Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - BIOLASE, INC | d301871d8k.htm |
 BIOLASE Technology,
Inc. Federico Pignatelli
Chairman, CEO & President
NASDAQ: BLTI
www.biolase.com
Orlando
|
February 9 –
12, 2012 •
Gaylord Palms Hotel & Convention Center
Exhibit 99.1 |
 Safe
Harbor Statement This presentation may contain forward-looking statements
that are based on our current expectations, estimates and projections about our
industry as well as management’s beliefs and assumptions. Words such as “anticipates,”
“expects,”
“intends,”
“plans,”
“believes,”
“seeks,”
“estimates,”
“may,”
“will,”
and variations of these words or similar
expressions are intended to identify forward-looking statements. These statements
include projections about our future earnings and margins and speak only as of
the date hereof. Such statements are based upon the information available to us
now and are subject to change. We will not necessarily inform you of such changes. These statements
are not guarantees of future performance and are subject to certain risks,
uncertainties and assumptions that are difficult to predict. Therefore our
actual results could differ materially and adversely from those expressed in any
forward-looking statements as a result of various factors. The important
factors which could cause actual results to
differ materially from those in the forward-looking statements include, among
others, a downturn or leveling off of
demand for our products due to the availability and pricing of competing products and
technologies, adverse international market or political conditions, a domestic
economic recession, the volume and pricing of product sales, our ability to
control costs, intellectual property disputes, the effects of natural disasters and other events beyond our
control and other factors including those detailed in BIOLASE’s filings with the
Securities and Exchange Commission including its prior filings on Form 10-K
and 10-Q. PAGE
2 |
 BIOLASE is Revolutionizing Surgery in Medicine
•
The Er,Cr:YSGG (2780nm) laser crystal has the highest level of absorption in water in
human tissue. •
This absorption creates an expansion and vaporization of the water molecules causing a
biological ablation of human tissue with very little trauma or bleeding.
•
Tooth enamel ~5% water, dentin and bone ~25% water, soft tissue ~80% water, and the
eye ~90% water. How? Through the science of WaterLase! PAGE 3
Conventional Surgical Devices include the scalpel, high speed drill,
electrosurge, electric bone saws, and heat generating lasers.
…BIOLASE is NOT Just Another Medical Device Company!
|
 BIOLASE
is
the
World
Leader
in
Laser
Dentistry…
…and the Dental Community is the Largest Medical Community in the
World!
PAGE 4
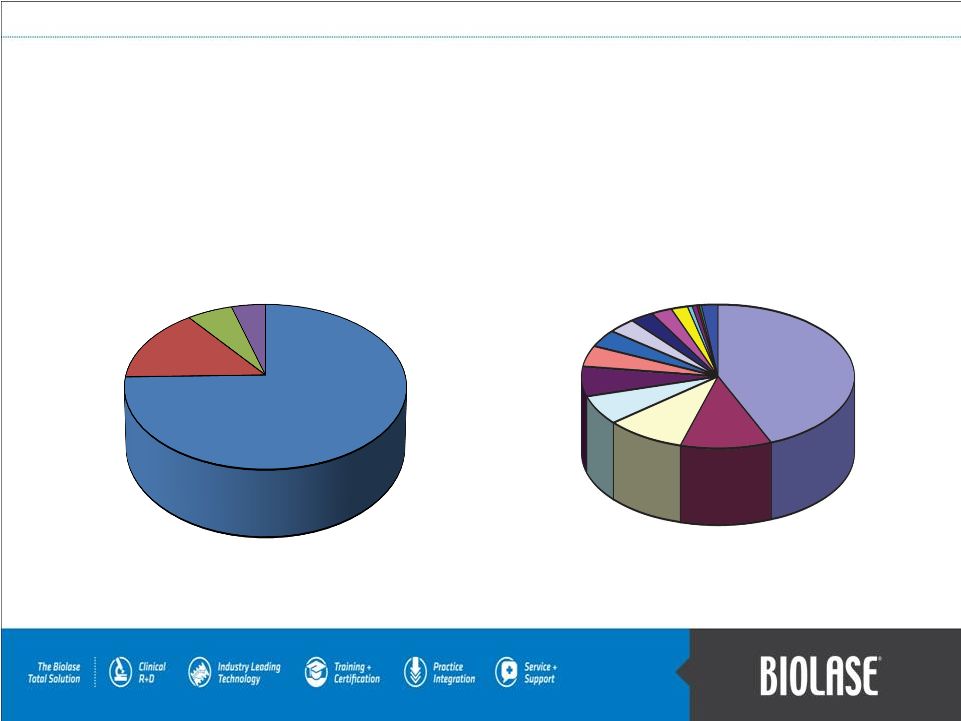
BIOLASE 45%
BIOLASE 45%
Source: iData Research Inc.
