Attached files
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number 0-21937
CERUS CORPORATION
(Exact name of registrant as specified in its charter)
| Delaware | 68-0262011 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 2550 Stanwell Dr. Concord, California |
94520 | |
| (Address of principal executive offices) | (Zip Code) |
(925) 288-6000
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class |
Name of Each Exchange on Which Registered | |
| Common Stock, par value $0.001 per share | The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act:
Preferred Share Purchase Rights
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K, (§229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, or a non-accelerated filer. See definition of “accelerated filer and large accelerated filer” in Rule 12b-2 of the Exchange Act. (Check one):
Large accelerated filer ¨ Accelerated filer x Non-accelerated filer ¨ Smaller reporting company ¨
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The approximate aggregate market value of the common stock held by non-affiliates of the registrant as of the last business day of the registrant’s most recently completed second fiscal quarter, based upon the closing sale price of the registrant’s common stock listed on the Nasdaq Global Market, was $118.7 million. (1)
As of February 22, 2012, there were 54,213,000 shares of the registrant’s common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s definitive proxy statement in connection with the registrant’s 2012 Annual Meeting of Stockholders, to be filed with the Securities and Exchange Commission pursuant to Regulation 14A not later than 120 days after the end of the fiscal year ended December 31, 2011, are incorporated by reference into Part III of this Annual Report on Form 10-K.
(1) Based on a closing sale price of $3.00 per share on June 30, 2011. Excludes 8.0 million shares of the registrant’s common stock held by executive officers, directors and affiliates at June 30, 2011.
Table of Contents
Table of Contents
This Annual Report on Form 10-K contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934, as amended, that involve risks and uncertainties. The forward-looking statements are contained principally in Item 7, “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and in Item 1A, “Risk Factors.” These statements involve known and unknown risks, uncertainties and other factors which may cause our actual results, performance or achievements to be materially different from any future results, performances or achievements expressed or implied by the forward-looking statements. Examples of forward-looking statements include, but are not limited to, statements about our estimates regarding the sufficiency of our cash resources, our ability to commercialize and achieve market acceptance of the INTERCEPT Blood System, the anticipated progress of our research, development and clinical programs, our ability to manage cost increases associated with pre-clinical and clinical development for the INTERCEPT Blood System, our ability to obtain and maintain regulatory approvals of the INTERCEPT Blood System, the ability of our products to inactivate pathogens that may emerge in the future, and our ability to protect our intellectual property and operate our business without infringing upon the intellectual property rights of others. In some cases, you can identify forward-looking statements by terms such as “anticipate,” “will,” “believe,” “estimate,” “expect,” “plan,” and similar expressions intended to identify such forward-looking statements. Forward-looking statements reflect our current views with respect to future events, are based on assumptions, and are subject to risks and uncertainties. There can be no assurance that these statements will prove to be correct. Certain important factors could cause actual results to differ materially from those discussed in such statements, including our need for additional financing, whether our pre-clinical and clinical data or data from commercial use will be considered sufficient by regulatory authorities to grant marketing approval for our products, market acceptance of our products, reimbursement, development and testing of additional configurations of our products, regulation by domestic and foreign regulatory authorities, our limited experience in sales, marketing and regulatory support for the INTERCEPT Blood System, our reliance on Fenwal and third parties to manufacture certain components of the INTERCEPT Blood System, incompatibility of our platelet system with some commercial platelet collection methods, our need to complete certain of our product components’ commercial design, more effective product offerings by, or clinical setbacks of, our competitors, product liability, our use of hazardous materials in the development of our products, business interruption due to earthquake, our limited operating history and expectation of continuing losses, protection of our intellectual property rights, volatility in our stock price, legal proceedings, on-going compliance with the requirements of the Sarbanes-Oxley Act of 2002 and other factors discussed below and under the caption “Risk Factors,” in Item 1A of this Annual Report on Form 10-K and in our other documents filed with the Securities and Exchange Commission. We discuss many of these risks in this Annual Report on Form 10-K in greater detail in the section entitled “Risk Factors” under Part I, Item 1A below. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Also, forward-looking statements represent our estimates and assumptions only as of the date of this Annual Report on Form 10-K. You should read this Annual Report on Form 10-K and the documents that we incorporate by reference in and have filed as exhibits to this Annual Report on Form 10-K, completely and with the understanding that our actual future results may be materially different from what we expect. Except as required by law, we assume no obligation to update any forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in any forward-looking statements, even if new information becomes available in the future.
| Item 1. | Business |
Overview
We are a biomedical products company focused on commercializing the INTERCEPT Blood System to enhance blood safety. The INTERCEPT Blood System, which is based on our proprietary technology for controlling biological replication, is designed to inactivate blood-borne pathogens in donated blood components intended for transfusion.
1
Table of Contents
We have worldwide rights for our INTERCEPT Blood System for three blood components: platelets, plasma and red blood cells. The INTERCEPT Blood System for platelets, or platelet system, and the INTERCEPT Blood System for plasma, or plasma system, have received CE marks and are being marketed and sold in a number of countries in Europe, The Commonwealth of Independent States, or CIS, the Middle East and selected countries in other regions around the world. We sell both the platelet and plasma systems using our direct sales force and through distributors. In addition, we are developing and plan to perform the required clinical trials for our INTERCEPT Blood System for red blood cells, or red blood cell system, for approval in Europe. Subject to the availability of adequate funding from partners, government grants and/or capital markets, we intend to complete development activities for the red blood cell system necessary for regulatory approval in Europe and we may seek regulatory approval of our products in the United States.
We were incorporated in California in 1991 and reincorporated in Delaware in 1996. Our wholly-owned subsidiary, Cerus Europe B.V., was formed in The Netherlands in 2006. Information regarding our revenue, net loss, and total assets for the last three fiscal years can be found in the consolidated financial statements and related notes found elsewhere in this Annual Report on Form 10-K.
Product Development
Background
The INTERCEPT Blood System is designed to broadly target and inactivate blood-borne pathogens, such as viruses (for example, HIV, West Nile, SARS, hepatitis B and C), bacteria and parasites, as well as potentially harmful white blood cells, while preserving the therapeutic properties of platelet, plasma and red blood cell transfusion products. The INTERCEPT Blood System inactivates a broad array of pathogens and has the potential to reduce the risk of transfusion related transmission of pathogens for which testing is not completely effective or is not currently performed. We believe that the INTERCEPT Blood System also has the potential to inactivate most new pathogens before they are identified and before tests are developed and adopted commercially to detect their presence in donated blood.
Products, Product Candidates and Development Activities
We have worldwide commercial rights for all INTERCEPT Blood System products. The following table identifies our products and product development programs and their current status:
| Product or Product
Under |
Product or Development Status | |
| INTERCEPT Blood |
• Commercialized in a number of countries in Europe, the CIS, the Middle East and selected countries in other regions around the world • United States: Phase III clinical trial completed; seeking United States Food and Drug Administration, or FDA, concurrence on an additional Phase III clinical trial | |
| INTERCEPT Blood |
• Commercialized in a number of countries in Europe, the CIS, the Middle East and selected countries in other regions around the world • United States: Orphan drug designation for Thrombotic Thrombocytopenic Purpura; Phase III clinical trials completed; seeking clarity on clinical pathway with the FDA | |
| INTERCEPT Blood |
• Phase I clinical trial completed in 2010; preparing for initiation of Phase III clinical trials to support CE Mark approval in Europe • United States: Seeking clarity on clinical pathway via Special Protocol Assessment process with the FDA | |
2
Table of Contents
INTERCEPT Blood System for Platelets
The platelet system is designed to inactivate blood-borne pathogens in platelets donated for transfusion. The platelet system has received CE mark approval in Europe and is marketed and sold in a number of countries in Europe, the CIS, the Middle East and selected countries in other regions around the world. Separate approvals for use of INTERCEPT-treated platelet products have been obtained in France and Switzerland. In Germany and Austria, where approvals must be obtained by individual blood centers for use of INTERCEPT-treated platelets, several centers have obtained such approvals. Many countries outside of Europe accept the CE mark and have varying additional administrative or regulatory processes before the platelet system can be made commercially available. In general, these processes do not require additional clinical trials. In addition to regulatory approvals, some potential customers, including the largest branch of the German Red Cross, may desire to conduct their own clinical studies before adopting the platelet system.
We completed a Phase III clinical trial of the platelet system in the United States in March 2001 and a supplemental analysis of data from this trial in 2005, and submitted this information along with several other modules of our pre-market approval application, or PMA, to the FDA. The FDA has indicated that the clinical trial data and supplemental analysis are not sufficient to support a PMA and we will therefore need to conduct and complete an additional Phase III clinical trial before we can complete our regulatory submission to the FDA. In November 2009, we and the FDA presented a proposed clinical trial protocol for a second Phase III clinical trial for platelets to the FDA’s Blood Product Advisory Committee, or BPAC. The outcome of that meeting was the support for the design and endpoints of a clinical trial for INTERCEPT-treated platelets, subject to changes regarding the sensitivity of the safety endpoint. We are currently in discussions with the FDA regarding further details of the proposed additional Phase III clinical trial. However, until the final study size and design requirements are determined, we will not be able to assess the feasibility of an additional Phase III clinical trial. Currently, we have no plans to initiate such a clinical trial unless adequate funding is secured.
INTERCEPT Blood System for Plasma
The plasma system is designed to inactivate blood-borne pathogens in plasma donated for transfusion. The plasma system has received CE mark approval in Europe and is marketed and sold in a number of countries in Europe, the CIS, the Middle East and selected countries in other regions around the world. Separate approvals for use of INTERCEPT-treated plasma products have been obtained in France and Switzerland. In Germany and Austria, approvals must be obtained by individual blood centers for use of INTERCEPT-treated plasma. One such center in Germany has received such an approval. Many countries outside of Europe accept the CE mark and have varying additional administrative or regulatory processes before the plasma system can be made commercially available. In general, these processes do not require additional clinical trials. In addition to regulatory approvals, some potential customers may desire to conduct their own clinical studies before adopting the plasma system.
We have completed Phase IIIa, Phase IIIb and Phase IIIc clinical trials of the plasma system in the United States, reports for which were filed with the FDA during 2005. We have not submitted any applications for regulatory approval of the plasma system in the United States. We have received orphan drug status by the FDA Office of Orphan Products Development for INTERCEPT-treated plasma for the treatment of thrombotic thrombocytopenic purpura, or TTP. Although we have completed Phase III clinical trials in this patient population, the FDA may require supportive supplemental data collected in commercial use in TTP patients receiving INTERCEPT-treated plasma in Europe, or they may require us to complete additional Phase III clinical trials, before approval would be granted. We are seeking clarity on the clinical pathway with the FDA, and we do not yet know if the FDA will require any additional clinical trials. If additional clinical trials are required for approval, we will likely only initiate such trials if adequate funding can be secured.
INTERCEPT Blood System for Red Blood Cells
The red blood cell system is designed to inactivate blood-borne pathogens in red blood cells donated for transfusion. In 2008, we completed a series of in vitro and in vivo tests with the red blood cell system. In
3
Table of Contents
addition, we initiated a Phase I clinical trial of the red blood cell system in the fourth quarter of 2008 and completed the trial in early 2010, successfully meeting the clinical trial’s primary endpoint of red cell recovery measured twenty-four hours after transfusion. In order to obtain CE mark approval, we have submitted a clinical trial application to European regulators for a proposed Phase III clinical trial for acute anemia patients in Europe. If the clinical trial application is approved, we expect to enroll and conduct a Phase III clinical trial for acute anemia patients in Europe using INTERCEPT-treated red blood cells beginning in 2012. We are also planning to prepare a clinical trial application for a Phase III clinical trial for chronic anemia patients in Europe. We expect to conduct a further process validation study in Europe prior to commencement of such trials.
Previously, we terminated Phase III clinical trials for acute and chronic anemia for a prior generation of the red blood cell system. The trials were terminated due to the detection of antibody reactivity to INTERCEPT-treated red blood cells in two patients in the study for chronic anemia. The antibody eventually cleared and the patients had no adverse health consequences. After unblinding the data from the original Phase III clinical trials, we found that we had met the primary end-point in the clinical trial for acute anemia. Prior to commencing the Phase I clinical trial in 2008, we evaluated the antibodies detected in the original Phase III clinical trials and developed process changes to diminish the likelihood of antibody reactivity in red blood cells treated with our modified process. There were no adverse events associated with INTERCEPT-treated red blood cells evident in the 2008 trial. Based on the results from the 2008 clinical trial, we plan to conduct the planned acute anemia Phase III clinical trials in Europe using the modified process, if our clinical trial application is approved by European regulators.
In the United States, we believe that the FDA will likely require us to complete an additional recovery and lifespan study and at least one additional Phase III clinical trial before we would be able to potentially obtain approval for INTERCEPT-treated red blood cells in the United States. We are seeking clarity on the clinical pathway for the red blood cell system through the Special Protocol Assessment, or SPA, process with the FDA by seeking concurrence from the FDA on the trial protocol for the potential additional Phase III clinical trial. A SPA is an agreement between the sponsor and the FDA indicating that the sponsor’s proposed trial protocol, including clinical endpoints and statistical analyses, are acceptable to support regulatory approval of the treatment being evaluated. Even if we are able to reach agreement with the FDA on a SPA for an additional Phase III clinical trial evaluating the red blood cell system, we would only initiate such a trial if adequate funding can be secured.
Additional information regarding our interactions with the FDA, BPAC and potential future clinical development of the INTERCEPT Blood System in the United States design can be found under “Item 1A—Risk Factors” of this Annual Report on Form 10-K, under the risk factor titled “Our products, blood products treated with the INTERCEPT Blood System and we are subject to extensive regulation by domestic and foreign authorities. If our preclinical and clinical data are not considered sufficient by a country’s regulatory authorities to grant marketing approval, we will be unable to commercialize our products and generate revenue in that country. Our red blood cell system requires extensive additional testing and development.”
Information regarding our revenues for the years ended December 31, 2011, 2010 and 2009 can be found in “Item 7—Management’s Discussion and Analysis of Financial Condition and Results of Operations”, and “Item 15(a)—Exhibits and Financial Statement Schedules—Financial Statements” of this Annual Report on Form 10-K.
INTERCEPT Blood System Technology
Both our platelet system and plasma system employ the same technology. Platelet or plasma components collected from blood donors are transferred into plastic INTERCEPT disposable kits and are mixed with our proprietary compound, amotosalen, which has an affinity for nucleic acid.
The disposable kits are then placed in an illumination device, or illuminator, where the mixture is exposed to ultra-violet A, or UVA, light. If pathogens such as viruses, bacteria or parasites are present in the platelet or
4
Table of Contents
plasma components, the energy from the UVA light causes the amotosalen to bond with the nucleic acid of the pathogens. The ability of amotosalen to form both cross-links between strands of nucleic acid and links to single nucleic acid strands results in a strong chemical bond between the amotosalen and the nucleic acid of the pathogens. The presence of these bonds is designed to prevent replication of the nucleic acid within pathogens, effectively inactivating the pathogens. A high level of inactivation has been demonstrated in a broad range of pathogens studied by us and others in laboratory testing. For instance, INTERCEPT has demonstrated inactivation of a number of single stranded nucleic acid-based viruses such as HIV, hepatitis B, hepatitis C (using a model virus), West Nile, chikungunya, and certain influenza viruses.
Since platelets and plasma do not rely on nucleic acid for therapeutic efficacy, the INTERCEPT Blood System is designed to preserve the therapeutic function of the platelet and plasma components when used in human transfusions.
Following the inactivation process, residual amotosalen and by-products are reduced by more than 99% through use of a compound adsorption device, which is an integrated component of the disposable kit. We have performed extensive toxicology testing on the residual amotosalen and its by-products and good safety margins have been demonstrated. Any remaining amotosalen which may be transfused is rapidly excreted by humans.
Leukocytes, also known as white blood cells, are typically present in platelet and plasma components collected for transfusion and can cause adverse transfusion reactions as well as an often fatal disease called graft-versus host disease. Leukocytes, like pathogens, rely on nucleic acid for replication and cellular function. The INTERCEPT Blood System, with its combination of the amotosalen and UVA light, is designed to inactivate leukocytes in the same manner it inactivates pathogens.
Like the platelet and plasma systems, the red blood cell system acts by using an additive compound to form bonds with nucleic acid in pathogens that may be present in donated red blood cell collections. The red blood cell system is designed to preserve the therapeutic qualities of the red blood cells, which like platelets and plasma, do not rely on nucleic acid for their cellular function. The red blood cell system uses another of our proprietary compounds, S-303. Unlike the platelet and plasma systems, the chemical bonds from S-303 are not triggered by UVA light, but instead, by the pH level of the red blood cell components. After mixture with the red blood cell components in plastic disposable kits, S-303 is designed to rapidly break down into a form that is no longer chemically reactive with nucleic acid. As with the platelet and plasma systems, a high level of inactivation in a broad range of pathogens has been demonstrated with the red blood cell system in the clinical setting.
By treating blood components with INTERCEPT within a day of collection, the inactivation of bacteria prevents bacterial growth that could create increased risk of inflammatory response or dangerous levels of endotoxins. Extensive clinical testing has been done on platelet and plasma products treated with the INTERCEPT Blood System, as well as post-marketing haemovigilance studies of the treated blood products in routine use.
We believe that, due to their mechanisms of action, the platelet system, plasma system, and red blood cell system will potentially inactivate blood-borne pathogens that have not yet been tested with our systems, including emerging and future threats to the blood supply. We do not claim, however, that our INTERCEPT Blood System will inactivate all pathogens, including prions, and our inactivation claims are limited to those contained in our product specifications.
Collaborations
Baxter International, Inc. and Fenwal, Inc.
We collaborated with Baxter International, Inc., or Baxter, on the development and commercialization of the INTERCEPT Blood System commencing in 1993. We obtained exclusive worldwide commercialization rights to the red blood cell system from Baxter in February 2005. In February 2006, we entered into a restructuring of our agreements with Baxter pursuant to which we obtained exclusive worldwide commercialization rights to the
5
Table of Contents
platelet and plasma systems, excluding certain Asian countries where the commercialization rights had been licensed to BioOne Corporation, or BioOne. We also agreed to pay Baxter royalties on future INTERCEPT Blood System product sales at royalty rates that vary by product: 10% of product sales for the platelet system, 3% of product sales for the plasma system, 5% of product sales for the red blood cell system, and 6.5% on sales of UVA illuminators. In March 2007, Baxter sold its transfusion therapies business, the unit of Baxter that has performed many of the manufacturing and supply chain activities related to our relationship with Baxter, to Fenwal, Inc., or Fenwal. Fenwal has assumed Baxter’s rights and obligations under our agreements.
BioOne
In June 2004, we and Baxter entered into a definitive agreement with BioOne, which we refer to as the 2004 agreement, for the commercialization of our platelet system in specified parts of Asia. In June 2005, we and Baxter entered into a definitive agreement with BioOne, which we refer to as the 2005 agreement, for the commercialization of our plasma system in specified parts of Asia. Under the terms of both the 2004 agreement and 2005 agreement, BioOne was responsible for seeking regulatory approvals for and commercializing the platelet and plasma system in Japan, China, Taiwan, South Korea, Thailand, Vietnam and Singapore. Under those agreements, BioOne received exclusive marketing and distribution rights in each of those countries. In March 2007, Baxter transferred its rights and obligations with regard to BioOne to Fenwal.
In August 2010, we completed an acquisition of certain assets of BioOne, including the commercialization rights that both Fenwal and we granted to BioOne for both the platelet and plasma systems. Concurrent with the acquisition, Fenwal and we terminated the commercialization rights that we and Fenwal had granted to BioOne. As a consequence of the termination, and pursuant to a pre-existing agreement with Fenwal, our commercialization rights to the platelet and plasma systems under our 2005 and 2006 agreements with Baxter became worldwide. As consideration for the acquired BioOne assets, we relinquished all shares we held in BioOne valued at approximately $0.3 million and issued 1,172,357 shares of our common stock to BioOne valued at approximately $3.4 million, of which 937,886 shares were issued at the close of the acquisition on August 24, 2010 and the remaining 234,471 shares were issued six months from the close of the acquisition date on February 25, 2011.
United States Armed Forces
In February 2001, we were awarded a cooperative agreement with the Army Medical Research Acquisition Activity division of the Department of Defense, or DoD. In total, we have been awarded an aggregate of $36.4 million under awards and cooperative agreements with the DoD, all of which were for the continued funding of projects to develop our pathogen inactivation technologies for the improved safety and availability of blood that may be used by the United States Armed Forces for medical transfusions. Under the terms of the cooperative agreements, we are conducting research on the inactivation of infectious pathogens in blood, including unusual viruses, bacteria and parasites that are of concern to the United States Armed Forces. This funding supports advanced development of our red blood cell system.
Investment in Aduro BioTech
In November 2007, we spun-off our former immunotherapy business to Anza Therapeutics, Inc., or Anza Therapeutics. In exchange for our contribution of tangible and intangible assets to Anza Therapeutics, we received preferred stock representing an equity interest of approximately 20% of Anza Therapeutics’ preferred equity. We were informed in February 2009 that Anza Therapeutics had ceased operations.
In July 2009, we entered into a three-way license agreement with Anza Therapeutics and Aduro BioTech, or Aduro, and separate agreements with each of Anza Therapeutics and Aduro, which we refer to collectively as the Assignment Agreements. In November 2009, Anza Therapeutics transferred all of its intellectual property to Aduro pursuant to the terms of the Assignment Agreements. In exchange for agreeing to the transfer and relinquishing our shares in Anza Therapeutics and releasing any claims against Anza Therapeutics, we received
6
Table of Contents
$0.8 million in cash, preferred stock representing 10% of Aduro’s capital, and a 1% royalty fee on any future sales resulting from the transferred technology. In April 2011, Aduro completed a subsequent round of financing, issuing Series B preferred stock and as a result, reduced our ownership in Aduro to less than 3%. Since receiving preferred stock in Aduro, we have carried our investment in Aduro at zero on our consolidated balance sheet as we have no basis to believe that we will receive any economic benefit from our equity ownership in Aduro as we believed that Aduro’s technology platforms, which were largely based on Anza Therapeutics’ in-process development programs, had a high risk of failure.
William Greenman, our President and Chief Executive Officer, is on the Board of Directors of Aduro. Mr. Greenman does not represent Cerus on Aduro’s Board of Directors.
Manufacturing and Supply
We are responsible for the full management and control of the supply chain for the INTERCEPT illuminators and certain other components of the platelet and plasma disposable kits. We have used, and intend to continue to use, third parties to manufacture and supply the devices, disposable kits and inactivation compounds that make up the INTERCEPT Blood System for use in clinical trials and for commercialization. We rely solely on Fenwal for the manufacture of INTERCEPT Blood System disposable kits and on contract manufacturers for the production of inactivation compounds, compound adsorption components of the disposable kits and UVA illuminators used in the INTERCEPT Blood System. We currently do not have alternate manufacturers for the components in our products beyond those that we currently rely on.
In December 2008, we amended our manufacturing and supply agreement with Fenwal. Under the amended agreement, Fenwal is obligated to sell, and we are obligated to purchase, finished disposable kits for the platelet and plasma systems for both clinical and commercial use. The agreement permits us to purchase platelet and plasma kits from third-party manufacturers provided that we meet certain annual minimum purchase obligations to Fenwal. We are responsible for developing and delivering to Fenwal our proprietary inactivation compounds and adsorption media for incorporation into the final system configuration. The term of the amended manufacturing and supply agreement with Fenwal extends through December 31, 2013, and is automatically renewed for one year terms, subject to termination by either party upon thirty months prior written notice, in the case of Fenwal, or twenty-four months prior written notice, in our case. We and Fenwal each have normal and customary termination rights, including termination for material breach.
Components of compound adsorption devices used in platelet and plasma disposable kits are manufactured by Porex Corporation, or Porex. In 2007, we and Porex entered into an agreement for the manufacture of such components, which expires in December 2012. We do not currently have alternate manufacturers validated for the manufacture of compound adsorption devices and will need to either identify and validate alternate suppliers for the manufacture of compound adsorption devices or agree with Porex on either an amended supply agreement or new agreement. We also have contracts with suppliers of raw materials used to make the compound adsorption devices, which includes such companies as Brotech Corporation d/b/a Purolite Company, or Purolite. We entered into the supplier agreement with Purolite in 2007, which extends through December 2013, and will automatically renew each year, unless terminated by either party upon providing at least two year prior written notice.
Pursuant to a contract that we and NOVA Biomedical Corporation, or NOVA, entered into in September 2008, NOVA has begun manufacturing illuminators for us. The term of the NOVA agreement extends through September 2013 and is automatically renewable for one year terms, subject to termination by either party upon twelve months prior written notice.
In September 2011, we amended our manufacturing and supply agreement with Ash Stevens, Inc., or Ash Stevens, for the synthesis of amotosalen, the inactivation compound used in our platelet and plasma systems. Under this amended agreement, we are not subject to minimum annual purchase requirements. However, if specified quantities of amotosalen are not purchased in any year, we are required to pay a maintenance fee of up
7
Table of Contents
to $50,000 for such year. In the past, we have incurred these penalties. The term of the amended manufacturing and supply agreement with Ash Stevens extends through December 31, 2015 and will automatically renew thereafter for a period of two years, unless terminated by either party upon providing at least one year prior written notice, in our case, or at least two years prior written notice, in the case of Ash Stevens.
We and our contract manufacturers, including Fenwal and NOVA, purchase certain raw materials for our disposable kits, inactivation compounds, materials and parts associated with compound adsorption devices and UVA illuminators from a limited number of suppliers. Some of our suppliers require minimum annual purchase amounts. While we believe that there are alternative sources of supply for such materials, parts and devices, we have not validated or qualified any alternate manufacturers. As such, establishing additional or replacement suppliers for any of the raw materials, parts and devices, if required, will likely not be accomplished quickly and could involve significant additional costs and potential regulatory reviews.
Marketing, Sales and Distribution
The market for the INTERCEPT Blood System is dominated by a relatively small number of blood collection organizations. Many of these organizations are national blood transfusion services or Red Cross organizations who collect, store and distribute virtually all of their respective nations’ blood and blood component supplies. The largest European markets for our products are in Germany, France, and England.
In Germany, decisions on product adoption are made on a regional or blood center-by-blood center basis. While obtaining CE marks allow us to sell the platelet and plasma systems to blood centers in Germany, blood centers in Germany must still obtain both local manufacturing approval and national marketing authorization from the Paul Ehrlich Institute before being allowed to sell platelet and plasma components treated with the INTERCEPT Blood System to transfusing hospitals and physicians. To date, several blood centers in Germany have received such requisite approvals and authorizations for the platelet system and/or the plasma system.
In France, broad product adoption is dependent on a central decision by the Etablissement Francais du Sang, or EFS, and then on a broad-based national supply contract being awarded. In 2011, we entered into a two-year contract with the EFS to supply platelet and plasma disposable kits. The contract contains two one-year renewal options and provides for minimum and maximum purchase commitments.
In England, decisions on product adoption are centralized in the National Blood Service. We understand that the National Blood Service has decided to implement bacterial detection testing for platelets before considering pathogen inactivation.
Our ability to successfully commercialize our products will depend in part on the availability of adequate reimbursement for product costs and related treatment of blood components from governmental authorities and private health care insurers (including health maintenance organizations), which are increasingly attempting to contain health care costs by limiting both the extent of coverage and the reimbursement rate for new tests and treatments.
We maintain a wholly-owned subsidiary, Cerus Europe B.V., headquartered in The Netherlands, which focuses its efforts on marketing and selling the INTERCEPT Blood System in a number of countries in Europe, the CIS, the Middle East and selected countries in other regions around the world. We also have a small scientific affairs group in the United States and The Netherlands that supports the commercialization efforts.
Competition
We believe that the INTERCEPT Blood System has certain competitive advantages over competing blood-borne pathogen inactivation methods that are either on the market or in development. The INTERCEPT Blood System is designed for use in blood centers, which allows for integration with current blood collection, processing and storage procedures. Certain competing products currently on the market, such as solvent
8
Table of Contents
detergent-treated plasma, use centralized processing that takes blood products away from the blood center in order to be treated at a central facility before being shipped back out to the blood centers or hospitals for ultimate transfusion. In Europe, several companies, including Grifols S.A., Octapharma AG and MacoPharma International, are developing or selling commercial pathogen inactivation systems or services to treat fresh frozen plasma. CaridianBCT (becoming TerumoBCT) has developed a pathogen inactivation system for blood products and has been issued CE marks for a pathogen reduction system for both platelets and plasma. We understand that CaridianBCT is also developing a pathogen inactivation system for whole blood. In addition to direct competition from other pathogen inactivation methods, we encounter indirect competition from other approaches to blood safety, including methods of testing blood products for bacterial and viral pathogens. Some of these indirect competitors have mature, well-established products and more resources than we have. Further discussion of the major competitors to our blood product business can be found under “Item 1A—Risk Factors” of this Annual Report on Form 10-K, under the risk factor entitled “If our competitors develop superior products to ours or market their products more effectively than we market our products, our commercial opportunities could be reduced or eliminated.”
We believe that the primary competitive factors in the market for pathogen inactivation of blood products include the breadth and effectiveness of pathogen inactivation processes, the amount of demonstrated reduction in transfusion related adverse events subsequent to adopting pathogen inactivation technology, robustness of treated blood components upon transfusion, ease of use, the scope and enforceability of patent or other proprietary rights, product value relative to perceived risk, product supply and marketing and sales capability. In addition, we believe the length of time required for products to be developed and to receive regulatory and, in some cases, reimbursement approval are also important competitive factors. We believe that the INTERCEPT Blood System will compete favorably with respect to these factors, although there can be no assurance that it will be able to do so. Our success will depend in part on our ability to respond quickly to medical and technological changes and customer demand through the development and introduction of new products.
Patents, Licenses and Proprietary Rights
Our success depends in part on our ability to obtain patents, to protect trade secrets, to operate without infringing upon the proprietary rights of others and to prevent others from infringing on our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing United States and foreign patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business. As of December 31, 2011, we owned approximately 24 issued or allowed United States patents and approximately 72 issued or allowed foreign patents related to the INTERCEPT Blood System. Our patents expire at various dates between 2012 and 2027. In addition, we have pending United States patent applications and have filed corresponding patent applications under the Patent Cooperation Treaty. We have a license from Fenwal to United States and foreign patents relating to the INTERCEPT Blood System, which expire at various dates between 2017 and 2024. Proprietary rights relating to our planned and potential products will be protected from unauthorized use by third parties only to the extent that they are covered by valid and enforceable patents or are effectively maintained as trade secrets. The laws of certain foreign countries do not protect our intellectual property rights to the same extent as do the laws of the United States.
Seasonality
Our business is dependent on the marketing and commercialization of the INTERCEPT Blood System to customers such as blood banks, hospitals, distributors and other health care providers that have a need for a pathogen inactivation system to treat blood products for transfusion. Since our customers’ needs are not based on seasonal trends, seasonality does not have a material effect on our business.
Inventory Requirements and Product Return Rights
Our platelet and plasma systems have received regulatory approval for two-year shelf lives. Illuminators and replacement parts do not have regulated expiration dates. We own work-in-process inventory for certain
9
Table of Contents
components of INTERCEPT disposable kits, finished INTERCEPT disposable kits, illuminators, and certain replacement parts for our illuminators. Our supply chain for certain of these components, held as work-in-process on our consolidated balance sheet, may potentially take over one year to complete production before being utilized in finished disposable kits. We maintain an inventory balance based on our current sales projections, and at each reporting period, we evaluate whether our work-in-process inventory would be consumed for production of finished units in order to sell to existing and prospective customers within the next twelve-month period. It is not customary for our production cycle for inventory to exceed twelve months. Instead, we use our best judgment to factor in lead times for the production of our finished units to meet our current demands. If actual results differ from those estimates, work-in-process inventory could potentially accumulate for periods exceeding one year. Inventory is recorded at the lower of cost, determined on a first in, first out basis, or market value. We use significant judgment to analyze and determine if the composition of our inventory is obsolete, slow-moving, or unsalable and frequently review such determinations. Our limited history and limited experience with manufacturing and selling the INTERCEPT Blood System limits the amount of historical data we have to perform such analysis. Generally, we write-down specifically identified obsolete, slow-moving, or known unsalable inventory that has no alternative use to net realizable value in the period that it is first recognized, by using a number of factors, including product expiration dates, open and unfulfilled orders, and sales forecasts. The write-down of our inventory to net realizable value establishes a new cost basis and will be maintained even if certain circumstances suggest that the inventory is recoverable in subsequent periods.
We sell the INTERCEPT Blood System directly to blood banks, hospitals, universities, and government agencies, as well as to distributors in certain regions. Generally, our contracts with our customers do not provide for open return rights, except within a reasonable time after receipt of goods in the case of defective or non-conforming product.
Customers and Financial Information About Geographic Areas
At December 31, 2011, we had two customers that each accounted for more than 10% of our outstanding trade receivables, which cumulatively represented approximately 58% of our outstanding trade receivables. In addition, we had three significant customers that each accounted for more than 10% of our total product revenue, which cumulatively represented 57% of our total product revenue and 61% of our total product revenue for the years ended December 31, 2011 and 2009, respectively, while we had four significant customers that each accounted for more than 10% of our total product revenue, which cumulatively represented 67% of our total product revenue for the year ended December 31, 2010. The loss of any one of these customers would have an adverse impact on our business. To date, we have not experienced collection difficulties from these customers. For additional details about these customers for the years ended December, 2011, 2010 and 2009, as well as information regarding our net revenues by geographical location and location of our long-lived assets, see Note 19 in the Notes to Consolidated Financial Statements under “Item 15(a)—Consolidated Financial Statements and Supplementary Data—Financial Statements—Financial Statements” of this Annual Report on Form 10-K.
Research and Development Expenses
A significant portion of our operating expenses is related to research and development and we intend to maintain our strong commitment to research and development. We have incurred total research and development expenses of $7.2 million, $5.2 million and $6.4 million for the years ended December 31, 2011, 2010 and 2009, respectively. See Note 2 in the Notes to Consolidated Financial Statements under “Item 15(a)—Consolidated Financial Statements and Supplementary Data—Financial Statements” of this Annual Report on Form 10-K for costs and expenses related to research and development, and other financial information for the years ended December 31, 2011, 2010 and 2009.
Government Regulation
We and our products are comprehensively regulated in the United States by the FDA and, in some instances, by state and local governments, and by comparable governmental authorities in other countries.
10
Table of Contents
Our European investigational plan has been based on the INTERCEPT Blood System being categorized as Class III drug/device combinations under the Medical Device Directives, or the MDD, of the European Union. The European Union requires that medical devices affix the CE mark, an international symbol of adherence to quality assurance standards and compliance with the MDD. We initially received the CE mark for our platelet system and separately for our plasma system in 2002 and 2006, respectively. We will need to obtain a CE mark extension in our name from European Union regulators for both our platelet and plasma systems every five years. The CE mark for the platelet system is effective through May 2012 while the CE mark for the plasma system is effective through September 2016. A separate CE mark certification must be received for the red blood cell system to be sold in the European Union and in other countries recognizing the CE mark. In addition, France, Switzerland, Germany, and Austria require separate approvals for INTERCEPT-treated blood products.
The FDA regulates drugs, medical devices and biologics under the Federal Food, Drug, and Cosmetic Act and other laws, including, in the case of biologics, the Public Health Service Act. These laws and implementing regulations govern, among other things, the development, testing, manufacturing, record keeping, storage, labeling, advertising, promotion and pre-market clearance or approval of products subject to regulation. The steps required before a medical device may be approved for marketing in the United States pursuant to a PMA include:
| • | preclinical laboratory and animal tests; |
| • | submission to the FDA of an investigational device exemption for human clinical testing, which must become effective before human clinical trials may begin; |
| • | appropriate tests to show the product’s safety; |
| • | adequate and well-controlled human clinical trials to establish the product’s safety and efficacy for its intended indications; |
| • | submission to the FDA of a PMA; and |
| • | FDA review of the PMA in order to determine, among other things, whether the product is safe and effective for its intended uses. |
The FDA will require a PMA for each of the INTERCEPT systems for platelets, plasma and red blood cells because the FDA considers the INTERCEPT Blood System a biological medical device. The FDA Center for Biologics Evaluation and Research, or CBER, is principally responsible for regulating the INTERCEPT Blood System. However, before the FDA determines whether to approve our blood safety products, we expect our PMA to be reviewed by the Blood Products Advisory Committee, or BPAC, an advisory committee convened by and reporting to the FDA. Should the FDA ask questions to BPAC, we expect BPAC will answer those questions and make recommendations to the FDA.
