Attached files
| file | filename |
|---|---|
| 8-K - TENGION, INC. FORM 8-K - TENGION INC | tengion8k.htm |

Regenerative medicine brought to life
®
May 2012
Exhibit 99.1

Forward-looking Statements
Certain statements in this presentation may constitute forward-looking statements within the meaning of the
Private Securities Litigation Reform Act of 1995. Although Tengion believes that these statements are based
upon reasonable assumptions within the bounds of its knowledge of its business and operations, there are a
number of factors that may cause actual results to differ from these statements.
Private Securities Litigation Reform Act of 1995. Although Tengion believes that these statements are based
upon reasonable assumptions within the bounds of its knowledge of its business and operations, there are a
number of factors that may cause actual results to differ from these statements.
For instance, there can be no assurance that: (i) the Company will be able to successfully enroll patients in its
clinical trials, including its phase I clinical trial for the Neo-Urinary Conduit; (ii) patients enrolled in the
Company's clinical trials will not experience adverse events related to the Company's product candidates,
which could delay clinical trials or cause the Company to terminate the development of a product candidate;
(iii) the results of the clinical trial for the Neo-Urinary Conduit will support further development of that
product candidate; (iv) the market opportunity data and physician and payer feedback for the Neo-Urinary
Conduit derived from the report prepared by L.E.K. Consulting LLC accurately predict the potential
commercial opportunity for the Neo-Urinary Conduit; (v) data from the Company's ongoing preclinical
studies, including its proposed GLP program for the Neo-Kidney Augment, will continue to be supportive of
advancing such preclinical product candidates; and (vi) the Company will be able to progress its product
candidates that are undergoing preclinical testing, including the Neo-Kidney Augment, into clinical trials and
that the Company will be successful in designing such clinical trials in a manner that supports the
development of such product candidate; and (vii) the Company will be able enter into strategic partnerships
on favorable terms, if at all, or obtain the capital it needs to develop its product candidates and continue its
operations.
clinical trials, including its phase I clinical trial for the Neo-Urinary Conduit; (ii) patients enrolled in the
Company's clinical trials will not experience adverse events related to the Company's product candidates,
which could delay clinical trials or cause the Company to terminate the development of a product candidate;
(iii) the results of the clinical trial for the Neo-Urinary Conduit will support further development of that
product candidate; (iv) the market opportunity data and physician and payer feedback for the Neo-Urinary
Conduit derived from the report prepared by L.E.K. Consulting LLC accurately predict the potential
commercial opportunity for the Neo-Urinary Conduit; (v) data from the Company's ongoing preclinical
studies, including its proposed GLP program for the Neo-Kidney Augment, will continue to be supportive of
advancing such preclinical product candidates; and (vi) the Company will be able to progress its product
candidates that are undergoing preclinical testing, including the Neo-Kidney Augment, into clinical trials and
that the Company will be successful in designing such clinical trials in a manner that supports the
development of such product candidate; and (vii) the Company will be able enter into strategic partnerships
on favorable terms, if at all, or obtain the capital it needs to develop its product candidates and continue its
operations.
For additional factors which could cause actual results to differ from expectations, reference is made to the
reports filed by the Company with the Securities and Exchange Commission under the Securities Exchange Act
of 1934, as amended. The forward-looking statements in this presentation are made only as of the date
hereof and the Company disclaims any intention or responsibility for updating predictions or expectations in
this presentation.
reports filed by the Company with the Securities and Exchange Commission under the Securities Exchange Act
of 1934, as amended. The forward-looking statements in this presentation are made only as of the date
hereof and the Company disclaims any intention or responsibility for updating predictions or expectations in
this presentation.
2
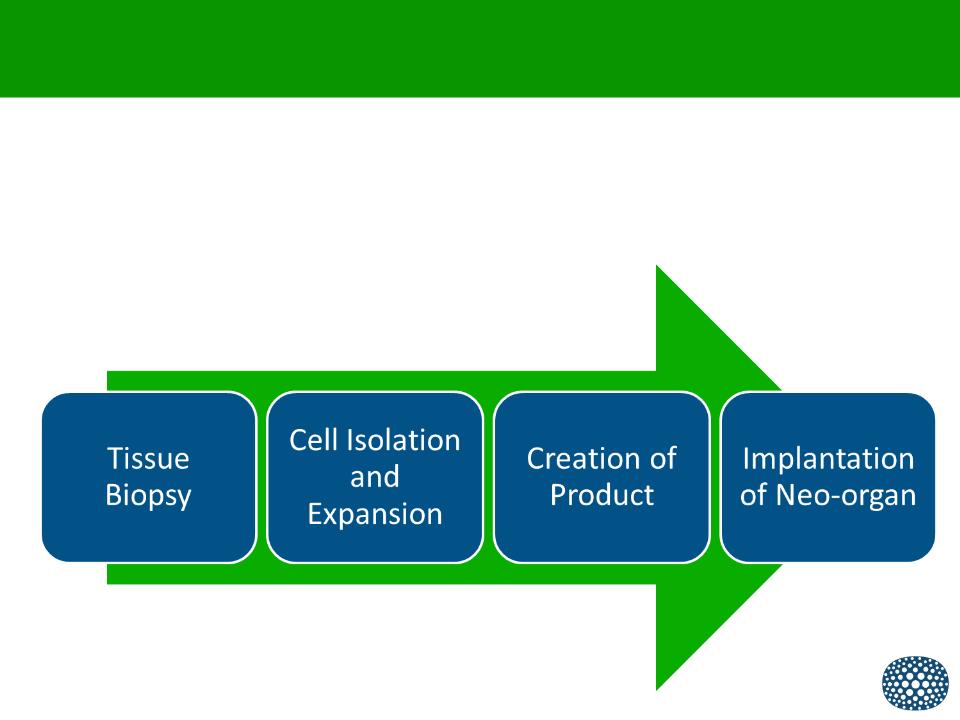
Delivering Organ Regeneration
Novel technology platform that:
•Utilizes selected populations of regenerative cells
•Harness these cells to create specific neo-organs
•Has the potential to produce these neo-organs on a commercial scale
3

