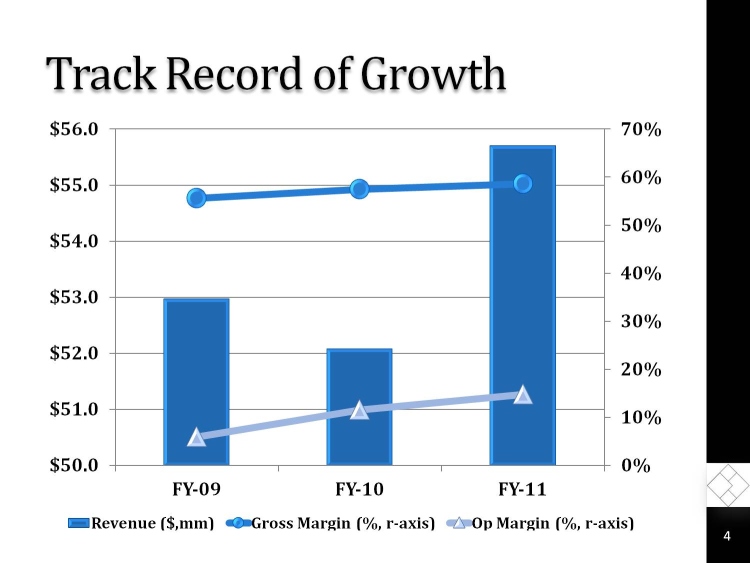
Track Record of Growth
FY-09
FY-10
FY-11
Revenue ($,mm)
Gross Margin (%, r-axis)
Op Margin (%, r-axis)
0%
10%
20%
30%
40%
50%
60%
70%
$50.0
$51.0
$52.0
$53.0
$54.0
$55.0
$56.0
FY-09
FY-10
FY-11
Revenue ($,mm)
Gross Margin (%, r-axis)
Op Margin (%, r-axis)
0%
10%
20%
30%
40%
50%
60%
70%
$50.0
$51.0
$52.0
$53.0
$54.0
$55.0
$56.0
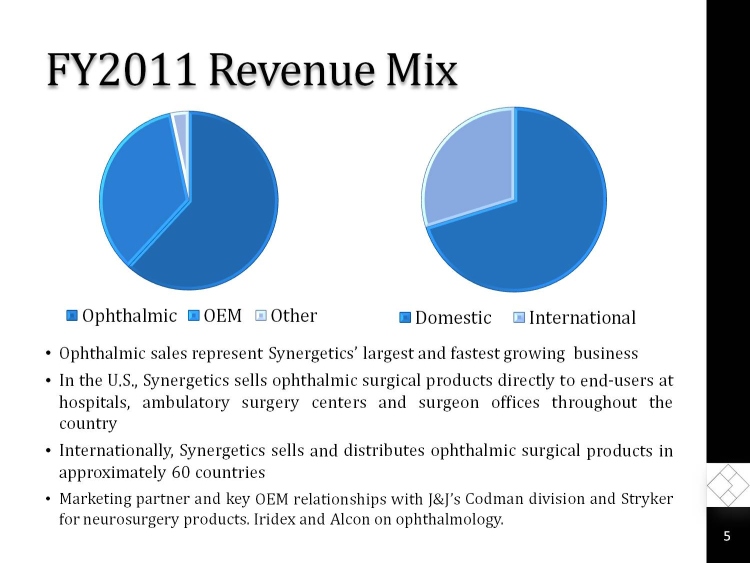
FY2011 Revenue Mix
Ophthalmic
OEM
Other
Domestic
International
Ophthalmic sales represent Synergetics largest and fastest growing business
In the U.S., Synergetics sells ophthalmic surgical products directly to end-users at hospitals, ambulatory surgery centers and surgeon offices throughout the country
Internationally, Synergetics sells and distributes ophthalmic surgical products in approximately 60 countries
Marketing partner and key OEM relationships with J&Js Codman division and Stryker for neurosurgery products. Iridex and Alcon on ophthalmology.
