Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - SIGMA LABS, INC. | Financial_Report.xls |
| EX-32.1 - EXHIBIT 32.1 - SIGMA LABS, INC. | v306578_ex32-1.htm |
| EX-21.1 - EXHIBIT 21.1 - SIGMA LABS, INC. | v306578_ex21-1.htm |
| EX-23.1 - EXHIBIT 23.1 - SIGMA LABS, INC. | v306578_ex23-1.htm |
| EX-32.2 - EXHIBIT 32.2 - SIGMA LABS, INC. | v306578_ex32-2.htm |
| EX-31.2 - EXHIBIT 31.2 - SIGMA LABS, INC. | v306578_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - SIGMA LABS, INC. | v306578_ex31-1.htm |
| EX-10.12 - EXHIBIT 10.12 - SIGMA LABS, INC. | v306578_ex10-12.htm |
| EX-10.14 - EXHIBIT 10.14 - SIGMA LABS, INC. | v306578_ex10-14.htm |
| EX-10.13 - EXHIBIT 10.13 - SIGMA LABS, INC. | v306578_ex10-13.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
______________________
FORM 10-K
______________________
| x | ANNUAL REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
| ¨ | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from ______ to ______
Commission file number: 33-2783-S
SIGMA LABS, INC.
(Exact name of Registrant as specified in its charter)
______________________
| Nevada | 82-0404220 | |
| (State or other jurisdiction of | (I.R.S. Employer | |
| incorporation or organization) | Identification Number) | |
| 223 East Palace Avenue, Suite B | ||
| Santa Fe, New Mexico 87501 | ||
| (Address of principal executive offices) | ||
| (505) 438-2576 | ||
| Issuer’s telephone number: |
Securities registered under Section 12(b) of the Act: None.
Securities registered under Section 12(g) of the Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No þ
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No þ
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and, (2) has been subject to such filing requirements for the past 90 days.
Yes þ No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes þ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein and, will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer ¨ | Non-accelerated filer ¨ (Do not check if a smaller reporting company) |
Smaller reporting company þ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes ¨ No þ
State the aggregate market value of the voting and non-voting common equity held by non-affiliates computed by reference to the price at which the common equity was last sold, or the average bid and asked price of such common equity, as of the last business day of the registrant’s most recently completed second fiscal quarter. $2,641,194.
The outstanding number of shares of common stock as of March 30, 2012 was 429,667,400.
Documents incorporated by reference: None.
Table of Contents
Form 10-K
| Page | ||
| PART I | 1 | |
| ITEM 1. | BUSINESS. | 1 |
| ITEM 1A | RISK FACTORS. | 14 |
| ITEM 1B. | UNRESOLVED STAFF COMMENTS. | 24 |
| ITEM 2. | PROPERTIES. | 24 |
| ITEM 3. | LEGAL PROCEEDINGS. | 25 |
| ITEM 4. | MINE SAFETY DISCLOSURES. | 25 |
| PART II | 25 | |
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS, AND ISSUER PURCHASES OF EQUITY SECURITIES. | 25 |
| ITEM 6. | SELECTED FINANCIAL DATA. | 26 |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. | 27 |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. | 30 |
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. | 30 |
| ITEM 9A. | CONTROLS AND PROCEDURES. | 30 |
| ITEM 9B. | OTHER INFORMATION | 31 |
| PART III | 31 | |
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE. | 31 |
| ITEM 11. | EXECUTIVE COMPENSATION. | 34 |
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. | 35 |
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS. | 36 |
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES. | 37 |
| PART IV | 38 | |
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. | 38 |
DISCLOSURE REGARDING FORWARD-LOOKING STATEMENTS
This Report, including any documents which may be incorporated by reference into this Report, contains “Forward-Looking Statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements other than statements of historical fact are “Forward-Looking Statements” for purposes of these provisions, including any projections of revenues or other financial items, any statements of the plans and objectives of management for future operations, any statements concerning proposed new products or services, any statements regarding future economic conditions or performance, and any statements of assumptions underlying any of the foregoing. All Forward-Looking Statements included in this document are made as of the date hereof and are based on information available to us as of such date. We assume no obligation to update any Forward-Looking Statement. In some cases, Forward-Looking Statements can be identified by the use of terminology such as “may,” “will,” “expects,” “plans,” “anticipates,” “intends,” “believes,” “estimates,” “potential,” or “continue,” or the negative thereof or other comparable terminology. Although we believe that the expectations reflected in the Forward-Looking Statements contained herein are reasonable, there can be no assurance that such expectations or any of the Forward-Looking Statements will prove to be correct, and actual results could differ materially from those projected or assumed in the Forward-Looking Statements. Future financial condition and results of operations, as well as any Forward-Looking Statements are subject to inherent risks and uncertainties, including any other factors referred to in our press releases and reports filed with the Securities and Exchange Commission. All subsequent Forward-Looking Statements attributable to the Company or persons acting on its behalf are expressly qualified in their entirety by these cautionary statements. Additional factors that may have a direct bearing on our operating results are described under “Risk Factors” and elsewhere in this report.
Introductory Comment
Our predecessor, Framewaves, Inc., was a shell company, as that term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended, immediately prior to the closing of the Reorganization (as defined below in the discussion captioned “Business – The Reorganization”). Throughout this Annual Report on Form 10-K, unless otherwise indicated or the context otherwise requires, the term “Framewaves” refers to our predecessor shell-entity prior to consummation of the Reorganization; the term “B6 Sigma” refers to B6 Sigma, Inc., a Delaware corporation and the operating company acquired in connection with the Reorganization; and the terms the “Company,” “Sigma,” “we,” “us” and “our” refers to Sigma Labs, Inc. (f/k/a Framewaves, Inc.) together with B6 Sigma, Inc., a wholly owned subsidiary of the Company following completion of the Reorganization.
PART I
| ITEM 1. | BUSINESS. |
Summary
Prior to the closing of the Reorganization, Framewaves was a shell corporation with no ongoing operations focused on seeking a business opportunity. In September 2010, Framewaves entered into a share exchange agreement with B6 Sigma and its shareholders. Pursuant to the share exchange agreement, Framewaves acquired all of the issued and outstanding capital stock of B6 Sigma in exchange for shares of Framewaves common stock. In connection with the closing of the Reorganization, the shareholders of Framewaves approved a 150:1 forward stock split, and a change of the name of the corporation to “Sigma Labs, Inc.” Additionally, following completion of the Reorganization, B6 Sigma became our wholly owned subsidiary and we conduct our operations through B6 Sigma.
| 1 |
As described below under the discussion captioned “Recent Developments,” effective as of December 31, 2011, we acquired Sumner & Lawrence Limited (dba Sumner Associates) ("Sumner") and La Mancha Company ("La Mancha"), private consulting companies that provide consulting services to the public and private sector, respectively, especially with regard to emerging technologies and alternative applications of established technologies. In connection with our acquisition of Sumner and La Mancha, we issued an aggregate of 35,000,000 shares of our common stock to their former stockholders.
B6 Sigma is a company that specializes in the development and commercialization of novel and unique manufacturing and materials technologies. It is the belief of our management that some of these technologies will fundamentally redefine conventional practice by embedding quality assurance into the manufacturing processes in real time. In addition, the Company anticipates that its core technologies will enable its clientele to combine advanced manufacturing protocols with novel materials to achieve breakthrough product potential in many industries including aerospace, defense, oil and gas, prosthetic implants, sporting goods, and power generation.
Certain members of our management team at B6 Sigma are uniquely qualified scientists with broad backgrounds in manufacturing and materials technologies. In the past, these members of our management team have worked with some of the largest defense contractors in the world, in such varied projects as advanced armor and anti-armor systems, hypervelocity projectile launch systems, advanced reactive munitions and nuclear weapons stewardship programs.
Our business plan and current principal business activities include the continued development and eventual commercialization of our current suite of technologies, which are described elsewhere in this Annual Report on Form 10-K. Our strategy is to leverage our manufacturing and materials knowledge, experience and capabilities through the following means: (i) identify, develop and commercialize manufacturing and materials technologies designed to improve manufacturing/quality control practices, and create innovative products in a variety of industries; and (ii) provide consulting services in respect of our manufacturing and materials technology expertise to third parties that have needs in developing next-generation technologies for materials and manufacturing projects. We are presently engaged in a variety of activities in which we seek to commercialize technologies and products in the following industry sectors:
| · | In process quality assurance for manufacturing; |
| · | Aerospace and defense manufacturing; |
| · | Active protection systems for defending light armored vehicles; |
| · | Advanced materials for munitions; |
| · | Advanced materials for sporting goods; |
| · | Advanced Manufacturing Technologies; and |
| · | Dental Implant and biomedical prosthetics technologies. |
We expect to generate revenues primarily by marketing and selling our manufacturing and materials technologies. Our continued development in fiscal 2011 of our “In Process Quality Assurance” or “IPQA® technology, and munitions technologies will enable us to commercialize these technologies in the remainder of 2012. We will continue to refine those and our other technologies, including our dental implant biomedical prosthetics technology, for commercialization during fiscal 2012. However, we presently make no sales of these technologies and generate no revenues therefrom. Since its inception, B6 Sigma has generated revenues primarily from consulting services it provides to third parties.
Corporate History
Framewaves, Inc., a Nevada corporation, was incorporated in December 1985 as “Messidor Limited.” In December 2000, the corporation’s shareholders approved a name change to “Framewaves, Inc.” At the same time, the shareholders also approved the acquisition of Corners, Inc., a Nevada corporation (“Corners”), which was originally intended to be used as an operating subsidiary as part of the corporation’s business strategy to actively pursue the custom framing business. Ultimately, the corporation decided to pursue a different business opportunity.
| 2 |
B6 Sigma, Inc., a Delaware corporation, was incorporated in February 2010. Four members of our current management team worked together at Technology Management Company, Inc., a New Mexico corporation (“TMC”), before leaving to form B6 Sigma. Pursuant to an asset purchase agreement, B6 Sigma acquired certain assets from a division of TMC in exchange for the surrender of certain securities of TMC previously issued to the founders of B6 Sigma. The assets acquired include equipment, contracts, licenses and intellectual property relating to our IPQA® technology. See further discussion of our IPQA® technology under “Products and Services.”
On September 13, 2010, Framewaves entered into a share exchange agreement with B6 Sigma and the shareholders of B6 Sigma pursuant to which it acquired all of the issued and outstanding shares of B6 Sigma. Following the closing of the transactions contemplated by the share exchange agreement, B6 Sigma became a wholly owned subsidiary of the Company and its operations now comprise our sole business activity.
Our principal executive offices are located at 223 East Palace Avenue, Suite B, Santa Fe, New Mexico 87501, and our current telephone number at that address is (505) 438-2576. Our website address is www.sigmalabsinc.com. We do not incorporate the information on our website into this annual report, and you should not consider such information part of this annual report.
The Reorganization
On September 13, 2010, Framewaves entered into a share exchange agreement (“Share Exchange Agreement”) with B6 Sigma and the holders of all of the issued and outstanding capital stock of B6 Sigma (collectively, the “B6 Sigma Shareholders”). The transactions contemplated by the Share Exchange Agreement are hereinafter collectively referred to as the “Reorganization.” Pursuant to the Share Exchange Agreement, Framewaves issued to the B6 Sigma Shareholders 234,917,400 (post-split) shares (the “Reorganization Shares”) of its common stock, $0.001 par value per share, in exchange for all of the issued and outstanding capital stock of B6 Sigma. In connection with the Reorganization, B6 Sigma acquired 110,700,000 (post-split) shares of Framewaves common stock from three shareholders of Framewaves for the cash sum of $195,000, and simultaneously cancelled all such shares (such transactions, collectively, the “Stock Cancellation”). In addition, as a condition to the closing of the Reorganization, B6 Sigma also closed a private offering of $1,000,000 of its common stock contemporaneous with the closing of the Reorganization. In connection with the Reorganization, the Chief Executive Officer (and also a director) of Framewaves resigned and the officers and directors of B6 Sigma were elected to serve as officers and directors of the Company.
Following issuance of the Reorganization Shares to the B6 Sigma Shareholders and the Stock Cancellation, Framewaves had 313,067,400 (post-split) shares of its common stock issued and outstanding. In connection with the closing of the Reorganization, the shareholders of Framewaves approved a 150:1 forward stock split, and a change of the name of the corporation to “Sigma Labs, Inc.” Additionally, following completion of the Reorganization, B6 Sigma became a wholly owned subsidiary of the Company and its operations now comprise our sole business activity.
Recent Developments
During the fourth quarter of 2011 and the first quarter of 2012, the Company announced important developments which are outlined below.
| 3 |
· Acquisition of Sumner and La Mancha. On December 27, 2011, we announced that we had entered into an Exchange Agreement and Plan of Reorganization (the “Exchange Agreement”), with all of the stockholders of Sumner and La Mancha, New Mexico corporations incorporated in 1985 and 1982, respectively. On December 31, 2011, pursuant to the terms of the Exchange Agreement, Sigma Labs, Inc. acquired from the former stockholders of Sumner and La Mancha all of the outstanding common stock of Sumner and La Mancha in exchange for an aggregate of 35,000,000 shares of our common stock. The terms of the Exchange Agreement are described in Form 8-Ks, filed by Sigma Labs, Inc. with the Securities and Exchange Commission ("SEC") on December 27, 2011 and January 6, 2012, respectively, each of which is incorporated herein by reference.
Sumner, based in Santa Fe, New Mexico, provides consulting services to the public sector, especially with regard to emerging technologies and alternative applications of established technologies. Sumner holds ongoing contracts with government agencies and the appropriate levels of security clearance for those contracts. Sumner's current clients include, but are not limited to, the State Department, the Department of Defense, the Department of Energy, various military services and affiliated agencies, the National Laboratories, and contractors to these organizations. La Mancha is engaged in a similar line of business as Sumner, except that La Mancha provides consulting services primarily to the private sector.
Following our acquisition of Sumner, Richard Mah, our Chief Executive Officer and a member of our Board of Directors, and James Stout, our Treasurer and Chairman of our Board of Directors, were appointed as members of the Board of Directors of Sumner.
· Patent Filings. In 2011, Sigma Labs filed the following two patent applications with the U.S. Patent and Trademark Office:
| · | Medical Implants with Enhanced Osseointegration, US Patent Application 61/559,991, 2011; and |
| · | Adaptive Multi-Mode Control for Friction Stir Welding, US Patent Application 61/527,902, 2011. |
The Company anticipates that these patent filings will further strengthen its position in IPQA as well as enable new product development opportunities in advanced rapid-healing dental implants.
Overview of Business
B6 Sigma is an early-stage company that specializes in the development and commercialization of novel manufacturing and materials technology solutions. We believe that our primary manufacturing solutions technology, which we refer to as “In Process Quality Assurance” or “IPQA®,” will redefine conventional manufacturing practices primarily by embedding quality assurance protocols in real-time manufacturing processes, thereby reducing the need for and cost of post-manufacturing quality assurance processes. Additionally, we expect the materials solutions technology we are developing will be beneficial to manufacturers and other businesses that seek to improve the most relevant characteristics of the materials used in their production processes or other business operations. For example, we are working with the United States Army in connection with the development of a new munitions technology we refer to as Advanced Reactive Materials and Structures or “ARMS,” the goal of which is to either reduce the weight of current munitions by 50%, or improve the explosive power of munitions by 50%, or both. Additionally, we are developing in the area of advanced biomedical materials advanced materials technology with the objective of improving the “heal time” of dental implants by as much as 50%.
We expect to generate revenues primarily by marketing and deploying our technology solutions to businesses that seek to improve their production processes and/or manipulate and improve the most functional characteristics of the materials and other input components used in their business operations. Our management anticipates that the Company’s technology solutions will allow its clientele to combine advanced manufacturing with novel materials to achieve breakthrough product potential in many industries including the following industries: aerospace, defense, oil and gas, prosthetic implants, sporting goods, and power generation. We are currently investigating and pursuing application of our IPQA® and other technologies in some of these markets, and we anticipate growth in both the breadth and depth of IPQA® applications in the future.
| 4 |
We anticipate that our primary business focus will be in the (i) deployment and implementation of our IPQA® technology to all appropriate manufacturing businesses, and (ii) development and commercialization of additional breakthrough technologies and innovations in the materials and manufacturing sciences. We will continue to expand our operations in this regard, including investigating additional opportunities for applications of our technology as well as undertaking further development efforts towards the commercialization of various technologies we have identified.
Our board of directors and management comprise scientists and business professionals with extensive experience in the energy and advanced manufacturing/advanced materials technology market. These individuals have worked with some of the largest defense contractors in the world in varied projects such as advanced armor and anti-armor systems, hypervelocity projectile launch systems, advanced reactive munitions and nuclear weapons stewardship programs. These individuals collectively possess over 100 years of experience working in the advanced manufacturing and materials technology space. As such, we believe we possess the resident expertise to provide consulting services to other companies regarding their manufacturing operations, or to companies seeking to improve the design of their products by using alternative next-generation materials or improving certain characteristics of the original input material, on a fee for services basis. Accordingly, in addition to our primary business focus, we intend to generate revenues by providing such consulting services to businesses seeking the same. Such consulting services may not necessarily involve deployment of our own technologies and may be limited to consulting with respect to the development, exploitation or improvement of the client’s own technology.
