Attached files
| file | filename |
|---|---|
| EXCEL - IDEA: XBRL DOCUMENT - INTEGRATED BIOPHARMA INC | Financial_Report.xls |
| EX-32.2 - INTEGRATED BIOPHARMA INC | exhibit32_2.htm |
| EX-32.1 - INTEGRATED BIOPHARMA INC | exhibit32_1.htm |
| EX-31.1 - INTEGRATED BIOPHARMA INC | exhibit31_1.htm |
| EX-31.2 - INTEGRATED BIOPHARMA INC | exhibit31_2.htm |
UNITED STATES SECURITIES AND EXCHANGE COMMISSION
Washington D.C. 20549
____________
FORM 10-Q
x Quarterly Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the quarterly period ended December 31, 2011
OR
o Transition Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
For the transition period from to
|
Commission File Number 001-31668
|
INTEGRATED BIOPHARMA, INC.
(Exact name of registrant, as specified in its charter)
|
Delaware
|
22-2407475
|
|
(State or other jurisdiction of
|
(I.R.S. Employer
|
|
incorporation or organization)
|
Identification No.)
|
|
225 Long Ave., Hillside, New Jersey
|
07205
|
|
(Address of principal executive offices)
|
(Zip Code)
|
(888) 319-6962
|
(Registrant’s telephone number, including Area Code)
|
Not Applicable
(Former name, former address and former fiscal year, if changed since last report)
Indicate by check mark whether the Registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities and Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the Registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer
|
Accelerated filer
|
Non-accelerated filer
|
Smaller reporting company þ
|
Indicate by check whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act).
Yes o No x
Applicable only to Corporate Issuers:
The number of shares outstanding of each of the issuer’s class of common stock, as of the latest practicable date:
| Class | Outstanding at February 21, 2012 |
| Common Stock, $0.002 par value | 20,980,174 Shares |
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
FORM 10-Q QUARTERLY REPORT
For the Three and Six Months Ended December 31, 2011
INDEX
|
Page
|
||
|
Part I. Financial Information
|
||
|
Item 1.
|
Condensed Consolidated Statements of Operations for the Three and Six Months Ended December 31, 2011 and 2010 (unaudited)
|
2
|
|
Condensed Consolidated Balance Sheets as of December 31, 2011 and June 30, 2011 (unaudited)
|
3
|
|
|
Condensed Consolidated Statements of Changes in Stockholders’ Deficiency for the Six Months Ended December 31, 2011 (unaudited)
|
4
|
|
|
Condensed Consolidated Statements of Cash Flows for the Six Months Ended December 31, 2011 and 2010 (unaudited)
|
5
|
|
|
Notes to Condensed Consolidated Statements
|
6
|
|
|
Item 2.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operations
|
14
|
|
Item 3.
|
Quantitative and Qualitative Disclosures about Market Risk
|
21
|
|
Item 4.
|
Controls and Procedures
|
21
|
|
Part II. Other Information
|
||
|
Item 1.
|
Legal Proceedings
|
22
|
|
Item 1A.
|
Risk Factors
|
22
|
|
Item 2.
|
Unregistered Sales of Equity Securities and Use of Proceeds
|
22
|
|
Item 3.
|
Defaults Upon Senior Securities
|
22
|
|
Item 5.
|
Other Information
|
22
|
|
Item 6.
|
Exhibits
|
22
|
|
Other
|
||
|
Signatures
|
23
|
|
Disclosure Regarding Forward-Looking Statements
Certain statements in this Quarterly Report on Form 10-Q may constitute “forward-looking” statements as defined in Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), Section 21E of the Securities Act of 1934, as amended (the “Exchange Act”), the Private Securities Litigation Reform Act of 1995 (the “PSLRA”) or in releases made by the Securities and Exchange Commission, all as may be amended from time to time. Such forward-looking statements involve known and unknown risks, uncertainties and other factors which may cause the actual results, performance or achievements of Integrated BioPharma, Inc. (the “Company”) or industry results, to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. Statements that are not historical fact are forward-looking statements. Forward-looking statements can be identified by, among other things, the use of forward-looking language, such as the words, “plan”, “believe”, “expect”, “anticipate”, “intend”, “estimate”, “project”, “may”, “will”, “would”, “could”, “should”, “seeks”, or “scheduled to”, or other similar words, or the negative of these terms or other variations of these terms or comparable language, or by discussion of strategy or intentions. These cautionary statements are being made pursuant to the Securities Act, the Exchange Act and the PSLRA with the intention of obtaining the benefits of the “safe harbor” provisions of such laws. The Company cautions investors that any forward-looking statements made by the Company are not guarantees or indicative of future performance. Important assumptions and other important factors that could cause actual results to differ materially from those forward-looking statements with respect to the Company, include, but are not limited to, the risks and uncertainties affecting its businesses described in Items 1 and 1A of the Company’s Annual Report filed on Form 10-K for the year ended June 30, 2011 and in registration statements and other securities filings by the Company. Although the Company believes that its plans, intentions and expectations reflected in or suggested by such forward-looking statements are reasonable, actual results could differ materially from a projection or assumption in any of the forward-looking statements and are subject to change due inherent risks and uncertainties. The forward-looking statements contained in this Quarterly Report on Form 10-Q are made only as of the date hereof and the Company does not have or undertake any obligation to update or revise any forward-looking statements whether as a result of new information, subsequent events or otherwise, unless otherwise required by law.
1
ITEM 1. FINANCIAL STATEMENTS

2
3

4
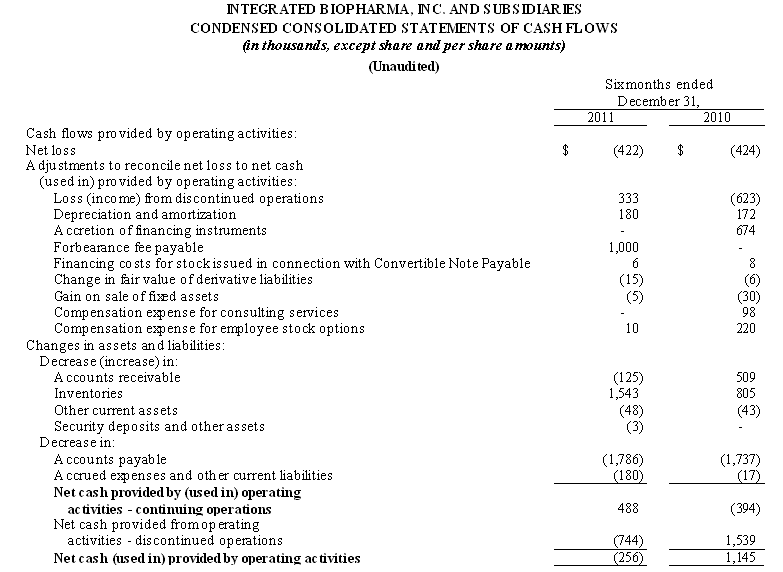
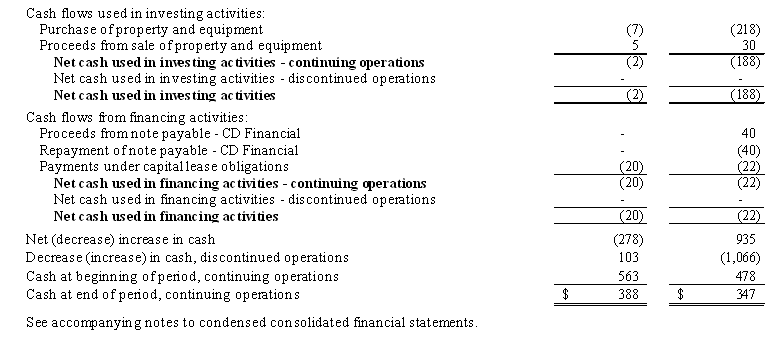
5
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2011 AND JUNE 30, 2011 AND FOR THE
THREE AND SIX MONTHS ENDED DECEMBER 31, 2011 AND 2010
(in thousands, except share and per share amounts)
(Unaudited)
Note 1. Principles of Consolidation and Basis of Presentation
Basis of Presentation of Interim Financial Statements
The accompanying condensed financial statements for the interim periods are unaudited and include the accounts of Integrated BioPharma, Inc., a Delaware corporation (together with its subsidiaries, the “Company”). The interim condensed financial statements have been prepared in conformity with Rule 10-01 of Regulation S-X of the Securities and Exchange Commission (“SEC”) and therefore do not include information or footnotes necessary for a complete presentation of financial position, results of operations and cash flows in conformity with accounting principles generally accepted in the United States of America. However, all adjustments (consisting only of normal recurring adjustments) which are, in the opinion of management, necessary for a fair presentation of the financial position and operating results for the periods presented have been included. These condensed financial statements should be read in conjunction with the financial statements and notes thereto, together with Management’s Discussion and Analysis of Financial Condition and Results of Operations, contained in the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2011 (“Form 10-K”), as filed with the SEC. The June 30, 2011 balance sheet was derived from audited financial statements, but does not include all disclosures required by accounting principles generally accepted in the United States of America. The results of operations for the three and six months ended December 31, 2011 are not necessarily indicative of the results for the full fiscal year ending June 30, 2012 or for any other period.