Ophthalmic
OEM
Other
Domestic
International
Ophthalmic sales represent Synergetics largest and fastest growing business
In the U.S., Synergetics sells ophthalmic surgical products directly to end-users at hospitals, ambulatory surgery centers and surgeon offices throughout the country
Internationally, Synergetics sells and distributes ophthalmic surgical products in approximately 60 countries
Marketing partner and key OEM relationships with J&Js Codman division and Stryker for neurosurgery products. Iridex and Alcon on ophthalmology.

Overall Strategy
1.Drive accelerating growth in Ophthalmology
2.Manage OEM neurosurgery business for stable growth and strong cash flows
3.Deliver improving profitability through enterprise-wide lean initiatives
4.Demonstrate solid financial performance
1.Drive accelerating growth in Ophthalmology
2.Manage OEM neurosurgery business for stable growth and strong cash flows
3.Deliver improving profitability through enterprise-wide lean initiatives
4.Demonstrate solid financial performance

Recent Events
1.Implementation of corporate-wide lean initiatives
2.Project Restore to improve cost structure
3.Transition of Neurosurgery business to OEM marketing partners from direct sales
4.Alcon settlement and discontinuation of supply agreement
1.Implementation of corporate-wide lean initiatives
2.Project Restore to improve cost structure
3.Transition of Neurosurgery business to OEM marketing partners from direct sales
4.Alcon settlement and discontinuation of supply agreement

Ophthalmic Surgical Market
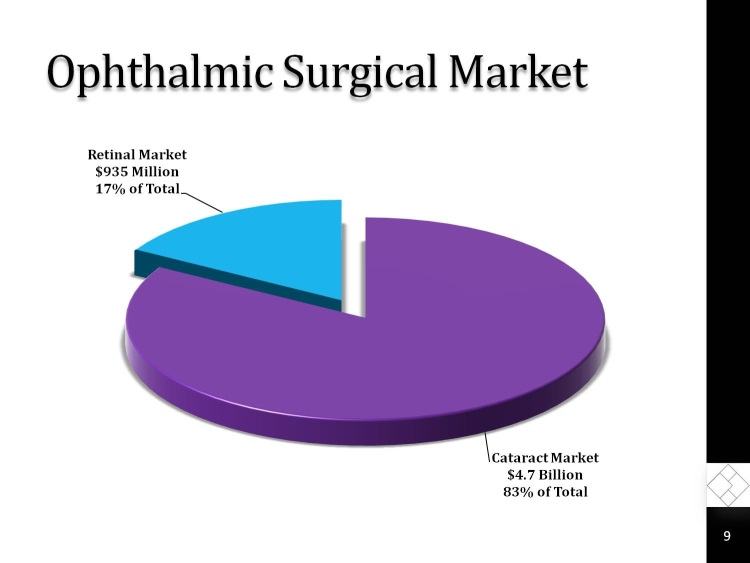
Ophthalmic Surgical Market
Retinal Market $935 Million 17% of Total
Cataract Market $4.7 Billion 83% of Total
Retinal Market $935 Million 17% of Total
Cataract Market $4.7 Billion 83% of Total
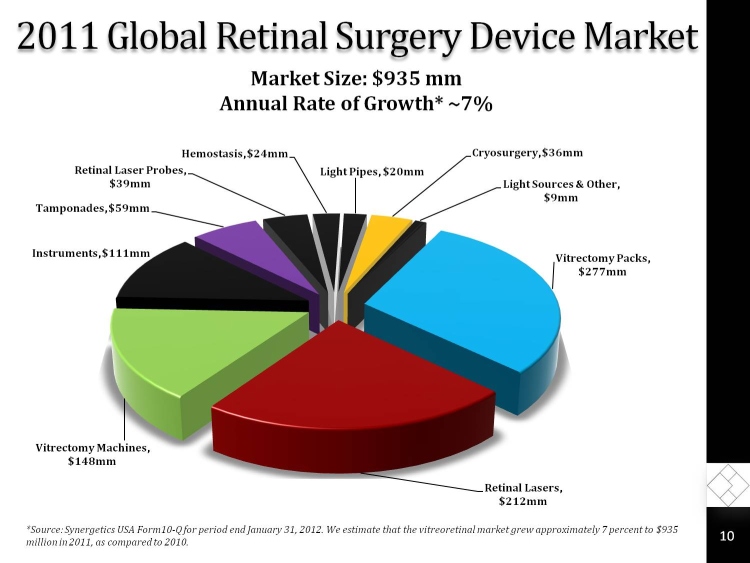
2011 Global Retinal Surgery Device Market
Market Size: $935 mm Annual Rate of Growth* ~7%
Vitrectomy Packs, $277mm
Retinal Lasers, $212mm
Vitrectomy Machines, $148mm
Instruments, $111mm
Tamponades, $59mm
Retinal Laser Probes, $39mm
Hemostasis, $24mm
Light Pipes, $20mm
Cryosurgery, $36mm
Light Sources & Other, $9mm
*Source: Synergetics USA Form10-Q for period end January 31, 2012. We estimate that the vitreoretinal market grew approximately 7 percent to $935 million in 2011, as compared to 2010.