Additionally, some members of our management team have worked at or with United States Department of Energy (“DOE”) national laboratories (including the Los Alamos National Laboratory (“LANL”) and Sandia National Laboratory (“SNL”)) over the last 30 years. Due to their work with the DOE, members of our management team have developed extensive relationships with the DOE and its network of national laboratories. Accordingly, we expect to leverage these relationships in connection with licensing and developing technologies created at such national laboratories for commercialization in the private sector. The DOE’s national laboratories possess a rich history of technological innovation, including in the following subject areas:
| · | Nuclear Weapons |
| · | Atomic energy |
| · | The supercomputer |
| · | Artificial intelligence |
| · | Advanced materials technology |
| · | The Human Genome Project |
| · | New research on the cause of Mad Cow Disease and potential transfer to humans |
| · | AIDS / HIV vaccines |
| · | Flow cytometry for rapidly studying human cells and pharma research |
| · | New computed tomography (CT) scanning for medicine, inspection, and security |
| · | New detectors for early-stage cancer |
| 5 |
Products and Services
In Process Quality Assurance – IPQA®
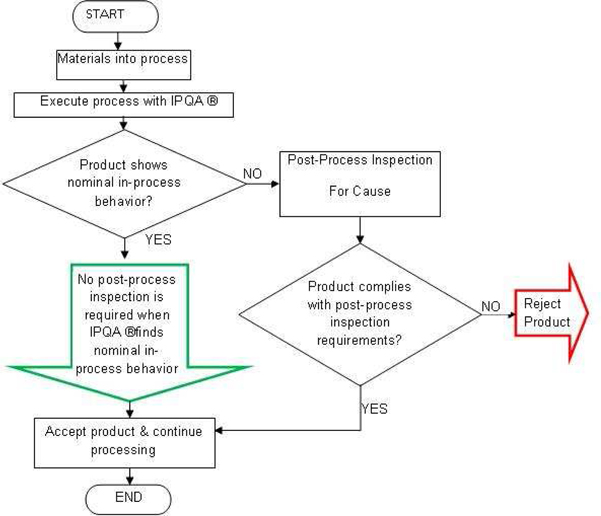
Under current manufacturing practices, it can take up to ten times longer to inspect a product than it takes to make it. Further, current inspection techniques at best only identify non-compliant products; they do not assist in determining the cause of the inspection failure. IPQA® refers to our proprietary method of product inspection frequency reduction for cause. It is a manufacturing solution that enables manufacturers to identify and determine the cause of manufacturing defects early in the manufacturing process, thereby reducing labor and materials waste and assuring product quality with a minimum of costly and time-consuming post-process inspection (see flowchart below). Our IPQA® solution observes and interprets process physical behavior during production runs to accept the largest possible fraction of process output immediately following completion of the manufacturing process. This acceptance occurs without further post-process inspection for product with acceptable in-process physical behavior. This acceptable behavior is similar to that of a known acceptable product, i.e., “nominal” product, is observed and characterized during process qualification and validation. The remnant fraction of product with rejectable in-process physical behaviors, i.e., “off-nominal” product, may be further post-process inspected via default stand-alone inspection methods. Since IPQA® occurs during the manufacturing process and reduces post-process inspection time and expense, we believe that our IPQA® solution is superior to conventional post-process inspection practices.
Our IPQA® technology combines hardware and software to observe, interpret and process physical behavior during production runs. Management believes that this technology solution will fundamentally redefine conventional manufacturing quality practices. For example, Boeing and Airbus are both moving towards welded aircraft structures in their manufacturing processes. Accordingly, the weld quality of aircraft structures will now be a critical concern. It is not economical to inspect millions of welds using traditional 100% post-process inspection methods. We believe that IPQA® provides an economical alternative that aircraft manufacturers such as Boeing will find very valuable in helping to solve this problem for future commercial aircraft production. IPQA® represents our only commercialized technology at this time. Please see the discussion below under “Technology and Solutions under Development” for a discussion of additional technologies we are currently developing or investigating for possible development.
| 6 |
Technology and Solutions in Development
IPQA for Additive Manufacturing ("AM")
Current aerospace practice for both commercial and military aircraft production is to “machine” large titanium parts out of either monolithic forgings or thick plate stock. This is done because the parts must have outstanding mechanical properties, and because there is a tradition of using this approach in the historical manufacturing base which dates back to an era when the US as well as Europe had significant primary metalworking industries such as rolling mills, forging presses, etc.
Additionally, primary metal prices were low historically and therefore the metal costs in the past were not a large part of the input/materials cost, and therefore historically there was no financial incentive to reduce materials utilization. Several factors have changed this historical paradigm in aerospace manufacturing, including the following:
| · | High metals commodity prices with no signs of long-term reduction; |
| · | High energy costs that dramatically increase cost of production, e.g., metals processing and machining away metal; |
| · | Demise of the primary metals working industry worldwide and therefore a critical shortage of qualified suppliers for large plates and forgings; and |
| 7 |
| · | Demise of skilled worker base that could support the historical approach to aerospace manufacturing. |
With all the above-mentioned factors, the actual demand for titanium in the aerospace market is increasing because of the increasing use of carbon composites which require titanium, and not aluminum, as the metal mating material for compatibility reasons.
Based on the foregoing, our management is of the opinion that demand exists for a new metals production technology called “additive manufacturing.” Additive manufacturing (AM) results in very efficient metal utilization for parts made on-demand, and utilizes a wide variety of rapid prototyping methods. "Additive manufacturing" is the generic moniker for these technologies. The use of our IPQA technology (or real-time nondestructive inspection) for Additive Manufacturing is what management believes will be our competitive advantage or unique-selling point that will allow us to enter the emerging market place for metal parts made using Additive Manufacturing.
One business model management consists of the formation of an additive manufacturing component production facility in collaboration with various business partners and large aerospace original equipment makers ("OEMs"). Through such collaborative efforts, management anticipates being able to sell AM parts at a price of $500 per pound and an internal cost of production of $200 - $250 per pound. A typical additive manufacturing machine tool may be able to produce 50 pounds per day, and therefore projected revenues based on an assumed 8-hour shift could be as high as $25,000. However, the machine tool will not have 100% utilization due to maintenance time and downtime to allow for part change-out, part cooling, etc. Therefore, with 75% utilization of an AM machine tool, effective revenue potential per day is closer to $12,500. Assuming 20 days of production per month, projected monthly revenues of $250,000 and annual revenues of over $2,000,000 for a single additive manufacturing machine tool are possible. Management is of the belief that current and future aerospace production could support the introduction of up to 20-30 additional additive manufacturing machine tools over the next five years. A typical component produced using Additive Manufacturing is shown in the photograph below. It is a fuel injector part designed with a complex geometry that is difficult to make using conventional manufacturing practices.

| 8 |
Upon commercialization of this technology, we expect to be the prime contractor for developing an additive manufacturing process for titanium components to build up parts layer by layer, thereby saving significant raw materials and manufacturing costs. This process is expected to help save millions of dollars per plane for military and commercial aircraft alike. The use of lightweight composite materials is the future of commercial airliners and is being heavily used in both the Boeing 787 as well as the Airbus A380. Basically, composite materials enable far lighter aircraft with associated fuel savings of tens to hundreds of millions of dollars over the lifecycle of the aircraft. However, titanium “skeleton” parts must still be used for critical structures such as wing spars and various fuselage and wing components. The problem with current titanium parts however is that they can cost up to $2000 per pound of metal going on wing. This exorbitant price prevents the widespread use of titanium fabrications, and therefore limits the cost-effective use of composites, thus impacting all of commercial aviation. We believe that this proposed technology of implementing IPQA for AM is at the forefront of a revolution in aerospace manufacturing as well as other industries that may have a need for such technology.
In 2010, the United States Air Force (“USAF”) awarded us a 2 year, $750,000 contract to adapt our IPQA® technology to the application of AM components fabricated for USAF's new Joint Strike Fighter F-35 program. We engaged an OEM supplier of AM equipment to perform certain consulting and other services on this project. We have also worked for the USAF on another AM project concerning the enhancement of gas metal arc welding processes for certified repair work on titanium components at facilities in the US and globally.
Dental Implant Technology
We believe that the key innovations in the future of dental implants are in the field of nanotechnology; specifically, to enhance rapid healing of implants and reduce failure rates as well as patient discomfort.
The greatest risk of failure of a dental implant is during the osseo-integration phase, or when the bone adopts the implant and bone tissue grow in close proximity to the metal implant. It has been found in numerous studies that when the surfaces immediately adjacent to the bone tissue are nano-structured, the rate of osteo-integration is increased and the time required for the implant to be integrated into the bone tissue is cut down significantly. The benefits of such technology extend to both dental professionals and patients. For one, it represents a significant advantage for all patients since the time it takes to heal would be cut down significantly and the implant could be used far sooner. Also, this would help dentists contain costs on failed implants and further reduce the overhead associated with patients’ “chair time,” which is not always fully recovered if there are too many visits required to execute any given procedure.
We have developed a unique solution to the problem of how to nano-structure a dental implant and speed up the healing time. Using Cold-War technology originally developed for the nuclear weapons program, we can create a favorable nano-texturing effect right at the surface of dental implants – precisely where it is needed.
We are in discussions with Omega Ti Implants of Albuquerque-NM (“Omega Ti”) and Alpha Omega Power Technologies of Albuquerque-NM to enter into a joint venture. In connection therewith, a new entity, named Osseostat, LLC, was formed for the sole purpose of commercializing our patent-pending rapid – healing dental implant technology. The earliest we expect to commercialize such technology is fourth quarter of 2012, although there is no assurance that it will be commercialized at all. Omega Ti has already received approval from the United States Food and Drug Administration in connection with its nano-structure implants for human use — a technology related to our patent-pending dental implant technology. They have conducted trials on material where the entire implant was made up of nano-grains. This material is very expensive, however, and there is currently no reliable source for bulk quantities. As such, our method of just "nano-texturing" the surface is particularly attractive since it could potentially be used on any implant made by any implant manufacturer (as long as it is titanium).
| 9 |
Also, other prosthetic markets exist beyond dental implants including hips, knees, and the plethora of smaller titanium components such as plates, screws, and other devices used to reconstruct bones or joints after either serious injury or natural aging. Through this innovative and advanced materials processing technology, we expect to significantly impact the dental implant market first, and then grow the business to include other bio-prosthetic devices that could benefit from rapid bone healing. Omega Ti has undertaken initial marketing efforts and sales campaigns in India as well since the Asian markets for implants represents the fastest growing sector of the market worldwide. Through our partnership with Omega Ti, we would anticipate access to the Chinese, Indian, Korean and Taiwanese markets Omega Ti plans to penetrate, as well as utilize Omega Ti’s existing domestic distribution channels.
Working with Omega Ti, we are currently performing pre-clinical evaluations of our dental implants in Canada. The results so far have shown that our implants are able to fully integrate with the human jaw in a period of four weeks. We are also in discussions with the University of New Mexico about the planning and performance of a human clinical trial. This would be an important step towards the full commercialization and approval of our medical device for sale in the United States, North America, Europe, and Asia. We expect the clinical trial to be underway by the third quarter of 2012.
Advanced Munitions Technology
For 21st century threats to security worldwide, the United States and its allies need new classes of effective weapons capable of limiting the potential harm to civilian populations. We are presently working to develop two different technologies to meet these modern threats.
Bonded Advanced Munitions. Bonded Advanced Munitions or “BAM™” is the first of such munitions technology that we are developing. BAM represent unique combinations of high density and high reactivity metals that are suitable for air-to-air defense, missile defense, ship defense, or defending buildings and structures against car bomb attacks.
Advanced Reactive Materials and Structures. The second technology is Advanced Reactive Materials and Structures or “ARMS™,” and is well suited to the battlefield of the future which will heavily rely on the use and deployment of Unmanned Aerial Vehicles (UAVs) or drones. Currently, UAVs are being used not just for surveillance but for interdiction strikes as well. The weapons used today were designed for much larger manned aircraft. ARMS would allow twice the explosive power in an equivalent weight, or more importantly for UAVs, would allow a 50% reduction in weapons weight with equivalent explosive power. This would allow for the design and deployment of new generations of munitions specifically tuned for UAVs. We have been recently awarded a contract by the United States Army in connection with funding development of the early proof of concept of this technology.
Various Customers, Projects and Prospects
Since inception, we have worked and are currently working with various companies to implement our range of technology solutions, some of which are presented in the table below. Our Management believes that our present activities serving such a diverse range of clients will operate to (i) cultivate a customer base and name recognition among domestic and international aerospace and defense firms; (ii) enable us to understand first-hand the market trends and needs in the various industries we intend to serve with our technology solutions; and (iii) allow us to deploy new products with valuable customer input and feedback before proceeding with broader market commercialization.
 |
Boeing is considering using IPQA® as a key enabling quality assurance tool for accepting (or possibly “shipping”) product, with the potential to save a significant volume of metals per aircraft, through more efficient utilization, resulting in significant savings per aircraft. |
| 10 |
 |
KUKA is planning to put IPQA® onto their friction welding machine tools which will make parts for aerospace companies, both engine makers and air-framers. Members of our management team have worked with KUKA since 2006 and expect to broadly diffuse IPQA® into their product lines of production machines tools for many industries. |
 |
We are currently working with ACB to potentially put IPQA® units onto their machine tools for linear friction welding. |
 |
The world-famous Nevada Test Site, currently managed by NSTec LLC, is where the United States conducted hundreds of nuclear tests during the Cold War from 1951 until 1992. Our scientists and engineers have teamed with NSTec LLC to provide a range of engineering/consulting services including the creation of new technical centers focusing on nuclear security in the post-911 era. |
 |
We assisted this major European jet engine maker in developing advanced processes and materials for next-generation aero-engines. Snecma is part of the SAFRAN Group, which is a market leader in aerospace, defense and security applications. |
 |
Pratt and Whitney was the first engine company to use friction welded fan blades on military aircraft, and now our manufacturing experts have helped them further innovate by creating new repair techniques with IPQA® for expensive rotor parts. |
 |
Honeywell aerospace systems has asked us to develop and implement an IPQA® solution for the manufacture of next-generation components for small engines that would go onto commuter and business jets. We are currently assisting Honeywell during their process development and pre-production trials using linear friction welding. |
 |
We have assisted ALCOA with advanced sensing of welding processes in aluminum alloys. The quality control aspects of the welding are critical for ALCOA, and we were specifically sought out by ALCOA on account of our unique and enabling sensing and control technology, namely IPQA®. |
| 11 |
 |
We have demonstrated the welding of titanium armor panels for the US Army using MIG welding and by monitoring the quality of the welds using IPQA®. The technology may be very useful for both commercial and military vehicle welding of all kinds as both the military and the private sector have a renewed interest in lighter vehicles that can carry more or that get better gas mileage. |
 |
We are assisting the DOE’s Los Alamos National Laboratory with the implementation of IPQA® and other technologies into the nuclear materials handling arena. |
Markets
We intend to market our manufacturing quality technology solutions to manufacturers seeking to reduce inspection/quality assurance costs by incorporating real-time quality assurance protocols in their manufacturing practices. Presently, our efforts in this regard are focused in the aerospace/aircraft manufacturing industry, and we expect the primary markets for our IPQA® (and IPQA-based AM) solution to be manufacturers based in the United States and Europe.
Similarly, we expect to market or license any materials technology solutions we develop to companies seeking to improve the design of their products by using alternative next-generation materials or improving certain characteristics of the original input material. In the advanced munitions space, we expect the primary market space for any technology we develop to be exclusively domestic (i.e., U.S.) in part due to applicable law regulating business involved in this industry. With respect to our dental implant technology under development, we expect to market in the United States as well as (via our partnership with Omega Ti) to the Chinese, Indian, Korean and Taiwanese dental implant markets.
Competition
We believe our technologies will be beneficial to several industries, including aerospace, defense, oil and gas, prosthetic implants, sporting goods, and power generation. However, developments by others may render our current and proposed technologies noncompetitive or obsolete, or we may be unable to keep pace with technological developments or other market factors. Additionally, our competitive position may be materially affected by our ability to develop or successfully commercialize certain technologies that we have identified for commercialization. Other general external factors may also impact the ability of our products to meet expectations or effectively compete, including pricing pressures.
We anticipate some of our principal competitors in the United States will include Alliant Techsystems Inc. and Energetic Materials and Processes, Inc., both of which are businesses focused on developing materials technology solutions in the advanced munitions market; and Straumann AG and BioMet 3I, companies that specialize in developing dental implants that heal rapidly. We believe that many of our competitors have significantly greater research and development capabilities than we do, as well as substantially more sales, marketing and financial and managerial resources. These entities represent significant competition for us. In addition, acquisitions of, or investments in, competing companies by large corporations could increase such competitors’ research, financial, manufacturing and other resources.
| 12 |
Intellectual Property
We regard our trademarks, domain names, trade secrets, in-licensed technologies, process knowledge, and other intellectual property as critical to our success. We rely on trademark and other intellectual property law, and confidentiality agreements and license agreements with employees, partners, and others to protect our intellectual assets. We have obtained one utility patent pending and two provisional patents pending with respect to our IPQA technology, in addition to filing a new patent application in 2011 for our IPQA technology. Also, as mentioned previously, in 2011 we filed a patent application pertaining to the advanced dental implant technology. There is no guarantee that the patents for which we have applied will offer adequate protection under applicable law.
We also rely on technologies that we license from third parties for further development. For example, we are presently developing technology that was originally licensed from the United States Department of Energy. If we succeed in developing such in-licensed technologies for commercialization, we expect to protect any interests in such further developed technology via a combination of intellectual property law (trademarks, patents, etc.) and confidentiality and non-disclosure agreements with partners and collaborators.
Government Regulation
Our business activities are subject to a variety of federal, state and local laws and regulations. For example, as a company involved with the development of munitions technology, we are required to comply with applicable provisions of the International Traffic in Arms Regulations, as well as register with the US Department of State’s Directorate of Defense Trade Controls. These regulations are aimed at preventing the inadvertent disclosure of munitions related data or the export of technical knowledge to foreign countries. The work we do with governmental units may also be subject to laws respecting the confidentiality of any classified or national security information we receive during the course of our activities under any government contract.
