Attached files
| file | filename |
|---|---|
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | a12-2202_18k.htm |
| EX-99.1 - EX-99.1 - AMAG PHARMACEUTICALS, INC. | a12-2202_1ex99d1.htm |
Exhibit 99.2
|
|
AMAG Pharmaceuticals 1 J.P. Morgan Healthcare Conference January 2012 |
|
|
Forward Looking Statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Any statements contained herein which do not describe historical facts, including but not limited to, statements regarding the expected timing of regulatory decisions on our marketing authorization application for Feraheme(R) in the EU, including the expected timing of a decision from CHMP; statements regarding the expected commercial launch of Feraheme(R) in Canada and the EU; statements regarding any potential milestone payments and royalties we may receive under our collaboration agreement with Takeda with respect to regulatory approval and commercial launch of Feraheme(R) in any territory and the expected magnitude and timing thereof; our expectation to complete enrollment in our global Feraheme(R) IDA clinical development program in Jan 2012; our expectation that we will file our sNDA for the broad IDA indication in 2012 and commercially launch Feraheme(R) in the IDA indication in 2013; our expected 2011 and 2012 year-end cash, cash equivalents and investments balance; statements regarding our plan to bring the company to cash flow break-even in 2012; statements regarding the potential incremental Feraheme(R) sales potential and market opportunity associated with Feraheme(R) geographic and label expansion; our expected fourth quarter 2011 financial results, including our expected total revenues, Feraheme(R) product revenues, our expected gross-to-net adjustment, and our expected operating expenses for the quarter; statements regarding our 2012 financial outlook, including our expected 2012 Feraheme(R) revenue growth, our expectations regarding our 2012 operating expenses, including our expected decrease in R&D expenses, and our expectation to receive $33 million in milestone payments; the expected magnitude of the restructuring charges we expect to incur in connection with our recently-announced restructuring plan; and our expectation to begin the Feraheme(R) CKD registrational program in China in 2012; are forward-looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward