Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - AUXILIUM PHARMACEUTICALS INC | d278959d8k.htm |
 1
JP Morgan 2012
(NASDAQ: AUXL)
Exhibit 99.1 |
 2
Safe Harbor Statement
We will make various remarks relating to our current plans, potential future events, and what we
believe to be the prospects for the Company in this presentation that constitute
forward-looking statements for purposes of the Safe Harbor provisions under the Private
Securities Litigation Reform Act of 1995. Today our forward-looking statements will cover,
among other things, the Company’s expected financial performance, results and strategic
priorities; marketing strategies for XIAFLEX for Dupuytren’s contracture; the potential for
XIAFLEX to change the treatment paradigm and become the standard of care for the treatment of
Dupuytren’s; the size of the Dupuytren’s market; the effect of new procedure codes on
the reimbursement process and XIAFLEX growth; the effect of the identified leading indicators
on the success of the XIAFLEX launch and future net revenues; additional Phase IV clinical trials for XIAFLEX; peer to peer
dialogue programs for U.S. physicians and our U.S. patient education campaign; Pfizer’s ability
to commercialize XIAPEX for Dupuytren’s contracture in EU and Eurasian markets; Asahi
Kasei Pharma's ability to develop, register for approval and commercialize XIAFLEX for
Dupuytren's contracture and Peyronie's disease in Japan; the potential for XIAFLEX to be used in
multiple indications, including for the treatment of Peyronie’s, cellulite, frozen
shoulder syndrome and canine and human lipomas, the effectiveness of XIAFLEX for any such
indications, and the success of efforts to advance any such new indications; the design and
methodology, and the timing to report results, of the phase III trials for XIAFLEX for the
treatment of Peyronie’s; the growth of the testosterone replacement therapy market, and the
growth opportunity for Testim; business development efforts and opportunities to build out the
Company’s pipeline; our ability to maximize the value of XIAFLEX and Testim, deliver on
our current pipeline, build out our pipeline and create shareholder value. Actual results may
differ materially from those reflected in these forward-looking statements due to various factors,
including general financial, economic, regulatory and political conditions affecting the
biotechnology and pharmaceutical industries and those discussed in Auxilium's Annual Report on
Form 10-K for the year ended December 31, 2010 and Quarterly Report on Form 10-Q for the quarter ended September 30,
2011 under the heading “Risk Factors,” which are on file with the Securities and Exchange
Commission (the “SEC”) and may be accessed electronically by means of the SEC’s
home page on the Internet at http://www.sec.gov or by means of the Company’s home page on the
Internet at http://www.auxilium.com under the heading “For Investors - SEC
Filings.” There may be additional risks that the Company does not presently know or
that the Company currently believes are immaterial which could also cause actual results to differ from those
contained in the forward-looking statements. Given these risks and uncertainties, any or all
of these forward-looking statements may prove to be incorrect. Therefore, you should
not rely on any such factors or forward-looking statements. In addition, forward-looking statements
provide the Company’s expectations, plans or forecasts of future events and views as of the date
of this presentation. The Company anticipates that subsequent events and developments
will cause the Company’s assessments to change. However, while the Company may elect
to update these forward-looking statements at some point in the future, the Company specifically disclaims any obligation to do
so. These forward-looking statements should not be relied upon as representing the
Company’s assessments as of any date subsequent to the date of this presentation. |
 3
Auxilium –
A Company Overview
•
Fully integrated specialty biopharmaceutical company, ~550 employees
including a sales force of ~250
•
2010 global net revenues of $211.4 million; CAGR of 39.4% over past six
years
•
2011
revenue
guidance
of
$254
–
271
million
•
Testim®
•
XIAFLEX®
›
Testosterone replacement therapy gel for treatment of hypogonadism, or low
testosterone
›
Launched in U.S. in 2003 and EU in 2005
›
Paradigm changing treatment for Dupuytren's contracture, launched in US/EU in
2010/11
›
Top-line results of pivotal trials in Peyronie’s disease expected in Q2
2012 ›
Represents a pipeline within a product (Frozen Shoulder syndrome, cellulite,
lipomas) |
 4
Auxilium –
Vision and Growth Strategy
Deliver on Current Pipeline
Maximize Value of Testim
and XIAFLEX
Build Out Pipeline in Specialty
Therapeutic Areas
Shareholder
Value
Our Vision Is to Become a Rapidly Growing, Profitable and
Sustainable Bio-Pharmaceutical Company |
 5
Auxilium –
Vision and Growth Strategy
Deliver on Current Pipeline
Maximize Value of Testim
and XIAFLEX
Build Out Pipeline in Specialty
Therapeutic Areas
Shareholder
Value
Our Vision Is to Become a Rapidly Growing, Profitable and
Sustainable Bio-Pharmaceutical Company |
 6
Taking Advantage of Global Opportunities
Through Licensing Partnerships
U.S.
