Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - Ocata Therapeutics, Inc. | Financial_Report.xls |
| EX-31.1 - EX-31.1 - Ocata Therapeutics, Inc. | v302543_ex31-1.htm |
| EX-32.1 - EX-32.1 - Ocata Therapeutics, Inc. | v302543_ex32-1.htm |
| EX-23.1 - EX-23.1 - Ocata Therapeutics, Inc. | v302543_ex23-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from _____________ to _____________
Commission file number 0-50295
ADVANCED CELL TECHNOLOGY, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 87-0656515 | |
|
(STATE OR OTHER JURISDICTION OF INCORPORATION OR ORGANIZATION) |
(I.R.S. EMPLOYER IDENTIFICATION NO.) |
33 Locke Drive, Marlborough, Massachusetts 01752
(508) 756-1212
(Address and telephone number, including area code, of registrant’s principal executive offices)
Securities registered pursuant to Section 12(b) of the Act:
None.
(Title of Class)
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.001 par value per share
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer ¨ | Accelerated filer x |
| Non-accelerated filer ¨ | Smaller reporting company ¨ |
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act.) Yes ¨ No S
The aggregate market value of the registrant’s Common Stock held by non-affiliates of the registrant (based upon the closing price of $0.19 for the registrant’s Common Stock as of June 30, 2011) was approximately $280 million (based on 1,473,991,085 shares of common stock outstanding and held by non-affiliates on such date). Shares of the registrant’s Common Stock held by each executive officer and director and by each entity or person that, to the registrant’s knowledge, owned 10% or more of the registrant’s outstanding Common Stock as of June 30, 2011 have been excluded in that such persons may be deemed to be affiliates of the registrant. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
The number of outstanding shares of the registrant’s Common Stock, $0.001 par value, was 2,029,049,544 shares as of February 7, 2012.
ADVANCED CELL TECHNOLOGY, INC.
2011 ANNUAL REPORT ON FORM 10-K
TABLE OF CONTENTS
| PART I | 3 | |
| Item 1. Business | 3 | |
| Item 1A. Risk Factors | 16 | |
| Item 1B. Unresolved Staff Comments | 31 | |
| Item 2. Properties | 31 | |
| Item 3. Legal Proceedings | 31 | |
| Item 4. [Reserved] | 34 | |
| PART II | 34 | |
| Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities | 34 | |
| Item 6. Selected Financial Data | 36 | |
| Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operation | 36 | |
| Item 7A. Quantitative and Qualitative Disclosures About Market Risk | 45 | |
| Item 8. Financial Statements and Supplementary Data | F-1 | |
| Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure | 46 | |
| Item 9A. Controls and Procedures | 46 | |
| Item 9B. Other Information | 49 | |
| PART III | 49 | |
| Item 10. Directors, Executive Officers and Corporate Governance | 49 | |
| Item 11. Executive Compensation | 54 | |
| Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters | 58 | |
| Item 13. Certain Relationships and Related Transactions, and Director Independence | 60 | |
| Item 14. Principal Accounting Fees and Services | 61 | |
| PART IV | 61 | |
| Item 15. Exhibits and Financial Statement Schedules | 61 |
| 2 |
CAUTIONARY STATEMENT RELATING TO FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K and the information incorporated by reference includes ‘‘forward-looking statements’’ All statements regarding our expected financial position and operating results, our business strategy, our financing plans and the outcome of any contingencies are forward-looking statements. Any such forward-looking statements are based on current expectations, estimates, and projections about our industry and our business. Words such as ‘‘anticipates,’’ ‘‘expects,’’ ‘‘intends,’’ ‘‘plans,’’ ‘‘believes,’’ ‘‘seeks,’’ ‘‘estimates,’’ or variations of those words and similar expressions are intended to identify such forward-looking statements. Forward-looking statements are subject to risks and uncertainties that could cause actual results to differ materially from those stated in or implied by any forward-looking statements.
PART I
Item 1. Business
Overview
Advanced Cell Technology, Inc., a Delaware corporation (the “Company”, “we”, “us”, or “our”) is a biotechnology company focused on developing and commercializing human embryonic, iPS and adult stem cell technology in the emerging field of regenerative medicine.
We were incorporated in Nevada under the name Two Moon Kachinas Corp. on May 18, 2000. On December 30, 2004, we filed an amendment to our articles of incorporation to change our corporate name to A.C.T. Holdings, Inc. On January 31, 2005, we completed the acquisition of Advanced Cell Technology, Inc., a Delaware corporation (prior to the Reincorporation (as defined below), “ACT”), pursuant to the terms of an Agreement and Plan of Merger dated January 3, 2005. As a result of the transaction, we terminated our kachina doll business and succeeded to the business operations and research efforts of ACT in the field of biotechnology. On June 17, 2005, we filed an amendment to our articles of incorporation to change our corporate name to Advanced Cell Technology, Inc. On November 18, 2005, we consummated a merger with and into our wholly-owned subsidiary ACT (the “Reincorporation”). As a result of the Reincorporation, we became a Delaware corporation.
We have acquired, developed and maintain a portfolio of patents and patent applications which, along with know-how and trade secrets, form the proprietary base for our research and development efforts in the area of embryonic, iPS and adult stem cell research. We believe that our intellectual property portfolio is one of the strongest in the field. Our team includes some of the world's leading scientists in the field of stem cell research and development, and experts in regulatory affairs and conducting clinical trials. We believe our technology base, combined with our know-how and experience both in the science and regulatory oversight of cell therapies, provides us with a strong competitive advantage and should facilitate the successful development and commercialization of products for use in the treatment of a wide array of chronic, degenerative diseases and in regenerative repair of a variety of acute diseases, such as trauma, myocardial infarction and burns.
Our belief that our intellectual property represents one of the strongest portfolios in the field is supported by:
• The early and consistent pace of filing, and the breadth of the large number of filings in the portfolio.
• The relative immaturity of this field of study.
• The limited number of truly competitive portfolios of intellectual property.
Regenerative medicine is a new and emerging field of study involving development of medical therapies based on advances in stem cell and the creation of differentiated cells and tissues in culture for use in transplantations. We have developed and maintain a broad intellectual property (IP) portfolio, with ownership or exclusive licensing of 35 issued patents and over 170 patent applications in the field of regenerative medicine and related areas. Our intellectual property includes patent rights and applications for specific applications of stem cell technology in producing retinal pigment epithelium (RPE) cells, hemangioblasts, myoblast stem cells and numerous methods and compositions for the use of these technologies and derived cells in treating retinal and other eye disease, inflammatory and autoimmune diseases, heart disease, as well as to provide agents for wound healing and replacement of blood components.
| 3 |
Although we have strong competitors in this field, we believe our intellectual property portfolio compares favorably with those of our competition based upon its size, focus and filing dates. With respect to the focus of our human embryonic stem cell portfolio, we believe that the manufacturing processes for generating therapeutic cell preparations and the use of the those preparations for treating diseases or otherwise repairing or replacing failing tissues will prove to be one of the technological keys to successful development of stem cell therapies. As described above, our intellectual property includes patent rights and applications for specific applications of stem cell technology. In addition, we have succeeded in deriving human embryonic cell (“hESC”) lines without destroying the donor embryo through our proprietary single blastomere derivation technology. We own or have a license to numerous other technologies directed to generating stem cell lines, including somatic cell nuclear transfer, parthenogenesis, transdifferentiation, induced pluripotency and dedifferentiation.
Our research efforts to date in human embryonic technologies include both clinical, pre-clinical and basic research efforts. In November and December 2010 we received approval for two Investigational New Drug (IND) Applications we filed with the US Food and Drug Administration (FDA) to initiate Phase I/II multicenter studies using embryonic stem cell derived retinal pigment epithelial (RPE) cells to treat patients with Stargardt’s Macular Dystrophy (SMD) in one study and patients with dry Age-related Macular Degeneration (dry AMD) in the other study. In September 2011, we received approval from U.K. Medicines and Healthcare products Regulatory Agency (MHRA) to conduct an SMD clinical trial in the United Kingdom. To date, three SMD and one dry AMD patient have been treated in the U.S. trials, and one SMD patient has been treated in the U.K. trial. These RPE cells used in these trials are derived from embryonic stem cells the company developed using our proprietary blastomere derivation techniques.
The Company has also secured Food and Drug Administration (FDA) clearance to proceed to a Phase II Clinical Trial for its Myoblast program for the treatment of heart failure, and the trial is currently being developed. We believe that the company's myoblast technology has demonstrated that a myoblast transplantation treatment is feasible and safe in clinical trials conducted to date and that the technology could address the large market potential presented by heart failure. The stem cells used in this clinical program are our autologous adult stem cells.
The Company's Hemangioblast program for the treatment of Diseases and Disorders of Circulatory and Vascular System is in preclinical development. These precursor cells derived from human embryonic stem (ES) cells can be used to achieve vascular repair in animal models of vascular injury.
We are focused on leveraging our key assets, including our intellectual property, our scientific team, our facilities and our capital, to accelerate the advancement of our stem cell technologies. In addition, we continue to pursue strategic collaborations with members of academia, industry and foundations to further accelerate the pace of our research efforts.
The Field of Regenerative Medicine
The emerging field of treatment called "regenerative medicine" or "cell therapy" refers to treatments that are founded on the concept of producing new cells to replace malfunctioning or damaged cells as a vehicle to treat disease, degeneration and injury. Our focus is the development of effective methods to generate replacement cells from both human embryonic and adult stem cells.
Many significant and currently untreatable human diseases arise from the loss or malfunction of specific cell types in the body. This is especially true of diseases associated with aging such as Alzheimer's disease, Parkinson's disease, type II diabetes, heart failure, osteoarthritis, macular degeneration, and aging of the immune system, known as immunosenescence. This is also true for medical conditions resulting from damage to cells due to acute disease, such as trauma, infarction and burns. We believe that replacing damaged or malfunctioning cells with fully functional ones may be a useful therapeutic strategy in treating many of these diseases and conditions.
A stem cell is a cell that has the ability to branch out and change, or differentiate, into two or more different cell types. Stem cells are self-renewing primitive cells that have the ability to develop into functional, differentiated cells. In general, there are two broad categories of stem cells: adult stem cells and embryonic stem cells. Adult stem cells are derived from various tissues in the human body. Because they can branch out into many different cell types, they are referred to as "multipotent." Multipotent means these cells develop into multiple, but not all, types of cells in the body.
| 4 |
Embryonic stem cells, referred to as ES cells, which are derived from pre-implantation embryos, are unique because they are "totipotent," which means that they can develop into all cells and tissues in the body, and they self-renew indefinitely in their undifferentiated state. The ability of ES cells to divide indefinitely in the undifferentiated state without losing pluripotency is a unique characteristic that distinguishes them from all other stem cells discovered to date in humans.
Our business is focused on both the development and commercialization of adult stem cell therapies and therapies based on cells derived from ES cells or other potentially totipotent stem cell technologies.
Our adult stem cell-based products are specifically targeted at therapies for heart and other cardiovascular disease and are at a more advanced stage of development than our human ES cell based technologies. Our first human ES cell-based product, retinal pigmented epithelial cells, have entered Phase I clinical trials and several patients have already been treated. We believe retinal pigmented epithelial cells technologies have potentially broader and more powerful applications with respect to a wide range of diseases.
Human ES Cell Programs
Since the discovery of the human ES cell, medical researchers worldwide have generally recognized the significance of this new technology and have begun to focus research on the translation of this discovery into important new therapies. Specifically, researchers have focused on several key challenges including:
• isolating and purifying cell lines,
• growing stable cell lines in culture for long periods without mutations,
• manufacturing cell lines in numbers sufficient for therapy,
• differentiating ES cells into all of the cell types desired for therapies, and
• solving the potential rejection of ES cells used in therapies due to immuno-incompatibility with the patient.
We believe that solving the potential rejection of hESC-derived cells and tissues in patients is the greatest scientific obstacle to developing successful therapeutics. Our research and technologies are focused on solving this obstacle by creating stem cell therapeutics with compatible tissues or which can be used in immunopriveleged or immunosuppressed sites of transplantation in patients. Compatible tissues are referred to as being histocompatible.
We believe the potential markets for regenerative medicine and stem cell therapies are large. The table below summarizes the potential United States patient populations which we believe may be amenable to cell or organ transplantation and represent target markets for products generated through our regenerative medicine technology.
POTENTIAL U.S. PATIENT POPULATIONS FOR CELL-BASED THERAPIES
| Medical Condition | Number of Patients* | |
| Cardiovascular disease | 70 million * | |
| Autoimmune disease | 50 million * | |
| Diabetes | 18 million | |
| Osteoporosis | 10 million | |
| Cancer | 10 million | |
| Alzheimer's disease | 4.5 million | |
| Parkinson's disease | 1 million | |
| Burns (severe) | 1.1 million | |
| Spinal-cord injuries | 0.25 million | |
| Birth defects | 0.15 million/year | |
| Macular Degeneration | 10 million |
*These estimates are based on patient estimates published by the following organizations from April 2005 to the present: the American Heart Association, the American Autoimmune Related Diseases Association, SEER (Surveillance, Epidemiology and End Result), American Burn Association, March of Dimes, the Alzheimer's Association, the Alzheimer's Disease Education & Referral Center (National Institute on Aging), the National Institutes of Health's National Institute on Neurological Disorders and Stroke, the Foundation for Spinal Cord Injury Prevention, Care & Cure, the Centers for Disease Control and Prevention, the American Association of Diabetes Educators, the Northwest Parkinson's Foundation and the Parkinson's Action Network, American Macular Degeneration Foundation.
| 5 |
Our Human Embryonic Stem Cell Technologies
In certain countries, the use of human embryonic stem cells has raised certain ethical, legal and social issues previously rooted in the fact that the initial discovered methods for generating human ES cells required the destruction of preimplantation embryos in order to isolate a cellular fraction called the inner cell mass (or “ICM”). We have developed an alternative to ICM-derivation of hESC - a method which utilizes single cell biopsy to remove a single blastomere from a 4-8 cell preembryo in a manner which does not result in the destruction of the preembryo. While the overall process for deriving hESC lines from single blastomeres is proprietary to ACT, and covered by an issued U.S. patent, the single cell biopsy technique is one that has been used routinely for more than decade by in vitro fertilization clinics as part of a process called preimplantation genetic diagnostics (PGD). In those clinics, single cells are removed from 4-8 cell pre-embryos and tested for genetic and chromosomal abnormalities, and embryos which pass PGD screening can then be used for implantation, suggesting to us that the single cell biopsy process is not only non-destructive, but may further be considered as a process which does not subject the preembryo to any undue risk of harm.
In August 2001, then-President George Bush set guidelines for federal funding of research on embryonic stem cells from human embryos created by in-vitro fertilization, referred to as IVF, limiting funding to just 60 lines. However, in March 2009, President Barack Obama issued an executive order opening the door to a significant increase in federal funding for ES cell research. That led to the National Institutes of Health (NIH) to promulgate new guidelines for registering hESC lines for federal eligibility. Between the time the proposed guidelines were published for comment, and the final guidelines were promulgated as rule later in 2009, the NIH changed the definition of “human Embryonic Stem Cell” to require that the stem cells must be derived from the inner cell mass of an embryos. This definitional change, which the NIH admitted in 2010 was a mistake in retrospect, has nevertheless restricted eligibility of hESC lines for federal funding to only ICM-derived hESC lines and as such has excluded our single blastomere-derived lines for eligibility. While we continue to work with the NIH towards promulgation of revised guidelines that remove this definitional limitation, research using our hESC lines does not currently qualify for federally-funded grants. It should be noted that this limitation is only with respect to obtaining federal funding of research, and not a limitation on our ability to use our own money or grants from other third parties to advance our research, nor does it prohibit the marketing of our hESC-derived therapeutics if and when such products may be approved by the FDA.
In addition to the allogeneic sourced approaches we have followed for certain of our cell therapy product opportunities, we have also maintained a strategic focus on producing pluripotent cell lines that are histocompatible with the patients in which the cells are to be injected or transplanted. We have numerous proprietary technologies that we believe will generate histocompatible, pluripotent stem cells for patient-specific application, including both techniques for generating hESC lines as well as induced Pluripotency (iPS) techniques. These various cell derivation techniques may help to improve the potential for effective use as transplants for a wider range of diseases and degenerative disorders in human patients. If successfully developed, our cellular reprogramming and pluripotent stem cell technologies will make it possible to produce cells that have the proliferative capacity of ES cells, have specific therapeutic application, and are immunologically compatible with the patient.
All of our non-ES cell technologies are at the level of basic research or in the pre-clinical stage of development.
Our Cell Therapy Research Programs
Regenerative medicine requires that stem cells, from whatever source derived, be differentiated, or re-differentiated, into specific body cell types and then physically transplanted into a patient. Differentiation into tissues such as retinal or corneal tissues, cardiac muscle, blood, and other tissues occurs spontaneously in ES cells being cultured in a dish. Successful application of stem cell technology will require developing appropriate manufacturing controls over the specific kinds of cells into which stem cells differentiate. Control of differentiation and the culture and growth of stem and differentiated cells are important current areas of research for us. We intend to continue to pursue differentiation approaches both in-house and through collaborations with other researchers who have particular interests in, and skills related to, cellular differentiation. These efforts include using both animal and human stem cell lines. Our research in this area includes projects focusing on developing many different cell types that may be used in the future to treat a wide range of diseases. As an example, our researchers have generated stable retinal pigment epithelium, or RPE, cell lines for use in our clinical retinal program and are working on projects to generate stable cell lines with particular focus on blood lineage and vascular epithelial cell lines from hemangioblast cells.
| 6 |
Retinal Pigment Epithelium Program. In November, 2006 we published data demonstrating human ES cell-derived RPE cells were capable of rescuing visual function in Royal College of Surgeon rats. Following the publication of that data, we entered into a pre-clinical development collaboration with Casey Eye Institute at Oregon Health & Science University. The purpose of the collaboration was to conduct dosage and safety studies in preparation for IND and Phase I human clinical trials. As mentioned, in November 2009 we filed an Investigational New Drug (IND) Application with the US Food and Drug Administration (FDA) to initiate a Phase I/II multicenter study using hESC-derived RPE cells to treat patients with Stargardt’s Macular Dystrophy (SMD). We also filed IND Application with the FDA to initiate a Phase I/II multicenter study using the same hESC-derived RPE cells to treat patients with Age-related Macular Degeneration. In November and December 2010 we received approval for both of these IND Applications. In September 2011, we received approval from U.K. Medicines and Healthcare products Regulatory Agency (MHRA) to conduct an SMD clinical trial in the United Kingdom. To date, three SMD and one dry AMD patient have been treated in the U.S. trials, and one SMD patient has been treated in the U.K. trial. These RPE cells use in these trials are derived from embryonic stem cells the company developed using our proprietary blastomere derivation techniques. Preliminary results for the first dry AMD and first SMD patient were first published on January 24, 2012 in the online version of the Lancet. See www.thelancet.com and doi:10.1016/S0140-6736(12)60028-2.
Hemangioblast Program. Hemangioblasts are a newly-characterized stem cell capable of differentiating into both hematopoietic, meaning blood cell-forming, and angiogenic, meaning blood vessel endothelium-forming, cells. We believe it will be possible to utilize hemangioblast cells to repair age-related endothelial dysfunction associated with numerous significant age-related diseases, including cardiovascular disease, stroke, and perhaps even cancer, as well as correct ischemic conditions, such as peripheral ischemia associated with diabetes. In 2006 we successfully derived hemangioblast cells generated from the company's blastomere-derived hESC lines. In 2007, we published data reporting that through utilization of hemangioblast based therapy we generated function in vivo with respect to the repair of ischemic retinal vasculatures and restoration of blood flow in ischemic limbs. In addition, we also reported increased survival rates of animals suffering from myocardial infarction. The hemangioblast program is currently in preclinical development.
Adult Stem Cell Program
Our adult stem cell-based program is developing an autologous myoblast transplantation therapy delivered using a minimally invasive catheter injection system to restore cardiac function in patients with advanced heart disease. The key target for the therapy will be heart failure patients with New York Heart Association ("NYHA") scores Class II to IV. The company's therapy could also benefit patients supported on ventricular assistance devices and potential additional indications, such as acute myocardial infarction, peripheral artery disease, and non-cardiac tissue repair. Currently available treatment options for heart failure patients are inadequate and can only slow the progression of heart failure; none can halt or reverse the process. We believe our autologous myoblast transplantation therapy uses patented myoblast compositions for catheter delivery to the heart offering repair of the disease in heart failure patients and for those end-stage disease patients on ventricular assistance device support.
These indications represent a significant unmet medical need and hold significant potential for clinical approval.
Our transplantation therapy involves extraction through simple biopsy from a patient's thigh of myoblasts, which are non-embryonic, skeletal muscle stem cells, which can be expanded in culture and injected back into damaged and scarred regions of the heart. This therapy promotes repair of damaged cardiac tissue by autologous cells, thereby avoiding immune rejection as each patient receives their own cells. Skeletal muscle, unlike heart muscle, can repair itself after injury. Skeletal muscle contains immature myoblasts that can fuse with surrounding myoblasts or with damaged muscle fibers to regenerate contractile skeletal muscle. In experimental models, our researchers have demonstrated that skeletal myoblasts can be transplanted into an infarcted myocardium with the subsequent development of elongated, striated cells characteristic of both skeletal and cardiac muscle. Our Phase I clinical studies have demonstrated the efficacy of this therapy on a preliminary basis.
We have received FDA approval to proceed with our Phase II clinical trial, to evaluate the applications for myoblast transplantation in slowing and/or reversing the impact of heart failure.
| 7 |
Our Intellectual Property
Our research and development is supported by a broad intellectual property portfolio. We currently own or have exclusive licenses to over 35 patents and have over 170 patent applications pending worldwide in the field of regenerative medicine and stem cell therapy. In the past two years, the United States Patent and Trademark office has granted several of our patents covering the methods we use to derive and produce our RPE cell therapy product that is currently being used in ongoing clinical trials in the United States and United Kingdom. We also have non-exclusive rights to a portfolio of patents and patent applications that support our core intellectual property.
Our success will likely depend upon our ability to preserve our proprietary technologies and operate without infringing the proprietary rights of other parties. However, we may rely on certain proprietary technologies and know-how that are not patentable. We protect such proprietary information, in part, by the use of confidentiality agreements with our employees, consultants and certain of our contractors.
| 8 |
We maintain a disciplined patent policy and, when appropriate, seek patent protection for inventions in our core technologies and in ancillary technologies that support our core technologies or which we otherwise believe will provide us with a competitive advantage. We pursue this strategy by filing patent applications for discoveries we make, either alone or in collaboration with scientific collaborators and strategic partners, including with respect to our RPE cell therapy program and the methods we use to derive and produce our RPE cell therapy product. Typically, although not always, we file patent applications both in the United States and in select international markets. In addition, we plan to obtain licenses or options to acquire licenses to patent filings from other individuals and organizations that we anticipate could be useful in advancing our research, development and commercialization initiatives and our strategic business interests.
The following table identifies the issued patents we own or license that we believe currently support our products and technology platform.
Owned by Advanced Cell Technology, Inc.
|
Patent Number |
Country |
Filing Date |
Issue Date |
Expiration Date* |
Title | |||||
| 7838727 | United States | 11/4/2005 | 11/23/2010 | 3/29/2026 | DERIVATION OF EMBRYONIC STEM CELLS | |||||
| 7893315 | United States | 5/3/2007 | 2/22/2011 | 11/4/2025 | DERIVATION OF EMBRYONIC STEM CELLS AND EMBRYONIC-DERIVED CELLS | |||||
| 7736896 | United States | 7/20/2005 | 6/15/2010 | 1/11/2026 | MODALITIES FOR THE TREATMENT OF DEGENERATIVE DISEASES OF THE RETINA | |||||
| 516236 | New Zealand | 6/30/2000 | 4/7/2005 | 6/30/2020 | CYTOPLASMIC TRANSFER TO DE-DIFFERENTIATE RECIPIENT CELLS | |||||
| 2002322522 | Australia | 7/18/2002 | 5/17/2010 | 7/18/2022 | METHODS AND COMPOSITIONS FOR CELL THERAPY | |||||
| 6808704 | United States | 9/6/2000 | 10/26/2004 | 2/18/2021 | METHOD FOR GENERATING IMMUNE-COMPATIBLE CELLS AND TISSUES USING NUCLEAR TRANSFER TECHNIQUES | |||||
| 783162 | Australia | 9/6/2000 | 1/12/2006 | 9/6/2020 | METHOD FOR GENERATING IMMUNE-COMPATIBLE CELLS AND TISSUES USING NUCLEAR TRANSFER TECHNIQUES | |||||
| 265679 | Mexico | 9/6/2000 | 4/3/2009 | 9/6/2020 | METHOD FOR GENERATING IMMUNE-COMPATIBLE CELLS AND TISSUES USING NUCLEAR TRANSFER TECHNIQUES | |||||
| 536786 | New Zealand | 11/24/2004 | 1/11/2007 | 9/6/2020 | METHOD FOR GENERATING IMMUNE-COMPATIBLE CELLS AND TISSUES USING NUCLEAR TRANSFER TECHNIQUES | |||||
| 782385 | Australia | 10/13/2000 | 11/3/2005 | 10/13/2020 | METHODS OF PRODUCING DIFFENTIATED PROGENITOR CELLS AND LINEAGE-DEFECTIVE EMBRYONIC STEM CELLS | |||||
| 518191 | New Zealand | 10/13/2000 | 5/10/2004 | 10/13/2020 | METHODS OF PRODUCING DIFFENTIATED PROGENITOR CELLS AND LINEAGE-DEFECTIVE EMBRYONIC STEM CELLS |
| 9 |
| 531844 | New Zealand | 9/6/2000 | 12/8/2005 | 9/6/2020 | TELOMERE RESTORATION AND EXTENSION OF CELL LIFE-SPAN IN ANIMALS CLONED FROM SENESCENT SOMATIC CELLS | |||||
| 7910369 | United States | 8/24/2005 | 3/22/2011 | 10/10/2025 | NOVEL CULTURE SYSTEMS FOR EX VIVO DEVELOPMENT | |||||
| 7621606 | United States | 8/27/2002 | 11/24/2009 | 8/27/2022 | TRANS-DIFFERENTIATION AND RE-DIFFERENTIATION OF SOMATIC CELLS AND PRODUCTION OF CELLS FOR CELL THERAPIES | |||||
| 7794704 | United States | 1/24/2005 | 9/14/2010 | 1/11/2026 |
METHODS FOR PRODUCING ENRICHED POPULATIONS OF HUMAN RETINAL PIGMENT EPITHELIUM CELLS FOR TREATMENT OF RETINAL DEGENERATION | |||||
| 7795025 | United States | 7/21/2006 | 9/14/2010 | 1/11/2026 |
METHODS FOR PRODUCING ENRICHED POPULATIONS OF HUMAN RETINAL PIGMENT EPITHELIUM CELLS | |||||
| 2005207042 | Australia | 1/24/2005 | 12/23/2010 | 1/24/2025 | MODALITIES FOR THE TREATMEANT OF DEGENERATIVE DISEASES OF THE RETINA | |||||
| ZL200580007359.0 | China | 1/24/2005 | 6/29/2011 | 1/24/2025 | MODALITIES FOR THE TREATMEANT OF DEGENERATIVE DISEASES OF THE RETINA | |||||
| 548929 | New Zealand | 1/24/2005 | 2/25/2011 | 1/24/2025 | MODALITIES FOR THE TREATMEANT OF DEGENERATIVE DISEASES OF THE RETINA | |||||
| 7696404 | United States | 12/27/2002 | 4/13/2010 | 11/29/2020 | EMBRYONIC OR STEM-LIKE CELL LINES PRODUCED BY CROSS SPECIES NUCLEAR TRANSPLANTATION… | |||||
| ZL00818200.0 | China | 12/20/2000 | 10/18/2006 | 12/20/2020 | METHOD TO PRODUCE CLONED EMBRYOS AND ADULTS FROM CULTURED CELLS | |||||
| 519347 | New Zealand | 12/20/2000 | 11/11/2004 | 12/20/2020 | METHOD TO PRODUCE CLONED EMBRYOS AND ADULTS FROM CULTURED CELLS | |||||
| 8017393 | United States | 4/13/2007 | 9/13/2011 | 4/13/2026 | HEMANGIO-COLONY FORMING CELLS | |||||
| 5453366 | United States | 3/15/1993 | 9/26/1995 | 9/26/2012 | METHOD OF CLONING BOVINE EMBRYOS | |||||
| 5496720 | United States | 2/10/1993 | 3/5/1996 | 3/5/2013 | PARTHENOGENIC OOCYTE ACTIVATION | |||||
| 6194202 | United States | 3/4/1996 | 2/27/2001 | 2/10/2013 | PARTHENOGENIC OOCYTE ACTIVATION | |||||
| 6077710 | United States | 10/21/1998 | 6/20/2000 | 2/10/2013 | PARTHENOGENIC OOCYTE ACTIVATION | |||||
| 6680199 | United States | 5/22/2000 | 1/20/2004 | 2/10/2013 | IN VITRO ACTIVATION OF MAMMALIAN OOCYTES |
| 10 |
Owned by Advanced Cell Technology, Inc.'s wholly-owned subsidiary Mytogen, Inc.
|
Patent Number |
Country |
Filing Date |
Issue Date |
Expiration Date* |
Title | |||||
| 6673604 | United States | 7/24/2000 | 1/6/2004 | 7/24/2020 | MUSCLE CELLS AND THEIR USE IN CARDIAC REPAIR** | |||||
| 6432711 | United States | 11/1/1994 | 8/13/2002 | 8/13/2019 | EMBRYONIC STEM CELLS CAPABLE OF DIFFERENTIATING INTO DESIRED CELL LINES |
University of Massachusetts Exclusive License to Advanced Cell Technology, Inc.
|
Patent Number |
Country |
Filing Date |
Issue Date |
Expiration Date* |
Title | |||||
| 7951591 | United States | 2/27/2003 | 5/31/2011 | 7/31/2022 | GYNOGENETIC OR ANDROGENETIC PRODUCTION OF PLURIPOTENT CELLS AND CELL LINES, AND USE THEREOF TO PRODUCE DIFFERENTIATED CELLS AND TISSUES | |||||
| 782846 | Australia | 10/27/2000 | 12/15/2005 | 10/27/2020 | GYNOGENETIC OR ANDROGENETIC PRODUCTION OF PLURIPOTENT CELLS AND CELL LINES, AND USE THEREOF TO PRODUCE DIFFERENTIATED CELLS AND TISSUES | |||||
| ZL00816098.8 | China | 10/27/2000 | 2/6/2009 | 10/27/2020 | GYNOGENETIC OR ANDROGENETIC PRODUCTION OF PLURIPOTENT CELLS AND CELL LINES, AND USE THEREOF TO PRODUCE DIFFERENTIATED CELLS AND TISSUES | |||||
| 149175 | Israel | 10/27/2000 | 3/31/2011 | 10/27/2020 | GYNOGENETIC OR ANDROGENETIC PRODUCTION OF PLURIPOTENT CELLS AND CELL LINES, AND USE THEREOF TO PRODUCE DIFFERENTIATED CELLS AND TISSUES | |||||
| 518365 | New Zealand | 10/27/2000 | 8/12/2004 | 10/27/2020 | GYNOGENETIC OR ANDROGENETIC PRODUCTION OF PLURIPOTENT CELLS AND CELL LINES, AND USE THEREOF TO PRODUCE DIFFERENTIATED CELLS AND TISSUES |
* Actual patent expiration dates may differ from the dates listed herein including due to patent term adjustments pursuant to 35 U.S.C. § 154(b) and 37 C.F.R. §§ 1.702-1.705.
| 11 |
The fundamental consequence of patent expiration is that the invention covered by that patent will enter the public domain. However, the expiration of patent protection, or anticipated patent protection, for the bulk of our portfolio is not scheduled to begin for approximately ten to fifteen years. Due to the rapid pace of technology development in this field, and the volume of intellectual property we anticipate will be generated over the next decade, it is unlikely that the expiration of any existing patents or patent rights would have an adverse effect on our business. In addition, we continue to file new patent applications as refinements to our products are made and clinical results are generated. Due to our current stage of development, our existing patent portfolio is not currently supporting a marketed product, so we will not suffer from any reduction in product revenue from patent expiration. Any actual products that we develop are expected to be supported by intellectual property covered by granted patents or current patent applications that, if granted, would not expire for 20 years from the date first filed. For example, the granted United States patents covering our RPE cell therapy product do not begin to expire until 2025. Due to the early stage of our business, we differ from, for example, the pharmaceutical industry where the loss of a key significant patent can result in contemporaneous loss of products, programs or revenues. As our table demonstrates, our business is at the front end of the patent protection spectrum and is not expected to be significantly impacted in the near term by expiration of existing patents or patents issued in response to existing applications.
Research and License Agreements
Collaborative Agreements
On June 21, 2011, we entered into a definitive collaborative agreement with Roslin Cells LTD ("Roslin Cells") of Scotland. We will work together to establish a bank of Good Manufacturing Practice (GMP)-grade human embryonic stem cell (hESC) lines using our patented, proprietary "single-cell blastomere" technique for deriving hESC lines without destroying embryos. Stem cell lines from the resulting bank will be made available for both research and commercial purposes. Our agreement with Roslin Cells is intended to address a number of practical and ethical issues facing the field, and should make it easier for researchers to explore the enormous potential of this exciting science for the future benefit of patients.
Under the terms of the agreement, the hESC lines will be created and banked in compliance with the regulations of both the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Roslin Cells will be responsible for maintaining the banked hESC lines, and it is anticipated that the banked hESC lines can be ushered expeditiously from laboratory settings directly into clinical programs, thereby speeding translational research. Roslin Cells will promote access to the hESC lines to both academic and commercial entities, and will establish a straightforward license which should enable third parties to have a predictable path to commercialization, at the time they choose to use the cells for discovery and preclinical research. We will continue to control any licenses to commercialization of products for the eye. We will share proceeds from these licenses, including milestone and royalty payments with Roslin Cells.
Licenses of Intellectual Property to Us
The following summarizes technology licensed to us. None of our technology that we use in our current clinical programs use any licensed technology.
UMass License - On February 1, 2002 and April 16, 1996, we entered into exclusive license agreements (indefinite license period) with the University of Massachusetts. The 1996 Agreement has been amended by amendments dated September 1, 1997, May 31, 2000 and September 19, 2002. Pursuant to these agreements, the University of Massachusetts, referred to as UMass, exclusively licensed to us certain biological materials, patent rights and related technology for commercialization in specified fields. The license agreements require us to use diligent efforts to develop licensed products and licensed services and require us to pay certain royalties, minimum annual royalties, milestone payments and sublicense income to UMass. UMass received 73,263 shares of common stock of ACT as partial consideration of the license granted. In 2008, we fell behind on our payments of all UMass license fees and as such faced termination of the UMass license agreements. In April 27, 2011, we executed an Amendment of Exclusive License Agreements with UMass under which the outstanding license payments were brought current through payment to UMass of cash and stock. As part of the amendment, UMass agreed that the underlying exclusive license to the Company was considered to be in continual full force and effect since its original execution date.
| 12 |
2002 License - Under the 2002 license, UMass licenses to us certain patent rights relating to the cloning of non-human animals for use in connection with the development, manufacture and sale of products and services in the field of non-human animals for agriculture, companion animals, research and diagnostic products, non-human and human therapeutics, and neutraceuticals, except production of immunoglobulin in the blood of Bos taurus and Bos indicus. We are required to pay royalties to UMass ranging from 1.5% to 2.0% based on the covered product or service. We agreed to pay minimum royalty payments of $15,000 on the first and second anniversary of the agreement, $20,000 on the third anniversary, $25,000 on the fourth anniversary, and increasing to $45,000 on the fifth anniversary and for each year thereafter. We also agreed to make milestone payments to UMass of up to $1,630,000 upon the achievement of various development and commercialization milestones. Finally, we have agreed to pay UMass 18% of all sublicense income.
1996 License - The 1996 license covers certain patent rights, biological materials and know-how related to the cloning of non-human animals and cells for use in cell fields except the production of immunoglobulin in the blood of Bos taurus and Bos indicus. We are required to pay royalties ranging from 2.5% to 4.5% on net sales of products and services covered by the license, and minimum royalty payments in the amount of $15,000 per year (beginning on the later of the fourth year after the effective date of the agreement or the completion of certain clinical trials) for net sales on products and services for use in human therapeutics, and $30,000 per year (beginning in the third year after the effective date of the agreement) for net sales on products and services for all uses other than in human therapeutics. UMass agreed to waive minimum royalty payments during any calendar year in which we fund research at UMass in the aggregate amount of $300,000. There are no milestone payments. We agreed to pay UMass 18% of all sublicense income except for equity. With respect to equity, we are required to pay UMass an amount equal to 10% of the total equity we receive for any transfer of rights under the 1996 license.
Both the 2002 agreement and the 1996 agreement, as amended, remain in effect until all issued patents within the patent rights licensed under the agreement have expired, or for a period ten years after the effective date of the agreement if no patents have issued within that ten-year period. Each party has the right to terminate the agreement upon the occurrence of a material uncured breach. We also have the right to terminate at any time for any reason with ninety days' written notice.
Wake Forest License - On January 26, 2001, we entered into a materials and research data license agreement with Wake Forest University (indefinite license period), pursuant to which WFU granted to us a worldwide, exclusive, royalty-free, perpetual and irrevocable right and license to use certain data and stem cells and stem cell cultures created by us from biological materials provided by WFU to us for specified purposes only. The agreement allows us to utilize certain primate skin cells and ovary materials produced by WFU and transferred to us pursuant to an agreement relating to the transfer of biological materials. There are no milestone payments. There are no royalty requirements unless we desire to negotiate a commercial license for use of the biological materials provided to us by WFU. WFU received 60,000 shares of common stock of ACT Group, Inc., a now dissolved Delaware corporation referred to hereinafter as ACT Group. We have agreed to provide WFU samples of stem cells for WFU's research, education and teaching purposes and we have a first option to obtain an exclusive license to any intellectual property rights claimed by WFU in connection with the use of such stem cells. The term of the license granted is perpetual and irrevocable absent a breach by us.
GenVec Agreement - On December 28, 2005, Mytogen and GenVec, Inc. entered into a patent assignment and security agreement (indefinite period). Under the agreement, as amended on July 31, 2007, GenVec assigned certain agreements and intellectual property to Mytogen, and retained a royalty-free non-exclusive license, with the right to grant sublicenses, to practice the intellectual property in connection with products, processes or services developed or provided by GenVec other than autologous and allogenic skeletal myoblasts for cardiac therapy. Under the original agreement, Mytogen granted a security interest in the assigned intellectual property, but the security interest was released in the amendment to the agreement. Under the agreement, as amended, Mytogen must use commercially reasonable efforts to commercialize the assigned intellectual property, including by spending specified amounts in support of research and development in support of such commercialization; Mytogen must pay GenVec one-half of the first milestone payment (anticipated to be two million U.S. dollars) received by Mytogen under the Terumo Agreement; and Mytogen must also pay GenVec four percent (4%) of the net sales revenue from sales or other provision of products, processes or services covered by the agreement.
| 13 |
Exclusive Licenses of Intellectual Property by Us
The following summarizes licenses from us to third parties.
Exeter Life Sciences License - On October 22, 2003, we entered into an exclusive license with Exeter Life Sciences, Inc. (indefinite license period), pursuant to which we exclusively licensed to Exeter certain technology and patent rights for use in the fields of agriculture, endangered species, companion animals and equine animals. The license also grants Exeter a right of first negotiation to any improvement patents that are obtained by us that relate to the licensed intellectual property or which are useful, necessary or required to develop or manufacture certain animals, cells or tissues within the defined fields of use.
Under the agreement, we license rights to certain patent rights and technology useful for the fields of use of non-human animals for agriculture, endangered animals and companion animals; excluding production of such animals for the primary purpose of producing human and non-human animal therapeutics and human healthcare products, including without limitation the production of biopharmaceutical agents in milk, such as proteins, peptides and polypeptides for pharmaceutical, neutraceutical or other use, and excluding the production of immunoglobulin in the blood of Bos taurus and Bos indicus.
Lifeline License - On May 14, 2004, we entered into three license agreements (indefinite license periods) with Lifeline Cell Technology, formerly known as PacGen Cellco, LLC; the licenses were subsequently amended in August 2005. Pursuant to the license agreements, as amended, we licensed to Lifeline, on an exclusive or non-exclusive basis, as applicable, certain know-how and patent rights for, among other things, the research, development, manufacture and sale of human cells for cell therapy in the treatment of human diabetes and liver diseases, and retinal diseases and retinal degenerative diseases. The license agreements require milestone payments up to $1.75 million in the aggregate. The agreement requires Lifeline to meet minimum research and development requirements. The licenses continue until expiration of the last valid claim within the licensed patent rights. Either party may terminate the agreements for an uncured breach, and Lifeline may terminate the agreement at any time with 30 days' notice.
Start Licensing License - On August 30, 2006, we entered into a Settlement and License Agreement with UMass and Start Licensing, Inc. (indefinite license period). See description of this agreement above. Pursuant to this agreement, we granted Start Licensing a worldwide, exclusive, fully paid-up and royalty-free license, with the right to grant sublicenses, to certain patent rights for use in connection with all uses and applications in non-human animals. The agreement was reached in connection with the settlement of the patent interference actions. The terms of the agreement also includes an initial payment to us, which has been made, and certain milestone payments. In addition, under the agreement, Start, Geron Corporation and Roslin Institute ("Roslin") each agree not to sue us under certain patent applications owned by Roslin.
Terumo Agreement - Diacrin, Inc. and Terumo Corporation entered into a development and license agreement on September 4, 2002 (indefinite license period); the agreement was transferred to Mytogen on December 28, 2005. Under the agreement, the parties agreed to collaborate to develop and commercialize products in the field described as autologous skeletal myoblasts for cardiac therapy (and conditionally allogenic skeletal myoblasts for cardiac therapy) in Japan and such other Asian countries as the parties may agree. This agreement is no longer in effect as of December 31, 2010.
