Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - ZOGENIX, INC. | d279320d8k.htm |
 ®
ZOGENIX UPDATE
January 2012
NASDAQ: ZGNX
Exhibit 99.1 |
 2
Forward Looking Statements
Zogenix cautions you that statements included in this presentation that are not a
description of historical facts are forward- looking statements. Words such
as “believes,” “anticipates,” “plans,” “expects,” “indicates,” “will,” “intends,” “potential,”
“suggests,” “assuming,” “designed” and similar
expressions are intended to identify forward looking statements. These
statements are based on Zogenix’s current beliefs and expectations. These
forward-looking statements include statements regarding: the ability to
successfully commercialize Sumavel DosePro and its continued sales growth; the progress and timing of
clinical trials for Zohydro and planned development of Relday; the potential for, and
timing of, an NDA submission for Zohydro and an IND submission for Relday; the
potential for Zohydro to be the first approved oral, single-entity extended release
formulation of hydrocodone; the expansion of Zogenix’s existing sales force; the
potential to broaden the application of the DosePro technology; and the
potential market penetration of Sumavel DosePro and Zohydro. The inclusion of forward-looking
statements should not be regarded as a representation by Zogenix that any of its
plans will be achieved. Actual results may differ from those set forth in this
presentation due to the risk and uncertainties inherent in Zogenix’s business, including, without
limitation: the market potential for migraine treatments, and Zogenix’s ability
to compete within that market; inadequate therapeutic efficacy or unexpected
adverse side effects relating to Sumavel DosePro that could adversely affect
commercialization, or that could result in recalls or product liability claims;
Zogenix’s dependence on its collaboration with Astellas Pharma US, Inc.
to promote Sumavel DosePro; the progress and timing of Zogenix’s clinical trials; the potential that earlier
clinical trials may not be predictive of future results; the potential for Zohydro to
receive regulatory approval on a timely basis or at all; the potential for
adverse safety findings relating to Zohydro to delay or prevent regulatory approval or commercialization;
the ability of Zogenix and its licensors to obtain, maintain and successfully enforce
adequate patent and other intellectual property protection of its products and
product candidates and the ability to operate its business without infringing the
intellectual property rights of others; the inherent risks of clinical development of
Relday and Zogenix's dependence on its collaboration with DURECT Corporation
to develop Relday; and other risks described in Zogenix’s filings with the SEC, including
the prospectus filed with the SEC on September 15, 2011. You are cautioned not to
place undue reliance on these forward- looking statements, which speak only
as of the date hereof, and Zogenix undertakes no obligation to revise or update this
presentation to reflect events or circumstances after the date hereof. All
forward-looking statements are qualified in their entirety by this
cautionary statement. This caution is made under the safe harbor provisions of Section 21E of the Private Securities