These condensed consolidated financial statements reflect the classification as discontinued operations of our branded proprietary nutraceutical business which is operated by AgroLabs, Inc. (“AgroLabs”), a wholly owned subsidiary of the Company, which distributes for sale through major mass market, grocery, drug and vitamin retailers, healthful nutritional products under the following brands: Naturally Noni, Naturally Pomegranate, Pomegranate with ACAI and Reservatrol, Coconut Water, Naturally Aloe, Aloe Pure, Naturally Thai Mangosteen, Peaceful Sleep, Green Envy, 1st Choice Multi-Vitamin, ACAI Extra, ACAI Immune, ACAI Cleanse, and other products which are being introduced into the market. These are referred to as our branded proprietary nutraceutical business and/or products. (See Note 3. Discontinued Operations).
Nature of Operations
The Company is engaged primarily in manufacturing, distributing, marketing and sales of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily in the United States. The Company was previously known as Integrated Health Technologies, Inc. and, prior to that, as Chem International, Inc. The Company was reincorporated in its current form in Delaware in 1995. The Company continues to do business as Chem International, Inc. with certain of its customers and certain vendors.
The Company’s nutraceutical business includes: InB:Manhattan Drug Company, Inc. (“Manhattan Drug”), which manufactures vitamins and nutritional supplements for sale to distributors, multilevel marketers and specialized health-care providers and The Vitamin Factory, which sells private label Manhattan Drug products, as well as our AgroLabs products, through the Internet.
The Company also distributes fine natural chemicals through its wholly-owned subsidiary IHT Health Products, Inc. and is a distributor of certain raw materials for DSM Nutritional Products, Inc.
Significant Accounting Policies
There have been no material changes during fiscal year 2012 in the Company’s significant accounting policies to those previously disclosed in the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2011.
6
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2011 AND JUNE 30, 2011 AND FOR THE
THREE AND SIX MONTHS ENDED DECEMBER 31, 2011 AND 2010
(in thousands, except share and per share amounts)
(Unaudited)
Investment in iBio, Inc. - Restricted. The Company accounts for its investment in iBio, Inc. (“iBio”) on the cost basis as it retained approximately 6% of its interest in iBio (1,266,706 shares of common stock) (the “iBio Stock”) at the time of the spin-off of this subsidiary in August 2008. The Company reviews its investment in iBio for impairment and records a loss when there is deemed to be an impairment of the investment. There was no impairment charge recorded in the six months ended December 31, 2011 and 2010. Pursuant to the Forbearance Agreement with respect to the Notes Payable, proceeds from any sales of the iBio Stock were to be used to repay a portion of the outstanding principal of the Notes Payable. (See Note 2. Liquidity and Going Concern and Note 6. Debt). No shares of iBio were sold pursuant to the Forbearance Agreement prior to the expiration thereof (See Note 2. Liquidity and Going Concern and Note 6. Debt).
Supplemental Statement of Cash Flows

Earnings Per Share. Basic earnings per common share amounts are based on the weighted average number of common shares outstanding. Diluted earnings per share amounts are based on the weighted average number of common shares outstanding, plus the incremental shares that would have been outstanding upon the assumed exercise of all potentially dilutive stock options, warrants and convertible preferred stock, subject to anti-dilution limitations using the treasury stock method.
Stock options and warrants to purchase or otherwise obtain shares of common stock were not included in the fully diluted computation for earnings per share as their exercise prices were greater than the market price of the common shares as of December 31, 2011 and 2010.
Note 2. Liquidity and Going Concern
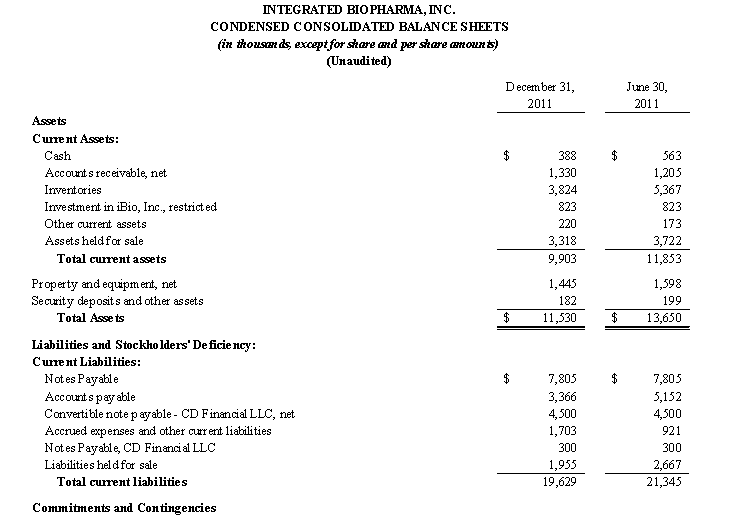
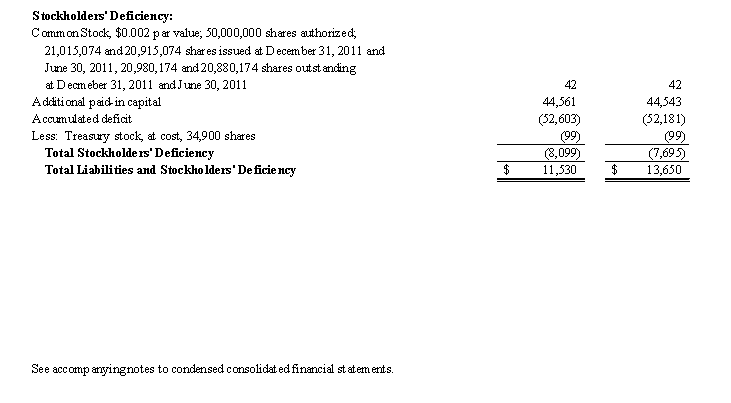
The Company’s condensed consolidated financial statements have been prepared assuming that it will continue as a going concern. The Company has cumulative historical net losses and cash outflows from operations. For the fiscal year ended June 30, 2011, the Company had cash provided from operating activities of $390. As of December 31, 2011, the Company had cash of $446 (inclusive of cash of $58 included in assets from discontinued operations) and a working capital deficit of $9,726, which is primarily attributable to the Notes Payable in the outstanding principal amount of $7,805, which matured on November 15, 2009 and are currently in default (See Note 6. Debt), the Convertible Note Payable in the outstanding principal amount of $4,500, which matured on February 21, 2011, which are secured by pledges of substantially all the Company’s assets (See Note 6. Debt). These factors raise substantial doubt as to the Company’s ability to continue as a going concern. The accompanying condensed consolidated financial statements do not include any adjustments that might result from these uncertainties.
