Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Vitacost.com, Inc. | Financial_Report.xls |
| EX-32.1 - EXHIBIT 32.1 - Vitacost.com, Inc. | v302200_ex32-1.htm |
| EX-23.2 - EXHIBIT 23.2 - Vitacost.com, Inc. | v302200_ex23-2.htm |
| EX-31.2 - CERTIFICATION - Vitacost.com, Inc. | v302200_ex31-2.htm |
| EX-21.1 - EXHIBIT 21.1 - Vitacost.com, Inc. | v302200_ex21-1.htm |
| EX-23.1 - EXHIBIT 23.1 - Vitacost.com, Inc. | v302200_ex23-1.htm |
| EX-31.1 - CERTIFICATION - Vitacost.com, Inc. | v302200_ex31-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| (Mark One) | ||
| S | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
Or
| £ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-34468
VITACOST.COM, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 37-1333024 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification Number) |
| 5400 Broken Sound Blvd., NW, Suite 500 Boca Raton, Florida |
33487 | |
| (Address of principal executive offices) | (Zip Code)) |
Registrant’s telephone number, including area code: (561) 982-4180
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class: | Name of Each Exchange on Which Registered | |
| Common Stock, $0.00001 par value | The NASDAQ Stock Market LLC (NASDAQ Global Market) |
Securities registered pursuant to Section 12(g) of the Act:
Preferred Stock Rights
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large Accelerated Filer o | Accelerated Filer x | Non-Accelerated Filer o | Smaller Reporting Company o |
Indicate by check mark whether the registrant is a shell company as defined in Rule 12-b-2 of Exchange Act. Yes o No x
As of June 30, 2011, the last business day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of voting and non-voting equity held by non-affiliates of the registrant was approximately $61.8 million. Shares of the registrant’s common stock held by each executive officer and director have been excluded in that such persons may be deemed to be affiliates. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 12, 2012, the registrant has 33,228,021 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
None
VITACOST.COM,
INC. FORM 10-K
TABLE OF CONTENTS
| Page | |||||
| PART I | |||||
|
Item 1. Business |
1 | ||||
|
Item 1A. Risk Factors |
11 | ||||
|
Item 1B. Unresolved Staff Comments |
20 | ||||
|
Item 2. Properties |
20 | ||||
|
Item 3. Legal Proceedings |
20 | ||||
|
Item 4. Mine Safety Disclosures |
20 | ||||
| PART II | |||||
|
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities |
21 | ||||
|
Item 6. Selected Financial Data |
23 | ||||
|
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations |
24 | ||||
|
Item 7A. Quantitative and Qualitative Disclosures About Market Risk |
30 | ||||
|
Item 8. Financial Statements and Supplementary Data |
31 | ||||
|
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
51 | ||||
|
Item 9A Controls and Procedures |
51 | ||||
|
Item 9B. Other Information |
51 | ||||
| PART III | |||||
|
Item 10. Directors, Executive Officers, and Corporate Governance |
52 | ||||
|
Item 11. Executive Compensation |
55 | ||||
|
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
66 | ||||
|
Item 13. Certain Relationships, Related Transactions and Director Independence |
68 | ||||
|
Item 14. Principal Accountant Fees and Services |
69 | ||||
| PART IV | |||||
|
Item 15. Exhibits and Financial Statement Schedules |
70 | ||||
| Signatures | 71 | ||||
| Exhibit 31.1 Certification of CEO Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 | |||||
| Exhibit 31.2 Certification of CFO Pursuant to Section 302 of Sarbanes-Oxley Act of 2002 | |||||
| Exhibit 32.1 Certification of CEO and CFO Pursuant to 18 U.S.C. Section 1350, as Adopted | |||||
| i |
CAUTIONARY NOTE REGARDING FORWARD LOOKING STATEMENTS
This Annual Report on Form 10-K contains trends, analyses and other forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended and Section 21E of the Securities Exchange Act of 1934, as amended. Forward-looking statements include statements concerning our plans, objectives, goals, strategies, future events, future revenue or performance, capital expenditures, financing needs and other information that is not historical information. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “could,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “intend,” “potential,” “continue,” “seek” or the negative of these terms or other comparable terminology or by discussions of strategy.
All forward-looking statements, including, without limitation, our examination of historical operating trends, are based upon our current expectations and various assumptions and we do not assume any obligation to update any of these statements. We believe there is a reasonable basis for our expectations and beliefs, but they are inherently uncertain. We may not realize our expectations and our beliefs may not prove correct. Actual results could differ materially from those described or implied by such forward-looking statements and are subject to change due to the inherent risks and uncertainties, such as those disclosed or incorporated by reference in our filings with the SEC. Important factors that could cause our actual results, performance and achievements, or industry results to differ materially from the forward-looking statements are set forth in this Annual Report on Form 10-K under Part I, Item 1a — Risk Factors and Part II, Item 7 — Management’s Discussion and Analysis of Financial Condition and Results of Operation, under the heading “Factors Influencing Future Operating Results” and include, among others:
| • | the current global economic climate; |
| • | significant competition in our industry; |
| • | unfavorable publicity or consumer perception of our products on the Internet; |
| • | the incurrence of material product liability and product recall costs; |
| • | costs of compliance and our failure to comply with government regulations; |
| • | our inability to successfully defend intellectual property claims; |
| • | our failure to keep pace with the changing demands and preferences of our customers for new products; |
| • | disruptions in our manufacturing system, including our information technology systems, or losses of manufacturing certifications; and |
| • | the lack of long-term experience with human consumption of some of our products with innovative ingredients. |
Forward-looking statements in this Annual Report on Form 10-K speak only as of the date hereof, and forward-looking statements in documents attached that are incorporated by reference speak only as of the date of those documents. We do not undertake any obligations to update or release any revisions to any forward-looking statement or to report any events or circumstances after the date hereof or to reflect the occurrence of unanticipated events, except as required by law.
| ii |
PART I
Item 1. Business.
Vitacost.com, Inc.
We are a leading online retailer, based on annual sales volume, of health and wellness products, including dietary supplements such as vitamins, minerals, herbs and other botanicals, amino acids and metabolites (which we refer to as “vitamins and dietary supplements”), as well as cosmetics, natural personal care products, pet products, sports nutrition and health foods. We sell these products directly to consumers primarily through our website, www.vitacost.com. We strive to offer our customers the broadest selection of healthy living products, while providing superior customer service and timely and accurate delivery.
We offer our customers a selection of over 34,000 Stock Keeping Units (“SKUs”) from over 1,600 third-party brands, such as New Chapter, Nature’s Way, Twinlab, Source Naturals, Jarrow Formulas, Jason, Desert Essence, Atkins, Bob’s Red Mill, BSN, Optimum Nutrition, USP Labs and MuscleTech in addition to our own proprietary brands, Cosmeceutical Sciences Institute (“CSI”), Best of All, and Smart Basics. Beginning in March 2011 we began transitioning our largest proprietary brand, Nutraceutical Sciences Institute (“NSI”), to a new Vitacost brand and completed the majority of this transition by year-end 2011. We support our operations through our contact center, our manufacturing and testing facility and our two distribution centers, delivering what we believe are industry-leading customer satisfaction results. Our website allows customers to easily browse and typically purchase products at prices lower than manufacturers’ suggested retail prices. Our website also serves as an educational resource for consumers seeking information on healthy living and the attributes of health and wellness supplements.
Our success is driven primarily by our ability to attract new customers and grow our product offerings. Our customers are typically individuals seeking value in their purchases of health and wellness products. Our active customer base, which we define as customers who have purchased from us within the last twelve months, has steadily increased from approximately 270,000 at the end of 2005 to approximately 1.4 million as of December 31, 2011. On average, our customers make purchases from us two to three times a year and during 2011, approximately 72% of our orders were placed by repeat customers.
Corporate Information
We were incorporated in Delaware in May 1994 and began operations as a catalog retailer of third-party vitamins and supplements under the name Nature’s Wealth Company. In 1999, we launched Vitacost.com and introduced our proprietary vitamins and supplements under our NSI brand. In 2000, we began operations under the name Vitacost.com, Inc. (the “Company”, “Vitacost”, “Vitacost.com”). In September 2009 the Company completed its Initial Public Offering.
Industry Overview
The expansion of the Internet has benefited retailers by improving methods of communication, delivery of content and ease of commerce. At the same time, consumers are leveraging online resources to make informed healthcare, dietary and nutritional choices and related purchasing.
Online Commerce. The Internet’s rapid expansion continues to increase its influence over communication, content and commerce. According to Forrester, U.S. online retail sales were $202 billion in 2011, a 14% increase from 2010. Forrester projects online retail sales to grow at a 10% CAGR to $327 billion by 2016. We believe several factors will contribute to this increase including convenience, expanded range of available products and services, improved security and electronic payment technology, increased access to broadband Internet connections and widespread consumer confidence and acceptance of the Internet as a means of commerce.
U.S. Dietary Supplement Market. According to the Nutrition Business Journal’s Direct Selling Report 2011, U.S. sales of dietary supplements (including vitamins, herbs, meal supplements and sports nutrition and specialty supplements) grew 4.3% to $28.1 billion in 2010. NBJ is forecasting U.S. sales of dietary supplements to grow at a 4.0% rate per year for the next seven years reaching $36.1 billion in 2017. Steady growth reflects customers’ purchases of these natural products to protect their health and ward off more expensive medical visits and prescription drugs. The dietary supplement industry is highly fragmented with products sold through multiple channels including retailers such as mass merchants, grocery stores, drug stores and specialty retailers, as well as through direct mail, catalogs, multi-level marketers and the Internet. U.S. sales of dietary supplements through the Internet grew significantly faster than the overall category increasing approximately 16% in 2010 to $1.3 billion and accounted for an estimated 4.8% of the total U.S. dietary supplement category. According to the NBJ Direct Selling Report 2011, internet sales of dietary supplements are expected to grow at a 13% CAGR over the next seven years, reaching $3.2 billion by 2017.
| 1 |
Our Value Proposition
We strive to offer our customers the broadest product selection of healthy living products at the best value, while providing superior customer service and timely and accurate delivery.
Broad Third-Party and Proprietary Product Selection. We offer approximately 34,000 SKUs representing over 1,600 brands, including nationally-recognized third-party brands and our proprietary brands. Our product selection is designed to appeal to a variety of demographic groups, including those seeking health maintenance and general well-being, baby boomers, the elderly and those with specific health concerns or goals. As consumer preferences evolve, our proprietary brands team works to develop new proprietary products and reformulate existing products to anticipate and fulfill expected consumer demand.
Consistently Superior Value. We offer vitamins, dietary supplements and health and wellness products at savings to our customers with prices typically lower than manufacturers’ suggested retail prices. We provide even greater savings to our customers through proprietary products.
User-Friendly Shopping Experience. Our website is designed to attract natural search traffic while providing a convenient, educational, secure and efficient shopping experience. Products are cross-indexed to allow consumers to easily locate and compare products when searching by brand, ingredient or keyword.
Customer Service Center. Our customer service representatives take orders and answer product and technical questions through our toll-free telephone number. Customers are also able to reach our customer service representatives via email or the live chat feature on our website. We seek to respond within 24 hours to all email requests received between Monday and Friday.
Accurate, Timely and Efficient Order Fulfillment. We operate two highly automated distribution centers, which use wireless, paperless systems to achieve efficient, quality order fulfillment and distribution. Orders are received and picked on high-speed automated lines using pick-to-light, a-frame and carousel technologies.
Growth Initiatives
Our growth strategy is based on the following key initiatives:
| · | Increase Brand and Company Awareness: We are focused on increasing customer awareness of Vitacost.com, including our vast healthy living and proprietary brand offerings. To facilitate this, during 2011, we transitioned our legacy NSI VMHS brand to a new Vitacost label to increase brand and overall Company awareness. In January, 2012, we unveiled a fresh new brand identity, complete with a new logo and corporate slogan, “take the cost out of healthy living” and launched a national radio and print ad campaign. In addition, we re-launched our website with a cleaner look and feel and improved usability features. We are also expanding our online content through a broader initiative to develop a deeper connection to our customers. |
| · | Expand Customer Base: New customer additions continue to be a top priority, as we market consumable products and our customers have high repeat order rates and lifetime values. We believe future top-line growth will stem from an expanded customer base and we are increasing our touch points on the internet to target customers directly where and how they shop. This is being achieved by participating in online marketplaces, such as Amazon.com, growing our network of affiliates, and becoming more effective at search engine marketing and search engine optimization. In addition, we are leveraging our referral networks to attract more customers to our site with the launch of ‘Refer a Friend’ in October of 2011. We also increased our focus on attracting mobile customers with the relaunch of our mobile application which offers an enhanced technology platform designed to improve the mobile user experience. |
| · | Expand Product Offerings: We continue to increase our product offerings of both proprietary and third party products to drive traffic to our site and increase basket size as we strive to become the leading online destination of health and wellness products. We continue to add brands and line extensions in our core VMHS categories as well as in faster growing healthy living categories such as food, sports nutrition/body building, and personal care. During 2011, we added over 5,200 net new SKUs, broken down as follows: 2,099 in VMHS, 1,273 in personal care, 886 in food, 462 in sports nutrition/body building, and 500 in other categories such as household products and pets. We ended fiscal year 2011 with over 34,000 SKUs. |
| · | Increasing Lifetime Value by Increasing Frequency of Purchases and Improving Customer Retention: As we continue to diversify our product offerings in the healthy living space, there is a renewed effort to educate customers on the breadth of our product offerings to increase total basket size and the frequency of purchases in order to drive greater lifetime value. We are also focused on increasing lifetime value per customer by improving customer retention through the use of targeted, personalized emails and promotional offers as well as offering an improved overall customer experience. |
| · | International Expansion: We currently ship products internationally to countries including Canada, Hong Kong, Japan, Taiwan and the United Kingdom, despite limited marketing efforts outside of the United States. We are now beginning to focus on creating an enhanced in-country experience for customers by increasing awareness of our international shipping destinations and improving the overall web and shipping experience for international customers. International represents a large, growing opportunity for us with international accounting for only 4% of total orders in 2011. |
| · | Expand and Optimize Distribution: We are in the process of increasing capacity of our distribution network to support higher level of sales growth. We believe increased capacity will also lead to an improved customer experience as order processing and delivery times are reduced. Construction on a third distribution center is expected to commence in the second half of 2012 with completion expected in the early part of 2013. We are currently in the process of completing the expansion of our existing distribution centers. |
| · | Improve Operating Efficiencies: We are focused on improving operating efficiencies by reducing costs at our existing distribution centers through improved labor productivity and benefits from new technology to reduce special handling costs. Finally, we expect to gain sales leverage of our fixed cost structure as sales continue to accelerate. |
| 2 |
Products
We provide online shoppers with one of the broadest selections of high-quality health and wellness products, including dietary supplements such as vitamins, minerals, herbs and other botanicals, amino acids and metabolites, as well as cosmetics, organic body and personal care products, pet products, sports nutrition and health foods. We offer products in a wide range of potency levels and dosage forms such as tablets, capsules, vegi-capsules, softgels, gelcaps, liquids and powders. Our focus on providing a broad selection enables our customers to purchase products from preferred, trusted brands through a single, comprehensive source.
We offer products that encompass four main categories: Vitamins, Minerals, Herbs and Supplements; Bodybuilding and Sports Nutrition; Personal Care; and Natural and Organic Food.
Vitamins, Minerals, Herbs and Supplements. VMHS products are generally taken to maintain or improve health and address specific health conditions. The Food and Drug Administration (“FDA”) classifies these products under the term “dietary supplements.” In this category, we offer our Vitacost products as well as third-party brands such as Nature’s Way, Twinlab, Jarrow, Carlson and Rainbow Light. Vitamin and mineral products include multi-vitamins, lettered vitamins, such as Vitamin A, C, D, E, and B-complex, along with minerals such as calcium, magnesium, chromium and zinc. These products help prevent deficiencies that can occur when diet alone does not provide all of the necessary vitamins and minerals. Herbal products include whole herbs, standardized extracts, herb combination formulas and teas. Herbs offer a natural solution to address specific health concerns. Certain herbs can be taken to help support specific body systems, such as ginkgo to support brain activity and milk thistle to help maintain proper liver function, as well as other less common herbs such as holy basil for stress relief, turmeric for inflammation support and black cohosh for menopause support. Supplements include essential fatty acids, probiotics, anti-oxidants, phytonutrients and condition-specific formulas. Certain supplements, such as greens, fiber and soy proteins, are taken for added support during various life stages and are intended to supplement vital nutrients absent in an individual’s diet. For example, conjugated linoleic acid (“CLA”) is a naturally-occurring fatty acid that, when used as part of a regular diet and exercise program, supports healthy weight management. Flax seed oils and folic acid are specifically useful during pregnancy. Super anti-oxidants, such as Resveratrol, are taken to fight free radicals. High ORAC (oxygen radical absorptive capacity) fruit concentrates like pomegranate and blueberry are taken to supplement levels of natural nutrients not available in modern diets.
Bodybuilding and Sport Products. Sports nutrition products are used in conjunction with cardiovascular conditioning, weight training and sports activities. Major categories in sports nutrition include protein and weight gain powders, meal replacements, nutrition bars, sport drinks and pre and post-workout supplements to either increase energy or enhance recovery after exercise. We offer bodybuilding and sports products from third parties such as Optimum Nutrition, CytoSport and BSN as well as our Vitacost branded sports nutrition products.
Natural Personal Care Products. Natural personal care products consist of a variety of natural products for skin, body, hair and oral health. We offer hundreds of natural personal care products from category leaders such as JASON and Kiss My Face, as well as our CSI branded products. These products appeal to allergen conscious and environmentally conscious consumers seeking products that are made without harsh chemicals and additives.
Natural and Organic Food. Natural and organic food consists of diet and weight management products, as well as organic and specialty products such as organic peanut butter, gluten free foods and low mercury tuna and salmon. We offer third-party brands such as Atkins, Kashi, Eden Foods and Amy’s Organic, as well as our Best of All natural food products and Walker Diet weight management products.
In 2011 and 2010, our proprietary brands accounted for approximately 23% and 27% of our net sales, respectively. Our proprietary brands include:
| • | Vitacost. Our Vitacost brand is our largest proprietary brand. Through Vitacost, we offer our line of Synergy multivitamins, which is available in over 30 formulations. We also offer dietary supplements including minerals, herbs, amino acids, anti-oxidants and others. |
| • | Cosmeceutical Sciences Institute. Under our CSI brand, we market and sell health and beauty products such as facial cleanser, facial and body moisturizing creams and lotions, and other beauty and skincare products. |
| • | Best of All. Under our Best of All brand, we market and sell organic food products such as banana chips, trail mix, almonds, cashews and more. |
| • | Smart Basics. Under our Smart Basics brand, we market and sell organic fruit juices and extracts and related dietary supplements. |
| • | Walker Diet. Under our Walker Diet brand, we market and sell low carb powders used to assist in weight loss and management. |
| 3 |
Merchandising & New Product Development
We believe we carry most major domestic brands of vitamins, dietary supplements and minerals, as well as many smaller specialty brands. We sell most of our suppliers’ most popular product lines. We also offer our proprietary brands based on our own formulations. We currently stock approximately 34,000 SKUs at each distribution center. Currently, no single SKU represents more than 2% of our net sales. In developing new proprietary products, our proprietary department, together with members of our management team:
| • | review our sales data and customer feedback of third-party SKUs to determine whether we should develop a comparable proprietary product; and |
| • | perform a cost-benefit analysis for the manufacture and sale of any new proprietary product. |
Marketing
Our marketing strategy is designed to increase brand awareness and drive highly targeted new and repeat customers to our website. We use a multi-channel approach which includes broad reach advertising, search engine marketing, email campaigns, partnerships, customer referral and affiliate programs to acquire and retain our customer base.
Online Marketing. We make our website available via keywords and shopping feeds on internet search engines including but not limited to Google, Bing, Nextag and Shopzilla. Banner advertisements on display networks are also used to drive traffic to our website. In addition, we sell our products through Amazon.com while operating an affiliate program aimed at creating brand awareness through websites that participate in the LinkShare network.
Email Campaigns. Our email marketing campaigns distribute information on new arrivals, promotional discounts and product information to customers.
Direct Mail and Promotional Inserts. We have scaled back direct mail marketing initiatives and are focused on creating efficiencies with our online initiatives. Direct mail is used on a limited basis to remind key customer segments to reorder.
Customer referrals. We launched a ‘Refer-A-Friend’ program in the fourth quarter of 2011 to further drive customers to our site. Any existing customer can ‘refer’ a new customer to Vitacost and both are rewarded with a $10 credit to shop on our website.
Partnerships. We are developing partnerships with organizations that have access to large customer bases that are potential Vitacost.com customers or that will improve the brand image and awareness of Vitacost.com.
Manufacturing
Proprietary Manufacturing. We have designed and implemented full-featured solid dose (capsule and tablet) manufacturing and packaging operations at our North Carolina facility, which provide efficient high speed production and aggregate capacities in excess of our present demand requirements. Under our present configuration, we produce or package over 60% of our proprietary products in-house. In 2011, we produced over 360 million tablets and capsules and packaged over three million bottles.
Our manufacturing lines include hard-shell encapsulation machines. Tableting operations include automatic tablet compression machines with a computerized high volume coating system. We ensure precise adherence to our formulations through computer-controlled bin blending systems, and remove under-filled capsules during production through an integrated capsule reject system. Additionally, all manufactured products are subject to inspection through one of two inspection lines.
We package finished products on a packaging line which starts with an automatic bottle un-scrambler and ionized air rinse prior to filling. We use a counting system with a capacity of up to 120 bottles per minute which individually measures tablet dimensions as tablets are counted and disbursed into the bottle.
Contract Manufacturing. All of our proprietary products not in the solid dose category, including softgels, liquids and powders, are manufactured by pre-selected contract manufacturers specializing in the respective dosage form. As of December 31, 2011, our third party manufacturers provided approximately 40% of total proprietary products and 100% of finished soft gel, liquid and powder products. Each of our contract manufacturers is required to maintain high standards of quality control consistent with federal regulatory guidelines and to manufacture our products according to our strict specifications. We have implemented vendor qualification programs for all of our suppliers and manufacturers, including full analytical testing of the products we purchase.
Raw Materials. All raw materials and ingredients for our proprietary products are selected for purchase from a group of third-party suppliers specializing in raw material manufacturing and processing and specialty distribution. We maintain multiple supply and purchasing relations throughout the raw materials marketplace to provide an uninterrupted supply for our manufacturing requirements whenever possible. We employ similar strategies throughout the supply chain operations, leveraging our production volume in all raw materials procurement operations.
Quality Control. Our quality assurance unit establishes process controls and documents and tests every stage of the manufacturing process to ensure we meet product specifications and that finished dietary supplements contain the correct ingredients, purity, strength, and composition in compliance with FDA regulations. We test incoming raw materials and finished goods to ensure they meet or exceed FDA and U.S. Pharmacopeia standards including quantitative and qualitative assay and microbial and heavy metal contamination. Additionally, we perform ingredient analysis and assay using procedures which include High Performance Liquid Chromatography, Ultraviolet/Visible Spectroscopy, Near Infra-Red Spectroscopy, and Inductively Coupled Plasma Mass Spectrometry.
| 4 |
Our plant quality and production standards are designed to meet or exceed the latest FDA regulations. To ensure the highest quality, our manufacturing operations are audited by NSF International for independent cGMP (current Good Manufacturing Practice) certification. NSF International is an independent, not-for-profit organization which offers programs and services to augment and support the work of regulatory officials around the country, including standards development, product testing and certification, as well as onsite audits and inspections. Our NSF certification indicates that NSF has reviewed our operations and determined that our operations comply with FDA and NSF cGMP standards and protocols. As part of its certification and compliance program, NSF conducts random compliance audits of our operations not less than two times per year.
Customer Service
We strive to offer outstanding customer service with each customer’s complete satisfaction as our goal. To achieve this goal, we maintain a fully staffed contact center, to respond to customers via incoming calls, e-mails and live-chat while providing accurate and timely shipping, all driven by our 5-Star Guarantee. We believe our customer service initiatives allow us to establish and maintain long-term customer relationships and facilitate repeat visits and purchases.
Fully Staffed Contact Center. Our contact center operations serve as the primary customer service contact between our customers and Vitacost. We operate a contact center in Lexington, North Carolina and utilize a third-party call center primarily for after-hours support. Contact center agents are available to answer customer questions and to accept customer orders. Both contact center locations use identical ERP systems to provide a seamless customer experience through our toll-free telephone numbers. These contact centers are staffed with specialists who receive regular training so that they can effectively and efficiently field questions from current and prospective consumers. Our specialists are also trained not to answer questions that should be directed to a customer’s physician, such as questions relating to drug interactions. In order to provide consistency, speed and accuracy, our specialists use an internal, standardized question and answer database in responding to customer inquiries. Our specialists also have access to real time inventory data to know if a product is in stock to properly manage customer expectations.
Our 5-Star Guarantee. Our 5-Star Guarantee makes it easy, convenient and safe for customers to purchase our products. Under the Guarantee we:
| • | Offer vitamins, supplements, organic products, body care and natural health products at everyday low prices, providing savings off retail 365 days a year, with no minimums, no memberships and no hidden charges. |
| • | Ensure the potency and quality of our vitamin products while providing the highest quality supplements and other natural health products. |
| • | Provide a 30-Day Money-Back guarantee for all our products. |
| • | Guarantee a safe, secure online shopping experience through the use of state-of-the-art Secure Socket Layer 128-bit encryption on our website. |
| • | Maintain one of the largest selections of vitamins, supplements and health products available anywhere, with approximately 34,000 items and more than 1,600 brands from which to choose. |
During 2011, we were named in the top 10 in the ‘Spring 2011 Top 100 Online Retail Satisfaction Index’ published by ForeSee Results for the second year in a row. ForeSee Results’ customer satisfaction ratings are done using the American Customer Satisfaction Index methodology, a sophisticated scientific formula developed at the University of Michigan that predicts consumer spending behavior. We also received an ELITE rating for customer service from STELLAService, an independent, third-party company that rates the customer service performance of online retailers. STELLAService ratings are performed using 300 unique customer service metrics and features for each online retailer across all areas of the shopping experience evaluating usability of the site, shipping, delivery and returns, and overall customer support.
Technology and Operations
Our website is supported by a technology infrastructure that is designed to provide a superior customer experience, including speed, ease of use and security. Our technology infrastructure allows us to monitor our website and services in real time, scale to size as required and balance traffic geographically across multiple sites. We also track and manage our manufacturing processes, inventory, order fulfillment, customer service and marketing through state-of-the-art technologies that allow us to condense and distribute customer and sales data as part of our business intelligence model. From this data, our marketing and product development teams are able to analyze and project market trends and consumer demand.
Our technology infrastructure uses highly scalable, fully fault tolerant enterprise-class technology. Coupled with the use of virtual and cloud-based capabilities that provide redundant coverage and virtually eliminate the risk of downtime, our infrastructure provides a set of strategic, high-availability systems that we believe rival those of larger companies. We maintain strategic partnerships with vendors to ensure that we can rapidly deploy new products and information technology solutions that we believe are key to our success.
We maintain three secure internal data centers that support product development, quality control and office and distribution center infrastructure. Our data centers are also collocated through a third-party provider with locations in Atlanta and Seattle for redundancy, which has provided us with 100% service availability since 2007.
| 5 |
We follow rigorous industry standards to protect our internal operations and the personal information we collect from our customers. We do not sell or disclose the personal information of our customers. We continue to maintain and upgrade our technology framework that can support high levels of security while meeting the compliance requirements of Payment Card Industry (“PCI”) security standards. We are considered a “sender” under the CAN-SPAM Act and comply with the applicable aspects thereof.
We have installed technologically advanced finished goods inventory control systems to track our finished goods from receipt through shipment. All items are barcoded to facilitate electronic tracking allowing us to tie each SKU number back to our inventory control, shipping and sales departments. Our inventory control system analyzes and automatically reorders a majority of the products we sell, minimizing out-of-stock situations. We consistently evaluate low volume items in order to minimize losses due to product expiration or obsolescence as well as to efficiently manage our capital and warehouse space.
Competition
The dietary supplement, natural health and wellness market is large, growing, competitive and highly fragmented. Our competition includes multi-level marketers, online VMHS specialty and mass retailers, and extends offline to brick and mortar stores including but not limited to grocery, membership clubs, specialty and mass retailers. We believe the following are the principal competitive factors in our market:
| • | Competitive pricing |
| • | Selection and availability of product |
| • | Reliability and speed of delivery |
| • | Website ease of use |
| • | Customer service and support |
We believe we compete favorably, however the nature and extent to which our competitors implement various pricing and promotional activities in response to increasing competition and our response to these competitive actions, could adversely affect our profitability.
Trademark and Other Intellectual Property
We rely on a combination of patent, copyright and trademark and trade secret laws, confidentiality procedures and contractual provisions to protect our proprietary rights with respect to our technology and proprietary information. We have applied for or registered all relevant trademarks with the U.S. Patent and Trademark Office (USPTO), including our Vitacost, Nutraceutical Sciences Institute, Cosmeceutical Sciences Institute, Best of All, Walker Diet, Smart Basics, Take the Cost out of Healthy Living, Momonomics and Wellness Times trademarks, among others. We believe our trademarks to be valuable and are identified strongly with our brands. Issuance of a federally registered trademark creates a rebuttable presumption of ownership of the mark; however, it is subject to challenge by others claiming first use in the mark in some or all of the areas in which it is used. We have also applied for foreign protection of certain of our trademarks in the European and Asian markets in which we operate and have registered Vitacost, NSI and Nutraceutical Sciences Institute in certain countries in these regions.
Federally registered trademarks have a perpetual life, as long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. We believe our patents and trademarks are valuable and provide us certain benefits in marketing our products. We intend to actively protect our patents, trademarks, trade secrets and other intellectual property.
We have obtained a number of U.S. patents on some product formulations and have applications pending for others. In designing our product formulations, we have attempted to blend an optimal combination of nutrients which appear to have beneficial impact based upon scientific literature. However, because formal clinical studies have in most instances not been conducted by us to validate the intended health benefits of the nutrients, we are generally prohibited by the FDA from making disease treatment and prevention claims in the promotion of products using these formulations. While we seek broad coverage for our patents, there is always a risk that an alteration to the formulation may provide sufficient basis for a competitor to avoid infringement claims by us. In addition, our issued patents expire over the next several years and we cannot provide any assurance that any patents will be issued from pending applications or that any issued patents will adequately protect our intellectual property. We intend to reevaluate each of our registered U.S. patents for strength and value as renewal dates approach.
Government Regulation
We are subject to federal and state consumer protection laws, including laws protecting the privacy of consumer non-public information and regulations prohibiting unfair and deceptive acts and trade practices. In particular, under federal and state financial privacy laws and regulations, we must provide:
| • | notice to consumers of our policies on sharing non-public information with third parties; |
| • | advance notice of any changes to our policies; and |
| • | with limited exceptions, provide consumers the right to prevent sharing of their non-public personal information with unaffiliated third parties. |
| 6 |
Furthermore, the growth and demand for online commerce could result in more stringent consumer protection laws that impose additional compliance burdens on online retailers. These consumer protection laws could result in substantial compliance costs and could interfere with the conduct of our business.
There is currently great uncertainty in many states whether or how existing laws governing issues such as property ownership, sales and other taxes, and libel and personal privacy applies to the Internet and commercial online retailers. These issues may take years to resolve. For example, tax authorities in a number of states, as well as a Congressional advisory commission, are currently reviewing the appropriate tax treatment of companies engaged in online commerce, and new state tax regulations may subject us to additional state sales and income taxes. New legislation or regulation, the application of laws and regulations from jurisdictions whose laws do not currently apply to our business, or a change in application of existing laws and regulations to the Internet and commercial online services could result in significant additional taxes on our business. These taxes could have an adverse effect on our results of operations.
Our products are subject to extensive regulation in the U.S. and abroad. As applied to products Vitacost sells, FDA enforces the Federal Food, Drug and Cosmetic Act (“FDCA”), and related regulations, which govern the identity, purity, quality, strength, and composition of dietary supplements and regulate the formulation, manufacture, packaging, labeling, holding, sale, and distribution of dietary supplements, foods, cosmetics, medical devices, animal foods and drugs, and over-the-counter (“OTC”) allopathic and homeopathic human drugs (collectively “FDA regulated products”). FDA prohibits the distribution and/or sale of misbranded and/or adulterated FDA regulated products. In particular, FDA evaluates dietary supplements to determine the intention of the manufacturer or distributor through promotion, label or labeling claims which cause the products to be unapproved new drugs.
The Federal Trade Commission, or FTC, enforces the Federal Trade Commission Act, or FTCA, and related regulations, which govern the advertising and advertising acts and practices associated with the promotion and sale of these products to prevent misleading or deceptive claims.
The U.S. Postal Inspection Service enforces federal laws governing fraudulent use of the mail. Regulation of certain aspects of the dietary supplement business at the federal level is also governed by the Consumer Product Safety Commission (“CPSC”) (e.g., concerning the presence of adulterated substances, such as toxic levels of lead or iron, that render products unsafe for consumption and require a CPSC ordered recall), the Department of Agriculture (e.g., for products that are intended for ingestion as dietary supplements for animals) and the Environmental Protection Agency (e.g., in the methods of disposal used for certain dietary ingredients, such as colloidal silver).
The manufacture, packaging, labeling, holding, sale, and distribution of dietary supplements are also subject to extensive local, state, and foreign government regulation. For example, under the European Union Directive, only dietary supplements listed in Annex II to that directive or otherwise ruled saleable in Europe by the European Union may be sold in Europe subject to EU restrictions on dosage amounts, forms, label claims and advertising. The Bureau of Customs and Border Patrol (“CBP”), a division of the Department of Homeland Security, also regulates shipments containing dietary ingredients, dietary supplements, cosmetics, drugs, biologics, and medical devices and engages in enforcement activity in concert with the FDA to block the import or export of articles deemed adulterated or otherwise unlawful for sale in the United States (imports) or in the non-U.S. country to which articles are addressed. CBP holds on articles or demands for recall can interfere with the timely delivery of products to market and can result in regulatory fines and penalties.
The FDCA has been amended several times affecting provisions that concern dietary ingredients and dietary supplements, including by the Dietary Supplement Health and Education Act of 1994 (“DSHEA”). DSHEA formally defined what may be sold as a dietary supplement, defined statements of nutritional support and the conditions under which they may lawfully be used, and included provisions that permit the FDA to regulate manufacturing practices and labeling claims peculiar to dietary supplements. “Dietary supplements” are defined as vitamins, minerals, herbs, other botanicals, amino acids and other dietary substances that are used to supplement the diet, as well as concentrates, constituents, extracts, metabolites, or combinations of such dietary ingredients. Generally, under DSHEA, dietary ingredients that were on the market before October 15, 1994 may be used in dietary supplements without notifying the FDA. However, a “new” dietary ingredient (i.e., a dietary ingredient that was not marketed in the U.S. before October 15, 1994) must be the subject of a new dietary ingredient notification submitted to the FDA unless the ingredient has been “present in the food supply as an article used for food” without having been “chemically altered.” A new dietary ingredient notification must provide the FDA with evidence of a “history of use or other evidence of safety” which establishes that use of the dietary ingredient “will reasonably be expected to be safe.” A new dietary ingredient notification must be submitted to the FDA at least 75 days before the new dietary ingredient can be marketed. There can be no assurance that the FDA will accept evidence purporting to establish the safety of any new dietary ingredients that we may want to market, and the FDA’s refusal to accept such evidence could prevent the marketing of such dietary ingredients.
