Attached files
| file | filename |
|---|---|
| 8-K - SUCAMPO PHARMACEUTICALS, INC. 8-K - Sucampo Pharmaceuticals, Inc. | a50165708.htm |
Exhibit 99.1

14th Annual 14 BIO CEO & Investor Conference Stanley G. Miele Senior Vice President, Sucampo Pharmaceuticals, Inc. President, Sucampo Pharma Americas, Inc. February 13, 2012

Forward-looking statements contained in this presentation are based on Sucampo’s assumptions and events expectations concerning future events. They are subject to significant business, economic and competitive risks and uncertainties that could cause actual results to differ materially from those reflected in the forward-looking statements. Sucampo’s forward-looking statements could be affected by numerous foreseeable and unforeseeable events and developments such as regulatory delays, the failure of clinical trials, the inability to fund drug development initiatives, competitive products and other factors identified in the “Risk Factors” section of Sucampo’s Annual Report on Form 10-K and other periodic reports filed with the Securities and Exchange Commission. While Sucampo may elect to update these statements at some point in the future Sucampo specifically disclaims any obligation to do so, whether as a result of new information, future events or otherwise. In light of the significant uncertainties inherent in the forward-looking information in this presentation, you are cautioned not to place undue reliance on these forward-looking statements. 2
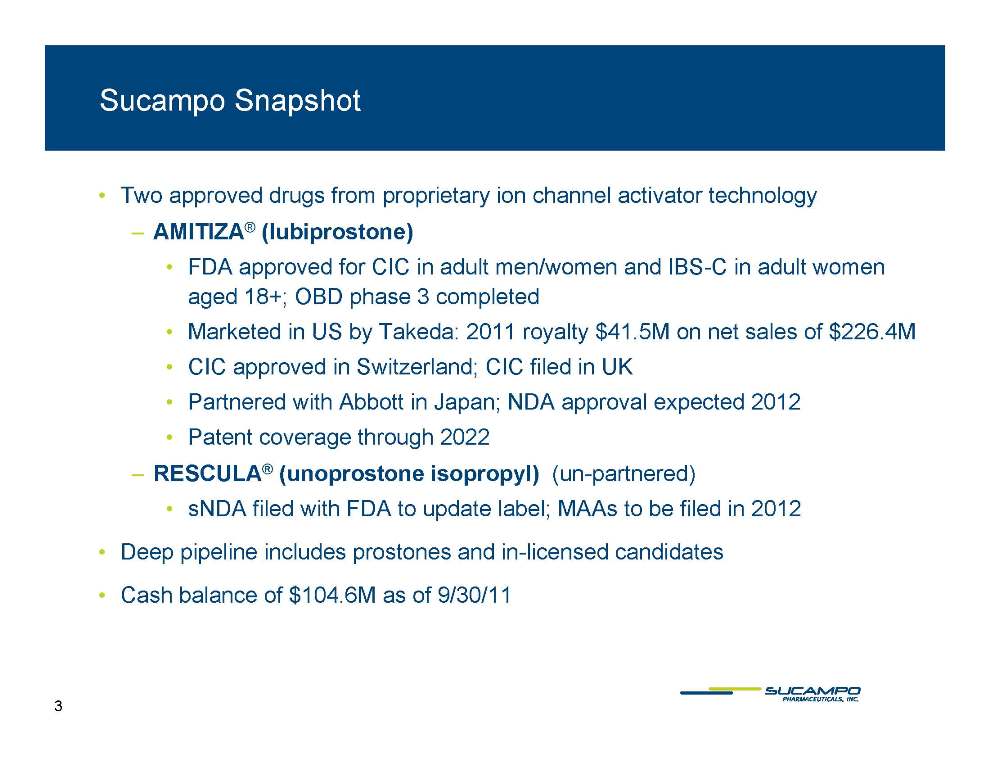
Sucampo Snapshot • Two approved drugs from proprietary ion channel activator technology – AMITIZA ® (lubiprostone) • FDA approved for CIC in adult men/women and IBS-C in adult women aged 18+; OBD phase 3 completed • Marketed in Takeda: royalty 41 5M 226 4M US by 2011 $41.5M on net sales of $226.4M • CIC approved in Switzerland; CIC filed in UK • Partnered with Abbott in Japan; NDA approval expected 2012 2022 • Patent coverage through – RESCULA® (unoprostone isopropyl) (un-partnered) • sNDA filed with FDA to update label; MAAs to be filed in 2012 • Deep pipeline includes prostones and in-licensed candidates • Cash balance of $104.6M as of 9/30/11 3 The 15-PGDH Converts Naturally-Occurring
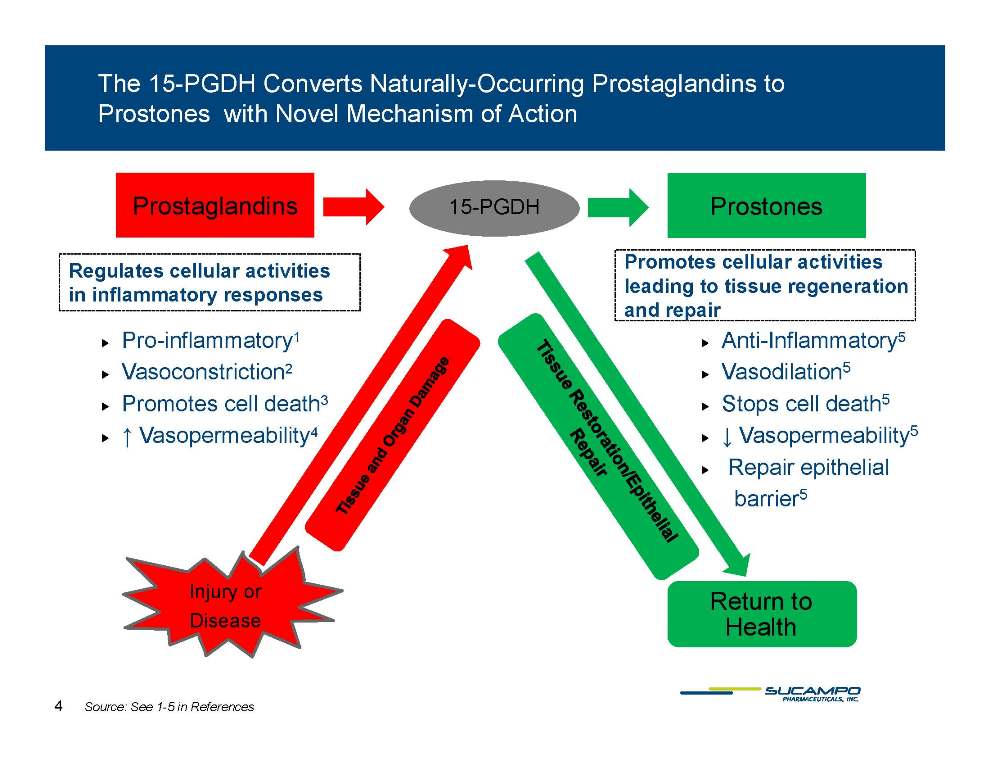
The 15-PGDH Converts Naturally-Occurring Prostaglandins to Prostones with Novel Mechanism of Action 15-PGDH Prostones Prostaglandins Promotes cellular activities leading to tissue regeneration and repair Regulates cellular activities in inflammatory responses �� Pro-inflammatory1 �� Vasoconstriction2 �� Promotes cell death3 ↑ Vasopermeabilit 4 �� Anti-Inflammatory5 �� Vasodilation5 �� Stops cell death5 ↓ Vasopermeabilit 5 �� Vasopermeability4 �� Vasopermeability5 �� Repair epithelial barrier5 Injury or Disease Return to Health Source: See 1-5 in References 4 Tissue and Organ Damage Tissue Restoration/Epithelial Repair 15-PGDH
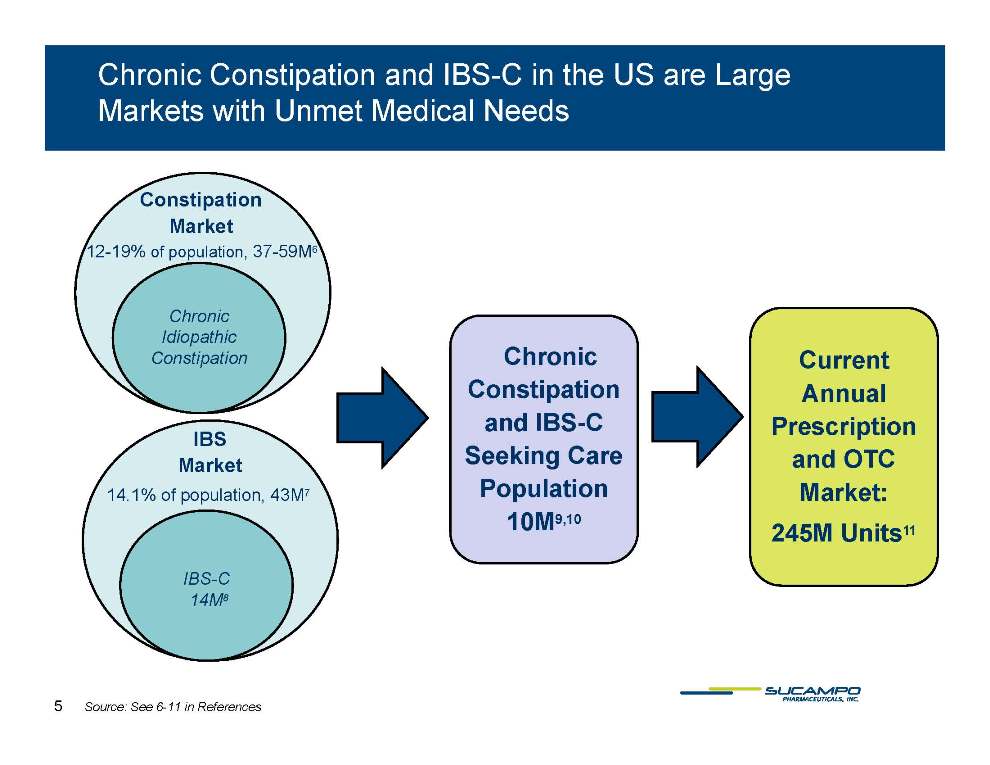
Chronic Constipation and IBS-C in the US are Large Markets with Unmet Medical Needs Constipation Market 12-19% of population, 37-59M6 Chronic Idiopathic Chronic Constipation and IBS-C Current Annual Prescription Constipation IBS Market Seeking Care Population 10M9,10 and OTC Market: 245M 11 14.1% of population, 43M7 10M Units IBS-C 14M8 5 Source: See 6-11 in References
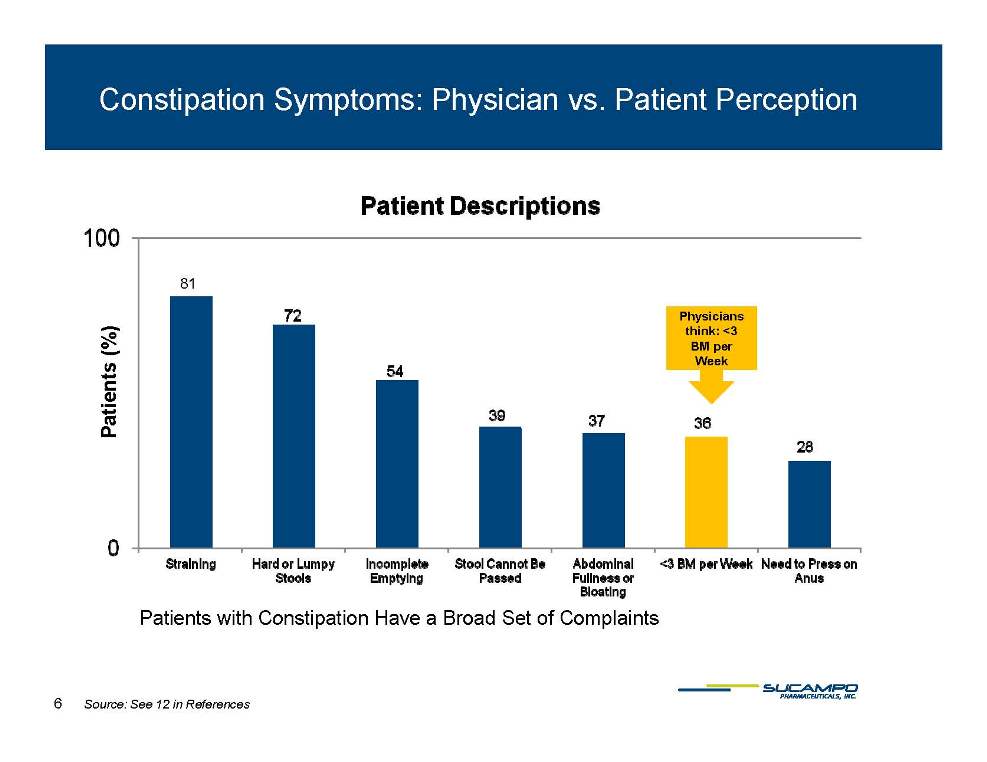
Constipation Symptoms: Physician vs. Patient Perception Physicians think: <3 81 %) BM per Week Patients (% P Patients with Constipation Complaints Have a Broad Set of 6 Source: See 12 in References
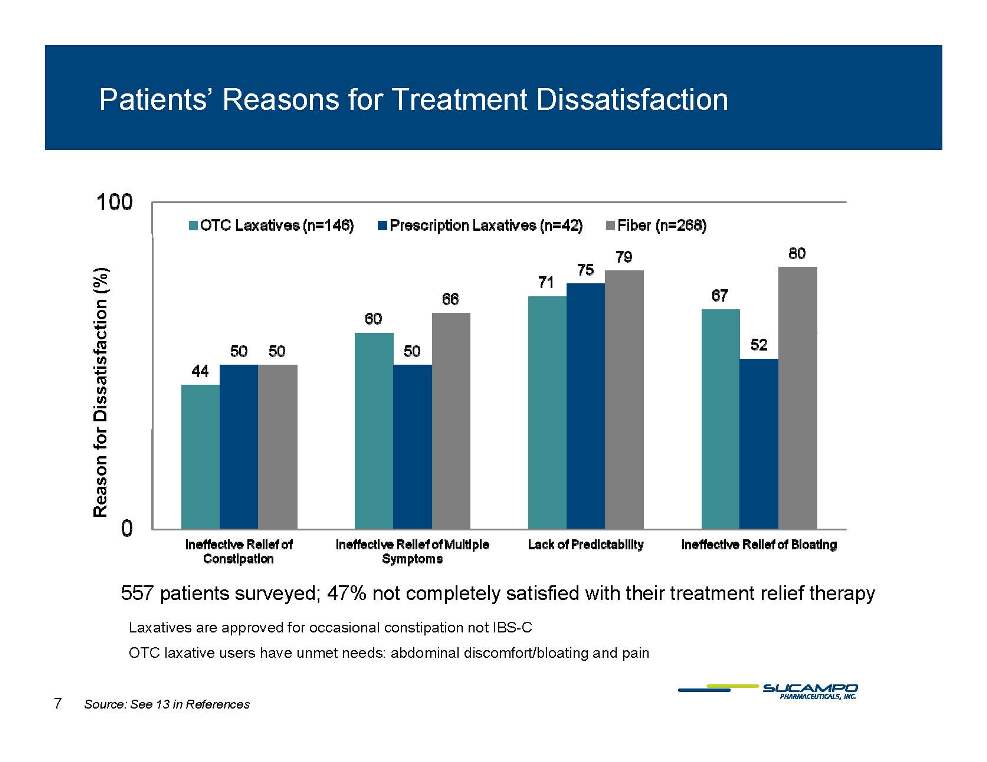
Patients’ Reasons for Treatment Dissatisfaction ction (%) Dissatisfac Reason for 557 patients surveyed; 47% not completely satisfied with their treatment relief therapy f SC Laxatives are approved for occasional constipation not IBS-C OTC laxative users have unmet needs: abdominal discomfort/bloating and pain 7 Source: See 13 in References