Additionally, with respect to our work with government agencies, our sales are driven by pricing based on costs incurred to produce products or perform services under contracts with the U.S. government. U.S. government contracts generally are subject to Federal Acquisition Regulations (“FAR”), agency-specific regulations that implement or supplement FAR, such as the DoD’s Defense Federal Acquisition Regulations (“DFAR”) and other applicable laws and regulations. These regulations impose a broad range of requirements, many of which are unique to government contracting, including various procurement, import and export, security, contract pricing and cost, contract termination and adjustment, and audit requirements. A contractor’s failure to comply with these regulations and requirements could result in reductions of the value of contracts, contract modifications or termination, and the assessment of penalties and fines and could lead to suspension or debarment from government contracting or subcontracting for a period of time. In addition, government contractors are also subject to routine audits and investigations by U.S. government agencies such as the Defense Contract Audit Agency (“DCAA”). These agencies review a contractor’s performance, cost structure, and compliance with applicable laws, regulations, and standards. The DCAA also reviews the adequacy of, and a contractor’s compliance with, its internal control systems and policies, including the contractor’s purchasing, property, estimating, compensation, and information systems.
Employees
The Company currently employs 7 full-time employees and 3 part-time employees. Newly acquired Sumner and La Mancha together employ 4 full-time employees and 2 part-time employees. We are not a party to any collective bargaining agreements.
| 13 |
| ITEM 1A | RISK FACTORS. |
An investment in our securities involves a high degree of risk. You should carefully consider the risks described below before deciding to invest in or maintain your investment in our Company. The risks described below are not intended to be an all-inclusive list of all of the potential risks relating to an investment in our securities. If any of the following or other risks actually occur, our business, financial condition or operating results and the trading price or value of our securities could be materially and adversely affected.
Risks Related to Our Business and Industry
We have a limited operating history, which makes it difficult to evaluate an investment in the Company.
Since we recently commenced business operations, it can be expected that we will continue to incur significant operating expenses and will experience significant losses in the foreseeable future. There is no assurance that any revenues we generate will be sufficient for us to become profitable or thereafter maintain profitability. As a result, the Company cannot predict when, if ever, it might achieve profitability and cannot be certain that it will be able to sustain profitability, if achieved. Our lack of an operating history may make it difficult for you to evaluate our business prospects in connection with an investment in our securities.
We face many of the risks normally associated with a new business.
Because we have had a little under two years of operations, we face all the risks inherent in a new business, including the expenses, difficulties, complications and delays frequently encountered in connection with conducting new operations. These uncertainties include establishing our internal organization structure, developing our brand name, raising capital to meet our working capital requirements and developing a customer base, among others. If we are not effective in addressing these risks, we will not be able to operate profitably in the future, and we may not have adequate working capital to meet our obligations as they become due.
The Company’s audited financial statements express substantial doubt about its ability to continue as a going concern.
Our audited financial statements for the period ended December 31, 2011, have been prepared assuming that it will continue as a going concern. However, our auditors have expressed substantial doubt about our ability to continue as a going concern because as of the date of the audited statements, we had generated limited revenues and had not achieved profitable operations. The Company’s ability to continue as a going concern is subject to its ability to finance its operations by generating and sustaining profits and/or obtaining necessary funding from outside sources. We have only recently commenced operations, and expect to continue to experience significant losses in the foreseeable future. There can be no assurance that we will ever achieve (or sustain) profitability, or successfully secure outside financing. Accordingly, there can be no assurance about our ability to continue as a going concern.
We have limited financial resources and may need to raise significant additional capital to continue our operations.
We will require significant financial resources to fund our current and future business operations. It is possible that our capital resources will be insufficient to fund all of such requirements and that the Company may be required to obtain additional capital in the future. In doing so, the Company may seek to access the capital markets to fund its capital needs. However, there can be no assurance that we will be able to secure such additional financing, or that we will do so on terms favorable to the Company. In addition, the current global financial crisis has exacerbated the difficulty of obtaining credit on favorable terms or at all, especially by companies with limited operating histories such as ours. Failure to obtain such additional funds as and when we need them, or securing such financing on unfavorable terms, may significantly impair our ability to continue operations.
| 14 |
Any additional financing we may undertake could result in dilution to existing stockholders.
Any additional financings we undertake in the future may be obtained through one or more transactions involving the issuance of our capital stock, which will dilute (either economically or in percentage terms) the ownership interests of our stockholders.
Our business may be adversely affected by the global economic downturn.
The global economy is currently in a pronounced economic downturn. Global financial markets are continuing to experience disruptions, including severely diminished liquidity and credit availability, declines in consumer confidence, declines in economic growth, increases in unemployment rates, and uncertainty about economic stability. Given these uncertainties, there is no assurance that there will not be further deterioration in the global economy, the global financial markets and consumer confidence. Any economic downturn generally could cause a drop in government spending and business investment, which would have a material adverse effect on our business. Further, as a result of the current global economic situation, there may be a disruption or delay in performance by the Company’s third-party contractors and suppliers. If such third parties are unable to adequately satisfy their contractual commitments to us in a timely manner, our business could be adversely affected.
If we fail to hire a chief financial officer, we may be unable to implement and monitor financial controls sufficient to ensure maximum profitability and compliance with applicable regulatory requirements.
We currently have no Chief Financial Officer (“CFO”) and it is unlikely we will hire a CFO in the near future due to the expense of employing a CFO and our limited capital resources. James Stout, our Chairman and Treasurer, presently acts as our principal accounting officer. Due to our limited internal organizational structure, our financial controls may be ineffective. Accordingly, unless we obtain the services of a qualified CFO, we may be unable to implement and monitor financial controls sufficient to ensure maximum profitability and compliance with applicable regulatory requirements. Such regulatory requirements include, among others, certifications and protocols set forth in the Sarbanes Oxley Act of 2002 and related laws and regulations governing accounting, and financial and auditing standards and practices designed to ensure accurate and transparent financial information regarding the financial health and prospects of companies.
We are not subject to certain reporting requirements under the federal securities laws – accordingly, our stockholders do not have the benefit of certain disclosures prior to voting on material transactions or the benefit of reviewing information regarding our officers’ and directors’ stock ownership and their transactions involving our securities.
We are currently subject to SEC reporting requirements under Section 15(d) of the Exchange Act of 1934, as amended (the “Exchange Act”). Because we have not filed a registration statement under Section 12 of the Exchange Act, we are not subject to the SEC’s proxy rules and related information requirements of the Exchange Act. Further, our officers, directors and stockholders owning 10% or more of our outstanding capital stock are not required to file reports with the SEC concerning their stock ownership and stock trading activity under Section 16 of the Exchange Act, which provides for timely disclosure of insider transactions. Accordingly, our shareholders do not have the benefit of (i) certain disclosures required under the SEC’s proxy rules in connection with their approval of certain corporate actions (e.g., significant acquisitions and election of directors); and (ii) disclosures about our officers’ and directors’ ownership of and their transactions involving the Company’s securities.
| 15 |
We could incur significant damages if we are unable to adequately discharge our contractual obligations.
Our failure to comply with contract requirements or to meet our clients’ performance expectations on a contract could materially and adversely affect our financial performance and our reputation. This, in turn, would impact our ability to compete for new clients and contracts. Our failure to meet contractual obligations could also result in substantial actual and consequential damages under the terms of such contracts. In addition, some of our contracts require us to indemnify clients for our failure to meet performance standards and/or contain liquidated damages provisions and financial penalties related to performance failures. Although we do have liability insurance, the policy limits may not be adequate to provide protection against all such potential liabilities.
We have financial exposure on our fixed-price contracts because we are required to complete a project even if the costs exceed the revenues we generate on such fixed-price contract.
We presently provide and expect to provide services under fixed-price and performance-based arrangements. Generally, under our fixed-price contracts, we receive a specified fee regardless of our cost to perform under such contracts (compared with performance-based contracts under which we earn fees on a per-transaction basis). If we underestimate the cost to complete a contract, we will still be required to complete the work specified under such contract, which could result in a loss to us. To earn a profit on these fixed-price contracts, we must accurately estimate costs involved and assess the probability of meeting the specified objectives, realizing the expected units of work or completing individual transactions, within the contracted time period. We expect to recognize revenues on these contracts, including a portion of estimated profit, as costs are incurred. Therefore, if a contract is cancelled or renegotiated after work has been performed, previously recognized revenue would be reversed and charged to earnings at that time. Reversals of previously recognized revenue could adversely affect our financial results. In addition, we expect to review these contracts quarterly and adjust revenues to reflect our current expectations as to the total anticipated costs of each contract. These adjustments may affect the timing and amount of revenue recognized and could adversely affect our financial results.
Requests for Proposals (RFPs) to secure government contracts are time consuming to prepare and our ability to successfully respond to RFPs will impact our operations.
A substantial portion of our clients will be state or local government authorities. To market our services to government clients, we will likely be required to respond to Request for Proposals or “RFPs.” To do so effectively, we must estimate accurately our cost structure for servicing a proposed contract, the time required to establish operations and likely terms of the proposals submitted by competitors. We must also assemble and submit a large volume of information within an RFP’s rigid timetable. Our ability to respond successfully to RFPs will greatly impact our business. There is no assurance that we will be awarded any contracts through the RFP process, or that our submitted RFPs will result in profitable contracts.
Our government clients may terminate our contracts prior to completion, which could result in revenue shortfalls and reduce profitability or cause losses on government contracts.
Many of our contracts with government agencies contain initial or base periods of one or more years, as well as option periods typically covering more than half of the contract’s initial duration. However, our government clients are under no obligation to exercise the option to extend the contract term. The profitability of some of our contracts could be adversely impacted if such options are not exercised and the contract term is not extended accordingly. Additionally, our contracts will likely contain provisions permitting a government client to terminate the contract on short notice, with or without cause. The unexpected termination of significant contracts could result in significant revenue shortfalls. If revenue shortfalls occur and are not offset by corresponding reductions in expenses, our business could be adversely affected. We cannot anticipate if, when or to what extent a client might terminate its contracts with us.
| 16 |
We are subject to government audits and our failure to comply with applicable laws, regulations and standards that could subject us to civil and criminal penalties and administrative sanctions.
The government agencies we contract with have the authority to audit and investigate our contracts with them. As part of that process, a government agency may review our performance on a contract, our pricing practices, our cost structure and our compliance with applicable laws, regulations and standards. If the agency determines that we have improperly allocated costs to a specific contract, we will not be reimbursed for those costs and we will be required to refund the amount of any such costs that have been previously reimbursed. If a government audit identifies improper activities by us or we otherwise determine that these activities have occurred, we could be subject to civil and criminal penalties and administrative sanctions, including termination of contracts, forfeitures of profits, suspension of payments, fines and suspension or disqualification from doing business with the government. Any adverse determination could adversely impact our ability to bid for Requests for Proposals (RFPs) in one or more jurisdictions.
Unions may interfere with our ability to obtain contracts.
Our success will depend in part on our ability to win profitable contracts to administer and manage programs that may have been previously administered by government employees. Many government employees, however, belong to labor unions with considerable financial resources and lobbying networks. Unions have in the past and are likely to continue to apply political pressure on legislators and other officials seeking to outsource government programs. Union opposition may result in fewer opportunities for us to service government agencies.
We rely on our relationship with government agencies to obtain contracts.
To facilitate our ability to prepare bids in response to RFPs, we expect to rely in part on establishing and maintaining relationships with officials of various government entities and agencies. These relationships will enable us to provide informal input and advice to the government entities and agencies prior to the development of an RFP. We also expect to engage marketing consultants, including lobbyists, to establish and maintain relationships with elected officials and appointed members of government agencies. The effectiveness of these consultants may be reduced or eliminated if a significant political change occurs. We may be unable to successfully manage our relationships with government entities and agencies and with elected officials and appointees and any failure to do so may adversely affect our ability to bid successfully for RFPs.
We have significant competition in bidding for government contracts from large national and international organizations.
The government contracting industry is subject to intense competition. Many of our competitors are national and international in scope and have greater resources than we do. Substantial resources could enable certain competitors to “low bid” on government RFPs or take other measures in an effort to gain market share. In addition, we may be unable to compete for a certain large government contract because we may not be able to meet an RFP’s requirement to obtain and post a large cash performance bond. Also, in some geographic areas, we face competition from smaller consulting firms with established reputations and political relationships. There is no assurance that we will compete successfully against our existing or any new competitors.
| 17 |
We may not be able to effectively control and manage our growth, which would negatively impact our operations.
We have operated our current line of business for a little over two years, and we expect to grow in the near future as our business develops and becomes established. If our business grows as we anticipate, it will be necessary for us to manage our expansion in an orderly fashion. Any significant growth in our activities or in the market for our services will require extension of our managerial, operational, marketing and other resources. Future growth will also impose significant additional responsibilities upon the members of management to identify, recruit, maintain, integrate, and motivate new employees. Our failure to manage growth effectively may lead to operational inefficiencies that will have a negative effect on our profitability. Additionally, if our growth comes at the expense of providing quality service and generating reasonable profits, our ability to successfully bid for contracts and our profitability will be adversely affected. We cannot assure investors that we will be able to effectively manage any future growth we may experience.
Failure to obtain adequate insurance coverage could put the Company at risk for uninsured losses.
We do currently have liability insurance. Some or all of the Company’s customers may require insurance as a requirement to conduct business with the Company. We may be unable to obtain or maintain adequate liability insurance on acceptable terms, if at all, and there is a risk that our insurance will not provide adequate coverage against our potential losses. Additionally, there are certain types of losses that may not be insurable at a cost that the Company can afford or at all. Claims or losses in excess of any insurance coverage we may obtain, or the lack of insurance coverage, could put the Company at risk of loss for any uninsured loss, which would have a material adverse effect on our business and financial condition.
We are dependent on our senior executive officers and other key personnel, loss of which could harm our business.
The Company depends on its senior executive officers as well as key scientific and other personnel. The loss of any of these individuals could harm the Company’s business and significantly delay or prevent the achievement of business objectives. In addition, our delivery of services will be labor-intensive: when the Company is awarded a government contract, we may need to quickly hire project leaders and case management personnel. The additional staff may also create a concurrent demand for increased administrative personnel. The success of our business will require that we attract, develop, motivate and retain:
| · | experienced and innovative executive officers; |
| · | senior managers who have successfully managed or designed government services programs in the public sector; and |
| · | Information technology professionals who have designed or implemented complex information technology projects |
Innovative, experienced and technically proficient individuals are in great demand and are likely to remain a limited resource. We may be unable to continue to attract and retain desirable executive officers and senior managers. Our inability to hire sufficient personnel on a timely basis or the loss of significant numbers of executive officers and senior managers could adversely affect our business.
Because we have limited capital resources, we expect to be dependent on cash flow and payments from customers in order to meet our expense obligations.
A number of factors may cause our revenues, cash flow and operating results to vary from quarter to quarter, including the following:
| · | the progression of contracts; |
| 18 |
| · | the levels of revenues earned on fixed-price and performance-based contracts (including any adjustments in expectations for revenue recognition on fixed-price contracts); |
| · | the commencement, completion or termination of contracts during any particular quarter; |
| · | the schedules of government agencies for awarding contracts; and |
| · | the term of awarded contracts and potential acquisitions. |
Changes in the volume of activity and the number of contracts commenced, completed or terminated during any quarter may cause significant variations in our cash flow from operations because a significant portion of our expenses are fixed. Fixed expenses include, rent, payroll, insurance, employee benefits, taxes and other administrative costs and overhead. Moreover, we expect to incur significant operating expenses during the start-up and early stages of large contracts and typically do not receive corresponding payments in that same quarter.
We may make acquisitions in the future that we are unable to effectively manage given our limited resources.
We may choose to grow our business by continuing to acquire other entities. We may be unable to manage businesses that we have acquired or integrate them successfully without incurring substantial expenses, delays or other problems that could negatively impact our results of operations. Moreover, business combinations involve additional risks, including:
| · | diversion of management’s attention; |
| · | loss of key personnel; |
| · | our becoming significantly leveraged as a result of the incurrence of debt to finance an acquisition; |
| · | assumption of unanticipated legal or financial liabilities; |
| · | unanticipated operating, accounting or management difficulties in connection with the acquired entities; |
| · | amortization of acquired intangible assets, including goodwill; and |
| · | dilution to existing shareholders and our earnings per share. |
Also, client dissatisfaction or performance problems with an acquired firm could materially and adversely affect our reputation as a whole. Further, the acquired businesses may not achieve the revenues and earnings we anticipated.
The Company must keep up with new and rapidly evolving technologies.
Some of the Company’s activities involve developing products or processes that are based upon new, rapidly evolving technologies. The ability to commercialize these technologies could fail for a variety of reasons, both within and outside of the Company’s control.
| 19 |
Our success depends upon our ability to protect our intellectual property rights.
Our success in part depends on the Company's ability to maintain the proprietary nature of our technology and other trade secrets. To do so, we will be required to prosecute and maintain patents, obtain new patents and pursue trade secret and other intellectual property protection. We have obtained one utility patent pending and two provisional patents pending with respect to our IPQA technology, in addition to filing a new patent application in 2011 for our IPQA technology. Also, as mentioned above, in 2011 we filed a patent application pertaining to the advanced dental implant technology. However, the efforts we have taken to protect our proprietary rights may not be sufficient or effective. Our business is also subject to the risk that our issued patents will not provide us with significant competitive advantages if, for example, a competitor were to independently develop or obtain similar or superior technologies. Prosecuting infringement claims can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that a patent owned by us is not valid or is unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that the Company’s patents do not cover its technology. An adverse determination of any litigation or defense proceedings could put one or more of our patents at risk of being invalidated or interpreted narrowly and could put the Company’s patent applications at the risk of not issuing. Any significant impairment of our intellectual property rights could harm our business or our ability to compete. The unauthorized use of our intellectual property could make it more expensive to do business and harm our operating results.