Pharming Technologies B.V. License - On February 26, 2008, we entered into a License Agreement with Pharming Technologies B.V., referred to as Pharming, pursuant to which we exclusively licensed to Pharming certain patents including oocyte activation patents for all uses and applications in or related to non-human animals (indefinite license period). We retained all use and applications of such patents in or related to humans. This agreement is no longer in effect as of December 31, 2010.
Transition Holdings, Inc. - On December 18, 2008, we entered into a license agreement with an Ireland-based investor, Transition Holdings Inc. (“Transition”), for certain of our non-core technology (indefinite license period). This license was terminated effective February 9, 2011.
| 14 |
Stem Cell & Regenerative Medicine International, Inc. - On December 1, 2008, the Company and CHA Bio & Diostech Co., Ltd. (“CHA”), a leading Korean-based biotechnology company focused on the development of stem cell technologies, formed an international joint venture. The new company, Stem Cell & Regenerative Medicine International, Inc. (“SCRMI”), will develop human blood cells and other clinical therapies based on our Hemangioblast Program, one of our core technologies. SCRMI has agreed to pay the Company fee of $500,000 for an exclusive, worldwide, license to the Hemangioblast Program (indefinite license period). On July 21, 2011, the Company and CHA entered into a binding term sheet to restructure certain aspects of SCRMI. Under the terms of the binding Term Sheet, SCRMI exclusively licensed the rights to the hemangioblast program to ACT for North America (United States and Canada) and to CHA Biotech for Korea and Japan. Further, under the terms of the agreement, ten (10) SCRMI scientists involved in hemangioblast research have been reassigned to ACT. The ownership in SCMRI remains largely unchanged between ACT and CHA Biotech, with the joint venture ceasing internal research activity and transitioning to a licensing entity.
CHA – On March 31, 2009, we entered into a licensing agreement (indefinite license period) under which we have licensed our retinal pigment epithelium (“RPE”) technology, for the treatment of diseases of the eye, to CHA for development and commercialization exclusively in Korea. We are eligible to receive up to $1.9 million in fees based upon achieving certain milestones, including us making an IND submission to the US FDA to commence clinical trials in humans using the technology, which we currently plan to do during the second half of 2009. We received an up-front fee of $250,000 and additional consideration under the agreement in the amount of $850,000. Under the terms of the agreement, CHA will incur all of the cost associated with RPE clinical trials in Korea.
CHA – On May 21, 2009, we have entered into a licensing agreement (indefinite license period) under which we will license our proprietary single blastomere technology, which has the potential to generate stable cell lines, including retinal pigment epithelium (RPE) cells for the treatment of diseases of the eye, to CHA for development and commercialization exclusively in Korea. We received a $300,000 up-front license fee, and received an additional $300,000 in December 2009. We believe there are some 200 different retinal diseases that may be impacted by this stem cell derived therapy including macular degeneration. Age-related macular degeneration (AMD) affects more than 30 million people worldwide and is the leading cause of blindness in people over 60 years of age in the United States (Source: Foundation For Fighting Blindness).
Embroyme Sciences, Inc. – In 2008, we entered into a license agreement (indefinite license period) whereby we licensed to Embryome Sciences certain cell processing technologies, including the technology licensed from Kirin Beer. We received an up-front payment of $470,000 and will receive royalties from future sales of product that utilizes the technologies from the licenses.
Nonexclusive Licenses of Intellectual Property by Us
We have entered into numerous nonexclusive license agreements pursuant to which we have granted non-exclusive rights to various parties to use certain patent rights in defined fields. These licenses generally provide for commercialization of our intellectual property and typically contain minimum royalties, milestones and continuing royalties based upon percentages of revenue.
Regulations
In addition to safety regulations enforced by the FDA, we are also subject to regulations under the Occupational Safety and Health Act, the Environmental Protection Act, the Toxic Substances Control Act and other present and potential future and federal, state, local, and foreign regulations.
Outside the United States, we will be subject to regulations that govern the import of drug products from the United States or other manufacturing sites and foreign regulatory requirements governing human clinical trials and marketing approval for our products. The requirements governing the conduct of clinical trials, product licensing, pricing and reimbursements vary widely from country to country.
The United States Congress, several states and foreign countries have considered legislation banning or restricting human application of ES cell-based and nuclear transfer based technologies. No assurance can be given regarding future restrictions or prohibitions that might affect our technology and business. In addition, we cannot assure you that future judicial rulings with respect to nuclear transfer technology or human ES cells will not have the effect of delaying, limiting or preventing the use of nuclear transfer technology or ES cell-based technology or delaying, limiting or preventing the sale, manufacture or use of products or services derived from nuclear transfer technology or ES cell-derived material. Any such legislative or judicial development would harm our ability to generate revenues and operate profitably.
| 15 |
For additional information about governmental regulations that will affect our planned and intended business operations, see "RISK FACTORS " beginning below.
Employees
As of February 7, 2012, we had 30 full-time employees, of whom twelve hold Ph.D. or M.D. degrees. Eighteen employees are directly involved in research and development activities and twelve are engaged in business development and administration. We also use the services of numerous outside consultants in business and scientific matters. We believe that we have good relations with our employees and consultants.
Item 1A. RISK FACTORS
An investment in the Company’s common stock involves a high degree of risk. You should carefully consider the risks described below as well as other information provided to you in this prospectus, including information in the section of this document entitled “Forward Looking Statements.” The risks and uncertainties described below are not the only ones facing us. Additional risks and uncertainties not presently known to us or that we currently believe are immaterial may also impair our business operations. If any of the following risks actually occur, our business, financial condition or results of operations could be materially adversely affected, the value of our common stock could decline, and you may lose all or part of your investment.
Risks Relating to the Company’s Early Stage of Development
Our business is at an early stage of development and we may not develop therapeutic products that can be commercialized.
We do not yet have any product candidates in late-stage clinical trials or in the marketplace. Our potential therapeutic products will require extensive preclinical and clinical testing prior to regulatory approval in the United States and other countries. We may not be able to obtain regulatory approvals in some cases (see REGULATORY RISKS), or even enter clinical trials, for some of our products, or commercialize any products. Our therapeutic and product candidates may prove to have undesirable and unintended side effects or other characteristics adversely affecting their safety, efficacy or cost-effectiveness that could prevent or limit their use. Any product using any of our technology may fail to provide the intended therapeutic benefits, or achieve therapeutic benefits equal to or better than the standard of treatment at the time of testing or production. In addition, we will need to determine whether any of our potential products can be manufactured in commercial quantities or at an acceptable cost. Our efforts may not result in a product that can be or will be marketed successfully. Physicians may not prescribe our products, and patients or third party payors may not accept our products. For these reasons we may not be able to generate revenues from commercial production.
We have limited clinical testing, regulatory, manufacturing, marketing, distribution and sales capabilities which may limit our ability to generate revenues.
Due to the relatively early stage of our therapeutic products, regenerative medical therapies and stem cell therapy-based programs, we have not yet invested significantly in regulatory, manufacturing, marketing, distribution or product sales resources. We cannot assure you that we will be able to invest or develop any of these resources successfully or as expediently as necessary. The inability to do so may inhibit or harm our ability to generate revenues or operate profitably.
We have a history of operating losses and we may not achieve future revenues or operating profits.
We have generated modest revenue to date from our operations. Historically we have had net operating losses each year since our inception. As of December 31, 2011, we have an accumulated deficit of $253,805,438 and a stockholders’ deficit of $46,123,844. We incurred net losses of $72,795,119 and $54,373,332 for the years ended December 31, 2011 and 2010, respectively. We have limited current potential sources of income from licensing fees and the Company does not generate significant revenue outside of licensing non-core technologies. Additionally, even if we are able to commercialize our technologies or any products or services related to our technologies it is not certain that they will result in revenue or profitability.
| 16 |
We have a limited operating history on which investors may evaluate our operations and prospects for profitable operations.
If we continue to suffer losses as we have in the past, investors may not receive any return on their investment and perhaps their entire investment. Our prospects must be considered speculative in light of the risks, expenses and difficulties frequently encountered by companies in their early stages of development, particularly in light of the uncertainties relating to the new, competitive and rapidly evolving markets in which we anticipate we will operate. To attempt to address these risks, we must, among other things, further develop our technologies, products and services, successfully implement our research, development, marketing and commercialization strategies, respond to competitive developments and attract, retain and motivate qualified personnel. A substantial risk is involved in investing in us because, as an early stage company we have fewer resources than an established company, our management may be more likely to make mistakes at such an early stage, and we may be more vulnerable operationally and financially to any mistakes that may be made, as well as to external factors beyond our control.
Risks Relating to Technology
We are dependent on new and unproven technologies.
Our risks as an early stage company are compounded by our heavy dependence on emerging and sometimes unproven technologies. If these technologies do not produce satisfactory results, our business may be harmed. Additionally some of our technologies and significant potential revenue sources involve ethically sensitive and controversial issues which could become the subject of legislation or regulations that could materially restrict our operations and, therefore, harm our financial condition, operating results and prospects for bringing our investors a return on their investment.
Over the last two years we have narrowed our potential product pool to focusing on our Retinal Program as well as the applications of our iPS technology, which will limit our revenue sources.
Our human embryonic stem cell program includes research, preclinical and clinical products including two U.S. and one European phase I trials using our RPE cells; our myoblast program has received FDA clearance to proceed to Phase II human clinical trials; our Hemangioblast program is in the preclinical development stage, and the Company doesn’t foresee having a commercial product until clinical trials are completed. We have identified the programs that we are working to get into the clinical testing phase. We have narrowed the scope of our developmental focus to our Retinal Program and those related therapies, our blastomere program and, as part of our recently established partnership with CHA, developing products in the hemangioblast/immunology arena (see DESCRIPTION OF BUSINESS Section of prospectus). As a result of our narrower product focus we have fewer revenue sources. Our emphasis on fewer programs may hinder our business if these programs are not successful. Although our adult stem cell myoblast program has been approved for a Phase II clinical trial, we have suspended that program as we work to find a suitable development partner for the next phase of clinical trials. As a result of our emphasis on our eye programs and our hemangioblast programs, our ability to progress as a company is more significantly hinged on the success of fewer programs and thus, a setback or adverse development relating to any one of them could potentially have a significant impact on share price as well as an inhibitory effect on our ability to raise additional capital. We cannot guarantee that we will be able to successfully develop our retinal, hemangioblast, single blastomere, embryonic stem cell, iPS cell or myoblast technologies or that such development will result in products or services with any significant commercial utility. We anticipate that the commercial sale of such products or services, and royalty/licensing fees related to our technology, would be our primary sources of revenues. If we are unable to develop our technologies, investors will likely lose their entire investment in us.
We may not be able to commercially develop our technologies and proposed product lines, which, in turn, would significantly harm our ability to earn revenues and result in a loss of investment.
Our ability to commercially develop our technologies will be dictated in large part by forces outside our control which cannot be predicted, including, but not limited to, general economic conditions, the success of our research and pre-clinical and field testing, the availability of collaborative partners to finance our work in pursuing applications of cell therapy technologies and technological or other developments in the biomedical field which, due to efficiencies, technological breakthroughs or greater acceptance in the biomedical industry, may render one or more areas of commercialization more attractive, obsolete or competitively unattractive. It is possible that one or more areas of commercialization will not be pursued at all if a collaborative partner or entity willing to fund research and development cannot be located. Our decisions regarding the ultimate products and/or services we pursue could have a significant adverse effect on our ability to earn revenue if we misinterpret trends, underestimate development costs and/or pursue wrong products or services. Any of these factors either alone or in concert could materially harm our ability to earn revenues or could result in a loss of any investment in us.
| 17 |
If we are unable to keep up with rapid technological changes in our field or compete effectively, we will be unable to operate profitably.
We are engaged in activities in the biotechnology field, which is characterized by extensive research efforts and rapid technological progress. If we fail to anticipate or respond adequately to technological developments, our ability to operate profitably could suffer. We cannot assure you that research and discoveries by other biotechnology, agricultural, pharmaceutical or other companies will not render our technologies or potential products or services uneconomical or result in products superior to those we develop or that any technologies, products or services we develop will be preferred to any existing or newly-developed technologies, products or services.
Risks Related to Intellectual Property
Our business is highly dependent upon maintaining licenses with respect to key technology.
Several of the key patents we utilize are licensed to us by third parties. These licenses are subject to termination under certain circumstances (including, for example, our failure to make minimum royalty payments or to timely achieve spending, development and commercialization benchmarks). The loss of any of such licenses, or the conversion of such licenses to non-exclusive licenses, could harm our operations and/or enhance the prospects of our competitors.
Certain of these licenses also contain restrictions, such as limitations on our ability to grant sublicenses that could materially interfere with our ability to generate revenue through the licensing or sale to third parties of important and valuable technologies that we have, for strategic reasons, elected not to pursue directly. The possibility exists that in the future we will require further licenses to complete and/or commercialize our proposed products. We cannot assure you that we will be able to acquire any such licenses on a commercially viable basis.
Certain parts of our technology are not protectable by patent.
Certain parts of our know-how and technology are not patentable. To protect our proprietary position in such know-how and technology, we require all employees, consultants, advisors and collaborators to enter into confidentiality and invention ownership agreements with us. We cannot assure you, however, that these agreements will provide meaningful protection for our trade secrets, know-how or other proprietary information in the event of any unauthorized use or disclosure. Further, in the absence of patent protection, competitors who independently develop substantially equivalent technology may harm our business.
Patent litigation presents an ongoing threat to our business with respect to both outcomes and costs.
We have previously been involved in patent interference litigation, and it is possible that further litigation over patent matters with one or more competitors could arise. We could incur substantial litigation or interference costs in defending ourselves against suits brought against us or in suits in which we may assert our patents against others. If the outcome of any such litigation is unfavorable, our business could be materially adversely affected. To determine the priority of inventions, we may also have to participate in interference proceedings declared by the United States Patent and Trademark Office, which could result in substantial cost to us. Without additional capital, we may not have the resources to adequately defend or pursue this litigation.
We may not be able to protect our proprietary technology, which could harm our ability to operate profitably.
The biotechnology and pharmaceutical industries place considerable importance on obtaining patent and trade secret protection for new technologies, products and processes. Our success will depend, to a substantial degree, on our ability to obtain and enforce patent protection for our products, preserve any trade secrets and operate without infringing the proprietary rights of others. We cannot assure you that:
| · | we will succeed in obtaining any patents in a timely manner or at all, or that the breadth or degree of protection of any such patents will protect our interests, |
| 18 |
| · | the use of our technology will not infringe on the proprietary rights of others, |
| · | patent applications relating to our potential products or technologies will result in the issuance of any patents or that, if issued, such patents will afford adequate protection to us or not be challenged invalidated or infringed, and |
| · | patents will not issue to other parties, which may be infringed by our potential products or technologies. |
| · | we will continue to have the financial resources necessary to prosecute our existing patent applications, pay maintenance fees on patents and patent applications, or file patent applications on new inventions. |
We are aware of certain patents that have been granted to others and certain patent applications that have been filed by others with respect to iPS cells and embryonic stem cells, and other stem cell technologies. The fields in which we operate have been characterized by significant efforts by competitors to establish dominant or blocking patent rights to gain a competitive advantage, and by considerable differences of opinion as to the value and legal legitimacy of competitors' purported patent rights and the technologies they actually utilize in their businesses.
Patents obtained by other persons may result in infringement claims against us that are costly to defend and which may limit our ability to use the disputed technologies and prevent us from pursuing research and development or commercialization of potential products.
A number of other pharmaceutical, biotechnology and other companies, universities and research institutions have filed patent applications or have been issued patents relating to cell therapies, stem cells, and other technologies potentially relevant to or required by our expected products. We cannot predict which, if any, of such applications will issue as patents or the claims that might be allowed. We are aware that a number of companies have filed applications relating to the generation, formulation and uses of various stem cells. We are also aware of a number of patent applications and patents claiming use of stem cells and other modified cells to treat disease, disorder or injury.
If third party patents or patent applications contain claims infringed by either our licensed technology or other technology required to make and use our potential products and such claims are ultimately determined to be valid, there can be no assurance that we would be able to obtain licenses to these patents at a reasonable cost, if at all, or be able to develop or obtain alternative technology. If we are unable to obtain such licenses at a reasonable cost, we may not be able to develop some products commercially. We may be required to defend ourselves in court against allegations of infringement of third party patents. Patent litigation is very expensive and could consume substantial resources and create significant uncertainties. And adverse outcome in such a suit could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties, or require us to cease using such technology.
Compliance with some of our license agreements.
Maintaining certain of our license agreements (for in-licensed technology) requires that we pay annual maintenance fees and/or meet particular development or spending milestones. If we are unable to be in compliance with our license agreements, the license may be terminated and our business may be harmed.
We may not be able to adequately defend against piracy of intellectual property in foreign jurisdictions.
Considerable research in the areas of stem cells, cell therapeutics and regenerative medicine is being performed in countries outside of the United States, and a number of potential competitors are located in these countries. The laws protecting intellectual property in some of those countries may not provide adequate protection to prevent our competitors from misappropriating our intellectual property. Several of these potential competitors may be further along in the process of product development and also operate large, company-funded research and development programs. As a result, our competitors may develop more competitive or affordable products, or achieve earlier patent protection or product commercialization than we are able to achieve. Competitive products may render any products or product candidates that we develop obsolete.
| 19 |
Regulatory Risks
We cannot market our product candidates until we receive regulatory approval.
We must comply with extensive government regulations in order to obtain and maintain marketing approval for our products in the United States and abroad. The process of obtaining regulatory approval is lengthy, expensive and uncertain. In the United States, the FDA imposes substantial requirements on the introduction of biological products and many medical devices through lengthy and detailed laboratory and clinical testing procedures, sampling activities and other costly and time-consuming procedures. Satisfaction of these requirements typically takes several years and the time required to do so may vary substantially based upon the type and complexity of the biological product or medical device.
In addition, product candidates that we believe should be classified as medical devices for purposes of the FDA regulatory pathway may be determined by the FDA to be biologic products subject to the satisfaction of significantly more stringent requirements for FDA approval. Any difficulties that we encounter in obtaining regulatory approval may have a substantial adverse impact on our business and cause our stock price to significantly decline.
We cannot assure you that we will obtain FDA or foreign regulatory approval to market any of our product candidates for any indication in a timely manner or at all.
If we fail to obtain regulatory approval of any of our product candidates for at least one indication, we will not be permitted to market our product candidates and may be forced to cease our operations.
Even if some of our product candidates receive regulatory approval, these approvals may be subject to conditions, and we and our third party manufacturers will in any event be subject to significant ongoing regulatory obligations and oversight.
Even if any of our product candidates receives regulatory approval, the manufacturing, marketing and sale of our product candidates will be subject to stringent and ongoing government regulation. Conditions of approval, such as limiting the category of patients who can use the product, may significantly impact our ability to commercialize the product and may make it difficult or impossible for us to market a product profitably. Changes we may desire to make to an approved product, such as cell culturing changes or revised labeling, may require further regulatory review and approval, which could prevent us from updating or otherwise changing an approved product. If our product candidates are approved by the FDA or other regulatory authorities for the treatment of any indications, regulatory labeling may specify that our product candidates be used in conjunction with other therapies.
Once obtained, regulatory approvals may be withdrawn and can be expensive to maintain.
Regulatory approval may be withdrawn for a number of reasons, including the later discovery of previously unknown problems with the product. Regulatory approval may also require costly post-marketing follow-up studies, and failure of our product candidates to demonstrate sufficient efficacy and safety in these studies may result in either withdrawal of marketing approval or severe limitations on permitted product usage. In addition, numerous additional regulatory requirements relating to, among other processes, the labeling, packaging, adverse event reporting, storage, advertising, promotion and record-keeping will also apply. Furthermore, regulatory agencies subject a marketed product, its manufacturer and the manufacturer's facilities to continual review and periodic inspections. Compliance with these regulatory requirements are time consuming and require the expenditure of substantial resources.
If any of our product candidates is approved, we will be required to report certain adverse events involving our products to the FDA, to provide updated safety and efficacy information and to comply with requirements concerning the advertisement and promotional labeling of our products. As a result, even if we obtain necessary regulatory approvals to market our product candidates for any indication, any adverse results, circumstances or events that are subsequently discovered, could require that we cease marketing the product for that indication or expend money, time and effort to ensure full compliance, which could have a material adverse effect on our business.
If our products do not comply with applicable laws and regulations our business will be harmed.
Any failure by us, or by any third parties that may manufacture or market our products, to comply with the law, including statutes and regulations administered by the FDA or other U.S. or foreign regulatory authorities, could result in, among other things, warning letters, fines and other civil penalties, suspension of regulatory approvals and the resulting requirement that we suspend sales of our products, refusal to approve pending applications or supplements to approved applications, export or import restrictions, interruption of production, operating restrictions, closure of the facilities used by us or third parties to manufacture our product candidates, injunctions or criminal prosecution. Any of the foregoing actions could have a material adverse effect on our business.
| 20 |
Our products may not be accepted in the marketplace.
If we are successful in obtaining regulatory approval for any of our product candidates, the degree of market acceptance of those products will depend on many factors, including:
| · | Our ability to provide acceptable evidence and the perception of patients and the healthcare community, including third party payors, of the positive characteristics of our product candidates relative to existing treatment methods, including their safety, efficacy, cost effectiveness and/or other potential advantages, |
| · | The incidence and severity of any adverse side effects of our product candidates, |
| · | The availability of alternative treatments, |
| · | The labeling requirements imposed by the FDA and foreign regulatory agencies, including the scope of approved indications and any safety warnings, |
| · | Our ability to obtain sufficient third party insurance coverage or reimbursement for our products candidates, |
| · | The inclusion of our products on insurance company coverage policies, |
| · | The willingness and ability of patients and the healthcare community to adopt new technologies, |
| · | The procedure time associated with the use of our product candidates, |
| · | Our ability to manufacture or obtain from third party manufacturers sufficient quantities of our product candidates with acceptable quality and at an acceptable cost to meet demand, and |
| · | Marketing and distribution support for our products. |
We cannot predict or guarantee that physicians, patients, healthcare insurers, third party payors or health maintenance organizations, or the healthcare community in general, will accept or utilize any of our product candidates. Failure to achieve market acceptance would limit our ability to generate revenue and would have a material adverse effect on our business. In addition, if any of our product candidates achieve market acceptance, we may not be able to maintain that market acceptance over time if competing products or technologies are introduced that are received more favorably or are more cost-effective.
Risks Related to Domestic Governmental Regulation
Restrictions on the use of human embryonic stem cells, and the ethical, legal and social implications of that research, could prevent us from developing or gaining acceptance for commercially viable products in these areas.
Some of our most important programs involve the use of stem cells that are derived from human embryos. The use of human embryonic stem cells gives rise to ethical, legal and social issues regarding the appropriate derivation of these cells. In the event that our research related to human embryonic stem cells becomes the subject of adverse commentary or publicity, our business could be harmed or otherwise substantially impaired, and the market price for our common stock could be significantly harmed. Some political and religious groups have voiced opposition to our technology and practices. We use stem cells derived from human embryos that have been created for in vitro fertilization procedures but are no longer desired or suitable for that use and are donated with appropriate informed consent for research use. Many research institutions, including some of our scientific collaborators, have adopted policies regarding the ethical use of human embryonic tissue. These policies may have the effect of limiting the scope of research conducted using human embryonic stem cells, thereby impairing our ability to conduct research in this field.
| 21 |
Governmental regulations and laws could change.
There can be no assurance that our operations will not be restricted by any future legislative or administrative efforts by politicians or groups opposed to the development of hES cell technology or nuclear transfer technology. Additionally, the scope of the Dickey–Wicker Amendment, a 13-year-old ban on federal funding for activity related to the harm or destruction of an embryo, is under review by the Federal courts. Judicial review of this or other federal or state laws could result in a more restrictive interpretation of those laws than is previously the case, and may limit or require us to terminate certain of our research and therapeutic programs.
Because we or our collaborators must obtain regulatory approval to market our products in the United States and other countries, we cannot predict whether or when we will be permitted to commercialize our products.
Federal, state and local governments in the United States and governments in other countries have significant regulations in place that govern many of our activities. We are or may become subject to various federal, state and local laws, regulations and recommendations relating to safe working conditions, laboratory and manufacturing practices, the experimental use of animals and the use and disposal of hazardous or potentially hazardous substances used in connection with our research and development work. The preclinical testing and clinical trials of the products that we or our collaborators develop are subject to extensive government regulation that may prevent us from creating commercially viable products from our discoveries. In addition, the sale by us or our collaborators of any commercially viable product will be subject to government regulation from several standpoints, including manufacturing, advertising and promoting, selling and marketing, labeling, and distributing.
If, and to the extent that, we are unable to comply with these regulations, our ability to earn revenues will be materially and negatively impacted. The regulatory process, particularly in the biotechnology field, is uncertain, can take many years and requires the expenditure of substantial resources. Biological drugs and non-biological drugs are rigorously regulated. In particular, proposed human pharmaceutical therapeutic product candidates are subject to rigorous preclinical and clinical testing and other requirements by the FDA in the United States and similar health authorities in other countries in order to demonstrate safety and efficacy. We may never obtain regulatory approval to market our proposed products.
Our products may not receive FDA approval, which would prevent us from commercially marketing our products and producing revenues.
The FDA and comparable government agencies in foreign countries impose substantial regulations on the manufacture and marketing of pharmaceutical products through lengthy and detailed laboratory, pre-clinical and clinical testing procedures, sampling activities and other costly and time-consuming procedures. Satisfaction of these regulations typically takes several years or more and varies substantially based upon the type, complexity and novelty of the proposed product. We cannot assure you that FDA approvals for any products developed by us will be granted on a timely basis, if at all. Any such delay in obtaining, or failure to obtain, such approvals could have a material adverse effect on the marketing of our products and our ability to generate product revenue.
The government maintains certain rights in technology that we develop using government grant money and we may lose the revenues from such technology if we do not commercialize and utilize the technology pursuant to established government guidelines.
Certain of our and our licensors' research have been or are being funded in part by government grants. In connection with certain grants, the U.S. government retains rights in the technology developed with the grant. These rights could restrict our ability to fully capitalize upon the value of this research.
Risks Related to International Regulation
We may not be able to obtain required approvals in other countries.
The requirements governing the conduct of clinical trials and cell culturing and marketing of our product candidates outside the United States vary widely from country to country. Foreign approvals may take longer to obtain than FDA approvals and can require, among other things, additional testing and different clinical trial designs. Foreign regulatory approval processes generally include all of the risks associated with the FDA approval processes. Some foreign regulatory agencies also must approve prices of the products. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may negatively impact the regulatory process in others. We may not be able to file for regulatory approvals and may not receive necessary approvals to market our product candidates in any foreign country. If we fail to comply with these regulatory requirements or fail to obtain and maintain required approvals in any foreign country, we will not be able to sell our product candidates in that country and our ability to generate revenue will be adversely affected.
| 22 |
Financial Risks
We may not be able to raise the required capital to conduct our operations and develop and commercialize our products.
We require substantial additional capital resources in order to conduct our operations and develop and commercialize our products and run our facilities. We will need significant additional funds or a collaborative partner, or both, to finance the research and development activities of our therapies and potential products. Accordingly, we are continuing to pursue additional sources of financing. Our future capital requirements will depend upon many factors, including:
| · | The continued progress and cost of our research and development programs, |
| · | The progress with pre-clinical studies and clinical trials, |
| · | The time and costs involved in obtaining regulatory clearance, |
| · | The costs in preparing, filing, prosecuting, maintaining and enforcing patent claims, |
| · | The costs of developing sales, marketing and distribution channels and our ability to sell the therapies/products if developed, |
| · | The costs involved in establishing manufacturing capabilities for commercial quantities of our proposed products |
| · | Competing technological and market developments, |
| · | Market acceptance of our proposed products, |
| · | The costs for recruiting and retaining employees and consultants, and |
| · | The costs for educating and training physicians about our proposed therapies/products. |
Additional financing through strategic collaborations, public or private equity financings or other financing sources may not be available on acceptable terms, or at all. Additional equity financing could result in significant dilution to our shareholders. Further, if additional funds are obtained through arrangements with collaborative partners, these arrangements may require us to relinquish rights to some of our technologies, product candidates or products that we would otherwise seek to develop and commercialize on our own. If sufficient capital is not available, we may be required to delay, reduce the scope of or eliminate one or more of our programs or potential products, any of which could have a material adverse effect on our financial condition or business prospects.
Risks Relating to Our Debt Financings
There are a large number of shares underlying our debt in full, and warrants. The sale of these shares may depress the market price of our common stock.
As of December 31, 2011, on an aggregated basis our debt and preferred stock financings may result in being converted into 5,810,750 shares of our common stock, and outstanding warrants and options that may be converted into approximately 113,557,706 shares of our common stock.
Sales of a substantial number of shares of our common stock in the public market could adversely affect the market price for our common stock and make it more difficult for you to sell shares of our common stock at times and prices that you feel are appropriate.
Risks Related to Third Party Reliance
We depend on third parties to assist us in the conduct of our preclinical studies and clinical trials, and any failure of those parties to fulfill their obligations could result in costs and delays and prevent us from obtaining regulatory approval or successfully commercializing our product candidates on a timely basis, if at all.
| 23 |
We engage consultants and contract research organizations to help design, and to assist us in conducting, our preclinical studies and clinical trials and to collect and analyze data from those studies and trials. The consultants and contract research organizations we engage interact with clinical investigators to enroll patients in our clinical trials. As a result, we depend on these consultants and contract research organizations to perform the studies and trials in accordance with the investigational plan and protocol for each product candidate and in compliance with regulations and standards, commonly referred to as "good clinical practice", for conducting, recording and reporting results of clinical trials to assure that the data and results are credible and accurate and the trial participants are adequately protected, as required by the FDA and foreign regulatory agencies. We may face delays in our regulatory approval process if these parties do not perform their obligations in a timely or competent fashion or if we are forced to change service providers.
We depend on our collaborators to help us develop and test our proposed products, and our ability to develop and commercialize products may be impaired or delayed if collaborations are unsuccessful.
Our strategy for the development, clinical testing and commercialization of our proposed products requires that we enter into collaborations with corporate partners, licensors, licensees and others. We are dependent upon the subsequent success of these other parties in performing their respective responsibilities and the continued cooperation of our partners. Under agreements with collaborators, we may rely significantly on such collaborators to, among other things:
| · | Design and conduct advanced clinical trials in the event that we reach clinical trials; |
| · | Fund research and development activities with us; |
| · | Pay us fees upon the achievement of milestones; and |
| · | Market with us any commercial products that result from our collaborations. |
Our collaborators may not cooperate with us or perform their obligations under our agreements with them. We cannot control the amount and timing of our collaborators’ resources that will be devoted to our research and development activities related to our collaborative agreements with them. Our collaborators may choose to pursue existing or alternative technologies in preference to those being developed in collaboration with us.
The development and commercialization of potential products will be delayed if collaborators fail to conduct these activities in a timely manner, or at all. In addition, our collaborators could terminate their agreements with us and we may not receive any development or milestone payments.
If we do not achieve milestones set forth in the agreements, or if our collaborators breach or terminate their collaborative agreements with us, our business may be materially harmed.
Our reliance on the activities of our non-employee consultants, research institutions, and scientific contractors, whose activities are not wholly within our control, may lead to delays in development of our proposed products.
We rely extensively upon and have relationships with scientific consultants at academic and other institutions, some of whom conduct research at our request, and other consultants with expertise in clinical development strategy or other matters. These consultants are not our employees and may have commitments to, or consulting or advisory contracts with, other entities that may limit their availability to us. We have limited control over the activities of these consultants and, except as otherwise required by our collaboration and consulting agreements to the extent they exist, can expect only limited amounts of their time to be dedicated to our activities. These research facilities may have commitments to other commercial and non-commercial entities. We have limited control over the operations of these laboratories and can expect only limited amounts of time to be dedicated to our research goals.
Preclinical & Clinical Product Development Risks
We have limited experience in conducting and managing preclinical development activities, clinical trials and the application process necessary to obtain regulatory approvals.
Our limited experience in conducting and managing preclinical development activities, clinical trials and the application process necessary to obtain regulatory approvals might prevent us from successfully designing or implementing a preclinical study or clinical trial. If we do not succeed in conducting and managing our preclinical development activities or clinical trials, or in obtaining regulatory approvals, we might not be able to commercialize our product candidates, or might be significantly delayed in doing so, which will materially harm our business.
| 24 |
Our ability to generate revenues from any of our product candidates will depend on a number of factors, including our ability to successfully complete clinical trials, obtain necessary regulatory approvals and implement our commercialization strategy. In addition, even if we are successful in obtaining necessary regulatory approvals and bringing one or more product candidates to market, we will be subject to the risk that the marketplace will not accept those products. We may, and anticipate that we will need to, transition from a company with a research and development focus to a company capable of supporting commercial activities and we may not succeed in such a transition.
Because of the numerous risks and uncertainties associated with our product development and commercialization efforts, we are unable to predict the extent of our future losses or when or if we will become profitable.
Our failure to successfully commercialize our product candidates or to become and remain profitable could depress the market price of our common stock and impair our ability to raise capital, expand our business, diversify our product offerings and continue our operations.
None of the products that we are currently developing has been approved for marketing by the FDA or any similar regulatory authority in any foreign country. Our approach of using cell-based therapy for the treatment of Retinal disease (we are beginning with a treatment for Startgardt’s disease, for which we filed an IND with the FDA) is risky and unproven and no products using this approach have received regulatory approval in the United States or Europe.
We believe that no company has yet been successful in its efforts to obtain regulatory approval in the United States or Europe of a cell-based therapy product for the treatment of retinal disease or degeneration in humans. Cell-based therapy products, in general, may be susceptible to various risks, including undesirable and unintended side effects, unintended immune system responses, inadequate therapeutic efficacy or other characteristics that may prevent or limit their approval by regulators or commercial use. Many companies in the industry have suffered significant setbacks in advanced clinical trials, despite promising results in earlier trials. If our clinical trials are unsuccessful or significantly delayed, or if we do not complete our clinical trials, we will not receive regulatory approval for or be able to commercialize our product candidates.
Our lead product candidates, our therapeutic Retinal programs for Startgardt’s disease and Dry AMD have recently started Phase I Clinical Trials and have not yet received market approval from the FDA or any similar foreign regulatory authority for any indication.
We cannot market any product candidate until regulatory agencies grant approval or licensure. In order to obtain regulatory approval for the sale of any product candidate, we must, among other requirements, provide the FDA and similar foreign regulatory authorities with preclinical and clinical data that demonstrate to the satisfaction of regulatory authorities that our product candidates are safe and effective for each indication under the applicable standards relating to such product candidate. The preclinical studies and clinical trials of any product candidates must comply with the regulations of the FDA and other governmental authorities in the United States and similar agencies in other countries. Our therapeutic Retinal programs may never receive market approval from the FDA or any similar foreign regulatory authority.
We may experience numerous unforeseen events during, or even if approved for clinical trials, as a result of, the clinical trial process that could delay or prevent regulatory approval and/or commercialization of our product candidates, including the following:
| · | The FDA or similar foreign regulatory authorities may find that our product candidates are not sufficiently safe or effective or may find our cell culturing processes or facilities unsatisfactory, |
| · | Officials at the FDA or similar foreign regulatory authorities may interpret data from preclinical studies and clinical trials differently than we do, |
| · | Our clinical trials may produce negative or inconclusive results or may not meet the level of statistical significance required by the FDA or other regulatory authorities, and we may decide, or regulators may require us, to conduct additional preclinical studies and/or clinical trials or to abandon one or more of our development programs, |
| 25 |
| · | The FDA or similar foreign regulatory authorities may change their approval policies or adopt new regulations, |
| · | There may be delays or failure in obtaining approval of our clinical trial protocols from the FDA or other regulatory authorities or obtaining institutional review board approvals or government approvals to conduct or continue clinical trials at current or prospective sites, |
| · | We, or regulators, may suspend or terminate our clinical trials because the participating patients are being exposed to unacceptable health risks or undesirable side effects, |
| · | We may experience difficulties in managing multiple clinical sites, |
| · | Enrollment in our clinical trials for our product candidates may occur more slowly than we anticipate, or we may experience high drop-out rates of subjects in our clinical trials, resulting in significant delays, |
| · | We may be unable to manufacture or obtain from third party manufacturers sufficient quantities of our product candidates for use in clinical trials, and |
| · | Our product candidates may be deemed unsafe or ineffective, or may be perceived as being unsafe or ineffective, by healthcare providers for a particular indication. |
Any delay of regulatory approval will harm our business.
Risks Related to Competition
The market for therapeutic stem cell products is highly competitive.
We expect that our most significant competitors will be fully integrated pharmaceutical companies and more established biotechnology companies. These companies are developing stem cell-based products and they have significantly greater capital resources in research and development, manufacturing, testing, obtaining regulatory approvals, and marketing capabilities. Many of these potential competitors are further along in the process of product development and also operate large, company-funded research and development programs. As a result, our competitors may develop more competitive or affordable products, or achieve earlier patent recognition and filings.
The biotechnology industries are characterized by rapidly evolving technology and intense competition. Our competitors include major multinational pharmaceutical companies, specialty biotechnology companies and chemical and medical products companies operating in the fields of regenerative medicine, cell therapy, tissue engineering and tissue regeneration.
Many of these companies are well-established and possess technical, research and development, financial and sales and marketing resources significantly greater than ours. In addition, certain smaller biotech companies have formed strategic collaborations, partnerships and other types of joint ventures with larger, well established industry competitors that afford these companies' potential research and development and commercialization advantages. Academic institutions, governmental agencies and other public and private research organizations are also conducting and financing research activities which may produce products directly competitive to those we are developing. Moreover, many of these competitors may be able to obtain patent protection, obtain FDA and other regulatory approvals and begin commercial sales of their products before we do.
In the general area of cell-based therapies (including both allogeneic and autologous cell therapies), we compete with a variety of companies, most of whom are specialty biotechnology companies, such as, Genzyme Corporation, StemCells, Inc., Aastrom Biosciences, Inc., Viacell, Inc., Biotime, Inc., ISCO, MG Biotherapeutics, Pfizer, Celgene, BioHeart, Inc., Baxter Healthcare, Osiris Therapeutics and Cytori.
Each of these companies is well-established and have substantial technical and financial resources compared to us. However, as cell-based products are only just emerging as medical therapies, many of our direct competitors are smaller biotechnology and specialty medical products companies. These smaller companies may become significant competitors through rapid evolution of new technologies. Any of these companies could substantially strengthen their competitive position through strategic alliances or collaborative arrangements with large pharmaceutical or biotechnology companies.
| 26 |
The diseases and medical conditions we are targeting have no effective long-term therapies. Nevertheless, we expect that our technologies and products will compete with a variety of therapeutic products and procedures offered by major pharmaceutical companies. Many pharmaceutical and biotechnology companies are investigating new drugs and therapeutic approaches for the same purposes, which may achieve new efficacy profiles, extend the therapeutic window for such products, alter the prognosis of these diseases, or prevent their onset.
We believe that our products, when and if successfully developed, will compete with these products principally on the basis of improved and extended efficacy and safety and their overall economic benefit to the health care system.
Competition for any stem cell products that we may develop may be in the form of existing and new drugs, other forms of cell transplantation, ablative and simulative procedures, and gene therapy. We believe that some of our competitors are also trying to develop stem and progenitor cell-based technologies. We expect that all of these products will compete with our potential stem cell products based on efficacy, safety, cost and intellectual property positions. We may also face competition from companies that have filed patent applications relating to the use of genetically modified cells to treat disease, disorder or injury. In the event our therapies should require the use of such genetically modified cells, we may be required to seek licenses from these competitors in order to commercialize certain of our proposed products, and such licenses may not be granted.
If we develop products that receive regulatory approval, they would then have to compete for market acceptance and market share. For certain of our potential products, an important success factor will be the timing of market introduction of competitive products. This timing will be a function of the relative speed with which we and our competitors can develop products, complete the clinical testing and approval processes, and supply commercial quantities of a product to market. These competitive products may also impact the timing of clinical testing and approval processes by limiting the number of clinical investigators and patients available to test our potential products.
Our competition includes both public and private organizations and collaborations among academic institutions and large pharmaceutical companies, most of which have significantly greater experience and financial resources than we do.
Private and public academic and research institutions also compete with us in the research and development of therapeutic products based on human embryonic and adult stem cell technologies. In the past several years, the pharmaceutical industry has selectively entered into collaborations with both public and private organizations to explore the possibilities that stem cell therapies may present for substantive breakthroughs in the fight against disease.
The biotechnology and pharmaceutical industries are characterized by intense competition. We compete against numerous companies, both domestic and foreign, many of which have substantially greater experience and financial and other resources than we have. Several such enterprises have initiated cell therapy research programs and/or efforts to treat the same diseases targeted by us.
Companies such as Pfizer, Genzyme Corporation, StemCells, Inc., Aastrom Biosciences, Inc. and Viacell, Inc., as well as others, many of which have substantially greater resources and experience in our fields than we do, are well situated to effectively compete with us. Any of the world's largest pharmaceutical companies represents a significant actual or potential competitor with vastly greater resources than ours. These companies hold licenses to genetic selection technologies and other technologies that are competitive with our technologies. These and other competitive enterprises have devoted, and will continue to devote, substantial resources to the development of technologies and products in competition with us.
Many of our competitors have significantly greater experience than we have in the development, pre-clinical testing and human clinical trials of biotechnology and pharmaceutical products, in obtaining FDA and other regulatory approvals of such products and in manufacturing and marketing such products.
| 27 |
Accordingly our competitors may succeed in obtaining FDA approval for products more rapidly or effectively than we can. Our competitors may also be the first to discover and obtain a valid patent to a particular stem cell technology which may effectively block all others from doing so. It will be important for us or our collaborators to be the first to discover any stem cell technology that we are seeking to discover. Failure to be the first could prevent us from commercializing all of our research and development affected by that discovery. Additionally, if we commence commercial sales of any products, we will also be competing with respect to manufacturing efficiency and sales and marketing capabilities, areas in which we have no experience.
