Attached files
Exhibit 99.4

Baxter International Inc.
Synovis and Baxter:
Saving and Sustaining Lives - Together
December 13, 2011
Baxter Confidential - For Internal Use Only
1

Our Discussion Today
• An Introduction to Baxter
• Our Common Values
• Strategic Fit
• What Happens Next
• Discussion and Q&A
Baxter Confidential - For Internal Use Only
2

Trust,
Mutual Respect,
High Communication
High Communication
and a Spirit of Cooperation
What Synovis Stands For…
Baxter Confidential - For Internal Use Only
3

What Baxter Stands For…
Saving and Sustaining Lives Worldwide
Baxter Confidential - For Internal Use Only
4

Introducing Baxter
Baxter is a global, diversified healthcare company applying innovative science to
develop specialty therapeutics and medical products that save and sustain
patients’ lives.
develop specialty therapeutics and medical products that save and sustain
patients’ lives.
Every day, our products and services help treat thousands of people around the
world with some of the most complex medical conditions — like hemophilia,
immune disorders, end-stage kidney disease, or challenging surgical procedures.
world with some of the most complex medical conditions — like hemophilia,
immune disorders, end-stage kidney disease, or challenging surgical procedures.
Baxter Confidential - For Internal Use Only
5
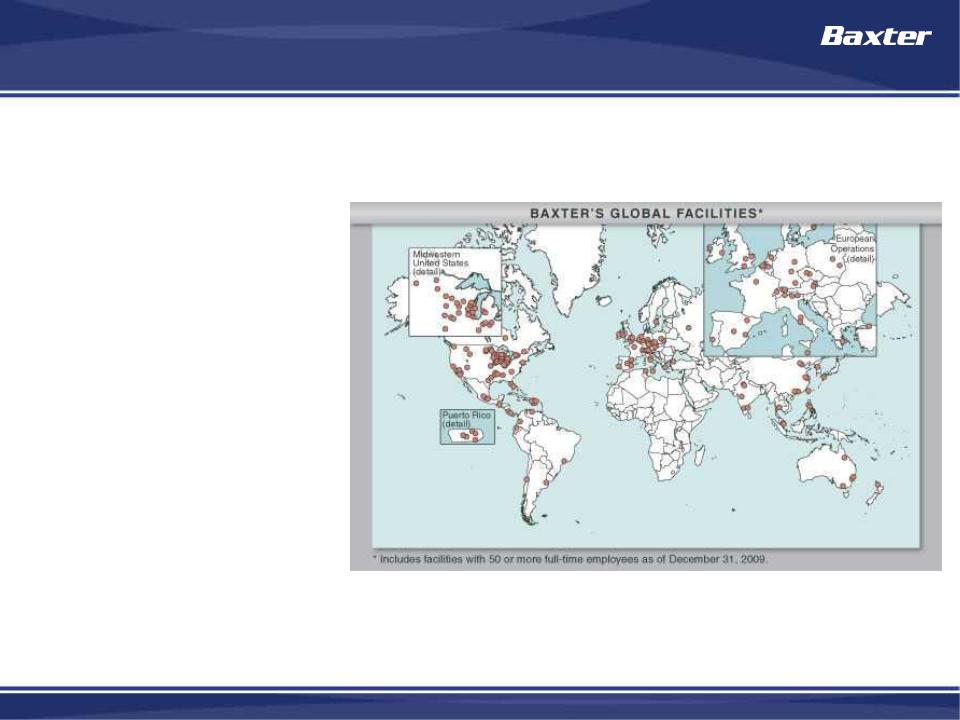
Global Healthcare Company
• Approximately 48,000
employees in more than
60 countries
employees in more than
60 countries
• Manufacturing facilities in
27 countries
27 countries
• Products sold in more
than 100 countries
than 100 countries
• More than half of sales
and earnings come from
outside the United States
and earnings come from
outside the United States
Baxter is an international company with a strong global brand and broad
geographic reach:
geographic reach:
Baxter Confidential - For Internal Use Only
6

At Baxter…
• Recognized and trusted worldwide
• A preferred partner in improving the quality of and access to healthcare
• An innovator in science and technology
• Committed to quality and excellence
• Focused on sustained financial strength
• A rewarding place to work and develop
• A socially responsible member of our communities
…We aspire to build a truly great company by being:
Baxter Confidential - For Internal Use Only
7
Baxter - 80 Years Strong
• Strong values-based culture; based in Midwest
• Significant R&D investment
• Passionate team with significant depth of talent
and expertise
and expertise
• Extensive global presence and network
• Global brand equity that is highly respected
throughout the industry
throughout the industry
• Significant global capabilities in manufacturing,
distribution and direct selling
distribution and direct selling
• Strong patient and customer relationships
• Solid scientific and technological expertise
Baxter Confidential - For Internal Use Only
8

