Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - DELCATH SYSTEMS, INC. | d352485d8k.htm |
 Investor Presentation
(NASDAQ: DCTH)
May 2012
Exhibit 99.1 |
 2
DELCATH SYSTEMS, INC
Forward-looking Statements
This presentation contains forward-looking statements within the meaning of the Safe Harbor
Provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statement are
subject to certain risks and uncertainties that can cause actual results to differ materially
from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating
to: the benefits of the Gen 2 CHEMOSAT system and market acceptance of the same, patient outcomes
using the Gen 2 CHEMOSAT system, agreements with additional early launch centers in Europe, our
ability to manufacture CHEMOSAT systems and the time required to build inventory and establish
commercial operations in Europe, adoption, use and resulting sales, if any, for the CHEMOSAT
system in the EEA, our ability to successfully commercialize the chemosaturation system and the potential of the
chemosaturation system as a treatment for patients with cancers in the liver, acceptability of the
Phase III clinical trial data by the FDA, our ability to address the issues raised in the
Refusal to File letter received from the FDA and the timing of our re-submission of our NDA,
re-submission and acceptance of the Company's NDA by the FDA, approval of the Company's NDA for the treatment of
metastatic melanoma to the liver, adoption, use and resulting sales, if any, in the United States,
approval of the CHEMOSAT system in foreign markets, approval of the current or future
chemosaturation system for other indications and/or with other chemotherapeutic agents, actions
by the FDA or other foreign regulatory agencies, our ability to obtain reimbursement for the CHEMOSAT system, our
ability to successfully enter into distribution and strategic partnership agreements in foreign
markets and the corresponding revenue associated with such foreign markets, uncertainties
relating to the timing and results of research and development projects and future clinical
trials, acceptance of our IND amendment, submission and publication of the Phase II and III clinical trial data, the timing and
use, if any, of the line of credit from SVB and our ability to access this facility, and uncertainties
regarding our ability to raise additional capital and obtain financial and other resources for
any research, development and commercialization activities. These factors, and others, are
discussed from time to time in our filings with the Securities and Exchange Commission including our annual
report on Form 10-K and our reports on Forms 10-Q and 8-K. You should not place undue
reliance on these forward-looking statements, which speak only as of the date they are
made. We undertake no obligation to publicly update or revise these forward- looking
statements to reflect events or circumstances after the date they are made. |
 3
DELCATH SYSTEMS, INC
Our Mission
Concentrating the Power of Chemotherapy for Disease Control in the Liver
•
We are a cancer therapy company
•
Our technology offers the opportunity to gain control of tumors
in the liver
•
The liver is a site where uncontrolled disease is often life-limiting
or leads to withdrawal of systemic treatments in favor of
palliative care
•
We plan on being a fully-integrated company and are building
the infrastructure to develop and commercialize our products in
Europe and North America
o
In Europe our first product is approved and we have begun selling it
•
We believe that our first product, CHEMOSAT, may extend the
lives of a large number of cancer patients |
 4
DELCATH SYSTEMS, INC
Existing Liver Cancer Treatments Have Significant Limitations
The Problem
•
Metastatic disease to the liver, brain or lungs is often the life-
limiting location of solid tumors
o
In contrast to the brain and lungs, where systemic chemotherapy
and radiation can exert some degree of local control, tumors in the
liver are not particularly responsive to chemotherapy and radiation
therapy
•
Existing treatments to control tumors in the liver include:
Surgical resection
Radioembolization (SIRT)
Chemoembolization (TACE)
Radiofrequency ablation (RFA), Microwave, Cryoablation
Hepatic arterial infusion (HAI)
Systemic chemotherapy |
 5
DELCATH SYSTEMS, INC
Existing Liver Cancer Treatments Have Limitations
Unmet Medical Need Exists for More Effective Liver Cancer Treatments
Treatment
Advantages
Disadvantages
Systemic
–
Non-invasive
–
Repeatable
–
Systemic toxicities
–
Limited efficacy in liver
Regional
(e.g., Isolated Hepatic Perfusion)
–
Therapeutic effect
–
Targeted
–
Invasive/limited repeatability
–
Multiple treatments are
required but not possible
Focal
(e.g. surgery, radioembolization,
chemoembolization, radio
frequency ablation)
–
Partial removal or
treatment of tumors
–
Only 10% to 20% resectable
–
Invasive and/or limited
repeatability
–
Treatment is limited by
tumor size, number of
lesions and location
–
“See a tumor, treat a tumor” |
 6
DELCATH SYSTEMS, INC
Concentrating the Power of Chemotherapy for Disease Control in the Liver
Our Solution
•
Our proprietary CHEMOSAT system isolates the liver
circulation, delivers an ultra-high concentration of
chemotherapy (melphalan) to the liver and filters most of the
chemotherapy out of the blood prior to returning it to the patient
•
The procedure typically takes approximately two hours to
complete and involves a team including the interventional
radiologist and perfusionist
•
CHEMOSAT (Gen 2) has demonstrated minimal systemic
toxicities and impact to blood components in initial commercial
use and may complement systemic therapy
•
CHEMOSAT
has
been
used
on
approximately
200
patients to
date through clinical development and early commercial launch
|
 7
DELCATH SYSTEMS, INC
Chemosaturation
1.
