Attached files
| file | filename |
|---|---|
| EX-21 - EXHIBIT 21 - NOVELION THERAPEUTICS INC. | d293106dex21.htm |
| EX-11 - EXHIBIT 11 - NOVELION THERAPEUTICS INC. | d293106dex11.htm |
| EX-23 - EXHIBIT 23 - NOVELION THERAPEUTICS INC. | d293106dex23.htm |
| EX-31.2 - EXHIBIT 31.2 - NOVELION THERAPEUTICS INC. | d293106dex312.htm |
| EX-32.1 - EXHIBIT 32.1 - NOVELION THERAPEUTICS INC. | d293106dex321.htm |
| EX-31.1 - EXHIBIT 31.1 - NOVELION THERAPEUTICS INC. | d293106dex311.htm |
| EXCEL - IDEA: XBRL DOCUMENT - NOVELION THERAPEUTICS INC. | Financial_Report.xls |
| EX-32.2 - EXHIBIT 32.2 - NOVELION THERAPEUTICS INC. | d293106dex322.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended December 31, 2011
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
Commission File No. 0-17082
QLT Inc.
(Exact Name of Registrant as Specified in its Charter)
| British Columbia, Canada | N/A | |
| (State or Other Jurisdiction of Incorporation or Organization) |
(I.R.S. Employer Identification No.) | |
| 101—887 Great Northern Way, Vancouver, B.C., Canada |
V5T 4T5 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code: (604) 707-7000
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Name of each exchange on which registered | |
| Common Shares, without par value | The Nasdaq Stock Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the Registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act.
Yes ¨ No x
Indicate by check mark if the Registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act.
Yes ¨ No x
Indicate by check mark whether the registrant: (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months and (2) has been subject to such filing requirements for the past 90 days.
Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files. Yes x No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of the registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the Registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See definition of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer |
¨ |
Accelerated filer |
x | |||
| Non-accelerated filer |
¨ |
Smaller Reporting Company |
¨ | |||
Indicate by check mark whether the Registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
As of June 30, 2011, the aggregate market value of the common shares held by non-affiliates of the registrant (based on the last reported sale price of the common shares of U.S. $7.21, as reported on the NASDAQ Stock Market) was approximately U.S. $251,991,396.
As of February 17, 2012 the registrant had 48,954,680 outstanding common shares.
DOCUMENTS INCORPORATED BY REFERENCE
Parts of the Company’s definitive proxy statement (to be filed pursuant to Regulation 14A within 120 days after Registrant’s fiscal year end of December 31, 2011) for its annual meeting to be held on May 24, 2012, are incorporated by reference in this Form 10-K in response to Part III, Items 10, 11, 12, 13 and 14.
Table of Contents
Note regarding references to QLT
Throughout this Annual Report on Form 10-K (this “Report”), the words “we,” “us,” “our,” “the Company” and “QLT” refer to QLT Inc. and our wholly owned subsidiaries, QLT Plug Delivery, Inc., QLT Therapeutics, Inc. and QLT Ophthalmics, Inc., unless stated otherwise.
Note regarding Currency and Accounting Standards
In this Report all dollar amounts are in U.S. dollars, except where otherwise stated, and financial reporting is made in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”).
We use the U.S. dollar as our reporting currency. For the year ended December 31, 2009 the Canadian dollar was the functional currency for the parent company, QLT Inc., and the U.S. dollar was the functional currency for our U.S. subsidiaries. On January 1, 2010, the functional currency of QLT Inc. was changed to the U.S. dollar as a result of the change in our business related to the receipt of exclusive U.S. rights to the Visudyne® patents from Novartis.
Note regarding Exchange Rates
The table below shows relevant exchange rates which approximate the noon buying rates in New York City as reported by the Federal Reserve Bank of New York for cable transfers expressed in Canadian dollars for the five most recent fiscal years of the Company.
| 2011 | 2010 | 2009 | 2008 | 2007 | ||||||||||||||||
| High |
$ | 1.0605 | $ | 1.0776 | $ | 1.2995 | $ | 1.2971 | $ | 1.1852 | ||||||||||
| Low |
0.9448 | 0.9960 | 1.0289 | 0.9717 | 0.9168 | |||||||||||||||
| Average |
0.9887 | 1.0298 | 1.1412 | 1.0660 | 1.0734 | |||||||||||||||
| Period End |
1.0168 | 1.0009 | 1.0461 | 1.2240 | 0.9881 | |||||||||||||||
Note regarding Trademarks
The following words used in this Report are trademarks:
| • | Aczone® is a registered trademark of Allergan, Inc. |
| • | Atrigel® is a registered trademark of TOLMAR Therapeutics, Inc. |
| • | Avastin® is a registered trademark of Genentech, Inc. |
| • | Eligard® is a registered trademark of Sanofi S.A. |
| • | Eylea® is a registered trademark of Regeneron Pharmaceuticals Inc. |
| • | Lucentis® is a registered trademark of Genentech, Inc. |
| • | Patanol® is a registered trademark of Alcon Research, Ltd. |
| • | Visudyne® is a registered trademark of Novartis AG |
| • | Xalatan® is a registered trademark of Pfizer Health AB |
Any words used in this Report that are trademarks but are not referred to above are the property of their respective owners.
2
Table of Contents
QLT INC.
ANNUAL REPORT ON FORM 10-K
DECEMBER 31, 2011
| 5 | ||||
| 5 | ||||
| 6 | ||||
| 6 | ||||
| 6 | ||||
| 8 | ||||
| 14 | ||||
| 14 | ||||
| 14 | ||||
| 15 | ||||
| 15 | ||||
| 17 | ||||
| 17 | ||||
| 22 | ||||
| 23 | ||||
| 23 | ||||
| 23 | ||||
| 24 | ||||
| 24 | ||||
| 37 | ||||
| 37 | ||||
| 37 | ||||
| 37 | ||||
| 38 | ||||
| 42 | ||||
| Item7.MANAGEMENT’S DISCUSSION AND ANAYLYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATION |
45 | |||
| Item 7A. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
65 | |||
| 65 | ||||
| Item 9. CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
93 | |||
3
Table of Contents
| 93 | ||||
| 95 | ||||
| Item 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
96 | |||
| 96 | ||||
| 97 | ||||
| Item 13. CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
97 | |||
| 97 | ||||
| 98 | ||||
4
Table of Contents
PART I
QLT is a biotechnology company dedicated to the development and commercialization of innovative ocular products that address the unmet medical needs of patients and clinicians worldwide. We are focused on developing our synthetic retinoid program for the treatment of certain inherited retinal diseases, developing our proprietary punctal plug drug delivery system, as well as U.S. marketing of our commercial product, Visudyne®, for the treatment of wet age related macular degeneration (“wet AMD”).
Products, Revenues and Other Sources of Funds
We have one commercial product, Visudyne, which utilizes light-activated photodynamic therapy (“PDT”) to treat the eye disease known as wet AMD, the leading cause of blindness in people over the age of 50 in North America and Europe. Visudyne is also used for the treatment of subfoveal choroidal neovascularization (“CNV”) secondary to pathologic myopia, or severe near-sightedness, and presumed ocular histoplasmosis. Visudyne was co-developed by QLT and Novartis Pharma AG (“Novartis”) and is marketed and sold in over 80 countries worldwide. Total revenue from the sale of Visudyne for each of the fiscal years ended December 31, 2011, 2010 and 2009 was $42.2 million, $44.7 million and $42.1 million, respectively.
On January 1, 2010, we received from Novartis the exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. As a result, we operate a direct marketing and sales force through our U.S. subsidiary, QLT Ophthalmics, Inc., and have rights to all end-user revenue derived from Visudyne sales in the U.S. Novartis continues to market and sell Visudyne for ophthalmic use outside the U.S. and pays us a royalty on net sales of the product.
On October 1, 2009, we divested the Eligard® line of products to TOLMAR Holding, Inc. (“Tolmar”) as part of the sale of all of the shares of our U.S. subsidiary, QLT USA, Inc. (“QLT USA”). Pursuant to the stock purchase agreement, we are entitled to future consideration payable quarterly in amounts equal to 80% of the royalties paid under the license agreement with Sanofi Synthelabo Inc. (“Sanofi”) for the commercial marketing of Eligard in the U.S. and Canada, and the license agreement with MediGene Aktiengesellschaft (“MediGene”), which, effective March 1, 2011, was assigned to Astellas Pharma Europe Ltd. (“Astellas”), for the commercial marketing of Eligard in Europe. The estimated fair value of the expected future quarterly payments is reflected as Contingent Consideration on our Consolidated Balance Sheet. We are entitled to these quarterly payments until the earlier of our receipt of $200.0 million or October 1, 2024. As of December 31, 2011, we had received an aggregate $86.1 million of contingent consideration. See Eligard® Contingent Consideration below.
Research and Development
Our current research and development efforts are focused on:
QLT091001 orphan drug program for the treatment of Leber Congenital Amaurosis and Retinitis Pigmentosa. We are currently conducting Phase Ib clinical proof-of-concept studies of QLT091001, a synthetic retinoid replacement therapy for 11-cis-retinal, a key biochemical component of the visual retinoid cycle, in patients with Leber Congenital Amaurosis (“LCA”) and Retinitis Pigmentosa (“RP”). In May 2011, we announced positive preliminary results from the analysis of data from 12 of the 14 LCA subjects. The preliminary results for the full cohort of 14 LCA subjects and updated longer term visual function data on previously reported LCA subjects were presented in October 2011 at the 2011 American Academy of Ophthalmology (AAO) annual meeting. We continue to monitor and analyze LCA subject follow-up and have initiated retreatment in subjects, as needed, in a retreatment study. We are also continuing our dialogue with the U.S. Food and Drug Administration (“FDA”) and the European Medicines Agency (“EMA”), with respect to the design and protocol requirements for a potential pivotal trial for this indication in 2012. Target enrolment in the RP cohort is now completed, with 17 subjects treated to date at investigator sites in Canada, the U.S. and Europe. We expect to report preliminary endpoint data from the RP cohort in the first quarter of 2012.
QLT091001 has received orphan drug designations for the treatment of LCA and RP by the EMA, and for the treatment of LCA and RP due to inherited mutations in the LRAT and RPE65 genes by the FDA. The drug has also been granted two Fast Track designations by the FDA for the treatment of the LRAT and RPE65 genetic mutations in both LCA and RP. On May 31, 2011, the United States Patent and Trademark Office issued a key patent related to this program, covering various methods of use of QLT091001 in the treatment of diseases associated with an endogenous 11-cis-retinal deficiency, expiring on July 27, 2027, including the period of Patent Term Adjustment. See Our Products in Development - QLT091001 - Synthetic Retinoid Program below.
5
Table of Contents
Punctal Plug Drug Delivery System for the treatment of Glaucoma. We are developing a minimally invasive, proprietary punctal plug drug delivery system with the goal of delivering a variety of drugs topically to the eye through controlled sustained release to the tear film. Our first product candidate is targeting the treatment of glaucoma and ocular hypertension through the sustained delivery of latanoprost in our punctal plug delivery system (“L-PPDS”). On August 29, 2011, we announced positive data from our Phase II clinical study on the safety and efficacy of the L-PPDS in subjects with glaucoma and ocular hypertension after four weeks of treatment with the L-PPDS. The trial featured simultaneous placement of latanoprost-eluting punctal plugs in both the upper and lower puncta for delivery of a daily drug load with a goal of enabling a 5 mmHg or greater reduction in intraocular pressure. We recently initiated two additional Phase II L-PPDS clinical trials to further evaluate the safety, efficacy and duration of effect of the L-PPDS, including assessment of the effect of tearing, latanoprost dosage and the single versus double plug approaches. We are also conducting a separate device study in volunteers to further study the longer term handling, comfort and retention of upper and lower punctal plug iterations. If the dosing and device-only clinical trials are successful, our goal is to commence Phase III clinical development activities for the L-PPDS in 2013.
We believe this platform technology may be suited for delivery of other types of molecules to the tear film of the eye in order to treat anterior segment diseases of the eye. This could include other medications for glaucoma, or other diseases or conditions of the eye including allergy, dry eye and ocular inflammation. Further development work in this program is ongoing with formulation development work underway for new product candidates in the glaucoma and anti-inflammatory areas. See Our Products in Development - Punctal Plug Drug Delivery System below.
Business Strategy
Our goal is to build a leading ocular-focused biotechnology company dedicated to the development and commercialization of innovative ocular products that address the unmet medical needs of patients and clinicians worldwide. We believe our strong cash position, the revenue we generate from sales of Visudyne in the U.S. from our direct sales efforts, the royalties we collect from Novartis from the sale of Visudyne outside the U.S. and the future contingent consideration payable to us from the commercial sale of Eligard in the U.S., Canada and Europe will help enable us to meet our goals. We intend to use these funds and our current cash to fund our research and development efforts, and to build our development pipeline through our pursuit of strategic acquisitions or in-licensing opportunities in the ocular field. We will also seek to drive growth in U.S. sales of Visudyne and, in the future, sales of our products now in development, by continuing to invest in our commercial sales force. Our growth will depend on the success of our research and development efforts, our ability to leverage our clinical expertise and U.S. sales and marketing infrastructure, our ability to acquire and develop future product opportunities, the strength of our commercial product, Visudyne, and the liquidity provided by the contingent consideration earned from Eligard sales.
During the 12 month period commencing December 16, 2010 and ended December 15, 2011, pursuant to a normal course issuer bid, we repurchased through the facilities of the NASDAQ Stock Market (“NASDAQ”), and immediately cancelled, an aggregate 2.7 million of our issued and outstanding common shares at an average price of $6.99 per share, for a total cost of $18.9 million. See Note 12 - Share Capital in Notes to the Consolidated Financial Statements.
Eligard® Contingent Consideration
Our product portfolio previously included the Eligard line of products approved for the palliative treatment of advanced prostate cancer. Eligard incorporates a luteinizing hormone-releasing hormone agonist, known as leuprolide acetate, with the Atrigel® drug delivery system. The Atrigel drug delivery system allows for sustained delivery of leuprolide acetate for periods ranging from one to six months.
On October 1, 2009, we divested the Eligard line of products as part of the sale of all of the shares of our U.S. subsidiary, QLT USA, to Tolmar for up to an aggregate $230.0 million plus cash on hand of $118.3 million. Pursuant to the stock purchase agreement, we received $20.0 million on closing and $10.0 million on October 1, 2010, and we are entitled to future consideration payable on a quarterly basis in amounts equal to 80% of the royalties paid under the license agreement with Sanofi for the commercial marketing of Eligard in the U.S. and Canada, and the license agreement with Astellas (formerly with MediGene), for the commercial marketing of Eligard in Europe. The estimated fair value of these expected future quarterly payments is reflected as Contingent Consideration on our Consolidated Balance Sheet. We are entitled to these quarterly payments until the earlier of our receipt of the additional $200.0 million or October 1, 2024. As of December 31, 2011, we had received an aggregate $86.1 million of contingent consideration and had up to $113.9 million remaining to be received. While we expect to receive the full amount of contingent consideration in the next three to four years, our continued receipt of contingent consideration under the stock purchase agreement is dependent upon sales of Eligard by Sanofi and Astellas, which could vary significantly due to competition, manufacturing difficulties and other factors. See Item 1A. Risk Factors.
Visudyne® - Our Approved Product
Visudyne is a photosensitizer that we co-developed with Novartis for the treatment of subfoveal CNV due to wet AMD, the leading cause of blindness in people over the age of 50 in North America and Europe. Since its commercialization, Visudyne has been used in more than two million treatments worldwide. For the year ended December 31, 2011, Visudyne worldwide net sales were $90.3 million, which included $21.0 million in U.S. sales. Sales of Visudyne in 2011 resulted in $28.4 million of net product revenue and $13.9 million of royalties from Novartis. See Commercialization Rights and Revenue below.
6
Table of Contents
Photodynamic Therapy
Visudyne utilizes our patented PDT technology. PDT is a minimally invasive medical procedure that utilizes photosensitizers (light-activated drugs) to treat a range of diseases associated with rapidly growing tissue, such as the formation of solid tumors and abnormal blood vessels. PDT is a two-step process. First, the photosensitizer is administered to the patient by intravenous infusion or other means, depending on the condition being treated. Second, a pre-determined dose of non-thermal light is delivered at a particular wavelength to the target site to interact with the photosensitizer. The photosensitizer traps energy from the light and causes oxygen found in cells to convert to a highly energized form called “singlet oxygen” that causes cell death by disrupting normal cellular functions. Because the photosensitizer and light have no effect unless combined, PDT is a relatively selective treatment that minimizes damage to normal surrounding tissue and allows for multiple courses of therapy. For ocular PDT applications, non-thermal lasers provide the necessary intensity of light required.
Wet AMD
Wet AMD is an eye disease characterized by the growth of abnormal blood vessels under the macula, which is the central part of the retina. These abnormal vessels often leak blood and fluid, raising the macula from its normal position in the eye and, over time, causing photoreceptor damage, the formation of scar tissue and a loss of central vision. Although the progression of the disease varies by patient, without treatment, the majority of patients with wet AMD become legally blind in the affected eye within approximately two years following the onset of the disease. According to the 2010 Report on the Retina Pharmaceuticals Market, there are approximately 18 million wet AMD patients worldwide, with approximately 1.4 million patients in the U.S., 3.0 million patients in Europe and, in the aggregate, 5.2 million patients in Japan and China.
Approved Indications
Visudyne is approved for the following indications:
Predominantly Classic CNV in AMD. Visudyne has been approved for marketing for predominantly classic subfoveal CNV in AMD in over 80 countries, including the U.S., Canada, Japan, Australia, New Zealand and the European Union (“EU”) countries.
Minimally Classic CNV in AMD. Visudyne has been approved for minimally classic CNV in Japan.
Occult CNV in AMD. Visudyne has been approved for the occult form of CNV in over 29 countries, including Japan, Australia, New Zealand and Switzerland. Visudyne was previously approved in the EU for the occult form of CNV. However, in April 2007, after reviewing the results of a second confirmatory Visudyne occult study, the Committee for Human Medicinal Products (“CHMP”) recommended to the European Commission (“EC”) that the indication be removed in the EU. In June 2007, the EC adopted the CHMP recommendation.
CNV due to Pathologic Myopia. Pathologic myopia (“PM”) is a degenerative form of near-sightedness that occurs largely in persons aged 30 to 50 and can result in CNV. We have received regulatory approval of Visudyne for the treatment of subfoveal CNV due to PM in 40 countries, including the U.S., Canada and the EU.
CNV due to Presumed Ocular Histoplasmosis Syndrome. Presumed ocular histoplasmosis syndrome (“OHS”) is a condition caused by a fungal infection endemic to certain areas in the central and eastern U.S. It can lead to severe, irreversible vision loss and is a leading cause of blindness in adults who have lived in geographic areas where the soil mold Histoplasma capsulatum is found. Visudyne is approved for the treatment of subfoveal CNV secondary to OHS in the U.S. and Canada.
Commercialization Rights and Revenue
In 1994, we entered into the PDT Product Development, Manufacturing and Distribution Agreement (“PDT Agreement”) with Novartis for the worldwide development and commercialization of PDT products, including Visudyne, for eye diseases. Under the PDT Agreement, we manufactured and supplied Visudyne and Novartis was responsible for worldwide marketing and distribution. We and Novartis shared the net revenues from product sales after deductions for marketing costs and manufacturing costs (including any third-party royalties), all calculated according to a formula set out in our agreement.
On October 16, 2009, we substantially restructured our business arrangement with Novartis by amending and restating the PDT Agreement pursuant to the Amended and Restated PDT Product Development, Manufacturing and Distribution Agreement (the “Amended PDT Agreement”). Under the Amended PDT Agreement, effective January 1, 2010, we and Novartis ceased joint development of Visudyne and all net revenue and cost sharing associated with the manufacturing and commercialization of Visudyne.
7
Table of Contents
Under the Amended PDT Agreement, we received exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. and retain all end-user revenue derived from U.S. sales. Novartis has retained distribution, sales and marketing rights to Visudyne for ophthalmic use outside the U.S. and pays us a royalty of 20% of net sales outside the U.S. until December 31, 2014, and thereafter will pay us 16% of net sales, until the expiry of the Amended PDT Agreement on December 31, 2019. We continue to manufacture and supply Visudyne exclusively to Novartis for sales outside the U.S. at a pre-specified price pursuant to a manufacturing and supply agreement with Novartis also expiring on December 31, 2019.
The Amended PDT Agreement sets forth the obligations of each party in connection with payment of third-party royalties related to Visudyne during the term and following expiry of the Amended PDT Agreement. Under the Amended PDT Agreement, QLT and Novartis also released each other from all open claims we may have had against the other, including any in connection with our previous litigation with Massachusetts Eye and Ear Infirmary (“MEEI”) and the General Hospital Corporation, doing business as Massachusetts General Hospital (“MGH”).
Novartis may terminate the Amended PDT Agreement for convenience in its entirety or on a country-by-country basis on 12 months prior written notice. In the event Novartis terminates the Amended PDT Agreement or either party ceases to commercialize Visudyne in their respective territories (and in the case of Novartis in any country in its territory), the other party will have the exclusive right, at its option, to commercialize Visudyne in that country or territory, as the case may be. Under the terms of the Amended PDT Agreement, QLT and Novartis indemnify each other in connection with certain matters relating to each party’s respective contractual obligations.
We commit significant resources to research and development opportunities in the field of ophthalmology. The following table sets forth our clinical development programs:
| Product/Indication |
Status/Development Stage | |
| QLT091001 |
||
| Leber Congenital Amaurosis (LCA) and Retinitis Pigmentosa (RP) |
Phase Ib proof-of-concept studies ongoing:
• LCA cohort treatment completed; follow-up ongoing. LCA retreatment study initiated.
• RP cohort target enrolment and treatment completed. RP retreatment study initiated. | |
| Latanoprost Punctal Plug Drug Delivery System (L-PPDS) |
||
| Glaucoma and Ocular Hypertension |
Phase II clinical study completed in August 2011.
Two Phase II clinical studies initiated in December 2011. | |
| Olopatadine Punctal Plug Drug Delivery System (O-PPDS) |
||
| Allergic Conjunctivitis |
Phase II proof-of-principle study completed in February 2011 demonstrating inconclusive data. Development has been suspended. | |
QLT091001 - Synthetic Retinoid Program
We are developing QLT091001, a synthetic retinoid compound for the potential treatment of certain inherited retinal degenerative diseases.
8
Table of Contents
Under the terms of a co-development agreement we entered into with Retinagenix LLC (“Retinagenix”) in April 2006, we obtained an exclusive, worldwide license and sub-license under certain intellectual property rights owned or controlled by Retinagenix related to the synthetic retinoid compound under development, and are responsible for using commercially reasonable and diligent efforts to develop and commercialize in certain major markets and other markets as we reasonably determine, one or more products covered by the licensed rights or developed using such licensed rights for use in diagnosing, treating or preventing certain human diseases and conditions. We are also responsible for committing certain annual funding to support research and development of such products.
Milestone and Royalty Obligations; Term
Pursuant to the co-development agreement, Retinagenix is eligible to receive, in the case of the first target indication for such products, $1.0 million upon initiation of the first pivotal trial and up to a total of an additional $11.5 million upon the achievement of other specified development or regulatory milestones and, for each of up to two additional indications, up to a total of $9.0 million upon achievement of specified development or regulatory milestones. If we commercialize such products, we will also pay Retinagenix royalties of between 4% and 6% of net sales, subject to reduction under certain specified circumstances. Retinagenix is also eligible to receive up to a total of $15.0 million upon achievement of specified cumulative sales milestones for such products. The term of our co-development agreement with Retinagenix expires on the later of the expiration of 10 years after first commercial sale of licensed products, or the expiration, lapse or abandonment of all licensed patents. Retinagenix can terminate the agreement earlier if we fail in any material respect to meet our diligence requirements, and we may terminate the agreement for convenience. Each party may terminate the agreement for uncured material breach by the other party.
Initial Target Indications
Leber Congenital Amaurosis and Retinitis Pigmentosa. LCA and RP are inherited, progressive, retinal degenerative diseases that arise from genetic mutations of enzymes or proteins required in the biochemistry of vision. LCA is characterized by abnormalities such as roving eye movements and sensitivity to light, and manifests in severe vision loss from birth. Both rod and cone photoreceptors are affected in LCA. Eye examinations of infants with LCA reveal normal appearing retinas; however, low level of retinal activity, measured by electroretinography, indicates very little visual function. RP is a set of hereditary retinal diseases demonstrating clinical features similar to LCA. RP is also characterized by degeneration of rod and cone photoreceptors, but it presents with a more variable loss of vision in late childhood to adulthood. Deficits in dark adaptation and peripheral vision are particular hallmarks of RP. LCA and RP diseases result from genetic mutations, including retinal pigment epithelium protein 65 (RPE65) or lecithin:retinol acyltransferase (LRAT), which result in an inadequate production of 11-cis-retinal, an essential component of the visual retinoid cycle. QLT091001 is a replacement therapy for 11-cis retinal.
QLT091001 has received orphan drug designations for the treatment of LCA and RP by the EMA, and for the treatment of LCA and RP due to inherited mutations in the LRAT and RPE65 genes by the FDA. These designations provide market exclusivity in the applicable jurisdiction after a product is approved for 10 years in the EU and seven years in the U.S., respectively. Orphan drug designation in the EU can also provide an additional two years of market exclusivity for pediatric orphan drug designated drug products. QLT091001 has also been granted two Fast Track designations by the FDA for the treatment of the LRAT and RPE65 genetic mutations in both LCA and RP. The FDA’s Fast Track is a process designed to facilitate the development and expedite the review of drugs that are intended for the treatment of serious diseases and fill an unmet medical need. See Government Regulation - Orphan Drug Regulation and Government Regulation - Fast Track Designations below.
Current Treatment. There are no FDA or EMA approved therapeutic treatments for LCA or RP.
Potential Patient Populations. Given the very low prevalence in these ultra-orphan drug indications, there is limited epidemiological data available to determine definitively the potential patient population for treatment with QLT091001. According to current epidemiological estimates, LCA affects approximately one in 81,000 newborns worldwide, of which approximately 10% carry the inherited deficiencies of either RPE65 or LRAT. Based on market research, we estimate the treatment-eligible LCA patient population for QLT091001 at 1,000 to 2,000 patients worldwide. RP is currently estimated to affect at least 300,000 individuals worldwide, of which approximately 20% - 30% are autosomal recessive (arRP). It is currently estimated that less than 3% of autosomal recessive RP patients carry the inherited deficiencies of either RPE65 or LRAT. Thus, the potential treatment eligible RP patient population for QLT091001 is currently estimated to be 2,000 to 3,000 patients worldwide.
LCA and RP Phase Ib Study. We are nearing completion of the post-treatment follow-up phase of an ongoing Phase Ib open-label clinical proof-of-concept trial to evaluate the safety profile and effects of a single seven-day course of treatment with QLT091001 on various parameters of retinal function and quality of life in patients with LCA and RP due to inherited deficiency of RPE65 or LRAT. In the study, LCA and RP subjects received daily oral doses of QLT091001 for seven days with post-treatment follow-up at regular intervals for as long as visual function or subjective improvements are observed or until the patient is enrolled in a retreatment protocol.
The study evaluated changes in several visual function parameters, including best-corrected visual acuity (“VA”) and visual field (“VF”) over the duration of the treatment and post-treatment follow-up. Visual acuity measures the acuteness or clearness of an individual’s central vision, expressed in the study as number of letters or number of lines read on a visual acuity eye chart. Visual field measures an
9
Table of Contents
individual’s entire scope or width of (central + peripheral) vision, expressed in the study as retinal areas. Peripheral vision is important in day-to-day mobility, whereas central vision reflects the ability to read and do fine vision work. Various medical conditions such as LCA, RP and glaucoma are characterized by debilitating loss of visual field.
LCA Cohort Results. 14 LCA subjects, aged six to 38 years (nine pediatric, five adult) with either LRAT (seven subjects) or RPE65 (seven subjects) mutations were administered QLT091001 for seven days at a single investigator center at the Montreal Children’s Hospital in Montreal, Canada. Twelve subjects received a dose of 40 mg/m2/day, while two subjects received a lower dose of 10 mg/m2/day. The subjects had baseline visual acuities across a range of vision categories from “Near Blindness” to “Near Normal,” including one subject with visual acuity in only the Hand Motion category, and no detectable visual field. Visual acuity categorization was based on ICD-9-CM criteria (International Classification of Diseases, 9th Revision – Clinical Modification). In May 2011, we announced positive preliminary results from the analysis of data from 12 of the 14 LCA subjects. The full 14 subject LCA cohort was presented in October 2011 at the 2011 American Academy of Ophthalmology (AAO) annual meeting. The study demonstrated that treatment with QLT091001 resulted in rapid, clinically meaningful improvement in either or both VA and VF in eight of the 14 subjects. The preliminary results from the LCA study are described below.
Of the 14 LCA subjects treated in the study, data from 13 subjects were included in a multi-level mixed effects model to assess effects on VF. One subject did not have detectable fields at baseline and, therefore, no data were available for analysis. After treatment with QLT091001, improvement in VF was as follows:
| • | average increases in VF from baseline across 9 days – 13 months of follow-up were: 2 - 10% for larger baseline retinal areas (near normal fields); and 30 - 38% for smaller baseline retinal areas (abnormal fields); |
| • | six subjects in a longer term cohort across 9 – 13 months of follow-up demonstrated increases in VF ranging between 40 - 50% from baseline; and |
| • | VF’s that had a mid-range size at baseline, reflecting a medium stage of VF loss, demonstrated clinically meaningful improvements in retinal area of 27 - 180% from baseline, averaged across time. |
Of the 14 LCA subjects treated in the study, 11 subjects (40 mg/m2/day) could be tested for VA improvement (excludes subjects unable to read a vision chart). Of these 11 subjects, five subjects showed notable improvements in VA as follows:
| • | two subjects showed a sustained improvement after treatment (up to six and 14 months, respectively) of 3 - 6 lines of vision or 15 - 30 letters; |
| • | two subjects showed a 1 - 2 line or 5 - 10 letters improvement; and |
| • | one subject who was initially unable to read the vision chart (“off-chart”) at baseline was able to read “on-chart” of several (1 -5) letters after nine days post-treatment. |
The responses were maintained up to 12 months in three of these five subjects.
During the post-treatment follow-up period, four subjects (40 mg/m2/day) showed an upward change in ICD-9-CM VA vision categories from the “Near Blindness” or “Severe Low Vision” categories towards the “Moderate Low Vision” and “Near Normal” vision categories. A number of subjects (10 of 14 subjects) also reported subjective improvements in activities of daily living, including dark adaptation and the ability to see in low-light environments.
The study treatment was well-tolerated, with mild to moderate adverse events observed, including transient headache and photophobia, a reversible increase in triglyceride levels, and decrease in high density lipoprotein (HDL). Further analyses of additional anatomic (OCT, optical coherence tomography) and retinal sensitivity (ERG, electroretinograms) tests are ongoing to further understand the potential impacts of treatment with QLT091001, and the optimal baseline characteristics of responders for further study purposes. The database for this study remains open and results are preliminary until all analysis and longer-term follow-up for subjects are completed.
We continue to monitor LCA subject follow-up and have begun retreatment of the LCA subjects, as needed. Discussions with the FDA and the EMA concerning the design and protocol requirements for the initiation of a potential pivotal trial of QLT091001 for the treatment of LCA in 2012 are ongoing.
RP Cohort Enrolment. In the second and third quarters of 2011, we received clearance from the FDA for our Investigational New Drug Application (“IND”), and also clearances from the U.K. Medicines and Healthcare Products Regulatory Agency, the Centrale Commissie Mensgebonden Onderzoek (CCMO) (The Netherlands) and the Federal Institute for Drugs and Medical Devices (Germany) for our Clinical Trial Applications, allowing us to proceed with the expansion of investigator sites in the U.S., U.K., The Netherlands and Germany. Target enrolment in the RP cohort is now completed, with 17 subjects treated at investigator sites in Canada, the U.S. and Europe. We expect to report preliminary endpoint data from the RP patient cohort in the first quarter of 2012.
10
Table of Contents
Retreatment and Safety/Dosing Studies. We have initiated two separate retreatment studies in LCA and RP subjects, respectively, to provide retreatment for subjects treated in the initial Phase Ib open-label study, to examine the safety, efficacy and tolerability of repeat dosing cycles of QLT091001 administered over seven days. Pre-clinical and clinical studies are also ongoing to further evaluate the safety and tolerability of QLT091001. Data from an ongoing clinical safety study in healthy volunteers to test intermittent, multiple oral dosing cycles of QLT091001 are expected in 2012.
Punctal Plug Drug Delivery System
Our proprietary punctal plug drug delivery technology is a minimally invasive drug delivery system we are developing with the goal of delivering a variety of drugs to the eye through controlled sustained release to the tear film. The platform technology builds upon a well-known ocular device, punctal plugs, which are placed in the tear duct, or punctum, of the eye in a relatively simple and minimally invasive office procedure routinely performed by an ophthalmologist or optometrist. Punctal plugs have been traditionally used to block the drainage of tears in patients with dry eye syndrome. Our goal is to develop a drug delivery system comprising a proprietary punctal plug designed to be retained for the desired treatment duration and a proprietary drug delivery core that can be tailored to deliver a wide range of therapeutic agents over different time periods.
We believe this drug delivery platform has the potential to address a broad range of ocular diseases that are currently being treated with eye drops, including glaucoma, allergy, dry eye and ocular inflammation. Our development efforts are currently focused on the Phase II clinical development of our first drug and target indication, latanoprost for the treatment of glaucoma and ocular hypertension. We are also conducting pre-clinical formulation development work for new product candidates in the glaucoma and anti-inflammatory areas.
Current therapies using eye drops typically require high drug doses due to the relatively inefficient method of drug delivery. The periodic dosing of an eye drop results in pulsatory dosing, meaning the drug concentration peaks in the eye shortly after administration and then diminishes with time until it is restored with administration of the next eye drop. Delivery of therapeutics via the punctal plug delivery system could result in lower and more stable drug dosing over a sustained period of time which, if achieved, could be a desirable alternative to daily treatment with eye drops.
Milestone and Royalty Obligations
We acquired the punctal plug drug delivery technology as a result of the acquisition of ForSight Newco II, Inc. (“ForSight”) by our U.S. subsidiary, 3088923, Inc., pursuant to the terms of a merger agreement dated October 8, 2007. Under the terms of the merger agreement, we have agreed to use commercially reasonable efforts to develop and commercialize in certain major markets a specified number of punctal plug products claimed under the patents we acquired in the ForSight acquisition and from which pharmaceutical preparations are delivered to the eye. We have also agreed to pay the former ForSight stockholders royalties equal to 10% of net sales of such products made by us or our licensees, subject to reduction under certain specified conditions. The total amount of royalties payable by us are capped on a product by product basis at certain specified aggregate amounts. In addition, if we grant licenses to unaffiliated third parties with respect to the intellectual property acquired as part of the acquisition of ForSight for punctal plug products, we have agreed to pay the former ForSight stockholders 10% of any payments received from such third parties in consideration for such license in the form of up-front fees, maintenance fees, milestone payments and similar fees or payments, and a specified percentage of any amounts recovered in any action to enforce the licensed patents. Also, to the extent we develop and commercialize such products, we have agreed to pay the former ForSight stockholders $5.0 million upon initiation of the first Phase III pivotal clinical trial for a punctal plug product that delivers latanoprost, and $20.0 million upon the first commercial sale in the United States of a first punctal plug product that delivers latanoprost that meets certain clinical or regulatory criteria. The former ForSight stockholders are also eligible to receive $20.0 million upon the first commercial sale of the first additional punctal plug product covered by ForSight’s intellectual property rights and $15.0 million upon the first commercial sale of each additional punctal plug product covered by ForSight’s intellectual property rights thereafter.
Initial Drug and Target Indication (L-PPDS)
Latanoprost for Glaucoma and Ocular Hypertension. The first indication we are pursuing for our drug delivery system is the treatment of glaucoma and ocular hypertension by the sustained release of latanoprost, a prostaglandin analog. Glaucoma is a disease of the optic nerve involving a progressive loss of retinal ganglion cells that is often associated with elevated intraocular pressure (“IOP”) that results in a reduced or diminished visual field over time. Latanoprost has been previously approved by the FDA for reducing IOP in patients with glaucoma or ocular hypertension. If successful, our punctal plug drug delivery system, when inserted into the punctum in the eye and retained for the desired treatment duration, will release a relatively steady stream of latanoprost into the tear film.
11
Table of Contents
Current Treatment and Patient Compliance. Over 90% of glaucoma patients requiring treatment are treated with topical medications, such as eye drops, four to six percent receive surgery and, on average, each diagnosed patient has multiple visits to his or her eye physician each year. Due to the chronic, progressive nature of the disease, compliance with topical eye drop medications in glaucoma patients is crucial for effective management of the disease. Compliance with existing glaucoma medications is generally acknowledged to be low, with approximately half of treated patients in the U.S. not refilling their prescription after the first six months of therapy. Patient non-compliance can be due to a number of factors, including the asymptomatic nature of the disease and the difficulties patients face in proper administration of, and adherence to prescribed dosing of, the eye drops. Patient non-compliance is a major obstacle in glaucoma treatment, and can lead to progression of disease and irreversible loss of vision.
Potential Market. Glaucoma is a chronic, life-long disease that affects approximately 2.7 million individuals in the U.S. and approximately 60 - 80 million individuals worldwide. According to the Glaucoma Research Foundation, glaucoma is the second leading cause of blindness in the Western World. In 2010, IMS Health estimated that sales in major markets for topical glaucoma treatments, including eye drops, aggregated approximately $4.6 billion annually (including a $2.0 billion U.S. market), despite the poor compliance associated with the current eye drop therapies. Prostaglandin analogs, such as latanoprost, represent the largest segment of the prescribed glaucoma medication market. In the U.S., latanoprost is marketed under the trade name Xalatan® and other generic brand names. Starting in the first quarter of 2011, patent protection on Xalatan expired in the U.S. and certain other major markets, which has led to generic products entering these markets. The generic competition has led to reduced prices of latanoprost in the U.S. and may reduce the market size (in dollars) for topical glaucoma treatments in the U.S. and other countries.
Latanoprost Punctal Plug Drug Delivery System (L-PPDS). Our goal is to develop a punctal plug drug delivery system that provides an effective, convenient and reliable treatment alternative to eye drops for glaucoma patients that could ultimately improve patient compliance with their medication and hence the long-term outcome for their disease. In addition to development of the L-PPDS, ancillary development activities are ongoing to optimize the final L-PPDS product, including development of an insertion tool to simplify the in-office insertion procedure for ophthalmologists and optometrists and a simple home-use detection system that would allow patients to confirm the presence of the punctal plugs between office visits. We believe that, if successful, the application of latanoprost using the punctal plug drug delivery system could potentially replace, in whole or in part, existing eye drop therapies used in the treatment of glaucoma.
2011 L-PPDS Phase II Trial. In August 2011, we completed a Phase II multicenter study to investigate the safety and efficacy of the L-PPDS utilizing simultaneous placement of punctal plugs in the upper and lower puncta containing a combined total of 141 µg of latanoprost over a four week testing period in 95 (Intent to Treat (ITT)) subjects with open-angle glaucoma (“OAG”) or ocular hypertension (“OH”). The study utilized a later stage proprietary prototype punctal plug in the lower punctum and an early stage prototype modified commercially available punctal plug in the upper punctum. The overall drug development objective for this study was to determine if double plugging could generate a mean reduction in IOP of 5 mmHg or greater.
The primary endpoint in the Phase II study was the mean change in IOP from baseline (measured as mmHg) at two weeks. Secondary endpoints were the mean change in IOP from baseline at four weeks and mean percentage change in IOP from baseline at two weeks and four weeks. The mean IOP at baseline was 25.8 mmHg. After two weeks of L-PPDS treatment, IOP showed a statistically significant mean change from baseline of -6.2 mmHg (95% C.I. -6.8, -5.6) (n=70). At the end of two weeks, 73% of subjects showed an IOP reduction vs. baseline of 5 mmHg or greater and 51% of subjects showed a reduction of 6 mmHg or greater. The mean percentage change in IOP from baseline at two weeks was -24.3%, which was statistically significant (95% C.I. -26.7, -21.9).
After four weeks of L-PPDS treatment, IOP showed a statistically significant mean change from baseline of -5.7 mmHg (95% C.I. -6.5, -4.9) (n=53). At the end of four weeks, 60% of subjects showed an IOP reduction vs. baseline of 5 mmHg or greater and 47% of subjects showed a reduction of 6 mmHg or greater. The mean percentage change in IOP from baseline at four weeks was also statistically significant at -22.3% (95% C.I. -25.4, -19.2). Results reported in the earlier Phase II L-PPDS studies using different L-PPDS plug designs and doses (44 µg, 81 µg and 95 µg of latanoprost) in the lower punctum only did not achieve these levels of IOP reduction over a four week treatment period.
The L-PPDS was well tolerated over the testing period with adverse events (AEs) similar to those reported for commercial punctal plugs. The majority of AEs were ocular in nature, with tearing reported as the most frequent. No associated AEs were serious. As assessed by subjective scoring by study subjects, tearing was rated for L-PPDS treated eyes at week four as occasional - 22%, mild - 31%, or moderate - 32%. Few subjects experienced any discomfort related to the punctal plugs with most patients having either no awareness or mild awareness of the punctal plugs by week four (87% of eyes for L-PPDS subjects). A total of 42 subjects were not included in the ITT analysis at week four, with 34 of these due to losses of the early stage prototype upper plug, as expected. During the study, five subjects discontinued due to AEs, and three subjects discontinued for other reasons. All subjects were included in the safety analysis.
Current L-PPDS Phase II Clinical Trials. Following the positive four week data from the L-PPDS Phase II clinical trial, in December 2011, we initiated two additional Phase II clinical trials at multiple investigator sites in the U.S. to further evaluate the safety, efficacy and duration of effect of the L-PPDS. The studies are designed to address questions raised from the L-PPDS Phase II trial completed in 2011, including assessment of the effect of tearing, latanoprost dosage and the single versus double plug approaches, and to evaluate longer duration of sustained release. The study designs utilize variable plug placement (in either or both upper and lower
12
Table of Contents
punctum) of the L-PPDS containing different doses of latanoprost up to a total 190 µg of latanoprost over a 12-week period, and address two primary study objectives: (i) a reduction in baseline IOP greater than 5 mmHg at various time points between 30 and 90 days, and (ii) to determine the optimal plug placement for IOP lowering effects to optimize the design of next stage clinical trials. Enrolment in these trials is ongoing. Results and analysis from these trials are expected in the second half of 2012.
Second Drug and Target Indication (O-PPDS)
Olopatadine for Allergic Conjunctivitis. The second indication we pursued for treatment with our punctal plug drug delivery system was the treatment of allergic conjunctivitis through the sustained release of olopatadine, an antihistamine, into the tear film. Allergic conjunctivitis is an allergic response on the surface of the eye due to an immune reaction to allergy-causing substances such as pollen spores or pet dander.
O-PPDS Clinical Trial. On February 9, 2011 we announced the results of a randomized, placebo-controlled, double-masked Phase II proof-of-principle study investigating the short-term safety and efficacy of the O-PPDS in subjects with seasonal allergic conjunctivitis. The data demonstrated that there were no significant differences noted between the O-PPDS and placebo-PPDS subjects with respect to reduction in the signs and symptoms of allergic conjunctivitis with both cohorts showing similar improvements. Internal study controls of the olopatadine (Patanol®) versus placebo eye drop comparison also failed to show a difference. The equivocal results support the notion that the environmental exposure chamber (EEC) model utilized in the conduct of the trial was not sufficiently sensitive to adequately demonstrate the potential benefit of the O-PPDS in patients suffering from allergic conjunctivitis.