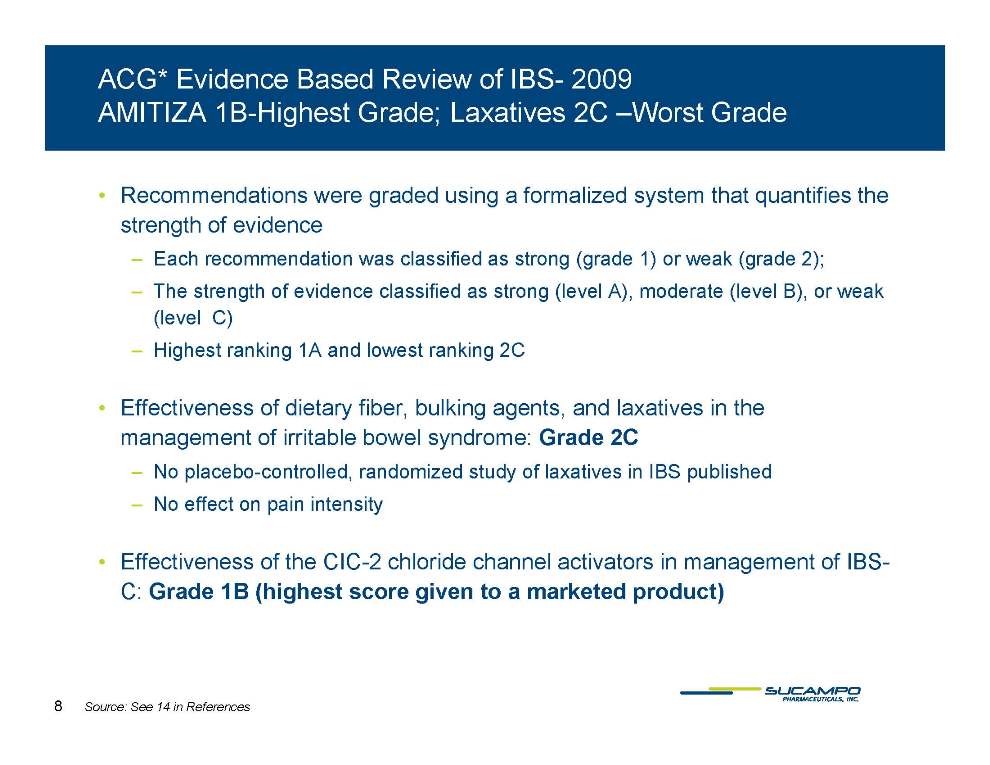
AMITIZA 1B-Highest Grade; Laxatives 2C –Worst Grade • Recommendations were graded using a formalized system that quantifies the strength of evidence – Each recommendation was classified as strong (grade 1) or weak (grade 2); – The strength of evidence classified as strong (level A), moderate (level B), or weak (level C) – Highest ranking 1A and lowest ranking 2C • Effectiveness of dietary fiber, bulking agents, and laxatives in the management of irritable bowel syndrome: Grade – No placebo-controlled, randomized study of laxatives in IBS published – No effect on pain intensity • Effectiveness of the CIC-2 chloride channel activators in management of IBSC: Grade 1B (highest score given to a marketed product) 8 Source: See 14 in References
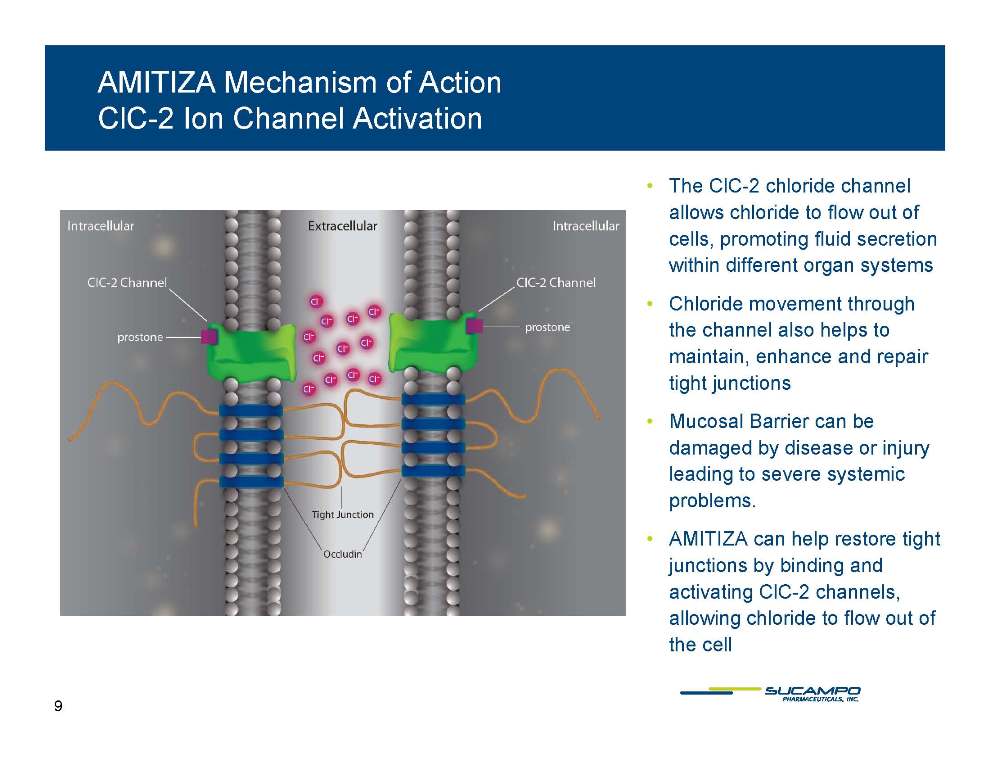
AMITIZA Mechanism of Action ClC-2 Ion Channel Activation • The ClC-2 chloride channel allows chloride to flow out of cells promoting fluid cells, secretion within different organ systems • Chloride movement through the channel helps to also maintain, enhance and repair tight junctions • Mucosal Barrier can be damaged by disease or injury leading to severe systemic problems. • AMITIZA can help restore tight junctions by binding and activating ClC-2 channels, allowing chloride to flow out of the cell 9
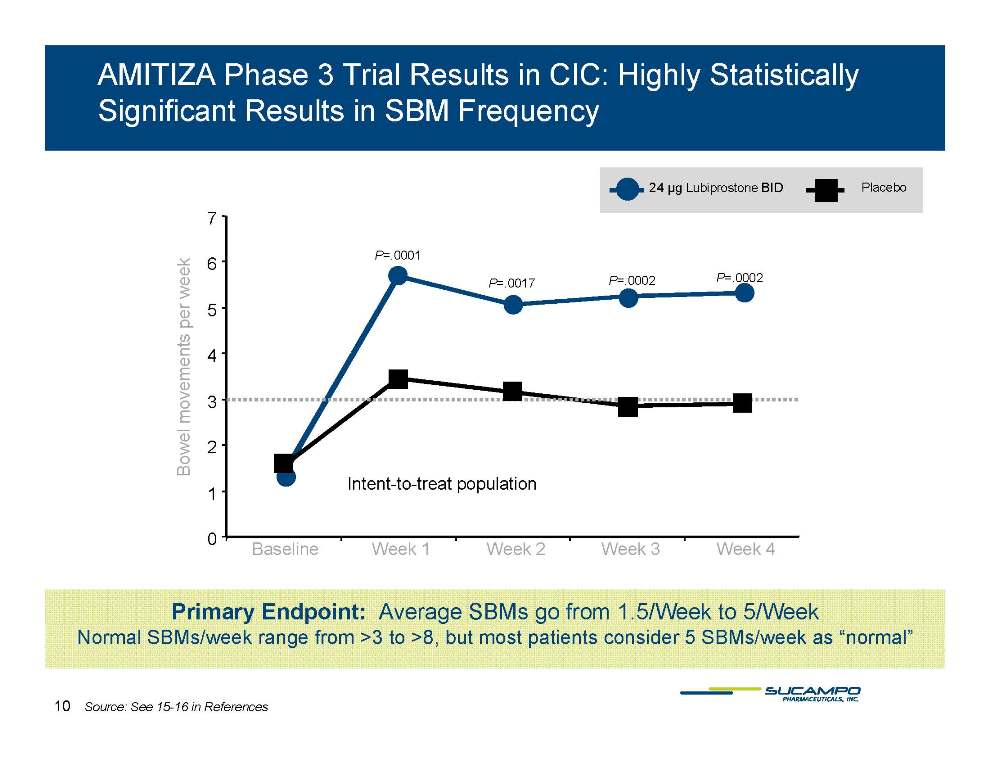
AMITIZA Phase 3 Trial Results in CIC: Highly Statistically Significant Results in SBM Frequency 24 µg Lubiprostone BID Placebo 7 P=.0001 P=.0017 P=.0002 P=.0002 5 6 s per week 3 4 l movements Intent-to-treat population 1 2 Bowel 0 Baseline Week 1 Week 2 Week 3 Week 4 Primary Endpoint: Average SBMs go from 1.5/Week to 5/Week Normal SBMs/week range from >3 to >8, but most patients consider 5 SBMs/week as “normal” 10 Source: See 15-16 in References
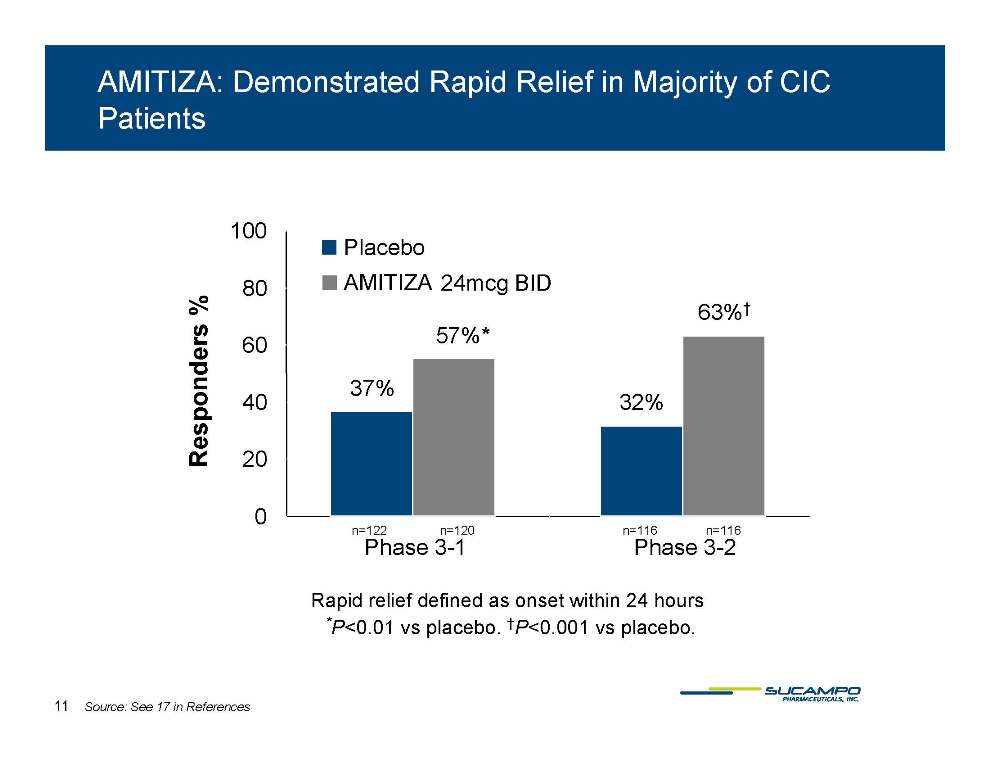
AMITIZA: Demonstrated Rapid Relief in Majority of CIC Patients 100 57%* 63%† 80 s % Placebo AMITIZA 24mcg BID 37% 57% 40 60 Responders 32% 0 20 122 120 116 116 Phase 3-1 Phase 3-2 n=n=n=n=Rapid relief defined as onset within 24 hours *P<0 01 placebo †P<0 001 placebo 0.01 vs placebo. 0.001 vs placebo. 11 Source: See 17 in References
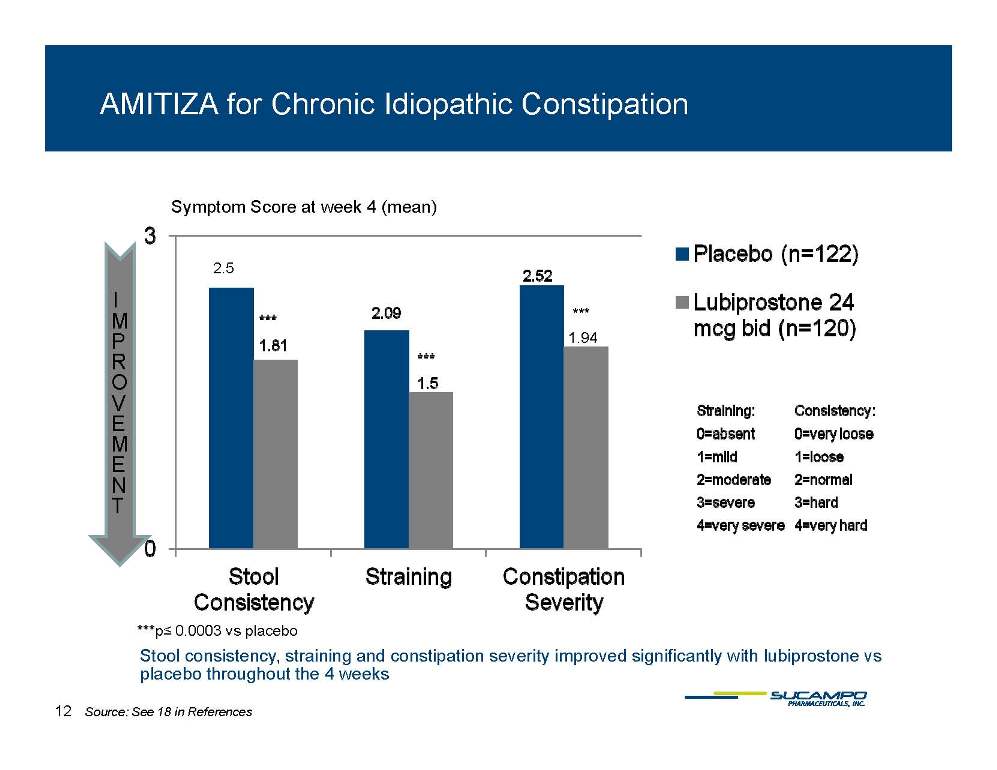
AMITIZA for Chronic Idiopathic Constipation Symptom Score at week 4 (mean) IM P 2.5 *** 1 94 R OVE 1.94 ME NT ***p≤ 0 0003 Stool consistency, straining and constipation severity improved significantly with lubiprostone vs placebo throughout the 4 weeks 0.0003 vs placebo 12 Source: See 18 in References
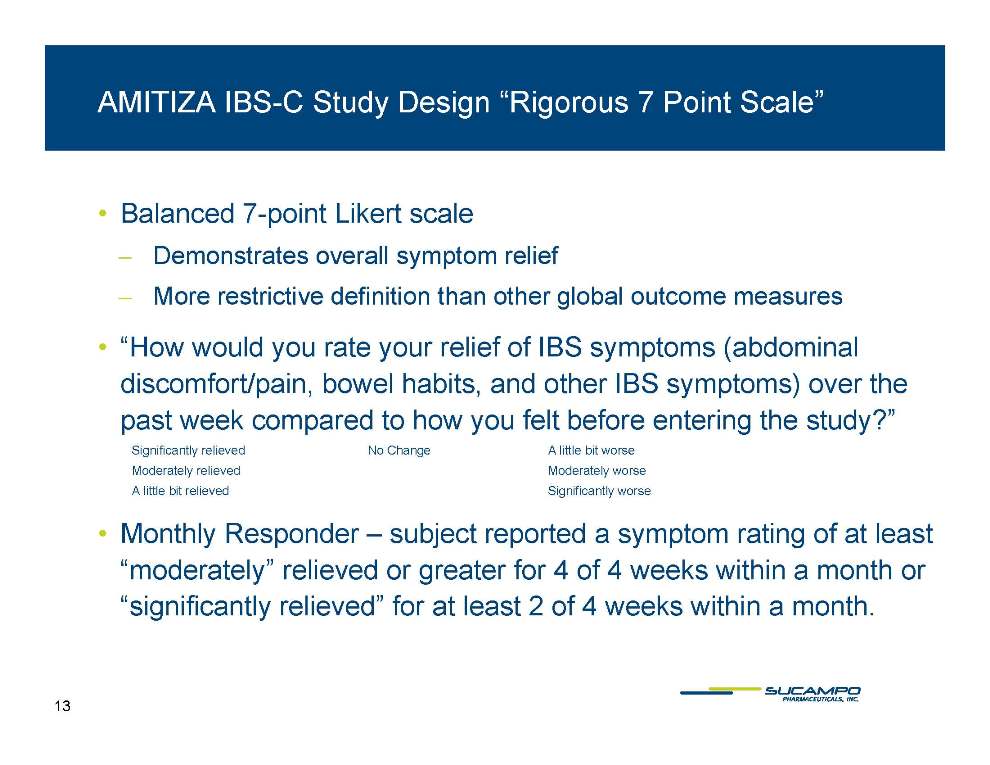
AMITIZA IBS-C Study Design “Rigorous 7 Point Scale” • Balanced 7-point Likert scale Demonstrates overall symptom relief ̶ More restrictive definition than other global outcome measures • “How would you rate your relief of IBS symptoms (abdominal discomfort/pain, bowel habits, and other IBS symptoms) over the past week compared to how you felt before entering the study?” Significantly relieved No Change A little bit worse Moderately relieved Moderately worse A little bit relieved Significantly worse Monthly Responder – subject reported a symptom rating of at least “moderately” relieved or greater for 4 of 4 weeks within a month or “significantly relieved” for at least 2 of 4 weeks within a month. 13
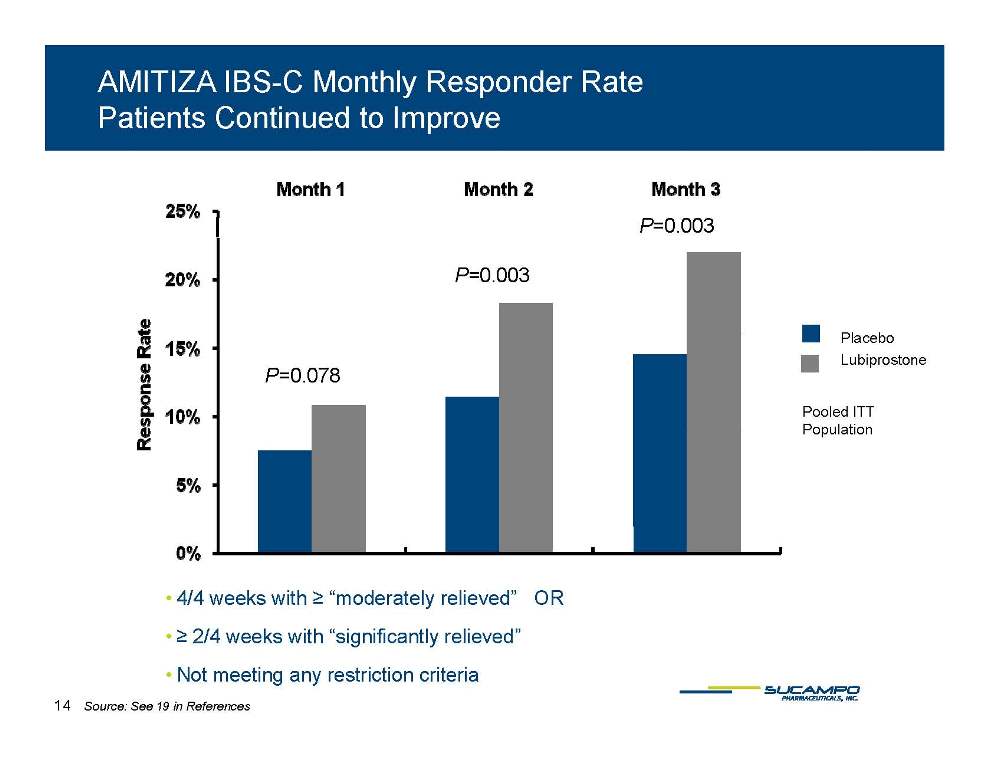
AMITIZA IBS-C Monthly Responder Rate Patients Continued to Improve P=0.003 P=0.003 Pl b P=0.078 Pooled ITT Placebo Lubiprostone Population • 4/4 weeks with ≥ “moderately relieved” OR • ≥ 2/4 weeks with “significantly relieved” • Not meeting any restriction criteria 14 Source: See 19 in References
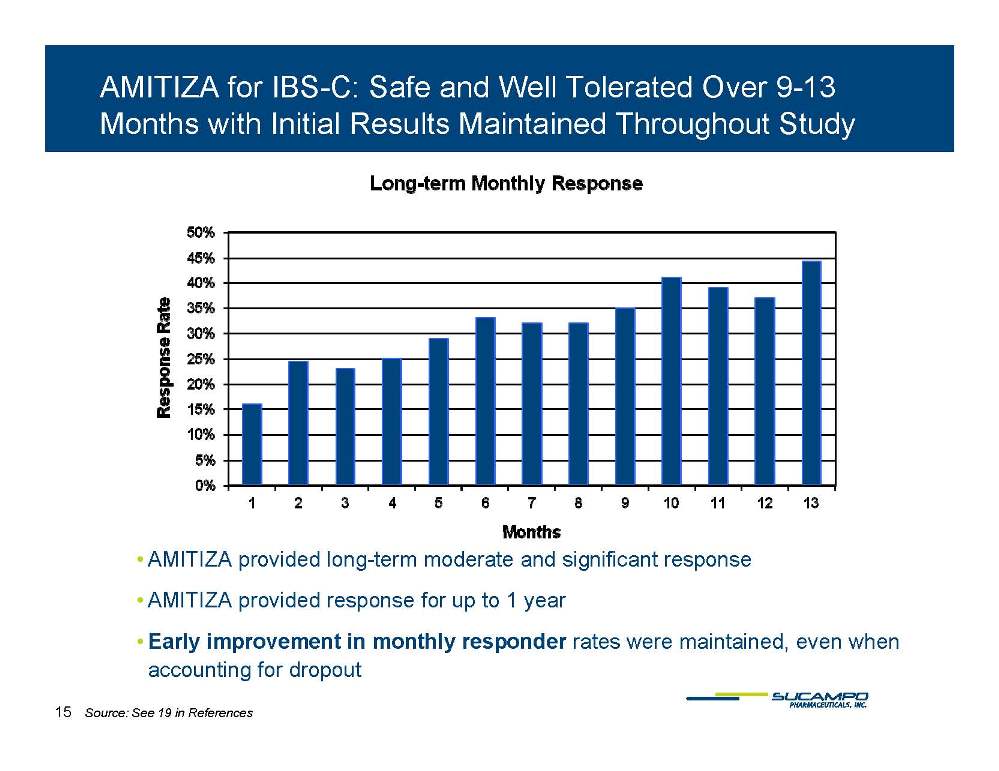