General Risks Relating to Our Business
We are subject to litigation that will be costly to defend or pursue and uncertain in its outcome.
Our business may bring us into conflict with our licensees, licensors, or others with whom we have contractual or other business relationships, or with our competitors or others whose interests differ from ours. If we are unable to resolve those conflicts on terms that are satisfactory to all parties, we may become involved in litigation brought by or against us. That litigation is likely to be expensive and may require a significant amount of management's time and attention, at the expense of other aspects of our business. The outcome of litigation is always uncertain, and in some cases could include judgments against us that require us to pay damages, enjoin us from certain activities, or otherwise affect our legal or contractual rights, which could have a significant adverse effect on our business. See "LEGAL PROCEEDINGS" in this prospectus for a more complete discussion of currently pending litigation against the Company.
We may not be able to obtain third-party patient reimbursement or favorable product pricing, which would reduce our ability to operate profitably.
Our ability to successfully commercialize certain of our proposed products in the human therapeutic field may depend to a significant degree on patient reimbursement of the costs of such products and related treatments at acceptable levels from government authorities, private health insurers and other organizations, such as health maintenance organizations. We cannot assure you that reimbursement in the United States or foreign countries will be available for any products we may develop or, if available, will not be decreased in the future, or that reimbursement amounts will not reduce the demand for, or the price of, our products with a consequent harm to our business. We cannot predict what additional regulation or legislation relating to the health care industry or third-party coverage and reimbursement may be enacted in the future or what effect such regulation or legislation may have on our business. If additional regulations are overly onerous or expensive, or if health care related legislation makes our business more expensive or burdensome than originally anticipated, we may be forced to significantly downsize our business plans or completely abandon our business model.
Our products are likely to be expensive to manufacture, and they may not be profitable if we are unable to control the costs to manufacture them.
Our products are likely to be significantly more expensive to manufacture than most other drugs currently on the market today. Our present manufacturing processes produce modest quantities of product intended for use in our ongoing research activities, and we have not developed processes, procedures and capability to produce commercial volumes of product. We hope to substantially reduce manufacturing costs through process improvements, development of new science, increases in manufacturing scale and outsourcing to experienced manufacturers. If we are not able to make these or other improvements, and depending on the pricing of the product, our profit margins may be significantly less than that of most drugs on the market today. In addition, we may not be able to charge a high enough price for any cell therapy product we develop, even if they are safe and effective, to make a profit. If we are unable to realize significant profits from our potential product candidates, our business would be materially harmed.
Our current source of revenues depends on the stability and performance of our sub-licensees.
Our ability to collect royalties on product sales from our sub-licensees will depend on the financial and operational success of the companies operating under a sublicense. Revenues from those licensees will depend upon the financial and operational success of those third parties. We cannot assure you that these licensees will be successful in obtaining requisite financing or in developing and successfully marketing their products. These licensees may experience unanticipated obstacles including regulatory hurdles, and scientific or technical challenges, which could have the effect of reducing their ability to generate revenues and pay us royalties.
| 28 |
We depend on key personnel for our continued operations and future success, and a loss of certain key personnel could significantly hinder our ability to move forward with our business plan.
Because of the specialized nature of our business, we are highly dependent on our ability to identify, hire, train and retain highly qualified scientific and technical personnel for the research and development activities we conduct or sponsor. The loss of one or more certain key executive officers, or scientific officers, would be significantly detrimental to us. In addition, recruiting and retaining qualified scientific personnel to perform research and development work is critical to our success. Our anticipated growth and expansion into areas and activities requiring additional expertise, such as clinical testing, regulatory compliance, manufacturing and marketing, will require the addition of new management personnel and the development of additional expertise by existing management personnel. There is intense competition for qualified personnel in the areas of our present and planned activities, and there can be no assurance that we will be able to continue to attract and retain the qualified personnel necessary for the development of our business. The failure to attract and retain such personnel or to develop such expertise would adversely affect our business.
Our insurance policies may be inadequate and potentially expose us to unrecoverable risks.
Any significant insurance claims would have a material adverse effect on our business, financial condition and results of operations. Insurance availability, coverage terms and pricing continue to vary with market conditions. We endeavor to obtain appropriate insurance coverage for insurable risks that we identify, however, we may fail to correctly anticipate or quantify insurable risks, we may not be able to obtain appropriate insurance coverage, and insurers may not respond as we intend to cover insurable events that may occur. We have observed rapidly changing conditions in the insurance markets relating to nearly all areas of traditional corporate insurance. Such conditions have resulted in higher premium costs, higher policy deductibles, and lower coverage limits. For some risks, we may not have or maintain insurance coverage because of cost or availability.
We have limited product liability insurance, which may leave us vulnerable to future claims we will be unable to satisfy.
The testing, manufacturing, marketing and sale of human therapeutic products entail an inherent risk of product liability claims, and we cannot assure you that substantial product liability claims will not be asserted against us. We have limited product liability insurance. In the event we are forced to expend significant funds on defending product liability actions, and in the event those funds come from operating capital, we will be required to reduce our business activities, which could lead to significant losses. We cannot assure you that adequate insurance coverage will be available in the future on acceptable terms, if at all, or that, if available, we will be able to maintain any such insurance at sufficient levels of coverage or that any such insurance will provide adequate protection against potential liabilities. Whether or not a product liability insurance policy is maintained in the future, any product liability claim could harm our business or financial condition.
We presently have members of management and other key employees located in various locations throughout the country which adds complexities to the operation of the business.
Presently, we have members of management and other key employees located in both California and Massachusetts, which adds complexities to the operation of our business.
We face risks related to compliance with corporate governance laws and financial reporting standards.
The Sarbanes-Oxley Act of 2002, as well as related new rules and regulations implemented by the Securities and Exchange Commission and the Public Company Accounting Oversight Board, require changes in the corporate governance practices and financial reporting standards for public companies. These new laws, rules and regulations, including compliance with Section 404 of the Sarbanes-Oxley Act of 2002 relating to internal control over financial reporting, referred to as Section 404, have materially increased our legal and financial compliance costs and made some activities more time-consuming and more burdensome.
| 29 |
Risks Relating to Our Common Stock
Stock prices for biotechnology companies have historically tended to be very volatile.
Stock prices and trading volumes for many biotechnology companies fluctuate widely for a number of reasons, including but not limited to the following factors, some of which may be unrelated to their businesses or results of operations:
| · | Clinical trial results, |
| · | The amount of cash resources and ability to obtain additional funding, |
| · | Announcements of research activities, business developments, technological innovations or new products by companies or their competitors, |
| · | Entering into or terminating strategic relationships, |
| · | Changes in government regulation, |
| · | Disputes concerning patents or proprietary rights, |
| · | Changes in revenues or expense levels, |
| · | Public concern regarding the safety, efficacy or other aspects of the products or methodologies being developed, |
| · | Reports by securities analysts, |
| · | Activities of various interest groups or organizations, |
| · | Media coverage, and |
| · | Status of the investment markets. |
This market volatility, as well as general domestic or international economic, market and political conditions, could materially and adversely affect the market price of our common stock and the return on your investment.
A significant number of shares of our common stock have become available for sale and their sale could depress the price of our common stock.
Substantially all of our common stock is freely tradeable in the equity markets.
We may also sell a substantial number of additional shares of our common stock in connection with a private placement or public offering of shares of our common stock (or other series or class of capital stock to be designated in the future). The terms of any such transactions would likely require us to register the resale of any shares of capital stock issued or issuable in the transaction.
Sales of a substantial number of shares of our common stock under any of the circumstances described above could adversely affect the market price for our common stock and make it more difficult for you to sell shares of our common stock at times and prices that you feel are appropriate.
We do not intend to pay cash dividends on our common stock in the foreseeable future.
Any payment of cash dividends will depend upon our financial condition, results of operations, capital requirements and other factors and will be at the discretion of our board of directors. We do not anticipate paying cash dividends on our common stock in the foreseeable future. Furthermore, we may incur additional indebtedness that may severely restrict or prohibit the payment of dividends.
| 30 |
Our common stock is subject to "penny stock" regulations and restrictions on initial and secondary broker-dealer sales.
The Securities and Exchange Commission (SEC) has adopted regulations which generally define "penny stock" to be any listed, trading equity security that has a market price less than $5.00 per share or an exercise price of less than $5.00 per share, subject to certain exemptions. Penny stocks are subject to certain additional oversight and regulatory requirements. Brokers and dealers affecting transactions in our common stock in many circumstances must obtain the written consent of a customer prior to purchasing our common stock, must obtain information from the customer and must provide disclosures to the customer. These requirements may restrict the ability of broker-dealers to sell our common stock and may affect your ability to sell your shares of our common stock in the secondary market.
As an issuer of “penny stock,” the protection provided by the federal securities laws relating to forward looking statements does not apply to us.
Although federal securities laws provide a safe harbor for forward-looking statements made by a public company that files reports under the federal securities laws, this safe harbor is not available to issuers of penny stocks. As a result, the Company will not have the benefit of this safe harbor protection in the event of any legal action based upon a claim that the material provided by the Company contained a material misstatement of fact or was misleading in any material respect because of the Company’s failure to include any statements necessary to make the statements not misleading. Such an action could hurt our financial condition.
Item 1B. Unresolved Staff Comments
None.
Item 2. Properties
Our headquarters are located in Marlboro, Massachusetts, where we lease approximately 12,257 square foot of office and laboratory facilities. The monthly rent for this property is $14,330. The lease term is from April 1, 2010 through June 30, 2015. We also lease approximately 700 square feet of corporate office space in Santa Monica, CA. The lease for our Santa Monica office terminates on February 28, 2013. The monthly rent for this space is $2,170.
Item 3. Legal Proceedings
On August 9, 2011, Advanced Cell Technology Inc. (the “Company”) entered into a settlement agreement and mutual release (the “Settlement Agreement”) with Midsummer Investment, Ltd. and Midsummer Small Cap Master, Ltd. (collectively, “Midsummer”).
Pursuant to the Settlement Agreement, upon tender by Midsummer to the Company of warrants held by Midsummer to purchase a total of 20,319,731 shares of the Company’s common stock (the “Warrants”), and duly executed notices of exercise (deemed to occur upon execution of the Settlement Agreement), the Company, to settle errors involving warrant issuances to Midsummer, agreed to (i) deliver to Midsummer an aggregate of 36,000,000 shares of the Company’s common stock (the “Current Shares”), as an exercise of the Warrants in respect of a partial exercise of Warrants, (ii) undertake to issue 30,585,774 additional shares of the Company’s common stock (the “Future Shares”), as an exercise of the remainder of the Warrants within ten days of the date that the Company shall have sufficient authorized and unissued shares of Common Stock (“Authorized Share Increase”) which are not otherwise reserved for issuance for other purposes to enable the Company to issue all of the Future Shares and (iii) issue 3,058,577 shares of the Company’s common stock (the “Additional Future Shares”) for every calendar month elapsed between the date of delivery of the Current Shares and the date following delivery of the Future Shares. The Company and Midsummer provided mutual general releases. Advanced Cell Technology delivered to Midsummer a total of 30,585,774 future shares and 15,292,885 additional future shares for a total of 45,878,659 shares on January 31, 2012, in accordance with the August 9, 2011 Settlement Agreement.
In connection with the foregoing, the Company relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended, for transactions not involving a public offering.
On or about September 16, 2011, Alpha Capital Anstalt (“Alpha Capital”), a Liechtenstein corporation with its principal place of business in Vaduz, Liechtenstein (“plaintiff”), filed an action against the Company in the United States District Court for the Southern District of New York, Case No. 11 CIV 6458. Plaintiff alleges that it is or was a holder of various convertible notes and warrants issued by the Company, and that by reason of certain transactions between the Company and JMJ Financial, Inc. during 2010, the exercise and conversion prices in plaintiff’s convertible notes and warrants should have been reset. Plaintiff demands a preliminary and permanent injunction directing that the Company deliver to it at least 39,514,859 shares of its common stock, as well as monetary damages in an amount to be determined at trial.
| 31 |
On October 14, 2011, the United States District Court for the Southern District of New York entered an order granting plaintiff Alpha Capital’s motion for a preliminary injunction and preliminary declaratory relief in the lawsuit entitled Alpha Capital Anstalt v. Advanced Cell Technology, Inc., Case No. 11 CIV 6458 (S.D.N.Y. filed Sept. 16, 2011). In its motion, Alpha Capital sought an order directing the Company to deliver to it at least 39,514,859 shares of its common stock in accordance with the terms of its warrants and convertible promissory notes. The court's October 14, 2011 order directed the Company to hold in escrow 39,514,859 shares of its common stock pending the entry of a preliminary injunction, and directed Alpha Capital to submit a proposed form of order to the court by October 27, 2011. On November 1, 2011, we issued the 39,514,859 shares to Alpha Capital. On November 23, 2011, we answered Alpha Capital’s Complaint and asserted affirmative defenses. On December 12, 2011, Alpha and we submitted a Civil Case Management Plan and Scheduling Order and discovery has since commenced. Despite receiving the 39,514,859 shares on November 1, 2011 as a result of its preliminary injunction, Alpha Capital continues to seek damages against the Company. We intend to contest this case vigorously.
On October 17, 2011, Black Mountain Equities, Inc. (“BME”) filed its Complaint against us in the United States District Court for the Southern District of New York, Case No. 11 CIV 7305. On October 28, 2011, BME moved for preliminary declaratory relief and for a preliminary injunction directing us to deliver immediately at least 18,000,000 shares of our common stock to BME. On November 9, 2011, this preliminary injunction was granted and the court directed us to immediately deliver 18,000,000 shares of its common stock to BME and ordered BME to place all proceeds from the sale of our stock into an interest-earning client escrow account held by its counsel. On December 15, 2011, we answered BME’s initial Complaint and asserted counterclaims, disputing BME’s contention that it was owed 18,000,000 shares. On December 29, 2011, BME filed an Amended Complaint. On January 17, 2012, we answered the Amended Complaint and asserted revised counterclaims. Discovery will begin in this case shortly.
In its Amended Complaint, BME argues that it made a cashless exercise of warrants issued by us by delivering a Notice of Exercise, asking for 18,000,000 shares of our common stock, based on a reduced exercise price and increased warrant share amount. In its counterclaims, we argue that even assuming arguendo that the exercise price of the warrants should have been reset as a result of certain JMJ Financial, Inc. transactions, BME would still only be entitled to 7,331,445 shares. Based on this calculation, we argue that BME should return to us no less than 10,668,555 shares of the stock it received on November 15, 2011 pursuant to its preliminary injunction. We intend to contest this case vigorously.
The shares that we issued to BME were issued in reliance upon the exemption from registration set forth in Section 3(a)(9) of the Securities Act of 1933, as amended, as well as Section 4(2) of the Securities Act.
On December 7, 2011, we entered into settlement agreements with certain holders of convertible promissory notes and warrants that were issued between 2005 and 2010. The settlement agreements relate to claims that the holders may have against us regarding the assertion that the conversion price of the notes and the exercise price of the warrants should have been adjusted as a result of certain transactions between the Company and JMJ Financial, Inc. during 2010.
We have entered into settlement agreements with 41 holders of the notes and/or warrants. Not included in these settlements are 4 holders that could not be reached and 3 other holders in active litigation with the Company. Pursuant to the settlement agreements, we agreed to issue an aggregate of 239,601,630 shares of our common stock to the settling holders.
Because at the time of the settlement agreement we did not have a sufficient number of authorized but unissued shares of common stock to issue all of the shares of common stock pursuant to the settlement agreements, we agreed to seek approval from our stockholders to amend our certificate of incorporation to increase our authorized common stock to accommodate the shares of common stock we agreed to issue pursuant to the settlement agreements. This approval was obtained on January 24, 2012 and we amended our certificate of incorporation to increase our authorized stock on January 24, 2012.
Pursuant to the settlement agreements, we were required to issue the shares of our common stock to the settling holders within ten business following the date we amended our certificate of incorporation to increase our authorized shares of common stock. The settlement agreements include a mutual release of claims that is effective upon the delivery of the common stock. On January 31, 2012, the Company issued a total of 239,601,630 shares of common stock to settling holders pursuant to the December 7, 2011 settlement agreements.
| 32 |
On December 15, 2011, the United States District Court for the Southern District of New York entered an order granting plaintiff Cranshire Capital Master Fund, Ltd.’s (“Cranshire”) motion for a preliminary injunction in the lawsuit entitled Cranshire Capital Master Fund, Ltd. v. Advanced Cell Technology, Inc., Case No. 11 CIV 8755 (S.D.N.Y. filed December 1, 2011). Cranshire claims in its lawsuit that the exercise price of warrants that we issued to Cranshire between 2005 and 2010 should have been adjusted as a result of certain transactions between our company and JMJ Financial, Inc. during 2010. The court’s December 15, 2011 order directed us to deliver 10,730,265 shares of our common stock to Cranshire. The court’s determination of the number of shares was based on a conversion price of $0.0392 per share. We issued the 10,730,265 shares to Cranshire on December 16, 2011. On February 24, 2012, we entered into an agreement with Cranshire to settle all outstanding claims against our company. Pursuant to the agreement, we are to issue Cranshire (1) an additional 1,949,735 of common stock, (2) plus the quotient of (x) $276,000 divided by (y) 90% of the closing price of common stock on the trading day immediately preceding the entry of the court order. The estimated number of shares of common stock to be issued based on an $0.11 share price at February 24, 2011 is 4,737,614.
The shares that we issued to Cranshire were issued in reliance upon the exemption from registration set forth in Section 3(a)(9) of the Securities Act of 1933, as amended, as well as Section 4(2) of the Securities Act.
On October 13, 2011, CAMOFI Master LDC and CAMHZN Master LDC (the “CAMOFI Parties”) filed a Complaint, CAMOFI Master LDC, et al. v. Advanced Cell Technology, Inc., Index No. 652816-2011 (Supreme Court of New York). We answered the Complaint and asserted affirmative defenses on November 18, 2011. Discovery has commenced in this case. In their Complaint, the CAMOFI Parties argue that as a result of the transactions between us and JMJ Financial, Inc. Gemini Master Fund, Ltd. and Midsummer Investment, Ltd. respectively, the exercise prices in their Warrants and Debentures should have been reset. Consequently, the CAMOFI Parties argue that they have been denied the right to receive, in total, at least 130,795,594 shares of the Company’s common stock, which has allegedly resulted in losses to the CAMOFI Parties of at least $22,265,951. We intend to contest this case vigorously.
Two warrant holders filed substantively identical actions against ACT and Wilmington Trust, N.A., the Administrator with Will Annexed of the Estate of William Mackay Caldwell, IV, Deceased (“Caldwell”), in the United States District Court for the District of Massachusetts: Gary D. Aronson v. Advanced Cell Technology, Inc., et al., Case No.: 1:11-CV-11492-NMG, filed August 23, 2011; and John S. Gorton, as Trustee of the John S. Gorton Separate Property Trust, Dated 3/3/1993 v. Advanced Cell Technology, Inc., et al., Case No.: 1:11-CV-11515-NMG, filed August 25, 2011. Substantively identical Amended Complaints were then filed: in Aronson, on October 13, 2011; and in Gorton, on November 2, 2011. These Amended Complaints allege claims for federal securities fraud against ACT and Caldwell, and breach of contract against ACT, purportedly based on separate Warrants To Purchase Securities (the “Warrants”) executed by Plaintiffs and ACT in September 2005. Specifically, Plaintiffs allege that ACT, contrary to the terms of the Warrants, (1) issued Equity Units (as defined therein) to Gunnar Engstrom and William Woodward during the Warrants’ Pricing Period (May 1, 2005 to January 15, 2009) for less than the exercise price stated in the Warrants ($2.20 per share), thereby triggering an automatic reduction of the exercise price and a concomitant increase of the number of ACT shares purchasable under the Warrants; and (2) failed to notify Plaintiffs of the issuance of the Equity Units that purportedly triggered adjustments under the Warrants; and that ACT (3) made material misrepresentations or omissions of fact related thereto. After settlement negotiations failed to resolve these matters, and Defendants agreed to waive formal service of the Amended Complaints, ACT and Caldwell separately moved to dismiss both Plaintiffs’ Amended Complaints, arguing that: (1) Plaintiffs failed to allege any fraudulent misrepresentation or omission by ACT in connection with the Warrants and Plaintiffs failed to allege any actionable breach of the Warrants, for the simple reason that the complained-of issuances of Equity Units took place outside the Warrants’ Pricing Period; (2) even if Plaintiffs had properly alleged fraud, the Amended Complaints do not give rise to the strong inference of scienter needed to satisfy the rigorous pleading requirements of the Private Securities Litigation Reform Act, 15 U.S.C. § 78u-4; (3) Plaintiffs’ securities-fraud claims are barred by the two-year statute of limitations and five-year statute of repose applicable to securities-fraud claims, 28 U.S.C. § 1658(b)(1), (2); (4) Plaintiffs failed to allege reliance and loss causation, both necessary elements of any securities-fraud claim; and (5) Plaintiffs failed to allege a cognizable request for preliminary injunctive relief. The Defendants’ Motions to Dismiss are fully briefed in Aronson, and have been filed and served in the Gorton matter. Both Defendants requested oral argument in both cases, which are pending before Honorable Nathaniel M. Gorton, United States District Judge for the District of Massachusetts. District Judge Gorton has referred the Motions to Dismiss in both actions to Honorable Judith G. Dein, United States Magistrate Judge for the District of Massachusetts, for a report and recommendation. Oral arguments have not yet been scheduled.
| 33 |
Item 4. [Reserved]
Not applicable.
PART II
Item 5. Market for the Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information
Our common stock is quoted on the OTCBB under the symbol "ACTC.OB." For the periods indicated, the following table sets forth the high and low bid prices per share of our common stock. These prices represent inter-dealer quotations without retail markup, markdown, or commission and may not necessarily represent actual transactions.
| High | Low | |||||||
| Fiscal Year 2011 | Bid | Bid | ||||||
| First Quarter | $ | 0.26 | $ | 0.12 | ||||
| Second Quarter | $ | 0.21 | $ | 0.17 | ||||
| Third Quarter | $ | 0.19 | $ | 0.13 | ||||
| Fourth Quarter | $ | 0.16 | $ | 0.07 | ||||
| High | Low | |||||||
| Fiscal Year 2010 | Bid | Bid | ||||||
| First Quarter | $ | 0.12 | $ | 0.08 | ||||
| Second Quarter | $ | 0.10 | $ | 0.07 | ||||
| Third Quarter | $ | 0.09 | $ | 0.05 | ||||
| Fourth Quarter | $ | 0.27 | $ | 0.04 | ||||
Trades of our common stock are subject to Rule 15g-9 of the Securities and Exchange Commission, known as the Penny Stock Rule. This rule imposes requirements on broker/dealers who sell securities subject to the rule to persons other than established customers and accredited investors. For transactions covered by the rule, brokers/dealers must make a special suitability determination for purchasers of the securities and receive the purchaser's written agreement to the transaction prior to sale. The Securities and Exchange Commission also has rules that regulate broker/dealer practices in connection with transactions in "penny stocks." Penny stocks generally are equity securities with a price of less than $5.00, other than securities registered on certain national securities exchanges or quoted on the NASDAQ system, provided that current price and volume information with respect to transactions in that security is provided by the exchange or system. The Penny Stock Rules requires a broker/dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document prepared by the Commission that provides information about penny stocks and the nature and level of risks in the penny stock market. The broker/dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker/dealer and its salesperson in the transaction, and monthly account statements showing the market value of each penny stock held in the customer's account. The bid and offer quotations, and the broker/dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer's confirmation. These disclosure requirements have the effect of reducing the level of trading activity in the secondary market for our common stock. As a result of these rules, investors may find it difficult to sell their shares.
Stock Price Performance Graph
A five-year comparison of the performance of our common stock with a broad equity market index and a peer group is set forth below. The broad equity market index used is the Nasdaq Composite Index and the peer group is the Dow Jones U.S. Biotechnology Index. The below comparison assumes $100 was invested on January 1, 2006 and dividends are reinvested for all years ending December 31.
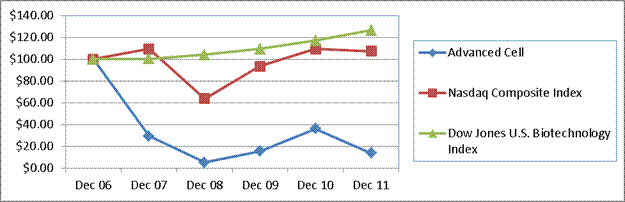
Holders
As of February 7, 2012, there were approximately 228 shareholders of record of our common stock.
Dividends
We have never paid cash dividends and have no plans to do so in the foreseeable future. Our future dividend policy will be determined by our board of directors and will depend upon a number of factors, including our financial condition and performance, our cash needs and expansion plans, income tax consequences, and the restrictions that applicable laws, our current preferred stock instruments, and our future credit arrangements may then impose.
| 34 |
Currently under Delaware law, unless further restricted in its certificate of incorporation, a corporation may declare and pay dividends out of surplus, or if no surplus exists, out of net profits for the fiscal year in which the dividend is declared and/or the preceding fiscal year (provided that the amount of capital of the corporation is not less than the aggregate amount of the capital represented by the issued and outstanding stock of all classes having a preference upon the distribution of assets).
Recent Sales of Unregistered Securities; Uses of Proceeds From Registered Securities
Recent Sales of Unregistered Securities
On November 2, 2011, we issued a board member 500,000 shares of common stock valued at $90,000 as compensation for board services. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
On November 2, 2011, we issued 39,514,859 shares to Alpha Capital as a result of a preliminary injunction from the court. The shares were recorded as finance costs and valued at $4,947,800. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
On November 14, 2011, we issued a board member 13,846 shares of common stock valued at $2,492 as compensation for board services. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
On November 15, 2011, we issued 18,000,000 shares to Black Mountain Equities as a result of a preliminary injunction from the court. The shares were recorded as finance costs and valued at $1,615,062. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
On December 15, 2011, we issued 24,615,385 shares of common stock in exchange for promissory notes of $2,000,000 and $400,000. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
On December 16, 2011, we issued 10,730,265 shares to Cranshire Capital Master Fund, Ltd. as a result of a preliminary injunction from the court. The shares were recorded as finance costs and valued at $1,073,027. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
On December 30, 2011, we issued various board members and an executive officer 2,550,000 shares of common stock valued at $207,667 as compensation for board services. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
During the year ended December 31, 2011, we issued 3,252,066 shares of common stock for the cashless exercise of 5,516,943 warrants not described above. The warrants were executed in accordance with their terms.
During the year ended December 31, 2011, we received $3,377,715 from the cash exercise of 34,225,302 warrants.
On January 30, 2012, the Company issued 48,878,659 shares of common stock to Midsummer based on the August 9, 2011 settlement agreement. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
| 35 |
On January 31, 2012, the Company issued 239,601,630 shares of common stock pursuant to the settlement agreement with certain holders of convertible promissory notes and warrants that were issued between 2005 and 2010. In connection with the foregoing, we relied on the exemption from registration provided by Section 4(2) of the Securities Act of 1933, as amended (the “Securities Act”) for transactions not involving a public offering.
Use of Proceeds from Registered Securities
Performance Graph
Item 6. Selected Financial Data
| For the Year Ended December 31, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (restated) | ||||||||||||||||||||
| Revenue | $ | 506,419 | $ | 725,044 | $ | 1,415,979 | $ | 787,106 | $ | 647,349 | ||||||||||
| Net loss | (72,795,119 | ) | (54,373,332 | ) | (36,758,208 | ) | (33,903,513 | ) | (15,898,725 | ) | ||||||||||
| Net loss per common share: | ||||||||||||||||||||
| Basic | $ | (0.05 | ) | $ | (0.04 | ) | $ | (0.07 | ) | $ | (0.14 | ) | $ | (0.26 | ) | |||||
| Diluted | $ | (0.05 | ) | $ | (0.04 | ) | $ | (0.07 | ) | $ | (0.14 | ) | $ | (0.26 | ) | |||||
| As of December 31, | ||||||||||||||||||||
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| (restated) | ||||||||||||||||||||
| Total assets | $ | 15,185,326 | $ | 19,054,152 | $ | 5,088,008 | $ | 2,577,778 | $ | 8,607,045 | ||||||||||
| Long-term debt: | ||||||||||||||||||||
| 2005 Convertible debenture and embedded derivatives, net of discounts | $ | - | $ | - | $ | - | $ | 85,997 | $ | 1,276,871 | ||||||||||
| 2006 Convertible debenture and embedded derivative, fair value | - | - | - | 1,993,354 | 3,047,491 | |||||||||||||||
| 2007 Convertible debenture and embedded derivatives, fair value | - | - | - | 7,706,344 | 3,482,542 | |||||||||||||||
| 2008 Convertible debenture and embedded derivatives, fair value | - | - | - | 4,066,505 | - | |||||||||||||||
| Convertible promissory notes and embedded derivatives, fair value | - | - | - | 1,757,470 | - | |||||||||||||||
| Amended and restated convertible debentures, net of discounts | - | - | 7,605,107 | - | - | |||||||||||||||
| Convertible promissory notes, net of discounts | - | 2,780 | 744,417 | - | - | |||||||||||||||
| 2009 Convertible promissory notes, net of discounts | 129,643 | 132,680 | 281,271 | - | - | |||||||||||||||
| Total Long-term debt | $ | 129,643 | $ | 135,460 | $ | 8,630,795 | $ | 15,609,670 | $ | 7,806,904 | ||||||||||
| Total liabilities | 59,880,044 | 41,434,801 | 50,262,896 | 38,506,762 | 30,133,775 | |||||||||||||||
| Redeemable preferred stock | $ | 1,429,126 | $ | 1,272,441 | $ | 908,195 | $ | - | $ | - | ||||||||||
| Toal stockholders' deficit | 46,123,844 | 23,653,090 | 46,083,083 | 35,928,984 | 21,526,730 | |||||||||||||||
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations
Certain statements in this annual report on Form 10-K that are not historical in fact constitute “forward-looking statements.” Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” and similar expressions are intended to identify forward-looking statements. Such forward-looking statements involve known and unknown risks, uncertainties and other factors based on the Company’s estimates and expectations concerning future events that may cause the actual results of the Company to be materially different from historical results or from any results expressed or implied by such forward-looking statements. These risks and uncertainties, as well as the Company’s critical accounting policies, are discussed in more detail under “Management’s Discussion and Analysis—Critical Accounting Policies” and in periodic filings with the Securities and Exchange Commission. The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future events or otherwise.
You should read the following discussion of our financial condition and results of operations together with the audited financial statements and the notes to the audited financial statements included in this annual report. This discussion contains forward-looking statements that reflect our plans, estimates and beliefs. Our actual results may differ materially from those anticipated in these forward-looking statements.
| 36 |
Executive Level Overview
We are a biotechnology company focused on developing and commercializing human stem cell technology in the emerging fields of regenerative medicine and stem cell therapy.
Critical Accounting Estimates
The discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make certain estimates and assumptions that affect the reported amounts of assets and liabilities, disclosure of any contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods. We regularly review our estimates and assumptions, which are based upon historical experience, as well as current economic conditions and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of certain assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates and assumptions.
We believe that the following critical accounting policies are affected by significant judgments and estimates used in the preparation of our consolidated financial statements.
Deferred Issuance Cost— Payments, either in cash or share-based payments, made in connection with the sale of debentures are recorded as deferred debt issuance costs and amortized using the effective interest method over the lives of the related debentures. The weighted average amortization period for deferred debt issuance costs is 48 months.
Fair Value Measurements — For certain financial instruments, including cash and cash equivalents, prepaid expenses, accounts payable, accrued expenses and notes payable, the carrying amounts approximate fair value due to their relatively short maturities.
On January 1, 2008, we adopted ASC 820-10, “Fair Value Measurements and Disclosures.” ASC 820-10 defines fair value, and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The carrying amounts reported in the consolidated balance sheets for receivables and current liabilities each qualify as financial instruments and are a reasonable estimate of their fair values because of the short period of time between the origination of such instruments and their expected realization and their current market rate of interest. The three levels of valuation hierarchy are defined as follows:
| · | Level 1 inputs to the valuation methodology are quoted prices for identical assets or liabilities in active markets. |
| · | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| · | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
We analyze all financial instruments with features of both liabilities and equity under ASC 480, “Distinguishing Liabilities From Equity” and ASC 815, “Derivatives and Hedging.” Derivative liabilities are adjusted to reflect fair value at each period end, with any increase or decrease in the fair value being recorded in results of operations as adjustments to fair value of derivatives. The effects of interactions between embedded derivatives are calculated and accounted for in arriving at the overall fair value of the financial instruments. In addition, the fair values of freestanding derivative instruments such as warrant and option derivatives are valued using the Black-Scholes model.
| 37 |
We did not identify any other non-recurring assets and liabilities that are required to be presented in the consolidated balance sheets at fair value in accordance with ASC 815.
Revenue Recognition— Our revenues are generated from license and research agreements with collaborators. Licensing revenue is recognized on a straight-line basis over the shorter of the life of the license or the estimated economic life of the patents related to the license. License fee revenue begins to be recognized in the first full month following the effective date of the license agreement. Deferred revenue represents the portion of the license and other payments received that has not been earned. Costs associated with the license revenue are deferred and recognized over the same term as the revenue. Reimbursements of research expense pursuant to grants are recorded in the period during which collection of the reimbursement becomes assured, because the reimbursements are subject to approval.
Stock Based Compensation— We record stock-based compensation in accordance with ASC 718, “Compensation – Stock Compensation.” ASC 718 requires companies to measure compensation cost for stock-based employee compensation at fair value at the grant date and recognize the expense over the employee’s requisite service period. We recognize in the statement of operations the grant-date fair value of stock options and other equity-based compensation issued to employees and non-employees.
RESULTS OF OPERATIONS
Comparison of the Years Ended December 31, 2011 and 2010
| 2011 | 2010 | |||||||||||||||
| % of | % of | |||||||||||||||
| Amount | Revenue | Amount | Revenue | |||||||||||||
| Revenue | $ | 506,419 | 100.0 | % | $ | 725,044 | 100.0 | % | ||||||||
| Cost of revenue | 343,950 | 67.9 | % | 216,600 | 29.9 | % | ||||||||||
| Gross profit | 162,469 | 32.1 | % | 508,444 | 70.1 | % | ||||||||||
| Research and development expenses | 10,021,863 | 1979.0 | % | 8,439,343 | 1164.0 | % | ||||||||||
| Grant reimbursements | (68,639 | ) | -13.6 | % | (977,917 | ) | -134.9 | % | ||||||||
| General and administrative expenses | 11,025,459 | 2177.1 | % | 15,506,191 | 2138.7 | % | ||||||||||
| Change in estimate of accrued liabilities | - | 0.0 | % | (1,263,009 | ) | -174.2 | % | |||||||||
| Loss on settlement of litigation | 294,144 | 58.1 | % | 11,132,467 | 1535.4 | % | ||||||||||
| Non-operating income (expense) | (51,684,761 | ) | -10205.9 | % | (22,044,701 | ) | -3040.5 | % | ||||||||
| Net loss | $ | (72,795,119 | ) | -14374.5 | % | $ | (54,373,332 | ) | -7499.3 | % | ||||||
Revenue
Revenue relates to license fees and royalties collected that are being amortized over the period of the license granted, and are therefore typically consistent between periods. The decrease in revenue during the year ended December 31, 2011, was due to license agreements that were terminated in 2011 that were recognized in 2010 revenue.
Research and Development Expenses and Grant Reimbursements
Research and development expenses (“R&D”) consists mainly of facility costs, payroll and payroll related expenses, research supplies and costs incurred in connection with specific research grants, and for scientific research. R&D expenditures increased from $8,439,343 in 2010 to $10,021,863 for 2011. The increase in R&D expenditures during the 2011 as compared to 2010 was primarily due to compensation increase of approximately $1,800,000, clinical trials increases of approximately $401,000, offset by decreases in legal expenses of approximately $325,000 and outside services of approximately $441,000.
| 38 |
Our research and development expenses consist primarily of costs associated with basic and pre-clinical research exclusively in the field of human stem cell therapies and regenerative medicine, with focus on development of our technologies in cellular reprogramming, reduced complexity applications, and stem cell differentiation. These expenses represent both pre-clinical development costs and costs associated with non-clinical support activities such as quality control and regulatory processes. The cost of our research and development personnel is the most significant category of expense; however, we also incur expenses with third parties, including license agreements, sponsored research programs and consulting expenses.
We do not segregate research and development costs by project because our research is focused exclusively on human stem cell therapies as a unitary field of study. Although we have three principal areas of focus for our research, these areas are completely intertwined and have not yet matured to the point where they are separate and distinct projects. The intellectual property, scientists and other resources dedicated to these efforts are not separately allocated to individual projects, since the research is conducted on an integrated basis.
We expect that research and development expenses will increase in the foreseeable future as we add personnel, expand our pre-clinical research, continue clinical trial activities, and increase our regulatory compliance capabilities. The amount of these increases is difficult to predict due to the uncertainty inherent in the timing and extent of progress in our research programs, and initiation of clinical trials. In addition, the results from our basic research and pre-clinical trials, as well as the results of trials of similar therapeutics under development by others, will influence the number, size and duration of planned and unplanned trials. As our research efforts mature, we will continue to review the direction of our research based on an assessment of the value of possible commercial applications emerging from these efforts. Based on this continuing review, we expect to establish discrete research programs and evaluate the cost and potential for cash inflows from commercializing products, partnering with others in the biotechnology or pharmaceutical industry, or licensing the technologies associated with these programs to third parties.
We believe that it is not possible at this stage to provide a meaningful estimate of the total cost to complete our ongoing projects and bring any proposed products to market. The use of human embryonic stem cells as a therapy is an emerging area of medicine, and it is not known what clinical trials will be required by the FDA in order to gain marketing approval. Costs to complete could vary substantially depending upon the projects selected for development, the number of clinical trials required and the number of patients needed for each study. It is possible that the completion of these studies could be delayed for a variety of reasons, including difficulties in enrolling patients, delays in manufacturing, incomplete or inconsistent data from the pre-clinical or clinical trials, and difficulties evaluating the trial results. Any delay in completion of a trial would increase the cost of that trial, which would harm our results of operations. Due to these uncertainties, we cannot reasonably estimate the size, nature nor timing of the costs to complete, or the amount or timing of the net cash inflows from our current activities. Until we obtain further relevant pre-clinical and clinical data, we will not be able to estimate our future expenses related to these programs or when, if ever, and to what extent we will receive cash inflows from resulting products.
General and Administrative Expenses
General and administrative expenses for 2011 compared to 2010 decreased by $4,480,732 to $11,025,459 in 2011. This expense decrease was primarily a result of decrease in compensation and stock issued for services from the prior year. During 2010, we issued shares of our stock to our Chief Executive Officer and directors and issued stock options to employees, for a total increase in G&A salaries, bonuses and option compensation of $10.8 million. During 2011, the compensation expense decreased by approximately $4,800,000. Our legal fees increased by approximately $321,000 due to the litigation surrounding the debenture and warrant holders of our 2005 through 2008 debentures.
| 39 |
Change in Estimate of Accrued Liabilities
In the year ended December 31, 2011 the Company did not recognize any gain or loss from the change in estimate of accrued liabilities. We recognized income of $1,263,009 related to reversals in our estimates of accrued liabilities during the year ended December 31, 2010. This amount relates to prior accrued liabilities where our estimate was adjusted based on new information as it became available. This amount has been separately classified in operating expenses in the accompanying consolidated statement of operations.
Loss on Settlement of Litigation
In 2010, we settled a lawsuit with an investor, whereby the Company delivered to the investor 49,220,665 shares of its common stock. Further, on September 30, 2010, under the terms of a final settlement and mutual release with the same investor, we exchanged a new convertible debenture to the investor in exchange for the investor’s outstanding convertible debenture. The terms of the new convertible debenture are the same as the amended and restated debentures, except that the amounts under the debenture are due and payable on or before December 31, 2010 and June 30, 2011. Concurrently with the settlement and release, all common stock purchase warrants previously issued to the investor were cancelled (23,701,263 warrants in total) and the legal actions were dismissed. We recorded a loss on settlement in the amount of $3,132,300 during the year ended December 31, 2010 in the accompanying statement of operations.
On December 22, 2010, Optimus CGII, Ltd. (“Optimus”) purchased a claim previously brought against the Company in a civil action by Alexandria Real Estate-79/96 Charlestown Navy Yard (“ARE”). In that action, ARE alleged that it was unable to relet the premises and therefore seeking rent for the vacated premises since September 2008. ARE also sought certain clean-up and storage expenses. On December 23, 2010, Optimus and the Company settled the claim in the amount of $8,000,167. During December 2010, we issued 55,688,368 shares of the Company’s common stock to Optimus in full settlement of this claim. Accordingly, we recognized loss on settlement in the amount of $8,000,167 in our accompanying consolidated statements of operations for the year ended December 31, 2010. This settlement ended all claims previously brought against the Company by ARE, and Optimus as bona fide claimant.
Other Income (Expense)
Other income (expense) consisted of the following:
| 2011 | 2010 | $ Change | % Change | |||||||||||||
| Interest income | 35,114 | 16,724 | 18,390 | 110 | % | |||||||||||
| Interest expense and late fees | (1,510,693 | ) | (11,726,120 | ) | 10,215,427 | -87 | % | |||||||||
| Finance cost | (60,834,170 | ) | (4,332,277 | ) | (56,501,893 | ) | 1304 | % | ||||||||
| Adjustments to fair value of derivatives | 11,444,988 | (6,209,898 | ) | 17,654,886 | -284 | % | ||||||||||
| Gain (loss) on disposal of fixed assets | - | 9,500 | (9,500 | ) | -100 | % | ||||||||||
| Gain on forgiveness of debt | - | 197,370 | (197,370 | ) | -100 | % | ||||||||||
| Losses attributable to equity method investment | (820,000 | ) | - | (820,000 | ) | 100 | % | |||||||||
| Total non-operating income (expense) | (51,684,761 | ) | (22,044,701 | ) | (29,640,060 | ) | ||||||||||
Interest expense decreased $10,215,427 due to the debentures that were redeemed during 2010. The average outstanding debt during 2010 was approximately $10,240,000 compared to 2011 of approximately $288,000.
