Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a12-5232_18k.htm |
Exhibit 99.1
|
|
Rick E Winningham, CEO February 16, 2012 Theravance, Theravance’s logo and Medicines That Make a Difference are registered trademarks of Theravance, Inc. © 2012 Theravance, Inc. |
|
|
Safe Harbor This presentation contains certain “forward-looking” statements as that term is defined in the Private Securities Litigation Reform Act of 1995 regarding, among other things, statements relating to goals, plans, objectives and future events. Theravance intends such forward-looking statements to be covered by the safe harbor provisions for forward-looking statements contained in Section 21E of the Exchange Act and the Private Securities Litigation Reform Act of 1995. The words “may”, “will”, “should”, “could”, “would”, “plan”, “anticipate”, “believe”, “estimate”, “intend”, “goal,” “project”, “potential”, “expect”, “consistent”, “supportive”, “target” and “promising” and similar expressions are intended to identify such forward-looking statements. Examples of such statements include statements relating to the status and timing of clinical studies, data analysis and communication, statements regarding the potential benefits and mechanisms of action of drug candidates, statements concerning the timing of seeking regulatory approval of our product candidates, statements concerning enabling capabilities of Theravance's approach to drug discovery and its proprietary insights, statements concerning expectations for product candidates through development and commercialization and projections of revenue, expenses and other financial items. These statements are based on the current estimates and assumptions of the management of Theravance as of the date of this presentation and are subject to risks, uncertainties, changes in circumstances, assumptions and other factors that may cause the actual results of Theravance to be materially different from those reflected in its forward-looking statements. Important factors that could cause actual results to differ materially from those indicated by such forward-looking statements include, among others, risks related to delays or difficulties in commencing or completing clinical studies, risks related to the potential that results of clinical or non-clinical studies indicate product candidates are unsafe or ineffective, our dependence on third parties in the conduct of our clinical studies, delays or failure to achieve regulatory approvals for product candidates, risks of relying on third-party manufacturers for the supply of our product and product candidates and risks of collaborating with third parties to develop and commercialize products. These and other risks are described in greater detail under the heading "Risk Factors" contained in Theravance’s Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission (SEC) on November 2, 2011 and the risks discussed in our other period filings with SEC. Given these uncertainties, you should not place undue reliance on these forward-looking statements. Theravance assumes no obligation to update its forward-looking statements. 2 |