In order to support our PMA for the INTERCEPT Blood System, we have conducted various types of studies, including toxicology studies to evaluate product safety, laboratory and animal studies to evaluate product effectiveness and human clinical trials to evaluate the safety, tolerability and effectiveness of treated blood components. Since assuming responsibility for regulatory approval of the INTERCEPT Blood System in the United States in 2006, we have used the same modular process for our PMA application for the platelet system that Baxter had used in the United States. The content, order and submission timing of the modules must be approved by the FDA, and a modular PMA application cannot be approved until all modules have been submitted to, reviewed by and accepted by the FDA.
We completed a Phase III clinical trial of the platelet system in the United States in March 2001 and a supplemental analysis of data from this trial in 2005. We submitted this information along with several other modules of our PMA, to the FDA. The FDA has indicated that the clinical trial data and supplemental analysis are not sufficient to support a PMA and we will therefore need to conduct and complete an additional Phase III clinical trial before we can complete our regulatory submission to the FDAl. In November 2009, we and the FDA
11
Table of Contents
presented a proposed clinical trial protocol for a second Phase III clinical trial for platelets to the BPAC. The outcome of that meeting was the support for the design and endpoints of a clinical trial for INTERCEPT-treated platelets, subject to changes regarding the sensitivity of the safety endpoint. We are currently in discussions with the FDA regarding further details of the proposed additional Phase III clinical trial. Currently, we have no plans to initiate such a clinical trial unless adequate funding can be secured.
We have completed Phase IIIa, Phase IIIb and Phase IIIc clinical trials of the plasma system in the United States, reports for which were filed with the FDA during 2005. We have not submitted any applications for regulatory approval of the plasma system in the United States. INTERCEPT-treated plasma was recently granted orphan drug status by the FDA Office of Orphan Products Development for the treatment of TTP. Although we have completed Phase III clinical trials in this patient population, the FDA may require supportive supplemental data collected in commercial use in TTP patients receiving INTERCEPT-treated plasma in Europe, or they may require us to complete additional Phase III clinical trials, before approval would be granted. We are seeking clarity on the clinical pathway with the FDA, and we do not yet know if the FDA will require any additional clinical trials. If additional clinical trials are required for approval, we will likely only initiate such trials if adequate funding can be secured.
The FDA inspects the facilities at which products are manufactured and will not permit clinical studies with a product or approve a product unless compliance with current Good Manufacturing Practice or Quality System Regulation requirements is satisfactory. The facilities of the principal third-party suppliers that manufacture our products are not currently FDA-qualified.
In addition to regulating our blood safety products, CBER also regulates the blood collection centers and the blood products that they prepare using the INTERCEPT Blood System. If our products were to be approved by the FDA, US-based blood centers will be required to obtain site-specific licenses prior to engaging in interstate transport of blood components processed using the INTERCEPT Blood System. Any delay in obtaining these licenses would adversely impact our ability to sell products in the United States.
We believe that, in deciding whether the INTERCEPT Blood System is safe and effective, regulatory authorities have taken, and are expected to take, into account whether it adversely affects the therapeutic efficacy of blood components as compared to the therapeutic efficacy of blood components not treated with the system. Data from human clinical studies must demonstrate the safety of treated blood components and their therapeutic comparability to untreated blood components. In addition, regulatory authorities will weigh the system’s safety, including potential toxicities of the inactivation compounds, and other risks against the benefits of using the system in a blood supply that has become safer. We have conducted many toxicology studies designed to demonstrate the INTERCEPT Blood System’s safety. There can be no assurance that regulatory authorities will not require further toxicology or other studies of our products. Based on discussions with the FDA and European regulatory authorities, we believe that data only from laboratory and animal studies, not data from human clinical studies, will be required to demonstrate the system’s efficacy in inactivating pathogens. In light of these criteria, our clinical trial programs for the INTERCEPT Blood System consist of studies that differ from typical Phase I, Phase II and Phase III clinical studies.
The INTERCEPT Blood System for red blood cells preclinical and clinical studies have been conducted using prototype system disposables and devices. In addition to the clinical trials, a number of manufacturing and validation activities must be completed before we could sell the red blood cell product.
Further discussion of our regulatory and clinical trial status can be found in under “Item 1A—Risk Factors” of this Annual Report on Form 10-K, under the risk factor titled: “Our products, blood products treated with the INTERCEPT Blood System and we are subject to extensive regulation by domestic and foreign authorities. If our preclinical and clinical data are not considered sufficient by a country’s regulatory authorities to grant marketing approval, we will be unable to commercialize our products and generate revenue in that country. Our red blood cell system requires extensive additional testing and development.”
12
Table of Contents
Health Care Reimbursement and Reform
Our ability to commercialize our products successfully will depend in part on the extent to which appropriate reimbursement levels for the cost of the products and related treatment are obtained. The recent United States healthcare reform act and ongoing cost saving efforts in the United States and in other regions of the world may have an impact on our ability to profitably commercialize the INTERCEPT Blood System in the United States and elsewhere.
Employees
As of December 31, 2011, we had 82 employees, 24 of whom were engaged in research and development and 58 in selling, general and administrative activities. Of the 58 employees engaged in selling, general, and administrative activities, 30 were employed by our European subsidiary, Cerus Europe B.V. None of our employees are covered by collective bargaining agreements, and we believe that our relationship with our employees is good.
Available Information
We maintain a website at www.cerus.com; however, information found on our website is not incorporated by reference into this report. We make available free of charge on or through our website our annual report on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934, as amended, or Exchange Act, as soon as reasonably practicable after we electronically file such material with, or furnish it to, the Securities Exchange Commission.
Financial Information
Our financial information including our consolidated balance sheets, results of operations, statements of cash flows, statements of stockholders’ equity and the related footnotes thereto, can be found under “Item 15—Exhibits and Financial Statement Schedules” in Part IV of this Annual Report on Form 10-K.
| Item 1A. | Risk Factors |
Our business faces significant risks. If any of the events or circumstances described in the following risks actually occurs, our business may suffer, the trading price of our common stock could decline and our financial condition or results of operations could be harmed. These risks should be read in conjunction with the other information set forth in this report. The risks and uncertainties described below are not the only ones facing us. There may be additional risks faced by our business. Other events that we do not currently anticipate or that we currently deem immaterial also may adversely affect our financial condition or results of operations.
The INTERCEPT Blood System may not achieve broad market acceptance.
We must address issues and concerns from broad constituencies involved in the healthcare system, from blood centers to patients, transfusing physicians, hospitals, private and public sector payors, regulatory bodies and public health authorities. We may be unable to demonstrate to these constituencies that the INTERCEPT Blood System is safe, effective and economical or that the benefits of using the INTERCEPT Blood System products justify their cost.
Use of the platelet system results in some processing loss of platelets. If the loss of platelets leads to increased costs for our customers, our customers or prospective customers believe that the loss of platelet reduces the efficacy of the transfusion unit, or our process requires changes in blood center or clinical regimens, prospective customers may not adopt our platelet system. Certain studies have indicated that transfusion of conventionally prepared platelets may yield higher post-transfusion platelet counts (according to a measurement
13
Table of Contents
called “corrected count increment”) and may be more effective than transfusion of INTERCEPT-treated platelets. While certain studies also demonstrate that INTERCEPT-treated platelets retain therapeutic function comparable to conventional platelets, customers may choose not to adopt our platelet system due to considerations relating to corrected count increment or efficacy.
The INTERCEPT Blood System does not inactivate all known pathogens, and the inability of the INTERCEPT Blood System to inactivate certain pathogens may limit its market acceptance. For example, due to the biology of certain non-lipid enveloped viruses, including the hepatitis A virus, our products have not been demonstrated to be effective in the inactivation of these viruses. In addition, for human parvovirus B-19, which is also a non-lipid-enveloped virus, our testing has not demonstrated a high level of inactivation. Although we have shown high levels of inactivation of a broad spectrum of lipid-enveloped viruses, some customers may choose not to adopt our products based on considerations concerning inability to inactivate, or limited inactivation, of certain non-lipid-enveloped viruses. Similarly, although our product has been demonstrated to effectively inactivate spore-forming bacteria, our products have not been shown to be effective in inactivating bacterial spores, once formed. In addition, since prions do not contain nucleic acid, our products do not inactivate prions. While transmission of prions has not been a major problem in blood transfusions, and we are not aware of any competing products that inactivate prions, the inability to inactivate prions may limit market acceptance of our products.
We have conducted studies of our products in both in vitro and in vivo environments using well-established tests that are accepted by regulatory bodies. When an in vitro test was not generally available or not well-established, we conducted in vivo studies in mammalian models to predict human responses. Although we have no reason to believe that the in vitro and in vivo studies are not predictive of actual results in humans, we cannot be certain that the results of these in vitro and in vivo studies accurately predict the actual results in humans in all cases. To the extent that actual results in human patients differs from the results of our in vitro or in vivo testing, market acceptance of our products may be negatively impacted.
Furthermore, due to limitations of detective tests, we cannot exclude that a sufficient quantity of pathogen or pathogens may still be present in active form which could present a risk of infection to the transfused patient. Such uncertainty may limit the market acceptance of our products.
If customers experience operational or technical problems with the use of INTERCEPT Blood System products, market acceptance may be reduced. For example, if adverse events arise from incomplete inactivation of pathogens, improper processing or user error, or if testing of INTERCEPT-treated blood samples fails to reliably confirm pathogen inactivation, whether or not directly attributable to the INTERCEPT Blood System, customers may refrain from purchasing the products. In addition, there is a risk that further studies we or others may conduct will show results inconsistent with previous studies. Should this happen, potential customers may delay or choose not to adopt our products, and existing customers may cease use of our products.
Market acceptance of our products is affected by blood center budgets and the availability of reimbursement from governments, managed care payors, such as insurance companies, or other third parties. In many cases, due to the structure of the blood products industry, we will have little control over budget and reimbursement discussions, which generally occur between blood centers and national or regional ministries of health and private payors. Even if a particular blood center is prepared to adopt the INTERCEPT Blood System, their hospital customers may not accept, or may not have the budget to purchase, INTERCEPT-treated blood products. Since blood centers would likely not eliminate the practice of screening donors or testing blood for pathogens prior to transfusion, even after implementing our products some blood centers may not be able to identify enough cost offsets to afford to purchase our products. Budgetary concerns may be further exacerbated by the economic austerity programs implemented in European countries, which may limit the adoption of new technologies, including our products. Furthermore, it is difficult to predict the reimbursement status of newly approved, novel medical device products. In certain countries, governments have issued regulations relating to the pricing and profitability of medical products and medical products companies. Health care reform in the United States has also placed downward pressure on the pricing of medical products.
14
Table of Contents
Product adoption in Europe and other regions may be negatively affected because we do not have United States Food and Drug Administration, or FDA, approval for any of our products. In addition, if we do not achieve widespread product adoption in key European countries, adoption in other countries may be affected.
The market for the INTERCEPT Blood System is highly concentrated with few customers, including often-dominant regional or national blood collection entities. Even if our products receive regulatory approval and reimbursement is available, failure to effectively market, promote, distribute, price or sell our products to any of these large customers could significantly delay or even diminish potential product revenue in those geographies. The market for our pathogen inactivation systems in the United States is highly concentrated, dominated by a small number of blood collection organizations. In many countries in Western Europe and in Japan, various national blood transfusion services or Red Cross organizations collect, store and distribute virtually all of their respective nations’ blood and blood components supply. In Europe, the largest markets for our products are in Germany, France, and England. In Germany, decisions on product adoption and subsequent reimbursement are made on a regional or even blood center-by-blood center basis, but depend on both local approvals and centralized regulatory approvals from the Paul Ehrlich Institute, or PEI. Product characteristics relating to platelet dose of INTERCEPT-treated platelets that have received marketing authorization from the PEI may be incompatible with market requirements. Some potential customers may await further safety information or additional studies before choosing whether to adopt our products. Customers or prospective customers may conduct and complete their own clinical trials before adopting our products. For instance, we understand that the largest group of blood centers in Germany will not purchase our products on a routine basis until and unless that group completes a clinical trial using our products. We cannot predict the final trial design, number of transfusions, enrollment duration, estimated time it will take to complete such a trial, or trial outcome. While INTERCEPT-treated platelets and plasma have received in-country regulatory approval and reimbursement rates have been established in France, adoption throughout France has been limited to certain blood centers. Decisions on product adoption in England are centralized with the National Blood Service and we understand that the National Blood Service has decided to implement bacterial detection testing for platelets before considering pathogen inactivation. The Japanese Red Cross controls a significant majority of blood transfusions in Japan and exerts a high degree of influence on the adoption and use of blood safety measures in Japan. The Japanese Red Cross has been reviewing preclinical and clinical data on pathogen inactivation of blood over a number of years and has yet to make a formal determination to adopt any pathogen inactivation approach. We understand that the Japanese Red Cross has begun formal evaluation of a competing technology. Before the Japanese Red Cross considers our products, we understand that we may need to commit to making certain product configuration changes in order to allow the INTERCEPT Blood System to integrate with the collection platforms of the Japanese Red Cross.
Adverse market and economic conditions may exacerbate certain risks affecting our business.
Sales of our products are dependent on reimbursement from government health administration authorities, distribution partners and other organizations. As a result of adverse conditions affecting the global economies and credit and financial markets, including the current sovereign debt crisis in certain countries in Europe and disruptions due to political instability or otherwise, these organizations may be unable to satisfy their reimbursement obligations, or may delay payment for the INTERCEPT Blood System. In addition, political and economic instability in Europe may diminish the value of the Euro, which could reduce our reported product revenue.
15
Table of Contents
Our products, blood products treated with the INTERCEPT Blood System and we are subject to extensive regulation by domestic and foreign authorities. If our preclinical and clinical data are not considered sufficient by a country’s regulatory authorities to grant marketing approval, we will be unable to commercialize our products and generate revenue in that country. Our red blood cell system requires extensive additional testing and development.
Our products, both those sold commercially and those under development are subject to extensive and rigorous regulation by local, state and federal regulatory authorities in the United States and by foreign regulatory bodies. These regulations are wide-ranging and govern, among other things:
| • | development; |
| • | testing; |
| • | manufacturing; |
| • | labeling; |
| • | storage; |
| • | pre-market clearance or approval; |
| • | sales and distribution; |
| • | use standards and documentation; |
| • | post-launch surveillance; |
| • | quality; |
| • | advertising and promotion; and |
| • | reimbursement. |
Our products must satisfy rigorous standards of safety and efficacy and we must adhere to quality standards regarding manufacturing and customer-facing business processes before the FDA and international regulatory authorities can approve them for commercial use. For our product candidates, we must provide the FDA and international regulatory authorities with preclinical, clinical and manufacturing data demonstrating that our products are safe, effective and in compliance with government regulations before the products can be approved for commercial sale. The process of obtaining FDA and other required regulatory approvals is expensive and uncertain, and typically takes a number of years. We may continue to encounter significant delays or excessive costs in our efforts to secure necessary approvals or licenses, or we may not be successful at all.
Clinical trials are particularly expensive and have a high risk of failure. Any of our product candidates may fail in the testing phase or may not achieve results sufficient to attain market acceptance, which could prevent us from achieving profitability. We do not know whether we will begin and conduct planned clinical trials on schedule, if at all. Significant delays in clinical testing could materially impact our clinical trials. Criteria for regulatory approval in blood safety indications are evolving with competitive advances in the standard of care against which new product candidates are judged, as well as with changing market needs and reimbursement levels. Clinical trial design, including enrollment criteria, endpoints, and anticipated label claims are thus subject to change, even if original objectives are being met. In addition to the reasons stated above, clinical trials can be delayed for a variety of reasons, including delays in obtaining regulatory approval to commence a study, delays in reaching agreement on acceptable clinical study agreement terms with prospective clinical sites, delays in obtaining institutional review board approval to conduct a study at a prospective clinical site and delays in recruiting subjects to participate in a study. We do not know whether any clinical trials will result in marketable products. Typically, there is a high rate of failure for product candidates in preclinical and clinical trials and products emerging from any successful trial may not reach the market for several years.
Enrollment criteria for certain of our clinical trials may be quite narrow. For instance, clinical trials previously conducted using INTERCEPT-treated plasma for patients with thrombotic thrombocytopenic purpura
16
Table of Contents
lasted approximately four years due in part, to the difficulties associated with enrolling qualified patients. Consequently, we may be unable to recruit suitable patients into clinical trials on a timely basis, if at all. We cannot rely on interim results of trials to predict their final results, and acceptable results in early trials might not be repeated in later trials. Any trial may fail to produce results satisfactory to the FDA or foreign regulatory authorities. In addition, preclinical and clinical data can be interpreted in different ways, which could delay, limit or prevent regulatory approval. Negative or inconclusive results from a preclinical study or clinical trial or adverse medical events during a clinical trial could cause a preclinical study or clinical trial to be repeated, require other studies to be performed or cause a program to be terminated, even if other studies or trials relating to a program are successful.
Outside the United States, regulations vary by country, including the requirements for approvals or clearance to market, the time required for regulatory review and the sanctions imposed for violations. In addition to CE mark documentation, countries outside the European Union may require clinical data submissions, registration packages, import licenses or other documentation.
In May 2007, we obtained a CE mark extension in our name from European Union regulators for our platelet system and will need to obtain an extension every five years. We or our customers may also be required to conduct additional testing in order to obtain regulatory approval in countries that do not recognize the CE mark as being adequate for commercializing the INTERCEPT Blood System in those countries. The level of additional product testing varies by country, but could be expensive or take a long time to complete. In addition, regulatory agencies are able to withdraw or suspend previously issued approvals.
We completed our Phase III clinical trial of the platelet system in the United States in March 2001 and submitted data from this trial, along with several other modules of our pre-market approval application, to the FDA. Based on discussions with the FDA, we performed an additional blinded analysis of the clinical trial data, under the direction of an independent expert physician panel, to determine if apparent differences between treatment groups in the category of pulmonary adverse events reported in the study were attributable to discrepancies in safety results. The reassessment of primary patient records by the expert physician panel showed no statistically significant differences between groups. This reassessment differed from the earlier analysis of adverse events that was based on clinical trial case report forms and had shown statistically significant differences in specific pulmonary events. We submitted a report of the analysis to the FDA for review. We understand that our reassessment of our previously completed Phase III clinical trial data will not be sufficient to address the FDA’s questions. In November 2009, we and the FDA presented a proposed clinical trial protocol for a second Phase III clinical trial for platelets to the FDA’s Blood Product Advisory Committee, or BPAC. Although the BPAC agreed with the proposed trial design, safety endpoints and efficacy endpoints, we believe we will need to reach agreement with the FDA on the means necessary to satisfy the BPAC’s request for more stringent safety margins than we had proposed. In order to meet the more stringent safety margins, we may need to enroll and collect data from more patients than what we had initially proposed to BPAC. Until the final study size and design requirements are determined, we will not be able to assess the feasibility of a second Phase III clinical trial. The dimensions of such a Phase III clinical trial may be prohibitive due either to prospective cost, availability of patients in the target population, or logistics. We have no plans to initiate such a trial unless adequate funding is secured. The additional Phase III clinical trial will need to be completed and data submitted to the FDA before we can complete our regulatory submission.
In September 2011, we obtained a CE mark extension in our name from European Union regulators for our plasma system and final French approval of INTERCEPT-treated plasma in May 2007. In February 2011, the first approval for use of INTERCEPT-treated plasma was obtained from the Paul Ehrlich Institute by a blood center in Germany. In some countries, including several in Europe, we or our customers may be required to perform additional clinical studies or submit manufacturing and marketing applications in order to obtain regulatory approval.
We have completed Phase IIIa, Phase IIIb and Phase IIIc clinical trials of the plasma system, in the United States, reports for which were filed with the FDA during 2005. We have not submitted any applications for
17
Table of Contents
regulatory approval of the plasma system in the United States or any other regions other than in Europe. INTERCEPT-treated plasma was recently granted orphan drug status by the FDA Office of Orphan Products Development for the treatment of thrombotic thrombocytopenic purpura. Although we have completed Phase III clinical trials in this patient population, the FDA may require us to compile supplemental data collected from commercial use in Europe or to complete additional Phase III clinical trials before approval would be granted. Should the FDA require us to complete additional clinical trials, we will likely need to secure adequate funding before we would initiate any such trials.
Before the FDA determines whether to approve the INTERCEPT Blood System products, we expect our approval applications to be reviewed by BPAC. Should the FDA ask BPAC questions, we expect BPAC to answer those questions and make recommendations to the FDA. Even if BPAC were to recommend approval of one or more of our products, the FDA would not necessarily have to approve those products. If BPAC were to answer FDA questions recommending against approval of one or more of our products, the FDA would have to take into consideration the points of concern raised by BPAC which could affect the approval of the products.
If our product candidates receive approval for commercial sale in the United States, their marketing and manufacturing will be subject to continuing FDA and other regulatory requirements, such as requirements to comply with Good Manufacturing Practice, or GMP, and ISO 13485, a quality management system standard applicable to the products we sell in Europe. The failure to comply with these requirements on an ongoing basis could result in delaying or precluding commercialization efforts in certain geographies, including the United States, and could result in an enforcement action, which could harm our business. The current manufacturing sites we rely upon for producing the platelet and plasma system products for international distribution and sale are not FDA-qualified facilities. It will require both time and expense to obtain such qualification.
The FDA will require, and other regulatory authorities may also require, a post-marketing clinical study, which can involve significant expense. Governments or regulatory authorities may impose new regulations or other changes or we may discover that we are subject to additional regulations that could further delay or preclude regulatory approval and subsequent adoption of our potential products. We cannot predict the impact of adverse governmental regulation that might arise from future legislative or administrative action.
We have conducted many toxicology studies to demonstrate the INTERCEPT platelet and plasma systems’ safety, and we have conducted and plan to conduct toxicology studies for the INTERCEPT red blood cell system throughout the product development process. At any time, the FDA and other regulatory authorities may require further toxicology or other studies to further demonstrate our products’ safety, which could delay commercialization. In addition, the FDA or foreign regulatory authorities may alter guidance at any time as to what constitutes acceptable clinical trial endpoints or trial design, which may necessitate a redesign of our product or proposed clinical trials and cause us to incur substantial additional expense or time in attempting to gain regulatory approval. We believe the FDA and other regulatory authorities are likely to weigh the potential risks of using our pathogen inactivation products against the incremental benefits, which may be difficult or impossible to quantify. We expect the FDA will require us to demonstrate a very low level of potential side effects in the proposed second Phase III trial of the platelet system.
As a result of the termination of Phase III clinical trials of our red blood cell system due to the detection of antibody reactivity to red blood cells treated with the INTERCEPT red blood cell system in two patients in the chronic arm of the trials, we have been conducting additional research and development activities on our red blood cell system to reduce the potential for antibody reactivity to treated red blood cells. Based upon an internal evaluation of the results from these additional research activities as well as additional in vitro and in vivo studies and after consulting with regulatory authorities, we initiated a new Phase I clinical trial in the fourth quarter of 2008 to test modifications to the red blood cell system. That new Phase I clinical trial was completed in early 2010, successfully meeting our primary endpoint of red cell recovery measured twenty-four hours after transfusion. In addition to red cell recovery, we also measured red cell lifespan, measured as the half-life of red cells circulating in transfusion recipients. INTERCEPT-treated red blood cells fell within the established normal reference range for red blood cells. Non-treated red cells were above the established normal reference range.
18
Table of Contents
We plan to initiate a Phase III clinical trial for acute anemia patients in Europe upon acceptance the proposed clinical trial application by European regulators. We are also planning to prepare a clinical trial application for a Phase III clinical trial for chronic anemia patients in Europe. We expect to conduct a further process validation study in Europe prior to commencement of such trials.We understand that the FDA will likely require us to complete an additional recovery and lifespan study and at least one additional Phase III clinical trial before we would be able to potentially obtain approval for INTERCEPT-treated red blood cells in the United States. Such studies could prolong development of the red blood cell program. Significantly lower lifespan for INTERCEPT-treated red blood cells compared to non-treated red blood cells may limit our ability to obtain regulatory approval for the product. We also understand that the planned Phase III clinical trial in Europe may be sufficient to receive CE mark but it would need to be supplemented by additional Phase III clinical trials for approval in certain countries in Europe, including in France and Germany. These additional Phase III clinical trials will likely need to demonstrate equivalency of INTERCEPT-treated red blood cells compared to conventional red blood cells. A number of trial design issues that could impact efficacy, regulatory approval and market acceptance will need to be resolved prior to the initiation of further clinical trials. We will also need to complete a number of in vitro studies, finalize development of the final commercial configuration of the red blood cell system and manufacture and validate sufficient quantities of the final red blood cell system prior to receiving regulatory approvals in Europe or the United States. Many of these activities will require capital beyond that which we currently have. If we are unsuccessful in advancing a modified red blood cell system through clinical trials, resolving process and product design issues or in obtaining subsequent regulatory approvals and acceptable reimbursement rates, we may never realize a return on our research and development expenses incurred to date in the red blood cell system program. Regulatory delays can also materially impact our product development costs. If we experience delays in testing, conducting trials or approvals, our product development costs will increase.
Regulatory agencies may limit the uses, or indications, for which any of our products are approved. For example, we believe that the INTERCEPT Blood System products will be able to claim the inactivation of particular pathogens only to the extent we have laboratory data to support such claims. After regulatory approval for the initial indications, further studies may be necessary to gain approval for the use of the product for additional indications.
In addition to the regulatory requirements applicable to us and to our products, there are regulatory requirements in several countries around the world, including the United States, Germany, Canada, Austria, and Australia, and other countries, applicable to our prospective customers of INTERCEPT Blood System products, the blood centers that process and distribute blood and blood products. In those countries, blood centers and other customers are required to obtain approved license supplements from the appropriate regulatory authorities in each country before making available blood products processed with our pathogen inactivation systems to hospitals and transfusing physicians. Our customers may lack the resources or capability to obtain such regulatory approvals. These requirements or regulators’ delays in approving license applications or supplements may deter some blood centers from using our products. Blood centers that do submit applications or supplements for manufacturing and sale may face disapproval or delays in approval that could provide further delay or deter them from using our products. The regulatory impact on potential customers could slow or limit the potential sales of our products.
In August 2010, in connection with our acquisition of certain assets from BioOne, we regained the rights to commercialize the platelet and plasma systems in Japan, China, Taiwan, South Korea, Vietnam, Thailand, and Singapore. Regulatory authorities in these countries may require, among other requirements, that our platelet and plasma systems to be widely adopted commercially in Europe or approved by the FDA before the platelet and plasma systems are considered for approval.
19
Table of Contents
We have limited experience operating a global commercial organization. We rely on third parties to market, sell, distribute and maintain our products and to maintain customer relationships in certain countries.
We are responsible for sales, marketing, distribution, maintenance and regulatory support of the INTERCEPT Blood System worldwide. If we fail in our efforts to develop or maintain such internal competencies or establish acceptable relationships with third parties on a timely basis, our ability to commercialize the INTERCEPT Blood System may be irreparably harmed.
We have a wholly-owned subsidiary, headquartered in The Netherlands, dedicated primarily to selling and marketing the platelet and plasma systems in geographies where the INTERCEPT platelet and plasma systems are approved or can be imported through the import license process. We will need to maintain and continue to increase our competence in a number of functions, including sales, marketing, regulatory, inventory and logistics, customer service, credit and collections, risk management, and quality assurance systems. Many of these competencies require compliance with European Union and local standards and practices, with which we have limited experience.
We have entered into distribution agreements, generally on a geographically exclusive basis, with distributors in countries where we have limited abilities to commercialize our products directly. We rely on these distributors to obtain any necessary in-country regulatory approvals, market and sell the INTERCEPT Blood System, provide customer and technical product support, maintain inventories, and adhere to our quality system in all material respects, among other activities. While our contracts generally require distributors to exercise diligence, these distributors may fail to commercialize the INTERCEPT Blood System in their respective territories. They may fail to sell product inventory they have purchased from us to end customers. Initial purchases of illuminators or disposable kits by these third parties may not lead to follow-on purchases of disposable platelet and plasma system kits. We have limited visibility into the identity and requirements of blood banking customers these distributors may have. Accordingly, we may be unable to ensure our distributors properly maintain illuminators sold or provide quality technical services to the blood banking customers to which they sell. Agreements with our distributors typically require the distributor to maintain quality standards that are compliant with standards generally accepted for medical devices. We may be unable to ensure that our distributors are compliant with such standards. Distributors may irreparably harm relationships with local existing and prospective customers and our standing with the blood banking community in general. We may have little recourse, short of termination, in the event that a distributor fails to execute according to our expectations and contractual provisions.
Our manufacturing supply chain exposes us to significant risks.
INTERCEPT platelet and plasma disposable kits are manufactured and assembled by Fenwal. Fenwal has agreed, through a supply agreement signed with us in December 2008, to manufacture disposable kits for the platelet and plasma systems for us. After 2013, Fenwal may terminate the supply agreement, provided that Fenwal shall have provided us thirty months prior notice of termination. Fenwal is our sole supplier for manufacture of these products. Fenwal may fail to manufacture an adequate supply of disposable kits or to do so on a cost effective basis, which would subject us to loss of revenue and reduced contribution margin if production of INTERCEPT disposable kits is produced at a facility that also produces Fenwal-branded products. Should production for Fenwal’s own products decline, our products may absorb more overhead, which would negatively impact our gross margins.
We also have contracts with independent suppliers, including Ash Stevens for the manufacture of amotosalen, our proprietary compound for inactivating pathogens, Porex for the manufacture of components of the compound adsorption devices, and NOVA Biomedical Corporation, or NOVA, for the manufacture of illuminators and certain components of the INTERCEPT Blood System. These independent suppliers are our sole suppliers for such components.
Our agreement with Porex expires on December 31, 2012. Prior to the expiration of that contract, we will need to negotiate an amendment or enter into a new contract with Porex, or identify, validate and contract with an
20
Table of Contents
alternate supplier for such components of the compound adsorption devices. Failure to reach agreement with Porex or an alternate supplier would impact our ability to manufacture INTERCEPT disposable kits and supply existing and prospective customers. We also have contracts with other companies who are our sole suppliers of raw materials used to make compound adsorption devices.
Facilities at which the INTERCEPT Blood System or its components are manufactured may cease operations for planned or unplanned reasons, causing at least temporary interruptions in supply. We do not have qualified suppliers beyond those on whom we currently rely, and we understand that Fenwal relies substantially on sole suppliers of certain materials for our products. If we need to or choose to identify and qualify alternate suppliers, the process will be time consuming and costly. Even a temporary failure to supply adequate numbers of INTERCEPT Blood System components may cause an irreparable loss of customer goodwill. If we conclude that supply of the INTERCEPT Blood System or components from Fenwal and others is uncertain, we may choose to build and maintain inventories of raw materials, work-in-process components, or finished goods, which would consume capital resources and may cause our supply chain to be less efficient.
Currently, we have depleted our inventory of saleable illuminators and have instructed NOVA to begin manufacture of new illuminators to supply customer demand. Should NOVA have difficulties manufacturing sufficient quantities of illuminators in a timely manner, we may not be able to supply customer demand or provide replacement illuminators to existing customers. Some components of the illuminators are no longer manufactured, which will require us to identify and qualify replacement components and may require that we conduct additional studies, which could include clinical trials, to demonstrate equivalency or validate any required design or component changes. Future supply of illuminators is limited to availability of components, some of which are in short supply or are no longer manufactured. We will likely need to redesign the illuminators used in the platelet and plasma systems. Such redesign may be expensive and lead to regulatory delays in obtaining approvals to market the redesigned device.
Fenwal manufactures our platelet and plasma systems in facilities that are not FDA-approved. In order to be used in clinical studies or sold in the United States, our products would be required to be manufactured in FDA-approved facilities. FDA validation of manufacturing facilities, whether owned by Fenwal or by other parties, will be costly and time-consuming.
If we attempt to establish alternate manufacturers, we will be dependent on Fenwal to transfer know-how relevant to the manufacture of the INTERCEPT Blood System; however, certain of Fenwal’s materials, manufacturing processes and methods are proprietary to Fenwal. We may be unable to establish alternate sources of supply to Fenwal, NOVA, or other suppliers without having to redesign certain elements of the platelet and plasma systems. Such redesign may be costly, time consuming and require further regulatory review. Fenwal is not obligated to provide support for development and testing of improvements or changes we may make to the INTERCEPT Blood System. We may be unable to identify, select, and qualify such manufacturers or those third parties able to provide support for development and testing activities on a timely basis or enter into contracts with them on reasonable terms, if at all. Raw material and component suppliers may not meet quality specifications we have set, which would cause a disruption in supply and may lead to lost sales and irreparable damage to our customer relationships. In 2011, non-conformities in certain components caused delays in manufacturing of INTERCEPT kits. Should similar or other non-conformities occur in the future, we may be unable to manufacture products to meet customer demands. Moreover, the inclusion of components manufactured by new suppliers could require us to seek new or updated approvals from regulatory authorities, which could result in delays in product delivery. We may not receive any such required regulatory approvals.
In the event of a failure by Fenwal or other manufacturers to perform their obligations to supply components of the INTERCEPT Blood System to us, damages recoverable by us may be insufficient to compensate us for the full loss of business opportunity. Many of our supply agreements contain limitations on incidental and consequential damages that we may recover. A supplier’s potential liability in the event of non-performance may not be sufficient to compel the supplier to continue to act in conformity with our agreements.
21
Table of Contents
Our product supply chain requires us to purchase certain components in minimum quantities and may result in a production cycle of more than one year. Significant disruptions to any of the steps in our supply chain process may result in longer productions cycles which could lead to inefficient use of cash.
We are in the early stages of commercializing the INTERCEPT Blood System. As such, we have limited experience overseeing the manufacture of INTERCEPT illuminators and disposable kits and may encounter unforeseen manufacturing difficulties which, at a minimum, may lead to higher than anticipated costs, scrap rates or delays in manufacturing products. In addition, we may not receive timely or accurate demand information from distributors or may not accurately forecast demand ourselves for the INTERCEPT Blood System. As a result, we may carry excess work-in-process or finished goods inventory, which would consume capital resources and may become obsolete, or our inventory may be inadequate to meet customer demand. We have entered into certain public tenders, some which call for us to maintain certain minimum levels of inventory. If our suppliers fail to produce components or our finished products satisfactorily, timely, at acceptable costs, and in sufficient quantities, we may incur delays, shortfalls and additional expenses, or non-compliance with certain public tenders which may in turn result in permanent harm to our customer relations or loss of customers. Our platelet and plasma system disposables have received regulatory approval for two-year shelf lives. We and our distributors may be unable to ship product to customers prior to the expiration of product shelf life, which would require that we destroy or consume the outdated inventory in product demonstration activities. Product expiration may in turn lead to elevated product demonstration costs or reduced gross margins.
The platelet system is not compatible with some commercial platelet collection methods.
The equipment and materials used to collect platelets vary by manufacturer and by geographic region. Platelets may be collected from a single donor by apheresis using an automated collection machine. Apheresis devices currently used in the United States and European markets differ, among other characteristics, in their ability to collect platelets in reduced volumes of plasma. Platelet concentrates may also be prepared from whole blood by pooling together platelets from multiple donors. There are two commonly used methods for preparing whole blood platelets: the buffy coat method, which is used extensively in Europe, and the pooled random donor method, which is used in the United States. Our system for platelets is designed to work with platelets collected and stored in storage solutions, called Intersol and SSP+, and for platelets suspended in plasma.