Litigation Reform Act of 1995.
This presentation also contains estimates, projections and other information
concerning our industry, our business, and the markets for Sumavel DosePro,
Zohydro, Relday and other drugs, as well as data regarding market research, estimates and
forecasts prepared by our management. Information that is based on estimates,
forecasts, projections, market research or similar methodologies is inherently
subject to uncertainties and actual events or circumstances may differ materially from events and
circumstances reflected in this information. In some cases, we do not refer to
the source from which this data is derived. |
 Sources:
Wolters
Kluwer
Pharma
Solutions,
Source
®
PHAST
Institution/Retail
(12
months
ended
December
2010);
industry
sources
1
st
Needle-Free,
Commercial Product
Acute Migraines and
Cluster Headache
Commercializing and Developing Products for Treatment of
Central Nervous System (CNS) Disorders and Pain
$3.5B Market
Expanding Commercial Initiatives
with PCPs in 2012
$13B Market
US Market Unpartnered
$16B Market
Worldwide Market Rights
1
st
Oral, Single-Entity
Hydrocodone, 12-Hour
Extended Release for
Chronic Pain (no APAP)
1
st
Once-Monthly,
Needle-Free
Subcutaneous
Antipsychotic
Relday™
GROWING SALES
PREPARING NDA
IND STUDIES IN 2012
3 |
 4
Goals Completed Since IPO
SUMAVEL DosePro
Continued US Script Growth
Direct-to-Patient Initiatives
Phase IV Data Publication
“Innovation”
Award from
Drug Delivery Partnerships
EU Approvals
Building on Track Record of
Successful Execution
Zohydro
Seeking First Licensee for Biologic
Relday
DosePro Technology
$31.5m financing
$60.0m Follow-on offering
Financing
Completed Enrollment of Phase 3
Efficacy Study 801
Announced Positive Phase 3
Efficacy Top–Line Results for Study
801
Last Patient Completion for Safety
Study 802
Pre-NDA Meeting with FDA
in
Q4 2011
Anticipate NDA Submission early in
Q2 2012
Completed Licensing Deal with
DURECT
Anticipate IND Submission in 1H
2012 |
 5
1
st
Single Use, Needle-Free,
Commercial Product Marketed
for Treating Acute Migraine and
Cluster Headaches |
 6
75%
of
Patients
Are Women
The
9th
Leading Cause
of Disability
in Women Globally
7th
Most Costly Disease
to US Employers
30 Million People in the U.S. Suffer Migraines**
Symptoms and Nature of Episodes Vary Widely
Disabling Headache Pain, 4-72 Hours in Duration
$25 B Annual Estimated Cost to Employers
63% Suffer
1 Attack per
Month
25% Have 1 Attack per
Week 48% Upon Morning Wakening (between 4 -9 am)
29% Reported Vomiting as a Symptom of Migraine Attacks
Treatment Depends on Type of Episode
Migraine Frequency, Severity, Speed of Onset, and Previous
Response to Medication Determine Treatment
25% Have
2 Active Prescriptions
Oral
Triptans
Often
Prescribed
as
1
st
Line
Therapy
Sources:
NHF(2010),
World
Health
Organization
(2000),
Goetzel
et
all,
JOEM
(2004),
International
Headache
Society
(www.ihs-headache.org), Thomson
Medstat (2006), Lipton et al, Neurology (2002), Fox and Davis, Headache (1998),
Lipton et al, Neurology (2001), Boston Healthcare Associates, Inc. (2007) |
 7
SUMAVEL DosePro:
A Differentiated Migraine Therapy
COMPLETE
Pain Free
EFFECTIVE
Pain Relief
EASY TO USE
FAST
Pain Relief in as little as