|
|
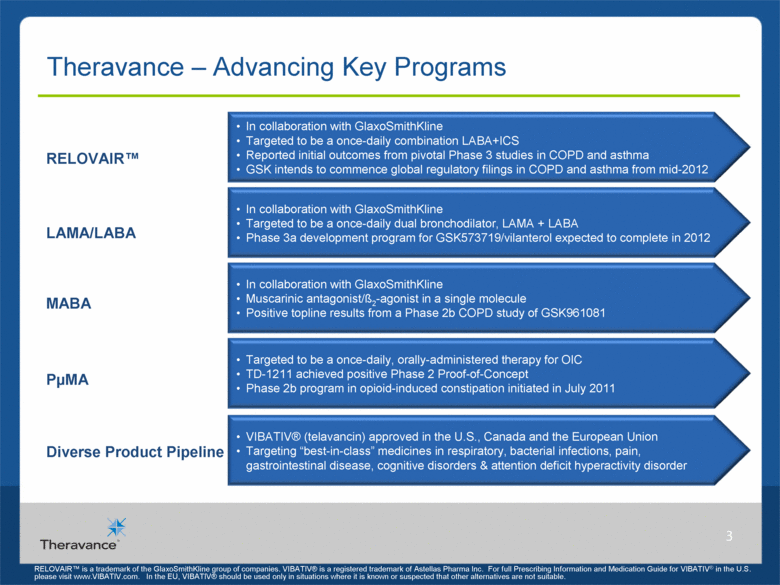
Theravance – Advancing Key Programs 3 PµMA MABA RELOVAIR™ In collaboration with GlaxoSmithKline Targeted to be a once-daily combination LABA+ICS Reported initial outcomes from pivotal Phase 3 studies in COPD and asthma GSK intends to commence global regulatory filings in COPD and asthma from mid-2012 Diverse Product Pipeline LAMA/LABA In collaboration with GlaxoSmithKline Muscarinic antagonist/ß2-agonist in a single molecule Positive topline results from a Phase 2b COPD study of GSK961081 In collaboration with GlaxoSmithKline Targeted to be a once-daily dual bronchodilator, LAMA + LABA Phase 3a development program for GSK573719/vilanterol expected to complete in 2012 Targeted to be a once-daily, orally-administered therapy for OIC TD-1211 achieved positive Phase 2 Proof-of-Concept Phase 2b program in opioid-induced constipation initiated in July 2011 VIBATIV® (telavancin) approved in the U.S., Canada and the European Union Targeting “best-in-class” medicines in respiratory, bacterial infections, pain, gastrointestinal disease, cognitive disorders & attention deficit hyperactivity disorder RELOVAIR™ is a trademark of the GlaxoSmithKline group of companies. VIBATIV® is a registered trademark of Astellas Pharma Inc. For full Prescribing Information and Medication Guide for VIBATIV® in the U.S. please visit www.VIBATIV.com. In the EU, VIBATIV® should be used only in situations where it is known or suspected that other alternatives are not suitable. |
|
|
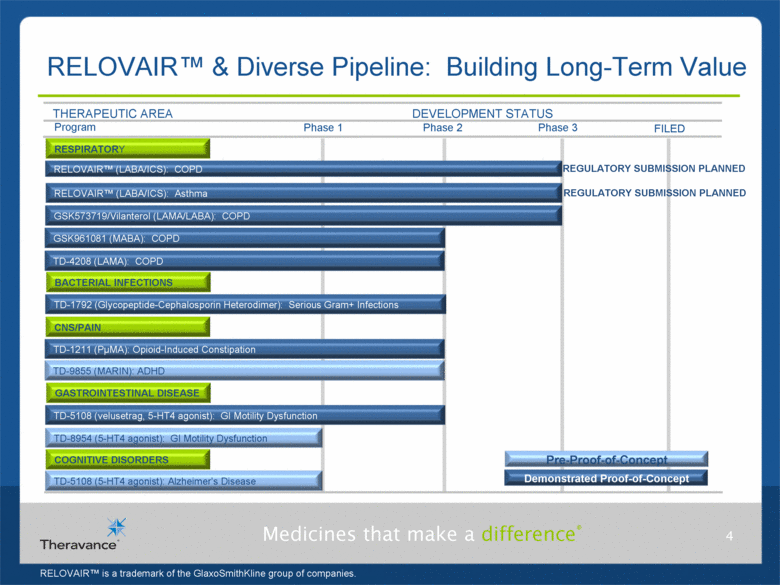
RELOVAIR™ & Diverse Pipeline: Building Long-Term Value 4 Phase 3 THERAPEUTIC AREA DEVELOPMENT STATUS Phase 1 Phase 2 FILED BACTERIAL INFECTIONS Demonstrated Proof-of-Concept TD-1792 (Glycopeptide-Cephalosporin Heterodimer): Serious Gram+ Infections RESPIRATORY GASTROINTESTINAL DISEASE TD-5108 (velusetrag, 5-HT4 agonist): GI Motility Dysfunction COGNITIVE DISORDERS TD-8954 (5-HT4 agonist): GI Motility Dysfunction TD-1211 (PµMA): Opioid-Induced Constipation GSK961081 (MABA): COPD RELOVAIR™ (LABA/ICS): Asthma RELOVAIR™ (LABA/ICS): COPD TD-5108 (5-HT4 agonist): Alzheimer’s Disease Pre-Proof-of-Concept CNS/PAIN TD-9855 (MARIN): ADHD Program GSK573719/Vilanterol (LAMA/LABA): COPD TD-4208 (LAMA): COPD RELOVAIR™ is a trademark of the GlaxoSmithKline group of companies. REGULATORY SUBMISSION PLANNED REGULATORY SUBMISSION PLANNED |
|
|
