Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - CardioNet, Inc. | Financial_Report.xls |
| EX-32 - EX-32 - CardioNet, Inc. | a2207476zex-32.htm |
| EX-31.1 - EX-31.1 - CardioNet, Inc. | a2207476zex-31_1.htm |
| EX-23.1 - EX-23.1 - CardioNet, Inc. | a2207476zex-23_1.htm |
| EX-31.2 - EX-31.2 - CardioNet, Inc. | a2207476zex-31_2.htm |
Use these links to rapidly review the document
TABLE OF CONTENTS
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
ý |
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the fiscal year ended December 31, 2011 |
||
OR |
||
o |
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
|
For the transition period from N/A to N/A |
||
Commission file number: 0-10961
CardioNet, Inc.
(Exact name of registrant as specified in its charter)
| DELAWARE (State or other jurisdiction of incorporation or organization) |
94-2573850 (I.R.S. Employer Identification No.) |
|
227 Washington Street Conshohocken, Pennsylvania (Address of principal executive offices) |
19428 (Zip Code) |
(610) 729-7000
(Registrant's telephone number, including area code)
Not Applicable
(Former name, former address and former fiscal year, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
| Title of Each Class | Name of Each Exchange on Which Registered | |
|---|---|---|
| Common Stock, $0.001 par value | NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes o No ý
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes o No ý
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ý No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 229.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ý No o
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant's knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of "large accelerated filer," "accelerated filer," and "smaller reporting company" in Rule 12b-2 of the Exchange Act. (Check one):
| Large accelerated filer o | Accelerated filer ý | Non-accelerated filer o (Do not check if a smaller reporting company) |
Smaller reporting company o |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes o No ý
The aggregate market value of the registrant's common stock held by non-affiliates of the registrant was $89,252,445 based on the closing sale price at which the common stock was last sold on June 30, 2011, the last business day of the registrant's most recently completed second fiscal quarter.
As of February 16, 2012, 24,550,398 shares of the registrant's common stock were outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Certain information contained in the registrant's definitive Proxy Statement for the 2012 annual meeting of stockholders is incorporated by reference into Part III of this Form 10-K.
CardioNet, Inc.
Annual Report on Form 10-K
For The Fiscal Year Ended December 31, 2011
2
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
This document includes certain forward-looking statements within the meaning of the "Safe Harbor" provisions of the Private Securities Litigation Reform Act of 1995 regarding, among other things, our growth prospects, the prospects for our products and our confidence in the Company's future. These statements may be identified by words such as "expect," "may," "anticipate," "possible," "estimate," "potential," "intend," "plan," "believe," "forecast," "promises" and other words and terms of similar meaning. Such forward-looking statements are based on current expectations and involve inherent risks and uncertainties, including important factors that could delay, divert, or change any of them, and could cause actual outcomes and results to differ materially from current expectations. These factors include, among other things, the effect of the Biotel acquisition on our business operations and financial results, effectiveness of our efforts to address operational initiatives, including cost savings initiatives that affect our business, changes to insurance coverage, relationships with our government and commercial payors and reimbursement levels for our products, the success of our sales and marketing initiatives, our ability to attract and retain talented executive management and sales personnel, our ability to identify acquisition candidates, acquire them on attractive terms and integrate their operations into our business, the commercialization of new products, market factors, internal research and development initiatives, partnered research and development initiatives, competitive product development, changes in governmental regulations and legislation, the continued consolidation of payors, acceptance of our new products and services and patent protection, adverse regulatory action and litigation success, as well as the risks discussed in Item 1A of this report entitled Risk Factors. Except as required by law, we undertake no obligation to publicly update any forward-looking statement, whether as a result of new information, future events, or otherwise
CardioNet, Inc. (the "Company," "CardioNet," "we" or "us"), a Delaware corporation, provides continuous, real-time ambulatory outpatient management solutions for monitoring relevant and timely clinical information regarding an individual's health. The Company was initially incorporated in California in 1994, and re-incorporated in Delaware in connection with its initial public offering in March 2008. In September 1999, the Company began its focus on helping physicians more rapidly diagnose and more effectively manage therapy for patients with cardiovascular disease. Since that time, the Company has developed a proprietary integrated patient management platform that incorporates a wireless data transmission network, internally developed software, Food and Drug Administration (FDA) cleared algorithms and medical devices, and a 24-hour digital monitoring service center. The Company is currently focused on the diagnosis and monitoring of cardiac arrhythmias, or heart rhythm disorders, through its core Mobile Cardiac Outpatient Telemetry™ (MCOT™), event and Holter services.
In February 2002, the Company first received FDA 510(k) clearance for its MCOT™ device. Since its initial FDA clearance, the Company has released multiple generations of its MCOT™ device, most recently its C5 model. MCOT™ automatically detects a patient's cardiac rhythm irregularities and transmits electrocardiogram (ECG) data to a continuously monitored information center. Our primary MCOT™ monitoring center was opened in Conshohocken, PA in July 2002. In December 2011, the Company expanded its operations by opening a second monitoring center in San Francisco, CA. The CardioNet Monitoring Center provides analysis and response for all incoming ECG data. Currently, the Company provides all cardiac arrhythmia monitoring services for MCOT™ at these two locations.
CardioNet's MCOT™ service incorporates a lightweight patient-worn sensor attached to electrodes that capture two-channel ECG data, measuring electrical activity of the heart, on a compact wireless handheld monitor. The monitor analyzes incoming heartbeat-by-heartbeat information from the sensor
3
on a real-time basis by applying proprietary algorithms designed to detect arrhythmias. When the monitor detects an arrhythmic event, it automatically transmits the ECG to the CardioNet Monitoring Center, even in the absence of symptoms noticed by the patient. At the CardioNet Monitoring Center, which operates 24 hours a day and 7 days per week, experienced certified cardiac monitoring specialists analyze the sent data; respond to urgent events and report results in the manner prescribed by the physician. The MCOT™ device employs two-way wireless communications, enabling continuous transmission of patient data to the CardioNet Monitoring Center and permitting physicians to remotely adjust monitoring parameters and request previous ECG data from the memory stored in the monitor. The MCOT™ device has the capability of storing 21 days of continuous ECG data, in contrast to a maximum of 10 minutes for a typical event monitor, and a maximum of 24 hours for a typical Holter monitor.
We believe that MCOT™'s continuous monitoring is a fundamental advancement in arrhythmia monitoring. Our system transformed an industry that has historically relied on memory-constrained, intermittent digital or analog tape recorders, such as event and Holter monitors, by offering a platform that captures continuous real-time patient data, leading to immediate potential lifesaving diagnoses. The drawbacks of the existing event and Holter technologies include the failure to provide real-time data, limited recording capability, frequent inaccurate diagnoses and an inability to monitor patient compliance and interaction. We believe these drawbacks lead to suboptimal diagnostic yields, adversely impacting clinical outcomes and health care costs. In a randomized clinical trial, MCOT™ detected clinically significant arrhythmias nearly three times as often as traditional loop event monitors in patients who had previously experienced negative or inconclusive Holter monitoring.
Since our commercial introduction of MCOT™ in February 2002, physicians have enrolled over 500,000 patients in our MCOT™ services. Through December 31, 2011, we marketed our solution in 49 states and have secured direct contracts with 356 commercial payors, which we estimate that, when combined with our Medicare participation, represents more than 200 million covered lives. We receive reimbursement for the monitoring services provided to patients from Medicare and the third-party commercial payors.
The American Medical Association ("AMA") has established billing codes applicable to the Category I Current Procedural Terminology ("CPT") code for Mobile Cardiovascular Telemetry. These billing codes allow for automated claims adjudication, substantially simplifying the reimbursement process for physicians and payors compared to the previous process. Reimbursement was previously obtained through non-specific billing codes which require various narratives that, in most cases, involve semi-automated or manual processing, as well as additional review by payors. The Centers for Medicare and Medicaid Services ("CMS") has established reimbursement rates that cover MCOT™.
Beginning in March 2007, the Company began offering event and Holter monitoring services upon its acquisition of PDSHeart Inc. By offering these additional services, CardioNet is effectively able to deliver all cardiac-related diagnostic monitoring services, giving us a market competitive advantage by allowing physicians and clinics to obtain services from one source.
On December 21, 2010, the Company completed the acquisition of Biotel Inc., and its wholly owned subsidiaries, Braemar, Inc. and Agility Centralized Research Services, Inc. The acquisition gives the Company the ability to develop, manufacture, test market medical devices and related software, and market such products to medical companies, clinics and hospitals. Additionally, the acquisition gives the Company access to established customer relationships, entry into the clinical trial service business as a Clinic Research Organization (CRO) and the ability to diversify its product and service offerings.
4
Industry Overview
Overview of Cardiac Arrhythmias
A cardiac arrhythmia is categorized as a temporary or sustained abnormal heart rhythm that is caused by a disturbance in the electrical signals in the chambers of the heart. Proper transmission of electrical signals to the heart is necessary to ensure effective heart function. There are two main categories of arrhythmias: tachycardia, meaning too fast a heartbeat; and bradycardia, meaning too slow a heartbeat.
Arrhythmias affect more than four million people annually in the United States. According to the American Heart Association, arrhythmias result in more than 780,000 hospitalizations and contribute to approximately 480,000 deaths each year. A number of factors can contribute to arrhythmias including cardiovascular disease, high blood pressure, diabetes, smoking, excessive consumption of alcohol or caffeine, drug abuse or stress. An arrhythmia may be a symptom of serious cardiovascular disease and, if left undiagnosed and untreated, can lead to stroke, other serious complications or even death. Examples of arrhythmias and their consequences include:
- •
- Atrial fibrillation. The most prevalent arrhythmia is
atrial fibrillation, an arrhythmia that affects approximately 2.2 million Americans and is characterized by a rapid, irregular quivering of the upper chambers of the heart. According to the
American Heart Association, approximately 15% to 20% of the estimated 700,000 strokes that occur annually in the United States are attributable to atrial fibrillation and people with atrial
fibrillation are approximately five times more likely to have a stroke.
- •
- Ventricular Tachycardia. Ventricular tachycardia is a
potentially life-threatening arrhythmia initiated in the lower chambers of the heart. It can interfere with the ability of the heart to pump blood and may degenerate into ventricular
fibrillation requiring CPR and defibrillation. It can occur with or without apparent heart disease.
- •
- Syncope. While not an arrhythmia, syncope, or fainting, many times results from an arrhythmia. It is the temporary loss of consciousness because of a sudden decline in blood flow to the brain that may be the result of tachycardia or bradycardia. Syncope accounts for 1% to 3% of emergency room visits and up to 6% of hospital admissions each year in the United States.
The ability to diagnose or rule out an arrhythmia as a symptom of a cardiac condition is important both to treat those patients with serious cardiovascular diseases as well as to identify those patients that may not require further medical attention.
Evolution of Traditional Arrhythmia Monitoring Technologies
Arrhythmias may be diagnosed either in a physician's office or other health care facility or remotely by monitoring a patient's heart rhythm. Typically, physicians will administer a resting ECG that monitors the electrical impulses in a patient's heart and if it is determined that a patient needs to be monitored for a longer period of time to produce a diagnosis, the physician will typically prescribe an ambulatory cardiac monitoring device, such as a Holter monitor or an event monitor.
Some physicians own their own ambulatory cardiac monitoring devices and directly provide monitoring services to their patients, while other physicians outsource the services to third party providers. In the wake of increasing legal and compliance requirements surrounding ambulatory cardiac monitoring, including a 2003 Medicare decision requiring 24 hour per day monitoring stations, the increasing trend is for physicians and hospitals to outsource their monitoring needs to third party providers.
If either the Holter monitor or event monitor are negative or inconclusive and the physician still suspects an arrhythmia as the cause of the symptom, the physician may decide to prescribe additional,
5
more expensive testing or hospitalize the patient in a telemetry unit (continuously attended ECG monitoring). In-hospital telemetry is expensive and therefore is only utilized selectively and for short time periods, and the monitored data is often not reflective of real-life cardiac activity.
Holter Monitors
A Holter monitor, first used in 1961, is an ambulatory cardiac monitoring device that is generally worn by a patient for a one-day or two-day period in order to record continuous ECG data. The magnetic or digital storage, or other medium containing the data recorded by this device, is then delivered by hand, mail or internet for processing and analysis by the physician or a third party service provider. Despite the advent of newer technologies, Holter monitoring continues to be used today for patients whose suspected arrhythmia is believed to occur many times during the course of a day. However, for a patient that has an unpredictable or intermittent arrhythmia, a Holter may not provide clinically useful information due to the insufficient duration of the monitoring period. In addition, as a result of the typical one- to three-day reporting delay and the lack of real-time physician notification, patients may not receive timely diagnosis of their condition. Any artifact, or noise, in the data will not be discovered until the test is analyzed. A 2005 Frost & Sullivan study reported that Holters have been found to be effective in diagnosing arrhythmias only 10% of the time.
Event Monitors
Beginning in the 1980s, a new category of ambulatory cardiac monitoring devices called event monitors emerged, with the most common type referred to as manual-trigger loop event monitors. An event monitor records several minutes of ECG activity at a time and then begins overwriting the memory, a process referred to as memory loop recording. The memory loop event monitor continuously records and stores the previous 60 seconds of ECG signal in internal loop memory. When a patient becomes symptomatic, the patient activates the monitor which stores the 60 seconds of existing loop memory and an additional 30 seconds of ECG signal following patient activation. The stored data is considered one cardiac event and provides physicians a snapshot of the ECG signal recorded immediately before and during a patient's symptoms. Non-loop event monitors are kept with the patient at all times. When a patient experiences symptoms, non-loop event monitors will typically record and store 30 seconds of ECG signal immediately following activation and placement in direct contact with the patient's chest. Event monitors have limited memory, usually less than 10 minutes, and can generally store data concerning between one and six cardiac events. The patient must transmit the event data to the monitoring center, typically by phone, and then erase the memory. Event monitors provide advantages over Holter monitors given that they are worn over a period of up to 30 days, instead of the one- to two-day period. However, event monitors have significant shortcomings. Manual-trigger loop event monitors capture only cardiac events associated with symptoms detectable by the patient and not asymptomatic cardiac events. In our experience, only 15% to 20% of clinically significant cardiac events are symptomatic, meaning that the patient can feel them as they occur. Other drawbacks of manual-trigger loop event monitors include the limited data storage, the lack of trend data, and poor patient compliance relating to the requirement that the patient must both trigger and transmit events.
A new type of event monitoring device was introduced in 1999 called the auto-detect loop event monitor. The auto-detect loop event monitor also records using a short memory loop and event storage capability, capturing several minutes of heart activity at a time before starting over, but incorporates basic algorithms that look at fast, slow or irregular heart rates and, in some instances, pauses to automatically detect certain asymptomatic arrhythmias. Similar to manual-trigger loop event monitors, the auto-detect loop event monitor requires the patient to call in and transmit the event data telephonically to either the physician's office or a monitoring center. The latest development in auto-detect loop event monitoring is referred to as auto-detect/auto-send. Auto-detect/auto-send loop
6
event monitors have the ability to send captured event data to a monitoring center via wireless access, instead of requiring patients to manually transmit event data. Patients do not have the ability to correlate symptoms to the event via the monitor and are required to carry a diary and make contact with the monitoring center to report symptoms. These monitors still continue to suffer from limited data storage and limited algorithm capabilities. To our knowledge, randomized prospective peer reviewed clinical trials have not yet been conducted to demonstrate any improvement in diagnostic yield between the standard loop monitors and the newer auto-trigger or auto-trigger/auto-send monitors.
MCOT™ Solution
We believe that there is a significant opportunity for new arrhythmia monitoring solutions that exploit the convergence of wireless, low power microelectronic and software technologies to address the shortcomings of traditional Holter and event monitors. We believe that existing technologies have drawbacks including the inability to detect asymptomatic events, failure to provide real-time data, memory constraints, frequent inaccurate diagnoses and an inability to monitor patient compliance and interaction. These drawbacks often lead to suboptimal diagnostic yields, adversely impacting clinical outcomes and health care costs.
We have developed an ambulatory, continuous and real-time arrhythmia monitoring solution that we believe represents a significant advancement over event and Holter monitoring. CardioNet's MCOT™ service incorporates a patient-worn sensor attached to leads that captures ECG data and communicates wirelessly with a compact monitor that analyzes incoming information by applying proprietary algorithms designed to detect arrhythmias and eliminate data noise. When the monitor detects an arrhythmic event, it automatically transmits the ECG data to the CardioNet Monitoring Center, where experienced certified cardiac monitoring specialists analyze the sent data, respond to urgent events and report results in the manner prescribed by the physician. The MCOT™ monitor, on average, is worn by the patient for a period of approximately 14 days. The C5 generation MCOT™ device received FDA 510(k) clearance in April 2010 and was released in December 2011. The C5 devices include a variety of product enhancements over previous generations of CardioNet monitoring devices. Some of these enhancements include the following:
- •
- additional processing capabilities to operate multiple sensors;
- •
- enhanced clinical applications;
- •
- additional memory space, faster processing, and simplified software upgrades and retrieval;
- •
- waterproofing;
- •
- support for international transmission capabilities; and
- •
- advanced remote operation.
MCOT™ results in a high diagnostic yield of clinically significant arrhythmias, allowing for real-time detection and analysis as well as timely intervention and treatment. In a randomized 300-patient clinical study conducted in March 2007, MCOT™ detected clinically significant arrhythmias nearly three times as often as traditional loop event monitors in patients who had previously experienced negative or non-diagnostic Holter monitoring.
MCOT™ Monitoring Overview
Initiation of Service
A physician prescribing MCOT™ for a patient completes an enrollment form that describes the length of time during which the patient should be monitored, together with patient-specific monitoring
7
thresholds and response parameters. Once the patient has been enrolled, a CardioNet representative contacts the patient to coordinate delivery and schedule a telephonic patient education session, or the patient accesses educational instruction through the Company's proprietary online software, to learn the use of the MCOT™ device.
Monitoring
A lightweight sensor (worn as a pendant or on a belt clip) attached to leads records two channels of ECG. The sensor constantly communicates wirelessly with the monitor, a compact handheld unit which can be tucked into a pocket or purse. The monitor analyzes incoming information from the sensor on a real-time basis by applying proprietary algorithms designed to detect arrhythmias.
When the monitor detects an arrhythmic event (defined by the values prescribed by the patient's physician), it transmits the ECG to the CardioNet Monitoring Center, even in the absence of symptoms noticed by the patient and without patient interaction. In instances when patients experience a symptom, they select their symptom and the contemporaneous activity level through the monitor's touch screen. Once completed, the monitor automatically transmits the event to the CardioNet Monitoring Center for review. When at home, the patient can place the monitor in a base station, which allows recharging and enables automated data transmission through the standard telephone line in the patient's home.
The monitor allows two-way wireless communications, enabling the CardioNet Monitoring Center to adjust device parameters, "check in" on the patient and pull previous ECG data, over standard telephone lines and through cellular coverage. Most other ambulatory devices on the market, such as most event monitors, only support one-way transmissions.
Central Monitoring Station/Data Transmission Network
At the CardioNet Monitoring Center in Conshohocken, PA, an Independent Diagnostic Testing Facilities (IDTFs) certified by Medicare, and San Francisco, CA, soon to be enrolled as an IDTF, we employ experienced certified cardiac monitoring specialists analyze the sent data, respond to urgent events and report results in the manner prescribed by the physician and monitor patient compliance. The CardioNet Monitoring Center operates 24 hours a day, 7 days per week. The data transmission is accomplished through (i) a wireless cell phone modem in the monitor or (ii) through the telephone line modem in the base station.
Physician Notification
When prescribing MCOT™, physicians will pre-prescribe the criteria for when they wish to be notified by the Monitoring Center regarding a significant arrhythmic event. The notification is based on the patient's ECG and symptoms and can occur any time, 24 hours a day, 7 days a week. Physicians can review the data via fax or over the internet. Reports have been designed to allow rapid review of results, graphing related data and trends. The following is a summary of the types of reports we provide:
Daily Report—includes a heart rate trending chart; charts describing the frequency and duration of atrial fibrillation (atrial fibrillation data is trended over the length of service); a summary of ECG activity from the prior 24 hours, including urgent ECG's; a description of symptoms and associated activity level if reported by patient; and clinical indicators demonstrating trending of arrhythmias.
Urgent Report—when a patient's ECG and/or symptom meets pre-prescribed physician notification criteria, the physician is notified immediately and provided with the relevant ECG
8
data, along with the symptoms and activity if reported by the patient. Physicians are also allowed to revise notification criteria if applicable.
Fetch Report—provides ECG data from the monitor at the request of the physician for any period during the previous 21 days.
End of Service Summary Report—at the completion of the patient's monitoring, a report is prepared describing the length of the monitoring service and all reports that were prepared for the patient during the monitoring service.
Other Arrhythmia Monitoring Services
In addition to MCOT™, we offer event, Holter and pacemaker monitoring services, positioning us as a "one-stop shop" for arrhythmia monitoring solutions. We provide cardiologists and electrophysiologists who prefer to use a single source of arrhythmia monitoring services with a full spectrum of solutions, ranging from our differentiated MCOT™ services to event and Holter monitoring.
Our event monitoring services provides physicians with the flexibility to prescribe both memory loop event monitors and non-loop event monitors. The patient transmits stored event data telephonically to one of two event monitoring centers in Minnesota or Pennsylvania, where our trained cardiac technicians analyze the data, generate a report of the findings and return the results back to the physician. Our two event monitoring centers are distinct from the CardioNet Monitoring Center. We provided event monitoring services to approximately 60,000 patients in 2011.
During the monitoring period, the Holter monitor stores an image of the electrical impulses of every heartbeat or irregularity in either digital format on an internal compact flashcard or in analog format on a standard cassette tape located inside the monitor. Approximately 95% of our Holter devices use digital flashcard technology. At the conclusion of the monitoring period, the patient returns to the physician office to have the monitor disconnected. The stored data is mailed or sent electronically through a secure web transfer to our Holter lab where our trained cardiac technicians analyze the data, generate a report of the findings and return the results back to the physician. Our Holter lab is distinct from the CardioNet Monitoring Center. We provided Holter monitoring services to approximately 58,000 patients in 2011.
Future Uses for MCOT™ Monitoring Platform
We believe that our integrated patient monitoring platform can be utilized for future applications in multiple markets beyond arrhythmia monitoring. We believe that we have growth opportunities in clinical trial monitoring, where we can leverage our FDA-cleared algorithms for uses such as specific cardiac data required in clinical trials, and in comprehensive disease management for congestive heart failure, diabetes and other diseases. We believe that our technology could also be used to create "instant telemetry beds" in hospitals, particularly in rural hospitals, step-down units or skilled nursing facilities, to help cope with acute nursing shortages by reducing the number of nurses needed to oversee ECG monitoring. In addition, the significant capital equipment costs associated with in-facility based cardiac telemetry (continuously attended ECG monitoring) could potentially be avoided through the use of MCOT™.
We designed MCOT™ to connect sensors and analysis devices on the patient's body (which could include ECG, weight, blood pressure, glucose and others) to a monitoring center through the use of a wireless data transmission network. Our advanced technology allows the patient system to be housed in a small, portable, non-invasive package that requires limited patient involvement and compliance. The extended monitoring period and portability of MCOT™ enables the capture and analysis of real-life patient activity through sophisticated patient information management systems and the transmission of
9
such data. Our wireless data transmission network incorporates the proprietary technologies we generated after we invested $250 million in capital and nine years in product development, including internally developed proprietary software and FDA cleared algorithms, technologically advanced medical devices and 24 hour digital monitoring service centers.
Wireless Data Transmission Network
MCOT™ makes use of multiple communication networks to transmit ECG data to the technicians in the CardioNet Monitoring Center in real time. When a patient experiences an event that meets the notification criteria that is predetermined by the physician, the monitor automatically transmits data wirelessly to the CardioNet Monitoring Center on a real-time basis. Pursuant to our agreement with nPhase, all data is sent from the monitor directly to nPhase. nPhase has both a primary and backup data center for high availability. nPhase immediately forwards the transmission to our CardioNet Monitoring Center. The CardioNet Monitoring Center is equipped with primary and backup data centers that are fully integrated with nPhase's primary and backup data centers so that data can be easily routed through a number of paths in the event of an emergency. When data is received by the CardioNet Monitoring Center, it is processed by our technicians in order of severity and time received. We have agreed with nPhase that they will be our exclusive provider of communication services for MCOT™ through the expiration of the agreement in September 30, 2014 and the agreement automatically renews for successive periods of one year each, unless terminated by either party with at least 90 days advance notice to the other party. nPhase may terminate the agreement if certain conditions occur, including if we fail to maintain an agreed upon number of active cardiac monitoring devices on the nPhase network or in the event that we begin to utilize the services of a provider of monitoring and communication services other than nPhase. Pursuant to the agreement, we are required to indemnify nPhase for all claims resulting from the provision of our services.
Research and Development
For the years ended December 31, 2011, 2010, and 2009, we spent $5.7 million, $4.9 million, and $5.8 million, respectively, on research and development expenses. We intend to continue to develop proof of superiority of our technology through clinical data. The three primary sources of clinical data that we have used to date to illustrate the clinical value of MCOT™ include: (1) a randomized 300-patient clinical study; (2) our cumulative actual monitoring experience from our databases; and (3) other published studies.
Randomized Clinical Study
We completed a 17 center, 300-patient randomized clinical trial in March 2007 that was CardioNet sponsored. We believe this study represents the largest randomized study comparing two noninvasive arrhythmia monitoring methods. The study was designed to evaluate patients who were suspected to have an arrhythmic cause underlying their symptoms, but who were a diagnostic challenge given that they had already had a non-diagnostic 24-hour Holter monitoring session or four hours of telemetry within 45 days prior to enrollment. Patients were randomized to either MCOT™ or to a loop event monitor for up to 30 days. Of the 300 patients who were randomized, 266 patients who completed a minimum of 25 days of monitoring were analyzed (134 patients using MCOT™ and 132 patients using loop event monitors).
Patient inclusion criteria included a high clinical suspicion of a malignant arrhythmia and symptoms of syncope, pre-syncope or severe palpitations occurring less frequently than once per 24 hours. Exclusion criteria included severe heart failure (as denoted by New York Heart Association Class IV), myocardial infarction (heart attack) within the prior three months, candidacy for or recent heart valve surgery, and a history of certain sustained tachycardias called ventricular tachycardia or ventricular fibrillation.
10
The primary endpoint was the confirmation or exclusion of a probable arrhythmic cause of the patient's symptoms, defined as "diagnosis." Study investigators classified any arrhythmias during the monitoring period as being either "clinically significant" or "clinically insignificant." "Confirmation" was based on investigators' assessment of the likelihood that a clinically significant arrhythmia caused the patient's presenting symptoms. "Exclusion" of a probable arrhythmic cause was determined if any reported symptoms were not associated with an arrhythmia. Monitoring was considered "non-diagnostic," or non-conclusive, if patients remained asymptomatic during the monitoring period with either no arrhythmia or only a clinically insignificant arrhythmia document. The study concluded that the primary endpoint was met.
