Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - ASSEMBLY BIOSCIENCES, INC. | Financial_Report.xls |
| EX-23.1 - EXHIBIT 23.1 - ASSEMBLY BIOSCIENCES, INC. | v305263_ex23-1.htm |
| EX-32.1 - EXHIBIT 32.1 - ASSEMBLY BIOSCIENCES, INC. | v305263_ex32-1.htm |
| EX-31.2 - EXHIBIT 31.2 - ASSEMBLY BIOSCIENCES, INC. | v305263_ex31-2.htm |
| EX-31.1 - EXHIBIT 31.1 - ASSEMBLY BIOSCIENCES, INC. | v305263_ex31-1.htm |
| EX-32.2 - EXHIBIT 32.2 - ASSEMBLY BIOSCIENCES, INC. | v305263_ex32-2.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Transition Period from __________ to __________
Commission File Number 001-35005
VENTRUS BIOSCIENCES, INC.
(Exact name of registrant specified in its charter)
| Delaware | 2834 | 20-8729264 |
|
(State or Other Jurisdiction of Incorporation or Organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
99 Hudson Street, 5th Floor
New York, New York 10013
(Address of Principal Executive Offices)
(646) 706-5208
(Telephone Number, Including Area Code)
Securities Registered Pursuant to Section 12(b) of the Exchange Act:
|
Title of Each Class |
Name of Exchange on which Registered | |
| Common Stock, $0.001 Par Value | Nasdaq Capital Market |
Securities Registered Pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act.
Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No £
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a smaller reporting company. See definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer x | |||
|
Non-accelerated filer ¨ (Do not check if a smaller reporting company) |
Smaller reporting company ¨ |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting stock held by non-affiliates of the registrant, as of June 30, 2011, was approximately $92.2 million. Such aggregate market value was computed by reference to the closing price of the common stock as reported on the Nasdaq Capital Market on June 30, 2011. For purposes of making this calculation only, the registrant has defined affiliates as including only directors and executive officers and shareholders holding greater than 10% of the voting stock of the registrant as of June 30, 2011.
As of March 8, 2012 there were 12,406,406 shares of the registrant’s common stock, $0.001 par value, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain portions of t he Company’s definitive Proxy Statement for its 2012 Annual Meeting of Stockholders are incorporated herein by reference, as indicated in Part III.
VENTRUS BIOSCIENCES, INC.
TABLE OF CONTENTS
| Page | ||
| PART I | 1 | |
| Item 1. | Business | 1 |
| Item 1A. | Risk Factors | 42 |
| Item 1B. | Unresolved Staff Comments | 61 |
| Item 2. | Properties | 61 |
| Item 3. | Legal Proceedings | 61 |
| Item 4. | Mine Safety Disclosures | 61 |
| PART II | 62 | |
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 62 |
| Item 6. | Selected Financial Data | 64 |
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operation | 65 |
| Item 7A. | Quantitative and Qualitative Disclosures about Market Risk | 72 |
| Item 8. | Financial Statements and Supplementary Data | 72 |
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 72 |
| Item 9A. | Controls and Procedures | 72 |
| Item 9B. | Other Information | 73 |
| PART III | 74 | |
| Item 10. | Directors, Executive Officers and Corporate Governance | 74 |
| Item 11. | Executive Compensation | 74 |
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 87 |
| Item 13. | Certain Relationships and Related Transactions, and Director Independence | 88 |
| Item 14. | Principal Accounting Fees and Services | 88 |
| Item 15. | Exhibits, Financial Statement Schedules | 88 |
| Financial Statements | F-1 | |
| i |
This report contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. These forward-looking statements are subject to risks and uncertainties, including those set forth under “Item 1A. Risk Factors” and “Cautionary Statement” included in “Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations” and elsewhere in this report, that could cause actual results to differ materially from historical results or anticipated results.
PART I
Item 1. Business
Overview
We are a development stage specialty pharmaceutical company currently focused on the development of late-stage prescription drugs for gastrointestinal disorders, specifically hemorrhoidal disease, anal fissures and fecal incontinence. Major pharmaceutical progress has been made in the gastrointestinal therapeutic areas of gastroesophageal reflux, peptic ulcer disease and inflammatory bowel disease. However, many major gastrointestinal disorders still lack medical treatments. We are pursuing treatments for three of the 10 most prevalent gastrointestinal disorders in the U.S. We estimate that the patient population of these three disorders is almost 30.0 million people in the U.S., based on the data we cite for each indication in this report.
We are not aware of any prescription drug treatments for hemorrhoids or fecal incontinence that have been approved by the U.S. Food and Drug Administration, or FDA for these indications, yet there currently are approximately 21.7 million Americans suffering from symptomatic hemorrhoids in the past year, and approximately 7.0 million from fecal incontinence. While there are approximately 1.1 million office visits per year for anal fissures in the U.S., we are aware of only one drug that has received FDA approval for the treatment of pain associated with anal fissures; Rectiv received approval in late June 2011, and is expected to come to market in the first quarter of 2012. Rectiv is effective in reducing the pain from anal fissures, but moderate and severe headaches are a frequent side effect of this drug whose active ingredient is nitroglycerin. Our lead product VEN 309 (iferanserin) is a new chemical entity, or NCE, for the topical treatment of symptomatic internal hemorrhoids. In seven clinical studies between 1993 and 2003 involving 359 patients, VEN 309 demonstrated good tolerability and no severe adverse events, and statistically significant improvements in bleeding, itchiness and pain. Beginning in 2008, we have had extensive discussions with the FDA under a Special Protocol Assessment, or SPA, process, for our first pivotal U.S. trial of VEN 309 for the treatment of symptomatic internal hemorrhoids. While we decided not to pursue an agreement letter, we received many recommendations from the FDA concerning the major and important elements of the trial during this process and we incorporated these into our protocol. To avoid delays and without having reached agreement with FDA on the SPA, we proceeded to file the protocol to our existing investigational new drug application, or IND, with the FDA in July 2011 and began enrolling and dosing patients in August 2011. We own all rights, title and interest in VEN 309.
Our additional product candidate portfolio consists of two in-licensed late-stage drugs. VEN 307 (diltiazem) is intended to treat pain associated with anal fissures and VEN 308 (phenylephrine) is intended to treat fecal incontinence. These candidates are two molecules that were previously approved and are currently marketed for other indications and that have been formulated into our proprietary topical treatments for these new gastrointestinal indications.
Diltiazem was first approved in 1982 in oral form for the treatment of angina and high blood pressure. It has been prescribed in the U.S. for millions of patients in oral dosages typically from 240 mg to 360 mg per day. In contrast, daily doses of VEN 307 for treatment of anal fissures will range from 15 mg to 45 mg. Because of the extensive patient exposure to diltiazem as a cardiovascular agent and the wide safety margin as a low dose topical therapy, we intend to develop the topical formulation as a Section 505(b)(2) new drug application, or NDA, based on our discussions with the FDA at our pre-IND meeting in August 2007.
| 1 |
Phenylephrine has been available since the early 1940s in oral and nasal form for the treatment of nasal congestion. It has also been used as a topical ophthalmic agent since 1936. Phenylephrine is prescribed more than 17 million times per year in the U.S., with 99% of the prescriptions being for cough/cold oral preparations. The typical oral dosing is 40 mg to 60 mg per day. Because of the extensive patient exposure to phenylephrine, we intend to develop VEN 308 as a topical formulation through a Section 505(b)(2) NDA.
In August 2007, we had a pre-IND meeting with the FDA concerning VEN 307 for the treatment of pain from anal fissures where we discussed necessary preclinical testing and product formulation to support an IND, established what clinical safety database would be required, and established that the next clinical studies needed for approval were two pivotal Phase III trials, preceded (if conducted in the U.S.) by three short-term dermal toxicology studies using final drug product formulation. In June 2007, we had a pre-IND meeting with the FDA concerning VEN 308 for the treatment of fecal incontinence associated with ileal pouch anal anastomosis (IPAA) where it was established that the next clinical study in the program should be a Phase IIb trial where multiple doses will be assessed and that existing toxicology data are sufficient to support Phase II testing. We have not had further meetings with the FDA on either VEN 307 or VEN 308 since the meetings in 2007. Beginning in February 2009, the development of the three products, VEN 307, VEN 308 and VEN 309, was delayed due to a lack of financial resources prior to the completion of our initial public offering in December 2010. We have used and are using the proceeds from that offering, as well as the proceeds from our July 2011 registered public offering of our common stock, to continue the development of VEN 309 and VEN 307 and we are using a portion of the proceeds from the July 2011 offering to fund the two pivotal Phase III trials for VEN 309.
Our Pipeline
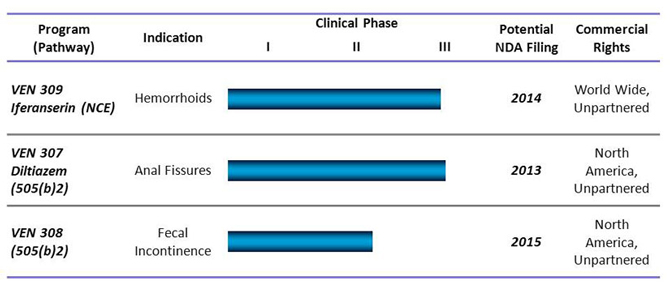
Our Products and Development Strategy
Our three late-stage product candidates are:
Iferanserin ointment (VEN 309) for the topical treatment of symptomatic internal hemorrhoids. Hemorrhoids, which are characterized by the inflammation and swelling of veins around the anus or lower rectum, can cause bleeding, itching, pain and difficulty defecating. VEN 309, an NCE formulated as an ointment for intra-anal application, has highly selective, antagonistic activity against peripheral 5HT 2A receptors involved in clotting and the contraction of arteries and veins, two events believed to be associated with hemorrhoid formation. By limiting 5HT 2A receptor activity, VEN 309 improves the flow of blood out of the dilated veins that comprise the hemorrhoid, thereby reducing bleeding, itchiness and pain. As reported a survey of 10,000 adult consumers in the U.S. conducted on our behalf by Princeton Brand Econometrics, a consumer market research company, symptomatic hemorrhoids have affected approximately 21.7 million people in the past year and approximately 6.7 million adults on any given day in the U.S. Despite such a high prevalence, we are not aware of any FDA-approved prescription drugs for the treatment of hemorrhoids. While there are commonly used prescription drugs in the U.S. for hemorrhoids, such as Anusol®, none have been approved by the FDA or have been designated by the FDA as safe and effective for this indication. Various combination products (such as the Preparation H® line of products) are available in the U.S. over-the-counter, or OTC, under the FDA’s OTC monograph rule. The great majority of these OTC treatments provide only temporary relief from the symptoms of hemorrhoids, but do not address the cause of hemorrhoids. The mechanism of action of these treatments is either generally anti-inflammatory, such as steroids, or acting as a protective coating on the hemorrhoid or acting as local anesthetics, in the case of most of the OTC products, or unknown, in the case of herbal remedies, and we are not aware of any clinical trials published in medical journals on the efficacy or safety of any topical or oral drug currently marketed in the U.S. for the treatment of hemorrhoids. We believe VEN 309 to be more effective than the currently available conventional hemorrhoid topical or oral drug therapies and more attractive than surgical procedures, which are the only currently validated treatment options.
| 2 |
We originally licensed VEN 309 from Sam Amer & Co., Inc., or Amer, who had developed VEN 309 through Phase II trials and up to readiness for Phase III trials in the U.S. and Europe. On November 14, 2011, we acquired all rights, title and interest to VEN 309 from Amer. VEN 309 is covered for composition of matter in patents that will expire in August 2015 in the U.S. and February 2018 elsewhere. If approved by the FDA, VEN 309 will receive five years of data exclusivity in the U.S. as an NCE under the Hatch-Waxman Act and 10 years from the date of approval in Europe. We filed a new concentration range patent in August 2010, which, if issued, would grant patent protection until 2030 and prevent substitutable generic competition.
Our initial Phase III trial for VEN 309 (ClinicalTrials.gov Identifier: NCT01355874) is a multicenter double-blind randomized placebo-controlled parallel treatment group trial, consisting of three arms with a double-blind portion and an open-label extension portion consisting of:
Double blind part
| · | Approximately 600 male or female patients aged 18 – 75 years (200 patients per arm) recruited at up to approximately 70 sites in the U.S., randomized 1:1:1 ratio to: |
| º | Arm 1: placebo ointment twice daily intra-anally for 14 days; |
| º | Arm 2: iferanserin ointment twice daily for 14 days; |
| º | Arm 3: iferanserin ointment twice daily for 7 days followed by placebo ointment twice daily for 7 days; |
| · | After 14 days treatment, patients will be followed up at Day 28; |
| · | Inclusion criteria include symptomatic grade I to III internal hemorrhoids, bleeding from hemorrhoids every day for the two days immediately preceding the day that they are randomized and study medication applied, with pain or itching accompanying the bleeding for the two days; and |
| · | Exclusion criteria include: grade IV hemorrhoids; thrombosed internal or external hemorrhoids; prior or current history of heart disease or depression; use of laxatives, anticoagulants, over-the-counter anti-hemorrhoidal agents, topical steroids, suppositories of any kind, non-steroidal anti-inflammatory drugs (NSAIDs), Cox-2 inhibitors, and other drugs and conditions including potential inhibitors of CYP2D6 such as SSRI drugs. |
The endpoints for the double-blind part of the trial are:
| · | Primary: Proportion of patients with cessation of bleeding by Day 7 that persists for the remainder of the treatment period (through Day 14); and |
| · | Key Secondary: Proportion of patients with cessation of pain and/or itching by Day 7 that persists for the remainder of the treatment period (through Day 14). |
| 3 |
Open Label part
After the 28 day double blind portion of the trial, patients will be followed quarterly for one year and treated with active drug if they have a recurrence at any time during that period. We will assess time to first recurrence, and the overall recurrence rate over one year, and will be able to observe the unblinded response to treatment of recurrence during this part of the trial.
Although we did not obtain an SPA agreement with the FDA, we believe that our modeling of the endpoint definitions as proposed by the FDA using the German Phase IIb trial data, confirm a projected power of > 99% for the primary endpoint and > 95% for the key secondary endpoints for our proposed Phase III trial.
We filed the protocol to our existing IND with the FDA in July 2011 and began enrolling and dosing patients in August 2011, and estimate we will complete enrollment approximately in April 2012. We anticipate reporting the top line data from our ongoing U.S. Phase III trial of VEN 309 in hemorrhoids in June of 2012.
Diltiazem cream (VEN 307), a topical treatment for the relief of pain associated with anal fissures. Anal fissures are small tears or cuts in the skin that lines the anus. They can be extremely painful, cause bleeding and often require surgery, which itself can have unsatisfactory outcomes. In 2010, it was estimated by SDI Health LLC that there were approximately 1.1 million office visits per year for anal fissures. At present, we are aware of only one FDA-approved drug for the treatment of anal fissures. Rectiv (nitroglycerin) ointment 0.4%, for the treatment of moderate to severe pain associated with chronic anal fissures, received FDA approval in late June 2011, and is expected to come to market in the first quarter of 2012. Topical nitroglycerin, the active ingredient in Rectiv, also has been compounded by pharmacists to treat anal fissures, but has a substantially higher rate of side effects than topical diltiazem, notably moderate and severe headaches, which also are experienced with Rectiv. We also are aware of limited use of Botox as an injection into the anal sphincter to treat this condition. Several topical forms of nifedipine, a calcium-channel blocker, also are used to treat pain from anal fissures. Diltiazem cream, also a calcium-channel blocker, however, is currently used as the preferred treatment prior to surgery by many gastroenterologists across the U.S. in a version that must be specially mixed, or compounded, for each patient in the pharmacy. Compounded diltiazem is currently listed in the U.S. and E.U. anal fissure treatment guidelines as a preferred agent prior to attempting surgery. Neither compounded diltiazem nor nifedipine, however, is FDA-approved for the relief of pain associated with anal fissures nor is the cost typically reimbursed by Medicare or health insurance plans. We expect that VEN 307, if approved by FDA and Rectiv would be reimbursable under Medicare and health insurance plans. When applied topically for the treatment of anal fissures, diltiazem, which has been used for decades for hypertension and angina, dilates the blood vessels supplying the region, reduces anal sphincter tone, and thereby substantially decreases pain. In the majority of multiple clinical trials conducted against placebo or topical nitroglycerin conducted between 1999 and 2002 by various researchers in investigator initiated trials, diltiazem cream significantly reduced the pain associated with anal fissures.
Our product, VEN 307, is a pre-mixed and pre-packaged proprietary formulation of diltiazem that when applied topically yields lower blood levels (at one-tenth the amount) than the lowest oral dose used for cardiovascular treatment. We believe these low blood levels improve the safety profile and lower the risk of side effects. We have potential to capture immediate market share if VEN 307 is approved due to the familiarity of gastroenterologists with the current use of diltiazem to treat anal fissures, its ease of prescription as a pre-formulated FDA-approved product with no need for compounding necessary at the pharmacy, and the expected ability for patients to be reimbursed through their health insurance plans or Medicare. We have licensed the exclusive North American rights to VEN 307 for the topical treatment of anal fissures from S.L.A. Pharma, our development partner, who has completed early-stage clinical trials, toxicology studies and manufacturing for VEN 307 up to the end of Phase II. VEN 307 is covered by a method of use in a patent that will expire in February 2018.
In August 2007, we had a pre-IND meeting with the FDA concerning VEN 307 for the treatment of pain from anal fissures where we addressed necessary preclinical testing and product formulation to support an IND, established what clinical safety database would be required, and that the next clinical studies needed for approval were two pivotal Phase III trials, preceded (if conducted in the U.S.) by three short-term dermal toxicology studies using final drug product formulation. Prior to conducting any clinical Phase III trials in the U.S., we must complete three short-term dermal toxicology studies and file an IND for FDA approval. We plan to employ a two-pronged development strategy for VEN 307. While S.L.A. Pharma is conducting the first Phase III VEN 307 clinical trial in the E.U. which completed enrollment in December 2011 and is anticipated to be reporting data in May 2012, we intend to initiate development of a different formulation of VEN 307 with new intellectual property in the form of an extended release formulation. There are several proven methodologies for extended release topical formulations, and we believe that diltiazem is readily druggable in this regard. We intend to assess three to four alternatives preclinically with multiple contractors, and then assess absorption and effect on the internal anal sphincter (IAS) pressure with the most promising candidate, while we file Patent Cooperation Treaty applications for the specific technology combined with diltiazem for all formulations that are technically feasible.
| 4 |
S.L.A. Pharma began enrollment in the VEN 307 Phase III trial in November 2010 and completed enrollment of 465 patients at 32 sites in Europe in December 2011. Patients were treated for two months and then observed without treatment for one month in a randomized 1:1:1 double blind study that compares treatments of fiber plus 2% VEN 307 and fiber plus 4% VEN 307 to fiber plus placebo. The primary endpoint is reduction of pain upon defecation averaged across the fourth week of treatment, using a validated numerical rating scale for pain. Patients used daily diaries and were observed for one week prior to randomization to ensure sufficient pain prior to randomization. We expect initial top-line data from the VEN 307 EU Phase III study to be available in May 2012.
If there is successful completion of and satisfactory data from the E.U. trial, we will make the final decision on which formulation to pursue depending on several factors, including whether the new formulation is clinically superior, our access to capital, clinical and regulatory considerations, and our assessment of the then-current state of our intellectual property estate. If the new U.S. developed formulation is superior as demonstrated by sufficient data and the other factors are met, we plan to file an IND for the new formulation of VEN 307 and then initiate two pivotal trials in parallel in order to complete the NDA for an estimated FDA submission in 2014. If the new formulation is not superior, from the clinical. CMC and intellectual property perspectives, we plan to finish clinical development utilizing the current formulation which would require three short-term dermal toxicology studies and one additional pivotal Phase III trial in the U.S. We believe that continuing with the current formulation could result in an NDA submission in 2013 but would expect to continue to pursue other lifecycle options for VEN 307. We intend to use a portion of our current resources to continue the development of VEN 307.
Phenylephrine gel (VEN 308) for the treatment of fecal incontinence associated with ileal pouch anal anastomosis, an FDA orphan indication. Ileal pouch anal anastomosis, or IPAA, is a surgical procedure used as part of a colectomy, which is a surgical treatment for patients with ulcerative colitis. Fecal incontinence resulting from dysfunctional sphincter tone is a common consequence of this procedure. Patients with IPAA, secondary to a total colectomy, tend to have a high incidence of fecal incontinence, up to 30%, according to a 1987 study conducted by Dr. John Pemberton and others at the Mayo Medical School. According to a U.S. community based epidemiology study (Nelson et al., JAMA, 1995), 2.2% of the U.S. population suffer from fecal incontinence, which we estimate to be approximately 7.0 million people, based on 2009 Census Bureau population estimates. The surgery associated with IPAA can weaken sphincters and muscles necessary for continence and therefore can result in incontinence. About 30% of patients with ulcerative colitis, a form of inflammatory bowel disease which has a prevalence of 700,000 patients in the U.S. (according to Datamonitor 2008) will have had a colectomy, almost always an IPAA procedure (according to McGlauchlin and Clark, Practical Gastroenterology, 8/2008). IPAA-related fecal incontinence is considered an orphan indication by the FDA and the European Medicines Agency, or EMEA. In 2006, the total population of patients with IPAA-related fecal incontinence in the U.S. was estimated to be 50,000 to 100,000, according to IMS Health, Inc. Currently, there are few options available to treat this problem, consisting of OTC bulk laxatives, fiber diets, Imodium, which is a treatment for diarrhea, and invasive surgical procedures. In addition, Solesta, an injectable inert bulking agent product, was approved as a device by the FDA in May 2011 for the treatment of fecal incontinence in adult patients who have failed conservative therapy. Solesta is injected submucosally around the anal sphincter and consequently has to be administered in an outpatient setting by qualified physicians. In addition, Norgine is conducting a European Phase II program with NRL001, a suppository formulation of an alpha adrenergic stimulating agent for the treatment of fecal incontinence. We are not aware of any FDA-approved drugs for fecal incontinence. In multiple investigator initiated clinical trials with patients suffering from IPAA-associated fecal incontinence, topical phenylephrine significantly (and in some patients, dramatically) improved patient bowel control. In clinical trials with other forms of incontinence, improvements were also observed following application of topical phenylephrine, depending on the cause of the incontinence.
| 5 |
Our product, VEN 308, is a gel formulation of phenylephrine. Applied topically, VEN 308 increases anal sphincter tone, thereby improving fecal incontinence in patients where sphincter tone is the major cause of their symptoms, such as post-IPAA surgery. We believe VEN 308 has significant advantages over the limited treatment options currently available for fecal incontinence associated with IPAA, including but not limited to, increased efficacy and/or reduced invasiveness. We have licensed the exclusive North American rights to VEN 308 from S.L.A. Pharma who developed the specific formulation of phenylephrine for the topical use in fecal incontinence and developed the manufacturing method. S.L.A. Pharma’s previous partner, Solvay, conducted important pharmacokinetic studies. We currently do not expect to spend any time or resources developing VEN 308 in the short term. VEN 308 is covered by a patent that will expire in December 2017. If approved by the FDA, VEN 308 will receive seven years of data exclusivity in the U.S. under the Orphan Drug Act.
The FDA has granted VEN 308 orphan status for the treatment of IPAA-related fecal incontinence. In the U.S., orphan drug designation is given to a drug intended to treat a rare disease of condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the U.S. Assuming sufficient resources in the future and positive results from a Phase IIb dose ranging trial in the U.S. in support of the orphan indication of IPAA-related fecal incontinence that we intend to undertake, we would expect to submit an orphan NDA for VEN 308 for this indication. Orphan status provides seven years of data exclusivity in the U.S. from the date of approval for a specific indication.
Our Development Efforts
We own VEN 309 (but prior to November 14, 2011, in-licensed it from Amer) and in-license our two other product candidates from S.L.A Pharma. All clinical trials to date have been conducted either by the licensor, the licensor’s previous partners or by independent investigators, as have the preclinical studies and product formulation activities. Since the time we licensed these products, we have focused our efforts on establishing and clarifying the regulatory pathway for late phase clinical trials and regulatory approval, on establishing the contract manufacturing capacity and methods necessary to allow late phase clinical trials to proceed, and on initiating late phase trials, preclinical toxicology and human pharmacology studies with our products, all of which will be conducted by contracted third parties under our direction. These development efforts have not required many employees and we have historically operated with only a limited number of employees with the expertise necessary to progress our product candidates down the development path outlined above. This helps us contain our operating costs.
Subsequent to the completion of our initial public offering in late December 2010, we began hiring a few employees and contracting with three individuals or entities to complete our staffing needs for our initial Phase III trial of VEN 309. Throughout 2011, we added several other employees. We also have contracted with contract research organizations to assist us in our Phase III trials for VEN 309. However, we remain dependent on the availability and competency of the third parties with whom we have contracted and with whom we plan to contract for the continued development of our product candidates.
Our Strategy
Our objective is to develop and commercialize highly differentiated products to address unmet medical needs of the lower gastrointestinal tract. We are developing our product candidates to treat hemorrhoids, anal fissures and fecal incontinence. Currently, there are no FDA-approved prescription drugs in the U.S. for the treatment of hemorrhoids. One product (Rectiv, a topical nitroglycerin) was approved by the FDA in June 2011 and we expect this product to be launched by Aptalis in 2012. There are no FDA-approved prescription drugs for the treatment of incontinence, but Solesta, a hyaluronic acid dermal filler, was approved as a device by the FDA in 2011 for intra-anal injection for fecal incontinence. We expect Salix Pharmaceuticals to launch this product in 2012.
To achieve this objective, we intend to:
| · | complete one of two planned pivotal Phase III trials in the U.S. of VEN 309 for the treatment of hemorrhoids, that began in August 2011 and for which enrollment is expected to be complete around April 2012 and for which top line results are expected around June 2012; |
| 6 |
| · | assuming positive data from the initial Phase III trial for VEN 309, conduct an additional pivotal Phase III trial as well as a Phase III double blind recurrence trial. Assuming acceptable results from these clinical trials, as well as from clinical pharmacology and other, non-clinical activities, such as carcinogenicity and toxicology studies, prepare and file an NDA for VEN 309 for the treatment of hemorrhoids in 2014; |
| · | assuming VEN 309 is approved by the FDA, and because there are no FDA-approved prescription drug competitors in the U.S., we intend to commercialize the product in the U.S. using either our own sales force or through an agreement with a suitable partner and to license the product for sale outside of the U.S.; |
| · | assuming receipt of positive data from an ongoing European Phase III trial of VEN 307, expected in May 2012, conduct one pivotal trial with the existing three times per day formulation or two parallel pivotal trials with a to-be-identified twice daily formulation as well as short-term dermal toxicology studies for VEN 307, with the goal to prepare and file an NDA for a Phase III trial of VEN 307 for the topical treatment of pain associated with anal fissures in 2013; |
| · | assuming VEN 307 is approved by the FDA, and because topical diltiazem is already used by colorectal surgeons in the U.S., we intend to engage our own gastrointestinal specialty sales force and marketing staff to commercialize this product and/or engage a suitable partner in the U.S. and to license it for sale in Canada; and |
| · | pending the outcome of the ongoing Phase III trials in VEN 309 and VEN 307, and the availability of additional capital, develop a final formulation of VEN 308 and advance that product through Phase IIB studies. |
History of Operations
We hired Dr. Russell Ellison, our Chief Executive Officer and Chief Medical Officer, and David Barrett, our Chief Financial Officer, in December 2010 upon the completion of our initial public offering. From June 2010 until they were hired, Dr. Ellison and Mr. Barrett served as consultants because our only business activities during that time consisted of maintaining our licenses with S.L.A. Pharma and Amer, and activities connected with our initial public offering. From late December 2010 through February 2011, we completed the staffing for our planned development of VEN 309, by adding a clinician, two clinical project managers, a head of manufacturing, and an executive assistant on a contract or permanent employment basis. We have used these consultancy arrangements to conserve our resources.
Although incorporated in 2005, we began active operations in the spring of 2007 upon the licensing of VEN 307 and VEN 308 by Paramount BioSciences from S.L.A. Pharma. Shortly thereafter, we hired Thomas Rowland as our chief executive officer (who was then one of our directors), Dr. Terrance Coyne as our chief medical officer, and Dr. John Dietrich as our vice president of clinical operations, as well as other employees. Due to our lack of capital, Drs. Coyne and Dietrich resigned in February 2009. Mr. Rowland resigned as our chief executive officer in February 2009, but he continued to act as our president from the date of his resignation in February 2009 until May 2010. Simultaneously with the resignation of Dr. Dietrich, we entered into a consulting agreement with him whereby he provided consultation on manufacturing, preclinical and clinical aspects of our drug programs on an as-needed basis. These arrangements with Mr. Rowland and Dr. Dietrich allowed us to continue minimal operations following their resignations until June 2010 when we contracted with Dr. Ellison and Mr. Barrett. In January 2011, we renewed the consulting agreement with Dr. Dietrich. Effective September 1, 2011, we hired Mr. Rowland as our Chief Business Officer.
| 7 |
Our Management
Our management team consists of: Russell H. Ellison, Chief Executive Officer and Chairman of the Board of Directors, who has over 30 years of experience in the pharmaceutical industry, including serving as vice president — medical affairs and Chief Medical Officer of Roche Laboratories, Inc., USA. and of Sanofi-Synthelabo, USA; David J. Barrett, Chief Financial Officer, previously chief financial officer of Neuro-Hitech, Inc., a publicly traded pharmaceutical company with development stage and marketed products; and Thomas Rowland, Chief Business Officer, who was hired effective September 1, 2011, has over 20 years of experience in the pharmaceutical industry, most of which was in the gastrointestinal area, and was our founding chief executive officer. Beginning in January 2011, we have increased the number of our employees to seven and have long-term contracts with seven consultants on manufacturing, preclinical and clinical aspects of our drug programs. We also have contracted with three contract research organizations to assist in our drug development plans. We use these consulting agreements to avoid the costs customarily associated with employees until our financial resources allow us to hire additional employees. We believe that the addition of these employees and consultants to the Ventrus team will help us advance our product candidates to the next stage of development.
Corporate History and Information
We were incorporated in Delaware in October 2005 under the name South Island Biosciences, Inc. and changed our name to Ventrus Biosciences, Inc. in April 2007. We began operations in April 2007 upon the acquisition of the licenses to VEN 307 and VEN 308 and the hiring of a development team. We acquired the license to VEN 309 in March 2008. We acquired the licenses to VEN 307, VEN 308 and VEN 309 from Paramount Bioscience, LLC and also borrowed funds from Paramount and one or more of its affiliates. Our largest stockholder, Dr. Lindsay Rosenwald, is the Chairman, Chief Executive Officer and sole stockholder of Paramount. We conducted operations until March 2009 when we terminated our employees due to a lack of financial resources. We retained the services of our then executive team through consulting agreements, pursuant to which those individuals, from February 2009 to June 2010, conducted minimal activities consisting of maintaining the licenses to our product candidates and business development and financing activities. We completed a series of convertible note financings in February, April and May of 2010 that provided us funds to hire as consultants our current chief executive officer and chief financial officer and undertake our initial public offering. The completion of our initial public offering in December 2010 and the related exercise of the underwriters’ over-allotment option in January 2011 raised approximately $17.5 million in net proceeds. In July 2011, we raised approximately $47.5 million in net proceeds in a registered public offering. We have used a portion of those net proceeds to resume the development of VEN 309 and VEN 307, including hiring employees, contracting with consultants, contracting with contract research organizations to assist us in executing and monitoring our Phase III trials for VEN 309 for the treatment of internal hemorrhoids, and contracting with manufacturers of clinical trial supplies for those studies.
IFERANSERIN OINTMENT (VEN 309)
Background on hemorrhoids
Incidence and prevalence
Hemorrhoids are a common anal disorder, characterized by bleeding, itching, pain, swelling, tenderness and difficulty defecating. Based on information from an article entitled The Prevalence of Hemorrhoids and Chronic Constipation by J. Johanson and A. Sonnenberg published in Gastroenterology (1990; 98: 380 – 386), the point prevalence of symptomatic hemorrhoids in the U.S. population currently is approximately 4.4%, representing approximately 12.5 million cases based on 2009 population data published by the U.S. Census Bureau. The prevalence of hemorrhoids peaks in adults aged 45 to 65 years.
According to IMS Health, Inc. (2009), 4.2 million prescriptions are written per year in the U.S. for hemorrhoid products and 22 million units per year are sold in the U.S. for the OTC hemorrhoid products. If VEN 309 receives FDA approval in the U.S., we expect our competition for patient use and physician prescribing will be these drugs which have not been approved by the FDA and, to our knowledge, lack any clinical trial data supporting their efficacy and safety. In Europe it appears that, from our discussions with experts and staff from other companies, many products exist, differently from country to country, and are mostly herbal extracts and mixtures in topical and systemic forms which are either prescribed or available over-the-counter. We do not have market data concerning these products in Europe, other than product acceptance market research, nor is their precise regulatory status clear to us.
| 8 |
Patho-physiology of hemorrhoids
Hemorrhoids are symptomatic abnormalities of normal vascular structures in the anal canal that are manifested by dilation of the local arteries and veins due to constriction and partial obstruction of the exiting colonic veins. Although the exact mechanism for hemorrhoid formation is not clear, the progressive occlusion of venous exit vessels (e.g., as seen in straining during defecation, heavy lifting and pregnancy) is thought to produce stretching of the vessels in the hemorrhoidal plexus combined with vascular stasis. This stasis could cause exposure of the blood to collagen, which in turn causes platelet clumping with the release of the platelet’s artery and vein constricting contents, including serotonin, which via stimulation of the 5HT 2 receptor causes localized constriction of the exit veins, where most of the vascular smooth muscles are, and, in combination with other factors, causes a cascade effect producing clot formation. These events result in additional stasis of the blood, perpetuating and further worsening the situation. As hemorrhoids worsen, the trapped blood forms piles (protruding skin folds filled with static and thrombosed blood), initially above the pectinate line (internal hemorrhoids) and then below the pectinate line (external hemorrhoids). The classification of internal hemorrhoid grades by Banov is accepted by most specialists. This system consists of four grades and symptoms: first degree (grade I): hemorrhoids bleed but do not protrude; second degree (grade II): hemorrhoids protrude but reduce on their own; third degree (grade III): hemorrhoids protrude and require manual re-insertion; and fourth degree (grade IV): hemorrhoids protrude and cannot be manually re-inserted.
The cardinal symptom and most common manifestation of internal hemorrhoids is bleeding. Bleeding is often the only sign in grade I hemorrhoids, but it can also be accompanied by other symptoms as the hemorrhoids further enlarge, such as discomfort, itching, prolapse, and fecal soilage.
Current treatments
Despite the high prevalence of hemorrhoids, we are not aware of any FDA-approved prescription drugs for the treatment of hemorrhoids in the U.S. While there are commonly used prescription drugs for hemorrhoids in the U.S., such as Anusol, none have been approved by the FDA nor been designated by the FDA as safe and effective. Various combination products (such as the Preparation H line of products) are available in the U.S. under the FDA’s OTC monograph rule. The great majority of these treatments provide only temporary relief from the symptoms of hemorrhoids and do not address the cause of hemorrhoids. The mechanism of action of these treatments is either generally anti-inflammatory, such as steroids, or acting as a protective coating on the hemorrhoid or acting as local anesthetics, in the case of most of the OTC products, or unknown, in the case of herbal remedies, and we are not aware of any clinical trials published in medical journals on the efficacy or safety of any topical or oral drug currently marketed in the U.S. for the treatment of hemorrhoids. Patients with persistent symptoms, especially bleeding, usually require an invasive procedure. The most common is rubber band ligation, which involves banding the internal hemorrhoid for four to seven days. Other procedures are the injection of a sclerosing agent, electrocoagulation, light therapy and hemorrhoidectomy. Most physicians treating hemorrhoids start with conservative therapy consisting of diet modification, fiber, sitz baths and stool softeners. In addition to this conservative therapy, physicians might prescribe topical steroids. The only other alternatives are invasive procedures and/or surgery. Because of the lack of effective prescription products, most hemorrhoid patients will use over-the-counter preparations or the prescription drugs available, which are similar to the over-the-counter treatment, but formulated with a higher dose of topical steroid.
By contrast, our product VEN 309 has highly selective, antagonistic activity against peripheral 5-HT 2A receptors (5HT 2A >5HT 2C >>5HT 2B ) involved in clotting and the contraction of arteries and veins, two events believed to be associated with hemorrhoid formation. By limiting 5-HT 2A receptor activity, VEN 309 improves the flow of blood out of the dilated veins that comprise the hemorrhoid, thereby reducing bleeding, itchiness and pain. We believe that the potential for side effects is likely to be limited because iferanserin is topically applied and iferanserin does not enter the brain to affect 5HT 2 CNS receptors, at the exposures seen with topical application. In multiple clinical trials, iferanserin ointment significantly reduced bleeding, pain and itchiness compared to placebo with minimal adverse effects. As a result, we believe VEN 309 to be more effective and/or less invasive than the currently available conventional hemorrhoid topical therapies and more attractive than surgical procedures, which are the only currently validated treatment options.
| 9 |
IFERANSERIN OINTMENT (VEN 309) DEVELOPMENT
Background on Iferanserin
The early proof of concept for the utilization of a 5-HT 2A antagonist for the treatment of hemorrhoid was developed by Sam Amer PhD, a former director of research and development at Bristol-Myers Squibb Company. Dr. Amer explored the potential application of serotonin drugs, which would not enter the brain at therapeutic concentrations, for use in various venous conditions. After successful preclinical and clinical experiments, Dr. Amer filed a method of use patent covering this molecule in 1992. Dr. Amer subsequently separated the S-isomer from this racemic mixture and filed new composition of matter patents for the S-isomer in 1998. Also in 1998, the early stage product was licensed to Tsumura, a Japanese company. Tsumura conducted over 350 pre-clinical and six clinical studies, but we believe was not able to continue development due to financial difficulty and returned the product to Dr. Amer. Upon the return, Dr. Amer’s company, Sam Amer & Co., Inc., or Amer, conducted a double-blind, placebo controlled, multi-center Phase IIb trial in Europe. After the successful completion of that study in 2003, Novartis Pharmaceuticals licensed iferanserin from Amer to be part of its gastroenterology portfolio strategy. Novartis improved the iferanserin manufacturing processes and completed important toxicology and metabolite studies. In 2005, Novartis’ lead gastroenterology product, Zelnorm TM was experiencing increased FDA scrutiny on the safety of that product, which would ultimately lead to its eventual withdrawal from the market. We believe that with the impending loss of their lead gastroenterology product, Novartis decided to dissolve the gastrointestinal franchise. In 2005, Novartis returned iferanserin to Amer. According to Amer, no safety or clinical issues were ever communicated as reasons for the return.
On February 5, 2008, in conjunction with Amer, we held an end of Phase II meeting with the FDA, to confirm the U.S. regulatory status and pathway to an NDA for iferanserin ointment where it was agreed that the product may enter late-stage Phase III development. In March 2008, we licensed exclusive worldwide rights to develop and market iferanserin ointment for the treatment of anorectal disorders from Amer. On November 14, 2011, we acquired all rights, title and interest in VEN 309 from Amer.
Mechanism of action on iferanserin
Iferanserin has selective antagonistic activity against 5-HT 2 receptors, especially against those involved in contraction of vascular smooth muscle and platelet aggregation (clotting), the 5HT 2A receptors. It is a particularly potent high-affinity antagonist of 5HT 2A , has less affinity for and is a moderate antagonist of 5HT 2C and has considerably less affinity for 5HT 2B receptors. In a specific validated model, iferanserin did not demonstrate any agonism activity at 5HT 2B receptors, but did demonstrate moderate antagonistic activity. Unlike other 5HT 2 receptor antagonists, iferanserin’s 5HT 2 receptor antagonism, clinically, is entirely peripheral, meaning it occurs outside the central nervous system because iferanserin does not cross the bloodbrain barrier except in extremely high exposures far above those seen with topical application. Studies conducted in 1997 and 1998 by Amer in rats addressed the potential effects of iferanserin on impaired rectal mucosal blood flow and increased peripheral vascular resistance after administration of serotonin or thrombin. At doses of 3 mg/kg and above administered intrarectally, iferanserin improved rectal mucosal blood flow and normalized the peripheral vascular resistance. Iferanserin had minimal effects on arterial blood pressure.
Preclinical safety
Iferanserin has been extensively tested in multiple preclinical models. The iferanserin exposure from dosing in humans topically using 0.5% applied twice daily (the dose to be used in our planned studies) ranges from 1/17th to 1/88th of the exposure that produces toxicity and from 1/45th to 1/85th of the exposure that produces cardiovascular effects in animal toxicology studies and 1/60th – 1/100th of the exposure that produces these effects in vitro. In addition, iferanserin exhibits low systemic exposure, with less than 10% bioavailability, based on a pre-clinical rat study.
| 10 |
Clinical trials and patent status
A total of seven clinical trials with iferanserin were completed by Amer (excluding Japan) and Tsumura in Japan between 1993 and 2003. One Phase I trial and one Phase II trial were completed using the racemic mixture of iferanserin. After the successful Phase II proof-of-principle trial, the licensor, Amer, separated the R- and S-isomers (the two active components of most small molecule pharmaceuticals), determined that the primary activity was focused in the S-isomer and filed a patent claiming this isomer. The patent issued in the U.S. and other countries and expires in 2015. In the U.S., the patent was filed with Dr. Amer as the inventor and in all foreign countries with Amer as the assignee. After the development of the S-isomer in the mid 1990s and the patent filing in 1998, the remaining trials — two Phase I trials, two Phase II trials, and one Phase III trial — were all conducted with the S-isomer product. This development progression (racemic to S-isomer) is a common pharmaceutical practice, enabling companies to use the purest form of the molecule in late-stage clinical trials.
On November 14, 2011, we acquired from Amer all rights to all intellectual property related to VEN 309 previously owned by or assigned to Amer as well as to any new improvements owned by or assigned to Amer. Different concentrations of a drug are separately patentable under certain circumstances. Because of unexpected differences between concentrations of the product that were observed in the clinical program (i.e., that 0.5% concentration is superior to a 0.25% and a higher 1.0% concentration in the comprehensive reduction in hemorrhoid symptoms), which data, to our knowledge, have not been previously published, we filed in August 2010 a patent claiming our specific concentration range (among other claims) which, we believe, if issued, could provide patent protection for 20 additional years. Dr. Amer is the inventor in this U.S. application and the assignee in the patent application (we now own all of the rights to VEN 309). However the original S-isomer patent could be challenged by a third party and invalidated, and the concentration patent may never issue and even if issued could be challenged by a third party, in which case we would still have five years of U.S. data exclusivity from the date of approval under the Hatch-Waxman Act.