Canada
EU
Japan
R.O.W.
Exploring
Opportunities |
 7
•
1% testosterone gel indicated for testosterone replacement therapy (TRT)
in adult males for hypogonadism
•
Launched in U.S. in 2003 and EU in 2005
•
TRT market value has grown at 25.4% CAGR since 2005
•
> 8 years of use with established safety and efficacy
›
~115M daily doses since launch
›
16 clinical studies involving ~1,800 patients
•
Applied once daily at 5-10mg
›
90% stay on starting dose of 5mg (one tube)
›
Simple application process and dosing
•
Patent protected through 2025
Testim –
Product and Market Overview |
 8
TRT Landscape is Changing
•
New competition helping increase overall market in 2011
-
17% y/y prescription growth in 3Q11
•
Aggressive strategy in select managed care plans has
helped Testim minimize market share loss to new
players
•
Despite loss of market share to new entrants, Testim
revenues through September 2011 have increased 8% |
 9
Testim Strategy Focuses on the Top TRT
Prescribers
13%
~156K
~3,200K
~700K
50%
Testim Market Share
72%
Source: IMS Prescriber Plan Trak 12 month rolling estimates through November
2011 0
20
40
60
80
100
120
140
160
180
0
500
1000
1500
2000
2500
3000
3500
TRT Prescribers
Gel TRx
Testim TRx
~ 21% total gel TRT market share
~ 31% market share in targets
~ 12% market share in non-targets
target prescribers
non-target prescribers |
 10
XIAFLEX -
XIAFLEX -
A Paradigm-Changing, Minimally
A Paradigm-Changing, Minimally
Invasive Treatment
Invasive Treatment
•
Approved to treat adults with Dupuytren’s contracture when a
cord can be felt
•
A mixture of collagenases injected directly into Dupuytren's cord
•
Commercial launch in U.S. in March 2010
•
Pfizer launch of XIAPEX®
in Europe in April 2011
•
Asahi pursuing development in Japan
•
Long protection period: Drug product composition patent (2028),
Biologic Exclusivity (2022) & Orphan Drug protection (2017)
|
 11
•
Excessive collagen causing reduced elasticity in fascia of hand with nodules or
pits as an early, active presentation
•
Rope-like cords develop in the finger and result in contractures affecting
quality of life and daily activities
•
Fasciectomy is an invasive treatment with high potential for complications
›
Results can be unpredictable and may require long recovery time of 6-8 weeks,
with intensive physical therapy
›
Recurrence of the disease can require additional surgeries
›
Surgical complication rate increases dramatically for retreated joints, primarily
due to presence of scar tissue
›
Only
~25%
of
diagnosed
patients
estimated
to
receive
surgery
annually
Dupuytren’s
Contracture
-
A
Debilitating
Disease
Pictures courtesy of Dr. Clayton Peimer
1.