looking statements. Such risks and uncertainties include: (1) uncertainties regarding our ability to successfully compete in the intravenous iron replacement market both in the U.S. and outside the U.S., (2) uncertainties regarding our ability to successfully and timely complete our clinical development programs and obtain regulatory approval for Feraheme(R) in new indications and in territories outside of the U.S., including the European Union, (3) the fact that significant safety or drug interaction problems could arise with respect to Feraheme(R), (4) the possibility that our audited fourth quarter and full-year 2011 financial results, including Feraheme(R) net revenues, operating expenses, and year-end cash, cash equivalents and investments balances, will differ materially from our current unaudited estimates, (5) uncertainties regarding our ability to manufacture Feraheme(R), (6) uncertainties relating to our patents and proprietary rights, (7) uncertainties regarding the outcome of our evaluation of strategic alternatives, including the risk that the process will not result in a transaction, and (8) other risks identified in our Securities and Exchange Commission filings, including our Annual Report on Form 10-K for the year ended December 31, 2010 and our Quarterly Report on Form 10-Q for the three and nine months ended September 30, 2011. We caution you not to place undue reliance on any forward-looking statements, which speak only as of the date they are made. We disclaim any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. 2 |
|
|
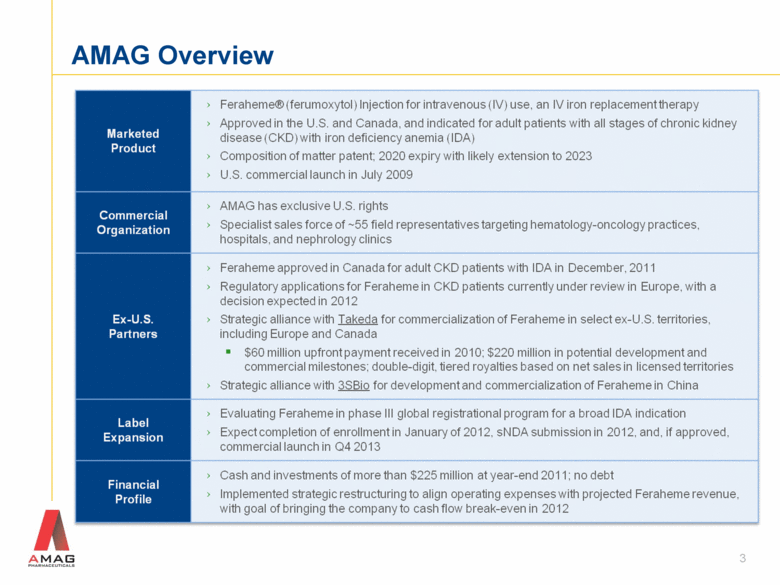
AMAG Overview Marketed Product›Feraheme® (ferumoxytol) Injection for intravenous (IV) use, an IV iron replacement therapy Approved in the U.S. and Canada, and indicated for adult patients with all stages of chronic kidney disease (CKD) with iron deficiency anemia (IDA) Composition of matter patent; 2020 expiry with likely extension to 2023 U.S. commercial launch in July 2009 Commercial Organization AMAG has exclusive U.S. rights Specialist sales force of ~55 field representatives targeting hematology-oncology practices, hospitals, and nephrology clinics Ex-U.S. Partners Feraheme approved in Canada for adult CKD patients with IDA in December, 2011 Regulatory applications for Feraheme in CKD patients