The Company has defaulted on all of its outstanding debt instruments in the aggregate amount of $12,605 (see Note 6. Debt) by failing to repay them on their respective scheduled maturity dates. The Notes Payable and Convertible Note Payable are secured by pledges of substantially all of the Company’s assets.
7
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2011 AND JUNE 30, 2011 AND FOR THE
THREE AND SIX MONTHS ENDED DECEMBER 31, 2011 AND 2010
(in thousands, except share and per share amounts)
(Unaudited)
The Company defaulted on the $7,805 outstanding principal amount of its Notes Payable, issued by the Company under that certain Securities Purchase Agreement, dated as of February 21, 2008, by failing to repay the Notes Payable on the scheduled maturity date of November 15, 2009. The Company’s failure to repay the Notes Payable on the scheduled maturity date constituted an Event of Default under the Notes Payable and triggered the right of the holders of the Notes Payable (the “Note Payable Holders”) to give the Company a notice (an “Acceleration Notice”) to accelerate the payment of all unpaid principal and accrued and unpaid interest (including interest accruing at the default rate). The Notes Payable are secured by a pledge of substantially all of the Company’s assets. As of February 21, 2012, the Company has not repaid the Notes Payable.
On October 4, 2011, the Company and the Collateral Agent for the holders of the Notes Payable entered into a Forbearance Agreement (the "Forbearance Agreement") extending, among other things, the due date until December 31, 2011 (See Note 6. Debt). Several extensions of the Forbearance Agreement were granted from December 31, 2011 through February 8, 2012; however, on February 8, 2012, the forbearance period under the Forbearance Agreement expired. As of February 21, 2012, the Company remains in default under the Notes Payable (See Note 6. Debt). In addition, pursuant to the Forbearance Agreement, a $1.0 million forbearance fee is due and payable at the end of the forbearance period under the Forbearance Agreement. Such fee is included in accrued expenses and other liabilities in the condensed consolidated balance sheets as of December 31, 2011. The Company is negotiating with the Collateral Agent regarding a further forbearance, however, there can be no assurance that any further forbearance will be granted.
The Company defaulted on the $4,500 million outstanding principal amount of its Convertible Note Payable, issued by the Company under that certain Securities Purchase Agreement, dated as of February 21, 2008, by failing to repay the Convertible Note Payable on the scheduled maturity date of February 21, 2011. The Company’s failure to repay the Convertible Note Payable on the scheduled maturity date constituted an Event of Default under the Convertible Note Payable and triggered the right of the holder of the Convertible Note Payable to give the Company a notice (“CD Acceleration Notice”) to accelerate the payment of all unpaid principal and accrued and unpaid interest (including interest accruing at the default rate, if any). The Convertible Note Payable is secured by a pledge of substantially all of the Company’s assets, which pledge is subordinated to the security interest held by the Note Payable Holders. As of February 21, 2012, the Company has not repaid the Convertible Note Payable or the default interest on the Convertible Note Payable. The holder of the Convertible Note Payable, CD Financial, LLC (“CD Financial”) is a significant shareholder of the Company and has not made any payment demands on the Company with respect to the Convertible Note Payable, nor has it converted the Convertible Note Payable into common shares of the Company.
There can be no assurance that the Company will be able to repay, restructure or amend the Notes Payable prior to the receipt of an Acceleration Notice or the Convertible Note Payable prior to receipt by the Company of a CD Acceleration Notice. In the interim, the Company has continued to make timely interest payments to the Note Payable Holders at the non default rate of 8% per annum and is exploring its strategic alternatives, which may include business divestitures, developing business and sales strategies to increase operating income, the sale of some or all of the Company’s assets or operating subsidiaries and/or capital restructuring plans.
As a result of the events of default that arose based upon the Company’s failure to pay each of the Notes Payable at maturity, the Note Payable Holders have the right to give the Company an Acceleration Notice. As a result of the event of default that arose based upon the Company’s failure to repay the Convertible Note Payable at maturity, CD Financial has the right to give the Company a CD Acceleration Notice. Each acceleration notice would (i) accelerate the payment of all unpaid principal and accrued and unpaid interest (including default interest (if any); and (ii) require the Company to pay an amount equal to the sum of all of the respective amounts described in the preceding clause (i) in same day funds on the payment date specified in such notice. If the Company is unable to raise additional capital, sell certain assets or successfully refinance the full outstanding amount of the Notes Payable and the Convertible Note Payable upon acceptable terms, it would have a material adverse effect on the Company, including the possible foreclosure by the holders of the Notes Payable and/or the Convertible Note Payable of all or some of the Company’s assets, which would impact the Company’s ability to continue as a going concern.
8
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2011 AND JUNE 30, 2011 AND FOR THE
THREE AND SIX MONTHS ENDED DECEMBER 31, 2011 AND 2010
(in thousands, except share and per share amounts)
(Unaudited)
Note 3. Assets and Liabilities Held for Sale and Discontinued Operations
The Company is seeking to sell the net assets of AgroLabs. Accordingly, the Company classified the business assets and liabilities and the results of operations of its branded proprietary nutraceutical business as discontinued operations. The operations of AgroLabs for the current and prior periods and the associated results of operations, financial position and cash flows are separately reported as discontinued operations for all periods presented.
The net assets classified as discontinued operations were comprised of the following:
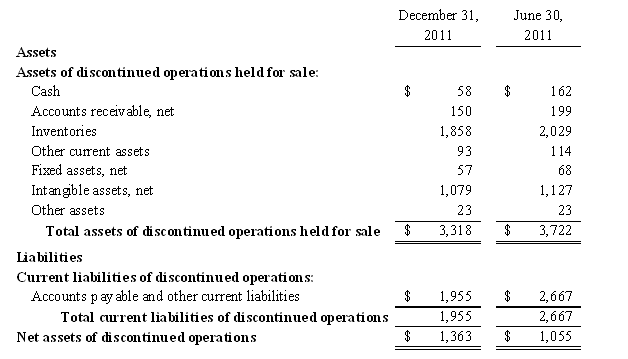
The Company’s net sales, gross profit and results of operations classified as discontinued operations were as follows:

9
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2011 AND JUNE 30, 2011 AND FOR THE
THREE AND SIX MONTHS ENDED DECEMBER 31, 2011 AND 2010
(in thousands, except share and per share amounts)
(Unaudited)
Note 4. Inventories
Inventories are stated at the lower of cost or market using the first-in, first-out method and consist of the following as of:
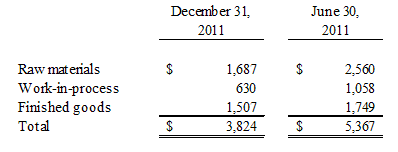
Note 5. Property and Equipment, net
Property and equipment, net consists of the following as of:
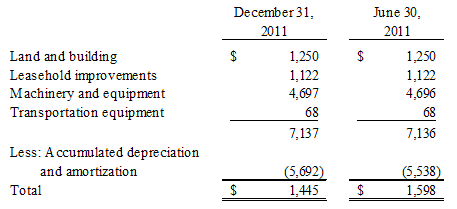
Depreciation and amortization expense was $80 and $77 for the three months ended December 31, 2011 and 2010, respectively and $160 and $152 for the six months ended December 31, 2011 and 2010, respectively. In the six months ended December 31, 2011 and 2010, the Company disposed of fully depreciated machinery and equipment in the amount of $6 and $137, respectively, for a gain of $5 and $30, respectively.