We may be sued by third parties who claim that we have infringed their intellectual property rights.
We may be exposed to future litigation by third parties based on claims that our research, development and commercialization activities infringe the intellectual property rights of third parties to which the Company does not hold licenses or other rights, or that we have misappropriated the trade secrets of others. Any litigation or claims against us, whether or not valid, could result in substantial costs, and could place a significant strain on our financial and human resources. In addition, if successful, such claims could cause the Company to pay substantial damages. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation.
Our services are subject to government regulation, changes in which may have an adverse effect on the Company.
Our business activities subject us to a variety of federal, state and local laws and regulations. For example, we are required to comply with applicable provisions of the International Traffic in Arms Regulations, as well as other export controls and laws governing the manufacture and distribution of munitions technology. Changes in the laws and regulations applicable to our business activities may have an adverse effect on our operations and profitability by making it more expensive and less profitable for us to do business. Additionally, the market for our services depends largely on federal and state legislative programs. These programs can be modified or amended at any time by acts of federal and state governments. Further, if additional programs are not proposed or enacted, or if previously enacted programs are challenged, repealed or invalidated, our growth strategy could be adversely impacted.
Our Bylaws contain provisions indemnifying our officers and directors against all costs, charges, and expenses incurred by them.
Our Bylaws contain provisions with respect to the indemnification of our officers and directors against all costs, charges, and expenses, including an amount paid to settle an action or satisfy a judgment, actually and reasonably incurred by an officer or director, including an amount paid to settle an action or satisfy a judgment in a civil, criminal, or administrative action or proceeding to which he is made a party by reason of being or having been one of our directors or officers.
| 20 |
Our Bylaws do not contain anti-takeover provisions, which could result in a change of our management and directors if there is a takeover of us.
We do not currently have a shareholder rights plan or any anti-takeover provisions in our Bylaws. Without any anti-takeover provisions, there is no deterrent for a takeover of our company, which may result in a change in our management and directors.
Our operating costs could be higher than we expect, and this could reduce our future profitability.
In addition to general economic conditions, market fluctuations and international risks, significant increases in operating, development and implementation costs could adversely affect our company due to numerous factors, many of which are beyond our control.
Our existing directors, officers and key employees hold a substantial amount of our common stock and may be able to prevent other shareholders from influencing significant corporate decisions.
As of March 30, 2012, our directors and executive officers beneficially owned approximately 30% of our outstanding common stock. These shareholders, if they act together, may be able to direct the outcome of matters requiring approval of the shareholders, including the election of our directors and other corporate actions such as:
| · | our merger with or into another company; |
| · | a sale of substantially all of our assets; and |
| · | amendments to our articles of incorporation. |
The decisions of these shareholders may conflict with our interests or those of our other shareholders.
Risks Related to Our Common Stock
We do not foresee paying cash dividends in the foreseeable future and, as a result, our investors’ sole source of gain, if any, will depend on capital appreciation, if any.
We do not plan to declare or pay any cash dividends on our shares of common stock in the foreseeable future and currently intend to retain any future earnings for funding growth of the Company’s business. As a result, investors should not rely on an investment in our securities if they require the investment to produce dividend income. Capital appreciation, if any, of our shares may be investors’ sole source of gain for the foreseeable future.
Our securities are considered highly speculative.
Our securities must be considered highly speculative, generally because of our limited operating history. We have neither generated any material revenues nor have we realized a profit from our operations to date and there is no assurance that we will operate on a profitable basis. Since we have not generated any material revenues and have only limited capital, we expect that we will need to raise additional monies through the sale of our equity securities or debt in order to continue our business operations.
| 21 |
Our stock is thinly traded, so you may be unable to sell your shares at or near the quoted bid prices if you need to sell a significant number of your shares.
Although the shares of our common stock are quoted on the OTC Bulletin Board, there has been very limited trading in our shares, meaning that the number of persons interested in purchasing our common shares at any given time is relatively small or non-existent. This situation is attributable to a number of factors, including the fact that we are a new company, our business is still in the development stage, and that we are a small company which is unknown to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume. Even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven, early stage company such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable. As a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common shares will develop or be sustained, or that current trading levels will be sustained. Due to these conditions, we can give you no assurance that you will be able to sell your shares if you need money or otherwise desire to liquidate your shares, or that any such sale would be at or near ask prices.
The price of our common stock could be highly volatile.
It is likely that our common stock will be subject to price volatility, low volumes of trades, and large spreads in bid and ask prices quoted by market makers. Due to the low volume of shares that may be traded on any trading day, persons buying or selling in relatively small quantities may easily influence prices of our common stock. This low volume of trades could also cause the price of our stock to fluctuate greatly, with large percentage changes in price occurring in any trading day session. Holders of our common stock may also not be able to liquidate their investment readily or may be forced to sell at depressed prices due to low volume trading. If high spreads between the bid and ask prices of our common stock exist at the time of a purchase, the price of the common stock would need to appreciate substantially on a relative percentage basis for an investor to recoup an investment in our shares. Broad market fluctuations and general economic and political conditions may also adversely affect the market price of our common stock. No assurance can be given that an orderly and active market in our common stock will develop or be sustained. If an orderly and active market does not develop, holders of our common stock may be unable to sell their shares, if at all.
Our common stock may be considered a “penny stock,” and thereby be subject to additional sale and trading regulations that may make it more difficult to sell.
Our common stock may be a “penny stock” if it meets one or more of the following conditions (i) the stock trades at a price less than $5.00 per share; (ii) it is not traded on a “recognized” national exchange; (iii) it is not quoted on the Nasdaq Capital Market, or even if so, has a price less than $5.00 per share; or (iv) is issued by a company that has been in business less than three years with net tangible assets less than $5 million.
The principal result or effect of being designated a “penny stock” is that securities broker-dealers participating in sales of our common stock will be subject to the “penny stock” regulations set forth in Rules 15-2 through 15g-9 promulgated under the Exchange Act. For example, Rule 15g-2 requires broker-dealers dealing in penny stocks to provide potential investors with a document disclosing the risks of penny stocks and to obtain a manually signed and dated written receipt of the document at least two business days before effecting any transaction in a penny stock for the investor’s account. Moreover, Rule 15g-9 requires broker-dealers in penny stocks to approve the account of any investor for transactions in such stocks before selling any penny stock to that investor. This procedure requires the broker-dealer to (i) obtain from the investor information concerning his or her financial situation, investment experience and investment objectives; (ii) reasonably determine, based on that information, that transactions in penny stocks are suitable for the investor and that the investor has sufficient knowledge and experience as to be reasonably capable of evaluating the risks of penny stock transactions; (iii) provide the investor with a written statement setting forth the basis on which the broker-dealer made the determination in (ii) above; and (iv) receive a signed and dated copy of such statement from the investor, confirming that it accurately reflects the investor’s financial situation, investment experience and investment objectives. Compliance with these requirements may make it more difficult and time consuming for holders of our common stock to resell their shares to third parties or to otherwise dispose of them in the market or otherwise.
| 22 |
If securities or industry analysts do not publish research or reports or publish unfavorable research about our business, the price and trading volume of our common stock could decline.
The future trading market for our common stock will be influenced in part by any research and reports that securities or industry analysts publish about us or our business. We do not currently have and may never obtain research coverage by securities and industry analysts. If no securities or industry analysts commence coverage of us the trading price for our common stock and other securities would be negatively affected. In the event we obtain securities or industry analyst coverage, if one or more of the analysts who covers us downgrades our securities, the price of our securities would likely decline. If one or more of these analysts ceases to cover us or fails to publish regular reports on us, interest in the purchase of our securities could decrease, which could cause the price of our common stock and other securities and their trading volume to decline.
If we are deemed to be an issuer of “penny stock”, the protection provided by the federal securities laws relating to forward-looking statements will not apply to us.
Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, if we are a penny stock, we will not have the benefit of this safe harbor protection in the event of any legal action based upon a claim that the material provided by us contained a material misstatement of fact or was misleading in any material respect because of our failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
Financial Industry Regulatory Authority (FINRA) sales practice requirements may also limit a stockholder’s ability to buy and sell our common stock.
FINRA has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
We may incur significant costs to ensure compliance with U.S. corporate governance and accounting requirements.
We may incur significant costs associated with our public company reporting requirements, costs associated with applicable corporate governance requirements, including requirements under the Sarbanes-Oxley Act of 2002, and other rules implemented by the Securities and Exchange Commission. We expect all of these applicable rules and regulations to significantly increase our legal and financial compliance costs and to make some activities more time consuming and costly. We also expect that these applicable rules and regulations may make it more difficult and more expensive for us to obtain director and officer liability insurance and we may be required to accept reduced policy limits and coverage or incur substantially higher costs to obtain the same or similar coverage. As a result, it may be more difficult for us to attract and retain qualified individuals to serve on our board of directors or as executive officers.
| 23 |
If we fail to maintain effective internal controls over financial reporting, the price of our common stock may be adversely affected.
As a public reporting company, we are required to establish and maintain appropriate internal controls over financial reporting. Failure to establish those controls, or any failure of those controls once established, could adversely impact our public disclosures regarding our business, financial condition or results of operations. Any failure of these controls could also prevent us from maintaining accurate accounting records and discovering accounting errors and financial frauds.
Rules adopted by the SEC pursuant to Section 404 of the Sarbanes-Oxley Act of 2002 require annual assessment of our internal control over financial reporting. The standards that must be met for management to assess the internal control over financial reporting as effective are complex, and require significant documentation, testing and possible remediation to meet the detailed standards. We may encounter problems or delays in completing activities necessary to make an assessment of our internal control over financial reporting. If we cannot assess our internal control over financial reporting as effective, investor confidence and share value may be negatively impacted. In addition, management’s assessment of internal controls over financial reporting may identify weaknesses and conditions that need to be addressed in our internal controls over financial reporting or other matters that may raise concerns for investors. Any actual or perceived weaknesses and conditions that need to be addressed in our internal control over financial reporting (including those weaknesses identified in our periodic reports), or disclosure of management’s assessment of our internal controls over financial reporting may have an adverse impact on the price of our common stock.
Obtaining additional capital through the sale of common stock will result in dilution of equity interests.
We plan to raise additional funds in the future by issuing additional shares of common stock or other securities, which may include securities such as convertible debentures, warrants or preferred stock that are convertible into common stock. Any such sale of common stock or other securities will lead to further dilution of the equity ownership of existing holders of our common stock. Additionally, the existing options, warrants and conversion rights may hinder future equity offerings, and the exercise of those options, warrants and conversion rights may have an adverse effect on the value of our stock. If any such options, warrants or conversion rights are exercised at a price below the then current market price of our shares, then the market price of our stock could decrease upon the sale of such additional securities. Further, if any such options, warrants or conversion rights are exercised at a price below the price at which any particular shareholder purchased shares, then that particular shareholder will experience dilution in his or her investment.
| ITEM 1B. | UNRESOLVED STAFF COMMENTS. |
Not applicable.
| ITEM 2. | PROPERTIES. |
On February 29, 2012, we terminated our lease for our prior office space located at 3900 Paseo del Sol, Santa Fe, New Mexico 87507, unit A341, after such lease was extended from November 1, 2011 until February 29, 2012. Thus, on March 1, 2012, we entered into a lease with Russ Hedrick dba Hedrick Group LLC for our office space located at 223 East Palace Avenue, Suite B, Santa Fe, New Mexico 87501. The office space consists of 400 square feet. We leased the property for a term of one year for a lump sum payment at signing of $8,500. The term of the lease expires on February 28, 2013, unless earlier terminated in accordance with the lease. On October 17, 2011, we entered into a new lease commencing November 1, 2011 for our development lab space located at 3900 Paseo del Sol, Santa Fe, New Mexico 87507, unit A201-202. Such property is leased at a monthly rate of $700, and consists of 807.2 square feet. The term of the lease expires on October 31, 2012, unless earlier terminated in accordance with the lease.
| 24 |
Sumner leases a 1,500 square foot facility located at 100 Cienega, Suites D and E, Santa Fe, New Mexico 87501. The lease for this space, which Sumner and La Mancha share, was entered into with Russ Hedrick dba Hedrick Group LLC on January 15, 2012 and expires on January 14, 2013.
We believe that our facilities are suitable for our current needs.
| ITEM 3. | LEGAL PROCEEDINGS. |
Neither Sigma Labs, Inc., nor any of its subsidiaries are currently a party to any legal proceedings. However, we may occasionally become subject to legal proceedings and claims that arise in the ordinary course of our business. It is impossible for us to predict with any certainty the outcome of pending disputes, and we cannot predict whether any liability arising from pending claims and litigation will be material in relation to our consolidated financial position or results of operations.
| ITEM 4. | MINE SAFETY DISCLOSURES. |
Not Applicable.
PART II
| ITEM 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED SHAREHOLDER MATTERS, AND ISSUER PURCHASES OF EQUITY SECURITIES. |
Market Information
Prior to the Reorganization, our predecessor entity, Framewaves, Inc., had shares of its common stock quoted on the OTC Bulletin Board under the symbol “FWAV.” To our knowledge, there was limited or no trading in such shares prior to the Reorganization.
Since the Reorganization, shares of our common stock have been quoted on the OTC Bulletin Board under the symbol “SGLB.” Additionally, during 2010, there were many days in which no shares were traded. While trading has increased in our stock in 2011, that trading has been limited and sporadic. The highest and lowest closing prices per share for our common stock (on a split adjusted basis) from the date of closing the Reorganization on September 13, 2010 through December 31, 2010 is $0.14 and $0.05, respectively.
The following table sets forth the range of closing prices for our common stock for the quarters indicated. Such quotations reflect inter-dealer prices, without retail mark-up, mark-down or commission and may not necessarily represent actual transactions.
| Fiscal Year Ended December 31, 2011 | High Bid | Low Bid | ||||||
| First Quarter | $ | 0.25 | $ | 0.07 | ||||
| Second Quarter | $ | 0.15 | $ | 0.01 | ||||
| Third Quarter | $ | 0.02 | $ | 0.01 | ||||
| Fourth Quarter | $ | 0.01 | $ | 0.01 | ||||
Shareholders
As of March 30, 2012, there were approximately 586 shareholders of record.
| 25 |
Dividends
We have not paid any dividends on our common stock to date and do not anticipate that we will pay dividends in the foreseeable future. Any payment of cash dividends on our common stock in the future will be dependent upon the amount of funds legally available, our earnings, if any, our financial condition, our anticipated capital requirements and other factors that the Board of Directors may think are relevant. However, we currently intend for the foreseeable future to follow a policy of retaining all of our earnings, if any, to finance the development and expansion of our business and, therefore, do not expect to pay any dividends on our common stock in the foreseeable future.
Securities Authorized For Issuance Under Equity Compensation Plans
The following table contains information regarding our equity compensation plans as of December 31, 2011:
| Plan Category | Number of Securities to be issued upon exercise of outstanding options, warrants and rights (a) | Weighted average price of outstanding options, warrants and rights (b) | Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a) (c) | |||||||||
| Equity Compensation Plans Approved by Security Holders(1) | 0 | 0 | 11,000,000 | |||||||||
| Equity Compensation Plans Not Approved by Security Holders | N/A | $ | N/A | N/A | ||||||||
(1) On March 9, 2011, the Company's Board of Directors approved the Company's 2011 Equity Incentive Plan, which was approved on March 31, 2011 by holders of at least a majority of the issued and outstanding shares of common stock of the Company. As of December 31, 2011, the Company issued an aggregate of 20,000,000 shares of the Company's common stock, subject to restrictions, pursuant to the Company's 2011 Equity Incentive Plan.
Recent Issuances Of Unregistered Securities
Not applicable.
Repurchase of Shares
We did not repurchase any of our shares during the fourth quarter of the fiscal year covered by this report.
| ITEM 6. | SELECTED FINANCIAL DATA. |
Not applicable to a “smaller reporting company” as defined in Item 10(f)(1) of SEC Regulation S-K.
| 26 |
| ITEM 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS. |
Critical Accounting Policies
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires management to make estimates and assumptions that affect the reported assets, liabilities, sales and expenses in the accompanying financial statements. Critical accounting policies are those that require the most subjective and complex judgments, often employing the use of estimates about the effect of matters that are inherently uncertain. Such critical accounting policies, including the assumptions and judgments underlying them, are disclosed in Note 1 to the Consolidated Financial Statements included in this Annual Report. However, we do not believe that there are any alternative methods of accounting for our operations that would have a material affect on our financial statements.
Recent Acquisition of Sumner and La Mancha
On December 31, 2011, we acquired 100% of the outstanding capital stock of Sumner & Lawrence Limited (dba Sumner Associates, Inc.) ("Sumner") and La Mancha Company. We paid an aggregate of $350,000 for Sumner and La Mancha, through the issuance of an aggregate of 35,000,000 shares of our common stock to the five former stockholders of such corporations. The results of Sumner's and La Mancha's operations during fiscal 2011 have not been included in the Company’s consolidated results of operations.
Results Of Operations
Year Ended December 31, 2011 Compared to the Year Ended December 31, 2010
We expect to generate revenues primarily by marketing and deploying our technology solutions to businesses that seek to improve their production processes and/or manipulate and improve the most functional characteristics of the materials and other input components used in their business operations. However, we presently make no sales of these technologies. B6 Sigma was formed February 5, 2010 and had not yet generated revenue from services rendered or from other sources from that date through the end of the first quarter of 2011. For the period from our inception through December 31, 2010 ("fiscal 2010"), we generated $498,504 in revenues, as compared to $797,354 in revenues that we generated during the fiscal year ended December 31, 2011 ("fiscal 2011"). The revenues we generated during fiscal 2010 and fiscal 2011 were primarily generated from engineering consulting services we provided to third parties during these periods.
