Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Vericel Corp | Financial_Report.xls |
| EX-23.1 - EX-23.1 - Vericel Corp | a11-31698_1ex23d1.htm |
| EX-21 - EX-21 - Vericel Corp | a11-31698_1ex21.htm |
| EX-31.2 - EX-31.2 - Vericel Corp | a11-31698_1ex31d2.htm |
| EX-32.1 - EX-32.1 - Vericel Corp | a11-31698_1ex32d1.htm |
| EX-31.1 - EX-31.1 - Vericel Corp | a11-31698_1ex31d1.htm |
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-K
|
x |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
for the fiscal year ended December 31, 2011
or
|
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File Number 0-22025
Aastrom Biosciences, Inc.
(Exact name of registrant as specified in its charter)
|
Michigan |
|
94-3096597 |
|
(State or other jurisdiction of |
|
(I.R.S. Employer |
|
incorporation or organization) |
|
Identification No.) |
24 Frank Lloyd Wright Drive,
P. O. Box 376,
Ann Arbor, MI 48106
(Address of principal executive offices, including zip code)
Registrant’s telephone number, including area code: (800) 556-0311
Securities registered pursuant to Section 12(b) of the Act:
|
|
Title of Class |
|
Name of Each Exchange on Which Registered |
|
|
|
Common Stock (No par value) |
|
The NASDAQ Stock Market, Inc. |
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes o No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer - o |
|
Accelerated filer - x |
|
|
|
|
|
Non-accelerated filer - o |
|
Smaller reporting company - o |
|
(Do not check if a smaller reporting company) |
|
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No x
The aggregate market value of the registrant’s Common Stock, no par value (“Common Stock”), held by non-affiliates of the registrant (based on the closing sales price of the Common Stock as reported on the NASDAQ Capital Market) on June 30, 2011 was approximately $105,712,991. This computation excludes shares of Common Stock held by directors, officers and each person who holds 5% or more of the outstanding shares of Common Stock, since such persons may be deemed to be affiliates of the registrant. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
As of March 13, 2012, 38,755,642 shares of Common Stock, no par value, were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
|
Document |
|
Form 10-K Reference |
|
Proxy Statement for the Annual Meeting of Shareholders scheduled for May 3, 2012 |
|
Items 10, 11, 12, 13 and 14 of Part III |
AASTROM BIOSCIENCES, INC.
ANNUAL REPORT ON FORM 10-K
|
|
|
Page |
|
|
1 | |
|
1 | ||
|
13 | ||
|
22 | ||
|
22 | ||
|
23 | ||
|
23 | ||
|
|
24 | |
|
24 | ||
|
27 | ||
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations |
28 | |
|
37 | ||
|
38 | ||
|
Changes in and Disagreements With Accountants on Accounting and Financial Disclosure |
54 | |
|
54 | ||
|
55 | ||
|
|
56 | |
|
56 | ||
|
56 | ||
|
Security Ownership of Certain Beneficial Owners and Management, and Related Shareholder Matters |
56 | |
|
Certain Relationships and Related Transactions, and Director Independence |
56 | |
|
56 | ||
|
|
57 | |
|
57 | ||
|
58 | ||
|
59 | ||
|
62 | ||
Except for the historical information presented, the matters discussed in this Report, including our product development and commercialization goals and expectations, our plans and anticipated timing and results of clinical development activities, potential market opportunities, revenue expectations and the potential advantages and applications of our products and product candidates under development, include forward-looking statements that involve risks and uncertainties. Our actual results may differ significantly from the results discussed in the forward-looking statements. Factors that could cause or contribute to such differences include, but are not limited to, those discussed under the caption “Risk Factors.” Unless the context requires otherwise, references to “we,” “us,” “our” and “Aastrom” refer to Aastrom Biosciences, Inc.
Change in Fiscal Year End
In 2010, our Board of Directors approved the change in our fiscal year end from June 30 to December 31. The change became effective at the end of the quarter ended December 31, 2010. All references to “years”, unless otherwise noted, refer to the 12-month fiscal year, which prior to July 1, 2010, ended on June 30, and beginning with December 31, 2010, ends on December 31, of each year.
General Information
We were incorporated in 1989 and are a regenerative medicine company focused on the development of innovative cell therapies to repair or regenerate damaged or diseased tissues. We are developing patient-specific, expanded multicellular therapies for use in the treatment of severe, chronic ischemic cardiovascular diseases. We believe ixmyelocel-T (the new generic name approved by the U.S. Food and Drug Administration (FDA) and United States Adopted Names (USAN) Council in March 2011 for our multicellular therapy) is a disease modifying therapy with multi-functional properties including: tissue remodeling, immuno-modulation and the promotion of angiogenesis. Our proprietary cell-manufacturing technology enables the manufacture of multicellular therapies, expanded from an adult’s own bone marrow, and delivered directly to damaged tissues. Preclinical and clinical data suggest that ixmyelocel-T may be effective in treating patients with severe, chronic ischemic cardiovascular diseases such as CLI. Preliminary data utilizing ixmyelocel-T in dilated cardiomyopathy (DCM) have provided indications of efficacy and safety. Nearly 200 patients have been treated in recent clinical trials using ixmyelocel-T (over 400 patients safely treated since our inception). We recently released positive Phase 2b data from our RESTORE-CLI trial and launched our pivotal Phase 3 REVIVE trial in CLI in February 2012. We also plan to start a randomized, placebo-controlled, double-blinded Phase 2b trial in DCM by mid-2012.
Our Therapy
Ixmyelocel-T is a patient specific, expanded multicellular therapy developed using our proprietary, automated processing system. Ixmyelocel-T is a product derived from an adult’s own bone marrow but it is significantly enhanced compared with the original bone marrow. Our process enhances the patient’s bone marrow mononuclear cells by expanding the mesenchymal stromal cells and alternatively activated macrophages while retaining many of the hematopoietic cells. The manufacture of our patient specific, expanded multicellular therapies is done under current Good Manufacturing Practices (cGMP) and current Good Tissue Practices (cGTP) guidelines required by the FDA.
Our therapy has several features that we believe are primarily responsible for success in treating adult patients with severe, chronic cardiovascular diseases:
Patient specific (autologous) — we start with the patient’s own cells, which are accepted by the patient’s immune system allowing the cells to integrate into existing functional tissues. This characteristic of our therapy, we believe, eliminates both the risk of rejection and the risk of having to use immunosuppressive therapy pre- or post-therapy. Our data also suggests that ixmyelocel-T provides the potential for long-term engraftment and tissue repair.
Expanded — we begin with a small amount of bone marrow from the patient (up to 60 ml) and significantly expand the number of certain cell types, primarily CD90+ (mesenchymal stromal cells or MSCs) and CD14+auto+ (alternatively activated
macrophages) to far more than are present in the patient’s own bone marrow (up to 200 times the number of certain cell types compared with the starting bone marrow aspirate). Ixmyelocel-T is derived from the patient’s own bone marrow but it is significantly enhanced compared with the starting bone marrow.
Multicellular — we believe the multiple cell types in ixmyelocel-T, which are normally only found in bone marrow but in smaller quantities, possess the key functions required for tissue remodeling, immuno-modulation and the promotion of angiogenesis.
Minimally invasive — our procedure for taking bone marrow (an “aspirate”) can be performed in an out-patient setting and takes approximately 15 minutes. For diseases such as CLI, the administration of ixmyelocel-T is performed in an out-patient setting (e.g. a physician’s office) in a one-time, approximately 20 minute procedure.
Safe — bone marrow and bone marrow-derived therapies have been used safely and efficaciously in medicine for over three decades. Our product, ixmyelocel-T, a bone marrow-derived, patient specific, expanded multicellular therapy leverages this body of scientific study and medical experience.
Our therapy is produced at our cell manufacturing facility in the United States, located at our headquarters in Ann Arbor, Michigan.
The following graphic summarizes the treatment process:
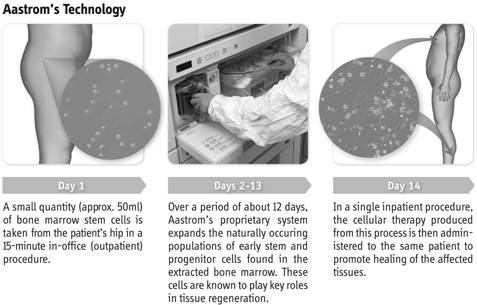
Clinical Development Programs
Our clinical development programs are focused on addressing areas of high unmet medical needs in severe, chronic ischemic cardiovascular diseases. We have completed a successful Phase 2b clinical trial in CLI. We have reached agreement with the FDA on CMC which has allowed us to launch our pivotal Phase 3 REVIVE clinical trial in the first quarter of 2012 with a protocol approved by FDA through the Special Protocol Assessment (SPA) process. Our CLI development program has also received Fast Track Designation from the FDA. We have completed our Phase 1/2 clinical trials in DCM and plan to begin a randomized, placebo-controlled, double-blinded Phase 2b trial by mid-2012. Our DCM development program has received Orphan Disease Designation from the FDA.
The following summarizes the status of our active clinical programs:

Results to date in our clinical trials may not be indicative of results obtained from subsequent patients enrolled in those trials or from future clinical trials. Further, our future clinical trials may not be successful or we may not be able to obtain the required Biologic License Application (BLA) approval to commercialize our products in the United States in a timely fashion, or at all. See “Risk Factors”.
Critical Limb Ischemia
Background
CLI is the most serious and advanced stage of peripheral arterial disease (PAD). PAD is a chronic atherosclerotic disease that progressively restricts blood flow in the limbs and can lead to serious medical complications. This disease is often associated with other serious clinical conditions including hypertension, cardiovascular disease, hyperlipidemia, diabetes, obesity and stroke. CLI is used to describe patients with the most severe forms of PAD: those with chronic ischemia-induced pain (even at rest) or tissue loss (ulcers or gangrene) in the limbs, often leading to amputation and death. Many CLI patients are considered “no option” patients as they have exhausted all other treatment options with the exception of amputation. The one-year and four-year mortality rates for no option CLI patients that progress to amputation are approximately 25% and 70%, respectively. Ixmyelocel-T, our disease modifying therapy with multiple functions, has shown significant promise in the treatment of CLI patients with existing tissue loss and no option for revascularization. Currently, there are an estimated 250,000 no option CLI patients in the U.S.
Phase 2b Clinical Program — RESTORE CLI
Our U.S. Phase 2b RESTORE-CLI program was a multi-center, randomized, double-blind, placebo-controlled clinical trial. This clinical trial was designed to evaluate the safety and efficacy of ixmyelocel-T in the treatment of patients with CLI and no option for revascularization. It was the largest multi-center, randomized, double-blind, placebo-controlled cellular therapy study ever conducted in no option CLI patients. We completed enrollment of this trial in February 2010 with a total of 86 patients at 18 sites across the United States, with the last patient treated in March 2010. These patients were followed for a period of 12 months after treatment. In addition to assessing the safety of our product, efficacy endpoints included time to first occurrence of treatment failure — the trial’s primary efficacy end-point — (defined as major amputation, all-cause mortality, doubling in wound surface area and de novo gangrene), amputation-free survival (defined as major amputation and all-cause mortality), major amputation rates, level of amputation, wound healing, patient quality of life and pain scores. The primary purpose of the trial was to assess performance of our therapy and, if positive, prepare for a Phase 3 program.
Results to date of the RESTORE-CLI trial have included two planned interim analyses and a final 12-month report:
· In June 2010, we reported interim results at the Society of Vascular Surgery Meeting. The interim analysis included the six-month results for the first 46 patients enrolled in the trial and twelve-month results for the first 30 patients enrolled in the
trial. Results of this analysis demonstrated that the study achieved both its primary safety endpoint and primary efficacy endpoint of time to first occurrence of treatment failure. The results related to the primary endpoint were statistically significant (p=0.0053). Analysis of the data for amputation free survival, a secondary efficacy endpoint which the study was not powered to demonstrate, were positive and showed a statistically significant reduction in event rates in favor of our therapy (p=0.038). Other endpoints measured (e.g., major amputation rate, complete wound healing, change in Wagner wound scale) showed encouraging trends, but did not reach statistical significance at the interim analysis.
· In November 2010, we presented six-month data on all 86 patients enrolled in the trial and twelve-month data on the first 72 patients at the VEITHsymposium™ non-CME satellite session. Results of this analysis showed that the study again achieved both its primary safety endpoint and primary efficacy endpoint of time to first occurrence of treatment failure. The findings related to time to first occurrence of treatment failure were statistically significant (p=0.0132). Further analyses showed a clinically meaningful reduction of 56% in treatment failure events. Analysis of the data for amputation-free survival, showed a clinically meaningful reduction in event rates of 24%, but did not show statistical significance (p=0.5541).
· In November 2011, the final 12-month data on all patients from the RESTORE-CLI trial were presented at the American Heart Association Scientific Sessions. Patients in the treatment arm showed a 62% reduction in risk relative to placebo in the primary efficacy endpoint of time to first occurrence of treatment failure (p=0.0032). While the study was not powered to show statistical significance in the secondary endpoint of amputation free survival, results from a subgroup of 45 patients with wounds at baseline (the approximate profile of the Phase 3 patient population) showed a positive trend in this measure (21% ixmyelocel-T treated vs 44% control event rate; p=0.0802). The study also met the primary safety endpoint with no meaningful differences between the treated and control groups.
Phase 3 Clinical Program — REVIVE
In February 2012, we began screening patients in the pivotal Phase 3 REVIVE clinical trial for patients with CLI and no option for revascularization. The first patient is expected to be randomized and aspirated in March 2012. Leading up to the launch of the REVIVE pivotal trial, we received Fast Track Designation from the FDA for use of ixmyelocel-T for CLI in October 2010 and reached agreement with the FDA on a Special Protocol Assessment (SPA) in July 2011. The Phase 3 REVIVE No Option Trial that we agreed to with the FDA under the SPA process includes 594 no option CLI patients with tissue loss (ulcers and gangrene) at baseline. Patients will be randomized 1:1 and followed for 12 months for the primary efficacy endpoint of amputation-free survival. Patients will be followed for an additional 6 months for safety. We anticipate that enrollment will occur at approximately 80 sites across the U.S.
Dilated Cardiomyopathy
Background
DCM is a severe, chronic cardiovascular disease that leads to enlargement of the heart, reducing the pumping function of the heart to the point that blood circulation is impaired. Patients with DCM typically present with symptoms of congestive heart failure, including limitations in physical activity and shortness of breath. It is currently estimated that there are approximately 125,000 ischemic DCM patients in the U.S. There are two types of DCM: ischemic and non-ischemic. Ischemic DCM, the most common form representing an estimated 60% of all DCM patients, is associated with atherosclerotic cardiovascular disease. Patient prognosis depends on the stage and cause of the disease but is typically characterized by a very poor quality of life and a high mortality rate.
Current treatments for DCM patients include both heart transplantation and left ventricular assist devices (LVADs). There are less than 2,500 heart transplantations in the U.S. each year, many DCM patients are not eligible, and they’re expensive at an estimated cost of over $750,000. LVADs are also expensive at an estimated cost of over $175,000 and have a mortality rate of 50% at 2 years.
In February 2007, the FDA granted Orphan Drug Designation to ixmyelocel-T for the treatment of DCM. Our DCM development program is currently in Phase 2. We recently completed follow up on two U.S. Phase 1/2 trials investigating surgical and catheter-based delivery for our product in the treatment of DCM in reporting stages. We plan to initiate a randomized, placebo-controlled, double-blinded Phase 2b trial using catheter delivery for 60 — 80 ischemic DCM patients in the U.S. in mid-2012.
Surgical Trial Program — DCM
We completed enrollment of 40 DCM patients in the IMPACT-DCM clinical trial in January 2010 and the final patient was treated in March 2010. Participants in the IMPACT-DCM clinical trial were required to have New York Heart Association (NYHA) functional class III or IV heart failure, a left ventricular ejection fraction (LVEF) of less than or equal to 30% (60-75% is typical for a healthy person), and meet other eligibility criteria, including optimized medical therapy. Patients were randomized in an approximate 3:1 ratio of treatment to control group. Patients in the treatment group received our therapy through direct injection into the heart muscle during minimally invasive-surgery (involving a chest incision of approximately 2 inches). The primary objective of this study was to assess the safety of ixmyelocel-T in patients with DCM. Efficacy measures include cardiac dimensions and tissue mass, cardiac function (e.g. cardiac output, LVEF, cardiopulmonary exercise testing parameters), cardiac perfusion and viability, as well as other efficacy endpoints. NYHA functional class and quality of life are also assessed. Patients were followed for 12 months after treatment.
Six-month data from the IMPACT-DCM interim analysis were presented at The Sixth International Conference on Cell Therapy for Cardiovascular Disease in January 2011. Results indicated that ixmyelocel-T is safe and showed that serious adverse events were associated with the surgical procedure and not the cellular therapy. Adverse events after the initial peri-operative period were roughly equal between the control and treatment groups. Efficacy findings include positive trends in clinical endpoints, quality of life, functional, and structural parameters in the treatment group as compared with the control group.
Twelve-month data on all 40 patients enrolled in the IMPACT-DCM trial were presented at the 15th Annual Heart Failure Society of America Scientific Meeting in September 2011. Results were consistent with the six-month interim analysis, indicating that ixmyelocel-T is safe and showed that serious adverse events were associated with the surgical procedure and not the therapy. Efficacy results were also consistent with the six-month results and demonstrated promising efficacy results in patients with ischemic DCM.
Catheter Trial Program — DCM
The Catheter-DCM clinical trial was designed to explore catheter-based direct injection delivery of ixmyelocel-T to treat DCM patients. This multi-center, randomized, controlled, prospective, open-label, Phase 2 study enrolled approximately 11 patients with ischemic DCM and 10 patients with non-ischemic DCM at clinical sites across the United States. Participants met the same criteria as stated above for the IMPACT-DCM surgical trial. The first patient was enrolled into the trial in April 2010 and enrollment concluded in December 2010 with 21 patients enrolled.
In September 2011, we reported results from a six-month interim analysis of patients treated in the Catheter-DCM Phase 2 trial. In this analysis, efficacy parameters were consistent with those seen in the IMPACT-DCM trial results. In addition, the adverse event profile suggests that catheter administration of ixmyelocel-T is safe and appears to cause fewer adverse events compared to surgical administration. We expect to report 12-month results from the Catheter-DCM Phase 2 trial in Q2 2012. We plan to launch a randomized, placebo-controlled, double-blind Phase 2b trial in mid-2012 in approximately 60 — 80 ischemic DCM patients in the U.S. and using catheter administration.
Production
Cell Manufacturing and Cell Production Components
We operate a centralized cell manufacturing facility in Ann Arbor, Michigan. The facility supports the current U.S. clinical trials and has sufficient capacity, with minor modifications, to supply our early commercialization requirements. We may establish and operate larger commercial-scale cell manufacturing facilities for the U.S. market in the future to accommodate potential market growth.
We have established relationships with manufacturers that are registered with the FDA as suppliers of medical products to produce various components of our patented cell manufacturing system.
We have established relationships with various third parties who manufacture and/or supply certain components, equipment, disposable devices and other materials used in our cell manufacturing process to develop our cell products, as well as our final
assemblies, component parts, subassemblies and associated spare parts used in the instrumentation platform of our cell production system.
There can be no assurance that we will be able to continue our present arrangements with our manufacturers and/or suppliers, supplement existing relationships or establish new relationships, or that we will be able to identify and obtain certain components, equipment, disposable devices, other materials, including ancillary materials that are necessary to develop our product candidates or that are used in our cell manufacturing and cell production components processes. Our dependence upon third parties for the supply and manufacture of such items could adversely affect our ability to develop and deliver commercially feasible cell products on a timely and competitive basis. See “Risk Factors.”
Our Arrangement with Vention Medical (formerly ATEK)
In October 2010, we entered into a contract manufacturing and supply agreement (Supply Agreement) with ATEK Medical, LLC (ATEK) for the manufacture of our proprietary cell cassette for use in our manufacturing process. In November 2011, ATEK was purchased by Vention Medical (Vention) and currently operates as a division of Vention. There have been no changes to the terms of the Supply Agreement as a result of this purchase.
Pursuant to the terms of the Supply Agreement, we have granted Vention the exclusive right to manufacture our proprietary cell cassette, which includes assembly, labeling, packaging and sterilization. Vention will be responsible for obtaining all of our approved components pertaining to the cassettes and we are obligated to order and purchase the cassettes from Vention on an agreed upon schedule and in agreed upon quantities. In addition, we will provide Vention with reasonable engineering support to initiate and ramp up manufacturing of the cassettes and will supply all manufacturing equipment.
The Supply Agreement has an initial term of four years and will terminate automatically without notice unless prior to that time the term is extended by mutual written consent delivered at least six months prior to the termination date. The minimum term extension is generally to be no less than two years.
The Supply Agreement provides that we may discontinue the manufacture of the cassettes at our sole discretion. In such event, we agree to use commercially best efforts to notify Vention at least 120 days prior to our intention to discontinue manufacture of the cassettes. Failure to provide such notice will not be a breach of the Supply Agreement, but without such notice, we agree to purchase from Vention (i) certain finished goods that are in usable condition and (ii) certain components or raw materials inventory or work in process in each case to the extent convertible into finished cassettes.
We or Vention may terminate the Supply Agreement if the other party materially defaults in the performance of any provision of the Supply Agreement and, should any such default occur, then the non-defaulting party may give written notice to the defaulting party that if the default is not cured within 45 days, the Supply Agreement will be terminated. If the non-defaulting party gives such notice and the default is not cured during the 45 day period, then the Supply Agreement shall automatically terminate at the end of such period unless an extension is mutually agreed to by Vention and us. In addition to other remedies, either party may terminate the Supply Agreement at any time if either of us breach our respective confidentiality obligations under the Supply Agreement, in which case termination shall be effective immediately upon receipt of notice from the non-breaching party of the breach and of termination. Either party may immediately terminate the Supply Agreement by written notice if the other party is or becomes insolvent, appoints or has appointed a receiver for all or substantially all of its assets, or makes an assignment for the benefit of its creditors. In addition, either party may terminate the Supply Agreement by written notice if the other party files a voluntary petition, or has filed against it an involuntary petition, for bankruptcy and such petition is not dismissed within 90 days.
Upon termination of the Supply Agreement, Vention agrees to provide reasonable technical support at Vention’s published engineering rates for the transfer of manufacturing technology to an alternative manufacturer chosen by us to conduct final manufacture, package and test of the cassettes in the event that Vention, for a period of 150 days from the date of receipt of the associated purchase order, is unable to manufacture all of our orders for any reason, or if Vention fails or refuses to meet our orders for cassettes pursuant to the terms of the Supply Agreement.
There can be no assurance that we will be able to continue our present arrangement with Vention. Our dependence upon our arrangement with Vention for the supply and manufacture of our proprietary cell cassette could adversely affect our ability to develop and deliver commercially feasible cell products on a timely and competitive basis. See “Risk Factors.”
Research & Development
Our therapy is produced from the patient’s bone marrow using Aastrom’s proprietary manufacturing system. The product is composed of a mixture of cell types normally found in bone marrow but at different quantities. For example, the mesenchymal stromal cells, identified with the CD90+ cell surface marker, as well as monocytes and activated macrophages, identified with CD14 marker, are expanded approximately 50 and 200 fold, respectively, while other CD45+ mononuclear cells from the bone marrow remain during the manufacturing process. We have demonstrated in the laboratory that the cells in our therapy are capable of multiple biological activities thought to play a critical role in repairing diseased and damaged tissues. These activities include aspects of tissue remodeling, promotion of angiogenesis and resolution of inflammation. In addition to these properties demonstrated in vitro, we have also shown that the therapy increases blood perfusion in both rat and mouse models of critical limb ischemia. In addition to these initial preclinical observations, we have on-going preclinical studies designed to further characterize the mechanism of action of our product in the treatment of cardiovascular diseases. These data support our current clinical-stage research where we are exploring the use of our therapies to regenerate tissue in patients with CLI and DCM.
In addition, our proprietary cell manufacturing system has demonstrated the capability to produce other types of cells. In the future, we may continue to explore the application of our manufacturing technology for the production of other cell types where there are potential opportunities to collaborate in the development of new cell therapies.
Patents and Proprietary Rights
Our success depends in part on our ability, and the ability of our licensors, to obtain patent protection for our products and processes. We have exclusive rights to approximately 25 unexpired issued U.S. patents. 15 of these are material patents that protect our cellular therapy. We own 8 of these patents and 6 of these patents have been licensed exclusively from the University of Michigan; 5 of the University of Michigan patents will expire in 2012. These patents present various claims relating to (i) the composition of our cellular therapy, (ii) methods to manufacture or administer the cellular therapy, and (iii) the bioreactor device (the Aastrom Replicell System) that is used to make our product. The number of U.S. patents of each type with expiration range is listed in the table below. Patents expiring in 2012 are not included in the table.
|
Patent Type |
|
Number |
|
Expiry (Years) |
|
|
Composition of Matter |
|
2 |
|
2 and 17 |
|
|
Methods |
|
1 |
|
17 |
|
|
Bioreactor Device |
|
7 |
|
2 — 3 |
|
Certain patent equivalents to the U.S. patents have also been issued in other jurisdictions including Australia, Japan, the Republic of Korea and Canada and under the European Patent Convention. In addition, we have filed applications for patents in the United States and equivalent applications in certain other countries claiming other aspects of our cell products and manufacturing processes. An important patent that protects the composition of the cellular therapy directly, “Mixed cell populations for tissue repair and separation technique for cell processing” (US Patent 7,871,605), was issued in January 2011 and will expire in 2029. A divisional application of 7,871,605 for administration of this composition to patients was allowed by the USPTO in January 2012 and is expected to be issued in the second quarter of 2012; this patent is categorized under Methods in the table above. Patents that protect our automated bioreactor device and culture system expire in 2015, but we will continue to rely on trade secrets and un-patentable know-how.
The validity and breadth of claims in medical technology patents involve complex legal and factual questions and, therefore, may be highly uncertain. No assurance can be given that any patents based on pending patent applications or any future patent applications by us, or our licensors, will be issued, that the scope of any patent protection will exclude competitors or provide competitive advantages to us, that any of the patents that have been or may be issued to us or our licensors will be held valid if subsequently challenged or that others will not claim rights in or ownership of the patents and other proprietary rights held or licensed by us. Furthermore, there can be no assurance that others have not developed or will not develop similar products, duplicate any of our products or design around any patents that have been or may be issued to us or our licensors. Since patent applications in the U.S. are maintained in secrecy until they are published 18 months after filing, we also cannot be certain that others did not first file applications
for inventions covered by our and our licensors’ pending patent applications, nor can we be certain that we will not infringe any patents that may be issued to others on such applications.