currently under review in Europe, with a decision expected in 2012›Strategic alliance with Takeda for commercialization of Feraheme in select ex-U.S. territories, including Europe and Canada $60 million upfront payment received in 2010; $220 million in potential development and commercial milestones; double-digit, tiered royalties based on net sales in licensed territories Strategic alliance with 3SBio for development and commercialization of Feraheme in China Label Expansion›Evaluating Feraheme in phase III global registrational program for a broad IDA indication›Expect completion of enrollment in January of 2012, sNDA submission in 2012, and, if approved, commercial launch in Q4 2013 Financial Profile Cash and investments of more than $225 million at year-end 2011; no debt Implemented strategic restructuring to align operating expenses with projected Feraheme revenue, with goal of bringing the company to cash flow break-even in 2012 3 |
|
|
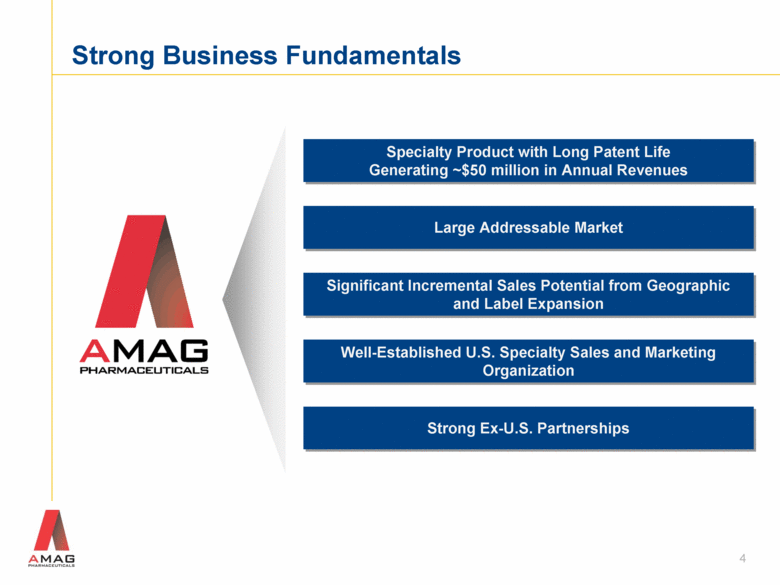
Strong Business Fundamentals 4 Specialty Product with Long Patent Life Generating ~$50 million in Annual Revenues Significant Incremental Sales Potential from Geographic and Label Expansion Large Addressable Market Strong Ex-U.S. Partnerships Well-Established U.S. Specialty Sales and Marketing Organization |
|
|
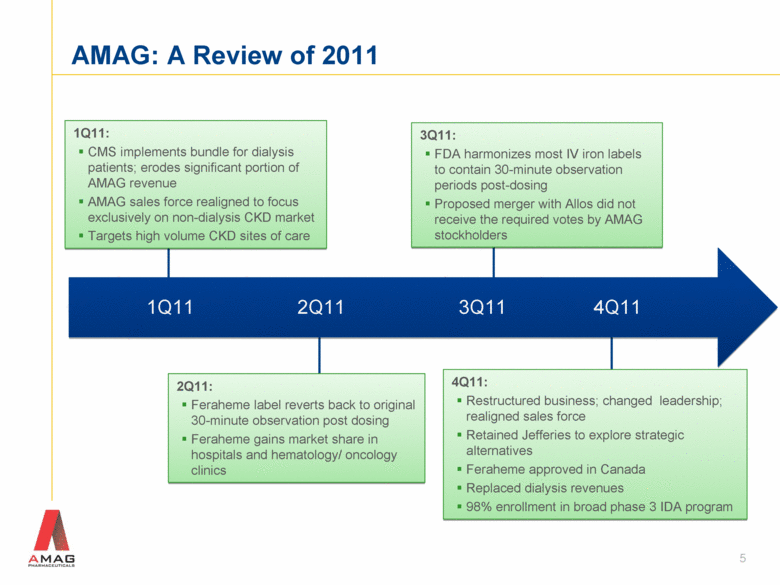
3Q11: FDA harmonizes most IV iron labels to contain 30-minute observation periods post-dosing Proposed merger with Allos did not receive the required votes by AMAG stockholders 1Q11: CMS implements bundle for dialysis patients; erodes significant portion of AMAG revenue AMAG sales force realigned to focus exclusively on non-dialysis CKD market Targets high volume CKD sites of care 1Q11 2Q11 3Q11 4Q11 2Q11: Feraheme label reverts back to original 30-minute observation post dosing Feraheme gains market share in hospitals and hematology/ oncology clinics 4Q11: Restructured business; changed leadership; realigned sales force Retained Jefferies to explore strategic alternatives Feraheme approved in Canada Replaced dialysis revenues 98% enrollment in broad phase 3 IDA program AMAG: A Review of 2011 5 |
|
|
Market Opportunity & Commercial Progress 6 |
|
|
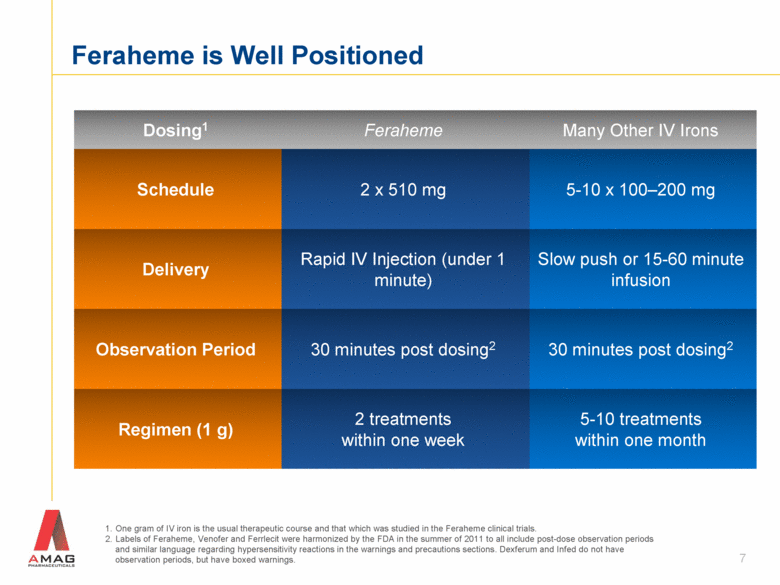
