Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Orexigen Therapeutics, Inc. | d280254d8k.htm |
 Exhibit 99.1 |
 2
Forward-Looking Statements
Forward-Looking Statements
This presentation contains forward-looking statements about Orexigen Therapeutics, Inc.
Words such as "believes," "anticipates," "plans,"
"expects,"
"indicates,"
"will,"
"intends,"
"potential,"
"suggests,"
"assuming,"
"designed,“
“probability”
and
similar
expressions
are
intended
to
identify
forward-looking
statements.
These
statements
are
based
on
the
Company's
current
beliefs
and
expectations.
These forward-looking statements include statements regarding: a clear and feasible path
forward for Contrave ®; the protocol for, and the timing and feasibility of, the
Contrave cardiovascular outcomes trial (CVOT); the initiation of enrollment for the CVOT in
second quarter of 2012; the expected rate of enrollment of the CVOT; the probability of success
of the CVOT; the potential for, and timing of, resubmission and approval of an NDA based
on interim results of the CVOT; the prospects for ultimate approval of an NDA for
Contrave; the potential to maintain the Company’s existing North American collaboration with Takeda Pharmaceuticals; estimates
of
the
potential
market
for
Contrave;
and
the
sufficiency
of
the
Company’s
existing
cash
to
fund
the
Company’s
operations
through
potential approval of Contrave in 2014. The inclusion of forward-looking statements should
not be regarded as a representation by Orexigen that any of its plans will be achieved.
Actual results may differ from those set forth in this presentation due to the risk and
uncertainties inherent in Orexigen’s business, including, without limitation: the
uncertainty of the FDA approval process, including requirements for additional clinical
and non-clinical studies or other commitments prior to the submission and approval of an NDA for
Contrave; Orexigen's ability to demonstrate that the risk of major adverse cardiovascular
events in overweight and obese subjects treated with Contrave does not adversely affect