Sources: SUMAVEL DosePro Prescribing Information
minutes
98%
Used SUMAVEL DosePro
During Migraine Attacks
on Their First Try at Home
of Patients Correctly |
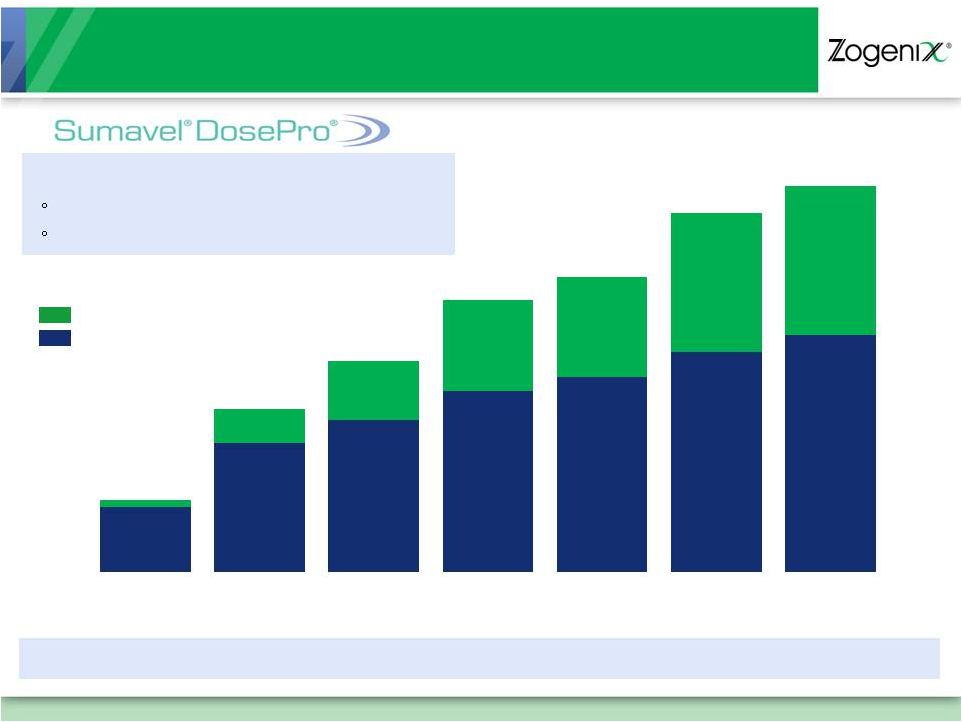 8
Growing Prescriptions Since Launch
$1.9M
Net Sales
$4.2M
Net Sales
$5.7M
Net Sales
$6.8M
Net Sales
$7.5M
Net Sales
$8.7M
Net Sales
Refill Prescriptions
New Prescriptions
*Revenue recognition methodology changed beginning Q3 2011
**Sources:
Wolters
Kluwer
Pharma
Solutions,Source®
PHAST
Prescription
Monthly,
January
2010
-
November
2011,and
Wolters
Kluwer
Pharma
Solutions,
Source®
LaunchTrac,
WE
10/7-WE
12/23
vs
WE 7/8-WE 9/23
$8.8M
Net Sales*
Q/Q Script Growth Rate
127% 29%
29%
8% 22%
6%*
3,207
6,332
7,443
8,872
9,568
10,806
11,549
327
1,674
2,907
4,487
4,910
6,822
7,109
Q1 2010
Q2 2010
Q3 2010
Q4 2010
Q1 2011
Q2 2011
Q3 2011
~100,000 Total Prescriptions**
38% Refill Rate (3Q 2011)
Total Prescriptions up 15% in first 12 weeks of Q4 2011 vs. first 12 weeks of Q3
2011** January 2010 –
November 2011
8 |
 9
Amended SUMAVEL DosePro Co-Promotion Agreement
Astellas agreement will end March 31, 2012
Beginning 2Q 2012, Zogenix will assume full responsibility for the
continued commercialization of the brand
–
Evaluating potential co-promotion partners who could complement the
Zogenix sales force efforts
–
Detailed transition plan to ensure uninterrupted access and service to
physicians within the Astellas segment
–
Astellas to receive tail payments which are estimated to be approximately $3.6
million in July 2013 and $2.1 million in July 2014*
Zogenix expects minimal impact on brand cash flow in 2012
–
Recent growth driven primarily by Zogenix sales force
–
Zogenix’s 95 reps can cover prescribers representing ~85% of the volume unit
demand by adding ~5 new doctors each to sales coverage
–
Lower expenses starting 2Q 2012 due to elimination of Astellas service fee
* These amounts are estimates and actuals will be based on the final sales results
for the 12 months ending March 31, 2012 |
 10
SUMAVEL DosePro Growth Drivers
Sales Force Expansion
95 reps calling on neurologists, pain specialists
and high Rx PCPs beginning in 4Q 2011
Evaluating new co-promotion opportunities
to supplement Zogenix U.S. sales team
Migraine Toolbox Launch
Helps patients choose
most
appropriate therapy
Supports updated label on dosing 1 hour
before or after other sumatriptan forms
Supported by published Phase 4 data
Direct-To-Patient Initiatives
WebMD online and print campaign
Real Age e-mail series
Future Product Enhancements
Sound suppression for improved patient
experience
Anticipate sNDA submission of 4mg dose line
extension by end of 2012 |
 11
Choosing the Right Tools for Specific Migraine Attacks
SUMAVEL DosePro Migraine
TOOLBOX
A comprehensive new patient starter
kit with relevant product information
for physician, patient and pharmacist,
including a free sample, $15 co-pay
card, carrying case, training material,
support resources, video access
and a migraine journal. |
 12
SUMAVEL DosePro Phase IV Study:
Improves Treatment Satisfaction and Patient Confidence*
Improvement in Overall Treatment
Satisfaction versus oral triptans
(p=0.0007)
Substantial Improvement in
Confidence to treat repeated
attacks after using SUMAVEL DosePro
(65% confident/very confident vs.
