Attached files
| file | filename |
|---|---|
| 8-K - 8-K - SYNERGY PHARMACEUTICALS, INC. | a11-32331_18k.htm |
Exhibit 99.1
|
|
Corporate Presentation December 2011 PK |
|
|
Certain statements made in this presentation are forward-looking. Such statements are indicated by words such as “expect,” “should,” “anticipate” and similar words indicating uncertainty in facts and figures. Although Synergy believes that the expectations reflected in such forward-looking statements are reasonable, it can give no assurance that such expectations reflected in such forward-looking statements will prove to be correct. As discussed in the Synergy Pharmaceuticals Annual Report on Form 10-K for the year ended December 31, 2010, and other periodic reports, as filed with the Securities and Exchange Commission, actual results could differ materially from those projected in the forward-looking statements as a result of the following factors, among others: uncertainties associated with product development, the risk that products that appeared promising in early clinical trials do not demonstrate efficacy in larger-scale clinical trials, the risk that Synergy will not obtain approval to market its products, the risks associated with dependence upon key personnel and the need for additional financing. Forward-Looking Information |
|
|
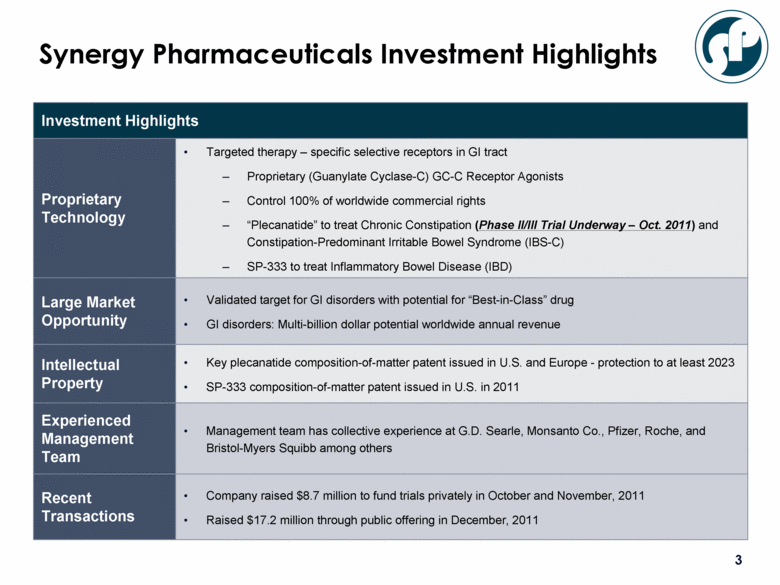
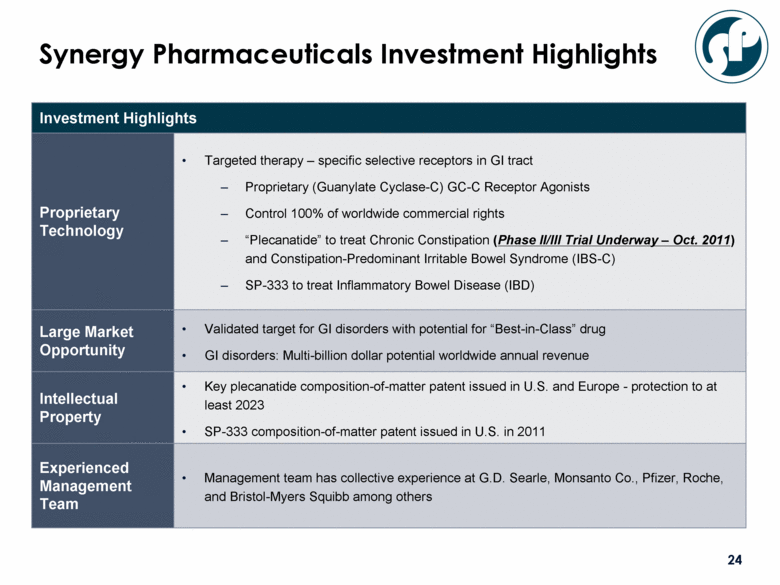
Investment Highlights Proprietary Technology Targeted therapy – specific selective receptors in GI tract Proprietary (Guanylate Cyclase-C) GC-C Receptor Agonists Control 100% of worldwide commercial rights “Plecanatide” to treat Chronic Constipation (Phase II/III Trial Underway – Oct. 2011) and Constipation-Predominant Irritable Bowel Syndrome (IBS-C) SP-333 to treat Inflammatory Bowel Disease (IBD) Large Market Opportunity Validated target for GI disorders with potential for “Best-in-Class” drug GI disorders: Multi-billion dollar potential worldwide annual revenue Intellectual Property Key plecanatide composition-of-matter patent issued in U.S. and Europe - protection to at least 2023 SP-333 composition-of-matter patent issued in U.S. in 2011 Experienced Management Team Management team has collective experience at G.D. Searle, Monsanto Co., Pfizer, Roche, and Bristol-Myers Squibb among others Recent Transactions Company raised $8.7 million to fund trials privately in October and November, 2011 Raised $17.2 million through public offering in December, 2011 Synergy Pharmaceuticals Investment Highlights |