An investigator IND for iferanserin was filed with the FDA in November 1991 and transferred to Amer as the sponsor in January 1994, which was transferred to us in April 2008, and remains open.
Trial Results
Overall safety
In the seven clinical studies of iferanserin conducted by Amer and Tsumura in 359 individuals, of whom 220 were exposed to iferanserin, the adverse effects, at least possibly related to the iferanserin administration, were mostly gastrointestinal (diarrhea, lower abdominal discomfort, residual stools, and anal irritation). These events were considered mild by the investigators and required no medical treatment. There were no serious adverse events judged by the investigator as related to iferanserin and no mortality in these studies. There was one report of exacerbation of atopic dermatitis requiring observation in hospital with an uncertain relationship to iferanserin.
Clinical Pharmacology in Normal Volunteers (Phase I)
Two clinical pharmacology studies were conducted in Japan by Tsumura in 1998 and 1999 in 18 healthy volunteers exposed to a single dose and in six healthy volunteers exposed to six days of dosing with the 1% preparation. Three mild adverse events where the drug could not be ruled out were observed in three patients in the single dose group and four mild adverse events were observed in three patients in the multi-dose group. There is no accumulation of the drug on twice daily dosing and the half life at one and six days is 1.6 hours. Peak concentrations are similar at one and six days and well below the lowest exposure where toxicity was observed in toxicology experiments in animals.
One patient was identified as having a very compromised activity of an enzyme, CYP2D6, and the maximum concentration of the drug in this patient was three times the maximum observed in the other patients and the total exposure (AUC) was 17 times that observed in the other patients. However, these exposures to the drug were still well below the lowest exposures where toxicity was observed in animal toxicology experiments, and this patient did not experience any adverse events.
| 11 |
As is typical of several modern drugs for depression such as Fluoxetine and older drugs such as tri-cyclic anti-depression agents and other drugs extensively prescribed, iferanserin is an inhibitor of the enzyme CYP2D6 and is at least partially dependant on this enzyme for its metabolism. Therefore, kinetic interactions with other drugs that are potent inhibitors of CYP2D6 and/or are highly dependent on CYP2D6 for their metabolism are possible. There are several of these drugs and most are psychiatric medications, and one is tamoxifen. We will exclude patients from the clinical trials who are taking such drugs, and will be conducting extensive drug-drug interaction studies as part of our clinical pharmacology program to clarify which drugs could be affected by or could affect iferanserin. We initiated these trials at the beginning of 2012 . The research is expected to allow narrowing of the exclusion criteria with respect to drugs acting on CYP2D6 for future studies.
Proof-of-concept trial (U.S.)
A double-blind, placebo-controlled trial of 26 patients conducted by Amer that was completed in August 1992 and published in August 1994 was the first clinical trial to test the activity of the racemic mixture of iferanserin. Topical 1% iferanserin ointment was applied three times daily for five days to calculate the effect on bleeding and other symptoms in patients with grade I to III external hemorrhoids. Treatment produced statistically significant improvements in ease of defecation, throbbing, fullness, bleeding and tenderness. Itchiness and pain were also reduced following treatment. These positive treatment effects started immediately after treatment and were maintained throughout the study.
Early Phase II dose-ranging trial (Japan)
Topical iferanserin ointment, in twice-a-day doses of 0.25%, 0.5%, and 1.0%, was provided to 72 patients for 14 days to treat symptomatic internal and mixed internal/external hemorrhoids. A total of 68 patients were evaluable for analysis: 23 patients in the 0.25% dose group, 24 patients in the 0.5% dose group, and 21 patients in the 1.0% dose group.
There was a significant change in ease of defecation between dose groups by Day 7 but no other differences in improvements of symptoms among the three dose groups. Anal discomfort and pain persistence improved with increasing dose on a visual analog scale, or VAS, of pain. For the symptom of bleeding, a significant difference between dose levels ( P = 0.016) and a paired comparison statistical analysis showed that the 0.5% dose was more effective than either the 0.25% dose or the 1.0% dose. By Day 14, hemorrhoid swelling was reduced in the 0.5% dose group (41%) and the 1.0% dose group (43%). A review of patient diaries revealed that all symptoms started improvement on Day 1, with improvement peaking at Day 7 and being maintained to Day 14. Comparison of all doses showed, unexpectedly, that the 0.5% dose provided the most consistent improvements.
There were 45 adverse events, but only five (11%) were judged as related to iferanserin ointment. These iferanserin-related adverse events were mostly mild diarrhea or lower abdominal discomfort, which required no medical treatment. Laboratory tests were generally normal, with the exception of one case of mild elevation of total bilirubin one month after trial completion, which required no therapy. Further evaluation of metabolites revealed no relationship to adverse events. The unexpected and novel finding that 0.5% concentration is superior to both a lower (0.25%) and higher (1.0%) concentration supports our patent claiming a specific concentration range that we filed in August 2010, which, if issued will expire in 2030.
Late Phase II trial (Japan)
A double-blind, placebo-controlled trial was conducted by Tsumura Company with three different concentrations of iferanserin ointment (0.25%, 0.5% and 1.0%) administered twice daily for four weeks for treatment of 104 patients with grade I to III internal hemorrhoids. The trial was completed in July 2002. Inclusion criteria required a minimal degree of either bleeding or prolapse. The primary endpoint was physician-rated size reduction of the hemorrhoids; secondary endpoints included subjective symptoms as assessed by patient diaries and VAS. By day 28, compared with placebo, the concentrations of 0.5% and 1.0% of iferanserin showed the most consistent improvements across groups for secondary symptoms, such as bleeding, pain severity and duration, and ease of defecation.
| 12 |
Phase IIb trial (E.U.)
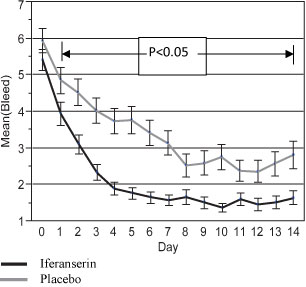
Based on the results of the two Tsumura Phase II trials, a double-blind, randomized, placebo-controlled trial was conducted by Amer at five sites in Germany to evaluate the safety and efficacy of topical iferanserin ointment for the treatment of internal hemorrhoids. Patients with grades I – III internal hemorrhoids with bleeding episodes of at least every other day for two weeks prior to enrollment were eligible for the study. We refer to this trial as the “German Phase IIb trial.” Participants were instructed to self-administer two grams of either placebo ointment or 0.5% iferanserin ointment into the anal canal twice-a-day (12 hours apart) for 14 days. At the end of each treatment day patients were instructed to complete a patient diary and record the following symptoms: bleeding, itching, pain, tenderness, fullness, throbbing, gas and difficulty of a bowel movement, with bleeding being the primary endpoint for the study. All symptoms were recorded on a scale of 1 – 10, with 1 indicating the absence of the symptom and 10 denoting the worst symptom. The patients were contacted by telephone 45 days after completion of treatment to determine their general health and hemorrhoid status. Adverse events were recorded by the patients. Patients who had complete 14 day diaries for efficacy endpoints and identified to a treatment group were included in the statistical analysis. The patient assessment scores for hemorrhoid bleeding at the end of seven and 14 days of treatment were the primary efficacy endpoints for the study. Secondary endpoints included the effects of treatment on itching, pain, tenderness, feeling of fullness, throbbing, gas and difficulty of defecation. Statistical analysis consisted of two-sided two-sample t-tests, with a p value of p 0.05 being considered statistically significant. Secondary analyses included the difference in assessment score per day. One hundred and eleven patients were evaluable for the primary endpoint of bleeding and 60 patients were evaluable for itching and 40 patients were evaluable for pain.
For the primary endpoint of bleeding, the difference in the scores on Day 0 between the two groups was not significant. There was a rapid and substantial decrease in the report of hemorrhoid-associated bleeding in the iferanserin group. The significant difference in bleeding scores between the groups started on Day 1 and remained significant until the end of the treatment period (Day 14) ( Figure 1). The primary endpoint of patient assessment scores for hemorrhoid bleeding at the end of seven and 14 days of treatment were significant with p values of p < 0.0001 and p < 0.0075, respectively.
Figure 1. Mean daily bleeding scores
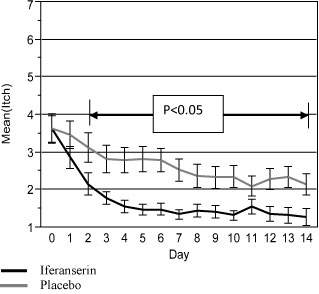
There was no difference in the itching scores on Day 0 between the two groups. As with bleeding, iferanserin produced a rapid, sustained reduction in itching. The significant difference in itching scores between the groups started on Day 2 and remained significant until the end of the treatment period (Day 14 (Figure 2)). The secondary endpoint of patient assessment scores for hemorrhoid itching at the end of seven and 14 days of treatment were significant with p values of p < 0.0008 and p < 0.0207, respectively.
| 13 |
Figure 2: Mean daily itching score
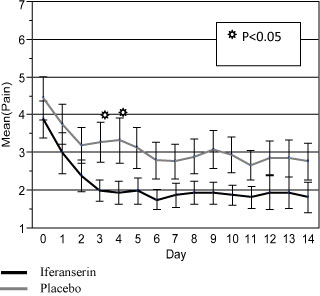
Finally, compared with placebo, iferanserin ointment significantly reduced pain (p < 0.05) by Day 3 (Figure 3). The effect of iferanserin was not significantly different from placebo on either Day 7 or Day 14, possibly due to the low number of patients with pain at baseline. Drug treatment was well tolerated in this trial. The rate of adverse events were similar in both treatment groups, and there were no serious adverse events. The majority of the adverse events were gastrointestinal related.
Figure 3: Mean daily pain scores
| 14 |
In this Phase IIb double-blind, randomized, placebo-controlled trial of 121 patients with grades I to III internal hemorrhoids, iferanserin provided rapid and sustained improvements of the main symptoms of this disorder: bleeding, itching and pain. Maximal improvements of symptoms, compared to baseline, occurred by Days 3 – 7 and were maintained to Day 14 at the end of the trial.
In order to determine the sample size and statistical power for our first pivotal Phase III trial, we have modeled the potential performance of the primary and secondary endpoints which were proposed by the FDA and which we will be using in that trial, using data from the German Phase IIb trial, because the principal elements of the German Phase IIb trial are substantially similar to our first Phase III trial. These endpoints are defined as:
| • | Primary: Proportion of patients with cessation of bleeding by Day 7 that persists for the remainder of the treatment period (through Day 14); and |
| • | Key Secondary: Proportion of patients with cessation of pain and/or itching by Day 7 that persists for the remainder of the treatment period (through Day 14) |
Applying the proposed statistical methodology and primary endpoint for our Phase III trial to the data from the German Phase IIb trial, the difference between the proportion of patients responding to treatment as defined by the new endpoint definition for cessation of bleeding in the VEN 309 arm (57% responders) and the placebo arm (20% responders) was considerable with p < 0.0001 ( Figure 4 ). Similarly, analyses of the key secondary endpoints of pain and/or itching also showed considerable differences between VEN 309 and placebo (itching: 59% response to VEN 309 versus 32% response to placebo, p < 0.034; pain: 50% response to VEN 309 versus 18% to placebo, p < 0.032).
Figure 4. Analysis of the proposed endpoint for the Phase III trial using the data from the German Phase IIb trial.
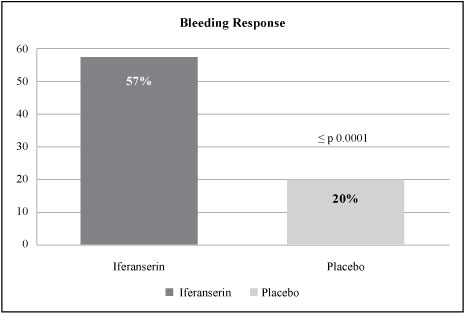
| 15 |
Iferanserin ointment (VEN 309) development plan
Overview
We had an end-of-Phase II meeting in February 2008 with the FDA and had several interactions with the FDA during an SPA process that we were engaged in with the FDA for several iterations. In these interactions, the FDA advised us that they consider VEN 309 to be a chronic repeated use product and as such, based on our preclinical and clinical data to date, the FDA advised us the following elements were required for an NDA submission:
| • | a total safety database of 1,500 patients exposed to iferanserin, a proportion of which need to be followed for repeat use for six months and 12 months (standard International Conference on Harmonization recommendation); |
| • | two pivotal Phase III trials, for the treatment of an episode of symptomatic hemorrhoids, and one double blind Phase III trial to determine the safety and efficacy of the treatment of recurrent episodes (which we might be able to combine with one of the pivotal trials depending on the recurrence rate and/or the ability to pre-identify patients who are likely to have recurrence); |
| • | a clinical pharmacology program consisting of a thorough QT study (standard for most drugs), drug-drug interaction studies, and pharmacology in special populations; and |
| • | as is usual for chronic or repeated use drugs, carcinogenicity studies in two species exposed for 104 weeks, preceded by a dose ranging study, and six months toxicology in rats and nine months in dogs. |
As the carcinogenicity study (including the prior dose ranging study) can take up to 40 months to complete, we intend to conduct the Phase III trials sequentially as this will not delay the program, will conserve funds, allow an assessment of the recurrence rate, and allow adjustments (for example, increased sample size) to the second Phase III study to optimize its potential. We initiated the first patient randomized into the first Phase III trial in August 2011 and expect to complete enrollment around April 2012, and we expect that data to be available around June 2012. We initiated the dose ranging part of the carcinogenicity studies in 2011, and plan to initiate the carcinogenicity studies in 2012.
First Pivotal Phase III trial
We originally filed, in June 2008, an SPA with the FDA to ensure its explicit agreement with our first pivotal Phase III protocol for VEN 309, using the 0.5% dose. Due to lack of funds we could not follow up or complete the process but were able to resume with another filing in March 2010 and made several filings thereafter based on the responses received from the FDA. In February 2011, we had a Type A meeting with the FDA when we accepted their new proposal for the endpoints in the trial and clarified statistical and other protocol elements. We refiled the protocol under the SPA and received the FDA’s response in May 2011 in which they accepted the changes but also proposed the addition of a third arm in the trial to study the safety and efficacy of seven days of treatment, in addition to the 14-day treatment arm and placebo arm that we had proposed in the original protocol. We agreed with the FDA to include the third arm because when we analyzed the Phase IIb German trial that compared iferanserin given twice daily for 14 days with placebo, using the proposed Phase III endpoints, we observed that the majority of iferanserin-treated patients started their response by Day 3. This raises the possibility that iferanserin therapy may require a shorter duration of treatment to show adequate efficacy to stop the bleeding, itching and pain associated with hemorrhoids. We believe that if this regimen proves to be effective, it could be even more acceptable to patients.
| 16 |
In late June 2011, we received a response to our last SPA submission of the revised protocol with the addition of the third arm, which, with the revised statistical plan, appeared to be acceptable to the FDA. However, in its response, the FDA requested that additional information be included in the protocol pertaining to some details of the study, and therefore did not issue an agreement letter for the SPA. The FDA’s recommendations included adding a standardized methodology to the protocol to assess patients’ comprehension of symptoms and symptom terms, such as “anus” or “anal-rectal area”; addressing the possibility that women in menses may not be able to determine whether the source of their bleeding is from hemorrhoids; and adding more clarity to the protocol regarding maintenance of blinding while preserving accurate dosing in the seven-day treatment arm. In addition, the FDA recommended adding a stratification to the efficacy analysis and a rewording for better clarity of the endpoint definition. None of these recommendations affected the previous recommendations from the FDA for the endpoints, overall statistical powering and subject number, and the overall clinical design. Inasmuch as we incorporated these latest changes into the protocol, in order to maintain our timelines for the trial, we filed the protocol to our existing IND with the FDA in July 2011, and decided to not continue to pursue the SPA process.
Following the progressive feedback from the FDA, the double-blinded randomized trial design of the three arms with a double blind portion and an open label portion consists of:
Double blind part
| • | 600 patients (200 patients per arm) recruited at up to 70 sites in the U.S., randomized 1:1:1 to: |
| º | Arm 1: placebo ointment twice daily intra-anally for two weeks; |
| º | Arm 2: iferanserin ointment twice daily for two weeks; and |
| º | Arm 3: iferanserin ointment twice daily for one week followed by placebo ointment twice daily for one week; |
| • | After 14 days treatment, patients will be followed up at Day 28; |
| • | Inclusion criteria to include patients with symptomatic grade I to III internal hemorrhoids, bleeding from hemorrhoids every day for the two days immediately preceding the day that they are randomized and study medication applied, with pain or itching accompanying the bleeding for the two days; and |
| • | Exclusion criteria to exclude patients with grade IV hemorrhoids; thrombosed internal or external hemorrhoids; laxatives, anticoagulants, over-the-counter anti-hemorrhoidal agents, topical steroids, suppositories of any kind, non-steroidal anti-inflammatory drugs (NSAIDs), Cox-2 inhibitors, and other drugs and conditions including potent inhibitors of CYP2D6 such as fluoxitene. |
For the double-blind part of the trial, where patients are treated twice daily for two weeks and then followed up on Day 28, the definitions for the endpoints are:
| • | Primary: Proportion of patients with cessation of bleeding by Day 7 that persists for the remainder of the treatment period (through Day 14); and |
| • | Key Secondary: Proportion of pati ents with cessation of pain and/or itching by Day 7 that persists for the remainder of the treatment period (through Day 14). |
Open Label part
After the 28 day double blind portion of the trial, patients will be followed quarterly for one year and treated with active drug if they have a recurrence at any time during that period. We will assess time to first recurrence, and the overall recurrence rate over one year, and will be able to observe the unblinded response to treatment of recurrence during this part of the trial.
We believe that our modeling of the endpoint definitions as proposed by the FDA using the German Phase IIb trial data, confirm a projected power of > 99% for the primary endpoint and > 95% for the key secondary endpoints for our proposed Phase III trial. All of our clinical study sites will be using central institutional review boards, or IRBs, with rapid review times. We initiated the study in August 2011, and estimate we will complete enrollment approximately in April 2012, and that data from the double blind part of the study will be available around June 2012.
| 17 |
While we believe that we addressed all of the FDA’s comments, we did not pursue the SPA and it was not agreed to by the FDA. The recommendations made by the FDA on the protocol to date and that we implemented are not binding on the FDA.
Subsequent Development
After the results of the Phase III trial are available and assuming the results are positive, and contingent on the availability of additional capital, we intend to:
| • | continue the carcinogenicity study, conduct the six- and nine-month chronic toxicology studies and conduct a substantially similar Phase III trial and a double blind recurrence trial (or combine this trial with the second pivotal Phase III trial, depending on the early recurrence rate observed in the first pivotal trial) which will also provide adequate numbers of patients exposed for the safety database; and |
| • | complete the clinical pharmacology program, which will include extensive drug-drug interaction studies to clarify the CYP2D6 interactions and a “thorough QT study” to test the arrythmogenic potential, which studies are routinely required by the FDA. |
We will also explore the feasibility of lifecycle options for follow-on products such as different formulations, which could be developed for launch after approval of the original VEN 309 product.
We expect that the earliest we will be able to file an NDA with the FDA will be 2014, and the earliest the product could be approved in the U.S. would be in 2015. However, the Phase III trial may not meet the primary endpoint, or unexpected safety problems could arise, or even if the trial is successful we may not be able to obtain more capital for other reasons, in which case we may not be able to complete the development of the product.
Supply of clinical trial product
We have identified qualified sources for the active pharmaceutical ingredient, or API, of VEN 309, a qualified source for drug product, and a qualified source for packaging and labeling as well as a source for the tubes and applicators for our Phase III trials for VEN 309. We have had an agent-in-plant at the manufacturing sites for the API and drug product to monitor quality and performance. Supplies have been produced to 10% of anticipated commercial lots to ensure that bridging studies will not be necessary for commercial supply and that the specifications are the same as used for the Phase II2b trial in Germany. The suppliers of the API and drug product are foreign, and the packaging and labeling source is in the U.S. Deliveries began in August 2011 and allowed us to begin the first Phase III trial in August 2011.
Commercial summary for iferanserin (VEN 309)
Market research regarding hemorrhoids
Market research conducted in 2001 by Amer with both patients and physicians shows a significant dissatisfaction with current treatment options and the need for a product that relieves multiple hemorrhoidal symptoms. In a survey conducted with 57 hemorrhoid patients, average satisfaction with current prescription treatment was rated at 6.0 on a 10-point scale. The most desired treatment effects of a new hemorrhoidal medication that patients described would be “fast onset,” and “bleeding cessation.” The most frequent hemorrhoidal symptoms these patients reported experiencing were itching (79%), bleeding (77%) and pain (68%).
A research study conducted by Amer of 40 physicians (30 primary-care physicians, five proctologists, and five colon and rectal surgeons) evaluated their satisfaction with current treatment for hemorrhoidal treatment on a 10-point scale. The level of satisfaction with current treatment for reducing bleeding was 6.4; for relieving itch, 7.1; and for reducing pain, 6.8. The physicians indicated that the most desirable treatment effects of new hemorrhoidal medication would be “fast onset (2 to 3 days)” and “multi-symptom relief.” Another research study of 98 physicians showed that most physicians would replace their current first line therapy with iferanserin ointment, if it is approved.
| 18 |
In 2011, we commissioned Princeton Brand Econometrics, or PBE, to conduct a landmark omnibus survey of hemorrhoid consumers and patients. From these data, PBE developed a predictive model to forecast physician and patient behavior in response to various product profiles and promotional levels. Results from the market research show that the hemorrhoid market is potentially large, patients are seeking solutions, and respond strongly to the VEN 309 product concept. Of the 10,202 adult consumers surveyed, 1,125 patients reported having hemorrhoids within the last two years, which represents approximately 11% of the U.S. adult population (25.8 million of 234 million people). In addition to the 11% two year prevalence, 9%, 6% and 3% reported having hemorrhoids within the past one year (21.7 million people), one month (14 million people) and on the day of survey (6.7 million people) respectively. Of the entire group of hemorrhoid patients surveyed, 85% reported having had treatment at some point; from the treatment subset, 86% reported using OTC and 14% using prescription products as their last treatment. 10% of all hemorrhoid patients surveyed had had an invasive procedure (banding, injection, surgery) at some time, of which 61% had surgery, and 75% of patients who had had an invasive procedure reported a recurrence. When exposed to the VEN 309 product concept, 88% of those surveyed who had hemorrhoidal symptoms on the day of survey stated they would request a prescription on their next office visit (using factoring by PBE this estimates that 80% probably would request a prescription). Of the entire sample of consumers who had had hemorrhoids at any time in the past two years, 66% would fill a prescription at a thirty-five dollar out of pocket co-pay; of those earning more than $50,000 per year, 78% would fill a prescription at a $35 out of pocket co-pay.
Seven hundred and ninety-five health care providers, or HCPs, were also surveyed. Based on these data and prescriber–level data from Wolters Kluwer, PBE estimates that 170,000 HCPs directly generate 4 mm prescriptions for intra-anal/intra-rectal steroids and 2 mm recommendations for OTC products; approximately 21,000 HCPs account for 50% of this activity. When exposed to the VEN 309 product concept and a range of patient co-pay scenarios, HCPs showed a high willingness to prescribe and a minimal co-pay sensitivity; the probability that they would write a prescription in response to a patient request ranged from .88 to .92 (factored by PBE).
DILTIAZEM CREAM (VEN 307)
Background on anal fissure
Incidence and prevalence
Anal fissure, which is a crack in the skin of the anal canal that results from reduced blood supply to the area and/or from increased sphincter tone, is a common anal disorder characterized by severe anal pain and bleeding with or after bowel movements. Because there have been no approved pharmacological treatments for anal fissure, many cases progress to surgery because of the severe pain. There are no formal epidemiology studies for anal fissure, but its prevalence has been estimated indirectly. When 1,500 unselected neurological inpatients were screened in studies between 1990 and 1998 conducted in the U.S. by Dr. Wolfgang Jost, the prevalence of anal fissure was estimated at 1.6% in males and 2.2% in females. By extrapolation to the 2009 U.S. adult population, we estimate that the general prevalence rate is 1.9%, with approximately 4.3 million current cases. In 2010, it was estimated by SDI Health LLC that there were approximately 1.1 million office visits per year for anal fissures.
Physiology of anal fissure
Although hypertonia, or an increase in tightness of muscle tone, of the internal anal sphincter, or IAS, is associated with anal fissure, its contribution to the cause of anal fissure remains unclear. Hypertonia of the IAS does, however, contribute to chronic anal fissure. Anatomical, angiographic, and blood-flow studies have shown that the vascular supply of the anal epithelium, or tissue lining the anus, is very poor in the posterior midline, the anal area most commonly affected by fissures. Thus, it is possible that decreased anodermal blood supply to this area contributes to the pain and ischemia, or decrease in the blood supply, of traumatized anal epithelium, perpetuating ulceration and preventing healing. Whether the primary event for anal fissure is hypertonia of the IAS or decreased blood supply, hypertonia itself reduces vascular perfusion in the anal area. This reduction of vascular perfusion has been compared with that associated with ischemic pain in the lower limbs.
| 19 |
Current treatments
The clinical goal in treating anal fissures is to reduce the pain associated with the fissure long enough for it to heal naturally and prevent the patient from having to resort to surgery. Currently, most physicians start treatment with diet modification, fiber, sitz baths and stool softeners. If these conservative treatments fail, physicians proceed to pharmacologic therapy, prescribing topical steroids or by directing special pharmacies to create compounded topical formulations by mixing raw diltiazem, and in some cases nifedipine, another calcium-channel blocker, or nitroglycerin, into a cream, ointment or gel for topical use by fissure patients. If these pharmacologic treatments fail to manage the pain, physicians consider, and often perform, surgery. In some instances, physicians initially prescribe pharmacologic therapy in addition to conservative treatments; in other instances because of the severe pain, they initially perform surgery.
The purpose of surgery is to reduce hypertonia of the IAS by either manual dilatation or lateral sphincterotomy. Both procedures are highly successful in relieving the pain and promoting healing of fissures. Although a relatively simple and effective surgical procedure, lateral sphincterotomy is also associated with short-term mild-to-moderate fecal incontinence. This is not an insignificant adverse effect and can become permanent or at least chronic in a fairly high percentage of patients. Studies have shown 6 – 8% of patients had incontinence to flatus or minor fecal soiling at a time greater than five years after surgery. In another study, at a mean follow-up time of 66.6 months (range 30 – 84 months), 10% of patients who had a lateral internal sphincterotomy were incontinent.
Over the last decades, Cellegy Pharmaceuticals, Inc., a drug developer (acquired by ProStrakan Group plc, which is a wholly owned subsidiary of Kyowa Hakko Kirin Co. Ltd.), attempted to gain FDA approval for the topical treatment of anal fissures with nitroglycerin, an agent that reduces IAS and anal fissure pain. Early attempts to develop nitroglycerin utilizing a healing endpoint failed as it was discovered most fissures will heal naturally if the patient can endure the pain for the first several weeks of the disorder. However, it was discovered during development that lowering IAS hypertonia did have a significant benefit in reducing the pain associated with anal fissures. Cellegy’s subsequent multiple pivotal studies with pain as a primary endpoint demonstrated a 33% reduction in pain scores in patients with baseline pain score > 48 (1 – 100 mm on the visual analog scale, or VAS). However because Cellegy did not use minimum pain scores as an inclusion criteria, the overall effect was diluted to 22%. In addition, 63% of subjects reported headaches, which is a known systemic side effect of nitroglycerin. The FDA denied its approval, concluding that the risk benefit ratio for nitroglycerin as topical treatment for anal fissure pain was not favorable due to the modest overall effect and high incidence of systemic side effects. Subsequently Cellegy (now ProStrakan) conducted an additional clinical trial in anal fissures which was filed with the FDA in 2009. ProStrakan received a complete response letter for this new NDA in April 2010, because of issues with statistical significance, according to ProStrakan. However, ProStrakan filed a response to these concerns and, in late June 2011, received approval for the product (Rectiv, a 0.4% concentration of nitroglycerin in ointment) to be applied twice daily for the treatment of pain associated with chronic anal fissures, for up to three weeks duration. This product has been marketed in the U.K. and other European countries and elsewhere since 2007. The professional label in Europe, which is a summary of product characteristics, lists headaches as being very common with a 63% incidence of which 45% were moderate or severe, in three pivotal trials. The U.S. label lists headaches as occurring in 64% of patients, with 938 headaches occurring in 79 patients, in one pivotal trial.
We have planned a clinical program that focuses on pain as the primary endpoint and includes only patients who have adequate pain scores on entry into the studies, which we believe will avoid the modest effects seen in these earlier studies. In addition, based on results of previously published trials (such as Kocher et al. 2002; see Table 1 below), we believe that the side effects of diltiazem cream are likely to be substantially less than those observed with topical nitroglycerin, which primarily were headaches.
| 20 |
DILTIAZEM CREAM (VEN 307) DEVELOPMENT
Background on diltiazem
Diltiazem, a calcium-channel blocker, was first approved in 1982 in oral form for the treatment of angina and high blood pressure. It has been prescribed in the U.S. for millions of patients in oral dosages typically from 240 mg to 360 mg per day. In contrast, daily doses of VEN 307 for treatment of anal fissures will range from 15 mg to 45 mg. Because of the extensive patient exposure to diltiazem as a cardiovascular agent and the wide safety margin as a low dose topical therapy, we intend to develop the topical formulation as a Section 505(b)(2) NDA, as agreed with the FDA at our pre-IND meeting in August 2007. This special NDA procedure, known as a “section 505(b)(2) application” or a “paper NDA,” allows an applicant to seek approval on the basis of a combination of a prior approval of a similar product or published literature, and some new clinical studies conducted or sponsored by the applicant. Section 505(b)(2) applications are often used for changes in a drug that require clinical investigations and thus cannot be handled through the generic drug process, such as a new indication or change in dosage or route of administration.
Compounded diltiazem (prepared by the pharmacist, for each patient, using a general cream base and diltiazem from oral formulations) is currently listed in the U.S. and E.U. anal fissure treatment guidelines as a preferred agent prior to attempting surgery. According to advice we have received from members of our scientific advisory board, who are experts in gastroenterology and gastrointestinal surgery, compounded diltiazem is utilized by many colorectal and gastroenterology specialists each year for the treatment of anal fissures and, according to these experts, has also reduced the number of surgeries required. As a result, awareness and utilization of diltiazem as an effective treatment for anal fissures is high among physicians that treat this disorder. However, compounded diltiazem for anal fissure is not an FDA-approved use nor is it an FDA-approved product, and as such, the cost is not typically reimbursed by Medicare or health insurance plans. Data on unit and dollar volumes of compounded preparations are not routinely collected and not available to us.
The use of diltiazem for the treatment of anal fissures was first discovered at St. Mark gastroenterology teaching hospital in London. Professors Kamm and Phillips filed the original method of use patent applications in 1997 in the Great Britain Patent Office. In 1998, a PCT International Application was filed designating the U.S. as National Phase country and which is the current patent application in the U.S. In 2001, North American rights were licensed to Solvay Pharmaceuticals, SA. During the time that Solvay held the rights, it improved the manufacturing processes and formulation and conducted important pharmacokinetic studies. In 2004, the new CEO of Solvay Pharmaceuticals refocused the R&D strategy on CNS and cardio-metabolic programs, discontinuing gastroenterology and women’s health projects. Consequently, in 2005, the license rights to diltiazem cream were returned to S.L.A. Pharma. From 2005 to the March 2007 licensing by Paramount BioSciences, S.L.A. Pharma focused on regulatory and manufacturing priorities, preparing diltiazem for further development.
We have the potential to capture immediate market share if VEN 307 is approved due to its known efficacy and the current use of the compounded version. We expect that VEN 307 will be highly competitive with the compounded version because of the ease of prescription (already formulated, and approved by the FDA), with no need for compounding at the pharmacy, and because VEN 307 should be eligible for reimbursement under Medicare and other health plans, which the compounded version is not. For these reasons, we believe that the use of the compounded form of diltiazem will greatly decrease if VEN 307 is approved.
In August 2007, we acquired North American rights to diltiazem from Paramount BioSciences, which previously acquired rights from S.L.A. Pharma in the United Kingdom for developing and marketing a proprietary diltiazem cream for relief of pain associated with anal fissures. We incurred a liability to Paramount BioSciences in the amount of $1,087,876, which represented the fees Paramount BioSciences had paid through August 2007 for both VEN 307 and VEN 308. Paramount BioSciences had acquired the S.L.A. rights in March 2007 and began working with us immediately to advance the development of these assets while an asset transfer agreement was finalized. S.L.A. Pharma is developing diltiazem cream for the European market and S.L.A. Pharma began a Phase III clinical trial in the E.U. in November 2010. We are financially supporting the E.U. trial and are obligated to make the following payments to S.L.A. Pharma for VEN 307 development milestones.
| 21 |
| Amount Due | Date Due | Fee Description | ||
| $41,500/monthly | Monthly beginning October 1, 2010, and continuing until S.L.A. Pharma is no longer managing the development program for VEN 307. | Project management fees for VEN 307. | ||
| $400,000 | Upon receipt of a quality controlled final study report of the Phase III trial for VEN 307 in Europe. | Development expense for VEN 307. |
On November 1, 2011, the U.S. Patent and Trademark Office, or PTO, issued U.S. Patent No. 8,048,875 with claims directed to the use of VEN 307 as a topical treatment for the relief of pain associated with anal fissures. The U.S. patent expires in February 2018. A continuation application was filed on July 8, 2011 claiming priority to U.S. Patent No. 8,048,875 with claims directed to additional uses of VEN 307 for related indications. If the continuation application is issued as a patent, it will also expire in February 2018. If approved by the FDA, VEN 307 will receive three years of data exclusivity in the U.S. under the Hatch-Waxman Act.
In August 2007, we concluded a pre-IND meeting with the FDA in anticipation of our IND submission for permission to initiate Phase III trials in the U.S. This meeting also afforded us an opportunity to gain agreement on the key design issues of the studies (including the one which S.L.A. Pharma is implementing) and additional information required for an approval of an NDA. We anticipate the availability of data from the S.L.A Phase III trial in the second quarter of 2012 and, if the E.U. trial is successful, we plan to initiate the U.S. pivotal program by the second half of 2012, contingent on the availability of additional capital. We expect to collaborate closely with S.L.A. Pharma in order to leverage clinical data for different regulatory agencies and to rationalize manufacturing capacity.
Mechanism of action
The mechanism of action for topical diltiazem cream was demonstrated in human pharmacodynamic studies that showed an anal maximal resting pressure, or MRP, reduction of 28% that was sustained for 3 – 5 hours. This MRP reduction is believed to decrease the pain associated with anal fissures by normalizing internal anal sphincter pressure, which improves vascular blood supply and reduces ischemic pain.
Preclinical safety
Studies have been conducted in rabbits and guinea pigs to assess the topical safety of diltiazem cream. Clinicians treated rabbits in and around the anus with 2% diltiazem or placebo cream twice daily for 90 days to evaluate the chronic safety of the product. Although exterior anal tissue showed an increase in erythema, or redness of the skin, and edema, or accumulation of fluid beneath the skin, the clinicians concluded that these effects were due to the application procedure, to a possible reaction to latex gloves or to both. There were no histological findings. In this study, topical 2% diltiazem cream had no other adverse effects. Clinicians used guinea pigs to assess the potential for 2% diltiazem cream to elicit contact sensitization, or skin reaction to the application. This study did not demonstrate any sensitization potential of the diltiazem cream in guinea pigs.
Investigator-initiated clinical studies (studies sponsored by individual clinicians)
The investigator studies conducted with diltiazem cream applied topically in the perianal area in normal subjects and in patients with anal fissures are summarized in Table 1. These studies were conducted by independent investigators and not by us or any partner of ours. The year the study was published is given in the column headed “Study.”
| 22 |
Table 1. Summary of Investigator-initiated clinical studies.
| Study | Condition, treatment, dosage |
Study design, endpoints |
Efficacy | Adverse events | ||||
| Carapeti, E.A., et al, Gut, 45:719 – 722, 1999 |
10 normal subjects; placebo (PBO) or diltiazem (DTZ) gel (0.1%, 0.5%, 1%, 2%, 5%, and 10%) | DTZ or PBO gel applied once to anal margin; maximum resting anal pressure (MRP) and anodermal blood flow measured starting 1 hour after treatment | DTZ decreased MRP at concentrations of 1% and higher, maximum decrease of 28% at 2% gel, no further effect of 5% or 10%; effect at 2% lasted 3 – 5 hours; no change in blood flow | No local or systemic adverse events (AEs) reported | ||||
| Carapeti, E.A., et al, Dis Colon rectum, 43:1359 – 1362, 2000 |
15 patients with chronic anal fissures (CAF); 2% DTZ gel, three times-per-day (TID) for 8 weeks | DTZ gel applied to anal margin; MRP, anodermal blood flow and healing rate monitored every 2 weeks, daily diary cards for worst pain (scale of 0 – 10) of the day | Fissures healed in 67% of subjects; significant decrease in MRP and pain (decreased from 5.5 pretreatment to 1 post-treatment); no effect on blood flow | No AEs | ||||
| Bhardwaj, R., et al, Annual Meeting of British Association of Colon proctologists, Brighton, United Kingdom, 2000 |
44 patients with CAF, 2% DTZ gel, TID for 8 weeks | 27 patients assessed at 2 months, 15 patients evaluated at 4 months (included 9 who had healed at 2 months and remained healed); assessed for healing, pain, rectal bleeding, MRP | Fissures healed in 56% of subjects at 2 months, 73% at 4 months; pain abolished in 88%, bleeding in 92%; MRP decreased by 24% at 2 months | 1 patient had minor incontinence to flatus |
| 23 |
| Study | Condition, treatment, dosage |
Study design, endpoints |
Efficacy | Adverse events | ||||
| Jonas, M., et al, Dis Colon rectum, 44:1074 – 1078, 2001 |
50 patients with CAF, 24 treated with oral DTZ (60 mg), 26 with topical DTZ (2% gel), twice per day (BID) for 8 weeks | DTZ gel applied 1cm inside anus and to anal margin; pain, bleeding, perianal irritation (all 3 measured on a scale of 1 – 100 mm), MRP, healing monitored every 2 weeks | Fissures healed in 38% of subjects (oral) vs. 65% (topical) (9 in each group had previously failed on glyceryl trinitrate (GTN); 7 of these healed on topical vs. 1 on oral DTZ); both oral and topical DTZ decreased MRP; pain, bleeding and irritation reduced by both formulations (pain went from 70 to 7 after 8 weeks on oral, from 68 to 3 on topical) | No AEs in topical group; AEs reported in 8 patients on oral DTZ (headaches, nausea and/or vomiting, rash, decreased sense of taste and smell) | ||||
| Knight, J,S., et al, Br J Surg, 88:553 – 556, 2001 |
71 patients with CAF, 2% DTZ gel, BID, additional 8 – 12 weeks for subjects who did not heal on original regimen | DTZ applied perianally; healing monitored; | 75% healed after 2 – 3 months, a total of 89% healed after a median duration of 9 weeks (range of 2 – 16 weeks); after a median of 32 weeks follow-up (range 14 – 67 weeks) 66% symptom-free, 17% had mild symptoms, and 7% had reoccurrence | 4 patients reported perianal dermatitis, 1 reported headache | ||||
| Griffin, N., et al, Colorectal Dir, 4:430 – 435, 2002 |
47 patients with CAF who failed topical GTN, 2% DTZ cream, BID for 8 weeks | Treatment administered in anal verge; daily diary for pain, bleeding and itching (scale of 0 – 100); healing monitored | Fissures healed in 48% of subjects; pain and bleeding decreased after 8 weeks, no effect on itching; 2 patients relapsed after median duration of follow-up 45 weeks (range 23 – 54) | 1 patient developed a local perianal rash; up to 25% reported increased perianal itch | ||||
| DasGupta, R., et al, Colorectal Dir, 4:20 – 22, 2002 |
23 patients with CAF, 2% DTZ gel, TID for up to 12 weeks | DTZ applied to lower half of anal canal, healing monitored | Fissures healed in 48% of subjects, in a median of 8 weeks (range 1 – 12 weeks); of 8 who had previously failed GTN, 6 (75%) healed; no recurrences at 3 months | No AEs |
| 24 |
| Study | Condition, treatment, dosage |
Study design, endpoints |
Efficacy | Adverse events | ||||
| Kocher, H.M., et al, Br J Surg, 89:413 – 417, 2002 |
60 patients with CAF, 0.2% GTN ointment (29 patients) or 2% DTZ cream (31 patients), BID for 6 – 8 weeks | DTZ or GTN applied to anal verge, monitored every 3 weeks for healing; pain recorded on VAS (0 – 100) scale | At 8 weeks fissures healed or improved in 12 and 13 patients, respectively, after GTN (86%) vs. 8 (healed) and 16 (improved) after DTZ (77%); both decreased pain to approximately same extent; at 12 weeks 2 GTN patients had recurred vs. none in the DTZ group | 21/29 GTN subjects (72%) reported AEs vs. 13/31 (42%) in DTZ group; 17/29 in GTN group had headaches, vs. 8/31 of DTZ patients | ||||
| Bielecki, K., et al, Colorectal Dir, 5:256 – 257, 2003 |
43 patients with CAF, 0.5% GTN ointment (21 patients) or 2% DTZ ointment (22 patients), BID for 8 weeks | Patients monitored 3 times during treatment | Fissures healed in 86% of GTN, 86% of subjects with DTZ, 3 failures in each group | Mainly headache in 7 GTN patients (33%), no AEs reported in DTZ patients | ||||
| Shrivastava, U.K., et al, Surg Today, 37:482 – 485, 2007 |
90 patients with CAF; 2% DTZ ointment (30 patients), 0.2% GTN ointment (30 patients), BID; no treatment (30 patients) | Treatments applied BID to anus, patients monitored for healing and pain (VAS) twice 2 per week then every 2 weeks | Fissures healed in 80%, 73% and 33% for DTZ, GTN and control subjects, respectively; mean time for healing 6.6 weeks, 7.0 weeks and 7.6 weeks for DTZ, GTN and controls, respectively; pain decreased by 75% for DTZ, 59% for GTN and 29% for controls at 6 weeks; recurrence rate 12.5%, 32% and 50% for DTZ, GTN and controls, respectively | No AEs in DTZ patients, 67% of GTN patients had headaches |
DTZ = diltiazem; GTN = glyceryltrinitrate (nitroglycerin)
Clinical trials of diltiazem cream sponsored by S.L.A. Pharma
In 2004 and 2005, S.L.A. Pharma assessed the pharmacokinetic profile of topical diltiazem cream over a four-day period in subjects with anal fissure. Clinical dosing was completed in November 2005 and published in January 2007. Clinicians treated patients with eight doses of either 2%, 4%, or 8% diltiazem cream. Clinicians administered a single dose perianally on Day 1, followed by doses three times a day on Days 2 and 3, followed by another single dose on Day 4. The clinicians collected blood over 24 hours on days 1 and 4. Maximum blood levels and area under the curve increased with the dose, and there appeared to be accumulation of diltiazem in blood on Day 4 after multiple dosing. The time to maximum blood levels was five to seven hours, and the plasma half-life was less than 12 hours. However, the maximum amount of diltiazem that was absorbed was much less (at least five-fold less) than observed after oral dosing. Side effects, such as anal irritation, headache, and nausea, were mild.
| 25 |
Blood pressure was measured at the following times after the single dose on Days 1 and 4: predose, 15, 30 and 45 minutes and one, one and a half, two, four and eight hours after dosing. The relatively small maximum mean decreases (mmHg) in blood pressure in patients receiving 2%, 4% and 8% cream (3 – 4 patients per group) by Day 4 ranged from 4 to 8mmHg systolic blood pressure, or SBP, and 4 to 6 mmHg diastolic blood pressure, or DBP. The changes were, in general, transient and asymptomatic and blood pressure had returned to at or near baseline by the next reading. There was no clear dose-related effect among the 2%, 4% and 8% creams with respect to decreases in blood pressure. In clinical trials with oral diltiazem for hypertension, the patients receiving placebo had mean decreases of blood pressure from 2 to 4 mmHg.