Current Development Status. Development work on the O-PPDS program was suspended in February 2011. We have no current plans to restart development work on this product candidate.
Punctal Plug Devices
We are developing proprietary punctal plug prototypes for the L-PPDS and other product candidates that are designed to be retained in the punctum for a desired treatment duration. The selection of an optimal final upper and/or lower punctal plug design for the L-PPDS will converge with the identification of a dose formulation and delivery system that, if successful in Phase II, would be taken into later-stage clinical trials. To date, we have studied the safety, tolerability, comfort, ease of handling and retention of numerous prototype plug designs in over 800 subjects. The objective of these studies is to identify a safe and comfortable punctal plug design for each of the upper and lower puncta that is easily administered and demonstrates an optimal 90% retention over 90 days. We continue to make progress towards this goal through ongoing clinical dosing studies and device-only studies.
Lower Punctal Plug Device Results. In 2011, the Phase II L-PPDS clinical trial in glaucoma demonstrated achievement of 95% retention at four weeks (53 subjects) utilizing a later stage proprietary punctal plug in the lower punctum. Data on safety, tolerability and comfort are described above.
Upper Punctal Plug Device Results. In 2011, we began testing proprietary prototype punctal plug designs for insertion in the upper punctum. The Phase II L-PPDS clinical trial utilized the first early stage prototype upper punctal plug which was based on a modified commercially available plug. This modified commercial plug achieved 45% retention at four weeks (53 subjects). Following these clinical trial results, in 2011 we conducted a short-term (four week) multicenter, device-only evaluation study on healthy volunteers to evaluate the safety, tolerability, comfort, ease of handling and insertion/removal, and retention of a new proprietary upper punctal plug. The data from this study to date demonstrated 81% retention at four weeks (27 eyes). Final analysis of data from this study is expected to be reported in 2012.
Current Punctal Plug Device Development. In conjunction with the ongoing Phase II L-PPDS trials in glaucoma, we are conducting a multicenter, device-only feasibility study to evaluate the safety, tolerability, comfort, tearing, ease of handling and insertion/removal, and retention of prototype upper and lower punctal plug designs in healthy volunteers with up to 12 weeks of follow-up. An investigational insertion tool and plug detector system will also be evaluated in this study.
Visudyne
In view of the importance of understanding the clinical significance of the use of Visudyne in combination with other therapies for the treatment of wet AMD, we and Novartis have separately conducted studies comparing the safety and efficacy of Visudyne in combination with an anti-VEGF drug either as bi-therapy (Visudyne plus an anti-VEGF) or triple therapy (Visudyne plus an anti-VEGF and a steroid) under the RADICAL and SUMMIT (consisting of the DENALI, MONT BLANC and EVEREST studies) clinical trial programs, respectively. The results of these studies were reported in 2009 and 2010.
From time to time, we may support investigator-sponsored studies that evaluate Visudyne alone or in combination with other products for the treatment of ocular diseases.
13
Table of Contents
We contract with qualified third parties to manufacture and supply Visudyne. We maintain internal quality control, quality assurance and regulatory affairs to oversee the activities of these third party manufacturers. Visudyne is currently manufactured in stages by several contract facilities located in the U.S., Europe and Japan. We have supply agreements with Nippon Fine Chemical Co., Ltd., JHP Pharmaceuticals, LLC (previously Parkedale Pharmaceuticals Co., Ltd.), Jubilant HollisterStier LLC (previously Hollister-Stier Laboratories LLC), and Axyntis S.A.S. (previously Orgapharm S.A.S.) for manufacturing activities in the commercial production of Visudyne. The key starting materials for the Visudyne manufacturing process are secured by long-term supply agreements or through inventory safety stocking. We believe that our current inventory of materials and outsourced manufacturing arrangements will allow us to meet our near and long-term manufacturing needs for Visudyne. Our manufacturing and supply agreements have terms expiring on various dates beginning in December 2012 and continuing through December 2016. We believe, but cannot assure, that we will be able to renew these agreements as they expire or will enter into arrangements with suitable alternate suppliers and manufacturers as needed. However, if we are not able to renew these agreements or find suitable replacements, our business may be harmed.
Certain clinical and preclinical materials and active pharmaceutical ingredients (“API”) used in our products in development are currently produced at our facilities in Vancouver, British Columbia. We also utilize a small number of third party contractors to manufacture and supply certain materials, API and devices for our products in development, and expect to continue to do so for our commercial needs.
We and our contract manufacturers and suppliers are subject to the FDA’s current Good Manufacturing Practices (“cGMP”) regulations and other rules and regulations prescribed by regulatory authorities outside the U.S. We rely on our contract manufacturers and suppliers to comply with cGMP requirements and other applicable standards.
Product Sales, Marketing and Distribution
United States Distribution
Effective January 1, 2010, pursuant to the Amended PDT Agreement with Novartis, we received exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. As a result, we operate a small U.S.-based marketing, sales and distribution organization through our wholly-owned U.S. subsidiary, QLT Ophthalmics, Inc. (“QOI”).
Our sales and marketing efforts are focused primarily on retinal specialists in private practice or at medical centres or hospitals in the U.S. We follow typical pharmaceutical company sales and marketing practices, such as sales representative visits to physicians, advertisements, public relations and other methods. QOI provides reimbursement support, a patient assistance program and customer service programs related to Visudyne, including a product-specific website at www.visudyne.com, and other order, delivery and fulfillment services. Our patient assistance program offers support for U.S. resident patients who are advised by their physician that they need to be treated with Visudyne, but who do not have sufficient insurance for the product and qualify under the household income guidelines and other criteria for the program.
We also provide on-going education and scientific-based information to physicians at their request through Field Medical Scientists at our wholly-owned U.S. subsidiary, QLT Therapeutics, Inc.
Visudyne is sold in the U.S. by QOI to specialty wholesale distributors who then distribute the product directly to end-user customers. Our specialty wholesale distributors for Visudyne in the U.S. are ASD Specialty Healthcare, Inc. d/b/a Besse Medical (“Besse”) and Priority Healthcare Distribution, Inc. d/b/a CuraScript SD Specialty Distribution (“CuraScript”). The distribution agreement with Besse automatically renews each year on December 31st for successive one year periods unless either party provides notice that it does not intend to renew at least 120 days before the end of the current term. The distribution agreement with CuraScript has a term expiring on May 31, 2012. We believe that on expiry of these agreements we will be able to enter into further agreements with these or other parties for distribution of our product, however, in the event we are unable to do so on terms acceptable to us or at all, our business may be harmed.
Rest of World Distribution
Visudyne is marketed and sold outside the U.S. by Novartis. Under the Amended PDT Agreement and a manufacturing and supply agreement with Novartis, we manufacture Visudyne and supply the product at a pre-specified price exclusively to Novartis for distribution outside the U.S. Novartis also pays us a royalty on net sales of Visudyne outside the U.S. See Visudyne - Our Approved Product above.
Medical Lasers Required for Visudyne Therapy
Visudyne therapy requires a physician to deliver a dose of non-thermal light at a particular wavelength to target tissue in the eye in order to activate the photosensitizer. We have historically relied on a limited number of third party medical device companies in the U.S., Europe, Japan and elsewhere to manufacture, sell and service the diode laser systems commercially approved for use in Visudyne therapy.
14
Table of Contents
However, with the decline in Visudyne sales over the last several years due to the emergence of competing therapies, the commercial supply and technical support for laser light delivery devices has significantly declined, particularly in the U.S. where third party commercial operations supporting these laser devices has declined or been discontinued altogether, and the supply of laser devices has been depleted.
The expected lifecycle of the laser systems for Visudyne therapy is approximately five to 10 years, which is heavily dependent on the number of patients treated and normal maintenance and care. We believe the majority of the lasers currently on the market have reached or will in the coming years reach the end of their lifecycle and, therefore, we expect many of these lasers will need significant upgrades or will need to be replaced. If customers decide not to maintain their lasers or invest in a new laser, for example, due to the associated costs and/or the availability of other competitive therapies that do not require a medical device, or if demand for repair or replacement of lasers exceeds the commercial supply and maintenance available in the U.S., Europe or elsewhere, Visudyne sales will be materially adversely affected.
In an effort to provide direct support to the commercial supply of laser light delivery devices in the U.S., we have engaged third parties to assist in developing and manufacturing a QLT proprietary laser. If approved by the FDA for use in Visudyne therapy through the supplemental premarket approval (“PMA”) process, we anticipate this product could be available for marketing and sale by QOI as early as 2013. We also continue to evaluate other ways in which we can support the commercial supply and maintenance of laser light delivery devices in the U.S. because of Visudyne’s dependence on these products.
Financial Information about Segments and Geographic Areas
We operate in one segment and the geographic information required herein is contained in Note 18 - Segment Information in Notes to the Consolidated Financial Statements and is incorporated by reference herein.
Patents, Trademarks and Proprietary Rights
We seek to protect our proprietary technology by obtaining patents to the extent we consider it advisable, and by taking contractual measures and other safeguards to protect our trade secrets and innovative ideas. We currently own or have acquired rights to a number of patents and patent applications for the technologies utilized in our commercial products and products in development in the U.S., Canada and other jurisdictions.
Our policy is to file patent applications on a worldwide basis in those jurisdictions where we consider it beneficial, depending on the subject matter and our commercialization strategy. The most significant patents owned or licensed by us are described below.
Visudyne
Verteporfin - UBC License. Verteporfin, the active ingredient in Visudyne, is protected by granted patents in major markets. These patents are owned by the University of British Columbia (“UBC”) and exclusively licensed to us. We entered into a license agreement with UBC in 1988 that granted us a worldwide, exclusive, royalty-bearing license to know-how and patents relating to porphyrin derivatives, including verteporfin, with the right to sublicense. The license was amended and restated in December 2007 and terminates upon the expiration of all of the licensed patents. UBC has the right to terminate the license upon, among other things, our bankruptcy, winding up, liquidation or otherwise ceasing to carry on our business (other than a disposition of all or substantially all of our assets to another party), our material breach of the requirement to obtain and maintain insurance pursuant to the terms of the license, or our material default under or our failure to comply with a material term of the licence which is not remedied within 30 days.
Verteporfin Patent Rights. U.S. Patent No. 5,095,030, which covered verteporfin, expired on September 9, 2011. In Europe, verteporfin is covered by European Patent 0352076, for which we applied for and received an extension of patent term until July 18, 2014. In Japan, verteporfin is covered by JP 2137244, for which we applied for and received an extension of patent term until July 19, 2014.
Verteporfin lipid-based formulation. We own or exclusively license patents covering the Visudyne drug product relating to the lipid-based formulation of verteporfin. U.S. Patent No. 5,214,036, which was owned by UBC and exclusively licensed to us, expired on May 25, 2010. U.S. Patent No. 5,707,608 expires on August 2, 2015, with foreign equivalents expiring in 2016. U.S. Patent No. 6,074,666 expired on February 5, 2012, with foreign equivalents expiring in 2013. In addition to these patents, we own or license several patents and patent applications covering alternative formulations of verteporfin.
Approved Uses of Visudyne. We own or license patents covering certain approved uses of Visudyne. U.S. Patent No. 5,756,541, expiring on March 11, 2016, with foreign equivalents expiring in 2017, is co-owned by Novartis and us and covers methods of using Visudyne to improve visual acuity in subjects having unwanted ocular neovasculature. U.S. Patent No. 5,798,349, which expires on August 25, 2015, and its Canadian equivalent expiring on March 14, 2014, is co-owned by MGH, MEEI and us, and covers methods of treating AMD using Visudyne. U.S. Patent No. 5,770,619, which expires on November 20, 2012, with foreign equivalents expiring in 2013, is owned by
15
Table of Contents
UBC and exclusively licensed to us and covers methods of using Visudyne to treat neovasculature involving a reduced interval between drug and light administration. In addition to these patents covering on-label uses of Visudyne, we own or license several other patent applications relating to alternative methods of using Visudyne in the treatment of ocular diseases, including AMD.
MGH License. We have an exclusive worldwide license from MGH for its rights in U.S. Patent No. 5,798,349 and to all foreign equivalents relating to methods of treating AMD using verteporfin, which MGH owns jointly with us and MEEI. These rights are non-exclusive if exclusive rights are not available in any foreign jurisdiction. The term of the MGH license continues on a country by country basis for so long as any such patent right remains in effect. The U.S. patent expires on August 25, 2015. Under the MGH license, we were required to pay a 0.5% royalty to MGH based on Visudyne sales in the U.S. and Canada. Pursuant to the terms of a settlement agreement and amendment to license agreement dated November 24, 2009 related to a legal action that MGH had launched against us in February 2009, we paid MGH an aggregate $20.0 million, constituting payment in full of all past and future royalty obligations and the license is now fully paid up. The payment was also in satisfaction and settlement of any obligations we had, may have, or may have had in connection with the subject matter of the MGH litigation.
Photosensitizers. We own or license additional patent applications relating to PDT and methods of using photosensitizers.
Punctal Plug Drug Delivery System
In connection with our acquisition of ForSight Newco II, Inc. (now named QLT Plug Delivery, Inc.) in October 2007, we acquired a number of patent applications covering different aspects of the punctal plug technology, including punctal plug designs and devices, methods of making punctal plugs and uses thereof for delivering therapeutic drugs to the eye for treating eye diseases, including glaucoma. Patents issuing from these patent applications will expire between 2024 and 2028, not including any possible patent term extensions that may be available. To further expand and strengthen our intellectual property portfolio, we have filed and continue to file additional patent applications on punctal plugs and devices, methods of manufacturing punctal plugs and uses thereof in those jurisdictions where we consider it beneficial, depending on the subject matter and our commercialization strategy.
QLT091001 - Synthetic Retinoid
Pursuant to our co-development agreement with Retinagenix, we have an exclusive, worldwide sub-license to patents and patent applications relating to various synthetic retinoids and uses thereof, including in the treatment of LCA and RP. These patents and patent applications are owned by the University of Washington, which has licensed the patents and patent applications to Retinagenix, and are sub-licensed to us by Retinagenix.
On May 31, 2011, the United States Patent and Trademark Office issued a key patent related to this program, covering various methods of use of QLT091001 in the treatment of diseases associated with an endogenous 11-cis-retinal deficiency, expiring on July 7, 2027, including the period of Patent Term Adjustment. The molecule in QLT091001 is not eligible for composition of matter protection per se, as it was previously known in the scientific community. To further expand and strengthen our intellectual property portfolio, we have filed and continue to file additional patent applications on synthetic retinoids, and uses thereof in those jurisdictions where we consider it beneficial, depending on the subject matter and our commercialization strategy. If QLT091001 is approved by the FDA and the EMA for marketing in the U.S. and EU, we plan to apply for any patent term extensions and regulatory exclusivities that are available to us under applicable law. See Government Regulation - Market Exclusivity and Government Regulation - Additional Regulatory Issues below.
In addition to any protection our patent portfolio may offer, QLT091001 has received orphan drug designations for the treatment of LCA and RP by the EMA, and for the treatment of LCA and RP due to inherited mutations in the LRAT and RPE65 genes by the FDA. If a drug is ultimately approved as an orphan drug, it receives an extended period of market exclusivity, subject to certain limitations based on the jurisdiction. See Government Regulation - Orphan Drug Regulation below.
Other Patents, Trademarks and Proprietary Rights
In addition to patent protection, we also rely on trade secrets, proprietary know-how and continuing technological innovation to develop and maintain a competitive position in our product areas.
We require our collaborative licensees and potential business collaborators, investigators, employees and consultants who might have access to or be provided with proprietary information to sign confidentiality agreements.
Our patent position and proprietary technologies are subject to certain risks and uncertainties. Although a patent has a statutory presumption of validity, the issuance of a patent is not conclusive as to its validity or as to enforceability of its claims. Accordingly, there can be no assurance that our patents will afford legal protection against competitors, nor can there be any assurance that the patents will not be infringed by others, nor that others will not obtain patents that we would need to license.
16
Table of Contents
Unpatented trade secrets, improvements, confidential know-how and continuing technological innovation are important to our scientific and commercial success. Although we attempt to and will continue to protect our proprietary information through reliance on trade secret laws and the use of confidentiality agreements with our collaborators, investigators, employees and consultants and other appropriate means, there can be no assurance these measures will effectively prevent disclosure of our proprietary information or that others will not develop independently or obtain access to the same or similar information or that our competitive position will not be affected adversely thereby.
We have included information about risks and uncertainties relating to protection of our proprietary information under Item 1A. Risk Factors.
Visudyne is sold around the world under a brand-name trademark which we are authorized to use by Novartis. We own several registered trademarks in the U.S. and Canada and in other jurisdictions.
The pharmaceutical and biotechnology industries are characterized by rapidly evolving technology and intense competition. Our competitors include major pharmaceutical and biopharmaceutical companies, many of which have financial, technical and marketing resources significantly greater than ours and substantially greater experience in developing products, conducting preclinical and clinical testing, obtaining regulatory approvals, manufacturing and marketing. In addition, many biopharmaceutical companies have formed collaborations with large, established pharmaceutical companies to support research, development and commercialization of products that may be competitive with our products. Academic institutions, government agencies and other public and private research organizations also are conducting research activities and seeking patent protection and may commercialize products on their own or through joint ventures. The existence of these products, or other products or treatments of which we are not aware, or products or treatments that may be developed in the future, may adversely affect the marketability of products developed by us.
Visudyne
In June 2006, Genentech, Inc. (“Genentech”) commercially launched Lucentis®, an anti-VEGF antibody, in the U.S. for the treatment of neovascular wet AMD. Novartis, Genentech’s marketing partner for Lucentis outside of the U.S., began marketing Lucentis in early 2007 in the EU and in early 2009 in Japan for the treatment of wet AMD. Although not approved for this use, Avastin® (bevacizumab) from Genentech is also being used off-label extensively by physicians for the treatment of wet AMD. Lucentis and Avastin have had a material negative impact on Visudyne sales and are expected to continue to be competitive with Visudyne as those products become available in other countries or are used off-label. For example, in January 2012, Lucentis was approved for marketing in China, one of the principal markets for Visudyne.
In addition, in November 2011, Regeneron Pharmaceuticals, Inc. commercially launched Eylea® (aflibercept injection), a VEGF binding protein also known as VEGF Trap-Eye, in the U.S. for the treatment of neovascular wet AMD. We are also aware of submissions for marketing authorization for Eylea in Europe and Japan and a Phase III clinical trial in China. In addition, we are aware of other competitive therapies for wet AMD that are currently in various stages of development. One of these therapies, EpiRad90™, is a product under development by NeoVista Inc., and has been CE marked in the EU for marketing and is in Phase III clinical trials in the U.S. for the treatment of wet AMD. EpiRad90 was recently reported to have failed to meet its primary endpoint in a large global clinical study. The impact of the results of this study on subsequent approvals and/or market penetration of EpiRad90 is not yet known. Further, one of our Visudyne competitors is also our collaborator. Novartis, which, pursuant to the Amended PDT Agreement, has the marketing and sales rights to our Visudyne product outside of the U.S., also has rights to market Lucentis® outside of the U.S.
The research and development, preclinical studies and clinical trials, and ultimately, the manufacturing, marketing and labelling of our products, are subject to extensive regulation by the FDA and other regulatory authorities in the U.S. and other countries. The U.S. Food, Drug and Cosmetic Act and its regulations govern, among other things, the testing, manufacturing, safety, efficacy, labelling, packaging, storage, record keeping, approval, clearance, distribution, export and import, advertising and promotion of our products. Preclinical studies, clinical trials and the regulatory clearance and approval process can take years and may require the expenditure of substantial resources. If regulatory approval or clearance of a product is granted, the approval or clearance may include significant limitations on the indicated uses for which the product may be marketed.
FDA Regulation - Approval of Drug Products
Visudyne is regulated in the U.S. as a drug. Our other products in development will also be regulated as drugs or, in the case of our punctal plug drug delivery system, drug/device combination products, under U.S. law. The steps ordinarily required before a drug, or certain kinds of drug/device combination products, may be marketed in the U.S. include:
17
Table of Contents
| • | preclinical testing; |
| • | submission of an IND to the FDA, which must become effective before human clinical trials may commence; |
| • | adequate and well-controlled human clinical trials to establish the safety and efficacy of the drug for its proposed intended use; |
| • | validation and approval of the manufacturing facilities and process; |
| • | submission of a new drug application (“NDA”) or abbreviated new drug application (“ANDA”) to the FDA; and |
| • | FDA approval of the application, including approval of all labelling. |
The testing and approval process requires substantial time, effort and financial resources and we cannot be certain that any approvals of our product candidates will be granted on a timely basis, if at all.
Preclinical tests include evaluation of product chemistry and formulation as well as in vitro and animal studies to assess the potential safety and efficacy of the product. The results of preclinical testing are submitted as part of an IND to the FDA. A 30-day waiting period after the filing of each new IND is required prior to the commencement of clinical testing in humans. In addition, the FDA may, at any time during this 30-day period, or anytime thereafter, impose a clinical hold on proposed or ongoing clinical trials. If the FDA imposes a clinical hold, clinical trials cannot commence or recommence without FDA authorization.
Clinical trials to support NDAs are typically conducted in three sequential phases, but the phases may overlap. In Phase I, the initial introduction of the drug into healthy human subjects or patients, the drug is tested to assess metabolism, pharmacokinetics, drug interaction, bioavailability and bioequivalence, pharmacodynamics and safety, including side effects associated with increasing doses. Phase II usually involves studies in a limited patient population to:
| • | assess the efficacy of the drug in specific, targeted indications; |
| • | assess dosage tolerance and optimal dosage; and |
| • | identify possible adverse effects and safety risks. |
If a compound is found to be potentially effective and to have an acceptable safety profile in Phase II evaluations, Phase III trials are undertaken to further demonstrate clinical efficacy and to further test for safety within an expanded patient population at multiple study sites.
After successful completion of the required clinical testing, the results of preclinical studies and clinical studies, along with descriptions of the manufacturing process, proposed labelling and other relevant information, are submitted to the FDA as part of an NDA. Under the Prescription Drug User Fee Act, the FDA aims to review the NDA within 10 months if it is a standard application, or within six months if it is a priority review application. If the FDA evaluations of the NDA and the manufacturing facilities are favorable, the FDA may issue either an approval letter or a “complete response letter” that identifies the deficiencies in the NDA that must be corrected in order to secure final FDA approval of the NDA. When, and if, those deficiencies have been addressed to the FDA’s satisfaction, the FDA will issue an approval letter. Approval may be conditioned on the sponsor’s agreement to undertake Phase IV post-approval studies to further assess the drug’s safety and effectiveness, or on the development of a Risk Evaluation and Mitigation Strategy (“REMS”) that limits the labeling, distribution or promotion of a drug product.
FDA Regulation - Clearance and Approval of Medical Devices
Visudyne requires a laser for light activation of the drug substance after injection. These lasers are classified as Class III devices and PMA applications must be filed for review with the FDA. A PMA application must demonstrate that the laser is safe and effective for the proposed use, and must be supported by extensive data, including data from preclinical studies and human clinical studies. A PMA must contain a full description of the device and its components, a full description of the methods, facilities, and controls used for manufacturing the device, and proposed labeling. The FDA has 180 days to review a complete PMA that has been accepted for filing, although the review more often occurs over a significantly longer period of time.
After FDA review and panel recommendation, the FDA may then issue an approval letter, an approvable letter, a not approvable letter or an order denying approval. Approval of a device may be conditioned on the sponsor’s agreement to conduct some form of post-market surveillance when necessary to protect the public health or provide additional safety and effectiveness data for the device. Once an approval letter is received, the holder of the approved PMA must submit annual reports and any PMA supplements for review and approval by the FDA before making any changes that affect the safety or effectiveness of the device. Due to the current lack of supply and commercial marketing of approved lasers for use with Visudyne, in particular in the U.S., we are developing a proprietary PDT laser for use with Visudyne. We plan to seek FDA approval for this device by submission through the PMA supplement process in the U.S.
18
Table of Contents
FDA Regulation - Post-Approval Requirements
Even if regulatory clearances or approvals for our products are obtained, our products and the facilities manufacturing our products are subject to continued review and periodic inspections by the FDA. Each U.S. drug and device-manufacturing establishment must be registered with the FDA. U.S. manufacturing establishments are subject to biennial inspections by the FDA and must comply with the FDA’s current good manufacturing practices (“cGMPs”) if the facility manufactures drugs, and quality system regulations (“QSRs”) if the facility manufactures devices. In complying with cGMPs and QSRs, manufacturers must expend funds, time and effort in the area of production and quality control to ensure full technical compliance. The FDA stringently applies regulatory standards for manufacturing.
The FDA also regulates labelling and promotional activities. Further, we must report adverse events involving our drugs and devices to the FDA under regulations issued by the FDA. Failure to maintain compliance with regulatory requirements may result in administrative or judicial actions, such as fines, warning letters, holds on clinical studies, product recalls or seizures, product detention or refusal to permit the import or export of products, refusal to approve pending applications or supplements, restrictions on marketing or manufacturing, injunctions, or civil or criminal penalties.
EU Regulation - Approval of Medicinal Products
Visudyne is regulated in the EU as a medicinal product. Our other products in development will also be regulated as medicinal products in the EU. In the European Economic Area (“EEA”) (which comprises the 27 Member States of the EU plus Norway, Iceland and Liechtenstein), medicinal products can only be commercialized after obtaining a marketing authorization (or “MA”). There are two types of marketing authorizations:
The Community MA, which is issued by the European Commission through the Centralised Procedure, based on the opinion of the Committee for Medicinal Products for Human Use (“CHMP”) of the EMA, and which is valid throughout the entire territory of the EEA. The Centralised Procedure is compulsory for medicinal products that contain a new active substance and are indicated for the treatment of AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and other immune dysfunctions, and viral diseases, as well as for orphan medicinal products and products developed through certain biotechnological processes. The Centralised Procedure is optional for products containing a new active substance not yet authorized in the EEA, or for products that constitute a significant therapeutic, scientific or technical innovation, or whose approval is in the interest of public health in the EU. In the Centralised Procedure applications are submitted directly to the EMA. The EMA’s CHMP then has 210 days (not including stop-clocks) to adopt an opinion on whether the medicine should be marketed or not. The CHMP’s opinion is then transmitted to the European Commission, which has the ultimate authority for granting the Community MA.
National MAs, which are issued by the competent authorities of the Member States of the EEA and only cover their respective territory, are available for products not falling within the mandatory scope of the Centralised Procedure. Where a product has already been authorized for marketing in a Member State of the EEA (the so-called Reference Member State), this National MA can be recognized in other Member States through the Mutual Recognition Procedure. If the product has not received a National MA in any Member State at the time of application, it can be approved simultaneously in various Member States through the Decentralised Procedure.
Under the Mutual Recognition Procedure, within 90 days of receipt of a valid application, the Reference Member State provides an updated assessment report together with the approved summary of product characteristics (“SPC”), labelling and package leaflet to the Member States where the applicant seeks recognition of its original MA (the Concerned Member States). After receipt of these documents, the Concerned Member States have another 90 days to recognise the decision of the Reference Member State and the approved SPC, package leaflet and labelling and grant a harmonised National MA. The Concerned Member States may, however, refuse to recognize the Reference Member State´s MA on grounds of a potential serious risk to public health, in which case the points of disagreement are referred to the Coordination Group for Mutual Recognition and Decentralised Procedures – Human (the “CMDh”). The CMDh will try to reach a consensus between the Reference Member State and the objecting Concerned Member States within 60 days, and if an agreement is not reached the matter will be referred to the EMA’s CHMP for arbitration.
Under the Decentralised Procedure, an identical dossier is submitted to the competent authorities of each of the Member States in which the MA is sought, one of which is selected by the applicant as the Reference Member State. The competent authority of the Reference Member State prepares a draft assessment report (a draft SPC), and a draft of the labelling and package leaflet, which are sent to the other Member States (i.e. the Concerned Member States) for their approval. If the Concerned Member States raise no objections, based on a potential serious risk to public health, to the assessment, SPC, labelling or packaging proposed by the Reference Member State, the product is subsequently granted a National MA in all the Member States (i.e., in the Reference Member State and the Concerned Member States). In case of disagreement, a procedure before the CMDh and/or the CHMP similar to the one described above must be followed.
19
Table of Contents
Under all the above described procedures, before granting the MA, the EMA or the competent authorities of the Member States of the EEA make an assessment of the risk-benefit balance of the product on the basis of scientific criteria concerning its quality, safety and efficacy.
Further, under EU Regulation No. 1901/2006 on medicinal products for pediatric use, as amended, companies that wish to market their products in the EEA are required to submit the results of pediatric studies with their marketing authorization application. These studies must be undertaken in compliance with a pediatric investigation plan (“PIP”) that must be approved in advance by the EMA’s Pediatric Committee (“PDCO”). The obligation to provide the results of pediatric studies with the marketing authorization application can be waived for certain products or product classes if: (i) they are likely to be ineffective or unsafe in part or all of the pediatric population; (ii) the disease or condition for which they are intended occurs only in adult populations; or (iii) the specific product does not represent a significant therapeutic benefit over existing treatments for pediatric patients. If justified, applicants can obtain a deferral of the obligation to conduct pediatric studies until a time after the submission of the marketing authorization application, for instance, because it is appropriate to conduct studies in adults prior to initiating studies in children or when studies in children will take longer to conduct than studies in adults. A marketing authorization holder whose application incorporated the results of pediatric studies conducted in compliance with an approved PIP can be eligible for a six-month extension to the patent protection afforded to its product. This extended patent protection period does not apply to orphan medicinal products, which can benefit instead from a twelve-year period of marketing exclusivity.
Market Exclusivity
In the U.S., the Drug Price Competition and Patent Term Restoration Act of 1984 (also known as the Hatch-Waxman Act) established abbreviated FDA approval procedures for drugs that are shown to be equivalent to proprietary drugs previously approved by the FDA through its NDA process. Approval to market and distribute generic drugs is obtained by filing an abbreviated new drug application, or ANDA with the FDA, which must demonstrate that the product is bioequivalent to the innovator drug, rather than independently demonstrating the safety and effectiveness of the product through the submission of preclinical and clinical data. Innovator drugs are protected from generic competition through patent exclusivity, and the holder of an NDA for an innovator drug may also be entitled to a period of non-patent market exclusivity, during which the FDA cannot approve an ANDA. If the innovator drug is a new chemical entity, the FDA may not accept an ANDA for a bioequivalent product for up to five years following approval of the NDA for the new chemical entity. If the innovator drug is not a new chemical entity but the holder of the NDA conducted clinical trials essential to approval of the NDA or a supplement thereto, the FDA may not approve an ANDA for a bioequivalent product before expiration of three years. Additionally, a six-month period of market exclusivity may be added to existing patent and market exclusivity periods if the innovator drug is studied for pediatric indications.
In the EEA, Regulation (EC) No. 726/2004/EC and Directive 2001/83/EC (each as amended) have established a harmonized approach to data and marketing exclusivity (known as the 8 + 2 + 1 formula). The approach permits eight years of data exclusivity and 10 years of marketing exclusivity. An additional non-cumulative one-year period of marketing exclusivity is possible if during the data exclusivity period (the first eight years of the 10-year marketing exclusivity period), the MA holder obtains an authorization for one or more new therapeutic indications that are deemed to bring a significant clinical benefit compared to existing therapies.
The data exclusivity period begins on the date of the product’s first MA in the EU and prevents generics from relying on the marketing authorization holder’s pharmacological, toxicological, and clinical data for a period of eight years. After eight years, a generic product application may be submitted and generic companies may rely on the marketing authorization holder’s data. However, a generic cannot launch until two years later (or a total of 10 years after the first marketing authorization in the EU of the innovator product), or three years later (or a total of 11 years after the first MA in the EU of the innovator product) if the MA holder obtains marketing authorization for a new indication with significant clinical benefit within the eight-year data exclusivity period.
The 8 + 2 + 1 exclusivity scheme applies to products that have been authorized in the EU by either the EMA through the Centralized Procedure or the competent authorities of the Member States of the EEA (under the Decentralised, or Mutual Recognition procedures).
Orphan Drug Regulation
In the U.S., the Orphan Drug Act provides financial incentives to drug manufacturers to develop and manufacture drugs for the treatment of rare diseases, currently defined as a disease or condition that affects fewer than 200,000 individuals in the U.S. If a product that has an orphan drug designation subsequently receives the first FDA approval for the indication for which it has such designation, the product is entitled to orphan exclusivity, i.e., the FDA may not approve any other applications to market the same drug for the same indication for a period of seven years following marketing approval, except in certain very limited circumstances, such as if the later product is shown to be clinically superior to the orphan product or a market shortage occurs. The market exclusivity granted by the FDA would not, however, prevent other drug manufacturers from obtaining approval of the same compound for other indications, including another orphan indication, or the use of other types of drugs for the same orphan indication. The Orphan Drug Act also allows for other financial incentives to promote the development of these orphan designated drugs, including regulatory guidance, FDA fee reductions and tax credits related to the development expenses. Orphan drug designation generally does not confer any special or preferential treatment in the regulatory review process. Moreover, if a drug designated as an orphan product receives marketing approval for an indication broader than what is designated, it may not be entitled to orphan product exclusivity. Further, although obtaining orphan drug designation can be advantageous, there is no assurance that we will successfully develop or receive regulatory approval to market the product, nor can there be any assurance that we will be granted orphan drug designation for additional diseases or that orphan drug exclusivity will provide us with a material commercial advantage.
20
Table of Contents
Legislation similar to the Orphan Drug Act has been enacted in other countries outside of the U.S., including the EU. The orphan legislation in the EU is available for products that are (i) intended for the diagnosis, prevention or treatment of chronic debilitating or life-threatening conditions that affect five or fewer out of 10,000 persons in the EU, or (ii) that are intended for the diagnosis, prevention or treatment of a life-threatening, seriously debilitating or serious and chronic condition in the EU, and without incentives it is unlikely that their commercialization in the EU would generate sufficient return, provided that (iii) there is no satisfactory method of diagnosis, prevention or treatment of the condition in question that has been authorized in the EU, or if such a method exists, the medicinal product will be of significant benefit to those affected by the condition. The EU market exclusivity period is for ten years, although that period can be reduced to six years if, at the end of the fifth year, available evidence establishes that the requirements for orphan drug designation are no longer fulfilled, e.g., because the product is sufficiently profitable not to justify maintenance of market exclusivity. The market exclusivity may be extended to 12 years if sponsors complete a pediatric investigation plan agreed upon with the EMA’s PDCO. Marketing authorization applicants for orphan designated drugs can benefit from protocol assistance and significant fee reductions in the EMA authorization procedure.
Fast Track Designation
The FDA’s Fast Track program is intended to facilitate the development and review of drugs that are intended to treat a serious or life-threatening condition and demonstrate the potential to address unmet medical needs for the condition. Under the program, the sponsor of a new drug may request that the FDA designate the drug for a specific indication as a Fast Track product concurrent with or after the IND is filed for the product candidate. A drug that receives Fast Track designation may be eligible for more frequent meetings with the FDA to discuss the drug’s development; more frequent written correspondence from the FDA about the design of the proposed clinical trials; eligibility for accelerated approval, i.e., approval of an effect on a surrogate or substitute endpoint; and rolling review, meaning the sponsor may submit its NDA in sections rather than wait until the entire NDA is complete. Most drugs with Fast Track designation are likely to become eligible for a Priority Review, which provides for FDA review of an NDA within a six-month time frame from the time the complete NDA is accepted for filing, as opposed to the ten-month time frame for a Standard Review. The FDA grants Priority Review for products that offer major advances in treatment, or provide a treatment where no adequate therapy exists.
Healthcare Fraud and Abuse Laws
We are subject to various U.S. federal and state laws pertaining to health care “fraud and abuse,” including anti-kickback laws and false claims laws. For example, anti-kickback laws make it illegal for a prescription drug manufacturer to solicit, offer, receive or pay any remuneration in exchange for, or to induce, the referral of business, including the purchase or prescription of a particular drug. Due to the breadth of the statutory provisions and the increasing attention being given to them by law enforcement authorities, it is possible that certain of our practices may be challenged under anti-kickback or similar laws.
False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment to third party payors (including Medicare and Medicaid), claims for reimbursed drugs or services that are false or fraudulent, claims for items or services not provided as claimed, or claims for medically unnecessary items or services. The Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) created new federal statutes to prevent healthcare fraud and false statements relating to healthcare matters. The healthcare fraud statute prohibits knowingly and wilfully executing a scheme to defraud any healthcare benefit program, including private payors. The false statements statute prohibits knowingly and wilfully falsifying, concealing or covering up a material fact or making any materially false, fictitious or fraudulent statement in connection with the delivery of or payment for healthcare benefits, items or services. In addition, legislation passed in March 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively referred to as “PPACA”), among other things, amends the intent requirement of the federal Anti-Kickback Statute and the applicable criminal healthcare fraud statutes. Pursuant to the statutory amendment, a person or entity no longer needs to have actual knowledge of this statute or specific intent to violate it in order to have committed a violation. The federal Anti-Kickback Statute is broad, and despite a series of narrow safe harbors, prohibits many arrangements and practices that are lawful in businesses outside of the healthcare industry. Our sales and marketing activities for Visudyne may be subject to scrutiny under these laws. Violations of fraud and abuse laws may be punishable by criminal and/or civil sanctions, including fines and civil monetary penalties, as well as the possibility of exclusion from federal and state health care programs (including Medicare and Medicaid).
Additional Regulatory Issues
Under the Drug Price Competition and Patent Term Restoration Act of 1984, under certain conditions, a patent that claims a product, use or method of manufacture covering drugs and certain other products may be eligible for limited patent term extension for a period of up to five years as a compensation for patent term lost during drug development and the FDA regulatory review process. However, this extension cannot be extended beyond 14 years from the drug’s approval date. The scope of rights during this period of extension is
21
Table of Contents
generally limited to the product that was subject to regulatory delay. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves applications for patent term extension. We intend to seek the benefits of this statute, but there can be no assurance that we will be able to obtain any such benefits. Analogous protection in the majority of EU countries may also be available to us to provide periods of patent term extension in various EU countries.
Various aspects of our business and operations are regulated by a number of other governmental agencies, including the U.S. Occupational Safety and Health Administration.
Third-Party Coverage and Reimbursement
U.S. governmental and private insurance programs, such as Medicare, Medicaid, health maintenance organizations and private insurers, known collectively as third-party payors, fund the cost of a significant portion of medical care in the U.S. Under certain U.S. governmental insurance programs, a healthcare provider is reimbursed a fixed sum for services and products, including drugs used during the course of rendering healthcare services to patients, and governmental imposed limits on reimbursement to hospitals, physicians and other health care providers have significantly impacted spending budgets and purchasing patterns. Private third-party reimbursement plans are also developing increasingly sophisticated methods of controlling health care costs through re-design of benefits and exploration of more cost-effective methods of delivering health care. In general, these governmental and private measures have caused health care providers to be more selective in the purchase of medical products.
In the U.S., Visudyne is reimbursed by governmental and private third-party payors. Because many payors model their policies after the Medicare program, which covers eligible elderly and disabled individuals, this payor’s policies are particularly significant for Visudyne. Under Medicare Part B, for example, separate payment is made for outpatient drugs that are furnished “incident to” a physician’s service if they satisfy certain criteria, including approval by the FDA, they are not usually self-administered by the patient and they are deemed reasonable and necessary for a medically accepted diagnosis or treatment. Visudyne is covered under Medicare as well as other governmental programs, such as those administered by the Department of Veterans’ Affairs and state drug assistance programs.
In the second half of 2010, we assumed responsibility from Novartis with respect to Visudyne as it relates to governmental programs that impose reporting and rebate responsibilities on drug manufacturers. These programs subject us to governmental audits and penalties and/or sanctions if we are found to be out of compliance with the applicable responsibilities. Under the Medicare program, manufacturers are responsible for reporting on a quarterly basis their average sales price (“ASP”) for certain outpatient drugs, which would include Visudyne. Under Medicare Part B, providers receive reimbursement based on a payment methodology that is derived from a percentage of manufacturers’ weighted ASP for unique billing codes. In addition, under the Medicaid Drug Rebate Program, manufacturers must report certain pricing for covered outpatient drugs and also are responsible for a rebate to state governments for those covered drugs based on utilization by patients enrolled in their state Medicaid program. We pay certain states a rebate for each unit of Visudyne reimbursed by Medicaid. The amount of the rebate is set by law as a minimum percentage of the average manufacturer price (“AMP”) of Visudyne, or if it is greater, the difference between AMP and the best price available from us. The AMP and best price are reported by manufacturers using formulas that take into account certain outpatient sales and associated price concessions, among other things. Related to our participation in the Medicaid rebate program for Visudyne is a requirement that we extend comparable discounts under the Public Health Service (“PHS”) pharmaceutical pricing program to a variety of covered entities, such as community health clinics and other qualifying entities that receive health services grants from the PHS, as well as hospitals that serve a disproportionate share of low-income patients.
We also make Visudyne available to authorized users of the Federal Supply Schedule (“FSS”). As a result of the Veterans Health Care Act of 1992, U.S. federal law requires that we offer deeply discounted FSS contract pricing for purchases by the U.S. Department of Veterans Affairs, the U.S. Department of Defense, the U.S. Coast Guard and the PHS (including the Indian Health Service) in order for U.S. federal funding to be available for reimbursement of Visudyne under the Medicaid program or purchase of Visudyne by these four federal agencies and certain federal grantees. FSS pricing to these four U.S. federal agencies must be equal to or less than the Federal Ceiling Price, which is calculated based on a formula that uses a percentage of the prior year’s weighted average price paid to the manufacturer by wholesalers for drug product ultimately sold to non-federal purchasers, also known as the Non-Federal Average Manufacturer Price.
Both within and outside the U.S., significant uncertainty exists as to the reimbursement status of both existing and newly approved health care products, and we cannot provide assurance that continued or adequate third-party reimbursement will be available. There have been, and we expect will continue to be, proposed and adopted healthcare reform measures that impacted or may impact our business. For example, PPACA provides for significant changes in the way healthcare is financed by both governmental and private insurers. Key provisions of PPACA specific to the pharmaceutical industry, among others, include the following:
22
Table of Contents
| • | An annual, non-deductible fee on any entity that manufactures or imports certain branded prescription drugs and biologic agents into the U.S., apportioned among these entities according to their market share in certain federal government healthcare programs (excluding sales of any drug or biologic product marketed for an orphan indication), beginning in 2011; |
| • | An increase, from 15.1% to 23.1%, in the rebates a manufacturer must pay under the Medicaid Drug Rebate Program, retroactive to January 1, 2010; |
| • | Extension of manufacturers’ Medicaid rebate liability to covered drugs dispensed to individuals who are enrolled in Medicaid managed care organizations, effective March 23, 2010; |
| • | Expansion of the entities eligible for discounts under the Public Health Service pharmaceutical pricing program, effective January 2010; |
| • | New (Sunshine) requirements to report certain financial arrangements with physicians and others, including reporting any “transfer of value” made or distributed to prescribers and other healthcare providers and reporting any investment interests held by physicians and their immediate family members during each calendar year, expected to begin at some point in 2012, with public disclosure reporting expected to follow in 2013; |
| • | A new requirement to annually report drug samples that manufacturers and distributors provide to physicians, effective April 1, 2012; |
| • | A licensure framework for follow-on biologic products; and |
| • | A new Patient-Centered Outcomes Research Institute to oversee, identify priorities in, and conduct comparative clinical effectiveness research, along with funding for such research. |
Although we cannot predict their full impact, we anticipate that PPACA, as it is implemented, as well as other healthcare reform measures that may be adopted in the future, may result in more rigorous coverage and reimbursement criteria that may negatively impact product price potential and could adversely affect our profits and our business generally.