|
|
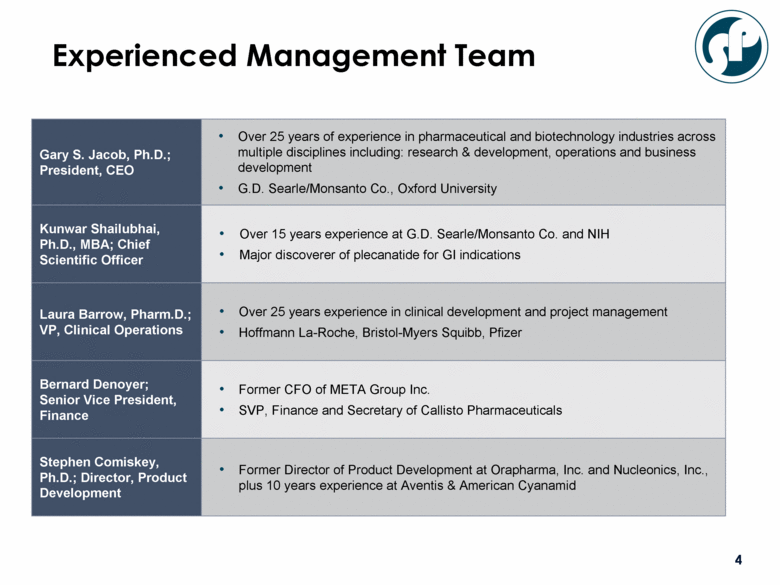
Gary S. Jacob, Ph.D.; President, CEO Over 25 years of experience in pharmaceutical and biotechnology industries across multiple disciplines including: research & development, operations and business development G.D. Searle/Monsanto Co., Oxford University Kunwar Shailubhai, Ph.D., MBA; Chief Scientific Officer Over 15 years experience at G.D. Searle/Monsanto Co. and NIH Major discoverer of plecanatide for GI indications Laura Barrow, Pharm.D.; VP, Clinical Operations Over 25 years experience in clinical development and project management Hoffmann La-Roche, Bristol-Myers Squibb, Pfizer Bernard Denoyer; Senior Vice President, Finance Former CFO of META Group Inc. SVP, Finance and Secretary of Callisto Pharmaceuticals Stephen Comiskey, Ph.D.; Director, Product Development Former Director of Product Development at Orapharma, Inc. and Nucleonics, Inc., plus 10 years experience at Aventis & American Cyanamid Experienced Management Team |
|
|
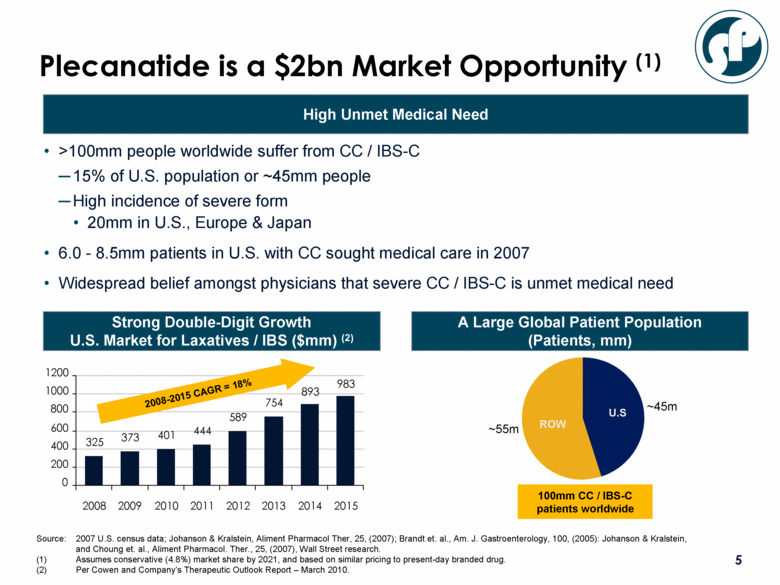
Plecanatide is a $2bn Market Opportunity (1) >100mm people worldwide suffer from CC / IBS-C 15% of U.S. population or ~45mm people High incidence of severe form 20mm in U.S., Europe & Japan 6.0 - 8.5mm patients in U.S. with CC sought medical care in 2007 Widespread belief amongst physicians that severe CC / IBS-C is unmet medical need Source: 2007 U.S. census data; Johanson & Kralstein, Aliment Pharmacol Ther, 25, (2007); Brandt et. al., Am. J. Gastroenterology, 100, (2005): Johanson & Kralstein, and Choung et. al., Aliment Pharmacol. Ther., 25, (2007), Wall Street research. (1) Assumes conservative (4.8%) market share by 2021, and based on similar pricing to present-day branded drug. (2) Per Cowen and Company’s Therapeutic Outlook Report – March 2010. A Large Global Patient Population (Patients, mm) High Unmet Medical Need Strong Double-Digit Growth U.S. Market for Laxatives / IBS ($mm) (2) ~45m ~55m ROW U.S 100mm CC / IBS-C patients worldwide 325 373 401 444 589 754 893 983 0 200 400 600 800 1000 1200 2008 2009 2010 2011 2012 2013 2014 2015 2008-2015 CAGR = 18% |
|
|
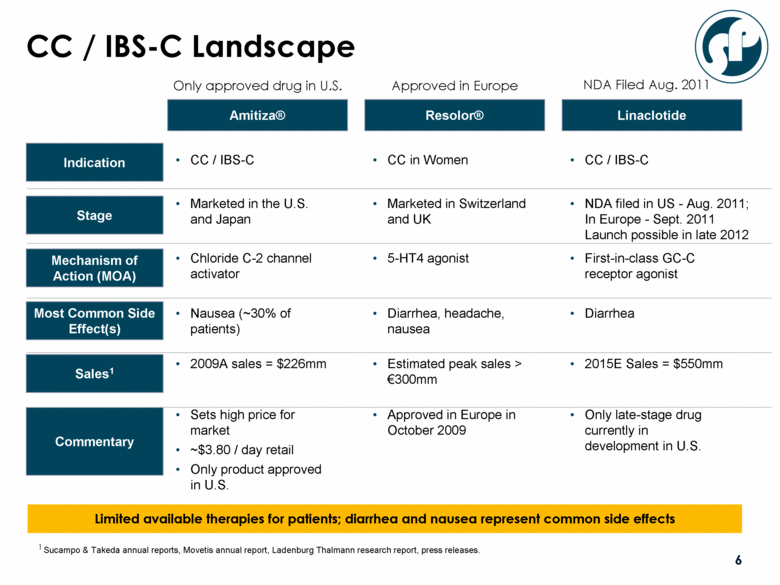