We rely on certain licenses granted by the University of Michigan for certain patent rights. If we breach such agreements or otherwise fail to comply with such agreements, or if such agreements expire or are otherwise terminated, we may lose our rights in such patents.
We also rely on trade secrets and un-patentable know-how that we seek to protect, in part, by confidentiality agreements. It is our policy to require our employees, consultants, contractors, manufacturers, outside scientific collaborators and sponsored researchers and other advisors to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information developed or made known to the individual during the course of the individual’s relationship with us is to be kept confidential and not disclosed to third parties except in specific limited circumstances. We also require signed confidentiality or material transfer agreements from any company that is to receive our confidential information. In the case of employees, consultants and contractors, the agreements generally provide that all inventions conceived by the individual while rendering services to us shall be assigned to us as the exclusive property of Aastrom. There can be no assurance, however, that these agreements will not be breached, that we would have adequate remedies for any breach, or that our trade secrets or un-patentable know-how will not otherwise become known or be independently developed by competitors.
Our success will also depend in part on our ability to develop commercially viable products without infringing the proprietary rights of others. We do not believe any of our currently contemplated products or processes infringe any existing valid issued patent. However, the results of patent litigation are unpredictable, and no assurance can be given that patents do not exist or could not be filed which would have an adverse effect on our ability to market our products or maintain our competitive position with respect to our products. If our technology components, designs, products, processes or other subject matter are claimed under other existing U.S. or foreign patents, or are otherwise protected by third party proprietary rights, we may be subject to infringement actions. In such event, we may challenge the validity of such patents or other proprietary rights or we may be required to obtain licenses from such companies in order to develop, manufacture or market our products. There can be no assurances that we would be able to obtain such licenses or that such licenses, if available, could be obtained on commercially reasonable terms. Furthermore, the failure to either develop a commercially viable alternative or obtain such licenses could result in delays in marketing our proposed products or the inability to proceed with the development, manufacture or sale of products requiring such licenses, which could have a material adverse effect on our business, financial condition and results of operations. If we are required to defend ourselves against charges of patent infringement or to protect our proprietary rights against third parties, substantial costs will be incurred regardless of whether we are successful. Such proceedings are typically protracted with no certainty of success. An adverse outcome could subject us to significant liabilities to third parties and force us to curtail or cease our development and sale of our products and processes.
Certain of our and our licensors’ research has been or is being funded in part by the Department of Commerce and by a Small Business Innovation Research Grant obtained from the Department of Health and Human Services. As a result of such funding, the U.S. Government has certain rights in the technology developed with such funding. These rights include a non-exclusive, fully paid-up, worldwide license under such inventions for any governmental purpose. In addition, the U.S. Government has the right to require us to grant an exclusive license under any of such inventions to a third party if the U.S. Government determines that: (i) adequate steps have not been taken to commercialize such inventions; (ii) such action is necessary to meet public health or safety needs; or (iii) such action is necessary to meet requirements for public use under federal regulations. Additionally, under the federal Bayh Dole Act, a party which acquires an exclusive license for an invention that was partially funded by a federal research grant is subject to the following government rights: (i) products using the invention which are sold in the United States. are to be manufactured substantially in the United States, unless a waiver is obtained; (ii) the government may force the granting of a license to a third party who will make and sell the needed product if the licensee does not pursue reasonable commercialization of a needed product using the invention; and (iii) the U.S. Government may use the invention for its own needs.
Sales and Marketing
We currently do not have the sales or marketing resources required to fully commercialize our therapeutic products. We intend to advance our programs to a point where we can evaluate the options to seek a development and/or commercialization partnership, or to make the investment to complete development and commercialize a product alone. We may also choose to undertake some pilot level of sales and marketing activity while seeking a commercial partnership.
Government Regulation
Our research and development activities and the manufacturing and marketing of our products are subject to the laws and regulations of governmental authorities in the United States and other countries in which our products will be marketed. Specifically, in the United States, the FDA, among other activities, regulates new product approvals to establish safety and efficacy of these products. Governments in other countries have similar requirements for testing and marketing. In the United States, in addition to meeting FDA regulations, we are also subject to other federal laws, such as the Occupational Safety and Health Act and the Environmental Protection Act, as well as certain state laws.
Our cell products will be regulated as somatic cell therapies/biologics/pharmaceuticals. With this classification, commercial production of our products will need to occur in registered/licensed facilities in compliance with Good Manufacturing Practice (GMP) for biologics (cellular products) or drugs.
Regulatory Process
Our products are subject to regulation as biological products under the Public Health Service Act and the Food, Drug and Cosmetic Act. Different regulatory requirements may apply to our products depending on how they are categorized by the FDA under these laws. The FDA has indicated that it intends to regulate products based on our technology as licensed biologics through the Center for Biologics Evaluation and Research. As current regulations exist, the FDA will require regulatory approval for certain human cellular- or tissue-based products, including our cell products, through a BLA submission.
Approval of new biological products is a lengthy procedure leading from development of a new product through preclinical and clinical testing. This process takes a number of years and the expenditure of significant resources. There can be no assurance that our product candidates will ultimately receive regulatory approval.
Regardless of how our product candidates are regulated, the Federal Food, Drug, and Cosmetic Act and other Federal and State statutes and regulations govern or influence the research, testing, manufacture, safety, labeling, storage, record-keeping, approval, distribution, use, product reporting, advertising and promotion of such products. Noncompliance with applicable requirements can result in civil penalties, recall, injunction or seizure of products, refusal of the government to approve or clear product approval applications or to allow us to enter into government supply contracts, withdrawal of previously approved applications and criminal prosecution.
Product Approval
In order to obtain FDA approval of a new medical product, sponsors must submit proof of safety and efficacy. In most cases, such proof entails extensive preclinical studies and clinical trials. The testing, preparation of necessary applications and processing of those applications by the FDA is expensive and may take several years to complete. There can be no assurance that the FDA will act favorably or in a timely manner in reviewing submitted applications, and we may encounter significant difficulties or costs in our efforts to obtain FDA approvals, in turn, which could delay or preclude us from marketing any products we may develop. The FDA may also require post-marketing testing and surveillance of approved products, or place other conditions on the approvals. These requirements could cause it to be more difficult or expensive to sell the products, and could therefore restrict the commercial applications of such products. Product approvals may be withdrawn if compliance with applicable regulations is not maintained or if problems occur following commercialization. For patented technologies, delays imposed by the governmental approval process may materially reduce the period during which we will have the exclusive right to exploit such technologies.
If clinical trials of a proposed medical product are required, the manufacturer or distributor of a drug or biologic will have to submit an IND application with the FDA prior to commencing human clinical trials. The submission must be supported by data, typically including the results of preclinical and laboratory testing. Following submission of the IND, the FDA has 30 days to review the application and raise safety and other clinical trial issues. If we are not notified of objections within that period, clinical trials may be initiated, and human clinical trials may commence at a specified number of investigational sites with the number of patients approved by the FDA. We have submitted several INDs for our cell products, and we have conducted clinical trials under these INDs.
Our products will be regulated by the FDA as a licensed biologic, although there can be no assurance that the FDA will not choose to regulate this product in a different manner in the future. The FDA categorizes human cell- or tissue-based products as either minimally manipulated or more than minimally manipulated, and has determined that more than minimally manipulated products require clinical trials to demonstrate product safety and efficacy and the submission of a BLA for marketing authorization. For products that may be regulated as biologics, the FDA requires: (i) preclinical laboratory and animal testing; (ii) submission to the FDA of an IND application, which must be approved prior to the initiation of human clinical trials; (iii) adequate and well-controlled clinical trials to establish the safety and efficacy of the product for its intended use; (iv) submission to the FDA of a BLA; and (v) review and approval of the BLA as well as inspections of the manufacturing facility by the FDA prior to commercial marketing of the product.
We conduct preclinical testing for internal use and as support for submissions to the FDA. Preclinical testing generally includes various types of in-vitro laboratory evaluations of our products as well as animal studies to assess the safety and the functionality of the product. Clinical trials are identified by phases (i.e., Phase 1, Phase 2, Phase 3, etc.). Depending on the type of preclinical and/or clinical data available, the trial sponsor will submit a request to the FDA to initiate a specific phase study (e.g., a Phase 1 trial represents an initial study in a small group of patients to test for safety and other relevant factors; a Phase 2 trial represents a study in a larger number of patients to assess the safety and efficacy of a product; and, Phase 3 trials are initiated to establish safety and efficacy in an expanded patient population at multiple clinical trial sites).
The results of the preclinical tests and clinical trials are submitted to the FDA in the form of a BLA for marketing approval. The testing, clinical trials and approval process are likely to require substantial time and effort and there can be no assurance that any approval will be granted on a timely basis, if at all. Additional animal studies or clinical trials may be requested during the FDA review period that may delay marketing approval. After FDA approval for the initial indications, further clinical trials may be necessary to gain approval for the use of the product for additional indications. The FDA requires that adverse effects be reported to the FDA and may also require post-marketing testing to monitor for adverse events, which can involve significant expense.
Under current requirements, facilities manufacturing biological products for commercial distribution must be licensed. To accomplish this, an establishment registration must be filed with the FDA. In addition to the preclinical studies and clinical trials, the BLA includes a description of the facilities, equipment and personnel involved in the manufacturing process. An establishment registration/license is granted on the basis of inspections of the applicant’s facilities in which the primary focus is on compliance with GMPs and the ability to consistently manufacture the product in the facility in accordance with the BLA. If the FDA finds the results of the inspection unsatisfactory, it may decline to approve the BLA, resulting in a delay in production of products.
As part of the approval process for human biological products, each manufacturing facility must be registered and inspected by the FDA prior to marketing approval. In addition, state agency inspections and approvals may also be required for a biological product to be shipped out of state.
Commercial Strategy
We are currently focused on utilizing our technology to produce expanded, patient specific multicellular products for use in severe, chronic ischemic cardiovascular indications. At such time as we satisfy applicable regulatory approval requirements, we expect the sales of our cell-based products to constitute nearly all of our product sales revenues.
We do not expect to generate positive cash flows from our consolidated operations for at least the next several years and then only if we achieve significant product sales. Until that time, we expect that our revenue sources from our current activities will consist of only minor sales of our cell products and manufacturing supplies to our academic collaborators, grant revenue, research funding and potential licensing fees or other financial support from potential future corporate collaborators.
We expect that we will need to raise significant additional funds or pursue strategic transactions or other strategic alternatives in order to complete our product development programs, complete clinical trials needed to market our products, and commercialize our products. To date, we have financed our operations primarily through public and private sales of our equity securities, and we expect to continue to seek to obtain the required capital in a similar manner. As a development stage company, we have never been profitable and do not anticipate having net income unless and until significant product sales commence. With respect to our current activities, this is not likely to occur until we obtain significant additional funding, complete the required clinical trials for regulatory approvals,
and receive the necessary approvals to market our products. Through December 31, 2011, we have accumulated a net loss of approximately $240,880,000. We cannot provide any assurance that we will be able to achieve profitability on a sustained basis, if at all, obtain the required funding, obtain the required regulatory approvals, or complete additional corporate partnering or acquisition transactions.
We believe, based on our current projections of cash utilization, our available cash and cash equivalents of approximately $5,500,000 as of December 31, 2011 and the net proceeds of $37,800,000 from the Series B financing that closed in March 2012 is adequate to finance our planned operations at least until December 31, 2012. The Series B preferred stock consist of Series B-1 non-voting convertible preferred stock, which we issued at the closing, and Series B-2 voting convertible preferred stock. The Series B-1 preferred stock will not be entitled to vote on matters on which the common shareholders are generally entitled to vote. The Series B-2 preferred stock will be entitled to vote with the holders of the common stock as a single class, with each share of Series B-2 voting convertible preferred stock having the number of votes equal to the number of shares of common stock issuable upon conversion of such Series B-2 preferred stock. Any holder of Series B-1 preferred stock may exchange its shares for shares of Series B-2 preferred stock on a one-for-one basis if the shareholder approval required in accordance with Nasdaq Marketplace Rule 5635(b) has been obtained and subject to certain terms and limitations. The Series B preferred stock will, with respect to dividend rights and rights on liquidation, winding-up and dissolution, rank on parity with any other class or series of Aastrom capital stock that we may issue in the future which is designated as being on parity with the Series B preferred stock, and rank senior to our common stock and Series A preferred stock. The Series B preferred stock is convertible, at the option of the holder thereof at any time after the five year anniversary of the closing of the offering, into shares of our common stock at a conversion price of $3.25 per share of common stock, subject to the Company obtaining any shareholder approval required in accordance with Nasdaq Marketplace Rule 5635(b). At any time after the five year anniversary of issuance, Aastrom may elect to convert any or all outstanding shares of Series B preferred stock into shares of our common stock, subject to certain limitations. Dividends on the Series B preferred stock will be cumulative and compound daily, at a rate of 11.5% per annum, payable upon conversion, liquidation, redemption or other similar events, and payable in Series B preferred stock until the five year anniversary of issuance. Following the five year anniversary of issuance and until the earlier of the tenth anniversary of the closing of the offering and the date no Series B preferred stock remain outstanding, dividends will accrue at a rate of 8% per annum and will be payable in cash or Series B preferred stock, at our option. Unless prohibited by Michigan law governing distributions to shareholders, the Series B-1 preferred stock shall be redeemable at the option of holder of the Series B-1 preferred stock commencing at any time after the five year anniversary of issuance, liquidation, winding up, dissolution or other similar events, subject to certain terms and limitations.
However, we will need to raise a significant amount of additional funds in order to complete our product development programs, complete clinical trials needed to market our products, and commercialize these products. We cannot be certain that such funding will be available on favorable terms, if at all. Some of the factors that will impact our ability to raise additional capital and our overall success include: the rate and degree of progress of our product development, the rate of regulatory approval to proceed with clinical trial programs, the level of success achieved in clinical trials, fulfillment of the requirements for marketing authorization from regulatory bodies in the United States and other countries, the liquidity and market volatility of our equity securities, regulatory and manufacturing requirements and uncertainties, technological developments by competitors, the U.S. economic conditions regarding the availability of investment capital and other factors. If we cannot raise such funds, we may not be able to develop or enhance products, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements, which would likely have a material adverse impact on our business, financial condition and results of operations.
Competitive Environment
The biotechnology and medical device industries are characterized by rapidly evolving technology and intense competition. Our competitors include major multi-national medical device companies, pharmaceutical companies, biotechnology companies and stem cell companies operating in the fields of tissue engineering, regenerative medicine, cardiac, vascular, orthopedics and neural medicine. Many of these companies are well-established and possess technical, research and development, financial, and sales and marketing resources significantly greater than ours. In addition, many of our smaller potential competitors have formed strategic collaborations, partnerships and other types of joint ventures with larger, well established industry competitors that afford these companies potential research and development and commercialization advantages in the technology and therapeutic areas currently being pursued by us. Academic institutions, governmental agencies and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those being commercialized by us. Moreover, many of these competitors may be able to obtain patent protection, obtain FDA and other regulatory approvals and begin commercial sales of their products before us.
Our potential commercial products address a broad range of existing and emerging therapeutic markets, in which cell-based therapy is a new and as of yet, unproven, commercial strategy. In a large part, we face primary competition from existing medical devices and drug products. Some of our competitors have longer operating histories and substantially greater resources. These include companies such as Baxter International, Inc. (Baxter), Biomet, Inc., Johnson & Johnson, Inc., Miltenyi Biotec, Medtronic, Inc. (Medtronic), and others.
In the general area of cell-based therapies, we potentially compete with a variety of companies, most of whom are specialty medical products or biotechnology companies. Some of these, such as Baxter, Johnson & Johnson, Medtronic and Miltenyi Biotec are well-established and have substantial technical and financial resources compared to ours. However, as cell-based products are only just emerging as viable medical therapies, many of our most direct competitors are smaller biotechnology and specialty medical products companies. These include Advanced Cell Technology, Inc., Cytomedix, Inc. (formerly Aldagen, Inc.), Arteriocyte Medical Systems, Inc., Athersys, Inc., Bioheart, Inc., Cytori Therapeutics, Inc., Genzyme Corporation, Terumo Medical Corporation (formerly Harvest Technologies Corporation), Mesoblast, Osiris Therapeutics, Inc., Pluristem, Inc. and others.
Employees
As of December 31, 2011, we employed 71 individuals on a full-time equivalent basis. A significant number of our management and professional employees have had prior experience with pharmaceutical, biotechnology or medical product companies. None of our employees are covered by collective bargaining agreements, and management considers relations with our employees to be good.
Executive Officers
|
Name |
|
Position |
|
Age |
|
Executive |
|
Timothy M. Mayleben |
|
President and Chief Executive Officer |
|
51 |
|
2009 |
|
Ronnda L. Bartel, Ph.D. |
|
Chief Scientific Officer |
|
53 |
|
2010 |
|
Sharon M. Watling, Pharm.D. |
|
Vice President Clinical Development |
|
48 |
|
2011 |
|
Brian D. Gibson |
|
Vice President of Finance |
|
33 |
|
2011 |
Timothy M. Mayleben — Mr. Mayleben joined Aastrom as a member of the Company’s Board of Directors in June 2005, and has served as our President and Chief Executive Officer since December 2009. Mr. Mayleben was formerly an advisor to life science and healthcare companies through his advisory and investment firm, ElMa Advisors. Prior to this, he served as the President and Chief Operating Officer and a Director of NightHawk Radiology Holdings, Inc. Mr. Mayleben was also formerly the Chief Operating Officer of Esperion Therapeutics, which later became a division of Pfizer Global Research & Development. He joined Esperion in late 1998 as Chief Financial Officer. While at Esperion, Mr. Mayleben led the raising of more than $200 million in venture capital and institutional equity funding and later negotiated the acquisition of Esperion by Pfizer in December 2003. Prior to joining Esperion, Mr. Mayleben held various senior and executive management positions at Transom Technologies, Inc., now part of Electronic Data Systems, Inc., and Applied Intelligent Systems, Inc., which was acquired by Electro-Scientific Industries, Inc. in 1997. Mr. Mayleben holds a Masters of Business Administration, with distinction, from the J.L. Kellogg Graduate School of Management at Northwestern University, and a Bachelor of Business Administration degree from the University of Michigan Ross School of Business. He is on the Advisory Board for the Wolverine Venture Fund and serves as a director for several private life science companies.
Ronnda L. Bartel, Ph.D. — Dr. Bartel joined Aastrom in 2006 and is responsible for research, development and manufacturing and engineering operations. Dr. Bartel has more than 20 years of research and product development experience and most recently was Executive Director, Biological Research at MicroIslet and Vice president, Scientific Development at StemCells, Inc. Earlier in her career, she was Senior Principal Scientist, Cell Biology at Advanced Tissue Sciences and was involved in the development and approval of two of the first three cell based products approved by the FDA. She has also worked as Senior Director, Science and Technology at SRS Capital, LLC evaluating life science investments and has also held positions in clinical development, drug delivery, business development and manufacturing. Dr. Bartel holds a Ph.D. in Biochemistry from the University of Kansas, completed postdoctoral work at the University of Michigan and received a B.A. in Chemistry and Biology from Tabor College.
Sharon M. Watling, Pharm.D — Dr. Watling joined Aastrom in February 2010 and is responsible for clinical development and clinical operations. She has over 12 years of experience in clinical development, with an emphasis on translational science, clinical development, and clinical strategies. Her industry career started in late stage development within Warner-Lambert/Parke Davis and evolved while at Pfizer to include an early clinical leadership role in cardiovascular-metabolic diseases. Following Pfizer, she was site leader and senior director, clinical development at Metabasis, Inc. Most recently, she served as the research and development strategy leader at Cognigen Corporation, working with multiple companies to incorporate modeling and simulation practices into their development strategies. Prior to industry, she was an intensive care unit clinical specialist at various academic institutions. Dr. Watling holds a Pharm.D. from the University of Michigan College of Pharmacy.
Brian D. Gibson — Mr. Gibson joined Aastrom in July 2010 and is Vice President of Finance. He brings more than 11 years of finance and accounting experience to Aastrom. Prior to joining Aastrom, Mr. Gibson was a senior manager at PricewaterhouseCoopers, with broad experience in multiple industries, including life sciences and healthcare. Mr. Gibson holds a BA in accounting with high honor from the Eli Broad College of Business at Michigan State University and is a certified public accountant.
Available Information
Additional information about Aastrom is contained at our website, www.aastrom.com. Information on our website is not incorporated by reference into this report. We make available on our website free of charge our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K as soon as reasonably practicable after those reports are filed with the Securities and Exchange Commission. Our reports filed with the Securities and Exchange Commission are also made available to read and copy at the SEC’s Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. You may obtain information about the Public Reference Room by calling the SEC at 1-800-SEC-0330. Reports filed with the SEC are also made available on its website at www.sec.gov. The following Corporate Governance documents are also posted on our website: Code of Business Conduct and Ethics, Code of Ethics for Senior Financial Officers, Board Member Attendance at Annual Meetings Policy, Director Nominations Policy, Shareholder Communications with Directors Policy and the Charters for each of the Committees of the Board of Directors.
Our operations and financial results are subject to various risks and uncertainties, including those described below, that could adversely affect our business, financial condition, results of operations, cash flows, and trading price of our common stock. The risks and uncertainties described below are not the only ones we face. There may be additional risks and uncertainties that are not known to us or that we do not consider to be material at this time. If the events described in these risks occur, our business, financial condition, and results of operations would likely suffer.
Risks Related to our Business
Our past losses and expected future losses cast doubt on our ability to operate profitably.
We were incorporated in 1989 and have experienced substantial operating losses since inception. As of December 31, 2011, we have incurred a cumulative net loss totaling approximately $240,880,000 and we have continued to incur losses since that date. These losses have resulted principally from costs incurred in the research and development (including clinical trials) of our cell culture technologies and our cell manufacturing system, general and administrative expenses, and the prosecution of patent applications. We expect to continue to incur significant operating losses over the next several years and at least until, and probably after, product sales increase, primarily owing to our research and development programs, including preclinical studies and clinical trials, and the establishment of marketing and distribution capabilities necessary to support commercialization efforts for our products. We cannot predict with any certainty the amount of future losses. Our ability to achieve profitability will depend, among other things, on successfully completing the development of our product candidates, timely initiation and completion of clinical trials, obtaining regulatory approvals, establishing manufacturing, sales and marketing arrangements with third parties, maintaining supplies of key manufacturing components, acquisition and development of complementary activities and raising sufficient cash to fund our operating activities. Therefore, we may not be able to achieve or sustain profitability.
We may not be able to raise the required capital to conduct our operations and develop and commercialize our products.
Despite the proceeds we received from our March 2012 financing, we will require substantial additional capital resources in order to conduct our operations, complete our product development programs, complete our clinical trials needed to market our products (including a Phase 3 clinical trial for CLI), and commercialize these products and cell manufacturing facilities. In order to grow and expand our business, to introduce our new product candidates into the marketplace and to acquire or develop complementary business activities, we will need to raise a significant amount of additional funds. We will also need significant additional funds or a collaborative partner, or both, to finance the research and development activities of our cell product candidates for additional indications. Accordingly, we are continuing to pursue additional sources of financing.
Our future capital requirements will depend upon many factors, including:
|
· |
continued scientific progress in our research, clinical and development programs; |
|
|
|
|
· |
costs and timing of conducting clinical trials and seeking regulatory approvals; |
|
|
|
|
· |
competing technological and market developments; |
|
|
|
|
· |
avoiding infringement and misappropriation of third-party intellectual property; |
|
· |
obtaining valid and enforceable patents that give us a competitive advantage; |
|
|
|
|
· |
our ability to establish additional collaborative relationships; |
|
|
|
|
· |
our ability to effectively launch a commercial product; |
|
|
|
|
· |
the effect of commercialization activities and facility expansions, if and as required; and |
|
|
|
|
· |
complementary business acquisition or development opportunities. |
In November 2010, we terminated the common stock purchase agreement with Fusion Capital Fund II entered into June 2009. As a result, we no longer have access to the potential funding from Fusion Capital under that agreement. We entered into an At the Market Sales Agreement (ATM) on June 16, 2011, which allows us to raise approximately $20,000,000 through sales of our common stock from time to time. However, there are certain factors, such as volume of trading in our stock and the stock price, which limit the amount that can be raised in a short period of time through the ATM. Regardless of the usage of the ATM, we need to raise additional capital in order to fund the phase 3 clinical trial of ixmyelocel-T for CLI, complete our product development programs, complete clinical trials needed to market our products and commercialize these products. We believe that with our existing cash and cash equivalents and the net proceeds of $37,800,000 from the financing that closed in March 2012, we have adequate liquidity to finance our operations, including development of our products and product candidates, through at least December 31, 2012. While our budgeted cash usage and operating plan through December 31, 2012 does not currently contemplate taking additional actions to reduce the use of cash over that period, we could, if necessary, delay or forego certain budgeted discretionary expenditures such as anticipated hiring plans or certain non-critical research and development expenditures, as well as slow down or delay certain clinical trial activity such that we believe that we will have sufficient cash on hand through at least December 31, 2012.
Notwithstanding the proceeds we received from our March 2012 financing, we will need to raise additional funds in order to complete our product development programs, complete clinical trials needed to market our products (including clinical trials for our CLI and DCM programs), and commercialize these products. Because of our long-term funding requirements, we may try to access the public or private equity markets if conditions are favorable to complete a financing, even if we do not have an immediate need for additional capital at that time, or whenever we require additional operating capital. In addition, we may seek collaborative relationships, incur debt and access other available funding sources. This additional funding may not be available to us on reasonable terms, or at all. Some of the factors that will impact our ability to raise additional capital and our overall success include:
|
· |
the rate and degree of progress for our product development; |
|
|
|
|
· |
the rate of regulatory approval to proceed with clinical trial programs; |
|
|
|
|
· |
the level of success achieved in clinical trials; |
|
|
|
|
· |
the requirements for marketing authorization from regulatory bodies in the United States and other countries; |
|
|
|
|
· |
the liquidity and market volatility of our equity securities; and |
|
|
|
|
· |
regulatory and manufacturing requirements and uncertainties, technological developments by competitors. |
If adequate funds are not available in the future, we may not be able to develop or enhance our products, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements and we may be required to delay or terminate research and development programs, curtail capital expenditures, and reduce business development and other operating activities. Should the financing we require to sustain our working capital needs be unavailable or prohibitively expensive when we require it, the consequences could have a material adverse effect on our business, operating results, financial condition and prospects.
Failure to obtain and maintain required regulatory approvals would severely limit our ability to sell our products.
We must obtain the approval of the FDA before commercial sales of our cell product candidates may commence in the United States, which we believe will ultimately be the largest market for our products. We will also be required to obtain additional approvals from various foreign regulatory authorities to initiate sales activities of cell products in those jurisdictions. If we cannot demonstrate the safety, purity and potency of our product candidates, including our cell product candidates, produced in our production system, the FDA or other regulatory authorities could delay or withhold regulatory approval of our product candidates.