Anticipated Benefits of Our Regenerative Medicine Platform
• Regenerate native-like function and structure
• Reduce healthcare costs for organ failure while improving
medical outcomes
medical outcomes
– Avoid the risk of organ rejection
– Eliminate the burden and costs of immunosuppression
• Provide meaningful clinical safety and efficacy advances
versus existing alternatives
versus existing alternatives
– Reduce or eliminate adverse effects of existing procedures
– Shorten surgical and recovery time
• Improve quality of life for patients and their families
4

Investment Highlights
• Biotechnology company focused on tissue and organ regeneration using
a patient’s own cells
a patient’s own cells
– Strong IP, know-how, and trade secrets around core process
• Neo-Urinary ConduitTM: our goal is to improve upon standard of care for
bladder cancer patients requiring cystectomy
bladder cancer patients requiring cystectomy
– Currently in a phase 1 clinical trial
• Neo-Kidney AugmentTM: our goal is to augment renal function and delay
progression of kidney failure
progression of kidney failure
– Preclinical proof-of-concept established in four animal studies
• New team focused on key value creating objectives
– Neo-Urinary Conduit: complete enrollment of phase 1 human proof-of-
concept trial in 2012
concept trial in 2012
– Neo-Kidney Augment: file IND during 1H 2013; expect initial data from phase
1 human proof-of-concept trial in 2014
1 human proof-of-concept trial in 2014
5

Neo-Urinary Conduit
6

Neo-Urinary Conduit: Large Market Opportunity
|
Estimated 2012 Urinary Diversion Surgical Procedures*
|
13,100
15,200
7
*L.E.K. and company estimates
Number of procedures growing at ~3% per annum

Our Neo-Urinary Conduit Offers Advantages
• Potentially shorter, less complex and standardized surgical
procedure
procedure
• Improved recovery times
• Avoids side effects associated with bowel tissue resection
and urine absorption
and urine absorption
₋ Infection
₋ Excess mucus formation
₋ Cancer
8

Positive Initial Payer Comments
• Payers believe a $40,000 price for Neo-Urinary Conduit is
reasonable
reasonable
• Incremental cost savings
– Reduction is surgery and hospitalization time
– Reduction in complications
– Quality of life improvements
• Limited incremental exposure per payer
• Older population likely covered by Medicare
• Other autologous therapies have comparable prices
9
Based on expected product profile

Tengion’s Neo-Urinary Conduit
10
• Regenerates native-like urinary tissue with
no bowel resection or urine absorption-
related metabolic disorders
no bowel resection or urine absorption-
related metabolic disorders
• Created using patient’s own cells
– Cells obtained from simple fat biopsy
– Cells grown and placed on biodegradable
scaffold
scaffold
– Implanted at time of cystectomy (removal
of bladder cancer)
of bladder cancer)
• Successful preclinical large animal studies
– Standardized surgical procedure
– Regenerated native-like urinary tissue
structure and function
structure and function
• U.S. regulatory pathway: Biologics
Licensing Application (BLA)
Licensing Application (BLA)