Increased FDA enforcement could lead the FDA to challenge dietary ingredients already on the market as “illegal” under the FDCA because of the failure to file a new dietary ingredient notification or because the substance may be one found to be the subject of an investigational new drug application for which clinical trials have commenced and been publicized.
| 7 |
The FDA generally prohibits labeling a dietary supplement with any “health claim” (i.e., any statement associating a nutrient with risk-reduction, but not treatment, of a disease or health-related condition), unless the claim is pre-approved by the FDA. The FDA prohibits entirely disease diagnosis, prevention and treatment claims when made for a dietary supplement. However, “statements of nutritional support,” including so-called “structure/function claims,” are permitted to be included in labeling for dietary supplements without FDA pre-approval. Such statements may describe how a particular dietary ingredient affects the structure, function or general well-being of the body, or the mechanism of action by which a dietary ingredient may affect the structure, function or well-being of the body, but such statements may not state that a dietary supplement will reduce the risk or incidence of a disease unless such claim has been reviewed and approved by the FDA. A company that uses a statement of nutritional support in labeling must possess evidence substantiating that the statement is truthful and not misleading. Such statements must be submitted to the FDA no later than thirty days after first marketing the product with the certification that they possess the necessary evidence and must be accompanied by an FDA mandated label disclaimer that “This statement has not been evaluated by the FDA. This product is not intended to diagnose, treat, cure or prevent any disease.” There can be no assurance; however, that the FDA will not determine that a particular statement of nutritional support that we want to use is an unacceptable disease claim or an unauthorized nutrient-disease relationship claim otherwise permitted with FDA approval as a “health claim.” Such a determination might prevent the use of such a claim.
In addition, DSHEA provides that certain “third-party literature,” such as a reprint of a peer-reviewed scientific publication linking a particular dietary ingredient with health benefits, may “in connection with the sale of a dietary supplement to consumers” be exempt from labeling regulation. However, the FDA has adopted an “intent to use” doctrine whereby such literature even if exempt from labeling may nonetheless form the basis for an agency determination that the literature in context reveals a company intent to sell a dietary ingredient or dietary supplement as a drug, thereby rendering the supplement an unlawful, unapproved new drug. Because the “intent to use” doctrine is predicated on a subjective assessment of all facts and circumstances associated with the promotion and sale of a dietary supplement, we cannot know whether any particular piece of literature otherwise exempt from labeling will be deemed by the FDA unlawful for use in association with the sale of the dietary ingredient or dietary supplement.
As authorized by the FDCA, the FDA has adopted and implemented Good Manufacturing Practices (“GMPs”), specifically for dietary supplements. These GMPs impose extensive process controls on the manufacture, holding, labeling, packaging, and distribution of dietary supplements and the components of dietary supplements. They require that every dietary supplement be made in accordance with a master manufacturing record with all dietary ingredients verified by identity testing before use, that each step in manufacture, holding, labeling, packaging, and distribution be defined with written standard operating procedures, monitored, and documented, and that any deviation in manufacture, holding, labeling, packaging, or distribution be contemporaneously documented, assessed by a quality control expert, and corrected through documented corrective action steps (whether through an intervention that restores the product to the specifications in the master manufacturing record or to document destruction of the non-conforming product). The GMPs are designed to ensure documentation, including testing results that confirm the identity, purity, quality, strength, and composition of finished dietary supplements. In addition, GMPs require a company to make and keep written records of every product complaint that is related to GMPs. The written record of the product complaint must include the following: the name and description of the dietary supplement; the batch, lot, or control number of the dietary supplement, if available; the date the complaint was received and the name, address, or telephone number of the person making the complaint, if available; the nature of the complaint, including, if known, how the product was used; the reply to the complainant, if any; and findings of the company’s quality control investigation and follow-up action taken when an investigation is performed. The regulations directly affect all who manufacture the dietary supplements we sell and our own manufacture, holding, labeling, packaging, and distribution of dietary supplements. The FDA may deem any dietary supplement adulterated, whether presenting a risk of illness or injury or not, based on a failure to comply with any one or more process controls in the GMP regulations. If deemed adulterated, a dietary supplement may not be lawfully sold and may have to be recalled from the market. It is possible that the FDA will find one or more of the process controls implemented by us, by our contract manufacturers, or by those whose dietary supplements we sell to be inadequate and, thus, requiring corrective action, requiring any one or more of the dietary supplements we sell to be unlawful for sale, or resulting in a judicial order that may impair our ability to manufacture, market, and sell dietary supplements.
The FDA also requires adverse event notices on labels and serious adverse event reporting for all supplements and OTC drugs. An “adverse event” is defined by statute to include “any health-related event associated with the use of a dietary supplement that is adverse.” While all adverse event complaints received must be recorded in accordance with the cGMPs discussed above, only serious adverse events must be reported to FDA. A “serious adverse event” is an adverse event that: results in death, a life-threatening experience, inpatient hospitalization, a persistent or significant disability or incapacity, or a congenital anomaly or birth defect; or requires, based on reasonable medical judgment, a medical or surgical intervention to prevent an outcome described above. When a manufacturer, packer, or distributor whose name appears on the product label of a dietary supplement receives any report of a serious adverse event associated with the use of the dietary supplement in the United States, the company must submit a “serious adverse event report” on MedWatch Form 3500A. The report must be filed within 15 business days of receipt of information regarding the adverse event. All adverse event reports, whether serious or not, must be recorded and kept in company records under the GMP rules. A company must maintain records of each report of any adverse event (both serious and non-serious) for a minimum of 6 years. These records should include any documents related to the report, including: the company’s serious adverse event report to the FDA with attachments; any new medical information about the serious adverse event received; all reports to the FDA of new medical information related to the serious adverse event; and any communications between the company and any other person(s) who provided information related to the adverse event.
The regulation of dietary supplements may increase or become more restrictive in the future. There can be no assurance that, if more stringent statutes are enacted for dietary supplements, or if more stringent regulations are promulgated, we will be able to comply with such statutes or regulations without incurring substantial expense.
The FDA regulates the formulation, manufacturing, packaging, labeling and distribution of all OTC drugs. It allows OTC allopathic drug products to be sold without premarket approval so long as the products comply with FDA’s “monograph” system that specifies active drug ingredients that are generally recognized as safe and effective for particular uses. If an OTC drug is not in compliance with the applicable FDA monograph, the product generally cannot be sold without first obtaining FDA approval of a new drug application, which can be a long and expensive procedure.
| 8 |
The homeopathic OTC drugs that we sell are regulated by FDA as non-prescription, over-the-counter drugs. These products must generally meet the manufacturing standards set forth in the Homeopathic Pharmacopeia of the United States (HPUS) and the limitations for OTC ingredients. Claims made for them must not deviate from those contained in specific homeopathic treatises recognized by the FDA as appropriate for use. The products must be labeled in compliance with FDA regulations for all OTC drugs. If these requirements are not met, the FDA can consider the products unapproved new drugs and prohibit their sale.
Cosmetics are not subject to pre-market approval by the FDA, but the products, their ingredients and their label and labeling content, are regulated by the FDA, and it is the burden of those who sell cosmetics to ensure that they are safe for uses as directed. The FDA prohibits certain ingredients from being contained in cosmetic products that are authorized only for drug use or are deemed adulterated. In addition, the labeling of cosmetic products is subject to the requirements of the FDCA, the Fair Packaging Labeling Act and other FDA regulations. The FDA limits cosmetic product claims to those of beautification and enhancement to the external appearance of the skin. Structure/function claims are generally prohibited for cosmetic products as are disease prevention and treatment claims. It is possible that cosmetic product ingredients now commonly in use that are derived from nanotechnology may be restricted or prohibited in future. It is also possible that claims now commonly in use concerning cosmetic reduction in the external appearance of aging, the effect of cosmetic ingredients on fine lines and wrinkles, or on other aspects of appearance may in the future be deemed prohibited, implied disease treatment claims.
Animal foods are also regulated by FDA and each state’s department of agriculture. Those regulators generally defer to a product’s compliance with the “American Association of Feed Control Officials” annual manual that sets out labeling requirements, approved ingredients, and nutrient needs for particular animal types including companion animals. FDA generally exercises enforcement discretion and allows some animal foods to make structure function claims. However, there is no legal authority in the FDCA for animal foods to make those types of claims. It is possible in the future that FDA may consider all structure/function claims prohibited.
Medical devices that we sell are OTC and not subject to pre-market approval but may be subject to a notification requirement (called a 510k) or a premarket compliance requirement, like an OTC monograph. Manufacturers of medical devices must register and list their products with FDA annually whether they are located domestically or overseas. All medical devices must be manufactured under good manufacturing standards called “quality assurance” standards set by FDA. Medical devices must be labeled in accordance with FDA’s general device labeling requirements and whatever particular label requirements FDA may designate for that type of device. When a company receives a positive response to a 510k notification, if the company promotes the product with any deviation from the labeling and claims that were submitted to the agency, the agency may consider the product misbranded. Similarly, if the company changes the design or performance of the device in any way from the notification then FDA may consider the product adulterated.
Conventional food manufacturing, labeling and distribution is also under FDA jurisdiction. Manufacturers must ensure their products are produced in a clean environment to prevent adulteration by contamination and must manufacture their products with either approved food additives or ingredients that are “generally recognized as safe” (GRAS). Conventional foods must meet FDA’s labeling requirements including a Nutrition Facts statement. Conventional foods may make structure/function statements, like dietary supplements but unlike dietary supplements are not required to file notice of those statements with FDA. Like dietary supplements, infant formulas are regulated by FDA as a type of food with their own requirements for manufacturing and labeling. Infant formulas, however, are required to go through a preapproval process, manufacturing inspection prior to product launch, and are held to a nutritional sufficiency standard unlike other foods due to the nature of this type of product.
Conventional foods and dietary supplements must also comply with the Organic Act (for the designation of organic ingredients) enforced by the US Department of Agriculture and the Food Allergen Labeling and Consumer Protection Act of 2004 (“FALCPA”) enforced by the FDA. Under the Organic Act there are specific requirements for the certification of ingredients as “organic” and requirements on how to use the term “organic” in labeling, whether for an ingredient or a complete product. USDA has also issued an enforcement policy applying the use of the term “organic” to cosmetics and their ingredients even though there is no law authorizing that use. Under FALCPA, all packaged foods (including supplements) containing any of the eight identified major food allergens (milk, egg, fish, crustacean shellfish, tree nuts, wheat, peanuts, and soybeans) must declare such allergens, at least once, by their common or usual name in the ingredient list or in a “CONTAINS” statement immediately following the ingredient list in bold. A packaged food “contains” an allergen for the purposes of the law when any intentionally added ingredient contains an allergen. Thus, even if an allergen is present only in a coloring or flavoring, FALCPA applies. Likewise, the law applies even if an allergen is present only in a small amount of an “incidental” (but intentionally added, non-cross-contact) additive, like a releasing agent. Disclosure in this case is required even though such an ingredient usually could be omitted altogether from the ingredients list. If a manufacturer believes that no allergen is present there is a preapproval process to seek an exception. Failure to comply with the FALCPA may lead to civil sanctions, criminal penalties, or both under the FDCA. In addition, the FDA is authorized to seize non-conforming products controlled by a company and issue a recall of products already on the market.
The FDA has broad authority to enforce the provisions of the FDCA concerning all of the products it regulates, including powers to issue a public “warning letter” to a company to quarantine and prohibit the sale of products deemed adulterated or misbranded, to publicize information about illegal products, to request a voluntary recall of illegal products from the market, to request that the Department of Justice initiate a seizure action, an injunction action or a criminal prosecution in U.S. courts, and to seek disgorgement from a federal court of all proceeds received from the sale of products deemed misbranded or adulterated.
The FTC exercises jurisdiction over the advertising of dietary supplements, OTC drugs, medical devices, and cosmetics. In recent years, the FTC has instituted numerous enforcement actions against dietary supplement companies for making false or misleading advertising claims and for failing to adequately substantiate claims made in advertising. These enforcement actions have often resulted in consent decrees and the payment of civil penalties and/or restitution by the companies involved. The FTC also regulates other aspects of consumer purchases including, but not limited to, promotional offers of savings compared policies, telemarketing, continuity plans, and “free” offers.
| 9 |
We are also subject to regulation under various state, local and international laws that include provisions governing, among other things, the formulation, manufacturing, packaging, labeling, advertising and distribution of dietary supplements, animal foods, medical devices, conventional foods, and OTC drugs. For example, under Proposition 65 in the State of California, a list of substances are deemed to pose a risk of carcinogenicity or birth defects at or above certain levels. If any such ingredient exceeds the permissible levels in a dietary supplement, cosmetic, or drug, the product may be lawfully sold in California only if accompanied by a prominent warning label alerting consumers that the product contains an ingredient linked to cancer or birth defect risk. Private attorney general actions as well as California attorney general actions may be brought against non-compliant parties and can result in substantial costs and fines. In additional California has a law called the “Consumers Legal Remedies Act” (Cal. Civ. Code § 1750 et seq) that allows private parties to assert a class action claim for false or deceptive advertising. It is typically asserted in combination with claims for false advertising and unfair competition under the California Business and Professions Code. California law firms specializing in these type of consumer class action claims have recently been targeting dietary supplement and OTC homeopathic drug makers and sellers of products sold in California, claiming injury based on the products’ failure to deliver results as claimed in product labeling and promotion.
Government regulations in foreign countries may prevent or delay the introduction, or require the reformulation, of certain of our products. Compliance with such foreign governmental regulations is generally the responsibility of our distributors in those countries. These distributors are independent contractors whom we do not control.
In addition, from time to time in the future, we may become subject to additional laws or regulations administered by the FDA, the FTC, or by other federal, state, local or foreign regulatory authorities, to the repeal of laws or regulations that we generally consider favorable, such as DSHEA, or to more stringent interpretations of current laws or regulations. We are not able to predict the nature of such future laws, regulations, repeals or interpretations, and we cannot predict what effect additional governmental regulation, if and when it occurs, would have on our business in the future. Such developments could, however, require reformulation of certain products to meet new standards, recalls or discontinuance of certain products not able to be reformulated, additional record-keeping requirements, increased documentation of the properties of certain products, additional or different labeling, additional scientific substantiation, additional personnel or other new requirements. Any such developments could have a material adverse effect on our business.
Europe. The European Union (“EU”) is responsible for the development of legislation governing foods, nutritional supplements, and medicines sold in Europe. Member States of the EU (“Member States”) are authorized to develop local legislation governing these products, provided such legislation is not more restrictive than the legislation promulgated by the EU. Member States are responsible for enforcement of the applicable legislation. In 2002, the EU established a process for Member States to bring this regulating legislation in line with a published directive of the EU, which addressed the labeling and marketing of vitamins and minerals, what nutrients are permitted or not permitted and other packaging requirements. In 2004, the EU established standards for the manufacture and marketing of herbal medicines with the Traditional Herbal Medicinal Products Directive. This requires, among other things, manufacturers of herbal medicinal products to comply with Pharmaceutical Group Standards, and only requires proof of safety, not efficacy. Registration of products is subject to a phase-in period which began in October 2005, and which terminated in April 2011, at which point all herbal medicinal products must be registered with Member States.
In 2006, the EU adopted its Commission Directive 2006/37/EC, amending its Directive 2002/46/EC. Under the amended directive, only nutrients listed in Annex II, or approved by subsequent order of the EU, may be lawfully sold in Member States. The EU also regulates labels, labeling, and advertising associated with the promotion and sale of dietary supplements in Europe. These regulations may make it unlawful for us to sell in Europe certain products lawfully labeled and sold in the United States, adversely affecting the finances of the business.
In the United Kingdom, the principal governing legislation is the Food Safety Act of 1990 (governing safety of food products) and the Medicines Act of 1968 (governing licensing and sale of medicine). Further guidance is provided by numerous Statutory Instruments addressing the formulation, purity, packaging, advertising and labeling of such products. Medicinal products are regulated and enforced by the Medicines and Healthcare Products Regulatory Agency (MHRA), an agency of the Department of Health. The MHRA determines if an herbal remedy is medicinal by virtue of its “presentation” or “function.” Food products are regulated by the Food Standard Agency (FSA), which reports to the Department of Health and to the Department of Environment, Food and Rural Affairs. Vitamin and mineral supplements and soup products with herbal ingredients are generally considered food supplements and are subject to the purview of the FSA. Additional legislative standards have been adopted in the other EU countries, typically similar in scope to the UK. The regulatory scheme in Canada is similar but not identical to that of the U.S. concerning medicines and healthcare products or material health products and is regulated by Health Canada.
Employees
As of December 31, 2011, we had 629 full-time and 2 part time employees. We employed 128 full-time employees at our Florida corporate headquarters, 351 full-time employees and 2 part-time employees at our North Carolina facility and 150 full-time employees at our Nevada facility. Additionally, from time to time, we hire temporary contract employees. None of our employees are covered by a collective bargaining agreement and we are unaware of any union organizing efforts. We consider our relationship with our employees to be good.
| 10 |
Geographic Information
During our last three years, substantially all of our revenue was generated within North America, and all of our long-lived assets are located within the United States.
Available Information
Our Web site is www.vitacost.com. The information posted on our website is not incorporated into this Annual Report on Form 10-K. We have made available through our Web site, free of charge, our Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and amendments to those reports, as soon as reasonably practicable after we electronically file such materials with, or furnish them to, the Securities and Exchange Commission. In addition, they are available directly on the website for the Securities and Exchange Commission.
Item 1A. Risk Factors
We operate in a rapidly changing environment that involves significant risks, a number of which are beyond our control. In addition to the other information contained in this Annual Report on Form 10-K, the following discussion highlights some of these risks and the possible impact of these factors on our business, financial condition and future results of operations. If any of the following risks actually occur, our business, financial condition or results of operations may be adversely impacted. In addition, these risks and uncertainties may impact the forward-looking statements described elsewhere in this Form 10-K and in the documents incorporated herein by reference. They could affect our actual results of operations, causing them to differ materially from those expressed in forward-looking statements.
Risks Relating to Our Business
We may incur product liability claims, which could increase our costs and/or materially adversely affect our business, reputation, financial condition or results of operations.
As a retailer, formulator and manufacturer of products designed for human consumption or use on or in the body, we are subject to product liability claims if the use of our products, whether manufactured by us or by our third-party manufacturer, is alleged to have resulted in illness or injury or if our products include inadequate instructions or warnings. Our products generally consist of vitamins, minerals, herbs and other ingredients that are classified as foods, OTC drugs, dietary supplements, medical devices, and cosmetics and generally are not subject to pre-market regulatory approval or clearance in the U.S. by the FDA or other governmental authorities. Our products could contain spoiled or contaminated substances, and some of our products contain ingredients that do not have long histories of human consumption. Previously unknown adverse reactions resulting from human consumption of these ingredients could occur. In addition, some of our products are produced by third-party manufacturers. As a distributor of products manufactured by third parties, we may also be liable for various product liability claims for products that we do not manufacture. We could be subject to product liability claims, including among others, that our products include insufficient instructions for use or inadequate warnings concerning possible side effects or interactions with other substances. Any product liability claim against us could result in increased costs and, therefore, adversely affect our reputation with our customers, which in turn could materially adversely affect our business, financial condition or results of operations.
Our network and communications systems are vulnerable to system interruption and damage, which could limit our ability to operate our business and could have a material adverse effect on our business, financial condition or results of operations.
Our ability to receive and fulfill orders promptly and accurately is critical to our success and largely depends on the efficient and uninterrupted operation of our computer and communications hardware and software systems. We experience periodic system interruptions that impair the performance of our transaction systems or make our website inaccessible to our customers. These systems interruptions may prevent us from efficiently accepting and fulfilling orders, sending out promotional emails and other customer communications in a timely manner, introducing new products and features on our website, promptly responding to customers, or providing services to third parties. Frequent or persistent interruptions in our services could cause current or potential customers to believe that our systems are unreliable, which could cause them to avoid our website, drive them to our competitors, and harm our reputation. To minimize future system interruptions, we must continue to add software and hardware and to improve our systems and network infrastructure to accommodate increases in website traffic and sales volume and to replace aging hardware and software. We may be unable to promptly and effectively upgrade and expand our systems and integrate additional functionality into our existing systems. In addition, upgrades to our system may cause existing systems to fail or operate incorrectly. Any unscheduled interruption in our services could result in fewer orders, additional operating expenses, or reduced customer satisfaction, any of which would harm our business, financial condition and operating results. In addition, the timing and cost of upgrades to our systems and infrastructure may substantially affect our ability to maintain profitability.
Our systems and operations and those of our suppliers and Internet service providers, are vulnerable to damage or interruption from fire, flood, earthquakes, power loss, server failure, telecommunications and Internet service failure, acts of war or terrorism, computer viruses and denial-of-service attacks, physical or electronic break-ins, sabotage, human error and similar events. Any of these events could lead to system interruptions, order fulfillment delays, and loss of critical data for us, our suppliers, or our Internet service providers, and could prevent us from accepting and fulfilling customer orders. Any significant interruption in the availability or functionality of our website or our customer processing, distribution, or communications systems, for any reason, could seriously harm our business, financial condition, and operating results. The occurrence of any of these factors could have a material adverse effect on our business, financial condition or results of operations.
| 11 |
Unfavorable publicity or consumer acceptance of our products or of nutritional supplements generally could reduce our sales.
We are highly dependent upon consumer acceptance of the safety, efficacy and quality of our products, as well as similar products distributed by other companies. Consumer acceptance of products can be significantly influenced by scientific research or findings, national media attention and other publicity about product use. A product may be received favorably, resulting in high sales associated with that product that may not be sustainable as consumer preferences change. In addition, recent studies have challenged the safety or benefit of certain nutritional supplements and dietary ingredients. Future scientific research or publicity could be unfavorable to our industry or any of our particular products and may not be consistent with earlier favorable research or publicity. A future research report or publicity that is perceived by our consumers as less than favorable or that questions earlier favorable research or publicity could have a material adverse effect on our ability to generate revenue. Adverse publicity in the form of published scientific research, statements by regulatory authorities or otherwise, whether or not accurate, that associates consumption of our products or any other similar products with illness or other adverse effects, or that questions the benefits of our or similar products, or that claims that such products are ineffective could have a material adverse effect on our business, reputation, financial condition or results of operations.
If we lose or are unable to obtain key personnel, our business, financial condition or results of operations could be materially adversely affected.
Our success depends to a significant degree upon the continued contributions of our executive officers and other key personnel. Although we have employment agreements with our executive officers, we cannot guarantee that such persons will remain affiliated with us. If any of our key personnel were to cease their affiliation with us, our operating results could suffer. Further, we do not maintain key person life insurance on any of our executive officers. If we lose or are unable to obtain the services of key personnel, our business, financial condition or results of operations could be materially and adversely affected.
If we are unable to effectively manage our growth plan, we could be unable to implement our business strategy.
Our growth plan requires significant management time and operational and financial resources. There is no assurance that we have the operational and financial resources to manage our growth. In addition, rapid growth in our headcount and operations may place a significant strain on our management and our administrative, operational and financial infrastructure. Failure to adequately manage our growth could have a material adverse effect on our business, financial condition or results of operations.
An unexpected interruption or shortage in the supply or significant increase in the cost of raw materials could limit our ability to manufacture our products, which could reduce our sales and our margins.
An unexpected interruption of supply or a significant increase in the cost of raw materials, whether to us or to our contract manufacturers for any reason, such as regulatory requirements, import restrictions, loss of certifications, disruption of distribution channels as a result of weather, terrorism or acts of war, or other events, could result in significant cost increases and/or shortages of our products. Our inability to obtain a sufficient amount of products or to pass through higher cost of products we offer could have a material adverse effect on our business, financial condition or results of operations.
We rely on third-party carriers as part of our inventory fulfillment and order delivery processing, and these third parties may fail to meet shipping schedules or requirements which could limit our ability to manufacture our products, which could reduce our sales and our margins.
We cannot control all of the factors that might affect our timely and cost-effective procurement of products from our vendors and delivery of our products to our customers. We rely on third-party carriers both for the delivery of raw materials and inventory and for the shipment of our products to our customers. Consequently, we are subject to risks of these carriers, including increased fuel costs, security concerns, labor disputes, union organizing activity and inclement weather. Any disruption in the ability of these carriers to timely deliver raw materials to us and products to our customers could damage our reputation and brand and result in customer dissatisfaction. This could, in turn, materially and adversely affect our business, financial condition or results of operations.
The current global economic climate could adversely affect our industry and, therefore, restrict our future growth.
The current global economic climate could negatively affect our sales because many consumers consider the purchase of our products discretionary. If the markets for our products significantly deteriorate due to the economic climate, our business, financial condition or results of operations could be materially and adversely affected.
Instability in financial markets could adversely affect our ability to access capital markets which could limit our ability to fund our operating costs if we do not generate sufficient cash from operations.
As a result of current economic conditions, the availability of capital has been severely restricted. From time to time, we may access debt or equity capital markets. Any restriction on our ability to access capital markets could limit our ability to pursue our growth strategy and could materially adversely affect our business, financial condition or results of operations.
We depend upon certain third-party suppliers and manufacturers; if these suppliers or manufacturers do not provide us materials or products when and as needed and we are unable to efficiently obtain alternative supply sources, we could be unable to manufacture our products, and our business, financial condition or results of operations may be materially adversely affected.
| 12 |
We rely upon third-party suppliers for certain ingredients and raw materials. The principal ingredients or raw materials required in our operations are vitamins, minerals, herbs and packaging components. We purchase these materials from third-party suppliers located in the U.S., Japan, China, India, Italy, Spain, France and Germany. Furthermore, although we manufacture many of our proprietary products in-house, we engage third-party manufacturers to produce our proprietary products that are in the form of soft-gels, liquids and powders. Disruption in the operations of any such third-party supplier or manufacturer can occur for a number of reasons, many of which are beyond our control, such as regulatory requirements, import restrictions, loss of certifications, power interruptions, fires, hurricanes, drought or other climate-related events, war or other events. If any of our third-party suppliers or manufacturers become unable or unwilling to continue to provide us supplies or products in the required volumes and quality levels or in a timely manner, we would be required to identify and obtain acceptable replacement supply or product sources. If we are unable to efficiently obtain alternative sources, our business, financial condition or results of operations may be materially adversely affected.
The content of our website and direct mailing pieces could expose us to significant liability which could reduce our profits.
Because we post product information and other content on our website and in our direct mailing pieces, we face potential liability for, among other things, copyright infringement, patent infringement, trademark infringement, defamation, unauthorized practice of medicine, false or misleading advertising and other claims based on the nature and content of the materials we post. Although we maintain general liability insurance, our insurance may not cover potential claims of this type or may not be adequate to indemnify us for all liability that may be imposed. Any imposition of liability that is not covered by insurance, or is in excess of insurance coverage, could materially adversely affect our business, financial condition or results of operations.
We depend primarily upon search engines and other online sources to increase traffic to our website, and need to convert this traffic into customers in a cost-effective manner; our failure to do so could reduce our sales.
Our success depends on our ability to attract visitors to our website and convert them into customers in a cost-effective manner. We utilize search engines and other online sources as a means to direct traffic to our website. Our website is included in search results as a result of both paid search listings, where we purchase specific search terms that result in the inclusion of our website in the search result, and algorithmic searches that depend upon the searchable content in our website. Search engines and other online sources revise their algorithms from time to time in an attempt to optimize their search results.
If one or more of the search engines or other online sources which we use to direct traffic to our website were to modify its general methodology for how it displays our website, fewer visitors may visit our website, which could have a material adverse effect on our business and results of operations. Further, if any free search engine which we use to direct traffic to our website begins charging fees for listing or placement, or if one or more of the search engines or other online sources on which we rely for purchased listings, modifies or terminates its relationship with us, the traffic to our website could decrease and our expenses could increase which could have a material adverse effect on our business, financial condition or results of operations.
We could be harmed by data loss or other security breaches.
Our servers are vulnerable to computer viruses, physical or electronic break-ins and similar disruptions, which could lead to interruptions and delays in our service and operations as well as loss, misuse or theft of data. Any attempts by hackers to disrupt our service or our internal systems, if successful, could harm our business, be expensive to remedy and damage our reputation. Although we have developed systems and processes that are designed to protect customer information and prevent data loss and other security breaches, such measures cannot provide absolute security. In addition, we rely on third party technology and systems in certain aspects of our businesses, including encryption and authentication technology to securely transmit confidential information. Any significant disruption to our service or internal computer systems could adversely affect our business and results of operations.
We may not be able to maintain our domain name, which may result in confusion to existing and new customers and lost sales and, therefore, could have a material adverse effect on our business, financial condition or results of operations.
Maintaining our Internet domain name is critical to our success. Under current domain name registration practices, no other entity may obtain an identical domain name but can obtain a similar or identical name with a different suffix, such as “.net” or “.org,” or with a different country designation, such as “.jp” for Japan.
We have not registered our domain name with each of the suffixes or jurisdictions available. As a result, third parties may use domain names that are similar to our domain name, which may result in confusion to existing and new customers and lost sales. Failure to maintain our domain name’s uniqueness could have a material adverse effect on our business, financial condition or results of operations.
If we experience product recalls, we may incur significant and unexpected costs and damage to our reputation and, therefore, could have a material adverse effect on our business, financial condition or results of operations.
We may be subject to product recalls, withdrawals or seizures if any of the products we formulate, manufacture or sell are believed to cause injury or illness or if we are alleged to have violated governmental regulations in the manufacture, labeling, promotion, sale or distribution of our products. A recall, withdrawal or seizure of any of our products could materially and adversely affect consumer confidence in our brands and lead to decreased demand for our products. In addition, a recall, withdrawal or seizure of any of our products would require significant management attention, would likely result in substantial and unexpected expenditures and could materially adversely affect our business, financial condition or results of operations.
Our inability to safely and efficiently conduct manufacturing operations or comply with health and safety regulations could materially and adversely affect our business, financial condition or results of operations.
Manufacturing a significant portion of our products at our manufacturing facility in Lexington, North Carolina concentrates our risk in the event there is any significant disruption in our operations or shutdown of this facility. Further, our operations are subject to environmental and health and safety laws and regulations, and some of our operations require environmental permits and controls to prevent and limit pollution of the environment. Any disruptions in our manufacturing operations would have a material adverse effect on our business, financial condition or results of operations and we could incur significant costs as a result of violations of, or liabilities under, environmental laws and regulations, or to maintain compliance with such environmental laws, regulations, or permit requirements.
| 13 |
Complying with new and existing government regulation, both in the U.S. and abroad, could significantly increase our costs and limit our ability to manufacture our products.
The processing, formulation, manufacturing, packaging, labeling, advertising, distribution and sale of our products are subject to regulation by several U.S. federal agencies, including the FDA, the FTC, the Postal Service, the Consumer Product Safety Commission, the Department of Agriculture and the Environmental Protection Agency, as well as various state, local and international laws and agencies of the localities in which our products are sold. Government regulations may prevent or delay the introduction or require the reformulation of our products.
The FDA regulates, among other things, the manufacture, composition, safety, labeling, marketing and distribution of dietary supplements (including vitamins, minerals, herbs, and other dietary ingredients for human use), OTC drugs (allopathic and homeopathic), conventional foods, infant formulas, animal foods, medical devices, and cosmetics. The FDA may not accept the evidence of safety we present for new dietary supplements we wish to market, or they may determine that a particular dietary supplement or ingredient that we currently market presents an unacceptable health risk. If that occurs, we could be required to cease distribution of and/or recall supplements or products containing that ingredient. In July 2011 the FDA introduced a draft Guidance Document called “Guidance for Industry: Dietary Supplements: New Dietary Ingredient Notifications and Related Issues” relating to pre-market safety notifications. In it FDA identified standards of proof for safety and ‘grandfathering’ that would impose new burdens on the dietary supplement industry and may impact the legality of some of our existing products. FDA has accepted industry comments on the guidance but has not yet published its response or otherwise finalized the draft.
The FDA may also determine that certain advertising and promotional claims, statements or activities are not in compliance with applicable laws and regulations and may determine that a particular statement is an unacceptable drug claim or an unauthorized version of a food or dietary supplement “health claim.” Failure to comply with FDA or other regulatory requirements could prevent us from marketing particular dietary supplement products or subject us to administrative, civil or criminal penalties.
Legislation has been introduced in the U.S. Senate seeking to provide the FDA with increased authority to regulate dietary supplements and increase labeling requirements with respect to dietary supplements.
The FTC exercises jurisdiction over the advertising of dietary supplements and has instituted numerous enforcement actions against dietary supplement companies for failing to have adequate substantiation for claims made in advertising or for using false or misleading advertising claims. The FTC routinely polices the market for deceptive dietary supplement advertising and accepts and reviews complaints from the public concerning such advertising.
The FTC also regulates deceptive advertising claims and promotional offers of savings compared to “regular” prices. The National Advertising Division (“NAD”), of the Council of Better Business Bureaus oversees an industry-sponsored self-regulatory system that permits competitors to resolve disputes over advertising claims, including promotions for savings off of regular prices. The NAD has no enforcement authority of its own but may refer promotions to the FTC that the NAD views as violating FTC guidelines or rules. Violations of these orders could result in substantial monetary penalties.
In Europe, non-compliance by us or others of relevant legislation can result in regulators bringing administrative or, in some cases, criminal proceedings. For example, in the U.K., it is common for regulators, including the Medicines and Healthcare Products Regulatory Agency Enforcement & Intelligence Group, to prosecute retailers and manufacturers for non-compliance with legislation governing foodstuffs and medicines. European Union (EU) regulations and directives are implemented and enforced by individual member states and, so, enforcement priorities and applicable law can occur in multiple countries at one time. Failure by us, the manufacturers or suppliers to comply with applicable legislation could result in prosecution and have a material adverse effect on our business, financial condition and results of operations.
Europe has adopted broad regulations and directives on health and nutrition claims. These regulations cover claims that can be made for foods (including supplements). Certain claims, such as those regarding general well-being, behavioral functions and weight-loss, may be prohibited or require prior approval. Unless subject to derogation, products that include certain claims cannot be lawfully marketed in EU member states absent preapproval. Applicable derogations under EU directives can enlarge the period within which we may seek approval for products containing claims. An approval must proceed through the European Food Safety Authority (EFSA), and the process includes the submission of a detailed dossier in support of the product claims. Lengthy delays within the new EU framework have been reported. This may severely impact our European marketing and expansion efforts. We are also anticipating the implementation of an EU directive relating to food supplements, which could significantly impact the formulation of our products. The implementation of this directive is expected to include dosage restrictions for certain vitamin and mineral supplements and may lead to some of our products being recalled or discontinued.