In fiscal 2011, B6 Sigma generated $797,354 in revenues from consulting contracts. Specifically, we generated:
| · | $30,149 in revenues in connection with consulting contracts with Honeywell International, Inc. concerning the application of our IPQA technology to the development of next-generation manufacturing technology of aero-engine components; |
| · | $421,087 in revenues in connection with a consulting contract with the US Air Force concerning the application of our IPQA technology to Additive Manufacturing using aero-frame materials; |
| · | $77,050 in revenues with respect to a consulting contract with the US Navy concerning the application of our IPQA technology to Additive Manufacturing for aero-frame components; |
| 27 |
| · | $9,196 in revenues in connection with a consulting agreement with Alcoa, Inc. concerning the application of our IPQA technology to the development of next-generation joining technology for oil and gas materials; |
| · | $99,487 in revenues in connection with a contract with Aerojet, a GenCorp Inc. (NYSE: GY) company to supply reactive materials for development testing of munition case liners for the US Army; |
| · | $75,311 in revenues in connection with a consulting contract with Los Alamos National Laboratory to supply upgraded machine controls technology for packaging of nuclear materials for long-term storage and disposition; |
| · | $7,820 in revenues in connection with a contract with Messier-Bugatti-Dowty concerning the application of our IPQA technology for next-generation landing gear materials joining; |
| · | $26,404 in revenues in connection with a contract with Manufacturing Technologies, Inc. concerning application of machine health monitoring for a rotary friction welding; and |
| · | $50,850 in revenue in connection with a contract with Pratt & Whitney concerning application of our IPQA technology for development of next-generation joining technology for aero-engine components. |
Our general and administrative expenses for fiscal 2011 were $426,519, as compared to $407,358 in fiscal 2010. Our payroll expenses for fiscal 2011 were $650,181, as compared to $394,029 for fiscal 2010. Our expenses relating to non-cash compensation for fiscal 2011 were $219,500, as compared to $0 for fiscal 2010.
General and administrative expenses principally include organizational expenses and outside services fees, the largest component of which consists of services in connection with our obligations as an SEC reporting company, in addition to other legal and accounting fees. The net increase in general and administrative expenses, payroll expenses and non-cash compensation expenses in fiscal 2011 as compared to fiscal 2010 is principally the result of increased outside services costs, payroll obligations and incentive compensation associated with our growing operations, and for the purpose of expanding the same. The Company incurred $105,000 of non-cash compensation expenses during 2011, resulting from the vesting of 5,250,000 shares of the 20,000,000 shares of Company common stock, subject to restrictions, issued to five of our employees pursuant to the Company’s 2011 Equity Incentive Plan.
We expect our general and administrative expenses to increase significantly for the remainder of 2012, as we continue to actively pursue our business plans, develop our technology, and increase our operations and marketing. We expect our payroll and non-cash compensation expenses to increase as we continue to grow our business. We also expect our expenses to increase substantially on a consolidated basis in 2012 as a result of our acquisition on December 31, 2011 of Sumner and La Mancha.
Our net loss for fiscal 2011 increased overall and totaled $910,129, as compared to $421,946 for fiscal 2010. This increase in fiscal 2011 is primarily the result of the increased payroll expenses and non-cash compensation expenses related to our increased operations. For example, the Company incurred $219,500 of non-cash stock compensation expenses in fiscal 2011, as compared to no such expenses in fiscal 2010.
| 28 |
Liquidity And Capital Resources
As of December 31, 2011, we had $653,113 in cash and a working capital surplus of $770,579, as compared with $226,268 in cash and a working capital surplus of $362,497 as of December 31, 2010. B6 Sigma was formed February 5, 2010 and had not yet generated revenue from services rendered or from other sources from that date through the end of the first quarter of 2011. Effective April 15, 2011, in a private placement offering with accredited investors, we sold an aggregate of 55,875,000 shares of our common stock, for aggregate net proceeds of $1,011,765. We plan to obtain additional funding through private sales of equity and/or debt securities.
We plan to generate revenues primarily by marketing and selling our manufacturing and materials technologies. However, for the period from our inception through fiscal 2011, we generated revenues and financed our operations primarily from engineering consulting services we provided during this period and through private sales of our common stock.
Our continued development in fiscal 2011 of our IPQA® and munitions technologies will enable us to commercialize these technologies in the remainder of 2012. We will continue to refine those and our other technologies, including our dental implant biomedical prosthetics technology, for commercialization during fiscal 2012. However, until commercialization of such technologies, we plan to fund our development activities and operating expenses by providing consulting services concerning our areas of expertise, i.e., materials and manufacturing quality technologies, and through the use of proceeds from sales of our securities.
We also expect our revenues to increase on a consolidated basis as a result of consulting contracts that we and Sumner have. As of March 31, 2012, B6 Sigma has two active consulting contracts with respect to which we expect to perform and generate up to $158,227 in revenues in fiscal 2012. As of March 31, 2012, Sumner has three active consulting contracts, which Sumner expects to perform and generate up to $580,100 of revenues in fiscal 2012. La Mancha has no active consulting contracts.
Some of these consulting contracts are fixed price contracts, for which we will receive a specified fee regardless of our cost to perform under such contract. In connection with entering into these fixed-contract consulting arrangements, we are required to estimate our costs of performance. To actually earn a profit on these contracts, we must accurately estimate costs involved and assess the probability of meeting the specified objectives, realizing the expected units of work or completing individual transactions, within the contracted time period. Accordingly, if we under-estimate the cost to complete a contract, we remain obligated to complete the work based on our initial cost estimate, which would reduce the amount of profit actually earned under the contract. Similarly, some of Sumner's consulting contracts are fixed price contracts with the same cost considerations faced by the Company, as described above.
We have no credit lines or facilities as of March 31, 2012, nor have we ever had a credit facility since our inception.
Based on the funds we have as of March 31, 2012 and the proceeds that we, Sumner and La Mancha expect to receive under our respective consulting agreements and from private offerings of the Company's stock, we believe that we will have sufficient funds to pay our respective administrative and other operating expenses during 2012. Until we are able to generate significant revenues from sales of our technologies, our ability to continue to fund our liquidity and working capital needs will be dependent upon revenues from existing and future consulting contracts of the Company, Sumner and La Mancha, and proceeds received from sales of the Company's securities.
Inflation and changing prices have had no effect on our continuing operations over our two most recent fiscal years.
We have no off-balance sheet arrangements as defined in Item 303(a) of Regulation S-K.
| ITEM 7A | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
Not applicable to a “smaller reporting company.”
| 29 |
| ITEM 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA. |
Financial Statements are referred to in Item 15, listed in the Index to Financial Statements and filed and included elsewhere herein as a part of this Annual Report on Form 10-K.
| ITEM 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE. |
None.
| ITEM 9A. | CONTROLS AND PROCEDURES. |
Evaluation of Disclosure Controls and Procedures
Rule 15d-15(e) under the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), defines the term “disclosure controls and procedures” as those controls and procedures designed to ensure that information required to be disclosed by a company in the reports that it files or submits under the Exchange Act is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission rules and forms and that such information is accumulated and communicated to the company’s management, including its principal executive and principal financial officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Based upon an evaluation of the effectiveness of our disclosure controls and procedures performed by our management, with the participation of our Chief Executive Officer and Principal Accounting Officer, as of the end of the period covered by this annual report, our management concluded that our disclosure controls and procedures were not effective as a result of a weakness in the design of internal control over financial reporting identified below.
Management’s Annual Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 15d-15(f) under the Exchange Act. Our management, with the participation of our Chief Executive Officer and Principal Accounting Officer, conducted an evaluation of the effectiveness of our control over financial reporting based on the framework in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”). Based on management’s evaluation under the framework, management has concluded that our internal control over financial reporting was not effective as of December 31, 2011.
In connection with the preparation of our financial statements for the year ended December 31, 2011, our management identified a deficiency, identified below, that management considered to be a material weakness in the effectiveness of our internal control over financial reporting related to our accounting for certain issuances of the Company's common stock, subject to restrictions, pursuant to the Company’s 2011 Equity Incentive Plan.
Pursuant to standards established by the Public Company Accounting Oversight Board, a “material weakness” is a “deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the company’s annual or interim financial statements will not be prevented or detected on a timely basis.”
| 30 |
For the quarterly period ended June 30, 2011, we reported expenses relating to non-cash compensation of $400,000, resulting from the Company’s April 2011 issuance, to five employees, of an aggregate of 20,000,000 shares of Company common stock, subject to restrictions, pursuant to the Company’s 2011 Equity Incentive Plan. However, only an aggregate of 5,250,000 shares of the 20,000,000 shares of such restricted stock had vested as of the year ended December 31, 2011, resulting in an aggregate non-cash compensation expense of $105,000. Thus, our expenses reported for the quarterly period ended June 30, 2011 and for the nine months ended September 30, 2011, respectively, were overstated by $303,000. Correspondingly, our net loss reported for the same period was overstated by $303,000.
Having completed our review and evaluation of our accounting for the issuance of the foregoing 20,000,000 shares of our common stock, subject to restrictions, we believe that the weakness described above has been remedied. We intend to closely monitor the vesting schedule applicable to the foregoing and any future issuances by the Company on an ongoing basis.
We continuously seek to improve and strengthen our control processes to ensure that all of our controls and procedures are adequate and effective. Any failure to implement and maintain improvements in the controls over our financial reporting could cause us to fail to meet our reporting obligations under the Securities and Exchange Commission’s rules and regulations. Any failure to improve our internal controls to address the weakness we have identified could also cause investors to lose confidence in our reported financial information, which could have a negative impact on the trading price of our common stock.
This annual report does not include an attestation report of the Company’s registered public accounting firm regarding internal control over financial reporting. Management’s report was not subject to attestation by our registered public accounting firm pursuant to Securities and Exchange Commission rules that permit us to provide only management’s report in this annual report.
There have been no changes in our internal controls over financial reporting during the fourth quarter of the year ended December 31, 2011 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting.
| ITEM 9B. | OTHER INFORMATION |
On December 15, 2011, pursuant to an unwritten employment arrangement with the Company, Richard Mah and James Stout, Chief Executive Officer and Treasurer of the Company, respectively, agreed to reduce their respective monthly salary from $2,461 to $0, effective January 1, 2012.
On December 15, 2011, pursuant to an unwritten employment arrangement with the Company, Mark J. Cola and Vivek R. Dave, President and Chief Operating Officer, and Executive Vice President of the Company, respectively, agreed to reduce their monthly salary from $11,856 and $12,029, respectively, to $9,484.80 and $9,623.20, effective January 1, 2012. On March 1, 2012, Messrs. Cola and Dave agreed with the Company to further reduce their respective salary to $6,000 per month each, effective retroactively to February 16, 2012.
PART III
| ITEM 10. | DIRECTORS, EXECUTIVE OFFICERS, AND CORPORATE GOVERNANCE. |
The following table sets forth the name, age and position held by each of our executive officers and directors as of December 31, 2011.
| Name | Age | Position | ||
| Richard Mah | 64 | Chief Executive Officer and Director | ||
| James Stout | 73 | Chairman of the Board and Treasurer | ||
| Mark Cola | 52 | President, Chief Operating Officer and Director | ||
| Dr. Vivek Dave | 43 | Executive Vice President and Director | ||
| Valerie V. Vekkos | 54 | Secretary and Director |
Each director will serve until the next annual meeting of the stockholders of the Company or until his or her successor is elected and qualified.
| 31 |
Business Experience of Directors and Management
The following describes the significant business experience of our directors and executive officers:
Richard Mah was appointed as Chief Executive Officer and a director of the Company in September 2010. In January 2012, Mr. Mah was appointed as a director of Sumner & Lawrence Limited (dba Sumner Associates, Inc.), a wholly-owned subsidiary of the Company. From 2006 through April 2010, Mr. Mah served as Vice President of TMC Corporation, an international project management and business services company. Prior thereto, Mr. Mah worked for Los Alamos National Laboratory (“LANL”) as the Associate Laboratory Director for Weapons Engineering and Manufacturing. Mr. Mah was the senior executive responsible for overseeing the engineering and manufacturing aspects of the nuclear weapons program to fulfill the stockpile stewardship mission at LANL. In over 30 years at the Laboratory, Mr. Mah managed two non-nuclear materials technology research groups in metallurgy and in polymers and coatings and has had oversight of four large research facilities. In addition, from 2004 to 2006, Mr. Mah reported to the University of California’s Office of the President as the Acquisition Manager in the successful competition for the LANL contract competition.
Mr. Mah has received the Federal Laboratory Consortium special award for excellence; the LANL distinguished Performance Award; the United States Department of Energy Award of Excellence in acknowledgement of his management and engineering accomplishments; and in 2006 he was given a special University of California award for his performance in winning the Los Alamos National Laboratory contract. He has also been recognized for his commitment to employees with a Diversity Award and an Outstanding Mentor Award. Mr. Mah earned a B.S. in Theoretical and Applied Mechanics and an M.S. in Metallurgical Engineering from the University of Illinois. He is a registered professional engineer through the state of California.
James Stout was appointed as Chairman of the Board and Treasurer of the Company in September 2010. In January 2012, Mr. Stout was appointed as a director of Sumner & Lawrence Limited (dba Sumner Associates, Inc.), a wholly-owned subsidiary of the Company. Mr. Stout also has served as a director of TechSource, Inc., a private scientific and technical consulting firm based in Los Alamos, New Mexico, since December 2005. From 2006 through April 2010, Mr. Stout served as Vice President of TMC Corporation. During the prior 10 years, he served as President of The Stout Group, Inc., a private consulting firm specializing in national security issues at the National Laboratories and military commands, including service as an advisor to the Strategic Advisory Group of the Commander of the U.S. Strategic Command. Mr. Stout also served for six years as a member of the Los Alamos National Laboratory Director’s Senior Advisory Group. Mr. Stout’s experience also includes over 30 years at the United States Department of Energy culminating with his appointment to the position of Chief Counsel, Albuquerque Operations Office. In this capacity, he served in a variety of roles concerning operation of the Nuclear Weapons Complex, including that of lead negotiator for the contracts for operation of the Berkeley, Los Alamos and Livermore National Laboratories. Mr. Stout served with the United States Air Force from 1963 to 1966. He received a J.D. from Drake University in 1963, a B.S. in Business Administration from the State University of Iowa in 1960, and is admitted to the New Mexico State Bar and the Iowa State Bar.
Mark Cola was appointed as President, Chief Operating Officer and a director of the Company in September 2010. From June 2006 through April 2010, Mr. Cola served as Director of Operations for the Beyond6 Sigma Division of TMC Corporation. In addition, Mr. Cola has over 28 years of experience in the aerospace and nuclear industries, including with Rockwell International, SPECO Division of Kelsey-Hayes Co., Westinghouse in the Naval Nuclear Reactors Program, Houston Lighting & Power, and within the NNSA Weapons Complex at Los Alamos National Laboratory. He has also worked as a Research Engineer at Edison Welding Institute and for Thermadyne’s Stoody Division, a leading manufacturer of wear-resistant materials.
| 32 |
At Beyond6 Sigma, Mr. Cola worked with a wide range of clients ranging from aerospace to defense systems. His expertise is in manufacturing process development, friction welding, light alloys such as titanium and aluminum, mechanical, physical and welding metallurgy, and nickel-based super-alloys for harsh environments. Mr. Cola served as the Technical Co-Chairman for the inaugural National Nuclear Security Administration Future Technologies Conference held in May 2004, and he is a principal reviewer for the American Welding Society’s Welding Journal. Mr. Cola earned a B.S. in Metallurgical Engineering and an M.S. in Welding Engineering from Ohio State University.
Dr. Vivek Dave was appointed as Executive Vice President and a director of the Company in September 2010. From 2006 through April 2010, Dr. Dave served as Director of Business Development for the Beyond6 Sigma Division of TMC Corporation. Prior thereto, Dr. Dave worked at Pratt & Whitney / United Technologies (UTC), where he developed experience working closely with manufacturing clients to resolve production quality issues. Dr. Dave also worked within the National Nuclear Security Administration Weapons Program at the Los Alamos National Laboratory (“LANL”), at which he held various technical and managerial positions including group leader of a manufacturing technology development group as well as director of the Los Alamos Manufacturing Sciences Institute.
At Beyond6 Sigma, Dr. Dave has worked with a wide range of clients ranging from renewable energy to defense systems. His expertise is in solid state joining, materials engineering, fusion welding, electron beam processing, reduced order process modeling, and designing manufacturing processes. Dr. Dave served as a co-organizer of the inaugural Small Lot Intelligent Manufacturing Conference held in 2003. Further, in 2001, he was awarded the Achieved American Welding Society Award for Best Original Contribution to Brazing Technology. Dr. Dave earned Ph.D. in Materials Engineering and an M.S. in Materials Engineering from the Massachusetts Institute of Technology. He also received a B.S. (with honors) in Engineering and Applied Science from the California Institute of Technology.
Valerie V. Vekkos was appointed as Secretary and a director of the Company in September 2010. Ms. Vekkos currently provides consulting services to small and start-up businesses through Zephyr Equities, Inc., a corporation she owns and controls. She has been providing such consulting services to corporate clients since 1999. In addition, from April 2009 to August 2011, Ms. Vekkos served as the President and a director of CT Holdings, Inc., a publicly held shell company. Ms. Vekkos earned a J.D. from University of San Diego, School of Law and a B.A. in Economics from Rowan University (formerly Glassboro State College). In addition, Ms. Vekkos is admitted to the following State Bars: California, New Jersey and Pennsylvania. She has also held a California Real Estate Broker’s License since 2004.