2009 US Overall Dental Laser Market: Hard
& Soft Tissue
PAGE 4
BIOLASE 80%
BIOLASE 80%
Source: Biolase Technology Licensing Reports
2009 US Dental Laser Market:
Hard Tissue |
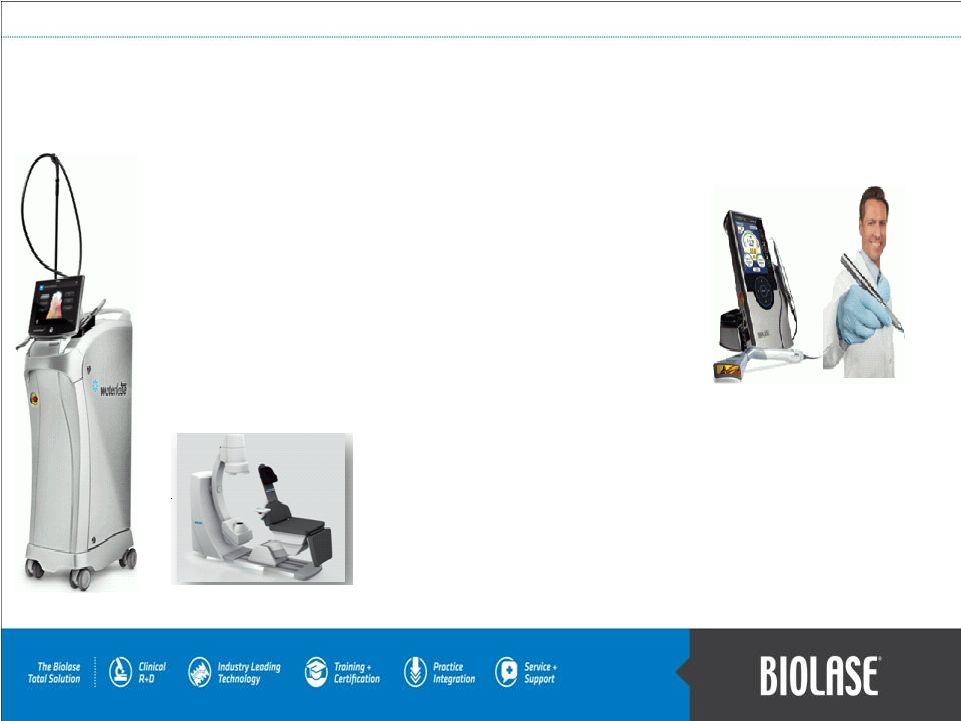 Medical:
The
Diolase
10
offers
high
intensity
laser
therapy
in
the palm of your hand for pain management.
Digital Imaging:
equipment, including cone beam 3-D dental imaging and
CAD/CAM digital dental impressions.
Diode
soft-tissue
dental
lasers:
Our
soft
tissue
lasers
include the ezLase total diode solution, which offers power
and portability, and the revolutionary iLase, a portable
diode laser with no foot pedal, power cord, or external
controls.
Current Line of BIOLASE Products
PAGE 5
All-tissue dental lasers:
revolutionary WaterLase iPlus, and the WaterLase MD Turbo.
Our
all-tissue
lasers
include
our
new
flagship
laser,
the
We
offer
a
full
line
of
digital
imaging |
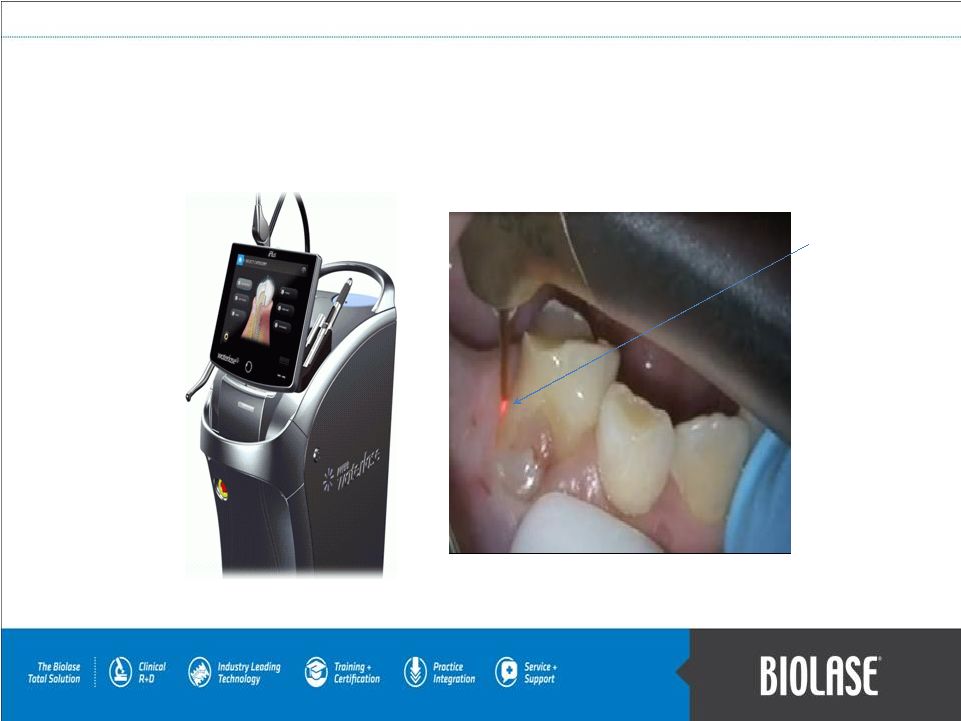 Alternative and Advanced Hi-Tech
Dentistry Atraumatic Pediatric Tooth Extraction
The red or green
aiming beam on the
WaterLase does not
cut the tissue or
generate any heat.