Dosing1 Feraheme Many Other IV Irons Schedule 2 x 510 mg 5-10 x 100–200 mg Delivery Rapid IV Injection (under 1 minute) Slow push or 15-60 minute infusion Observation Period 30 minutes post dosing2 30 minutes post dosing2 Regimen (1 g) 2 treatments within one week 5-10 treatments within one month One gram of IV iron is the usual therapeutic course and that which was studied in the Feraheme clinical trials. Labels of Feraheme, Venofer and Ferrlecit were harmonized by the FDA in the summer of 2011 to all include post-dose observation periods and similar language regarding hypersensitivity reactions in the warnings and precautions sections. Dexferum and Infed do not have observation periods, but have boxed warnings. Feraheme is Well Positioned 7 |
|
|
2.4 Million Adults with Diagnosis of IDA Between 200,000 and 250,000 are Treated with IV Iron4 U.S. Census U.S. Renal Data System, USRDS 2010 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2010: 41-42. Fishbane, S. et al. Iron Indices in CKD in the NHANES 1998-2004. Clin J Am Soc Nephrol. 2009 January; 4(1): 57–61. Company Estimates Current Focus 33 Million U.S. Adults with CKD1,2 400,000 Dialysis Patients2 >360,000 (90%) Treated with IV Iron for Iron Deficiency2 Patients receive 2.5 to 3 grams a year, leading to an annual U.S. dialysis IV iron market of ~1 million grams 16 Million Adults with Stage 3 - 5 CKD2 >1.6 Million have Diagnosis of CKD and Iron Deficiency Anemia3,4 Between 200,000 and 250,000 are Treated with IV Iron4 Typically administered at hematology infusion center, hospital outpatient infusion center or nephrology clinic; patients receive ~1.5 grams of IV iron per year IV Iron Therapy in Iron Deficiency Anemia 8 >4 Million U.S. Adults with Non-CKD IDA ~13.5 Million U.S. Adults with Oncology, GI or GYN Disorders Concomitant with IDA |
|
|
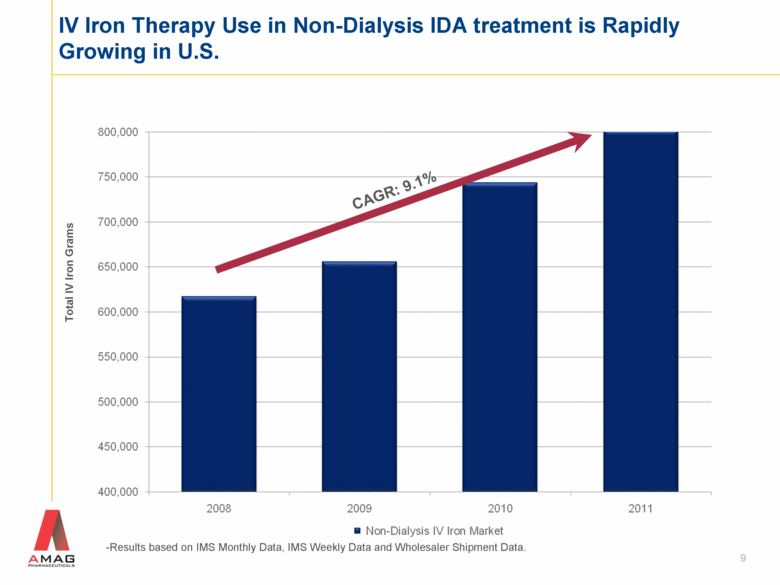
9 IV Iron Therapy Use in Non-Dialysis IDA treatment is Rapidly Growing in U.S. -Results based on IMS Monthly Data, IMS Weekly Data and Wholesaler Shipment Data. Total IV Iron Grams CAGR: 9.1% |
|
|
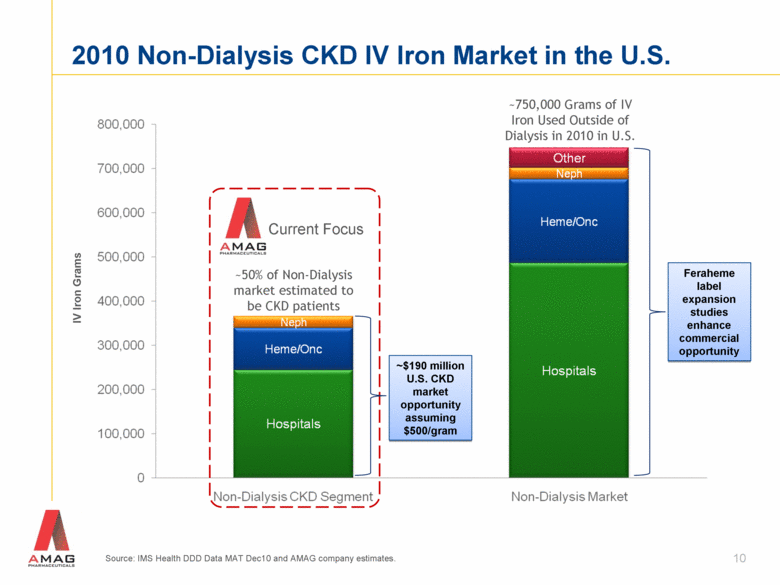
Source: IMS Health DDD Data MAT Dec10 and AMAG company estimates. ~750,000 Grams of IV Iron Used Outside of Dialysis in 2010 in U.S. IV Iron Grams ~50% of Non-Dialysis market estimated to be CKD patients 2010 Non-Dialysis CKD IV Iron Market in the U.S. Current Focus 10 ~$190 million U.S. CKD market opportunity assuming $500/gram Feraheme label expansion studies enhance commercial opportunity |