Neo-Urinary Conduit vs. Standard of Care
Non-Continent Urinary Diversion Conduit
Neo-Urinary Conduit
11

Phase 1 Trial Overview
• Open label study to define surgical procedure and safety
• Preliminary efficacy assessment: Structural integrity and
conduit patency at 1 year post implant
conduit patency at 1 year post implant
• Will enroll up to 10 patients with primary bladder cancer
requiring cystectomy
requiring cystectomy
• Expect to complete enrollment by the end of 2012
• Sequential enrollment of initial patients allows for real-time
optimization of the surgical procedure and post-surgical care
optimization of the surgical procedure and post-surgical care
– Goals for the new surgical procedure
• Standardize attachment of ureters
• Vascularize the Neo-Urinary Conduit
• Standardize stoma procedure
– Patients enrolled at Johns Hopkins Hospital and University of Chicago
Medical Center
Medical Center
12

Phase 1 Trial Progress: Translation and Regeneration
• Four of 10 patients enrolled so far
• Results from patients 1-3 provided a
framework for an updated
procedure for the fourth patient
framework for an updated
procedure for the fourth patient
– Ureteral attachment utilizes a widely
accepted procedure
accepted procedure
– Modified stoma ostomy to improve
stoma patency
stoma patency
– Vascularization procedure utilizes widely
accepted source (omentum)
accepted source (omentum)
• Histological evidence of urinary
tissue regeneration where there was
appropriate blood supply
tissue regeneration where there was
appropriate blood supply
Human Ureter
Normal Tissue
Regenerated Tissue
Human Neo-
Urinary
Conduit
Urinary
Conduit
13

Next Steps for the Neo-Urinary Conduit
• Expect to implant the 10th patient by the end of 2012
– Anticipate one-year data available for all patients during 4Q 2013
• Begin phase 2/3 clinical trial during 2H 2013
– Randomized, non-blinded 2-arm trial
– N = 250-300 (150 NUC vs 100-150 standard of care)
– Approximately 3 years to enroll with 1-year follow-up
– Data likely applicable to EU filing
• Engage in strategic alliance discussions
• Continue working with multiple experts from:
– Baylor, Brigham & Women’s, Johns Hopkins, Memorial Sloan
Kettering, University of Chicago, and University of Michigan
Kettering, University of Chicago, and University of Michigan
14

Neo-Kidney Augment
15

Neo-Kidney Augment Market Opportunity
• Intended to prevent or delay the need for dialysis or
transplantation
transplantation
• An estimated 26 million adults in the United States have
chronic kidney disease (CKD)
chronic kidney disease (CKD)
– 100,000 new dialysis patients each year in the US
– 350,000 currently on dialysis
– 20% annual mortality
– $77,000 annual cost per patient
• $39 billion in direct US costs annually for end stage kidney
disease
disease
16

Tengion’s Neo-Kidney Augment
• Intended to prevent or delay dialysis or kidney
transplantation by increasing renal function in patients with
advanced CKD
transplantation by increasing renal function in patients with
advanced CKD
– Neo-Kidney Augment may catalyze the regeneration of functional
kidney tissue
kidney tissue
• Utilizes a patient’s own renal cells
– Obtained by needle biopsy
– Cells formulated in a hydrogel for ease of manufacturing and delivery
• Designed for laparoscopic injection of Neo-Kidney Augment
into diseased kidney
into diseased kidney
• U.S. regulatory pathway: Biologics Licensing Application
(BLA)
(BLA)
17
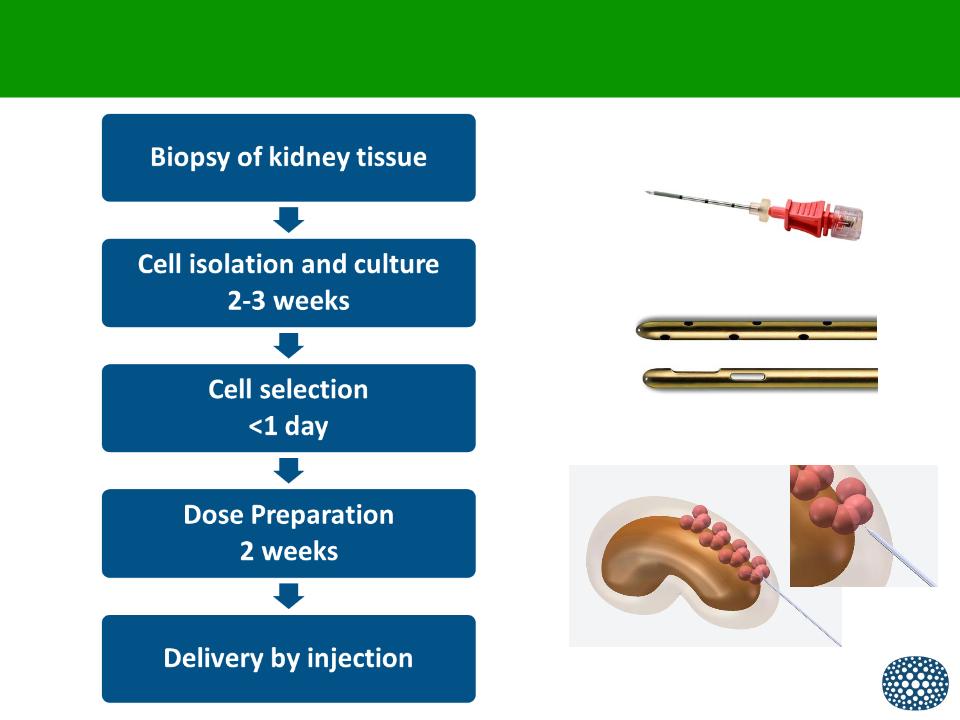
Efficient and Scalable Manufacturing Process
18

