Attached files
| file | filename |
|---|---|
| 8-K/A - 8-K/A - Prestige Consumer Healthcare Inc. | v309197_8ka.htm |
| EX-23.1 - EXHIBIT 23.1 - Prestige Consumer Healthcare Inc. | v309197_ex23-1.htm |
| EX-99.2 - EXHIBIT 99.2 - Prestige Consumer Healthcare Inc. | v309197_ex99-2.htm |
Exhibit 99.1
The North American Divested Brands of GlaxoSmithKline Plc
Report and Combined Financial Statements
For the years ended 31 December 2011, 31 December 2010 and 31 December 2009
The North American Divested Brands of GlaxoSmithKline Plc
| Index | |
| Independent Auditor’s Report | 3 |
| Combined Statements of Revenue and Direct Operating Expenses | 4 |
| Combined Statements of Net Assets to be Sold | 5 |
| Notes to statements | 6 |
| 1. Background | 6 |
| 2. Basis of preparation and accounting policies | 7 |
| 3. Intangible assets | 14 |
| 4. Related party transactions | 16 |
| 5. Post balance sheet events | 17 |
| 6. Commitments and Contingencies | 17 |
| 2 |

Report of Independent Registered Public Accounting Firm
To: the Directors of GlaxoSmithKline Plc
We have audited the accompanying Combined Statement of Net Assets to be Sold of The North American Divested Brands of GlaxoSmithKline Plc (the ‘Divested Brands’) as of 31 December 2011, 31 December 2010 and 31 December 2009, the related Combined Statement of Revenue and Direct Operating Expenses for the years ended 31 December 2011, 31 December 2010 and 31 December 2009 and associated footnotes (collectively referred to as the “Combined Financial Statements”). The Combined Financial Statements are the responsibility of GlaxoSmithKline Plc’s management. Our responsibility is to express an opinion on the Combined Financial Statements based on our audit.
We conducted our audit in accordance with auditing standards generally accepted in the United States of America. Those standards require that we plan and perform the audit to obtain reasonable assurance that the Combined Financial Statements are free of material misstatement. An audit also includes examining, on a test basis, evidence supporting the amounts and disclosures in the Combined Financial Statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall Combined Financial Statements presentation. We believe that our audit provides a reasonable basis for our opinion.
The Combined Financial Statements were prepared to present the net assets to be sold of the Divested Brands and the revenue and direct operating expenses pursuant to the basis of preparation described in Note 2 of the Combined Financial Statements, and are not intended to be a complete presentation of the Divested Brands’ financial position, operating results or cash flows.
In our opinion, the Combined Financial Statements referred to above present fairly, in all material respects, the net assets to be sold of the Divested Brands as of 31 December 2011, 31 December 2010 and 31 December 2009 and the Divested Brands’ revenue and direct operating expenses for the years ended 31 December 2011, 31 December 2010 and 31 December 2009 in conformity with the basis of preparation described in Note 2.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Chartered Accountants
London
9 March 2012

PricewaterhouseCoopers LLP, 1 Embankment Place, London WC2N 6RH
T: +44 (0) 20 7583 5000, F: +44 (0) 20 7212 4652, www.pwc.co.uk
PricewaterhouseCoopers LLP is a limited liability partnership registered in England with registered number OC303525. The registered office of
PricewaterhouseCoopers LLP is 1 Embankment Place, London WC2N 6RH.PricewaterhouseCoopers LLP is authorised and regulated by the Financial Services Authority for designated investment business.
| 3 |
The North American Divested Brands of GlaxoSmithKline Plc
Combined Statements of Revenue and Direct Operating Expenses
For the years ended 31 December 2011, 31 December 2010 and 31 December 2009
| Note | 12/31/2011 | 12/31/2010 | 12/31/2009 | |||||||||||||
| $ | 000 | s | $ | 000 | s | $ | 000 | s | ||||||||
| Revenue | 206,154 | 207,342 | 215,991 | |||||||||||||
| Cost of sales | (65,186 | ) | (64,676 | ) | (65,270 | ) | ||||||||||
| Gross profit | 140,968 | 142,666 | 150,721 | |||||||||||||
| Selling, general and administration | (56,974 | ) | (59,719 | ) | (72,987 | ) | ||||||||||
| Research and development | - | - | (6 | ) | ||||||||||||
| Amortisation and impairment | 3 | (550 | ) | (10,311 | ) | (550 | ) | |||||||||
| Other operating income/(expenses) | 4 | 648 | (295 | ) | (611 | ) | ||||||||||
| Excess of revenue over direct operating expenses | 84,092 | 72,341 | 76,567 | |||||||||||||
The accompanying notes on pages 6 to 17 are an integral part of these Combined Financial Statements.