Family Relationships
There are no family relationships among any of the executive officers and directors.
Involvement in Certain Legal Proceedings
There have been no events under any bankruptcy act, no criminal proceedings and no judgments, injunctions, orders or decrees material to the evaluation of the ability and integrity of any director, executive officer, promoter or control person of the Company during the past ten years. The Company is not aware of any legal proceedings in which any director, nominee, officer or affiliate of the Company, any owner of record or beneficially of more than five percent of any class of voting securities of the Company, or any associate of any such director, nominee, officer, affiliate of the Company, or security holder is a party adverse to the Company or any of its subsidiaries or has a material interest adverse to the Company or any of its subsidiaries.
| 33 |
Code of Ethics
Our Board of Directors has adopted a code of ethics that applies to our officers, directors and employees (“Code of Ethics”). A copy of our Code of Ethics will be furnished without charge to any person upon written request. Requests should be sent to: Secretary, Sigma Labs, Inc., 223 East Palace Avenue, Suite B, Santa Fe, New Mexico 87501.
Nominations of Directors
There are no material changes to the procedures by which security holders may recommend nominees to our Board of Directors.
Board Committees
Pursuant to our Bylaws, our Board of Directors may establish committees of one or more directors from time-to-time, as it deems appropriate. Our common stock is quoted on the OTC Bulletin Board under the symbol “SGLB.” The OTC Bulletin Board does not maintain any standards requiring us to establish or maintain an audit, nominating or compensation committee. As of March 31, 2012, our Board of Directors does not maintain any audit, nominating or compensation committee, or any other committees.
| ITEM 11. | EXECUTIVE COMPENSATION. |
The Company has no compensatory plans or arrangements whereby any executive officer would receive payments from the Company or a third party upon his resignation, retirement or termination of employment, or from a change in control of the Company or a change in the officer’s responsibilities following a change in control. The Company has not entered into any written employment agreements, change-of-control, severance or similar agreements with any of our directors or executive officers.
Summary Compensation Table.
The following table sets forth certain information concerning the compensation for services rendered to us in all capacities for the fiscal years ended December 31, 2010 and 2011 of all persons who served as our principal executive officer and principal accounting officer during the fiscal year ended December 31, 2011, and to each executive officer, other than our principal executive officer, who earned over $100,000 during the fiscal year ended December 31, 2011 (collectively, the "named executive officers”). No other executive officers of the Company earned annual compensation during the fiscal year ended December 31, 2011 that exceeded $100,000.
| 34 |
Summary Compensation Table
| Name and Principal Position | Fiscal Year Ended 12/31 | Salary Paid or Accrued ($) | Bonus Paid or Accrued ($) | Stock Awards ($) | Option Awards ($) | All Other Compensation ($) | Total ($) | |||||||||||||||||||
| Richard Mah | 2011 | 28,968 | 0 | 0 | 0 | 0 | 28,968 | |||||||||||||||||||
| CEO and Director (Principal | 2010 | 14,384 | 0 | 0 | 0 | 0 | 14,384 | |||||||||||||||||||
| Executive Officer) | ||||||||||||||||||||||||||
| Mark Cola | 2011 | 138,990 | 0 | 0 | 0 | 0 | 138,990 | |||||||||||||||||||
| President, Chief Operating | 2010 | 104,117 | 0 | 0 | 0 | 0 | 104,117 | |||||||||||||||||||
| Officer, and Director | ||||||||||||||||||||||||||
| Vivek R. Dave | 2011 | 134,499 | 0 | 0 | 0 | 0 | 134,499 | |||||||||||||||||||
| Executive Vice President and Director | 2010 | 98,558 | 0 | 0 | 0 | 0 | 98,558 | |||||||||||||||||||
| James Stout | 2011 | 28,968 | 0 | 0 | 0 | 0 | 28,968 | |||||||||||||||||||
| Treasurer and Chairman of the Board (Principal Accounting Officer) | 2010 | 14,384 | 0 | 0 | 0 | 0 | 14,384 | |||||||||||||||||||
Equity Awards
There were no options, warrants or other security awards outstanding for any named executive officer as of December 31, 2011.
Unwritten Employment Arrangements with Executive Officers
Each of our executive officers has entered into an "at will" unwritten employment arrangement with the Company.
Under Richard Mah's and James Stout's respective employment arrangement, from October 11, 2010 until December 31, 2011, each were entitled to receive a monthly salary of $2,461. As a cost saving measure, on December 15, 2011, Messrs. Mah and Stout agreed with the Company to reduce their respective salary to $0, effective January 1, 2012. Actual amounts paid to Messrs. Mah and Stout are as set forth in the table above.
Under Mark J. Cola's and Vivek R. Dave's respective employment arrangement, Mr. Cola was entitled to receive a monthly salary of $11,856 in 2010, commencing on April 17, 2010, and in 2011, and Dr. Dave was entitled to receive a monthly salary of $12,029 in 2010, commencing on April 17, 2010, and in 2011. As a cost saving measure, (i) on December 15, 2011, Messrs. Cola and Dave agreed with the Company to reduce their salary to $9,484.80 and $9,623.20, respectively, per month, effective January 1, 2012, and (ii) on March 1, 2012, Messrs. Cola and Dave agreed with the Company to further reduce their respective salary to $6,000 per month each, effective retroactively to February 16, 2012. Actual amounts paid to Messrs. Cola and Dave are as set forth in the table above.
Under their respective employment arrangements, all executive officers are eligible to receive medical and dental benefits, life insurance, and long term and short term disability coverage. Further, Messrs. Mah, Stout, Cola and Dave are eligible to participate in the Company's 2011 Equity Incentive Plan as employees and directors of the Company. The Company has not agreed to pay bonuses to any executive officer.
| ITEM 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The following table sets forth certain information regarding beneficial ownership of our common stock as of March 30, 2012 (a) by each person known by us to own beneficially 5% or more of any class of our common stock, (b) by each of our named executive officers and each of our directors and (c) by all executive officers and directors of this company as a group. As of March 30, 2012, there were 429,667,400 shares of our common stock issued and outstanding. Unless otherwise noted, we believe that all persons named in the table have sole voting and investment power with respect to all the shares beneficially owned by them.
| 35 |
| Name and Address of Beneficial Owner (1) | Shares Beneficially Owned (2) | Percent of Class | ||||||
| Certain Beneficial Owners: | ||||||||
| Richard Mah and Mary Mah, as joint tenants | 28,500,000 | (3) | 6.63 | % | ||||
| James Stout and Sally Stout, as joint tenants | 28,014,750 | (4) | 6.52 | % | ||||
| Directors/Named Executive Officers: | ||||||||
| Mark Cola | 32,016,000 | 7.45 | % | |||||
| Dr. Vivek Dave | 32,016,000 | 7.45 | % | |||||
| Richard Mah | 31,000,200 | (3) | 7.21 | % | ||||
| James Stout | 30,514,800 | (4) | 7.10 | % | ||||
| Valerie V. Vekkos | 5,001,000 | (5) | 1.16 | % | ||||
| All Named Executive Officers and Directors as a group (5 persons) | 130,548,000 | 30.38 | % | |||||
(1) Unless otherwise indicated, the address of each person listed is c/o Sigma Labs, Inc., 223 East Palace Avenue, Suite B, Santa Fe, New Mexico 87501.
(2) For purposes of this table, shares are considered beneficially owned if the person directly or indirectly has the sole or shared power to vote or direct the voting of the securities or the sole or shared power to dispose of or direct the disposition of the securities. Shares are also considered beneficially owned if a person has the right to acquire beneficial ownership of the shares within 60 days of March 30, 2012.
(3) Consists of 28,500,000 shares of common stock which are owned by Richard Mah and Mary Mah as joint tenants, with the shared power to vote or dispose of such shares.
(4) Consists of 28,014,750 shares of common stock which are owned by James Stout and Sally Stout as joint tenants, with the shared power to vote or dispose of such shares.
(5) Consists of 2,001,000 shares owned of record by Valerie V. Vekkos, as Trustee of the Valerie V. Vekkos Trust, UAD 09/20/1994, and 3,000,000 shares owned by Zephyr Equities, Inc., a corporation owned and controlled by Valerie V. Vekkos.
Equity Compensation Plans
On March 9, 2011, the Company's Board of Directors approved the Company's 2011 Equity Incentive Plan, which was approved on March 31, 2011 by holders of at least a majority of the issued and outstanding shares of common stock of the Company. See the discussion of the 2011 Equity Incentive Plan under the caption "Securities Authorized For Issuance Under Equity Compensation Plans" included under Item 5, Part II of this Annual Report on Form 10-K.
| ITEM 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS. |
Certain Relationships and Related Transactions
On February 11, 2010, B6 Sigma, Inc. entered into a stock purchase agreement with Cletha Walstrand, Esq., as representative of certain shareholders of Framewaves, Inc. (in such capacity, the “Representative”), pursuant to which B6 Sigma agreed to acquire from the Representative 110,700,000 shares of Framewaves’ common stock in exchange for a cash payment of $195,000. The transactions contemplated by the Stock Purchase Agreement closed concurrently with the closing of the Reorganization. On or about February 23, 2010, the Representative, on behalf of certain Framewaves shareholders, entered into a stock purchase agreement with certain individuals pursuant to which the Representative sold an aggregate of 58,200,000 shares of Framewaves common stock to said individuals for aggregate consideration of $30,000.
| 36 |
On March 1, 2010, B6 Sigma entered into a consulting agreement with Zephyr Equities, Inc., a corporation owned and controlled by Valerie V. Vekkos, the Secretary and a director of the Company, to assist B6 Sigma and the Company with SEC reporting and other regulatory obligations and provide corporate secretarial and corporate governance services as required. Under the consulting agreement, which expired on February 22, 2012, we paid Zephyr Equities, Inc. $3,500 per month. As of February 22, 2012, the Company has retained the consulting services of Zephyr Equities, Inc. on a month-to-month basis in exchange for a monthly payment to Zephyr Equities, Inc. of $2,500. Additionally, on January 4, 2011 and April 8, 2011, respectively, the Company issued Zephyr Equities, Inc. 1,000,000 shares and 2,000,000 shares of the Company's common stock, valued at an aggregate of $60,000, as consideration for services rendered.
Under James Stout's unwritten employment arrangement with the Company, as described in Item 11, above, under "Unwritten Employment Arrangements with Executive Officers," from September 2010 until December 31, 2011, Mr. Stout received a monthly salary of $2,461. Effective January 1, 2012, as a cost saving measure, the Company no longer pays Mr. Stout any salary. Under his employment arrangement, Mr. Stout is eligible to receive medical and dental benefits, life insurance, and long term and short term disability coverage. Further, Mr. Stout is eligible to participate in the Company's 2011 Equity Incentive Plan.
Director Independence
Our common stock is traded on the OTC Bulletin Board under the symbol “SGLB.OB.” The OTC Bulletin Board electronic trading platform does not maintain any standards regarding the “independence” of the directors on our company’s Board of Directors, and we are not otherwise subject to the requirements of any national securities exchange or an inter-dealer quotation system with respect to the need to have a majority of our directors be independent.
In the absence of such requirements, we have elected to use the definition for “director independence” under the NASDAQ stock market’s listing standards, which defines an “independent director” as “a person other than an officer or employee of us or its subsidiaries or any other individual having a relationship, which in the opinion of our Board of Directors, would interfere with the exercise of independent judgment in carrying out the responsibilities of a director.” The definition further provides that, among others, employment of a director by us (or any parent or subsidiary of ours) at any time during the past three years is considered a bar to independence regardless of the determination of our Board of Directors.
All of our Board members, except Ms. Vekkos, are employee-directors and therefore not deemed “independent” under the NASDAQ Stock Market’s listing standards. Although a majority of our Board of Directors is not “independent” under NASDAQ’s listing standards, due to their combined business and financial experience and because our common stock is not currently listed on any of the NASDAQ stock markets, we believe that our employee-directors can competently perform the functions required of them as directors of the Company.
| ITEM 14. | PRINCIPAL ACCOUNTING FEES AND SERVICES. |
Audit Fees
The aggregate fees accrued by Pritchett, Siler & Hardy, P.C. during the fiscal year ended December 31, 2011 for professional services for the audit of our financial statements and the review of financial statements included in our SEC filings was $32,924.
| 37 |
Audit-Related Fees
Pritchett, Siler & Hardy, P.C. did not provide and did not bill and it was not paid any fees for, audit-related services in the fiscal year ended December 31, 2011. Audit-related fees represent fees for assurance and related services that are reasonably related to the performance of the audit or review of our financial statements and not reported above under “Audit Fees.”
Tax Fees
Pritchett, Siler & Hardy, P.C. did not provide, and did not bill and was not paid any fees for tax compliance, tax advice, and tax planning services for the fiscal year ended December 31, 2011.
All Other Fees
Pritchett, Siler & Hardy, P.C. did not provide, and did not bill and were not paid any fees for, any other services in the fiscal years ended December 31, 2011.
PART IV
| ITEM 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES. |
Our financial statements and related notes thereto are listed and included in this Annual Report beginning on page F-1. The following documents are furnished as exhibits to this Form 10-K. Exhibits marked with an asterisk are filed herewith. The remainder of the exhibits previously have been filed with the Commission and are incorporated herein by reference.
| Number | Exhibit | |
| 2.1 | Share Exchange Agreement effective August 23, 2010, among Framewaves, Inc., B6 Sigma Group, Inc. and the shareholders thereof (filed as Exhibit 2.1 to the Company’s Current Report on Form 8-K filed September 17, 2010, and incorporated herein by reference). | |
| 3.1 | Amended and Restated Articles of Incorporation of the Company (filed as Exhibit 3.1 to the Company’s Current Report on Form 8-K/A filed September 17, 2010, and incorporated herein by reference). | |
| 3.2 | Certificate of Correction to Amended and Restated Articles of Incorporation, as filed with the Nevada Secretary of State on May 25, 2011 (filed as Exhibit 3.2 to the Company’s Current Report on Form 8-K filed June 1, 2011, and incorporated herein by reference). | |
| 3.3 | Amended Bylaws of the Company (filed as Exhibit 3.2 to the Company’s Annual Report on Form 10-KSB for the fiscal year ended December 31, 2000, and incorporated herein by reference). | |
| 4.1 | Form of Convertible Note of B6 Sigma, Inc. (filed as Exhibit 4.1 to the Company’s Current Report on Form 8-K/A filed November 12, 2010, and incorporated herein by reference). | |
| 10.1 | Consulting Agreement dated March 1, 2010 between B6 Sigma, Inc. and Zephyr Equities, Inc. (filed as Exhibit 10.1 to the Company’s Current Report on Form 8-K/A filed November 12, 2010, and incorporated herein by reference). | |
| 10.2 | Asset Purchase Agreement dated April 17, 2010 between B6 Sigma, Inc. and Technology Management Company, Inc. (filed as Exhibit 10.2 to the Company’s Current Report on Form 8-K/A filed November 12, 2010, and incorporated herein by reference). | |
| 10.3 | Escrow Deposit Agreement dated April 14, 2010, between B6 Sigma, Inc. and Signature Bank (filed as Exhibit 10.6 to the Company’s Current Report on Form 8-K filed November 12, 2010, and incorporated herein by reference). | |
| 10.4 | Form of B6 Sigma, Inc. Subscription Agreement (filed as Exhibit 10.7 to the Company’s Current Report on Form 8-K/A filed November 12, 2010, and incorporated herein by reference). | |
| 10.5 | Office Lease, dated September 30, 2010, between Santa Fe Business Incubator, Inc. and B6 Sigma, Inc. (filed as Exhibit 10.8 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2010, and incorporated herein by reference). |
| 38 |
| Number | Exhibit | |
| 10.6 | Development Lease, dated September 30, 2010, between Santa Fe Business Incubator, Inc. and B6 Sigma, Inc. (filed as Exhibit 10.9 to the Company’s Annual Report on Form 10-K for the fiscal year ended December 31, 2010, and incorporated herein by reference). | |
| 10.7 | 2011 Equity Incentive Plan adopted by the Board of Directors as of March 9, 2011 (filed as Exhibit 10.1 to the Company's Form 10-Q, filed on May 16, 2011, for the period ended March 31, 2011, and incorporated herein by reference). | |
| 10.8 | Form of Subscription Agreement between participants in April 2011 private placement and Sigma Labs, Inc. (filed as Exhibit 10.2 to the Company's Form 10-Q, filed on May 16, 2011, for the period ended March 31, 2011, and incorporated herein by reference). | |
| 10.9 | Placement Agent Agreement dated February 9, 2011 between Hudson Valley Capital Management Corp. and Sigma Labs, Inc. (filed as Exhibit 10.3 to the Company's Form 10-Q, filed on May 16, 2011, for the period ended March 31, 2011, and incorporated herein by reference). | |
| 10.10 | Form of Warrant issued to placement agent in April 2011 private placement to purchase shares of common stock of Sigma Labs, Inc. (filed as Exhibit 10.4 to the Company's Form 10-Q, filed on May 16, 2011, for the period ended March 31, 2011, and incorporated herein by reference). | |
| 10.11 | Exchange Agreement and Plan of Reorganization by and among Sigma Labs, Inc. and all Stockholders of Sumner & Lawrence Limited (dba Sumner Associates, Inc.) and La Mancha Company (filed as Exhibit 10.1 to the Company's Current Report on Form 8-K filed on December 27, 2011, and incorporated herein by reference). | |
| 10.12 | Lease, dated March 1, 2012, between Sigma Labs, Inc. and Russ Hedrick dba Hedrick Group LLC.* | |
| 10.13 | Development Lease, dated October 17, 2011, between Santa Fe Business Incubator, Inc. and B6 Sigma, Inc.* | |
| 10.14 | Summary of unwritten employment arrangements with executive officers.* | |
| 14.1 | Framewaves, Inc. Code of Ethics and Business Conduct (filed as Exhibit 99.3 to the Company’s Annual Report on Form 10-KSB for the fiscal year ended December 31, 2002, and incorporated herein by reference). | |
| 21.1 | Subsidiaries of Sigma Labs, Inc.* | |
| 23.1 | Consent of Pritchett, Siler & Hardy, P.C.* | |
| 31 | Certificate of principal executive officer and principal accounting officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002.* | |
|
32
|
Certificate of principal executive officer and principal accounting officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* | |
| 101.INS | XBRL Instance Document | |
| 101.SCH | XBRL Taxonomy Extension Schema | |
| 101.CAL | XBRL Taxonomy Extension Calculation | |
| 101.DEF | XBRL Taxonomy Extension Definition | |
| 101.LAB | XBRL Taxonomy Extension Label | |
| 101.PRE | XBRL Taxonomy Extension Presentation | |
| * Filed herewith. | ||
| 39 |
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| SIGMA LABS, INC. | ||
| April 16, 2012 | By: | /s/ Richard Mah |
| Richard Mah | ||
| Chief Executive Officer (Principal Executive Officer) | ||
| April 16, 2012 | By: | /s/ James Stout |
| James Stout | ||
| Treasurer (Principal Accounting Officer) | ||
In accordance with the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Richard Mah | Chief Executive Officer (Principal | April 16, 2012 | ||
| Richard Mah | Executive Officer) and Director | |||
| /s/ James Stout | Chairman of Board of Directors and | April 16, 2012 | ||
| James Stout | Treasurer (Principal Accounting Officer) | |||
| /s/ Mark Cola | President, Chief Operating Officer and | April 16, 2012 | ||
| Mark Cola | Director | |||
| /s/ Vivek Dave | Executive Vice President and Director | April 16, 2012 | ||
| Vivek Dave | ||||
| /s/ Valerie V. Vekkos | Secretary and Director | April 16, 2012 | ||
| Valerie V. Vekkos |
| 40 |
Sigma Labs, Inc.
