Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - PROGENICS PHARMACEUTICALS INC | Financial_Report.xls |
| EX-23.1 - EXHIBIT 23.1 CONSENT OF PRICEWATERHOUSECOOPERS - PROGENICS PHARMACEUTICALS INC | ex23_112312011.htm |
| EX-12.1 - EXHIBIT 12.1 RATIO - PROGENICS PHARMACEUTICALS INC | ex12_112312011.htm |
| EX-32.1 - EXHIBIT 32.1 CERTIFICATION - PROGENICS PHARMACEUTICALS INC | ex32_112312011.htm |
| EX-31.1 - EXHIBIT 31.1 CERTIFICATION - PROGENICS PHARMACEUTICALS INC | ex31_112312011.htm |
| EX-31.2 - EXHIBIT 31.2 CERTIFICATION - PROGENICS PHARMACEUTICALS INC | ex31_212312011.htm |
| EX-32.2 - EXHIBIT 32.2 CERTIFICATION - PROGENICS PHARMACEUTICALS INC | ex32_212312011.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
______________
FORM 10-K
(Mark One)
|
x
|
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the fiscal year ended December 31, 2011
|
|
|
Or
|
|
|
¨
|
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
|
|
For the transition period from ___________ to ___________
|
Commission File No. 000-23143
______________
PROGENICS PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
|
Delaware
|
13-3379479
|
|
(State or other jurisdiction of
incorporation or organization)
|
(I.R.S. Employer Identification Number)
|
______________
777 Old Saw Mill River Road
Tarrytown, NY 10591
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code: (914) 789-2800
Securities registered pursuant to Section 12(b) of the Act:
Title of each
class Name of each exchange on which
registered
Common Stock, par value $0.0013 per
share The NASDAQ Stock Market LLC
|
Securities registered pursuant to Section 12(g) of the Act:
|
None
|
______________
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90
days. Yes
x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Website, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such
files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form
10-K.
¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Act:
|
Large Accelerated Filer ¨
|
Accelerated Filer x
|
|
Non-accelerated Filer ¨ (Do not check if a smaller reporting company)
|
Smaller Reporting Company ¨
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange
Act). Yes o No x
The aggregate market value of the voting and non-voting stock held by non-affiliates of the registrant on June 30, 2011, based upon the closing price of the Common Stock on The NASDAQ Stock Market LLC on that date of $7.18 per share, was $144,724,374 (1).
|
(1)
|
Calculated by excluding all shares that may be deemed to be beneficially owned by executive officers, directors and five percent stockholders of the Registrant, without conceding that any such person is an “affiliate” of the Registrant for purposes of the Federal securities laws.
|
As of March 5, 2012, a total of 33,861,834 shares of Common Stock, par value $.0013 per share, were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Specified portions of the Registrant’s definitive proxy statement to be filed in connection with solicitation of proxies for its 2012 Annual Meeting of Shareholders are hereby incorporated by reference into Part III of this Form 10-K where such portions are referenced.
|
PART I
|
||
|
Item 1.
|
1
|
|
|
Item 1A.
|
10
|
|
|
Item 1B.
|
18
|
|
|
Item 2.
|
18
|
|
|
Item 3.
|
18
|
|
|
Item 4.
|
18
|
|
|
PART II
|
||
|
Item 5.
|
19
|
|
|
Item 6.
|
20
|
|
|
Item 7.
|
21
|
|
|
Item 7A.
|
33
|
|
|
Item 8.
|
33
|
|
|
Item 9.
|
33
|
|
|
Item 9A.
|
34
|
|
|
Item 9B.
|
34
|
|
|
PART III
|
||
|
Item 10.
|
35
|
|
|
Item 11.
|
35
|
|
|
Item 12.
|
35
|
|
|
Item 13.
|
35
|
|
|
Item 14.
|
35
|
|
|
PART IV
|
||
|
Item 15.
|
36
|
|
|
F-1
|
||
|
S-1
|
||
|
E-1
|
||
PART I
This document and other public statements we make may contain statements that do not relate strictly to historical fact, any of which may be forward-looking statements within the meaning of the U.S. Private Securities Litigation Reform Act of 1995. When we use the words “anticipates,” “plans,” “expects” and similar expressions, we are identifying forward-looking statements. Forward-looking statements involve known and unknown risks and uncertainties which may cause our actual results, performance or achievements to be
materially different from those expressed or implied by forward-looking statements. While it is impossible to identify or predict all such matters, these differences may result from, among other things, the inherent uncertainty of the timing and success of, and expense associated with, research, development, regulatory approval and commercialization of our products and product candidates, including the risks that clinical trials will not commence or proceed as planned; products appearing promising in early trials will not demonstrate efficacy or safety in larger-scale trials; clinical trial data on our products and product candidates will be unfavorable; our products will not receive marketing approval from regulators or, if approved, do not gain sufficient market acceptance to justify development and commercialization costs;
competing products currently on the market or in development might reduce the commercial potential of our products; we, our collaborators or others might identify side effects after the product is on the market; or efficacy or safety concerns regarding marketed products, whether or not originating from subsequent testing or other activities by us, governmental regulators, other entities or organizations or otherwise, and whether or not scientifically justified, may lead to product recalls, withdrawals of marketing approval, reformulation of the product, additional pre-clinical testing or clinical trials, changes in labeling of the product, the need for additional marketing applications, declining sales or other adverse events.
We are also subject to risks and uncertainties associated with the actions of our corporate, academic and other collaborators and government regulatory agencies, including risks from market forces and trends; potential product liability; intellectual property, litigation, environmental and other risks; the risk that we may not be able to enter into favorable collaboration or other relationships or that existing or future relationships may not proceed as planned; the risk that current and pending patent protection for our products may be invalid, unenforceable or challenged, or fail to provide adequate market exclusivity, or that our rights to in-licensed intellectual property may be terminated
for our failure to satisfy performance milestones; the risk of difficulties in, and regulatory compliance relating to, manufacturing products; and the uncertainty of our future profitability.
Risks and uncertainties also include general economic conditions, including interest and currency exchange-rate fluctuations and the availability of capital; changes in generally accepted accounting principles; the impact of legislation and regulatory compliance; the highly regulated nature of our business, including government cost-containment initiatives and restrictions on third-party payments for our products; trade buying patterns; the competitive climate of our industry; and other factors set forth in this document and other reports filed with the U.S. Securities and Exchange Commission (SEC). In particular, we cannot assure you that
RELISTOR® will be commercially successful or be approved in the future in other formulations, indications or jurisdictions, or that any of our other programs will result in a commercial product.
We do not have a policy of updating or revising forward-looking statements and we assume no obligation to update any statements as a result of new information or future events or developments. It should not be assumed that our silence over time means that actual events are bearing out as expressed or implied in forward-looking statements.
Available Information
We file annual, quarterly and current reports, proxy statements and other documents with the SEC under the Securities Exchange Act of 1934. The SEC maintains an Internet website that contains reports, proxy and information statements and other information regarding issuers, including Progenics, that file electronically with the SEC. You may obtain documents that we file with the SEC at http://www.sec.gov, and read and copy them at the SEC’s Public Reference Room at 100 F Street NE, Washington, DC 20549. You may obtain information on operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. We also make available our annual,
quarterly and current reports and proxy materials on http://www.progenics.com.
Additional information concerning Progenics and its business may be available in press releases or other public announcements and quarterly and current reports and documents filed with the SEC.
Progenics Pharmaceuticals is dedicated to the development of innovative medicines to treat disease. Our focus is on the treatment of cancer.
RELISTOR. Our first commercial drug is RELISTOR®, for the treatment of opioid induced constipation (OIC) in patients with advanced illnesses, such as cancer. OIC is the constipation that often arises when patients take opioids for pain relief. RELISTOR is the only prescription medicine approved in the United States to treat this form of constipation. RELISTOR subcutaneous injection is now approved in the U.S. and over 50 other counties around the world. In the U.S. RELISTOR is marketed by our
commercial partner Salix Pharmaceuticals, a leading specialty pharmaceutical company focusing on gastrointestinal diseases; it is sold outside the U.S. by sublicensees of Salix. Our partner Ono Pharmaceutical is currently developing subcutaneous RELISTOR for Japan.
Together with Salix we have applied to the U.S. Food and Drug Administration to expand the population that can be treated with subcutaneous RELISTOR to include patients taking opioids for non-cancer pain, and who suffer from OIC as a result. This population includes patients taking opioids for conditions such as back pain or joint pain. The FDA’s action date on this marketing application under the Prescription Drug User Fee Act (PDUFA) is April 27, 2012.
We also recently announced results from a Phase 3 clinical test of an oral form of RELISTOR, in which the efficacy of oral methylnaltrexone was comparable to that reported in clinical studies of subcutaneous methylnaltrexone in subjects with chronic, non-cancer pain, and the overall observed safety profile in patients treated was comparable to placebo.
PSMA ADC. Our lead oncology product candidate under development is PSMA ADC, which we are currently testing in a Phase 1 trial in men with advanced prostate cancer. PSMA ADC is an antibody-drug conjugate which targets prostate specific membrane antigen (PSMA), a protein found on the surface of prostate cancer tumor cells. This ADC combines our own proprietary antibody to PSMA with auristatin E (MMAE), a potent cytotoxic drug. MMAE and the conjugation technology for PSMA ADC is licensed to us by Seattle Genetics, a clinical stage biotechnology company focusing on monoclonal antibody-based therapies for cancer and autoimmune diseases. We
expect that the ongoing Phase 1 trial of PSMA ADC will be completed in 2012 and if the results are successful we plan then to commence a Phase 2 trial of PSMA ADC in advanced prostate cancer. PSMA is also expressed on cells in the growing blood vessels, or neovasculature, that supplies many other forms of cancers. We have not yet explored in clinical trials the potential of PSMA ADC for treatment of these other cancers.
PI3K. As a part of our work in oncology, we are conducting preclinical development of novel multiplex phosphoinositide 3-kinase (PI3K) inhibitors. We believe these compounds may be effective in blocking signaling pathways that are critical in the growth of aggressive cancers, particularly RAS-mutated tumors.
Future Plans and Recent Events. With our focus on the development of medicines to treat cancer, we are seeking opportunities to expand our oncology pipeline through in-licensing and acquisitions. We have discontinued work on programs outside of this focus and are working to out-license them.
On March 14, 2012, Progenics and company founder Paul J. Maddon entered into an agreement providing for his retirement as Chief Science Officer. He will continue as a member of the Progenics Board and a Vice Chairman. In connection with Dr. Maddon’s retirement and termination of his employment agreement, Progenics has agreed to pay him an amount equal to $1,789,333 and provide other benefits under the agreement.
Here is a summary of our current RELISTOR efforts and our product candidate pipeline:
| RELISTOR® | ||||||||
|
Marketed commercial product
|
Approved indication
|
Status
|
||||||
|
RELISTOR-Subcutaneous injection
|
Treatment of OIC in advanced-illness patients receiving palliative care when laxative therapy has not been sufficient
|
Marketed in the U.S., E.U., Canada, Australia and elsewhere; Licensed to Salix Pharmaceuticals worldwide other than Japan
|
||||||
|
Clinical product candidates
|
Proposed indication
|
Status
|
||||||
|
RELISTOR-Subcutaneous injection
|
Treatment of OIC in patients with non-cancer pain
|
PDUFA action date of April 27, 2012 for pending sNDA
|
||||||
|
RELISTOR-Subcutaneous injection
|
Treatment of OIC (Japan)
|
Development being conducted by Ono Pharmaceutical
|
||||||
|
RELISTOR-Oral
|
Treatment of OIC
|
Phase 3 testing completed
|
||||||
|
ONCOLOGY
|
||||||||
|
Clinical product candidate
|
Proposed indication
|
Status
|
||||||
|
PSMA ADC
|
Prostate cancer
|
Phase 1 in advanced prostate cancer
|
||||||
|
Research
|
Proposed indication
|
Status
|
||||||
|
Multiplex PI3K inhibitor compounds
|
Cancer
|
Discovery research
|
||||||
Please note:
RELISTOR is a registered trademark. In this document, RELISTOR refers to methylnaltrexone – the active ingredient of RELISTOR - as it has been and is being developed and commercialized by or in collaboration with Progenics. Subcutaneous RELISTOR has received regulatory marketing approval for specific indications, and references to RELISTOR do not imply that any other form or possible use of the drug has received such approval. The approved U.S. label for RELISTOR also provides that use of RELISTOR beyond four months has not been studied. Full U.S. prescribing information is available at www.RELISTOR.com. Other approved labels for RELISTOR apply in ex-US markets.
In summarizing the status of our commercialization and product candidates:
(i) Research and discovery means initial research related to specific molecular targets, synthesis of new chemical entities, assay development or screening for identification and optimization of lead compound(s). This work precedes pre-clinical investigations, which involves lead compound(s) undergoing toxicology, formulation and other testing in preparation for clinical trials.
|
(ii)
|
Phase 1-3 clinical trials are safety and efficacy tests in humans:
|
1: Initial evaluation of safety in humans; study method of action and metabolization.
2: Evaluation of safety, dosing and activity or efficacy; continue safety evaluation.
3: Larger scale evaluation of safety, efficacy and dosage.
RELISTOR
Opioid-based medications such as morphine and codeine are used to control moderate-to-severe pain in patients receiving palliative care, undergoing surgery, experiencing chronic pain or with other medical conditions. Opioids relieve pain by interacting with receptors located in the brain and spinal cord, but also activate receptors in the gut, often resulting in constipation, referred to as opioid-induced constipation or OIC. As a result of OIC, many patients may stop or reduce their opioid therapy, opting to endure pain in order to obtain relief from their OIC and its associated side effects.
RELISTOR, the first approved treatment for OIC that addresses the underlying mechanism of this condition, is a selective, peripherally acting, mu-opioid-receptor antagonist that decreases the constipating side effects induced by opioid pain medications in the gastrointestinal tract without diminishing the ability of these medications to relieve pain. Relief of OIC is an important need that is not adequately met by any other approved drug or intervention. Because of its chemical composition, RELISTOR has restricted access to the blood-brain barrier to enter the central nervous system, where pain is perceived. Outside the central nervous system, RELISTOR competes with opioid pain medications for binding sites on
opioid receptors, displacing the pain medications only in the periphery and selectively “turning off” the constipating effects of those medications on the gastrointestinal tract without affecting pain relief occurring in the central nervous system.
Under our 2011 License Agreement, Salix is responsible for further developing and commercializing subcutaneous RELISTOR, including completing clinical development necessary to support regulatory marketing approvals for potential new indications and formulations of the drug. Through December 31, 2011, we have received under this Agreement a $60.0 million upfront cash payment and $0.2 million in respect of Salix ex-U.S. sublicensee revenue and are eligible to receive (i) up to $40.0 million upon U.S. marketing approval for subcutaneous RELISTOR in non-cancer pain patients, (ii) up to $50.0 million upon U.S. marketing approval of an oral formulation of RELISTOR, (iii) up to $200.0 million of commercialization milestone
payments upon achievement of specified U.S. sales targets, (iv) royalties ranging from 15 to 19 percent of net sales by Salix and its affiliates, and (v) 60% of any upfront, milestone, reimbursement or other revenue (net of costs of goods sold, as defined, and territory-specific research and development expense reimbursement) Salix receives from sublicensees outside the U.S. In the event that either marketing approval is subject to a Black Box Warning or Risk Evaluation and Mitigation Strategy (REMS), payment of a portion of the milestone amount would be deferred, and subject, to achievement of the first commercialization milestone.
Subcutaneous RELISTOR. RELISTOR has been approved by regulatory authorities in the U.S., countries in the European Union, Canada and Australia since 2008 for treatment of OIC in advanced-illness patients receiving palliative care when laxative therapy has not been sufficient. Marketing applications are pending elsewhere throughout the world. Salix, Progenics, and Progenics’ former collaborator Wyeth have transitioned U.S., European and other marketing authorizations and are transitioning additional commercialization outside the U.S. and Japan. Salix has secured distribution and marketing partners for RELISTOR in the European territory
and has licensed Link Medical Products Pty Limited for distribution in Australia, New Zealand, South Africa and certain other markets in Asia. Salix is continuing efforts to secure additional distribution partners and/or sublicensees.
RELISTOR net sales and related royalties earned through the end of 2011 are set forth below. Our recognition of royalty revenue for financial reporting purposes is explained in Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A) and our financial statements included elsewhere in this document. Royalties in 2011 are based on net sales reported by Salix; royalties through September 30, 2010 were based on net sales reported by Wyeth.
|
First
Quarter
|
Second
Quarter
|
Third
Quarter
|
Fourth
Quarter
|
Full
Year
|
|||||
|
(in thousands)
|
|||||||||
|
2011:
|
|||||||||
|
Net Sales
|
$3,300
|
$5,200
|
$9,700
|
$8,800
|
$27,000
|
||||
|
Royalties Earned
|
-
|
527
|
1,240
|
1,279
|
3,046
|
||||
|
2010:
|
|||||||||
|
Net Sales
|
$4,200
|
$3,800
|
$4,100
|
$4,000
|
$16,100
|
||||
|
Royalties Earned
|
625
|
581
|
620
|
-
|
1,826
|
||||
|
2009:
|
|||||||||
| Net Sales | $1,900 | $3,200 | $3,300 | $3,900 | $12,300 | ||||
| Royalties Earned | 280 | 487 | 497 | 589 | 1,853 | ||||
| 2008: | |||||||||
|
Net Sales
|
n.a
|
$2,100
|
$800
|
$1,500
|
$4,400
|
||||
|
Royalties Earned
|
n.a
|
321
|
117
|
227
|
665
|
||||
Progenics began developing RELISTOR in 2001 and continued development and commercialization worldwide except Japan with Wyeth Pharmaceuticals (now a Pfizer Inc. subsidiary) pursuant to a 2005 collaboration agreement that was terminated in October 2009. Under our 2008 License Agreement, Ono began clinical testing of RELISTOR subcutaneous injection in June 2009. See Licenses – RELISTOR. In addition to our pending sNDA for subcutaneous RELISTOR in non-cancer pain patients, we have also received U.S., E.U. and Canadian approvals to market RELISTOR in pre-filled syringes, which are designed to ease preparation and administration for patients and caregivers,
and expect Salix to begin distributing RELISTOR in this presentation later this year.
Oral RELISTOR. As noted above, we and Salix recently announced top-line data from a phase 3 trial of oral RELISTOR in patients with non-cancer pain.
Oncology
Through PSMA Development Company LLC, our wholly owned subsidiary, we conduct research and development programs directed at prostate specific membrane antigen, or PSMA, a protein that is abundantly expressed on the surface of prostate cancer cells as well as cells in the newly formed blood vessels of many other solid tumors. The principal focus of these efforts is our fully human monoclonal ADC, which utilizes technology licensed to us from Sloan-Kettering Institute for Cancer Research and Seattle Genetics, and is designed to deliver a
chemotherapeutic agent to cancer cells by targeting the three-dimensional structure of the PSMA protein on these cells and binding to and internalizing within the cell. We believe a PSMA-directed therapy may have application in prostate cancer and solid tumors of other types of cancer. We are conducting a phase 1 clinical trial of PSMA ADC for the treatment of prostate cancer which we expect will be completed in 2012, and if the results are successful we plan then to commence a Phase 2 trial of PSMA ADC in advanced prostate cancer.
We also recently presented data from preclinical studies of novel multiplex phosphoinositide 3-kinase (PI3K) inhibitors -- synthetic, small-molecule compounds identified by us that in laboratory studies have blocked both PI3K, a key regulator of one molecular signaling pathway, and MNK, an oncogenic kinase in the Ras pathway. We believe simultaneously blocking these interlinked cellular pathways may provide a strategy to combat some of the most aggressive forms of cancer.
We are seeking to in-license or acquire opportunities in the oncology field and related supportive, diagnostic and/or other areas that are complementary to these ongoing initiatives and our oncology focus generally.
Licenses
Following is a summary of significant license agreements under which we have in- and/or out-licensed rights to use certain technologies and materials related to RELISTOR and product candidates in our pipeline.
RELISTOR. Under our License Agreement, Salix Pharmaceuticals is responsible for further developing and commercializing subcutaneous RELISTOR worldwide other than Japan, including completing clinical development necessary to support regulatory marketing approvals for potential new indications and formulations, and marketing and selling the product. Salix is marketing RELISTOR directly through its specialty sales force in the U.S., and outside the U.S., directly through distribution and marketing partners and sublicensing regional companies. Among the rights we have licensed to Salix
are our exclusive rights to develop and commercialize methylnaltrexone, the active ingredient of RELISTOR, which we in-licensed from the University of Chicago and for which we are obligated to make milestone and royalty payments to the University. Salix is paying us royalties ranging from 15 to 19 percent on its net sales of RELISTOR as well as 60% of any upfront, milestone, reimbursement or other revenue (net of costs of goods sold, as defined, and territory-specific research and development expense reimbursement) it receives from sublicensees in respect of any country outside the U.S.
We have licensed to Ono Pharmaceutical the rights to subcutaneous RELISTOR in Japan, where Ono is responsible for developing and commercializing subcutaneous RELISTOR, including conducting clinical development to support regulatory marketing approval and will own the subcutaneous filings and approvals relating to RELISTOR. We received a $15.0 million upfront payment from Ono, and are entitled to receive up to an additional $20.0 million upon achievement of development milestones. Ono is also obligated to pay us royalties and commercialization milestones on sales of subcutaneous RELISTOR in Japan. Ono has the option to acquire the rights to develop and
commercialize other formulations of RELISTOR in Japan, on terms to be negotiated separately. Supervision of and consultation with respect to Ono’s development and commercialization responsibilities are carried out by joint committees. The Ono License contains, among other terms, provisions which permit termination by either party upon the occurrence of certain events.
PSMA. PSMA Development Company LLC has a collaboration agreement with Seattle Genetics under which SGI has granted it an exclusive worldwide license to SGI’s proprietary ADC technology. PSMA LLC has the right to use this technology, which is based in part on technology licensed by SGI from third parties, to link chemotherapeutic agents to PSMA LLC’s monoclonal antibodies that target prostate specific membrane antigen utilizes technology licensed to us from Sloan-Kettering. PSMA LLC is responsible for research, product development, manufacturing and commercialization of all
products, and may sublicense the ADC technology to a third party manufacturer. PSMA LLC is obligated to make maintenance and milestone payments aggregating up to $14.3 million and to pay royalties to SGI and its licensors, as applicable, on a percentage of net sales. The SGI agreement terminates at the latest of (i) the tenth anniversary of the first commercial sale of each licensed product in each country or (ii) the latest date of expiration of patents underlying the licensed products. PSMA LLC may terminate the agreement upon advance written notice, and SGI may terminate if PSMA LLC fails to cure a breach of an SGI in-license within a specified time period after written notice. In addition, either party may terminate the agreement after written notice upon an uncured breach or in the event of bankruptcy of the other party. As of December 31, 2011, PSMA LLC has paid approximately $3.8
million under this agreement, including $1.0 million in milestone payments.
PSMA LLC also has a worldwide exclusive licensing agreement with Abgenix (now Amgen Fremont, Inc.) to use its XenoMouse® technology for generating fully human antibodies to PSMA. PSMA LLC is obligated to make development and commercialization milestone payments with respect to products incorporating an antibody generated utilizing the XenoMouse technology. As of December 31, 2011, PSMA LLC has paid $0.9 million under this agreement and is obligated to pay up to an additional $6.3 million if certain milestones are met, along
with royalties based upon net sales of antibody products, if any. This agreement may be terminated, after an opportunity to cure, by Abgenix for cause upon 30 days prior written notice; PSMA LLC has the right to terminate upon 30 days prior written notice. The agreement continues until the later of the expiration of the XenoMouse technology patents that may result from pending patent applications or seven years from the first commercial sale of the products.
Patents and Proprietary Technology
Protection of our intellectual property rights is important to our business. In addition to seeking U.S. patent protection for many of our inventions, we generally file patent applications in Canada, Japan, European countries that are party to the European Patent Convention and additional foreign countries on a selective basis in order to protect the inventions that we consider to be important to the development of our foreign business. Generally, patents issued in the U.S. are effective for either (i) 20 years from the earliest asserted filing date, if the application was filed on or after June 8, 1995, or (ii) the longer of 17 years from the date of issue or 20 years from the earliest asserted filing date, if the
application was filed prior to that date.
In certain instances, the U.S. patent term can be extended up to a maximum of five years to recapture a portion of the term during which the FDA regulatory review was being conducted. The duration of foreign patents varies in accordance with the provisions of applicable local law, although most countries provide for patent terms of 20 years from the earliest asserted filing date and allow patent extensions similar to those permitted in the U.S.
We also rely on trade secrets, proprietary know-how and continuing technological innovation to develop and maintain a competitive position in our product areas. We generally require our employees, consultants and corporate partners who have access to our proprietary information to sign confidentiality agreements.
Information with respect to our patent portfolio regarding our therapeutic and research programs, as of year-end 2011, is set forth below.
|
RELISTOR and
|
Number of Patents
|
Expiration
|
Number of Patent Applications
|
|||||||
|
Product Candidates
|
U.S.
|
International
|
Dates (1)
|
U.S.
|
International
|
|||||
|
RELISTOR
|
8
|
30
|
2015-2028
|
22
|
179
|
|||||
|
Oncology (PSMA; PI3K)
|
7
|
25
|
2013-2026
|
6
|
20
|
|||||
|
Other
|
-
|
-
|
-
|
1
|
4
|
|||||
|
(1)
|
Patent term extensions and pending patent applications may extend the period of patent protection afforded our products and product candidates under development.
|
Patents may not enable us to preclude competitors from commercializing drugs in direct competition with our products, and consequently may not provide us with any meaningful competitive advantage. See Risk Factors.
We are aware of intellectual property rights held by third parties that relate to products or technologies we are developing. For example, we are aware of others investigating methylnaltrexone and other peripheral opioid antagonists as well as PSMA or related compounds, and of patents and applications held or filed by others in those areas. While the validity of issued patents, patentability of claimed inventions in pending applications and applicability of any of them to our programs are uncertain, patent rights asserted against us could adversely affect our ability to commercialize or collaborate with others regarding our products.
Research, development and commercialization of a biopharmaceutical product often require choosing between alternative development and optimization routes at various stages in the development process. Preferred routes depend upon subsequent discoveries and test results and cannot be identified with certainty at the outset. There are numerous third-party patents in our field, and we may need to obtain a license under a patent in order to pursue the preferred development route of one or more of our product candidates. The need to obtain a license would decrease the ultimate profitability of the applicable product. If we cannot negotiate a license, we might have to pursue a less desirable development route or terminate
the entire program altogether.
Government Regulation
Progenics and its product candidates are subject to comprehensive regulation by the U.S. FDA and comparable authorities in other countries. Pharmaceutical regulation currently is a topic of substantial interest in lawmaking and regulatory bodies in the U.S. and internationally, and numerous proposals exist for changes in FDA and non-U.S. regulation of pre-clinical and clinical testing, safety, effectiveness, approval, manufacture, labeling, marketing, export, storage, recordkeeping, advertising, promotion and other aspects of biologics, small molecule drugs and medical devices, many of which, if adopted, could significantly alter our business and the current regulatory structure described below. See
Risk Factors.
FDA Regulation. FDA approval of our product candidates, including a review of the manufacturing processes and facilities used to produce them, are required before they may be marketed in the U.S. This process is costly, time consuming and subject to unanticipated delays, and a drug candidate may fail to progress at any point.
None of our product candidates other than RELISTOR has received marketing approval from the FDA or any other regulatory authority. The process required by the FDA before product candidates may be approved for marketing in the U.S. generally involves:
· pre-clinical laboratory and animal tests;
· submission to and favorable review by the FDA of an IND (Investigational New Drug) application before clinical trials may begin;
· adequate and well-controlled human clinical trials to establish the safety and efficacy of the product for its intended indication (animal and other nonclinical studies also are typically conducted during each phase of human clinical trials);
· submission to the FDA of a marketing application; and
· FDA review of the marketing application in order to determine, among other things, whether the product is safe and effective for its intended uses.