| 4 |
The North American Divested Brands of GlaxoSmithKline Plc
Combined Statements of Net Assets to be Sold
As of 31 December 2011, 31 December 2010 and 31 December 2009
| Note | 12/31/2011 | 12/31/2010 | 12/31/2009 | |||||||||||||
| $ | 000 | s | $ | 000 | s | $ | 000 | s | ||||||||
| Non-current assets | ||||||||||||||||
| Intangible assets | 3 | 211,339 | 212,134 | 221,928 | ||||||||||||
| Total non-current assets | 211,339 | 212,134 | 221,928 | |||||||||||||
| Current assets | ||||||||||||||||
| Finished goods inventories | 12,511 | 12,816 | 21,481 | |||||||||||||
| Total current assets | 12,511 | 12,816 | 21,481 | |||||||||||||
| Net assets to be sold | 223,850 | 224,950 | 243,409 | |||||||||||||
The accompanying notes on pages 6 to 17 are an integral part of these Combined Financial Statements.
| 5 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Combined Financial Statements
For the years ended 31 December 2011, 31 December 2010 and 31 December 2009
| 1 | Background |
The GlaxoSmithKline Plc (“GSK” or “Group”) Consumer Healthcare business operates in three key areas: over the counter (“OTC”) medicines, oral healthcare and nutritional healthcare. The North American Divested Brands of GlaxoSmithKline Plc (“Divested Brands”) operate as part of the Consumer Healthcare business of GSK. GSK, its subsidiaries and associated undertakings, constitute a major global healthcare group engaged in the creation, discovery, development, manufacture and marketing of pharmaceutical and consumer health-related products.
In April 2011, GSK identified certain non-core, OTC brands that it intended to divest as the Group focuses its Consumer Healthcare business around a portfolio of priority brands and the emerging markets.
On 20 December 2011 Prestige Brands Holdings Inc (the “Purchaser”) entered into two sale and purchase agreements (the “Agreements”) with GSK to acquire the Divested Brands in the respective countries where these Divested Brands are sold.
The first arrangement covers the outright transfer of the respective brands from the completion date for all products besides Debrox and Gly-oxide. This agreement closed on 31 January 2012.
The second agreement relates to the transfer and transitional supply arrangement for Debrox and Gly-oxide anticipated to close on or before 1 May 2012. These brands are supplied to GSK under licence from Sanofi Aventis. The agreement provides for the transfer of the licences for these brands and the transitional supply arrangement of the brands to the Purchaser.
Included in the scope of this transaction are the following brands and the respective countries where these Divested Brands are sold.
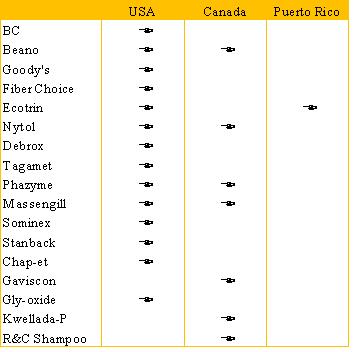
| 6 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
| 2 | Basis of preparation and accounting policies |
The Divested Brands and related net assets to be sold are not within separate legal entities and historically GSK has not maintained separate records for the Divested Brands. The Combined Statements of Net Assets to be Sold and Combined Statements of Revenue and Direct Operating Expenses, including the accompanying notes (collectively referred to as the “Combined Financial Statements”) have been derived from the consolidated financial statements and the underlying accounting records of GSK. The accounting policies herein are reflective of those used for the historical GSK consolidated financial statements unless stated otherwise.