ISOLATE
2.
SATURATE
3.
FILTRATE
The Delcath CHEMOSAT System
Improved disease control in the liver
Treats entire liver (macro and micro)
Controls systemic toxicities
Allows for over 100x effective dose
escalation at tumor site
Repeatable & minimally invasive,
Complements systemic therapy
Minimally Invasive, Repeatable Liver Procedure That Could Complement Systemic
Therapy Note: Image not to scale. |
 8
DELCATH SYSTEMS, INC
Concentrating the Power of Chemotherapy for Disease Control in the Liver
The Data
•
We conducted a randomized Phase 3 study under a Special
Protocol Assessment (“SPA”) using Generation 1 of our
chemosaturation system in patients with melanoma (ocular and
cutaneous) metastatic to the liver
•
Melanoma liver metastases are relatively homogeneous
regardless of origin
•
Liver metastases are typically the life-limiting aspect of the
disease
•
Melanoma is notoriously insensitive to systemic chemotherapy
and our study was a great demonstration of our technology’s
potential in a challenging histology |
 9
DELCATH SYSTEMS, INC
Phase III Clinical Trial Design
Randomized to CS
92 patients: ocular
or cutaneous melanoma
Best Alternative Care (BAC)
Investigator and patient decision
(any and all treatments)
CS/Melphalan
Treat every 4 weeks x 4 rounds
(responders
can receive up to 6 rounds)
Cross-over
Primary Trial Endpoint
•
Statistically significant difference in Hepatic Progression
Free Survival (“hPFS”): p < 0.05
•
Over 80% of Oncologic drugs approved by FDA between
2005
–
2007
on
endpoints
other
than
overall
survival
Modeled hPFS for Trial Success:
7.73 months (CS)
vs.
4 months (BAC)
Secondary Trial Endpoints
•
Hepatic
response
and
duration
of
hepatic
response
•
Overall
response
and
duration
of
overall
response
•
Overall
Survival
–
Diluted
by
Cross
Over
•
SAP calls for analysis of various patient cohorts
Pre-CS (Baseline)
Post-CS (22+ Months)
Hepatic Response –
Metastatic Melanoma
Fully Powered, 93 Patient, Randomized, Multi-Center NCI Led Study
CS = ChemoSaturation (CHEMOSAT) |
 10
DELCATH SYSTEMS, INC
Phase 3 Hepatic Progression-free Survival (ITT)
Hazard Ratio: 0.35
(CI: 0.23-0.54)
0
5 10 15
20
25
30 35
Months
CS-PHP
BAC
8.0
1.6
p<0.0001
1.0
Survival probability
0.8
0.6
0.4
0.2
0.0
CS-PHP Demonstrated a 5x Improvement in Primary Endpoint of hPFS
|
 11
DELCATH SYSTEMS, INC
Phase 3 Overall Progression-free Survival (ITT)
Hazard Ratio: 0.36
(CI: 0.23-0.57)
0
5 10 15
20
25
30 35
Months
CS-PHP
BAC
6.7
1.6
p<0.0001
1.0
Survival probability
0.8
0.6
0.4
0.2
0.0
CS also Demonstrated a Highly Statistically Significant Improvement in Overall
PFS |
 12
DELCATH SYSTEMS, INC
Overall survival (ITT population)
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
12
36
0
24
48
Time (months)
60
p-value = 0.4170
BAC
PHP
Censored observations
BAC vs PHP
55% Cross-over
HR=1.20 (CI: 0.78-1.85)
Overall Survival Confounded By Crossover Study Design
9.8
10.0 |
 13
DELCATH SYSTEMS, INC
Overall survival (ITT population)
Time (months)
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
12
36
0
24
48
60
4.1
PHP randomized
BAC crossover*
BAC only*
Censored observations
PHP randomized vs PHP crossover vs BAC only
9.8
13.1
Overall Survival Tail PHP Treated Patients
* Similar patient characteristics and demographics between BAC crossover and BAC
only |
 14
DELCATH SYSTEMS, INC
Overall survival (ITT population)
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
12
36
0
24
48
60
11.4
Total PHP incl. crossover
BAC only
Censored observations
Time (months)
Total PHP vs BAC only
4.1
Overall Survival Tail For Treated Patients |
 15
DELCATH SYSTEMS, INC
Positive Phase III Results*
•
Primary endpoint exceeded, p value = 0.0001, hazard ratio of .35
o
Treatment arm shows 5x median hepatic progression free (hPFS) survival compared to
control arm o
CS/PHP median hPFS of 8.0 months compared to 1.6 months for BAC
o
86% overall clinical benefit (CR + PR + SD)
•
Secondary endpoints support results
o
OS Secondary endpoint –
No difference in Kaplan-Meier curves due to cross over treatment response (9.8
months compared to 10.0 months)
o
CS/PHP median overall PFS of 6.7 months vs. 1.6 months for BAC
•
OS exploratory cohort analysis favorable
o
Median survival of 9.8 months for treatment arm compared to 4.1 months
non-crossover BAC patients o
Median survival of 11.4 months for all patients treated with melphalan, including
crossover o
8 CS/PHP-treated patients and 2 BAC-treated patients still alive as of
4/2012 •
Gen 1 Safety profile –
expected and consistent with currently approved labeling
for melphalan
o
30-day deaths on PHP: 3/44 patients (6.8%)
1 Neutropenic Sepsis (2.3%); 1 Hepatic Failure 2.5% (95% tumor burden); 1 gastric
perforation o
30-day deaths on BAC: 3/49 patients (6.1%)
Trial Outcomes Favorable and Consistent with Special Protocol Assessment
* Updated Investigator results presented at 2011 ECCO/ESMO Annual Meeting.