Market Size: $935 mm Annual Rate of Growth* ~7%
Vitrectomy Packs, $277mm
Retinal Lasers, $212mm
Vitrectomy Machines, $148mm
Instruments, $111mm
Tamponades, $59mm
Retinal Laser Probes, $39mm
Hemostasis, $24mm
Light Pipes, $20mm
Cryosurgery, $36mm
Light Sources & Other, $9mm
*Source: Synergetics USA Form10-Q for period end January 31, 2012. We estimate that the vitreoretinal market grew approximately 7 percent to $935 million in 2011, as compared to 2010.
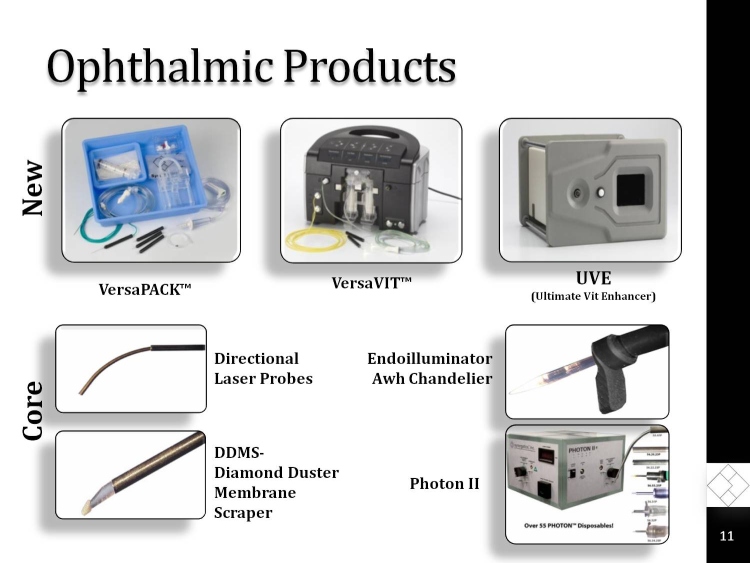
Ophthalmic Products
Core
New
VersaPACK
UVE
(Ultimate Vit Enhancer)
VersaVIT
Directional Laser Probes
DDMS-
Diamond Duster Membrane Scraper
Endoilluminator Awh Chandelier
Photon II
Core
New
VersaPACK
UVE
(Ultimate Vit Enhancer)
VersaVIT
Directional Laser Probes
DDMS-
Diamond Duster Membrane Scraper
Endoilluminator Awh Chandelier
Photon II

VersaPACK: Compelling Value Proposition
VersaPACK is our first product for the $275 million vitrectomy pack market
Compelling value proposition to retinal surgeons
Competitively priced vs. other packs
Compatible with existing competitive vitrectomy machines
Enables continued use of 1st gen machines thus avoiding large capital expenditure
Estimated 200,000 vitrectomies performed yearly (U.S.)
High margin, recurring disposable product
VersaPACK is our first product for the $275 million vitrectomy pack market
Compelling value proposition to retinal surgeons
Competitively priced vs. other packs
Compatible with existing competitive vitrectomy machines
Enables continued use of 1st gen machines thus avoiding large capital expenditure
Estimated 200,000 vitrectomies performed yearly (U.S.)