the product candidate’s benefit-risk profile; the potential for FDA’s planned 2012
public
advisory
committee
meeting
on
obesity
drug
development
to
result
in
additional
NDA
approval
requirements
for
Contrave
as
well as post-approval commitments; Orexigen’s dependence on Takeda Pharmaceuticals for
aspects of the development and commercialization of Contrave; reliance on third parties
to manufacture and supply Contrave and assist with the conduct of the CVOT; the
potential for adverse safety findings relating to Contrave; intense competition in the obesity marketplace and the potential
for new products to emerge that provide different or better therapeutic alternatives for
obesity and weight loss compared to Contrave; and other risks described in the Company's
filings with the SEC. You are cautioned not to place undue reliance on these
forward-looking statements, which speak only as of the date hereof, and Orexigen undertakes
no obligation to revise or update this presentation to reflect events or circumstances
after the date hereof. Further information regarding these and other risks is included
under
the
heading
"Risk
Factors"
in
Orexigen’s
Quarterly
Report
on
Form
10-Q,
which
was
filed
with
the
SEC
on
November
10,
2011
and
is
available
from
the
SEC's
website
(www.sec.gov)
and
on
the
Company’s
website
(www.orexigen.com)
under
the
heading
"Investor Relations”. All forward-looking statements are qualified in their
entirety by this cautionary statement. This caution is made under the safe harbor
provisions of Section 21E of the Private Securities Litigation Reform Act of 1995. |
 3
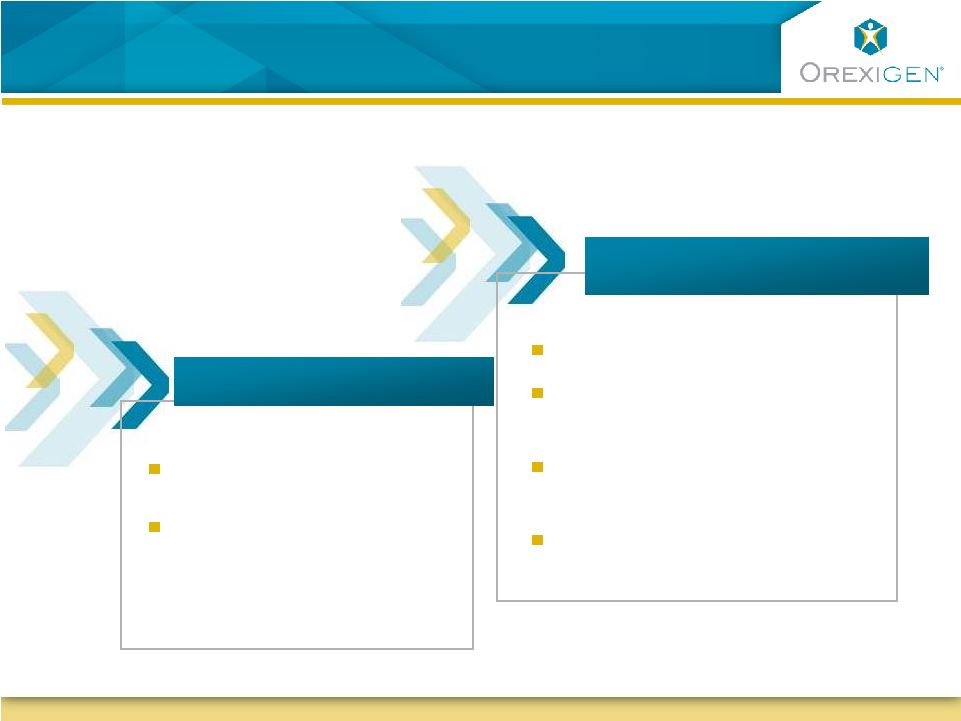
Large, growing market
Unmet need, limited
competition
Differentiated product
profile
Skilled partner with heavy
resourcing
Keys to Commercial Success
Keys to Commercial Success
Orexigen: Poised to Deliver
Orexigen: Poised to Deliver
Clear path to approval
High probability of
technical success
Keys to Regulatory Success
Keys to Regulatory Success |
 |
 5
Regulatory Direction is Clear