41% at baseline)
Rapid and sustained pain relief and
freedom from pain
Tolerability of treatment same as
baseline therapy (mainly oral triptans)
* Cady, R. et al (2011) Headache
Study design: >200 patients who were less than very satisfied with current
triptan therapy treated >650 migraine attacks with Sumavel DosePro.
Pre-study triptan use was predominantly oral forms (99% patients) with
only 17% of patients reporting recent experience with injectable sumatriptan.
Pain
Relief
Pain
Free
24 hours
81.3%
72.3%
1 –
24 hour sustained
66.3%
35.4%
Pain Relief %
Pain Free % |
 Oral,
Single-Entity (without acetaminophen),
Extended Release Hydrocodone
for Treating Chronic Pain
1
st
13 |
 14
Effective 12-hour pain relief without acetaminophen
Single-Entity Hydrocodone,
Multiple Strengths (10-50 mg)
Uses Alkermes’
Proprietary
SODAS Delivery System to
Enhance Hydrocodone
Release Profile
Schedule II product
Allows clinicians to prescribe the RIGHT dose of hydrocodone
by simplifying conversion from IR to ER and
reducing the risk of accidental acetaminophen toxicity
1
st
1
st
Oral, extended-release, single-
entity hydrocodone
Consistent 12-hour pain relief
Improved convenience
and
Acetaminophen-free
hydrocodone product
Reduces risk of liver toxicity
Unrestricted therapeutic dosing
(hydrocodone bitartrate)
compliance |
 15
Zohydro in the Marketplace
Large market characterized by dose escalation and opioid rotation
Hydrocodone
is
the
only
opioid
not
currently
available
in
ER
form
and
available
only
in
combination
with
APAP/NSAID;
FDA
advisory
committee
noted
single
hydrocodone would be logical substitute
FDA asked manufacturers to limit acetaminophen to 325 mg in combination products and
will
require
updated
labeling
information
warning
about
the
risk
of
severe
liver
injury
Drug
Sales (M)
IR TRx
Count (M)
ER TRx
Count (M)
% ER
ER Products
Sales
(M)
Oxycodone
$6,823
46.1
6.9
13%
OxyContin (including generics)
$3,645
Morphine
$1,172
1.8
5.6
76%
Avinza, Kadian,
Embeda,
MS Contin + Generic ERs
$1,127
Oxymorphone
$480
0.2
0.8
81%
Opana ER
$394
Hydromorphone
$170
2.5
0.02
1%
Exalgo
$18
Hydrocodone
$3,222
128.3
n/a
n/a
entity
formulation
of
Sources:
Wolters
Kluwer
Pharma
Solutions,
Source
®
PHAST
Institution/Retail,
12
months
ended
December
2010 |
 16
Pivotal Phase 3 Efficacy Study (Study 801) Met Endpoints
Significant
difference
in
chronic
pain
relief
(lower
back)
vs.