High margin, recurring disposable product
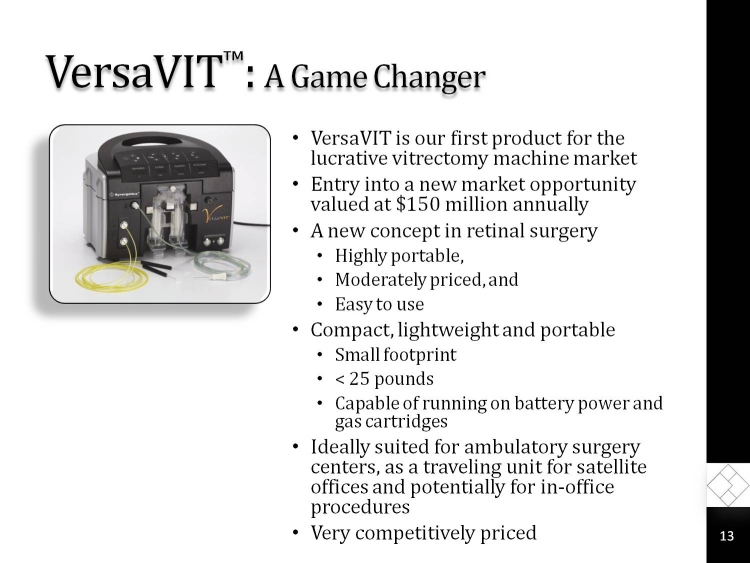
VersaVIT: A Game Changer
VersaVIT is our first product for the lucrative vitrectomy machine market
Entry into a new market opportunity valued at $150 million annually
A new concept in retinal surgery
Highly portable,
Moderately priced, and
Easy to use
Compact, lightweight and portable
Small footprint
< 25 pounds
Capable of running on battery power and gas cartridges
Ideally suited for ambulatory surgery centers, as a traveling unit for satellite offices and potentially for in-office procedures
Very competitively priced
VersaVIT is our first product for the lucrative vitrectomy machine market
Entry into a new market opportunity valued at $150 million annually
A new concept in retinal surgery
Highly portable,
Moderately priced, and
Easy to use
Compact, lightweight and portable
Small footprint
< 25 pounds
Capable of running on battery power and gas cartridges
Ideally suited for ambulatory surgery centers, as a traveling unit for satellite offices and potentially for in-office procedures
Very competitively priced

VersaVIT vs. the Competition
VersaVIT vs. ACCURUS
(25lbs vs. 90lbs)
CONSTELLATION Vision System
CONSTELLATION Vision System and ACCURUS are registered trademarks of Alcon Laboratories a division of Novartis
VersaVIT vs. ACCURUS
(25lbs vs. 90lbs)
CONSTELLATION Vision System
CONSTELLATION Vision System and ACCURUS are registered trademarks of Alcon Laboratories a division of Novartis

Ophthalmology Product Video

Neurosurgery Market

Neurosurgery Overview
Best-in-class neurosurgical technologies
Ultrasonic aspirators
Disposable tips and tubing
Electrosurgical generators
Disposable bipolar forceps
Strong OEM partnerships
J&Js Codman division distributes our electrosurgical generators and bipolar forceps
Stryker distributes our ultrasonic aspirator disposables
Multi-year OEM contracts with Codman and Stryker provide high visibility