|
|
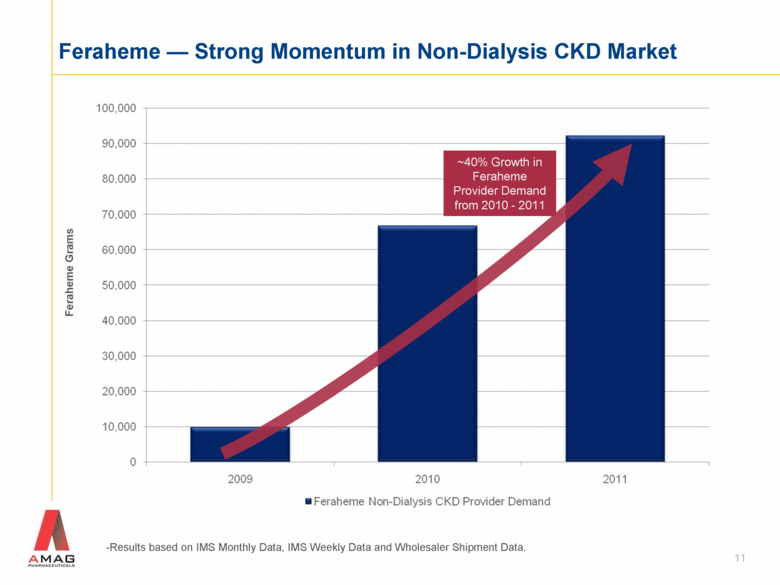
11 Feraheme — Strong Momentum in Non-Dialysis CKD Market -Results based on IMS Monthly Data, IMS Weekly Data and Wholesaler Shipment Data. Feraheme Grams ~40% Growth in Feraheme Provider Demand from 2010 - 2011 |
|
|
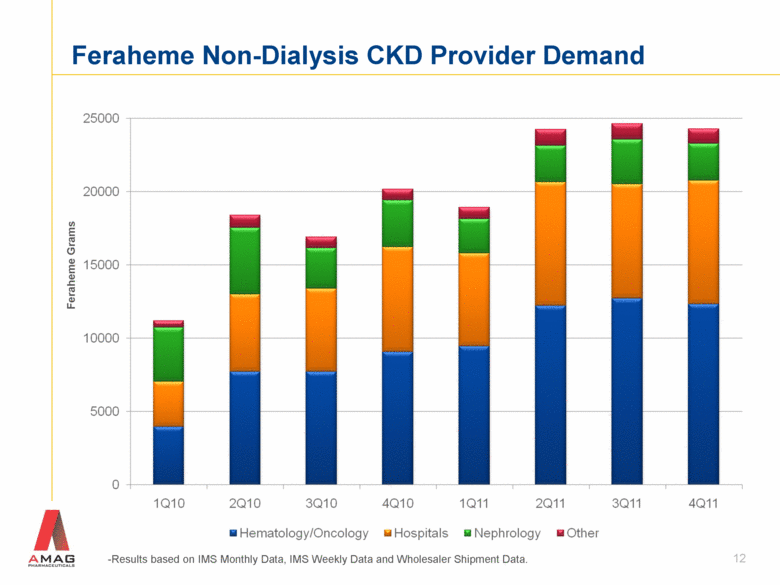
Feraheme Non-Dialysis CKD Provider Demand 12 Feraheme Grams -Results based on IMS Monthly Data, IMS Weekly Data and Wholesaler Shipment Data. |
|
|
Enhance commercial leadership at AMAG Hired VP of commercial operations in January 2012 Hired VP of commercial analytics in January 2012 Optimize Feraheme’s pricing strategy Minimize opportunistic discounting Stabilize Feraheme ASP Grow Feraheme CKD IDA Market Share Hematology/Oncology Maintain existing users through traditional rep-driven and non-personal marketing communications Drive adoption of non-users through traditional rep-driven efforts and through GPO and oncology physician network access Hospitals Pull-through existing hospital efforts; leverage existing hospital contracts Establish new hospital accounts; focus on institutions with large outpatient infusion centers Maintain Feraheme as market leader in Nephrology segment Emphasize clinical benefits of Feraheme 2012 Key Commercial Imperatives 13 |
|
|
Growth Opportunities 14 |
|
|
collaboration in place for EU and numerous ex-U.S. territories Takeda is a leading specialty pharmaceutical company with significant commercial capabilities AMAG leads clinical development activities in the licensed territories and regulatory filings in EU Takeda leads regulatory filings in all other countries AMAG supplies commercial and clinical material to Takeda Takeda leads commercialization activities in the licensed territories Potential Milestones for AMAG from Takeda Alliance: $33 million in milestones expected in 2012 upon IDA CKD approval and commercial launch in EU and Canada Significant milestone upon broad IDA approval in EU Attractive tiered, double-digit royalties on product sales in licensed regions Additional milestone payments for achievement of commercial sales targets collaboration in China Chinese CKD registrational program expected to begin in 2012 Strong Partnerships to Expand Opportunity Outside U.S. 15 |
|
|