The testing, manufacture, marketing and sale of human pharmaceutical products entail significant inherent risks of allegations of product defects. The use of our products in clinical trials and the sale of such products may expose us to liability claims alleged to result from the use of such products. These claims could be made directly by patients or consumers, healthcare providers or others selling the products. In addition, we are subject to the inherent risk that a governmental authority may require the recall of our product. We currently carry clinical trials and product liability insurance in amounts that we consider to be consistent with industry standards to insure against certain claims that could arise during the clinical studies or commercial use of our products. Such coverage and the amount and scope of any coverage obtained in the future may be inadequate to protect us in the event of a successful product liability claim, and there can be no assurance that the amount of such insurance can be increased, renewed or both. A successful product liability claim could materially adversely affect our business, financial condition or results of operations.
Further, liability claims relating to the use of our products or a product recall could negatively affect our ability to obtain or maintain regulatory approval for our products. We have agreed to indemnify our distributors against certain potential liabilities relating to the manufacture and sale of Visudyne. See Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources.
Research and Development Costs
A significant portion of our operating expenses is related to research and development. During the years ended December 31, 2011, 2010 and 2009, our total company-sponsored research and development expenses were $43.5 million, $33.5 million and $28.6 million, respectively. See Our Products in Development above and Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
As of February 17, 2012, we had approximately 188 employees, 110 of whom were engaged in research, development, clinical and regulatory affairs, medical devices, manufacturing, distribution, quality control and assurance, process development and medical affairs, and 78 of whom were engaged in administration, business development, finance, information technology, human resources, legal and sales. None of our employees belong to a labour union and we consider our relationship with our employees to be good.
23
Table of Contents
QLT was formed in 1981 under the laws of the Province of British Columbia. Our principal executive office is located at 887 Great Northern Way, Suite 101, Vancouver, British Columbia, Canada, and our telephone number is 604-707-7000.
We make available free of charge on or through our Internet website our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after they are electronically filed with, or furnished to, the SEC. Our internet address is www.qltinc.com. This website address is intended to be an inactive, textual reference only; none of the material on this website is part of this Report. Copies of our annual reports on Form 10-K will be furnished without charge to any person who submits a written request directed to the attention of our Secretary, at our offices located at 887 Great Northern Way, Suite 101, Vancouver, B.C., Canada V5T 4T5. The SEC maintains an Internet site (http://www.sec.gov) that contains reports, proxy and information statements, and other information regarding issuers that file electronically with the SEC.
| Item 1A. | RISK FACTORS |
In addition to the other information included in this Report, you should consider carefully the following factors, which describe the risks, uncertainties and other factors that may materially and adversely affect our business, products, financial condition and operating results. There are many factors that affect our business and our results of operations, some of which are beyond our control. The following is a description of important factors that may cause our actual results of operations in future periods to differ materially from those currently expected or discussed in forward-looking statements set forth in this Report relating to our financial results, operations and business prospects. Except as required by law, we undertake no obligation to update any such forward-looking statements to reflect events or circumstances after the date on which it is made.
Our commercial efforts are dependent upon obtaining regulatory approval for our product candidates. The regulatory approval process is costly and lengthy and we may not be able to successfully obtain all required regulatory approvals. If we fail to obtain all required regulatory approvals, our business may suffer.
As part of the regulatory approval process, we must conduct, at our own expense, preclinical studies and clinical trials on humans for each product candidate. Generally, in order to gain approval for a product, we must provide the FDA and other applicable regulatory authorities with clinical data that adequately demonstrate the safety and efficacy of that product for the intended disease or condition applied for in the NDA or respective regulatory file. We expect the number and size of clinical trials that the regulatory authorities will require will vary depending on the product candidate, the disease or condition the product is being developed to address, the expected size of the patient population and regulations applicable to the particular product. The length of time necessary to complete clinical trials and to submit an application for marketing approval varies significantly and may be difficult to predict. Further, the approval procedure varies among countries and can involve different testing or data review. Drug development is a long, expensive and uncertain process, and delay or failure can occur at any stage in our clinical trials. Product candidates that appear promising in research or development may be delayed or fail to reach later stages of development or the market for several reasons, including:
| • | preclinical studies may show the product to be toxic or lack efficacy in animal models; |
| • | the interim or final results of clinical trials are inconclusive, negative, or not as favorable as results of previous trials, or show the product candidate to be less effective than desired; |
| • | the FDA, EMA or other regulatory authorities do not permit us to proceed with a clinical trial protocol or a clinical trial, or place a clinical trial on hold; |
| • | the data and safety monitoring committee of a clinical trial recommends that a trial be placed on hold or suspended; |
| • | our trial design, although approved, is inadequate for demonstration of safety and/or efficacy; |
| • | the FDA, EMA or other regulatory authorities determine that any study endpoints used in clinical trials are not sufficient for movement into a next stage clinical trial or for product approval; |
| • | delay in or failure to enroll or retain a sufficient number of patients, or difficulty diagnosing, identifying and recruiting suitable patients, including, for example, due to the rarity of the disease being studied; |
| • | patients die or experience adverse side effects or events for a variety of reasons, including those related to our product candidates or due to the advanced stage of their disease or medical problems, which may or may not be related to our product candidates; |
| • | inability to attract or retain personnel with appropriate expertise; |
| • | third party clinical investigators do not perform our clinical trials on our anticipated schedule or consistent with the clinical trial protocol or other third-party organizations do not perform data collection and analysis in a timely or accurate manner; |
| • | difficulties formulating the product; |
| • | inability to manufacture sufficient quantities of the product candidate which conform to design and performance specifications; |
| • | our clinical trial expenditures are constrained by our budgetary considerations; |
| • | changes in governmental regulations, policies or administrative actions; or |
24
Table of Contents
| • | regulatory inspections of our clinical trials or manufacturing facilities, which may, among other things, require us to undertake corrective action or suspend or terminate our clinical trials if investigators find us not to be in compliance with regulatory requirements. |
For example, a significant challenge for our clinical trials of QLT091001 for the treatment of LCA and RP has been and will likely continue to be patient recruitment due to the small population of patients with these conditions, and in particular with the specific genetic mutations causing LCA and RP we are currently investigating. The challenge in recruiting subjects from this small population is further exacerbated by the lack of public awareness of such conditions and resulting delay in (or lack of) available genetic testing and diagnosis. Further, patients may be discouraged from enrolling in our clinical trials if the trial protocol requires them to undergo extensive procedures to assess the safety and effectiveness of our products, or they may be persuaded to participate in contemporaneous trials of competitive products. Delay in, or failure of, enrolment of sufficient patients or failure of patients to continue to participate in a study may cause an increase in costs and delays or result in the failure of the trial. Our clinical trial costs will also increase if we have material delays in our clinical trials for other reasons or if we need to perform more or larger clinical trials than anticipated.
From time to time, we engage in discussions with the FDA, EMA and other regulatory authorities to determine the regulatory requirements for our development programs. These discussions may include deliberations on number and size of clinical trials, definition of patient population, study end points and safety. The final determination on these matters by the applicable regulatory authority may be difficult to predict in particular where there are no approved precedents to establish drug development norms in a particular class of drug disease area, such as orphan drug development, and may be different, including more onerous, than we anticipated, which may delay, limit or prevent approval of our product candidates.
In addition, the FDA, EMA and other regulatory authorities have substantial discretion in deciding whether any of our product candidates should be granted approval for the treatment of the particular disease or condition. Even if we believe that a clinical trial or trials has demonstrated the safety and efficacy of any of our product candidates, the results may not be satisfactory to the regulator. Preclinical and clinical data can be interpreted by regulators in different ways, which could delay, limit or prevent regulatory approval of our product candidates.
Even if regulatory authorities approve our products or product candidates for the treatment of the diseases or conditions we are targeting, our products may not be marketed or commercially successful. If our products are not marketed or commercially successful, it would seriously harm our ability to generate revenue.
The successful commercialization of our technology, and in particular our punctal plug drug delivery technology and synthetic retinoid, QLT091001, is crucial for our success. Successful product commercialization in the pharmaceutical industry is highly uncertain and very few product commercialization initiatives or research and development projects progress through all phases of development and/or produce a successful commercial product. Even if our products or product candidates are successfully developed, receive all necessary regulatory approvals and are commercially produced, there are number of risks and uncertainties involved in commercializing a product in this industry including the following:
| • | negative safety or efficacy data from post-approval marketing experience or production-quality problems could cause sales of our products to decrease or a product to be recalled; |
| • | negative safety or efficacy data from clinical studies conducted by any party could cause sales of our products to decrease or a product to be recalled; |
| • | we may face significant or unforeseen difficulties or expenses in manufacturing our products, which may only become apparent when scaling-up the manufacturing to commercial scale; |
| • | we may need to obtain licenses under third-party patents which can be costly, or may not be available at all; |
| • | our intellectual property rights could be challenged by third parties or we could be found to be infringing on intellectual property rights of third parties; |
| • | small patient populations may impact distribution and marketing strategy, which may increase our distribution, marketing and per-patient or per-treatment costs; |
| • | we may be unable to obtain or maintain sufficient market share at a price high enough to justify commercialization of the product; and |
| • | effectiveness of our distribution and marketing strategy, including establishing and maintaining key relationships with distributors and suppliers. |
Numerous other factors may impact market acceptance and demand for our products, including but not limited to:
| • | size of the target populations for a product and ability to identify and reach such target populations with the product; |
| • | our pricing decisions, including a decision to increase or decrease the price of a product, and the pricing decisions of our competitors; |
25
Table of Contents
| • | formulation of products in a manner in which they are marketable or subject to appropriate third-party coverage or reimbursement, or the inability to obtain appropriate third-party coverage or reimbursement for any other reason; |
| • | availability and rate of market penetration by competing products; |
| • | relative convenience and ease of administration; |
| • | perceived safety or efficacy relative to other available therapies, including efficacy data from clinical studies conducted by a party showing similar or improved treatment benefit at a lower dose or shorter duration of therapy; and |
| • | acceptance in the medical community and target patient populations to new products, treatment paradigms or standards of care. |
If we are unsuccessful in dealing with any of these risks, or if we are unable to successfully commercialize our product candidates for some other reason, it would seriously harm our ability to generate revenue.
If we fail to comply with ongoing regulatory requirements, it will materially harm our business.
The regulatory clearance process is lengthy, expensive and uncertain. We may not be able to obtain, continue to obtain, or maintain necessary regulatory clearances or approvals on a timely basis, or at all, for Visudyne or any of our product candidates in development, and delays in receipt or failure to receive such clearances or approvals, the loss of previously received clearances or approvals, or failure to comply with existing or future regulatory requirements could have a material adverse effect on our business and our financial condition.
Visudyne and our product candidates are subject to extensive and rigorous regulation for safety, efficacy and quality by the U.S. federal government, principally the FDA, and by state and local governments and by foreign regulatory authorities in jurisdictions in which Visudyne and our product candidates are or may be sold or used in clinical development. Product labeling, manufacturing, adverse event reporting, pricing rules and restrictions, storage, distribution, advertising and promotion, and record keeping are some of the ongoing regulatory requirements to which products are subject. Our or any of our licensees’ failure to comply with applicable requirements could result in sanctions being imposed on us and/or our licensees. These sanctions include, among other things, product recalls or seizures, warning letters, fines, price rebates, total or partial suspension of production and/or distribution, refusals to permit products to be imported into or exported out of the U.S. or elsewhere, FDA or other regulatory agency refusal to grant approval of drugs or to allow us to enter into governmental supply contracts, withdrawals of previously approved marketing applications and enforcement actions, including injunctions and civil or criminal prosecution. Regulators can also withdraw a product’s approval under some circumstances, such as the failure to comply with regulatory requirements or unexpected safety issues.
The FDA often requires post-marketing testing and surveillance to monitor the effects of approved products. The FDA, EMA or other regulatory authorities may condition approval of our product candidates on the completion of such post-marketing clinical studies. These post-marketing studies may suggest that a product causes undesirable side effects or may present a risk to the patient. If data produced from post-marketing studies suggest that one of our approved products may present a risk to safety, the government authorities could withdraw our product approval, suspend production or place other marketing restrictions on our products. If we are unable to sell our products because we have failed to maintain regulatory approval or have to expend significant resources having to address compliance issues, our revenue, financial conditions or results of operations may be materially adversely affected.
We, our contract manufacturers and distributors, all of our suppliers, as well as the suppliers of the medical lasers required for Visudyne therapy, are also subject to numerous federal, state and local laws relating to such matters as safe working conditions, manufacturing practices, environmental protection, fire hazard control and disposal of hazardous or potentially hazardous substances. In addition, advertising and promotional materials relating to medical devices and drugs are, in certain instances, subject to regulation by the FDA, the Federal Trade Commission and other regulatory agencies in other jurisdictions. We, our contract manufacturers and distributors, suppliers and laser suppliers may be required to incur significant costs to comply with such laws and regulations in the future, and such laws or regulations may materially harm our business. Unanticipated changes in existing regulatory requirements, the failure of us, or any of these manufacturers, suppliers or laser suppliers to comply with such requirements or the adoption of new requirements could materially harm our business.
Product development is a long, expensive and uncertain process, and we may terminate one or more of our development programs. If we terminate a development program or product candidate, or if we decide to modify or continue a development program that does not succeed, our prospects may suffer and we may incur significant expenses that could adversely affect our financial condition or results of operations.
We may determine that certain programs or product candidates do not have sufficient potential to warrant the continued allocation of resources to them. Accordingly, we may elect to terminate or modify one or more of our programs, which could include changing our clinical or business model for further development, including by attempting to extract or monetize value from the program by either selling, out-licensing or potentially partnering part or all of the program. If we terminate and seek to monetize part or all of a program in which we have invested significant resources, or we modify a program and expend further resources on it, and subsequently fail to achieve our intended goals, our prospects may suffer, as we will have expended resources on a program that may not provide a suitable return, if
26
Table of Contents
any, on our investment and we may have missed the opportunity to allocate those resources to potentially more productive uses. In addition, in the event of a termination of a product candidate or program, we may incur significant expenses and costs associated with the termination of the program, which could adversely affect our financial condition or results of operations.
If we do not achieve our projected development goals in the timeframes we expect and announce, marketing approval and commercialization of our product candidates may be delayed and the credibility of our management may be adversely affected and, as a result, our stock price may decline.
For strategic and operational planning purposes, we estimate the timing of the accomplishment of various scientific, clinical, regulatory and other product development goals. These milestones may include the commencement or completion of scientific studies and clinical trials and the submission of regulatory filings. From time to time, we publicly announce the expected timing of some of these milestones. All of these milestones are based on a variety of assumptions. The actual timing of these milestones can vary dramatically compared to our estimates, in many cases for reasons beyond our control. If we do not meet these milestones as publicly announced, the market approval and commercialization of our product candidates may be delayed and the credibility of our management may be adversely affected and, as a result, our stock price may decline.
We face intense competition, which may limit our commercial opportunities and our ability to generate revenues.
The biopharmaceutical industry is highly competitive and is characterized by rapidly evolving technology. Competition in our industry occurs on many fronts, including developing and bringing new products to market before others, developing new technologies to improve existing products, developing new products to provide the same benefits as existing products at less cost, developing new products to provide benefits superior to those of existing products, and acquiring or licensing complementary or novel technologies from other pharmaceutical companies or individuals.
We face intense competition against Visudyne, as well as our technology under clinical development. We may be unable to contend successfully with current or future competitors. Our competitors include major pharmaceutical and biopharmaceutical companies, such as Genentech and Novartis, many of which are large, well-established companies with access to financial, technical and marketing resources significantly greater than ours and substantially greater experience in developing and manufacturing products, conducting preclinical and clinical testing and obtaining regulatory approvals. Our competitors may develop or acquire new or improved products to treat the same conditions that our products treat, or may make technological advances that reduce their cost of production so that they may engage in price competition through aggressive pricing policies to secure a greater market share to our detriment. Our commercial opportunities will be reduced or eliminated if our competitors develop or acquire and market products that are more effective, have fewer or less severe adverse side effects, or are less expensive than our products. Competitors also may develop or acquire products that make our current or future products obsolete.
For example, in connection with Visudyne, we face competition primarily from Lucentis®, Avastin® and Eylea® for the treatment of wet AMD. We expect these products to continue to be competitive with Visudyne as they become available in additional markets. We are also aware of other competitive therapies for wet AMD in various stages of development. See Item 1. Business – Competition – Visudyne.
In connection with our technology under clinical development, including our punctal plug drug delivery technology and synthetic retinoid, our competitors may develop or obtain patent protection for products earlier than us, design around patented technology developed by us, obtain regulatory approval for such products before us, or develop more effective or less expensive products than us. Any of these competitive products or events could have a significant negative impact on our business and financial results, including reductions in our market share, revenues and gross margins.
Our commercial success depends in part on our ability and the ability of our licensors to obtain and maintain patent protection on our products and technologies, to preserve trade secrets, and to operate without infringing the proprietary rights of others.
We have applied for and will continue to apply for patents for certain aspects of Visudyne and our product candidates and technology. We have patents and patent applications covering, among other things, compositions, pharmaceutical formulations, processes for manufacturing compounds and/or methods of treating diseases by administering therapeutic compounds. Such applications may not result in the issuance of any patents, and any patents now held or that may be issued may not provide us with a preferred position with respect to any product or technology.
We may not be able to obtain patent protection on aspects of our product candidates and technology. For example, while U.S. Patent No. 7,951,841 was issued on May 31, 2011 covering various methods of use of QLT091001 in the treatment of diseases associated with an endogenous 11-cis-retinal deficiency until 2027, the molecule in QLT091001 is not eligible for composition of matter protection in the U.S. or elsewhere because it was previously known in the scientific community. Therefore, we may not be able to prevent competitors from commercializing the molecule in QLT091001 for the treatment of diseases that fall outside of the scope of our patents protecting this program.
27
Table of Contents
Patents issued or licensed to us may be challenged, invalidated or circumvented successfully, or may have expired or expire in the near future. For example, some of the key U.S patents protecting Visudyne have expired or will expire in the near future, with the remaining unexpired patents having varying scope and duration. To the extent a preferred position is conferred by patents we own or license, upon expiry of such patents, or if such patents are successfully challenged, invalidated or circumvented, our preferred position may be lost. We may not be able to develop and successfully launch more advanced replacement products and/or technologies before our patents expire. If we fail to develop and successfully launch new products prior to the expiry of patents for our products, our revenue from those products could decline significantly.
Further, upon the expiry of, or successful challenge to, our patents covering a product, including Visudyne, generic competitors may introduce a generic version of that product, thereby adversely affecting our revenue and profitability. Generic competitors typically sell their products at prices much lower than those charged by the innovative pharmaceutical or biotechnology companies who have incurred substantial expenses associated with the research and development of the drug product. As a result of this possible increase in competition, we may need to lower our prices in order to maintain sales of our products or we may lose a competitive advantage and marketability of our products and technologies. In the case of Visudyne, the loss of a preferred position may have a material adverse effect on our business, cash flow, results of operations and financial position.
Patents issued or licensed to us may be infringed by the products or processes of other parties. The cost of enforcing our patent rights against infringers, if such enforcement is required, could be significant, and the time demands could interfere with our normal operations.
It is also possible that a court may find us to be infringing validly issued patents of third parties. In that event, in addition to the cost of defending the underlying suit for infringement, we may have to pay license fees and/or damages and may be enjoined from conducting certain activities. Obtaining licenses under third-party patents can be costly, and such licenses may not be available at all. Under such circumstances, we may need to materially alter our products or processes, may be unable to launch a product or may lose the right to continue to manufacture and sell a product entirely or for a period of time.
Unpatented trade secrets, improvements, confidential know-how and continuing technological innovation are important to our scientific and commercial success. Although we attempt to, and will continue to attempt to, protect our proprietary information through reliance on trade secret laws and the use of confidentiality agreements with our collaborators, contract manufacturers, licensees, clinical investigators, employees and consultants and other appropriate means, these measures may not effectively prevent disclosure of or access to our proprietary information, and, in any event, others may develop independently, or obtain access to, the same or similar information.
If we fail to obtain or maintain orphan drug designation for some of our product candidates, our competitive position may be harmed.
Since the extent and scope of our patent protection for QLT091001 is limited, orphan drug designation is especially important for this product candidate. QLT091001 has received orphan drug designations for the treatment of LCA and RP by the EMA, and for the treatment of LCA and RP due to inherited mutations in the LRAT and RPE65 genes by the FDA. These designations provide market exclusivity in the applicable jurisdiction for ten years and seven years, respectively, if a product is approved. This market exclusivity does not, however, pertain to indications outside of the scope of our patents, nor does it prevent other types of drugs from receiving orphan designations or approvals in these same indications. Further, even after an orphan drug is approved, the FDA can subsequently approve another drug for the same condition if the FDA concludes that the new drug is clinically superior to the orphan product or a market shortage occurs. While we may seek orphan drug designations for other indications (including for the treatment of an indication due to a specific inherited genetic mutation), there is no assurance we will receive such orphan drug designations or, even if we do, that such orphan drug designations will provide us with a commercial advantage.
Receipt of the consideration for the sale of QLT USA, including the Eligard product line, is largely dependent on the success of Eligard in the U.S., Canada and in Europe, which is not within our control. If we do not receive all or a material portion of the contingent consideration, our cash position will suffer. Furthermore, an unfavorable change in the fair value of our contingent consideration as a result of changes in estimates related to amount and timing of future cash flows, and the applicable discount rate may adversely impact our earnings.
Under the stock purchase agreement with Tolmar for the sale of QLT USA, including its Eligard product line, we are entitled to receive future consideration payable on a quarterly basis in amounts equal to 80% of the royalties paid under the license agreement with Sanofi for the commercial marketing of Eligard in the U.S. and Canada, and the license agreement with Astellas (formerly with MediGene) for the commercial marketing of Eligard in Europe until the earlier of receiving the full $200.0 million or October 1, 2024. These payments represent and will for some time represent a substantial source of our funds. Since our receipt of contingent consideration under the stock purchase agreement depends, in large part, on the success of Eligard, our receipt of contingent consideration may also be adversely affected by, among other things:
| • | lower than expected Eligard sales; |
28
Table of Contents
| • | product manufacturing or supply interruptions or recalls; |
| • | the development of competitive products, including generics, by other companies that compete with Eligard; |
| • | marketing or pricing actions by competitors or regulatory authorities; |
| • | changes in foreign exchange rates; |
| • | changes in the reimbursement or substitution policies of third-party payors; |
| • | changes in or withdrawal of regulatory approvals; |
| • | disputes relating to patents or other intellectual property rights; |
| • | the commercial efforts of Eligard marketing licensees; |
| • | disputes with Eligard marketing licensees; and |
| • | changes in laws or regulations that adversely affect the ability to market Eligard. |
Furthermore, the fair value of our contingent consideration reflected on our balance sheet is based on future Eligard sales estimated by us utilizing external market research to estimate market size, to which we apply market share and pricing assumptions based on historical sales data and expected future competition. We do not have access to the Sanofi and Astellas sales forecasts for Eligard and, therefore, our forecasts of Eligard sales may be inaccurate.
As of December 31, 2011, we had received an aggregate $86.1 million of contingent consideration. While there are operational covenants in the stock purchase agreement intended to ensure that we receive the total consideration provided for under the stock purchase agreement prior to October 1, 2024, we do not have a security interest in the license agreements and there is no guarantee that we will actually receive the total consideration. If we do not ultimately receive all or a material portion of the consideration provided for under the stock purchase agreement due to the risks noted above or for any other reason, our cash position will suffer.
Visudyne is currently our only commercial product. Accordingly, any decrease in sales of Visudyne would harm our business.
Visudyne is our only commercial product and, accordingly, any decrease in Visudyne product sales would harm our business and cause our financial results to fall below expectations. In 2010, worldwide sales of Visudyne decreased 14.2% from the prior year, primarily due to continued competition from alternative therapeutics for AMD. While worldwide sales of Visudyne in 2011 decreased only 0.4% from 2010, we cannot provide assurance that Visudyne sales will not continue to decrease. Visudyne may be rendered obsolete or uneconomical by competitive changes, including generic competition. Visudyne sales could also be adversely affected by other factors, including:
| • | product manufacturing or supply interruptions or recalls; |
| • | the development of competitive products by other companies; |
| • | developing and maintaining effective sales and marketing capabilities; |
| • | marketing or pricing actions by our competitors or regulatory authorities; |
| • | changes in foreign exchange rates; |
| • | changes in the reimbursement by third-party payors; |
| • | changes in or withdrawal of regulatory approval for or the labelling of Visudyne; |
| • | reliance on the laser devices used in Visudyne therapy; |
| • | disputes relating to patents or other intellectual property rights; |
| • | disputes with our licensees; |
| • | shifting medical community support for treatments; and |
| • | governmental actions or changes in laws and regulations that adversely affect the ability to market Visudyne. |
In the field of PDT, Visudyne therapy is dependent on the continued supply of, and technical support for, complementary laser light delivery devices. If these laser light delivery devices are not available for use with Visudyne, our revenues from the sale of Visudyne will be materially adversely affected.
Visudyne therapy requires a physician to deliver a dose of non-thermal light at a particular wavelength to target tissue in the eye in order to activate the photosensitizer. We currently rely on a limited number of third-parties to manufacture, sell and service the lasers required for Visudyne therapy and, therefore, we are dependent on the continued operation and success of these medical device companies, unless and until we are able to develop an effective proprietary laser light delivery device in time to replace the third-party lasers. The medical device companies supply such lasers to treating physicians directly, and we do not have a supply or distribution agreement with these companies for the supply or servicing of such devices. We cannot guarantee that these medical device companies will continue to support their laser device operations in the U.S., Europe or elsewhere, or that we could successfully develop an effective proprietary laser light delivery device or find other medical device companies to supply laser devices to physicians at acceptable prices, or at all. If these medical device companies cease to carry on business, or no longer supply and/or service lasers approved for Visudyne therapy, and we or other medical device manufacturers and medical devices approved for Visudyne therapy do not fill this void, sales of Visudyne and our revenues from the sale of Visudyne will be materially adversely affected.
29
Table of Contents
Portable diode lasers that have been commercially approved for use with Visudyne are available for sale in the U.S., Europe and elsewhere, including a Carl Zeiss-Meditec diode laser that is commercially available in Japan. However, with the decline in Visudyne sales over the last few years due to the emergence of competing therapies, the commercial supply and technical support for laser light delivery devices has also significantly declined, particularly in the U.S. where third party commercial operations supporting these laser devices has declined or been discontinued altogether and commercial supply for new laser devices has been depleted. Further, the expected lifecycle of the current third-party laser light delivery devices for Visudyne therapy is approximately five to 10 years, which is heavily dependent on number of patients treated and normal maintenance and care. We believe the majority of the lasers currently on the market have reached or will in the coming years reach the end of their lifecycle and, therefore, we expect many of these lasers will need significant upgrades or will need to be replaced. If customers decide not to maintain their lasers or invest in a new laser, for example, due to the associated costs and/or due to the availability of other competitive therapies that do not require a medical device, or if demand for repair or replacement of lasers exceeds the commercial supply and maintenance available in the U.S., Europe or elsewhere, sales of Visudyne and our revenues from the sale of Visudyne will be materially adversely affected.
The commercialization of our products is substantially dependent on our ability to establish and maintain effective sales and marketing capabilities. If we are unable to establish and maintain effective sales and marketing capabilities, or fail to enter into agreements with third parties to sell and market our products, our ability to generate revenues from the sale of our products, including Visudyne, may be harmed.
In order to commercialize our products, or any of our product candidates that may be approved for commercial sale, we must establish and maintain an effective sales and marketing infrastructure or enter into collaborations with partners able to perform these services for us. We have invested and intend to continue investing in our sales and marketing activities in the U.S. to help grow our business, including establishing an in-house sales and marketing organization for U.S. sales of Visudyne through our wholly-owned subsidiary, QLT Ophthalmics, Inc. However, we cannot assure you that our sales force will be sufficient in size, scope or effectiveness to compete successfully in the marketplace and maintain adequate sales levels in the U.S. for Visudyne or for any of our future products. We also cannot guarantee that, if we elect to use third-party providers to sell our products, whether within or outside the U.S., we will be able to enter into and maintain marketing or distribution agreements with third-party providers on acceptable terms, if at all. In addition, we currently have no sales and marketing capabilities in territories outside the U.S. If we are unable to successfully establish and/or expand our capabilities to sell and market our products outside the U.S. either through our own capabilities or by entering into collaborations with partners, we will have difficulty globally commercializing our products. In any of these events, our ability to generate revenues may be harmed.
The marketing and sale of Visudyne in the U.S. is subject to extensive regulation and aggressive government enforcement. Failure to comply with applicable laws and regulations could have a material adverse effect on our business.
Our activities relating to the sale and marketing of Visudyne in the U.S. are subject to extensive regulation under the U.S. Federal Food, Drug and Cosmetic Act and other federal statutes and associated regulations. These laws and regulations limit the types of marketing claims and other communications we can make regarding marketed products. We are also subject to various U.S. federal and state laws pertaining to healthcare “fraud and abuse,” including anti-kickback and false claims laws. Anti-kickback laws prohibit payments of any kind intended to induce physicians or others either to purchase or arrange for or recommend the purchase of healthcare products or services, including the selection of a particular prescription drug. These laws make certain business practices that are relatively common in other industries illegal in our industry. False claims laws prohibit anyone from knowingly and willingly presenting, or causing to be presented for payment to third-party payors, including Medicare and Medicaid, claims for reimbursed drugs or services that are false or fraudulent. Federal and state governments have asserted very broad interpretations of these laws against pharmaceutical manufacturers, even though these manufacturers did not directly submit claims for reimbursement to government payors. In addition, regulation is not static and regulatory authorities, including the FDA, evolve in their staff interpretations and practices and may impose more stringent requirements than currently in effect, which may adversely affect our sales and marketing efforts. Violations of the above laws may be punishable by criminal and/or civil sanctions against the Company and/or our senior officers, including fines and civil monetary penalties, as well as the possibility of exclusion from federal health care programs, including Medicare and Medicaid. Many pharmaceutical and biotechnology companies have in recent years been the target of lawsuits and investigations alleging violations of government regulation, including claims asserting violations of the federal False Claims Act, the federal anti-kickback statute, and other violations in connection with off-label promotion of products, pricing, and government price reporting. The interpretation of these laws as applied to particular sales and marketing practices continues to evolve, and it is possible that our sales and marketing practices might be challenged. Further, although we expect to take measures to prevent potential challenges, we cannot guarantee that such measures will protect us from future challenges, lawsuits or investigations. Even if such challenges are without merit, they could cause adverse publicity, divert management attention and be costly to respond to, and thus could have a material adverse effect on our business, including impact on our stock price.
30
Table of Contents
Our commercial success depends in part on the success of third parties to market our products, including Visudyne.
Our strategy for the development and commercialization of our products, including our current product Visudyne, has included entering into marketing arrangements with third parties, and we may in the future implement a similar strategy with respect to any future products. However, the amount and timing of resources to be devoted to these activities generally are not under our control. For example, under the Amended PDT Agreement, Novartis is responsible for marketing and sales of Visudyne outside of the U.S. A significant portion of our revenue depends on the efforts of Novartis to market and sell Visudyne outside of the U.S., and our growth is dependent in part on the success of Novartis in performing its responsibilities. Further, Novartis is not prevented from commercializing non-PDT products that could be competitive with Visudyne. Novartis entered into a license arrangement with Genentech in which Novartis has been granted a license to the rights outside of the U.S. to Lucentis, a product that has been approved for the treatment of wet AMD, and is a competing product to Visudyne.
To the extent such third parties do not perform adequately under our agreement with them, or do not comply with applicable laws or regulations in performing their obligations, the development and commercialization of our products, including Visudyne, may be delayed, may become more costly to us or may be terminated in the applicable territory, and may require us to expend significant amounts of time and money to find new collaborators and structure alternative arrangements.
Our revenues depend on coverage and reimbursement from third party payors and pricing, and if third party payors reduce or refuse coverage or reimbursement or if prices are reduced, the use and sales of our products will suffer, we may not increase our market share, and our revenues and profitability will suffer.
The continuing efforts of governmental and third-party payors to contain or reduce the costs of healthcare may negatively affect the sale of Visudyne and our product candidates. Our ability to commercialize Visudyne and our product candidates successfully will depend, in part, on the timeliness of and the extent to which adequate coverage and reimbursement for the cost of such products and related treatments is obtained from government health administration authorities, private health insurers and other organizations in the U.S. and foreign markets. Product sales, attempts to gain market share or introductory pricing programs of our competitors could require us to lower our prices, which could adversely affect our results of operations. We may be unable to set or maintain price levels sufficient to realize an appropriate return on our investment in product development. We may also be subject to price reductions as a result of government pricing rules and regulations that may impact both our financial condition and future revenues. Significant uncertainty exists as to the coverage and reimbursement status of newly approved therapeutic products or newly approved product indications.
In both the U.S. and some non-U.S. jurisdictions, there have been a number of legislative and regulatory proposals to change the healthcare system in ways that could affect our ability to sell our products profitably. In the U.S., new legislation and regulations have been and likely will continue to be proposed and adopted at the federal and state levels. For example, effective January 2004, the Medicare Prescription Drug, Improvement and Modernization Act, directs the Secretary of the Department of Health and Human Services (“HHS”) to contract with procurement organizations, who would purchase physician-administered drugs from manufacturers, provide them to physicians and also bill the Medicare program. The competitive acquisition program was postponed for 2009 and has not yet been resumed, but could result in reductions to future amounts that will be paid for physician-administered drugs.
In March 2010, the President of the United States signed the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act (collectively, the “PPACA”), which makes significant changes to the way healthcare is financed by both federal and state governmental and private insurers and directly impacts the pharmaceutical and medical device industries. The PPACA extends health insurance to more individuals and includes, among other things, annual, non-deductible fees that went into effect in 2011 for entities that manufacture or import certain prescription drugs and biologics. This fee is calculated based upon each organization’s percentage share of total branded prescription drug sales to U.S. government programs (such as Medicare, Medicaid and Veterans’ Affairs and Public Health Service discount programs). In addition, the PPACA changes the computations used to determine Medicaid rebates owed by manufacturers under the Medicaid Drug Rebate Program by redefining the average manufacturer’s price (“AMP”), effective October 1, 2010, and by using 23.1% instead of 15% of AMP for most branded drugs and 13% instead of 11% of AMP for generic drugs, effective January 1, 2010. The PPACA also extends the discounts under the Public Health Service pharmaceutical pricing program to additional providers, including critical access hospitals, rural referral centers and sole community hospitals. To further facilitate the government’s efforts to coordinate and develop comparative clinical effectiveness research, the PPACA establishes a new Patient-Centered Outcomes Research Institute to oversee and identify priorities in such research. The manner in which the comparative research results would be used by third-party payors is uncertain.
A number of state governors have strenuously opposed certain of the PPACA’s provisions, and initiated lawsuits challenging its constitutionality. These challenges are pending final adjudication in several jurisdictions, including the United States Supreme Court. Congress has also proposed a number of legislative initiatives, including possible repeal of the PPACA. At this time, it remains unclear whether there will be any changes made to the PPACA. In addition, other legislative changes have been proposed and adopted since the PPACA was enacted. Most recently, on August 2, 2011, the President signed into law the Budget Control Act of 2011, which, among other things, creates the Joint Select Committee on Deficit Reduction to recommend proposals in spending reductions to Congress. The Joint
31
Table of Contents
Select Committee did not achieve a targeted deficit reduction of at least $1.2 trillion for the years 2013 through 2021, triggering the legislation’s automatic reduction to several government programs. This includes aggregate reductions to Medicare payments to providers of up to 2% per fiscal year, starting in 2013. While we have made certain estimates with regard to determining how, and the extent to which, the PPACA fees and rebates will impact our business, until the PPACA or any future legislation is fully implemented, their full impact on our business cannot be determined.
The reimbursement environment for Visudyne may continue to change in the future and become more challenging due to, among other reasons, new policies of the current U.S. administration. The result may be a reduction in the pricing of or demand for Visudyne. To the extent that private insurers or managed care programs follow governmental coverage and payment developments, the adverse effects of changes to governmental programs may be magnified. At this time, a few states have also enacted health care reform legislation, and the federal government and individual state governments continue to consider health care reform policies and legislation. We cannot predict how future measures would impact Visudyne or our future products. Our results of operations could be materially adversely affected by measures to reduce Medicare drug coverage, or by any other future healthcare reform measures that would reduce amounts that other governmental or private insurers will pay for drugs.
Our applications or re-applications for coverage and reimbursement for any of our products may not result in approvals and our current reimbursement approvals for Visudyne and our other products may be reduced or reversed in whole or in part. If we were to have coverage or reimbursement reduced or reversed, the market for the affected product may be materially impaired and could materially harm our business and future revenues from that product. For example, while we believe that the results seen in the Visudyne in occult (“VIO”) study did not contradict results seen in prior studies, because the VIO study failed to meet its primary endpoint, there is a risk that reimbursement for Visudyne in the occult form of wet AMD could be re-evaluated in the U.S. and elsewhere by the applicable governmental authorities. In April 2007, after reviewing the results in the VIO study, the Committee for Human Medicinal Products (“CHMP”) recommended to the European Commission that the indication of the use of Visudyne in the treatment of occult CNV, secondary to AMD be removed in Europe. In June 2007, the European Commission adopted the recommendation by CHMP to remove the indication of Visudyne in the treatment of occult CNV in the EU. As a result, reimbursement for Visudyne in the occult form of wet AMD has ceased in most European countries.
If our supply of Visudyne does not match demand, our business and future revenue could be materially adversely impacted.
A significant decrease or delay in the supply of raw materials, intermediates or finished product could hinder our ability to timely distribute Visudyne, which could materially harm our business and adversely impact our future revenue.
If we are unable to manufacture and supply Visudyne to satisfy our customers’ or licensees’ orders, we may lose those orders and our customers or licensees may cancel other orders and seek damages or exercise other remedies permitted under their agreement with us. In addition, customers or licensees may decide to stock and sell competing products, which in turn could cause a loss of our market share and materially adversely affect our revenues. Numerous factors could cause interruptions in the supply of our finished products, including damage or destruction to the storage facilities containing our product inventory and related raw materials and intermediates, shortages in raw material required by our manufacturers, changes in our sources for raw materials or manufacturing, the failure of our manufacturers to comply with FDA and foreign regulatory authorities requirements for the manufacture of our product or our product specifications, our failure to timely locate and obtain regulatory approval for additional or replacement manufacturers as needed, and conditions affecting the cost and availability of raw materials and manufacturing processes. If the supply of our products is inadequate to meet demand or interrupted, our sales and market share could decrease.
We maintain levels of inventory based upon various factors including our forecasted demand for products, anticipated commercial launch of new products, minimum contractual requirements with third party suppliers and as we consider appropriate for supply chain management and security. Some of our inventory has a limited “shelf life.” If our inventory exceeds forecasted demand, or if we are unable to use our inventory or use it during its shelf life due to delay in or failure to launch a product, withdrawal of a product from the market, impact of generic competition, or delays in, or termination of agreements for, the marketing and sale of our products by third parties, our inventory may be written-down, possibly materially, in market value. For example, in order to mitigate certain risks in our supply chain, we have committed to purchasing additional intermediate material used in the production of Visudyne from one of our suppliers. Our commitment to purchase the intermediate material may result in our inventory exceeding forecasted demand, which may result in a material write-down of our inventory. Further, we may not be able to resell our inventory at a price equal to its full value or recovery of our costs, or at all. If we are unable to sell all of our inventory or sell our inventory at an appropriate price, our business may be materially harmed and our revenues adversely impacted. At December 31, 2011, we carried $13.1 million of Visudyne raw materials, intermediates and finished goods.
We rely on third-party manufacturers and specialty wholesale distributors and other service providers for the manufacture and distribution of Visudyne, and currently expect to rely on third-party manufacturers and distributors for the manufacture and distribution of our future commercial products. Any difficulties with such third parties could delay future revenues from our sales of Visudyne or our future commercial products.
32
Table of Contents
We rely on several third parties in the U.S., Europe and Japan to manufacture Visudyne, and we currently intend to rely on third parties to manufacture our punctal plug drug delivery system and synthetic retinoid product candidates for use in later stage clinical trials and, if commercialized, to manufacture our punctal plug drug delivery system and synthetic retinoid products in commercial quantities. If we are unable to maintain agreements on favorable terms with any of our contract manufacturers or our contract manufacturers fail to supply required materials or comply with regulatory requirements, or if we fail to timely locate and obtain regulatory approval for additional or replacement manufacturers as needed, it could impair or prevent our ability to deliver Visudyne or our future commercial products on a timely basis, or at all, which in turn would materially and adversely harm our business and financial results.
We are responsible for the distribution, marketing and sale of Visudyne in the U.S.; however, we rely on third parties to distribute Visudyne in the U.S. to end-user customers. We may also rely on third parties to distribute our future commercial products to end-user customers. If we are unable to continue to secure and maintain the necessary agreements with third parties to accomplish this in a timely manner on terms favorable to us or at all, supply of Visudyne in the U.S. or supply of our future commercial products may be adversely affected.
In addition, if any such third party service providers fail to meet their respective contractual commitments, we may not be able to supply or continue to supply commercial quantities of Visudyne or any of our future commercial products. Further, any loss of a manufacturer or distributor or any difficulties that could arise in the manufacturing or distribution process, including any disputes with third party service providers, could significantly affect our inventories and supply of Visudyne or our future commercial products available for sale.
Furthermore, our ability to commercialize Visudyne or any of our future commercial products depends, in large part, on our ability to have such products manufactured at a competitive cost and in accordance with FDA and other foreign regulatory requirements, including FDA cGMPs, and our product specifications, which could significantly adversely affect our product inventories and our ability to have product available for commercial sale. We do not have full control over our contract manufacturers’ compliance with these regulations and standards. Our contract manufacturers’ manufacturing and quality procedures may not achieve or maintain compliance with applicable FDA and other foreign regulatory standards or product specifications, and, even if they do, we may be unable to produce or continue to produce commercial quantities of Visudyne or our future commercial products at an acceptable cost or margin.
If current manufacturing processes are modified, or the source or location of our product supply is changed (voluntarily or involuntarily), regulatory authorities will require us to demonstrate that the material produced from the modified or new process or facility is equivalent to the material used in the clinical trials or products previously approved. Any such modifications to the manufacturing process or supply may not achieve or maintain compliance with the applicable regulatory requirements or our product specifications. In many cases, prior approval by regulatory authorities may be required before any changes can be instituted.
If our contract manufacturers produce one or more product batches that do not conform to FDA or other regulatory requirements, or our product specifications, or if they introduce changes to their manufacturing processes, our manufacturing expenses may increase materially, our product inventories may be reduced to unacceptable levels or entirely for an uncertain and potentially significant amount of time, we may lose market share, and/or our ability to meet demand for Visudyne or our future commercial products may be materially and adversely impacted, which may cause us to lose potential revenue and become subject to claims from our licensees.