|
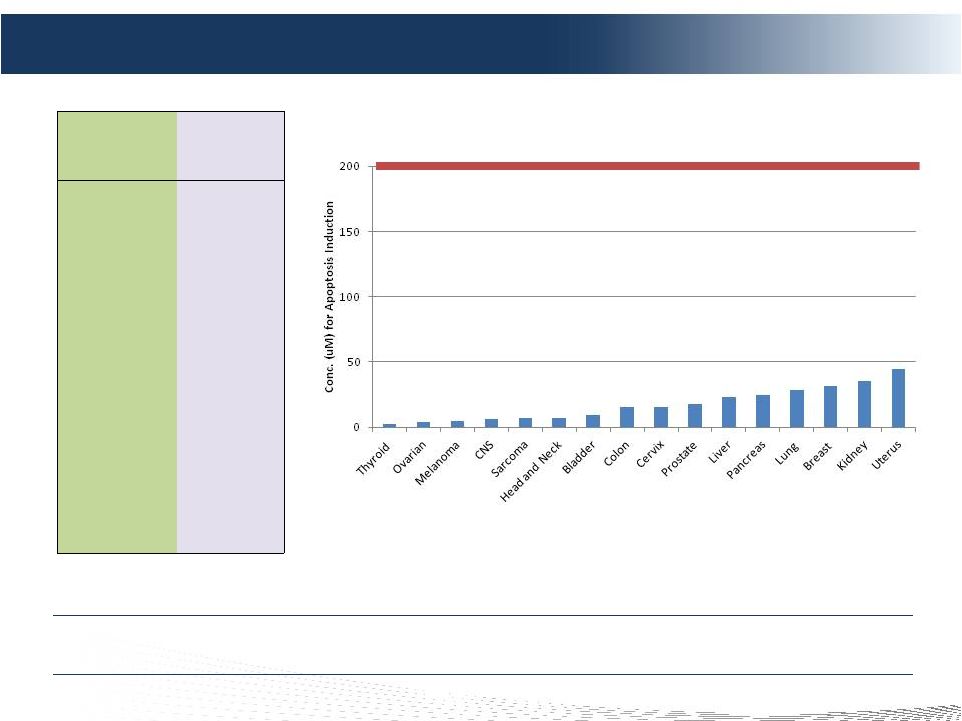 16
DELCATH SYSTEMS, INC
Melphalan Sensitivity: In Vitro Tumor Cell Lines Study
Cancer Origin
(Cell lines)
Apoptosis
Induction
(uM)
Thyroid
2.54
Ovarian
4.31
Melanoma
4.53
CNS
6.40
Sarcoma
6.68
Head and Neck
6.78
Bladder
9.50
Colon
15.12
Cervix
15.16
Prostate
17.55
Liver
23.23
Pancreas
25.00
Lung
28.60
Breast
31.82
Kidney
35.30
Uterus
44.60
We Believe CHEMOSAT Will Be Effective On a Wide Variety of Histologies
Melphalan Sensitivity-
Apoptosis Induction
Melphalan
Conc.
achieved
during
CHEMOSAT
=
~
192
uM
Tissue of Origin for Cell Line |
 17
DELCATH SYSTEMS, INC
Our Opportunity
•
At the concentrations of melphalan we are achieving in the
liver, we believe CHEMOSAT will be effective on a wide variety
of histologies
•
We believe that physicians are recognizing the broad
applicability of CHEMOSAT, based on early experience and
their interest in testing our technology with melphalan in a
variety of tumor histologies
•
CE Mark approved broad indication
•
Large global market opportunity with pharmaceutical-like gross
margin
Concentrating the Power of Chemotherapy for Disease Control in the Liver
|
 18
DELCATH SYSTEMS, INC
CHEMOSAT -
Potential Multi-Billion Dollar Market
8,708
6,563
4,085
8,212
7,202
33,966
19,861
33,953
52,143
5,585
7,671
99,749
0
25,000
50,000
75,000
100,000
125,000
150,000
175,000
200,000
USA
EU
APAC
$7 Billion Annual Global Opportunity with Pharmaceutical-Like Gross
Margins Sources: LEK Consulting, GLOBOCAN, Company estimates.
*TPM for initial U.S. labeled indication only.