Pre-clinical tests include laboratory evaluation of product chemistry and animal studies to gain preliminary information about a product’s pharmacology and toxicology and to identify safety problems that would preclude testing in humans. Products must generally be manufactured according to current Good Manufacturing Practices, and pre-clinical safety tests must be conducted by laboratories that comply with FDA good laboratory practices regulations.
Results of pre-clinical tests are submitted to the FDA as part of an IND which must become effective before clinical trials may commence. The IND submission must include, among other things, a description of the sponsor’s investigational plan; protocols for each planned study; chemistry, manufacturing and control information; pharmacology and toxicology information and a summary of previous human experience with the investigational drug. Unless the FDA objects to, makes comments or raises questions concerning an IND, it becomes effective 30 days following submission, and initial clinical studies may begin. Companies often obtain affirmative FDA approval, however, before beginning such studies.
Clinical trials involve the administration of the investigational new drug to healthy volunteers or to individuals under the supervision of a qualified principal investigator. Clinical trials must be conducted in accordance with the FDA’s Good Clinical Practice requirements under protocols submitted to the FDA that detail, among other things, the objectives of the study, parameters used to monitor safety and effectiveness criteria to be evaluated. Each clinical study must be conducted under the auspices of an Institutional Review Board, which considers, among other things, ethical factors, safety of human subjects, possible liability of the institution
and informed consent disclosure which must be made to participants in the trial.
Clinical trials are typically conducted in three sequential phases, which may overlap. During phase 1, when the drug is initially administered to human subjects, the product is tested for safety, dosage tolerance, absorption, metabolism, distribution and excretion. Phase 2 involves studies in a limited population to evaluate preliminarily the efficacy of the product for specific, targeted indications, determine dosage tolerance and optimal dosage and identify possible adverse effects and safety risks.
When a product candidate is found in phase 2 evaluation to have an effect and an acceptable safety profile, phase 3 trials are undertaken in order to further evaluate clinical efficacy and test for safety within an expanded population. Safety studies are conducted in accordance with the FDA’s International Conference on Harmonization (ICH) Guidelines. Phase 2 results do not guarantee a similar outcome in phase 3 trials. The FDA may suspend clinical trials at any point in this process if it concludes that clinical subjects are being exposed to an unacceptable health risk.
A New Drug Application, or NDA, is an application to the FDA to market a new drug. A Biologic License Application, or BLA, is an application to market a biological product. The new drug or biological product may not be marketed in the U.S. until the FDA has approved the NDA or issued a biologic license. The NDA must contain, among other things, information on chemistry, manufacturing and controls; non-clinical pharmacology and toxicology; human pharmacokinetics and bioavailability; and clinical
data. The BLA must contain, among other things, data derived from nonclinical laboratory and clinical studies which demonstrate that the product meets prescribed standards of safety, purity and potency, and a full description of manufacturing methods. Supplemental NDAs (sNDAs) are submitted to obtain regulatory approval for additional indications for a previously approved drug.
The results of the pre-clinical studies and clinical studies, the chemistry and manufacturing data, and the proposed labeling, among other things, are submitted to the FDA in the form of an NDA or BLA. The FDA may refuse to accept the application for filing if certain administrative and content criteria are not satisfied, and even after accepting the application for review, the FDA may require additional testing or information before approval of the application, in either case based upon changes in applicable law or FDA policy during the period of product development and FDA regulatory review. The applicant’s analysis of the results of clinical studies is subject to review and interpretation by the FDA, which
may differ from the applicant’s analysis, and in any event, the FDA must deny an NDA or BLA if applicable regulatory requirements are not ultimately satisfied. If regulatory approval of a product is granted, such approval may be made subject to various conditions, including post-marketing testing and surveillance to monitor the safety of the product, or may entail limitations on the indicated uses for which it may be marketed. Finally, product approvals may be withdrawn if compliance with regulatory standards is not maintained or if problems occur following initial marketing.
Both before and after approval is obtained, a product, its manufacturer and the sponsor of the marketing application for the product are subject to comprehensive regulatory oversight. Violations of existing or newly-adopted regulatory requirements at any stage, including the pre-clinical and clinical testing process, the approval process, or thereafter, may result in various adverse consequences, including FDA delay in approving or refusal to approve a product, withdrawal of an approved product from the market or the imposition of criminal penalties against the manufacturer or sponsor. Later discovery of previously unknown problems may result in restrictions on the product, manufacturer or sponsor, including
withdrawal of the product from the market.
Regulation Outside the U.S. Whether or not FDA approval has been obtained, approval of a pharmaceutical product by comparable government regulatory authorities in foreign countries must be obtained prior to marketing the product there. The approval procedure varies from country to country, and the time required may be longer or shorter than that required for FDA approval. The requirements for regulatory approval by governmental agencies in other countries prior to commercialization of products there can be rigorous, costly and uncertain, and approvals may not be granted on a timely basis or at all.
In European Union countries, Canada, Australia and Japan, regulatory requirements and approval processes are similar in principle to those in the United States. Regulatory approval in Japan requires that clinical trials of new drugs be conducted in Japanese patients. Depending on the type of drug for which approval is sought, there are currently two potential tracks for marketing approval in the E.U. countries: mutual recognition and the centralized procedure. These review mechanisms may ultimately lead to approval in all E.U. countries, but each method grants all participating countries some decision-making authority in product approval. The centralized procedure, which is mandatory for biotechnology derived
products, results in a recommendation in all member states, while the E.U. mutual recognition process involves country-by-country approval.
In other countries, regulatory requirements may require additional pre-clinical or clinical testing regardless of whether FDA approval has been obtained. This is the case in Japan, where Ono is responsible for developing and commercializing the subcutaneous form of RELISTOR and where trials are required to involve patient populations which we and our other collaborators have not examined in detail. If the particular product is manufactured in the U.S., we must also comply with FDA and other U.S. export provisions. In most countries outside the U.S., coverage, pricing and reimbursement approvals are also required which may affect the profitability of the affected product.
Other Regulation. In addition to regulations enforced by the FDA, we are also subject to regulation under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act, the Resource Conservation and Recovery Act and various other current and potential future federal, state or local regulations. Biopharmaceutical research and development involves the controlled use of hazardous materials, chemicals, viruses and various radioactive compounds. Even strict compliance with safety procedures for storing, handling, using and disposing of such materials prescribed by applicable regulations cannot
completely eliminate the risk of accidental contaminations or injury from these materials, which may result in liability for resulting legal and regulatory violations as well as damages.
Manufacturing
Under our License Agreement, Salix is responsible for the manufacture and supply, at its expense, of all active pharmaceutical ingredient (API) and finished and packaged products for its RELISTOR commercialization efforts, including contracting with contract manufacturing organizations (CMOs) for supply of RELISTOR API and subcutaneous and oral finished drug product. See Risk Factors.
Having closed our biologics pilot production facilities in 2011, we plan to engage third-party CMOs for manufacturing additional clinical trial supplies of our PSMA monoclonal antibody and to continue using CMOs for other portions of the PSMA ADC manufacturing process. If we are unable to arrange for satisfactory CMO services, or otherwise were to determine to establish a new manufacturing capacity, we would need to expand our manufacturing staff and facilities or obtain new facilities. In order to establish a full-scale commercial manufacturing facility for any of our product candidates, we would need to spend substantial additional funds, hire and train
significant numbers of employees and comply with the extensive FDA regulations applicable to such a facility.
Sales and Marketing
We from time to time seek strategic collaborations and other funding support for product candidates in our pipeline. We expect that we would market other products for which we obtain regulatory approval through co-marketing, co-promotion, licensing and distribution arrangements with third-party collaborators, and might also consider contracting with professional detailing and sales organizations to perform promotional and/or medical-scientific support functions for them. See Risk Factors.
Competition
Competition in the biopharmaceutical industry is intense and characterized by ongoing research and development and technological change. We face competition from many for-profit companies and major universities and research institutions in the U.S. and abroad. We face competition from companies marketing existing products or developing new products for diseases targeted by our technologies. Many of our competitors have substantially greater resources, experience in conducting pre-clinical studies and clinical trials and obtaining regulatory approvals for their products, operating experience, research and development and marketing capabilities and production
capabilities than we do. Our products and product candidates under development may not compete successfully with existing products or products under development by other companies, universities and other institutions. Drug manufacturers that are first in the market with a therapeutic for a specific indication generally obtain and maintain a significant competitive advantage over later entrants and therefore, the speed with which industry participants move to develop products, complete clinical trials, approve processes and commercialize products is an important competitive factor.
RELISTOR is the first FDA-approved product for any indication involving OIC. We are, however, aware of products in pre-clinical or clinical development that target the side effects of opioid pain therapy. Adolor Corporation (acquired by Cubist Pharmaceuticals in 2011) markets ENTEREG® (alvimopan) for the treatment of postoperative ileus, and has completed a phase 2 study of a compound for opioid-bowel dysfunction in chronic-pain patients. Sucampo Pharmaceuticals, Inc., in collaboration with Takeda Pharmaceutical Company Limited, markets
AMITIZA® (lubiprostone), a selective chloride channel activator, for chronic idiopathic (non-opioid related) constipation, and recently completed a phase 3 clinical trial of this drug in opioid-induced bowel dysfunction. A Nektar Therapeutics-AstraZeneca PLC collaboration is conducting phase 2 studies of an oral peripheral mu-opioid receptor antagonist in patients with OIC and a related combination product is in early stage development. Alkermes, Inc. has completed a phase 2 clinical study of an oral peripherally-restricted opioid antagonist, and has a combination product in preclinical testing. Theravance, Inc. has completed phase 2 clinical testing of an oral peripheral mu-opioid antagonist. In Europe, Mundipharma International Limited markets
TARGIN® (oxycodone/naloxone), a combination of an opioid and a systemic opioid antagonist, and Movetis NV, which has recently been acquired by Shire plc, is conducting a phase 3 clinical trial with prucalopride in patients with constipation induced by opioid based pain medications.
Radiation and surgery are two traditional forms of treatment for prostate cancer. If the disease spreads, hormone (androgen) suppression therapy is often used to slow the cancer’s progression, but this form of treatment can eventually become ineffective. We are aware of several competitors who are developing alternative treatments for castrate-resistant prostate cancer, some of which are directed against PSMA, including Zytiga® (abiraterone acetate), Medivation, Inc.’s MDV3100, and Algeta ASA’s
Alpharadin® (radium-223 chloride).
Recent evidence suggests that activation of complementary oncogenic pathways can confer resistance to PI3K inhibition, requiring co-administration of agents targeting these “resistance” pathways. We are aware of several competitors who are developing small molecule PI3K inhibitors that co-target additional oncogenic pathways.
A significant amount of research in the biopharmaceutical field is carried out at academic and government institutions. An element of our research and development strategy has been to in-license technology and product candidates from academic and government institutions. These institutions are sensitive to the commercial value of their findings and pursue patent protection and negotiate licensing arrangements to collect royalties for use of technology they develop. They may also market competitive commercial products on their own or in collaboration with competitors and compete with us in recruiting highly qualified scientific personnel, which may result in
increased costs or decreased availability of technology or product candidates from these institutions to other industry participants.
Competition with respect to our technologies and products is based on, among other things, product efficacy, safety, reliability, method of administration, availability, price and clinical benefit relative to cost; timing and scope of regulatory approval; sales, marketing and manufacturing capabilities; collaborator capabilities; insurance and other reimbursement coverage; and patent protection. Competitive position in our industry also depends on a participant’s ability to attract and retain qualified personnel, obtain patent protection or otherwise develop proprietary products or processes, and secure sufficient capital resources for the typically substantial period between technological conception and
commercial sales.
Product Liability
The testing, manufacturing and marketing of our product candidates and products involves an inherent risk of product liability attributable to unwanted and potentially serious health effects. To the extent we elect to test, manufacture or market product candidates and products independently, we bear the risk of product liability directly. We maintain product liability insurance coverage in the amount of $10.0 million per occurrence, subject to a deductible and a $10.0 million aggregate limitation. Where local statutory requirements exceed the limits of our existing insurance or local policies of insurance are required, we maintain additional clinical trial liability insurance to meet these requirements. This
insurance is subject to deductibles and coverage limitations. The availability of and cost of maintaining insurance may change over time.
Human Resources
At December 31, 2011, we had 105 full-time employees, 21 of whom hold Ph.D. degrees, three of whom hold M.D. degrees and two of whom hold both Ph.D. and M.D. degrees. At that date, 78 employees were engaged in research and development, medical, regulatory affairs and manufacturing related activities and 27 were engaged in finance, legal, administration and business development. We consider our relations with our employees to be good. None of our employees is covered by a collective bargaining agreement.
Item 1A. Risk Factors
Overview; Significance of our RELISTOR collaborations and our focus on oncology.
Our business and operations entail a variety of serious risks and uncertainties. Our business is inherently risky. We are subject to the risks of failure inherent in the development of product candidates based on new technologies. We, or our RELISTOR collaborators, must complete successfully clinical trials and obtain regulatory approvals for potential commercial products. Once approved, commercial products sales are subject to general and industry-specific local and international economic pressures such as those experienced worldwide over the last five years. Our product candidates other than RELISTOR are in pre-clinical or early clinical development. As an oncology-focused research and development
strategy, these risks continue to be significant, and may increase to the extent the oncology space becomes more competitive or less favored in the commercial marketplace. We now rely on Salix to complete development and obtain regulatory approvals for additional formulations of and indications for RELISTOR, and in the Japanese market, we rely on Ono to conduct successful clinical trials and obtain regulatory approvals. The research and development programs on which we are now focusing involve novel approaches to human therapeutics. There is little precedent for the successful commercialization of products based on our technologies, and there are a number of technological challenges that we must overcome to complete most of our development efforts. We may not be able successfully to develop further any of our products.
In addition to the risks we face in our research and development activities and our business as a publicly held commercial enterprise devoted to developing and commercializing high-technology consumer products, the transitioning of RELISTOR to our new partner Salix has presented us with new risks. Major risks we face in both our own research and development efforts and Salix’s and Ono’s development and commercialization efforts for RELISTOR include the following:
We are dependent on Salix, Ono and other business partners to develop and commercialize RELISTOR in their respective areas, exposing us to significant risks.
We are and will be dependent upon Salix, Ono and any other business partner(s) with which we may collaborate in the future to perform and fund development, including clinical testing of RELISTOR, make related regulatory filings and manufacture and market products, including for new indications and in new formulations, in their respective territories. Revenues from the sale of RELISTOR now depend entirely upon the efforts of Salix and its sublicensees, which have significant discretion in determining the efforts and resources they apply to sales of RELISTOR. Ono will have similar discretion with respect to sales in Japan. Neither may be effective in obtaining approvals for new indications and/or formulations,
marketing existing or future products, or arranging for necessary sublicense or distribution relationship. Our business relationships with Salix, Ono and other partners may not be scientifically, clinically or commercially successful. For example, Salix is a larger pharmaceutical company than Progenics with a variety of marketed products. Unlike Wyeth and Pfizer, however, Salix is not a large diversified pharmaceutical company and does not have resources commensurate with such companies. Salix has its own corporate objectives, which may not be consistent with our best interests, and may change its strategic focus or pursue alternative technologies in a manner that results in reduced or delayed revenues to us. Changes of this nature might also occur if Salix were acquired or if its management changed.
We may have future disagreements with Salix and Ono concerning product development, marketing strategies, manufacturing and supply issues, and rights relating to intellectual property. Both of them have significantly greater financial and managerial resources than we do, which either could draw upon in the event of a dispute. Disagreements between either of them and us could lead to lengthy and expensive litigation or other dispute-resolution proceedings as well as extensive financial and operational consequences to us, and have a material adverse effect on our business, results of operations and financial condition.
We are subject to extensive regulation, which can be costly and time consuming and can subject us to unanticipated fines and delays.
Our business and products are subject to comprehensive regulation by the FDA and comparable authorities in other countries. These agencies and other entities regulate the pre-clinical and clinical testing, safety, effectiveness, approval, manufacture, labeling, marketing, export, storage, recordkeeping, advertising, promotion and other aspects of our products. If we violate regulatory requirements at any stage, whether before or after marketing approval is obtained, we may be subject to forced removal of a product from the market, product seizure, civil and criminal penalties and other adverse consequences. We cannot guarantee that approvals of proposed products, processes or facilities will be granted on a timely
basis, or at all. If we experience delays or failures in obtaining approvals, commercialization of our product candidates will be slowed or stopped. Even if we obtain regulatory approval, the approval may include significant limitations on indicated uses for which the product could be marketed or other significant marketing restrictions. Under our License Agreement, we are dependent on Salix for compliance with these regulations as they apply to RELISTOR.
Our products may face regulatory, legal or commercial challenges even after approval.
Even if a product receives regulatory approval:
· It might not obtain labeling claims necessary to make the product commercially viable (in general, labeling claims define the medical conditions for which a drug product may be marketed, and are therefore very important to the commercial success of a product), or may be required to carry Black Box or other warnings that adversely affect its commercial success.
· Approval may be limited to uses of the product for treatment or prevention of diseases or conditions that are relatively less financially advantageous to us than approval of greater or different scope, or subject to an FDA-imposed Risk Evaluation and Mitigation Strategy (REMS) that limits the sources from and conditions under which it may be dispensed.
· Side effects identified after the product is on the market might hurt sales or result in product recalls or withdrawals from the market.
· Efficacy or safety concerns regarding a marketed product, or manufacturing or other problems, may lead to a recall, withdrawal of marketing approval, reformulation of the product, additional pre-clinical testing or clinical trials, changes in labeling, the need for additional marketing applications, declining sales or other adverse events. These potential consequences may occur whether or not the concerns originate from subsequent testing or other activities by us, governmental regulators, other entities or organizations or otherwise, and whether or not they are scientifically justified. If products lose previously received
marketing and other approvals, our financial results would be adversely affected.
· We or our collaborators will be subject to ongoing FDA obligations and continuous regulatory review, and might be required to undertake post-marketing trials to verify the product’s efficacy or safety or other regulatory obligations.
Competing products in development may adversely affect acceptance of our products.
We are aware of a number of products and product candidates described in this Annual Report under Business – Competition which compete or may potentially compete with RELISTOR. Any of these approved products or product candidates, or others which may be developed in the future may achieve a significant competitive advantage relative to RELISTOR, and, in any event, the existing or future marketing and sales capabilities of these competitors may impair Salix’s and/or Ono’s ability to compete effectively in the market.
We are also aware of competitors which are developing alternative treatments for disease targets to which our research and development programs are directed, any of which – or others which may be developed in the future – may achieve a significant competitive advantage relative to any product we may develop.
Developing product candidates may require us to obtain additional financing. Our access to capital funding is uncertain.
We expect to continue to incur significant development expenditures for our product candidates, and do not have committed external sources of funding for most of these projects. These expenditures will be funded from cash on hand, or we may seek additional external funding for them, most likely through collaborative, license or royalty financing agreements with one or more pharmaceutical companies, securities issuances or government grants or contracts. We cannot predict when we will need additional funds, how much we will need, the form any financing may take (such as securities issuance or royalty or other financing), or whether additional funds will be available at all, especially in light of current conditions
in global credit and financial markets. Our need for future funding will depend on numerous factors, such as the availability of new product development projects or other opportunities which we cannot predict, and many of which are outside our control. We cannot assure you that any currently-contemplated or future initiatives for funding our product candidate programs will be successful.
Our access to capital funding is always uncertain. Stresses in international markets are still affecting access to capital. We may not be able at the necessary time to obtain additional funding on acceptable terms, or at all. Our inability to raise additional capital on terms reasonably acceptable to us would seriously jeopardize our business.
If we raise funds by issuing and selling securities, it may be on terms that are not favorable to existing stockholders. If we raise funds by selling equity securities, current stockholders will be diluted, and new investors could have rights superior to existing stockholders. Raising funds by selling debt securities often entails significant restrictive covenants and repayment obligations.
If we are unable to negotiate collaborative agreements, our cash burn rate could increase and our rate of product development could decrease.
We may not be successful in negotiating additional collaborative arrangements with pharmaceutical and biotechnology companies to develop and commercialize product candidates and technologies. If we do not enter into new collaborative arrangements, we would have to devote more of our resources to clinical product development and product-launch activities, seeking additional sources of capital, and our cash burn rate would increase or we would need to take steps to reduce our rate of product development.
If testing does not yield successful results, our products will not be approved.
Regulatory approvals are necessary before product candidates can be marketed. To obtain them, we or our collaborators must demonstrate a product’s safety and efficacy through extensive pre-clinical and clinical testing. During this process, we may find that, for example, results of pre-clinical studies are inconclusive or not indicative of results in human clinical trials, or that potential products do not have the desired efficacy or have undesirable side effects or other characteristics that preclude marketing approval or limit their potential commercial use if approved. We, our collaborators or regulators may also suspend or terminate clinical trials if we or they believe that the participating subjects are
being exposed to unacceptable health risks, or after reviewing test results, we or our collaborators may abandon projects which we previously believed to be promising.
Clinical testing is very expensive and can take many years. Results attained in early human clinical trials may not be indicative of results in later clinical trials. In addition, many of our investigational or experimental drugs are at an early stage of development, and successful commercialization of early stage product candidates requires significant research, development, testing and approvals by regulators, and additional investment. Our products in the research or pre-clinical development stage may not yield results that would permit or justify clinical testing. Our failure to demonstrate adequately the safety and efficacy of a product under development would delay or prevent marketing approval, which could
adversely affect our operating results and credibility.
Setbacks in clinical development programs could adversely affect us.
We and our collaborators continue to conduct clinical trials, including trials of RELISTOR and other drug candidates. If the results of these or future trials are not satisfactory, we or our collaborators encounter problems enrolling subjects, clinical trial supply issues or other difficulties arise, or we or our collaborators experience setbacks in developing drug formulations, including raw material-supply, manufacturing or stability difficulties, the entire development program for that product or candidate could be adversely affected, resulting in delays in trials or regulatory filings for further marketing approval. Conducting additional clinical trials or making significant revisions to the clinical development
plan would lead to delays in regulatory filings. If clinical trials indicate a serious problem with the safety or efficacy of a product or candidate, we or our collaborators may stop development or commercialization of affected products. Since RELISTOR is our only approved product, any setback of these types with respect to it could have a material adverse effect on our business, results of operations and financial condition.
Ono is conducting required clinical trials with Japanese patients to obtain regulatory approval of RELISTOR in Japan. There can be no assurance that these clinical trials will yield results adequate for that regulatory approval.
If the results of current or future clinical studies of our product candidates are not satisfactory, we would need to reconfigure our clinical trial programs to conduct additional trials or abandon the program involved.
Clinical trials often take longer than expected.
Projections that we publicly announce of commencement and duration of clinical trials may not be certain. For example, we have experienced clinical trial delays in the past as a result of slower than anticipated enrollment. These delays may recur. Delays can be caused by, among other things, deaths or other adverse medical events; regulatory or patent issues; interim or final results of ongoing clinical trials; failure to enroll clinical sites as expected; competition for enrollment from clinical trials; scheduling conflicts with participating clinicians and institutions; disagreements, disputes or other matters arising from collaborations; our inability to obtain necessary funding; or manufacturing
problems.
We have limited experience in conducting clinical trials, and we rely on others to conduct, supervise or monitor some or all aspects of some of our clinical trials. In addition, certain clinical trials for our product candidates may be conducted by government-sponsored agencies, and consequently will be dependent on governmental participation and funding. Under our License Agreement with Salix, Salix generally has responsibility for conducting RELISTOR clinical trials, including all trials outside of the United States other than Japan, where Ono has the responsibility for clinical trials. We have less control over the timing and other aspects of these clinical trials than if we conducted them entirely on our
own.
Our product candidates may not obtain regulatory approvals needed for marketing.
None of our product candidates other than RELISTOR has been approved by applicable regulatory authorities for marketing. The process of obtaining FDA and foreign regulatory approvals often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. We have had only limited experience in filing and pursuing applications and other submissions necessary to gain marketing approvals. Products under development may never obtain marketing approval from the FDA or other regulatory authorities necessary for commercialization.
The commercial success of our products will depend upon their acceptance by the medical community and third party payors as clinically useful, cost effective and safe. If health care providers believe that patients can be managed adequately with alternative, currently available therapies, they may not prescribe our products, especially if the alternative therapies are viewed as more effective, as having a better safety or tolerability profile, as being more convenient to the patient or health care providers or as being less expensive. For pharmaceuticals administered in an institutional setting, the ability of the institution to be adequately reimbursed could also play a significant role in demand for our products.
Even if our products obtain marketing approval, they may not achieve market acceptance. If any of our products do not achieve market acceptance, we will likely lose our entire investment in that product.
Market acceptance of approved products such as RELISTOR also is affected by the timing of regulatory approvals, product launches and reimbursement programs for existing and expanded uses by our collaborators or generic, over-the-counter or other competitors; price increases for the product and relative prices of competing products; product development efforts for new indications; availability of sufficient commercial quantities of the product; success in arranging for necessary sublicense or distribution relationships; and general and industry-specific local and international economic pressures such as those experienced worldwide over the last five years.
Marketplace acceptance depends in part on competition in our industry, which is intense.
The extent to which any of our products achieves market acceptance will depend on competitive factors. Competition in our industry is intense, and it is accentuated by the rapid pace of technological development. There are currently marketed products that will compete with the product candidates that we are developing. There are product candidates in pre-clinical or clinical development that target the side effects of opioid pain therapy, and a marketed product for the treatment of post-operative ileus could compete with RELISTOR. Many of our competitors have substantially greater research and development capabilities and experience and greater
manufacturing, marketing, financial and managerial resources than we do. These competitors may develop products that are superior to those we are developing and render our products or technologies non-competitive or obsolete. If our product candidates receive marketing approval but cannot compete effectively in the marketplace, our operating results and financial position would suffer. Competition with respect to our technologies and products is based on, among other things, product efficacy, safety, reliability, method of administration, availability, price and clinical benefit relative to cost; timing and scope of regulatory approval; sales, marketing and manufacturing capabilities; collaborator capabilities; insurance and other reimbursement coverage; and patent protection. Competitive disadvantages in any of these factors could materially
harm our business and financial condition.
If we or our collaborators are unable to obtain sufficient quantities of the raw and bulk materials needed to make our products, development and commercialization of our product candidates could be slowed or stopped.
Salix or Ono may not be able to fulfill manufacturing obligations for RELISTOR, either on their own or through third-party suppliers. Our existing arrangements with suppliers for our other product candidates may not result in the supply of sufficient quantities of our product candidates needed to accomplish our clinical development programs, and we may not have the right and in any event do not currently have the capability to manufacture these products if our suppliers are unable or unwilling to do so. We currently arrange for supplies of critical raw materials used in production of our product candidates from single sources. We do not have long-term contracts with any of these suppliers. Any delay or disruption in
the availability of raw materials would slow or stop product development and commercialization of the relevant product. A delay or disruption of supplies of RELISTOR would have a material adverse effect on the RELISTOR franchise, and therefore on our business as a whole.
Manufacturing resources could limit or adversely affect our ability to commercialize products.
Under our License Agreement, Salix is responsible for obtaining supplies of RELISTOR, including contracting with contract manufacturing organizations (CMOs) for supply of RELISTOR active pharmaceutical ingredient (API) and subcutaneous and oral finished drug product. These arrangements may not be on optimally-advantageous terms, and as a result of our royalty and other interests in RELISTOR’s commercial success will subject us to risks that the counterparties may not perform optimally in terms of quality or reliability.