Finally, even if we obtain regulatory approval of a product, that approval may be subject to limitations on the indicated uses for which it may be marketed. Even after granting regulatory approval, the FDA and regulatory agencies in other countries continue to review and inspect marketed products, manufacturers and manufacturing facilities, which may create additional regulatory burdens. Later discovery of previously unknown problems with a product, manufacturer or facility may result in restrictions on the product or manufacturer, including a withdrawal of the product from the market. Further, regulatory agencies may establish additional regulations that could prevent or delay regulatory approval of our products.
Our product development programs are based on novel technologies and are inherently risky.
We are subject to the risks of failure inherent in the development of products based on new technologies. The novel nature of our therapeutics creates significant challenges in regard to product development and optimization, manufacturing, government regulation, third party reimbursement and market acceptance. For example, if regulatory agencies have limited experience in approving cellular therapies for commercialization, the development and commercialization pathway for our therapies may be subject to increased uncertainty, as compared to the pathway for new conventional drugs.
Any changes in the governmental regulatory classifications of our products could prevent, limit or delay our ability to market or develop our products.
The FDA establishes regulatory requirements based on the classification of a product. Because our product development programs are designed to satisfy the standards applicable to biological licensure for our cellular products, any change in the regulatory classification or designation would affect our ability to obtain FDA approval of our products. Each of these cell products is, under current regulations, regulated as a biologic, which requires a BLA.
Our inability to complete our product development activities successfully would severely limit our ability to operate or finance operations.
In order to commercialize our cell product candidates in the United States, we must complete substantial clinical trials and obtain sufficient safety, purity and potency results to support required registration approval and market acceptance of our cell product candidates. We may not be able to successfully complete the development of our product candidates, or successfully market our technologies or product candidates. We, and any of our potential collaborators, may encounter problems and delays relating to research and development, regulatory approval and intellectual property rights of our technologies and product candidates. Our research and development programs may not be successful, and our cell culture technologies and product candidates may not facilitate the production of cells outside the human body with the expected results. Our technologies and cell product candidates may not prove to be safe and efficacious in clinical trials, and we may not obtain the requisite regulatory approvals for our technologies or product candidates and the cells produced in such products. If any of these events occur, we may not have adequate resources to continue operations for the period required to resolve any issues delaying commercialization and we may not be able to raise capital to finance our continued operation during the period required for resolution of any such issues.
We must successfully complete our clinical trials to be able to market certain of our products.
To be able to market therapeutic cell products in the United States, we must demonstrate, through extensive preclinical studies and clinical trials, the safety and efficacy of our processes and product candidates. If our clinical trials are not successful, our products may not be marketable. The results of early stage clinical trials do not ensure success in later clinical trials, and interim results are not necessarily predictive of final results.
Our ability to complete our clinical trials in a timely manner depends on many factors, including the rate of patient enrollment. Patient enrollment can vary with the size of the patient population, the proximity of suitable patients to clinical sites, perceptions of the utility of cell therapy for the treatment of certain diseases, and the eligibility criteria for the study. For example, patients enrolling in our studies need to provide an adequate amount of bone marrow to process and expand for injection and some patients may not be able to provide sufficient starting material despite our study inclusion and exclusion criteria designed to prevent this. Bone marrow is an inherently variable starting material. We have experienced delays in patient accrual in our previous clinical trials. If we experience
future delays in patient enrollment, we could experience increased costs and delays associated with clinical trials, which would impair our product development programs and our ability to market our products.
Furthermore, the FDA monitors the progress of clinical trials and it may suspend or terminate clinical trials at any time due to patient safety or other considerations.
Our research programs are currently directed at improving product functionality for certain clinical indications, improving product shelf life, and decreasing the cost of manufacturing our products. These production process changes may alter the functionality of our cells and require various additional levels of experimental and clinical testing and evaluation. Any such testing could lengthen the time before these products would be commercially available.
Even if successful clinical results are reported for a product from a completed clinical trial, this does not mean that the results will be sustained over time, or will be sufficient for a marketable or regulatory approvable product.
We will rely on third parties to conduct some of our clinical trials, and their failure to perform their obligations in a timely or competent manner may delay development and commercialization of our product candidates.
We have engaged and we may use clinical research organizations to assist in conduct of our clinical trials. There are numerous alternative sources to provide these services. However, we may face delays outside of our control if these parties do not perform their obligations in a timely or competent fashion or if we are forced to change service providers. Any third party that we hire to conduct clinical trials may also provide services to our competitors, which could compromise the performance of their obligations to us. If we experience significant delays in the progress of our clinical trials, the commercial prospects for product candidates could be harmed and our ability to generate product revenue would be delayed or prevented. In addition, we and any provider that we retain will be subject to Good Clinical Practice, or GCP requirements. If GCP and other regulatory requirements are not adhered to by us or our third-party providers, the development and commercialization of our product candidates could be delayed.
Any failure of such CRO to successfully accomplish clinical trial monitoring, data collection, safety monitoring and data management and the other services it provides for us in a timely manner and in compliance with regulatory requirements could have a material adverse effect on our ability to complete clinical development of our products and obtain regulatory approval. Problems with the timeliness or quality of the work of a CRO may lead us to seek to terminate the relationship and use an alternate service provider. However, making such changes may be costly and may delay our trials, and contractual restrictions may make such a change difficult or impossible. Additionally, it may be difficult to find a replacement organization that can conduct our trials in an acceptable manner and at an acceptable cost.
Failure of third parties, including Vention Medical (formerly ATEK Medical, LLC), to manufacture or supply certain components, equipment, disposable devices and other materials used in our cell manufacturing process would impair our cell product development.
We rely on third parties, including Vention Medical (Vention), to manufacture and/or supply certain of our devices/manufacturing equipment and to manufacture and/or supply certain components, equipment, disposable devices and other materials used in our cell manufacturing process to develop our cell products. Vention is our sole supplier of cell cassettes for which it would be difficult to obtain alternate sources of supply on a short-term basis. If any of our manufacturers or suppliers fails to perform their respective obligations, or if our supply of certain components, equipment, disposable devices and other materials is limited or interrupted, it could impair our ability to manufacture our products, which would delay our ability to conduct our clinical trials or market our product candidates on a timely and cost-competitive basis, if at all.
In addition, we may not be able to continue our present arrangements with our suppliers, supplement existing relationships, establish new relationships or be able to identify and obtain the ancillary materials that are necessary to develop our product candidates in the future. Our dependence upon third parties for the supply and manufacture of these items could adversely affect our ability to develop and deliver commercially feasible products on a timely and competitive basis.
Manufacturing of our cell products in centralized facilities may increase the risk that we will not have adequate quantities of our cell products for clinical programs.
We are subject to regulatory compliance and quality assurance requirements at our production site in Ann Arbor, Michigan. This site could be subject to ongoing, periodic, unannounced inspection by regulatory agencies to ensure strict compliance with GMP regulations and other governmental regulations. We do not have redundant cell manufacturing sites. In the event our cell production facility is damaged or destroyed or is subject to regulatory restrictions, our clinical trial programs and other business prospects would be adversely affected.
Even if we obtain regulatory approvals to sell our products, lack of commercial acceptance could impair our business.
We will be seeking to obtain regulatory approvals to market our cell products for tissue repair treatments. Even if we obtain all required regulatory approvals, we cannot be certain that our products and processes will be accepted in the marketplace at a level that would allow us to operate profitably. Our products may be unable to achieve commercial acceptance for a number of reasons, such as the availability of alternatives that are less expensive, more effective, or easier to use; the perception of a low cost-benefit ratio for the product amongst physicians and hospitals; or an inadequate level of product support from ourselves or a commercial partner. Our technologies or product candidates may not be employed in all potential applications being investigated, and any reduction in applications would limit the market acceptance of our technologies and product candidates, and our potential revenues.
The market for our products will be heavily dependent on third party reimbursement policies.
Our ability to successfully commercialize our product candidates will depend on the extent to which government healthcare programs, such as Medicare and Medicaid, as well as private health insurers, health maintenance organizations and other third party payers will pay for our products and related treatments.
Reimbursement by third party payers depends on a number of factors, including the payer’s determination that use of the product is safe and effective, not experimental or investigational, medically necessary, appropriate for the specific patient and cost-effective. Reimbursement in the United States or foreign countries may not be available or maintained for any of our product candidates. If we do not obtain approvals for adequate third party reimbursements, we may not be able to establish or maintain price levels sufficient to realize an appropriate return on our investment in product development. Any limits on reimbursement from third party payers may reduce the demand for, or negatively affect the price of, our products. For example, in the past, published studies suggested that stem cell transplantation for breast cancer, which constituted a significant portion of the overall stem cell therapy market at the time, may have limited clinical benefit. The lack of reimbursement for these procedures by insurance payers has negatively affected the marketability of our products in this indication in the past.
Managing and reducing health care costs has been a general concern of federal and state governments in the United States and of foreign governments. In addition, third party payers are increasingly challenging the price and cost-effectiveness of medical products and services, and many limit reimbursement for newly approved health care products. In particular, third party payers may limit the indications for which they will reimburse patients who use any products that we may develop. Cost control initiatives could decrease the price for products that we may develop, which would result in lower product revenues to us.
Use of animal-derived materials could harm our product development and commercialization efforts.
Some of the manufacturing materials and/or components we use in, and are critical to, implementation of our technology involve the use of animal-derived products, including fetal bovine serum. Suppliers or regulatory changes may limit or restrict the availability of such materials for clinical and commercial use. We currently purchase all of our fetal bovine sera from protected herds in Australia and New Zealand. These sources are considered to be the safest and raise the least amount of concern from the global regulatory agencies. If, for example, the so-called “mad cow disease” occurs in New Zealand or in Australia, it may lead to a restricted supply of the serum currently required for our product manufacturing processes. Any restrictions on these materials would impose a potential competitive disadvantage for our products or prevent our ability to manufacture our cell products. The FDA has issued draft regulations for controls over bovine materials. These proposed regulations do not appear to affect our ability to purchase the manufacturing materials we currently use. However, the FDA may issue final regulations that could affect our operations. Our
inability to develop or obtain alternative compounds would harm our product development and commercialization efforts. There are certain limitations in the supply of certain animal-derived materials, which may lead to delays in our ability to complete clinical trials or eventually to meet the anticipated market demand for our cell products.
Given our limited internal manufacturing, sales, marketing and distribution capabilities, we need to develop increased internal capability or collaborative relationships to manufacture, sell, market and distribute our products.
We have only limited internal manufacturing, sales, marketing and distribution capabilities. As market needs develop, we intend to establish and operate commercial-scale manufacturing facilities, which will need to comply with all applicable regulatory requirements. We will also need to develop new configurations of our cell manufacturing system for these facilities to enable processes and cost efficiencies associated with large-scale manufacturing. Establishing these facilities will require significant capital and expertise. We may need to make such expenditures when there are significant uncertainties as to the market opportunity. Any delay in establishing, or difficulties in operating, these facilities will limit our ability to meet the anticipated market demand for our cell products. We intend to get assistance to market some of our future cell products through collaborative relationships with companies with established sales, marketing and distribution capabilities. Our inability to develop and maintain those relationships would limit our ability to market, sell and distribute our products. Our inability to enter into successful, long-term relationships could require us to develop alternate arrangements at a time when we need sales, marketing or distribution capabilities to meet existing demand. We may market one or more of our products through our own sales force. Our inability to develop and retain a qualified sales force could limit our ability to market, sell and distribute our cell products.
If we do not keep pace with our competitors and with technological and market changes, our products will become obsolete and our business may suffer.
The markets for our products are very competitive, subject to rapid technological changes, and vary for different candidates and processes that directly compete with our products. Our competitors may have developed, or could in the future develop, new technologies that compete with our products or even render our products obsolete. As an example, in the past, published studies have suggested that hematopoietic stem cell therapy use for bone marrow transplantation, following marrow ablation due to chemotherapy, may have limited clinical benefit in the treatment of breast cancer, which was a significant portion of the overall hematopoietic stem cell transplant market. This resulted in the practical elimination of this market for our cell-based product for this application.
Our cell manufacturing system is designed to improve and automate the processes for producing cells used in therapeutic procedures. Even if we are able to demonstrate improved or equivalent results, the cost or process of treatment and other factors may cause researchers and practitioners to not use our products and we could suffer a competitive disadvantage. Finally, to the extent that others develop new technologies that address the targeted application for our products, our business will suffer.
The current credit and financial market conditions may exacerbate certain risks affecting our business.
We rely upon third parties for certain aspects of our business, including collaboration partners, wholesale distributors, contract clinical trial providers, contract manufacturers and third-party suppliers. Because of the recent tightening of global credit and the volatility in the financial markets, there may be a delay or disruption in the performance or satisfaction of commitments to us by these third parties, which could adversely affect our business.
If we cannot attract and retain key personnel, our business may suffer.
Our success depends in large part upon our ability to attract and retain highly qualified scientific and management personnel. We face competition for such personnel from other companies, research and academic institutions and other entities. Further, in an effort to conserve financial resources, we have implemented reductions in our work force on three previous occasions, most recently in fiscal 2008. As a result of these and other factors, we may not be successful in hiring or retaining key personnel. Our inability to replace any key employee could harm our operations.
Risks Related to Intellectual Property
If our patents and proprietary rights do not provide substantial protection, then our business and competitive position will suffer.
Our success depends in large part on our ability to develop or license intellectual property rights to protect our proprietary products and technologies. This involves complex legal, scientific, and factual questions and uncertainties. We rely upon patent, trade secret, copyright and contract laws to protect proprietary technology and trademark law to protect brand identities. However, we cannot assure you that any patent applications filed by, assigned to, or licensed to us will be granted, and that the scope of any of our issued or licensed patents will be sufficiently broad to offer meaningful protection. In addition, our issued patents or patents licensed to us could be successfully challenged, invalidated, held to be unenforceable, or circumvented so that our patent rights would not create an effective competitive barrier. We also cannot assure you that the inventors of the patents and applications that we own or license were the first to invent or the first to file on the inventions, or that a third party will not claim ownership in one of our patents or patent applications. We cannot assure you that a third party does not have or will not obtain patents that dominate the patents we own or license now or in the future. Furthermore, we rely on exclusive, world-wide licenses relating to the production of human cells granted to us by the University of Michigan. If we materially breach such agreements or otherwise fail to materially comply with such agreements, or if such agreements expire or are otherwise terminated by us, we may lose our rights under the patents held by the University of Michigan. At the latest, each of these licenses will terminate when the patent underlying the license expires. The first of these underlying patents will expire on March 21, 2012. Once the patents expire, third parties may be able to practice the inventions covered by those patents and thus compete with us.
Patent law relating to the scope of claims in the biotechnology field is evolving and our patent rights in this country and abroad are subject to this uncertainty.
We also rely on trade secrets and un-patentable know-how that we seek to protect, in part, by confidentiality agreements with our employees, consultants, suppliers and licensees. These agreements may be breached, and we might not have adequate remedies for any breach. Our competitors may also independently develop technologies substantially equivalent or superior to ours. If this were to occur, our business and competitive position would suffer.
Intellectual property litigation could harm our business.
Our success will also depend in part on our ability to develop commercially viable products without infringing the proprietary rights of others. Our cell processing system and cell compositions utilize a wide variety of technologies and we can give no assurance that we have identified or can identify all inventions and patents that may be infringed by development and manufacture of our cell compositions. Although we have not been subject to any filed infringement claims, patents could exist or could be filed which would prohibit or limit our ability to market our products or maintain our competitive position. In the event of an intellectual property dispute, we may be forced to litigate. Such litigation is typically protracted and the results are unpredictable. Intellectual property litigation would divert management’s attention from developing our products and would force us to incur substantial costs regardless of whether we are successful. An adverse outcome could subject us to significant liabilities to third parties including treble damages and the opposing party’s attorney fees, and force us to pay significant license fees and royalties or cease the development and sale of our products and processes.
We have hired and will continue to hire individuals who have experience in cell culture and cell based therapeutics and may have confidential trade secret or proprietary information of third parties. We caution these individuals not to use or reveal this third-party information, but we cannot assure you that these individuals will not use or reveal this third-party information. Thus, we could be sued for misappropriation of proprietary information and trade secrets. Such claims are expensive to defend and could divert our attention and could result in substantial damage awards and injunctions that could have a material adverse effect on our business, financial condition or results of operations.
We may need to initiate lawsuits to protect or enforce our patents or other proprietary rights, which would be expensive and, if unsuccessful, may cause us to lose some of our intellectual property rights.
To protect or enforce our patent rights, it may be necessary for us to initiate patent litigation proceedings against third parties, such as infringement suits or interference proceedings. These lawsuits would be expensive, take significant time and would divert management’s attention from other business concerns. These lawsuits could put our patents at risk of being invalidated, held unenforceable, or interpreted narrowly, and our patent applications at risk of not being issued. Further, these lawsuits may provoke the defendants to assert claims against us. The patent position of biotechnology firms is highly uncertain, involves complex legal and factual questions and recently has been the subject of much litigation. We cannot assure you that we will prevail in any of such suits or proceedings or that the damages or other remedies awarded to us, if any, will be commercially valuable.
The government maintains certain rights in technology that we develop using government grant money and we may lose the revenues from such technology if we do not commercialize and utilize the technology pursuant to established government guidelines.
Certain of our and our licensors’ research have been or are being funded in part by government grants. As a result of such funding, the U.S. Government has established guidelines and has certain rights in the technology developed with the grant. These rights include a non-exclusive, fully paid-up, worldwide license under such inventions for any governmental purpose. In addition, the U.S. Government has the right to require us to grant an exclusive license under any of such inventions to a third party if the U.S. Government determines that: (i) adequate steps have not been taken to commercialize such inventions; (ii) such action is necessary to meet public health or safety needs; or (iii) such action is necessary to meet requirements for public use under federal regulations. Additionally, under the federal Bayh Dole Act, a party which acquires an exclusive license for an invention that was partially funded by a federal research grant is subject to the following government rights: (i) products using the invention which are sold in the United States are to be manufactured substantially in the United States, unless a waiver is obtained; (ii) the government may force the granting of a license to a third party who will make and sell the needed product if the licensee does not pursue reasonable commercialization of a needed product using the invention; and (iii) the U.S. Government may use the invention for its own needs. If we fail to meet these guidelines, we would lose our exclusive rights to these products, and we would lose potential revenue derived from the sale of these products.
Potential product liability claims could affect our earnings and financial condition.
We face an inherent business risk of exposure to product liability claims in the event that the manufacture and/or use of our products during clinical trials, or after commercialization, results in adverse events. As a result, we may incur significant product liability exposure, which could exceed existing insurance coverage. We may not be able to maintain adequate levels of insurance at reasonable cost and/or reasonable terms. Excessive insurance costs or uninsured claims would increase our operating loss and adversely affect our financial condition.
Risks Related to an Investment in our Common Stock
Our stock price has been volatile and future sales of substantial numbers of our shares could have an adverse effect on the market price of our shares.
The market price of shares of our common stock has been volatile, ranging in closing price between $1.79 and $3.27 during the period ended December 31, 2011. The price of our common stock may continue to fluctuate in response to a number of events and factors, such as:
|
|
· |
clinical trial results; |
|
|
|
|
|
|
· |
the amount of our cash resources and our ability to obtain additional funding; |
|
|
|
|
|
|
· |
announcements of research activities, business developments, technological innovations or new products by us or our competitors; |
|
|
|
|
|
|
· |
entering into or terminating strategic relationships; |
|
|
|
|
|
|
· |
regulatory developments in both the United States and abroad; |
|
|
|
|
|
|
· |
disputes concerning patents or proprietary rights; |
|
|
|
|
|
|
· |
changes in our revenues or expense levels; |
|
|
|
|
|
|
· |
public concern regarding the safety, efficacy or other aspects of the products or methodologies we are developing; |
|
|
|
|
|
|
· |
news or reports from other stem cell, cell therapy or regenerative medicine companies; |
|
|
|
|
|
|
· |
reports by securities analysts; |
|
|
|
|
|
|
· |
status of the investment markets; |
|
|
|
|
|
|
· |
concerns related to management transitions; and |
|
|
|
|
|
|
· |
delisting from the NASDAQ Capital Market. |
Any of these events may cause the price of our shares to fall, which may adversely affect our business and financing opportunities. In addition, the stock market in general and the market prices for biotechnology companies in particular have experienced significant volatility recently that often has been unrelated to the operating performance or financial conditions of such companies. These broad market and industry fluctuations may adversely affect the trading price of our stock, regardless of our operating performance or prospects.
The sale of our common stock through future equity offerings may cause dilution and could cause the price of our common stock to decline.
We have registered $100,000,000 of securities for public sale pursuant to our registration statement on Form S-3 declared effective in July 2011. In addition, we registered $75,000,000 of securities for public sale pursuant to our registration statement on Form S-3 filed in November 2010. In December 2010, we offered 10,000,000 shares of common stock and warrants to purchase up to 10,000,000 shares of common stock under such registration statement and pursuant to a prospectus supplement first made available on December 10, 2010. Additionally, we entered into an At the Market Sales Agreement (ATM) on June 16, 2011, which allows us to raise approximately $20,000,000 through sales of our common stock from time to time under such registration statement. However, there are certain factors, such as volume of trading in our stock and the stock price, which limit the amount that can be raised in a short period of time through the ATM.
Sales of our common stock offered through future equity offerings may result in substantial dilution to the interests of other holders of our common stock. The sale of a substantial number of shares of our common stock to investors, or anticipation of such sales, could make it more difficult for us to sell equity or equity-related securities in the future at a time and at a price that we might otherwise wish to effect sales.
Eastern Capital Limited, the investor in Aastrom's March 2012 financing, holds a large percentage of our outstanding capital stock and, should they choose to, or should we elect to, convert their capital stock into shares of voting stock, will have significant influence over the outcome of corporate actions requiring shareholder approval; and such shareholder's priorities for our business may be different from other shareholders.
All of our outstanding Series B-1 non-voting preferred stock, representing a significant amount of our outstanding capital stock on a fully-converted basis, is held by Eastern Capital Limited, the investor in our March 2012 financing. These shares are exchangeable for shares of Series B-2 voting preferred stock and, in March 2017, are convertible into shares of our common stock. If we or Eastern Capital elect to exchange or convert its shares of Series B-1 Preferred Stock into a series of our voting shares, based solely on the number of shares of Series B-1 Preferred Stock that Eastern Capital purchased at the closing of the transaction, Eastern Capital will have beneficial ownership of up to approximately twenty-four percent (24%) (calculated on an as converted to common stock basis and excluding any shares that will accrue as a dividend on the shares of Series B-1 Preferred) of our voting securities based on the approximately fifty-one million shares of common stock and Series B-1 Preferred Stock outstanding as of the date of this report. Furthermore, in connection with the March 2012 financing, we amended our Shareholder Rights Plan to allow Eastern Capital to acquire beneficial ownership of up to 49.9% of the Company's outstanding securities without being deemed an "Acquiring Person" for purposes of our Shareholder Rights Plan. As a result of their current ownership and their ability to acquire more of our securities, they will be able to significantly influence the outcome of any financing transaction or other matter submitted to our shareholders for approval, including the election of directors, any merger, consolidation or sale of all or substantially all of our assets or any other significant corporate transaction. The interests of Eastern Capital may differ from the interests of our other shareholders. For example, Eastern Capital could delay or prevent a change of control of Aastrom even if such a change of control would benefit Aastrom's other shareholders. The significant concentration of stock ownership may adversely affect the trading price of Aastrom's common stock due to our investors’ perception that conflicts of interest may exist or arise.
In addition, the shares of Series B-1 Preferred Stock and the shares of Series B-2 Preferred Stock which may be issued upon exchange of the shares of Series B-1 Preferred Stock have certain rights, preferences and privileges that rank senior to the shares of our common stock. For example, the shares of Series B-1 Preferred Stock and Series B-2 Preferred Stock are entitled to receive a liquidation preference prior to any payment being made to holders of common stock upon a voluntary or involuntary liquidation, dissolution or winding up of the Company, or we experience a change of control. Furthermore, if the shares of Series B-1 Preferred Stock are never exchanged for shares of Series B-2 Preferred Stock and/or converted into shares of our common stock, at any time after March 2017, we may be required to redeem the then outstanding shares of Series B-1 Preferred Stock and any dividend shares accrued thereon at a price equal to the greater of (A) $3,250 (subject to adjustments for stock splits and similar events) plus all accrued dividends and (B) the then fair market value of a share of common stock multiplied by the number of shares of common stock into which such share of Series B-1 Preferred Stock is then convertible. Such redemption would be completed in three annual installments beginning not more than 120 days after we receive a request for redemption. The requirement for us to redeem Eastern Capital’s shares of Series B-1 Preferred Stock in cash could diminish our working capital, the consequences of which could have a material adverse effect on our business, operating results, financial condition and prospects.
Our corporate documents and Michigan law contain provisions that may make it more difficult for us to be acquired.
Our Board of Directors has the authority, without shareholder approval, to issue additional shares of preferred stock and to fix the rights, preferences, privileges and restrictions of these shares without any further vote or action by our shareholders. Michigan law contains a provision that makes it more difficult for a 10% shareholder, or its officers, to acquire a company. This authority, together with certain provisions of our charter documents, may have the effect of making it more difficult for a third party to acquire, or of discouraging a third party from attempting to acquire, control of our company. This effect could occur even if our shareholders consider the change in control to be in their best interest. We have adopted a shareholder rights plan, the purpose of which is, among other things, to enhance our Board’s ability to protect shareholder interests and to ensure that shareholders receive fair treatment in the event any coercive takeover attempt of our company is made in the future. The shareholder rights plan could make it more difficult for a third party to acquire, or could discourage a third party from acquiring, our company or a large block of our company’s common stock.
Forward-looking statements
This report, including the documents that we incorporate by reference, contains forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act. Any statements about our expectations, beliefs, plans, objectives, assumptions or future events or performance are not historical facts and may be forward-looking. These statements are often, but are not always, made through the use of words or phrases such as “anticipates,” “estimates,” “plans,” “projects,” “trends,” “opportunity,” “comfortable,” “current,” “intention,” “position,” “assume,” “potential,” “outlook,” “remain,” “continue,” “maintain,” “sustain,” “seek,” “achieve,” “continuing,” “ongoing,” “expects,” “management believes,” “we believe,” “we intend” and similar words or phrases, or future or conditional verbs such as “will,” “would,” “should,” “could,” “may,” or similar expressions. Accordingly, these statements involve estimates, assumptions and uncertainties which could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified in their entirety by reference to the factors discussed throughout this report, and in particular those factors listed under the section “Risk Factors.”