Diversified Portfolio and Expertise
• Diversified healthcare model provides
competitive advantage and enhances
company’s ability to innovate
competitive advantage and enhances
company’s ability to innovate
• Broad portfolio of medical devices,
biotechnology and pharmaceutical products
and therapies
biotechnology and pharmaceutical products
and therapies
• Diverse technology platforms across the
organization are leveraged to create unique
products to address unmet medical needs
organization are leveraged to create unique
products to address unmet medical needs
• Core technical competencies include drug
delivery, medical plastics, protein
development and manufacturing, separation
and purification, and sterilization
delivery, medical plastics, protein
development and manufacturing, separation
and purification, and sterilization
BioScience
Medical Products
Baxter Confidential - For Internal Use Only
9

BioScience Business
2010 Sales: $5.7 billion
Baxter has been working in specialty biologic therapies for 80 years,
and is a market leader in the areas of blood-based disorders for
hemophilia and biotherapeutics for immune deficiencies and other
conditions. Baxter’s BioScience franchises:
and is a market leader in the areas of blood-based disorders for
hemophilia and biotherapeutics for immune deficiencies and other
conditions. Baxter’s BioScience franchises:
•Hemophilia
•BioTherapeutics
•BioSurgery
•Vaccines
Baxter Confidential - For Internal Use Only
10

BioScience Business Product Offerings
BioScience produces
recombinant and plasma-based
therapies that are used to treat:
recombinant and plasma-based
therapies that are used to treat:
• Hemophilia and other bleeding
disorders
disorders
• Immune disorders
• Hypoalbuminemia and
hypovolemia
hypovolemia
• Alpha-1 antitrypsin deficiency
• Vaccines for seasonal and
pandemic influenza, tick-borne
encephalitis, and meningococcal
C meningitis
pandemic influenza, tick-borne
encephalitis, and meningococcal
C meningitis
BioScience also produces:
Baxter Confidential - For Internal Use Only
11

BioScience’s Mission is Built Upon our Strengths as an Organization
We deliver innovative specialty therapies through strong,
global brands that improve patients’ lives
global brands that improve patients’ lives
Baxter Confidential - For Internal Use Only
12
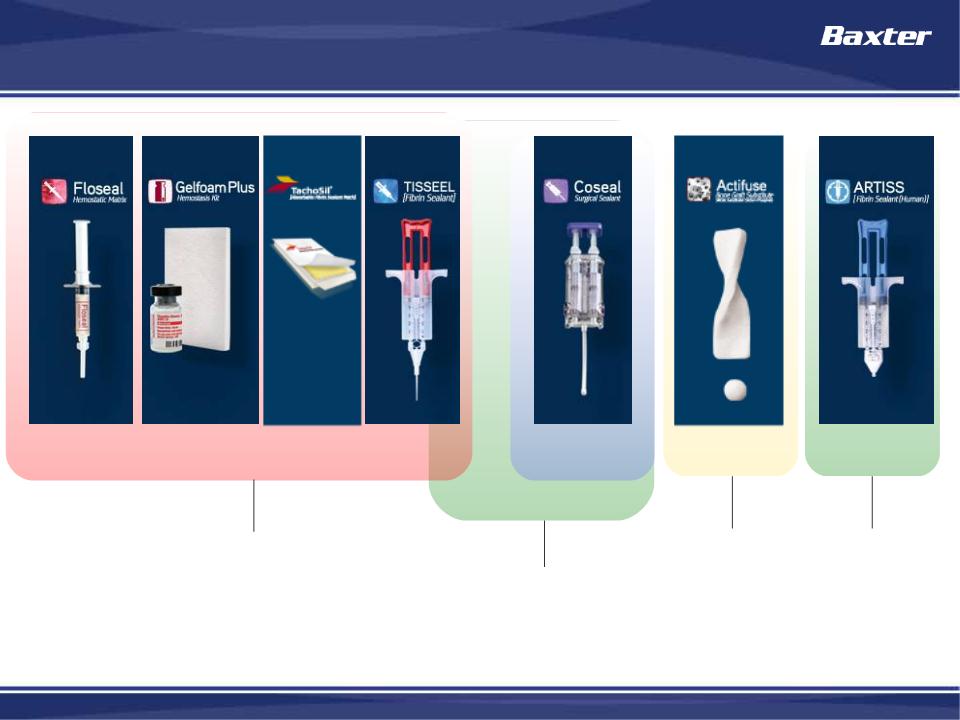
BioSurgery Franchise - Key Products
FLOSEAL
Gelfoam Plus
TISSEEL
Hemostasis
(stop bleeding)
Tissue
Sealing
Tissue
Fixation
COSEAL
ACTIFUSE
ARTISS
Tissue
Healing
TACHOSIL
Baxter Confidential - For Internal Use Only
13

Baxter BioSurgery
• Strong growth and global presence
• Commercial expertise
• Investing for the future
Baxter Confidential - For Internal Use Only
14

Synovis Strengths
• Synovis has a solid business with 26-year track
record
record
• Synovis’ deep insights in biological material
technology and surgical medical devices
technology and surgical medical devices
• Synovis’ product mix is complementary to
Baxter’s commercial product line and technical
capabilities in the BioSurgery space
Baxter’s commercial product line and technical
capabilities in the BioSurgery space
Baxter Confidential - For Internal Use Only
15