CC / IBS-C Landscape Sucampo & Takeda annual reports, Movetis annual report, Ladenburg Thalmann research report, press releases. Amitiza® Resolor® Linaclotide Only late-stage drug currently in development in U.S. Indication Stage Mechanism of Action (MOA) Most Common Side Effect(s) Sales1 Commentary 2015E Sales = $550mm Diarrhea First-in-class GC-C receptor agonist NDA filed in US - Aug. 2011; In Europe - Sept. 2011 Launch possible in late 2012 CC / IBS-C Approved in Europe in October 2009 Estimated peak sales > €300mm Diarrhea, headache, nausea 5-HT4 agonist Marketed in Switzerland and UK CC in Women Sets high price for market ~$3.80 / day retail Only product approved in U.S. 2009A sales = $226mm Nausea (~30% of patients) Chloride C-2 channel activator Marketed in the U.S. and Japan CC / IBS-C Limited available therapies for patients; diarrhea and nausea represent common side effects Only approved drug in U.S. Approved in Europe NDA Filed Aug. 2011 1 |
|
|
Mechanism of Action Clinical Data Regulatory & Clinical Plan Intellectual Property Competitive Positioning |
|
|
Guanylate Cyclase C Receptor Agonists Physiological mechanism Cross section of the GI tract Activation of GC-C receptors stimulates synthesis of cyclic GMP, activating cystic fibrosis transmembrane conductance regulator (CFTR) Activated CFTR secretes Cl-, HCO3- and fluid into the intestinal lumen. Secretion of fluid into intestine is critical for normal digestion 2 3 Uroguanylin (UroG) activates Guanylate Cyclase-C (GC-C) receptors on the lumenal side of the gut 1 1 2 3 4 Plecanatide binds to GC-C receptors, promoting spontaneous bowel movement (SBM) and reducing abdominal discomfort and bloating 4 Plecanatide |
|
|
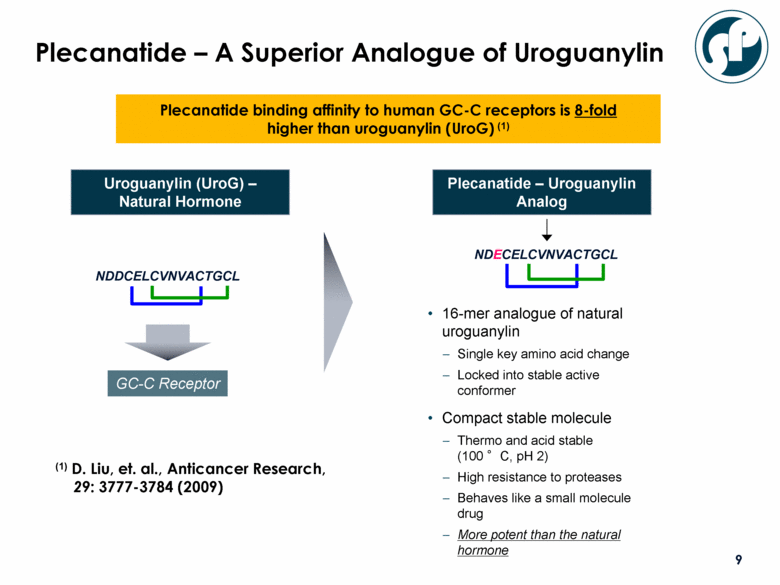
Plecanatide – A Superior Analogue of Uroguanylin 16-mer analogue of natural uroguanylin Single key amino acid change Locked into stable active conformer Compact stable molecule Thermo and acid stable (100 °C, pH 2) High resistance to proteases Behaves like a small molecule drug More potent than the natural hormone NDDCELCVNVACTGCL NDECELCVNVACTGCL GC-C Receptor Uroguanylin (UroG) – Natural Hormone Plecanatide – Uroguanylin Analog Plecanatide binding affinity to human GC-C receptors is 8-fold higher than uroguanylin (UroG) (1) (1) D. Liu, et. al., Anticancer Research, 29: 3777-3784 (2009) |
|
|
Mechanism of Action Clinical Data Regulatory & Clinical Plan Intellectual Property Competitive Positioning |
|
|
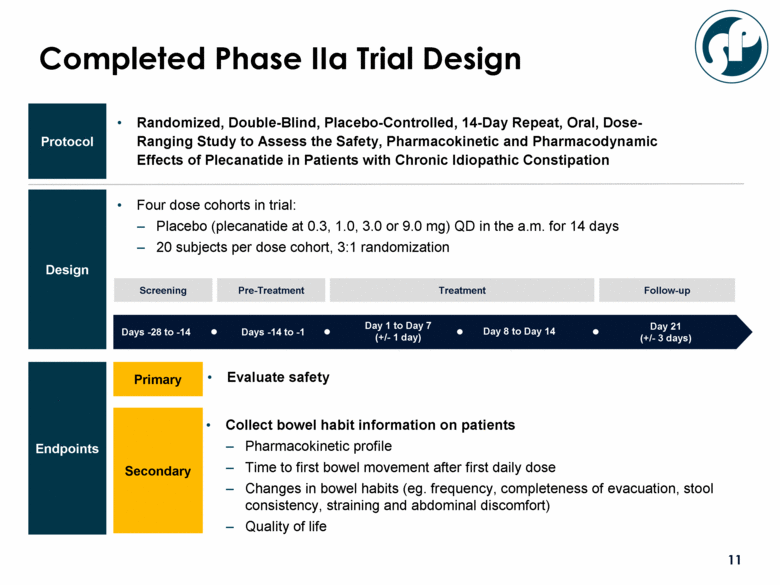