AMITIZA for IBS-C: Safe and Well Tolerated Over 9-13 Months with Initial Results Maintained Throughout Study • AMITIZA provided long-term moderate and significant response • AMITIZA provided response for up to 1 year • Early improvement in monthly responder rates were maintained, even when accounting for dropout 15 Source: See 19 in References
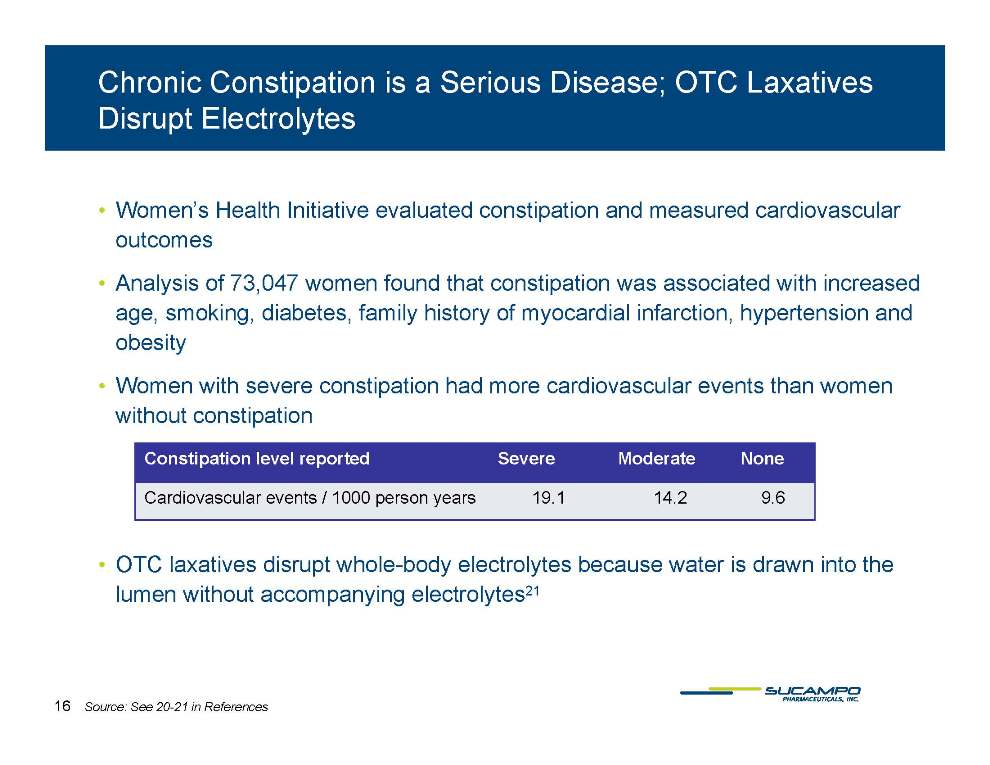
Chronic Constipation is a Serious Disease; OTC Laxatives Disrupt Electrolytes • Women’s Health Initiative evaluated constipation and measured cardiovascular outcomes • Analysis of 73,047 women found that constipation was associated with increased age, smoking, diabetes, family history of myocardial infarction, hypertension and b it obesity • Women with severe constipation had more cardiovascular events than women without constipation Constipation level reported Severe Moderate None Cardiovascular events / 1000 person years 19.1 14.2 9.6 • OTC laxatives disrupt whole-body electrolytes because water is drawn into the lumen without accompanying electrolytes21 16 Source: See 20-21 in References
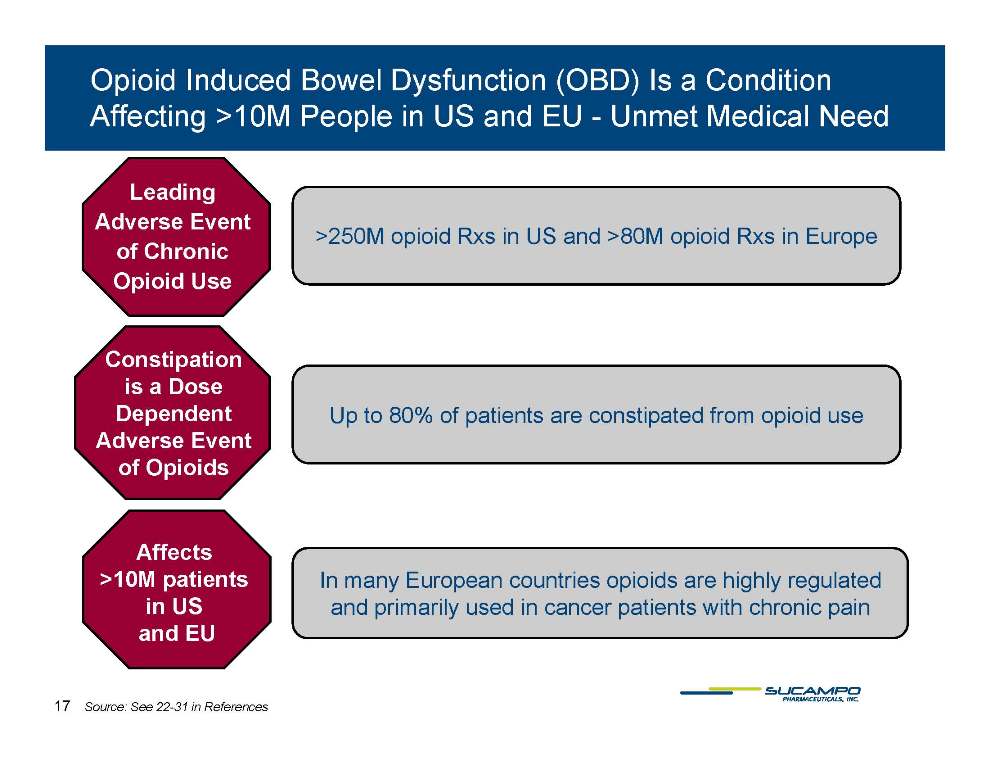
Opioid Induced Bowel Dysfunction (OBD) Is a Condition Affecting >10M People in US and EU - Unmet Medical Need >250M opioid Rxs opioid Rxs Europe Leading Adverse Event in US and >80M in of Chronic Opioid Use Constipation is a Dose Dependent Up to 80% of patients are constipated from opioid use Adverse Event of Opioids Affects >10M patients in US and EU In many European countries opioids are highly regulated and primarily used in cancer patients with chronic pain Affects >10M patients in US 17 Source: See 22-31 in References and EU
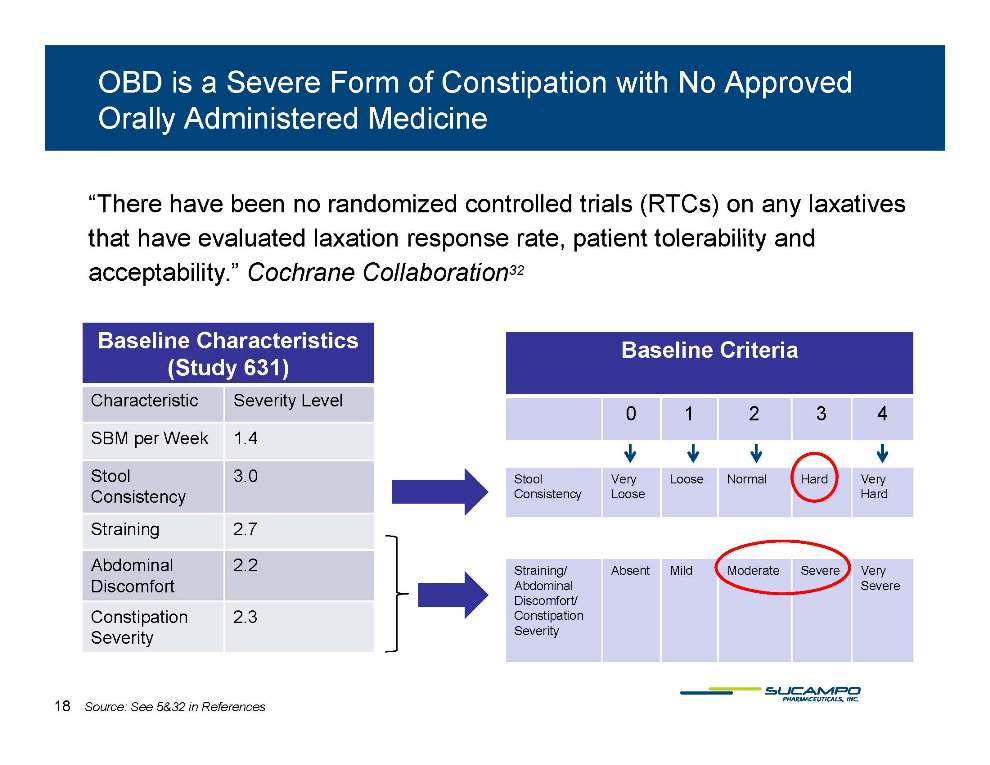
OBD is a Severe Form of Constipation with No Approved Orally Administered Medicine “There have been no randomized controlled trials (RTCs) on any laxatives that rate have evaluated laxation response rate, patient tolerability and acceptability.” Cochrane Collaboration32 Baseline Characteristics (Study 631) Characteristic Severity Level Baseline Criteria 0 1 2 3 4 SBM per Week 1.4 Stool Consistency 3.0 Straining 2 7 Stool Consistency Very Loose Loose Normal Hard Very Hard 2.7 Abdominal Discomfort 2.2 Constipation 2.3 Straining/Abdominal Discomfort/Constipation Absent Mild Moderate Severe Very Severe Severity Severity 18 Source: See 5&32 in References

AMITIZA: Expansion Strategy Well Underway; Results of Third Phase 3 Trial in OBD • Third Phase 3 Trial (OBD1033) design: Primary endpoint: Overall SBM response rate in non-cancer, non-methadone pain Patients Randomized and treated ~440 patients in a placebo-controlled, multi-center trial Almost the same protocol as used in the previous phase 3 trial (OBD0631) reported at 2010 2010, except for FDA-requested new primary endpoint and exclusion of patients on methadone. One 24-mcg gel capsule of lubiprostone or placebo twice each day, over 12 weeks Met Primary Endpoint of at least 9 weeks with > 3 SBMs/week and on all non-missing treatment weeks having > 1 SBM over baseline, p=0.035 Aim to File sNDA in 2Q12 • Data to be submitted to an appropriate medical conference and peer-reviewed publication 19 Source: See 33 in References
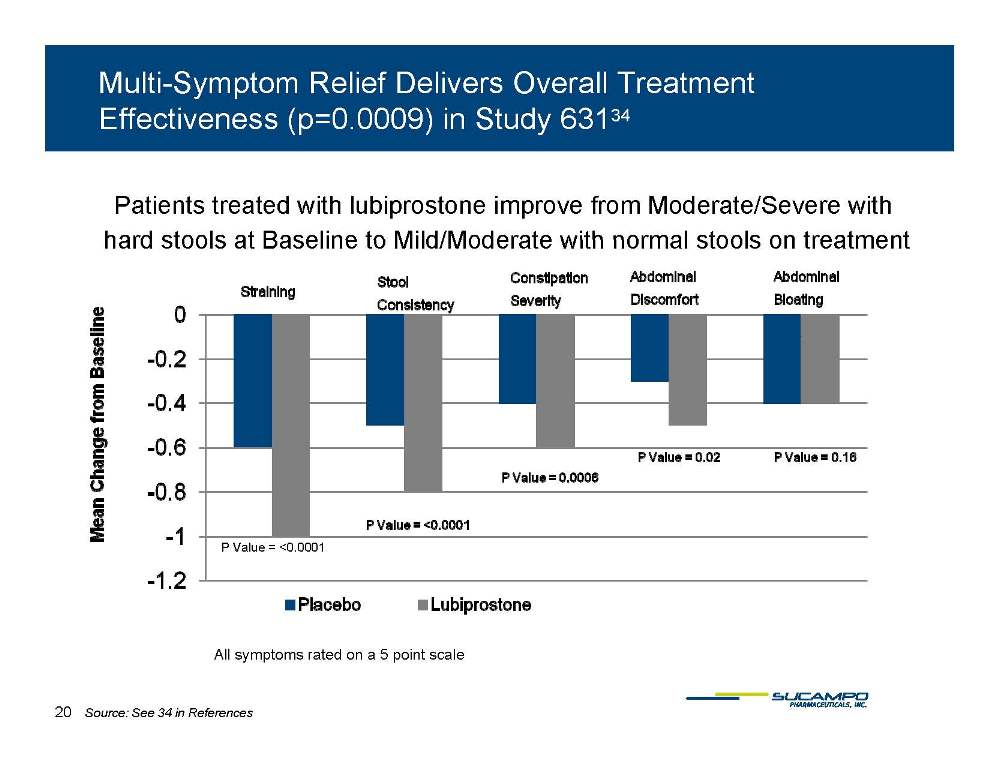
Multi-Symptom Relief Delivers Overall Treatment Effectiveness (p=0.0009) in Study 63134 Patients treated with lubiprostone improve from Moderate/Severe with hard stools at Baseline to Mild/Moderate with normal stools on treatment P Value = <0.0001 All symptoms rated on a 5 point scale 20 Source: See 34 in References
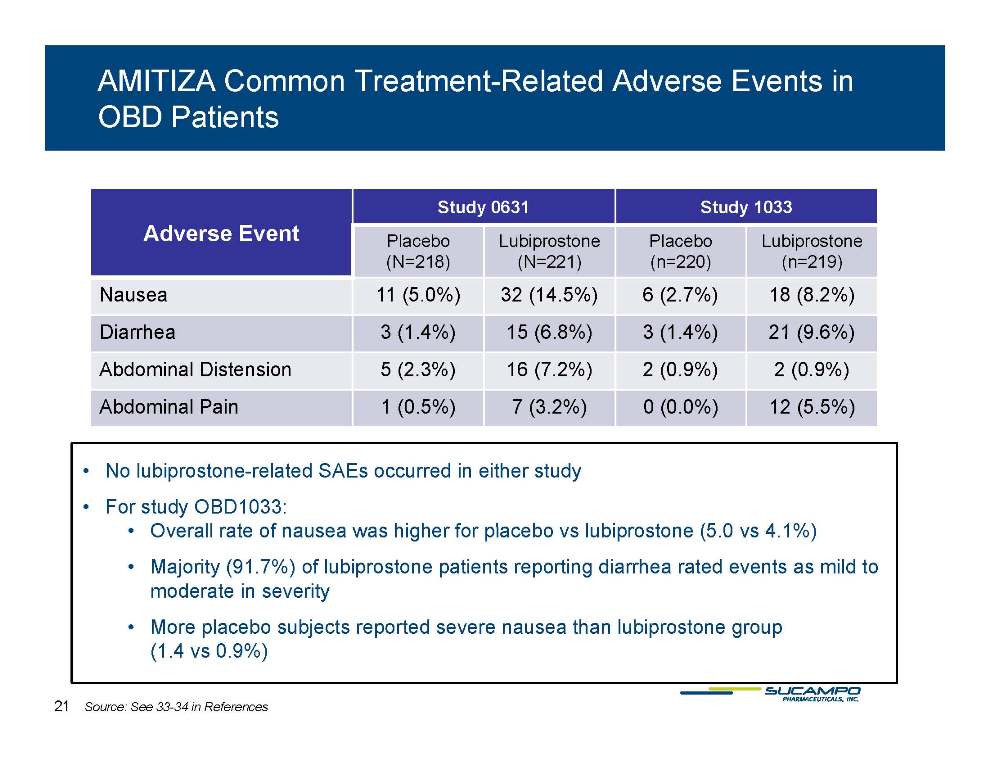