We depend on key management, scientific and technical personnel, and if we do not attract and retain these key personnel, we may not be able to manage our growth, and our business could be adversely affected.
Our future success depends in significant part on the contributions of our executive officers and scientific and technical personnel. The loss of the services of one or more key individuals may significantly delay or prevent achievement of our scientific or business objectives. Competition for qualified and experienced personnel in the life sciences field is generally intense, and we rely heavily on our ability to attract and retain qualified personnel in order to successfully implement our scientific and business objectives. While our headquarters are located in Vancouver, B.C., Canada, historically, many of our executive and life science personnel candidates reside outside of Canada. As a result, we can face significant challenges recruiting executives and life science personnel. In the event we seek to recruit key executives or key scientific, technical or commercial operations personnel, we may not be able to successfully attract or retain skilled and qualified personnel on acceptable terms, or at all, and even if our recruitment efforts are successful, we may incur significant relocation costs. The failure to attract or retain key executives and personnel could impact our operations, including failure to achieve targets and advance our clinical development programs.
The future growth of our business may depend in part on our ability to successfully identify, acquire on favorable terms, and assimilate technologies, products or businesses.
From time to time, we may engage in negotiations to expand our operations and market presence by future product, technology or other acquisitions, in-licensing and business combinations, joint ventures or other strategic alliances with other companies. We may not be successful in identifying, initiating or completing such negotiations. Competition for attractive product acquisition or alliance targets can be intense, and we may not succeed in completing such transactions on terms that are acceptable to us. Even if we are successful in these negotiations, these transactions create risks, including:
| • | difficulties in and costs associated with assimilating the operations, technologies, personnel and products of an acquired business; |
33
Table of Contents
| • | assumption of known or unknown liabilities or other unanticipated events or circumstances; |
| • | acquired / in-licensed technology may not be successfully developed and commercialized; |
| • | the potential disruption to our ongoing business; and |
| • | the potential negative impact on our earnings and cash position. |
Any of these risks could harm our ability to achieve anticipated levels of profitability for acquired businesses or technology or to realize other anticipated benefits of the transaction.
We have in the past and may in the future acquire one or more technologies, products or products in development that we believe will complement our business. However, we may not ultimately be able to effectively capitalize on the acquisition of such technologies, products or products in development. For example, in December 2009, we acquired QLT091568, a prodrug of a beta adrenergic antagonist, from Othera Pharmaceuticals, Inc. and its wholly-owned subsidiary, Othera Holding, Inc., but following formulation and development work in 2010 and consideration of our research and development priorities, we discontinued development of QLT091568. We may not be able to effectively integrate acquired technologies, products or products in development into our business and any such acquisition could bring additional risks, exposures and challenges to our company.
We may become involved in legal proceedings from time to time and if there is an adverse outcome in our litigation or other legal actions, our business may be harmed.
We may become involved in legal actions in the ordinary course of our business. Litigation may result in verdicts against us, including excessive verdicts, which may include a judgment with a significant monetary award, as occurred in 2008 in the litigation with MEEI, including the possibility of punitive damages, a judgment that certain of our patent or other intellectual property rights are invalid or unenforceable and, as occurred in 2006 in the litigation with TAP Pharmaceuticals in the U.S., the risk that an injunction could be issued preventing the manufacture, marketing and sale of our products that are the subject of the litigation.
In addition, we may become involved in disputes or legal actions as a result of our strategic corporate restructuring undertaken in 2008 and 2009. Under our strategic restructuring, we divested Eligard (as part of the sale of QLT USA), Aczone® and Atrigel and sold the land and building comprising our Canadian headquarters. We also restructured our agreement with Novartis under the Amended PDT Agreement pursuant to which, among other things, we obtained exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. Transactions such as these may result in disputes regarding representations and warranties, indemnities, future payments or other matters, and we may not realize some or all of the anticipated benefits of these transactions.
If disputes are resolved unfavorably, our financial condition and results of operations may be adversely affected. Additionally, any litigation, whether or not successful, may damage our reputation. Furthermore, we will have to incur substantial expense in defending these lawsuits and the time demands of such lawsuits could divert management’s attention from ongoing business concerns and interfere with our normal operations.
In addition, the testing, manufacturing, marketing and sale of human pharmaceutical products entail significant inherent risks of allegations of product liability. Our use of such products in clinical trials and our sale of Visudyne or our product candidates and related medical devices expose us to liability claims allegedly resulting from the use of these products or devices. These risks exist even with respect to those products or devices that are approved for commercial sale by the FDA or applicable foreign regulatory authorities and manufactured in facilities licensed and regulated by those regulatory authorities.
Our current insurance may not provide coverage or adequate coverage against potential claims, losses or damages resulting from such litigation. We also cannot be certain that our current coverage will continue to be available in the future on reasonable terms, if at all. If we were found liable for any claims in excess of our coverage or outside of our coverage, the cost and expense of such liability could materially harm our business and financial condition.
Our use of hazardous materials exposes us to the risk of environmental liabilities, and we may incur substantial additional costs to comply with environmental laws.
Our research, development and manufacturing activities involve the controlled use of hazardous chemicals, primarily flammable solvents, corrosives, and toxins. The biologic materials include microbiological cultures, animal tissue and serum samples. Some experimental and clinical materials include human source tissue or fluid samples. We are subject to federal, state/provincial and local government regulation in the use, storage, handling and disposal of hazardous and radioactive materials. If any of these materials resulted in contamination or injury, or if we fail to comply with these regulations, we could be subject to fines and other liabilities, and any such liabilities could exceed our resources. Our insurance may not provide adequate coverage against potential claims or losses related to our use of any such materials, and we cannot be certain that our current insurance coverage will continue to be available on reasonable terms, if at all. In addition, any new regulation or change to an existing regulation could require us to implement costly capital or operating improvements for which we have not budgeted.
34
Table of Contents
Our provision for income taxes and effective income tax rate may vary significantly and may adversely affect our results of operations and cash resources.
Significant judgment is required in determining our provision for income taxes. Various internal and external factors may have favorable or unfavorable effects on our future provision for income taxes, income taxes receivable, and our effective income tax rate. These factors include, but are not limited to, changes in tax laws, regulations and/or rates, results of audits by tax authorities, changing interpretations of existing tax laws or regulations, changes in estimates of prior years’ items, the impact of transactions we complete, future levels of research and development spending, changes in the overall mix of income among the different jurisdictions in which we operate, and changes in overall levels of income before taxes. Furthermore, new accounting pronouncements or new interpretations of existing accounting pronouncements (such as those described in Note 3 - Significant Accounting Policies in Notes to the Consolidated Financial Statements) can have a material impact on our effective income tax rate.
We file income tax returns and pay income taxes in jurisdictions where we believe we are subject to tax. In jurisdictions in which we do not believe we are subject to tax and therefore do not file income tax returns, we can provide no certainty that tax authorities in those jurisdictions will not subject one or more tax years (since our inception) to examination. Tax examinations are often complex as tax authorities may disagree with the treatment of items reported by us, the result of which could have a material adverse effect on our financial condition and results of operations.
We have incurred losses from continuing operations for the last five years and expect to continue to incur losses for the foreseeable future.
We generated net losses for the fiscal years ended December 31, 2011 and 2010. Although we earned net income for the fiscal years ended December 31, 2009 and 2008, we incurred a loss from continuing operations in both of those years. Further, we incurred a net loss for the year ended December 31, 2007. Our accumulated deficit at December 31, 2011 was approximately $528.1 million. We expect our research and development expenses to increase in the future due to increased clinical development costs primarily related to our punctal plug drug delivery system and synthetic retinoid product candidates. As a result, we expect to continue to incur net losses for the foreseeable future and these losses may increase from current levels. We are uncertain when or if we will be able to achieve or sustain profitability. If we are unable to achieve or sustain profitability in the future, our stock price may decline.
Our operating results may fluctuate, which may cause our financial results to be below expectations and the market price of our securities to decline.
Our operating results may fluctuate from period to period for a number of reasons, some of which are beyond our control. A revenue shortfall or increase in operating expenses could arise from any number of factors, such as:
| • | higher than expected operating expenses as a result of increased costs associated with the research and development or commercialization of our product candidates; |
| • | lower than expected revenues from sales of Visudyne; |
| • | changes in pricing, pricing strategies or reimbursement levels for Visudyne; |
| • | seasonal fluctuations, particularly in the third quarter due to decreased demand for Visudyne in the summer months; |
| • | fluctuations in currency exchange rates; |
| • | costs of operating an internal sales and marketing team and infrastructure for the distribution, sale and marketing of Visudyne in the U.S.; and |
| • | increased operating expenses as a result of product, technology or other acquisitions or business combinations. |
Even a relatively small revenue shortfall may cause a period’s results to be below our expectations or projections, which in turn may cause the market price of our securities to drop significantly and the value of your investment to decline.
The market price of our common shares is extremely volatile and the value of an investment in our common shares could decline.
The market prices for securities of biotechnology companies, including QLT, have been and are likely to continue to be extremely volatile. As a result, investors in companies such as ours often buy at high prices only to see the price drop substantially a short time later, resulting in an extreme drop in value in the holdings of these investors. Trading prices of the securities of many biotechnology companies, including us, have experienced extreme price and volume fluctuations which have, at times, been unrelated to the operating performance of the companies whose securities were affected. Some of the factors that may cause volatility in the price of our securities include:
| • | announcements of technological innovations or new products by us or our competitors; |
| • | results of our research and development programs; |
| • | litigation commenced against us; |
35
Table of Contents
| • | regulatory developments or delays concerning our products; |
| • | quarterly variations in our financial results; |
| • | business and product market cycles; |
| • | fluctuations in customer requirements; |
| • | the availability and utilization of manufacturing capacity and our ability to continue to supply Visudyne; |
| • | the timing and amounts of contingent consideration or royalties paid to us by third parties; and |
| • | issues with the safety or effectiveness of our products. |
The price of our common shares may also be adversely affected by the estimates and projections of the investment community, general economic and market conditions, and the cost of operations in our product markets. Due to general economic conditions and the recent worldwide economic downturn, extreme price and volume fluctuations occur in the stock market from time to time that can particularly affect the prices of biotechnology companies. These extreme fluctuations are sometimes unrelated or disproportionate to the actual performance of the affected companies.
We may need additional capital in the future, and our prospects for obtaining it are uncertain.
Although our divestment of non-core assets in 2008 and 2009 generated significant cash, we have not yet received, and may not ultimately ever receive, all of the contingent consideration payable to us for the sale of QLT USA and, going forward, our business may not generate the cash necessary to fund our operations and anticipated growth. The amount required to fund our operating expenses will depend on many factors, including the status of competitive products, the success of our research and development programs, the extent and success of any collaborative research arrangements, and the results of product, technology or other acquisitions or business combinations. We could seek additional funds in the future from a combination of sources, including product licensing, joint development, sale of assets and other financing arrangements. In addition, we may issue debt or equity securities if we determine that additional cash resources could be obtained under favorable conditions or if future development funding requirements cannot be satisfied with available cash resources. The availability of financing will depend on a variety of factors such as market conditions, the general availability of credit and the availability of credit to our industry, the volume of trading activities, our credit ratings and credit capacity, as well as the possibility that customers or lenders could develop a negative perception of our long- or short-term financial prospects if we incur large investment losses or if the level of our business activity decreases due to a market downturn. Disruptions, uncertainty or volatility in the capital and credit markets may also limit our access to capital required to operate our business. As a result of any or all of these factors, we may not be able to successfully obtain additional financing on favourable terms, or at all.
We believe that we were a passive foreign investment company for the taxable year ended December 31, 2011, which could result in adverse United States federal income tax consequences to U.S. Holders and may deter certain U.S. investors from purchasing our stock, which could have an adverse impact on our stock price.
Based on the price of our common shares and the composition of our assets, we believe that we were a “passive foreign investment company” (“PFIC”) for United States federal income tax purposes for the taxable year ended December 31, 2011. We believe that we were also a PFIC for the taxable years ended December 31, 2008 through 2010, and we may be a PFIC in future years. A non-U.S. corporation generally will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which, after applying relevant look-through rules with respect to the income and assets of subsidiaries, either 75% or more of its gross income is “passive income” or 50% or more of the average value of its assets consists of assets that produce, or are held for the production of, passive income. If we were a PFIC for any taxable year during a U.S. Holder’s holding period for our common shares, certain adverse United States federal income tax consequences could apply to such U.S. Holder, as that term is defined in Item 7, Management’s Discussion and Analysis of Financial Condition and Results of Operations—Certain Canadian and U.S. Federal Income Tax Information for U.S. Residents—U.S. Federal Income Tax Information—U.S. Holders. In addition, our PFIC status may deter certain U.S. investors from purchasing our stock, which could have an adverse impact on our stock price.
If we fail to manage our exposure to global financial, securities market and foreign exchange risk successfully, our operating results and financial statements could be materially impacted.
The primary objective of our investment activities is to preserve principal while at the same time maintaining liquidity and maximizing yields without significantly increasing risk. To achieve this objective, our cash equivalents are high credit quality, liquid, money market instruments. If the carrying value of our investments exceeds the fair value, and the decline in fair value is deemed to be other-than-temporary, we will be required to write down the value of our investments, which could materially harm our results of operations and financial condition. Moreover, the performance of certain securities in our investment portfolio may correlate with the credit condition of governments, government agencies, financial institutions and corporate issuers. If the credit environment were to become unstable, as it did in the second half of 2008 and throughout much of 2009, we might incur significant realized, unrealized or impairment losses associated with these investments.
36
Table of Contents
Prior to January 1, 2010, the Canadian dollar was QLT Inc.’s functional currency, while the U.S. dollar was our reporting currency. As a result, U.S. dollar-denominated monetary assets and liabilities held by us were revalued and gave rise to foreign currency gains (or losses). During the first quarter of 2010, the functional currency of QLT Inc. was changed from the Canadian dollar to the U.S. dollar as a result of the change in our business related to the receipt of exclusive U.S. rights to the Visudyne patents from Novartis. As a result, since 2010, to the extent that foreign currency-denominated (i.e., non-USD) monetary assets do not equal the amount of our foreign currency-denominated monetary liabilities, foreign currency gains or losses could arise and materially impact our financial statements.
Any of these events could have a significant negative impact on our business and financial results, including reductions in our market share and gross margins.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
None.
| Item 2. | PROPERTIES |
In conjunction with the sale of our land and building, on August 29, 2008 we entered into a five-year lease with Discovery Parks Holdings Ltd., an affiliate of Discovery Parks Trust (“Discovery Parks”), a private Canadian trust that designs and builds research facilities for the benefit of the people of British Columbia, Canada. Under the agreement, we currently lease approximately 67,000 square feet of office space in Vancouver, British Columbia, where our head office, certain research facilities and pilot manufacturing facility are located.
In connection with our U.S. operations, we lease approximately 10,800 square feet of space at a facility in Menlo Park, California for a term expiring in 2013, to support certain of our commercial and development operations.
| Item 3. | LEGAL PROCEEDINGS |
From time to time we are involved in legal proceedings arising in the ordinary course of business. There are currently no material pending legal proceedings.
| Item 4. | MINE SAFETY DISCLOSURES |
Not applicable.
37
Table of Contents
PART II
| Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Common Share Information
Our common stock is traded in Canada on the TSX under the symbol “QLT” and in the U.S. on the NASDAQ under the symbol “QLTI.” The following table sets out, for the periods indicated, the high and low closing sales prices of the common shares, as reported by the TSX and NASDAQ.
| The Toronto Stock Exchange | The NASDAQ Stock Market | |||||||||||||||
| High | Low | High | Low | |||||||||||||
| (CAD$) | (CAD$) | (U.S.$) | (U.S.$) | |||||||||||||
| 2011 |
||||||||||||||||
| Fourth Quarter |
$ | 7.69 | $ | 6.61 | $ | 7.45 | $ | 6.47 | ||||||||
| Third Quarter |
7.77 | 5.62 | 7.57 | 5.65 | ||||||||||||
| Second Quarter |
8.01 | 6.58 | 8.52 | 6.75 | ||||||||||||
| First Quarter |
8.37 | 6.44 | 8.50 | 6.53 | ||||||||||||
| 2010 |
||||||||||||||||
| Fourth Quarter |
$ | 7.33 | $ | 5.60 | $ | 7.33 | $ | 5.43 | ||||||||
| Third Quarter |
6.56 | 5.73 | 6.41 | 5.47 | ||||||||||||
| Second Quarter |
6.68 | 5.31 | 6.46 | 5.25 | ||||||||||||
| First Quarter |
5.46 | 4.77 | 5.32 | 4.48 | ||||||||||||
The last reported sale price of the common shares on the TSX and on the NASDAQ on February 17, 2012 was CAD $8.00 and U.S. $8.01, respectively.
As of February 17, 2012, there were 1,429 registered holders of our common shares, 1,286 of whom were residents of the U.S. Of the total 48,954,680 common shares outstanding, the portion held by registered holders resident in the U.S. was 42,140,904 or 86.08%.
38
Table of Contents
Share Price Performance Graph
The graph below compares cumulative total shareholder return on the common shares of QLT for the last five fiscal years with the total cumulative return of the S&P/TSX Composite Index and the NASDAQ Biotechnology Index over the same period.
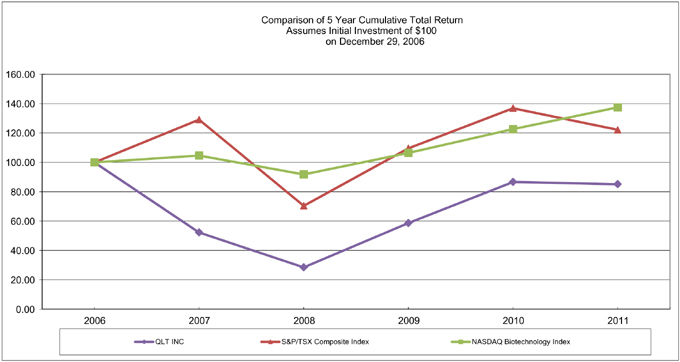
| Dec. 29, 2006 |
Dec. 31, 2007 |
Dec. 31, 2008 |
Dec. 31, 2009 |
Dec. 31, 2010 |
Dec. 30, 2011 |
|||||||||||||||||||
| QLT Total Return |
100.00 | 52.24 | 28.48 | 58.62 | 86.64 | 85.10 | ||||||||||||||||||
| S&P/TSX Composite Index |
100.00 | 129.11 | 70.40 | 109.54 | 136.86 | 122.17 | ||||||||||||||||||
| NASDAQ Biotechnology Index |
100.00 | 104.64 | 91.77 | 106.42 | 122.62 | 137.42 | ||||||||||||||||||
The graph above assumes $100 invested on December 29, 2006 in common shares of QLT and in each index. The returns shown above are historical and not indicative of future price performance.
The foregoing graph and chart shall not be deemed to be incorporated by reference by any general statement incorporating by reference this Report into any filing under the Securities Act of 1933 as amended, or under the Exchange Act, except to the extent we specifically incorporate this information by reference, and shall not otherwise be deemed filed under those Acts.
Dividend Policy
We have not declared or paid any dividends on our common shares since inception. The declaration of dividend payments is at the sole discretion of our Board of Directors. The Board of Directors may declare dividends in the future depending upon numerous factors that ordinarily affect dividend policy, including the results of our operations, our financial position and general business conditions.
39
Table of Contents
Equity Plans
We incorporate information regarding the securities authorized for issuance under our equity compensation plans into this section by reference to the section entitled “Securities Authorized for Issuance Under Equity Compensation Plans” in the proxy statement for our 2012 Annual Meeting of Shareholders.
Recent Sales of Unregistered Securities
None.
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
During the 12-month period commencing December 16, 2010 and ended December 15, 2011, pursuant to a normal course issuer bid, we repurchased through the facilities of the NASDAQ, and immediately cancelled, an aggregate 2,700,817 of our issued and outstanding common shares at an average price of $6.99 per share, for a total cost of $18.9 million.
The following table sets forth information regarding our purchases of common shares on a monthly basis during the three months ended December 31, 2011:
| ISSUER PURCHASES OF EQUITY SECURITIES |
||||||||||||||||
| Period |
Total Number of Shares Purchased |
Average Price Paid per Share |
Total Number of Shares Purchased as Part of Publicly Announced Plans or Programs |
Maximum Number of Shares that May Yet Be Purchased Under the Plans or Programs |
||||||||||||
| October 1, 2011 through October 31, 2011 |
— | — | — | 1,423,468 | ||||||||||||
| November 1, 2011 through November 30, 2011 |
270,400 | $ | 6.97 | 270,400 | 1,153,068 | |||||||||||
| December 1, 2011 through December 31, 2011 |
238,600 | $ | 6.71 | 238,600 | — | (1) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
509,000 | $ | 6.85 | 509,000 | — | (1) | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | The normal course issuer bid expired on December 15, 2011. |
Exchange Controls and Other Limitations Affecting Holders of Common Shares
There is no limitation imposed by Canadian law or the Notice of Articles or Articles of the Company on the right of non-residents to hold or vote common shares in the Company, other than those imposed by the Investment Canada Act (Canada) (the “Investment Act”). Generally speaking, the Investment Act establishes the following two principal procedures for certain investments involving Canadian businesses, as defined by the Investment Act, by an individual, government or agency thereof, corporation, partnership, trust or joint venture that is not a “Canadian,” as defined in the Investment Act (a “non-Canadian”): either the filing of an application for review which, except in certain limited circumstances, must be filed before closing and the non-Canadian cannot complete its investment until the Minister responsible for the Investment Act has determined that the investment is “likely to be of net benefit to Canada,” or the filing of a notice, which must be filed within 30 days after the completion of the investment. A notice is not subject to substantive review and is required for investments that involve either the establishment of a new Canadian business or that involve an acquisition of control of a Canadian business but the prescribed thresholds for review are not exceeded. Subject to the possible application of the national security provisions, the Investment Act does not apply to investments in existing Canadian businesses that do not result in an acquisition of control, as defined under the Investment Act.
A direct investment by a non-Canadian to acquire control of a Canadian business is a reviewable investment where the value of the assets of the corporation, based on the corporation’s fiscal year immediately preceding the investment, is CAD$5 million or more. Higher limits apply for direct acquisitions by or from World Trade Organization (“WTO”) member country investors, as described below.
40
Table of Contents
The acquisition of a majority of the voting interests of an entity or of a majority of the undivided ownership interests in the voting shares of an entity that is a corporation is deemed to be acquisition of control of that entity. The acquisition of less than a majority but one-third or more of the voting shares of a corporation or of an equivalent undivided ownership interest in the voting shares of the corporation is presumed to be acquisition of control of that corporation unless it can be established that, on the acquisition, the corporation is not controlled in fact by the acquirer through the ownership of voting shares. The acquisition of less than one-third of the voting shares of a corporation or of an equivalent undivided ownership interest in the voting shares of the corporation is deemed not to be acquisition of control of that corporation. Certain transactions in relation to common shares in the Company would be exempt from review from the Investment Act, including:
| (a) | acquisition of common shares by a person in the ordinary course of that person’s business as a trader or dealer in securities; |
| (b) | acquisition of control of the Company in connection with the realization of security granted for a loan or other financial assistance and not for any purpose related to the provisions of the Investment Act; and |
| (c) | acquisition of control of the Company by reason of an amalgamation, merger, consolidation or corporate reorganization following which the ultimate direct or indirect control in fact of the Company, through the ownership of voting interests, remains unchanged. |
The Investment Act was amended with the Act to Implement the Agreement Establishing the WTO to provide for special review thresholds for WTO member country investors. Under the Investment Act, a direct investment in common shares of the Company by a non-Canadian who is a WTO investor (as defined in the Investment Act) would be reviewable only if it were an investment to acquire control of the Company and the value of the assets of the Company was equal to or greater than a specified amount (the Review Threshold). The Review Threshold is expected to rise to CAD $330 million for transactions closing in 2012. This amount is subject to an annual adjustment on the basis of a prescribed formula in the Investment Act to reflect inflation and real growth within Canada.
On March 12, 2009, the Government passed the Budget Implementation Act, 2009, (Bill C-10) in Parliament, pursuant to which the Minister of Industry can, within a prescribed period, require the review of an investment by a non-Canadian (even one that does not amount to an acquisition of control, and/or does not meet the Review Threshold) on grounds of whether it is likely to be injurious to national security. Ultimately, Cabinet can prohibit the completion of an investment, or require divestment of control of a completed investment, or impose terms and conditions on an investment where the investment is injurious to national security.
See also Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations – Certain Canadian and U.S. Federal Income Tax Information for U.S. Residents – U.S. Federal Income Tax Information.
41
Table of Contents
| Item 6. | SELECTED FINANCIAL DATA |
Annual Financial Data
(Recast due to discontinued operations)
| Year Ended December 31, |
2011 | 2010(1) | 2009(2) | 2008(3) | 2007(4) | |||||||||||||||
| (In thousands of U.S. dollars except per share information) | ||||||||||||||||||||
| CONSOLIDATED STATEMENT OF OPERATIONS DATA |
||||||||||||||||||||
| Total revenues |
$ | 42,228 | $ | 44,697 | $ | 42,106 | $ | 48,312 | $ | 67,746 | ||||||||||
| Research and development expenses |
43,533 | 33,485 | 28,590 | 29,568 | 37,510 | |||||||||||||||
| Purchase of in-process research and development |
— | — | 7,517 | — | 42,865 | |||||||||||||||
| (Loss) from continuing operations |
(30,416 | ) | (17,539 | ) | (36,220 | ) | (9,247 | ) | (125,537 | ) | ||||||||||
| Net (loss) income |
(30,416 | ) | (17,539 | ) | 99,434 | 134,891 | (109,997 | ) | ||||||||||||
| Basic and diluted net (loss) income per common share: |
||||||||||||||||||||
| Continuing operations |
(0.61 | ) | (0.33 | ) | (0.64 | ) | (0.12 | ) | (1.68 | ) | ||||||||||
| Discontinued operations |
— | — | 2.41 | 1.93 | 0.21 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net (loss) income |
(0.61 | ) | (0.33 | ) | 1.77 | 1.81 | (1.47 | ) | ||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| CONSOLIDATED BALANCE SHEET DATA |
||||||||||||||||||||
| Cash and cash equivalents |
$ | 205,597 | $ | 209,478 | $ | 188,114 | $ | 165,395 | $ | 30,564 | ||||||||||
| Short-term investment securities |
— | — | — | — | 96,167 | |||||||||||||||
| Working capital |
247,332 | 255,495 | 257,610 | 281,966 | 49,583 | |||||||||||||||
| Total assets |
344,544 | 387,783 | 419,637 | 491,758 | 548,987 | |||||||||||||||
| Convertible debt |
— | — | — | — | 172,500 | |||||||||||||||
| Total shareholders’ equity |
329,005 | 372,944 | 404,454 | 343,230 | 219,823 | |||||||||||||||
For all years presented there were no cash dividends per common share.
42
Table of Contents
Quarterly Financial Data(5)
Set out below is selected unaudited consolidated financial information for each of the fiscal quarters of 2011 and 2010.
| Three Months Ended |
December 31 | September 30 | June 30 | March 31 | ||||||||||||
| (In thousands of U.S. dollars except per share information) | ||||||||||||||||
| 2011 |
||||||||||||||||
| Total revenues |
$ | 11,063 | $ | 9,581 | $ | 12,685 | $ | 8,900 | ||||||||
| Gross profit |
8,070 | 6,908 | 9,274 | 7,562 | ||||||||||||
| Research and development expenses |
11,199 | 11,282 | 11,319 | 9,734 | ||||||||||||
| Net (loss) from continuing operations |
(6,617 | ) | (9,106 | ) | (6,145 | ) | (8,549 | ) | ||||||||
| Net (loss) |
(6,617 | ) | (9,106 | ) | (6,145 | ) | (8,549 | ) | ||||||||
| Basic and diluted net (loss) per common share |
||||||||||||||||
| Continuing operations |
(0.13 | ) | (0.18 | ) | (0.12 | ) | (0.17 | ) | ||||||||
| Net (loss) |
(0.13 | ) | (0.18 | ) | (0.12 | ) | (0.17 | ) | ||||||||
|
2010(1) |
||||||||||||||||
| Total revenues |
$ | 10,020 | $ | 8,585 | $ | 12,376 | $ | 13,716 | ||||||||
| Gross profit |
6,629 | 6,716 | 8,984 | 7,164 | ||||||||||||
| Research and development expenses |
10,709 | 8,101 | 7,329 | 7,347 | ||||||||||||
| Net (loss) income from continuing operations |
(19,230 | ) | (697 | ) | (1,015 | ) | 3,403 | |||||||||
| Net (loss) income |
(19,230 | ) | (697 | ) | (1,015 | ) | 3,403 | |||||||||
| Basic and diluted net (loss) income per common share |
||||||||||||||||
| Continuing operations |
(0.38 | ) | (0.01 | ) | (0.02 | ) | 0.06 | |||||||||
| Net (loss) income |
(0.38 | ) | (0.01 | ) | (0.02 | ) | 0.06 | |||||||||
| (1) | For the year ended December 31, 2010, our net loss reflects a tax provision of $10.9 million which was primarily due to a net increase in our valuation allowance resulting from lack of sufficient evidence available to support our continued recognition of certain future tax benefits. |
| (2) | On October 1, 2009, the Eligard product line was divested as part of the sale of all of the shares of our U.S. subsidiary, QLT USA. See Item 1. Business - Eligard® Contingent Consideration. We recognized a pre-tax gain of $107.4 million related to this transaction within discontinued operations for 2009. |
| (3) | Assets related to Aczone were sold by QLT USA to Allergan Sales, LLC in July 2008 for cash consideration of $150.0 million, pursuant to the terms of a purchase agreement executed on June 6, 2008. We recognized a pre-tax gain of $118.2 million related to this transaction within discontinued operations for 2008. |
| On August 25, 2008, QLT USA entered into an exclusive license agreement with Reckitt Benckiser Pharmaceuticals Inc. for QLT USA’s Atrigel sustained-release drug delivery technology, except for certain rights being retained by us and our prior licensees, including rights retained for use with the Eligard products. We recognized a pre-tax gain of $16.7 million related to this transaction within discontinued operations for 2008. |
| On August 29, 2008, we completed the sale of our land and building comprising our corporate headquarters and the adjacent undeveloped parcel of land in Vancouver to Discovery Parks for CAD$65.5 million. We recognized a pre-tax gain of $21.7 million related to this transaction in our results for 2008. |
| As a result of an evaluation of QLT USA’s prior earnings history, expected future earnings, and gains recorded on the divestiture of Aczone and out-licensing of Atrigel, we concluded that a valuation allowance was no longer required on substantially all of QLT USA’s tax assets. We recognized a reduction of our valuation allowance of $54.7 million within discontinued operations and $4.8 million within continuing operations in the third quarter of 2008. |
| (4) | In July 2007, the U.S. District Court for the District of Massachusetts entered judgment in the lawsuit brought against us by MEEI in connection with U.S. Patent No. 5,789,349. The District Court found that we were liable under Massachusetts state law for unfair trade practices, but that such violation was not knowing or willful, and determined that we should pay MEEI 3.01% of past, present and future worldwide Visudyne net sales, plus interest and legal fees. As a result, we recorded a charge of $110.2 million in our results for 2007. |
43
Table of Contents
| On October 18, 2007, we completed the acquisition of ForSight Newco II, Inc. (now QLT Plug Delivery, Inc.) for aggregate consideration of $42.3 million. The acquired in-process R&D charge of $42.9 million related to the proprietary ocular punctal plug drug delivery system in development. |
| (5) | The basic and diluted income (loss) per share are determined separately for each quarter. Consequently, the sum of the quarterly amounts may differ from the annual amounts disclosed in the consolidated financial statements as a result of using different weighted average numbers of shares outstanding. |
44
Table of Contents
| Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following information should be read in conjunction with the accompanying 2011 consolidated financial statements and notes thereto, which are prepared in accordance with U.S. generally accepted accounting principles (“U.S. GAAP”). All of the following amounts are expressed in U.S. dollars unless otherwise indicated.
SPECIAL NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Report contains forward-looking statements within the meaning of the United States Private Securities Litigation Reform Act of 1995 and “forward looking information” within the meaning of the Canadian securities legislation which are based on our current expectations and projections. Words such as “anticipate”, “project”, “believe”, “expect”, “forecast”, “outlook”, “plan”, “intend”, “estimate”, “should”, “may”, “assume”, “continue”, and variations of such words or similar expressions are intended to identify our forward-looking statements and forward-looking information. Such statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of QLT to be materially different from the results of operations or plans expressed or implied by such forward-looking statements and forward-looking information. Many such risks, uncertainties and other factors are taken into account as part of our assumptions underlying the forward-looking statements and forward-looking information.
The following factors, among others, including those described under Item 1A. Risk Factors, could cause our future results to differ materially from those expressed in the forward-looking statements and forward-looking information:
| • | the anticipated timing, cost and progress of the development of our technology and clinical trials; |
| • | the anticipated timing of regulatory submissions for products and product candidates; |
| • | the anticipated timing for receipt of, and our ability to maintain, regulatory approvals for products and product candidates; |
| • | our ability to successfully develop and commercialize our programs, including our punctal plug drug delivery system and synthetic retinoid program; |
| • | existing governmental laws and regulations and changes in, or the failure to comply with, governmental laws and regulations; |
| • | the scope, validity and enforceability of our and third party intellectual property rights; |
| • | the anticipated timing for receipt of, and our ability to maintain, orphan drug designations for our product candidates, particularly our synthetic retinoid; |
| • | receipt of all or part of the contingent consideration pursuant to the stock purchase agreement entered into with TOLMAR Holding, Inc. (“Tolmar”), which is based on anticipated levels of future sales of Eligard®; |
| • | levels of future sales of Visudyne®, including the impact of competition, loss of patent protection and limited commercial supply and technical support for laser devices required for Visudyne therapy; |
| • | our ability to effectively market and sell Visudyne and any future products; |
| • | the ability of Novartis to effectively market and sell Visudyne in countries outside the U.S.; |
| • | our expectations regarding Visudyne label changes and reimbursement; |
| • | the anticipated timing for receipt of, and our ability to maintain, reimbursement approvals for our products and product candidates, including reimbursement under U.S. governmental and private insurance programs; |
| • | our continued ability to supply Visudyne to our customers; |
| • | our reliance on contract manufacturers and suppliers to manufacture Visudyne at competitive prices and in accordance with the U.S. Food and Drug Administration and other local and foreign regulatory requirements as well as our product specifications; |
| • | our reliance on our specialty wholesale distributors to distribute Visudyne in accordance with regulatory requirements and the terms of our agreements; |
| • | our expectations regarding future tax liabilities as a result of our restructuring transactions, changes in estimates of prior years’ tax items and results of tax audits by tax authorities; and |
| • | unanticipated future operating results. |
Although we believe that the assumptions underlying the forward-looking statements and forward-looking information contained herein are reasonable, any of the assumptions could be inaccurate, and therefore such statements and information included in this Report may not prove to be accurate. In light of the significant uncertainties inherent in the forward-looking statements and forward-looking information included herein, the inclusion of such statements and information should not be regarded as a representation by us or any other person that the results or conditions described in such statements and information or our objectives and plans will be achieved. Any forward-looking statement and forward-looking information speaks only as of the date on which it is made. Except to fulfill our obligations under the applicable securities laws, we undertake no obligation to update any such statement or information to reflect events or circumstances occurring after the date on which it is made.
Financial guidance is contained in our earnings press release issued on February 23, 2012 which can be found on SEDAR at www.sedar.com and EDGAR at www.sec.gov. Information contained in the earnings press release and related Material Change Report and Current Report on Form 8-K filed therewith shall not be deemed “filed” for purposes of Section 18 of the Exchange Act, and is not incorporated by reference herein.
45
Table of Contents
Overview
QLT was formed in 1981 under the laws of the Province of British Columbia and is a biotechnology company dedicated to the development and commercialization of innovative ocular products that address the unmet medical needs of patients and clinicians worldwide. We are focused on developing our synthetic retinoid program for the treatment of certain inherited retinal diseases, developing our proprietary punctal plug drug delivery system, as well as U.S. marketing of our commercial product, Visudyne®, for the treatment of wet age related macular degeneration (“wet AMD”).
Products, Revenues and Other Sources of Funds
We have one commercial product, Visudyne, which utilizes light-activated photodynamic therapy (“PDT”) to treat the eye disease known as wet AMD, the leading cause of blindness in people over the age of 50 in North America and Europe. Visudyne is also used for the treatment of subfoveal choroidal neovascularization secondary to pathologic myopia, or severe near-sightedness, and presumed ocular histoplasmosis. Visudyne was co-developed by QLT and Novartis Pharma AG (“Novartis”) and is marketed and sold in over 80 countries worldwide.
On January 1, 2010, we received from Novartis the exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. As a result, we operate a direct marketing and sales force through our U.S. subsidiary, QLT Ophthalmics, Inc., and have rights to all end-user revenue derived from Visudyne sales in the U.S. Novartis continues to market and sell Visudyne for ophthalmic use outside the U.S. and pays us a royalty on net sales of the product.
On October 1, 2009, we divested the Eligard® line of products to Tolmar as part of the sale of all of the shares of our U.S. subsidiary, QLT USA, Inc. (“QLT USA”). Pursuant to the stock purchase agreement, we are entitled to future consideration payable quarterly in amounts equal to 80% of the royalties paid under the license agreement with Sanofi Synthelabo Inc. (“Sanofi”) for the commercial marketing of Eligard in the U.S. and Canada, and the license agreement with MediGene Aktiengesellschaft (“MediGene”), which, effective March 1, 2011, has been assigned to Astellas Pharma Europe Ltd. (“Astellas”) for the commercial marketing of Eligard in Europe. The estimated fair value of the expected future quarterly payments is reflected as Contingent Consideration on our Consolidated Balance Sheet. We are entitled to these quarterly payments until the earlier of our receipt of $200.0 million or October 1, 2024. As of December 31, 2011, we had received an aggregate $86.1 million of contingent consideration. See Item 1. Business - Eligard® Contingent Consideration.
Research and Development
We devote significant resources to research and development programs in various stages of development. See Item 1. Business - Overview - Research and Development.
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
The preparation of financial statements in conformity with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting periods presented. Significant estimates are used for, but not limited to, provisions for non-completion of inventory, provision for excess inventory, the fair value of contingent consideration, allocation of costs to manufacturing under a standard costing system, allocation of overhead expenses to research and development, stock-based compensation, and provisions for taxes, tax assets and tax liabilities. Actual results may differ from estimates made by management. The significant accounting policies which we believe are the most critical to aid in fully understanding and evaluating our reported financial results include those which follow:
Revenue Recognition
With respect to Visudyne, under the terms of the Amended and Restated PDT Product Development, Manufacturing and Distribution Agreement (the “Amended PDT Agreement”) with Novartis, on January 1, 2010 we received from Novartis the exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. As a result, we have established a commercial presence in the U.S., operating a direct marketing and sales force through our U.S. subsidiary, QLT Ophthalmics, Inc., and have rights to all end-user revenue derived from U.S. Visudyne sales. Novartis continues to market and sell Visudyne outside the U.S. and now pays us a royalty on net sales of the product, instead of the 50% share of Novartis’ net proceeds from Visudyne sales that was payable to us under the previous PDT Product Development, Manufacturing and Distribution Agreement (the “PDT Agreement”) with Novartis. We utilize contract manufacturers for Visudyne production and are responsible for product supply to Novartis and our U.S. specialty wholesale distributors.
46
Table of Contents
Net Product Revenue
Net product revenues for periods after 2009 have been derived from sales of Visudyne to distributors in the U.S. and to Novartis outside the U.S., plus reimbursement of certain costs from Novartis. We recognize revenue from the sale of Visudyne when persuasive evidence of an arrangement exists, delivery has occurred, the end selling price of Visudyne is fixed or determinable, and collectibility is reasonably assured. For U.S. Visudyne sales, provisions for certain vendor charge-backs, discounts, Medicaid rebates, distributor fees and product returns are accounted for as a reduction of revenue in the same period the related revenue is recorded. Rebates, vendor charge-backs and discounts are estimated based on contractual terms, historical experience, and projected market conditions. Product returns are estimated based on historical experience and business trends. We recognize revenue upon delivery, when title and risk of loss passes to Novartis and other distributors.
Significant categories of gross-to-net sales adjustments related to U.S. Visudyne sales are described below:
Vendor Charge-backs
We participate in prime vendor programs with government entities whereby pricing on products is extended below wholesaler acquisition cost to participating entities. These entities purchase products through wholesalers at the lower prime vendor price and the wholesalers then charge us the difference between their acquisition cost and the lower prime vendor price. We record prime vendor charge-backs as a liability in an amount equal to our estimate of charge-back claims attributable to a sale. We determine our estimate of the prime vendor charge-backs based on the level of inventory in the distribution channel and actual experience regarding prime vendor charge-backs.
Discounts
We offer cash discounts as an incentive for prompt payment. We record cash discounts by reducing accounts receivable by the full amount of the discount. We consider payment performance and adjust the accrual to reflect actual experience.
Medicaid Rebates
We participate in state government-managed Medicaid programs as well as certain other qualifying federal and state government programs whereby discounts and rebates are provided to participating state and local government entities. We account for these rebates by establishing a liability in an amount equal to our estimate of rebate claims attributable to a sale. We determine our estimate of rebates primarily based on historical experience regarding rebates, as well as considering any new information regarding changes in the Medicaid programs, regulations and guidelines that would impact the amount of the rebates. We consider outstanding Medicaid claims, Medicaid payments and levels of inventory in the distribution channel and adjust the accrual periodically throughout each quarter to reflect actual experience.
Distributor Fees
We pay distributor fees to our U.S. specialty wholesale distributors. We account for distributor fees by establishing a liability in an amount equal to fees owing on product sales. We determine our estimate based on the level of inventory in the distribution channel and adjust the accrual to reflect actual experience.
Product Returns
We account for product returns by establishing an accrual in an amount equal to our estimate of sales recognized for which the related products are expected to be returned. Product returns are recorded by reducing accounts receivable by the full amount of the reserve. We determine our estimate of the product return accrual primarily based on historical experience, but also consider other factors such as the level of inventory in the distribution channel and estimated shelf life.
Through December 31, 2009, our agreement with Novartis provided that the calculation of total revenue for the sale of Visudyne be composed of three components: (1) an advance on the cost of inventory sold to Novartis, (2) an amount equal to 50% of Novartis’ net proceeds from Visudyne sales to end-customers (determined according to a contractually agreed definition), and (3) the reimbursement of other specified costs incurred and paid for by us. Under the calculation of revenue noted above, this occurred when Novartis sold Visudyne to its end customers.
47
Table of Contents
Royalties
Under the Amended PDT Agreement, Novartis is required to pay us a royalty of 20% of net sales outside the U.S. until December 31, 2014, and 16% of net sales thereafter, until the expiry of the Amended PDT Agreement on December 31, 2019. We recognize royalties when product is sold by Novartis to end customers based on royalty rates specified in the Amended PDT Agreement with Novartis. Royalties are based on net product sales (gross sales less discounts, allowances and other items) and calculated based on information supplied to us by Novartis.
Inventory and Cost of Sales
For periods after 2009, Visudyne cost of sales, consisting of expenses related to the production of bulk Visudyne, royalty expense on Visudyne sales and ongoing damages related to the Massachusetts Eye and Ear Infirmary (“MEEI”) judgment, are charged against earnings in the period that we sell to distributors in the U.S. and to Novartis outside the U.S. Through December 31, 2009, Visudyne cost of sales were charged against earnings in the period that Novartis sold to end customers.