Attractive operating margins
High barriers to entry
Expanding OEM platform complements our strategic focus
Packaging Mitosol, a drug used in glaucoma surgery, for Mobius TherapeuticsTM
Best-in-class neurosurgical technologies
Ultrasonic aspirators
Disposable tips and tubing
Electrosurgical generators
Disposable bipolar forceps
Strong OEM partnerships
J&Js Codman division distributes our electrosurgical generators and bipolar forceps
Stryker distributes our ultrasonic aspirator disposables
Multi-year OEM contracts with Codman and Stryker provide high visibility
Attractive operating margins
High barriers to entry
Expanding OEM platform complements our strategic focus
Packaging Mitosol, a drug used in glaucoma surgery, for Mobius TherapeuticsTM
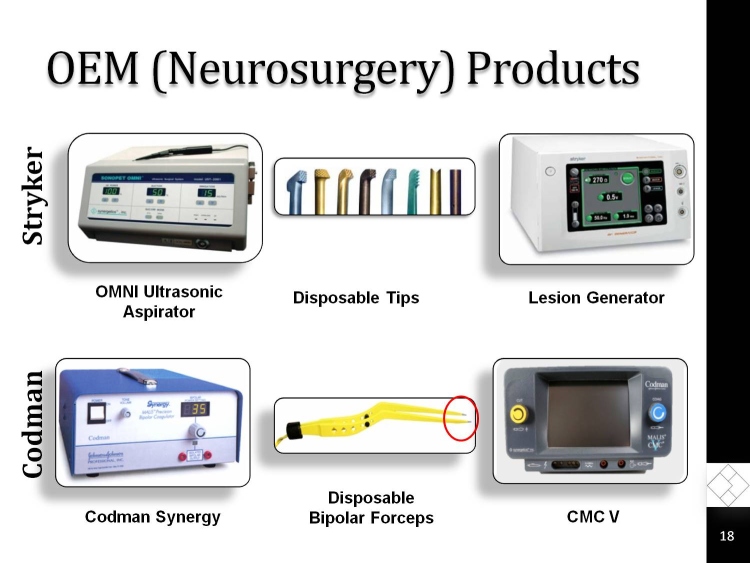
OEM (Neurosurgery) Products
Codman
Stryker
Lesion Generator
OMNI Ultrasonic Aspirator
Disposable Tips
Codman Synergy
Disposable
Bipolar Forceps
CMC V
Codman
Stryker
Lesion Generator
OMNI Ultrasonic Aspirator
Disposable Tips
Codman Synergy
Disposable
Bipolar Forceps
CMC V

Neurology Product Video

Financials
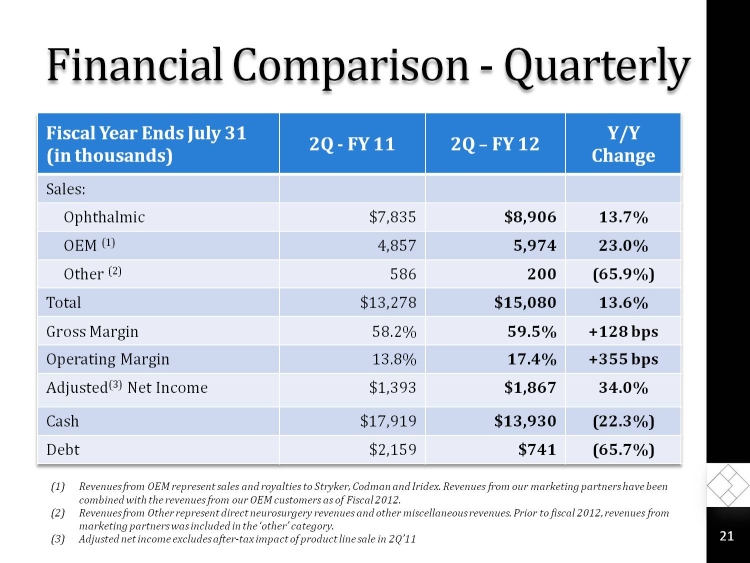
Financial Comparison - Quarterly
Fiscal Year Ends July 31
(in thousands)
2Q - FY 11
2Q FY 12
Y/Y Change
Sales:
Ophthalmic
$7,835
$8,906
13.7%
OEM (1)
4,857
5,974
23.0%
Other (2)
586
200
(65.9%)
Total
$13,278
$15,080
13.6%
Gross Margin
58.2%
59.5%
+128 bps
Operating Margin
13.8%
17.4%
+355 bps
Adjusted(3) Net Income
$1,393
$1,867
34.0%
Cash
$17,919
$13,930
(22.3%)
Debt
$2,159
$741
(65.7%)
(1)Revenues from OEM represent sales and royalties to Stryker, Codman and Iridex. Revenues from our marketing partners have been combined with the revenues from our OEM customers as of Fiscal 2012.
(2)Revenues from Other represent direct neurosurgery revenues and other miscellaneous revenues. Prior to fiscal 2012, revenues from marketing partners was included in the other category.