We engage third parties for manufacturing product candidates and means of administration for them, which may not be optimally cost-effective. In doing so, we also do not control many aspects of the manufacturing process, including compliance with cGMP and other regulatory requirements. We may not be able to obtain adequate supplies from third-party manufacturers in a timely fashion for development or commercialization purposes, and commercial quantities of products may not be available from contract manufacturers at acceptable costs.
In order to commercialize our product candidates successfully, we or our collaborators would need to be able to manufacture or arrange for the manufacture of products in commercial quantities, in compliance with regulatory requirements, at acceptable costs and in a timely manner. Manufacture of our product candidates can be complex, difficult to accomplish even in small quantities, difficult to scale-up for large-scale production and subject to delays, inefficiencies and low yields of quality products. The cost of manufacturing some of our products may make them prohibitively expensive. If adequate supplies of any of our product candidates or related materials are not available on a timely basis or at all, our
clinical trials could be seriously delayed, since these materials are time consuming to manufacture and cannot be readily obtained from third-party sources.
If we were to decide to establish a commercial-scale manufacturing facility in the future, we would require substantial additional funds and be required to hire and train significant numbers of employees and comply with applicable regulations.
We are dependent on patents and other intellectual property rights. The validity, enforceability and commercial value of these rights are highly uncertain.
We must obtain, maintain and enforce patent and other rights to protect our intellectual property. The patent position of biotechnology and pharmaceutical firms is highly uncertain and involves many complex legal and technical issues. There is no clear policy involving the breadth of claims allowed, or the degree of protection afforded, under patents in this area. Accordingly, patent applications owned by or licensed to us may not result in patents being issued. We are aware of others who have patent applications or patents containing claims similar to or overlapping those in our patents and patent applications. Because of these considerations and potential post-issuance events discussed below, it is generally
difficult to determine the relative strength or scope of a biotechnology patent position in absolute terms at any given time. Patents that we own or license may not enable us to preclude competitors from commercializing drugs, and consequently may not provide us with any meaningful competitive advantage.
We own or have licenses to a number of issued patents. The issuance of a patent, however, is not conclusive as to its validity or enforceability, which can be challenged in litigation. Our patents may be successfully challenged. We may incur substantial costs in litigation seeking to uphold the validity of patents or to prevent infringement. If the outcome of litigation is adverse to us, third parties may be able to use our patented invention without payment to us. Third parties may also avoid our patents through design innovation.
Most of our product candidates, as well as RELISTOR, incorporate to some degree intellectual property licensed from third parties. We can lose the right to patents and other intellectual property licensed to us if the related license agreement is terminated due to a breach by us or otherwise. Our ability, and that of our collaboration partners, to commercialize products incorporating licensed intellectual property would be impaired if the related license agreements were terminated.
The license agreements from which we derive or out-license intellectual property provide for various royalty, milestone and other payment, commercialization, sublicensing, patent prosecution and enforcement, insurance, indemnification and other obligations and rights, and are subject to certain reservations of rights. While we generally have the right to defend and enforce patents licensed by us, either in the first instance or if the licensor chooses not to do so, we must usually bear the cost of doing so. Under our License Agreement, Salix generally has control over defense and enforcement of our RELISTOR patents. With respect to Japan, Ono has certain limited rights to prosecute, maintain and enforce relevant
intellectual property. With most of our in-licenses, the licensor bears the cost of engaging in all of these activities, although we may share in those costs under specified circumstances.
We also rely on unpatented technology, trade secrets and confidential information. Third parties may independently develop substantially equivalent information and techniques or otherwise gain access to our technology or disclose our technology, and we may be unable to effectively protect our rights in unpatented technology, trade secrets and confidential information. We require each of our employees, consultants and advisors to execute a confidentiality agreement at the commencement of an employment or consulting relationship with us. These agreements may, however, not provide effective protection in the event of unauthorized use or disclosure of confidential information.
If we do not achieve milestones or satisfy conditions regarding some of our product candidates, we may not maintain our rights under related licenses.
We are required to make substantial cash payments, achieve milestones and satisfy other conditions, including filing for and obtaining marketing approvals and introducing products, to maintain rights under our intellectual property licenses. Due to the nature of these agreements and the uncertainties of research and development, we may not be able to achieve milestones or satisfy conditions to which we have contractually committed, and as a result may be unable to maintain our rights under these licenses. If we do not comply with our license agreements, the licensors may terminate them, which could result in our losing our rights to, and therefore being unable to commercialize, related products.
If we infringe third-party patent or other intellectual property rights, we may need to alter or terminate a product development program.
There may be patent or other intellectual property rights belonging to others that require us to alter our products, pay licensing fees or cease certain activities. If our products infringe patent or other intellectual property rights of others, the owners of those rights could bring legal actions against us claiming damages and seeking to enjoin manufacturing and marketing of the affected products. If these legal actions are successful, in addition to any potential liability for damages, we could be required to obtain a license in order to continue to manufacture or market the affected products. We may not prevail in any action brought against us, and any license required under any rights that we infringe may not
be available on acceptable terms or at all. We are aware of intellectual property rights held by third parties that relate to products or technologies we are developing. For example, we are aware of other groups investigating PSMA or related compounds, monoclonal antibodies directed at targets relevant to PSMA ADC, and methylnaltrexone and other peripheral opioid antagonists, and of patents held, and patent applications filed, by these groups in those areas. While the validity of these issued patents, patentability of these pending patent applications and applicability of any of them to our programs are uncertain, if asserted against us, any related patent or other intellectual property rights could adversely affect our ability to commercialize our products.
Research, development and commercialization of a biopharmaceutical often require choosing between alternative development and optimization routes at various stages in the development process. Preferred routes depend on subsequent discoveries and test results and cannot be predicted with certainty at the outset. There are numerous third-party patents in our field, and we may need to obtain a license under a patent in order to pursue the preferred development route of one or more of our products or product candidates. The need to obtain a license would decrease the ultimate profitability of the applicable product. If we cannot negotiate a license, we might have to pursue a less desirable development route or terminate
the program altogether.
We are dependent upon third parties for a variety of functions. These arrangements may not provide us with the benefits we expect.
We rely in part on third parties to perform a variety of functions. We are party to numerous agreements which place substantial responsibility on clinical research organizations, consultants and other service providers for the development of our products. We also rely on medical and academic institutions to perform aspects of our clinical trials of product candidates. In addition, an element of our research and development strategy has been to in-license technology and product candidates from academic and government institutions in order to minimize investments in early research. We have entered into agreements under which we are now dependent on Ono and Salix, respectively, for the commercialization and development
of RELISTOR. We may not be able to maintain our relationships with them, or establish new ones for RELISTOR or other drug candidates on beneficial terms. We may not be able to enter new arrangements without undue delays or expenditures, and these arrangements may not allow us to compete successfully.
We lack sales and marketing infrastructure and related staff, which will require significant investment to establish and in the meantime may make us dependent on third parties for their expertise in this area.
We have no established sales, marketing or distribution infrastructure. If we receive marketing approval for a pharmaceutical product, significant investment, time and managerial resources will be required to build the commercial infrastructure required to market, sell and support it. Should we choose to commercialize a product directly, we may not be successful in developing an effective commercial infrastructure or in achieving sufficient market acceptance. Alternatively, we may choose to market and sell products through distribution, co-marketing, co-promotion or licensing arrangements with third parties. We may also consider contracting with a third party professional pharmaceutical detailing and sales
organization to perform the marketing function for one or more products. To the extent that we enter into distribution, co-marketing, co-promotion, detailing or licensing arrangements for the marketing and sale of product candidates, any revenues we receive will depend primarily on the efforts of third parties. We will not control the amount and timing of marketing resources these third parties devote to our products.
We are exposed to product liability claims, and in the future may not be able to obtain insurance against claims at a reasonable cost or at all.
Our business exposes us to product liability risks, which are inherent in the testing, manufacturing, marketing and sale of pharmaceutical products. We may not be able to avoid product liability exposure. If a product liability claim is successfully brought against us, our financial position may be adversely affected. We are indemnified by Salix under our License Agreement for product liability exposure arising from its marketing and sales of RELISTOR, and maintain our own product liability insurance coverage in the amount of $10.0 million per occurrence, subject to a deductible and a $10.0 million annual aggregate limitation and other clinical trial or other insurance as required by contract and local laws.
Pursuant to our Transition Agreement, we released Wyeth from its indemnification responsibility for product liability exposure arising from its marketing and sales of RELISTOR.
Product liability insurance for the biopharmaceutical industry is generally expensive, when available at all, and may not be available to us at a reasonable cost in the future. Our current insurance coverage and indemnification arrangements may not be adequate to cover claims brought against us, and are in any event subject to the insuring or indemnifying entity discharging its obligations to us.
We handle hazardous materials and must comply with environmental laws and regulations, which can be expensive and restrict how we do business. If we are involved in a hazardous waste spill or other accident, we could be liable for damages, penalties or other forms of censure.
Our research and development work and manufacturing processes involve the use of hazardous, controlled and radioactive materials. We are subject to federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of these materials. Despite procedures that we implement for handling and disposing of these materials, we cannot eliminate the risk of accidental contamination or injury. In the event of a hazardous waste spill or other accident, we could be liable for damages, penalties or other forms of censure. We may be required to incur significant costs to comply with environmental laws and regulations in the future.
If we lose key management and scientific personnel on whom we depend, our business could suffer.
We are dependent upon our key management and scientific personnel, the loss of whom could require us to identify and engage qualified replacements, and could cause our management and operations to suffer in the interim. Competition for qualified employees among companies in the biopharmaceutical industry is intense. Future success in our industry depends in significant part on the ability to attract, retain and motivate highly skilled employees, which we may not be successful in doing.
If health care reform measures are enacted, our operating results and our ability to commercialize products could be adversely affected.
In recent years, there have been numerous proposals to change the health care system in the U.S. and in other jurisdictions. Some of these proposals have included measures that would change the nature of and regulatory requirements relating to drug discovery, clinical testing and regulatory approvals, limit or eliminate payments for medical procedures and treatments, or subject the pricing of pharmaceuticals to government control. Outside the U.S., and particularly in the E.U., the pricing of prescription pharmaceuticals is subject to governmental control. In addition, as a result of the trend towards managed health care in the U.S., as well as legislative proposals to reduce government insurance programs,
third-party payors are increasingly attempting to contain health care costs by limiting both coverage and the level of reimbursement of new drug products, including relatively expensive products which may be perceived to provide relatively limited benefits to patients with potentially terminal conditions. Consequently, significant uncertainty exists as to the reimbursement status of newly approved health care products.
If we or any of our collaborators succeed in bringing one or more of our product candidates to market, third party payors may establish and maintain price levels insufficient for us to realize an appropriate return on our investment in product development. Significant changes in the health care system in the U.S. or elsewhere, including changes resulting from adverse trends in third-party reimbursement programs, could have a material adverse effect on our operating results and our ability to raise capital and commercialize products.
A substantial portion of our past funding has come from federal government grants and research contracts. We cannot rely on these grants or contracts as a continuing source of funds.
A portion of our revenues to date has been derived from federal government grants and research contracts. During the last three years, we generated revenues from awards made to us by the NIH to partially fund some of our programs. Most of these resources have been directed toward candidates which we have discontinued work on or are now seeking to out-license. In any event, we cannot rely on grants or additional contracts as a continuing source of funds, as funds available under these grants and/or contracts must be applied toward the research and development programs specified by the government rather than for all our programs generally, and are subject to adjustment based on the results of periodic audits. The
government’s obligation to make payments under these grants and/or contracts is subject to appropriation by the U.S. Congress for funding in each year, which is subject to being scaled back due to budgetary constraints.
We have a history of operating losses.
Progenics has incurred substantial losses since its founding. A large portion of our revenues has historically consisted of upfront and milestone payments from licensing transactions. While we reported a profit in 2011 as a result of the upfront payment we received from Salix, the timing and amount of such transactions is highly unpredictable and uncertain. We have derived no significant revenues from product sales and have only in the last several years derived revenue from royalties. We may not achieve significant product sales or royalty revenue for a number of years, if ever. We expect to incur operating losses in the future, which could increase significantly if we expand our clinical trial programs and other
product development efforts. Our ability to achieve and sustain profitability is dependent in part on obtaining regulatory approval for and then commercializing our products, either alone or with others. We may not be able to develop and commercialize products beyond subcutaneous RELISTOR. Our operations may not be profitable even if any of our other products under development are commercialized.
Our stock price has a history of volatility and may be affected by selling pressure. You should consider an investment in our stock as risky and invest only if you can withstand a significant loss.
Our stock price has a history of significant volatility. At times, our stock price has been volatile even in the absence of significant news or developments. The stock prices of biotechnology companies and securities markets generally have been subject to dramatic price swings in recent years, and financial and market conditions during that period have resulted in widespread pressures on securities of issuers throughout the world economy. Factors that may have a significant impact on the market price of our common stock include the results of clinical trials and pre-clinical studies undertaken by us or others; delays, terminations or other changes in development programs;
developments in marketing approval efforts; developments in collaborator or other business relationships, particularly regarding RELISTOR, PSMA ADC or other significant products or programs; technological innovation or product announcements by us, our collaborators or our competitors; patent or other proprietary rights developments; governmental regulation; changes in reimbursement policies or health care legislation; safety and efficacy concerns about products developed by us, our collaborators or our competitors; our ability to fund ongoing operations; fluctuations in our operating results; purchases we may make under our 2008 share repurchase program, or discontinuation of any such purchases; and general market conditions.
Sales of substantial numbers of shares of common stock could cause a decline in the market price of our stock. We require substantial external funding to finance our research and development programs and may seek such funding through the issuance and sale of our common stock. We have in place a shelf registration statement which may be used for the issuance of up to $100.0 million of our common stock, preferred stock, debt securities, warrants and other rights units to investors, as well as registration statements registering shares issuable pursuant to our equity compensation plans. Sales of our securities pursuant to these registration statements could cause the market price or our stock to decline. Any sales by
existing stockholders or holders of options, or other rights, may have an adverse effect on our ability to raise capital and may adversely affect the market price of our common stock.
Our principal stockholders are able to exert significant influence over matters submitted to stockholders for approval.
At 2011 year end, our directors and executive officers and stockholders affiliated with Tudor Investment Corporation together beneficially owned or controlled approximately one-fifth of our outstanding shares of common stock and our five largest other stockholders beneficially owned or controlled in the aggregate approximately two-fifths of our shares. Should these parties choose to act together, they could exert significant influence in determining the outcome of corporate actions requiring stockholder approval and otherwise control our business. This control could have the effect of delaying or preventing a change in control of the Company and, consequently, could adversely affect our stock price.
Anti-takeover provisions may make removal of our Board and/or management more difficult, discouraging hostile bids for control that may be beneficial to our stockholders.
Our Board is authorized, without further stockholder action, to issue from time to time shares of preferred stock in one or more designated series or classes. The issuance of preferred stock, as well as provisions in some outstanding stock options that provide for acceleration of exercisability upon a change of control, and Section 203 and other provisions of the Delaware General Corporation Law could make the takeover or the removal of our Board or management more difficult; discourage hostile bids for control in which stockholders may receive a premium for their shares; and otherwise dilute the rights of common stockholders and depress the market price of our stock.
Item 1B. Unresolved Staff Comments
There were no unresolved SEC staff comments regarding our periodic or current reports under the Exchange Act as of December 31, 2011.
Item 2. Properties
As of December 31, 2011, we occupy in total approximately 106,149 square feet of laboratory, manufacturing and office space on a single campus in Tarrytown, New York, under lease agreements expiring in June 2012 and December 2020. In addition to rents due under these arrangements, we are obligated to pay additional facilities charges, including utilities, taxes and operating expenses.
Item 3. Legal Proceedings
We are not a party to any material legal proceedings. From time to time we may be subject or a party to claims or legal proceedings arising in the ordinary course of business, none of which we currently believe will have a material adverse effect on our financial condition or results of operations.
Item 4. Not Applicable
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Price Range of Common Stock
Our common stock is quoted on The NASDAQ Stock Market LLC under the symbol PGNX. The following table sets forth, for the periods indicated, the high and low sales price per share of the common stock, as reported on NASDAQ.
|
High
|
Low
|
|||||
|
2011:
|
Fourth quarter
|
$
|
9.19
|
$
|
5.01
|
|
|
Third quarter
|
7.93
|
4.50
|
||||
|
Second quarter
|
8.69
|
5.97
|
||||
|
First quarter
|
6.50
|
5.32
|
||||
|
2010:
|
Fourth quarter
|
5.69
|
4.41
|
|||
|
Third quarter
|
5.72
|
4.00
|
||||
|
Second quarter
|
7.00
|
4.25
|
||||
|
First quarter
|
5.50
|
4.16
|
On March 5, 2012, the last sale price for our common stock, as reported by The NASDAQ Stock Market LLC, was $9.01. There were approximately 135 holders of record of our common stock as of that date.
Comparative Stock Performance Graph
The graph below compares, for the past five years, the cumulative stockholder return on our common stock with the cumulative stockholder return of (i) the Nasdaq Stock Market (U.S.) Index and (ii) the Nasdaq Pharmaceutical Index, assuming an investment in each of $100 on December 31, 2006.
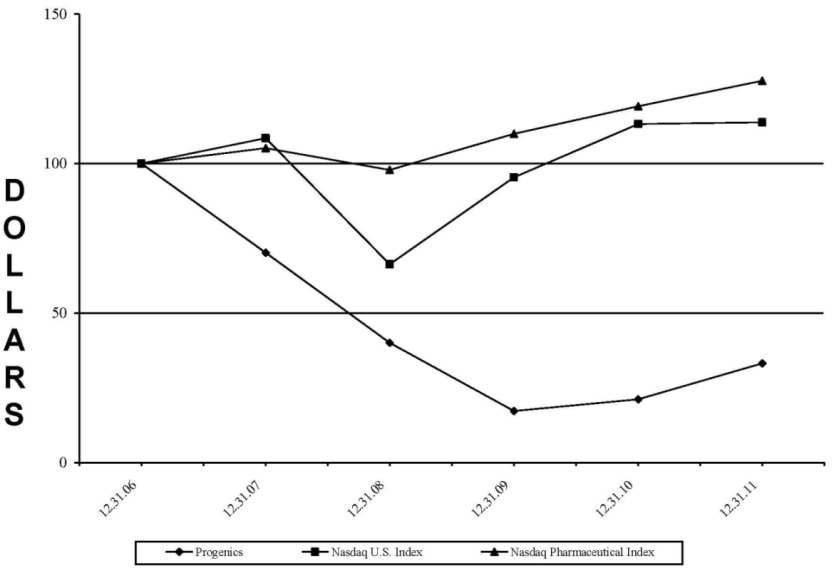
Dividends
We have not paid any dividends since the Company’s inception and currently anticipate that all earnings, if any, will be retained for development of our business and that no dividends on our common stock will be declared in the foreseeable future.
Item 6. Selected Financial Data
The selected financial data presented below as of December 31, 2011 and 2010 and for each of the three years in the period ended December 31, 2011 are derived from our audited financial statements, included elsewhere herein. The selected financial data presented below with respect to the balance sheet data as of December 31, 2009, 2008 and 2007 and for each of the two years in the period ended December 31, 2008 are derived from our audited financial statements not included herein. The data set forth below should be read in conjunction with Management’s Discussion and Analysis of Financial Condition and Results of Operations and the Financial Statements and related Notes included elsewhere herein.
|
Years Ended December 31,
|
||||||||||||||||||||
|
2011
|
2010
|
2009
|
2008
|
2007
|
||||||||||||||||
|
(in thousands, except per share data)
|
||||||||||||||||||||
|
Statement of Operations Data:
|
||||||||||||||||||||
|
Revenues:
|
||||||||||||||||||||
|
Collaboration revenue
|
$ | 76,764 | $ | 1,413 | $ | 44,351 | $ | 59,885 | $ | 65,455 | ||||||||||
|
Royalty income
|
3,046 | 1,826 | 2,372 | 146 | - | |||||||||||||||
|
Research grants and contract
|
4,810 | 4,573 | 1,968 | 7,460 | 10,075 | |||||||||||||||
|
Other revenues
|
176 | 140 | 256 | 180 | 116 | |||||||||||||||
|
Total revenues
|
84,796 | 7,952 | 48,947 | 67,671 | 75,646 | |||||||||||||||
|
Expenses:
|
||||||||||||||||||||
|
Research and development
|
53,183 | 50,640 | 49,798 | 82,290 | 95,234 | |||||||||||||||
|
License fees – research and development
|
578 | 1,270 | 1,058 | 2,830 | 942 | |||||||||||||||
|
General and administrative
|
18,248 | 22,832 | 25,106 | 28,834 | 27,901 | |||||||||||||||
|
Royalty expense
|
405 | 241 | 237 | 15 | - | |||||||||||||||
|
Depreciation and amortization
|
2,066 | 2,853 | 5,078 | 4,609 | 3,027 | |||||||||||||||
|
Total expenses
|
74,480 | 77,836 | 81,277 | 118,578 | 127,104 | |||||||||||||||
|
Operating income (loss)
|
10,316 | (69,884 | ) | (32,330 | ) | (50,907 | ) | (51,458 | ) | |||||||||||
|
Other income:
|
||||||||||||||||||||
|
Interest income
|
65 | 64 | 1,481 | 6,235 | 7,770 | |||||||||||||||
|
Gain on sale of marketable securities
|
- | - | 237 | - | - | |||||||||||||||
|
Total other income
|
65 | 64 | 1,718 | 6,235 | 7,770 | |||||||||||||||
|
Net income (loss) before income taxes
|
10,381 | (69,820 | ) | (30,612 | ) | (44,672 | ) | (43,688 | ) | |||||||||||
|
Income tax benefit
|
- | 95 | - | - | - | |||||||||||||||
|
Net income (loss)
|
$ | 10,381 | $ | (69,725 | ) | $ | (30,612 | ) | $ | (44,672 | ) | $ | (43,688 | ) | ||||||
|
Per share amounts on net income (loss):
|
||||||||||||||||||||
|
Basic
|
$ | 0.31 | $ | (2.14 | ) | $ | (0.98 | ) | $ | (1.48 | ) | $ | (1.59 | ) | ||||||
|
Diluted
|
$ | 0.31 | $ | (2.14 | ) | $ | (0.98 | ) | $ | (1.48 | ) | $ | (1.59 | ) | ||||||
|
December 31,
|
||||||||||||||||||||
|
2011
|
2010
|
2009
|
2008
|
2007
|
||||||||||||||||
|
(in thousands)
|
||||||||||||||||||||
|
Balance Sheet Data:
|
||||||||||||||||||||
|
Cash and cash equivalents
|
$ | 70,105 | $ | 47,918 | $ | 90,903 | $ | 56,186 | $ | 10,423 | ||||||||||
|
Marketable and auction rate securities
|
3,332 | 3,608 | 5,293 | 85,188 | 159,947 | |||||||||||||||
|
Working capital
|
65,890 | 42,207 | 95,388 | 85,983 | 102,979 | |||||||||||||||
|
Total assets
|
80,110 | 62,738 | 113,613 | 157,833 | 189,539 | |||||||||||||||
|
Deferred revenue – current
|
204 | - | - | - | - | |||||||||||||||
|
Deferred revenue – long term
|
162 | - | - | - | 9,131 | |||||||||||||||
|
Other liabilities – long term
|
1,497 | 1,635 | - | 266 | 359 | |||||||||||||||
|
Total stockholders’ equity
|
71,801 | 51,308 | 107,607 | 119,369 | 147,499 | |||||||||||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations (MD&A)
Overview
General. Progenics Pharmaceuticals is dedicated to the development of innovative medicines to treat disease. Our focus is on the treatment of cancer.
Our first commercial drug is RELISTOR®, for the treatment of opioid induced constipation (OIC) in patients with advanced illnesses, such as cancer. OIC is the constipation that often arises when patients take opioids for pain relief. RELISTOR is the only prescription medicine approved in the United States to treat this form of constipation. RELISTOR subcutaneous injection is now approved in the U.S. and over 50 other counties around the world. In the U.S. RELISTOR is marketed by our commercial partner Salix Pharmaceuticals, a leading specialty pharmaceutical company focusing on gastrointestinal diseases; it is sold outside the U.S.
by sublicensees of Salix. Our partner Ono Pharmaceutical is currently developing subcutaneous RELISTOR for Japan. Our current principal sources of revenue from operations are upfront, commercialization milestone, royalty and revenue-sharing payments from Salix’s RELISTOR operations.
Together with Salix we have applied to the U.S. Food and Drug Administration to expand the population that can be treated with subcutaneous RELISTOR to include patients taking opioids for non-cancer pain, and who suffer from OIC as a result. This population includes patients taking opioids for conditions such as back pain or joint pain. The FDA’s action date on this marketing application under the Prescription Drug User Fee Act (PDUFA) is April 27, 2012. We also recently announced results from a Phase 3 clinical test of an oral form of RELISTOR, in which the efficacy of oral methylnaltrexone was comparable to that reported in clinical studies of subcutaneous methylnaltrexone in subjects with chronic,
non-cancer pain, and the overall observed safety profile in patients treated was comparable to placebo.
Our lead oncology product candidate is PSMA ADC, a fully human monoclonal antibody-drug conjugate (ADC) directed against prostate specific membrane antigen (PSMA), a protein found at high levels on the surface of prostate cancer cells and also on the neovasculature of a number of other types of solid tumors. We are conducting a phase 1 clinical trial of PSMA ADC for the treatment of prostate cancer which we expect will be completed in 2012, and if the results are successful we plan then to commence a Phase 2 trial of PSMA ADC in advanced prostate cancer. As a part of our work in oncology, we are also conducting preclinical development of novel multiplex
phosphoinositide 3-kinase (PI3K) inhibitors. We believe these compounds may be effective in blocking signaling pathways that are critical in the growth of aggressive cancers, particularly RAS-mutated tumors. With our focus on the development of medicines to treat cancer, we are seeking opportunities to expand our oncology pipeline through in-licensing and acquisitions. We have discontinued work on programs outside of this focus and are working to out-license them.
Our sources of revenues for the year ended December 31, 2011 have been payments under our collaboration agreements, including $76.8 million from upfront payments and collaboration reimbursements and $3.0 million from royalties, and $4.8 million from research grants from NIH related to our oncology and virology programs. Salix, Progenics and former collaborator Wyeth have transitioned U.S., European and other marketing authorizations and are transitioning additional commercialization outside of the U.S. and Japan. Salix has secured distribution and marketing partners for RELISTOR in Europe and has granted a license to Link Medical Products Pty Limited for distribution in Australia, New Zealand, South Africa and
certain other markets in Asia. Royalty income in 2011 is based only on net sales reported by Salix; royalty income through September 30, 2010 was based on net sales reported by Wyeth. Salix is continuing its efforts to secure additional distribution partners and/or sublicensees. To date, our product sales have consisted solely of limited revenues from the sale of research reagents and we expect that those sales will not significantly increase over current levels in the near future.
A majority of our expenditures to date have been for research and development activities. During 2011, expenses for our RELISTOR research program were $23.2 million compared to $23.3 million in 2010 and $7.8 million in 2009. Expenses for our cancer and HIV research programs were $18.2 million and $3.8 million, respectively, during 2011 compared to (i) $14.7 million and $5.5 million, respectively in 2010 and (ii) $20.1 million and $11.8 million, respectively, in 2009. Our expenses and reimbursement revenue related to RELISTOR in the future will depend on the amount of research and development work we perform upon requests by Salix or Ono. We also expect to incur a significant amount of development expenses for our
oncology programs as these programs progress. We expect future expenses related to research and development for which we receive reimbursement from the NIH, including the HIV program, to decline to the extent we out-license these programs.