EU: Initial target countries of Germany, UK, Italy, France, Spain, Netherlands,
Ireland. APAC: Initial target countries of China, Japan, S. Korea, Taiwan,
Australia. Assumes 2.5 treatments per patient.
Assumes EU ASP of $15K; US ASP of $25K; APAC ASP of $5K.
55,389
$2.2 B
8,708*
$2.6 B
189,943
$2.2 B
HCC
CRC
Melanoma
NET |
 19
DELCATH SYSTEMS, INC
Approved (CE Mark Device)
Regulatory Filing (NDA) Planned in August, 2012
Application
Submitted/
Planned
–
Mutual
Recognition
of
European
CE Mark in 2012-2013
Global Commercialization Status
On The Cusp of Addressing A Multi-Billion Dollar Global Market in Next Two
Years |
 20
DELCATH SYSTEMS, INC
European Commercialization Strategy
Strategy:
•
Focus efforts in 7 Target Countries (EU 5 + Netherlands & Ireland)
•
8-10 leading EU cancer centers as initial training centers
•
Validate business model and demonstrate scalability
•
Push and Pull marketing and selling strategy
Tactics & Execution:
•
Educate medical oncologists via contract organization
•
Sell to hospital-based interventional radiologists, surgeons and C-suite
decision makers with combination of direct sales and distributors
•
Hospitals procure melphalan from third parties and physicians use at their
discretion •
Establish European patient education & awareness programs (PR, website)
•
Leverage existing new technology reimbursement channels, while pursuing permanent
procedure reimbursement via Health Technology Assessment (HTA)
•
Clinical trials to generate additional data for CRC and HCC to support revenue
ramp up Currently In Initial Training and Marketing Phase
|
 21
DELCATH SYSTEMS, INC
Patient Referral Path
Interventional
Radiologist
Offers chemosaturation
procedure
Patient
Primary
Care
Medical
Oncologist
Offers systemic therapy
Surgical
Oncologist
Offers resection or
other focal therapy
Chemosaturation
Diagnosis
of Cancer
Identification
of
liver
involvement
with no
improvement from systemic
therapy
When liver disease is
controlled
patients return to the Medical
Oncologist for additional systemic therapy
|
 22
DELCATH SYSTEMS, INC
CHEMOSAT Training and Marketing Commenced in Europe
Continue Training and Marketing Centers Roll-Out
•
Entered training and marketing agreements with leading cancer centers in
Europe o
Institute of European Oncology (IEO), Milan, Italy
o
Johann Wolfgang Goethe (JWG) University Hospital, Frankfurt, Germany
o
University Medical Center Schleswig-Holstein, Kiel Campus, Germany
o
Institut Gustave Roussy ("IGR"), Villejuif, France
o
Instituto Oncologico Baselga (IOB), Grupo Quiron, Barcelona, Spain
o
Istituto Nazionale Tumori Fondazione G. Pascale in Naples, Italy
o
Netherlands Cancer Institute-Antoni van Leeuwenhoek Hospital
o
Universidad de Navarra Pamplona, Spain
•
Training completed and first patients treated at IEO and JWG University Hospital,
Frankfurt, Germany
o
Cutaneous melanoma, ocular melanoma, gastric cancer, and breast cancer liver
metastases •
Agreements with additional leading cancer centers expected in Target Countries in
2012 •
Selected Quintiles to support EU launch
•
We are establishing direct sales force in UK, Germany and Netherlands and seeking
exclusive distributors in Italy, France and Spain
|
 23
DELCATH SYSTEMS, INC
European Reimbursement Considerations
•
No centralized pan-European medical device reimbursement body –
regional and national systems
•
Devices typically reimbursed under Diagnosis Related Groups (DRG) as
part of a procedure
•
Working
with
reimbursement
specialists
to
develop
a
plan
in
each
of
our
key markets
•
Immediate reimbursement plans:
o
Utilize existing codes where permitted until permanent reimbursement
established (e.g. Italy)
o
Apply
for
funding
under
existing
New
Technology
Payment
programs
(e.g.