Because the factors referred to in the preceding paragraph could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements we make, you should not place undue reliance on any such forward-looking statements. Further, any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any forward-looking statement or statements to reflect events or circumstances after the date on which such statement is made or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. These forward-looking statements include statements regarding:
· potential strategic collaborations with others;
· future capital needs;
· adequacy of existing capital to support operations for a specified time;
· product development and marketing plan;
· features and successes of our cellular therapies;
· manufacturing and facility capabilities;
· clinical trial plans and anticipated results, including the publication thereof;
· anticipation of future losses;
· replacement of manufacturing sources;
· commercialization plans; or
· revenue expectations and operating results.
Item 1B. Unresolved Staff Comments
Not applicable.
We lease approximately 30,000 square feet of office, manufacturing and research and development space in Ann Arbor, Michigan under a lease agreement. This lease was entered into in January 2007 and covers a period of six years, beginning on the date we occupied the new space in May 2007. This lease also includes two five-year options to extend the term to 2018 and 2023, respectively. We believe that our facilities are adequate for our current needs. Additional facilities may be required to support expansion for
research and development activities or to assume manufacturing operations that are currently fulfilled through contract manufacturing relationships.
We are currently not party to any material legal proceedings, although from time to time we may become involved in disputes in connection with the operation of our business.
Item 4. Mine Safety Disclosures
Not applicable.
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchase of Equity Securities
Our common stock is currently quoted on the NASDAQ Capital Market under the symbol “ASTM”. The following table sets forth the high and low closing prices per share of common stock as reported on the NASDAQ Stock Market. Prices per share of our common stock have been adjusted for the eight-for-one reverse stock split on February 18, 2010 on a retroactive basis.
Price Range of Common Stock
|
|
|
High |
|
Low |
| ||
|
Year ended December 31, 2010 |
|
|
|
|
| ||
|
First Quarter |
|
$ |
2.72 |
|
$ |
1.43 |
|
|
Second Quarter |
|
1.88 |
|
1.34 |
| ||
|
Third Quarter |
|
1.61 |
|
1.40 |
| ||
|
Fourth Quarter |
|
4.20 |
|
1.44 |
| ||
|
Year ended December 31, 2011 |
|
|
|
|
| ||
|
First Quarter |
|
$ |
3.25 |
|
$ |
2.06 |
|
|
Second Quarter |
|
3.27 |
|
2.48 |
| ||
|
Third Quarter |
|
2.89 |
|
2.01 |
| ||
|
Fourth Quarter |
|
2.75 |
|
1.79 |
| ||
As of March 13, 2012 there were approximately 540 holders of record of the common stock. We have never paid any cash dividends on our common stock and we do not anticipate paying such cash dividends in the foreseeable future. We currently anticipate that we will retain all future earnings, if any, for use in the development of our business.
Comparison of Shareholder Return
Set forth below is a line graph comparing changes in the cumulative total return on Aastrom’s common stock, a broad market index (the NASDAQ Composite Index), a peer group consisting of the following regenerative medicine companies: Advanced Cell Technology, Inc., Athersys, Inc., Cytori Therapeutics, International Stem Cell Corporation, Neostem, Inc., Osiris Therapeutics, Inc., Pluristem Therapeutics Inc., StemCells, Inc. and Tengion, Inc., for the period commencing on December 31, 2006 and ending on December 31, 2011 (1). The new peer group is the old peer group with the addition of Pluristem Therapeutics Inc.
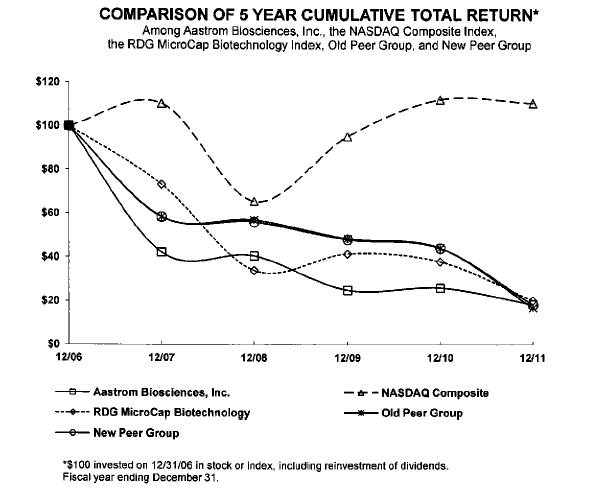
|
Aastrom/Index |
|
12/31/06 |
|
12/31/07 |
|
12/31/08 |
|
12/31/09 |
|
12/31/10 |
|
12/31/11 |
|
|
Aastrom Biosciences, Inc. |
|
100.00 |
|
42.28 |
|
40.65 |
|
25.00 |
|
26.02 |
|
18.50 |
|
|
NASDAQ Composite |
|
100.00 |
|
110.38 |
|
65.58 |
|
95.27 |
|
112.22 |
|
110.58 |
|
|
RDG MicroCap Biotechnology |
|
100.00 |
|
73.24 |
|
34.01 |
|
41.57 |
|
37.98 |
|
20.23 |
|
|
Old Peer Group |
|
100.00 |
|
58.35 |
|
57.01 |
|
48.49 |
|
44.20 |
|
16.94 |
|
|
New Peer Group |
|
100.00 |
|
58.48 |
|
56.12 |
|
48.04 |
|
44.06 |
|
18.30 |
|
(1) Assumes that $100 was invested on December 31, 2006 in Aastrom’s common stock and each index, and that all dividends were reinvested. No cash dividends have been declared on Aastrom’s common stock. Shareholder returns over the indicated period should not be considered indicative of future shareholder returns.
Equity Compensation Plan Information as of December 31, 2011
The following table sets forth information as of December 31, 2011 with respect to compensation plans (including individual compensation arrangements) under which equity securities are authorized for issuances:
|
|
|
Number of Securities |
|
Weighted Average |
|
Number of Securities |
| |
|
Equity compensation plans approved by security holders (employees and directors)(1) |
|
8,116,081 |
|
$ |
2.42 |
|
408,527 |
(2) |
(1) The material features of these securities are described in Note 3 of the Consolidated Financial Statements.
(2) Shares issuable under the 2009 Omnibus Incentive Plan.
Item 6. Selected Financial Data
The following selected financial data should be read in conjunction with our financial statements and the notes thereto, and with Item 7, “Managements’ Discussion and Analysis of Financial Condition and Results of Operations.” The statement of operations data has been derived from and should be read in conjunction with our audited financial statements and notes thereto included elsewhere in this Annual Report on Form 10-K. As previously announced, in 2010, we changed our fiscal year end to December 31 from June 30. The change became effective at the end of the quarter ended December 31, 2010. All references to “years”, unless otherwise noted, refer to the 12-month fiscal year, which prior to July 1, 2010, ended on June 30, and beginning with December 31, 2010, ends on December 31 of each year.
The following tables include selected consolidated summary financial data for the year ended December 31, 2011 and each of the last five years.
|
|
|
Year ended June 30, |
|
Six Months |
|
Year ended |
|
March 24, 1989 |
| |||||||||||||
|
|
|
2007 |
|
2008 |
|
2009 |
|
2010 |
|
2010 |
|
2011 |
|
2011 |
| |||||||
|
Statement of Operations Data (in thousands, except per share amounts): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Revenues: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Product sales and rentals |
|
$ |
94 |
|
$ |
208 |
|
$ |
182 |
|
$ |
89 |
|
$ |
9 |
|
$ |
18 |
|
$ |
1,877 |
|
|
Research and development agreements |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
2,105 |
| |||||||
|
Grants |
|
591 |
|
314 |
|
— |
|
— |
|
244 |
|
— |
|
9,901 |
| |||||||
|
Total revenues |
|
685 |
|
522 |
|
182 |
|
89 |
|
253 |
|
18 |
|
13,883 |
| |||||||
|
Costs and expenses: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
Cost of product sales and rentals (1) |
|
29 |
|
56 |
|
112 |
|
34 |
|
2 |
|
4 |
|
3,041 |
| |||||||
|
Research and development |
|
11,443 |
|
15,249 |
|
11,289 |
|
12,658 |
|
8,609 |
|
21,330 |
|
190,705 |
| |||||||
|
Selling, general and administrative |
|
8,682 |
|
6,436 |
|
4,950 |
|
5,201 |
|
3,265 |
|
7,724 |
|
84,848 |
| |||||||
|
Total costs and expenses |
|
20,154 |
|
21,741 |
|
16,351 |
|
17,893 |
|
11,876 |
|
29,058 |
|
278,594 |
| |||||||
|
Loss from operations |
|
(19,469 |
) |
(21,219 |
) |
(16,169 |
) |
(17,804 |
) |
(11,623 |
) |
(29,040 |
) |
(264,711 |
) | |||||||
|
Other income (expense): |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||||
|
(Increase) decrease in fair value of warrants |
|
1,248 |
|
4,632 |
|
(115 |
) |
3,171 |
|
(7,500 |
) |
9,329 |
|
12,289 |
| |||||||
|
Other income |
|
— |
|
— |
|
— |
|
— |
|
— |
|
— |
|
1,249 |
| |||||||
|
Interest income |
|
1,875 |
|
1,170 |
|
296 |
|
115 |
|
40 |
|
53 |
|
10,772 |
| |||||||
|
Interest expense |
|
— |
|
(84 |
) |
(73 |
) |
(40 |
) |
(5 |
) |
(10 |
) |
(479 |
) | |||||||
|
Net loss |
|
$ |
(16,346 |
) |
$ |
(15,501 |
) |
$ |
(16,061 |
) |
$ |
(14,558 |
) |
$ |
(19,088 |
) |
$ |
(19,668 |
) |
$ |
(240,880 |
) |
|
Net loss applicable to common shares |
|
$ |
(16,346 |
) |
$ |
(15,501 |
) |
$ |
(16,061 |
) |
$ |
(14,558 |
) |
$ |
(19,088 |
) |
$ |
(19,668 |
) |
$ |
(240,880 |
) |
|
Net loss per common share (basic and diluted) |
|
$ |
(1.09 |
) |
$ |
(.96 |
) |
$ |
(0.90 |
) |
$ |
(0.59 |
) |
$ |
(0.65 |
) |
$ |
(0.51 |
) |
|
| |
|
Weighted average number of common shares outstanding (basic and diluted) |
|
14,940 |
|
16,140 |
|
17,877 |
|
24,729 |
|
29,186 |
|
38,627 |
|
|
| |||||||
|
|
|
Year ended June 30, |
|
Six Months December 31, |
|
Year Ended |
| ||||||||||||
|
|
|
2007 |
|
2008 |
|
2009 |
|
2010 |
|
2010 |
|
2011 |
| ||||||
|
Balance Sheet Data (in thousands): |
|
|
|
|
|
|
|
|
|
|
|
|
| ||||||
|
Cash, cash equivalents and short-term investments |
|
$ |
28,325 |
|
$ |
22,462 |
|
$ |
17,000 |
|
$ |
19,119 |
|
$ |
31,248 |
|
$ |
5,530 |
|
|
Working capital |
|
25,742 |
|
21,545 |
|
15,572 |
|
13,847 |
|
1,835 |
|
(14,495 |
) | ||||||
|
Total assets |
|
32,848 |
|
26,217 |
|
19,276 |
|
20,531 |
|
32,827 |
|
7,739 |
| ||||||
|
Long-term debt |
|
1,536 |
|
1,229 |
|
784 |
|
305 |
|
255 |
|
80 |
| ||||||
|
Deficit accumulated during the development stage |
|
(156,972 |
) |
(172,473 |
) |
(188,534 |
) |
(203,092 |
) |
(222,180 |
) |
(241,848 |
) | ||||||
|
Total shareholders’ equity |
|
27,316 |
|
22,916 |
|
17,284 |
|
14,781 |
|
2,922 |
|
(12,971 |
) | ||||||
(1) Cost of product sales and rentals for the period from Inception to December 31, 2011 include a charge of $2,239,000 for excess inventories.
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Change in Fiscal Year End
In 2010, our Board of Directors approved the change in our fiscal year end from June 30 to December 31. The change became effective at the end of the quarter ended December 31, 2010. All references to “years”, unless otherwise noted, refer to the 12-month fiscal year, which prior to July 1, 2010, ended on June 30, and beginning with December 31, 2010, ends on December 31, of each year. All amounts presented for the six months ended December 31, 2009, the twelve months ended December 31, 2010 and the six months ended December 31, 2011 are unaudited.
Overview
We were incorporated in 1989 and are a regenerative medicine company focused on the development of innovative cell therapies to repair or regenerate damaged or diseased tissues. We are developing patient-specific, expanded multicellular therapies for use in the treatment of severe, chronic ischemic cardiovascular diseases. We believe ixmyelocel-T (the new generic name approved by the U.S. Food and Drug Administration (FDA) and United States Adopted Names (USAN) Council in March 2011 for our multicellular therapy) is a disease modifying therapy with multi-functional properties including: tissue remodeling, immuno-modulation and the promotion of angiogenesis. Our proprietary cell-manufacturing technology enables the manufacture of multicellular therapies, expanded from an adult’s own bone marrow, and delivered directly to damaged tissues. Preclinical and clinical data suggest that ixmyelocel-T may be effective in treating patients with severe, chronic ischemic cardiovascular diseases such as CLI. Preliminary data utilizing ixmyelocel-T in dilated cardiomyopathy (DCM) have provided indications of efficacy and safety. Nearly 200 patients have been treated in recent clinical trials using ixmyelocel-T (over 400 patients safely treated since our inception). We recently released positive Phase 2b data from our RESTORE-CLI trial and launched our pivotal Phase 3 REVIVE trial in CLI in February 2012. We also plan to start a randomized, placebo-controlled, double-blinded Phase 2b trial in DCM by mid-2012.
Our Therapy
Ixmyelocel-T is a patient specific, expanded multicellular therapy developed using our proprietary, automated processing system. Ixmyelocel-T is a product derived from an adult’s own bone marrow but it is significantly enhanced compared with the original bone marrow. Our process enhances the patient’s bone marrow mononuclear cells by expanding the mesenchymal stromal cells and alternatively activated macrophages while retaining many of the hematopoietic cells. The manufacture of our patient specific, expanded multicellular therapies is done under current Good Manufacturing Practices (cGMP) and current Good Tissue Practices (cGTP) guidelines required by the FDA.
Our therapy has several features that we believe are primarily responsible for success in treating adult patients with severe, chronic cardiovascular diseases:
Patient specific (autologous) — we start with the patient’s own cells, which are accepted by the patient’s immune system allowing the cells to integrate into existing functional tissues. This characteristic of our therapy, we believe, eliminates both the risk of rejection and the risk of having to use immunosuppressive therapy pre- or post-therapy. Our data also suggests that ixmyelocel-T provides the potential for long-term engraftment and tissue repair.
Expanded — we begin with a small amount of bone marrow from the patient (up to 60 ml) and significantly expand the number of certain cell types, primarily CD90+ (mesenchymal stromal cells or MSCs) and CD14+auto+ (alternatively activated macrophages) to far more than are present in the patient’s own bone marrow (up to 200 times the number of certain cell types compared with the starting bone marrow aspirate). Ixmyelocel-T is derived from the patient’s own bone marrow but it is significantly enhanced compared with the starting bone marrow.
Multicellular — we believe the multiple cell types in ixmyelocel-T, which are normally only found in bone marrow but in smaller quantities, possess the key functions required for tissue remodeling, immuno-modulation and the promotion of angiogenesis.
Minimally invasive — our procedure for taking bone marrow (an “aspirate”) can be performed in an out-patient setting and takes approximately 15 minutes. For diseases such as CLI, the administration of ixmyelocel-T is performed in an out-patient setting (e.g. a physician’s office) in a one-time, approximately 20 minute procedure.
Safe — bone marrow and bone marrow-derived therapies have been used safely and efficaciously in medicine for over three decades. Our product, ixmyelocel-T, a bone marrow-derived, patient specific, expanded multicellular therapy leverages this body of scientific study and medical experience.
Our therapy is produced at our cell manufacturing facility in the United States, located at our headquarters in Ann Arbor, Michigan.
Clinical Development Programs
Our clinical development programs are focused on addressing areas of high unmet medical needs in severe, chronic ischemic cardiovascular diseases. We have completed a successful Phase 2b clinical trial in CLI. We have reached agreement with the FDA on CMC which has allowed us to launch our pivotal Phase 3 REVIVE clinical trial in the first quarter of 2012 with a protocol approved by FDA through the Special Protocol Assessment (SPA) process. Our CLI development program has also received Fast Track Designation from the FDA. We have completed our Phase 1/2 clinical trials in DCM and plan to begin a randomized, placebo-controlled, double-blinded Phase 2b trial in mid-2012. Our DCM development program has received Orphan Disease Designation from the FDA.
Results to date in our clinical trials may not be indicative of results obtained from subsequent patients enrolled in those trials or from future clinical trials. Further, our future clinical trials may not be successful or we may not be able to obtain the required Biologic License Application (BLA) approval to commercialize our products in the United States in a timely fashion, or at all. See “Risk Factors”.
Critical Limb Ischemia
Background
CLI is the most serious and advanced stage of peripheral arterial disease (PAD). PAD is a chronic atherosclerotic disease that progressively restricts blood flow in the limbs and can lead to serious medical complications. This disease is often associated with other serious clinical conditions including hypertension, cardiovascular disease, hyperlipidemia, diabetes, obesity and stroke. CLI is used to describe patients with the most severe forms of PAD: those with chronic ischemia-induced pain (even at rest) or tissue loss (ulcers or gangrene) in the limbs, often leading to amputation and death. Many CLI patients are considered “no option” patients as they have exhausted all other treatment options with the exception of amputation. The one-year and four-year mortality rates for no option CLI patients that progress to amputation are approximately 25% and 70%, respectively. Ixmyelocel-T, our disease modifying therapy with multiple functions, has shown significant promise in the treatment of CLI patients with existing tissue loss and no option for revascularization. Currently, there are an estimated 250,000 no option CLI patients in the U.S.
Phase 2b Clinical Program — RESTORE CLI
Our U.S. Phase 2b RESTORE-CLI program was a multi-center, randomized, double-blind, placebo-controlled clinical trial. This clinical trial was designed to evaluate the safety and efficacy of ixmyelocel-T in the treatment of patients with CLI and no option for revascularization. It was the largest multi-center, randomized, double-blind, placebo-controlled cellular therapy study ever conducted in no option CLI patients. We completed enrollment of this trial in February 2010 with a total of 86 patients at 18 sites across the United States, with the last patient treated in March 2010. These patients were followed for a period of 12 months after treatment. In addition to assessing the safety of our product, efficacy endpoints included time to first occurrence of treatment failure — the trial’s primary efficacy end-point — (defined as major amputation, all-cause mortality, doubling in wound surface area and de novo gangrene), amputation-free survival (defined as major amputation and all-cause mortality), major amputation rates, level of amputation, wound healing, patient quality of life and pain scores. The primary purpose of the trial was to assess performance of our therapy and, if positive, prepare for a Phase 3 program.
Results to date of the RESTORE-CLI trial have included two planned interim analyses and a final 12-month report:
· In June 2010, we reported interim results at the Society of Vascular Surgery Meeting. The interim analysis included the six-month results for the first 46 patients enrolled in the trial and twelve-month results for the first 30 patients enrolled in the trial. Results of this analysis demonstrated that the study achieved both its primary safety endpoint and primary efficacy endpoint of time to first occurrence of treatment failure. The results related to the primary endpoint were statistically significant (p=0.0053). Analysis of the data for amputation free survival, a secondary efficacy endpoint which the study was not powered to demonstrate, were positive and showed a statistically significant reduction in event rates in favor of our therapy (p=0.038). Other endpoints measured (e.g., major amputation rate, complete wound healing, change in Wagner wound scale) showed encouraging trends, but did not reach statistical significance at the interim analysis.
· In November 2010, we presented six-month data on all 86 patients enrolled in the trial and twelve-month data on the first 72 patients at the VEITHsymposium™ non-CME satellite session. Results of this analysis showed that the study again achieved both its primary safety endpoint and primary efficacy endpoint of time to first occurrence of treatment failure. The findings related to time to first occurrence of treatment failure were statistically significant (p=0.0132). Further analyses showed a clinically meaningful reduction of 56% in treatment failure events. Analysis of the data for amputation-free survival, showed a clinically meaningful reduction in event rates of 24%, but did not show statistical significance (p=0.5541).
· In November 2011, the final 12-month data on all patients from the RESTORE-CLI trial were presented at the American Heart Association Scientific Sessions. Patients in the treatment arm showed a 62% reduction in risk relative to placebo in the primary efficacy endpoint of time to first occurrence of treatment failure (p=0.0032). While the study was not powered to show statistical significance in the secondary endpoint of amputation free survival, results from a subgroup of 45 patients with wounds at baseline (the approximate profile of the Phase 3 patient population) showed a positive trend in this measure (21% ixmyelocel-T treated vs 44% control event rate; p=0.0802). The study also met the primary safety endpoint with no meaningful differences between the treated and control groups.
Phase 3 Clinical Program — REVIVE
In February 2012, we began screening patients in the pivotal Phase 3 REVIVE clinical trial for patients with CLI and no option for revascularization. The first patient is expected to be randomized and aspirated in March 2012. Leading up to the launch of the REVIVE pivotal trial, we received Fast Track Designation from the FDA for use of ixmyelocel-T for CLI in October 2010 and reached agreement with the FDA on a Special Protocol Assessment (SPA) in July 2011. The Phase 3 REVIVE No Option Trial that we agreed to with the FDA under the SPA process includes 594 no option CLI patients with tissue loss (ulcers and gangrene) at baseline. Patients will be randomized 1:1 and followed for 12 months for the primary efficacy endpoint of amputation-free survival. Patients will be followed for an additional 6 months for safety. We anticipate that enrollment will occur at approximately 80 sites across the U.S.
Dilated Cardiomyopathy
Background
DCM is a severe, chronic cardiovascular disease that leads to enlargement of the heart, reducing the pumping function of the heart to the point that blood circulation is impaired. Patients with DCM typically present with symptoms of congestive heart failure, including limitations in physical activity and shortness of breath. It is currently estimated that there are approximately 125,000 ischemic DCM patients in the U.S. There are two types of DCM: ischemic and non-ischemic. Ischemic DCM, the most common form representing an estimated 60% of all DCM patients, is associated with atherosclerotic cardiovascular disease. Patient prognosis depends on the stage and cause of the disease but is typically characterized by a very poor quality of life and a high mortality rate.
Current treatments for DCM patients include both heart transplantation and left ventricular assist devices (LVADs). There are less than 2,500 heart transplantations in the U.S. each year, many DCM patients are not eligible, and they’re expensive at an estimated cost of over $750,000. LVADs are also expensive at an estimated cost of over $175,000 and have a mortality rate of 50% at 2 years.
In February 2007, the FDA granted Orphan Drug Designation to ixmyelocel-T for the treatment of DCM. Our DCM development program is currently in Phase 2. We recently completed follow up on two U.S. Phase 1/2 trials investigating surgical and catheter-based delivery for our product in the treatment of DCM in reporting stages. We plan to initiate a randomized, placebo-controlled, double-blinded Phase 2b trial using catheter delivery for 60 — 80 ischemic DCM patients in the U.S. in mid-2012.
Surgical Trial Program — DCM
We completed enrollment of 40 DCM patients in the IMPACT-DCM clinical trial in January 2010 and the final patient was treated in March 2010. Participants in the IMPACT-DCM clinical trial were required to have New York Heart Association (NYHA) functional class III or IV heart failure, a left ventricular ejection fraction (LVEF) of less than or equal to 30% (60-75% is typical for a healthy person), and meet other eligibility criteria, including optimized medical therapy. Patients were randomized in an approximate 3:1 ratio of treatment to control group. Patients in the treatment group received our therapy through direct injection into the heart muscle during minimally invasive-surgery (involving a chest incision of approximately 2 inches). The primary objective of this study was to assess the safety of ixmyelocel-T in patients with DCM. Efficacy measures include cardiac dimensions and tissue mass, cardiac function (e.g. cardiac output, LVEF, cardiopulmonary exercise testing parameters), cardiac perfusion and viability, as well as other efficacy endpoints. NYHA functional class and quality of life are also assessed. Patients were followed for 12 months after treatment.
Six-month data from the IMPACT-DCM interim analysis were presented at The Sixth International Conference on Cell Therapy for Cardiovascular Disease in January 2011. Results indicated that ixmyelocel-T is safe and showed that serious adverse events were associated with the surgical procedure and not the cellular therapy. Adverse events after the initial peri-operative period were roughly equal between the control and treatment groups. Efficacy findings include positive trends in clinical endpoints, quality of life, functional, and structural parameters in the treatment group as compared with the control group.
Twelve-month data on all 40 patients enrolled in the IMPACT-DCM trial were presented at the 15th Annual Heart Failure Society of America Scientific Meeting in September 2011. Results were consistent with the six-month interim analysis, indicating that ixmyelocel-T is safe and showed that serious adverse events were associated with the surgical procedure and not the therapy. Efficacy results were also consistent with the six-month results and demonstrated promising efficacy results in patients with ischemic DCM.
Catheter Trial Program — DCM
The Catheter-DCM clinical trial was designed to explore catheter-based direct injection delivery of ixmyelocel-T to treat DCM patients. This multi-center, randomized, controlled, prospective, open-label, Phase 2 study enrolled approximately 11 patients with ischemic DCM and 10 patients with non-ischemic DCM at clinical sites across the United States. Participants met the same criteria as stated above for the IMPACT-DCM surgical trial. The first patient was enrolled into the trial in April 2010 and enrollment concluded in December 2010 with 21 patients enrolled.
In September 2011, we reported results from a six-month interim analysis of patients treated in the Catheter-DCM Phase 2 trial. In this analysis, efficacy parameters were consistent with those seen in the IMPACT-DCM trial results. In addition, the adverse event profile suggests that catheter administration of ixmyelocel-T is safe and appears to cause fewer adverse events compared to surgical administration. We expect to report 12-month results from the Catheter-DCM Phase 2 trial in Q2 2012. We plan to launch a randomized, placebo-controlled, double-blind Phase 2b trial in mid-2012 in approximately 60 — 80 ischemic DCM patients in the U.S. and using catheter administration.
Critical Accounting Policies and Estimates
The preparation of our consolidated financial statements in accordance with U.S. generally accepted accounting principles (GAAP) requires management to make estimates and assumptions that affect the reported amounts of assets, liabilities, net revenues and expenses, and related disclosures. We believe our estimates and assumptions are reasonable; however, actual results and the timing of the recognition of such amounts could differ from these estimates.
There are accounting policies that we believe are significant to the presentation of our consolidated financial statements. The most significant accounting policies relate to stock-based compensation and warrants.