In order to address the entire market in the United States and Japan, we would need to develop and test additional configurations of the platelet system. We estimate that the majority of platelets used in the United States are collected by apheresis, though a significant minority is prepared from pooled random donor platelets derived from whole blood collections. In order to gain regulatory approvals for a pathogen inactivation system compatible with random donor platelets, we will need to perform additional product development and testing, including additional clinical trials. Similarly, to achieve market acceptance in certain geographies, we may be required to design, develop and test new product configurations for the platelet and plasma systems. These development activities would increase our costs significantly, and may not be successful.
Other manufacturers supplying blood component collection platforms to the market may resist our efforts to make the INTERCEPT Blood System compatible with their platforms and may have competing pathogen inactivation technologies. Attaining compatibility with collection platforms manufactured by others may require adaptations to either the INTERCEPT Blood System or to the collection platforms, which may be difficult to engineer, expensive to implement and test, require additional clinical trials, cause delays in regulatory approval and/or be commercially unattractive to pursue. These development activities will increase our costs significantly, and may not be successful. Market acceptance of the INTERCEPT Blood System may be delayed until the system receives regulatory approval for use on such other equipment, if required.
22
Table of Contents
We have used prototype components in our preclinical studies and clinical trials of the INTERCEPT red blood cell system and have not completed the components’ commercial design. We will be required to identify and enter into agreements with third parties to manufacture the red blood cell system.
Our red blood cell system that was used in our preclinical studies and Phase I red blood cell trial was a prototype of the system to be used in the final products. As a result, we plan to perform additional preclinical and clinical studies using the commercial versions of the systems to demonstrate the acceptability of the commercial configuration and the equivalence of the prototypes and the commercial products, which may increase our expenses and delay the commercialization of our products. We may determine that the red blood cell system may not be commercially feasible from potential customers’ perspectives. If we fail to develop commercial versions of the INTERCEPT red blood cell system on schedule, our potential revenue would be delayed or diminished and our potential competitors may be able to bring products to market before we do.
In addition, the design and engineering effort required to complete the final commercial product will likely be substantial and time-consuming. As with any complex development effort, we expect to encounter design, engineering and manufacturing issues. Such issues have previously arisen, sometimes unexpectedly, and solutions to these issues have not always been readily forthcoming. Additional unforeseen design, engineering and manufacturing issues may arise in the future, which could increase the development cost and delay commercialization of our products.
We will need to identify and contract with manufacturers who can develop processes to manufacture components and the compounds used in the red blood cell system. For commercial manufacturing, we will need to demonstrate to regulatory authorities that the commercial scale manufacturing processes comply with government regulations and that the compounds are equivalent to originally licensed compounds. It may be difficult to economically manufacture the red blood cell system on a commercial scale.
If our competitors develop superior products to ours or market their products more effectively than we market our products, our commercial opportunities could be reduced or eliminated.
We expect our products to encounter significant competition. The INTERCEPT Blood System products compete with other approaches to blood safety currently in use, and may compete with future products that may be developed by others. Our success will depend in part on our ability to respond quickly to customer and prospective customer needs and medical and technological changes brought about by the development and introduction of new products. Competitors’ products or technologies may make our products obsolete or non-competitive before we are able to generate any significant revenue. In addition, competitors or potential competitors may have substantially greater financial and other resources than we have. They may also have greater experience in preclinical testing, human clinical trials and other regulatory approval procedures. If competitors’ products experience significant problems, customers and potential customers may question the safety and efficacy of all pathogen inactivation technologies, including the INTERCEPT Blood System. Such questions and concerns may impair our ability to market and sell the INTERCEPT Blood System.
Several companies have, or are developing, technologies that are, or in the future may be, the basis for products that will directly compete with or reduce the market for our pathogen inactivation systems. A number of companies are specifically focusing on alternative strategies for pathogen inactivation in platelets and plasma. These alternative strategies may be more effective in inactivating certain types of pathogens from blood products, including non-lipid-enveloped pathogens, such as hepatitis A virus, which our products have not demonstrated an ability to inactivate, or human parvovirus B-19, for which our products have not demonstrated a high level of inactivation. While our products can effectively inactivate a broad spectrum of pathogens in blood components, including more robust inactivation of many pathogens than has been shown by other companies, market acceptance of our products may be reduced if customers determine that competitor’s products inactivate a broader range of pathogens that are of particular interest to the transfusion medicine community. In addition, customers and prospective customers may believe that our competitor’s products are safer or more cost effective than INTERCEPT Blood System products. In Europe, several companies, including Grifols S.A., Octapharma
23
Table of Contents
AG and MacoPharma International, are developing or selling commercial pathogen inactivation systems or services to treat fresh frozen plasma. CaridianBCT (becoming TerumoBCT) has developed a pathogen inactivation system for blood products and has been issued CE marks for a pathogen reduction system for both platelets and plasma. We understand that CaridianBCT is also developing a pathogen inactivation system for whole blood. CaridianBCT’s product candidate, if successful, may offer competitive advantages over our INTERCEPT Blood System. CaridianBCT was recently acquired by Terumo Corporation, a large Japanese-based, multinational corporation with more mature products and relationships than we have. Our ability to commercialize our products in certain markets, particularly Japan, may be negatively affected by Terumo’s resources and their pre-existing relationships with regulators and customers. Other companies developing competing products may also offer and sell other blood-banking products and services. As a result, competitors may have pre-existing long-term relationships with customers and may be able to offer synergies for both pathogen inactivation and non-pathogen inactivation products that we are unable to offer.
New methods of testing whole blood for specific pathogens have been approved by the FDA and in Europe, as have tests for bacteria in platelets. Other companies are marketing rapid, point-of-care bacterial tests, and developing synthetic blood product substitutes and products to stimulate the growth of platelets. Development and commercialization of any of these or other related technologies could limit the potential market for our products.
We may be liable and we may need to withdraw our products from the market if our products harm people. We may be liable if an accident occurs in our controlled use of hazardous materials. Our insurance coverage may be inadequate to offset losses we may incur.
We are exposed to potential liability risks inherent in the testing and marketing of medical devices and pharmaceutical products. We may be liable if any of our products cause injury, illness or death. Although we will have completed rigorous preclinical and clinical safety testing prior to marketing our products, there may be harmful effects caused by our products that we are unable to identify in preclinical or clinical testing. In particular, unforeseen, rare reactions or adverse side effects related to long-term use of our products may not be observed until the products are in widespread commercial use. Because of the limited duration and number of patients receiving blood components treated with the INTERCEPT Blood System products in clinical trials, it is possible that harmful effects of our products not observed in clinical and preclinical testing could be discovered after a marketing approval has been received. For example, in cases where we have obtained regulatory approval for our products, we have demonstrated pathogen inactivation to specified levels based on well-established tests. However, there is no way to determine, after treatment by our products, whether our products have completely inactivated all of the pathogens that may be present in blood components. There is also no way to determine whether any residual amount of a pathogen remains in the blood component treated by our products, and there is no way to exclude that such residual amount would be enough to cause disease in the transfused patient. For ethical reasons, we cannot conduct human testing to determine whether an individual who receives a transfusion of a blood component containing a pathogen that was inactivated using the INTERCEPT Blood System might show positive results if tested for an antibody against that pathogen. While we believe, based on the clinical experience of our scientists, that the level of inactivated pathogens would likely be too small to induce a detectable antibody response in diagnostic tests, we cannot exclude that a transfused patient might show positive results if tested for an antibody against that pathogen. We could be subject to a claim from a patient that tests positive, even though that patient did not contract a disease. Later discovery of problems with a product, manufacturer or facility may result in additional restrictions on the product or manufacturer, including withdrawal of the product from the market. We are subject to risks and costs of product recall, which include not only potential out-of-pocket costs, but also potential interruption to our supply chain. In such an event, our customer relations would be harmed and we would incur unforeseen losses.
We maintain product liability insurance, but do not know whether the insurance will provide adequate coverage against potential liabilities. If we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities or be required to limit commercialization of our products.
24
Table of Contents
Our research and development activities involve the controlled use of hazardous materials, including certain hazardous chemicals, radioactive materials and infectious pathogens, such as HIV and hepatitis viruses. Although we believe that our safety procedures for handling and disposing of hazardous materials are adequate and comply with regulatory requirements, we cannot eliminate the risk of accidental contamination or injury. If an accident occurs, we could be held liable for any damages that result.
If we fail to obtain the capital necessary to fund our future operations or if we are unable to generate positive cash flows from our operations, we will need to curtail planned development or sales and commercialization activities.
Our near-term capital requirements are dependent on various factors, including operating costs and working capital investments associated with commercializing the INTERCEPT Blood System, costs associated with pursuing regulatory approval in geographies where we do not currently sell our platelet and plasma systems, costs associated with planning and conducting studies and clinical development of our red blood cell system, timing and magnitude of payments under grants from the United States government, and costs related to creating, maintaining and defending our intellectual property. Our long-term capital requirements will also be dependent on competitive developments, clinical development and regulatory factors. Until we are able to generate a sufficient amount of product revenue and generate positive net cash flows from operations, meeting our long-term capital requirements is in large part subject to access to public and private equity and debt capital markets, as well as to additional collaborative arrangements with partners or government grants, augmented by cash generated from operations and interest income earned on the investment of our cash balances and short-term investments. We believe that cash received from product sales and government grants, our available cash balances, and access to debt will be sufficient to meet our capital requirements for at least the next twelve months. If our assumptions prove to be incorrect, we could consume our available capital resources sooner than we currently expect.
We have borrowed and in the future may borrow capital from institutional and commercial banking sources. Potential borrowings may include restrictive covenants, including covenants that restrict the operation of our business, liens on assets, high effective interest rates and repayment provisions that reduce cash resources and limit future access to capital markets. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. To the extent that we raise additional funds through collaboration or partnering arrangements, we may be required to relinquish some of our rights to product revenues, our technologies or rights to market and sell our products in certain geographies, or grant licenses on terms that are not favorable to us.
As a result of economic conditions and general global economic uncertainty and other factors, we do not know whether additional capital will be available when needed, or that, if available, we will be able to obtain additional capital on reasonable terms. If we are unable to raise additional capital due to disruptions to the global credit and financial markets and general economic uncertainty or other factors, we may need to curtail planned development or commercialization activities. In addition, we will need to obtain additional funds to complete development activities for the red blood cell system necessary for regulatory approval in Europe, and we do not plan on conducting any additional clinical trials of the platelet or the plasma systems in the United States unless and until we can obtain sufficient additional funding.
Historically, we have received significant awards in funding under cooperative agreements with the DoD for the INTERCEPT Blood System. Access to federal grants and cooperative agreements is subject to the authorization of funds and approval of our research plans by various organizations within the federal government, including the United States Congress. The general economic environment, coupled with tight federal budgets, has led to a general decline in the amount available for government funding. There is no assurance that we will be awarded future federal grants and cooperative agreements for the INTERCEPT Blood System.
25
Table of Contents
We have only a limited operating history, and we expect to continue to generate losses.
We may never achieve a profitable level of operations. Our development and selling, general, and administrative expenses have resulted in substantial losses each year since our inception with the exception of the year ended December 31, 2005. The platelet and plasma systems are not yet approved in the United States or in many other countries around the world. The red blood cell system is in clinical development and may never emerge from the clinical development stage as a marketed product. We may be required to reduce the sales price for our products in order to make our products economically attractive to our customers and to governmental and private payors, which may reduce or altogether eliminate our gross profit on sales. At our present sales levels of the platelet and plasma systems, our costs to manufacture, distribute, market, sell, support and administer the systems are in excess of revenue. Contribution from product sales is unlikely to exceed the costs we incur in research, development, and commercialization of the INTERCEPT Blood System for near-term. We expect our losses to continue at least until the INTERCEPT Blood System achieves more significant market acceptance. To the extent that we reach agreement on a clinical pathway with the FDA for any or all of our products and if we choose to pursue such opportunities, we would expect to incur substantial costs which could extend the period during which we expect to operate at a loss.
We have issued debt containing certain covenants that we may be unable to comply with. Our operations may not provide sufficient cash to meet the repayment obligations of the note.
In September 2011, we entered into a loan and security agreement, or Credit Agreement, for $10.0 million, of which we immediately borrowed $5.0 million and subsequently drew an additional $2.3 million from the revolving line of credit under the Credit Agreement. The Credit Agreement is secured by all our current and future assets, except for intellectual property and 35% of our investment in our subsidiary, Cerus Europe B.V. The Credit Agreement requires that we comply with certain customary and routine covenants, including the requirement to maintain a minimum cash balance of $2.5 million and achieve minimum revenue levels, which are measured monthly based on a six-month trailing basis and must be at least 75% of the pre-established future projected revenues for the trailing six-month period. If we are unable to increase our revenues to comply with the covenants in the Credit Agreement, the lender may call the note which would require us to repay the principal of the note sooner than we have anticipated. In the event that the note was called due to non-compliance with the covenants, we may be unable to pay back the principal, which would allow the lender to liquidate collateralized assets. This in turn, would harm our business.
In addition, our operations may not reach the levels needed to meet the scheduled repayment obligations of the note. If we are unable to meet the scheduled repayment obligations of the note using our available cash, we may be forced to liquidate other assets, refinance the notes or issue equity securities to raise the necessary cash to meet our obligations. There is no assurance that we would be able to sufficiently or timely liquidate assets to meet the note’s repayment obligations or that we would be able to refinance the notes or issue equity, in which case our business would be significantly harmed and may force us into bankruptcy.
Virtually all of our research and development activities and the significant majority of our general and administrative activities are performed in or managed from a single site that may be subject to lengthy business interruption in the event of a severe earthquake. We also may suffer loss of computerized information and may be unable to make timely filings with regulatory agencies in the event of catastrophic failure of our data storage and backup systems.
Virtually all of our research and development activities and the significant majority of our general and administrative activities are performed in or managed from our facilities in Concord, California, which are within an active earthquake fault zone. Should a severe earthquake occur, we might be unable to occupy our facilities or conduct research and development and general and administrative activities in support of our business and products until such time as our facilities could be repaired and made operational. Our property and casualty and business interruption insurance in general does not cover losses caused by earthquakes. While we have taken certain measures to protect our scientific, technological and commercial assets, a lengthy or costly disruption due to an earthquake would have a material adverse effect on us. We have also taken measures to limit damage that
26
Table of Contents
may occur from the loss of computerized data due to power outage, system or component failure, or corruption of data files. However, we may lose critical computerized data, which may be difficult or impossible to recreate, which may harm our business. We may be unable to make timely filings with regulatory agencies in the event of catastrophic failure of our data storage and backup systems, which may subject us to fines or adverse consequences, up to and including loss of our abilities to conduct business.
We may not be able to protect our intellectual property or operate our business without infringing intellectual property rights of others.
Our commercial success will depend, in part, on obtaining and maintaining patent protection on our products and successfully defending our products against third-party challenges. Our technology will be protected from unauthorized use only to the extent that it is covered by valid and enforceable patents or effectively maintained as trade secrets. As a result, our success depends in part on our ability to:
| • | obtain patents; |
| • | protect trade secrets; |
| • | operate without infringing upon the proprietary rights of others; and |
| • | prevent others from infringing on our proprietary rights. |
We cannot be certain that our patents or patents that we license from others will be enforceable and afford protection against competitors. Our patents or patent applications, if issued, may be challenged, invalidated or circumvented. Our patent rights may not provide us with proprietary protection or competitive advantages against competitors with similar technologies. Others may independently develop technologies similar to ours or independently duplicate our technologies. For example, a United States patent issued to a third-party covers methods to remove psoralen compounds from blood products. We have reviewed the patent and believe there exists substantial questions concerning its validity. We cannot be certain, however, that a court would hold the patent to be invalid or not infringed by our platelet or plasma systems, if and when those products are sold in the United States. As a result, in order to commercialize our platelet or plasma systems in the United States, we may be required to obtain a license from the owner of the patent, which we may not be able to do at a reasonable cost or at all. Our patents expire at various dates between 2012 and 2027. Recent patent applications will, if granted, result in patents with later expiration dates. In addition, we have a license from Fenwal to United States and foreign patents relating to the INTERCEPT Blood System, which expire at various dates from 2017 to 2024. Due to the extensive time required for development, testing and regulatory review of our potential products, our patents may expire or remain in existence for only a short period following commercialization. This would reduce or eliminate any advantage of the patents.
We cannot be certain that we were the first to make the inventions covered by each of our issued patents or pending patent applications or that we were the first to file patent applications for such inventions. We may need to license the right to use third-party patents and intellectual property to continue development and commercialization of our products. We may not be able to acquire such required licenses on acceptable terms, if at all. If we do not obtain such licenses, we may need to design around other parties’ patents, or we may not be able to proceed with the development, manufacture or sale of our products.
Our patents do not cover all of the countries in which we are selling, and planning to sell, our products. We will not be able to prevent potential competitors from using our technology in countries where we do not have patent coverage.
We may face litigation to defend against claims of infringement, assert claims of infringement, enforce our patents, protect our trade secrets or know-how or determine the scope and validity of others’ proprietary rights. Patent litigation is costly. In addition, we may require interference proceedings before the United States Patent and Trademark Office to determine the priority of inventions relating to our patent applications. Litigation or interference proceedings could be expensive and time consuming, and we could be unsuccessful in our efforts to enforce our intellectual property rights.
27
Table of Contents
We may rely, in certain circumstances, on trade secrets to protect our technology. However, trade secrets are difficult to protect. We protect our proprietary technology and processes, in part, by confidentiality agreements with employees and contractors. These agreements may be breached and we may not have adequate remedies for any breach or our trade secrets may otherwise become known or be independently discovered by competitors. To the extent that our employees, consultants or contractors use intellectual property owned by others, disputes also may arise as to the rights in related or resulting know-how and inventions.
As our international operations grow, we may be subject to adverse fluctuations in exchange rates between the United States dollar and foreign currencies.
Our international operations are subject to risks typical of an international business, including, among other factors: differing political, economic, and regulatory climates, different tax structures, and foreign exchange volatility. We do not currently enter into any hedging contracts to normalize the impact of foreign exchange fluctuations. As a result, our future results could be materially affected by changes in these or other factors.
Product sales of our blood safety products are typically made in Europe and generally are invoiced to customers in Euros. In addition, we purchase finished disposable kits for our platelet and plasma systems and incur certain operating expenses in Euros and other foreign currencies. Our exposure to foreign exchange rate volatility is a direct result of our product sales, cash collection and expenses to support our international operations. Foreign exchange rate fluctuations are recorded as a component of interest (expense) and other, net on our consolidated statements of operations. Significant fluctuations in the volatility of foreign currencies relative to the United States dollar may materially affect our results of operations. Currently we do not have any near-term plans to enter into a formal hedging program to mitigate the effects of foreign currency volatility.
The market price of our stock may be highly volatile.
The market prices for our securities and those of other emerging medical device and biotechnology companies have been, and may continue to be, volatile. For example, during the period from January 1, 2009 to December 31, 2011, the sale price of our common stock as quoted on the Nasdaq Global Market fluctuated within a range from a low of $0.59 to a high of $4.01. Announcements may have a significant impact on the market price of our common stock. Such announcements may include:
| • | decisions regarding reimbursement and commercial adoption by customers, national blood services or governmental bodies; |
| • | biological or medical discoveries; |
| • | technological innovations discovered or new commercial services offered by us or our competitors; |
| • | developments concerning proprietary rights, including patents and litigation matters; |
| • | regulatory developments; |
| • | status of development partnerships; |
| • | dilution from future issuances of common stock, including common stock issued pursuant to our At-The Market Issuance Sales Agreement, with MLV & Co. LLC, or through the exercise of warrants and vested stock options; |
| • | debt financings, with terms that may not be viewed favorably by stockholders; |
| • | public concern as to the safety of new technologies; |
| • | general market conditions; |
| • | comments made by analysts, including changes in analysts’ estimates of our financial performance; and |
| • | quarterly fluctuations in our revenue and financial results. |
28
Table of Contents
We may fail to comply fully with elements of the Sarbanes-Oxley Act of 2002. Our failure to maintain effective internal controls in accordance with Section 404 of this Act could have a material adverse effect on our stock price.
Section 404 of the Sarbanes-Oxley Act of 2002 requires annual management assessments of the effectiveness of our internal controls over financial reporting and a report by our independent registered public accountants attesting to the effectiveness of our internal controls. These requirements extend to the operations of our subsidiary in Europe. If we fail to maintain the adequacy of our internal controls over financial reporting, as such standards are modified, supplemented or amended from time to time, we may not be able to ensure that we can conclude in future periods that we have effective internal controls over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002. If we cannot favorably assess, or our independent registered public accountants are unable to provide an unqualified attestation report on the effectiveness of our internal controls over financial reporting, investor confidence in the reliability of our financial reports may be adversely affected, which could have a material adverse effect on our stock price.
Provisions of our charter documents, our stockholder rights plan and Delaware law could make it more difficult for a third party to acquire us, even if the offer may be considered beneficial by our stockholders.
Provisions of the Delaware General Corporation Law could discourage potential acquisition proposals and could delay, deter or prevent a change in control. The anti-takeover provisions of the Delaware General Corporation Law impose various impediments to the ability of a third party to acquire control of us, even if a change in control would be beneficial to our existing stockholders. In addition, Section 203 of the Delaware General Corporation Law, unless its application has been waived, provides certain default anti-takeover protections in connection with transactions between the company and an “interested stockholder” of the company. Generally, Section 203 prohibits stockholders who, alone or together with their affiliates and associates, own more than 15% of the subject company from engaging in certain business combinations for a period of three years following the date that the stockholder became an interested stockholder of such subject company without approval of the board or the vote of two-thirds of the shares held by the independent stockholders. Our board of directors has also adopted a stockholder rights plan, or “poison pill,” which would significantly dilute the ownership of a hostile acquirer. Additionally, provisions of our amended and restated certificate of incorporation and bylaws could deter, delay or prevent a third party from acquiring us, even if doing so would benefit our stockholders, including without limitation, the authority of the board of directors to issue, without stockholder approval, preferred stock with such terms as the board of directors may determine.
| Item 1B. | Unresolved Staff Comments |
None.
| Item 2. | Properties |
Our corporate headquarters, which include our principal executive offices, are located in Concord, California. This leased facility includes laboratory space for blood safety research and supports general administrative, marketing and technical support functions. We also lease a facility in Amersfoort, the Netherlands, which is used for selling and administrative functions. We believe that our current facilities will be adequate for the foreseeable future. The following table summarizes the properties we lease and their location, size, term and primary functions as of December 31, 2011.
| Location |
Square Footage |
Lease Expiration Date |
Expiration if Renewal Options Exercised |
Primary Functions | ||||||
| Concord, CA, United States |
36,029 | November 2019 | Not applicable | Administrative, marketing, technical support and research | ||||||
| Amersfoort, The Netherlands |
7,300 | January 2013 | January 2018 | Sales and administrative | ||||||
29
Table of Contents
Subsequent to December 31, 2011, we entered into an amended lease with the landlord for our office in The Netherlands. By way of entering into the amended lease, we exercised an option to extend the lease for an additional five years following the lease expiration of January 2013. We may terminate the lease no earlier than January 2016.
| Item 3. | Legal Proceedings |
None.
| Item 4. | Mine Safety Disclosures |
Not applicable.
30
Table of Contents
PART II
| Item 5. | Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities |
Our common stock is traded on the Nasdaq Global Market under the symbol “CERS.” The following table sets forth, for the periods indicated, the high and low intra-day sales prices for our common stock as reported by the Nasdaq Global Market:
| High | Low | |||||||
| Year Ended December 31, 2011: |
||||||||
| First Quarter |
$ | 3.68 | $ | 2.40 | ||||
| Second Quarter |
$ | 3.16 | $ | 2.62 | ||||
| Third Quarter |
$ | 3.07 | $ | 1.92 | ||||
| Fourth Quarter |
$ | 3.15 | $ | 1.94 | ||||
| Year Ended December 31, 2010: |
||||||||
| First Quarter |
$ | 3.14 | $ | 1.76 | ||||
| Second Quarter |
$ | 3.71 | $ | 2.42 | ||||
| Third Quarter |
$ | 4.01 | $ | 2.80 | ||||
| Fourth Quarter |
$ | 3.95 | $ | 2.11 | ||||
On February 22, 2012, the last reported sale price of our common stock on the Nasdaq Global Market was $3.57 per share. On February 22, 2012, we had approximately 171 holders of record of common stock. We have not declared or paid dividends on our common stock and do not intend to pay cash dividends on our common stock in the foreseeable future.
31
Table of Contents
Stock Performance Graph (1)
The following graph shows the total stockholder return of an investment of $100 in cash (and the reinvestment of any dividends thereafter) on December 31, 2006 and tracked the performance through December 31, 2011 for (i) our common stock, (ii) the NASDAQ Biotechnology Stocks Index, (iii) the Amex Biotech Index, and (iv) the NASDAQ Stock Market (United States) Index. Our stock price performance shown in the graph below is based upon historical data and is not indicative of future stock price performance.
Comparison of 5-year Cumulative Total Return on Investment
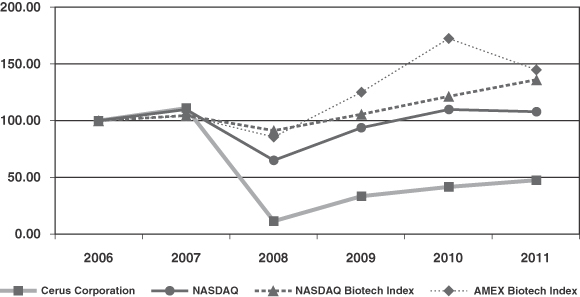
| December 31, | ||||||||||||||||||||||||
| 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | |||||||||||||||||||
| Cerus Corporation |
$ | 100.00 | $ | 111.09 | $ | 11.95 | $ | 33.96 | $ | 41.98 | $ | 47.78 | ||||||||||||
| NASDAQ Biotech Index |
100.00 | 104.58 | 91.38 | 105.66 | 121.52 | 135.86 | ||||||||||||||||||
| AMEX Biotech Index |
100.00 | 104.28 | 85.80 | 124.91 | 172.04 | 144.70 | ||||||||||||||||||
| NASDAQ |
100.00 | 109.81 | 65.29 | 93.95 | 109.84 | 107.86 | ||||||||||||||||||
| (1) | The graph and certain other information furnished under this Part II Item 5 of this Annual Report on Form 10-K shall not be deemed to be “soliciting material” or to be “filed” with the Commission or subject to Regulation 14A or 14C, or to the liabilities of Section 18 of the Exchange Act of 1934, as amended. |
32
Table of Contents
| Item 6. | Selected Financial Data |
The following table summarizes certain selected financial data for the five years ended December 31, 2011, which has been derived from audited consolidated financial statements. The information presented below may not be indicative of future results and should be read in conjunction with “Item 7—Management’s Discussion and Analysis of Financial Condition and Results of Operations,” and the consolidated financial statements and notes thereto included elsewhere in this Annual Report on Form 10-K.
| Years Ended December 31, | ||||||||||||||||||||
| (in thousands, except per share amounts) |
2011 | 20101 | 20092 | 2008 | 20073 | |||||||||||||||
| Consolidated Statements of Operations Data: |
||||||||||||||||||||
| Revenue: |
||||||||||||||||||||
| Product revenue |
$ | 30,602 | $ | 21,677 | $ | 16,751 | $ | 15,518 | $ | 8,015 | ||||||||||
| Government grants and cooperative agreements |
2,442 | 1,432 | 1,231 | 989 | 3,029 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenue |
33,044 | 23,109 | 17,982 | 16,507 | 11,044 | |||||||||||||||
| Cost of product revenue |
18,535 | 12,046 | 12,580 | 9,668 | 5,228 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Gross profit |
14,509 | 11,063 | 5,402 | 6,839 | 5,816 | |||||||||||||||
| Operating expenses (gains): |
||||||||||||||||||||
| Research and development |
7,178 | 5,195 | 6,372 | 10,205 | 14,957 | |||||||||||||||
| Selling, general and administrative |
23,053 | 21,577 | 21,867 | 27,164 | 24,575 | |||||||||||||||
| Amortization of intangible assets |
202 | 67 | 0 | 0 | 0 | |||||||||||||||
| Acquisition related costs and impairment of long-term investments in related parties, net1,2 |
0 | 182 | 1,536 | 0 | 9,450 | |||||||||||||||
| Settlement gain2 |
0 | 0 | (1,381 | ) | 0 | 0 | ||||||||||||||
| Restructuring charges2 |
0 | 0 | 841 | 0 | 0 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total operating expenses |
30,433 | 27,021 | 29,235 | 37,369 | 48,982 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from operations |
(15,924 | ) | (15,958 | ) | (23,833 | ) | (30,530 | ) | (43,166 | ) | ||||||||||
| Non-operating income (expense), net |
(1,058 | ) | (953 | ) | (302 | ) | 1,349 | 4,066 | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from continuing operations |
(16,982 | ) | (16,911 | ) | (24,135 | ) | (29,181 | ) | (39,100 | ) | ||||||||||
| Discontinued operations: |
||||||||||||||||||||
| Loss from discontinued operations |
0 | 0 | 0 | 0 | (5,820 | ) | ||||||||||||||
| Loss from sale of discontinued operations |
0 | 0 | 0 | 0 | (384 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Loss from discontinued operations |
0 | 0 | 0 | 0 | (6,204 | ) | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (16,982 | ) | $ | (16,911 | ) | $ | (24,135 | ) | $ | (29,181 | ) | $ | (45,304 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Basic loss per common share: |
||||||||||||||||||||
| Loss from continuing operations |
$ | (0.35 | ) | $ | (0.42 | ) | $ | (0.69 | ) | $ | (0.90 | ) | $ | (1.23 | ) | |||||
| Loss from discontinued operations |
$ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | (0.19 | ) | |||||||||
| Net loss |
$ | (0.35 | ) | $ | (0.42 | ) | $ | (0.69 | ) | $ | (0.90 | ) | $ | (1.42 | ) | |||||
| Diluted loss per common share: |
||||||||||||||||||||
| Loss from continuing operations |
$ | (0.35 | ) | $ | (0.42 | ) | $ | (0.69 | ) | $ | (0.90 | ) | $ | (1.23 | ) | |||||
| Loss from discontinued operations |
$ | 0 | $ | 0 | $ | 0 | $ | 0 | $ | (0.19 | ) | |||||||||
| Net loss |
$ | (0.35 | ) | $ | (0.42 | ) | $ | (0.69 | ) | $ | (0.90 | ) | $ | (1.42 | ) | |||||
| Weighted average common shares outstanding used for calculating loss per common share: |
||||||||||||||||||||
| Basic |
48,050 | 40,300 | 34,750 | 32,430 | 31,870 | |||||||||||||||
| Diluted |
48,050 | 40,300 | 34,750 | 32,430 | 31,870 | |||||||||||||||
| December 31, | ||||||||||||||||||||
| (in thousands) |
2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||||||
| Consolidated Balance Sheets Data: |
||||||||||||||||||||
| Cash, cash equivalents and short-term investments |
$ | 25,784 | $ | 30,009 | $ | 19,931 | $ | 22,578 | $ | 56,850 | ||||||||||
| Working capital |
18,625 | 22,052 | 19,446 | 29,145 | 55,582 | |||||||||||||||
| Total assets |
45,367 | 48,167 | 34,491 | 47,339 | 78,209 | |||||||||||||||
| Debt—non-current |
4,697 | 3,131 | 0 | 0 | 0 | |||||||||||||||
| Capital lease obligations—non-current |
0 | 6 | 15 | 0 | 2 | |||||||||||||||
| Other non-current liabilities |
1,243 | 1,595 | 115 | 163 | 0 | |||||||||||||||
| Accumulated deficit |
(443,935 | ) | (426,953 | ) | (410,042 | ) | (385,907 | ) | (356,726 | ) | ||||||||||
| Total stockholders’ equity |
$ | 18,313 | $ | 23,732 | $ | 21,448 | $ | 34,278 | $ | 59,887 | ||||||||||
| (1) | The statements of operations data for the year ended December 31, 2010 included (i) acquisition related costs of $0.5 million related to our acquisition of certain assets of BioOne in August 2010 and (ii) a gain of $0.3 million associated with relinquishing our shares in BioOne as part of the consideration for the acquisition of BioOne. See Note 3 to Consolidated Financial Statements under Part IV to this Annual Report on Form 10-K. |
| (2) | The statements of operations data for the year ended December 31, 2009 included (i) an impairment charge of $2.3 million related to our investment in BioOne (see Note 9 in the Notes to Consolidated Financial Statements under Part IV to this Annual Report on Form 10-K), (ii) a gain of $0.8 million associated with relinquishing our shares in Anza Therapeutics (see Note 9 in the Notes to Consolidated Financial Statements under Part IV to this Annual Report on Form 10-K), (iii) a settlement gain of $1.4 million associated with certain transition services provided by Baxter in 2006 (see Note 17 in the Notes to Consolidated Financial Statements under Part IV to this Annual Report on Form 10-K), and (iv) a charge of $0.8 million related to an approved restructuring plan (see Note 11 in the Notes to Consolidated Financial Statements under Part IV to this Annual Report on Form 10-K). |
| (3) | The statements of operations data for the year ended December 31, 2007 has been reclassified to reflect the treatment of our former immunotherapy business as a discontinued operation. The reclassifications to discontinued operations had no impact on net loss. |
33
Table of Contents
| Item 7. | Management’s Discussion and Analysis of Financial Condition and Results of Operations |
This discussion and analysis should be read in conjunction with our audited consolidated financial statements and the accompanying notes thereto included in this Annual Report on Form 10-K for the year ended December 31, 2011. Operating results for the year ended December 31, 2011 are not necessarily indicative of results that may occur in future periods.
Overview
Since our inception in 1991, we have devoted substantially all of our efforts and resources to the research, development, clinical testing and commercialization of the INTERCEPT Blood System and, from 2001 until late 2007, immunotherapies for cancer and infectious disease. The INTERCEPT Blood System is designed for three blood components. The INTERCEPT Blood System for platelets, or platelet system, and our INTERCEPT Blood System for plasma, or plasma system, have received CE marks and are being marketed and sold in a number of countries in Europe, The Commonwealth of Independent States, or CIS, the Middle East and selected countries in other regions around the world. In addition, we are developing and plan to perform the required clinical trials for our INTERCEPT Blood System for red blood cells, or red blood cell system, in Europe. Subject to the availability of adequate funding from partners, government grants and/or capital markets, we intend to complete development activities for the red blood cell system necessary for regulatory approval in Europe and we may seek regulatory approval of our products in the United States.
Our near-term capital requirements are dependent on various factors, including operating costs and working capital investments associated with commercializing the INTERCEPT Blood System, costs associated with pursuing regulatory approval in geographies where we do not currently sell our platelet and plasma systems, costs associated with planning and conducting studies and clinical development of our red blood cell system, timing and magnitude of payments under grants from the United States government, and costs related to creating, maintaining and defending our intellectual property. Our long-term capital requirements will also be dependent on competitive developments, clinical development and regulatory factors. We believe that cash received from product sales and government grants, our available cash balances and access to debt will be sufficient to meet our capital requirements for at least the next twelve months. If our assumptions prove to be incorrect, we could consume our available capital resources sooner than we currently expect.
We may borrow additional capital from institutional and commercial banking sources to fund future growth outside of our credit agreement with Comerica Bank, as described below, on terms that may include restrictive covenants, which may comprise of covenants that restrict the operation of our business, liens on assets, high effective interest rates and repayment provisions that reduce cash resources and limit future access to capital markets. To the extent that we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. To the extent that we raise additional funds through collaboration or partnering arrangements, we may be required to relinquish some of our rights to our technologies or rights to market and sell our products in certain geographies, or grant licenses on terms that are not favorable to us. If we are unable to raise additional capital due to disruptions to the global credit and financial markets and general economic uncertainty or other factors, we may need to curtail planned development or commercialization activities.