S.L.A. Pharma compared the effect of 2% diltiazem cream with 0.2% glyceryltrinitrate cream in subjects with chronic anal fissure. This study was completed in January 2001 and published in October 2001. Clinicians applied the preparations in and around the anus twice daily for six weeks. Nine of the 31 patients treated with diltiazem and three of the 29 patients treated with glyceryltrinitrate withdrew from the study by eight weeks. In the diltiazem group, 26% of the patients experienced healed fissures and 52% of patients experienced improved fissures. In the glyceryltrinitrate group, 41% of patients experienced healed fissures and 45% of patients experienced improved fissures. There was no significant difference in the healing rates between the groups. Both treatments resulted in a significant decrease in pain. Four weeks after the end of treatment, no fissures recurred in patients treated with diltiazem, but fissures recurred in two patients treated with glyceryltrinitrate. Compared with 18 treatment-emergent adverse events reported by 13 patients (42%) receiving diltiazem, there were 33 adverse events reported by 21 patients (72%) receiving glyceryltrinitrate. Eight patients receiving diltiazem complained of nine headaches, 17 patients receiving glyceryltrinitrate complained of 20 headaches.
Similar to the early glyceryltrinitrate, or GTN, development program that found healing to be a difficult and inappropriate endpoint for registration trials, S.L.A. Pharma also pursued a healing endpoint strategy in early development. In an exploratory trial sponsored by S.L.A. Pharma that was completed in February 2002 and published in February 2003, the effects of 2% diltiazem cream on healing rates were compared with placebo cream in patients with severe chronic anal fissure. Thirty-one patients were randomized to each treatment group. Creams were applied twice daily for eight weeks. At the end of eight weeks, there was no difference in the healing rates between patients receiving diltiazem (10%) and patients receiving placebo (19%). No difference was observed in the secondary endpoints, including pain, which is likely due to the assessment being made only at the end of the study, not daily as in the other trials, which showed a positive outcome in these endpoints. Fifteen patients receiving diltiazem reported 28 adverse events and 12 patients receiving placebo received 18 adverse events. Seven patients receiving diltiazem and three patients receiving placebo reported a rash or pruritus, or itchiness. Headaches were reported in the same number of patients in both treatment groups.
Summary of studies to date
The topical application of diltiazem cream provides pain relief associated with anal fissure and has also been found to be associated with healing. The effects of diltiazem cream are comparable to those observed for treatment of anal fissure with topical application of GTN, but diltiazem cream is much better tolerated. Based on currently available data and discussion with the FDA, we think it is clear that relief of pain associated with anal fissures is the preferred clinical endpoint. Our belief is supported by the study by U.K. Shrivastava, et al., published in Surgery Today, 37:482 – 485, 2007 (see Table 1 above), which compared GTN and diltiazem perianally compared with standard care alone. In this trial, pain decreased by 75% for diltiazem compared with 29% for controls at six weeks. In almost all studies with either GTN or diltiazem where pain was measured, results are consistent whereas with healing as an endpoint results are variable.
Our belief that relief of pain associated with anal fissures is the preferred clinical endpoint is further supported by market research that identified clinicians’ primary treatment goal as pain relief. Importantly, the diltiazem mechanism of action for pain relief is to reduce IAS pressure which addresses the underlying cause of anal fissure pain.
| 26 |
Diltiazem cream (VEN 307) development plan
In August 2007, we had a pre-IND meeting with the FDA concerning VEN 307 for the treatment of pain from anal fissures where it was established that next clinical studies needed for approval were two pivotal Phase III trials, preceded (if conducted in the U.S.) by three short-term dermal toxicology studies. Prior to conducting clinical Phase III trials in the U.S., we must complete three short-term dermal toxicology studies and file an IND for FDA approval. We plan to employ a two-pronged development strategy for VEN 307. While S.L.A. Pharma is conducting the first Phase III clinical trial in the E.U. which is anticipated to be complete in May 2012, we have initiated development of a superior formulation with new intellectual property in the form of an extended release formulation. There are several proven methodologies for extended release topical formulations, and we believe that diltiazem is readily drugable in this regard. We intend to assess three to four alternatives preclinically with multiple contractors, and then assess absorption and effect on IAS pressure with the most promising, while we will file PCP applications for the specific technology combined with diltiazem for all formulations that are technically feasible.
S.L.A. Pharma has completed enrollment of 465 patients at 34 sites in Europe in the Phase III study that it began in November 2010. Patients will be treated for two months in a randomized 1:1:1 double blind study that compares treatments of fiber plus 2% VEN 307 and fiber plus 4% VEN 307 to fiber plus placebo. The primary endpoint is reduction of pain upon defecation during the fourth week of treatment, using the validated numerical rating scale for pain. Patients will use daily diaries and will be observed for one week prior to randomization to ensure sufficient pain prior to randomization.
If there is successful completion of the E.U. trial, we will make the final decision on which formulation to pursue depending on several factors, including whether the new formulation is clinically superior, our access to capital, clinical and regulatory considerations, and our assessment of the then-current state of our intellectual property estate. If the new U.S. developed formulation is superior and the other factors are met, we plan to initiate two pivotal trials in parallel in order to complete the NDA for an estimated FDA submission in 2014. If the new formulation is not superior, and/or we judge the existing formulation to be patentable, we plan to finish clinical development utilizing the current formulation which would require one additional pivotal Phase III trial in the U.S., and expect to continue to pursue other lifecycle options such as combination with other drugs. This development pathway could result in an NDA submission in 2013. In addition, we intend to compare VEN 307 with Rectiv either in our next Phase III trial(s) as an additional arm or in a separate Phase IV trial to enable a direct comparison of side effects, particularly headache, by physicians.
Supply of clinical trial product
S.L.A. Pharma currently is conducting a Phase III trial in Europe for VEN 307 for the treatment of anal fissures. S.L.A. Pharma has established clinical supply sources for its trials. We would expect to contract with those sources for our planned clinical trials and also plan to identify sources for clinical supplies located in the U.S. prior to conducting clinical trials in the U.S.
Commercial summary for diltiazem cream (VEN 307)
Eidetics, a Boston-based research company, conducted quantitative market research in 2003 and reported that on average primary-care physicians see 23 anal fissure patients per month, gastroenterologists see 17 anal fissure patients per month, and colon and rectal surgeons see 31 anal fissure patients per month. Physicians in all three medical specialties indicated that there is a significant unmet need for therapeutic choices for anal fissure. Only 8% of primary-care physicians, 5% of gastroenterologists, and 27% of colon and rectal surgeons reported being “very satisfied” with current treatment options. All three medical specialties reported failure rates exceeding 50% for current first-line therapy in patients with anal fissure. Given this unmet medical need and the absence of other approved drugs in the U.S., we believe that up to two million patients per year could benefit from treatment with VEN 307.
| 27 |
PHENYLEPHRINE GEL (VEN 308)
Background on fecal incontinence
Incidence and prevalence
According to a U.S. community based epidemiology study (Nelson et al., JAMA, 1995), 2.2% of U.S. adults suffer from fecal incontinence, which we estimate to be approximately seven million people, based on 2009 Census Bureau population estimates.
The IPAA orphan population
Patients with an ileal pouch anal anastomosis, or IPAA, secondary to a total colectomy, tend to have a high incidence of fecal incontinence, up to 30%, according to a 1987 study conducted by Dr. John Pemberton and others at the Mayo Medical School. The surgery associated with IPAA can weaken sphincters and muscles necessary for continence and therefore can result in incontinence. About 30% of patients with ulcerative colitis, a form of inflammatory bowel disease which has a prevalence of 700,000 patients in the U.S. (according to Datamonitor 2008) will have had a colectomy, almost always an IPAA procedure (according to McGlauchlin and Clark, Practical Gastroenterology, 8/2008). IPAA-related fecal incontinence is considered an orphan indication by the FDA and the European Medicines Agency, or EMEA. Patients who undergo ileal pouch anal anastomosis are prone to fecal incontinence. In 2006, the total population of patients with IPAA-related fecal incontinence in the U.S. was estimated to be 50,000 to 100,000, according to IMS Health, Inc.
The FDA has granted VEN 308 orphan status for the treatment of IPAA-related fecal incontinence. In the U.S., orphan drug designation is given to a drug intended to treat a rare disease of condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the U.S.
Physiology of fecal incontinence
Continence is a complex physiological action that requires the presence of a series of anatomical barriers preventing the movement of feces through the anus. The puborectalis muscle works with the internal and external anal sphincters to control continence. If any of these three barriers are dysfunctional, incontinence can occur in a wide range of severity. Specifically, anal sphincter weakness has long been associated with fecal incontinence. Abnormal fibrosis, reduced elasticity, insensitivity to norepinephrine and spontaneous relaxation are associated with anal sphincter weakness.
Current treatments
To our knowledge, there are no FDA-approved drugs for the treatment of fecal incontinence. Most physicians start with conservative therapy, which consists of diet modification, sitz baths and over-the-counter antidiarrheal medication. In addition to conservative therapy, physicians might prescribe antidiarrheal medication or recommend surgery.
The most common surgical procedure is sphincteroplasty for patients with physical injury to the anal sphincter. Success rates for this type of surgery are low and most of the benefit decreases with time. Solesta is an injectable inert bulking agent product approved as a device by the FDA in May 2011 for the treatment of fecal incontinence in adult patients who have failed conservative therapy. Solesta is injected submucosally around the anal sphincter and consequently has to be administered in an outpatient setting by qualified physicians. In addition, Norgine plans to conduct a European Phase I trial with NRL001, a suppository formulation of an alpha adrenergic stimulating agent for the treatment of fecal incontinence, which is anticipated to start in Europe in early 2011.
| 28 |
Background on phenylephrine
Phenylephrine has been available since the early 1940s in oral and nasal form for the treatment of nasal congestion. It has also been used as a topical ophthalmic agent since 1936. Phenylephrine is prescribed more than 17 million times per year in the U.S., with 99% of the prescriptions being for cough/cold oral preparations. The typical oral dosing is 40 mg to 60 mg per day. Because of the extensive patient exposure to phenylephrine, we intend to develop the topical formulation as a Section 505(b)(2) NDA. The use of phenylephrine for the treatment of fecal incontinence was first discovered at St. Mark gastroenterology teaching hospital in London. Professors Kamm and Phillips filed the original method of use patents in 1996. In 1997, phenylephrine patent application and rights were assigned to S.L.A. Pharma. In 2001, S.L.A. Pharma licensed North American rights to Solvay Pharmaceuticals, SA. During the time that Solvay held the rights, it improved the manufacturing processes and formulation and conducted important pharmacokinetic studies. In 2004, the new CEO of Solvay Pharmaceuticals refocused its R&D strategy on CNS and cardio-metabolic programs, discontinuing gastroenterology and women’s health projects. Consequently, in 2005, the licensed rights to phenylephrine gel were returned to S.L.A. Pharma. From 2005 to the March 2007 licensing by Paramount BioSciences, S.L.A. Pharma focused on regulatory and manufacturing priorities, preparing the asset for further development.
In August 2007, we acquired North American rights to phenylephrine gel from Paramount BioSciences, which previously acquired rights from S.L.A. Pharma in the United Kingdom in March 2001 for developing and marketing a proprietary phenylephrine gel for the treatment of fecal incontinence. We incurred a liability to Paramount BioSciences of $1,087,876, which represented the fees Paramount BioSciences had paid through August 2007 for both VEN 307 and VEN 308. Paramount BioSciences had acquired the S.L.A. rights in March 2007 and began working with Ventrus immediately to advance the development of these assets while an asset transfer agreement was finalized.
We expect to collaborate closely with S.L.A. Pharma to leverage clinical data for different regulatory agencies and to rationalize manufacturing capacity.
Our total payment obligation for VEN 308 will not exceed $1,200,000. S.L.A. Pharma has billed us for, and we have paid, $973,500 of services through December 31, 2011. This leaves $226,500 in possible additional payments. However, we currently have no further payment obligations for VEN 308 unless we agree with S.L.A. Pharma to additional services outside the scope of the agreement.
Mechanism of action (MOA)
The MOA for topical phenylephrine gel is to increase resting anal sphincter pressure, thus increasing patient bowel control. Phenylephrine gel’s MOA makes it an attractive candidate for any patient population that suffers from incontinence characterized as leaking/seeping fecal incontinence.
Preclinical safety
A mouse lymph node assay conducted by S.L.A. Pharma did not show phenylephrine hydrochloride to be a sensitizer (meaning a chemical that induces an allergic reaction after repeated exposure) because the drug was not associated with any type of delayed hypersensitization. In another S.L.A. Pharma study, contact sensitization potential, as measured in guinea pigs, under the conditions of the study, a 20% gel was considered to be a strong sensitizer to guinea pig skin. A 28-day study by S.L.A. Pharma in rabbits, in which 10% and 20% phenylephrine gel (900 mg) was applied three times each day to the dorsum, demonstrated mild inflammation which may have been exacerbated by animals biting the site of application. These studies were primarily conducted at St. Mark’s Hospital in the U.K. in the 1990s.
| 29 |
Investigator-initiated clinical studies
A number of investigator studies have been conducted with phenylephrine applied topically for the treatment of fecal incontinence and are summarized in Table 2. These studies were conducted by independent investigators and not by us or any partner of ours. The year the study was published is given under the column headed “Study.” One of these studies was conducted in patients with IPAA-related fecal incontinence. In one specific randomized controlled trial, phenylephrine significantly reduced the incontinence score ( P = 0.015) and improved subjective measures ( P = 0.04) compared with placebo. For some patients in this study, phenylephrine totally eliminated nocturnal episodes of fecal incontinence. No patient discontinued treatment during the study due to side effects. Studies in patients whose incontinence was more related to factors other than anal sphincter tone (many patients in the passive fecal incontinence studies) showed less response. As a result, our development plan will initially focus on the orphan IPAA indication.
Table 2. Investigator-initiated studies of topical phenylephrine gel for treatment of fecal incontinence.
| Study | Condition, treatment, dosage | Summary of results | ||
| Carapeti, E.A., et al, Br J Surg. 86:267 – 270, 1999 |
Normal subjects, phenylephrine gel (5%, 10%, 20%, 30%) applied once to anal verge |
Resting anal pressure increased by 8% to 33%, effect lasted for median of 7 hours, no change in pulse | ||
| Carapeti, E.A., et al, Dis Colon rectum, 43:1059 – 1063, 2000 |
IPAA-related FI, 10% phenylephrine or placebo gel, 2 times/day for 4 weeks | 50% (6/12) of phenylephrine subjects improved vs 8% (1/12) placebo, 33% had cessation of FI on phenylephrine, 0% on placebo, phenylephrine increased anal pressure. No reported side effects. | ||
| Carapeti, E.A., et al, Br J Surg, 87:35 – 42, 2000 |
Passive FI, 10% phenylephrine vs placebo cream, 2 times/day for 4 weeks | No effect of phenylephrine or placebo on incontinence or anal pressure, 17% of phenylephrine and 6% of placebo patients had > 75% improvement | ||
| Cheetham, M.J., et al, 2000 | Passive FI, 20% phenylephrine or placebo gel, 2 times/day for 4 weeks | No effect of phenylephrine or placebo on incontinence, anal pressure, blood pressure, or pulse rate | ||
| Sasse, K.L., et al, Dis Colon rectum, 43:A2, 2000 |
FI, 10% phenylephrine cream, 24 weeks | Increased anal pressure, improved incontinence | ||
| Cheetham, M.J., et al, Gut, 43:356 – 359, 2001 |
Passive FI, placebo or phenylephrine gel (10%, 20%, 30%, or 40%) as single application | Anal pressure increased in dose-related manner after phenylephrine, no effect on pulse, transient perianal burning | ||
| Mutch, M.G., et al, 2002 | Passive FI, 10% phenylephrine cream, 3 times/day for 30 days | Phenylephrine improved incontinence score, anal pressure, and anal sphincter length |
FI = fecal incontinence; IPAA = ileal pouch anal anastomosis
Clinical trials
Solvay Pharmaceuticals assessed the safety and pharmacokinetic profile of intra-anal and perianal application of phenylephrine gel in healthy volunteers in 2004 in a study completed in March 2004 and published in May 2004. The phenylephrine gel was applied as a single dose either intra-anally at doses of 5, 10, 25, 50, or 100 mg, or perianally at doses of 100, 200, or 400 mg. Blood samples were collected out to 24 hours after dosing.
| 30 |
Perianal application of phenylephrine gel resulted in much less absorption than intra-anal application: at a perianal dose of 400 mg, blood levels were comparable to what was seen after intra-anal treatment with 10 mg to 25 mg.
Intra-anal application of phenylephrine was associated with increased blood pressure that lasted for approximately three hours, whereas these effects were not seen with perianal treatment. The most frequent side effects were headache and goosebumps after intra-anal application of phenylephrine gel which were not seen with perianal application, and anal/rectal pain after perianal application of phenylephrine gel.
Summary of studies to date
Topical phenylephrine gel has demonstrated efficacy for the treatment of fecal incontinence associated with IPAA. Pharmacokinetic studies have shown a superiority of perianal dosing which yielded low systemic absorption while still providing the desired local therapeutic effect. No hemodynamic effects where observed when phenylephrine gel was administered perianally at up to eight times the therapeutic dose. Therefore, further development of the drug will focus solely on perianal application.
Phenylephrine gel (VEN 308) development plan
Based on pre-IND meetings with the FDA in 2007, we are planning to initiate the U.S. Phase IIb dose ranging trial in support of the orphan indication of IPAA-related fecal incontinence. Pending the outcomes of our ongoing Phase III trials for VEN 309 and VEN 307 and assuming the availability of sufficient capital, we may start this trial in 2012 to finalize the dose and clinical endpoints. We expect to conclude VEN 308 development and submit the orphan NDA in 2015. Orphan status provides seven years exclusivity from the date of approval during which time we will pursue several potential lifecycle opportunities.
Supply of clinical trial product
At this time, we are not actively pursuing the development of VEN 308 and have not undertaken any clinical supply activities for VEN 308.
Commercial summary for phenylephrine gel (VEN 308)
Quantitative market research conducted in 2003 by Eidetics reported that, on average, primary-care physicians see 23 fecal incontinence patients per month, gastroenterologists see 20 fecal incontinence patients per month, and colon and rectal surgeons see 14 fecal incontinence patients per month. Physicians categorize fecal incontinence according to its underlying cause. This market research was not designed to eliminate double counting of referred patients and has not been used in calculating commercial potential. However, these data do indicate the volume of patients seen at each type of practice irrespective of whether the same patient has been seen by another physician, and any one of these physicians can initiate a prescription for the product.
Physicians in all three medical specialties indicated that there is a significant unmet need for therapeutic choices for fecal incontinence. Only 4% of primary-care physicians, 3% of gastroenterologists, and 7% of colon and rectal surgeons reported being “very satisfied” with current treatment options.
License Agreements & Intellectual Property
General
Our goal is to obtain, maintain and enforce patent protection for our products, formulations, processes, methods and other proprietary technologies, preserve our trade secrets and operate without infringing on the proprietary rights of other parties, both in the U.S. and in other countries. Our policy is to actively seek to obtain, where appropriate, the broadest intellectual property protection possible for our current product candidates and any future product candidates, proprietary information and proprietary technology through a combination of contractual arrangements and patents, both in the U.S. and abroad. However, patent protection may not afford us with complete protection against competitors who seek to circumvent our patents.
| 31 |
We also depend upon the skills, knowledge, experience and know-how of our management and research and development personnel, as well as that of our advisors, consultants and other contractors. To help protect our proprietary know-how, which is not patentable, and for inventions for which patents may be difficult to enforce, we currently rely and will in the future rely on trade secret protection and confidentiality agreements to protect our interests. To this end, we require all of our employees, consultants, advisors and other contractors to enter into confidentiality agreements that prohibit the disclosure of confidential information and, where applicable, require disclosure and assignment to us of the ideas, developments, discoveries and inventions important to our business.
License Agreements — VEN 309
In March 2008, we entered into an Exclusive License Agreement with Amer whereby we acquired exclusive patent rights to iferanserin (the Amer Technology) for the topical treatment of any anorectal disorders. Pursuant to the Exclusive License Agreement, we paid Amer a monthly fee of $15,000. If and when we complete our Phase III trial for VEN 309, this monthly fee would decrease to $7,500. If and when we file an NDA for VEN 309 with the FDA, this monthly fee would cease. We also were required to make future milestone payments totaling up to $20 million upon the achievement of various milestones related to regulatory or commercial events. In the event that the Amer Technology is commercialized, we were obligated to pay to Amer annual royalties based upon sales of the product in the U.S. market and outside the U.S.
On June 5, 2011, we entered into an asset purchase agreement with Amer to acquire all rights, title and interest to VEN 309. We paid $500,000 on execution and paid $12 million for the asset at closing, which took place on November 14, 2011. We also paid Amer $50,000 on execution and paid Amer $5,000 per month for consulting services through the closing. Closing was subject to our raising net proceeds of a certain minimum amount as well as customary closing conditions. Closing was also subject to, in respect of the first pivotal Phase III trial and any recurrence treatment for VEN 309, the absence through November 10, 2011 of any serious severe adverse events that are life threatening with a risk of serious morbidity that have occurred in one or more subjects receiving VEN 309 which are either determined to be at least probably caused by VEN 309 or have been disclosed by us in a public securities filing. The purchase agreement contains customary indemnification provisions. We are obligated to pay milestone payments as follows: $1.5 million upon the one year anniversary of FDA approval of our planned NDA for VEN 309; $750,000 upon the attainment of $20 million in cumulative net sales of VEN 309; $1.5 million upon the attainment of $50 million in cumulative net sales; $3.0 million upon the attainment of $75 million in cumulative net sales; and $3.75 million upon regulatory approval for over-the-counter sale of VEN 309. Upon commercialization, we will pay Amer royalties of between 3.0% and 4.0% for net sales in the U.S., depending on the level of net sales in the U.S., and between 1.0% and 1.33% for sales outside of the U.S., depending on the level of gross sales outside the U.S., which, in addition to an approximately 50% reduction in the $20 million aggregate milestone payments under the Exclusive License Agreement, represents an approximately 66% decrease in the royalty fees due to Amer under the Exclusive License Agreement. We will pay Amer a minimum royalty of 50% of the royalties on the forecasted annual net sales in the U.S. and 50% of the royalties on the forecasted annual gross sales outside the U.S. Amer, Dr. Amer and his wife are prohibited for a period of five years after the closing from, directly or indirectly, owning an interest in, managing, operating, joining, controlling or participating in the ownership, management, operation or control of any profit or non-profit business or organization other than Ventrus that conducts research, develops, formulates, tests, produces, licenses, commercializes, manufactures or distributes a product incorporating VEN 309 or any other product which has the function of affecting the 5HT 2A receptor. The non-compete covers the United Sates and its territories and any other jurisdiction in the world where a patent has issued for iferanserin. Upon the closing of the acquisition, the Exclusive License Agreement with Amer terminated.
There are four patent filings for VEN 309, all of which, except as noted below, were filed in the name of Dr. Sam Amer as the inventor and Amer as the assignee (we now own all of the rights to VEN 309). The patent filing for the VEN 309 compound that we are developing was filed in the U.S. (No. 5,780,487), Europe (Germany, Great Britain, France, Switzerland and Spain) (No. EP 0973741), Japan (No. 520835/98), Norway (No. 19994181) and Korea (No. 10-997007763). Patents have been granted in the U.S. and Europe, while applications are pending in Norway, Japan and Korea. The U.S. patent will expire on August 7, 2015 and all foreign patents will expire on January 23, 2018, if all maintenance fees are timely paid.
| 32 |
The second patent filing is for the treatment of hemorrhoids with 5-HT 2 antagonists and has been filed in the U.S. (No. 5,266,571), Europe (Germany, Great Britain, Austria, Greece, France, Portugal, Luxemburg, Ireland, Spain, Denmark, Switzerland, Belgium, Sweden, and Netherland) (No. EP 0684816), Japan (No. 2807092) and Korea (No. 0278522), in all of which the patent has been granted. The U.S. patent will expire on January 9, 2012 and all foreign patents will expire on February 19, 2013, if all maintenance fees are timely paid.
The third patent filing for VEN 309 is for 5-HT 2 receptor antagonist compositions useful in treating venous conditions. This patent has been filed in the U.S. only (No. 5,605,902) and has been granted. The patent will expire on January 9, 2012, if all maintenance fees are timely paid.
The fourth patent filing for VEN is a concentration of range patent for the treatment of internal and external hemorrhoids that was filed in the U.S. (No. 12/860,974) and internationally on August 23, 2010 (No. PCT/US2010/046260). This patent is still pending. If issued, any patent will expire on August 23, 2030.
Finally, we will have data exclusivity for our NDA for VEN 309 in the U.S. for five years from the time of approval under the Hatch-Waxman Act.
We are solely responsible for the prosecution of the patent for VEN 309.
License Agreements — VEN 307 and 308
In March 2007, pursuant to an Exclusive License Agreement, S.L.A. Pharma granted Paramount BioSciences, Inc., or PBS, an exclusive, royalty-bearing license to sell, make, use and import diltiazem for treatment, through topical administration, of anal fissures and phenylepherine for treatment, through topical administration, of fecal incontinence in the U.S., Canada and Mexico. Pursuant to the Exclusive License Agreement, PBS was obligated to form a company to develop the technologies referenced in the Exclusive License Agreement and issue to S.L.A. Pharma that number of shares equal to 5% of such company’s outstanding common stock as of the effective date of the Exclusive License Agreement. To satisfy this obligation, PBS formed our company and we issued 18,401 shares of our common stock to S.L.A. Pharma in August 2007. In the event we closed an equity financing with gross proceeds of not less than $5,000,000 and the 18,401 shares issued to S.L.A. Pharma did not have a fair market value at least equal to $500,000 (calculated by multiplying the number of shares by the price per share paid in the financing), we were required to issue to S.L.A. Pharma that number of additional shares of our common stock so that, when added to the 18,401 shares initially issued, the new and old shares would have a fair market value equal to $500,000 (based on the price per share paid in the financing). As a result, upon the closing of our initial public offering on December 22, 2010, based on the initial offering price of $6.00, we issued S.L.A. Pharma 64,933 shares of our common stock.
In August 2007, pursuant to an Assignment and Assumption Agreement, PBS sold all of its rights in and arising out of the Exclusive License Agreement with S.L.A. Pharma to us for $1,087,876. The corresponding U.S. and foreign patents and applications for the two compounds have been licensed to us under the Assignment and Assumption Agreement (the technology referred to collectively as the Compound Technology). As consideration in part for the rights to the Compound Technology, an initial licensing fee of $250,000 was paid to S.L.A. Pharma and $50,000 for reimbursement of clinical development costs incurred by S.L.A. Pharma (these amounts were paid by PBS and was included in the consideration paid by us to PBS in connection with the Assignment and Assumption Agreement). In the event that the Compound Technology is commercialized, we are obligated to pay to S.L.A. Pharma annual royalties ranging from the mid to upper single digit percentages, based upon net sales of the product. In addition, we are required to make payments to S.L.A. Pharma up to an aggregate amount of $20 million upon the achievement of various milestones related to regulatory events. Should we make any improvements regarding the Compound Technology, we are required to grant S.L.A. Pharma licenses to use such improvements.
| 33 |
We also are required to reimburse S.L.A. Pharma for clinical development costs associated with the technology development of both VEN 307 and VEN 308. Our total payment obligation for these development costs for VEN 307 will not exceed $4,200,000. From August 2007 through December 31, 2011, we made $4,200,000 of such payments. Additionally, upon receipt of a quality controlled final study report for the Phase III trial for VEN 307 in Europe (anticipated in the second quarter of 2012), the cap on the amount of payments we must make to S.L.A. Pharma in respect of VEN 307 development costs will be increased to $4,600,000, and we must pay S.L.A. Pharma $400,000. S.L.A. Pharma has not provided the services for this additional work and therefore we have not recorded any additional expenses.
From August 2007 through December 31, 2011, we had paid $973,500 in project management fees to S.L.A. Pharma relating to the development of VEN 308. These project management fees were terminated effective October 1, 2010. We do not expect to continue developing VEN 308 in the short term and therefore do not expect to make any additional payments.
Our future known payment obligations to S.L.A. Pharma are as follows.
| Amount Due | Date Due | Fee Description | ||
| $41,500/monthly | Monthly beginning October 1, 2010, and continuing until S.L.A. Pharma is no longer managing the development program for VEN 307. | Project management fees for VEN 307. | ||
| $400,000 | Upon receipt of a quality controlled final study report of the Phase III trial for VEN 307 in Europe. | Development expense for VEN 307. |
We issued an additional 2,016 shares of our common stock to S.L.A. Pharma pursuant to the terms of the fourth amendment to the license agreement entered into in December 2009 and issued a warrant to purchase 13,605 shares of our common stock at an exercise price of $1.24 per share pursuant to the terms of the sixth amendment entered into on August 30, 2010. The sixth amendment benefited us by providing for an extension of the next $600,000 development fee, due September 30, 2010 to December 31, 2010 and the cancellation of all future VEN 308 monthly project management fees of $41,500 per month beginning after September 30, 2010, resulting in significant short term savings.
The Exclusive License Agreement with S.L.A. Pharma is terminable by us for any reason upon 90 days’ written notice, and by either party in the event of a material breach or default of the Exclusive License Agreement or either party becomes bankrupt or insolvent. In the event that we are not current in our payments under the license agreement, S.L.A. Pharma may terminate the Exclusive License Agreement if we have not brought the payments current within three business days of receipt of notice from S.L.A. Pharma. If the Exclusive License Agreement is terminated in any of these situations, we would have no further payment obligations to S.L.A. Pharma. In the event we have a “change in control” prior to the completion of the Phase III trial for VEN 307 and we terminate the Exclusive License Agreement within 30 days of the change in control, we must pay the balance of all payments (including the contingent $500,000 and $400,000 payments) owed for VEN 307 even if S.L.A. Pharma has not actually incurred those costs. In the event we have a “change in control” after the completion of the Phase III trial for VEN 307 and we terminate the Exclusive License Agreement within 30 days of the change in control, we must pay the balance of all payments (including the contingent $500,000 and $400,000 payments) owed for VEN 307 even if S.L.A. Pharma has not actually incurred those costs plus any other development expenses mutually agreed upon, but excluding the $41,500 monthly payments for VEN 307 and any monthly payments that might have been agreed to and initiated for VEN 308. A “change in control” is defined as a merger or other reorganization of our company in which our stockholders prior to the transaction do not own a majority of the voting stock of the surviving or successor entity, the sale by one or more of our stockholders of a majority of our voting securities, or the sale of all or substantially all of our assets related to VEN 307 and VEN 308. A “change in control” does not include a bona fide financing transaction in which voting control transfers to one or more persons or entities who acquire our securities in the transaction.
| 34 |
The U.S. patent for VEN 307 for topical treatment of pain associated with anal fissures was filed with the PTO on August 12, 1999 (No. 09/335,928) and a notice of allowance was issued by the PTO on May 26, 2011. A patent application was filed under the Patent Treaty Cooperation Act on February 23, 1998, entered the national stage in Canada on August 23, 1999 and a patent was issued on November 11, 2006 (No. 2,281,755). The expiration date for the patent in both the U.S. and Canada is February 23, 2018, if all maintenance fees are paid. The patent was filed in the name of Michael A. Kamm and Robin K. S. Phillips as the inventors and S.L.A. Pharma as the assignee.
The U.S. patent expires in February 2018. If approved, VEN 307 will have three years of data exclusivity in the U.S. under the Hatch-Waxman Act.
A patent application for VEN 308 for fecal incontinence was filed under the Patent Treaty Cooperation Act on December 23, 1997, entered the national stage in the U.S. on August 24, 1999 and in Canada on June 18, 1999. A patent was issued in the U.S. on October 21, 2003 (No. 6,635,678) and in Canada on March 18, 2008 (No. 2,275,663). The expiration date for the patent in both the U.S. and Canada is December 23, 2017, if all maintenance fees are paid. The patent was filed in the name of Michael A. Kamm and Robin K. S. Phillips as the inventors and S.L.A. Pharma as the assignee.
Under the S.L.A. Pharma Exclusive License Agreement, we are also responsible for the costs of prosecution of the patents, as well as any new patent filings for the licensed products. While we will pay these costs, S.L.A. Pharma will retain ownership of the patents although we will have the rights to license the technology underlying the patents for the duration of the Exclusive License Agreement.
Competition
As of the date of this report, we believe that there are no FDA-approved drug products that compete with VEN 309 and VEN 308 nor are we aware of any products that could potentially compete against any of our products for which FDA approval is currently being sought. However, a competing product could be filed for FDA approval in the future. Further, non-FDA-approved products could be introduced in the future that could compete with our planned products.
In late June 2011, ProStrakan Group plc received approval for Rectiv, a 0.4% concentration of nitroglycerin in ointment to be applied intra- and peri-anally twice daily for the treatment of pain associated with chronic anal fissures for a duration of up to three weeks. The U.S. label (professional package insert) for Rectiv lists headache occurring in 64% of patients with 938 headaches occurring in 79 patients, in the one pivotal trial described. In January 2012, Aptalis Pharma announced that it had signed an exclusive license agreement with ProStrakan Group plc to market Rectiv in the U.S.
The American Gastroenterology Association, in a technical review of anal fissure management in 2003 (Madoff, R.D. & Fleshman, J.W. (2003) AGA Technical Review on the Diagnosis and Care of Patients With Anal Fissure , Gastroenterology , 124, 235–245) states, “Based on the relatively limited data available to date, topical anal fissure therapy with calcium-channel blockers appears to be roughly as effective as treatment with topical nitrates. Moreover, the side effect profile of topical calcium-channel blockers appears superior, specifically with respect to fewer reported headaches.” Rectiv is a topical nitrate.
In the U.S., topical nitroglycerin, compounded in a twice daily ointment has been used for over a decade while diltiazem cream has been in use for approximately five to seven years. Solvay Pharmaceuticals Inc., the original licensee for VEN 307 in the U.S., commissioned in 2003 an extensive quantitative market research study by Eidetics in 206 general practitioners, gastroenterologists and colorectal surgeons. In 2003, compounded topical nitroglycerin had been in use for several years but diltiazem cream had not yet seen appreciable use. The product profile presented to physicians described equivalent efficacy of diltiazem to nitroglycerin for pain relief and healing, but also described meaningful differences in headache incidence, and this is the comparative profile we expect if VEN 307 is approved. In response to this comparative profile, diltiazem was the preferred prescription treatment for anal fissures with 35% overall preference share for topical diltiazem, 23% for topical hydrocortisone and 14% for topical nitroglycerin.
| 35 |
Topical nitroglycerin has also been marketed in the U.K. and other European countries and elsewhere as Rectogesic TM since 2007 while at the same time diltiazem cream, though not approved, has been used on a named patient basis or compounded. The professional label in Europe for Rectiv marketed as “Rectogesic” lists headaches as being very common with a 63% incidence of which 45% were moderate or severe. Indeed, the Association of Coloproctology of Great Britain and Ireland in their guidelines of 2008 (Cross, K.L.R., et al., (2008), The Management of Anal Fissure: ACPGBI Position Statement , Colorectal Disease, 10 (Suppl. 3), 1-7) states that, “ Topical diltiazem has similar efficacy to GTN (nitroglycerin) but with fewer side effects and should be recommended as first line treatment in the management of anal fissure”.
Based on results of previously published trials (such as Kocher et al. 2002 and Shrivastava 2007, see Table 1 above under the heading “Diltiazem Cream (VEN 309) Development – Investigator-initiated clinical studies (studies sponsored by individual clinicians)”, we believe that the efficacy of diltiazem cream is likely to be similar to Rectiv in the relief of pain from chronic anal fissures while we believe that the side effects, particularly moderate and severe headaches, are likely to be substantially less than those observed with topical nitroglycerin, and we expect to observe this in our subsequent trials some or all of which are expected to have a comparative arm of Rectiv. Consequently, considering existing professional society views in the U.S. and the U.K., even though VEN 307 is not yet approved in those countries, and considering existing data (some of which is directly comparative) on both products, we believe that, if approved, VEN 307 will be highly competitive with Rectiv.
In addition, in 2011, an Israeli company, RDD Pharma Ltd., completed in Israel a 20 patient single-arm open label study of the effect of coated suppositories of nifedipine on pain and healing in the treatment of chronic anal fissures.
Our industry is highly competitive and subject to rapid and significant technological change. Our potential competitors include large pharmaceutical and biotechnology companies, specialty pharmaceutical and generic drug companies, academic institutions, government agencies and research institutions. We believe that key competitive factors that will affect the development and commercial success of our product candidates are efficacy, safety and tolerability profile, reliability, convenience of dosing, price and reimbursement.
Many of our potential competitors have substantially greater financial, technical and human resources than we do and significantly greater experience in the discovery and development of product candidates, obtaining FDA and other regulatory approvals of products and the commercialization of those products. Accordingly, our competitors may be more successful than we may be in obtaining FDA approval for drugs and achieving widespread market acceptance. Our competitors’ drugs may be more effective, or more effectively marketed and sold, than any drug we may commercialize and may render our product candidates obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our product candidates. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the indications we are targeting could render our drugs non-competitive or obsolete.
Government Regulation
General
The production, distribution, and marketing of products employing our technology, and its development activities, are subject to extensive governmental regulation in the U.S. and in other countries. In the U.S., our products are regulated as drugs and are subject to the Federal Food, Drug, and Cosmetic Act, as amended, and the regulations of the FDA, as well as to other federal, state, and local statutes and regulations. These laws, and similar laws outside the U.S., govern the clinical and preclinical testing, manufacture, safety, effectiveness, approval, labeling, distribution, sale, import, export, storage, recordkeeping, reporting, advertising, and promotion of our products. Product development and approval within this regulatory framework, if successful, will take many years and involve the expenditure of substantial resources. Violations of regulatory requirements at any stage may result in various adverse consequences, including the FDA’s and other health authorities’ delay in approving or refusal to approve a product. Violations of regulatory requirements also may result in enforcement actions.
| 36 |
The following provides further information on legal and regulatory matters that have the potential to affect our operations or future marketing of products.
Research, Development and Product Approval Process
The research, development, and approval process in the U.S. and elsewhere is intensive and rigorous and generally takes many years to complete. The typical process the FDA requires before a therapeutic drug may be marketed in the U.S. includes:
| • | preclinical laboratory and animal tests performed under the FDA’s Good Laboratory Practices regulations, or GLPs; |
| • | submission to the FDA of an application for an IND, which must become effective before human clinical trials may commence; |
| • | preliminary human clinical studies to evaluate the drug and its manner of use; |
| • | adequate and well-controlled human clinical trials to establish whether the drug is safe and effective for its intended uses; |
| • | FDA review of whether the facility in which the drug is manufactured, processed, packed, or held meets standards designed to assure the product’s continued quality; and |
| • | submission of a marketing application to the FDA, and review and approval of the application by the FDA. |
During preclinical testing, studies are performed with respect to the chemical and physical properties of candidate formulations. These studies are subject to GLP requirements. Biological testing is typically done in animal models to demonstrate the activity of the compound against the targeted disease or condition and to assess the apparent effects of the new product candidate on various organ systems, as well as its relative therapeutic effectiveness and safety. An IND must be submitted to the FDA and become effective before studies in humans may commence.
Clinical trial programs in humans generally follow a three-phase process. Typically, Phase I trials are conducted in small numbers of healthy volunteers or, on occasion, in patients afflicted with the target disease. Phase I trials are conducted to determine the metabolic and pharmacological action of the product candidate in humans and the side effects associated with increasing doses, and, if possible, to gain early evidence of effectiveness. In Phase II, studies are generally conducted in larger groups of patients having the target disease or condition in order to validate clinical endpoints, and to obtain preliminary data on the effectiveness of the product candidate and optimal dosing. This phase also helps determine further the safety profile of the product candidate. In Phase III, large-scale clinical trials are generally conducted in patients having the target disease or condition to provide sufficient data for the statistical proof of effectiveness and safety of the product candidate as required by U.S. and foreign regulatory agencies.