Finance costs increased by $56,501,893 primarily due to the warrant and debenture settlements that occurred during the year. We have issued approximately 126.2 million shares related to settlements during 2011 and issued approximately 285.5 million shares on January 31, 2012 and February 7, 2012 which were accrued for as finance costs during the year ended December 31, 2011. We anticipate having to issue approximately an additional 135.5 million shares related to debenture settlements that were accrued for as finance costs at December 31, 2011.
| 40 |
Adjustment to fair value of derivatives changed from a loss of $6,209,898 in 2010 to a gain of $11,444,988 during 2011. The change of $17,654,886 is due to the fluctuation in our share price. At December 31, 2009 the share price was $0.09 and at December 31, 2010, the share price was $0.21. This increase in share price increased the derivative liability and we recorded a loss on the adjustment of the derivative liabilities. The share price at December 31, 2011 decreased from the December 31, 2010 share price of $0.21 to $0.08. This decrease in share price decreased the value of the derivative liability and we recorded a gain on the adjustment of the derivative liabilities.
Comparison of the Years Ended December 31, 2010 and 2009
| 2010 | 2009 | |||||||||||||||
| % of | % of | |||||||||||||||
| Amount | Revenue | Amount | Revenue | |||||||||||||
| Revenue | $ | 725,044 | 100.0 | % | $ | 1,415,979 | 195.3 | % | ||||||||
| Cost of revenue | 216,600 | 29.9 | % | 500,899 | 69.1 | % | ||||||||||
| Gross profit | 508,444 | 70.1 | % | 915,080 | 126.2 | % | ||||||||||
| Research and development expenses | 8,439,343 | 1164.0 | % | 3,531,540 | 487.1 | % | ||||||||||
| Grant reimbursements | (977,917 | ) | -134.9 | % | (136,840 | ) | -18.9 | % | ||||||||
| General and administrative expenses | 15,506,191 | 2138.7 | % | 3,439,085 | 474.3 | % | ||||||||||
| Change in estimate of accrued liabilities | (1,263,009 | ) | -174.2 | % | - | 0.0 | % | |||||||||
| Loss on settlement of litigation | 11,132,467 | 1535.4 | % | 4,903,949 | 676.4 | % | ||||||||||
| Non-operating income (expense) | (22,044,701 | ) | -3040.5 | % | (25,935,554 | ) | -3577.1 | % | ||||||||
| Net loss | $ | (54,373,332 | ) | -7499.3 | % | $ | (36,758,208 | ) | -5069.8 | % | ||||||
Revenue
Revenue relates to license fees and royalties collected that are being amortized over the period of the license granted, and are therefore typically consistent between periods. The decrease in revenue during the year ended December 31, 2010, was due to license agreements that were terminated in 2009 that were recognized in 2009 revenue. During 2009, we recognized approximately $382,000 in license fee revenue for licenses that were terminated in 2009. Further, we received $2,600,000 in license fees in 2009, and of that we recognized an additional $231,000 in license fee revenues during the year ended December 31, 2009.
Research and Development Expenses and Grant Reimbursements
Research and development expenses (“R&D”) consists mainly of facility costs, payroll and payroll related expenses, research supplies and costs incurred in connection with specific research grants, and for scientific research. R&D expenditures increased from $3,531,540 in 2009 to $8,439,343 for 2010. The increase in R&D expenditures during the 2010 as compared to 2009 because during 2010, the US Food and Drug Administration (“FDA”) cleared our Investigational New Drug (“IND”) application to immediately initiate a Phase I/II multicenter clinical trial using retinal cells derived from human embryonic stem cells (hESCs) to treat patients with Stargardt’s Macular Dystrophy (SMD), one of the most common forms of juvenile macular degeneration in the world. The decision removes the clinical hold that the FDA had placed on the trial. Stargardt’s Macular Dystrophy causes progressive vision loss, usually starting in children between 10 to 20 years of age. Eventually, blindness results from photoreceptor loss associated with degeneration in the pigmented layer of the retina, called the retinal pigment epithelium (RPE).
The Phase I/II trial will be a prospective, open-label study that is designed to determine the safety and tolerability of the RPE cells following sub-retinal transplantation to patients with advanced SMD. A total of twelve patients will be enrolled in the study at multiple clinical sites. The sites which are currently under consideration are the Jules Stein Eye Institute at UCLA (headed by Dr. Steven Schwartz); the Casey Eye Institute in Portland, Oregon (headed by Dr. Peter Francis of the Oregon Health Sciences University); the University of Massachusetts Memorial Medical Center in Worcester, Massachusetts (headed by Dr. Shalesh Kaushal, Chair of the Department of Ophthalmology); the UMDNJ – New Jersey Medical School in Newark, New Jersey (headed by Dr. Marco Zarbin, Chair, Institute of Ophthalmology and Visual Science); additional sites may be considered.
| 41 |
Further, in January 2011, the FDA cleared our IND application to treat Dry Age-Related Macular Degeneration (“AMD”) using retinal pigment epithelial (RPE) cells derived from human embryonic stem cells (hESCs). ACT is now permitted to initiate a Phase I/II multicenter clinical trial to treat patients with Dry AMD, the most common form of macular degeneration in the world. There are currently no treatments available for this prevalent disease of an aging global population. Dry AMD, representing a substantial global market opportunity and afflicts between 10-15 million Americans.
Our research and development expenses consist primarily of costs associated with basic and pre-clinical research exclusively in the field of human stem cell therapies and regenerative medicine, with focus on development of our technologies in cellular reprogramming, reduced complexity applications, and stem cell differentiation. These expenses represent both pre-clinical development costs and costs associated with non-clinical support activities such as quality control and regulatory processes. The cost of our research and development personnel is the most significant category of expense; however, we also incur expenses with third parties, including license agreements, sponsored research programs and consulting expenses.
We do not segregate research and development costs by project because our research is focused exclusively on human stem cell therapies as a unitary field of study. Although we have three principal areas of focus for our research, these areas are completely intertwined and have not yet matured to the point where they are separate and distinct projects. The intellectual property, scientists and other resources dedicated to these efforts are not separately allocated to individual projects, since the research is conducted on an integrated basis.
We expect that research and development expenses will increase in the foreseeable future as we add personnel, expand our pre-clinical research, continue clinical trial activities, and increase our regulatory compliance capabilities. The amount of these increases is difficult to predict due to the uncertainty inherent in the timing and extent of progress in our research programs, and initiation of clinical trials. In addition, the results from our basic research and pre-clinical trials, as well as the results of trials of similar therapeutics under development by others, will influence the number, size and duration of planned and unplanned trials. As our research efforts mature, we will continue to review the direction of our research based on an assessment of the value of possible commercial applications emerging from these efforts. Based on this continuing review, we expect to establish discrete research programs and evaluate the cost and potential for cash inflows from commercializing products, partnering with others in the biotechnology or pharmaceutical industry, or licensing the technologies associated with these programs to third parties.
We believe that it is not possible at this stage to provide a meaningful estimate of the total cost to complete our ongoing projects and bring any proposed products to market. The use of human embryonic stem cells as a therapy is an emerging area of medicine, and it is not known what clinical trials will be required by the FDA in order to gain marketing approval. Costs to complete could vary substantially depending upon the projects selected for development, the number of clinical trials required and the number of patients needed for each study. It is possible that the completion of these studies could be delayed for a variety of reasons, including difficulties in enrolling patients, delays in manufacturing, incomplete or inconsistent data from the pre-clinical or clinical trials, and difficulties evaluating the trial results. Any delay in completion of a trial would increase the cost of that trial, which would harm our results of operations. Due to these uncertainties, we cannot reasonably estimate the size, nature nor timing of the costs to complete, or the amount or timing of the net cash inflows from our current activities. Until we obtain further relevant pre-clinical and clinical data, we will not be able to estimate our future expenses related to these programs or when, if ever, and to what extent we will receive cash inflows from resulting products.
General and Administrative Expenses
General and administrative expenses for 2010 compared to 2009 increased by $12,067,106 to $15,506,191 in 2010. This expense increase was primarily a result of shares of our stock issued to our Chief Executive Officer and directors, and stock options issued to employees, for a total increase in G&A salaries, bonuses and option compensation of $10.8 million. Further, legal fees were higher in 2010 because we retained council to defend the Company in legal matters (see “Commitments and Contingencies” footnote to our accompanying consolidated financial statements, as well as the “Legal Proceedings” section filed in this Form 10-K).
Change in Estimate of Accrued Liabilities
We recognized income of $1,263,009 related to reversals in our estimates of accrued liabilities during the year ended December 31, 2010. This amount relates to prior accrued liabilities where our estimate was adjusted based on new information as it became available. This amount has been separately classified in operating expenses in the accompanying consolidated statement of operations.
| 42 |
Loss on Settlement of Litigation
In 2010, we settled a lawsuit with an investor, whereby the Company delivered to the investor 49,220,665 shares of its common stock. Further, on September 30, 2010, under the terms of a final settlement and mutual release with the same investor, we exchanged a new convertible debenture to the investor in exchange for the investor’s outstanding convertible debenture. The terms of the new convertible debenture are the same as the amended and restated debentures, except that the amounts under the debenture are due and payable on or before December 31, 2010 and June 30, 2011. Concurrently with the settlement and release, all common stock purchase warrants previously issued to the investor were cancelled (23,701,263 warrants in total) and the legal actions were dismissed. We recorded a loss on settlement in the amount of $3,132,300 during the year ended December 31, 2010 in its accompanying statement of operations.
On December 22, 2010, Optimus CGII, Ltd. (“Optimus”) purchased a claim previously brought against the Company in a civil action by Alexandria Real Estate-79/96 Charlestown Navy Yard (“ARE”). In that action, ARE alleged that it was unable to relet the premises and therefore seeking rent for the vacated premises since September 2008. ARE also sought certain clean-up and storage expenses. On December 23, 2010, Optimus and the Company settled the claim in the amount of $8,000,167. During December 2010, we issued 55,688,368 shares of the Company’s common stock to Optimus in full settlement of this claim. Accordingly, we recognized loss on settlement in the amount of $8,000,167 in our accompanying consolidated statements of operations for the year ended December 31, 2010. This settlement ended all claims previously brought against the Company by ARE, and Optimus as bona fide claimant.
In 2009, we settled $505,199 in accounts payable through the issuance of 39,380,847 shares of our common stock. We recorded a loss on settlement of $4,793,949 in our accompanying statements of operations for the year ended December 31, 2009.
On June 30, 2009, an investor submitted a conversion notice in the principal amount of $150,000 into 7,500,000 shares of common stock at $0.02 per share. At that time, we did not have sufficient authorized shares to satisfy this conversion notice. On July 6, 2009, by means of a settlement between the two parties, we agreed to deliver the 7,500,000 shares of our common stock no later than September 25, 2009. We delivered the 7,500,000 shares on September 25, 2009. Further, we agreed to provide the investor with an additional $110,000 principal, which is to be upon the same terms and conditions as the original 2008 debenture. Accordingly, we recognized a loss on settlement in the amount of $110,000 during the year ended December 31, 2009.
Other Income (Expense)
Other income (expense), net, for 2010 and 2009 was ($22,044,701) and ($25,935,554), respectively. The change of ($3,890,853) is primarily due to an increase of $2,626,586 in finance costs during 2010 and an increase in interest expense of $2,535,313. Adjustments to fair value of derivative liabilities during 2010 was ($6,209,898) compared to $23,103,668 in 2009. In periods when the share price increases, the derivative securities become more attractive to exercise or in-the-money, and therefore the value of the derivative liabilities increases. Additionally, in 2009, we recognized charges related to repricing derivative liabilities in the amount of ($30,316,708). These repricing charges were incurred in connection with the modification of our debt during 2009. We also recognized $8,200,984 in loss on extinguishment of convertible debentures and note, relating to the modification of our debt during 2009.
Interest expense including late fees was $11,726,120 and $9,190,807, for the years ended 2010 and 2009, respectively. The increase in interest expense of $2,535,313 is due to the additional debt that was issued in 2010. Further, the interest expense in 2010 was greater than in 2009 because we amortized remaining debt discounts on the 2005-2008 debentures. These debentures were repaid in full by December 31, 2010.
LIQUIDITY AND CAPITAL RESOURCES
Cash Flows
The following table sets forth a summary of our cash flows for the periods indicated below:
| 43 |
| Year Ended December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Net cash used in operating activities | $ | (13,627,287 | ) | $ | (8,782,932 | ) | $ | (5,142,778 | ) | |||
| Net cash used in investing activities | (36,830 | ) | (219,998 | ) | (7,538 | ) | ||||||
| Net cash provided by financing activities | 10,877,715 | 22,353,501 | 6,872,250 | |||||||||
| Net increase (decrease) in cash and cash equivalents | (2,786,402 | ) | 13,350,571 | 1,721,934 | ||||||||
| Cash and cash equivalents at the end of the period | $ | 13,103,007 | $ | 15,889,409 | $ | 2,538,838 | ||||||
Cash used in operating activities changed from $8,782,932 in 2010 to $13,627,287 in 2011. The change arose from changes in net income after adjusting for non-cash items, as well as less cash received from license agreements. Cash used in operating activities in 2009 was $5,142,778. The increase in cash used in operating activities from 2009 to 2010 is primarily attributable to the decrease in accrued interest during 2010, offset by income after adjusting for non-cash items as well as differences in cash received from license agreements and changes in our accounts payable.
Cash used in investing activities was $36,830, $219,998 and $7,538 in 2011, 2010 and 2009, respectively, consisting of property and equipment purchases.
Cash generated by financing activities in 2011, 2010 and 2009 arose from proceeds from new convertible debt and preferred stock that we successfully raised. We also received $3,377,715 in 2011 upon exercises of warrants.
As of December 31, 2011, we have $13,103,007 in cash, approximately $60 million in liabilities, and $42,342,877 in negative working capital. Of the $60 million in liabilities, approximately $50.9 million will be paid with common stock per the settlement agreements and approximately $4 million is related to derivative liabilities representing the fair value of our warrants, options and beneficial conversion options.
During 2011, we received the following amounts:
| · | $3.4 million as a result of cash exercises of warrants. |
| · | $7.5 million through the sale of our Series C preferred stock; |
We plan to fund our operations for the next twelve months primarily from the following financings:
| · | As of December 31, 2011, $1,581,834 is available to us upon the sale of our Series A-1 preferred stock for a maximum placement commitment of $5 million. |
| · | As of December 31, 2011, $13.5 million is available to us upon the sale of our Series C preferred stock for a maximum placement commitment of $25 million. |
| · | We continue to repay our debt financings in shares of common stock, enabling us to use our cash resources to fund our operations. |
On a longer term basis, we have no expectation of generating any meaningful revenues from our product candidates for a substantial period of time and will rely on raising funds in capital transactions to finance our research and development programs. Our future cash requirements will depend on many factors, including the pace and scope of our research and development programs, the costs involved in filing, prosecuting and enforcing patents, and other costs associated with commercializing our potential products. We intend to seek additional funding primarily through public or private financing transactions, and, to a lesser degree, new licensing or scientific collaborations, grants from governmental or other institutions, and other related transactions. If we are unable to raise additional funds, we will be forced to either scale back or business efforts or curtail our business activities entirely. We anticipate that our available cash and expected income will be sufficient to finance most of our current activities for at least twelve months from the date we file these financial statements, although certain of these activities and related personnel may need to be reduced. We cannot assure you that public or private financing or grants will be available on acceptable terms, if at all. Several factors will affect our ability to raise additional funding, including, but not limited to, the volatility of our common stock.
Contractual Obligations
At December 31, 2011, our significant contractual obligations were as follows:
| 44 |
| Less than | One to | Three to | More Than | |||||||||||||||||
| One Year | Three Years | Five Years | Five Years | Total | ||||||||||||||||
| Operating lease obligations | 195,340 | 342,940 | 84,650 | - | 622,930 | |||||||||||||||
| Convertible debt | - | 287,785 | - | - | 287,785 | |||||||||||||||
| Total | $ | 195,340 | $ | 630,725 | $ | 84,650 | $ | - | $ | 910,715 | ||||||||||
Off-Balance Sheet Arrangements
We do not maintain any off-balance sheet arrangements, transactions, obligations or other relationships with unconsolidated entities that would be expected to have a material current or future effect upon our financial condition or results of operations.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Our exposure to market risk is limited primarily to interest income sensitivity, which is affected by changes in the general level of U.S. interest rates, particularly because a significant portion of our investments are in short-term debt securities issued by the U.S. government and institutional money market funds. The primary objective of our investment activities is to preserve principal. Due to the nature of our marketable securities, we believe that we are not exposed to any material market risk. We do not have any derivative financial instruments or foreign currency instruments. If interest rates had varied by 10% in the year ended December 31, 2011, it would not have had a material effect on our results of operations or cash flows for that period.
| 45 |
Item 8. Financial Statements and Supplementary Data
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
Advanced Cell Technology, Inc. and subsidiary
We have audited the accompanying consolidated balance sheets of Advanced Cell Technology, Inc. and subsidiary as of December 31, 2011 and 2010, and the related consolidated statements of operations, stockholders’ deficit and cash flows for each of the three years in the period ended December 31, 2011. These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of Advanced Cell Technology, Inc. and subsidiary as of December 31, 2011 and 2010, and the results of their operations and their cash flows for each of the three years in the period ended December 31, 2011, in conformity with U.S. generally accepted accounting principles.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Advanced Cell Technology, Inc. and subsidiary’s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission, and our report dated March 1, 2012 expressed an unqualified opinion on the effectiveness of Advanced Cell Technology, Inc. and subsidiary’s internal control over financial reporting.
/s/ SingerLewak LLP
Los Angeles, California
March 1, 2012
| F-1 |
ADVANCED CELL TECHNOLOGY, INC. AND SUBSIDIARY
CONSOLIDATED BALANCE SHEETS
AS OF DECEMBER 31, 2011 AND 2010
| December 31, | December 31, | |||||||
| 2011 | 2010 | |||||||
| ASSETS | ||||||||
| CURRENT ASSETS: | ||||||||
| Cash and cash equivalents | $ | 13,103,007 | $ | 15,889,409 | ||||
| Deferred royalty fees, current portion | 62,435 | 91,598 | ||||||
| Prepaid expenses | 241,248 | - | ||||||
| Total current assets | 13,406,690 | 15,981,007 | ||||||
| Property and equipment, net | 154,771 | 185,102 | ||||||
| Deferred royalty fees, less current portion | 232,652 | 295,089 | ||||||
| Deposits | 14,766 | 14,766 | ||||||
| Deferred costs, net of amortization of $4,854,556 and $4,152,812, respectively | 1,376,447 | 2,578,188 | ||||||
| TOTAL ASSETS | $ | 15,185,326 | $ | 19,054,152 | ||||
| LIABILITIES AND STOCKHOLDERS' DEFICIT | ||||||||
| CURRENT LIABILITIES: | ||||||||
| Accounts payable | $ | 2,128,562 | $ | 1,982,743 | ||||
| Accrued expenses | 2,538,545 | 4,971,304 | ||||||
| Accrued settlement | 34,155,552 | 3,205,856 | ||||||
| Loss contingency accrual | 16,704,169 | - | ||||||
| Deferred revenue, current portion | 222,739 | 506,418 | ||||||
| 2009 Convertible promissory notes, current portion, net of discounts of $0 and $19,229, respectively | - | 132,680 | ||||||
| Embedded conversion option liabilities, current portion | - | 537,249 | ||||||
| Deferred joint venture obligations, current portion | - | 6,870 | ||||||
| Total current liabilities | 55,749,567 | 11,343,120 | ||||||
| Convertible promissory notes, less current portion, net of discounts of $158,142 and $122,463, respectively | 129,643 | 2,780 | ||||||
| Embedded conversion option liabilities, less current portion | 253,530 | 482,686 | ||||||
| Warrant and option derivative liabilities | 1,671,047 | 27,307,218 | ||||||
| Deferred revenue, less current portion | 2,076,257 | 2,298,997 | ||||||
| Total liabilities | 59,880,044 | 41,434,801 | ||||||
| Series A-1 redeemable preferred stock, $0.001 par value; 50,000,000 shares authorized, 113 abd 113 shares issued and outstanding; aggregate liquidation value, net of discounts: $1,472,262 and $1,349,657, respectively | 1,429,126 | 1,272,441 | ||||||
| Commitments and contingencies | ||||||||
| STOCKHOLDERS' DEFICIT: | ||||||||
| Preferred stock, Series B; $0.001 par value; 50,000,000 shares authorized, 1,000 and 1,000 shares issued and outstanding | 1 | 1 | ||||||
| Preferred stock, Series C; $0.001 par value; 50,000,000 shares authorized, 1,150 and 400 shares issued and outstanding | 1 | - | ||||||
| Common stock, $0.001par value; 1,750,000,000 shares authorized, 1,743,569,255, and 1,439,826,362 shares issued and outstanding | 1,743,569 | 1,439,826 | ||||||
| Additional paid-in capital | 229,319,208 | 166,033,976 | ||||||
| Promissory notes receivable, net of discount of $4,278,016 and $3,322,630, respectively | (23,381,185 | ) | (10,177,370 | ) | ||||
| Accumulated deficit | (253,805,438 | ) | (180,949,523 | ) | ||||
| Total stockholders' deficit | (46,123,844 | ) | (23,653,090 | ) | ||||
| TOTAL LIABILITIES AND STOCKHOLDERS' DEFICIT | $ | 15,185,326 | $ | 19,054,152 | ||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-2 |
ADVANCED CELL TECHNOLOGY, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF OPERATIONS
FOR THE YEARS ENDED DECEMBER 31, 2011, 2010 AND 2009
| 2011 | 2010 | 2009 | ||||||||||
| Revenue (License fees and royalties) | $ | 506,419 | $ | 725,044 | $ | 1,415,979 | ||||||
| Cost of Revenue | 343,950 | 216,600 | 500,899 | |||||||||
| Gross profit | 162,469 | 508,444 | 915,080 | |||||||||
| Operating expenses: | ||||||||||||
| Research and development | 10,021,863 | 8,439,343 | 3,531,540 | |||||||||
| Grant reimbursements | (68,639 | ) | (977,917 | ) | (136,840 | ) | ||||||
| General and administrative expenses | 11,025,459 | 15,506,191 | 3,439,085 | |||||||||
| Change in estimate of accrued liabilities | - | (1,263,009 | ) | - | ||||||||
| Loss on settlement of litigation | 294,144 | 11,132,467 | 4,903,949 | |||||||||
| Total operating expenses | 21,272,827 | 32,837,075 | 11,737,734 | |||||||||
| Loss from operations | (21,110,358 | ) | (32,328,631 | ) | (10,822,654 | ) | ||||||
| Non-operating income (expense): | ||||||||||||
| Interest income | 35,114 | 16,724 | 4,661 | |||||||||
| Interest expense and late fees | (1,510,693 | ) | (11,726,120 | ) | (9,190,807 | ) | ||||||
| Finance cost | (60,834,170 | ) | (4,332,277 | ) | (1,705,691 | ) | ||||||
| Adjustments to fair value of derivatives | 11,444,988 | (6,209,898 | ) | 23,103,668 | ||||||||
| Gain (loss) on disposal of fixed assets | - | 9,500 | - | |||||||||
| Gain on forgiveness of debt | - | 197,370 | 598,425 | |||||||||
| Loss on extinguishment of convertible debentures and note | - | - | (8,200,984 | ) | ||||||||
| Charges related to repricing derivative liabilities | - | - | (30,316,708 | ) | ||||||||
| Loss on warrant re-pricing | - | - | (83,680 | ) | ||||||||
| Losses attributable to equity method investment | (820,000 | ) | - | (144,438 | ) | |||||||
| Total non-operating income (expense) | (51,684,761 | ) | (22,044,701 | ) | (25,935,554 | ) | ||||||
| Loss before income tax | (72,795,119 | ) | (54,373,332 | ) | (36,758,208 | ) | ||||||
| Income tax | - | - | - | |||||||||
| Net loss | $ | (72,795,119 | ) | $ | (54,373,332 | ) | $ | (36,758,208 | ) | |||
| Weighted average shares outstanding : | ||||||||||||
| Basic and diluted | 1,582,095,095 | 1,218,190,921 | 521,343,094 | |||||||||
| Loss per share: | ||||||||||||
| Basic and diluted | $ | (0.05 | ) | $ | (0.04 | ) | $ | (0.07 | ) | |||
The accompanying notes are an integral part of these consolidated financial statements.
| F-3 |
ADVANCED CELL TECHNOLOGY, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF STOCKHOLDERS' DEFICIT
FOR THE YEARS ENDED DECEMBER 31, 2011, 2010 AND 2009
| Additional | Promissory | Total | ||||||||||||||||||||||||||||||||||||||
| Series B Preferred Stock | Series C Preferred Stock | Common Stock | Paid-in | Notes | Accumulated | Stockholders' | ||||||||||||||||||||||||||||||||||
| Shares | Amount | Shares | Amount | Shares | Amount | Capital | Receivable, net | Deficit | Deficit | |||||||||||||||||||||||||||||||
| Balance December 31, 2008 | - | $ | - | - | $ | - | 429,448,381 | $ | 429,448 | $ | 53,459,172 | $ | - | $ | (89,817,604 | ) | $ | (35,928,984 | ) | |||||||||||||||||||||
| Convertible debentures redemptions | - | - | - | - | 63,009,884 | 63,010 | 5,965,243 | - | 6,028,253 | |||||||||||||||||||||||||||||||
| Debt and preferred stock conversions | - | - | - | - | 104,412,687 | 104,413 | 9,299,147 | - | 9,403,560 | |||||||||||||||||||||||||||||||
| Option compensation charges | - | - | - | - | 817,444 | - | 817,444 | |||||||||||||||||||||||||||||||||
| Issuance of stock in settlement of accounts payable | - | - | - | - | 39,380,847 | 39,381 | 5,259,767 | - | 5,299,148 | |||||||||||||||||||||||||||||||
| Issuance of stock in payment of debt issue costs for preferred stock credit facility | - | - | - | - | 24,900,000 | 24,900 | 4,706,100 | - | 4,731,000 | |||||||||||||||||||||||||||||||
| Issuance of common stock for legal services | - | - | - | - | 375,000 | 375 | 37,875 | - | 38,250 | |||||||||||||||||||||||||||||||
| Issuance of common stock on cashless warrant exercise | - | - | - | - | 2,122,495 | 2,122 | 284,332 | - | 286,454 | |||||||||||||||||||||||||||||||
| Net loss for the year ended December 31, 2009 | - | - | - | - | (36,758,208 | ) | (36,758,208 | ) | ||||||||||||||||||||||||||||||||
| Balance December 31, 2009 | - | $ | - | - | $ | - | 663,649,294 | $ | 663,649 | $ | 79,829,080 | $ | - | $ | (126,575,812 | ) | $ | (46,083,083 | ) | |||||||||||||||||||||
| Redemptions of convertible debentures | - | - | - | - | 144,311,100 | 144,311 | 9,582,742 | - | - | 9,727,053 | ||||||||||||||||||||||||||||||
| Conversions of convertible debentures | - | - | - | - | 34,822,169 | 34,822 | 3,379,286 | - | - | 3,414,108 | ||||||||||||||||||||||||||||||
| Conversions of Series A-1 preferred stock | - | - | - | - | 6,206,961 | 6,207 | 614,489 | - | - | 620,696 | ||||||||||||||||||||||||||||||
| Conversions of amended convertible promissory notes | - | - | - | - | 211,916,152 | 211,916 | 9,545,273 | - | - | 9,757,189 | ||||||||||||||||||||||||||||||
| Common stock issued on exercise of warrants | - | - | - | - | 36,390,745 | 36,391 | 12,805,631 | - | - | 12,842,022 | ||||||||||||||||||||||||||||||
| Common stock issued to executives for compensation | - | - | - | - | 107,051,697 | 107,052 | 9,527,601 | - | - | 9,634,653 | ||||||||||||||||||||||||||||||
| Common stock issued to directors for board compensation | - | - | - | - | 16,773,597 | 16,774 | 1,543,439 | - | - | 1,560,213 | ||||||||||||||||||||||||||||||
| Common stock issued for settlements | - | - | - | - | 120,875,143 | 120,875 | 13,760,283 | - | - | 13,881,158 | ||||||||||||||||||||||||||||||
| Issuance of stock for financing costs | - | - | - | - | 1,959,142 | 1,959 | 396,552 | - | - | 398,511 | ||||||||||||||||||||||||||||||
| Issuance of Series B preferred stock | 1,000 | 1 | - | - | - | - | 9,999,999 | - | - | 10,000,000 | ||||||||||||||||||||||||||||||
| Common stock issued upon exercise of Series B preferred stock warrants | - | - | - | - | 95,870,362 | 95,870 | 9,884,893 | (9,980,763 | ) | - | - | |||||||||||||||||||||||||||||
| Dividends on Series B preferred stock | - | - | - | - | - | - | 196,986 | - | (196,986 | ) | - | |||||||||||||||||||||||||||||
| Issuance of Series C preferred stock | - | - | 400 | - | - | - | 4,000,000 | - | - | 4,000,000 | ||||||||||||||||||||||||||||||
| Accretion of note receivable discount | - | - | - | - | - | - | - | (196,607 | ) | 196,607 | - | |||||||||||||||||||||||||||||
| Option compensation charges | - | - | - | - | - | - | 967,722 | - | - | 967,722 | ||||||||||||||||||||||||||||||
| Net loss for year ended December 31, 2010 | - | - | - | - | - | - | - | - | (54,373,332 | ) | (54,373,332 | ) | ||||||||||||||||||||||||||||
| Balance December 31, 2010 | 1,000 | $ | 1 | 400 | $ | - | 1,439,826,362 | $ | 1,439,826 | $ | 166,033,976 | $ | (10,177,370 | ) | $ | (180,949,523 | ) | $ | (23,653,090 | ) | ||||||||||||||||||||
| Convertible debenture redemptions | - | - | - | - | 1,519,077 | 1,519 | 150,390 | - | - | 151,909 | ||||||||||||||||||||||||||||||
| Shares issued for compensation | - | - | - | - | 15,571,152 | 15,571 | 2,658,389 | - | - | 2,673,960 | ||||||||||||||||||||||||||||||
| Shares issued for accrued liabilities | - | - | - | - | 23,205,895 | 23,206 | 2,998,693 | - | - | 3,021,899 | ||||||||||||||||||||||||||||||
| Common stock issued for settlements recorded as financing costs | - | - | - | - | 133,645,953 | 133,646 | 22,029,270 | - | - | 22,162,916 | ||||||||||||||||||||||||||||||
| Warrant exercises | - | - | - | - | 37,477,368 | 37,478 | 10,246,139 | - | - | 10,283,617 | ||||||||||||||||||||||||||||||
| Option exercises | - | - | - | - | 1,386,126 | 1,386 | 196,276 | - | - | 197,662 | ||||||||||||||||||||||||||||||
| Shares issued for services | - | - | - | - | 2,381,406 | 2,381 | 473,519 | - | - | 475,900 | ||||||||||||||||||||||||||||||
| Accrued dividends on Series B and C Preferred Stock | - | - | - | - | - | - | 1,432,661 | - | (1,432,661 | ) | - | |||||||||||||||||||||||||||||
| Accretion of note receivable discount Series B and C Preferred Stock | - | - | - | - | - | - | - | (1,371,865 | ) | 1,371,865 | - | |||||||||||||||||||||||||||||
| Option compensation charges | - | - | - | - | - | - | 3,856,502 | - | - | 3,856,502 | ||||||||||||||||||||||||||||||
| Issuance of Series C preferred stock | - | - | 750 | 1 | - | - | 7,499,999 | - | - | 7,500,000 | ||||||||||||||||||||||||||||||
| Issuance of Common Stock to Series C Preferred Stock holder for note receivable | - | - | - | - | 73,796,597 | 73,797 | 9,786,161 | (9,859,958 | ) | - | - | |||||||||||||||||||||||||||||
| Common stock issued upon exercise of Series C Preferred Stock warrants and issuance of note receivable | - | - | - | - | 14,759,319 | 14,759 | 1,957,233 | (1,971,992 | ) | - | - | |||||||||||||||||||||||||||||
| Net loss for the year ended December 31, 2011 | - | - | - | - | - | - | - | - | (72,795,119 | ) | (72,795,119 | ) | ||||||||||||||||||||||||||||
| Balance December 31, 2011 | 1,000 | $ | 1 | 1,150 | $ | 1 | 1,743,569,255 | $ | 1,743,569 | $ | 229,319,208 | $ | (23,381,185 | ) | $ | (253,805,438 | ) | $ | (46,123,844 | ) | ||||||||||||||||||||
The accompanying notes are an integral part of these consolidated financial statements
| F-4 |
ADVANCED CELL TECHNOLOGY, INC. AND SUBSIDIARY
CONSOLIDATED STATEMENTS OF CASH FLOWS
FOR THE YEARS ENDED DECEMBER 31, 2011, 2010 AND 2009
| 2011 | 2010 | 2009 | ||||||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||||||
| Net loss | $ | (72,795,119 | ) | $ | (54,373,332 | ) | $ | (36,758,208 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: | ||||||||||||
| Depreciation | 67,161 | 138,050 | 291,472 | |||||||||
| Amortization of deferred charges | 960,224 | 91,600 | 363,399 | |||||||||
| Amortization of deferred revenue | - | (725,044 | ) | (1,415,979 | ) | |||||||
| Redeemable preferred stock dividend accrual | 122,605 | 95,883 | 123,609 | |||||||||
| Stock based compensation | 3,856,501 | 967,721 | 817,444 | |||||||||
| Amortization of deferred issuance costs | - | 617,568 | 3,535,245 | |||||||||
| Amortization of discounts | - | 12,443,112 | 4,134,693 | |||||||||
| Adjustments to fair value of derivatives | (11,444,988 | ) | 6,209,898 | (23,103,668 | ) | |||||||
| Shares of common stock issued for services | 475,900 | 11,194,866 | 38,250 | |||||||||
| Shares of common stock issued for compensation | 2,673,960 | 55,168 | - | |||||||||
| Non-cash financing costs | 60,834,170 | 3,375,745 | 1,704,535 | |||||||||
| Loss on settlement of litigation | 294,144 | 11,132,467 | 4,903,949 | |||||||||
| Gain on forgiveness of debt | - | (197,370 | ) | (598,425 | ) | |||||||
| (Gain) Loss on disposal of fixed assets | - | (9,500 | ) | - | ||||||||
| Amortization of deferred joint venture obligations | - | (56,602 | ) | (86,574 | ) | |||||||
| Loss on extinguishment of debt | - | - | 8,200,984 | |||||||||
| Charges related to repricing derivative liabilities | - | - | 30,316,708 | |||||||||
| Loss attributable to investment in joint venture | - | - | 144,438 | |||||||||
| Repricing of 2006 and 2007 convertible debentures and warrants | - | - | 83,680 | |||||||||
| Warrants issued for consulting services | 834,443 | - | 130,663 | |||||||||
| (Increase) / decrease in assets: | ||||||||||||
| Accounts receivable | - | - | 261,504 | |||||||||
| Prepaid expenses | (241,248 | ) | 9,054 | 23,422 | ||||||||
| Increase / (decrease) in current liabilities: | ||||||||||||
| Accounts payable and accrued expenses | 734,960 | 97,784 | (2,915,249 | ) | ||||||||
| Accrued interest | - | - | 1,311,330 | |||||||||
| Deferred revenue | - | 150,000 | 3,350,000 | |||||||||
| Net cash used in operating activities | (13,627,287 | ) | (8,782,932 | ) | (5,142,778 | ) | ||||||
| CASH FLOWS FROM INVESTING ACTIVITIES | ||||||||||||
| Purchases of property and equipment | (36,830 | ) | (207,402 | ) | (5,368 | ) | ||||||
| Payment of lease deposits | - | (12,596 | ) | (2,170 | ) | |||||||
| Net cash used in investing activities | (36,830 | ) | (219,998 | ) | (7,538 | ) | ||||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||||||
| Proceeds from exercise of warrants and options | 3,377,715 | 719,636 | - | |||||||||
| Proceeds from issuance of convertible debentures | - | 1,685,000 | - | |||||||||
| Proceeds from convertible promissory notes | - | 5,880,000 | 4,284,250 | |||||||||
| Proceeds from issuance of preferred stock | 7,500,000 | 14,068,865 | 2,588,000 | |||||||||
| Net cash provided by financing activities | 10,877,715 | 22,353,501 | 6,872,250 | |||||||||
| NET INCREASE (DECREASE) IN CASH AND CASH EQUIVALENTS | (2,786,402 | ) | 13,350,571 | 1,721,934 | ||||||||
| CASH AND CASH EQUIVALENTS, BEGINNING BALANCE | 15,889,409 | 2,538,838 | 816,904 | |||||||||
| CASH AND CASH EQUIVALENTS, ENDING BALANCE | $ | 13,103,007 | $ | 15,889,409 | $ | 2,538,838 | ||||||
| CASH PAID FOR: | ||||||||||||
| Interest | $ | - | $ | - | $ | - | ||||||
| Income taxes | $ | - | $ | 5,353 | $ | 970 | ||||||
| SUPPLEMENTAL DISCLOSURE OF NON-CASH FINANCING ACTIVITIES: | ||||||||||||
| Issuance of 1,519,077, 144,311,100 and 63,009,884 shares of common stock in redemption of debt | $ | 151,909 | $ | 9,727,053 | $ | 6,028,253 | ||||||
| Issuance of 0 , 246,738,321 and 87,739,641 shares of common stock in conversion of debt | $ | - | $ | 13,171,297 | $ | 7,736,256 | ||||||
| Issuance of 0, 6,206,961 and 16,673,046 shares of common stock in conversion of preferred stock | $ | - | $ | 620,696 | $ | 1,667,304 | ||||||
| Issuance of note receivable on issuance of shares and exercise of warrants for 88,555,916, 95,870,362, and 0 shares of common stock | $ | 13,800,000 | $ | 13,500,000 | $ | - | ||||||
| Record note receivable discount related to Series B and Series C preferred stock | $ | 1,968,050 | $ | 3,519,238 | $ | - | ||||||
| Issuance of 0 , 120,875,143 and 39,380,847 shares of common stock in settlement of litigation | $ | - | $ | 13,881,158 | $ | 5,299,148 | ||||||
| Issuance of 2,381,406, 0 and 375,000 shares of common stock for services | $ | 475,900 | $ | - | $ | 38,250 | ||||||
| Issuance of 2,571,152, 16,773,597, and 0 shares of common stock in payment of board fees | $ | 389,454 | $ | 1,560,213 | $ | - | ||||||
| Issuance of 13,000,000, 107,051,697, and 0 shares of common stock in payment of executive compensation | $ | 2,284,505 | $ | 9,634,653 | $ | - | ||||||
| Issuance of 0, 1,959,142 and 0 shares of common stock in payment of financing costs | $ | - | $ | 398,511 | $ | - | ||||||
| Series B and Series C preferred stock dividend | $ | 1,432,661 | $ | 196,986 | $ | - | ||||||
| Interest accreted on promissory notes receivable | $ | 1,371,865 | $ | 196,607 | $ | - | ||||||
| Issuance of 0, 0 and 24,900,000 shares of common stock for convertible preferred stock issuance costs | $ | - | $ | - | $ | 4,731,000 | ||||||
| Issuance of 3,252,066, 32,589,112 and 2,122,495 shares of common stock for cashless exercise of warrants | $ | 1,156,861 | $ | 12,188,685 | $ | 286,454 | ||||||
| Issuance of 126,232,953, 0, and 0 shares of common stock for debenture settlement | $ | 18,662,916 | $ | - | $ | - | ||||||
| Issuance of 636,126, 0 and 0 shares of common stock for exercise of options | $ | 160,162 | $ | - | $ | - | ||||||
| Issuance of 30,618,895, 0, and 0 shares of common stock for liabilities | $ | 6,521,899 | $ | - | $ | - | ||||||
The accompanying notes are an integral part of these consolidated financial statements.
| F-5 |
ADVANCED CELL TECHNOLOGY, INC. AND SUBSIDIARY
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
December 31, 2011, 2010 and 2009
| 1. | ORGANIZATIONAL MATTERS |
Organization and Nature of Business
Advanced Cell Technology, Inc. (the “Company”) is a biotechnology company, incorporated in the state of Delaware, focused on developing and commercializing human embryonic and adult stem cell technology in the emerging fields of regenerative medicine. Principal activities to date have included obtaining financing, securing operating facilities, and conducting research and development. The Company has no therapeutic products currently available for sale and does not expect to have any therapeutic products commercially available for sale for a period of years, if at all. These factors indicate that the Company’s ability to continue its research and development activities is dependent upon the ability of management to obtain additional financing as required.
| 2. | SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES |
Basis of Presentation —The Company follows accounting standards set by the Financial Accounting Standards Board (“FASB”). The accompanying consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“GAAP”). References to GAAP issued by the FASB in these footnotes are to the FASB Accounting Standards Codification,™ sometimes referred to as the Codification or ASC.
Principles of Consolidation — The accounts of the Company and its wholly-owned subsidiary Mytogen, Inc. (“Mytogen”) are included in the accompanying consolidated financial statements. All intercompany balances and transactions were eliminated in consolidation.
Segment Reporting —ASC 280, “Segment Reporting” requires use of the “management approach” model for segment reporting. The management approach model is based on the way a company’s management organizes segments within the company for making operating decisions and assessing performance. The Company determined it has one operating segment. Disaggregation of the Company’s operating results is impracticable, because the Company’s research and development activities and its assets overlap, and management reviews its business as a single operating segment. Thus, discrete financial information is not available by more than one operating segment.