We recognize product revenues from the sale of our platelet and plasma systems in Europe, the CIS, the Middle East, and certain other countries around the world. Our product revenues increased by $8.9 million during the year ended December 31, 2011 compared to the December 31, 2010 primarily as a result of an increase in volume sales to existing customers and sales to new customers due to increased market penetration and customer adoption of the INTERCEPT Blood System in Europe, the CIS, and the Middle East. Although our revenues have grown over time, if we are unable to gain widespread commercial adoption in markets where our blood safety products are approved for commercialization, we anticipate that we may have difficulties achieving profitability. As such, we may never achieve a profitable level of operations in the future as we must conduct significant research, development, preclinical and clinical evaluation, commercialization and regulatory compliance activities for our product candidates, which, together with anticipated selling, general and administrative expenses, are expected to result in substantial losses.
34
Table of Contents
In addition to the product revenues from sales of our platelet and plasma systems, we recognize revenue from government grants and cooperative agreements. Historically, we have received significant awards in funding under cooperative agreements with the United States Department of Defense, or DoD, for the INTERCEPT Blood System. Any such funding is subject to the authorization of funds and approval of our research plans by various organizations within the federal government, including the United States Congress. In August 2011, we were awarded a $2.1 million grant from the DoD to support the development of our red blood cell system. We recognize revenue associated with this award as qualified costs are incurred for reimbursement over the performance period of one year from the date of issuance. The general economic environment, coupled with tight federal budgets, has led to a general decline in the amount available for government funding. There is no assurance that we will be awarded future federal grants and cooperative agreements for the INTERCEPT Blood System.
In 2007, we spun-off our immunotherapy business, and in 2009 entered into agreements to license the immunotherapy technologies to Aduro BioTech, or Aduro. In connection with those agreements, we received and currently hold preferred shares representing less than 10% of Aduro’s capital. Pursuant to these license agreements, we will obtain a 1% royalty fee on any future sales resulting from certain technology. In April 2011, Aduro completed a subsequent round of financing, issuing Series B preferred stock and as a result, reduced our ownership in Aduro to less than 3%. Since receiving preferred stock in Aduro, we have carried our investment in Aduro at zero on our consolidated balance sheet as we have no basis to believe that we will receive any economic benefit from our equity ownership in Aduro as we believed that Aduro’s technology platforms, which were largely based on the in-process development programs of Anza Therapeutics Inc., or Anza Therapeutics, had a high risk of failure.
We pay royalties to Fenwal Inc., or Fenwal, on future INTERCEPT Blood System product sales under certain agreements which arose from the sale of the transfusion therapies division of Baxter International Inc., or Baxter, in 2007 to Fenwal, at rates of 10% of net sales for our platelet system, 3% of net sales for our plasma system, 5% of net sales for our red blood cell system, and 6.5% on net sales of illumination devices, or illuminators. We also pay Fenwal certain costs associated with the amended manufacturing and supply agreement we executed with Fenwal in December 2008 for the manufacture of INTERCEPT finished disposable kits for our platelet and plasma systems through December 31, 2013. Under the amended manufacturing and supply agreement, we pay Fenwal a set price per disposable kit, which is established annually, plus a fixed surcharge per disposable kit. In addition, volume driven manufacturing overhead will be paid or refunded if actual manufacturing volumes are higher or lower than the annually estimated production volumes. We are also obligated to provide certain disposable kit components at no cost to Fenwal under the amended manufacturing and supply agreement. This required us to enter into manufacturing and supply arrangements with certain other manufacturers for those components, some of which contain minimum purchase commitments. As a result, our supply chain for certain of these components, held as work-in-process on our consolidated balance sheets, may potentially take over one year to complete production before being utilized in finished disposable kits.
In August 2010, we completed an acquisition of certain assets of BioOne Corporation, or BioOne, including the commercialization rights that both Baxter (later Fenwal) and we granted to BioOne for both the platelet and plasma systems. Concurrent with the acquisition, Fenwal and we terminated the commercialization rights we and Fenwal granted to BioOne. As a consequence of the termination, and pursuant to a pre-existing agreement with Fenwal, our commercialization rights to the platelet and plasma systems under our 2005 and 2006 agreements with Baxter became worldwide. As consideration for the acquired BioOne assets, we relinquished all shares we held in BioOne valued at approximately $0.3 million and issued 1,172,357 shares of our common stock to BioOne valued at approximately $3.4 million, of which 937,886 shares were issued at the close of the acquisition on August 24, 2010 and the remaining 234,471 shares were issued six months from the close of the acquisition date on February 25, 2011. Accordingly, at the acquisition date, we recorded the fair value of the assets acquired, consisting of commercialization rights in Asia of $2.0 million and illuminators of $0.4 million, with the excess of the purchase price over the fair value of the asset acquired was recorded as goodwill of $1.3 million. The recognition of goodwill was attributable to the buyer-specific value derived by us as a result of acquiring the commercialization rights in certain Asian countries in order to complete the global commercialization rights for our platelet and plasma systems.
35
Table of Contents
In June 2011, we entered into an At-The-Market Issuance Sales Agreement, or Sales Agreement, with MLV & Co. LLC, or MLV, that provides for the issuance and sale of shares of our common stock over the term of the Sales Agreement having an aggregate offering price of up to $20.0 million from time to time through MLV as our sales agent. During the year ended December 31, 2011, approximately 3.5 million shares of our common stock were sold under the Sales Agreement for aggregate net proceeds of $9.7 million. Subsequent to the year ended December 31, 2011 through the date of filing this Annual Report on Form 10-K, we sold approximately 3.0 million of additional shares of common stock for aggregate net proceeds of approximately $9.1 million.
In September 2011, we entered into a loan and security agreement, or Credit Agreement, with Comerica Bank, or Comerica, which provides for a growth capital loan of up to $8.0 million, or Growth Capital Loan, and a formula based revolving line of credit of up to $4.0 million plus any unused amounts from the Growth Capital Loan. Under the Credit Agreement, we are limited to an aggregate borrowing of up to $10.0 million at any time. We pledged all current and future assets, excluding our intellectual property and 35% of our investment in our subsidiary, Cerus Europe B.V., as security for borrowings under the Credit Agreement. We are also required to maintain compliance with certain customary and routine financial covenants, including maintaining a minimum cash balance with Comerica and achieving certain minimum revenue levels. Concurrent with the execution of the Credit Agreement, we borrowed $5.0 million of the Growth Capital Loan, substantially all of which was used to repay our prior debt with Oxford Finance Corporation, or Oxford, with the remainder used for general corporate purposes. In addition, we drew $2.3 million from the revolving line of credit in 2011.
Critical Accounting Policies and Management Estimates
The preparation of financial statements requires us to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosures of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates, including those related to revenue recognition, inventory valuation, accrued liabilities, valuation and impairment of purchased intangibles and goodwill, valuation of warrants, non-cash stock compensation assumptions, and income taxes. We base our estimates on historical experience and on various other assumptions that we believe to be reasonable under the circumstances, the results of which form our basis for making judgments about the carrying value of assets and liabilities that are not readily apparent from other sources. Actual results may differ from those estimates under different assumptions or conditions.
We believe the following critical accounting policies require us to make significant judgments and estimates used in the preparation of our financial statements:
•Revenue—We recognize revenue in accordance with ASC Topic 605-25, “Revenue Recognition—Arrangements with Multiple Deliverables,” as applicable. Revenue is recognized when (i) persuasive evidence of an agreement with the funding party exists; (ii) services have been rendered or product has been delivered; (iii) pricing is fixed or determinable; and (iv) collection is probable.
Revenue related to product sales is generally recognized when we fulfill our obligations for each element of an agreement. For all sales of our INTERCEPT Blood System products, we use a binding purchase order and signed sales contract as evidence of a written agreement. We sell INTERCEPT Blood System for platelets and plasma directly to blood banks, hospitals, universities, government agencies, as well as to distributors in certain regions. Generally, our contracts with customers do not provide for open return rights, except within a reasonable time after receipt of goods in the case of defective or non-conforming product. Deliverables and the units of accounting vary according to the provisions of each purchase order or sales contract. For revenue arrangements with multiple elements, we evaluate whether the delivered elements have stand-alone value to the customer. Prior to adoption of ASU No. 2009-13, consideration received was allocated to elements that were identified as discrete units of accounting based on the relative fair value method. Beginning January 1, 2011, consideration received is allocated to elements that are identified as discrete units of accounting based on the best estimated selling price. We have determined that vendor specific objective evidence is not discernable due to our limited
36
Table of Contents
history of selling our products and variability in our pricing. Since our products are novel and unique and are not sold by others, third-party evidence of selling price is unavailable. Freight costs charged to customers are recorded as a component of revenue under ASC Topic 605, “Accounting for Shipping and Handling Fees and Costs” and value-added-taxes, or VAT, that we invoice to our customers and remit to governments, are recorded on a net basis, which excludes such VAT from product revenue.
Revenue related to the cost reimbursement provisions under development contracts is recognized as the costs on the projects are incurred. We receive certain United States government grants and contracts that support research in defined research projects. These grants generally provide for reimbursement of approved costs incurred as defined in the various grants.
•Inventory—We own certain components of INTERCEPT disposable kits in the form of work-in-process inventory and finished goods, UVA illuminators, and certain replacement parts for our illuminators. Our supply chain for certain of these components, held as work-in-process on our consolidated balance sheet, can potentially take over one year to complete production before being utilized in finished disposable kits. We maintain an inventory balance based on our current sales projections, and at each reporting period, we evaluate whether our work-in-process inventory would be consumed for production of finished units in order to sell to existing and prospective customers within the next twelve-month period. It is not customary for our production cycle for inventory to exceed twelve months. Instead, we use our best judgment to factor in lead times for the production of our finished units to meet our current demands. If actual results differ from those estimates, work-in-process inventory could potentially accumulate for periods exceeding one year.
Under our manufacturing and supply agreement with Fenwal, our carrying value of INTERCEPT disposable kits is dependent on an annually set price. In addition, at the end of each year, volume driven manufacturing overhead is either paid or refunded by or to us if manufacturing volumes are higher or lower than the anticipated manufacturing volumes at the time the price is established. As a result, manufacturing overhead can fluctuate and requires us to use judgment in accruing the manufacturing overhead. In addition, we use judgment in determining whether the manufacturing overhead is a cost of our inventory and recoverable when product is sold. We use significant judgment and evaluate manufacturing variances incurred during periods of abnormally low production by considering a variety of factors including the reasons for low production volumes, anticipated future production levels that correlate to and offset volumes experienced during abnormally low production cycles and contractual requirements. We record manufacturing variances incurred during periods without production as a component of “Cost of product revenue” on our consolidated statements of operations.
Inventory is recorded at the lower of cost, determined on a first in, first-out basis, or market value. Our platelet and plasma system disposable kits generally have a two-year shelf life from the date of manufacture. Illuminators and replacement parts do not have regulated expiration dates. We use significant judgment to analyze and determine if the composition of our inventory is obsolete, slow-moving, or unsalable and frequently review such determinations. Our limited history selling the INTERCEPT Blood System limits the amount of historical data we have to perform this analysis. Generally, we write-down specifically identified unusable, obsolete, slow-moving, or known unsalable inventory that has no alternative use in the period that it is first recognized by using a number of factors including product expiration dates, open and unfulfilled orders, and sales forecasts. Any write-down of our inventory to net realizable value establishes a new cost basis and will be maintained even if certain circumstances suggest that the inventory is recoverable in subsequent periods. Costs associated with the write-down of inventory are recorded in “Cost of product revenue” on our consolidated statements of operations.
•Accrued expenses—We record accrued liabilities for expenses related to certain contract research activities and development services, including those related to clinical trials, preclinical safety studies and external laboratory studies, as well as development activities being performed by third parties. Some of those accrued liabilities are based on estimates because billings for these activities may not occur on a timely basis consistent with the performance of the services. Specifically, accruals for clinical trials require us to make estimates surrounding costs
37
Table of Contents
associated with patients at various stages of the clinical trial, pass through costs to clinical sites, contract research organization costs including fees, database development, and reporting costs, among others.
•Goodwill and Intangible assets—In August 2010, we acquired certain assets from BioOne. We accounted for the acquisition as a business combination in accordance with ASC Topic 805, “Business Combinations.” In connection with the acquisition, we used significant judgment, including, but not limited to, judgments as to cash flows, discount rates, and economic lives, in identifying the assets acquired and in determining the fair values to record the purchased assets on our consolidated balance sheet. In addition, under ASC Topic 805, we were required to assess the fair value of the non-controlling interest that we held in BioOne prior to the acquisition. We determined that a considerable amount of the purchase consideration was goodwill, which represents value unique to us as the holder of worldwide rights to the INTERCEPT Blood System. We may be unable to realize the recorded value of the acquired assets and our assumptions may prove to be incorrect, which may require us to write-down or impair the value of the assets if and when facts and circumstances indicate a need to do so. We perform an impairment test on our goodwill annually on August 31 of each fiscal year or more frequently if indicators of impairment exist. The test for goodwill impairment is a two-step process. The first step compares the fair value of each reporting unit with the respective carrying amount, including goodwill. We have determined that we operate in one reporting unit and estimate the fair value of our one reporting unit using the enterprise approach under which we consider our quoted market capitalization as reported on the Nasdaq Global Market. We consider quoted market prices that are available in active markets to be the best evidence of fair value. We also consider other factors, which include future forecasted results, the economic environment and overall market conditions. If the fair value of the reporting unit exceeds the carrying amount, goodwill of the reporting unit is not considered impaired and, therefore, the second step of the impairment test is unnecessary. The second step, which is used to measure the amount of impairment loss, compares the implied fair value of each reporting unit’s goodwill with the respective carrying amount of that goodwill. If the carrying amount of the reporting unit’s goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess. On August 31, 2011, we performed our annual review of goodwill as described above and determined that goodwill was not impaired. We will perform an impairment test on our intangible assets by continually monitoring events and changes in circumstances that could indicate carrying amounts of our intangible assets may not be recoverable. When such events or changes in circumstances occur, we assess recoverability by determining whether the carrying value of such assets will be recovered through the undiscounted expected future cash flows. If the expected undiscounted future cash flows are less than the carrying amount of these assets, we then measure the amount of the impairment loss based on the excess of the carrying amount over the fair value of the assets. No events or changes in circumstances arose during the year ended December 31, 2011 which would require us to test the recoverability of our intangible assets.
•Warrants—In August 2009 and November 2010, we issued warrants to purchase 2.4 million and 3.7 million shares of common stock, respectively. The material terms of the warrants were identical under each issuance except for the exercise price, date issued and expiration date. We classified the warrants as a liability on our consolidated balance sheets as the warrants contain certain material terms which require us (or our successor) to purchase the warrants for cash in an amount equal to the value of the unexercised portion of the warrants (as determined in accordance with the Black-Scholes option pricing model) in connection with certain change of control transactions. In addition, we may also be required to pay cash to a warrant holder under certain circumstances if we are unable to timely deliver the shares acquired upon warrant exercise to such holder.
The fair value of these outstanding warrants is calculated using the binomial-lattice option-pricing model and is adjusted accordingly at each reporting period. The binomial-lattice option-pricing model requires that we use significant assumptions and judgment to determine appropriate inputs to the model. Some of the assumptions that we rely on include the probability of a change of control occurring, the volatility of our stock over the life of the warrant and assumptions and inputs used to value the warrants under the Black-Scholes model should a change of control occur.
Gains and losses from warrant revaluation are recorded in “Gain from revaluation of warrant liability” on the consolidated statements of operations. Upon the exercise or modification to remove the provisions which
38
Table of Contents
require the warrants to be treated as a liability, the fair value of the warrants will be reclassified from a liability to stockholders’ equity on our consolidated balance sheets and no further adjustment to the fair value would be made in subsequent periods.
•Stock-based compensation—We issue stock-based awards to our employees, contractors and members of our Board of Directors, as strategic, long-term incentives. We also maintain an active employee stock purchase plan within the meaning of Section 423(b) of the Internal Revenue Code. We record stock-based compensation expense for employee awards in accordance with ASC Topic 718, “Compensation—Stock Compensation.” We use the Black-Scholes option pricing model to determine the grant-date fair value of stock-based awards. The Black-Scholes option pricing model requires that we use assumptions regarding a number of complex and subjective variables to determine appropriate inputs to the model, which include the expected term of the grants, actual and projected employee stock option exercise behaviors, including forfeitures, our expected stock price volatility, the risk-free interest rate and expected dividends. The grant-date fair value of stock-based awards is then recognized as stock-based compensation expense on a straight-line basis over the requisite service period, which is the vesting period, and is adjusted for estimated forfeitures. To the extent that stock options contain performance criteria for vesting, stock-based compensation is recognized once the performance criteria are probable of being achieved. We continue to apply the provisions of ASC Topic 505-50, “Equity Based Payment to Non-Employees” for our stock-based awards issued to non-employees. Under the provisions, the measurement date at which the fair value of the stock-based award is measured to be the earlier of (i) the date at which a commitment for performance by the grantee to earn the equity instrument is reached or (ii) the date at which the grantee’s performance is complete. We recognize stock-based compensation expense for the fair value of the vested portion of the non-employee awards in our consolidated statements of operations.
•Income taxes—Since our inception, we have accumulated significant net operating losses and research and development credits that may be used in future periods to offset future taxable income. We currently estimate that we may not be able to utilize all of our deferred tax assets. In addition, we may not generate future taxable income prior to the expiration of our net operating loss carry forwards and research and development credits. Timing and significance of any estimated future taxable income is highly subjective and is beyond the control of management due to uncertainties in market conditions, economic environments in which we operate, and timing of regulatory approval of our products. We do not recognize tax positions that do not have a greater than 50% likelihood of being recognized upon review by a taxing authority having full knowledge of all relevant information. Use of a valuation allowance is not an appropriate substitute for the derecognition of a tax position. We did not have any recorded liabilities for unrecognized tax benefits at December 31, 2011 or 2010. We recognize accrued interest and penalties related to unrecognized tax benefits in our income tax expense. To date, we have not recognized any interest and penalties in our consolidated statements of operations, nor have we accrued for or made payments for interest and penalties. We continue to carry a full valuation allowance on all of our deferred tax assets. Although we believe it more likely than not that a taxing authority would agree with our current tax positions, there can be no assurance that the tax positions we have taken will be substantiated by a taxing authority if reviewed. Our tax years 2007 through 2011 remain subject to examination by the taxing jurisdictions.
Results of Operations
Years Ended December 31, 2011, 2010 and 2009
Revenue
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Product revenue |
$ | 30,602 | $ | 21,677 | $ | 16,751 | 41 | % | 29 | % | ||||||||||
| Government grants and cooperative agreements |
2,442 | 1,432 | 1,231 | 71 | % | 16 | % | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total revenue |
$ | 33,044 | $ | 23,109 | $ | 17,982 | 43 | % | 29 | % | ||||||||||
|
|
|
|
|
|
|
|||||||||||||||
39
Table of Contents
Product revenue increased by $8.9 million in the year ended December 31, 2011 compared to the year ended December 31, 2010 primarily as a result of an increase in sales volume of our disposable platelet and plasma system kits sold to existing customers due to increased market penetration and customer adoption of the INTERCEPT Blood System in Europe, the CIS, and the Middle East, which was partially offset by a decline in the sales volume of our illuminators. Product revenue increased by $4.9 million in the year ended December 31, 2010 compared to the year ended December 31, 2009, which was driven by an increase in the number of disposable platelet and plasma system kits sold to customers in Europe, the CIS, and the Middle East and to a lesser extent, an increase in sales volumes of our UVA illuminators.
We anticipate product revenue for both our platelet and plasma systems will continue to increase in future periods as the INTERCEPT Blood System gains market acceptance in geographies where commercialization efforts are underway. The historical results may not be indicative of INTERCEPT Blood System revenue in the future.
Revenue from government grants and cooperative agreements increased by $1.0 million in the year ended December 31, 2011 compared to the year ended December 31, 2010. This increase was partly attributable to higher reimbursable development efforts related to our red blood cell system during the year ended December 31, 2011 compared to the corresponding period of 2010. In addition, in August 2011, we were awarded a new grant by the DoD totaling $2.1 million. Revenue from government grants and cooperative agreements for the year ended December 31, 2010 increased by $0.2 million compared to year ended December 31, 2009. The increase was primarily due to an increase in the red blood cell system development activities reimbursed under awards with the DoD.
Cost of Product Revenue
Our cost of product revenue consists of the cost of the INTERCEPT Blood System inventory sold, royalties payable to Fenwal for product sales, provisions for obsolete, slow-moving and unsaleable product, certain order fulfillment costs, and to the extent applicable, costs for idle facilities. Inventory is accounted for on a first-in, first-out basis.
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Cost of product revenue |
$ | 18,535 | $ | 12,046 | $ | 12,580 | 54 | % | (4 | )% | ||||||||||
Cost of product revenue increased by $6.5 million in the year ended December 31, 2011 compared to the year ended December 31, 2010 primarily due to a higher number of disposable kits sold and higher scrap rate for certain components manufactured during 2011 compared to 2010. Cost of product revenue decreased by $0.5 million to $12.0 million in the year ended December 31, 2010 compared to the year ended December 31, 2009. Despite the higher volume of products sold, the decrease in the cost of product revenue was due to lower manufacturing overhead variances capitalized as a result of increased production volumes during 2010. In addition, we had lower costs for obsolete, slow moving and scrapped inventory during the year ended December 31, 2010 compared to the corresponding period of 2009.
Our realized gross margins on product sales were 39% in the year ended December 31, 2011, down from 44% in the year ended December 31, 2010, and up from 25% in the year ended December 31, 2009. 2011 gross margins were negatively impacted compared to 2010 by non-routine period costs, including higher scrap rates associated with certain components manufactured. We have a limited history manufacturing these components and as such, have limited means to predict the frequency and magnitude of events leading to higher than expected scrap. 2010 gross margins were positively impacted compared to 2009 due to incurring certain costs in 2009 as a result of abnormally low manufacturing volumes.
40
Table of Contents
Changes in our gross margins are affected by various factors, including manufacturing and supply chain costs, the mix of product sold, and the mix of customers to which product is sold. Generally, we offer our distributors tiered volume discounts of varying magnitudes, depending on their purchase commitments. Our gross margins may be impacted in the future based on all of these criteria.
We expect to maintain inventory levels that will be sufficient to meet forecasted demand for a relatively short time period and plan to manufacture at levels above those produced in 2011. Manufacturing disposable kits at levels above the levels produced in 2011 should result in a continuing lower per unit cost of goods sold when the product is ultimately sold; however, actual manufacturing levels may differ from our assumptions.
Research and Development Expenses
Our research and development expenses include salaries and related expenses for our scientific personnel, non-cash stock based compensation, payments to consultants, costs to prepare and conduct preclinical and clinical trials, third-party costs for development activities, certain regulatory costs, infrastructure, and laboratory chemicals and supplies.
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Research and development |
$ | 7,178 | $ | 5,195 | $ | 6,372 | 38 | % | (18 | )% | ||||||||||
Research and development expenses increased by $2.0 million in the year ended December 31, 2011 compared to the year ended December 31, 2010 primarily due to increased costs related to our efforts to further advance the development of our red blood cell system program. Research and development expenses decreased by $1.2 million in the year ended December 31, 2010 compared to the year ended December 31, 2009 due to reduced research and development activities driven primarily by our March 2009 restructuring plan and the associated reduction in force. Of the total research and development expenses incurred, non-cash stock-based compensation represented $0.5 million, $0.4 million and $0.5 million for the years ended December 31, 2011, 2010 and 2009, respectively.
We anticipate our research and development spending will continue to increase over the near term to further our red blood cell system development efforts in Europe and will increase over the longer term if we are able to secure sufficient additional capital to pursue regulatory approval for our products in the United States. Due to the inherent uncertainties and risks associated with developing biomedical products, including, but not limited to, intense and changing government regulation, uncertainty of future pre-clinical and clinical trial results and uncertainty associated with manufacturing, it is not possible to reasonably estimate the costs to complete these research and development projects. We face numerous risks and uncertainties associated with the successful completion of our research and development projects; which is discussed in further detail under “Item 1A – Risk Factors” in Part I of this Annual Report on Form 10-K.
Selling, General, and Administrative Expenses
Selling, general, and administrative expenses include salaries and related expenses for administrative personnel, non-cash stock based compensation, expenses for our commercialization efforts in Europe and elsewhere, expenses for accounting, tax, and internal control, legal and facility related expenses, and insurance premiums.
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Selling, general and administrative |
$ | 23,053 | $ | 21,577 | $ | 21,867 | 7 | % | (1 | )% | ||||||||||
Selling, general, and administrative expenses increased by $1.5 million in the year ended December 31, 2011 compared to the year ended December 31, 2010 primarily due to increased spending related to the
41
Table of Contents
expansion of our marketing efforts in Europe. Selling, general, and administrative expenses decreased by $0.3 million in the year ended December 31, 2010 compared to the year ended December 31, 2009 primarily due to decreased personnel costs and lower marketing and public affairs costs driven primarily by our March 2009 restructuring plan and the associated reduction in force as well as our continued emphasis on controlling costs. Of the total selling, general, and administrative expenses incurred, non-cash stock-based compensation represented $1.4 million, $1.5 million, and $1.6 million for the years ended December 31, 2011, 2010 and 2009, respectively.
We anticipate that we will be focused on tightly managing growth in our selling, general, and administrative spending over the coming year, as part of a larger effort to focus our resources and conserve cash.
Amortization of Intangible Assets
Amortization of intangible assets relates to a license to commercialize the INTERCEPT Blood System in certain Asian countries in connection with our acquisition of certain assets from BioOne. The BioOne transaction was accounted for as a business combination under ASC Topic 805, “Business Combination,” which assigned a fair value of $2.0 million to the intangible assets in August 2010. These intangible assets are being amortized over an estimated useful life of ten years and will be reviewed for impairment as facts and circumstances arise.
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Amortization of intangible assets |
$ | 202 | $ | 67 | $ | 0 | 201 | % | 100 | % | ||||||||||
Amortization of intangible assets increased by $0.1 million in the year ended December 31, 2011 compared to the year ended December 31, 2010, and in the year ended December 31, 2010 compared to the year ended December 31, 2009, as the acquisition of purchased intangible assets related to our license to commercialize the INTERCEPT Blood System in certain Asian countries occurred during the second half of 2010.
We expect that the amortization of our intangible assets to remain relatively consistent in future periods, unless facts and circumstances arise which may result in our intangible assets being impaired.
Acquisition Related Costs and Impairment of Long-term Investment in Related Parties, Net
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Gain from long-term investment in related party—Anza Therapeutics |
$ | 0 | $ | 0 | $ | (804 | ) | 0 | % | (100 | )% | |||||||||
| Acquisition related costs and impairment of long-term investments in related party—BioOne |
0 | 182 | 2,340 | (100 | )% | (92 | )% | |||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Acquisition related costs and impairment of long-term investments in related parties, net |
$ | 0 | $ | 182 | $ | 1,536 | (100 | )% | (88 | )% | ||||||||||
|
|
|
|
|
|
|
|||||||||||||||
During the year ended December 31, 2010, we incurred acquisition related costs of $0.5 million related to our acquisition of certain assets of BioOne in August 2010. In addition, we relinquished all BioOne shares that we held as part of the consideration for certain of these assets and recognized a gain of $0.3 million during the year ended December 31, 2010, which represented the difference between the assumed fair value of the pre-acquisition non-controlling equity interest of BioOne and the carrying value. We carried our 13% investment in BioOne at zero as we had previously fully impaired our BioOne investment of $2.3 million during the year ended December 31, 2009 since we determined that certain factors were present to support our position that our BioOne investment was not recoverable. These factors included, but were not limited to, third-party investor interest and participation in recent equity offerings at current pricing, business outlook of BioOne and available financial information.
42
Table of Contents
During the year ended December 31, 2009, we also recognized a gain of $0.8 million, which represented the difference between the cash received and the carrying value of our non-controlling equity interest in Anza Therapeutics as we relinquished our shares in Anza Therapeutics, released any claims against them and agreed to the transfer of all of Anza Therapeutics’ intellectual property to Aduro in 2009.
Settlement Gain
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Settlement gain |
$ | 0 | $ | 0 | ($ | 1,381 | ) | 0 | % | (100 | )% | |||||||||
In December 2009, we and Baxter International Inc., or Baxter, entered into a settlement agreement regarding disputed amounts for certain transition services provided in 2006 by Baxter in conjunction with the transfer of commercialization rights to us. In consideration for agreeing to the settlement, with both parties waiving all rights and obligations, we eliminated the disputed amounts from our consolidated balance sheet of $4.7 million in payment obligations to Baxter and $2.8 million in receivables due from Baxter, and agreed to pay Baxter $0.5 million, resulting in us recording a gain of $1.4 million during the year ended December 31, 2009.
Restructuring Charges
Restructuring charges comprised of one-time termination benefits, facility consolidation and related moving costs.
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Restructuring charges |
$ | 0 | $ | 0 | $ | 841 | 0 | % | (100 | )% | ||||||||||
In March 2009, pursuant to the Board of Directors’ approval, we began implementing a plan to focus resources on commercializing the INTERCEPT Blood System in Europe, to consolidate facilities, and to reduce our cost structure. During the year ended December 31, 2009, we incurred costs for one-time termination benefits for employee positions that were eliminated under the restructuring plan. During the year ended December 31, 2009, we also incurred costs associated with consolidating facilities and certain other costs associated with the restructuring plan. Most of the costs accrued as one-time termination benefits as of March 31, 2009 were paid by December 31, 2009 and any remaining costs were paid by December 31, 2010.
Non-Operating Expense, Net
Non-operating expense, net consists of mark-to-market adjustments related to the calculated fair value of our outstanding warrants, foreign exchange loss, interest charges incurred on our debt, interest earned from our short-term investment portfolio, and other non-operating gains and losses.
| Years Ended December 31, | % Change | |||||||||||||||||||
| (in thousands, except percentages) |
2011 | 2010 | 2009 | 2011 to 2010 |
2010 to 2009 |
|||||||||||||||
| Gain from revaluation of warrant liability |
$ | 486 | $ | 39 | $ | 63 | 1,146 | % | (38 | )% | ||||||||||
| Foreign exchange loss |
(529 | ) | (816 | ) | (611 | ) | (35 | )% | 34 | % | ||||||||||
| Interest expense |
(964 | ) | (689 | ) | (10 | ) | 40 | % | 6,790 | % | ||||||||||
| Other income (expense), net |
(51 | ) | 513 | 256 | (110 | )% | 100 | % | ||||||||||||
|
|
|
|
|
|
|
|||||||||||||||
| Total non-operating expense, net |
$ | (1,058 | ) | $ | (953 | ) | $ | (302 | ) | 11 | % | 216 | % | |||||||
|
|
|
|
|
|
|
|||||||||||||||
43
Table of Contents
Warrant liability
In August 2009 and November 2010, we issued warrants to purchase an aggregate of 2.4 million and 3.7 million shares of common stock, respectively, in connection with offerings of our common stock. The fair value of these outstanding warrants, which uses the binomial-lattice option-pricing model, is classified as a liability on the consolidated balance sheets and is adjusted at each subsequent reporting period, until such time the instruments are exercised or otherwise modified to remove the provisions which require this treatment. Upon the exercise or modification to remove the provisions which require the warrants to be treated as a liability, the fair value of the warrants will be reclassified from liabilities to stockholders’ equity and no further adjustment to the fair value would be made in subsequent periods. Further changes in stock price will result in similar adjustment as needed.
Gain from revaluation of warrant liability increased by $0.4 million in the year ended December 31, 2011 compared to the year ended December 31, 2010 primarily due to the change in our underlying stock price, as compared to the strike price of the warrants. Gain from revaluation of warrant liability was relatively consistent in the year ended December 31, 2010 compared to the year ended December 31, 2009.
Foreign exchange loss
Foreign exchange loss improved by $0.3 million in the year ended December 31, 2011 compared to the year ended December 31, 2010, which was primarily attributable to favorable foreign currency variations over that time period between the Euro and U.S. dollar, our functional currency. Foreign exchange loss declined by $0.2 million in the year ended December 31, 2010 compared to the year ended December 31, 2009, which was primarily attributable to unfavorable foreign currency variations between the Euro and U.S. dollar.
Interest expense
Interest expense increased by $0.3 million in the year ended December 31, 2011 compared to the year ended December 31, 2010 primarily due to the acceleration of the closing cost and fees associated with the repayment of our prior debt and interest incurred from borrowings on our prior credit facility, and to a lesser extent, from the financing of leasehold improvements for our headquarters. Interest expense increased by $0.7 million in the year ended December 31, 2010 compared to the year ended December 31, 2009 primarily due to interest incurred from borrowings on our prior credit facility that was entered into in March 2010,
Other income (expense), net
Other income (expense), net decreased by $0.6 million in the year ended December 31, 2011 compared to the year ended December 31, 2010, and increased by $0.3 million in the year ended December 31, 2010 compared to the year ended December 31, 2009, primarily due to income from two therapeutic tax credits received during 2010.
We expect to earn interest income at market rates in proportion to the marketable securities balances we maintain. We generally hold such investments until such time as we liquidate them to meet an operating cash need. Interest paid on our investment portfolio may decrease and the value of certain securities we hold may decline, which could negatively affect our financial condition, cash flow and reported earnings.
Liquidity and Capital Resources
In recent years, our sources of capital have primarily consisted of public offerings and private placements of equity securities, debt instruments, United States government grants and cooperative agreements, and contribution from product sales, net of expenses and interest income.
44
Table of Contents
At December 31, 2011, we had cash, cash equivalents and short-term investments of $25.8 million. Our cash equivalents and short-term investments primarily consist of marketable debt securities, which primarily include money market instruments and, to a lesser extent, United States government agency securities, and are classified as available-for-sale.
Operating Activities
Net cash used in operating activities was $15.6 million for the year ended December 31, 2011 compared to $14.3 million during the year ended December 31, 2010. The increase in net cash used in operating activities was primarily due to changes in our operating assets and liabilities, primarily related to accounts receivable due to the timing of cash collections from our customers.
Net cash used in operating activities was $14.3 million for the year ended December 31, 2010 compared to $14.5 million during the year ended December 31, 2009. The decrease in net cash used in operating activities was primarily due to higher revenues, improved gross margins and lower operating expenses, offset by changes in our operating assets and liabilities, notably increases in accounts receivable balances and lower accrued expenses.
Investing Activities
Net cash provided by investing activities during the year ended December 31, 2011 was $0.6 million compared to $0.1 million net cash used in investing activities during the year ended December 31, 2010. The increase in investing activities was primarily due to purchasing less furniture, equipment and leasehold improvements during the year ended December 31, 2011 as compared to the year ended December 31, 2010, in which we incurred costs related to the consolidation and improvement of our facilities. This change was offset by a decrease in our investment activities during the year ended December 31, 2011 as the proceeds received from the maturities of our existing investments are generally reinvested in money market funds with original maturities of less than 90 days.
Net cash used in investing activities during the year ended December 31, 2010 was $0.1 million compared to $9.3 million of net cash provided by investing activities during the year ended December 31, 2009. The decrease in investing activities was primarily due to fewer maturities of short-term investments and higher purchases of furniture, equipment and leasehold improvements during the year ended December 31, 2010 compared to the year ended December 31, 2009. During 2010, we relocated our headquarters and capitalized leasehold improvements associated with the leasehold build-out.
Financing Activities
Net cash provided by financing activities were during the year ended December 31, 2011 was $11.6 million compared to $26.0 million during the year ended December 31, 2010. The decrease in net cash provided by financing activities was primarily due to lower cash proceeds received from common stock offerings and the repayment of our prior debt associated with a growth capital facility agreement with Oxford, which was issued in March 2010. Our common stock offerings during the year ended December 31, 2011 related to sales of our common stock pursuant to the Sales Agreement in which we sold approximately 3.5 million shares of our common stock for aggregate net proceeds of $9.7 million, of which $0.4 million was received in the first quarter of 2012. The repayment of our prior debt was a result of using substantially all of the proceeds we received from the Credit Agreement in September 2011. In addition, we received $2.3 million during the year ended December 31, 2011, as we drew down on our revolving line of credit.