AMITIZA Common Treatment-Related Adverse Events in OBD Patients Study 0631 Study 1033 Placebo Lubiprostone Placebo Lubiprostone Adverse Event (N=218) (N=221) (n=220) (n=219) Nausea 11 (5.0%) 32 (14.5%) 6 (2.7%) 18 (8.2%) Diarrhea 3 1 4%) 15 (6 8%) 3 1 4%) 21 (9 6%) (1.4%) 6.8%) (1.4%) 9.6%) Abdominal Distension 5 (2.3%) 16 (7.2%) 2 (0.9%) 2 (0.9%) Abdominal Pain 1 (0.5%) 7 (3.2%) 0 (0.0%) 12 (5.5%) • No lubiprostone-related SAEs occurred in either study • For study OBD1033: 5 0 4 1%) • Overall rate of nausea was higher for placebo vs lubiprostone (5.0 vs 4.1%) • Majority (91.7%) of lubiprostone patients reporting diarrhea rated events as mild to moderate in severity placebo • More subjects reported severe nausea than lubiprostone group (1.4 vs 0.9%) 21 Source: See 33-34 in References

Terms of Sucampo’s AMITIZA Agreement with Takeda • Takeda shall exert best efforts to promote, market, and sell and to maximize net sales revenue AMITIZA in the US and Canada • Sucampo’s tiered annual Sucampo s royalty rate: 18% to 26% of net sales • Sucampo earned $20M in upfront and $130M in development milestone payments, as of Sept. 30, 2011 • We are disappointed by our partner’s performance • Arbitration hearing was held in December 2011; expect decision by April 30, 2012 but it is not known how long thereafter the arbitration proceedings will conclude 22
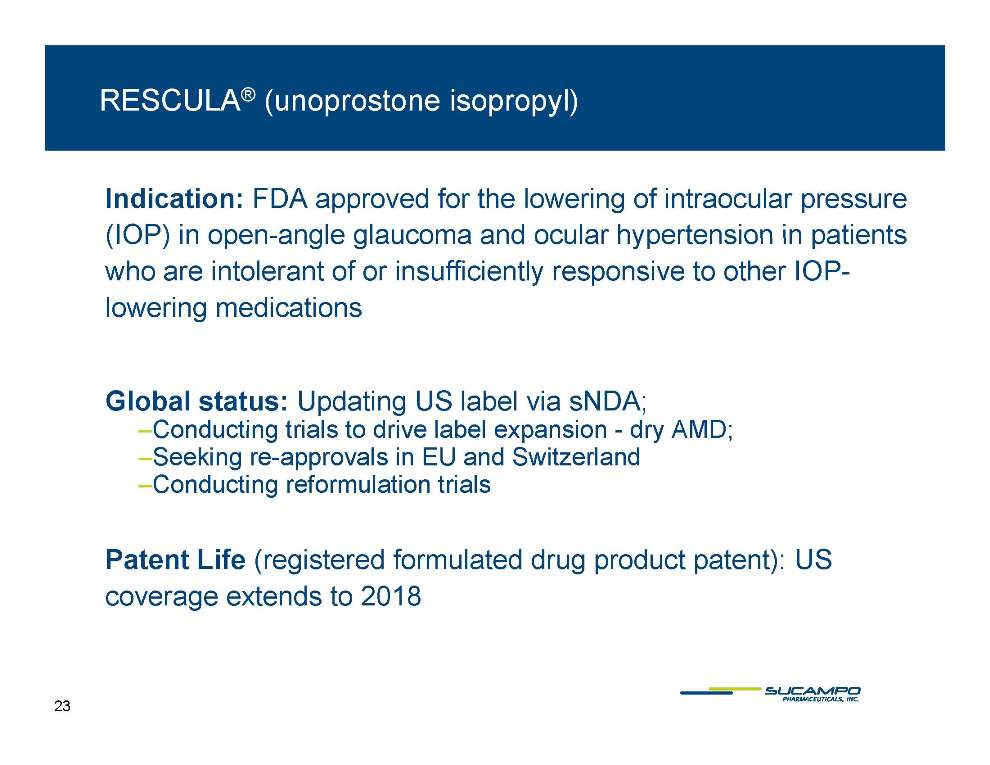
RESCULA® (unoprostone isopropyl) Indication: FDA approved for the lowering of intraocular pressure (IOP) in open-angle open glaucoma and ocular hypertension in patients who are intolerant of or insufficiently responsive to other IOPlowering medications Global status: Updating US label via sNDA; –Conducting - AMD; trials to drive label expansion dry –Seeking re-approvals in EU and Switzerland –Conducting reformulation trials Patent Life (registered formulated drug product patent): US coverage extends to 2018 23

US Glaucoma Market Overview • On the surface, large, mature “satisfied” Rx market ̶2.5M patients35, 19.2M TRxs, 67% of the market is generic36 ̶IOP is associated with slowing the progression of visual field degeneration ̶Limited new products vs. reformulations • In reality, unsatisfied patients IOP improvement does not guarantee visual field maintenance̶ Compliance and adherence are unmet needs • 50% of new patients drop off therapy within one year of initiation ̶Prostaglandins are inflammatory agents which depolarize cell membranes• #1 hyperemia37 reason for discontinuation of prostaglandins is 24 Source: See 35-39 in References

Current AAO/AOA Treatment Guidelines • AAO Guidelines40 for treatment of POAG: – identify and treat POAG and to To preserve visual function while minimizing adverse effects of therapy, thereby enhancing the patient’s health and quality of life. • “The ophthalmologist should consider the balance between side effects and effectiveness in choosing a regimen of maximal effectiveness and tolerance to achieve the desired IOP reduction for each patient.” – The goals of managing patients with POAG are to achieve the following: • Stable visual fields • Stable optic nerve/retinal nerve fiber layer status • Controlled IOP in the target pressure range • Maintenance of quality of life • Objective of AOA Guidelines41: – To provide the patient with their individualized target pressure, with the understanding that multiple treatments may be necessary as glaucoma is a progressive degenerative disease 25 Source: See 40-41 in References
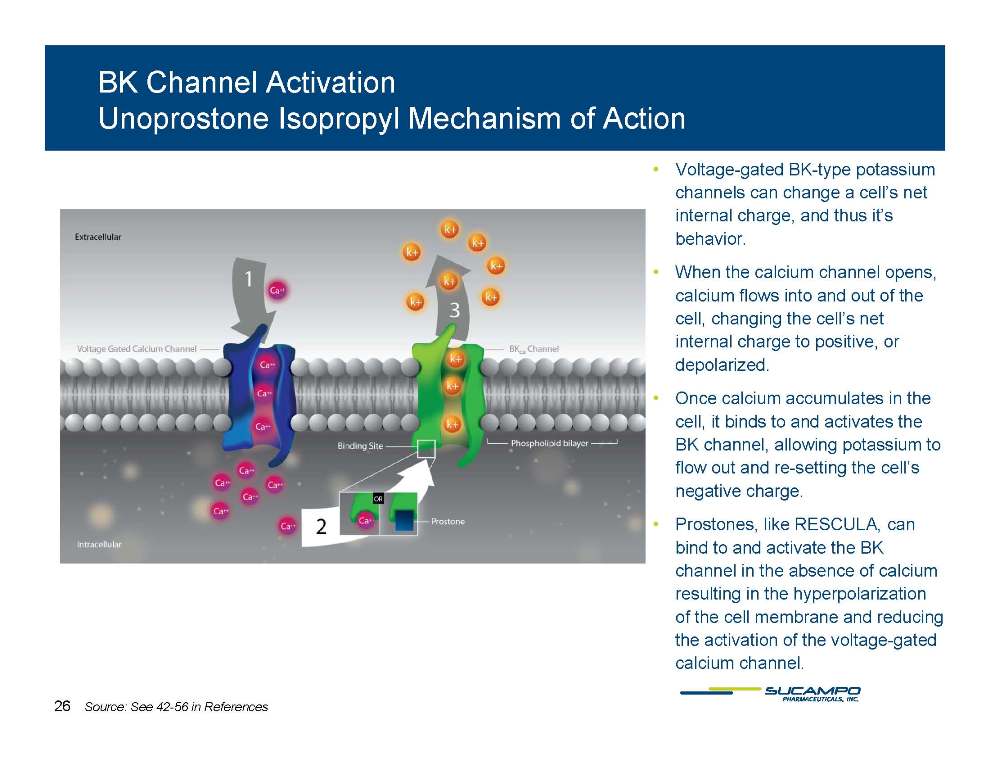
BK Channel Activation Unoprostone Isopropyl Mechanism of Action • Voltage-gated BK-type potassium channels can change a cell’s net internal charge, and thus it’s behavior behavior. • When the calcium channel opens, calcium flows into and out of the cell, changing the cell’s net internal charge to positive, or depolarized. • Once calcium accumulates in the cell, it binds to and activates the BK channel, allowing potassium to flow out and re-setting the cell’s negative charge. • Prostones RESCULA Prostones, like RESCULA, can bind to and activate the BK channel in the absence of calcium resulting in the hyperpolarization of the cell membrane and reducing the activation of the voltage-gated calcium channel. 26 Source: See 42-56 in References