placebo
(p=0.008)
based on average mean changes in daily pain intensity (Numeric Rating Scale)
Key secondary endpoints support Zohydro efficacy
–
Significantly higher proportion of patients experiencing improvement in pain
relief
from beginning to end of study
–
Significantly improved overall satisfaction (p<0.001)
Safe and generally well tolerated
–
Adverse event profile consistent with approved oral, extended release opioid
products 68
48
31
23
30% improvement
50% improvement
Zohydro
Placebo
p<0.001
p<0.001 |
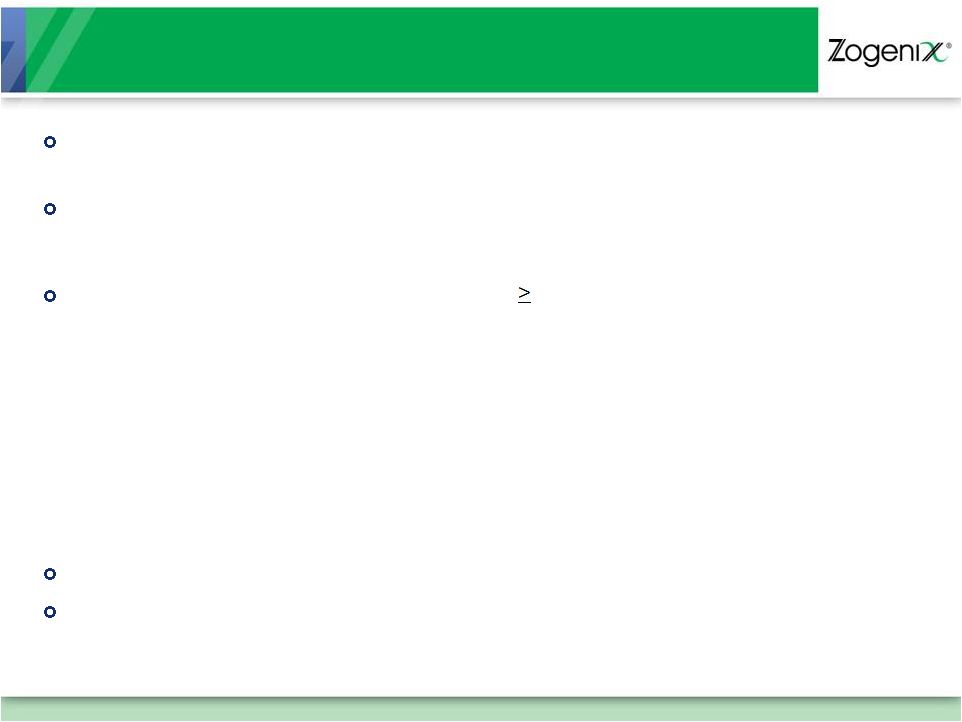 17
Summary of Safety (Study 801)
Safe and generally well tolerated
–
Adverse event profile consistent with approved oral, extended release opioid
products Overall % of subjects experienced an AE
–
C/T Phase = 33.7%
–
Treatment Phase = 28.8%
Most Commonly reported Adverse Events ( 2%)
–
Constipation
–
Nausea
–
Somnolence
–
Vomiting
–
Diarrhea
–
Insomnia
–
Fatigue
–
Headache
–
Dizziness
–
Dry mouth
< 3% of patients withdrew due to an adverse event
AEs representative of those experienced with other opioid products
Zohydro Phase 3 Trial |
 18
Zohydro NDA Submission Expected Early 2Q 2012
More than 1,000 Patients with Chronic Pain Treated with Zohydro
Phase 3 open label safety study (Study 802) completed; fulfills
database requirements for 505(b)(2) NDA
–
300 patients completed six months of exposure
–
100 patients completed 12 months of exposure
All NDA-required Phase 1 clinical trials and toxicology studies
completed
REMS program in preparation to meet ER opioid standards
CMC work with Alkermes on track to support NDA and
commercial scale production from established US manufacturing
facility
Completed Pre-NDA meeting Q4 2011; NDA filing anticipated
early Q2 2012 |
 19
Zohydro Key Commercial Events and Goals
Expand Zogenix Sales Team from 95
Reps to ~ 250 Reps to Support Zohydro
Launch and SUMAVEL DosePro
2013
Develop Marketing Communications and Launch Campaign
2012
Physician Advisory Boards
Branding and Market Research
Payer/Pricing Research
APAP data dissemination
2011
95
250 |
 20
Relday
(risperidone)
DosePro Needle-Free Delivery System
TM
Once-monthly risperidone
available as a needle-free
subcutaneous injection for
treatment of schizophrenia
1
st |
 21
Relday Needle-Free SubQ Injection for Schizophrenia
Proprietary long-acting injection formulation
of risperidone (Durect’s SABER technology)
deliverable by DosePro
Potential Benefits
–
Consistent, sustained drug release over one
month per injection
–
High drug loading facilitates low injection
volume and subcutaneous dosing
–
Prefilled, easy-to-use delivery system, no
reconstitution required
–
No needle
Completed pre-IND
meeting;
505(b)(2) NDA strategy
World-wide marketing rights provide
potential partnering opportunities
2H 2011
IND Enabling Studies
Early 2012
Submit IND to FDA
Begin Human
Clinical Trials
Ex-US Partner with PK
Data -
before Phase 3
Potential to leverage DosePro manufacturing capacity
with volume and lower SUMAVEL DosePro COGs |
 22
Relday Addresses Treatment Needs
Long-acting injections reduces the risk of noncompliance compared with
oral treatments