Eric Prystowsky, a member of our board of directors, is the chief editor of the Journal of Cardiovascular Electrophysiology in which the study was published. Dr. Prystowsky recused himself from the journal's review of the study and a guest editor was chosen who selected the reviewers and oversaw the entire review process, which was blinded to Dr. Prystowsky.
The study specifically compared the success of MCOT™ against loop event monitors in detecting patients afflicted with atrial fibrillation because of the prevalence of asymptomatic episodes that occur in cases of atrial fibrillation and the difficulty of diagnosis. Diagnosis and treatment of atrial fibrillation is important because it can lead to many other medical problems, including stroke. The study concluded that MCOT™ provided a significantly higher diagnostic yield, approximately three times as likely to detect an arrhythmic event, compared to traditional loop event monitoring, including such monitoring designed to automatically detect certain arrhythmias.
CardioNet's Monitoring Experience
In January 2005, we completed a study of the first 100 patients who used CardioNet's MCOT™ service. 51% of such patients were diagnosed with clinically significant arrhythmias. 53% of patients who had previously been tested without successful diagnosis using Holter or event monitors were diagnosed with clinically significant arrhythmias by MCOT™. Of the 100 patients, 34% of patients experienced a change of management by their physician as a result of their diagnosis using MCOT™. Of those, 15% were implanted with pacemakers, 6% were implanted with cardioverter-defibrillators and 12% were prescribed ablations.
Other Studies
MCOT™ has been cited and referenced in a total of 35 publications and abstracts, including the aforementioned 300-patient randomized clinical trial.
Business Strategy
Our goal is to expand our position as the leading provider of ambulatory, continuous and real-time outpatient monitoring services by establishing our proprietary integrated technology and service offering as the standard of care for multiple health care markets. The key elements of the business strategy by which we intend to achieve these goals include:
- •
- Enhance Product Capabilities, Introduce New Products and Establish Complimentary Product Offerings
through Acquisitions or Joint Ventures. We intend to grow the business through acquiring and licensing technologies and collaborating
with third parties to offer new and complimentary product offerings. We believe there are opportunities to leverage these capabilities through select technology or company acquisitions, as well as
joint ventures that contribute to our goals of growth and market expansion.
- •
- Continue to Educate the Market on the Benefits of Our MCOT™ Solution. We intend to continue to educate cardiologists and electrophysiologists on the benefits of using MCOT™ to meet their
11
- •
- Leverage Monitoring Platform to New Market
Opportunities. We believe that MCOT™ is a platform that can be leveraged for applications in multiple markets. While our
initial focus has been on arrhythmia diagnosis and monitoring, we intend to expand into new market areas such as cardiac monitoring for clinical trials, comprehensive disease management for congestive
heart failure, diabetes and other diseases that require outpatient or ambulatory monitoring and management. We believe that our technology could also be used to create "instant telemetry beds" in
hospitals, particularly in rural hospitals, step-down units or skilled nursing facilities to help cope with acute nursing shortages by reducing the number of nurses needed to oversee ECG
monitoring and reduce capital equipment costs.
- •
- Capitalize on Clinical Trial Results and Other Publications to Enhance Payor Relationships. At year-end 2007, we had contracts with 152 commercial payors representing over 110 million covered lives. Our efforts since year-end 2007 have resulted in an additional 204 contracts, bringing our total to 356 commercial payors and Medicare as of December 31, 2011. We estimate that this represents more than 200 million covered lives. We are using evidence from clinical trials, along with subsequent publications, to both drive continued physician adoption of our solution and to attempt to secure contracts with additional commercial payors.
arrhythmia monitoring needs, stressing the increased diagnostic yield and their ability to use the clinically significant data to make timely interventions and guide more effective treatments.
Sales and Marketing
We market our arrhythmia monitoring solutions, including MCOT™, primarily to cardiologists and electrophysiologists, who are the physician specialists who most commonly diagnose and manage patients with arrhythmias. We attend trade shows and medical conferences to promote MCOT™ and to meet medical professionals with an interest in performing research and reporting their results in peer-reviewed medical journals and at major medical conferences. The trade shows and conferences we attend are related to organizations such as the Heart Rhythm Society, American College of Cardiology (ACC), numerous regional ACC chapter events, Society of Thoracic Surgeons, and the annual Boston Atrial Fibrillation Conference. We also sponsor peer-to-peer educational opportunities and participate in targeted public relations opportunities.
Segment Information
We aggregate our operations into two reportable business segments: service and products. The patient service business segment's principal focus is on the diagnosis and monitoring of cardiac arrhythmias, or heart rhythm disorders, through its core Mobile Cardiac Outpatient Telemetry (MCOT™), event and Holter services. The product business segment, which was developed through the Biotel acquisition in December 2010, focuses on the development, manufacturing, testing and marketing of medical devices and related software to medical companies, clinics and hospitals. Financial information about our business segments is provided in Note 14 to the consolidated financial statements in Part II, Item 8. "Financial Statements and Supplementary Data" of the Report.
Reimbursement
Our services are billed to government and commercial payors using specific codes describing those services. Those codes are part of the CPT coding system which was established by the American Medical Association to describe services provided by physicians and other suppliers. Physicians select the code that best describes the medical services being prescribed. In addition to receiving reimbursement from Medicare at rates that are set nationally and adjusted for certain regional indices, the Company enters into contracts with commercial payors to receive reimbursement at specified rates for our technical services. Such contracts typically provide for an initial term of between one and three
12
years and provide for automatic renewal. Either party can typically terminate these contracts by providing between 60 to 120 days prior notice to the other party at any time following the end of the initial term of the agreement. The contracts provide for an agreed upon reimbursement rate, which in some instances is tied to the rate of reimbursement we receive from Medicare. Pursuant to these contracts, we generally agree to indemnify our commercial payors for damages arising in connection with the performance of our obligations thereunder.
In addition to receiving reimbursement from government and commercial payors, the Company has direct arrangements with physicians who purchase our event, Holter and pacemaker monitoring services and then submit claims for these services directly to commercial and government payors. In some cases, patients may pay out-of-pocket on a fee for service basis.
Competition
Although we believe that we have a leading market share in the mobile cardiac arrhythmia monitoring industry, the market in which we operate is fragmented and characterized by a large number of smaller regional service providers. We believe that the principal competitive factors that impact the success of our cardiac monitoring solutions include some or all of the following:
- •
- quality of the algorithm used to detect symptoms;
- •
- quality of clinical data;
- •
- ease of use and reliability of cardiac monitoring solutions for patients and physicians;
- •
- technology performance, innovation, flexibility and range of application;
- •
- timeliness and clinical relevance of new product introductions;
- •
- quality and availability of customer support services;
- •
- size, experience, knowledge and training of sales and marketing staff;
- •
- brand recognition and reputation;
- •
- relationships with referring physicians, hospitals, managed care organizations and other third party payors;
- •
- reporting capabilities; and
- •
- perceived value.
We believe that we compete favorably based on the factors described above. However, our industry is evolving rapidly and is becoming increasingly competitive and the basis on which we compete may change over time. In addition, if companies with substantially greater resources than ours enter our market, we will face increased competition.
Intellectual Property
To protect our proprietary rights, we rely on a combination of trademark, copyright, patent, trade secret and other intellectual property laws, employment, confidentiality and invention assignment agreements with our employees and contractors, and confidentiality agreements and protective contractual provisions with our partners and other third parties.
13
Patents. As of December 31, 2011, we had 21 issued U.S. patents and 25 issued foreign patents relating to functionality of individual components of our MCOT™ device, operation of the total monitoring system, communication methodologies, control of data in the system, algorithms for ECG detection and analysis, and monitoring methods. We are in the process of applying for additional patents relating to various aspects of our technology, including our proprietary ECG detection algorithm. As of December 31, 2011, we had 46 U.S., foreign and international patent applications on file relating to various aspects of our technology.
Trademarks and Copyrights. As of December 31, 2011, we had 5 trademark registrations in the United States for a variety of word marks and slogans. Our trademarks are an integral part of our business and include, among others, the registered trademark CardioNet®, and the unregistered trademarks Mobile Cardiac Outpatient Telemetry™ and MCOT™. We also have a significant amount of copyright-protected materials, including among other things, software textual material.
In addition, we also seek to maintain certain intellectual property and proprietary know-how as trade secrets, and generally require our partners to execute non-disclosure agreements prior to any substantive discussions or disclosures of our technology or business plans. Our business and competitive positions are dependent in part upon our ability to protect our proprietary technology and our ability to avoid infringing the patents or proprietary rights of others.
Government Regulation
The health care industry is highly regulated, with no guarantee that the regulatory environment in which we operate will not change significantly and adversely in the future. We believe that health care legislation, rules, regulations and interpretations will change, and we expect to modify our agreements and operations in response to these changes.
U.S. Food and Drug Administration. The monitors and sensors that comprise part of the MCOT™ service are regulated by the FDA as a medical device under the Federal Food, Drug, and Cosmetic Act. The basic regulatory requirements that manufacturers of medical devices distributed in the U.S. must comply with are Premarket Notification 510(k), unless exempt, or Premarket Approval ("PMA"); establishment registration, medical device listing, quality system regulation, labeling requirements and medical device reporting.
The algorithms we use in the MCOT™ service maintain FDA 510(k) clearance as a Class II device. On October 28, 2003, the FDA issued a draft guidance document entitled: "Class II Special Controls Guidance Document: Arrhythmia Detector and Alarm." In addition to conforming to the general requirements of the Federal Food, Drug, and Cosmetic Act, including the premarket notification requirements described above, all of our 510(k) submissions address the specific issues covered in this special controls guidance document.
Failure to comply with applicable regulatory requirements can result in enforcement action by the FDA, which may include certain sanctions, such as fines, injunctions and civil penalties, recall or seizure of our MCOT™ devices and intellectual property, operating restrictions, partial suspension or total shutdown of production; withdrawal of 510(k) clearance of new components or algorithms, withdrawal of 510(k) clearance already granted to one or more of our existing components or algorithms, and criminal prosecution.
Health Care Fraud and Abuse. In the United States, there are state and federal anti-kickback laws that generally prohibit the payment or receipt of kickbacks, bribes or other remuneration in exchange for the referral of patients or other health care-related business. Federal legislation, such as the Physician Payments Sunshine Act of 2009, also has been proposed that would require disclosure to the federal government of payments to physicians. Anti-kickback laws constrain our sales, marketing and promotional activities by limiting the kinds of financial arrangements we may have with physicians,
14
medical centers, and others in a position to purchase, recommend or refer patients for our cardiac monitoring services or other products or services we may develop and commercialize. Due to the breadth of some of these laws, it is possible that some of our current or future practices might be challenged under one or more of these laws.
Furthermore, federal and state false claims laws prohibit anyone from presenting, or causing to be presented, claims for payment to third party payors that are false or fraudulent. Violations may result in substantial civil penalties, including treble damages, and criminal penalties, including imprisonment, fines and exclusion from participation in federal health care programs. The federal False Claims Act also contains "whistleblower" or "qui tam" provisions that allow private individuals to bring actions on behalf of the government alleging that the defendant has defrauded the government. Various states have enacted laws modeled after the federal False Claims Act, including "qui tam" provisions, and some of these laws apply to claims filed with commercial insurers. Any violations of anti-kickback and false claims laws could have a material adverse effect on our business, financial condition and results of operations.
The Patient Protection and Affordable Care Act. On March 23, 2010, the Patient Protection and Affordable Care Act was signed into law and on March 30, 2010, the Health Care and Education Reconciliation Act of 2010 was signed into law. Together, the two measures make the most sweeping and fundamental changes to the United States health care system since the creation of Medicare and Medicaid. The Health Care Reform laws include a large number of health-related provisions to take effect over the next four years, including expanding Medicaid eligibility, requiring most individuals to have health insurance, establishing new regulations on health plans, establishing health insurance exchanges, requiring manufacturers to report payments or other transfers of value made to physicians and teaching hospitals, modifying certain payment systems to encourage more cost-effective care and a reduction of inefficiencies and waste, and by including new tools to address fraud and abuse.
Health Insurance Portability and Accountability Act of 1996 (HIPAA). The Health Insurance Portability and Accountability Act was enacted by the United States Congress in 1996. Numerous state and federal laws govern the collection, dissemination, use and confidentiality of patient and other health information, including the administrative simplification provisions of HIPAA. Historically, state law has governed confidentiality issues and HIPAA preserves these laws to the extent they are more protective of a patient's privacy or provide the patient with more access to his or her health information. As a result of the implementation of the HIPAA regulations, many states are considering revisions to their existing laws and regulations that may or may not be more stringent or burdensome than the federal HIPAA provisions. HIPAA applies directly to covered entities, which include health plans, health care clearinghouses and many health care providers. These HIPAA rules' standards are concerned primarily with the privacy of information when it is used and/or disclosed; confidentiality, integrity and availability of electronic health information; and the content and format of certain identified electronic health care transactions. The laws governing health care information impose civil and criminal penalties for their violation and can require substantial expenditures of financial and other resources for information technology system modifications and for implementation of operational compliance.
Medicare. Medicare is a federal program administered by the Centers for Medicare and Medicaid Services ("CMS") through fiscal intermediaries and carriers. The Medicare program provides qualified persons with health care benefits that cover the major costs of medical care within prescribed limits, subject to certain deductibles and co-payments. The Medicare program has established guidelines for local and national coverage determinations and reimbursement of certain equipment, supplies and services. The methodology for determining coverage status and the amount of Medicare reimbursement varies based upon, among other factors, the setting in which a Medicare beneficiary received health care items and services.
15
The Medicare program is subject to statutory and regulatory changes, retroactive and prospective rate adjustments, administrative rulings, interpretations of policy, intermediary determinations, and government funding restrictions. All of these restrictions may materially increase or decrease the rate of program payments to health care facilities and other health care suppliers and practitioners, including those paid for our cardiac monitoring services. Any changes in federal legislation, regulations and policy affecting Medicare coverage and reimbursement relative to our cardiac monitoring services could have an adverse effect on our performance.
Our facilities in Pennsylvania and Minnesota are enrolled as IDTFs, and we intend to enroll our San Francisco facility as an IDTF, which is defined by CMS as an entity independent of a hospital or physician's office in which diagnostic tests are performed by licensed or certified non-physician personnel under appropriate physician supervision. Medicare has set certain performance standards that every IDTF must meet in order to obtain or maintain their billing privileges. Specifically, an IDTF is required to: (i) operate its business in compliance with all applicable federal and state licensure and regulatory requirements for the health and safety of patients; (ii) provide complete and accurate information on its enrollment application, and report certain changes, within 30 calendar days, to the designated fee-for-service contractor on the Medicare enrollment application; (iii) maintain a physical facility on an appropriate site, that is not an office box or a commercial mail box that contains space for equipment appropriate for the services designated on the enrollment application, and both business and current medical records storage within the office setting of the IDTF; (iv) have all applicable diagnostic testing equipment, with the physical site maintaining a catalog of portable diagnostic testing equipment, including the equipment's serial number; (v) maintain a primary business phone under the name of the designated business, which is located at the designated site of the business, or within the home office of the mobile IDTF units; (vi) have a comprehensive liability insurance policy of at least $0.3 million per location, covering both the place of business and all customers and employees of the IDTF, and carried by a non-relative owned company; (vii) agree not to directly solicit patients and to accept only those patients referred for diagnostic testing by an attending physician, who is furnishing a consultation or treating a beneficiary for a specific medical problem and who uses the results in the management of the beneficiary's specific medical problem; (viii) answer beneficiaries' questions and respond to their complaints; (ix) openly post the Medicare standards for review by patients and the public; (x) disclose to the government any person having ownership, financial, or control interest or any other legal interest in the supplier at the time of enrollment or within 30 days of a change; (xi) have its testing equipment calibrated and maintained per equipment instructions and in compliance with applicable manufacturers suggested maintenance and calibration standards; (xii) have technical staff on duty with the appropriate credentials to perform tests and produce the applicable federal or state licenses or certifications of the individuals performing these services; (xiii) have proper medical record storage and be able to retrieve medical records upon request from CMS or its fee-for-service contractor within two business days; and (xiv) permit CMS, including its agents, or its designated fee-for-service contractors, to conduct unannounced, on-site inspections to confirm the IDTFs compliance with these standards.
Environmental Regulation. We use materials and products regulated under environmental laws, primarily in manufacturing and the sterilization processes. While it is difficult to quantify, we believe the ongoing cost of compliance with environmental protection laws and regulations will not have a material impact on our business, financial position or results of operations.
Product Liability and Insurance
The design, manufacture and marketing of medical devices and services of the types we produce entail an inherent risk of product liability claims. In addition, we provide information to health care providers and payors upon which determinations affecting medical care are made, and claims may be made against us resulting from adverse medical consequences to patients resulting from the information
16
we provide. To protect ourselves from product liability claims, we maintain professional liability and general liability insurance on a "claims made" basis. Insurance coverage under such policies is contingent upon a policy being in effect when a claim is made, regardless of when the events which caused the claim occurred. While, as of the date of this Report, a product liability claim has never been made against us and we believe our insurance policies are adequate in amount and coverage for our current operations, there can be no assurance that the coverage maintained by us is sufficient to cover all future claims. In addition, there can be no assurance that we will be able to obtain such insurance on commercially reasonable terms in the future.
Manufacturing
Our Chester, PA, Phoenix, AZ, and Eagan, MN facilities provide space for our production and in-house depot repair operations, product upgrading, packaging, storage and shipping. We believe that our manufacturing facilities will be sufficient to meet our manufacturing needs for the foreseeable future. Our facilities located in San Diego, CA and Eagan, MN are responsible for product specifications and development under FDA guidelines.
We believe our manufacturing operations are in compliance with regulations mandated by the FDA. We are subject to unannounced inspections by the FDA and we successfully completed a routine audit by the FDA in December 2011 with no significant findings noted or warnings issued. In June 2009, our San Diego and Chester facilities received ISO 13485:2003 certification, and in July 2009 we registered our Chester facility with the FDA. Our Phoenix facility received ISO 13485:2003 certification in July 2010 and became FDA-registered in October 2010. Additionally, our Eagan, MN facility, which was acquired through the Biotel acquisition in December 2010, is registered with the FDA and is ISO 13485:2003 certified. ISO 13485 is a quality system standard used by medical companies providing design, development, manufacturing, installation and servicing.
Manufacturing of our monitors, sensors and bases is provided by a limited number of electronics manufacturing service providers. However, we believe that there are ample other capable suppliers available should we choose to supplement our current service providers' capabilities and capacity. Our production group provides system test and product release activities.
There are a number of critical components and sub-assemblies in the monitors, sensors and bases that compose part of our MCOT™ service. The vendors for these materials are qualified through stringent evaluation and testing of their performance. We implement a strict no change policy with our contract manufacturer to ensure that no components are changed without our approval.
Employees
As of December 31, 2011, we employed 665 full-time employees. None of our employees are represented by a collective bargaining agreement. We consider our relationship with our employees to be good.
Corporate Governance and Internet Address
The Company emphasizes the importance of professional business conduct and ethics through its corporate governance initiatives. The Company's Board of Directors has adopted a code of business conduct and ethics that applies to all employees, directors and officers, including the Company's principal executive officer and principal financial officer. Our corporate governance information and materials, including our Code of Business Conduct and Ethics, are posted on the corporate governance section of our website at www.cardionet.com. Our Board regularly reviews corporate governance developments and modifies these materials and practices as warranted. To the extent we make amendments to or grant waivers from our Code of Business Conduct and Ethics in the future, we intend to disclose the amendments and waivers on the corporate governance section of our website.
17
The information contained on our website, or on other websites linked to our website, is not part of this document. Reference in this Report to our website is an inactive text reference only.
Available Information
We file electronically with the U.S. Securities and Exchange Commission our annual reports on Form 10-K, quarterly reports on Form 10-Q, current reports on Form 8-K and amendments to those reports filed or furnished pursuant to Section 13(a) or 15(d) of the Securities Exchange Act of 1934. We make available on our website at http://www.cardionet.com, free of charge, copies of these reports as soon as reasonably practicable after we electronically file such material with, or furnish it to the SEC. Further copies of these reports are located at the SEC's Public Reference Room at 100 F Street, NE, Washington, D.C. 20549. Information on the operation of the Public Reference Room can be obtained by calling the SEC at 1-800-SEC-0330. The SEC maintains a website that contains reports, proxy and information statements, and other information regarding our filings, at http://www.sec.gov.
Risks related to our business and industry
We have a history of net losses and future profitability is uncertain.
We have incurred net losses from our inception. For the years ended December 31, 2011 and 2010, we realized net losses of $61.4 million and $19.9 million, respectively. As of December 31, 2011, we had total accumulated deficit of approximately $174.3 million. Although we have initiated plans to reduce our operating losses and achieve profitability, we may continue to incur losses if we are not able to execute our plans. If we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis.
Our business is dependent upon physicians prescribing our services; if we fail to obtain those prescriptions, our revenue could fail to grow and could decrease.
The success of our business is dependent upon physicians prescribing our services. Our success in obtaining prescriptions will be directly influenced by a number of factors, including:
- •
- the ability of the physicians with whom we work to obtain sufficient reimbursement and be paid in a timely manner for the
professional services they provide in connection with the use of our arrhythmia monitoring solutions;
- •
- continuing to assert ourselves as a comprehensive arrhythmia monitoring services provider;
- •
- our ability to educate physicians regarding the benefits of MCOT™ over alternative diagnostic monitoring
solutions; and
- •
- the clinical efficacy of MCOT™.
If we are unable to educate physicians regarding the benefits of MCOT™ and obtain sufficient prescriptions for our services, revenue from the provision of our arrhythmia monitoring solutions could potentially decrease.
We and the physicians with whom we work are dependent upon reimbursement for the fees associated with our services; the absence or inadequacy of reimbursement would cause our revenue to fail to grow, or could cause our revenue to decrease.
We receive reimbursement for our services from commercial payors and from Medicare Part B carriers where the services are performed on behalf of CMS. The Medicare Part B carriers in each
18
state change from time to time, which may result in changes in coverage for our services, increased administrative burden and reimbursement delays.
In addition, our prescribing physicians receive reimbursement for professional interpretation of the information provided by our products and services from commercial payors or Medicare carriers. The efficacy, safety, performance and cost-effectiveness of our products and services, on a stand- alone basis and relative to competing services, will determine the availability and level of reimbursement we and our prescribing physicians receive. Our ability to successfully contract with payors is critical to our business because physicians and their patients will select arrhythmia monitoring solutions other than ours in the event that payors refuse to adequately reimburse our technical fees and physicians' professional fees.
The national reimbursement rate set by CMS for our mobile cardiovascular telemetry service is subject to continuing change and any reductions in reimbursement levels would decrease our revenues and adversely affect our results of operations and financial condition.
Reimbursement to healthcare providers, including the Company, is subject to continuing change in policies by CMS. Reimbursement from governmental payors is subject to statutory and regulatory changes, retroactive rate adjustments, administrative rulings and other policy changes, all of which could materially decrease the range of services or the rate for which we are reimbursed. Reimbursement under the Medicare program for our services is subject to the physician fee schedule that is typically updated annually.
The amounts paid under the physician fee schedule are based on geographically adjusted relative value units, or RVUs, for each procedure or service, adjusted by a budget neutrality adjustor, and multiplied by an annually determined conversion factor. Historically, the formula used to calculate the fee schedule conversion factor resulted in significant decreases in payment levels. However, in every year from 2004 through 2011, Congress has intervened multiple times to freeze or increase the conversion factor.
Using the relative value formula and values currently in place, the Company's national rate is approximately $734 per service, effective January 1, 2012. This is a decrease of less than 1% from the Company's national carrier rate of $739 per service that was established by CMS in 2011. Congress recently passed legislation that freezes the Medicare reimbursement rates for 2012, avoiding a decrease in payment levels. If Congress does not intervene again to freeze or increase rates for 2013, Medicare reimbursement rates would be reduced significantly, having a materially adverse affect on our business and results of operations.
Reductions in the Medicare reimbursement rates applicable to our services may lead to pressure from insurance carriers to reduce our commercial pricing.
We have experienced declines in our Medicare reimbursement rates for MCOT™ over the past several years. As a result, we received substantial pressure from commercial payors to reduce our contractual reimbursement rates. Average commercial reimbursement rates have declined significantly from 2009 to 2011. We expect to experience some fluctuations in its average commercial reimbursement rates due to payor mix, as well as contract negotiations for new and existing payors. Over time we expect commercial payors may transition from commercial pricing to the CMS national rate. A decrease in commercial pricing would adversely affect our financial results.
19
We may experience difficulty in obtaining reimbursement for our services from commercial payors that consider our technology to be experimental and investigational, which would adversely affect our revenue and operating results.
Many commercial payors refuse to enter into contracts to reimburse the fees associated with medical devices or services that such payors determine to be "experimental and investigational". Commercial payors typically label medical devices or services as "experimental and investigational" until such devices or services have demonstrated product superiority evidenced by a randomized clinical trial. We completed a clinical trial in March 2007 that showed that MCOT™ provided higher diagnostic yield than traditional loop event monitoring. Prior to our clinical trial, MCOT™ was labeled "experimental and investigational" by several commercial payors. Since the trial was published in March 2007 we have obtained contracts with several of these commercial payors that previously labeled MCOT™ as "experimental and investigational". We have not obtained contracts with certain remaining commercial payors, however, and these payors have informed us that they do not believe the data from this trial justifies the removal of the experimental designation. As a result, these commercial payors may refuse to reimburse the technical and professional fees associated with MCOT™.
If commercial payors or Medicare decide not to reimburse our services or the related services provided by physicians, or the rates of such reimbursement change, or if we fail to properly administer claims, our revenue could fail to grow and could decrease.
Reimbursement by Medicare is highly regulated and subject to change; our failure to comply with applicable regulations, could decrease our revenue and may subject us to penalties or have an adverse impact on our business.
The Medicare program is administered by CMS, which imposes extensive and detailed requirements on medical services providers, including, but not limited to, rules that govern how we structure our relationships with physicians, how and when we submit reimbursement claims, how we operate our monitoring facilities and how and where we provide our arrhythmia monitoring solutions. Our failure to comply with applicable Medicare rules could result in discontinuing our reimbursement under the Medicare payment program, our being required to return funds already paid to us, civil monetary penalties, criminal penalties and/or exclusion from the Medicare program.
We have significant outstanding accounts receivables; failure to liquidate these receivables may lead to additional bad debt expense being recorded and could have a materially adverse effect on our operating results.
We continue to execute on several strategic initiatives to collect on outstanding receivable accounts. While we have realized improvements in collection rates and our days sales outstanding (DSO), and believe we will continue to see improvements in the foreseeable future, there is no guarantee that collection rates will remain at current levels or improve. A failure to liquidate receivables may have a materially adverse impact on our financial results.
A reduction in sales of our services or a loss of one or more of our key commercial payors would adversely affect our business and operating results.