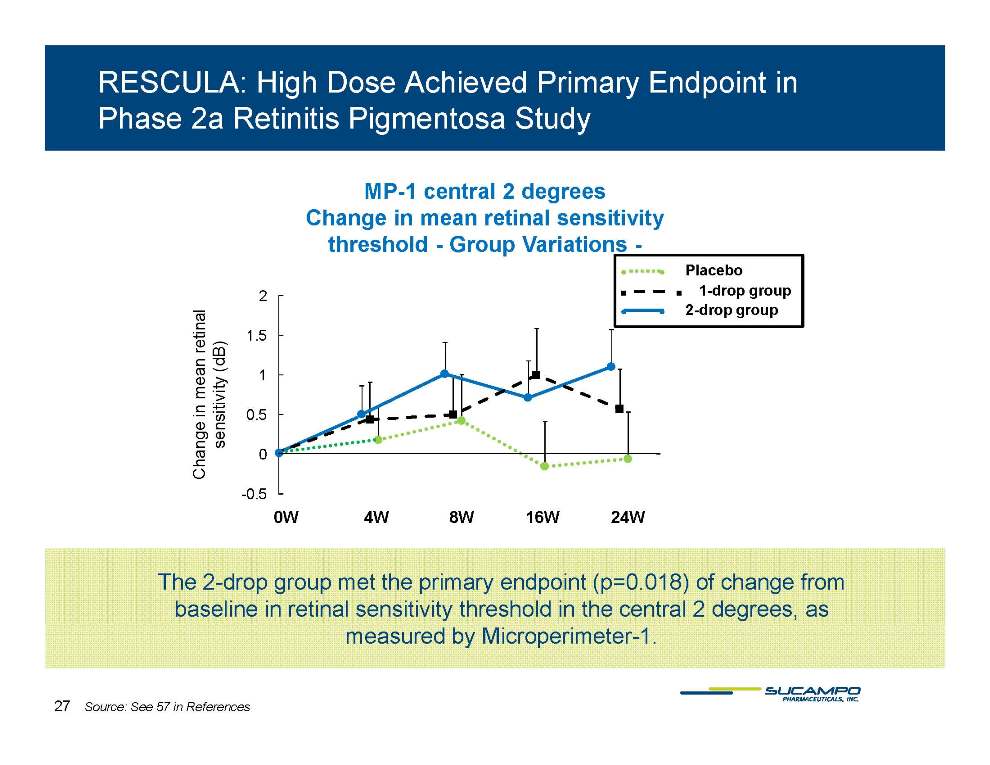
RESCULA: High Dose Achieved Primary Endpoint in Phase 2a Retinitis Pigmentosa Study MP-1 central 2 degrees Change in mean retinal sensitivity threshold - Group Variations – 2 ____S.E. 1 5 2 inal ● ● Placebo ■ ■ ■ 1-drop group ● ● 2-drop group Mean_ S.E. 0.5 1 1.5 UF- 021 1_ 2_ _ UF- 021 1_ 1_ _ _____0.5 1 1.5 e in mean ret nsitivity (dB) -0.5 0 -0.5 0 Chang se 0W 4W 8W 16W 24W The 2-drop group met the primary endpoint (p=0.018) of change from baseline in retinal sensitivity threshold in the central 2 degrees, as measured by Microperimeter-1. 27 Source: See 57 in References Proprietary Platform Technology:
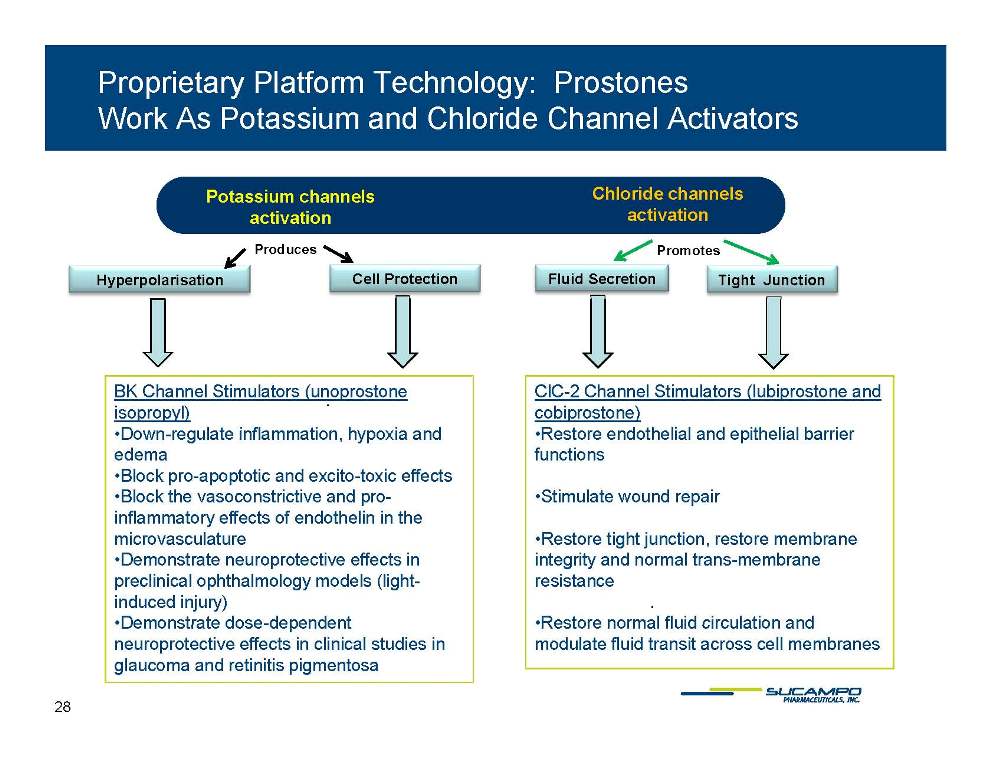
Proprietary Platform Technology: Prostones Work As Potassium and Chloride Channel Activators Chloride channels activation Potassium channels activation Hyperpolarisation Cell Protection Fluid Secretion Tight Junction Produces Promotes . BK Channel Stimulators (unoprostone isopropyl) •Down regulate inflammation hypoxia ClC-2 Channel Stimulators (lubiprostone and cobiprostone) •Restore endothelial and epithelial Down-inflammation, and edema •Block pro-apoptotic and excito-toxic effects •Block the vasoconstrictive and proinflammatory effects of endothelin in the barrier functions •Stimulate wound repair . microvasculature •Demonstrate neuroprotective effects in preclinical ophthalmology models (lightinduced injury) •Demonstrate dose-dependent •Restore tight junction, restore membrane integrity and normal trans-membrane resistance •Restore normal fluid neuroprotective effects in clinical studies in glaucoma and retinitis pigmentosa circulation and modulate fluid transit across cell membranes 28
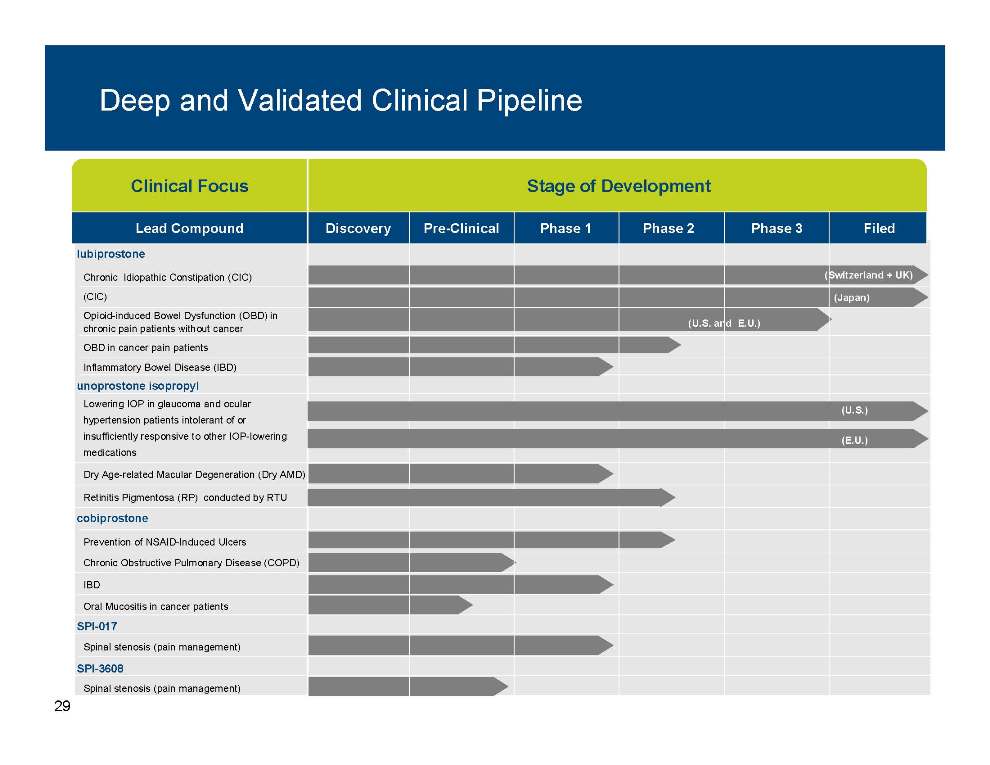
Deep and Validated Clinical Pipeline Clinical Focus Stage of Development Lead Compound Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 Filed lubiprostone Chronic Idiopathic Constipation (CIC) (CIC) Opioid-induced Bowel Dysfunction (OBD) in chronic pain patients without cancer (U.S. and E.U.) (Japan) (Switzerland + UK) unoprostone isopropyl OBD in cancer pain patients Inflammatory Bowel Disease (IBD) Lowering IOP in glaucoma and ocular hypertension patients intolerant of or (U.S.) cobiprostone insufficiently responsive to other IOP-lowering medications Dry Age-related Macular Degeneration (Dry AMD) Retinitis Pigmentosa (RP) conducted by RTU (E.U.) SPI 017 Prevention of NSAID-Induced Ulcers Chronic Obstructive Pulmonary Disease (COPD) IBD Oral Mucositis in cancer patients SPI-Spinal stenosis (pain management) SPI-3608 Spinal stenosis (pain management) 29
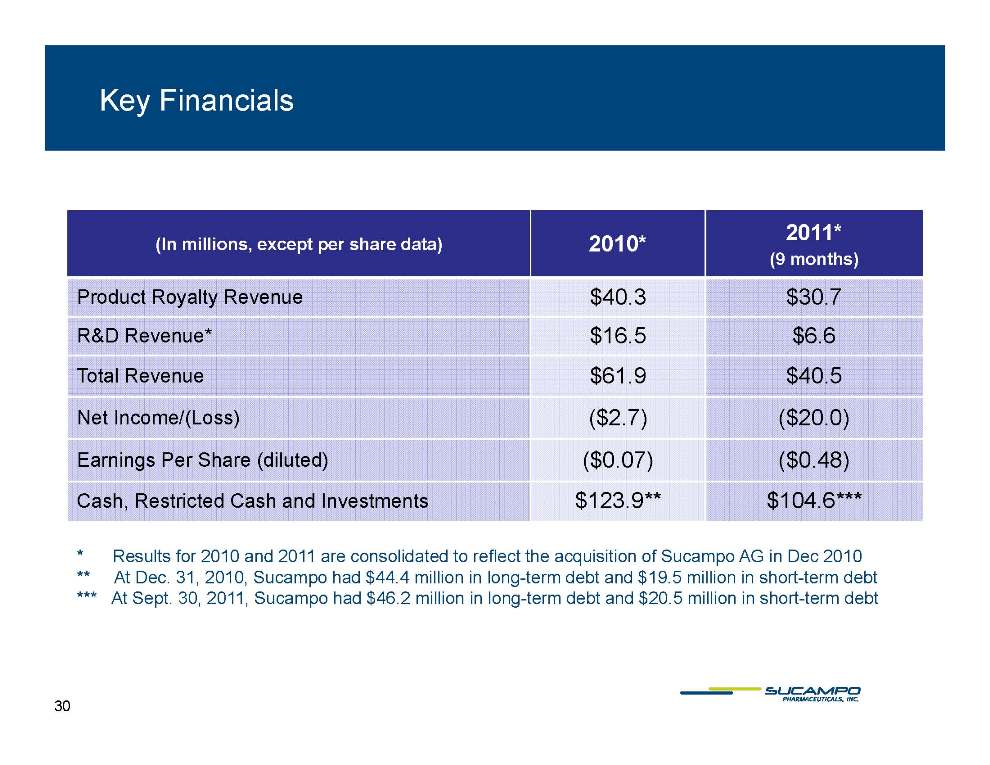
Key Financials 2010* 2011* In millions, except per share data) 2011 (9 months) Product Royalty Revenue $40.3 $30.7 Revenue* $16 5 $6 6 R&D Revenue 16.5 6.6 Total Revenue $61.9 $40.5 Net Income/(Loss) ($2.7) ($20.0) Earnings Per Share (diluted) ($0.07) ($0.48) Cash, Restricted Cash and Investments $123.9** $104.6*** * Results for 2010 and 2011 are consolidated to reflect the acquisition of Sucampo AG in Dec 2010 ** At Dec. 31, 2010, Sucampo had $44.4 million in long-term debt and $19.5 million in short-term debt *** At Sept. 30, 2011, Sucampo had $46.2 million in long-term debt and $20.5 million in short-term debt 30