Use of Estimates — These consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States and, accordingly, require management to make estimates and assumptions that affect the reported amounts of assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. Specifically, the Company’s management has estimated variables used to calculate the Black-Scholes option pricing model used to value derivative instruments as discussed below under “Fair Value Measurements”. In addition, management has estimated the expected economic life and value of the Company’s licensed technology, the Company’s net operating loss for tax purposes, share-based payments for compensation to employees, directors, consultants and investment banks, and the useful lives of the Company’s fixed assets and its accounts receivable allowance. Actual results could differ from those estimates.
Reclassifications — Certain prior year financial statement balances have been reclassified to conform to the current year presentation.
| F-6 |
Cash and Cash Equivalents — Cash equivalents are comprised of certain highly liquid investments with maturities of three months or less when purchased. The Company maintains its cash in bank deposit accounts, which at times, may exceed federally insured limits. The Company has not experienced any losses related to this concentration of risk. As of December 31, 2011 and December 31, 2010, the Company had deposits in excess of federally-insured limits totaling $12,037,949 and $15,399,150, respectively.
Accounts Receivable — The Company periodically assesses its accounts receivable for collectability on a specific identification basis. If collectability of an account becomes unlikely, the Company records an allowance for that doubtful account. Once the Company has exhausted efforts to collect, management writes off the account receivable against the allowance it has already created. The Company does not require collateral for its trade accounts receivable.
Property and Equipment — The Company records its property and equipment at historical cost. The Company expenses maintenance and repairs as incurred. Upon disposition of property and equipment, the gross cost and accumulated depreciation are written off and the difference between the proceeds and the net book value is recorded as a gain or loss on sale of assets. In the case of certain assets acquired under capital leases, the assets are recorded net of imputed interest, based upon the net present value of future payments. Assets under capital lease are pledged as collateral for the related lease.
The Company provides for depreciation over the assets’ estimated useful lives as follows:
| Machinery & equipment | 4 years | |
| Computer equipment | 3 years | |
| Office furniture | 4 years | |
| Leasehold improvements | Lesser of lease life or economic life | |
| Capital leases | Lesser of lease life or economic life |
Equity Method Investment — The Company follows ASC 323 “Investments-Equity Method and Joint Ventures” in accounting for its investment in the joint venture. In the event the Company’s share of the joint venture’s net losses reduces the Company’s investment to zero, the Company will discontinue applying the equity method and will not provide for additional losses unless the Company has guaranteed obligations of the joint venture or is otherwise committed to provide further financial support for the joint venture. If the joint venture subsequently reports net income, the Company will resume applying the equity method only after its share of that net income equals the share of net losses not recognized during the period the equity method was suspended.
Deferred Costs — Consists of the following:
| (a) | Payments, either in cash or share-based, made in connection with the sale of debentures which are amortized using the effective interest method over the lives of the related debentures. These deferred issuance costs are charged to financing costs when and if the related debt instrument is retired or converted early. The weighted average amortization period for deferred debt issuance costs is 48 months. |
| (b) | Payments made to secure commitments under certain financing arrangements. These amounts are recognized in financing costs ratably over the period of the financing arrangements, and are recognized in financing costs immediately if the arrangement is cancelled, forfeited or the utility of the arrangement to the company is otherwise compromised. |
| (c) | Payments made to financial institutions and consulting firms in order to provide financing related services. These costs are being amortized over the terms of the related agreements. |
Intangible and Long-Lived Assets— The Company follows ASC 360-10, “Property, Plant, and Equipment,” which established a “primary asset” approach to determine the cash flow estimation period for a group of assets and liabilities that represents the unit of accounting for a long-lived asset to be held and used. Long-lived assets to be held and used are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. The carrying amount of a long-lived asset is not recoverable if it exceeds the sum of the undiscounted cash flows expected to result from the use and eventual disposition of the asset. Long-lived assets to be disposed of are reported at the lower of carrying amount or fair value less cost to sell. Through December 31, 2011, the Company had not experienced impairment losses on its long-lived assets.
| F-7 |
Fair Value of Financial Instruments — For certain financial instruments, including cash and cash equivalents, prepaid expenses, accounts payable, accrued expenses and notes payable, the carrying amounts approximate fair value due to their relatively short maturities.
Fair Value Measurements — The Company applies the provisions of ASC 820-10, “Fair Value Measurements and Disclosures.” ASC 820-10 defines fair value, and establishes a three-level valuation hierarchy for disclosures of fair value measurement that enhances disclosure requirements for fair value measures. The carrying amounts reported in the consolidated balance sheets for cash and cash equivalents and current liabilities each qualify as financial instruments and are a reasonable estimate of their fair values because of the short period of time between the origination of such instruments and their expected realization and their current market rate of interest. The three levels of valuation hierarchy are defined as follows:
| · | Level 1 inputs to the valuation methodology are quoted prices for identical assets or liabilities in active markets. |
| · | Level 2 inputs to the valuation methodology include quoted prices for similar assets and liabilities in active markets, and inputs that are observable for the asset or liability, either directly or indirectly, for substantially the full term of the financial instrument. |
| · | Level 3 inputs to the valuation methodology are unobservable and significant to the fair value measurement. |
The Company analyzes all financial instruments with features of both liabilities and equity under ASC 480, “Distinguishing Liabilities From Equity” and ASC 815, “Derivatives and Hedging.” Derivative liabilities are adjusted to reflect fair value at each period end, with any increase or decrease in the fair value being recorded in results of operations as adjustments to fair value of derivatives. The effects of interactions between embedded derivatives are calculated and accounted for in arriving at the overall fair value of the financial instruments. In addition, the fair values of freestanding derivative instruments such as warrant and option derivatives are valued using the Black-Scholes model.
The Company uses Level 2 inputs for its valuation methodology for the warrant derivative liabilities and embedded conversion option liabilities as their fair values were determined by using the Black-Scholes option pricing model based on various assumptions. The Company’s derivative liabilities are adjusted to reflect fair value at each period end, with any increase or decrease in the fair value being recorded in results of operations as adjustments to fair value of derivatives.
At December 31, 2011, the Company identified the following assets and liabilities that are required to be presented on the balance sheet at fair value:
| Fair Value Measurements at | ||||||||||||||||
| Fair Value | December 31, 2011 | |||||||||||||||
| As of | Using Fair Value Hierarchy | |||||||||||||||
| Derivative Liabilities | December 31, 2011 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Warrant derivative liabilities | $ | 1,671,047 | $ | - | 1,671,047 | - | ||||||||||
| Embedded conversion option liabilities | 253,530 | - | 253,530 | - | ||||||||||||
| $ | 1,924,577 | $ | - | 1,924,577 | - | |||||||||||
At December 31, 2010, the Company identified the following assets and liabilities that are required to be presented on the balance sheet at fair value:
| F-8 |
| Fair Value Measurements at | ||||||||||||||||
| Fair Value | December 31, 2010 | |||||||||||||||
| As of | Using Fair Value Hierarchy | |||||||||||||||
| Derivative Liabilities | December 31, 2010 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Warrant derivative liabilities | $ | 27,307,218 | $ | - | 27,307,218 | - | ||||||||||
| Embedded conversion option liabilities | 1,019,935 | - | 1,019,935 | - | ||||||||||||
| $ | 28,327,153 | $ | - | 28,327,153 | - | |||||||||||
For the years ended December 31, 2011, 2010 and 2009, the Company recognized a gain (loss) of $11,444,988, ($6,209,898), and $23,103,668, respectively, for the changes in the valuation of derivative liabilities.
The Company did not identify any non-recurring assets and liabilities that were recorded at fair value during the periods presented.
Revenue Recognition and deferred Revenue — The Company’s revenues are primarily generated from license and research agreements with collaborators. Licensing revenue is recognized on a straight-line basis over the shorter of the life of the license or the estimated economic life of the patents related to the license.
License fee revenue begins to be recognized in the first full month following the effective date of the license agreement. Deferred revenue represents the portion of the license and other payments received that has not been earned. Costs associated with the license revenue are deferred and recognized over the same term as the revenue. Reimbursements of research expense pursuant to grants are recorded in the period during which collection of the reimbursement becomes assured, because the reimbursements are subject to approval.
In some cases, the company is entitled to receive royalty payments from licensees. In such cases, the company recognizes the royalties when they are earned and collectability of those royalty payments is reasonably assured.
In connection with its license agreements, the Company recorded $506,419, $418,166 and $553,448 in license fee revenue for the years ended December 31, 2011, 2010 and 2009, respectively, in its accompanying consolidated statements of operations, and the remainder of the license fee has been accrued in deferred revenue at December 31, 2011 and 2010, respectively.
Research and Development Costs — Research and development costs consist of expenditures for the research and development of patents and technology, which cannot be capitalized. The Company’s research and development costs consist mainly of payroll and payroll related expenses, research supplies and research grants. Reimbursements of research expense pursuant to grants are recorded in the period during which collection of the reimbursement becomes assured, because the reimbursements are subject to approval. Research and development costs are expensed as incurred.
Share-Based Compensation — The Company records stock-based compensation in accordance with ASC 718, “Compensation – Stock Compensation.” ASC 718 requires companies to measure compensation cost for stock-based employee compensation at fair value at the grant date and recognize the expense over the employee’s requisite service period. The Company recognizes in the statement of operations the grant-date fair value of stock options and other equity-based compensation issued to employees and non-employees. There were 91,800,285 options outstanding as of December 31, 2011.
Income Taxes — Deferred income taxes are provided using the liability method whereby deferred tax assets are recognized for deductible temporary differences and operating loss and tax credit carryforwards, and deferred tax liabilities are recognized for taxable temporary differences. Temporary differences are the differences between the reported amounts of assets and liabilities and their tax bases. Deferred tax assets are reduced by a valuation allowance when, in the opinion of management, it is more likely than not that some portion or all of the deferred tax assets will not be realized. Deferred tax assets and liabilities are adjusted for the effects of the changes in tax laws and rates of the date of enactment.
| F-9 |
When tax returns are filed, it is highly certain that some positions taken would be sustained upon examination by the taxing authorities, while others are subject to uncertainty about the merits of the position taken or the amount of the position that would be ultimately sustained. The benefit of a tax position is recognized in the financial statements in the period during which, based on all available evidence, management believes it is more likely than not that the position will be sustained upon examination, including the resolution of appeals or litigation processes, if any. Tax positions taken are not offset or aggregated with other positions. Tax positions that meet the more-likely-than-not recognition threshold are measured as the largest amount of tax benefit that is more than 50 percent likely of being realized upon settlement with the applicable taxing authority. The portion of the benefits associated with tax positions taken that exceeds the amount measured as described above is reflected as a liability for unrecognized tax benefits in the accompanying balance sheets along with any associated interest and penalties that would be payable to the taxing authorities upon examination.
Applicable interest and penalties associated with unrecognized tax benefits are classified as additional income taxes in the statements of operations.
Net Loss Per Share — Earnings per share is calculated in accordance with the ASC 260-10, “Earnings Per Share.” Basic earnings-per-share is based upon the weighted average number of common shares outstanding. Diluted earnings-per-share is based on the assumption that all dilutive convertible shares and stock options were converted or exercised. Dilution is computed by applying the treasury stock method. Under this method, options and warrants are assumed to be exercised at the beginning of the period (or at the time of issuance, if later), and as if funds obtained thereby were used to purchase common stock at the average market price during the period.
At December 31, 2011, 2010 and 2009, approximately 119,000,000, 190,000,000 and 395,000,000 potentially dilutive shares, respectively, were excluded from the shares used to calculate diluted earnings per share as their inclusion would be anti-dilutive.
Concentrations and Other Risks — Currently, the Company’s revenues and accounts receivable are concentrated on a small number of customers. The following table shows the Company’s concentrations of its revenue for those customers comprising greater than 10% of total license revenue for the years ended December 31, 2011, 2010 and 2009.
| Year Ended | ||||||||||||
| December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Exeter Life Sciences, Inc. | 24 | % | 17 | % | * | |||||||
| START Licensing, Inc. | 13 | % | 14 | % | * | |||||||
| International Stem Cell Corporation | 15 | % | 23 | % | 10 | % | ||||||
| Transition Holdings, Inc. | * | * | 14 | % | ||||||||
| CHA Biotech and SCRMI | 26 | % | 18 | % | * | |||||||
| Lifeline | 13 | % | * | * | ||||||||
| Genzyme Transgenics Corporation | * | * | 28 | % | ||||||||
*License revenue earned during the period was less than 10% of total license revenue.
Other risks include the uncertainty of the regulatory environment and the effect of future regulations on the Company’s business activities. As the Company is a biotechnology research and development company, there is also the attendant risk that someone could commence legal proceedings over the Company’s discoveries. Acts of God could also adversely affect the Company’s business.
| F-10 |
Recent Accounting Pronouncements
In January 2010, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No. 2010-06, Improving Disclosures about Fair Value Measurements (“ASU No. 2010-06”). The new standard addresses, among other things, guidance regarding activity in Level 3 fair value measurements. Portions of ASU No. 2010-06 that relate to the Level 3 activity disclosures became effective for the annual reporting period beginning after December 15, 2010. The adoption of this ASU did not have an impact on the Company’s consolidated financial statements.
On March 5, 2010, the FASB issued ASU No. 2010-11 Derivatives and Hedging Topic 815 “Scope Exception Related to Embedded Credit Derivatives.” This ASU clarifies the guidance within the derivative literature that exempts certain credit related features from analysis as potential embedded derivatives requiring separate accounting. The ASU specifies that an embedded credit derivative feature related to the transfer of credit risk that is only in the form of subordination of one financial instrument to another is not subject to bifurcation from a host contract under ASC 815-15-25, “Derivatives and Hedging — Embedded Derivatives — Recognition.” All other embedded credit derivative features should be analyzed to determine whether their economic characteristics and risks are “clearly and closely related” to the economic characteristics and risks of the host contract and whether bifurcation is required. The ASU became effective for the Company on July 1, 2010. The adoption of this ASU did not have an impact on the Company’s consolidated financial statements.
In May 2011, the FASB issued ASU 2011-04 which was issued to provide a consistent definition of fair value and ensure that the fair value measurement and disclosure requirements are similar between U.S. GAAP and IFRS. ASU 2011-04 changes certain fair value measurement principles and enhances the disclosure requirements particularly for Level 3 fair value measurements. This guidance is effective for the Company beginning on January 1, 2012. The adoption of ASU 2011-04 is not expected to significantly impact the Company’s consolidated financial statements.
In June 2011, the Financial Accounting Standards Board (FASB) issued Accounting Standards Update (ASU) 2011-05, Presentation of Comprehensive Income. ASU 2011-05 revises the manner in which entities present comprehensive income in their financial statements. The new guidance removes the presentation options in Accounting Standards Codification (ASC) 220, Comprehensive Income, and requires entities to report components of comprehensive income in either (1) a continuous statement of comprehensive income or (2) two separate but consecutive statements. The ASU does not change the items that must be reported in other comprehensive income. In December 2011, the FASB issued ASU 2011-12 which defers the requirement in ASU 2011-05 that companies present reclassification adjustments for each component of accumulated other comprehensive income in both net income and other comprehensive income on the face of the financial statements. ASU 2011-05 is effective for fiscal years and interim reporting periods within those years beginning after December 15, 2011, with early adoption permitted. The adoption of ASU 2011-05, as amended by ASU 2011-12, is not expected to significantly impact the Company’s consolidated financial statements.
In September 2011, the FASB issued ASU 2011-08 which provides an entity the option to first assess qualitative factors to determine whether it is necessary to perform the current two-step test for goodwill impairment. If an entity believes, as a result of its qualitative assessment, that it is more-likely-than-not that the fair value of a reporting unit is less than its carrying amount, the quantitative impairment test is required. Otherwise, no further testing is required. The revised standard is effective for the Company for its annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. The adoption of ASU 2011-08 is not expected to significantly impact the Company’s consolidated financial statements.
| F-11 |
| 3. | SETTLEMENT AND CANCELATION OF LICENSE AGREEMENT |
On December 18, 2008, the Company entered into a license agreement with Transition Holdings, Inc. for certain of the Company’s non-core technology. Under the agreement, the Company received $2,000,000, less wire fees. The Company further received $1,500,000 in 2009. The Company had initially recorded the transactions as deferred revenue and was amortizing the revenue over its 17-year patent useful life. In December 2010, the Company received notice that Transition Holdings, Inc. was disputing the nature of the arrangement, and subsequently entered into a settlement arrangement with Transition Holdings, Inc. As a result of this settlement, the Company reclassified the unamortized license fee in the amount of $3,205,856 from deferred revenue to accrued settlement. On February 15, 2011, the Company issued 7,413,000 shares as payment in full and recorded a loss on settlement of $294,144.
| 4. | INVESTMENT IN JOINT VENTURE |
On December 1, 2008, the Company and CHA Bio & Diostech Co., Ltd. formed an international joint venture. The new company, Stem Cell & Regenerative Medicine International, Inc. (“SCRMI”), will develop human blood cells and other clinical therapies based on the Company’s hemangioblast program, one of the Company’s core technologies. Under the terms of the agreement, the Company purchased upfront a 33% interest in the joint venture, and will receive another 7% interest upon fulfilling certain obligations under the agreement over a period of 3 years. The Company’s contribution includes (a) the uninterrupted use of a portion of its leased facility at the Company’s expense, (b) the uninterrupted use of certain equipment in the leased facility, and (c) the release of certain of the Company’s research and science personnel to be employed by the joint venture. In return, for a 60% interest, CHA has agreed to contribute $150,000 cash and to fund all operational costs in order to conduct the hemangioblast program. Effective May 1, 2010, the Company was no longer obligated to provide laboratory space to SCRMI, and the Company holds a 40% interest in the joint venture and CHA Bio & Diostech, Ltd. owns a 60% interest. The two partners to the joint venture are in negotiations on further funding of the joint venture, but there can be no assurances that an agreement will be reached. Any financial statement impact at this time is unclear should an agreement not be reached.
The Company has agreed to collaborate with the joint venture in securing grants to further research and development of its technology. Additionally, SCRMI has agreed to pay the Company a fee of $500,000 for an exclusive, worldwide license to the Hemangioblast Program. The Company recorded $29,412, $29,412 and $29,412 in license fee revenue for the years ended December 31, 2011, 2010 and 2009, respectively, in its accompanying consolidated statements of operations, and the balance of unamortized license fee of $410,539 and $439,951 is included in deferred revenue in the accompanying consolidated balance sheets at December 31, 2011 and 2010, respectively.
On July 15, 2011, the Company and CHA Biotech entered into a binding term sheet, with the expectation of entering into a future definitive agreement, in which the joint venture was realigned around both product development rights and research responsibilities. Under the terms of the binding term sheet, SCRMI exclusively licensed the rights to the Hemangioblast Program to the Company for United States and Canada and expanded the jurisdictional scope of the license to CHA Biotech to include Japan (in addition to South Korea, which was already exclusively licensed to CHA Biotech). As part of the agreement, the scientists at SCRMI involved in the Hemangioblast Program were transferred to the Company, and SCRMI discontinued its research activity and became solely a licensing entity. The Company is obligated to meet a minimal research spending requirement of $6.75 million by July 31, 2014 in order to maintain its exclusive license, up to the point of filing an investigational new drug for a therapeutic product. Intellectual property rights created by the Company in the course of our research are subject to a non-exclusive license to CHA Biotech for Japan and South Korea, and to SCRMI to be sub-licensable under certain circumstances for countries other than the United States, Canada, Japan and South Korea. Pursuant to the agreement, the Company paid $820,000 to SCRMI which is recorded to “losses attributable to equity method investments.”
| F-12 |
The following table is a summary of key financial data for the joint venture as of and for the years ended December 31, 2011, 2010 and 2009:
| December 31, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Current assets | $ | 194,349 | $ | 611,843 | $ | 737,760 | ||||||
| Noncurrent assets | $ | 1,082,778 | $ | 855,372 | $ | 501,744 | ||||||
| Current liabilities | $ | 294,469 | $ | 1,203,941 | $ | 863,436 | ||||||
| Noncurrent liabilities | $ | 2,459,785 | $ | 1,439,394 | $ | 488,297 | ||||||
| Net revenue | $ | 417,382 | $ | 76,672 | $ | 26,775 | ||||||
| Net loss | $ | (574,713 | ) | $ | (1,852,336 | ) | $ | (1,526,851 | ) | |||
| 5. | PROPERTY AND EQUIPMENT |
Property and equipment consisted of the following at December 31, 2011 and 2010:
| December 31, | December 31, | |||||||
| 2011 | 2010 | |||||||
| Machinery & equipment | $ | 1,488,527 | $ | 1,488,527 | ||||
| Computer equipment | 449,893 | 449,893 | ||||||
| Office furniture | 82,822 | 76,201 | ||||||
| Leasehold improvements | 311,592 | 281,383 | ||||||
| Capital leases | 51,235 | 51,235 | ||||||
| 2,384,069 | 2,347,239 | |||||||
| Accumulated depreciation | (2,229,298 | ) | (2,162,137 | ) | ||||
| Property and equipment, net | $ | 154,771 | $ | 185,102 | ||||
Depreciation expense for the years ended December 31, 2011, 2010 and 2009 amounted to $67,161, $138,050 and $291,472, respectively.
| 6. | ACCRUED SETTLEMENT |
Midsummer Investment, Ltd
On August 9, 2011, the Company entered into a Settlement Agreement and Mutual Release (“Settlement Agreement”) with Midsummer Investment, Ltd and Midsummer Small Cap Master, Ltd. (collectively, “Midsummer”). Pursuant to the Settlement Agreement, upon tender by Midsummer to the Company of warrants held by Midsummer to purchase a total of 20,319,730 shares of the Company’s common stock (the “Warrants”), and duly executed notices of exercise (deemed to occur upon execution of the Settlement Agreement), the Company, to settle errors involving warrant issuances to Midsummer, agreed to (i) deliver to Midsummer an aggregate of 36,000,000 shares of the Company’s common stock (the “Current Shares”), as an exercise of the Warrants in respect of a partial exercise of Warrants, (ii) undertake to issue 30,585,774 additional shares of the Company’s common stock (the “Future Shares”), as an exercise of the remainder of the Warrants within ten days of the date that the Company shall have sufficient authorized and unissued shares of Common Stock (“Authorized Share Increase”) which are not otherwise reserved for issuance for other purposes to enable the Company to issue all of the Future Shares and (iii) issue 3,058,577 shares of the Company’s common stock (the “Additional Future Shares”) for every calendar month elapsed between the date of delivery of the Current Shares and the date following delivery of the Future Shares. The Company and Midsummer provided mutual general releases.
The Company calculated the fair value of the warrants on August 9, 2011 using the stock price on that date, the exercise price per the warrant agreement, an expected term based on the expiration date of the warrant, volatility of 165%, and a discount rate of 0.33%. Based on these assumptions the fair value of the warrants to purchase 20,319,730 shares of common stock was $3,039,090 which represents a $409,082 decrease in fair value from June 30, 2011. The decrease of $409,082 is recorded in the consolidated statement of operations as “adjustments to fair value of derivatives.”
| F-13 |
The shares to be issued were valued at $0.17 which is the share price on the date of the agreement. Per the Settlement Agreement, the Company issued 36,000,000 shares on August 12, 2011. The Company issued the Future Shares of 30,585,774 and the Additional Future Shares of 15,292,885 in January 2012. The Company estimated the required liability at December 31, 2011 to be $7,799,373 based on 30,585,774 Future Shares and 15,292,885 Additional Future Shares valued at $0.17 per share of common stock which is recorded as “accrued settlement” on the accompanying consolidated balance sheets. The Company recorded $10,880,282 as “finance costs” in the consolidated statements of operations related to the 36,000,000 Initial shares, the 30,585,774 Future Shares, the 15,292,885 Additional Future Shares, offset by the $3,039,090 fair value of the warrant derivative liability.
Alpha Capital
On October 14, 2011, the United States District Court for the Southern District of New York entered an order granting plaintiff Alpha Capital Anstalt's ("Alpha Capital") motion for a preliminary injunction and preliminary declaratory relief in the lawsuit entitled Alpha Capital Anstalt v. Advanced Cell Technology, Inc., Case No. 11 CIV 6458 (S.D.N.Y. filed Sept. 16, 2011). The lawsuit is described in the current report on Form 8-K filed by the Company with the Securities and Exchange Commission on September 22, 2011. In its motion, Alpha Capital sought an order directing the Company to deliver to it at least 39,514,859 shares of its common stock in accordance with the terms of its warrants and convertible promissory notes. The court's October 14, 2011 order directed the Company to hold in escrow 39,514,859 shares of its common stock pending the entry of a preliminary injunction, and directed Alpha Capital to submit a proposed form of order to the court by October 27, 2011.
On November 1, 2011, the Company issued the 39,514,859 shares to Alpha Capital. The Company recorded $4,947,800 as “finance cost” on the accompanying consolidated statements of operations and balance sheets, respectively which represents the fair value of the 39,514,859 shares of common stock valued at $0.14 per share less the fair value of the warrant derivative liability of $584,280. The $0.14 common stock fair value was the share price on the date of the preliminary injunction.
On November 23, 2011, the Company answered Alpha Capital’s Complaint and asserted affirmative defenses. On December 12, 2011, the Company and Alpha submitted a Civil Case Management Plan and Scheduling Order and discovery has since commenced. Despite receiving the 39,514,859 shares on November 1, 2011 as a result of its preliminary injunction, Alpha Capital continues to seek damages against the Company. The Company’s Management intends to contest this case vigorously.
Black Mountain Equities
On November 9, 2011, the United States District Court for the Southern District of New York entered an order granting plaintiff Black Mountain Equities, Inc. ("Black Mountain") motion for a preliminary injunction and preliminary declaratory relief in the lawsuit entitled Black Mountain Equities, Inc., v. Advanced Cell Technology, Inc., Case No. 11 CIV 7305, filed on October 17, 2011. In its motion, Black Mountain sought an order directing the Company to deliver to it at least 18,000,000 shares of its common stock in accordance with the terms of its warrants and convertible promissory notes. The court's November 9, 2011 order directed the Company to hold in escrow 18,000,000 shares of its common stock pending the entry of a preliminary injunction.
On November 15, 2011, the Company issued the 18,000,000 shares to Black Mountain. The Company recorded $1,615,062 as “finance cost” and “accrued settlement” on the accompanying consolidated statements of operations and balance sheets, respectively which represents the fair value of the 18,000,000 shares of common stock valued at $0.11 per share less the fair value of the warrant derivative liability of $364,938. The $0.11 common stock fair value was the share price on the date of the preliminary injunction.
| F-14 |
On December 15, 2011, the Company answered BME’s initial Complaint and asserted counterclaims, disputing BME’s contention that it was owed 18,000,000 shares. On December 29, 2011, BME filed an Amended Complaint. On January 17, 2012, the Company answered the Amended Complaint and asserted revised counterclaims. Discovery will begin in this case shortly. In its Amended Complaint, BME argues that it made a cashless exercise of warrants issued by the Company by delivering a Notice of Exercise, asking for 18,000,000 shares of the Company’s common stock, based on a reduced exercise price and increased warrant share amount. In its counterclaims, the Company argues that even assuming arguendo that the exercise price of the warrants should have been reset as a result of certain JMJ Financial, Inc. transactions, BME would still only be entitled to 7,331,445 shares. Based on this calculation, the Company argues that BME should return to the Company no less than 10,668,555 shares of the stock it received on November 15, 2011 pursuant to its preliminary injunction. The Company’s Management intends to contest this case vigorously.
Cranshire Master Fund
On December 15, 2011, the United States District Court for the Southern District of New York entered an order granting plaintiff Cranshire Capital Master Fund, Ltd.’s (“Cranshire”) motion for a preliminary injunction in the lawsuit entitled Cranshire Capital Master Fund, Ltd. v. Advanced Cell Technology, Inc., Case No. 11 CIV 8755 (S.D.N.Y. filed December 1, 2011). Cranshire asserts that as a result of the transactions between the Company and JMJ, the exercise price of its warrants should have been decreased to $.0353 and the total number of warrant shares issuable upon exercise should have been increased from 6,918,197 to 19,598,292. Based upon these figures, Cranshire asserted that its December 2010 warrant exercise should have resulted in an additional 12,680,094 shares. Cranshire asserts claims for damages, in an amount to be determined at trial, for the Company's alleged failure to deliver the shares and to provide proper notice of reduction in exercise price and conversion price. On December 2, 2011, Cranshire moved for preliminary declaratory relief and for a preliminary injunction directing the Company to deliver immediately at least 12,680,094 shares of its common stock to Cranshire. At the hearing on December 15, 2011, Cranshire changed its argument, contending that the exercise price should have been decreased to $.027 (as opposed to $.0353) and that, consequently, it was entitled to 18,000,000 shares (as opposed to 12,660,094 shares). On December 15, 2011, the court granted a preliminary injunction and directed the Company to deliver to Cranshire 10,730,265 shares of the Company's common stock. The parties will begin discovery shortly. Management intends to contest this case vigorously if a reasonable settlement cannot be achieved.
The Company issued the 10,730,265 shares to Cranshire on December 16, 2011. The Company recorded $1,073,027 as “finance cost” and “accrued settlement” on the accompanying consolidated statements of operations and balance sheets, respectively which represents the fair value of the 10,730,265 shares of common stock valued at $0.10 per share less. The $0.10 common stock fair value was the share price on the date of the preliminary injunction. See note 19, Subsequent Events, regarding the February 24, 2012 settlement.
Global Settlement
On December 7, 2011, the Company entered into settlement agreements with 40 holders of convertible promissory notes and warrants that were issued between 2005 and 2010. The settlement agreements relate to claims that the holders may have against the Company regarding the assertion that the conversion price of the notes and the exercise price of the warrants should have been adjusted as a result of certain transactions between the Company and JMJ Financial, Inc. during 2010. Pursuant to the settlement agreements, the Company agreed to issue an aggregate of 239,601,630 shares of common stock to the settling holders.
At the time of settlement, the Company did not have a sufficient number of authorized but unissued shares of common stock to issue all of the shares of common stock that the Company agreed to issue to settling holders pursuant to the settlement agreements. On January 24, 2012, the Company’s shareholders approved the increase in authorized shares to 2,750,000,000. The Company issued the 239,601,630 shares on January 30, 2012.
| F-15 |
The Company recorded $22,724,947 as “finance cost” and “accrued settlement” on the accompanying consolidated statements of operations and balance sheets, respectively which represents the fair value of the 239,601,630 shares of common stock valued at $0.11 per share less. The $0.11 common stock fair value was the share price on the date of the settlement agreement.
| 7. | LOSS CONTINGENCY ACCRUAL |
The Company was not able to reach a settlement agreement with all of holders of convertible promissory notes and warrants that were issued between 2005 and 2010. The Company will continue to negotiate with the holders and anticipates that the number of shares to be issued will be similar to the settlements that have already been finalized as of December 31, 2011.
The Company recorded $16,704,169 as “finance cost” and “loss contingency accrual” on the accompanying consolidated statements of operations and balance sheets, respectively which represents the fair value of the shares of common stock valued at $0.13 per share less the fair value of the warrant derivative liability of $810,077. The $0.13 common stock fair value was determined by estimating the share price at the date of settlement.
| 8. | CONVERTIBLE PROMISSORY NOTES |
2010 JMJ Convertible Promissory Notes
During 2010, the Company issued three convertible promissory notes to JMJ Financial, for a total of $3,000,000 available to receive in cash, for a principal sum of $3,850,000, which included an original issue discount of $850,000. The notes bear a one-time interest charge of 10% on the principal sum. The holder may at its election convert all or part of these notes into shares of the Company's common stock at the conversion rate of the lesser of: (a) $0.10 per share, or (b) 85% of the average of the three lowest trade prices in the 20 trading days prior to the conversion. During 2010, the Company received the entire $3,000,000 on these notes. Of the $3,850,000 borrowed, the Company converted $3,562,215 into 76,465,706 shares of common stock during 2010. The notes mature on March 30, 2013.
As of December 31, 2011 and 2010, the convertible promissory notes were convertible at the option of the holders into a total of 4,303,863 shares, subject to anti-dilution and other customary adjustments. The fair value of the embedded conversion option was $227,547 and $628,919 as of December 31, 2011 and 2010, respectively. The decrease in the fair value of this liability was $401,372 and $7,778,168 during the years ended December 31, 2011 and 2010, respectively, which was recorded through the results of operations as an adjustment to fair value of derivatives. The assumptions used in the Black-Scholes option pricing model at December 31, 2011 are as follows: (1) dividend yield of 0%; (2) expected volatility of 160%, (3) risk-free interest rate of 0.12%, and (4) expected life of 1.25 years.
Interest expense from amortization of debt discounts related to the JMJ Convertible Promissory Notes for the years ended December 31, 2011, 2010 and 2009 was $126,863, $6,410,552 and $1,220,220, respectively.
2009 Convertible Promissory Notes
On November 12, 2009, the Company entered into a subscription agreement (the “Subscription Agreement”) with certain subscribers (the “Subscribers”) pursuant to which the Company sold certain original issue discount promissory notes (“2009 Convertible Promissory Notes”). The 2009 Convertible Promissory Notes are convertible at the option of the holder into shares of the Company’s common stock at a conversion price of $0.10.
The initial closing under the Subscription Agreement occurred on November 12, 2009, pursuant to which, the Company sold 2009 Convertible Promissory Notes (“First Close Notes”) in the principal amount of $1,662,000 for a purchase price of $1,385,000. In addition, on November 13, 2009, the Company sold 2009 Convertible Promissory Notes in the principal amount of $441,000 for a purchase price of $367,500 (including $67,500 previously owed to a subscriber for legal services). The closing that occurred on November 13, 2009 was deemed part of the initial closing, such that, pursuant to the initial closing under the Subscription Agreement, the Company sold 2009 Convertible Promissory Notes in the aggregate principal amount of $2,103,000 for an aggregate purchase price of $1,752,500.
| F-16 |
On February 18, 2010, the Company completed the second closing, issuing additional debentures (“Second Close Debentures”), under the same terms of the initial closing, in the principal amount of up to $2,076,451 for a purchase price of $1,730,375 (including $45,375 previously owed to a subscriber for legal services).
The Company was required to redeem the 2009 Convertible Promissory Notes monthly commencing in May 2010 under the first closing and September 2010 under the second closing, in the amount of 14.28% of the initial principal amount of the 2009 Convertible Promissory Notes, in cash or common stock at the Company’s option, until the 2009 Convertible Promissory Notes were paid in full. The maturity date of the 2009 Convertible Promissory Notes, first close is November 12, 2010, and March 1, 2011 under the second close.
During the year ended December 31, 2011, the Company issued 1,519,077 shares of common stock for debt of $151,909. As of December 31, 2011 and 2010, the outstanding debt related to the 2009 Convertible Promissory Notes is $0 and $132,680 (net of discount of $19,229), respectively.
Interest expense from amortization of debt discounts related to the 2009 Convertible Promissory Notes for the years ended December 31, 2011, 2010 and 2009 was $19,229, $3,878,950 and $281,271, respectively.
| 9. | Series A-1 REDEEMABLE Convertible Preferred Stock |
On March 3, 2009, the Company entered into a $5 million credit facility (“Facility”) with a life sciences fund. Under the terms of the agreement, the Company may draw down funds, as needed, from the investor through the issuance of Series A-1 redeemable convertible preferred stock, par value $.001, at a basis of 1 share of Series A-1 redeemable convertible preferred stock for every $10,000 invested. The preferred stock pays dividends, in kind of preferred stock, at an annual rate of 10%, matures in four years from the initial drawdown date, and is convertible into common stock at $0.75 per share at the option of the holder.
However, in the event the closing price of the common stock during the 5 trading days following the notice to convert falls below 75% of the average of the closing bid price in the 5 trading days prior to the closing date, the investor may, at its option, and without penalty, decline to purchase the applicable put shares on the closing date.
The Company is required to keep available out of its authorized but unissued shares of common stock, such number of shares sufficient to effect a conversion of all then outstanding shares of the Series A-1 redeemable convertible preferred stock.
The Series A-1 redeemable preferred stock has been classified within the mezzanine section between liabilities and equity in the consolidated balance sheets because it is considered conditionally redeemable. The embedded conversion option has been recorded as a derivative liability in the Company’s consolidated balance sheets, and changes in the fair value each reporting period are reported in adjustments to fair value of derivatives in the consolidated statements of operations.
The outstanding balance at December 31, 2011 and 2010 was $1,130,165, and is convertible into 1,506,887 shares of the Company’s common stock. The Company values the conversion option initially when each draw takes place (see section entitled “Conversion Option” in this footnote below). As of December 31, 2011, the Company has drawn $3,418,166 of the $5,000,000 commitment.
| F-17 |
The following table summarizes the Series A-1 redeemable convertible preferred stock outstanding at December 31, 2011 and 2010:
| December 31, | December 31, | |||||||
| 2011 | 2010 | |||||||
| Principal due | $ | 1,130,165 | 1,130,165 | |||||
| Accrued dividend | 342,098 | 219,492 | ||||||
| Debt discount | (43,137 | ) | (77,216 | ) | ||||
| Non-current portion | $ | 1,429,126 | 1,272,441 | |||||
| Aggregate liquidation value* | $ | 1,472,262 | 1,349,657 | |||||
* Represents the sum of principal due and accrued dividends.
The dividends are accrued at a rate of 10% per annum, and the Company records the accrual as interest expense in its consolidated statements of operations in the period incurred. The Company recorded accrued dividends on the Series A-1 redeemable convertible preferred stock of $122,605, $219,492 and $123,609 for the years ended December 31, 2011, 2010 and 2009, respectively, which is recorded as interest expense in the accompanying consolidated statements of operations.
Redemption Rights
Upon the earlier of (i) the fourth anniversary of the issuance date, and (ii) the occurrence of a major transaction, each holder shall have the right, to require the Company to redeem all or a portion of such holder’s share of Series A-1 preferred stock, at a price per share equal to the Series A-1 liquidation value. The Company has the option to pay the redemption price in cash or in shares of its common stock. The Company shall have the right to redeem all or a portion of the shares of Series A-1 redeemable preferred stock, at any time at a price per share of Series A-1 redeemable preferred stock equal to 100% of the Series A-1 liquidation value.
Termination and Liquidation Rights
The Company may terminate this agreement and its right to initiate future draw-downs by providing 30 days advanced written notice to the investor, subject to certain limitations.
Upon any liquidation, dissolution or winding up of the Company, the holders of the Series A-1 redeemable convertible preferred stock shall first be entitled to be paid out of the assets of the Company available for distribution (subject to certain limitations) to its stockholders an amount with respect to each share of Series A-1 redeemable convertible preferred stock equal to $10,000, plus any accrued by unpaid dividends.
Conversion Option:
The embedded conversion option was valued at $25,983 and $212,447 at December 31, 2011 and 2010, respectively, at fair value using the Black-Scholes model. The decrease in the fair value of the embedded conversion option liability of $186,464, $392,400 and $2,094,924 for the years ended December 31, 2011, 2010 and 2009, respectively, was recorded through the results of operations as an adjustment to fair value of derivatives.
The assumptions used in the Black-Scholes model to value the embedded conversion option at December 31, 2011 were as follows: (1) dividend yield of 0%; (2) expected volatility of 160%, (3) risk-free interest rate of 0.12%, and (4) expected life of 1.27 years.
Commitment fee and expenses
For providing investor relations services in connection with the Series A-1 redeemable convertible preferred stock credit facility, the Company issued a consultant 24,900,000 shares of its common stock on February 9, 2009. The Company valued the issuance of these shares at $4,731,000 based on a closing price of $0.19 on February 9, 2009 and recorded the value of the shares as deferred financing costs on the date they were issued. Beginning on the date of the first draw-down on April 6, 2009 (the loan maturity date is 4 years after the initial draw-down), the Company amortizes these fees over the term of the Series A-1 redeemable convertible preferred stock facility which represents the implied term of the investor relations contract.
| F-18 |
The Company also incurred a non-refundable commitment fee to the holder of this convertible preferred stock facility in the amount of $250,000. The initial fee went into delinquency and was modified on October 19, 2009. (See modification section in the footnote below.)
Beginning on the date of the first draw-down on April 6, 2009 (the loan maturity date is 4 years after the initial draw-down), the Company amortizes the deferred issuance costs ratably over the term of the Series A-1 redeemable convertible preferred stock facility.
Interest expense from amortization of the debt discount and deferred costs for the years ended December 31, 2011, 2010 and 2009 was $445,521, $137,753 and $3,778,850, respectively.
Modification of Series A-1 Convertible Redeemable Preferred Stock:
On October 19, 2009, the Company entered into two letter agreements with Volation, pursuant to which (i) the Company reduced the conversion price of its existing outstanding Series A-1 convertible preferred stock issued to Volation to $.10 per share resulting in 22,880,000 shares of Common Stock upon conversion, (ii) the Company issued Volation 2,500,000 shares of its Common Stock at $0.10 per share in payment of an outstanding commitment fee, and (iii) Volation waived the delinquency in non-payment of the $250,000 commitment fee required pursuant to the preferred stock purchase agreement between the Company and Volation. The commitment fee was paid during the year ended December 31, 2010 by reducing the proceeds paid by the Series A-1 Preferred Stock investors by the amount of the commitment fee.
During 2010, the Company issued 6,206,961 shares of common stock for the conversion of $620,696 of Series A-1 redeemable convertible preferred stock.
| 10. | SERIES B PREFERRED STOCK |
On November 2, 2009 (“Effective Date”), the Company entered into a preferred stock purchase agreement with Optimus Life Sciences Capital Partners, LLC (“Investor” or “Optimus”). Pursuant to the purchase agreement, the Company agreed to sell, and the Investor agreed to purchase, in one or more purchases from time to time at the Company’s sole discretion, (i) up to 1,000 shares of Series B preferred stock at a purchase price of $10,000 per share, for an aggregate purchase price of up to $10,000,000, and (ii) five-year warrants to purchase shares of the Company’s common stock with an aggregate exercise price equal to 135% of the purchase price paid by the Investor, at an exercise price per share as follows:
| · | On the sixth (6th) Trading Day following the Tranche Notice Date, the Exercise Price of the Optimus Warrant shall be adjusted to equal the VWAP for the 5 trading days beginning on and including the Tranche Notice Date (as so adjusted, the “Adjusted Exercise Price”); and |
| · | If the Adjusted Exercise Price results in additional Warrant Shares being issuable to the Holder, such additional shares shall be delivered to the Holder within one Trading Day following the Adjustment Date. If the Adjusted Exercise Price results in less Warrant Shares being issuable to the Holder, the excess Warrant Shares shall be returned by the Holder to the Company within one Trading Day following on the Adjustment Date. |
The Warrants were issued in replacement of a five-year warrant to purchase 119,469,027 shares of common stock with an exercise price per share of $0.113 the Company issued on the Effective Date.