A small number of commercial payors represent a significant percentage of our revenue. In the year ended December 31, 2011, our top 10 commercial payors by revenue accounted for approximately 60% of our total revenue. Our agreements with these commercial payors typically allow either party to the contract to terminate the contract by providing between 60 and 120 days prior written notice to the other party at any time following the end of the initial term of the contract. Our commercial payors may elect to terminate or not to renew their contracts with us for any reason and, in some instances can unilaterally change the reimbursement rates they pay. In the event any of our key commercial
20
payors terminate their agreements with us, elect not to renew or enter into new agreements with us upon expiration of their current agreements, or do not renew or establish new agreements on terms as favorable as are currently contracted, our business, operating results and prospects would be adversely affected.
We have a concentration of risk related to the accounts receivable from one customer. Failure to fully collect outstanding balances from this customer, or a combination of other customers, may adversely affect our results of operations.
As of December 31, 2011, we have balances owed to us from one customer representing approximately 16% of our total gross accounts receivable. We maintain an allowance for doubtful accounts based on the aging of outstanding receivables, as well as for any specific instances we become aware of that may preclude us from reasonably assuring collection on outstanding balances. Determining the allowance for doubtful accounts is judgmental in nature and often involves the use of significant estimates. A determination that requires a change in our estimates could have a materially adverse effect on our financial condition and operating results.
Consolidation of commercial payors could result in payors eliminating coverage of MCOT™ services or reduced reimbursement rates for MCOT™.
When payors combine their operations, the combined company may elect to reimburse MCOT™ services at the lowest rate paid by any of the participants in the consolidation. If one of the payors participating in the consolidation does not reimburse for MCOT™ at all, the combined company may elect not to reimburse for MCOT™. Our reimbursement rates tend to be lower for larger payors. As a result, as payors consolidate, our average reimbursement rate may decline.
If we do not have enough MCOT™ monitors or sensors or experience delays in manufacturing, we may be unable to fill prescriptions in a timely manner, physicians may elect not to prescribe MCOT™, and our revenue and growth prospects could be harmed.
When a physician prescribes MCOT™ to a patient, our customer service department begins the patient hook-up process, which includes procuring a monitor, sensors and base from our distribution department and sending them to the patient. While our goal is to provide each patient with a monitor, sensors and base in a timely manner, we have experienced and may, in the future, experience delays due to the availability of monitors, primarily when converting to a new generation of monitor or in connection with the increase in prescriptions following potential acquisitions of other companies.
We may also experience shortages of monitors, sensors or bases due to manufacturing difficulties. Multiple suppliers provide the components used in our MCOT™ devices, but our facilities in Chester, PA and Phoenix, AZ are registered and approved by the FDA, as the ultimate manufacturer of MCOT™ devices. Our manufacturing operations could be disrupted by fire, earthquake or other natural disaster, a labor-related disruption, failure in supply or other logistical channels, electrical outages or other reasons. If there were a disruption to our facilities in Chester or Phoenix, we would be unable to manufacture MCOT™ devices until we have restored and re-qualified our manufacturing capability or developed alternative manufacturing facilities.
Our success in obtaining future prescriptions from physicians is dependent upon our ability to promptly deliver monitors, sensors and bases to our patients, and a failure in this regard would have an adverse effect on our revenue and growth prospects.
21
If we or our suppliers fail to achieve or maintain regulatory approval of manufacturing facilities, our growth could be limited and our business could be harmed.
We currently assemble the monitors, sensors and bases for MCOT™ in Chester, PA and Phoenix, AZ. We manufacture event and Holter monitors in our Eagan, MN facility. Monitors used for pacemaker services are purchased from third parties. In order to maintain compliance with FDA and other regulatory requirements, our manufacturing facilities must be periodically re-evaluated and qualified under a quality system to ensure they meet production and quality standards. Suppliers of components and products used to manufacture MCOT™, event and Holter devices, and the manufacturers of the monitors used in pacemaker services must also comply with FDA regulatory requirements, which often require significant resources and subject us and our suppliers to potential regulatory inspections and stoppages. If we or our suppliers do not maintain regulatory approval for our manufacturing operations, our business could be adversely affected.
Our dependence on a limited number of suppliers may prevent us from delivering our devices on a timely basis.
We currently rely on a limited number of suppliers of components for MCOT™, event and Holter devices. If these suppliers became unable to provide components in the volumes needed or at an acceptable price, we would have to identify and qualify acceptable replacements from alternative sources of supply. The process of qualifying suppliers is lengthy. Delays or interruptions in the supply of our requirements could limit or stop our ability to provide sufficient quantities of devices on a timely basis, meet demand for our services, which could have a material adverse effect on our business, financial condition and results of operations.
We have outstanding lawsuits, the outcome of which is uncertain.
We are subject to material legal proceedings as described in Item 3, "Legal Proceedings." In addition to our existing lawsuits, other lawsuits may be brought against us. We may be required to defend such lawsuits, thus incurring expenses which we may not be able to bear, or which we may not be successful in defending.
We could be subject to medical liability or product liability claims, which may not be covered by insurance and which would adversely affect our business and results of operations.
The design, manufacture and marketing of services of the types we provide entail an inherent risk of product liability claims. Any such claims against us may require us to incur significant defense costs, irrespective of whether such claims have merit. In addition, we provide information to health care providers and payors upon which determinations affecting medical care are made, and claims may be made against us resulting from adverse medical consequences to patients resulting from the information we provide. In addition, we may become subject to liability in the event that the monitors and sensors we use fail to correctly record or transfer patient information or if we provide incorrect information to patients or health care providers using our services. We have also agreed to indemnify nPhase for any claims resulting from the provision of our services. If we incur one or more significant claims against us, if we are required to indemnify nPhase as a result of the provision of our services, or if we are required to undertake remedial actions in response to any such claims, such claims or actions would adversely affect our business and results of operations.
Our liability insurance is subject to deductibles and coverage limitations. In addition, our current insurance may not continue to be available to us on acceptable terms, if at all, and, if available, the coverages may not be adequate to protect us against any future claims. If we are unable to obtain insurance at an acceptable cost or on acceptable terms with adequate coverage or otherwise protect
22
against any claims against us, we will be exposed to significant liabilities, which may adversely affect our business and results of operations.
Our acquisition of other companies or technologies in the future could prove difficult to integrate and may disrupt our business and harm our operating results and prospects.
Acquisitions, in which we may engage in the future, involve risks associated with our assumption of the liabilities of an acquired company, which may be liabilities that we were or are unaware of at the time of the acquisition, potential write-offs of acquired assets and potential loss of the acquired company's key employees or customers.
We may encounter difficulties in successfully integrating our operations, technologies, services and personnel with that of the acquired company, and our financial and management resources may be diverted from our existing operations. Offices in multiple states create a strain on our ability to effectively manage our operations and key personnel. If we elect to consolidate our facilities, we may lose key personnel unwilling to relocate to the consolidated facility, may have difficulty hiring appropriate personnel at the consolidated facility and may have difficulty providing continuity of service through the consolidation.
Physician and patient satisfaction or performance problems with an acquired business, technology, service or device could also have a material adverse effect on our reputation. Additionally, potential disputes with the seller of an acquired business or its employees, suppliers or customers and amortization expenses related to intangible assets could adversely affect our business, operating results and financial condition. If we fail to properly evaluate and execute acquisitions, our business may be disrupted and our operating results and prospects may be harmed.
Interruptions or delays in telecommunications systems or in the data services provided to us by nPhase or the loss of our wireless or data services could impair the delivery of MCOT™ services.
The success of MCOT™ is dependent upon our ability to store, retrieve, process and manage data and to maintain and upgrade our data processing and communication capabilities. The MCOT™ monitors rely on a third party wireless carrier to transmit data over its data network during times that the monitor is removed from its base. All data sent by our monitors via this wireless data network or via landline is routed directly to nPhase data centers and subsequently routed to our monitoring center. We are dependent upon this third party wireless carrier to provide data transmission and data hosting services to us through our agreement with nPhase. We do not have an agreement with the third party wireless carrier, and although we have an agreement with nPhase that has a termination date in September 2014, nPhase may terminate its agreement with us if certain conditions occur. We have no control over the status of the agreement between nPhase and the wireless carrier. If we fail to maintain our relationship with nPhase, or if we lose wireless carrier services, we would be forced to seek alternative providers of data transmission and data hosting services, which might not be available on commercially reasonable terms or at all.
As we expand our commercial activities, an increased burden will be placed upon our data processing systems and the equipment upon which they rely. Interruptions of our data networks, or the data networks of nPhase, for any extended length of time, loss of stored data or other computer problems could have a material adverse effect on our business and operating results. Frequent or persistent interruptions in our arrhythmia monitoring services could cause permanent harm to our reputation and could cause current or potential users of MCOT™ or prescribing physicians to believe that our systems are unreliable, leading them to switch to our competitors. Such interruptions could result in liability, claims and litigation against us for damages or injuries resulting from the disruption in service.
23
Our systems are vulnerable to damage or interruption from earthquakes, floods, fires, power loss, telecommunication failures, terrorist attacks, computer viruses, break-ins, sabotage, and acts of vandalism. Despite any precautions that we may take, the occurrence of a natural disaster or other unanticipated problems could result in lengthy interruptions in these services. We do not carry business interruption insurance to protect against losses that may result from interruptions in service as a result of system failures. Moreover, the communications and information technology industries are subject to rapid and significant changes, and our ability to operate and compete is dependent on our ability to update and enhance the communication technologies used in our systems and services.
If our competitors are able to develop or market monitoring solutions that are more effective, or gain greater acceptance in the marketplace than our solutions, our commercial opportunities will be reduced or eliminated.
The market for arrhythmia monitoring solutions is evolving rapidly and becoming increasingly competitive. Our industry is highly fragmented and characterized by a small number of large providers and a large number of smaller regional service providers. These third parties compete with us in marketing to payors and prescribing physicians, recruiting and retaining qualified personnel, acquiring technology and developing solutions complementary to our programs. In addition, as companies with substantially greater resources than ours enter our market, we will face increased competition. If our competitors are better able to develop and patent arrhythmia monitoring solutions than us, or develop more effective or less expensive arrhythmia monitoring solutions that render our solutions obsolete or non-competitive, or deploy larger or more effective marketing and sales resources than ours, our business will be harmed and our commercial opportunities will be reduced or eliminated.
We operate in an intensely competitive industry, and our failure to respond quickly to technological developments and incorporate new features into our products could harm our ability to compete.
We operate in an intensely competitive industry that experiences rapid technological developments, changes in industry standards, changes in patient requirements, and frequent new product introductions and improvements. If we are unable to respond quickly and successfully to these developments, we may lose our competitive position, and our products or technologies may become uncompetitive or obsolete. To compete successfully, we must maintain a successful research and development effort, develop new products and production processes, and improve our existing products and processes at the same pace or ahead of our competitors. Our research and development efforts are aimed at solving increasingly complex problems, as well as creating new technologies, and we do not expect that all of our projects will be successful. If our research and development efforts are unsuccessful, our future results of operations could be materially harmed.
If we do not obtain and maintain adequate protection for our intellectual property, the value of our technology and devices may be adversely affected.
Our business and competitive positions are dependent in part upon our ability to protect our proprietary technology. To protect our proprietary rights, we rely on a combination of trademark, copyright, patent, trade secret and other intellectual property laws, employment, confidentiality and invention assignment agreements with our employees and contractors, and confidentiality agreements and protective contractual provisions with other third parties. We attempt to protect our intellectual property position by filing trademark applications and U.S., foreign and international patent applications related to our proprietary technology, inventions and improvements that are important to the development of our business.
We do not believe that any single patent, trademark or other intellectual property right of ours, or combination of our intellectual property rights, is likely to prevent others from competing with us using a similar business model. There are many issued patents and patent applications held by others in our industry and the electronics field. Our competitors may independently develop technologies that are
24
substantially similar or superior to our technologies, or design around our patents or other intellectual property to avoid infringement. In addition, we may not apply for a patent relating to products or processes that are patentable, we may fail to receive any patent for which we apply or have applied, and any patent owned by us or issued to us could be circumvented, challenged, invalidated, or held to be unenforceable, or rights granted thereunder may not adequately protect our technology or provide a competitive advantage to us. If a third-party challenges the validity of any patents or proprietary rights of ours, we may become involved in intellectual property disputes and litigation that would be costly and time-consuming.
Although third parties may infringe on our patents and other intellectual property rights, we may not be aware of any such infringement, or we may be aware of potential infringement but elect not to seek to prevent such infringement or pursue any claim of infringement, and the third party may continue its potentially infringing activities. Any decision whether or not to take further action in response to potential infringement of our patent or other intellectual property rights may be based on a variety of factors, such as the potential costs and benefits of taking such action, and business and legal issues and circumstances. Litigation of claims of infringement of a patent or other intellectual property rights may be costly and time-consuming, may divert the attention of key Company personnel, and may not be successful or result in any significant recovery of compensation for any infringement or enjoining of any infringing activity. Litigation or licensing discussions may also involve or lead to counterclaims that could be brought by a potential infringer to challenge the validity or enforceability of our patents and other intellectual property.
To protect our trade secrets and other proprietary information, we generally require our employees, consultants, contractors and outside collaborators to enter into written nondisclosure agreements. These agreements, however, may not provide adequate protection to prevent any unauthorized use, misappropriation or disclosure of our trade secrets, know-how or other proprietary information. These agreements may be breached, and we may not become aware of, or have adequate remedies in the event of, any such breach. Also, others may independently develop the same or substantially equivalent proprietary information and techniques or otherwise gain access to our trade secrets.
Our ability to market our services may be impaired by the intellectual property rights of third parties.
Our success is dependent in part upon our ability to avoid infringing the patents or proprietary rights of others. Our industry and the electronics field are characterized by a large number of patents, patent filings and frequent litigation based on allegations of patent infringement. Competitors may have filed applications for, or have been issued, patents, and may obtain additional patents and proprietary rights related to devices, services or processes that we compete with. We may not be aware of all of the patents or patent applications potentially adverse to our interests that may have been filed or issued to others.
U.S. patent applications may be kept confidential while pending in the Patent and Trademark Office. If other companies have or obtain patents relating to our products or services, we may be required to obtain licenses to those patents or to develop or obtain alternative technology. We may not be able to obtain any such licenses on acceptable terms, or at all. Any failure to obtain such licenses could impair or foreclose our ability to make, use, market or sell our products and services.
Based on the litigious nature of our industry and the electronics field and the fact that we may pose a competitive threat to some companies who own or control various patents, it is possible that one or more third parties may assert a patent infringement claim seeking damages and to enjoin the manufacture, use, sale and marketing of our products and services. If a third-party asserts that we have infringed on its patent or proprietary rights, we may become involved in intellectual property disputes and litigation that would be costly and time-consuming and could impair or foreclose our ability to make, use, market or sell our products and services.
25
Lawsuits may have already been filed against us without our knowledge. Additionally, we may receive notices from other third parties suggesting or asserting that we are infringing their patents and inviting us to license such patents. We do not believe that we are infringing on any other party's patents or that a license to any such patents is necessary. Should litigation over such patents arise, we intend to vigorously defend against any allegation of infringement.
If we are found to infringe on the patent or intellectual property rights of others, we may be required to pay damages, stop the infringing activity or obtain licenses or rights to the patents or other intellectual property in order to use, manufacture, market or sell our products and services. Any required license may not be available to us on acceptable terms or at all. If we succeed in obtaining such licenses, payments under such licenses would reduce any earnings from our products. In addition, licenses may be non-exclusive and, accordingly, our competitors may have access to the same technology as that which may be licensed to us. If we fail to obtain a required license or are unable to alter the design of our product candidates to make a license unnecessary, we may be unable to manufacture, use, market or sell our products and services, which could significantly affect our ability to achieve, sustain or grow our commercial business.
If we fail to obtain and maintain necessary FDA clearances, our business will be adversely affected.
The monitors, sensors and bases that we manufacture and use as part of our MCOT™ service are classified as medical devices and are subject to extensive regulation by the FDA. Further, we maintain establishment registration with the FDA as a distributor of medical devices. FDA regulations govern manufacturing, labeling, promotion, distribution, importing, exporting, shipping and sale of these devices. Our MCOT™ devices, including our C3 and C5 monitors, and our arrhythmia detection algorithms have "510(k) clearance" status from the FDA. Modifications to our MCOT™ devices or our algorithms that could significantly affect safety or effectiveness, or that could constitute a significant change in intended use, would require a new clearance from the FDA. If in the future we make changes to our MCOT™ devices or our algorithms, the FDA could determine that such modifications require new FDA clearance, and we may not be able to obtain such FDA clearances timely, or at all.
We are subject to continuing regulation by the FDA, including quality regulations applicable to the manufacture of our MCOT™ devices and various reporting regulations, as well as regulations that govern the promotion and advertising of medical devices. The FDA could find that we have failed to comply with one of these requirements, which could result in a wide variety of enforcement actions, ranging from a warning letter to one or more severe sanctions. These sanctions could include fines, injunctions and civil penalties; recall or seizure of MCOT™ devices; operating restrictions, partial suspension or total shutdown of production; refusal to grant 510(k) clearance of new components or algorithms; withdrawing 510(k) clearance already granted to one or more of our existing components or algorithms; and criminal prosecution. Any of these enforcement actions could be costly and significantly harm our business, financial condition and results of operations.
Enforcement of federal and state laws regarding privacy and security of patient information may adversely affect our business, financial condition or operations.
The use and disclosure of certain health care information by health care providers and their business associates have come under increasing public scrutiny. Recent federal standards under the Health Insurance Portability and Accountability Act of 1996, or HIPAA, establish rules concerning how individually-identifiable health information may be used, disclosed and protected. Historically, state law has governed confidentiality issues, and HIPAA preserves these laws to the extent they are more protective of a patient's privacy or provide the patient with more access to his or her health information. As a result of the implementation of the HIPAA regulations, many states are considering revisions to their existing laws and regulations that may or may not be more stringent or burdensome than the federal HIPAA provisions. We must operate our business in a manner that complies with all
26
applicable laws, both federal and state, and that does not jeopardize the ability of our customers to comply with all applicable laws. We believe that our operations are consistent with these legal standards. Nevertheless, these laws and regulations present risks for health care providers and their business associates that provide services to patients in multiple states. Because these laws and regulations are recent, and few have been interpreted by government regulators or courts, our interpretations of these laws and regulations may be incorrect. If a challenge to our activities is successful, it could have an adverse effect on our operations, may require us to forego relationships with customers in certain states and may restrict the territory available to us to expand our business. In addition, even if our interpretations of HIPAA and other federal and state laws and regulations are correct, we could be held liable for unauthorized uses or disclosures of patient information as a result of inadequate systems and controls to protect this information or as a result of the theft of information by unauthorized computer programmers who penetrate our network security. Enforcement of these laws against us could have a material adverse effect on our business, financial condition and results of operations.
We may be subject, directly or indirectly, to federal and state health care fraud and abuse laws and regulations and, if we are unable to fully comply with such laws, could face substantial penalties.
Our operations may be directly or indirectly affected by various broad state and federal health care fraud and abuse laws, including the Federal Healthcare Programs' Anti-Kickback Statute, which prohibits any person from knowingly and willfully offering, paying, soliciting or receiving remuneration, directly or indirectly, to induce or reward either the referral of an individual for an item or service, or the ordering, furnishing or arranging for an item or service, for which payment may be made under federal health care programs, such as the Medicare and Medicaid programs. For some of our services, we directly bill physicians, who in turn bill payors. Although we believe such payments are proper and in compliance with laws and regulations, we may be subject to claims asserting that we have violated these laws and regulations. If our past or present operations are found to be in violation of these laws, we or our officers may be subject to civil or criminal penalties, including large monetary penalties, damages, fines, imprisonment and exclusion from Medicare and Medicaid program participation. If enforcement action were to occur, our business and results of operations could be adversely affected.
The operation of our call centers and monitoring facilities is subject to rules and regulations governing IDTFs and state licensure requirements; failure to comply with these rules could prevent us from receiving reimbursement from Medicare and some commercial payors.
We have call centers and monitoring facilities in Pennsylvania, Minnesota and San Francisco that analyze the data obtained from arrhythmia monitors and report the results to physicians. In order for us to receive reimbursement from Medicare and some commercial payors, we must have a call center certified as an IDTF. Certification as an IDTF requires that we follow strict regulations governing how the center operates, such as requirements regarding the experience and certifications of the technicians who review data transmitted from our monitors. These rules and regulations vary from location to location and are subject to change. If they change, we may have to change the operating procedures at our monitoring facilities and call centers, which could increase our costs significantly. If we fail to obtain and maintain IDTF certification, our services may no longer be reimbursed by Medicare and some commercial payors, which could have a material adverse impact on our business.
We may be subject to federal and state false claims laws which impose substantial penalties.
Many of the physicians and patients who use our services file claims for reimbursement with government programs such as Medicare and Medicaid. As a result, we may be subject to the federal False Claims Act if we knowingly "cause" the filing of false claims. Violations may result in substantial civil penalties, including treble damages. The federal False Claims Act also contains "whistleblower" or
27
"qui tam" provisions that allow private individuals to bring actions on behalf of the government alleging that the defendant has defrauded the government. In recent years, the number of suits brought in the medical industry by private individuals has increased dramatically. Various states have enacted laws modeled after the federal False Claims Act, including "qui tam" provisions, and some of these laws apply to claims filed with commercial insurers. We are unable to predict whether we could be subject to actions under the federal False Claims Act, or the impact of such actions. However, the costs of defending claims under the False Claims Act, as well as sanctions imposed under the False Claims Act, could adversely affect our results of operations.
Changes in the health care industry or tort reform could reduce the number of arrhythmia monitoring solutions ordered by physicians, which could result in a decline in the demand for our solutions, pricing pressure and decreased revenue.
Changes in the health care industry directed at controlling health care costs or perceived over-utilization of arrhythmia monitoring solutions could reduce the volume of solutions ordered by physicians. If more health care cost controls are broadly instituted throughout the health care industry, the volume of cardiac monitoring solutions could decrease, resulting in pricing pressure and declining demand for our services, which could harm our operating results. In addition, it has been suggested that some physicians order arrhythmia monitoring solutions, even when the services may have limited clinical utility, primarily to establish a record for defense in the event of a claim of medical malpractice against the physician. Legal changes increasing the difficulty of initiating medical malpractice cases, known as tort reform, could reduce the amount of our services prescribed as physicians respond to reduced risks of litigation, which could harm our operating results.
Legislation and policy changes reforming the United States healthcare system may have a material adverse effect on our operating results and financial condition.
On March 23, 2010, the Patient Protection and Affordable Care Act was signed into law and on March 30, 2010, the Health Care and Education Reconciliation Act of 2010 was signed into law. Together, the two measures make the most sweeping and fundamental changes to the United States health care system since the creation of Medicare and Medicaid. The Health Care Reform laws include a large number of health-related provisions to take effect over the next few years, including expanding Medicaid eligibility, requiring most individuals to have health insurance, establishing new regulations on health plans, establishing health insurance exchanges, requiring manufacturers to report payments or other transfers of value made to physicians and teaching hospitals, and modifying certain payment systems to encourage more cost-effective care.
In addition, various healthcare reform proposals have also emerged at the state level. We cannot predict the effect that newly enacted laws or any future legislation or regulation will have on us. However, the implementation of new legislation and regulation may lower reimbursements for our products, reduce medical procedure volumes and adversely affect our business.
We experienced an impairment of goodwill in 2011 that had a material negative impact on our operating results. Further write-offs of the value of our goodwill or intangible assets could adversely affect our results of operations.
Current accounting rules require that goodwill and certain intangible assets be assessed for impairment annually, or when events arise that might indicate impairment, using fair value measurement techniques. When the carrying amount of a reporting unit exceeds its fair value, a goodwill impairment test is performed to measure the amount of the impairment loss, if any. Determining the fair value of goodwill is judgmental in nature and often involves the use of significant estimates and assumptions.
28
At December 31, 2011, the Company performed its required annual impairment test of goodwill. Based on this impairment test, the Company determined that its product unit was not impaired. However, as a result of the impairment test, the Company determined that impairment may exist in the patient services reporting unit due primarily to the fact that the market price of the Company's stock has been suppressed for a prolonged period of time. Therefore, the Company performed Step 2 of the goodwill impairment analysis on its patient services reporting unit.
Based on the impairment analysis performed as of December 31, 2011, we recorded an impairment charge of $46.0 million to reduce the carrying value of goodwill in our patient services segment. The remaining carrying value of goodwill after impairment is $3.4 million for the Company's products operating segment. If a determination is made in the future requiring the write-off of a significant portion of goodwill or intangible assets, it could have a further material adverse effect on our financial position and results of operations.
Tax requirements and audits could impact our results of operations.
We are subject to the tax laws of various jurisdictions. Our results of operations could be materially affected with a change in tax law or in the interpretation of tax law. This also includes the risk of changes in tax rates and the risk of failure to comply with procedures required by the taxing authorities. Failure to manage our tax strategies could lead to an additional tax charge. Any material disagreement with taxing authorities could result in cash expenditures and adversely affect our results of operations and financial position.
Our annual operating results and stock price may be volatile or may decline regardless of our operating performance.
The market price for our common stock has been and is likely to continue to be volatile, and may fluctuate significantly in response to a number of factors, most of which we cannot control, including:
- •
- changes in reimbursement rates or policies by payors;
- •
- adoption of our services by physicians;
- •
- changes in Medicare rules or regulations;
- •
- the development of increased competition for arrhythmia monitoring solutions;
- •
- price and volume fluctuations in the overall stock market;
- •
- changes in operating performance and stock market valuations of other early stage companies generally;
- •
- changes in the competitive landscape of the market for our services, including technological innovations by our
competitors and new entrants to the market;
- •
- the financial projections we may provide to the public, any changes in these projections or our failure to meet these
projections;
- •
- changes in financial estimates by any securities analysts who follow our common stock, our failure to meet these estimates
or failure of those analysts to initiate or maintain coverage of our common stock;
- •
- ratings downgrades by any securities analysts who follow our common stock;
- •
- the public's response to press releases or other public announcements by us or third parties, including our filings with the SEC, regulatory matters relating to governmental entities including Medicare, the FDA, and the Department of Justice, and announcements relating to payor
29
- •
- market conditions or trends in our industry or the economy as a whole;
- •
- the development and sustainability of an active trading market for our common stock;
- •
- future sales of our common stock by our officers, directors and significant stockholders;
- •
- other events or factors, including those resulting from war, incidents of terrorism, natural disasters or responses to
these events; and
- •
- changes in accounting principles.
reimbursement decisions, product development, litigation and intellectual property impacting us or our business;
In addition, the stock markets, and in particular the Nasdaq Global Market, have experienced considerable price and volume fluctuations that have affected and continue to affect the market prices of equity securities of many health care companies. Stock prices of many health care companies have fluctuated in a manner unrelated or disproportionate to the operating performance of those companies. In the past, stockholders have instituted securities class action litigation following periods of market volatility. If we were involved in securities litigation, we could incur substantial costs, and our resources and the attention of management could be diverted from our business.
If we need to raise additional funding in the future, we may be unable to raise such capital when needed, or at all, and the terms of such capital may be adverse to our stockholders.