The tissue is cut
biologically, with little
or no pain, by
energizing water at
the molecular level.
Using BIOLASE WaterLase Systems, including our new WaterLase iPlus System:
PAGE 6
The iPlus features an
intuitive, touch-based
computer screen,
similar to a tablet
personal computer.
It is designed to
allow for remote
servicing and
software upgrades. |
 WaterLase High Speed Cutting
PAGE 7 |
 In 2011:
Single Product Company 1.
Moved from exclusive distribution with
Schein to direct sales and multi-distributor
model.
2.
Launched iPlus which EQUALS the speed of
a traditional dental drill.
3.
Globalized distribution with approvals in
Canada, Korea, Russia, Taiwan, & others.
4.
Launched digital imaging products.
5.
Growing revenues over 100%.
6.
Continuing to expand global sales footprint.
In 2012: Broaden Platform and Product Portfolio
Becoming a Globally Diversified Platform Company
1.
Diversify revenue base to expand
addressable markets and reduce investor
risk.
2.
Launch 2 to 3 new dental products in
2Q12, including lasers and imaging.
3.
Enter or begin clinical development &
approval in: ophthalmology, orthopedics,
pain management, podiatry, and aesthetics.
4.
Re-launch revised iLase with new
distribution partners.
5.
Reset investor expectations…
,,,under promise, over deliver.
PAGE 8 |
 Why BIOLASE WaterLase
Technology in Dentistry? Traditional high-speed drills
work
by
FRICTION
which
creates
HEAT
which
causes
PAIN.
This
PAIN
necessitates
injections
of
ANESTHESIA
which
results
in
PAIN
and
NUMB
LIPS.
In addition, the lack of feeling from the numbing injections can
lead to THERMAL DAMAGE
which can lead to PULPAL DEATH and result in a ROOT CANAL.
The traditional high-speed drill
also causes VIBRATION,
which
causes
PAIN
and leads to
MICROFRACTURES. These MICROFRACTURES allow BACTERIA to penetrate the tooth
which causes further DECAY and the FRACTURING of teeth.
High speed drills
also
require
the
use
of
BURS
which
even
after
autoclaving
have a 15% chance of carrying pathogenic micro-organisms. Needles
and files
can
also
carry
bacteria.
That
means
in
traditional
dentistry
the
patient
has
a
1
in
6
chance
of
cross
contamination.
WaterLase energy
does not create heat or vibration so there is NO PAIN. Further, the
WaterLase is bactericidal, antiviral and essentially
eliminates
the
risk
of
cross
contamination.
PAGE 9 |
 Why Dentists Should
Buy an iPlus System? •
WaterLase technology enables traditional dentists to perform procedures that they
currently refer out to specialists or current WaterLase dentists, for
example: •
Gingivectomy = $160
•
Perio Treatment = $375
•
Hard-tissue Crown Lengthening = $520
•
Herpetic or Aphthous Ulcer = $308
•
Frenectomy = $355
•
With no anesthesia, the WaterLase also increases efficiency and allows the dentist to
work in multiple quadrants in a single visit.
•
This equates to $250-$750 per day in additional revenue. These procedures
are easy to learn and training is included in the cost..
Monthly Lease Payment
New Monthly Revenue
Generated
Approx. $1,000
$5,000 –
$15,000
PAGE 10
A dentists’
return on investment can
be between 500% and 1,500%!
…WaterLase’s Tremendous Return on Investment! |
 Current
Status: BIOLASE
has
a
tremendous
IP
portfolio
with
284
issued
and
pending
patents,
70%
of
which
are related to our core WaterLase (Er,Cr:YSGG) technology and medical lasers.
Filing
costs
and
maintenance
are
closely
monitored.
Funding
for
redundant
patents
and
patents
with
a
low-
probability for issuance are stopped.