The accompanying Combined Financial Statements were prepared to present the net assets to be sold pursuant to the two Agreements between GSK and Prestige Brands Holdings Inc signed on 20 December 2011 and the related revenue and direct operating expenses. The basis of preparation describes how the Combined Financial Statements have been prepared in conformity with International Accounting Standards (“IAS”), International Financial Reporting Standards (“IFRS”) and related interpretations, as issued by the International Accounting Standards Board (“IASB”) as applicable to the items included in these Combined Financial Statements. These Combined Financial Statements are not intended to be a complete presentation of assets, revenues and expenses of the Divested Brands.
The Combined Statements of Net Assets to be Sold have been prepared on a basis which includes only those assets which are directly attributable to the Divested Brands and are identified in the Agreements as being transferred to the Purchaser as described in Clause 2.1 of the Agreements. No liabilities, contingent or otherwise are being assumed by the Purchaser. The Combined Statements of Revenue and Direct Operating Expenses include revenue and expenses that are directly attributable to the Divested Brands and certain allocations of other direct expenses incurred by GSK attributable to the Divested Brands as discussed below.
GSK maintains all debt and notes payable on a consolidated basis to fund and manage operations; accordingly, debt and related interest expense were not allocated to the Divested Brands. GSK also maintains its tax functions on a consolidated basis; accordingly, tax expense was not allocated to the Divested Brands.
Historically GSK has not maintained separate financial records for the Divested Brands and, as such, it is impracticable for GSK to identify operating or financing cash flows associated with the Divested Brands. There were no acquisitions or disposals of intangible assets during the years ended 31 December 2009, 2010 and 2011 respectively and therefore no investing cash flows associated with the Divested Brands are presented.
Accounting convention
The Combined Financial Statements have been prepared using the historical cost convention.
The Combined Financial Statements are reported in United States Dollars (“USD”). It is assumed for the preparation of these Combined Financial Statements that the functional currency for revenue, expenses and assets is the same as that previously adopted by GSK. Any exchange differences arising on re-stating functional currencies of the Canadian business to a US dollar presentation currency are recognised under IFRS in other comprehensive income and, consequently, are not presented as part of these Combined Financial Statements. It is also assumed that all foreign currency transactions were settled in local markets at the rate in force at the date the transaction arose and, as such, no transactional exchange differences have been recognised or presented in these Combined Financial Statements.
| 7 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
Revenue, cost of sales, selling, general and administration expenses, research and development and other operating income and expenses were derived as follows:
Revenue
Data has been derived from underlying accounting records without adjustment, including gross sales, discounts on invoices and other discounts.
Revenue is recognised in the Combined Statements of Revenue and Direct Operating Expenses when all of the following have taken place: (i) goods or services are supplied or made available to external customers against orders received, (ii) title and risk of loss is passed to the customer, (iii) reliable estimates can be made of relevant deductions and (iv) all relevant obligations have been fulfilled, such that the earnings process is regarded as being complete. Revenue represents net invoice value after the deduction of discounts and allowances given and accruals for estimated future rebates and returns. The methodology and assumptions used to estimate rebates and returns are monitored and adjusted regularly in the light of contractual and legal obligations, historical trends, past experience and projected market conditions. Sales taxes are excluded from revenue.
The top three customers accounted for 21%, 8%, 6% of revenue for the year ended 31 December 2011, 22%, 7%, 6% of revenue for the year ended 31 December 2010 and 25%, 7% and 6% of revenue for the year ended 2009. No other customer individually accounted for more than 5% of revenue in any of the three years ended 31 December 2011. The top five customers of Divested Brands accounted for 41%, 43% and 45% of total revenue for the years ended 31 December 2011, 2010 and 2009, respectively.
Cost of sales
Data has been derived from the underlying accounting records at transfer cost for products sourced from GSK and its affiliates, which approximates the actual manufacturing cost to the Group. Transfer cost for products sourced externally reflects actual cost to the Group.
Selling, general and administration expenses
Comprises: (i) Consumer Healthcare selling and distribution expenses allocated on the basis of revenue, (ii) Consumer Healthcare other marketing expenses allocated on the basis of advertising and promotion spend, (iii) administration expenses including Consumer Healthcare finance, information technology, legal and human resource costs allocated on the basis of revenue or other methodologies which management believes are reasonable. Expenses have been derived from the underlying accounting records of GSK and the Consumer Healthcare business.
