Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a12-12596_1ex99d1.htm |
| EX-99.4 - EX-99.4 - Innoviva, Inc. | a12-12596_1ex99d4.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a12-12596_1ex99d2.htm |
| 8-K - 8-K - Innoviva, Inc. | a12-12596_18k.htm |
Exhibit 99.3
Poster No. D69
Effect of fluticasone furoate (FF)/vilanterol (VI) administered once daily on 24h pulmonary function in patients with COPD: a randomized, three-way, incomplete block, crossover study
Boscia JA,(1) Pudi KK,(2) Zvarich MT,(3) Sanford L,(4) Crim C(3)
(1)CU Pharmaceutical Research, Union, SC, USA; (2)Upstate Pharmaceutical Research, Greenville, SC, USA; (3)Respiratory Medicines Development Center, GlaxoSmithKline, Research Triangle Park, NC, USA; (4)Quantitative Sciences Division, GlaxoSmithKline, Uxbridge, UK
INTRODUCTION
· Available inhaled corticosteroid/long-acting beta2 agonist (ICS/LABA) combinations for COPD require twice-daily administration.
· Once-daily treatment could improve adherence and simplify treatment in chronic diseases such as COPD.(1)
· VI, an inhaled LABA with 24-h activity,(2) provides clinically relevant 24-h improvement in lung function in COPD.(3) FF is an ICS still active at 24h with demonstrated efficacy in asthma.(4)–(8)
· The combination FF/VI is being developed in a new dry powder inhaler (DPI) for the treatment of COPD(9) and asthma with the potential for once-daily dosing.
OBJECTIVES
· To evaluate the 24-h spirometric effect of FF/VI compared with placebo in patients with COPD.
METHODS
· Phase III, multicenter, randomized, double-blind, placebo-controlled, incomplete block, crossover study.
· Eligible patients were aged >40 years, had COPD, a post-bronchodilator FEV1 of <70% predicted, FEV1/FVC ratio of <0.70, current or prior history of >10 pack-years of cigarette smoking, and a score of >2 on the Modified Medical Research Council (mMRC) Dyspnea 0–4 Scale.
· A schematic of the study design is shown in Figure 1. Each treatment was inhaled once a day in the morning using a new DPI.
Figure 1. Study schematic.
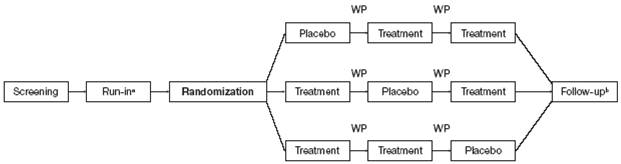
Treatment was FF/VI 50/25mcg, FF/VI 100/25mcg, FF/VI 200/25mcg.
Each patient received two of three doses of FF/VI and placebo.
(a) Run-in period was 2 weeks; during this time all patients received placebo to establish a stable baseline. Each treatment period lasted 28 days.
(b) Follow-up was 7 days after the last treatment day.
WP = washout period (each 2 weeks).
Efficacy endpoints and safety measures
· Primary: time-adjusted (weighted mean) area under the FEV1-vs-time curve over 24h at the end of each 28-day treatment period (Period Days 28–29)
· analysis was performed using a mixed model with period, dose regimen, period baseline FEV1 and mean baseline FEV1 fitted as fixed effects and subject as a random coefficient.
· Secondary (1) 0–25h serial FEV1 at Period Days 28–29
· analysis was the same as used for primary analysis but with the addition of a categorical fixed effect for nominal time relative to dosing on Period Day 28 and a dose regimen by time interaction term.
· Secondary (2) change from treatment period baseline in trough FEV1 (Period Day 29)
· analysis was the same as used for the primary analysis.
· Safety assessments: incidence of adverse events (AEs), pneumonias and exacerbations; vital signs; QTc calculated by Fridericia formula (QTcF); heart rate; clinical laboratory evaluations, including serum glucose and potassium levels; oropharyngeal examinations; and 0–24h serum cortisol at Period Days 28–29.
RESULTS
· Fifty-four patients were randomized; all received at least one dose of study drug (intent-to-treat [ITT] population) and 78% completed all three treatment periods and follow-up.