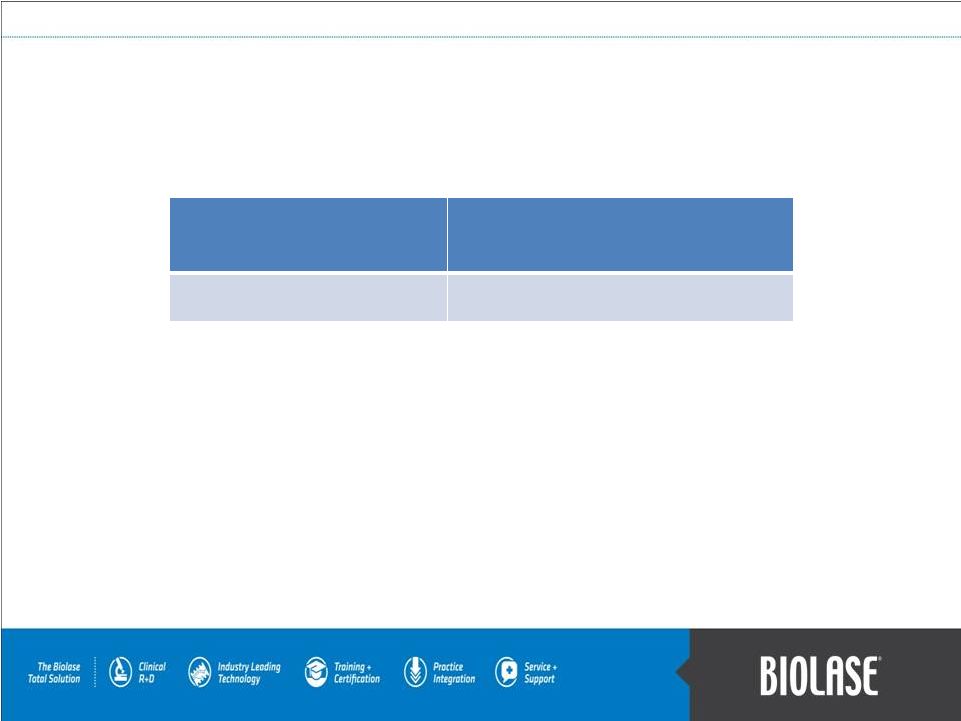
Patent Portfolio
Issued
Pending
Total
U.S.
84
53
137
International
76
71
147
Total
160
124
284
PAGE 11 |
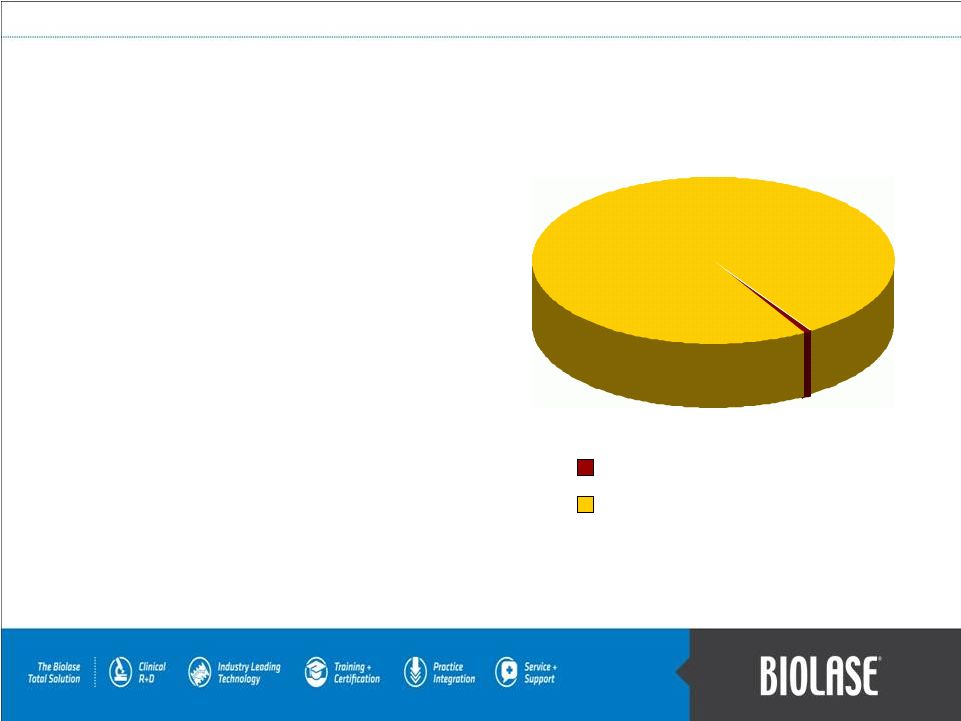 $50B to
$100B WaterLase Market Opportunity The WaterLase’s global market opportunity is
immense… because the dental community is the largest medical
community in the world.
•
176,000 dentists in the US and Canada.
1
•
Over 1.2 million dentists in 134 countries and growing
rapidly in emerging economies.
2
•
18,000 systems in over 11,000 practices.
•
Every 1% of further market penetration, just with the
WaterLase, is equal to an opportunity of well over $600
million in revenues!
1. American Dental Association. 2. World Federation of Dentistry. 3.
1998-present over 17,300 BIOLASE systems sold. PAGE 12
Our current market penetration is approximately
3% in the US and slightly less than 1% internationally!
Estimated total global market of 1,200,000,
which is growing rapidly due to new markets
such as China, India, and Indonesia.
18,000
3
systems sold worldwide. |
 1. Dental
Health Magazine 2. Dental Burs and Endodontic Files: Are Routine Sterilization Procedures Effective?: Archie
Morrison, DDS, MS, FRCD(C); Susan Conrod, DDS JCDA •
www.cda-adc.ca/jcda •
February 2009, Vol. 75, No. 1. 3.
Contaminated dental instruments: Smith A, Dickson M, Aitken J, Bagg J.J Hosp Infect. 2002
Jul;51(3):233-5. 4. The antimicrobial efficacy of the erbium,
chromium:yttrium-scandium-gallium-garnet laser with radial emitting tips on root canal
dentin walls infected with Enterococcus faecalis: Wanda Gordon, DMD, Vahid A. Atabakhsh, DDS,
Fernando Meza, DMD, Aaron Doms, DDS, Roni Nissan, DMD, Ioana Rizoiu, MS and Roy H.
