Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - OMNI BIO PHARMACEUTICAL, INC. | c26736e8vk.htm |
| EX-99.1 - EXHIBIT 99.1 - OMNI BIO PHARMACEUTICAL, INC. | c26736exv99w1.htm |
Exhibit 99.2

| Noble Financial Capital Markets' Eighth Annual Equity Conference Hollywood, FL January 18, 2012 Pharmaceutical, Inc. (OTC:OMBP) 1 |

| Forward-Looking Statements Except for the historical information contained herein, the matters discussed in this presentation are forward-looking statements. The forward-looking statements in this presentation are based on information available to us as of the date any such statements are made and we assume no obligation to update these forward-looking statements. These statements are subject to risks and uncertainties that could cause actual results to differ materially from those described in the statements. These risks and uncertainties include the risks described from time to time in our reports filed with the Securities and Exchange Commission, including our most recent annual report on Form 10-K, subsequent quarterly reports on Form 10-Q and current reports on Form 8-K, all of which are available at www.sec.gov. We refer you to the "Forward- Looking Statements" and "Risk Factors" sections of these filings. 2 |

| 2012 Omni Bio Controlling Inflammation and Autoimmunity 3 |

| Antioxidant Mimetic (BMX-001) Program Alpha-1-Antitrypsin (AAT) Program Largest Shareholder 25% now 40% after next funding round BioMimetix Treatment of Cancer and Radiation Therapy Plasma-derived AAT Treatment of Autoimmune Diseases Omni Bio Synthetic AAT 4 |
| CEO: James D. Crapo CFO: Robert C. Ogden BMX-001 - 2010 Patent Licensed from Duke University BioMimetix Scientists: James D. Crapo, M.D. Irwin Fridovich, Ph.D. Ines Batinic-Haberle, Ph.D. Ivan Spasovic, Ph.D. David Warner, M.D. Jon Piganelli, Ph.D. Rebecca Oberley-Deegan, Ph.D. National Jewish Health Duke University Duke University Duke University Duke University University of Pittsburgh National Jewish Health Drug Development Leader: Shayne Gad, Ph.D. Antioxidant Program 5 |

| CEO: James D. Crapo CFO: Robert C. Ogden Fc-AAT - 2003 and 2011 Patent Applications License pending from University of Colorado Method of Use Patents - 2000-2011 OmniBio Founding Scientist, Omni Bio Chief Scientific Officer, Omni Bio Chair, SAC , Omni Bio University of Michigan Ben Gurion University YdbY Biotech Virginia Commonwealth University Fred Hutchinson Cancer Center Alpha-1-Antitrypsin Program Leland Shapiro, M.D. Charles A. Dinarello, M.D. Michael Iseman, M.D. Pavan Reddy, M.D. Eli Lewis, Ph.D. Soohyun Kim, Ph.D. Antonio Abbate, MD Joachim Deeg, MD 6 Scientists: |

| Two Potential New Drugs Synthetic Human Fc-AAT Fusion protein possessing the body's most powerful anti-inflammatory molecule - AAT Metalloporphyrin antioxidant mimicking the body's most powerful antioxidant enzyme BMX-001 7 |

| BioMimetix Controlling Inflammation Using Mangano Porphyrin Antioxidant Mimetics BioMimetix is a private corporation funded by its largest stockholder, OmniBio 8 |

| A Mangano Porphyrin Antioxidant Mimetic N N N N N N N N R R Mn + + + + + R R 9 |

| Mimetics Protect against Lung Radiation Damage Histology 16 weeks post 28 Gy hemithoracic RT Mimetic (MnTE-2-PyP), 6 mg/kg s.c. for 2 weeks after RT B Gauter-Fleckenstein et al., Free Rad Biol Med 44:982, 2008 Control (no RT, PBS) RT + PBS RT + Mimetic Hematoxylin & Eosin 10 |

| Radiation-Induced Injury to the GI Tract Radiation Field - 100 KvP spread out Bragg peak, incorporating the rectum 21.5 Gy single dose Mimetic (MnTE-2-PyP): 5mg/kg i.p. 1 hour prior or post irradiation and then weekly 11 |
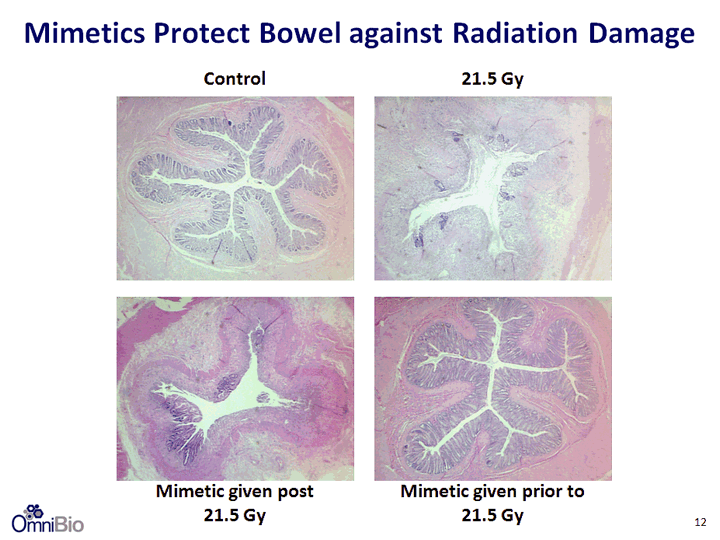
| Control 21.5 Gy Mimetic given post 21.5 Gy Mimetic given prior to 21.5 Gy Mimetics Protect Bowel against Radiation Damage 12 |