Regulatory Direction is Clear
Complete NDA already reviewed by FDA
–
Efficacy threshold has been met
–
General safety & tolerability profile established
–
Positive Ad-Com
A single approval deficiency of theoretical CV risk was identified in CRL
Clarity on the requirement to satisfactorily address the CRL was
obtained through a formal dispute resolution process
–
Approval obtainable based on acceptable interim analysis
–
Key trial elements and risk exclusion criteria were defined and not subject
to change based on upcoming obesity Advisory Committee
Subsequent protocol discussions with FDA maintained alignment and
clarity on path to approval |
 6
Key Elements for CVOT Trial
Key Elements for CVOT Trial
Primary endpoint ITT analysis of MACE (CV death, MI, stroke)
Exclude a doubling of MACE at interim to obtain approval
Exclude a 40% increase in MACE at final to remain on market
Real-world test of Contrave-
long term exposure to study drug is only
in those experiencing benefit from therapy
–
Patients with limited weight loss or increased blood pressure discontinue
study drug at Week 16, but remain in ITT analysis
Enroll patient population targeting a background MACE rate of 1.5%
per year |
 7
Large But Streamlined Trial
Large But Streamlined Trial
Sole focus to address the single approval
deficiency in CRL
Excluding low CV risk requires large
sample size, but limited information per
subject
–
No ongoing labs
–
Infrequent visits
–
Minimal data collection
Cost per patient lower than traditional
clinical development programs
World-class advisors assembled to steer
the study
Total cost of
Contrave CVOT to
interim analysis
and approval is
estimated at
~$100M
*
*based on Orexigen current estimates (01/09/12) |
 8
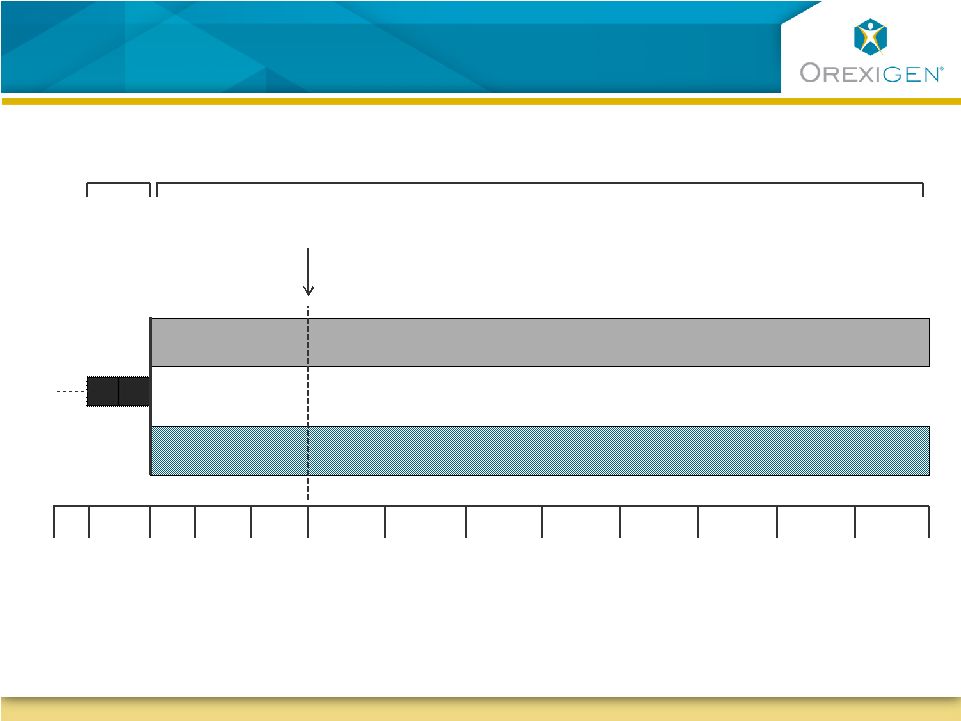
CV Outcomes Study Design
CV Outcomes Study Design
Contrave32 + Weight Management Program
Placebo + Weight Management Program
Wk
208
Wk
182
Wk
130
Wk
104
Wk
78
Wk
52
Wk
26
Wk
16
Wk
156
Wk
8
Wk
2
Day
1
Wk
-2
Evaluation of appropriateness of treatment
Lead-in
Treatment Period |