The Company agreed to pay to the Investor a commitment fee of $500,000, at the earlier of the closing of the first Tranche or the six month anniversary of the effective date, payable at the Company’s election in cash or common stock valued at 90% of the volume weighted average price of the Company’s common stock on the five trading days preceding the payment date. The $500,000 commitment fee was outstanding and was recorded in accrued expenses in the Company’s consolidated balance sheet at December 31, 2009. During 2010, the Company issued 50 shares of preferred stock as payment for the commitment fee.
| F-19 |
During 2010, the Company delivered tranche notices to Optimus Life Sciences Capital Partners, LLC for delivery of a total of 1,000 shares under the Series B preferred stock for funding in the amount of $10,000,000 ($9,485,000 in cash proceeds, $500,000 of commitment fee applied, and $15,000 in legal fees).
During 2010, in connection with the funding, the Company issued 95,870,362 shares of its common stock upon exercise of the same number of warrants, which were granted simultaneously with the Company’s tranche notices. During 2010, the Company received secured promissory notes in the amount of $13,500,000 to settle the warrant exercise.
Dividends
Commencing on the date of the issuance of any shares of Series B preferred stock, Holders of Series B preferred stock will be entitled to receive dividends on each outstanding share of Series B preferred stock, which will accrue in shares of Series B preferred stock at a rate equal to 10% per annum from the issuance date compounded annually. Accrued dividends will be payable upon redemption of the Series B preferred stock. Accrued dividends were $1,229,538 and $196,986 at December 31 2011 and 2010, respectively.
Redemption Rights
Upon or after the fourth anniversary of the initial issuance date, the Company will have the right, at the Company’s option, to redeem all or a portion of the shares of the Series B preferred stock, at a price per share equal to 100% of the Series B liquidation value. The preferred stock may be redeemed at the Company’s option, commencing 4 years from the issuance date at a price per share of (a) $10,000 per share plus accrued but unpaid dividends (the “Series B Liquidation Value”), or, at a price per share of : (x) 127% of the Series B Liquidation Value if redeemed on or after the first anniversary but prior to the second anniversary of the initial issuance date, (y) 118% of the Series B Liquidation Value if redeemed on or after the second anniversary but prior to the third anniversary of the initial issuance date, and (z) 109% of the Series B Liquidation Value if redeemed on or after the third anniversary but prior to the fourth anniversary of the initial issuance date.
Liquidation Rights
The preferred shares shall, with respect to dividend, rights upon liquidation, winding-up or dissolution, rank: (i) senior to the Company’s common stock, and any other class or series of preferred stock of the Company, except Series A-1 Convertible Preferred Stock which shall rank senior in right of liquidation and pari passu with respect to dividends; and (ii) junior to all existing and future indebtedness of the Company. If the Company determines to liquidate, dissolve or wind-up its business, it must redeem the Series B preferred stock at the prices set forth above. Upon any liquidation, dissolution or winding up of the Company the Holders of Series B preferred stock shall be first entitled to be paid out of the assets of the Company available for distribution to its stockholders an amount with respect to each share of Series B preferred stock equal to $10,000, plus any accrued and unpaid dividends.
The Company has classified the Series B redeemable preferred stock in the equity section in its consolidated balance sheets.
Related Secured Promissory Notes Receivable:
In accordance with the terms of the Series B preferred stock agreement, Optimus issued to the Company a secured promissory note in consideration for receiving warrants under each tranche. The value of each secured promissory note equals the value of the warrants that Optimus received. Interest on the notes accrues at 2% per year, compounding annually if the interest remains unpaid at the end of each year. The note is secured by freely tradable marketable securities belonging to Optimus. Each promissory note matures on the fourth anniversary of its issuance.
In the event the Company redeems all or a portion of any shares of Series B preferred stock held by Optimus, the Company will be permitted to offset the full amount of such proceeds against amounts outstanding under the promissory notes. Accordingly, the Company included the discounted value of the secured promissory notes as a separate component of stockholders’ deficit at December 31, 2011 and 2010.
| F-20 |
The value of the secured promissory notes in the accompanying consolidated balance sheet was $11,207,935, net of discounts of $2,537,499 and accrued interest of $245,434 at December 31, 2011, reflecting a face value of $13,500,000. The value of the secured promissory notes in the accompanying consolidated balance sheet was $10,177,370, net of discounts of $3,361,952 and accrued interest of $39,322 at December 31, 2010, reflecting a face value of $13,500,000. The Company determined that a 10% discount is appropriate, in order to consistently reflect the Company’s cost of borrowing under the terms of the underlying Series B preferred stock that permits offset. The Company recorded an initial discount on the promissory notes in the amount of $3,519,238 during the year ended December 31, 2010. The Company accretes interest at 10% over the respective four-year terms of the promissory notes.
During the years ended December 31, 2011 and 2010, the Company accreted interest on the promissory notes in the amount of $1,227,173 and $196,607, respectively, which was recorded in accumulated deficit during the periods then ended. The Company recorded dividends on its Series B preferred stock during the years ended December 31, 2011 and 2010 of $1,229,538 and $196,986, respectively. The accrued dividends are offset by the accretion of the note receivable discount.
As of December 31, 2011 and 2010, 1,000 shares of Series B preferred stock were outstanding.
| 11. | SERIES C PREFERRED STOCK |
On December 30, 2010 (the “Series C Effective Date”), the Company entered into a securities purchase agreement (the “Series C Purchase Agreement”) with Socius CG II, Ltd., a Bermuda exempted company (“Socius”). Pursuant to the Series C Purchase Agreement:
| · | The Company agreed to sell, and Socius agreed to purchase, in one or more purchases from time to time (each such purchase, a “Series C Tranche”) in the Company’s sole discretion (subject to the conditions set forth therein), (i) up to 2,500 shares of Series C Preferred Stock (the “Series C Preferred Shares”) at a purchase price of $10,000 per share, for an aggregate purchase price of up to $25,000,000, and (ii) a two-year warrant (the “Socius Warrant”) obligating Socius to purchase shares of the Company’s common stock (the “Common Stock” ) with an aggregate exercise price equal to 20% of the purchase price paid by Socius for the Series C Preferred Shares sold in each Series C Tranche, at an exercise price per share equal to the closing bid price of the Company’s Common Stock on the date the Company provides notice of such Series C Tranche (the “Series C Tranche Notice”). On each date that the Company delivers a Series C Tranche Notice to Socius, Socius shall also become obligated, pursuant to a right automatically vesting on such Series C Tranche Notice date, to purchase that number of shares of Common Stock (such shares of Common Stock the “Additional Investment Shares”) equal in dollar amount to 100% of the Series C Tranche amount set forth in the Series C Tranche Notice at a price per share equal to the closing bid price of the Common Stock on the Series C Tranche Notice date. |
| ● | The Series C Purchase Agreement requires that, when the Company requests Socius to purchase a tranche of Series C Preferred Shares, the mandatory purchase by Socius of the related Additional Investment Shares must occur no later than sixty (60) calendar days following the Series C Tranche Notice date. |
| ● | The Socius Warrant was issued to Socius on December 30, 2010 (the “Closing Date”) simultaneous with entering into the Series C Purchase Agreement. The Socius Warrant was issued with an initial exercise price per warrant is of $0.16 per share and for a total of up to 31,250,000 shares, subject to adjustment as described therein. On January 10, 2011, Socius and the Company entered into a letter agreement in which the parties agreed that, following arms-length negotiations and notwithstanding anything to the contrary in the Socius Warrant, that the initial number of shares issuable under the Socius Warrant, subject to the adjustment mechanism set forth therein, was equal to 30,000,000. |
| ● | As required by the Purchase Agreement, the Socius Warrant must be exercised for such number of shares of Common Stock equal in amount to 20% of the cumulative purchase price paid by Socius for the Series C Preferred Shares. The maximum amount of Series C Preferred Stock that Socius may become obligated to purchase under all Series C Tranches is $25,000,000. Assuming the maximum drawdown of $25,000,000 by the Company under the Series C Purchase Agreement, Socius would be required to exercise the Socius Warrant to purchase 20% of this total dollar amount, or $5,000,000 worth of shares of Common Stock. |
| F-21 |
| ● | The Letter Agreement modified the Socius Warrant only with respect to the initial number of underlying shares and expressly provides that, except as so modified, the Socius Warrant shall remain unchanged and shall continue in full force and effect. |
| ● | At the initial closing pursuant to the Series C Purchase Agreement, which occurred on the Closing Date, (i) Socius purchased 400 Preferred Shares and the Company received gross proceeds of $4,000,000 (ii) the Company delivered to Socius an initial warrant (the “Initial Warrant”) obligating Socius to purchase shares of Common Stock with an aggregate purchase price of $800,000, which shall be automatically exercisable on the date a registration statement for the resale of all shares of Common Stock issuable pursuant to the Series C Purchase Agreement is declared effective (which effectiveness occurred on April 13, 2011), with delivery of such shares made to Socius on the trading day immediately following the exercise date at a per-share price equal to the closing bid price of the Common Stock on the delivery date, and (iii) Socius became obligated to purchase additional shares of Common Stock equal in aggregate dollar amount to $4,000,000 ( such shares of Common Stock the “Initial Investment Shares”), with delivery of such shares made to Socius on the trading day immediately following the date the registration statement is declared effective at a price per share equal to the closing bid price of Common Stock on the delivery date. |
| · | The Company agreed to pay to Socius a commitment fee of $1,250,000 (the “Commitment Fee”), at the earlier of the closing of the first Series C Tranche or the six month anniversary of the Series C Effective Date. This Commitment Fee is payable solely at the Company’s election, in cash or in the alternative, in shares of common stock valued at 88% of the volume weighted average price of the Company’s Common Stock on the five trading days preceding the payment date. If the Company elects to pay the Commitment Fee in shares of Common Stock, no cash payment would be due as the issuance of shares would satisfy the Commitment Fee obligation in full. The Company issued 7,562,008 shares of common stock on June 30, 2011 as full payment of the Commitment Fee. |
| · | The Company agreed to use its best efforts to file within 60 days of the Series C Effective Date, and cause to become effective as soon as possible thereafter, a registration statement with the Securities and Exchange Commission for the resale of all shares of Common Stock issuable pursuant to the Series C Purchase Agreement, including the shares of Common Stock underlying the Socius Warrant, shares of the Common Stock issuable upon exercise of the Initial Warrant, shares of Common Stock issuable as Initial Investment Shares, shares of Common Stock issuable as Additional Investment Shares, and shares of Common Stock issuable in payment of the Commitment Fee. |
| ● | In the event that Socius does not comply with its obligations under the Series C Purchase Agreement (including its obligations to exercise the Socius Warrant), the Series C Purchase Agreement provides that, in addition to being entitled to exercise all rights provided therein or granted by law, the Company would be entitled to seek specific performance by Socius under the Series C Purchase Agreement and the Socius Warrant. |
On December 30, 2010, in accordance with the purchase agreement, the Company filed a certificate of designations for the Series C preferred stock with the Secretary of State of the state of Delaware. As previously reported, pursuant to the Certificate of Designations, the preferred shares shall, with respect to dividend, rights upon liquidation, winding-up or dissolution, rank: (i) senior to the Company’s common stock, and any other class or series of preferred stock of the Company (collectively, with any warrants, rights, calls or options exercisable for or convertible into such preferred stock, the “Junior Securities”); provided, however, the Series A-1 convertible preferred stock and Series B preferred stock (together, the “Senior Securities”) shall rank senior in right of redemption, liquidation, and dividends; and (ii) junior to all existing and future indebtedness of the Company.
On June 16, 2011, the Company delivered the second Series C Tranche notice to Socius for delivery of a total of 400 shares under the Series C preferred stock for funding in the amount of $4,000,000.
| F-22 |
On September 22, 2011, the Company delivered the third Series C Tranche notice to Socius for delivery of a total of 150 shares under the Series C preferred stock for funding in the amount of $1,500,000.
On December 15, 2011, the Company delivered the fourth Series C Tranche notice to Socius for delivery of a total of 200 shares under the Series C preferred stock for funding in the amount of $2,000,000.
Dividends
Commencing on the date of the issuance of any shares of Series C preferred stock, holders of Series C preferred stock will be entitled to receive dividends on each outstanding share of Series C preferred stock, which will accrue in shares of Series C preferred stock at a rate equal to 6% per annum from the issuance date compounded annually. Accrued dividends will be payable upon redemption of the Series C preferred stock. Accrued dividends were $400,110 and $0 at December 31, 2011 and 2010, respectively.
Redemption Rights
Upon or after the fourth anniversary of the initial issuance date, the Company will have the right, at the Company’s option, to redeem all or a portion of the shares of the Series C preferred stock, at a price per share equal to 100% of the Series C liquidation value. The preferred stock may be redeemed at the Company’s option, commencing 4 years from the issuance date at a price per share of (a) $10,000 per share plus accrued but unpaid dividends (the “Series C Liquidation Value”), or, at a price per share of : (x) 136% of the Series C Liquidation Value if redeemed prior to the first anniversary of the initial issuance date, (y) 127% of the Series C Liquidation Value if redeemed on or after the second anniversary but prior to the third anniversary of the initial issuance date, and (z) 109% of the Series C Liquidation Value if redeemed on or after the third anniversary but prior to the fourth anniversary of the initial issuance date.
Termination and Liquidation Rights
If the Company determines to liquidate, dissolve or wind-up its business, it must redeem the Series C preferred stock at the prices set forth above. Upon any liquidation, dissolution or winding up of the Company, the Holders of Series C preferred stock shall be first entitled to be paid out of the assets of the Company available for distribution to its stockholders an amount with respect to each share of Series C preferred stock equal to $10,000, plus any accrued and unpaid dividends.
Related Secured Promissory Notes Receivable:
In accordance with the terms of the Series C preferred stock agreement, on April 14, 2011 and associated with the first Series C Tranche notice which occurred on December 31, 2010, Socius issued to the Company a secured promissory note of $4,000,000 for 22,222,222 shares of common stock and issued a secured promissory note of $800,000 for the exercise of warrants for 4,444,444 shares of common stock. On June 16, 2011 and associated with the second Series C Tranche notice, Socius issued to the Company a secured promissory note of $4,000,000 for 21,390,374 shares of common stock and issued a secured promissory note of $800,000 for the exercise of warrants for 4,278,075 shares of common stock. On September 22, 2011 and associated with the third Series C Tranche notice, Socius issued to the Company a secured promissory note of $1,500,000 for 9,671,180 shares of common stock and issued a secured promissory note of $300,000 for the exercise of warrants for 1,934,236 shares of common stock. On December 15, 2011 and associated with the fourth Series C Tranche notice, Socius issued to the Company a secured promissory note of $2,000,000 for 20,512,821 shares of common stock and issued a secured promissory note of $400,000 for the exercise of warrants for 4,102,564 shares of common stock. Interest on the notes accrues at 2% per year, compounding annually if the interest remains unpaid at the end of each year. The note is secured by freely tradable marketable securities belonging to Socius. Each promissory note matures on the fourth anniversary of its issuance.
In the event the Company redeems all or a portion of any shares of Series C preferred stock held by Socius, the Company will be permitted to offset the full amount of such proceeds against amounts outstanding under the promissory notes. Accordingly, the Company included the discounted value of the secured promissory notes as a separate component of stockholders’ deficit at December 31, 2011 and 2010.
| F-23 |
The value of the secured promissory notes in the accompanying consolidated balance sheet was $12,173,251, net of discounts of $1,740,516 and accrued interest of $113,767 at December 31, 2011, reflecting a face value of $13,800,000. The Company determined that a 6% discount is appropriate, in order to consistently reflect the Company’s cost of borrowing under the terms of the underlying Series C preferred stock that permits offset. The Company recorded an initial discount on the promissory notes in the amount of $1,968,050 during the year ended December 31, 2011. The Company accretes interest at 6% over the respective four-year terms of the promissory notes.
During the years ended December 31, 2011 and 2010 the Company accreted interest on the promissory note in the amount of $341,301 and $0, respectively, which was recorded in accumulated deficit during the periods then ended. The Company recorded dividends on its Series C preferred stock during the years ended December 31, 2011 and 2010 of $400,110 and $0, respectively. The accrued dividends are offset by the accretion of the note receivable discount.
The Company has classified the Series C redeemable preferred stock in the equity section in its consolidated balance sheets. As of December 31, 2011 and 2010, 1,150 and 400 shares of Series C preferred stock were outstanding, respectively.
| 12. | WARRANT SUMMARY |
Warrant Activity
A summary of warrant activity for the year ended December 31, 2011 is presented below:
| Weighted | ||||||||||||||||
| Weighted | Average | Aggregate | ||||||||||||||
| Average | Remaining | Intrinsic | ||||||||||||||
| Number of | Exercise | Contractual | Value | |||||||||||||
| Warrants | Price $ | Life (in years) | (000)$ | |||||||||||||
| Outstanding, December 31, 2010 | 134,931,242 | 0.12 | 3.54 | |||||||||||||
| Granted | 20,575,780 | 0.20 | ||||||||||||||
| Exercised | (132,870,434 | ) | 0.08 | |||||||||||||
| Forfeited/Canceled | (879,167 | ) | 1.61 | |||||||||||||
| Outstanding, December 31, 2011 | 21,757,421 | 0.18 | 2.88 | - | ||||||||||||
| Exercisable, December 31, 2011 | 21,757,421 | 0.18 | 2.88 | - | ||||||||||||
The aggregate intrinsic value in the table above is before applicable income taxes and is calculated based on the difference between the exercise price of the warrants and the quoted price of the Company’s common stock as of the reporting date.
| F-24 |
The following table summarizes information about warrants outstanding and exercisable at December 31, 2011:
| Warrants Outstanding and Exercisable | ||||||||||||||
| Weighted | Weighted | |||||||||||||
| Average | Average | |||||||||||||
| Exercise | Number | Remaining | Exercise | |||||||||||
| Price $ | of Shares | Life (Years) | Price $ | |||||||||||
| .10 - .11 | 15,916,785 | 2.59 | 0.10 | |||||||||||
| .20 - .30 | 1,630,000 | 4.00 | 0.25 | |||||||||||
| .38-.39 | 1,330,636 | 5.57 | 0.39 | |||||||||||
| .40-.45 | 2,065,000 | 2.06 | 0.42 | |||||||||||
| 0.70 | 815,000 | 4.00 | 0.70 | |||||||||||
| 21,757,421 | ||||||||||||||
During the year ended December 31, 2011, the Company issued 3,413,016 warrants to consultants. The warrants were 3 and 5 year warrants with an exercise price ranging from $0.10 to $0.70. The fair value of the warrants at the date of issuance was approximately $660,000 which was recorded as consulting expense in the accompanying consolidated financial statements. The Company used the Black-Scholes option pricing model to value the warrants using the following assumptions: (1) dividend yield of 0%; (2) expected volatility of 165-170%, (3) risk-free interest rate of 0.81- 2.01%, and (4) expected life of 3-5 years.
During the year ended December 31, 2011, the Company extended the expiration date of 2,403,445 warrants that had expired on December 31, 2010. The fair value of the warrants at the extension date was approximately $470,000 which was recorded as financing costs in the accompanying consolidated statement of operations. The Company used the Black-Scholes option pricing model to value the warrants using the following assumptions: (1) dividend yield of 0%; (2) expected volatility of 170%, (3) risk-free interest rate of 1.02%, and (4) expected life of 4 years.
During the year ended December 31, 2011, the Company issued to Socius 14,759,319 warrants which were exercised immediately through Socius issuing the Company a note receivable as discussed in Note 9.
During the year ended December 31, 2011, there were 34,225,302 warrants exercised for $3,377,715 in cash and 83,885,817 warrants exercised using the cashless exercise provision or legal settlements with the company.
| 13. | STOCKHOLDERS’ EQUITY TRANSACTIONS |
During the year ended December 31, 2011, the Company issued 1,519,077 shares related to the convertible debenture redemptions for the principal amount of $151,909.
During the year ended December 31, 2011, the Company issued 636,126 shares for the exercise of 1,500,000 non-employee options and relieved the derivative liability for $160,161.
During the year ended December 31, 2011, the Company issued 750,000 shares for the exercise of 750,000 employee options.
On January 1, 2011, the Company issued 1,630,000 shares of common stock in connection with consulting agreements. The agreements are for one year unless terminated by either party. The Company recognized consulting expense of $342,300 related to these agreements during the year ended December 31, 2011.
On January 11, 2011, per Gary Rabin’s employment agreement, the Company issued 5,000,000 shares of restricted common stock. The Company valued the shares at $0.14 per share for a value of $700,000. The Company was to amortize this expense over the earlier of one year or the naming of a new CEO. On July 1, 2011, Gary Rabin was named CEO; therefore, the Company fully amortized the $700,000 as of July 1, 2011. During the year ended December 31, 2011 the Company recorded $700,000 as payroll expense in the accompanying consolidated statement of operations.
| F-25 |
On February 11, 2011 and June 15, 2011, the Company entered into an agreement with Gemini Master Fund (“Gemini”), whereby, the Company issued 20,000,000 shares and 1,987,829 shares, respectively to settle errors involving warrant issuances to Gemini. The Company relieved the warrant liability and recorded a financing cost of $2,401,282 in the accompanying consolidated statements of operations.
On February 14, 2011, the Company issued Robert Lanza 12,421,101 shares of common stock. The Company had granted these shares to Mr. Lanza during 2010 and had recorded the compensation expense and an accrued liability for $1,117,899 as of December 31, 2010. The Company relieved the accrued liability with the issuance of the shares.
On February 15, 2011, the Company issued 3,222,786 shares of common stock valued at $654,000 to Optimus CG II Ltd, which was related to the true-up of shares issued on conversion of warrants. The Company has recorded the expense as a finance cost and the accrued liability in the 2010 consolidated financial statements. The Company relieved the accrued liability with the issuance of the shares.
On February 15, 2011, the Company issued Transition Holdings, Ltd. 7,413,000 shares of common stock for full settlement of the licensing agreements between Transition Holdings, Ltd. and the Company. The Company had recorded the value of the shares in the December 31, 2010 consolidated financial statements as advances payable. The Company relieved the advances payable with the issuance of the shares.
On February 16, 2011, the Company issued a board member 406,324 shares of common stock valued at $73,138 as compensation for board services.
On April 13, 2011, the Company issued 26,666,666 shares of common shares in exchange for promissory notes of $4,000,000 and $800,000 as discussed in Note 9.
On May 16, 2011, the Company issued 751,406 shares of common stock valued at $133,600 to University of Massachusetts for licensing rights.
On June 16, 2011, the Company issued 25,668,449 shares of common stock in exchange for promissory notes of $4,000,000 and $800,000 as discussed in Note 9.
On June 30, 2011, the Company issued 7,562,008 shares of common stock related to the $1,250,000 commitment fee for the Series C first and second tranche as discussed in Note 9.
Effective July 1, 2011, the Company entered into an amended and restated employment agreement with Gary Rabin. Per the agreement, the Company agreed to issue 10,000,000 shares of restricted stock which vests in equal installments on the last day of each calendar quarter commencing on July 31, 2011 and ending on December 31, 2013. As of December 31, 2011, 2,000,000 shares have been issued. The Company valued the 10,000,000 shares at $0.185 per share for a value of $1,850,000 which will be amortized over 30 months. During the years ended December 31, 2011 and 2010, the Company recorded $370,000 and $0 as payroll expense in the accompanying consolidated statements of operations.
On August 8, 2011, the Company entered into a new employment agreement with Robert Lanza. Per the agreement, the Company agreed to issue 15,000,000 shares of restricted stock with 6,000,000 shares vesting immediately and the remaining 9,000,000 shares vesting over a 21 months period beginning on January 31, 2012. As of December 31, 2011, 6,000,000 shares have been issued. The Company valued the 15,000,000 shares at $0.1571 per share for a value of $2,356,500 which will be amortized through September 30, 2013. During the years ended December 31, 2011 and 2010, the Company recorded $1,214,504 and $0 as payroll expense in the accompanying consolidated statements of operations.
On August 9, 2011, the Company entered into a settlement agreement and mutual release with Midsummer Investment, Ltd. and Midsummer Small Cap Master, Ltd. (collectively, “Midsummer”). Pursuant to the agreement, the Company issued to Midsummer 36,000,000 shares on August 12, 2011. The shares were recorded as finance costs and valued $6,120,000..
| F-26 |
On September 2, 2011, the Company issued a board member 100,982 shares of common stock valued at $16,157 as compensation for board services.
On September 22, 2011, the Company issued 11,605,416 shares of common stock in exchange for promissory notes of $1,500,000 and $300,000 as discussed in Note 9.
On November 2, 2011, the Company issued a board member 500,000 shares of common stock valued at $90,000 as compensation for board services.
On November 2, 2011, the Company issued 39,514,859 shares to Alpha Capital as a result of a preliminary injunction from the court. The shares were recorded as finance costs and valued at $4,947,800.
On November 14, 2011, the Company issued a board member 13,846 shares of common stock valued at $2,492 as compensation for board services.
On November 15, 2011, the Company issued 18,000,000 shares to Black Mountain Equities as a result of a preliminary injunction from the court. The shares were recorded as finance costs and valued at $1,615,062.
On December 15, 2011, the Company issued 24,615,385 shares of common stock in exchange for promissory notes of $2,000,000 and $400,000 as discussed in Note 9.
On December 16, 2011, the Company issued 10,730,265 shares to Cranshire Capital Master Fund, Ltd. as a result of a preliminary injunction from the court. The shares were recorded as finance costs and valued at $1,073,027.
On December 30, 2011, the Company issued various board members and an executive officer 2,550,000 shares of common stock valued at $207,667 as compensation for board services.
During the year ended December 31, 2011, the Company issued 3,252,066 shares of common stock for the cashless exercise of 5,516,943 warrants not described above. The warrants were executed in accordance with their terms.
During the year ended December 31, 2011, the Company received $3,377,715 from the cash exercise of 34,225,302 warrants.
| 14. | STOCK-BASED COMPENSATION |
Stock Plans
| Options/Shares | ||||||||||||
| Options/Shares | Options | Available | ||||||||||
| Stock Plan | Issued | Outstanding | For Grant | |||||||||
| 2004 Stock Plan | 2,492,000 | 70,000 | 308,000 | |||||||||
| 2004 Stock Plan II | 1,301,161 | 1,071,161 | - | |||||||||
| 2005 Stock Plan | 95,169,650 | 90,659,124 | 155,841,383 | |||||||||
| 98,962,811 | 91,800,285 | 156,149,383 | ||||||||||
| F-27 |
Stock Option Activity
A summary of option activity for the years ended December 31, 2011 is presented below:
| Weighted | ||||||||||||||||
| Weighted | Average | Aggregate | ||||||||||||||
| Average | Remaining | Intrinsic | ||||||||||||||
| Number of | Exercise | Contractual | Value | |||||||||||||
| Options | Price | Life (in years) | (000) | |||||||||||||
| Outstanding, December 31, 2009 | 28,486,119 | 0.32 | 8.09 | 33 | ||||||||||||
| Granted | 19,890,000 | 0.11 | ||||||||||||||
| Exercised | - | - | ||||||||||||||
| Forfeited/canceled | - | - | ||||||||||||||
| Outstanding, December 31, 2010 | 48,376,119 | $ | 0.23 | 7.56 | $ | 3,825 | ||||||||||
| Granted | 46,207,499 | - | ||||||||||||||
| Exercised | (2,250,000 | ) | - | |||||||||||||
| Forfeited/canceled | (533,333 | ) | - | |||||||||||||
| Outstanding, December 31, 2011 | 91,800,285 | $ | 0.23 | 8.19 | $ | 2 | ||||||||||
| Vested and expected to vest at December 31, 2011 | 86,920,122 | $ | 0.23 | 8.14 | $ | 2 | ||||||||||
| Exercisable, December 31, 2011 | 54,260,570 | $ | 0.25 | 7.46 | $ | 2 | ||||||||||
The aggregate intrinsic value in the table above is before applicable income taxes and is calculated based on the difference between the exercise price of the options and the quoted price of the Company’s common stock as of the reporting date.
The following table summarizes information about stock options outstanding and exercisable at December 31, 2011.
| Options Outstanding | Options Exercisable | |||||||||||||||||||||||||
| Weighted | Weighted | Weighted | Weighted | |||||||||||||||||||||||
| Average | Average | Average | Average | |||||||||||||||||||||||
| Exercise | Number | Exercise | Remaining | Number | Exercise | Remaining | ||||||||||||||||||||
| Price | of Shares | Price | Life (Years) | of Shares | Price | Life (Years) | ||||||||||||||||||||
| $ | 0.05 | 70,000 | $ | 0.05 | 2.62 | 70,000 | $ | 0.05 | 2.62 | |||||||||||||||||
| 0.09 | 11,890,000 | 0.09 | 8.10 | 5,573,348 | 0.09 | 8.10 | ||||||||||||||||||||
| 0.10 - 0.157 | 35,751,273 | 0.13 | 8.57 | 26,272,106 | 0.12 | 8.22 | ||||||||||||||||||||
| 0.185 - 0.21 | 26,735,835 | 0.19 | 8.65 | 12,991,939 | 0.20 | 7.78 | ||||||||||||||||||||
| 0.25 - 0.45 | 11,071,161 | 0.36 | 8.88 | 3,071,161 | 0.33 | 7.24 | ||||||||||||||||||||
| 0.85 | 5,604,099 | 0.85 | 3.09 | 5,604,099 | 0.85 | 3.09 | ||||||||||||||||||||
| $ | 1.35 - 2.48 | 677,917 | $ | 2.04 | 3.86 | 677,917 | $ | 2.04 | 3.86 | |||||||||||||||||
| 91,800,285 | 54,260,570 | |||||||||||||||||||||||||
The assumptions used in calculating the fair value of options granted using the Black-Scholes option- pricing model for options granted during the years ended December 31, 2011 and 2010 are as follows:
| December 31, | December 31, | December 31, | ||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Risk-free interest rate | 0.02 - 3.47 | % | 2.3 - 2.8 | % | 2.3 - 3.4 | % | ||||||
| Expected life of the options | 0.2 - 9 years | 5 - 7 years | 5 - 10 years | |||||||||
| Expected volatility | 160 - 180 | % | 175 - 180 | % | 185 | % | ||||||
| Expected dividend yield | 0 | % | 0 | % | 0 | % | ||||||
| Expected forfeitures | 13 | % | 13 | % | 13 | % | ||||||
| F-28 |
As of December 31, 2011, total unrecognized stock-based compensation expense related to nonvested stock options was approximately $5,274,000, which is expected to be recognized over a weighted average period of approximately 1.76 years.
| 15. | COMMITMENTS AND CONTINGENCIES |
Estate of William Caldwell
The Company has received a copy of a Creditor’s Claim (the “Claim”) in the amount of $27,909,706 made with the Estate of William Caldwell (“Decedent”), who at the time of his death was the Chief Executive Officer and Chairman of the Board of Directors of the Company. The Claim states that Decedent’s liability arises under a cause of action that the Claimant intends to file in Federal court against the Company for violations of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), including Section 10(b) of the Exchange Act and the rules promulgated thereunder. As of the date of the filing of this report, the Company is not aware of any action commenced against it by the Claimant.
In the Claim, the Claimant alleges that in September 2005, he entered into a Settlement Agreement with the Company pursuant to which he received a warrant to purchase shares of the Company’s Common Stock. In the Claim, the Claimant makes several allegations against the Company including that in reliance on misinformation provided to him by the Decedent he exercised his warrant to purchase the Company’s Common Stock at an inflated price and received fewer shares than he was owed by the Company under the terms of his warrant, that the Company breached the Claimant’s warrant by not timely issuing stock after the warrant was exercised, and that the Company failed to provide proper notice of certain events that allegedly triggered the Claimant’s purported rights to additional shares under the warrant. Claimant previously brought an action against the Company, in October 2007, with respect to a dispute over the interpretation of the anti-dilution provisions of the warrant but withdrew this action the day before the trial date.
Pursuant to the employment agreement between the Company and the Decedent, the Company has to indemnify and hold Decedent harmless from costs, expenses or liability arising out of or relating to any acts or decisions made by Decedent in the course of his employment to the same extent that the Company indemnifies and holds harmless other officers and directors of the company in accordance with the Company’s established policies. Our directors and officers are indemnified by our bylaws against amounts actually and necessarily incurred by them in connection with the defense of any action, suit or proceeding in which they are a party by reason of being or having been directors or officers of the Company. Our certificate of incorporation provides that none of our directors or officers shall be personally liable for damages for breach of any fiduciary duty as a director or officer involving any act or omission of any such director or officer. Insofar as indemnification for liabilities arising under the Securities Act of 1933, as amended, may be permitted to such directors, officers and controlling persons pursuant to the foregoing provisions, or otherwise, the Company has been advised that in the opinion of the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities, other than the payment by the Company of expenses incurred or paid by such director, officer or controlling person in the successful defense of any action, suit or proceeding, is asserted by such director, officer or controlling person in connection with the securities being registered, the Company will, unless in the opinion of counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act and will be governed by the final adjudication of such issue.
The Company determined that an accrual was necessary at December 31, 2011, which is included in the “loss contingency accrual” amount on the accompanying consolidated balance sheets. See Note 7.
| F-29 |
Camofi Master LOC
Camofi Master LOC and Camzhn Master LOC (the "Camofi Parties") filed their Complaint on October 13, 2011. In their Complaint, the Camofi Parties argue that as a result of the transactions between the Company and JMJ, Gemini Master Fund, Ltd. and Midsummer Investment, Ltd. respectively, the exercise prices in their Warrants and debentures should have been reduced. Consequently, the Camofi Parties argue that they have been denied the right to receive, in total, at least 130,795,594 shares of the Company's common stock, which has allegedly resulted in losses to the Camofi Parties of at least $22,265,951. The Camofi Parties also seek unspecified damages, in an amount to be proven at trial, based upon the Company’s alleged failure to lower the conversion price of the debentures and to provide proper notice of reduction in exercise price and conversion price. On November 18, 2011, the Company answered the Complaint and asserted affirmative defenses. Discovery has commenced in this case. Management intends to contest this case vigorously if a reasonable settlement cannot be achieved.
See Note 6 “Accrued Settlement” and Note 7 “Loss Contingency Accrual”
Employment Contracts
The Company has entered into employment contracts with certain executives and research personnel. The contracts provide for salaries, bonuses and stock option grants, along with other employee benefits.
Leases
On January 29, 2010, the Company signed a new lease to move from its Worcester facility to a new 10,607 square-foot facility in Marlboro, Massachusetts. The lease term is from April 1, 2010 through June 30, 2015. Monthly base rent was $12,817 and $12,596 for 2011 and 2010, respectively. The Company amended the lease effective March 1, 2011 adding an additional 1,650 square feet with an increase in monthly rent of $1,513. The Company renewed its site in Los Angeles, California through February 28, 2013. Monthly base rent is $2,170. Annual minimum lease payments are as follows:
| 2012 | $ | 195,340 | ||
| 2013 | 173,640 | |||
| 2014 | 169,300 | |||
| 2015 | 84,650 | |||
| $ | 622,929 |
Rent expense recorded in the financial statements for the years ended December 31, 2011, 2010 and 2009 was approximately $201,000, $281,000 and $134,000, respectively.
| 16. | INCOME TAXES |
The items accounting for the difference between income taxes computed at the federal statutory rate and the provision for income taxes were as follows:
| 2011 | 2010 | 2009 | ||||||||||
| Statutory federal income tax rate | (34 | )% | (34 | )% | (34 | )% | ||||||
| State income taxes, net of federal taxes | (6 | )% | (6 | )% | (6 | )% | ||||||
| Non-includable items | 29 | % | 18 | % | (25 | )% | ||||||
| Increase in valuation allowance | 12 | % | 22 | % | 65 | % | ||||||
| Effective income tax rate | - | - | - | |||||||||
| F-30 |
Significant components of deferred tax assets and (liabilities) are as follows:
| 2011 | 2010 | |||||||
| Deferred tax assets: | ||||||||
| Net operating loss carryforwards | $ | 43,707,906 | $ | 36,704,171 | ||||
| Depreciation | 141,001 | 175,487 | ||||||
| Capitalized R&D expenses | 566,788 | 559,375 | ||||||
| Deferred revenue | 1,392,669 | 1,595,743 | ||||||
| Losses from joint venture | 350,857 | 64,498 | ||||||
| Professional fees paid in stock | 1,253,223 | 1,101,535 | ||||||
| Stock-based compensation | 2,078,675 | 1,412,064 | ||||||
| Reversal of unpaid liabilities | 1,184,298 | 1,184,298 | ||||||
| Valuation allowance | (50,675,417 | ) | (42,797,171 | ) | ||||
| Net deferred tax asset | $ | - | $ | - | ||||
The Company files income tax returns in the U.S. federal jurisdiction, and various state jurisdictions. With few exceptions, the Company is no longer subject to U.S. federal, state and local income tax examinations by tax authorities for years before 2003.
At December 31, 2011, the Company had federal and state net operating loss carry forwards available to offset future taxable income of approximately $103 million and $94 million respectively. These carry forwards will begin to expire in the years ending December 31, 2025 and December 31, 2015, respectively. These net operating losses are subject to various limitations on utilization based on ownership changes in the prior years under Internal Revenue Code Section 382. The Company is in the process of analyzing the impact of the ownership changes but management does not believe they will have a material impact on the Company’s ability to utilize the net operating losses in the future.
The Company periodically evaluates the likelihood of the realization of deferred tax assets, and adjusts the carrying amount of the deferred tax assets by the valuation allowance to the extent the future realization of the deferred tax assets is not judged to be more likely than not. The Company considers many factors when assessing the likelihood of future realization of its deferred tax assets, including its recent cumulative earnings experience by taxing jurisdiction, expectations of future taxable income or loss, the carryforward periods available to the Company for tax reporting purposes, and other relevant factors.
At December 31, 2011, based on the weight of available evidence, including cumulative losses in recent years and expectations of future taxable income, the Company determined that it was more likely than not that its deferred tax assets would not be realized and have a $50.7 million valuation allowance associated with its deferred tax assets.
The components of income tax expense are as follows:
| 2011 | 2010 | 2009 | ||||||||||
| Current federal income tax | $ | - | $ | - | $ | - | ||||||
| Current state income tax | - | - | - | |||||||||
| Deferred taxes | 7,878,246 | 4,309,420 | 3,520,340 | |||||||||
| Valuation allowance | (7,878,246 | ) | (4,309,420 | ) | (3,520,340 | ) | ||||||
| $ | - | $ | - | $ | - | |||||||
Future changes in the unrecognized tax benefit will have no impact on the effective tax rate due to the existence of the valuation allowance. The Company estimates that the unrecognized tax benefit will not change significantly within the next twelve months. The Company will continue to classify income tax penalties and interest as part of general and administrative expense in its consolidated statements of operations. There were no interest or penalties accrued as of December 31, 2011, 2010 or 2009.
| F-31 |
The following table summarizes the open tax years for each major jurisdiction:
| Open Tax | ||
| Jurisdiction | Years | |
| Federal | 2003 – 2010 | |
| States | 2003 - 2010 |
| 17. | GRANT RECEIVED |
On November 2, 2010, the Company received a $977,917 grant under the Patient Protection and Affordable Care Act of 2010 (PPACA). The grant was related to four of the Company’s projects: the Blastomere Program, the Myoblast Program, the RPE Program for Stargardt’s Disease, and the iPS Program. The grants were for $244,479.25 each, for a total grant of $977,917, and are exempt from federal income taxes. The Company recognized $68,639 and $977,917 as a grant reimbursement in its accompanying consolidated statements of operations during the years ended December 31, 2011 and 2010, respectively.
| 18. | RELATED PARTY TRANSACTIONS |
On January 31, 2012, the Shapiro Family Trust received 5,532,198 shares of the Company’s common stock upon cashless exercise of the warrants in connection with the 2005-2008 convertible debentures and in accordance with the December 7, 2011 global settlement agreement. Dr. Shapiro, one of the Company’s directors, may be deemed the beneficial owner of the securities owned by the Shapiro Family Trust.
On January 31, 2012, PDPI, LLC received 11,204,101 of the Company’s common stock upon cashless exercise of warrants in accordance with the December 7, 2011 global settlement agreement. Mr. Rabin, the Company’s Chief Executive Office and Chairman of the Board of Directors, has a 33.33% equity interest in the entity.
| 19. | SUBSEQUENT EVENTS |
On January 24, 2012, the Company filed a Certificate of Amendment to the Certificate of Incorporation with the Secretary of State of the State of Delaware to increase the number of authorized shares of common stock by 1,000,000,000 shares to a total of 2,750,000,000 shares of common stock. On January 24, 2012, the shareholders approved the Certificate of Amendment.
On January 30, 2012, the Company issued 48,878,659 shares of common stock to Midsummer based on the August 9, 2011 settlement agreement. At the date of issuance, the Company decreased the accrued settlement by approximately $7,800,000 with a corresponding increase to stockholders’ equity.
On January 31, 2012, the Company issued 239,601,630 shares of common stock pursuant to the settlement agreement with certain holders of convertible promissory notes and warrants that were issued between 2005 and 2010. At the date of issuance, the Company decreased the accrued settlement by approximately $26,360,000 with a corresponding increase to stockholders’ equity.