Dupuytren’s
Disease
–
Tubiana,
LeClerq,
Hurst,
Badalamente,
Mackin
2.
SDI Claims Data Based Projections
3.
Medicare
Data
Based
Projections
(BESS
database
used,
Medicare
5%
database
also
used
to
validate
numbers)
1
•
Annually, estimates of 70K surgeries and 300K new diagnoses
1,2,3 |
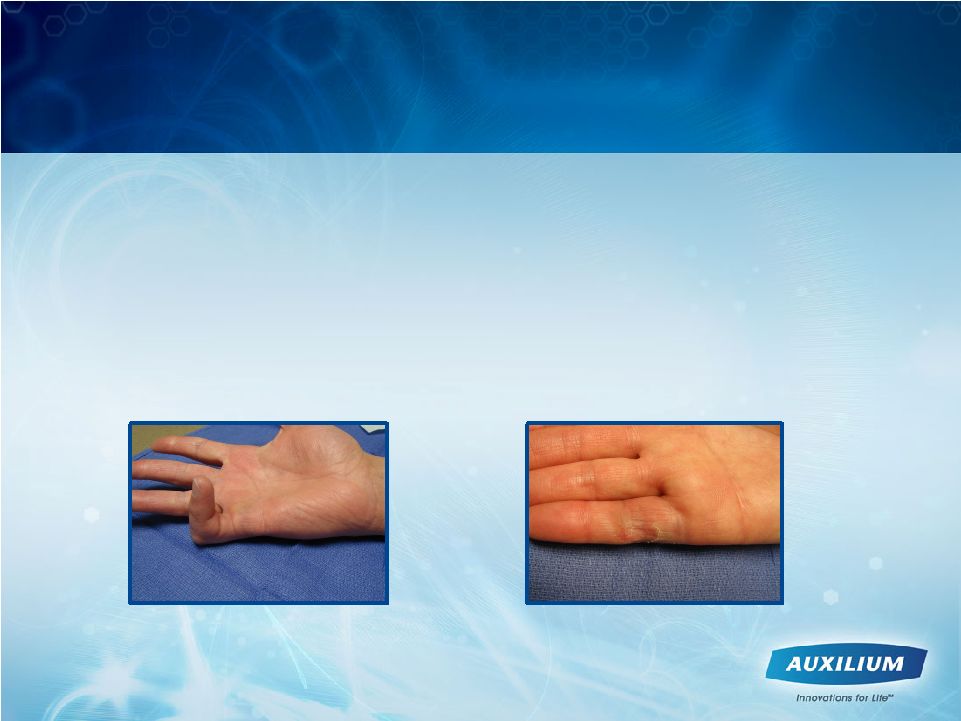 12
XIAFLEX -
Provides Surgeons With A
Minimally Invasive Tool
Pre-XIAFLEX injection
30 days following XIAFLEX injection
•
Simple injection and manipulation procedure
•
Established mechanism of actions and selective for specific types of collagen
•
Efficient use of surgeon’s time
•
No active physical therapy requirement
•
Limited occurrence of scar tissue at XIAFLEX injection site*
•
First-line minimally invasive treatment maintains optionality if disease
recurs *Blazar, ASSH 2011 poster |
 Adoption of XIAFLEX is Growing
~58% of enrolled sites have ordered XIAFLEX
13
2399
2511
2596
2668
2764
2819
2884
2973
3059
3163
3242
3312
1758
1844
1936
2001
2076
2133
2179
2249
2322
2395
2522
840
902
983
1047
1099
1155
1202
1243
1287
1351
1412
1458
PHYSICIAN ENROLLMENTS
SITES ENROLLED
UNIQUE SITES ORDERING
2462 |
 14
Steady Growth in Experienced Sites
Cumulative Injections per Site |
 15
Strategies for Establishing XIAFLEX as the
Standard of Care
Motivate Physicians
•
Compelling clinical efficacy and safety
data
•
Facilitate peer-to-peer dialogue
•
Activate a critical mass of experienced
prescribers
•
Sample program for XIAFLEX-naïve physicians
•
Multi-cord phase IV clinical study initiated
Activate Patients
•
Patient copay assistance
program
d
•
Direct Response TV campaign in 7 ready
markets
Streamline Reimbursement
•
Additional
wins
with
managed
care
–
96%
of
covered
lives
have access to
XIAFLEX
•
2012 XIAFLEX growth should be helped modestly by new
CPT codes |
 16
Auxilium
–
Vision
and
Growth
Strategy
Deliver on Current Pipeline
Maximize Value of Testim