Canadian regulatory approval was granted in December 2011 Feraheme is expected to be launched in Canada in 2012 Marketing Authorization Application is under review by the European Medicines Agency AMAG recently requested, and was granted, a 60-day extension to the filing timeline in order to fully respond to the final set of questions from the Agency to support the optimal label in the E.U. AMAG currently expects a decision from the Committee for Medicinal Products for Human Use (CHMP) in the first half of 2012 $33 million in milestones expected in 2012 upon IDA CKD approval and commercial launch in EU and commercial launch in Canada Ex-US CKD IDA Expansion of Feraheme 16 |
|
|
Global phase III registrational program for Feraheme in patients with IDA, regardless of the underlying cause, 98% enrolled Phase III, double-blind, placebo-controlled trial Primary endpoint: mean change in hemoglobin from Baseline to Week 5 [Day 35] Target enrollment: 800 patients; enrollment nearing completion Randomized 3:1 (600 Feraheme, 200 Placebo) Open-label extension study of 500 – 800 patients planned to assess retreatment over 6-month period Phase III, open-label, active-controlled trial comparing ferumoxytol with IV iron sucrose Primary endpoint: mean change in hemoglobin from Baseline to Week 5 [Day 35] Fully enrolled with >600 patients Randomized 2:1 (400 Feraheme, 200 iron sucrose) Pursuing Broad IDA Label 17 |
|
|
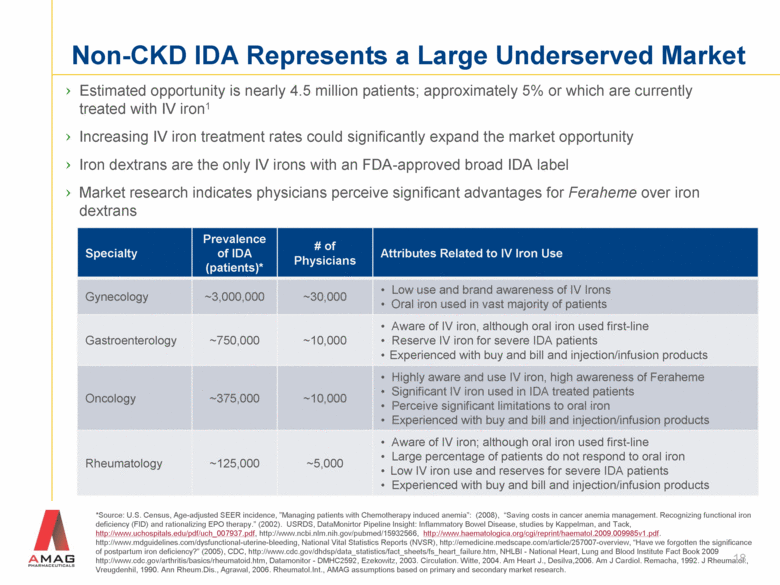
Non-CKD IDA Represents a Large Underserved Market Estimated opportunity is nearly 4.5 million patients; approximately 5% or which are currently treated with IV iron1 Increasing IV iron treatment rates could significantly expand the market opportunity Iron dextrans are the only IV irons with an FDA-approved broad IDA label Market research indicates physicians perceive significant advantages for Feraheme over iron dextrans *Source: U.S. Census, Age-adjusted SEER incidence, ”Managing patients with Chemotherapy induced anemia”: (2008), “Saving costs in cancer anemia management. Recognizing functional iron deficiency (FID) and rationalizing EPO therapy.” (2002). USRDS, DataMonirtor Pipeline Insight: Inflammatory Bowel Disease, studies by Kappelman, and Tack, http://www.uchospitals.edu/pdf/uch_007937.pdf, http://www.ncbi.nlm.nih.gov/pubmed/15932566, http://www.haematologica.org/cgi/reprint/haematol.2009.009985v1.pdf. http://www.mdguidelines.com/dysfunctional-uterine-bleeding, National