Questions?
Baxter Confidential - For Internal Use Only
16

Proxy Information
In connection with the proposed acquisition and required shareholder approval, Synovis will file with the U.S.
Securities and Exchange Commission (SEC) a proxy statement. The proxy statement will be mailed to the
shareholders of Synovis. Synovis’ shareholders are urged to read the proxy statement and other relevant materials
when they become available because they will contain important information about the acquisition and Synovis.
Investors and security holders may obtain free copies of these documents (when they are available) and other
documents filed with the SEC at the SEC’s web site at www.sec.gov. In addition, investors and security holders may
obtain free copies of the documents filed with the SEC by Synovis by going to Synovis’ Investor Information page on
its corporate website at www.synovislife.com.
Synovis and its officers and directors may be deemed to be participants in the solicitation of proxies from Synovis’
shareholders with respect to the acquisition. Information about Synovis’ executive officers and directors and their
ownership of Synovis stock is set forth in the proxy statement for the Synovis 2011 Annual Meeting of Shareholders,
which was filed with the SEC on January 18, 2011. Investors and security holders may obtain more detailed
information regarding the direct and indirect interests of Synovis and its executive officers and directors in the
acquisition by reading the preliminary and definitive proxy statements regarding the merger, which will be filed with
the SEC.
In addition, Baxter and its officers and directors may be deemed to have participated in the solicitation of proxies from
Synovis’ shareholders in favor of the approval of the merger. Information concerning Baxter’s directors and executive
officers is set forth in the proxy statement for the Baxter 2011 Annual Meeting of Stockholders, which was filed with
the SEC on March 18, 2011, and other reports filed with the SEC. These documents are available free of charge at
the SEC’s web site at www.sec.gov or by going to the Reports & Financials page of the Investors tab on Baxter’s
corporate website at www.baxter.com.
Securities and Exchange Commission (SEC) a proxy statement. The proxy statement will be mailed to the
shareholders of Synovis. Synovis’ shareholders are urged to read the proxy statement and other relevant materials
when they become available because they will contain important information about the acquisition and Synovis.
Investors and security holders may obtain free copies of these documents (when they are available) and other
documents filed with the SEC at the SEC’s web site at www.sec.gov. In addition, investors and security holders may
obtain free copies of the documents filed with the SEC by Synovis by going to Synovis’ Investor Information page on
its corporate website at www.synovislife.com.
Synovis and its officers and directors may be deemed to be participants in the solicitation of proxies from Synovis’
shareholders with respect to the acquisition. Information about Synovis’ executive officers and directors and their
ownership of Synovis stock is set forth in the proxy statement for the Synovis 2011 Annual Meeting of Shareholders,
which was filed with the SEC on January 18, 2011. Investors and security holders may obtain more detailed
information regarding the direct and indirect interests of Synovis and its executive officers and directors in the
acquisition by reading the preliminary and definitive proxy statements regarding the merger, which will be filed with
the SEC.
In addition, Baxter and its officers and directors may be deemed to have participated in the solicitation of proxies from
Synovis’ shareholders in favor of the approval of the merger. Information concerning Baxter’s directors and executive
officers is set forth in the proxy statement for the Baxter 2011 Annual Meeting of Stockholders, which was filed with
the SEC on March 18, 2011, and other reports filed with the SEC. These documents are available free of charge at
the SEC’s web site at www.sec.gov or by going to the Reports & Financials page of the Investors tab on Baxter’s
corporate website at www.baxter.com.
Baxter Confidential - For Internal Use Only
17

Safe Harbor
This material includes forward-looking statements concerning a definitive agreement between Baxter
International Inc. and Synovis Life Technologies, Inc. pursuant to which Baxter will acquire Synovis,
including expectations with respect to the closing of the transaction and its financial impact on
Baxter. The statements are based on assumptions about many important factors, including the
following, which could cause actual results to differ materially from those in the forward-looking
statements: the result of the review of the proposed transactions by various regulatory agencies, and
any conditions imposed on the companies in connection with consummation of the transactions
described herein; approval of the merger by the shareholders of Synovis; satisfaction of various other
conditions to the closing of the transactions described herein; and other risks identified in Baxter’s
and Synovis's respective reports filed with the SEC, including Baxter's annual report on Form 10-K
for the year ended December 31, 2010 and Synovis's annual report on Form 10-K for the year ended
October 31, 2010. Neither Baxter nor Synovis undertakes to update its forward-looking statements.
International Inc. and Synovis Life Technologies, Inc. pursuant to which Baxter will acquire Synovis,
including expectations with respect to the closing of the transaction and its financial impact on
Baxter. The statements are based on assumptions about many important factors, including the
following, which could cause actual results to differ materially from those in the forward-looking
statements: the result of the review of the proposed transactions by various regulatory agencies, and
any conditions imposed on the companies in connection with consummation of the transactions
described herein; approval of the merger by the shareholders of Synovis; satisfaction of various other
conditions to the closing of the transactions described herein; and other risks identified in Baxter’s
and Synovis's respective reports filed with the SEC, including Baxter's annual report on Form 10-K
for the year ended December 31, 2010 and Synovis's annual report on Form 10-K for the year ended
October 31, 2010. Neither Baxter nor Synovis undertakes to update its forward-looking statements.
−