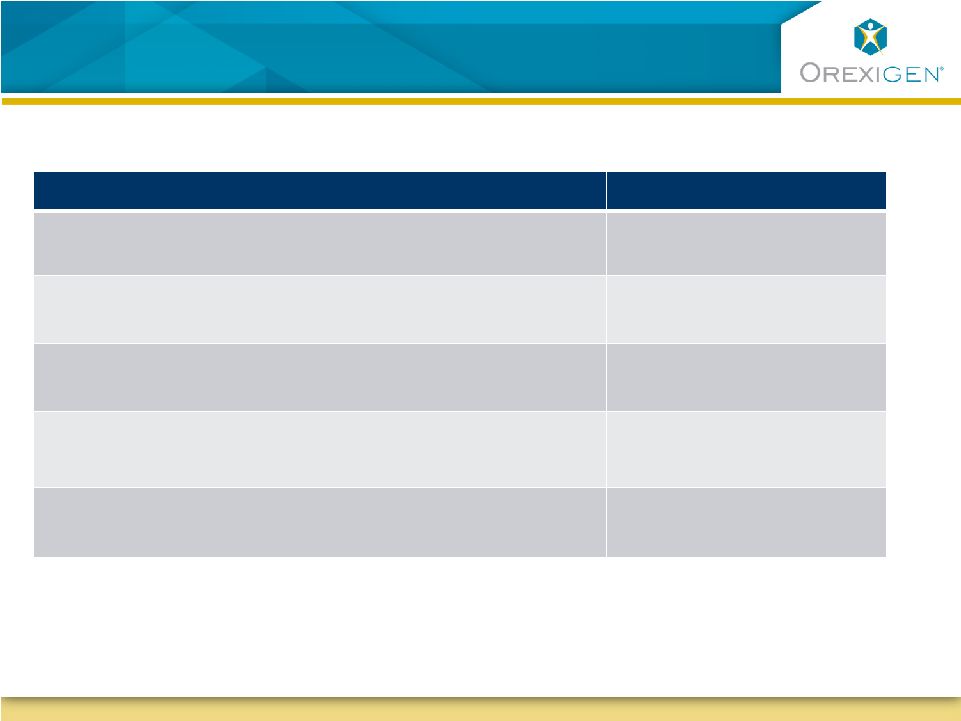 9
Timeline Assumptions that Lead to a Potential
2014 Approval
Timeline Assumptions that Lead to a Potential
2014 Approval
Development Milestone
Estimated Time
First Patient Visit
Late Q2 2012
Enroll Trial and Accrue 87 CV Events for Interim
Analysis
18-20 months
Time to Prepare Resubmission
3-4 months
Regulatory Review (Type II)
6 months
Potential Approval
H2 2014 |
 10
Probability of Success of CVOT is High
Probability of Success of CVOT is High
Hurdle for approval is not based on demonstrating CV benefit, but
instead requires excluding a doubling of CV risk in non-inferiority
design
Bupropion well characterized in >50M patients over >25 years
–
Large patient registries and AERS have not identified evidence of
increased CV events
Risk Engine modeling of 1-year Contrave Phase 3 data predicts
reduced
10-year CV risk compared to placebo
Trial focus on responders to drug therapy allows real-world test
–
Long term exposure to study drug is only in those experiencing benefit
from therapy |
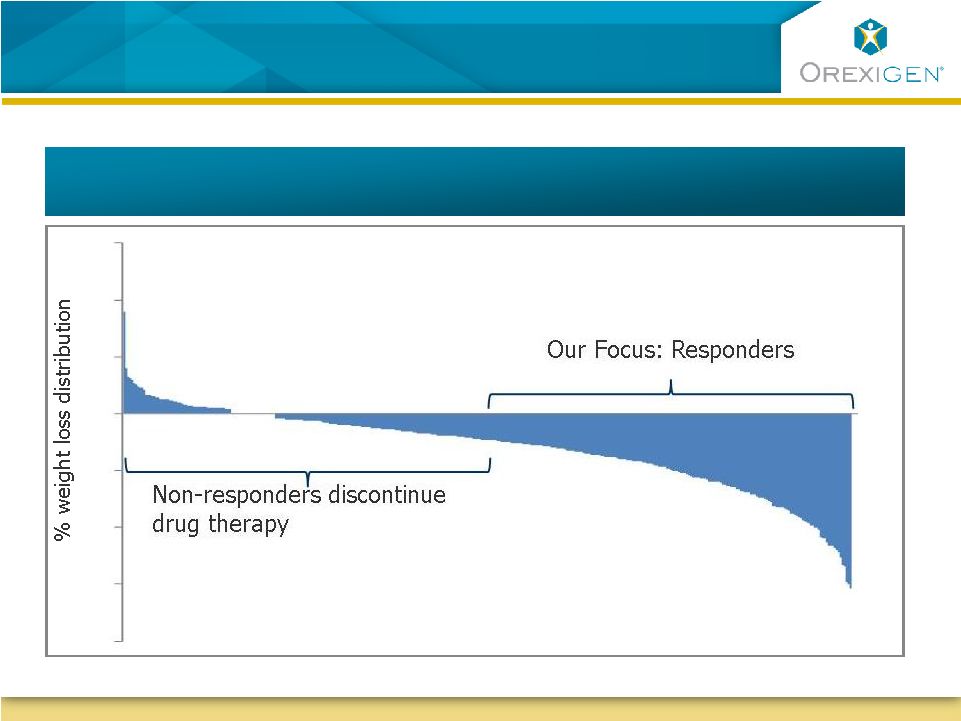 11
-
40
-
30
-
20
-
10
0
10
20
30
Our Focus is on Responders to Contrave Therapy
Our Focus is on Responders to Contrave Therapy
Illustrative : Typical distribution of weight loss response to obesity therapy
|
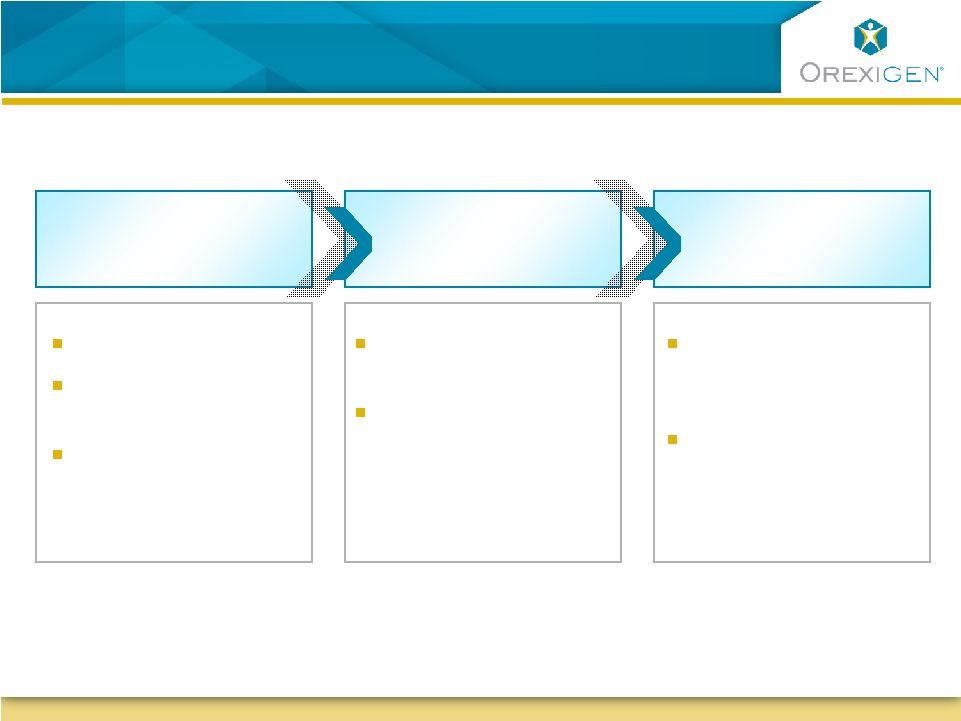     12
Proposed Treatment Algorithm Identifies Early
Responders for Continued Long-term Therapy
Proposed Treatment Algorithm Identifies Early
Responders for Continued Long-term Therapy
Appropriate BMI
Motivated to Adhere
to Lifestyle Change
Inadequate
Response to Diet &
Exercise
Select Responders
for Continued
Treatment
Reinforce Adherence
to Appropriate Use
Evaluate Adherence
to Appropriate Use
Assess Weight & BP
Response to
Therapy
Screening
16-Week
Therapeutic Trial
Long-Term
Therapy |
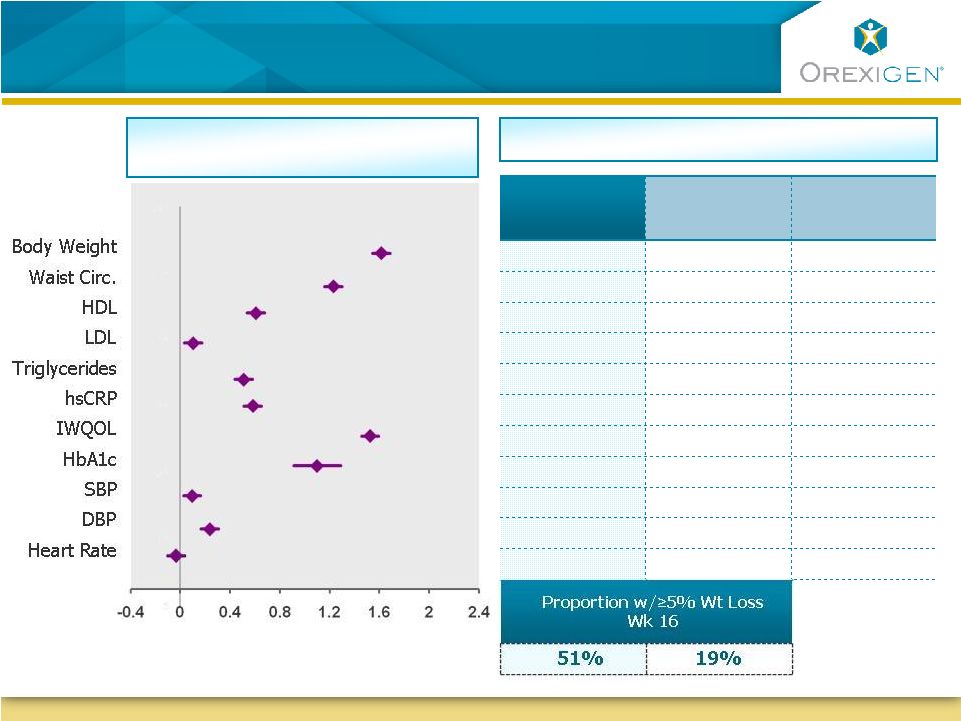 13
Contrave Responders Achieve Improvements Across
Multiple Cardiometabolic Parameters
Contrave Responders Achieve Improvements Across
Multiple Cardiometabolic Parameters
Improvement
(Standardized Mean Change from Baseline)