We utilize a standard costing system, which includes a reasonable allocation of overhead expenses, to account for inventory and cost of sales, with adjustments being made periodically to reflect current conditions. Losses on manufacturing purchase commitments are immediately charged to cost of sales. Our standard costs are estimated based on management’s best estimate of annual production volumes and material costs. Overhead expenses comprise direct and indirect support activities related to manufacturing and involve costs associated with activities such as quality inspection, quality assurance, supply chain management, safety and regulatory. Overhead expenses are allocated to inventory at various stages of the manufacturing process under a standard costing system. Unallocated overheads are recognized as cost of sales in the period in which they are incurred. For periods after 2009, overhead was allocated to cost of sales as Visudyne was sold to our distributors in the U.S. and to Novartis outside the U.S. Through December 31, 2009, overhead was allocated to cost of sales as Visudyne was sold by Novartis to third parties. We record a provision for the non-completion of product inventory based on our history of batch completion. The provision is calculated at each stage of the manufacturing process. There are three areas within our inventory costing system that require significant management judgment and estimates: (i) annual production volume, (ii) overhead allocation, and (iii) provision for non-completion of product inventory and obsolete inventory. These three areas are described below:
(i) Annual Production Volume. We estimate our normal production volume at the beginning of the year in order to arrive at a per unit allocation of fixed costs. Our estimate of normal production volume is based on our forecast of product sales and is updated periodically.
(ii) Overhead Allocation. We estimate our overhead expenses in the beginning of the year in order to arrive at a per unit allocation of overhead. Our estimate of overhead expenses is based on historical experience and the projected production volume. We update our estimate on a periodic basis based on the latest information. Overhead expenses are allocated to inventory at various stages of the manufacturing process under a standard costing system. Unallocated overheads are recognized as cost of sales in the period in which they are incurred.
(iii) Provisions for Non-completion of Inventory and Obsolete Inventory. We record a provision for the non-completion of product inventory based on our history of batch completion to provide for the potential failure of inventory batches to pass quality inspection. The provision is calculated at each stage of the manufacturing process. We estimate our non-completion rate based on past production and adjust our provision based on actual production volume. A batch failure may utilize a significant portion of the provision as a single completed batch currently costs up to $1.6 million, depending on the stage of production.
While we believe our standard costs are reliable, actual production costs and volume changes may impact inventory, cost of sales, and the absorption of production overheads.
Inventory that is obsolete or expired is written down to its market value if lower than cost. Inventory quantities are regularly reviewed and provisions for excess or obsolete inventory are recorded primarily based on our forecast of future demand and market conditions. If actual market conditions differ from those we have assumed, if there is a sudden and significant decrease in demand for our products, if there is a decrease in our long-term sales forecast, or if there is a higher incidence of inventory obsolescence due to a rapid change in technology, we may be required to take additional provision for excess or obsolete inventory. Further, in order to mitigate certain risks in our supply chain, we have committed to purchase additional intermediate material used in the production of Visudyne. Our commitment to purchase the intermediate material may result in our inventory exceeding forecasted demand, which may result in a material write-down of our inventory. If our future Visudyne demand were to drop by as much as 10%, there would be no impact on our reserve for obsolescence.
Stock-Based Compensation
ASC topic 718 requires stock-based compensation to be recognized as compensation expense in the statement of earnings based on their fair values on the date of the grant, with the compensation expense recognized over the period in which a grantee is required to provide service in exchange for the stock award. Compensation expense recognition provisions are applicable to new awards and to any awards modified, repurchased or cancelled after the adoption date.
48
Table of Contents
We use the Black-Scholes option pricing model to estimate the value of our stock option awards at each grant date. The Black-Scholes option pricing model was developed for use in estimating the value of traded options that have no vesting restrictions and are fully transferable. In addition, option pricing models require the input of highly subjective assumptions including the expected stock price volatility. We project expected volatility and expected life of our stock options based upon historical and other economic data trended into future years. The risk-free interest rate assumption is based upon observed interest rates appropriate for the terms of our stock options.
For the year ended December 31, 2011, stock based compensation of $3.0 million was expensed as follows: $1.5 million to research and development costs, $1.4 million to selling, general and administrative costs, and $0.1 million to cost of sales. The weighted average assumptions used for options granted during 2011 included a volatility factor of 48.8%, a 3.7 year term until exercise, and a 2.1% risk-free interest rate.
For the year ended December 31, 2010, stock based compensation of $2.5 million was expensed as follows: $1.3 million to research and development costs, $1.0 million to selling, general and administrative costs, and $0.2 million to cost of sales. The weighted average assumptions used for options granted during 2010 included a volatility factor of 53.8%, a 3.7 year term until exercise, and a 2.3% risk-free interest rate.
Research and Development
Research and development (“R&D”) costs are expensed as incurred and consist of direct and indirect expenditures, including a reasonable allocation of overhead expenses associated with our various R&D programs. Overhead expenses comprise general and administrative support provided to the R&D programs and involve costs associated with support activities such as rent, facility maintenance, utilities, office services, information technology, legal, accounting and human resources. Significant management judgment is required in the selection of an appropriate methodology for the allocation of overhead expenses. Our methodology for the allocation of overhead expenses utilizes the composition of our workforce as the basis for our allocation. Specifically, we determine the proportion of our workforce that is dedicated to R&D activities and allocate to R&D expense the equivalent proportion of overhead expenses. We consider this method the most reasonable method of allocation based on the nature of our business and workforce. Changes in the composition of our workforce and the types of support activities are factors that can influence our allocation of overhead expenses. Costs related to the acquisition of development rights for which no alternative use exists are classified as research and development and expensed as incurred. Patent application, filing and defense costs are also expensed as incurred.
Income Taxes
Income taxes are reported using the asset and liability method, whereby deferred tax assets and liabilities are recognized for the future tax consequences attributable to: (i) differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and (ii) operating loss and tax credit carryforwards using applicable enacted tax rates. An increase or decrease in these tax rates will increase or decrease the carrying value of future net tax assets resulting in an increase or decrease to net income. Income tax credits, such as investment tax credits, are included as part of the provision for income taxes. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations. Various internal and external factors may have favorable or unfavorable effects on our future effective tax rate. These factors include, but are not limited to, changes in tax laws, regulations and/or rates, changing interpretations of existing tax laws or regulations, changes in estimates of prior years’ items, results of tax audits by tax authorities, future levels of research and development spending, changes in estimates related to repatriation of undistributed earnings of foreign subsidiaries, changes in financial statement presentation related to discontinued operations, and changes in overall levels of pre-tax earnings. The realization of our deferred tax assets is primarily dependent on generating sufficient capital gains and taxable income prior to expiration of any loss carry forward balance. A valuation allowance is provided when it is more likely than not that a deferred tax asset will not be realized. The assessment of whether or not a valuation allowance is required often requires significant judgment including the long-range forecast of future taxable income and the evaluation of tax planning initiatives. Adjustments to the deferred tax valuation allowances are made to earnings in the period when such assessments are made.
We record tax benefits for all years subject to examination based upon management’s evaluation of the facts, circumstances and information available at the reporting date. There is inherent uncertainty in quantifying income tax positions. We have recorded tax benefits for those tax positions where it is more likely than not that a tax benefit will be sustained upon ultimate settlement with a taxing authority that has full knowledge of all relevant information. For those income tax positions where it is not more likely than not that a tax benefit will be sustained, no tax benefit has been recognized in the financial statements. See Note 14 - Income Taxes in Notes to the Consolidated Financial Statements.
49
Table of Contents
Contingent Consideration
Contingent consideration arising from the sale of QLT USA is measured at fair value. The contingent consideration is revalued each reporting period and changes are included in continuing operations.
To estimate the fair value of contingent consideration at December 31, 2011, we used a discounted cash flow model based on estimated timing and amount of future cash flows, discounted using a cost of capital of 9.0% determined by management after considering available market and industry information. Future cash flows were estimated by utilizing external market research to estimate market size, to which we applied market share, pricing, and foreign exchange assumptions based on historical sales data, expected competition and current exchange rates. If the discount rate were to increase by 1%, the contingent consideration would decrease by $1.3 million, from $99.9 million to $98.6 million. If estimated future sales of Eligard were to decrease by 10%, the contingent consideration would decrease by $1.2 million, from $99.9 million to $98.7 million. Additionally, the fair value change in contingent consideration is positively impacted by the passage of time since the remaining cash flows are closer to collection, thereby increasing their present value.
Recently Issued and Recently Adopted Accounting Standards
See Note 3 - Significant Accounting Policies in Notes to the Consolidated Financial Statements for a discussion of new accounting standards.
COMPARISON OF YEARS ENDED DECEMBER 31, 2011 AND 2010
The following table sets out our net loss for the years ended December 31, 2011 and 2010.
| (In thousands of U.S. dollars, except per share data) |
Year ended December 31, 2011 |
Year ended December 31, 2010 |
||||||
| Net loss |
$ | (30,416 | ) | $ | (17,539 | ) | ||
| Basic and diluted net loss per common share |
$ | (0.61 | ) | $ | (0.33 | ) | ||
Detailed discussion and analysis of our results of operations are as follows:
Revenues
Net Product Revenue
Net product revenue for the years ended December 31, 2011 and 2010 was determined as follows:
| (In thousands of U.S. dollars) |
Year ended December 31, 2011 |
Year ended December 31, 2010 |
||||||
| U.S. Visudyne sales by QLT |
$ | 21,022 | $ | 22,597 | ||||
| Visudyne sales to Novartis |
5,924 | 6,988 | ||||||
| Add: Royalties and other costs reimbursed to QLT(1) |
1,430 | 1,508 | ||||||
|
|
|
|
|
|||||
| Net product revenue from Visudyne sales |
$ | 28,376 | $ | 31,093 | ||||
|
|
|
|
|
|||||
| (1) | “Royalties and other costs reimbursed to QLT” |
| This represents amounts we receive from Novartis in reimbursement for actual royalty expenses we incur with third party licensors for sales of Visudyne outside of the U.S. and certain administrative expenses we incur on behalf of Novartis under the Amended PDT Agreement. |
Under the terms of the Amended PDT Agreement, on January 1, 2010 we received from Novartis the exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. As of January 1, 2010, Novartis continues to market and sell Visudyne outside the U.S. We utilize contract manufacturers for Visudyne production and are responsible for product supply to Novartis and other distributors. Details of our revenue recognition accounting policy are described in Note 3 – Significant Accounting Policies in Notes to the Consolidated Financial Statements.
For the year ended December 31, 2011, net product revenue from Visudyne decreased by $2.7 million, or 8.7%, to $28.4 million compared to $31.1 million for the year ended December 31, 2010. The decrease was primarily due to the recognition of $5.0 million of previously deferred
50
Table of Contents
revenue in the prior period and lower Visudyne sales in the U.S., partially offset by higher product shipments to Novartis. The deferred revenue was recognized following the commencement of the Amended PDT Agreement on January 1, 2010 and related to inventory previously shipped to Novartis for sales outside the U.S.
The tables below summarize end-user Visudyne sales by dollar amount and by percentage for the years ended December 31, 2011 and 2010, respectively. Under the Amended PDT Agreement, Visudyne is sold by QLT in the U.S. and by Novartis outside the U.S. (for which we earn a 20% royalty on net sales). See Note 3 - Significant Accounting Policies in Notes to the Consolidated Financial Statements.
| (In thousands of U.S. dollars) |
Year ended December 31, 2011 |
Year ended December 31, 2010 |
||||||
| U.S. |
$ | 21,022 | $ | 22,597 | ||||
| Europe |
25,528 | 26,243 | ||||||
| Rest of World |
43,729 | 41,778 | ||||||
|
|
|
|
|
|||||
| Worldwide |
$ | 90,279 | $ | 90,618 | ||||
|
|
|
|
|
|||||
| Year ended December 31, 2011 |
Year ended December 31, 2010 |
|||||||
| U.S. |
23 | % | 25 | % | ||||
| Europe |
28 | % | 29 | % | ||||
| Rest of World |
49 | % | 46 | % | ||||
|
|
|
|
|
|||||
| Worldwide |
100 | % | 100 | % | ||||
|
|
|
|
|
|||||
An analysis of the gross-to-net sales adjustments for the years ended December 31, 2011 and 2010, respectively, is summarized below:
| (In thousands of U.S. dollars) |
Distributor Fees and Discounts |
Contractual Adjustments |
Returns | Total | ||||||||||||
| Balance at January 1, 2010 |
$ | — | $ | — | $ | — | $ | — | ||||||||
| Provisions relating to sales |
989 | 1,315 | 398 | 2,702 | ||||||||||||
| Payments/returns relating to sales |
(778 | ) | (1,092 | ) | (80 | ) | (1,950 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance at December 31, 2010 |
211 | 223 | 318 | 752 | ||||||||||||
| Provisions relating to sales |
975 | 1,223 | 235 | 2,433 | ||||||||||||
| Payments/returns relating to sales |
(991 | ) | (1,270 | ) | (114 | ) | (2,375 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Balance at December 31, 2011 |
$ | 195 | $ | 176 | $ | 439 | $ | 810 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
The worldwide sale of Visudyne is our only source of revenue. While we have sought to protect our proprietary Visudyne technology by, among other things, obtaining and licensing patents in the U.S., Europe, Japan, and other jurisdictions, because Visudyne is a mature pharmaceutical product, some of the key U.S. patents protecting it have expired or will expire in the near future. For example, two of the U.S. patents (U.S. Patent Nos. 5,214,036 and 6,074,666) covering lipid-based formulations of the compound verteporfin (the active ingredient in Visudyne) expired on May 25, 2010 and February 5, 2012, respectively, and the U.S. patent (U.S. Patent No. 5,095,030) covering the compound verteporfin expired on September 9, 2011. See Item 1. Business – Patents, Trademarks and Proprietary Rights for a more detailed discussion of our Visudyne patent portfolio. Generally, once all patent protection expires, market exclusivity is lost. As is inherent in the biopharmaceutical industry, the loss of market exclusivity can result in significant competition from market entry of generic versions of products, which can have a significant adverse effect on product revenues. However, the effect of the expiration of one or more patents covering a biopharmaceutical product will depend upon a number of factors, such as the extent to which the product is protected by other patents in a relevant jurisdiction, the nature of the market and the position of the product in it, the growth or decline of the market and the complexities and economics of the process for manufacture of the product. Based upon our consideration of these and other factors as they relate specifically to Visudyne, we believe that the expiration of the U.S. patents covering lipid-based formulations of verteporfin and the expiration of the U.S. patent covering the compound verteporfin are unlikely to have a material adverse effect on our revenues over the next several years since, among other things:
| • | we own and license additional U.S. patents covering lipid-based formulations of verteporfin and approved uses of Visudyne that provide additional patent protection for Visudyne and do not expire until, at the latest, 2015 and 2016, respectively; |
51
Table of Contents
| • | in Europe and Japan, the basic patents covering the verteporfin compound do not expire until 2014 and additional patents and patent applications relating to lipid-based formulations of verteporfin and approved uses of Visudyne do not expire until, at the latest, 2016 and 2017, respectively; and |
| • | relative to the sale of many other biopharmaceutical products, Visudyne does not generate significant revenue and the manufacturing process is complex, which may make Visudyne a less desirable generic target. |
Royalties
Under the Amended PDT Agreement, Novartis continues to market and sell Visudyne outside the U.S. and pays us a 20% royalty on net sales of the product outside the U.S. For the year ended December 31, 2011, royalty revenues increased by $0.2 million, or 1.8%, to $13.9 million compared to $13.6 million for the same period in 2010. The increase was in line with higher Visudyne sales outside the U.S.
Costs and Expenses
Cost of Sales
For the year ended December 31, 2011, cost of sales of $10.4 million decreased $4.8 million, or 31.5%, compared to $15.2 million for the same period in 2010. The decrease was primarily due to the inclusion of certain charges in the prior period, whereas there were no similar charges in the current period. The prior period charges included a $4.0 million charge for cost of sales associated with the recognition of deferred revenue, a $1.4 million charge for a loss on a manufacturing purchase commitment related to the supply of intermediate material used in the production of Visudyne, and higher overhead expenses. The decrease attributable to the prior period charges was partially offset by higher costs of sales in the current period associated with higher product shipments to Novartis.
Research and Development
R&D expenditures increased 30.0% to $43.5 million for the year ended December 31, 2011 compared to $33.5 million in the same period in 2010. The increase was primarily due to higher spending on our synthetic retinoid program, offset by lower spending on the punctal plugs programs and no spending on QLT091568 (Beta Blocker Glaucoma Program).
The magnitude of future R&D expenses is highly variable. Numerous events can happen to an R&D project prior to it reaching any particular milestone that can significantly affect future spending and activities related to the project. These events include:
| • | inability to design devices to function as expected; |
| • | delays or inability to formulate an active ingredient in an appropriate concentration to deliver effective doses of a drug; |
| • | changes in governmental regulations, policies or administrative actions; |
| • | introduction of competing technologies and treatments; |
| • | unexpected safety issues; |
| • | patent application, maintenance and enforcement issues; |
| • | inability to operate without infringing the proprietary rights of others; |
| • | changes in the commercial marketplace; |
| • | delay in or failure to enroll or retain a sufficient number of patients, or difficulty diagnosing, identifying and recruiting suitable patients; |
| • | inadequate trial design to demonstrate safety and/or efficacy; |
| • | delay in study or program progression, including study site, Institutional Review Board and regulatory delays; |
| • | positive trial results that may result in increased spending; |
| • | failure to meet favorable study endpoints; |
| • | inability to develop cost-effective manufacturing methods that comply with regulatory standards; |
| • | inability to manufacture sufficient quantities of the product candidate which conform to design and performance specifications; |
| • | inability to attract or retain personnel with appropriate expertise; |
| • | uncertainties related to collaborative arrangements; |
| • | environmental risks; and |
| • | other factors referenced under Item 1A. Risk Factors. |
52
Table of Contents
We may also undertake new R&D projects that may significantly affect our future spending and activities.
R&D expenditures by therapeutic area were as follows:
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Ocular |
||||||||||||
| Punctal Plug Drug Delivery System |
$ | 18,928 | $ | 20,752 | $ | 23,254 | ||||||
| QLT091001 |
23,042 | 9,483 | 1,918 | |||||||||
| QLT091568 |
— | 1,716 | — | |||||||||
| Visudyne |
1,563 | 1,277 | 2,844 | |||||||||
|
|
|
|
|
|
|
|||||||
| Ocular Total |
43,533 | 33,228 | 28,016 | |||||||||
| Dermatology and Other |
— | 257 | 574 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 43,533 | $ | 33,485 | $ | 28,590 | |||||||
|
|
|
|
|
|
|
|||||||
Total costs incurred through December 31, 2011 with respect to our significant products under development in the ocular therapeutic areas are: $81.1 million with respect to our Punctal Plug Drug Delivery System, $51.3 million with respect to QLT091001, and $1.7 million with respect to QLT091568 (we discontinued development of QLT091568 in October 2010). In addition, we have incurred $113.2 million in ocular-related Visudyne R&D costs since 2000, the year Visudyne was commercially launched and the year in which our current accounting system that tracks R&D costs associated with our products was implemented.
The status of significant products in development is as follows:
| Product/Indication |
Status/Development Stage | |
| QLT091001 |
||
| Leber Congenital Amaurosis (LCA) and Retinitis Pigmentosa (RP) |
Phase Ib proof-of-concept studies ongoing: | |
| • LCA cohort treatment completed; follow-up ongoing. LCA retreatment study initiated. | ||
| • RP cohort target enrolment and treatment completed. RP retreatment study initiated. | ||
| Latanoprost Punctal Plug Drug Delivery System (L-PPDS) |
||
| Glaucoma and Ocular Hypertension |
Phase II clinical study completed in August 2011.
Two Phase II clinical studies initiated in December 2011. | |
| Olopatadine Punctal Plug Drug Delivery System (O-PPDS) |
||
| Allergic Conjunctivitis |
Phase II proof-of-principle study completed in February 2011 demonstrating inconclusive data. Development has been suspended. | |
See Item 1. Business - Research and Development and Item 1. Business - Our Products In Development for a description of our significant development programs.
Selling, General and Administrative Expenses
For the year ended December 31, 2011, selling, general and administration (“SG&A”) expenses increased 23.4% to $25.7 million compared to $20.8 million for the same period in 2010. The increase was primarily due to increased spending on commercial operations.
53
Table of Contents
Investment and Other Income (Expense)
Net Foreign Exchange (Losses) Gains
For the years ended December 31, 2011 and 2010, net foreign exchange (losses) gains represent the impact of foreign exchange fluctuations on our monetary assets and liabilities that are denominated in currencies other than the U.S. dollar (principally the Canadian dollar). See Liquidity and Capital Resources – Interest and Foreign Exchange Rates below.
Interest Income
For the year ended December 31, 2011, interest income decreased 63.3% to $0.7 million compared to $1.8 million for the same period in 2010. The decrease occurred primarily because the prior period included $0.7 million of interest earned on the note receivable from Tolmar which was collected on October 1, 2010 in connection with the sale of QLT USA to Tolmar on October 1, 2009.
Fair Value Change in Contingent Consideration
As part of the sale of all of the shares of QLT USA to Tolmar, we are entitled to receive up to $200.0 million in consideration payable on a quarterly basis in amounts equal to 80% of the royalties paid under the license agreements with each of Sanofi and Astellas (formerly with MediGene) for the commercial marketing of Eligard in the U.S., Canada, and Europe. At December 31, 2011, there was $113.9 million remaining to be paid to us by Tolmar. The fair value of this amount, $99.9 million, is reported as Contingent Consideration on our Consolidated Balance Sheet, and is estimated using a discounted cash flow model. Contingent consideration is revalued at each reporting period and is positively impacted each period by the passage of time, since all remaining expected cash flows move closer to collection, thereby increasing their present value. The fair value change in contingent consideration is also impacted by the projected amount and timing of expected future cash flows and by the cost of capital used to discount these cash flows.
For the year ended December 31, 2011, the fair value change in contingent consideration decreased by $6.4 million to $10.1 million compared to $16.5 million for the same period in 2010. Approximately $3.5 million of the decrease occurred because the fair value change diminishes each period as the outstanding balance owed to us decreases. Additionally, the fair value change in 2010 benefited from increases in the cash flow projections (primarily due to higher demand forecasted in Europe) and from higher-than-projected cash collections throughout the year.
Other Gains
For the year ended December 31, 2011, other gains included $0.4 million related to an R&D tax grant and a $0.3 million milestone related to the sale and out-license of certain non-core assets in 2010.
For the year ended December 31, 2010, other gains included $0.3 million related to the sale and out-license of certain non-core assets and $0.2 million related to receipt of an R&D tax grant.
Income Taxes
The provision for income taxes was $2.8 million for the year ended December 31, 2011, compared to $10.9 million for the same period in 2010. The change in the effective tax rate was primarily because the provision for 2010 reflected the application of a valuation allowance against Canadian tax assets due to an intra-entity transfer of intellectual property and the resulting shift of related future development expenditures to the acquiring entity. During 2011, as insufficient evidence exists to support current or future realization of the tax benefits associated with such development expenditures as well as certain other current period expenditures, the benefit of certain tax assets has not been recognized in the current periods. The $2.8 million provision for income taxes relates primarily to a drawdown of the contingent consideration tax asset associated with the current period gain on the fair value change, income taxes associated with our mix of income allocable to our activities in the U.S., as well as the reversal of a prepaid tax asset set up at December 31, 2010 in relation to profits on intercompany sales that had not been sold to third parties at that time. The latter is not expected to be a recurring item in the near future.
The net 2011 deferred tax asset of $2.7 million was largely the result of contingent consideration, certain loss carryforwards and other temporary differences against which a valuation allowance was not applied.
As of December 31, 2010, we had a valuation allowance against specifically identified tax assets. The valuation allowance is reviewed periodically and if management’s assessment of the “more likely than not” criterion for accounting purposes changes, the valuation allowance is adjusted accordingly. See Note 14 - Income Taxes in Notes to the Consolidated Financial Statements.
54
Table of Contents
COMPARISON OF YEARS ENDED DECEMBER 31, 2010 AND 2009
The following table sets out our net (loss) income for the years ended December 31, 2010 and 2009.
| (In thousands of U.S. dollars, except per share data) |
Year ended December 31, 2010 |
Year ended December 31, 2009 |
||||||
| Net (loss) income |
$ | (17,539 | ) | $ | 99,434 | |||
| Basic and diluted net (loss) income per common share |
$ | (0.33 | ) | $ | 1.77 | |||
Detailed discussion and analysis of our results of operations are as follows:
Revenues
Net Product Revenue
Under the terms of the Amended PDT Agreement, on January 1, 2010 we received from Novartis the exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. As of January 1, 2010, Novartis continues to market and sell Visudyne outside the U.S. and pays us a royalty on net sales of the product outside the U.S. instead of the 50% share of Novartis’ net proceeds from Visudyne sales that was payable under the previous PDT Agreement. Novartis is still responsible for royalties reimbursed to QLT for Visudyne sales outside the U.S., and certain other costs reimbursed to QLT. We utilize contract manufacturers for Visudyne production and are responsible for product supply to Novartis and other distributors. Details of our revenue recognition accounting policy are described in Note 3 – Significant Accounting Policies in Notes to the Consolidated Financial Statements.
Net product revenue for the year ended December 31, 2010 was determined as follows:
| (In thousands of U.S. dollars) |
Year ended December 31, 2010 |
|||
| U.S. Visudyne sales by QLT |
$ | 22,597 | ||
| Visudyne sales to Novartis |
6,988 | |||
| Add: Royalties reimbursed to QLT(1) |
1,374 | |||
| Add: Other costs reimbursed to QLT(2) |
134 | |||
|
|
|
|||
| Net product revenue from Visudyne sales |
$ | 31,093 | ||
|
|
|
|||
Net product revenue for the year ended December 31, 2009 was determined as follows:
| (In thousands of U.S. dollars) |
Year ended December 31, 2009 |
|||
| Worldwide Visudyne sales by Novartis |
$ | 105,657 | ||
| Less: Marketing and distribution costs(3) |
(34,346 | ) | ||
| Less: Inventory costs(4) |
(6,031 | ) | ||
| Less: Royalties to third parties(5) |
(2,276 | ) | ||
|
|
|
|||
| Novartis’ net proceeds from Visudyne sales |
$ | 63,004 | ||
|
|
|
|||
| QLT’s 50% share of Novartis’ net proceeds from Visudyne sales |
$ | 31,502 | ||
| Add: Advance on inventory costs from Novartis(6) |
5,608 | |||
| Add: Royalties reimbursed to QLT(1) |
2,272 | |||
| Add: Other costs reimbursed to QLT(2) |
2,724 | |||
|
|
|
|||
| Net product revenue from Visudyne sales |
$ | 42,106 | ||
|
|
|
|||
55
Table of Contents
| (1) | “Royalties reimbursed to QLT” |
| For 2010, this represented amounts we received from Novartis in reimbursement for actual royalty expenses we incurred with third party licensors for sales of Visudyne outside of the U.S. For 2009, this represented the amounts we received from Novartis in reimbursement for the actual royalty expenses we incurred with third party licensors on worldwide Visudyne sales. |
| (2) | “Other costs reimbursed to QLT” |
| For 2010, this represented certain administrative expenses we incurred on behalf of Novartis under the Amended PDT Agreement. For 2009, this represented reimbursement by Novartis to us of our portion of the marketing and distribution costs described in (3) below. Our marketing and distribution costs included marketing support, legal and administrative expenses that we incurred in support of Visudyne sales. |
| (3) | “Marketing and distribution costs” |
| This represented Novartis’ cost of marketing, promoting, and distributing Visudyne, as well as certain specified costs incurred and paid for by QLT, determined in accordance with the PDT Agreement between QLT and Novartis. The costs incurred by Novartis were related to its sales force, advertising expenses, marketing, and certain administrative overhead costs. The costs incurred by us included marketing support, legal and administrative expenses that we incurred in support of Visudyne sales. |
| (4) | “Inventory costs” |
| This represented Novartis’ cost of goods sold related to Visudyne. It included the cost of bulk Visudyne we shipped to Novartis and our previous provisions for certain excess or obsolete inventory, losses on manufacturing purchase commitments, plus Novartis’ packaging and labelling costs, freight, custom duties and inventory obsolescence. |
| (5) | “Royalties to third parties” |
| This represented the royalty expenses we incurred and charged to Novartis pursuant to the PDT Agreement between QLT and Novartis. The amounts were calculated by us based on specified royalty rates from license agreements with our licensors of certain Visudyne patent rights. |
| (6) | “Advance on inventory costs from Novartis” |
| This represented the amount that Novartis advanced to us for shipments of bulk Visudyne and reimbursement for inventory obsolescence. The price of the Visudyne shipments was determined based on the PDT Agreement between QLT and Novartis and represented our actual costs of producing Visudyne. |
For the year ended December 31, 2010, net product revenue from Visudyne decreased by $11.0 million, or 26%, to $31.1 million compared to $42.1 million for the year ended December 31, 2009. A direct comparison of net product revenue between 2009 and 2010 is difficult because the composition of net product revenue changed significantly under the terms of the Amended PDT Agreement, which became effective on January 1, 2010. In 2009, net product revenue consisted entirely of our 50% share of Novartis’ net proceeds from worldwide Visudyne sales, plus certain cost reimbursements from Novartis to us. Under the Amended PDT Agreement, net product revenue consists primarily of end-user sales in the U.S. (where we sell Visudyne ourselves), and it also includes product shipments that we make to Novartis for their Visudyne sales outside the U.S. market, and certain cost reimbursements from Novartis related to sales outside the U.S. Importantly, in 2009, all of QLT’s revenue related to worldwide Visudyne sales was reflected in net product revenue, while in 2010 we also had revenue from royalties of $13.6 million (associated with Novartis’ sales of Visudyne outside the U.S. - see Royalties section below) which is not included in net product revenue. In summary, the change in determination of net product revenue under the Amended PDT agreement was the primary reason for the decline in net product revenue from 2009 to 2010. In addition, net product revenue dropped as a result of a 14% decline in Visudyne sales (presented below) from 2009 to 2010, which was primarily the result of lower end-user demand due to competing therapies. These decreases were partially offset by the recognition of $5.0 million of deferred revenue related to inventory previously shipped to Novartis for sales of Visudyne outside the U.S. in 2010.
The tables below summarize end-user Visudyne sales by dollar amount and by percentage for the years ended December 31, 2010 and 2009, respectively. Beginning January 1, 2010, under the Amended PDT Agreement, Visudyne is sold by QLT in the U.S. and by Novartis outside the U.S. As of January 1, 2010, we earn a 20% royalty on net sales of Visudyne made by Novartis outside the U.S. In 2009, Visudyne was sold solely by Novartis on a worldwide basis, and our revenue was derived from our 50% share of Novartis’ net proceeds from Visudyne sales. See Note 3 - Significant Accounting Policies in Notes to the Consolidated Financial Statements.
56
Table of Contents
| (In thousands of U.S. dollars) |
Year ended December 31, 2010 |
Year ended December 31, 2009 |
||||||
| U.S. |
$ | 22,597 | $ | 30,809 | ||||
| Europe |
26,243 | 29,639 | ||||||
| Rest of World |
41,778 | 45,209 | ||||||
|
|
|
|
|
|||||
| Worldwide |
$ | 90,618 | $ | 105,657 | ||||
|
|
|
|
|
|||||
| Year ended December 31, 2010 |
Year ended December 31, 2009 |
|||||||
| U.S. |
25 | % | 29 | % | ||||
| Europe |
29 | % | 28 | % | ||||
| Rest of World |
46 | % | 43 | % | ||||
|
|
|
|
|
|||||
| Worldwide |
100 | % | 100 | % | ||||
|
|
|
|
|
|||||
Royalties
Under the Amended PDT Agreement, Novartis continues to market and sell Visudyne outside the U.S. and, effective January 1, 2010, pays us a 20% royalty on net sales of the product outside the U.S. For the year ended December 31, 2010, we earned $13.6 million of royalties under the Amended PDT Agreement.
Costs and Expenses
Cost of Sales
For the year ended December 31, 2010, cost of sales of $15.2 million decreased $5.0 million, or 24.7%, compared to $20.2 million for the same period in 2009. The decrease was primarily due to an inventory write-down of $8.1 million in 2009 partially offset by $4.0 million in 2010 cost of sales associated with the recognition of deferred revenue related to inventory previously shipped to Novartis for sales outside the U.S.
Research and Development
R&D expenditures increased 17.1% to $33.5 million for the year ended December 31, 2010 compared to $28.6 million in the same period in 2009. The increase was due to higher spending on our synthetic retinoid program and QLT091568, as well as strengthening of the Canadian dollar relative to the U.S. dollar, offset by lower spending on the punctal plug program and Visudyne combination studies.
Selling, General and Administrative Expenses
For the year ended December 31, 2010, SG&A expenses increased 13.5% to $20.8 million compared to $18.3 million for the same period in 2009. The increase was primarily due to the launch of a direct marketing and sales force for Visudyne in the U.S. and the strengthening of the Canadian dollar relative to the U.S. dollar, partially offset by a $0.6 million charge for capital tax in 2009.
Litigation
For the year ended December 31, 2010, there was no litigation expense. For the year ended December 31, 2009, litigation expense of $20.7 million included a $20.0 million payment to Massachusetts General Hospital (“MGH”) pursuant to a settlement agreement and amendment to license agreement, $0.3 million related to reimbursement of certain MEEI legal costs, as awarded by the Court of Appeals for the First Circuit in connection with the previous MEEI patent litigation, and $0.3 million in connection with the reimbursement of legal fees, accounting fees and other amounts to resolve issues not material to QLT or its business.
Purchase of In-process Research and Development (“IPR&D”)
For the year ended December 31, 2010, there were no acquisitions of in-process research and development. On December 30, 2009, we acquired QLT091568 (formerly OT-730), a type of beta blocker under investigation for the treatment of glaucoma, from privately-held Othera Pharmaceuticals, Inc. and its wholly-owned subsidiary, Othera Holding, Inc. for a one-time cash payment of $7.5 million. The purchase was an acquisition of assets and as QLT091568 had not reached technological feasibility and had no alternative future use at the time of purchase, we allocated the aggregate consideration of $7.5 million to IPR&D. We discontinued development of QLT091568 in October 2010.
57
Table of Contents
Restructuring Charge
For the year ended December 31, 2010, there was no charge for restructuring. For the year ended December 31, 2009 we recorded a $0.3 million adjustment to our restructuring accrual related to severance, termination benefits and other costs as we completed the final activities associated with the 2008 restructuring.
Investment and Other Income (Expense)
Net Foreign Exchange Gains (Losses)
For the year ended December 31, 2010, net foreign exchange gains comprised gains from the impact of foreign exchange fluctuations on our monetary assets and liabilities that are denominated in currencies other than the U.S. dollar (principally the Canadian dollar). In prior periods, the Canadian dollar was the functional currency of QLT Inc., the Canadian parent company, and the U.S. dollar was the functional currency for our U.S. subsidiaries. In 2009, the net foreign currency gain of $7.0 million included a foreign exchange gain of $11.7 million related to the intercompany debt settlement upon the sale of QLT USA, partially offset by a foreign currency loss of $5.5 million on the revaluation of contingent consideration. Effective January 1, 2010, we changed the functional currency for QLT Inc. to the U.S. dollar thereby eliminating foreign exchange gains (losses) on intercompany debt and contingent consideration.
Interest Income
For the year ended December 31, 2010, interest income decreased 57.7% to $1.8 million compared to $4.3 million for the same period in 2009. The decrease occurred primarily because 2009 included $2.7 million of interest earned on tax refunds partially offset by a $0.5 million favorable variance related to interest earned on the note receivable from Tolmar in 2010.
Interest Expense
For the year ended December 31, 2010, there was no interest expense. For the year ended December 31, 2009, $1.8 million of interest expense was entirely related to interest expense on the post judgment accrued liability associated with the MEEI patent litigation damage award.
Fair Value Change in Contingent Consideration
As part of the sale of all of the shares of QLT USA to Tolmar, we are entitled to receive up to $200.0 million in consideration payable on a quarterly basis in amounts equal to 80% of the royalties paid under the license agreements with each of Sanofi and Astellas (formerly with MediGene) for the commercial marketing of Eligard in the U.S., Canada, and Europe. At December 31, 2010, there was $154.6 million remaining to be paid to us by Tolmar. The fair value of this amount, $130.6 million, was reported as Contingent Consideration on our Consolidated Balance Sheet, and was estimated using a discounted cash flow model. Contingent consideration is revalued at each reporting period and is positively impacted each period by the passage of time, since all remaining expected cash flows move closer to collection, thereby increasing their present value. The fair value change in contingent consideration is also impacted by the projected amount and timing of expected future cash flows and by the cost of capital used to discount these cash flows.
For the year ended December 31, 2010, the fair value change in contingent consideration increased by $13.2 million to $16.5 million compared to $3.3 million for the same period in 2009. The increase occurred primarily because 2010 included the impact of a full year related to the passage of time, whereas 2009 only included three months related to the passage of time since the shares of QLT USA were divested on October 1, 2009. In addition, in 2010 there was a favorable impact on the contingent consideration due to an increase in our cash flow projections and a positive impact due to a decrease in the cost of capital used to discount these cash flows.
Other Gains
For the year ended December 31, 2010, other gains included $0.3 million related to the out-license and sale of certain non-core assets and $0.2 million related to receipt of an R&D tax grant.
Income from Discontinued Operations
There was no income from discontinued operations for the year ended December 31, 2010. The income from discontinued operations in 2009 was largely due to the gain on the sale of QLT USA on October 1, 2009.
58
Table of Contents
Income Taxes
The provision for income taxes was $10.9 million for the year ended December 31, 2010, compared to a recovery of income taxes of $5.3 million for the same period in 2009. The provision for 2010 was primarily the result of the application of a valuation allowance against the Canadian tax assets due to an intra-entity transfer of intellectual property and the resulting shift of related future development expenditures to the acquiring entity. Since insufficient evidence existed to support our future realization of the tax benefits of the acquiring entity, a valuation allowance was applied. The recovery of income taxes for 2009 was largely the result of the litigation charge related to the MGH settlement.
The net 2010 deferred tax asset of $6.3 million was largely the result of contingent consideration, certain research and development credits as well as other temporary differences against which a valuation allowance was not applied.
As of December 31, 2009, we had a valuation allowance against specifically identified tax assets. The valuation allowance is reviewed periodically and if management’s assessment of the “more likely than not” criterion for accounting purposes changes, the valuation allowance is adjusted accordingly. See Note 14 - Income Taxes in Notes to the Consolidated Financial Statements.
LIQUIDITY AND CAPITAL RESOURCES
General
In 2012 and foreseeable future periods, we expect our cash resources and working capital, cash flow from operations, cash from the collection of the contingent consideration, and other available financing resources to be sufficient to fund current product research and development, operating requirements, liability requirements, acquisition and licensing activities, milestone payments, and potential repurchases of our common shares.
If adequate capital is not available, our business could be materially and adversely affected. Factors that may affect our future capital requirements include: the status of competitors and their intellectual property rights; levels of future sales of Eligard and our receipt of contingent consideration under the QLT USA stock purchase agreement with Tolmar; levels of future sales of Visudyne; the progress of our R&D programs, including preclinical and clinical testing; future share repurchases; fluctuating or increasing manufacturing requirements; the timing and cost of obtaining regulatory approvals; the levels of resources that we devote to the development of manufacturing and other support capabilities; technological advances; the cost of filing, prosecuting and enforcing our patent claims and other intellectual property rights; the expiration of our Visudyne patents and potential loss of market exclusivity for Visudyne; pre-launch costs related to commercializing one of our products in development; acquisition and licensing activities; milestone payments and receipts; and our ability to establish collaborative arrangements with other organizations.
Sources and Uses of Cash
We finance operations, product development and capital expenditures primarily through existing cash, proceeds from our commercial operations, sales of assets and interest income.
For the year ended December 31, 2011, we used $16.6 million in cash from operations as compared to generating $4.4 million for the same period in 2010. The $21.0 million negative cash flow variance is primarily attributable to:
| • | A negative operating cash flow variance from higher operating and inventory related expenditures of $13.2 million; |
| • | A negative operating cash flow variance from proceeds related to the fair value change in contingent consideration of $6.4 million; |
| • | A negative operating cash flow variance from lower net tax refunds of $4.8 million; |
| • | A negative operating cash flow variance from lower investment and other income of $0.6 million; and |
| • | A positive operating cash flow variance from higher cash receipts from product sales and royalties of $4.0 million. |
During the year ended December 31, 2011, cash flows provided by investing activities consisted of proceeds on collection of the contingent consideration of $30.6 million and proceeds from collection of the mortgage receivable of $2.0 million, offset by capital expenditures of $3.3 million.
For the year ended December 31, 2011, our cash flows used in financing activities consisted primarily of common shares repurchased for $18.8 million including share repurchase costs, offset by $2.2 million received for the issuance of common shares related to the exercise of stock options.
59
Table of Contents
In the first quarter of 2012, we received the remaining CAD $6.0 million from Discovery Parks Holdings Ltd., an affiliate of Discovery Parks Trust, related to the interest-only second mortgage held on our corporate headquarters, which was due on or before August 29, 2012.
Interest and Foreign Exchange Rates
We are exposed to market risk related to changes in interest and foreign currency exchange rates, each of which could adversely affect the value of our current assets and liabilities. At December 31, 2011, we had $205.6 million in cash and cash equivalents and our cash equivalents had an average remaining maturity of approximately 17 days. If market interest rates were to increase immediately and uniformly by one hundred basis points from levels at December 31, 2011, the fair value of the cash equivalents would decline by an immaterial amount due to the short remaining maturity period.
The functional currency of QLT Inc. and its U.S. subsidiaries is the U.S. dollar, therefore our U.S. dollar-denominated cash and cash equivalents holdings do not result in foreign currency gains or losses in operations. To the extent that QLT Inc. holds a portion of its monetary assets and liabilities in Canadian dollars, we are subject to translation gains and losses. These translation gains and losses are included in operations for the period.
At December 31, 2011, we had no outstanding forward foreign currency contracts.