and XIAFLEX
Build Out Pipeline in Specialty
Therapeutic Areas
Shareholder
Value
Our Vision Is to Become a Rapidly Growing, Profitable and
Sustainable Bio-Pharmaceutical Company |
 17
XIAFLEX -
Pipeline Opportunities
LATE RESEARCH
PRE-CLINICAL PHASE I PHASE II PHASE III
* Right to exercise option for exclusive global license from BioSpecifics
Technologies Corp. PEYRONIE’S DISEASE
FROZEN SHOULDER SYNDROME
CANINE LIPOMA*
HUMAN LIPOMA*
CELLULITE*
Top-line phase III
data in 2Q12
Top-line phase IIa
data in 1H13
Clinical trials to
start soon
Clinical trials to
start soon
Begin phase Ib in
1Q12, data in
2H12
EXPECTED
TIMELINE |
 18
•
Scarring
phenomenon
affecting
the
tunica
albuginea
1
•
Plaques
show
excessive
collagen
deposition
2
•
Potential symptoms:
›
Pain with erection, penile curvature/deviation, penile shortening,
indentations, and/or erectile dysfunction
›
May have difficulty with sexual intercourse, loss of self-esteem and
depression
•
Prevalence of Peyronie’s disease is estimated to be
approximately 5% in adult men
3,4,5
›
The
average
age
of
disease
onset
is
53
years
6
•
High association with other diseases such as:
›
Diabetes, erectile dysfunction, Dupuytren’s contracture, plantar
fascial
contracture,
tympanosclerosis,
gout,
and
Paget’s
disease
•
Surgery is a treatment of last resort and most patients
are treated first-line with unapproved medical therapy
Peyronie’s
Disease:
A
Devastating
Disorder
1
Smith BH. Am J Clin Pathol. 1966;45:670-678.
2
Somers KD, Dawson DM. J Urol. 1997;157:311-315.
3
Bella A. Peyronie’s Disease J Sex Med 2007;4:1527–1538
4
Lue TF, et al. Summary of the recommendations on sexual dysfunctions in men. J Sex
Med 2004;1:6–23. 5
Mulhall JP, et al. Subjective and objective analysis of the prevalence of
Peyronie’s disease in a population of men presenting for prostate cancer screening. J Urol
2004;171:2350–3.
6
Lindsay MB, J Urol.
1991;146:1007-1009.