Note 6. Debt
Notes Payable
On February 21, 2008, the Company entered into a Securities Purchase Agreement with Imperium Master Fund, LTD. and three other parties (collectively “Imperium”), which Securities Purchase Agreement was amended on October 14, 2008 (as so amended, the “SPA”), pursuant to which the Company issued, and Imperium purchased from the Company, an aggregate $7,000 in principal of senior secured notes (“Notes Payable”). The Notes Payable matured on November 15, 2009 and bear interest at the rate of 8.0%. Interest is payable monthly. As of December 31 and June 30, 2011, accrued interest of $54 and $47, respectively, is included in accrued expenses and other current liabilities in the condensed consolidated balance sheets.
An additional premium of $805 was granted due to the amendment noted above. The initial additional premium of $805 was subject to interest payments commencing October 1, 2011. In addition, pursuant to the Forbearance Agreement, a $1.0 million forbearance fee is due and payable at the end of the forbearance period under the Forbearance Agreement. Such fee is included in accrued expenses and other liabilities in the condensed consolidated balance sheets as of December 31, 2011.
10
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2011 AND JUNE 30, 2011 AND FOR THE
THREE AND SIX MONTHS ENDED DECEMBER 31, 2011 AND 2010
(in thousands, except share and per share amounts)
(Unaudited)
The Notes Payable are secured by a pledge of substantially all of the Company’s assets.
On October 4, 2011, the Company and the Collateral Agent for the Note Payable Holders, entered into a Forbearance Agreement. The Forbearance Agreement provided that the Collateral Agent shall forbear from exercising rights and remedies arising from the occurrence of Specified Defaults (as defined in the Forbearance Agreement), including the Company’s failure to repay the Notes Payable which are due and payable. Several extensions of the Forbearance Agreement were granted from December 31, 2011 through February 8, 2012; however, on February 8, 2012, the forbearance period under the Forbearance Agreement expired. As of February 21, 2012, the Company remains in default under the Notes Payable. The Company is negotiating with the Collateral Agent regarding a further forbearance, however, there can be no assurance that any further forbearance will be granted.
The Company and the Collateral Agent continue to discuss the options available to the Company to repay the Notes Payable among other strategic options, including retaining an investment banker to assist the Company in selling its propriety nutraceutical business, to repay the Notes Payable under agreeable terms.
Convertible Note Payable – CD Financial, LLC
On February 21, 2008, the Company entered into a Securities Purchase Agreement (the “CD SPA”) with CD Financial, a significant shareholder of the Company, pursuant to which the Company issued and CD Financial purchased from the Company, a Convertible Senior Secured Note in the principal amount of $4,500 (the “Convertible Note Payable”). The Convertible Note Payable matured on February 21, 2011 and bears interest at the rate of 9.5%. Interest is payable monthly. The Convertible Note Payable is secured by a pledge of substantially all of the Company’s assets.
In March 2009, the Company and CD Financial entered into an oral agreement to suspend the cash interest payments on the Convertible Note Payable until the Company returned to positive cash flows in its operations. In this oral agreement, CD Financial agreed not to give any default notices or increase interest rates due to such default (the default interest rate as defined in the Convertible Note Payable is 18%). The Company resumed interest payments on the Convertible Note Payable in August of 2009. In March 2010, CD Financial orally agreed to defer the interest owed for April 2010, in the amount of $36, until the Company returned to positive cash flows to assist the Company in meeting its short term cash flow requirements. The Company has made timely monthly interest payments, beginning with the May 2010 monthly interest obligation. As of February 21, 2012, the Company is in default under the Convertible Note Payable for the nonpayment of the principal balance due on February 21, 2011.
As of December 31 and June 30, 2011, accrued interest of $254 with respect to the Convertible Note Payable for each period is included in accrued expenses and other current liabilities in the condensed consolidated balance sheets, including interest in arrears of $217 in each period.
The Convertible Note Payable may be converted, at any time and at the holder’s option, into shares of the Company’s common stock under the terms and conditions set forth in the CD SPA. The conversion price is a formula that bases the conversion price on the greater of (i) 90% of the average Volume Weighted Average Price (the "VWAP") market price of the Company’s common stock for 20 trading days immediately preceding the conversion date and (ii) $2.00, subject to adjustment in the event of a stock dividend, stock split or combination, reclassification or similar event and upon certain issuances below the conversion price.
11
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2011 AND JUNE 30, 2011 AND FOR THE
THREE AND SIX MONTHS ENDED DECEMBER 31, 2011 AND 2010
(in thousands, except share and per share amounts)
(Unaudited)
Also, in accordance with the CD SPA, the Company will issue and deliver to CD Financial, for no additional consideration, 50,000 shares of common stock, on a quarterly basis in arrears, until the Convertible Note Payable has been repaid in full, after which the Company's obligations to issue shares of common stock will no longer be applicable.
Debt discount of $333 and $640, respectively, in the three and six months ended December 31, 2010 was recorded in interest expense in condensed consolidated statement of operations.
Notes Payable, CD Financial LLC
On November 24, 2009, Manhattan Drug, a wholly owned subsidiary of the Company, entered into a $300 promissory note (the “CD Note”) with CD Financial. The CD Note matured on November 24, 2010 and bears interest at the rate of 5%, which interest is payable quarterly. As of December 31, 2011, the Company is in default under the CD Note as a result of the Company’s failure to repay the CD Note on its scheduled maturity date. Interest is paid quarterly. The CD Note is expected to remain outstanding until the Company satisfies the Company’s obligations under the Notes Payable. (See Note 6. Debt – Notes Payable).
On July 29, 2010, Manhattan Drug entered into a second promissory note in the amount of $40 (the “CD $40 Note”) with CD Financial. The CD $40 Note matured on October 29, 2010 and bore interest at the rate of 5%. Manhattan Drug repaid the CD $40 Note on October 29, 2010. Interest was accrued monthly and was paid on maturity.
The weighted average interest rate paid on the Company’s outstanding debt was 8.56% and 8.49% in the three months ended December 31, 2011 and 2010, respectively and 8.46% and 8.49% for the six months ended December 31, 2011 and 2010, respectively.
Note 7. Significant Risks and Uncertainties
(a) Major Customers. For the three and six months ended December 31, 2011, approximately 81% and 80%, respectively, of total net sales were derived from one customer as compared to approximately 89% and 88% of total net sales derived from two customers in the three and six months ended December 31, 2010, respectively. Accounts receivable as of December 31, 2011 from the one customer represented approximately 54% of total accounts receivable. The loss of this customer would have an adverse affect on the Company’s operations. Major customers are those customers who account for more than 10% of net sales.
(b) Other Business Risks. Approximately 57% the Company’s employees, located in its New Jersey facility, are covered by a union contract. The contract was renewed in August 2010 for an additional one year term, and was extended in August 2011 to December 31, 2011. In December 2011, the union verbally agreed to continue operating under the terms of the existing contract until the Company completes the refinancing of its material debt (see Note 6. Debt).
Note 8. Commitments and Contingencies
(a) Leases
Related Party Leases. Warehouse and office facilities are leased from Vitamin Realty, which is 90% owned by the Chairman of the Company’s Board of Directors, a director and majority shareholder and certain of his family members. The lease provides for minimum annual rental payment of $324 through May 31, 2015 plus increases in real estate taxes and building operating expenses. On July 1, 2004, the Company leased an additional 24,810 square feet of warehouse space on a month-to month basis.
12
INTEGRATED BIOPHARMA, INC. AND SUBSIDIARIES
NOTES TO THE CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
AS OF DECEMBER 31, 2011 AND JUNE 30, 2011 AND FOR THE
THREE AND SIX MONTHS ENDED DECEMBER 31, 2011 AND 2010
(in thousands, except share and per share amounts)
(Unaudited)
Rent expense for the three months ended December 31, 2011 and 2010 on these leases were $172 and $167, respectively, and $355 and $368 for the six months ended December 31, 2011 and 2010, respectively, and are included in cost of sales in the accompanying Condensed Consolidated Statements of Operations. As of December 31, 2011 and June 30, 2011, the Company had an outstanding obligation of $686 and $738, respectively, included in accounts payable in the accompanying Condensed Consolidated Balance Sheet.