GSK management believes that the Consumer Healthcare allocations included within the Combined Statements of Revenue and Direct Operating Expenses are reasonable; however, these allocated expenses are not necessarily indicative of expenses that would have been incurred by the Divested Brands on a standalone basis.
Also included are direct expenses for advertising and promotion, which have been sourced from the underlying accounting records.
Expenditure for goods and services is recognised when supplied, in accordance with contractual terms. Expenses are recorded when an obligation exists for a future liability in respect of a past event and where the amount of the obligation can be reliably estimated and will be transferred to the buyer within the Agreements. Advertising and promotion expenditure is charged to the Combined Statements of Revenue and Direct Operating Expenses as incurred. Inbound shipment costs on purchases and distribution expenses on sales to customers are included in selling, general and administrative expenditure. Purchases from other GSK entities for the Divested Brands are recorded on the date of shipment from the manufacturer as risks and rewards are considered to be transferred on that date.
| 8 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
Research and development
Research and development expenditure is charged to the Combined Statements of Revenue and Direct Operating Expenses in the period in which it is incurred. Research and development costs are directly attributable to the brands and contain no allocations. Research and development costs incurred by GSK Corporate are not allocated as they are not directly related to the Divested Brands.
Other operating income and expenses
Other operating income and expenses includes Consumer Healthcare allocated costs covering bad debt expense and other sundry items which are allocated on the basis of revenue.
Certain expenses and income, including corporate overhead, interest income, interest expense, restructuring costs and income taxes are not included in the accompanying Combined Statements of Revenue and Direct Operating Expenses, as they are not historically allocated to individual businesses and are not directly associated with revenue generating operations of the Divested Brands. Corporate overhead expenses include expenses incurred for insurance, legal, finance, human resources and executive management functions.
Product liability
Product liability costs included in these Combined Financial Statements are directly attributable to the brands and contain no allocations. No product liability costs are incurred by the Consumer Healthcare business of GSK which are not allocated to the brands. Product liability costs incurred by GSK Corporate are not allocated to the Divested Brands as they are not directly related to the Divested Brands. No provisions related to product liability are included in the Combined Statements of Net Assets to be Sold as historical product liabilities for sale prior to completion are not being assumed by the Purchaser.
In respect of product liability claims related to certain products, an expense is recorded when there is evidence of claims and settlements to enable management to make a reliable estimate of the amount required to cover unasserted claims. In certain cases, an actuarial technique is used to determine this estimate.
Intangible assets
Intangible assets are stated at cost less provisions for amortisation and impairment.
Acquired brands are valued independently, as part of the fair value of businesses acquired from third parties, where the brand has a value which is substantial and long-term and where the brands are either contractual or legal in nature or can be sold separately from the rest of the businesses acquired.
Brands are amortised over their estimated useful lives of up to 20 years, except where they are considered indefinite life brands.
Intangible assets included in the Combined Financial Statements are specific to the Divested Brands and do not therefore include any allocated balances.
| 9 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
Amortisation and impairment
The carrying values of intangible assets which have a definitive life are reviewed for impairment when there is an indication that the asset value might be impaired. Intangible assets with indefinite useful lives are tested for impairment annually and if there was any indication of impairment during the year. Any provision for impairment is charged to the Combined Statements of Revenue and Direct Operating Expenses in the year concerned. Impairment losses are reversed if there has been a change in the estimates used to determine recoverable amounts and only to the extent that the revised recoverable amounts do not exceed the carrying values that would have existed, net of amortisation, had no impairments been recognised.
Impairment testing is performed using post tax discount rate and post tax cash flows as deferred tax balances and a standalone tax rate is not available due to the limited nature of these Combined Financial Statements. Refer to Note 3 for a sensitivity analysis which includes the impact on the fair value of the Fiber Choice asset as a result of an increase or decrease in the discount rate by 1%.
Inventories
Inventories are included in the Combined Statements of Net Assets to be Sold at the lower of cost and net realisable value. Cost is determined on a first in, first out basis and been derived from the underlying accounting records at transfer cost for products sourced from GSK and its affiliates, which approximates the actual manufacturing cost to the Group. Transfer cost for products sourced externally reflects actual cost to the Group.
Inventory data has been derived from the underlying accounting records and includes direct costs of the Divested Brands only, no allocations have been included.