(3)Adjusted net income excludes after-tax impact of product line sale in 2Q11
Fiscal Year Ends July 31
(in thousands)
2Q - FY 11
2Q FY 12
Y/Y Change
Sales:
Ophthalmic
$7,835
$8,906
13.7%
OEM (1)
4,857
5,974
23.0%
Other (2)
586
200
(65.9%)
Total
$13,278
$15,080
13.6%
Gross Margin
58.2%
59.5%
+128 bps
Operating Margin
13.8%
17.4%
+355 bps
Adjusted(3) Net Income
$1,393
$1,867
34.0%
Cash
$17,919
$13,930
(22.3%)
Debt
$2,159
$741
(65.7%)
(1)Revenues from OEM represent sales and royalties to Stryker, Codman and Iridex. Revenues from our marketing partners have been combined with the revenues from our OEM customers as of Fiscal 2012.
(2)Revenues from Other represent direct neurosurgery revenues and other miscellaneous revenues. Prior to fiscal 2012, revenues from marketing partners was included in the other category.
(3)Adjusted net income excludes after-tax impact of product line sale in 2Q11
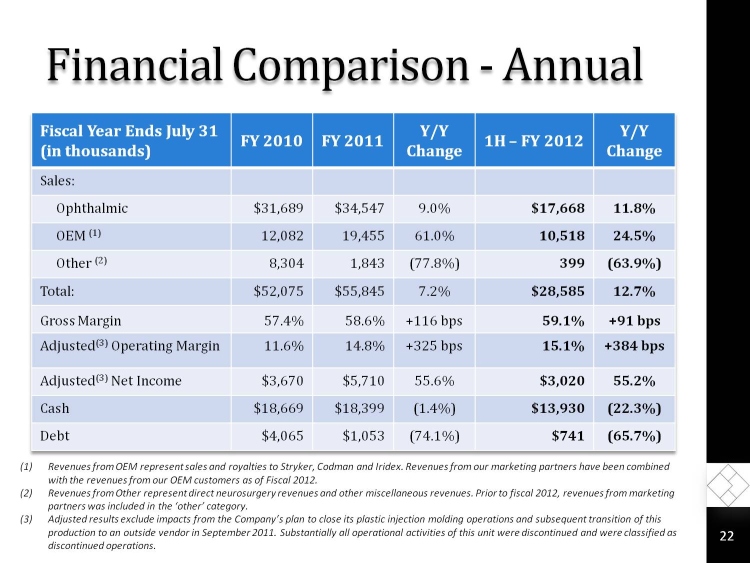
Financial Comparison - Annual
Fiscal Year Ends July 31
(in thousands)
FY 2010
FY 2011
Y/Y Change
1H FY 2012
Y/Y Change
Sales:
Ophthalmic
$31,689
$34,547
9.0%
$17,668
11.8%
OEM (1)
12,082
19,455
61.0%
10,518
24.5%
Other (2)
8,304
1,843
(77.8%)
399
(63.9%)
Total:
$52,075
$55,845
7.2%
$28,585
12.7%
Gross Margin
57.4%
58.6%
+116 bps
59.1%
+91 bps
Adjusted(3) Operating Margin
11.6%
14.8%
+325 bps
15.1%
+384 bps
Adjusted(3) Net Income
$3,670
$5,710
55.6%
$3,020
55.2%
Cash
$18,669
$18,399
(1.4%)
$13,930
(22.3%)
Debt
$4,065
$1,053
(74.1%)
$741
(65.7%)
(1)Revenues from OEM represent sales and royalties to Stryker, Codman and Iridex. Revenues from our marketing partners have been combined with the revenues from our OEM customers as of Fiscal 2012.
(2)Revenues from Other represent direct neurosurgery revenues and other miscellaneous revenues. Prior to fiscal 2012, revenues from marketing partners was included in the other category.
(3)Adjusted results exclude impacts from the Companys plan to close its plastic injection molding operations and subsequent transition of this production to an outside vendor in September 2011. Substantially all operational activities of this unit were discontinued and were classified as discontinued operations.