We believe that the net proceeds from our initial public offering, together with our existing cash and cash equivalents and investment balances, will be sufficient to meet our anticipated cash requirements for the foreseeable future. However, our future funding requirements will depend on many factors, including:
- •
- the results of our operations;
- •
- the reimbursement rates associated with our products and services;
- •
- our ability to secure contracts with additional commercial payors providing for the reimbursement of our services;
- •
- the costs associated with manufacturing and building our inventory of our current and future generation monitors;
- •
- the costs of hiring additional personnel and investing in infrastructure to support future growth;
- •
- the costs of undertaking future strategic initiatives, such as acquisitions or joint ventures;
- •
- the emergence of competing technologies and products and other adverse market developments;
- •
- the costs of preparing, filing, prosecuting, maintaining and enforcing patent claims and other intellectual property
rights or defending against claims of infringement by others; and
- •
- actions taken by the FDA, CMS and other regulatory authorities affecting MCOT™ and competitive products.
If we decide to raise additional capital in the future, such capital may not be available on reasonable terms, or at all. If we raise additional funds by issuing equity securities, substantial dilution to existing stockholders would likely result. If we raise additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and financial ratios that may restrict our ability to operate our business.
30
Future sales of our common stock may depress our stock price.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares intend to sell shares, could reduce the market price of our common stock. As of December 31, 2011, we had 24,534,601 outstanding shares of vested common stock. In addition, we have outstanding 2,468,991 options and restricted stock units (RSUs) to purchase shares of our common stock that will become exercisable over the next four years. If exercised, these options and RSUs would result in additional shares becoming available for sale upon expiration of the lock-up agreements.
Anti-takeover provisions in our charter documents and Delaware law might deter acquisition bids for us that our stockholders might consider favorable.
Our amended and restated certificate of incorporation and bylaws contain provisions that may make the acquisition of our Company more difficult without the approval of our Board of Directors. These provisions:
- •
- establish a classified board of directors so that not all members of our board are elected at one time;
- •
- authorize the issuance of undesignated preferred stock, the terms of which may be established and shares of which may be
issued without stockholder approval, and which may include rights superior to the rights of the holders of common stock;
- •
- prohibit stockholder action by written consent, which requires all stockholder actions to be taken at a meeting of our
stockholders;
- •
- provide that the board of directors is expressly authorized to make, alter, or repeal our bylaws; and
- •
- establish advance notice requirements for nominations for elections to our board or for proposing matters that can be acted upon by stockholders at stockholder meetings.
In addition, because we are incorporated in Delaware, we are subject to Section 203 of the Delaware General Corporation Law which, subject to certain exceptions, prohibits stockholders owning in excess of 15% of our outstanding voting stock from merging or combining with us. These anti-takeover provisions and other provisions under Delaware law could discourage, delay or prevent a transaction involving a change of control of our Company, even if doing so would benefit our stockholders. These provisions could also discourage proxy contests and make it more difficult for our stockholders to elect directors of their choosing and cause us to take other corporate actions such stockholders desire.
If securities or industry analysts publish inaccurate or unfavorable research about our business, our stock price and trading volume could decline.
The trading market for our common stock will depend in part on the research and reports that securities or industry analysts publish about us or our business. If one or more of the analysts who covers us downgrades our stock or publishes inaccurate or unfavorable research about our business, our stock price would likely decline. If one or more of these analysts ceases coverage of us or fails to publish reports on us regularly, demand for our stock could decrease, which could cause our stock price and trading volume to decline.
We do not expect to pay any cash dividends for the foreseeable future.
The continued expansion of our business may require substantial funding. Accordingly, we do not anticipate that we will pay any cash dividends on shares of our common stock for the foreseeable
31
future. Any determination to pay dividends in the future would be at the discretion of our Board of Directors and will depend upon our results of operations, financial condition, contractual restrictions, restrictions imposed by applicable law and other factors our Board of Directors deems relevant. Accordingly, realization of a gain of investment from our stock will depend on the appreciation of the price of our common stock, which may never occur. Investors seeking cash dividends in the foreseeable future should not purchase our common stock.
General economic conditions, which are largely out of our control, may adversely affect our financial condition and results of operations.
Our operations may be affected by changes in general economic conditions. Recessionary economic cycles, higher interest rates, inflation, higher levels of unemployment, changes in the laws or industry regulations or other economic factors may adversely affect the demand for our products. Additionally, these economic factors and changes in laws and regulations may adversely affect our financial condition and results of operations.
Economic conditions may materially and adversely affect our investment portfolio.
We have invested our excess cash in money market funds and corporate debt securities issued by banks and corporations. The interest paid on these types of investments and the value of certain securities may decline due to general market conditions, such as the recent volatility in credit markets and the national and global financial crisis. If there is continued and expanded disruption in the credit markets, our investment portfolio could be adversely affected. There is no assurance that the financial or credit markets will improve in the near term, or that the national and worldwide economic conditions will not worsen.
Item 1B. Unresolved Staff Comments
None.
We lease facilities in the following locations :
- •
- 55,000 square feet of space for our headquarters and service center in Conshohocken, PA, under an agreement that expires
in December 2013
- •
- 12,000 square feet of space dedicated to research and development, various IT functions, and engineering activities in San
Diego, CA, under an agreement that expires in November 2014
- •
- 10,000 square feet of space for our distribution operation in Chester, PA, under an agreement that expires in October 2012
- •
- 11,000 square feet of space for our distribution operation in Phoenix, AZ, under an agreement that expires in April 2015
- •
- 50,000 square feet of space for our event and Holter monitoring, as well as product production in Eagan, MN, under an
agreement that expires in January 2017
- •
- 1,225 square feet of space for contract research monitoring in Bannockburn, IL, under an agreement that expires in April
2013
- •
- 7,000 square feet for space for our MCOT™ monitoring facility in San Francisco, CA, under an agreement that expires in March, 2019
We believe that our existing facilities are adequate to meet our current needs, and that suitable additional alternative spaces will be available in the future on commercially reasonable terms.
32
On September 25, 2009, LifeWatch Services, Inc., and Card Guard Scientific Survival, Ltd., the licensee and owner, respectively, of U.S. Patent Nos. 7,542,878 B2 ("the '878 Patent") and 5,730,143 ("the '143 Patent") commenced an action LifeWatch Patent Matter against CardioNet's wholly owned subsidiary, Braemar Inc. ("Braemar"), and one of its customers, eCardio Diagnostics, LLC ("eCardio"), in Federal District Court for the Northern District of Illinois, File No. 09-CV-6001, alleging that Braemar and eCardio had infringed the '878 and '143 Patents. The Supply Agreement between Braemar and eCardio provides that Braemar will hold eCardio harmless from any liability it incurs in connection with a claim that Braemar's products violate the intellectual property rights or infringe upon any patent of a third party. Braemar and eCardio have denied the allegations. Since the action was commenced, the Plaintiffs have dismissed their claims relating to alleged infringement of the '878 Patent, Card Guard dropped out of the action, and LifeWatch has continued to pursue its claims relating to the alleged infringement of the '143 Patent. The '143 Patent has been in reexamination proceedings since February 19, 2010. On February 1, 2011, the U.S. Patent Office indicated that the claims as amended during the reexamination will be issued. The Company believes that LifeWatch's claims under the original '143 Patent and under the soon-to-issue amended patent are without merit and intends to defend the litigation vigorously. Consistent with the accounting for contingent liabilities, no accrual has been recorded in the financial statements.
On August 25, 2011, the Company received a Civil Investigative Demand ("CID") issued by the U.S. Department of Justice, Western District of Washington. The CID states that it was issued in the course of an investigation under the federal false claims act and seeks documents for the period January 1, 2007 through the date of the CID. The CID indicates that the investigation concerns allegations that the Company may have used inappropriate diagnosis codes when submitting claims for payment to Medicare for its real-time, outpatient cardiac monitoring services. The Company is cooperating with the government's request and is in the process of providing information in response to the CID. The Company is unable to predict what action, if any, might be taken in the future by the Department of Justice or other governmental authorities as a result of this investigation or what impact, if any, the outcome of this matter might have on the Company's business, financial position or results of operations. The Company cannot reasonably estimate the range of loss, if any, that may result from this matter. Consistent with the accounting for contingent liabilities, no accrual has been recorded in the financial statements.
On December 12, 2011 the Company announced that it has reached a preliminary agreement to settle the West Palm Beach Police Pension Fund putative class action litigation filed in California Superior Court, San Diego County, which asserted claims against the Company for violations of Sections 11, 12 and 15 of the Securities Act of 1933, and is more fully described in the Company's Quarterly Report on Form 10-Q for the third quarter and nine months ended September 30, 2011. The preliminary agreement is subject to certain conditions, including court approval of a final settlement agreement. The parties filed a stipulation of settlement and plaintiff filed a motion for preliminary approval on January 6, 2012. Under the terms of the preliminary agreement, in consideration for the settlement and release of all defendants, the amount of $7.25 million will be paid by or on behalf of the defendants (of which management expects approximately $6 million will be covered by insurance). The court issued an order preliminarily approving the settlement on January 13, 2012 and set June 22, 2012 as the date for the final fairness hearing.
Item 4. Mine Safety Disclosures
Not Applicable.
33
Item 5. Market for Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
Market Information for Common Stock
Our common stock has been traded on the Nasdaq Global Market under the symbol "BEAT" since March 19, 2008. The following table sets forth the range of high and low sale prices of our common stock for the periods indicated.
2011
Quarter Ended
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
December 31, 2011 |
$ | 3.16 | $ | 2.20 | |||
September 30, 2011 |
5.28 | 2.84 | |||||
June 30, 2011 |
5.66 | 4.42 | |||||
March 31, 2011 |
4.96 | 4.25 | |||||
2010
Quarter Ended
|
High | Low | |||||
|---|---|---|---|---|---|---|---|
December 31, 2010 |
$ | 5.83 | $ | 4.01 | |||
September 30, 2010 |
5.16 | 4.20 | |||||
June 30, 2010 |
9.98 | 5.48 | |||||
March 31, 2010 |
7.72 | 5.48 | |||||
As of February 16, 2012, there were 24,550,398 shares of our common stock outstanding. Also as of that date, we had approximately 66 holders of record, including multiple beneficial holders at depositories, banks and brokers included as a single holder in the single "street" name of each respective depository, bank or broker.
Share Repurchases
We did not repurchase any of our equity securities during 2011 or 2010.
Dividends
We have never declared or paid any cash dividends on our capital stock. We currently intend to retain all available funds and any future earnings to support our operations and finance the growth and development of our business. We do not intend to pay cash dividends on our common stock for the foreseeable future. Any future determination related to dividend policy will be made at the discretion of our Board of Directors.
Stock Performance Graph
The graph below compares the total stockholder return of an investment of $100 on March 19, 2008 (the first day of trading of our common stock on the Nasdaq Stock Exchange) through December 31, 2011 for (i) our common stock (ii) The Nasdaq Health Care Index and (iii) The Russell 2000 Index. Each of the three measures of cumulative total return assumes reinvestment of dividends, if any. The stock price performance show on the graph below is based on historical data and is not indicative of future stock price performance.
34
Comparison of Cumulative Total Return
Among CardioNet, Inc., The NASDAQ Health Care Index
and The Russell 2000 Index
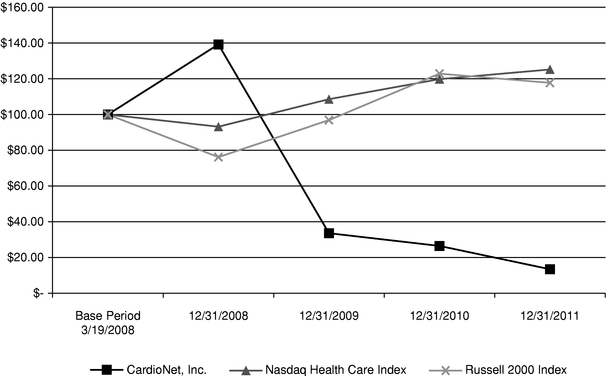
Company/Index
|
Base Period 3/19/2008 |
12/31/2008 | 12/31/2009 | 12/31/2010 | 12/31/2011 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CardioNet, Inc. |
$ | 100.00 | $ | 139.27 | $ | 33.56 | $ | 26.44 | $ | 13.39 | ||||||
Nasdaq Health Care Index |
$ | 100.00 | $ | 93.19 | $ | 108.59 | $ | 119.81 | $ | 125.22 | ||||||
Russell 2000 Index |
$ | 100.00 | $ | 76.17 | $ | 96.87 | $ | 122.89 | $ | 117.75 | ||||||
The foregoing graph and chart shall not be deemed incorporated by reference by any general statement incorporating by reference this Annual Report on Form 10-K into any filing under the Securities Act of 1933, as amended, or under the Securities Exchange Act of 1934, as amended, except to the extent we specifically incorporate this information by reference, and shall not otherwise be deemed filed under those acts.
Item 6. Selected Financial Data
The selected financial data set forth below are derived from our consolidated financial statements. The statement of operations for the years ended December 31, 2011, 2010 and 2009, and the balance sheet data at December 31, 2011 and 2010 are derived from our audited consolidated financial statements included elsewhere in this report. The statement of operations data for the years ended December 31, 2008 and 2007 and the balance sheet data at December 2009, 2008 and 2007 are derived from our audited consolidated financial statements which are not included herein.
35
The following selected financial data should be read in conjunction with the Consolidated Financial Statements and related notes thereto in Item 8 and "Management's Discussion and Analysis of Financial Condition and Results of Operation" in Item 7 of this report.
| |
Year ended December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||
| |
in thousands, except per share data |
|||||||||||||||
Statement of Operations Data: |
||||||||||||||||
Revenues: |
||||||||||||||||
Patient service revenue |
$ | 106,853 | $ | 119,924 | $ | 140,233 | $ | 119,764 | $ | 72,357 | ||||||
Product revenue |
12,169 | — | 388 | 690 | 635 | |||||||||||
Total revenues |
119,022 | 119,924 | 140,621 | 120,454 | 72,992 | |||||||||||
Cost of revenues: |
||||||||||||||||
Patient service cost of revenue |
42,258 | 47,492 | 48,688 | 39,913 | 25,526 | |||||||||||
Product cost of revenue |
6,818 | — | — | — | — | |||||||||||
Total cost of revenues |
49,076 | 47,492 | 48,688 | 39,913 | 25,526 | |||||||||||
Gross profit |
69,946 |
72,432 |
91,933 |
80,541 |
47,466 |
|||||||||||
Operating expenses: |
||||||||||||||||
Goodwill Impairment |
45,999 | — | — | — | — | |||||||||||
General and administrative |
35,011 | 34,657 | 39,153 | 27,607 | 19,397 | |||||||||||
Sales and marketing |
27,821 | 29,338 | 34,656 | 21,111 | 15,968 | |||||||||||
Bad debt expense |
12,080 | 18,578 | 19,982 | 13,253 | 8,077 | |||||||||||
Research and development |
5,698 | 4,897 | 5,810 | 3,999 | 3,782 | |||||||||||
Integration, restructuring and other charges |
4,659 | 4,654 | 12,981 | 4,880 | — | |||||||||||
Total operating expenses |
131,268 | 92,124 | 112,585 | 70,850 | 47,224 | |||||||||||
(Loss) income from operations |
(61,322 | ) | (19,692 | ) | (20,649 | ) | 9,691 | 242 | ||||||||
Other income (expense): |
||||||||||||||||
Interest income |
144 | 97 | 190 | 1,167 | 1,621 | |||||||||||
Interest expense |
— | (3 | ) | (12 | ) | (170 | ) | (2,221 | ) | |||||||
Total other income (expense) |
144 | 94 | 178 | 997 | (600 | ) | ||||||||||
(Loss) income before provision from income taxes |
$ | (61,178 | ) | $ | (19,598 | ) | $ | (20,471 | ) | $ | 10,688 | $ | (358 | ) | ||
Provision for income taxes |
244 | 262 | 5 | 1,483 | — | |||||||||||
Net (loss) income |
$ | (61,422 | ) | $ | (19,860 | ) | $ | (20,476 | ) | $ | 9,205 | $ | (358 | ) | ||
Dividends on and accretion of mandatorily redeemable convertible preferred stock |
— | — | — | (2,597 | ) | (8,346 | ) | |||||||||
Net (loss) income applicable to common shares |
$ | (61,422 | ) | $ | (19,860 | ) | $ | (20,476 | ) | $ | 6,608 | $ | (8,704 | ) | ||
Net (loss) income per common share: |
||||||||||||||||
Basic |
$ | (2.51 | ) | $ | (0.82 | ) | $ | (0.86 | ) | $ | 0.36 | $ | (2.89 | ) | ||
Diluted |
$ | (2.51 | ) | $ | (0.82 | ) | $ | (0.86 | ) | $ | 0.29 | $ | (2.89 | ) | ||
Weighted average number of shares outstanding: |
||||||||||||||||
Basic |
24,425,318 | 24,109,085 | 23,771,368 | 18,348,594 | 3,011,699 | |||||||||||
Diluted |
24,425,318 | 24,109,085 | 23,771,368 | 22,658,813 | 3,011,699 | |||||||||||
36
| |
As of December 31, | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||
| |
in thousands |
|||||||||||||||
Balance Sheet Data: |
||||||||||||||||
Cash and cash equivalents |
$ | 18,531 | $ | 18,705 | $ | 49,152 | $ | 58,171 | $ | 18,091 | ||||||
Short-term available-for-sale investments |
27,953 | 26,779 | — | — | — | |||||||||||
Working capital |
57,177 | 60,634 | 75,383 | 84,003 | 29,375 | |||||||||||
Total assets |
94,975 | 156,692 | 168,322 | 165,773 | 103,040 | |||||||||||
Total debt |
— | — | — | 72 | 2,743 | |||||||||||
Total mandatorily redeemable convertible preferred stock |
— | — | — | — | 115,302 | |||||||||||
Total shareholders' equity |
77,997 | 134,928 | 149,353 | 150,117 | (26,865 | ) | ||||||||||
Item 7. Management's Discussion and Analysis of Financial Condition and Results of Operation
You should read the following discussion and analysis of our financial condition and results of our operations in conjunction with our consolidated financial statements and the related notes to those statements included elsewhere in this report. This discussion contains forward-looking statements reflecting our current expectations that involve risks and uncertainties. Our actual results and the timing of events may differ materially from those contained in these forward-looking statements due to a number of factors, including those discussed in the section entitled "Risk Factors," and elsewhere in this prospectus. We are on a calendar year end, and except where otherwise indicated below, "2011" refers to the year ended December 31, 2011, "2010" refers to the year ended December 31, 2010 and "2009" refers to the year ended December 31, 2009.
Overview
Company Background
CardioNet is a leading provider of ambulatory, continuous, real-time outpatient management solutions for monitoring relevant and timely clinical information regarding an individual's health. The Company's efforts have initially been focused on the diagnosis and monitoring of cardiac arrhythmias, or heart rhythm disorders, with a solution that it markets as Mobile Cardiac Outpatient Telemetry™ (MCOT™). The Company actively began developing its product platform in April 2000, and since that time, has devoted substantial resources in advancing its patient monitoring solutions. The platform successfully integrates a wireless data transmission network, internally developed software, FDA 510(k)-cleared algorithms and medical devices with 24-hour monitoring. The Company also provides event, Holter and pacemaker monitoring.
In December 2010, the Company completed the acquisition of Biotel Inc., and its wholly owned subsidiaries, Braemar, Inc. and Agility Centralized Research Services, Inc. Braemar is engaged in the manufacture and sale of event and Holter medical devices, as well as the repair of such devices. Braemar's customers include distributors and other resellers, physicians, clinics and hospitals. Agility is involved primarily in contract research monitoring services. Its customers include universities, hospitals, physicians, and private companies that are involved in the research and testing of pharmaceuticals, products and medical procedures. The acquisition gives the Company access to established customer relationships, entry into the clinical trade service business and the ability to diversify its product and service offerings.
37
nPhase Supplier Agreement
The Company established a relationship with nPhase, formerly Qualcomm Inc., in May 2003. nPhase is the sole provider of wireless cellular data connectivity solutions, data hosting and queuing services for the Company's monitoring network. The Company has no fixed or minimum financial commitment as it relates to network usage or volume activity. However, if the Company fails to maintain an agreed-upon number of active cardiac monitoring devices on the nPhase network or it utilizes the monitoring and communications services of a provider other than nPhase, the Company may be subject to penalties and nPhase has the right to terminate its relationship with the Company.
Reimbursement
The Company is dependent on reimbursement for its patient services by government and commercial insurance payors. Medicare reimbursement rates for the Company's event, Holter and pacemaker monitoring services have been established nationally by the Centers for Medicare and Medicaid Services ("CMS") for many years, and fluctuate periodically based on the annually published CMS rate table.
The American Medical Association ("AMA") established CPT codes covering MCOT™ services which became effective on January 1, 2009. At that time, Highmark Medicare Services ("HMS") was responsible for setting the Medicare reimbursement rate on behalf of CMS for MCOT™ services. Reimbursement prior to the use of the MCOT™ specific CPT codes was obtained through non-specific billing codes. Effective September 1, 2009, HMS reduced the Medicare reimbursement rate for MCOT™ services to $754 per service, a reduction of approximately 33%. In November 2010, CMS announced that it would establish a national rate $739 per service for MCOT™, effective January 1, 2011. Based on the information currently available, the national rate for the Company's MCOT™ services will be approximately $734 per service beginning on January 1, 2012.
In addition to government reimbursement through Medicare, the Company has entered into contracts with commercial insurance carriers for its MCOT™, event, Holter and pacemaker monitoring services. As of December 31, 2011, we have 356 contracts with commercial payors that cover all of our monitoring services compared to 304 at December 31, 2010. We have reimbursement contracts representing approximately 65% of the current estimated total of over 200 million covered lives for Medicare and commercial insurance carriers in the United States. In addition, we have approximately 52 contracts with commercial payors that pertained only to event, Holter and pacemaker services. The majority of the remaining covered lives are insured by a relatively small number of large commercial insurance companies that have deemed MCOT™ to be experimental in nature and do not currently reimburse for services.
Commercial reimbursement pricing for our services has declined over the past three years. Commercial pricing is affected by numerous factors, including the current Medicare reimbursement rates, competitive pressures, our ability to successfully negotiate favorable terms in our agreements and the perceived value and effectiveness of our services.
Critical Accounting Policies and Estimates
The discussion and analysis of our financial condition and results of operations are based on our financial statements, which we have prepared in accordance with generally accepted accounting principles. The preparation of these financial statements requires us to make estimates and assumptions that affect the reported amount of assets and liabilities, revenues and expenses, and related disclosures. We base our estimates and judgments on historical experience and on various other factors that we believe to be reasonable under the circumstances; however, actual results may differ from these estimates. We review our estimates and judgments on an ongoing basis.
38
We believe that the following accounting policies and estimates are most critical to a full understanding and evaluation of our reported financial results. Our significant accounting policies are more fully described in Note 2 to our consolidated financial statements.
Revenue Recognition
The Company recognizes approximately 90% of its revenue from patient monitoring services, derived from its MCOT™, event, Holter and pacemaker services. The Company receives a significant portion of its revenue from third party commercial payors and governmental entities. It also receives reimbursement directly from patients through co-pays, deductibles and self-pay arrangements.
Revenue from the Medicare program is based on reimbursement rates set by CMS. Revenue from contracted commercial payors is recorded at the negotiated contractual rate. Revenue from non-contracted commercial payors is recorded at net realizable value based on historical payment patterns. Adjustments to the estimated net realizable value, based on final settlement with the third party payors, are recorded upon settlement. If the Company does not have consistent historical information regarding collectability from a given payor, revenue is recognized when cash is received. Unearned amounts are appropriately deferred until service is performed.
For the years ended December 31, 2011, 2010 and 2009, revenue from Medicare as a percentage of the Company's total revenue was 33%, 35% and 36%, respectively.
Revenue received from the sale of products, product repair and supplies is recognized when shipped, or as service is completed. Unearned amounts are appropriately deferred until service is performed.
Accounts Receivable
Accounts receivable are recorded at the time revenue is recognized, net of contractual allowances and are presented on the balance sheet net of allowance for doubtful accounts. The ultimate collection of accounts receivable may not be known for several months after services have been provided and billed. The Company records bad debt expense based on the aging of the receivable using historical Company- specific data. The percentages and amounts used to record bad debt expense and the allowance for doubtful accounts are supported by various methods and analyses, including current and historical cash collections, and the aging of specific receivables. Because of continuing changes in the health care industry and third party reimbursement, it is possible that our estimates could change, which could have a material impact on our operations and cash flows.
The Company will write-off receivables when the likelihood for collection is remote, the receivables have been fully reserved, and when the Company believes collection efforts have been fully exhausted and it does not intend to devote additional resources in attempting to collect. The Company performs write-offs on a quarterly basis. The Company wrote off $14.0 million and $29.2 million of receivables for the years ended December 31, 2011 and 2010, respectively. The impact was a reduction of gross receivables and a reduction in the allowance for doubtful accounts. Additionally, the Company recorded bad debt expense of $12.1 million and $18.6 million for the years ended December 31, 2011 and 2010, respectively.
Stock Based Compensation
ASC 718, Compensation—Stock Compensation, addresses the accounting for share-based payment transactions in which an enterprise receives employee services in exchange for (a) equity instruments of the enterprise or (b) liabilities that are based on the fair value of the enterprise's equity instruments or that may be settled by the issuance of such equity instruments. ASC 718 requires that an entity measure the cost of equity-based service awards based on the grant-date fair value of the award and
39
recognize the cost of such awards over the period during which the employee is required to provide service in exchange for the award (the vesting period). ASC 718 requires that an entity measure the cost of liability-based service awards based on current fair value that is re-measured subsequently at each reporting date through the settlement date. The Company accounts for equity awards issued to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees.
We estimate the fair value of our share-based awards to employees and directors using the Black-Scholes option valuation model. The Black-Scholes option valuation model requires the use of certain subjective assumptions. The most significant of these assumptions are our estimates of the expected volatility of the market price of our stock and the expected term of the award. We base our estimates of expected volatility on a group of similar entities whose stock prices are publicly available. The expected term represents the period of time that stock-based awards granted are expected to be outstanding. Other assumptions used in the Black-Scholes option valuation model include the risk-free interest rate and expected dividend yield. The risk-free interest rate for periods pertaining to the contractual life of each option is based on the U.S. Treasury yield of a similar duration in effect at the time of grant. We have never paid, and do not expect to pay, dividends in the foreseeable future. The fair value of our stock-based awards was estimated at the date of grant using the following assumptions:
| |
Year Ended December 31, |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | |||||||
Expected volatility |
62.0 | % | 65.0 | % | 54.0 | % | ||||
Expected term (in years) |
6.25 | 6.25 | 6.25 | |||||||
Weighted-average risk-free interest rate |
2.48 | % | 2.29 | % | 2.23 | % | ||||
Expected dividends |
0.0 | % | 0.0 | % | 0.0 | % | ||||
Weighted-average grant date fair value per share |
$ | 2.82 | $ | 3.95 | $ | 10.26 | ||||
ASC 718 requires forfeitures to be estimated at the time of grant and revised, if necessary, in subsequent periods if actual forfeitures differ from estimates. Forfeitures are estimated based on our historical experience and distanct groups of employees that have similar historical forfeiture behavior are considered for expense recognition.
