Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - SPECTRUM PHARMACEUTICALS INC | d268802d8k.htm |
| EX-99.1 - EXHIBIT 99.1 - SPECTRUM PHARMACEUTICALS INC | d268802dex991.htm |
Exhibit 99.2

COMPANY CONTACTS
| Paul Arndt | Shiv Kapoor | |
| Senior Manager, Investor Relations | Vice President, Strategic Planning & Investor Relations | |
| 702-835-6300 | 702-835-6300 |
IMPRESSIVE ZEVALIN® DATA HIGHLIGHTED AT 53RD ANNUAL MEETING OF THE AMERICAN SOCIETY OF HEMATOLOGY; SPECTRUM TO INCREASE CLINICAL EFFORTS TO EXPAND ZEVALIN INDICATIONS
| • | Groundbreaking ZEVALIN Data In Follicular Non-Hodgkin’s Lymphoma Presented From a Phase 2 Study In Newly Diagnosed Patients Who Have Never Received Any Prior Treatment For Their Disease |
| • | 93% Overall Response Rate and 82% Complete Response Rate Observed |
| • | Spectrum To Initiate Clinical Trial To Confirm These Results in 2012 |
| • | Trial Results Could Lead to Paradigm Shift Using ZEVALIN In Follicular Non-Hodgkin’s Lymphoma as First Line Treatment |
| • | Spectrum Has Recently Initiated a Registrational Study Based on Encouraging Data In Diffused Large B-Cell Lymphoma (DLBCL) |
| • | First Patient Will Be Enrolled in Early 2012 |
| • | Encouraging Data From ZEVALIN In Several Indications Highlighted In a Total of 19 Oral and Poster Presentations |
HENDERSON, Nevada – December 12, 2011 – Spectrum Pharmaceuticals (NasdaqGS: SPPI), a biotechnology company with fully integrated commercial and drug development operations with a primary focus in hematology and oncology, announced today results from several clinical trials further expanding the body of evidence supporting the safety and efficacy of ZEVALIN (ibritumomab tiuxetan) Injection for intravenous use. The data were presented at the 53rd Annual Meeting of the American Society of Hematology (ASH), held December 10-13, 2011 in San Diego, California.
Results from multiple studies of ZEVALIN were presented in nineteen abstracts. Five of these papers were selected for oral presentation by an expert committee of ASH. Encouraging data were seen in diverse patient groups, including those with newly diagnosed follicular lymphoma, relapsed/refractory follicular lymphoma, marginal zone lymphoma and patients who have received autologous or allogeneic transplantation. Additionally, there were four abstracts related to using ZEVALIN for consolidation after response to chemotherapy. All studies presented at this year’s ASH meeting were investigator-sponsored studies.
“We are encouraged to see that the excitement surrounding ZEVALIN continues to grow in the lymphoma community. The number of abstracts at this year’s ASH conference and the quality of the data demonstrate the growing interest of the lymphoma experts in ZEVALIN and the promise of ZEVALIN to improve upon the standard treatment for these patients,” said Rajesh C. Shrotriya, MD, Chairman, Chief Executive Officer, and President of Spectrum Pharmaceuticals. “The ASH abstract reviewing committee selected five of the 19 papers for oral presentation and singled out one presentation – Abstract #100 – as being of particular clinical interest due to its groundbreaking results in follicular lymphoma patients who have never been treated. We believe the time has come to evaluate ZEVALIN in the front-line setting.”
“Based on the results presented today it is clear that ZEVALIN used in the front-line setting has the potential to alter the current paradigm of follicular lymphoma treatment,” said Stephanie A. Gregory, M.D., FACP the Elodia Kehm Chair of Hematology, Professor of Medicine, and Director of the Section of Hematology at Rush University Medical Center in Chicago, Illinois. “It is exciting to see that a single injection of this anti-lymphoma targeted therapy could potentially obviate the need for additional therapies, significantly improving quality of life. The high Complete Response rates suggest that there could be a meaningful impact on overall survival. We look forward to future clinical development.”
11500 S. Eastern Ave., Ste. 240 • Henderson, Nevada 89052 • Tel: 702-835-6300 • Fax: 702-260-7405 • www.sppirx.com • NASDAQ: SPPI
Spectrum’s clinical development program for ZEVALIN includes, among other initiatives, a Phase 3 study in Diffuse Large B-Cell Lymphoma and a trial to evaluate ZEVALIN in previously untreated follicular non-Hodgkin’s lymphoma patients. The company expects to start both these trials in 2012.
Following are summaries of the key ZEVALIN abstracts presented at ASH.