· Table 1 shows patient demographic characteristics and screening lung function.
Table 1. Patient demographics and lung function at screening (ITT population).
|
Variable |
|
Total (N=54) |
|
|
|
|
|
Age, years |
|
57.9 (9.24) |
|
|
|
|
|
Female, n (%) |
|
29 (54) |
|
|
|
|
|
Race, n (%) |
|
|
|
White/Caucasian/European heritage |
|
48 (89) |
|
African American/African heritage |
|
6 (11) |
|
|
|
|
|
Smoking status at screening, n (%) |
|
|
|
Current smoker |
|
45 (83) |
|
Former smoker |
|
9 (17) |
|
|
|
|
|
Duration of COPD, n (%) |
|
|
|
<1 year |
|
6 (11) |
|
>1 to <5 years |
|
19 (35) |
|
>5 to <10years |
|
17 (31) |
|
>10 years |
|
12 (22) |
|
|
|
|
|
Type of COPD, n (%) |
|
|
|
Chronic bronchitis(a) |
|
35 (65) |
|
Emphysema(a) |
|
34 (63) |
|
|
|
|
|
Patients with >1 exacerbation in the 12 months prior to screening, n (%) |
|
|
|
Requiring oral/systemic corticosteroids and/or antibiotics (but not involving hospitalization) |
|
4 (7) |
|
Requiring hospitalization |
|
4 (7) |
|
|
|
|
|
Dyspnea score on mMRC Dyspnea Scale, n (%) |
|
|
|
2 |
|
29 (54) |
|
3 |
|
24 (44) |
|
4 |
|
1 (2) |
|
|
|
|
|
COPD medication taken at screening, n (%) |
|
|
|
Any COPD medication |
|
36 (67) |
|
Short-acting beta2 agonist |
|
34 (63) |
|
Long-acting anticholinergic |
|
5 (9) |
|
Short-acting anticholinergic |
|
5 (9) |
|
Oxygen |
|
2 (4) |
|
ICS + LABA(b) |
|
1 (2) |
|
|
|
|
|
Lung function at screening |
|
|
|
Post-bronchodilator % predicted FEV1 |
|
49.8 (10.61) |
|
Post-bronchodilator FEV1/FVC, % |
|
52.9 (9.52) |
|
Percent reversibility FEV1(c) |
|
8.8 (16.97) |
|
FEV1 reversibility, mL(c) |
|
90.7 (227.12) |
|
Patients with reversible(d) FEV1, n (%) |
|
14 (26) |
Data presented as mean (SD) unless otherwise stated
(a)Patients could select both chronic bronchitis and emphysema;
(b)salmeterol xinafoate plus fluticasone propionate;
(c)N=53;
(d)Reversibility was defined as an increase in FEV1 of >12% and >200mL following administration of albuterol/salbutamol
SD = standard deviation
Efficacy
· All three strengths of FF/VI demonstrated significantly higher 0–24h weighted mean FEV1 than placebo over Period Days 28–29. The effect was not ICS-dose related; adjusted mean improvements in FEV1 for FF/VI compared with placebo were 220–236mL (all p<0.001; Figure 2).
· Adjusted mean improvements against placebo in change from period baseline serial FEV1 measures (Period Days 28–29) were observed at each timepoint and with each strength of FF/VI over the 0–25h period (147–301mL; p<0.001 vs placebo; Figure 3a and 3b), indicating sustained bronchodilation.
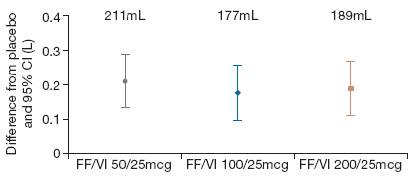
· Analysis of change from period baseline in trough FEV1 at the end of the 28-day treatment periods showed both statistically and clinically significantly greater values for all three strengths of FF/VI compared with placebo (adjusted mean improvements, 177–211mL; all p<0.001; Figure 4).
Figure 2. Weighted mean treatment differences from placebo in 0–24h weighted mean FEV1 (L) over Period Days 28–29 (ITT population).