Four dose cohorts in trial: Placebo (plecanatide at 0.3, 1.0, 3.0 or 9.0 mg) QD in the a.m. for 14 days 20 subjects per dose cohort, 3:1 randomization Protocol Design Completed Phase IIa Trial Design Days -28 to -14 Days -14 to -1 Day 1 to Day 7 (+/- 1 day) Day 21 (+/- 3 days) Screening Pre-Treatment Follow-up Treatment Day 8 to Day 14 Primary Secondary Collect bowel habit information on patients Pharmacokinetic profile Time to first bowel movement after first daily dose Changes in bowel habits (eg. frequency, completeness of evacuation, stool consistency, straining and abdominal discomfort) Quality of life Evaluate safety Randomized, Double-Blind, Placebo-Controlled, 14-Day Repeat, Oral, Dose-Ranging Study to Assess the Safety, Pharmacokinetic and Pharmacodynamic Effects of Plecanatide in Patients with Chronic Idiopathic Constipation Endpoints |
|
|
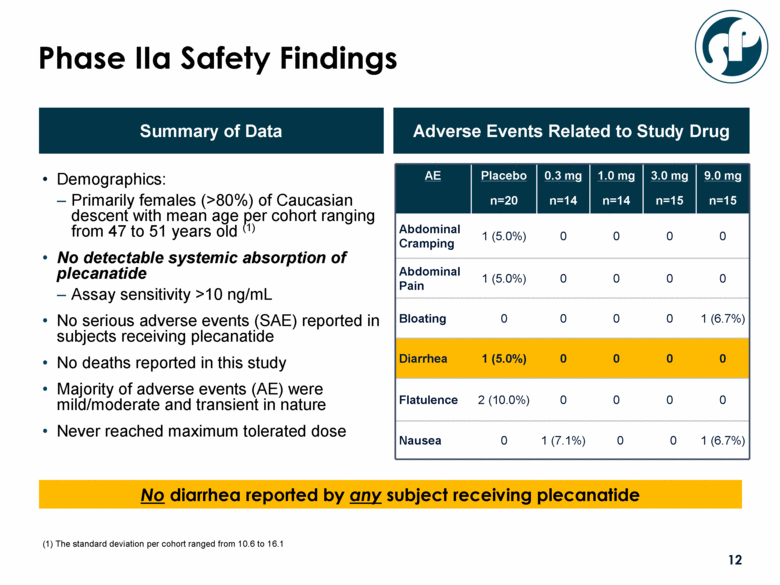
Demographics: Primarily females (>80%) of Caucasian descent with mean age per cohort ranging from 47 to 51 years old (1) No detectable systemic absorption of plecanatide Assay sensitivity >10 ng/mL No serious adverse events (SAE) reported in subjects receiving plecanatide No deaths reported in this study Majority of adverse events (AE) were mild/moderate and transient in nature Never reached maximum tolerated dose Summary of Data Adverse Events Related to Study Drug AE Placebo n=20 0.3 mg n=14 1.0 mg n=14 3.0 mg n=15 9.0 mg n=15 Abdominal Cramping 1 (5.0%) 0 0 0 0 Abdominal Pain 1 (5.0%) 0 0 0 0 Bloating 0 0 0 0 1 (6.7%) Diarrhea 1 (5.0%) 0 0 0 0 Flatulence 2 (10.0%) 0 0 0 0 Nausea 0 1 (7.1%) 0 0 1 (6.7%) Phase IIa Safety Findings No diarrhea reported by any subject receiving plecanatide (1) The standard deviation per cohort ranged from 10.6 to 16.1 |
|
|
Phase IIa Study Findings and Conclusions More than 50% of patients on plecanatide had 1st bowel movement within 7 hours vs. 16 hours for placebo Plecanatide also demonstrates increase in number of spontaneous and complete spontaneous bowel movements Median Time to First Bowel Movement after Administration of First Daily Dose Change From Baseline in Bristol Stool Score BSFS 0 5 10 15 20 25 Placebo n=20 0.3 mg n=13 1 mg n=14 3 mg n=15 9 mg n=15 All Tx n=57 Median Time to First BM (Hours) Plecanatide Dose (mg) 0.0 0.5 1.0 1.5 2.0 2.5 Placebo n=20 0.3 mg n=13 1 mg n=14 3 mg n=15 9 mg n=15 All Tx n=57 Average Difference in BSFS Plecanatide Dose (mg) |
|
|
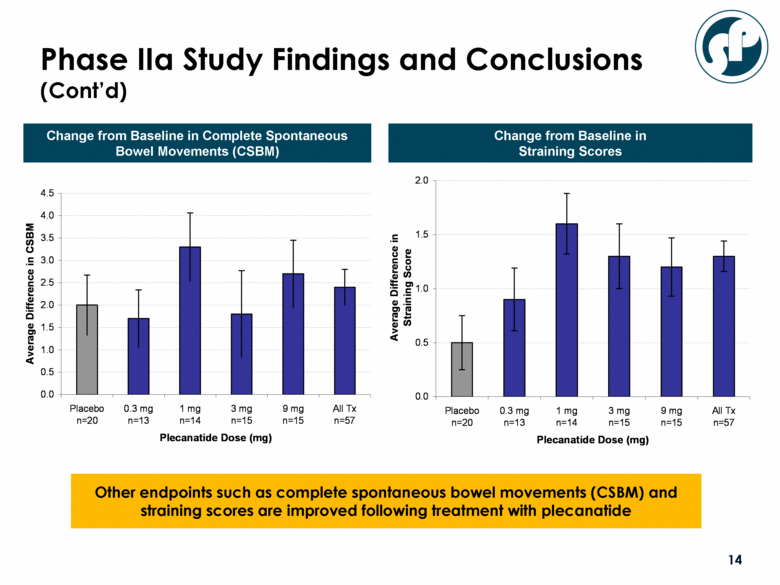