Legal and other disputes
No provision for the costs of legal disputes are included in the Combined Statements of Net Assets to be Sold as the liability for any existing litigation claims will not be assumed by the Purchaser. An expense is recorded for the anticipated settlement costs of legal or other disputes against the Divested Brands where an outflow of resources is considered probable and a reliable estimate can be made of the likely outcome. The Divested Brands may become involved in legal proceedings, in respect of which it is not possible to make a reliable estimate of the expected financial effect, if any, that could result from ultimate resolution of the proceedings. In these cases, appropriate disclosures about such cases are included but no expenses are included in the Combined Financial Statements.
Key accounting judgements and estimates
In preparing the Combined Financial Statements, management is required to make estimates and assumptions that affect the amounts of assets, liabilities, revenue and expenses reported in the Combined Financial Statements. Actual amounts and results could differ from those estimates. The following are considered to be the key accounting judgements and estimates made:
Revenue
Revenue is recognised when title and risk of loss is passed to the customer, reliable estimates can be made of relevant deductions and all relevant obligations have been fulfilled, such that the earnings process is regarded as being complete.
Gross revenue is reduced by rebates, discounts, allowances and product returns given or expected to be given, which vary by product arrangements and contractual arrangements. These arrangements with customers are dependent upon the submission of claims some time after the initial recognition of the sale.
| 10 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
Amounts are recorded at the time of sale for the estimated rebates, discounts or allowances payable or returns to be made, based on available market information and historical experience. Since the amounts are estimated they may not fully reflect the final outcome, and the amounts are subject to change dependent upon, amongst other things, the types of customer and product sales mix.
The level of expense is reviewed and adjusted regularly in the light of contractual and legal obligations, historical trends, past experience and projected market conditions. Market conditions are evaluated using wholesaler and other third-party analyses, market research data and internally generated information. Future events could cause the assumptions on which the expenses are based to change, which could affect the future results of the Divested Brands. There are no accruals, product liability claims or liabilities transferred to the Purchaser of the Divested Brands.
In respect of product liability claims related to certain products, an expense is recorded when there is evidence of claims made and settlements to enable management to make a reliable estimate of the amount required to cover unasserted claims. In certain cases, an actuarial technique is used to determine this estimate.
Legal and other disputes
The Divested Brands provide for anticipated settlement expenses where an outflow of resources is considered probable and a reliable estimate may be made of the likely outcome of the dispute and legal and other expenses arising from claims against the Divested Brands.
GSK management having taken legal advice, records legal expense after taking into account the relevant facts and circumstances of each matter and in accordance with accounting requirements.
The Divested Brands may become involved in legal proceedings, in respect of which it is not possible to make a reliable estimate of the expected financial effect, if any, that could result from ultimate resolution of the proceedings, for which no expense is recorded. At 31 December 2011, 31 December 2010 and 31 December 2009 management believes no material provisions for legal and other disputes were required.
The ultimate liability for legal claims may vary from the amounts provided and is dependent upon the outcome of litigation proceedings, investigations and possible settlement negotiations. The position could change over time, however, litigation claims and assessments are not assumed by the Purchaser.
Intangible assets
Where intangible assets are acquired by the Divested Brands from third parties, the costs of acquisition are capitalised. Brands acquired with businesses are capitalised independently where they are separable and have an expected life of more than one year. Brands are amortised on a straight-line basis over their estimated useful lives, not exceeding 20 years, except where the end of the useful economic life cannot be foreseen. Where brands are not amortised, they are subject to annual impairment tests in accordance with the assumptions set out in note 3. Patents are amortised from the point at which they are available for use, over their estimated useful lives, which may include periods of non-exclusivity.
Estimated useful lives are reviewed annually and impairment tests are undertaken if events occur which call into question the carrying values of the assets. Both initial valuations and valuations for subsequent impairment tests are based on established market multiples or risk-adjusted future cash flows discounted using appropriate interest rates reflecting GSK’s risk profile. These future cash flows are based on business forecasts and are therefore inherently judgemental. Future events could cause the assumptions used in these impairment analyses to change with a consequent adverse effect on the future results of the Divested Brands.
| 11 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
Implementation of new accounting standards
With effect from 1 January 2011, the Divested Brands have implemented IAS 24 (Revised) ‘Related party disclosures’ and IFRIC 19 ‘Extinguishing financial liabilities with equity instruments’ and minor amendments to a number of other accounting standards. There is no material impact on the Combined Financial Statements on application of these standards.