NUB in Germany and HAS in France)
o
Other oncology therapies currently reimbursed, despite lacking randomized
data
Reimbursement Mechanisms in Place to Support Commercial Launch
|
 24
DELCATH SYSTEMS, INC
International Strategy beyond EU and US
•
Leverage CE Mark to obtain reciprocal regulatory approvals for CHEMOSAT
System in other international markets
•
International regulatory submissions status:
Application
submitted
and
expected
approvals
in
Australia
-
2012 (approved)
Hong Kong
-
2012
S. Korea
-
2013
Singapore
-
2013
Brazil
-
2013
Intend
to
submit
applications
Israel
Canada
Mexico/Argentina
Russia
India
Japan
China and Taiwan
•
Utilize
3
rd
party
melphalan
and
doxorubicin
available
to
physicians
Combination of Strategic Partnerships and Specialty Distributors
|
 25
DELCATH SYSTEMS, INC
U.S. FDA Regulatory Status
•
Pre-NDA submission meeting with FDA conducted in January 2012
o
Satisfied with FDA response
o
Addressed RTF related issues
Manufacturing plant inspection timing
Product and sterilization validation
Additional statistical analysis clarification
Additional safety data
•
Completed data entry and monitoring
o
Completed data migration to new FDA compliant CDISC database
o
Created new Case Report Form (CRF)
•
We will lock database on May 25, 2012
•
Plan to file NDA submission in Mid-August 2012
•
Initiated
dialogue
with
FDA
to
discuss
optimal
approval
path
for
Gen 2
Progress on Track to Submit NDA |
 26
DELCATH SYSTEMS, INC
U.S. Commercialization Strategy
•
Initial focus on leading cancer centers and referring community hospitals
•
Educate Medical Oncologists via Medical Science Liaison (MSL)
•
Direct strategy to sell to Interventional Radiologists and Surgeons: 12
sales territories ultimately expanding to as many as 60 territories as
revenues ramp
•
5 Clinical Specialists initially to support site initiation and training
•
Utilize top centers from Phase III trial as Centers of Excellence for
training and support
•
Intend to seek chemosaturation specific codes based upon value
proposition relative to other cancer therapies
Direct Sales Channels Supplemented with Contract MSLs
|
 27
DELCATH SYSTEMS, INC
Clinical Development Program
•
Goal:
o
Expand indications for HCC and mCRC with US registration trials
o
Generate robust clinical data to support commercialization
•
Submitted IND Amendment to include Gen 2 in Expanded Access
Program (EAP), compassionate use and all future clinical trials
o
Assuming IND Amendment accepted, initiate EAP for metastatic melanoma in September
2012 •
Planned Clinical Trials (first patient enrolled in 2013)
o
HCC
Global Phase 2 randomized 1L CHEMOSAT Melphalan vs. Sorafenib
US
registration
–
Global
Phase
3
Randomized
2L
CHEMOSAT
Melphalan
vs.
BSC
for
Sorafenib Failure
Asia Phase 3 Randomized 2L CHEMOSAT Doxorubicin vs. BSC for Sorafenib
Failure o
mCRC
Global Phase 2 Signal Seeking/Safety 2L CHEMOSAT Melphalan
US
Registration
–
Global
Phase
3
Randomized
2L
CHEMOSAT
Melphalan
vs.
Approved
Alternatives
Establish CHEMOSAT as the Standard of Care (SOC) for Disease Control in the
Liver |
 28
DELCATH SYSTEMS, INC
2012 Milestones
•
First patients have been treated with CHEMOSAT Melphalan in Europe -
Done
•
Execute contract for MSL services in EU –
1Q 2012 (Quintiles was selected to
support EU launch of CHEMOSAT) -
Done
•
Secure agreements with 6-8 leading cancer centers in EU -
Done
•
Obtain CE Mark for Gen 2 CHEMOSAT Melphalan -
Done
•
US NDA submission in August 2012
•
Submission for publications of Phase III data and mNET arm of Phase II data
– 2H 2012
•
First
patients
enrolled
in
EAP
–
September
2012
•
Submit and seek approval of CE Mark for CHEMOSAT Doxorubicin –
2H 2012
•
Potential Asia strategic partnership –
dedicated BD with China a top priority |
 29
DELCATH SYSTEMS, INC
Financial Update
Cash & Cash Equivalents:
$20.8 million at March 31, 2012
Financing Program
$4.8 million raised via At-The-Market (ATM)
equity offering program in Q1 2012
Working Capital Line of Credit
$20.0 million credit facility
Cash Spend:
$14.7 million in Q1 2012
Debt:
None
Shares Outstanding:
49.8
million
(57.5
million
fully
diluted
1
)
Institutional Ownership
22%
Market Capitalization
$155 million as of March 31, 2012
Avg. Daily Volume (3 mo.)
400,000
1) As of March 31, 2012 fully diluted includes an additional 5.0 million options at $4.96 and 2.7
million warrants at $3.03 |
 30
DELCATH SYSTEMS, INC
Team
Executive
Title
Prior Affiliation(s)
Years of
Experience
Eamonn Hobbs
President and CEO
AngioDynamics, E-Z-EM
31
Graham Miao, Ph.D.
EVP & CFO
D&B, Pagoda Pharma, Schering-
Plough, Pharmacia, JP Morgan
22
Krishna Kandarpa,
M.D., Ph.D.
CMO and EVP, R&D
Harvard, MIT(HST), Cornell,
UMass
32
Agustin Gago
EVP, Global Sales
AngioDynamics, E-Z-EM
30
Jennifer Simpson,
Ph.D.
EVP, Global Marketing
Eli Lilly (ImClone), Johnson &
Johnson (Ortho Biotech)
22
Peter Graham, J.D.
EVP, General Counsel &
Global Human Resources
Bracco, E-Z-EM
17
David McDonald
EVP, Business Development
AngioDynamics, RBC Capital
Markets
29
John Purpura
EVP, Regulatory Affairs &
Quality Assurance
E-Z-EM, Sanofi-Aventis
28
Harold Mapes
EVP, Global Operations
AngioDynamics, Mallinkrodt
26
J. Chris Houchins
SVP, Clinical and Medical
Affairs
Arno, Schering-Plough, Pfizer,
Pharmacia, GD Searle
21
Bill Appling
SVP Medical Device R&D
AngioDynamics
26
Dan Johnston, Ph.D.