Phase IIa Study Findings and Conclusions (Cont’d) Other endpoints such as complete spontaneous bowel movements (CSBM) and straining scores are improved following treatment with plecanatide Change from Baseline in Complete Spontaneous Bowel Movements (CSBM) Change from Baseline in Straining Scores 0.0 0.5 1.0 1.5 2.0 2.5 3.0 3.5 4.0 4.5 Placebo n=20 0.3 mg n=13 1 mg n=14 3 mg n=15 9 mg n=15 All Tx n=57 Plecanatide Dose (mg) Average Difference in CSBM 0.0 0.5 1.0 1.5 2.0 Placebo n=20 0.3 mg n=13 1 mg n=14 3 mg n=15 9 mg n=15 All Tx n=57 Plecanatide Dose (mg) Average Difference in Straining Score |
|
|
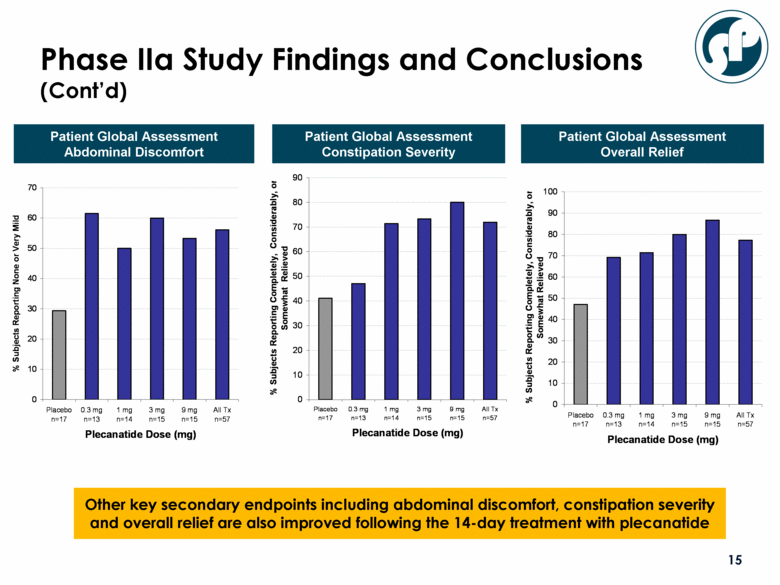
Other key secondary endpoints including abdominal discomfort, constipation severity and overall relief are also improved following the 14-day treatment with plecanatide Patient Global Assessment Abdominal Discomfort Patient Global Assessment Constipation Severity Patient Global Assessment Overall Relief Phase IIa Study Findings and Conclusions (Cont’d) 0 10 20 30 40 50 60 70 Placebo n=17 0.3 mg n=13 1 mg n=14 3 mg n=15 9 mg n=15 All Tx n=57 Plecanatide Dose (mg) % Subjects Reporting None or Very Mild 0 10 20 30 40 50 60 70 80 90 100 Placebo n=17 0.3 mg n=13 1 mg n=14 3 mg n=15 9 mg n=15 All Tx n=57 Plecanatide Dose (mg) % Subjects Reporting Completely, Considerably, or Somewhat Relieved 0 10 20 30 40 50 60 70 80 90 Placebo n=17 0.3 mg n=13 1 mg n=14 3 mg n=15 9 mg n=15 All Tx n=57 Plecanatide Dose (mg) % Subjects Reporting Completely, Considerably, or Somewhat Relieved |
|
|
Plecanatide Clinical Summary: Efficacy in CIC Consistent with GC-C Receptor Agonists Increases the number of CSBMs & SBMs/week Doses > 1 mg resulted in 50% of patients experiencing their 1st bowel movement within 7 hours vs. 16 hours for placebo Improves straining scores, stool consistency, abdominal pain & QoL measures No detectable plasma concentrations (LOD: 10ng/ml) Majority of adverse events mild/moderate and transient: Abdominal pain & bloating, nausea Mild to moderate in intensity No reported diarrhea in constipation patients receiving plecanatide , to date |
|
|
Mechanism of Action Clinical Data Regulatory & Clinical Plan Intellectual Property Competitive Positioning |
|
|
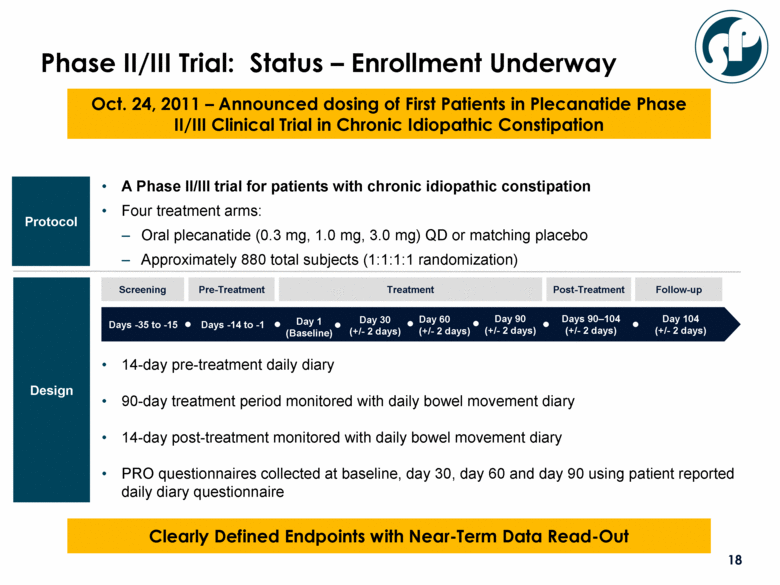