Abstract #99 – Phase 2 Study with R-FND Followed by ZEVALIN and Rituximab Maintenance for Untreated High-Risk Follicular Lymphoma
Follicular lymphoma patients with high-risk features using the Follicular Lymphoma International Prognostic Index (FLIPI) have an expected 5-year survival of approximately 50% with conventional chemotherapy. The incorporation of anti-CD20 monoclonal antibody therapy has improved results in this poor risk subgroup. The authors have previously demonstrated that R-FND (rituximab, fludarabine, mitoxantrone, and dexamethasone) is an effective regimen for indolent lymphoma, capable of inducing molecular remissions. Extended dosing of rituximab following induction, and consolidation or improvement of response with ZEVALIN can increase progression free survival rates for patients with advanced follicular lymphoma. This is the first report of a chemo immunotherapy approach followed by both RIT consolidation and rituximab maintenance.
Untreated patients with Grade 1-3 follicular lymphoma with high risk disease (FLIPI score >=3) who had adequate hematologic function and advanced stage (3/4) disease were eligible for study entry. Patients received rituximab (375mg/m2 days 1 and 8 of cycle 1, and day 1 of subsequent cycles) fludarabine (25mg/m2 days 1-3), mitoxantrone (10mg/m2 day 1), and dexamethasone (20mg days 1-5) for four 28 day cycles. RIT was given 12-16 weeks following R-FND following hematologic recovery. Six weeks following RIT, patients received rituximab 375mg/m2 every two months for one year. The primary objective of the study was to determine the PFS rates based on 1999 International Working Group criteria. The secondary objectives included assessing the safety and tolerance of ZEVALIN and maintenance rituximab after R-FND, assessing the CR and overall response rates, and determining the overall survival following treatment.
Forty-nine patients were enrolled and 47 received treatment between October 2004 and April 2009. Forty-six patients were eligible for efficacy analysis. The median age was 61 (37-78), 80% had bone marrow involvement, and all had stage 3/4 disease. Twenty four (51%) patients had bulky disease (>5cm) and 42 (91%) had elevated ß2M. Thirty-six patients completed all planned courses of treatment. Eight patients did not receive RIT, two due to Neutropenia after R-FND. One patient had progressive disease while on treatment. Following R-FND, the complete (CR+CRu) and partial response rates were 87% and 13%. With RIT consolidation, the CR rate increased to 91%. At a median follow up of 50 months, the projected five-year overall survival and PFS rates were 93% and 74%. Toxicity was mainly hematologic. Grade >= 3 neutropenia and thrombocytopenia occurred in 57% and 35% of patients, respectively. Thirty-seven patients required growth factors and 17 patients required transfusions. The median time to hematologic recovery following RIT was 10 weeks. The most common non-hematologic adverse events (>=Grade 3) were fatigue (17%), dyspnea (13%), and myalgia (11%). There were 3 cases of myelodysplasia (MDS), one in a patient who did not receive FIT.
Conclusions: The combination of R-FND followed by RIT intensification and rituximab maintenance results in OS and PFS outcomes that are better than traditional combinations in this high risk population. Given the potential for serious toxicity (eg. MDS) seen in this trial and other intensive treatment strategies, this approach may be most appropriate in high-risk FLIPI patients whose outlook with standard therapy is poor.
Abstract #100 – Safety and Efficacy of 90-Y Ibritumomab Tiuxetan (ZEVALIN®) for Untreated Follicular Non-Hodgkin’s Lymphoma Patients, an Italian Cooperative Study
11500 S. Eastern Ave., Ste. 240 • Henderson, Nevada 89052 • Tel: 702-835-6300 • Fax: 702-260-7405 • www.sppirx.com • NASDAQ: SPPI
ZEVALIN combines the targeting advantage of monoclonal antibody with the radiosensitivity of follicular lymphoma. Previous studies showed that ZEVALIN was safe and highly effective in relapsed/refractory indolent NHL, irrespective to prior treatment with rituximab.
Based on these results, we designed a multicenter trial to evaluate the safety and the efficacy of “upfront” single-agent ZEVALIN in follicular lymphoma. The primary endpoint was the incidence of responses in terms of overall remission rate (ORR) and complete remissions (CR). The secondary endpoints were the treatment safety by monitoring hematology and biochemistry parameters as well as adverse events.
Fifty patients, with a median age of 59 years (range, 35-81), were treated. Forty-eight percent had bone marrow involvement (<25%) and 14% an elevated LDH. Thirty-four percent of patients had high risk FLIPI. Forty-six patients were also assessed by qualitative and quantitative PCR for Bcl2/IgH or IgH clonal rearrangement, for total 30 cases PCR-positive (65.2%).