Robust Preclinical Data for Neo-Kidney Augment
• Significant effects in aggressive rodent models of diabetic kidney failure
and CKD - results published in top-tier journals
and CKD - results published in top-tier journals
– Diabetic kidney failure model
• Uncontrolled diabetic, obese, hypertensive rodent model of kidney failure
• Slowed kidney disease progression and improved survival at 1 year
– Renal mass reduction model
• Increased functional kidney mass, slowed progression, improved survival at
6 months
6 months
– Human regenerative cells in acute/chronic renal disease
• Demonstrated regeneration of functional kidney tissue using human renal
cells
cells
• Reversed kidney failure at 3 months
• Consistent observations in large animal model of CKD
– Effects seen as early as 7 weeks, with persistent effects reported at 9 months
19

Regenerative Cells Improve Kidney Function and Extend Survival
Renal Cells improve kidney
function over time
function over time
|
ZSF1 Groups
|
1-Year
Survival
|
|
Untreated
|
20% (1/5)
|
|
OB ZSF1
+ NKA |
100% (5/5)
|
Effects beyond 1 year of age in renal diseased diabetic rat
20
Renal Cells support survival
beyond 50% mortality time
point for OB ZSF1
beyond 50% mortality time
point for OB ZSF1
34 weeks of age (16 weeks post treatment)
p = 0.0038

Human Kidney Tissue Regeneration in Nude Rats
Human-derived cells
prevent renal failure in
CKD Nude Rats for 3
months
prevent renal failure in
CKD Nude Rats for 3
months
Human-derived cells
improve CKD Nude Rat
nephron function
improve CKD Nude Rat
nephron function
Delayed progression of CKD and stabilized renal function
21
*

Multiple Mechanisms of Action in CKD
• Implanted regenerative cells persist for at least 6 months
• Regeneration of functional renal tissue
- Regenerative and resident cells regenerate, replace and repair renal
structures post-implantation
structures post-implantation
• Attenuation of inflammatory and fibrosis pathways
- Molecular evidence of key pathway reduction:
• TGFb >50%
• PAI-1 >50%
• Fibronectin 50%
22

Next Steps for Neo-Kidney Augment
• Commence GLP studies to support U.S. IND filing in 1H 2013
– 6 month ZSF1 rat study (diabetic kidney failure)
– 3 month canine safety study
– 6 month repeat dose canine study
• Parallel track European approach
– Define pathway during 2H 2012 to European First-in-Human
• Planning Advanced Therapy Medicinal Products (ATMP) pathway
• Continue working with multiple experts from:
– Harvard, Karolinska Institute, Mario Negri Institute, UNC-Chapel
Hill
Hill
23

• Technology protected by issued patent protection plus recent applications pending
— 28 U.S. and 97 international patents and patent applications for regeneration of multiple
organs and tissues
organs and tissues
— Core patents cover composition, design and methods of manufacture*
Significant IP and Barriers to Entry
= Patent Applications
= Issued Patents
* Individual patents may cover multiple elements
24
CELLS
DEVICE /
MATERIAL
PROCESS
THX USE
• Technology platform also includes know-how, trade secrets and integrated capabilities
for the discovery, development and manufacturing of multiple product candidates
for the discovery, development and manufacturing of multiple product candidates
Neo-Urinary Conduit
Neo-Kidney Augment
CELLS
DEVICE /
MATERIAL
PROCESS
THX USE