Cash provided by financing activities during the year ended December 31, 2010 was $26.0 million compared to $12.2 million during the year ended December 31, 2009. The increase in cash provided by financing activities was primarily due to higher cash proceeds received from common stock offerings and proceeds from the issuance of debt during the year ended December 31, 2010.
45
Table of Contents
Working Capital
Working capital decreased to $18.6 million at December 31, 2011, from $22.1 million at December 31, 2010, primarily due to lower balances in cash and investments, which were used for our operations, net increases in the combined total for our accounts payable and accrued liabilities balances as a result of the timing of payments to our vendors, and increases in the current portion of our debt, as we entered into a new credit facility. This was partially offset by increases in accounts receivable due to timing of cash collections from our customers, increases in inventory levels in order to be able to fulfill anticipated future customer demand for our products coupled with the management of our supply chain and decreases in our warrant liability.
Working capital increased to $22.1 million at December 31, 2010, from $19.4 million at December 31, 2009, primarily due to higher cash, cash equivalents and short-term investments, and accounts receivable. This was partially offset by decreases in inventory and increases in warrant liabilities and the current portion of debt.
Capital Requirements
Our near-term capital requirements are dependent on various factors, including operating costs and working capital investments associated with commercializing the INTERCEPT Blood System, costs associated with pursuing regulatory approval in geographies where we do not currently sell our platelet and plasma systems, costs associated with planning and conducting studies and clinical development of our red blood cell system, timing and magnitude of payments under grants from the United States government, and costs related to creating, maintaining and defending our intellectual property. Our long-term capital requirements will also be dependent on competitive developments, clinical developments and regulatory factors. Until we are able to generate a sufficient amount of product revenue and generate positive net cash flows from operations, meeting our long-term capital requirements is in large part subject to access to public and private equity and debt capital markets, as well as to additional collaborative arrangements with partners or government grants, augmented by cash generated from operations and interest income earned on the investment of our cash balances and short-term investments. We believe that cash received from product sales and government grants, our available cash balances and access to debt will be sufficient to meet our capital requirements for at least the next twelve months. If our assumptions prove to be incorrect, we could consume our available capital resources sooner than we currently expect.
We may borrow additional capital from institutional and commercial banking sources to fund future growth outside of our credit agreement with Comerica Bank, as described below, on terms that may include restrictive covenants, which may comprise of covenants that restrict the operation of our business, liens on assets, high effective interest rates and repayment provisions that reduce cash resources and limit future access to capital markets. To the extent we raise additional capital by issuing equity securities, our stockholders may experience substantial dilution. To the extent that we raise additional funds through collaboration or partnering arrangements, we may be required to relinquish some of our rights to our technologies or rights to market and sell our products in certain geographies, or grant licenses on terms that are not favorable to us. The disruptions to the global credit and financial markets and general economic uncertainty has generally made equity and debt financing more difficult to obtain and the terms less favorable to the companies seeking to raise financing. As a result of these and other factors, we do not know whether additional capital will be available when needed, or that, if available, we will be able to obtain additional capital on reasonable terms. If we are unable to raise additional capital due to disruptions to the global credit and financial markets and general economic uncertainty or other factors, we may need to curtail planned development or commercialization activities.
Other Information
Historically, we have received significant awards in funding under cooperative agreements with the DoD for the INTERCEPT Blood System. Any such funding is subject to the authorization of funds and approval of our research plans by various organizations within the federal government, including the United States Congress. The
46
Table of Contents
general economic environment, coupled with tight federal budgets, has led to a general decline in the amount available for government funding. We are unsure whether government funding will be available to us in the future, or if available, at what levels.
In June 2011, we entered into the Sales Agreement with MLV that provides for the issuance and sale of shares of our common stock over the term of the Sales Agreement having an aggregate offering price of up to $20.0 million from time to time through MLV as our sales agent. Future issuances and sales of shares of common stock by us under the Sales Agreement are subject to the continuing effectiveness of our shelf registration statements on Form S-3 that we filed with the SEC in December 2011. Sales of our common stock through MLV will be made on the Nasdaq Global Market by means of ordinary brokers’ transactions at market prices, in block transactions or as otherwise agreed by us and MLV. Subject to the terms and conditions of the Sales Agreement, MLV will use commercially reasonable efforts to sell our common stock from time to time, based upon our instructions (including any price, time or size limits or other customary parameters or conditions we may impose). We are not obligated to make any sales of common stock under the Sales Agreement. The offering of shares of our common stock pursuant to the Sales Agreement will terminate upon the earlier of (1) the sale of all common stock subject to the Sales Agreement and (2) termination of the Sales Agreement. The Sales Agreement may be terminated by MLV or us at any time upon 10 days notice to the other party, or by MLV at any time in certain circumstances, including our undergoing a material adverse change. We pay MLV an aggregate commission rate equal to 3% of the gross proceeds of the sales price per share of any common stock sold through MLV under the Sales Agreement. During the year ended December 31, 2011, approximately 3.5 million shares of our common stock were sold under the Sales Agreement for aggregate net proceeds of $9.7 million. Subsequent to the year ended December 31, 2011 through the date of filing this Annual Report on Form 10-K, we sold approximately 3.0 million of additional shares of common stock for aggregate net proceeds of approximately $9.1 million.
In December 2011, we filed a shelf registration statement on Form S-3 to offer and sell up to $150.0 million of common stock, preferred stock, warrants, and/or debt securities, less amounts sold under the Sales Agreement following the effectiveness of the shelf registration statement. The registration statement was declared effective in January 2012 and expires in January 2015.
Commitments and Off-Balance Sheet Arrangements
Off-balance sheet arrangements
We did not have any off-balance sheet arrangements as of December 31, 2011 or 2010.
Commitments
The following summarizes our commitments at December 31, 2011:
| (in thousands) |
Total | Less than 1 year |
1 - 3 years |
4 - 5 years |
After 5 years |
|||||||||||||||
| Minimum purchase requirements |
$ | 3,668 | $ | 3,518 | $ | 100 | $ | 50 | $ | 0 | ||||||||||
| Operating leases |
1,575 | 706 | 869 | 0 | 0 | |||||||||||||||
| Other commitments |
1,366 | 367 | 293 | 287 | 419 | |||||||||||||||
| Debt |
8,318 | 677 | 6,061 | 1,580 | 0 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total contractual obligations |
$ | 14,927 | $ | 5,268 | $ | 7,323 | $ | 1,917 | $ | 419 | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Minimum purchase requirements
Our minimum purchase commitments include certain components of our INTERCEPT Blood System which we purchase from third party manufacturers and supply to Fenwal at no cost for use in manufacturing finished disposable kits.
47
Table of Contents
Operating leases
We generally lease our office facilities and certain equipment under non-cancelable operating leases with initial terms in excess of one year that require us to pay operating costs, property taxes, insurance and maintenance. These facility leases generally contain renewal options and provisions adjusting the lease payments if those renewal options are exercised. Our lease payments have increased as we exercised a ten year extension option on December 10, 2009 to extend the term of our Concord California lease. However, we have the right to early terminate the Concord California lease, which may occur as early as January 2015. Our facility leases qualify as operating leases under ASC Topic 840, “Leases” and as such, are not included on our consolidated balance sheets.
Other commitments
Our other commitments primarily consist of obligations for landlord financed leasehold improvements, which are in addition to the operating leases we have for office and laboratory space. We pay for the financed leasehold improvements as a component of rent and are required to reimburse our landlords over the remaining life of the respective leases. If we exercise our right to early terminate the Concord California lease, which may occur as early as January 2015, we would be required to pay for any remaining portion of the landlord financed leasehold improvements at such time. At December 31, 2011, we had an outstanding liability of $0.9 million related to these leasehold improvements.
Debt
In September 2011, we entered into a Credit Agreement with Comerica. The Credit Agreement provides for a growth capital loan of up to $8.0 million, or Growth Capital Loan, and a formula based revolving line of credit, or RLOC, of up to $4.0 million plus any unused amounts from the Growth Capital Loan. Under the Credit Agreement, we are limited to an aggregate borrowing of up to $10.0 million at any time. We pledged all current and future assets, excluding our intellectual property and 35% of our investment in our subsidiary, Cerus Europe B.V., as security for borrowings under the Credit Agreement.
Concurrent with the execution of the Credit Agreement, in September 2011, we borrowed and issued $5.0 million of the Growth Capital Loan, or Growth Capital Loan A, substantially all of which was used to repay our prior debt with Oxford, with the remainder used for general corporate purposes. Growth Capital Loan A, which matures in 48 months, bears a fixed interest rate of 6.37%, with interest–only payments due for the first twelve months, followed by equal principal and interest payments for the remaining 36 months.
We may draw up to an additional $3.0 million of the Growth Capital Loan, or Growth Capital Loan B, between December 31, 2011 and June 30, 2012. Growth Capital Loan B will bear a fixed interest rate based on the higher of (i) 6.25% or (ii) 6.00% plus the three month LIBOR rate at the date of draw, with interest–only payments due for the first six months followed by equal principal and interest payments for the remaining 36 months. As of December 31, 2011, we have not drawn down any amounts under the Growth Capital Loan B.
In September 2011, we paid a commitment fee of $40,000 and loan fees of $50,000, which were recorded as a discount to our Growth Capital Loan A and are being amortized as a component of interest expense using the effective interest method over the term of the Growth Capital Loan A (discount was based on an implied interest rate of 7.07%). We will also be required to make a final payment fee of 1% of the amounts drawn under Growth Capital Loan A and Growth Capital Loan B due on the earlier of (i) prepayment of the Growth Capital Loan or (ii) the maturity of the Growth Capital Loan. The final payment fee will be accreted to interest expense using the effective interest method over the life of the Growth Capital Loan A and B upon draw.
The Credit Agreement also provides for a RLOC of up to $4.0 million plus any unused amounts from the Growth Capital Loan B, or the RLOC Loan Amount. The amount available under the RLOC, which is available to us until September 30, 2013, is limited to the lesser of (i) 80% of eligible trade receivables or (ii) the RLOC
48
Table of Contents
Loan Amount. In December 2011, we drew down $2.3 million under the RLOC. The RLOC will bear a floating rate based on the lender’s prime rate plus 1.50%, with interest—only payments due each month. At December 31, 2011, the floating rate of the RLOC was at 4.75%. We will be required to repay the principal drawn from the RLOC at the end of the RLOC term. In September 2011, we incurred a commitment fee of $20,000, and will pay the same commitment fee at each annual anniversary of the RLOC. As of December 31, 2011, we have $2.3 million outstanding under the RLOC.
We are required to maintain compliance with certain customary and routine financial covenants under the Credit Agreement. Throughout the term of the Credit Agreement, we are also obligated to meet certain conditions which include maintaining a minimum cash balance of $2.5 million at Comerica and achieving minimum revenue levels, which are measured monthly based on a six-month trailing basis and must be at least 75% of the pre-established future projected revenues for the trailing six-month period. Non-compliance with the covenants could result in the principal of the note becoming due and payable. As of December 31, 2011, we were in compliance with the financial covenants as set forth in the Credit Agreement.
Financial Instruments
We maintain an investment portfolio of various securities, which are classified as available-for-sale and, consequently, are recorded on the consolidated balance sheet at fair value with unrealized gains or losses reported as a separate component of stockholders’ equity. Our investment policy is to manage our marketable securities portfolio to preserve principal and liquidity while maximizing the return on the investment portfolio to assist us in funding our operations. We did not have any unrealized gains at December 31, 2011. Unrealized gains totaled $0.1 million at December 31, 2010.
We invest our cash, cash equivalents and short-term investments in a variety of financial instruments, consisting primarily of high credit, high liquidity United States government agency securities, money market funds and interest-bearing accounts with financial institutions. Our money market funds are classified as Level 1 in the fair value hierarchy, in which quoted prices are available in active markets, as the maturity of money market funds are relatively short and the carrying amount is a reasonable estimate of fair value. Our available-for-sale securities related to United States government agencies and corporate debt securities are classified as Level 2 in the fair value hierarchy, which uses observable inputs to quoted market prices, benchmark yields, reported trades, broker/dealer quotes or alternative pricing sources with reasonable levels of price transparency. We maintain portfolio liquidity by ensuring that the securities have active secondary or resale markets. Certain of the investments in our portfolio are subject to general market risk and more specifically, the United States mortgage industry and financial institutions. We did not record any other-than-temporary impairment losses during the years ended December 31, 2011, 2010 and 2009. The current global economic crisis has had, and may continue to have, a negative impact on the market values of the investments in our investment portfolio. There can be no assurance that the markets for these securities will not deteriorate further or that the institutions that these investments are with will be able to meet their debt obligations at the time we may need to liquidate such investments or until such time as the investments mature.
| Item 7A. | Quantitative and Qualitative Disclosures About Market Risk |
Interest Rate Risk
Of our cash, cash equivalents, and short-term investments balance of $25.8 million at December 31, 2011, approximately 99% had original maturity dates of less than 90 days, and the remaining 1% had original maturities more than one year. We do not believe our exposure to interest rate risk to be material given the short-term nature of our investment portfolio and the relatively flat yields in high credit, fixed-income investments and the consistent yields we have experienced and anticipate experiencing across our portfolio.
Our exposure to market rate risk for changes in interest rates relates primarily to our investment portfolio. We do not use derivative financial instruments in our investment portfolio. By policy, we place our investments
49
Table of Contents
with high quality debt security issuers, limit the amount of credit exposure to any one issuer and limit duration by restricting the term for single securities and for the portfolio as a whole. Our investments are held and managed by a third-party capital management adviser that in turn, utilizes a combination of active market quotes and where necessary, proprietary pricing models as well as a subscribed pricing service, in order to estimate fair value.
We invest our cash, cash equivalents and short-term investments in a variety of financial instruments, consisting primarily of high credit, high liquidity money market instruments, United States government agency securities, and interest-bearing accounts with financial institutions. We maintain portfolio liquidity by ensuring that the securities have active secondary or resale markets. Certain of the investments in our portfolio are subject to general market risk and, to a lesser extent, the United States mortgage industry. While we believe that we will be able to recognize the fair value of these instruments when they mature or we sell them, there can be no assurance that the markets for these securities will not deteriorate further or that the institutions that these investments are with will be able to meet their debt obligations.
We account for our short-term investments in accordance with ASC Topic 320, “Accounting for Certain Investments in Debt and Equity Securities.” All our cash, cash equivalents and short-term investments are recorded as current assets on our consolidated balance sheets as they are classified as available-for-sale. Securities with original maturities of less than 90 days from the purchase date are classified as cash equivalents. The table below presents the amounts and weighted interest rates of our cash, cash equivalents and marketable securities at December 31, 2011:
| (in thousands, except percentages) |
Fair Value | Weighted Average Interest Rate |
||||||
| Cash and cash equivalents (0 – 90 days(1) ) |
$ | 25,497 | 0.17 | % | ||||
| Short-term investments (91 days – 1 year(1) ) |
0 | 0.00 | % | |||||
| Short-term investments (1 year – 3 years(1) ) |
0 | 0.00 | % | |||||
| Short-term investments (3 years – 5 years(1) ) |
287 | 5.00 | % | |||||
|
|
|
|||||||
| Total cash, cash equivalents and short-term investments |
$ | 25,784 | 0.22 | % | ||||
|
|
|
|||||||
| (1) | Based on original contractual maturity date |
Foreign Currency Risk
Our international operations are subject to risks typical of an international business, including, among other factors: differing political, economic, and regulatory climates, different tax structures, and foreign exchange volatility. We do not currently enter into any hedging contracts to normalize the impact of foreign exchange fluctuations. As a result, our future results could be materially impacted by changes in these or other factors.
Product sales for our blood safety products are predominantly made in Europe and generally are invoiced to customers in Euros. In addition, we incur operating expenses, including payment for finished goods inventory of disposable kits for the platelet and plasma systems. These inventory purchases and operating expenses are generally paid in Euros and, to a much lesser degree, other foreign currencies. Our exposure to foreign exchange rate volatility is a direct result of our product sales, cash collection and expenses to support our international operations. Foreign exchange rate fluctuations are recorded as a component of non-operating expense, net on our consolidated statements of operations. Significant fluctuations in the volatility of foreign currencies relative to the United States dollar may materially impact our results of operations. A 10% change in foreign currency exchange rates for our accounts receivable, accounts payable and accrued liabilities that are denominated in foreign currencies at December 31, 2011 would have negatively impacted our annual financial results by $0.1 million. Currently we do not have any near-term plans to enter into a formal hedging program to mitigate the effects of foreign currency volatility.
50
Table of Contents
| Item 8. | Financial Statements and Supplementary Data |
Our consolidated financial statements, together with related notes and reports of Ernst & Young LLP, independent registered public accounting firm, are listed in Item 15(a) and included herein.
| Item 9. | Changes in and Disagreements with Accountants on Accounting and Financial Disclosure |
None.
| Item 9A. | Controls and Procedures |
Evaluation of Disclosure Controls and Procedures. Our chief executive officer and chief accounting officer are responsible for establishing and maintaining “disclosure controls and procedures” (as defined in Rule 13a-15(e) and Rule 15d-15(e), promulgated under the Securities Exchange Act of 1934, as amended) for our company. Based on their evaluation of our disclosure controls and procedures as of December 31, 2011, our chief executive officer and chief accounting officer have concluded that our disclosure controls and procedures were effective.
Changes in Internal Control over Financial Reporting. During the last quarter of our fiscal year ended December 31, 2011, there were no changes in our internal control over financial reporting during the period covered by this report that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
Limitations on the Effectiveness of Controls. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. Our disclosure controls and procedures are designed to provide reasonable assurance of achieving their objectives, and the chief executive officer and chief accounting officer have concluded that these controls and procedures are effective at the “reasonable assurance” level.
Management’s Report on Internal Control over Financial Reporting. Management’s assessment of the effectiveness of our internal control over financial reporting as of December 31, 2011, is discussed in the Management’s Report on Internal Control over Financial Reporting included on page 58.
Attestation Report of Independent Registered Public Accounting Firm. Ernst & Young LLP, independent registered public accounting firm, has issued an audit report on our internal control over financial reporting, which report is included on page 59.
| Item 9B. | Other Information |
None.
51
Table of Contents
PART III
Certain information required by Part III is omitted from this Annual Report on Form 10-K since we intend to file our definitive proxy statement for our 2012 annual meeting of stockholders, or the Proxy Statement, pursuant to Regulation 14A of the Securities Exchange Act of 1934, as amended, not later than 120 days after the end of the fiscal year covered by this Annual Report on Form 10-K, and certain information to be included in the proxy statement is incorporated herein by reference.
| Item 10. | Directors, Executive Officers and Corporate Governance |
Information required by this item regarding executive officers, directors and nominees for directors, including information with respect to our audit committee and audit committee financial expert, and the compliance of certain reporting persons with Section 16(a) of the Securities Exchange Act of 1934, as amended, will be included in the Proxy Statement and is incorporated herein by reference.
Code of Ethics
We have adopted the Cerus Corporation Code of Business Conduct and Ethics, or Ethics Code, that applies to all of our officers, directors and employees. The Ethics Code is available on our website at www.cerus.com on the “Corporate Governance” page of the section entitled “Investors.” If we make any substantive amendments to the Ethics Code or grant any waiver from a provision of the Ethics Code to any executive officer or director, we intend to promptly disclose the nature of the amendment or waiver as required by applicable laws. To satisfy our disclosure requirements, we may post any waivers of or amendments to the Ethics Code on our website in lieu of filing such waivers or amendments on a Form 8-K.
Our employees are required to report any conduct that they believe in good faith to be an actual or apparent violation of the Ethics Code. The Audit Committee of our Board of Directors has established procedures to receive, retain and address complaints regarding accounting, internal accounting controls or auditing matters and to allow for the confidential and anonymous submission by employees of related concerns.
| Item 11. | Executive Compensation |
The information required by this item is incorporated herein by reference to our Proxy Statement.
| Item 12. | Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters |
The information required by this item is incorporated herein by reference to our Proxy Statement.
| Item 13. | Certain Relationships and Related Transactions, and Director Independence |
The information required by this item is incorporated herein by reference to our Proxy Statement.
| Item 14. | Principal Accountant Fees and Services |
The information required by this item is incorporated herein by reference to our Proxy Statement.
52
Table of Contents
PART IV
| Item 15. | Exhibits and Financial Statement Schedules |
The following documents are being filed as part of this Annual Report on Form 10-K:
| (a) | Financial Statements. |
Other information is omitted because it is either presented elsewhere, is inapplicable or is immaterial as defined in the instructions.
| (b) | Exhibits. |
| Exhibit |
Description of Exhibit | |
| 2.1(25)† | Asset Purchase and Redemption Agreement by and between Cerus Corporation and BioOne Corporation, dated as of August 24, 2010. | |
| 3.1(5) | Amended and Restated Certificate of Incorporation of Cerus Corporation | |
| 3.2(24) | Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Cerus Corporation. | |
| 3.3(12) | Amended and Restated Bylaws of Cerus Corporation. | |
| 4.1(1) | Specimen Stock Certificate. | |
| 4.2(19) | Rights Agreement, dated as of November 3, 1999, as amended as of August 6, 2001, between Cerus Corporation and Wells Fargo Bank, N.A. (formerly known as Norwest Bank Minnesota, N.A.). | |
| 4.3(21) | Amendment to Rights Agreement, dated as of October 28, 2009, between Cerus Corporation and Wells Fargo Bank, N.A. (which includes the form of Rights Certificate as Exhibit B thereto). | |
| 4.4(20) | Form of 2009 Warrant to Purchase Common Stock. | |
| 4.5(26) | Form of 2010 Warrant to Purchase Common Stock. | |
| Supply and/or Manufacturing Agreements | ||
| 10.1(10)† | Supply Agreement, dated December 19, 2007, by and between Cerus Corporation and Brotech Corporation d/b/a Purolite Company. | |
| 10.2(10)† | Supply and Manufacturing Agreement, dated March 1, 2008, by and between Cerus Corporation and Porex Corporation. | |
| 10.3(15)† | Amended and Restated Manufacturing and Supply Agreement, dated December 12, 2008, by and between Cerus Corporation and Fenwal, Inc. | |
| 10.4(15)† | Manufacturing and Supply Agreement, dated September 30, 2008, by and between Cerus Corporation and NOVA Biomedical Corporation. | |
53
Table of Contents
| Exhibit |
Description of Exhibit | |
| 10.5(32)† | Amended and Restated Supply Agreement, dated as of September 1, 2011, between Cerus Corporation and Ash Stevens Inc. | |
| Loan and Security Agreement | ||
| 10.6(27)† | Loan and Security Agreement, by and between Cerus Corporation and Oxford Finance Corporation, dated March 31, 2010. | |
| 10.7(28) | First Amendment to Loan and Security Agreement, by and between Cerus Corporation and Oxford Finance Corporation, dated March 3, 2011. | |
| 10.8(32)† | Loan and Security Agreement, dated as of September 30, 2011, by and between Cerus Corporation and Comerica Bank. | |
| Real Estate Lease Agreements | ||
| 10.9(6) | Standard Industrial/Commercial Single-Tenant Lease-Net, dated October 12, 2001 between Cerus Corporation and California Development, Inc. | |
| 10.10(13) | Second Amendment to Standard Industrial/Commercial Single-Tenant Lease-Net, dated as of September 18, 2008 between Cerus Corporation and California Development, Inc. | |
| 10.11(22) | Letter to California Development, Inc. exercising option to extend the lease term from the Second Amendment to Standard Industrial/Commercial Single-Tenant Lease-Net, dated as of September 18, 2008 between Cerus Corporation and California Development, Inc. | |
| Employment Agreements or Offer Letters | ||
| 10.12(9)* | Offer Letter to Gail Schulze, dated October 15, 2007. | |
| 10.13(14)* | Amended and Restated Employment Agreement with Claes Glassell, dated December 19, 2008. | |
| 10.14(29)* | Employment Letter, by and between Cerus corporation and William M. Greenman, dated May 12, 2011. | |
| 10.15(23)* | Employment Letter, by and between Cerus Corporation and Laurence Corash, dated March 2, 2010. | |
| 10.16(14)* | Amended and Restated Employment Agreement with Howard G. Ervin, dated December 22, 2008. | |
| 10.17(19)* | Employment Letter for Kevin D. Green dated May 1, 2009. | |
| 10.18* | Employment Agreement for Caspar Hogeboom dated March 6, 2006. | |
| 10.19* | Promotion Letter for Caspar Hogeboom dated December 11, 2009 and executed on September 21, 2010. | |
| 10.20* | Addendum to Employment Agreement for Caspar Hogeboom dated February 17, 2011. | |
| 10.21* | Healthcare Contribution Letter for Caspar Hogeboom dated December 18, 2007. | |
| 10.22* | Home Telephone and Internet Expenses Letter for Caspar Hogeboom dated January 11, 2012. | |
| Stock Plans and Related Forms | ||
| 10.23(1)* | 1996 Equity Incentive Plan. | |
| 10.24(1)* | Form of Incentive Stock Option Agreement under the 1996 Equity Incentive Plan. | |
| 10.25(1)* | Form of Nonstatutory Stock Option Agreement under the 1996 Equity Incentive Plan. | |
| 10.26(1)* | 1996 Employee Stock Purchase Plan. | |
54
Table of Contents
| Exhibit |
Description of Exhibit | |
| 10.27(3)* | 1998 Non-Officer Stock Option Plan. | |
| 10.28(4)* | 1999 Equity Incentive Plan, adopted April 30, 1999, approved by stockholders July 2, 1999. | |
| 10.29(7)* | 1999 Non-Employee Directors’ Stock Option Sub-Plan, amended December 4, 2002. | |
| 10.30(11)* | 2008 Equity Incentive Plan, approved by stockholders June 2, 2008. | |
| 10.31(31)* | 2008 Equity Incentive Plan, as amended, reapproved by stockholders June 1, 2011. | |
| 10.32(16)* | Form of Restricted Stock Unit Agreement under the 1999 Equity Incentive Plan, as amended. | |
| Other Compensatory Plans or Agreements | ||
| 10.33(23)* | Bonus Plan for Senior Management of Cerus Corporation, as amended March 3, 2010. | |
| 10.34(16)* | Cerus Corporation Change of Control Severance Benefit Plan, as amended. | |
| 10.35(18)* | Form of Severance Benefits Agreement. | |
| 10.36 * | 2011 and 2012 Executive Officer Compensation Arrangements. | |
| 10.37 * | Non-Employee Director Compensation Policy. | |
| Other Material Agreements | ||
| 10.38(30) | At-The-Market-Issuance Sales Agreement, dated June 3, 2011, by and between Cerus Corporation and MLV & Co. LLC. | |
| 10.39(33) | Amendment to At-The-Market-Issuance Sales Agreement, dated January 4, 2012, by and between Cerus Corporation and MLV & Co. LLC. | |
| 10.40 (1) | Form of Indemnity Agreement entered into between Cerus Corporation and each of its directors and executive officers. | |
| 10.41(17) | Form of Amended and Restated Indemnity Agreement, adopted April 24, 2009. | |
| 10.42(20) | Form of Subscription Agreement. | |
| 10.43 (2) | Series B Preferred Stock Purchase Agreement, dated as of June 30, 1998, by and between Cerus Corporation and Baxter Healthcare Corporation. | |
| 10.44(22)† | Restructuring Agreement, dated as of February 2, 2005, by and among Cerus Corporation, Baxter Healthcare S.A. and Baxter Healthcare Corporation. | |
| 10.45(22)† | License Agreement, dated as of February 2, 2005, by and among Cerus Corporation, Baxter Healthcare S.A. and Baxter Healthcare Corporation. | |
| 10.46(8)† | Commercialization Transition Agreement, dated as of February 12, 2006, by and among Cerus Corporation, Baxter Healthcare S.A. and Baxter Healthcare Corporation. | |
| 12.1 | Computation of Earnings to Fixed Charges | |
| 21.1 | List of Registrant’s subsidiaries. | |
| 23.1 | Consent of Independent Registered Public Accounting Firm. | |
| 24.1 | Power of Attorney (see signature page). | |
| 31.1 | Certification of the Principal Executive Officer of Cerus Corporation pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 31.2 | Certification of the Principal Financial Officer of Cerus Corporation pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.1 (35) | Certification of the Principal Executive Officer and Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
55
Table of Contents
| Exhibit |
Description of Exhibit | |
| 101.INS(34) | XBRL Instance Document | |
| 101.SCH(34) | XBRL Taxonomy Extension Schema Document | |
| 101.CAL(34) | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF(34) | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB(34) | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE(34) | XBRL Taxonomy Extension Presentation Linkbase Document | |
| † | Certain portions of this exhibits are subject to a confidential treatment order. |
| * | Compensatory Plan. |
| (1) | Incorporated by reference to the like-described exhibit to the Registrant’s Registration Statement on Form S-1 (File No. 333-11341) and amendments thereto. |
| (2) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on July 22, 1998. |
| (3) | Incorporated by reference to the like-described exhibit to the Registrant’s Registration Statement on Form S-8, dated March 24, 1999. |
| (4) | Incorporated by reference to the like-described exhibit to the Registrant’s Registration Statement on Form S-8, dated August 4, 1999. |
| (5) | Incorporated by reference to the like-described exhibit to the Registrant’s Registration Statement on Form 8-K, dated November 12, 1999. |
| (6) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2001. |
| (7) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2003. |
| (8) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2006. |
| (9) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2007. |
| (10) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2008. |
| (11) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 6, 2008. |
| (12) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 19, 2008. |
| (13) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended September 30, 2008. |
| (14) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 23, 2008. |
| (15) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2008. |
| (16) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2009. |
| (17) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 30, 2009. |
| (18) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 1, 2009. |
| (19) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended June 30, 2009. |
56
Table of Contents
| (20) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 20, 2009. |
| (21) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on October 30, 2009. |
| (22) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2009. |
| (23) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on March 8, 2010. |
| (24) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended June 30, 2010. |
| (25) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 30, 2010. |
| (26) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on November 12, 2010. |
| (27) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2010. |
| (28) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2011. |
| (29) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 18, 2011. |
| (30) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 6, 2011. |
| (31) | Incorporated by reference to the like-described exhibit to Amendment No. 1 to the Registrant’s Quarterly Report on Form 10-Q/A, for the quarter ended June 30, 2011. |
| (32) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended September 30, 2011. |
| (33) | Incorporated by reference to the like-described exhibit to Amendment No. 1 to the Registrant’s Registration Statement on Form S-3/A, filed with the SEC on January 6, 2012. |
| (34) | Furnished herewith. Pursuant to applicable securities laws and regulations, the Registrant is deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and is not subject to liability under any anti-fraud provisions of the federal securities laws as long as the Registrant has made a good faith attempt to comply with the submission requirements and promptly amends the interactive data files after becoming aware that the interactive data files fails to comply with the submission requirements. These interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise are not subject to liability under these sections. |
| (35) | This certification accompanies the Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission, and is not incorporated by reference into any filing of the Registrant’s under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing. |
57
Table of Contents
MANAGEMENT’S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
Management is responsible for establishing and maintaining effective internal control over the Company’s financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act of 1934, as amended. Management assessed the effectiveness of the Company’s internal control over financial reporting as of December 31, 2011. In making this assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”) in Internal Control—Integrated Framework. Based on this assessment, management has concluded that, as of December 31, 2011, the Company’s internal control over financial reporting is effective.
The Company’s independent registered public accounting firm, Ernst & Young LLP, has audited the effectiveness of internal control over financial reporting as of December 31, 2011. Ernst and Young LLP’s attestation report on internal control over financial reporting is included herein.
The Company’s internal control system was designed to provide reasonable assurance to the Company’s management and Board of Directors regarding the preparation and fair presentation of published financial statements. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation. Accordingly, our internal control systems are designed to provide reasonable, not absolute, assurance that the objectives of our internal control systems are met and, as set forth above, our principal executive officer and principal financial officer have concluded, based on their evaluation as of the end of the period covered by this Annual Report on Form 10-K, that our internal control over financial reporting was effective. To provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external reporting purposes in accordance with generally accepted accounting principles, we continue to implement, improve and refine our disclosure controls and procedures and our internal control over financial reporting.
58
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Cerus Corporation
We have audited Cerus Corporation’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Cerus Corporation’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Cerus Corporation maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Cerus Corporation as of December 31, 2011, and 2010, and the related consolidated statements of operations, stockholders’ equity, and cash flows for each of the three years in the period ended December 31, 2011, and our report dated March 5, 2012, expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Jose, California
March 5, 2012
59
Table of Contents
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
The Board of Directors and Stockholders of Cerus Corporation
We have audited the accompanying consolidated balance sheets of Cerus Corporation as of December 31, 2011, and 2010, and the related consolidated statements of operations, stockholders’ equity and cash flows for each of the three years in the period ended December 31, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of Cerus Corporation at December 31, 2011, and 2010, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2011, in conformity with United States generally accepted accounting principles.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Cerus Corporation’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organization of the Treadway Commission and our report dated March 5, 2012 expressed an unqualified opinion thereon.
/s/ ERNST & YOUNG LLP
San Jose, California
March 5, 2012
60
Table of Contents
CONSOLIDATED BALANCE SHEETS
(in thousands, except per share amounts)
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| ASSETS | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 25,497 | $ | 28,948 | ||||
| Short-term investments |
287 | 1,061 | ||||||
| Accounts receivable, net of allowance of $5 and $51 at December 31, 2011 and 2010, respectively |
6,096 | 4,792 | ||||||
| Inventories |
6,444 | 5,957 | ||||||
| Prepaid expenses |
810 | 681 | ||||||
| Other current assets |
605 | 316 | ||||||
|
|
|
|
|
|||||
| Total current assets |
39,739 | 41,755 | ||||||
| Non-current assets: |
||||||||
| Property and equipment, net |
2,032 | 2,390 | ||||||
| Goodwill |
1,316 | 1,316 | ||||||
| Intangible assets, net |
1,748 | 1,950 | ||||||
| Restricted cash |
303 | 305 | ||||||
| Other assets |
229 | 451 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 45,367 | $ | 48,167 | ||||
|
|
|
|
|
|||||
| LIABILITIES AND STOCKHOLDERS’ EQUITY | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 4,680 | $ | 3,230 | ||||
| Accrued liabilities |
5,825 | 6,003 | ||||||
| Deferred revenue |
111 | 248 | ||||||
| Debt—current |
2,519 | 1,747 | ||||||
| Capital lease obligations—current |
0 | 10 | ||||||
| Warrant liability |
7,979 | 8,465 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
21,114 | 19,703 | ||||||
| Non-current liabilities: |
||||||||
| Debt—non-current |
4,697 | 3,131 | ||||||
| Capital lease obligations—non-current |
0 | 6 | ||||||
| Other non-current liabilities |
1,243 | 1,595 | ||||||
|
|
|
|
|
|||||
| Total liabilities |
27,054 | 24,435 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.001 par value 5,000 shares authorized, issuable in series; 3 shares issued and outstanding at both December 31, 2011 and 2010; aggregate liquidation preference of $9,496 at December 31, 2011 and 2010 |
9,496 | 9,496 | ||||||
| Common stock, $0.001 par value 112,500 shares authorized; 51,211 and 47,329 shares issued and outstanding at December 31, 2011 and 2010, respectively |
51 | 47 | ||||||
| Additional paid-in capital |
452,701 | 441,034 | ||||||
| Accumulated other comprehensive income |
0 | 108 | ||||||
| Accumulated deficit |
(443,935 | ) | (426,953 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
18,313 | 23,732 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 45,367 | $ | 48,167 | ||||
|
|
|
|
|
|||||
See accompanying Notes to Consolidated Financial Statements.