Stock-Based Compensation — Calculating stock-based compensation expense requires the input of highly subjective assumptions. We apply the Black-Scholes option-pricing model to determine the fair value of our stock options. Inherent in this model are assumptions related to expected stock-price volatility, option life, risk-free interest rate and dividend yield. We estimate the volatility of our common stock at the date of grant based on historical volatility. We estimate the expected life of options that vest solely on service using the “simplified method” provided for in the Securities and Exchange Commission Staff Accounting Bulletin No. 110. The “simplified method” is permitted for estimating the expected term of “plain-vanilla” stock options for which the historical stock option exercise experience is likely not indicative of future exercise patterns. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected life of the options. The dividend rate is based on our historical rate, which we anticipate to remain at zero. The assumptions used in calculating the fair value of stock options represent our best estimates, however; these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and different assumptions are used, the stock-based compensation expense could be materially different in the future. In addition, we are required to estimate the expected forfeiture rate and only recognize expense for those stock options and restricted stock awards and units expected to vest over the service period. We estimate the forfeiture rate considering the historical experience of our stock-based awards. If the actual forfeiture rate is different from the estimate, we adjust the expense accordingly.
Warrants — Warrants that could require cash settlement or have anti-dilution price protection provisions are recorded as liabilities at their estimated fair value at the date of issuance, with subsequent changes in estimated fair value recorded in other income (expense) in our statement of operations in each subsequent period. In general, warrants with anti-dilution provisions are measured using the Monte Carlo valuation model, while the others are measured using the Black-Scholes valuation model. Both of the methodologies are based, in part, upon inputs for which there is little or no observable market data, requiring the Company to develop its own assumptions. Inherent in both of these models are assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. We estimate the volatility of our common stock at the date of issuance, and at each subsequent reporting period, based on historical volatility that matches the expected remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of the warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on our historical rate, which we anticipate to remain at zero. For those warrants valued using a Monte Carlo model, we estimate the probability and timing of potential future financings and fundamental transactions, as applicable. The assumptions used in calculating the estimated fair value of the warrants represent our best estimates; however, these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and different assumptions are used, the warrant liability and the change in estimated fair value could be materially different.
The summary of significant accounting policies should be read in conjunction with our consolidated financial statements and related notes and this discussion of our results of operations.
Results of Operations
Total revenues decreased to $18,000 for the year ended December 31, 2011 from $253,000 for the twelve months ended December 31, 2010. Total revenues decreased to $9,000 for the six months ended December 31, 2011 from $253,000 for the six months ended December 31, 2010. The decreases in both periods are due to the non-recurring proceeds from the Qualifying Therapeutic Discovery Project in November 2010. Total revenues increased to $253,000 for the six months ended December 31, 2010 from $89,000 for the six months ended December 31, 2009. The increase is due to proceeds from the Qualifying Therapeutic Discovery Project in November 2010. Total revenues decreased to $89,000 in fiscal 2010 from $182,000 in fiscal 2009 due to the declines in volume of cell production sales for investigator sponsored clinical trials in Spain and limited cell manufacturing supplies to a research institute in the United States. At such time as we satisfy applicable regulatory approval requirements, we expect the sales of our cell-based products will constitute nearly all of our product sales revenues.
Total costs and expenses increased to $29,058,000 for the year ended December 31, 2011 from $21,279,000 for the twelve months ended December 31, 2010. Total costs and expenses increased to $15,282,000 for the six months ended December 31, 2011 from $11,876,000 for the six months ended December 31, 2010. The increases in both periods are due to increased cash compensation and non-cash stock based compensation associated with an increase in headcount, as well as increased CRO costs and increased consulting costs due to preparations for the Phase 3 CLI program. Total costs and expenses increased to $11,876,000 for the six months ended December 31, 2010 from $8,490,000 for the six months ended December 31, 2009 due to preparation for our Phase 3 CLI program
and increased consulting and non-cash stock-based compensation expense. Total costs and expenses increased to $17,893,000 in fiscal 2010 from $16,351,000 in fiscal 2009 due to increased clinical activity related to our DCM and CLI programs.
Research and development expenses increased to $21,330,000 for the year ended December 31, 2011 from $15,073,000 for the twelve months ended December 31, 2010 due to increased cash compensation and non-cash stock based compensation associated with an increase in headcount, as well as increased CRO costs and increased consulting costs due to preparations for the Phase 3 CLI program. These amounts include non-cash stock-based compensation of $2,164,000 for the year ended December 31, 2011 compared to $670,000 for the twelve months ended December 31, 2010 due to increased headcount and higher fair value of options granted. Research and development expenses increased to $11,654,000 for the six months ended December 31, 2011 from $8,609,000 for the six months ended December 31, 2010 due to increased headcount and clinical activity related to DCM and CLI programs. These amounts include non-cash stock-based compensation of $1,123,000 for the six months ended December 31, 2011 compared to $549,000 for the six months ended December 31, 2010. Research and development expenses increased to $8,609,000 for the six months ended December 31, 2010 from $6,194,000 for the six months ended December 31, 2009 due to preparation for our Phase 3 CLI program. These amounts include non-cash stock-based compensation of $549,000 for the six months ended December 31, 2010, compared to $361,000 for the six months ended December 31, 2009. In fiscal 2010, research and development expenses increased to $12,658,000 from $11,289,000 in fiscal 2009 due to increased clinical activity related to our DCM and CLI programs. These amounts include non-cash stock-based compensation of $484,000 for fiscal 2010, compared to $579,000 for fiscal 2009.
Our major ongoing research and development programs are focused on the clinical development of our technology platform for treatment of severe, chronic cardiovascular diseases. The following table summarizes the approximate allocation of cost for our research and development projects (in thousands):
|
|
|
12 Months Ended December 31, |
| ||||
|
|
|
2010 |
|
2011 |
| ||
|
|
|
|
|
|
| ||
|
Critical Limb Ischemia |
|
$ |
6,799 |
|
$ |
15,842 |
|
|
Dilated Cardiomyopathy |
|
8,191 |
|
5,452 |
| ||
|
Other |
|
83 |
|
36 |
| ||
|
Total research and development expenses |
|
$ |
15,073 |
|
$ |
21,330 |
|
|
|
|
Six Months Ended December 31, |
| |||||||
|
|
|
2009 |
|
2010 |
|
2011 |
| |||
|
|
|
|
|
|
|
|
| |||
|
Critical Limb Ischemia |
|
$ |
2,456 |
|
$ |
4,092 |
|
$ |
9,337 |
|
|
Dilated Cardiomyopathy |
|
3,673 |
|
4,494 |
|
2,307 |
| |||
|
Other |
|
65 |
|
23 |
|
10 |
| |||
|
Total research and development expenses |
|
$ |
6,194 |
|
$ |
8,609 |
|
$ |
11,654 |
|
|
|
|
Year Ended June 30, |
| ||||
|
|
|
2009 |
|
2010 |
| ||
|
|
|
|
|
|
| ||
|
Critical Limb Ischemia |
|
$ |
5,334 |
|
$ |
5,163 |
|
|
Dilated Cardiomyopathy |
|
5,510 |
|
7,370 |
| ||
|
Other |
|
445 |
|
125 |
| ||
|
Total research and development expenses |
|
$ |
11,289 |
|
$ |
12,658 |
|
Selling, general and administrative expenses increased to $7,724,000 for the year ended December 31, 2011 from $6,204,000 for the twelve months ended December 31, 2010 due to an increase in non-cash stock-based compensation, and legal and consulting expenses. Stock-based compensation included in selling, general and administrative expenses increased to $1,753,000 for the year ended December 31, 2011 from $730,000 for the twelve months ended December 31, 2010. Selling, general and administrative expenses increased to $3,626,000 for the six months ended December 31, 2011 from $3,265,000 for the six months ended December 31, 2010. Stock-based compensation included in selling, general and administrative expenses
increased to $933,000 for the six months ended December 31, 2011 from $494,000 for the six months ended December 31, 2010. Selling, general and administrative expenses increased to $3,265,000 for the six months ended December 31, 2010 from $2,262,000 for the six months ended December 31, 2009 due to an increase in non-cash stock-based compensation and consulting costs. Stock-based compensation included in selling, general and administrative expenses increased to $494,000 for the six months ended December 31, 2010 from a net reversal of expense of $11,000 for the six months ended December 31, 2009. In fiscal 2010, selling, general and administrative expenses increased to $5,201,000 from $4,950,000 in fiscal 2009 due to increased cash compensation costs and increased legal and consulting costs offset by lower non-cash stock-based compensation expense. Stock-based compensation expense included in selling, general and administrative expenses decreased to $225,000 in fiscal 2010 from $783,000 in fiscal 2009. Stock-based compensation expense for the six months ended December 31, 2009 and for fiscal 2010 was impacted by the reversal of previously recognized expense for options that were forfeited in excess of our estimated rate of forfeiture. Approximately $279,000 of the reversal was for certain options held by George W. Dunbar that were forfeited when he stepped down as Chief Executive Officer, President and Chief Financial Officer in December 2009.
Non-cash income (expense) from the change in fair value of warrants was $9,329,000 for the year ended December 31, 2011 compared to ($4,593,000) for the twelve months ended December 31, 2010. Non-cash income (expense) from the change in fair value of warrants was $10,540,000 for the six months ended December 31, 2011 compared to ($7,500,000) for the six months ended December 31, 2010. The fluctuations in both periods are due to changes in fair value of our stock price. Non-cash income (expense) from the change in fair value of warrants was ($7,500,000) for the six months ended December 31, 2010 compared to $264,000 for the six months ended December 31, 2009. For fiscal 2010, warrant income (expense) was $3,171,000 compared to ($115,000) in fiscal 2009. The fluctuations are due to the issuance of warrants in the January 2010 and December 2010 financings, as well as changes in the fair value of our warrant liability resulting from changes in the fair value of our common stock. Fluctuations in the fair value of warrants in future periods could result in significant non-cash adjustments to the consolidated financial statements, however any income or expense recorded will not impact our cash and cash equivalents, operating expenses or cash flows.
Interest income was $53,000 in the year ended December 31, 2011 compared to $106,000 for the twelve months ended December 31, 2010. Interest income was $16,000 in the six months ended December 31, 2011 compared to $40,000 for the six months ended December 31, 2010. Interest income was $40,000 in the six months ended December 31, 2010 compared to $49,000 for the six months ended December 31, 2009. In fiscal 2010, interest income was $115,000 compared to $296,000 in fiscal 2009. The fluctuations in interest income are due primarily to corresponding changes in the levels of cash, cash equivalents and short-term investments combined with interest rate changes during the periods.
Our net loss was $19,668,000, or $0.51 per share for the year ended December 31, 2011 compared to $25,534,000, or $0.90 per share for the twelve months ended December 31, 2010. The net loss for the six months ended December 31, 2011 was $4,723,000, or $0.12 per share, compared to $19,088,000, or $0.65 per share for the six months ended December 31, 2010. Our net loss was $19,088,000, or $0.65 per share for the six months ended December 31, 2010 compared to $8,112,000, or $0.38 per share for the six months ended December 31, 2009. In fiscal 2010, our net loss was $14,558,000, or $0.59 per share, compared to $16,061,000, or $0.90 per share in fiscal 2009. The changes in net loss are primarily due to the non-cash fluctuations in the fair value of warrants, in addition to the changes in research and development expenses and selling, general and administrative expense as described in more detail above. Loss per share comparisons were also impacted by the issuance of 10,000,000 shares of common stock in December 2010 and 6,510,000 shares of common stock in January 2010.
Because of the uncertainties of clinical trials and the evolving regulatory requirements applicable to our products, estimating the completion dates or cost to complete our major research and development programs would be highly speculative and subjective. The risks and uncertainties associated with developing our products, including significant and changing governmental regulation and the uncertainty of future clinical study results, are discussed in greater detail in the “Any changes in the governmental regulatory classifications of our products could prevent, limit or delay our ability to market and develop our products,” “Our inability to complete our product development activities successfully would severely limit our ability to operate or finance operations,” and “We must successfully complete our clinical trials to be able to market certain of our products,” sections under the heading “Risk Factors” in Item 1A of this report. The lengthy process of seeking regulatory approvals for our product candidates, and the subsequent compliance with applicable regulations, will require the expenditure of substantial resources. Any failure by us to obtain, or any delay in obtaining, regulatory approvals could cause our research and development expenditures to increase and, in turn, have a material adverse effect on our results of operations. We cannot be certain when any net cash inflow from products validated under our major research and development project, if any, will commence.
We have not generated any net taxable income since our inception and therefore have not paid any federal income taxes since inception. We issued shares of common stock in prior years, which resulted in multiple ownership changes under relevant taxation rules (Section 382 of the Internal Revenue Code). Consequently, pursuant to these taxation rules, the utilization of net operating loss and tax credit loss and tax carryforwards may be significantly limited in future periods, even if we generate taxable income. Such limitations may result in our carryforwards expiring before we can utilize them. At December 31, 2011, we had generated cumulative U.S. federal tax net operating loss and tax credit carryforwards of $156,542,000 and $1,600,000, respectively, which will expire in various periods through 2030 if not utilized. Our ability to utilize our net operating loss and tax credit carryforwards may become subject to further annual limitation in the event of future changes in ownership under the taxation rules.
Liquidity and Capital Resources
We are currently focused on utilizing our technology to produce patient specific cell-based products for use in regenerative medicine applications. At such time as we satisfy applicable regulatory approval requirements, we expect the sales of our cell-based products to constitute nearly all of our product sales revenues.
We do not expect to generate positive cash flows from our consolidated operations for at least the next several years and then only if we achieve significant product sales. Until that time, we expect that our revenue sources from our current activities will consist of only minor sales of our cell products and manufacturing supplies to our academic collaborators, grant revenue, research funding and potential licensing fees or other financial support from potential future corporate collaborators.
We expect that we will need to raise significant additional funds or pursue strategic transactions or other strategic alternatives in order to complete our product development programs, complete clinical trials needed to market our products, and commercialize our products. To date, we have financed our operations primarily through public and private sales of our equity securities, and we expect to continue to seek to obtain the required capital in a similar manner. As a development stage company, we have never been profitable and do not anticipate having net income unless and until significant product sales commence. With respect to our current activities, this is not likely to occur until we obtain significant additional funding, complete the required clinical trials for regulatory approvals, and receive the necessary approvals to market our products. Through December 31, 2011, we had accumulated a net loss of approximately $240,880,000. We cannot provide any assurance that we will be able to achieve profitability on a sustained basis, if at all, obtain the required funding, obtain the required regulatory approvals, or complete additional corporate partnering or acquisition transactions.
We have financed our operations since inception primarily through public and private sales of our equity securities, which, from inception through December 31, 2011, have totaled approximately $228,877,000 and, to a lesser degree, through grant funding, payments received under research agreements and collaborations, interest earned on cash, cash equivalents, and short-term investments, and funding under equipment leasing agreements. These financing sources have generally allowed us to maintain adequate levels of cash and other liquid investments.
Our combined cash and cash equivalents totaled $5,530,000 at December 31, 2011, a decrease of $25,718,000 from December 31, 2010. The primary uses of cash during the year ended December 31, 2011 were for employee related expenses and preparations for the Phase 3 CLI program.
Our combined cash, cash equivalents and short-term investments totaled $31,248,000 at December 31, 2010, an increase of $12,129,000 from June 30, 2010. During the six months ended December 31, 2010, the primary source of cash, and cash equivalents and short-term investments was from the sale of our equity securities in December 2010 with net proceeds of $20,600,000, in addition to $1,074,000 from the exercise of stock purchase warrants and stock options. The primary uses of cash, cash equivalents and short-term investments during the six months ended December 31, 2010 included $9,252,000 for our operations and working capital requirements, and $305,000 in capital expenditures.
Our combined cash, cash equivalents and short-term investments totaled $19,119,000 at June 30, 2010, an increase of $2,119,000 from June 30, 2009. During the year ended June 30, 2010, the primary source of cash, cash equivalents and short-term investments was from the sale of our equity securities in January 2010 with net proceeds of $12,432,000, in addition to $5,094,000 of equity securities issued pursuant to the June 2009 agreement with Fusion Capital. The primary uses of cash, cash equivalents and short-term
investments during the year ended June 30, 2010 included $15,085,000 to finance our operations and working capital requirements, and $120,000 in capital expenditures.
Our cash and cash equivalents included short-term investments which include certificates of deposit with original maturities of less than twelve months.
Our future cash requirements will depend on many factors, including continued scientific progress in our research and development programs, the scope and results of clinical trials, the time and costs involved in obtaining regulatory approvals, the costs involved in filing, prosecuting and enforcing patents, competing technological and market developments, costs of possible acquisition or development of complementary business activities and the cost of product commercialization. We do not expect to generate positive cash flows from operations for at least the next several years due to the expected spending for research and development programs and the cost of commercializing our product candidates. We intend to seek additional funding through research and development agreements or grants, distribution and marketing agreements and through public or private debt or equity financing transactions. Successful future operations are subject to several technical and risk factors, including our continued ability to obtain future funding, satisfactory product development, obtaining regulatory approval and market acceptance for our products.
In order to complete our Phase 3 CLI trial, grow and expand our business, introduce our product candidates into the marketplace and possibly acquire or develop complementary business activities, we will need to raise additional funds. We will also need additional funds or a collaborative partner, or both, to finance the research and development activities of our product candidates for the expansion of additional cell types. We expect that our primary sources of capital for the foreseeable future will be through collaborative arrangements and through the public or private sale of our equity or debt securities. There can be no assurance that such collaborative arrangements, or any public or private financing, will be available on acceptable terms, if at all, or can be sustained. Several factors will affect our ability to raise additional funding, including, but not limited to, market volatility of our common stock, continued stock market listing and economic conditions affecting the public markets generally or some portion or the entire technology sector.
In October 2008, we entered into a common stock purchase agreement with Fusion Capital Fund II, LLC (âFusion Capitalâ) pursuant to which we were entitled to sell up to $15,000,000 of our common stock to Fusion Capital. In April 2009, we concluded the sales of the registered shares under this common stock purchase agreement. Under this purchase agreement we issued 2,836,583 shares of common stock for net proceeds of approximately $8,600,000. In connection with entering into this common stock purchase agreement, we issued to Fusion Capital 242,040 shares of our common stock as a commitment fee. We also issued to Fusion Capital an additional 139,229 shares as a pro rata commitment fee.
In June 2009, we entered into a $30,000,000 common stock purchase agreement with Fusion Capital. Pursuant to the purchase agreement with Fusion Capital, we had the right to sell to Fusion Capital up to $30,000,000 of our common stock over a 25-month period, which began on July 1, 2009. Such sales were to be made from time to time in amounts between $100,000 and $4,000,000, depending on certain conditions as set forth in the agreement. In consideration for entering into the purchase agreement, we issued 181,530 shares of our common stock to Fusion Capital as an initial commitment fee. During fiscal 2010, 1,718,538 shares of our common stock (including 51,432 shares related to its commitment fee) were issued to Fusion Capital for net proceeds of approximately $5,100,000. No additional shares were issued to Fusion Capital subsequent to October 2009, and we terminated the agreement with Fusion Capital in November 2010.
On January 21, 2010, we completed the sale of 6,509,637 units (including 740,387 units sold to the underwriter pursuant to the exercise of its over-allotment option) at a public offering price of $2.08 per unit. Each unit consisted of (i) one share of our common stock, (ii) a Class A warrant to purchase 0.75 of a share of our common stock at an exercise price of $2.52 per share (as adjusted from $2.97 per share for the anti-dilution provision triggered in the December 2010 financing) and (iii) a Class B warrant to purchase 0.50 of a share of our common stock at an exercise price of $2.08 per share. We received approximately $12,400,000 in net proceeds from the sale of the units (including the partially exercised option of the over-allotment), after underwriting discounts and commissions and other offering expenses.
The 6,509,637 units consist of an aggregate of 6,509,637 shares of our common stock, Class A warrants to purchase an aggregate of 4,882,228 shares of our common stock and Class B warrants to purchase an aggregate of 3,254,818 shares of our common stock. The Class A warrants are exercisable for a five year period commencing on July 21, 2010. The Class B warrants were exercisable at any time from January 21, 2010 through July 21, 2010 and expired unexercised.
On December 15, 2010, we completed the sale of 10,000,000 units at a public offering price of $2.25 per unit. Each unit consisted of one share of our common stock and a warrant to purchase one share of our common stock at an exercise price of $3.22 per share. We received approximately $20,600,000 in net proceeds from the sale of the units, after underwriting discounts and commissions and other offering expenses. The warrants to purchase 10,000,000 shares of our common stock are exercisable for a five year period commencing on December 15, 2010.
On March 9, 2012, we completed the sale of 12,308 shares of Series B convertible preferred stock at an offering price of $3,250 per share. We received approximately $37,800,000 in net proceeds from the sale of the shares, after offering expenses. On March 9, 2012, we completed the sale of 12,308 shares of Series B convertible preferred stock at an offering price of $3,250 per share. We received approximately $37,800,000 in net proceeds from the sale of the shares, after offering expenses. The Series B preferred stock consists of Series B-1 non-voting convertible preferred stock, which we issued at the closing and Series B-2 voting convertible preferred stock. The Series B-1 non-voting convertible preferred stock will not be entitled to vote on matters on which the common shareholders are generally entitled to vote. The Series B-2 preferred stock will be entitled to vote with the holders of the common stock as a single class, with each share of Series B-2 voting convertible preferred stock having the number of votes equal to the number of shares of common stock issuable upon conversion of such Series B-2 preferred stock. Any holder of Series B-1 preferred stock may exchange its shares for shares of Series B-2 preferred stock on a one-for-one basis if the shareholder approval required in accordance with Nasdaq Marketplace Rule 5635(b) has been obtained and subject to certain terms and limitations. The Series B preferred stock will, with respect to dividend rights and rights on liquidation, winding-up and dissolution, rank on parity with any other class or series of Aastrom capital stock that we may issue in the future which is designated as being on parity with the Series B preferred stock, and rank senior to our common stock and Series A preferred stock. The Series B preferred stock is convertible, at the option of the holder thereof at any time after the five year anniversary of the closing of the offering, into shares of our common stock at a conversion price of $3.25 per share of common stock, subject to the Company obtaining any shareholder approval required in accordance with Nasdaq Marketplace Rule 5635(b). At any time after the five year anniversary of issuance, Aastrom may elect to convert any or all outstanding shares of Series B preferred stock into shares of our common stock, subject to certain limitations. Dividends on the Series B preferred stock will be cumulative and compound daily, at a rate of 11.5% per annum, payable upon conversion, liquidation, redemption or other similar events, and payable in Series B preferred stock until the five year anniversary of issuance. Following the five year anniversary of issuance and until the earlier of the tenth anniversary of the closing of the offering and the date no Series B preferred stock remain outstanding, dividends will accrue at a rate of 8% per annum and will be payable in cash or Series B preferred stock, at our option. Unless prohibited by Michigan law governing distributions to shareholders, the Series B-1 preferred stock shall be redeemable at the option of holder of the Series B-1 preferred stock commencing at any time after the five year anniversary of issuance, liquidation, winding up, dissolution or other similar events, subject to certain terms and limitations.
We believe that we will have adequate liquidity to finance our operations, including development of our products and product candidates, via our cash and cash equivalents on hand as of December 31, 2011, and the net proceeds of $37,800,000 from the financing that closed in March 2012, until at least December 31, 2012. While our budgeted cash usage and operating plan for 2012 does not currently contemplate taking additional actions to reduce the use of cash over the next twelve months, we could, if necessary, delay or forego certain budgeted discretionary expenditures such as anticipated hiring plans or certain non-critical research and development expenditures. In addition, we could slow down or delay certain clinical trial activity (without jeopardizing our Phase 3 clinical trial for CLI) such that we will have sufficient cash on hand until at least December 31, 2012. These estimates are based on certain assumptions which could be negatively impacted by the matters discussed under this heading and under the caption “Risk Factors,” in Item 1A of this report.
If we cannot raise necessary funding in the future, we may not be able to develop or enhance products, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements, which would have a material adverse impact on our business, financial condition and results of operations. See “Risk Factors” and “Notes to Consolidated Financial Statements” included herein.
Off-Balance Sheet Arrangements
We do not have any off-balance sheet arrangements or relationships with unconsolidated entities or financial partnerships, such as entities often referred to as structured finance or special purpose entities.
Long-Term Contractual Obligations and Commitments
The following table summarizes future estimated cash payments to be made under existing contractual obligations (in thousands):
|
|
|
|
|
Payments Due by Period |
| ||||||||||||||
|
Contractual Obligations |
|
Total |
|
2012 |
|
2013 |
|
2014 |
|
2015 |
|
More then |
| ||||||
|
Operating leases |
|
$ |
1,502 |
|
$ |
1,124 |
|
$ |
378 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Capital leases |
|
80 |
|
40 |
|
34 |
|
6 |
|
— |
|
— |
| ||||||
|
Total |
|
$ |
1,582 |
|
$ |
1,164 |
|
$ |
412 |
|
$ |
6 |
|
$ |
— |
|
$ |
— |
|
New Accounting Standards
Not applicable.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
As of December 31, 2011, we would not expect our operating results or cash flows to be affected to any significant degree by the effect of a sudden change in market interest rates or credit conditions on our securities portfolio.
Our vendors in countries outside the U.S. are typically paid in Euro. However, such expenditures have not been significant to date. Accordingly, we are not directly exposed to significant market risks from currency exchange rate fluctuations. We believe that the interest rate risk related to our accounts receivable is not significant. We manage the risk associated with these accounts through periodic reviews of the carrying value for non-collectability and establishment of appropriate allowances. We do not enter into hedging transactions and do not purchase derivative instruments.
Item 8. Financial Statements and Supplementary Data
|
|
|
Page |
|
|
39 | |
|
Consolidated Balance Sheets as of December 31, 2010 and December 31, 2011 |
|
40 |
|
|
41 | |
|
|
42 | |
|
|
43 | |
|
|
44 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders
of Aastrom Biosciences, Inc.:
In our opinion, the accompanying consolidated balance sheets and the related consolidated statements of operations, of shareholders’ equity (deficit) and comprehensive loss and of cash flows present fairly, in all material respects, the financial position of Aastrom Biosciences, Inc. and its subsidiaries (a development stage company) at December 31, 2011 and December 31, 2010, and the results of their operations and their cash flows for the year ended December 31, 2011, for the six month period ended December 31, 2010, for the years ended June 30, 2010 and 2009, and, cumulatively, for the period from March 24, 1989 (Inception) to December 31, 2011 in conformity with accounting principles generally accepted in the United States of America. Also in our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (COSO). The Company’s management is responsible for these financial statements, for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in Management’s Report on Internal Control over Financial Reporting appearing under Item 9A. Our responsibility is to express opinions on these financial statements and on the Company’s internal control over financial reporting based on our audits, which were integrated audits as of December 31, 2011, June 30, 2010 and June 30, 2009. We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audits to obtain reasonable assurance about whether the financial statements are free of material misstatement and whether effective internal control over financial reporting was maintained in all material respects. Our audits of the financial statements included examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements, assessing the accounting principles used and significant estimates made by management, and evaluating the overall financial statement presentation. Our audit of internal control over financial reporting included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audits also included performing such other procedures as we considered necessary in the circumstances. We believe that our audits provide a reasonable basis for our opinions.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
/s/ PricewaterhouseCoopers LLP
Detroit, Michigan
March 15, 2012
AASTROM BIOSCIENCES, INC.