Results: The ORR was 93% (45/48) with a CR rate of 82% (41/48). Twenty-six patients, who were PCR-positive at diagnosis, were assessed at week 14. Twenty out of 26 (77%) became PCR-negative. After a median follow up of 24 months, the 2-year EFS for all patients was 85 %; moreover, 15 patients (55%), who were PCR-positive at diagnosis, maintain PCR negativity.
As expected, the main toxicity was moderate myelosuppression, with 30% and 26% of patients developing Grade 3/4 neutropenia and thrombocytopenia, respectively. Very few patients required platelets transfusion (4%) or growth factor use (6%). None of the patients experienced grade 3/4 non hematologic toxicity.
In conclusion, ZEVALIN is a highly effective and safe treatment for newly diagnosed follicular lymphoma patients. In the near future, the role of radioimmunotherapy—i.e. including optimal sequencing with chemotherapy—should be established in randomized studies.
Abstract #101 A Systematic Review and Meta-Analysis of Radioimmunotherapy Consolidation for Untreated Patients with Follicular Lymphoma
Background: The disease course of follicular lymphoma is characterized by multiple relapses and progressively shorter response durations with subsequent therapies. As a result, numerous treatment strategies have been developed to reduce the risk of progression including consolidation with transplantation, radio-immunotherapy (RIT), or maintenance therapy with rituximab (R). At present, the optimal therapeutic strategy for follicular patients remains undefined. R maintenance and RIT with an anti-CD20 antibody linked to iodine-131 (I131 Tositumomab) or to ZEVALIN have emerged as well tolerated treatments following induction. To quantify the benefits of consolidative RIT, we conducted a systematic review of the literature and a meta-analysis of selected studies.
Methods: As part of a broader review, we searched the Cochrane Central Register of Controlled Trials (Cochrane Library Issue, 2011), MEDLINE (1/1966-6/2011), American Society of Hematology Annual Meeting abstracts (2004-2010), and American Society of Clinical Oncology Annual Meeting abstracts (2007-2010). Each database was searched using combinations of the term ‘follicular lymphoma’ and the terms for treatment regimens. Inclusion criteria for studies were as follows: 1) reports on phase 2/3 studies; 2) n³30; 3) previously untreated patients 4) treatment with RIT targeted at the CD20 antigen following an induction regimen; 5) original reporting in English of the following treatment outcome measures for patients with follicular lymphoma: CR/CR-unconfirmed, OR, and at least one form of survival data. Extracted data included pre-treatment disease status, patient characteristics, treatment regimen, progression free survival (PFS), overall survival (OS), complete response (CR) and overall response (OR). Pooled estimates of the CR rate, OR rate, 2-year PFS and 5-year PFS for patients treated with consolidative RIT were computed using DerSimonian and Laird random effects models.
11500 S. Eastern Ave., Ste. 240 • Henderson, Nevada 89052 • Tel: 702-835-6300 • Fax: 702-260-7405 • www.sppirx.com • NASDAQ: SPPI
Results: Over 1136 records were reviewed with 8 studies meeting inclusion criteria with 556 patients. Between 1998 and 2007, patients were accrued at multiple sites in all but one study. Median ages ranged from 49-57 years with 41-61% male subjects, among the studies reporting gender. A weighted average of 97.2% of patients had stage 3/4 disease with 73-98% pts having grade 1/2 disease, among those studies reporting histology. Among studies reporting this information, 19-44% of patients had abnormal LDH values, and 25-100% had bulky lymph nodes. CR rates ranged from 51% to 97%, 2-year PFS ranged from 65% to 86%, and 5-year PFS ranged from 38% to 67%. The pooled estimates of the CR rate and OR rate following consolidative RIT were 78% (95% CI 66%-87%) and 98% (95% CI 92.9%-99.5%), respectively (Figure A). The pooled estimates for the 2-year and 5-year PFS were 77.0% (95% CI 70.5-82.4%) and 56.0% (95% CI 41.9-69.2%), respectively (Figure B).
Conclusions: This analysis suggests that consolidative RIT is beneficial to patients with previously untreated follicular lymphoma with meaningful CR rates and 5-year PFS. In addition, consolidative RIT compares favorably to maintenance therapy with rituximab given after chemotherapy (ECOG 1496) in both 2-year PFS (77.0% vs. 73.5%) and 5-year PFS (56.0% vs. 46.4%), and needs to be compared to maintenance R following R-chemotherapy induction.