On February 13, 2012, the Company issued 5,183,374 shares of common stock pursuant to the settlement agreement with RHP Master Fund, Ltd related to the convertible promissory notes and warrants that were issued between 2005 and 2010. At the date of issuance, the Company decreased the loss contingency accrual by approximately $674,000 with a corresponding increase to stockholders’ equity.
On February 24, 2012, the Company entered into an agreement with Cranshire to settle all outstanding claims against the Company. Pursuant to the agreement, the Company is to issue Cranshire (1) an additional 1,949,735 of common stock, (2) plus the quotient of (x) $276,000 divided by (y) 90% of the closing price of common stock on the trading day immediately preceding the entry of the court order. The estimated number of shares of common stock to be issued based on an $0.11 share price at February 24, 2011 is 4,737,614. The Company has accrued approximately $521,000 which has been recorded in the accompanying consolidated financial statements as finance cost and loss contingency accrual.
20. SELECTED QUARTERLY DATA (UNAUDITED)
| Quarterly Periods Ended | ||||||||||||||||
| March 31, | June 30, | September 30, | December 31, | |||||||||||||
| 2011 | 2011 | 2011 | 2011 | |||||||||||||
| Revenue | $ | 153,688 | 153,688 | 132,805 | $ | 66,238 | ||||||||||
| Gross profit (loss) | $ | 130,788 | (127,812 | ) | 116,155 | $ | 43,338 | |||||||||
| Loss from operations | $ | (4,835,655 | ) | (3,611,811 | ) | (6,895,786 | ) | $ | (5,767,106 | ) | ||||||
| Other income (expense) | $ | 1,493,618 | (1,208,338 | ) | (45,625,243 | ) | $ | (6,344,798 | ) | |||||||
| Net income (loss) | $ | (3,342,037 | ) | (4,820,149 | ) | (52,521,029 | ) | $ | (12,111,904 | ) | ||||||
| Basic and diluted earnings (loss) per share | $ | (0.00 | ) | $ | (0.00 | ) | $ | (0.03 | ) | $ | (0.02 | ) | ||||
| F-32 |
| Quarterly Periods Ended | ||||||||||||||||
| March 31, | June 30, | September 30, | December 31, | |||||||||||||
| 2010 | 2010 | 2010 | 2010 | |||||||||||||
| Revenue | $ | 205,158 | $ | 205,158 | $ | 205,158 | $ | 109,570 | ||||||||
| Gross profit | $ | 138,508 | $ | 138,508 | $ | 138,508 | $ | 92,920 | ||||||||
| Loss from operations | $ | (14,975,316 | ) | $ | (1,124,886 | ) | $ | (5,406,563 | ) | $ | (10,821,866 | ) | ||||
| Other income (expense) | $ | (2,874,317 | ) | $ | 4,055,285 | $ | 7,342,278 | $ | (30,567,947 | ) | ||||||
| Net income (loss) | $ | (17,849,633 | ) | $ | 2,930,399 | $ | 1,935,715 | $ | (41,389,813 | ) | ||||||
| Basic and diluted earnings (loss) per share | $ | (0.03 | ) | $ | 0.00 | $ | 0.00 | $ | (0.03 | ) | ||||||
| F-33 |
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
(a) Evaluation of Disclosure Controls and Procedures.
Our Chief Executive Officer (Principal Executive Officer and Principal Financial Officer) has evaluated our disclosure controls and procedures as of December 31, 2011 and have concluded that these disclosure controls and procedures are effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Act is recorded, processed, summarized and reported within the time periods specified in the Commission’s rules and forms and is accumulated and communicated to our management, including the Chief Executive Officer, as appropriate to allow timely decisions regarding required disclosure.
(b) Management’s Report on Internal Control Over Financial Reporting.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in Exchange Act Rules 13a-15(f). Our management conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control – Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Based on this evaluation under the framework in Internal Control – Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2011.
Our internal control over financial reporting as of December 31, 2011, has been audited by SingerLewak LLP, an independent registered public accounting firm, as stated in their report which is included herein.
| 46 |
(c) Changes in Internal Controls Over Financial Reporting.
There were no changes in our internal control over financial reporting that occurred during the fourth quarter of 2011 that have materially affected or are reasonably likely to materially affect our internal control over financial reporting.
| 47 |
Report of Independent Registered Public Accounting Firm
To the Board of Directors and Stockholders
Advanced Cell Technology, Inc. and subsidiary
We have audited Advanced Cell Technology, Inc. and subsidiary's internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. Advanced Cell Technology, Inc. and subsidiary’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, and testing and evaluating the design and operating effectiveness of internal control based on the assessed risk. Our audit also included performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (a) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (b) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (c) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Advanced Cell Technology, Inc. and subsidiary maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control — Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of Advanced Cell Technology, Inc. and subsidiary as of December 31, 2011 and 2010 and the related consolidated statements of operations, stockholders’ deficit and cash flows for each of the three years in the period ended December 31, 2011, and our report dated March 1, 2012 expressed an unqualified opinion.
/s/ SingerLewak LLP
Los Angeles, California
March 1, 2012
| 48 |
Item 9B. Other Information
None.
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
DIRECTORS AND EXECUTIVE OFFICERS OF THE REGISTRANT
Our directors are elected at the annual meeting of shareholders to hold office until the annual meeting of shareholders for the ensuing year or until their successors have been duly elected and qualified. Officers are elected annually by the Board of Directors and serve at the discretion of the Board. The directors and executive officers of the Company are as follows. Our executive officers, key employees and directors are described below. There are no family relationships among our executive officers or directors.
| Name | Age | Position | ||
| Gary Rabin | 46 | Chief Executive Officer and Chairman of the Board of Directors | ||
| Robert P. Lanza M.D. | 56 | Chief Scientific Officer | ||
| Alan C. Shapiro, Ph.D. | 66 | Member of the Board of Directors | ||
| Robert Langer, Sc.D. | 63 | Member of the Board of Directors | ||
| Zohar Loshitzer | 56 | Member of the Board of Directors | ||
| Gregory D. Perry | 51 | Member of the Board of Directors |
Gary Rabin has served as a director since December 2007 and as our Chief Executive Officer and Chairman of the Board since December 2010. Prior to joining ACT as CEO, Mr. Rabin had a twenty three year career in finance that primarily encompassed investment management and capital raising targeting small-cap and emerging growth companies. Until November 2010, he was the Managing Partner of GR Advisors LLC, a long/short hedge fund focused on the media and communications industry. From 2003 until July 2007, he was a Portfolio Manager at MAC Investment Management, LLC ("MAC"), at long/short hedge funds where he focused on communications, healthcare services, energy and special situations. Prior to that, he was Managing Director and Co-Head of the Media and Telecom Investment Banking Group at CIBC World Markets ("CIBC"), where he was responsible for all corporate finance and M&A, financial restructurings, and principal investing activities (both debt and equity) within the sector. Before joining CIBC, Mr. Rabin served in an operating capacity at a broadband services company when he was Chief Strategy Officer of CAIS Internet, Inc. ("CAIS"). At CAIS, he was responsible for raising over $500 million of financing commitments in both the public equity markets and from his relationships at Kohlberg, Kravis Roberts & Co., Qwest Communications, Cisco, Nortel, 3Com and Microsoft. Mr. Rabin has also started and served as Managing Director and Head of the Global Telecom Investment Banking Group at ING Barings Furman Selz, and was a founder of the telecom group at UBS Securities. He began his career in finance in 1987, and concentrated on energy, utilities, and metals until 1993. Throughout his career, Mr. Rabin has been responsible for building and developing businesses. Mr. Rabin earned an AB in Economics from the University of Michigan. Mr. Rabin’s long career as a senior manager in both the investment banking community and as a senior financial executive qualifies him to be a member of the Board of Directors of Advance Cell Technology, Inc.
Robert P. Lanza, M.D. has been our Chief Scientific Officer since October 2007. Dr. Lanza has over 20 years of research and industrial experience in the areas of tissue engineering and transplantation medicine. Before joining us in 1998, from 1990 to 1998, Dr. Lanza was Director of Transplantation Biology at BioHybrid Technologies, Inc., where he oversaw that company's xenotransplantation and bioartificial pancreas programs. He has edited or authored sixteen books, including Principles of Tissue Engineering (2d ed. co-edited with R. Langer and J. Vacante), Yearbook of Cell and Tissue Transplantation, One World The Health & Survival of the Human Species in the Twenty-First Century, and Xeno: The Promise of Transplanting Animal Organs into Humans (co-authored with D.K.C. Cooper). Dr. Lanza received his B.A. and M.D. Degrees from the University of Pennsylvania, where he was both a University Scholar and Benjamin Franklin Scholar. Dr. Lanza is not an officer or director of any other reporting company.
| 49 |
Alan C. Shapiro, Ph.D. has served as director since 2005. He adds more than 30 years' experience in corporate and international financial management to the Company. Dr. Shapiro is currently the Ivadelle and Theodore Johnson Professor of Banking and Finance at the Marshall School of Business, University of Southern California, where he previously served as the Chairman of the Department of Finance and Business Economics, Marshall School of Business. Prior to joining the University of Southern California, Dr. Shapiro taught as an Assistant Professor at the University of Pennsylvania, Wharton School of Business, and has been a visiting professor at Yale University, UCLA, the Stockholm School of Economics, University of British Columbia, and the U.S. Naval Academy. Dr. Shapiro has published over 50 articles in such academic and professional journals as the Journal of Finance, Harvard Business Review, and the Journal of Business, among many others. He frequently serves as an expert witness in cases involving valuation, economic damages, international finance, takeovers, and transfer financing through Trident Consulting Group LLC. He received his B.A. in Mathematics from Rice University, and a Ph.D. in Economics from Carnegie Mellon University. Dr. Shapiro is a trustee of Pacific Corporate Group's Private Equity Fund. Dr. Shapiro’s board experience on multiple public company boards, his recognized expertise as a highly sought after financial advisor and his career as a professor and Chair in the field of Finance and Administration qualifies him as a valued member of Advanced Cell Technology’s Board of Directors.
Robert S. Langer, Sc.D. has served as a director since October 2011. Since 2005, he has been an Institute Professor (there are 14 Institute Professors at MIT; being an Institute Professor is the highest honor that can be awarded to a faculty member). Dr. Langer has written approximately 1,120 articles and has nearly 800 issued or pending patents. His many awards include the National Medal of Science, Charles Stark Draper Prize (considered the engineering Nobel Prize), Albany Medical Center Prize (largest US medical prize) and the Lemelson-MIT prize, for being “one of history’s most prolific inventors in medicine.” Langer is one of the very few individuals ever elected to the Institute of Medicine, the National Academy of Engineering, and the National Academy of Sciences. Dr. Langer also serves on the board of directors of Fibrocell Science, Inc. Dr. Langer’s medical and scientific knowledge and experience qualify him to serve as a director of the Company.
Zohar Loshitzer has served as a director since November 2011. He is currently the CEO of Presbia, Inc. As a principal in Los Angeles-based private equity firm Orchard Capital, he has held leadership positions in several of its portfolio companies, including Presbia. Previously, Mr. Loshitzer served as the president, CEO and founder of Universal Telecom Services (UTS), which provides high-quality, competitively priced voice and data telecommunications solutions to emerging markets. Mr. Loshitzer oversaw the company’s operations and its critical relationships with key foreign entities, mainly in the Indochina region. He is one of the founders of J2 Global Communications (NASDAQ: JCOM), and a co-founder and former managing director of Life Alert Emergency Response, Inc., currently serves as a managing director of Orchard Telecom, Inc., and currently serve as a board member of Environmental Solutions Worldwide Inc. Environmental Solutions Worldwide (ESW) is a publicly traded company (OTCBB: ESWW) engaged through its wholly owned subsidiaries in the design and development. The ESW Group of Companies currently manufactures and markets a diverse line of proprietary catalytic emission conversion, control and support products and technologies for the International Transportation, Construction and Utility markets. and has served as a board member to MAI Systems Corporation, an AMEX-listed company. Earlier in his career, Mr. Loshitzer worked in the aerospace industry at the R&D lab of Precision Instruments, a division of IAI (Israel Aircraft Industries).Mr. Loshitzer’s focuses on helping grow companies from startups to global enterprises. Under his leadership, company infrastructures have been dramatically scaled and offerings broadened while maintaining a strong culture of innovation. Mr. Loshitzer holds a degree in Electrical & Electronic Engineering from Ort Syngalowski College in Israel. Mr. Loshitzer’s finance and business management knowledge and experience qualifies him to serve as a director of the Company.
Gregory D. Perry has served as a director since December 2011. He is currently the Executive V.P. and CFO at ImmunoGen which he joined in January 2009 as Senior Vice President and Chief Financial Officer and was promoted to his current position in March 2011. Before joining ImmunoGen, Mr. Perry was CFO of Elixir Pharmaceuticals, Inc., where he was extensively involved in partnering and fundraising activities. Prior to Elixir, he was CFO of Domantis, Ltd., an antibody-related therapeutics company acquired by GlaxoSmithKline in 2006. Previously, Mr. Perry was Senior Vice President of Finance and CFO at Transkaryotic Therapies, Inc. (TKT) until its acquisition by Shire plc. in 2005. Before joining TKT in 2003, Mr. Perry held positions of increasing responsibility during his five years at PerkinElmer, Inc., rising to Senior Vice President, Finance and Business Development, Life Sciences. Prior to PerkinElmer, Mr. Perry spent the early part of his career at General Electric, joining the company’s financial management program in 1982 and departing in 1996 as Vice President and CFO, GE Medical Systems – Europe, after numerous promotions. Mr. Perry’s pharmaceutical industry knowledge and experience qualifies him to serve as a director of the Company.
| 50 |
CORPORATE GOVERNANCE
General
We believe that good corporate governance is important to ensure that the Company is managed for the long-term benefit of our stockholders. This section describes key corporate governance practices that we have adopted.
Board of Directors Meetings and Attendance
The Board of Directors has responsibility for establishing broad corporate policies and reviewing our overall performance rather than day-to-day operations. The primary responsibility of our Board of Directors is to oversee the management of our company and, in doing so, serve the best interests of the company and our stockholders. The Board of Directors selects, evaluates and provides for the succession of executive officers and, subject to stockholder election, directors. It reviews and approves corporate objectives and strategies, and evaluates significant policies and proposed major commitments of corporate resources. Our Board of Directors also participates in decisions that have a potential major economic impact on our company. Management keeps the directors informed of company activity through regular communication, including written reports and presentations at Board of Directors and committee meetings.
We have no formal policy regarding director attendance at the annual meeting of stockholders. The Board of Directors held one meeting in 2011. All board members were present at the meeting.
Board Committees
Our Board of Directors has established an Audit Committee, a Compensation Committee and a Nominating and Corporate Governance Committee. The members of each committee are appointed by our Board of Directors, upon recommendation of the Nominating Committee, and serve one-year terms. Each of these committees operates under a charter that has been approved by the Board of Directors. The charter for each committee is available on our website. The Audit Committee met four times during 2011. The Compensation Committee met two times during 2011. The Nominating Committee met one time during 2011.
Audit Committee
The Audit Committee's responsibilities include:
| · | Monitoring the integrity of the Company's financial reporting process and systems of internal controls regarding finance, accounting and legal compliance. |
| · | Monitoring the independence and performance of the Company's internal and independent auditors. |
| · | Monitoring compliance by the Company with legal and regulatory requirements. |
| · | Facilitating open communication among the Company's independent auditors, internal auditors, employees, management, and the Board. |
Dr. Shapiro, Mr. Rabin, Mr. Perry and Mr. Loshitzer serve on our Audit Committee. Dr. Shapiro serves as chair of the Audit Committee. The Board of Directors has determined that Dr. Shapiro is an "audit committee financial expert" as defined in Item 401(e) of Regulation S-B. The Board has determined that Dr. Shapiro meets the additional independence requirements of Rule 10A-3 under the Securities Exchange Act of 1934.
Compensation Committee
The Compensation Committee's responsibilities include:
| · | Reviewing and recommending approval of the compensation of our executive officers, |
| · | Overseeing the evaluation of our senior executives, |
| · | Reviewing and making recommendations to the Board of Directors regarding incentive compensation and equity-based plans, |
| · | Administering our stock incentive plans, and |
| · | Reviewing and making recommendations to the Board of Directors regarding director compensation. |
| 51 |
The members of the Compensation Committee are Dr. Shapiro, Mr. Rabin, Mr. Perry and Mr. Loshitzer.
Nominating Committee
The Nominating Committee's responsibilities include:
| · | Identifying individuals qualified to become board members; |
| · | Recommending to the Board the persons to be nominated for election as directors and to each of the board's committees; |
| · | Reviewing and making recommendations to the Board with respect to senior management succession planning; and |
| · | Overseeing an annual evaluation of the Board. |
The members of the Nominating Committee are Dr. Shapiro and Mr. Rabin.
Changes in Nominating Procedures
None.
Director Candidates
The process followed by the Nominating Committee to identify and evaluate director candidates includes requests to board members and others for recommendations, meetings from time to time to evaluate biographical information and background material relating to potential candidates and interviews of selected candidates by members of the Nominating Committee and the Board.
In considering whether to recommend any particular candidate for inclusion in the Board's slate of recommended director nominees, the Nominating Committee applies certain criteria, including
| · | The candidate's honesty, integrity and commitment to high ethical standards, |
| · | Demonstrated financial and business expertise and experience, |
| · | Understanding of our company, its business and its industry, |
| · | Actual or potential conflicts of interest, and |
| · | The ability to act in the interests of all stockholders. |
The Nominating Committee does not assign specific weights to particular criteria and no particular criterion is a prerequisite for each prospective nominee. We believe that the backgrounds and qualifications of our directors, considered as a group, should provide a significant breadth of experience, knowledge and abilities that will allow our Board to fulfill its responsibilities.
The Nominating Committee will consider director candidates recommended by stockholders or groups of stockholders who have owned more than 5% of our common stock for at least a year as of the date the recommendation is made. Stockholders may recommend individuals to the Nominating Committee for consideration as potential director candidates by submitting their names, together with appropriate biographical information and background materials and a statement as to whether the stockholder or group of stockholders making the recommendation has beneficially owned more than 5% of our common stock for at least a year as of the date such recommendation is made, to the Nominating Committee, c/o Corporate Secretary, Advanced Cell Technology, Inc., 33 Locke Drive, Marlboro, Massachusetts. Assuming that appropriate biographical and background material have been provided on a timely basis, the Committee will evaluate stockholder-recommended candidates by following substantially the same process, and applying substantially the same criteria, as it follows for candidates submitted by others.
| 52 |
Communicating with the Directors
The Board will give appropriate attention to written communications that are submitted by stockholders, and will respond if and as appropriate. The chair of the Audit Committee is primarily responsible for monitoring communications from stockholders and for providing copies or summaries to the other directors as he considers appropriate.
Communications are forwarded to all directors if they relate to important substantive matters and include suggestions or comments that the chair of the Audit Committee considers to be important for the directors to know. In general, communications relating to corporate governance and corporate strategy are more likely to be forwarded than communications relating to ordinary business affairs, personal grievances and matters as to which we tend to receive repetitive or duplicative communications.
Stockholders who wish to send communications on any topic to the Board should address such communications to the Board of Directors, c/o Corporate Secretary, Advanced Cell Technology, Inc., 33 Locke Drive, Marlborough, Massachusetts, 01752. You should indicate on your correspondence that you are an Advanced Cell Technology, Inc. stockholder.
Anyone may express concerns regarding questionable accounting or auditing matters or complaints regarding accounting, internal accounting controls or auditing matters to the Audit Committee by calling (508) 756-1212. Messages to the Audit Committee will be received by the chair of the Audit Committee and our Corporate Secretary. You may report your concern anonymously or confidentially.
Board Leadership Structure and Role in Risk Oversight
Although we have not adopted a formal policy on whether the Chairman and Chief Executive Officer positions should be separate or combined, we have traditionally determined that it is in the best interests of the Company and its shareholders to combine these roles. Mr. Caldwell served as our Chairman from January 2005 until December 13, 2010. From December 14, 2010 and currently, Gary Rabin serves as our Chairman and Chief Executive Officer. Due to the small size and early stage of the Company, we believe it is currently most effective to have the Chairman and Chief Executive Officer positions combined.
Our Audit Committee is primarily responsible for overseeing our risk management processes on behalf of our board of directors. The Audit Committee receives and reviews periodic reports from management, auditors, legal counsel, and others, as considered appropriate regarding our company’s assessment of risks. In addition, the Audit Committee reports regularly to the full Board of Directors, which also considers our risk profile. The Audit Committee and the full Board of Directors focus on the most significant risks facing our company and our company’s general risk management strategy, and also ensure that risks undertaken by our Company are consistent with the Board’s appetite for risk. While the Board oversees our company’s risk management, management is responsible for day-to-day risk management processes. We believe this division of responsibilities is the most effective approach for addressing the risks facing our company and that our Board leadership structure supports this approach.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires the Company's directors, executive officers and persons who own more than 10% of the Company's stock (collectively, "Reporting Persons") to file with the SEC initial reports of ownership and changes in ownership of the Company's common stock. Reporting Persons are required by SEC regulations to furnish the Company with copies of all Section 16(a) reports they file. To the Company's knowledge, based solely on its review of the copies of such reports received or written representations from certain Reporting Persons that no other reports were required, the Company believes that during its fiscal year ended December 31, 2011, all Reporting Persons timely complied with all applicable filing requirements, except that Form 3s were not timely filed for Dr. Langer, Mr. Perry, and Mr. Loshitzer and have since been filed.
Code of Ethics
We have adopted a code of business conduct and ethics that applies to our directors, officers (including our principal executive officer, principal financial officer, principal accounting officer or controller, or persons performing similar functions) as well as our employees. A copy of our code of business conduct and ethics is available on our website at www.advancedcell.com under "Investors—Corporate Governance." We intend to post on our website all disclosures that are required by applicable law, the rules of the Securities and Exchange Commission or OTCBB listing standards concerning any amendment to, or waiver from, our code of business conduct and ethics.
| 53 |
ITEM 11. EXECUTIVE COMPENSATION
COMPENSATION DISCUSSION AND ANALYSIS
This section describes the compensation program for our executive officers. In particular, this section focuses on our 2011 compensation program and related decisions.
The Board of Directors has established a Compensation Committee, the majority of which are independent outside directors which approves all compensation and awards to executive management. The members of the Compensation Committee have extensive executive level experience in other companies and bring a perspective of reasonableness to compensation matters with our Company. In addition, the Compensation Committee compares executive compensation practices of similar companies at similar stages of development.
The objectives of our compensation program are as follows:
| · | Reward performance that drives substantial increases in shareholder value, as evidenced through both future operating profits and increased market price of our common shares; and |
| · | Attract, hire and retain well-qualified executives. |
The compensation level of our executives generally reflects their unique position and incentive to positively affect our future operating performance and shareholder value. Part of the compensation of our executives is from equity compensation, primarily through stock option grants or restricted stock awards.
Specific salary and bonus levels, as well as the amount and timing of equity incentive grants, are determined informally and judgmentally, on an individual-case basis, taking into consideration each executive's unique talents and experience as they relate to our needs. With respect to equity compensation, the Compensation Committee approves all option grants, generally based on the recommendation of the president and chief executive officer. Executive compensation is paid or granted pursuant to each executive's compensation agreement. Compensation adjustments are made occasionally based on changes in an executive's level of responsibility or on changed local and specific executive employment market conditions. Based on these factors the Compensation Committee approved the execution of employment agreement with the Company’s only two executive officers.
With respect to the 5,000,000 stock options and 5,000,000 shares of the Company’s common stock awarded to Mr. Rabin, the exercise price was the price of the Company’s common stock on the day that Board approved the grant of the options. With respect to the amount of the stock and options the Board approved the grant because it believed that this was fair in light of the contributions of Mr. Rabin, and the Board believed the shares would provide sufficient incentive for Mr. Rabin to perform services as Interim Chairman and Chief Executive Officer.
A performance bonus was awarded to Mr. Rabin on April 15, 2011, $207,692 was awarded. A retention bonus was awarded to Mr. Rabin on August 5, 2011, $41,667 was awarded. On July 1, 2011, (1) 10,000,000 restricted shares of common stock (2) a non-qualified option to purchase 10,000,000 shares of common stock with an exercise price per share equal to the fair market value on the date of grant, (3) a non-qualified option to purchase 5,000,000 shares of common stock with a price per share equal to $0.30; and (4) a non-qualified option to purchase 5,000,000 shares of common stock with a price per share equal to $0.45 were granted to Mr. Rabin. Bonuses and options were awarded to Mr. Rabin in accordance with his employment Agreement.
On January 10, 2011, the Company granted Dr. Lanza 1,783,333 with a share price equal to the Company’s stock price as of the closing trading date the Agreement was signed. On July 1, 2011, the Company granted Dr. Lanza (1) 15,000,000 restricted shares of common stock , and (2) a non- qualified option to purchase 15,000,000 shares of common stock with an exercise price equal per share equal to the Company’s stock price as of the close of trading date the Agreement was signed.
Risk Management Considerations
In response to the ongoing global economic recession, in 2011 the compensation committee considered the incentives under our executive compensation program and whether they introduced or encouraged excessive risk taking or other behaviors by our executives that could have a negative impact on our business. The compensation committee determined that our executive compensation program provides an appropriate balance of incentives and that it does not encourage our executives to take excessive risks or otherwise create risks that are reasonably likely to have a material adverse effect on us.
| 54 |
Summary Compensation Table
The following table summarizes the annual compensation paid to our named executive officers for the three years ended December 31, 2011, 2010, and 2009:
| Stock | Option | All Other | ||||||||||||||||||||||||||
| Salary | Bonus | Awards | Awards | Comp | Total | |||||||||||||||||||||||
| Name and Principal Position | Year | ($) | ($) | ($) | ($) | ($) | ($) | |||||||||||||||||||||
| Gary Rabin | 2011 | 490,000 | 649,359 | 1,070,000 | 1,378,405 | - | 3,587,764 | |||||||||||||||||||||
| Chief Executive Officer | 2010 | 18,461 | 40,000 | - | 686,896 | 115,692 | (1) | 861,049 | ||||||||||||||||||||
| Principle Financial Officer, and Chairman of the Board of Directors | ||||||||||||||||||||||||||||
| Robert P. Lanza, M.D., | 2011 | 407,500 | 255,000 | 1,214,504 | 1,615,904 | - | 3,492,908 | |||||||||||||||||||||
| Chief Scientific Officer | 2010 | 375,000 | 50,000 | 2,717,298 | - | - | 3,142,298 | |||||||||||||||||||||
| 2009 | 311,250 | 81,250 | - | 441,665 | 1,524 | (2) | 835,689 | |||||||||||||||||||||
| William M. Caldwell, IV | 2010 | 586,667 | 240,000 | 8,035,254 | - | - | 8,861,921 | |||||||||||||||||||||
| Former Chief Executive Officer, | 2009 | 417,500 | 140,000 | - | 210,866 | 1,879 | (2) | 770,245 | ||||||||||||||||||||
Please see the assumptions relating to the valuation of our stock option awards which are contained in Notes to audited Financial Statements included in this 10K.
| (1) | This amount represents the amount earned by Mr. Rabin in his capacity as a director for the Company until December 14, 2010. |
| (2) | This amount represents a life insurance premium paid by the Company for the named executive officer. |
Employment Agreements
Employment Agreement with Gary Rabin
Effective July 1, 2011, the Company entered into an amended and restated employment agreement with Gary H. Rabin (the “Rabin Agreement”). Pursuant to the Rabin Agreement, the parties agreed as follows:
| · | Mr. Rabin will serve as the Company’s chief executive officer and chief financial officer for a term commencing on July 1, 2011 until December 31, 2013 (subject to earlier termination as provided therein). |
| · | The Company will pay Mr. Rabin a base salary of $500,000 per year, through December 31, 2011, which amount shall increase at the end of each year of the Rabin Agreement, by an amount determined by the board, but by not less than 5% per year. |
| · | The Company agreed to pay Mr. Rabin a retention bonus of $41,667 within 10 days of execution of the Rabin Agreement. |
| · | The Company shall pay Mr. Rabin an annual incentive bonus, which will be calculated by reference to the 10-day volume weighted average price of the Company’s common stock, as set forth therein. |
| · | The Company shall pay Mr. Rabin a performance bonus in amount (not less than $100,000 per year) to be determined by the Compensation Committee of the Board of Directors. |
| 55 |
| · | The Company agreed to issue to Mr. Rabin, upon execution of the Rabin Agreement, (i) 10,000,000 shares of common stock, (ii) an option to purchase 10,000,000 shares of common stock with an exercise price equal to fair market value on the date of grant, (iii) an option to purchase 5,000,000 shares of common stock with an exercise price of $0.30, and (iv) an option to purchase 5,000,000 shares of common stock with an exercise price of $0.45. The options will vest, and the shares will no longer be subject to the Company’s right to repurchase for aggregate consideration of $1.00, in equal installments on the last day of each calendar quarter commencing on July 1, 2011 and ending on December 31, 2013. |
| · | If Mr. Rabin’s employment under the Rabin Agreement were to be terminated by the Company without cause (as defined therein), the Company will pay Mr. Rabin (in addition to unpaid base salary, performance bonus and incentive bonus to the date of termination), a lump sum equal to the aggregate installments of base salary in effect on the date of termination and otherwise payable in respect of the period commencing on the date immediately subsequent to the date of termination and ending on the earlier to occur of the first anniversary of such date and December 31, 2013. |
Employment Agreement with Robert P. Lanza, M.D.
Effective July 1, 2011, the Company entered into an amended and restated employment agreement with Robert Lanza (the “Lanza Agreement”). Pursuant to the Lanza Agreement, the parties agreed as follows:
| · | Dr. Lanza will continue serve as the Company’s chief scientific officer for a term commencing on July 1, 2011 until September 30, 2013 (subject to earlier termination as provided therein, and extension by mutual written agreement). |
| · | The Company will pay Dr. Lanza a base salary of $440,000 per year, which amount shall increase at the end of each year of the Lanza Agreement, by an amount determined by the board, but by not less than 5% per year. The Company may also pay Dr. Lanza annual bonuses in in the Company’s sole discretion. |
| · | The Company agreed to issue to Dr. Lanza, upon execution of the Lanza Agreement, (i) 15,000,000 shares of common stock (of which 6,000,000 shares will vest on the date of grant, with the balance of 9,000,000 shares vesting in equal installments on the last day of each month commencing on January 31, 2012 and ending on September 30, 2013), (ii) an option to purchase 15,000,000 shares of common stock with an exercise price equal to the closing price on the date of execution (of which 6,000,000 options will vest on the date of grant, with the balance of 9,000,000 options vesting in equal installments on the last day of each month commencing on January 31, 2012 and ending on September 30, 2013). |
| · | If Dr. Lanza’s employment under the Lanza Agreement were to be terminated by the Company without cause (as defined therein), the Company will pay Dr. Lanza severance equal to one year base salary. |
Stock Option Grants Under Our Stock Option Plans
On July 1, 2011, (1) 10,000,000 restricted shares of common stock (2) a non-qualified option to purchase 10,000,000 shares of common stock with an exercise price per share equal to the fair market value on the date of grant, (3) a non-qualified option to purchase 5,000,000 shares of common stock with a price per share equal to $0.30; and (4) a non-qualified option to purchase 5,000,000 shares of common stock with a price per share equal to $0.45 were granted to Mr. Rabin. Bonuses and options were awarded to Mr. Rabin in accordance with his employment Agreement. Shares granted were under the 2005 stock option plan.
On January 10, 2011, the Company granted Dr. Lanza 1,783,333 with a share price equal to the Company’s stock price as of the closing trading date the Agreement was signed. On July 1, 2011, the Company granted Dr. Lanza (1) 15,000,000 restricted shares of common stock , and (2) a non- qualified option to purchase 15,000,000 shares of common stock with an exercise price equal per share equal to the Company’s stock price as of the close of trading date the Agreement was signed. Shares granted were under the 2005 stock option plan.
| 56 |
Outstanding Equity Awards at December 31, 2011
| Option Awards | Stock Awards | |||||||||||||||||||||||
| Number | Market | |||||||||||||||||||||||
| Number of | Number of | of shares | value of | |||||||||||||||||||||
| Securities | Securities | or units | shares or | |||||||||||||||||||||
| Underlying | Underlying | Option | Option | of stock | units of stock | |||||||||||||||||||
| Unexercised | Unexercised | Exercise | Expiration | that have | that have | |||||||||||||||||||
| Options (#) | Options (#) | Price | Date | not vested | not vested | |||||||||||||||||||
| Name | Exercisable | Unexercisable | ($) | ($) | # | $ | ||||||||||||||||||
| Gary Rabin | 5,000,000 | (1) | - | 0.140 | 12/29/2020 | 8,000,000 | (9) | 640,000 | ||||||||||||||||
| Chief Executive Officer | 2,000,000 | (2) | 8,000,000 | 0.185 | 7/1/2021 | |||||||||||||||||||
| and Chairman | 1,000,000 | (2) | 4,000,000 | 0.30 | 7/1/2021 | |||||||||||||||||||
| 1,000,000 | (2) | 4,000,000 | 0.45 | 7/1/2021 | ||||||||||||||||||||
| Robert P. Lanza, M.D., | ||||||||||||||||||||||||
| Chief Scientific Officer | 500,000 | (3) | - | 0.85 | 1/31/2015 | 9,000,000 | (10) | 720,000 | ||||||||||||||||
| 250,000 | (4) | - | 2.20 | 9/15/2015 | ||||||||||||||||||||
| 4,000,000 | (5) | - | 0.21 | 2/7/2018 | ||||||||||||||||||||
| 5,350,000 | (6) | - | 0.098 | 11/13/2019 | ||||||||||||||||||||
| 1,713,956 | (7) | 69,377 | 0.20 | 1/10/2021 | ||||||||||||||||||||
| 6,000,000 | (8) | 9,000,000 | 0.157 | 8/8/2021 | ||||||||||||||||||||
| (1) | These options held by Mr. Rabin vested in full as of July 1, 2011. |
| (2) | These options held by Mr. Rabin vest in equal installment on the last day of each calendar quarter commencing on July 1, 2011 and ending December 31, 2013. |
| (3) | These options held by Dr. Lanza vested in full as of January 31, 2009. |
| (4) | These options held by Dr. Lanza vested in full as of December 31, 2006. |
| (5) | These options held by Dr. Lanza vested in full as of February 7, 2010. |
| (6) | These options held by Dr. Lanza vested in full as of November 13, 2010. |
| (7) | These options held by Dr. Lanza originally vested evenly over three years but vesting was accelerated when Dr. Lanza signed a new employment agreement in 2011. Under the new vesting schedule the options will be fully vested as of March 31, 2012. |
| (8) | These options held by Dr. Lanza vest as follows: 6,000,000 vest immediately with remaining 9,000,000 vesting in 21 equal installments on the last day of each month beginning on January 31, 2012 and ending on September 30, 2013. |
| (9) | These shares were granted to Mr. Rabin under his employment contract and vest on the last day of each calendar quarter through December 31, 2013. The value is based on the closing market price of $0.08. |
| (10) | These shares were granted to Mr. Lanza under his employment contract and vest on the last day of each calendar quarter through September 30, 2013. The value is based on the closing market price of $0.08. |
| 57 |
Pension Benefits
We do not have any plan which provides for payments or other benefits at, following, or in connection with retirement.
Non-qualified Deferred Compensation
We do not have any defined contribution or other plan which provides for the deferral of compensation on a basis that is not tax-qualified.
DIRECTOR COMPENSATION
| Fees Earned | Stock | Option | All Other | |||||||||||||||||||||
| or Paid in Cash | Awards | Awards | Comp | Total | ||||||||||||||||||||
| Name and Principal Position | Year | ($) | ($) | ($) | ($) | ($) | ||||||||||||||||||
| Alan C. Shapiro, Ph.D. | 2011 | 63,625 | 108,000 | 89,360 | - | 260,985 | ||||||||||||||||||
| Robert Langer, Sc.D. | 2011 | 32,000 | 210,925 | 124,417 | - | 367,342 | ||||||||||||||||||
| Zohar Loshitzer | 2011 | 12,000 | 22,000 | 9,385 | - | 43,385 | ||||||||||||||||||
| Gregory D. Perry | 2011 | 12,000 | 14,167 | 3,905 | - | 30,072 | ||||||||||||||||||
Director Compensation Arrangements
Non-executive members of the Company's Board of Directors receive (1) an initial grant of 100,000 shares of common stock, (2) an annual grant of 100,000 shares of common stock (this number has been increased to 200,000 for 2008), (3) an annual retainer of $40,000 (payable quarterly) and (4) a cash payment for attendance at each board meeting in the amount of $1,500 for in-person meetings and $1,000 for telephonic meetings. Regarding members of the Company's Audit Committee, the Chair receives a payment of $1,500 per meeting and the regular members receive $1,000 per meeting. With respect to the Company's Compensation Committee and the Company's Nominating and Corporate Governance Committee, the Chair receives a payment of $1,125 per meeting and the regular members receive $750 per meeting. Each director is entitled to receive payment of the directors' fees in the form of shares of the Company's Common Stock valued at 150% of the actual directors' fees due and payable. The fee structure for the directors was established and approved by the Compensation Committee and ratified by the full Board of Directors.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
Beneficial Ownership of Directors, Officers and 5% Stockholders
The following table sets forth certain information regarding the beneficial ownership of our Common Stock as of February 7, 2012. On such date, 2,029,049,544 shares of common stock were outstanding.
Beneficial ownership is determined in accordance with the applicable rules of the Securities and Exchange Commission and includes voting or investment power with respect to shares of our common stock. In computing the number of shares of common stock beneficially owned by a person and the percentage ownership of that person, we deemed as outstanding shares of common stock subject to options or warrants held by that person that are currently exercisable or exercisable within 60 days of February 7, 2012. We did not deem these shares outstanding, however, for the purpose of computing the percentage ownership of any other person. The information set forth below is not necessarily indicative of beneficial ownership for any other purpose, and the inclusion of any shares deemed beneficially owned in this table does not constitute an admission of beneficial ownership of those shares.
| 58 |
Unless otherwise indicated, to our knowledge, all persons named in the table have sole voting and investment power with respect to their shares of common stock, except, where applicable, to the extent authority is shared by spouses under applicable state community property laws.
The following table sets forth information regarding beneficial ownership of our capital stock as of February 7, 2012 by:
| · | 5% or greater stockholders; |
| · | Each of our directors and named executive officers; and |
| · | All of our directors and executive officers, as a group. |
| Number of | ||||||||
| Shares | ||||||||
| Beneficially | ||||||||
| Name and Address(1) of Beneficial Owner | Owned | Percentage | ||||||
| 5% or Greater Stockholders | ||||||||
| None | ||||||||
| Directors and Named Executive Officers | ||||||||
| Gary Rabin | 30,822,130 | (2) | 1.51 | % | ||||
| Robert P. Lanza, M.D. | 46,412,336 | (3) | 2.27 | % | ||||
| Alan C. Shapiro, Ph.D. | 23,164,785 | (4) | 1.14 | % | ||||
| Robert Langer, Sc.D. | 2,016,667 | (5) | * | |||||
| Zohar Loshitzer | 266,666 | (6) | * | |||||
| Gregory D. Perry | 183,334 | (7) | * | |||||
| Directors and Executive Officers as a Group ( 6 Persons) | 102,865,918 | 5.04 | % | |||||
(1) Unless otherwise indicated, the address of the beneficial owner is 33 Locke Drive, Marlboro, Massachusetts 01752.
| (2) | Includes (i) indirect ownership of 3,734,700 shares representing 33% of the shares that PDPI, LLC was issued on January 31, 2012 as part of the global settlement with former and current debenture and warrant holders for which Mr. Rabin disclaims beneficial ownership, (ii) 11,000,000 subject to stock options that are currently exercisable or exercisable within 60 days of February 7, 2012. |
| (3) | Includes 19,169,047 shares subject to stock options that are currently exercisable or exercisable within 60 days of February 7, 2012. |
| (4) | Includes (i) 22,564,785 shares subject to convertible debentures, board fees, common stock grant held by The Shapiro Family Trust and of which Dr. Shapiro may be deemed the beneficial owner, (ii) 600,000 shares subject to stock options that are currently exercisable or exercisable within 60 days of February 7, 2012. |
| (5) | Includes 791,677 shares subject to stock options that are currently exercisable or exercisable within 60 days of February 7, 2012. |
| (6) | Includes 83,333 shares subject to stock options that are currently exercisable or exercisable within 60 days of February 7, 2012. |
| (7) | Includes 41,667 shares subject to stock options that are currently exercisable or exercisable within 60 days of February 7, 2012. |
There are no arrangements known to the Company, including any pledge by any person of securities of the Company, the operation of which may at a subsequent date result in a change in control of the Company.
| 59 |
Securities Authorized for Issuance Under Equity Compensation Plans
The following table shows information with respect to each equity compensation plan under which the Company's common stock is authorized for issuance as of the fiscal year ended December 31, 2011
EQUITY COMPENSATION PLAN INFORMATION
| Number of | ||||||||||||
| securities | ||||||||||||
| remaining | ||||||||||||
| available | ||||||||||||
| Number of | for issuance | |||||||||||
| securities | under | |||||||||||
| to be issued | Weighted | equity | ||||||||||
| upon | average | compensation | ||||||||||
| exercise of | exercise price of | plans | ||||||||||
| outstanding | outstanding | (excluding | ||||||||||
| options, | options, | securities | ||||||||||
| warrants and | warrants and | reflected | ||||||||||
| Plan Category | rights | rights | in column (a)) | |||||||||
| (a) | (b) | (c) | ||||||||||
| Equity compensation plans approved by security holders | 91,800,285 | (1) | $ | 0.23 | 156,149,383 | (2) | ||||||
| Equity compensation plans not approved by security holders | 5,873,511 | (3) | $ | 0.34 | - | |||||||
| Total | 97,673,796 | 156,149,383 | ||||||||||
| 1) | Awards for 2,492,000 options have been issued under the Advanced Cell Technology, Inc. 2004 Stock Option Plan I ("2004 Plan 1"), 1,301,161 options have been issued under the Advanced Cell Technology, Inc. 2004 Stock Option Plan II ("2004 Plan 2" and together with the 2004 Plan I, the "2004 ACT Plans"), and 95,169,650 options have been issued under the 2005 Stock Plan. |
| 2) | This number included 308,000 shares available under the 2004 Plan I, 0 shares available under the 2004 Plan II and 155,841,383 shares available under the 2005 Stock Plan. |
| 3) | The number reflects the aggregate number of shares underlying compensatory warrants that have been issued and continue to be outstanding as of December 31, 2011. Each warrant was part of a separate equity compensation arrangement. |
Item 13. Certain Relationships and Related Transactions, and Director Independence
None of the following parties has, during the year ended December 31, 2011, had any material interest, direct or indirect, in any transaction with us or in any presently proposed transaction that has or will materially affect us, other than as noted in this section:
| · | Any of our directors or officers, |
| · | Any person proposed as a nominee for election as a director, |
| · | Any person who beneficially owns, directly or indirectly, shares carrying more than 5% of the voting rights attached to our outstanding shares of common stock, |
| · | Any of our promoters, and |
| · | Any relative or spouse of any of the foregoing persons who has the same house as such person. |
| 60 |
Board Determination of Independence
The Company complies with the standards of "independence" prescribed by rules set forth by the National Association of Securities Dealers ("NASD"). Accordingly, a director will only qualify as an "independent director" if, in the opinion of our Board of Directors, that person does not have a material relationship with our company which would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. A director who is, or at any time during the past three years, was employed by the Company or by any parent or subsidiary of the Company, shall not be considered independent. Accordingly, Dr. Alan Shapiro, Mr. Robert Langer, Mr. Zohar Loshitzer and Mr. Gregory Perry meet the definition of "independent director" under Rule 4200(A)(15) of the NASD Manual; Mr. Rabin does not.