VP, Pharmaceutical R&D
Pfizer, Wyeth
11 |
 31
DELCATH SYSTEMS, INC
Appendices |
 32
DELCATH SYSTEMS, INC
Appendix I
Intellectual Property |
 33
DELCATH SYSTEMS, INC
Intellectual Property
•
Patent Protection
o
7 issued U.S. patents, 10 foreign patents issued and 4 pending
o
Primary device patent set to expire August 2016
o
Up to 5 years of patent extension post FDA approval
•
Trade Secret Protection
o
Developed improved filter media via new manufacturing processes
•
FDA Protection
o
Orphan Drug Designation granted for melphalan in the treatment of ocular
melanoma, cutaneous melanoma and metastatic neuroendocrine tumors,
as well as for doxorubicin in the treatment of HCC
Provides 7 years of marketing exclusivity post FDA approval
o
Additional Orphan Drug applications to be filed for other drugs and
indications, including melphalan for HCC and CRC
Multiple Levels of Protection |
 34
DELCATH SYSTEMS, INC
Appendix II
CHEMOSAT Market Opportunity
by Disease and Target Counties |
 35
DELCATH SYSTEMS, INC
•
Europe
–
Largest
near-term
opportunity
•
CRC –
Largest opportunity worldwide
•
Melanoma
–
Largest
opportunity
is
in
US
•
China-
Largest opportunity for HCC
Market
Opportunity
defined
as
Total
Potential
Market
(TPM)
for
CHEMOSAT
®
1.Primary cancer incidence
2.Adjusted for predominant disease in the liver (primary
or metastatic cancer)
3.Adjusted
for
addressable
patients
via
Delcath
CHEMOSAT
®
Market Opportunity by Disease (patients) |
 36
DELCATH SYSTEMS, INC
Europe Market by Disease –
Device Only
Germany
(Direct)
UK
(Direct)
France
(Indirect)
Italy
(Indirect)
Spain
(Indirect)
Netherlands
(Direct)
Ireland
(Direct)
Total
Potential
(patients)
Potential
Market
($ MM)
1,2,3
Total Potential Market #Patients
Ocular
Melanoma
404
297
295
285
197
79
19
1,576
$ 62
Cutaneous
Melanoma
1,625
994
753
801
360
379
73
4,987
$ 206
CRC
9,902
5,300
5,475
7,281
4,016
1,644
335
33,953
$1,339
HCC
(Primary)
1,637
720
1,514
2,597
1,087
82
35
7,671
$277
NET
1,783
1,336
1,353
1,299
974
360
98
7,202
$ 281
TOTAL
15,351
8,647
9,389
12,263
6,634
2,545
560
55,389
$ 2,166
Europe Presents Significant Potential Market Opportunity
Sources: LEK Consulting, GLOBOCAN, Company estimates.
1) Assumes 2.5 treatments per patient.
2) Assumes ASP of ~$15K USD.
3) Assumes mix of direct sales and distributors. |
 37
DELCATH SYSTEMS, INC
US Market by Disease –
Device and Drug Combination
Liver Metastasis
Potential Market
# Patients
Potential Market
# Procedures
Potential Market
($MM)
1,2
Ocular
Melanoma
1,685
4,213
$ 105
Cutaneous
Melanoma
7,023
17,557
$ 439
TOTAL MELANOMA
(Initial Expected Label)
8,708
21,770
$ 544
CRC
19,861
49,653
$ 1,241
HCC (Primary)
5,586
13,964
$ 349
NET
8,212
20,530
$ 513
OTHER TOTAL
(Potential Label Expansion)
33,659
84,147
$ 2,104
TOTAL
42,367
105,917
$ 2,648
Sources: LEK Consulting, GLOBOCAN, Company estimates.
1) Assume 2.5 treatments per patient.
2) Estimated ASP of $25K. |
 38
DELCATH SYSTEMS, INC
APAC Market by Disease
China
(Device)
S. Korea
(Device)
Japan
(Device)
Taiwan
(Device)
Australia
(Device)
Total
Potential
(patients)
Potential
Market
($MM)
1,2
Total Potential Market #Patients
HCC
(Primary)
85,780
3,258
8,296
2,152
263
99,749
$ 1,156
Other
CRC
31,127
3,245
14,298
1,441
2,031
52,143
$ 642
NET
29,197
1,048
2,759
500
462
33,966
$ 393
Ocular
Melanoma
1,765
66
175
31
96
2,134
$ 25
Cutaneous
Melanoma
382
43
136
246
1,144
1,951
$ 23
OTHER
TOTAL
62,472
4,403
17,368
2,218
3,733
90,194
$ 1,083
TOTAL
148,104
7,661
25,665
4,370
3,996
189,943
$ 2,239
APAC Target Markets Represent Over $2 Billion Potential Market Opportunity
Sources: LEK Consulting, GLOBOCAN, Company estimates.
1) Assume 2.5 treatments per patient.