At December 31, 2011, we held $70.1 million in cash and cash equivalents, an increase of $22.2 million from $47.9 million at December 31, 2010. We expect that this amount will be sufficient to fund operations as currently anticipated beyond one year. We may require additional funding in the future, and if we are unable to conclude favorable collaboration, license, asset sale, capital raising or other financing transactions, we will have to reduce, delay or eliminate spending on some current operations, and/or reduce salary and other overhead expenses, to extend our remaining operations. We expect funding from the NIH to decline in the future to the extent we out-license existing NIH-funded programs not in the
oncology area. We expect to incur operating losses during the near term. At December 31, 2011, cash, cash equivalents and auction rate securities increased $21.9 million to $73.4 million from $51.5 million at December 31, 2010.
RELISTOR. RELISTOR has been approved by regulatory authorities in the U.S., countries in the European Union, Canada and Australia since 2008 for treatment of OIC in advanced-illness patients receiving palliative care when laxative therapy has not been sufficient. Marketing applications are pending elsewhere throughout the world.
Under our 2011 License Agreement, Salix is responsible for further developing and commercializing subcutaneous RELISTOR, including completing clinical development necessary to support regulatory marketing approvals for potential new indications and formulations of the drug. Through December 31, 2011, we have received under this Agreement a $60.0 million upfront cash payment and $0.2 million in respect of Salix ex-U.S. sublicensee revenue and are eligible to receive (i) up to $40.0 million upon U.S. marketing approval for subcutaneous RELISTOR in non-cancer pain patients, (ii) up to $50.0 million upon U.S. marketing approval of an oral formulation of RELISTOR, (iii) up to $200.0 million of commercialization milestone
payments upon achievement of specified U.S. sales targets, (iv) royalties ranging from 15 to 19 percent of net sales by Salix and its affiliates, and (v) 60% of any upfront, milestone, reimbursement or other revenue (net of costs of goods sold, as defined, and territory-specific research and development expense reimbursement) Salix receives from sublicensees outside the U.S. In the event that either marketing approval is subject to a Black Box Warning or Risk Evaluation and Mitigation Strategy (REMS), payment of a portion of the milestone amount would be deferred, and subject, to achievement of the first commercialization milestone.
RELISTOR was previously developed and commercialized worldwide except Japan with Wyeth pursuant to a 2005 collaboration agreement that was terminated in October 2009. Salix, Progenics, and Progenics’ former collaborator Wyeth have transitioned U.S., European and other marketing authorizations and are transitioning additional commercialization outside the U.S. and Japan. Salix has secured distribution and marketing partners for RELISTOR in the European territory and has licensed Link Medical Products Pty Limited for distribution in Australia, New Zealand, South Africa and certain other markets in Asia. Salix is continuing efforts to secure additional distribution partners and/or sublicensees. Royalty income in
2011 is based on net sales reported by Salix; royalty income through September 30, 2010 was based on net sales reported by Wyeth. Under the Transition Agreement, Wyeth paid us $10.0 million in six quarterly installments through January 2011. Wyeth also provided financial resources for the development of a multi-dose pen for subcutaneous RELISTOR for which we recognized $1.6 million in 2011 and $1.2 million in 2010.
Together with Salix we have applied to the U.S. Food and Drug Administration to expand the population that can be treated with subcutaneous RELISTOR to include patients taking opioids for non-cancer pain, and who suffer from OIC as a result. This population includes patients taking opioids for conditions such as back pain or joint pain. We have also received U.S., E.U. and Canadian approvals to market RELISTOR in pre-filled syringes, which are designed to ease preparation and administration for patients and caregivers, and expect Salix to introduce that product in 2012. In return for our October 2008 out-license to Ono Pharmaceutical of the rights to subcutaneous RELISTOR in Japan, we received an upfront payment of
$15.0 million and the right to receive potential milestones, upon achievement of development responsibilities by Ono, of up to $20.0 million, commercial milestones and royalties on sales by Ono of subcutaneous RELISTOR in Japan. Ono also has the option to acquire from us the rights to develop and commercialize in Japan other formulations of RELISTOR on terms to be negotiated separately. Ono may request us to perform activities related to its development and commercialization responsibilities beyond our participation in joint committees and specified technology transfer related tasks which will be at its expense, and reimbursable at the time we perform these services.
Royalty and milestone payments will depend on success in development and commercialization of RELISTOR, which is dependent on many factors, such as the actions of Salix and Ono and any other business partner(s) with which we may collaborate, decisions by the FDA and other regulatory bodies, the outcome of clinical and other testing of RELISTOR, and our own efforts. Many of these matters are outside our control. In particular, we cannot guarantee that Salix will be successful in furthering the development and commercialization of the RELISTOR franchise.
Oncology. We recently announced preliminary data from a phase 1 clinical trial of a fully human monoclonal ADC directed against PSMA for the treatment of prostate cancer and recently presented data from preclinical studies of novel multiplex phosphoinositide 3-kinase (PI3K) inhibitors for the treatment of cancer.
Results of Operations (amounts in thousands unless otherwise noted)
Revenues:
Our sources of revenue during the years ended December 31, 2011, 2010 and 2009, included our License Agreement with Salix, Transition Agreement with Wyeth, our License Agreement with Ono, our research grants from the NIH and, to a small extent, our sale of research reagents.
|
Sources of Revenue
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent Change
|
||||||||||
|
Collaboration revenue
|
$ 76,764
|
$ 1,413
|
$ 44,351
|
5,333%
|
(97%)
|
|||||
|
Royalty income
|
3,046
|
1,826
|
2,372
|
67%
|
(23%)
|
|||||
|
Research grants
|
4,810
|
4,573
|
1,968
|
5%
|
132%
|
|||||
|
Other revenues
|
176
|
140
|
256
|
26%
|
(45%)
|
|||||
|
$ 84,796
|
$ 7,952
|
$ 48,947
|
966%
|
(84%)
|
||||||
Collaboration revenue:
Salix Collaboration. During the year ended December 31, 2011, we recognized $75,091 of revenue from Salix, which includes $59,634 from the $60,000 upfront cash payment under the License Agreement, $225 in respect of Salix ex-U.S. sublicensee revenue and $15,232 as reimbursement of our expenses, including $2,172 of manufacturing supplies, in accordance with the License Agreement. As of December 31, 2011, $204 and $162 are recorded in deferred revenue – current and long-term, respectively.
Wyeth Collaboration. During the years ended December 31, 2011 and 2010, we recognized $1,630 and $1,383, respectively, of revenue from Wyeth, as reimbursement of our expenses under the 2009 Transition Agreement.
During the years ended December 31, 2010 and 2009, we recognized $1,383 and $29,298, respectively, of revenue from Wyeth, consisting of (i) $0 and $14,562, respectively, of the $60,000 upfront payment we received upon entering into our 2005 collaboration, (ii) $0 and $4,736, respectively, as reimbursement of our development expenses, and (iii) $1,383 and $10,000, respectively, as reimbursement of our expenses under the 2009 Transition Agreement.
Ono Collaboration. During the years ended December 31, 2011 and 2010, we recognized $43 and $30, respectively, of reimbursement revenue for activities requested by Ono.
During the years ended December 31, 2010 and 2009, we recognized $30 and $53, respectively, of reimbursement revenue for activities requested by Ono and in 2009 recognized the $15,000 upfront payment as revenue, due to satisfying our performance obligations.
Royalty income. We began earning royalties from net sales by Wyeth of subcutaneous RELISTOR in June 2008. Under our 2009 Transition Agreement, Wyeth continued to distribute RELISTOR in the U.S. until April 1, 2011, in Europe until October 1, 2011, and in Australia until December 1, 2011, at which times Salix assumed those responsibilities. Royalties due to us during 2011 are attributable only to Salix net sales in those territories from the date in which Salix assumed responsibility, the basis for 2011
royalty income. From April 1, 2011 to December 31, 2011, we earned royalty income of $3,046 based on net sales of RELISTOR reported by Salix or its sublicensees.
During the years ended December 31, 2010 and 2009, royalties of $1,826 and $1,853, respectively, were owed to us based on the net sales of RELISTOR reported by Wyeth and we recognized royalty revenue of $1,826 and $2,372, respectively. During the fourth quarter of 2010, no royalties were payable to us.
| RELISTOR Net Sales Reported by Collaborators | ||||||
|
Years Ended December 31,
|
||||||
|
2011
|
2010
|
2009
|
||||
|
U.S.
|
$ 21,500
|
$ 9,500
|
$ 7,100
|
|||
|
Ex-U.S.
|
5,500
|
6,600
|
5,200
|
|||
|
Global
|
$ 27,000
|
$ 16,100
|
$ 12,300
|
|||
Research grants. During the years ended December 31, 2011 and 2010, we recognized $4,810 and $4,573, respectively, as revenue from federal government grants by the NIH to partially offset costs related to our research and development programs. The increase in grant revenue resulted from new grant awards and higher reimbursable expenses in 2011 than in 2010.
During the years ended December 31, 2010 and 2009, we recognized $4,573 and $1,968, respectively, as revenue from federal government grants primarily by the NIH to partially offset costs related to our research and development programs. The increase in grant revenue resulted from new grants awarded in June 2009 and September 2010, and the $733 received as part of the U.S. government’s qualifying therapeutic discovery project.
We expect revenue from the NIH to decline in the future to the extent we out-license existing NIH-funded programs not in the oncology area.
Other revenues, primarily from orders for research reagents, increased to $176 for the year ended December 31, 2011 from $140 for the year ended December 31, 2010 and decreased from $256 for the year ended December 31, 2009.
Expenses:
Research and Development Expenses include scientific labor, clinical trial costs, supplies, product manufacturing costs, consulting, license fees, royalty payments and other operating expenses. Research and development expenses increased to $54,166 for the year ended December 31, 2011 from $52,151 for the year ended December 31, 2010, and from $51,093 for the year ended December 31, 2009. During 2011, the increase in research and development expenses over those in 2010 was primarily due to higher (i) clinical trial costs from activities related to oral methylnaltrexone phase 3 study and regulatory filing fees for the submission of the sNDA for subcutaneous
RELISTOR and (ii) purchases of manufacturing supplies on behalf of Salix, partially offset by a decrease in consulting expenses for RELISTOR and lower compensation expenses. Compensation expenses decreased in 2011 compared to 2010 primarily from lower share-based compensation partially offset by higher salaries and benefits. See Liquidity and Capital Resources – Uses of Cash, for details of the changes in these expenses by project. Primarily in 2011 and 2009, Salix and Wyeth reimbursed us for development expenses we incurred related to RELISTOR. Portions of our expenses related to our HIV, HCV and PSMA programs are funded through grants from the NIH (see Revenues- Research Grants). The changes in research and development expense, by category of expense, are as
follows:
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Salaries and benefits
|
$18,658
|
$18,469
|
$21,576
|
1%
|
(14%)
|
||||
2011 vs. 2010 Salaries and benefits increased due to accrued severance expenses related to headcount reduction and higher accrued bonus expense, partially offset by a decrease in salary expenses due to a decline in average headcount to 104 from 138 for the years ended December 31, 2011 and 2010, respectively, in the research and development departments.
2010 vs. 2009 Salaries and benefits decreased due to a decline in average headcount to 138 from 175 for the years ended December 31, 2010 and 2009, respectively, in the research and development departments.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
||||||
|
Percent change
|
||||||||||
|
Share-based compensation
|
$4,499
|
$5,091
|
$7,225
|
(12%)
|
(30%)
|
|||||
2011 vs. 2010 Share-based compensation decreased for the year ended December 31, 2011 compared to the year ended December 31, 2010 due to lower restricted stock and employee stock purchase plan expenses, partially offset by an increase in stock option plan expenses.
2010 vs. 2009 Share-based compensation decreased for the year ended December 31, 2010 compared to the year ended December 31, 2009 due to lower stock option plan, restricted stock and employee stock purchase plan expenses.
For the year ended December 31, 2011, share-based compensation included restricted stock and option plan expenses from (i) accelerated vesting of outstanding awards to non-management employees in connection with a change in program eligibility and termination of the Company’s employee stock purchase plans (which we expect to result in a decline in future share-based compensation), and (ii) a shift in headcount from general and administrative departments to research and development. See Critical Accounting Policies − Share-Based Payment Arrangements.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Clinical trial costs
|
$10,099
|
$7,056
|
$2,198
|
43%
|
221%
|
||||
2011 vs. 2010 Clinical trial costs increased primarily due to higher expenses for (i) RELISTOR ($3,385), from increased clinical trial expenses including activities related to oral methylnaltrexone phase 3 study and regulatory filing fees for the submission of the sNDA for subcutaneous RELISTOR, partially offset by decreases in expenses for Cancer ($218) and HIV ($122), all for the year ended December 31, 2011 compared to the year ended December 31, 2010.
2010 vs. 2009 Clinical trial costs increased primarily due to higher expenses for (i) RELISTOR ($5,224), from increased clinical trial activities for oral methylnaltrexone phase 3 study and (ii) Cancer ($352), partially offset by a decrease in expenses for HIV ($718), due to a decline in PRO 140 clinical trial activities, all for the year ended December 31, 2010 compared to the year ended December 31, 2009.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Laboratory and manufacturing supplies
|
$4,203
|
$2,388
|
$3,011
|
76%
|
(21%)
|
||||
2011 vs. 2010 Laboratory and manufacturing supplies increased due to higher expenses for (i) RELISTOR ($1,562), primarily due to purchases of manufacturing supplies on behalf of Salix, (ii) Cancer ($166), resulting from increase in expenses for PSMA ADC, and (iii) Other projects ($199), partially offset by a decrease in HIV ($112), all for the year ended December 31, 2011 compared to the year ended December 31, 2010.
2010 vs. 2009 Laboratory and manufacturing supplies decreased due to lower expenses for (i) Cancer ($485), due to reduced expenses for PSMA ADC, (ii) HIV ($136), resulting from a decline in the purchases of manufacturing supplies and (iii) Other ($631), partially offset by an increase in RELISTOR ($629), due to higher expenses for the multi-dose pen, all for the year ended December 31, 2010 compared to the year ended December 31, 2009.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Contract manufacturing and subcontractors
|
$6,713
|
$6,853
|
$8,040
|
(2%)
|
(15%)
|
||||
2011 vs. 2010 Contract manufacturing and subcontractors decreased due to lower expenses for (i) Cancer ($288), resulting from a decline in manufacturing expenses for PSMA ADC, (ii) RELISTOR ($2), due to lower contract manufacturing expenses for the multi-dose pen, and (iii) Other projects ($72), partially offset by increases in HIV ($222), for HIV Vaccine, all for the year ended December 31, 2011 compared to the year ended December 31, 2010.
2010 vs. 2009 Contract manufacturing and subcontractors decreased due to lower (i) Cancer expenses ($2,287), resulting from a decline in manufacturing expenses for PSMA ADC, (ii) Other ($962) and (iii) HIV expenses ($362), resulting from a decline in manufacturing expenses for PRO 140, partially offset by an increase in RELISTOR expenses ($2,424), due to higher contract manufacturing expenses for the multi-dose pen, all for the year ended December 31, 2010 compared to the year ended December 31, 2009.
These expenses are related to the conduct of clinical trials, including manufacture by third parties of drug materials, testing, analysis, formulation and toxicology services, and vary as the timing and level of such services are required.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Consultants
|
$1,270
|
$3,310
|
$1,007
|
(62%)
|
229%
|
||||
2011 vs. 2010 Consultants expenses decreased due to lower expenses for RELISTOR ($2,082), HIV ($41) and Other projects ($1), partially offset by an increase in Cancer ($84), all for the year ended December 31, 2011 compared to the year ended December 31, 2010.
2010 vs. 2009 Consultants expenses increased due to higher expenses for RELISTOR ($2,478) and Cancer ($41), partially offset by decreases in consultants expenses for HIV ($160) and Other projects ($56), all for the year ended December 31, 2010 compared to the year ended December 31, 2009.
These expenses are related to the monitoring of clinical trials as well as the analysis of data from completed clinical trials and vary as the timing and level of such services are required.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
License fees
|
$578
|
$1,270
|
$1,058
|
(54%)
|
20%
|
||||
2011 vs. 2010 License fees decreased primarily due to lower expenses for HIV ($664) and RELISTOR ($28), all for the year ended December 31, 2011 compared to the year ended December 31, 2010.
2010 vs. 2009 License fees increased primarily due to higher expenses for HIV ($428), partially offset by lower expenses for Cancer ($149) and RELISTOR ($67), all for the year ended December 31, 2010 compared to the year ended December 31, 2009.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Royalty expense
|
$405
|
$241
|
$237
|
68%
|
2%
|
||||
2011 vs. 2010 We recognized $405 and $241, respectively, of royalty expenses during the years ended December 31, 2011 compared to the same period in 2010, due to increased net sales of RELISTOR in 2011.
2010 vs. 2009 We incurred $241 and $185, respectively, of royalty costs and recognized $241 and $237, respectively, of royalty expenses during the years ended December 31, 2010 and 2009.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Other operating expenses
|
$7,741
|
$7,473
|
$6,741
|
4%
|
11%
|
||||
2011 vs. 2010 Other operating expenses increased for the year ended December 31, 2011 compared to the year ended December 31, 2010, primarily due to higher expenses for rent ($237), insurance ($89) and travel ($20), partially offset by a decrease in facilities ($78).
2010 vs. 2009 Other operating expenses increased for the year ended December 31, 2010 compared to the year ended December 31, 2009, primarily due to higher expenses for rent ($1,015) and insurance ($6), partially offset by a decrease in facilities ($193), travel ($5) and other operating expenses ($91).
General and Administrative Expenses decreased to $18,248 for the year ended December 31, 2011 from $22,832 for the year ended December 31, 2010 and from $25,106 for the year ended December 31, 2009, as follows:
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Salaries and benefits
|
$7,228
|
$8,086
|
$8,257
|
(11%)
|
(2%)
|
||||
2011 vs. 2010 Salaries and benefits decreased for the year ended December 31, 2011 compared to the same period in 2010, due to a decline in average headcount to 32 from 39, in the general and administrative departments, and lower accrued bonus expense, partially offset by accrued severance expenses related to headcount reduction.
2010 vs. 2009 Salaries and benefits decreased for the year ended December 31, 2010 compared to the same period in 2009, due to a decline in average headcount to 39 from 49, in the general and administrative departments, partially offset by higher bonus expense.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Share-based compensation
|
$1,863
|
$4,424
|
$5,761
|
(58%)
|
(23%)
|
||||
2011 vs. 2010 Share-based compensation decreased due to lower restricted stock, stock option and employee stock purchase plans expenses for the year ended December 31, 2011 compared to the year ended December 31, 2010.
2010 vs. 2009 Share-based compensation decreased due to lower restricted stock, stock option and employee stock purchase plans expenses for the year ended December 31, 2010 compared to the year ended December 31, 2009.
For the year ended December 31, 2011, share-based compensation included restricted stock and option plan expenses from (i) accelerated vesting of outstanding awards to non-management employees in connection with a change in program eligibility and termination of the Company’s employee stock purchase plans (which we expect to result in a decline in future share-based compensation), and (ii) a shift in headcount from general and administrative departments to research and development. See Critical Accounting Policies − Share-Based Payment Arrangements.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Consulting and professional fees
|
$4,389
|
$5,843
|
$6,696
|
(25%)
|
(13%)
|
||||
2011 vs. 2010 Consulting and professional fees decreased due to lower patent ($1,203), legal ($497) and other fees ($13), which were partially offset by an increase in consulting ($137), tax accounting ($63) and audit fees ($59), all for the year ended December 31, 2011 compared to the year ended December 31, 2010.
2010 vs. 2009 Consulting and professional fees decreased due to lower patent ($785), audit and compliance ($176), legal ($33) and other fees ($21), which were partially offset by an increase in consulting ($147) and public relations fees ($15), all for the year ended December 31, 2010 compared to the year ended December 31, 2009.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Other operating expenses
|
$4,768
|
$4,479
|
$4,392
|
6%
|
2%
|
||||
2011 vs. 2010 Other operating expenses increased due to higher expenses for rent ($82), taxes ($59), computer software ($87) and other operating expenses ($122), partially offset by decreases in recruiting ($51) and travel ($10), all for the year ended December 31, 2011 compared to the year ended December 31, 2010.
2010 vs. 2009 Other operating expenses increased due to higher expenses for rent ($341) and recruiting ($106), partially offset by a decrease in investor relations ($79), taxes ($35), conferences and seminars ($27), travel ($23) and other operating expenses ($196), all for the year ended December 31, 2010 compared to the year ended December 31, 2009.
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Depreciation and amortization
|
$2,066
|
$2,853
|
$5,078
|
(28%)
|
(44%)
|
||||
2011 vs. 2010 Depreciation and amortization expense decreased to $2,066 for the year ended December 31, 2011 from $2,853 for the year ended December 31, 2010, primarily due to lower leasehold improvement amortization expenses.
2010 vs. 2009 Depreciation and amortization expense decreased to $2,853 for the year ended December 31, 2010 from $5,078 for the year ended December 31, 2009, primarily due to lower capital expenditures in 2009.
Other income:
|
2011
|
2010
|
2009
|
2011 vs. 2010
|
2010 vs. 2009
|
|||||
|
Percent change
|
|||||||||
|
Interest income
|
$65
|
$64
|
$1,481
|
2%
|
(96%)
|
||||
2011 vs. 2010 Interest income increased to $65 for the year ended December 31, 2011 from $64 for the year ended December 31, 2010. For the years ended December 31, 2011 and 2010, investment income remained unchanged at $65, while amortization of premiums, net of discounts, was $0 and ($1) for years ended December 31, 2011 and 2010, respectively.
2010 vs. 2009 Interest income decreased to $64 for the year ended December 31, 2010 from $1,481 for the year ended December 31, 2009. For the years ended December 31, 2010 and 2009, investment income decreased to $65 from $2,075, respectively, due to lower interest rates for cash equivalents, lower average balance of cash equivalents and marketable securities in 2010 than in 2009. Amortization of premiums, net of discounts, was ($1) and ($594) for years ended December 31, 2010 and 2009, respectively.
Interest income, as reported, is primarily the result of investment income from our marketable and auction rate securities, decreased by the amortization of premiums we paid or increased by the amortization of discounts we received for those securities. Other income also includes $237 of gains from the sale of marketable securities in 2009.
Income Taxes:
For the year ended December 31, 2011 we recognized $10,381 in pre-tax income primarily as a result of the $60,000 Salix upfront cash payment. Our taxable income for the year is expected to be offset fully with net operating loss carry-forwards. For the years ended December 31, 2010 and 2009, we had losses both for book and tax purposes. We received a federal tax refund of $95 in 2010 from new legislation permitting the carryback of NOLs to 2005 as well as permitting the suspension of limitations on alternative minimum tax NOL utilization.
Net Income (Loss):
Our net income was $10,381 for the year ended December 31, 2011, and our net losses were $69,725 for the year ended December 31, 2010 and $30,612 for the year ended December 31, 2009.
Liquidity and Capital Resources
We have to date funded operations principally through payments received from private placements of equity securities, public offerings of common stock, collaborations, grants and contracts, royalties, interest on investments, proceeds from the exercise of outstanding options and warrants, and through September 30, 2011, sales of our common stock under our two employee stock purchase plans (Purchase Plans) which were terminated during 2011.
Under the Salix License Agreement, we received through December 31, 2011, a $60,000 upfront cash payment and $225 in respect of Salix ex-U.S. sublicensee revenue and are eligible to receive development and commercialization milestone payments plus royalties on net sales and 60% of any upfront, milestone, reimbursement or other revenue (net of costs of goods sold, as defined, and territory-specific research and development expense reimbursement) Salix receives from ex-U.S. sublicensees.
Our expenses and reimbursement revenue related to RELISTOR have declined substantially in the second half of 2011, since Salix assumed direct responsibility for expenses under third-party contracts we have assigned to it. Under the Salix License Agreement, we are reimbursed for Salix approved full-time equivalents (FTE) and third-party development expenses incurred and paid by us after February 3, 2011. For the year ended December 31, 2011, we incurred $23,197 of RELISTOR related expenses for which we have received reimbursements from Salix and Wyeth totaling $14,659 and $1,630, respectively, through December 31, 2011, and in respect of which we expect to receive $58 during the first quarter of 2012.
At December 31, 2011, we held $70,105 in cash and cash equivalents, an increase of $22,187 from $47,918 at December 31, 2010. We expect that this amount will be sufficient to fund operations as currently anticipated beyond one year. In addition, at December 31, 2011 and 2010, our investment in auction rate securities classified as long-term assets on the Consolidated Balance Sheets amounted to $3,332 and $3,608, respectively.
We may require additional funding in the future, and if we are unable to enter into favorable collaboration, license, asset sale, capital raising or other financing transactions, we will have to reduce, delay or eliminate spending on some current operations, and/or reduce salary and other overhead expenses, to extend our remaining operations.
Our cash flow from operating activities was positive for the year ended December 31, 2011, due to the receipt in 2011 of a $60,000 Salix upfront payment, $225 in respect of Salix ex-U.S. sublicensee revenue and $16,289 in reimbursement payments from Salix and Wyeth, partially offset by expenditures on our research and development programs and general and administrative costs. Our cash flow from operating activities was negative for the years ended December 31, 2010 and 2009 due primarily to the excess of expenditures on our research and development programs and general and administrative costs over cash received from collaborators and government grants to fund such programs, as described below. See
Risk Factors.
During the third quarter of 2011, we put in place a shelf registration statement with the SEC which may be used for the issuance of up to $100.0 million of common stock, preferred stock, debt securities, warrants, other rights and units. We do not have any current plans for issuing securities under this registration statement, which may be used up to three years from its effective date.
Sources of Cash
Operating Activities. During the year ended December 31, 2011 we received $79,998 under our collaborations, consisting of (i) $60,000 Salix upfront cash payment and $225 in respect of Salix ex-U.S. sublicensee revenue (ii) $14,659 in reimbursement payments under the Salix License Agreement, (iii) $1,767 in royalties from Salix, (iv) $3,317 under the Transition Agreement with Wyeth and (v) $30 under the License Agreement with Ono. During the years ended December 31, 2010 and 2009, we received $10,351 and $6,385, respectively, from Wyeth, consisting of (i) $0 and $3,172 as reimbursements payments under the 2005 Wyeth collaboration, (ii) $7,906
and $1,666 under the Transition Agreement, (iii) $2,415 and $1,494 in royalties and (iv) $30 and $53, respectively under the License Agreement with Ono. Reimbursements under the 2005 collaboration have ceased as a result of its termination.
Under our License Agreement with Ono, we are entitled to receive potential milestone payments, upon achievement of development milestones by Ono, of up to $20,000, commercial milestones and royalties on sales of subcutaneous RELISTOR in Japan. Ono is also responsible for development and commercialization costs for subcutaneous RELISTOR in Japan.
We have partially funded research programs through awards from the NIH. For the years ended December 31, 2011, 2010 and 2009, we received $5,178, $4,315 and $2,865, respectively, of revenue from all of our NIH awards including the $733 received as part of the federal government’s qualifying therapeutic discovery project. We expect funding from the NIH to decline in the future to the extent we out-license existing NIH-funded programs not in the oncology area.
Changes in Accounts receivable and Accounts payable for the years ended December 31, 2011, 2010 and 2009 resulted from the timing of receipts from the NIH, Salix, Wyeth and Ono, and payments made to trade vendors in the normal course of business.
Other than amounts to be received from Salix and Ono, we have no committed external sources of capital. Other than revenues from RELISTOR, we expect no significant product revenues for a number of years, as it will take at least that much time, if ever, to bring our product candidates to the commercial marketing stage.
Investing Activities. We redeem money market funds and use proceeds from maturities to provide funding for operations. Of $70,105 in cash and cash equivalents, $64,068 are guaranteed by the U.S. Treasury or Federal Deposit Insurance Corporation’s guarantee program. Our auction rate securities of $3,332 include $2,392 of securities collateralized by student loan obligations subsidized by the U.S. government. These investments, while rated investment grade by the Standard & Poor’s and Moody’s rating agencies and predominantly having scheduled maturities greater than ten years, are heavily concentrated in the U.S.
financial sector.