Fiscal Year Ends July 31
(in thousands)
FY 2010
FY 2011
Y/Y Change
1H FY 2012
Y/Y Change
Sales:
Ophthalmic
$31,689
$34,547
9.0%
$17,668
11.8%
OEM (1)
12,082
19,455
61.0%
10,518
24.5%
Other (2)
8,304
1,843
(77.8%)
399
(63.9%)
Total:
$52,075
$55,845
7.2%
$28,585
12.7%
Gross Margin
57.4%
58.6%
+116 bps
59.1%
+91 bps
Adjusted(3) Operating Margin
11.6%
14.8%
+325 bps
15.1%
+384 bps
Adjusted(3) Net Income
$3,670
$5,710
55.6%
$3,020
55.2%
Cash
$18,669
$18,399
(1.4%)
$13,930
(22.3%)
Debt
$4,065
$1,053
(74.1%)
$741
(65.7%)
(1)Revenues from OEM represent sales and royalties to Stryker, Codman and Iridex. Revenues from our marketing partners have been combined with the revenues from our OEM customers as of Fiscal 2012.
(2)Revenues from Other represent direct neurosurgery revenues and other miscellaneous revenues. Prior to fiscal 2012, revenues from marketing partners was included in the other category.
(3)Adjusted results exclude impacts from the Companys plan to close its plastic injection molding operations and subsequent transition of this production to an outside vendor in September 2011. Substantially all operational activities of this unit were discontinued and were classified as discontinued operations.

Investment Rationale
Serving growing ophthalmic and neurosurgery markets with leading technologies
Retinal surgery a compelling segment of ophthalmology
High barriers to entry and limited competition
New product introductions to help drive acceleration in revenue growth
Business model favors high margin disposables and leverages off our capital equipment
Lean initiatives fueling improving operating margins
Serving growing ophthalmic and neurosurgery markets with leading technologies
Retinal surgery a compelling segment of ophthalmology
High barriers to entry and limited competition
New product introductions to help drive acceleration in revenue growth
Business model favors high margin disposables and leverages off our capital equipment
Lean initiatives fueling improving operating margins

Management Team
David M. Hable President, CEO
Over 30 years of progressive responsibility in sales, marketing, new business development and general management in the medical device industry. 20+ years with J&J/Codman.
Pamela Boone Executive Vice President, CFO
Previously served as CFO, VP and Corporate Controller for Maverick Tube Corporation. 25 years of financial expertise.
Jerry Malis, M.D. Executive Vice President, CSO
Served as President, CEO and Chairman of Valley Forge. Over 40 years of industry experience. Published over 50 articles in the biological science, electronics and engineering fields. Issued ten U.S. patents.
Michael Fanning Vice President, Domestic Sales
Over 20 years in sales and management roles, working in service, medical device and manufacturing sectors.
Jason Stroisch Vice President, International Sales & Marketing
Over 15 years in the medical device industry covering engineering, international sales and marketing management roles.
Joan Kraus Vice President, Regulatory Affairs / Quality Assurance
Over 20 years in quality systems and process improvement roles working in healthcare, manufacturing, distribution and service sectors.
David M. Hable President, CEO
Over 30 years of progressive responsibility in sales, marketing, new business development and general management in the medical device industry. 20+ years with J&J/Codman.
Pamela Boone Executive Vice President, CFO
Previously served as CFO, VP and Corporate Controller for Maverick Tube Corporation. 25 years of financial expertise.
Jerry Malis, M.D. Executive Vice President, CSO
Served as President, CEO and Chairman of Valley Forge. Over 40 years of industry experience. Published over 50 articles in the biological science, electronics and engineering fields. Issued ten U.S. patents.
Michael Fanning Vice President, Domestic Sales
Over 20 years in sales and management roles, working in service, medical device and manufacturing sectors.
Jason Stroisch Vice President, International Sales & Marketing
Over 15 years in the medical device industry covering engineering, international sales and marketing management roles.
Joan Kraus Vice President, Regulatory Affairs / Quality Assurance
Over 20 years in quality systems and process improvement roles working in healthcare, manufacturing, distribution and service sectors.
OPHTHALMOLOGY
QUALITY. PERFORMANCE. INNOVATION.
NEUROSURGERY
QUALITY. PERFORMANCE. INNOVATION.
NEUROSURGERY