Item 14. Principal Accounting Fees and Services
The following table summarizes the fees of our current independent registered public accounting firm, SingerLewak LLP, billed to us for each of the last three fiscal years for audit services and billed to us in each of the last three years for other services:
| Fee Category | 2011 | 2010 | 2009 | |||||||||
| Audit Fees | $ | 231,000 | $ | 215,000 | $ | 213,859 | ||||||
| Audit Related Fees | $ | 36,500 | $ | 33,102 | $ | 12,000 | ||||||
| Tax Fees | $ | - | $ | - | $ | - | ||||||
| All Other Fees | $ | - | $ | - | $ | - | ||||||
Audit fees consist of aggregate fees billed for professional services rendered for the audit of the Company's annual financial statements and review of the interim financial statements included in quarterly reports or services that are normally provided by the independent auditor in connection with statutory and regulatory filings or engagements for the fiscal years ended December 31, 2011, 2010 and 2009.
Audit related fees consist of aggregate fees billed for assurance and related services that are reasonably related to the performance of the audit or review of the Company's financial statements and are not reported under "Audit Fees." These fees include review of registration statements and participation at meetings of the audit committee.
Tax fees consist of aggregate fees billed for professional services for tax compliance, tax advice and tax planning.
All other fees consist of aggregate fees billed for products and services provided by the independent auditor, other than those disclosed above. These fees include services related to certain accounting research and assistance with a regulatory matter.
The Company's policy is to pre-approve all audit and permissible non-audit services provided by the independent auditors. These services may include audit services, audit-related services, tax services and other services. Pre-approval is generally provided for up to one year and any pre-approval is detailed as to the particular service or category of services and is generally subject to a specific budget. The independent auditors and management are required to periodically report to the audit committee regarding the extent of services provided by the independent auditors in accordance with this pre-approval, and the fees for the services performed to date. To the extent that additional services are necessary beyond those specifically budgeted for, the audit committee and management pre-approve such services on a case-by-case basis. All services provided by the independent auditors were approved by the Audit Committee.
PART IV
Item 15. Exhibits and Financial Statement Schedules
(a)(1) Financial Statements
The following is a list of the Financial Statements included in Item 8 of Part II of this Report.
| Page | ||
| Report of Independent Registered Public Accounting Firm | F-1 | |
| Consolidated Balance Sheets as of December 31, 2011 and December 31, 2010 | F-2 | |
| Consolidated Statements of Operations for the Years Ended December 31, 2011, 2010 and 2009 | F-3 | |
| Consolidated Statements of Stockholders’ Deficit for the Years Ended December 31, 2011, 2010 and 2009 | F-4 | |
| Consolidated Statements of Cash Flows for the Years Ended December 31, 2011, 2010 and 2009 | F-5 | |
| Notes to Financial Statements | F-6 |
| 61 |
(a)(2) Financial Statement Schedules
Schedules not included herein are omitted because they are inapplicable or not required or because the required information is given in the financial statements and notes thereto.
(b)
The exhibits required by this item and included in this report or incorporated herein by reference are as follows:
|
Exhibit Number |
Description | |
| 2.1 | Agreement and Plan of Merger between the Compny, A.C.T. Acquisition Corp. and ACT, dated as of January 3, 2005 (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on January 4, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 2.2 | Agreement and Plan of Merger between Advanced Cell Technology, Inc., a Nevada corporation, and Advanced Cell Technology, Inc., a Delaware corporation, dated as of November 18, 2005 (previously filed as Exhibit 2.1 to the Registrant's Current Report on Form 8-K filed on November 21, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 2.2 | Agreement and Plan of Merger between Advanced Cell Technology, Inc., a Delaware corporation, and ACT, dated as of November 18, 2005 (previously filed as Exhibit 2.2 to the Registrant's Current Report on Form 8-K filed on November 21, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 3.1 | Certificate of Incorporation of the Company (previously filed as Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on November 21, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 3.1.1 | Certificate of Amendment to Articles of Incorporation dated April 1, 2004 (previously filed as Exhibit 3.1.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 3.1.2 | Certificate of Amendment to Articles of Incorporation dated December 30, 2004 (previously filed as Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on January 4, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 3.1.3 | Certificate of Amendment to Articles of Incorporation dated June 23, 2005 (previously filed as Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on June 22, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 3.1.4 | Certificate of Amendment to Articles of Incorporation dated July 6, 2005 (previously filed as Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on July 7, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 3.15 | Certificate of Amendment to Certificate of Incorporation dated September 15, 2009 (previously filed as Exhibit 3.15 to S-1 filed November 18, 2009 and herein incorporated by reference). | |
| 3.16 | Certificate of Designation of Series B Preferred Stock 2005 (previously filed as Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on November 12, 2009 (File No. 000-50295) and incorporated by reference herein). | |
| 3.17 | Certificate of Designation of Series C Preferred Stock (previously filed as exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on January 3, 2011 and incorporated herein by reference). |
| 62 |
| 3.18 | Certificate of Amendment to Certificate of Incorporation (previously filed as exhibit 3.1 to the Registrant’s Current Report on Form 8-K filed on January 24, 2012 and incorporated herein by reference). | |
| 3.2 | Bylaws of the Company (previously filed as Exhibit 3.2 to the Registrant's Current Report on Form 8-K filed on November 21, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 3.2.1 | Amendment to Bylaws of the Company (previously filed as Exhibit 3.1 to the Registrant's Current Report on Form 8-K filed on December 29, 2004 (File No. 000-50295) and incorporated by reference herein). | |
| 4.1 | Specimen Stock Certificate (previously filed as Exhibit 4.1 to the Registrant's Current Report on Form 8-K filed on November 21, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.2 | Form of $0.05 Warrant to Purchase Common Stock of ACT. ACT issued warrants in this form for the purchase of an aggregate of 900,000 shares, including a warrant to purchase 250,000 shares of ACT common stock to Andwell, LLC, an entity affiliated with William Caldwell, IV, the Chief Executive Officer and a director of the Company (previously filed as Exhibit 4.2 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.3 | Form of $0.25 Warrant to Purchase Common Stock of ACT. ACT issued warrants in this form for the purchase of an aggregate of 1,954,000 shares, including (i) a warrant to purchase 236,000 shares of ACT common stock to Andwell, LLC, an entity affiliated with William Caldwell, IV, the Chief Executive Officer and a director of the Company, (ii) a warrant to purchase 75,000 shares of ACT common stock to Rocket Ventures, an entity affiliated with Jonathan Atzen, a Senior Vice President and the General Counsel of the Company (previously filed as Exhibit 4.3 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.4 | $0.25 Warrant to Purchase Common Stock of the Company issued to Gunnar Engstrom (previously filed as Exhibit 4.4 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.5 | Form of $0.85 Warrant to Purchase Common Stock of ACT (previously filed as Exhibit 4.5 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.6 | Form of $1.27 Warrant to Purchase Common Stock of ACT (previously filed as Exhibit 4.6 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.7 | Form of $2.00 Warrant to Purchase Common Stock of ACT (previously filed as Exhibit 4.7 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.8 | Form of Subscription Agreement to Purchase Series A Convertible Preferred Units of ACT (previously filed as Exhibit 4.8 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.9 | Form of Share Purchase Agreement to purchase common stock of Two Moons Kachinas Corp. ("TMOO"), the predecessor to the Company (previously filed as Exhibit 4.9 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.10 | Form of Lock-Up Agreement entered into by certain sellers of TMOO common stock (previously filed as Exhibit 4.10 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 4.11 | Form of Lock-Up Agreement entered into by certain buyers of TMOO common stock (previously filed as Exhibit 4.11 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). |
| 63 |
| 4.12 | Investor's Rights Agreement between ACT and Avian Farms, Inc. dated December 31, 1998 (previously filed as Exhibit 4.12 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 9.1 | Form of Voting Agreement for shares of common stock of ACT held by certain parties effective as of January 31, 2005 (previously filed as Exhibit 9.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000- 50295) and incorporated by reference herein). | |
| 10.1 | Exclusive Development and License Agreement between GTC Biotherapeutics (f/k/a as Genzyme Transgenics Corporation) and ACT dated June 8, 1999 (previously filed as Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000- 50295) and incorporated by reference herein). | |
| 10.2 | Exclusive License Agreement dated April 16, 1996 between the University of Massachusetts and ACT as amended on September 1, 1997, May 31, 2000 and September 19, 2002 (previously filed as Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.3 | Materials and Research Data License Agreement dated January 26, 2001 between Wake Forest University and ACT (previously filed as Exhibit 10.3 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.3.1 | July 1, 2002 Assignment to Wake Forest University Health Sciences (previously filed as Exhibit 10.3.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.4 | Exclusive License Agreement dated February 1, 2002 between the University of Massachusetts and ACT (previously filed as Exhibit 10.4 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.5 | Non-Exclusive Sublicense Agreement between ACT and Infigen, Inc. dated August 1, 2003 (previously filed as Exhibit 10.5 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.6 | Non-Exclusive License Agreements, dated January 1, 2001 between ACT and PPL Therapeutics (Scotland) Limited (previously filed as Exhibit 10.6 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.7 | Nonexclusive License Agreement dated May 1, 2001 between ACT and Immerge BioTherapeutics, Inc. (previously filed as Exhibit 10.7 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.8 | Nonexclusive License and Sponsored Research Agreement dated June 29, 2001 between ACT and Charles River Laboratories, Inc. (previously filed as Exhibit 10.8 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.9 | Non-Exclusive Sublicense Agreement between Cyagra, Inc., ACT, ACT Group and Goyaike, S.A. dated November 20, 2001 (previously filed as Exhibit 10.9 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.10 | Exclusive Sublicense Agreement between ACT, ACT Group and Cyagra, Inc. dated June 28, 2002 (previously filed as Exhibit 10.10 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.11 | Non-Exclusive License Agreement dated November 8, 2002 between ACT and Merial Limited (previously filed as Exhibit 10.11 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). |
| 64 |
| 10.12 | Non-Exclusive Sublicense Agreement between ACT and Infigen, Inc. dated August 1, 2003 (previously filed as Exhibit 10.12 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.13 | Exclusive License Agreement dated October 22, 2003 between ACT and Exeter Life Sciences, Inc. (previously filed as Exhibit 10.13 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.13.1 | Letter of Intent between ELS and ACT dated March 16, 2003 (previously filed as Exhibit 10.13.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.13.2 | Sponsored Research Agreement (previously filed as Exhibit 10.13.2 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.14 | Non-Exclusive License Agreement dated January 4, 2002 between ACT and Genetic Savings & Clone (previously filed as Exhibit 10.14 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.15 | Non-Exclusive License Agreement dated February 3, 2004 between ACT and Pureline Genetics (previously filed as Exhibit 10.15 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.16 | Non-Exclusive License Agreement dated February 3, 2004 between ACT and First Degree Genetics (previously filed as Exhibit 10.6 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.17 | Non-Exclusive License Agreement dated February 3, 2004 between ACT and One Degree Genetics (previously filed as Exhibit 10.17 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.18 | Option to License Intellectual Property dated December 31, 2003 between ACT and PacGen Cellco, LLC (previously filed as Exhibit 10.18 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.18.1 | First Amendment to Option to License Intellectual Property dated February 13, 2004 (previously filed as Exhibit 10.18.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.19 | Exclusive License Agreement (Infigen IP) dated May 14, 2004 between ACT and PacGen Cellco, LLC (previously filed as Exhibit 10.19 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.19.1 | First Amendment to Exclusive License Agreement (Infigen IP) dated August 25, 2005. | |
| 10.20 | Exclusive License Agreement (UMass IP) dated May 14, 2004 between ACT and PacGen Cellco, LLC (previously filed as Exhibit 10.20 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.20.1 | First Amendment to Exclusive License Agreement (UMass IP) dated August 25, 2005, previously filed and incorporated by reference herein. | |
| 10.21 | Exclusive License Agreement (ACT IP) dated May 14, 2004 between ACT and PacGen Cellco, LLC (previously filed as Exhibit 10.21 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.21.1 | First Amendment to Exclusive License Agreement (ACT IP) dated August 25, 2005, previously filed and incorporated by reference herein. |
| 65 |
| 10.22 | Agreement to Amend ACT/CELLCO License Agreements dated September 7, 2004 ACT and PacGen Cellco, LLC (previously filed as Exhibit 10.22 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.23 | Indemnification Agreement of David Merrell to certain buyers of TMOO common stock dated December 31, 2004 (previously filed as Exhibit 10.23 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.24 | Convertible Promissory Note to ACT Group, Inc. dated July 12, 2002 in the amount of $1,000,000 (previously filed as Exhibit 10.24 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.25 | Promissory Note issued by ACT to Pierce Atwood LLP dated January 2005 in the amount of $150,000 (previously filed as Exhibit 10.25 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.26 | Promissory Note issued by ACT to Pierce Atwood dated July 1, 2003 in the amount of $339,000 (previously filed as Exhibit 10.26 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.27 | Promissory Note issued by ACT to Rothwell, Figg, Ernst & Manbeck, P.C. dated July 8, 2003 in the amount of $272,108 (previously filed as Exhibit 10.27 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.28 | Forbearance and Stock Purchase Agreement Among Avian Farms, Inc., ACT Group, Inc., ACT and Cima Biotechnology, Inc., dated July 16, 1999, as amended December 23, 1999 (previously filed as Exhibit 10.28 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.29 | Securityholders' Agreement among ACT, ACT Group, Cyagra, Inc. and Goyaike S.A. dated November 20, 2001 (previously filed as Exhibit 10.29 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.30.1 | Securityholders' Agreement among ACT, ACT Group, Cyagra, Inc. and Goyaike S.A. dated July 1, 2002 (previously filed as Exhibit 10.30.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.30.2 | Collaboration Agreement and Technology License (previously filed as Exhibit 10.30.2 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.30.3 | Separation Agreement among ACT, ACT Group, Cyagra, Inc. and Goyaike S.A. (previously filed as Exhibit 10.30.3 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.31 | Membership Interest Exchange and Asset Sale Agreement dated May 31, 2000, by and among ACT and Hematech, LLC, et al. (previously filed as Exhibit 10.31 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.31.1 | Buyout Option Agreement dated May 31, 2000 between Hematech, LLC and ACT (previously filed as Exhibit 10.31.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.32 | Space Sublease Agreement dated November, 2004, between BioReliance and ACT, for 381 Plantation Street, Worcester, MA 01605 (previously filed as Exhibit 10.32 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). |
| 66 |
| 10.33 | Advanced Cell Technology, Inc. 2004 Stock Option Plan. Pursuant to this option plan, ACT issued options to purchase an aggregate 2,604,000 shares, including (i) options to purchase 1,500,000 shares of ACT common stock to Michael West, the Chairman of the Board of Directors and the Chief Scientific Officer of the Company, and (ii) options to purchase 750,000 shares of ACT common stock to Robert Lanza, the Vice President of Medical and Scientific Development of the Company (previously filed as Exhibit 10.33 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000- 50295) and incorporated by reference herein). | |
| 10.34 | Advanced Cell Technology, Inc. 2004 Stock Option Plan II. Pursuant to this option plan, ACT issued options to purchase an aggregate 1,301,161 shares, including (i) options to purchase 651,161 shares of ACT common stock to William Caldwell, IV, the Chief Executive Officer and a director of the Company, and (ii) options to purchase 240,000 shares of ACT common stock to Robert Peabody, a director of the Company (previously filed as Exhibit 10.34 to the Registrant's Quarterly Report on Form 10- QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.35 | A.C.T. Holdings, Inc. 2005 Stock Option Plan (previously filed as Appendix A to the Registrant's preliminary proxy statement on Form PRE-14A filed on May 10, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.36 | Form of Incentive Stock Option Agreement (previously filed as Exhibit 10.36 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.37 | Form of Nonqualified Stock Option Agreement (previously filed as Exhibit 10.37 to the Registrant's Quarterly Report on Form 10- QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.38 | Employment Agreement between ACT and William M. Caldwell, IV dated December 31, 2004 (previously filed as Exhibit 10.38 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.39 | Employment Agreement between ACT and Michael D. West dated December 31, 2004 (previously filed as Exhibit 10.39 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.39.1 | Amendment No. 1 to Employment Agreement between ACT and Michael D. West dated August 1, 2005 (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on August 5, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.40 | Employment Agreement between ACT and Robert Lanza dated February 1, 2005 (previously filed as Exhibit 10.40 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.41 | Employment Agreement between the Registrant, ACT and James G. Stewart dated March 13, 2005 (previously filed as Exhibit 10.41 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.41.1 | Amendment to Employment Agreement between the Registrant and James G. Stewart dated September 16, 2005 (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on September 22, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.42 | Employment Agreement between ACT and Robert Peabody dated February 9, 2005 (previously filed as Exhibit 10.42 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.43 | Employment Agreement between ACT and Jonathan Atzen dated April 1, 2005 (previously filed as Exhibit 10.43 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). |
| 67 |
| 10.44 | Employment Agreement between ACT and Irina Klimanskaya dated October 1, 2003 (previously filed as Exhibit 10.44 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.45 | Employment Agreement between ACT and Sadhana Agarwal dated April 1, 2004 (previously filed as Exhibit 10.45 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.46 | Employment Agreement between ACT and James Murai dated February 17, 2005 (previously filed as Exhibit 10.46 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.47 | Employment Agreement between ACT and David Larocca dated February 9, 2005 (previously filed as Exhibit 10.47 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.48 | Consulting Agreement between ACT and William M. Caldwell, IV dated October 1, 2004 (previously filed as Exhibit 10.48 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.49 | Consulting Agreement between ACT and Jonathan Atzen dated January 14, 2005 (previously filed as Exhibit 10.49 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.50 | Consulting Agreement between ACT and Stephen Price dated December 31, 2004 (previously filed as Exhibit 10.50 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.50.1 | Consulting Agreement between ACT and Stephen Price dated April 28, 2005 (previously filed as Exhibit 10.50.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.51 | Consulting Agreement between ACT and Chad Griffin dated April 1, 2005 (previously filed as Exhibit 10.51 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.52 | Consulting Agreement between ACT and James Stewart dated January 14, 2005 (previously filed as Exhibit 10.52 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.53 | Settlement Agreement between ACT and Gunnar Engstrom dated January 28, 2005 (previously filed as Exhibit 10.53 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.54 | Confidentiality and Nondisclosure Agreement dated February 3, 1999 between ACT and Robert Lanza, M.D. (previously filed as Exhibit 10.54 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.55 | Consulting Agreement dated September 29, 1997 between ACT and Dr. James Robl (previously filed as Exhibit 10.55 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.56 | Consulting Agreement dated January 23, 1998 between ACT and Dr. James Robl (previously filed as Exhibit 10.56 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.57 | Final Settlement Agreement dated August 6, 1999 between Infigen, Inc., ACT and Steven Stice (previously filed as Exhibit 10.57 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). |
| 68 |
| 10.58 | Letter Agreement dated April 20, 2000 between ACT and Dr. Steven L. Stice (previously filed as Exhibit 10.58 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.59 | Master Laboratory Services Agreement dated as of January 4, 2001 between White Eagle Laboratories, Inc. and ACT (previously filed as Exhibit 10.59 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.60 | Master Study Agreement dated as of December 4, 2000 between Biomedical Research Models, Inc. and ACT (previously filed as Exhibit 10.60 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.61 | Agreement Relating to the Transfer of Biological Materials dated as of February 3, 2000 between Wake Forest University and ACT (previously filed as Exhibit 10.61 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.62 | Materials Transfer Agreement dated February 16, 2000 between ACT, B.C. Cancer Agency and Dr. Peter Lansdorp (previously filed as Exhibit 10.62 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.63 | Materials Transfer Agreement dated January 19, 2000 between ACT, IPK and Anna Wobus (previously filed as Exhibit 10.63 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.64 | Materials Transfer Agreement dated February 23, 2000 between ACT, Philip Damiani and Carlos T. Moraes (previously filed as Exhibit 10.64 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.65 | Material Transfer Agreement dated January 6, 1997 between ACT, University of Massachusetts, University of Colorado and Curtis R. Freed (previously filed as Exhibit 10.65 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000- 50295) and incorporated by reference herein). | |
| 10.66 | Material Transfer Agreement dated March 20, 2000 between ACT, Charlotte Farin and Peter Farin (previously filed as Exhibit 10.66 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.67 | Sponsored Research Agreement dated as of May 15, 2000 between Carl H. Lindner, Jr. Family Center for Research of Endangered Wildlife (CREW) and ACT (previously filed as Exhibit 10.67 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.68 | Sponsored Research Agreement dated as of August 9, 2000 between Cornell University and ACT (previously filed as Exhibit 10.68 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.69 | Sponsored Research Agreement dated as of December 1, 1999 between ACT and the University of Massachusetts Amherst (previously filed as Exhibit 10.69 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.69.1 | Amendment No. 1 to Agreement dated December 1, 1999 (previously filed as Exhibit 10.69.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.70 | Sponsored Research Agreement dated August 1, 1999 between ACT and UMass (D. Good) (previously filed as Exhibit 10.70 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). |
| 69 |
| 10.71 | Term Sheet for Non-Exclusive License Agreement dated as of December 23, 2000 between Immerge BioTherapeutics, Inc. and ACT, as amended by First Amendment to Term Sheet dated March 14, 2001 (previously filed as Exhibit 10.71 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.72 | Withdrawal, Termination, Assignment and Assumption Agreement dated March 14, 2001 by and among ACT, BioTransplant, Inc., Immerge BioTherapeutics, Inc. and Infigen, Inc. (previously filed as Exhibit 10.72 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.73 | Consulting Agreement between ACT and Karen Chapman dated January 15, 2005 (previously filed as Exhibit 10.73 to the Registrant's Quarterly Report on Form 10-QSB filed on May 23, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.74 | Research Collaboration Agreement between ACT and The Burnham Institute dated May 23, 2005 (previously filed as Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-QSB filed on August 15, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.75 | Securities Purchase Agreement dated September 15, 2005 (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.76 | Registration Rights Agreement dated September 15, 2005 (previously filed as Exhibit 10.2 to the Registrant's Current Report on Form 8-K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.77 | Form of Common Stock Purchase Warrant (previously filed as Exhibit 10.3 to the Registrant's Current Report on Form 8-K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.78 | Form of Amortizing Convertible Debenture (previously filed as Exhibit 10.4 to the Registrant's Current Report on Form 8-K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.79 | Form of Lock-up Agreement (previously filed as Exhibit 10.5 to the Registrant's Current Report on Form 8-K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.80 | Settlement Agreement dated September 14, 2005 (previously filed as Exhibit 10.6 to the Registrant's Current Report on Form 8-K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.81 | Form of Convertible Promissory Note (Unsecured) (previously filed as Exhibit 10.7 to the Registrant's Current Report on Form 8- K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.82 | Form of Warrant to Purchase Securities (previously filed as Exhibit 10.8 to the Registrant's Current Report on Form 8-K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.83 | Agreement between Advanced Cell Technology, Inc., Advanced Cell, Inc. and A.C.T. Group, Inc. dated September 15, 2005 (previously filed as Exhibit 10.9 to the Registrant's Current Report on Form 8-K filed on September 19, 2005 (File No. 000-50295) and incorporated by reference herein). | |
| 10.84 | Agreement between Capital Financial Media, LLC and Advanced Cell Technology, Inc., dated February 9, 2006 (previously filed as Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-QSB filed on May 15, 2006 (File No. 000-50295) and incorporated by reference herein). |
| 70 |
| 10.85 | Sublease Agreement between Avigen, Inc. and Advanced Cell Technology, Inc., dated November 29, 2005. (previously filed as Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-QSB filed on May 15, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.86 | Exclusive Sublicense Agreement between Advanced Cell Technology, Inc. and TranXenoGen, Inc., dated March 29, 2006 (previously filed as Exhibit 10.3 to the Registrant's Quarterly Report on Form 10-QSB filed on May 15, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.87 | Non-Exclusive License Agreement between Kirin Beer Kabushiki Kaisha, Aurox, LLC, Hematech, LLC, and Kirin SD, Inc., and Advanced Cell Technology, Inc., dated May 9, 2006 (previously filed as Exhibit 10.1 to the Registrant's Quarterly Report on Form 10-QSB filed on August 11, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.88 | Exclusive License Agreement between Kirin Beer Kabushiki Kaisha, Aurox, LLC, Hematech, LLC, and Kirin SD, Inc., and Advanced Cell Technology, Inc., dated May 9, 2006 (previously filed as Exhibit 10.2 to the Registrant's Quarterly Report on Form 10-QSB filed on August 11, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.89 | Purchase Agreement between Kirin SD, Inc. and Advanced Cell Technology, Inc., dated May 9, 2006(previously filed as Exhibit 10.3 to the Registrant's Quarterly Report on Form 10-QSB filed on August 11, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.90 | Consulting Agreement between Advanced Cell Technology, Inc. and James G. Stewart, dated August 17, 2006 (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on August 18, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.91 | Securities Purchase Agreement dated August 30, 2006 (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on September 8, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.92 | Registration Rights Agreement dated September 15, 2005 (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on September 8, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.93 | Form of Common Stock Purchase Warrant (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on September 8, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.94 | Form of Amortizing Convertible Debenture (previously filed as Exhibit 10.4 to the Registrant's Current Report on Form 8-K filed on September 8, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.95 | Form of Lock-up Agreement (previously filed as Exhibit 10.5 to the Registrant's Current Report on Form 8-K filed on September 8, 2006 (File No. 000-50295) and incorporated by reference herein). | |
| 10.96 | Amendment No. 1, dated as of January 11, 2007, to the Securities Purchase Agreement, dated August 30, 2006, the Amortizing Convertible Debenture, dated September 6, 2006, and the Registration Rights Agreement, dated August 30, 2006 (previously filed as Exhibit 10.97 to the Registrant's Registration Statement on Form SB-2 filed on January 26, 2007 (File No. 333-140265) and incorporated by reference herein). | |
| 10.97 | Amendment No. 1, dated as of January 11, 2007, to the Securities Purchase Agreement, the Amortizing Convertible Debenture, and the Registration Rights Agreement, each dated August 30, 2006 (previously filed as Exhibit 10.97 to the Registrant's Registration Statement on Form SB-2 filed on January 26, 2007 (File No. 333-140265) and incorporated by reference herein). | |
| 10.98 | Patent Assignment Agreement between Advanced Cell Technology, Inc. and Infigen, Inc., dated February 5, 2007 (previously filed as Exhibit 10.98 to the Registrant's Post-Effective Amendment No. 3 to its Registration Statement on Form SB-2 filed on March 28, 2007 and incorporated by reference herein). |
| 71 |
| 10.99 | Employment Agreement between Advanced Cell Technology, Inc. and Pedro Huertas, M.D., Ph.D., dated February 5, 2007 (previously filed as Exhibit 10.99 to the Registrant's Post-Effective Amendment No. 3 to its Registration Statement on Form SB-2 filed on March 28, 2007 and incorporated by reference herein). | |
| 10.100 | Research Services Agreement between Advanced Cell Technology, Inc. and Oregon Health & Science University, dated February 5, 2007 (previously filed as Exhibit 10.100 to the Registrant's Post-Effective Amendment No. 3 to its Registration Statement on Form SB-2 filed on March 28, 2007 and incorporated by reference herein). | |
| 10.101 | Agreement and Plan of Merger by and among Advanced Cell technology, Inc., ACT Acquisition Sub, Inc., Mytogen, Inc. and certain shareholders of Mytogen, Inc., dated as of July 31, 2007 (previously filed as exhibit 10.101 to the Amendment No. 1 to the Registrant’s 10-KSB for the year ended December 31, 2007 filed with the SEC on June 30, 2008 and incorporated by reference herein). | |
| 10.102 | Escrow Agreement by and among Advanced Cell Technology, Inc. and certain former shareholders of Mytogen, Inc., dated as of September 20, 2007 (previously filed as exhibit 10.102 to the Amendment No. 1 to the Registrant’s 10-KSB for the year ended December 31, 2007 filed with the SEC on June 30, 2008 and incorporated by reference herein) | |
| 10.103 | Securities Purchase Agreement dated August 31, 2007 (previously filed as Exhibit 10.1 to the Registrant's Current Report on Form 8-K filed on September 7, 2007 (File No. 000-50295) and incorporated by reference herein). | |
| 10.104 | Registration Rights Agreement dated August 31, 2007 (previously filed as Exhibit 10.2 to the Registrant's Current Report on Form 8-K filed on September 7, 2007 (File No. 000-50295) and incorporated by reference herein). | |
| 10.105 | Form of Common Stock Purchase Warrant (previously filed as Exhibit 10.3 to the Registrant's Current Report on Form 8-K filed on September 7, 2007 (File No. 000-50295) and incorporated by reference herein). | |
| 10.106 | Form of Amortizing Convertible Debenture (previously filed as Exhibit 10.4 to the Registrant's Current Report on Form 8-K filed on September 7, 2007 (File No. 000-50295) and incorporated by reference herein). | |
| 10.107 | Form of Security Agreement dated August 31, 2007 (previously filed as Exhibit 10.5 to the Registrant's Current Report on Form 8-K filed on September 7, 2007 (File No. 000-50295) and incorporated by reference herein). | |
| 10.108 | Form of Subsidiary Guaranty dated August 31, 2007 (previously filed as Exhibit 10.6 to the Registrant's Current Report on Form 8-K filed on September 7, 2007 (File No. 000-50295) and incorporated by reference herein). | |
| 10.109 | Form of Lock-up Agreement (previously filed as Exhibit 10.7 to the Registrant's Current Report on Form 8-K filed on September 7, 2007 (File No. 000-50295) and incorporated by reference herein). | |
| 10.110 | Amended and Restated Consulting Agreement, dated as of September 19, 2007 by and between Advanced Cell Technology, Inc., through its wholly owned subsidiary Mytogen, Inc., and Dib, LLC. (previously filed as Exhibit 10.110 to the Registrant's Registration Statement on Form SB-2 filed on October 1, 2007 and incorporated by reference herein). | |
| 10.111 | Employment Agreement, dated as of September 20, 2007, by and between Advanced Cell technology, Inc., and Jonathan Dinsmore. (previously filed as Exhibit 10.111 to the Registrant's Registration Statement on Form SB-2 filed on October 1, 2007 and incorporated by reference herein). | |
| 10.112 | Nomination Agreement, dated September 20, 2007, by and between Advanced Cell Technology, Inc. and Anthem Ventures Fund, LP. (previously filed as Exhibit 10.112 to the Registrant's Registration Statement on Form SB-2 filed on October 1, 2007 and incorporated by reference herein). |
| 72 |
| 10.113 | Securities Purchase Agreement dated March 31, 2008, by and among the Company and the investors party thereto (previously filed as Exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.114 | Security Agreement dated March 31, 2008, by and among the Company and the investors party thereto (previously filed as Exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.115 | Form of Common Stock Purchase Warrant issued in connection with March 31, 2008 Securities Purchase Agreement (previously filed as Exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
|
10.116 |
Form of Amortizing Convertible Debenture issued in connection with March 31, 2008 Securities Purchase Agreement (previously filed as Exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.117 | Subsidiary Guarantee dated March 31, 2008 (previously filed as Exhibit 10.5 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.118 | Convertible Note, dated as of March 17, 2008, issued by the Company to PDPI LLC (previously filed as Exhibit 10.6 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.119 |
Bridge Note, dated as of March 17, 2008, issued by the Company to The Shapiro Family Trust Dated September 25, 1989 (previously filed as Exhibit 10.7 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.120 |
License Agreement, dated as of February 25, 2008, by and between the Company and Pharming Technologies B.V (previously filed as Exhibit 10.8 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.121 |
Convertible Promissory Note A, dated as of February 15, 2008, issued by the Company to JMJ Financial (previously filed as Exhibit 10.9 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.122 | Convertible Promissory Note B , dated as of February 15, 2008, issued by the Company to JMJ Financial, and Amendment to Convertible Promissory Note B, dated as of March 17, 2008 (previously filed as Exhibit 10.10 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.123 |
Secured & Collateralized Promissory Note, dated as of February 15, 2008, issued by JMJ Financial to the Company (previously filed as Exhibit 10.11 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.124 |
Collateral & Security Agreement, dated as of February 15, 2008, by and between the Company and JMJ Financial (previously filed as Exhibit 10.12 to the Registrant’s Quarterly Report on Form 10-Q filed on July 15, 2008 and incorporated herein by reference). | |
| 10.125 | Consent, Amendment and Exchange Agreement, dated as of July 29, 2009, by and between the Company and the holders named on the signature pages thereto (previously filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on August 4, 2009 and incorporated herein by reference). | |
| 10.126 | Consent, Amendment and Exchange Agreement, dated as of July 29, 2009, by and between the Company and the senior noteholders named on the signature pages thereto (previously filed as Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on August 4, 2009 and incorporated herein by reference). |
| 73 |
| 10.127 | Preferred Stock Purchase Agreement, dated November 2, 2009, between Advanced Cell Technology, Inc, and Optimus Capital Partners, LLC, dba Optimus Life Sciences Capital Partners, LLC (previously filed as exhibit to the Registrant’s S-1 filed on November 18, 2009 and incorporated herein by reference) | |
| 10.128 | Warrant, dated November 2, 2009 (previously filed as exhibit to the Registrant’s S-1 filed on November 18, 2009 and incorporated herein by reference) | |
| 10.129 | Subscription Agreement, dated November 12, 2009 (previously filed as exhibit to the Registrant’s S-1 filed on November 18, 2009 and incorporated herein by reference) | |
| 10.130 | Form of Class A Warrant (previously filed as exhibit to the Registrant’s S-1 filed on November 18, 2009 and incorporated herein by reference) | |
| 10.131 | Form of Class B Warrant (previously filed as exhibit to the Registrant’s S-1 filed on November 18, 2009 and incorporated herein by reference) | |
| 10.132 | Form of Additional Investment Right (previously filed as exhibit to the Registrant’s S-1 filed on November 18, 2009 and incorporated herein by reference) | |
| 10.133 | Employment Agreement, dated October 1, 2009, between the Company and Robert P. Lanza (previously filed as Exhibit 10.1 to the Registrant’s Current Report on Form 8-K filed on November 17, 2009 and incorporated herein by reference). | |
| 10.134 | Form of Note (previously filed as exhibit to the Registrant’s S-1 filed on November 18, 2009 and incorporated herein by reference) | |
| 10.135 | Employment Agreement, dated February 18, 2010, between the Company and William Caldwell | |
| 10.136 | Promissory Note, dated January 19, 2010, issued to JMJ Financial (previously filed as exhibit 10.1 to the Registrant’s Quarterly Report on Form 10-Q filed on May 7, 2010 and incorporated herein by reference). | |
| 10.137 | Promissory Note, dated March 30, 2010, in principal amount of $600,000, issued to JMJ Financial (previously filed as exhibit 10.2 to the Registrant’s Quarterly Report on Form 10-Q filed on May 7, 2010 and incorporated herein by reference). | |
| 10.138 | Promissory Note, dated March 30, 2010, in principal amount of $1,200,000, issued to JMJ Financial (previously filed as exhibit 10.3 to the Registrant’s Quarterly Report on Form 10-Q filed on May 7, 2010 and incorporated herein by reference). | |
| 10.139 | Promissory Note, dated March 30, 2010, in principal amount of $1,700,000, issued to JMJ Financial (previously filed as exhibit 10.4 to the Registrant’s Quarterly Report on Form 10-Q filed on May 7, 2010 and incorporated herein by reference). | |
| 10.140 | Letter Agreement, dated March 30, 2010, between the Company and JMJ Financial (previously filed as exhibit 10.5 to the Registrant’s Quarterly Report on Form 10-Q filed on May 7, 2010 and incorporated herein by reference). | |
| 10.141 |
Registration Rights Agreement, dated March 30, 2010, between the Company and JMJ Financial (previously filed as exhibit 10.6 to the Registrant’s Quarterly Report on Form 10-Q filed on November 9, 2010 and incorporated herein by reference). | |
| 10.142 | Settlement Agreement and Mutual Release between the Company and Bristol Investment Fund, Ltd and Bristol Capital, LLC (previously filed as exhibit 99.1 to the Registrant’s Quarterly Report on Form 10-Q filed on May 7, 2010 and incorporated herein by reference). | |
| 10.143 | Form of Warrant for Series C Preferred transaction (previously filed as exhibit 4.1 to the Registrant’s Current Report on Form 8-K filed on January 3, 2011 and incorporated herein by reference). |
| 74 |
| 10.144 | Form of Initial Warrant for Series C Preferred transaction (previously filed as exhibit 4.2 to the Registrant’s Current Report on Form 8-K filed on January 3, 2011 and incorporated herein by reference). | |
| 10.145 | Securities Purchase Agreement, dated as of December 30, 2010, by and among Advanced Cell Technology, Inc. and Socius CG II Ltd. (previously filed as exhibit 99.1 to the Registrant’s Current Report on Form 8-K filed on January 3, 2011 and incorporated herein by reference). | |
| 10.146 | Letter Agreement, dated December 30, 2010, by and among Advanced Cell Technology, Inc. and Optimus CG II, Ltd. (previously filed as exhibit 99.2 to the Registrant’s Current Report on Form 8-K filed on January 3, 2011 and incorporated herein by reference). | |
| 10.147 | Employment Agreement, dated December 14, 2010, between the Company and Gary Rabin ( filed as exhibit 10.147 to 10-K filed March 17, 2011 and incorporated herein by reference). | |
| 10.148 | Settlement Agreement and Mutual Release between the Company and Transition Holdings, Ltd. dated February 9, 2011 ( filed as exhibit 10.148 to 10-K filed March 17, 2011 and incorporated herein by reference). | |
| 10.149 | Settlement Agreement and Mutual Release between the Company and Gemini Master Fund, Ltd. dated February 11, 2011 ( filed as exhibit 10.149 to 10-K filed March 17, 2011 and incorporated herein by reference). | |
| 10.150 | Settlement Agreement and Mutual Release between the Company and Midsummer Investment, Ltd. and Midsummer Small Cap Master, Ltd. (previously filed as Exhibit 10.150 to the Registrant’s Current Report on Form 8-K filed on August 17, 2011 and incorporated herein by reference). | |
| 10.151 | Amended and Restated Employment Agreement, dated July 1, 2011, by and between the Company and Robert P. Lanza (previously filed as Exhibit 10.2 to the Registrant’s Current Report on Form 8-K filed on August 17, 2011 and incorporated herein by reference). | |
| 10.152 | Amended and Restated Employment Agreement, dated July 1, 2011, by and between the Company and Gary H. Rabin (previously filed as Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed on August 17, 2011 and incorporated herein by reference). | |
| 10.153 | Settlement Agreement and Mutual Release Form used between the Company and several counter parties (previously filed as Exhibit 10.3 to the Registrant’s Current Report on Form 8-K filed on December 12, 2011 and incorporated herein by reference). | |
| 23.1 | Consent of Independent Registered Public Accounting Firm | |
| 31.1 | Certification of the Principal Executive Officer and Principal Financial Officer Pursuant to Rule 13a-14 of the Securities Exchange Act of 1934 | |
| 32.1 | Certification of Principal Executive Officer and Principal Financial Officer Pursuant to Section 1350 |
| 75 |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
ADVANCED CELL TECHNOLOGY, INC. |
|||
| Dated: March 1, 2012 | By: | /s/ Gary Rabin | |
| Gary Rabin | |||
| Chief Executive Officer and Chairman | |||
|
(Principal Executive Officer, Principal Financial Officer and Principal Accounting Officer) |
|||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
| /s/ Gary Rabin | March 1, 2012 | |
| Gary Rabin | ||
| Chief Executive Officer and | ||
| Chairman of the Board of Directors | ||
| (Principal Executive Officer, Principal | ||
| Financial Officer and Principal Accounting Officer | ||
| /s/ Robert Langer | March 1, 2012 | |
| Robert Langer | ||
| Director | ||
| /s/ Alan Shapiro | March 1, 2012 | |
| Alan Shapiro | ||
| Director | ||
| /s/ Gregory D. Perry | March 1, 2012 | |
| Gregory D. Perry | ||
| Director | ||
| /s/ Zohar Loshitzer | March 1, 2012 | |
| Zohar Loshitzer | ||
| Director |
| 76 |