MABA (GSK961081) Program 5 Muscarinic antagonist and ß2-agonist in a single molecule Potential for monotherapy and triple mechanism in a single inhaler when combined with an inhaled corticosteroid (ICS) for the treatment of COPD Discovered through Theravance’s insights on secondary binding sites on both the ß2 and muscarinic receptors Theravance has no cost obligation on the program All doses of GSK961081 („081) achieved primary endpoint in Phase 2b study in COPD Evaluating „081 initially as BID fixed-dose combination with fluticasone propionate (FP) outside the US Efficient pathway to market In the US, further discussion with FDA is required QD/BID and Phase 3 timing dependent upon successful completion of Phase 3 enabling studies |
|
|
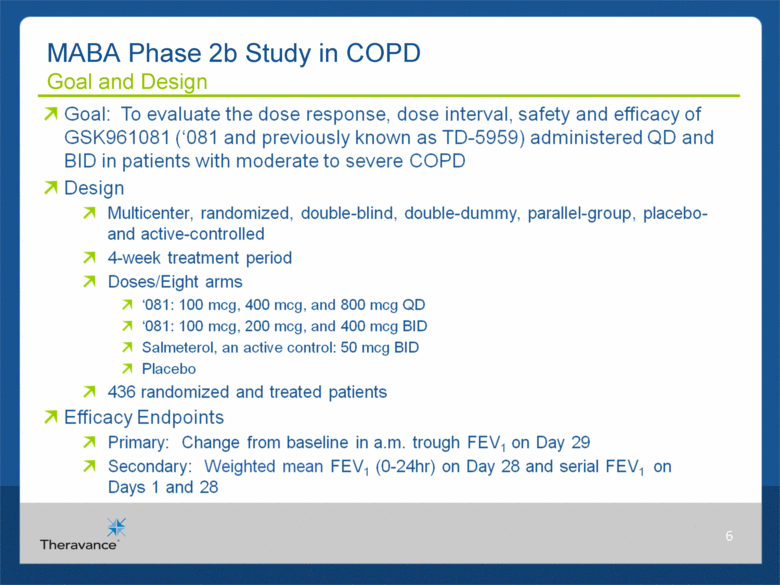
MABA Phase 2b Study in COPD Goal and Design 6 Goal: To evaluate the dose response, dose interval, safety and efficacy of GSK961081 („081 and previously known as TD-5959) administered QD and BID in patients with moderate to severe COPD Design Multicenter, randomized, double-blind, double-dummy, parallel-group, placeboand active-controlled 4-week treatment period Doses/Eight arms „081: 100 mcg, 400 mcg, and 800 mcg QD „081: 100 mcg, 200 mcg, and 400 mcg BID Salmeterol, an active control: 50 mcg BID Placebo 436 randomized and treated patients Efficacy Endpoints Primary: Change from baseline in a.m. trough FEV1 on Day 29 Secondary: Weighted mean FEV1 (0-24hr) on Day 28 and serial FEV1 on Days 1 and 28 |
|
|
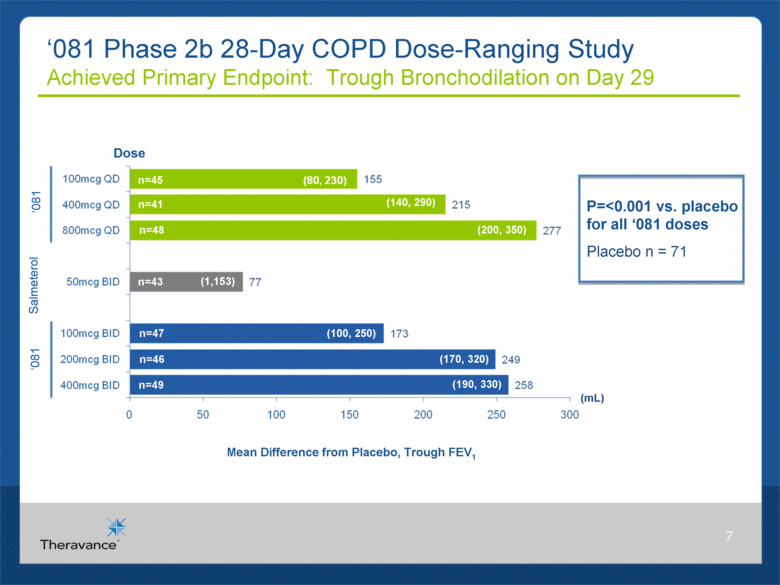
7 ‘081 Phase 2b 28-Day COPD Dose-Ranging Study Achieved Primary Endpoint: Trough Bronchodilation on Day 29 Mean Difference from Placebo, Trough FEV1 (mL) n=49 Dose n=45 n=47 n=41 n=46 n=48 n=43 (140, 290) (200, 350) (190, 330) (1,153) (100, 250) (170, 320) ‘081 ‘081 Salmeterol P=<0.001 vs. placebo for all ‘081 doses Placebo n = 71 (mL) |
|
|
MABA Phase 2b Positive Results in COPD Summary Efficacy ‘081 QD/BID achieved statistically significant difference from placebo in mean change from baseline in morning trough FEV1 on Day 29 Weighted mean FEV1 (0-24hr) on Day 28 and serial FEV1 on Day 1 and Day 28, demonstrated statistically significant differences vs. placebo for all doses of ‘081 at all time points except one (100mcg QD at 12h on Day 1) All ‘081 doses produced numerically greater improvements in bronchodilation compared to salmeterol* Safety ‘081 was generally well-tolerated Incidences of adverse events (AEs) and drug-related AEs were similar across treatments with no apparent dose-related effect Most frequently reported AEs were headache, cough, and dysgeusia One serious AE was reported (biliary colic) in the 400mcg QD ‘081 treatment group, but was not considered related to study drug *No prespecified comparison versus ‘081 was conducted in this study 8 |
|
|
THANK YOU Theravance, Theravance’s logo and Medicines That Make a Difference are registered trademarks of Theravance, Inc. © 2012 Theravance, Inc. |