Contractual Obligations
In the normal course of business, we enter into purchase commitments related to daily operations. In addition, we have entered into operating lease agreements related to office space, vehicles and office equipment. The minimum annual commitments related to these agreements are as follows:
| (in thousands of U.S. dollars) |
Payments due by period | |||||||||||||||||||
| Contractual Obligations(1) |
Total | Less than 1 year | 1-3 years | 3-5 years | More than 5 years | |||||||||||||||
| Operating Leases (2) |
$ | 3,649 | $ | 2,122 | $ | 1,458 | $ | 69 | $ | — | ||||||||||
| Purchase Obligations (3) (4) |
13,298 | 9,660 | 3,438 | 200 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 16,947 | $ | 11,782 | $ | 4,896 | $ | 269 | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| (1) | At December 31, 2011, we had approximately $1.7 million of long-term liabilities associated with uncertain tax positions. At this time, we are unable to make a reasonably reliable estimate of the timing of future payments, if any, due to uncertainties in the timing of future outcomes of tax audits that may arise. As a result, uncertain tax liabilities are not included in the table above. |
| (2) | Operating leases comprise our long-term leases of office space, photocopiers and motor vehicles. In conjunction with the sale of our land and building, in September 2008 we entered into a five-year operating lease with Discovery Parks for office and laboratory space. In connection with our U.S. operations, in May 2009 we entered into a four-year operating lease with AMB Property, L.P, to support certain of our commercial and development operations. |
| (3) | Purchase obligations comprise $5.3 million in ongoing research contracts with third-party organizations, $4.0 million in minimum purchase requirements under our agreement with our supplier for the supply of an intermediate material used in the production of Visudyne, and $4.0 million in other outstanding purchase commitments related to the normal course of business. Although all of our material research contracts with third-party organizations are cancelable, we do not intend to cancel such contracts. These amounts reflect commitments based on existing contracts and do not reflect any future modifications to, or terminations of, existing contracts or anticipated or potential new contracts. |
| (4) | We have also committed to make potential future milestone payments to third parties as part of our licensing, development, and purchase agreements. Payments under these arrangements generally become due and payable upon achievement of certain developmental, regulatory or commercial milestones. The achievement of these milestones is not probable and therefore, not included in the table above. |
60
Table of Contents
Off-Balance Sheet Arrangements
In connection with the sale of assets and businesses, we provide indemnities with respect to certain matters, including product liability, patent infringement, contractual breaches and misrepresentations, and we provide other indemnities to third parties under the clinical trial, license, service, manufacturing, supply, distribution and other agreements that we enter into in the normal course of our business. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnities are generally subject to threshold amounts, specified claims periods and other restrictions and limitations.
Except as described above and the contractual arrangements described in the Contractual Obligations section above, we do not have any off-balance sheet arrangements that have, or are reasonably likely to have, a current or future material effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that is material to investors.
CERTAIN CANADIAN AND U.S. FEDERAL INCOME TAX INFORMATION FOR U.S. RESIDENTS
The following is a summary of certain Canadian and U.S. federal income tax considerations applicable to holders of common shares of the Company. These tax considerations are stated in brief and general terms and are based on Canadian and U.S. law currently in effect. There are other potentially significant Canadian and U.S. federal income tax considerations and provincial, state and local income tax considerations with respect to ownership and disposition of the common shares which are not discussed herein. The tax considerations relative to ownership and disposition of the common shares may vary from shareholder to shareholder depending on the shareholder’s particular status. Accordingly, shareholders and prospective shareholders are encouraged to consult with their tax advisors regarding tax considerations which may apply to the particular situation.
Canadian Federal Tax Information
The following is a general summary of the principal Canadian federal income tax considerations generally applicable to a holder of common shares of the Company who, at all relevant times, for purposes of the Income Tax Act (Canada) (the “Canadian Tax Act”) (i) is not, or is not deemed to be, a resident of Canada, (ii) holds the common shares as capital property, (iii) deals at arm’s length with, and is not affiliated with, the Company and (iv) does not and will not use or hold, and is not and will not be deemed to use or hold, common shares of the Company in connection with carrying on a business in Canada (a “Non-Resident Holder”). Special rules, which are not discussed in this summary, may apply to a Non-Resident Holder that is an insurer carrying on business in Canada and elsewhere. Common shares of the Company will generally be considered to be capital property to a holder thereof, unless the shares are held in the course of carrying on a business or were acquired in a transaction considered to be an adventure in the nature of trade.
Dividends paid, deemed to be paid, or credited on the common shares held by Non-Resident Holders will generally be subject to Canadian withholding tax at the rate of 25% of the gross amount of the dividend unless the rate is reduced by an applicable income tax convention or treaty. The Canada-U.S. Income Tax Convention (1980) (the “Convention”) provides that the withholding tax rate on dividends paid on the common shares to U.S. residents who qualify for the benefit of the Convention will generally be reduced to 15% of the gross amount of the dividend.
A Non-Resident Holder will generally not be subject to Canadian income tax in respect of any gain realized on the disposition of common shares unless the common shares constitute “taxable Canadian property” to such Non-Resident Holder and such Non-Resident Holder is not entitled to relief under an applicable income tax treaty or convention. Generally, provided the common shares are then listed on a designated stock exchange for purposes of the Canadian Tax Act (which includes the TSX and the NASDAQ), the common shares will not be “taxable Canadian property” to a Non-Resident Holder unless, at any particular time during the 60-month period immediately preceding the disposition (i) 25% or more of the issued shares of any class or series of the capital stock of the Company were owned by such Non-Resident Holder, by persons with whom the Non-Resident Holder did not deal at arm’s length, or any combination thereof and (ii) the shares derived more than 50% of their fair market value directly or indirectly from one or any combination of real or immovable property situated in Canada, Canadian resource properties or timber resource properties (as defined in the Canadian Tax Act), or options in respect of, or interests or rights in any of the foregoing. A gain realized upon the disposition of the common shares by a U.S. resident who qualifies for the benefits of the Convention that is otherwise subject to Canadian tax may be exempt from Canadian tax under the Convention.
Where the common shares are disposed of by way of an acquisition of such common shares by the Company, other than a purchase in the open market in the manner in which common shares normally would be purchased by any member of the public in the open market, the amount paid by the Company in excess of the paid-up capital of such common shares will be treated as a dividend and will be subject to non-resident withholding tax as described above.
61
Table of Contents
U.S. Federal Income Tax Information
Special U.S. federal income tax rules apply to “U.S. Holders” (as defined below) of stock of a “passive foreign investment company” (a “PFIC”). As previously disclosed, the Company believes, but cannot offer any assurance, that it was classified as a PFIC for one or more taxable years prior to 2000, and that it was not a PFIC during any of the taxable years from the taxable year ended December 31, 2000 through the taxable year ended December 31, 2007. The Company further believes that it was a PFIC for the taxable years ended December 31, 2008 through 2011, which significantly impacts the U.S. federal income tax consequences to U.S. Holders. The Company is uncertain regarding its potential PFIC status for the taxable year ending December 31, 2012. The Company’s actual PFIC status for a given taxable year will not be determinable until the close of such year and, accordingly, no assurances can be given regarding the Company’s PFIC status in 2012 or any future year. See further discussion of the PFIC rules below. In addition, the following assumes that the common shares are held as a capital asset within the meaning of Section 1221 of the Internal Revenue Code of 1986, as amended (the “Code”).
This summary is of a general nature only and is not intended for non-U.S. Holders. Furthermore, it is not intended to constitute, and should not be construed to constitute, legal or tax advice to any particular U.S. Holder, and it does not address U.S. federal income tax considerations that may be relevant to U.S. Holders that are subject to special treatment under U.S. federal income tax law. U.S. Holders are urged to consult their own tax advisors as to the tax consequences in their particular circumstances.
U.S. Holders
A “U.S. Holder” is a holder of the Company’s common shares that is (i) an individual who is a citizen or resident of the United States for U.S. federal income tax purposes; (ii) a corporation (or other entity taxed as a corporation for U.S. federal income tax purposes) created or organized under the laws of the United States, any U.S. state or the District of Columbia; (iii) an estate the income of which is subject to U.S. federal income taxation regardless of the income’s source; or (iv) a trust (a) if a U.S. court is able to exercise primary supervision over the trust’s administration and one or more U.S. persons, as defined under Section 7701(a)(30) of the Code, have authority to control all of the trust’s substantial decisions; or (b) that was in existence on August 20, 1996, was treated as a U.S. person under the Code on the previous day and has a valid election in effect under applicable U.S. Treasury regulations to be treated as a U.S. person.
Sale or Other Disposition of Common Shares
Subject to different treatment pursuant to the PFIC rules discussed below, if a U.S. Holder engages in a sale, exchange or other taxable disposition of such U.S. Holder’s common shares, (i) such U.S. Holder will recognize gain or loss equal to the difference between the amount realized by such U.S. Holder and such U.S. Holder’s adjusted tax basis in the common shares, (ii) any such gain or loss will be capital gain or loss, and (iii) such capital gain or loss will be long-term capital gain or loss if the holding period of the common shares exceeds one year as of the date of the sale. Such gain generally is treated as U.S. source gain for U.S. foreign tax credit limitation purposes.
If the Company purchases common shares from a U.S. Holder, such transaction will be treated as a taxable sale or exchange of the common shares by the U.S. Holder if the transaction meets certain conditions under U.S. federal income tax rules, or otherwise will be treated as a distribution by the Company in respect of the U.S. Holder’s common shares, as described below.
Distributions on Common Shares
Subject to different treatment pursuant to the PFIC rules discussed below, a distribution with respect to our common shares generally will be treated as a dividend, taxable as ordinary income, to the extent of the Company’s current or accumulated earnings and profits, as determined under U.S. federal income tax principles. In general, to the extent that the amount of the distribution exceeds the Company’s current and accumulated earnings and profits, the excess first will be treated as a tax-free return of capital that will reduce the holder’s tax basis in the holder’s common shares, and to the extent of any remaining portion in excess of such tax basis, the excess will be taxable as capital gain. Any such capital gain will be long-term capital gain if the U.S. Holder has held the common shares for more than one year at the time of the distribution. However, under proposed U.S. Treasury regulations regarding the treatment of PFICs, a purchase of common shares from a U.S. Holder by the Company that does not qualify as a “sale or exchange” under U.S. federal income tax rules, and hence is treated as a distribution, is in fact treated as a distribution in full for PFIC purposes regardless of whether there are any earnings and profits.
A dividend received by a corporate U.S. Holder generally will not be eligible for a dividends-received deduction. In addition, a dividend received by an individual U.S. Holder will not qualify for the 15% reduced maximum rate if the Company is a PFIC in the year in which the dividend is paid or in the preceding year.
62
Table of Contents
Dividends will constitute foreign source income for foreign tax credit limitation purposes. The limitation on foreign taxes eligible for credit is calculated separately with respect to specific classes of income. For this purpose, dividends distributed by the Company with respect to our common shares will constitute “passive category income” or, in the case of certain U.S. Holders, “general category income.”
Passive Foreign Investment Company
A non-U.S. corporation generally will be classified as a PFIC for U.S. federal income tax purposes in any taxable year in which, after applying relevant look-through rules with respect to the income and assets of subsidiaries, either 75% or more of its gross income is “passive income” (the income test) or 50% or more of the average value of its assets consists of assets that produce, or are held for the production of, passive income (the asset test). For this purpose, passive income generally includes, among other things, dividends, interest, certain rents and royalties and gains from the disposition of passive assets.
The Company believes that it qualified as a PFIC for 2008 through 2011. Please be aware that the Company’s status as a PFIC can have significant adverse tax consequences for U.S. Holders.
A U.S. Holder that holds common shares while the Company is a PFIC may be subject to increased tax liability upon the sale, exchange or other disposition of the common shares or upon the receipt of certain distributions, regardless of whether the Company is a PFIC in the year in which such disposition or distribution occurs. These adverse tax consequences will not apply, however, if (i) a U.S. Holder timely filed and maintained (and in certain cases, continues to maintain), or timely files and maintains, as the case may be, a qualified electing fund (“QEF”) election to be taxed annually on the U.S. Holder’s pro rata portion of the Company’s earnings and profits, (ii) the U.S. Holder timely made or makes, as the case may be, a mark-to-market election as described below, or (iii) a U.S. Holder is eligible to make a “purging” election and timely does so, as described below.
The adverse tax consequences include:
| (a) | “Excess distributions” by the Company are subject to the following special rules. An excess distribution generally is the excess of the amount a PFIC distributes to a shareholder during a taxable year over 125% of the average amount it distributed to the shareholder during the three preceding taxable years or, if shorter, the part of the shareholder’s holding period before the taxable year. Distributions with respect to the common shares made by the Company during the taxable year to a U.S. Holder that are excess distributions must be allocated ratably to each day of the U.S. Holder’s holding period. The amounts allocated to the current taxable year and to taxable years prior to the first year in which the Company was classified as a PFIC are included as ordinary income in a U.S. Holder’s gross income for that year. The amount allocated to each other prior taxable year is taxed as ordinary income at the highest tax rate in effect for the U.S. Holder in that prior year (without offset by any net operating loss for such year) and the tax is subject to an interest charge at the rate applicable to deficiencies in income taxes (the “special interest charge”). |
| (b) | The entire amount of any gain realized upon the sale or other disposition of the common shares will be treated as an excess distribution made in the year of sale or other disposition and as a consequence will be treated as ordinary income and, to the extent allocated to years prior to the year of sale or disposition, will be subject to the special interest charge described above. |
QEF Election. A U.S. Holder of stock in a PFIC may make a QEF election with respect to such PFIC to elect out of the tax treatment discussed above. Generally, a QEF election, on U.S. Internal Revenue Service (“IRS”) Form 8621, should be made with the filing of a U.S. Holder’s U.S. federal income tax return for the first taxable year for which both (i) the U.S. Holder holds common shares of the Company, and (ii) the Company was a PFIC. A U.S. Holder that timely makes a valid QEF election with respect to a PFIC will generally include in gross income for a taxable year (i) as ordinary income, such holder’s pro rata share of the corporation’s ordinary earnings for the taxable year, and (ii) as long-term capital gain, such holder’s pro rata share of the corporation’s net capital gain for the taxable year. However, the QEF election is available only if such PFIC provides such U.S. Holder with certain information regarding its earnings and profits as required under applicable U.S. Treasury regulations. The Company will provide, upon request, all information and documentation that a U.S. Holder making a QEF election is required to obtain for U.S. federal income tax purposes (e.g., the U.S. Holder’s pro rata share of ordinary income and net capital gain, and a “PFIC Annual Information Statement” as described in applicable U.S. Treasury regulations, which will be made available on the Company’s website).
Deemed Sale Election. If the Company is a PFIC for any year during which a U.S. Holder holds common shares, but the Company ceases in a subsequent year to be a PFIC (which could occur, for example, if the Company were a PFIC for 2011 but is not a PFIC for 2012), then a U.S. Holder can make a “purging” election, in the form of a deemed sale election, for such subsequent year in order to avoid the adverse PFIC tax treatment described above that would otherwise continue to apply because of the Company having previously been a PFIC. If such election is timely made, the U.S. Holder would be deemed to have sold the common shares held by the holder at their fair market value, and any gain from such deemed sale would be taxed as an excess distribution (as described above). The basis of the common shares would be increased by the gain recognized, and a new holding period would begin for the common shares for purposes of the PFIC rules. The U.S. Holder would not recognize any loss incurred on the deemed sale, and such a loss would not result in
63
Table of Contents
a reduction in basis of the common shares. After the deemed sale election, the U.S. Holder’s common shares with respect to which the deemed sale election was made would not be treated as shares in a PFIC, unless the Company subsequently becomes a PFIC. A U.S. Holder may also be able to make a deemed sale election with respect to the Company’s subsidiaries that are PFICs, if any. The rules regarding deemed sale elections are very complex. U.S. Holders are strongly urged to consult their tax advisors about the deemed sale election with regard to the Company and any subsidiaries.
Mark-to-Market Election. Alternatively, a U.S. Holder of “marketable stock” (as defined below) in a PFIC may make a mark-to-market election for such stock to elect out of the adverse PFIC tax treatment discussed above. If a U.S. Holder makes a mark-to-market election for shares of marketable stock, the holder will include in income each year an amount equal to the excess, if any, of the fair market value of the shares as of the close of the holder’s taxable year over the holder’s adjusted basis in such shares. A U.S. Holder is allowed a deduction for the excess, if any, of the adjusted basis of the shares over their fair market value as of the close of the taxable year. However, deductions are allowable only to the extent of any net mark-to-market gains on the shares included in the holder’s income for prior taxable years. Amounts included in a U.S. Holder’s income under a mark-to-market election, as well as gain on the actual sale or other disposition of the shares, are treated as ordinary income. Ordinary loss treatment also applies to the deductible portion of any mark-to-market loss on the shares, as well as to any loss realized on the actual sale or disposition of the shares, to the extent that the amount of such loss does not exceed the net mark-to-market gains previously included for such shares. A U.S. Holder’s basis in the shares will be adjusted to reflect any such income or loss amounts. However, the special interest charge and related adverse tax consequences described above for non-electing holders may continue to apply on a limited basis if the U.S. Holder makes the mark-to-market election after such holder’s holding period for the shares has begun.
The mark-to-market election is available only for “marketable stock,” which is stock that is traded in other than de minimis quantities on at least 15 days during each calendar quarter on a qualified exchange or other market, as defined in applicable U.S. Treasury regulations. The Company’s common shares are listed on the TSX and quoted on NASDAQ, each of which constitutes a “qualified exchange or other market” under applicable U.S. Treasury regulations. U.S. Holders of common shares are urged to consult their tax advisors as to whether the common shares would qualify for the mark-to-market election.
Subsidiary PFICs. To the extent any of the Company’s subsidiaries is also a PFIC, a U.S. Holder will also be deemed to own shares in such lower-tier PFIC and could incur a liability for the deferred tax and special interest charge described above if either (i) the Company receives a distribution from, or disposes of all or part of its interest in, the lower-tier PFIC, or (ii) the U.S. Holder disposes of all or part of such holder’s common shares. In addition, the mark-to-market election cannot be made for a subsidiary of a PFIC if the stock of such subsidiary is not itself marketable stock.
Recent Legislation. Under legislation enacted in 2010, unless otherwise provided by the U.S. Treasury, each U.S. Holder of a PFIC is required to file an annual report on IRS Form 8621 containing such information as the U.S. Treasury may require. Until guidance is issued implementing the new reporting requirements, a U.S. Holder of a PFIC will be required to file IRS Form 8621 only for each taxable year in which such shareholder receives distributions from the PFIC, recognizes gain on a disposition of the PFIC stock, or makes a “reportable election.” In addition, for taxable years beginning after March 18, 2010, legislation enacted in 2010 requires certain U.S. Holders who are individuals to file IRS Form 8938 reporting information relating to an interest in our common shares, subject to certain exceptions (including an exception for common shares held in accounts maintained by certain financial institutions and an exception applicable if the aggregate value of all “specified foreign financial assets” (as defined in the Code) does not exceed $50,000). U.S. Holders should consult their tax advisors regarding any reporting requirements that may apply to them and the effect, if any, of this legislation on their ownership and disposition of our common shares.
THE APPLICABILITY AND CONSEQUENCES OF THE PFIC RULES ARE EXCEEDINGLY COMPLEX. IN ADDITION, THE FOREGOING SUMMARY DOES NOT ADDRESS ALL OF THE POTENTIAL U.S. FEDERAL INCOME TAX CONSEQUENCES WITH RESPECT TO PFIC STATUS THAT MAY BE RELEVANT TO A PARTICULAR INVESTOR IN LIGHT OF SUCH INVESTOR’S PARTICULAR CIRCUMSTANCES OR THAT MAY BE RELEVANT TO INVESTORS THAT ARE SUBJECT TO SPECIAL TREATMENT UNDER U.S. FEDERAL INCOME TAX LAW. ACCORDINGLY, INVESTORS ARE STRONGLY URGED TO CONSULT THEIR TAX ADVISORS REGARDING THE APPLICATION OF THE PFIC RULES TO THEM AND THE ADVISABILITY OF MAKING ANY OF THE ELECTIONS DESCRIBED ABOVE.
Outstanding Share Data
As of February 17, 2012, there were 48,954,680 common shares issued and outstanding for a total of $458.3 million in share capital. As of February 17, 2012, we had 6,018,370 stock options outstanding under the QLT 2000 Incentive Stock Option Plan (of which 4,353,852 were exercisable) at a weighted average exercise price of Cdn $5.44 per share. Each stock option is exercisable for one common share.
64
Table of Contents
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
See Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations - Liquidity and Capital Resources.
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
65
Table of Contents
REPORT OF INDEPENDENT REGISTERED CHARTERED ACCOUNTANTS
To the Board of Directors and Shareholders of QLT Inc.
We have audited the accompanying consolidated balance sheets of QLT Inc. and subsidiaries (the “Company”) as of December 31, 2011 and 2010 and the related consolidated statements of operations, comprehensive (loss) income, cash flows and changes in shareholders’ equity for each of the years in the three year period ended December 31, 2011. Our audits also included the financial statement schedule listed in the Index at Item 15. These financial statements and financial statement schedule are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements and financial statement schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, such consolidated financial statements present fairly, in all material respects, the financial position of QLT Inc. and subsidiaries as of December 31, 2011 and 2010, and the results of their operations and their cash flows for each of the years in the three year period ended December 31, 2011, in conformity with accounting principles generally accepted in the United States of America. Also, in our opinion, such financial statement schedule, when considered in relation to the basic consolidated financial statements taken as a whole, presents fairly, in all material respects, the information set forth therein.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the Company’s internal control over financial reporting as of December 31, 2011, based on the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 23, 2012 expressed an unqualified opinion on the Company’s internal control over financial reporting.
/s/ DELOITTE & TOUCHE LLP
Independent Registered Chartered Accountants
Vancouver, Canada
February 23, 2012
66
Table of Contents
CONSOLIDATED BALANCE SHEETS
| As at December 31, |
2011 | 2010 | ||||||
| (In thousands of U.S. dollars) | ||||||||
| ASSETS |
||||||||
| Current assets |
||||||||
| Cash and cash equivalents |
$ | 205,597 | $ | 209,478 | ||||
| Accounts receivable (Note 4) |
9,985 | 10,659 | ||||||
| Current portion of contingent consideration (Notes 15 and 16) |
34,669 | 36,520 | ||||||
| Income taxes receivable |
321 | 61 | ||||||
| Inventories (Note 5) |
1,938 | 3,324 | ||||||
| Current portion of deferred income tax assets (Note 14) |
1,351 | 3,643 | ||||||
| Current portion of mortgage receivable (Note 6) |
5,874 | 2,004 | ||||||
| Prepaid and other |
1,404 | 2,958 | ||||||
|
|
|
|
|
|||||
| 261,139 | 268,647 | |||||||
| Property, plant and equipment (Note 7) |
4,731 | 3,035 | ||||||
| Deferred income tax assets (Note 14) |
1,350 | 2,700 | ||||||
| Mortgage receivable (Note 6) |
— | 6,013 | ||||||
| Long-term inventories and other assets (Note 9) |
12,046 | 13,319 | ||||||
| Contingent consideration (Notes 15 and 16) |
65,278 | 94,069 | ||||||
|
|
|
|
|
|||||
| $ | 344,544 | $ | 387,783 | |||||
|
|
|
|
|
|||||
| LIABILITIES |
||||||||
| Current liabilities |
||||||||
| Accounts payable |
$ | 6,099 | $ | 6,031 | ||||
| Income taxes payable |
29 | 716 | ||||||
| Accrued liabilities (Note 10) |
7,679 | 6,405 | ||||||
|
|
|
|
|
|||||
| 13,807 | 13,152 | |||||||
| Uncertain tax position liabilities (Note 14) |
1,732 | 1,687 | ||||||
|
|
|
|
|
|||||
| 15,539 | 14,839 | |||||||
|
|
|
|
|
|||||
| COMMITMENTS AND GUARANTEES (NOTE 17) |
||||||||
| CONTINGENCIES (NOTE 19) |
||||||||
| SHAREHOLDERS’ EQUITY |
||||||||
| Share capital (Note 12) |
||||||||
| Authorized |
||||||||
| 500,000,000 common shares without par value |
||||||||
| 5,000,000 first preference shares without par value, issuable in series |
||||||||
| Issued and outstanding |
||||||||
| Common shares |
458,118 | 479,998 | ||||||
| December 31, 2011 –48,927,742 shares |
||||||||
| December 31, 2010 –51,154,392 shares |
||||||||
| Additional paid in-capital |
296,003 | 287,646 | ||||||
| Accumulated deficit |
(528,085 | ) | (497,669 | ) | ||||
| Accumulated other comprehensive income |
102,969 | 102,969 | ||||||
|
|
|
|
|
|||||
| 329,005 | 372,944 | |||||||
|
|
|
|
|
|||||
| $ | 344,544 | $ | 387,783 | |||||
|
|
|
|
|
|||||
See the accompanying Notes to the Consolidated Financial Statements.
67
Table of Contents
CONSOLIDATED STATEMENTS OF OPERATIONS
| Year ended December 31, |
2011 | 2010 | 2009 | |||||||||
| (In thousands of U.S. dollars except per share information) | ||||||||||||
| Revenues |
||||||||||||
| Net product revenue (Note 13) |
$ | 28,376 | $ | 31,093 | $ | 42,106 | ||||||
| Royalties (Note 3) |
13,852 | 13,604 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| 42,228 | 44,697 | 42,106 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Costs and expenses |
||||||||||||
| Cost of sales |
10,414 | 15,204 | 20,198 | |||||||||
| Research and development |
43,533 | 33,485 | 28,590 | |||||||||
| Selling, general and administrative |
25,683 | 20,808 | 18,337 | |||||||||
| Depreciation |
1,433 | 1,202 | 1,403 | |||||||||
| Litigation (Note 19) |
— | — | 20,662 | |||||||||
| Purchase of in-process research and development (Note 8) |
— | — | 7,517 | |||||||||
| Restructuring recovery |
— | — | (263 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| 81,063 | 70,699 | 96,444 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(38,835 | ) | (26,002 | ) | (54,338 | ) | ||||||
| Investment and other income (expense) |
||||||||||||
| Net foreign exchange (losses) gains |
(147 | ) | 363 | 7,003 | ||||||||
| Interest income |
673 | 1,834 | 4,339 | |||||||||
| Interest expense |
— | — | (1,848 | ) | ||||||||
| Fair value change in contingent consideration (Notes 15 and 16) |
10,078 | 16,493 | 3,279 | |||||||||
| Other gains |
629 | 674 | 28 | |||||||||
|
|
|
|
|
|
|
|||||||
| 11,233 | 19,364 | 12,801 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Loss from continuing operations before income taxes |
(27,602 | ) | (6,638 | ) | (41,537 | ) | ||||||
| (Provision for) recovery of income taxes (Note 14) |
(2,814 | ) | (10,901 | ) | 5,317 | |||||||
|
|
|
|
|
|
|
|||||||
| Loss from continuing operations |
$ | (30,416 | ) | $ | (17,539 | ) | $ | (36,220 | ) | |||
|
|
|
|
|
|
|
|||||||
| Income from discontinued operations, net of income taxes (Note 15) |
— | — | 135,654 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (loss) income |
$ | (30,416 | ) | $ | (17,539 | ) | $ | 99,434 | ||||
|
|
|
|
|
|
|
|||||||
| Basic and diluted net (loss) income per common share |
||||||||||||
| Continuing operations |
$ | (0.61 | ) | $ | (0.33 | ) | $ | (0.64 | ) | |||
| Discontinued operations |
— | — | 2.41 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (loss) income |
$ | (0.61 | ) | $ | (0.33 | ) | $ | 1.77 | ||||
|
|
|
|
|
|
|
|||||||
| Weighted average number of common shares outstanding (thousands) |
||||||||||||
| Basic |
50,105 | 52,382 | 56,194 | |||||||||
| Diluted |
50,105 | 52,382 | 56,194 | |||||||||
See the accompanying Notes to the Consolidated Financial Statements.
68
Table of Contents
CONSOLIDATED STATEMENTS OF COMPREHENSIVE (LOSS) INCOME
| Year ended December 31, |
2011 | 2010 | 2009 | |||||||||
| (In thousands of U.S. dollars) | ||||||||||||
| Net (loss) income |
$ | (30,416 | ) | $ | (17,539 | ) | $ | 99,434 | ||||
| Other comprehensive income: |
||||||||||||
| Change in foreign currency translation adjustment |
— | — | 5,763 | |||||||||
|
|
|
|
|
|
|
|||||||
| Other comprehensive income |
— | — | 5,763 | |||||||||
|
|
|
|
|
|
|
|||||||
| Comprehensive (loss) income |
$ | (30,416 | ) | $ | (17,539 | ) | $ | 105,197 | ||||
|
|
|
|
|
|
|
|||||||
See the accompanying Notes to the Consolidated Financial Statements.
69
Table of Contents
CONSOLIDATED STATEMENTS OF CASH FLOWS
| Year ended December 31, |
2011 | 2010 | 2009 | |||||||||
| (In thousands of U.S. dollars) | ||||||||||||
| Cash flows (used in) provided by operating activities |
||||||||||||
| Net (loss) income |
$ | (30,416 | ) | $ | (17,539 | ) | $ | 99,434 | ||||
| Adjustments to reconcile net (loss) income to net cash from operating activities |
||||||||||||
| Depreciation |
1,433 | 1,202 | 1,403 | |||||||||
| Write-down of inventory |
— | — | 8,114 | |||||||||
| Write-down of fixed assets, investments and deposit |
— | — | 448 | |||||||||
| Share-based compensation |
2,973 | 2,494 | 2,161 | |||||||||
| Unrealized foreign exchange (gains) losses |
131 | (331 | ) | 2,290 | ||||||||
| Interest on restricted cash |
— | — | (295 | ) | ||||||||
| Interest earned on note receivable |
— | (741 | ) | (250 | ) | |||||||
| Deferred income taxes |
3,661 | 12,872 | (5,510 | ) | ||||||||
| Gain on sale of long-lived assets |
(269 | ) | (425 | ) | — | |||||||
| Gain on sale of discontinued operations |
— | — | (107,363 | ) | ||||||||
| Changes in non-cash operating assets and liabilities |
||||||||||||
| Appeal bond collateral |
— | — | 124,622 | |||||||||
| Long-term deposits and other assets |
2,139 | 3,115 | 5,599 | |||||||||
| Accounts receivable |
1,029 | (1,186 | ) | 8,143 | ||||||||
| Inventories |
2,118 | 1,006 | (833 | ) | ||||||||
| Accounts payable |
236 | 2,040 | (1,920 | ) | ||||||||
| Income taxes receivable / payable |
(937 | ) | 5,553 | 47,371 | ||||||||
| Accrued restructuring charge |
— | — | (743 | ) | ||||||||
| Other accrued liabilities |
1,324 | 633 | (124,306 | ) | ||||||||
| Deferred revenue |
— | (4,244 | ) | (1,723 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| (16,578 | ) | 4,449 | 56,642 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash provided by investing activities |
||||||||||||
| Net proceeds from sale of property, plant & equipment |
19 | 108 | 116 | |||||||||
| Net proceeds from sale of investment in QLT USA |
— | 10,000 | 16,477 | |||||||||
| Purchase of property, plant and equipment |
(3,317 | ) | (1,589 | ) | (1,094 | ) | ||||||
| Proceeds from sale of long-lived assets |
— | 317 | — | |||||||||
| Proceeds from mortgage receivable |
2,004 | 3,822 | — | |||||||||
| Proceeds from contingent consideration(1) |
30,641 | 20,490 | 5,162 | |||||||||
|
|
|
|
|
|
|
|||||||
| 29,347 | 33,148 | 20,661 | ||||||||||
|
|
|
|
|
|
|
|||||||
| Cash used in financing activities |
||||||||||||
| Common shares repurchased, including fees |
(18,839 | ) | (17,246 | ) | (55,911 | ) | ||||||
| Issuance of common shares |
2,182 | 910 | 125 | |||||||||
|
|
|
|
|
|
|
|||||||
| (16,657 | ) | (16,336 | ) | (55,786 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Effect of exchange rate changes on cash and cash equivalents |
7 | 103 | 1,202 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (decrease) increase in cash and cash equivalents |
(3,881 | ) | 21,364 | 22,719 | ||||||||
| Cash and cash equivalents, beginning of year |
209,478 | 188,114 | 165,395 | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents, end of year |
$ | 205,597 | $ | 209,478 | $ | 188,114 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplementary cash flow information: |
||||||||||||
|
|
|
|
|
|
|
|||||||
| Interest paid |
$ | — | $ | — | $ | 1,832 | ||||||
| Income taxes paid |
994 | 2,002 | 6,305 | |||||||||
|
|
|
|
|
|
|
|||||||
Non-cash investing activity:
| (1) | On October 1, 2009, all of the shares of QLT USA were sold to TOLMAR Holding, Inc. (“Tolmar”) for up to an aggregate $230.0 million, plus cash on hand of $118.3 million. The purchase price included contingent consideration of $200.0 million which had a fair value of $156.2 million on October 1, 2009, representing a non-cash investing activity. |
70
Table of Contents
During the year ended December 31, 2011, proceeds received on collection of the contingent consideration totalled $40.7 million (2010 - $37.0 million). Approximately $30.6 million (2010 - $20.5 million) of the proceeds were included within cash provided by investing activities. The remaining $10.1 million (2010 - $16.5 million) of the proceeds were recorded in the Statement of Operations as the fair value change in contingent consideration and were therefore reflected in the net (loss) income line item within cash (used in) provided by operating activities.
See the accompanying Notes to the Consolidated Financial Statements.
71
Table of Contents
CONSOLIDATED STATEMENTS OF CHANGES IN SHAREHOLDERS’ EQUITY
| Common Shares | Additional Paid-in |
Accumulated | Accumulated Other Comprehensive |
Total Shareholders’ |
||||||||||||||||||||
| Shares | Amount | Capital | Deficit | Income (Loss) | Equity | |||||||||||||||||||
| (All amounts except share and per share information are expressed in thousands of U.S. dollars) | ||||||||||||||||||||||||
| Balance at January 1, 2009 |
74,620,328 | $ | 702,221 | $ | 123,367 | $ | (579,564 | ) | $ | 97,206 | $ | 343,230 | ||||||||||||
| Exercise of stock options, for cash, at prices ranging from CAD $2.44 to CAD $3.73 per share and U.S. $3.78 per share |
35,751 | 170 | (45 | ) | — | — | 125 | |||||||||||||||||
| Stock-based compensation |
— | — | 2,266 | — | — | 2,266 | ||||||||||||||||||
| Common share repurchase |
(20,866,790 | ) | (196,368 | ) | 150,004 | — | — | (46,364 | ) | |||||||||||||||
| Other comprehensive income: |
||||||||||||||||||||||||
| Cumulative translation adjustment from application of U.S. dollar reporting |
— | — | — | — | 5,763 | 5,763 | ||||||||||||||||||
| Net income |
— | — | — | 99,434 | — | 99,434 | ||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2009 |
53,789,289 | $ | 506,023 | $ | 275,592 | $ | (480,130 | ) | $ | 102,969 | $ | 404,454 | ||||||||||||
| Exercise of stock options, for cash, at prices ranging from CAD $2.44 to CAD $6.27 per share |
265,585 | 1,243 | (333 | ) | — | — | 910 | |||||||||||||||||
| Stock-based compensation |
— | — | 2,365 | — | — | 2,365 | ||||||||||||||||||
| Common share repurchase |
(2,900,482 | ) | (27,268 | ) | 10,022 | — | — | (17,246 | ) | |||||||||||||||
| Net loss |
— | — | — | (17,539 | ) | — | (17,539 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2010 |
51,154,392 | $ | 479,998 | $ | 287,646 | $ | (497,669 | ) | $ | 102,969 | $ | 372,944 | ||||||||||||
| Exercise of stock options, for cash, at prices ranging from CAD $2.44 to CAD $7.20 per share |
451,867 | 3,226 | (931 | ) | — | — | 2,295 | |||||||||||||||||
| Stock-based compensation |
— | — | 3,021 | — | — | 3,021 | ||||||||||||||||||
| Common share repurchase |
(2,678,517 | ) | (25,106 | ) | 6,267 | — | — | (18,839 | ) | |||||||||||||||
| Net loss |
— | — | — | (30,416 | ) | — | (30,416 | ) | ||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| Balance at December 31, 2011 |
48,927,742 | $ | 458,118 | $ | 296,003 | $ | (528,085 | ) | $ | 102,969 | (1) | $ | 329,005 | |||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
| (1) | At December 31, 2011, our accumulated other comprehensive income is entirely related to historical cumulative translation adjustments from the application of U.S. dollar reporting when the functional currency of QLT Inc. was the Canadian dollar. See Note 3 - Significant Accounting Policies. |
See the accompanying Notes to the Consolidated Financial Statements.
72
Table of Contents
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
QLT is a biotechnology company dedicated to the development and commercialization of innovative ocular products that address the unmet medical needs of patients and clinicians worldwide. We are focused on developing our synthetic retinoid program for the treatment of certain inherited retinal diseases, developing our proprietary punctal plug delivery system, as well as U.S. marketing of our commercial product, Visudyne®, for the treatment of wet age related macular degeneration (“wet AMD”).
1. BASIS OF PRESENTATION
These consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America. All amounts herein are expressed in U.S. dollars unless otherwise noted.
On October 1, 2009, the Eligard® product line was divested as part of the sale of all of the shares of our wholly owned U.S. subsidiary, QLT USA, Inc. (“QLT USA”). In accordance with the accounting standard for discontinued operations, the results of operations related to this disposition have been excluded from continuing operations and reported as discontinued operations for 2009.
2. PRINCIPLES OF CONSOLIDATION
These consolidated financial statements include the accounts of QLT and its subsidiaries, all of which are wholly owned. All intercompany transactions have been eliminated.
3. SIGNIFICANT ACCOUNTING POLICIES
Use of Estimates
The preparation of financial statements in accordance with generally accepted accounting principles requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of revenue and expenses during the reporting periods presented. Significant estimates are used for, but not limited to, provisions for non-completion of inventory, provision for excess inventory, the fair value of contingent consideration, allocation of costs to manufacturing under a standard costing system, allocation of overhead expenses to research and development, stock-based compensation, provisions for taxes, tax assets and liabilities. Actual results may differ from estimates made by management.
Reporting Currency and Functional Currency Change
The U.S. dollar is the reporting currency and functional currency for QLT Inc. and our subsidiaries. For 2009 and prior periods, the Canadian dollar was the functional currency for QLT Inc. As a result of the change in our business related to the receipt of exclusive U.S. rights to the Visudyne patents from Novartis Pharma AG (“Novartis”), effective January 1, 2010, we changed the functional currency for QLT Inc., the Canadian parent company, to the U.S. dollar. The U.S. dollar reflects the currency of the economic environment in which QLT Inc. operates as a result of significant U.S. dollar denominated revenues, expenditures, and cash flows. Past translation gains and losses from the application of the U.S. dollar as the reporting currency while the Canadian dollar was the functional currency of QLT Inc. are included as part of the cumulative foreign currency translation adjustment, which is reported as a component of shareholders’ equity under accumulated other comprehensive income (loss).
For periods commencing January 1, 2010, monetary assets and liabilities denominated in foreign currencies are translated into U.S. dollars using exchange rates in effect at the balance sheet date. Opening balances related to non-monetary assets and liabilities are based on prior period translated amounts, and nonmonetary assets and nonmonetary liabilities incurred after January 1, 2010 are translated at the approximate exchange rate prevailing at the date of the transaction. Revenue and expense transactions are translated at the approximate exchange rate in effect at the time of the transaction. Foreign exchange gains and losses are included in income or loss for the period.
Segment Information
We operate in one industry segment, which is the business of developing, manufacturing, and commercializing opportunities in ophthamology. Our chief operating decision maker reviews our operating results on an aggregate basis and manages our operations as a single operating segment. Our segment information does not include the results of businesses classified as discontinued operations.
73
Table of Contents
Cash and Cash Equivalents
Cash equivalents include highly liquid investments with insignificant interest rate risk and original maturities of three months or less at the date of purchase. We report available-for-sale investments, if any, at fair value and include any unrealized holding gains and losses in shareholders’ equity.
Inventories
Raw materials and supplies inventories are carried at the lower of actual cost and net realizable value. Finished goods and work-in-process inventories are carried at the lower of weighted average cost and net realizable value. We record a provision for non-completion of product inventory to provide for potential failure of inventory batches in production to pass quality inspection. We estimate our non-completion rate based on past production and adjust our provision quarterly based on actual production volume and actual non-completion experience. Inventory that is obsolete or expired is written down to its market value if lower than cost. Inventory quantities are regularly reviewed and provisions for excess or obsolete inventory are recorded primarily based on our forecast of future demand and market conditions. We classify inventories that we do not expect to convert or consume in the next year as non-current based upon an analysis of market conditions such as sales trends, sales forecasts, sales price, and other factors.
Long-lived and Intangible Assets
We depreciate property, plant and equipment using the straight-line method over their estimated economic lives, which range from three to five years. Determining the economic lives of plant and equipment requires us to make significant judgments that can materially impact our operating results.
We periodically evaluate our long-lived assets for potential impairment. We perform these evaluations whenever events or changes in circumstances suggest that the carrying amount of an asset or group of assets is not recoverable. If impairment recognition criteria are met, we charge impairments of the long-lived assets to operations.
In accounting for acquisitions, we allocate the purchase price to the fair value of the acquired tangible and intangible assets, including in-process research and development (“IPR&D”). We generally determine the value of acquired intangible assets and IPR&D using a discounted cash flow model, which requires us to make assumptions and estimates about, among other things: the time and investment that is required to develop products and technologies; our ability to develop and commercialize products before our competitors develop and commercialize products for the same indications; the amount of revenue to be derived from the products; and appropriate discount rates to use in the analysis. Use of different estimates and judgments could yield materially different results in our analysis, and could result in materially different asset values.
Property, Plant and Equipment
Leasehold improvements are depreciated over the expected useful life but in no case longer than the lease term, except when the lease renewal has been determined to be reasonably assured and failure to renew the lease imposes a penalty on the Company. Property, plant and equipment are recorded at cost and amortized as follows:
| Method | Years | |||||
| Office furnishings, fixtures and other |
Straight-line | 5 | ||||
| Research and commercial manufacturing equipment |
Straight-line | 3-5 | ||||
| Computer hardware and operating system |
Straight-line | 3-5 | ||||
Revenue Recognition
With respect to Visudyne, under the terms of the Amended and Restated PDT Product Development, Manufacturing and Distribution Agreement with Novartis (“Amended PDT Agreement”), on January 1, 2010 we received from Novartis the exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. As a result, we have established a commercial presence in the U.S., operating a direct marketing and sales force through one of our U.S. subsidiaries, QLT Ophthalmics, Inc., and have rights to all end-user revenue derived from U.S. Visudyne sales. Novartis continues to market and sell Visudyne for ophthalmic use outside the U.S. and now pays us a royalty on net sales of the product outside the U.S., instead of the 50% share of Novartis’ net proceeds from Visudyne sales that was payable under the previous PDT Product Development, Manufacturing and Distribution Agreement. We utilize contract manufacturers for Visudyne production and are responsible for product supply to Novartis and our U.S. specialty wholesale distributors.
Net Product Revenue
Net product revenues for periods after 2009 have been derived from sales of Visudyne to distributors in the U.S. and to Novartis outside the U.S., plus reimbursement of certain costs from Novartis. We recognize revenue from the sale of Visudyne when persuasive evidence of an arrangement exists, delivery has occurred, the end selling price of Visudyne is fixed or determinable, and collectibility is reasonably assured. For U.S. Visudyne sales, provisions for certain vendor charge-backs, discounts, Medicaid rebates, distributor fees and product returns are accounted for as a reduction of revenue in the same period the related revenue is recorded. Rebates, vendor charge-backs, and
74
Table of Contents
discounts are estimated based on contractual terms, historical experience, and projected market conditions. Distributor fees are estimated based on the level of inventory in the distribution channel and adjusted to reflect actual experience. Product returns are estimated based on historical experience and business trends. We recognize revenue upon delivery, when title and risk of loss passes to Novartis and other distributors.
Through December 31, 2009, our agreement with Novartis provided that the calculation of total revenue for the sale of Visudyne be composed of three components: (1) an advance on the cost of inventory sold to Novartis, (2) an amount equal to 50% of Novartis’ net proceeds from Visudyne sales to end-customers (determined according to a contractually agreed definition), and (3) the reimbursement of other specified costs incurred and paid for by us. Under the calculation of revenue noted above, this occurred when Novartis sold Visudyne to its end customers.