(a clinical development stage company)
(in thousands)
|
|
|
December 31, |
| ||||
|
|
|
2010 |
|
2011 |
| ||
|
ASSETS |
|
|
|
|
| ||
|
CURRENT ASSETS: |
|
|
|
|
| ||
|
Cash and cash equivalents |
|
$ |
31,248 |
|
$ |
5,530 |
|
|
Receivables, net |
|
25 |
|
9 |
| ||
|
Other current assets |
|
426 |
|
636 |
| ||
|
Total current assets |
|
31,699 |
|
6,175 |
| ||
|
PROPERTY AND EQUIPMENT, NET |
|
1,128 |
|
1,564 |
| ||
|
Total assets |
|
$ |
32,827 |
|
$ |
7,739 |
|
|
|
|
|
|
|
| ||
|
LIABILITIES AND SHAREHOLDERS’ EQUITY (DEFICIT) |
|
|
|
|
| ||
|
CURRENT LIABILITIES: |
|
|
|
|
| ||
|
Accounts payable and accrued expenses |
|
$ |
2,900 |
|
$ |
2,963 |
|
|
Accrued employee benefits |
|
796 |
|
1,042 |
| ||
|
Current portion of long-term debt |
|
214 |
|
40 |
| ||
|
Warrant liabilities |
|
25,954 |
|
16,625 |
| ||
|
Total current liabilities |
|
29,864 |
|
20,670 |
| ||
|
LONG-TERM DEBT |
|
41 |
|
40 |
| ||
|
COMMITMENTS AND CONTINGENCIES (Notes 7 and 8) |
|
|
|
|
| ||
|
SHAREHOLDERS’ EQUITY (DEFICIT): |
|
|
|
|
| ||
|
Common Stock, no par value; shares authorized — 62,500 and 150,000 respectively; shares issued and outstanding — 38,616 and 38,635, respectively |
|
225,102 |
|
228,877 |
| ||
|
Deficit accumulated during the development stage |
|
(222,180 |
) |
(241,848 |
) | ||
|
Total shareholders’ equity (deficit) |
|
2,922 |
|
(12,971 |
) | ||
|
Total liabilities and shareholders’ equity (deficit) |
|
$ |
32,827 |
|
$ |
7,739 |
|
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
AASTROM BIOSCIENCES, INC.
(a clinical development stage company)
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except per share amounts)
|
|
|
|
|
|
|
Six Month Period |
|
Year Ended |
|
March 24, 1989 |
| |||||
|
|
|
Year Ended June 30, |
|
December 31, |
|
December 31, |
|
December 31, |
| |||||||
|
|
|
2009 |
|
2010 |
|
2010 |
|
2011 |
|
2011 |
| |||||
|
REVENUES: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Product sales and rentals |
|
$ |
182 |
|
$ |
89 |
|
$ |
9 |
|
$ |
18 |
|
$ |
1,877 |
|
|
Research and development agreements |
|
— |
|
— |
|
— |
|
— |
|
2,105 |
| |||||
|
Grants |
|
— |
|
— |
|
244 |
|
— |
|
9,901 |
| |||||
|
Total revenues |
|
182 |
|
89 |
|
253 |
|
18 |
|
13,883 |
| |||||
|
COSTS AND EXPENSES: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Cost of product sales and rentals |
|
112 |
|
34 |
|
2 |
|
4 |
|
3,041 |
| |||||
|
Research and development |
|
11,289 |
|
12,658 |
|
8,609 |
|
21,330 |
|
190,705 |
| |||||
|
Selling, general and administrative |
|
4,950 |
|
5,201 |
|
3,265 |
|
7,724 |
|
84,848 |
| |||||
|
Total costs and expenses |
|
16,351 |
|
17,893 |
|
11,876 |
|
29,058 |
|
278,594 |
| |||||
|
LOSS FROM OPERATIONS |
|
(16,169 |
) |
(17,804 |
) |
(11,623 |
) |
(29,040 |
) |
(264,711 |
) | |||||
|
OTHER INCOME (EXPENSE): |
|
|
|
|
|
|
|
|
|
|
| |||||
|
(Increase) decrease in fair value of warrants |
|
(115 |
) |
3,171 |
|
(7,500 |
) |
9,329 |
|
12,289 |
| |||||
|
Other income |
|
— |
|
— |
|
— |
|
— |
|
1,249 |
| |||||
|
Interest income |
|
296 |
|
115 |
|
40 |
|
53 |
|
10,772 |
| |||||
|
Interest expense |
|
(73 |
) |
(40 |
) |
(5 |
) |
(10 |
) |
(479 |
) | |||||
|
Total other income (expense) |
|
108 |
|
3,246 |
|
(7,465 |
) |
9,372 |
|
23,831 |
| |||||
|
NET LOSS |
|
$ |
(16,061 |
) |
$ |
(14,558 |
) |
$ |
(19,088 |
) |
$ |
(19,668 |
) |
$ |
(240,880 |
) |
|
NET LOSS PER SHARE (Basic and Diluted) |
|
$ |
(0.90 |
) |
$ |
(0.59 |
) |
$ |
(0.65 |
) |
$ |
(0.51 |
) |
|
| |
|
Weighted average number of common shares outstanding (Basic and Diluted) |
|
17,877 |
|
24,729 |
|
29,186 |
|
38,627 |
|
|
| |||||
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
AASTROM BIOSCIENCES, INC.
(a clinical development stage company)
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY (DEFICIT) AND COMPREHENSIVE LOSS
(In thousands, except per share data)
|
|
|
Preferred Stock |
|
Common Stock |
|
Deficit |
|
Total |
| ||||||||
|
|
|
Shares |
|
Amount |
|
Shares |
|
Amount |
|
Stage |
|
Equity (Deficit) |
| ||||
|
BALANCE, MARCH 24, 1989 (Inception) |
|
— |
|
$ |
— |
|
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Net loss and comprehensive loss |
|
|
|
|
|
|
|
|
|
(187,566 |
) |
(187,566 |
) | ||||
|
Issuance of common stock for cash, services and license rights |
|
|
|
|
|
149 |
|
2,336 |
|
|
|
2,336 |
| ||||
|
Issuance of Series A through Series E Preferred Stock for cash, net of issuance costs of $342 |
|
9,452 |
|
34,218 |
|
|
|
|
|
|
|
34,218 |
| ||||
|
Issuance of Series E Preferred Stock at $17.00 per Share |
|
206 |
|
3,500 |
|
|
|
(3,500 |
) |
|
|
— |
| ||||
|
Exercise of stock options and stock purchase warrants, and issuance of stock under Employee Stock Purchase Plan |
|
|
|
|
|
1,088 |
|
7,293 |
|
|
|
7,293 |
| ||||
|
Issuance of Stock Purchase Rights for cash in September 1995 and March 1996 |
|
|
|
|
|
|
|
3,500 |
|
|
|
3,500 |
| ||||
|
Principal payment received under shareholder note Receivable |
|
|
|
|
|
|
|
31 |
|
|
|
31 |
| ||||
|
Initial public offering of common stock at $56.00 per share, net of issuance costs of $2,865 |
|
|
|
|
|
406 |
|
19,885 |
|
|
|
19,885 |
| ||||
|
Conversion of preferred stock |
|
(11,866 |
) |
(55,374 |
) |
2,720 |
|
55,374 |
|
|
|
— |
| ||||
|
Compensation expense related to stock options and warrants granted |
|
|
|
|
|
|
|
8,379 |
|
|
|
8,379 |
| ||||
|
Issuance of 5.5% Convertible Preferred Stock at $5.00 per share, net of issuance costs of $1,070 |
|
2,200 |
|
9,930 |
|
|
|
|
|
|
|
9,930 |
| ||||
|
Issuance of 1998 Series I Convertible Preferred Stock at $1,000 per share, net of issuance costs of $460 |
|
5 |
|
4,540 |
|
5 |
|
149 |
|
|
|
4,689 |
| ||||
|
Issuance of 1999 Series III Convertible Preferred Stock at $1,000 per share, net of issuance costs of $280 |
|
3 |
|
2,720 |
|
6 |
|
90 |
|
|
|
2,810 |
| ||||
|
Issuance of common stock, net of issuance costs of $10,215 |
|
|
|
|
|
15,491 |
|
110,377 |
|
|
|
110,377 |
| ||||
|
Issuance of restricted stock, net of cancellations |
|
|
|
|
|
54 |
|
— |
|
|
|
— |
| ||||
|
Issuance of stock under Direct Stock Purchase Plan |
|
|
|
|
|
94 |
|
943 |
|
|
|
943 |
| ||||
|
Dividends and yields on preferred stock |
|
|
|
466 |
|
19 |
|
502 |
|
(968 |
) |
— |
| ||||
|
Repurchase and retirement of common shares outstanding |
|
|
|
|
|
(4 |
) |
(73 |
) |
|
|
(73 |
) | ||||
|
BALANCE, JUNE 30, 2009 |
|
— |
|
— |
|
20,028 |
|
205,286 |
|
(188,534 |
) |
16,752 |
| ||||
|
Net loss and comprehensive loss |
|
|
|
|
|
|
|
|
|
(14,558 |
) |
(14,558 |
) | ||||
|
Compensation expense related to stock options and restricted stock awards and units granted |
|
|
|
|
|
— |
|
710 |
|
|
|
710 |
| ||||
|
Issuance of common stock, net of issuance costs of $1,265 |
|
|
|
|
|
8,228 |
|
11,877 |
|
|
|
11,877 |
| ||||
|
BALANCE, JUNE 30, 2010 |
|
— |
|
— |
|
28,256 |
|
217,873 |
|
(203,092 |
) |
14,781 |
| ||||
|
Net loss and comprehensive loss |
|
|
|
|
|
|
|
|
|
(19,088 |
) |
(19,088 |
) | ||||
|
Exercise of stock options and stock purchase warrants |
|
|
|
|
|
360 |
|
1,498 |
|
|
|
1,498 |
| ||||
|
Compensation expense related to stock options and restricted stock awards and units granted |
|
|
|
|
|
— |
|
1,043 |
|
|
|
1,043 |
| ||||
|
Issuance of common stock, net of issuance costs of $1,944 |
|
|
|
|
|
10,000 |
|
4,688 |
|
|
|
4,688 |
| ||||
|
BALANCE, DECEMBER 31, 2010 |
|
— |
|
— |
|
38,616 |
|
225,102 |
|
(222,180 |
) |
2,922 |
| ||||
|
Net loss and comprehensive loss |
|
|
|
|
|
|
|
|
|
(19,668 |
) |
(19,668 |
) | ||||
|
Exercise of stock options |
|
|
|
|
|
19 |
|
32 |
|
|
|
32 |
| ||||
|
Compensation expense related to stock options granted |
|
|
|
|
|
|
|
3,743 |
|
|
|
3,743 |
| ||||
|
BALANCE, DECEMBER 31, 2011 |
|
— |
|
$ |
— |
|
38,635 |
|
$ |
228,877 |
|
$ |
(241,848 |
) |
$ |
(12,971 |
) |
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
AASTROM BIOSCIENCES, INC.
(a clinical development stage company)
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands)
|
|
|
|
|
|
|
Six Month Period |
|
Year Ended |
|
March 24, 1989 |
| |||||
|
|
|
Year Ended June 30, |
|
December 31, |
|
December 31, |
|
December 31, |
| |||||||
|
|
|
2009 |
|
2010 |
|
2010 |
|
2011 |
|
2011 |
| |||||
|
OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net loss |
|
$ |
(16,061 |
) |
$ |
(14,558 |
) |
$ |
(19,088 |
) |
$ |
(19,668 |
) |
$ |
(240,880 |
) |
|
Adjustments to reconcile net loss to net cash used for operating activities: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Depreciation and amortization |
|
704 |
|
592 |
|
259 |
|
647 |
|
7,498 |
| |||||
|
Loss on property held for resale |
|
— |
|
— |
|
— |
|
— |
|
110 |
| |||||
|
Amortization of discounts and premiums on investments |
|
(30 |
) |
— |
|
— |
|
— |
|
(1,704 |
) | |||||
|
Stock compensation expense |
|
1,362 |
|
710 |
|
1,043 |
|
3,743 |
|
13,885 |
| |||||
|
Increase (decrease) in fair value of warrant liabilities |
|
115 |
|
(3,171 |
) |
7,500 |
|
(9,329 |
) |
(12,289 |
) | |||||
|
Inventory write downs and reserves |
|
— |
|
— |
|
— |
|
— |
|
2,240 |
| |||||
|
Stock issued pursuant to license agreement |
|
— |
|
— |
|
— |
|
— |
|
3,300 |
| |||||
|
Provision for losses on accounts receivable |
|
— |
|
— |
|
— |
|
— |
|
204 |
| |||||
|
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Receivables |
|
(40 |
) |
42 |
|
(9 |
) |
16 |
|
(258 |
) | |||||
|
Inventories |
|
(1 |
) |
1 |
|
— |
|
— |
|
(2,335 |
) | |||||
|
Other current assets |
|
592 |
|
72 |
|
(43 |
) |
(210 |
) |
(616 |
) | |||||
|
Accounts payable and accrued expenses |
|
(54 |
) |
896 |
|
976 |
|
63 |
|
2,731 |
| |||||
|
Accrued employee benefits |
|
(392 |
) |
331 |
|
110 |
|
246 |
|
1,042 |
| |||||
|
Net cash used for operating activities |
|
(13,805 |
) |
(15,085 |
) |
(9,252 |
) |
(24,492 |
) |
(227,072 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Organizational costs |
|
— |
|
— |
|
— |
|
— |
|
(73 |
) | |||||
|
Purchase of short-term investments |
|
— |
|
(5,000 |
) |
— |
|
— |
|
(217,041 |
) | |||||
|
Maturities of short-term investments |
|
6,000 |
|
— |
|
5,000 |
|
— |
|
218,745 |
| |||||
|
Property and equipment purchases |
|
(35 |
) |
(120 |
) |
(305 |
) |
(1,031 |
) |
(7,217 |
) | |||||
|
Proceeds from sale of property held for resale |
|
— |
|
— |
|
— |
|
— |
|
400 |
| |||||
|
Net cash provided by (used for) investing activities |
|
5,965 |
|
(5,120 |
) |
4,695 |
|
(1,031 |
) |
(5,186 |
) | |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Net proceeds from issuance of preferred stock |
|
— |
|
— |
|
— |
|
— |
|
51,647 |
| |||||
|
Net proceeds from issuance of common stock and warrants |
|
8,534 |
|
17,526 |
|
21,805 |
|
32 |
|
184,708 |
| |||||
|
Payments received for stock purchase rights |
|
— |
|
— |
|
— |
|
— |
|
3,500 |
| |||||
|
Restricted cash used as compensating balance |
|
259 |
|
277 |
|
— |
|
— |
|
— |
| |||||
|
Proceeds from long-term debt |
|
— |
|
— |
|
— |
|
— |
|
751 |
| |||||
|
Payments on long-term debt |
|
(445 |
) |
(479 |
) |
(119 |
) |
(227 |
) |
(2,800 |
) | |||||
|
Other, net |
|
— |
|
— |
|
— |
|
— |
|
(18 |
) | |||||
|
Net cash provided by (used for) financing activities |
|
8,348 |
|
17,324 |
|
21,686 |
|
(195 |
) |
237,788 |
| |||||
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS |
|
508 |
|
(2,881 |
) |
17,129 |
|
(25,718 |
) |
5,530 |
| |||||
|
CASH AND CASH EQUIVALENTS AT BEGINNING OF PERIOD |
|
16,492 |
|
17,000 |
|
14,119 |
|
31,248 |
|
— |
| |||||
|
CASH AND CASH EQUIVALENTS AT END OF PERIOD |
|
$ |
17,000 |
|
$ |
14,119 |
|
$ |
31,248 |
|
$ |
5,530 |
|
$ |
5,530 |
|
|
|
|
|
|
|
|
|
|
|
|
|
| |||||
|
SUPPLEMENTAL CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
|
|
|
| |||||
|
Interest paid |
|
$ |
73 |
|
$ |
40 |
|
$ |
11 |
|
$ |
16 |
|
$ |
491 |
|
|
Equipment acquired under capital lease obligations |
|
$ |
— |
|
$ |
— |
|
$ |
69 |
|
$ |
52 |
|
$ |
1,295 |
|
The accompanying Notes to Consolidated Financial Statements are an integral part of these statements.
AASTROM BIOSCIENCES, INC.
(a clinical development stage company)
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
1. Organization and Summary of Significant Accounting Policies
Aastrom Biosciences, Inc. was incorporated in March 1989 (Inception), began employee-based operations in 1991, and is in the development stage. The Company operates its business in one reportable segment — research and product development involving the development of patient specific cell products for use in regenerative medicine.
Successful future operations are subject to several technical hurdles and risk factors, including satisfactory product development, timely initiation and completion of clinical trials, regulatory approval and market acceptance of the Company’s products and the Company’s continued ability to obtain future funding.
The Company is subject to certain risks related to the operation of its business and development of its products and product candidates. The Company believes that it will have adequate liquidity to finance its planned operations, including development of its products and product candidates, via its cash and investments on hand as of December 31, 2011 and the net proceeds of $37,800,000 from the financing that closed in March 2012, see note 10, until at least until December 31, 2012. While the Company’s budgeted cash usage and operating plan for 2012 does not currently contemplate taking additional actions to reduce the use of cash over the next twelve months, the Company could, if necessary, delay or forego certain budgeted discretionary expenditures such as anticipated hiring plans or certain non-critical research and development expenditures, as well as slow down or delay certain clinical trial activity (without jeopardizing our Phase 3 clinical trial for CLI). On a longer-term basis, the Company will need to raise additional funds in order to complete its product development programs, complete clinical trials needed to market its products, and commercialize these products. The Company cannot be certain that such funding will be available on favorable terms, if at all. Some of the factors that will impact the Company’s ability to raise additional capital and its overall success include: the rate and degree of progress for its product development, the rate of regulatory approval to proceed with clinical trial programs, the level of success achieved in clinical trials, the requirements for marketing authorization from regulatory bodies in the United States and other countries, the liquidity and market volatility of the Company’s equity securities, regulatory and manufacturing requirements and uncertainties, technological developments by competitors, and other factors. If the Company cannot raise such funds, it may not be able to develop or enhance products, take advantage of future opportunities, or respond to competitive pressures or unanticipated requirements, which would likely have a material adverse impact on the Company’s business, financial condition and results of operations.
Principles of Consolidation — The consolidated financial statements include the accounts of Aastrom and its wholly-owned subsidiary, Aastrom Biosciences GmbH, located in Berlin, Germany and Aastrom Biosciences, SL, located in Barcelona, Spain (collectively, the Company). All inter-company transactions and accounts have been eliminated in consolidation.
Fiscal Year Change — On November 11, 2010, the Board of Directors approved the change in the Company’s fiscal year end from June 30 to December 31. The change became effective at the end of the quarter ended December 31, 2010. All references to “years”, unless otherwise noted, refer to the 12-month fiscal year, which prior to July 1, 2010, ended on June 30, and beginning with January 1, 2011, ends on December 31, of each year. In addition, the Company presents the Consolidated Statement of Operations and Consolidated Statement of Cash Flows for the six month transition period ended December 31, 2010.
Cash and Cash Equivalents — Cash and cash equivalents include cash and highly liquid short-term investments with original maturities of three months or less.
Fair Value Measurements — Fair value is the amount that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants. As such, fair value is determined based upon assumptions that market participants would use in pricing an asset or liability. Fair value measurements are rated on a three-tier hierarchy as follows:
· Level 1 inputs: Quoted prices (unadjusted) for identical assets or liabilities in active markets;
· Level 2 inputs: Inputs, other than quoted prices included in Level 1 that are observable either directly or indirectly; and
· Level 3 inputs: Unobservable inputs for which there is little or no market data, which require the reporting entity to develop its own assumptions.
In many cases, a valuation technique used to measure fair value includes inputs from multiple levels of the fair value hierarchy described above. The lowest level of significant input determines the placement of the entire fair value measurement in the hierarchy.
See Note 5 for disclosures related to the fair value of the Company’s warrants. The Company does not have any other assets or liabilities on the balance sheet as of December 31, 2011 that are measured at fair value.
Diversity of Credit Risk — The Company has established guidelines relative to diversification and maturities of its investments in an effort to limit risk. These guidelines are periodically reviewed and modified to take advantage of trends in yields and interest rates. The Company has not experienced any losses on its cash equivalents.
Property and Equipment — Property and equipment is recorded at cost and depreciated or amortized using the straight-line method over the estimated useful life of the asset (primarily three to five years) or the underlying lease term for leasehold improvements, whichever is shorter. When assets are disposed of, the cost and accumulated depreciation are removed from the accounts. Repairs and maintenance are charged to expense as incurred.
Revenue Recognition — The Company’s revenue can be generated from grants and research agreements, collaborative agreements, licensing fees and product sales. Revenue from grants and research agreements is recognized on a cost reimbursement basis consistent with the performance requirements of the related agreement. Revenue from collaborative agreements is recognized when the scientific or clinical results stipulated in the agreement have been met and there are no ongoing obligations on the Company’s part. Revenue from product sales is recognized when title to the product transfers and there are no remaining obligations that will affect the customer’s final acceptance of the sale. Revenue from licensing fees under licensing agreements is recognized when there are no future performance obligations remaining with respect to such revenues. Payments received before all obligations are fulfilled are classified as deferred revenue.
Research and Development Costs — Research and development costs are expensed as incurred. These costs include direct research and development costs such as salaries, clinical trial expenses, consulting fees and other expenses that are specific to the Company’s research and development programs, as well as an allocation of indirect costs such as facility expenses, human resources and information technology expenses.
Stock-Based Compensation — Calculating stock-based compensation expense requires the input of highly subjective assumptions. We apply the Black-Scholes option-pricing model to determine the fair value of our stock options. Inherent in this model are assumptions related to expected stock-price volatility, option life, risk-free interest rate and dividend yield. We estimate the volatility of our common stock at the date of grant based on historical volatility. We estimate the expected life of options that vest solely on service using the “simplified method” provided for in the Securities and Exchange Commission Staff Accounting Bulletin No. 110. The “simplified method” is permitted for estimating the expected term of “plain-vanilla” stock options for which the historical stock option exercise experience is likely not indicative of future exercise patterns. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected life of the options. The dividend rate is based on our historical rate, which we anticipate to remain at zero. The assumptions used in calculating the fair value of stock options represent our best estimates, however these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and different assumptions are used, the stock-based compensation expense could be materially different in the future. In addition, we are required to estimate the expected forfeiture rate and only recognize expense for those stock options expected to vest over the service period. We estimate the forfeiture rate considering the historical experience of our stock-based awards. If the actual forfeiture rate is different from the estimate, we adjust the expense accordingly.
Income Taxes — Deferred tax assets are recognized for deductible temporary differences and tax credit carryforwards and deferred tax liabilities are recognized for taxable temporary differences. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
Net Loss Per Share — Net loss per common share is computed using the weighted-average number of common shares outstanding during the period. Common equivalent shares are not included in the diluted per share calculation where the effect of their inclusion would be anti-dilutive. The aggregate number of common equivalent shares (related to options and warrants) that have been excluded from the computations of diluted net loss per common share for the years ended June 30, 2009 and 2010 was approximately 2,228,200 and 12,200,500, respectively, and 19,599,700 for the six month transition period ended December 31, 2010 and 23,382,190 for the year ended December 31, 2011.
Use of Estimates — The preparation of financial statements in accordance with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reported period. Actual results could differ from those estimates.
Financial Instruments — The Company’s financial instruments include cash equivalents and receivables for which the current carrying amounts approximate market value based upon their short-term nature.
Warrants — Warrants that could require cash settlement or have anti-dilution price protection provisions are recorded as liabilities at their estimated fair value at the date of issuance, with subsequent changes in estimated fair value recorded in other income (expense) in our statement of operations in each subsequent period. In general, warrants with anti-dilution provisions are measured using the Monte Carlo valuation model, while the others are measured using the Black-Scholes valuation model. Both of the methodologies are based, in part, upon inputs for which there is little or no observable market data, requiring the Company to develop its own assumptions. The assumptions used in calculating the estimated fair value of the warrants represent our best estimates, however these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and different assumptions are used, the warrant liability and the change in estimated fair value could be materially different.
Long-Lived Assets — The Company reviews its long-lived assets for impairment whenever an event or change in circumstances indicates that the carrying values of an asset may not be recoverable. If such an event or change in circumstances occurs and potential impairment is indicated because the carrying values exceed the estimated future undiscounted cash flows of the asset, the Company would measure the impairment loss as the amount by which the carrying value of the asset exceeds its fair value. No significant events or changes in circumstances were identified by the Company that would indicate that the carrying value of an asset was not recoverable for any of the periods presented in the accompanying financial statements.
2. Selected Balance Sheet Information
Property and Equipment (in thousands):
|
|
|
December 31, |
|
December 31, |
| ||
|
|
|
2010 |
|
2011 |
| ||
|
Machinery and equipment |
|
$ |
2,729 |
|
$ |
3,041 |
|
|
Furniture and fixtures |
|
469 |
|
678 |
| ||
|
Computer software |
|
410 |
|
410 |
| ||
|
Computer equipment |
|
414 |
|
467 |
| ||
|
Office equipment |
|
75 |
|
105 |
| ||
|
Leasehold improvements |
|
922 |
|
998 |
| ||
|
|
|
5,019 |
|
5,699 |
| ||
|
Less accumulated depreciation |
|
(3,891 |
) |
(4,135 |
) | ||
|
|
|
$ |
1,128 |
|
$ |
1,564 |
|
Accounts Payable and Accrued Expenses (in thousands):
|
|
|
December 31, |
|
December 31, |
| ||
|
|
|
2010 |
|
2011 |
| ||
|
Accounts payable |
|
$ |
2,083 |
|
$ |
2,239 |
|
|
Accrued clinical trial expense |
|
281 |
|
— |
| ||
|
Other accruals |
|
536 |
|
724 |
| ||
|
|
|
$ |
2,900 |
|
$ |
2,963 |
|
Accrued Employee Benefits (in thousands):
|
|
|
December 31, |
|
December 31, |
| ||
|
|
|
2010 |
|
2011 |
| ||
|
Vacation pay and other |
|
$ |
239 |
|
$ |
398 |
|
|
Bonus |
|
522 |
|
644 |
| ||
|
Severance |
|
35 |
|
— |
| ||
|
|
|
$ |
796 |
|
$ |
1,042 |
|
3. Stock-Based Compensation
Stock Option and Equity Incentive Plans
The Company has historically had various stock incentive plans and agreements that provide for the issuance of nonqualified and incentive stock options as well as other equity awards. Such awards may be granted by the Company’s Board of Directors to certain of the Company’s employees, directors and consultants. Options granted under these plans expire no later than ten years from the date of grant, and other than those granted to non-employee directors, generally become exercisable over a four-year period, under a graded-vesting methodology, following the date of grant. The Company generally issues new shares upon the exercise of stock options.