2) Estimated ASP of ~$5K. |
 39
DELCATH SYSTEMS, INC
Appendix III
CHEMOSAT Melphalan Phase I and II |
 40
DELCATH SYSTEMS, INC
Melphalan Dosing & Background
•
Well understood, dose dependant, tumor preferential, alkylating cytotoxic agent
that demonstrates little to no hepatic toxicity
•
Manageable systemic toxicities associated with Neutropenia and
Thrombocytopenia
•
Drug dosing 12x higher than FDA-approved dose via systemic IV
chemotherapy •
Dose delivered to tumor is over 100x higher than that of systemic IV
chemotherapy
Type
Dosing (mg/kg)
Multiple Myeloma (label)
0.25
Chemoembolization
0.62
Surgical Isolated Hepatic Perfusion (IHP)
1.50
Myeloablation
2.50-3.50
Chemosaturation (PHP)
3.00
An Established Drug for Liver Cancer Therapy |
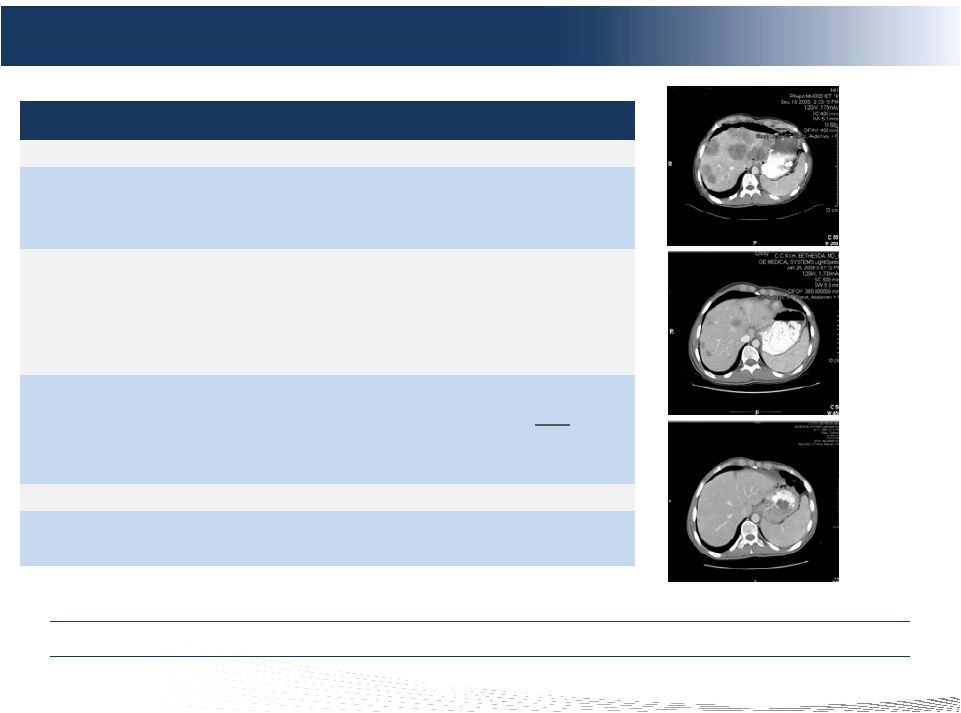 41
DELCATH SYSTEMS, INC
Phase II NCI Trial –
Metastatic Neuroendocrine Cohort
Pre-CS
(Baseline)
Post-CS #2
(+4 Months)
Post-CS #1
(+6 Weeks)
Compelling Clinical Data in Attractive mNET Market
Phase II mNET Tumor Cohort (n=24)*
Number (n)
Primary Tumor Histology
Carcinoid
4
Pancreatic Islet Cell
20
Response
Not Evaluable (Toxicity, Incomplete Treatment, Orthotopic Liver
Transplantation)
4
Progressive Disease
2
Minor Response / Stable Disease
4
Partial
Response
(30.0%
-
99.0%
Tumor
Reduction)
13
Complete Response (No Evidence of Disease)
1
Objective Tumor Response
14
Objective Tumor Response Rate
70%
Duration (months)
Median Hepatic PFS
15.5
Overall Survival After CS
30.4
*Presentation at ECCO/ESMO 2011 annual meeting. |
 42
DELCATH SYSTEMS, INC
Phase II NCI Trial –
HCC Cohort
•
Hepatocellular
carcinoma
(HCC)
is
the
most
common
primary
cancer
of
the liver, with approximately 749,000* new cases diagnosed worldwide
annually
•
Nine patients with tumors of hepatobiliary origin: five HCC patients and
four cholangiocarcinoma patients
•
Both groups received CHEMOSAT procedures and had positive efficacy
signals
•
The responses were especially encouraging in the HCC group and
consisted of confirmed partial response or durable stable disease
•
Safety profile –
expected and consistent with pivotal US Phase III
melanoma trial
•
Intend to invest in new HCC trials with CHEMOSAT
Encouraging Initial Positive Signal for Primary Liver Cancer
*Source: GLOBOCAN |
 43
DELCATH SYSTEMS, INC
•
Substantial clinical evidence of benefit of using melphalan to treat
mCRC via isolated hepatic perfusion (IHP) procedure
o
Over 800 patients treated in 15 studies since 1998
o
Patients treated only once
o
Median
response
rate
of
47%
(range
29%-76%)
1
•
Delcath
Phase
II
NCI
Chemosaturation
Trial
–
mCRC
Cohort
o
Challenges enrolling at NCI
o
16 patients treated since 2004
o
Inconclusive
efficacy
due
to
advanced
disease
status
(generally
5
th
or
6
th
line)
o
Safety profile –
expected and consistent with pivotal FDA Phase III
melanoma trial
•
Intend to invest in new mCRC trials with CHEMOSAT Melphalan
Strong Rationale for Using CHEMOSAT with Melphalan to Treat mCRC
1) van Iersel LB, Koopman M, Van D, V, et al. Ann Oncol. 2010;21:1662-7.