Royalties
Under the Amended PDT Agreement, Novartis is required to pay us a royalty of 20% of net sales outside the U.S. until December 31, 2014, and 16% of net sales thereafter, until the expiry of the Amended PDT Agreement on December 31, 2019. We recognize royalties when product is sold by Novartis to end customers based on royalty rates specified in the Amended PDT Agreement with Novartis. Royalties are based on net product sales (gross sales less discounts, allowances and other items) and calculated based on information supplied to us by Novartis.
Inventory and Cost of Sales
For periods after 2009, Visudyne cost of sales, consisting of expenses related to the production of bulk Visudyne, royalty expense on Visudyne sales and ongoing damages related to the Massachusetts Eye and Ear Infirmary judgment (See Note 19 – Contingencies), are charged against earnings in the period that we sell to distributors in the U.S. and to Novartis outside the U.S. Through December 31, 2009, Visudyne cost of sales were charged against earnings in the period that Novartis sold to end customers.
We utilize a standard costing system, which includes a reasonable allocation of overhead expenses, to account for inventory and cost of sales, with adjustments being made periodically to reflect current conditions. Losses on manufacturing purchase commitments are immediately charged to cost of sales. Our standard costs are estimated based on management’s best estimate of annual production volumes and material costs. Overhead expenses comprise direct and indirect support activities related to manufacturing and involve costs associated with activities such as quality inspection, quality assurance, supply chain management, safety and regulatory. Overhead expenses are allocated to inventory at various stages of the manufacturing process under a standard costing system. Unallocated overheads are recognized as cost of sales in the period in which they are incurred. For periods after 2009, overhead was allocated to cost of sales as Visudyne was sold to our distributors in the U.S. and to Novartis outside the U.S. Through December 31, 2009, overhead was allocated to cost of sales as Visudyne was sold by Novartis to third parties.
Stock-Based Compensation
ASC topic 718 requires stock-based compensation to be recognized as compensation expense in the statement of earnings based on their fair values on the date of the grant, with the compensation expense recognized over the period in which a grantee is required to provide service in exchange for the stock award. Compensation expense recognition provisions are applicable to new awards and to any awards modified, repurchased or cancelled after the adoption date. We recognize compensation expense based on the estimated grant date fair value method using the Black-Scholes valuation model, adjusted for estimated forfeitures. When estimating forfeitures, we consider voluntary termination behaviors as well as trends of actual option forfeitures.
The Company has a Directors’ Deferred Share Unit Plan (“DDSU Plan”) for non-employee directors. We recognize compensation expense for Deferred Share Units (“DSUs”) based on the market price of the Company’s stock. A vested DSU is convertible to cash only, and the obligations for future settlement of DSUs are accrued as compensation expense as the DSUs vest and are included in accrued liabilities. Each reporting period, obligations are revalued for changes in the market value of QLT’s common shares. See Note 12 – Share Capital.
Research and Development
Research and development costs, including certain acquired in-process research and development related to acquired assets or group of assets that do not meet the definition of a business under applicable accounting standards, are expensed as incurred. These costs generally consist of direct and indirect expenditures, including a reasonable allocation of overhead expenses, associated with our various research and development programs. Overhead expenses comprise general and administrative support provided to the research and development programs and involve costs associated with support activities such as rent, facility maintenance, utilities, office services, information technology, legal, accounting and human resources. Patent application, filing and defense costs are expensed as incurred.
Income Taxes
Income taxes are reported using the asset and liability method, whereby deferred tax assets and liabilities are recognized for the future tax consequences attributable to: (i) differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases, and (ii) operating loss and tax credit carryforwards using applicable enacted tax rates. An increase or decrease in
75
Table of Contents
these tax rates will increase or decrease the carrying value of future net tax assets resulting in an increase or decrease to net income. Income tax credits, such as investment tax credits, are included as part of the provision for income taxes. The realization of our deferred tax assets is primarily dependent on generating sufficient capital gains and taxable income prior to expiration of any loss carry forward balance. A valuation allowance is provided when it is more likely than not that a deferred tax asset may not be realized. Changes in valuation allowances are included in our tax provision, or within discontinued operations in the period of change.
Derivative Financial Instruments
Periodically, we may enter into foreign exchange contracts to manage exposure to currency rate fluctuations related to our expected future net earnings and cash flows. The foreign exchange contracts are not designated as hedging instruments and, as a result, all foreign exchange contracts are marked to market and the resulting gains and losses are recorded in the statement of income in each reporting period. There were no foreign exchange contracts outstanding at December 31, 2011.
Discontinued Operations
We consider assets to be held for sale when management approves and commits to a formal plan to actively market the assets for sale. Upon designation as held for sale, the carrying value of the assets is recorded at the lower of the carrying value or the estimated fair value. We cease to record depreciation or amortization expense at that time.
The results of operations, including the gain on disposal for businesses classified as held for sale are excluded from continuing operations and reported as discontinued operations for all periods presented. We do not expect any continuing involvement with these businesses following their sales.
Contingent Consideration
Contingent consideration arising from the sale of QLT USA is measured at fair value. The contingent consideration is revalued at each reporting period and changes are included in continuing operations.
Contingencies Related to Legal Proceedings
We record a liability in the consolidated financial statements for actions when a loss is considered probable and the amount can be reasonably estimated. If the loss is not probable or a range cannot reasonably be estimated, no liability is recorded in the consolidated financial statements.
Net (Loss) Income Per Common Share
Basic net (loss) income per common share is computed using the weighted average number of common shares outstanding during the period. Diluted net (loss) income per common share is computed in accordance with the treasury stock method, which uses the weighted average number of common shares outstanding during the period and also includes the dilutive effect of potentially issuable common stock from outstanding stock options.
The following table sets out the computation of basic and diluted net (loss) income per common share:
| (In thousands of U.S. dollars, except per share data) |
2011 | 2010 | 2009 | |||||||||
| Numerator: |
||||||||||||
| Loss from continuing operations |
$ | (30,416 | ) | $ | (17,539 | ) | $ | (36,220 | ) | |||
| Income from discontinued operations, net of income taxes |
— | — | 135,654 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (loss) income |
$ | (30,416 | ) | $ | (17,539 | ) | $ | 99,434 | ||||
|
|
|
|
|
|
|
|||||||
| Denominator: (thousands) |
||||||||||||
| Weighted average common shares outstanding |
50,105 | 52,382 | 56,194 | |||||||||
| Effect of dilutive securities: |
||||||||||||
| Stock options |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Diluted weighted average common shares outstanding |
50,105 | 52,382 | 56,194 | |||||||||
|
|
|
|
|
|
|
|||||||
| Basic and diluted net (loss) income per common share: |
||||||||||||
| Continuing operations |
$ | (0.61 | ) | $ | (0.33 | ) | $ | (0.64 | ) | |||
| Discontinued operations |
— | — | 2.41 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net (loss) income |
$ | (0.61 | ) | $ | (0.33 | ) | $ | 1.77 | ||||
|
|
|
|
|
|
|
|||||||
Excluded from the calculation of diluted net (loss) income per common share for the year ended December 31, 2011 were 6,048,197 shares (in 2010 - 6,100,101 shares, in 2009 - 4,833,050 shares) related to stock options because their effect was anti-dilutive.
76
Table of Contents
Fair Value of Financial Assets and Liabilities
The carrying values of cash and cash equivalents, trade receivables and payables, contingent consideration and the mortgage receivable approximate fair value. We estimate the fair value of our financial instruments using the market approach. The fair values of our financial instruments reflect the amounts that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date (exit price).
Advertising and Promotional Expenses
We expense advertising and promotional costs in the period in which they are incurred. For years ended December 31, 2011 and 2010, advertising and promotional expenses related to U.S. Visudyne sales totaled approximately $0.3 million and $0.6 million, respectively. For 2009 and prior years, Novartis was responsible for all marketing costs.
Recently Issued Accounting Standards
In May 2011, the FASB issued ASU 2011-04, Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRSs. ASU No. 2011-04 generally provides a uniform framework for fair value measurements and related disclosures between U.S. GAAP and International Financial Reporting Standards (“IFRS”). Additional disclosure requirements in the update include: (1) for Level 3 fair value measurements, quantitative information about unobservable inputs used, a description of the valuation processes used by the entity, and a qualitative discussion about the sensitivity of the measurements to changes in the unobservable inputs; (2) for an entity’s use of a nonfinancial asset that is different from the asset’s highest and best use, the reason for the difference; (3) for financial instruments not measured at fair value but for which disclosure of fair value is required, the fair value hierarchy level in which the fair value measurements were determined; and (4) the disclosure of all transfers between Level 1 and Level 2 of the fair value hierarchy. ASU 2011-04 will be effective for interim and annual periods beginning on or after December 15, 2011, which for us will be our 2012 first quarter. We are currently evaluating the impact that ASU 2011-04 will have on our financial statements.
Recently Adopted Accounting Standards
In October 2009, the FASB issued ASU 2009-13, Multiple Deliverable Revenue Arrangements. This statement provides principles for allocation of consideration among its multiple-elements, allowing more flexibility in identifying and accounting for separate deliverables under an arrangement. The EITF introduces an estimated selling price method for valuing the elements of a bundled arrangement if vendor-specific objective evidence or third-party evidence of selling price is not available, and significantly expands related disclosure requirements. This standard is effective on a prospective basis for revenue arrangements entered into or materially modified in fiscal years beginning on or after June 15, 2010. Alternatively, adoption may be on a retrospective basis, and early application is permitted. The prospective adoption of this standard on January 1, 2011, did not have a material impact on our financial condition, results of operations or cash flows.
In March 2010, the FASB ratified the EITF final consensus on Issue ASC 2010-17, Milestone Method of Revenue Recognition. The guidance in this consensus allows the milestone method as an acceptable revenue recognition methodology when an arrangement includes substantive milestones. The guidance provides a definition of a substantive milestone and should be applied regardless of whether the arrangement includes single or multiple deliverables or units of accounting. The scope of this consensus is limited to transactions involving milestones relating to research and development deliverables. The guidance includes enhanced disclosure requirements about each arrangement, individual milestones and related contingent consideration, information about substantive milestones and factors considered in the determination. The consensus is effective prospectively to milestones achieved in fiscal years, and interim periods within those years, after June 15, 2010. Early application and retrospective application are permitted. The prospective adoption of this standard on January 1, 2011, did not have a material impact on our financial condition, results of operations or cash flows.
In December 2010, the FASB issued ASU 2010-27, Fees Paid to the Federal Government by Pharmaceutical Manufacturers. ASU 2010-27 provides guidance on the recognition and classification of the annual fee imposed by the Patient Protection and Affordable Care Act as amended by the Health Care and Education Affordability Reconciliation Act on pharmaceutical companies that sell branded prescription drugs or biologics to specified government programs in the United States. Under this guidance, the annual fee should be estimated and recognized in full as a liability upon the first qualifying sale with a corresponding deferred cost that is amortized to operating expense using a straight-line method of allocation unless another method better allocates the fee over the calendar year in which it is payable. The annual fee is not deductible for federal income tax purposes. This guidance became effective for calendar years beginning after December 31, 2010. The prospective adoption of this standard on January 1, 2011, did not have a material impact on our financial condition, results of operations or cash flows.
In June 2011, the FASB issued ASU 2011-05, Presentation of Comprehensive Income, which amends existing guidance by allowing only two options for presenting the components of net income and other comprehensive income: (1) in a single continuous financial statement, statement of comprehensive income or (2) in two separate but consecutive financial statements, consisting of an income statement followed by a separate statement of other comprehensive income. Also, items that are reclassified from other comprehensive income to net income must be presented on the face of the financial statements. In December 2011, the FASB issued ASU No. 2011-12, Deferral of the Effective Date for Amendments to the Presentation of Reclassifications of Items Out of Accumulated Other Comprehensive Income in Accounting Standards Update No. 2011-05, which defers the requirement within ASU 2011-05 to present on the face of the financial
77
Table of Contents
statements the effects of reclassifications out of accumulated other comprehensive income on the components of net income and other comprehensive income for all periods presented. During the deferral, entities should continue to report reclassifications out of accumulated other comprehensive income consistent with the presentation requirements in effect prior to the issuance of ASU 2011-05. These ASU’s require retrospective application, and are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011 (for us this will be our 2012 first quarter), with early adoption permitted. Our adoption of these standards in the fourth quarter of 2011 did not have a material impact on our financial condition, results of operations or cash flows.
4. ACCOUNTS RECEIVABLE
| (In thousands of U.S. dollars) |
2011 | 2010 | ||||||
| Visudyne |
$ | 8,877 | $ | 10,139 | ||||
| Trade and other |
1,108 | 520 | ||||||
|
|
|
|
|
|||||
| $ | 9,985 | $ | 10,659 | |||||
|
|
|
|
|
|||||
At December 31, 2011 and 2010, accounts receivable related to Visudyne represents amounts due from our distributors in the U.S. for sale of bulk Visudyne to them, and amounts due from Novartis relating to the 20% royalties due on net sales outside the U.S. as well as reimbursement of specified royalty and other costs. Our principal U.S. wholesale distributor of Visudyne is ASD Speciality Healthcare, Inc. and our other distributor is Priority Healthcare Distribution, Inc.
5. INVENTORIES
| (In thousands of U.S. dollars) |
2011 | 2010 | ||||||
| Raw materials and supplies |
$ | 50 | $ | 74 | ||||
| Work-in-process |
26,098 | 28,045 | ||||||
| Finished goods |
171 | 274 | ||||||
| Provision for excess inventory |
(11,077 | ) | (11,077 | ) | ||||
| Provision for non-completion of inventory |
(2,185 | ) | (2,185 | ) | ||||
|
|
|
|
|
|||||
| $ | 13,057 | $ | 15,131 | |||||
| Long-term inventory (See Note 9) |
(11,119 | ) | (11,807 | ) | ||||
|
|
|
|
|
|||||
| Current inventory |
$ | 1,938 | $ | 3,324 | ||||
|
|
|
|
|
|||||
Our provision for excess inventory of $11.1 million, which was substantially applied against our long-term inventory, has been determined based on our forecast of future Visudyne demand. During the year ended December 31, 2011, there was no charge against this provision.
During the years ended December 31, 2011 and 2010, there were no charges against the provision for non-completion of product inventory.
6. MORTGAGE RECEIVABLE
Under the terms of the original mortgage agreement, our mortgage receivable was due on August 29, 2010 and comprised a two-year, 6.5% interest-only, second mortgage in the amount of CAD $12.0 million related to the sale of our land and building to Discovery Parks Holdings Ltd., an affiliate of Discovery Parks Trust (“Discovery Parks”), in 2008. Effective August 29, 2010, we entered into an amended mortgage agreement with Discovery Parks, pursuant to which we received payment of CAD $4.0 million on August 30, 2010. The remaining mortgage receivable of CAD $8.0 million comprised a 7.5% interest-only second mortgage, of which CAD $1.0 million was received in each of the first and second quarters of 2011 and the remaining $6.0 million was received in the first quarter of 2012.
78
Table of Contents
7. PROPERTY, PLANT AND EQUIPMENT
| 2011 | ||||||||||||
| Accumulated | Net | |||||||||||
| (In thousands of U.S. dollars) |
Cost | Depreciation | Book Value | |||||||||
| Leasehold improvements |
$ | 484 | $ | 205 | $ | 279 | ||||||
| Office furnishings, fixtures, and other |
551 | 177 | 374 | |||||||||
| Research equipment |
5,031 | 1,914 | 3,117 | |||||||||
| Commercial manufacturing equipment |
2,277 | 2,233 | 44 | |||||||||
| Computer hardware and operating system |
13,300 | 12,383 | 917 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 21,643 | $ | 16,912 | $ | 4,731 | |||||||
|
|
|
|
|
|
|
|||||||
| 2010 | ||||||||||||
| Accumulated | Net | |||||||||||
| (In thousands of U.S. dollars) |
Cost | Depreciation | Book Value | |||||||||
| Leasehold improvement |
$ | 460 | $ | 110 | $ | 350 | ||||||
| Office furnishings, fixtures, and other |
352 | 103 | 249 | |||||||||
| Research equipment |
2,494 | 1,281 | 1,213 | |||||||||
| Commercial manufacturing equipment |
2,320 | 2,239 | 81 | |||||||||
| Computer hardware and operating system |
12,957 | 11,815 | 1,142 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 18,583 | $ | 15,548 | $ | 3,035 | |||||||
|
|
|
|
|
|
|
|||||||
8. ACQUISITIONS AND DIVESTITURES
Acquisition of OT-730
On December 30, 2009, we acquired intellectual property related to QLT091568 (formerly OT-730), including patent applications and other intellectual property, FDA filings, data, and inventory, from privately-held Othera Pharmaceuticals, Inc. and its wholly-owned subsidiary, Othera Holding, Inc. for a one-time cash payment of $7.5 million. The purchase of QLT091568 did not qualify as a business in accordance with ASC 805. QLT091568, a type of beta blocker under investigation for the treatment of glaucoma, had not reached technological feasibility and did not have an alternative future use when acquired. Accordingly, we allocated the aggregate consideration of $7.5 million to IPR&D and charged the amount to expense. We discontinued development of QLT091568 in October 2010.
Sale of QLT USA
On October 1, 2009, we divested our Eligard product line as part of the sale of all of the shares of our U.S. subsidiary, QLT USA, for up to an aggregate $230.0 million, plus cash on hand of $118.3 million. See Note 15 – Discontinued Operations.
9. LONG-TERM INVENTORIES AND OTHER ASSETS
| (In thousands of U.S. dollars) |
2011 | 2010 | ||||||
| Inventory, net of provisions (See Note 5) |
$ | 11,119 | $ | 11,807 | ||||
| Other |
927 | 1,512 | ||||||
|
|
|
|
|
|||||
| $ | 12,046 | $ | 13,319 | |||||
|
|
|
|
|
|||||
79
Table of Contents
10. ACCRUED LIABILITIES
| (In thousands of U.S. dollars) |
2011 | 2010 | ||||||
| Royalties |
$ | 1,122 | $ | 1,225 | ||||
| Compensation |
4,756 | 3,780 | ||||||
| DDSU compensation |
1,801 | 1,400 | ||||||
|
|
|
|
|
|||||
| $ | 7,679 | $ | 6,405 | |||||
|
|
|
|
|
|||||
11. FOREIGN EXCHANGE FACILITY
We have a foreign exchange facility for the sole purpose of entering into foreign exchange contracts. The facility allows us to enter into a maximum of $50.0 million in spot or forward foreign exchange contracts for terms up to 15 months. The facility requires security in the form of cash or money market instruments based on the contingent credit exposure for any outstanding foreign exchange transactions. At December 31, 2011 and 2010, there was no collateral pledged as security for this facility, as we had no outstanding foreign exchange transactions.
12. SHARE CAPITAL
| (a) | Authorized Shares |
There were no changes to the authorized share capital of QLT for the years ended December 31, 2011 and 2010.
| (b) | Share Buy-Back Programs |
On December 8, 2010, we announced that our Board of Directors authorized the repurchase of up to 3,615,285 of our issued and outstanding common shares, being 10% of our public float as of December 9, 2010, over a 12-month period commencing December 16, 2010 under a normal course issuer bid. All purchases were effected in the open market through the facilities of the NASDAQ, and in accordance with regulatory requirements. All common shares repurchased were cancelled. Total purchases under this program were 2,700,817 common shares at an average price of $6.99 per share, for a total cost of $18.9 million.
On October 27, 2009, we announced that our Board of Directors authorized the repurchase of up to 2,731,534 of our common shares, being 5% of our issued and outstanding common shares, over a 12-month period commencing November 3, 2009 under a normal course issuer bid. In May 2010, the normal course issuer bid was increased to repurchase up to 4,700,060 shares, representing 10% of our public float. All purchases under the bid were effected in the open market through the facilities of the NASDAQ, and in accordance with all regulatory requirements. Total purchases under this program were 3,744,972 shares at an average price of $5.59 per share, for a total cost of $20.9 million.
We repurchased 2,678,517 common shares at a cost of $18.7 million during 2011, compared to 2,900,482 common shares at a cost of $17.1 million during 2010 (not including stock repurchase fees for both years). We retired all of these shares as they were acquired. In connection with this retirement, we recorded an increase in additional paid-in capital of $6.3 million in 2011 and $10.0 million in 2010.
| (c) | Stock Options |
We currently maintain one equity compensation plan, the QLT 2000 Incentive Stock Option Plan (“QLT Plan”), which provides for the issuance of common shares to directors, officers, employees and consultants of QLT and its affiliates. The QLT Plan was amended and restated with shareholder approval effective May 5, 2009. No financial assistance is provided by us to the participants under the QLT Plan. Below is a summary of the principal terms of the QLT Plan:
Share Reserve. We have reserved an aggregate of 7.8 million common shares for issuance under the QLT Plan. Common shares with respect to options that are not exercised in full will be available for grant under subsequent options under the plan. In addition, the number of common shares reserved for issuance to any one person shall not, in the aggregate, exceed five percent of the issued and outstanding common shares (on a non-diluted basis). The Compensation Committee of our Board of Directors will not grant options to a director who is not an employee or executive of QLT where such grant will result in such director being awarded options with an aggregate grant value in excess of CAD $100,000 in any one year. At December 31, 2011, options to purchase an aggregate total of 6.0 million common shares were outstanding under the QLT Plan and exercisable in the future at prices ranging between CAD $2.44 and CAD $8.48 per common share.
80
Table of Contents
Administration. The Compensation Committee administers the QLT Plan.
Eligibility. The directors, officers, employees and consultants of QLT or our affiliated companies, who are or will be, in the opinion of the Compensation Committee, important for our growth and success and whose participation in the QLT Plan will, in the opinion of the Compensation Committee, accomplish the purposes of the QLT Plan, are eligible to participate in the QLT Plan.
Grant and Exercise of Options. Subject to the terms of the QLT Plan, the Compensation Committee may grant to any eligible person one or more options as it deems appropriate. The Compensation Committee may also impose such limitations or conditions on the exercise or vesting of any option as it deems appropriate.
An option will expire automatically on the earlier of (i) the date on which such option is exercised in respect of all of the common shares that may be purchased under the QLT Plan, and (ii) the date fixed by the Compensation Committee as the expiry date of such option, which date will not be more than five years from the date of grant. Options that would otherwise expire during “black out” periods established by QLT will not expire until the tenth business day after the earlier of the end of such black out period or, provided the black out period has ended, the expiry date.
Early termination of stock options in the event of termination of service, death or disability are subject to the specific terms of each applicable option agreement.
Exercise Price. The exercise price of options granted will be determined by the Compensation Committee, but will in no event be less than the closing price on the Toronto Stock Exchange immediately preceding the grant.
Vesting. Vesting of options occurs monthly over 36 months, subject to such altered vesting schedules as the Compensation Committee or our Board may determine. For senior managers, executive officers and directors and excluding some situations of retirement and service periods over 20 years, 50% of any unvested options vest on the termination of employment without cause, and 100% of unvested options vest on the occurrence of a change of control.
Transferability. No option may be transferred or assigned except by will or by operation of the laws of devolution or distribution and descent or pursuant to a qualified domestic relations order, as defined by the U.S. Internal Revenue Code of 1986 and may be exercised only by an optionee during his or her lifetime.
Amendments or Termination. The QLT Plan will terminate on March 1, 2019 or such earlier date as the Compensation Committee determines. The Compensation Committee has the right at any time to suspend or terminate the QLT Plan. The QLT Plan explicitly specifies: (i) the type of amendments that can be made to the QLT Plan by the Compensation Committee without the approval of the shareholders, and (ii) the type of amendments that the Compensation Committee may not make to the QLT Plan or any specific option grant without shareholder or applicable optionee, as the case may be, approval. Amendments that may be made without shareholder approval include altering vesting and termination provisions or the mechanics of exercise, changing the persons eligible for stock options and effecting formal minor or technical modifications or corrections to the QLT Plan.
81
Table of Contents
Stock option activity with respect to our QLT Plan is presented below.
| (In CAD dollars) |
Number of Options | Weighted Average Exercise Price |
Weighted Average Remaining Contractual Term (Years) |
|||||||||
| Outstanding at January 1, 2009 |
5,086,487 | $ | 7.95 | |||||||||
| Granted |
1,338,500 | 2.85 | ||||||||||
| Exercised |
(16,197 | ) | 3.35 | |||||||||
| Forfeited and expired |
(581,978 | ) | 17.49 | |||||||||
|
|
|
|
|
|||||||||
| Outstanding at December 31, 2009 |
5,826,812 | $ | 5.84 | |||||||||
| Granted |
1,390,600 | 6.26 | ||||||||||
| Exercised |
(265,585 | ) | 3.56 | |||||||||
| Forfeited and expired |
(851,726 | ) | 9.74 | |||||||||
|
|
|
|
|
|||||||||
| Outstanding at December 31, 2010 |
6,100,101 | $ | 5.49 | |||||||||
| Granted |
1,430,900 | 7.19 | ||||||||||
| Exercised |
(451,867 | ) | 4.98 | |||||||||
| Forfeited and expired |
(1,030,937 | ) | 8.36 | |||||||||
|
|
|
|
|
|
|
|||||||
| Outstanding at December 31, 2011 |
6,048,197 | $ | 5.44 | 2.44 | ||||||||
| Exercisable at December 31, 2011 |
4,246,394 | $ | 4.99 | 1.81 | ||||||||
Stock option activity with respect to all other previous option plans of the Company are presented below:
| (In U.S. dollars) |
Number of Options | Weighted Average Exercise Price |
||||||
| Outstanding at January 1, 2009 |
255,207 | $ | 9.52 | |||||
| Granted |
— | — | ||||||
| Exercised |
(19,554 | ) | 3.78 | |||||
| Forfeited and expired |
(157,182 | ) | 10.94 | |||||
|
|
|
|
|
|||||
| Outstanding at December 31, 2009 |
78,471 | $ | 8.13 | |||||
| Granted |
— | — | ||||||
| Exercised |
— | — | ||||||
| Forfeited and expired |
(78,471 | ) | 8.13 | |||||
|
|
|
|
|
|||||
| Outstanding at December 31, 2010 |
— | $ | — | |||||
|
|
|
|
|
|||||
As of December 31, 2011, we maintain one equity compensation plan, the QLT Plan. The number of options issued and outstanding under the QLT Plan was 12% of the issued and outstanding common shares.
We used the Black-Scholes option pricing model to estimate the value of the options at each grant date, using the following weighted average assumptions:
82
Table of Contents
| 2011 | 2010 | 2009 | ||||||||||
| Annualized volatility |
48.8 | % | 53.8 | % | 53.8 | % | ||||||
| Risk-free interest rate |
2.1 | % | 2.3 | % | 1.8 | % | ||||||
| Expected life (years) |
3.7 | 3.7 | 3.6 | |||||||||
| Dividends |
— | — | — | |||||||||
The Black-Scholes option pricing model was developed for use in estimating the value of traded options that have no vesting restrictions and are fully transferable. In addition, option pricing models require the input of highly subjective assumptions including the expected stock price volatility. We project expected volatility and expected life of our stock options based upon historical and other economic data trended into future years. The risk-free interest rate assumption is based upon observed interest rates appropriate for the terms of our stock options.
The weighted average grant date fair value of stock options granted during the years ended December 31, 2011, 2010 and 2009 was as follows:
| (In CAD dollars) |
2011 | 2010 | 2009 | |||||||||
| Weighted average grant date fair value of stock options granted |
$ | 2.78 | $ | 2.62 | $ | 1.18 | ||||||
The aggregate intrinsic value of options outstanding and exercisable at December 31, 2011, 2010 and 2009 was as follows:
| (In thousands of CAD dollars) |
2011 | 2010 | 2009 | |||||||||
| Aggregate intrinsic value of options outstanding |
$ | 12,406 | $ | 13,458 | $ | 6,154 | ||||||
| Aggregate intrinsic value of options exercisable |
10,903 | 8,533 | 2,187 | |||||||||
The intrinsic value of stock options exercised and the related cash received from exercise of stock options during the years ended December 31, 2011, 2010 and 2009 was as follows:
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Intrinsic value of stock options exercised |
$ | 1,022 | $ | 687 | $ | 42 | ||||||
| Cash from exercise of stock options |
2,295 | 910 | 125 | |||||||||
At December 31, 2011, total unrecognized compensation cost related to non-vested stock options granted prior to that date was $4.2 million, which is expected to be recognized over 36 months with a weighted-average period of 1.9 years. Upon option exercise, we issue new shares of stock. The total share-based compensation cost of stock options capitalized as part of inventory for the years ended December 31, 2011, 2010 and 2009 was negligible.
The impact on our results of operations of recording stock option compensation for the years ended December 31, 2011, 2010, and 2009 was as follows:
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Cost of sales |
$ | 101 | $ | 163 | $ | 31 | ||||||
| Research and development |
1,470 | 1,308 | 939 | |||||||||
| Selling, general and administrative |
1,402 | 1,023 | 1,208 | |||||||||
| Discontinued operations |
— | — | (17 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Share option compensation expense |
2,973 | 2,494 | 2,161 | |||||||||
| Related income tax benefits |
(162 | ) | (136 | ) | (48 | ) | ||||||
| (d) | Deferred Share Units |
We have a DDSU Plan for non-employee directors. Under the DDSU Plan, at the discretion of the Board of Directors, non-employee directors can receive all or a percentage of their equity-based compensation in the form of DSUs, each of which has a value equal to the closing price of QLT’s common shares on the Toronto Stock Exchange on the trading day immediately prior to the Board approval of
83
Table of Contents
the grant. A vested DSU is convertible into cash only (no share is issued), and is automatically converted after the non-employee director ceases to be a member of the Board unless the director is removed from the Board for just cause. The DSUs vest in equal amounts over 36 months beginning on the first day of the first month after the grant date. The value of a vested DSU, when converted to cash, is equivalent to the closing price of a QLT common share on the date the conversion takes place.
DSU activity is presented below.
| Number of Options | ||||
| Outstanding at January 1, 2009 |
157,500 | |||
| Granted |
52,500 | |||
| Redeemed |
— | |||
| Cancelled |
— | |||
| Outstanding at December 31, 2009 |
210,000 | |||
| Granted |
60,000 | |||
| Redeemed |
— | |||
| Cancelled |
— | |||
| Outstanding at December 31, 2010 |
270,000 | |||
| Granted |
60,000 | |||
| Redeemed |
— | |||
| Cancelled |
— | |||
| Outstanding at December 31, 2011 |
330,000 | |||
| Vested at December 31, 2011 |
250,937 | |||
The obligation to pay the cash amount is recorded as a liability in our financial statements and is marked-to-market in each reporting period. See Note 4 – Accrued Liabilities. No cash payment for the DDSU Plan was made for the years ended December 31, 2011, 2010 and 2009. The impact on our results of operations of recording DSU compensation for the years ended December 31, 2011, 2010 and 2009 was as follows:
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Research and development |
$ | 120 | $ | 173 | $ | 194 | ||||||
| Selling, general and administrative |
322 | 457 | 254 | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred share unit compensation expense |
$ | 442 | $ | 630 | $ | 448 | ||||||
|
|
|
|
|
|
|
|||||||
13. NET PRODUCT REVENUE
Under the terms of the Amended PDT Agreement with Novartis, on January 1, 2010, we received from Novartis the exclusive U.S. rights to the Visudyne patents to sell and market Visudyne in the U.S. In the year ended December 31, 2010, previously deferred revenue of $5.0 million related to inventory previously shipped to Novartis for sales outside the U.S. was recognized as revenue.
Net product revenue for the years ended December 31, 2011 and 2010 was determined as follows:
| (In thousands of U.S. dollars) |
2011 | 2010 | ||||||
| U.S. Visudyne sales by QLT |
$ | 21,022 | $ | 22,597 | ||||
| Visudyne sales to Novartis |
5,924 | 6,988 | ||||||
| Add: Royalties and other costs reimbursed to QLT |
1,430 | 1,508 | ||||||
|
|
|
|
|
|||||
| Net product revenue from Visudyne sales |
$ | 28,376 | $ | 31,093 | ||||
|
|
|
|
|
|||||
84
Table of Contents
Net product revenue for the year ended December 31, 2009 was determined as follows:
| (In thousands of U.S. dollars) |
2009 | |||
| Visudyne sales by Novartis |
$ | 105,657 | ||
| Less: Marketing and distribution costs |
(34,346 | ) | ||
| Less: Inventory costs |
(6,031 | ) | ||
| Less: Royalties to third parties |
(2,276 | ) | ||
|
|
|
|||
| Novartis’ net proceeds from Visudyne sales |
$ | 63,004 | ||
|
|
|
|||
| QLT’s 50% share of Novartis’ net proceeds from Visudyne sales |
$ | 31,502 | ||
| Add: Advance on inventory costs from Novartis |
5,608 | |||
| Add: Royalties reimbursed to QLT |
2,272 | |||
| Add: Other costs reimbursed to QLT |
2,724 | |||
|
|
|
|||
| Revenue from Visudyne sales |
$ | 42,106 | ||
|
|
|
|||
The tables below summarize end-user Visudyne sales for the years ended December 31, 2011, 2010 and 2009. Beginning January 1, 2010, under the Amended PDT Agreement, Visudyne is sold by QLT in the U.S., and by Novartis outside the U.S. (for which we earn a 20% royalty on net sales). Prior to 2010, Visudyne was sold solely by Novartis on a worldwide basis, and our revenue was derived from our 50% share of Novartis’ net proceeds from Visudyne sales.
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| U.S. |
$ | 21,022 | $ | 22,597 | $ | 30,809 | ||||||
| Europe |
25,528 | 26,243 | 29,639 | |||||||||
| Rest of World |
43,729 | 41,778 | 45,209 | |||||||||
|
|
|
|
|
|
|
|||||||
| Worldwide |
$ | 90,279 | $ | 90,618 | $ | 105,657 | ||||||
|
|
|
|
|
|
|
|||||||
| 2011 | 2010 | 2009 | ||||||||||
| U.S. |
23 | % | 25 | % | 29 | % | ||||||
| Europe |
28 | % | 29 | % | 28 | % | ||||||
| Rest of World |
49 | % | 46 | % | 43 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Worldwide |
100 | % | 100 | % | 100 | % | ||||||
|
|
|
|
|
|
|
|||||||
14. INCOME TAXES
Loss from continuing operations before income taxes was as follows:
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Canada |
$ | (28,931 | ) | $ | 15,330 | $ | (15,624 | ) | ||||
| United States and other |
1,329 | (21,968 | ) | (25,913 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Loss from continuing operations before income taxes |
$ | (27,602 | ) | $ | (6,638 | ) | $ | (41,537 | ) | |||
|
|
|
|
|
|
|
|||||||
The components of the (provision for) recovery of income taxes were as follows:
85
Table of Contents
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Canada |
$ | (2,748 | ) | $ | (11,501 | ) | $ | 5,156 | ||||
| United States and other |
(66 | ) | 600 | 161 | ||||||||
|
|
|
|
|
|
|
|||||||
| (Provision for) recovery of income taxes |
$ | (2,814 | ) | $ | (10,901 | ) | $ | 5,317 | ||||
|
|
|
|
|
|
|
|||||||
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Current income taxes |
$ | 857 | $ | 1,971 | $ | 530 | ||||||
| Deferred income taxes |
(3,671 | ) | (12,872 | ) | 4,787 | |||||||
|
|
|
|
|
|
|
|||||||
| (Provision for) recovery of income taxes |
$ | (2,814 | ) | $ | (10,901 | ) | $ | 5,317 | ||||
|
|
|
|
|
|
|
|||||||
Differences between our statutory income tax rates and our effective income tax rates applied to the pre-tax income consisted of the following:
| (In thousands of U.S. dollars, except as noted) |
2011 | 2010 | 2009 | |||||||||
| Loss from continuing operations before income taxes |
$ | (27,602 | ) | $ | (6,638 | ) | $ | (41,537 | ) | |||
| Canadian statutory tax rates |
26.50 | % | 28.50 | % | 30.00 | % | ||||||
|
|
|
|
|
|
|
|||||||
| Expected income tax recovery |
$ | 7,315 | $ | 1,892 | $ | 12,461 | ||||||
| Net increase in valuation allowance |
(12,817 | ) | (22,791 | ) | (11,179 | ) | ||||||
| Non-taxable portion of capital gains |
1,401 | 5,850 | 849 | |||||||||
| Tax (cost) recovery of undistributed earnings of affiliates |
— | (82 | ) | 1,613 | ||||||||
| Foreign tax rate differences |
(238 | ) | 2,511 | 2,537 | ||||||||
| Investment tax credits |
3,278 | 2,904 | 1,626 | |||||||||
| Stock options |
(778 | ) | (574 | ) | (547 | ) | ||||||
| Future tax rate reductions |
(583 | ) | (427 | ) | (1,385 | ) | ||||||
| Other |
(392 | ) | (184 | ) | (658 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| (Provision for) recovery of income taxes |
$ | (2,814 | ) | $ | (10,901 | ) | $ | 5,317 | ||||
|
|
|
|
|
|
|
|||||||
The $2.8 million provision for income taxes relates primarily to a drawdown of the contingent consideration tax asset associated with the current period gain on the fair value change, income taxes associated with our mix of income allocable to our activities in the U.S., as well as the reversal of a prepaid tax asset set up at December 31, 2010 in relation to profits on intercompany sales that had not been sold to third parties at that time. The latter is not expected to be a recurring item in the future.
86
Table of Contents
Deferred tax assets and liabilities
The tax effects of temporary differences that give rise to significant components of the deferred income tax assets and deferred income tax liabilities are presented below:
| (In thousands of U.S. dollars) |
2011 | 2010 | ||||||
| Deferred tax assets |
||||||||
| Net operating loss carryforwards |
$ | 24,547 | $ | 14,927 | ||||
| Contingent consideration |
1,739 | 3,073 | ||||||
| Research and development tax credit carryforwards |
6,150 | 4,978 | ||||||
| Capital loss carryforwards |
35,723 | 35,699 | ||||||
| Depreciable and amortizable assets |
10,090 | 11,037 | ||||||
| Provision for excess and non-completion of inventory |
3,315 | 3,315 | ||||||
| Other temporary differences |
1,602 | 1,660 | ||||||
|
|
|
|
|
|||||
| Total gross deferred income tax assets |
$ | 83,166 | $ | 74,689 | ||||
| Less: valuation allowance |
(80,465 | ) | (68,346 | ) | ||||
|
|
|
|
|
|||||
| Total deferred income tax assets |
$ | 2,701 | $ | 6,343 | ||||
| Less: current portion |
(1,351 | ) | (3,643 | ) | ||||
|
|
|
|
|
|||||
| Net long-term portion of deferred income tax assets |
$ | 1,350 | $ | 2,700 | ||||
|
|
|
|
|
|||||
| Deferred tax liabilities |
||||||||
| Tax cost of undistributed earnings |
(27 | ) | (82 | ) | ||||
|
|
|
|
|
|||||
| Total deferred income tax liabilities |
$ | (27 | ) | $ | (82 | ) | ||
|
|
|
|
|
|||||
| Net deferred income tax assets |
$ | 2,674 | $ | 6,261 | ||||
|
|
|
|
|
|||||
At December 31, 2011, our valuation allowance increased primarily because there was insufficient evidence to support current or future realization of the tax benefits associated with our current period development expenditures as well as certain other expenditures. The valuation allowance is reviewed periodically and if the assessment of the “more likely than not” criterion changes, the valuation allowance is adjusted accordingly. There may be limitations on the utilization of our accumulated net operating losses and federal and state tax credit carryforwards as a result of changes in control that have occurred. There may also be an inability to utilize a significant amount of our accumulated net operating losses, capital loss carryforwards and federal and state tax credit carryforwards to the extent future changes in control occur for tax purposes.
At December 31, 2011, we had approximately $75.4 million of total operating loss carryforwards, $40.1 million of which relate to Canada and the balance of $35.3 million relate to our U.S. subsidiaries. The loss carryforwards expire at various dates through 2031. We also had approximately $6.2 million of federal and state research and development credits available for carryforward of which approximately $1.2 million were generated by our U.S. subsidiaries. The research and development credit carryforwards expire at various dates through 2031. We also had approximately $282.7 million of capital loss carryforwards which carryforward indefinitely. The deferred tax benefit of these loss carryforwards and research and development credits is ultimately subject to final determination by taxation authorities.
87
Table of Contents
The following table summarizes the activity related to our unrecognized tax benefits:
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Balance as at January 1 |
$ | 1,687 | $ | 1,489 | $ | 766 | ||||||
| Increases related to current year tax positions |
— | — | 365 | |||||||||
| Changes in tax positions of a prior period |
45 | 198 | 358 | |||||||||
| Settlements |
— | — | — | |||||||||
| Lapse of Statute of Limitations |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Balance as at December 31 |
$ | 1,732 | $ | 1,687 | $ | 1,489 | ||||||
|
|
|
|
|
|
|
|||||||
The total amount of unrecognized tax benefits at December 31, 2011 that, if recognized, would not impact the effective tax rate is $1.7 million.
We recognize potential accrued interest and penalties related to unrecognized tax benefits within our income tax provision. Only an inconsequential amount of interest and penalties has been accrued and is included as a component of the uncertain tax position liabilities.
We do not currently expect any significant increases or decreases to our unrecognized tax benefits within 12 months of the reporting date.
QLT Inc. and its subsidiaries file income tax returns and pay income taxes in jurisdictions where we believe we are subject to tax. In jurisdictions in which QLT Inc. and its subsidiaries do not believe we are subject to tax and therefore do not file income tax returns, we can provide no certainty that tax authorities in those jurisdictions will not subject one or more tax years (since inception of QLT Inc. or its subsidiaries) to examination. Further, while the statute of limitations in each jurisdiction where an income tax return has been filed generally limits the examination period, as a result of loss carryforwards, the limitation period for examination generally does not expire until several years after the loss carryforwards are utilized. Other than routine audits by tax authorities for tax credits and tax refunds that we claim, we are not aware of any other material income tax examination currently in progress by any taxing jurisdiction. Our major tax jurisdictions are Canada and the U.S. With few exceptions, QLT Inc. and its subsidiaries should not be subject to Canadian income tax examinations in respect of taxation years before 2007 and U.S. income tax examinations in respect of taxation years before 2008.
15. DISCONTINUED OPERATIONS
On October 1, 2009, we divested the Eligard product line as part of the sale of all of the shares of our U.S. subsidiary, QLT USA, to Tolmar for up to an aggregate $230.0 million plus cash on hand of $118.3 million. Pursuant to the stock purchase agreement, we received $20.0 million on closing and $10.0 million on October 1, 2010 and we are entitiled to future consideration payable on a quarterly basis in amounts equal to 80% of the royalties paid under the license agreements with Sanofi Synthelabo Inc. for the commercial marketing of Eligard in the U.S. and Canada, and the license agreement with MediGene Aktiengesellschaft which, effective March 1, 2011, has been assigned to Astellas Pharma Europe Ltd. for the commercial marketing of Eligard in Europe. The estimated fair value of these expected future quarterly payments is reflected as Contingent Consideration on our Consolidated Balance Sheet. We are entitled to these payments until the earlier of our receipt of $200.0 million or October 1, 2024. As of December 31, 2011, we had received an aggregate $86.1 million of contingent consideration. We expect to receive the remaining $113.9 million on a quarterly basis, over the next three to four years. The contingent consideration payments are not generated from a migration or continuation of activities and therefore are not direct cash flows of the divested business. We have not had any continuing involvement with this business following its sale.