New accounting requirements
The following new and amended accounting standards and International Financial Reporting Interpretations Committee (“IFRIC”) interpretations have been issued by the IASB and are likely to affect future financial information produced by the Divested Brands, although, in their current forms, none is expected to have a material impact on the Combined Financial Statements of the Divested Brands.
IFRS 9 ‘Financial instruments’ was first issued in November 2009 and amended in October 2010 and will be effective from 1 January 2015. The standard will eventually replace IAS 39 and covers the classification, measurement and de-recognition of financial assets and financial liabilities. The IASB intends to expand IFRS 9 to add new requirements for impairment and hedge accounting at future dates.
An amendment to IFRS 7 ‘Disclosures – Transfers of financial assets’ was issued in October 2010 and will be effective from 1 January 2012. The amendment requires additional disclosures regarding the risk exposures relating to transfers of financial assets.
An amendment to IAS 12 ‘Deferred tax: recovery of underlying assets’ was issued in December 2010 and will be effective from 1 January 2012. The amendment requires that the deferred tax on non-depreciated assets measured using the revaluation model should be calculated on a sale basis.
IFRS 10 'Consolidated financial statements' was issued in May 2011 and will be effective from 1 January 2013. The standard builds on existing principles by identifying the concept of control as the determining factor in whether an entity should be included within the consolidated financial statements. The standard provides additional guidance to assist in determining control where this is difficult to assess.
IAS 27 (Revised 2011), ‘Separate financial statements’ was issued in May 2011 and will be effective from 1 January 2013. The revised standard includes the provisions on separate financial statements that are left after the control provisions of IAS 27 have been included in the new IFRS 10.
IFRS 11 'Joint arrangements' was issued in May 2011 and will be effective from 1 January 2013. The standard provides for a more realistic reflection of joint arrangements by focusing on the rights and obligations of the arrangement, rather than its legal form. Proportional consolidation of joint ventures is no longer allowed.
IAS 28 (Revised 2011) ‘Investments in associates and joint ventures’ was issued in May 2011 and will be effective from 1 January 2013. The revised standard now includes the requirements for joint ventures, as well as associates, to be equity accounted following the issue of IFRS 11.
IFRS 12 'Disclosure of interests in other entities' was issued in May 2011 and will be effective from 1 January 2013. The standard includes disclosure requirements for all forms of interests in other entities, including joint arrangements, associates, special purpose vehicles and other off balance sheet vehicles.
| 12 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
IFRS 13 ‘Fair value measurement’ was issued in May 2011 and will be effective from 1 January 2013. The standard explains how to measure fair value and aims to enhance fair value disclosures; it does not say when to measure fair value or require additional fair value measurements.
An amendment to IAS 1 ‘Presentation of Items of Other Comprehensive Income’ was issued in June 2011 and will be effective from 1 January 2013. The amendments improved the consistency and clarity of the presentation of items of other comprehensive income (OCI).