Before proceeding with a study, sponsors may seek a written agreement from the FDA regarding the design, size, and conduct of a clinical trial. This is known as a Special Protocol Assessment, or SPA. Among other things, SPAs can cover clinical studies for pivotal trials whose data will form the primary basis to establish a product’s efficacy. SPAs help establish up-front agreement with the FDA about the adequacy of a clinical trial design to support a regulatory approval, but the agreement is not binding if new circumstances arise. There is no guarantee that a study will ultimately be adequate to support an approval even if the study is subject to an SPA.
| 37 |
U.S. law requires that studies conducted to support approval for product marketing be “adequate and well controlled.” In general, this means that either a placebo or a product already approved for the treatment of the disease or condition under study must be used as a reference control. Studies must also be conducted in compliance with good clinical practice requirements, and informed consent must be obtained from all study subjects.
The clinical trial process for a new compound can take 10 years or more to complete. The FDA may prevent clinical trials from beginning or may place clinical trials on hold at any point in this process if, among other reasons, it concludes that study subjects are being exposed to an unacceptable health risk. Trials may also be prevented from the beginning or may be terminated by institutional review boards, which must review and approve all research involving human subjects. Side effects or adverse events that are reported during clinical trials can delay, impede, or prevent marketing authorization. Similarly, adverse events that are reported after marketing authorization can result in additional limitations being placed on a product’s use and, potentially, withdrawal of the product from the market.
Following the completion of clinical trials, the data are analyzed to determine whether the trials successfully demonstrated safety and effectiveness and whether a product approval application may be submitted. In the U.S., if the product is regulated as a drug, a new drug application, or NDA, must be submitted and approved before commercial marketing may begin. The NDA must include a substantial amount of data and other information concerning the safety and effectiveness of the compound from laboratory, animal, and human clinical testing, as well as data and information on manufacturing, product quality and stability, and proposed product labeling. This special NDA procedure, known as a “section 505(b)(2) application” or a “paper NDA,” allows an applicant to seek approval on the basis of a combination of a prior approval of a similar product or published literature, and some new clinical studies conducted or sponsored by the applicant. Section 505(b)(2) applications are often used for changes in a drug that require clinical investigations and thus cannot be handled through the generic drug process, such as a new indication or change in dosage form or route of administration.
Each domestic and foreign manufacturing establishment, including any contract manufacturers we may decide to use, must be listed in the NDA and must be registered with the FDA. The application generally will not be approved until the FDA conducts a manufacturing inspection, approves the applicable manufacturing process for the drug product, and determines that the facility is in compliance with cGMP requirements.
Each NDA submitted for FDA approval is usually reviewed for administrative completeness and reviewability within 45 to 60 days following submission of the application. If deemed complete, the FDA will “file” the NDA, thereby triggering substantive review of the application. The FDA can refuse to file any NDA that it deems incomplete or not properly reviewable. The FDA has established performance goals for the review of NDAs — six months for priority applications and 10 months for standard applications. However, the FDA is not legally required to complete its review within these periods, and these performance goals may change over time. Moreover, the outcome of the review, even if generally favorable, typically is not an actual approval but an “action letter” that describes additional work that must be done before the application can be approved. The FDA’s review of an application may involve review and recommendations by an independent FDA advisory committee. Even if the FDA approves a product, it may limit the approved therapeutic uses for the product as described in the product labeling, require that warning statements be included in the product labeling, require that additional studies be conducted following approval as a condition of the approval, impose restrictions and conditions on product distribution, prescribing, or dispensing in the form of a risk management plan, or otherwise limit the scope of any approval.
Significant legal and regulatory requirements also apply after FDA approval to market under an NDA. These include, among other things, requirements related to adverse event and other reporting, product advertising and promotion and ongoing adherence to cGMPs, as well as the need to submit appropriate new or supplemental applications and obtain FDA approval for certain changes to the approved product, product labeling, or manufacturing process. The FDA also enforces the requirements of the Prescription Drug Marketing Act which, among other things, imposes various requirements in connection with the distribution of product samples to physicians.
| 38 |
The regulatory framework applicable to the production, distribution, marketing, and/or sale, of our products may change significantly from the current descriptions provided herein in the time that it may take for any of our products to reach a point at which an NDA is approved.
Overall research, development, and approval times depend on a number of factors, including the period of review at FDA, the number of questions posed by the FDA during review, how long it takes to respond to the FDA’s questions, the severity or life-threatening nature of the disease in question, the availability of alternative treatments, the availability of clinical investigators and eligible patients, the rate of enrollment of patients in clinical trials, and the risks and benefits demonstrated in the clinical trials.
Orphan Drugs
Under the Orphan Drug Act, special incentives exist for companies to develop products for rare diseases or conditions, which are defined to include those diseases or conditions that affect fewer than 200,000 people in the U.S. Companies may request that the FDA grant a drug orphan designation prior to approval. Products designated as orphan drugs are eligible for special grant funding for research and development, FDA assistance with the review of clinical trial protocols, potential tax credits for research, reduced filing fees for marketing applications, and a special seven-year period of market exclusivity after marketing approval. Orphan drug exclusivity prevents FDA approval of applications by others for the same drug and the designated orphan disease or condition. The FDA may approve a subsequent application from another entity if the FDA determines that the application is for a different drug or different use, or if the FDA determines that the subsequent product is clinically superior, or that the holder of the initial orphan drug approval cannot assure the availability of sufficient quantities of the drug to meet the public’s need. A grant of an orphan designation is not a guarantee that a product will be approved. If a sponsor receives orphan drug exclusivity upon approval, there can be no assurance that the exclusivity will prevent another entity or a similar drug from receiving approval for the same or other uses.
Other U.S. Regulatory Requirements
In the U.S., the research, manufacturing, distribution, sale, and promotion of drug and biological products are potentially subject to regulation by various federal, state, and local authorities in addition to the FDA, including the Centers for Medicare and Medicaid Services, other divisions of the U.S. Department of Health and Human Services (for example, the U.S. Department of Justice and individual U.S. Attorney offices within the Department of Justice, and state and local governments. For example, sales, marketing, and scientific/educational grant programs must comply with the anti-fraud and abuse provisions of the Social Security Act, the False Claims Act, the privacy provision of the Health Insurance Portability and Accountability Act, and similar state laws, each as amended. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veterans Health Care Act of 1992, each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection, unfair competition, and other laws. Moreover, we are now, and in the future may become subject to, additional federal, state, and local laws, regulations, and policies relating to safe working conditions, laboratory practices, the experimental use of animals, and/or the use, storage, handling, transportation, and disposal of human tissue, waste, and hazardous substances, including radioactive and toxic materials and infectious disease agents used in conjunction with our research work.
Foreign Regulatory Requirements
We and our collaborative partners may be subject to widely varying foreign regulations, which may be quite different from those of the FDA, governing clinical trials, manufacture, product registration and approval, and pharmaceutical sales. Whether or not FDA approval has been obtained, we or our collaboration partners must obtain a separate approval for a product by the comparable regulatory authorities of foreign countries prior to the commencement of product marketing in these countries. In certain countries, regulatory authorities also establish pricing and reimbursement criteria. The approval process varies from country to country, and the time may be longer or shorter than that required for FDA approval. In addition, under current U.S. law, there are restrictions on the export of products not approved by the FDA, depending on the country involved and the status of the product in that country.
| 39 |
Reimbursement and Pricing Controls
In many of the markets where we or our collaborative partners would commercialize a product following regulatory approval, the prices of pharmaceutical products are subject, by law, to direct price controls and to drug reimbursement programs with varying price control mechanisms. Public and private health care payors control costs and influence drug pricing through a variety of mechanisms, including through negotiating discounts with the manufacturers and through the use of tiered formularies and other mechanisms that provide preferential access to certain drugs over others within a therapeutic class. Payors also set other criteria to govern the uses of a drug that will be deemed medically appropriate and therefore reimbursed or otherwise covered. In particular, many public and private health care payors limit reimbursement and coverage to the uses of a drug that are either approved by the FDA or that are supported by other appropriate evidence (for example, published medical literature) and appear in a recognized drug compendium. Drug compendia are publications that summarize the available medical evidence for particular drug products and identify which uses of a drug are supported or not supported by the available evidence and whether or not such uses have been approved by the FDA. For example, in the case of Medicare coverage for physician-administered oncology drugs, the Omnibus Budget Reconciliation Act of 1993, with certain exceptions, prohibits Medicare carriers from refusing to cover unapproved uses of an FDA-approved drug if the unapproved use is supported by one or more citations in the American Hospital Formulary Service Drug Information, the American Medical Association Drug Evaluations, or the U.S. Pharmacopoeia Drug Information. Another commonly cited compendium, for example under Medicaid, is the DRUGDEX Information System.
Available Information
Our website address is www.ventrusbio.com. Information on our website is not incorporated herein by reference. We make available free of charge through our website our press releases, Annual Report on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after electronically filed with or furnished to the Securities and Exchange Commission.
Employees
As of December 31, 2011, we had seven employees and had contracted with seven consultants on manufacturing, preclinical and clinical aspects of our drug programs. We use consulting agreements to avoid the costs customarily associated with employees to save resources.
Our activities to date have consisted of establishing and clarifying the regulatory pathway for the late phase clinical trials and regulatory approval of our product candidates, primarily VEN 309 and VEN 307, and on establishing the contract manufacturing capacity and methods, and study start up procedures necessary to allow the first Phase III clinical trial of VEN 309 to proceed. All of these planned trials will be conducted by third parties. All clinical trials to date have been conducted either by the licensor, the licensor’s previous partners or by independent investigators, as have the preclinical studies and product formulation activities. Consequently, we have needed only a few employees with medical expertise and drug development experience and a limited number of administrative employees.
| 40 |
Executive Officers
As of the date of this Report, our executive officers are Dr. Russell H. Ellison, our President and Chief Executive Officer, David J. Barrett, our Chief Financial Officer and Thomas Rowland, our Chief Business Officer. Information for each is provided below.
| Name | Age (as of 02/29/12) |
Business Experience For Last Five Years | ||
|
Russell H. Ellison, M.D., M.Sc.
|
64 | Dr. Ellison joined us as a director, Chief Executive Officer and Chief Medical Officer in June 2010. He was elected Chairman of our Board in January 2011. From July 2007 to January 2010, Dr. Ellison served as Executive Vice President of Paramount Biosciences LLC, a global pharmaceutical development and healthcare investment firm. From October 2005 until June 2007, Dr. Ellison served as the Vice President of Clinical Development of Fibrogen, Inc., a privately held biotechnology company. From August 2002 to December 2004, Dr. Ellison served as Vice President of Medical Affairs and Chief Medical Officer of Sanofi-Synthelabo, USA, a pharmaceutical company. From May 1997 to August 2002, Dr. Ellison served as Vice President, Medical Affairs and Chief Medical Officer of Hoffman-La Roche, Inc., a pharmaceutical company. From July 2007 until December 2010, Dr. Ellison served as a director of CorMedix, Inc., a pharmaceutical company that went public in March 2010. He currently serves as a director of several privately held development stage biotechnology companies. Dr. Ellison holds an M.D. from the University of British Columbia and an M.Sc. (with distinction) from The London School of Tropical Medicine and Hygiene. | ||
| David J. Barrett | 35 | Mr. Barrett joined us as Chief Financial Officer in July 2010. From April 2006 to September 2009, Mr. Barrett served as Chief Financial Officer of Neuro-Hitech, Inc., a publically traded company focused on developing, marketing and distributing branded and generic pharmaceutical products. From September 2003 to April 2006, Mr. Barrett was the Chief Financial Officer /Vice President of Finance of Overture Asset Managers and Overture Financial Services, which, at the time, was a start-up asset management firm that assembled investment products and platforms to distribute turnkey and unbundled investment solutions to financial intermediaries and institutional investors. From July 1999 to September 2003, Mr. Barrett was employed as a Manager at Deloitte & Touche, LLP. Mr. Barrett received his B.S. in Accounting and Economics in May of 1998 and his M.S. in Accounting in May of 1999 from the University of Florida. He is a certified public accountant. |
| 41 |
| Name | Age (as of 02/29/12) |
Business Experience For Last Five Years | ||
| Thomas Rowland | 45 |
Mr. Rowland joined Ventrus Biosciences in April 2007 as our Chief Executive Officer, a position he held until February 2009. From March 2009 to June 2010, he served as our Acting President. On September, 2011, Mr. Rowland rejoined Ventrus as our Chief Business Officer. Prior to Ventrus, Mr. Rowland was founder and principal of his own consulting firm, consulting to various pharmaceutical and biotechnology companies in the areas of business development, marketing and launch preparation. He continued these consulting services from March 2009 to January 2010. In February 2010, Mr. Rowland became Vice President of Commercial Development for Alnara Pharmaceuticals, Inc., which was acquired by Eli Lilly and Company. Mr. Rowland currently is a Product Director for Eli Lilly. Mr. Rowland started consulting in 2006 after serving as Vice President of the Gastroenterology and Women’s Health Business Unit at Solvay Pharmaceuticals, Inc., where he oversaw all commercial operations for the over $250 million and 250 person unit. Prior to being named Vice President, Mr. Rowland successfully led the turnaround of the gastrointestinal franchise, returning the franchise to positive sales growth, record sales and profitability. Mr. Rowland had the commercial responsibilities for the gastroenterology franchise at Solvay from 2000 to 2005. During that time Mr. Rowland was the commercial lead in the licensing of diltiazem and phenylephrine from S.L.A. Pharma. Mr. Rowland’s responsibilities included the commercial assessment and strategic guidance of diltiazem and phenylephrine. The Solvay R&D department, however, was responsible for the development of diltiazem and phenylephrine and Mr. Rowland was not part of that department. Mr. Rowland’s initial work in the gastroenterology therapeutic area started when he joined Scandipharm, Inc. in 1998 as Director of Marketing to assist in the company turnaround which resulted in the sale of the company to Axcan Pharmaceuticals. Mr. Rowland started his career in 1990 at UCB Pharma, Inc. where he spent over eight years in various positions of increasing responsibility including sales, market research and product management. Throughout his career, Mr. Rowland has participated in numerous successful new product and line extension launches. In addition, in senior management roles, he has contributed to several product, franchise and company turnarounds. Mr. Rowland earned his B.S. in Finance from Metropolitan State University in Denver, Colorado in 1989. Mr. Rowland served on our Board of Directors from April 2007 until January 17, 2012.
|
Item 1A. Risk Factors
This report contains forward-looking statements that involve risks and uncertainties. Our actual results could differ materially from those discussed in this report. Factors that could cause or contribute to these differences include, but are not limited to, those discussed below and elsewhere in this report and in any documents incorporated in this report by reference.
If any of the following risks, or other risks not presently known to us or that we currently believe to not be significant, develop into actual events, then our business, financial condition, results of operations or prospects could be materially adversely affected. If that happens, the market price of our common stock could decline, and stockholders may lose all or part of their investment.
| 42 |
Risks Related to Our Business
We have a limited operating history and a history of operating losses, and expect to incur significant additional operating losses.
We were established in October 2005, began active operations in the spring of 2007 and have only a limited operating history. Therefore, there is limited historical financial information upon which to base an evaluation of our performance. Our prospects must be considered in light of the uncertainties, risks, expenses, and difficulties frequently encountered by companies in their early stages of operations. We have generated losses since we began operations and, as of December 31, 2011, we had a deficit accumulated during the development stage of $57.9 million. We expect to incur substantial additional losses over the next several years as our research, development, pre-clinical testing, and clinical trial activities increase. The amount of future losses and when, if ever, we will achieve profitability are uncertain. We have no products that have generated any commercial revenue, do not expect to generate revenues from the commercial sale of products unless and until our product candidates are approved by the FDA for sale, and might never generate revenues from the sale of products.
We are not currently profitable and might never become profitable.
We have a history of losses and expect to incur substantial losses and negative operating cash flow for the foreseeable future, and we might never achieve or maintain profitability. Even if we succeed in developing and commercializing one or more product candidates, we expect to incur substantial losses for the foreseeable future. We also expect to continue to experience negative cash flow and to incur significant operating and capital expenditure for the foreseeable future. We anticipate that our expenses will increase substantially in the foreseeable future as we:
| · | continue to undertake preclinical development and clinical trials for product candidates; |
| · | seek regulatory approvals for product candidates; |
| · | implement additional internal systems and infrastructure; and |
| · | hire additional personnel. |
As a result, we will need to generate significant revenues in order to achieve and maintain profitability. Our ability to generate revenue and achieve profitability will depend on, among other things:
| · | successful completion of animal tests, which are referred to as pre-clinical studies, as well as human tests, which are referred to as clinical trials, for our product candidates; |
| · | obtaining necessary regulatory approvals from the FDA and international regulatory agencies; |
| · | establishing manufacturing, sales, and marketing arrangements with third parties; and |
| · | raising sufficient funds to finance our activities. |
We might not succeed at any of these undertakings. If we are unsuccessful at some or all of these undertakings, our business, prospects, and results of operations might be materially adversely affected.
We have no approved products.
To date, we have no approved product on the market and have generated no product revenues. Unless and until we receive approval from the FDA and other regulatory authorities for our product candidates, we cannot sell our drugs and will not have product revenues. Therefore, for the foreseeable future, we will have to fund all of our operations and capital expenditures from cash on hand, any licensing fees and any future securities offerings or debt financings. We intend to devote substantially all of our resources to the development of VEN 309 and VEN 307. In the event we do not obtain regulatory approval of either of these product candidates, our business will be materially and adversely affected.
| 43 |
We are a development-stage company and might not be able to commercialize any product candidates.
We are a development-stage company and have not demonstrated our ability to perform the functions necessary for the successful commercialization of any product candidates. The successful commercialization of any product candidates will require us to perform a variety of functions, including:
| · | continuing to undertake preclinical development and clinical trials; |
| · | participating in regulatory approval processes; |
| · | formulating and manufacturing products; and |
| · | conducting sales, marketing and distribution activities. |
Our development of current and future product candidates is subject to the risks of failure and delay inherent in the development of new pharmaceutical products and products based on new technologies, including:
| · | delays in product development, clinical testing, or manufacturing; |
| · | unplanned expenditures in product development, clinical testing, or manufacturing; |
| · | failure of a product candidate to demonstrate acceptable safety and efficacy; |
| · | failure to receive regulatory approvals; |
| · | emergence of superior or equivalent products; |
| · | inability to manufacture and sell on our own, or through any others, product candidates on a commercial scale or at a financially viable cost; and |
| · | failure to achieve market acceptance. |
Because of these risks, our research and development efforts might not result in any commercially viable products. If we do not successfully complete a significant portion of these development efforts, obtain required regulatory approvals, and have commercial success with any approved products, our business, financial condition and results of operations will be materially harmed.
We will need additional financing to fund our activities in the future and complete the development of our product candidates.
We anticipate that we will incur operating losses for the foreseeable future. We expect that our current resources will provide us with sufficient capital to fund our operations to develop VEN 309 through two pivotal Phase III trials. However, we might consume our available capital before that time if, for example, we are not efficient in developing our product candidates and conducting clinical trials or if regulatory requirements change.
Moreover, we believe we will require substantial funds in the future to support our operations. We anticipate that to complete the clinical trial process to obtain the approval of our product candidates will cost approximately $20 million for VEN 307, $15 million for VEN 308 and $40 million for VEN 309. We might seek equity or debt financings in the future to fund our operations. However, there is no assurance that we will be successful in raising the additional capital we need to fund our business plan on terms that are acceptable to us, or at all. If we do not succeed in raising additional funds on acceptable terms, we might be unable to initiate or complete clinical trials or obtain approval of any product candidate from the FDA and other regulatory authorities. In addition, we could be forced to discontinue product development, forego sales and marketing efforts, sacrifice attractive business opportunities, cease operations entirely and sell or otherwise transfer all or substantially all of our remaining assets.
| 44 |
We are dependent on a license relationship for VEN 307 and VEN 308.
We have acquired, by license from S.L.A. Pharma, the rights to VEN 307 and VEN 308, which are critical to our business, and we might enter into additional licenses in the future. The license with S.L.A. Pharma contains, and we expect that any future licenses will contain, provisions requiring up-front, milestone, and royalty payments to the licensor. If we fail to comply with these obligations to a licensor, that licensor might have the right to terminate the license on relatively short notice, in which event we would not be able to commercialize drug candidates or technologies that were covered by the license. Also, the milestone and other payments associated with licenses will make it less profitable for us to develop our drug candidates than if we owned the technology ourselves.
We did not continue to pursue a Special Protocol Assessment, or SPA, for VEN 309 and the FDA may not find the pivotal trials we conduct for VEN 309 to be sufficient to support approval.
In order not to delay the start of our Phase III trial for VEN 309 for the treatment of internal hemorrhoids, we chose not to reach agreement with FDA on a SPA and proceeded instead with the trial without an agreement letter on the SPA from the FDA. As a result, none of the recommendations made by the FDA on the major and important elements of the protocol to date and that we have implemented are binding on the FDA, which could result in delays in or failure to obtain approval of the NDA we plan to file for VEN 309. Further, in addition to our two pivotal Phase III trials for VEN 309, the FDA will also require that we complete various additional clinical trials and non-clinical testing, such as a Phase III recurrence trial and carcinogenicity and toxicology testing, and our discussions with the FDA from 2008 to date do not cover the detailed design or conduct of these additional trials and testing. As a result, we cannot assure that the pivotal trials and other studies we conduct will be sufficient to support approval of any NDA we file with respect to VEN 309.
The results of our Phase III trial for VEN 309 might not be as expected, which expectations are based on our post hoc analysis of an earlier study.
We have modeled the potential performance of the endpoints suggested by the FDA for our Phase III trial for VEN 309 using data from a prior double-blind Phase IIb trial of VEN 309 conducted in Germany that was very similar in all major respects to the Phase III trial we began conducting in August 2011. While we believe this post hoc analysis provided illustrative information, there are some differences related to patient inclusion/exclusion criteria and clinical endpoints and there could be unknown differences related to physician characteristics and study conduct between the studies that could possibly result in different outcomes. Accordingly, the successful results in the prior study might not be an indicator of success in our Phase III trials.
We have had negative cash flows from operations and might not be able to generate sufficient cash to meet our substantial obligations to S.L.A. Pharma, which could result in the termination of our license or put substantial burdens on our financial position.
We license two of our product candidates, VEN 307 and VEN 308, from S.L.A. Pharma, a Swiss corporation, and have obligations related to VEN 308 and to fund S.L.A. Pharma’s development efforts for VEN 307 in the E.U., all of which are set forth in the chart below.
| Amount Due | Date Due | Fee Description | ||
| $41,500/monthly | Monthly beginning October 1, 2010, and continuing until S.L.A. Pharma is no longer managing the development program for VEN 307. | Project management fees for VEN 307. |
| 45 |
| Amount Due | Date Due | Fee Description | ||
| $400,000 | Upon receipt of a quality controlled final study report of the Phase III trial for VEN 307 in Europe | Development expense for VEN 307 |
Our ability to make the payments required under the S.L.A. Pharma license agreement depends on our ability to generate cash in the future. We expect to experience negative cash flow for the foreseeable future as we fund our operating losses and capital expenditures. In the event that we are not current in our payments under the license agreement, S.L.A. Pharma may terminate the license agreement if we have not brought the payments current within three business days of receipt of notice from S.L.A. Pharma. Further, if we commercialize a product candidate, we must pay S.L.A. Pharma annual royalties ranging from the mid to upper single digit percentages, based upon net sales of the product. We also are required to make future milestone payments totaling up to $20 million upon the achievement of various milestones related to regulatory events for both VEN 307 and VEN 308, the earliest of which is not anticipated until 2015. In the event we breach these obligations, we could lose our rights to VEN 307 or VEN 308, or both, depending on the breach, which would have a material adverse effect on our business and prospects.
Corporate and academic collaborators might take actions to delay, prevent, or undermine the success of our products.
Our operating and financial strategy for the development, clinical testing, manufacture, and commercialization of drug candidates heavily depends on collaborating with corporations, academic institutions, licensors, licensees, and other parties. However, there can be no assurance that we will successfully establish these collaborations. In addition, should a collaboration be terminated, replacement collaborators might not be available on attractive terms, or at all. The activities of any collaborator will not be within our control and might not be within our power to influence. There can be no assurance that any collaborator will perform its obligations to our satisfaction or at all, that we will derive any revenue or profits from these collaborations, or that any collaborator will not compete with us. If any collaboration is not successful, we might require substantially greater capital to undertake development and marketing of our proposed products and might not be able to develop and market these products effectively, if at all. In addition, a lack of development and marketing collaborations might lead to significant delays in introducing proposed products into certain markets and/or reduced sales of proposed products in such markets.
We rely on data provided by our collaborators and others that has not been independently verified and could prove to be false, misleading, or incomplete.
We rely on third-party vendors, scientists, and collaborators to provide us with significant data and other information related to our projects, clinical trials, and our business. If these third parties provide inaccurate, misleading, or incomplete data, our business, prospects, and results of operations could be materially adversely affected.
We rely exclusively on third parties to formulate and manufacture our product candidates.
While we have contracted with a highly experienced head of manufacturing to oversee the manufacture of our clinical trial supplies, we do not have and do not intend to establish our own manufacturing facilities. Consequently, we lack the physical plant to formulate and manufacture our own product candidates, which are currently being manufactured entirely by commercial third parties, albeit under close supervision by our contractors. If any product candidate we might develop or acquire in the future receives FDA approval, we will rely on one or more third-party contractors to manufacture our products. If, for any reason, we become unable to rely on our current source or any future source to manufacture our product candidates, either for clinical trials or, at some future date, for commercial quantities, then we would need to identify and contract with additional or replacement third-party manufacturers to manufacture compounds for preclinical, clinical and commercial purposes. We might not be successful in identifying additional or replacement third-party manufacturers, or in negotiating acceptable terms with any that we do identify. If we are unable to secure and maintain third-party manufacturing capacity, the development and sales of our products and our financial performance might be materially affected.
| 46 |
In addition, before any of our collaborators can begin to commercially manufacture our product candidates, each must obtain regulatory approval of the manufacturing facility and process. Manufacturing of drugs for clinical and commercial purposes must comply with the FDA’s Current Good Manufacturing Practices, or cGMPs, and applicable non-U.S. regulatory requirements. The cGMP requirements govern quality control and documentation policies and procedures. Complying with cGMP and non-U.S. regulatory requirements will require that we expend time, money, and effort in production, recordkeeping, and quality control to assure that the product meets applicable specifications and other requirements. Our contracted manufacturing facilities must also pass a pre-approval inspection prior to FDA approval. Failure to pass a pre-approval inspection might significantly delay FDA approval of our products. If any of our collaborators fails to comply with these requirements, it would be subject to possible regulatory action which could limit the jurisdictions in which we are permitted to sell our products. As a result, our business, financial condition, and results of operations might be materially harmed.
Our reliance on a limited number of third-party manufacturers exposes us to the following risks:
| · | We might be unable to identify manufacturers for commercial supply on acceptable terms or at all because the number of potential manufacturers is limited and the FDA must approve any replacement contractor. This approval would generally require compliance inspections. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our products after receipt of FDA approval, if any. |
| · | Our third-party manufacturers might be unable to formulate and manufacture our drugs in the volume and of the quality required to meet our clinical and commercial needs, if any. |
| · | Our contract manufacturers might not perform as agreed or might not remain in the contract manufacturing business for the time required to supply our clinical trials or to successfully produce, store and distribute our products. |
| · | Currently, our contract manufacturers are all foreign, which increases the risk of shipping delays and adds the risk of import restrictions. |
| · | Drug manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state agencies to ensure strict compliance with cGMP and other government regulations and corresponding foreign standards. We do not have complete control over third-party manufacturers’ compliance with these regulations and standards although we have agents in plant that monitor the production process. |
| · | If any third-party manufacturer makes improvements in the manufacturing process for our products, we might not own, or might have to share, the intellectual property rights to the innovation with our licensors. |
| · | We might compete with other companies for access to these manufacturers’ facilities and might be subject to manufacturing delays if the manufacturers give other clients higher priority than us. |
Each of these risks could delay our clinical trials or the approval, if any, of our product candidates by the FDA or the commercialization of our product candidates and could result in higher costs or deprive us of potential product revenues. As a result, our business, financial condition, and results of operations might be materially harmed.
| 47 |
Preclinical and clinical trials required for our product candidates are expensive and time-consuming, and their outcome is uncertain.
In order to obtain FDA approval to market a new drug product, we must demonstrate safety and effectiveness in humans. To meet these requirements, we must conduct extensive preclinical testing and sufficient adequate and well-controlled clinical trials. Conducting clinical trials is a lengthy, time consuming, and expensive process. The length of time might vary substantially according to the type, complexity, novelty, and intended use of the product candidate, and often can be several years or more per trial. Delays associated with products for which we are directly conducting preclinical or clinical trials might cause us to incur additional operating expenses. The commencement and rate of completion of clinical trials might be delayed by many factors, including, for example:
| · | inability to manufacture sufficient quantities of qualified materials under cGMP for use in clinical trials; |
| · | slower than expected rates of patient recruitment; |
| · | failure to recruit a sufficient number of patients; |
| · | modification of clinical trial protocols; |
| · | changes in regulatory requirements for clinical trials; |
| · | the lack of effectiveness during clinical trials; |
| · | the emergence of unforeseen safety issues; |
| · | delays, suspension, or termination of clinical trials by the institutional review board responsible for overseeing the study at a particular study site; and |
| · | government, institutional review board or other regulatory delays or clinical holds requiring suspension or termination of the trials. |
We still must complete pharmacological and toxicity testing for VEN 309. In addition, because VEN 309 may be used as a chronic treatment, we are also required to complete long-term carcinogenicity testing. If any of this testing demonstrates meaningful toxicity, it could delay or prevent us from obtaining regulatory approval of VEN 309.
The results from preclinical testing and early clinical trials are not necessarily predictive of results obtained in later clinical trials. Accordingly, even if we obtain or have obtained positive results from preclinical or early clinical trials, we might not achieve the same success in future clinical trials. For example, although positive results have been observed in earlier clinical trials of each of VEN 309, VEN 307 and VEN 308, there is no assurance that any of our future clinical trials will be successful. Clinical trials might not provide statistically significant data supporting a product candidate’s safety and effectiveness to meet the requisite regulatory approvals.
We intend to rely on one or more contract research organizations, or CROs, to conduct our clinical trials for VEN 309 and VEN 307. We will be highly dependent on these CROs to conduct our trials in accordance with the requirements of the FDA and good scientific practice. In the event the CROs fail to perform their duties in such a fashion, we may not obtain regulatory approval for any of our product candidates.
The failure of clinical trials to demonstrate safety and effectiveness for the desired indications could harm the development of that product candidate and other product candidates. This failure could cause us to abandon a product candidate and could delay development of other product candidates. Any delay in, or termination of, our clinical trials would delay the filing of our NDAs with the FDA and, ultimately, our ability to commercialize our product candidates and generate product revenues. Any change in, or termination of, our clinical trials could materially harm our business, financial condition, and results of operation.
| 48 |
Existing and unforeseen safety issues could hinder the development of our product candidates and their adoption, if approved.
VEN 309, like numerous other drugs, is dependent on the CYP2D6 enzyme for its metabolism. An important property of CYP2D6 is that its activity is affected by genetic variability in individuals, including individuals who are CYP2D6 deficient and that its activity can be reduced by certain drugs. If this enzyme is inhibited by other medications being taken by a patient or the patient has a genetically reduced amount or a deficiency of the enzyme, and the patient takes VEN 309, the patient might have a higher level of iferanserin in his or her blood and might experience side effects although we are unaware of what the side effects might be. One patient in one of our Phase I trials had a genetic reduction of this enzyme and did experience substantially higher levels of VEN 309 in his blood. However, no side effects were observed in this patient. There are several well known drugs that also are dependent on CYP2D6, including several antidepressants as well as tamoxifen. We might restrict the use of VEN 309 in patients taking medications that inhibit or are dependent on the CYP2D6 enzyme, depending on the outcome of clinical drug-drug interaction clinical studies that we have initiated. VEN 309 has demonstrated arrythmogenic potential in in vitro (hERG channel) studies at exposures 60-100 times the topical 0.5% twice daily dose being studied in humans. We expect to conduct an arrhythmia clinical study (“thorough QT study”) as part of our Phase III clinical pharmacology program, which studies are routinely required by the FDA. Even though VEN 309 has a wide safety margin in this area, we cannot be certain of the outcome of this study, and demonstration of clinically meaningful arrhythmia risks could compromise or prevent the approvability of the product in major markets.
Both VEN 307 and VEN 308 have been safely used extensively for decades when given orally at much higher exposures (blood levels) than currently under study in the topical application of VEN 307 and VEN 308. Despite these safety records, other safety issues could arise during testing of our products, which might delay testing or prevent further development entirely. If a product is approved, any limitation on use that might be necessary could hinder its adoption in the marketplace. In addition, if any product is approved, it could be used against any instructions that we publish that limit its use, which could subject us to litigation.
If we cannot compete successfully for market share against other drug companies, we might not achieve sufficient product revenues and our business will suffer.
If our product candidates receive FDA approval, they will compete with a number of existing and future drugs and therapies developed, manufactured and marketed by others. Existing or future competing drugs might provide greater therapeutic convenience or clinical or other benefits for a specific indication than our products, or might offer comparable performance at a lower cost. If our products fail to capture and maintain market share, we might not achieve sufficient product revenues and our business will suffer.
We might compete against fully integrated pharmaceutical companies and smaller companies that are collaborating with larger pharmaceutical companies, academic institutions, government agencies and other public and private research organizations. Many of these competitors, either alone or together with their collaborative partners, operate larger research and development programs or have substantially greater financial resources than we do, as well as significantly greater experience in:
| · | developing drugs; |
| · | undertaking pre-clinical testing and human clinical trials; |
| · | obtaining FDA and other regulatory approvals of drugs; |
| · | formulating and manufacturing drugs; and |
| · | launching, marketing and selling drugs. |
We might not obtain the same resources and experience as our competitors. If we are unable to perform these tasks effectively and efficiently, our results of operations might be materially adversely affected.
| 49 |
Developments by competitors might render our products or technologies obsolete or non-competitive.
The pharmaceutical and biotechnology industries are intensely competitive. We might compete with organizations that are developing treatments for the indications that our products target.
To our knowledge, there is currently only one FDA-approved drug for the treatment of anal fissures. Rectiv, a topical nitroglycerin treatment, was approved in late June 2011 by the FDA, and is expected to come to market in the first quarter of 2012. For the treatment of fecal incontinence, Solesta, an injectable therapy developed by Oceana Therapeutics, was approved as a device by the FDA in 2011 and is expected to come to market in 2012. To our knowledge, there are no other products approved or in development although there are two non-drug products in development. For the treatment of hemorrhoids, some physicians are known to prescribe topical steroids, although such treatment has not been approved by the FDA for this indication. Further, many hemorrhoid sufferers use Wyeth’s Preparation H or similar products for symptomatic relief (active ingredients can vary by country but generally include glycerin, phenylephrine HCl, pramoxine HCl, white petrolatum, shark liver oil and/or witch hazel). No data are publicly available regarding the clinical efficacy of this or other over-the-counter symptomatic treatments for hemorrhoids. Finally, there are surgical devices being studied for the treatment of hemorrhoids. If our competitors develop effective treatments for anal fissure, fecal incontinence or hemorrhoids and successfully commercialize those treatments, our business and prospects might be materially harmed.
If we are not able to develop collaborative marketing relationships with licensees or partners, or create an effective internal sales, marketing, and distribution capability, we might be unable to market our products successfully.
To market our products, we will have to establish our own marketing and sales force or out-license our product candidates to, or collaborate with, larger firms with experience in marketing and selling pharmaceutical products. There can be no assurance that we will be able to successfully establish our own marketing capabilities or establish marketing, sales, or distribution relationships with third parties; that such relationships, if established, will be successful; or that we will be successful in gaining market acceptance for our products. To the extent that we enter into any marketing, sales, or distribution arrangements with third parties, our product revenues will be lower than if we marketed and sold our products directly, and any revenues we receive will depend upon the efforts of such third parties. If we are unable to establish such third-party sales and marketing relationships, or choose not to do so, we will have to establish our own in-house capabilities. Although our employees have extensive experience in the commercialization of drug products, we, as a company, have no experience in marketing or selling pharmaceutical products and currently have no sales, marketing, or distribution infrastructure. To market any of our products directly, we would need to develop a marketing, sales, and distribution force that both has technical expertise and the ability to support a distribution capability. To establish our own marketing, sales, and distribution capacity would significantly increase our costs, and require substantial additional capital. In addition, there is intense competition for proficient sales and marketing personnel, and we might not be able to attract individuals who have the qualifications necessary to market, sell, and distribute our products. There can be no assurance that we will be able to establish internal marketing, sales, or distribution capabilities.
Physicians and patients might not accept and use our drugs.
Even if the FDA approves one of our product candidates, physicians and patients might not accept and use it. Acceptance and use of our products will depend upon a number of factors, including:
| • | perceptions by members of the health care community, including physicians, about the safety and effectiveness of our product; |
| • | cost-effectiveness of our product relative to competing product or therapies; |
| • | availability of reimbursement for our product from government or other healthcare payors; and |
| • | effective marketing and distribution efforts by us and our licensees and distributors, if any. |
| 50 |
If our current product candidates are approved, we expect sales to generate substantially all of our revenues for the foreseeable future, and as a result, the failure of these products to find market acceptance would harm our business and would require us to seek additional financing.
Our ability to generate product revenues will be diminished if our products sell for inadequate prices or patients are unable to obtain adequate levels of reimbursement.
Our ability to commercialize our products, alone or with collaborators, will depend in part on the extent to which reimbursement will be available from:
| • | government and health administration authorities; |
| • | private health maintenance organizations and health insurers; and |
| • | other healthcare payors. |
Significant uncertainty exists as to the reimbursement status of newly approved healthcare products. Healthcare payors, including Medicare, are challenging the prices charged for medical products and services. Government and other healthcare payors increasingly attempt to contain healthcare costs by limiting both coverage and the level of reimbursement for drugs. Even if our product candidates are approved by the FDA, insurance coverage might not be available, and reimbursement levels might be inadequate, to cover our products. If government and other healthcare payors do not provide adequate coverage and reimbursement levels for our products, once approved, market acceptance of such products could be reduced.
Proposals to modify the current health care system in the U.S. to improve access to health care and control its costs are continually being considered by the federal and state governments. In March 2010, the U.S. Congress passed landmark healthcare reform legislation. We cannot predict what impact on federal reimbursement policies and regulatory compliance landscape this legislation will have in general or on our business specifically. Members of the U.S. Congress and some state legislatures are seeking to overturn at least portions of the legislation and the U.S. Supreme Court is scheduled to hear in March 2012 a case challenging the constitutionality of the legislation. We expect continued judicial and legislative review and assessment of this legislation and possibly alternative health care reform proposals. We cannot predict judicial results or whether new proposals will be made or adopted, when they may be adopted or what impact they may have on us if they are adopted.
Health administration authorities in countries other than the U.S. may not provide reimbursement for our products at rates sufficient for us to achieve profitability, or at all. Like the U.S., these countries could adopt health care reform proposals and could materially alter their government-sponsored health care programs by reducing reimbursement rates.
Any reduction in reimbursement rates under Medicare or private insurers or foreign health care programs could negatively affect the pricing of our products. If we are not able to charge a sufficient amount for our products, then our margins and our profitability will be adversely affected.
If we lose key management or scientific personnel, cannot recruit qualified employees, directors, officers, or other significant personnel or experience increases in our compensation costs, our business might materially suffer.
We are highly dependent on the services of our Chairman, Chief Executive Officer and acting Chief Medical Officer, Dr. Russell H. Ellison and our Chief Business Officer, Thomas Rowland. Our employment agreements with Dr. Ellison and Mr. Rowland do not ensure the retention of either. This is also true for our other management team members, both present and future.
| 51 |
Furthermore, our future success also depends, in part, on our ability to identify, hire, and retain additional management team members as our operations grow. We expect to experience intense competition for qualified personnel and might be unable to attract and retain the personnel necessary for the development of our business. Finally, we do not currently maintain, nor do we intend to obtain in the future, “key man” life insurance that would compensate us in the event of the death or disability of any of the members of our management team.
If we cannot enforce non-compete and confidentiality provisions applicable to our employees and consultants, our business might materially suffer.
We include a non-compete provision in any employment agreement we enter into with an employee including Dr. Ellison and Mr. Rowland, that runs during the term of the agreement and for six months after termination, and up to one year after termination if Mr. Rowland voluntarily resigns without good reason (as defined in his employment agreement). This non-compete provision was also included in employment agreements with our former chief medical officer and chief scientific officer, which have lapsed.
We include a confidentiality provision in any employment or consulting agreement we enter into with an employee or a consultant. The confidentiality provision runs during the term of the agreement and thereafter without limit. As a result, the confidentiality provisions contained in the employment agreements with our former chief medical officer and chief scientific officer remain in effect and are in effect under all of our current consulting agreements.
For future employees with whom we do not enter into an employment agreement, we will enter into a confidentiality agreement with the same provisions described above.
To be able to enforce these non-compete and confidentiality provisions we would need to know of any breach and have sufficient funds to enforce the provisions. We cannot assure you that we would know of or be able to afford enforcement of any breach. In addition, such provisions are subject to state law and interpretation by courts, which could limit the scope and duration of these provisions. Any limitation on or non-enforcement of these non-compete and confidentiality provisions could have an adverse effect on our business.
If we are unable to hire additional qualified personnel, our ability to grow our business might be harmed.
At December 31, 2011, we had seven employees, seven consultants and three contract research organizations with whom we have contracted to carry out our business plan. While we believe this will provide us with sufficient staffing to develop VEN 309 and VEN 307 through the fourth quarter of 2013, we will need to hire or contract with additional qualified personnel with expertise in clinical research and testing, government regulation, formulation and manufacturing and sales and marketing to commercialize VEN 309 and VEN 307 and to develop VEN 308. We compete for qualified individuals with numerous biopharmaceutical companies, universities and other research institutions. Competition for these individuals is intense, and we cannot be certain that our search for such personnel will be successful. Attracting and retaining qualified personnel will be critical to our success.
We might not successfully manage our growth.
Our success will depend upon the expansion of our operations and the effective management of our growth, which will place a significant strain on our current and future management and other administrative and operational resources. To manage this growth, we must expand our facilities, augment our operational, financial and management systems and hire and train additional qualified personnel. If we are unable to manage our growth effectively, our business would be harmed.
We might seek to develop our business through acquisitions of or investment in new or complementary businesses, products or technologies, and the failure to manage these acquisitions or investments, or the failure to integrate them with our existing business, could have a material adverse effect on us.