61
Table of Contents
CONSOLIDATED STATEMENTS OF OPERATIONS
(in thousands, except per share amounts)
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Revenue: |
||||||||||||
| Product revenue |
$ | 30,602 | $ | 21,677 | $ | 16,751 | ||||||
| Government grants and cooperative agreements |
2,442 | 1,432 | 1,231 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
33,044 | 23,109 | 17,982 | |||||||||
| Cost of product revenue |
18,535 | 12,046 | 12,580 | |||||||||
|
|
|
|
|
|
|
|||||||
| Gross profit |
14,509 | 11,063 | 5,402 | |||||||||
| Operating expenses (gains): |
||||||||||||
| Research and development |
7,178 | 5,195 | 6,372 | |||||||||
| Selling, general and administrative |
23,053 | 21,577 | 21,867 | |||||||||
| Amortization of intangible assets |
202 | 67 | 0 | |||||||||
| Acquisition related costs and impairment of long-term investments in related parties, net |
0 | 182 | 1,536 | |||||||||
| Settlement gain |
0 | 0 | (1,381 | ) | ||||||||
| Restructuring charges |
0 | 0 | 841 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total operating expenses |
30,433 | 27,021 | 29,235 | |||||||||
|
|
|
|
|
|
|
|||||||
| Loss from operations |
(15,924 | ) | (15,958 | ) | (23,833 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Non-operating expense, net: |
||||||||||||
| Gain from revaluation of warrant liability |
486 | 39 | 63 | |||||||||
| Foreign exchange loss |
(529 | ) | (816 | ) | (611 | ) | ||||||
| Interest expense |
(964 | ) | (689 | ) | (10 | ) | ||||||
| Other income (expense), net |
(51 | ) | 513 | 256 | ||||||||
|
|
|
|
|
|
|
|||||||
| Total non-operating expense, net |
(1,058 | ) | (953 | ) | (302 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (16,982 | ) | $ | (16,911 | ) | $ | (24,135 | ) | |||
|
|
|
|
|
|
|
|||||||
| Net loss per common share: |
||||||||||||
| Basic |
$ | (0.35 | ) | $ | (0.42 | ) | $ | (0.69 | ) | |||
| Diluted |
$ | (0.35 | ) | $ | (0.42 | ) | $ | (0.69 | ) | |||
| Weighted average common shares outstanding used for calculating net loss per common share: |
||||||||||||
| Basic |
48,050 | 40,300 | 34,750 | |||||||||
| Diluted |
48,050 | 40,300 | 34,750 | |||||||||
See accompanying Notes to Consolidated Financial Statements.
62
Table of Contents
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(in thousands)
| Preferred Stock | Common Stock | Additional Paid-in Capital |
Accumulated Other Comprehensive Income |
Accumulated Deficit |
Total Stockholders’ Equity |
|||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | |||||||||||||||||||||||||||||
| Balance at December 31, 2008 |
3 | $ | 9,496 | 32,544 | $ | 33 | $ | 410,444 | $ | 212 | $ | (385,907 | ) | $ | 34,278 | |||||||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||||||||||
| Net loss |
0 | 0 | 0 | 0 | 0 | 0 | (24,135 | ) | (24,135 | ) | ||||||||||||||||||||||
| Net change in unrealized loss on investments |
0 | 0 | 0 | 0 | 0 | (154 | ) | 0 | (154 | ) | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Comprehensive loss |
(24,289 | ) | ||||||||||||||||||||||||||||||
| Issuance of common stock from public offering, net of expenses of $3,865 |
0 | 0 | 6,000 | 6 | 9,329 | 0 | 0 | 9,335 | ||||||||||||||||||||||||
| Issuance of common stock from exercise of stock options and purchases from ESPP |
0 | 0 | 134 | 0 | 78 | 0 | 0 | 78 | ||||||||||||||||||||||||
| Stock-based compensation |
0 | 0 | 0 | 0 | 2,046 | 0 | 0 | 2,046 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2009 |
3 | 9,496 | 38,678 | 39 | 421,897 | 58 | (410,042 | ) | 21,448 | |||||||||||||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||||||||||
| Net loss |
0 | 0 | 0 | 0 | 0 | 0 | (16,911 | ) | (16,911 | ) | ||||||||||||||||||||||
| Net change in unrealized gain on investments |
0 | 0 | 0 | 0 | 0 | 50 | 0 | 50 | ||||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Comprehensive loss |
(16,861 | ) | ||||||||||||||||||||||||||||||
| Issuance of common stock from public offering, net of expenses of $1,710 |
0 | 0 | 8,306 | 8 | 16,940 | 0 | 0 | 16,948 | ||||||||||||||||||||||||
| Issuance of common stock from exercise of stock options and purchases from ESPP |
0 | 0 | 345 | 0 | 369 | 0 | 0 | 369 | ||||||||||||||||||||||||
| Stock-based compensation |
0 | 0 | 0 | 0 | 1,828 | 0 | 0 | 1,828 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2010 |
3 | 9,496 | 47,329 | 47 | 441,034 | 108 | (426,953 | ) | 23,732 | |||||||||||||||||||||||
| Components of comprehensive loss: |
||||||||||||||||||||||||||||||||
| Net loss |
0 | 0 | 0 | 0 | 0 | 0 | (16,982 | ) | (16,982 | ) | ||||||||||||||||||||||
| Net change in unrealized loss on investments |
0 | 0 | 0 | 0 | 0 | (108 | ) | 0 | (108 | ) | ||||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||||||
| Comprehensive loss |
(17,090 | ) | ||||||||||||||||||||||||||||||
| Issuance of common stock from public offering, net of expenses of $420 |
0 | 0 | 3,701 | 4 | 9,674 | 0 | 0 | 9,678 | ||||||||||||||||||||||||
| Issuance of common stock from exercise of stock options and purchases from ESPP |
0 | 0 | 181 | 0 | 143 | 0 | 0 | 143 | ||||||||||||||||||||||||
| Stock-based compensation |
0 | 0 | 0 | 0 | 1,850 | 0 | 0 | 1,850 | ||||||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
| Balance at December 31, 2011 |
3 | $ | 9,496 | 51,211 | $ | 51 | $ | 452,701 | $ | 0 | $ | (443,935 | ) | $ | 18,313 | |||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||||
See accompanying Notes to Consolidated Financial Statements.
63
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
(in thousands)
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Operating activities |
||||||||||||
| Net loss |
$ | (16,982 | ) | $ | (16,911 | ) | $ | (24,135 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
922 | 853 | 866 | |||||||||
| Stock-based compensation |
1,850 | 1,828 | 2,046 | |||||||||
| Gain from revaluation of warrant liability |
(486 | ) | (39 | ) | (63 | ) | ||||||
| Loss (gain) on sale of fixed assets |
(114 | ) | 46 | 102 | ||||||||
| Impairment of long-term investment in related party |
0 | 0 | 2,340 | |||||||||
| Gain from operating settlement |
0 | 0 | (1,381 | ) | ||||||||
| Gain on non-controlling equity interest |
0 | (315 | ) | 0 | ||||||||
| Non-cash interest expense |
5 | 152 | 0 | |||||||||
| Changes in operating assets and liabilities, net of effects of acquired business: |
||||||||||||
| Accounts receivable |
(1,304 | ) | (1,167 | ) | 696 | |||||||
| Inventories |
(487 | ) | 2,020 | 3,402 | ||||||||
| Other assets |
(13 | ) | 99 | 97 | ||||||||
| Accounts payable |
1,450 | (1,193 | ) | 102 | ||||||||
| Accrued restructuring |
0 | (113 | ) | 113 | ||||||||
| Accrued liabilities |
(336 | ) | 569 | 1,368 | ||||||||
| Deferred revenue |
(137 | ) | (97 | ) | (100 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(15,632 | ) | (14,268 | ) | (14,547 | ) | ||||||
| Investing activities |
||||||||||||
| Purchases of furniture, equipment and leasehold improvements |
(158 | ) | (1,692 | ) | (191 | ) | ||||||
| Sales (purchases) of certain other assets |
55 | (11 | ) | 37 | ||||||||
| Purchases of investments |
0 | 0 | (499 | ) | ||||||||
| Sales of investments |
0 | 88 | 499 | |||||||||
| Maturities of investments |
666 | 1,545 | 9,477 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
563 | (70 | ) | 9,323 | ||||||||
| Financing activities |
||||||||||||
| Net proceeds from equity incentive plans |
143 | 370 | 78 | |||||||||
| Net proceeds from public offering |
9,273 | 19,291 | 12,135 | |||||||||
| Proceeds from landlord provided leasehold incentives |
0 | 1,561 | 0 | |||||||||
| Proceeds from revolving line of credit |
2,300 | 0 | 0 | |||||||||
| Proceeds from debt, net of discount |
4,910 | 4,811 | 0 | |||||||||
| Payments on debt and landlord provided leasehold incentives |
(5,008 | ) | (34 | ) | (5 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
11,618 | 25,999 | 12,208 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
(3,451 | ) | 11,661 | 6,984 | ||||||||
| Cash and cash equivalents, beginning of period |
28,948 | 17,287 | 10,303 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of period |
$ | 25,497 | $ | 28,948 | $ | 17,287 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental disclosures: |
||||||||||||
| Common stock issued in connection with the acquisition of certain assets of BioOne |
$ | 0 | $ | 3,423 | $ | 0 | ||||||
| Cash paid for interest |
$ | 1,024 | $ | 600 | $ | 4 | ||||||
See accompanying Notes to Consolidated Financial Statements.
64
Table of Contents
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2011
Note 1. Nature of Operations and Basis of Presentation
Cerus Corporation (the “Company”) was incorporated in September 1991, and is developing and commercializing the INTERCEPT Blood System, which is designed to enhance the safety of blood components through pathogen inactivation. The Company has worldwide commercialization rights for the INTERCEPT Blood System for platelets, plasma and red blood cells.
The Company sells its INTERCEPT platelet and plasma systems in Europe, the Commonwealth of Independent States (“CIS”) countries, the Middle East and selected countries in other regions around the world. The Company conducts significant research, development, testing and regulatory compliance activities on its product candidates that, together with anticipated selling, general, and administrative expenses, are expected to result in substantial additional losses, and the Company may need to adjust its operating plans and programs based on the availability of cash resources. The Company’s ability to achieve a profitable level of operations will depend on successfully completing development, obtaining additional regulatory approvals and achieving widespread market acceptance of its products. There can be no assurance that the Company will ever achieve a profitable level of operations.
Note 2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include those of Cerus Corporation and its subsidiary, Cerus Europe B.V. (collectively hereinafter “Cerus” or the “Company”) after elimination of all intercompany accounts and transactions. These consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”) and pursuant to the rules and regulations of the Securities and Exchange Commission (“SEC”).
Use of Estimates
The preparation of financial statements requires management to make estimates, assumptions and judgments that affect the reported amounts of assets, liabilities, revenue and expenses, and related disclosures of contingent assets and liabilities. On an ongoing basis, management evaluates its estimates, which are based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances. Actual results may differ from those estimates under different assumptions or conditions.
Revenue
The Company recognizes revenue in accordance with ASC Topic 605-25, “Revenue Recognition—Arrangements with Multiple Deliverables,” as applicable. Revenue is recognized when (i) persuasive evidence of an agreement with the funding party exists; (ii) services have been rendered or product has been delivered; (iii) pricing is fixed or determinable; and (iv) collection is probable. The Company’s main sources of revenues for the years ended December 31, 2011, 2010 and 2009 were product revenue from sales of the INTERCEPT Blood System for platelets and plasma (“platelet and plasma systems”) and United States government grants and awards.
Revenue related to product sales is generally recognized when the Company fulfills its obligations for each element of an agreement. For all sales of our INTERCEPT Blood System products, the Company uses a binding purchase order and signed sales contract as evidence of a written agreement. The Company sells its platelet and
65
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
plasma systems directly to blood banks, hospitals, universities, government agencies, as well as to distributors in certain regions. Generally, the Company’s contracts with its customers do not provide for open return rights, except within a reasonable time after receipt of goods in the case of defective or non-conforming product. Deliverables and the units of accounting vary according to the provisions of each purchase order or sales contract. For revenue arrangements with multiple elements, the Company evaluates whether the delivered elements have stand-alone value to the customer. Effective January 1, 2011, the Company adopted the revised guidance in Accounting Standard Update (“ASU”) No. 2009-13, which was issued by the Financial Accounting Standards Board (“FASB,”) in October 2009 related to revenue recognition in accordance with the Accounting Standards Codification (“ASC”) Topic 605-25, “Revenue Recognition—Arrangements with Multiple Deliverables,” on a prospective basis for applicable transactions originating or materially modified after December 31, 2010. Under the revised guidance, companies must assess whether or not revenue arrangements with multiple deliverables exist under the revised guidance, how the deliverables should be separated and how the consideration should be allocated to the elements. Prior to adoption of ASU No. 2009-13, consideration received was allocated to elements that were identified as discrete units of accounting based on the relative fair value method. Beginning January 1, 2011, upon the adoption of ASU No. 2009-13, consideration received is allocated to elements that are identified as discrete units of accounting based on the best estimated selling price. The Company has determined that vendor specific objective evidence is not discernable due to the Company’s limited history of selling its products and variability in its pricing. Since the Company’s products are novel and unique and are not sold by others, third-party evidence of selling price is unavailable. The adoption of the revised guidance did not have a material impact on the Company’s consolidated results of operations for the year ended December 31, 2011 nor is it currently anticipated to have a material impact on future periods.
At December 31, 2011 and 2010, the Company had $0.1 million and $0.2 million, respectively, of short-term deferred revenue on its consolidated balance sheets related to future performance obligations. Freight costs charged to customers are recorded as a component of revenue under ASC Topic 605, “Accounting for Shipping and Handling Fees and Costs.” Value-added-taxes (“VAT”) that the Company invoices to its customers and remits to governments, are recorded on a net basis, which excludes such VAT from product revenue.
Revenue related to the cost reimbursement provisions under development contracts is recognized as the costs on the projects are incurred. The Company receives certain United States government grants and contracts that support research in defined research projects. These grants generally provide for reimbursement of approved costs incurred as defined in the various grants.
Research and Development Expenses
The Company receives certain United States government grants that support the Company’s efforts in defined research projects. These grants generally provide for reimbursement of approved costs incurred as defined in the various grants. Revenue associated with these grants is recognized as costs under each grant are incurred. In addition, in accordance with ASC Topic 730, “Accounting for Research and Development Expenses,” research and development expenses are charged to expense when incurred. Research and development expenses include salaries and related expenses for scientific personnel, payments to consultants, supplies and chemicals used in in-house laboratories, costs of research and development facilities, depreciation of equipment and external contract research expenses, including clinical trials, preclinical safety studies, other laboratory studies, process development and product manufacturing for research use.
The Company’s use of estimates in recording accrued liabilities for research and development activities (described previously in this Note under the heading “Use of Estimates”) affects the amounts of research and
66
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
development expenses recorded and revenue recorded from development funding and government grants and collaborative agreements. Actual results may differ from those estimates under different assumptions or conditions.
Cash, Cash Equivalents and Short-Term Investments
The Company considers all highly liquid investments with original maturities of three months or less from the date of purchase to be classified as cash equivalents. Investments with original maturities of greater than three months but less than one year from the date of purchase as well as available-for-sale investments with original maturities of greater than one year from the date of purchase are classified as short-term investments. These investments primarily consist of marketable debt securities, which include money market instruments and United States government agency securities, and are classified as available-for-sale.
In accordance with ASC Topic 320, “Accounting for Certain Investments in Debt and Equity Securities,” the Company has classified all debt securities as available-for-sale at the time of purchase and reevaluates such designation as of each balance sheet date. Available-for-sale securities are carried at estimated fair value based on quoted market prices. Unrealized gains and losses derived by changes in the estimated fair value of available-for-sale securities are recorded in “Accumulated other comprehensive income” on the Company’s consolidated balance sheets. Realized gains and losses from the sale or maturity of available-for-sale investments are recorded in “Other income (expense), net” on the Company’s consolidated statements of operations. The cost of securities sold is based on the specific identification method. The Company reports the amortization of any premium and accretion of any discount resulting from the purchase of debt securities as a component of interest expense.
The Company also reviews all of its marketable securities on a regular basis to evaluate whether any security has experienced an other-than-temporary decline in fair value. Other-than-temporary declines in market value are recorded in “Other income (expense), net” on the Company’s consolidated statements of operations.
As of December 31, 2011, the Company also maintained a certificate of deposit for approximately $0.2 million with a domestic bank. The Company holds this certificate of deposit for any potential decommissioning resulting from the Company’s possession of radioactive material. The certificate of deposit is held to satisfy the financial surety requirements of the California Department of Health Services and is recorded in “Restricted cash” on the Company’s consolidated balance sheets at December 31, 2011 and 2010.
Concentration of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash equivalents, short-term investments and accounts receivable.
Substantially all of the Company’s cash, cash equivalents and short-term investments are maintained pursuant to the Company’s investment policy at a major financial institution of high credit standing. The Company monitors the financial credit worthiness of the issuers of its investments and limits the concentration in individual securities and type of investments that exist within its investment portfolio. Generally, all of the Company’s remaining investments carry high credit quality ratings, which is in accordance with its investment policy. At December 31, 2011, the Company does not believe there is significant financial risk from non-performance by the issuers of the Company’s cash equivalents and short-term investments.
67
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Concentrations of credit risk with respect to trade receivables exist. However, in connection with the Company’s revolving line of credit, as discussed in Note 12 in the Notes to Consolidated Financial Statements, the Company purchased a credit insurance policy that mitigates some of its credit risk, as the policy will pay either the Company or its lender on eligible claims filed on its outstanding receivables. On a regular basis, including at the time of sale, the Company performs credit evaluations of its customers. Generally, the Company does not require collateral from its customers to secure accounts receivable. To the extent that the Company determines specific invoices or customer accounts may be uncollectible, the Company reserves against the accounts receivable on its consolidated balance sheets and records a charge on its consolidated statements of operations.
The Company recorded minimal amounts for allowances for potentially uncollectible accounts receivable at December 31, 2011 and approximately $0.1 million at December 31, 2010.
The Company had two and four customers that each accounted for more than 10% of the Company’s outstanding trade receivables, which cumulatively represented approximately 58% and 63% of the Company’s outstanding trade receivables, at December 31, 2011 and 2010, respectively. To date, the Company has not experienced collection difficulties from these customers.
Inventories
Inventory consisted of work-in-process and finished goods only. Finished goods include INTERCEPT disposable kits, UVA illumination devices (“illuminators”), and certain replacement parts for the illuminators. Platelet and plasma systems’ disposable kits generally have a two-year life from the date of manufacture. Illuminators and replacement parts do not have regulated expiration dates. Work-in-process includes certain components that are manufactured over a protracted length of time, which can exceed one year, before being incorporated and assembled by Fenwal, Inc. (“Fenwal”) into the finished INTERCEPT disposable kits. The Company maintains an inventory balance based on its current sales projections, and at each reporting period, the Company evaluates whether its work-in-process inventory would be consumed for production of finished units in order to sell to existing and prospective customers within the next twelve-month period. It is not customary for the Company’s production cycle for inventory to exceed twelve months. Instead, the Company uses its best judgment to factor in lead times for the production of its finished units to meet the Company’s current demands. If actual results differ from those estimates, work-in-process inventory could potentially accumulate for periods exceeding one year. At December 31, 2011 and 2010, the Company classified its work-in-process inventory as a current asset on its consolidated balance sheets based on its evaluation that the work-in-process inventory would be consumed for production and subsequently sold within each respective subsequent twelve-month period.
Inventory is recorded at the lower of cost, determined on a first-in, first-out basis, or market value. The Company uses significant judgment to analyze and determine if the composition of its inventory is obsolete, slow-moving or unsalable and frequently reviews such determinations. The Company’s limited history selling the INTERCEPT Blood System limits the amount of historical data the Company has to perform this analysis. Generally, the Company writes-down specifically identified unusable, obsolete, slow-moving, or known unsalable inventory that has no alternative use in the period that it is first recognized by using a number of factors including product expiration dates, open and unfulfilled orders, and sales forecasts. Any write-down of its inventory to net realizable value establishes a new cost basis and will be maintained even if certain circumstances suggest that the inventory is recoverable in subsequent periods. Costs associated with the write-down of inventory are recorded in “Cost of product revenue” on the Company’s consolidated statements of operations. At December 31, 2011 and 2010, the Company had $0.6 million and $0.4 million, respectively, reserved for potential obsolete or expiring product.
68
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Property and Equipment, net
Property and equipment is comprised of furniture, equipment, information technology hardware and software and is recorded at cost. At the time the property and equipment is ready for its intended use, it is depreciated on a straight-line basis over the estimated useful lives of the assets (generally three to five years). Leasehold improvements are amortized on a straight-line basis over the shorter of the lease term or the estimated useful lives of the improvements.
Goodwill and Intangible Assets, net
Goodwill and intangible assets, net is derived at the time of a business acquisition, in which the Company assigns the total consideration transferred to the acquired assets based on each asset’s fair value and any residual amount becomes goodwill, an indefinite life intangible asset. Intangible assets, net, which include a license for the right to commercialize the INTERCEPT Blood System in Asia, are subject to periodic amortization over the estimated useful life of ten years. The amortization of the Company’s intangible assets, net, is recorded in “Amortization of intangible assets” on the Company’s consolidated statements of operations.
Goodwill is not amortized but instead is subject to an impairment test performed on an annual basis, or more frequently if events or changes in circumstances indicate that goodwill may be impaired. The Company evaluates goodwill on an annual basis on August 31 of each fiscal year. The test for goodwill impairment is a two-step process. The first step compares the fair value of each reporting unit with its respective carrying amount, including goodwill. The Company has determined that it operates in one reporting unit and estimates the fair value of its one reporting unit using the enterprise approach under which it considers the quoted market capitalization of the Company as reported on the Nasdaq Global Market. The Company considers quoted market prices that are available in active markets to be the best evidence of fair value. The Company also considers other factors, which include future forecasted results, the economic environment and overall market conditions. If the fair value of the reporting unit exceeds its carrying amount, goodwill of the reporting unit is not considered impaired and, therefore, the second step of the impairment test is unnecessary. The second step, which is used to measure the amount of impairment loss, compares the implied fair value of each reporting unit’s goodwill with the respective carrying amount of that goodwill. If the carrying amount of the reporting unit’s goodwill exceeds the implied fair value of that goodwill, an impairment loss is recognized in an amount equal to that excess.
See Note 8 in the Notes to Consolidated Financial Statements for further information regarding the Company’s impairment analysis and the valuation of goodwill and intangible assets, net.
Long-lived Assets
The Company evaluates its long-lived assets for impairment by continually monitoring events and changes in circumstances that could indicate carrying amounts of its long-lived assets may not be recoverable. When such events or changes in circumstances occur, the Company assesses recoverability by determining whether the carrying value of such assets will be recovered through the undiscounted expected future cash flows. If the expected undiscounted future cash flows are less than the carrying amount of these assets, the Company then measures the amount of the impairment loss based on the excess of the carrying amount over the fair value of the assets. The Company did not recognize impairment charges related to its long-lived assets during the years ended December 31, 2011, 2010 and 2009.
69
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Foreign Currency Remeasurement
The functional currency of the Company’s foreign subsidiary is the United States dollar. Monetary assets and liabilities denominated in foreign currencies are remeasured in United States dollars using the exchange rates at the balance sheet date. Non-monetary assets and liabilities denominated in foreign currencies are remeasured in United States dollars using historical exchange rates. Revenues and expenses are remeasured using average exchange rates prevailing during the period. Remeasurements are recorded in the Company’s consolidated statements of operations. The Company recorded foreign currency losses of $0.5 million, $0.8 million and $0.6 million during the years ended December 31, 2011, 2010 and 2009, respectively.
Stock-Based Compensation
The Company accounts for stock-based compensation in accordance with ASC Topic 718, “Compensation —Stock Compensation.” Stock-based compensation expense is measured at the grant-date based on the fair value of the award and is recognized as expense on a straight-line basis over the requisite service period, which is the vesting period, and is adjusted for estimated forfeitures. To the extent that stock options contain performance criteria for vesting, stock-based compensation is recognized once the performance criteria are probable of being achieved.
For stock-based awards issued to non-employees, the Company follows ASC Topic 505-50, “Equity Based Payment to Non-Employees” and considers the measurement date at which the fair value of the stock-based award is measured to be the earlier of (i) the date at which a commitment for performance by the grantee to earn the equity instrument is reached or (ii) the date at which the grantee performance is complete. The Company recognizes stock-based compensation expense for the fair value of the vested portion of the non-employee stock-based awards in its consolidated statements of operations.
See Note 15 in the Notes to Consolidated Financial Statements for further information regarding the Company’s stock-based compensation assumptions and expenses.
Warrant Liability
In August 2009 and November 2010, the Company issued warrants to purchase an aggregate of 2.4 million and 3.7 million shares of common stock, respectively. The material terms of the warrants were identical under each issuance except for the exercise price, date issued and expiration date. The Company classified the warrants as a liability on its consolidated balance sheets as the warrants contain certain material terms which require the Company (or its successor) to purchase the warrants for cash in an amount equal to the value of the unexercised portion of the warrants (as determined in accordance with the Black-Scholes option pricing model) in connection with certain change of control transactions. In addition, the Company may also be required to pay cash to a warrant holder under certain circumstances if the Company is unable to timely deliver the shares acquired upon warrant exercise to such holder.
The fair value of these outstanding warrants is calculated using the binomial-lattice option-pricing model and is adjusted accordingly at each reporting period. The binomial-lattice option-pricing model requires that the Company uses significant assumptions and judgment to determine appropriate inputs to the model. Some of the assumptions that the Company relies on include the probability of a change of control occurring, the volatility of the Company’s stock over the life of the warrant and assumptions and inputs used to value the warrants under the Black-Scholes model should a change of control occur.
Gains and losses from warrant revaluation are recorded in “Gain from revaluation of warrant liability” on the consolidated statements of operations. During the year ended December 31, 2011, the Company recorded
70
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
non-cash gains of $0.5 million associated with changes in the fair value of the warrants, and recorded non-cash gains of less than $0.1 million during each of the years ended December 31, 2010 and 2009. Upon the exercise or modification to remove the provisions which require the warrants to be treated as a liability, the fair value of the warrants will be reclassified from a liability to stockholders’ equity on the Company’s consolidated balance sheets and no further adjustment to the fair value would be made in subsequent periods.
See Note 14 in the Notes to Consolidated Financial Statements for further information regarding the Company’s valuation of warrant liability.
Other Comprehensive Income (Loss)
The components of comprehensive loss included net loss and other comprehensive income (loss). The Company’s only component of other comprehensive income (loss) for the years ended December 31, 2011, 2010 and 2009 consisted of unrealized gains or losses from the Company’s available-for-sale short-term investments. Other comprehensive income (loss) is reported as a separate component of stockholders’ equity.
Income Taxes
The Company accounts for income taxes using an asset and liability approach in accordance with ASC Topic 740 “Accounting for Income Taxes.” Under this method, deferred tax assets and liabilities are determined based on differences between the financial reporting and tax bases of assets and liabilities and are measured using the enacted tax rates and laws that will be in effect when the differences are expected to reverse. ASC Topic 740 requires derecognition of tax positions that do not have a greater than 50% likelihood of being recognized upon review by a taxing authority having full knowledge of all relevant information. Use of a valuation allowance as described in ASC Topic 740 is not an appropriate substitute for the derecognition of a tax position. The Company did not have any recorded liabilities for unrecognized tax benefits at both December 31, 2011 and 2010. The Company recognizes accrued interest and penalties related to unrecognized tax benefits in its income tax expense. To date, the Company has not recognized any interest and penalties in its consolidated statements of operations, nor has its accrued for or made payments for interest and penalties. The Company continues to carry a full valuation allowance on all of its deferred tax assets. Although the Company believes it more likely than not that a taxing authority would agree with its current tax positions, there can be no assurance that the tax positions the Company has taken will be substantiated by a taxing authority if reviewed. The Company’s tax years 2007 through 2011 remain subject to examination by the taxing jurisdictions.
Net Loss Per Share
Basic loss per share is computed by dividing net loss by the weighted average number of common shares outstanding for the period. Diluted loss per share uses the same weighted average number of common shares outstanding for the period as calculated for the basic loss per share as the inclusion of any commons stock equivalents would be anti-dilutive. If the Company earned net income, diluted earnings per share would assume conversion of all potentially dilutive securities, such as stock options, convertible preferred stock, ESPP rights, warrants and restricted stock units.
71
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
The following table sets forth the reconciliation of the numerator and denominator used in the computation of basic and diluted net loss per common share for the years ended December 31, 2011, 2010 and 2009 (in thousands, except per share amounts):
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Numerator for Basic and Diluted: |
||||||||||||
| Net loss |
$ | (16,982 | ) | $ | (16,911 | ) | $ | (24,135 | ) | |||
| Denominator: |
||||||||||||
| Basic weighted average number of common shares outstanding |
48,050 | 40,300 | 34,750 | |||||||||
| Effect of dilutive potential common shares resulting from convertible preferred stock, stock options, restricted stock units, warrants and ESPP rights |
0 | 0 | 0 | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted weighted average number of common shares outstanding |
48,050 | 40,300 | 34,750 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss per common share: |
||||||||||||
| Basic |
$ | (0.35 | ) | $ | (0.42 | ) | $ | (0.69 | ) | |||
| Diluted |
$ | (0.35 | ) | $ | (0.42 | ) | $ | (0.69 | ) | |||
The table below presents common shares underlying stock options, convertible preferred stock, ESPP rights, warrants and restricted stock units that are excluded from the diluted net loss per common share due to their anti-dilutive effect for the years ended December 31, 2011, 2010 and 2009 (shares in thousands):
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Weighted average of anti-dilutive common shares |
13,595 | 9,867 | 7,662 | |||||||||
Guarantee and Indemnification Arrangements
The Company recognizes the fair value for guarantee and indemnification arrangements issued or modified by the Company after December 31, 2002. In addition, the Company monitors the conditions that are subject to the guarantees and indemnifications in order to identify if a loss has occurred. If the Company determines it is probable that a loss has occurred, then any such estimable loss would be recognized under those guarantees and indemnifications. Some of the agreements that the Company is a party to contain provisions that indemnify the counter party from damages and costs resulting from claims that the Company’s technology infringes the intellectual property rights of a third party or claims that the sale or use of the Company’s products have caused personal injury or other damage or loss. The Company has not received any such requests for indemnification under these provisions and has not been required to make material payments pursuant to these provisions.
The Company generally provides for a one-year warranty on certain of its INTERCEPT blood-safety products covering defects in materials and workmanship. The Company accrues costs associated with warranty obligations when claims become known and are estimable. There have been very few warranty costs incurred through December 31, 2011, and the Company is unaware of any future warranty claims. Accordingly, at December 31, 2011 and 2010, the Company had not accrued for any potential future warranty costs.
Fair Value of Financial Instruments
The Company applies the provisions of fair value relating to its financial assets and liabilities. The carrying amounts of accounts receivables, accounts payable, and other accrued liabilities approximate their fair value due
72
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
to the relative short-term maturities. Based on the borrowing rates currently available to the Company for loans with similar terms, the Company believes the fair value of its debt approximates their carrying amounts. The Company measures and records certain financial assets and liabilities at fair value on a recurring basis, including its available-for-sale securities and warrant liability. The Company classifies instruments within Level 1 if quote prices are available in active markets, which include its money market funds as the maturity of money market funds are relatively short and the carrying amount is a reasonable estimate of fair value. The Company classifies instruments in Level 2 if the instruments are valued using observable inputs to quoted market prices, benchmark yields, reported trades, broker/dealer quotes or alternative pricing sources with reasonable levels of price transparency. These instruments include the Company’s available-for-sale securities related to United States government agencies and corporate debt securities. The available-for-sale securities are held by a custodian who obtains investment prices from a third party pricing provider that uses standard inputs to models which vary by asset class. The Company classifies instruments in Level 3 if one or more significant inputs or significant value drivers are unobservable, which include its warrant liability. The Company assesses any transfers among fair value measurement levels at the end of each reporting period.
See Note 4 and 14 in the Notes to Consolidated Financial Statements for further information regarding the Company’s valuation on financial instruments.
New Accounting Pronouncements
In May 2011, the FASB issued updated fair value measurement guidance under ASU No. 2011-04 “Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs,” surrounding changes in the valuation premise of highest and best use of an asset, the application of premiums and discounts, and enhanced disclosure requirements. Under ASU No. 2011-04, the measurement of fair value of financial instruments will primarily be measured at the level of the unit of account whereas it was historically able to utilize the valuation premise of highest and best use of an asset, which can be applied primarily to measuring the fair value of nonfinancial assets only going forward. In addition, the application of blockage factors and other premiums and discounts in a fair value measurement will be prohibited in the valuation of all fair value levels of the hierarchy. The new disclosure requirements include, but are not limited to, further qualitative and quantitative discussions regarding level 3 fair value measurements, specifically significant unobservable inputs used, description of the valuation processes and sensitivity analysis, the disclosure of any transfers and the reasons thereof between levels 1 and 2, and the determination of assets and liabilities that are not recorded at fair value to be categorized under the fair value hierarchy. The updated fair value measurement guidance is effective for interim and annual periods beginning after December 15, 2011, which will begin for the Company on January 1, 2012. The Company does not anticipate that the additional disclosure requirements will have a material impact on the consolidated financial statements.
In June 2011, the FASB issued ASU No. 2011-05 “Presentation of Comprehensive Income,” which eliminates the presentation of other comprehensive income from the consolidated statements of stockholders’ equity. Instead, companies would have the option to display net income and other comprehensive income in two separate, but consecutive statements or combine net income and other comprehensive income in one continuous statement, which would be referred to the consolidated statements of comprehensive income. The new presentation requirements under this guidance are effective for interim and annual periods beginning after December 15, 2011, which will begin for the Company on January 1, 2012, and retrospective application is required for all periods presented. In December 2011, the FASB issued ASU No. 2011-12 “Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05,” which defers only the presentation of
73
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
reclassification adjustments from accumulated other comprehensive income to net income. The reclassification adjustment presentation requirement under ASU No. 2011-12 is effective for interim and annual periods beginning after December 15, 2012, which will begin for the Company on January 1, 2013. The Company does not anticipate that the additional disclosure requirements under both ASU Nos. 2011-05 and 2011-12 will have a material impact on the consolidated financial statements. The Company expects to adopt the presentation for net income and other comprehensive income in two separate, but consecutive statements.
In September 2011, the FASB issued ASU No. 2011-08 “Testing Goodwill for Impairment,” which allows a company to test goodwill for impairment by first assessing the qualitative factors, which has also been updated under this guidance, to determine whether it is more likely than not that the fair value of a reporting unit is less than the carrying amount. If a company determines that it is more likely than not that the fair value of a reporting unit is less than the carrying amount, a company must then proceed with performing the quantitative two-step process to test goodwill for impairment; otherwise, goodwill is not considered impaired and no further testing is warranted. Under ASU No. 2011-08, the qualitative assessment is optional, such that a company has the choice to perform the goodwill impairment test under the original quantitative two-step approach only. The optional qualitative procedure for testing goodwill impairment under this guidance is effective for interim and annual periods beginning after December 15, 2011, which will begin for the Company on January 1, 2012; however, early adoption is permitted. The Company does not anticipate that the update to this accounting standard will have a material impact on the consolidated financial statements.
Note 3. Acquisition
On August 24, 2010, the Company acquired certain assets of BioOne, a privately held Japanese company established to develop technologies to improve the safety of blood products in Asia. The assets included the commercialization licenses that the Company had granted to BioOne for both the platelet and plasma systems, illuminators held as saleable inventory and demonstration illuminators. No liabilities were assumed.