Index to Financial Statements
| Page | |
| Financial Statements: | |
| Report of Independent Registered Public Accounting Firm – Pritchett, Siler & Hardy, P.C. | F-1 |
| Consolidated Balance Sheets | F-2 |
| Statements of Operations | F-3 |
| Statement of Stockholders’ Equity | F-4 |
| Statements of Cash Flows | F-5 |
| Notes to Consolidated Financial Statements | F-6 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
Board of Directors
Sigma Labs, Inc. and Subsidiaries
Santa Fe, New Mexico
We have audited the accompanying consolidated balance sheets of Sigma Labs, Inc. and Subsidiaries, as of December 31, 2011 and 2010 and the related consolidated statements of operations, stockholders’ equity and cash flows for each of the years in the two-year period ended December 31, 2011. Sigma Labs, Inc. and Subsidiaries’ management is responsible for these consolidated financial statements. Our responsibility is to express an opinion on these consolidated financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. Our audit included consideration of internal control over financial reporting as a basis for designing audit procedures that are appropriate in the circumstances, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Sigma Labs, Inc. and Subsidiaries as of December 31, 2011 and 2010 and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2011, in conformity with accounting principles generally accepted in the United States of America.
The accompanying consolidated financial statements have been prepared assuming Sigma Labs, Inc. and Subsidiaries will continue as a going concern. As discussed in Note 3 to the consolidated financial statements, Sigma Labs, Inc. and Subsidiaries was only recently formed and has not yet achieved profitable operations. These factors raise substantial doubt about the ability of the Company to continue as a going concern. Management’s plans in regards to these matters are also described in Note 3. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
PRITCHETT, SILER & HARDY, P.C.
Salt Lake City, Utah
April 16, 2012
| F-1 |
| Sigma Labs, Inc. and Subsidiaries | ||
| Consolidated Balance Sheets | ||
| December 31, 2011 and 2010 | ||
| December 31, 2011 | December 31, 2010 | |||||||
| ASSETS | ||||||||
| Current Assets | ||||||||
| Cash | $ | 653,113 | $ | 226,268 | ||||
| Accounts Receivable, net | 263,973 | 180,855 | ||||||
| Other Assets | 28,195 | 370 | ||||||
| Total Current Assets | 945,281 | 407,493 | ||||||
| Furniture and Equipment, net | 31,674 | 42,778 | ||||||
| Noncurrent Assets | ||||||||
| Intangible Assets, net | 299,241 | 25,083 | ||||||
| Total Noncurrent Assets | 299,241 | 25,083 | ||||||
| TOTAL ASSETS | $ | 1,276,196 | $ | 475,354 | ||||
| LIABILITIES AND STOCKHOLDERS' EQUITY | ||||||||
| Current Liabilities | ||||||||
| Accounts Payable | 153,852 | 44,996 | ||||||
| Accrued Expenses | 20,850 | - | ||||||
| Total Current Liabilities | 174,702 | 44,996 | ||||||
| TOTAL LIABILITIES | 174,702 | 44,996 | ||||||
| Stockholders' Equity | ||||||||
| Preferred Stock, $0.001 par; 10,000,000 shares authorized; | ||||||||
| None issued and outstanding | - | - | ||||||
| Common Stock, $0.001 par; 750,000,000 shares authorized; | ||||||||
| 313,067,400 shares issued and outstanding | - | 313,067 | ||||||
| 429,667,400 shares issued and outstanding | 429,667 | - | ||||||
| Additional Paid-In Capital | 2,298,902 | 539,237 | ||||||
| Deferred Compensation | (295,000 | ) | - | |||||
| Retained Earnings (Deficit) | (1,332,075 | ) | (421,946 | ) | ||||
| Total Stockholders' Equity | 1,101,494 | 430,358 | ||||||
| TOTAL LIABILITIES AND STOCKHOLDERS' EQUITY | $ | 1,276,196 | $ | 475,354 |
The accompanying notes are an integral part of these financial statements
| F-2 |
| Sigma Labs, Inc. and Subsidiaries |
| Consolidated Statements of Operations |
| Years Ended December 31, 2011 and 2010 |
| Years Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| INCOME | ||||||||
| Services | $ | 797,354 | $ | 498,504 | ||||
| Total Revenue | 797,354 | 498,504 | ||||||
| COST OF SERVICE REVENUE | 412,396 | 107,063 | ||||||
| GROSS PROFIT | 384,958 | 391,441 | ||||||
| EXPENSES | ||||||||
| General & Administration | 426,519 | 407,358 | ||||||
| Payroll Expense | 650,181 | 394,029 | ||||||
| Non-cash Stock Compensation | 219,500 | - | ||||||
| Total Expenses | 1,296,200 | 801,387 | ||||||
| OTHER INCOME (EXPENSE) | ||||||||
| Interest Income | 1,236 | - | ||||||
| Sale of Assets | - | 5,000 | ||||||
| Interest Expense | (123 | ) | (17,000 | ) | ||||
| Total Other Income (Expense) | 1,113 | (12,000 | ) | |||||
| INCOME (LOSS) BEFORE INCOME TAXES | (910,129 | ) | (421,946 | ) | ||||
| Current Income Tax Expense | - | - | ||||||
| Deferred Income Tax Expense | - | - | ||||||
| Net Income (Loss) | $ | (910,129 | ) | $ | (421,946 | ) | ||
| Loss per Common Share - Basic and Diluted | $ | (0.00 | ) | $ | (0.00 | ) | ||
| Weighted Average Number of Shares | ||||||||
| Outstanding - Basic and Diluted | 370,568,975 | 230,359,070 | ||||||
The accompanying notes are an integral part of these financial statements
| F-3 |
| Sigma Labs, Inc. and Subsidiaries |
| Statement of Stockholders' Equity |
| Years Ended December 31, 2011 and 2010 |
| Common Stock Shares | Common Stock Amount | Additional Paid in Capital | Deferred Compensation | Retained Earnings (Deficit) | Total Stockholders' Equity | ||||||||||||||||||
| Balance February 5, 2010 | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||
| Stock issued for cash | 184,092,000 | 184,092 | (152,788 | ) | - | - | 31,304 | |||||||||||||||||
| Stock issued for note payable | 15,807,900 | 15,808 | 300,192 | - | - | 316,000 | ||||||||||||||||||
| Stock issued for cash of $618,200 | ||||||||||||||||||||||||
| and assets valued at $81,800 | 35,017,500 | 35,017 | 664,983 | - | - | 700,000 | ||||||||||||||||||
| Rounding shares issued | 900 | 1 | (1 | ) | - | - | - | |||||||||||||||||
| Re-capitalization of Company | 188,849,100 | 188,849 | (188,849 | ) | - | - | - | |||||||||||||||||
| Stock purchased for cancellation | (110,700,000 | ) | (110,700 | ) | (84,300 | ) | - | - | (195,000 | ) | ||||||||||||||
| Net loss for the year ended December 31, 2010 | (421,946 | ) | (421,946 | ) | ||||||||||||||||||||
| Balance December 31, 2010 | 313,067,400 | 313,067 | 539,237 | - | (421,946 | ) | 430,358 | |||||||||||||||||
| Shares issued in noncash transactions | 25,725,000 | 25,725 | 488,775 | (295,000 | ) | - | 219,500 | |||||||||||||||||
| Shares issued for cash in a private offering | 55,875,000 | 55,875 | 955,890 | - | - | 1,011,765 | ||||||||||||||||||
| Warrants issued for offering costs (7,931,250 shares) | - | - | - | - | - | - | ||||||||||||||||||
| Shares issued in an acquisition | 35,000,000 | 35,000 | 315,000 | - | - | 350,000 | ||||||||||||||||||
| Net loss for the year ended December 31, 2011 | (910,129 | ) | (910,129 | ) | ||||||||||||||||||||
| Balance December 31, 2011 | 429,667,400 | $ | 429,667 | $ | 2,298,902 | $ | (295,000 | ) | $ | (1,332,075 | ) | $ | 1,101,494 | |||||||||||
The accompanying notes are an integral part of these financial statements
| F-4 |
| Sigma Labs, Inc. and Subsidiaries |
| Consolidated Statements of Cash Flows |
| Years Ended December 31, 2011 and 2010 |
| Years Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| OPERATING ACTIVITIES | ||||||||
| Net Income (Loss) | $ | (910,129 | ) | $ | (421,946 | ) | ||
| Adjustments to reconcile Net Income (Loss) to Net Cash used by operations: | ||||||||
| Noncash Expenses: | ||||||||
| Amortization | 1,011 | 717 | ||||||
| Depreciation | 20,151 | 13,222 | ||||||
| Stock Compensation | 219,500 | - | ||||||
| Non-cash Expense | - | 16,000 | ||||||
| Change in assets and liabilities: | ||||||||
| (Increase) Decrease in Accounts Receivable | 70,220 | (180,855 | ) | |||||
| (Increase) in Other Assets | (27,825 | ) | (370 | ) | ||||
| (Decrease) Increase in Accounts Payable | 32,224 | 44,996 | ||||||
| Increase In Accrued Expenses | 19,379 | - | ||||||
| NET CASH (USED) BY OPERATING ACTIVITIES | (575,469 | ) | (528,236 | ) | ||||
| INVESTING ACTIVITIES | ||||||||
| Purchase of Furniture and Equipment | (8,904 | ) | - | |||||
| Purchase of Intangible Assets | (13,160 | ) | - | |||||
| NET CASH (USED) BY INVESTING ACTIVITIES | (22,064 | ) | - | |||||
| FINANCING ACTIVITIES | ||||||||
| Proceeds from Note Payable | - | 300,000 | ||||||
| Net Proceeds from Sale of Common Stock | 1,011,765 | 649,504 | ||||||
| Cash Acquired (Paid) in Reorganization | 12,613 | (195,000 | ) | |||||
| NET CASH PROVIDED BY FINANCING ACTIVITIES | 1,024,378 | 754,504 | ||||||
| NET CASH INCREASE FOR PERIOD | 426,845 | 226,268 | ||||||
| CASH AT BEGINNING OF PERIOD | 226,268 | - | ||||||
| CASH AT END OF PERIOD | $ | 653,113 | $ | 226,268 | ||||
| Supplemental Disclosure for Cash Flow Information | ||||||||
| Cash paid during the period for: | ||||||||
| Interest | $ | - | $ | 1,000 | ||||
| Income Taxes | $ | - | $ | - | ||||
| Supplemental Schedule of Noncash Investing and Financing Activities: | ||||||||
| For the year ended December 31, 2011 | ||||||||
| 5,725,000 shares of common stock issued for consulting services at $0.02 per share | ||||||||
| 20,000,000 shares of common stock issued for employee equity plan at $0.02 per share | ||||||||
| The Company issued 7,931,250 warrants valued at $158,625 as a stock offering cost | ||||||||
| For the year ended December 31, 2010 | ||||||||
| Shareholders of the Company contributed equipment and patents valued at $81,800 | ||||||||
| The Company converted notes payable of $300,000 and accrued interest of $16,000 to common stock. | ||||||||
The accompanying notes are an integral part of these financial statements
| F-5 |
SIGMA LABS, INC. AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
DECEMBER 31, 2011
NOTE 1 – Summary of Significant Accounting Policies
Nature of Business – On September 13, 2010 Sigma Labs, Inc., formerly named Framewaves, Inc., a Nevada corporation, acquired 100% of the shares of B6 Sigma, Inc. by exchanging 6.67 shares of Framewaves, Inc. restricted common stock for each issued and outstanding share of B6 Sigma, Inc. The acquisition has been accounted for as a “reverse purchase”, and accordingly the operations of Framewaves, Inc. prior to the date of acquisition have been eliminated.
B6 Sigma, Inc., incorporated February 5, 2010, was founded by a group of scientists, engineers and businessmen to develop and commercialize novel and unique manufacturing and materials technologies. A Company trademark, In Process Quality Assurance (IPQA®), is a technology that management believes will fundamentally redefine manufacturing practices by embedding quality assurance in the manufacturing processes in real time. Management also anticipates that the Company’s core competencies will allow its clientele to combine advanced manufacturing with novel material to achieve breakthrough product potential in many industries including
aerospace, defense, oil and gas, prosthetic implants, sporting goods, and power generation.
On December 31, 2011, Sigma Labs, Inc. acquired 100% of the shares of Sumner & Lawrence Limited (“Sumner”), a New Mexico Corporation, and La Mancha Company, a New Mexico Corporation, by issuing 35,000,000 shares of Sigma Labs, Inc. common stock for all issued and outstanding shares of Sumner and La Mancha Company. The operations of Sumner and La Mancha Company prior to the date of acquisition have been eliminated.
Sumner is a small business with a broad spectrum of scientific disciplines that provides consulting services to the public sector, especially with regard to emerging technologies, alternative applications of established technologies, and assessment of development and maintenance programs for strategic technologies. The Company’s principal product is scientific and technological knowledge, gained through academic discipline, research activities and application experience. Sumner formed in 1985, expanded in 1993 with the addition of retired senior scientists and technical managers from the Los Alamos National Laboratory. The Company offers consulting services that are based on sound science, an unprejudiced perspective and multi-disciplined capabilities at reasonable rates. Sumner holds ongoing contracts with government agencies that provide a framework of audited fees and burden, as well as appropriate levels of security clearance. Major clients include the State Department, the Department of Defense, the Department of Energy, various military services and affiliated agencies, the National Laboratories, and contractors to these organizations.
La Mancha Company is a small business with a broad spectrum of scientific disciplines that provides consulting services to the private sector, especially with regard to emerging technologies, alternative applications of established technologies, and assessment of development and maintenance programs for strategic technologies. The Company’s principal product is scientific and technological knowledge, gained through academic discipline, research activities and application experience. La Mancha, formed in 1982, expanded in 1993 with the addition of retired scientists and technical managers from the Los Alamos National Laboratory. The Company offers consulting services that are based on sound science, an unprejudiced perspective and multi-disciplined capabilities at reasonable rates. La Mancha Company’s primary work is to provide risk assessment consulting as well as technical and management consulting, often in the international environment. The firm maintains extensive contacts, both public and private, in Latin America, Europe, Asia and the Middle East, as well as with top levels of the U.S. Government.
| F-6 |
Reclassification – Certain amounts in prior-period financial statements have been reclassified for comparative purposes to conform to presentation in the current-period financial statements.
Principles of Consolidation – The consolidated financial statements for December 31, 2011 include the accounts of Sigma Labs, Inc., B6 Sigma, Inc., Sumner and La Mancha Company. All significant intercompany balances and transactions have been eliminated.
Property and Equipment – Property and equipment are stated at cost. Expenditures for major renewals and betterments that extend the useful lives of property and equipment are capitalized upon being placed in service. Expenditures for maintenance and repairs are charged to expense as incurred. Depreciation is computed using the straight-line method over the estimated useful lives of the assets. The estimated life has been determined to be three years unless a unique circumstance exists, which is then fully documented as an exception to the policy.
Fair Value of Financial Instruments – The Company estimates that the fair value of all financial instruments does not differ materially from the aggregate carrying values of its financial instruments recorded in the accompanying consolidated balance sheets because of the short-term maturity of these financial instruments.
Income Taxes – The Company accounts for income taxes in accordance with ASC Topic No. 740, “Accounting for Income Taxes.”