We might consider opportunities to acquire or invest in other technologies, products and businesses that might enhance our capabilities or complement our current product candidates. Potential and completed acquisitions and strategic investments involve numerous risks, including potential problems or issues associated with the following:
| 52 |
| • | assimilating the purchased technologies, products or business operations; |
| • | maintaining uniform standards, procedures, controls and policies; |
| • | unanticipated costs associated with the acquisition or investment; |
| • | diversion of our management’s attention from our preexisting business; |
| • | maintaining or obtaining the necessary regulatory approvals or complying with regulatory standards; and |
| • | adverse effects on existing business operations. |
We have no current commitments with respect to any acquisition or investment in other technologies or businesses. We do not know if we will identify suitable acquisitions, whether we will be able to successfully complete any acquisitions, or whether we will be able to successfully integrate any acquired product, technology or business into our business or retain key personnel, suppliers or collaborators.
Our ability to successfully develop our business through acquisitions would depend on our ability to identify, negotiate, complete and integrate suitable target businesses or technologies and obtain any necessary financing. These efforts could be expensive and time consuming and might disrupt our ongoing operations. If we are unable to efficiently integrate any acquired business, technology or product into our business, our business and financial condition might be adversely affected.
Risks Related to Our Regulatory and Legal Environment
We are subject to extensive and costly government regulation.
Product candidates employing our technology are subject to extensive and rigorous domestic government regulation including regulation by the FDA, the Centers for Medicare and Medicaid Services, other divisions of the U.S. Department of Health and Human Services, the U.S. Department of Justice, state and local governments, and their respective foreign equivalents. The FDA regulates the research, development, preclinical and clinical testing, manufacture, safety, effectiveness, record-keeping, reporting, labeling, storage, approval, advertising, promotion, sale, distribution, import, and export of pharmaceutical products. The FDA regulates small molecule chemical entities, whether administered orally, topically or by injection, as drugs, subject to an NDA, under the Federal Food, Drug, and Cosmetic Act. If products employing our technologies are marketed abroad, they will also be subject to extensive regulation by foreign governments, whether or not they have obtained FDA approval for a given product and its uses. Such foreign regulation might be equally or more demanding than corresponding U.S. regulation.
Government regulation substantially increases the cost and risk of researching, developing, manufacturing, and selling our products. The regulatory review and approval process, which includes preclinical testing and clinical trials of each product candidate, is lengthy, expensive, and uncertain. We or our collaborators must obtain and maintain regulatory authorization to conduct clinical trials and approval for each product we intend to market, and the manufacturing facilities used for the products must be inspected and meet legal requirements. Securing regulatory approval requires submitting extensive preclinical and clinical data and other supporting information for each proposed therapeutic indication in order to establish the product’s safety and efficacy for each intended use. The development and approval process might take many years, requires substantial resources, and might never lead to the approval of a product.
Even if we are able to obtain regulatory approval for a particular product, the approval might limit the intended medical uses for the product, limit our ability to promote, sell, and distribute the product, require that we conduct costly post-marketing surveillance, and/or require that we conduct ongoing post-marketing studies. Material changes to an approved product, such as, for example, manufacturing changes or revised labeling, might require further regulatory review and approval. Once obtained, any approvals might be withdrawn, including, for example, if there is a later discovery of previously unknown problems with the product, such as a previously unknown safety issue.
| 53 |
If we, our collaborators, or our contract manufacturers fail to comply with applicable regulatory requirements at any stage during the regulatory process, such noncompliance could result in, among other things, delays in the approval of applications or supplements to approved applications; refusal of a regulatory authority, including the FDA, to review pending market approval applications or supplements to approved applications; untitled letter or warning letters; fines; import and export restrictions; product recalls or seizures; injunctions; total or partial suspension of production; civil penalties; withdrawals of previously approved marketing applications or licenses; recommendations by the FDA or other regulatory authorities against governmental contracts; and/or criminal prosecutions.
We might not obtain the necessary U.S. or worldwide regulatory approvals to commercialize any product candidate.
We cannot assure you that we will receive the approvals necessary to commercialize for sale any of our product candidates, or any product candidate we acquire or develop in the future. We will need FDA approval to commercialize our product candidates in the U.S. and approvals from the FDA-equivalent regulatory authorities in foreign jurisdictions to commercialize our product candidates in those jurisdictions. In order to obtain FDA approval of any product candidate, we must submit to the FDA an NDA demonstrating that the product candidate is safe for humans and effective for its intended use. This demonstration requires significant research, pre-clinical studies, and clinical trials. Satisfaction of the FDA’s regulatory requirements typically takes many years, depends upon the type, complexity and novelty of the product candidate and requires substantial resources for research, development and testing. We cannot predict whether our research and clinical approaches will result in drugs that the FDA considers safe for humans and effective for their indicated uses. The FDA has substantial discretion in the drug approval process and might require us to conduct additional pre-clinical and clinical testing, perform post-marketing studies or otherwise limit or impose conditions on any approval we obtain. For example, in late April 2011, the FDA proposed that we include an additional one week treatment arm in our pivotal Phase III trials for VEN 309 to evaluate whether patients could be fully treated within seven days, in addition to the 14-day period we proposed testing. We agreed with the FDA and added the third arm, which increased the costs of the pivotal study.
The approval process might also be delayed by changes in government regulation, future legislation or administrative action or changes in FDA policy that occur prior to or during our regulatory review. Delays in obtaining regulatory approvals might:
| • | delay commercialization of, and our ability to derive product revenues from, our product candidates; |
| • | impose costly procedures on us; and |
| • | diminish any competitive advantages that we might otherwise enjoy. |
Even if we comply with all FDA requests, the FDA might ultimately reject one or more of our NDAs. We cannot be sure that we will ever obtain regulatory approval for our product candidates. Failure to obtain FDA approval of our product candidates will severely undermine our business by leaving us without a saleable product, and therefore without any source of revenues, until another product candidate could be developed or obtained. There is no guarantee that we will ever be able to develop or acquire another product candidate.
In foreign jurisdictions, we must receive approval from the appropriate regulatory authorities before we can commercialize any drugs. The risks associated with foreign regulatory approval processes are similar to the risks associated with the FDA approval procedures described above. We cannot assure you that we will receive the approvals necessary to commercialize our product candidates for sale outside the U.S.
| 54 |
Even if approved, our products will be subject to extensive post-approval regulation.
Once a product is approved, numerous post-approval requirements apply. Among other things, the holder of an approved NDA is subject to ongoing FDA oversight monitoring and reporting obligations, including obligations to monitor and report adverse events and instances of the failure of a product to meet the specifications in the NDA. Application holders must submit new or supplemental applications and obtain FDA approval for changes to the approved product, product labeling, or manufacturing process. Application holders also must submit advertising and other promotional material to the FDA and report on ongoing clinical trials. The FDA also has the authority to require changes in the labeling of approved drug products and to require post-marketing studies.
Advertising and promotional materials must comply with FDA rules in addition to other applicable federal and state laws. The distribution of product samples to physicians must comply with the requirements of the Prescription Drug Marketing Act. Manufacturing facilities remain subject to FDA inspection and must continue to adhere to the FDA’s cGMP requirements. Application holders must obtain FDA approval for product, manufacturing, and labeling changes, depending on the nature of the change. Sales, marketing, and scientific/educational grant programs, among other activities, must comply with the anti-fraud and abuse provisions of the Social Security Act, the False Claims Act, and similar state laws, each as amended. Pricing and rebate programs must comply with the Medicaid rebate requirements of the Omnibus Budget Reconciliation Act of 1990 and the Veteran’s Health Care Act of 1992, each as amended. If products are made available to authorized users of the Federal Supply Schedule of the General Services Administration, additional laws and requirements apply. All of these activities are also potentially subject to federal and state consumer protection and unfair competition laws.
Depending on the circumstances, failure to meet these post-approval requirements can result in criminal prosecution, fines, injunctions, recall or seizure of products, total or partial suspension of production, denial or withdrawal of pre-marketing product approvals, or refusal to allow us to enter into supply contracts, including government contracts. In addition, even if we comply with FDA and other requirements, new information regarding the safety or effectiveness of a product could lead the FDA to modify or withdraw product approval.
We face the risk of product liability claims and might not be able to obtain insurance.
Our business exposes us to the risk of product liability claims that are inherent in the development of drugs. If the use of one or more of our or our collaborators’ drugs harms people, we might be subject to costly and damaging product liability claims brought against us by clinical trial participants, consumers, health care providers, pharmaceutical companies or others selling our products. Our inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could prevent or inhibit the commercialization of pharmaceutical products we develop. We currently do not carry clinical trial insurance or product liability insurance for VEN 307. We obtained such insurance prior to beginning the Phase III trial for VEN 309. We cannot predict all of the possible harms or side effects that might result and, therefore, the amount of insurance coverage we hold now or in the future might not be adequate to cover all liabilities we might incur. We intend to expand our insurance coverage to include the sale of commercial products if we obtain marketing approval for our drug candidates in development, but we might be unable to obtain commercially reasonable product liability insurance for any products approved for marketing. If we are unable to obtain insurance at an acceptable cost or otherwise protect against potential product liability claims, we will be exposed to significant liabilities, which might materially and adversely affect our business and financial position. If we are sued for any injury allegedly caused by our or our collaborators’ products, our liability could exceed our total assets and our ability to pay the liability. A successful product liability claim or series of claims brought against us would decrease our cash and could cause the value of our common stock to decrease.
We might be exposed to liability claims associated with the use of hazardous materials and chemicals.
Our research, development and manufacturing activities and/or those of our third-party contractors might involve the controlled use of hazardous materials and chemicals. Although we will strive to have our safety procedures, and those of our contractors, for using, storing, handling and disposing of these materials comply with federal, state and local laws and regulations, we cannot completely eliminate the risk of accidental injury or contamination from these materials. In the event of such an accident, we could be held liable for any resulting damages, and any liability could materially adversely affect our business, financial condition and results of operations. In addition, the federal, state and local laws and regulations governing the use, manufacture, storage, handling and disposal of hazardous or radioactive materials and waste products might require us to incur substantial compliance costs that could materially adversely affect our business, financial condition and results of operations. We currently do not carry hazardous materials liability insurance. We intend to obtain such insurance in the future if necessary.
| 55 |
Risks Related to Our Intellectual Property
Our patent for the concentration range of VEN 309 may not issue and our existing composition of matter patent covering VEN 309 could be invalidated.
Different concentrations of a drug are separately patentable under certain circumstances. Because of unexpected differences between concentrations of the product that were observed in the clinical program (i.e. that 0.5% concentration is superior to a 0.25% and a higher 1.0% concentration in the comprehensive reduction in hemorrhoid symptoms), which data have not been previously published, on August 23, 2010, we filed method of use patent applications in the U.S. and internationally for VEN 309, claiming a specific concentration range. The patent, if issued, could be considered new art and provide patent protection for 20 additional years. However, if our existing composition of matter patent for VEN 309 is challenged by a third party and invalidated, and the concentration patent is never issued and even if issued is challenged by a third party, we would have only five years of U.S. data exclusivity under the Hatch-Waxman Act from the time VEN 309 is approved.
Our business depends on protecting our intellectual property.
If we and our licensor S.L.A. Pharma do not obtain protection for our respective intellectual property rights, our competitors might be able to take advantage of our research and development efforts to develop competing drugs.
Our success, competitive position and future revenues, if any, depend in part on our ability and the abilities of our licensors to obtain and maintain patent protection for our products, methods, processes and other technologies, to preserve our trade secrets, to prevent third parties from infringing on our proprietary rights and to operate without infringing the proprietary rights of third parties. To date, we hold some exclusive patent rights, including rights under U.S. patents and patent applications as well as rights under foreign patents and patent applications. We anticipate filing additional patent applications both in the U.S. and in other countries, as appropriate. However, the patent process is subject to numerous risks and uncertainties, and there can be no assurance that we will be successful in protecting our products by obtaining and defending patents. These risks and uncertainties include the following:
| • | Our patent rights might be challenged, invalidated, or circumvented, or otherwise might not provide any competitive advantage; |
| • | Our competitors, many of which have substantially greater resources than we do and many of which might make significant investments in competing technologies, might seek, or might already have obtained, patents that will limit, interfere with, or eliminate our ability to make, use, and sell our potential products either in the U.S. or in international markets; |
| • | As a matter of public policy regarding worldwide health concerns, there might be significant pressure on the U.S. government and other international governmental bodies to limit the scope of patent protection both inside and outside the U.S. for disease treatments that prove successful; and |
| • | Countries other than the U.S. might have less restrictive patent laws than those upheld by U.S. courts, allowing foreign competitors the ability to exploit these laws to create, develop, and market competing products. |
In addition, the U.S. Patent and Trademark Office and patent offices in other jurisdictions have often required that patent applications concerning pharmaceutical and/or biotechnology-related inventions be limited or narrowed substantially to cover only the specific innovations exemplified in the patent application, thereby limiting the scope of protection against competitive challenges. Thus, even if we or our licensors are able to obtain patents, the patents might be substantially narrower than anticipated.
| 56 |
In addition to patents, we also rely on trade secrets and proprietary know-how. Although we take measures to protect this information by entering into confidentiality and inventions agreements with our employees, scientific advisors, consultants, and collaborators, we cannot provide any assurances that these agreements will not be breached, that we will be able to protect ourselves from the harmful effects of disclosure if they are breached, or that our trade secrets will not otherwise become known or be independently discovered by competitors. If any of these events occurs, or we otherwise lose protection for our trade secrets or proprietary know-how, the value of this information may be greatly reduced.
Patent and other intellectual property protection is crucial to the success of our business and prospects, and there is a substantial risk that such protections will prove inadequate. Our business and prospects will be harmed if these protections prove insufficient.
Our non-compete with Amer, Dr. Sam Amer and his wife may not be enforceable.
As a condition to our purchase of VEN 309 from Amer, each of Amer, Dr. Sam Amer and his wife entered into a five-year non-compete agreement with us. The non-compete applies to the U.S. and its territories and anywhere else in the world where a patent has issued for VEN 309 and prohibits Amer, Dr. Amer and/or his wife, directly or indirectly, from owning an interest in, managing, operating, joining, controlling or participating in the ownership, management, operation or control of any profit or non-profit business or organization that conducts research, develops, formulates, tests, produces, licenses, commercializes, manufactures or distributes a product incorporating VEN 309 or any product which has the function of affecting the 5HT 2A receptor. The enforceability of non-competes is a matter of state law and courts generally look with disfavor on non-competes that are not narrowly drawn. California is particularly strict with the limitations that may be imposed by non-compete agreements and the geographic scope must be limited to the entity’s or individual’s “scope of business”. While we believe that the non-compete has been drafted to comply with California law, we cannot be certain that it will be enforced. However, Amer, Dr. Amer and his wife could challenge the non-compete in court or choose to violate it in which event we would have to sue to enforce it. Either situation would be costly, might distract the attention of our management and the court might not uphold the non-compete. Further, the milestone and royalty payments we must pay Amer are not contingent on compliance with the non-compete. If Amer, Dr. Amer and/or his wife competed against us in developing a product incorporating VEN 309, it could have a material adverse effect on our business.
We rely on trade secret protections through confidentiality agreements with our employees, customers and other parties, and the breach of these agreements could adversely affect our business and prospects.
We rely on trade secrets, which we seek to protect, in part, through confidentiality and non-disclosure agreements with our employees, collaborators, supplies, and other parties. There can be no assurance that these agreements will not be breached, that we would have adequate remedies for any such breach or that our trade secrets will not otherwise become known to or independently developed by our competitors. We might be involved from time to time in litigation to determine the enforceability, scope and validity of our proprietary rights. Any such litigation could result in substantial cost and divert management’s attention from our operations.
If we infringe the rights of third parties we might have to forgo selling our future products, pay damages, or defend against litigation.
If our product candidates, methods, processes and other technologies infringe the proprietary rights of other parties, we could incur substantial costs and we might have to:
| • | obtain licenses, which might not be available on commercially reasonable terms, if at all; |
| • | abandon an infringing product candidate; |
| • | redesign our products or processes to avoid infringement; |
| • | stop using the subject matter claimed in the patents held by others; | |
| 57 |
| • | pay damages; and/or |
| • | defend litigation or administrative proceedings which might be costly whether we win or lose, and which could result in a substantial diversion of our financial and management resources. |
Any of these events could substantially harm our earnings, financial condition and operations.
Risks Related to Our Common Stock
There are interlocking relationships among us and certain affiliates of Paramount Biosciences, LLC, which might present potential conflicts of interest.
Dr. Lindsay Rosenwald is the Chairman, Chief Executive Officer and sole stockholder of Paramount BioCapital, Inc., or Paramount, and is the sole member of Paramount BioSciences, LLC. We acquired the rights to VEN 307 and VEN 308 from Paramount BioSciences who had licensed them from S.L.A. Pharma. Dr. Rosenwald individually and through entities he controls beneficially owned as of December 31, 2011 approximately 7.8% of our issued and outstanding common stock, excluding any shares issuable upon the exercise of warrants.
In consideration of his guaranteeing the $800,000 promissory note we issued to Israel Discount Bank of New York in September 2010, we entered into a letter agreement with Dr. Rosenwald whereby Dr. Rosenwald had the right to attend our board meetings, which right he had not exercised since May 2011, and to appoint two directors to our board. Dr. Rosenwald had never exercised his right to appoint those directors. This agreement was terminated effective February 22, 2012.
As of December 31, 2011, we owed Paramount Corporate Development, LLC, an affiliate of Dr. Rosenwald’s, $100,000 for services previously rendered and for which there is no due date.
Generally, Delaware corporate law, under which we are governed, requires that any transactions between us and any of our affiliates be on terms that, when taken as a whole, are substantially as favorable to us as those then reasonably obtainable from a person who is not an affiliate in an arms-length transaction. We believe that the terms of our relationships with Dr. Rosenwald, Paramount BioSciences and their affiliates satisfy the requirement of Delaware law, but in the event that one or more parties challenges the fairness of such terms, we might have to expend substantial resources in resolving the challenge, and we can make no guarantees as to the result.
None of our affiliates, Paramount BioSciences, its affiliates or Dr. Rosenwald is obligated pursuant to any agreement or understanding with us to make any additional products or technologies available to us, nor can there be any assurance, and we do not expect and purchasers of our common stock should not expect, that any biomedical or pharmaceutical product or technology identified by such affiliates, Paramount BioSciences, its affiliates or Dr. Rosenwald in the future will be made available to us. In addition, certain of our current officers and directors or certain of any officers or directors hereafter appointed or elected might from time to time serve as officers or directors of other biopharmaceutical or biotechnology companies. There can be no assurance that such other companies will not have interests in conflict with our own.
Dr. Rosenwald could exert influence on our board of directors and the management of our company.
As of December 31, 2011, Dr. Rosenwald and his affiliates beneficially owned approximately 7.8% of our issued and outstanding capital stock, excluding any shares issuable upon the exercise of warrants. As a result, Dr. Rosenwald and his affiliates could exert influence on the election of our board of directors and the outcome of issues submitted to our stockholders, including any merger, consolidation, or sale of all or substantially all of our assets. The interests of Dr. Rosenwald and his affiliates might not coincide with the interests of other holders of our capital stock. This concentration of ownership may harm the value of our common stock by, among other things:
| • | delaying, deferring or preventing a change in control of our company; |
| 58 |
| • | impeding a merger, consolidation, takeover or other business combination involving our company; or |
| • | causing us to enter into transaction or agreements that are not in the best interests of all stockholders. |
We might not be able to maintain the listing of our common stock on the NASDAQ Capital Market.
Our common stock is listed on the NASDAQ Capital Market under the symbol “VTUS.” We might not be able to maintain the listing standards of that exchange. If we fail to maintain the listing requirements, our common stock might trade on the OTC Bulletin Board or in the “pink sheets” maintained by Pink OTC Markets, Inc. These alternative markets are generally considered to be markets that are less efficient and less broad than the NASDAQ Capital Market.
The price of our common stock might fluctuate significantly, and you could lose all or part of your investment.
Since we went public on December 22, 2010, the price of our common stock has fluctuated between $6.00 and $21.00. Volatility in the market price of our common stock might prevent you from being able to sell your shares of our common stock at or above the price you paid for such shares. The trading price of our common stock might be volatile and subject to wide price fluctuations in response to various factors, including:
| • | results of our clinical trials and other studies; |
| • | availability of capital; |
| • | future sales of our common stock; |
| • | sale of shares of our common stock by our significant stockholders or members of our management; |
| • | additions or departures of key personnel; |
| • | investor perceptions of us and the pharmaceutical industry; |
| • | issuance of new or changed securities analysts’ reports or recommendations, or the announcement of any changes to our credit rating; |
| • | success or failure of our product candidates; |
| • | introduction of new products or announcements of significant contracts, acquisitions or capital commitments by us or our competitors; |
| • | threatened or actual litigation and government investigations; |
| • | legislative, political or regulatory developments; |
| • | the overall performance of the equity markets; |
| • | actual or anticipated fluctuations in our quarterly financial and operating results; |
| • | general economic conditions; |
| • | changes in interest rates; and |
| • | changes in accounting standards, policies, guidance, interpretations or principles. |
| 59 |
These and other factors might cause the market price of our common stock to fluctuate substantially, which might limit or prevent investors from readily selling their shares of our common stock and might otherwise negatively affect the liquidity of our common stock. In addition, in recent years, the stock market has experienced significant price and volume fluctuations. This volatility has had a significant impact on the market price of securities issued by many companies across many industries. The changes frequently appear to occur without regard to the operating performance of the affected companies. Accordingly, the price of our common stock could fluctuate based upon factors that have little or nothing to do with our company, and these fluctuations could materially reduce our share price.
The requirements of being a public company adds to our operating costs and might strain our resources and distract our management.
As a public company, we face increased legal, accounting, administrative and other costs and expenses not faced by private companies. We are subject to the reporting requirements of the Securities Exchange Act of 1934, which requires that we file annual, quarterly and current reports with respect to our business and financial condition, and the rules and regulations implemented by the SEC, the Sarbanes-Oxley Act of 2002, and the NASDAQ Capital Market, each of which imposes additional reporting and other obligations on public companies. These rules and regulations increase our legal and financial compliance costs and make some activities more time-consuming and costly, although we are currently unable to estimate these costs with any degree of certainty. Complying with these requirements might divert management’s attention from other business concerns, which could have a material adverse effect on our prospects, business, and financial condition.
Additionally, the expenses incurred by public companies generally for reporting and corporate governance purposes have been increasing. These increased costs will require us to divert a significant amount of money that we could otherwise use to develop our product candidates or otherwise expand our business. If we are unable to satisfy our obligations as a public company, we could be subject to delisting of our common stock, fines, sanctions and other regulatory action and potentially civil litigation.
We do not intend to pay dividends for the foreseeable future and our stock may not appreciate in value.
We currently intend to retain our future earnings, if any, to finance the operation and growth of our business and do not expect to pay any cash dividends in the foreseeable future. As a result, the success of an investment in shares of our common stock will depend upon any future appreciation in its value. There is no guarantee that shares of our common stock will appreciate in value or that the price at which our stockholders have purchased their shares will be able to be maintained.
Several provisions of the Delaware General Corporation Law and our amended and restated certificate of incorporation and bylaws could discourage, delay or prevent a merger or acquisition, which could adversely affect the market price of our common stock.
Several provisions of the Delaware General Corporation Law and our amended and restated certificate of incorporation and bylaws could discourage, delay or prevent a merger or acquisition that stockholders may consider favorable, and the market price of our common stock could be reduced as a result. These provisions include:
| • | “blank check” preferred stock; |
| • | prohibiting us from engaging in a “business combination” with an “interested stockholder” for a period of three years after the date of the transaction in which the person became an interested stockholder unless certain provisions are met; |
| • | prohibiting cumulative voting in the election of directors; |
| • | limiting the persons who may call special meetings of stockholders; |
| 60 |
| • | establishing advance notice requirements for nominations for election to our board of directors or for proposing matters that can be acted on by stockholders at stockholder meetings; and |
| • | the ability of our board of directors to increase its size and fill vacancies. |
We will need additional financing to fund our activities in the future, which likely will dilute our stockholders.
We anticipate that we will incur operating losses for the foreseeable future. Additionally, we believe we will require substantial funds in the future to support our operations. We expect to seek equity or debt financings in the future to fund our operations. The issuance of additional equity securities, or convertible debt or other derivative securities, likely will dilute some if not all of our then existing stockholders, depending on the financing terms.
Shares eligible for registration for future sale, if and when sold may adversely affect the market price of our common stock, as the future market sale of a substantial amount of outstanding stock in the public marketplace could reduce the price of our common stock.
Holders of an aggregate of approximately 925,000 shares of our common stock issuable upon the exercise of warrants are entitled to rights to register the shares held by them under the Securities Act pursuant to registration rights granted to the holders of these securities. We intend to file in the near future a registration statement covering the resale of these shares. Any substantial sale of common stock by these holders after this offering may have an adverse effect on the market price of our common stock.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
We occupy space on the 5 th floor at 99 Hudson Street, New York, New York 10013. We rent this space pursuant to a lease that runs until June 2012. We believe our current facilities are suitable and adequate for our activities until such time as we hire a significant number of additional employees or consultants. We intend to renew the lease prior to its expiration.
Item 3. Legal Proceedings
We are not a party to any legal proceedings and we are not aware of any claims or actions pending or threatened against us. In the future, we might from time to time become involved in litigation relating to claims arising from our ordinary course of business.
Item 4. Mine Safety Disclosures
Not applicable.
| 61 |
PART II
Item 5. Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Our common stock is traded under the symbol “VTUS” and is quoted on the NASDAQ Capital Market. The following table sets forth the high and low sales prices for shares of our common stock, as reported by NASDAQ for the periods indicated.
| 2010 | ||||||||
| High | Low | |||||||
| First Quarter* | $ | — | $ | — | ||||
| Second Quarter* | $ | — | $ | — | ||||
| Third Quarter* | $ | — | $ | — | ||||
| Fourth Quarter | $ | 7.71 | $ | 6.00 | ||||
| 2011 | ||||||||
| High | Low | |||||||
| First Quarter | $ | 11.98 | $ | 5.75 | ||||
| Second Quarter | $ | 21.00 | $ | 11.02 | ||||
| Third Quarter | $ | 15.10 | $ | 7.84 | ||||
| Fourth Quarter | $ | 9.94 | $ | 6.96 | ||||
| * | Our common stock began trading on the NASDAQ Capital Market on December 17, 2010, on a “when-issued” basis. On December 23, 2010, the first trading day after the distribution, “when-issued” trading with respect to our common stock ended and “regular way” trading began. As a result, our stock was not listed in the first three quarters of 2010 and only listed for 10 trading days in the fourth quarter of 2010. |
On March 8, 2012, the closing price for the common stock as reported on the NASDAQ Capital Market was $10.79.
As of March 8, 2012, there were 125 stockholders of record, which excludes stockholders whose shares were held in nominee or street name by brokers.
Performance Graph
The following graph compares our cumulative total stockholder return from December 31, 2010 with those of the Nasdaq Composite Index and the Nasdaq Biotech Index and assumes that all dividends were reinvested. The graph assumes that U.S. $100 was invested on December 31, 2010 in (1) our common stock, (2) the Nasdaq Biotech Index and (3) the Nasdaq Composite Index. The measurement points utilized in the graph consist of the last trading day in each calendar year, which closely approximates the last day of our respective fiscal year. The historical stock performance presented below is not intended to and may not be indicative of future stock performance.
| 62 |
| Company/Peer Company/ Market | 12/31/2011 | 12/31/2010 | ||||||
| Ventrus Biosciences | $ | 120.97 | $ | 100.00 | ||||
| NASDAQ Biotechnology Index | $ | 111.81 | $ | 100.00 | ||||
| NASDAQ Composite Index | $ | 98.20 | $ | 100.00 | ||||
Dividend Policy
We currently do not plan to declare dividends on shares of our common stock in the foreseeable future. We expect to retain our future earnings, if any, for use in the operation and expansion of our business. The payment of cash dividends in the future, if any, will be at the discretion of our board of directors and will depend upon such factors as earnings levels, capital requirements, our overall financial condition and any other factors our board deems relevant.
Equity Compensation Plans
The information required by Item 4 of Form 10-K regarding equity compensation plans is incorporated herein by reference to “Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters” in this report.
| 63 |
Item 6. Selected Financial Data
The following selected consolidated financial data should be read in conjunction with “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and the financial statements and the notes thereto included elsewhere in this report.
Statement of Operations Data:
| Year Ended December 31, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Operating expenses | $ | 24,404 | $ | 4,766 | $ | 3,340 | $ | 7,164 | $ | 4,501 | ||||||||||
| Loss from operating | (24,404 | ) | (4,766 | ) | (3,340 | ) | (7,164 | ) | (4,501 | ) | ||||||||||
| Interest income | 76 | 6 | - | 13 | - | |||||||||||||||
| Interest expense | (419 | ) | (10,530 | ) | (1,199 | ) | (1,635 | ) | (67 | ) | ||||||||||
| Net loss | (24,747 | ) | (15,290 | ) | (4,539 | ) | (8,786 | ) | (4,568 | ) | ||||||||||
Balance Sheet Data:
| As of December 31, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (in thousands) | ||||||||||||||||||||
| Total assets | $ | 46,646 | $ | 14,616 | $ | 166 | $ | 210 | $ | 2,146 | ||||||||||
| Deferred financing costs, net | - | 26 | 69 | - | - | |||||||||||||||
| Total stockholders’ equity (deficiency) | 44,131 | 11,626 | (13,362 | ) | (8,907 | ) | (4,382 | ) | ||||||||||||
| 64 |
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation
Overview
We are a specialty pharmaceutical company currently focused on the development and commercialization of late-stage prescription drugs for gastrointestinal disorders, specifically hemorrhoid disease, anal fissures and fecal incontinence. We have in-licensed all of the products in our current pipeline.
We have several proprietary product candidates, one of which we own and two of which we have licensed that are in clinical development that address large market opportunities, including our most advanced product candidates, VEN 309 and VEN 307. VEN 309, a topical form of iferanserin which blocks a specific serotonin receptor (5HT 2A ), is being developed for the topical treatment of symptomatic hemorrhoids, where it can reduce the bleeding, itchiness, and pain associated with the condition. Approximately 12.5 million people in the U.S. currently suffer from symptomatic hemorrhoids and we are not aware of any FDA-approved prescription drugs for this condition. VEN 307 is a pre-mixed and pre-packaged proprietary topical formulation of the drug diltiazem which we are developing for the treatment of anal fissures. We estimate that over four million people in the U.S. currently suffer from anal fissures and that there are approximately 1.1 million office visits per year and yet, to our knowledge, there is only one drug with FDA approval for this condition.
We previously met with the FDA regarding our plans for the development of VEN 309, VEN 307 and VEN 308. We initiated one of two pivotal Phase III clinical trials in the U.S. with VEN 309 in August 2011 and intend to initiate a long-term carcinogenicity study. Depending on our assessment of the data generated by the Phase III trial, which is expected around June 2012, as well as on other factors, including our access to capital, clinical and regulatory considerations, and our assessment of the then-current state of our intellectual property estate, we intend to initiate and conduct the second Phase III trial, and a double blind recurrence trial which, together with the first study, a clinical pharmacology program, one 9-month and one 6-month toxicology study, and the carcinogenicity study (which we plan to complete after the second trial) will comprise the data needed to be able to submit an NDA to the FDA and analogous filings to authorities in Europe and Japan, which we anticipate could occur in 2014.
Our development partner for VEN 307, S.L.A. Pharma, began conducting a Phase III clinical trial with VEN 307 in Europe in November 2010. Enrollment was completed in December 2011 and data is expected in May 2012. At the same time, we are conducting a formulation program with contract manufacturers to create a new, improved formulation of topical diltiazem, with new intellectual property protections. We expect to receive the data from the first Phase III trial in Europe around May 2012 and aim to have important information from our formulation program around that time. Depending on our assessment of the data generated by this trial and on whether the new formulation is superior to the existing version, as well as on other factors, including our access to capital, clinical and regulatory considerations, and our assessment of the then-current state of our intellectual property estate, we intend to initiate either one additional Phase III trial in the U.S. with the existing formulation or two additional Phase III clinical trials in the U.S. with the new formulation, to be run in parallel. We anticipate that both program options could provide sufficient data for an NDA submission to the FDA in 2013.
Since our inception, we have had no revenue from product sales, and have funded our operations principally through debt financings, our initial public offering in 2010 and a public offering of our common stock in July 2011. Our operations to date have been primarily limited to organizing and staffing our company, licensing our product candidates, developing clinical trials for our product candidates, establishing manufacturing for our product candidates, maintaining and improving our patent portfolio and raising capital. We have generated significant losses to date, and we expect to continue to generate losses as we progress towards the commercialization of our product candidates, including VEN 307 and VEN 309. As of December 31, 2011, we had a deficit accumulated during the development stage of $67,529,084. Because we do not generate revenue from any of our product candidates, our losses will continue as we advance our product candidates towards regulatory approval and eventual commercialization. As a result, our operating losses are likely to be substantial over the next several years. We are unable to predict the extent of any future losses or when we will become profitable, if at all.
| 65 |
We believe that our existing cash will be sufficient to fund our projected operating requirements into the third quarter of 2013, while we anticipate receiving data from the key clinical trials with VEN 309 around June 2012 and VEN 307 in the second quarter of 2012. Until we can generate a sufficient amount of product revenue, if ever, we expect to finance future cash needs through public or private equity offerings, debt financings or corporate collaborations and licensing arrangements.
Financial Operations Overview
Critical Accounting Policies
Our management’s discussion and analysis of our financial condition and results of operations is based on our financial statements, which have been prepared in accordance with U.S. generally accepted accounting principles, or GAAP. Our significant accounting policies are more fully described in Note 2 to the December 31, 2011 audited financial statements included in this report. The following accounting policies are critical to fully understanding and evaluating our financial results.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires our management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the disclosure of contingent liabilities at the date of the financial statements as well as the reported revenue, if any, and expenses during the reporting periods. On an ongoing basis, management evaluates their estimates and judgments. Management bases estimates on historical experience and on various other factors that they believe are reasonable under the circumstances, the results of which form the basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results might differ from these estimates under different assumptions or conditions.
Stock-Based Compensation
We account for stock options granted to employees, measured at grant date, based on the estimated fair value of the award, which is recognized as expense over the employee’s requisite service period on a straight-line basis. We account for stock options and warrants granted to non-employees on a fair value basis. The initial non-cash charge to operations for nonemployee options and warrants with vesting are revalued at the end of each reporting period based upon the change in the fair value of the options and recognized as consulting expense over the related service period. For the purpose of valuing options and warrants granted to employees and non-employees, we use the Black-Scholes option pricing model. To determine the risk-free interest rate, we utilize the U.S. Treasury yield curve in effect at the time of grant with a term consistent with the expected term of the awards. We estimate the expected life of the options granted based on anticipated exercises in the future periods assuming the success of our business model as currently forecasted. For warrants and non-employee options, we use the contractual term of the warrant, the length of the note or option as the expected term. The expected dividend yield reflects our current and expected future policy for dividends on our common stock. The expected stock price volatility for our stock options will be calculated by examining historical volatilities for publicly traded industry peers as we do not now and for the near future will not have any significant trading history for our common stock. Forfeiture rates will be calculated based on the expected service period for our employees.
Research and Development Expense
Research and development expenses consist primarily of costs associated with: (i) internal costs associated with our development activities; (ii) payments we make to third party contract research organizations, contract manufacturers, and consultants; (iii) technology and intellectual property license costs; and (iv) patent reimbursements. All research and development is expensed as incurred. License fees and pre-approved milestone payments due under each research and development arrangement that are paid prior to regulatory approval are expensed when the license is entered into or the milestone is achieved.
| 66 |
Conducting a significant amount of research and development is central to our business model. Since our inception on October 7, 2005 to December 31, 2011, we incurred $39,529,243 in research and development expenses. Product candidates in later-stage clinical development generally have higher development costs than those in earlier stages of development, primarily due to the significantly increased size and duration of the clinical trials. Included in research and development expense is the purchase price we paid in 2011 for VEN 309, discussed below.
We extended into an asset purchase agreement with Amer (the “Purchase Agreement”) to acquire all rights, title and interest to VEN 309 (including patents, know how, other research materials and other indications), iferanserin inventory for use in clinical trials and a non-compete agreement. We paid $500,000 on execution of the agreement and $12 million at closing, which took place on November 14, 2011. Additionally, we paid Amer $50,000 on execution and $5,000 per month through the closing for the consulting service. Upon the closing of the acquisition, the Exclusive License Agreement that we had previously entered into with Amer terminated.
As part of the Purchase Agreement, the milestone payments from the original Exclusive License Agreement were reduced to an aggregate of $10.5 million as follows: $1.5 million upon the one year anniversary of FDA approval of our planned NDA for VEN 309; $750,000 upon the attainment of $20 million in cumulative net sales of VEN 309; $1.5 million upon the attainment of $50 million in cumulative net sales; $3.0 million upon the attainment of $75 million in cumulative net sales; and $3.75 million upon regulatory approval for over-the-counter sale of VEN 309. These aggregate milestone payments represent an approximately 50% reduction in the $20 million aggregate milestone payments under the Exclusive License Agreement.
Further, the royalties payable to Amer upon commercialization also were reduced to between 3.0% and 4.0% of net sales in the U.S., based on the level of net sales in the U.S., and between 1.0% and 1.33% of sales outside of the U.S., based on the level of gross sales outside the U.S. These royalty rates represent an approximately 66% decrease in the royalty fees that would have been due to Amer under the Exclusive License Agreement. We will pay Amer a minimum royalty of 50% of the royalties on the forecasted annual net sales in the U.S. and 50% of the royalties on the forecasted annual gross sales outside the U.S.
The Purchase Agreement also prohibits Amer, Dr. Amer and his wife for a period of five years after November 14, 2012 from directly or indirectly, owning an interest in, managing, operating, joining, controlling or participating in the ownership, management, operation or control of any profit or non-profit business or organization other than us that conducts research, develops, formulates, tests, produces, licenses, commercializes, manufactures or distributes a product incorporating VEN 309 or any other product which has the function of affecting the 5HT 2A receptor. The non-compete covers the United Sates and its territories and any other jurisdiction in the world where a patent has issued for iferanserin. We determined that the non-compete agreement had minimal value due to the age of Dr. and Mrs. Amer. Accordingly, we allocated the entire purchase price to in-process research and development and concluded that there is no alternative future use for these assets. Therefore, the entire $12.5 million has been recorded as a research and development expense in the statement of operations.
We plan to increase our research and development expenses for the foreseeable future in order to complete development of our two most advanced product candidates, VEN 309 and VEN 307. The following table summarizes the research and development expenses related to our two most advanced product candidates and other projects. The table reflects expenses directly attributable to each development candidate, which are tracked on a project basis.
| 67 |
| YE 2009 | YE 2010 | YE 2011 | Period from October 7, 2005 (inception) to December 31, 2011 | |||||||||||||
| VEN 307 | $ | 155,000 | $ | 1,309,501 | $ | 1,921,922 | $ | 5,723,923 | ||||||||
| VEN 309 | $ | 2,734,147 | $ | 379,237 | $ | 22,230,856 | $ | 30,670,839 | ||||||||
| Other | $ | 53,845 | $ | 161,928 | $ | 1,124,904 | $ | 3,134,481 | ||||||||
The process of conducting pre-clinical studies and clinical trials necessary to obtain FDA approval is costly and time consuming. The probability of success for each product candidate and clinical trial may be affected by a variety of factors, including, among others, the quality of the product candidate’s early clinical data, investment in the program, competition, manufacturing capabilities and commercial viability. As a result of the uncertainties discussed above, the uncertainty associated with clinical trial enrollments and the risks inherent in the development process, we are unable to determine with certainty the duration and completion costs of current or future clinical stages of our product candidates or when, or to what extent, we will generate revenues from the commercialization and sale of any of our product candidates. Development timelines, probability of success and development costs vary widely. Based on their current status, we anticipate that to complete the clinical trial process and commercialize our product candidates will cost approximately $20 million for VEN 307, $15 million for VEN 308 and $40 million for VEN 309. These estimates could change significantly depending on the progress, timing and results of non-clinical and clinical trials. We will need to raise additional funds in order to fully complete the development of VEN 307 and VEN 309.
Off-Balance Sheet Arrangements
Since our inception, we have not engaged in any off-balance sheet arrangements, including the use of structured finance, special purpose entities or variable interest entities.
Results of Operations
Comparison of the Years Ended December 31, 2011 and December 31, 2010
Research and Development Expense
Research and development expense was $25,277,682 for the year ended December 31, 2011, an increase of $23,427,016, or 1265%, from $1,850,666 for same period in 2010. The primary reason for the increase was our ability to increase development of VEN 309, which commenced after we received the proceeds from our initial public offering. We have incurred higher development costs due to initiation of the Phase III clinical trial as well as product development and manufacturing costs to support the clinical study. Additionally, the Company expensed $12,500,000 relating to the acquisition of title and rights to VEN 309 from Sam Amer.
General and Administrative Expense
General and administrative expense consists primarily of salaries, consulting fees and other related costs, professional fees for legal services and accounting services, insurance and travel expenses, as well as the option expense associated with the grants of options to our employees and directors in 2011. We expect that our general and administrative expenses will increase as we add additional personnel to continue our development plans and comply with the reporting obligations applicable to public companies.
General and administrative, or G & A, expense was $8,724,391 for the year ended December 31, 2011, an increase of $5,808,801, or approximately 199%, from $2,915,590 for the year ended December 31, 2010. We had limited operations and related operating expenses in the first half of 2010 due to the lack of funds. We began increasing our operating activities in the second half of 2010. The largest G&A expense incurred in the year 2011 was associated with stock-based compensation expense for employees, consultants and directors which increased by $3,550,080 as well as G & A salaries of $1,331,211 which did not exist in 2010.
| 68 |
Interest Expense
Interest expense was $418,991 for the year ended December 31, 2011, a decrease of $10,111,108, or 2.413%, from $10,530,099 for the year ended December 31, 2010. The decrease was primarily due to not incurring certain one-time charges in 2011, which we incurred in 2010. The one time charges in 2010 consisted of $6,001,496 associated with the conversion of convertible notes and $2,484,927 associated with amortization of debt discount on warrants issued with the 2010 notes. Interest expense paid or payable in cash was $1,329,925 for the year ended December 31, 2010. Interest paid or payable in cash was $277,324 for the year ended December 31, 2011.