Stevens, DDS, MS JADA 2007; 138(7): 992-1002. 5. The Bacterial
Challenge: Time to React. A call to narrow the gap between multi-drug resistant
bacteria in the EU and the development of new antibacterial agents., ECDC/EMEA Joint
Working Group, Stockholm, Sweden, September, 2009. Warning:
Patients Urged to Ask their Dentists
About the Risk of Cross Contamination!
Cross contamination is a huge threat:
1,2,3,4
•
The CDC defines cross-contamination as the act
of spreading bacteria and viruses from one surface
to another. Blood-borne viruses have the ability to
live on objects and surfaces for as long as a week.
•
Sterilization techniques used for dental burs used
with
high
speed
drill
and
endodontic
files
are
not
effective. This is a serious health threat!
•
Many bacteria are becoming more and more
resistant to antibiotics.
5
PAGE 13 |
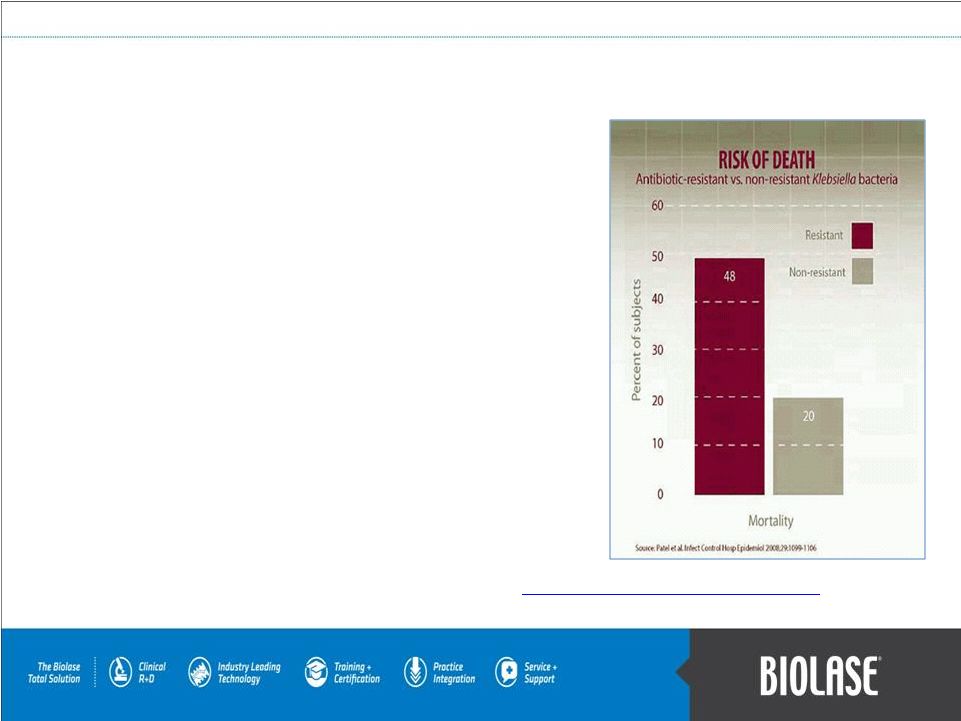 The
Bacterial Challenge Antibiotic resistance adversely impacts the health of
millions of patients every year. Of the patients receiving antibiotics, 50% receive unnecessary
or
redundant
therapy,
resulting
in
overuse
of
antibiotics.
Resistance to antibiotics is high among bacteria that cause serious
infections in humans and reaches 25% or more in several
EU Member States.
Each
year,
about
99,000
patients
in
the
US
die
from
hospital
acquired
infections
and 25,000 patients in the EU
die from an infection related
to multidrug-resistant bacteria.
Infections due to these multidrug-resistant bacteria result in extra
healthcare costs and productivity losses of at least EUR 1.5 billion in
the EU each year.
Mortality rates of one resistant strain MRSA in the U.S. exceeded
the combined
death
toll
of
AIDS,
tuberculosis,
and
hepatitis
B.
1. Centers for Disease Control and Prevention. Delivering safe care for patients: all
healthcare providers play a role., November 15, 2011. Source: http://www.cdc.gov/getsmart/healthcare/learn-from-others/factsheets/hc_providers.html. 2. The Bacterial Challenge:
Time to React. A call to narrow the gap between multi-drug resistant bacteria in the
EU and the development of new antibacterial agents., ECDC/EMEA Joint Working Group, Stockholm, Sweden, September, 2009. 3. Klevens RM, Edwards JR, Richards CL, Horan TC.
Estimating Health-Care Infections and Deaths in US Hospitals, 2002. Public Helth
Reports 2007; 122: 160-166. 1
2
2
2
1
3 |
 WaterLase Tips:
•
Smooth tip surface does not harbor debris or bacteria like
abrasive surface of burs or files.
•
YSGG laser energy is bacteriacidal.