Corporate
25
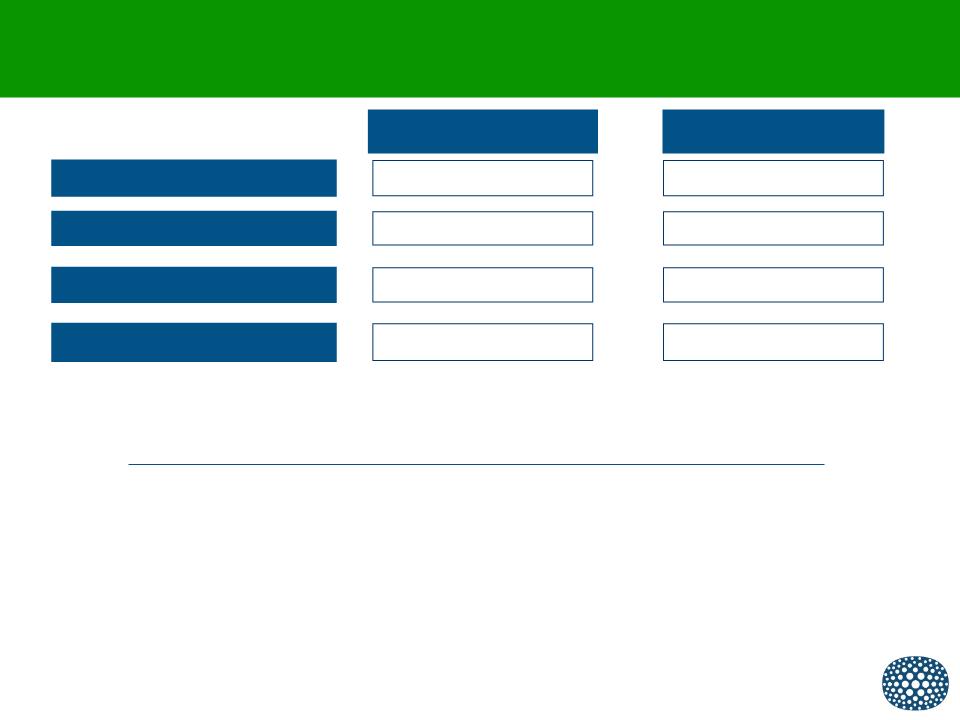
Financial Overview and Current Stockholders
March 31, 2012
Cash and Investments
Long-Term Debt
Adjusted Net Loss
Shares Outstanding *
$8.9 M
$4.5 M
$4.1 M
24.5 M
December 31, 2011
$15.3 M
$5.0 M
$19.1 M
23.8 M
Current cash expected to last until September 2012
* Potential for reverse stock split in range of one-for-six to one-for-ten if stockholders approve proposal at May 2012 Annual Meeting
Current stockholders (based on most recent public filings)
HealthCap Venture Capital Great Point Partners Empery Asset Mgmt
Medtronic, Inc. Deerfield Management Safeguard Scientifics
Oak Investment Partners Quaker BioVentures Kingsbrook
26

Upcoming Milestones
|
Milestone
|
Expected
|
|
Neo-Urinary Conduit
|
|
|
Complete enrollment of phase 1
clinical trial |
End of 2012
|
|
Initiate phase 2/3 study
|
2H 13
|
|
Obtain 1-year data from phase 1
clinical trial |
2H 13
|
|
Neo-Kidney Augment
|
|
|
Define European regulatory pathway
|
2H 12
|
|
IND filing
|
1H 13
|
27

Investment Highlights
• Biotechnology company focused on tissue and organ regeneration using
a patient’s own cells
a patient’s own cells
– Strong IP, know-how, and trade secrets around core process
• Neo-Urinary ConduitTM: our goal is to improve upon standard of care for
bladder cancer patients requiring cystectomy
bladder cancer patients requiring cystectomy
– Currently in a phase 1 clinical trial
• Neo-Kidney AugmentTM: our goal is to augment renal function and delay
progression of kidney failure
progression of kidney failure
– Preclinical proof-of-concept established in four animal studies
• New team focused on key value creating objectives
– Neo-Urinary Conduit: complete enrollment of phase 1 human proof-of-
concept trial in 2012
concept trial in 2012
– Neo-Kidney Augment: file IND during 1H 2013; expect initial data from phase
1 human proof-of-concept trial in 2014
1 human proof-of-concept trial in 2014
28