We recognized a pre-tax gain of $107.4 million related to this transaction. The assets sold included $31.7 million of income tax assets, $14.9 million of accounts receivable, $10.6 million of inventory, $1.3 million of fixed and other assets, and $23.1 million of goodwill. Liabilities of $7.3 million were assumed by Tolmar. See Note 16 - Financial Instruments and Concentration of Credit Risk for further information on fair value of contingent consideration.
In accordance with the accounting standard for discontinued operations, the results of operations related to QLT USA have been excluded from continuing operations and reported as discontinued operations for 2009.
88
Table of Contents
Operating results of QLT USA included in discontinued operations are summarized as follows:
| (In thousands of U.S. dollars) |
January 1, 2009
- October 1, 2009 |
|||
| Net product revenue |
$ | 28,625 | ||
| Royalties |
32,148 | |||
|
|
|
|||
| 60,773 | ||||
|
|
|
|||
| Operating income |
29,174 | |||
| Other gains |
1,828 | |||
| Gain on sale of discontinued operations |
107,363 | |||
|
|
|
|||
| Income for discontinued operations before income taxes |
138,365 | |||
| Provision for income taxes |
(2,711 | ) | ||
|
|
|
|||
| Net income from discontinued operations |
$ | 135,654 | ||
|
|
|
|||
16. FINANCIAL INSTRUMENTS AND CONCENTRATION OF CREDIT RISK
We have various financial instruments that must be measured under the fair value standard including cash and cash equivalents, the mortgage receivable and, from time to time, forward currency contracts. The mortgage is recorded as a receivable and is carried at amortized cost. Based on market information, the book value of our mortgage receivable approximates fair value. We received full payment of the mortgage receivable in the first quarter of 2012. Our financial assets and liabilities are measured using inputs from the three levels of the fair value hierarchy.
The following tables provide information about our assets and liabilities that are measured at fair value on a recurring basis as of December 31, 2011 and 2010 and indicate the fair value hierarchy of the valuation techniques we utilized to determine such fair value:
| Carrying Value December 31, |
Fair Value Measurements at December 31, 2011 | |||||||||||||||
| (In thousands of U.S .dollars) |
2011 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Assets: |
||||||||||||||||
| Cash and cash equivalents |
$ | 205,597 | $ | 205,597 | $ | — | $ | — | ||||||||
| Contingent consideration(1) |
99,947 | — | — | 99,947 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 305,544 | $ | 205,597 | $ | — | $ | 99,947 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Carrying Value December 31, |
Fair Value Measurements at December 31, 2010 | |||||||||||||||
| (In thousands of U.S .dollars) |
2010 | Level 1 | Level 2 | Level 3 | ||||||||||||
| Assets: |
||||||||||||||||
| Cash and cash equivalents |
$ | 209,478 | $ | 209,478 | $ | — | $ | — | ||||||||
| Contingent consideration(1) |
130,589 | — | — | 130,589 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 340,067 | $ | 209,478 | $ | — | $ | 130,589 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | To estimate the fair value of contingent consideration at December 31, 2011 and 2010, we used a discounted cash flow model based on estimated timing and amount of future cash flows, discounted using a cost of capital of 9.0% determined by management after considering all available market and industry information. Future cash flows were estimated by utilizing external market research to estimate market size, to which we applied market share, pricing and foreign exchange assumptions based on historical sales data, expected competition and current exchange rates. If the discount rate were to increase by 1%, the contingent consideration at December 31, 2011 would decrease by $1.3 million, from $99.9 million to $98.6 million. If estimated future sales of Eligard were to decrease by 10%, the contingent consideration at December 31, 2011 would decrease by $1.2 million, from $99.9 million to $98.7 million. |
89
Table of Contents
The following table represents a reconciliation of our asset (contingent consideration) measured and recorded at fair value on a recurring basis, using significant unobservable inputs (Level 3):
| (In thousands of U.S. dollars) |
Level 3 | |||
| Balance at January 1, 2010 |
$ | 151,078 | ||
| Transfers to Level 3 |
— | |||
| Settlements |
(36,982 | ) | ||
| Fair value change in contingent consideration |
16,493 | |||
|
|
|
|||
| Balance at December 31, 2010 |
$ | 130,589 | ||
| Transfers to Level 3 |
— | |||
| Settlements |
(40,720 | ) | ||
| Fair value change in contingent consideration |
10,078 | |||
|
|
|
|||
| Balance at December 31, 2011 |
$ | 99,947 | ||
|
|
|
|||
We purchase goods and services primarily in U.S. dollars and Canadian dollars, and earn most of our revenues in U.S. dollars.
At December 31, 2011 and 2010, we had no outstanding forward foreign currency contracts. The net unrealized loss in respect of foreign currency contracts for the year ended December 31, 2009 was approximately $0.1 million which was included in our results of operations.
Other financial instruments that potentially subject us to concentration of credit risk include our cash, cash equivalents, accounts receivable, contingent consideration, and mortgage receivable. To limit our credit exposure in regards to cash and cash equivalents, we deposit our cash with high quality financial institutions and the primary goals of our treasury policy are capital preservation and liquidity. Our treasury policy limits investments to certain money market securities issued by governments, financial institutions and corporations with investment-grade credit ratings, and places restrictions on maturities and concentration by issuer.
In the U.S., we rely on ASD Specialty Healthcare, Inc. (our principal U.S. wholesale distributor of Visudyne) and Priority Healthcare Distribution, Inc. to distribute Visudyne to end-user customers. If we are unable to continue to secure and maintain the necessary agreements with our wholesale distributors to accomplish this in a timely manner on terms favorable to us or at all, supply of Visudyne in the U.S. may be adversely affected. Our accounts receivable, as at December 31, 2011, comprised approximately 38% (2010 - 38%) owing from Novartis, approximately 44% (2010 - 48%) owing from ASD Specialty Healthcare, Inc. and approximately 7% (2010 - 8%) owing from Priority Healthcare Distribution, Inc. We have a high degree of customer concentration in our business. Novartis accounted for 50% of our total revenues and our two wholesale distributors accounted for the other 50% of our total revenues.
17. COMMITMENTS AND GUARANTEES
In conjunction with the sale of our land and building on August 29, 2008, we entered into a five-year operating lease with Discovery Parks for office and laboratory space. We have two options to renew this lease for an additional five years each, at the greater of the current contractual lease rate or the fair market value at the time of each renewal.
We are committed to pay a portion of the actual operating expenses under our lease agreements for office space. These operating expenses are not included in the table below. Estimated operating lease payments for office space, motor vehicles and office equipment payable over the next five years are as follows:
| (In thousands of U.S. dollars) | ||||
| Year ending December 31, |
||||
| 2012 |
$ | 2,122 | ||
| 2013 |
1,370 | |||
| 2014 |
89 | |||
| 2015 |
68 | |||
| 2016 and thereafter |
— | |||
|
|
|
|||
| Total |
$ | 3,649 | ||
|
|
|
|||
Rent expense amounted to $2.2 million in 2011, $2.0 million in 2010, and $1.6 million in 2009.
90
Table of Contents
In connection with the sale of assets and businesses, we provided indemnities with respect to certain matters, including product liability, patent infringement, contractual breaches and misrepresentations, and we provide other indemnities to third parties under the clinical trial, license, service, manufacturing, supply, distribution and other agreements that we enter into in the normal course of our business. If the indemnified party were to make a successful claim pursuant to the terms of the indemnification, we would be required to reimburse the loss. These indemnifications are generally subject to threshold amounts, specified claims periods and other restrictions and limitations. As at December 31, 2011, no amount has been accrued in relation to indemnities.
Milestone and Royalty Obligations
We have committed to make potential future milestone payments to third parties as part of our various licensing, development and purchase agreements. Payments under these arrangements generally become due and payable only upon achievement of certain development, regulatory or commercial milestones. The achievement of these milestones has not occurred as of December 31, 2011 and has not been recorded in our financial statements.
QLT091001. Under the terms of a co-development agreement we entered into with Retinagenix LLC (“Retinagenix”) in April 2006, we obtained an exclusive, worldwide license and sub-license under certain intellectual property rights owned or controlled by Retinagenix related to the synthetic retinoid compound under development, and are responsible for using commercially reasonable and diligent efforts to develop and commercialize in certain major markets and other markets as we reasonably determine, one or more products covered by the licensed rights or developed using such licensed rights for use in diagnosing, treating or preventing certain human diseases and conditions. Pursuant to the agreement, we have agreed to pay, in the case of the first target indication for such products, $1.0 million upon initiation of the first pivotal trial and up to a total of an additional $11.5 million upon the achievement of other specified development or regulatory milestones and, for each of up to two additional indications, up to a total of $9.0 million upon achievement of specified development or regulatory milestones. If we commercialize such products, we will also pay Retinagenix royalties of between 4% and 6% of net sales, subject to reduction under certain specified circumstances. Retinagenix is also eligible to receive up to a total of $15.0 million upon achievement of specified cumulative sales milestones for such products.
Punctal Plug Drug Delivery System. We acquired the punctal plug drug delivery technology as a result of the acquisition of ForSight Newco II, Inc. (“ForSight”) by our U.S. subsidiary pursuant to the terms of a merger agreement dated October 8, 2007. Under the terms of the merger agreement, we have agreed to use commercially reasonable efforts to develop and commercialize in certain major markets a specified number of punctal plug products claimed under the patents we acquired in the ForSight acquisition and from which pharmaceutical preparations are delivered to the eye. We have also agreed to pay the former ForSight stockholders royalties equal to 10% of net sales of such products made by us or our licensees, subject to reduction under certain specified conditions. The total amount of royalties payable by us are capped on a product by product basis at certain specified aggregate amounts. In addition, if we grant licenses to unaffiliated third parties with respect to the intellectual property acquired as part of the acquisition of ForSight for punctal plug products, we have agreed to pay the former ForSight stockholders 10% of any payments received from such third parties in consideration for such license in the form of up-front fees, maintenance fees, milestone payments and similar fees or payments, and a specified percentage of any amounts recovered in any action to enforce the licensed patents. Also, to the extent we develop and commercialize such products, we have agreed to pay the former ForSight stockholders $5.0 million upon initiation of the first Phase III pivotal clinical trial for a punctal plug product that delivers latanoprost, and $20.0 million upon the first commercial sale in the United States of a first punctal plug product that delivers latanoprost that meets certain clinical or regulatory criteria. The former ForSight stockholders are also eligible to receive $20.0 million upon the first commercial sale of the first additional punctal plug product covered by ForSight’s intellectual property rights and $15.0 million upon the first commercial sale of each additional punctal plug product covered by ForSight’s intellectual property rights thereafter.
18. SEGMENT INFORMATION
We operate in one industry segment, which is the business of developing, manufacturing, and commercializing opportunities in ophthalmology. Our chief operating decision makers review our operating results on a company-wide basis and manage our operations as a single operating segment. Our segment information does not include the results of businesses classified as discontinued operations.
91
Table of Contents
Details of our revenues and property, plant and equipment by geographic segments are as follows:
Revenues(1)
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| U.S. |
$ | 21,022 | $ | 22,597 | $ | 16,394 | ||||||
| Europe |
11,555 | 12,817 | 10,960 | |||||||||
| Canada |
361 | 619 | 2,526 | |||||||||
| Japan |
3,981 | 3,760 | 8,301 | |||||||||
| Other |
5,309 | 4,904 | 3,925 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 42,228 | $ | 44,697 | $ | 42,106 | |||||||
|
|
|
|
|
|
|
|||||||
Property, plant and equipment
| (In thousands of U.S. dollars) |
2011 | 2010 | 2009 | |||||||||
| Canada |
$ | 4,071 | $ | 2,570 | $ | 1,977 | ||||||
| U.S. |
660 | 465 | 620 | |||||||||
|
|
|
|
|
|
|
|||||||
| $ | 4,731 | $ | 3,035 | $ | 2,597 | |||||||
|
|
|
|
|
|
|
|||||||
| (1) | Revenues are attributable to a geographic segment based on the location of the customer. With effect from 2010, under the Amended PDT Agreement, Visudyne is sold by QLT in the U.S., and by Novartis outside the U.S. Previously, Visudyne was sold solely by Novartis on a worldwide basis, and they contributed all of our total revenues. |
19. CONTINGENCIES
From time to time we are involved in legal proceedings arising in the ordinary course of business. There are currently no material pending legal proceedings.
The following is a description of a legal action against us that was settled by the parties in the last fiscal quarter of 2009.
Following the 2008 judgment of the U.S. District Court for the District of Massachusetts in favor of Massachusetts Eye and Ear Infirmary (“MEEI”), under which we were ordered to pay MEEI, among other things, 3.01% of all past, present and future worldwide net sales of Visudyne, on February 12, 2009, the General Hospital Corporation, doing business as Massachusetts General Hospital (“MGH”), launched a legal action against us in the Superior Court of the Commonwealth of Massachusetts alleging that it entered into a written agreement with us that required us to pay MGH the same amount that we pay MEEI on net sales of Visudyne. At the time of the action, our license agreement with MGH provided for a 0.5% royalty payable to MGH for Visudyne sales in the U.S. and Canada, and was subject to a most-favored-nations provision which would have required us to adjust the royalty rate upward had we entered into a license agreement with MEEI for the same rights at a higher rate.
On November 24, 2009, we announced that we reached a settlement with MGH. Under the terms of the settlement agreement and amendment to license agreement, we paid MGH $20.0 million, constituting payment in full for all past and future royalty obligations and in satisfaction and settlement of any obligations we had, may have, or may have had to MGH in connection with the subject matter of the lawsuit. The action was dismissed with prejudice on December 1, 2009.
92
Table of Contents
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
| Item 9A. | CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
We maintain a set of disclosure controls and procedures designed to ensure that information required to be disclosed in filings made pursuant to the Securities Exchange Act of 1934 is recorded, processed, summarized and reported within the time periods specified and in accordance with the Securities and Exchange Commission’s rules and forms and is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer. Our principal executive and financial officers have evaluated our disclosure controls and procedures as of the end of the period covered by this report and concluded that our disclosure controls and procedures were effective, at the reasonable assurance level, in timely alerting them to material information required to be included in our periodic SEC reports.
It should be noted that the design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, regardless of how remote. However, our Chief Executive Officer and Chief Financial Officer have concluded that our disclosure controls and procedures are effective under circumstances where our disclosure controls and procedures should reasonably be expected to operate effectively.
Management’s Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting, as such term is defined in the U.S. Securities Exchange Act of 1934, Rules 13a-15(f). Under the supervision and with the participation of our management, including our principal executive officer and principal financial officer, we conducted an evaluation of the effectiveness of our internal control over financial reporting based on the framework in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
Based on our evaluation under the framework in Internal Control—Integrated Framework, our management concluded that our internal control over financial reporting was effective as of December 31, 2011.
Deloitte & Touche LLP, the independent registered chartered accountants that audited our December 31, 2011 consolidated annual financial statements, has issued an attestation report on our internal control over financial reporting.
Changes in Internal Control over Financial Reporting
There were no changes in our internal controls during our last fiscal quarter that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
93
Table of Contents
REPORT OF INDEPENDENT REGISTERED CHARTERED ACCOUNTANTS
To the Board of Directors and Shareholders of QLT Inc.
We have audited the internal control over financial reporting of QLT Inc. and subsidiaries (the “Company”) as of December 31, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission. The Company’s management is responsible for maintaining effective internal control over financial reporting and for its assessment of the effectiveness of internal control over financial reporting, included in the accompanying Management’s Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the Company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed by, or under the supervision of, the company’s principal executive and principal financial officers, or persons performing similar functions, and effected by the company’s board of directors, management, and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of the inherent limitations of internal control over financial reporting, including the possibility of collusion or improper management override of controls, material misstatements due to error or fraud may not be prevented or detected on a timely basis. Also, projections of any evaluation of the effectiveness of the internal control over financial reporting to future periods are subject to the risk that the controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, the Company maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on the criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission.
We have also audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated financial statements and financial statement schedule as of and for the year ended December 31, 2011 of the Company and our report dated February 23, 2012 expressed an unqualified opinion on those consolidated financial statements and financial statement schedule.
/s/ DELOITTE & TOUCHE LLP
Independent Registered Chartered Accountants
Vancouver, Canada
February 23, 2012
94
Table of Contents
None
95
Table of Contents
PART III
The Information required by Items 10 through 14 of Part III of this Report are incorporated by reference from the proxy statement for use in connection with the Company’s annual meeting of shareholders to be held on May 24, 2012.
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required for this Item is incorporated by reference from the information set forth under the headings “Director Nomination Process,” “Audited Consolidated Financial Statements and Additional Information,” “Report of the Audit and Risk Committee,” “Section 16(a) Beneficial Ownership Reporting Compliance,” “Corporate Code of Ethics and Code of Exemplary Conduct,” “Election of Directors” and “Board of Directors and Board Committees” in our definitive proxy statement for our annual meeting of shareholders to be held on May 24, 2012.
We have a code of business conduct and ethics that applies to our Board of Directors and all of our executives and employees. We also have a code of exemplary conduct for senior financial officers that applies to our principal executive officer, all senior financial managers, including the principal financial and accounting officer, our treasurer, controller, internal legal counsel, corporate secretary, and all other company officers. Information regarding our codes is available on our website at www.qltinc.com, and is incorporated by reference to the information set forth under the heading “Corporate Code of Ethics and Code of Exemplary Conduct” in our definitive proxy statement for our annual meeting of shareholders to be held on May 24, 2012. Information on our website is not incorporated by reference and does not form a part of this Report. We intend to satisfy the disclosure requirements under Item 10 of Form 8-K regarding any amendment to, or a waiver from, a provision of our codes by posting such amendment or waiver on our website. Copies of our annual reports on Form 10-K will be furnished without charge to any person who submits a written request directed to the attention of our Secretary, at our offices located at 887 Great Northern Way, Suite 101, Vancouver, B.C, Canada V5T 4T5.
OUR EXECUTIVE OFFICERS
The following table sets forth information about our executive officers as of February 17, 2012. The executive officers listed below serve in their respective capabilities at the discretion of our Board of Directors.
| Name |
Age | Position | ||||
| Robert L. Butchofsky |
50 | President and Chief Executive Officer | ||||
| Cameron R. Nelson |
46 | Senior Vice President, Finance and Chief Financial Officer | ||||
| Linda M. Lupini |
52 | Senior Vice President, Human Resources and Organizational Development | ||||
| Alexander R. Lussow |
49 | Senior Vice President, Commercial Operations and Business Development | ||||
| Suzanne M. Cadden |
52 | Senior Vice President, Development | ||||
| Item 11. | EXECUTIVE COMPENSATION |
The information required for this Item is incorporated by reference from the information set forth under the headings “2012 Independent Director Compensation Program,” “Equity-Based Compensation,” “Compensation Committee Interlocks and Insider Participation,” “Compensation Committee Report,” “Executive Compensation,” “2011 Summary Compensation Table,” “Grants of Plan Based-Awards for the Fiscal Year Ended December 31, 2011 Table,” “Potential Payments Upon Termination or Change-in-Control,” “Outstanding Equity Awards at 2011 Fiscal Year-End Table,” “Option Exercises and Stock Vested Table,” “Compensation Discussion and Analysis” and “Post-Employment Compensation and Change in Control Arrangements” in our definitive proxy statement for our annual meeting of shareholders to be held on May 24, 2012.
96
Table of Contents
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
Equity Compensation Plan Information
The following table sets out information regarding our common stock that may be issued upon the exercise of options, warrants and other rights granted to employees, consultants or directors under all of our existing equity compensation plans, as of December 31, 2011:
| (a) | (b) | (c) | ||||||||||
| PLAN CATEGORY |
Number of securities to be issued upon exercise of outstanding options, warrants and rights |
Weighted average exercise price of outstanding options, warrants and rights |
Number of
securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||||
| Equity compensation plans approved by security holders (QLT 2000 Incentive Stock Option Plan) |
6,048,197 | (1) | $ | 5.35 | (2) | 649,336 | ||||||
|
|
|
|
|
|
|
|||||||
| Total |
6,048,197 | $ | 5.35 | 649,336 | ||||||||
| (1) | The QLT 2000 Incentive Stock Option Plan, as amended and restated effective May 5, 2009, provides for the issuance of common stock to employees, consultants, officers and directors of QLT and its affiliates. |
| (2) | Options were granted/priced in Canadian dollars at a weighted average exercise price of CAD $5.44 but have been converted to U.S. dollars for disclosure purposes using the December 31, 2011 period end rate published by the Federal Reserve Bank of New York: U.S.$1.00 = CAD$1.0168. |
Other information required for this Item is incorporated by reference from the information set forth under the heading “Security Ownership of Certain Beneficial Owners and Management” in our definitive proxy statement for our annual meeting of shareholders to be held on May 24, 2012.
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The information required for this Item is incorporated by reference from the information set forth under the headings “Indebtedness of Directors and Executive Officers,” “Potential Payments upon Termination or Change in Control,” “Interest of Certain Persons in Material Transactions,” “Independence of Directors” and “Independence of Committee Members” in our definitive proxy statement for use in connection with the annual meeting of shareholders to be held on May 24, 2012.
| Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required for this Item is incorporated by reference from the information set forth under the heading “Report of the Audit and Risk Committee” in our definitive proxy statement for use in connection with the annual meeting of shareholders to be held on May 24, 2012.
97
Table of Contents
PART IV
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
Financial Statements
| (i) | The following financial statement documents are included as part of Item 8 to this Form 10-K. |
Reports of Independent Registered Chartered Accountants
Consolidated Balance Sheets
Consolidated Statements of Operations
Consolidated Statements of Comprehensive (loss) Income
Consolidated Statements of Cash Flows
Consolidated Statements of Changes in Shareholders’ Equity
Notes to the Consolidated Financial Statements
| (ii) | Schedules required by Article 12 of Regulation S-X: |
Except for Schedule II – Valuation and Qualifying Accounts, all other schedules have been omitted because they are not applicable or not required, or because the required information is included in the consolidated financial statements or notes thereto.
Schedule II - Valuation and Qualifying Accounts for the Years ended December 31, 2011, 2010 and 2009.
Provision for non-completion of inventory
(In thousands of U.S. dollars)
| Year |
Balance at beginning of year |
Additions charged to costs and expenses |
Charged
/ (credited) to other accounts(1) |
Write-offs and provision reduction |
Balance at end of year |
|||||||||||||||
| 2011 |
$ | 2,185 | $ | — | $ | — | $ | — | $ | 2,185 | ||||||||||
| 2010 |
2,185 | — | — | — | 2,185 | |||||||||||||||
| 2009 |
1,874 | — | 311 | — | 2,185 | |||||||||||||||
| (1) | Foreign currency translation adjustments. |
Provision for excess inventory
(In thousands of U.S. dollars)
| Year |
Balance at beginning of year |
Additions charged to costs and expenses |
Charged
/ (credited) to other accounts(1) |
Write-offs and provision reduction |
Balance at end of year |
|||||||||||||||
| 2011 |
$ | 11,077 | $ | — | $ | — | $ | — | $ | 11,077 | ||||||||||
| 2010 |
11,560 | — | — | (483 | ) | 11,077 | ||||||||||||||
| 2009 |
2,471 | 8,114 | 975 | — | 11,560 | |||||||||||||||
| (1) | Foreign currency translation adjustments. |
Deferred tax asset valuation allowance
(In thousands of U.S. dollars)
| Year |
Balance at beginning of year |
Additions charged to costs and expenses |
Write-offs and provision reduction |
Balance at end of year |
||||||||||||
| 2011 |
$ | 68,346 | $ | 13,689 | $ | (1,570 | ) | $ | 80,465 | |||||||
| 2010 |
58,669 | 17,540 | (7,863 | ) | 68,346 | |||||||||||
| 2009 |
9,843 | 48,826 | — | 58,669 | ||||||||||||
98
Table of Contents
Exhibits
The exhibits filed with this Report are set forth in the Exhibit Index.
99
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
Dated: February 23, 2012
| QLT INC. | ||
| By: | /s/ Robert L. Butchofsky | |
| Robert L. Butchofsky, President and Chief Executive Officer | ||
| (Principal Executive Officer) | ||
| By: | /s/ Cameron R. Nelson | |
| Cameron R. Nelson, Senior Vice President, Finance and Chief Financial Officer | ||
| (Principal Financial and Accounting Officer) | ||
100
Table of Contents
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS:
That the undersigned officers and directors of QLT Inc. do hereby constitute and appoint Robert L. Butchofsky and Cameron R. Nelson, and each of them, the lawful attorney and agent or attorneys and agents with power and authority to do any and all acts and things and to execute all instruments which said attorneys and agents, or either of them, determine may be necessary or advisable or required to enable QLT Inc. to comply with the Securities Exchange Act of 1934, as amended, and any rules or regulations or requirements of the Securities and Exchange Commission in connection with this Form 10-K Annual Report. Without limiting the generality of the foregoing power and authority, the powers granted include the power and authority to sign the names of the undersigned officers and directors in the capacities indicated below to this Form 10-K or amendments or supplements thereto, and each of the undersigned hereby ratifies and confirms all that said attorneys and agents or either of them, shall do or cause to be done by virtue hereof. This Power of Attorney may be signed in several counterparts.
IN WITNESS WHEREOF, each of the undersigned has executed this Power of Attorney on behalf of the Registrant and in the capacities and on the dates indicated.
| Signatures |
Title |
Date | ||
| /s/ Robert L. Butchofsky |
President, Chief Executive Officer and Director | February 23, 2012 | ||
| Robert L. Butchofsky | (Principal Executive Officer) | |||
| /s/ Cameron R. Nelson |
Senior Vice President, Finance and Chief Financial Officer | February 23, 2012 | ||
| Cameron R. Nelson | (Principal Financial and Accounting Officer) | |||
| /s/ C. Boyd Clarke |
Chairman of the Board of Directors and Director | February 23, 2012 | ||
| C. Boyd Clarke | ||||
| /s/ Bruce L.A. Carter |
Director | February 23, 2012 | ||
| Bruce L.A. Carter | ||||
| /s/ Peter A. Crossgrove |
Director | February 23, 2012 | ||
| Peter A. Crossgrove | ||||
| /s/ Kathryn E. Falberg |
Director | February 23, 2012 | ||
| Kathryn E. Falberg | ||||
| /s/ Ian J. Massey |
Director | February 23, 2012 | ||
| Ian J. Massey | ||||
| /s/ Joseph L. Turner |
Director | February 23, 2012 | ||
| Joseph L. Turner | ||||
| /s/ L. Jack Wood |
Director | February 23, 2012 | ||
| L. Jack Wood | ||||
101
Table of Contents
Exhibit Index
The exhibits listed below are filed as part of this Report. References under the caption “Location” to exhibits or other filings indicate that the exhibit or other filing has been filed, that the indexed exhibit and the exhibit or other filing referred to are the same and that the exhibit or other filing referred to is incorporated by reference. Management contracts and compensatory plans or arrangements filed as exhibits to this Report are identified by an asterisk. The Commission file number for our Exchange Act filings referenced below is 0-17082.
| Exhibit |
Description |
Location | ||
| 2.1 | Asset Purchase Agreement dated December 20, 2006 by and among QLT USA, Inc., Tolmar, Inc. and Dillford Company S.A. (1) | Exhibit 2.1 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006. | ||
| 2.2 | Agreement and Plan of Merger dated June 14, 2004 by and among QLT Inc., Aspen Acquisition Corp. and Atrix Laboratories, Inc. | Annex A to the Company’s Joint Proxy Statement/Prospectus on Form S-4 dated October 14, 2004. | ||
| 2.3 | Agreement and Plan of Merger dated October 8, 2007 by and among QLT Inc., 3088923, Inc., ForSight Newco II, Inc. and the Stockholders Representatives named therein.(1) | Exhibit 2.1 to the Company’s Current Report on Form 8-K dated October 8, 2007 and filed with the Commission on October 11, 2007. | ||
| 2.4 | Purchase Agreement dated June 6, 2008 by and between QLT USA, Inc. and Allergan Sales, LLC. | Exhibit 2.1 to the Company’s Current Report on Form 8-K dated June 6, 2008 and filed with the Commission on June 10, 2008. | ||
| 2.5 | Stock Purchase Agreement dated October 1, 2009 between QLT Inc. and TOLMAR Holding, Inc. | Exhibit 2.1 to the Company’s Current Report on Form 8-K dated October 1, 2009 and filed with the Commission on October 7, 2009. | ||
| 3.0 | Articles of QLT Inc. dated May 25, 2005. | Exhibit 3.2 to the Company’s Current Report on Form 8-K dated May 25, 2005 and filed with the Commission on June 1, 2005. | ||
| 4.1 | Shareholder Rights Plan Agreement, as amended and restated, dated April 8, 2005 between QLT Inc. and ComputerShare Trust Company of Canada. | Exhibit 4.1 to the Company’s Current Report on Form 8-K dated April 8, 2005 and filed with the Commission on April 13, 2005. | ||
| 4.2 | Registration Rights Agreement, as amended and restated, dated December 17, 2004 by and among QLT Inc., Elan International Services, Ltd. and Elan Pharmaceutical Investments III, Ltd. | Exhibit 4.2 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2005. | ||
| 10.1 | Agreement dated April 8, 1982 by and among Quadra Logic Technologies Inc., Dr. Julia Levy and the University of British Columbia. | Exhibit to the Company’s Registration Statement on Form F-1 (File No. 33-31222) filed with the Commission on September 25, 1989. | ||
| 10.2 | Agreement dated January 15, 1988 by and among Quadra Logic Technologies Inc., Dr. David Dolphin and the University of British Columbia. | Exhibit to the Company’s Annual Report on Form 20-F for the year ended December 31, 1988. | ||
| 10.3 | Royalty Adjustment and Stock Option Agreement dated August 10, 1989 between Quadra Logic Technologies Inc. and Dr. David Dolphin. | Exhibit to the Company’s Amendment No. 1 to the Registration Statement on Form F-1 dated November 6, 1989. | ||
| 10.4 | Royalty Agreement dated December 15, 1987 between Quadra Logic Technologies Inc. and Dr. David Dolphin. | Exhibit to the Company’s Amendment No. 1 to the Registration Statement on Form F-1 dated November 6, 1989. | ||
| 10.5* | 1998 QLT Incentive Stock Option Plan. | Exhibit 10.68 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1998. | ||
| 10.6* | 2000 QLT Incentive Stock Option Plan (as amended in 2002); (formerly numbered 10.70). | Exhibit to the Company’s Registration Statement on Form S-8 filed with the Commission on September 20, 2002. | ||
102
Table of Contents
| Exhibit |
Description |
Location | ||
| 10.7* | Employment Agreement dated December 18, 2001 between QLT Inc. and Paul J. Hastings. | Exhibit 10.77 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002. | ||
| 10.8* | Employment Agreement dated May 19, 2000 between QLT Inc. and Alain Curaudeau. | Exhibit 10.80 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2002. | ||
| 10.9 | PDT Product Development, Manufacturing and Distribution Agreement dated July 1, 1994 between Quadra Logic Technologies Inc. and CIBA Vision AG, Hettlingen (now Novartis Pharma AG). | Exhibit to the Company’s Annual Report on Form 10-K for the year ended December 31, 1995. | ||
| 10.10 | BPD-MA Verteporfin Supply Agreement dated March 12, 1999 between QLT PhotoTherapeutics Inc. and Parkedale Pharmaceuticals, Inc. (1) | Exhibit 10.54 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1998. | ||
| 10.11 | BPD-MA Presome Supply Agreement dated February 6, 2008 between QLT PhotoTherapeutics Inc. and Nippon Fine Chemical Co., Ltd. (1) | Exhibit 10.55 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1998. | ||
| 10.12 | BPD-MA Supply Agreement dated December 11, 1998 between QLT PhotoTherapeutics Inc. and Raylo Chemicals Limited. (1) | Exhibit 10.56 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1998. | ||
| 10.13 | License Agreement dated December 8, 1998 between QLT PhotoTherapeutics Inc. and The General Hospital Corporation. (1) | Exhibit 10.63 to the Company’s Annual Report on Form 10-K for the year ended December 31, 1998. | ||
| 10.14 | Amending Agreement to PDT Product Development, Manufacturing and Distribution Agreement dated July 23, 2001 between QLT Inc. and Novartis Ophthalmics AG. (1) | Exhibit 10.74 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2002. | ||
| 10.15 | Amending Agreement to PDT Product Development, Manufacturing and Distribution Agreement dated July 22, 2003 between QLT Inc. and Novartis Ophthalmics AG (now a division of Novartis Pharma AG). | Exhibit 10.77 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2003. | ||
| 10.16 | Agreement and Plan of Merger dated June 14, 2004 by and among QLT Inc., Aspen Acquisition Corp. and Atrix Laboratories, Inc.. | See Exhibit 2.2 above. | ||
| 10.17 | License and Royalty Agreement dated August 8, 2000 between Atrix Laboratories, Inc. and Pfizer Inc. | Exhibit 99.3 to Atrix Laboratories, Inc.’s Current Report on Form 8-K dated August 8, 2000 and filed with the Commission on September 7, 2000. | ||
| 10.18 | Collaboration, Development and Supply Agreement dated August 28, 2000 between Atrix Laboratories, Inc. and Sandoz, Inc. (formerly Geneva Pharmaceuticals, Inc.) | Exhibit 10.13 to Atrix Laboratories, Inc.’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2000. | ||
| 10.19 | Collaboration, License and Supply Agreement dated December 8, 2000 between Atrix Laboratories, Inc. and Sanofi-Synthelabo Inc. as amended through February 15, 2007. (1) | Exhibit 10.19 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006. | ||
| 10.20 | Collaboration, License and Supply Agreement dated April 4, 2001 between Atrix Laboratories, Inc. and MediGene as amended through May 17, 2006. (1) | Exhibit 10.20 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006. | ||
103
Table of Contents
| Exhibit |
Description |
Location | ||
| 10.21* | Separation Letter Agreement with Michael J. Doty dated April 15, 2005. | Exhibit 10.1 to the Company’s Current Report on Form 8-K dated April 15, 2005 and filed with the Commission on April 21, 2005. | ||
| 10.22* | QLT Inc. 2005 Cash Incentive Plan dated May 10, 2005. | Exhibit 10.01 to the Company’s Current Report on Form 8-K dated May 10, 2005 and filed with the Commission on May 16, 2005. | ||
| 10.23* | Deferred Share Unit Plan For Non-Employee Directors. | Exhibit 10.32 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005. | ||
| 10.24* | Change of Control Agreement dated August 8, 2005 between QLT Inc. and Cameron Nelson. | Exhibit 10.34 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2005. | ||
| 10.25* | Letter Agreement dated September 23, 2005 between QLT Inc. and Paul J. Hastings. | Exhibit 10.1 to the Company’s Current Report on Form 8-K dated September 23, 2005 and filed with the Commission on September 26, 2005. | ||
| 10.26* | Employment Agreement dated September 26, 2005 between QLT Inc. and Robert L. Butchofsky. | Exhibit 10.35 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2005. | ||
| 10.27* | Change of Control Agreement dated September 26, 2005 between QLT Inc. and Robert L. Butchofsky. | Exhibit 10.36 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2005. | ||
| 10.28* | Employment Agreement dated November 8, 2005 between QLT Inc. and Cameron Nelson. | Exhibit 10.37 to the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2005. | ||
| 10.29* | Consultancy Agreement dated December 7, 2005 between QLT Inc. and Dr. Mohammad Azab. | Exhibit 10.40 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2005. | ||
| 10.30* | Employment Agreement dated December 9, 2005 between QLT USA, Inc. and Michael R. Duncan. | Exhibit 10.41 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2005. | ||
| 10.31* | Change of Control Agreement dated December 9, 2005 between QLT USA, Inc. and Michael R. Duncan. | Exhibit 10.42 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2005. | ||
| 10.32* | Form of Stock Option Agreement for stock option grants to senior employees and executive officers. | Exhibit 10.43 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2006. | ||
| 10.33* | Employment Agreement dated May 31, 2006 between QLT Inc. and Peter J. O’Callaghan. | Exhibit 10.44 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2006. | ||
| 10.34* | Change of Control Agreement dated May 31, 2006 between QLT Inc. and Peter J. O’Callaghan. | Exhibit 10.45 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2006. | ||
| 10.35 | Settlement, Release and Patent License dated February 9, 2007 by and among QLT USA, Inc., Takeda Pharmaceutical Company Limited, Wako Pure Chemical Industries, Ltd., TAP Pharmaceutical Products Inc., Abbott Laboratories, Limited – Laboratories Abbott, Limited and Sanofi-Synthelabo, Inc. | Exhibit 10.35 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006. | ||
104
Table of Contents
| Exhibit |
Description |
Location | ||
| 10.36 | Eligard Manufacturing and Supply Agreement dated December 22, 2006 between QLT USA, Inc. and Tolmar, Inc. (1) | Exhibit 10.36 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006. | ||
| 10.37 | Amended and Restated Contribution Agreement dated February 9, 2007 between QLT USA, Inc. and Sanofi-Synthelabo, Inc. (1) | Exhibit 10.37 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2006. | ||
| 10.38 | Settlement Agreement dated March 2, 2007 between QLT Inc. and Massachusetts Eye and Ear Infirmary.(1) | Exhibit 10.4 to the Company’s Quarterly Report on Form 10-Q for the quarter ended March 31, 2007. | ||
| 10.39* | Separation Letter Agreement with Alain Curaudeau dated January 18, 2008. | Exhibit 10.1 to the Company’s Current Report on Form 8-K dated January 18, 2008 and filed with the Commission on January 25, 2008. | ||
| 10.40 | Amended and Restated License Agreement dated December 14, 2007 between QLT Inc. and the University of British Columbia.(1) | Exhibit 10.40 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2007. | ||
| 10.41* | 2007 Cash Incentive Bonus Plan for Executive Officers. | Item 1.01 of the Company’s Current Report on Form 8-K dated February 27, 2007 and filed with the Commission on March 3, 2007. | ||
| 10.42* | 2007 Cash Incentive Compensation Structure for Executive Officers. | Item 5.02 of the Company’s Current Report on Form 8-K dated May 17, 2007 and filed with the Commission on May 23, 2007. | ||
| 10.43* | 2007 Base Salary for Chief Executive Officer. | Item 5.02 of the Company’s Current Report on Form 8-K dated April 16, 2007 and filed with the Commission on April 20, 2007. | ||
| 10.44 | QLT Guarantee dated June 6, 2008. | Exhibit 10.44 to the Company’s Current Report on Form 8-K dated June 6, 2008 and filed with the Commission on June 10, 2008. | ||
| 10.45 | Sale and Purchase Agreement dated May 15, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Inc. as amended by each of: an Amending Agreement dated July 4, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Inc., an Amended and Restated Sale and Purchase Agreement dated July 11, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Inc., a Third Amending Agreement dated July 16, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Inc., a Fourth Amending Agreement dated July 18, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Inc., a Fifth Amending Agreement dated July 23, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Inc., a Sixth Amending Agreement dated July 25, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Inc., a Second Amended and Restated Sale and Purchase Agreement dated July 30, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Inc. and an Eighth Amending Agreement dated August 7, 2008 by and among QLT Inc., 560677 B.C. Ltd., 630321 B.C. Ltd. and Discovery Parks Holdings Ltd. | Exhibit 10.45 to the Company’s Quarterly Report on Form 10-Q for the quarter ended June 30, 2008. | ||
105
Table of Contents
| Exhibit |
Description |
Location | ||
| 10.46 | License Agreement dated August 25, 2008 by and between QLT USA, Inc. and Reckitt Benckiser Pharmaceuticals Inc. (1) | Exhibit 10.1 to the Company’s Amended Current Report on Form 8-K/A dated August 25, 2008 and filed with the Commission on September 3, 2008. | ||
| 10.47 | Amendment No. 8 to BPD-MA Presome Supply Agreement dated December 29, 2008 by and between QLT Inc. and Nippon Fine Chemical Co., Ltd. (1) | Exhibit 10.47 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2008. | ||
| 10.48 | Amended and Restated PDT Product Development, Manufacturing and Distribution Agreement dated October 16, 2009 between QLT Inc. and Novartis Pharma AG. | Exhibit 10.48 to the Company’s Current Report on Form 8-K dated October 16, 2009 and filed with the Commission on October 22, 2009. | ||
| 10.49* | 2000 QLT Incentive Stock Option Plan (as amended and restated effective May 5, 2009). | Exhibit 10.1 to the Company’s Registration Statement on Form S-8 filed on October 14, 2009. | ||
| 10.50* | Employment Agreement dated April 12, 2010 between QLT Inc. and Dr. Dipak Panigrahi. | Exhibit 10.50 to the Company’s Current Report on Form 8-K dated April 29, 2010 and filed with the Commission on May 5, 2010. | ||
| 10.51* | Change of Control Agreement dated April 12, 2010 between QLT Inc. and Dr. Dipak Panigrahi. | Exhibit 10.51 to the Company’s Current Report on Form 8-K dated April 29, 2010 and filed with the Commission on May 5, 2010. | ||
| 10.52 | Distribution Services Agreement dated effective as of January 1, 2010 by and between QLT Ophthalmics, Inc. and ASD Specialty Healthcare, Inc. d/b/a Besse Medical. (1) | Exhibit 10.52 to the Company’s Quarterly Report on Form 10-Q/A (Amendment No. 1) dated November 1, 2010. | ||
| 10.53* | Amendment Agreement to Employment Agreement dated February 3, 2011 between QLT Inc. and Dr. Dipak Panigrahi. | Exhibit 10.53 to the Company’s Current Report on Form 8-K dated January 31, 2011 and filed with the Commission on February 4, 2011. | ||
| 10.54 | Co-Development Agreement dated effective as of April 4, 2006 by and between QLT Inc. and Retinagenix, LLC, as amended by letter agreements dated August 10, 2006, September 11, 2008 and October 20, 2010 between QLT Inc. and Retinagenix, LLC. (1) | Exhibit 10.54 to the Company’s Annual Report on Form 10-K for the year ended December 31, 2010. | ||
| 10.55* | Employment Agreement dated September 14, 2010 between QLT Inc. and Suzanne M. Cadden. | Exhibit 10.55 to the Company’s Current Report on Form 8-K dated September 14, 2011 and filed with the Commission on September 19, 2011. | ||
| 10.56* | Change of Control Agreement dated September 15, 2011 between QLT Inc. and Suzanne M. Cadden. | Exhibit 10.56 to the Company’s Current Report on Form 8-K dated September 14, 2011 and filed with the Commission on September 19, 2011. | ||
| 11 | Statement re: computation of per share earnings. | Filed herewith. | ||
| 21 | Subsidiaries of QLT Inc. | Filed herewith. | ||
| 23 | Consent of Deloitte & Touche LLP. | Filed herewith. | ||
106
Table of Contents
| Exhibit |
Description |
Location | ||
| 31.1 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002: Robert L. Butchofsky, President and Chief Executive Officer. | Filed herewith. | ||
| 31.2 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 302 of the Sarbanes-Oxley Act of 2002: Cameron R. Nelson, Senior Vice President, Finance, and Chief Financial Officer. | Filed herewith. | ||
| 32.1 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002: Robert L. Butchofsky, President and Chief Executive Officer. | Filed herewith. | ||
| 32.2 | Certification pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002: Cameron R. Nelson, Senior Vice President, Finance, and Chief Financial Officer. | Filed herewith. | ||
Notes:
| * | Denotes executive compensation plans or arrangements. |
| (1) | Certain portions of this exhibit have been omitted and filed separately with the Commission pursuant to a grant of confidential treatment under Rule 24b-2 promulgated under the Securities Exchange Act of 1934, as amended. |
107