A Phase II/III trial for patients with chronic idiopathic constipation Four treatment arms: Oral plecanatide (0.3 mg, 1.0 mg, 3.0 mg) QD or matching placebo Approximately 880 total subjects (1:1:1:1 randomization) Protocol Design Phase II/III Trial: Status – Enrollment Underway Days -35 to -15 Days -14 to -1 Day 1 (Baseline) Day 30 (+/- 2 days) Day 104 (+/- 2 days) Days 90–104 (+/- 2 days) Day 90 (+/- 2 days) Day 60 (+/- 2 days) Clearly Defined Endpoints with Near-Term Data Read-Out Screening Pre-Treatment Follow-up Treatment Post-Treatment 14-day pre-treatment daily diary 90-day treatment period monitored with daily bowel movement diary 14-day post-treatment monitored with daily bowel movement diary PRO questionnaires collected at baseline, day 30, day 60 and day 90 using patient reported daily diary questionnaire Oct. 24, 2011 – Announced dosing of First Patients in Plecanatide Phase II/III Clinical Trial in Chronic Idiopathic Constipation |
|
|
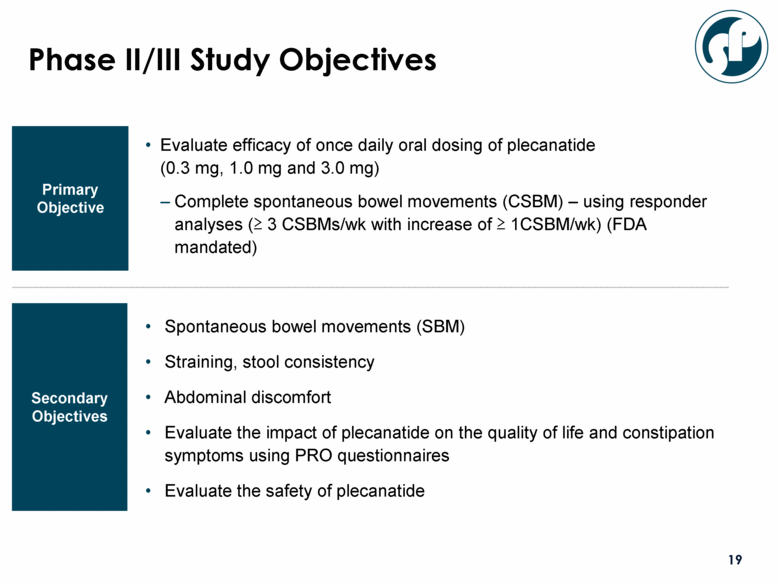
Phase II/III Study Objectives Evaluate efficacy of once daily oral dosing of plecanatide (0.3 mg, 1.0 mg and 3.0 mg) Complete spontaneous bowel movements (CSBM) – using responder analyses (> 3 CSBMs/wk with increase of > 1CSBM/wk) (FDA mandated) Primary Objective Secondary Objectives Spontaneous bowel movements (SBM) Straining, stool consistency Abdominal discomfort Evaluate the impact of plecanatide on the quality of life and constipation symptoms using PRO questionnaires Evaluate the safety of plecanatide |
|
|
Near-Term Milestones Value Catalysts Began Phase II/III trial in October, 2011; Increased awareness within GI Community Key IP include plecanatide "composition of matter" patents in U.S., EU; and SP-333 U.S. “composition-of-matter” patent issued in 2011 Control 100% of worldwide rights Plecanatide potential as “best-in-class" in multi-billion U.S. market 2011 2012 2013 2014 EOP2 Meeting for CC Plan for Plecanatide NDA Filing File SP-333 IND Value Inflection Points 2ND Plecanatide Ph. III CC Pivotal Trial Top Line Data Initiate SP-333 Phase I Trial in Volunteers Oct., 2011 - Began Plecanatide Ph. II/III CC Trial Top Line Data Plecanatide IBS-C Ph. IIb Trial Top Line Data |
|
|
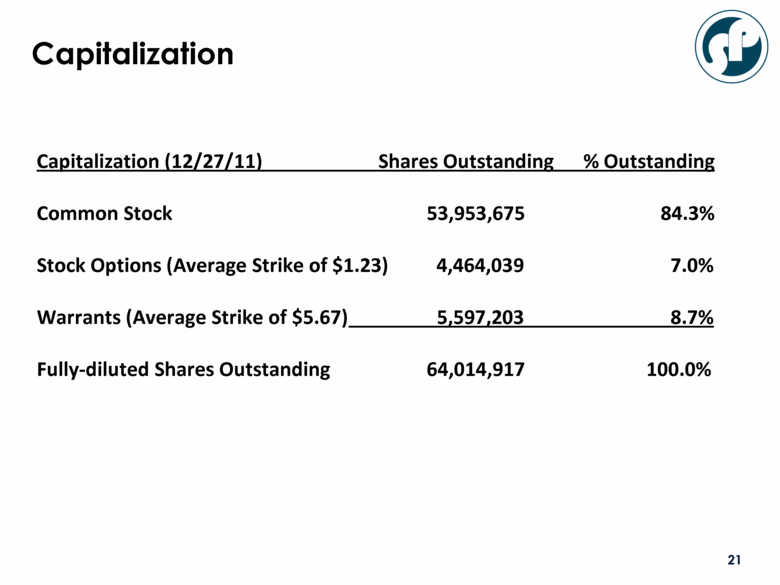
Capitalization Capitalization (12/27/11) Shares Outstanding % Outstanding Common Stock 53,953,675 84.3% Stock Options (Average Strike of $1.23) 4,464,039 7.0% Warrants (Average Strike of $5.67) 5,597,203 8.7% Fully-diluted Shares Outstanding 64,014,917 100.0% |
|
|
Mechanism of Action Clinical Data Regulatory & Clinical Plan Intellectual Property Competitive Positioning |
|
|
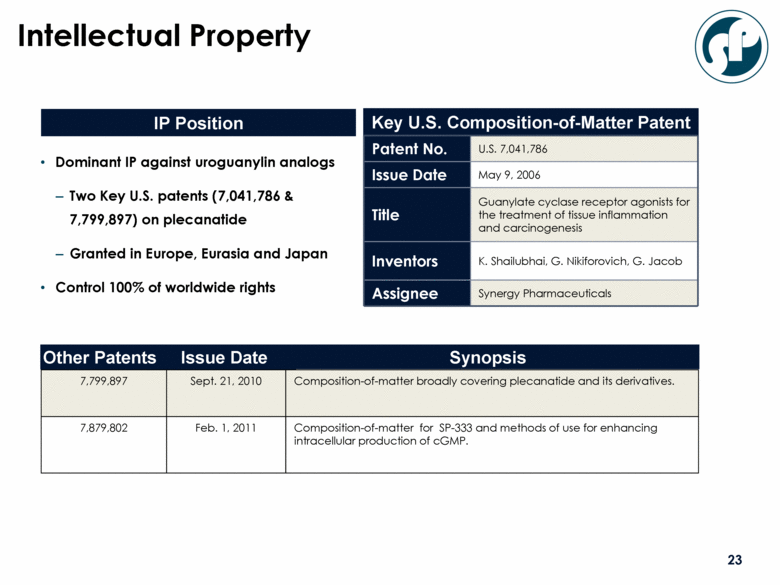