Contrave32 Standardized Mean
Change from Baseline at 1 Year
Change from Baseline at 1 Year
Contrave
Contrave
Responder
Responder
N=1038
N=1038
Placebo
Responder
N=254
All Placebo
N=1319
-11.3%
-8.6%
-2.4%
-9.5 cm
-7.2 cm
-3.5 cm
5.2 mg/dL
2.9 mg/dL
0.0 mg/dL
-2.5 mg/dL
1.7 mg/dL
0.0 mg/dL
-16.0%
-9.2%
-2.8%
-38.9%
-36.2%
-14.5%
14.7
12.3
8.3
-1.0%
-0.6%
-0.1%
-0.9 mm Hg
-2.8 mm Hg
-1.6 mm Hg
-1.6 mm Hg
-2.6 mm Hg
-1.3 mm Hg
0.2 bpm
-2.3 bpm
-0.2 bpm |
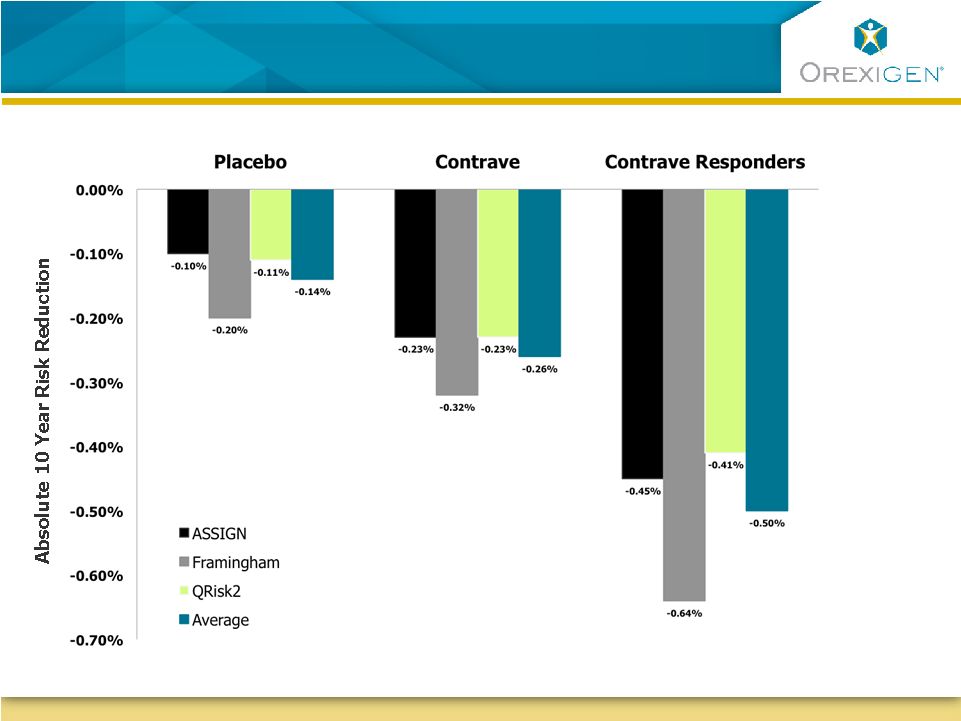 14
Risk Engine Modeling Suggests Long-term CVD Risk
Reduction on Contrave
Risk Engine Modeling Suggests Long-term CVD Risk
Reduction on Contrave |
 |
 16
Contrave Poised for Commercial Success
Contrave Poised for Commercial Success
Market Size / Market Growth
Market Size / Market Growth
Unmet Need / Limited Competition
Unmet Need / Limited Competition
Differentiated Product Profile
Differentiated Product Profile
Blockbuster Building Blocks
Blockbuster Building Blocks
Heavy Resourcing
Heavy Resourcing |
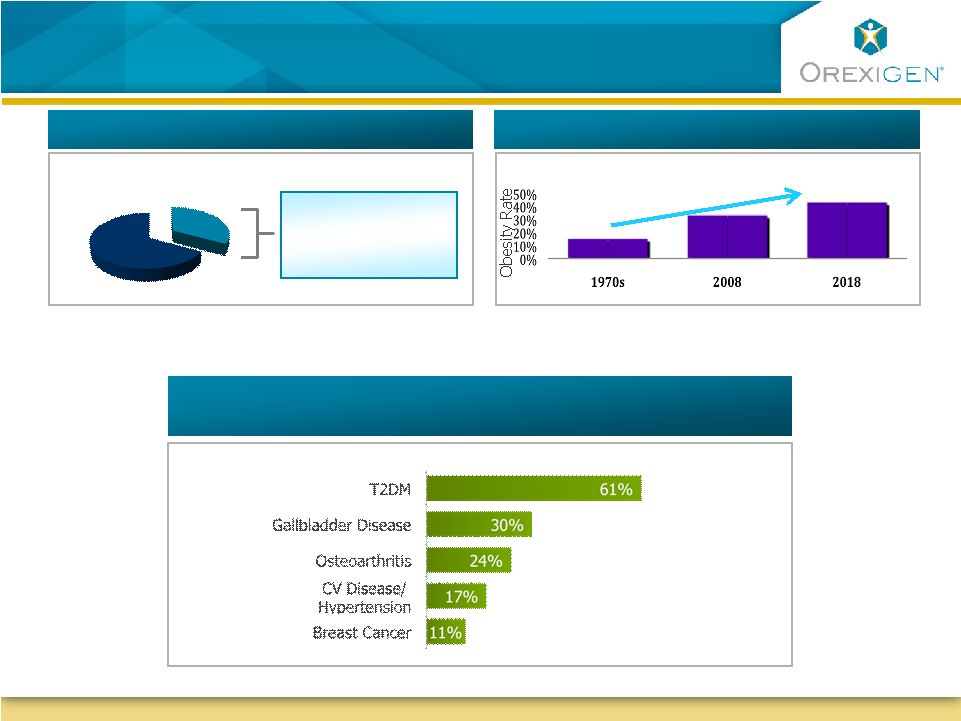 17
103M
US Obesity Rate Growth
Blockbuster Market Growth
Blockbuster Market Growth
Serious Disease Attributable to Obesity
Serious Disease Attributable to Obesity
Obesity: A Large Untapped Market in Need of
Solutions
Obesity: A Large Untapped Market in Need of
Solutions
Source: Wolf, AM, et al. Obes. Res. 1998; 6(2) 97-106
Source: CDC (2010)
Blockbuster Market Size
Blockbuster Market Size
Source: CDC (2009)
US Population
~ 1 in 3 People
in the US
are Obese |
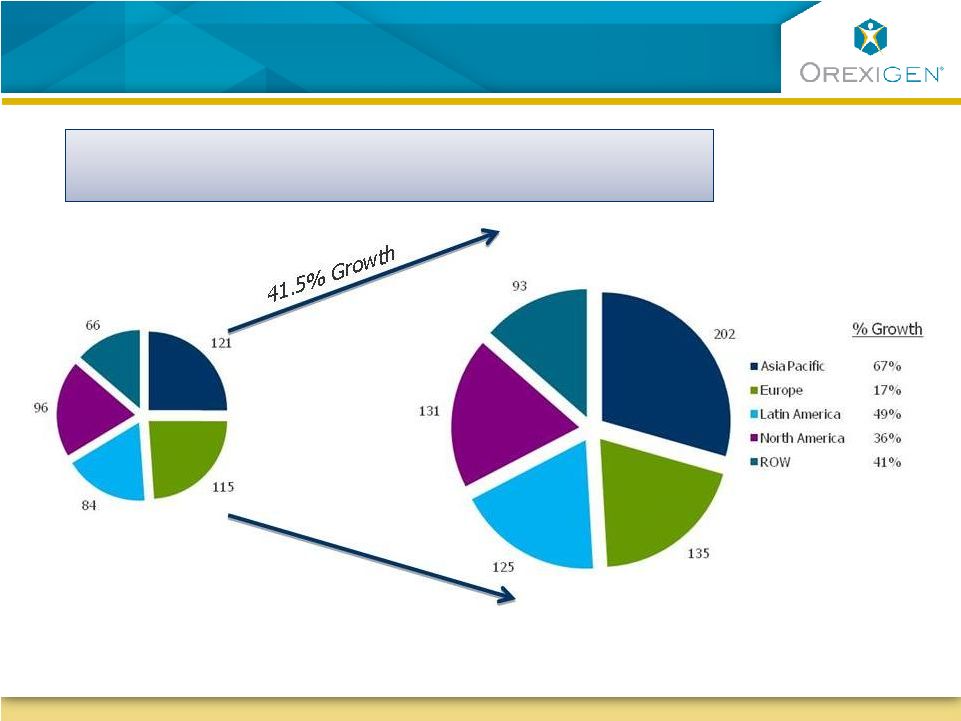 18
Obesity Epidemic is Projected to Grow Globally
Obesity Epidemic is Projected to Grow Globally
n= ~484M people
n= ~484M people
n= ~685M people
n= ~685M people
2010
2010
Source: EuroMonitor, 2010; Timely Data Resources 2010
Source: EuroMonitor, 2010; Timely Data Resources 2010
2020
2020 |
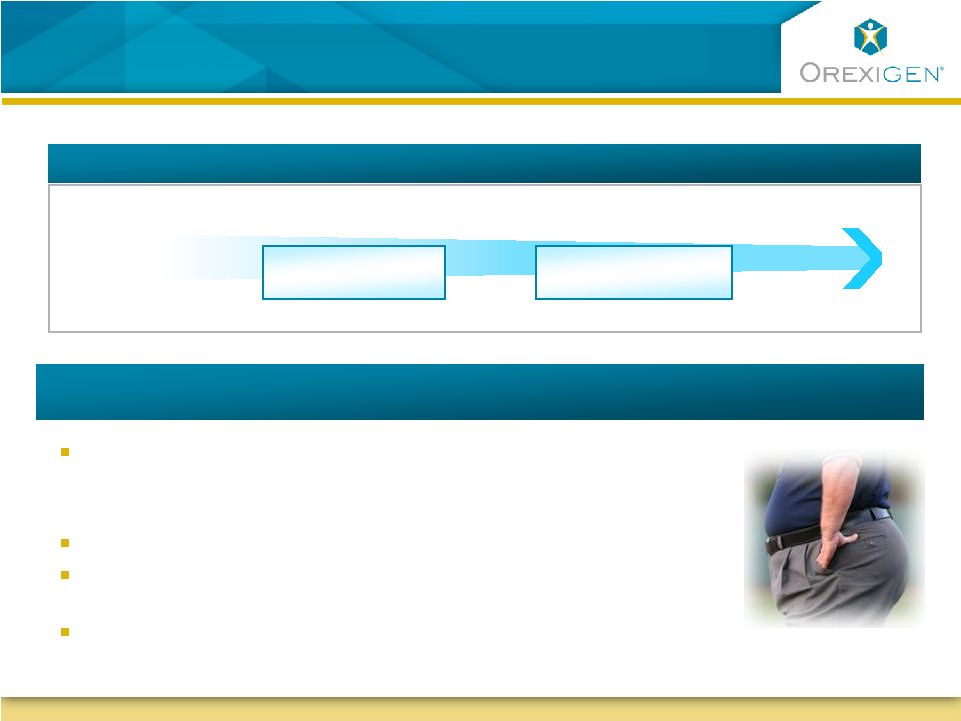 19
Significant Unmet Need in the Market
Significant Unmet Need in the Market
Treatment Options Needed
Treatment Options Needed
T2DM, driven by obesity, is an epidemic