Vital Statistics Reports (NVSR), http://emedicine.medscape.com/article/257007-overview, “Have we forgotten the significance of postpartum iron deficiency?” (2005), CDC, http://www.cdc.gov/dhdsp/data_statistics/fact_sheets/fs_heart_failure.htm, NHLBI - National Heart, Lung and Blood Institute Fact Book 2009 http://www.cdc.gov/arthritis/basics/rheumatoid.htm, Datamonitor - DMHC2592, Ezekowitz, 2003. Circulation. Witte, 2004. Am Heart J., Desilva,2006. Am J Cardiol. Remacha, 1992. J Rheumatol., Vreugdenhil, 1990. Ann Rheum.Dis., Agrawal, 2006. Rheumatol.Int., AMAG assumptions based on primary and secondary market research. 18 Specialty Prevalence of IDA (patients)* # of Physicians Attributes Related to IV Iron Use Gynecology ~3,000,000 ~30,000 Low use and brand awareness of IV Irons Oral iron used in vast majority of patients Gastroenterology ~750,000 ~10,000 Aware of IV iron, although oral iron used first-line Reserve IV iron for severe IDA patients Experienced with buy and bill and injection/infusion products Oncology ~375,000 ~10,000 Highly aware and use IV iron, high awareness of Feraheme Significant IV iron used in IDA treated patients Perceive significant limitations to oral iron Experienced with buy and bill and injection/infusion products Rheumatology ~125,000 ~5,000 Aware of IV iron; although oral iron used first-line Large percentage of patients do not respond to oral iron Low IV iron use and reserves for severe IDA patients Experienced with buy and bill and injection/infusion products |
|
|
Source: IMS Health DDD Data MAT Dec10 and AMAG company estimates. IV Iron Grams ~$190 million current U.S. CKD market opportunity assuming $500/gram ~$375 million current U.S. CKD and IDA market opportunity assuming $500/gram CKD1 CKD1 IDA Treated Today1 CKD1 IDA Potential1 ~$550 million potential U.S. market opportunity assuming a 5% increase in IV iron treatment rates for broader non-CKD patients with IDA at $500/gram Expanded Market Opportunity from Broad IDA Label 19 Broad IDA label would open access to 50% of the current U.S. non-dialysis IV iron market IDA Treated Today1 |
|
|