Other Lease Commitments. The Company has entered into certain non-cancelable operating lease agreements expiring up through May 31, 2015, related to office and warehouse space, equipment and vehicles.
The minimum rental commitment for long-term non-cancelable leases is as follows:
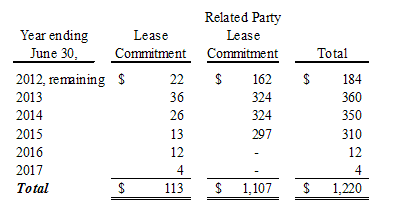
Total rent expense, including real estate taxes and maintenance charges, was approximately $267 and $261 for the three months ended December 31, 2011 and 2010, respectively, and $543 and $555 for the six months ended December 31, 2011 and 2010, respectively. Rent expense is included in cost of sales, selling and administrative expenses and income (loss) from discontinued operations in the accompanying Condensed Consolidated Statements of Operations. The Company has a remaining lease commitment of $108 ($93 remaining in the fiscal year ending June 30, 2012) relating to operations classified as discontinued.
(b) Legal Proceedings.
The Company is subject, from time to time, to claims by third parties under various legal theories. The defense of such claims, or any adverse outcome of any such claims, could have a material adverse effect on the Company’s liquidity, financial condition and cash flows. The Company believes that there are no significant legal matters pending that would have a material effect on the Company’s liquidity, financial condition or cash flows.
Note 9. Related Party Transactions
See Note 6. Debt, for related party securities transactions.
See Note 8(a). Leases for related party lease transactions.
13
Item 2. MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANICAL CONDITION AND RESULTS OF OPERATION
Certain statements set forth under this caption constitute “forward-looking statements.” See “Disclosure Regarding Forward-Looking Statements” on page 1 of this Quarterly Report on Form 10-Q for additional factors relating to such statements. The following discussion should also be read in conjunction with the condensed consolidated financial statements of the Company and Notes thereto included herein and the Company’s Annual Report on Form 10-K for the fiscal year ended June 30, 2011.
The Company is engaged primarily in the manufacturing, distributing, marketing and sales of vitamins, nutritional supplements and herbal products. The Company’s customers are located primarily throughout the United States.
Business Outlook
Our future results of operations and the other forward-looking statements contained in this Quarterly Report on Form 10-Q, including this MD&A, involve a number of risks and uncertainties—in particular, the statements regarding our goals and strategies, new product introductions, plans to cultivate new businesses, pending divestitures, future economic conditions, revenue, pricing, gross margin and costs, the tax rate, and potential legal proceedings. We are focusing our efforts to improve operational efficiency and reduce spending that may have an impact on expense levels and gross margin. In addition to the various important factors discussed above, a number of other important factors could cause actual results to differ significantly from our expectations. See the risks described in “Risk Factors” in Part II, Item 1A of this Quarterly Report on Form 10-Q.
In the third quarter of our fiscal year ended June 30, 2011, as a result of our ongoing negotiations with the Note Payable Holders (See Note 6 to Condensed Consolidated Financial Statements – Debt), we determined, in order to repay the Notes Payable, which matured on November 15, 2009, and to effect the Company’s strategic business plan to revert to our original core business of solid dosage manufacturing, to seek to sell the assets of AgroLabs, Inc., (“AgroLabs”), a wholly owned subsidiary of the Company, in order to repay a portion of the defaulted balance of the Notes Payable with the proceeds of such sale. Accordingly, we classified the business assets, liabilities and results of operations of our branded proprietary nutraceutical business as discontinued operations. As such, the operations of AgroLabs for the current and prior periods presented in the financial statements contained in this Quarterly Report on Form 10-Q and the associated results of operations, financial position and cash flows are separately reported as discontinued operations for all periods presented.
Our financial results are substantially dependent on net sales. Net sales are partly dependent on the mix of contract manufactured products and other nutraceutical sales, which are difficult to forecast. The net sales from our branded proprietary neutraceutical business are included in loss from discontinued operations and continue to influence our financial results. The varied sales pricing among our products and promotional support in the form of consumer coupons and other sales price allowances, along with the mix of products sold, affects the average selling price that we will realize and has a large impact on our revenue and gross margins in the operations of AgroLabs. Net sales in our operations of AgroLabs, classified as discontinued operations, is also affected by: the timing of new product introductions and the demand for and market acceptance of our products; actions taken by our competitors, including new product offerings and introductions, marketing programs and pricing pressures, and our response to such actions; our ability to respond quickly to consumer tastes and needs; and the availability of sufficient raw materials and production lead-time from suppliers to meet demand. Factors that could cause demand to be different from our expectations include: customer acceptance of our products and our competitors products; changes in customer order patterns, including order returns; changes in the level of inventory at customers; and changes in business and economic conditions, including conditions in the credit market that could affect consumer confidence and result in lower than expected demand for our products.
We believe that we have the product offerings, established and developing business relationships, facilities, personnel, and competitive and financial resources in place for business success; however, future revenue, costs, gross margins, and profits are all influenced by a number of factors, including those discussed above, all of which are inherently difficult to forecast.
14
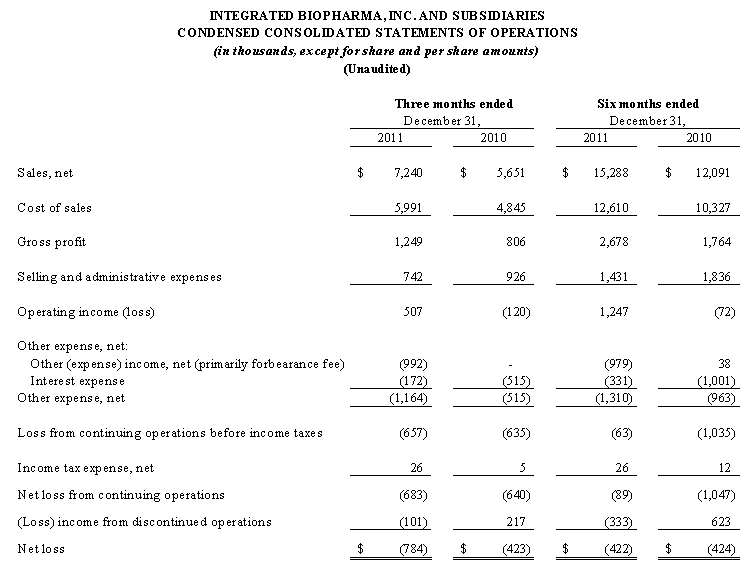
For the three months ended December 31, 2011, our net sales from continuing operations increased by $1.6 million or 28.0% to $7.2 million from $5.7 million for the three months ended December 31, 2010 and for the six months ended December 31, 2011, our net sales from continuing operations increased by $3.2 million or 26.4% to $15.3 million from $12.1 million for the six months ended December 31, 2010. Our operating income increased from a loss of $0.1 million and $0.07 million for the three and six months ended December 31, 2010, respectively, to operating income of $0.5 million and $1.2 million for the three and six months ended December 31, 2011, respectively. Also, in the three and six months ended December 31, 2011, our gross profit increased by approximately $0.4 million and $0.9 million from the three and six months ended December 31, 2010 and we cut our selling and administrative expenses by approximately $0.2 million and $0.4 million, respectively. We continue to focus on our core businesses and on maintaining our cost structure in line with our sales.