We have received all scheduled interest payments on the auction rate securities we hold at December 31, 2011. We will not realize cash in respect of the principal amount of these securities until the issuer calls or restructures the underlying security, the underlying security matures and is paid, or a buyer outside the auction process emerges.
We monitor markets for our investments, but cannot guarantee that additional losses will not be required to be recorded. Valuation of securities is subject to uncertainties that are difficult to predict, such as changes to credit ratings of the securities and/or the underlying assets supporting them, default rates applicable to the underlying assets, underlying collateral value, discount rates, counterparty risk, ongoing strength and quality of market credit and liquidity and general economic and market conditions. We do not believe the carrying values of our investments are other than temporarily impaired and therefore expect the positions will eventually be liquidated without significant loss.
Our money market funds are purchased and, in the case of auction rate securities, sold by third-party brokers in accordance with our investment policy guidelines. Our brokerage account requires that all securities be held to maturity unless authorization is obtained from us to sell earlier. In fact, we had a history of holding all securities to maturity prior to the second quarter of 2009, when we decided to sell a portion of our securities which had scheduled maturities between the fourth quarter of 2009 and the third quarter of 2010. The proceeds from these sales were $24.8 million, resulting in a gain of $0.2 million.
We expect to recover the amortized cost of all of our investments at maturity. Because we do not anticipate having to sell these securities in order to operate our business and believe it is not more likely than not that we will be required to sell these securities before recovery of principal, we do not consider these securities to be other than temporarily impaired at December 31, 2011.
Financing Activities. During the years ended December 31, 2011, 2010 and 2009, we received cash of $3,726, $3,896 and $4,874, respectively, from the exercise of stock options and from the sale of our common stock under our Purchase Plans. The amount of cash we receive from these sources fluctuates commensurate with headcount levels and changes in the price of our common stock on the grant date for options exercised, and on the sale date for shares sold under the Purchase Plans.
Unless we obtain regulatory approval from the FDA for additional product candidates and/or enter into agreements with corporate collaborators with respect to our additional technologies, we will be required to fund our operations in the future through sales of common stock or other securities, royalty or other financing agreements and/or grants and government contracts. Adequate additional funding may not be available to us on acceptable terms or at all. Our inability to raise additional capital on terms reasonably acceptable to us may seriously jeopardize the future success of our business.
Uses of Cash
Operating Activities. The majority of our cash has been used to advance our research and development programs. Our total expenses for research and development from inception through December 31, 2011 have been approximately $636.0 million. For various reasons, including the early stage of certain of our programs, the timing and results of our clinical trials, our dependence in certain instances on third parties, many of which are outside of our control, we cannot estimate the total remaining costs to be incurred and timing to complete all our research and development programs.
For the years ended December 31, 2011, 2010 and 2009, research and development costs incurred, by project, were as follows:
|
2011
|
2010
|
2009
|
||||||||||
|
RELISTOR
|
$ | 23,197 | $ | 23,325 | $ | 7,759 | ||||||
|
Cancer
|
18,168 | 14,690 | 20,127 | |||||||||
|
HIV
|
3,772 | 5,447 | 11,787 | |||||||||
|
Other programs
|
9,029 | 8,689 | 11,420 | |||||||||
|
Total
|
$ | 54,166 | $ | 52,151 | $ | 51,093 | ||||||
We may require additional funding to continue our research and product development programs, conduct pre-clinical studies and clinical trials, fund operating expenses, pursue regulatory approvals for our product candidates, file and prosecute patent applications and enforce or defend patent claims, if any, and fund product in-licensing and any possible acquisitions.
Investing Activities. During the years ended December 31, 2011, 2010 and 2009, we have spent $226, $2,171 and $901, respectively, on capital expenditures. These expenditures have been primarily related to leasehold improvements and the purchase of laboratory equipment for our research and development projects.
Contractual Obligations
Our funding requirements, both for the next 12 months and beyond, will include required payments under operating leases and fixed and contingent payments under our licensing and collaboration agreements. The following table summarizes our contractual obligations as of December 31, 2011 for future payments under these agreements:
|
Payments due by Year-end
|
||||||||||||||||||||
|
Total
|
2012
|
2013-2014 | 2015-2016 |
Thereafter
|
||||||||||||||||
|
(in millions)
|
||||||||||||||||||||
|
Operating leases
|
$ | 23.1 | $ | 2.5 | $ | 4.8 | $ | 5.0 | $ | 10.8 | ||||||||||
|
License and collaboration agreements:
|
||||||||||||||||||||
|
- Fixed payments
|
2.4 | 0.2 | 0.5 | 0.6 | 1.1 | |||||||||||||||
|
- Contingent payments (1)
|
85.4 | 2.4 | 2.3 | - | 80.7 | |||||||||||||||
|
Total
|
$ | 110.9 | $ | 5.1 | $ | 7.6 | $ | 5.6 | $ | 92.6 | ||||||||||
_______________
|
(1)
|
Based on assumed achievement of milestones covered under each agreement, the timing and payment of which is highly uncertain.
|
We periodically assess the scientific progress and merits of each of our programs to determine if continued research and development is commercially and economically viable. Certain of our programs have been terminated due to the lack of scientific progress and prospects for ultimate commercialization. Because of the uncertainties associated with research and development in these programs, the duration and completion costs of our research and development projects are difficult to estimate and are subject to considerable variation. Our inability to complete research and development projects in a timely manner or failure to enter into collaborative agreements could significantly increase capital requirements and
adversely affect our liquidity.
Our cash requirements may vary materially from those now planned because of results of research and development and product testing, changes in existing relationships or new relationships with licensees, licensors or other collaborators, changes in the focus and direction of our research and development programs, competitive and technological advances, the cost of filing, prosecuting, defending and enforcing patent claims, the regulatory approval process, manufacturing and marketing and other costs associated with the commercialization of products following receipt of regulatory approvals and other factors.
The above discussion contains forward-looking statements based on our current operating plan and the assumptions on which it relies. There could be deviations from that plan that would consume our assets earlier than planned.
Off-Balance Sheet Arrangements and Guarantees
We have no obligations under off-balance sheet arrangements and do not guarantee the obligations of any other unconsolidated entity.
Critical Accounting Policies
We prepare our financial statements in conformity with accounting principles generally accepted in the United States of America. Our significant accounting policies are disclosed in Note 2 to our financial statements included in this Annual Report on Form 10-K for the year ended December 31, 2011. The selection and application of these accounting principles and methods requires us to make estimates and assumptions that affect the reported amounts of assets, liabilities, revenues and expenses, as well as certain financial statement disclosures. On an ongoing basis, we evaluate our estimates. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the
circumstances. The results of our evaluation form the basis for making judgments about the carrying values of assets and liabilities that are not otherwise readily apparent. While we believe that the estimates and assumptions we use in preparing the financial statements are appropriate, these estimates and assumptions are subject to a number of factors and uncertainties regarding their ultimate outcome and, therefore, actual results could differ from these estimates.
We have identified our critical accounting policies and estimates below. These are policies and estimates that we believe are the most important in portraying our financial condition and results of operations, and that require our most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. We have discussed the development, selection and disclosure of these critical accounting policies and estimates with the Audit Committee of our Board of Directors.
Revenue Recognition. We recognize revenue from all sources based on the provisions of the SEC’s Staff Accounting Bulletin (SAB) No. 104 (SAB 104) and ASC 605 Revenue Recognition.
In October 2009, the FASB updated ASC 605 Revenue Recognition by specifying how to separate deliverables in multiple-deliverable arrangements, and how to measure and allocate arrangement consideration to one or more units of accounting. Under ASC 605, the delivered item(s) are separate units of accounting, provided (i) the delivered item(s) have value to a collaborator on a stand-alone basis, and (ii) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item(s) is considered probable and substantially in our control. We adopted this update on January 1, 2011.
Royalty revenue is recognized based upon net sales of related licensed products. Royalty revenue is recognized in the period the sales occur, provided that the royalty amounts are fixed or determinable, collection of the related receivable is reasonably assured and we have no remaining performance obligations under the arrangement providing for the royalty.
Amounts not expected to be recognized within one year of the balance sheet date are classified as long-term deferred revenue. The estimate of the classification of deferred revenue as short-term or long-term is based upon the periods in which we expect to perform joint committee services.
Share-Based Payment Arrangements. Our share-based compensation to employees includes non-qualified stock options, restricted stock and shares issued under our Purchase Plans, which are compensatory under ASC 718 Compensation – Stock Compensation. We account for share-based compensation to non-employees, including non-qualified stock options and restricted stock, in accordance with ASC 505 Equity.
The fair value of each non-qualified stock option award is estimated on the date of grant using the Black-Scholes option pricing model. The model requires input assumptions with respect to (i) expected volatility of our common stock, which is based upon the daily quoted market prices on The NASDAQ Stock Market LLC over a period equal to the expected term, (ii) the period of time over which employees, officers, directors and non-employee consultants are expected to hold their options prior to exercise, (iii) zero expected dividend yield due to never having paid dividends and not expecting to pay dividends in the future, and (iv) risk-free interest rates for periods within the expected term of the options, which are
based on the U.S. Treasury yield curve in effect at the time of grant.
Historical volatilities are based upon daily quoted market prices of our common stock on The NASDAQ Stock Market LLC over a period equal to the expected term of the related equity instruments. We rely only on historical volatility since it provides the most reliable indication of future volatility. Future volatility is expected to be consistent with historical; historical volatility is calculated using a simple average calculation; historical data is available for the length of the option’s expected term and a sufficient number of price observations are used consistently. Since our stock options are not traded on a public market, we do not use implied volatility.
The expected term of options granted represents the period of time that options granted are expected to be outstanding based upon historical data related to exercise and post-termination cancellation activity. The expected term of stock options granted to our Chief Executive Officer (CEO), Chief Science Officer (CSO) and non-employee directors and consultants are calculated separately from stock options granted to employees and other officers.
We apply a forfeiture rate to the number of unvested awards in each reporting period in order to estimate the number of awards that are expected to vest. Estimated forfeiture rates are based upon historical data on vesting behavior of employees. We adjust the total amount of compensation cost recognized for each award, in the period in which each award vests, to reflect the actual forfeitures related to that award. Changes in our estimated forfeiture rate will result in changes in the rate at which compensation cost for an award is recognized over its vesting period.
Changes in the assumptions used to compute the fair value of the option awards are likely to affect the fair value of the non-qualified stock option awards and the amount of compensation expense recognized in future periods. A higher volatility, longer expected term and higher risk-free rate increases the resulting compensation expense recognized in future periods as compared to prior periods. Conversely, a lower volatility, shorter expected term and lower risk-free rate decreases the resulting compensation expense recognized in future periods as compared to prior periods.
For performance-based stock option awards vesting of a defined portion of each award will occur earlier if a defined performance condition is achieved; more than one condition may be achieved in any period. We estimate the probability of achievement of each performance condition and use those probabilities to determine the requisite service period of each award. The requisite service period for the award is the shortest of the explicit or implied service periods. In the case of the CSO's options, the explicit service period is nine years and 11 months from the respective grant dates. The implied service periods related to the performance conditions are the estimated times for each performance condition to be
achieved. Thus, compensation expense will be recognized over the shortest estimated time for the achievement of performance conditions for that award (assuming that the performance conditions will be achieved before the cliff vesting occurs). For performance and market-based stock option awards to our CEO (consisting of options in 2010) and CSO (consisting of options in 2010 and 2009 and options and restricted stock in 2008), vesting occurs on the basis of the achievement of specified performance or market-based milestones. The options have an exercise price equal to the closing price on our common stock on the date of grant. The awards are valued using a Monte Carlo simulation model and the expense related to these grants will be recognized over the shortest estimated time for the achievement of the performance or market conditions. On July 1, 2011, we granted an option to our CEO
which vests on the basis of the achievement of specified performance-based milestones. The option has an exercise price equal to the closing price of our common stock on the date of grant. The award is valued using the Black-Scholes option pricing model and the expense related to this grant will be recognized during the period in which one of the performance milestones is achieved. The awards will not vest unless one of the performance milestones is achieved or the market condition is met. Changes in the estimate of probability of achievement of any performance or market condition will be reflected in compensation expense of the period of change and future periods affected by the change.
Research and Development Expenses Including Clinical Trial Expenses. Clinical trial expenses, which are included in research and development expenses, represent obligations resulting from our contracts with various clinical investigators and clinical research organizations in connection with conducting clinical trials for our product candidates. Such costs are expensed as incurred, and are based on the total number of patients in the trial, the rate at which the patients enter the trial and the period over which the clinical investigators and clinical research organizations to provide services. We believe that this method best approximates
the efforts expended on a clinical trial with the expenses we record. We adjust our rate of clinical expense recognition if actual results differ from our estimates. In addition to clinical trial expenses, we estimate the amounts of other research and development expenses, for which invoices have not been received at the end of a period, based upon communication with third parties that have provided services or goods during the period. Such estimates are subject to change as additional information becomes available.
Fair Value Measurements. Our available-for-sale investment portfolio consists of money market funds and auction rate securities, and is recorded at fair value in the accompanying Consolidated Balance Sheets in accordance with ASC 320 Investments – Debt and Equity Securities. The change in the fair value of these investments is recorded as a component of other comprehensive income (loss).
We continue to monitor markets for our investments and consider the impact, if any, of market conditions on the fair market value of our investments.
We expect to recover the amortized cost of all of our investments at maturity. Currently, we do not anticipate having to sell these securities in order to operate our business and we believe that it is not more likely than not that we will be required to sell these securities before recovery of principal. We do not believe the carrying values of our investments are other than temporarily impaired and therefore expect the positions will eventually be liquidated without significant loss.
Valuation of securities is subject to uncertainties that are difficult to predict, such as changes to credit ratings of the securities and/or the underlying assets supporting them, default rates applicable to the underlying assets, underlying collateral value, discount rates, counterparty risk, ongoing strength and quality of market credit and liquidity and general economic and market conditions. The valuation of the auction rate securities we hold is based on an internal analysis of timing of expected future successful auctions, collateralization of underlying assets of the security and credit quality of the security.
Impact of Recently Issued Accounting Standards
In June 2011, the FASB issued ASU No. 2011-05, which requires that comprehensive income and the related components be presented in a single continuous statement or in two separate but consecutive statements. The ASU is effective for fiscal years, and interim periods within those years, beginning after December 15, 2011, except for the deferral of the effective date related to the presentation of reclassification of items out of accumulated other comprehensive income under ASU No. 2011-12, which was issued in January 2012. We are currently evaluating the effect this ASU will have on our consolidated financial statements.
In May 2011, the FASB issued ASU No. 2011-04, which is the result of joint efforts by the FASB and International Accounting Standards Board to develop a single, converged fair value framework. The converged guidance specifies how to measure fair value and what disclosures to provide about fair value measurements. The ASU is effective for interim and annual periods beginning after December 15, 2011. We are currently evaluating the effect this ASU will have on our consolidated financial statements.
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Our primary investment objective is to preserve principal. Our available-for-sale investments consist of money market funds and auction rate securities, all of which had interest rates that were variable and totaled $67,400 at December 31, 2011. As a result, we do not believe that we have a material exposure to interest-rate risk.
At December 31, 2011, we continue to hold approximately $3,332 (4.9% of assets measured at fair value) of auction rate securities, in respect of which we have received all scheduled interest payments. The principal amount of these remaining auction rate securities will not be accessible until the issuer calls or restructures the underlying security, the underlying security matures and is paid or a buyer outside the auction process emerges.
We continue to monitor the market for auction rate securities and consider the impact, if any, of market conditions on the fair market value of our investments. We believe that the failed auctions experienced to date are not a result of the deterioration of the underlying credit quality of these securities, although valuation of them is subject to uncertainties that are difficult to predict, such as changes to credit ratings of the securities and/or the underlying assets supporting them, default rates applicable to the underlying assets, underlying collateral value, discount rates, counterparty risk, ongoing strength and quality of market credit and liquidity, and general economic and market conditions. We do not
believe the carrying values of these auction rate securities are other than temporarily impaired and therefore expect the positions will eventually be liquidated without significant loss.
The valuation of the auction rate securities we hold is based on an internal analysis of timing of expected future successful auctions, collateralization of underlying assets of the security and credit quality of the security. We re-evaluated the valuation of these securities as of December 31, 2011 and the temporary impairment amount decreased $24 from $292 at December 31, 2010 to $268. A 100 basis point increase to our internal analysis would result in a $36 increase in the temporary impairment of these securities as of the year ended December 31, 2011.
Item 8. Financial Statements and Supplementary Data
See page F-1, Index to Consolidated Financial Statements.
Item 9. Changes in and Disagreements With Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain disclosure controls and procedures that are designed to ensure that information required to be disclosed in our Exchange Act reports is recorded, processed, summarized and reported within the timelines specified in the SEC’s rules and forms, and that such information is accumulated and communicated to our management, including our CEO and Chief Financial Officer (CFO), as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating the disclosure controls and procedures, management recognized that any controls and procedures, no matter how well designed and operated, can only provide reasonable assurance of achieving the desired control objectives, and in
reaching a reasonable level of assurance, management necessarily was required to apply its judgment in evaluating the cost-benefit relationship of possible controls and procedures. We have a Disclosure Committee consisting of members of our senior management which monitors and implements our policy of disclosing material information concerning the Company in accordance with applicable law.
As required by SEC Rule 13a-15(e), we carried out an evaluation, under the supervision and with the participation of our management, including our CEO and our CFO, of the effectiveness of the design and operation of our disclosure controls and procedures as of the end of the period covered by this report. Based on the foregoing, our CEO and CFO concluded that our current disclosure controls and procedures, as designed and implemented, were effective at the reasonable assurance level.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting, as such term is defined in the Exchange Act Rules 13a-15(f) and 15d-15(f) during our fiscal quarter ended December 31, 2011 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Management’s Report on Internal Control Over Financial Reporting
Internal control over financial reporting is a process designed by, or under the supervision of, our principal executive and principal financial officers and effected by our Board, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. Our management is responsible for establishing and maintaining adequate internal control over financial reporting and it includes policies and procedures that:
· Pertain to the maintenance of records that in reasonable detail accurately and fairly reflect the transactions and dispositions of our assets;
· Provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorization of our management and directors; and
· Provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Management has used the framework set forth in the report entitled Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, known as COSO, to evaluate the effectiveness of our internal control over financial reporting. Management has concluded that our internal control over financial reporting was effective as of December 31, 2011. The effectiveness of our internal control over financial reporting as of December 31, 2011 has been audited by
PricewaterhouseCoopers LLP, an independent registered public accounting firm, as stated in their report which appears herein.
Item 9B. Other Information
None.
PART III
The information required by the Form 10-K Items listed in the following table will be included under the respective headings specified for such Items in our definitive proxy statement for our 2012 Annual Meeting of Stockholders to be filed with the SEC:
|
Item of Form 10-K
|
Location in 2012 Proxy Statement
|
|
Item 10. Directors, Executive Officers and Corporate Governance
|
Election of Directors.
Board and Committee Meetings.
Executive Officers of the Company.
Section 16(a) Beneficial Ownership Reporting and Compliance.
Code of Business Ethics and Conduct.*
*The full text of our code of business ethics and conduct is available on our website (http://www.progenics.com/documents.cfm).
|
|
Item 11. Executive Compensation
|
Executive Compensation.
Compensation Committee Report.
Compensation Committee Interlocks and Insider Participation.
|
|
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
Equity Compensation Plan Information.
Security Ownership of Certain Beneficial Owners and Management.
|
|
Item 13. Certain Relationships and Related Transactions, and Director Independence
|
Certain Relationships and Related Transactions.
Affirmative Determinations Regarding Director Independence and Other Matters.
|
|
Item 14. Principal Accounting Fees and Services
|
Fees Billed for Services Rendered by our Independent Registered Public Accounting Firm.
Pre-approval of Audit and Non-Audit Services by the Audit Committee.
|
PART IV
Item 15. Exhibits, Financial Statement Schedules
The following documents or the portions thereof indicated are filed as a part of this Report.
|
|
(a)
|
Documents filed as part of this Report:
|
|
|
Consolidated Financial Statements of Progenics Pharmaceuticals, Inc.:
|
Report of Independent Registered Public Accounting Firm
Consolidated Balance Sheets at December 31, 2011 and 2010
Consolidated Statements of Operations for the years ended December 31, 2011, 2010 and 2009
Consolidated Statements of Stockholders’ Equity and Comprehensive Income (Loss) for the years ended December 31, 2011, 2010 and 2009
Consolidated Statements of Cash Flows for the years ended December 31, 2011, 2010 and 2009
Notes to Consolidated Financial Statements
|
|
(b)
|
Financial Statement Schedules
|
All financial statement schedules referred to in Item 12-01 of Regulation S-X are inapplicable and therefore have been omitted.
|
|
(c)
|
Item 601 Exhibits
|
Those exhibits required to be filed by Item 601 of Regulation S-K are listed in the Exhibit Index immediately following the signature page hereof and preceding the exhibits filed herewith, and such listing is incorporated herein by reference.
PROGENICS PHARMACEUTICALS, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Page
|
|
|
|
F-2
|
|
Financial Statements:
|
|
|
|
F-3
|
|
|
F-4
|
|
Consolidated Statements of Stockholders’ Equity and Comprehensive Loss for the years ended December 31, 2011, 2010 and 2009
|
F-5
|
|
|
F-6
|
|
F-7
|
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders of
Progenics Pharmaceuticals, Inc.
In our opinion, the consolidated financial statements listed in the index appearing under Item 15(a) present fairly, in all material respects, the financial position of Progenics Pharmaceuticals, Inc. and its subsidiaries at December 31, 2011 and 2010, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2011 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control -
Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our integrated audits. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of
material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our
opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted
accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
New York, New York
March 15, 2012
CONSOLIDATED BALANCE SHEETS
(in thousands, except for par value and share amounts)
| December 31, | |||||||
| 2011 | 2010 | ||||||
|
Assets
|
|||||||
|
Current assets:
|
|||||||
|
Cash and cash equivalents
|
$
|
70,105
|
$
|
47,918
|
|||
|
Accounts receivable
|
1,516
|
2,283
|
|||||
|
Other current assets
|
919
|
1,801
|
|||||
|
Total current assets
|
72,540
|
52,002
|
|||||
|
Auction rate securities
|
3,332
|
3,608
|
|||||
|
Fixed assets, at cost, net of accumulated depreciation and amortization
|
4,038
|
5,878
|
|||||
|
Other assets
|
200
|
1,250
|
|||||
|
Total assets
|
$
|
80,110
|
$
|
62,738
|
|||
|
Liabilities and Stockholders’ Equity
|
|||||||
|
Current liabilities:
|
|||||||
|
Accounts payable and accrued expenses
|
$
|
6,331
|
$
|
9,683
|
|||
|
Deferred revenue - current
|
204
|
-
|
|||||
|
Other current liabilities
|
115
|
112
|
|||||
|
Total current liabilities
|
6,650
|
9,795
|
|||||
|
Deferred revenue – long term
|
162
|
-
|
|||||
|
Other liabilities
|
1,497
|
1,635
|
|||||
|
Total liabilities
|
8,309
|
11,430
|
|||||
|
Commitments and contingencies (Note 9)
|
|||||||
|
Stockholders’ equity:
|
|||||||
|
Preferred stock, $.001 par value; 20,000,000 shares authorized; issued and outstanding – none
|
-
|
-
|
|||||
|
Common stock, $.0013 par value; shares authorized – 80,000,000 in 2011 and 40,000,000 in 2010; issued – 34,046,409 in 2011 and 33,325,802 in 2010
|
44
|
43
|
|||||
|
Additional paid-in capital
|
463,440
|
453,353
|
|||||
|
Accumulated deficit
|
(388,674
|
)
|
(399,055
|
)
|
|||
|
Accumulated other comprehensive loss
|
(268
|
)
|
(292
|
)
|
|||
|
Treasury stock, at cost (200,000 shares in 2011 and 2010)
|
(2,741
|
)
|
(2,741
|
)
|
|||
|
Total stockholders’ equity
|
71,801
|
51,308
|
|||||
|
Total liabilities and stockholders’ equity
|
$
|
80,110
|
$
|
62,738
|
|||
The accompanying notes are an integral part of the financial statements.
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except for loss per share data)
|
Years Ended December 31,
|
||||||||||
|
2011
|
2010
|
2009
|
||||||||
|
Revenues:
|
||||||||||
|
Collaboration revenue
|
$
|
76,764
|
$
|
1,413
|
$
|
44,351
|
||||
|
Royalty income
|
3,046
|
1,826
|
2,372
|
|||||||
|
Research grants
|
4,810
|
4,573
|
1,968
|
|||||||
|
Other revenues
|
176
|
140
|
256
|
|||||||
|
Total revenues
|
84,796
|
7,952
|
48,947
|
|||||||
|
Expenses:
|
||||||||||
|
Research and development
|
53,183
|
50,640
|
49,798
|
|||||||
|
License fees – research and development
|
578
|
1,270
|
1,058
|
|||||||
|
General and administrative
|
18,248
|
22,832
|
25,106
|
|||||||
|
Royalty expense
|
405
|
241
|
237
|
|||||||
|
Depreciation and amortization
|
2,066
|
2,853
|
5,078
|
|||||||
|
Total expenses
|
74,480
|
77,836
|
81,277
|
|||||||
|
Operating income (loss)
|
10,316
|
(69,884
|
)
|
(32,330
|
)
|
|||||
|
Other income:
|
||||||||||
|
Interest income
|
65
|
64
|
1,481
|
|||||||
|
Gain on sale of marketable securities
|
-
|
-
|
237
|
|||||||
|
Total other income
|
65
|
64
|
1,718
|
|||||||
|
Net income (loss) before income taxes
|
10,381
|
(69,820
|
)
|
(30,612
|
)
|
|||||
|
Income tax benefit
|
-
|
95
|
-
|
|||||||
|
Net income (loss)
|
$
|
10,381
|
$
|
(69,725
|
)
|
$
|
(30,612
|
)
|
||
|
Net income (loss) per share - basic
|
$
|
0.31
|
$
|
(2.14
|
)
|
$
|
(0.98
|
)
|
||
|
Weighted-average shares - basic
|
33,375
|
32,590
|
31,219
|
|||||||
|
Net income (loss) per share - diluted
|
$
|
0.31
|
$
|
(2.14
|
)
|
$
|
(0.98
|
)
|
||
|
Weighted-average shares - diluted
|
33,494
|
32,590
|
31,219
|
|||||||
The accompanying notes are an integral part of the financial statements.