Comparison of the Years Ended December 31, 2010 and December 31, 2009
Research and Development Expense
Research and development expense was $1,850,666 for the year ended December 31, 2010, a decrease of $1,092,324, or 59%, from $2,942,992 for the year ended December 31, 2009. The primary reason for the decrease was the contractual payment of approximately $1,600,000 that we expensed in 2009. The decrease was offset by the expense from the issuance of a warrant to purchase shares of our common stock issued to S.L.A. Pharma in August 2010 and additional shares of common stock issued to S.L.A. Pharma in December 2010 as a result of our initial public offering share price. These issuances were at a discount to the market price and therefore the warrants had a significant value. We expect to incur higher development costs in the future due to initiation of the Phase III clinical trial for VEN 307 as well as product development and manufacturing costs to support the clinical study.
General and Administrative Expense.
General and administrative expense was $2,915,590 for the year ended December 31, 2010, an increase of $2,518,352, or 634%, from $397,238 for the year ended December 31, 2009. The increase was primarily due to $2,298,782 of compensation expense related to options granted to our employees and directors in 2010. In addition, professional fees increased by approximately $210,000, or 98%, over 2009 due to the use of consultants to oversee our operations and prepare us for being a public company.
Interest Expense
Interest expense in 2010 consisted of interest incurred on the 5% related parties’ promissory notes from October 2005 to June 2008, the 8% related parties’ promissory notes from July 2008 to December 2010, the 10% Paramount Credit Partners notes from January 2009 to June 2010, the 8% senior convertible notes from December 2007 to December 2008, the 10% senior convertible notes from December 2008 to December 2010, the 8% 2010 senior convertible notes from February 2010 to December 2010, our letter of credit borrowings and interest due on our license fee payments. Additionally, interest expense included the beneficial conversion feature of conventional convertible debt that was converted below market value as well as amortization of debt discount and deferred financing costs, as well as the debt discount for warrants issued in connection with debt financings.
Liquidity and Capital Resources
Sources of Liquidity
As a result of our significant research and development expenditures and the lack of any FDA-approved products to generate product sales revenue, we have not been profitable and have generated operating losses since we were incorporated in October 2005. We have funded our operations through December 31, 2011 principally with debt (which in connection with the initial public offering, all of the convertible notes, and accrued interest thereon, were converted into common stock) and equity financing, including raising approximately $15.2 million in net proceeds in our initial public offering, which closed on December 22, 2010, and approximately $2.4 million in net proceeds upon the exercise on January 7, 2011 of the over-allotment option granted to the underwriter of our initial public offering. In addition, in July 2011, we raised $47.6 million in net proceeds in a registered public offering of our common stock.
| 69 |
On January 31, 2012, we filed a shelf registration statement with the Securities and Exchange Commission, or SEC, under which we may offer shares of our common stock and preferred stock, various series of debt securities and/or warrants to purchase any of such securities, either individually or in units, in one or more offerings, up to a total dollar amount of $100,000,000. The registration statement became effective as of February 10, 2012. As part of the shelf registration statement, we included a prospectus for an at-the-market common equity sales program for the sale of up to $20,000,000 of our common stock. No securities have been offered or sold pursuant to the shelf registration statement.
Net Cash Used in Operating Activities
Net cash used in operating activities was $25,068,831 for the year ended December 31, 2011 to fund our research and development program build out and general and administrative expenses. The net loss of $34,344,730 for the year ended December 31, 2011 was greater than cash used in operating activities by $9,275,899. The primary reason for the difference is attributed to a stock-based compensation charge of $6,973,766
Net Cash Used in Investing Activities
Net cash used in investing activities was $11,964 for the year ended December 31, 2011. The cash used was for computer equipment for new employees that we purchased in the first quarter.
Net Cash Provided by Financing Activities
Net cash provided by financing activities was $47,485,175 for the year ended December 31, 2011. Net cash provided by financing activities during the year ended December 31, 2011 consisted of the sale of common stock pursuant to the exercise of the over-allotment option issued to the underwriters of our IPO, through which we received net proceeds of $2,420,776. Additionally, we received $47,568,047 from our underwritten public offering in July 2011. We also received $288,732 from the exercise of warrants in 2011. Net cash provided by financing activities for the 12 months was reduced by $800,000 for the repayment of a promissory note and line of credit due to the Israel Discount Bank in the first quarter of 2011, repayment of Paramount Credit Partners, LLC note of $1,573,000, and $419,380 for repayment of debt facilities.
Funding Requirements
We expect to incur losses for the foreseeable future. We expect to incur increasing research and development expenses. We expect that our general and administrative expenses will also increase as we expand our finance and administrative staff, add infrastructure, and incur additional costs related to being a public company, including directors’ and officers’ insurance, investor relations programs, and increased professional fees. Our future capital requirements will depend on a number of factors, including the timing and outcome of clinical trials and regulatory approvals, the costs involved in preparing, filing, prosecuting, maintaining, defending, and enforcing patent claims and other intellectual property rights, the acquisition of licenses to new products or compounds, the status of competitive products, the availability of financing, and our success in developing markets for our product candidates.
Based on our cash position at December 31, 2011, and our analysis of our future development costs, we estimate our expected future expenditures related to product development, through our planned first pivotal Phase III trial for VEN 309 and the ongoing Phase III trial for VEN 307 in Europe, as follows:
| · | costs to complete the double blind portion of the Phase III clinical trial of VEN 309 in the treatment of hemorrhoids, carcinogenicity testing and developing new intellectual property: $20,000,000; |
| 70 |
| · | costs to complete the double blind portion of the Phase III clinical trial of VEN 307 in the treatment of anal fissures: $10,000,000; |
| · | payment to S.L.A. Pharma of our licensing obligations for VEN 307 of $41,500 per month until the filing of an NDA with the FDA, and $400,000 in development costs upon receipt of a quality controlled final study report for the Phase III clinical trial. |
We believe that our existing cash and cash equivalents will be sufficient to enable us to fund our operating expenses and capital expenditure requirements into the third quarter of 2013. We have based these estimates on assumptions that may prove to be wrong, and we could use our available capital resources sooner than we currently expect, which would cause us to require additional capital earlier. Because of the numerous risks and uncertainties associated with the development and commercialization of our product candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials.
We do not anticipate that we will generate product revenue for at least the next several years. In the absence of additional funding, we expect our continuing operating losses to result in increases in our cash used in operations over the next several quarters and years.
We may need to finance our future cash needs through public or private equity offerings, debt financings or corporate collaboration and licensing arrangements. We do not currently have any commitments for future external funding. We may need to raise additional funds more quickly if one or more of our assumptions prove to be incorrect or if we choose to expand our product development efforts more rapidly than we presently anticipate, and we may decide to raise additional funds even before we need them if the conditions for raising capital are favorable. We may seek to sell additional equity or debt securities or obtain a bank credit facility or other financing vehicle. The sale of additional equity or debt securities, if convertible, could result in dilution to our stockholders. The incurrence of indebtedness would result in increased fixed obligations and could also result in covenants that would restrict our operations.
Additional equity or debt financing or corporate collaboration and licensing arrangements may not be available on acceptable terms, if at all. If adequate funds are not available, we may be required to delay, reduce the scope of or eliminate our research and development programs, reduce our planned commercialization efforts or obtain funds through arrangements with collaborators or others that may require us to relinquish rights to certain product candidates that we might otherwise seek to develop or commercialize independently.
Cautionary Statement
We operate in a highly competitive environment that involves a number of risks, some of which are beyond our control. The following statement highlights some of these risks. For more detail, see “Item 1A. Risk Factors”.
Statements contained in this Form 10-K that are not historical facts, are or might constitute forward-looking statements under the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Although we believe the expectations reflected in such forward-looking statements are based on reasonable assumptions, our expectations might not be attained. Forward-looking statements involve known and unknown risks that could cause actual results to differ materially from expected results. Factors that could cause actual results to differ materially from our expectations expressed in the report include, among others: risks related to the costs, timing, regulatory review and results of our studies and clinical trials; our ability to obtain FDA approval of our product candidates; differences between historical studies on which we have based our planned clinical trials and actual results from our trials; our anticipated capital expenditures, our estimates regarding our capital requirements, and our need for future capital; our liquidity and working capital requirements; our expectations regarding our revenues, expenses and other results of operations; the unpredictability of the size of the markets for, and market acceptance of, any of our products, including VEN 309; our ability to sell any approved products and the price we are able realize; our need to obtain additional funding and our ability to obtain future funding on acceptable terms; our ability to retain and hire necessary employees and to staff our operations appropriately; our ability to compete in our industry and innovation by our competitors; our ability to stay abreast of and comply with new or modified laws and regulations that currently apply or become applicable to our business; estimates and estimate methodologies used in preparing our financial statements; the future trading prices of our common stock and the impact of securities analysts’ reports on these prices; and the risks set out in our filings with the SEC.
| 71 |
Item 7A. Quantitative and Qualitative Disclosures about Market Risk
Our primary exposure to market risk is interest income sensitivity, which is affected by changes in the general level of U.S. interest rates.
We do not believe that our cash and equivalents have significant risk of default or illiquidity. Under our current investment policies, we invest our cash and cash equivalents in money market funds which invest in short-term U.S. Treasury securities with insignificant rates of return. While we believe our cash and equivalents do not contain excessive risk, we cannot provide absolute assurance that in the future our investments will not be subject to adverse changes in market value. In addition, we maintain significant amounts of cash and equivalents at one or more financial institutions that are in excess of federally insured limits.
Inflation generally affects us by increasing our cost of labor and clinical trial costs. We do not believe that inflation has had a material effect on our results of operations during 2009, 2010 or 2011.
Our purchases of raw materials and finished goods are denominated in U.S. dollars. Consequently, we have not considered it necessary to use foreign currency contracts or other derivative instruments to manage changes in currency rates. We do not now, nor do we plan to, use derivative financial instruments for speculative or trading purposes. However, these circumstances might change.
Item 8. Financial Statements and Supplementary Data
The financial statements required to be filed pursuant to this Item 8 are appended to this report. An index of those financial statements is found on page F-1.
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosue
Not applicable.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
We maintain a system of disclosure controls and procedures, as defined in Exchange Act Rule 13a-15(e),which is designed to provide reasonable assurance that information, which is required to be disclosed in our reports filed pursuant to the Securities and Exchange Act of 1934, as amended (the “Exchange Act”), is accumulated and communicated to management in a timely manner. At the end of the period covered by this report, we carried out an evaluation, under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Exchange Act Rule 13a-15(b). Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer concluded that our disclosure controls and procedures as of the end of the period covered by this report were effective.
| 72 |
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rule 13a-15(f). Our internal control over financial reporting is designed to provide reasonable assurance to our management and board of directors regarding the preparation and fair presentation of published financial statements. A control system, no matter how well designed and operated, can only provide reasonable, not absolute, assurance that the objectives of the control system are met. Because of these inherent limitations, management does not expect that our internal controls over financial reporting will prevent all error and all fraud. Under the supervision and with the participation of our management, including our Chief Executive Officer and our Chief Financial Officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on our evaluation under the framework in Internal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2011.
The effectiveness of our internal control over financial reporting as of December 31, 2011, has been audited by EisnerAmper LLP, an independent registered public accounting firm, as stated in their report which is included on page F-3 herein.
Changes in Internal Control over Financial Reporting
During 2010 and 2011, we took the following measures to address the material weaknesses that we had identified in 2010 and improve our periodic financial statement reporting process:
| · | hired a permanent Chief Financial Officer in December 2010 (who had previously been serving as a consultant) to strengthen our internal staffing and technical expertise in financial accounting and reporting; |
| · | upgraded our accounting software system in the first quarter of 2011 |
| · | limited access to the accounting and information systems and related data to strengthen segregation of duties; |
| · | implemented in the fourth quarter of 2010 procedures and controls in the financial statement close process to improve the accuracy and timeliness of the preparation of quarterly and annual financial statements; |
| · | hired a controller in April 2011; |
| · | began third party proofing of our quarterly and annual financial statements during the second half of 2011; and |
| · | implemented recommendations of our outside consultants regarding our processes and controls. |
Our audit committee, board of directors and management discussed among themselves and with EisnerAmper LLP the material weaknesses identified in fiscal year 2010 and assigned the highest priority to correct the issue. We actively sought the guidance and expertise of external consultants to help evaluate and recommend a stronger internal control structure. We hired outside consultants after the second quarter of fiscal year 2011 who have reviewed and tested our internal controls. Based on recommendations by the consultants, management implemented additional procedures and refined controls. As of December 31, 2011, we believe we have effectively remediated the previously identified material weaknesses.
Other than the matters discussed above, there were no other significant changes in our internal control over financial reporting or in other factors that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Item 9B. Other Information
Not applicable.
| 73 |
PART III
Item 10. Directors, Executive Officers and Corporate Governance
We have adopted a written code of ethics and business conduct that applies to our directors, executive officers and all employees. We intend to disclose any amendments to, or waivers from, our code of ethics and business conduct that are required to be publicly disclosed pursuant to rules of the SEC by filing such amendment or waiver with the SEC. This code of ethics and business conduct can be found in the corporate governance section of our website, www.ventrusbio.com .
The other information required by this Item is incorporated by reference to the information under the sections captioned “Proposal No. 1 - Election of Directors,” “Section 16(A) Beneficial Ownership Reporting Compliance,” and “Corporate Governance and Board Matters.”
Item 11. Executive Compensation
Director Compensation
Our board of directors consists of five individuals. Four directors are independent as defined by Nasdaq rules: Anthony E. Altig, Mark Auerbach, Dr. Joseph Felder and Myron Holubiak. Our fifth director is Russell H. Ellison, who also is our President, Chief Executive Officer and Chairman of the Board.
The following table shows the compensation earned by each of our non-employee directors for the year ended December 31, 2011
Non-Employee Director Compensation in Fiscal 2011
| Name (1) | Fees Earned or Paid in Cash | Option Awards ($)(2)(3) | All Other Compensation ($) | Total ($) | ||||||||||||
| Mark Auerbach | $ | 30,000 | $ | 60,274 | -0- | $ | 90,274 | |||||||||
| Joseph Felder | 28,750 | 60,274 | -0- | 89,024 | ||||||||||||
| Myron Holubiak | 28,750 | 60,274 | -0- | 89,024 | ||||||||||||
| (1) | As of December 31, 2011, our non-employee directors held options to purchase the following number of shares of our common stock: Mr. Auerbach, 45,000 shares; Dr. Felder, 47,016 shares; and Mr. Holubiak, 45,000 shares. |
| (2) | Thomas Rowland was hired as our Chief Business Officer on September 1, 2011. He resigned from our Board of Directors on January 17, 2012. Mr. Rowland is not included in this table; the options he received as a director are disclosed in the column titled “Option Awards” and the directors fees he received are disclosed in the column titled “All Other Compensation” in the Summary Compensation Table below. |
| (3) | The reported amount in the table above of the stock option grants made in 2011 represents the aggregate grant date fair value of the options computed in accordance with FASB ASC Topic 718. |
In November 2010, our Board of Directors established the non-employee director compensation structure, which consists of cash and equity compensation. Upon a director’s first election to the Board, he or she will be granted an option to purchase 35,000 shares of our common stock. Each director will be granted an option annually for his or her prior year’s service on the Board in an amount to be determined by the Board. The grant to a director, who, at the time of the grant, has served less than a full year prior to the date of grant, will be pro-rated for that portion of the year actually served.
| 74 |
For most of 2011, the cash compensation will consist of an annual cash fee of $5,000. Committee members received an additional $5,000, the Chairs of the Nominating and Governance Committee and the Compensation Committee each received an additional $2,500 and the Chair of the Audit Committee received an additional $5,000. The cash compensation component was not effective until such time as we had raised cumulative net proceeds from equity financings (including our initial public offering) and partnership and licensing transactions of $20.0 million. This contingency was met on July 19, 2011, when we closed on our underwritten public offering.
In November 2011, our Board of Directors approved a change in non-employee director compensation after a review or our director compensation practice by an independent compensation consultant that compared our practice to those of comparable small public companies in the industry. Directors receive a grant of 10,000 options annually. Upon joining the Board of Directors, a new director will be granted 35,000 stock options; in the next calendar year, that director will receive no options; and in the next calendar year he will receive the 10,000 annual grant of options to all directors. Each non-employee director receives an annual cash fee of $40,000, payable quarterly. The chairman of the Audit Committee receives an additional annual cash fee of $5,000. This new cash compensation became effective in the fourth quarter of 2011.
In November 2011, our Board of Directors granted to each non-employee director a grant of 10,000 fully-vested stock options to compensate the non-employee directors for services provided in 2011.
EXECUTIVE COMPENSATION
Compensation Discussion and Analysis
Overview
This Compensation Discussion and Analysis explains our compensation philosophy, policies and practices with respect to our Chief Executive Officer, Chief Financial Officer and Chief Business Officer who are our only executive officers and who we refer to as our named executive officers. Our Board of Directors has delegated responsibility for creating and reviewing the compensation of our entire senior management team, including our named executive officers, to the Compensation Committee of our Board of Directors. The role of the Compensation Committee is to oversee our compensation and benefit plans and policies, to administer our equity incentive plans and to review and make recommendations to our Board of Directors, generally on an annual basis, regarding all compensation decisions for our named executive officers.
Compensation Objectives
Objectives of Executive Compensation Program
The Compensation Committee of our Board of Directors has responsibility for establishing and monitoring our executive compensation program. The primary objectives of the Compensation Committee with respect to executive compensation are to attract, retain and motivate executive officers who will make important contributions to the achievement of our business goals and success. The Compensation Committee believes that the most effective executive compensation program will reward the achievement of annual, long-term and strategic goals of our company. Our executive compensation program has been designed to link short- and long-term cash and equity incentives to the achievement of measurable corporate and individual performance objectives, and to align executives’ incentives with stockholder value creation. To achieve these objectives, the Compensation Committee has recommended that we maintain, and expects to continue to recommend further implementation of, compensation plans that tie a substantial portion of our named executive officers’ overall compensation to our research, development, and operational performance.
| 75 |
The Compensation Committee, with the input of management, develops our compensation plans by utilizing publicly available compensation data for national and regional companies in the biopharmaceutical industry. The Compensation Committee also considers competitive market practices based on the experience of the members of the Compensation Committee. While disparities in market capitalization, size, product pipeline and other factors may exist, we believe that the practices of national, regional and other companies in the biopharmaceutical industry provide us with appropriate comparative compensation guidance, because these companies operate in our same industry, tend to have similar organizational structures and tend to compete with us for executives and other employees. We select companies against which to measure our compensation practices in an informal manner and have not established a definitive group of peer companies against which we measure ourselves. The companies we select at any time depend on the data that is available to us, publically or otherwise, at the time we review our compensation practices.
Based on these overall objectives and philosophy, the Compensation Committee has designed an executive compensation program that generally seeks to bring base salaries and total executive compensation in line with the companies at a similar stage of clinical development represented in the compensation data we review. Our program allows the Compensation Committee to determine each component of an executive’s compensation based on a number of factors, including (a) the executive’s overall experience and skills (with an emphasis on particular industry experience), (b) the executive’s position and responsibilities in comparison to other executives at the company and (c) the demand within our market for the executive’s skills relative to other executives in our industry.
We have also implemented an annual performance program, under which annual corporate goals are proposed by management and approved by the Compensation Committee at the start of each calendar year. These corporate goals include the achievement of qualitative operational goals and predefined research and development milestones. Each goal is weighted as to importance by the Compensation Committee. The individual performance of our named executive officers is based on the level of achievement of a combination of corporate goals and goals related to their respective areas of responsibility. Annual bonuses granted to our named executive officers are tied to the achievement of these corporate goals. The Board of Directors, generally based on a recommendation of the Compensation Committee, approves all salary increases, as well as bonuses and stock option awards, if any, for named executive officers. Annual base salary increases and annual bonuses, to the extent granted, are generally implemented during the first calendar quarter of the year.
Components of our Executive Compensation Program
The principal components of our executive compensation program are base salary, annual bonus, and long-term incentives. Our Compensation Committee believes that each component of executive compensation must be evaluated and determined with reference to competitive market data, individual and corporate performance, our recruiting and retention goals, internal equity and consistency, and other information we deem relevant. We believe that in the biopharmaceutical industry stock option awards are a primary motivator in attracting and retaining executives, in addition to salary and cash incentive bonuses.
The components of our compensation package are set forth below.
Base Salary
We provide base salaries for our named executive officers to compensate them for their services rendered during the fiscal year. Base salaries for our named executive officers have been established based on their position and scope of responsibilities, their prior experience and training, and competitive market compensation data we review for similar positions in our industry.
Base salaries are reviewed periodically and may be increased for merit reasons based on the executive’s performance, for retention reasons or if the base salary is not competitive to salaries paid by comparative companies for similar positions. Additionally, we may adjust base salaries throughout the year for promotions or other changes in the scope or breadth of an executive’s role or responsibilities.
| 76 |
Annual Bonus
A significant element of the cash compensation of our named executive officers is an annual performance-based cash bonus. A named executive officer’s target bonus is generally set as a percentage of base salary to reward strong performance and retain his or her employment in a competitive labor market. In the case of Dr. Ellison and Mr. Rowland, their employment agreements provide an annual bonus of up to 50% and 20% of their base salary, respectively. For 2011, the Board of Directors established for Mr. Barrett a bonus of up to 25% of his salary. In August 2011, Mr. Barret's employment was amended to provide an annual bonus of up to 25% of his base salary. Bonuses are based on the achievement of significant company goals, including research, clinical development, financial, business development and operational milestones, with specific goals tailored to the executive officer’s area of responsibility. The performance goals generally are determined by our Compensation Committee in the first quarter of the calendar year but the bonuses are determined at the time bonuses are paid. Additionally, the Board of Directors or the Compensation Committee may increase or decrease an executive’s bonus payment (above or below the target) based on its assessment of the company’s and an executive’s individual performance during a given year. For 2011, annual bonuses were based on achievement of company goals related to development of VEN 309 and VEN 307, business development/strategic planning for VEN 309, VEN 307 and VEN 308, corporate development and financial operations. Each officer's potential bonus was weighted differently for each set of goals, depending on his respective area of responsibility. The development goals for VEN 309 and VEN 307 were met only partially, primarily due to a delay in enrollment for the Phase III trial for VEN 309. The strategic planning/business development goals were missed slightly in that no strategic plan was produced for VEN 308. All corporate development goals were met. For these three goals combined, Dr. Ellison achieved 10%, 18% and 10%, respectively, out of possible 50%, 20% and 10% weightings (for an overall 48% achievement, excluding financial operations goals, as explained below), Mr. Barrett achieved 7%, 18% and 10% out of a possible 10%, 20% and 10%, respectively (overall achievement of 65%, excluding financial operations goals), and Mr. Rowland achieved 7%, 68% and 10% out of a possible 10%, 75% and 10%, respectively (overall achievement of 90%, excluding financial operations goals). For the goal related to the development of VEN 309, Mr. Ellison voluntarily recommended that he receive no credit for the trial enrollment portion because the decision affecting enrollment was his responsibility and not within the other officers’ oversight and Dr. Ellison did not believe the other officers should be penalized. The resulting bonuses for Dr. Ellison, Mr. Barrett and Mr. Rowland were $90,000, $41,250 and $14,167, respectively; Mr. Rowland's bonus was prorated due to his having been hired on September 1, 2011. Because so much of the financial operations goal is related to meeting Sarbanes-Oxley goals, particularly requirements for internal controls over financial reporting due to be reported in our Annual Report on Form 10-K for the year ended December 31, 2011, the Board determined to defer consideration of that goal until after the Form 10-K has been filed.
Long-term Incentives
Our equity-based long-term incentive program is designed to align our named executive officers’ long-term incentives with stockholder value creation. We believe that long-term participation by our executive officers in equity-based awards is a critical factor in the achievement of long-term company goals and business objectives. Our 2010 Plan allows the grant to executive officers of stock options, as well as other forms of equity incentives, as part of our overall compensation program. Grants of options to our executive officers other than our Chief Executive Officer are recommended by the Chief Executive Officer and finalized by the Compensation Committee and/or the Board of Directors. Grants of options to our Chief Executive Officer are made by the Compensation Committee and/or the Board of Directors.
Dr. Ellison's and Mr. Barrett's employment agreements each originally contained a provision for an first incentive bonus of $250,000 in the event that our market capitalization exceeds $100 million for a period of 30 consecutive trading days with an average trading volume of the common stock during such period of at least 100,000 shares per trading day and a second incentive bonus of $500,000 in the event that our market capitalization exceeds $250 million for a period of 30 consecutive trading days with an average trading volume of the common stock during such period of at least 100,000 shares per trading day. The first threshold was met in August 2011 and this bonus was paid in September 2011. On August 24, 2011, we amended Dr. Ellison’s and Mr. Barrett’s employment agreements to provide that if the second market capitalization threshold is attained, the second market capitalization bonus will be paid in a combination of shares of our common stock worth $300,000 and $200,000 in cash. The valuation of the shares of common stock was determined by the closing price of our common stock as reported on NASDAQ on August 24, 2011 ($9.85), and resulted in a share amount to which each of Dr. Ellison and Mr. Barrett is entitled if the market capitalization threshold is attained of 30,457 shares of our common stock.
| 77 |
Other Compensation
We maintain broad-based benefits and perquisites that are provided to all eligible employees, including health insurance, life and disability insurance, dental insurance and paid vacation.
Severance and Change in Control Benefits
If the severance payments called for in the agreements for Dr. Ellison, Mr. Barrett and Mr. Rowland had been triggered on December 31, 2011, we would have been obligated to make the following payments:
| Name | Cash Payment Per Month (# of months paid) |
Benefits ($ per month) and (# of months paid) |
Number of Options that Would Vest (Market Value)(1) |
||||||||||
| Russell H. Ellison | $ | 31,250 | (6 mos) | $ | 0 | (6 mos) | (2) | 573,599 ($1,152,934) | |||||
| David J. Barrett | $ | 20,833 | (6 mos) | $ | 670 | (6 mos) | 305,920 ($614,899) | ||||||
| Thomas Rowland | $ | 20,833 | (6 mos) | $ | 1,919 | (6 mos) | 285,000 ($467,850) | ||||||
| (1) | The market value equals the difference between $8.01, the fair market value of the shares that could be acquired based on the closing sale price per share of our common stock on the Nasdaq Capital Market on December 30, 2011 (the last trading day of 2011), and the exercise prices for the underlying stock options. |
| (2) | Dr. Ellison does not participate in our health insurance plan. |
Pursuant to the stock option agreements between us and the named executive officers, in the event of a merger or other change in control of our company (as defined above), vesting of outstanding stock options will automatically accelerate.
Tax and Accounting Considerations
U.S. federal income tax generally limits the tax deductibility of compensation we pay to our named executive officers to $1.0 million in the year the compensation becomes taxable to the executive officers. There is an exception to the limit on deductibility for performance-based compensation that meets certain requirements. Although deductibility of compensation is preferred, tax deductibility is not a primary objective of our compensation programs. Rather, we seek to maintain flexibility in how we compensate our executive officers so as to meet a broader set of corporate and strategic goals and the needs of stockholders, and as such, we may be limited in our ability to deduct amounts of compensation from time to time. Accounting rules require us to expense the cost of our stock option grants. Because of option expensing and the impact of dilution on our stockholders, we pay close attention to, among other factors, the type of equity awards we grant and the number and value of the shares underlying such awards.
Pension Benefits
We do not maintain any qualified or non-qualified defined benefit plans. As a result, none of our named executive officers participate in or have account balances in qualified or non-qualified defined benefit plans sponsored by us. Our Compensation Committee may elect to adopt qualified or non-qualified benefit plans in the future if it determines that doing so is in our best interests.
| 78 |
Nonqualified Deferred Compensation
None of our named executive officers participate in or have account balances in nonqualified defined contribution plans or other non-qualified deferred compensation plans maintained by us. Our Compensation Committee may elect to provide our officers and other employees with non-qualified defined contribution or other non-qualified deferred compensation benefits in the future if it determines that doing so is in our best interests.
Summary Compensation Table
The following table sets forth all compensation earned in the fiscal years ended December 31, 2011, 2010 and 2009 by our named executive officers.
| Name and Principal Position | Year | Salary ($) | Bonus ($) | Stock awards ($) | Option awards (1) ($) | Non-equity incentive plan compensation (2) ($) | All other compensation ($) | Total ($) | ||||||||||||||||||||||||
| Russell H. Ellison, M.D. | 2011 | $ | 375,000 | $ | 325,000 | $ | - | $ | - | $ | 90,000 | $ | - | $ | 790,000 | |||||||||||||||||
| President and Chief | 2010 | - | - | - | 2,785,384 | - | 210,000 | (3) | 2,995,384 | |||||||||||||||||||||||
| Executive Officer | 2009 | - | - | - | - | - | - | - | ||||||||||||||||||||||||
| David J. Barrett | 2011 | $ | 250,000 | $ | 250,000 | $ | - | $ | - | $ | 41,250 | $ | - | $ | 541,250 | |||||||||||||||||
| Chief Financial Officer | 2010 | - | - | - | 1,485,541 | - | 105,000 | (3) | 1,590,541 | |||||||||||||||||||||||
| 2009 | - | - | - | - | - | - | - | |||||||||||||||||||||||||
| Thomas Rowland(4) | 2011 | $ | 83,333 | $ | - | $ | - | $ | 1,282,143 | $ | 15,000 | $ | 16,667 | (5) | $ | 1,397,143 | ||||||||||||||||
| Chief Business Officer | 2010 | - | - | - | 168,653 | - | - | 168,653 | ||||||||||||||||||||||||
| 2009 | 50,000 | - | - | - | - | 62,500 | (6) | 112,500 | ||||||||||||||||||||||||
| (1) | The reported amounts represent the grant date fair value of the award, computed in accordance with FASB ASC Topic 718. |
| (2) | Non-equity incentive plan compensation represents amounts paid as annual performance awards. |
| (3) | Consists of consulting fees. Prior to December 20, 2010, Dr. Ellison and Mr. Barrett served pursuant to consulting contracts and were not employees. |
| (4) | Mr. Rowland resigned in February 2009 and was rehired on September 1, 2011. |
| (5) | Consists of director’s fees. |
| (6) | Option granted when Mr. Rowland was a director. |
| (6) | Consists of severance payments. |
| 79 |
Grants of Plan-Based Awards
The following table provides information regarding grants of plan-based awards made to our named executive officers in 2011. The only plan-based awards granted were stock options; no non-equity awards were granted. All stock options granted to our named executive officers are incentive stock options, to the extent permissible under the Internal Revenue Code. The exercise price per share of each stock option granted to our named executive officers was equal to the fair market value of our common stock as determined in good faith by our Board of Directors on the date of the grant. All stock options listed below were granted under our 2010 Equity Incentive Plan.
| Estimated future payouts
under non-equity incentive plan awards | Estimated future payouts
under equity incentive plan awards | All other stock awards: number of shares of | All other option awards: number of securities underlying | Exercise or base price of option | Grant date fair value of stock and option | |||||||||||||||||||||||||||||||||||||||
| Name | Grant
date | Threshold
($) | Target
($) | Maximum
($) | Threshold
(#) | Target
(#) | Maximum
(#) | stock or
units (#) | options (#) (1) | awards
($/Sh) | awards (2) ($) | |||||||||||||||||||||||||||||||||
| Russell H. Ellison, M.D. | N.A. | $ | - | $ | 187,500 | $ | - | - | - | - | - | - | $ | - | $ | - | ||||||||||||||||||||||||||||
| David J. Barrett | N.A. | - | 62,500 | - | - | - | - | - | - | $ | - | $ | - | |||||||||||||||||||||||||||||||
| Thomas Rowland (3) | 1/25/11 | - | 16,665 | - | - | - | - | - | 250,000 | (4) | $ | 6.42 | $ | 1,282,143 | ||||||||||||||||||||||||||||||
| (1) | The named executive officers were each granted the number of options provided next to their names in the table. The option grant to Mr. Rowland vests one-third on the date of the grant and in three equal installments thereafter on the first, second and third anniversary grant dates. |
| (2) | The grant date fair value of the restricted stock and option awards is calculated in accordance with FASB ASC Topic 718. |
| (3) | Mr. Rowland resigned as our President and Chief Executive Officer in February 2009 and was rehired as our Chief Business Officer on September 1, 2011. |
| (4) | This award is a non-incentive stock option granted when Mr. Rowland was a director of the company and in charge of business development for the company in that role. |
Outstanding Equity Awards at December 31, 2011
The following table contains certain information concerning unexercised options for the named executive officers as of December 31, 2011.
| Name | Grant date | Number of securities underlying unexercised options exercisable(#) | Number of securities underlying unexercised options unexercisable(#) | Option exercise price ($) | Option expiration date | |||||||||||||||
| Russell H. Ellison, M.D.(1) | 12/22/10 | 382,399 | 191,200 | $ | 6.00 | 12/22/20 | ||||||||||||||
| David J. Barrett(2) | 12/22/10 | 203,948 | 101,974 | $ | 6.00 | 12/22/20 | ||||||||||||||
| Thomas Rowland (3) | 1/25/11 | 83,333 | 166,667 | $ | 6.42 | 1/25/21 | ||||||||||||||
| (1) | In respect of the awards granted to Dr. Ellison, one-third of the options vest on the grant date, one-third vest on the first anniversary of the grant date and one-third vest on the second anniversary of the grant date. |
| 80 |
| (2) | In respect of the awards granted to Mr. Barrett, one-third of the options vest on the grant date, one-third vest on the first anniversary of the grant date and one-third vest on the second anniversary of the grant date. |
| (3) | In respect of the awards granted to Mr. Rowland, one-third of the options vested on the date of grant and the remaining amount vests in three equal installments on the first, second and third anniversary dates of the grant date. |
Option Exercises
None of our named officers exercised any stock options during the year ended December 31, 2011.
Option Repricings
We did not engage in any repricings or other modifications to any of our named executive officers’ outstanding options during the year ended December 31, 2011.
Employment Arrangements
Chief Executive Officer
Dr. Ellison serves as our Chief Executive Officer pursuant to an amended and restated employment agreement, which became effective upon the closing of our initial public offering on December 22, 2010. The agreement has a term of three years. The employment agreement provides for a base salary of $375,000 per year, a guaranteed bonus of $75,000 per year and an annual performance-based bonus of up to 50% of his base salary based on financial, clinical development and business milestones established by the Board. The agreement also provides a first incentive bonus of $250,000 in the event that our market capitalization exceeds $100 million for a period of 30 consecutive trading days with an average trading volume of the common stock during such period of at least 100,000 shares per trading day. This threshold was met in August 2011 and this bonus was paid in September 2011. The agreement also originally provided for a second incentive bonus of $500,000 in the event that our market capitalization exceeds $250 million for a period of 30 consecutive trading days with an average trading volume of the common stock during such period of at least 100,000 shares per trading day. On August 24, 2011, we amended Dr. Ellison’s employment agreement to provide that if the second market capitalization threshold is attained, the second market capitalization bonus will be paid in a combination of shares of our common stock worth $300,000 and $200,000 in cash. The valuation of the shares of common stock was determined by the closing price of our common stock as reported on NASDAQ on August 24, 2011 ($9.85), and resulted in a share amount to which Dr. Ellison is entitled if the market capitalization threshold is attained of 30,457 shares of our common stock.
Also, pursuant to the agreement, at the completion of our initial public offering, Dr. Ellison received a grant of options to purchase 573,599 shares of our common stock at the initial public offering price of $6.00. This amount was equal to 7.5% of our fully diluted capitalization on that date, giving effect to all shares issuable under all convertible notes, warrants and options outstanding on such date, but excluding shares reserved for grants not issued under our 2007 Stock Incentive Plan. One third of the options vested on grant, one-third vests one year later and the remainder vests two years after grant.
Under the employment agreement, Dr. Ellison is prohibited for six months after termination from (a) engaging in any business within a restricted territory that develops or commercializes prescription drugs for the specific disease treatment of hemorrhoids, anal fissures, and fecal incontinence or other products that compete with products we are developing or selling at the time of his termination, (b) soliciting or working within a restricted territory with our competitors or any other entity that could benefit from Dr. Ellison’s use of our confidential information, (c) becoming financially interested with one of our competitors within a restricted territory, (d) soliciting or accepting business from our competitors, and (e) inducing any employee or consultant of ours to terminate employment or a contractual relationship with us, provided that these provisions will not apply if we do not renew the agreement.
Set forth below is a description of the potential payments we will need to make upon termination of Dr. Ellison’s employment or upon a change in control of our company.
| 81 |
Termination due to Death, Disability or Change of Control
If Dr. Ellison’s employment is terminated as a result of his death, disability or change of control, we must pay him or his estate, as applicable, his then current base salary for a period of six months following the date of termination and any earned but unpaid incentive bonus and expense reimbursement through the date of his death or disability. All stock options that are scheduled to vest on the next succeeding anniversary of the effective date of the agreement will be accelerated and deemed to have vested as of the termination date. All stock options that have not vested or been deemed to have vested as of the date of termination will be forfeited. Stock options that have vested as of Dr. Ellison’s termination will remain exercisable for 360 days following his termination.
Termination by us For Cause or by Dr. Ellison without Good Reason
If we terminate Dr. Ellison for cause (as defined in the agreement) or if he terminates without good reason (as defined in the agreement), we must pay his then current base salary through the date of his termination and any expense reimbursement amounts owed through the date of termination.
Termination by us for other than Cause or by Dr. Ellison for Good Reason
If Dr. Ellison’s employment is terminated by us other than for cause or by Dr. Ellison for good reason (as defined in the agreement), then we must pay Dr. Ellison his then current base salary and all fringe benefits for a period of six months following such termination, any accrued but unpaid bonus, and any expense reimbursement amounts owed through the date of termination. All stock options granted to Dr. Ellison that are scheduled to vest by the end of the term of the employment agreement will be accelerated and deemed to have vested as of the termination date. Stock options that have vested as of Dr. Ellison’s termination will remain exercisable for 360 days following his termination.
In the agreement, the term “change in control” is defined generally as the acquisition by any person of more than 50% of the voting power of our then outstanding securities, and/or the merger or consolidation of our company or the sale of any or substantially all of our assets.
In the agreement, the term “cause” is defined generally as follows: (i) willful failure, disregard or continuing refusal by Dr. Ellison to perform his duties; (ii) willful, intentional or grossly negligent act by Dr. Ellison that injures, in a material way, our business or reputation; (iii) insubordination by Dr. Ellison with respect to lawful directions received from our Board of Directors; (iv) indictment for any felony or a misdemeanor involving moral turpitude; (v) Dr. Ellison engaging in some form of harassment prohibited by law; (vi) any misappropriation or embezzlement of our property; (vii) willful violation of the noncompetition, non-solicitation and confidentiality provisions of the agreement; and/or (viii) breach by Dr. Ellison of any other provision of the agreement that, if capable of being cured, is not cured by him within 30 days.
In the agreement, the term “good reason” is defined generally as: (i) the assignment to Dr. Ellison of duties materially inconsistent with his position and duties as chief executive officer; (ii) any reduction by us of Dr. Ellison’s compensation or benefits; and/or (iii) any requirement that he relocate outside a 30 mile radius of New York, New York.
From June 2010 to December 22, 2010, Dr. Ellison served as our Chief Executive Officer pursuant to a consulting agreement, pursuant to which he was paid $30,000 per month.
| 82 |
Chief Financial Officer
Mr. Barrett serves as our Chief Financial Officer pursuant to an amended and restated employment agreement that became effective upon the closing of our initial public offering on December 22, 2010. The agreement has a term of three years. The employment agreement provides for base salary of $250,000 per year. The agreement also provides a first incentive bonus of $250,000 in the event that our market capitalization exceeds $100 million for a period of 30 consecutive trading days with an average trading volume of the common stock during such period of at least 100,000 shares per trading day. This threshold was met in August 2011 and this bonus was paid in September 2011. The agreement also originally provided for a second incentive bonus of $500,000 in the event that our market capitalization exceeds $250 million for a period of 30 consecutive trading days with an average trading volume of the common stock during such period of at least 100,000 shares per trading day. On August 24, 2011, we amended Mr. Barrett’s employment agreement to provide that if the second market capitalization threshold is attained, the second market capitalization bonus will be paid in a combination of shares of our common stock worth $300,000 and $200,000 in cash. The valuation of the shares of common stock was determined by the closing price of our common stock as reported on NASDAQ on August 24, 2011 ($9.85), and resulted in a share amount to which Mr. Barrett is entitled if the market capitalization threshold is attained of 30,457 shares of our common stock.
Also, pursuant to the agreement, at the completion of our initial public offering, Mr. Barrett received a grant of options to purchase 305,920 shares of our common stock at the initial public offering price of $6.00. This amount was equal to 4.0% of our fully diluted capitalization on the date the employment agreement became effective, giving effect to all shares issuable under all convertible notes, warrants and options outstanding on such date, but excluding shares reserved for grants not issued under our 2010 Equity Incentive Plan. One-third of the options vested on grant, one-third vests one year later and the remainder vests two years after grant.
Under the employment agreement, Mr. Barrett is prohibited for six months after termination from (a) engaging in any business within a restricted territory that develops or commercializes prescription drugs for the specific disease treatment of hemorrhoids, anal fissures, and fecal incontinence or other products that compete with products we are developing or selling at the time of his termination, (b) soliciting or working within a restricted territory with our competitors or any other entity that could benefit from Mr. Barrett’s use of our confidential information, (c) becoming financially interested with one of our competitors within a restricted territory, (d) soliciting or accepting business from our competitors, and (e) inducing any employee or consultant of ours to terminate employment or a contractual relationship with us.
Set forth below is a description of the potential payments we will need to make upon termination of Mr. Barrett’s employment or upon a change in control of our company.
Termination due to Death, Disability or Change of Control
If Mr. Barrett’s employment is terminated as a result of his death, disability or change of control, we must pay him or his estate, as applicable, his then current base salary for a period of six months following the date of termination and any earned but unpaid incentive bonus and expense reimbursement through the date of his death or disability. All stock options that are scheduled to vest on the next succeeding anniversary of the effective date of the agreement will be accelerated and deemed to have vested as of the termination date. All stock options that have not vested or been deemed to have vested as of the date of termination will be forfeited. Stock options that have vested as of Mr. Barrett’s termination will remain exercisable for 360 days following his termination.