Goodwill and Acquired Intangible Assets
Goodwill is the excess of the purchase price of an acquired business over the amounts assigned to assets acquired and liabilities assumed in a business combination. In accordance with ASC 350, Intangibles—Goodwill and Other, goodwill is reviewed for impairment annually, or when events arise that could indicate that impairment exists. The provisions of ASC 350 require that the Company perform a two-step impairment test. In the first step, the Company compares the fair value of its reporting units to the carrying value of the reporting units. If the carrying value of the net assets assigned to the reporting units exceeds the fair value of the reporting units, then the second step of the impairment test is performed in order to determine the implied fair value of the reporting units' goodwill. If the carrying value of the reporting units' goodwill exceeds its implied fair value, an impairment loss equal to the difference is recorded.
For the purpose of performing its goodwill impairment analysis, the Company considers its business to be comprised of two reporting units, patient services and products. The Company calculates the fair value of the reporting units utilizing a weighting of the income and market approaches. The income approach is based on a discounted cash flow methodology that includes assumptions for, among other things, forecasted income, cash flow, growth rates, income tax rates, expected tax benefits and long-term discount rates, all of which require significant judgment. The market approach utilizes the Company's market data as well as market data from publicly traded companies that are similar to the Company. There are inherent uncertainties related to these factors and the judgment applied in the
40
analysis. The Company believes that the combination of an income and a market approach provides a reasonable basis to estimate the fair value of its reporting units.
The analysis performed in the third quarter of 2011 did not indicate goodwill impairment on either reporting unit. At December 31, 2011, the Company determined that is product unit was not impaired. However, due to the market price of the Company's stock trading lower than its book value for a prolonged period, the Company was required to acknowledge that impairment may exist and had to perform Step 2 of the goodwill impairment analysis on its patient services reporting unit.
The Step 2 analysis was performed by allocating the fair value of the patient services reporting unit to the identifiable assets, including unrecorded intangible assets, and liabilities. This allocation is performed as if the reporting unit had been acquired in a business combination, and assumes the purchase price was equivalent to the fair value. The residual of the fair value of the reporting unit after allocation is the implied fair value of goodwill. This value is then compared to the carrying value of the reporting unit's goodwill. If the implied fair value of goodwill is less than the carrying value, impairment exists and a charge is recorded in the amount of the difference. As a result of the Company's analysis, an impairment of $46.0 million was recorded for the year ended December 31, 2011 related to the patient services reporting unit. This charge has no effect on the Company's operations, cash balances or cash flows.
Statements of Operations Overview
Revenue
Our principal source of revenue is patient revenue from cardiac monitoring services. The amount of revenue generated is based on the number of patients enrolled through physician prescriptions and the rates reimbursed to us by commercial payors, patients and Medicare. Consistent with the economic life cycle of a premium service that is introduced and achieves successful market penetration, we expect MCOT™ pricing to decline over time due to competition and the introduction of new technologies.
Event, Holter and pacemaker monitoring services utilize widely accepted technologies, and we expect the price to remain relatively constant or with slight declines in the long-term.
In 2011, as a result of the Biotel acquisition, revenue included the sale of cardiac monitoring products to third-party distributors and service providers.
Gross Profit
Gross profit consists of revenue less the cost of revenue. Cost of revenue includes:
- •
- salaries and benefits for personnel providing various services and customer support to physicians and patients including
patient education, monitoring services, distribution services (scheduling, packaging and delivery of the devices to the patients), device repair and maintenance, and quality assurance;
- •
- cost of patient-related services provided by third-party subcontractors including device transportation to and from the
patient and cellular airtime charges related to transmission of ECGs to the CardioNet Monitoring Center;
- •
- consumable supplies sent to patients along with the durable components of MCOT™ devices;
- •
- depreciation on our medical devices; and
- •
- cost of materials and labor related to the manufacture of our products and product repair services.
41
We expect multiple factors to influence our gross profit margins in the foreseeable future. If reimbursement rates decline, it would have an adverse effect on our gross profit margin. Payor mix is unpredictable and dependent on the insurance coverage of patients that are prescribed our services. We expect to continue to achieve efficiencies in cost of revenues through process improvements, as well as from a reduction in the cost of our devices. These factors will have a favorable impact on our gross profit margins. While these factors could be offsetting, it is difficult to predict how they will influence our gross profit margins.
General and Administrative
General and administrative expense consists primarily of salaries and benefits related to general and administrative personnel, stock-based compensation, management bonus, professional fees primarily related to legal and audit fees, facilities expenses and the related overhead.
Sales and Marketing
Sales and marketing expense consists primarily of salaries, benefits, and commissions related to sales, marketing and contracting personnel. Also included are marketing programs such as trade shows and advertising campaigns.
Research and Development
Research and development expense consists primarily of salaries and benefits of personnel as well as subcontractors who work on new product development and sustaining engineering of our existing products.
Integration, Restructuring and Other Charges
From time to time, we incur integration, restructuring and other charges. These costs are related to strategic acquisitions, cost reduction programs, reorganizations and facility closures, as well as other costs that are not considered part of our ongoing business operations.
Results of Operations
Years Ended December 31, 2011 and 2010
Revenue. Total revenue for the year ended December 31, 2011 decreased to $119.0 million from $119.9 million for the year ended December 31, 2010, a decrease of $0.9 million, or 0.8%. Patient services revenue decreased $13.1 million due to slightly lower MCOT™ volume and contracted reimbursement rates partially offset by increased event and Holter volumes. Substantially offsetting the patient services revenue decline was the inclusion of revenue resulting from our Biotel acquisition of $12.2 million.
Gross Profit. Gross profit decreased to $69.9 million for the year ended December 31, 2011 from $72.4 million for the year ended December 31, 2010. The decrease of $2.5 million was due to the decreased revenues as well as higher cost of sales resulting from the acquisition of Biotel partially offset by cost reductions implemented during 2011. Gross profit as a percentage of revenue declined to 58.8% for the year ended December 31, 2011 compared to 60.4% for the year ended December 31, 2010 due to the addition of the lower margin Biotel business.
Goodwill Impairment. The Company incurred a charge of $46.0 million to reduce the carrying value of goodwill associated with the patient services unit. The impairment was driven by a suppressed market price which the Company believes is a result of current market conditions as well as market reaction to the ongoing Department of Justice inquiry. This charge had no effect on the Company's operations, cash balances or cash flows.
42
General and Administrative Expense. General and administrative expense was $35.0 million for the year ended December 31, 2011 compared to $34.7 million for the year ended December 31, 2010. The increase of $0.3 million, or 1.0%, was due primarily to the inclusion of Biotel expenses of $3.0 million partially offset by decreases in outside services of $1.5 million, and $1.2 million of other expenses. As a percent of total revenues, general and administrative expense was 29.4% for the year ended December 31, 2011 compared to 28.9% for the year ended December 31, 2010.
Sales and Marketing Expense. Sales and marketing expense was $27.8 million for the year ended December 31, 2011 compared to $29.3 million for the year ended December 31, 2010. The decrease of $1.5 million, or 5.2%, was primarily related to a $1.2 million decrease in outside services related to training and the contract sales organization and $0.3 million of other expenses. As a percentage of total revenues, sales and marketing expense was 23.4% for the year ended December 31, 2011 compared to 24.5% for the year ended December 31, 2010.
Bad Debt Expense. Bad debt expense was $12.1 million for the year ended December 31, 2011 compared to $18.6 million for the year ended December 31, 2010. The decrease of $6.5 million, or 35.0%, was primarily a result of improved cash collections due to process improvements during 2011 and lower patient services revenue. The bad debt expense recorded was based on an evaluation of historical collection experience and a review of outstanding accounts receivable, by age, by payor class. As a percentage of total revenues, bad debt expense was 10.1% for the year ended December 31, 2011 compared to 15.5% for the year ended December 31, 2010.
Research and Development Expense. Research and development expense was $5.7 million for the year ended December 31, 2011 compared to $4.9 million for the year ended December 31, 2010. The increase of $0.8 million, or 16.4%, was largely due to costs incurred in the development of our next generation MCOT™ device, C5, which was launched in December 2011 as well as the inclusion of expenses related to the acquisition of Biotel. As a percent of total revenue, research and development expense was 4.8% for the year ended December 31, 2011 compared to 4.1% for the year ended December 31, 2010.
Integration, Restructuring and Other Charges. The Company incurred integration, restructuring and other charges of $4.7 million for the year ended December 31, 2011. Restructuring and integration costs of $1.0 million were related to severances and other costs largely associated with the acquisition of Biotel. Other charges incurred were for legal fees of $1.4 million related to the settlement of the class action lawsuit, $1.1 million for professional services related to strategic initiatives, $1.0 million related to other ongoing litigation and miscellaneous charges of $0.2 million. Integration, restructuring and other charges were 3.9% of total revenues for the year ended December 31, 2011.
Integration, restructuring and other charges were $4.7 million for the year ended December 31, 2010. The restructuring costs related to the 2010 restructuring plan were $2.1 million of severance and employee related costs as well as $1.4 million of other charges. The 2010 restructuring plan included the consolidation of the Company's sales and service organizations, the closure of the Company's event monitoring facility in Georgia and consolidation with its monitoring facilities in Pennsylvania and Minnesota, and an overall reduction of administrative costs company-wide. Additionally, the Company incurred other charges of $1.2 million for the year ended December 31, 2010, including legal costs related to the Company's defense of the class-action and Biotel lawsuits. Integration, restructuring and other charges were 3.9% of total revenues for the year ended December 31, 2010.
Other Income. Net interest income was $0.1 million for the years ended December 31, 2011 and 2010. The Company had additional interest income and realized gains on available-for-sale investments, all of which were primarily offset by amortization of bond premiums during 2011.
43
Income Taxes. The Company's effective tax rate was (0.40)% for the year ended December 31, 2011 and was (1.34)% for the year ended December 31, 2010. The tax expense resulted from certain state taxes that are based on gross receipts rather than income. Additionally, the Company recognized tax expense related to the reconciliation of its prior year provision to tax return filed during 2011 and 2010.
Net Loss. The Company incurred a net loss of $61.4 million for the year ended December 31, 2011 compared to a net loss of $19.9 million for the year ended December 31, 2010.
Years Ended December 31, 2010 and 2009
Revenue. Total revenue for the year ended December 31, 2010 decreased to $119.9 million from $140.6 million for the year ended December 31, 2009, a decrease of $20.7 million, or 14.7%. MCOT™ revenue decreased $19.0 million due to a decrease in MCOT™ reimbursement rates totaling $25.4 million. The decrease in reimbursement rates was offset by an increase in volume in 2010 compared to 2009, or $4.7 million. Additionally, there was a decrease in event, Holter and other revenue of $1.7 million for the year ended December 31, 2010 compared to the year ended December 31, 2009, due to both volume and price declines.
Gross Profit. Gross profit decreased to $72.4 million for the year ended December 31, 2010 from $91.9 million for the year ended December 31, 2009. The decrease of $19.5 million was due to a decrease in revenue offset by $3.8 million of lower cost of sales resulting from our Company-wide cost reduction initiatives for the year ended December 31, 2010 compared to the year ended December 31, 2009. Gross profit as a percentage of revenue declined to 60.4% for the year ended December 31, 2010 compared to 65.4% for the year ended December 31, 2009 due to pricing pressures, partially offset by operational efficiencies.
General and Administrative Expense. General and administrative expense was $34.6 million for the year ended December 31, 2010 compared to $39.1 million for the year ended December 31, 2009. The decrease of $4.5 million, or 11.5%, was due primarily to our cost reduction efforts resulting in a decrease in stock compensation expense of $2.2 million, professional fees of $0.7 million, payroll costs of $1.1 million and other costs of $0.5 million. As a percent of total revenues, general and administrative expense was 28.9% for the year ended December 31, 2010 compared to 27.8% for the year ended December 31, 2009.
Sales and Marketing Expense. Sales and marketing expense was $29.3 million for the year ended December 31, 2010 compared to $34.7 million for the year ended December 31, 2009. The decrease of $5.4 million, or 15.3%, was due to lower payroll, travel and stock based compensation costs primarily resulting from Company-wide cost reduction efforts in 2010. As a percentage of total revenues, sales and marketing expense was 24.5% for the year ended December 31, 2010 compared to 24.6% for the year ended December 31, 2009.
Bad Debt Expense. Bad debt expense was $18.6 million for the year ended December 31, 2010 compared to $20.0 million for the year ended December 31, 2009. The decrease of $1.4 million, or 7.0%, was due to lower gross receivable balances moving into older aging brackets with higher reserve percentages, which was primarily a result of improved cash collections during 2010. The bad debt expense we recorded was based upon an evaluation of our historical collection experience and a review of outstanding accounts receivable, by age, for our various payor classes. As a percentage of total revenues, bad debt expense was 15.5% for the year ended December 31, 2010 compared to 14.2% for the year ended December 31, 2009.
Research and Development Expense. Research and development expense was $4.9 million for the year ended December 31, 2010 compared to $5.8 million for the year ended December 31, 2009. The
44
decrease of $0.9 million, or 15.7%, was largely due to lower consulting costs. As a percent of total revenue, research and development expense was 4.1% for the year ended December 31, 2010 compared to 4.1% for the year ended December 31, 2009.
Integration, Restructuring and Other Charges. The Company incurred restructuring costs of $3.5 million and other charges of $1.2 million for the year ended December 31, 2010. The restructuring costs included $2.1 million of severance and employee related costs and $1.4 million of other charges related to the 2010 restructuring plan. The 2010 restructuring plan included the consolidation of the Company's sales and service organizations, the closure of the Company's event monitoring facility in Georgia and consolidation with its monitoring facilities in Pennsylvania and Minnesota, and an overall reduction of administrative costs company-wide. The other charges related to legal costs and other miscellaneous items. Integration, restructuring and other charges were 3.9% of total revenues for the year ended December 31, 2010.
Integration, restructuring and other charges were $13.0 million for the year ended December 31, 2009. The Company incurred a one-time charge of $9.8 million related to the cancellation of unvested stock options held by certain of the Company's executive officers, severance expenses of $2.0 million related to the departure of certain executives, including the Company's former Chief Executive Officer, in the first quarter of 2009 and $1.2 million of costs associated with the 2009 restructuring plan activities that were initiated in the third quarter of 2009. These costs were offset slightly by a realized gain of $0.2 million from insurance proceeds related to the Conshohocken fire in 2008. Integration, restructuring and other charges were 9.2% of total revenues for the year ended December 31, 2009.
Other Income. Net interest income was $0.1 million for the year ended December 31, 2010, a decrease of $0.1 million, or 47.2% from $0.2 million for the year ended December 31, 2009. The Company had additional interest income and realized gains on available-for-sale investments, all of which were primarily offset by amortization of bond premiums during 2010.
Income Taxes. The Company's effective tax rate was (1.34)% for the year ended December 31, 2010, compared to an effective tax rate of zero for the year ended December 31, 2009. The tax expense resulted from certain state taxes that are based on gross receipts rather than income. Additionally, the Company recognized tax expense related to the reconciliation of its prior year provision to tax return filed during 2010.
Net Loss. The Company incurred a net loss of $19.9 million for the year ended December 31, 2010 compared to a net loss of $20.5 million for the year ended December 31, 2009.
Liquidity and Capital Resources
As of December 31, 2011, our principal source of liquidity was cash and cash equivalents of $18.5 million, available-for-sale investments of $28.0 million and net accounts receivable of $21.0 million. The Company has no short or long-term debt and does not anticipate needing to secure financing from external sources for cash to operate the business. The Company had working capital of $57.2 million as of December 31, 2011. We believe that our existing cash and cash equivalents balances will be sufficient to meet our anticipated cash requirements for the foreseeable future.
The Company generated $5.0 million of cash from operations for the year ended December 31, 2011, primarily through revenue and improved cash collection efforts. Cash was used primarily to fund the Company's net working capital requirements of $4.1 million. Additionally, the Company had $62.1 million of non-cash items related to the goodwill impairment charge, depreciation, amortization and stock compensation expense during the twelve month period.
The Company used $4.0 million to invest in medical devices for use in its ongoing operations for the year ended December 31, 2011. In addition, the Company used $49.7 million for the purchase of
45
available-for-sale securities, which was partially offset by the sale or maturity of available-for-sale securities of $47.9 million for the year ended December 31, 2011. The Company believes that the available-for-sale investments can be converted to cash in a short period of time, if needed, as all of our securities will mature within one year.
If the Company determines that it needs to raise additional capital, such capital may not be available on reasonable terms, or at all. If the Company raises additional funds by issuing equity securities, its existing stockholders' ownership will be diluted. If the Company raises additional funds by incurring debt financing, the terms of the debt may involve significant cash payment obligations as well as covenants and specific financial ratios that may restrict its ability to operate the business.
Contractual Obligations and Commitments
The following table describes our long-term contractual obligations and commitments as of December 31, 2011:
| |
(in thousands) Payments due by period |
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Contractual obligations
|
Total | 2012 | 2013 | 2014 | 2015 | 2016 | Beyond | |||||||||||||||
Operating lease obligations |
$ | 9,063 | $ | 2,933 | $ | 2,973 | $ | 1,026 | $ | 715 | $ | 687 | $ | 729 | ||||||||
As of December 31, 2011, the Company is bound under facility leases and several office equipment leases that are included in the table above. From time to time, we may enter into contracts or purchase orders with third parties under which we may be required to make payments. Our payment obligations under certain agreements will depend on, among other things, the progress of our development programs. Therefore, we are unable at this time to estimate with certainty the potential future costs we will incur under these agreements or purchase orders.
Recent Accounting Pronouncements
In May 2011, the Financial Accounting Standards Board (FASB) issued accounting standards update (ASU) 2011-04, Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS. The new guidance results in a consistent definition of fair value and common requirements for measurement of and disclosure about fair value between U.S. Generally Accepted Accounting Principles (GAAP) and International Financial Reporting Standards (IFRS). The ASU is effective for interim and annual periods beginning on or after December 15, 2011, with early adoption prohibited. The new guidance changes certain fair value measurement principles and disclosure requirements. The Company does not expect the amendments to have a material impact on its results of operations, cash flows, or financial position.
In June 2011, the FASB issued ASU 2011-05, Comprehensive Income (Topic 220): Presentation of Comprehensive Income. The ASU is effective for interim and annual periods beginning after December 15, 2011, with early adoption permitted. The new guidance eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholder's equity and states that an entity has the option to present the total of comprehensive income, the components of income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. Additionally, entities are required to present on the face of the financial statements reclassification adjustments for items that are reclassified from other comprehensive income to net income in the statement(s) where the components of net income and the components of other comprehensive are presented. This ASU will change the financial statement presentation of comprehensive income but the Company does not expect the amendments to have a material impact on its results of operations, cash flows, or financial position.
46
In July 2011, the FASB issued ASU 2011-07, Health Care Entities (Topic 954): Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts for Certain Health Care Entities. The ASU is effective for interim and annual periods beginning on or after December 15, 2011, with early adoption prohibited. The new guidance changes certain presentation and disclosure requirements for Patient Service Revenue. The Company does not expect the amendments to have a material impact on its results of operations, cash flows, or financial position, as well as does not expect it to have a change in the presentation of the consolidated financial statements.
In September 2011, the FASB issued ASU 2011-08, Intangibles—Goodwill and Other (Topic 350): Testing Goodwill for Impairment. The ASU is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011, with early adoption permitted. The new guidance allows an entity the option to first assess qualitative factors to determine whether existence of events or circumstances lead to a determination that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the qualitative assessment leads to the determination that the fair value of the reporting unit is not less than the carrying value, then performing a two-step impairment test is no longer necessary. The Company does not expect the amendments to have a material impact on its results of operations, cash flows, or financial position.
Off-Balance Sheet Arrangements
As of December 31, 2011 and 2010, we did not have any relationships with unconsolidated entities or financial partnerships, such as entities referred to as structured finance or special purpose entities, which would have been established for the purpose of facilitating off-balance sheet arrangements or other contractually narrow or limited purposes.
Item 7A. Quantitative and Qualitative Disclosures About Market Risk
Our cash and cash equivalents as of December 31, 2011 were $18.5 million, and consisted primarily of cash and money market funds with maturities of less than 90 days. We also invest our excess funds, which were $28.0 million as of December 31, 2011, in available-for-sale securities. The Company earned approximately $0.7 million in interest during 2011. A decrease in interest rates of 100 basis points would not have a material impact on the Company's financial results. The primary objective of our investment activities is to preserve our capital for the purpose of funding operations while, at the same time, maximizing the income we receive from our investments without significantly increasing risk. To achieve this objective, our investment policy allows us to maintain a portfolio of cash equivalents and short term investments in a variety of securities including money market funds and corporate debt securities. Due to the short term nature of our investments, we believe we have no material exposure to interest rate risk.
47
Item 8. Financial Statements and Supplementary Data
Report of Independent Registered Public Accounting Firm
The
Board of Directors and Shareholders
CardioNet, Inc.
We have audited the accompanying consolidated balance sheets of CardioNet, Inc. as of December 31, 2011 and 2010, and the related consolidated statements of operations, cash flows, and shareholders' equity for each of the three years in the period ended December 31, 2011. Our audits also included the financial statement schedule listed at Item 15(a). These financial statements and schedule are the responsibility of the Company's management. Our responsibility is to express an opinion on these financial statements and schedule based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the consolidated financial position of CardioNet, Inc. at December 31, 2011 and 2010, and the consolidated results of its operations and its cash flows for each of the three years in the period ended December 31, 2011, in conformity with U.S. generally accepted accounting principles. Also, in our opinion, the related financial statement schedule, when considered in relation to the basic financial statements taken as a whole, presents fairly in all material respects the information set forth therein.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), CardioNet, Inc.'s internal control over financial reporting as of December 31, 2011, based on criteria established in "Internal Control—Integrated Framework" issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated February 23, 2012 expressed an unqualified opinion thereon.
| /s/ Ernst & Young LLP |
Philadelphia,
Pennsylvania
February 23, 2012
48
CARDIONET, INC.
CONSOLIDATED BALANCE SHEETS
(In thousands, except shares and per share amounts.)
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | |||||
Assets |
|||||||
Current assets: |
|||||||
Cash and cash equivalents |
$ | 18,531 | $ | 18,705 | |||
Short-term available-for-sale investments |
27,953 | 26,779 | |||||
Accounts receivable, net of allowance for doubtful accounts of $9,889 and $11,779 at December 31, 2011 and 2010, respectively |
21,028 | 24,978 | |||||
Other receivables |
1,564 | 3,041 | |||||
Inventory |
2,009 | 1,461 | |||||
Prepaid expenses and other current assets |
1,511 | 3,086 | |||||
Total current assets |
72,596 | 78,050 | |||||
Property and equipment, net |
15,041 | 22,000 | |||||
Intangible assets, net |
2,545 | 3,764 | |||||
Goodwill |
3,363 | 49,362 | |||||
Other assets |
1,430 | 3,516 | |||||
Total assets |
$ | 94,975 | $ | 156,692 | |||
Liabilities and shareholders' equity |
|||||||
Current liabilities: |
|||||||
Accounts payable |
$ | 4,094 | $ | 7,127 | |||
Accrued expenses |
10,453 | 9,881 | |||||
Deferred revenue |
872 | 408 | |||||
Total current liabilities |
15,419 | 17,416 | |||||
Deferred tax liability |
705 | 3,191 | |||||
Deferred rent |
854 | 1,157 | |||||
Total liabilities |
16,978 | 21,764 | |||||
Shareholders' equity |
|||||||
Common stock—$.001 par value as of December 31, 2011 and 2010; 200,000,000 shares authorized as of December 31, 2011 and 2010; 24,534,601 and 24,251,170 shares issued and outstanding at December 31, 2011 and 2010, respectively |
25 | 24 | |||||
Paid-in capital |
252,261 | 247,747 | |||||
Accumulated other comprehensive (loss) income |
(16 | ) | 8 | ||||
Accumulated deficit |
(174,273 | ) | (112,851 | ) | |||
Total shareholders' equity |
77,997 | 134,928 | |||||
Total liabilities and shareholders' equity |
$ | 94,975 | $ | 156,692 | |||
See accompanying notes.
49
CARDIONET, INC.
CONSOLIDATED STATEMENTS OF OPERATIONS
(In thousands, except shares and per share amounts.)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | |||||||
Revenues: |
||||||||||
Patient service revenue |
$ | 106,853 | $ | 119,924 | $ | 140,233 | ||||
Product revenue |
12,169 | — | 388 | |||||||
Total revenues |
119,022 | 119,924 | 140,621 | |||||||
Cost of revenue: |
||||||||||
Patient service cost of revenue |
42,258 | 47,492 | 48,688 | |||||||
Product cost of revenue |
6,818 | — | — | |||||||
Total cost of revenues: |
49,076 | 47,492 | 48,688 | |||||||
Gross profit |
69,946 | 72,432 | 91,933 | |||||||
Operating expenses: |
||||||||||
Goodwill impairment |
45,999 | — | — | |||||||
General and administrative |
35,011 | 34,657 | 39,153 | |||||||
Sales and marketing |
27,821 | 29,338 | 34,656 | |||||||
Bad debt expense |
12,080 | 18,578 | 19,982 | |||||||
Research and development |
5,698 | 4,897 | 5,810 | |||||||
Integration, restructuring and other charges |
4,659 | 4,654 | 12,981 | |||||||
Total operating expenses |
131,268 | 92,124 | 112,582 | |||||||
Loss from operations |
(61,322 | ) | (19,692 | ) | (20,649 | ) | ||||
Other income (expense): |
||||||||||
Interest income |
144 | 97 | 190 | |||||||
Interest expense |
— | (3 | ) | (12 | ) | |||||
Total other income |
144 | 94 | 178 | |||||||
Loss before income taxes |
(61,178 | ) | (19,598 | ) | (20,471 | ) | ||||
Provision for income taxes |
244 | 262 | 5 | |||||||
Net loss |
(61,422 | ) | (19,860 | ) | (20,476 | ) | ||||
Net loss per common share: |
||||||||||
Basic and diluted |
$ | (2.51 | ) | $ | (0.82 | ) | $ | (0.86 | ) | |
Weighted average number of common shares outstanding: |
||||||||||
Basic and diluted |
24,425,318 | 24,109,085 | 23,771,368 | |||||||
See accompanying notes.
50
CARDIONET, INC.
CONSOLIDATED STATEMENTS OF CASH FLOWS
(In thousands, except shares and per share amounts.)