•
Single-use, disposable tips that work without the need to
contact tissue. Also eliminates accidental sticks with
contaminated burs.
WaterLase MD™
reduced E. faecalis 2.86
times more effectively than NaOCl³
Burs and Endo Files:
•
15% of “sterilized”
burs and up to 76% of “sterilized”
endodontic files carry pathogenic micro-organisms.
•
Complex and rugged bur surface difficult to sterilize.
•
Autoclaving fails 15% of the time to decontaminate burs¹ •Only 32% of endodontic engine-driven files are replaced after
every
case
and
36%
after
every
other
case.
4
BIOLASE is entering a new dental category utilizing WaterLase technology as it
essentially eliminates the risk of cross contamination and contagion:
PAGE 15
Eliminating the Risk of Cross Contamination
1, 2
1. Dental Burs and Endodontic Files: Are Routine Sterilization Procedures Effective?: Archie
Morrison, DDS, MS, FRCD(C); Susan Conrod, DDS JCDA • www.cda-adc.ca/jcda • February 2009, Vol. 75, No. 1. 2. Contaminated dental
instruments: Smith A, Dickson M, Aitken J, Bagg J.J Hosp Infect. 2002
Jul;51(3):233-5. 3. The antimicrobial efficacy of the erbium, chromium:yttrium-scandium-gallium-garnet laser with radial emitting tips on root canal dentin
walls infected with Enterococcus faecalis: Wanda Gordon, DMD, Vahid A. Atabakhsh, DDS, Fernando
Meza, DMD, Aaron Doms, DDS, Roni Nissan, DMD, Ioana Rizoiu, MS and Roy H. Stevens, DDS, MS JADA 2007; 138(7): 992-1002
4. Dentaltown Research: Endodontics; Survey, October 2011, dentaltown.com.
3 |
 Patented
laser toothbrush for home use: •
BIOLASE is currently developing a laser toothbrush for consumer home-use in
connection with a license agreement with The Proctor and Gamble Company
(“P&G”). •
Research indicates that a laser toothbrush for home-use will whiten teeth,
disinfect teeth and gums, bio- stimulate
teeth
and
gums,
relieve
pain,
and
perform
photodynamic
therapy.
New lasers:
•
BIOLASE expects to offer additional all-tissue WaterLase products in 2012 that
complement the iPlus. •
Planning to re-launch its handheld diode laser with several new options and
applications. •
Currently engineering more powerful diode soft-tissue lasers for launch in 2012.
New Imaging products:
•
BIOLASE expects to offer additional imaging products in 2012 that complement its
current products. Further Opportunities to Expand in Dentistry
PAGE 16 |
 •
iPlus sales will continue grow rapidly as new products ramp enhancing revenue
visibility. •
Disposables and consumables will continue to represent ~20% of total sales.
•
New products will expand BIOLASE’s addressable markets 2 to 3 times.
•
BIOLASE
will
re-launch
its
handheld
diode
laser
with
new
distributor
partners
in
early
2012.
2011 (Projected)
2012 (Projected)
WaterLase Systems
~60%
~50%
Diode Systems
~19%
~15%
Consumables &
Other
~20%
~20%
New
~1%
~15%
BIOLASE will diversify in 2012 and expand product sales beyond dentistry.
Diversification in 2012
PAGE 17 |
 With our
patented WaterLase and diode technologies, we have created technological platforms
that have the ability to extend far outside of dentistry. We expect to greatly
expand our addressable markets in the coming years which, we believe, has the
potential to substantially increase our revenues. Each of these
potential markets represents a multi-billion dollar opportunity.
Ophthalmology:
We
currently
hold
14
issued
and
19
pending
U.S.
and
International
patents
in
four
patent families in the field of ophthalmology, giving us a wide range of applications
and coverage. Our patented
technology
has
the
capability
to
restore
the
elasticity
of
the
eye
and
allow
it
to
return
to
normal
function, eliminating presbyopia. Management has established a new subsidiary,
OCULASE, which will own and develop BIOLASE’s ophthalmic assets and
technologies. In 2012, we expect approval to market
treatments
for
glaucoma
and
dry
eyes.
We
expect
approval
to
market
WaterLase
technology
for
presbyopia internationally in 2013 and in the United States in 2014.
2012: Expanding Beyond Dentistry
PAGE 18 |
 Aesthetics:
We
have
various
FDA
approvals
for
applications
in
dermatology,
plastic
surgery,
and oculoplastics and are currently investigating options for entering these markets in
2012. Pain
Management:
We
anticipate
launching
a
new
deep-tissue
hand
piece
and
upgraded
laser
for
pain
therapy in 2012 which will coincide with a new marketing campaign.
Podiatry:
We
have
found
that
our
Diolase
10
technology
is
very
effective
in
the
treatment
of
nail
fungus
and we are completing the clinical and regulatory requirements necessary to enter the
market in 2012. Orthopedics:
We
are
working
with
several
key
manufacturers
and
universities
to
provide
solutions
that
are
not
currently
available.
We
are
investigating
opportunities
for
several
orthopedic
applications
and
anticipate filing several 510(k) applications over the next 12 months.