Critical Accounting Policies and Estimates
There have been no changes to our critical accounting policies in the six months ended December 31, 2011. Critical accounting policies and the significant estimates made in accordance with them are regularly discussed by management with our Audit Committee. Those policies are discussed under “Critical Accounting Policies” in our “Management’s Discussion and Analysis of Financial Condition and Results of Operations” included in Item 7 of our Annual Report on Form 10-K for the year ended June 30, 2011.
Results of Operations
Our results from operations in the following table, sets forth the income statement data of our results as a percentage of net sales for the periods indicated:

15
For the six months ended December 31, 2011 compared to the six months ended December 31, 2010
Sales, net. Sales, net, for the six months ended December 31, 2011 and 2010 were $15.3 million and $12.1 million, respectively, an increase of 26%, and are comprised of the following:

For the six months ended December 31, 2011, approximately 80% of total net sales were derived from one customer and as compared to approximately 88% of total net sales were derived from two customers in the six months ended December 31, 2010. The loss of this customer would have an adverse affect on our operations.
The increase in our net sales is the result of an increase of approximately $2.9 million in our contract manufacturing product sales primarily from increased sales to one of our major customers in the amount of $3.0 million. The remaining nutraceutical product lines had net sales increases of approximately $0.3 million compared to the prior period.
Cost of sales. Cost of sales increased by $2.3 million to $12.6 million for the six months ended December 31, 2011 as compared to $10.3 million for the six months ended December 31, 2010. Cost of sales decreased as a percentage of sales to 82.5% for the six months ended December 31, 2011 as compared to 85.4% for the six months ended December 31, 2010. The increase in cost of sales was primarily the result of increased sales. The cost of sales as a percentage of sales decreased as we benefited from spreading the fixed manufacturing costs over an increased amount of products manufactured and sold to customers.
Selling and Administrative Expenses. Selling and administrative expenses were $1.4 million for the six months ended December 31, 2011, as compared to $1.8 million for the six months ended December 31, 2010, a decrease of $0.4 million or 22.0%. As a percentage of sales, net, selling and administrative expenses were 9.4% for the six months ended December 31, 2011 and 15.2% for the prior comparable period.
The net decrease in selling and administrative expenses of $0.4 million was mainly due to a decrease in our stock compensation expense, which decreased by $0.3 million primarily due to the significant decrease in the market value of our common stock at the measurement date of the stock option grants (the market value of our common stock is one of several factors used in determining the fair value of the stock compensation at the time of the award and ultimate expense to our consolidated financial statements), and also due to no stock options being granted in the past two fiscal years ended June 30, 2011.
Other expense, net. Other expense, net was approximately $1.3 million for the six months ended December 31, 2011 compared to $1.0 million for the six months ended December 31, 2010, an increase of $0.3 million. The forbearance fee of $1.0 million incurred under the Forbearance Agreement represents substantially all of the other expense, net in the six months ended December 31, 2011 (see Note 6 to the Condensed Consolidated Financial Statements – Debt) and interest expense of $1.0 million represents substantially all of the other expense, net in the six months ended December 31, 2010. The decrease in interest expense of approximately $0.6 million is primarily attributable to the lack of any accretion on our Convertible Note Payable in the six months ended December 31, 2011, compared to approximately $0.6 million of accretion of our Convertible Note Payable in the six months ended December 31, 2010 and the increase of $1.0 million in other expense, net is attributable to the forbearance fee incurred pursuant to the Forbearance Agreement in the six months ended December 31, 2011 compared to no such fee in the six months ended December 31, 2010.
Federal and state income tax, net. For the six months ended December 31, 2011 and December 31, 2010, we had minimal amounts of state tax expenses. We continue to provide and maintain a full reserve on our deferred tax assets as it has been determined that based upon past losses, the Company’s liquidity concerns and the current economic environment, that it is “more likely than not” that the Company’s deferred tax assets may not be realized.
16
(Loss) income from discontinued operations. In the third quarter of our fiscal year ended June 30, 2011, as a result of our ongoing negotiations with the Note Payable Holders (see Note 6 to Condensed Consolidated Financial Statements – Debt), we determined, in order to repay the Notes Payable, which matured on November 15, 2009, and to effect the Company’s strategic business plan to revert to our original core business of solid dosage manufacturing, to seek to sell the assets of AgroLabs in order to repay a portion of the defaulted balance of the Notes Payable with the proceeds of such sale. Accordingly, we classified the business assets, liabilities and results of operations of our branded proprietary nutraceutical business as discontinued operations. The loss from these discontinued operations was approximately $0.3 million for the six months ended December 31, 2011 compared to net income from these operations of $0.6 million for the six months ended December 31, 2010, a decrease of $0.9 million.
Net loss. Although we had a net loss of approximately $0.4 million in each of the six month periods ended December 31, 2011 and 2010, our operating income increased by $1.3 million which was offset by increases in other expense, net of $0.3 million and a decrease in the net income (loss) from discontinued operations of $1.0 million.
For the three months ended December 31, 2011 compared to the three months ended December 31, 2010
Sales, net. Sales, net, for the three months ended December 31, 2011 and 2010 were $7.2 million and $5.7 million, respectively, an increase of 28%, and are comprised of the following:
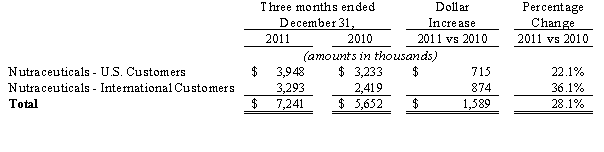
For the three months ended December 31, 2011, approximately 81% of total net sales were derived from one customer and as compared to approximately 89% of total net sales were derived from two customers in the three months ended December 31, 2010. The loss of this customer would have an adverse affect on our operations.
The increase in our net sales is the result of an increase of approximately $1.4 million in our contract manufacturing product sales primarily from increased sales to one of our major customers in the amount of $1.5 million. The remaining nutraceutical product lines had net sales increases of approximately $0.2 million compared to the prior period.
Cost of sales. Cost of sales increased by $1.1 million to $6.0 million for the three months ended December 31, 2011 as compared to $4.8 million for the three months ended December 31, 2010. Cost of sales decreased as a percentage of sales to 82.7% for the three months ended December 31, 2011 as compared to 85.7% for the three months ended December 31, 2010. The increase in cost of sales was primarily the result of increased sales. The cost of sales as a percentage of sales decreased as we benefited from spreading the fixed manufacturing costs over an increased amount of products manufactured and sold to customers.
Selling and Administrative Expenses. Selling and administrative expenses were $0.7 million for the three months ended December 31, 2011, as compared to $0.9 million for the three months ended December 31, 2010, a decrease of $0.2 million or 19.8%. As a percentage of sales, net, selling and administrative expenses were 10.3% for the three months ended December 31, 2011 and 16.4% for the prior comparable period.
17
The net decrease in selling and administrative expenses of $0.2 million was mainly due to a decrease in our stock compensation expense, which decreased by $0.1 million primarily due to the significant decrease in the market value of our common stock at the measurement date of the stock option grants (the market value of our common stock is one of several factors used in determining the fair value of the stock compensation at the time of the award and ultimate expense to our consolidated financial statements), and also due to no stock options being granted in the past two fiscal years ended June 30, 2011.
Other expense, net. Other expense, net was approximately $1.2 million for the three months ended December 31, 2011 compared to $0.5 million for the three months ended December 31, 2010, an increase of $0.7 million. Interest expense and the forbearance fee incurred pursuant to the Forbearance Agreement represent substantially all of the other expense, net in each of the three month periods ended December 31, 2011 and 2010 (See Note 6 to the Condensed Consolidated Financial Statements - Debt). The decrease in interest expense is the result of accretion on the Convertible Note Payable of approximately $0.3 million in the three months ended December 31, 2010 with no such accretion charge in the three months ended December 31, 2011. The increase in other expense, net was primarily attributable to the $1.0 million forbearance fee payable to the Notes Payable holders under the Forbearance Agreement incurred in the three months ended December 31, 2011, with no such charge in the three months ended December 31, 2010 (See Note 6 to the Condensed Consolidated Financial Statements - Debt).