–
1 in 3 adults in the U.S. May have diabetes by 2050 (CDC)
–
Associated with premature death & physical/psychosocial consequences
Weight loss of 5-10% confers significant benefits
Lifestyle change is critical, but weight loss difficult to
achieve/maintain
Large gaps in current treatment paradigm
Majority of Obese are Untreated in an Environment of Limited Treatment Options
BMI
Distribution
of US Pop:
Morbidly Obese
Obese
Overweight
~ 113K People
Treated w/ Surgery
~ 2 Million People
Treated w/ Rxs
Source: Walters Kluwer 2009; Am. J. Surg. 2010
Significant Unmet Need –
Limited Competition |
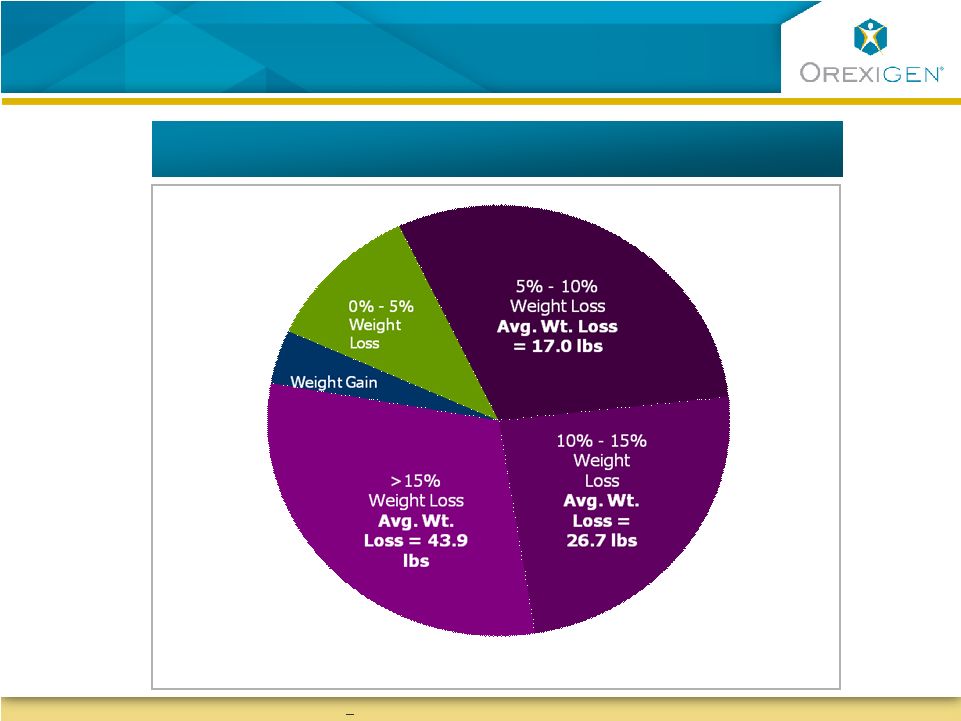 20
Contrave Responders Achieved Significant Weight
Loss
Contrave Responders Achieved Significant Weight
Loss
Responders Maintained Meaningful Weight Loss
Responders Maintained Meaningful Weight Loss
Represents one year data for responders (patients with
>5% weight loss at week 16 per recommended treatment protocol)
|
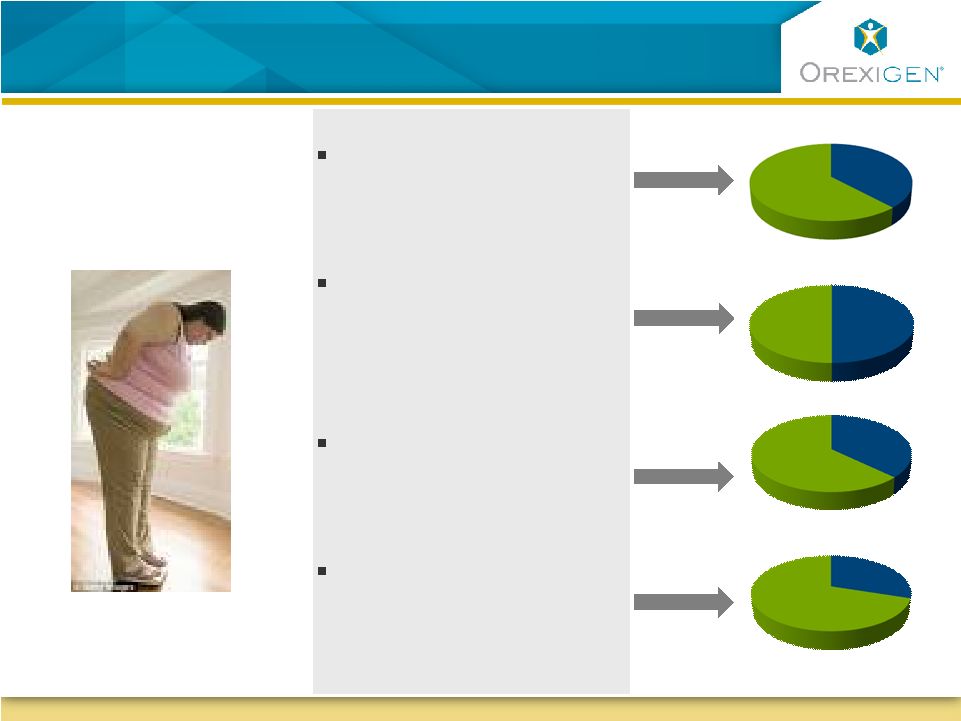 21
Contrave is Uniquely Positioned for Significant
Segments of the Obese Population
Contrave is Uniquely Positioned for Significant
Segments of the Obese Population
Has diabetes and/or
dyslipidemia
Reports having
uncontrollable food
cravings
Is depressed or has
depressive symptoms
Is female, many of
child bearing age
A Typical
Obese Patient:
Sources: Orexigen quantitative market research 2009; IMS NDTI audit data, 6 months ending
3/09 63%
50%
72%
62% |
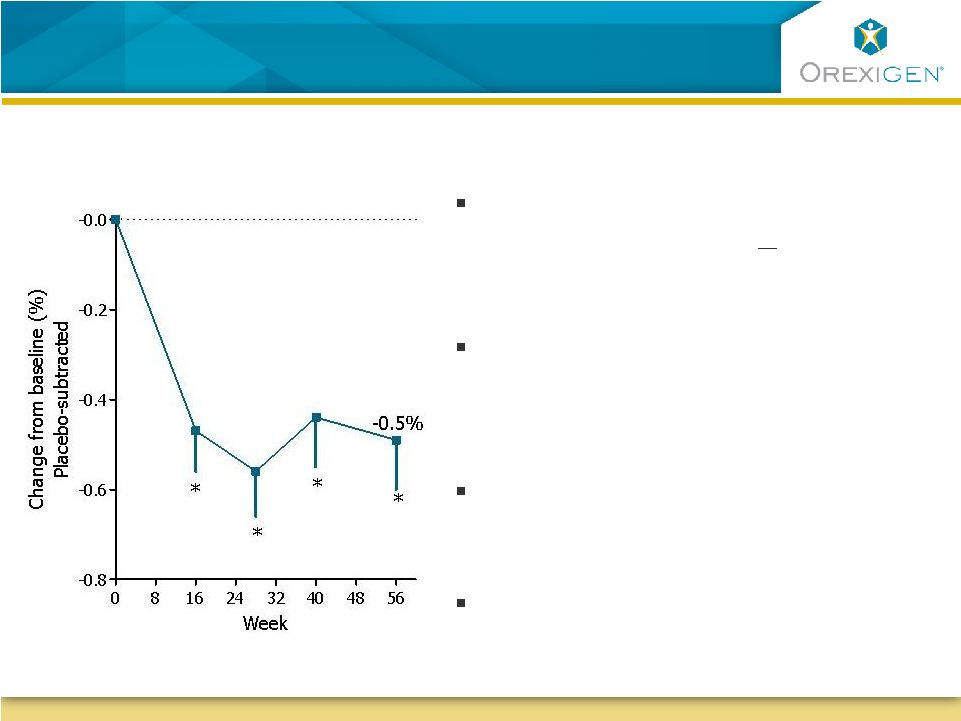 22
Patients With Diabetes On Contrave Achieve
Improvements In Glycemic Control with Weight Loss
Patients With Diabetes On Contrave Achieve
Improvements In Glycemic Control with Weight Loss
HbA1c, change from baseline
ITT-LOCF (Mean baseline A1C = 8.0%)
Half the Contrave patients who
completed therapy lost >5% body
weight
Half the Contrave patients who
completed therapy achieved ADA
HbA1c guideline of <7%
As little as 5% weight loss has been
shown to reduce mortality risk by 25%
1
Patients on Contrave experienced a
reduction in rescue medications