Key Value Drivers in 2012 AMITIZA • US • File OBD sNDA in 2Q12; request priority review • Anticipate decision in Takeda arbitration by April 30, 2012 • Switzerland • Pricing negotiations for approved CIC indication ongoing • Japan • Expect Japanese regulatory approval decision in August 2012, and pricing decision in 3Q12, launch in 4Q12 (CIC patient population in Japan: >25M) • EU • Expect approval of MAA in UK for CIC in 3Q12, to be followed by mutual recognition procedure for EU; approval will trigger filing of in UK and Switzerland RESCULA • Expect data from exploratory trial in dry AMD patients • US: Anticipate approval of sNDA for glaucoma indication in US (updated MOA) • EUROPE: Re-approval filings in EU and Switzerland 31

Appendix
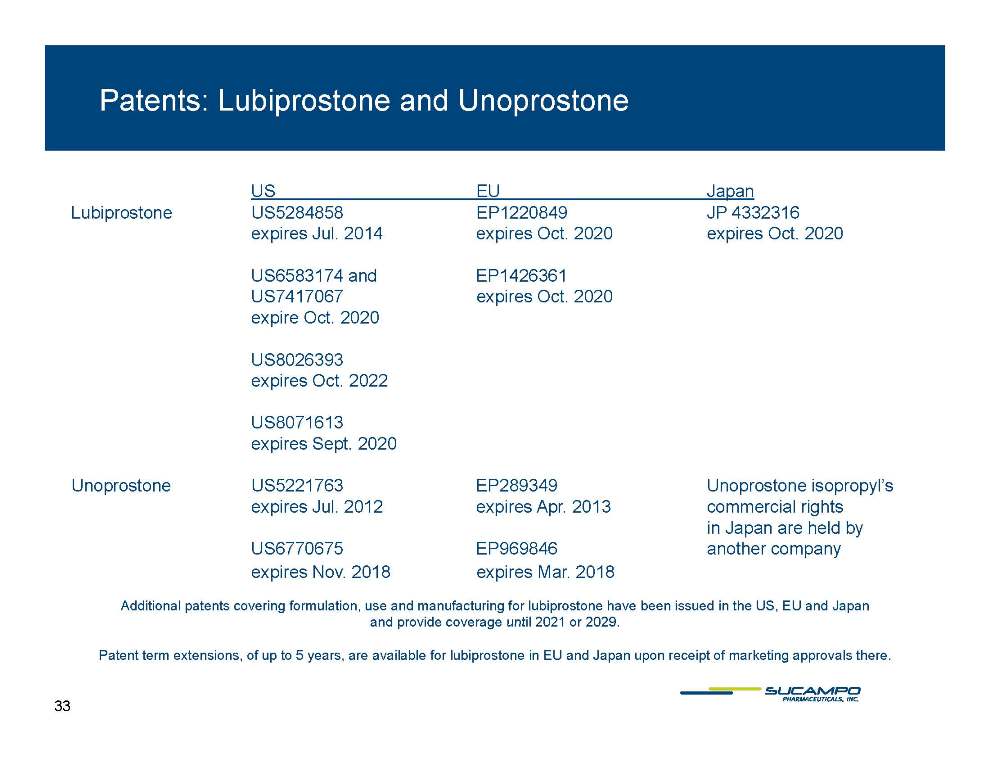
Patents: Lubiprostone and Unoprostone US EU Japan Lubiprostone US5284858 EP1220849 JP 4332316 expires Jul. expires Oct. 2020 expires Oct. 2020 2014 US6583174 and EP1426361 US7417067 expires Oct. 2020 expire Oct. 2020 US8026393 expires Oct. 2022 US8071613 expires Sept. 2020 Unoprostone US5221763 EP289349 Unoprostone isopropyl’s expires Jul. 2012 expires Apr. 2013 commercial rights in Japan are held by US6770675 EP969846 another company expires Nov. 2018 expires Mar. 2018 Additional patents covering formulation, use and manufacturing for lubiprostone have been issued in the US, EU and Japan provide coverage until 2029 and 2021 or 2029. Patent term extensions, of up to 5 years, are available for lubiprostone in EU and Japan upon receipt of marketing approvals there. 33
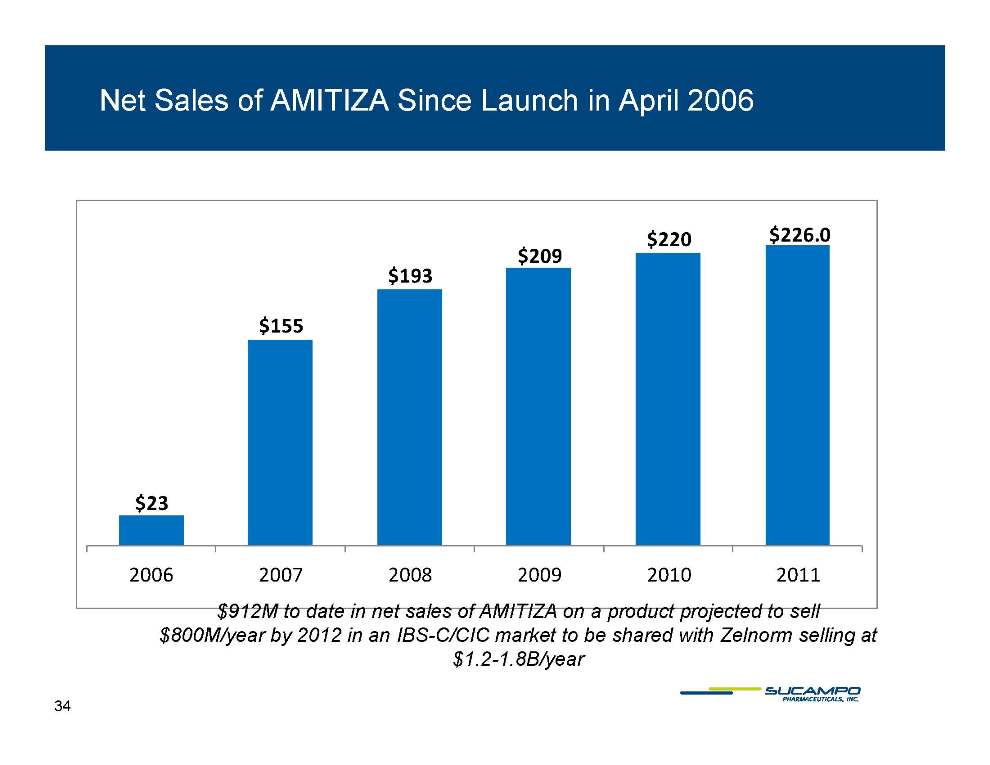
Net Sales of AMITIZA Since Launch in April 2006 $220 $226 0 $ 155 $193 $209 226.0 $23 2006 2007 2008 2009 2010 2011 $912M to date in net sales of AMITIZA on a product projected to sell $800M/year by 2012 in an IBS-C/CIC market to be shared with Zelnorm selling at $1.2-1.8B/year 34
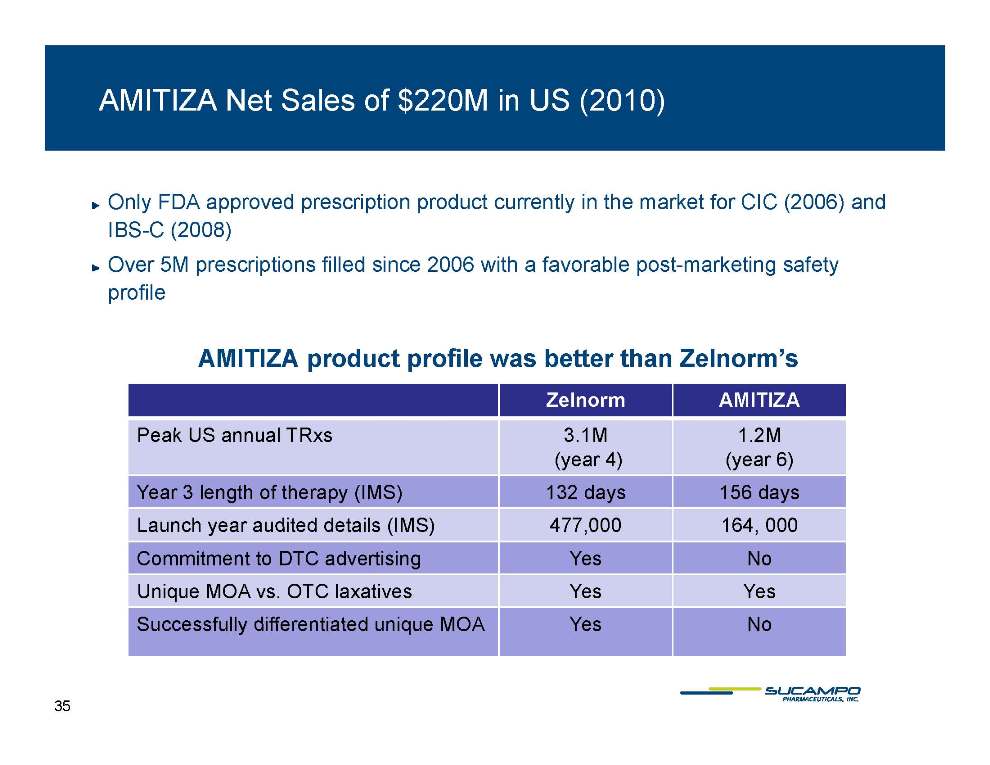
AMITIZA Net Sales of $220M in US (2010) Only FDA approved prescription product currently in the market for CIC (2006) and IBS-C ( 2008) ) Over 5M prescriptions filled since 2006 with a favorable post-marketing safety profile AMITIZA product profile was better than Zelnorm’s Zelnorm AMITIZA P kUS lTR 3 1M 1 2M Peak US annual TRxs 3.1M (year 4) 1.2M (year 6) Year 3 length of therapy (IMS) 132 days 156 days year audited details 477 000 164 Launch (IMS) 477,000 164, 000 Commitment to DTC advertising Yes No Unique MOA vs. OTC laxatives Yes Yes Yes No Successfully differentiated unique MOA 35
RESCULA: Exploring Potential with Phase 2a Study in Dry AMD • Purpose: To study choroidal blood low following administration of unoprostone isopropyl vs. placebo • Design: ̶A single-center, double-masked, randomized, placebo-controlled, crossover design study in 28 dry AMD patients ̶ Administer two doses (Day 1 and 8); 14 day follow-up period ̶Choroidal blood flow measured by laser doppler flowmetery • Study initiated in May 2011, expecting results in 1Q12 36
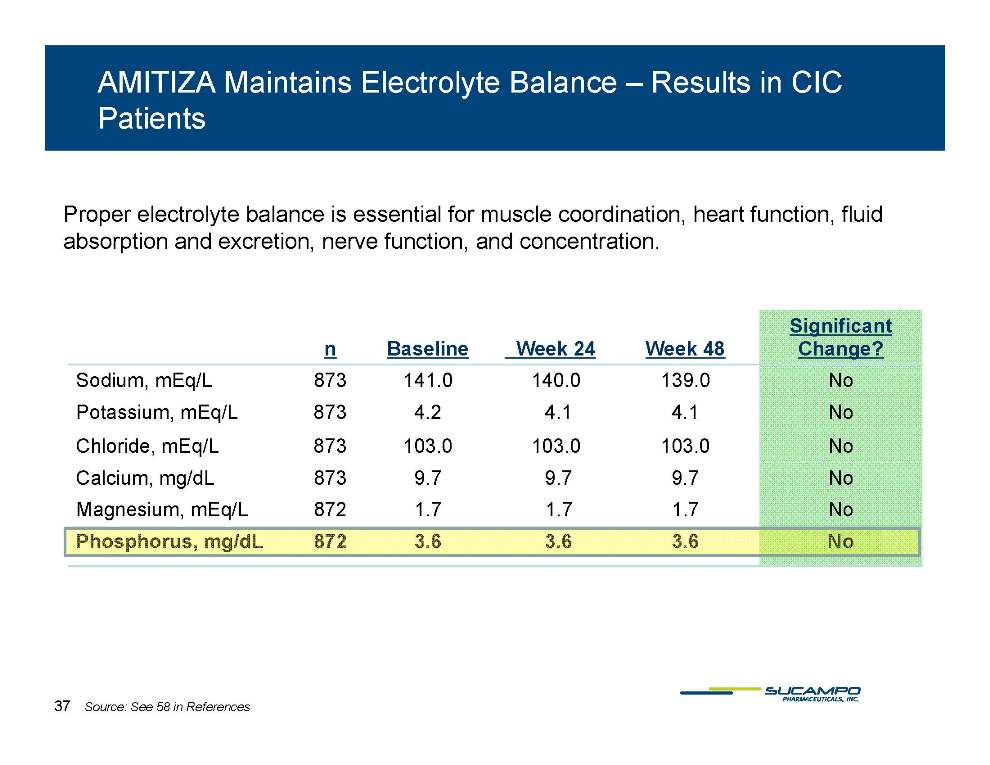
AMITIZA Maintains Electrolyte Balance – Results in CIC Patients Proper electrolyte balance is essential for muscle coordination, heart function, fluid absorption excretion function concentration Significant and excretion, nerve function, and concentration. n Baseline Week 24 Week 48 g Change? Sodium, mEq/L 873 141.0 140.0 139.0 No Potassium, mEq/L 873 4.2 4.1 4.1 No Chloride, mEq/L 873 103.0 103.0 103.0 No Calcium, mg/dL 873 9.7 9.7 9.7 No Magnesium, mEq/L 872 1.7 1.7 1.7 No Phosphorus, mg/dL 872 3.6 3.6 3.6 No 37 Source: See 58 in References
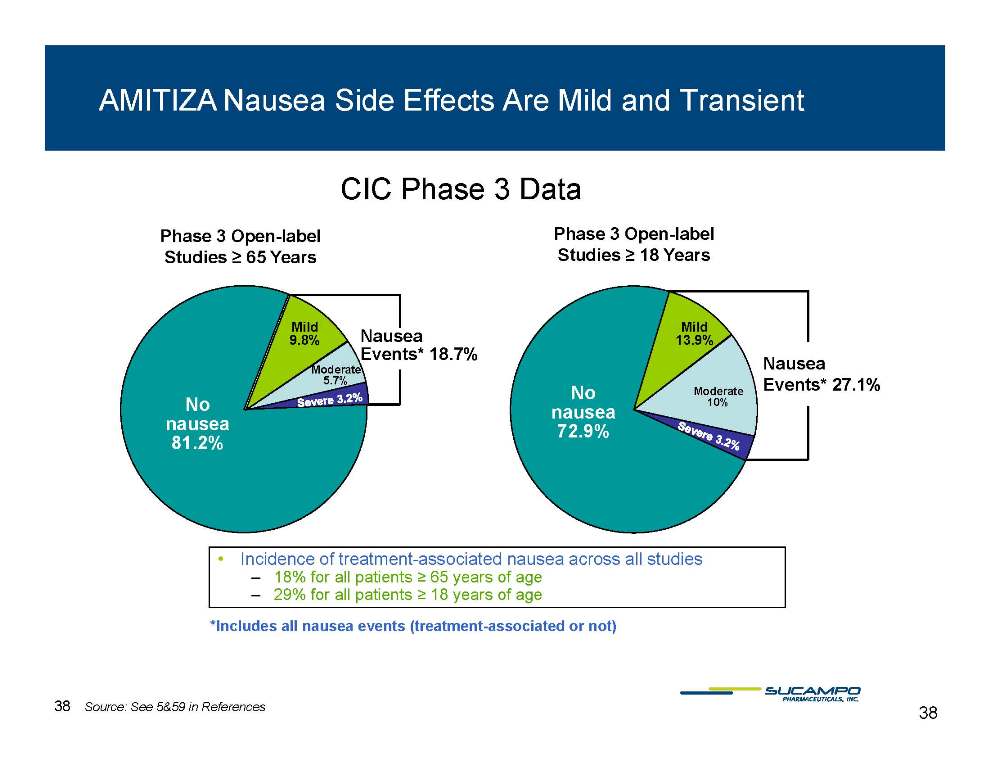
AMITIZA Nausea Side Effects Are Mild and Transient Phase 3 Open-label Phase 3 Open-label CIC Phase 3 Data Nausea Mild Studies ≥ 65 Years Mild Open Studies ≥ 18 Years Events* 18.7% No nausea 9.8% Moderate 5.7% Nausea Events* 27.1% No nausea 72 9% 13.9% Moderate 10% 81.2% 72.9% • Incidence of treatment-associated nausea across all studies – 18% for all patients ≥ 65 years of age – 29% for all patients ≥ 18 years of age *nausea events (treatment associated Includes all treatment-or not) 38 38 Source: See 5&59 in References