In December 2009, the shareholders approved the 2009 Omnibus Incentive Plan (the 2009 Plan). The 2009 Plan provides incentives through the grant of stock options, stock appreciation rights, restricted stock awards and restricted stock units. The exercise price of stock options granted under the 2009 Plan shall not be less than the fair market value of the Company’s common stock on the date of grant. The 2009 Plan replaced the 1992 Stock Option Plan, the 2001 Stock Option Plan and the Amended and Restated 2004 Equity Incentive Plan (the Prior Plans), and no new awards will be granted under the Prior Plans. However, the expiration or forfeiture of options previously granted under the Prior Plans will increase the awards available for issuance under the 2009 Plan.
As of December 31, 2011, there were 408,527 awards available for future grant under the 2009 Plan.
Service-Based Stock Options
During the period ended December 31, 2011, the Company granted 4,310,550 service-based options to purchase common stock. These were granted with exercise prices equal to the fair value of the Company’s stock at the grant date, vest over four years (other than 505,975 non-employee director options which vest over three years) and have lives of ten years. The weighted average grant-date fair value of service-based options granted during the years ended June 30, 2009 and 2010 was $2.08 and $1.33, respectively, $0.99 for the six month transition period ended December 31, 2010, and $1.54 for the year ended December 31, 2011.
The net compensation costs recorded for the service-based stock options related to employees and directors (including the impact of the forfeitures) were $1,292,000 and $698,000 for the years ended June 30, 2009 and 2010, respectively, $1,043,000 for the six month transition period ended December 31, 2010 and $3,743,000 for the year ended December 31, 2011.
The fair value of each service-based stock option grant for the reported periods is estimated on the date of the grant using the Black-Scholes option-pricing model using the weighted average assumptions noted in the following table.
|
|
|
Year Ended June 30, |
|
Six Month Period |
|
Year Ended |
| ||
|
Service-Based Stock Options |
|
2009 |
|
2010 |
|
2010 |
|
2011 |
|
|
Expected dividend rate |
|
0% |
|
0% |
|
0% |
|
0% |
|
|
Expected stock price volatility |
|
61.2% - 72.7% |
|
70.2% - 72.8% |
|
70.6% - 77.4% |
|
72.1% - 78.9% |
|
|
Risk-free interest rate |
|
2.0% - 3.3% |
|
2.4% - 3.1% |
|
1.5% - 2.1% |
|
1.4% - 2.7% |
|
|
Expected life (years) |
|
6.6 |
|
5.5 - 6.3 |
|
6.1 - 6.3 |
|
6.0 - 6.3 |
|
The following table summarizes the activity for service-based stock options for the indicated periods:
|
Service-Based Stock Options |
|
Options |
|
Weighted Average |
|
Weighted Average |
|
Aggregate |
| ||
|
Outstanding at June 30, 2008 |
|
1,066,898 |
|
$ |
10.48 |
|
7.8 |
|
$ |
1,000 |
|
|
Granted |
|
498,750 |
|
$ |
3.12 |
|
|
|
|
| |
|
Forfeited or expired |
|
(200,266 |
) |
$ |
10.88 |
|
|
|
|
| |
|
Outstanding at June 30, 2009 |
|
1,365,382 |
|
$ |
7.76 |
|
7.9 |
|
$ |
114,000 |
|
|
Granted |
|
2,508,525 |
|
$ |
2.02 |
|
|
|
|
| |
|
Forfeited or expired |
|
(590,727 |
) |
$ |
7.63 |
|
|
|
|
| |
|
Outstanding at June 30, 2010 |
|
3,283,180 |
|
$ |
3.40 |
|
8.6 |
|
$ |
2,750 |
|
|
Granted |
|
1,700,000 |
|
$ |
1.54 |
|
|
|
|
| |
|
Exercised |
|
(7,500 |
) |
$ |
1.80 |
|
|
|
$ |
3,000 |
|
|
Forfeited or expired |
|
(678,471 |
) |
$ |
4.85 |
|
|
|
|
| |
|
Outstanding at December 31, 2010 |
|
4,297,209 |
|
$ |
2.43 |
|
8.9 |
|
$ |
3,159,000 |
|
|
Granted |
|
4,310,550 |
|
$ |
2.31 |
|
|
|
|
| |
|
Exercised |
|
(19,688 |
) |
$ |
1.64 |
|
|
|
$ |
21,000 |
|
|
Forfeited or expired |
|
(508,404 |
) |
$ |
2.33 |
|
|
|
|
| |
|
Outstanding at December 31, 2011 |
|
8,079,667 |
|
$ |
2.38 |
|
8.4 |
|
$ |
674,000 |
|
|
Exercisable at December 31, 2011 |
|
2,487,332 |
|
$ |
3.01 |
|
7.1 |
|
$ |
281,000 |
|
As of December 31, 2011, there was approximately $4,373,000, of total unrecognized compensation cost related to non-vested service-based stock options granted under the 2009 Plan and the Prior Plans. That cost is expected to be recognized over a weighted-average period of 3.0 years.
The total fair value of stock options vested during the years ended June 30, 2009 and 2010 was $1,678,000 and $1,084,000, respectively, $622,000 for the six month transition period ended December 31, 2010, and $2,182,000 for the year ended December 31, 2011.
4. Shareholders’ Equity
In October 2008, the Company entered into a common stock purchase agreement with Fusion Capital Fund II, LLC (Fusion Capital) pursuant to which the Company was entitled to sell up to $15,000,000 of its common stock to Fusion Capital. In April 2009, the Company concluded the sales of the registered shares under this common stock purchase agreement. Under this purchase agreement the Company issued 2,836,583 shares of common stock for net proceeds of approximately $8,600,000. In connection with entering into this common stock purchase agreement, the Company issued to Fusion Capital 242,040 shares of its common stock as a commitment fee. The Company also issued to Fusion Capital an additional 139,229 shares as a pro rata commitment fee.
In June 2009, the Company entered into a $30,000,000 common stock purchase agreement with Fusion Capital. Pursuant to the purchase agreement with Fusion Capital, the Company had the right to sell to Fusion Capital up to $30,000,000 of its common stock over a 25-month period, which began on July 1, 2009. Such sales were to be made from time to time in amounts between $100,000 and $4,000,000, depending on certain conditions as set forth in the agreement. In consideration for entering into the purchase agreement, the Company issued 181,530 shares of its common stock to Fusion Capital as an initial commitment fee. During fiscal
2010, 1,718,538 shares of the Company’s common stock (including 51,432 shares related to its commitment fee) were issued to Fusion Capital for net proceeds of approximately $5,100,000. The Company terminated the agreement with Fusion Capital in November 2010.
On January 21, 2010, the Company completed the sale of 6,509,637 units (including 740,387 units sold to the underwriter pursuant to the exercise of its over-allotment option) at a public offering price of $2.08 per unit. Each unit consisted of (i) one share of the Company’s common stock, (ii) a Class A warrant to purchase 0.75 of a share of the Company’s common stock at an exercise price of $2.52 per share (as adjusted from $2.97 per share for the anti-dilution provision triggered in the December 2010 financing) and (iii) a Class B warrant to purchase 0.50 of a share of the Company’s common stock at an exercise price of $2.08 per share. The Company received approximately $12,400,000 in net proceeds from the sale of the units (including the partially exercised option of the over-allotment), after underwriting discounts and commissions and other offering expenses.
On December 15, 2010, the Company completed the sale of 10,000,000 units at a public offering price of $2.25 per unit. Each unit consisted of one share of the Company’s common stock and a warrant to purchase one share of the Company’s common stock at an exercise price of $3.22 per share. The Company received approximately $20,600,000 in net proceeds from the sale of the units, after underwriting discounts and commissions and other offering expenses. The warrants to purchase 10,000,000 shares of the Company’s common stock are exercisable for a five year period commencing on December 15, 2010.
On June 16, 2011, the Company entered into an At the Market Sales Agreement (ATM) with McNicoll, Lewis & Vlak LLC (MLV), pursuant to which the Company may sell shares of its common stock through MLV, as sales agent, in registered transactions from its shelf registration statement filed in November 2010, for aggregate proceeds of up to $20,300,000. Shares of common stock sold under the ATM are to be sold at market prices. The Company will pay up to 3% of the gross proceeds to MLV as a commission. As of December 31, 2011, the Company had not sold any shares under the ATM.
On August 11, 2011, the Board of Directors of the Company adopted a Shareholder Rights Plan, as set forth in the Shareholder Rights Agreement between the Company and the rights agent, the purpose of which is, among other things, to enhance the Board’s ability to protect shareholder interests and to ensure that shareholders receive fair treatment in the event any coercive takeover attempt of the Company is made in the future. The Shareholder Rights Plan could make it more difficult for a third party to acquire, or could discourage a third party from acquiring, the Company or a large block of the Company’s common stock. The following summary description of the Shareholder Rights Plan should be read in conjunction with the Company’s Shareholder Rights Plan, which was filed with the Securities and Exchange Commission as an exhibit to a Registration Statement on Form 8-A on August 12, 2011.
In connection with the adoption of the Shareholder Rights Plan, the Board of Directors of the Company declared a dividend distribution of one preferred stock purchase right (a “Right”) for each outstanding share of common stock to stockholders of record as of the close of business on August 15, 2011. In addition, one Right will automatically attach to each share of common stock issued between August 15, 2011 and the distribution date. The Rights currently are not exercisable and are attached to and trade with the outstanding shares of common stock. Under the Shareholder Rights Plan, the Rights become exercisable if a person or group becomes an “acquiring person” by acquiring 15% or more of the outstanding shares of common stock or if a person or group commences a tender offer that would result in that person owning 15% or more of the common stock. If a person or group becomes an “acquiring person,” each holder of a Right (other than the acquiring person and its affiliates, associates and transferees) would be entitled to purchase, at the then-current exercise price, such number of shares of the Company’s preferred stock which are equivalent to shares of common stock having a value of twice the exercise price of the Right. If the Company is acquired in a merger or other business combination transaction after any such event, each holder of a Right would then be entitled to purchase, at the then-current exercise price, shares of the acquiring company’s common stock having a value of twice the exercise price of the Right.
The Rights may be redeemed in whole, but not in part, at a price of $0.001 per Right (payable in cash, common stock or other consideration deemed appropriate by the Board of Directors) by the Board of Directors only until the earlier of (i) the time at which any person becomes an “acquiring person” or (ii) the expiration date of the Rights Agreement. Immediately upon the action of the Board of Directors ordering redemption of the Rights, the Right will terminate and thereafter the only right of the holders of Rights will be to receive the redemption price. The Rights will expire at the close of business on August 15, 2021, unless previously redeemed or exchanged by the Company as described above.
Dividends
No cash dividends have been declared or paid by the Company since its Inception.
5. Stock Purchase Warrants
The Company has historically issued warrants to purchase shares of the Company’s common stock in connection with certain of its common stock offerings. The following warrants were outstanding during the years ended June 30, 2009 and 2010, the six month transition period ended December 31, 2010, and the year ended December 31, 2011, and include provisions that could require cash settlement of the warrants or have anti-dilution price protection provisions requiring each to be recorded as liabilities of the Company at the estimated fair value at the date of issuance, with changes in estimated fair value recorded as non-cash income or expense in the Company’s statement of operations in each subsequent period:
(i) warrants to purchase an aggregate of 740,131 shares of the Company’s common stock, issued on October 17, 2007 in connection with the Company’s registered direct offering, exercisable from April 18, 2008 through April 17, 2013 at an exercise price of $12.72 per share, all of which remained outstanding as of December 31, 2011;
(ii) Class A warrants to purchase an aggregate of 4,882,228 shares of the Company’s common stock, issued on January 21, 2010 in connection with the Company’s registered public offering, exercisable for a five year period commencing on July 21, 2010 at an exercise price of $2.52 per share (as adjusted from $2.97 per share for the anti-dilution provision triggered in the December 2010 financing), 4,525,978 of which remained outstanding as of December 31, 2011; and
(iii) warrants to purchase an aggregate of 10,000,000 shares of the Company’s common stock, issued on December 15, 2010 in connection with the Company’s registered public offering, exercisable for a five year period commencing on December 15, 2010 at an exercise price of $3.22 per share, all of which remained outstanding as of December 31, 2011.
All of the warrants listed above could require net cash settlement in the event that registered shares are not available at the time of exercise of such warrant. The Class A warrants and the December 2010 warrants also contain anti-dilution provisions that adjust the exercise price of the warrant if the Company issues or sells, or is deemed to have issued or sold, any shares of its common stock or securities exercisable or convertible into shares of common stock for no consideration or for a consideration per share less than the applicable exercise price in effect immediately prior to the time of such issue or sale. In the event of such a subsequent issuance of common stock of the Company, (i) the exercise price of the Class A warrants would be adjusted to a point between the current exercise price per share of such Class A warrant and the price per share at which the new shares of common stock of the Company are being issued based on a weighted average calculation as outlined in the Class A warrant agreement, and (ii) the exercise price of the December 2010 warrants would be adjusted to the price per share at which the new shares of common stock of the Company are being issued. Notwithstanding the foregoing, there are certain issuances of the Company that would not trigger the anti-dilution provisions of the Class A warrants or the December 2010 warrants, including but not limited to, issuances under any duly authorized Company stock option, restricted stock plan or stock purchase plan whether now existing or hereafter approved by the Company and its stockholders in the future, or as an inducement grant to employees, consultants, directors or officers. The December 2010 warrants also contain a feature that allows the warrant holder to put the warrants back to the Company and receive cash in the event of a fundamental transaction, such as a change in control of the Company or a sale of all or substantially all of its assets. The value received by the warrant holder upon exercise of the put right is based on a Black-Scholes model using a defined set of inputs outlined in the December 2010 warrant agreement.
The Class A warrants and the December 2010 warrants are measured using the Monte Carlo valuation model, while the other warrants listed above are measured using the Black-Scholes valuation model. Both of the methodologies are based, in part, upon inputs for which there is little or no observable market data, requiring the Company to develop its own assumptions. The assumptions used in calculating the estimated fair value of the warrants represent the Company’s best estimates; however, these estimates involve inherent uncertainties and the application of management judgment. As a result, if factors change and different assumptions are used, the warrant liabilities and the change in estimated fair value of the warrants could be materially different.
Inherent in both the Monte Carlo and Black-Scholes valuation models are assumptions related to expected stock-price volatility, expected life, risk-free interest rate and dividend yield. The Company estimates the volatility of its common stock based on historical volatility that matches the expected remaining life of the warrants. The risk-free interest rate is based on the U.S. Treasury zero-coupon yield curve on the grant date for a maturity similar to the expected remaining life of the warrants. The expected life of the
warrants is assumed to be equivalent to their remaining contractual term. The dividend rate is based on the historical rate, which the Company anticipates to remain at zero.
The Monte Carlo model is used for the Class A warrants and the December 2010 warrants to appropriately value the potential future exercise price adjustments triggered by the anti-dilution provisions as well as the value of the put feature of the December 2010 warrants. These both require Level 3 inputs which are based on the Company’s estimates of the probability and timing of potential future financings and fundamental transactions. The other assumptions used by the Company are summarized in the following tables for warrants that were outstanding as of any of the balance sheet dates presented on our consolidated balance sheets:
|
October 2007 Warrants |
|
December 31, 2010 |
|
December 31, 2011 |
| ||
|
Closing stock price |
|
$ |
2.56 |
|
$ |
1.82 |
|
|
Expected dividend rate |
|
0 |
% |
0 |
% | ||
|
Expected stock price volatility |
|
100.3 |
% |
93.6 |
% | ||
|
Risk-free interest rate |
|
0.6 |
% |
0.1 |
% | ||
|
Expected life (years) |
|
2.25 |
|
1.25 |
| ||
|
January 2010 Class A Warrants |
|
December 31, 2010 |
|
December 31, 2011 |
| ||
|
Closing stock price |
|
$ |
2.56 |
|
$ |
1.82 |
|
|
Expected dividend rate |
|
0 |
% |
0 |
% | ||
|
Expected stock price volatility |
|
79.6 |
% |
86.1 |
% | ||
|
Risk-free interest rate |
|
2.0 |
% |
0.5 |
% | ||
|
Expected life (years) |
|
4.50 |
|
3.50 |
| ||
|
December 2010 Warrants |
|
December 31, 2010 |
|
December 31, 2011 |
| ||
|
Closing stock price |
|
$ |
2.56 |
|
$ |
1.82 |
|
|
Expected dividend rate |
|
0 |
% |
0 |
% | ||
|
Expected stock price volatility |
|
78.0 |
% |
83.6 |
% | ||
|
Risk-free interest rate |
|
2.0 |
% |
0.6 |
% | ||
|
Expected life (years) |
|
4.96 |
|
3.96 |
| ||
The following table summarizes the change in the estimated fair value of the Company’s warrant liabilities (in thousands):
|
Warrant Liabilities |
|
|
| |
|
Balance at June 30, 2010 |
|
$ |
3,010 |
|
|
Warrants issued |
|
15,868 |
| |
|
Warrants exercised |
|
(424 |
) | |
|
Increase in fair value |
|
7,500 |
| |
|
Balance at December 31, 2010 |
|
25,954 |
| |
|
Decrease in fair value |
|
(9,329 |
) | |
|
Balance at December 31, 2011 |
|
$ |
16,625 |
|
6. Income Taxes
A reconciliation of income taxes computed using the federal statutory rate to the taxes reported in our consolidated statements of operations is as follows (in thousands):
|
|
|
Year Ended June 30, |
|
Six Months |
|
December 31, |
| ||||||
|
|
|
2009 |
|
2010 |
|
2010 |
|
2011 |
| ||||
|
Loss before income taxes |
|
$ |
16,061 |
|
$ |
14558 |
|
$ |
19,088 |
|
$ |
19,668 |
|
|
Federal statutory rate |
|
34 |
% |
34 |
% |
34 |
% |
34 |
% | ||||
|
Taxes computed at federal statutory rate |
|
(5,461 |
) |
(4,950 |
) |
(6,490 |
) |
(6,687 |
) | ||||
|
Loss attributable to foreign operations |
|
437 |
|
116 |
|
— |
|
— |
| ||||
|
Warrants |
|
41 |
|
(1076 |
) |
2,550 |
|
(3,172 |
) | ||||
|
Nondeductible stock compensation |
|
— |
|
— |
|
— |
|
1,559 |
| ||||
|
Michigan business tax repeal |
|
— |
|
— |
|
— |
|
1,214 |
| ||||
|
Other |
|
79 |
|
136 |
|
397 |
|
336 |
| ||||
|
Change in valuation allowance |
|
4,904 |
|
5,776 |
|
3,543 |
|
6,750 |
| ||||
|
Reported income taxes |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
Deferred tax assets consist of the following (in thousands):
|
|
|
December 31, |
|
December 31, |
| ||
|
|
|
2010 |
|
2011 |
| ||
|
Net operating loss carryforwards |
|
$ |
46,533 |
|
$ |
53,224 |
|
|
Research and development credit carryforwards |
|
1,600 |
|
1,600 |
| ||
|
Employee benefits and stock compensation |
|
1,178 |
|
1,167 |
| ||
|
Other, net |
|
294 |
|
364 |
| ||
|
Total deferred tax assets |
|
49,605 |
|
56,355 |
| ||
|
Valuation allowance |
|
(49,605 |
) |
(56,355 |
) | ||
|
Net deferred tax assets |
|
$ |
— |
|
$ |
— |
|
As of December 31, 2011, the Company’s U.S. federal tax net operating loss and tax credit carryforwards are $156,542,000 and $1,600,000, respectively. These net operating loss carryforwards will expire between 2012 and 2031. The tax credit carryforwards will expire between 2022 and 2030. Effective January 1, 2012, the Michigan Business Tax (MBT) has been repealed. As a result of this repeal, the MBT net operating loss of $65,520,000 will not carry forward and has been derecognized.
The Company’s net operating losses are subject to the limitations imposed under section 382 of the Internal Revenue Code. These limits are triggered when a change in control occurs, and are computed based upon several variable factors including the share price of the Company’s common stock on the date of the change in control. A change in control is generally defined as a cumulative change of 50% or more in the ownership positions of certain stockholders during a rolling three year period. Based on common stock issuances over the Company’s history and the likelihood of additional issuances, it is possible that the use of the Company’s existing net operating losses will be limited. If a limitation occurs, it is likely that a significant portion of our net operating losses will expire unutilized regardless of the amount of future profitability.
Due to the historical losses incurred by the Company, a full valuation allowance for deferred tax assets, including the deferred tax assets for the aforementioned net operating losses and credits, has been provided since they are not more likely than not to be realized. If the Company achieves profitability, these deferred tax assets may be available to offset future income taxes. The increase in the valuation allowance was $3,543,000 and $6,750,000 for the six month transition period ended December 31, 2010 and the year ended December 31, 2011, respectively.
The Company assesses uncertain tax positions in accordance with the provisions of Financial Accounting Standards Board Accounting Standards Codification (ASC) 740-10-5, “Accounting for Uncertain Tax Positions.” This pronouncement prescribes a
recognition threshold and measurement methodology for recording within the financial statements uncertain tax positions taken, or expected to be taken, in the Company’s income tax returns. As of December 31, 2010 and December 2011, the Company had an unrecognized tax benefit of $2,100,000. There were no increases, decreases, or settlements related to uncertain tax positions in 2010 or 2011. Income tax expense would be reduced by zero if the gross unrecognized tax benefits were recognized due to the valuation allowance. It is not anticipated that the unrecognized tax benefits will significantly increase or decrease within the next twelve months.
The Company files U.S. federal and Michigan income tax returns. Due to the Company’s net operating loss carryforwards, Federal income tax returns from incorporation are still subject to examination. Michigan tax returns for the year ended June 30, 2007 and forward are subject to examination.
7. Licenses, Royalties and Collaborative Agreements and Commitments
University of Michigan — In August 1989, the Company entered into a research agreement with the University of Michigan (the University). In March 1992, and as provided for under the research agreement, the Company also entered into a license agreement for the technology developed under the research agreement. The license agreement, as amended, provides for a royalty to be paid to the University equal to 2% of net sales of products containing the licensed technology sold by the Company. Such royalties have been nominal since Inception. This license agreement will expire in 2014.
Corning Incorporated — In December 2002, the Company entered into an agreement with Corning Incorporated (Corning) that granted Corning an exclusive sublicense relating to the Company’s cell transfection technology. Under the terms of the agreement, the Company retains exclusive rights to the applications of the technologies involving cells for therapeutic applications. In addition, the agreement provides for future royalty payments on net sales of licensed products sold under the sublicense amounting to 5% of such sales up to $50,000,000. However, the Company does not expect to receive material revenue from this source for several years, if ever.
RealBio Technologies — In May 2009, the Company entered into an agreement with RealBio Technologies, Inc. (RealBio) that granted RealBio an exclusive license to utilize our technology outside of the Company’s core area of focus - human regenerative medicine. In return for this license, the Company received a minority equity interest in RealBio, which was not material as of December 31, 2011.
Manufacture, Supply and Other Agreements — The Company has entered into various agreements relating to the manufacture of its products and the supply of certain components. If the manufacturing or supply agreements expire or are otherwise terminated, the Company may not be able to identify and obtain ancillary materials that are necessary to develop its product and such expiration and termination could have a material effect on the Company’s business.
8. Commitments, Contingencies and Debt
During 2007 the Company entered into a new operating lease with Domino’s Farms Office Park, LLC, for approximately 30,000 square feet. This lease has a noncancelable term of six years, which began on May 14, 2007, and has two five-year market value renewals that the Company, at its option, can exercise six months prior to May 14, 2013 and May 14, 2018. The Company’s leased facility includes a Class 100,000 modular manufacturing clean room, laboratories and office space. The lease also provides the Company the right of first refusal on certain additional space.
As of December 31, 2011, future minimum payments related to our operating and capital leases and long-term debt are as follows (in thousands):
|
|
|
|
|
Payments Due by Period |
| ||||||||||||||
|
Contractual Obligations |
|
Total |
|
2012 |
|
2013 |
|
2014 |
|
2015 |
|
More then |
| ||||||
|
Operating leases |
|
$ |
1,502 |
|
$ |
1,124 |
|
$ |
378 |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
|
Capital leases |
|
80 |
|
40 |
|
34 |
|
6 |
|
— |
|
— |
| ||||||
|
Total |
|
$ |
1,582 |
|
$ |
1,164 |
|
$ |
412 |
|
$ |
6 |
|
$ |
— |
|
$ |
— |
|
Rent expense for the years ended June 30, 2009 and 2010, six month transition period ended December 31, 2010, year ended December 31, 2011 and for the period from March 24, 1989 (Inception) to December 31, 2011, was $1,153,000, $1,175,000, $548,000, $1,099,000, and $12,092,000 respectively.
9. Employee Savings Plan
The Company has a 401(k) savings plan that allows participating employees to contribute a portion of their salary, subject to annual limits and minimum qualifications. The Board may, at its sole discretion, approve Company matching contributions to the plan. The Company made contributions of $195,000 and $200,000 for the years ended June 30, 2009 and 2010, respectively, $88,000 for the six month transition period ended December 31, 2010, $159,000 for the year ended December 31, 2011 and $1,704,000 for the period from March 24, 1989 (Inception) to December 31, 2011.