In addition, an EU Directive governing product safety requires manufacturers to notify regulators about unsafe products and gives regulators in each member state the power to order product recalls. As a result, the number of product recalls in Europe has increased substantially. A product recall in Europe could have a material adverse effect on our business, financial condition and results of operations.
| 14 |
The Controlling the Assault of Non-Solicited Pornography and Marketing Act of 2003 imposes certain obligations on the senders of commercial emails, which could minimize the effectiveness of our email marketing campaign, and establishes financial penalties for non-compliance, which could increase the costs of our business and, could have a material adverse effect on our business, financial condition or results of operations.
In December 2003, the Controlling the Assault of Non-Solicited Pornography and Marketing Act of 2003, or the CAN-SPAM Act, was enacted. The CAN-SPAM Act establishes certain requirements for commercial email messages and penalizes commercial email message transmissions that are intended to deceive the recipient as to source or content. The CAN-SPAM Act, among other things, requires that senders of commercial emails allow recipients to opt out of receiving future emails from the sender. The ability of our customers to opt out of receiving commercial emails may minimize the effectiveness of our email marketing campaign. Moreover, non-compliance with the CAN-SPAM Act carries significant financial penalties. If we were found to be in violation of the CAN-SPAM Act, applicable state laws not preempted by the CAN-SPAM Act, or foreign laws regulating the distribution of commercial email, we could be required to pay penalties, which could have a material adverse effect on our business, result of operations, financial condition and cash flows.
Taxation risks could subject us to liability for past sales, increase our costs and cause our future sales to decrease.
We do not collect sales or other taxes on shipments of most of our goods into most states in the U.S. Currently, U.S. Supreme Court decisions restrict the imposition of obligations to collect state and local sales and use taxes with respect to sales made over the Internet. However, a number of states, as well as the U.S. Congress, have been considering initiatives that could limit or supersede the Supreme Court’s position regarding sales and use taxes on Internet sales. If any of these initiatives were successful, we could be required to collect sales and use taxes in additional states. The imposition by state and local governments of various taxes upon Internet commerce could create administrative burdens for us, reduce our competitive advantage over traditional retailers and decrease our future sales. Our warehousing and expected manufacturing centers, and any future expansion of them, along with other aspects of our evolving business, may result in additional sales and other tax obligations.
One or more states or foreign countries may seek to impose sales or other tax collection obligations on out-of-jurisdiction eCommerce companies. Effective June 2008, New York imposed such a sales tax obligation requirement on online retailers that use New York residents to directly or indirectly refer potential customers, via a link on an Internet website or otherwise, to the online retailer.
A successful assertion by one or more states or foreign countries that we should collect sales or other taxes on the sale of merchandise or services could result in substantial tax liabilities for past sales, decrease our ability to compete with traditional retailers and otherwise harm our business, financial condition or results of operations.
Unfavorable changes to government regulations, service interruptions or adverse consumer attitudes about online commerce could have a material adverse effect on our business, financial condition or results of operation by impeding the growth and use of the Internet and thereby decreasing revenue.
As the role and importance of online commerce has grown in the U.S., there have been continuing efforts to increase the legal and regulatory obligations and restrictions on companies conducting commerce through the Internet, primarily in the areas of taxation, consumer privacy, restrictions on imports and exports, customs, tariffs, user privacy, data protection, pricing, content, copyrights, distribution, electronic contracts and other communications, consumer protection, the provision of online payment services, broadband residential Internet access and the characteristics and quality of products and services, which could increase the cost of conducting business over the Internet. In addition, consumer unwillingness or inability to use the Internet to conduct business, due to adverse regulation, security concerns, service interruptions or otherwise, could materially reduce our growth. Governmental laws and regulations, service interruptions or adverse attitudes about online commerce could increase the costs and liabilities associated with our online commerce activities, increase the price of our product to consumers, or reduce traffic to our website. Unfavorable resolution of these issues could have a material adverse effect on our business, financial condition or results of operations.
We are subject to a number of risks related to credit card payments we accept which, if we fail to be in compliance with applicable credit card rules and regulations, will result in additional fees, fines and ultimately the revocation of the right to use the credit card company, which could have a material adverse effect on our business, financial condition or results of operations.
For credit and debit card payments, we pay interchange and other fees, which may increase over time and raise our operating costs and lower our profit margins. We are also subject to payment card association operating rules, certification requirements and rules governing electronic funds transfers, which could change or be reinterpreted to make it difficult or impossible for us to comply. If we fail to comply with these rules or requirements, we may be subject to fines and higher transaction fees and lose our ability to accept credit and debit card payments from our customers. In addition, we have and may continue to suffer losses as a result of orders placed with fraudulent credit and debit card data. We do not carry insurance against the risk of credit card fraud, so the failure to adequately control fraudulent credit card transactions could reduce our net revenue and our gross profit percentage. We have implemented technology to help us detect the fraudulent use of credit card information. Under current practices, a merchant is liable for fraudulent credit card transactions when the merchant does not obtain a cardholder’s signature. A failure to adequately control fraudulent credit card transactions would result in significantly higher credit card-related costs and could have a material adverse effect on our business, financial condition or results of operations.
| 15 |
We may incur significant costs to protect our customers’ personal information, and may incur liability if such personal information is misappropriated, which could increase our costs, harm our reputation and reduce our sales.
If our customers’ personal or credit card information is misappropriated by us or third parties that breach our network security, we could be subject to liability. This liability could include claims for unauthorized purchases with credit card information, impersonation or other similar fraud claims or damages for alleged violations of state or federal laws governing security protocols for the safekeeping of customers’ personal or credit card information. This liability could also include claims for other misuses of personal information, including unauthorized marketing purposes. These claims could result in litigation against us. Liability for misappropriation of this information could materially adversely affect our business, financial condition or operating results. In addition, the FTC and state agencies have been investigating various Internet companies regarding their use of customers’ personal information. We could incur additional expenses if new regulations regarding the use of personal information are introduced or if government agencies investigate our privacy practices.
We rely on encryption and authentication technology licensed from third parties to provide the security and authentication necessary to effect secure transmission of confidential information such as customer credit card numbers. We cannot provide assurance that advances in computer capabilities, new discoveries in the field of cryptography or other events or developments will not result in a compromise or breach of the algorithms that we use to protect our customers’ transaction data. If any such compromise of our security were to occur, it could harm our reputation, business, prospects, financial condition and results of operations. A party who is able to circumvent our security measures could misappropriate proprietary information or cause interruptions in our operations. We may be required to expend significant capital and other resources to protect against such security breaches or to resolve problems caused by such breaches. We cannot assure you that our security measures will prevent security breaches or that failure to prevent such security breaches will not harm our business, financial condition or results of operations.
Laws or regulations relating to privacy and data protection may materially adversely affect the growth of our Internet business or our marketing efforts, which could reduce our sales and cause us to incur significant costs.
We are subject to increasing regulation at the federal, state and international levels relating to privacy and the use of personal user information. For example, we are subject to various telemarketing laws that regulate the manner in which we may solicit future suppliers and customers. Such regulations, along with increased governmental or private enforcement, may increase the cost of growing our business. In addition, many jurisdictions have laws that limit the use of personal information gathered online or offline or require companies to establish privacy policies. The FTC has adopted regulations regarding the collection and use of personal identifying information obtained from children under thirteen years of age. Proposed legislation in this country and existing laws in foreign countries require companies to establish procedures to notify users of privacy and security policies, obtain consent from users for the collection and use of personal information, and/or provide users with the ability to access, correct and delete personal information stored by us. From time to time, Congress has proposed legislation regarding data security and privacy protection. Any enacted data protection regulations may restrict our ability to collect demographic and personal information, which could be costly or harm our marketing efforts, and could require us to implement new and potentially costly processes, procedures and/or protective measures.
Our failure to protect our intellectual property rights could reduce our sales and increase our costs.
We have applied for or registered all relevant trademarks with the U.S. Patent and Trademark Office, including our Vitacost, Nutraceutical Sciences Institute, Cosmeceutical Sciences Institute, Best of All, Walker Diet, Smart Basics, OcuPower, CardioLift, NeuroPower, ArthriPower and Mega EFA trademarks, among others. We believe our trademarks to be valuable and are identified strongly with our brands. Issuance of a federally registered trademark creates a rebuttable presumption of ownership of the mark; however, it is subject to challenge by others claiming first use in the mark in some or all of the areas in which it is used. We have also applied for foreign protection of certain of our trademarks in the European and Asian markets in which we operate and have registered Vitacost, NSI and Nutraceutical Sciences Institute in certain countries in these regions.
Federally registered trademarks have a perpetual life, as long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. We believe our patents and trademarks are valuable and provide us certain benefits in marketing our products. We intend to actively protect our patents, trademarks, trade secrets and other intellectual property.
We may in the future be subject to intellectual property litigation and infringement claims, which could cause us to incur significant expenses, be involved in protracted litigation or prevent us from manufacturing, selling or using some aspect of our products. Claims of intellectual property infringement may also require us to enter into costly royalty or license agreements. Alternatively, we may be unable to obtain necessary royalty or license agreements on terms acceptable to us, if at all. Claims that our technology or products infringe on intellectual property rights of others could be costly and would divert the attention of our management and key personnel, which in turn could materially adversely affect our business, financial condition or results of operations.
| 16 |
Third parties could use our trademarks as keywords in Internet search engine advertising programs, which may direct potential customers to competitors’ websites, which, in turn, could result in decreased sales and could harm our reputation.
Competitors and other third parties could purchase our trademarks and confusingly similar terms as keywords in Internet search engine advertising programs and in the resulting sponsored link advertisements which may divert potential customers to their websites. Preventing such unauthorized use is difficult. Further, the legal precedent on whether such activity infringes on our intellectual property varies significantly within the United States and in other countries. If we are unable to protect our trademarks or confusingly similar terms from such unauthorized use, competitors and other third parties could drive potential online customers away from our website, which could result in a loss of sales and have a material adverse effect on our business, financial condition or results of operations.
We operate in a highly competitive industry, and our failure to compete effectively could materially adversely affect our market share, financial condition and growth prospects.
The U.S. health products industry is a large and highly fragmented industry. Our competitors include specialty retailers, supermarkets, drugstores, mass merchants, multi-level marketing organizations, online merchants, mail-order companies and a variety of other participants in the industry. The principal elements of competition in the industry are price, selection and distribution channel offerings. We believe that the market is also highly sensitive to the introduction of new products, including various prescription drugs, which may rapidly capture a significant share of the market. In the United States, we also compete for sales with heavily advertised national brands manufactured by large pharmaceutical and food companies, as well as other retailers. In addition, as some products gain market acceptance, we experience increased competition for those products as more participants enter the market. Our manufacturing operations compete with manufacturers of third-party nutritional supplements. Certain of our competitors are larger than us and have longer operating histories, larger customer bases, greater brand recognition and greater resources for marketing, advertising and product promotion. They may be able to secure inventory from vendors on more favorable terms, operate with a lower cost structure or adopt more aggressive pricing policies. In addition, our competitors may be more effective and efficient in introducing new products. We may not be able to compete effectively, and our attempt to do so may require us to increase marketing and/or reduce our prices, which may result in lower margins. Failure to effectively compete could materially adversely affect our market share, financial condition and growth prospects.
Our failure to efficiently respond to changing consumer preferences and demand for new products and services could significantly harm our product sales, inventory management and customer relationships and our business, results of operations and financial condition could be materially adversely affected.
Our continued success depends, in part, on our ability to anticipate and respond to changing consumer trends and preferences. We may not be able to respond in a timely or commercially appropriate manner to these changes. Our failure to accurately predict these trends could negatively impact our inventory levels, sales and consumer opinion of us as a source for the latest products. The success of our new product offerings depends upon a number of factors, including our ability to:
| • | accurately anticipate customer needs, |
| • | innovate and develop new products, |
| • | successfully commercialize new products in a timely manner, |
| • | competitively price our products, |
| • | procure and maintain products in sufficient volumes and in a timely manner; and, |
| • | differentiate our product offerings from those of our competitors. |
If we do not introduce new products, make enhancements to existing products or maintain the appropriate inventory levels to meet customers’ demand in a timely manner, our business, results of operations and financial condition could be materially adversely affected.
Insurance coverage, even where available, may not be sufficient to cover losses we may incur, which could increase our costs and lower our profits.
Our business exposes us to the risk of liabilities arising out of our products and operations. For example, we may be liable for claims brought by users of our products or by employees, customers or other third parties for personal injury or property damage occurring in the course of our operations. Our operations are subject to closure and loss due to power outages, Internet and telephone line failures, work stoppages and acts of nature. We seek to minimize these risks through various insurance policies from third-party insurance carriers. However, our insurance coverage is subject to large individual claim deductibles, individual claim and aggregate policy limits and other terms and conditions. Our estimate of retained-insurance liabilities is subject to change as new events or circumstances develop that might materially impact the ultimate cost to settle these losses. We cannot assure you that our insurance will be sufficient to cover our losses. We do not view insurance, by itself, as a material mitigant to these business risks. Any losses that are not completely covered by our insurance could have a material adverse effect on our business, financial condition or results of operations.
| 17 |
The insurance industry has become more selective in offering certain types of liability insurance coverage, and we may not be able to maintain our existing coverage or obtain increased coverage in the future, which could increase our costs and reduce our profits.
The insurance industry has become more selective in offering certain types of insurance, including product liability, product recall and property casualty insurance. While we believe our current insurance policies provide us adequate coverage for our current business operations, there can be no assurance that we will be able to maintain such coverage or obtain comparable coverage on terms and conditions favorable to us, if at all. Further, as we expand our business, we expect to correspondingly increase our insurance coverage, and there can be no assurance that we will be able to obtain such increased coverage if and when needed.
Our manufacturing operations are located in a single location and our inventory is concentrated in two warehouse locations, which exposes us to the risk of natural disasters or other force majeure events. Losses at either location could materially adversely affect our manufacturing operations, product distributions, sales and consumer satisfaction.
We house our manufacturing operations and one of our two distribution warehouses at a facility located in Lexington, North Carolina. We also operate a distribution and warehouse facility located in Las Vegas, Nevada. Any significant disruption in either of these locations for any reason, such as a power failure, equipment breakdown, workforce disruption, or natural or similar disasters, could materially adversely affect our manufacturing operations, product distributions, sales and consumer satisfaction.
Our quarterly results of operations may fluctuate in the future. As a result, we may fail to meet or exceed the expectations of investors or securities analysts, which could cause our stock price to decline.
Our quarterly revenue and results of operations may fluctuate as a result of a variety of factors, many of which are outside of our control. If our quarterly revenue or results of operations fall below the expectations of investors or securities analysts, the price of our common stock could decline substantially. Fluctuations in our results of operations may be due to a number of factors, including, but not limited to, those listed below and identified throughout this “Risk Factors” section in this Annual Report:
| • | our ability to retain and increase sales to existing customers and attract new customers; |
| • | changes in the volume and mix of dietary supplements and health and wellness products sold in a particular quarter; |
| • | the timing and success of new dietary supplement introductions or reformulations by us or our competitors; |
| • | changes in our pricing policies or those of our competitors; |
| • | competition, including entry into the market by new competitors including traditional brick and mortar retailers and new product offerings by existing competitors; |
| • | the amount and timing of expenditures related to expanding our operations, research and development or introducing new products; |
| • | changes in the payment terms for our products and services; and |
| • | the purchasing cycles of our customers. |
Most of our expenses are relatively fixed in the short-term, and our expense levels are based in part on our expectations regarding future revenue levels. As a result, if revenue for a particular quarter is below our expectations, we may not be able to proportionally reduce operating expenses for that quarter, causing a disproportionate effect on our expected results of operations for that quarter.
If we are unable to sufficiently increase our revenue to offset increased costs as we expand our business, we may experience operating losses, net losses or negative cash flows.
We expect operating expenses and working capital requirements to increase substantially as we expand our business. We expect our costs of product development, sales and marketing, research and development, manufacturing and general and administrative expenses to increase substantially as a result of our growth. If we are unable to continue to sufficiently increase our revenue to offset these increased costs, we will not maintain profitability and may experience operating losses, net losses or negative cash flows.
Healthcare reform measures and related changes in applicable federal and state laws could materially adversely affect our business.
The efforts of governmental and third-party payers to contain or reduce the costs of healthcare may adversely affect our business, operating results, and financial condition. As part of the 2010 U.S. health care reform initiative, non-prescription products are no longer eligible for purchase using flexible spending accounts. We expect that there will continue to be a number of legislative and regulatory proposals aimed at changing the healthcare system, which could include restructuring the tax benefits available through flexible spending accounts and/or health savings accounts. If the laws or regulations are changed to limit further the tax benefits through these accounts, such a change could have a material adverse effect on our business by reducing revenues and eroding our margins.
Certain stockholders own a significant amount of our common stock, which could discourage an acquisition of Vitacost.com or make removal of incumbent management more difficult.
Great Hill Investors, LLC, Great Hill Equity Partners III, L.P. and Great Hill Equity Partners IV, L.P. (collectively, “GHP”) beneficially own approximately 19.16% of our outstanding stock. Because GHP owns a significant percentage of our capital stock, it could significantly influence all matters requiring approval by our stockholders, including the election of directors and the approval of mergers or other business combination transactions. Furthermore, because of the substantial equity stake in Vitacost.com, potential acquirers could decide not to merge with, or acquire us.
| 18 |
Risks Related to Our Common Stock
If we are unable to comply with rules and regulations of the NASDAQ Stock Market, our common stock could be delisted from NASDAQ.
On December 7, 2010, trading in our common stock was temporarily suspended by NASDAQ for failure to comply with rules and regulations relating to the timely filing of SEC reports. On December 21, 2010, we received a letter from The NASDAQ Stock Market indicating that based on its review of the Company, the NASDAQ staff had determined that continued listing of our securities on NASDAQ was no longer warranted. In accordance with the procedures set forth in the NASDAQ Listing Rules, we timely appealed the staff determination, and requested a hearing before a NASDAQ Hearings Panel (the “Panel”). The hearing was held on February 3, 2011. On February 28, 2011, we received a written notice from NASDAQ indicating that the Panel had determined to grant our request to remain listed on NASDAQ, subject to certain conditions, including that (1) on or before June 20, 2011, we become current in our filing obligations, and demonstrated compliance with all quantitative requirements for continued listing, and (2) on or before July 5, 2011, we have solicited proxies and held our annual meeting. On June 20, 2011, trading of our securities on NASDAQ resumed. On July 6, 2011, we received a letter from NASDAQ indicating that we had met the requirements of the Panel’s decision and the applicable listing requirements and that accordingly the Panel had determined to continue listing our securities on the NASDAQ Stock Market. If we are unable to comply with the NASDAQ requirements in the future, our common stock could again be suspended from trading or be delisted from NASDAQ. A trading suspension or delisting of our common stock could negatively impact us by reducing the liquidity and market price of our common stock and the number of investors willing to hold or acquire our common stock, which could negatively impact our stock price and ability to raise equity financing.
Volatility of our stock price could materially adversely affect an investment in our common stock.
The market price of our common stock could fluctuate significantly as a result of, among other things:
| • | quarterly and annual variations in our operating results, |
| • | the level and quality of research analyst coverage for our common stock, changes in financial estimates or investment recommendations by securities analysts following our business or failure to meet such estimates, |
| • | the financial disclosure we may provide to the public, any changes in such disclosure or our failure to meet such disclosure, |
| • | the public’s response to our press releases, our other public announcements and our filings with the Securities and Exchange Commission, |
| • | various market factors or perceived market factors, including rumors, whether or not correct, involving us, our customers, our suppliers or our competitors, |
| • | changes in accounting standards, policies, guidance, interpretations or principles, |
| • | sales of common stock by our directors, officers or significant stockholders, |
| • | introductions of new products or new pricing policies by us or by our competitors, |
| • | recruitment or departure of key personnel, |
| • | developments with respect to intellectual property rights, |
| • | acquisitions or strategic alliances by us or our competitors, |
| • | changes in shipping costs, |
| • | short sales, hedging and other derivative transactions in shares of our common stock, |
| • | the operating and stock price performance of other companies that investors may deem comparable to us, |
| • | broad market conditions and trends in the eCommerce industry and the economy as a whole; and, |
| • | other events or factors, including those resulting from war, incidents of terrorism or responses to such events. |
Fluctuations in the price of our common stock could contribute to the loss of all or part of a stockholder’s investment. Any of the factors listed above could have a material adverse effect on an investment in our common stock. In addition, stocks of Internet-related and eCommerce companies have historically experienced significant price and volume fluctuations that may have been unrelated or disproportionate to these companies’ operating performance. Public announcements by us or other such companies concerning, among other things, performance, accounting practices or legal problems could cause the market price of our common stock to decline regardless of our actual operating performance. We could be the subject of securities class action litigation due to future stock price volatility, which could divert management’s attention and materially adversely affect our results of operations.
| 19 |
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties.
We conduct our operations through three facilities:
| • | We lease 35,600 square feet of office space in Boca Raton, Florida which we use as our corporate headquarters. |
| • | We own a 224,000 square foot manufacturing and distribution facility located on 27 acres in Lexington, North Carolina. Our call center, east coast distribution center and manufacturing functions are conducted at this location. This facility includes approximately 12,000 square feet of office space and 53,000 square feet of manufacturing space. |
| • | We lease approximately 155,000 square feet in Las Vegas, Nevada that serves as our west coast distribution facility. |
See Note 7 of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for more information about our lease commitments.
Item 3. Legal Proceedings.
Refer to Note 11, Contingencies, of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for a discussion on the nature of the legal proceedings against us, which is incorporated herein by reference.
Item 4. Mine Safety Disclosures.
Not applicable.
| 20 |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters, and Issuer Purchases of Equity Securities.
Our common stock, par value $0.00001 per share, is listed on The NASDAQ Global Market under the symbol VITC and has been included for listing thereon since September 24, 2009. As previously reported, the NASDAQ halted trading of our stock from December 7, 2010 through June 17, 2011. Accordingly, the sales prices per share of our common stock during those periods reflect the closing price on December 7, 2010 of $5.70 a share.
The following table sets forth for the indicated periods the high and low sales prices per share for our common stock on the NASDAQ Global Market:
| High | Low | |||||||
| Year Ended December 31, 2011: | ||||||||
| Fourth quarter | $ | 6.60 | $ | 4.66 | ||||
| Third quarter | $ | 5.72 | $ | 4.33 | ||||
| Second quarter | $ | 5.70 | $ | 3.41 | ||||
| First quarter | $ | 5.70 | $ | 5.70 | ||||
| Year Ended December 31, 2010: | ||||||||
| Fourth quarter | $ | 6.94 | $ | 5.70 | ||||
| Third quarter | $ | 10.23 | $ | 5.60 | ||||
| Second quarter | $ | 13.19 | $ | 8.34 | ||||
| First quarter | $ | 13.02 | $ | 8.76 | ||||
Holders of Record
At March 12, 2012, our common stock was held by approximately 57 holders of record.
Dividends
We have never declared or paid any dividends on our common stock, and our Board of Directors does not contemplate doing so in the near future. Any decisions as to future payment of dividends will depend on our earnings and financial position and such other factors, as our Board of Directors deems relevant.
Equity Compensation Plan Information
The following is a summary of our equity compensation plans as of December 31, 2011:
| Number of Securities to Be Issued Upon Exercise of Outstanding Options, Warrants, and Rights | Weighted Average Exercise Price of Outstanding Options, Warrants, and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans | ||||||||||
| Equity compensation plans approved by security holders | 4,262,230 | $ | 5.69 | 6,531,514 | ||||||||
| Equity compensation plans not approved by security holders | - | $ | - | - | ||||||||
Additional information about our equity compensation plans is incorporated by reference from the Notes to our Consolidated Financial Statements included in this Form 10-K.
| 21 |
Performance Graph
The graph set forth below compares the cumulative total stockholder return on our common stock between September 24, 2009 and December 31, 2011, with the cumulative total return of the (i) Russell 2000 Index, (ii) the NASDAQ Internet Index and (iii) a custom selected Peer Group Index. This graph assumes the investment of $100 on September 24, 2009 in our common stock, the Russell 2000 Index, the NASDAQ Internet Index, and a custom selected Peer Group Index. The custom Peer Group Index consists of the following companies: Blue Nile, Shutterfly, Vitamin Shoppe, Overstock.com, Herbalife Ltd., 1-800-FLOWERS.COM, Nu Skin Enterprises, Whole Foods Market, US Auto Parts, GNC Holdings, Stamps.com, USANA, Pet Meds and NutriSystem. As previously reported, the NASDAQ halted trading of our stock from December 7, 2010 through June 17, 2011. Note that historic stock price performance is not necessarily indicative of future stock price performance.
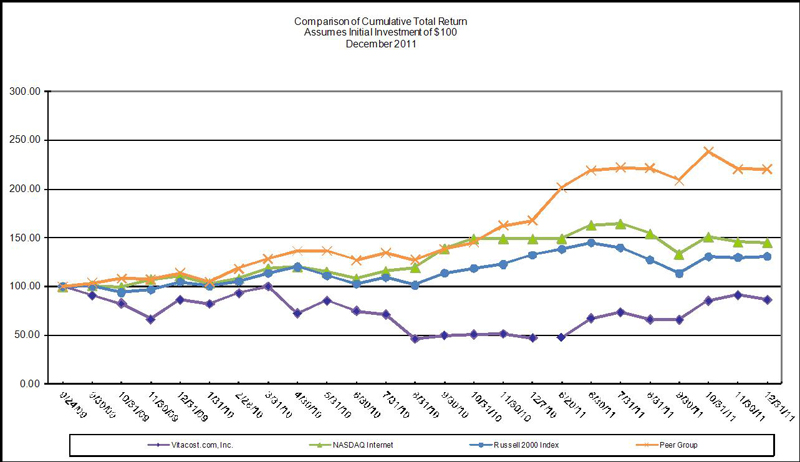
| 22 |
Item 6. Selected Financial Data
The selected consolidated financial data presented below has been derived from our consolidated financial statements. The statement of operations data for each of the fiscal years ended December 31, 2011, 2010, and 2009 and the balance sheet data at December 31, 2011 and 2010 are derived from our audited financial statements that are included in this annual report. The statement of operations data for the year ended December 31, 2008 and 2007 and the balance sheet data at December 31, 2009, 2008, and 2007 are derived from audited 2009, 2008 and 2007 financial statements that are not included in this annual report. The historical results presented below are not necessarily indicative of the results to be expected in any future period.
This information should be read in connection with Item 7 — Management’s Discussion and Analysis of Financial Condition and Results of Operations and our financial statements and the related notes included elsewhere in this annual report.
| Year Ended December 31, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (in thousands, except shares and per share data) | ||||||||||||||||||||
| Consolidated Statement of Operations Data: | ||||||||||||||||||||
| Net sales | $ | 260,523 | $ | 220,680 | $ | 191,807 | $ | 143,602 | $ | 99,290 | ||||||||||
| Cost of goods sold | 200,597 | 164,206 | 130,605 | 105,529 | 70,381 | |||||||||||||||
| Gross profit | 59,926 | 56,475 | 61,202 | 38,073 | 28,909 | |||||||||||||||
| Operating expenses: | ||||||||||||||||||||
| Fulfillment | 22,290 | 17,354 | 8,954 | 8,393 | 6,629 | |||||||||||||||
| Sales and marketing | 22,842 | 18,728 | 14,284 | 13,147 | 10,845 | |||||||||||||||
| General and administrative | 29,620 | 33,989 | 29,083 | 14,871 | 11,116 | |||||||||||||||
| Total operating expenses | 74,752 | 70,070 | 52,321 | 36,411 | 28,590 | |||||||||||||||
| Operating income (loss) | (14,825 | ) | (13,595 | ) | 8,881 | 1,662 | 319 | |||||||||||||
| Interest income (expense), net | 23 | (213 | ) | (401 | ) | (1,150 | ) | (515 | ) | |||||||||||
| Other income (expense), net | 24 | 38 | 250 | (13 | ) | 743 | ||||||||||||||
| Income tax benefit (expense) | (53 | ) | (1,421 | ) | (2,836 | ) | (482 | ) | 1,279 | |||||||||||
| Net income (loss) | $ | (14,831 | ) | $ | (15,191 | ) | $ | 5,894 | $ | 17 | $ | 1,826 | ||||||||
| Net income (loss) per share: | ||||||||||||||||||||
| Basic | $ | (0.53 | ) | $ | (0.55 | ) | $ | 0.24 | $ | - | $ | 0.08 | ||||||||
| Diluted | $ | (0.53 | ) | $ | (0.55 | ) | $ | 0.24 | $ | - | $ | 0.08 | ||||||||
| Weighted average number of shares outstanding | ||||||||||||||||||||
| Basic | 27,829,497 | 27,703,710 | 24,216,942 | 23,188,380 | 23,188,380 | |||||||||||||||
| Diluted | 27,829,497 | 27,703,710 | 24,674,248 | 23,975,068 | 24,087,978 | |||||||||||||||
| Consolidated Balance Sheet Data: | ||||||||||||||||||||
| Cash and cash equivalents | $ | 12,939 | $ | 11,952 | $ | 8,658 | $ | 61 | $ | 1 | ||||||||||
| Inventory | 34,822 | 29,828 | 28,097 | 21,663 | 14,710 | |||||||||||||||
| Working capital (deficit) | 13,201 | 22,377 | 49,599 | (5,990 | ) | (6,535 | ) | |||||||||||||
| Total assets | 90,629 | 99,464 | 107,679 | 46,869 | 36,662 | |||||||||||||||
| Total debt | - | 59 | 9,405 | 18,232 | 12,340 | |||||||||||||||
| Stockholders' equity | 48,807 | 62,186 | 74,500 | 6,965 | 6,153 | |||||||||||||||
| 23 |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
The following discussion and analysis of the Company’s financial condition and results of operations should be read in connection with Item 6, Selected Financial Data and Item 8, Financial Statements and Supplementary Data of this Annual Report on Form 10-K. The Company does not believe that its historical operating results will be indicative of future operating results. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. The Company’s actual results may differ materially from those anticipated in these forward-looking statements as a result of many factors, including but not limited to those under the headings “Risk Factors” and “Forward-Looking Information” of this Annual Report. We undertake no obligation to publicly update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Overview
We are a leading online retailer, based on annual sales volume, of health and wellness products, including dietary supplements such as vitamins, minerals, herbs and other botanicals, amino acids and metabolites (which we refer to as “vitamins and dietary supplements”), as well as cosmetics, natural personal care products, pet products, sports nutrition and health foods. We sell these products directly to consumers primarily through our website, www.vitacost.com. We strive to offer our customers the broadest selection of healthy living products, while providing superior customer service and timely and accurate delivery.
We offer our customers a selection of over 34,000 Stock Keeping Units (“SKUs”) from over 1,600 third-party brands, such as New Chapter, Nature’s Way, Twinlab, Source Naturals, Jarrow Formulas, Jason, Desert Essence, Atkins, Bob’s Red Mill, BSN, Optimum Nutrition, USP Labs and MuscleTech in addition to our own proprietary brands, Cosmeceutical Sciences Institute (“CSI”), Best of All, and Smart Basics. Beginning in March 2011 we began transitioning our largest proprietary brand, Nutraceutical Sciences Institute (“NSI”), to a new Vitacost brand and completed the majority of this transition by year-end 2011. We support our operations through our contact center, our manufacturing and testing facility and our two distribution centers, delivering what we believe are industry-leading customer satisfaction results. Our website allows customers to easily browse and typically purchase products at prices lower than manufacturers’ suggested retail prices. Our website also serves as an educational resource for consumers seeking information on healthy living and the attributes of health and wellness supplements.
Our success is driven primarily by our ability to attract new customers and grow our product offerings. Our customers are typically individuals seeking value in their purchases of health and wellness products. Our active customer base, which we define as customers who have purchased from us within the last twelve months, has steadily increased from approximately 270,000 at the end of 2005 to approximately 1.4 million as of December 31, 2011. On average, our customers make purchases from us two to three times a year and during 2011, approximately 72% of our orders were placed by repeat customers.
We were incorporated in Delaware in May 1994 and began operations as a catalog retailer of third-party vitamins and supplements under the name Nature’s Wealth Company. In 1999, we launched Vitacost.com and introduced our proprietary vitamins and supplements under our NSI brand. In 2000, we began operations under the name Vitacost.com, Inc. In September 2009 the Company completed its Initial Public Offering.
Recent Transactions
On February 16, 2012, we entered into a Purchase Agreement (the “Purchase Agreement”) by and among Vitacost, JHH Capital, LLC (an entity affiliated with Jeffrey Horowitz, our Chief Executive Officer, “JHH”), Great Hill Equity Partners III, L.P. (“Great Hill III”), Great Hill Equity Partners IV, L.P. (“Great Hill IV”), Great Hill Investors, LLC (“Great Hill Investors”), Freshford Partners, LP (“Freshford Partners”), Freshford Master Fund, Ltd (“Freshford Master Fund”) and Baron Small Cap Fund (“Baron” and, together with JHH, Great Hill III, Great Hill IV, Great Hill Investors, Freshford Partners, Freshford Master Fund, collectively, the “Investors”) pursuant to which the Investors purchased, and we sold, an aggregate of 4,920,288 shares of our common stock at a purchase price of $7.04 per share, and warrants to purchase an aggregate of 1,680,601 shares of our common stock for an aggregate purchase price of $34,848,903. The warrants have an exercise price of $7.04 per share and a term of four years. The proceeds from the financing will be used primarily to expand and optimize our distribution network. Of this amount, an estimated $15.0 million will be used to build and stock a third distribution center to support our high level of sales growth. Construction on the new facility is expected to commence in the second half of 2012 with completion expected in the early part of 2013. In addition, an estimated $5.0 million will be used to complete the expansion of our existing distribution center in Lexington, North Carolina and increase storage capacity at our other existing distribution center located in Las Vegas, Nevada. We intend to use the remaining proceeds for general operations.
Trends and Other Factors Affecting Our Business
We continue to experience increased levels of competition as the vitamin and supplement industry shifts towards a greater internet presence. This competitive environment continues to drive margin pressure as deep discounting results from aggressive customer acquisition and retention actions. We continue to respond to the increased levels of competition by expanding our product offerings through the introduction of new and diverse products, improving our customer service and support, improving our reliability and speed of delivery and by adjusting our product mix and pricing.
| 24 |
The vitamin and supplement industry continues to benefit from positive demographic trends, with an aging population, and trends affecting health and lifestyle preferences, with an increasingly health conscious consumer. In addition, the increasing cost of traditional healthcare has helped bolster demand for natural products to help ward off more expensive medical visits and prescription drugs. Changes in these trends and other factors that we may not foresee may also impact our business, including potential regulatory actions by the FDA and the FTC that may affect the viability of a certain products that we offer.
Critical Accounting Policies and Estimates
Our discussion and analysis of our financial condition and results of operations is based upon our consolidated financial statements, which have been prepared in accordance with generally accepted accounting principles of the U.S. (GAAP).
The preparation of these financial statements requires estimates and assumptions that affect the reported amounts of assets and liabilities, revenue and expenses and related disclosures of contingent assets and liabilities in the consolidated financial statements and accompanying notes. Critical accounting policies are those that are the most important to the portrayal of our financial condition and results of operations and require our most difficult, subjective and complex judgments as a result of the need to make estimates about the effect of matters that are inherently uncertain. While our significant accounting policies are described in more detail in the notes to our consolidated financial statements, our most critical accounting policies, discussed below, pertain to revenue recognition, income taxes, stock-based compensation, contingencies, inventories and goodwill. In applying such policies, we exercise our best judgment and best estimates. Actual results may differ from these estimates under different assumptions or conditions. For a further discussion of these Critical Accounting Policies and Estimates, as well as a description of our other significant accounting policies, see Note 1of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K.