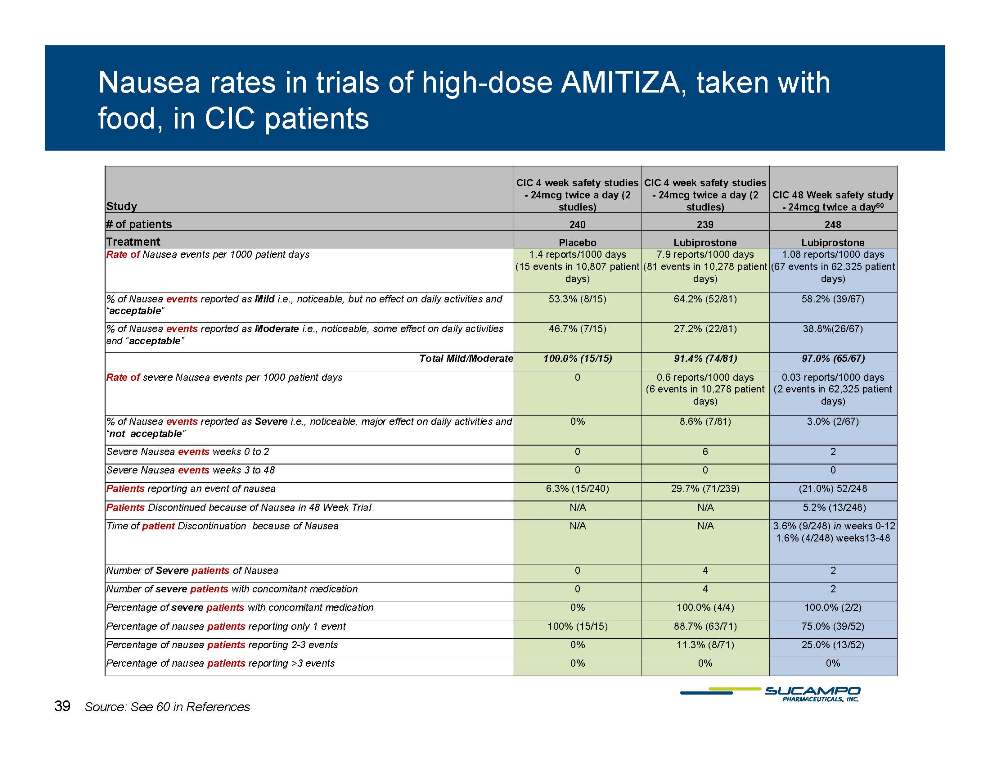
Nausea rates in trials of high-dose AMITIZA, taken with food, in CIC patients Study CIC 4 week safety studies - 24mcg twice a day (2 studies) CIC 4 week safety studies - 24mcg twice a day (2 studies) CIC 48 Week safety study - 24mcg twice a day60 # of patients 240 239 248 Treatment Placebo Lubiprostone Lubiprostone Rate of Nausea events per 1000 patient days 1.4 reports/1000 days (15 events in 10,807 patient days) 7.9 reports/1000 days (81 events in 10,278 patient days) 1.08 reports/1000 days (67 events in 62,325 patient days) % of Nausea events reported as Mild i.e., noticeable, but no effect on daily activities and “acceptable” 53.3% (8/15) 64.2% (52/81) 58.2% (39/67) % of Nausea events reported as Moderate i.e., noticeable, some effect on daily activities 46.7% (7/15) 27.2% (22/81) 38.8%(26/67) and “acceptable” Total Mild/Moderate 100.0% (15/15) 91.4% (74/81) 97.0% (65/67) Rate of severe Nausea events per 1000 patient days 0 0.6 reports/1000 days (6 events in 10,278 patient days) 0.03 reports/1000 days (2 events in 62,325 patient days) % of Nausea events reported as Severe i.e., noticeable, major effect on daily activities and “ t tbl” 0% 8.6% (7/81) 3.0% (2/67) not acceptable” Severe Nausea events weeks 0 to 2 0 6 2 Severe Nausea events weeks 3 to 48 Patients reporting an event of nausea 6.3% (15/240) 29.7% (71/239) (21.0%) 52/248 Patients Discontinued because of Nausea in 48 Week Trial N/A N/A 5.2% (13/248) Time patient N/A N/A 3 6% 248) in 0-12 of Discontinuation because of Nausea 3.6% (9/weeks 0 1.6% (4/248) weeks13-48 Number of Severe patients of Nausea 0 4 2 Number of severe patients with concomitant medication 0 4 2 Percentage of severe patients with concomitant medication 0% 100.0% (4/4) 100.0% (2/2) 88 7% 75 0% Percentage of nausea patients reporting only 1 event 100% (15/15) 88.7% (63/71) 75.0% (39/52) Percentage of nausea patients reporting 2-3 events 0% 11.3% (8/71) 25.0% (13/52) Percentage of nausea patients reporting >3 events 0% 0% 0% 39 Source: See 60 in References

References 1. Yang C et al.Prostaglandin E receptors as inflammatory therapeutic targets for therosclerosis. Life Sci. 2011 Jan 31; 88(5-6): 201-5. Epub 2010 Nov 26. 2. Wong SL et al. Prostaglandins in action indispensable roles of cyclooxygenase-1 and -2 in endothelium-dependent contractions. Adv Pharmacol. 2010;60:61-83. 3. Föller M. et al. Erythrocyte programmed cell death. IUBMB Life. 2008 Oct;60(10):661-8. 4. Kolaczkowska E et al. Enhanced early vascular permeability in gelatinase B (MMP-9)-deficient mice: putative contribution of COX- 1-derived PGE2 of macrophage origin. J Leukoc Biol. 2006 Jul;80(1):125-32. Epub 2006 May 9. 5. Sucampo Data on File 6. Higgins, P. D. R. et al. (2004) Epidemiology of Constipation in North America: A Systematic Review. American Journal of Gastroenterology, 99(4):750-9. 7. Hungin, A.P.S. et al. (2005) IBS in the United States:Prevalence, Symptom Patterns and Impact: Discussion. Alimentary Pharmacology & Therapeutic , 21(11):1365-1375. 8. Muller-Lissner, S. et al. (2001) Epidemiological Aspects of Irritable Bowel Syndrome in Europe and North America. Digestion, 64, 200-204 9. Chey, W. et al “Frequency and Bothersomeness of Symptoms, Health Care Seeking Behavior and Satisfaction with Therapy in IBS-C Patients Meeting ROME II Criteria: Results of a Population Based Survey.” 10 10. Schoenfield, P. “System Frequency, Health Care Seeking Behavior, and Satisfaction with Therapy among Chronic Constipation Patients: Results of a Population-Based Survey” 11. IMS NPA Data, Dec 2011, current Q. annualized 12. Pare et al, Am J Gastroenterol 2001; 96: 3130–7 13 & 13. Johanson Kralstein, Aliment Pharmacol Ther 2007; 25: 599–608 14. American College of Gastroenterology, Supplement 1, January 2009 15. Barish CF, et al Dig Dis Sci 2010; 55: 1090-1097 40