| 13 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
3 Intangible assets
| Licences and Patents | Indefinite Life brands | Total | ||||||||||
| $ | 000 | s | $ | 000 | s | $ | 000 | s | ||||
| Cost at 1 January 2009 | 5,500 | 216,937 | 222,437 | |||||||||
| Exchange adjustments | - | 1,382 | 1,382 | |||||||||
| Cost at 31 December 2009 | 5,500 | 218,319 | 223,819 | |||||||||
| Amortisation at 1 January 2009 | (1,341 | ) | - | (1,341 | ) | |||||||
| Charge for the year | (550 | ) | - | (550 | ) | |||||||
| Amortisation at 31 December 2009 | (1,891 | ) | - | (1,891 | ) | |||||||
| Net book value at 31 December 2009 | 3,609 | 218,319 | 221,928 | |||||||||
| Licences and Patents | Indefinite Life brands | Total | ||||||||||
| $ | 000 | s | $ | 000 | s | $ | 000 | s | ||||
| Cost at 1 January 2010 | 5,500 | 218,319 | 223,819 | |||||||||
| Exchange adjustments | - | 517 | 517 | |||||||||
| Cost at 31 December 2010 | 5,500 | 218,836 | 224,336 | |||||||||
| Amortisation at 1 January 2010 | (1,891 | ) | - | (1,891 | ) | |||||||
| Charge for the year | (550 | ) | - | (550 | ) | |||||||
| Amortisation at 31 December 2010 | (2,441 | ) | - | (2,441 | ) | |||||||
| Impairment at 1 January 2010 | - | - | - | |||||||||
| Impairment losses | - | (9,761 | ) | (9,761 | ) | |||||||
| Impairment at 31 December 2010 | - | (9,761 | ) | (9,761 | ) | |||||||
| Net book value at 31 December 2010 | 3,059 | 209,075 | 212,134 | |||||||||
| Licences and Patents | Indefinite Life brands | Total | ||||||||||
| $ | 000 | s | $ | 000 | s | $ | 000 | s | ||||
| Cost at 1 January 2011 | 5,500 | 218,836 | 224,336 | |||||||||
| Exchange adjustments | - | (245 | ) | (245 | ) | |||||||
| Cost at 31 December 2011 | 5,500 | 218,591 | 224,091 | |||||||||
| Amortisation at 1 January 2011 | (2,441 | ) | - | (2,441 | ) | |||||||
| Charge for the year | (550 | ) | - | (550 | ) | |||||||
| Amortisation at 31 December 2011 | (2,991 | ) | - | (2,991 | ) | |||||||
| Impairment at 1 January 2011 | - | (9,761 | ) | (9,761 | ) | |||||||
| Impairment losses | - | - | - | |||||||||
| Impairment at 31 December 2011 | - | (9,761 | ) | (9,761 | ) | |||||||
| Net book value at 31 December 2011 | 2,509 | 208,830 | 211,339 | |||||||||
| 14 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
The Licences and Patents relate to a Fiber Choice Patent acquired with the acquisition of CNS, Inc in 2006. The estimated useful life of the Patent is ten years from the acquisition date which is the date of expiration of the patent.
Indefinite life brands comprise a portfolio of consumer healthcare products primarily acquired with the acquisitions of Sterling Winthrop, Inc in 1994, Block Drug Company Inc in 2001 and CNS, Inc in 2006. The net book values of the major brands are as follows:
| 12/31/2011 | 12/31/2010 | 12/31/2009 | ||||||||||
| $ | 000 | s | $ | 000 | s | $ | 000 | s | ||||
| Gaviscon | 9,437 | 9,682 | 9,165 | |||||||||
| Fiber Choice | 45,839 | 45,839 | 55,600 | |||||||||
| BC | 89,240 | 89,240 | 89,240 | |||||||||
| Goody's | 29,701 | 29,701 | 29,701 | |||||||||
| Phazyme | 24,278 | 24,278 | 24,278 | |||||||||
| Nytol | 10,335 | 10,335 | 10,335 | |||||||||
| 208,830 | 209,075 | 218,319 | ||||||||||
Each of these brands is considered to have an indefinite life, given the strength and durability of the brand and the level of marketing support. The brands are in relatively similar stable and profitable market sectors, with similar risk profiles, and their size, diversification and market shares mean that the risk of market-related factors causing a reduction in the lives of the brands is considered to be relatively low. Management is not aware of any material legal, regulatory, contractual, competitive, economic or other factor which could limit their useful lives. Accordingly, these brands are not amortised.
Each brand is tested annually for impairment applying a value in use methodology, generally using five year post-tax cash flow forecasts (2009: four year post-tax cash flow forecasts) with a terminal value calculation and a discount rate equal to the GSK North American post-tax discount rate of 7%. The main assumptions include future sales price and volume growth, product contribution and the future expenditure required to maintain the product’s marketability and registration in the relevant jurisdictions. These assumptions are based on past experience and are reviewed as part of management’s budgeting and strategic planning cycle for changes in market conditions and sales erosion through competition. The terminal growth rates applied are between 0% and 2.5% and are management’s estimates of future long-term average growth rates of the relevant markets.