Phase II NCI Trial –
mCRC Cohort |
 44
DELCATH SYSTEMS, INC
Appendix IV
Published Phase I/II Studies of
Doxorubicin with PHP (percutaneous hepatic
perfusion) for HCC |
 45
DELCATH SYSTEMS, INC
CHEMOSAT Doxorubicin Development
•
Multiple published Phase I/II studies from MD Anderson Cancer Center
and Yale with percutaneous hepatic perfusion (PHP) and Kobe
University using doxorubicin show promising response rates for HCC*
•
Status:
o
First pass removal efficiency 95% in initial in vitro studies
o
Utilize new trade secret manufacturing process
o
Intend to file and seek CE Mark approval in 2H 2012
o
Plan to use CHEMOSAT Doxorubicin in Asia Phase III 2L
HCC trials
•
Expected Benefits:
o
Multiple treatments
o
Reduced systemic toxicity for improved safety profile
o
Concomitant therapy (complements systemic therapies)
Addressing the Large HCC Market Opportunity in China
|
 46
DELCATH SYSTEMS, INC
Phase I/II Studies of PHP-Doxorubicin For HCC
Delivered Safely in Multiple Studies with Promising Response Rates
1) Ku Y et al. Chir Gastroenterol 2003;19:370–376.
2) Curley SA et al. Ann Surg Oncol 1994;1:389–99.
3) Ravikumar TS et al. J Clin Oncol 1994;12:2723–36.
4) Hwu WJ et al. Oncol Res 1999;11:529–37.
No. of
pts
No. of
PHP/
pt
Disease stage
(tumor diameter)
Treatment
Median survival
(mo)
Response
Rates
Reference
HCC
(n=79)
CHM
(n=23)
1–4
1–2
IV A: n=66
IV B: n=13
All multiple
bilobar
Extrahepatic disease in 52%
Doxorubicin 60
–150 mg/m
2
Cisplatin
50–150 mg/m
2
Mitomycin C 50–200 mg/m
2
16
13
HCC pts
RR 64.5%
5-year
survival 20.3%
Kobe
1
Phase I/II
HCC
(n=11)
1–3
Mean
9.5 cm
Doxorubicin 60
–120 mg/m
2
6.5
13 (responders)
2 (non-responders)
RR 20%
MDACC
2
Phase I
HCC
(n=5)
CHM
(n=8)
Other
(n=8)
2–4
Extrahepatic disease in 17%
Doxorubicin 50
–120 mg/m
2
5-FU 1000–5000 mg/m
2
NR
RR 22%
Yale
3
Phase I
HCC
(n=7)
Other
(n=11)
1–10
NR
Doxorubicin 90
–120 mg/m
2
23 (responders)
8 (non-responders)
RR 58%
Yale
4
Phase I |
 47
DELCATH SYSTEMS, INC
Appendix V
Product Development Pipeline |
 Product Development Pipeline
•
Melanoma liver mets
•
Proprietary drug-melphalan &
CHEMOSAT
•
All liver cancers –
melphalan
•
Class III medical device
•
3
rd
party melphalan
•
Gen 2 melphalan CE Mark
•
CHEMOSAT for additional drugs
•
CHEMOSAT for other organs (lung
and brain)
•
mCRC and HCC indications
Initial Opportunity
Near Term (< 5 years)
Intermediate Term (> 5 years)
•
Doxorubicin system CE Mark
•
mCRC and HCC clinical trials
•
CHEMOSAT for additional drugs
•
CHEMOSAT for other organs (lung
and brain)
•
CHEMOSAT Melphalan in South
Korea, Japan
•
CHEMOSAT Doxorubicin in
China and Taiwan
•
3
rd
party doxorubicin
•
CHEMOSAT for additional drugs
•
CHEMOSAT for other organs (lung
and brain)
•
CHEMOSAT Melphalan in
Australia and Hong Kong
•
3
rd
party melphalan
Development Aligned to Address Significant Market Opportunity
48
DELCATH SYSTEMS, INC
E
U
A
S
I
A
U
S |
 2
DELCATH SYSTEMS, INC
©
2011 DELCATH SYSTEMS, INC. ALL RIGHTS RESERVED |