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY AND COMPREHENSIVE INCOME (LOSS)
For the Years Ended December 31, 2011, 2010 and 2009
(in thousands)
|
Common Stock
|
Additional
|
Accumulated
Other
|
Treasury Stock
|
|||||||||||||||||||||||||||||
|
Shares
|
Amount
|
Paid-In
Capital
|
Accumulated
Deficit
|
Comprehensive
Income (Loss)
|
Shares
|
Amount
|
Total
|
|||||||||||||||||||||||||
|
Balance at December 31, 2008
|
30,807 | $ | 40 | $ | 422,085 | $ | (298,718 | ) | $ | (1,297 | ) | (200 | ) | $ | (2,741 | ) | $ | 119,369 | ||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||||
|
Net loss
|
- | - | - | (30,612 | ) | - | - | - | (30,612 | ) | ||||||||||||||||||||||
|
Net unrealized gain on marketable and auction rate securities
|
- | - | - | - | 990 | - | - | 990 | ||||||||||||||||||||||||
|
Total comprehensive loss:
|
(29,622 | ) | ||||||||||||||||||||||||||||||
|
Compensation expenses for share-based payment arrangements
|
- | - | 12,986 | - | - | - | - | 12,986 | ||||||||||||||||||||||||
|
Issuance of restricted stock, net of forfeitures
|
266 | - | - | - | - | - | - | - | ||||||||||||||||||||||||
|
Sale of common stock under employee stock purchase plans and exercise of stock options
|
1,069 | 2 | 4,872 | - | - | - | - | 4,874 | ||||||||||||||||||||||||
|
Balance at December 31, 2009
|
32,142 | 42 | 439,943 | (329,330 | ) | (307 | ) | (200 | ) | (2,741 | ) | 107,607 | ||||||||||||||||||||
|
Comprehensive loss:
|
||||||||||||||||||||||||||||||||
|
Net loss
|
- | - | - | (69,725 | ) | - | - | - | (69,725 | ) | ||||||||||||||||||||||
|
Net unrealized gain on marketable and auction rate securities
|
- | - | - | - | 15 | - | - | 15 | ||||||||||||||||||||||||
|
Total comprehensive loss:
|
(69,710 | ) | ||||||||||||||||||||||||||||||
|
Compensation expenses for share-based payment arrangements
|
- | - | 9,515 | - | - | - | - | 9,515 | ||||||||||||||||||||||||
|
Issuance of restricted stock, net of forfeitures
|
173 | - | - | - | - | - | - | - | ||||||||||||||||||||||||
|
Sale of common stock under employee stock purchase plans and exercise of stock options
|
1,011 | 1 | 3,895 | - | - | - | - | 3,896 | ||||||||||||||||||||||||
|
Balance at December 31, 2010
|
33,326 | 43 | 453,353 | (399,055 | ) | (292 | ) | (200 | ) | (2,741 | ) | 51,308 | ||||||||||||||||||||
|
Comprehensive income:
|
||||||||||||||||||||||||||||||||
|
Net income
|
- | - | - | 10,381 | - | - | - | 10,381 | ||||||||||||||||||||||||
|
Net unrealized gain on auction rate securities
|
- | - | - | - | 24 | - | - | 24 | ||||||||||||||||||||||||
|
Total comprehensive income:
|
10,405 | |||||||||||||||||||||||||||||||
|
Compensation expenses for share-based payment arrangements
|
- | - | 6,362 | - | - | - | - | 6,362 | ||||||||||||||||||||||||
|
Forfeitures of restricted stock
|
(38 | ) | - | - | - | - | - | - | - | |||||||||||||||||||||||
|
Sale of common stock under employee stock purchase plans and exercise of stock options
|
758 | 1 | 3,725 | - | - | - | - | 3,726 | ||||||||||||||||||||||||
|
Balance at December 31, 2011
|
34,046 | $ | 44 | $ | 463,440 | $ | (388,674 | ) | $ | (268 | ) | (200 | ) | $ | (2,741 | ) | $ | 71,801 | ||||||||||||||
The accompanying notes are an integral part of the financial statements.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
|
Years Ended December 31,
|
|||||||||||
|
2011
|
2010
|
2009
|
|||||||||
|
Cash flows from operating activities:
|
|||||||||||
|
Net income (loss)
|
$
|
10,381
|
$
|
(69,725
|
)
|
$
|
(30,612
|
)
|
|||
|
Adjustments to reconcile net income (loss) to net cash provided by (used in) operating activities:
|
|||||||||||
|
Depreciation and amortization
|
2,066
|
2,853
|
5,078
|
||||||||
|
Write-off of fixed assets
|
-
|
-
|
334
|
||||||||
|
Amortization of discounts, net of premiums, on marketable securities
|
-
|
-
|
889
|
||||||||
|
Expenses for share-based compensation awards
|
6,362
|
9,515
|
12,986
|
||||||||
|
Gain on sale of marketable securities
|
-
|
-
|
(237
|
)
|
|||||||
|
Changes in assets and liabilities:
|
|||||||||||
|
Decrease (increase) in accounts receivable
|
767
|
5,239
|
(6,185
|
)
|
|||||||
|
Decrease (increase) in other current assets
|
882
|
(333
|
)
|
2,063
|
|||||||
|
Decrease (increase) in other assets
|
1,050
|
617
|
(1,667
|
)
|
|||||||
|
(Decrease) increase in accounts payable and accrued expenses
|
(3,352
|
)
|
3,847
|
(660
|
)
|
||||||
|
Increase (decrease) in deferred revenue - current
|
204
|
-
|
(31,645
|
)
|
|||||||
|
Increase (decrease) in other current liabilities
|
3
|
(58
|
)
|
113
|
|||||||
|
Increase in deferred revenue – long term
|
162
|
-
|
-
|
||||||||
|
(Decrease) increase in other liabilities
|
(138
|
)
|
1,635
|
(266
|
)
|
||||||
|
Net cash provided by (used in) operating activities
|
18,387
|
(46,410
|
)
|
(49,809
|
)
|
||||||
|
Cash flows from investing activities:
|
|||||||||||
|
Capital expenditures
|
(226
|
)
|
(2,171
|
)
|
(901
|
)
|
|||||
|
Sales/maturities of marketable and auction rate securities
|
300
|
1,700
|
80,233
|
||||||||
|
Decrease (increase) in restricted cash
|
-
|
-
|
320
|
||||||||
|
Net cash provided by (used in) investing activities
|
74
|
(471
|
)
|
79,652
|
|||||||
|
Cash flows from financing activities:
|
|||||||||||
|
Proceeds from the exercise of stock options and sale of common stock under the Employee Stock Purchase Plan
|
3,726
|
3,896
|
4,874
|
||||||||
|
Net cash provided by financing activities
|
3,726
|
3,896
|
4,874
|
||||||||
|
Net increase (decrease) in cash and cash equivalents
|
22,187
|
(42,985
|
)
|
34,717
|
|||||||
|
Cash and cash equivalents at beginning of period
|
47,918
|
90,903
|
56,186
|
||||||||
|
Cash and cash equivalents at end of period
|
$
|
70,105
|
$
|
47,918
|
$
|
90,903
|
|||||
The accompanying notes are an integral part of the financial statements.
F-6
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(amounts in thousands, except per share amounts or as otherwise noted)
1. Organization and Business
Progenics Pharmaceuticals, Inc. (“Progenics,” “we” or “us”) has been engaged in research and development for proprietary and in-licensed biotechnology product candidates in oncology, virology, supportive care and gastroenterology. In 2011, we licensed our first commercial product, RELISTOR® (methylnaltrexone bromide) subcutaneous injection, to Salix Pharmaceuticals, Inc., a leading gastrointestinal disease specialty company. Salix is marketing RELISTOR directly through its specialty sales force in the U.S. and sublicensing
the drug to regional companies elsewhere except Japan, where we have previously licensed to Ono Pharmaceutical Co., Ltd. the subcutaneous formulation of the drug. In addition to the FDA-approved indication for advanced illness patients, a supplemental New Drug Application (sNDA) for subcutaneous RELISTOR in non-cancer pain patients has an action date under the U.S. Prescription Drug User Fee Act (PDUFA) of April 27, 2012. We and Salix recently announced successful top-line data from the ongoing phase 3 trial of oral methylnaltrexone in non-cancer pain patients. Our current principal sources of revenue from operations are upfront, commercialization milestone, royalty and revenue-sharing payments from Salix’s RELISTOR operations.
Progenics also continues its research and development efforts for drug candidates focused on oncology. Our principal product candidate is PSMA ADC, a fully human monoclonal antibody-drug conjugate (ADC) directed against prostate specific membrane antigen (PSMA), a protein found at high levels on the surface of prostate cancer cells and also on the neovasculature of a number of other types of solid tumors. We expect that the ongoing Phase 1 trial of PSMA ADC will be completed in 2012 and if the results are successful we plan then to commence a Phase 2 trial of PSMA ADC in advanced prostate cancer.
Also in oncology, we are allocating additional financial and personnel resources to our pre-clinical development work on novel multiplex phosphoinositide 3-kinase (PI3K) inhibitors that may be effective in blocking signaling pathways that are critical in the growth of aggressive cancers. We are seeking to in-license or acquire opportunities in the oncology field and related supportive, diagnostic and/or other areas that are complementary to these ongoing initiatives and our oncology focus generally. As we have expanded our focus on oncology, we have terminated programs directed toward hepatitis C infection and human immunodeficiency virus (HIV) vaccine
therapy, and are working to out-license other programs not within the Company’s new focus.
Progenics commenced principal operations in 1988, became publicly traded in 1997 and throughout has been engaged primarily in research and development efforts, establishing corporate collaborations and related activities. Certain of our intellectual property rights are held by wholly owned subsidiaries. None of our subsidiaries other than PSMA Development Company LLC (PSMA LLC) had operations during the years ended December 31, 2011, 2010 or 2009. All of our operations are conducted at our facilities in Tarrytown, New York. We operate under a single research and development segment.
RELISTOR (methylnaltrexone bromide) subcutaneous injection is a first-in-class therapy for opioid-induced constipation (OIC) which we developed over the course of the last decade and since 2008 has been approved for sale in the United States and over 50 other countries worldwide, including countries in the European Union, Canada and Australia. Marketing applications are pending elsewhere throughout the world. Under our 2011 License Agreement, Salix is responsible for further developing and commercializing subcutaneous RELISTOR, including completing clinical development necessary to support regulatory marketing approvals for potential new indications and
formulations of the drug. Through December 31, 2011, we have received under this Agreement a $60.0 million upfront cash payment and $0.2 million in respect of Salix ex-U.S. sublicensee revenue and are eligible to receive (i) up to $40.0 million upon U.S. marketing approval for subcutaneous RELISTOR in non-cancer pain patients, (ii) up to $50.0 million upon U.S. marketing approval of an oral formulation of RELISTOR, (iii) up to $200.0 million of commercialization milestone payments upon achievement of specified U.S. sales targets, (iv) royalties ranging from 15 to 19 percent of net sales by Salix and its affiliates, and (v) 60% of any upfront, milestone, reimbursement or other revenue (net of costs of goods sold, as defined, and territory-specific research and development expense reimbursement) Salix receives from sublicensees outside the U.S. In the event that either marketing approval
is subject to a Black Box Warning or Risk Evaluation and Mitigation Strategy (REMS), payment of a portion of the milestone amount would be deferred, and subject, to achievement of the first commercialization milestone.
F-7
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
Progenics began developing RELISTOR in 2001 and continued development and commercialization worldwide except Japan with Wyeth Pharmaceuticals, now a Pfizer Inc. subsidiary, pursuant to a 2005 collaboration agreement that was terminated in October 2009.
Funding and Financial Matters. At December 31, 2011, we held $70.1 million in cash and cash equivalents which, we expect will be sufficient to fund operations as currently anticipated beyond one year. We may require additional funding in the future and if we are unable to enter into favorable collaboration, license, asset sale, capital raising or other financing transactions, we will have to reduce, delay or eliminate spending on some current operations, and/or reduce salary and other overhead expenses, to extend our remaining operations. At December 31, 2011, cash, cash equivalents and auction rate securities increased to $73.4 million from
$51.5 million at December 31, 2010.
In April 2008, our Board of Directors approved a share repurchase program to acquire up to $15.0 million of our outstanding common shares, under which we have $12.3 million remaining available. Purchases may be discontinued at any time. We did not repurchase any common shares during the years ended December 31, 2011, 2010 and 2009.
2. Summary of Significant Accounting Policies
Basis of Presentation
The consolidated financial statements have been prepared on the basis of accounting principles generally accepted in the United States of America (GAAP). The preparation of financial statements in conformity with GAAP requires management to make estimates and assumptions that affect the amounts reported in the financial statements and the accompanying notes. As additional information becomes available or actual amounts become determinable, the recorded estimates are revised and reflected in the operating results. Actual results could differ from those estimates.
Consolidation
The consolidated financial statements include the accounts of Progenics and PSMA LLC, as of and for the years ended December 31, 2011, 2010 and 2009. Inter-company transactions have been eliminated in consolidation.
Revenue Recognition
We recognize revenue from all sources based on the provisions of the SEC’s Staff Accounting Bulletin (SAB) No. 104 (SAB 104) and ASC 605 Revenue Recognition.
In October 2009, the FASB updated ASC 605 Revenue Recognition by specifying how to separate deliverables in multiple-deliverable arrangements, and how to measure and allocate arrangement consideration to one or more units of accounting. Under ASC 605, the delivered item(s) are separate units of accounting, provided (i) the delivered item(s) have value to a collaborator on a stand-alone basis, and (ii) if the arrangement includes a general right of return relative to the delivered item, delivery or performance of the undelivered item(s) is considered probable and substantially in our control. We adopted this update on January 1, 2011. In April 2010, the FASB issued a separate update to ASC 605 which provides
guidance on the criteria that should be met when determining whether the milestone method of revenue recognition is appropriate. This method is effective on a prospective basis for milestones achieved after January 1, 2011.
If we are involved in a steering or other committee as part of a multiple-deliverable arrangement, we assess whether our involvement constitutes a performance obligation or a right to participate. For those committees that are deemed obligations, we will evaluate our participation along with other obligations in the arrangement and will attribute revenue to our participation through the period of our committee responsibilities. We recognize revenue for payments that are contingent upon performance solely by our collaborator immediately upon the achievement of the defined event if we have no related performance obligations. Reimbursement of costs is recognized as revenue provided the provisions of ASC 605 Revenue
Recognition are met, the amounts are determinable and collection of the related receivable is reasonably assured.
Amounts received prior to satisfying the above revenue recognition criteria are recorded as deferred revenue on the accompanying Consolidated Balance Sheets. Amounts not expected to be recognized within one year of the balance sheet date are classified as long-term deferred revenue. The estimate of the classification of deferred revenue as short-term or long-term is based upon the period in which we expect to perform joint committee services.
F-8
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
Royalty revenue is recognized in the period the sales occur, provided that the royalty amounts are fixed or determinable, collection of the related receivable is reasonably assured and we have no remaining performance obligations under the arrangement providing for the royalty.
During the years ended December 31, 2011, 2010 and 2009, we also recognized revenue primarily from government research grants, which are used to subsidize a portion of certain of our research projects (Projects), exclusively from the National Institutes of Health (NIH). We also recognized revenue from the sale of research reagents during those periods. NIH grant revenue is recognized as efforts are expended and as related subsidized project costs are incurred. We perform work under the NIH grants on a best-effort basis. The NIH reimburses us for costs associated with projects in the fields of virology and cancer, including pre-clinical research, development and early clinical testing of a prophylactic vaccine
designed to prevent HIV from becoming established in uninfected individuals exposed to the virus, as requested by the NIH.
License Agreement with Salix – February 2011
Under our license agreement, Salix is responsible for further developing and commercializing subcutaneous RELISTOR worldwide other than Japan, including completing clinical development necessary to support regulatory marketing approvals for potential new indications and formulations. We have granted Salix an exclusive license of relevant know-how, patent rights and technology, assigned relevant third-party contracts, and performed substantially all of our other transition-related activities as of June 30, 2011. During the second quarter of 2011, we and Salix completed a number of tasks involved in enabling Salix to distribute RELISTOR in the U.S. and Europe, as well as clinical and regulatory development related
activities, and have agreed with Salix on research and development services we are to perform at Salix’s direction. We have not performed any significant research and development activities during the third and fourth quarters of 2011.
In consideration of the $60.0 million upfront payment from Salix, we are responsible for delivering to Salix an exclusive license of relevant know-how, patent rights and technology and serving on joint committees provided for in the License Agreement. These deliverables, which have stand-alone value and represent separate units of accounting, include (i) the exclusive license which was delivered for revenue recognition purposes during the 2011 second quarter, (ii) performing reimbursable development services at Salix’s direction during the 2011 second quarter, the period in which we and Salix finalized the development plan, and (iii) joint committee services, which we expect to perform through 2013. We
determined that the license has stand-alone value as the license was delivered to Salix for revenue recognition purposes in the second quarter of 2011 and Salix is responsible for continuing research and development.
We developed a best estimate of selling price for each deliverable as vendor-specific objective evidence and third-party evidence was not available. We allocated the best estimate of selling price, on a relative basis, to each of the three units of accounting as the $60.0 million upfront payment was the only payment from Salix which was fixed and determinable at the inception of the arrangement. As a result, $58.4 million, $1.1 million and $0.5 million was allocated to the license, reimbursable development services and our participation in the joint committees as provided in the License Agreement, respectively. We recognized $58.4 million for the license and relevant know-how, patent rights and technology and $1.1
million for the reimbursable development services, respectively, during the second quarter of 2011, the period in which we delivered these items and performed the development services. At December 31, 2011, the remaining deferred revenue of $0.4 million pertaining to joint committee services is recognized in collaboration revenue as such activities are performed in the future.
Transition Agreement with Wyeth – October 2009
Under the Transition Agreement, Wyeth’s license of Progenics’ technology under the original 2005 collaboration was terminated except as necessary for performance of its obligations during the transition period, and Wyeth returned the rights to RELISTOR that we had previously granted. During the transition, Wyeth was obligated to pay all costs of commercialization of subcutaneous RELISTOR, including manufacturing costs, and retained all proceeds from its sale of the products, subject to royalties due to us for sales prior to September 30, 2010. Decisions with respect to commercialization of the product during the transition period were made solely by Wyeth. We have no further obligations to Wyeth under
the 2005 collaboration agreement. As of the beginning of the fourth quarter of 2011, Salix assumed substantially all of Wyeth’s remaining ex-U.S. development and commercialization activities for RELISTOR worldwide ex-Japan.
F-9
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
Wyeth Collaboration Agreement – December 2005 to October 2009
The Wyeth collaboration agreement was in effect until October 2009, which includes periods covered by this report. The agreement included a non-refundable upfront license fee, reimbursement of development costs, research and development payments based upon our achievement of clinical development milestones, contingent payments based upon the achievement by Wyeth of defined events and royalties on product sales. In 2009, we recognized revenue from (i) research from January 1, 2009 to October 2009, (ii) the upfront license payment we received from Wyeth using the proportionate performance method and (iii) royalties.
Ono Agreement – October 2008
Ono is responsible for developing and commercializing subcutaneous RELISTOR in Japan, including conducting the clinical development necessary to support regulatory marketing approval. Ono will own the filings and approvals related to subcutaneous RELISTOR in Japan. In addition to the $15.0 million upfront payment from Ono, we are entitled to receive up to an additional $20.0 million, payable upon achievement by Ono of its development milestones. Ono is also obligated to pay to us royalties and commercialization milestones on sales by Ono of subcutaneous RELISTOR in Japan. Ono has the option to acquire from us the rights to develop and commercialize in Japan other formulations of RELISTOR, including intravenous and
oral forms, on terms to be negotiated separately. Ono may request us to perform activities related to its development and commercialization responsibilities, beyond our participation in joint committees and specified technology transfer-related tasks, at its expense payable at the time we perform such services. Revenue earned from activities we perform for Ono is recorded in collaboration revenue.
We recognized the upfront payment of $15.0 million, which we received from Ono in November 2008, as collaboration revenue during the first quarter of 2009, upon satisfaction of our performance obligations.
Research and Development Expenses
Research and development expenses include costs directly attributable to the conduct of research and development programs, including the cost of salaries, payroll taxes, employee benefits, materials, supplies, maintenance of research equipment, costs related to research collaboration and licensing agreements, the purchase of in-process research and development, the cost of services provided by outside contractors, including services related to our clinical trials, the full cost of manufacturing drug for use in research, pre-clinical development and clinical trials. All costs associated with research and development are expensed as incurred.
At each period end, we evaluate the accrued expense balance related to these activities based upon information received from the suppliers and estimated progress towards completion of the research or development objectives to ensure that the balance is reasonably stated. Such estimates are subject to change as additional information becomes available.
Use of Estimates
Significant estimates include useful lives of fixed assets, the periods over which certain revenues and expenses will be recognized, including collaboration revenue recognized from non-refundable up-front licensing payments and expense recognition of certain clinical trial costs which are included in research and development expenses, the amount of non-cash compensation costs related to share-based payments to employees and non-employees and the periods over which those costs are expensed and the likelihood of realization of deferred tax assets.
Patents
As a result of research and development efforts conducted by us, we have applied, or are applying, for a number of patents to protect proprietary inventions. All costs associated with patents are expensed as incurred.
F-10
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
Net Income (Loss) Per Share
We prepare our earnings per share (EPS) data in accordance with ASC 260 Earnings Per Share. Basic net income (loss) per share amounts have been computed by dividing net income (loss) by the weighted-average number of shares of common shares outstanding during the period. For the year ended December 31, 2011, we reported net income, and the computation of diluted earnings per share is based upon the weighted-average number of our common shares and dilutive effect, determined using the treasury stock method, of potential common shares outstanding including amounts of unrecognized compensation expense. For the years ended December 31, 2010 and 2009, we reported net losses and, therefore, potential common shares,
amounts of unrecognized compensation expense and windfall tax benefits have been excluded from diluted net loss per share since they would be anti-dilutive.
Concentrations of Credit Risk
Financial instruments that potentially subject Progenics to concentrations of credit risk consist of cash, cash equivalents, marketable and auction rate securities and receivables from Salix, Ono or the NIH. We invest our excess cash in money market funds. We have established guidelines that relate to credit quality, diversification and maturity and that limit exposure to any one issue of securities. We hold no collateral for these financial instruments.
Cash and Cash Equivalents
We consider all highly liquid investments which have maturities of three months or less, when acquired, to be cash equivalents. The carrying amount reported in the balance sheet for cash and cash equivalents approximates its fair value. Cash and cash equivalents subject us to concentrations of credit risk. At December 31, 2011 and 2010, we have invested approximately $64,068 and $43,958, respectively, in cash equivalents in the form of money market funds with one major investment company and held approximately $6,037 and $3,960, respectively, in a single commercial bank.
Auction Rate Securities
In accordance with ASC 320 Investments – Debt and Equity Securities, investments are classified as available-for-sale. Available-for-sale securities are carried at fair value, with the unrealized gains and losses reported in comprehensive income (loss). The amortized cost of debt securities is adjusted for amortization of premiums and accretion of discounts to maturity. Such amortization is included in interest income or expense. Realized gains and losses and declines in value judged to be other-than-temporary, if any, on available-for-sale securities are included in other income or expense. In computing realized gains and losses, we compute the cost of its investments on a specific identification basis. Such
cost includes the direct costs to acquire the securities, adjusted for the amortization of any discount or premium. The fair value of marketable securities has been estimated based on a three-level hierarchy for fair value measurements. Interest and dividends on securities classified as available-for-sale are included in interest income (see Note 3).
At December 31, 2011 and 2010, our investment in auction rate securities in the long term assets section of the Consolidated Balance Sheets amounted to $3,332 and $3,608, respectively. Valuation of securities is subject to uncertainties that are difficult to predict, such as changes to credit ratings of the securities and/or the underlying assets supporting them, default rates applicable to the underlying assets, underlying collateral value, discount rates, counterparty risk, ongoing strength and quality of market credit and liquidity and general economic and market conditions. The valuation of the auction rate securities we hold is based on an internal analysis of timing of expected future successful auctions,
collateralization of underlying assets of the security and credit quality of the security. We re-evaluated the valuation of these securities as of December 31, 2011 and the temporary impairment amount decreased $24 from $292 at December 31, 2010 to $268. All income generated from these investments was recorded as interest income (see Note 3).
F-11
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
Fair Value Measurements
In accordance with ASC 820 Fair Value Measurements and Disclosures, we use a three-level hierarchy for fair value measurements that distinguishes between market participant assumptions developed from market data obtained from sources independent of the reporting entity (observable inputs) and the reporting entity’s own assumptions about market participant assumptions developed from the best information available in the circumstances (unobservable inputs). The hierarchy level assigned to each security in our available-for-sale portfolio is based on our assessment of the transparency and reliability of the inputs used in the valuation of such instrument at the measurement date. The three hierarchy levels are
defined as follows:
● Level 1 - Valuations based on unadjusted quoted market prices in active markets for identical securities.
● Level 2 - Valuations based on observable inputs other than Level 1 prices, such as quoted prices for similar assets at the measurement date, quoted prices in markets that are not active or other inputs that are observable, either directly or indirectly.
● Level 3 - Valuations based on inputs that are unobservable and significant to the overall fair value measurement, and involve management judgment.
Other current assets are comprised of prepaid expenses, interest and other receivables of $919 and $1,801 at December 31, 2011 and 2010, respectively, which are expected to be settled within one year. Other assets of $1,250 at December 31, 2010 include $1,050, which represents the long term portions of amounts prepaid to a clinical research organization. Restricted cash of $200 at both December 31, 2011 and 2010, respectively, consists of collateral for a letter of credit securing lease obligations. We believe that carrying value of those assets approximates fair value.
Fixed Assets
Leasehold improvements, furniture and fixtures, and equipment are stated at cost. Furniture, fixtures and equipment are depreciated on a straight-line basis over their estimated useful lives. Leasehold improvements are amortized on a straight-line basis over the life of the lease or of the improvement, whichever is shorter. Costs of construction of long-lived assets are capitalized but are not depreciated until the assets are placed in service.
Expenditures for maintenance and repairs which do not materially extend the useful lives of the assets are charged to expense as incurred. The cost and accumulated depreciation of assets retired or sold are removed from the respective accounts and any gain or loss is recognized in operations. The estimated useful lives of fixed assets are as follows:
|
Computer equipment
|
3 years
|
|
Machinery and equipment
|
5-7 years
|
|
Furniture and fixtures
|
5 years
|
|
Leasehold improvements
|
Earlier of life of improvement or lease
|
Deferred Lease Liability and Incentive
Our lease agreements include fixed escalations of minimum annual lease payments and we recognize rental expense on a straight-line basis over the lease terms and record the difference between rent expense and current rental payments as deferred rent. Deferred lease incentive includes a construction allowance from our landlord which is amortized as a reduction to rental expense on a straight-line basis over the lease terms. As of December 31, 2011, the Consolidated Balance Sheets include deferred lease liability of $577 in Other liabilities and deferred lease incentive of $115 and $920 in Other current liabilities and Other liabilities, respectively. As of December 31, 2010, the Consolidated Balance Sheets include
deferred lease liability of $400 in Other liabilities and deferred lease incentive of $112 and $1,004 in Other current liabilities and Other liabilities, respectively.
F-12
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
Impairment of Long-Lived Assets
We periodically assess the recoverability of fixed assets and evaluate such assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. In accordance with ASC 360 Property, Plant, and Equipment – Impairment or Disposal of Long-Lived Assets, if indicators of impairment exist, we assess the recoverability of the affected long-lived assets by determining whether the carrying value of such assets can be recovered through undiscounted future operating cash flows. If the carrying amount is not recoverable, we measure the amount of any impairment by comparing the carrying value of the asset to market prices for similar assets. As a result of
closing our biologics pilot facilities in 2011, an impairment loss of $22 is included in Research and development expenses in our accompanying Consolidated Statement of Operations during the year ended December 31, 2011. No impairments occurred as of December 31, 2010 or 2009.
Income Taxes
We account for income taxes in accordance with the provisions of ASC 740 Income Taxes, which requires that we recognize deferred tax liabilities and assets for the expected future tax consequences of events that have been included in the financial statements or tax returns. Under this method, deferred tax assets and liabilities are determined on the basis of the difference between the tax basis of assets and liabilities and their respective financial reporting amounts (temporary differences) at enacted tax rates in effect for the years in which the temporary differences are expected to reverse. A valuation allowance is established for deferred tax assets for which realization is uncertain.
In accordance with ASC 718 Compensation – Stock Compensation and ASC 505 Equity, we have made a policy decision related to intra-period tax allocation, to account for utilization of windfall tax benefits based on provisions in the tax law that identify the sequence in which amounts of tax benefits are used for tax purposes (i.e., tax law ordering).