References Cont. 16. Johanson JF, et al Am J Gastroenterol. 2008:103:170-177 17. AMITIZA [package insert]. Bethesda, MD: Sucampo Pharma Americas, Inc.; 2011. 18. Johanson et al, Am J Gastroenterol 2008; 103: 170—7 19. Chey et al., 2012 Safety and patient outcomes with lubiprostone for up to 52 weeks in patients with irritable bowel syndrome with constipation. Aliment Pharmacol Therapeutics 20. Salmoirago-Blotcher E et al. Am J Med.2011 Aug:124(8):714-23. 21. Caprilli R, Frieri G, Marchetti G, Giambartolomei S. Ion transport in the colon. Minerva Gastroenterol Dietol. 1995 Dec;41(4):289- 301 22. Pappagallo, M. Prevalence and Mgmt of OBD. American Journal of Surgery, 2001 23. Panchal et a. OBD: prevalence, pathophysiology and burden. Int J Clin Pract 24. Hess, B. Attitude of Swiss Physicians towards OIC: A National Survey. European Journal of Internal Medicine, 2001 25. Reimer K et al. Meeting the Challenge of OIC in Cronic Pain MGMT—A Novel Approach. Pharmacology, 2009 26. Choung RS. OBD and Narcotic Bowel Syndrome: A Population-Based Study. AM J Gastroengerol 27. National Digestive Diseases Information Clearinghouse. Constipation. NIH Publication No. 07-2754, 2007. 28. Colluzzi, F. Opiod Therapy for Chronic non-Cancer Pain: Practice Guidelines for Initiation and Maintenance of Therapy. Minerva Anestesoil, 2007 29. Thomas J. OBD. J Pain and Symptom Mgmt. 200830. Sloots EJ, et al. Efficacy and Safety of Prucalopride in Patients with Chronic Non-cancer Pain Suffering from OIC. Dig Dis Sci, 2010. 31. Becker G. Novel opioid antagonists for oIBD and Postoperatived Ileus. Lancet, 2009. 32. The Cochrane Collaboration, 2011 Wiley Laxatives or Methylanaltrexone for the Mgmt of Constipation in Palliative Care Patients 33. Sucampo press release Feb. 2, 2012 41

References Cont. 34. Dr. Byron Cryer DDW 2010 , abstract 780 958 35. Arch Ophthalmology, 2004, 122:532-538 36. IMS Health 37. Catalina 2011 38. American Academy of Ophthalmology 39. Input from KOLs 40. 2010 AAO Preferred Practice Patterns for POAG 41. 2010 AOA Practice Guidelines 42. Yu DY et al. Invest Ophthalmol Vis Sci. 1994;35:4087-4099. 43. Kern TS. Exp Diabetes Res. 2007;2007:95013. 44 Hardy P et al Prostaglandins Acids 301 325 44. al. Leukot Essent Fatty Acids. 2005;72(5):301-325. 45. Alm A et al. Exp Eye Res. 2009;88:760-768. 46. Toris CB et al. Arch Ophthalmol. 2004;122:1782-1787. 47. Llobet A et al. News Physiol Sci. 2003;18:205-209 48. Kojima S et al. Nippon Ganka Bakkai Zasshi. 1997:101;605-610. 49. Makimoto Y et al. Jpn J Ophthalmol. 2002;46:31-35. 50. Kimura I et al. Jpn J Ophthalmol. 2005;49:287-293 51. Sugiyama T et al. Arch Ophthalmol. 2009;127:454-459 52. Inoue K et al. Clinical Ophthalmology 2011:5 1003-1005 53. Hayami K et al. Ophthalmic Res. 2001 Jul-Aug;33(4):203-9 54. Melamed S. Drugs Exp Clin Res 2002;28(2-3):63-73. 42

References Cont. 55. Ishida T al. Topical Monotherapy for Normal Tension Glaucoma-Comparison of Long-term Monotherapies in Maintaining Visual Field. Ophthalmology 47:1107-1112,2005. 56. ARVO 2011, Poster#4992,A416 57 Yamamoto S et al ARVO 2011 Microperimetry Shows Protection of Central Vision in with 57. S, al. 2011, Retinitis Pigmentosa Patients Treated UF-021: A Phase 2 Study. Poster # 4992, A416 58. NDA Filing 59. AMITIZA® (lubiprostone) [prescribing information]. Bethesda, Md: Sucampo Pharmaceuticals, Inc. 2008.\ 60 Lembo AJ al Long Term Safety and Lubiprostone ClC 2) Activator 60. Lembo, AJ, et al. Long-Effectiveness of Lubiprostone, a Chloride Channel (ClC-Activator, in Patients with Chronic Idiopathic Constipation. Dig Dis Sci (2011) 56:2639-2645 43