Revenue Recognition. Revenue from the sale of vitamins, nutritional supplements and other related products is recognized when all the following criteria are met: a customer executes an order, the sales price and the shipping and handling fee have been determined, credit card authorization has occurred and collection is reasonably assured, and the product has been received by the customer. Our internet sales are made to customers permitting certain limited rights of return for a 30-day period. It has been our experience that such returns have been insignificant.
Income Taxes. Income taxes are computed under an asset and liability approach whereby deferred tax assets and liabilities are recognized based on the difference between the financial statement amounts and the tax basis of assets and liabilities using tax rates in effect for the year in which the differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized as income in the period that includes the enactment date. The Company evaluates its deferred tax assets on a regular basis to determine if valuation allowances are required. In its evaluation, the Company considers taxable loss carryback availability, expectations of sufficient future taxable income, trends in earnings, existence of taxable income in recent years, the future reversal of temporary differences and the available tax planning strategies that could be implemented, if required. Valuation allowances are established based on the consideration of all available evidence using a more likely than not standard. The Company’s deferred tax assets for which it has not established a valuation allowance relate to amounts than can be realized through future reversals of existing taxable temporary differences. The Company’s deferred tax asset valuation allowance will be reversed if and when it becomes more likely than not that the Company can generate sufficient taxable income in the future to utilize the tax benefits of the related deferred tax assets.
Stock-based Compensation. We use a fair value-based method to recognize non-cash compensation expense for share-based transactions. Under the fair value recognition provisions, we recognize stock-based compensation net of an estimated forfeiture rate and only recognize compensation cost for those shares expected to vest on a straight line basis over the requisite service period of the award.
Contingencies. On an ongoing basis, we assess potential liabilities related to lawsuits or claims brought against us. While it is typically very difficult to determine the timing and ultimate outcome of such actions, we use our best judgment to determine if it is probable that we will incur an expense related to the settlement or final adjudication of such matters and whether a reasonable estimation of such probable loss, if any, can be made. In assessing probable losses, we take into consideration estimates of the amount of insurance recoveries, if any. We accrue a liability when we believe a loss is probable and the amount of loss can be reasonably estimated. Due to the inherent uncertainties related to the eventual outcome of litigation and potential insurance recoveries, it is possible that certain matters may be resolved for amounts materially different from any provisions or disclosures that we have previously made. For a further discussion of contingencies, see Note 11 of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K.
Inventory. Inventory, consisting principally of health and wellness products, is primarily stated at the lower of cost or market using the first in, first out (“FIFO”) method. Management determines the inventory reserve by regularly reviewing and evaluating individual inventory items and movement history. Inventory is written off when deemed obsolete or unsellable. Future factors, such as changes in consumer demand and reports in the media deemed negative to certain products we market and sell, could impact management’s inventory obsolescence reserve estimates.
Goodwill. The excess of purchase price over fair value of assets acquired and liabilities assumed in business combinations is classified as goodwill. We review goodwill for impairment at the reporting unit level annually or, when events or circumstances dictate, more frequently. We assess qualitative factors to determine whether it is more likely than not that the fair value of our reporting unit is less than its carrying amount. In performing the qualitative assessment, we assess relevant events and circumstances that may impact the fair value of our reporting unit, including the following: (i) macroeconomic conditions, (ii) industry and market considerations, (iii) overall financial performance, (iv) events affecting the reporting unit, (v) share price and (vi) recent fair value calculation for our reporting unit, if available. We performed this qualitative assessment as of December 31, 2011 as allowable per the newly issued authoritative guidance described under the heading Recently Adopted Accounting Standards in Note 1 of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K.
| 25 |
After assessing the above described events and circumstances, if we determine that it is more likely than not that the fair value of our reporting unit is greater than its carrying value, then no further testing is required. Otherwise, we would perform a two-step impairment test to evaluate goodwill. Under the first step, we compare the estimated fair value of the reporting unit with its carrying value. If the estimated fair value of the reporting unit is less than its carrying value, we will complete a second step to determine the amount of the goodwill impairment that we should record.
Based on our qualitative assessment, we concluded that it was more likely than not that the estimated fair value of our reporting unit exceeded its carrying value as of December 31, 2011 and thus, did not proceed to the two-step goodwill impairment test. No indicators of impairment exist primarily because our reporting unit’s fair value has consistently exceeded its carrying value by a significant margin.
Sources of Revenue
We derive our revenue principally through the sale of products and freight billed to customers associated with the shipment of products. For 2011, product net sales accounted for approximately 97% of our total net sales, with freight comprising the remainder.
Cost of Goods Sold and Operating Expenses
Cost of Goods Sold. Cost of goods sold consists primarily of the cost of the product sold and the cost of shipping the products to the customers.
Fulfillment. Fulfillment expenses include the costs of warehousing supplies, equipment, maintenance, employees, professional services and rent.
Sales and Marketing. Sales and marketing expenses include advertising and promotional expenditures, including third-party content license fees, traditional media advertising, print expenses and payroll related expenses for personnel engaged in marketing, sales, customer service, and website development and maintenance. We expense advertising costs as incurred.
General and Administrative. General and administrative expenses consist of management and executive compensation, information technology expenses, credit card fees, professional services and general corporate expenses.
Results of Operations
The following table sets forth certain consolidated statement of operations data as a percentage of net sales for the periods indicated:
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Net sales | 100.0 | % | 100.0 | % | 100.0 | % | ||||||
| Cost of goods sold | 77.0 | 74.4 | 68.1 | |||||||||
| Gross profit | 23.0 | 25.6 | 31.9 | |||||||||
| Operating expenses: | ||||||||||||
| Fulfillment | 8.5 | 7.9 | 4.7 | |||||||||
| Sales and marketing | 8.8 | 8.5 | 7.4 | |||||||||
| General and administrative | 11.4 | 15.4 | 15.2 | |||||||||
| Total operating expense | 28.7 | 31.8 | 27.3 | |||||||||
| Operating (loss) income | (5.7 | ) | (6.2 | ) | 4.6 | |||||||
| Net (loss) income | (5.7 | )% | (6.9 | )% | 3.1 | % | ||||||
| 26 |
Comparison of Year Ended December 31, 2011 to Year Ended December 31, 2010
| Year Ended | $ | % | ||||||||||||||
| December 31, | Increase | Increase | ||||||||||||||
| 2011 | 2010 | (Decrease) | (Decrease) | |||||||||||||
| Third-party products | $ | 190,541,774 | $ | 147,064,772 | $ | 43,477,003 | 29.6 | % | ||||||||
| Proprietary products | 60,972,816 | 59,159,197 | 1,813,619 | 3.1 | ||||||||||||
| Freight | 9,008,556 | 14,456,528 | (5,447,972 | ) | (37.7 | ) | ||||||||||
| Net sales | 260,523,146 | 220,680,497 | 39,842,649 | 18.1 | ||||||||||||
| Cost of goods sold | 200,596,556 | 164,205,657 | 36,390,899 | 22.2 | ||||||||||||
| Gross profit | $ | 59,926,590 | $ | 56,474,840 | $ | 3,451,750 | 6.1 | % | ||||||||
Net Sales. Net sales increased by $39.8 million, or 18.1%, to $260.5 million for 2011 compared to 2010. Net sales of third-party products increased $43.5 million or 29.6%, to $190.5 million for 2011. Third-party products include advertising and other fees earned from affiliate programs of an insignificant amount for 2011 and $0.8 million for 2010. Net sales of our proprietary products increased $1.8 million, or 3.1%, to $61.0 million, and freight decreased $5.4 million, or 37.7%, to $9.0 million for 2011 compared to 2010.
The increase in net sales was primarily the result of an increase in our customer base and number of shipped orders compared to the year ended December 31, 2010. We also expanded shipping and promotional offers in 2011, along with growing our network of affiliates. In July 2011, we also began distributing our products on Amazon.com. In February 2011, we extended our “free shipping” policy to include all orders above $49, prior to that only orders above $100 included “free shipping”. The change in our “free shipping” policy accounted for additional product sales, although it reduced freight revenue in 2011. Free shipping and other promotional offers are expected to continue for the foreseeable future.
Cost of Goods Sold. Cost of goods sold increased $36.4 million, or 22.2%, to $200.6 million for 2011 compared to 2010. As a percentage of net sales, cost of goods sold increased to 77.0% for 2011 from 74.4% for 2010. The increase in costs of goods sold is primarily due to a shift in product mix to higher cost third-party products partially offset by a reduction in our shipping costs per order, as we implemented a program to reduce shipping expenses in the fourth quarter of 2011. We expect to see this trend continue as third party product offerings continue to outpace new proprietary product launches.
Gross Profit. As a result of the changes discussed in net sales and cost of goods sold, gross profit increased $3.5 million, or 6.1%, to $59.9 million for 2011 compared to 2010 and gross profit as a percentage of net sales decreased to 23.0% for 2011 from 25.6% for 2010.
Fulfillment. Fulfillment expense increased $4.9 million, or 28.4%, to $22.3 million for 2011 compared to 2010. As a percentage of net sales, fulfillment expense increased to 8.5% for 2011 from 7.9% for 2010. The increase in fulfillment expense was primarily attributable to an increase in shipped orders in 2011 and expenses associated with a program to reduce our freight costs per shipment. Additionally, there were higher operating costs at our distribution centers as a result of certain operating inefficiencies, primarily attributable to difficulties related to optimizing our warehouse systems and a significant unanticipated shift to products requiring special handling (e.g. liquids and glass packaging) resulting in higher labor costs. These inefficiencies began in 2010 and continued through 2011. We expect improved fulfillment expense as a percentage of sales in the future as we continue to implement initiatives to boost efficiencies.
Sales and Marketing. Sales and marketing expense increased $4.1 million, or 22.0%, to $22.8 million for 2011. As a percentage of net sales, sales and marketing expense increased to 8.8% for 2011 from 8.5% for 2010. Included in the 2011 amount were $0.9 million in expenses consisting of the $0.7 million severance payment to our former Chief Marketing Officer and $0.2 million in recruiting fees related to the hiring of our new Chief Marketing officer. The remaining increase in sales and marketing expense of $3.2 million was primarily related to increases in online advertising, including fees paid to affiliates and Amazon.com associated with the expanded distribution of our products, employee compensation costs, and depreciation partially offset by decreases in print advertising and co-op advertising. We expect sales and marketing expenses to increase in 2012 as we continue to expand our marketing team and invest in marketing initiatives to drive additional growth in our customer base.
General and Administrative. General and administrative expenses decreased $4.4 million, or 12.9%, to $29.6 million for 2011. As a percentage of net sales, general and administrative expenses decreased to 11.4% for 2011 from 15.4% for 2010. Included in the 2011 amount were $2.9 million in expenses consisting of the following: $2.1 million in expenses primarily related to our equity capitalization issue and strategic review, $0.3 million related to a software litigation settlement offer, $0.2 million for severance to our former Chief Information Officer, $0.2 million in recruiting fees for the hiring of our current Chief Information Officer and Chief Financial Officer, and $0.1 million for an adjustment to the severance settlements of our former Chief Executive Officer and former Chief Financial Officer. Included in the 2010 amount were an estimated $10.6 million in expenses consisting of the following: $3.6 million in fees associated with our class action lawsuit, conclusion of the proxy solicitation and other matters, $3.5 million accrual for the settlement of our derivative lawsuit, $1.2 million in accrued severance to our former Chief Executive Officer, $1.1 million in accrued severance to our former Chief Financial Officer, $0.7 million in proxy reimbursement fees to Great Hill Partners and $0.5 million in professional services fees to our independent accountants. Excluding the aforementioned items in both periods, general and administrative expenses increased $3.3 million period-over-period. The increase period-over-period was primarily attributable to increased employee compensation costs of $2.9 million due to headcount growth in critical departments, increased credit card fees of $0.6 million due to higher sales and increased depreciation of $0.2 million; partially offset by a $0.4 million decrease in professional services fees. We expect to expand our administrative and information technology staff in 2012 to support our future growth.
| 27 |
Interest Expense. Interest expense decreased $0.4 million, or 99.2%, to an insignificant amount for 2011. The decrease was primarily attributable to the pay off of our notes payable and line of credit balances in 2010.
Income Tax Expense. Income tax expense decreased $1.4 million, or 96.3%, to $0.1 million for 2011. This was a result of reducing our net deferred tax assets to zero at December 31, 2010. Maintaining a valuation allowance on our deferred tax assets throughout 2011 resulted in an insignificant income tax expense for the year.
Comparison of Year Ended December 31, 2010 to Year Ended December 31, 2009
| Year Ended | $ | % | ||||||||||||||
| December 31, | Increase | Increase | ||||||||||||||
| 2010 | 2009 | (Decrease) | (Decrease) | |||||||||||||
| Third-party products (1) | $ | 147,064,772 | $ | 119,750,019 | $ | 27,314,753 | 22.8 | % | ||||||||
| Proprietary products | 59,159,197 | 58,480,009 | 679,188 | 1.2 | ||||||||||||
| Freight | 14,456,528 | 13,577,001 | 879,527 | 6.5 | ||||||||||||
| Net sales | 220,680,497 | 191,807,029 | 28,873,468 | 15.1 | ||||||||||||
| Cost of goods sold | 164,205,657 | 130,605,493 | 33,600,164 | 25.7 | ||||||||||||
| Gross profit | $ | 56,474,840 | $ | 61,201,536 | $ | (4,726,696 | ) | (7.7 | )% | |||||||
| (1) | Third-party product includes advertising and fees earned from affiliate programs of approximately $0.8 million and $1.9 million for the years ended December 31, 2010 and 2009, respectively. |
Net Sales. Net sales increased by $28.9 million, or 15.1%, to $220.7 million for fiscal year ended 2010. Sales of third-party products increased $27.3 million or 22.8%, sales of our proprietary products increased $0.7 million or 1.2%, and freight sales increased $0.9 million or 6.5% in 2010. The increase in net sales was primarily the result of an increase in our customer base, number of shipped orders and our average order value compared to the twelve months ended December 31, 2009. Sales growth was negatively impacted during the year by a shortage of our proprietary products that occurred during the first quarter 2010, and an increasingly competitive environment, which began in the latter part of the second quarter, as many of our competitors offered deep discounts. We responded with increased promotional activities, including “free shipping” and other discounts on both proprietary and third party products.
Cost of Goods Sold. Cost of goods sold increased $33.6 million, or 25.7%, to $164.2 million for 2010. As a percentage of net sales, cost of goods sold increased to 74.4% for 2010 from 68.1% for 2009. The increase is primarily due to a shift in product mix to higher cost third-party products and the use of various shipping discounts including “free shipping” as a promotional activity in response to the augmented competitive environment.
Gross Profit. As a result of the changes discussed in net sales and cost of goods sold, gross profit decreased $4.7 million, or 7.7%, to $56.5 million in 2010 and gross profit as a percentage of net sales decreased to 25.6% in 2010 from 31.9% in 2009.
Fulfillment. Fulfillment expense increased $8.4 million or 93.8%, to $17.4 million for 2010. As a percentage of net sales, fulfillment expense increased to 7.9% for 2010 from 4.7% for 2009. The increase in fulfillment expense as a percentage of net sales was primarily attributable to operating our expanded distribution center in Las Vegas, Nevada and higher labor costs at both our Las Vegas, Nevada and Lexington, North Carolina distribution centers as a result of operating inefficiencies. The operating inefficiencies were primarily attributable to temporary warehouse systems optimization problems and a significant unanticipated shift to products requiring special handling (e.g. liquids and glass packaging) resulting in higher labor costs. Additionally, these inefficiencies were exacerbated in the fourth quarter due to increased order volume. We understand the requirements to reduce these inefficiencies and have been making the appropriate modifications to address them.
Sales and Marketing. Sales and marketing expense increased $4.4 million, or 31.1%, to $18.7 million in 2010. As a percentage of net sales, sales and marketing expense increased to 8.5% for 2010 from 7.4% for 2009. Sales and marketing expenses increased due to a $2.5 million dollar increase in total internet advertising spending, a $1.3 million increase in total labor costs and a $0.9 million increase in total print spending, partially offset by $0.3 million in increased co-op advertising revenue.
General and Administrative. General and administrative expenses increased $4.9 million, or 16.9%, to $34.0 million in 2010. As a percentage of net sales, general and administrative expenses were virtually flat when compared to the twelve months ended December 31, 2009. Included in the 2010 amount were an estimated $10.6 million in expenses consisting of the following: $3.6 million in fees associated with the legal proceedings, the proxy solicitation and other matters, $3.5 million accrual for the settlement of the derivative lawsuit, $1.2 million in accrued severance to our former Chief Executive Officer, $1.1 million in accrued severance to our former Chief Financial Officer, $0.7 million in proxy reimbursement fees to Great Hill Partners and $0.5 million in professional services fees to our independent accountants. Included in the 2009 amount was $10.9 million in stock based compensation incurred in connection with our initial public offering. Excluding these items in both twelve month periods, general and administrative expenses increased $5.1 million year-over-year. This was primarily attributable to an overall increase of $4.1 million in on-going expenses, an increase of $1.4 million in costs associated with being a public company, an increase of $0.8 million in credit card fees due to higher sales, an increase of $0.7 million in higher labor costs, and an additional $0.2 million in stock-based compensation expense. This was partially offset by a decrease of $2.1 million in depreciation expense.
| 28 |
Interest Expense. Interest expense decreased $157,000, or 31.5%, to $341,000 for the year ended December 31, 2010, from $498,000 when compared to the same period in the prior year. The decrease was primarily a result of the capitalization of interest expense and the pay down of substantially all of our outstanding notes payable and line of credit balances.
Income Tax Expense. During 2010 our net deferred tax position, prior to the valuation allowance, increased by approximately $3.3 million. In our assessment of the need for a valuation allowance, in accordance with applicable guidance, we considered our cumulative pre-tax loss after three years, adjusting for permanent items and the potential for accumulated taxable losses in 2011, given our first quarter results. Accordingly, we considered our pattern of recent losses and potential losses in 2011 to be relevant to our analysis. As a result of our assessment, we concluded that a full valuation allowance of $6.3 million was needed as of December 31, 2010, and has been recorded in the accompanying consolidated financial statements.
Income tax expense decreased $1.4 million, or 49.9%, to $1.4 million for the year ended December 31, 2010. This was a result of recognizing a loss before income taxes of $13.8 million and a valuation allowance of $6.3 million for the year ended December 31, 2010 compared to a profit before income taxes of $8.7 million for the year ended December 31, 2009.
Historical Cash Flows
Comparison of Year Ended December 31, 2011 to Year Ended December 31, 2010
Net Cash (Used in) Provided by Operating Activities. For the year ended December 31, 2011, net cash used in operations was $7.0 million compared to net cash provided by operations of $4.1 million for the year ended December 31, 2010. The decrease in 2011 of $11.1 million was primarily attributable to the decrease in accrued expenses and increases in current assets including inventory and accounts receivables. This was partially offset by the decrease in other assets and the increase in deferred revenue.
Net Cash Provided By Investing Activities. For the year ended December 31, 2011, net cash provided by investing activities was $7.6 million compared to $7.1million for the year ended December 31, 2010. During 2011 we generated positive cash inflows primarily due to proceeds from the redemption of our available-for-sale securities. These investing cash inflows were partially offset by expenditures for property and equipment.
Net Cash Provided by (Used In) Financing Activities. For the year ended December 31, 2011, net cash provided by financing activities was $0.4 million compared to net cash used in financing activities of $7.9 million for the year ended December 31, 2010. During 2011 we received approximately $0.4 million in proceeds from the exercise of stock options. During 2010 we paid off our line of credit, notes payable, and our interest rate swaps.
Liquidity and Capital Resources
Since our formation through 2006, we have primarily funded our operations through the sale of equity securities and cash generated from operations. During 2007 and 2008, we funded operations and investments in manufacturing and distribution facilities, as well as the related equipment, primarily through loan agreements and cash generated from operations. In 2009, the Company received net proceeds of approximately $47.1 million from its initial public offering after deducting underwriting discounts, commissions and offering expenses. During 2011, we primarily funded operations from proceeds generated from the sale of available-for-sale securities and cash provided by operating activities.
Liquidity. The significant components of our working capital are cash and cash equivalents, inventory and accounts receivable, primarily from credit card processors, reduced by accounts payable and accrued expenses. Cash and cash equivalents consist of cash and money market accounts. The working capital characteristics of our business allow us to collect cash from sales to customers within a few business days of the related sale. At December 31, 2011, we had a working capital surplus of $13.2 million.
Amounts deposited with third party financial institutions exceed the Federal Deposit Insurance Corporation, or FDIC, and Securities Investor Protection Corporation, or SIPC, insurance limits, as applicable. These cash and cash equivalent balances could be impacted if the underlying financial institutions fail or are subjected to other adverse conditions in the financial markets. To date we have experienced no loss or lack of access to our cash and cash equivalents; however, we can provide no assurances that access to our invested cash and cash equivalents will not be impacted by adverse conditions in the financial markets.
Our future capital requirements will depend on many factors, including:
| • | the rate of our revenue growth, |
| • | the timing and extent of expenditures to enhance our website, network infrastructure, and transaction processing systems, |
| • | the extent of our advertising and marketing programs, |
| • | the levels of inventory we maintain; and |
| • | other factors relating to our business. |
| 29 |
We may require additional financing in the future in order to execute our operating plan. We cannot predict whether future financing, if any, will be in the form of equity, debt, or a combination of both. We may not be able to obtain additional funds on a timely basis, on acceptable terms, or at all.
Net cash used in operations for the year ended December 31, 2011 was approximately $7.0 million. Included in this amount were payments related to the settlement of our derivative lawsuit, equity capitalization issue, strategic review, proxy solicitation, severance paid to our former Chief Executive Officer, Chief Marketing Officer, and Chief Information Officer and recruiting fees for the hiring of our current Chief Marketing Officer, Chief Operating Officer, and Chief Information Officer. These amounts were partially offset by refunds of our estimated 2010 tax payments and certain freight charges. We do not expect the same level of these payments to continue going forward. The net effect of the aforementioned items reduced our cash flow from operations for the year ended December 31, 2011 by approximately $8.0 million. At December 31, 2011, we had approximately $12.9 million in cash and cash equivalents.
On February 16, 2012, we entered into the Purchase Agreement pursuant to which the Investors purchased, and we sold, an aggregate of 4,920,288 shares of our common stock at a purchase price of $7.04 per share, and warrants to purchase an aggregate of 1,680,601 shares of our common stock for an aggregate purchase price of $34,848,903. The warrants have an exercise price of $7.04 per share and a term of four years. The proceeds from the financing will be used primarily to expand and optimize our distribution network. Of this amount, an estimated $15.0 million will be used to build and stock a third distribution center to support our high level of sales growth. Construction on the new facility is expected to commence in the second half of 2012 with completion expected in the early part of 2013. In addition, an estimated $5.0 million will be used to complete the expansion of our existing distribution center in Lexington, North Carolina and increase storage capacity at our other existing distribution center located in Las Vegas, Nevada. We intend to use the remaining proceeds for general operations.
We believe that our cash and cash equivalents currently on hand, our net cash flows from operations, and the $34.8 million cash infusion as a result of the private placement offering will be sufficient to continue our operations for the next twelve months.
Aggregate Contractual Obligations. A summary of contractual cash obligations as of December 31, 2011 is as follows:
| Payments Due By Period | ||||||||||||||||||||
| Less Than | More Than | |||||||||||||||||||
| Total | 1 Year | 1 to 3 Years | 3 to 5 Years | 5 Years | ||||||||||||||||
| Operating Leases | $ | 7,534,036 | $ | 1,604,589 | $ | 3,360,402 | $ | 1,194,320 | $ | 1,374,725 | ||||||||||
| Total Contractual Obligations | $ | 7,534,036 | $ | 1,604,589 | $ | 3,360,402 | $ | 1,194,320 | $ | 1,374,725 | ||||||||||
We have various non-cancelable operating leases that expire in various years through August 2019 for the rental of office space. Rent expense totaled $2,131,573 and $2,137,137 for the years ended December 31, 2011 and 2010, respectively.
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements.
Recent Accounting Pronouncements
Refer to Note 1 of our consolidated financial statements included in Part II, Item 8 of this Annual Report on Form 10-K for a discussion of recently issued accounting standards applicable to us, which is incorporated herein by reference.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Market risk represents the risk of changes in the value of market risk sensitive instruments caused by fluctuations in interest rates, foreign exchange rates and commodity prices. Changes in these factors could cause fluctuations in the results of our operations and cash flows. However, we do not believe that a change in market interest rates would have a material effect on our results of operations or financial condition. Although we derive a minor portion of our sales outside of the U.S., all of our sales are denominated in U.S. dollars. We have limited exposure to financial market risks, including changes in interest rates and foreign currency exchange rates. Inflation generally affects us by increasing costs of raw materials, labor and equipment. We do not believe that inflation had any material effect on our results of operations in the periods presented in our financial statements.
| 30 |
Item 8. Financial Statements and Supplementary Data
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
| Reports of Independent Registered Public Accounting Firms | 32 | |||
| Consolidated Balance Sheets as of December 31, 2011 and 2010 | 34 | |||
| Consolidated Statements of Operations for the Years Ended December 31, 2011, 2010 and 2009 | 35 | |||
| Consolidated Statements of Stockholders’ Equity and Comprehensive Income for the Years Ended December 31, 2011, 2010 and 2009 | 36 | |||
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2011, 2010 and 2009 | 37 | |||
| Notes to Consolidated Financial Statements | 38 | |||
| 31 |
REPORT OF INDEPENDENT REGISTERED CERTIFIED PUBLIC ACCOUNTING FIRM
To the Shareholders and Board of Directors
of Vitacost.com, Inc.:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, stockholders' equity and comprehensive income, and cash flows present fairly, in all material respects, the financial position of Vitacost.com, Inc. and its subsidiary (the "Company") at December 31, 2011 and 2010, and the results of their operations and their cash flows for each of the two years in the period ended December 31, 2011, in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company's management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management's Report on Internal Control over Financial Reporting appearing under item 9A. Our responsibility is to express opinions on these financial statements and on the Company's internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
PricewaterhouseCoopers LLP
Miami, Florida
March 15, 2012
| 32 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Vitacost.com, Inc.
We have audited the accompanying consolidated statements of operations, stockholders’ equity and comprehensive income, and cash flows of Vitacost.com, Inc. and its subsidiary (the “Company”) for the year ended December 31, 2009. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audit provides a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects the results of operations of Vitacost.com, Inc. and its subsidiary and their cash flows for the year ended December 31, 2009 in conformity with U.S. generally accepted accounting principles.
/s/ McGladrey & Pullen, LLP
Fort Lauderdale, Florida
March 30, 2010
| 33 |
VITACOST.COM, INC.
CONSOLIDATED BALANCE SHEETS
| For the year ended December 31, | ||||||||
| Assets | 2011 | 2010 | ||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 12,938,584 | $ | 11,951,643 | ||||
| Securities available-for-sale | - | 10,912,392 | ||||||
| Accounts receivable, net | 2,168,742 | 440,033 | ||||||
| Other receivables | 264,130 | 1,087,311 | ||||||
| Inventory | 34,821,901 | 29,827,929 | ||||||
| Prepaid expenses | 1,912,532 | 1,361,230 | ||||||
| Other assets | 2,343,015 | 3,553,089 | ||||||
| Total current assets | 54,448,904 | 59,133,627 | ||||||
| Property and equipment, net | 33,628,990 | 38,011,314 | ||||||
| Goodwill | 2,200,000 | 2,200,000 | ||||||
| Intangible assets, net | 531 | 4,946 | ||||||
| Deposits | 125,243 | 114,308 | ||||||
| Restricted cash | 225,000 | - | ||||||
| Total assets | $ | 90,628,668 | $ | 99,464,195 | ||||
| Liabilities and Stockholders' Equity | ||||||||
| Current Liabilities | ||||||||
| Current maturities of notes payable | $ | - | $ | 58,888 | ||||
| Accounts payable | 30,249,651 | 23,892,044 | ||||||
| Deferred revenue | 4,572,521 | 2,134,305 | ||||||
| Accrued expenses | 6,425,278 | 10,671,865 | ||||||
| Total current liabilities | 41,247,450 | 36,757,102 | ||||||
| Deferred tax liability | 573,967 | 521,389 | ||||||
| Total liabilities | 41,821,417 | 37,278,491 | ||||||
| Commitments and contingencies | ||||||||
| Stockholders' Equity | ||||||||
| Preferred stock, par value $.00001 per share; authorized 25,000,000 shares; | ||||||||
| no shares issued and outstanding | - | - | ||||||
| Common stock, par value $.00001 per share; authorized 100,000,000 shares; | ||||||||
| 27,974,553 and 27,780,453 shares issued and outstanding at | ||||||||
| December 31, 2011 and December 31, 2010, respectively | 280 | 278 | ||||||
| Additional paid-in capital | 76,262,450 | 74,829,972 | ||||||
| Accumulated other comprehensive loss | - | (20,207 | ) | |||||
| Accumulated deficit | (27,455,479 | ) | (12,624,339 | ) | ||||
| Total stockholders' equity | 48,807,251 | 62,185,704 | ||||||
| Total liabilities and stockholders' equity | $ | 90,628,668 | $ | 99,464,195 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| 34 |
VITACOST.COM, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
| For the year ended December 31, | |||||||||||
| 2011 | 2010 | 2009 | |||||||||
| Net sales | $ | 260,523,146 | $ | 220,680,497 | $ | 191,807,029 | |||||
| Cost of goods sold | 200,596,556 | 164,205,657 | 130,605,493 | ||||||||
| Gross profit | 59,926,590 | 56,474,840 | 61,201,536 | ||||||||
| Operating expenses: | |||||||||||
| Fulfillment | 22,290,353 | 17,353,699 | 8,953,592 | ||||||||
| Sales and marketing | 22,841,611 | 18,727,532 | 14,283,715 | ||||||||
| General and administrative | 29,620,059 | 33,988,736 | 29,082,595 | ||||||||
| 74,752,023 | 70,069,967 | 52,319,902 | |||||||||
| Operating (loss) income | (14,825,433 | ) | (13,595,127 | ) | 8,881,634 | ||||||
| Other income (expense): | |||||||||||
| Interest income | 25,767 | 127,880 | 96,544 | ||||||||
| Interest expense | (2,885 | ) | (341,026 | ) | (497,934 | ) | |||||
| Other income | 23,989 | 38,063 | 249,811 | ||||||||
| 46,871 | (175,083 | ) | (151,579 | ) | |||||||
| (Loss) income before income taxes | (14,778,562 | ) | (13,770,210 | ) | 8,730,055 | ||||||
| Income tax expense | (52,578 | ) | (1,421,240 | ) | (2,836,066 | ) | |||||
| Net (loss) income | $ | (14,831,140 | ) | $ | (15,191,450 | ) | $ | 5,893,989 | |||
| Basic per share information: | |||||||||||
| Net (loss) income available to common stockholders | $ | (0.53 | ) | $ | (0.55 | ) | $ | 0.24 | |||
| Weighted average shares outstanding | 27,829,497 | 27,703,710 | 24,216,942 | ||||||||
| Diluted per share information: | |||||||||||
| Net (loss) income available to common stockholders | $ | (0.53 | ) | $ | (0.55 | ) | $ | 0.24 | |||
| Weighted average shares outstanding | 27,829,497 | 27,703,710 | 24,674,248 | ||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| 35 |
VITACOST.COM, INC.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY AND
COMPREHENSIVE INCOME
| Additional | Note Receivable | Accumulated Other | (Accumulated Deficit) | |||||||||||||||||||||||||
| Common Stock | Paid-In | from Exercise | Comprehensive | Retained | ||||||||||||||||||||||||
| Shares | Amount | Capital | of Options | Loss | Earnings | Total | ||||||||||||||||||||||
| Balance at December 31, 2008 | 23,188,380 | 232 | 11,457,241 | (1,165,625 | ) | - | (3,326,878 | ) | 6,964,970 | |||||||||||||||||||
| Comprehensive income: | ||||||||||||||||||||||||||||
| Net income | - | - | - | - | - | 5,893,989 | 5,893,989 | |||||||||||||||||||||
| Comprehensive income | 5,893,989 | |||||||||||||||||||||||||||
| Stock options exercised | 62,000 | 1 | 13,810 | - | - | - | 13,811 | |||||||||||||||||||||
| Stock repurchased and retired | (360,000 | ) | (4 | ) | (499,996 | ) | - | - | - | (500,000 | ) | |||||||||||||||||
| Repayment of note receivable | - | - | - | 1,165,625 | - | - | 1,165,625 | |||||||||||||||||||||
| Stock issued in initial public offering, net | 4,597,973 | 46 | 47,093,308 | - | - | - | 47,093,354 | |||||||||||||||||||||
| Stock based compensation expense | - | - | 11,831,331 | - | - | - | 11,831,331 | |||||||||||||||||||||
| Income tax benefit from stock options exercised | - | - | 2,036,562 | - | - | - | 2,036,562 | |||||||||||||||||||||
| Balance at December 31, 2009 | 27,488,353 | 275 | 71,932,256 | - | - | 2,567,111 | 74,499,642 | |||||||||||||||||||||
| Comprehensive income: | ||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | (15,191,450 | ) | (15,191,450 | ) | |||||||||||||||||||
| Unrealized loss related to securities available-for-sale | - | - | - | - | (20,207 | ) | - | (20,207 | ) | |||||||||||||||||||
| Comprehensive loss | (15,211,657 | ) | ||||||||||||||||||||||||||
| Stock options exercised | 292,100 | 3 | 851,013 | - | - | - | 851,016 | |||||||||||||||||||||
| Stock-based compensation expense | - | - | 1,463,413 | - | - | - | 1,463,413 | |||||||||||||||||||||
| Income tax benefit from stock options exercised | - | - | 583,290 | - | - | - | 583,290 | |||||||||||||||||||||
| Balance at December 31, 2010 | 27,780,453 | $ | 278 | $ | 74,829,972 | $ | - | $ | (20,207 | ) | $ | (12,624,339 | ) | $ | 62,185,704 | |||||||||||||
| Comprehensive income: | ||||||||||||||||||||||||||||
| Net loss | - | - | - | - | - | (14,831,140 | ) | (14,831,140 | ) | |||||||||||||||||||
| Realized gain related to securities available-for-sale | - | - | - | - | 20,207 | - | 20,207 | |||||||||||||||||||||
| Comprehensive loss | (14,810,933 | ) | ||||||||||||||||||||||||||
| Stock options exercised | 194,100 | 2 | 424,322 | - | - | - | 424,324 | |||||||||||||||||||||
| Stock-based compensation | ||||||||||||||||||||||||||||
| expense | - | - | 1,008,156 | - | - | - | 1,008,156 | |||||||||||||||||||||
| Balance at December 31, 2011 | 27,974,553 | $ | 280 | $ | 76,262,450 | $ | - | $ | - | $ | (27,455,479 | ) | $ | 48,807,251 | ||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements.