Uncertain tax positions are accounted for in accordance with ASC 740 Income Taxes, which prescribes a comprehensive model for the manner in which a company should recognize, measure, present and disclose in its financial statements all material uncertain tax positions that we have taken or expect to take on a tax return. ASC 740 applies to income taxes and is not intended to be applied by analogy to other taxes, such as sales taxes, value-add taxes, or property taxes. We review our nexus in various tax jurisdictions and our tax positions related to all open tax years for events that could change the status of our ASC 740 liability, if any, or require an additional liability to be recorded. Such events may be the
resolution of issues raised by a taxing authority, expiration of the statute of limitations for a prior open tax year or new transactions for which a tax position may be deemed to be uncertain. Those positions, for which management’s assessment is that there is more than a 50 percent probability of sustaining the position upon challenge by a taxing authority based upon its technical merits, are subjected to the measurement criteria of ASC 740. We record the largest amount of tax benefit that is greater than 50 percent likely of being realized upon ultimate settlement with a taxing authority having full knowledge of all relevant information. Any ASC 740 liabilities for which we expect to make cash payments within the next twelve months are classified as “short term.” In the event that we conclude that we are subject to interest and/or penalties arising from uncertain
tax positions, we will record interest and penalties as a component of income taxes (see Note 12).
Risks and Uncertainties
We have to date relied principally on external funding, collaborations with Salix, Wyeth and others, royalty and product revenue to finance our operations. There can be no assurance that our research and development will be successfully completed, that any products developed will obtain necessary marketing approval by regulatory authorities or that any approved products will be commercially viable. In addition, we operate in an environment of rapid change in technology, and we are dependent upon satisfactory relationships with our partners and the continued services of our current employees, consultants and subcontractors. We are also dependent upon Salix and Ono fulfilling their manufacturing obligations, either on
their own or through third-party suppliers. For the years ended December 31, 2011, 2010 and 2009, the primary sources of our revenues were Salix, Wyeth, Ono and research grant revenues from the NIH. Most of our grant revenues were directed toward candidates which we are now seeking to out-licenses as part of the reorientation or our research and development focus on oncology. There can be no assurance that revenues from Salix and Ono or from research awards will continue. Substantially all of our accounts receivable at December 31, 2011 and 2010 were from the above-named sources.
F-13
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
Comprehensive Income (Loss)
Comprehensive income (loss) represents the change in net assets of a business enterprise during a period from transactions and other events and circumstances from non-owner sources. Our comprehensive income (loss) includes net income (loss) adjusted for the change in net unrealized gain or loss on marketable and auction rate securities. The disclosures required by ASC 220 Comprehensive Income for the years ended December 31, 2011, 2010 and 2009 have been included in the Consolidated Statements of Stockholders’ Equity and Comprehensive Income (Loss). There was no income tax expense/benefit allocated to any component of Other Comprehensive Income (Loss) (see Note 12).
Impact of Recently Adopted Accounting Standards
In October 2009, the FASB updated ASC 605 Revenue Recognition by specifying how to separate deliverables in multiple-deliverable arrangements, and how to measure and allocate arrangement consideration to one or more units of accounting. We adopted this update on January 1, 2011 (see Note 2).
3. Fair Value Measurements
Our available-for-sale investments consist of money market funds and auction rate securities and are recorded at fair value in the accompanying Consolidated Balance Sheets in accordance with ASC 320 Investments – Debt and Equity Securities. The change in the fair value of these securities is recorded as a component of other comprehensive income (loss).
F-14
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
The following table presents our available-for-sale investments measured at fair value on a recurring basis, summarized by valuation hierarchy, as of December 31, 2011 and 2010:
|
Fair Value Measurements at December 31, 2011
|
||||||||||||
|
Balance at
December 31,
2011
|
Quoted Prices
in Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||
|
Money market funds
|
$
|
64,068
|
$
|
64,068
|
$
|
-
|
$
|
-
|
||||
|
Auction rate securities
|
3,332
|
-
|
-
|
3,332
|
||||||||
|
Total
|
$
|
67,400
|
$
|
64,068
|
$
|
-
|
$
|
3,332
|
||||
|
Fair Value Measurements at December 31, 2010
|
||||||||||||
|
Balance at
December 31,
2010
|
Quoted Prices
in Active
Markets for
Identical Assets
(Level 1)
|
Significant
Other
Observable
Inputs
(Level 2)
|
Significant
Unobservable
Inputs
(Level 3)
|
|||||||||
|
Money market funds
|
$
|
43,958
|
$
|
43,958
|
$
|
-
|
$
|
-
|
||||
|
Auction rate securities
|
3,608
|
-
|
-
|
3,608
|
||||||||
|
Total
|
$
|
47,566
|
$
|
43,958
|
$
|
-
|
$
|
3,608
|
||||
At December 31, 2011, we hold $3,332 in auction rate securities which are classified as Level 3. The fair value of these securities includes $2,392 of U.S. government subsidized securities collateralized by student loan obligations and $940 of investment company perpetual preferred stock. Auction rate securities are collateralized long-term instruments that were intended to provide liquidity through an auction process that resets interest rates at pre-determined intervals. We will not realize cash in respect of the principal amount of these securities until the issuer calls or restructures the security, the security reaches any scheduled maturity and is paid, or a buyer outside the auction process emerges. As of
December 31, 2011, we have received all scheduled interest payments on these securities, which, in the event of auction failure, are reset according to the contractual terms in the governing instruments.
The valuation of auction rate securities we hold is based on Level 3 unobservable inputs which consist of our internal analysis of (i) timing of expected future successful auctions, (ii) collateralization of underlying assets of the security and (iii) credit quality of the security. In re-evaluating the valuation of these securities as of December 31, 2011, the temporary impairment amount decreased $24 from $292 at December 31, 2010, to $268, which is reflected as a part of accumulated other comprehensive loss on our accompanying Consolidated Balance Sheets. These securities are held “available-for-sale” and the unrealized loss is included in accumulated other comprehensive loss. Due to the uncertainty
related to the liquidity in the auction rate security market and therefore when individual positions may be liquidated, we have classified these auction rate securities as long-term assets on our accompanying Consolidated Balance Sheets. We continue to monitor markets for our investments and consider the impact, if any, of market conditions on the fair market value of our investments. We do not believe the carrying values of our investments are other than temporarily impaired and therefore expect the positions will eventually be liquidated without significant loss.
F-15
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
For those of our financial instruments with significant Level 3 inputs (all auction rate securities), the following tables summarize the activities for the years ended December 31, 2011 and 2010:
|
Fair Value Measurements Using Significant
Unobservable Inputs
(Level 3)
|
||||||||
|
Description
|
2011
|
2010
|
||||||
|
Balance at beginning of period
|
$ | 3,608 | $ | 3,792 | ||||
|
Transfers into Level 3
|
- | - | ||||||
|
Total realized/unrealized gains (losses)
|
||||||||
|
Included in net income (loss)
|
- | - | ||||||
|
Included in comprehensive income (loss) (1)
|
24 | 16 | ||||||
|
Settlements
|
(300 | ) | (200 | ) | ||||
|
Balance at end of period
|
$ | 3,332 | $ | 3,608 | ||||
|
(1) Total amount of unrealized gains (losses) for the period included in other comprehensive loss attributable to the change in fair market value of related assets still held at the reporting date
|
$ | - | $ | - | ||||
The following tables summarize the amortized cost basis, the aggregate fair value and gross unrealized holding gains and losses at December 31, 2011 and 2010:
|
Amortized
|
Fair
|
Unrealized Holding
|
||||||||||||||||||
|
Cost Basis
|
Value
|
Gains
|
(Losses)
|
Net
|
||||||||||||||||
|
2011:
|
||||||||||||||||||||
|
Maturities greater than ten years:
|
||||||||||||||||||||
|
Auction rate securities
|
$ | 2,600 | $ | 2,392 | $ | - | $ | (208 | ) | $ | (208 | ) | ||||||||
|
Investments without stated maturity dates:
|
||||||||||||||||||||
|
Auction rate securities
|
1,000 | 940 | - | (60 | ) | (60 | ) | |||||||||||||
| $ | 3,600 | $ | 3,332 | $ | - | $ | (268 | ) | $ | (268 | ) | |||||||||
|
Amortized
|
Fair
|
Unrealized Holding
|
||||||||||||||||||
|
Cost Basis
|
Value
|
Gains
|
(Losses)
|
Net
|
||||||||||||||||
|
2010:
|
||||||||||||||||||||
|
Maturities greater than ten years:
|
||||||||||||||||||||
|
Auction rate securities
|
$ | 2,900 | $ | 2,668 | $ | - | $ | (232 | ) | $ | (232 | ) | ||||||||
|
Investments without stated maturity dates:
|
||||||||||||||||||||
|
Auction rate securities
|
1,000 | 940 | - | (60 | ) | (60 | ) | |||||||||||||
| $ | 3,900 | $ | 3,608 | $ | - | $ | (292 | ) | $ | (292 | ) | |||||||||
We compute the cost of its investments on a specific identification basis. Such cost includes the direct costs to acquire the securities, adjusted for the amortization of any discount or premium.
F-16
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
The following table shows the gross unrealized losses and fair value of our marketable securities with unrealized losses that are not deemed to be other-than-temporarily impaired, aggregated by investment category and length of time that individual securities have been in a continuous unrealized loss position, at December 31, 2011 and 2010.
2011:
|
Less than 12 Months
|
12 Months or Greater
|
Total
|
||||||||||||||||||||||
|
Description of Securities
|
Fair Value
|
Unrealized
Losses
|
Fair Value
|
Unrealized
Losses
|
Fair Value
|
Unrealized
Losses
|
||||||||||||||||||
|
Auction rate securities
|
$ | - | $ | - | $ | 3,332 | $ | (268 | ) | $ | 3,332 | $ | (268 | ) | ||||||||||
|
Total
|
$ | - | $ | - | $ | 3,332 | $ | (268 | ) | $ | 3,332 | $ | (268 | ) | ||||||||||
2010:
|
Less than 12 Months
|
12 Months or Greater
|
Total
|
||||||||||||||||||||||
|
Description of Securities
|
Fair Value
|
Unrealized
Losses
|
Fair Value
|
Unrealized
Losses
|
Fair Value
|
Unrealized
Losses
|
||||||||||||||||||
|
Auction rate securities
|
$ | - | $ | - | $ | 3,608 | $ | (292 | ) | $ | 3,608 | $ | (292 | ) | ||||||||||
|
Total
|
$ | - | $ | - | $ | 3,608 | $ | (292 | ) | $ | 3,608 | $ | (292 | ) | ||||||||||
Other-than-temporary impairment analysis on auction rate securities. The unrealized losses in our auction rate securities investments were the result of an internal analysis of timing of expected future successful auctions, collateralization of underlying assets of the security and credit quality of the security. At December 31, 2011 and 2010, there were two securities with a gross unrealized loss position of $268 and $292 ($3,332 and $3,608 of the total fair value), respectively.
The severity of the unrealized losses for auction rate securities at December 31, 2011 and 2010 was ranged from 6 percent and 8 percent below amortized cost, and the weighted average duration of the unrealized losses for these securities was 46 and 34 months, respectively.
We have evaluated our individual auction rate securities holdings for other-than-temporary impairment and determined that the unrealized losses as of December 31, 2011 and 2010 are attributable to uncertainty in the liquidity of the auction rate security market. Because we do not intend to sell these securities, and believe it is not more likely than not that we would be required to sell these securities before recovery of principal, we do not consider these securities to be other-than-temporarily impaired at December 31, 2011 and 2010.
4. Accounts Receivable
Our accounts receivable represent amounts due to Progenics from collaborators, royalties, research grants and the sales of research reagents. These amounts are considered to be short-term as they are expected to be collected within one year and we believe carrying value approximates fair value. Accounts receivable as of December 31, 2011 and 2010, consisted of the following:
|
2011
|
2010
|
||||||
|
Collaborators
|
$ | 77 | $ | 1,811 | |||
|
Royalties
|
1,279 | - | |||||
|
National Institutes of Health
|
100 | 468 | |||||
|
Other
|
60 | 4 | |||||
|
Total
|
$ | 1,516 | $ | 2,283 | |||
F-17
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
5. Fixed Assets
Fixed assets as of December 31, 2011 and 2010 consisted of the following:
|
2011
|
2010
|
||||||
|
Computer equipment
|
$
|
2,133
|
$
|
2,508
|
|||
|
Machinery and equipment
|
12,035
|
13,380
|
|||||
|
Furniture and fixtures
|
230
|
740
|
|||||
|
Leasehold improvements
|
11,526
|
13,354
|
|||||
|
Construction in progress and other
|
175
|
74
|
|||||
|
26,099
|
30,056
|
||||||
|
Less, accumulated depreciation and amortization
|
(22,061)
|
|
(24,178
|
)
|
|||
|
Total
|
$
|
4,038
|
$
|
5,878
|
|||
At December 31, 2011, $2.3 million of leasehold improvements, net were being amortized over periods of 1.3-10.8 years, under leases with terms through December 31, 2020. At December 31, 2010, $2.7 million of leasehold improvements, net were being amortized over periods of 10.0-10.8 years, under leases with terms through December 31, 2020.
6. Accounts Payable and Accrued Expenses
The carrying value of our accounts payable and accrued expenses approximates fair value, as it represents amounts due to vendors and employees, which will be satisfied within one year. Accounts payable and accrued expenses as of December 31, 2011 and 2010, consisted of the following:
|
2011
|
2010
|
||||||
|
Accrued consulting and clinical trial costs
|
$ | 1,637 | $ | 6,125 | |||
|
Accrued payroll and related costs
|
3,149 | 1,725 | |||||
|
Restructuring accrual
|
731 | - | |||||
|
Legal and professional fees
|
371 | 1,116 | |||||
|
Accounts payable
|
309 | 658 | |||||
|
Other
|
134 | 59 | |||||
|
Total
|
$ | 6,331 | $ | 9,683 | |||
7. Restructuring
In the third and fourth quarters of 2011, we reduced headcount resulting in a restructuring accrual of $1.3 million of severance and related benefits, which are being paid during the period from October 2011 through August 2012. We also incurred other exit and contract termination costs related to a lease amendment and consolidation of employees within reduced facility space and recorded equipment write-off of $8 related to a grant out-licensing. Other exit and contract termination costs are being paid during the period from October 2011 to March 31, 2012.
Activity in the restructuring accrual, which is included in accounts payable and accrued expenses in our Consolidated Balance Sheets and research and development and general and administrative expenses in the Consolidated Statements of Operations, is specified below.
|
Severance
and Related
Benefits
|
Other Exit
Costs
|
Contract
Termination
Costs
|
Total
Restructuring
Accrual
|
|||||||||||||
|
Balance at September 30, 2011
|
$ | 1,064 | $ | - | $ | - | $ | 1,064 | ||||||||
|
Additions
|
277 | 8 | 292 | 577 | ||||||||||||
|
Payments
|
(770 | ) | (2 | ) | (138 | ) | (910 | ) | ||||||||
|
Balance at December 31, 2011
|
$ | 571 | $ | 6 | $ | 154 | $ | 731 | ||||||||
F-18
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
8. Stockholders’ Equity
We are authorized to issue 80.0 million shares of Common Stock, par value $.0013, and 20.0 million shares of preferred stock, par value $.001. The Board of Directors has the authority to issue common and preferred shares, in series, with rights and privileges as determined by the Board of Directors.
In 2008, our Board of Directors approved a share repurchase program to acquire up to $15.0 million of our outstanding common shares. Purchases may be discontinued at any time. We did not repurchase any common shares during the years ended December 31, 2011, 2010 and 2009. We have $12.3 million remaining available for purchases under the program.
9. Commitments and Contingencies
a. Operating Leases
As of December 31, 2011, we leased office, manufacturing and laboratory space, under lease agreements expiring in June 2012 and December 2020.
Rental payments are recognized as rent expense on a straight-line basis over the term of the lease. In addition to rents due under these agreements, we are obligated to pay additional facilities charges, including utilities, taxes and operating expenses. We also lease certain office equipment under non-cancelable operating leases, which expire at various times through April 2013.
As of December 31, 2011, future minimum annual payments under all operating lease agreements are as follows:
|
Years ending
December 31,
|
Minimum
Annual Payments
|
||
|
2012
|
$ | 2,547 | |
|
2013
|
2,410 | ||
|
2014
|
2,410 | ||
|
2015
|
2,470 | ||
|
2016
|
2,532 | ||
|
Thereafter
|
10,777 | ||
|
Total
|
$ | 23,146 | |
Rental expense totaled approximately $3,475, $3,544 and $2,773 for the years ended December 31, 2011, 2010 and 2009, respectively. For the years ended December 31, 2011 and 2010, we recognized rent expense in excess of amounts paid of $63 and $181, respectively, due to the recognition of escalation clauses and lease incentives. For the year ended December 31, 2009 amounts paid exceeded rent expense by $154, due to the recognition of escalation clauses and lease incentives. Additional facility charges, including utilities, taxes and operating expenses, for the years ended December 31, 2011, 2010 and 2009 were approximately $4,033, $3,645 and $3,060, respectively.
F-19
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
b. Licensing, Service and Supply Agreements
Progenics has entered into intellectual property-based license and service agreements in connection with its product development programs. Progenics has recognized milestone, license and sublicense fees and supply costs, which are included in research and development expenses, totaling approximately $578, $1,266 and $788 for the years ended December 31, 2011, 2010 and 2009, respectively.
|
Agreement
|
Paid from
inception to
December 31,
2011
|
Future (1)
Commitments
|
Terms
|
|||
|
Progenics agreements with:
|
||||||
|
Lonza Sales AG
|
909
|
3,863
|
Annual license fee payments, milestones and royalties, as applicable, in respect of oncology and other products.
|
|||
|
PSMA LLC agreements with:
|
||||||
|
Seattle Genetics, Inc.
|
3,800
|
14,300
|
Milestone and periodic maintenance payments to use ADC technology to link chemotherapeutic agents to monoclonal antibodies that target prostate specific membrane antigen. ADC technology is based in part on technology licensed by SGI from third parties.
|
|||
|
Amgen Fremont, Inc. (formerly Abgenix)
|
850
|
6,250
|
Milestones and royalties to use XenoMouse® technology for generating fully human antibodies to PSMA LLC’s PSMA antigen.
|
|||
|
Former member of PSMA LLC
|
203
|
52,244
|
Annual minimum royalty payments and milestones to use technology related to PSMA.
|
|||
|
|
(1) Amounts based on known contractual obligations as specified in the respective license agreements, which are dependent on the achievement or occurrence of future milestones or events and exclude amounts for royalties which are dependent on future sales and are unknown.
|
In focusing on the development of medicines to treat cancer, we are seeking to out-license or terminate license and services agreements as to which we have paid $7,951 through December 31, 2011, and have future commitments of $9,690, which are dependent on the achievement or occurrence of future milestones or events.
As part of our research and development efforts, we enter into consulting agreements with external scientific specialists (Scientists). These agreements contain various terms and provisions, including fees to be paid by us and royalties, in the event of future sales, and milestone payments, upon achievement of defined events, payable by us. Certain Scientists are advisors to Progenics, including Stephen P. Goff, Ph.D. and David A. Scheinberg, M.D., Ph.D., both of whom are also members of our Board of Directors. Some Scientists have purchased our Common Stock or received stock options which are subject to vesting provisions. We have recognized expenses with regard to the consulting agreements of $87, $179 and $220
for the years ended December 31, 2011, 2010 and 2009, respectively. Those expenses include the fair value of stock options granted during 2011, 2010 and 2009, which were fully vested at grant date, of approximately $11, $42 and $83, respectively. Such amounts of fair value are included in research and development expense for each year presented (see Note 10).
d. Retirement Agreement
On March 14, 2012, Progenics and company founder Paul J. Maddon entered into an agreement providing for his retirement as Chief Science Officer. In connection with Dr. Maddon’s retirement and termination of his employment agreement, Progenics has agreed to pay him an amount equal to $1,789,333 and provide other benefits under the agreement.
10. Share-Based Payment Arrangements
Our share-based compensation to employees includes non-qualified stock options, restricted stock and shares issued under our Purchase Plans, which are compensatory under ASC 718 Compensation – Stock Compensation. During the second quarter of 2011, we accelerated the vesting of outstanding awards to non-management employees in connection with a change in program eligibility and termination of the Company’s employee stock purchase plans. We account for share-based compensation to non-employees, including non-qualified stock options and restricted stock, in accordance with ASC 505 Equity.
F-20
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
Compensation cost for share-based awards will be recognized in our financial statements over the related requisite service periods; usually the vesting periods for awards with a service condition. We have made an accounting policy decision to use the straight-line method of attribution of compensation expense, under which the grant date fair value of share-based awards will be recognized on a straight-line basis over the total requisite service period for the total award.
We have adopted three stock incentive plans, the 1989 Non-Qualified Stock Option Plan, the 1996 Amended Stock Incentive Plan and the 2005 Stock Incentive Plan (the Plans). Under each of these Plans as amended, up to 375, 5,000 and 7,450 shares of common stock, respectively, have been reserved for the issuance of awards to employees, consultants, directors and other individuals who render services to Progenics (collectively, Awardees). The Plans contain certain anti-dilution provisions in the event of a stock split, stock dividend or other capital adjustment as defined. The 1989 Plan provides for the Board, or the Compensation Committee (Committee) of the Board, to grant stock options to Awardees and to determine the
exercise price, vesting term and expiration date. The 1996 Plan and the 2005 Plan provide for the Board or Committee to grant to Awardees stock options, stock appreciation rights, restricted stock, performance awards or phantom stock, as defined (collectively, Awards). The Committee is also authorized to determine the term and vesting of each Award and the Committee may in its discretion accelerate the vesting of an Award at any time. Stock options granted under the Plans generally vest pro rata over three to ten years and have terms of ten to twenty years. Restricted stock issued under the 1996 Plan or 2005 Plan usually vests annually over three to five years, unless specified otherwise by the Committee. The exercise price of outstanding non-qualified stock options is usually equal to the fair value of our common stock on the date of grant. The exercise price of non-qualified stock
options granted from the 2005 Plan and incentive stock options (ISO) granted from the Plans may not be lower than the fair value of our common stock on the dates of grant. At December 31, 2011, 2010 and 2009, all outstanding stock options were non-qualified options. The 1989 and 1996 Plans terminated in April 1994 and October 2006, respectively, and the 2005 Plan will terminate in April 2015; options granted before termination of the Plans will continue under the respective Plans until exercised, cancelled or expired.
We apply a forfeiture rate to the number of unvested awards in each reporting period in order to estimate the number of awards that are expected to vest. Estimated forfeiture rates are based upon historical data on vesting behavior of employees. We adjust the total amount of compensation cost recognized for each award, in the period in which each award vests, to reflect the actual forfeitures related to that award. Changes in our estimated forfeiture rate will result in changes in the rate at which compensation cost for an award is recognized over its vesting period.
Under ASC 718 Compensation – Stock Compensation, the fair value of each non-qualified stock option award is estimated on the date of grant using the Black-Scholes option pricing model, which requires input assumptions noted in the following table. Ranges of assumptions for inputs are disclosed where the value of such assumptions varied during the related period. Historical volatilities are based upon daily quoted market prices of our common stock on The NASDAQ Stock Market LLC over a period equal to the expected term of the related equity instruments. We rely only on historical volatility since it provides the most reliable indication of future volatility. Future volatility is expected to be consistent with
historical; historical volatility is calculated using a simple average calculation; historical data is available for the length of the option’s expected term and a sufficient number of price observations are used consistently. Since our stock options are not traded on a public market, we do not use implied volatility. For the years ended December 31, 2011, 2010 and 2009 our expected term was calculated based upon historical data related to exercise and post-termination cancellation activity. Accordingly, for grants made to employees and directors and officers (excluding our CSO), we are using expected terms of 5.3 and 7.4 years, 5.3 and 7.3 years, and 5.3 and 7.3 years, respectively. The expected term of stock options granted to our CSO and non-employee consultants are calculated separately from stock options granted to employees and directors and officers and the expected term
for our CSO was 8 years for each of the years ended December 31, 2011, 2010 and 2009. Expected term for options granted to non-employee consultants was ten years, which is the contractual term of those options. We have never paid dividends and do not expect to pay dividends in the future. Therefore, our dividend rate is zero. The risk-free rate for periods within the expected term of the options is based on the U.S. Treasury yield curve in effect at the time of grant. The following table presents assumptions used in computing the fair value of option grants during 2011, 2010 and 2009:
|
2011
|
2010
|
2009
|
||||
|
Expected volatility
|
68% – 78%
|
68% – 87%
|
70% – 91%
|
|||
|
Expected dividends
|
zero
|
zero
|
zero
|
|||
|
Expected term (years)
|
5.3 – 10
|
5.3 – 10
|
5.3 – 10
|
|||
|
Weighted average expected term (years)
|
6.17
|
6.92
|
7.10
|
|||
|
Risk-free rate
|
0.77% – 2.97%
|
1.21% – 3.09%
|
1.78% – 3.22%
|
F-21
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
A summary of option activity under the Plans as of December 31, 2011 and changes during the year then ended is presented below:
|
Options
|
Shares
|
Weighted
Average
Exercise
Price
|
Weighted Average
Remaining
Contractual Term
(Yr.)
|
Aggregate
Intrinsic
Value
|
|||||
|
Outstanding at January 1, 2011
|
5,265
|
$ 14.20
|
|||||||
|
Granted
|
1,373
|
7.43
|
|||||||
|
Exercised
|
(170)
|
4.60
|
|||||||
|
Forfeited or expired
|
(871)
|
15.68
|
|||||||
|
Outstanding at December 31, 2011
|
5,597
|
$ 12.60
|
6.20
|
$ 6,371
|
|||||
|
Exercisable at December 31, 2011
|
3,489
|
$ 15.62
|
4.67
|
$ 2,552
|
The weighted average grant-date fair value of options granted under the Plans during the years ended December 31, 2011, 2010 and 2009 was $5.51, $3.10 and $3.39, respectively. The total intrinsic value of options exercised during the years ended December 31, 2011, 2010 and 2009 was $345, $0 and $41, respectively.
The options granted under the Plans, described above, include 33, 113, 38, 75, 145 and 113 non-qualified stock options granted to our CSO on July 1, 2002, 2003, 2004 and 2005, on July 3, 2006 and on July 2, 2007, respectively, which cliff vest after nine years and 11 months from the respective grant date. The July 1, 2002, 2003 and 2005 awards have fully vested. Vesting of a defined portion of each award will occur earlier if a defined performance condition is achieved; more than one condition may be achieved in any period. In accordance with ASC 718 Compensation – Stock Compensation, at the end of each reporting period, we will estimate the probability of achievement of each performance condition and will use
those probabilities to determine the requisite service period of each award. The requisite service period for the award is the shortest of the explicit or implied service periods. In the case of the CSO’s options, the explicit service period is nine years and 11 months from the respective grant dates. The implied service periods related to the performance conditions are the estimated times for each performance condition to be achieved. Thus, compensation expense will be recognized over the shortest estimated time for the achievement of performance conditions for that award (assuming that the performance conditions will be achieved before the cliff vesting occurs). On July 1, 2008, 2009 and 2010, we granted awards to our CSO (consisting of options in 2010 and 2009 and options and restricted stock in 2008) and to our CEO (consisting of options in 2010) which vest on the basis of the
achievement of specified performance or market-based milestones. The options have an exercise price equal to the closing price of our common stock on the date of grant. The awards are valued using a Monte Carlo simulation model. On July 1, 2011, we granted an option award to our CEO which vests on the basis of the achievement of specified performance-based milestones. The option has an exercise price equal to the closing price of our common stock on the date of grant. The award is valued using the Black-Scholes option pricing model. The expense related to the grants with performance and market-based milestones will be recognized over the shortest estimated time for the achievement of the performance or market conditions. The awards will not vest unless one of the milestones is achieved or the market condition is met. Changes in the estimate of probability of achievement of any
performance or market condition will be reflected in compensation expense of the period of change and future periods affected by the change.