Federal and state income tax, net. For the three months ended December 31, 2011 and December 31, 2010, we had minimal amounts of state tax expenses. We continue to provide and maintain a full reserve on our deferred tax assets as it has been determined that based upon past losses, the Company’s liquidity concerns and the current economic environment, that it is “more likely than not” that the Company’s deferred tax assets may not be realized.
(Loss) income from discontinued operations. In the third quarter of our fiscal year ended June 30, 2011, as a result of our ongoing negotiations with the Note Payable Holders (see Note 6 to Condensed Consolidated Financial Statements – Debt), we determined, in order to repay the Notes Payable, which matured on November 15, 2009, and to effect the Company’s strategic business plan to revert to our original core business of solid dosage manufacturing, to seek to sell the assets of AgroLabs in order to repay a portion of the defaulted balance of the Notes Payable with the proceeds of such sale. Accordingly, we classified the business assets, liabilities and results of operations of our branded proprietary nutraceutical business as discontinued operations. The loss from these discontinued operations was approximately $0.1 million for the three months ended December 31, 2011 compared to net income from these operations of $0.2 million for the three months ended December 31, 2010, a decrease of $0.3 million.
Net loss. We had a net loss of approximately $0.8 million for the three months ended December 31, 2011 compared to a net loss of approximately $0.4 million for the three months ended December 31, 2010. The increase in the net loss of approximately $0.4 million is primarily the result of increased operating income of $0.6 million offset by an increase in other expenses, net of $0.7 million, offset by a decrease in the net income (loss) from discontinued operations of $0.3 million.
Seasonality
The nutraceutical business tends to be seasonal. We have found that in our first fiscal quarter ending on September 30th of each year, orders for our branded proprietary nutraceutical products usually slow (absent the addition of new customers or a new product launch with a significant first time order), as buyers in various markets may have purchased sufficient inventory to carry them through the summer months. Conversely, in our second fiscal quarter, ending on December 31st of each year, orders for our products increase as the demand for our branded nutraceutical products seems to increase in late December to early January as consumers become health conscious as they enter the new year.
The Company believes that there are other non-seasonal factors that also may influence the variability of quarterly results including, but not limited to, general economic and industry conditions that affect consumer spending, changing consumer demands and current news on nutritional supplements. In addition, our recent growth has caused additional variability in our quarterly results. Accordingly, a comparison of the Company’s results of operations from consecutive periods is not necessarily meaningful, and the Company’s results of operations for any period are not necessarily indicative of future periods.
18
Liquidity, Going Concern and Capital Resources
The following table sets forth, for the periods indicated, the Company’s net cash flows used in operating, investing and financing activities, its period end cash and cash equivalents and other operating measures:
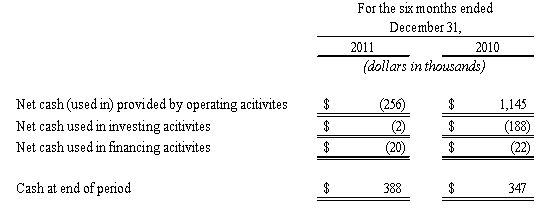
At December 31, 2011, our working capital deficit was approximately $9.7 million, an increase of approximately $0.2 million in our working capital deficit of approximately $9.5 million at June 30, 2011. Our current assets decreased by $1.9 million and our current liabilities decreased by $1.7 million, as we were able to pay our suppliers quicker as a result of our increased sales in our contract manufacturing business.
Net cash used in operating activities of $0.3 million in the six months ended December 31, 2011, includes our net loss of $0.4 million. After excluding the effects of non-cash expenses, including depreciation and amortization, compensation expense for employee stock options and consultants and changes in the fair value of derivative liabilities, the adjusted cash provided from operations before the effect of the changes in working capital components was $1.2 million. Cash was used in operations from our working capital assets and liabilities in the amount of approximately $0.8 million and was the result of an increase in accounts receivable of $0.1 million and a decrease in accounts payable and accrued expenses of $2.0, which was offset by an increase in inventory of $1.4 million. Net cash of $0.7 million was used in operating activities from our discontinued operations.
Net cash provided by operating activities of $1.1 million in the six months ended December 31, 2010, includes a net loss of $0.4 million. After excluding the effects of non-cash expenses, including, depreciation and amortization, compensation expense for employee stock options and consultants and changes in the fair value of derivative liabilities, the adjusted cash used in operations before the effect of the changes in working capital components was $64,000. Cash was used in operations from our working capital assets and liabilities in the amount of approximately $0.3 million and was primarily the result of a decrease in accounts payable and accrued expenses of $1.7 million offset by decreases in accounts receivable of $0.5 million and inventories of $0.9 million. Net cash of $1.5 million was provided by operating activities from our discontinued operations.
Cash used in investing activities was from purchase of machinery and equipment of $7,000, offset by the gain on the sale of fully depreciated machinery and equipment of $5,000 in the six months ended December 31, 2011, compared to the use of approximately $218,000 in the six months ended December 31, 2010 for the purchase of fixed assets offset by a gain on the sale of fully depreciated machinery and equipment of $30,000.
Cash used in financing activities was approximately $20,000 for the six months ended December 31, 2011 for payments under our capitalized lease obligation. Cash used by financing activities was approximately $62,000 for the six months ended December 31, 2010 which included a $40,000 repayment of funds borrowed in the same six months period under a short term promissory note entered into with CD Financial, a related party and the holder of our Convertible Note Payable, and the repayment of $22,000 under our capitalized lease obligations (See Note 6 to the Condensed Consolidated Financial Statements – Debt).
19
Our condensed consolidated financial statements have been prepared assuming that the Company will continue as a going concern. We have cumulative historical losses and cash outflows from operations. As of December 31, 2011, we had cash of $0.4 million (inclusive of $0.06 million of cash in assets from discontinued operations) and a working capital deficit of $9.7 million, which is primarily attributable to the Notes Payable in the amount of $7.8 million which matured on November 15, 2009, the Convertible Note Payable in the amount of $4.5 million, which matured on February 21, 2011, with respect to each of which we are in default. These factors raise substantial doubt as to our ability to continue as a going concern. The condensed consolidated financial statements included in this Quarterly Report on Form 10-Q do not include any adjustments that might result from this uncertainty.
As stated above, we have defaulted on substantially all of our outstanding debt instruments in the aggregate amount of $12.6 million by failing to repay them on their respective scheduled maturity dates. The Notes Payable and the Convertible Note Payable are secured by pledges of substantially all of the Company’s assets.
On October 4, 2011, we entered into a Forbearance Agreement (the “Forbearance Agreement”) with the Collateral Agent for the Note Payable Holders. The Forbearance Agreement provided that the Collateral Agent shall forbear from exercising rights and remedies arising from the occurrence of Specified Defaults (as defined in the Forbearance Agreement) including our failure to repay the Notes Payable which are due and payable. Several extensions of the Forbearance Agreement were granted from December 31, 2011 through February 8, 2012; however, on February 8, 2012, the forbearance period under the Forbearance Agreement expired. As of February 21, 2012, the Company remains in default under the Notes Payable and the forbearance fee of $1.0 million is payable under the Forbearance Agreement and has not yet been paid by the Company. The Company is negotiating with the Collateral Agent regarding a further forbearance, however, there can be no assurance that any further forbearance will be granted.
The Company and the Collateral Agent continue to discuss the options available to the Company to repay the Notes Payable, among other strategic options, including retaining an investment banker as contemplated under the Forbearance Agreement to assist the Company in selling its proprietary nutraceutical business, to repay the Notes Payable under agreeable terms.