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | |||||||
Operating activities |
||||||||||
Net loss |
$ | (61,422 | ) | $ | (19,860 | ) | $ | (20,476 | ) | |
Adjustments to reconcile net loss to net cash provided by operating activities: |
||||||||||
Goodwill Impairment |
45,999 | |||||||||
Depreciation |
10,913 | 11,696 | 10,053 | |||||||
Loss on the disposal of property & equipment |
— | 807 | 408 | |||||||
(Decrease) increase in deferred rent |
(303 | ) | (340 | ) | 532 | |||||
Provision for doubtful accounts |
12,080 | 18,578 | 19,982 | |||||||
Stock-based compensation |
4,006 | 3,945 | 16,625 | |||||||
Amortization of intangibles |
1,219 | 375 | 884 | |||||||
Amortization of investment premium |
561 | 421 | — | |||||||
Changes in operating assets and liabilities: |
||||||||||
Accounts receivable |
(6,653 | ) | (3,062 | ) | (21,436 | ) | ||||
Inventory |
(548 | ) | — | — | ||||||
Prepaid expenses and other current assets |
1,575 | — | (1,759 | ) | ||||||
Other assets |
2,086 | (2,043 | ) | 238 | ||||||
Accounts payable |
(3,033 | ) | (1,182 | ) | 3,322 | |||||
Accrued and other liabilities |
(1,450 | ) | 1,027 | (469 | ) | |||||
Net cash provided by operating activities |
5,030 | 10,362 | 7,904 | |||||||
Investing activities |
||||||||||
Purchases of property and equipment |
(3,954 | ) | (5,247 | ) | (19,938 | ) | ||||
Purchases of short-term available-for-sale investments |
(49,657 | ) | (36,942 | ) | — | |||||
Sale or maturity of short-term available-for-sale investments |
47,898 | 9,750 | — | |||||||
Acquisition of business, net of cash acquired |
— | (9,852 | ) | — | ||||||
Net cash used in investing activities |
(5,713 | ) | (42,291 | ) | (19,938 | ) | ||||
Financing activities |
||||||||||
Proceeds from issuance of common stock |
— | 10 | 36 | |||||||
Proceeds from the exercise of employee stock options and employee stock purchase plan contributions |
509 | 1,472 | 3,051 | |||||||
Repayment of debt |
— | — | (72 | ) | ||||||
Net cash provided by financing activities |
509 | 1,482 | 3,015 | |||||||
Net decrease in cash and cash equivalents |
(174 | ) | (30,447 | ) | (9,019 | ) | ||||
Cash and cash equivalents—beginning of period |
18,705 | 49,152 | 58,171 | |||||||
Cash and cash equivalents—end of period |
18,531 | $ | 18,705 | $ | 49,152 | |||||
Supplemental disclosure of cash flow information |
||||||||||
Cash paid for interest |
$ | — | $ | 3 | $ | 12 | ||||
Cash paid for taxes |
$ | 171 | $ | 692 | $ | 6,218 | ||||
See accompanying notes.
51
CARDIONET, INC.
CONSOLIDATED STATEMENTS OF
SHAREHOLDERS' EQUITY
(In thousands, except share amounts.)
| |
Shareholders' Equity | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Common Stock | |
Accumulated Other Comprehensive Income |
|
|
||||||||||||||
| |
Paid-in Capital |
Accumulated Deficit |
Total Shareholders' Equity |
||||||||||||||||
| |
Shares | Amount | |||||||||||||||||
Balance, December 31, 2008 |
23,477,137 | 24 | 222,608 | — | (72,515 | ) | 150,117 | ||||||||||||
Issuance/vesting of common stock |
129,618 | — | 1,026 | — | — | 1,026 | |||||||||||||
Exercise of stock options and purchase of shares related to the employee stock purchase plan |
353,124 | — | 3,051 | — | — | 3,051 | |||||||||||||
Stock based compensation |
— | — | 15,635 | — | — | 15,635 | |||||||||||||
Exercise of warrants |
5,526 | — | — | — | — | — | |||||||||||||
Net loss |
— | — | — | — | (20,476 | ) | (20,476 | ) | |||||||||||
Balance December 31, 2009 |
23,965,405 | 24 | 242,320 | — | (92,991 | ) | 149,353 | ||||||||||||
Issuance/vesting of common stock |
22,083 | — | 1,422 | — | — | 1,422 | |||||||||||||
Exercise of stock options and purchase of shares related to the employee stock purchase plan |
263,682 | — | 1,472 | — | — | 1,472 | |||||||||||||
Stock based compensation |
— | — | 2,533 | — | — | 2,533 | |||||||||||||
Comprehensive loss: |
|||||||||||||||||||
Net loss |
— | — | — | — | (19,860 | ) | (19,860 | ) | |||||||||||
Changes in unrealized gain on available-for-sale investments |
— | — | — | 8 | — | 8 | |||||||||||||
Total comprehensive loss |
— | — | — | — | — | (19,852 | ) | ||||||||||||
Balance December 31, 2010 |
24,251,170 | 24 | 247,747 | 8 | (112,851 | ) | 134,928 | ||||||||||||
Issuance/vesting of common stock |
112,824 | 1 | 1,593 | — | — | 1,594 | |||||||||||||
Exercise of stock options and purchase of shares related to the employee stock purchase plan |
170,607 | — | 515 | — | — | 515 | |||||||||||||
Stock based compensation |
— | — | 2,406 | — | — | 2,406 | |||||||||||||
Comprehensive loss: |
|||||||||||||||||||
Net loss |
— | — | — | — | (61,422 | ) | (61,422 | ) | |||||||||||
Changes in unrealized gain on available-for-sale investments |
— | — | — | (24 | ) | — | (24 | ) | |||||||||||
Total comprehensive loss |
— | — | — | — | — | (61,446 | ) | ||||||||||||
Balance December 31, 2011 |
24,534,601 | 25 | 252,261 | (16 | ) | (174,273 | ) | 77,997 | |||||||||||
See accompanying notes.
52
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
Years Ended December 31, 2011, 2010 and 2009
(In thousands,
except shares and per share amounts.)
1. Organization and Description of Business
CardioNet (the "Company") is a leading provider of ambulatory, continuous, real-time outpatient management solutions for monitoring relevant and timely clinical information regarding an individual's health. The Company's efforts have initially been focused on the diagnosis and monitoring of cardiac arrhythmias, or heart rhythm disorders, with a solution that it markets as Mobile Cardiac Outpatient Telemetry™ (MCOT™). The Company began actively developing its product platform in April 2000, and since that time, has devoted substantial resources in advancing its patient monitoring solutions. The platform successfully integrates a wireless data transmission network, internally developed software, FDA 510(k)-cleared algorithms and medical devices with 24-hour monitoring. The Company also provides event and Holter monitoring.
In December 2010, the Company completed the acquisition of Biotel Inc., and its wholly owned subsidiaries, Braemar, Inc. and Agility Centralized Research Services, Inc. The acquisition gives the Company the ability to develop, manufacture, test and market medical devices and related software to medical companies, clinics and hospitals. Additionally, the acquisition also gave the Company access to established customer relationships, entry into the clinical trial service business and the ability to diversify its product and service offerings.
2. Summary of Significant Accounting Policies
Principles of Consolidation
The accompanying consolidated financial statements include the accounts of the Company and its wholly owned subsidiaries. All significant intercompany transactions and balances have been eliminated in consolidation.
Use of Estimates
The preparation of financial statements in conformity with accounting principles generally accepted in the United States requires that management make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenue and expenses during the reporting period. Actual results may differ from those estimates.
Fair Value of Financial Instruments
The Company's financial instruments consist mainly of cash and cash equivalents, available-for-sale investments, accounts receivable, other current assets, accounts payable, deferred revenue and other current liabilities. The carrying value of these financial instruments approximates their fair value because of their short-term nature. The fair value of financial instruments is defined as the amount at which the instrument could be exchanged in a current transaction between willing parties.
Cash and Cash Equivalents
Cash and cash equivalents are held in U.S. financial institutions or in custodial accounts with U.S. financial institutions. Cash equivalents are defined as liquid investments and money market funds with
53
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
2. Summary of Significant Accounting Policies (Continued)
maturity from date of purchase of 90 days or less that are readily convertible into cash and have insignificant interest rate risk.
Available-for-Sale Investments
Marketable securities that do not meet the definition of cash and cash equivalents are classified as available-for-sale. Available-for-sale securities are carried at fair value, based on quoted market prices and observable inputs, with unrealized gains and losses, reported as a separate component of shareholders' equity. We classify securities as current or non-current assets on the consolidated balance sheet based on maturity dates. The amortized cost of debt securities is adjusted for amortization of premiums and accretions of discounts to maturity. Amortization of debt premiums and accretion of debt discounts are recorded in other income and expense. Realized gains and losses, and declines in value, that are considered to be other-than-temporary, are recorded in other income and expense. The cost of securities sold is based on specific identification.
Accounts Receivable and Allowance for Doubtful Accounts
Accounts receivable are recorded at the time revenue is recognized, net of contractual allowances, and are presented on the balance sheet net of allowance for doubtful accounts. The ultimate collection of accounts receivable may not be known for several months after services have been provided and billed. The Company records bad debt expense based on the aging of the receivable using historical Company- specific data. The percentages and amounts used to record bad debt expense and the allowance for doubtful accounts are supported by various methods and analyses, including current and historical cash collections, and the aging of specific receivables. Because of continuing changes in the health care industry and third party reimbursement, it is possible that the Company's estimates could change, which could have a material impact on the Company's operations and cash flows.
The Company writes off receivables when the likelihood for collection is remote, the receivables have been fully reserved, and when the Company believes collection efforts have been fully exhausted and it does not intend to devote additional resources in attempting to collect. The Company performs write-offs on a quarterly basis. The Company wrote off $13,970 and $29,195 of receivables for the years ended December 31, 2011 and 2010, respectively. The impact was a reduction of gross receivables and a reduction in the allowance for doubtful accounts. Additionally, the Company recorded bad debt expense of $12,080, $18,578, and 19,982 for the years ended December 31, 2011, 2010 and 2009, respectively. Based on collection experience in 2011, 2010 and 2009, bad debt expense included $2,074, $11,669, and $9,112 in 2011, 2010, and 2009, respectively, related to prior year's accounts receivable.
Concentrations of Credit Risk
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash, cash equivalents, short-term available-for-sale investments and accounts receivable. The Company maintains its cash and cash equivalents with high quality financial institutions to mitigate this risk. The Company has established guidelines to limit exposure to credit risk by placing investments with high quality financial institutions, diversifying the Company's investment portfolio and placing
54
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
2. Summary of Significant Accounting Policies (Continued)
investments with maturities that maintain safety and liquidity. The Company performs ongoing credit evaluations of its customers and generally does not require collateral. The Company records an allowance for doubtful accounts in accordance with the procedures described above. Past-due amounts are written off against the allowance for doubtful accounts when collections are believed to be unlikely and all collection efforts have ceased.
At December 31, 2011, 2010 and 2009, one customer accounted for 19%, 18% and 20%, respectively, of the Company's net accounts receivable.
Inventory
Inventory is valued at the lower of cost (using the average and first-in, first-out cost methods) or market (net realizable value or replacement cost). Company management periodically reviews inventory for specific future usage, and estimates of impairment of individual inventory items are recorded to reduce inventory to the lower of cost or market.
Property and Equipment
Property and equipment is recorded at cost. Depreciation is recorded over the estimated useful life of each class of depreciable assets (generally 2-5 years), and is computed using the straight-line method. Leasehold improvements are amortized over the shorter of the estimated asset life or term of the lease. Repairs and maintenance costs are charged to expense as incurred.
Impairment of Long-Lived Assets
The Company periodically evaluates the recoverability of the carrying value of its long-lived assets based on the criteria established in Accounting Standards Codification (ASC) 360, Property, Plant & Equipment. The Company considers historical performance and anticipated future results in its evaluation of potential impairment. Accordingly, when indicators of impairment are present, the Company evaluates the carrying value of these assets in relation to the operating performance of the business and the undiscounted cash flows expected to result from the use of these assets. Impairment losses are recognized when the sum of the expected future cash flows is less than the assets' carrying value.
Goodwill and Acquired Intangible Assets
Goodwill is the excess of purchase price of an acquired business over the amounts assigned to assets acquired and liabilities assumed in a business combination. In accordance with ASC 350, Intangibles—Goodwill and Other, goodwill is reviewed for impairment annually, or when events arise that could indicate that impairment exists. The provisions of ASC 350 require that the Company perform a two-step impairment test. In the first step, the Company compares the fair value of its reporting units to the carrying value of the reporting units. If the carrying value of the net assets assigned to the reporting units exceeds the fair value of the reporting units, then the second step of the impairment test is performed in order to determine the implied fair value of the reporting units'
55
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
2. Summary of Significant Accounting Policies (Continued)
goodwill. If the carrying value of the reporting units' goodwill exceeds its implied fair value, an impairment loss equal to the difference is recorded.
For the purpose of performing its goodwill impairment analysis, the Company considers its business to be comprised of two reporting units, patient services and products. The Company calculates the fair value of the reporting units utilizing a weighting of the income and market approaches. The income approach is based on a discounted cash flow methodology that includes assumptions for, among other things, forecasted income, cash flow, growth rates, income tax rates, expected tax benefits and long-term discount rates, all of which require significant judgment. The market approach utilizes the Company's market data as well as market data from publicly traded companies that are similar to the Company. There are inherent uncertainties related to these factors and the judgment applied in the analysis. The Company believes that the combination of an income and a market approach provides a reasonable basis to estimate the fair value of its reporting units.
Revenue Recognition
The Company recognizes approximately 90% of its revenue from patient monitoring services, derived from its MCOT™, event, Holter and pacemaker services. The Company receives a significant portion of its revenue from third party commercial payors and governmental entities. It also receives reimbursement directly from patients through co-pay, deductibles and self-pay arrangements.
Revenue from the Medicare program is based on reimbursement rates set by CMS. Revenue from contracted commercial payors is recorded at the negotiated contractual rate. Revenue from non-contracted commercial payors is recorded at net realizable value based on historical payment patterns. Adjustments to the estimated net realizable value, based on final settlement with the third party payors, are recorded upon settlement. If the Company does not have consistent historical information regarding collectability from a given payor, revenue is recognized when cash is received. Unearned amounts are appropriately deferred until service is performed.
For the years ended December 31, 2011, 2010 and 2009, reimbursement revenue from Medicare as a percentage of the Company's total revenue was 33%, 35% and 36%, respectively.
Revenue received from the sale of products, product repair and supplies is recognized when shipped, or as service is completed. Unearned amounts are appropriately deferred until service is performed.
Advertising Costs
Advertising costs are charged to expense as incurred. For the years ended December 31, 2011, 2010 and 2009, the Company incurred advertising costs of $218, $823 and $628, respectively.
Research and Development Costs
Research and development costs are charged to expense as incurred.
56
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
2. Summary of Significant Accounting Policies (Continued)
Net Loss
The Company computes net loss per share in accordance with ASC 260, Earnings Per Share. Basic net loss per share is computed by dividing net loss per share available to common shareholders by the weighted average number of common shares outstanding for the period, and excludes the effects of any potentially dilutive securities. Diluted earnings per share, if presented, would include the dilution that would occur upon the exercise or conversion of all potentially dilutive securities into common stock using the treasury stock or if converted methods, as applicable.
The following summarizes the potential outstanding common stock of the Company as of the end of each period:
| |
December 31, 2011 |
December 31, 2010 |
December 31, 2009 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Employee stock purchase plan estimated share options outstanding |
51,544 | 40,208 | 58,095 | |||||||
Common stock options and restricted stock units ("RSUs") outstanding |
2,468,991 | 2,102,376 | 1,575,645 | |||||||
Common stock options available for grant |
2,369,802 | 1,649,723 | 1,132,135 | |||||||
Common stock held by certain employees and unvested |
— | — | 9,583 | |||||||
Common stock |
24,534,601 | 24,251,170 | 23,965,405 | |||||||
Total |
29,315,655 | 28,043,477 | 26,740,863 | |||||||
Basic net loss per share is computed by dividing net loss by the weighted average number of fully vested common shares outstanding during the period. Diluted net loss per share is computed by giving effect to all potential dilutive common shares, including stock options, and RSUs.
The following table presents the calculation of historical basic and diluted net loss per share:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | |||||||
| |
(in thousands, except per share amounts) |
|||||||||
Numerator: |
||||||||||
Net loss |
$ | (61,422 | ) | $ | (19,860 | ) | $ | (20,476 | ) | |
Denominator: |
||||||||||
Weighted average common shares outstanding—Basic |
24,425,318 | 24,109,085 | 23,771,368 | |||||||
Dilutive effect of the Company's employee stock compensation plans |
— | — | — | |||||||
Weighted average shares used in computing diluted net loss per share |
24,425,318 | 24,109,085 | 23,771,368 | |||||||
Basic and diluted net loss per share |
$ | (2.51 | ) | $ | (0.82 | ) | $ | (0.86 | ) | |
57
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
2. Summary of Significant Accounting Policies (Continued)
If the outstanding options and unvested stock were exercised or converted into common stock, the result would be anti-dilutive for the years ended December 31, 2011, 2010 and 2009. Accordingly, basic and diluted net loss attributable to common stockholders per share are identical for these periods presented in the accompanying consolidated statements of operations.
Comprehensive Loss
Comprehensive loss consists of net loss for the period plus all changes to shareholders' equity that are not reflected in the consolidated statement of operations. The following summarizes the components of the Company's comprehensive loss:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | |||||||
Net loss |
$ | (61,422 | ) | $ | (19,860 | ) | $ | (20,476 | ) | |
Other comprehensive (loss) income: |
||||||||||
Unrealized gain (loss) on securities |
(24 | ) | 8 | — | ||||||
Total comprehensive loss |
$ | (61,446 | ) | $ | (19,852 | ) | $ | (20,476 | ) | |
Stock-Based Compensation
ASC 718, Compensation—Stock Compensation, addresses the accounting for share-based payment transactions in which an enterprise receives employee services in exchange for (a) equity instruments of the enterprise or (b) liabilities that are based on the fair value of the enterprise's equity instruments or that may be settled by the issuance of such equity instruments. ASC 718 requires that an entity measures the cost of equity-based service awards based on the grant-date fair value of the award and recognizes the cost of such awards over the period during which the employee is required to provide service in exchange for the award (the vesting period). ASC 718 requires that an entity measures the cost of liability-based service awards based on current fair value that is re-measured subsequently at each reporting date through the settlement date. The Company accounts for equity awards issued to non-employees in accordance with ASC 505-50, Equity-Based Payments to Non-Employees.
Income Taxes
The Company accounts for income taxes under the liability method, as described in ASC 740, Income Taxes. Deferred income taxes are recognized for the tax consequences of temporary differences between the tax and financial statement reporting bases of assets and liabilities. A valuation allowance for net deferred tax assets is provided unless realizability is judged by the Company to be more likely than not.
Certain Significant Risks and Uncertainties
Financial instruments that potentially subject the Company to concentrations of credit risk consist primarily of cash, cash equivalents, short-term available-for-sale investments and accounts receivable
58
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
2. Summary of Significant Accounting Policies (Continued)
balances. Cash and cash equivalents consist primarily of cash in bank accounts, as well as money market funds. Short-term available-for-sale investments consist of investments with high quality financial institutions. The Company diversifies its investment portfolio and places investments with maturities that maintain safety and liquidity.
Accounts receivable consist of amounts due to the Company from its normal business activities. The Company performs ongoing credit evaluations of its customers' financial condition and if applicable maintains an allowance for potential credit losses.
Segment Information
ASC 280, Segment Reporting, establishes standards for reporting information regarding operating segments in annual financial statements. Operating segments are identified as components of an enterprise for which separate discrete financial information is available for evaluation by the chief operating decision-maker, or decision-making group in making decisions on how to allocate resources and assess performance.
The Company aggregates its operations into two reportable business segments, services and products. The patient service business segment's principal focus is on the diagnosis and monitoring of cardiac arrhythmias, or heart rhythm disorders, through its core MCOT™, event and Holter services. The product business segment, which was developed through the Biotel acquisition in December 2010, focuses on the development, manufacturing, testing and marketing of medical devices and related software to medical companies, clinics and hospitals.
Recent Accounting Pronouncements
In January 2010, the Financial Accounting Standards Board (FASB) updated the disclosure requirements for fair value measurements. The updated guidance requires companies to disclose separately the investments that transfer in and out of Levels 1 and 2 and the reason for those transfers. Additionally, in the reconciliation for fair value measurements using significant unobservable inputs (Level 3), companies should separately present information about purchases, sales, issuances and settlements. In accordance with the new guidance, the Company is required to disclose transfers between category levels, as well as certain other disclosures related to Level 3 investments. No transfers were made into or out of the different category levels, nor did the Company categorize any of its investments as Level 3 as of December 31, 2011. The Company will continue to review our fair value inputs on a quarterly basis.
In May 2011, the FASB issued ASU 2011-04, Fair Value Measurement (Topic 820): Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS. The new guidance results in a consistent definition of fair value and common requirements for measurement of and disclosure about fair value between U.S. GAAP and International Financial Reporting Standards (IFRS). The ASU is effective for interim and annual periods beginning on or after December 15, 2011, with early adoption prohibited. The new guidance changes certain fair value measurement principles and disclosure requirements. The Company does not expect the amendments to have a material impact on its results of operations, cash flows, or financial position.
59
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
2. Summary of Significant Accounting Policies (Continued)
In June 2011, the FASB issued ASU 2011-05, Comprehensive Income (Topic 220): Presentation of Comprehensive Income. The ASU is effective for interim and annual periods beginning after December 15, 2011, with early adoption permitted. The new guidance eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholder's equity and states that an entity has the option to present the total of comprehensive income, the components of income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. Additionally, entities are required to present on the face of the financial statements reclassification adjustments for items that are reclassified from other comprehensive income to net income in the statement(s) where the components of net income and the components of other comprehensive are presented. This ASU will change the financial statement presentation of comprehensive income but the Company does not expect the amendments to have a material impact on its results of operations, cash flows, or financial position.
In July 2011, the FASB issued ASU 2011-07, Health Care Entities (Topic 954): Presentation and Disclosure of Patient Service Revenue, Provision for Bad Debts, and the Allowance for Doubtful Accounts for Certain Health Care Entities. The ASU is effective for interim and annual periods beginning on or after December 15, 2011, with early adoption prohibited. The new guidance changes certain presentation and disclosure requirements for Patient Service Revenue. The Company does not expect the amendments to have a material impact on its results of operations, cash flows, or financial position, as well as does not expect it to have a change in the presentation of the consolidated financial statements.
In September 2011, the FASB issued ASU 2011-08, Intangibles—Goodwill and Other (Topic 350): Testing Goodwill for Impairment. The ASU is effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011, with early adoption permitted. The new guidance allows an entity the option to first assess qualitative factors to determine whether existence of events or circumstances lead to a determination that it is more likely than not that the fair value of a reporting unit is less than its carrying amount. If the qualitative assessment leads to the determination that the fair value of the reporting unit is not more likely than not less than the carrying value, then performing a two-step impairment test is no longer necessary. The Company does not expect the amendments to have a material impact on its results of operations, cash flows, or financial position.
3. Available-for-Sale Investments
The Company invests its excess funds in securities issued by the United States government, corporations, banks, municipalities, financial holding companies and in money market funds comprised of these same types of securities. Cash and cash equivalents and available-for-sale investments are placed with high credit quality financial institutions. Additionally, we diversify our investment portfolio in order to maintain safety and liquidity. As of December 31, 2011, all of the investments will mature within one year. These investments are recorded at fair value, based on quoted market prices, with unrealized gains and losses reported as a separate component of stockholders' equity.
60
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
3. Available-for-Sale Investments (Continued)
Investments are classified as available-for-sale investments. At December 31, 2011, available-for-sale investments are detailed as follows:
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Short-term investments: |
|||||||||||||
Corporate debt securities |
$ | 20,012 | $ | 1 | $ | (18 | ) | $ | 19,995 | ||||
U.S. Treasury and agency debt securities |
7,957 | 1 | — | 7,958 | |||||||||
Total |
$ | 27,969 | $ | 2 | $ | (18 | ) | $ | 27,953 | ||||
At December 31, 2010, available-for-sale investments are detailed as follows:
| |
Amortized Cost |
Gross Unrealized Gains |
Gross Unrealized Losses |
Estimated Fair Value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Short-term investments: |
|||||||||||||
Corporate debt securities |
$ | 13,132 | $ | 2 | $ | (5 | ) | $ | 13,129 | ||||
U.S. Treasury and agency debt securities |
13,639 | 11 | — | 13,650 | |||||||||
Total |
$ | 26,771 | $ | 13 | $ | (5 | ) | $ | 26,779 | ||||
Net unrealized gains and losses on available-for-sale investments are included as a component of shareholders' equity and comprehensive loss until realized from a sale or other-than-temporary impairment. The Company recorded net unrealized losses for the year ended December 31, 2011 of $16, and recorded net unrealized gains for the years ended December 31, 2010 and 2009 of $8 and $0, respectively. Realized gains and losses from the sale of securities are determined on a specific identification basis. Purchases and sales of investments are recorded on their trade dates. The Company recorded realized gains for the years ended December 31, 2011, 2010 and 2009 of $1, $2 and $0, respectively. Dividend and interest income are recognized when earned. Interest income from available-for-sale investments for the years ended December 31, 2011, 2010 and 2009 were $144, 97, and 0, respectively, which were partially offset by amortization of investment premiums.
At December 31, 2011, the Company had 21 corporate debt securities and 6 U.S. Treasury and agency debt securities in its available-for-sale investment balance, of which 15 securities were in an unrealized loss position totaling $18. The unrealized losses relate to available-for-sale investments with a fair value of $14,654 at December 31, 2011. Based on the Company's intent to hold these investments for a reasonable period of time sufficient for a forecasted recovery of fair value, the Company does not consider these investments to be other-than-temporarily impaired at December 31, 2011.
4. Fair Value Measurements
ASC 820 defines fair value as an exit price that would be received from the sale of an asset or paid to transfer a liability in the principal or most advantageous market for the asset or liability in an
61
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
4. Fair Value Measurements (Continued)
orderly transaction between market participants on the measurement date. ASC 820 establishes a three-level hierarchy for disclosure that is based on the extent and level of judgment used to estimate the fair value of assets and liabilities.
- •
- Level 1—Valuations based on quoted prices for identical assets or liabilities in active markets at the
measurement date. Since valuations are based on quoted prices that are readily and regularly available in an active market, valuation of these products does not entail a significant degree of
judgment. Our Level 1 assets consist of cash and money market funds, as well as U.S. Treasury and agency debt securities.
- •
- Level 2—Valuations based on quoted prices for similar assets and liabilities in active markets; quoted
prices for identical or similar assets and liabilities in markets that are not active; or other inputs that are observable or can be corroborated by observable market data, such as alternative pricing
sources with reasonable levels of price transparency. Our Level 2 assets consist of fixed income securities such as corporate debt securities including commercial paper and corporate bonds.
- •
- Level 3—Valuations based on inputs that are unobservable and significant to the overall fair value measurement. We have not measured the fair value of any of our assets using Level 3 inputs.