Termination by us For Cause or by Mr. Barrett without Good Reason
If we terminate Mr. Barrett for cause (as defined in the agreement) or if he terminates without good reason (as defined in the agreement), we must pay his then current base salary through the date of his termination and any expense reimbursement amounts owed through the date of termination.
Termination by us for other than Cause or by Mr. Barrett for Good Reason
If Mr. Barrett’s employment is terminated (i) by us other than for cause or (ii) by Mr. Barrett for good reason (as defined in the agreement), then we must (1) continue to pay Mr. Barrett his then current base salary and all fringe benefits for a period of six months following such termination, (2) any expense reimbursement amounts owed through the date of termination, (3) pay any accrued but unpaid bonus and (4) all stock options granted to Mr. Barrett that are scheduled to vest by the end of the term of the employment agreement shall be accelerated and deemed to have vested as of the termination date. Stock options that have vested as of Mr. Barrett’s termination will remain exercisable for 360 days following his termination.
| 83 |
In the agreement, the term “change in control” is defined generally as the acquisition by any person of more than 50% of the voting power of our then outstanding securities; and/or the merger or consolidation of our company or the sale of any or substantially all of our assets.
In the agreement, the term “cause” is defined generally as follows: (i) willful failure, disregard or continuing refusal by Mr. Barrett to perform his duties; (ii) willful, intentional or grossly negligent act by Mr. Barrett that injures, in a material way, our business or reputation; (iii) insubordination by Mr. Barrett with respect to lawful directions received from our Board of Directors; (iv) indictment for any felony or a misdemeanor involving moral turpitude; (v) Mr. Barrett engaging in some form of harassment prohibited by law; (vi) any misappropriation or embezzlement of our property; (vii) willful violation of the noncompetition, nonsolicitation and confidentiality provisions of the agreement; and/or (viii) breach by Mr. Barrett of any other provision of the agreement that, if capable of being cured, is not cured by him within 30 business days.
In the agreement, the term “good reason” is defined generally as: (i) the assignment to Mr. Barrett of duties materially inconsistent with his position and duties as chief financial officer; (ii) any reduction by us of Mr. Barrett’s compensation or benefits; and/or (iii) any requirement that he relocate outside a 30 mile radius of New York, New York.
From June 2010 to December 22, 2010, Mr. Barrett served as our Chief Financial Officer pursuant to a consulting agreement, pursuant to which he was paid $15,000 per month.
Chief Business Officer
Mr. Rowland serves as our Chief Business Officer pursuant to an employment agreement that became effective on September 1,. 2011. The agreement runs until December 31, 2013. The employment agreement provides for base salary of $250,000 per year.
Under the employment agreement, Mr. Rowland is prohibited for six months after termination from (a) engaging in any business within a restricted territory that develops or commercializes prescription drugs for the specific disease treatment of hemorrhoids, anal fissures, and fecal incontinence or other products that compete with products we are developing or selling at the time of his termination, (b) soliciting or working within a restricted territory with our competitors or any other entity that could benefit from Mr. Rowland’s use of our confidential information, (c) becoming financially interested with one of our competitors within a restricted territory, (d) soliciting or accepting business from our competitors, and (e) inducing any employee or consultant of ours to terminate employment or a contractual relationship with us.
Set forth below is a description of the potential payments we will need to make upon termination of Mr. Rowland’s employment or upon a change in control of our company.
Termination due to Death, Disability or Change of Control
If Mr. Rowland’s employment is terminated as a result of his death, disability or change of control, we must pay him or his estate, as applicable, his then current base salary for a period of six months following the date of termination and any earned but unpaid incentive bonus and expense reimbursement through the date of his death or disability. All stock options that are scheduled to vest on the next succeeding anniversary of the effective date of the agreement will be accelerated and deemed to have vested as of the termination date. All stock options that have not vested or been deemed to have vested as of the date of termination will be forfeited. Stock options that have vested as of Mr. Rowland’s termination will remain exercisable for 360 days following his termination.
Termination by us For Cause or by Mr. Rowland without Good Reason
If we terminate Mr. Rowland for cause (as defined in the agreement) or if he terminates without good reason (as defined in the agreement), we must pay his then current base salary through the date of his termination and any expense reimbursement amounts owed through the date of termination.
| 84 |
Termination by us for other than Cause or by Mr. Rowland for Good Reason
If Mr. Rowland’s employment is terminated (i) by us other than for cause or (ii) by Mr. Rowland for good reason (as defined in the agreement), then we must (1) continue to pay Mr. Rowland his then current base salary and all fringe benefits for a period of six months following such termination, (2) any expense reimbursement amounts owed through the date of termination, (3) pay any accrued but unpaid bonus and (4) all stock options granted to Mr. Rowland that are scheduled to vest by the end of the term of the employment agreement shall be accelerated and deemed to have vested as of the termination date. Stock options that have vested as of Mr. Rowland’s termination will remain exercisable for 360 days following his termination.
In the agreement, the term “change in control” is defined generally as the acquisition by any person of more than 50% of the voting power of our then outstanding securities; and/or the merger or consolidation of our company or the sale of any or substantially all of our assets.
In the agreement, the term “cause” is defined generally as follows: (i) willful failure, disregard or continuing refusal by Mr. Rowland to perform his duties; (ii) willful, intentional or grossly negligent act by Mr. Rowland that injures, in a material way, our business or reputation; (iii) insubordination by Mr. Rowland with respect to lawful directions received from our Board of Directors; (iv) indictment for any felony or a misdemeanor involving moral turpitude; (v) Mr. Rowland engaging in some form of harassment prohibited by law; (vi) any misappropriation or embezzlement of our property; (vii) willful violation of the noncompetition, nonsolicitation and confidentiality provisions of the agreement; and/or (viii) breach by Mr. Rowland of any other provision of the agreement that, if capable of being cured, is not cured by him within 30 business days.
In the agreement, the term “good reason” is defined generally as: (i) the assignment to Mr. Rowland of duties materially inconsistent with his position and duties as chief financial officer; and/or (ii) any reduction by us of Mr. Rowland’s compensation or benefits.
Employee Benefit Plans
Our only employee benefit plan, other than customary health insurance, is our 2010 Equity Incentive Plan, which is discussed below.
2010 Equity Incentive Plan
Eligibility and Administration. All of our employees, directors and consultants are eligible to receive incentive awards under the 2010 Plan. Incentive awards under the 2010 Plan can include incentive stock options, nonstatutory stock options, stock appreciation rights, stock awards and restricted stock. The 2010 Plan can be administered by our Board of Directors or a committee appointed to administer the plan, and is currently administered by our Compensation Committee, referred to herein as the “administrator.” Subject to the restrictions of the 2010 Plan, the administrator determines who is granted incentive awards under the 2010 Plan, the terms granted, including the exercise price, the number of shares subject to the incentive award and the incentive award’s exercisability.
Stock Options. The 2010 Plan provides for the grant of “incentive stock options” within the meaning of Section 422 of the Internal Revenue Code of 1986, as amended, or the Code, solely to employees (including officers and employee directors), and nonstatutory stock options to employees, directors and consultants.
The exercise price of options granted under the 2010 Plan is determined on the date of grant, and in the case of incentive stock options must be at least 100% of the fair market value per share at the time of grant. The exercise price of any incentive stock option granted to an employee who owns stock possessing more than 10% of the voting power of our outstanding capital stock must equal, at least 110% of the fair market value of the common stock on the date of grant. The aggregate fair market value of common stock (determined as of the date of the option grant) for which incentive stock options may for the first time become exercisable by any individual in any calendar year may not exceed $100,000. Payment of the exercise price may be made by delivery of cash or a check, or, in the discretion of the administrator, the exercise price may be paid through any other form of consideration and method of payment permitted by law and the 2010 Plan, including the delivery of shares of already-owned shares of our common stock and the surrender of certain shares subject to the stock option.
| 85 |
Options granted to employees, directors and consultants under the 2010 Plan generally become exercisable in increments, based on the optionee’s continued employment or service with us, over a period of three years. The term of an incentive stock option may not exceed 10 years. Options granted under the 2010 Plan, whether incentive stock options or nonstatutory options, generally expire 10 years from the date of grant, except that incentive stock options granted to an employee who owns stock possessing more than 10% of the voting power of our outstanding capital stock are not exercisable for longer than five years after the date of grant.
Stock Appreciation Rights. A stock appreciation right, referred to as a SAR, is the right to receive, in cash or common stock, all or a portion of the difference between the fair market value of a share of our common stock at the time of exercise of the SAR and the exercise price of the SAR established by the administrator, subject to such terms and conditions set forth in a SAR agreement. A SAR may be granted in connection with a stock option or alone, without reference to any related stock option.
The exercise price of a SAR granted under the 2010 Plan is determined on the date of grant, and shall not be less than 100% of the fair market value of a share of our common stock on the date of grant. SARs granted under the 2010 Plan would generally become exercisable in increments, based on the recipient’s continued employment or service with us, over a period of four years. The term of a SAR may not exceed 10 years from the date of grant.
Stock Awards and Restricted Stock. Shares of common stock may be sold or awarded to participants under the 2010 Plan as an incentive for the performance of past or future services to us. The administrator may determine the purchase price to be paid for such stock, if any, and other terms of such purchase or award.
Transferability. Except for transfers made by will or the laws of descent and distribution in the event of the holder’s death, no incentive stock option may be transferred, pledged or assigned by the holder thereof. Stock options, SARs, restricted stock or other awards may be transferred, pledged or assigned by the holder thereof to “family members” (as defined in the 2010 Plan), or by will or the laws of descent and distribution in the event of the holder’s death. We are not required to recognize any attempted assignment of such rights by any participant that is not in compliance with the 2010 Plan. During a participant’s lifetime, an incentive stock option may be exercised only by him or her or by his or her guardian or legal representative.
Change of Control. Generally, in the event of our consolidation or merger with or into another corporation or a sale of all or substantially all of our assets, referred to herein as an “acquisition,” whereby the acquiring entity or our successor does not agree to assume the incentive awards or replace them with substantially equivalent incentive awards, all outstanding options, SARs, restricted stock or other stock rights will vest and will become immediately exercisable in full and, if not exercised on the date of the acquisition, will terminate on such date regardless of whether the participant to whom such stock rights have been granted remains in our employ or service or of any acquiring or successor entity. In the event of an acquisition in which the acquiring entity agrees to assume the incentive awards, and, 60 days prior to the acquisition or 180 days after the acquisition, the holder of an award is terminated as an employee or consultant other than for cause or the holder terminates his or her employment for good reason, then upon such termination any incentive award held by the holder will vest and will become immediately exercisable in full.
Amendment. Our Board of Directors may amend the 2010 Plan at any time or from time to time or may terminate the 2010 Plan without the approval of the stockholders, provided that stockholder approval will be required for any amendment to the 2010 Plan that (1) increases the total number of shares reserved thereunder, (2) changes the provisions regarding eligibility for incentive stock options, (3) changes the requirements that the exercise price of a stock option be set at the fair market value of our common stock at the time of grant, or (4) extends the expiration date of the 2010 Plan beyond 10 years. However, no action by our Board of Directors or stockholders may alter or impair any option previously granted under the 2010 Plan. Our Board may accelerate the exercisability of any option or waive any condition or restriction pertaining to such option at any time. The 2010 Plan will terminate in July 2020, unless terminated sooner by the Board.
| 86 |
REPORT OF COMPENSATION COMMITTEE
The Compensation Committee has reviewed and discussed the Compensation Discussion and Analysis contained in this report with management and, based on that review and discussion, the Compensation Committee recommended to the Board of Directors that the Compensation Discussion and Analysis be included in this report and in our proxy statement to be prepared for our 2012 annual meeting of stockholders.
| Submitted by: | THE COMPENSATION COMMITTEE OF THE BOARD OF DIRECTORS | |
| Myron Holubiak, Chairman | ||
| Mark Auerbach | ||
| Joseph Felder |
COMPENSATION COMMITTEE INTERLOCKS AND INSIDER PARTICIPATION
Mark Auerbach, Joseph Felder and Myron Holubiak served as members of our Compensation Committee during all of 2011. None of these individuals was at any time during 2011 or at any other time an officer or employee of Ventrus. No interlocking relationship exists between any member of our Compensation Committee and any member of any other company’s Board of Directors or Compensation Committee.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Equity Compensation Plans
The following table sets forth the indicated information as of December 31, 2011 with respect to our equity compensation plans:
| Plan Category | Number of Securities to be issued upon exercise of outstanding options, warrants and rights | Weighted-average exercise price of outstanding options, warrants and rights | Number of securities remaining available for future issuance under equity compensation plans | |||||||||
| Equity compensation plans approved by our shareholders: | ||||||||||||
| 2007 Stock Plan | $ | 2,016 | $ | 6.00 | -0- | |||||||
| 2010 Stock Plan: | 2,046,455 | $ | 6.40 | $ | 1,920,745 | |||||||
| Equity compensation plans not approved by our shareholders: | ||||||||||||
| 2008 Placement Agent Warrants | $ | 42,782 | $ | 12.40 | -0- | |||||||
| Licensor Warrants | $ | 13,605 | $ | 1.24 | -0- | |||||||
| Consultant Warrants | $ | 87,770 | $ | 6.22 | -0- | |||||||
| 2010 Placement Agent Warrants | $ | 89,000 | $ | 7.50 | -0- | |||||||
| Underwriter Warrants | $ | 197,200 | $ | 7.50 | -0- | |||||||
| Total | $ | 2,478,828 | $ | 6.60 | $ | 1,920,745 | ||||||
| 87 |
Our equity compensation plan consists of the 2007 Stock Plan and the 2010 Stock Plan, both of which were approved by our stockholders. Our equity compensation arrangements that have not been approved by our stockholders consist of warrants to purchase shares of our common stock issued to: Paramount BioCapital as placement agent in our 2008 common stock offering; S.L.A. Pharma to whom we issued a warrant for 13,605 shares as part of an amendment to the license agreement between us and S.L.A. Pharma for VEN 307 and VEN 308; three consultants; National Securities Corporation as placement agent in our 2010 convertible note offering; and the underwriters of our initial public offering.
The other information required by this Item is incorporated by reference to the information under the section captioned “Security Ownership of Certain Beneficial Owners and Management” contained in the proxy statement.
Item 13. Certain Relationships and Related Transactions, and Director Independence
The information required by this Item is incorporated by reference to the information under the section captioned “Certain Relationships and Related Transactions” and “Corporate Governance and Board Matters - Independence of Directors” contained in the proxy statement.
Item 14. Principal Accounting Fees and Services
The information required by this Item is incorporated by reference to the information under the section captioned “Audit and Audit Committee Matters” contained in the proxy statement.
Item 15. Exhibits, Financial Statement Schedules
(a) Exhibits. The following exhibits are filed as part of this registration statement:
|
Exhibit No. |
Description | |
| 1.1 | Form of Underwriting Agreement dated December 22, 2010. (1) | |
| 1.2 | Form of Underwriting Agreement | |
| 1.3 | Controlled Equity Offering Sales Agreement, dated January 30, 2012 between Ventrus Biosciences, Inc. and Cantor Fitzgerald & Co. (6) | |
| 3.1 | Amended and Restated Certificate of Incorporation dated November 11, 2010. (2) | |
| 3.2 | Amended and Restated Bylaws dated July 12, 2010. (5) | |
| 4.1 | Specimen of Common Stock Certificate. (3) | |
| 4.2 | Form of Convertible Promissory Note issued to investors between December 2007 and March 2008, as amended in December 14, 2009. (5) | |
| 4.3 | Form of Warrant issued to investors between June and September 2008. (5) | |
| 4.4 | Form of Convertible Promissory Note issued to Paramount BioSciences, LLC and Capretti Grandi, LLC in 2008 and 2009, as amended on December 21, 2009. (4) | |
| 4.5 | Warrants issued to Paramount Credit Partners, LLC on January 23, March 25, June 1 and June 24, 2009. (4) |
| 88 |
|
Exhibit No. |
Description | |
| 4.6 | Form of Convertible Promissory Note issued to investors and Paramount BioCapital, Inc. in February, March and April 2010. (5) | |
| 4.7 | Form of Convertible Promissory Note issued to investors in May 2010. (4) | |
| 4.8 | Form of Warrant issued to investors in February and March, 2010. (4) | |
| 4.9 | Form of Warrant issued to investors in May 2010. (4) | |
| 4.10 | Form of Placement Agent Warrant issued to Paramount BioCapital, Inc. on March 11, 2008. (5) | |
| 4.11 | Placement Agent Warrants issued to National Securities Corporation on February 26, March 31 and May 6, 2010, as amended October 28, 2010 and November 30, 2010. (1) | |
| 4.12 | Warrant issued to S.L.A. Pharm AG on August 30, 2010. (4) | |
| 4.13 | Form of underwriters warrant dated December 22, 2010. (1) | |
| 10.1* | Exclusive License Agreement dated March 23, 2007 by and between S.L.A. Pharma AG, and Paramount Biosciences, LLC, as amended on July 24, 2008, November 20, 2008, June 1, 2009, December 18, 2009 and June 24, 2010 and letter agreements dated October 27, 2008, November 20, 2008 and January 22, 2009. (2) | |
| 10.2 | Assignment and Assumption Agreement dated August 2, 2007, by and between Paramount Biosciences LLC and Ventrus Biosciences, Inc. (5) | |
| 10.3* | License Agreement dated March 10, 2008 by and between Sam Amer & Co., Inc. and Ventrus Biosciences, Inc., as amended on July 31, 2008, September 29, 2008, November 17, 2008, and letter agreements dated March 13, 2009, August 18, 2009, May 13, 2009 and December 15, 2009. (5) | |
| 10.4 | Amended and Restated Consulting Agreement dated July 19, 2010 between Russell H. Ellison and Ventrus Biosciences, Inc. (5) | |
| 10.5 | Amended and Restated Employment Agreement dated July 19, 2010 between Russell H. Ellison and Ventrus Biosciences, Inc. (5) | |
| 10.6 | Amended and Restated Consulting Agreement dated July 19, 2010 between David J. Barrett and Ventrus Biosciences, Inc. (5) | |
| 10.7 | 2007 Stock Incentive Plan. (5) | |
| 10.8 | Consulting Agreement dated March 1, 2009 between John Dietrich and Ventrus Biosciences, Inc. (4) | |
| 10.9 | Consulting Agreement dated May 11, 2010 between Timothy Hofer and Ventrus Biosciences, Inc. (4) | |
| 10.10 | Amendment No. 6, dated August 30, 2010, to Exclusive License Agreement between S.L.A. Pharma AG and Paramount BioSciences, LLC (assigned to Ventrus Biosciences). (4) | |
| 10.11 | Senior promissory notes issued by Ventrus Biosciences, Inc. to Paramount Credit Partners, LLC on January 23, March 25, June 1 and June 24, 2010 and Waiver Agreement and Amendment dated as of August 30, 2010. (3) | |
| 10.12 | Employment Agreement dated November 11, 2010 between David J. Barrett and Ventrus Biosciences, Inc. (2) | |
| 10.14 | 2010 Equity Incentive Plan. (4) | |
| 10.15 | Asset Purchase Agreement dated June 5, 2011 between Ventrus Biosciences, Inc. and Sam Amer & Co., Inc. (5) | |
| 10.16 | Amendment No. 7, dated June 6, 2011, to Exclusive License Agreement between S.L.A. Pharma AG and Paramount BioSciences, LLC (assigned to Ventrus Biosciences). (5) | |
| 10.17 | Amendment No. 1, dated August 24, 2011, to Employment Agreement between David J. Barrett and Ventrus Biosciences, Inc. (7) | |
| 10.18 | Amendment No. 1, dated August 24, 2011, to Amended and Restated Employment Agreement between Russell Ellison and Ventrus Biosciences, Inc. (7) | |
| 10.19 | Employment Agreement dated September 1, 2011 between Thomas Rowland and Ventrus Biosciences, Inc. (7) |
| 89 |
|
Exhibit No. |
Description | |
| 23.1 | Consent of EisnerAmper LLP, Independent Registered Public Accounting Firm. | |
| 31.1 | Certification of the Chief Executive Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 31.2 | Certification of the Chief Financial Officer Pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.1 | Certification of the Chief Executive Officer Pursuant to 18 U.S. C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 32.2 | Certification of the Chief Financial Officer Pursuant to 18 U.S. C. Section 1350 as Adopted Pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| * | Certain information in this exhibit has been omitted and filed separately with the Securities and Exchange Commission pursuant to a confidential treatment request. |
| (1) | Incorporated by reference to the exhibit filed in the Registrant’s Amendment No. 4 to Registration Statement on Form S-1 filed on December 6, 2010. |
| (2) | Incorporated by reference to the exhibit filed in the Registrant’s Amendment No. 3 to Registration Statement on Form S-1 filed on November 16, 2010. |
| (3) | Incorporated by reference to the exhibit filed in the Registrant’s Amendment No. 2 to Registration Statement on Form S-1 filed on October 29, 2010. |
| (4) | Incorporated by reference to the exhibit filed in the Registrant’s Amendment No. 1 to Registration Statement on Form S-1 filed on October 4, 2010. |
| (5) | Incorporated by reference to the exhibit filed in the Registrant’s Registration Statement on Form S-1 filed on July 20, 2010. |
| (6) | Incorporated by reference to the exhibit filed in the Registrant’s Registration Statement on Form S-3 filed on January 31, 2012. |
| (7) | Incorporated by reference to the exhibit filed in the Registrant’s Current Report on Form 8-K filed on August 25, 2011. |
| 90 |
SIGNATURES
In accordance with Section 13 or 15(d) of the Securities Exchange Act, the Registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
| VENTRUS BIOSCIENCES, INC. | |||
| Date: March 14, 2012 | By: | /s/ Russell H. Ellison | |
| Name: Russell H. Ellison | |||
| Title: Chief Executive Officer | |||
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed by the following persons on behalf of the registrant in the capacities and on the dates indicated.
| Signature | Title | Date | ||
| /s/ Russell H. Ellison | Chief Executive Officer | |||
| Russell H. Ellison | (Principal Executive Officer) and Director | March 14, 2012 | ||
| /s/ David J. Barrett | Chief Financial Officer | March 14, 2012 | ||
| David J. Barrett | (Principal Financial and Accounting Officer) | |||
| /s/ Anthony E. Altig | Director | March 14, 2012 | ||
| Anthony E. Altig | ||||
| /s/ Mark Auerbach | Director | March 14, 2012 | ||
| Mark Auerbach | ||||
| /s/ Joseph Felder | Director | March 14, 2012 | ||
| Joseph Felder | ||||
| /s/ Myron Z. Holubiak | Director | March 14, 2012 | ||
| Myron Z. Holubiak |
| S-1 |
FINANCIAL STATEMENTS
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
| Page | ||
| Reports of Independent Registered Public Accounting Firm | F-2 | |
| Balance Sheets as of December 31, 2011, and 2010 | F-4 | |
| Statements of Operations for the Years ended December 31, 2011, 2010, 2009 and the period from October 7, 2005 (Inception) to December 31, 2011 | F-5 | |
| Statements of Cash Flows for the Years ended December 31, 2011, 2010, 2009 and the period from October 7, 2005 (Inception) to December 31, 2011 | F-6 – F-8 | |
| Statement of Changes in Stockholders’ Equity (Deficiency) period from October 7, 2005 (Inception) to December 31, 2011 | F-9 – F-12 | |
| Notes to Financial Statements | F-13– F-28 |
| F-1 |
REPORTS OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders
Ventrus Biosciences, Inc.
We have audited the accompanying balance sheets of Ventrus Biosciences, Inc. (a development stage company) (the "Company") as of December 31, 2011 and 2010, and the related statements of operations, stockholders' equity (deficiency), and cash flows for each of the years in the three-year period ended December 31, 2011 and for the period from October 7, 2005 (inception) to December 31, 2011. The financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Ventrus Biosciences, Inc. as of December 31, 2011 and 2010, and the results of its operations and its cash flows for each of the years in the three-year period ended December 31, 2011 and for the period from October 7, 2005 (inception) to December 31, 2011, in conformity with accounting principles generally accepted in the United States of America.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Ventrus Biosciences, Inc.'s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO"), and our report dated March 13, 2012 expressed an unqualified opinion thereon.
/s/ EisnerAmper LLP
New York, New York
March 13, 2012
| F-2 |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Ventrus Biosciences, Inc.
We have audited Ventrus Biosciences, Inc.'s (a development stage company) (the "Company") internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control - Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO"). The Company's management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management's Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (i) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (ii) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (iii) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Ventrus Biosciences, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control - Integrated Framework issued by COSO.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Ventrus Biosciences, Inc. as of December 31, 2011 and 2010, and the related statements of operations, stockholders' equity (deficiency) and cash flows for each of the years in the three-year period ended December 31, 2011 and for the period from October 7, 2005 (inception) to December 31, 2011, and our report dated March 13, 2012 expressed an unqualified opinion thereon.
/s/ EisnerAmper LLP
New York, New York
March 13, 2012
| F-3 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Balance Sheets
| December 31, | December 31, | |||||||
| 2011 | 2010 | |||||||
| ASSETS | ||||||||
| Current assets: | ||||||||
| Cash and Cash Equivalents | $ | 36,975,434 | $ | 14,571,055 | ||||
| Other current assets | 62,129 | 18,915 | ||||||
| Total current assets | 37,037,563 | 14,589,970 | ||||||
| Computer equipment, net of depreciation of $28,432 and $27,260 | 8,218 | — | ||||||
| Deferred financing costs, net | — | 26,631 | ||||||
| Total assets | $ | 37,045,781 | $ | 14,616,601 | ||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: | ||||||||
| Borrowings under short-term note and line of credit | $ | — | $ | 419,380 | ||||
| Accounts payable | 2,342,074 | 312,896 | ||||||
| Accrued expenses | 171,000 | — | ||||||
| Term Note – bank | — | 800,000 | ||||||
| Interest payable – related party | — | 187,536 | ||||||
| Total current liabilities | 2,513,074 | 1,719,812 | ||||||
| Notes payable – Paramount Credit Partners, LLC (net of discount of $0 and $302,327) | — | 1,270,673 | ||||||
| Total liabilities | 2,513,074 | 2,990,485 | ||||||
| stockholders’ equity: | ||||||||
| Preferred stock, $.001 par value; 5,000,000 shares authorized, none issued | — | — | ||||||
| Common stock, $.001 par value; 50,000,000 authorized; 12,406,406 and 6,746,365 issued and outstanding | 12,406 | 6,746 | ||||||
| Additional paid-in capital | 102,049,385 | 44,803,724 | ||||||
| Deficit accumulated during the development stage | (67,529,084 | ) | (33,184,354 | ) | ||||
| Total stockholders’ equity | 34,532,707 | 11,626,116 | ||||||
| Total liabilities and stockholders’ equity | $ | 37,045,781 | $ | 14,616,601 | ||||
The accompanying notes are an integral part of these financial statements
| F-4 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Statements of Operations
| Period from | ||||||||||||||||
| October 7, | ||||||||||||||||
| Year Ended | Year Ended | Year Ended | 2005 (Inception) to | |||||||||||||
| December 31, | December 31, | December 31, | December 31, | |||||||||||||
| 2011 | 2010 | 2009 | 2011 | |||||||||||||
| Operating expenses: | ||||||||||||||||
| Research and development | $ | 25,277,682 | $ | 1,850,666 | $ | 2,942,992 | $ | 39,529,243 | ||||||||
| General and administrative | 8,724,391 | 2,915,590 | 397,238 | 14,245,068 | ||||||||||||
| Loss from operation | (34,002,073 | ) | (4,766,256 | ) | (3,340,230 | ) | (53,776,311 | ) | ||||||||
| Other income (expense): | ||||||||||||||||
| Interest income | 76,334 | 5,730 | 140 | 96,053 | ||||||||||||
| Interest expense: | ||||||||||||||||
| Beneficial conversion charge | — | (6,001,496 | ) | — | (6,001,496 | ) | ||||||||||
| Amortization of debt discount and warrants | (302,327 | ) | (2,484,927 | ) | (78,504 | ) | (2,865,758 | ) | ||||||||
| Interest expense | (116,664 | ) | (2,043,676 | ) | (1,120,811 | ) | (4,983,572 | ) | ||||||||
| Total other (expense) | (342,657 | ) | (10,524,369 | ) | (1,199,175 | ) | (13,754,773 | ) | ||||||||
| Net loss | $ | (34,344,730 | ) | $ | (15,290,625 | ) | $ | (4,539,405 | ) | $ | (67,529,084 | ) | ||||
| Basic and diluted net loss per common share | $ | (3.57 | ) | $ | (24.67 | ) | $ | (10.02 | ) | |||||||
| Weighted average common shares outstanding – basic and diluted | 9,613,900 | 619,923 | 445,040 | |||||||||||||
The accompanying notes are an integral part of these financial statements
| F-5 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Statements of Cash Flows
| Year ended December 31, 2011 | Year ended December 31, 2010 | Year ended December 31, 2009 | Period from October 7, 2005 (Inception) to December 31, 2011 | |||||||||||||
| Cash flows from operating activities: | ||||||||||||||||
Net loss | $ | (34,344,730 | ) | $ | (15,290,625 | ) | $ | (4,539,405 | ) | $ | (67,529,084 | ) | ||||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||||||
| Stock-based compensation | 2,982,949 | 2,356,087 | 123,758 | 5,940,271 | ||||||||||||
| Stock - based payments to consultants | 3,990,817 | — | — | 3,990,817 | ||||||||||||
| Stock issued in connection with license agreement | — | 389,597 | 25,000 | 414,825 | ||||||||||||
| Charge resulting from beneficial conversion feature | — | 6,001,496 | — | 6,001,496 | ||||||||||||
| Stock issued to vendor | — | — | 5,000 | 5,000 | ||||||||||||
| Warrants issued in connection with related party note conversion | — | 915,118 | — | 1,255,978 | ||||||||||||
| Amortization of deferred financing costs and debt discount | 328,958 | 2,327,193 | 116,952 | 3,466,010 | ||||||||||||
| Non-cash research and development | — | — | — | 1,087,876 | ||||||||||||
| Interest payable – 2007 Senior convertible notes | — | 611,266 | 573,708 | 1,598,104 | ||||||||||||
| Interest payable – 2010 Senior convertible notes | — | 354,269 | — | 354,269 | ||||||||||||
| Expenses paid on behalf of the Company satisfied through the issuance of notes | — | — | — | 227,910 | ||||||||||||
| Interest payable – related parties | — | 94,912 | 55,841 | 266,279 | ||||||||||||
| Interest payable – Paramount Credit Partners, LLC | — | 79,696 | 107,840 | 187,536 | ||||||||||||
| Depreciation | 3,746 | 12,525 | 7,511 | 31,006 | ||||||||||||
| Changes in operating assets and liabilities: | ||||||||||||||||
| Prepaid research and development | — | — | 800,000 | — | ||||||||||||
| Other current assets | (43,214 | ) | (16,396 | ) | 2,649 | (62,129 | ) | |||||||||
| Accounts payable and accrued expenses | 2,012,642 | (3,049,564 | ) | (762,773 | ) | 2,325,537 | ||||||||||
| Net cash used in operating activities | (25,068,832 | ) | (5,214,427 | ) | (3,483,919 | ) | (40,438,299 | ) | ||||||||
| F-6 |
| Year ended December 31, 2011 | Year ended December 31, 2010 | Year ended December 31, 2009 | Period from October 7, 2005 (Inception) to December 31, 2011 | |||||||||||||
| Cash flows from investing activities: | ||||||||||||||||
| Purchase of office and computer equipment | (11,964 | ) | — | (2,573 | ) | (39,224 | ) | |||||||||
| Cash flows from financing activities: | ||||||||||||||||
| Net Proceeds from initial public offering and other offerings | 49,988,823 | 15,184,344 | — | 65,173,167 | ||||||||||||
| Proceeds from private placement | — | — | — | 1,146,024 | ||||||||||||
| Proceeds from exercise of warrants | 288,732 | — | — | 288,732 | ||||||||||||
| Proceeds from 2010 Senior convertible notes | — | 3,425,000 | — | 3,425,000 | ||||||||||||
| Proceeds from notes payable to Paramount Credit Partners, LLC | — | — | 1,573,000 | 1,573,000 | ||||||||||||
| Repayment of Paramount Credit Partners, LLC Note | (1,573,000 | ) | — | — | (1,573,000 | ) | ||||||||||
| Proceeds from notes payable to related parties | — | 950,562 | 1,905,390 | 5,041,953 | ||||||||||||
| Repayment of notes payable – related party | — | — | — | (1,500,000 | ) | |||||||||||
| Proceeds from 2007 Senior convertible notes | — | — | — | 5,305,000 | ||||||||||||
| Payment for deferred financing costs | — | (755,092 | ) | (76,461 | ) | (1,431,603 | ) | |||||||||
| Proceeds from utilization of short-term note and line of credit | — | 99,380 | 150,000 | 419,380 | ||||||||||||
| Repayment of debt facilities | (419,380 | ) | — | — | (419,380 | ) | ||||||||||
| Proceeds from term note payable | — | 800,000 | — | 800,000 | ||||||||||||
| Repayment of term note payable | (800,000 | ) | — | — | (800,000 | ) | ||||||||||
| Proceeds from receipt of subscriptions | — | — | — | 4,684 | ||||||||||||
| Net cash provided by financing activities | 47,485,175 | 19,704,194 | 3,551,929 | 77,452,957 | ||||||||||||
| Net increase in cash and cash equivalents | 22,404,379 | 14,489,767 | 65,437 | 36,975,434 | ||||||||||||
| Beginning of period | 14,571,055 | 81,288 | 15,851 | — | ||||||||||||
| End of period | $ | 36,975,434 | $ | 14,571,055 | $ | 81,288 | $ | 36,975,434 | ||||||||
| F-7 |
| Year ended December 31, 2011 | Year ended December 31, 2010 | Year ended December 31, 2009 | Period from October 7, 2005 (Inception) to December 31, 2011 | |||||||||||||
| Supplemental schedule of non-cash financing activities: | ||||||||||||||||
| Warrants issued to placement agent | — | — | — | $ | 341,334 | |||||||||||
| Warrants issued to investors in connection with convertible notes | — | $ | 1,166,989 | — | $ | 1,166,989 | ||||||||||
| Debt discount on Paramount Credit Partners, LLC notes | $ | 302,327 | — | — | $ | 782,376 | ||||||||||
| Debt discount on 2010 senior convertible notes | — | — | — | $ | 1,468,254 | |||||||||||
| Related party notes and accrued interest converted to 2010 Senior convertible notes | — | $ | 2,192,433 | — | $ | 3,995,667 | ||||||||||
| Notes and accrued interest converted to common stock | — | $ | 14,003,158 | — | $ | 14,003,158 | ||||||||||
| Supplemental disclosure – cash paid for interest | $ | 277,324 | $ | 76,899 | $ | 344,974 | $ | 685,397 | ||||||||
The accompanying notes are an integral part of these financial statements
| F-8 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Statements of Changes in Stockholders’ Equity (Deficiency)
Period from October 7, 2005 (Inception) to December 31, 2011
| Common Stock | ||||||||||||||||||||
| Shares | Amount | Additional Paid-in Capital | Deficit Accumulated During the Development Stage | Total | ||||||||||||||||
| Issuance of common stock to founders and employees at $0.0124 per share in March and April 2007 | $ | 368 | $ | 4,196 | — | $ | 4,564 | |||||||||||||
| Issuance of common stock to founders and employees at $0.0124 per share in May and June 2007 | 9,677 | 10 | 110 | — | 120 | |||||||||||||||
| Issuance of common stock to licensor at $0.0124 per share in August 2007 | 18,401 | 18 | 210 | — | 228 | |||||||||||||||
| Stock-based compensation for the period from January to December 2007 | — | — | 16,655 | — | 16,655 | |||||||||||||||
| Warrants issued in connection with senior convertible notes in 2007 | — | — | 164,284 | — | 164,284 | |||||||||||||||
| Net loss | — | — | — | (4,567,894 | ) | (4,567,894 | ) | |||||||||||||
| Balance at December 31, 2007 | 396,090 | 396 | 185,455 | (4,567,894 | ) | (4,382,043 | ) | |||||||||||||
| Warrants issued in connection with senior convertible notes in January, February and March 2008 | — | — | 177,050 | — | 177,050 | |||||||||||||||
| Common stock issued in financing at $60.39 per share in June and September 2008 net of related costs | 18,977 | 19 | 929,438 | — | 929,457 | |||||||||||||||
| F-9 |
| Common Stock | ||||||||||||||||||||
| Shares | Amount | Additional Paid-in Capital | Deficit Accumulated During the Development Stage | Total | ||||||||||||||||
| Conversion of related party notes and interest payable at $60.39 per share in June 2008 | 29,861 | 30 | 1,803,204 | — | 1,803,234 | |||||||||||||||
| Warrants issued in connection with related party note conversion in June 2008 | — | — | 340,860 | — | 340,860 | |||||||||||||||
| Stock-based compensation for the period from January to December 2008 | — | — | 460,822 | — | 460,822 | |||||||||||||||
| Net loss | — | — | — | (8,786,430 | ) | (8,786,430 | ) | |||||||||||||
| Balance at December 31, 2008 | 444,928 | 445 | 3,896,829 | (13,354,324 | ) | (9,457,050 | ) | |||||||||||||
| Stock-based compensation for the period from January to December 2009 | — | — | 123,758 | — | 123,758 | |||||||||||||||
| Warrants issued in connection with Paramount Credit Partner LLC notes in January, March and June 2009 | — | — | 480,049 | — | 480,049 | |||||||||||||||
| Common Stock issued to licensor in December 2009 at $12.40 per share | 2,016 | 2 | 24,998 | — | 25,000 | |||||||||||||||
| Common Stock issued to vendor in December 2009 at $12.40 per share | 403 | — | 5,000 | — | 5,000 | |||||||||||||||
| Net loss | — | — | — | (4,539,405 | ) | (4,539,405 | ) | |||||||||||||
| Balance at December 31, 2009 | 447,347 | 447 | 4,530,634 | (17,893,729 | ) | (13,362,648 | ) | |||||||||||||
| Warrant issued to licensor in connection with amendment to the agreement in August 2010 | — | — | 161,552 | — | 161,552 | |||||||||||||||
| F-10 |
| Common Stock | ||||||||||||||||||||
| Shares | Amount | Additional Paid-in Capital | Deficit Accumulated During the Development Stage | Total | ||||||||||||||||
| Stock based compensation for the period from January to December 2010 | — | — | 2,194,535 | — | 2,194,535 | |||||||||||||||
| Conversion of notes and accrued interest to common stock in December 2010 at $4.20 per share | 3,334,085 | 3,334 | 13,999,824 | — | 14,003,158 | |||||||||||||||
| Beneficial conversion charge recorded on notes and interest converted to common stock in December 2010 | — | — | 6,001,496 | — | 6,001,496 | |||||||||||||||
| Common stock issued in IPO in December 2010, net of related costs at $6.00 per share | 2,900,000 | 2,900 | 15,181,444 | — | 15,184,344 | |||||||||||||||
| Fair value of warrants issued with Senior convertible notes in December 2010 | — | — | 2,344,708 | — | 2,344,708 | |||||||||||||||
| Common Stock issued to Licensor for amendment in December 2010 at $6.00 per share | 64,933 | 65 | 389,532 | — | 389,597 | |||||||||||||||
| Net loss | — | — | — | (15,290,625 | ) | (15,290,625 | ) | |||||||||||||
| Balance at December 31, 2010 | 6,746,365 | 6,746 | 44,803,724 | (33,184,354 | ) | 11,626,116 | ||||||||||||||
| Common Stock issued in January 2011 to fulfill over-allotment option from IPO, net of related costs | 435,000 | 435 | 2,420,341 | 2,420,776 | ||||||||||||||||
| Warrants exercised | 50,034 | 50 | 288,682 | 288,732 | ||||||||||||||||
| F-11 |
| Common Stock | ||||||||||||||||||||
| Shares | Amount | Additional Paid-in Capital | Deficit Accumulated During the Development Stage | Total | ||||||||||||||||
| Additional shares issued in January 2011 in connection with the December 22, 2010 conversion of notes into common stock | 7 | |||||||||||||||||||
| Shares issued in a stock offering in July 2011,net of related costs at $10.00 per share | 5,175,000 | 175 | 47,562,872 | 47,568,047 | ||||||||||||||||
| Stock- based compensation for the period from January 1 to December 31, 2011 to employees and directors | 2,982,949 | 2,982,949 | ||||||||||||||||||
| Stock-based payments for the period from January 1 to December 31, 2011 for options issued to consultants | 3,990,817 | 3,990,817 | ||||||||||||||||||
| Net Loss | (34,344,730 | ) | (34,344,730 | ) | ||||||||||||||||
| Balance at December 31, 2011 | 12,406,406 | $ | 12,406 | $ | 102,049,385 | $ | (67,529,084 | ) | $ | 34,532,707 | ||||||||||
| F-12 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Note 1 — Organization, Business and Basis of Presentation:
Organization and business:
Ventrus BioSciences Inc. (“Ventrus” or the “Company”) is a specialty pharmaceutical company focused on the late-stage development and commercialization of gastrointestinal products. Ventrus, formerly known as South Island BioSciences, Inc., was incorporated in the State of Delaware on October 7, 2005 and commenced operations in April 2007.
Basis of presentation:
The accompanying audited financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America.
The Company’s primary activities since incorporation have been organizational activities, including recruiting personnel, acquiring licenses for its pharmaceutical compound pipeline, performing business and financial planning, conducting clinical and nonclinical trials, performing research and development, and raising funds through the issuance of debt and common stock.
The Company is in the development stage and has funded its operations primarily through the issuance of equity and debt. The Company expects to continue to expend substantial amounts for continued product research, development, and commercialization activities for the foreseeable future. Management believes the Company’s funds are sufficient to continue operations through the third quarter of 2013. Management’s plans with respect to funding this development are to secure additional equity, if possible, and to secure additional strategic alliances that will provide available cash funding for operations. Continuation of the Company is dependent on its ability to obtain additional financing and, ultimately, on its ability to achieve profitable operations. There is no assurance, however, that such financing will be available or that the Company’s efforts ultimately will be successful.