| Mimetics Protect Normal Tissues and Inhibit Tumor Regrowth following Radiation Therapy Tumor Regrowth following Radiation Therapy Tumor Regrowth following Radiation Therapy 13 |

| Human Colon Cancer (HCT116) Growth Inhibition In Vivo Control Radiation Drug+Radiation 0 2 305 340 325 4 415 385 330 6 535 420 340 8 685 490 335 10 785 480 325 12 915 495 295 14 965 505 292.5 16 1015 515 290 18 560 295 20 595 345 22 642.5 377.5 24 710 410 26 760 460 28 815 515 30 1040 680 32 725 34 765 36 830 38 895 Courtesy of Ben Moeller & Mark Dewhirst Untreated Tumor Radiation- treated Radiation + Mimetic (MnTE-2-PyP) 14 |

| Plan for Clinical Development of BMX-001 Scale-up Synthesis Demonstration Batch cGMP Drug Product Preclinical Safety/Tox IND Human Safety Cancer/Radiation Protection Purity Efficacy Safety 100 grams Stability Characterize Impurities 1 Kilo Timetable 2012 2013 Clinical Trials 2014 15 |

| Batinic-Haberle, Rajic, Keir, Tovmasyan, Dewhirst and Bigner BMX-001 Enhances Therapy of Glioblastoma Sc xenografts, 3 x 1 Gy radiation, 5 x 5 mg/kg temozolomide and ~ 48 days 3.2 mg/kg bid sc of BMX-001 Control BMX-001 Temo Rad Temo+Rad BMX-001+Rad BMX-001+Temo BMX-001+Rad+Temo Tumor Volume Days Radiation + Temo Radiation + Temo + BMX-001 16 |

| Market Opportunities Cancer Radiation Therapy In 2004, nearly 1 million patients were treated with radiation therapy* This is a potential multi-billion dollar market *Source: American Society for Radiation Oncology 17 |

| Planned Therapeutic Targets BMX-001 Cancer / Radiation Therapy Glioblastoma Head and Neck Cancer Irradiation Prostate Cancer Irradiation CNS Indications Morphine Tolerance Stroke Spinal Cord Injury 18 |

| Alpha-1-Antitrypsin (AAT) The body's natural anti-inflammatory circulating protein Prescribed for emphysema in AAT deficient patients - $500M market (Grifols, Baxter, CSL Behring, Kamada) Administered once a week by infusion One of best safety records of any biological FDA approved - and marketed for > 20 years 19 |
| 20 |

| New Indications for AAT Therapy: A Broad Spectrum of Inflammatory and Immunological Diseases Type 1 Diabetes Lewis et al., PNAS 2005 Strom et al., PNAS 2005 Ma et al., Diabetologia 2010 Bone-marrow Transplantation Marcondes et al., Blood 2011 Tarawa et al., PNAS 2011 Acute Myocardial Infarction Toldo et al., J Mol Cell Cardiol 2011 Inflammatory Bowel Disease 21 |

| Alpha-1-Antitrypsin (AAT) (for treatment of multiple new indications) The Problem - Limited Supply and High Cost AAT is derived by purification from human blood (plasma) The Solution - Make a Synthetic Protein Multiple companies are working on creating synthetic AAT using recombinant technology 22 |