There can be no assurance that we will be able to repay, restructure or amend the Notes Payable prior to the receipt of an Acceleration Notice or the Convertible Note Payable prior to the receipt of a CD Acceleration Notice. In the interim, we have continued to make timely interest payments to the Note Payable Holders at the non default rate of 8% per annum and are exploring our strategic alternatives, which may include business divestitures, developing business and sales strategies to increase operating income, the sale of some or all of our assets or operating subsidiaries and/or capital restructuring plans.
As a result of the events of default that arose based upon our failure to pay each of the Notes Payable at maturity, the Note Payable Holders have the right to give us an Acceleration Notice. As a result of the event of default that arose based upon our failure to repay the Convertible Note Payable at maturity, CD Financial has the right to give us a CD Acceleration Notice. Each of the Acceleration Notice and the CD Acceleration Notice would (i) accelerate the payment of all unpaid principal, accrued and unpaid interest (including default interest (if any) on the Notes Payable and the Convertible Note Payable) and any other payment obligations (including the forbearance fee payable under the Forbearance Agreement) and (ii) require us to pay an amount equal to the sum of all of the respective amounts described in the preceding clause (i) in same day funds on the payment date specified in such notice. If we are unable to raise additional capital, sell certain assets or successfully refinance the full outstanding amount of the Notes Payable and the Convertible Note Payable upon acceptable terms, it would have a material adverse effect on us, including the possible foreclosure by the Note Payable Holders, and/or the Convertible Note Payable of all or some of our assets, and would impact our ability to continue as a going concern.
As of February 21, 2012, we have not repaid the holder of the Convertible Note Payable, CD Financial. CD Financial is a significant shareholder of the Company and has not made any payment demands on us with respect to the Convertible Note Payable, nor has it converted the Note Payable into common shares of the Company. In March 2009, we entered into an oral agreement with CD Financial to suspend the cash interest payments on the Convertible Note Payable until we return to positive cash flows in our operations. In this oral agreement, CD Financial agreed not to give any default notices and that default interest does not have to be accrued at the default interest rate.
20
Our total annual commitments at December 31, 2011 for long term non-cancelable leases of approximately $472,000 consists of obligations under operating leases for facilities and operating lease agreements for the rental of warehouse equipment, office equipment and automobiles, including $108,000 relating to our operations classified as discontinued.
Capital Expenditures
The Company's capital expenditures for the six months ended December 31, 2011 and 2010 were approximately $7,000 and $0.2 million, respectively. The Company has budgeted approximately $0.5 million for capital expenditures for fiscal 2012. The total amount is expected to be funded from cash provided from its operations and from lease financing.
Off-Balance Sheet Arrangements
The Company has no off-balance sheet arrangements.
Recent Accounting Pronouncement
None.
Impact of Inflation
The Company does not believe that inflation has significantly affected its results of operations.
Item 3. QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
Not applicable.
Item 4. CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Disclosure controls and procedures are controls and other procedures that are designed to ensure that information required to be disclosed by the Company in the reports it files or submits under the Securities Exchange Act of 1934, as amended (the “Exchange Act”) is recorded, processed, summarized, and reported within the time periods specified by the Commission’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to provide reasonable assurance that information required to be disclosed by the Company in the reports it files or submits under the Exchange Act is accumulated and communicated to management, including the Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Under the supervision and with the participation of management, including the Chief Executive Officer and Chief Financial Officer, the Company has evaluated the effectiveness of its disclosure controls and procedures (as such term is defined in Rule 13a-15(e) and 15d-15(e) under the Exchange Act) as of December 31, 2011, and, based upon this evaluation, the Chief Executive Officer and Chief Financial Officer have concluded that these controls and procedures are effective in providing reasonable assurance of compliance.
Changes in Internal Control over Financial Reporting
No change in our internal control over financial reporting occurred during the three and six months ended December 31, 2011 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
21
PART II – OTHER INFORMATION
Item 1. LEGAL PROCEEDINGS
None.
Item 1A. Risk Factors
The risks described in Item 1A, Risk Factors, in our Annual Report on Form 10-K for the year ended June 30, 2011, could materially and adversely affect our business, financial condition and results of operations. The risk factors discussed in that Form 10-K do not identify all risks that we face because our business operations could also be affected by additional factors that are not presently known to us or that we currently consider to be immaterial to our operations. There have been no material changes to our risk factors from those disclosed in our Form 10-K for the year ended June 30, 2011.
Item 2. UNREGISTERED SALES OF EQUITY SECURITIES AND USE OF PROCEEDS
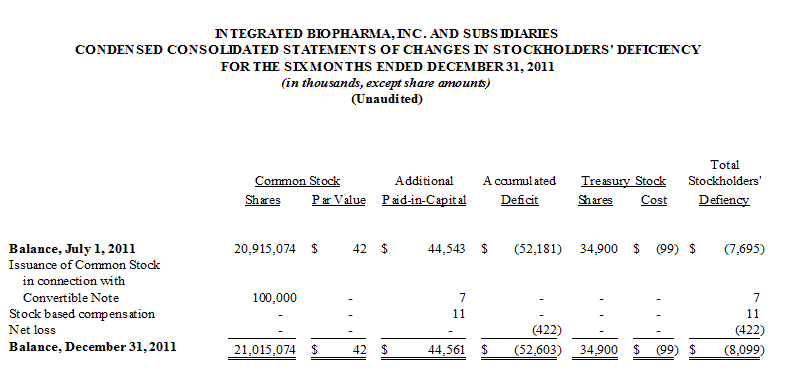
Effective November 21, 2011, the Company issued 50,000 shares of common stock to CD Financial. See Note 6 to the Condensed Consolidated Financial Statements – Debt.
Item 3. DEFAULTS UPON SENIOR SECURITIES
The Company is in default with respect to its $7.8 million outstanding principal amount of Notes Payable and its $4.5 million outstanding principal amount of Convertible Note Payable, as a result of the Company failing to repay the Notes Payable and Convertible Note Payable on their scheduled maturity dates. See Note 6 to the Condensed Consolidated Financial Statements – Debt.
Item 5. OTHER INFORMATION
None.
Item 6. EXHIBITS
(a) Exhibits
Exhibit
Number
|
31.1
|
Certification of pursuant to Section 302 of Section 302 of the Sarbanes-Oxley Act of 2002 by Chief Executive Officer.
|
|
31.2
|
Certification of pursuant to Section 302 of Section 302 of the Sarbanes-Oxley Act of 2002 by Chief Financial Officer.
|
|
32.1
|
Certification of periodic financial report pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 by Chief Executive Officer.
|
|
32.2
|
Certification of periodic financial report pursuant to Section 906 of the Sarbanes-Oxley Act of 2002 by Chief Financial Officer.
|
|
101
|
The following financial information from Integrated Biopharma, Inc.’s Quarterly Report on Form 10-Q for the quarter ended December 31, 2011, formatted in XBRL (eXtensible Business Reporting Language): (i) Condensed Consolidated Statements of Operations for the Three and Six Months ended December 31, 2011 and 2010, (ii) Condensed Consolidated Balance Sheets as of December 31, 2011 and June 30, 2011, (iii) Condensed Consolidated Statements of Changes in Stockholders’ Deficiency for the Six Months Ended December 31, 2011 (iv) Condensed Consolidated Statements of Cash Flows for the Six Months ended December 31, 2011 and 2010, and (v) the Notes to Condensed Consolidated Statements.
|
22
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Company has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
INTEGRATED BIOPHARMA, INC.
| Date: February 21, 2012 | By: /s/ E. Gerald Kay | |
| E. Gerald Kay, | ||
| President and Chief Executive Officer |
| Date: February 21, 2012 | By: /s/ Dina L. Masi | |
| Dina L. Masi, | ||
| Chief Financial Officer & Senior Vice President |