| 36 |
VITACOST.COM, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the year ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Cash Flows From Operating Activities | ||||||||||||
| Net (loss) income | $ | (14,831,140 | ) | $ | (15,191,450 | ) | $ | 5,893,989 | ||||
| Adjustments to reconcile net (loss) income to net cash | ||||||||||||
| provided by (used in) operating activities: | ||||||||||||
| Depreciation and amortization | 6,173,739 | 5,137,626 | 3,650,116 | |||||||||
| Amortization of premium on debt securities available-for-sale | 72,016 | 684,843 | - | |||||||||
| Change in fair value of interest rate swap | - | (8,843 | ) | (236,121 | ) | |||||||
| Realized gain on the sale of securities available-for-sale | (779 | ) | (6,635 | ) | - | |||||||
| Stock-based compensation expense | 1,008,156 | 1,463,413 | 11,831,330 | |||||||||
| Deferred taxes | 52,578 | 3,050,930 | (1,517,621 | ) | ||||||||
| Loss (gain) on disposition of property | ||||||||||||
| and equipment and other assets | 40,774 | (69,074 | ) | 89,767 | ||||||||
| Changes in assets and liabilities: | ||||||||||||
| (Increase) decrease in: | ||||||||||||
| Accounts receivable | (1,728,709 | ) | 295,322 | 107,168 | ||||||||
| Other receivables | 823,181 | (31,939 | ) | (409,921 | ) | |||||||
| Inventory | (4,993,972 | ) | (1,731,045 | ) | (6,434,138 | ) | ||||||
| Prepaid expenses | (551,302 | ) | 627,308 | (1,331,563 | ) | |||||||
| Other assets | 1,210,074 | (3,553,089 | ) | - | ||||||||
| Increase (decrease) in: | ||||||||||||
| Accounts payable | 7,569,546 | 5,839,549 | 2,282,586 | |||||||||
| Deferred revenue | 2,438,216 | 214,953 | (459,946 | ) | ||||||||
| Accrued expenses | (4,246,587 | ) | 7,389,389 | 661,716 | ||||||||
| Income taxes payable | - | (51,221 | ) | 21,969 | ||||||||
| Net cash (used in) provided by operating activities | (6,964,209 | ) | 4,060,037 | 14,149,331 | ||||||||
| Cash Flows From Investing Activities | ||||||||||||
| Proceeds from disposition of property, equipment | - | 232,595 | 1,032,429 | |||||||||
| Settlement of interest rate swap | - | (459,876 | ) | - | ||||||||
| Payments for the purchase of property and equipment | (3,039,713 | ) | (16,965,184 | ) | (7,423,883 | ) | ||||||
| (Increase) decrease in deposits | (10,935 | ) | 160,945 | (4,570,921 | ) | |||||||
| Increase in restricted cash | (225,000 | ) | - | - | ||||||||
| Proceeds from repayment of related party advance | - | - | 215,241 | |||||||||
| Purchases of securities available-for-sale | - | (27,457,228 | ) | (35,787,227 | ) | |||||||
| Proceeds from maturities of securities available-for-sale | 10,861,362 | 51,633,649 | - | |||||||||
| Net cash provided by (used in) investing activities | 7,585,714 | 7,144,901 | (46,534,361 | ) | ||||||||
| Cash Flows From Financing Activities | ||||||||||||
| Principal payments on notes payable | (58,888 | ) | (5,852,123 | ) | (812,457 | ) | ||||||
| Repayments on related party note payable | - | - | (2,000,000 | ) | ||||||||
| Net borrowings on line of credit | - | (3,458,183 | ) | (5,954,447 | ) | |||||||
| Repayments on capital lease obligation | - | (35,452 | ) | (60,589 | ) | |||||||
| Repayments of note receivable from exercise of stock options | - | - | 1,165,625 | |||||||||
| Proceeds from the exercise of stock options | 424,324 | 851,016 | 13,811 | |||||||||
| Tax benefit from stock based compensation | - | 583,290 | 2,036,564 | |||||||||
| Net proceeds from initial public offering | - | - | 47,093,354 | |||||||||
| Payments for redemption of common stock | - | - | (500,000 | ) | ||||||||
| Net cash provided by (used in) financing activities | 365,436 | (7,911,452 | ) | 40,981,861 | ||||||||
| Net increase in cash and cash equivalents | 986,941 | 3,293,486 | 8,596,831 | |||||||||
| Cash and cash equivalents: | ||||||||||||
| Beginning of year | 11,951,643 | 8,658,157 | 61,326 | |||||||||
| Ending of year | $ | 12,938,584 | $ | 11,951,643 | $ | 8,658,157 | ||||||
| Supplemental Disclosures of Cash Flow Information | ||||||||||||
| Cash payments for: | ||||||||||||
| Interest | $ | - | $ | 349,869 | $ | 734,055 | ||||||
| Income taxes | $ | - | $ | 1,461,330 | $ | 1,229,000 | ||||||
| Supplemental Schedule of Noncash Operating, Investing and Financing Activities | ||||||||||||
| Equipment purchased not yet paid (Note 3) | $ | (1,211,939 | ) | $ | 2,203,439 | $ | - | |||||
| Application of deposits towards purchases of equipment | $ | - | $ | 4,380,875 | $ | - | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| 37 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Nature of Business, Recent Developments, Significant Accounting Policies and Recent Accounting Guidance
Nature of Business
Vitacost.com, Inc. (“Vitacost” or the “Company”) is an internet-based retailer of nutritional supplements. Vitacost was incorporated in 1994 and began its internet-based retail activity in 1999. Vitacost sells an internally developed proprietary line of nutraceuticals as well as a wide selection of other manufacturers’ brand-name health and wellness products. The Company ships products from two distribution centers located in Lexington, North Carolina and Las Vegas, Nevada.
Recent Developments
On February 16, 2012, the Company entered into a Purchase Agreement (the “Purchase Agreement”) by and among Vitacost, JHH Capital, LLC (an entity affiliated with Jeffrey Horowitz, our Chief Executive Officer, “JHH”), Great Hill Equity Partners III, L.P. (“Great Hill III”), Great Hill Equity Partners IV, L.P. (“Great Hill IV”), Great Hill Investors, LLC (“Great Hill Investors”), Freshford Partners, LP (“Freshford Partners”), Freshford Master Fund, Ltd (“Freshford Master Fund”) and Baron Small Cap Fund (“Baron” and, together with JHH, Great Hill III, Great Hill IV, Great Hill Investors, Freshford Partners, Freshford Master Fund, collectively, the “Investors”) pursuant to which the Investors purchased, and Vitacost sold, an aggregate of 4,920,288 shares of the Company’s common stock at a purchase price of $7.04 per share, and warrants to purchase an aggregate of 1,680,601 shares of the Company’s common stock for an aggregate purchase price of $34,848,903. The warrants have an exercise price of $7.04 per share and a term of four years. The net proceeds will be allocated between common stock and warrants based on their relative fair values as of the purchase date.
Significant Accounting Policies
Principles of Consolidation:
The consolidated financial statements include the accounts of Vitacost and its wholly-owned subsidiary. All significant intercompany accounts and transactions have been eliminated in consolidation.
Revision to Previously Issued Financial Statements:
The Company misclassified its outstanding checks in its December 31, 2010 consolidated balance sheets as increases in accounts payable instead of reductions of cash and cash equivalents. These misclassifications also resulted in an overstatement of net cash provided by operating activities for the year ended December 31, 2010. The Company has revised its December 31, 2010 cash and cash equivalents, and accounts payable balances to properly reflect the outstanding checks as a reduction of cash and cash equivalents and its consolidated statements of cash flows for the year ended December 31, 2010. These revisions, which the Company concluded are not material, do not impact net sales, gross profit, operating loss, net loss, or the working capital surplus. A summary of the effect of the revisions on the consolidated balance sheet and the consolidated statement of cash flows is as follows:
| December 31, 2010 | ||||||||||||
| As previously | ||||||||||||
| reported | Adjustment | As revised | ||||||||||
| Cash and cash equivalents | 14,592,803 | (2,641,160 | ) | 11,951,643 | ||||||||
| Accounts payable | 26,533,204 | (2,641,160 | ) | 23,892,044 | ||||||||
| Year ended December 31, 2010 | ||||||||||||
| As previously | ||||||||||||
| reported | Adjustment | As revised | ||||||||||
| Increase (decrease) in accounts payable | 8,480,709 | (2,641,160 | ) | 5,839,549 | ||||||||
| Net cash provided by (used in) operating activities | 6,701,197 | (2,641,160 | ) | 4,060,037 | ||||||||
Reclassifications:
Reclassifications on the 2010 Consolidated Statements of Operations have been made to conform to the 2011 presentation.
| 38 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Accounting Estimates:
The preparation of the consolidated financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Actual results could differ from those estimates. Significant estimates inherent in the preparation of the accompanying consolidated financial statements include management’s forecast of future cash flows used as a basis to assess recoverability of long-lived assets, including intangible assets and goodwill; the reserve for inventory obsolescence, deferred revenue and assumptions used in the valuation of the Company’s stock-based compensation.
Cash and Cash Equivalents:
The Company considers all highly-liquid investments purchased with original maturities of three months or less at the date of purchase to be cash equivalents. The Company maintains its cash and cash equivalents in various bank deposit accounts which, at times, may exceed the federally-insured limits. The Company has not experienced any losses in such accounts. The Company believes it is not exposed to any significant credit risk on such accounts.
Restricted Cash:
Restricted cash consists of cash pledged as collateral to secure a vendor obligation.
Securities Available-for-Sale:
Available-for-sale securities consisted of investment grade municipal debt securities not classified as trading or held-to-maturity. Available-for-sale securities were stated at fair value, and unrealized holding gains and losses, net of the related deferred tax effect, were reported as a separate component of stockholders’ equity. Realized gains and losses were determined on the basis of the average cost of the securities sold. During the quarter ended June 30, 2011, the Company liquidated its portfolio of available-for-sale securities. The aggregate fair value of securities available-for-sale as of December 31, 2010 was $10,912,392, which approximated cost.
Accounts Receivable:
Accounts receivable consist primarily of amounts in transit from banks for customer credit card, debit card and electronic benefit transfer transactions that are generally processed by the banks within seven days of authorization. Based on the Company’s historical collection experience and a review of the status of accounts receivable, an allowance for doubtful accounts of $216,999 and $144,253 was recorded as of December 31, 2011 and 2010, respectively.
Inventory:
Inventory, consisting principally of health and wellness products, is primarily stated at the lower of cost or market using the first in, first out (“FIFO”) method. Management determines the inventory reserve by regularly reviewing and evaluating individual inventory items and movement history. Inventory is written off when deemed obsolete or unsellable. Based on its experiences, the Company recorded a reserve for obsolescence of approximately $483,000 and $603,000 at December 31, 2011 and 2010, respectively.
Property and Equipment:
Property and equipment is stated at cost less accumulated depreciation. Leasehold improvements are amortized using the straight-line method over the shorter of the estimated useful life of the assets or the lease term. Depreciation is computed using the straight-line method over the following estimated useful lives:
| 2011 Years | |
| Furniture, fixtures and equipment | 3 – 10 |
| Computers | 3 – 5 |
| Software | 3 – 5 |
| Leasehold and building improvements | 3 – 39 |
| Buildings | 39 |
Upon retirement or sale, the cost and accumulated depreciation are eliminated from the accounts and the gain or loss, if any, is included in the consolidated statements of operations.
| 39 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Goodwill:
The excess of purchase price over fair value of assets acquired and liabilities assumed in business combinations is classified as goodwill. The Company reviews goodwill for impairment at the reporting unit level annually or, when events or circumstances dictate, more frequently. The Company assesses qualitative factors to determine whether it is more likely than not that the fair value of its reporting unit is less than its carrying amount. In performing the qualitative assessment, the Company assesses relevant events and circumstances that may impact the fair value of its reporting unit, including the following: (i) macroeconomic conditions, (ii) industry and market considerations, (iii) overall financial performance, (iv) events affecting the reporting unit, (v) share price and (vi) recent fair value calculation for the reporting unit, if available. The Company performed this qualitative assessment as of December 31, 2011 as allowable per the newly issued authoritative guidance described under the heading Recently Adopted Accounting Standards below.
After assessing the above described events and circumstances, if the Company determines that it is more likely than not that the fair value of its reporting unit is greater than its carrying value, then no further testing is required. Otherwise, the Company would perform a two-step impairment test to evaluate goodwill. Under the first step, the Company compares the estimated fair value of the reporting unit with its carrying value. If the estimated fair value of the reporting unit is less than its carrying value, the Company will complete a second step to determine the amount of the goodwill impairment that should be recorded.
Based on the Company’s qualitative assessment, it has concluded that it was more likely than not that the estimated fair value of its reporting unit exceeded its carrying value as of December 31, 2011 and thus, did not proceed to the two-step goodwill impairment test. No indicators of impairment exist primarily because the Company’s reporting unit’s fair value has consistently exceeded its carrying value by a significant margin.
Long-Lived Assets:
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The Company evaluates potential impairment by comparing the carrying amount of the asset with the estimated undiscounted future cash flows associated with the use of the asset and its eventual disposition. Should the review indicate that the asset is not recoverable, the Company’s carrying value of the asset would be reduced to its estimated fair value, which is measured by future discounted cash flows.
Revenue Recognition and Shipping and Handling:
Revenue from the sale of vitamins, nutritional supplements and other related products is recognized when all the following criteria are met: a customer executes an order, the sales price and the shipping and handling fee have been determined, credit card authorization has occurred, collection is reasonably assured and the product has been received by the customer. The Company establishes a deferred revenue liability which represents orders that have been shipped but not yet received by the customer at the end of a given period. The Company’s sales are made to customers permitting certain limited rights of return for a 30-day period. It has been the Company’s experience that such returns have been insignificant. The Company recorded a reserve for returns of $184,000 and $148,000 at December 31, 2011 and 2010, respectively.
The Company formerly entered into agreements whereby third parties were able to advertise through the Company’s website. The Company was paid and recognized revenue based upon the contractual agreement, generally upon consummation of a sale by the third party advertiser. Revenue related to these agreements was $700,000 and $1,900,000 for the year ended December 31, 2010 and 2009, respectively.
Shipping and handling billed to customers is classified as revenue and totaled approximately $9,009,000, $14,457,000, and $13,576,000 for the years ended December 31, 2011, 2010, and 2009, respectively. Shipping and handling costs are expensed as incurred and recorded as a component of cost of goods sold. Shipping and handling expense totaled approximately $25,998,000, $22,963,000, and $16,654,000 for the years ended December 31, 2011, 2010, and 2009, respectively.
Operating Expenses:
The Company’s operating expenses are grouped into three categories: fulfillment, sales and marketing, and general and administrative. The following is a brief synopsis of each category:
Fulfillment Expenses:
Fulfillment expenses include the costs of warehousing supplies, equipment, maintenance, employees, professional services and rent.
Sales and Marketing Expenses:
Sales and marketing expenses include advertising and promotional expenditures, including third-party content license fees, traditional media advertising, print expenses and payroll related expenses for personnel engaged in marketing, sales, customer service, and website development and maintenance. We expense advertising costs as incurred.
| 40 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
General and Administrative Expenses:
General and administrative expenses consist of management and executive compensation, information technology expenses, credit card fees, professional services and general corporate expenses.
Earnings per Share
The Company computes earnings per share by dividing net income by the weighted-average number of common shares outstanding during the period. Diluted earnings per share is computed by giving effect to all potentially dilutive common shares, including stock options. The following table reconciles basic weighted-average shares outstanding to diluted weighted-average shares outstanding for the years ended December 31, 2011, 2010, and 2009:
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Weighted-average shares outstanding - basic | 27,829,497 | 27,703,710 | 24,216,942 | |||||||||
| Effect of dilutive securities | - | - | 457,306 | |||||||||
| Weighted-average shares outstanding - diluted | 27,829,497 | 27,703,710 | 24,674,248 | |||||||||
For periods where the Company reported income, common stock equivalents related to employee stock-based compensation are not included in the computation of diluted earnings per share to the extent that their exercise price exceeded market value, since the result would be antidilutive. For the periods where the Company reported losses, all common stock equivalents are excluded from the computation of diluted earnings per share, since the result would be antidilutive. Securities that could potentially dilute earnings per share in the future, but which were not included in the calculation of diluted earnings per share because to do so would have been antidilutive for the periods presented, are as follows:
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Antidilutive common stock equivalents excluded from diluted earnings per share | 2,581,567 | 2,341,188 | 431,000 | |||||||||
Stock-Based Compensation:
In September 2011, the Company obtained shareholder approval of the 2011 Incentive Compensation Plan (the “Plan”) which replaced the 2007 Stock Award Plan (the “2007 Plan”). The 2007 Plan, however, will continue to govern awards previously granted under it. The Plan’s share reserve includes the sum of 6,000,000 shares of the Company’s common stock, plus (i) any shares of its common stock which have been reserved but not issued pursuant to any awards granted under the 2007 Plan, and (ii) the number of shares subject to outstanding awards under the 2007 Plan that expire or otherwise terminate without having been exercised in full, or are forfeited to or repurchased by the Company. Under the terms of the Plan, options to purchase stock are granted at an exercise price that approximates the fair market value of the underlying shares at the time of the grant. Nonqualified options generally become exercisable on the date of grant and expire in ten years. Incentive stock options generally become exercisable over a five-year period and the maximum term of the option may not exceed ten years. Compensation expense related to stock options recognized in earnings in 2011, 2010, and 2009, was approximately $1,008,000, $1,463,000, and $11,831,000 on a pretax basis, respectively.
The Company recognizes compensation expense for stock awards based on their fair value and related tax effects over the requisite service period. The fair value of each option award is estimated on the date of grant using an option valuation method that uses the assumptions noted in the below table. The valuation technique incorporates assumptions for the expected volatility of the stock price, the expected term of the option, expected dividends, forfeitures and risk-free interest rate for the expected term of the option. As a result of its limited trading history, the Company uses expected volatilities based on the historical volatility of a sample of companies in a similar industry and of a comparable size as the Company. The expected term of the option is based on historical experience and represents the time period options actually remain outstanding. The risk-free interest rate takes into account the time-value of money. The risk-free rate for periods within the contractual life of the option is based on the U.S. Treasury yield curve in effect at time of grant. The Company estimates forfeitures based on historical pre-vesting forfeitures and revises those estimates in subsequent periods if actual forfeitures differ from those estimates.
| 41 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The fair value of each option granted was estimated using the following assumptions:
| 2011 | 2010 | 2009 | |
| Expected life - employees | N/A | 7 years | 8 years |
| Expected life - executives | 5.5 - 6.5 years | 5.75 - 7 years | 5 - 6 years |
| Volatility percentage | 62.6% - 63.9% | 67.9% - 68.8% | 67% - 78% |
| Risk free interest rate | 1.13% - 1.94% | 1.55% - 3.16% | 2.40% - 3.30% |
| Dividends | None | None | None |
Derivative Financial Instruments:
The Company’s risk management policy is to use derivative financial instruments, as appropriate, to manage the interest expense related to the Company’s debt with variable interest rates. These instruments historically have not been designated as hedges for financial reporting purposes; accordingly, gains and losses related to fair value are reflected in the statement of operations at each reporting date.
Fair Value of Financial Instruments:
The Financial Accounting Standards Board (“FASB”) issued authoritative guidance that defines fair value, establishes a framework and gives guidance regarding the methods used for measuring fair value, and expands disclosures about fair value measurements. The guidance applies to all assets and liabilities that are being measured and reported on a fair value basis. It requires new disclosure that establishes a framework for measuring fair value in GAAP and expands disclosure about fair value measurements. This guidance enables the reader of the financial statements to assess the inputs used to develop those measurements by establishing a hierarchy for ranking the quality and reliability of the information used to determine fair values. The guidance requires that assets and liabilities carried at fair value will be classified and disclosed in one of the following three categories:
Level 1: Quoted market prices in active markets for identical assets or liabilities.
Level 2: Observable market based inputs or unobservable inputs that are corroborated by market data.
Level 3: Unobservable inputs that are not corroborated by market data.
The Company’s investments in securities available-for-sale were valued based on observable market based inputs that were corroborated by market data and were therefore considered a level 2 investment within the fair value hierarchy.
The carrying amounts of other financial instruments, including cash, cash equivalents, accounts receivable, other receivables, accounts payable and short-term borrowings approximate fair value due to the short maturity of these instruments.
Income Taxes:
Deferred tax assets and liabilities are recognized for future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to reverse. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date.
When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The Company has determined that there were no material uncertain tax positions and accordingly no associated interest and penalties were required to be accrued at December 31, 2011 and 2010, respectively.
| 42 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Contingencies:
On an ongoing basis, the Company assesses potential liabilities related to lawsuits or claims brought against it. While it is typically very difficult to determine the timing and ultimate outcome of such actions, the Company’s management use their best judgment to determine if it is probable that it will incur an expense related to the settlement or final adjudication of such matters and whether a reasonable estimation of such probable loss, if any, can be made. In assessing probable losses, the Company takes into consideration estimates of the amount of insurance recoveries, if any. The Company accrues a liability when it believes a loss is probable and the amount of loss can be reasonably estimated. Due to the inherent uncertainties related to the eventual outcome of litigation and potential insurance recoveries, it is possible that certain matters may be resolved for amounts materially different from any provisions or disclosures that the Company has previously made.
Recent Accounting Guidance
Recently Adopted Accounting Guidance:
On January 1, 2011, the Company adopted provisions of the authoritative guidance related to disclosure requirements for fair value measurements. Specifically, the changes required a reporting entity to disclose, in the reconciliation of fair value measurements using significant unobservable inputs (Level 3), separate information about purchases, sales, issuances, and settlements. The adoption of this guidance did not have a material impact on the Company’s consolidated financial statements.
On January 1, 2011, the Company adopted provisions of the authoritative guidance related to the testing of goodwill for impairment. These changes required an entity to perform all steps in the test for a reporting unit whose carrying value is zero or negative if it is more likely than not that a goodwill impairment exists based on qualitative factors. This will result in the elimination of an entity’s ability to assert that such a reporting unit’s goodwill is not impaired and additional testing is not necessary despite the existence of qualitative factors that indicate otherwise. The adoption of these changes did not have an impact on the Company’s consolidated financial statements.
In September 2011, the Company adopted provisions of the authoritative guidance which permits an entity to first assess qualitative factors to determine if it is more likely than not that goodwill might be impaired and based on this assessment determine whether it is necessary to perform the two-step goodwill impairment test. This guidance is effective for the Company’s annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011 and early adoption was permitted. The Company early adopted this guidance when performing its annual goodwill impairment testing as of December 31, 2011. The adoption of this authoritative guidance did not have a material impact on the Company’s consolidated financial statements.
Recently Issued Accounting Guidance:
In May 2011, the FASB issued changes to fair value measurement and disclosure. These changes both clarify the FASB’s intent about the application of existing fair value measurement and disclosure requirements and amend certain principles or requirements for measuring fair value or for disclosing information about fair value measurements. The clarifying changes relate to the application of the highest and best use and valuation premise concepts, measuring the fair value of an instrument classified in a reporting entity’s shareholders’ equity, and disclosure of quantitative information about unobservable inputs used for Level 3 fair value measurements. The amendments relate to measuring the fair value of financial instruments that are managed within a portfolio; application of premiums and discounts in a fair value measurement; and additional disclosures concerning the valuation processes used and sensitivity of the fair value measurement to changes in unobservable inputs for those items categorized as Level 3, a reporting entity’s use of a nonfinancial asset in a way that differs from the asset’s highest and best use, and the categorization by level in the fair value hierarchy for items required to be measured at fair value for disclosure purposes only. These changes become effective for the Company on January 1, 2012 and they are not expected to have a material impact on the Company’s consolidated financial statements.
In June 2011, the FASB issued changes to the presentation of comprehensive income. These changes give an entity the option to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. The option to present components of other comprehensive income as part of the statement of changes in stockholders’ equity was eliminated. The items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income were not changed. Additionally, no changes were made to the calculation and presentation of earnings per share. In December 2011, the FASB issued an accounting standard update to defer the effective date of the specific requirement to present items that are reclassified out of accumulated other comprehensive income to net income alongside their respective components of net income and other comprehensive income. These changes become effective for the Company on January 1, 2012. The Company is currently evaluating these changes to determine which option will be chosen for the presentation of comprehensive income. Other than the change in presentation, they are not expected to have a material impact on the Company’s consolidated financial statements.
| 43 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
2. Inventory
Inventory consists of the following as of December 31, 2011 and 2010:
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Raw materials | $ | 2,958,588 | $ | 2,898,955 | ||||
| Work in process | 2,591,310 | 4,585,946 | ||||||
| Finished goods | 29,272,003 | 22,343,028 | ||||||
| $ | 34,821,901 | $ | 29,827,929 | |||||
3. Property and Equipment
Property and equipment consists of the following as of December 31, 2011 and 2010:
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Buildings and building improvements | $ | 12,427,794 | $ | 8,072,716 | ||||
| Furniture, fixtures and equipment | 21,594,159 | 20,830,220 | ||||||
| Computers | 2,708,724 | 2,334,427 | ||||||
| Software | 7,235,229 | 5,836,948 | ||||||
| Leasehold improvements | 2,619,611 | 2,379,597 | ||||||
| Land | 460,000 | 460,000 | ||||||
| 47,045,517 | 39,913,908 | |||||||
| Less accumulated depreciation | (14,994,892 | ) | (10,488,361 | ) | ||||
| 32,050,625 | 29,425,547 | |||||||
| Construction-in-progress | 1,578,365 | 8,585,767 | ||||||
| $ | 33,628,990 | $ | 38,011,314 | |||||
During 2011, the Company was released from its obligation to purchase $1,211,939 of equipment which was previously recorded in construction-in-progress.
At December 31, 2010, construction-in-progress primarily related to the expansion of the Company’s Lexington, North Carolina distribution facility of which the majority was placed into service during 2011.
4. Accrued Expenses
Accrued expenses consist of the following at December 31, 2011 and 2010:
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Salaries and bonus | $ | 2,322,234 | $ | 1,396,094 | ||||
| Professional services | 768,634 | 1,305,761 | ||||||
| Deferred rent | 605,623 | 508,604 | ||||||
| Advertising | 487,121 | 25,720 | ||||||
| Settlements (Note 11) | 288,500 | 3,500,000 | ||||||
| Severances | - | 2,266,544 | ||||||
| Leasehold improvement liability | 282,040 | 321,395 | ||||||
| Other | 1,671,126 | 1,347,747 | ||||||
| $ | 6,425,278 | $ | 10,671,865 | |||||
| 44 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
5. Notes Payable
The Company repaid in full the outstanding borrowings under its promissory notes and its line of credit as well as its interest rate swap obligations on December 13, 2010. The remaining debt at December 31, 2010 consisted of a note payable to a financial institution with an outstanding balance of approximately $59,000.
6. Stock Option Plan
A summary of the Company’s stock option activity for common stock for the years ended December 31, 2011, 2010 and 2009 is as follows:
| Incentive Options | ||||||||||||||||||||
| Shares | Weighted Average Exercise Price | Weighted Average Grant Date Fair Value | Weighted Average Remaining Contractural Life (Years) | Aggregate Intrinsic Value | ||||||||||||||||
| Outstanding, January 1, 2009 | 1,806,000 | $ | 5.14 | $ | 1.21 | 7.66 | - | |||||||||||||
| Granted | 16,000 | 10.31 | 7.85 | 9.59 | - | |||||||||||||||
| Exercised | (171,128 | ) | 2.66 | 2.66 | 4.41 | $ | 1,597,596 | |||||||||||||
| Cancelled | (154,192 | ) | 3.49 | 0.66 | 5.10 | - | ||||||||||||||
| Forfeited | (102,000 | ) | 4.56 | 2.91 | 7.07 | - | ||||||||||||||
| Outstanding, December 31, 2009 | 1,394,680 | $ | 5.74 | $ | 1.50 | 7.06 | $ | 6,428,418 | ||||||||||||
| Granted | 247,250 | 9.72 | 6.52 | 8.09 | - | |||||||||||||||
| Exercised | (190,300 | ) | 4.02 | 1.53 | 4.83 | $ | 1,526,270 | |||||||||||||
| Cancelled | - | - | - | - | - | |||||||||||||||
| Forfeited | (183,500 | ) | 7.86 | 2.85 | 9.53 | - | ||||||||||||||
| Outstanding, December 31, 2010 | 1,268,130 | $ | 6.47 | $ | 2.28 | 7.89 | $ | 204,970 | ||||||||||||
| Granted | - | - | - | - | - | |||||||||||||||
| Exercised | (109,100 | ) | 3.30 | 0.62 | 3.82 | $ | 305,480 | |||||||||||||
| Cancelled | - | - | - | - | - | |||||||||||||||
| Forfeited | (101,200 | ) | 9.05 | 5.23 | 7.42 | - | ||||||||||||||
| Outstanding, December 31, 2011 | 1,057,830 | $ | 6.55 | $ | 2.16 | 5.50 | $ | 908,835 | ||||||||||||
| Exercisable at December 31, 2010 | 925,121 | $ | 5.63 | $ | 1.26 | 5.93 | $ | 199,218 | ||||||||||||
| Exercisable at December 31, 2011 | 883,629 | $ | 6.04 | $ | 1.57 | 5.19 | $ | 908,835 | ||||||||||||
| Nonqualified Options | ||||||||||||||||||||
| Shares | Weighted Average Exercise Price | Weighted Average Grant Date Fair Value | Weighted Average Remaining Contractural Life (Years) | Aggregate Intrinsic Value | ||||||||||||||||
| Outstanding, January 1, 2009 | 916,400 | $ | 1.95 | $ | 0.41 | 5.15 | - | |||||||||||||
| Granted | 503,200 | 11.63 | 6.88 | 9.84 | - | |||||||||||||||
| Exercised | (60,400 | ) | 0.25 | 0.25 | 2.54 | $ | 709,738 | |||||||||||||
| Cancelled | - | - | - | - | - | |||||||||||||||
| Forfeited | (8,000 | ) | 7.50 | 2.40 | 7.30 | - | ||||||||||||||
| Outstanding, December 31, 2009 | 1,351,200 | $ | 5.59 | $ | 2.84 | 6.27 | $ | 5,561,204 | ||||||||||||
| Granted | 200,000 | 8.91 | 4.47 | 10.59 | - | |||||||||||||||
| Exercised | (101,800 | ) | 0.83 | 0.41 | 2.36 | $ | 873,806 | |||||||||||||
| Cancelled | - | - | - | - | - | |||||||||||||||
| Forfeited | - | - | - | - | - | |||||||||||||||
| Outstanding, December 31, 2010 | 1,449,400 | $ | 6.38 | $ | 3.23 | 6.25 | $ | 1,691,801 | ||||||||||||
| Granted | 1,840,000 | 4.42 | 2.77 | 10.19 | ||||||||||||||||
| Exercised | (85,000 | ) | 0.75 | 0.25 | 1.22 | $ | 427,124 | |||||||||||||
| Cancelled | - | - | - | - | - | |||||||||||||||
| Forfeited | - | - | - | - | - | |||||||||||||||
| Outstanding, December 31, 2011 | 3,204,400 | $ | 5.41 | $ | 3.04 | 8.23 | $ | 6,036,513 | ||||||||||||
| Exercisable at December 31, 2010 | 1,416,067 | $ | 6.32 | $ | 3.21 | 6.14 | $ | 1,691,801 | ||||||||||||
| Exercisable at December 31, 2011 | 1,697,938 | $ | 6.17 | $ | 3.25 | 6.56 | $ | 3,464,470 | ||||||||||||
| 45 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
As of December 31, 2011, 2010, and 2009, there was approximately $4,718,000, $2,067,000, and $1,026,000 of total unrecognized compensation cost, net of estimated forfeitures related to stock options granted under the Company’s stock plan, which is expected to be recognized over a weighted average period of 3.14 years.
7. Leases
Certain office space is leased under noncancelable operating leases expiring in various years through August 2016. Future minimum lease payments, by year, and in the aggregate, under operating leases are due as follows as of December 31, 2011:
| Operating | ||||
| Year Ending December 31, | Leases | |||
| 2012 | $ | 1,604,589 | ||
| 2013 | 1,700,293 | |||
| 2014 | 1,660,109 | |||
| 2015 | 588,335 | |||
| 2016 | 605,985 | |||
| Thereafter | 1,374,725 | |||
| Total minimum lease payments | $ | 7,534,036 | ||
The total rental expense included in the statements of operations for the years ended December 31, 2011, 2010, and 2009 were $2,131,573, $2,137,137, and $1,050,021, respectively.
8. Income Taxes
The Company files income tax returns in the U.S. federal jurisdiction and various state and local jurisdictions. Pursuant to applicable statutes of limitation, returns open for adjustment by the Internal Revenue Service (“IRS”) are the consolidated federal income tax returns for tax years ended December 31, 2007 through December 31, 2010. However, material amounts of net operating loss (“NOL”) carryforwards exist at the consolidated level, originating from the 1997 – 2000 and 2002 – 2005 tax years. These NOL carryforwards were fully utilized in 2009. Although the tax returns from the years of NOL origination prior to 2004 are not generally within the statute of limitations of the Internal Revenue Code, their use in 2009 will open the returns to audit exposure, specifically verification of the returns generating said NOLs. As such, the Company’s open years for consolidated federal returns are the tax years ended December 31, 1997 through December 31, 2010. This same range also represents the vast majority of open years for state returns. The IRS previously examined the Company’s 2008 federal income tax return, which resulted in no change to the Company’s tax return. The Company was recently notified by the Internal Revenue Service that it intends to examine the Company’s 2010 federal income tax return.
A number of years may elapse before an uncertain tax position is audited and finally resolved. Settlement of any particular position would usually require the use of cash. The resolution of a matter would be recognized as an adjustment to the provision for income taxes and the effective tax rate in the period of resolution. The Company has determined that there are no material uncertain tax positions and accordingly no associated interest and penalties are required to be accrued at December 31, 2011 and 2010, respectively.