Expect to complete enrollment in 1Q2012 R&D investment in program winds down in 2012 U.S. sNDA filing targeted for 2H2012 Determining commercialization strategy for various specialties (Gastro, Oncology, OB/GYN) Potential broad IDA approval in EU would result in a significant milestone payment from Takeda Broad IDA Program — Next Steps 20 |
|
|
Financial Overview 21 |
|
|
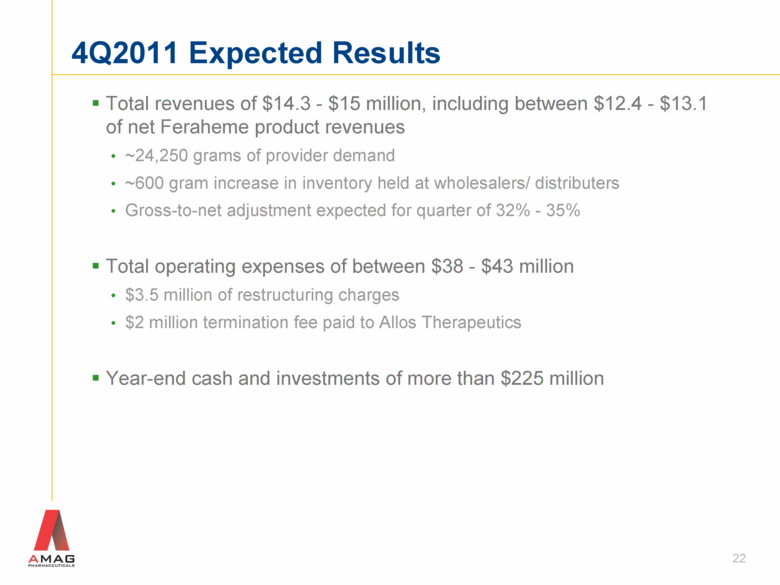
Total revenues of $14.3 - $15 million, including between $12.4 - $13.1 of net Feraheme product revenues ~24,250 grams of provider demand ~600 gram increase in inventory held at wholesalers/ distributers Gross-to-net adjustment expected for quarter of 32% - 35% Total operating expenses of between $38 - $43 million $3.5 million of restructuring charges $2 million termination fee paid to Allos Therapeutics Year-end cash and investments of more than $225 million 22 4Q2011 Expected Results |
|
|
2012 Milestones and Financial Outlook Clinical and regulatory milestones Complete enrollment of broad IDA phase 3 registration program Regulatory decisions in international markets on CKD filings Present/publish IDA program data Submit sNDA in US for broad IDA indication Commercial and business milestones Complete strategic alternatives process Support international launches Financial Outlook Low double-digit product revenue growth Expect total operating expenses, excluding COGS, of $90 – $100 million Expect to receive $33 million in milestones associated with potential regulatory approvals and commercial launches in the EU and Canada Committed to managing business to achieve cash flow neutrality, ending 2012 with $225 - $230 million of cash and investments, including milestone payments 23 |
|
|
Leveraging Core Assets to Create Long-term Stockholder Value Core Assets Strong balance sheet, with more than $225 million in cash and no debt Talented employee base, with a U.S. commercial footprint, including a sales force, focused on hematology/oncology and hospitals Feraheme, a marketed drug that generates ~$50 million a year in U.S. revenues Organic growth potential through geographic and label expansion Strategic Review Process Working with strategic advisors to evaluate all strategic options to enhance stockholder value and leverage AMAG’s core assets Managing expenses to ensure our balance sheet remains strong to allow us to thoroughly evaluate the various options 24 |
|
|
AMAG Pharmaceuticals 25 INVESTOR RELATIONS CONTACT: 617-498-3303 |