Growing US and EU market for long-acting injectable products -
exceeded $1.5 billion in 2010 (according to industry reports)
–
Risperdal Consta, 100 mg/month >$1000
Highly concentrated market with 13,000 prescribers of long-acting
injectable antipsychotics (US)
Relday is the only product in class that may satisfy all
criteria
highlighted in physician research conducted on our behalf
Attributes from
Physician Research
Risperdal
Consta
(risperidone)
Invega
Sustenna
(paliperidone)
Zyprexa
Relprevv
(olanzapine)
Relday
(risperidone)
1X Monthly
×
Subcutaneous
×
×
×
Needle-Free
×
×
×
Sources:
Goldine;
Wolters
Kluwer
Pharma
Solutions,
Source®
PHAST
Institution/Retail
(January
2010
–
December
2010),
BioStrategies
market
research
study |
 23
DosePro Technology:
New CNS Applications and Out Licensing Opportunities
Validated Delivery Option for Additional Medications,
Including Biologics and Small Molecules
DosePro Technology Platform
Internal Development
Injectable CNS Drug
Product Candidates
(RELDAY + TBA Pre-Clinical)
Second Generation
Technology
(1mL device)
Broaden Application
for Biologics
Technology Out Licensing
Enhance, Differentiate
or Extend Injectable
Product Lifecycles
Prototypes of Second Generation DosePro |
 24
Large Commercial Opportunities
*Includes Co-Pay Assistance ** Based on current pricing of branded ER
opioids †
Represents net revenue assuming market share equal to 1% of estimated TRx
1.28 Million TRx
$248 Million
†
122,000 TRx
$59 Million
†
48% of Episodes Upon Morning
Wakening (between 4 -9 am)
29% of Sufferers Reported Vomiting as a
Symptom of Migraine Attacks
30% of Patients Fail to Respond
to Oral or Nasal Triptans
Net Revenue/Dose: $69/Unit*
7 Units/TRx
Average TRx Value: $483 Net
1% Triptan TRx Share
128M TRx Hydrocodone only
(~30% for Chronic Use)
(excludes
78M TRx Other ER & IR Opioids) Net Revenue/Day: $7.75**
Average TRx: 25 Days
Average TRx Value: $194 Net
1% Hydrocodone TRx only Share
128 Million TRx per Year
12.2
Million
TRx
per
Year
Patient/
Market
Segments
Prescription
Values
Illustrative
Market Penetration
Sources:
Wolters
Kluwer
Pharma
Solutions,
Source
®
PHAST
Institution/Retail
(12
months
ended
December
2010) |
 25
Financial Summary
P&L
9 months ended September 30, 2011
($ Millions)
Financial Highlights
($ Millions)
Revenue
$
29.8
Operating Expense
$
85.5
Loss From Operations
$
55.8
Other (Income)/Loss
$ 4.4
Net Loss
$
60.2
Cash Flow Used In
Operations
$ 64.2
**Sum total may not appear to add due to rounding
Market Capitalization
(January 6, 2012)
~$184m
Cash Balance
At 12/31/11 (unaudited)
$ 56.5m
Debt
At 12/31/11
$30M Royalty Financing
$25M Term Loan
$10M Working Capital Line
-
Balance $5.2M |
 26
Continue U.S. Script Growth
Q1 2012 transition of Astellas
segment
Explore new co-promotion
opportunities
Sound Suppression
Improvements
4mg DosePro Line Extension
NDA
Submission
–
early
Q2
2012
Publish Pivotal Data
Explore partnership
Commercial Readiness
Expand Sales Force Upon
Approval
2
nd
Generation 1mL Device
3
rd
New CNS Product
File IND in 1H 2012
Initiate Phase 1 Study
Building on Track Record of
Successful Execution
Planned Goals
SUMAVEL DosePro
Relday
DosePro Technology
Zohydro |