As consideration for the acquired BioOne assets, the Company relinquished all shares of BioOne that had been held by the Company and issued 1,172,357 shares of the Company’s common stock to BioOne, of which 937,886 shares were issued at the close of the acquisition on August 24, 2010 and the remaining 234,471 shares were issued six months from the close of the acquisition date (February 25, 2011). The fair value of the Company’s common stock issued to BioOne on both dates was measured based on the closing price of the Company’s common stock on August 24, 2010, the date of acquisition, and was recorded as part of the total consideration.
The total value of the consideration provided was $3.7 million, of which approximately $3.4 million related to the fair value of the 1,172,357 shares of the Company’s common stock issued to BioOne and approximately $0.3 million related to the fair value of the Company’s non-controlling equity interest in BioOne relinquished as a result of the acquisition. The Company recognized a gain of $0.3 million, which represented the difference between the assumed fair value of the pre-acquisition non-controlling equity interest of BioOne and its carrying value. The Company carried its 13% investment in BioOne at zero as it had previously fully impaired its investment in BioOne. The assumed fair value of the pre-acquisition non-controlling equity interest was calculated by applying the Company’s 13% ownership investment in BioOne to the estimated fair value of the acquired assets (excluding goodwill) of $2.4 million as noted in the table below.
The Company also incurred acquisition related costs of $0.5 million, which were recorded as a component in “Acquisition related costs and impairment of long-term investment in related parties, net” on the consolidated statements of operations during the year ended December 31, 2010.
74
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
The BioOne acquisition was accounted for as an acquisition of a business in accordance with ASC Topic 805, “Business Combinations.” The Company allocated the acquired tangible and intangible assets based on their estimated fair values as of the acquisition date. The excess purchase price over the value of the net tangible and identifiable intangible assets was recorded as goodwill. The goodwill recognized is not expected to be deductible for income tax purposes. The factors that contributed to the recognition of goodwill included securing buyer-specific synergies to increase revenue and profits through the commercialization of the INTERCEPT Blood System worldwide. By acquiring these commercialization rights in certain Asian countries, the Company was able to complete the global commercialization rights for its platelet and plasma systems.
The following table summarizes the final allocation of fair value of assets acquired at the acquisition date (in thousands):
| Commercialization rights—Asia |
$ | 2,017 | ||
| Illuminators—inventory |
270 | |||
| Demonstration illuminators |
135 | |||
| Goodwill |
1,316 | |||
|
|
|
|||
| Total |
$ | 3,738 | ||
|
|
|
The commercialization rights in Asia represent the reacquisition of contractual rights originally granted to BioOne to market the Company’s products in certain countries in Asia. The contractual term of this original agreement was perpetual and the Company estimated the fair value of these acquired rights based on future expected cash flows to be generated over the expected life of the underlying technology. As a result, these intangible assets are subject to periodic amortization over the estimated useful life of ten years. The estimated fair value of inventory illuminators and demonstration illuminators was based on the expected sales price of the inventory, less reasonable profit margins.
The Company’s operating results included the impact of the BioOne acquisition beginning from the acquisition date. The pro forma disclosures for historical periods have not been presented as the impact of the BioOne acquisition was not significant to the results of operations of the Company since BioOne did not have any significant revenues or expenses due to their limited operating activities as a result of a deteriorating financial situation.
Note 4. Fair Value on Financial Instruments
The fair values of certain of the Company’s financial assets and liabilities were determined using the following inputs at December 31, 2011 (in thousands):
| Total | Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||||||
| Money market funds(1) |
$ | 8,683 | $ | 8,683 | $ | 0 | $ | 0 | ||||||||
| United States government agency securities(2) |
287 | 0 | 287 | 0 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total financial assets |
$ | 8,970 | $ | 8,683 | $ | 287 | $ | 0 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Warrant liability(3) |
$ | 7,979 | $ | 0 | $ | 0 | $ | 7,979 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total financial liabilities |
$ | 7,979 | $ | 0 | $ | 0 | $ | 7,979 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Included in cash and cash equivalents on the Company’s consolidated balance sheets. |
75
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
| (2) | Included in short-term investments on the Company’s consolidated balance sheets. |
| (3) | Included in current liabilities on the Company’s consolidated balance sheets. |
The fair values of certain of the Company’s financial assets and liabilities were determined using the following inputs at December 31, 2010 (in thousands):
| Total | Quoted Prices in Active Markets for Identical Assets (Level 1) |
Significant Other Observable Inputs (Level 2) |
Significant Unobservable Inputs (Level 3) |
|||||||||||||
| Money market funds(1) |
$ | 6,178 | $ | 6,178 | $ | 0 | $ | 0 | ||||||||
| Corporate debt securities(2) |
73 | 0 | 73 | 0 | ||||||||||||
| United States government agency securities(2) |
988 | 0 | 988 | 0 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total financial assets |
$ | 7,239 | $ | 6,178 | $ | 1,061 | $ | 0 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Warrant liability(3) |
$ | 8,465 | $ | 0 | $ | 0 | $ | 8,465 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total financial liabilities |
$ | 8,465 | $ | 0 | $ | 0 | $ | 8,465 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Included in cash and cash equivalents on the Company’s consolidated balance sheets. |
| (2) | Included in short-term investments on the Company’s consolidated balance sheets. |
| (3) | Included in current liabilities on the Company’s consolidated balance sheets. |
A reconciliation of the beginning and ending balances for warrant liability using significant unobservable inputs (Level 3) from December 31, 2009 to December 31, 2011 was as follows (in thousands):
| Balance at December 31, 2009 |
$ | 2,737 | ||
| Issuance of warrants |
5,767 | |||
| Decrease in fair value of warrants |
(39 | ) | ||
|
|
|
|||
| Balance at December 31, 2010 |
8,465 | |||
| Issuance of warrants |
0 | |||
| Decrease in fair value of warrants |
(486 | ) | ||
|
|
|
|||
| Balance at December 31, 2011 |
$ | 7,979 | ||
|
|
|
The Company did not have any transfers among fair value measurement levels during the years ended December 31, 2011 and 2010.
76
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Note 5. Cash, Cash Equivalents and Short-Term Investments
The following is a summary of cash, cash equivalents and short-term investments at December 31, 2011 (in thousands):
| December 31, 2011 | ||||||||||||
| Carrying Value | Gross Unrealized Gain |
Fair Value | ||||||||||
| Cash and cash equivalents: |
||||||||||||
| Cash |
$ | 16,814 | $ | 0 | $ | 16,814 | ||||||
| Money market funds |
8,683 | 0 | 8,683 | |||||||||
|
|
|
|
|
|
|
|||||||
| 25,497 | 0 | 25,497 | ||||||||||
| Short-term investments: |
||||||||||||
| United States government agency securities |
287 | 0 | 287 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total short-term investments |
287 | 0 | 287 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cash, cash equivalents and short-term investments |
$ | 25,784 | $ | 0 | $ | 25,784 | ||||||
|
|
|
|
|
|
|
|||||||
The following is a summary of cash, cash equivalents and short-term investments at December 31, 2010 (in thousands):
| December 31, 2010 | ||||||||||||
| Carrying Value | Gross Unrealized Gain |
Fair Value | ||||||||||
| Cash and cash equivalents: |
||||||||||||
| Cash |
$ | 22,770 | $ | 0 | $ | 22,770 | ||||||
| Money market funds |
6,178 | 0 | 6,178 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cash and cash equivalents |
28,948 | 0 | 28,948 | |||||||||
| Short-term investments: |
||||||||||||
| Corporate debt securities |
14 | 59 | 73 | |||||||||
| United States government agency securities |
939 | 49 | 988 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total short-term investments |
953 | 108 | 1,061 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total cash, cash equivalents and short-term investments |
$ | 29,901 | $ | 108 | $ | 30,009 | ||||||
|
|
|
|
|
|
|
|||||||
Cash equivalents and short-term investments at December 31, 2011 and 2010 consisted of the following by original contractual maturity (in thousands):
| December 31, 2011 | December 31, 2010 | |||||||||||||||
| Carrying Value |
Fair Value | Carrying Value |
Fair Value | |||||||||||||
| Due in one year or less |
$ | 8,683 | $ | 8,683 | $ | 6,178 | $ | 6,178 | ||||||||
| Due greater than three years and less than five years |
287 | 287 | 953 | 1,061 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total cash equivalents and short-term investments |
$ | 8,970 | $ | 8,970 | $ | 7,131 | $ | 7,239 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
77
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
The maturities of certain short-term investments were estimated primarily based upon assumed prepayment features and credit enhancement characteristics.
Gross realized gains from the sale or maturity of available-for-sale investments were minimal, $0 and $0.2 million during the years ended December 30, 2011, 2010 and 2009, respectively. The Company recorded minimal gross realized losses from the sale or maturity of available-for-sale investments during the year ended December 31, 2010 and did not record any gross realized losses during the years ended December 31, 2011 and 2009. The Company did not record losses on investments experiencing an other-than-temporary decline in fair value during the years ended December 31, 2011, 2010 and 2009.
Note 6. Inventories
Inventories at December 31, 2011 and 2010 consisted of the following (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Work-in-process |
$ | 2,742 | $ | 2,652 | ||||
| Finished goods |
3,702 | 3,305 | ||||||
|
|
|
|
|
|||||
| Total inventories |
$ | 6,444 | $ | 5,957 | ||||
|
|
|
|
|
|||||
Note 7. Property and Equipment, net
Property and equipment, net at December 31, 2011 and 2010 consisted of the following (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Leasehold improvements |
$ | 5,598 | $ | 5,470 | ||||
| Machinery and equipment |
1,682 | 1,652 | ||||||
| Demonstration equipment |
24 | 104 | ||||||
| Office furniture |
636 | 583 | ||||||
| Computer equipment |
525 | 488 | ||||||
| Computer software |
1,062 | 1,062 | ||||||
| Consigned demonstration equipment |
502 | 401 | ||||||
| Construction-in-progress |
38 | 134 | ||||||
|
|
|
|
|
|||||
| Total property and equipment, gross |
10,067 | 9,894 | ||||||
| Accumulated depreciation and amortization |
(8,035 | ) | (7,504 | ) | ||||
|
|
|
|
|
|||||
| Total property and equipment, net |
$ | 2,032 | $ | 2,390 | ||||
|
|
|
|
|
|||||
Depreciation and amortization expense related to property and equipment, net was $0.6 million, $0.6 million and $0.7 million for the years ended December 31, 2011, 2010 and 2009, respectively.
Note 8. Goodwill and Intangible Assets, net
Goodwill
During the year ended December 31, 2011, the Company did not dispose of or recognize additional goodwill. On August 31, 2011, the Company performed its annual review of goodwill. As described in Note 2
78
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
above, the Company applied the enterprise approach by reviewing the quoted market capitalization of the Company as reported on the Nasdaq Global Market to calculate the fair value. In addition, the Company considered its future forecasted results, the economic environment and overall market conditions. As a result of the Company’s assessment that its fair value of the reporting unit exceeded its carrying amount, the Company determined that goodwill was not impaired. Accordingly, at both December 31, 2011 and 2010, the carrying amount of goodwill was $1.3 million.
Intangible Assets, net
The following is a summary of intangible assets, net at December 31, 2011 (in thousands):
| December 31, 2011 | ||||||||||||
| Gross
Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||
| Acquisition-related intangible assets: |
||||||||||||
| License—INTERCEPT Asia |
$ | 2,017 | $ | (269 | ) | $ | 1,748 | |||||
|
|
|
|
|
|
|
|||||||
| Total intangible assets |
$ | 2,017 | $ | (269 | ) | $ | 1,748 | |||||
|
|
|
|
|
|
|
|||||||
The following is a summary of intangible assets, net at December 31, 2010 (in thousands):
| December 31, 2010 | ||||||||||||
| Gross
Carrying Amount |
Accumulated Amortization |
Net Carrying Amount |
||||||||||
| Acquisition-related intangible assets: |
||||||||||||
| License—INTERCEPT Asia |
$ | 2,017 | $ | (67 | ) | $ | 1,950 | |||||
|
|
|
|
|
|
|
|||||||
| Total intangible assets |
$ | 2,017 | $ | (67 | ) | $ | 1,950 | |||||
|
|
|
|
|
|
|
|||||||
The Company recognized $0.2 million and $0.1 million in amortization expense related to intangible assets for the years ended December 31, 2011 and 2010, respectively. During the years ended December 31, 2011 and 2010, there were no impairment charges recognized related to the acquired intangible assets.
At December 31, 2011, the expected annual amortization expense of the intangible assets, net is $0.2 million beginning with the year ending December 31, 2012 and each subsequent year thereafter through the year ending December 31, 2019, and $0.1 million for the year ending December 31, 2020.
Note 9. Long-Term Investments
At December 31, 2009, the Company held a 13% equity interest in the voting securities of BioOne, which was accounted for under the cost method. At December 31, 2009, the Company evaluated several criteria to determine whether facts and circumstances supported the carrying value of its investment in BioOne. These criteria included, but were not limited to, third-party investor interest and participation in recent equity offerings at current pricing, business outlook of BioOne and available financial information. Based on its evaluation of these criteria, the Company determined that there were no factors to support any carrying value of its investment in BioOne. As a result, during the year ended December 31, 2009, the Company completely impaired its investment in BioOne to zero and as such, recorded an impairment charge of $2.3 million as a component in “Acquisition related costs and impairment of long-term investments in related parties, net” on the consolidated
79
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
statements of operations. In connection with the BioOne acquisition in August 2010, the Company relinquished all BioOne shares that the Company held as part of the consideration for certain of these assets and recognized a gain of $0.3 million during the year ended December 31, 2010, which represented the difference between the assumed fair value of the pre-acquisition non-controlling equity interest of BioOne and the carrying value. The Company also incurred acquisition related costs of $0.5 million during the year ended December 31, 2010.
See Note 3 in the Notes to Consolidated Financial Statements for further information regarding the Company’s acquisition and valuation of BioOne.
During the year ended December 31, 2009, the Company also recognized a gain of $0.8 million, which represented the difference between the cash received and the carrying value of its non-controlling equity interest in Anza Therapeutics, Inc. (“Anza Therapeutics”), as the Company relinquished its shares in Anza Therapeutics, released any claims against them and agreed to the transfer of all of Anza Therapeutics’ intellectual property to Aduro BioTech (“Aduro”) in 2009. In addition, in connection with the agreements to license the immunotherapy technologies to Aduro in 2009, the Company received and held preferred shares representing less than 10% of Aduro’s capital. Pursuant to these license agreements, the Company will obtain a 1% royalty fee on any future sales resulting from certain technology. In April 2011, Aduro completed a subsequent round of financing, issuing Series B preferred stock and as a result, reduced its ownership in Aduro to less than 3%. Since receiving preferred stock in Aduro, the Company has carried its investment in Aduro at zero on its consolidated balance sheet as the Company has no basis to believe that it will receive any economic benefit from its equity ownership in Aduro as the Company believed that Aduro’s technology platforms, which were largely based on the in-process development programs of Anza Therapeutics, had a high risk of failure.
Note 10. Accrued Liabilities
Accrued liabilities at December 31, 2011 and 2010 consisted of the following (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Accrued compensation and related |
$ | 2,027 | $ | 1,861 | ||||
| Accrued inventory |
1,417 | 1,424 | ||||||
| Accrued contract expenses and other accrued expenses |
2,381 | 2,718 | ||||||
|
|
|
|
|
|||||
| Total accrued liabilities |
$ | 5,825 | $ | 6,003 | ||||
|
|
|
|
|
|||||
Note 11. Restructuring
In March 2009, pursuant to the approval by the Board of Directors, the Company implemented a restructuring plan to reduce its cost structure by reducing its workforce, focusing its resources on the commercialization of the INTERCEPT Blood System in Europe and consolidating its facilities, resulting in restructuring costs of $0.8 million, which were recorded in “Restructuring charges” on the Company’s consolidated statements of operations during the year ended December 31, 2009. Employees whose positions were impacted by this restructuring plan received one-time termination benefits, which included severance consideration, continuation of benefits, and transition assistance. Costs associated with the consolidation of its facilities included related moving costs. All restructuring costs were paid by December 31, 2010.
80
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Note 12. Debt
Debt at December 31, 2011 consisted of the following (in thousands):
| December 31, 2011 | ||||||||||||
| Principal | Unamortized Discount |
Total | ||||||||||
| Comerica—Growth Capital Loan A, due 2015 |
$ | 5,000 | $ | (84 | ) | $ | 4,916 | |||||
| Comerica—Revolving Line of Credit, due 2013 |
2,300 | 0 | 2,300 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total debt |
7,300 | (84 | ) | 7,216 | ||||||||
| Less: debt—current |
(2,554 | ) | 35 | (2,519 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Debt—non-current |
$ | 4,746 | $ | (49 | ) | $ | 4,697 | |||||
|
|
|
|
|
|
|
|||||||
Debt at December 31, 2010 consisted of the following (in thousands):
| December 31, 2010 | ||||||||||||
| Principal | Unamortized Discount |
Total | ||||||||||
| Oxford—Loan, due 2013 |
$ | 5,000 | $ | (122 | ) | $ | 4,878 | |||||
|
|
|
|
|
|
|
|||||||
| Total debt |
5,000 | (122 | ) | 4,878 | ||||||||
| Less: debt—current |
(1,822 | ) | 75 | (1,747 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Debt—non-current |
$ | 3,178 | $ | (47 | ) | $ | 3,131 | |||||
|
|
|
|
|
|
|
|||||||
Principal and interest payments on debt at December 31, 2011 are expected to be as follows for each of the following five years (in thousands):
| Year ended December 31, |
||||
| 2012 |
$ | 677 | ||
| 2013 |
4,226 | |||
| 2014 |
1,835 | |||
| 2015 |
1,580 | |||
| 2016 |
0 | |||
2011 Growth Capital Facility
In September 2011, the Company entered into a loan and security agreement (the “Credit Agreement”) with Comerica Bank (“Comerica”). The Credit Agreement provides for a growth capital loan of up to $8.0 million (“Growth Capital Loan”) and a formula based revolving line of credit (“RLOC”) of up to $4.0 million plus any unused amounts from the Growth Capital Loan. Under the Credit Agreement, the Company is limited to an aggregate borrowing of up to $10.0 million at any time. The Company pledged all current and future assets, excluding its intellectual property and 35% of the Company’s investment in its subsidiary, Cerus Europe B.V., as security for borrowings under the Credit Agreement.
Growth Capital Loan
Concurrent with the execution of the Credit Agreement, in September 2011, the Company borrowed $5.0 million of the Growth Capital Loan (“Growth Capital Loan A”), substantially all of which was used to repay the
81
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Company’s prior debt with Oxford Finance Corporation (“Oxford”), as discussed in further detail below, with the remainder used for general corporate purposes. Growth Capital Loan A, which matures in 48 months, bears a fixed interest rate of 6.37%, with interest–only payments due for the first twelve months, followed by equal principal and interest payments for the remaining 36 months.
The Company may draw up to an additional $3.0 million of the Growth Capital Loan (“Growth Capital Loan B”) between December 31, 2011 and June 30, 2012. Growth Capital Loan B will bear a fixed interest rate based on the higher of (i) 6.25% or (ii) 6.00% plus the three month LIBOR rate at the date of draw, with interest—only payments due for the first six months followed by equal principal and interest payments for the remaining 36 months. As of December 31, 2011, the Company had not drawn down any amounts under the Growth Capital Loan B.
In September 2011, the Company incurred a commitment fee of $40,000 and loan fees of $50,000, which were recorded as a discount to its Growth Capital Loan A and are being amortized as a component of interest expense using the effective interest method over the term of the Growth Capital Loan A (discount was based on an implied interest rate of 7.07%). The Company will also be required to make a final payment fee of 1% of the amounts drawn under Growth Capital Loan A and Growth Capital Loan B due on the earlier of (i) prepayment of the Growth Capital Loan or (ii) the maturity of the Growth Capital Loan. The final payment fee will be accreted to interest expense using the effective interest method over the life of the Growth Capital Loan A and B upon draw.
Revolving Line of Credit
The Credit Agreement also provides for a RLOC of up to $4.0 million plus any unused amounts from Growth Capital Loan B (“RLOC Loan Amount”). The amount available under the RLOC, which is available to the Company until September 30, 2013, is limited to the lesser of (i) 80% of eligible trade receivables or (ii) the RLOC Loan Amount. In December 2011, the Company drew down $2.3 million under the RLOC. The RLOC will bear a floating rate based on the lender’s prime rate plus 1.50%, with interest—only payments due each month. At December 31, 2011, the floating rate of the RLOC was at 4.75%. The Company will be required to repay the principal drawn from the RLOC at the end of the RLOC term. In September 2011, the Company incurred a commitment fee of $20,000, and will pay the same commitment fee at each annual anniversary of the RLOC. As of December 31, 2011, the Company had $2.3 million outstanding under the RLOC.
Compliance with Covenants
The Company is required to maintain compliance with certain customary and routine financial covenants under the Credit Agreement. Throughout the term of the Credit Agreement, the Company is also obligated to meet certain conditions which include maintaining a minimum cash balance of $2.5 million at Comerica and achieving minimum revenue levels, which are measured monthly based on a six-month trailing basis and must be at least 75% of the pre-established future projected revenues for the trailing six-month period. Non-compliance with the covenants could result in the principal of the note becoming due and payable. As of December 31, 2011, the Company was in compliance with the financial covenants as set forth in the Credit Agreement.
2010 Growth Capital Facility
In March 2010, the Company entered into a growth capital facility agreement with Oxford and immediately borrowed and issued a senior secured note for $5.0 million. The note carried a fixed interest rate of 12.04%, with
82
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
interest–only payments due for the first nine months and then equal principal and interest payments for an additional 30 months. In connection with the issuance of the note, the Company paid an upfront facility fee of $0.1 million and incurred closing costs of $0.1 million, which was recorded as a discount to the note and was amortized as a component of interest expense using the effective interest method over the term of the note (discount was based on an implied interest rate of 13.84%). In addition, the Company agreed to pay a $0.4 million closing fee upon maturity of the note, which was being accreted to interest expense using the effective interest method over the life of the note. For the year ended December 31, 2010, the Company also incurred a non-utilization fee of $0.1 million, which was recognized as an operating expense, as the Company had not drawn down on the additional $5.0 million available to be drawn between September 30, 2010 and December 31, 2010. The Company was required to maintain compliance with certain customary and routine financial covenants, which included generating minimum revenues at certain pre-established levels. At December 31, 2010, the Company was in compliance with financial covenants set forth in the growth capital facility.
In March 2011, the Company amended its growth capital facility with Oxford, which extended the availability of borrowing an additional $5.0 million through September 30, 2011 without incurring additional upfront facility fees and modified the covenant compliance requirements. In September 2011, the Company repaid the outstanding balance of the debt owed to Oxford using the proceeds received from the Credit Agreement as discussed in further detail above. The Company also accelerated and expensed the remaining closing cost and fees of $0.2 million to interest expense during the year ended December 31, 2011.
Note 13. Commitments and Contingencies
Operating Leases
The Company leases its office facilities, located in Concord, California and Amersfoort, The Netherlands, and certain equipment under non-cancelable operating leases with initial terms in excess of one year that require the Company to pay operating costs, property taxes, insurance and maintenance. The operating leases expire at various dates through 2019, with certain of the leases providing for renewal options, provisions for adjusting future lease payments, which is based on the consumer price index and the right to terminate the lease early, which may occur as early as January 2015. The Company’s leased facilities qualify as operating leases under ASC Topic 840, “Leases” and as such, are not included on its consolidated balance sheets.
Future minimum non-cancelable lease payments under operating leases as of December 31, 2011 are as follows (in thousands):
| Year ended December 31, |
||||
| 2012 |
$ | 706 | ||
| 2013 |
448 | |||
| 2014 |
421 | |||
|
|
|
|||
| Total minimum non-cancellable lease payments |
$ | 1,575 | ||
|
|
|
|||
Rent expense for office facilities was $0.7 million, $0.9 million and $1.4 million for the years ended December 31, 2011, 2010 and 2009, respectively.
Financed Leasehold Improvements
At December 31, 2010, the Company financed $1.1 million of leasehold improvements. The Company pays for the financed leasehold improvements as a component of rent and is required to reimburse its landlord over the
83
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
remaining life of the respective leases. If the Company exercises its right to early terminate the Concord California lease, which may occur as early as January 2015, the Company would be required to repay for any remaining portion of the landlord financed leasehold improvements at such time. At December 31, 2011, the Company had an outstanding liability of $0.9 million related to these leasehold improvements, of which $0.1 million was reflected in “Accrued liabilities” and $0.8 million was reflected in” ‘Other non-current liabilities” on the Company’s consolidated balance sheets.
Purchase Commitments
The Company is party to agreements with certain providers for certain components of INTERCEPT Blood System which the Company purchases from third party manufacturers and supplies to Fenwal at no cost for use in manufacturing finished disposable kits. Certain of these agreements require minimum purchase commitments from the Company. The Company has paid $3.6 million, $0.9 million and $1.2 million for goods under agreements which are subject to minimum purchase commitments during the years ended December 31, 2011, 2010 and 2009, respectively. As of December 31, 2011, the Company has future minimum purchase commitments under these agreements of $3.5 million for the year ending December 31, 2012 and less than $0.1 million for each subsequent year thereafter through December 31, 2015.
Note 14. Stockholders’ Equity
Series B Preferred Stock
Fenwal holds 3,327 shares of the Company’s Series B preferred stock. The Series B preferred stock has no voting rights, except with respect to the authorization of any class or series of stock having preference or priority over the Series B preferred stock as to voting, liquidation or conversion or with respect to the determination of fair market value of non-publicly traded shares received by the holder of Series B preferred stock in the event of a liquidation, or except as required by Delaware law. At any time, the holder may convert each share of Series B preferred stock into 100 shares of the Company’s common stock. If all shares of Series B preferred stock were converted to common stock, 332,700 shares of common stock would be issued, which represents less than 1% of the outstanding common stock of the Company at December 31, 2011. The Company has the right to redeem the Series B preferred stock prior to conversion for a payment of $9.5 million.
Common Stock and Associated Warrant Liability
In August 2009, the Company received net proceeds of approximately $12.1 million, after deducting placement agent’s fees and stock issuance costs of approximately $1.1 million, from a registered direct offering of 6.0 million units. Each unit sold consisted of one share of common stock and a warrant to purchase 4/10 of a share of common stock. Each unit was sold for $2.20, resulting in the issuance of 6.0 million shares of common stock and warrants to purchase 2.4 million shares of common stock, exercisable at an exercise price of $2.90 per share. The warrants issued in August 2009 (“2009 Warrants”) are exercisable for a period of five years from the issue date. The fair value on the date of issuance of the 2009 Warrants was determined to be $2.8 million using the binomial-lattice option valuation model and applying the following assumptions: (i) a risk-free rate of 2.48%, (ii) an expected term of 5.0 years, (iii) no dividend yield and (iv) a volatility of 77%.
In November 2010, the Company received net proceeds of approximately $19.7 million, after deducting underwriting discounts and commissions and stock issuance costs of approximately $1.3 million, from an underwritten public offering of 7.4 million units. Each unit sold consisted of one share of common stock and a warrant to purchase 1/2 of a share of common stock. Each unit was sold for $2.85, resulting in the issuance of
84
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
7.4 million shares of common stock and warrants to purchase 3.7 million shares of common stock, exercisable at an exercise price of $3.20 per share. The warrants issued in November 2010 (“2010 Warrants”) became exercisable on May 15, 2011 and are exercisable for a period of five years from the issue date. The fair value on the date of issuance of the 2010 Warrants was determined to be $5.8 million using the binomial-lattice option valuation model and applying the following assumptions: (i) a risk-free rate of 1.23%, (ii) an expected term of 5.0 years, (iii) no dividend yield and (iv) a volatility of 85%.
The fair value of the 2009 Warrants and 2010 Warrants was recorded on the consolidated balance sheets as a liability pursuant to “Accounting for Derivative Instruments and Hedging Activities” and “Accounting for Certain Financial Instruments with Characteristics of Both Liabilities and Equity” Topics of ASC and will be adjusted to fair value at each financial reporting date thereafter until the earlier of exercise or expiration, at which time, these warrants would be reclassified into stockholders’ equity. The Company classified the 2009 Warrants and 2010 Warrants as a liability as these warrants contain certain provisions that, under certain circumstances, which may be out of the Company’s control, could require the Company to pay cash to settle the exercise of the warrants or may require the Company to redeem the warrants.
The fair value of the warrants at December 31, 2011 and 2010 consisted of the following (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| 2009 Warrants |
$ | 3,010 | $ | 2,768 | ||||
| 2010 Warrants |
4,969 | 5,697 | ||||||
|
|
|
|
|
|||||
| Total warrant liability |
$ | 7,979 | $ | 8,465 | ||||
|
|
|
|
|
|||||
The fair value of the Company’s warrants was based on using the binomial-lattice option valuation model and using the following assumptions at December 31, 2011 and 2010:
| December 31, | ||||
| 2011 | 2010 | |||
| 2009 Warrants: |
||||
| Expected term (in years) |
2.65 | 3.65 | ||
| Estimated volatility |
74% | 70% | ||
| Risk-free interest rate |
0.36% | 1.52% | ||
| Expected dividend yield |
0% | 0% | ||
| 2010 Warrants: |
||||
| Expected term (in years) |
3.86 | 4.86 | ||
| Estimated volatility |
70% | 86% | ||
| Risk-free interest rate |
0.60% | 2.01% | ||
| Expected dividend yield |
0% | 0% | ||
For the year ended December 31, 2011, the Company recorded non-cash gains of $0.5 million and recorded non-cash gains of less than $0.1 million for each of the years ended December 31, 2010 and 2009, on its consolidated statements of operations within non-operating expense, net, due to the changes in fair value of the warrants. At December 31, 2011, no warrants had been exercised.
At-the-Market Agreement
In June 2011, the Company entered into an At-The-Market Issuance Sales Agreement (the “Sales Agreement”) with MLV & Co. LLC (“MLV”) that provides for the issuance and sale of shares of its common
85
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
stock over the term of the Sales Agreement having an aggregate offering price of up to $20.0 million through MLV. In conjunction with the Sales Agreement, MLV acts as the Company’s sales agent and receives compensation based on an aggregate of 3% of the gross proceeds on the sale price per share of its common stock. The issuance and sale of these shares by the Company pursuant to the Sales Agreement are deemed an “at-the-market” offering registered under the Securities Act. During the year ended December 31, 2011, approximately 3.5 million shares of the Company’s common stock were sold under the Sales Agreement for aggregate net proceeds of $9.7 million.
Stockholder Rights Plan
In October 2009, the Company’s Board of Directors adopted an amendment to its 1999 stockholder rights plan, commonly referred to as a “poison pill,” to reduce the exercise price, extend the expiration date and revise certain definitions under the plan. The stockholder rights plan is intended to deter hostile or coercive attempts to acquire the Company. The stockholder rights plan enables stockholders to acquire shares of the Company’s common stock, or the common stock of an acquirer, at a substantial discount to the public market price should any person or group acquire more than 15% of the Company’s common stock without the approval of the Board of Directors under certain circumstances. The Company has designated 250,000 shares of Series C Junior Participating preferred stock for issuance in connection with the stockholder rights plan.
Note 15. Stock-Based Compensation
Employee Stock Plans
Employee Stock Purchase Plan
The Company maintains an Employee Stock Purchase Plan (the “Purchase Plan”), which is intended to qualify as an employee stock purchase plan within the meaning of Section 423(b) of the Internal Revenue Code. Under the Purchase Plan, the Company’s Board of Directors may authorize participation by eligible employees, including officers, in periodic offerings. Although the Purchase Plan provides for an offering period to be no more than 27 months, the Company currently allows eligible employees to purchase shares of the Company’s common stock at the end of each six-month offering period at a purchase price equal to 85% of the lower of the fair market value per share on the start date of the offering period or the fair market value per share on the purchase date. The Purchase Plan, as amended by the Company’s stockholders, has authorized and provided for issuance an aggregate of 820,500 shares of common stock. At December 31, 2011, the Company had 137,687 shares available for future issuance.
2008 Equity Incentive Plan
The Company also maintains an equity compensation plan to provide long-term incentives for employees, contractors, and members of its Board of Directors. The Company currently grants equity awards from one plan, the 2008 Equity Incentive Plan (the “2008 Plan”). The 2008 Plan allows for the issuance of non-statutory and incentive stock options, restricted stock, restricted stock units, stock appreciation rights, other stock-related awards, and performance awards which may be settled in cash, stock, or other property. On June 1, 2011, the stockholders approved an amendment to the 2008 Plan to increase the aggregate number of shares of common stock authorized for issuance under the 2008 plan by 2,000,000 shares, such that the 2008 Plan has reserved for issuance an amount not to exceed 10,540,940 shares. Awards under the 2008 Plan generally have a maximum term of 10 years from the date of the award. The 2008 Plan generally requires options to be granted at 100% of the fair market value of the Company’s common stock subject to the option on the date of grant and will
86
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
generally vest over four years. Performance-based stock or cash awards granted under the 2008 Plan are limited to either 500,000 shares of common stock or $1.0 million per recipient per calendar year. The attainment of any performance-based awards granted shall be conclusively determined by a committee designated by the Company’s Board of Directors. At December 31, 2011, 100,000 performance-based stock options were outstanding, of which 50,000 were granted during the year ended December 31, 2008 and 50,000 were granted during the year ended December 31, 2011.
1996 Equity Incentive Plan, 1998 Non-Officer Stock Option Plan, and 1999 Equity Incentive Plan
The Company continues to have equity awards outstanding under its previous stock plans: 1998 Non-Officer Stock Option Plan and 1999 Equity Incentive Plan (collectively, the “Prior Plans”) and 1996 Equity Incentive Plan (the “1996 Plan”). Equity awards issued under the Prior Plans and the 1996 Plan continues to adhere to the terms of those respective stock plans and no further options may be granted under those previous plans. However, at June 2, 2008, any shares that remained available for future grants under the Prior Plans became available for issuance under the 2008 Plan.
At December 31, 2011, the Company had an aggregate of approximately 10.1 million shares of its common stock reserved for issuance under the 2008 Plan, the Prior Plans and the 1996 Plan, of which approximately 7.5 million shares were subject to outstanding options and other stock-based awards, and approximately 2.6 million shares were available for future issuance under the 2008 Plan.
Activity under the Company’s equity incentive plans related to stock options is set forth below (in thousands except weighted average exercise price):
| Number of Options Outstanding |
Weighted Average Exercise Price per Share |
|||||||
| Balances at December 31, 2008 |
5,063 | $ | 12.29 | |||||
| Granted |
2,229 | 1.11 | ||||||
| Forfeited |
(360 | ) | 3.28 | |||||
| Expired |
(363 | ) | 13.20 | |||||
| Exercised |
(4 | ) | 2.39 | |||||
|
|
|
|||||||
| Balances at December 31, 2009 |
6,565 | $ | 7.38 | |||||
| Granted |
981 | 2.90 | ||||||
| Forfeited |
(28 | ) | 3.03 | |||||
| Expired |
(309 | ) | 19.20 | |||||
| Exercised |
(202 | ) | 1.62 | |||||
|
|
|
|||||||
| Balances at December 31, 2010 |
7,007 | $ | 6.42 | |||||
| Granted |
2,169 | 2.36 | ||||||
| Forfeited |
(465 | ) | 2.45 | |||||
| Expired |
(1,237 | ) | 11.52 | |||||
| Exercised |
(112 | ) | 0.81 | |||||
|
|
|
|||||||
| Balances at December 31, 2011 |
7,362 | $ | 4.70 | |||||
|
|
|
|||||||
87
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Information regarding the Company’s stock options outstanding, stock options vested and expected to vest, and stock options exercisable at December 31, 2011, 2010 and 2009, was as follows (in thousands except weighted average exercise price and contractual term):
| Number of Shares |
Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (Years) |
Aggregate Intrinsic Value |
|||||||||||||
| Balances at December 31, 2011 |
||||||||||||||||
| Stock options outstanding |
7,362 | $ | 4.70 | 6.66 | $ | 4,065 | ||||||||||
| Stock options vested and expected to vest |
6,900 | $ | 4.86 | 6.47 | $ | 3,835 | ||||||||||
| Stock options exercisable |
4,058 | $ | 6.70 | 5.00 | $ | 1,902 | ||||||||||
| Balances at December 31, 2010 |
||||||||||||||||
| Stock options outstanding |
7,007 | $ | 6.42 | 6.22 | $ | 2,761 | ||||||||||
| Stock options vested and expected to vest |
6,705 | $ | 6.60 | 6.08 | $ | 2,610 | ||||||||||
| Stock options exercisable |
4,323 | $ | 8.93 | 4.83 | $ | 922 | ||||||||||
| Balances at December 31, 2009 |
||||||||||||||||
| Stock options outstanding |
6,565 | $ | 7.38 | 6.37 | $ | 1,891 | ||||||||||
| Stock options vested and expected to vest |
6,165 | $ | 7.74 | 6.18 | $ | 1,617 | ||||||||||
| Stock options exercisable |
3,901 | $ | 10.77 | 4.62 | $ | 220 | ||||||||||
The aggregate intrinsic value in the table above is calculated as the difference between the exercise price of the stock option and the Company’s closing stock price on the last trading day of each respective fiscal period. The total intrinsic value of options exercised for the years ended December 31, 2011, 2010 and 2009 was $0.2 million, $0.3 million and $0.0 million, respectively.