The Company adopted the provisions of ASC Topic No. 740, “Accounting for Income Taxes,” at the date of inception on February 5, 2010. As a result of the implementation of ASC Topic No. 740, the Company recognized no increase in the liability for unrecognized tax benefits.
The Company has no tax positions at December 31, 2011 and 2010 for which the ultimate deductibility is highly certain but for which there is uncertainty about the timing of such deductibility.
The Company recognizes interest accrued related to unrecognized tax benefits in interest expense and penalties in operating expenses. During the year ended December 31, 2011, the Company recognized no interest and penalties. The Company had no accruals for interest and penalties at December 31, 2011 and 2010. All tax years starting with 2008 are open for examination.
| F-7 |
Loss Per Share – The computation of loss per share is based on the weighted average number of shares outstanding during the period in accordance with ASC Topic No. 260, “Earnings Per Share.”
Allowance for Doubtful Accounts - The Company establishes an allowance for doubtful accounts to ensure accounts receivables are not overstated due to uncollectibility. Bad debt reserves are maintained based on a variety of factors, including the length of time receivables are past due and a detailed review of certain individual customer accounts. If circumstances related to customers change, estimates of the recoverability of receivables would be further adjusted. The allowance for doubtful accounts at December 31, 2011 and 2010 is $4,884 and $0, respectively.
Long-Lived and Intangible Assets – Long-lived assets and certain identifiable definite life intangibles to be held and used by the Company are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company continuously evaluates the recoverability of its long-lived assets based on estimated future cash flows and the estimated liquidation value of such long-lived assets, and provides for impairment if such undiscounted cash flows are insufficient to recover the carrying amount of the long-lived assets. If impairment exists, an adjustment is made to write the asset down to its fair value, and a loss is recorded as the difference between the carrying value and fair value. Fair values are determined based on quoted market values, discounted cash flows or internal and external appraisals, as applicable. Assets to be disposed of are carried at the lower of carrying value or estimated net realizable value. No impairment was recorded during the years ended December 31, 2011 and 2010.
Recently Enacted Accounting Standards – The FASB established the Accounting Standards Codification (“Codification” or “ASC”) as the source of authoritative accounting principles recognized by the FASB to be applied by nongovernmental entities in the preparation of financial statements in accordance with generally accepted accounting principles in the United States (“GAAP”). Rules and interpretive releases of the Securities and Exchange Commission (“SEC”) issued under authority of federal securities laws are also sources of GAAP for SEC registrants. Existing GAAP was not intended to be changed as a result of the Codification, and accordingly the change did not impact our financial statements. The ASC does change the way the guidance is organized and presented.
Accounting Standards Update (“ASU”) ASU’s No. 2009-2 through ASU No. 2011-12 which contain technical corrections to existing guidance or affect guidance to specialized industries or entities were recently issued. These updates have no current applicability to the Company or their effect on the financial statements would not have been significant.
Cash Equivalents - The Company considers all highly liquid investments with an original maturity of three months or less at date of purchase to be cash equivalents.
Concentration of Credit Risk - The Company maintains its cash in bank deposit accounts, which, at times, may exceed federally insured limits. The Company has not experienced any losses in such accounts and believes it is not exposed to any significant credit risk on cash and cash equivalents.
| F-8 |
Organization Expenditures – Organizational expenditures are expensed as incurred for Securities Exchange Commission (SEC) filings, but capitalized and amortized for income tax purposes.
Stock Based Compensation – The Company recognizes compensation costs to employees under ASC Topic No. 718, Compensation – Stock Compensation. Under ASC Topic No. 718, companies are required to measure the compensation costs of share-based compensation arrangements based on the grant-date fair value and recognize the costs in the financial statements over the period during which employees are required to provide services. Share based compensation arrangements include stock options, restricted share plans, performance based awards, share appreciation rights and employee share purchase plans. As such, compensation cost is measured on the date of grant at their fair value. Such compensation amounts, if any, are amortized over the respective vesting periods of the option grant.
Equity instruments issued to other than employees are recorded on the basis of the fair value of the instruments, as required by ASC Topic No. 505, Equity Based Payments to Non-Employees. In general, the measurement date is when either a (a) performance commitment, as defined, is reached or (b) the earlier of (i) the non-employee performance is complete or (ii) the instruments are vested. The measured value related to the instruments is recognized over a period based on the facts and circumstances of each particular grant as defined in the FASB Accounting Standards Codification.
Amortization - Utility patents are amortized over a 17 year period. Patents which are pending are not amortized. Customer contacts intangible asset is being amortized over a 3 year period.
Accounting Estimates - The preparation of financial statements in conformity with generally accepted accounting principles in the United States requires management to make estimates and assumptions that affect certain reported amounts of assets and liabilities, the disclosures of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimated by management.
Revenue Recognition – The Company’s revenue is derived primarily from providing services under contractual agreements. The Company recognizes revenue in accordance with ASC Topic No. 605 based on the following criteria: Persuasive evidence of an arrangement exists, services have been rendered, the price is fixed or determinable, and collectability is reasonably assured.
NOTE 2 – Capital Stock
Common Stock
The Company has authorized 750,000,000 shares of common stock, $0.001 par value.
| F-9 |
On September 13, 2010 the Company closed a share exchange transaction (the “Reorganization”) with the shareholders of B6 Sigma, Inc., a Delaware corporation (“B6 Sigma”), which resulted in B6 Sigma becoming a wholly-owned subsidiary of the Company. Each share of B6 Sigma, Inc. common stock outstanding as at the closing of the Reorganization was exchanged for 6.67 shares of the Company’s common stock. At the closing, B6 Sigma, Inc. also acquired and cancelled 110,700,000 (post-split) shares of the Company’s common stock from three shareholders for the sum of $195,000. Upon the closing of the Reorganization, the Company ceased to be a “Shell” company (as such term is defined in Rule 12b-2 under the Securities Exchange Act of 1934, as amended). As a condition to the closing of the Reorganization, B6 Sigma, Inc. also closed a private offering of $1,000,000 of its common stock contemporaneously with the closing of the Reorganization, which included the conversion of $300,000 of previously issued convertible notes and related interest by B6 Sigma, Inc. into the private offering of common stock.
Following issuance of the Reorganization shares to the B6 Sigma shareholders and the stock cancellation, the Company had 313,067,400 (post split) shares of its common stock issued and outstanding. In connection with the closing of the Reorganization, the shareholders of the Company approved a 150:1 forward stock split, and a change of the name of the corporation to Sigma Labs, Inc. Additionally, following completion of the Reorganization, B6 Sigma became a wholly owned subsidiary and its operations comprise the sole business activity.
On January 6, 2011, the Company issued an aggregate of 1,100,000 shares of the Company’s common stock to two consultants as noncash compensation for services rendered valued at $22,000 or $0.02 per share.
In January 2011, the Company commenced a private offering of up to 75,000,000 shares of common stock, $0.001 par value per share, at an issue price of $0.02 per share of common stock. On April 15, 2011, the Company closed the private offering, pursuant to which the Company issued 55,875,000 shares of the Company’s common stock. Gross proceeds amounted to $1,117,500.
Hudson Valley Capital Management Corp. (“Hudson”) acted as placement agent and received a total of $105,735 in commissions. The Company also issued to Hudson in connection with the offering five year warrants to purchase up to 7,931,250 shares of the Company’s common stock. Such warrants have an exercise price of $0.025 per share and are valued at $158,625. As of December 31, 2011 none of the warrants have been exercised. The direct cost associated with the stock offering has been reflected as a reduction to Additional Paid-in-Capital. Net proceeds from the sale of stock were $1,011,765.
The fair value of the warrants issued was estimated at the date of grant using the Black-Scholes option-pricing model with the following assumptions: risk free interest rate of 2.14%; Volatility of 470 and an expected life of five years. It is assumed that no dividends will be paid during the periods of calculation, resulting in a respective weighted-average fair value per warrant of $0.02. Management believes the resulting warrant values are reasonable.
| F-10 |
On March 9, 2011, our Board of Directors adopted an equity incentive plan, the 2011 Equity Incentive Plan (the “Equity Plan”). On March 31, 2011, the holders of at least a majority of the issued and outstanding shares of common stock of the Company approved the Equity Plan. Pursuant to the Equity Plan, the Company is authorized to grant options, restricted stock and stock appreciation rights to purchase up to 31,000,000 shares of common stock to its employees, officers, directors, consultants and advisors. The Equity Plan provides for awards of incentive stock options, non-statutory stock options, and rights to acquire restricted stock. Incentive stock options granted under the Equity Plan are intended to qualify as “incentive stock options” within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended (the “Code”). Non-statutory stock options granted under the Equity Plan are not intended to qualify as incentive stock options under the Code.
During April 2011, the Company issued to five employees an aggregate of 20,000,000 shares of the Company’s common stock, subject to restrictions, pursuant to the Equity Incentive Plan. Such shares are valued at the fair value of $400,000 or $0.02 per share. This compensation is being expensed over the vesting period. As of December 31, 2011, 5,250,000 of the shares had vested resulting in expense of $105,000 being recorded. The balance of unvested compensation cost expected to be recognized is $295,000 and is recorded as a reduction of stockholder’s equity. The unvested compensation is expected to be recognized over the weighted average period of approximately 2.5 years (through April 8, 2012).
In April 2011, the Company issued an aggregate of 3,625,000 shares of the Company’s common stock to one consultant and two professionals as noncash compensation for services rendered to the Company, which services were valued at $72,500 or $0.02 per share.
On May 16, 2011, the Company issued 1,000,000 shares of the Company’s common stock to a consultant as noncash compensation for services rendered valued at $20,000 or $0.02 per share.
On December 31, 2011, the Company issued 35,000,000 shares of the Company’s common stock to acquire 100% of the shares of Sumner and La Mancha Company.
Preferred Stock
The Company is authorized to issue 10,000,000 shares of preferred stock, $0.001 par value. There were none issued and outstanding at December 31, 2011 and 2010.
NOTE 3 – Going Concern
The Company was only recently formed and has not yet achieved profitable operations. The ability of the Company to continue as a going concern is dependent on expanding income opportunities. Management anticipates that additional contracts and their recent business acquisition will allow the Company to achieve profitable operations. There is no assurance that the Company will be successful in raising this additional capital or in achieving profitable operations. The financial statements do not include any adjustments that might result from the outcome of these uncertainties.
| F-11 |
NOTE 4 – Income Taxes
The Company accounts for income taxes in accordance with ASC Topic No. 740. This standard requires the Company to provide a net deferred tax asset or liability equal to the expected future tax benefit or expense of temporary reporting differences between book and tax accounting methods and any available operating loss or tax credit carryforwards.
The Company has available at December 31, 2011, unused operating loss carryforwards of approximately $1,263,924, which may be applied against future taxable income and which expire in various years through 2031. However, if certain substantial changes in the Company’s ownership should occur, there could be an annual limitation on the amount of net operating loss carryforward which can be utilized. The amount of and ultimate realization of the benefits from the operating loss carryforwards for income tax purposes is dependent, in part, upon the tax laws in effect, the future earnings of the Company and other future events, the effects of which cannot be determined. Because of the uncertainty surrounding the realization of the loss carryforwards, the Company has established a valuation allowance equal to the tax effect of the loss carryfowards of approximately $189,589 and $52,293 at December 31, 2011 and 2010, respectively, and, therefore, no deferred tax asset has been recognized for the loss carryforwards. The change in the valuation allowance is approximately $137,296 and $52,293 for the years ended December 31, 2011 and 2010, respectively.
Deferred tax assets are comprised of the following:
| 2011 | 2010 | |||||||
| Deferred tax assets: | ||||||||
| NOL carryover | $ | 189,589 | $ | 52,293 | ||||
| Valuation allowance | (189,589 | ) | (52,293 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
The reconciliation of the provision for income taxes computed at the U.S. federal statutory tax rate to the Company’s effective tax rate for the period ended December 31, 2011 and 2010 is as follows:
| 2011 | 2010 | |||||||
| Book Income | $ | (136,519 | ) | $ | (63,292 | ) | ||
| Organization costs | (777 | ) | 10,999 | |||||
| Valuation allowance | 137,296 | 52,293 | ||||||
| Tax at statutory rate | $ | - | $ | - | ||||
| F-12 |
NOTE 5 – Loss Per Share
The following data show the amounts used in computing loss per share and the effect on income and the weighted average number of shares of dilutive potential common stock for the period ended December 31, 2011 and December 31, 2010:
| Year Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| Loss from continuing | ||||||||
| Operations available to | ||||||||
| Common stockholders (numerator) | $ | (910,129 | ) | $ | (421,946 | ) | ||
| Weighted average number of | ||||||||
| common shares Outstanding | ||||||||
| used in loss per share during | ||||||||
| the Period (denominator) | 370,568,975 | 230,359,050 | ||||||
Dilutive loss per share was not presented as the Company had no common equivalent shares for all periods presented that would affect the computation of diluted loss per share or its effect is anti-dilutive.
NOTE 6 – Furniture and Equipment
The following is a summary of property and equipment, purchased, used and depreciated over a three-year period, less accumulated depreciation, as of December 31, 2011 and 2010:
| Year Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| Furniture and Fixtures | $ | 101,207 | $ | 56,000 | ||||
| Less: Accumulated Depreciation | (69,533 | ) | (13,222 | ) | ||||
| Net Property and Equipment | $ | 31,674 | $ | 42,778 | ||||
Depreciation expense on property and equipment was $20,151 and $13,222 for the periods ended December 31, 2011 and 2010.
| F-13 |
NOTE 7 – Intangible Assets
The Company’s intangible assets consist of Patents, Patent Pending Applications and Customer Contacts.
Provisional patent applications are not amortized until a patent has been granted. Once a patent is granted, the Company will amortize the related costs over the estimated useful life of the patent. If a patent application is denied, then the costs will be expensed at that time.
The customer contacts were acquired in a business acquisition on December 31, 2011 (See Note 10) and will be amortized over their estimated useful life of 3 years.
The following is a summary of definite-life intangible assets less accumulated amortization as of December 31, 2011 and 2010:
| Year Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| Provisional Patent Applications | $ | 21,760 | $ | 8,600 | ||||
| Utility Patents | 17,200 | 17,200 | ||||||
| Customer Contacts | 262,009 | - | ||||||
| Less: Accumulated Amortization | (1,728 | ) | (717 | ) | ||||
| Net Intangible Assets | $ | 299,241 | $ | 25,083 | ||||
Amortization expense on intangible assets was $1,011 and $717 for the years ended December 31, 2011 and 2010.
The estimated aggregate amortization expense for each of the succeeding years ending December 31 is as follows:
| 2012 | $ | 88,348 | ||
| 2013 | 88,348 | |||
| 2014 | 88,348 | |||
| 2015 | 1,012 | |||
| 2016 | 1,012 | |||
| Thereafter | 32,173 | |||
| $ | 299,241 |
| F-14 |
NOTE 8 – Commitments and Contingencies
Operating Leases – The Company leases office space under operating leases. Expense relating to these operating leases was $21,785 for the year ended December 31, 2011. The future minimum lease payments required under non-cancellable operating leases at December 31, 2011 was $9,570. Subsequent to year end, the Company entered into two new lease agreements with future minimum lease payments totaling $36,820. All the future minimum lease payments are currently due during 2012.
NOTE 9 – Concentrations
Revenues – During the years ended December 31, 2011 and 2010, the Company had the following significant customers. The loss of the revenues generated by these customers would have a significant effect on the operations of the Company.
| Customer | 2011 | 2010 | ||
| A | 53% | 65% | ||
| B | 12% | 0% | ||
| C | 0% | 13% |
Accounts Receivable – The Company had the following significant customers who accounted for more than 10% each of the Company’s accounts receivable balance at December 31, 2011 and 2010, respectively.
| Customer | 2011 | 2010 | ||
| A | 64% | 0% | ||
| B | 22% | 16% | ||
| C | 11% | 11% | ||
| D | 0% | 58% | ||
| E | 0% | 15% |
NOTE 10 – Acquisition of Sumner and La Mancha
On December 31, 2011, Sigma Labs acquired 100% of the issued and outstanding common stock of Sumner and La Mancha Company, both companies with services which complement its own. Information related to the acquisition is as follows:
Consideration paid:
| Equity instruments: | $ | 350,000 | ||
| (35,000,000 common shares of Sigma Labs) | ||||
| $ | 350,000 |
| F-15 |
The fair value of the 35,000,000 common shares issued as consideration was determined on the basis of the closing market price of Sigma Labs stock on the acquisition date. The excess cost over net assets acquired of $262,009 was recorded as an intangible asset and is being amortized over 3 years.
Assets acquired and liabilities assumed:
| Cash | $ | 12,613 | ||
| Accounts receivable, net | 153,338 | |||
| Property and equipment, net | 143 | |||
| Intangible – Customer Contacts | 262,009 | |||
| Accounts payable | (76,632 | ) | ||
| Accrued expenses | (1,471 | ) | ||
| $ | 350,000 |
Revenues and earnings:
The following unaudited pro forma summary presents the consolidated results of operations of the combined entities as if the business acquisition had occurred at the beginning of the year on January 1, 2011 and 2010, respectively:
| Year Ended December 31, | ||||||||
| 2011 | 2010 | |||||||
| Total Revenues (unaudited) | $1,899,773 | $2,202,205 | ||||||
| Net Income (Loss) (unaudited) | $ | (935,439 | ) | $ | (301,299 | ) | ||
| Earnings (Loss) per share | $ | (0.00 | ) | $ | (0.00 | ) | ||
NOTE 11 – Subsequent Events
The Company has evaluated subsequent events from the balance sheet date through the date the financial statements were issued and determined there are no items to disclose, except as noted below.
The Company entered into two office space lease agreements subsequent to year end. Total minimum future payments under these leases are $36,820.
| F-16 |