Intellectual Property IP Position Key U.S. Composition-of-Matter Patent Patent No. U.S. 7,041,786 Issue Date May 9, 2006 Title Guanylate cyclase receptor agonists for the treatment of tissue inflammation and carcinogenesis Inventors K. Shailubhai, G. Nikiforovich, G. Jacob Assignee Synergy Pharmaceuticals Synopsis Issue Date Other Patents 7,799,897 7,879,802 Sept. 21, 2010 Feb. 1, 2011 Composition-of-matter broadly covering plecanatide and its derivatives. Composition-of-matter for SP-333 and methods of use for enhancing intracellular production of cGMP. Dominant IP against uroguanylin analogs Two Key U.S. patents (7,041,786 & 7,799,897) on plecanatide Granted in Europe, Eurasia and Japan Control 100% of worldwide rights |
|
|
Investment Highlights Proprietary Technology Targeted therapy – specific selective receptors in GI tract Proprietary (Guanylate Cyclase-C) GC-C Receptor Agonists Control 100% of worldwide commercial rights “Plecanatide” to treat Chronic Constipation (Phase II/III Trial Underway – Oct. 2011) and Constipation-Predominant Irritable Bowel Syndrome (IBS-C) SP-333 to treat Inflammatory Bowel Disease (IBD) Large Market Opportunity Validated target for GI disorders with potential for “Best-in-Class” drug GI disorders: Multi-billion dollar potential worldwide annual revenue Intellectual Property Key plecanatide composition-of-matter patent issued in U.S. and Europe - protection to at least 2023 SP-333 composition-of-matter patent issued in U.S. in 2011 Experienced Management Team Management team has collective experience at G.D. Searle, Monsanto Co., Pfizer, Roche, and Bristol-Myers Squibb among others Synergy Pharmaceuticals Investment Highlights |
|
|
Appendix |
|
|
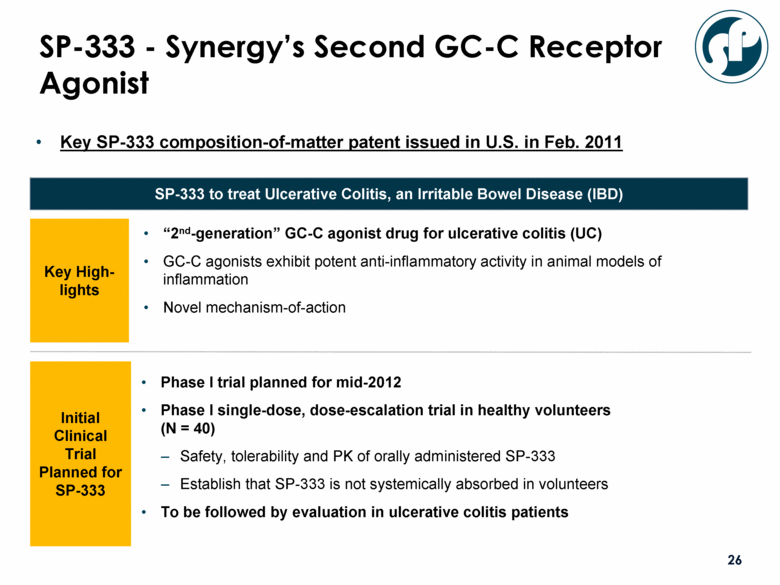
SP-333 - Synergy’s Second GC-C Receptor Agonist “2nd-generation” GC-C agonist drug for ulcerative colitis (UC) GC-C agonists exhibit potent anti-inflammatory activity in animal models of inflammation Novel mechanism-of-action SP-333 to treat Ulcerative Colitis, an Irritable Bowel Disease (IBD) Key High-lights Initial Clinical Trial Planned for SP-333 Phase I trial planned for mid-2012 Phase I single-dose, dose-escalation trial in healthy volunteers (N = 40) Safety, tolerability and PK of orally administered SP-333 Establish that SP-333 is not systemically absorbed in volunteers To be followed by evaluation in ulcerative colitis patients Key SP-333 composition-of-matter patent issued in U.S. in Feb. 2011 |
|
|