PAGE 19
2012:
Expanding
Beyond
Dentistry
(Continued) |
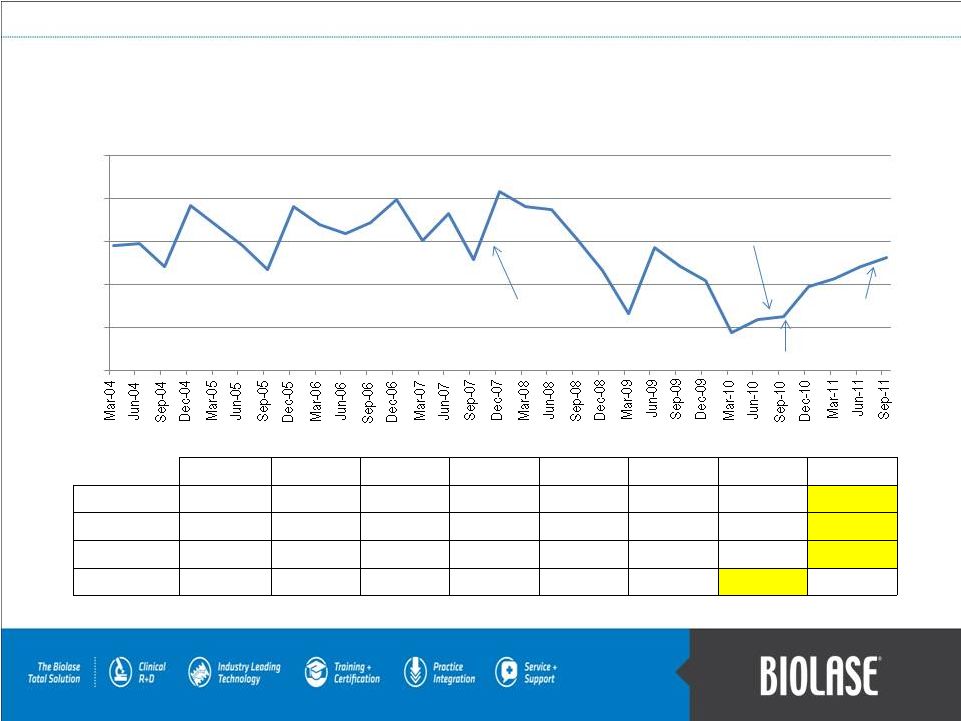 Quarterly Revenues (in Millions)
PAGE 20
Federico Pignatelli restored
as Chairman & CEO in Aug 2010
Record quarter with
Federico Pignatelli interim
CEO during Q407
2004
2005
2006
2007
2008
2009
2010
2011
Q1-Mar
$ 14.5
$ 16.8
$ 16.9
$ 15.1
$ 19.0
$ 6.6
$ 4.4
$ 10.6
Q2-Jun
$ 14.7
$ 14.5
$ 15.9
$ 18.2
$ 18.7
$ 14.3
$ 5.9
$ 12.1
Q3-Sep
$ 12.0
$ 11.7
$ 17.1
$ 12.8
$ 15.3
$ 12.1
$ 6.2
$ 13.1
Q4-Dec
$ 19.1
$ 19.0
$ 19.8
$ 20.8
$ 11.6
$ 10.4
$ 9.7
$-
$5.0
$10.0
$15.0
$20.0
$25.0
Direct sales ramp begins in Sep 2010
Over 100% revenue
growth to date in 2011
compared to 2010 |
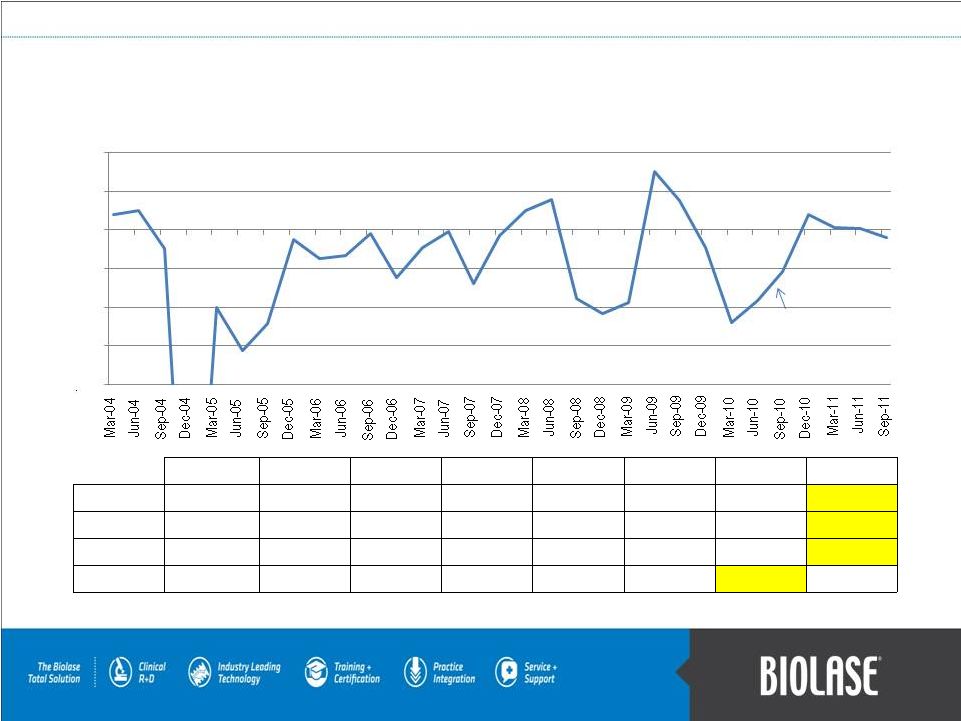 Quarterly Non-GAAP Net Income (in Millions)
2004
2005
2006
2007
2008
2009
2010
2011
Q1-Mar
$ 0.783
$ (4.015)
$ (1.462)
$ (0.890)
$ 0.985
$ (3.763)
$ (4.797)
$ 0.153
Q2-Jun
$ 1.006
$ (6.222)
$ (1.340)
$ (0.089)
$ 1.578
$ 3.020
$ (3.657)
$ 0.069
Q3-Sep
$ (0.962)
$ (4.820)
$ (0.167)
$ (2.765)
$ (3.551)
$ 1.534
$ (2.173)
$ (0.397)
Q4-Dec
$(23.257)
$ (0.479)
$ (2.466)
$ (0.272)
$ (4.338)
$ (0.888)
$ 0.787
Adjustments from net income (loss) include: depreciation and amortization,