1
Source: Diabetes Care, Vol 23, No.10 Oct (2000) |
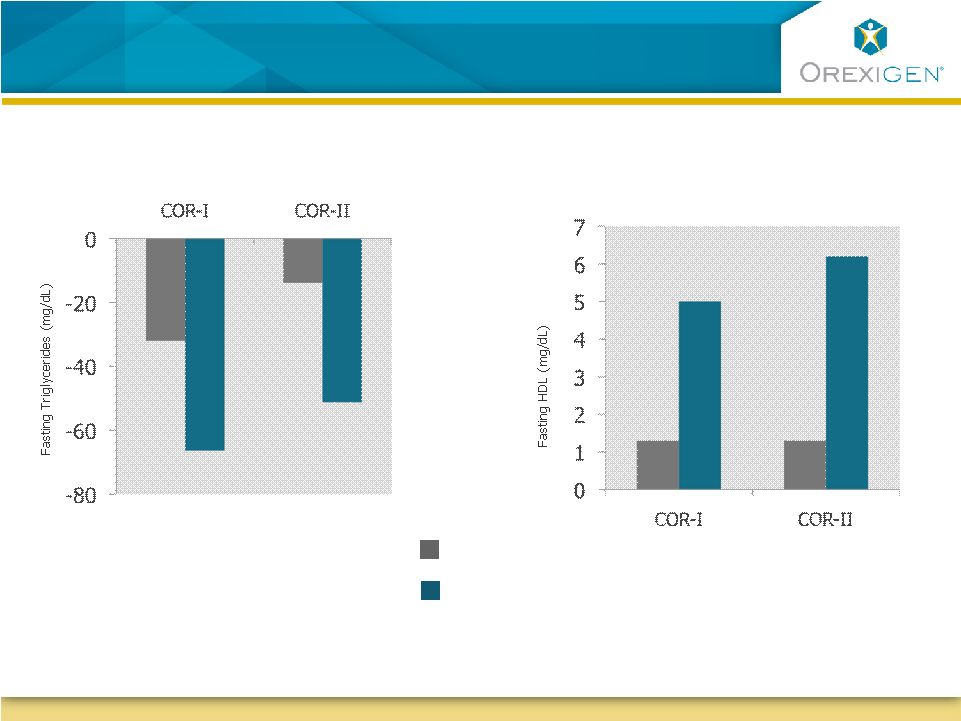 23
Patients with Dyslipidemia Experienced Clinically
Significant Improvement in Lipid Profiles
Patients with Dyslipidemia Experienced Clinically
Significant Improvement in Lipid Profiles
Triglycerides
HDL Cholesterol
Placebo
Contrave32
P < 0.05
P < 0.05
P < 0.05
P < 0.05
Data is for completers in high-risk subgroups. High risk is defined by ATPIII Guidelines;
Baseline 200-222mg/dl for TGs, 40-41 mg/dl for HDL
N=82
N=87
N=69
N=135
N=122
N=121
N=117
N=184 |
 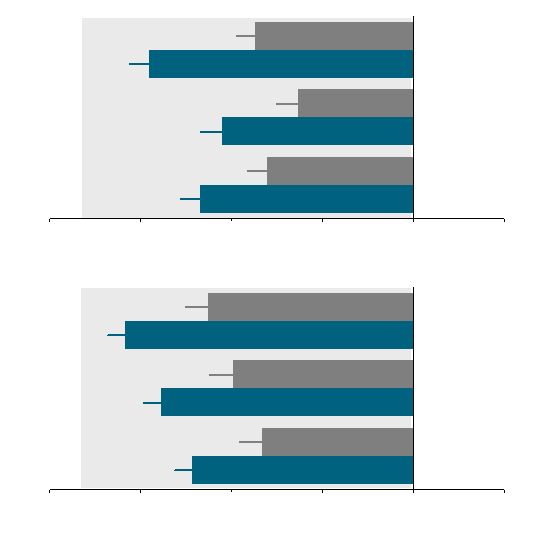 24
Contrave Patients Experienced Statistically Significant
Improvements in Eating Control
Contrave Patients Experienced Statistically Significant
Improvements in Eating Control
-20
-15
-10
-5
0
5
*
*
*
Change from baseline (mm)
-20
-15
-10
-5
0
5
*
*
*
Change from baseline (mm)
Less
More
Placebo (N=511)
Contrave32 (N=471)
How difficult has it been to control your eating?
How often have you eaten in response to food cravings?
How difficult has it been to resist any food cravings?
COR-I
Placebo (N=456)
Contrave32 (N=702)
COR-II
How difficult has it been to control your eating?
How often have you had food cravings?
How difficult has it been to resist any food cravings?
*P<0.05 vs. Placebo; ITT-LOCF; results were measured based on
measurements using 100mm VAS scale Less
More |
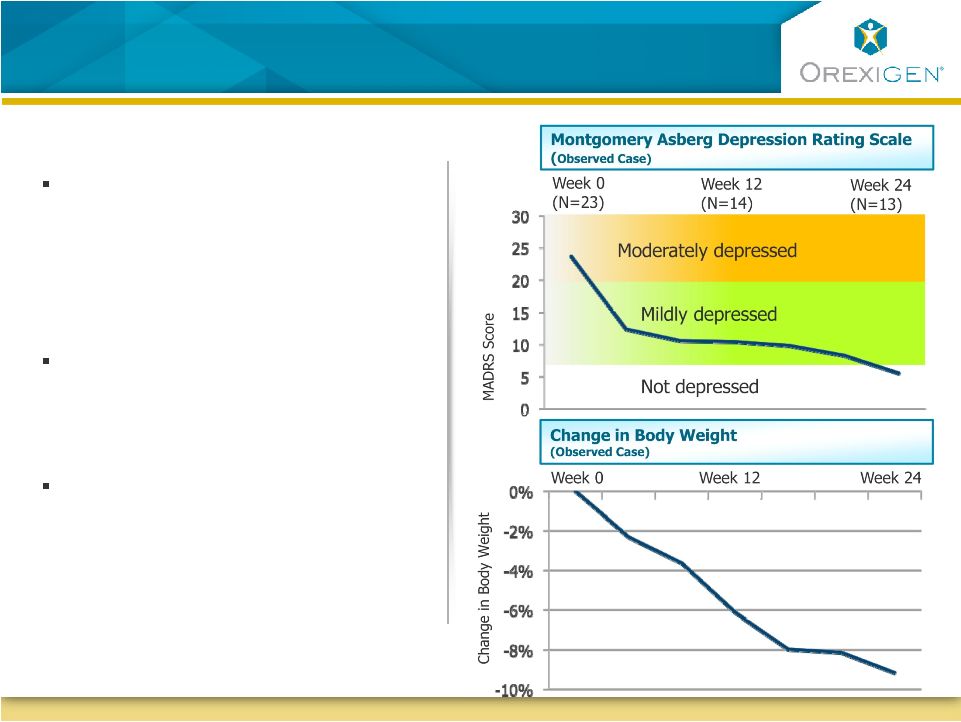 25
Contrave Has Shown Significant Weight Loss And
Improvement In Depressive Symptoms Within 24 Weeks
Contrave Has Shown Significant Weight Loss And
Improvement In Depressive Symptoms Within 24 Weeks
In a 24-week Phase 2 open-label
trial of obese depressed patients,
Contrave significantly
improved measures of
depression
At the same time,
patients who
completed the trial lost over
9% of their body weight
In addition, in a Control of Eating
Questionnaire, patients reported
decreases in hunger and cravings,
and less difficulty resisting cravings
Trial included 25 obese patients that met DSM-IV criteria for
major depression. |