7
Nyberg L, J Urol.128: 48, 1982
7 |
 19
Peyronie’s Phase IIb Efficacy Results (2009)
•
Penile curvature and domains of the patient reported outcomes (PRO) survey
were measured
›
Three dosing cycles –
up to six injections
›
Penile modeling was compared to no modeling
•
In all subjects, penile curvature and Peyronie’s disease bother, a PRO domain,
were statistically significantly improved vs. placebo
›
Other domains of the PRO trended toward significance
›
Curvature improvement was greatest during the dosing phase
›
Improvement in curvature continued through 36 weeks
•
In the modeling arm, curvature and Peyronie’s disease bother were
statistically significantly improved vs. placebo
›
Mean 35% improvement in curvature vs. placebo
›
Mean 41% improvement in Peyronie’s disease bother vs. placebo
•
In the non-modeling arm, curvature and Peyronie’s disease bother were not
statistically significantly improved vs. placebo
›
Greater
than
expected
placebo
effect
occurred
in
the
no
modeling
arm
›
5 of 16 placebo patients with <12 months since diagnosis had an apparent
spontaneous resolution of disease |
 20
Changes to the Phase IIb Trial Design Should
Increase the Chance of Success in Phase III
•
4 cycles of therapy in the Phase III trial
•
All patients receive modeling in the Phase III trial
•
All patients must have greater than 12 months of disease since
diagnosis to enter the Phase III trial
•
Active to placebo ratio of 2:1
Mean net improvements in phase IIb penile curvature
and bother domain for modeling arm would have met
statistical significance using phase III co-primary
endpoint requirements |
 21
Peyronie’s Disease: Pivotal Phase III Update
•
Active dosing phase complete
•
Double-blind patients complete 52 week follow-up
phase in March 2012
•
Top-line results should be available in 2Q12
•
sBLA filing expected by end of 2012
•
Potential commercial launch planned in late 2013 |
 22
Edematous Fibrosclerotic Panniculopathy
(EFP) Represents a Compelling Opportunity
•
There are currently no FDA-approved medical therapies
for EFP, commonly known as cellulite
•
Current non-surgical options haven’t shown benefit
›
OTC pills, creams and lotions
›
Unapproved mixtures of enzymes and fat dissolvers
›
Various devices, including lasers
•
Surgical approaches have shown some benefit, but are invasive
›
Liposuction removes fat cells
›
Subscision surgically disrupts collagen septae and underlying dermal
tissues
•
We believe XIAFLEX may enable physicians to enzymatically and
specifically lyse the connective septae, in a more targeted fashion than
subscision, possibly resulting in a smoother appearance to the skin surface
|
 23
Frozen Shoulder Syndrome: An Unmet
Medical Need
•
Current treatment options have limited results
›
Non-steroidal pain relievers
›
Oral steroids
›
Corticosteroid injections
›
Physical therapy
•
Manipulation of the shoulder under anesthesia, long-term intensive physical
therapy or arthroscopic surgery can disrupt the frozen capsule
•