Reconciliation of the federal statutory income tax rate to the Company’s effective tax rate is as follows:
| December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Taxes computed at federal tax rate | $ | (5,024,711 | ) | $ | (4,681,871 | ) | $ | 2,968,219 | ||||
| State income taxes, net of federal income tax benefit, excluding change in valuation allowance | (276,877 | ) | (236,609 | ) | 53,372 | |||||||
| Non-taxable income | (6,262 | ) | (27,200 | ) | (17,000 | ) | ||||||
| Meals and entertainment | 5,673 | 6,470 | 6,630 | |||||||||
| Incentive stock options | (34,371 | ) | 83,192 | 51,682 | ||||||||
| Other tax assets | 556,051 | (75,054 | ) | (226,837 | ) | |||||||
| Change in valuation allowance – state | 404,223 | 943,998 | - | |||||||||
| Change in valuation allowance - federal | 4,428,852 | 5,408,314 | - | |||||||||
| Income tax expense | $ | 52,578 | $ | 1,421,240 | $ | 2,836,066 | ||||||
| 46 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
The provision for income taxes charged to operations for the years ended December 31, 2011, 2010 and 2009, consists of the following:
| December 31, | ||||||||||||||||
| 2011 | 2010 | 2009 | ||||||||||||||
| Current tax expense (benefit) | $ | - | $ | (1,629,690 | ) | $ | 1,318,445 | |||||||||
| Deferred tax expense | 52,578 | 3,050,930 | 1,517,621 | |||||||||||||
| Income tax expense | $ | 52,578 | $ | 1,421,240 | $ | 2,836,066 | ||||||||||
The tax effects of temporary differences that give rise to significant components of deferred tax assets at December 31, 2011 and 2010 are presented below:
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforward | $ | 8,930,293 | $ | 2,643,774 | ||||
| Inventory | 375,274 | 364,597 | ||||||
| Accrued compensation | 392,690 | 144,040 | ||||||
| Stock-based compensation | 4,152,772 | 4,453,272 | ||||||
| Accrued expenses | 174,327 | 2,137,237 | ||||||
| Deferred revenue and reserves | 790,959 | 871,218 | ||||||
| 14,816,315 | 10,614,138 | |||||||
| Deferred tax liability: | ||||||||
| Depreciation and amortization | (4,204,895 | ) | (4,783,215 | ) | ||||
| Valuation allowance - state | (1,348,221 | ) | (943,998 | ) | ||||
| Valuation allowance - federal | (9,837,166 | ) | (5,408,314 | ) | ||||
| Deferred tax asset (liability), net | $ | (573,967 | ) | $ | (521,389 | ) | ||
The deferred tax amounts mentioned above have been classified on the accompanying consolidated balance sheets as follows:
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Deferred tax asset, net | ||||||||
| Current assets | $ | - | $ | |||||
| Noncurrent assets | - | |||||||
| $ | - | $ | - | |||||
| Deferred tax liability, net | ||||||||
| Noncurrent liability | $ | 573,967 | $ | 521,389 | ||||
The Company evaluates its deferred tax assets on a regular basis to determine if valuation allowances are required. In its evaluation, the Company considers taxable loss carryback availability, expectations of sufficient future taxable income, trends in earnings, existence of taxable income in recent years, the future reversal of temporary differences and the available tax planning strategies that could be implemented, if required. Valuation allowances are established based on the consideration of all available evidence using a more likely than not standard. Based on the Company’s evaluation, a valuation allowance of $11,185,387 was established against its net deferred tax assets as of December 31, 2011. The Company’s deferred tax assets for which it has not established a valuation allowance relate to amounts than can be realized through future reversals of existing taxable temporary differences. The Company’s valuation allowance will be reversed if and when it becomes more likely than not that the Company can generate sufficient taxable income in the future to utilize the tax benefits of the related deferred tax assets. The noncurrent deferred tax liability of $573,967 relates to the excess book basis over tax basis of our goodwill. Because goodwill has an indefinite life, the reversal of the book basis over tax basis is not considered a source of taxable income to support the realization of the Company’s
| 47 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
deferred tax assets. Subsequent revisions to the estimated net realizable value of the deferred tax asset or deferred tax liability could cause the provision for income taxes to vary significantly from period to period.
As of December 31, 2011, the Company had unused federal NOL carryforwards of approximately $24,500,000, which were generated during the year ended December 31, 2011 and 2010. As of December 31, 2011, the Company had Florida and North Carolina NOL carryforwards of approximately $7,700,000 and $6,700,000, respectively. The state NOL carryforwards, if not utilized, expire beginning December 2023.
For the years ended December 31, 2011 and 2010, employees exercised incentive stock options and sold the stock in disqualifying dispositions. For the year ended December 31, 2010 a portion of the tax benefit associated with the sale of the stock has been recognized as an income tax benefit. Generally, incentive stock options provide employees with significant tax benefits by allowing the employee to exercise stock options without being taxed on the intrinsic value on the exercise date provided the employee owns the stock for specified periods of time. Since the employer will not receive a tax benefit upon the exercise of incentive stock options, unless there is a disqualifying disposition, the compensation expense associated with incentive stock options is treated as a permanent difference between book and tax accounting and consequently affects the effective tax rate of the Company. For the year ended December 31, 2010, the disqualifying disposition of incentive stock options resulted in a related tax benefit of $583,290, which has been recorded as a reduction in income taxes payable and an increase in additional paid-in capital in the accompanying consolidated balance sheet; there were no tax benefits related to disqualifying disposition of incentive stock options during the year ended December 31, 2011.
9. Related Party Transactions
On June 17, 2008, the Company received an unsecured promissory note in the amount of $400,000 from a Board member. The note bore interest at the greater of one-month LIBOR plus 3.0% or 8.00% for the first six months and increased by 0.5% per month to a maximum interest rate of 13.0%. The loan was paid in full in June 2009. Interest expense on the loan during the year ended December 31, 2009 was approximately $19,000.
On July 15, 2008, the Company received an unsecured promissory note in the amount of $1,600,000 from a Board member. The note bore interest at the greater of one-month LIBOR plus 3.0% or 8.00% for the first six months and increased by 0.5% per month to a maximum interest rate of 13.0%. The loan was paid in full in December 2009. Interest expense on the loan during the year ended December 31, 2009 was approximately $92,000.
In September 2008, the Company made an unsecured loan of $215,241 to its Chief Operations Architect. The loan was interest free and was to be repaid in full upon a successful IPO or sale of his primary residence, but in no event later than September 2011. The loan was repaid in full in September 2009. Imputed interest for 2009 was not significant.
In October 2010, the Company entered into a stockholder agreement with Great Hill Investors, LLC, Great Hill Equity Partners III, L.P., and Great Hill Equity Partners IV, L.P., (collectively, “GHP”), whereby GHP agreed to various restrictions with respect to the voting, transfer and sale of shares of the Company’s common stock which it currently owns and with respect to any shares it may own in the future in excess of 30% of the Company’s outstanding common stock. In addition, the Company and Great Hill entered into a registration rights agreement, which provides Great Hill with certain demand, incidental and shelf registration rights subject to various exceptions and qualifications. Lastly, the Company agreed to reimburse Great Hill $700,000 for its reasonable and documented out-of-pocket expenses incurred in connection with a consent solicitation that occurred in 2010. The Company reimbursed $400,000 and $300,000 to Great Hill in 2011 and 2010, respectively.
The Company paid Board members a total of $117,970 and $55,240 in consulting fees during the years ended December 31, 2010 and 2009, respectively. Such amounts have been included in general and administrative expenses in the statements of operations. No consulting fee payments were made to Board members in 2011.
As discussed in Note 1, on February 16, 2012, the Company entered into the Purchase Agreement with the Investors for the purchase of shares of common stock and warrants to purchase shares of common stock of the Company for an aggregate purchase price of $34,848,903. Concurrently with entering into the Purchase Agreement, the Company entered into a registration rights agreement with Jeffrey Horowitz and JHH which provides Jeffrey Horowitz and JHH with demand, shelf and piggyback registration rights. The registration rights agreement further provides that if the Company enters into a registration rights agreement (or similar agreement) with any person or entity on more favorable terms than those contained in the registration rights agreement, the registration rights agreement will be amended to include such more favorable terms. Pursuant to the registration rights agreement, the Company has agreed to pay all of the registration costs and expenses incurred in connection with such demand, shelf and piggyback registrations.
| 48 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
10. Retirement Plan
The Company has a defined contribution plan which covers substantially all of the Company’s employees. Under the plan, the Company matches 100% of the first 3% of contributions and 50% of the next 2% of contributions. The Company’s contribution is based on a maximum of 5% of the employee’s contributions. The amount contributed to the plan by the Company amounted to $556,835, $251,546, and $196,649 for the years ended December 31, 2011, 2010, and 2009, respectively.
11. Contingencies
Securities Class Action:
On May 24, 2010, a punitive class action complaint was filed in the United States District Court for the Southern District of Florida against the Company and certain current and former officers and directors by a stockholder on behalf of herself and other stockholders who purchased Vitacost common stock between September 24, 2009 and April 20, 2010, captioned Miyahira v. Vitacost.com, Inc., Ira P. Kerker, Richard P. Smith, Stewart Gitler, Allen S. Josephs, David N. Ilfeld, Lawrence A. Pabst, Eran Ezra, and Robert G. Trapp, Case 9:10-cv-80644-KLR. After being appointed to represent the purported class of shareholders, the lead plaintiffs filed an amended complaint asserting claims under Sections 11, 12(a)(2), and 15 of the Securities Act of 1933 and Sections 10(b) and 20(a) of the Securities Exchange Act of 1934, as amended, and Rule 10b-5 promulgated thereunder against Vitacost, its current and former officers and directors, and the underwriters of its initial public offering (“IPO”). On December 12, 2011, the Court granted defendants’ motion to dismiss the complaint, and granted plaintiffs leave to amend.
On January 11, 2012 lead plaintiff filed its second amended complaint asserting claims under Sections 11, 12(a)(2), and 15 of the Securities Act of 1933 against Vitacost, its current and former officers and directors, and its underwriters. Lead plaintiff purports to bring its action on behalf of investors who purchased stock in connection with or traceable to the Company’s IPO between September 24, 2009 and April 20, 2010. The complaint alleges that defendants violated the federal securities laws during the period by, among other things, disseminating false and misleading statements and/or concealing material facts concerning the Company’s current and prospective business and financial results. The complaint also alleges that as a result of these actions the Company’s stock price was artificially inflated during the class period. The complaint seeks unspecified compensatory damages, costs, and expenses.
On February 24, 2012, defendants filed their motion to dismiss the second amended complaint. Lead plaintiff’s opposition is due on March 16, 2012, and defendants’ reply is due on April 6, 2012.
The Company records provisions in its consolidated financial statements for pending litigation when it determines that an unfavorable outcome is probable and the amount of loss can be reasonably estimated. As of December 31, 2011, the Company has concluded that it is not probable that a loss has been incurred and is unable to estimate the possible loss or range of loss that could result from an unfavorable verdict. Therefore, the Company has not provided any amounts in the consolidated financial statements for an unfavorable outcome. The Company believes, and has been so advised by counsel, that it has meritorious defenses to the complaint pending against it and will vigorously defend against it. It is possible that the Company’s consolidated balance sheets, statements of operations, or cash flows could be materially adversely affected by an unfavorable outcome.
Derivative Action and Shareholder Demand:
On July 16, 2010 the Company received a letter from a stockholder’s counsel demanding that our board of directors take action against certain current and former officers and directors. The factual allegations in the letter were similar to those in the class action lawsuit discussed above. On July 20, 2010, a complaint was filed by an alleged stockholder in the Circuit Court of the 15th Judicial Circuit for Palm Beach County, Florida, purportedly on the Company’s behalf. The suit named the Company’s then-directors and certain officers as defendants. The factual allegations in the lawsuit mirrored those in the class action lawsuit as well, and the claims were for breach of fiduciary duty, unjust enrichment, abuse of control, gross mismanagement, insider trading, and waste of corporate assets. The complaint sought disgorgement, restitution, costs, expenses and non-monetary equitable relief. In April 2011, the parties in the case, including the plaintiff and the stockholder who made a demand on the Company, reached an agreement to settle the matter, subject to court approval. As a result of the settlement, the Company has adopted or will adopt significant organizational reforms to strengthen its compliance and corporate governance. The settlement also provided that the Company would agree to pay plaintiffs’ counsels a total of $3.5 million in attorney’s fees which was paid in full in May 2011. On May 27, 2011, the Court issued an order and final judgment (the “Order”) approving the settlement of the derivative lawsuit. The Order also quieted title to all outstanding Vitacost shares of stock and stock options for all periods since 2004 through June 30, 2009 pursuant to the inherent equitable powers of the Court and the Uniform Commercial Code. The Order further provided that the Company’s certificate of incorporation, as amended, was the valid and effective certificate of incorporation of the Company, until validly amended in accordance with applicable Delaware law and the Company’s certificate of incorporation and bylaws.
| 49 |
VITACOST.COM, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Other matters:
In addition to the matters described above, the Company is involved in litigation and administrative proceedings primarily arising in the normal course of its business. During the year ended December 31, 2011, the Company accrued $288,500 related to a software litigation settlement offer. In the opinion of the Company, except as set forth above, its liability, if any, under any other pending litigation or administrative proceedings, even if determined adversely, would not materially affect its financial condition, results of operations or cash flows. Furthermore, the Company has not been the subject of any product liability litigation.
12. Quarterly Selected Financial Data
| Unaudited | ||||||||||||||||||||||||||||||||
(In Thousands, Except per Share Data) | First Quarter | Second Quarter | Third Quarter | Fourth Quarter | ||||||||||||||||||||||||||||
| 2011 | 2010 | 2011 | 2010 | 2011 | 2010 | 2011 | 2010 | |||||||||||||||||||||||||
| Net sales | $ | 63,762 | $ | 57,176 | $ | 65,891 | $ | 53,952 | $ | 63,456 | $ | 50,312 | $ | 67,414 | $ | 59,240 | ||||||||||||||||
| Operating (loss) income | (2,199 | ) | 3,788 | (3,667 | ) | (2,000 | ) | (5,393 | ) | (8,877 | ) | (3,566 | ) | (6,473 | ) | |||||||||||||||||
| Net (loss) income | (2,222 | ) | 2,505 | (3,700 | ) | (1,421 | ) | (5,341 | ) | (5,630 | ) | (3,568 | ) | (10,645 | ) | |||||||||||||||||
| (Loss) Earnings per share | ||||||||||||||||||||||||||||||||
| Basic | (0.08 | ) | 0.09 | (0.13 | ) | (0.05 | ) | (0.19 | ) | (0.20 | ) | (0.13 | ) | (0.38 | ) | |||||||||||||||||
| Diluted | $ | (0.08 | ) | $ | 0.09 | $ | (0.13 | ) | $ | (0.05 | ) | $ | (0.19 | ) | $ | (0.20 | ) | $ | (0.13 | ) | $ | (0.38 | ) | |||||||||
During the quarter ended September 30, 2011, the Company recorded an out of period adjustment to increase stock-based compensation expense by $424,048 related to a stock-based option grant that occurred in June 2011 under the Company’s 2007 Stock Award Plan, the terms of which provided for the immediate vesting of certain options under the grant. The adjustment was to expense the portion of the award which vested immediately at the grant date. The Company concluded that the adjustment was not material to the current or previously reported consolidated financial statements.
13. Stockholder Rights Plan
In March 2010, the Board of Directors approved the Preferred Stock Rights Agreement, dated as of March 24, 2010 (the “Rights Agreement”), by and between the Company and Mellon Investor Services LLC, under which stockholders of record on March 24, 2010 received rights (“Rights”) to purchase one one-thousandth of a share of Vitacost’s Series A Junior Participating Preferred Stock for each outstanding share of Vitacost’s common stock. The Rights were exercisable at an exercise price of $45, subject to adjustment. The Rights were to expire on the earlier of (1) March 24, 2015 or the thirtieth day following the Company’s 2012 annual meeting if the approval of the Company’s stockholders did not occur at such meeting or (2) redemption or exchange of the Rights.
On June 16, 2011, the Company entered into an Amendment to the Rights Agreement (the “Amendment”) that accelerated the final expiration date of the Rights to June 16, 2011.
14. Subsequent Events
On January 25, 2012, the Company’s former Interim Chief Financial Officer informed the Company of his resignation. His resignation did not involve any disagreement with the Company. On February 24, 2012, the Company and the former Interim Chief Financial Officer entered into an Employment Termination and General Release Agreement (the “Agreement”) setting forth the terms of his departure. Under the terms of the Agreement, his employment with the Company was terminated effective February 24, 2012. Subject to receipt by the Company of an unconditional release and any amounts owed by the former Interim Chief Financial Officer to the Company, the Company agreed to pay to him a lump sum payment of $225,000.
On January 25, 2012, the Company entered into an employment agreement (the “Employment Agreement”) with its Chief Financial Officer, outlining the terms of his employment with the Company. Under the terms of the Employment Agreement, he will receive a base salary of $285,000 per year and an annual bonus of up to 50% of his base salary plus a one-time $50,000 bonus which shall be paid on the first anniversary of his employment. Subject to the approval of the award grant by the Company’s Board of Directors, the Company will issue options to purchase 330,000 shares of the Company’s common stock. The options will vest 20% per year over five years. In addition, he is eligible to participate in the Company’s standard employee benefits programs available to senior executives. He joined the Company on February 27, 2012.
As discussed in Note 1, on February 16, 2012, the Company entered into the Purchase Agreement with the Investors for the purchase of shares of common stock and warrants to purchase shares of common stock of the Company for an aggregate purchase price of $34,848,903. Concurrently with entering into the Purchase Agreement, the Company entered into a Registration Rights Agreement with the Company’s Chief Executive Officer and JHH.
| 50 |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, conducted an evaluation of the effectiveness of our disclosure controls and procedures, as such term is defined in Exchange Act Rule 13a-15(e), as of the end of the period covered by this report. Based upon such evaluation, our Chief Executive Officer and Chief Financial Officer concluded that those controls and procedures are effective to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is accumulated and communicated to management, including our Chief Executive Officer and our Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure and are effective to provide reasonable assurance that such information is recorded, processed, summarized and reported within the time periods specified by the SEC’s rules and forms.
Management’s Report on Internal Control Over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Under the supervision and with the participation of our management, including our Chief Executive Officer and Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control — Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2011.
The effectiveness of our internal control over financial reporting as of December 31, 2011 has been audited by PricewaterhouseCoopers LLP, an independent registered certified public accounting firm, as stated in their report, which is included herein.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting identified in connection with the evaluation required by paragraph (d) of Exchange Act Rules 13a-15 during the year ended December 31, 2011 that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including the Chief Executive Officer and Chief Financial Officer, does not expect that our disclosure controls or internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well designed and operated, can provide only reasonable, not absolute, assurance that the control system’s objectives will be met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within Vitacost have been detected.
Item 9B. Other Information
None.
| 51 |
PART III
Item 10. Directors, Executive Officers and Corporate Governance
Our directors and executive officers and their ages as of March 15, 2012 are as follows:
| Name | Age | Position | ||
| Jeffrey J. Horowitz | 64 | Chief Executive Officer and Director | ||
| Brian D. Helman | 42 | Chief Financial Officer | ||
| David Zucker | 43 | Chief Marketing Officer | ||
| Robert Wegner | 43 | Chief Operating Officer | ||
| Nachiket Desai | 39 | Chief Information Officer | ||
| Mary L. Marbach | 45 | General Counsel and Corporate Secretary | ||
| Michael Kumin | 39 | Chairman of the Board of Directors | ||
| Christopher Gaffney | 49 | Director | ||
| Stuart Goldfarb | 57 | Director | ||
| Edwin J. Kozlowski | 63 | Director, Audit Committee Chairman | ||
| Robert G. Trapp, M.D. | 63 | Director |
Executive Officers
Jeffrey J. Horowitz was appointed Chief Executive Officer on February 17, 2011. He served as Interim Chief Executive Officer and a Director of Vitacost from August 2010 through February 17, 2011. Mr. Horowitz provided consulting services to Vitacost in 2010 prior to being appointed Interim Chief Executive Officer. Over the five years prior to joining Vitacost, Mr. Horowitz pursued personal interests. Mr. Horowitz founded Vitamin Shoppe, Inc. (“Vitamin Shoppe”) in 1977 and served as its President and Chief Executive Officer from 1977 to January 2000, during which time he oversaw the retail expansion from one store in 1977 to over 200 stores in 11 states. In addition, Mr. Horowitz expanded Vitamin Shoppe's business by establishing a catalog to solicit mail order sales in 1981 and pioneered the online vitamin sales industry in 1998 with the launch of VitaminShoppe.com. Mr. Horowitz also led Vitamin Shoppe during its initial public offering on The NASDAQ Stock Market in 1999. Mr. Horowitz served as President and Chief Executive Officer of VitaminShoppe.com, Inc. from July 1999 to January 2000. Mr. Horowitz served as Chairman of the Board of Directors of VitaminShoppe.com, Inc. from June 1999 to January 2000, as a Director of VitaminShoppe.com, Inc. from May 1999 to 2007 and as a Director of Vitamin Shoppe Industries Inc. from its inception to 2007. We believe Mr. Horowitz's knowledge and valuable insight into the health and wellness industry, his experience as the Chief Executive Officer of Vitamin Shoppe and his service on several boards of directors provide the requisite qualifications, skills, perspective and experience that make him well qualified to serve on our Board of Directors and as our Chief Executive Officer.
Brian D. Helman was appointed Chief Financial Officer on January 25, 2012. Mr. Helman has nearly 20 years of combined financial and accounting experience, and was most recently Chief Financial Officer of Intermedix Corporation, a leading provider of revenue cycle management solutions from 2010 to 2012. Prior to joining Intermedix, Mr. Helman was Chief Financial Officer of Rosetta Stone Inc., a leading provider of technology-enabled language learning solutions, from 2007 to 2010, where he directed all of the Company's financial activities in a rapid growth environment and led its initial public offering in 2009. Prior to joining Rosetta Stone Inc., Mr. Helman was Chief Financial Officer at NEON Systems from 2002 to 2006 and also held senior level financial positions at NetSpeak Corporation from 1996 to 2001. Mr. Helman is a Certified Public Accountant in Georgia and a member of the American Institute of Certified Public Accountants. He holds a B.S. in Finance from the University of Florida.
David Zucker was appointed Chief Marketing Officer on August 22, 2011. Mr. Zucker has over 20 years of e-commerce, marketing, and customer analytical experience, and was most recently Chief Marketing Officer of Gilt Group, Inc., an online lifestyle retailer offering highly coveted products and services from 2010 to 2011. He joined Gilt in November 2009 as the Vice President of Customer Marketing. During his tenure at Gilt, he developed and executed the personalization process and helped double membership and triple customers. Prior to joining Gilt Group, Inc., Mr. Zucker was the Global Director of Customer Relationship Management and Implementation at Dell Inc. and has also held senior positions at the Home Shopping Network, Martha Stewart Living Omnimedia and Priceline.com. Mr. Zucker holds a Ph.D. in Applied Economics from the University of Rhode Island, a M.S. in Applied Economics from West Virginia University and a B.S. in Biology from West Virginia University. He has also served as an adjunct professor at the University of South Florida and Rutgers University.
Robert Wegner was appointed Chief Operating Officer on August 4, 2011. Mr. Wegner has over 20 years of combined experience in operations, logistics and e-commerce and was formerly the Director of Operations, North America at Amazon.com from 2006 through 2010. He served in other senior and operational roles at Amazon from 1999 through 2006, managing software development efforts within Amazon’s supply chain systems and leading continuous improvement initiatives to increase operating leverage while scaling the fulfillment network. Prior to joining Amazon.com, Mr. Wegner spent over 10 years at United Parcel Service serving in various operational and logistical management positions. Mr. Wegner graduated Cum Laude from La Salle University with a B.S. in Business Administration and obtained his M.B.A. from La Salle University as well. He earned a Certificate in Computer Information Systems from the University of California, Berkeley and is knowledgeable in systems infrastructure, lean manufacturing principles and is Six Sigma certified.
| 52 |
Nachiket Desai was appointed Chief Information Officer on November 28, 2011. Mr. Desai has more than 15 years of industry experience with specialties in e-commerce, business intelligence, and leading research and development teams working in emerging and cutting edge technology. Most recently Mr. Desai was Vice President, Strategy, Enterprise Architecture and Business Intelligence at 1-800-Flowers.com, a leading online florist and gift shop. While at 1-800-Flowers.com, Mr. Desai was responsible for charting the overall technology strategy of the Company, including defining and transitioning to a new state-of-the-art e-commerce platform, revitalizing the analytics infrastructure, and establishing a Business Intelligence Competency Center. Prior to joining 1-800-Flowers, Mr. Desai was the Senior Information Technology Applications Architect, Performance Architect at MetLife from 2006 to 2007 and was Product Manager, Project Manager, Architect, Technical Lead and Developer at Triveni Digital Inc. from 2001 to 2006. He also held senior level positions at LG Software India, Accord Software and Systems, and at Innomedia Technologies. Mr. Desai holds a B.E. in Computer Science from Pune University in India.
Mary L. Marbach was appointed General Counsel in December, 2009 and our Corporate Secretary in July 2010. Ms. Marbach has been an attorney at the Company since July of 2009. Prior to joining the Company, Ms. Marbach was Senior Transactional Counsel at Imperial Finance and Trading, LLC in Boca Raton, Florida. Ms. Marbach was an associate at Greenberg Traurig, LLP in its Corporate and Securities Group in Boca Raton, Florida from 2002 through 2004. Prior to that, she was an associate at Morrison & Foerster, LLP in its Corporate & Securities Group in Palo Alto, California. Ms. Marbach has a B.S. from Syracuse University, an M.B.A. from the University of Miami, and a J.D. from Boston University School of Law. Ms. Marbach is a member of the State Bar of California and the State Bar of Florida.
Directors
Michael A. Kumin has served as Interim Chairman of the Board of Vitacost since July 2010. Mr. Kumin is a Partner with Great Hill Partners, (“GHP”), a Boston-based investment firm. Mr. Kumin has served in a number of positions since joining GHP in 2002, and is presently a Partner responsible for originating and evaluating investment opportunities in the media, internet, software, and business and consumer services sectors. Prior to joining GHP, Mr. Kumin held various executive and investing positions, including roles at both Apollo Management, L.P. and Goldman, Sachs, L.P. Mr. Kumin has served on the Board of Directors of Spark Networks since June 2006. Mr. Kumin serves and has served on the Boards of Directors of numerous private internet companies, including BuscaPé.com and IGN Entertainment. Mr. Kumin graduated with honors from the Woodrow Wilson School of Public & International Affairs at Princeton University with a Bachelor of Arts. We believe Mr. Kumin's extensive experience as a board member of both public and private growth-oriented technology companies, his knowledge of the media, internet, software, and business and consumer services sectors, and his wealth of expertise, particularly in the areas of strategy, mergers and acquisitions, and corporate finance, provide the requisite qualifications, skills, perspective and experience that make him well qualified to serve on our Board of Directors.
Christopher S. Gaffney has served as a Director of Vitacost since July 2010. Mr. Gaffney is a Managing Partner with, and one of the co-founders of GHP. Mr. Gaffney has been a Managing Partner at GHP since its founding in 1998, and in such position he shares responsibility for the general management, investment policy, fund raising and investor relations at GHP. Mr. Gaffney also continues to actively pursue new investments and manage portfolio companies. Prior to GHP, Mr. Gaffney served as an Associate, Principal and General Partner for Media/Communications Partners, a predecessor organization to GHP. Mr. Gaffney began his career as a commercial lending officer for the First National Bank of Boston in the specialized media lending unit. Mr. Gaffney has served on the Board of Directors of LECG Corporation, a publicly-traded provider of professional services, since December 2009. Mr. Gaffney previously served on the Boards of Directors of Spark Networks from November 2006 to October 2007, Incentra Solutions, Inc., a provider of information technology services that, at the time of Mr. Gaffney's service, traded on the OTC Bulletin Board, from August 2004 to July 2005, and Haights Cross Communications, Inc., an educational and library publisher with publicly-registered debt, from March 1997 to September 2007. Mr. Gaffney serves and has served on the Boards of Directors of numerous private companies, including BuscaPé.com and IGN. Mr. Gaffney is a summa cum laude graduate of Boston College with a degree in Accounting and Economics. We believe Mr. Gaffney's extensive experience in all phases of the lifecycles of growth companies, with a particular emphasis on companies in the media, internet, and business and consumer services sectors, gained as a Managing Partner at a well-known investment firm with $2.7 billion under management and his membership on numerous public and private company boards of directors provide the requisite qualifications, skills, perspective and experience that make him well qualified to serve on our Board of Directors.
Stuart Goldfarb has served as our Director since February 28, 2011. Mr. Goldfarb has more than 25 years of direct marketing, media and e-commerce experience. He currently serves on the board of Atrinsic, Inc., a marketer of direct-to-consumer subscription products and an internet search-marketing agency. Mr. Goldfarb served as the President and CEO of Direct Brands, Inc., the world’s largest direct marketer of music, DVDs, and books, from 2001 to 2009. Prior to joining Direct Brands, Inc., Mr. Goldfarb was President and CEO of bol.com, Bertelsmann’s premier online retailer of books and music, doing business in eighteen European and Asian countries. Before joining Bertelsmann, he was Vice Chairman of Value Vision International, a cable TV home shopping and e-commerce company and was also the former Executive Vice President, Worldwide Business Development at NBC, where he held various executive level positions. We believe Mr. Goldfarb’s experience as an executive at multiple online and ecommerce companies and his experience as a member of other boards of directors provide the requisite qualifications, skills, perspective and experience that make him well qualified to serve on our Board of Directors.
| 53 |
Edwin J. Kozlowski has served as our Director since February 28, 2011. Mr. Kozlowski has over 30 years of financial experience working in the retail and nutritional supplement industries. Mr. Kozlowski currently serves on the board and Executive Committee of Suncoast Centers, Inc., a social service, non-profit organization. Mr. Kozlowski served as Executive Vice President and Chief Operating Officer of Retail Ventures, Inc. from 2001 to 2004 and Senior Vice President and Chief Financial Officer — Chief Operating Officer of DSW in 2001. Prior to joining Retail Ventures, Inc., Mr. Kozlowski served as Executive Vice President and Chief Financial Officer of General Nutrition Companies, Inc. (“GNC”) from 1990 to 2000. From 1978 to 1990, Mr. Kozlowski held various management positions within GNC. He has also served in senior financial positions at H.K. Porter Company and Arthur Young, LLP. Mr. Kozlowski was an officer with the United States Secret Service and served in the United State Army. He received a Bachelor of Science in accounting from Robert Morris University in 1975. We believe that Mr. Kozlowski’s prior retail, operations, management and finance background and his role as the Chief Financial Officer of GNC and DSW provide the requisite qualifications, skills, perspective and experience that make him well qualified to serve on our Board of Directors.
Robert G. Trapp, M.D. has served as our Director since April 2007. Dr. Trapp has maintained a private practice in rheumatology in Springfield, Illinois since 1989. He was a faculty member at Southern Illinois University School of Medicine from 1981 – 1989 where he served as Chief of the Division of Rheumatology. He has been a principal investigator in more than 125 phase I, II and III clinical trials evaluating new therapies in the treatment of rheumatological diseases. Dr. Trapp is board certified in internal medicine and rheumatology. He is a Fellow of the American College of Physicians and a member of the American College of Rheumatology. He received a Bachelor of Arts from Earlham College and his M.D. from Northwestern University School of Medicine. We believe Dr. Trapp’s prior extensive service with our company and his medical expertise and knowledge of our products provide the requisite qualifications, skills, perspective and experience that make him well qualified to serve on our Board of Directors.
All directors hold office until their successors have been elected and qualified or until their earlier resignation or removal. Directors are elected annually. None of the directors are involved in certain legal proceedings covered under Item 401(f) of Regulation S-K. Our Board of Directors has determined that all of our directors except for Mr. Horowitz are “independent directors” and meet the independence requirements under the listing standards of The NASDAQ Stock Market.
There are no family relationships among any of our directors or executive officers.
Section 16(a) Beneficial Ownership Reporting Compliance
Our executive officers, directors, and stockholders with beneficial ownership of more than 10% of our common stock are required, under the Securities Exchange Act of 1934, as amended, to file reports of ownership of our common stock with the Securities and Exchange Commission. Based solely upon a review of the copies of reports furnished to us and written representations that no other reports were required to be filed during fiscal 2011, we are not aware of any late filings.
Code of Ethics
We have adopted a code of business conduct and ethics applicable to all of our directors, officers and employees. A copy of the full text of that code is available on our corporate website at www.vitacost.com . If any substantive amendments are made to the code of business conduct and ethics or any waiver is granted, we intend to satisfy the disclosure requirement under Item 5.05 of Form 8-K regarding such amendment to, or waiver from, a provision of this code of business conduct and ethics by posting such information on our website, at the address and location specified above, or as otherwise required by the NASDAQ Global Market.
Board Composition
Our board currently consists of six members. Our bylaws permit our board to establish by resolution the authorized number of directors, and six directors are currently authorized. At each annual meeting of stockholders, the terms of each of our incumbent directors expire and all members of our board of directors are elected. All directors elected at an annual meeting will be elected to serve from the time of election and qualification until the next annual meeting following such election.
Audit Committee
We have a separately-designated audit committee of our board of directors established in accordance with Section 3(a)(58)(A) of the Securities Exchange Act of 1934, as amended.
The audit committee of our board of directors recommends the appointment of our independent registered public accountant, reviews our internal accounting procedures and financial statements, and consults with and reviews the services provided by our independent registered public accountant, including the results and scope of their audit. The audit committee met nine times (including telephonic meetings) during 2011.
The audit committee is currently comprised of Edwin J. Kozlowski (chair), Stuart Goldfarb and Robert G. Trapp, M.D., each of whom is independent within the meaning of the requirements of the Sarbanes-Oxley Act of 2002 and applicable SEC and NASDAQ rules. Mr. Kozlowski is chairman of our audit committee as well as our audit committee financial expert, as currently defined under the SEC rules implementing the Sarbanes-Oxley Act of 2002. We believe that the composition and functioning of our audit committee complies with all applicable requirements of the Sarbanes-Oxley Act of 2002, The NASDAQ Stock Market LLC, and the SEC’s rules and regulations.
| 54 |
The audit committee operates under a written charter adopted by the board of directors, a copy of which is available under the “Investor Relations” section of our website, www.vitacost.com.
Director Nominations
There have been no material changes to the procedures by which security holders may recommend nominees to our board of directors since those procedures were described in our proxy statement for our 2011 annual meeting of stockholders.
Item 11. Executive Compensation
Compensation Discussion and Analysis
Overview
Our executive compensation program is designed to enable us to attract and retain key personnel and provide incentives that promote short and long-term financial growth and stability to enhance stockholder value based on a pay-for-performance model. Our compensation committee reviews and recommends to our Board of Directors the compensation program for our “Named Executive Officers” and oversees our executive compensation strategy. In 2011, our Named Executive Officers were, Jeffrey Horowitz, our Chief Executive Officer, Stephen E. Markert, Jr. our Interim Chief Financial Officer, David Zucker, our Chief Marketing Officer, Robert Wegner, our Chief Operating Officer, Nachiket Desai, our Chief Information Officer, Mary Marbach, our General Counsel and Corporate Secretary, Robert D. Hirsch, our former Vice President Information Technology and Chief Information Officer and Sonya L. Lambert, our former Chief Marketing Officer.
The discussion in this section describes compensation paid to our Named Executive Officers for services rendered to us in all capacities during our fiscal year ended December 31, 2011.
Our executive compensation program provides for the following elements:
| • | base salaries, which are designed to allow us to attract and retain qualified candidates, |
| • | incentive compensation, which provides additional cash compensation and is designed to support our pay-for-performance philosophy and align compensation with our corporate strategies and business and financial objectives, |
| • | equity compensation, principally in the form of stock options, which are granted to incentivize executive behavior that results in increased stockholder value; and, |
| • | a benefits package that is available to all of our employees. |
A detailed description of these components is provided below.
Elements of Our Executive Compensation Program
Base Salary . The base salary provides cash compensation for performing the essential elements of our executive positions. We strive to set our base salaries at levels which we believe are competitive in our market and provide our executives a level of compensation that permits them to focus their energies on job performance.