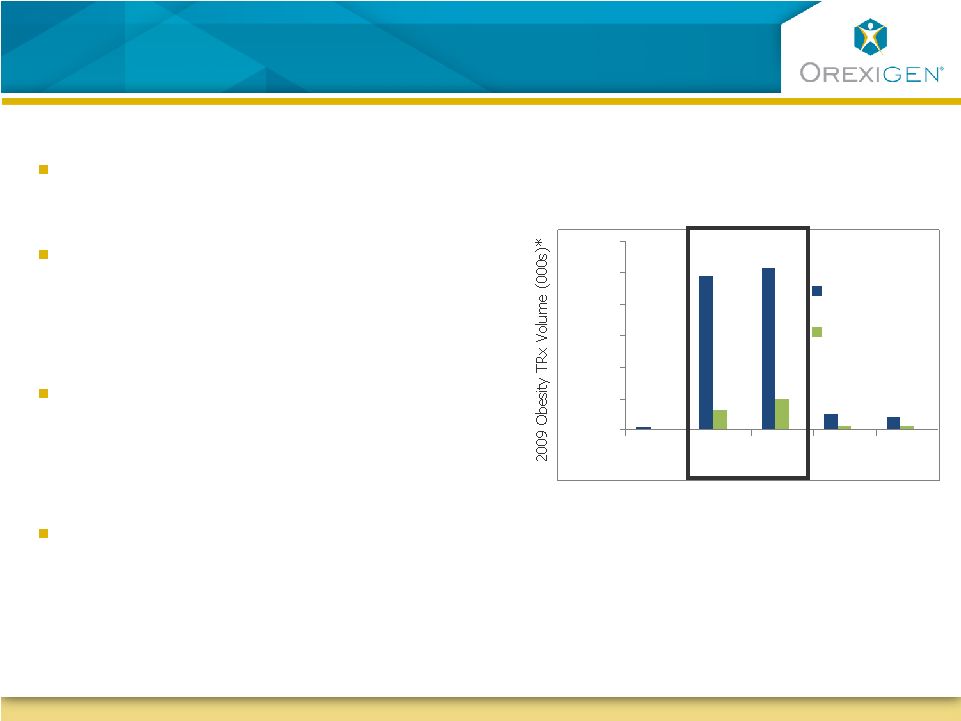 26
Contrave May Be The Logical Treatment Choice for
Women
Contrave May Be The Logical Treatment Choice for
Women
Source: Orexigen market research; IMS
Health, National Disease and Therapeutic Index, Obesity (2010) 18:
347-353
Women seek obesity therapy more than
men
Research has indicated that women tend
to crave food more than men, and
younger women tend to crave more than
older women (Pelchat, 1997)
Female gender is one of the few factors
that has been consistently associated with
an increased risk of depression among
obese individuals (Ma, 2010)
Majority of obesity Rx today go to women
under 60 years of age
Majority of the obesity Rxs today go
to women of child-bearing age
0
500
1,000
1,500
2,000
2,500
3,000
0-19
20-39
40-59
60-64
65+
Female
Male
Age (years) |
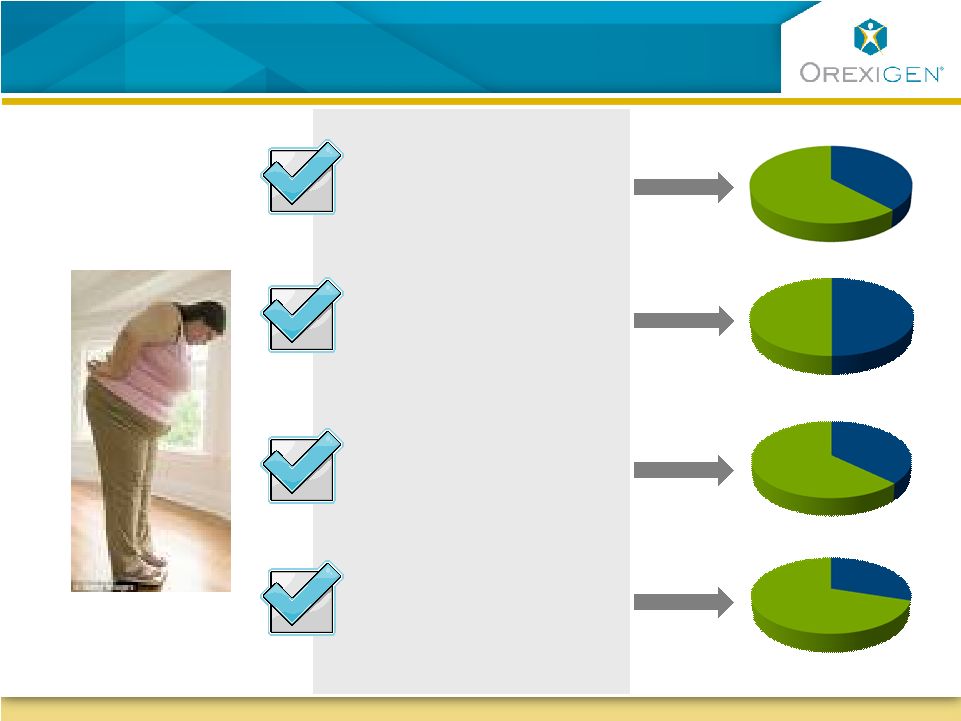 27
Contrave is Uniquely Positioned for Significant
Segments of the Obese Population
Contrave is Uniquely Positioned for Significant
Segments of the Obese Population
Has diabetes and/or
dyslipidemia
Reports having
uncontrollable food
cravings
Is depressed or has
depressive symptoms
Is female, many of
child bearing age
62%
63%
72%
50%
A Typical
Obese Patient: |
 28
SCALE
Top 15 Pharma
Significant U.S. Presence
Primary Care Focus
COMMITMENT
TO OBESITY
Partnership
with Amylin
Stated
Strategy
DOMAIN
EXPERIENCE
Leader in
Cardiometabolic
Care
Excellence in
Life Cycle
Management
SUCCESSFUL
BRAND BUILDER
Actos ~$3B in U.S.
Sales in 2009
Prevacid ~$3B in U.S.
Sales in 2009
Heavy Resourcing: Takeda is an Ideal Partner
Heavy Resourcing: Takeda is an Ideal Partner |
 29
Attractive Value Proposition
Attractive Value Proposition
Focused, experienced and capable team
World-class clinical advisory board to steer the study
Large capable partner in Takeda responsible for North American
commercialization
Significant revenue potential projected from lead product, Contrave
Retained ROW rights for future catalysts and upside
Sufficient cash to fund operations (~$145m) through potential
approval in 2014 |
 30 |