Restricted Stock Units
The Company has previously granted restricted stock units to the Company’s Chief Executive Officer, Senior Vice Presidents, and Vice Presidents in accordance with the Bonus Plan for Senior Management of Cerus Corporation. Subject to each grantee’s continued employment, the restricted stock units vest in three annual installments from the date of grant and are generally issuable at the end of the three-year vesting term.
88
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Activity under the Company’s equity incentive plans related to restricted stock units, which were made in connection with the Bonus Plan for Senior Management of Cerus Corporation, is set forth below (in thousands except weighted average grant-date fair value):
| Number of RSUs |
Weighted
Average Grant-Date Fair Value |
|||||||
| Balances at December 31, 2008 |
84,658 | $ | 6.80 | |||||
| Granted |
0 | 0.00 | ||||||
| Forfeited |
(6,485 | ) | 6.41 | |||||
| Vested |
(40,306 | ) | 7.19 | |||||
|
|
|
|||||||
| Balances at December 31, 2009 |
37,867 | $ | 6.45 | |||||
| Granted |
76,532 | 1.85 | ||||||
| Forfeited |
0 | 0.00 | ||||||
| Vested |
(25,999 | ) | 6.20 | |||||
|
|
|
|||||||
| Balances at December 31, 2010 |
88,400 | $ | 2.54 | |||||
| Granted |
0 | 0.00 | ||||||
| Forfeited |
(17,727 | ) | 1.85 | |||||
| Vested |
(37,378 | ) | 3.48 | |||||
|
|
|
|||||||
| Balances at December 31, 2011 |
33,295 | $ | 1.85 | |||||
|
|
|
|||||||
Stock-based Compensation Expense
Stock-based compensation expense recognized on the Company’s consolidated statements of operations for the years ended December 31, 2011, 2010 and 2009, was as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Stock-based compensation expense by caption: |
||||||||||||
| Research and development |
$ | 450 | $ | 376 | $ | 494 | ||||||
| Selling, general and administrative |
1,400 | 1,452 | 1,552 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total stock-based compensation expense |
$ | 1,850 | $ | 1,828 | $ | 2,046 | ||||||
|
|
|
|
|
|
|
|||||||
Stock-based compensation expense in the above table does not reflect any income taxes as the Company has experienced a history of net losses since its inception and has a full valuation allowance on its deferred tax assets. In addition, there was neither income tax benefits realized related to stock-based compensation expense nor any stock-based compensation costs capitalized as part of an asset during the years ended December 31, 2011, 2010 and 2009. The Company has also not recorded any stock-based compensation associated with performance-based stock options during the years ended December 31, 2011, 2010 and 2009 as the performance criteria was not probable of being achieved.
As of December 31, 2011, the Company expects to recognize the remaining unamortized stock-based compensation expense of $3.3 million related to non-vested stock options, net of estimated forfeitures, over an estimated remaining weighted average period of 2.78 years. As of December 31, 2011, the Company expects to
89
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
recognize the remaining unamortized stock-based compensation expense of less than $0.1 million related to non-vested restricted stock units, net of estimated forfeitures, over an estimated remaining weighted average period of 1.02 years.
Valuation Assumptions for Stock-based Compensation
The Company currently uses the Black-Scholes option pricing model to determine the grant-date fair value of stock options and employee stock purchase plan shares. The Black-Scholes option-pricing model is affected by the Company’s stock price, as well as assumptions regarding a number of complex and subjective variables, which include the expected term of the grants, actual and projected employee stock option exercise behaviors, including forfeitures, the Company’s expected stock price volatility, the risk-free interest rate and expected dividends. The Company recognizes the grant-date fair value of the stock award as stock-based compensation expense on a straight-line basis over the requisite service period, which is the vesting period, and is adjusted for estimated forfeitures.
Expected Term
The Company estimates the expected term for stock options based on grouping the population of stock options into discreet, homogeneous groups and then analyzing employee exercise and post-vesting termination behavior. The Company may also average the vesting term and the contractual term of the stock options, as illustrated in SAB 107 and SAB 110, if the Company is unable to obtain sufficient information for a particular homogeneous group of stock options. The expected term for the shares issuable under the employee stock purchase plan is the term of each purchase period, which is six months.
Estimated Forfeiture Rate
The Company estimates the forfeiture rate of stock options at the time of grant and revises those estimates in subsequent periods if actual forfeitures differ from those estimates. The Company uses historical data to estimate pre-vesting option forfeitures and records stock-based compensation expense only for those awards that are expected to vest. The Company estimates the historic pre-vesting forfeiture rates by groups that possess a degree of homogeneity regarding average time to vest and expected term.
Estimated Volatility
The Company estimates the volatility of its common stock by using historical volatility of its common stock. The Company has used significant judgment in making these estimates and will continue to monitor the availability of actively traded stock options on its common stock. The Company may also consider a combination of historical and implied volatility, or solely implied volatility, if the Company determines that sufficient actively traded stock options on its common stock exists.
Risk-Free Interest Rate
The Company uses the risk-free interest rate based on the yield derived from United States Treasury zero-coupon issues with remaining terms similar to the expected term on the stock options.
Expected Dividend Yield
The Company does not anticipate paying any cash dividends in the foreseeable future and therefore uses an expected dividend yield of zero.
90
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
The weighted average assumptions used to value the Company’s stock-based awards for the years ended December 31, 2011, 2010 and 2009, was as follows:
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Stock Options: |
||||||||||||
| Expected term (in years) |
5.30 | 5.40 | 5.69 | |||||||||
| Estimated volatility |
68 | % | 82 | % | 84 | % | ||||||
| Risk-free interest rate |
1.23 | % | 1.40 | % | 1.99 | % | ||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Employee Stock Purchase Plan Rights: |
||||||||||||
| Expected term (in years) |
0.50 | 0.50 | 0.50 | |||||||||
| Estimated volatility |
48 | % | 61 | % | 114 | % | ||||||
| Risk-free interest rate |
0.08 | % | 0.89 | % | 2.24 | % | ||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | ||||||
The weighted average grant-date fair value of stock options granted during the years ended December 31, 2011, 2010 and 2009, was $1.37, $1.94 and $0.76 per share, respectively. The weighted average grant-date fair value of restricted stock units granted during the year ended December 31, 2010 was $1.85 per share. The weighted average grant-date fair value of employee stock purchase rights during the years ended December 31, 2011, 2010 and 2009, was $0.68, $0.90 and $0.58 per share, respectively.
Note 16. Retirement Plan
The Company maintains a defined contribution savings plan (the “401(k) Plan”) that qualifies under the provisions of Section 401(k) of the Internal Revenue Code and covers all employees of the Company. Under the terms of the 401(k) Plan, employees may make pre-tax dollar contributions of up to 60% of their eligible pay up to a maximum cap established by the IRS. The Company may contribute a discretionary percentage of qualified individual employee’s salaries, as defined, to the 401(k) Plan. The Company has not contributed to the 401(k) Plan in the years ended December 31, 2011, 2010 and 2009.
Note 17. Development and License Agreements
Agreements with Fenwal, Related Party
In December 2009, the Company and Baxter International Inc. (“Baxter”) entered into a settlement agreement regarding disputed amounts for certain transition services provided in 2006 by Baxter in conjunction with the transfer of commercialization rights to the Company. In consideration for agreeing to the settlement, with both parties waiving all rights and obligations, the Company eliminated the disputed amounts from its consolidated balance sheet of $4.7 million in payment obligations to Baxter and $2.8 million in receivables due from Baxter, and agreed to pay Baxter $0.5 million, resulting in the Company recording a gain of $1.4 million during the year ended December 31, 2009. The $0.5 million payable to Baxter was recorded in “Accounts payable” on the Company’s consolidated balance sheet at December 31, 2009 and subsequently paid by the Company in 2010.
As a result of Baxter’s sale of its transfusion therapies division in 2007 to Fenwal, the Company has certain agreements with Fenwal which require the Company to pay royalties on future INTERCEPT Blood System product sales at royalty rates that vary by product: 10% of product sales for the platelet system, 3% of product sales for the plasma system, 5% of product sales for the red blood cell system, and 6.5% on sales of illuminators.
91
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
During the years ended December 31, 2011, 2010 and 2009, the Company made royalty payments to Fenwal of $2.2 million, $2.0 million and $0.9 million, respectively. At December 31, 2011 and December 31, 2010, the Company owed royalties to Fenwal of $0.7 million and $0.5 million, respectively.
In December 2008, the Company extended its agreement with Fenwal to manufacture finished disposable kits for the platelet and plasma systems through December 31, 2013. Under the amended manufacturing and supply agreement, the Company pays Fenwal a set price per kit, which is established annually, plus a fixed surcharge per kit. In addition, volume driven manufacturing overhead is to be paid or refunded if actual manufacturing volumes are lower or higher than the estimated production volumes. The Company made payments to Fenwal of $9.6 million, $8.6 million and $5.3 million relating to the manufacturing of the Company products during the years ended December 31, 2011, 2010 and 2009, respectively. At December 31, 2011 and December 31, 2010, the Company owed Fenwal $3.4 million and $2.3 million, respectively, for INTERCEPT disposable kits manufactured.
Cooperative Agreements with the United States Armed Forces
Since February 2001, the Company has received awards under cooperative agreements with the Army Medical Research Acquisition Activity division of the Department of Defense. The Company received these awards in order to develop its pathogen inactivation technologies for the improved safety and availability of blood that may be used by the United States Armed Forces for medical transfusions. Under the terms of the cooperative agreements, the Company is conducting research on the inactivation of infectious pathogens in blood, including unusual viruses, bacteria and parasites that are of concern to the United States Armed Forces. This funding supports advanced development of the Company’s red blood cell system. The Company recognized $2.4 million, $1.4 million and $1.2 million of revenue under these agreements during the years ended December 31, 2011, 2010 and 2009, respectively.
Note 18. Income Taxes
Deferred income taxes reflect the net tax effects of temporary differences between the carrying amounts of assets and liabilities for financial reporting purposes and the amounts used for income tax purposes at the enacted rates. The significant components of the Company’s deferred tax assets at December 31, 2011 and 2010 were as follows (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| Net operating loss carryforwards |
$ | 137,800 | $ | 132,600 | ||||
| Research and development credit carryforwards |
31,400 | 31,600 | ||||||
| Capitalized inventory costs |
500 | 700 | ||||||
| Inventory reserve |
200 | 200 | ||||||
| Capitalized research and development |
10,300 | 13,000 | ||||||
| Capitalized trademark |
200 | 300 | ||||||
| Capitalized revenue sharing rights |
600 | 900 | ||||||
| Deferred compensation |
4,200 | 3,600 | ||||||
| Accrued liabilities |
400 | 300 | ||||||
| Depreciation |
1,400 | 2,200 | ||||||
| Acqusition costs |
200 | 0 | ||||||
| Deferred tenant allowance |
200 | 0 | ||||||
| Capital loss carryforwards |
3,900 | 3,900 | ||||||
|
|
|
|
|
|||||
| Total deferred tax assets |
191,300 | 189,300 | ||||||
| Valuation allowance |
(191,300 | ) | (189,300 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | 0 | $ | 0 | ||||
|
|
|
|
|
|||||
92
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
The valuation allowance increased by $2.0 million, $7.2 million and $2.5 million for the years ended December 31, 2011, 2010 and 2009, respectively. The Company believes that, based on a number of factors, the available objective evidence creates sufficient uncertainty regarding the realizability of the deferred tax assets such that a full valuation allowance has been recorded. These factors include the Company’s history of net losses since its inception, the need for regulatory approval of the Company’s products prior to commercialization, expected near-term future losses and the absence of taxable income in prior carryback years. The Company expects to maintain a full valuation allowance until circumstances change. Undistributed earnings of the Company’s foreign subsidiary, Cerus Europe B.V., amounted to approximately $0.4 million at December 31, 2011. The earnings are considered to be permanently reinvested and accordingly, no deferred United States income taxes have been provided thereon. Upon distribution of those earnings in the form of dividends or otherwise, the Company would be subject to United States income taxes. At the Federal statutory income tax rate of 34%, this would result in taxes of approximately $0.2 million.
For the year ended December 31, 2011, the Company reported net losses of $17.0 million on its consolidated statement of operations and calculated taxable losses for both federal and state taxes. The difference between reported net loss and taxable loss are due to temporary differences between book accounting and the respective tax laws.
At December 31, 2011, the Company had net operating loss carryforwards of approximately $354.1 million for federal and $297.4 million for state income tax purposes. The Company also had research and development tax credit carryforwards of approximately $21.2 million for federal income tax purposes and approximately $15.4 million for state income tax purposes at December 31, 2011. The federal net operating loss and tax credit carryforwards expire between the years 2012 and 2031. The state net operating loss carryforwards expire between the years 2012 and 2031. The state research and development credits do not expire and are carryforward indefinitely until fully exhausted.
The utilization of net operating loss carryforwards, as well as research and development credit carryforwards, is limited by current tax regulations. These net operating loss carryforwards, as well as research and development credit carryforwards, will be utilized in future periods if sufficient income is generated. The Company believes it more likely than not that its tax positions would be recognized upon review by a taxing authority having full knowledge of all relevant information. The Company’s ability to utilize certain loss carryforwards and certain research credit carryforwards are subject to limitations pursuant to the ownership change rules of Internal Revenue Code Section 382.
Note 19. Segment, Customer and Geographic Information
The Company operates in only one segment, blood safety. The Company’s chief executive officer is the chief operating decision maker who evaluates performance based on the net revenues and operating loss of the blood safety segment. The Company considers the sale of all of its INTERCEPT Blood System products to be similar in nature and function, and any revenue earned from services are minimal.
The Company’s operations outside of the United States include a wholly-owned subsidiary headquartered in Europe. The Company’s operations in the United States are responsible for the research and development and global commercialization of the INTERCEPT Blood System, while operations in Europe are responsible for the commercialization efforts of the INTERCEPT platelet and plasma systems in Europe, the CIS, and the Middle East. Product revenues are attributed to each region based on the location of the customer, and in the case of non-product revenues, on the location of the collaboration partner.
93
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
The Company had the following significant customers that accounted for more than 10% of the Company’s total product revenue, all of which operate in a country outside of the United States, during the years ended December 31, 2011, 2010 and 2009 (in percentages):
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Etablissement Francais du Sang |
24 | % | 20 | % | 24 | % | ||||||
| Movaco, S.A. |
21 | % | 19 | % | 25 | % | ||||||
| Delrus Inc. |
12 | % | 16 | % | * | |||||||
| Service Francophone du Sang |
* | 12 | % | 12 | % | |||||||
| * | Represents an amount less than 10% of product revenue. |
The Company also recognized government grants and cooperative agreements revenue which represented 7% of total revenue, 6% of total revenue and 7% of total revenue, during the years ended December 31, 2011, 2010 and 2009, respectively.
Net revenues by geographical location was based on the location of the customer, in the case of product revenues, and in the location of the collaboration partner, in the case of non-product revenues, during the years ended December 31, 2011, 2010 and 2009 and was as follows (in thousands):
| Years Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Product Revenue: |
||||||||||||
| France |
$ | 7,385 | $ | 4,432 | $ | 4,134 | ||||||
| Spain and Portugal |
6,504 | 4,175 | 4,191 | |||||||||
| CIS |
3,754 | 3,383 | 1,006 | |||||||||
| Belgium |
3,703 | 3,710 | 3,142 | |||||||||
| Switzerland |
3,315 | 1,330 | 0 | |||||||||
| Other countries |
5,941 | 4,647 | 4,278 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total product revenue |
30,602 | 21,677 | 16,751 | |||||||||
| Government grants and cooperative agreements: |
||||||||||||
| United States |
2,442 | 1,432 | 1,231 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total government grants and cooperative agreements |
2,442 | 1,432 | 1,231 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
$ | 33,044 | $ | 23,109 | $ | 17,982 | ||||||
|
|
|
|
|
|
|
|||||||
Long-lived assets by geographical location, which consist of property and equipment, net, intangible assets, net, and certain other assets, at December 31, 2011 and 2010 were as follows (in thousands):
| December 31, | ||||||||
| 2011 | 2010 | |||||||
| United States |
$ | 3,299 | $ | 3,883 | ||||
| Europe |
650 | 797 | ||||||
|
|
|
|
|
|||||
| Total long-lived assets |
$ | 3,949 | $ | 4,680 | ||||
|
|
|
|
|
|||||
94
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
Note 20. Quarterly Financial Information (Unaudited)
The following tables summarize the Company’s quarterly financial information for the years ended December 31, 2011 and 2010 (in thousands except per share amounts):
| Three Months Ended | ||||||||||||||||
| March 31, 2011(1) |
June 30, 2011(1) |
September 30, 2011(1) |
December 31, 2011 |
|||||||||||||
| Revenue: |
||||||||||||||||
| Product revenue |
$ | 6,183 | $ | 6,753 | $ | 7,770 | $ | 9,896 | ||||||||
| Government grants and cooperative agreements |
436 | 0 | 1,479 | 527 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
6,619 | 6,753 | 9,249 | 10,423 | ||||||||||||
| Cost of product revenue |
3,529 | 4,074 | 4,726 | 6,206 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
3,090 | 2,679 | 4,523 | 4,217 | ||||||||||||
| Operating expenses: |
||||||||||||||||
| Research and development |
1,808 | 1,994 | 1,814 | 1,562 | ||||||||||||
| Selling, general and administrative |
5,528 | 6,207 | 5,380 | 5,938 | ||||||||||||
| Amortization of intangible assets |
50 | 51 | 51 | 50 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
7,386 | 8,252 | 7,245 | 7,550 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(4,296 | ) | (5,573 | ) | (2,722 | ) | (3,333 | ) | ||||||||
| Non-operating income (expense), net |
(802 | ) | (836 | ) | 4,982 | (4,402 | ) | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net income (loss) |
$ | (5,098 | ) | $ | (6,409 | ) | $ | 2,260 | $ | (7,735 | ) | |||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per common share: |
||||||||||||||||
| Basic |
$ | (0.11 | ) | $ | (0.13 | ) | $ | 0.05 | $ | (0.16 | ) | |||||
| Diluted |
$ | (0.11 | ) | $ | (0.13 | ) | $ | 0.05 | $ | (0.16 | ) | |||||
95
Table of Contents
CERUS CORPORATION
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS—(Continued)
December 31, 2011
| Three Months Ended | ||||||||||||||||
| March 31, 2010 |
June 30, 2010 |
September 30, 2010 |
December 31, 2010 |
|||||||||||||
| Revenue: |
||||||||||||||||
| Product revenue |
$ | 5,500 | $ | 5,690 | $ | 4,521 | $ | 5,965 | ||||||||
| Government grants and cooperative agreements |
222 | 245 | 470 | 496 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
5,722 | 5,935 | 4,991 | 6,461 | ||||||||||||
| Cost of product revenue |
3,158 | 2,934 | 2,324 | 3,630 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Gross profit |
2,564 | 3,001 | 2,667 | 2,831 | ||||||||||||
| Operating expenses (gains): |
||||||||||||||||
| Research and development |
1,250 | 1,244 | 1,282 | 1,419 | ||||||||||||
| Selling, general and administrative |
5,270 | 5,304 | 5,089 | 5,913 | ||||||||||||
| Amortization of intangible assets |
0 | 0 | 0 | 67 | ||||||||||||
| Acquisition related costs and impairment of long-term investments in related parties, net |
251 | 132 | (201 | ) | 0 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total operating expenses |
6,771 | 6,680 | 6,170 | 7,399 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Loss from operations |
(4,207 | ) | (3,679 | ) | (3,503 | ) | (4,568 | ) | ||||||||
| Non-operating income (expense), net |
(1,066 | ) | (1,880 | ) | (128 | ) | 2,120 | |||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (5,273 | ) | $ | (5,559 | ) | $ | (3,631 | ) | $ | (2,448 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss per common share: |
||||||||||||||||
| Basic |
$ | (0.14 | ) | $ | (0.14 | ) | $ | (0.09 | ) | $ | (0.06 | ) | ||||
| Diluted |
$ | (0.14 | ) | $ | (0.14 | ) | $ | (0.09 | ) | $ | (0.06 | ) | ||||
| (1) | The financial information for the first three quarters of 2011 have been restated from amounts previously reported in the Company’s quarterly reports on Form 10-Q for cost of product revenue, gross profit, loss from operations, total non-operating income (expense), net, and net loss. The adjustments made related to capitalized inventory costs, which should have been charged to cost of product revenue as products were sold. The Company has determined that although the corrections were immaterial to each of the quarters ended March 31, June 30, and September 30, 2011, the Company has decided to restate the amounts previously reported to better reflect the actual operating trends of the business for 2011. The adjustments primarily impacted the cost of product revenue by $0.1 million for each of the three months ended March 31, 2011, June 30, 2011 and September 30, 2011. In addition, there was no impact to loss per share as a result of these immaterial misstatements in each of the three months ended March 31, 2011, June 30, 2011 and September 30, 2011. |
96
Table of Contents
Pursuant to the requirement of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Concord, State of California, on the 5th day of March, 2012.
| CERUS CORPORATION | ||
| By: | /s/ WILLIAM M. GREENMAN | |
| William M. Greenman | ||
| President and Chief Executive Officer | ||
Each person whose signature appears below constitutes and appoints William M. Greenman and Kevin D. Green, his true and lawful attorney-in-fact and agent, each acting alone, with full power of substitution and resubstitution, for him and in his name, place and stead, in any and all capacities, to sign any or all amendments to the Annual Report on Form 10-K and to file the same, with all exhibits thereto, and all documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorney-in-fact and agent, full power and authority to do and perform each and every act and thing requisite and necessary to be done in and about the premises, as fully to all intents and purposes as he might or could do in person, hereby ratifying and confirming all that said attorney-in-fact and agent, or his substitute or substitutes, may lawfully do or cause to be done by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons in the capacities and on the dates indicated.
| Signature |
Title |
Date | ||
| /s/ WILLIAM M. GREENMAN William M. Greenman |
President, Chief Executive Officer and Director (Principal Executive Officer) |
March 5, 2012 | ||
| /S/ KEVIN D. GREEN Kevin D. Green |
Vice President, Finance and Chief Accounting Officer (Principal Financial and Accounting Officer) |
March 5, 2012 | ||
| /S/ B. J. CASSIN B. J. Cassin |
Chairman of the Board of Directors |
March 5, 2012 | ||
| /S/ TIMOTHY B. ANDERSON Timothy B. Anderson |
Director |
March 5, 2012 | ||
| /S/ LAURENCE M. CORASH, M.D. Laurence M. Corash, M.D. |
Director |
March 5, 2012 | ||
| /S/ BRUCE C. COZADD Bruce C. Cozadd |
Director |
March 5, 2012 | ||
| /S/ GAIL SCHULZE Gail Schulze |
Director |
March 5, 2012 | ||
| /S/ DANIEL N. SWISHER, JR. Daniel N. Swisher, Jr. |
Director |
March 5, 2012 | ||
97
Table of Contents
INDEX TO EXHIBITS
| Exhibit |
Description of Exhibit | |
| 2.1 (25)† | Asset Purchase and Redemption Agreement by and between Cerus Corporation and BioOne Corporation, dated as of August 24, 2010. | |
| 3.1 (5) | Amended and Restated Certificate of Incorporation of Cerus Corporation | |
| 3.2 (24) | Certificate of Amendment to the Amended and Restated Certificate of Incorporation of Cerus Corporation. | |
| 3.3 (12) | Amended and Restated Bylaws of Cerus Corporation. | |
| 4.1 (1) | Specimen Stock Certificate. | |
| 4.2 (19) | Rights Agreement, dated as of November 3, 1999, as amended as of August 6, 2001, between Cerus Corporation and Wells Fargo Bank, N.A. (formerly known as Norwest Bank Minnesota, N.A.). | |
| 4.3 (21) | Amendment to Rights Agreement, dated as of October 28, 2009, between Cerus Corporation and Wells Fargo Bank, N.A. (which includes the form of Rights Certificate as Exhibit B thereto). | |
| 4.4 (20) | Form of 2009 Warrant to Purchase Common Stock. | |
| 4.5 (26) | Form of 2010 Warrant to Purchase Common Stock. | |
| Supply and/or Manufacturing Agreements | ||
| 10.1 (10)† | Supply Agreement, dated December 19, 2007, by and between Cerus Corporation and Brotech Corporation d/b/a Purolite Company. | |
| 10.2 (10)† | Supply and Manufacturing Agreement, dated March 1, 2008, by and between Cerus Corporation and Porex Corporation. | |
| 10.3 (15)† | Amended and Restated Manufacturing and Supply Agreement, dated December 12, 2008, by and between Cerus Corporation and Fenwal, Inc. | |
| 10.4 (15)† | Manufacturing and Supply Agreement, dated September 30, 2008, by and between Cerus Corporation and NOVA Biomedical Corporation. | |
| 10.5 (32)† | Amended and Restated Supply Agreement, dated as of September 1, 2011, between Cerus Corporation and Ash Stevens Inc. | |
| Loan and Security Agreements | ||
| 10.6 (27)† | Loan and Security Agreement, by and between Cerus Corporation and Oxford Finance Corporation, dated March 31, 2010. | |
| 10.7 (28) | First Amendment to Loan and Security Agreement, by and between Cerus Corporation and Oxford Finance Corporation, dated March 3, 2011. | |
| 10.8 (32)† | Loan and Security Agreement, dated as of September 30, 2011, by and between Cerus Corporation and Comerica Bank. | |
| Real Estate Lease Agreements | ||
| 10.9 (6) | Standard Industrial/Commercial Single-Tenant Lease-Net, dated October 12, 2001 between Cerus Corporation and California Development, Inc. | |
| 10.10(13) | Second Amendment to Standard Industrial/Commercial Single-Tenant Lease-Net, dated as of September 18, 2008 between Cerus Corporation and California Development, Inc. | |
98
Table of Contents
| Exhibit |
Description of Exhibit | |
| 10.11(22) | Letter to California Development, Inc. exercising option to extend the lease term from the Second Amendment to Standard Industrial/Commercial Single-Tenant Lease-Net, dated as of September 18, 2008 between Cerus Corporation and California Development, Inc. | |
| Employment Agreements or Offer Letters | ||
| 10.12(9)* | Offer Letter to Gail Schulze, dated October 15, 2007. | |
| 10.13(14)* | Amended and Restated Employment Agreement with Claes Glassell, dated December 19, 2008. | |
| 10.14(29)* | Employment Letter, by and between Cerus corporation and William M. Greenman, dated May 12, 2011. | |
| 10.15(23)* | Employment Letter, by and between Cerus Corporation and Laurence Corash, dated March 2, 2010. | |
| 10.16(14)* | Amended and Restated Employment Agreement with Howard G. Ervin, dated December 22, 2008. | |
| 10.17(19)* | Employment Letter for Kevin D. Green dated May 1, 2009. | |
| 10.18 * | Employment Agreement for Caspar Hogeboom dated March 6, 2006. | |
| 10.19* | Promotion Letter for Caspar Hogeboom dated December 11, 2009 and executed on September 21, 2010. | |
| 10.20* | Addendum to Employment Agreement for Caspar Hogeboom dated February 17, 2011. | |
| 10.21* | Healthcare Contribution Letter for Caspar Hogeboom dated December 18, 2007. | |
| 10.22* | Home Telephone and Internet Expenses Letter for Caspar Hogeboom dated January 11, 2012. | |
| Stock Plans and Related Forms | ||
| 10.23(1)* | 1996 Equity Incentive Plan. | |
| 10.24(1)* | Form of Incentive Stock Option Agreement under the 1996 Equity Incentive Plan. | |
| 10.25(1)* | Form of Nonstatutory Stock Option Agreement under the 1996 Equity Incentive Plan. | |
| 10.26(1)* | 1996 Employee Stock Purchase Plan. | |
| 10.27(3)* | 1998 Non-Officer Stock Option Plan. | |
| 10.28(4)* | 1999 Equity Incentive Plan, adopted April 30, 1999, approved by stockholders July 2, 1999. | |
| 10.29(7)* | 1999 Non-Employee Directors’ Stock Option Sub-Plan, amended December 4, 2002. | |
| 10.30(11)* | 2008 Equity Incentive Plan, approved by stockholders June 2, 2008. | |
| 10.31(31)* | 2008 Equity Incentive Plan, as amended, reapproved by stockholders June 1, 2011. | |
| 10.32(16)* | Form of Restricted Stock Unit Agreement under the 1999 Equity Incentive Plan, as amended. | |
| Other Compensatory Plans or Agreements | ||
| 10.33(23)* | Bonus Plan for Senior Management of Cerus Corporation, as amended March 3, 2010. | |
| 10.34(16)* | Cerus Corporation Change of Control Severance Benefit Plan, as amended. | |
| 10.35(18)* | Form of Severance Benefits Agreement. | |
| 10.36* | 2011 and 2012 Executive Officer Compensation Arrangements. | |
| 10.37* | Non-Employee Director Compensation Policy. | |
| Other Material Agreements | ||
| 10.38(30) | At-The-Market-Issuance Sales Agreement, dated June 3, 2011, by and between Cerus Corporation and MLV & Co. LLC. | |
| 10.39(33) | Amendment to At-The-Market-Issuance Sales Agreement, dated January 4, 2012, by and between Cerus Corporation and MLV & Co. LLC. | |
99
Table of Contents
| Exhibit Number |
Description of Exhibit | |
| 10.40(1) | Form of Indemnity Agreement entered into between Cerus Corporation and each of its directors and executive officers. | |
| 10.41(17) | Form of Amended and Restated Indemnity Agreement, adopted April 24, 2009. | |
| 10.42(20) | Form of Subscription Agreement. | |
| 10.43(2) | Series B Preferred Stock Purchase Agreement, dated as of June 30, 1998, by and between Cerus Corporation and Baxter Healthcare Corporation. | |
| 10.44(22)† | Restructuring Agreement, dated as of February 2, 2005, by and among Cerus Corporation, Baxter Healthcare S.A. and Baxter Healthcare Corporation. | |
| 10.45(22)† | License Agreement, dated as of February 2, 2005, by and among Cerus Corporation, Baxter Healthcare S.A. and Baxter Healthcare Corporation. | |
| 10.46(8)† | Commercialization Transition Agreement, dated as of February 12, 2006, by and among Cerus Corporation, Baxter Healthcare S.A. and Baxter Healthcare Corporation. | |
| 12.1 | Computation of Earnings to Fixed Charges | |
| 21.1 | List of Registrant’s subsidiaries. | |
| 23.1 | Consent of Independent Registered Public Accounting Firm. | |
| 24.1 | Power of Attorney (see signature page). | |
| 31.1 | Certification of the Principal Executive Officer of Cerus Corporation pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 31.2 | Certification of the Principal Financial Officer of Cerus Corporation pursuant to Section 302 of the Sarbanes-Oxley Act of 2002. | |
| 32.1(35) | Certification of the Principal Executive Officer and Principal Financial Officer pursuant to Section 906 of the Sarbanes-Oxley Act of 2002. | |
| 101.INS(34) | XBRL Instance Document | |
| 101.SCH(34) | XBRL Taxonomy Extension Schema Document | |
| 101.CAL(34) | XBRL Taxonomy Extension Calculation Linkbase Document | |
| 101.DEF(34) | XBRL Taxonomy Extension Definition Linkbase Document | |
| 101.LAB(34) | XBRL Taxonomy Extension Label Linkbase Document | |
| 101.PRE(34) | XBRL Taxonomy Extension Presentation Linkbase Document | |
| † | Certain portions of this exhibits are subject to a confidential treatment order. |
| * | Compensatory Plan. |
| (1) | Incorporated by reference to the like-described exhibit to the Registrant’s Registration Statement on Form S-1 (File No. 333-11341) and amendments thereto. |
| (2) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on July 22, 1998. |
| (3) | Incorporated by reference to the like-described exhibit to the Registrant’s Registration Statement on Form S-8, dated March 24, 1999. |
| (4) | Incorporated by reference to the like-described exhibit to the Registrant’s Registration Statement on Form S-8, dated August 4, 1999. |
| (5) | Incorporated by reference to the like-described exhibit to the Registrant’s Registration Statement on Form 8-K, dated November 12, 1999. |
100
Table of Contents
| (6) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2001. |
| (7) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2003. |
| (8) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2006. |
| (9) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2007. |
| (10) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2008. |
| (11) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 6, 2008. |
| (12) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 19, 2008. |
| (13) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended September 30, 2008. |
| (14) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on December 23, 2008. |
| (15) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2008. |
| (16) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2009. |
| (17) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on April 30, 2009. |
| (18) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 1, 2009. |
| (19) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended June 30, 2009. |
| (20) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 20, 2009. |
| (21) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on October 30, 2009. |
| (22) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2009. |
| (23) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on March 8, 2010. |
| (24) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended June 30, 2010. |
| (25) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on August 30, 2010. |
| (26) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on November 12, 2010. |
| (27) | Incorporated by reference to the like-described exhibit to the Registrant’s Annual Report on Form 10-K, for the year ended December 31, 2010. |
| (28) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended March 31, 2011. |
| (29) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on May 18, 2011. |
| (30) | Incorporated by reference to the like-described exhibit to the Registrant’s Current Report on Form 8-K, filed with the SEC on June 6, 2011. |
| (31) | Incorporated by reference to the like-described exhibit to Amendment No. 1 to the Registrant’s Quarterly Report on Form 10-Q/A, for the quarter ended June 30, 2011. |
101
Table of Contents
| (32) | Incorporated by reference to the like-described exhibit to the Registrant’s Quarterly Report on Form 10-Q, for the quarter ended September 30, 2011. |
| (33) | Incorporated by reference to the like-described exhibit to Amendment No. 1 to the Registrant’s Registration Statement on Form S-3/A, filed with the SEC on January 6, 2012. |
| (34) | Furnished herewith. Pursuant to applicable securities laws and regulations, the Registrant is deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and is not subject to liability under any anti-fraud provisions of the federal securities laws as long as the Registrant has made a good faith attempt to comply with the submission requirements and promptly amends the interactive data files after becoming aware that the interactive data files fails to comply with the submission requirements. These interactive data files are deemed not filed or part of a registration statement or prospectus for purposes of Sections 11 or 12 of the Securities Act of 1933, as amended, are deemed not filed for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, and otherwise are not subject to liability under these sections. |
| (35) | This certification accompanies the Form 10-K to which it relates, is not deemed filed with the Securities and Exchange Commission, and is not incorporated by reference into any filing of the Registrant’s under the Securities Act of 1933, as amended, or the Securities Exchange Act of 1934, as amended (whether made before or after the date of the Form 10-K), irrespective of any general incorporation language contained in such filing. |
102