The fair value of the Company's financial assets subject to the disclosure requirements of ASC 820 was determined using the following levels of inputs at December 31, 2011:
Fair Value Measurements at December 31, 2011
| |
Level 1 | Level 2 | Level 3 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Assets: |
|||||||||||||
Cash |
$ | 10,622 | $ | — | $ | — | $ | 10,622 | |||||
Money market funds |
7,909 | — | — | 7,909 | |||||||||
Corporate debt securities |
— | 19,995 | — | 19,995 | |||||||||
U.S. Treasury and agency debt securities |
7,958 | — | — | 7,958 | |||||||||
Total |
$ | 26,489 | $ | 19,995 | $ | — | $ | 46,484 | |||||
62
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
4. Fair Value Measurements (Continued)
The fair value of the Company's financial assets subject to the disclosure requirements of ASC 820 was determined using the following levels of inputs at December 31, 2010:
Fair Value Measurements at December 31, 2010
| |
Level 1 | Level 2 | Level 3 | Total | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Assets: |
|||||||||||||
Cash |
$ | 12,681 | $ | — | $ | — | $ | 12,681 | |||||
Money market funds |
5,024 | — | — | 5,024 | |||||||||
Corporate debt securities |
— | 14,129 | — | 14,129 | |||||||||
U.S. Treasury and agency debt securities |
13,650 | — | — | 13,650 | |||||||||
Total |
$ | 31,355 | $ | 14,129 | $ | — | $ | 45,484 | |||||
5. Inventory
Inventory consists of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | |||||
Raw materials and supplies |
$ | 1,727 | $ | 1,218 | |||
Finished goods |
282 | 243 | |||||
Total inventories |
$ | 2,009 | $ | 1,461 | |||
Inventories, which include purchased parts, materials, direct labor and applied manufacturing overhead, are stated at the lower of cost or net realizable value, with cost determined by use of the first-in, first-out method.
6. Property and Equipment
Property and equipment consists of the following:
| |
|
December 31, | |||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Estimated Useful Life (Years) |
||||||||
| |
2011 | 2010 | |||||||
Cardiac monitoring devices and device parts and components |
3 - 5 | $ | 44,796 | $ | 44,529 | ||||
Computers and purchased software |
3 - 5 | 10,635 | 10,336 | ||||||
Equipment, tools and molds |
3 | 5,975 | 5,180 | ||||||
Furniture and fixtures |
3 | 3,078 | 2,819 | ||||||
Leasehold improvements |
Life of lease | 4,911 | 4,759 | ||||||
Total property and equipment, at cost |
69,395 | 67,623 | |||||||
Less accumulated depreciation |
(54,354 | ) | (45,623 | ) | |||||
Total property and equipment, net |
$ | 15,041 | $ | 22,000 | |||||
63
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
6. Property and Equipment (Continued)
In November 2011, the Company changed the estimated useful life of its C3 medical devices from three to four years. The Company performed an analysis based on accumulated field performance data, and concluded that due to superior product innovation and low failure rates, the estimated useful life is approximately four years. The change in estimate is accounted for prospectively, with the remaining net book value of the C3 devices being depreciated over the remaining useful life. The change in estimate resulted in higher pre-tax and net income of $460 for the year ended December 31, 2011. The impact on basic and diluted earnings per share for the year ended December 31, 2011 was $0.02.
Depreciation expense associated with property and equipment was $10,913, $11,696 and $10,053, for the years ended December 31, 2011, 2010 and 2009, respectively.
7. Goodwill and Intangible Assets
Goodwill was recognized at the time of the Biotel and PDSHeart acquisitions. The carrying amount of goodwill as of December 31, 2011 and 2010 was $3,663 and $49,362, respectively.
The changes in the carrying amounts of goodwill by segment were as follows:
| |
Reporting Segment | ||||||
|---|---|---|---|---|---|---|---|
| |
Patient Services |
Product | |||||
Balance at December 31, 2010 |
$ | 45,999 | $ | 3,363 | |||
Goodwill acquired during the year |
— | — | |||||
Purchase price allocation adjustments and other |
— | — | |||||
Impairments |
(45,999 | ) | — | ||||
Balance at December 31, 2011 |
$ | — | $ | 3,363 | |||
The gross carrying amounts and accumulated amortization of the Company's intangible assets as of December 31, 2011 and 2010 are as follows:
| |
|
December 31, | |||||||
|---|---|---|---|---|---|---|---|---|---|
| |
Estimated Useful Life (Years) |
||||||||
| |
2011 | 2010 | |||||||
Customer relationships |
6 - 10 | $ | 2,551 | 2,551 | |||||
Proprietary technology |
5 | 800 | 800 | ||||||
Signed backlog |
1 | 700 | 700 | ||||||
Total intangible assets, gross |
4,051 | 4,051 | |||||||
Customer relationships accumulated amortization |
(1,346 | ) | (987 | ) | |||||
Proprietary technology accumulated amortization |
(160 | ) | — | ||||||
Signed backlog accumulated amortization |
(700 | ) | — | ||||||
Total accumulated amortization |
(2,206 | ) | (987 | ) | |||||
Indefinite-lived trade name |
700 | 700 | |||||||
Total intangible assets, net |
$ | 2,545 | $ | 3,764 | |||||
64
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
7. Goodwill and Intangible Assets (Continued)
The estimated amortization expense for the next five years is summarized as follows at December 31, 2011:
2012 |
519 | |||
2013 |
307 | |||
2014 |
260 | |||
2015 |
260 | |||
2016 |
100 | |||
Total |
$ | 1,446 | ||
Amortization expense for the years ending December 31, 2011, 2010 and 2009 was $1,219, $375 and $884, respectively.
At December 31, 2011, the Company performed its required annual impairment test of goodwill. Based on this impairment test, the Company determined that its product unit was not impaired. However, as a result of the impairment test, the Company determined that impairment may exist in the patient services reporting unit. Therefore, the Company performed Step 2 of the goodwill impairment analysis on its patient services reporting unit.
The Step 2 analysis was performed by allocating the fair value of the patient services reporting unit to the identifiable assets, including unrecorded intangible assets and liabilities. This allocation is performed as if the reporting unit had been acquired in a business combination, and assumes the purchase price was equivalent to the fair value determined in Step 1 of the goodwill impairment test. The residual fair value of the reporting unit after allocation is the implied fair value of goodwill. This value is then compared to the carrying value of the reporting unit's goodwill. If the implied fair value of goodwill is less than the carrying value, impairment exists and a charge is recorded in the amount of the difference. As a result of the Company's analysis, an impairment of $45,999 was recorded for the year ended December 31, 2011 related to the patient services reporting unit.
8. Accrued Expenses
Accrued expenses consisted of the following:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | |||||
Accrued purchases |
$ | 461 | $ | 538 | |||
Accrued compensation |
6,583 | 6,470 | |||||
Accrued professional fees |
1,667 | 834 | |||||
2010 restructuring costs |
— | 463 | |||||
Other |
1,742 | 1,576 | |||||
Total |
$ | 10,453 | $ | 9,881 | |||
65
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
9. Integration, Restructuring and Other Charges
2011 Integration, Restructuring and Other Charges
For the year ended December 31, 2011, the Company incurred expenses related to restructuring, integration and other activities. A summary of these expenses is as follows:
Legal fees |
$ | 2,835 | ||
Biotel integration |
1,023 | |||
Professional fees |
639 | |||
Other charges |
162 | |||
Total |
$ | 4,659 | ||
Restructuring and integration costs of $1,023 were related to severances and other employee related costs associated with the acquisition of Biotel.
A summary of the reserve activity related to the Biotel integration as of December 31, 2011 is as follows:
| |
Initial Reserve Recorded |
Payments through December 31, 2011 |
Balance as of December 31, 2011 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Severance and employee related costs |
$ | 1,023 | $ | 723 | $ | 300 | ||||
The Company accounts for expenses associated with exit or disposal activities in accordance with ASC 420, Exit or Disposal Cost Obligations, and records the expenses in Integration, restructuring and other charges in its statement of operations, and records the related accrual in the Accrued liabilities line of its balance sheet.
Other Charges
Other charges were incurred for legal fees of $2,835 related to ongoing litigation, $639 related to professional services associated with transaction due diligence and $162 related to severance and other employee related costs.
2010 Integration, Restructuring and Other Charges
For the year ended December 31, 2010, the Company incurred expenses related to restructuring, integration and other activities. A summary of these expenses is as follows:
2010 restructuring |
$ | 3,523 | ||
Other charges |
1,131 | |||
Total |
$ | 4,654 | ||
66
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
9. Integration, Restructuring and Other Charges (Continued)
2010 Restructuring
During the first quarter of 2010, the Company undertook an initiative to streamline its sales and service organizations and reduce support costs company-wide. It also initiated plans to close its event monitoring facility in Georgia and consolidate it with the Company's monitoring facilities in Pennsylvania and Minnesota. The Company realized cost efficiencies by undertaking these initiatives.
The restructuring plan involved the elimination of approximately 100 positions. The restructuring activities were substantially complete as of December 31, 2010. No additional charges were incurred after December 31, 2010 related to this restructuring plan, and all remaining accruals related to the plan were paid in 2011.
Other Charges
The Company incurred other charges of $1,131 for the year ended December 31, 2010, including legal costs related to the Company's defense of class-action and Biotel lawsuits.
2009 Integration, Restructuring and Other Charges
For the year ended December 31, 2009, the Company incurred expenses related to restructuring, integration and other activities. A summary of these expenses is as follows:
2009 restructuring |
$ | 1,153 | ||
Costs associated with option cancellation |
9,818 | |||
PDSHeart integration |
(143 | ) | ||
Conshohocken fire |
(181 | ) | ||
Other costs |
2,334 | |||
Total |
$ | 12,981 | ||
2009 Restructuring
During the third quarter of 2009, the Company undertook an initiative to reduce support costs company-wide and initiated plans to move the majority of its manufacturing activities from San Diego to its facility in Chester, PA. The Company achieved a reduction in shipping and administrative costs by combining its manufacturing facilities into one location. Prior to the restructuring, devices were shipped to and from the San Diego location for production and maintenance before being deployed out of the Company's distribution facility in Pennsylvania.
Also during the third quarter of 2009, the Company closed its event monitoring facility in Florida and consolidated it with the Company's event monitoring facility in Georgia. The Company realized cost efficiencies by consolidating its event monitoring centers in the southeastern United States and by eliminating duplicative administrative costs.
67
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
9. Integration, Restructuring and Other Charges (Continued)
The restructuring plan involved the elimination of approximately 80 positions and the relocation of 15 employees. The restructuring was substantially completed as of December 31, 2009. The Company incurred restructuring expenses of $1,153 for the year ended December 31, 2009.
A summary of the reserve activity related to the 2009 restructuring plan as of December 31, 2009 is as follows:
| |
Initial Reserve Recorded |
Payments through December 31, 2009 |
Balance as of December 31, 2009 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
Severance and employee related costs |
$ | 1,014 | $ | 797 | $ | 217 | ||||
Other exit activity costs |
139 | 139 | — | |||||||
Total |
$ | 1,153 | $ | 936 | $ | 217 | ||||
The remaining accrual as of December 31, 2009 was paid in 2010.
Option Cancellation
On December 1, 2009, certain executive officers cancelled a portion of their remaining unvested stock options as of that date. No consideration was given in exchange for the cancellation, and no new options were granted. The Company incurred a one-time charge of $9,818 to recognize the remaining unamortized expense associated with the cancelled options. The Company has recognized this charge in accordance with the guidance in ASC 718, Compensation—Stock Compensation. This charge was recorded in the Integration, restructuring and other charges line in its statement of operations.
Other Costs
In January 2009, the Company incurred costs related to the departure of certain executive officers, including the former Chief Executive Officer. The costs include primarily severance and benefit payments. The expenses are included in the Integration, restructuring and other charges line in its statement of operations, and unpaid amounts are included in the Accrued expenses line in the balance sheet as accrued compensation.
10. Shareholders' Equity
Common Stock
As of December 31, 2011 and 2010, the Company was authorized to issue 200,000,000 shares of common stock. As of December 31, 2011 and 2010, the Company had 24,534,601 and 24,251,170 shares outstanding, respectively.
Preferred Stock
The Company maintains an unregistered blank check preferred stock class. As of December 31, 2011 and 2010, there are no shares authorized and outstanding.
68
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
10. Shareholders' Equity (Continued)
Stock Based Compensation
2008 Equity Incentive Plan
The Company's 2008 Equity Incentive Plan (the 2008 Option Plan) became effective on March 18, 2008. The Plan permits the Company's Board of Directors to grant incentive stock options to employees of the Company and non-qualified stock options, restricted stock, performance stock and other stock-based incentive awards to officers, directors, employees and consultants of the Company. On that date, the Company began granting options to purchase shares of common stock to employees, executives, directors and consultants. Under the terms of the 2008 Option Plan, all available shares in the 2003 Option Plan's share reserve automatically roll into the 2008 Option Plan. Any cancellations or forfeitures of granted options under the 2003 Option Plan also automatically roll into the 2008 Option Plan. Beginning on January 1, 2009, and each year thereafter, the number of options available to be granted under the plan will increase by the lesser of 4% of the total number of common shares outstanding or 1,500,000 shares.
The restrictions on restricted stock units issued under the plan lapse as follows: one third on the date of grant, one third on the first anniversary of the date of grant, and one third on the second anniversary of the date of grant. The restrictions on certain other restricted stock units issued under the plan lapse in full on the third anniversary of the date of grant. Options granted to certain officers of the Company in combination with restricted stock units, described above, under the Plan vest in three equal installments beginning on the third anniversary from the date of grant.
Options granted under the 2008 Option Plan have exercise prices not less than the fair market value at the date of grant and have an expiration date of no greater than ten years from the date of grant. There is no vesting schedule provided in the 2008 Option Plan, and vesting is determined by the Board of Directors on the date of grant. However, the Company's practice is to follow a four year vesting schedule such that 25% of the granted options vest on the anniversary date of grant, and the remaining options granted vest ratably over 36 months thereafter. No options have been granted with vesting schedules that differ from Company practice.
2008 Non-employee Directors' Stock Option Plan
The Company's 2008 Non-employee Directors' Stock Option Plan (the Directors' Plan) became effective March 18, 2008. Beginning on that date, all directors elected for the first time to the Board of Directors receive a fixed number of options. On the date of the annual meeting, and when directors are elected to a committee or a chair position of a committee, they will also receive a grant equal to a fixed number of options per the Directors' Plan. Options granted under the Directors' Plan have exercise prices not less than the fair market value at the date of grant, and have an expiration date of no greater than ten years from the date of grant. Initial and committee chair grants vest 33% on the first anniversary date of grant, and the balance vests ratably over 24 months. Annual grants vest ratably over 12 months from the date of grant.
69
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
10. Shareholders' Equity (Continued)
2003 Equity Incentive Plan
As of March 18, 2008 the Company no longer granted options to purchase shares of common stock to employees, executives, directors and consultants under the Company's 2003 Equity Incentive Plan (the 2003 Option Plan). Options granted under the 2003 Option Plan have exercise prices not less than the fair market value at date of grant for incentive stock options and not less than 85% of the fair market value at the date of grant for non-statutory options. The options generally expire ten years from the date of grant and generally vest 25% twelve months from the date of grant, and ratably over the next 36 months thereafter.
The 2003 Option Plan allows for employees to early exercise options on the first anniversary date of employment, regardless of the vested status of granted options. If an employee terminates prior to fully vesting in options that have been early exercised, the Company repurchases the common stock associated with unvested options at the original exercise price.
Option activity under all stock option plans is summarized as follows for the years ended December 31, 2011, 2010 and 2009:
| |
|
Options Outstanding | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Shares Available for Grant |
Number of Shares |
Weighted Average Exercise Price |
|||||||
Balance—December 31, 2008 |
340,935 | 1,635,205 | $ | 13.67 | ||||||
Additional shares authorized for grant |
1,024,921 | — | — | |||||||
Granted |
(1,569,276 | ) | 1,569,276 | $ | 10.26 | |||||
Cancelled/forfeited |
1,335,555 | (1,335,555 | ) | $ | 20.37 | |||||
Exercised |
— | (293,281 | ) | $ | 8.21 | |||||
Balance—December 31, 2009 |
1,132,135 | 1,575,645 | $ | 15.21 | ||||||
Additional shares authorized for grant |
1,194,094 | — | — | |||||||
Granted |
(1,034,663 | ) | 1,034,663 | $ | 6.70 | |||||
Cancelled/forfeited |
358,157 | (358,157 | ) | $ | 14.44 | |||||
Exercised |
— | (149,775 | ) | $ | 8.21 | |||||
Balance—December 31, 2010 |
1,649,723 | 2,102,376 | $ | 12.18 | ||||||
Additional shares authorized for grant |
1,207,210 | — | — | |||||||
Granted |
(724,333 | ) | 724,333 | $ | 4.67 | |||||
Cancelled/forfeited |
237,202 | (237,202 | ) | $ | 15.10 | |||||
Exercised |
— | (120,516 | ) | $ | 7.78 | |||||
Balance—December 31, 2011 |
2,369,802 | 2,468,991 | $ | 9.43 | ||||||
70
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
10. Shareholders' Equity (Continued)
A summary of total outstanding stock options as of December 31, 2011 is as follows:
| |
Options Outstanding | Options Exercisable | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Range of Exercise Price
|
Number Outstanding |
Weighted- Average Remaining Contractual Life (in years) |
Weighted- Average Exercise Price |
Number Exercisable |
Weighted- Average Remaining Contractual Life (in years) |
Weighted- Average Exercise Price |
|||||||||||||
$0.70 - $7.50 |
1,263,011 | 8.39 | $ | 5.63 | 369,285 | 7.51 | $ | 5.91 | |||||||||||
$7.51 - $15.00 |
104,446 | 7.09 | 9.50 | 83,157 | 6.81 | 9.66 | |||||||||||||
$15.01 - $22.50 |
315,764 | 7.15 | 18.74 | 315,764 | 7.15 | 18.74 | |||||||||||||
$22.51 - $31.18 |
159,030 | 6.58 | 29.15 | 159,030 | 6.58 | 29.15 | |||||||||||||
$0.70 - $31.18 |
1,842,251 | 7.95 | 10.12 | 927,236 | 7.17 | 14.60 | |||||||||||||
In addition, a summary of total outstanding RSU's as of December 31, 2011 is as follows:
Range of Grant Price
|
RSU's Outstanding |
|||
|---|---|---|---|---|
$4.24 - $6.75 |
272,340 | |||
$6.76 - $7.75 |
263,000 | |||
$7.76 - $17.75 |
51,932 | |||
$17.76 - $26.49 |
39,468 | |||
$4.24 - $26.49 |
626,740 | |||
The table below summarizes certain additional information with respect to our options:
(In thousands)
|
2011 | 2010 | 2009 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
Aggregate intrinsic value of options outstanding at year-end |
$ | 17 | $ | 141 | $ | 182 | ||||
Aggregate intrinsic value of options exercisable at year-end |
17 | 87 | 156 | |||||||
Aggregate market value of unvested stock awards at year-end |
12,156 | 15,751 | 16,475 | |||||||
Aggregate intrinsic value of options exercised during the year |
7 | 151 | 3,892 | |||||||
Aggregate market value of stock awards vested during the year |
11,154 | 8,936 | 5,624 | |||||||
As of December 31, 2011, 2010 and 2009, the Company has reserved shares of common stock for issuance as follows:
| |
December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | |||||||
Exercise of options available and grants of awards under equity plans |
4,838,793 | 3,752,099 | 2,707,780 | |||||||
The Company's loss before income taxes for the years ended December 31, 2011, 2010 and 2009 was $4,006, $3,945 and $16,625 lower, respectively, and the Company's after-tax net loss for years ended December 31, 2011, 2010 and 2009 was $3,990, $3,892 and $16,625 lower, respectively, as a result
71
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
10. Shareholders' Equity (Continued)
of stock-based compensation expense incurred. The impact of stock-based compensation expense was $(0.16) on the basic and diluted earnings per share for both the years ended December 31, 2011 and 2010. For the year ended December 31, 2009, the impact of stock-based compensation expense was $(0.70) on the basic and diluted earnings per share.
Total cash received from the exercise of stock options for the year ended December 31, 2011, 2010 and 2009 was $11, $665 and $935, respectively. The tax benefit realized from the exercise of nonqualified stock options for the year ended December 31, 2009 was $357. The tax benefit was fully reserved for through a tax valuation allowance.
The Company estimates the fair value of our share-based awards to employees and directors using the Black-Scholes option valuation model. The Black-Scholes option valuation model requires the use of certain subjective assumptions. The most significant of these assumptions are our estimates of the expected volatility of the market price of the Company's stock and the expected term of the award. The Company bases our estimates of expected volatility on a group of similar entities whose stock prices are publicly available. The expected term represents the period of time that stock-based awards granted are expected to be outstanding. Other assumptions used in the Black-Scholes option valuation model include the risk-free interest rate and expected dividend yield. The risk-free interest rate for periods pertaining to the contractual life of each option is based on the U.S. Treasury yield of a similar duration in effect at the time of grant. The Company has never paid, and do not expect to pay, dividends in the foreseeable future.
The fair value of the Company's stock-based awards was estimated at the date of grant using the following weighted average assumptions:
| |
Year Ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | |||||||
Expected volatility |
62.0 | % | 65.0 | % | 54.0 | % | ||||
Expected term (in years) |
6.25 | 6.25 | 6.25 | |||||||
Weighted-average risk-free interest rate |
2.48 | % | 2.29 | % | 2.23 | % | ||||
Expected dividends |
0.0 | % | 0.0 | % | 0.0 | % | ||||
Weighted-average grant date fair value per share |
$ | 2.82 | $ | 3.95 | $ | 10.26 | ||||
Based on the Company's historical experience of options that cancel before becoming fully vested, the Company has assumed an annualized forfeiture rate of 15% for all options. Under the true-up provision of ASC 718, the Company will record additional expense if the actual forfeiture rate is lower than estimated, and will record a recovery of prior expense if the actual forfeiture rate is higher than estimated.
Total compensation cost of options granted but not yet vested at December 31, 2011, 2010 and 2009 was approximately $3,615, $5,047 and $4,397, respectively. At December 31, 2011, 2010 and 2009, the weighted average remaining periods over which the above amounts are expected to be recognized were 2.62 years, 3.09 years, and 2.06 years, respectively. At December 31, 2011, 2,369,802 shares remained available for future grant under the Plan.
72
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
10. Shareholders' Equity (Continued)
A summary of the status of the Company's unvested stock options as of the respective balance sheet dates, and changes during years, is presented below:
| |
Number of Shares |
Weighted- Average Grant-Date Fair Value (per share) |
|||||
|---|---|---|---|---|---|---|---|
Unvested shares at December 31, 2009 |
1,170,392 | $ | 14.08 | ||||
Granted |
1,034,663 | $ | 6.70 | ||||
Vested |
(252,378 | ) | $ | 8.21 | |||
Cancelled/forfeited |
(358,157 | ) | $ | 14.44 | |||
Unvested shares at December 31, 2010 |
1,594,520 | $ | 9.88 | ||||
Granted |
724,333 | $ | 4.67 | ||||
Vested |
(422,031 | ) | $ | 7.78 | |||
Cancelled/forfeited |
(237,202 | ) | $ | 15.10 | |||
Unvested shares at December 31, 2011 |
1,659,620 | $ | 7.32 | ||||
Option Acceleration
On December 1, 2009, the Company accelerated the vesting of certain employees' unvested options that were deeply out-of-the-money. The acceleration was done because the Company believed that there was no longer a compensation incentive tied to Company performance, given the exercise price of the options that were accelerated. Consistent with ASC 718, the Company will continue to expense the accelerated options over the remaining service period. The Company does not have a static policy threshold to use for determining whether an option is deeply out-of-the-money. Rather, the Company believes that the determination should be made in light of current market conditions, probability of stock price recovery within the remaining service period, and historical volatility of the Company's stock price. For the purposes of this option acceleration, the Company determined that options that were out-of-the-money by 30% or more were deeply out-of-the-money. As a result of the option acceleration, approximately 309,000 previously unvested shares became fully vested on December 1, 2009. The Company incurred an expense associated with the options that were accelerated in the amount of $984, $1,269 and $75 for the years ended December 31, 2011, 2010 and 2009, respectively, which have been recorded in the General and administrative line of the consolidated statement of operations. The weighted average exercise price of the accelerated options is $19.87, and the average remaining service period is 1.15 years.
Option Cancellation
On December 1, 2009, certain executive officers of the Company cancelled approximately 707,000 of their unvested outstanding options. No consideration was given in exchange for the cancellation, and no new options were granted. The Company has recognized this charge in accordance with the guidance in ASC 718, Compensation—Stock Compensation. This charge was recorded in the Integration,
73
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
10. Shareholders' Equity (Continued)
restructuring and other charges line in the statement of operations. The weighted average exercise price of the cancelled options was $26.85 and the average service period remaining at the time of the cancellation was 3.02 years. The Company incurred a one-time charge of $9,818 to recognize the remaining unamortized expense associated with the cancelled options. The impact on basic and diluted earnings per share for the year ended December 31, 2009 was $(0.41).
Employee Stock Purchase Plan
In July 2008, the Company made available an employee stock purchase plan in which substantially all of the Company's full-time employees became eligible to participate effective March 18, 2008. Under the plan, employees may contribute through payroll deductions up to 15% of their compensation toward the purchase of the Company's common stock, or $21, whichever is lower. The price per share is equal to the lower of 85% of the fair market price on the first day of the offering period, or 85% of the fair market price on the day of purchase. Proceeds received from the issuance of shares are credited to stockholders' equity in the period that the shares are issued. Under the terms of the plan, a total of 238,000 shares of common stock have been reserved for issuance to employees. On March 17, 2011 and September 17, 2011, 77,822 shares and 85,093 shares, respectively, were purchased in accordance with the Employee Stock Purchase Plan (ESPP). Net proceeds to the Company from the issuance of shares of common stock under the ESPP for the year ended December 31, 2011 were $504. In January 2011, the number of shares available for grant was increased by 241,442, per the ESPP plan documents. At December 31, 2011, approximately 459,671 shares remain available for purchase under the ESPP. For the years ended December 31, 2011, 2010 and 2009, the Company incurred ESPP expenses of $201, $202, $614, respectively.
11. Income Taxes
The Company has net deferred income tax assets totaling $46,904 at December 31, 2011, consisting primarily of federal and state net operating loss and credit carryforwards. Due to uncertainty regarding the ultimate realization of these net operating loss and credit carryforwards and other deferred income tax assets, we have established a full valuation allowance for these assets and will recognize the benefits only as reassessment indicates the benefits are realizable. The determination of the required valuation allowance against net deferred tax assets was made without taking into account the deferred tax liabilities created from the book and tax differences on indefinite-lived assets.
The Company's provision for income taxes for 2011 of $244 primarily relates to certain state taxes based on gross receipts or modified gross receipts calculations properly included as income taxes. The Company performed an analysis to determine the extent to which it can use its net operating loss carryforwards in future periods, subject to certain limitations imposed by the Internal Revenue Code. The Company concluded that because of the Company's limited history of reporting a net profit, it cannot predict that the benefits of the net operating loss carryfowards will be realized in future periods, and therefore the Company continues to provide a full valuation allowance for deferred tax assets. The Company will perform a similar analysis during 2012 to reassess the estimated future realizability of net operating loss carryforwards.