Annual Bonus/Incentive Compensation. Our incentive compensation, in the form of cash payment, is intended to compensate our executives after the end of the calendar year for meeting our corporate and/or financial objectives and, in the case of certain executives, their individual performance objectives and to incentivize our executives to meet these objectives. We may set multiple objectives for an executive to achieve. A specific incentive payment may be earned for meeting one or more of such objectives. Further, our incentive compensation is intended to reward and incentivize our executives for exceeding their objectives. We may grant discretionary cash bonuses as an award to our executives for performance that is not necessarily rewarded by the incentive compensation or to attract new executives to join our management team. For those Named Executive Officers who received a bonus, their objectives were set by the appropriate executive or compensation committee. The potential incentive compensation of our Named Executive Officers in 2011 ranged from 50.0% to 68.8% of base salary.
Equity-Based Compensation. Generally, the goals of our equity based compensation are intended to align the interests of our Named Executive Officers with the interests of our stockholders. Our Named Executive Officers typically receive equity awards in the form of stock options that vest equally over a period of five years in an effort to encourage the long-term retention of our executives. We may, however, grant options or other awards that vest immediately as determined by our compensation committee to be consistent with our objectives. The exercise price of our stock option grants is the fair market value of our stock on the grant date. Also, our stock option awards typically provide for the acceleration of vesting of options in the event of a change in control of our company. We have not yet established policies for the timing of awarding stock option grants to our executives. The compensation committee intends to adopt a policy regarding the timing of grants in relation to the release of material information to our stockholders.
Benefits. We provide our Named Executive Officers other benefits generally available to our employees such as health benefits, a 401(k) plan and life and disability insurance. These benefits are intended to provide support to our executives and their families throughout various stages of their careers, and these core benefits are provided to all executives regardless of their individual performance levels. The 401(k) plan allows participants to defer their annual compensation, subject to the limitations set by the Internal Revenue Code, which was $16,500 per person for 2011. The executives' elective deferrals are immediately vested and non-forfeitable upon contribution to the 401(k) plan.
| 55 |
Determining the Amount of Each Element of Compensation
Base Salary. Generally, base salaries for our Named Executive Officers are established through negotiation when the executive is hired. Factors we considered in the negotiation are prior experience, qualifications, prior salary and our need for the particular qualifications of such executive. Adjustments in base salary are based on the executive's responsibilities, performance and their overall compensation package. We review our executives' base salaries annually taking into consideration the executive's level of responsibilities, performance, tenure and salaries of our comparable executive and an employee's overall compensatory arrangement. In the event of material changes in position, responsibilities or other factors, the compensation committee may consider changes in base pay during the year. Our Named Executive Officers' base salaries are reviewed and approved by our Board of Directors.
In 2011, our Compensation Committee, as approved by our Board of Directors, made the following increases in the base salaries of our executives:
| 2011 | 2010 | Percentage | ||||||||||||||
| Base Salary | Base Salary | Increase | Change | |||||||||||||
| Jeffrey Horowitz, | $ | 400,000 | $ | 400,000 | 0.0 | % | $ | - | ||||||||
| Chief Executive Officer | ||||||||||||||||
| Stephen E. Markert, Jr. | $ | 300,000 | $ | 235,000 | 27.7 | % | $ | 65,000 | ||||||||
| Interim Chief Financial Officer | ||||||||||||||||
| Mary L. Marbach, | $ | 200,000 | $ | 140,000 | 42.9 | % | $ | 60,000 | ||||||||
| General Counsel and Corporate Secretary | ||||||||||||||||
| Sonya L. Lambert, | $ | 225,000 | $ | 225,000 | 0.0 | % | $ | - | ||||||||
| Former Chief Marketing Officer | ||||||||||||||||
| Robert Hirsch, | $ | 178,000 | $ | 178,000 | 0.0 | % | $ | - | ||||||||
| Former Vice President Information Technology and Chief Information Officer | ||||||||||||||||
Annual Bonus/Incentive Compensation. Our incentive compensation is payable subsequent to completion of our annual audit and after it has been determined the goals have been achieved. Our Compensation Committee or appropriate executive has the authority to modify a bonus structure during the year if deemed appropriate. Examples of circumstances in which we may consider revising a bonus plan include acquisitions, mergers, divestitures, successful expansion of distribution or manufacturing capabilities and other material changes in our Company.
Our executive bonus plan for 2011 provides a potential bonus for each executive. In certain cases, bonuses were guaranteed in 2011 under our Named Executive Officers' employment agreements. In 2011, the potential awards were as follows:
| Name | Annual Maximum Bonus Opportunity | Maximum Bonus as a Percentage of Base Salary 2011 | ||||||
| Jeffrey Horowitz, | $ | 275,000 | 68.8 | % | ||||
| Chief Executive Officer | ||||||||
| Stephen E. Markert, Jr., | $ | 150,000 | 50.0 | % | ||||
| Interim Chief Financial Officer | ||||||||
| David Zucker, (1) | $ | 150,000 | 50.0 | % | ||||
| Chief Marketing Officer | ||||||||
| Robert Wegner, (1) | $ | 125,000 | 50.0 | % | ||||
| Chief Operating Officer | ||||||||
| Nachiket Desai, (1) | $ | 125,000 | 50.0 | % | ||||
| Chief Information Officer | ||||||||
| Mary L. Marbach, | $ | 100,000 | 50.0 | % | ||||
| General Counsel and Corporate Secretary | ||||||||
| (1) | Named Executives hired in 2011. |
| 56 |
Allocation of Equity Compensation Awards
In 2011, we granted stock options to our Named Executive Officers. Stock options were granted to our Chief Executive Officer (option to purchase 950,000 shares of our common stock), Chief Marketing Officer (option to purchase 300,000 shares of our common stock), Chief Operating Officer (Option to purchase 300,000 shares of our common stock), and Chief Information Officer (option to purchase 250,000 shares of our common stock).
Our Compensation Committee does not apply a rigid formula in allocating stock options to executives as a group or to any particular executive. Instead, our Compensation Committee exercises its judgment and discretion and considers, among other things, the role and responsibility of the executive, competitive factors, the amount of stock-based equity compensation already held by the executive, the non-equity compensation received by the executive and the total number of options to be granted to all participants during the year. Our Compensation Committee typically makes annual grants of equity awards to our Named Executive Officers in connection with its annual review of our employees' compensation. These options were granted pursuant to the terms of employment agreements entered into in 2011 with the executives listed above (See “Employment Agreements and Change in Control Agreements”).
Executive Equity Ownership
We believe it is important for our Named Executive Officers to have their interests aligned with our stockholders and, therefore, to be granted equity incentive awards. We have not, however, established specific stock retention and ownership guidelines for our executives.
Type of Equity Awards
Our stock award plans permits us to issue qualified and non-qualified stock options, stock appreciation rights, restricted stock, stock units, bonus stock, and other equity awards.
Severance and Change in Control Arrangements
For a description of the severance and change in control arrangements we have with our Named Executive Officers, see “Executive Compensation — Employment Agreements” and “Executive Compensation — Payments Upon Termination or Upon Change in Control.” The Compensation Committee believed that these arrangements were necessary to attract and retain our Named Executive Officers. The terms of each arrangement were determined in negotiation with the applicable Named Executive Officer in connection with the executive's hiring and were not based on any set formula.
Effect of Accounting and Tax Treatment on Compensation Decisions
In the review and establishment of our compensation programs, we consider the anticipated accounting and tax implications to us and our executives. In this regard, we may begin utilizing restricted stock and restricted stock units as additional forms of equity compensation incentives in response to changes in the accounting treatment of equity awards under the authoritative accounting guidance. While we consider the applicable accounting and tax treatment of alternative forms of equity compensation, these factors alone are not dispositive, and we also consider the cash and non-cash impact of the programs and whether a program is consistent with our overall compensation philosophy and objectives.
Section 162(m) of the Internal Revenue Code imposes a limit on the amount of compensation that we may deduct in any one year with respect to our Chief Executive Officer and each of our three highest compensated officers excluding our Chief Financial Officer, unless specific and detailed criteria are satisfied. Performance-based compensation, as defined in the Internal Revenue Code, is fully deductible if the programs are approved by stockholders and meet other requirements. In general, we have determined that we will not seek to limit executive compensation so that it is deductible under Section 162(m). However, from time to time, we monitor whether it might be in our interests to structure our compensation programs to satisfy the requirements of Section 162(m). We seek to maintain flexibility in compensating our executives in a manner designed to promote our corporate goals and, therefore, our compensation committee has not adopted a policy requiring all compensation to be deductible.
Role of Executives in Executive Compensation Decisions
Our Compensation Committee seeks input from our Chief Executive Officer when discussing the performance of, and compensation levels for, our Named Executive Officers other than himself. The Compensation Committee also works with the Chief Executive Officer and with our Chief Financial Officer in evaluating the financial, accounting, tax and retention implications of our various compensation programs. No Executive participates in deliberations relating to his or her own compensation.
| 57 |
Compensation Committee Report
The information contained in this report will not be deemed to be “soliciting material” or to be “filed” with the Securities and Exchange Commission, nor will such information be incorporated by reference into any future filing under the Securities Act or the Exchange Act, except to the extent that we specifically incorporate it by reference in such filing.
The Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis set forth in this Annual Report on Form 10-K with our management. Based on such review and discussions, the Compensation Committee has recommended to our board that the Compensation Discussion and Analysis be included in this Annual Report on Form 10-K.
Compensation Committee
Michael A. Kumin
Christopher S. Gaffney
Summary of Cash and Other Compensation
The Summary Compensation Table below presents information concerning the total compensation of our Named Executive Officers for the fiscal years ended December 31, 2011, 2010 and 2009. Mr. Zucker, Mr. Wegner, and Mr. Desai were not Named Executive Officers prior to 2011; therefore, their compensation information is not presented for fiscal 2009 and 2010. Mr. Horowitz and Mr. Markert were not Named Executive Officers prior to 2010; therefore, their compensation information is not presented for fiscal 2009.
Summary Compensation Table
| Salary | Bonus (1) | Option Awards (2) | Non-Equity Incentive Plan Compensation | All Other Compensation(3) | Total Compensation | |||||||||||||||||||||||
| Jeffrey Horowitz, (4) | 2011 | $ | 374,141 | $ | - | $ | 2,434,155 | $ | 275,000 | $ | 25,470 | $ | 3,108,765 | |||||||||||||||
| Chief Executive Officer | 2010 | $ | 153,846 | $ | - | $ | 894,000 | $ | 75,000 | $ | - | $ | 1,122,846 | |||||||||||||||
| 2009 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| Stephen E. Markert, Jr., | 2011 | $ | 276,021 | $ | - | $ | - | $ | 150,000 | $ | - | $ | 426,021 | |||||||||||||||
| Interim Chief Financial Officer | 2010 | $ | 37,058 | $ | - | $ | - | $ | 10,000 | $ | - | $ | 47,058 | |||||||||||||||
| 2009 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| David Zucker (5) | 2011 | $ | 108,892 | $ | - | $ | 789,000 | $ | 19,028 | $ | 17,576 | $ | 934,496 | |||||||||||||||
| Chief Marketing Officer | 2010 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||
| 2009 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| Robert Wegner, (5) | 2011 | $ | 93,165 | $ | - | $ | 789,000 | $ | 15,857 | $ | 36,523 | $ | 934,545 | |||||||||||||||
| Chief Operating Officer | 2010 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||
| 2009 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| Nachiket Desai (5) | 2011 | $ | 24,161 | $ | - | $ | 977,500 | $ | - | $ | - | $ | 1,001,661 | |||||||||||||||
| Chief Information Officer | 2010 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | |||||||||||||||
| 2009 | $ | - | $ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||||||||
| Mary Marbach, | 2011 | $ | 200,450 | $ | - | $ | - | $ | 100,000 | $ | - | $ | 300,450 | |||||||||||||||
| General Counsel and Corporate Secretary | 2010 | $ | 140,000 | $ | - | $ | 326,000 | $ | 40,000 | $ | - | $ | 506,000 | |||||||||||||||
| 2009 | $ | 31,877 | $ | 1,000 | $ | - | $ | 10,000 | $ | - | $ | 42,877 | ||||||||||||||||
| Sonya L. Lambert, (6) | 2011 | $ | 141,923 | $ | - | $ | - | $ | - | $ | 685,800 | $ | 827,723 | |||||||||||||||
| Former Chief Marketing Officer | 2010 | $ | 209,408 | $ | - | $ | - | $ | - | $ | - | $ | 209,408 | |||||||||||||||
| 2009 | $ | 154,735 | $ | 15,600 | $ | 565,600 | $ | 103,680 | $ | - | $ | 839,615 | ||||||||||||||||
| Robert D. Hirsch, (7) | 2011 | $ | 164,321 | $ | - | $ | - | $ | - | $ | 240,000 | $ | 404,321 | |||||||||||||||
| Former Vice President Information | 2010 | $ | 177,778 | $ | - | $ | - | $ | 31,710 | $ | - | $ | 209,488 | |||||||||||||||
| Technology and Chief Information Officer | 2009 | $ | 159,046 | $ | 11,700 | $ | 141,400 | $ | 36,240 | $ | - | $ | 348,386 | |||||||||||||||
| (1) | We report executive plan awards in the column titled “Non-Equity Incentive Plan Compensation.” |
| (2) | Amounts shown do not reflect compensation actually received by the Named Executive Officer. Instead, the amounts represent the aggregate grant date fair value related to option awards, granted in the year indicated, pursuant to Statement of Financial Accounting Standards Codification Topic 718. For a discussion of the valuation assumptions, see Note 1 to our consolidated financial statements. |
| 58 |
| (3) | Named Executive Officers who received personal benefits valued at $10,000 or more in the aggregate during the fiscal year ending December 31, 2011. The All Other Compensation listed for Messrs Horowitz, Zucker, and Wegner were travel, living and relocation expenses contractually agreed upon in their employment agreements. |
| (4) | Mr. Horowitz became Chief Executive Officer on February 17, 2011. Prior to his appointment as the Company's Chief Executive Officer, he served as the Company's Interim Chief Executive Officer from August 2010 through February 2011. |
| (5) | Named Executive hired in 2011. |
| (6) | On August 22, 2011, the Company announced Ms. Lambert’s separation from the Company as Chief Marketing Officer. Ms. Lambert received $685,800 in severance in connection with her separation from the Company as shown in the "All Other Compensation" column. |
| (7) | On November 28, 2011, the Company announced Mr. Hirsch’s separation from the Company as Vice President Information Technology and Chief Information Officer. Mr. Hirsch received $240,000 in severance in connection with his separation from the Company as shown in the "All Other Compensation" column. |
Stock and Option Award Grants and Exercises
The following table summarizes information concerning grants of plan-based awards made by us for services rendered during the fiscal year ended December 31, 2011 to each of the Named Executive Officers.
Grants of Plan-Based Awards
| All Other | ||||||||||||||||||||||||||||
| Estimated Future Payouts Under | Option Awards | Exercise or | Grant Date Fair | |||||||||||||||||||||||||
| Non- Equity Incentive Plan Awards | Number of Securities | Base Price of | Value of Stock | |||||||||||||||||||||||||
| Threshold | Target | Maximum | Underlying Options | Option Awards | and Options | |||||||||||||||||||||||
| Name | Grant Date | ($) | ($) | ($) | (#) | ($/Sh) (1) | Awards (2) | |||||||||||||||||||||
| Jeffrey Horowitz | 2011 | - | $ | 275,000 | - | - | - | - | ||||||||||||||||||||
| 6/22/2011 | - | - | - | 582,750 | $ | 3.75 | $ | 1,497,667 | ||||||||||||||||||||
| 9/12/2011 | - | - | - | 367,250 | $ | 4.33 | $ | 936,488 | ||||||||||||||||||||
| Stephen E. Markert, Jr. | 2011 | - | $ | 150,000 | - | - | - | - | ||||||||||||||||||||
| David Zucker | 2011 | - | $ | 150,000 | - | - | - | - | ||||||||||||||||||||
| 9/12/2011 | - | - | - | 300,000 | $ | 4.33 | $ | 789,000 | ||||||||||||||||||||
| Robert Wegner | 2011 | - | $ | 125,000 | - | - | - | - | ||||||||||||||||||||
| 9/12/2011 | - | - | - | 300,000 | $ | 4.33 | $ | 789,000 | ||||||||||||||||||||
| Nachiket Desai | 2011 | - | $ | 125,000 | - | - | - | - | ||||||||||||||||||||
| 12/9/2011 | - | - | - | 250,000 | $ | 6.44 | $ | 977,500 | ||||||||||||||||||||
| Mary Marbach | 2011 | - | $ | 100,000 | - | - | - | - | ||||||||||||||||||||
| (1) | The exercise price for the stock option was the closing price our common stock on the grant date, as reported on the NASDAQ Stock Market. |
| (2) | The grant date fair value of options is based on the fair value calculated pursuant to Statement of Financial Accounting Codification Topic 718. For a discussion of the valuation assumptions, see Note 1 to our consolidated financial statements. |
| 59 |
Outstanding Equity Awards at Fiscal Year End
| Name | Number of Securities Underlying Unexercised Options (1) Exercisable | Number of Securities Underlying Unexercised Options (1) Unexercisable | Option Exercise Price | Option Expiration Date | ||||||||||||
| Jeffrey Horowitz | 200,000 | - | $ | 8.910 | 8/1/2021 | |||||||||||
| 242,528 | 340,222 | (2) | $ | 3.750 | 6/21/2022 | |||||||||||
| 51,010 | 316,240 | (3) | $ | 4.330 | 9/11/2022 | |||||||||||
| David Zucker | 300,000 | (4) | $ | 4.330 | 9/11/2021 | |||||||||||
| Robert Wegner | 300,000 | (5) | $ | 4.330 | 9/11/2021 | |||||||||||
| Nachiket Desai | 250,000 | (6) | $ | 6.440 | 12/8/2021 | |||||||||||
| Mary Marbach | 10,000 | 40,000 | (7) | $ | 9.720 | 2/3/2019 | ||||||||||
| Sonya L. Lambert | 35,200 | - | $ | 3.750 | 3/4/2015 | |||||||||||
| 20,000 | - | $ | 3.750 | 3/31/2016 | ||||||||||||
| 80,000 | - | $ | 3.750 | 9/19/2016 | ||||||||||||
| 80,000 | - | $ | 7.500 | 12/30/2017 | ||||||||||||
| 80,000 | - | $ | 12.000 | 9/23/2019 | ||||||||||||
| Robert D. Hirsch | 80,000 | - | $ | 7.500 | 12/14/2018 | |||||||||||
| 20,000 | - | $ | 12.000 | 9/23/2019 | ||||||||||||
| (1) | The aggregate number of option awards outstanding at December 31, 2011 was 4,262,230. The aggregate number of option awards owned by our Named Executive Officers at December 31, 2011 was 2,445,200. |
| (2) | These options vest as follows: 10,632 on the 15th day of each month. |
| (3) | These options vest as follows: 10,202 on the 16th day of each month through December 16, 2012 and then 10,201 on the 16th day of each month through July 16, 2014. |
| (4) | These options vest as follows: 60,000 vest on each of September 12, 2012, 2013, 2014, 2015 and 2016, respectively. |
| (5) | These options vest as follows: 60,000 vest on each of September 12, 2012, 2013, 2014, 2015 and 2016, respectively. |
| (6) | These options vest as follows: 50,000 vest on each of December 9, 2012, 2013, 2014, 2015 and 2016, respectively. |
| (7) | These options vest as follows: 10,000 vest on each of February 2, 2012, 2013, 2014 and 2015, respectively. |
Pension Benefits
We do not maintain any defined benefit pension plans.
Nonqualified Deferred Compensation
We do not maintain any nonqualified deferred compensation plans.
| 60 |
Fiscal 2011 Potential Payments Upon Termination or Change In Control Table
If the Named Executive Officers' employment had been terminated on December 31, 2011 for any reason other than a change of control (as defined in the employment agreements entered into with each Named Executive Officer) or terminated pursuant to a change of control on December 31, 2011, each executive would have received the payments described in the following table.
| Name | Benefit | Termination with Cause | Termination without Cause | Termination due to Change in Control | Death | Disability | ||||||||||||||||||
| Jeffrey Horowitz | Continuation of Salary (1) | $ | - | $ | 400,000 | $ | 400,000 | $ | - | $ | - | |||||||||||||
| - | - | - | - | - | ||||||||||||||||||||
| $ | - | $ | 400,000 | $ | 400,000 | $ | - | $ | - | |||||||||||||||
| Stephen Markert | Continuation of Salary | $ | - | $ | 225,000 | $ | 225,000 | $ | - | $ | - | |||||||||||||
| - | - | - | - | - | ||||||||||||||||||||
| $ | - | $ | 225,000 | $ | 225,000 | $ | - | $ | - | |||||||||||||||
| David Zucker | Continuation of Salary (2)(3)(4) | $ | - | $ | 150,000 | $ | 150,000 | $ | 75,000 | $ | 150,000 | |||||||||||||
| - | - | - | - | - | ||||||||||||||||||||
| $ | - | $ | 150,000 | $ | 150,000 | $ | 75,000 | $ | 150,000 | |||||||||||||||
| Robert Wegner | Continuation of Salary (2)(3)(4) | $ | - | $ | 125,000 | $ | 125,000 | $ | 62,500 | $ | 125,000 | |||||||||||||
| - | - | - | - | - | ||||||||||||||||||||
| $ | - | $ | 125,000 | $ | 125,000 | $ | 62,500 | $ | 125,000 | |||||||||||||||
| Nachiket Desai | Continuation of Salary (2)(3)(4) | $ | - | $ | 125,000 | $ | 125,000 | $ | 62,500 | $ | 125,000 | |||||||||||||
| - | - | - | - | - | ||||||||||||||||||||
| $ | - | $ | 125,000 | $ | 125,000 | $ | 62,500 | $ | 125,000 | |||||||||||||||
| Mary Marbach | Continuation of Salary (2)(3)(4) | $ | - | $ | 100,000 | $ | 100,000 | $ | 50,000 | $ | 50,000 | |||||||||||||
| - | - | - | - | - | ||||||||||||||||||||
| $ | - | $ | 100,000 | $ | 100,000 | $ | 50,000 | $ | 50,000 | |||||||||||||||
| (1) | Payable over a 12 month period subject to executive executing and delivering to us a full and unconditional release and executive paying any and all amounts owed to us under any contract, agreement or loan document. |
| (2) | Termination without cause obligation payable in a lump sum within 30 days subject to executive executing and delivering to us a full and unconditional release and executive paying any and all amounts owed to us under any contract, agreement or loan document. |
| (3) | Change in control amount obligation in a single lump sum within 10 days of termination subject to executive executing and delivering to us a full and unconditional release and executive paying any and all amounts owed to us under any contract, agreement or loan document. |
| (4) | Death or disability obligation payable in a single lump sum within 30 days |
Employment Agreements and Change-In-Control Arrangements
Named Executive Officers
Jeffrey Horowitz
On February 17, 2011, in connection with his appointment as Chief Executive Officer, the Company and Mr. Horowitz entered into an employment agreement that has a four year term from its commencement date of August 16, 2010 and supersedes his previous consulting agreement with the Company. In consideration for Mr. Horowitz's services, we will pay Mr. Horowitz an annual salary of $400,000. For the period from August 16, 2010 through December 31, 2010, Mr. Horowitz was paid a bonus of $75,000 and for the 2011 calendar year, Mr. Horowitz will be paid a guaranteed bonus of $275,000. For the period from August 16, 2010 until the date of the agreement, Mr. Horowitz was paid a lump sum equal to the difference between the annual salary that would have been paid under the employment agreement and the amount he was paid pursuant to his consulting agreement for such period. Mr. Horowitz was also granted nonqualified stock options to purchase 950,000 shares of the common stock of the Company. The options will vest periodically and will be fully vested on August 15, 2014. Under the employment agreement, in the event Mr. Horowitz's employment is terminated by us without cause or by Mr. Horowitz for “good reason” (as such terms are defined in the employment agreement), Mr. Horowitz will be entitled to receive the following: severance equal to twelve months of Mr. Horowitz's annual salary or, if less, the salary that would have been paid had he remained employed through August 15, 2014; prorated annual bonus for the year of termination; and complete vesting of any stock options that had not yet vested. If Mr. Horowitz's employment is terminated due to his death or disability, he or his estate will be entitled to payment of a prorated annual bonus for the year of termination. Mr. Horowitz's receipt of the severance benefits discussed above is contingent on Mr. Horowitz signing and not revoking a release of claims against us.
| 61 |
Stephen E. Markert, Jr.
On October 19, 2010, we entered into an employment agreement with Stephen E. Markert, Jr., Interim Chief Financial Officer, which can be terminated by the employee or us upon 60 days written notice. Mr. Markert initially received a salary of $235,000 annually, and was eligible to earn discretionary bonuses and employee benefits commensurate with his position. On May 14, 2011, Mr. Markert’s salary was increased to $300,000 annually. Mr. Markert is also entitled to reimbursement of living expenses of up to $2,000 per month. Under his agreement, Mr. Markert is subject to a confidentiality, non-solicitation and non-competition agreement during the period he is employed for a one-year period following termination. On January 25, 2012, Mr. Markert informed us of his resignation as Interim Chief Financial Officer. On February 24, 2012, we and Mr. Markert entered into an Employment Termination and General Release Agreement (the “Agreement”) setting forth the terms of Mr. Markert’s departure. Under the terms of the Agreement, Mr. Markert’s employment was terminated effective February 24, 2012. Subject to receipt of an unconditional release and any amounts owed by Mr. Markert to us, we will pay to Mr. Markert a lump sum payment of $225,000.
David Zucker
On August 18, 2011, in connection with Mr. Zucker’s appointment as Chief Marketing Officer, we and Mr. Zucker entered into an employment agreement (the “Employment Agreement”), outlining the terms of Mr. Zucker’s employment. Under the terms of the Employment Agreement, Mr. Zucker will receive a base salary of $300,000 per year and an annual bonus equal to up to 50% of his base salary, which is guaranteed on a prorated basis for 2011. Subject to the approval of the award grant by our Board of Directors, we issued to Mr. Zucker options to purchase 300,000 shares of our common stock. The options will vest 20% per year over five years. In addition, Mr. Zucker receives $5,000 monthly for living and travel expenses and is eligible to participate in our standard employee benefits programs available to senior executives. The term of the Employment Agreement is one year, renewing automatically for additional one year periods unless the Employment Agreement is terminated by Mr. Zucker or us, in each case by 30 days’ written notice. In addition, we may terminate Mr. Zucker’s employment at any time, with or without cause, and Mr. Zucker may terminate his employment on 30 days’ written notice or immediately for good reason, as defined in the Employment Agreement. Under the Employment Agreement, in the event Mr. Zucker’s employment is terminated without cause, Mr. Zucker will be entitled to receive severance benefits equal to six months of his base salary, plus a prorated bonus for the year of termination. In the event that Mr. Zucker’s employment is terminated without cause or by Mr. Zucker for good reason during the twelve months following a change in control of the Company, then Mr. Zucker will be entitled to receive a bonus prorated through the date of his termination and a severance amount equal to six months of his current base salary. Mr. Zucker, or his estate, is also entitled to a prorated bonus and other benefits in the event that Mr. Zucker’s employment is terminated due to his death or disability.
Robert Wegner
On August 4, 2011, in connection with Mr. Wegner’s appointment as Chief Operating Officer, we and Mr. Wegner entered into an employment agreement (the “Employment Agreement”), outlining the terms of Mr. Wegner’s employment. Under the terms of the Employment Agreement, Mr. Wegner will receive a base salary of $250,000 per year and an annual bonus equal to up to 50% of his base salary, which is guaranteed on a prorated basis for 2011. Subject to the approval of the award grant by our Board of Directors, we issued to Mr. Wegner options to purchase 300,000 shares of our common stock. The options will vest 20% per year over five years. In addition, Mr. Wegner received $25,000 for relocation expenses and is eligible to participate in our standard employee benefits programs available to senior executives. The term of the Employment Agreement is one year, renewing automatically for additional one year periods unless the Employment Agreement is terminated by Mr. Wegner or us, in each case by 30 days’ written notice. In addition, we may terminate Mr. Wegner’s employment at any time, with or without cause, and Mr. Wegner may terminate his employment on 30 days’ written notice or immediately for good reason, as defined in the Employment Agreement. Under the Employment Agreement, in the event Mr. Wegner’s employment is terminated without cause, Mr. Wegner will be entitled to receive severance benefits equal to six months of his base salary, plus a prorated bonus for the year of termination. In the event that Mr. Wegner’s employment is terminated without cause or by Mr. Wegner for good reason during the twelve months following a change in control of the Company, then Mr. Wegner will be entitled to receive a bonus prorated through the date of his termination and a severance amount equal to six months of his current base salary. Mr. Wegner, or his estate, is also entitled to a prorated bonus and other benefits in the event that Mr. Wegner’s employment is terminated due to his death or disability. Mr. Wegner’s receipt of the severance benefits discussed above is contingent on Mr. Wegner signing and not revoking a release of claims against us.
Nachiket Desai
On November 28, 2011, in connection with Mr. Desai’s appointment as Chief Information Officer, we and Mr. Desai entered into an employment agreement (the “Employment Agreement”), outlining the terms of Mr. Desai’s employment. Under the terms of the Employment Agreement, Mr. Desai will receive a base salary of $250,000 per year and an annual bonus of up to 50% of his base salary. Subject to the approval of the award grant by our Board of Directors, we issued to Mr. Desai options to purchase 250,000 shares of our common stock. The options will vest 20% per year over five years. In addition, Mr. Desai will receive $5,000 per month for living and travel expenses for his first three months’ of employment, $25,000 in relocation assistance and is eligible to participate in our standard employee benefits programs available to senior executives. The term of the Employment Agreement is one year, renewing automatically for additional one year periods unless the Employment Agreement is terminated by Mr. Desai or us, in each case by 30 days’ written notice. In addition, we may terminate Mr. Desai’s employment at any time, with or without cause, and Mr. Desai may terminate his employment on 30 days’ written notice or immediately for good reason, as defined in the Employment Agreement. Under the Employment Agreement, in the event Mr. Desai’s employment is terminated without cause, Mr. Desai will be entitled to receive severance benefits equal to six months of his base salary, plus a prorated bonus for the year of termination. In the event that Mr. Desai’s employment is terminated without cause or by Mr. Desai for good reason during the twelve months following a change in control of the Company, then Mr. Desai will be entitled to receive a bonus prorated through the date of his termination and a severance amount equal to six months of his current base salary. Mr. Desai, or his estate, is also entitled to a prorated bonus and other benefits in the event that Mr. Desai’s employment is terminated due to his death or disability.
| 62 |
Sonya L. Lambert
Effective April 1, 2007, we entered into an employment, non-competition and proprietary rights agreement with Sonya L. Lambert, Chief Marketing Officer, for a one-year term, automatically renewable for additional one-year terms annually, unless otherwise terminated by the employee or us. Under the original agreement, Ms. Lambert earned a salary of $120,000 annually and was eligible to receive a marketing bonus based on performance (up to $21,600 per quarter), vacation and employee benefits commensurate with her position. If Ms. Lambert's employment is terminated by us without “cause” (as defined in the agreement), in addition to any compensation and benefits accrued through such termination, Ms. Lambert may, subject to her execution of a general release of claims against us, receive a lump-sum severance payment of up to 12 months' base salary plus a prorated portion of the performance bonuses, as well as up to 18 months of company-paid continuation medical benefits. If Ms. Lambert's employment terminates due to her death or disability, she or her estate will receive a lump sum payment equal to the sum of three months' base salary, accrued yet unused vacation pay and a prorated bonus, in addition to any compensation and benefits accrued through such termination. Under her agreement, Ms. Lambert was subject to a confidentiality, non-solicitation and non-competition agreement during the period she is employed and for a period thereafter. The confidentiality and non-competition provisions extend for a two-year period following termination and the non-solicitation provision extends for a period of one year following termination. For the year ended December 31, 2008, Ms. Lambert's base salary under the agreement was increased to $144,000.
On June 30, 2009, the agreement was amended to increase her annual base salary to $165,600 and to increase the maximum amount of the quarterly bonus she may receive to $25,920 per quarter. Further, pursuant to the amendment, effective as of the successful registration of our initial public offering: (i) so long as Ms. Lambert had not voluntarily resigned from her employment with us, all of Ms. Lambert's then-outstanding options, to the extent necessary, became fully vested and non-forfeitable; and (ii) so long as Ms. Lambert was then employed by us, Ms. Lambert was issued fully vested, non-forfeitable options to purchase 100,000 shares of our common stock at the initial public offering price (or 80,000 shares of our common stock after giving effect to the four-for-five reverse stock split of our common stock effected on September 17, 2009).
On March 22, 2010, the amended agreement was further amended to provide for a two-year term commencing on March 15, 2010, renewable upon mutual agreement by us and Ms. Lambert. The amendment further amended the agreement to provide that in the event of a termination by us of Ms. Lambert “without cause” (as defined in the agreement), then, in addition to any compensation and benefits accrued through such termination, and subject to her execution of a general release of claims against us, and subject to her compliance with her confidentiality, non-solicitation and non-competition agreement, Ms. Lambert is entitled to (i) a severance payment equal to the greater of (a) two times the sum of her then current base salary and the average of her prior two years' annual bonus, or (b) the amount she would be entitled to receive (e.g. base salary, bonus, vacation pay) for the remainder of the term as if she remained employed until the last day of such term, payable in 24 equal monthly payments and (ii) 18 months of company-paid continuation medical benefits. In the event of a termination by the Company without cause within the time periods specified above, and subject to her execution of a general release of claims against us, and subject to her compliance with her confidentiality, non-solicitation and non-competition agreement, then Ms. Lambert would be entitled to the severance payment of $685,800. The amendment further amended the agreement to provide that if the employment of Ms. Lambert is terminated following a “Change in Control” (as defined in the amendment), either by the Company without cause or by Ms. Lambert for “Good Reason” (as defined in the amendment), then Ms. Lambert is entitled to the following severance terms (in addition to any compensation and benefits accrued through such termination), subject to her execution of a general release of claims against us, and subject to her compliance with her confidentiality, non-solicitation and non-competition agreement: if terminated without cause or for Good Reason within 18 months after Change in Control, a lump sum payment equal to two times her then current base salary and two times the higher of (i) the average of the prior two years' annual bonus and (ii) last year's bonus. If the employment of Ms. Lambert is terminated following a Change in Control, either by the Company without cause or by Ms. Lambert for Good Reason, within the time periods specified above, subject to her execution of a general release of claims against us, and subject to her compliance with her confidentiality, non-solicitation and non-competition agreement, then, subject to the following sentence, Ms. Lambert would be entitled to the severance payment of $685,800. Notwithstanding the foregoing, however, the second amendment also provided for certain cutbacks of amounts owed to Ms. Lambert in the event such payments to be made to her on account of a Change in Control was deemed to be “excess parachute payments” as defined in Section 280G of the Code and, as a result, Ms. Lambert may not receive that total severance payment amount. Capitalized terms used in this paragraph with regard to certain employment agreements have the meaning ascribed to them in that certain employment agreement.