Due to adverse economic conditions, Fiber Choice experienced a decline in sales volume from 2008 to 2010. Management announced a significant price decrease in the fourth quarter of 2010, effective January 2011, to counter the volume drop and make the brand more competitive. The recoverable amount of the Fiber Choice brand was estimated, using the GSK value in use methodology, to be lower than its carrying value by $9,761,000. Consequently these Combined Financial Statements recognise an impairment loss in 2010 of $9,761,000 which was recorded in the Combined Statements of Revenue and Direct Operating Expenses within amortisation and impairment.
The estimate of value in use was determined using a post-tax discount rate of 7% specific to the US and the impairment loss was allocated entirely to the indefinite life brands. Impairment testing is performed using a post tax discount rate and post tax cash flows as deferred tax balances and a standalone tax rate is not available due to the limited nature of these Combined Financial Statements.
| 15 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
The table below shows the effects on the Fiber Choice impairment analysis if an alternative discount rate of 6% and 8% had been used, with all other assumptions remaining unchanged.
| Impairment Sensitivity Analysis Fiber Choice Discount Rate Sensitivity | ||||||||||||
| Discount Rate | Headroom/ (impairment) | |||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| $ | 000 | s | $ | 000 | s | $ | 000 | s | ||||
| 8% | (5,618 | ) | (12,168 | ) | (3,926 | ) | ||||||
| 6% | 8,271 | (308 | ) | 23,354 | ||||||||
| 4 | Related party transactions |
The Divested Brands enter into a significant number of transactions with GSK and affiliated entities for sale and purchase transactions and other support services.
Cost of sales
GSK entities supply the Divested Brands with inventory. These transactions have been recorded at transfer cost transfer cost for products sourced from GSK and its affiliates which approximates to the actual manufacturing cost to the Group. Amounts of $43,893,000, $42,776,000 and $41,764,000 are included in cost of sales in the Combined Statements of Revenue and Direct Operating Expenses for these transactions for the years ended 31 December 2011, 2010 and 2009, respectively.
Cost allocation
GSK and affiliated entities provide various services to the Consumer Healthcare business, including the Divested Brands. These services include selling and distribution, marketing, administration, and medical administration. Costs of these services are allocated to the Divested Brands based on the allocation methodology described under Note 2 – Basis of preparation and accounting policies. The total expenses allocated to the Divested Brands for these services were $24,497,000, $22,998,000 and $29,233,000 for the years ended 31 December 2011, 2010 and 2009, respectively and are included in selling, general and administration expenses in Combined Statements of Revenue and Direct Operating Expenses. Although the cost of these services cannot be quantified on a stand-alone basis, management believes that these allocations are reasonable.
The other operating income/(expenses) allocated to the Divested Brands were $648,000, $(295,000) and $(611,000) for the years ended 31 December 2011, 2010 and 2009, respectively and related to items such as bad debt expense and corporate cost allocation, franchise tax and sundry expenses and income. In the year ended 31 December 2011 there was a release of bad debt provision compared to bad debt expense being recognised in prior years.
The costs incurred by GSK’s corporate headquarters for services such as group insurance, legal, finance, human resources and the executive management functions are not allocated to the Divested Brands as they were not historically allocated to individual businesses and are not directly associated with revenue generating operations of the Divested Brands. Refer to Note 2 – Basis of preparation and accounting policies.
Remuneration of key management personnel
The Divested Brands operate as part of the overall Consumer Healthcare business within GSK and were historically not managed on a standalone basis, as a result there are no key management personnel identified for the Divested Brands.
| 16 |
The North American Divested Brands of GlaxoSmithKline Plc
Notes to Financial Statements
5 Post balance sheet events
These Combined Financial Statements are prepared in expectation of a sale of the Divested Brands in early 2012 as discussed in Note 2 - Basis of preparation and accounting policies.
The first agreement completed on 31 January 2012 for all brands excluding Debrox and Gly-oxide. The second agreement for Debrox and Gly-oxide is expected to complete on or before 1 May 2012.
There were no other subsequent events relevant to these Combined Financial Statements.
6 Commitments and Contingencies
The Divested Brands are involved in various legal matters and product liability claims arising in the ordinary course of business. Although the outcome of these matters cannot be presently determined, in the opinion of management, the disposition of these matters will not have a material adverse effect on the revenues or direct operating expenses of the Divested Brands.
| 17 |