74
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
11. Income Taxes (Continued)
Deferred taxes result from temporary differences between the carrying amounts of assets and liabilities used for financial reporting purposes and the amounts used for income tax purposes. The significant components of the Company's deferred tax assets and liabilities are as follows:
| |
December 31, | ||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | |||||
Deferred tax assets: |
|||||||
Net operating loss carryforwards |
$ | 32,574 | $ | 30,142 | |||
Research & development and AMT credit carryforwards |
4,578 | 3,716 | |||||
Stock option grants |
4,389 | 3,419 | |||||
Allowance for doubtful accounts |
3,896 | 4,674 | |||||
Goodwill and acquired intangibles |
— | 458 | |||||
Other, net |
2,172 | 1,938 | |||||
Total deferred tax assets |
47,609 | 44,347 | |||||
Less valuation allowance |
(47,142 | ) | (41,369 | ) | |||
Net deferred tax assets |
$ | 467 | $ | 2,978 | |||
Deferred tax liabilities: |
|||||||
Property, plant and equipment |
(197 | ) | (2,061 | ) | |||
Identified intangible assets |
(166 | ) | (875 | ) | |||
Indefinite lived intangible assets |
(269 | ) | (256 | ) | |||
Prepaid insurance |
(73 | ) | (42 | ) | |||
Total deferred tax liabilities |
$ | (705 | ) | $ | (3,234 | ) | |
Net deferred tax asset (liability) |
$ | (238 | ) | $ | (256 | ) | |
Reconciliations between expected income taxes computed at the federal rate of 35% for each of the years ended December 31, 2011, 2010 and 2009, and the provision for income taxes is as follows:
| |
Years ended December 31, | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | 2009 | |||||||
Income tax benefit at statutory rate |
$ | (21,412 | ) | $ | (6,855 | ) | $ | (6,999 | ) | |
State income tax benefit, net of federal benefit |
191 | 73 | 28 | |||||||
Stock-based compensation |
493 | 478 | 3,902 | |||||||
Goodwill Impairment |
16,100 | — | — | |||||||
Other |
(173 | ) | 326 | (629 | ) | |||||
Increase in valuation allowance |
5,045 | 6,240 | 3,703 | |||||||
Income tax provision |
$ | 244 | $ | 262 | $ | 5 | ||||
At December 31, 2011, the Company had federal net operating loss carryforwards of approximately $81,296, to offset future federal taxable income expiring in various years through 2030. At December 31, 2010, the Company had state net operating loss carryforwards of $72,971 which expire in various years starting in 2011.
75
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
11. Income Taxes (Continued)
The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences are deductible. The timing and manner in which the Company can utilize its net operating loss carryforward and future income tax deductions in any year may be limited by provisions of the Internal Revenue Code regarding the change in ownership of corporations. Such limitation may have an impact on the ultimate realization of the Company's carry forwards and future tax deductions. Section 382 of the Internal Revenue Code ("Section 382") imposes limitations on a corporation's ability to utilize net operating losses if it experiences an "ownership change." In general terms, an ownership change may result from transactions increasing the ownership of certain stockholders in the stock of a corporation by more than 50 percentage points over a three-year period. Any unused annual limitation may be carried over to later years, and the amount of the limitation may under certain circumstances be increased by the built-in gains in assets held by the Company at the time of the change that are recognized in the five-year period after the change. Currently, the Company's loss carryforwards are limited under Section 382.
The components of the Company's income tax provision are summarized as follows:
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | |||||
Current: |
|||||||
Federal |
$ | — | $ | 150 | |||
State |
231 | 112 | |||||
Total current provision for income taxes |
231 | 262 | |||||
Deferred: |
|||||||
Federal |
— | — | |||||
State |
13 | — | |||||
Total deferred provision for income taxes |
13 | — | |||||
Total provision for income taxes |
$ | 244 | $ | 262 | |||
The U.S. Internal Revenue Service concluded its examination of the Company's U.S. federal tax returns for all years through 2008. Because of net operating losses, the Company's U.S. federal tax returns for those years will remain subject to examination until the losses are utilized.
The Company does not have a tax reserve recorded for tax contingencies. As of December 31, 2011 and 2010, the Company has not identified any uncertain tax positions and therefore, it has no tax reserve recorded as of December 31, 2011 and 2010.
76
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
12. Commitments and Contingencies
Operating Leases
The Company leases its principal administrative and service facilities as well as office equipment under noncancelable operating leases expiring at various dates through 2019. The terms of the leases are renewable at the end of the lease term. Payments made under operating leases are charged to operations on a straight-line basis over the period of the lease. Differences between straight-line expense and cash payments are recognized in the Deferred rent line of the balance sheet. Rent expense was $2,713, $2,993and $2,619 for the years ended December 31, 2011, 2010 and 2009, respectively.
Future minimum lease payments under non-cancelable operating leases are summarized as follows at December 31, 2011:
2012 |
$ | 2,933 | ||
2013 |
2,973 | |||
2014 |
1,026 | |||
2015 |
715 | |||
2016 |
687 | |||
Thereafter |
729 | |||
|
$ | 9,063 | ||
The Company has an agreement with nPhase Incorporated (nPhase) whereby the Company has no fixed or minimum financial commitment, however, in the event the Company fails to maintain an agreed upon number of active cardiac monitoring devices on the nPhase network, nPhase has the right to terminate this agreement.
13. Employee Benefit Plan
The Company sponsors a 401(k) Retirement Savings Plan (the Plan) for all eligible employees who meet certain requirements. Participants may contribute, on a pretax basis, up to the maximum allowable amount pursuant to Section 401(k) of the Internal Revenue Code. The Company is not required to contribute to the Plan. In May 2009, the Company adopted an amendment to the Plan that allowed for an employer matching contribution of 100% of employee contributions, up to 3% of the employees' salary. For the years ended December 31, 2011, 2010 and 2009, the Company contributed $1,296, $1,134 and $0, respectively. Employer contributions vest immediately.
77
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
14. Segment Information
Summarized financial information concerning the Company's reportable segments is shown in the following table:
| |
Year Ended December 31, |
||||||
|---|---|---|---|---|---|---|---|
| |
2011 | 2010 | |||||
Revenues: |
|||||||
Patient service |
$ | 106,853 | $ | 119,924 | |||
Product |
12,169 | — | |||||
Total revenues |
119,022 | 119,924 | |||||
Loss before income taxes: |
|||||||
Patient service |
(60,222 | ) | (19,598 | ) | |||
Product |
(956 | ) | — | ||||
Total loss before income taxes |
(61,178 | ) | (19,598 | ) | |||
Depreciation and amortization: |
|||||||
Patient service |
10,762 | 12,878 | |||||
Product |
1,370 | — | |||||
Total depreciation and amortization |
12,132 | 12,878 | |||||
Capital expenditures: |
|||||||
Patient service |
3,616 | 5,247 | |||||
Product |
338 | — | |||||
Total capital expenditures |
3,954 | 5,247 | |||||
| |
December 31, 2011 |
December 31, 2010 |
|||||
|---|---|---|---|---|---|---|---|
Total assets: |
|||||||
Patient service |
82,451 | 142,114 | |||||
Product |
12,524 | 14,578 | |||||
Total assets |
94,975 | 156,692 | |||||
15. Legal Proceedings
On September 25, 2009, LifeWatch Services, Inc., and Card Guard Scientific Survival, Ltd., the licensee and owner, respectively, of U.S. Patent Nos. 7,542,878 B2 ("the '878 Patent") and 5,730,143 ("the '143 Patent") commenced an action LifeWatch Patent Matter against CardioNet's wholly owned subsidiary, Braemar Inc. ("Braemar"), and one of its customers, eCardio Diagnostics, LLC ("eCardio"), in Federal District Court for the Northern District of Illinois, File No. 09-CV-6001, alleging that Braemar and eCardio had infringed the '878 and '143 Patents. The Supply Agreement between Braemar and eCardio provides that Braemar will hold eCardio harmless from any liability it incurs in connection with a claim that Braemar's products violate the intellectual property rights or infringe upon
78
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
15. Legal Proceedings (Continued)
any patent of a third party. Braemar and eCardio have denied the allegations. Since the action was commenced, the Plaintiffs have dismissed their claims relating to alleged infringement of the '878 Patent, Card Guard dropped out of the action, and LifeWatch has continued to pursue its claims relating to the alleged infringement of the '143 Patent. The '143 Patent has been in reexamination proceedings since February 19, 2010. On February 1, 2011, the U.S. Patent Office indicated that the claims as amended during the reexamination will be issued. The Company believes that LifeWatch's claims under the original '143 Patent and under the soon-to-issue amended patent are without merit and intends to defend the litigation vigorously. Consistent with the accounting for contingent liabilities, no accrual has been recorded in the financial statements. The Company believes that the claims are without merit and intends to defend the litigation vigorously. The Company cannot reasonably estimate the range of loss, if any, that may result from this matter. Consistent with the accounting for contingent liabilities, no accrual has been recorded in the financial statements.
On December 12, 2011 the Company announced that it has reached a preliminary agreement to settle the West Palm Beach Police Pension Fund putative class action litigation filed in California Superior Court, San Diego County, which asserted claims against the Company for violations of Sections 11, 12 and 15 of the Securities Act of 1933. The preliminary agreement is subject to certain conditions, including court approval of a final settlement agreement. The parties filed a stipulation of settlement and joint plaintiff filed a motion for preliminary approval on January 6, 2012. Under the terms of the preliminary agreement, in consideration for the settlement and release of all defendants, the amount of $7,250 will be paid by or on behalf of the defendants (of which management expects approximately $6,000 will be covered by insurance). The court issued an order preliminarily approving the settlement on January 13, 2012 and set June 22, 2012 as the date for the final fairness hearing. The Company has recorded an accrual of $1,250 for the settlement of this litigation.
16. Civil Investigative Demand
On August 25, 2011, the Company received a Civil Investigative Demand ("CID") issued by the U.S. Department of Justice, Western District of Washington. The CID states that it was issued in the course of an investigation under the federal false claims act and seeks documents for the period January 1, 2007 through the date of the CID. The CID indicates that the investigation concerns allegations that the Company may have used inappropriate diagnosis codes when submitting claims for payment to Medicare for its real-time, outpatient cardiac monitoring services. The Company is cooperating with the government's request and is in the process of providing information in response to the CID. The Company is unable to predict what action, if any, might be taken in the future by the Department of Justice or other governmental authorities as a result of this investigation or what impact, if any, the outcome of this matter might have on the Company's business, financial position or results of operations. The Company cannot reasonably estimate the range of loss, if any, that may result from this matter. Consistent with the accounting for contingent liabilities, no accrual has been recorded in the financial statements.
79
CARDIONET, INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS (Continued)
Years Ended December 31, 2011, 2010 and 2009
(In thousands, except shares and per share amounts.)
17. Subsequent Events
On February 9, 2012, the Company purchased all of the outstanding shares of ECG Scanning and Medical Services for $5,880 in cash.
18. Quarterly Financial Data (Unaudited)
The following tables summarize the unaudited quarterly financial data for the last two fiscal years.
| |
First Quarter |
Second Quarter |
Third Quarter |
Fourth Quarter |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
(in thousands, except per share amount) |
||||||||||||
2011 |
|||||||||||||
Total revenues |
$ | 33,999 | $ | 31,637 | $ | 26,602 | $ | 26,784 | |||||
Gross profit |
20,347 | 18,619 | 14,350 | 16,630 | |||||||||
Integration, restructuring and other charges |
124 | 1,014 | 1,619 | 1,902 | |||||||||
Loss from operations |
(1,589 | ) | (3,038 | ) | (7,137 | ) | (49,558 | ) | |||||
Net loss |
(1,552 | ) | (3,006 | ) | (7,103 | ) | (49,761 | ) | |||||
Basic and diluted net loss per share |
$ | (0.06 | ) | $ | (0.12 | ) | $ | (0.29 | ) | $ | (2.03 | ) | |
2010 |
|||||||||||||
Total revenues |
$ | 31,816 | $ | 31,939 | $ | 27,486 | $ | 28,683 | |||||
Gross profit |
20,067 | 20,104 | 15,548 | 16,713 | |||||||||
Integration, restructuring and other charges |
1,945 | 1,128 | 859 | 722 | |||||||||
Loss from operations |
(5,435 | ) | (2,162 | ) | (7,504 | ) | (4,591 | ) | |||||
Net loss |
(5,431 | ) | (2,142 | ) | (7,470 | ) | (4,817 | ) | |||||
Basic and diluted net loss per share |
$ | (0.23 | ) | $ | (0.09 | ) | $ | (0.31 | ) | (0.20 | ) | ||
80
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
None.
Item 9A. Controls and Procedures
Evaluation of Disclosure Controls and Procedures
Prior to the filing of this Report on Form 10-K, an evaluation was performed under the supervision of and with the participation of the Company's management, including the Chief Executive Officer ("CEO") and Chief Financial Officer ("CFO"), of the effectiveness of the Company's disclosure controls and procedures. Based on the evaluation, the CEO and CFO have concluded that, as of December 31, 2011, the Company's disclosure controls and procedures are effective to ensure that information required to be disclosed by the Company in reports that it files or submits under the Exchange Act, is recorded, processed, summarized and reported within the time periods specified in SEC rules and forms, and is accumulated and communicated to the Company's management, as appropriate, to allow timely decisions regarding required disclosure. It should be noted that the design of any system of controls is based in part upon certain assumptions about the likelihood of future events, and there can be no assurance that any design will succeed in achieving its stated goals under all potential future conditions, regardless of how remote.
Changes in Internal Control over Financial Reporting
There were no changes in the Company's internal control over financial reporting (as defined in Section 240.13a-15(f) or 240.15d-15(f) of the Exchange Act) during our fourth fiscal quarter ended December 31, 2011 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
MANAGEMENT'S REPORT ON INTERNAL CONTROL OVER FINANCIAL REPORTING
The management of the Company is responsible for establishing and maintaining adequate internal control over financial reporting as defined in Rules 13a-15(f) and 15d-15(f) under the Securities Exchange Act. The Company's internal control over financial reporting is designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. The Company's internal control over financial reporting includes those policies and procedures that:
- (i)
- pertain
to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the
Company;
- (ii)
- provide
reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with U.S. generally
accepted accounting principles, and that receipts and expenditures of the Company are being made only in accordance with authorizations of management and directors of the Company; and
- (iii)
- provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the Company's assets that could have a material effect on the financial statements.
Because of inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
The Company's management assessed the effectiveness of the Company's internal control over financial reporting as of December 31, 2011. In making this assessment, management used the criteria
81
set forth by the Committee of Sponsoring Organizations of the Treadway Commission ("COSO") in Internal Control—Integrated Framework. Based on management's assessment and those criteria, management has concluded that the Company's internal control over financial reporting was effective as of December 31, 2011.
The effectiveness of the Company's internal control over financial reporting did not include the internal controls of Biotel Inc., which were included in the Company's consolidated financial statements for the year ended December 31, 2011. Management considers the acquisition of Biotel Inc. to be immaterial for consideration of internal controls for the year ended December 31, 2011.
The effectiveness of our internal control over financial reporting as of December 31, 2011 has been audited by Ernst & Young LLP, an independent registered public accounting firm, as stated in their report included in this Annual Report on form 10-K.
82
Report of Independent Registered Public Accounting Firm
The Board of Directors and Shareholders
CardioNet, Inc.
We have audited CardioNet, Inc.'s internal control over financial reporting as of December 31, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the "COSO criteria"). CardioNet, Inc.'s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management's Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company's internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company's internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company's internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company's assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, CardioNet, Inc. maintained, in all material respects, effective internal control over financial reporting as of December 31, 2011, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the consolidated balance sheets of CardioNet, Inc. as of December 31, 2011 and 2010 and the related consolidated statements of operations, cash flows and shareholders' equity for each of the three years in the period ended December 31, 2011 of CardioNet, Inc. and our report dated February 23, 2012 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Philadelphia,
Pennsylvania
February 23, 2012
83
Not applicable.
Item 10. Directors, Executive Officers and Corporate Governance
Information with respect to this Item is incorporated by reference from our definitive proxy statement in connection with the 2012 Annual Meeting of Stockholders, or the Proxy Statement, unless the Proxy Statement is not filed by April 30, 2012 (the first business day after the day that is 120 days after the last day of our 2011 fiscal year), in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
Item 11. Executive Compensation
Information with respect to this Item is incorporated by reference from the Proxy Statement unless the Proxy Statement is not filed by April 30, 2012, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
Information with respect to this Item is incorporated by reference from the Proxy Statement unless the Proxy Statement is not filed by April 30, 2012, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
Equity Compensation Plan Information
The following table presents the equity compensation plan information as of December 31, 2011:
| |
Equity Compensation Plan Information | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| |
Number of securities to be issued upon exercise of outstanding options, warrants, and rights |
Weighted-average exercise price of outstanding options, warrants and rights |
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|||||||
| |
(a) |
(b) |
(c) |
|||||||
Equity compensation plans approved by security holders: |
||||||||||
Employee and non-employee director stock option plans |
2,468,991 |
$ |
9.43 |
2,369,802 |
||||||
Employee stock purchase plan |
51,544 | $ | 2.54 | 408,127 | ||||||
Total |
2,520,535 | $ | 11.97 | 2,777,929 | ||||||
Item 13. Certain Relationships and Related Transactions, and Director Independence
Information with respect to this Item is incorporated by reference from the Proxy Statement unless the Proxy Statement is not filed by April 30, 2012, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
84
Item 14. Principal Accounting Fees and Services
Information with respect to this Item is incorporated by reference from the Proxy Statement unless the Proxy Statement is not filed by April 30, 2012, in which case we will amend this Form 10-K to provide the omitted information in accordance with the requirements of Instruction G to Form 10-K.
Item 15. Exhibits and Financial Statement Schedules
- (a)
- The
following financial statements, schedules and exhibits are filed as part of this report:
- 1.
- Financial Statements—The Financial Statements required by this item are listed on the Index to
Financial Statements in Part II, Item 8 of this report.
- 2.
- Financial Statement Schedules—
- •
- Schedule II—Valuation and Qualifying Accounts and Reserves; and
- •
- Other financial statement schedules are not included because they are not required or the information is otherwise shown
in the financial statements or notes thereto.
- 3.
- Exhibits—The exhibits listed on the accompanying Exhibit Index are filed as part of, or are
incorporated by reference into, this report.
- (b)
- See
Item 15(a)(3) above.
- (c)
- See Item 15(a)(2) above.
85
| |
Beginning Balance |
Additions Charged To Expense |
Deductions From Reserve |
Ending Balance |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Allowance for Doubtful Accounts |
|||||||||||||
Year ended December 31, 2011 |
11,779 | 12,080 | (13,970 | ) | 9,889 | ||||||||
Year ended December 31, 2010 |
22,396 | 18,578 | (29,195 | ) | 11,779 | ||||||||
Year ended December 31, 2009 |
14,426 | 19,982 | (12,012 | ) | 22,396 | ||||||||
86
| Exhibit Number |
Description | ||
|---|---|---|---|
| 2.1 | Merger Agreement, dated as of November 5, 2010, among Biotel Inc., Garden Merger Sub, Inc. and the Registrant. (Incorporated by reference to Exhibit 2.1 to the Registrant's Form 8-K filed November 12, 2010). | ||
3.1 |
Amended and Restated Certificate of Incorporation (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). |
||
3.2 |
Amended and Restated Bylaws (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). |
||
4.1 |
Form of Common Stock Certificate (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). |
||
10.1 |
Form of Indemnity Agreement (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). |
||
| 10.2 | (1) | Indemnity Agreement, dated as of May 8, 2009, by and between the Registrant and Rebecca W. Rimel. (Incorporated by reference to Exhibit 10.37 to the Registrant's Form 10-K filed February 23, 2010). | |
10.3 |
Indemnification Agreement of Ronald A. Ahrens, relating to service on the Board of Directors, effective August 19, 2008 (Incorporated by reference to Exhibit 10.5 to the Registrant's Form 10-Q filed November 7, 2008). |
||
10.4 |
Indemnification Agreement of Kirk E. Gorman, relating to service on the Board of Directors, effective August 19, 2008 (Incorporated by reference to Exhibit 10.6 to the Registrant's Form 10-Q filed November 7, 2008). |
||
| 10.5 | (1) | Indemnity Agreement by and between the Registrant and Fred A. Middleton. (Incorporated by reference to Exhibit 10.41 to the Registrant's Form 10-K, filed February 25, 2011). | |
| 10.6 | (1) | Indemnity Agreement by and between the Registrant and Eric N. Prystowsky. (Incorporated by reference to Exhibit 10.42 to the Registrant's Form 10-K, filed February 25, 2011). | |
| 10.7 | (1) | Indemnity Agreement by and between the Registrant and Robert J. Rubin. (Incorporated by reference to Exhibit 10.43 to the Registrant's Form 10-K, filed February 25, 2011). | |
| 10.8 | (1) | 2008 Equity Incentive Plan and Form of Stock Option Agreement thereunder (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). | |
| 10.9 | (1) | 2008 Non-Employee Directors' Stock Option Plan and Form of Stock Option Agreement thereunder (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). | |
| 10.10 | (1) | 2008 Employee Stock Purchase Plan and Form of Offering Document thereunder (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). | |
87
| Exhibit Number |
Description | ||
|---|---|---|---|
| 10.11 | (1) | Forms of Employee Innovations and Proprietary Rights Assignment Agreement (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). | |
10.12 |
Office Lease dated February 6, 2004 between the Registrant and Executive One Associates, as amended (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). |
||
10.13 |
Building Lease Agreement dated September 30, 2009, between the Registrant and EastGroup Properties, L.P. (Incorporated by reference to Exhibit 10.5 to the Registrant's Form 10-Q filed November 6, 2009). |
||
| 10.14 | † | Amendment No. 8 dated February 1, 2010 to the Communication Voice and Data Services Provider Agreement dated May 12, 2003 between the Company and nPhase, LLC (as successor to Qualcomm Incorporated), as amended (Incorporated by reference to the Registrant's Form 8-K, dated November 30, 2011. | |
| 10.15 | † | Purchase Agreement dated September 14, 2001 between the Registrant and Varian, Inc. (a wholly-owned subsidiary of Jabil Circuit, Inc.) (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). | |
| 10.16 | † | Consignment Inventory Agreement dated September 13, 2004 between the Registrant and Varian, Inc. (a wholly- owned subsidiary of Jabil Circuit, Inc.) (Incorporated by reference to the Registrant's registration statement on Form S-1 and amendments thereto (File No. 33-145547)). | |
10.17 |
Separation Agreement waiver and release by and between the Registrant and Randy Thurman, dated October 25, 2011 (Incorporated by reference to the Registrant's Form 8-K, dated October 27, 2011). |
||
| 10.18 | (1) | CardioNet, Inc. Long Term Incentive Plan (Incorporated by reference to Exhibit 10.2 to the Registrant's Form 8-K filed October 28, 2008). | |
| 10.19 | (1) | Compensation Program for Non-Employee Directors. (Incorporated by reference to Exhibit 99.5 to the Registrant's Form 8-K filed January 28, 2009). | |
| 10.20 | (1) | Employment Agreement, dated as of October 19, 2009, by and among the Registrant and Anna McNamara. (Incorporated by reference to Exhibit 10.35 to the Registrant's Form 10-K filed February 23, 2010). | |
| 10.21 | (1) | Employment Agreement, dated as of June 15, 2010, between Joseph H. Capper and the Registrant. (Incorporated by reference to Exhibit 99.2 to the Registrant's Form 8-K filed June 18, 2010). | |
| 10.22 | (1) | Employment Agreement, dated as of January 28, 2010, by and among the Registrant and Heather Getz. (Incorporated by reference to Exhibit 10.36 to the Registrant's Form 10-K filed February 23, 2010). | |
| 10.23 | (1) | Employment Agreement, dated as of December 7, 2010, between the Registrant and Daniel Wisniewski (Incorporated by reference to Exhibit 10.38 to the Registrant's Form 10-K, dated February 25, 2010). | |
| 10.24 | (1) | Employment Agreement dated as of February 7, 2011, between the Registrant and Peter Ferola (Incorporated by reference to Exhibit 10.1 to the Registrant's Form 10-Q dated May 6, 2011). | |
88
| Exhibit Number |
Description | ||
|---|---|---|---|
23.1 |
Consent of Independent Registered Public Accounting Firm.* |
||
31.1 |
Certification of Chief Executive Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities and Exchange Act of 1934, as amended.* |
||
31.2 |
Certification of Chief Financial Officer pursuant to Rules 13a-14(a) and 15d-14(a) promulgated under the Securities and Exchange Act of 1934, as amended.* |
||
32 |
Certification of Chief Executive Officer and Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.* |
||
101.INS |
XBRL Instance Document.** |
||
101.SCH |
XBRL Taxonomy Extension Schema Document.** |
||
101.CAL |
XBRL Taxonomy Extension Calculation Linkbase Document.** |
||
101.LAB |
Taxonomy Label Linkbase Document.** |
||
101.PRE |
XBRL Taxonomy Presentation Linkbase Document.** |
||
101.DEF |
XBRL Taxonomy Definition Linkbase Document.** |
||
- *
- Filed
herewith.
- †
- Confidential
treatment has been granted with respect to certain portions of this exhibit. Omitted portions have been filed separately with the
Securities and Exchange Commission.
- (1)
- Indicates
a management plan or compensatory plan or arrangement.
- **
- Furnished herewith. Pursuant to applicable securities laws and regulations, we are deemed to have complied with the reporting obligation relating to the submission of interactive data files in such exhibits and are not subject to liability under any anti-fraud provisions of the federal securities laws as long as we have made a good faith attempt to comply with the submission requirements and promptly amend the interactive data files after becoming aware that the interactive data files fail to comply with the submission requirements. Users of this data are advised that, pursuant to Rule 406T, these interactive data files are deemed not filed and otherwise are not subject to liability.
89
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
| Date: February 23, 2012 | CardioNet, Inc. | |||
By: |
/s/ JOSEPH H. CAPPER Joseph H. Capper President and Chief Executive Officer |
|||
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report has been signed below by the following persons on behalf of the Registrant and in the capacities and on the dates indicated.
Signature
|
Title
|
Date
|
||
|---|---|---|---|---|
| /s/ JOSEPH H. CAPPER Joseph H. Capper |
President and Chief Executive Officer (Principal Executive Officer) |
February 23, 2012 | ||
/s/ HEATHER C. GETZ Heather C. Getz, CPA |
Chief Financial Officer (Principal Financial and Accounting Officer) |
February 23, 2012 |
||
/s/ KIRK E. GORMAN Kirk E. Gorman |
Chairman and Director |
February 23, 2012 |
||
/s/ RONALD A. AHRENS Ronald A. Ahrens |
Director |
February 23, 2012 |
||
/s/ ERIC N. PRYSTOWSKY Eric N. Prystowsky, M.D. |
Director |
February 23, 2012 |
||
/s/ REBECCA RIMEL Rebecca Rimel |
Director |
February 23, 2012 |
||
/s/ ROBERT J. RUBIN Robert J. Rubin, M.D. |
Director |
February 23, 2012 |
90