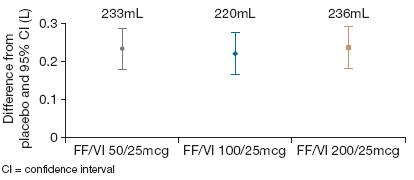
Figure 3. (a) Adjusted mean and (b) adjusted mean treatment differences from placebo for change from period baseline in serial 0–25h FEV1 (L) over Period Days 28–29 (ITT population).
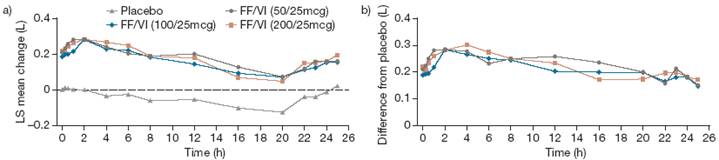
Figure 4. Adjusted mean differences from placebo in change from period baseline in trough FEV1 (L) at Period Day 29 (ITT population).
Safety
· FF/VI was well tolerated; the number of on-treatment AEs was low in each FF/VI group and was not ICS-dose dependent (3–4 [10–12%] with FF/VI; 2 [4%] with placebo) (Table 2).
· Two serious AEs were reported: one during the first washout period after treatment with placebo and one during the second washout period after treatment with FF/VI 50/25mcg (Table 2); neither was considered to be treatment related.
· No pneumonias were reported.
· No significant effects on vital signs, QTcF, heart rate, serum glucose or serum potassium levels were reported.
· Four cases of possible candidiasis were identified: one produced a positive culture (pre-FF/VI 200/25mcg treatment; period baseline assessment).
· Mean serum cortisol levels over Period Days 28–29 with FF/VI were similar to placebo (Figure 5). No significant effects on 0–24h weighted mean serum cortisol were seen (LS geometric mean ratio FF/VI to placebo 0.89–0.98; p=NS).
Figure 5. Raw geometric means for serial serum cortisol over Period Days 28–29 (nmol/L) (ITT population).
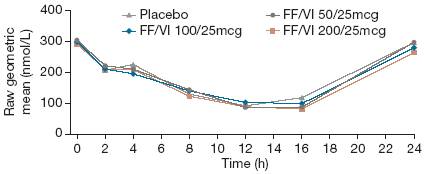
Table 2. Summary of AEs(a) reported by at least one patient in any treatment group (ITT population).
|
Variable |
|
Placebo |
|
FF/VI |
|
FF/VI |
|
FF/VI |
|
|
|
|
|
|
|
|
|
|
|
AEs on treatment, n (%) |
|
2 (4) |
|
4 (12) |
|
4 (12) |
|
3 (10) |
|
|
|
|
|
|
|
|
|
|
|
Infections and infestations |
|
|
|
|
|
|
|
|
|
Cellulitis |
|
0 |
|
0 |
|
1 (3) |
|
0 |
|
Rhinitis |
|
0 |
|
0 |
|
1 (3) |
|
0 |
|
Sinusitis |
|
0 |
|
1 (3) |
|
0 |
|
0 |
|
Upper respiratory tract infection |
|
0 |
|
0 |
|
1 (3) |
|
0 |
|
Urinary tract infection |
|
1 (2) |
|
0 |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Respiratory, thoracic and mediastinal disorders |
|
|
|
|
|
|
|
|
|
Epistaxis |
|
0 |
|
1 (3) |
|
0 |
|
0 |
|
Nasal congestion |
|
0 |
|
1 (3) |
|
0 |
|
0 |
|
Oropharyngeal pain |
|
1 (2) |
|
0 |
|
0 |
|
0 |
|
Pharyngeal inflammation |
|
0 |
|
0 |
|
0 |
|
1 (3) |
|
Postnasal drip |
|
1 (2) |
|
0 |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Gastrointestinal disorders |
|
|
|
|
|
|
|
|
|
Diarrhea |
|
0 |
|
0 |
|
1 (3) |
|
0 |
|
Vomiting |
|
0 |
|
0 |
|
1 (3) |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Injury, poisoning and procedural complications |
|
|
|
|
|
|
|
|
|
Caustic injury |
|
0 |
|
0 |
|
0 |
|
1 (3) |
|
|
|
|
|
|
|
|
|
|
|
Musculoskeletal and connective tissue disorders |
|
|
|
|
|
|
|
|
|
Muscle spasms |
|
0 |
|
0 |
|
0 |
|
1 (3) |
|
|
|
|
|
|
|
|
|
|
|
Nervous system disorders |
|
|
|
|
|
|
|
|
|
Headache |
|
0 |
|
1 (3) |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Vascular disorders |
|
|
|
|
|
|
|
|
|
Raynaud’s phenomenon |
|
0 |
|
1 (3) |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