Currently no FDA approved non-surgical therapies for Frozen Shoulder
syndrome •
We believe that XIAFLEX may act by thinning the thickened collagen capsule
allowing improved range of motion of the affected shoulder
Normal Shoulder
Frozen Shoulder |
 24
Auxilium-
Vision and Growth Strategy
Deliver on Current Pipeline
Maximize Value of Testim
and XIAFLEX
Build Out Pipeline in Specialty
Therapeutic Areas
Shareholder
Value
Our Vision Is to Become a Rapidly Growing, Profitable and
Sustainable Bio-Pharmaceutical Company |
 25
Business Development Efforts Focused on
Building Out Pipeline
•
Leverage current infrastructure of Testim
and XIAFLEX field forces with in-licensed
marketed products
•
Seeking phase II and later assets for
specialty markets (Urology, Orthopedics)
and Orphan diseases |
 26
Financials
Financials |
 27
3Q11 Net Revenues Up 24% over 3Q10
$53.6
$66.7
+24% |
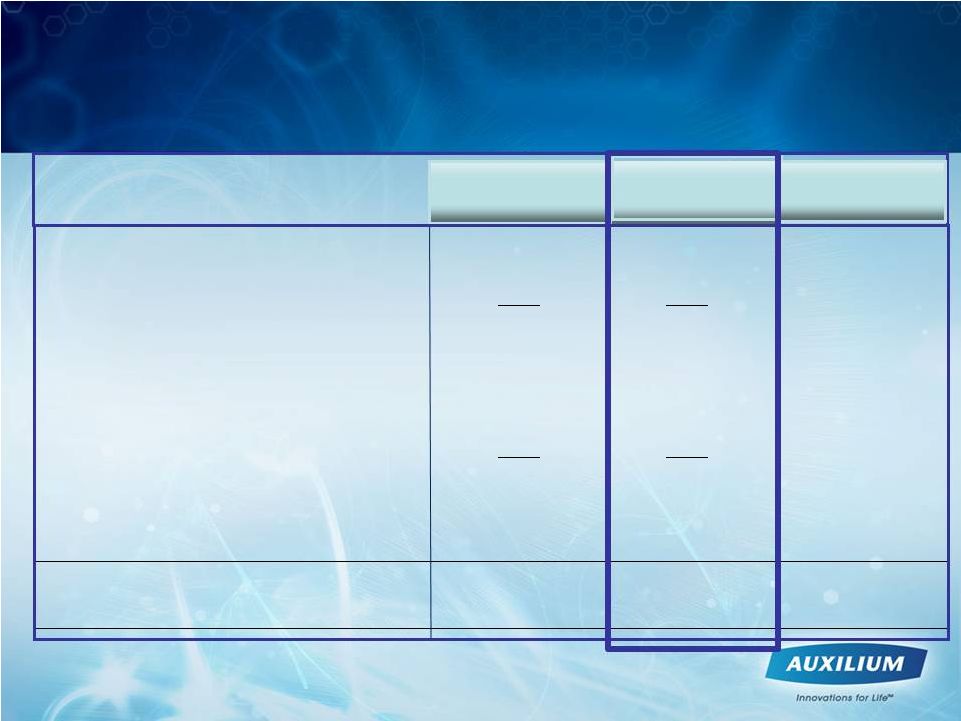 28
** Weighted average
common
shares
were
47,592,975
in
Q3
2011
and
47,933,447
in
Q3
2011.
Total revenues
$53.6
$66.7
+24%
Cost of goods sold*
11.6
13.5
+17%
Gross Profit
$42.1
$53.2
+26%
% of revenues
78%
80%
Operating expenses:
Research and development*
14.4
14.2
-2%
Sales, general and administrative*
40.3
43.3
+7%
Net loss
(12.8)
(4.1)
+68%
Net loss per common share**
(0.27)
(0.08)
+68%
*Stock based compensation expense
3.5
3.8
Cash and Cash Equivalents
$149.6
(amounts in millions, except per share amounts)
% change
Q3 2011
Q3 2010
Auxilium -
Q3 2011 Financial Results |
 29
Auxilium -
2011 Preliminary Net Revenue
•
Global net revenues should be above midpoint of previously
issued guidance of $254 to 271 million and slightly above
current 2011 sell side consensus of $263.9 million.
•
Global Testim net revenues should be above midpoint of
previously issued guidance of $200 to 210 million and slightly
above current 2011 sell side consensus of $206.5 million.
•
U.S. XIAFLEX net revenues will be below previously issued
guidance of $45 to 50 million and slightly below current 2011
consensus of $44.2 million. |
 30
Auxilium
-
Strategic
Priorities
to
Drive
Long -
Term Growth
•
Maximize Testim revenue growth in a changing competitive
environment
•
Establish XIAFLEX as the standard of care for Dupuytren’s contracture
in the U.S.
•
Support Pfizer’s launch of XIAPEX for the treatment of Dupuytren’s
contracture
•
Complete Peyronie’s Disease double-blind studies and announce
top- line data in 2Q12
•
Announce top-line phase Ib data for cellulite in 2H12 and phase IIa
data for Frozen Shoulder syndrome in 1H13
•
Work collaboratively with BTC as they initiate studies using XIAFLEX
|
 31
Auxilium -
Investment Rationale
•
Track record of developing and commercializing novel treatments for
unmet medical needs
•
Pipeline with five potential new XIAFLEX indications in development
•
Global commercialization through licensing partnerships
•
Opportunity to build urology, orthopedics and other specialty franchises
•
Strong intellectual property protection for assets
•
Solid financial position with no debt |