10. Subsequent Events
On March 9, 2012, the Company completed the sale of 12,308 shares of Series B convertible preferred stock at an offering price of $3,250 per share. We received approximately $37,800,000 in net proceeds from the sale of the shares, after offering expenses. The Series B preferred stock consists of Series B-1 non-voting convertible preferred stock, which was issued at the closing and Series B-2 voting convertible preferred stock. The Series B-1 non-voting convertible preferred stock will not be entitled to vote on matters on which the common shareholders are generally entitled to vote. The Series B-2 preferred stock will be entitled to vote with the holders of the common stock as a single class, with each share of Series B-2 voting convertible preferred stock having the number of votes equal to the number of shares of common stock issuable upon conversion of such Series B-2 preferred stock. Any holder of Series B-1 preferred stock may exchange its shares for shares of Series B-2 preferred stock on a one-for-one basis if the shareholder approval required in accordance with Nasdaq Marketplace Rule 5635(b) has been obtained and subject to certain terms and limitations. The Series B preferred stock will, with respect to dividend rights and rights on liquidation, winding-up and dissolution, rank on parity with any other class or series of the Company capital stock that we may issue in the future which is designated as being on parity with the Series B preferred stock, and rank senior to our common stock and Series A preferred stock. The Series B preferred stock is convertible, at the option of the holder thereof at any time after the five year anniversary of the closing of the offering, into shares of our common stock at a conversion price of $3.25 per share of common stock, subject to the Company obtaining any shareholder approval required in accordance with Nasdaq Marketplace Rule 5635(b). At any time after the five year anniversary of issuance, the Company may elect to convert any or all outstanding shares of Series B preferred stock into shares of our common stock, subject to certain limitations. Dividends on the Series B preferred stock will be cumulative and compound daily, at a rate of 11.5% per annum, payable upon conversion, liquidation, redemption or other similar events, and payable in Series B preferred stock until the five year anniversary of issuance. Following the five year anniversary of issuance and until the earlier of the tenth anniversary of the closing of the offering and the date no Series B preferred stock remain outstanding, dividends will accrue at a rate of 8% per annum and will be payable in cash or Series B preferred stock, at our option. Unless prohibited by Michigan law governing distributions to shareholders, the Series B-1 preferred stock shall be redeemable at the option of holder of the Series B-1 preferred stock commencing at any time after the five year anniversary of issuance, liquidation, winding up, dissolution or other similar events, subject to certain terms and limitations.
11. Quarterly Financial Data (Unaudited)
The following is a summary of the Company’s quarterly consolidated results of operations (unaudited) for the year ended December 31, 2011 and 2010. The sum of quarterly net loss per share, basic and diluted, may not equal total net loss per share, basic and diluted, due to variation in shares outstanding (in thousands, except for per share data).
|
Year Ended December 31, 2011 |
|
First |
|
Second |
|
Third |
|
Fourth Quarter |
|
Fiscal Year |
| |||||
|
Revenues |
|
$ |
9 |
|
$ |
— |
|
$ |
9 |
|
$ |
— |
|
$ |
18 |
|
|
Loss from operations |
|
(6,260 |
) |
(7,507 |
) |
(7,439 |
) |
(7,834 |
) |
(29,040 |
) | |||||
|
Net loss |
|
(4,988 |
) |
(9,957 |
) |
(1,934 |
) |
(2,789 |
) |
(19,668 |
) | |||||
|
Net loss per common share |
|
$ |
(0.13 |
) |
$ |
(0.26 |
) |
$ |
(0.05 |
) |
$ |
(0.07 |
) |
$ |
(0.51 |
) |
Net loss includes the (increase) / decrease in fair value of warrants of $1,254,000, ($2,465,000), $5,496,000, $5,044,000 and $9,329,000 for the first quarter, second quarter, third quarter, fourth quarter and fiscal year, respectively.
|
Year Ended December 31, 2010 |
|
First |
|
Second |
|
Third |
|
Fourth |
|
Fiscal Year |
| |||||
|
Revenues |
|
$ |
— |
|
$ |
— |
|
$ |
— |
|
$ |
253 |
|
$ |
253 |
|
|
Loss from operations |
|
(4,263 |
) |
(5,140 |
) |
(5,853 |
) |
(5,770 |
) |
(21,026 |
) | |||||
|
Net loss |
|
(2,679 |
) |
(3,767 |
) |
(5,932 |
) |
(13,156 |
) |
(25,534 |
) | |||||
|
Net loss per common share |
|
$ |
(0.10 |
) |
$ |
(0.13 |
) |
$ |
(0.21 |
) |
$ |
(0.44 |
) |
$ |
(0.90 |
) |
Net loss includes the (increase) / decrease in fair value of warrants of $1,559,000, $1,348,000, ($99,000), ($7,401,000) and ($4,593,000) for the first quarter, second quarter, third quarter, fourth quarter and fiscal year, respectively.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
There are none to report.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
The Company conducted an evaluation, under the supervision and with the participation of management, including the Company’s Chief Executive Officer and Chief Accounting Officer (“CEO and CAO”) of the effectiveness of the design and operation of the Company’s disclosure controls and procedures as defined in Rules 13a-15(e) and 15d-15(e) of the Securities and Exchange Act of 1934, as amended (the
“Exchange Act”). Based on that evaluation, the CEO and CAO have concluded that the Company’s disclosure controls and procedures were effective as of December 31, 2011, in ensuring that information related to the Company required to be disclosed in reports the Company files or submits under the Exchange Act is (i) recorded, processed, summarized and reported within the time periods specified in the SEC’s rules and forms, and (ii) accumulated and communicated to the Company’s management, including the CEO and CAO, to allow timely decisions regarding required disclosure.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting (as defined in Rule 13a-15(f) under the Exchange Act). Our internal control over financial reporting is a process designed under the supervision of our CEO and CAO to provide reasonable assurance regarding the reliability of financial reporting and the preparation of our financial statements for external purposes in accordance with generally accepted accounting principles. Management evaluated the effectiveness of our internal control over financial reporting using the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control — Integrated Framework. Management, under the supervision and with the participation of the CEO and CAO, assessed the effectiveness of our internal control over financial reporting as of December 31, 2011 and concluded that it was effective.
Our independent registered public accounting firm, PricewaterhouseCoopers LLP, has audited the effectiveness of our internal control over financial reporting as of December 31, 2011, and has expressed an unqualified opinion thereon in their report which appears under Item 8.
Changes in Internal Control over Financial Reporting
During our fourth quarter of fiscal 2011, there were no changes in our internal control over financial reporting (as such term is defined in Rules 13a-15(f) and 15d-15(f) of the Exchange Act) that have materially affected, or are reasonably likely to materially affect, the Company’s internal control over financial reporting.
Not applicable.
Certain information required by Part III is omitted from this Annual Report on Form 10-K, and is incorporated by reference to our definitive Proxy Statement to be filed with the Securities and Exchange Commission pursuant to Regulation 14A in connection with our 2012 Annual Meeting of Shareholders scheduled for May 3, 2012.
Item 10. Directors, Executive Officers and Corporate Governance
The information relating to our directors is incorporated by reference to the Proxy Statement as set forth under the caption “Election of Directors.” Information relating to our executive officers is set forth in Part I of this Report under the caption “Executive Officers.”
Information with respect to delinquent filings pursuant to Item 405 of Regulation S-K is incorporated by reference to the Proxy Statement as set forth under the caption “Section 16(a) Beneficial Ownership Reporting Compliance.”
Item 11. Executive Compensation
The information relating to executive compensation is incorporated by reference to the Proxy Statement under the caption “Executive Compensation and Related Information.”
Item 12. Security Ownership of Certain Beneficial Owners and Management, and Related Shareholder Matters
The information relating to ownership of our equity securities by certain beneficial owners and management is incorporated by reference to the Proxy Statement as set forth under the caption “Stock Ownership of Certain Beneficial Owners and Management.”
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information relating to certain relationships and related person transactions is incorporated by reference to the Proxy Statement under the caption “Certain Relationships and Related Party Transactions.”
Item 14. Principal Accountant Fees and Services
The information relating to principal accountant fees and services is incorporated by reference to the Proxy Statement under the caption “Ratification of Appointment of Independent Registered Public Accounting Firm.”
Item 15. Exhibits and Financial Statement Schedules
(a) The following documents are filed as part of this Annual Report on Form 10-K:
1. Financial Statements (see Item 8).
2. All information is included in the Financial Statements or Notes thereto.
3. Exhibits:
See Exhibit Index.
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
Date: March 15, 2012 |
|
|
|
AASTROM BIOSCIENCES, INC. |
|
|
|
|
|
/s/ TIMOTHY M. MAYLEBEN |
|
|
Timothy M. Mayleben |
|
|
President and Chief Executive Officer |
|
|
(Principal Executive Officer) |
Pursuant to the requirements of the Securities Exchange Act of 1934, this Annual Report on Form 10-K has been signed on behalf of the registrant on March 15, 2012 by the following persons in the capacities indicated.
|
Signature |
|
Title |
|
|
|
|
|
|
|
|
|
/s/ TIMOTHY M. MAYLEBEN |
|
President and Chief Executive Officer, Director |
|
Timothy M. Mayleben |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
|
|
/s/ BRIAN D. GIBSON |
|
Vice President of Finance, Chief Accounting Officer and |
|
Brian D. Gibson |
|
Treasurer |
|
|
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|
|
|
|
/s/ NELSON M. SIMS |
|
Lead Independent Director |
|
Nelson M. Sims |
|
|
|
|
|
|
|
|
|
|
|
/s/ RONALD M. CRESSWELL, PH.D. |
|
Director |
|
Ronald M. Cresswell, Ph.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ ALAN L. RUBINO |
|
Director |
|
Alan L. Rubino |
|
|
|
|
|
|
|
|
|
|
|
/s/ HAROLD C. URSCHEL, JR., M.D. |
|
Director |
|
Harold C. Urschel, Jr., M.D. |
|
|
|
|
|
|
|
|
|
|
|
/s/ ROBERT L. ZERBE, M.D. |
|
Director |
|
Robert L. Zerbe, M.D. |
|
|
|
Exhibit No. |
|
Description |
|
3.1 |
|
Restated Articles of Incorporation of Aastrom, filed as Exhibit 4.1 to Aastrom’s Current Report on Form 8-K filed on December 17, 2009, incorporated herein by reference. |
|
|
|
|
|
3.2 |
|
Certificate of Amendment to Restated Articles of Incorporation of Aastrom dated February 9, 2010, filed as Exhibit 3.2 to Aastrom’s Post Effective Amendment No. 1 to Form S-1 filed on March 31, 2010, incorporated herein by reference. |
|
|
|
|
|
3.3 |
|
Certificate of Amendment to Restated Articles of Incorporation of Aastrom dated March 22, 2011, attached as Exhibit 3.1 to Aastrom’s Current Report on Form 8-K filed on March 25, 2011, incorporated herein by reference. |
|
|
|
|
|
3.4 |
|
Certificate of Designation, Preferences and Rights, of Aastrom Biosciences, Inc. classifying and designating the Series A Junior Participating Cumulative Preferred Stock, attached as Exhibit 3.1 to the Company’s Current Report on Form 8-A filed on August 12, 2011, incorporated herein by reference. |
|
|
|
|
|
3.5 |
|
Certificate of Designations, Preferences and Rights, of Aastrom Biosciences, Inc. classifying and designating the Series B Non-Voting Convertible Preferred Stock and the Series B-2 Voting Convertible Preferred Stock, attached as Exhibit 3.1 to the Company’s Current Report on Form 8-K filed on March 9, 2012, incorporated herein by reference. |
|
|
|
|
|
3.6 |
|
Bylaws, as amended, attached as Exhibit 3.1 to Aastrom’s Current Report on Form 8-K filed on November 12, 2010, incorporated herein by reference. |
|
|
|
|
|
4.1 |
|
Form of Senior Indenture for Senior Debt Securities, filed as Exhibit 4.1 to the Company’s Registration Statement on Form S-3 filed on June 16, 2011 and incorporated herein by reference. |
|
|
|
|
|
4.2 |
|
Form of Indenture for Subordinated Debt Securities, filed as Exhibit 4.3 to the Company’s Registration Statement on Form S-3 filed on June 16, 2011 and incorporated herein by reference. |
|
|
|
|
|
4.3 |
|
Shareholder Rights Agreement, dated as of August 11, 2011, between Aastrom Biosciences, Inc. and Continental Stock Transfer & Trust Company, as Rights Agent, attached as Exhibit 4.3 to the Company’s Current Report on Form 8-A filed on August 12, 2011, incorporated herein by reference. |
|
|
|
|
|
4.4 |
|
Amendment to Shareholder Rights Agreement, dated as of March 9, 2012, between Aastrom Biosciences, Inc. and Continental Stock Transfer & Trust Company, as Rights Agent, attached as Exhibit 4.1 to the Company’s Current Report on Form 8-K filed on March 9, 2012, incorporated herein by reference. |
|
|
|
|
|
10.1 # |
|
Form of Indemnification Agreement, attached as Exhibit 10.1 to Aastrom’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference. |
|
|
|
|
|
10.2 # |
|
Amended and Restated 1992 Incentive and Non-Qualified Stock Option Plan and forms of agreements thereunder, attached as Exhibit 10.5 to Aastrom’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference. |
|
|
|
|
|
10.3 # |
|
Form of Employment Agreement, attached as Exhibit 10.8 to Aastrom’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference. |
|
|
|
|
|
10.4 |
|
License Agreement, dated March 13, 1992, between Aastrom and the University of Michigan and amendments thereto dated March 13, 1992, October 8, 1993 and June 21, 1995, attached as Exhibit 10.17 to Aastrom’s Registration Statement on Form S-1 (No. 333-15415), filed on November 1, 1996, incorporated herein by reference. |
|
|
|
|
|
10.5 # |
|
Aastrom Biosciences 2001 Stock Option Plan, attached as Exhibit 10.72 to Aastrom’s Annual Report on Form 10-K for the year ended June 30, 2002, incorporated herein by reference. |
|
|
|
|
|
10.6 |
|
Supply Agreement between Aastrom and Moll Industries, Inc., dated December 16, 2003, attached as Exhibit 10.77 to Aastrom’s Annual Report on Form 10-K for the year ended June 30, 2004, incorporated herein by reference. |
|
|
|
|
|
10.7 # |
|
2004 Equity Incentive Plan, attached as Exhibit 10.82 to Amendment No. 1 to Aastrom’s Quarterly Report on Form 10-Q/A for the quarter ended September 30, 2004, incorporated herein by reference. |
|
|
|
|
|
10.8 # |
|
Form of Option and Restricted Stock Award Agreements for Grants under 2004 Equity Incentive Plan, attached as Exhibit 10.84 to Aastrom’s Annual Report on Form 10-K for the year ended June 30, 2005, incorporated herein by reference. |
|
|
|
|
|
10.9 |
|
Amendment dated December 5, 2002 to License Agreement with the University of Michigan, attached as Exhibit 10.87 to Aastrom’s Annual Report on Form 10-K for the year ended June 30, 2005, incorporated herein by reference. |
|
|
|
|
|
10.10 # |
|
2004 Equity Incentive Plan, as amended, attached as Exhibit 99.1 to Aastrom’s Current Report on Form 8-K filed on November 8, 2006, incorporated herein by reference. |
|
10.11 # |
|
Forms of Grant Notice and Stock Option Agreement for Grants under 2004 Equity Incentive Plan, as amended, attached as Exhibit 99.2 to Aastrom’s Current Report on Form 8-K filed on November 8, 2006, incorporated herein by reference. |
|
|
|
|
|
10.12 |
|
Placement Agency Agreement, dated October 15, 2007, by and between the Company and BMO Capital Markets Corp., attached as Exhibit 10.1 to Aastrom’s Current Report on Form 8-K filed on October 16, 2007, incorporated herein by reference. |
|
|
|
|
|
10.13 |
|
Escrow Agreement, dated as of October 15, 2007, among the Company, BMO Capital Markets Corp. and The Bank of New York, attached as Exhibit 10.2 to Aastrom’s Current Report on Form 8-K filed on October 16, 2007, incorporated herein by reference. |
|
|
|
|
|
10.14 |
|
Form of Purchase Agreement, attached as Exhibit 10.3 to Aastrom’s Current Report on Form 8-K filed on October 16, 2007, incorporated herein by reference. |
|
|
|
|
|
10.15 |
|
Form of Warrant, attached as Exhibit 10.4 to Aastrom’s Current Report on Form 8-K filed on October 16, 2007, incorporated herein by reference. |
|
|
|
|
|
10.16 |
|
Standard Lease between Aastrom and Domino’s Farms Office Park, L.L.C. dated January 31, 2007., attached as Exhibit 10.96 to Amendment No. 1 to Aastrom’s Annual Report on Form 10-K for the year ended June 30, 2007, incorporated herein by reference. |
|
|
|
|
|
10.17 |
|
Common Stock Purchase Agreement, dated June 12, 2009, between Aastrom Biosciences, Inc. and Fusion Capital Fund II, LLC, attached as Exhibit 10.1 to Aastrom’s Current Report on Form 8-K filed on June 12, 2009, incorporated herein by reference. |
|
|
|
|
|
10.18 |
|
Registration Rights Agreement, dated June 12, 2009, between Aastrom Biosciences, Inc. and Fusion Capital Fund II, LLC, attached as Exhibit 10.2 to Aastrom’s Current Report on Form 8-K filed on June 12, 2009, incorporated herein by reference. |
|
|
|
|
|
10.19 # |
|
2009 Omnibus Incentive Plan, attached as Appendix II to Aastrom’s Proxy Statement filed on October 9, 2009, incorporated herein by reference. |
|
|
|
|
|
10.20 |
|
Class A Warrant Agreement, dated as of January 21, 2010, by and between the Registrant and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 27, 2010). |
|
|
|
|
|
10.21 |
|
Class B Warrant Agreement, dated as of January 21, 2010, by and between the Registrant and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.2 to the Company’s Current Report on Form 8-K filed with the SEC on January 27, 2010). |
|
|
|
|
|
10.22 |
|
Underwriting Agreement, dated as of January 15, 2010, and between the Registrant and Oppenheimer & Co. Inc. (incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed with the SEC on January 15, 2010). |
|
|
|
|
|
10.23 # |
|
Employment Agreement with Timothy M. Mayleben dated October 23, 2009 attached as Exhibit 99.3 to Aastrom’s Current Report on Form 8-K filed on October 27, 2009, incorporated herein by reference. |
|
|
|
|
|
10.24 # |
|
Employment Agreement with Scott C. Durbin dated June 7, 2010 attached as Exhibit 10.1 to Aastrom’s Current Report on Form 8-K filed on June 8, 2010, incorporated herein by reference. |
|
|
|
|
|
10.25 # |
|
Form of indemnification agreement entered into between the Company and each of its directors, including Timothy M. Mayleben, a director and the Company’s President and Chief Executive Officer, attached as Exhibit 10.1 to Aastrom’s Current Report on Form 8-K filed on August 31, 2010, incorporated herein by reference. |
|
|
|
|
|
10.26 |
|
Amended Code of Business Conduct and Ethics, attached as Exhibit 14.1 to Aastrom’s Current Report on Form 8-K filed on August 31, 2010, incorporated herein by reference. |
|
|
|
|
|
10.27* |
|
Contract Manufacturing and Supply Agreement, dated as of November 1, 2010, by and between Vention Medical (formerly ATEK Medical, LLC) and the Company. |
|
|
|
|
|
10.28 |
|
Warrant agreement, dated as of December 15, 2010, by and between the Registrant and Continental Stock Transfer & Trust Company (incorporated herein by reference to Exhibit 4.1 to the Company’s Current Report on Form 8-K filed with the SEC on December 16, 2010). |
|
|
|
|
|
10.29 |
|
Underwriting Agreement, dated as of December 10, 2010, and between the Registrant and Stifel, Nicolaus & |
|
|
|
Company, Incorporated, Needham & Company, LLC and Roth Capital Partners (incorporated herein by reference to Exhibit 1.1 to the Company’s Current Report on Form 8-K filed with the SEC on December 10, 2010). |
|
|
|
|
|
10.30# |
|
Amendment to the 2009 Omnibus Incentive Plan, dated March 21, 2011 (incorporated herein by reference to Exhibit 10.4 to the Company’s current Report on Form 8-K, filed on March 25, 2011). |
|
|
|
|
|
10.31# |
|
Employment Agreement with Ronnda L. Bartel, PhD, dated March 22, 2011 (incorporated herein by reference to Exhibit 10.1 to the Company’s current Report on Form 8-K, filed on March 25, 2011). |
|
|
|
|
|
10.32# |
|
Employment Agreement with Sharon Watling, PharmD, dated March 22, 2011 (incorporated herein by reference to Exhibit 10.2 to the Company’s current Report on Form 8-K, filed on March 25, 2011). |
|
|
|
|
|
10.33# |
|
Senior Executive Incentive Bonus Plan (incorporated herein by reference to Exhibit 10.3 to the Company’s current Report on Form 8-K, filed on March 25, 2011). |
|
|
|
|
|
10.34 |
|
At Market Issuance Sales Agreement, dated June 16, 2011, by and among Aastrom Biosciences, Inc. and McNicoll, Lewis & Vlak LLC (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC on June 16, 2011). |
|
|
|
|
|
10.35 |
|
Master Services Agreement by and between the Company and CRO, made and entered into as of September 23, 2011 (the “Master Services Agreement”) (incorporated herein by reference to Exhibit 10.1 to the Company’s Current Report on Form 8-K filed with the SEC November 22, 2011). |
|
|
|
|
|
10.36 |
|
Project Addendum to the Master Services Agreement, dated as of November 16, 2011 (incorporated herein by reference to Exhibit 10.2 to the Company’s Current Report on Form 8-K filed with the SEC November 22, 2011). |
|
|
|
|
|
10.37 |
|
Registration Rights Agreement, dated March 9, 2012, between Aastrom Biosciences, Inc. and Eastern Capital Limited, attached as Exhibit 10.2 to Aastrom’s Current Report on Form 8-K filed on March 9, 2012, incorporated herein by reference. |
|
|
|
|
|
21 |
|
Subsidiaries of Registrant. |
|
|
|
|
|
23.1 |
|
Consent of Independent Registered Public Accounting Firm. |
|
|
|
|
|
31.1 |
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
31.2 |
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
32.1 |
|
Certification of Chief Executive Officer and Chief Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. |
|
|
|
|
|
101.INS |
|
XBRL Instance Document |
|
|
|
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document |
|
|
|
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
|
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
|
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document |
# Management contract or compensatory plan or arrangement covering executive officers or directors of Aastrom.
* Confidential treatment status has been requested as to certain portions thereto, which portions are omitted and filed with Securities and Exchange Commission.
|
TERM |
|
DEFINITION |
|
Adverse Event |
|
Any adverse change in health or “side-effect” that occurs in a person participating in a clinical trial, from the time they consent to joining the trial until a pre-specified period of time after their treatment has been completed. |
|
Autologous (Patient Specific) |
|
Originating from the patient receiving treatment. (Aastrom uses only autologous cells) |
|
BLA — Biologics License Application |
|
An application containing product safety, efficacy and manufacturing information required by the FDA to market biologics products in the U.S. |
|
CBER — Center for Biologics Evaluation and Research |
|
Branch of the FDA that regulates biological products for disease prevention and treatment that are inherently more complex than chemically synthesized pharmaceuticals. |
|
CLI — Critical Limb Ischemia |
|
A vascular disease characterized by insufficient blood flow in the lower extremities that causes severe pain, tissue loss or both. |
|
CMC — Chemistry, Manufacturing, and Control |
|
The composition, manufacture, and control of the drug substance and the drug product. It is information on the identification, quality, purity, and strength of the investigational product. |
|
Controlled Clinical Trial |
|
A clinical study that compares patients receiving a specific treatment to patients receiving an alternate treatment for the condition of interest. The alternate treatment may be another active treatment, standard of care for the condition and/or a placebo (inactive) treatment. |
|
DCM — Dilated Cardiomyopathy |
|
A chronic cardiac disease where expansion of the patient’s heart reduces the pumping function to a point that the normal circulation of blood cannot be maintained. |
|
Double-Blind Clinical Trial |
|
Clinical trials in which neither the patient nor the physician know if the patient received the experimental treatment or a control/placebo. |
|
Ex vivo |
|
Outside the body |
|
FDA — Food & Drug Administration |
|
The U.S. FDA ensures that medicines, medical devices, and radiation-emitting consumer products are safe and effective. Authorized by Congress to enforce the Federal Food, Drug, and Cosmetic Act and several other public health laws, the agency monitors the manufacture, import, transport, storage, and sale of $1 trillion worth of goods annually. |
|
GMP — Good Manufacturing Practice |
|
GMP regulations require that manufacturers, processors, and packagers of drugs, medical devices, some food, and blood take proactive steps to ensure that their products are safe, pure, and effective. GMP regulations require a quality approach to manufacturing, enabling companies to minimize or eliminate instances of contamination, mix-ups, and errors. |
|
Hematopoietic Stem Cells |
|
Stem cells that give rise to all the blood cell types including myeloid (monocytes and macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes/platelets, dendritic cells), and lymphoid lineages (T-cells, B-cells, NK-cells). |
|
IMPACT-DCM |
|
Aastrom’s U.S. Phase 2 dilated cardiomyopathy clinical trial. |
|
IND — Investigational New Drug |
|
An application submitted to the FDA for a new drug or biologic that, if allowed, will be used in a clinical trial. |
|
Ischemia |
|
A shortage or inadequate flow of blood to a body part (commonly an organ or tissue) caused by a constriction or obstruction of the blood vessels supplying it. |
|
LVEF — Left Ventricular Ejection Fraction |
|
The fraction of blood pumped out of the left ventricle with each heart beat. |
|
Open-label Clinical Trial |
|
A trial in which both the treating physician and the patient know whether they are receiving the experimental treatment or control/placebo treatment. |
|
TERM |
|
DEFINITION |
|
Orphan Drug Designation |
|
“Orphan drug” refers to a drug or biologic that is intended for use in the treatment of a rare disease or condition. Orphan drug designation from the U.S. Food and Drug Association (FDA) qualifies the sponsor to receive certain benefits from the Government in exchange for developing the drug for a rare disease or condition. The drug must then go through the FDA marketing approval process like any other drug or biologic which evaluates for safety and efficacy. Usually a sponsor receives a quicker review time and lower application fees for an orphan product. |
|
Phase 1 Clinical Trial |
|
A Phase 1 trial represents an initial study in a small group of patients to test for safety and other relevant factors. |
|
Phase 2 Clinical Trial |
|
A Phase 2 trial represents a study in a moderate number of patients to assess the safety and efficacy of a product. |
|
Phase 2b Clinical Trial |
|
A Phase 2b trial is a moderately-sized Phase 2 trial that is more specifically designed assess the efficacy of a product than a Phase 2a trial. |
|
Phase 3 Clinical Trial |
|
Phase 3 studies are initiated to establish safety and efficacy in an expanded patient population at multiple clinical trial sites and are generally larger than trials in earlier phases of development. |
|
Progenitor Cells |
|
A “parent” cell that gives rise to a distinct cell lineage by a series of cell divisions. |
|
Prospective Clinical Trial |
|
A clinical trial in which participants are identified and then followed throughout the study going forward in time. |
|
Randomized Clinical Trial |
|
A clinical trial in which the participants are assigned randomly to different treatment groups. |
|
Somatic Cell |
|
Any of the cells responsible for forming the body of an organism such as internal organs, bones, skin, connective tissues and blood. |
|
Stem Cell |
|
Unspecialized (undifferentiated) cells that retain the ability to divide throughout a lifetime and give rise to more specialized (differentiated) cells which take the place of cells that die or are lost. In culture, these undifferentiated cells possess the ability to divide for indefinite periods in culture and may give rise to highly specialized cells. |