AEs during washout, n (%) |
|
3 (6) |
|
4 (12) |
|
0 |
|
1 (3) |
|
|
|
|
|
|
|
|
|
|
|
Musculoskeletal and connective tissue disorders |
|
|
|
|
|
|
|
|
|
Neck pain |
|
1 (2) |
|
1 (3) |
|
0 |
|
0 |
|
CREST syndrome |
|
0 |
|
1 (3) |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Infections and infestations |
|
|
|
|
|
|
|
|
|
Upper respiratory tract infection |
|
1 (2) |
|
1 (3) |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Nervous system disorders |
|
|
|
|
|
|
|
|
|
Headache |
|
1 (2) |
|
0 |
|
0 |
|
0 |
|
Transient ischemic attack |
|
0 |
|
1 (3)(b) |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Gastrointestinal disorders |
|
|
|
|
|
|
|
|
|
Nausea |
|
0 |
|
1 (3)(b) |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Metabolism and nutrition disorders |
|
|
|
|
|
|
|
|
|
Hyponatremia |
|
0 |
|
1 (3)(b) |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Vascular disorders |
|
|
|
|
|
|
|
|
|
Hypertension |
|
0 |
|
0 |
|
0 |
|
1 (3) |
|
|
|
|
|
|
|
|
|
|
|
AEs leading to permanent withdrawal from study, n (%) |
|
0 |
|
1 (3)(b) |
|
0 |
|
0 |
|
|
|
|
|
|
|
|
|
|
|
Serious AEs, n (%) |
|
1 (2) |
|
1 (3)(b) |
|
0 |
|
0 |
(a)AEs defined by the Medical Dictionary for Regulatory Activities: one patient could report more than one AE
(b)This was a single patient who experienced a serious AE of transient ischemic attack (with previous history of strokes), accompanied by AEs of nausea and hyponatremia, which occurred during the second washout period and led to withdrawal from treatment
During the run-in period, prior to randomization, one patient reported an upper respiratory tract infection; this AE was not considered to be treatment related
CONCLUSIONS
· FF/VI inhaled once daily in the morning for 28 days produced significant improvements in pulmonary function with a prolonged (>24h) duration of action and was well tolerated, supporting once-daily dosing in patients with COPD.
REFERENCES
(1) Saini SD, et al. Am J Manag Care 2009;15:e22–e33.
(2) Morrison V, et al. Am J Respir Crit Care Med 2010;181:A4453.
(3) Hanania NA, et al. Chest 2012 Jan 12 [Epub ahead of print].
(4) Busse WW, et al. Thorax 2012;67:35–41.
(5) Bleecker ER, et al. Eur Respir J 2010;36(Suppl. 54):204s.
(6) Bateman ED, et al. Resp Med 2012;106:642–650.
(7) Woodcock A, et al. Respir Res 2011;12:160.
(8) Woodcock A, et al. Respir Res 2011;12:132.
(9) Lötvall J, et al. BMJ Open 2012;2:e000370.
ACKNOWLEDGMENTS
· This study was sponsored by GlaxoSmithKline. ClinicalTrails.gov: NCT01072149; protocol number: HZC110946.
· Joseph A Boscia (presenting author) is on the speakers’ bureau of GlaxoSmithKline and was a principal investigator in this clinical trial, which was sponsored by GlaxoSmithKline and administered by his employer CU Pharmaceutical Research.
· Editorial support (in the form of writing assistance, assembling tables and figures, collating author comments, grammatical editing and referencing) was provided by Lisa Moore at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.
![]()
Presented at the Annual Conference of the American Thoracic Society (ATS), San Francisco, California, USA, May 18–23, 2012