Board of Directors Gabriele Cerrone, Chairman Founder of number of biotechnology and diagnostic companies: FermaVir, Inc. (Acquired by Inhibitex, Inc.) SIGA Technologies, Inc. Synergy Pharmaceuticals, Inc. Trovagene, Inc., a molecular diagnostic company Callisto Pharmaceuticals, Inc. Managing partner of Panetta Partners Ltd. Thomas H. Adams, Ph.D. Seasoned corporate executive and scientist with extensive medical technology experience. Co-founded Gen-Probe Inc. and served as Chairman and Chief Executive Officer Former Senior Vice President of Research and Development and Chief Technology Officer of Hybritech Co-founded Genta, Inc. and served as Chief Executive Officer Director of number of Biotechnology and Diagnostic Companies John Brancaccio Chief Financial Officer of Accelerated Technologies, Inc., an incubator for medical device companies. Former Chief Financial Officer of Memory Pharmaceuticals Corp. Former Chief Financial Officer/Chief Operating Officer of Eline Group, an entertainment and media company. Currently director of Alfacell Corporation, Xenomics, Inc. and Callisto Pharmaceuticals, Inc. Gary S. Jacob, Ph.D.; President & CEO Over 25 years experience in pharmaceutical and biotechnology industries across research & development, operations and business development. Monsanto Science Fellow, specializing in the field of glycobiology, and Director of Glycobiology at G.D. Searle Pharmaceuticals Inc. Former Manager of the G.D. Searle Glycobiology Group at Oxford University, England. Alan F. Joslyn, Ph.D. Currently Chief Executive Officer of Edusa Pharmaceuticals Former Senior Vice President of Research & Development at Penwest Pharmaceuticals. Held leadership positions at Johnson & Johnson, focused on development of GI products including: Propulsid®, Motilium®, Aciphex® and prucalopride. Chris McGuigan, Ph.D. Professor and Deputy Pro-Vice Chancellor, University of Cardiff, England Chairman of Departmental Research Committee and Director of Research, and Head of Medicinal Chemistry, Welsh School of Pharmacy Immediate Past President of International Society for Antiviral Research Melvin Spigelman, MD Currently CEO of the Global Alliance for TB Drug Development, a non-profit discovery and development organization focused on drugs to fight tuberculosis. Former President of Hudson-Douglas Ltd, a consulting company. Former Vice President and Head of R&D at Knoll Pharmaceuticals, a pharmaceutical unit of BASF Pharma. |