| N full-length human AAT hinge Omni Solution: Synthetically Produced Human Fc-AAT AAT AAT Fc Fc Fusion Protein -S-S- -S-S- Fc fragment of immunoglobulin 23 |
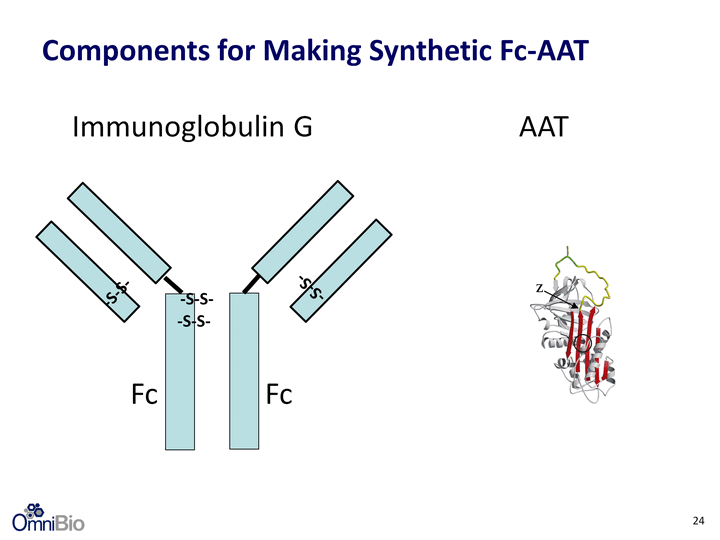
| AAT Immunoglobulin G Fc -S-S- -S-S- -S-S- -S-S- Fc Components for Making Synthetic Fc-AAT 24 |

| Fc -S-S- -S-S- Fc 100 to 1000 times more potent than plasma-derived AAT One-step Purification Novel Molecule - Patent Application Filed Fusion Protein - Fc-AAT 25 |

| pCAG.neo/huIgG1aFc AAT Gene Affinity Purification Fc-AAT Cell Culture one-step purification Production and Purification of Fc-AAT 26 |

| Comparison of Plasma-Derived AAT with Fc-AAT on Endotoxin-Induced IL-1^ (human adherent blood monocytes) endotoxin 500 250 125 5 2.5 1.25 0.65 0 100 200 300 400 500 Plasma-derived AAT (µg/mL) Synthetic Fc-AAT (µg/mL) 350-fold difference 100-fold difference Interleukin-1^ (pg/mL) 27 |

| Enhanced ease and speed of purification Reduced inactivation of AAT Less oxidation No polymerization Prolonged plasma and tissue half-life Targeting of AAT to myeloid cells (which have an Fc receptor) Possible Mechanisms of Enhanced Potency of Fc-AAT 28 |

| Plan for Clinical Development of Synthetic Fc-AAT Scale-up Synthesis Demonstration Batch cGMP Drug Product Preclinical Safety/Tox IND Human Safety Type 1 Diabetes Graft vs. Host Disease (GVHD) Myocardial Infarction Purity Efficacy Safety 100 grams Stability Characterize Impurities 1 Kilo Timetable 2012 2013 Clinical Trials 2014 29 |

| Fc-AAT Naturally occurring circulating protein Bound to Fc Produced in CHO cells Affinity Purification Enbrel Naturally occurring circulating protein Bound to Fc Produced in CHO cells Affinity Purification Enbrel is a Successful Model for the Clinical Development of Fc-AAT 30 |

| Omni - 2012 Human Clinical Trials Plasma-Derived AAT Type 1 Diabetes Placebo-controlled 12 week trial with 1 year follow-up Graft vs. Host Disease (GVHD) Open label trials of steroid-resistant GVHD 31 |

| Type 1 Diabetes Market Opportunity Annually, more than 15,000 children and 15,000 adults are diagnosed with Type 1 diabetes.* The rate of Type 1 diabetes incidence among children under the age of 14 is estimated to increase by 3% annually worldwide.* *Source: Juvenile Diabetes Research Foundation (JDRF) 32 |

| Develop BMX-001 to IND Develop synthetic Fc-AAT to an IND Clinical trials of plasma-derived AAT Type 1 diabetes Graft vs. host disease Form strategic partnership with an AAT manufacturer Omni - Objectives - 2012/13 33 |
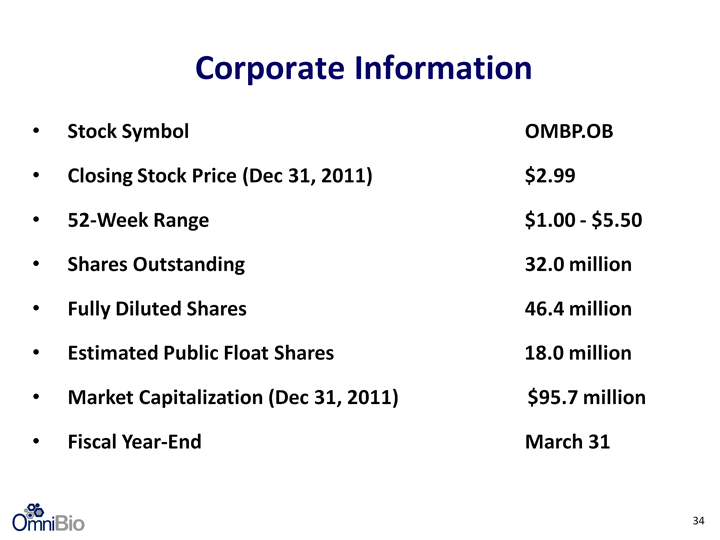
| Stock Symbol OMBP.OB Closing Stock Price (Dec 31, 2011) $2.99 52-Week Range $1.00 - $5.50 Shares Outstanding 32.0 million Fully Diluted Shares 46.4 million Estimated Public Float Shares 18.0 million Market Capitalization (Dec 31, 2011) $95.7 million Fiscal Year-End March 31 Corporate Information 34 |