Capital Resources:
The Company has not derived any revenue from product sales to date as our products have not been approved for sale by the U.S. Food and Drug Administration (“FDA”) or any foreign regulatory agency. Since inception, the Company’s operations have been financed primarily through the sale of equity securities, the proceeds from the exercise of warrants and stock options and issuance of debt. The Company has incurred losses from operations and negative cash flows since the inception and expects to continue to incur substantial losses for the foreseeable future as it continues product development. As a result, the Company may need to obtain additional funds to finance its operations in the future. In July 2011, the Company raised net proceeds of approximately $47,600,000 in a secondary offering of its equity securities. Until the Company can generate significant cash from its operations, it intends to obtain any additional funding it requires through strategic relationships, public or private equity or debt financings, or other arrangements and it cannot assure such funding will be available on reasonable terms, or at all. The Company currently has sufficient funds to meet its operating requirements and scheduled regulatory and development activities through the third quarter of 2013.
Note 2 — Summary of Significant Accounting Policies:
Cash and Cash Equivalents:
All highly liquid investments with maturities of three months or less at the time of purchase are considered to be cash equivalents. All of the Company’s cash equivalents have liquid markets and high credit ratings. The Company maintains its cash in bank deposit and other accounts, the balances of which, at times and at December 31, 2011, exceed federally insured limits.
| F-13 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Use of estimates:
The preparation of financial statements in conformity with accounting principles generally accepted in the United States of America requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and reported amounts of revenue and expenses during the reporting period. Actual results could differ from those estimates.
Significant estimates inherent in the preparation of the accompanying financial statements include the fair value of stock options and warrants granted to employees, consultants, directors, investors, licensors, placement agents and underwriters.
Computer equipment:
Computer equipment is stated at cost and depreciated using the straight-line method over the estimated useful life of the related assets of three years.
Stock-based compensation:
The Company’s share-based compensation cost is measured at grant date, using the Black-Scholes option pricing model to estimate the fair value of the award, and is recognized as expense over the employee’s requisite service period on a straight-line basis. The Company accounts for stock options and warrants granted to non-employees on a fair value basis which is estimated using the Black-Scholes option pricing model. The initial non-cash charge to operations for non-employee options and warrants with vesting are revalued at the end of each reporting period until vested and recognized as consulting expense over the related vesting period.
Warrants issued with convertible notes:
For the purpose of valuing the warrants issued with convertible notes (See Notes 3, and 8), the Company used the Black-Scholes option pricing model utilizing the assumptions noted in those Notes. To determine the risk-free interest rate, the Company utilized the U.S. Treasury yield curve in effect at the time of grant with a term consistent with the expected term of the Company’s awards. The Company estimated the expected life of the warrants is based on the full term of the warrant. The expected dividend yield reflects the Company’s current and expected future policy for dividends on its common stock. The expected stock price volatility for the Company’s stock options was calculated by examining historical volatilities for publicly traded industry peers as the Company did not have any trading history for its common stock at the time the grants were issued.
Warrants, or any other detachable instruments issued in connection with debt financing agreement not required to be recorded at fair value, are accounted for using the relative fair value method and allocated to additional paid-in capital and recorded as a reduction in the carrying value of the related debt. This discount is amortized to interest expense from the issuance date through the maturity date of the debt using the effective interest method.
Beneficial Conversion Feature:
When the conversion feature of conventional convertible debt provides for a rate of conversion that is below market value, this feature is characterized as a beneficial conversion feature (“BCF”). Prior to the determination of the BCF, the proceeds from the debt instrument are first allocated between the convertible debt and any detachable free standing instruments that are included, such as common stock warrants. The Company has disclosed the contingent nature of its BCFs (See Notes 3, and 8) and has recorded their effects.
| F-14 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Research and development:
Research and development expenses include personnel and facility-related expenses, third party contracted services including clinical trial costs, manufacturing and process development costs, research costs and other consulting services. Research and development costs are expensed as incurred. In instances where the Company enters into agreements with third parties for clinical trials, manufacturing and process development, research and other consulting activities, costs are expensed as services are performed. Amounts due under such arrangements may be either fixed fee or fee for service, and may include upfront payments, monthly payments, and payments upon the completion of milestones or receipt of deliverables.
The Company’s accruals for clinical trials are based on estimates of the services received and pursuant to contracts with the respective clinical trial centers and clinical research organizations. In the normal course of business, the Company contracts with third parties to perform various clinical trial activities in the ongoing development of potential products. The financial terms of these agreements are subject to negotiation and variation from contract to contract and may result in uneven payment flows. Payments under the contracts depend on factors such as the achievement of certain events, the successful enrollment of patients, and the completion of portions of the clinical trial or similar conditions. The objective of the Company’s accrual policy is to match the recording of expenses in its financial statements to the actual services received. As such, expense accruals related to clinical trials are recognized based on the estimate of the degree of completion of the event or events specified in the specific clinical study or trial contract.
Income taxes:
The Company’s income tax expense consists of current and deferred income tax expense or benefit. Current income tax expense or benefit is the amount of income taxes expected to be payable or refundable for the current year. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in income in the period that includes the enactment date. Valuation allowances are established when it is more likely than not that some or all of the deferred tax assets will not be realized.
Loss per common share:
Basic net loss per common share excludes dilution and is computed by dividing net loss by the weighted average number of common shares outstanding during the period. Diluted net loss per common share reflects the potential dilution that could occur if securities or other contracts to issue common stock were exercised or converted into common stock or resulted in the issuance of common stock that then shared in the earnings of the entity unless inclusion of such shares would be anti-dilutive. Since the Company has only incurred losses, basic and diluted net loss per share is the same. The number of potentially dilutive securities (options, warrants and convertible instruments) excluded from the diluted loss per share calculation at December 31, 2011, 2010 and 2009 was 3,002,898, 2,093,064 and 168,885, respectively.
Fair value measurements:
The carrying value of the Paramount Credit Partners, LLC notes approximated fair value due to the short-term nature of the notes and the related interest rates approximate market rates.
| F-15 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Note 3 — Related Party Transactions:
The following are descriptions of the Company’s related party transactions that have been entered into, modified, terminated, or were still in effect in 2011.
Consulting services:
Effective April 2007, the Company began accruing monthly fees for consulting services at a rate of $25,000 per month to Paramount Corporate Development, LLC (“Paramount”), which is an affiliate of Dr. Lindsay A. Rosenwald, M.D., a significant investor in and stockholder of the Company. This agreement was terminated as of August 31, 2008. For the period from October 7, 2005 (inception) through August 31, 2008, $425,000 was incurred under this arrangement. As of December 31, 2011 and 2010, the Company had $100,000 outstanding under this arrangement, which is included in accounts payable.
Notes payable:
During 2009, the Company issued four separate 10% promissory notes (collectively, the “PCP Notes”) to Paramount Credit Partners, LLC (“PCP”), an entity whose managing member is Dr. Rosenwald. Specifically, the PCP Notes consist of a note in the principal amount of $1,100,000 issued on January 23, 2009, a note in the principal amount of $100,000 issued on March 25, 2009, a note in the principal amount of $250,000 issued on June 1, 2009 and a note in the principal amount of $123,000 issued on June 24, 2009. Interest on the PCP Notes is payable quarterly, in arrears, and the principal matures on the earlier of (i) December 31, 2013 or (ii) the completion by the Company of a transaction, subsequent to the Company’s IPO, including an equity offering, sale of assets, licensing or strategic partnership, in which the Company raises at least $5,000,000 in gross cash proceeds. In addition, PCP received five-year warrants (“PCP Warrants”) to purchase 104,867 shares of common stock at an exercise price of $6.60. The Company allocated proceeds of $480,049 from the sale of the PCP Notes to the warrants at the time of issuance, which are recorded as a debt discount and reduced the carrying values of the PCP Notes. Such discount was being amortized to interest expense over the term of the PCP Notes. The fair value of the warrants granted was based on the following assumptions:
| Risk-free interest rate | 1.64% – 2.58 | % | ||
| Expected volatility | 104.11% – 110.89 | % | ||
| Expected life of warrants | 5 years | |||
| Expected dividend yield | 0 | % | ||
During 2010, PCP transferred the rights to an aggregate of $1,147,000 in principal amount of the PCP Notes and an aggregate of 76,553 of PCP Warrants to various employees of affiliates of PCP, none of whom are related parties. As a result, at December 31, 2010, PCP owned $426,000 in principal amount of the PCP Notes and 28,314 PCP Warrants.
On December 22, 2010, in connection with the completion of the IPO and pursuant to the terms of the warrants held by the purchasers of the convertible notes issued in 2010 (the “2010 Notes”), the above related party holders of 2010 Notes were issued 182,703 warrants with a per share exercise price of $6.60. Each of these warrants will expire on February 26, 2015. The Company valued these warrants at $915,118 using the Black-Scholes option pricing model, and the Company expensed the entire amount as interest expense in 2010. The fair value of the warrants granted was based on the following assumptions:
| Risk-free interest rate | 2.30% – 2.55 | % | ||
| Expected volatility | 124.46% – 129.05 | % | ||
| Expected life of warrants | 5 years | |||
| Expected dividend yield | 0 | % | ||
| F-16 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Line of Credit:
On July 19, 2011, the Company paid off these notes in full, and the remaining debt discount was fully charged to interest expense.
On December 3, 2008, the Company, PBS and various other private pharmaceutical companies in which Dr. Rosenwald is a significant investor and stockholder, entered into a loan agreement with Bank of America, N.A. for a line of credit of $2,000,000. PBS pledged collateral consisting of personal assets securing the Company’s and the other borrowers’ obligations to Bank of America, N.A. under the loan agreement. Interest on amounts borrowed under the line of credit was accrued and was payable on a monthly basis at an annual rate equal to the London Interbank Offered Rate (LIBOR) plus 1%. On November 10, 2009, the parties entered into Amendment No. 1 to the Loan Agreement, which extended the initial one-year term for an additional year, such that it was to mature on November 5, 2010, and reduced the aggregate amount available under the line of credit to $1,000,000. Under the loan agreement, the Company’s liability under the line of credit was several, not joint, with respect to the payment of all obligations there under. As of December 31, 2009, the amount borrowed by the Company under the Bank of America, N.A. line of credit was $320,000. In November 2010, the Company paid off the Bank of America, N.A. line of credit with proceeds from a promissory note issued to Israel Discount Bank of New York.
On September 23, 2010, the Company borrowed $800,000 from Israel Discount Bank of New York (“Israel Discount Bank”). The loan was personally guaranteed by Dr. Rosenwald. In consideration for the guarantee, the Company entered into a letter agreement with Dr. Rosenwald whereby Dr. Rosenwald has the right to attend meetings of the Company’s board of directors and to appoint two directors to the board. Dr. Rosenwald has not exercised his right to appoint these directors. If and when appointed, these directors would be subject to stockholder approval at the expiration of their terms. The rights granted to Dr. Rosenwald in connection with his guarantee continue until specified termination conditions.
On November 5, 2010, the Company borrowed an additional $420,000 from Israel Discount Bank of New York. The promissory note issued to Israel Discount Bank to evidence the loan is guaranteed by Dr. Rosenwald. The interest rate on the note is equal to the interest rate that Israel Discount bank will pay on the cash accounts at Israel Discount Bank maintained by Dr. Rosenwald and pledged to secure the note, plus 1%. At December 31, 2010 the interest rate was 1.45%. The note was due on demand or on November 4, 2011.
The Company repaid the Israel Discount Bank promissory notes in full in January 2011.
Placement Agent:
In connection with the offering of the 2010 Notes and related warrants, National Securities Corporation (“National”) and the Company entered into a placement agency agreement dated January 5, 2010, as amended on January 29, 2010, and a placement agency agreement dated April 14, 2010, as amended on April 30, 2010. Pursuant to these agreements, the Company paid National cash fees of $671,592, which consisted of placement agent commissions of $561,743 and non-accountable expense reimbursements of $109,849. In addition, the Company issued National warrants to purchase an aggregate of 89,000 shares of common stock, with an exercise price of $7.50. In addition, the Company paid National’s outside counsel $32,500 for its services as placement agent counsel. Dr. Lindsay A. Rosenwald beneficially owns, indirectly, a controlling interest in the parent holding company of National.
In connection with the Company’s IPO, National, Rodman & Renshaw (“Rodman”) and the Company entered into an underwriting agreement dated December 16, 2010, pursuant to which at closing on December 22, 2010, the Company paid National and Rodman cash fees of $1,662,400, which consisted of underwriting discounts of $1,261,500 and non-accountable expense reimbursements of $261,000. In addition, the Company issued to each of National and Rodman warrants to purchase an aggregate of 98,600 shares of common stock with an exercise price of $7.50.
The Company also granted National the exclusive right until May 6, 2011 to act as lead placement agent on the next private placement of the Company’s securities, or as lead managing underwriter on the initial public offering of the Company’s securities, with the compensation being paid to National with respect to such financing to be mutually agreed to by the parties in good faith with respect to such financing. The IPO satisfied this obligation.
| F-17 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Note 4 — Income Taxes:
There was no current or deferred income tax provision for the years ended December 31, 2011, 2010 and 2009.
The Company’s deferred tax assets as of December 31 consist of the following:
| 2011 | 2010 | |||||||
| Net operating loss | $ | 15,371,295 | $ | 8,255,000 | ||||
| Stock-based compensation | 4,395,743 | 1,291,000 | ||||||
| Research and development credits | 977,305 | 245,000 | ||||||
| Related Party Interest | - | 83,504 | ||||||
| Totals | 26,263,836 | 9,791,000 | ||||||
| Less: valuation allowance | (26,263,836 | ) | (9,791,000 | ) | ||||
| $ | — | $ | — | |||||
A valuation allowance is provided when it is more likely than not that some portion or all of the deferred tax assets will not be realized. The net increase in the total valuation allowance for the years ended December 31, 2011 and 2010 was $16,472,836 and $3,047,000, respectively. The tax benefit assumed the Federal statutory tax rate of 34% and a state and local tax rate of 10.53% and has been fully offset by the aforementioned valuation allowance.
At December 31, 2011, the Company had potentially utilizable Federal and state net operating loss tax carry-forwards of approximately $34,521,290 and R & D credit carryforward of approximately $977,300, both of which expire between 2027 and 2031.
An ownership change under Internal Revenue Code (“IRC”) Section 382 is likely to have occurred due to common stock issued in the IPO and debt conversions in December 2010. Due to the change in ownership provisions of the IRC, the availability of the Company’s net operating loss carry forwards may be subject to annual limitations against taxable income in future periods, which could substantially limit the eventual utilization of such carry forwards. The Company has not analyzed the historical or potential impact of its equity financings on beneficial ownership and therefore no determination has been made whether the net operating loss carry forward is subject to any IRC Section 382 limitation. To the extent there is a limitation, there would be a reduction in the deferred tax asset with an offsetting reduction in the valuation allowance.
| 2011 | 2010 | |||||||
| Statutory Federal tax rate | (34 | )% | (34 | )% | ||||
| Statutory state and local income taxes (net of Federal) | (11 | )% | (7 | )% | ||||
| Warrant amortization and beneficial conversion charges | (1 | )% | 19 | % | ||||
| Effect of valuation allowance | 46 | % | 22 | % | ||||
| Effective tax rate | — | % | — | % | ||||
| F-18 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Management believes that the Company does not have any uncertain tax positions that will result in a material impact on the Company’s financial statements. The Company files income tax returns in the U.S. federal and applicable state jurisdictions. There are currently no federal or state income tax examinations and therefore all years are statutorily open and subject to examination. If and when applicable, the Company will recognize interest and penalties as income tax expense.
Note 5 — Commitments:
Employment agreements:
Dr. Russell Ellison, the Company’s Chief Executive Officer has an employment agreement which provides for a base salary of $375,000 per year, a guaranteed bonus of $75,000 per year and an annual performance-based bonus of up to 50% of his base salary. The agreement also provides incentive bonuses of $250,000 and $500,000 in the event that the Company’s market capitalization exceeds specified levels. The first threshold was met and the $250,000 bonus was paid in the third quarter of 2011. The second threshold has not yet occurred.
Mr. David Barrett, the Chief Financial Officer has an employment agreement which provides for a base salary of $250,000 per year. The agreement also provides incentive bonuses of $250,000 and $500,000 in the event that the Company’s market capitalization exceeds specified levels. The first threshold was met and the $250,000 bonus was paid in the third quarter of 2011. The second threshold has not yet occurred.
On August 24, 2011, the above agreements were amended to provide that if the second market capitalization threshold is attained, the bonus of $500,000 will be paid in a combination of shares of the Company’s common stock worth $300,000 and $200,000 in cash. The number of the shares of common stock each would receive was determined by the closing price of the Company’s common stock as reported on NASDAQ on August 24, 2011 ($9.85), which could result in 30,457 shares being issued to each of Dr. Ellison and Mr. Barrett if the second market capitalization threshold is attained. In addition, the amendment to Mr. Barrett’s agreement provides that he will be eligible for an incentive cash bonus in the discretion of the Company’s Compensation Committee of up to 25% of his base salary. As of December 31, 2011, the second market capitalization threshold has not been attained.
On August 24, 2011, Ventrus entered into an employment agreement with Thomas Rowland that became effective on September 1, 2011 and pursuant to which Mr. Rowland serves as the Company’s Chief Business Officer. The employment agreement provides for a base salary of $250,000 per year and an annual performance-based bonus of up to 20% of his base salary.
Consulting Agreement:
Effective May 11, 2010, the Company entered into a one year consulting agreement with Timothy Hofer, pursuant to which Mr. Hofer provides the Company with consulting services focused on general business and company development. Mr. Hofer is a former employee of PBS, a related party. The Company did not renew the agreement at the end of its one year term.
Under the terms of the consulting agreement with Mr. Hofer and as compensation for his services thereunder, the Company granted Mr. Hofer a fully vested ten-year warrant to purchase 76,480 shares of the Company’s common stock, at an exercise price of $6.00 per share. The Company recognized $372,103 of consulting expense as of December 31, 2010 because the warrants were fully vested on that date.
| F-19 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Note 6 — Stockholders’ Transactions:
Common Stock Transactions:
During March and April 2007, the Company issued 368,012 shares of common stock to its founders for $4,564, or $0.0124 per share.
During May and June 2007, the Company issued 9,677 shares of common stock to its employees for $120, or $0.0124 per share. During August 2007, the Company issued 18,401 shares of common stock at $0.0124 per share in accordance with the license agreement between the Company and S.L.A. Pharma (see note 7). During 2007, the Company recorded $228 of stock-based research and development expense in connection with this license.
During June through September 2008, the Company issued 18,977 shares of common stock and 3,796 warrants at $60.39 per unit (consisting of a share of common stock with 20% warrant coverage) in connection with a private placement financing at $60.39 per unit. Each warrant has a seven-year term and an exercise price of $66.46. The Company raised $929,457 of net proceeds.
During July 2008, the Company issued 29,861 shares of common stock and 6,151 warrants at $60.39 per unit (consisting of a share of common stock with 20% warrant coverage) to related parties in connection with the conversion of amounts outstanding under certain promissory notes (see Note 3). Each warrant has a seven-year term and an exercise price of $66.46. The warrants had a fair value of $340,860 and were expensed on issuance since the promissory notes were converted.
The fair value of the warrants granted, mentioned in the preceding paragraph, was based on the following assumptions:
| Risk-free interest rate | 3.89 | % | ||
| Expected volatility | 128.18 | % | ||
| Expected life of warrants | 5 years | |||
| Expected dividend yield | 0 | % |
During December 2009, the Company issued 2,016 shares of common stock to S.L.A. Pharma pursuant to an amendment to the license agreement between the Company and S.L.A. Pharma, and 403 shares of common stock to a vendor, each at a value of $12.40 per share, recording an expense of $25,000 and $5,000 to research and development expense, respectively.
In connection with the Company’s IPO, all of the issued and outstanding convertible notes issued in 2007 and 2010 converted into shares of common stock pursuant to the terms of those notes. All principal and accrued interest on the 2007 and 2010 convertible notes converted at per share price of $4.20, which was 70% of the public offering price of $6.00 per share in the IPO, resulting in an aggregate of 1,642,802 shares of common stock issued upon conversion of the 2007 convertible notes and an aggregate of 1,421,834 shares of common stock issued upon conversion of the 2010 convertible notes. Also in connection with the IPO, and pursuant to their terms, the promissory notes issued to PBS and Capretti Grandi LLC, were converted at a per share price of $4.20, which was 70% of the public offering price of $6.00 per share in the IPO, resulting in an aggregate of 269,449 shares of common stock issued upon conversion of these notes.
| F-20 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
On December 22, 2010, the Company issued 2,900,000 shares of its common stock in an IPO at $6.00 per share and received net proceeds of $15,184,344, after deduction of underwriting discounts, commissions and other expenses related to the IPO.
Pursuant to the terms of the license agreement between the Company and S.L.A Pharma, the Company was obligated to issue to S.L.A. Pharma that number of additional shares of common stock so that, when added to the 18,401 shares initially issued, the new and old shares had an estimated fair market value equal to $500,000 (based on the price per share paid in the financing). The closing of the Company’s IPO triggered this obligation. As a result, the Company issued 64,933 shares of its common stock to S.L.A. Pharma on December 22, 2010. The Company valued the stock issuance to S.L.A. Parma at $389,597 and expensed the full amount to research and development expense as of December 31, 2010.
On January 7, 2011, the Company issued 435,000 shares of its common stock to fulfill the over-allotment option that it granted to the underwriters as part of the IPO and raised net proceeds of $2,420,776.
During the twelve months ended December 31, 2011, the Company issued an aggregate of 50,034 shares of common stock pursuant to the exercise of warrants with an average exercise price of $5.77.
On July 19, 2011, the Company issued 5,175,000 shares of its common stock in an underwritten public offering and raised net proceeds of $47,568,047.
Common Stock Options and Warrants:
In 2007, the Company established a stock incentive plan (the “2007 Plan”) under which incentive stock and/or options could be granted to officers, directors, consultants and key employees of the Company for the purchase of up to 483,871 shares of the Company’s common stock. The options could have a maximum term of ten years, vest over a period to be determined by the Company’s Board of Directors and have an exercise price at or above fair market value on the date of grant.
There were no options issued under the 2007 Plan in 2008 or 2009.
On May 11, 2010, the Company granted options to purchase 2,016 shares of its common stock to a director under the 2007 Plan with an exercise price of $6.00. The Company valued these options at $9,714 and expensed the full amount on the grant date since the options were fully vested.
The Company terminated the 2007 Plan in July 2010, but the 2,016 options granted under the 2007 Plan remain outstanding.
During 2007, the Company granted 12,903 warrants to various consultants with an exercise price of $7.69 per share. Each warrant granted during 2007 vests equally over a three-year period and has a seven-year term. During 2008, 1,613 of these warrants were forfeited due to the consultant’s relationship with the Company ending prior to the vesting period. All of the warrants that remain outstanding were fully vested at December 31, 2010.
On August 30, 2010, the Company issued a warrant to purchase 13,605 shares of its common stock with an exercise price of $1.24 per share to S.L.A. Pharma (see Note 7) pursuant to an amendment to the license agreement between the Company and S.L.A. Pharma. The warrant was fully vested at issuance and the Company recognized the full amount of $161,552 of stock-based research and development expense as of December 31, 2010. The fair value of the warrants granted and the related fair value adjustments at the end of each reporting period were based on the following assumptions:
| F-21 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
| 2007 | 2008 | 2009 | 2010 | |||||||||||||
| Risk-free interest rate | 4.00 | % | 1.55%-3.61 | % | 1.67%-2.69 | % | 0.75 | % | ||||||||
| Expected volatility | 65.55 | % | 104.78%-219.91 | % | 128.96%-163.74 | % | 113.31 | % | ||||||||
| Expected life of warrants (in years) | 7 years | 7 years | 7 years | 3 years | ||||||||||||
| Expected dividend yield | 0 | % | 0 | % | 0 | % | 0 | % | ||||||||
In August 2010, the Company’s stockholders approved the 2010 Equity Incentive Plan (the “2010 Plan”). In May 2011, the Company’s stockholders approved an amendment to the 2010 Plan to increase the shares reserved for issuance from 2,467,200 to 3,967,200 shares of the Company’s common stock. The 2010 Plan authorizes the Company to issue equity incentive awards in the form of shares, options or other awards based on Ventrus common stock as part of an overall compensation package to provide performance-based compensation to attract and retain qualified personnel.
In November 2010, the Company granted options to non-employee directors to purchase an aggregate of 160,000 shares under the 2010 Plan. In addition, under Dr. Ellison’s and Mr. Barrett’s respective employment agreements, in connection with the closing of the Company’s IPO, the Company granted to Dr. Ellison and Mr. Barrett options under the 2010 plan to purchase shares of the Company’s common stock with an exercise price of $6.00, which was equal to the initial public offering price per share, in an amount equal to 7.5% (573,599 shares) and 4.0% (305,920 shares), respectively, of the Company’s fully diluted capitalization on that date.
In addition to the options and warrants discussed above, in connection with the Company’s financings in 2007, 2008, 2009 and 2010, the Company issued warrants to investors and/or placement agents to purchase shares of common stock as well as certain consulting warrants (See Notes 3, and 8 ).
| F-22 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
A summary of the Company’s warrant activity and related information is as follows:
| Year Ended | Year Ended | Year Ended | ||||||||||||||||||||||
| December 31, 2011 | December 31, 2010 | December 31, 2009 | ||||||||||||||||||||||
| Weighted Average | Weighted Average | Weighted Average | ||||||||||||||||||||||
| Shares | Exercise Price | Shares | Exercise Price | Shares | Exercise Price | |||||||||||||||||||
| Outstanding at beginning of period | 1,013,291 | $ | 7.58 | 168,885 | $ | 11.43 | 64,018 | $ | 33.86 | |||||||||||||||
| Granted | - | $ | - | 844,405 | $ | 6.76 | 104,867 | $ | 6.60 | |||||||||||||||
| Exercised | 56,848 | $ | 5.08 | - | $ | 6.76 | - | - | ||||||||||||||||
| Outstanding at end of year | 956,443 | $ | 7.61 | 1,013,290 | $ | 7.58 | 168,885 | $ | 11.43 | |||||||||||||||
| Warrants exercisable at end of period | 956,443 | $ | 7.61 | 1,013,290 | $ | 7.58 | 168,885 | $ | 11.43 | |||||||||||||||
Included in the exercise of 56,848 warrants in 2011, are some warrants that were exercised utilizing a cashless exercise feature. In the aggregate, 50,034 shares were issued on the exercise of warrants and $288,732 was received in cash proceeds. All outstanding options have vested and no additional expense is expected to be recorded in the future years.
In 2011, the Company granted options to purchase 30,000 shares to three of its directors, options to purchase an aggregate of 552,440 shares to four employees and options to purchase an aggregate of 384,240 shares to seven consultants, all pursuant to the 2010 Plan with exercise prices at or greater than the then market value of the Company’s common stock ($6.00 - $15.77 per share).
A summary of the Company’s option activity and related information is as follows:
| Year Ended | Year Ended | |||||||||||||||||||||||
| December 31, 2011 | December 31, 2010 | |||||||||||||||||||||||
| Shares | Weighted Average Exercise Price | Aggregate Intrinsic Value | Shares | Weighted Average Exercise Price | Aggregate Intrinsic Value | |||||||||||||||||||
| Outstanding at beginning of period | 1,079,775 | $ | 6.01 | $ | - | - | - | - | ||||||||||||||||
| Granted | 966,680 | $ | 6.82 | $ | - | 1,079,775 | $ | 6.01 | $ | - | ||||||||||||||
| Outstanding at end of year | 2,046,455 | $ | 6.40 | $ | 4,092,910 | 1,079,775 | $ | 6.01 | $ | 657,989 | ||||||||||||||
| Options exercisable at end of period | 1,338,896 | $ | 6.19 | $ | 2,435,852 | 473,991 | $ | 6.00 | $ | 293,874 | ||||||||||||||
| Vested or expected to vest at December 31 | 2,046,455 | - | - | 1,079,775 | - | - | ||||||||||||||||||
| Shares available on December 31 for options that may be granted | 1,922,761 | - | - | 1,389,441 | - | - | ||||||||||||||||||
| F-23 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
The Company expects that all outstanding unvested options will vest. The fair value of the options granted for the year ended December 31, 2011 and 2010, was based on the following assumptions:
| 2011 | 2010 | |||||||
| Risk-free interest rate | 1.43%-3.03 | % | 1.84 | % | ||||
| Expected volatility | 88.05%-94.74 | % | 94.74 | % | ||||
| Expected life of options | 7 years | 7 years | ||||||
| Expected dividend yield | 0 | % | 0 | % | ||||
Estimated future stock-based compensation expense relating to unvested stock options (for consultants based on the fair value at December 31, 2011) is as follows:
| Calendar Years Ending December 31, | Future Stock Option Compensation Expense | |||
| 2012 | $ | 2,638,747 | ||
| 2013 | 1,028,650 | |||
| 2014 | 166,315 | |||
| Total estimated future stock-based compensation expense – stock options | $ | 3,833,712 | ||
The weighted average remaining contractual life of options outstanding at December 31, 2011 is approximately 9 years.
Stock-based compensation expensed to research and development expense for the years ended December 31, 2011, 2010 and 2009 was $1,124,904, $446,902 and $123,758, respectively. Stock-based compensation expensed to general and administrative expense for the years ended December 31, 2011, 2010 and 2009 was $5,848,862, $2,298,782 and $0, respectively.
Note 7 — License Agreements:
In March 2007, pursuant to an Exclusive License Agreement, S.L.A. Pharma, AG (“S.L.A. Pharma”) granted PBS a royalty-bearing license to sell, make and use diltiazem for treatment, through topical administration, of anal fissures and phenylepherine for treatment, through topical administration, of fecal incontinence in the United States, Canada and Mexico. Pursuant to the Exclusive License Agreement, PBS was obligated to form a company to develop the technologies referenced in the Exclusive License Agreement and issue a number of shares equal to 5% of such company’s outstanding common stock as of the effective date of the Exclusive License Agreement. On August 2, 2007, the Company issued 18,401 shares to S.L.A. Pharma to satisfy this obligation. In addition, the Company was obligated to issue to S.L.A. Pharma that number of additional shares of common stock so that the number of shares following specific transactions would have a fair market value equal to $500,000. On December 22, 2010, the Company issued S.L.A Pharma an additional 64,933 shares to satisfy this obligation.
| F-24 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
In August 2007, pursuant to an Assignment and Assumption Agreement, PBS sold all of its rights in and arising out of the Exclusive License Agreement with S.L.A. Pharma to Ventrus for $1,087,876. The corresponding U.S. and foreign patents and applications for the two compounds have been licensed to Ventrus under the Assignment and Assumption Agreement (the technology referred to collectively as the “Compound Technology”). As consideration in part for the rights to the Compound Technology, an initial licensing fee of $250,000 was paid to S.L.A. Pharma and $50,000 for reimbursement of clinical development costs incurred by S.L.A. Pharma (these amounts were paid by PBS and were included in the consideration paid by the Company to PBS in connection with the Assignment and Assumption Agreement). In the event that the Compound Technology is commercialized, the Company is obligated to pay to S.L.A. Pharma annual royalties, based upon net sales of the product. In addition, the Company is required to make payments to S.L.A. Pharma up to an aggregate amount of $20 million upon the achievement of various milestones related to regulatory events. Should the Company make any improvements regarding the Compound Technology, the Company is required to grant S.L.A. Pharma licenses to use such improvements.
As compensation for S.L.A. Pharma’s participation in the management and the development of the technologies, Ventrus is required to make separate payments to S.L.A. Pharma equal to $41,500 per month (“Monthly Payments”) for each of diltiazem and phenylephrine. Per the agreement, Ventrus’ obligation to make these monthly payments was to terminate upon a new drug application (“NDA”) filing. Pursuant to amendments to the Exclusive License Agreement, the Company, as of September 30, 2010, was no longer required to make additional payments for phenylephrine. At December 31, 2011, the Company had no amounts due to S.L.A. Pharma.
Ventrus is also required to reimburse S.L.A. Pharma for clinical development costs associated with the technology development of both diltiazem and phenylephrine. Ventrus’ total payment obligation for the diltiazem project is limited to $4,200,000. Ventrus made $4,200,000 of payments to S.L.A. Pharma from August 2007 through December 31, 2011. Both Ventrus and S.L.A. Pharma have agreed to add additional services outside the scope of the agreement for $400,000. The services have not yet been provided by S.L.A. Pharma. S.L.A. Pharma has been paid $600,000 of services for the phenylephrine project through December 31, 2011. S.L.A. Pharma did not provide Ventrus with any services for the phenylephrine project in 2010 or 2011 and management does not expect any services from SLA Pharma for the phenylephrine project in the foreseeable future.
On June 6, 2011, Ventrus further amended the Exclusive License Agreement with S.L.A. Pharma. The amendment eliminates its potential $800,000 milestone payment to S.L.A. Pharma for the development of diltiazem, previously payable upon the completion of enrollment into the Phase III clinical trial that S.L.A. Pharma is conducting in Europe. It also eliminates S.L.A. Pharma’s ability to terminate the license agreement at any time, with one month’s notice, in the event that Ventrus had failed to make a required payment and a third party wished to enter into a license agreement for diltiazem and phenylephrine, provided the termination would not have been effective if within that one-month period Ventrus paid all the then required payments under the agreement. Pursuant to the amendment, Ventrus must pay S.L.A. Pharma up to $1,000,000 in milestone payments, payable in four equal installments of $250,000 once specified thresholds of randomized patients are achieved in the Phase III trial for diltiazem that S.L.A. Pharma is conducting in Europe. The first two milestones were met and paid in the third quarter of 2011 and the third and fourth milestone was met and paid in the fourth quarter of 2011. As of December 31, 2011, Ventrus’ total remaining payment obligation for the phenylephrine project shall not exceed $400,000, consisting of to-be-agreed-upon services. Additionally, upon Ventrus’ receipt of a quality controlled final study report of the Phase III trial for diltiazem in Europe, Ventrus must pay S.L.A. Pharma $400,000 in development costs for diltiazem.
| F-25 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
In March 2008, Ventrus entered into an exclusive worldwide license agreement with Sam Amer & Co., Inc., a California company (“Amer”), whereby Ventrus acquired certain patent rights to iferanserin for the topical treatment of any anorectal disorders. Ventrus is obligated to pay Amer (i) a monthly consulting fee of $7,500 through May 2010, (ii) a license fee of $2,050,000, (iii) late fees of $7,500 per month starting July 2009 until the successful completion of the Phase III trials, (iv) interest payments totaling $595,000 and (v) additional late fees of $7,500 per month as an NDA was not submitted by September 2010. In addition, Ventrus may be required to make future milestone and royalty payments totaling up to $20 million upon the achievement of various milestones related to regulatory or commercial events. The license agreement is terminable by either party for cause and, upon 30 days notice in the event any safety, efficacy or regulatory issues prevent development or commercialization of iferanserin. Through November 14, 2011, the Company had made all contractual payments relating to the license agreement.
In December 2009, the Company and Amer supplemented the license agreement and added an additional licensing fee of $20,000 for six months. In April 2010, the Company and Amer agreed that the additional license would not be needed and, therefore, the Company was not required to pay the last two months.
On June 5, 2011, the Company entered into an agreement with Amer to acquire all rights, title and interest to iferanserin. Ventrus paid $500,000 on execution of the agreement. On November 14, 2011, the Company closed the acquisition and paid Amer an additional $12 million. Closing was subject to Ventrus raising net proceeds of a certain minimum amount, which the Company met upon its sale of common stock in July 2011. Closing was also subject to, in respect of the first pivotal Phase III trial and any recurrence treatment for iferanserin, the absence through November 10, 2011 of any serious severe adverse events, as defined. Ventrus will pay Amer royalties of between 3.0% and 4.0% on net annual sales in the U.S. and between 1.0% and 1.33% on gross annual sales outside the U.S., subject to a minimum annual royalty payment on both U.S. and ex-U.S. sales equal to (i) the royalty that would have been due had U.S. Net Sales (as defined in the Amer Purchase Agreement) equaled 50% of the U.S. Net Sales contained in the sales forecast provided by the Company to Amer for the applicable year and (ii) the royalty that would have been due had ex-U.S. Gross Sales (as defined in the Amer Purchase Agreement) equaled 50% of the ex-U.S. Gross Sales contained in the sales forecast provided by the Company to Amer for the applicable year.
We evaluated whether the cost of all or a portion of the assets purchased pursuant to the Amer Purchase Agreement should be measured at fair value and capitalized or charged to an expense account. In deciding the appropriate accounting treatment, we referenced “Working Draft of AICPA Accounting and Valuation Guide Assets Acquired to Be Used in Research and Development Activities” released on November 18, 2011. Because the assets purchased (1) do not meet the definition of a business combination and (2) do not have alternative future use since the assets acquired are contingent on further development and clinical risk, we determined that the entire purchase price of the asset be expensed immediately as in-process research and development.
| F-26 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
Note 8 — Private Placements:
2007 Senior convertible notes:
During 2007 and 2008, the Company issued 8% senior convertible notes in connection with a private placement in the aggregate principal amount of $5,305,000 (the “Bridge Notes”). The Bridge Notes were originally scheduled to mature on December 20, 2008, but the Company exercised its option to extend the maturity date to December 20, 2009, at an increased interest rate of 10%. The Company subsequently obtained the consent of the note holders to an additional extension of the maturity date of the Bridge Notes to September 10, 2010 and again to December 31, 2010. The Bridge Notes, plus all accrued interest thereon, would automatically convert into the same securities issued in the Company’s next Qualified Financing (as defined below), at a conversion price equal to 70% of the lowest per unit price paid for such securities in cash. The completion of the Company’s IPO triggered the automatic conversion of the Bridge Notes. Upon conversion, the Bridge Notes were automatically cancelled. The Company valued the beneficial conversion feature of the 2007 Notes at $2,957,187, which was recorded as interest expense after the Qualified Financing was completed. The Company computed the conversion feature to be $2,957,187 by dividing the amount of debt and interest ($6,899,770), which is convertible into common stock by the conversion rate (70%). From this amount ($9,856,957) the amount of debt and interest ($6,899,770) was subtracted to determine the amount by which the instrument if-converted exceeds the conversion amount ($2,957,187).
In connection with the offering of the Bridge Notes, Paramount Biocapital, Inc. (“PCI”) and the Company entered into a placement agency agreement dated October 9, 2007, pursuant to which the Company paid PCI and third party agents cash commissions of $243,600 and $19,250, respectively, for its services. The Company agreed to additional services by PCI during the 18-month period subsequent to March 11, 2008 which expired without any further amounts being paid. PCI is a related party to the Company since it is wholly-owned by Dr. Rosenwald.
In addition, PCI and third party agents received seven-year warrants (the “Placement Warrants”). The amount of shares and the exercise price were to be determined based on whether a qualified financing occurred on or before December 21, 2009. The qualified financing did not occur by such date and as a result the number of shares subject to the Placement Warrants is 42,782 shares, an amount equal to 10% of the principal amount of the Bridge Notes purchased, divided by $12.40, with an exercise price equal to $12.40. PCI subsequently transferred the Placement Warrants among its employees. The Company estimated the value of the Placement Warrants using the Black-Scholes option pricing model at approximately $341,000 and recorded them as deferred financing costs, which were amortized to interest expense over the term of the Bridge Notes. The fair value of the Placement Warrants granted was based on the following assumptions:
| Risk-free interest rate | 3.01% – 3.84 | % | ||
| Expected volatility | 63.69% – 123.73 | % | ||
| Expected life of warrants | 7 years | |||
| Expected dividend yield | 0 | % |
2010 Senior convertible notes:
In February, March, April and May 2010, the Company issued 8% senior convertible notes in connection with a private placement in the aggregate principal amount of $3,425,000 (the “2010 Notes”). The 2010 Notes matured on September 10, 2010. Upon the closing of a Qualified Financing (as defined below), the 2010 Notes plus any accrued but unpaid interest thereon were to convert into shares of the Company’s common stock at 70% of the price at which shares of common stock are sold in the Qualified Financing (the “IPO Price”). The completion of the Company’s IPO triggered the automatic conversion of the 2010 Notes. Upon conversion, the 2010 Notes were automatically cancelled.
| F-27 |
VENTRUS BIOSCIENCES, INC.
(A Development Stage Company)
Notes to Financial Statements
The Company valued the beneficial conversion feature of the 2010 Notes at $1,619,687, which was recorded as interest expense after the Qualified Financing was completed. The Company computed the conversion feature to be $1,619,687 by dividing the amount of debt and interest ($3,779,269), which is convertible into common stock by the conversion rate (70%). From this amount ($5,398,956) the amount of debt and interest ($3,779,269) was subtracted to determine the amount by which the instrument if-converted exceeds the conversion amount ($1,619,687).
Each 2010 Noteholder holds a warrant to purchase that number of shares of the Company’s common stock equal to 50% of the principal amount of the 2010 Notes purchased by it divided by the IPO Price at a per share exercise price equal to 110% of the IPO Price, subject to adjustment. Each of these warrants will expire and no longer be exercisable after February 26, 2015. In connection with the Company’s IPO, the number of shares of common stock issuable pursuant to these warrants is an aggregate of 285,417 shares with an exercise price of $6.60 per share. The Company valued these warrants at $1,429,590 using the Black-Scholes option pricing model and has expensed such amount as of December 31, 2010. The fair value of the warrants granted was based on the following assumptions:
| Risk-free interest rate | 2.02 | % | ||
| Expected volatility | 124 | % | ||
| Expected life of warrants | 5 years | |||
| Expected dividend yield | 0 | % |
On February 26, 2010, a 2010 Note in the aggregate principal amount of $2,192,433 and related warrant were issued to PBS for the cancellation of certain debt (as discussed in Note 3 above), which is not included in the $3,425,000 of aggregate principal amount of 2010 Notes issued in the private placement. Including such converted debt, the total aggregate principal amount of 2010 Notes was $5,617,433. In connection with the Company’s IPO, these 2010 Notes converted into an aggregate of 1,421,834 shares of common stock. Upon conversion, these 2010 Notes were automatically cancelled.
Note 9– Subsequent Event
On January 31, 2012, the Company filed a shelf registration statement with the Securities and Exchange Commission under which it may offer shares of its common stock and preferred stock, various series of debt securities and/or warrants to purchase any of such securities, either individually or in units, in one or more offerings, up to a total dollar amount of $100,000,000. Such registration statement became effective as of February 10, 2012. As part of the shelf registration statement, the Company included a prospectus for an at-the-market common equity sales program for the sale of up to $20,000,000 of common stock. No securities have been offered or sold pursuant to the shelf registration statement.
| F-28 |