At December 31, 2011, the estimated requisite service periods for the 2004, 2006, 2008, 2009, 2010 and 2011 awards, described above, were 2.5, 4.5, 1.75, 3.25, 3.25 and 3.0 years, respectively. For the years ended December 31, 2011, 2010 and 2009, the total compensation expense recognized for the performance-based options was $0.4 million, $1.1 million and $0.5 million, respectively.
F-22
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
A summary of the status of our outstanding restricted stock awarded under the Plans which has not yet vested as of December 31, 2011 and changes during the year then ended is presented below:
|
Restricted Stock Awards
|
Shares
|
Weighted
Average
Grant-Date
Fair Value
|
||||||
|
Nonvested at January 1, 2011
|
341 | $ | 9.46 | |||||
|
Granted
|
- | - | ||||||
|
Vested
|
(205 | ) | 11.05 | |||||
|
Forfeited
|
(38 | ) | 6.29 | |||||
|
Nonvested at December 31, 2011
|
98 | $ | 7.40 | |||||
Our two employee stock purchase plans (the Purchase Plans), the 1998 Employee Stock Purchase Plan (the Qualified Plan) and the 1998 Non-Qualified Employee Purchase Plan (the Non-Qualified Plan), as amended, provided for the issuance of up to 4,400 and 1,100 shares of common stock, respectively. Issuances of common stock under the Purchase Plans were terminated by the Company during the second quarter of 2011 and provided for the grant to all employees of options to use an amount equal to 25% of their quarterly compensation, as such percentage was determined by the Board of Directors prior to the date of grant, to purchase shares of our common stock at a price per share equal to the lesser of the fair market value of
the common stock on the date of grant or 85% of the fair market value on the date of exercise. Options were granted automatically on the first day of each fiscal quarter and expired six months after the date of grant. The Qualified Plan was not available to employees owning more than five percent of the common stock and imposed certain other quarterly limitations on the option grants. Options under the Non-Qualified Plan were granted to the extent that option grants were restricted under the Qualified Plan.
The fair value of shares purchased under the Purchase Plans was estimated on the date of grant in accordance with ASC 718 Compensation – Stock Compensation, via the same option valuation model used for options granted under the Plans, but with the following assumptions during 2011, 2010 and 2009:
|
2011
|
2010
|
2009
|
||||
|
Expected volatility
|
43% – 51%
|
45% – 72%
|
46% – 100%
|
|||
|
Expected dividends
|
zero
|
zero
|
zero
|
|||
|
Expected term
|
6 months
|
6 months
|
6 months
|
|||
|
Risk-free rate
|
0.06% – 0.22%
|
0.11% – 0.18%
|
0.00% – 0.38%
|
Purchases of common stock under the Purchase Plans during the years ended December 31, 2011, 2010 and 2009 are summarized as follows:
F-23
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
|
Qualified Plan
|
Non-Qualified Plan
|
||||||||||
|
Shares
Purchased
|
Price Range
|
Weighted
Average
Grant-Date
Fair Value
|
Shares
Purchased
|
Price Range
|
Weighted
Average
Grant-Date
Fair Value
|
||||||
|
2011
|
428
|
$4.62 – $5.65
|
$0.88
|
162
|
$4.62 – $5.65
|
$0.84
|
|||||
|
2010
|
802
|
$3.50 – $4.56
|
$0.94
|
208
|
$3.75 – $4.56
|
$0.96
|
|||||
|
2009
|
872
|
$3.37 – $9.13
|
$1.49
|
189
|
$3.98 – $9.13
|
$1.58
|
|||||
The total compensation expense of shares, granted to both employees and non-employees, under all of our share-based payment arrangements that was recognized in operations during the years ended December 31, 2011, 2010 and 2009 was:
|
2011
|
2010
|
2009
|
||||||||||
|
Recognized as:
|
||||||||||||
|
Research and Development
|
$ | 4,499 | $ | 5,091 | $ | 7,225 | ||||||
|
General and Administrative
|
1,863 | 4,424 | 5,761 | |||||||||
|
Total
|
$ | 6,362 | $ | 9,515 | $ | 12,986 | ||||||
No tax benefit was recognized related to such compensation cost because of the Company’s net operating losses and the related deferred tax assets were fully offset by valuation allowance. Accordingly, no amounts related to windfall tax benefits have been reported in cash flows from operations or cash flows from financing activities for the periods presented.
As of December 31, 2011, there was $6.6 million and $0.3 million of total unrecognized compensation cost related to non-vested stock options under the Plans and non-vested restricted shares, respectively. Those costs are expected to be recognized over weighted average periods of 2.4 years and 1.4 years, respectively. Cash received from exercises under all share-based payment arrangements for the year ended December 31, 2011 was $3.7 million. We issue new shares of our common stock upon share option exercise and share purchase.
In applying the treasury stock method for the calculation of diluted EPS, amounts of unrecognized compensation expense and windfall tax benefits are required to be included in the assumed proceeds in the denominator of the diluted EPS calculation unless they are anti-dilutive. We reported net income for the year ended December 31, 2011 and included the dilutive effect of unrecognized compensation expense in the assumed proceeds in the denominator of the diluted EPS calculation. We incurred net losses for the years ended December 31, 2010 and 2009 and, therefore, such amounts have not been included in the calculations for those periods since they would be anti-dilutive. As a result, basic and diluted EPS are the same
for the 2010 and 2009 periods. We have made an accounting policy decision to calculate windfall tax benefits/shortfalls, for purposes of diluted EPS calculation, excluding the impact of deferred tax assets. This policy decision will apply when we have net income and windfall tax benefits/shortfalls are realizable.
11. Employee Savings Plan
The terms of the amended and restated Progenics Pharmaceuticals 401(k) Plan (the Amended Plan), among other things, allow eligible employees to participate in the Amended Plan by electing to contribute to the Amended Plan a percentage of their compensation to be set aside to pay their future retirement benefits. During the three years ended December 31, 2011, we matched 50% of those employee contributions that are equal to 5%-8% of compensation and are made by eligible employees to the Amended Plan (the Matching Contribution). In addition, we may also make a discretionary contribution each year on behalf of all participants who are non-highly compensated employees. We made Matching Contributions of approximately
$597, $594 and $718 to the Amended Plan for the years ended December 31, 2011, 2010 and 2009, respectively. No discretionary contributions were made during those years.
F-24
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
12. Income Taxes
We account for income taxes using the liability method in accordance with ASC 740 Income Taxes. Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes.
There is no provision or benefit for federal or state income taxes for the years ended December 31, 2011, 2010 or 2009 other than a federal tax refund of $95 we received in 2010 from new legislation permitting the carryback of net operating losses (NOLs) to 2005 as well as permitting the suspension of limitations on alternative minimum tax NOL utilization. We have completed a calculation through March 31, 2011, under Internal Revenue Code Section 382, the results of which indicate that past ownership changes will limit utilization of NOLs in the future. Ownership changes subsequent to March 31, 2011, may further limit the future utilization of net operating loss and tax credit carry-forwards as defined by the
federal and state tax codes.
Deferred tax assets as of December 31, 2011 and 2010, consisted of the following:
|
2011
|
2010
|
|||||||
|
Depreciation and amortization
|
$ | 6,865 | $ | 6,881 | ||||
|
R&E tax credit carry-forwards
|
11,966 | 11,543 | ||||||
|
NYS investment tax credit carry-forwards
|
1,088 | 1,076 | ||||||
|
AMT credit carry-forwards
|
211 | 211 | ||||||
|
Net operating loss carry-forwards
|
85,110 | 95,848 | ||||||
|
Capitalized research and development expenditures
|
43,113 | 35,818 | ||||||
|
Stock compensation
|
13,789 | 13,942 | ||||||
|
Other items
|
3,600 | 2,776 | ||||||
| 165,742 | 168,095 | |||||||
|
Valuation allowance
|
(165,742 | ) | (168,095 | ) | ||||
| $ | - | $ | - | |||||
We do not recognize deferred tax assets considering our history of taxable losses and the uncertainty regarding our ability to generate sufficient taxable income in the future to utilize these deferred tax assets. For the year ended December 31, 2011, we had income for tax purposes and such amount was offset completely by our available net operating loss carry-forwards. For the year ended December 31, 2010, we incurred net losses for tax purposes. We recognized a full tax valuation against deferred taxes at December 31, 2011 and 2010.
The following is a reconciliation of income taxes computed at the Federal statutory income tax rate to the actual effective income tax provision during 2011, 2010 and 2009:
|
2011
|
2010
|
2009
|
||||
|
U.S. Federal statutory rate
|
35.0%
|
(34.0)%
|
(34.0)%
|
|||
|
State income taxes, net of Federal benefit
|
8.0
|
(5.1)
|
(4.6)
|
|||
|
Research and experimental tax credit
|
(4.1)
|
(1.7)
|
(4.0)
|
|||
|
Change in valuation allowance
|
(22.6)
|
38.2
|
40.4
|
|||
|
Effect of federal tax rate bracket change on valuation allowance
|
(34.8)
|
-
|
-
|
|||
|
Equity compensation
|
17.0
|
2.3
|
6.3
|
|||
|
Investment tax credit
|
(0.1)
|
0.1
|
(3.8)
|
|||
|
Other
|
1.6
|
0.1
|
(0.3)
|
|||
|
Income tax provision (benefit)
|
0.0%
|
(0.1)%
|
0.0%
|
As of December 31, 2011, we had available, for tax return purposes, unused NOLs of approximately $228.8 million, which will expire in various years from 2021 to 2030, $18.2 million of which were generated from deductions that, when realized, will reduce taxes payable and will increase paid-in-capital and are not reflected in our deferred tax assets above. Additionally, $11.4 million of the valuation allowance relates to NOLs attributable to excess tax deductions for equity compensation. When realized this will also be reflected as an increase to paid-in-capital.
F-25
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
We have reviewed our nexus in various tax jurisdictions and our tax positions related to all open tax years for events that could change the status of our ASC 740 Income Taxes liability, if any, or require an additional liability to be recorded. Such events may be the resolution of issues raised by a taxing authority, expiration of the statute of limitations for a prior open tax year or new transactions for which a tax position may be deemed to be uncertain. During the years ended December 31, 2011, 2010 and 2009, we had no unrecognized tax benefits resulting from tax positions during a prior or current period, settlements with taxing authorities or the expiration of the applicable statute of limitations. At
December 31, 2011, there were no amounts of unrecognized tax benefits that, if recognized, would affect the effective tax rate and there were no tax positions for which it is reasonably possible that the total amounts of unrecognized tax benefits will significantly increase or decrease within twelve months from the respective date. As of December 31, 2011, we are subject to federal and state income tax in the United States. Open tax years relate to years in which unused net operating losses were generated or, if used, for which the statute of limitation for examination by taxing authorities has not expired. Our open tax years extend back to 1995, with the exception of 1997, during which we reported net income. No amounts of interest or penalties were recognized in our Consolidated Statements of Operations or Consolidated Balance Sheets as of and for the years ended December 31, 2011,
2010 and 2009.
Our research and experimental (R&E) tax credit carry-forwards of approximately $12.0 million at December 31, 2011 expire in various years from 2012 to 2031. During the year ended December 31, 2011, research and experimental tax credit carry-forwards of approximately $81 expired.
13. Net Income (Loss) Per Share
Our basic net income (loss) per share amounts have been computed by dividing net income (loss) by the weighted-average number of common shares outstanding during the period. For the year ended December 31, 2011, we reported net income, and the computation of diluted earnings per share is based upon the weighted-average number of our common shares and dilutive effect, determined using the treasury stock method, of potential common shares outstanding. As of December 31, 2011, our 98 shares of unvested restricted stock outstanding have non-forfeitable rights to dividends. The allocation of 2011 net income to these participating securities pursuant to the two-class method is not material to both basic and diluted
earnings per share. For the years ended December 31, 2010 and 2009, we reported net losses and, therefore, potential common shares were not included since such inclusion would have been anti-dilutive. The calculations of net loss per share, basic and diluted, are as follows:
|
Net Income (Loss)
(Numerator)
|
Weighted Average
Common Shares
(Denominator)
|
Per Share
Amount
|
|||||
|
2011:
|
|||||||
|
Basic
|
$ 10,381
|
33,375
|
$ 0.31
|
||||
|
Dilutive effect of stock options
|
-
|
66
|
|||||
|
Dilutive effect of restricted stock
|
-
|
53
|
|||||
|
Diluted
|
$ 10,381
|
33,494
|
$ 0.31
|
||||
|
2010:
|
|||||||
|
Basic and diluted
|
$ (69,725
|
)
|
32,590
|
$ (2.14
|
)
|
||
|
2009:
|
|||||||
|
Basic and diluted
|
$ (30,612
|
)
|
31,219
|
$ (0.98
|
)
|
During 2011, 2010 and 2009, anti-dilutive common shares excluded from diluted per share amounts consist of the following:
|
2011
|
2010
|
2009
|
||||||
|
Weighted
Average
Number
|
Weighted
Average
Exercise Price
|
Weighted
Average
Number
|
Weighted
Average
Exercise Price
|
Weighted
Average
Number
|
Weighted
Average
Exercise Price
|
|||
|
Options
|
4,543
|
$ 14.92
|
5,037
|
$ 15.17
|
4,705
|
$ 17.48
|
||
|
Restricted stock
|
45
|
45
|
34
|
|||||
|
Total
|
4,588
|
5,082
|
4,739
|
|||||
F-26
PROGENICS PHARMACEUTICALS, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS ¾ continued
(amounts in thousands, except per share amounts or as otherwise noted)
14. Unaudited Quarterly Results (unaudited)
Summarized quarterly financial data during 2011 and 2010 are as follows:
|
2011 Quarter Ended
|
||||||||||||||||
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||||
|
Revenues
|
$ | 2,388 | $ | 74,407 | $ | 5,804 | $ | 2,197 | ||||||||
|
Net income (loss)
|
(22,927 | ) | 55,486 | (11,432 | ) | (10,746 | ) | |||||||||
|
Net income (loss) per share - basic
|
(0.69 | ) | 1.66 | (0.34 | ) | (0.32 | ) | |||||||||
|
Net income (loss) per share - diluted
|
(0.69 | ) | 1.64 | (0.34 | ) | (0.32 | ) | |||||||||
|
2010 Quarter Ended
|
||||||||||||||||
|
March 31
|
June 30
|
September 30
|
December 31
|
|||||||||||||
|
Revenues
|
$ | 1,523 | $ | 2,305 | $ | 1,967 | $ | 2,157 | ||||||||
|
Net loss
|
(18,583 | ) | (15,241 | ) | (17,101 | ) | (18,800 | ) | ||||||||
|
Net loss per share - basic and diluted
|
(0.58 | ) | (0.47 | ) | (0.52 | ) | (0.57 | ) | ||||||||
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
PROGENICS PHARMACEUTICALS, INC.
|
||
|
By:
|
/s/ MARK R. BAKER
|
|
|
Mark R. Baker
(Chief Executive Officer and Director)
|
||
Date: March 14, 2012
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant in the capacities and on the dates indicated.
|
Signature
|
Capacity
|
Date
|
|
|
/s/ PETER J. CROWLEY
|
Chairman
|
March 14, 2012
|
|
|
Peter J. Crowley
|
|||
|
/s/ PAUL J. MADDON
|
Vice Chairman, Chief Science
Officer and Director
|
March 14, 2012
|
|
|
Paul J. Maddon, M.D., Ph.D.
|
|||
|
/s/ MARK R. BAKER
|
Chief Executive Officer and Director
(Principal Executive Officer)
|
March 14, 2012
|
|
|
Mark R. Baker
|
|||
|
/s/ CHARLES A. BAKER
|
Director
|
March 14, 2012
|
|
|
Charles A. Baker
|
|||
|
/s/ KURT W. BRINER
|
Director
|
March 14, 2012
|
|
|
Kurt W. Briner
|
|||
|
/s/ MARK F. DALTON
|
Director
|
March 14, 2012
|
|
|
Mark F. Dalton
|
|||
|
/s/ STEPHEN P. GOFF
|
Director
|
March 14, 2012
|
|
|
Stephen P. Goff, Ph.D.
|
|||
|
/s/ DAVID A. SCHEINBERG
|
Director
|
March 14, 2012
|
|
|
David A. Scheinberg, M.D., Ph.D.
|
|||
|
/s/ NICOLE S. WILLIAMS
|
Director
|
March 14, 2012
|
|
|
Nicole S. Williams
|
|||
|
/s/ ROBERT A. MCKINNEY
|
Chief Financial Officer, Senior Vice President,
Finance & Operations
(Principal Financial and Accounting Officer)
|
March 14, 2012
|
|
|
Robert A. McKinney
|
EXHIBIT INDEX
|
Exhibit
|
||
|
Number *
|
Description
|
|
|
3.1(23)
|
Amended and Restated Certificate of Incorporation of the Registrant.
|
|
|
3.2(12)
|
Amended and Restated By-laws of the Registrant.
|
|
|
4.1(1)
|
Specimen Certificate for Common Stock, $0.0013 par value per share, of the Registrant.
|
|
|
10.1(1)
|
Form of Registration Rights Agreement.
|
|
|
10.2(1)
|
1989 Non-Qualified Stock Option Plan‡
|
|
|
10.3(1)
|
1993 Stock Option Plan, as amended‡
|
|
|
10.4(1)
|
1993 Executive Stock Option Plan‡
|
|
|
10.5(3)
|
Amended and Restated 1996 Stock Incentive Plan‡
|
|
|
10.6(12)
|
2005 Stock Incentive Plan‡
|
|
|
10.6.1(8)
|
Form of Non-Qualified Stock Option Award Agreement‡
|
|
|
10.6.2(8)
|
Form of Restricted Stock Award Agreement‡
|
|
|
10.6.3(14)
|
Amended 2005 Stock Incentive Plan ‡
|
|
|
10.6.4(16)
|
Form of Non-Qualified Stock Option Award Agreement ‡
|
|
|
10.6.5(16)
|
Form of Restricted Stock Award Agreement ‡
|
|
|
10.7(13)
|
Form of Indemnification Agreement‡
|
|
|
10.8(17)
|
Employment Agreement, dated December 31, 2007, between the Registrant and Dr. Paul J. Maddon‡
|
|
|
10.8.1(22)
|
First Amendment to Employment Agreement, dated March 31, 2011, between the Registrant and Dr. Paul J. Maddon‡
|
|
|
10.9(1)
|
Letter dated August 25, 1994 between the Registrant and Dr. Robert J. Israel‡
|
|
|
10.10(6)
|
Amended 1998 Employee Stock Purchase Plan‡
|
|
|
10.11(6)
|
Amended 1998 Non-qualified Employee Stock Purchase Plan‡
|
|
|
10.16(2)†
|
Development and License Agreement, dated April 30, 1999, between Protein Design Labs, Inc. and the Registrant.
|
|
|
10.16.1(9)
|
Letter Agreement, dated November 24, 2003, relating to the Development and License Agreement between Protein Design Labs, Inc. and the Registrant.
|
|
|
10.18(4)
|
Director Stock Option Plan‡
|
|
|
10.19(5)†
|
Exclusive Sublicense Agreement, dated September 21, 2001, between the Registrant and UR Labs, Inc.
|
|
|
10.19.1(7)
|
Amendment to Exclusive Sublicense Agreement, dated September 21, 2001, between the Registrant and UR Labs, Inc.
|
|
|
10.21.1
|
Amended and Restated Agreement of Lease, dated October 28, 2009, between BMR-Landmark at Eastview LLC and the Registrant.
|
|
|
10.23
|
Information concerning compensation of the Registrant’s non-employee directors is included in the Registrant’s proxy material for its 2011 Annual Meeting of Stockholders and is incorporated herein by reference.‡
|
|
|
10.25(10) †
|
Option and License Agreement, dated May 8, 1985, by and between the University of Chicago and UR Labs, Inc., as amended by (i) Amendment to Option and License Agreement, dated September 17, 1987, by and between the University of Chicago and UR Labs, Inc., (ii) Second Amendment to Option and License Agreement, dated March 3, 1989, by and among the University of Chicago, ARCH Development Corporation and UR Labs, Inc., and (iii) Letter Agreement Related to Progenics’ RELISTOR In-License dated, December 22, 2005, by and among the University of Chicago, acting on behalf of itself and ARCH Development Corporation, the Registrant, Progenics Pharmaceuticals Nevada, Inc. and Wyeth, acting through
its Wyeth Pharmaceuticals Division.
|
|
|
10.26(11)
|
Membership Interest Purchase Agreement, dated April 20, 2006, between the Registrant Inc. and Cytogen Corporation.
|
|
|
10.27(11) †
|
Amended and Restated PSMA/PSMP License Agreement, dated April 20, 2006, by and among the Registrant, Cytogen Corporation and PSMA Development Company LLC.
|
|
|
10.28(15)
|
Consulting Agreement, dated May 1, 1995, between Active Biotherapies, Inc. and Dr. David A. Scheinberg, M.D., Ph.D., as amended on June 13, 1995, as assigned to the Registrant, and as amended on January 1, 2001‡
|
|
|
10.29(18) †
|
License Agreement, dated as of October 16, 2008, by and among Ono Pharmaceutical Co., Ltd. and the Registrant.
|
|
|
10.30(18) †
|
Partial Termination and License Agreement, dated October 16, 2008, by and among Wyeth, acting through Wyeth Pharmaceuticals Division, Wyeth-Whitehall Pharmaceuticals, Inc. and Wyeth-Ayerst Lederle, Inc. and the Registrant and Progenics Pharmaceuticals Nevada, Inc.
|
|
10.31(18) †
|
Consent, Acknowledgment and Agreement, dated as of October 16, 2008, by and among Wyeth, acting through Wyeth Pharmaceuticals Division, Wyeth-Whitehall Pharmaceuticals, Inc. and Wyeth-Ayerst Lederle, Inc., the Registrant and Ono Pharmaceutical Co., Ltd.
|
|
|
10.32(18) †
|
2008 Agreement Related to Progenics’ MNTX In-License, dated October 16, 2008, by and among the University of Chicago, acting on behalf of itself and ARCH Development Corporation, the Registrant, Progenics Pharmaceuticals Nevada, Inc. and Ono Pharmaceutical Co., Ltd.
|
|
|
10.33(19) †
|
Termination and Transition Agreement, effective as of October 1, 2009, by and among Wyeth, acting through Wyeth Pharmaceuticals Division, Wyeth-Whitehall Pharmaceuticals, Inc., Wyeth-Ayerst Lederle, Inc., and AHP Manufacturing B.V., and the Registrant, Progenics Pharmaceuticals Nevada, Inc. and Excelsior Life Sciences Ireland Limited.
|
|
|
10.33.1(21) †
|
First Amendment to Termination and Transition Agreement, effective as of October 1, 2010, by and among Wyeth LLC, acting through Wyeth Pharmaceuticals Division, Wyeth-Whitehall Pharmaceuticals LLC, Wyeth-Ayerst Lederle LLC, and AHP Manufacturing B.V., and the Registrant, Progenics Pharmaceuticals Nevada, Inc. and Excelsior Life Sciences Ireland Limited.
|
|
|
10.34(20) †
|
Collaboration Agreement, effective June 14, 2005, by and between Seattle Genetics, Inc. and PSMA Development Company, LLC.
|
|
|
10.35(20) †
|
Collaboration Agreement, effective February 21, 2001, by and between Abgenix, Inc. and PSMA Development Company, LLC.
|
|
|
10.36(20) †
|
License Agreement, effective September 5, 2001, by and between AlphaVax Human Vaccines, Inc. and PSMA Development Company, LLC.
|
|
|
10.37(22) †
|
License Agreement dated as of February 3, 2011, by and between Salix Pharmaceuticals, Inc., the Registrant, Progenics Pharmaceuticals Nevada, Inc. and Excelsior Life Sciences Ireland Limited.
|
|
|
10.37.1(22) †
|
2010 Agreement Related to Progenics’ MNTX In-License, dated February 3, 2011, by and among the University of Chicago, acting on behalf of itself and ARCH Development Corporation, the Registrant, Progenics Pharmaceuticals Nevada, Inc. and Salix Pharmaceuticals, Inc.
|
|
|
12.1
|
Statement re computation of ratio of earnings (loss) to combined fixed charges and preferred stock dividends.
|
|
|
21.1(17)
|
Subsidiaries of the Registrant.
|
|
|
23.1
|
Consent of PricewaterhouseCoopers LLP.
|
|
|
31.1
|
Certification of Mark R. Baker, Chief Executive Officer of the Registrant pursuant to 13a-14(a) and Rule 15d-14(a) under the Securities Exchange Act of 1934, as amended.
|
|
|
31.2
|
Certification of Robert A. McKinney, Chief Financial Officer, Senior Vice President, Finance and Operations of the Registrant pursuant to 13a-14(a) and Rule 15d-14(a) under the Securities Exchange Act of 1934, as amended.
|
|
|
32.1
|
Certification of Mark R. Baker, Chief Executive Officer of the Registrant pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
32.2
|
Certification of Robert A. McKinney, Chief Financial Officer, Senior Vice President, Finance and Operations of the Registrant pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
*
|
Exhibits footnoted as previously filed have been filed as an exhibit to the document of the Registrant referenced in the footnote below, and are incorporated by reference herein.
|
|
|
(1)
|
Previously filed in Registration Statement on Form S-1, Commission File No. 333-13627.
|
|
|
(2)
|
Previously filed in Quarterly Report on Form 10-Q for the quarterly period ended June 30, 1999.
|
|
|
(3)
|
Previously filed in Registration Statement on Form S-8, Commission File No. 333-120508.
|
|
|
(4)
|
Previously filed in Annual Report on Form 10-K for the year ended December 31, 1999.
|
|
|
(5)
|
Previously filed in Annual Report on Form 10-K for the year ended December 31, 2002.
|
|
|
(6)
|
Previously filed in Registration Statement on Form S-8, Commission File No. 333-143671.
|
|
|
(7)
|
Previously filed in Current Report on Form 8-K filed on September 20, 2004.
|
|
|
(8)
|
Previously filed in Current Report on Form 8-K filed on July 8, 2008.
|
|
|
(9)
|
Previously filed in Annual Report on Form 10-K for the year ended December 31, 2004.
|
|
|
(10)
|
Previously filed in Annual Report on Form 10-K for the year ended December 31, 2005.
|
|
|
(11)
|
Previously filed in Quarterly Report on Form 10-Q for the quarterly period ending June 30, 2006.
|
|
|
(12)
|
Previously filed in Current Report on Form 8-K filed on May 13, 2005.
|
|
|
(13)
|
Previously filed in Quarterly Report on Form 10-Q for the quarterly period ending March 31, 2007.
|
|
|
(14)
|
Previously filed in Registration Statement on Form S-8, Commission File No. 333-143670.
|
|
|
(15)
|
Previously filed in Annual Report on Form 10-K/A for the year ended December 31, 2006.
|
|
|
(16)
|
Previously filed in Current Report on Form 8-K filed on July 8, 2008.
|
|
|
(17)
|
Previously filed in Annual Report on Form 10-K for the year ended December 31, 2007.
|
|
|
(18)
|
Previously filed in Annual Report on Form 10-K for the year ended December 31, 2008.
|
|
|
(19)
|
Previously filed in Annual Report on Form 10-K for the year ended December 31, 2009.
|
|
|
(20)
|
Previously filed in Amendment No. 2 to Annual Report on Form 10-K/A for the year ended December 31, 2009.
|
|
|
(21)
|
Previously filed in Annual Report on Form 10-K for the year ended December 31, 2010.
|
|
|
(22)
|
Previously filed in Quarterly Report on Form 10-Q for the quarter ended March 31, 2011.
|
|
|
(23)
|
Previously filed in Quarterly Report on Form 10-Q for the quarter ended June 30, 2011.
|
|
|
†
|
Confidential treatment granted as to certain portions omitted and filed separately with the Commission.
|
|
|
‡
|
Management contract or compensatory plan or arrangement.
|
|
E-3