On August 18, 2011 the Company and Ms. Lambert mutually agreed to the immediate termination of her employment with the Company as Chief Marketing Officer. In connection with the termination, Ms. Lambert received a severance payment of $685,800.
Robert D. Hirsch
On September 16, 2008, we entered into an employment, non-competition and proprietary rights agreement with Robert D. Hirsch, Vice President Information Technology and Chief Information Officer, for a one-year term, automatically renewable for additional one-year terms annually, unless otherwise terminated by the employee or us. The contract term has been automatically renewed each year since September 16, 2008, with the current one-year term continuing to September 17, 2011. Under the original agreement, Mr. Hirsch earned a salary of $145,000 annually, and was eligible to earn annual performance bonuses, vacation and employee benefits commensurate with his position. On the initial date of Mr. Hirsch's employment, he was also granted options to purchase up to 100,000 shares of our common stock at $6.00 per share (or 80,000 shares at $7.50 per share after giving effect to the four-for-five reverse stock split of our common stock affected on September 17, 2009). These options vest 20% each year on the anniversary date of Mr. Hirsch's employment agreement and are fully vested after five years. Each option has a term of nine years. Upon Mr. Hirsch's termination of employment for any reason, all unvested options are forfeited to us and all vested options must be exercised within 30 days and if not exercised during the 30-day period will be forfeited to us. If Mr. Hirsch's employment is terminated by us without “cause” (as defined in the agreement), in addition to any compensation and benefits accrued through such termination, Mr. Hirsch may, subject to his execution of a general release of claims against us, receive a lump-sum severance payment in an amount equal to the sum of two weeks' base salary for each year served, up to a total of six weeks' base salary, accrued yet unused vacation pay and a prorated portion of the annual performance bonus earned through the date of termination, as well as up to 18 months of employee-paid continuation medical benefits. If Mr. Hirsch's employment terminates due to his death or disability, he or his estate will receive a lump sum payment equal to the sum of three months' base salary accrued yet unused vacation pay and a prorated portion of the annual performance bonus earned through the date of termination, in addition to any compensation and benefits accrued through such termination. Under his agreement, Mr. Hirsch is subject to a confidentiality, non-solicitation and non-competition agreement during the period he is employed and for a period of two years thereafter.
| 63 |
On June 30, 2009, the agreement was amended to increase his annual base salary to $166,750. Further, pursuant to the amendment, effective as of the successful registration our initial public offering: (i) so long as Mr. Hirsch had not voluntarily resigned from his employment with us, all of Mr. Hirsch's then-outstanding options, to the extent necessary, became fully vested and non-forfeitable; and (ii) so long as Mr. Hirsch was then employed by us, Mr. Hirsch was issued fully vested, non-forfeitable options to purchase 25,000 shares of our common stock at the initial public offering price (or 20,000 shares of our common stock after giving effect to the four-for-five reverse stock split of our common stock effected on September 17, 2009).
On March 22, 2010, the amended agreement was further amended to provide that in the event of a termination by us of Mr. Hirsch “without cause” (as defined in the agreement), then, in addition to any compensation and benefits accrued through such termination, and subject to his execution of a general release of claims against us, and subject to his compliance with his confidentiality, non-solicitation and non-competition agreement, Mr. Hirsch is entitled to (i) a severance payment equal to the greater of (a) the sum of his then current base salary and the average of his prior two years' annual bonus, or (b) the amount he would be entitled to receive (e.g. base salary, bonus, vacation pay) for the remainder of the term as if he remained employed until the last day of such term, payable in 12 equal monthly payments and (ii) 18 months of company-paid continuation medical benefits. In the event of a termination by the Company without cause within the time periods specified above, and subject to his execution of a general release of claims against us, and subject to his compliance with his confidentiality, non-solicitation and non-competition agreement, then Mr. Hirsch would be entitled to the severance payment of $215,300. The amendment further amended the agreement to provide that if the employment of Mr. Hirsch is terminated following a “Change in Control” (as defined in the amendment), either by the Company without cause or by Mr. Hirsch for “good reason” (as defined in the amendment), then Mr. Hirsch is entitled to the following severance terms (in addition to any compensation and benefits accrued through such termination), subject to his execution of a general release of claims against us, and subject to his compliance with his confidentiality, non-solicitation and non-competition agreement: if terminated Without Cause or for Good Reason within 12 months after a Change in Control, a lump sum payment equal to his then current base salary and the higher of (i) the average of the prior two years' annual bonus and (ii) last year's bonus. If the employment of Mr. Hirsch is terminated following a Change in Control, either by the Company Without Cause or by Mr. Hirsch for Good Reason, within the time periods specified above, and subject to his execution of a general release of claims against us, and subject to his compliance with his confidentiality, non-solicitation and non-competition agreement, then, subject to the following sentence, Mr. Hirsch would be entitled to the severance payment of $234,740. Notwithstanding the foregoing, however, the second amendment also provided for certain cutbacks of amounts owed to Mr. Hirsch in the event such payments to be made to him on account of a Change in Control was deemed to be “excess parachute payments” as defined in Section 280G of the Code and, as a result, Mr. Hirsch may not receive that total severance payment amount. Capitalized terms used in this paragraph with regard to certain employment agreements have the meaning ascribed to them in that certain employment agreement.
On November 22, 2011, the Company and Mr. Hirsch mutually agreed to the immediate termination of his employment with the Company as Chief Information Officer. In connection with the termination, Mr. Hirsch received a severance payment of $240,000.
Mary L. Marbach
On December 2, 2009, we entered into an employment, non-competition and proprietary rights agreement with Mary L. Marbach, General Counsel, for a one-year term, automatically renewable for additional one-year terms annually, unless otherwise terminated by the employee or us. The current one-year term will continue until December 2, 2012. Ms. Marbach earns a salary of $200,000 annually, and is eligible to earn annual performance bonuses, vacation and employee benefits commensurate with her position. If Ms. Marbach’s employment is terminated by us without “cause” (as defined in her agreement), in addition to any compensation and benefits accrued through such termination, Ms. Marbach may, subject to her execution of a general release of claims against us, receive a lump-sum severance payment in an amount equal to six months’ of base salary, accrued yet unused vacation pay and a prorated portion of the annual performance bonus earned through the date of termination, as well as up to 18 months of employee-paid continuation medical benefits. If Ms. Marbach’s employment is terminated by us without “cause” or by Ms. Marbach for “good reason” (as defined in her agreement), in either case during the twelve month period immediately following a “change in control” (as defined in her agreement), then in lieu of the amounts specified in the preceding sentence, in addition to any compensation and benefits accrued through such termination, Ms. Marbach shall be entitled to a severance amount equal to 6 months of her then base salary. If Ms. Marbach’s employment terminates due to her death or disability, she or her estate will receive a lump sum payment equal to the sum of three months’ base salary, accrued yet unused vacation pay and a prorated portion of the annual performance bonus earned through the date of termination, in addition to any compensation and benefits accrued through such termination. Under her agreement, Ms. Marbach is subject to a confidentiality, non-solicitation and non-competition agreement during the period she is employed and for a period of two years thereafter.
| 64 |
Director Compensation
Directors who are also our employees are not separately compensated for their services as directors but are reimbursed for out-of-pocket expenses incurred in connection with providing board services. The following table summarizes compensation earned by our non-employee directors in 2011:
| Fees Earned or Paid in Cash | Option Awards | All Other Compensation | Total Compensation | |||||||||||||
| Michael A. Kumin, Chairman | $ | 25,000 | $ | 62,250 | $ | - | $ | 87,250 | ||||||||
| Edwin Kozlowski | $ | 20,000 | $ | 41,500 | $ | - | $ | 61,500 | ||||||||
| Robert G. Trapp, M.D. | $ | 15,000 | $ | 20,750 | $ | - | $ | 35,750 | ||||||||
| Christopher S. Gaffney | $ | 15,000 | $ | 20,750 | $ | - | $ | 35,750 | ||||||||
| Stuart Goldfarb | $ | 15,000 | $ | 20,750 | $ | - | $ | 35,750 | ||||||||
| Jeffrey M. Stibel (1) | $ | 2,500 | $ | - | $ | - | $ | 2,500 | ||||||||
| (1) | Mr. Stibel resigned from our board effective February 28, 2011. |
The aggregate number of shares subject to stock options outstanding at December 31, 2011 for each non-employee director was as follows:
|
Name |
Aggregate Number of Options Awards Outstanding as of December 31, 2011 | |||
| Michael A. Kumin, Chairman | 15,000 | |||
| Edwin Kozlowski | 10,000 | |||
| Robert G. Trapp, M.D. | 24,800 | |||
| Christopher S. Gaffney | 5,000 | |||
| Stuart Goldfarb | 5,000 | |||
Cash Compensation of Non-Employee Directors in Fiscal 2011
During fiscal 2011, each of our non-employee directors received an annual retainer of $15,000 for service on the board and any committee of the board, except for the Chairman of the board, who received an annual retainer of $25,000 in lieu of the standard annual retainer of $15,000 for his services in such capacity. Directors were also reimbursed for certain expenses they incurred in connection with attendance at board and committee meetings. The Chairman of the audit committee received the additional annual compensation of $5,000 for his services as Chairman of the audit committee in fiscal 2011. The cash retainers are paid quarterly.
Equity and Non-cash Compensation of Non-Employee Directors in Fiscal 2011
The current practice of our board is to approve the grant of an option to purchase 5,000 shares of our common stock for each director. The exercise price for all options granted is 100% of the fair market value of the shares on the grant date. The options vest immediately upon grant. The Chairman of the board is also granted of an option to purchase an additional 10,000 shares of our common stock and the chairman of the audit committee is granted an additional 5,000 shares of our common stock.
Compensation Committee Interlocks and Insider Participation
Our compensation committee currently consists of Messrs. Gaffney and Kumin. None of the members of our compensation committee has, at any time, served as an officer or employee of Vitacost. None of our executive officers currently serves, or in the past year has served, as a member of the board of directors or compensation committee of any entity that has one or more executive officers serving on our board of directors or compensation committee.
| 65 |
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
The following table sets forth certain information regarding the beneficial ownership of our common stock on March 12, 2012, by the following:
| • | each of our directors and executive officers listed in the “Summary Compensation Table” within this Annual Report on Form 10-K, |
| • | all of our directors and executive officers as a group; and, |
| • | each person known by us to beneficially own more than 5% of our outstanding common stock. |
Beneficial ownership is determined in accordance with the rules of the SEC. In computing the number of shares beneficially owned by a person and the percentage ownership of that person, shares of common stock subject to options or warrants held by that person that are currently exercisable or exercisable within 60 days of March 12, 2012 are deemed outstanding, but are not deemed outstanding for computing the percentage ownership of any other person.
Except as otherwise indicated and subject to applicable community property laws, each person named in the table has sole voting and investment power with respect to the shares set forth opposite such person's name.
| Number of | Percent | |||||||
| Shares | Beneficially | |||||||
| Beneficially | Owned | |||||||
| Name and Address of Beneficial Owner (1) | Owned | |||||||
| Directors and Executive Officers : | ||||||||
| Jeffrey J. Horowitz (2) | 3,936,517 | 11.8 | % | |||||
| Brian D. Helman | — | * | ||||||
| David A. Zucker | — | * | ||||||
| Robert Wegner (3) | 3,000 | * | ||||||
| Nachiket Desai | — | * | ||||||
| Mary L. Marbach (4) | 20,180 | * | ||||||
| Christopher S. Gaffney (5) | 6,367,713 | 19.16 | % | |||||
| Stuart Goldfarb(6) | 5,000 | * | ||||||
| Edwin J. Kozlowski (7) | 10,000 | * | ||||||
| Michael A. Kumin (8) | 35,630 | * | ||||||
| Robert G. Trapp, M.D. (9) | 758,926 | 2.3 | % | |||||
| All current directors and executive officers as a group (11) persons) (10) | 11,136,966 | 33.5 | % | |||||
| 5% Stockholders : | ||||||||
| Group comprised of Great Hill Investors, LLC, Great Hill Equity Partners III, L.P. and Great Hill Equity Partners IV, L.P. (11) | 6,362,713 | 19.15 | % | |||||
| Group comprised of Freshford Capital Management, LLC, Freshford GP, LLC and Freshford Partners, LP (12) | 3,483,160 | 10.5 | % | |||||
| Group comprised of Baron Capital Group, Inc., BAMCO, Inc., Baron Capital Management, Inc. and Ronald Baron (13) | 2,325,000 | 7.0 | % | |||||
| Jennison Associates, LLC and Prudential Financial, Inc. (14) | 3,098,654 | 9.3 | % | |||||
| Allen S. Josephs (15) | 2,082,197 | 6.3 | % | |||||
| JHH Capital, LLC (16) | 3,049,013 | 9.2 | % | |||||
| Royce & Associates, LLC (17) | 1,537,661 | 4.6 | % | |||||
* Less than 1%
| 66 |
.
| 1 | Except as otherwise indicated, each person may be reached at our Company’s corporate address at Vitacost.com Inc., 5400 Broken Sound Boulevard, NW, Suite 500, Boca Raton, FL 33487. |
| 2 | Mr. Horowitz owns 300,000 shares of Common Stock individually. Mr. Horowitz holds options to purchase 200,000 shares of Common Stock exercisable at $8.91 per share, all of which are immediately exercisable and are included in the table above. Mr. Horowitz also holds options to purchase 582,750 shares of Common Stock at an exercise price of $3.75, of which 295,686 are immediately exercisable or exercisable within 60 days of March 12, 2012 and are included in the table above. Mr. Horowitz also holds options to purchase 367,250 shares of Common Stock at an exercise price of $4.33, of which 91,818 are immediately exercisable or exercisable within 60 days of March 12, 2012 and are included in the table above. For additional information regarding Mr. Horowitz's beneficial ownership of shares of common stock, see note (16), below. |
| 3 | Mr. Wegner owns 3,000 shares of Common Stock individually. |
| 4 | Ms. Marbach owns 145 shares of Common Stock individually and 35 shares are held in trust for her minor child. Ms. Marbach holds options to purchase 50,000 shares of Common Stock exercisable at $9.72 per share, of which 20,000 are immediately exercisable or exercisable within 60 days of March 12, 2012. |
| 5 | Mr. Gaffney owns no shares of Common Stock individually. Mr. Gaffney holds options to purchase 5,000 shares of Common Stock at an exercise price of $3.75, all of which are immediately exercisable. For additional information regarding Mr. Gaffney’s beneficial ownership of shares of Common Stock, see note (11), below. |
| 6 | Mr. Goldfarb holds options to purchase 5,000 shares of Common Stock at an exercise price of $3.75, all of which are immediately exercisable. |
| 7 | Mr. Kozlowski holds options to purchase 10,000 shares of Common Stock at an exercise price of $3.75, all of which are immediately exercisable. |
| 8 | Mr. Kumin owns 20,630 shares of Common Stock. Mr. Kumin also holds options to purchase 15,000 shares of Common Stock at an exercise price of $3.75, all of which are immediately exercisable. |
| 9 | Dr. Trapp owns 734,126 shares of Common Stock and holds options to purchase 24,800 shares of Common Stock exercisable at the following prices: (i) 2,000 at $0.16 per share; (ii) 800 at $1.88 per share; (iii) 800 at $2.50 per share; (iv) 800 at $3.13 per share; (v) 10,400 at $7.50 per share; (vi) 5,000 at $12.00 per share, and (vii) 5,000 at $3.75 per share. All options are immediately exercisable. |
| 10 | Includes shares beneficially owned by all current directors and executive officers as of March 12, 2012. |
| 11 | Based on the statement on the Schedule 13D (Amendment No. 7) filed with the SEC on February 21, 2012, Mr. Gaffney, Matthew T. Vettel and John G. Hayes have reported shared voting and dispositive power with respect to all 6,362,713 shares of Common Stock. Great Hill Investors, LLC has reported shared voting and dispositive power with respect to 18,550 shares of Common Stock. Great Hill Equity Partners III, L.P. has reported shared voting and dispositive power with respect to 4,161,898 shares of Common Stock. Great Hill Partners GP III, L.P. has reported shared voting and dispositive power with respect to 4,161,898 shares of Common Stock. GHP III, LLC has reported shared and dispositive power with respect to 4,161,898 shares of Common Stock. Great Hill Equity Partners IV, L.P. has reported shared voting and dispositive power with respect to 2,182,165 shares of Common Stock. Great Hill Partners GP IV, L.P. has reported shared voting and dispositive power with respect to 2,182,265 shares of Common Stock. GHP IV, LLC has reported shared voting and dispositive power with respect to 2,182,265 shares of Common Stock. Pursuant to the Warrant to Purchase Common Stock of Vitacost.com, Inc., dated February 16, 2012, Great Hill Investors LLC, Great Hill Equity Partners III, L.P. and Great Hill Equity Partners IV, L.P. (collectively “Great Hill”) hold warrants to purchase 776,286 shares of Common Stock from Vitacost.com, Inc., however these warrants are only currently exercisable to the extent that the Great Hill’s proportionate share of outstanding Common Stock does not exceed 19.16%. GPIII is the sole general partner of GHEPIII, and the sole general partner of GPIII is GHPIII. The sole general partner of GHEPIV is GPIV, and the sole general partner of GPIV is GHPIV. Messrs. Gaffney and Hayes are managers of GHI, and managers of the general partners of GHPIII and GHPIV, and Mr. Vettel is a manager of GHPIII and GHPIV. GPIII may be deemed to indirectly beneficially own the shares of Common Stock beneficially owned by GHEPIII, and GHPIII may be deemed to indirectly beneficially own the shares of Common Stock beneficially owned by GHEPIII and that may be deemed indirectly beneficially owned by GPIII. GPIV may be deemed to indirectly beneficially own the shares of Common Stock beneficially owned by GHEPIV, and GHPIV may be deemed to indirectly beneficially own the shares of Common Stock beneficially owned by GHEPIV and that may be deemed indirectly beneficially owned by GPIV. Each of Messrs. Gaffney and Hayes may be deemed to indirectly beneficially own the shares of Common Stock beneficially owned by GHI, GHPIII and GHPIV, and Mr. Vettel may be deemed to indirectly beneficially own the shares of Common Stock beneficially owned by GHPIII and GHPIV. Each of Messrs. Gaffney, Hayes and Vettel, GHI, GHPIII and GHPIV disclaims beneficial ownership of such shares of Common Stock. The address of each of the above entities and individuals is One Liberty Square, Boston, Massachusetts 02109. |
| 12 | Based on the statement on the Amended Schedule 13G filed with the SEC on February 27, 2012, Freshford Capital Management, LLC has reported sole voting and dispositive power with respect to 894,475 shares of Common Stock and shared voting and dispositive power with respect to 2,588,685 shares of Common Stock. Freshford GP, LLC and Freshford Partners, LP have reported shared voting and dispositive power with respect to 2,588,685 shares of Common Stock. Each of Freshford Capital Management, LLC, Freshford GP, LLC and Freshford Partners, LP disclaims beneficial ownership of such shares of Common Stock, except to the extent of his pecuniary interest therein. The address of Freshford Capital Management, LLC, Freshford GP, LLC and Freshford Partners, LP is 10 Bank Street, Suite 675, White Plains, New York 10606. |
| 13 | Based on the statement on Amended Schedule 13G filed with the SEC on February 14, 2012, Baron Capital Group, Inc., BAMCO, Inc., Baron Small Cap Fund, and Ronald Baron have reported shared voting and dispositive power with respect to all such shares of Common Stock. The address of Baron Capital Group, Inc., BAMCO, Inc., Baron Small Cap Fund, and Ronald Baron is 767 Fifth Avenue, 49th Floor, New York, New York 10153. |
| 14 | Based on the statement on the Amended Schedule 13G filed with the SEC on February 13, 2012, Jennison Associates, LLC has reported sole voting power with respect to 3,094,544 shares of Common Stock and shared dispositive power with respect to 3,098,654 shares of Common Stock. The address of Jennison Associates, LLC is 466 Lexington Avenue, New York, New York, 10017. Based on the statement on the Amended Schedule 13G filed with the SEC on February 14, 2012, Prudential Financial, Inc. has reported sole voting and dispositive power with respect to 259,222 shares of Common Stock, shared voting power with respect to 1,415,519 shares of Common Stock, and shared dispositive power with respect to 2,839,432 shares of Common Stock. The address of Prudential Financial, Inc. is 751 Broad Street, Newark, New Jersey, 07102-3777. |
| 15 | Based on the statement on the Amended Schedule 13G filed with the SEC on February 8, 2012, Allen S. Josephs has reported sole voting and dispositive power with respect to 699,100 shares of Common Stock and shared voting and dispositive power with respect to 1,383,097 shares of Common Stock. The address of Allen S. Josephs is 7710 Kenway Place, Boca Raton, Florida 33433. |
| 16 | Based on the statement on the Schedule 13D filed with the SEC on February 16, 2012, Mr. Horowitz has reported sole voting and dispositive power with respect to 887,504 shares of Common Stock and shared voting and dispositive power with respect to 2,272,727 shares of Common Stock and warrants to purchase 776,286 shares of Common Stock from Vitacost.com, Inc. JHH Capital, LLC has reported shared voting and dispositive power with respect to 2,272,727 shares of Common Stock and warrants to purchase 776,286 shares of Common Stock from Vitacost.com, Inc.. Mr. Horowitz may be deemed to indirectly beneficially own the shares of common stock beneficially owned by JHH Capital, LLC. The address of JHH Capital, LLC is 5400 Broken Sound Blvd NW, Suite 500, Boca Raton, FL 33487. |
| 17 | Based on the statement on the Schedule 13G filed with the SEC on January 24, 2012, Royce & Associates, LLC has reported sole voting and dispositive power with respect to all such shares of Common Stock. The address of Royce & Associates, LLC is 745 Fifth Avenue, New York, NY 10151 |
| 67 |
Equity Compensation Plans
| Number of Securities to Be Issued Upon Exercise of Outstanding Options, Warrants, and Rights | Weighted Average Exercise Price of Outstanding Options, Warrants, and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans | ||||||||||
| Equity compensation plans approved by security holders | 4,262,230 | $ | 5.69 | 6,531,514 | ||||||||
| Equity compensation plans not approved by security holders | - | $ | - | - | ||||||||
Item 13. Certain Relationships, Related Transactions and Director Independence
Indemnification Agreements
Our certificate of incorporation and bylaws contain provisions that limit the liability of our directors and provide for indemnification of our officers and directors to the full extent permitted under Delaware law. In addition, we have entered into separate indemnification agreements with our directors and officers that could require us to, among other things, indemnify them against certain liabilities that may arise by reason of their status or service as directors or officers. Such provisions do not, however, affect liability for any breach of a director's duty of loyalty to us or our stockholders, liability for acts or omissions not in good faith or involving active and deliberate dishonesty or knowing violations of law or liability for transactions in which the director derived an improper personal benefit, among others.
Procedures for Related Party Transactions
Under our code of conduct and ethics, our employees, officers and directors are encouraged to avoid actual or apparent conflicts of interest between personal and corporate-related relationships. In particular, our employees, officers and directors should not participate in a personal business transaction with us in which they will receive a significant profit or gain, unless otherwise approved by our Board. Further, our employees, officers and directors should advise the Board of any prospective or existing potential conflict. Pursuant to its charter, our audit committee must then approve any related-party transactions reported to the Board. In approving or rejecting such proposed transactions, the audit committee considers the facts and circumstances available and deemed relevant to the audit committee, including the material terms of the transactions, risks, benefits, costs, availability of other comparable services or products and, if applicable, the impact on a director's independence. Our audit committee will approve only those transactions that, in light of known circumstances, are in, or are not inconsistent with, our best interests, as our audit committee determines in the good faith exercise of its discretion. A copy of our Code of Conduct and Ethics and Audit Committee Charter are available for review at the investor relations section of our corporate website at http://investor.vitacost.com .
Purchase Agreement
On February 16, 2012, we entered into a Purchase Agreement (the “Purchase Agreement”) by and among Vitacost, JHH Capital, LLC (an entity affiliated with Jeffrey Horowitz, our Chief Executive Officer, “JHH”), Great Hill Equity Partners III, L.P. (“Great Hill III”), Great Hill Equity Partners IV, L.P. (“Great Hill IV”), Great Hill Investors, LLC (“Great Hill Investors”), Freshford Partners, LP (“Freshford Partners”), Freshford Master Fund, Ltd (“Freshford Master Fund”) and Baron Small Cap Fund (“Baron” and, together with JHH, Great Hill III, Great Hill IV, Great Hill Investors, Freshford Partners, Freshford Master Fund, collectively, the “Investors”) pursuant to which the Investors purchased, and we sold, an aggregate of 4,920,288 shares of our common stock at a purchase price of $7.04 per share, and warrants to purchase an aggregate of 1,680,601 shares of our common stock for an aggregate purchase price of $34,848,903. The warrants have an exercise price of $7.04 per share and a term of four years.
Registration Rights Agreement
Concurrently with entering into the Purchase Agreement, we entered into a registration rights agreement with Jeffrey Horowitz and JHH which provides Jeffrey Horowitz and JHH with demand, shelf and piggyback registration rights. The registration rights agreement further provides that if we enter into a registration rights agreement (or similar agreement) with any person or entity on more favorable terms than those contained in the registration rights agreement, the registration rights agreement will be amended to include such more favorable terms. Pursuant to the registration rights agreement, we have agreed to pay all of the registration costs and expenses incurred in connection with such demand, shelf and piggyback registrations.
| 68 |
Item 14. Principal Accounting Fees and Services
PricewaterhouseCoopers LLP is the Company's principal accountant for the year ended December 31, 2011. The table below sets forth the audit fees, audit-related fees, and tax fees billed to the Company by PricewaterhouseCoopers LLP for the years ended December 31, 2011 and 2010, respectively.
| 2011(1) | 2010(2) | |||||||
| Audit fees | $ | 492,500 | $ | 532,500 | ||||
| Audit-related fees | - | - | ||||||
| Tax fees | - | - | ||||||
| $ | 492,500 | $ | 532,500 | |||||
For purposes of the preceding table, the professional fees are classified as follows:
| • |
Audit Fees — (1) The Company incurred $492,500 in fees for professional services rendered by PricewaterhouseCoopers in connection with the audit of the consolidated financial statements included in the Annual Report on Form 10 K, the review of the quarterly consolidated financial statements included in the Quarterly Report on Form 10 Q for the three, six and nine month periods ended March 31, 2011, June 30, 2011 and September 30, 2011, the assessment of the internal control assertions required by Section 404 of the Sarbanes-Oxley Act of 2002 and other SEC filings and accounting consultations on matters related to the annual audit or interim reviews. (2) The Company incurred $532,500 in fees for professional services rendered by PricewaterhouseCoopers in connection with the audit of the consolidated financial statements included in the Annual Report on Form 10 K, the review of the quarterly consolidated financial statements included in the Quarterly Report on Form 10 Q for the three and nine month period ended September 30, 2011, the assessment of the internal control assertions required by Section 404 of the Sarbanes-Oxley Act of 2002 and other SEC filings and accounting consultations on matters related to the annual audit or interim review. |
| • | Audit-Related Fees — The Company did not incur any Audit Related Fees by our principal accountants during 2011 and 2010. |
| • | Tax Fees — The Company did not incur any Tax Fees by our principal accountants during 2011 and 2010. |
Audit Committee Pre-Approval Policy
Our Audit Committee has reviewed and approved all of the fees charged by our principal accountants. The Audit Committee concluded that all services rendered during 2011 and 2010 by our principal accountants were consistent with maintaining their respective independence. As a matter of policy, we will not engage our principal accountants for non-audit services other than “audit-related services,” as defined by the SEC, certain tax services, and other permissible non-audit services as specifically approved by the chairperson of the Audit Committee and presented to the full Board at its next regular meeting. The policy also includes limits on hiring partners of, and other professionals employed by, the principal accountants to ensure that the SEC's auditor independence rules are satisfied.
Under the policy, the Audit Committee must pre-approve all services provided by our principal accountants and fees charged for these services including an annual review of audit fees, audit-related fees, tax fees, and other fees with specific dollar value limits for each category of service. The Audit Committee will also consider and, if appropriate, approve specific engagements on a case-by-case basis that are not otherwise pre-approved. Any proposed engagement that does not fit within the definition of a pre-approved service may be presented to the chairperson of the Audit Committee for approval.
| 69 |
PART IV
Item 15. Exhibits and Financial Statement Schedules
| (a) | The following documents are filed as part of this Report: |
| 1. | Financial Statements: The information concerning Vitacost’s financial statements, and Report of PricewaterhouseCoopers LLP, Independent Registered Public Accounting Firm required by this Item is incorporated by reference herein to the section of this Report in Item 8, entitled “Financial Statements and Supplementary Data.” |
| 2. | Financial Statement Schedule: The following financial statement schedule of Vitacost.com, Inc., for the fiscal years ended December 31, 2011, 2010 and 2009, is filed as part of this Report and should be read in conjunction with the Consolidated Financial Statements of Vitacost.com, Inc. |
Schedule II Valuation and Qualifying Accounts
Schedules not listed above have been omitted because they are not applicable or are not required or the information required to be set forth therein is included in the Consolidated Financial Statements or Notes thereto.
| 3. |
Exhibits: See Item 15(b) below. We have filed, or incorporated into this Report by reference, the exhibits listed on the accompanying Index to Exhibits immediately following the signature page of this Form 10-K. |
| (b) | Exhibits: |
We have filed, or incorporated into the Report by reference, the exhibits listed on the accompanying Index to Exhibits immediately following the signature page of this Form 10-K.
| (c) | Financial Statement Schedules: See Item 15(a), above. |
Item 15(A)(2) Financial Statement Schedule II
All schedules have been omitted as the requested information is inapplicable or the information is presented in the financial statements or related notes included as part of this Report.
| 70 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| VITACOST.COM, INC. | ||
| Dated: March 15, 2012 |
By: /s/ Jeffrey J. Horowitz | |
|
/s/ Brian D. Helman
|
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS , that each person whose signature appears below constitutes and appoints Jeffrey J. Horowitz and Brian D. Helman each as his attorney-in-fact, each with the power of substitution, for him in any and all capacities, to sign any amendments to this Report on Form 10-K, and to file the same, with exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, hereby ratifying and confirming all that each of said attorneys-in-fact, or his substitute or substitutes, may do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated:
| Signature | Title | Date | ||
| /s/ Jeffrey J. Horowitz Jeffrey J. Horowitz |
Chief Executive Officer (Principal Executive Officer) | March 15, 2012 | ||
|
/s/ Brian D. Helman |
|
Chief Financial Officer (Principal Financial Officer) |
March 15, 2012 | |
|
/s/ Michael Kumin |
Director (Interim Chairman of the Board) |
March 15, 2012 | ||
|
/s/ Christopher Gaffney |
Director |
March 15, 2012 | ||
|
/s/ Stuart Goldfarb |
Director |
March 15, 2012 | ||
|
/s/ Edwin J. Kozlowski |
Director |
March 15, 2012 | ||
|
/s/ Robert G. Trapp |
Director |
March 15, 2012 |
| 71 |
EXHIBIT INDEX
|
Exhibit |
Exhibits |
| 3.1(1) | Certificate of Incorporation of the Registrant |
| 3.2(1) | Bylaws of the Registrant |
| 4.1(2) | Specimen of Common Stock Certificate |
| 4.2†(3) | Form of Nonstatutory Stock Option Agreement |
| 4.3†(4) | Form of Incentive Stock Option Agreement |
| 4.4(5) | Stockholder Agreement dated October 8, 2010 by and between Vitacost.com, Inc. and Great Hill Investors, LLC, Great Hill Equity Partners III, L.P. and Great Hill Partners IV, L.P. |
| 4.5(5) | Registration Rights Agreement dated October 8, 2010 by and between Vitacost.com, Inc. and Great Hill Investors, LLC, Great Hill Equity Partners III, L.P. and Great Hill Partners IV, L.P. |
| 10.1†(3) | 2000 Stock Option Plan |
| 10.2†(6) | 2007 Stock Award Plan |
| 10.3†(7) | 2011 Incentive Compensation Plan |
| 10.4†(8) | Employment Agreement dated October 19, 2010 between Vitacost.com, Inc. and Stephen E. Markert, Jr. |
| 10.5†(9) | Employment Agreement dated February 16, 2011 between Vitacost.com, Inc. and Jeffery Horowitz |
| 10.6†(10) | Employment Agreement dated August 4, 2011 between Vitacost.com, Inc. and Robert Wegner |
| 10.7†(11) | Employment Agreement dated August 18, 2011 between Vitacost.com, Inc. and David Zucker |
| 10.8†(12) | Employment Agreement dated November 28, 2011 between Vitacost.com, Inc. and Nachiket Desai |
| 10.9†(13) | Employment Agreement dated January 25, 2012 between Vitacost.com, Inc. and Brian Helman |
| 10.10†(2) | Form of Indemnification Agreement between Vitacost.com, Inc. and each officer and director |
| 16.1(14) | Letter dated March 17, 2011 from McGladrey & Pullen, LLP to the Securities and Exchange Commission regarding change in certifying accountant |
| 21.1 | List of Subsidiaries of Registrant |
| 23.1 | Consent of Independent Registered Certified Public Accounting Firm |
| 23.2 | Consent of Independent Registered Public Accounting Firm |
| 31.1 | Certification of Chief Executive Officer Pursuant to Rule 13-14(a) of the Securities and Exchange Act of 1934 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 31.2 | Certification of Chief Financial Officer Pursuant to Rule 13-14(a) of the Securities and Exchange Act of 1934 as Adopted Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002 |
| 32.1 |
Certification of Chief Executive Officer and Chief Financial Officer Pursuant to 18 U.S.C. Section 1350, as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 |
| 101.INS | XBRL Instance Document |
| 101.SCH | XBRL Taxonomy Extension Schema Document |
| 101.CAL | XBRL Taxonomy Calculation Linkbase Document |
| 101.LAB | XBRL Taxonomy Extension Label Linkbase Document |
| 101.PRE | XBRL Taxonomy Extension Presentation Linkbase Document |
| 101.DEF | XBRL Taxonomy Extension Definition Linkbase Document |
| 72 |
| † | Denotes a management contract or compensatory plan or arrangement. |
| (1) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K12B filed with the Securities and Exchange Commission on September 28, 2011. |
| (2) | Filed as an Exhibit to Amendment No. 7 of Registrant’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on September 21, 2009. |
| (3) | Filed as an Exhibit to Registrant’s Registration Statement on Form S-1 filed with the Securities and Exchange Commission on June 20, 2007. |
| (4) | Filed as an Exhibit to Registrant’s Amendment No. 4 of Registration Statement on Form S-1 filed with the Securities and Exchange Commission on July 28, 2009. |
| (5) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 13, 2010. |
| (6) | Filed as an Exhibit to Registrant’s Amendment No. 3 of Registration Statement on Form S-1 filed with the Securities and Exchange Commission on June 12, 2009. |
| (7) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on September 8, 2011. |
| (8) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on October 19, 2010. |
| (9) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 22, 2011. |
| (10) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 9, 2011. |
| (11) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 22, 2011. |
| (12) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 28, 2011. |
| (13) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on January 31, 2012. |
| (14) | Filed as an Exhibit to Registrant’s Current Report on Form 8-K filed with the Securities and Exchange Commission on March 17, 2011. |
| 73 |