non-cash stock-based compensation expense, and interest expense. PAGE 21
$(8.0)
$(6.0)
$(4.0)
$(2.0)
$-
$2.0
$4.0
Federico Pignatelli restored
as Chairman & CEO in Aug 2010 |
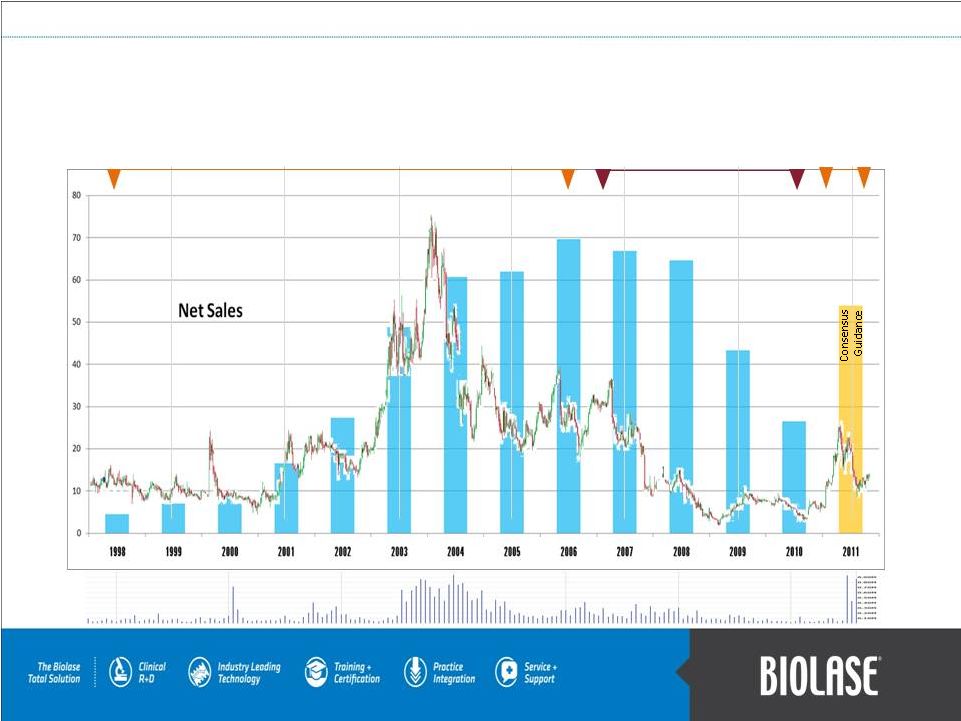 BLTI
Revenues
Chart & Financial History
1994-2006 Pignatelli
Chairman of the Board
Exclusive distribution partnership with
Henry Schein Aug 2006–Aug 2010
Aug 2010-Present
Pignatelli CEO &
Board Chairman
1998 FDA
Clearance
WaterLase
PAGE 22
Volume: Millions of Shares
20
15
10
5
BLTI
Share
Price |
 BIOLASE Technology,
Inc. Today Company Headquarters in Irvine, California
PAGE 23
•
We have over 180 employees worldwide.
•
We also have manufacturing capabilities in Floss, Germany.
•
We have sales offices in the U.S., Floss (Germany), Madrid
(Spain), Shanghai (China), and Mumbai (India), with expansion
planned in Dubai (UAE), and Rio de Janero (Brazil). We also
have training facilities in Charlotte, NC, and Irvine, CA.
BIOLASE Europe in Floss, Germany
•
Our 57,000 sq. ft. corporate HQ in Irvine houses finance &
administrative, sales, marketing, customer care, training,
manufacturing, and R&D. This facility can accommodate
growth to $250 million.
•
ISO 9001 certified and FDA GMP with clean room operations.
|