| Our Team Chief Executive Officer - James D. Crapo, MD - Professor of Medicine-National Jewish Hospital (1996-Present) - Chief, Division of Allergy, Critical Care and Respiratory Medicine-Duke University (1979-1996) - Chairman, Dept of Medicine, National Jewish Health (1996-2004) - Prior Experience as CEO of Aeolus (AOLS) and Chairman of Lifevantage (LFVN) - MD, University of Rochester Chief Scientific Officer - Charles A. Dinarello, MD - Professor of Medicine-University of Colorado Denver - Member of the National Academy of Sciences - World's fourth most-cited scientist for 20 years (1982-2003) - Numerous international awards : - 2008 Sheik Hamdan Prize (United Arab Emirates) - 2009 Crafoord Prize (Royal Swedish Academy of Sciences) - 2009 Albany Medical School Prize (largest prize in medicine in USA) - 2010 Paul Ehrlich Prize (Germany) and Novartis Prize in Immunology - MD, Yale University 35 |

| Our Team Chief Financial Officer - Robert C. Ogden, CPA 20 plus years in senior financial management positions Significant experience as CFO of NASDAQ and microcap companies CPA with major public accounting firm BS Commerce, University of Virginia Chairman of Scientific Advisory Board - Michael D. Iseman, MD Professor of Infectious Diseases-National Jewish Hospital Considered one of top experts in the world on tuberculosis 36 |

| Thank you for your interest Thank you for your interest! omnibiopharma.com (720) 488-4708 (Corporate & Investor Inquiries) 37 |

| Appendices 38 |

| Toll-4 Receptor Expression by Islet Macrophages in Response to LPS Fc-AAT vs. plasma-derived AAT Fc-AAT vs. plasma-derived AAT Fc-AAT vs. plasma-derived AAT Fc-AAT vs. plasma-derived AAT 500 µg/ml 500 ng/ml; 100 ng/mil LPS 10 ng/ml Control PBS Plasma Fc-AAT 39 |

| Toll-4 Receptor Expression by Islet Macrophages in Response to LPS Fc-AAT vs. plasma-derived AAT Fc-AAT vs. plasma-derived AAT Fc-AAT vs. plasma-derived AAT Fc-AAT vs. plasma-derived AAT ~1,000-Fold Difference 500 µg/ml 500 ng/ml; 100 ng/mil LPS 10 ng/ml Control PBS Plasma Fc-AAT 40 |

| * Plasma-Derived AAT Reduces Acute Myocardial Infarct Size Myocardial Infarct Size Myocardial Infarct Size Plasma-derived AAT at 2 mg/mouse/day x 7 days Toldo et al., J Mol Cell Cardiol, 51:244, 2011 AMI *† 41 |
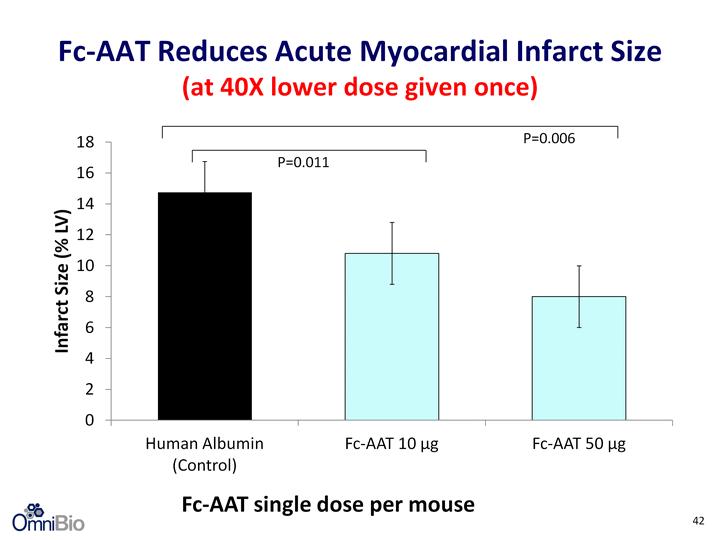
| Fc-AAT Reduces Acute Myocardial Infarct Size (at 40X lower dose given once) (at 40X lower dose given once) (at 40X lower dose given once) Fc-AAT single dose per mouse P=0.006 P=0.011 42 |