Abstract #102 Fractionated 90y Ibritumomab Tiuxetan (ZEVALIN®) Radioimmunotherapy As An Initial Therapy of Follicular Lymphoma – First Results From a Phase II Study in Patients Requiring Treatment According to GELF/BNLI Criteria
Background: Radioimmunotherapy (RIT) has proven to be highly active in relapsed follicular lymphoma and the best single agent efficacy results in frontline therapy of follicular lymphoma were obtained with Iodine-131 Tositumomab (Bexxar) (Kaminski et al NEJM 2005); albeit half of the patients had low tumor burden. In patients with higher tumor burden, using more than one fraction of RIT increases the overall radiation dose over that of a single fraction of treatment, thereby potentially improving both the response rates and survival (Illidge et al, Blood 2009).
Methods: We conducted an international, multicenter phase 2 trial to evaluate the efficacy and toxicity of Fractionated ZEVALIN RIT as an initial therapy of Follicular Lymphoma. Eligible patients had untreated follicular lymphoma (grade 1, 2, or 3a) and at least one criterion of high tumor burden - one lymphoma lesion greater than 7 cm or three separate nodes of 3 cm or more; symptomatic splenic enlargement; raised serum concentrations of either lactate dehydrogenase or ß2-microglobulin; compressive syndrome; or the presence of B symptoms. Treatment consisted of two doses of ZEVALIN (11.1 MBq/kg) given 8-12 weeks apart. Patients with greater than 20% bone marrow involvement (BM) with lymphoma received 4 weekly infusions Rituximab (375 mg/m2) and proceeded to fractionated RIT only if a repeat BM biopsy demonstrated clearing of lymphoma with less than or equal to 20% involvement. The primary endpoint was end of treatment response (EOR) of the intent-to-treat (ITT) population according to IWC 1999, assessed 12 weeks after last 90Y Ibritumomab tiuxetan infusion (21 weeks after treatment start). Secondary objectives were safety and progression free survival (PFS).
Results: 74 patients with a median age of 61 years (28-80), including 58 (78%) with stage 3/4 stage, 23 (31%) intermediate risk FLIPI 2 and 34 (46%) with high risk FLIPI 3-5; were included between June 2007 and June 2010 in 7 centers. Thirteen (18%) patients with >20% BM involvement required rituximab pre-treatment, 2/74 did not qualify for RIT, meaning 72 received the first ZEVALIN infusion and 55 (76%) completed the full treatment schedule. The 2nd infusion of RIT was withheld secondary to hematologic toxicity with 1st infusion (n=12, 17%) or human anti murine antibodies positive testing (n = 4; 5.6%) or other (n = 1, 1.4%). Two out of 72 patients did not have recorded response data and the EOR was 95.7% (67/70) with CR/CRu of 57.1% (40/71). Six patients subsequently improved response making an ORR of 97.1% (68/70) (95% CI 90.0%—99.7%), and CR/CRu of 64.3%% (45/70) (95% CI 51.9%—75.4%). For the subset of 17 patients who only received a single ZEVALIN infusion, ORR (CR/CRu) was 100% (76.5%). At a median follow-up of 1.52 years (range 0.13—3.69 years) the PFS is 67 %, 20 patients have progressed and 12 of these have required further treatment (8 chemotherapy, 2 radiotherapy, 2 other). Updated data with median follow-up of more than 2 years were presented.
11500 S. Eastern Ave., Ste. 240 • Henderson, Nevada 89052 • Tel: 702-835-6300 • Fax: 702-260-7405 • www.sppirx.com • NASDAQ: SPPI
Ten patients experienced at least one SAE during the treatment period, with 3 related to study treatment (one case of rigors associated with the first infusion of rituximab and 2 cases of neutropenic sepsis both associated with the second RIT dose. The most common toxicity was hematologic: after the first ZEVALIN dose, related G3-4 hematological AEs were transient neutropenia (20.8%, 18 days median duration) and thrombocytopenia (20.8%; 20 days median duration). After the second ZEVALIN dose, related G3-4 hematological AEs increased to 36.4% for neutropenia (31 days median duration), 14% for anemia (8/55 required transfusion) and 56.4% for thrombocytopenia (40 days median duration). There has been one case of MDS diagnosed 26 months after treatment and one death due to metastatic breast cancer diagnosed 9 months post last dose of ZEVALIN.
Conclusion: Fractionated RIT using ZEVALIN is an effective frontline treatment of advanced-stage follicular lymphoma in patients with high tumor burden requiring treatment and delivers high response rates. The treatment was well tolerated by patients with few infectious episodes and AE’s and manageable hematologic toxicity.
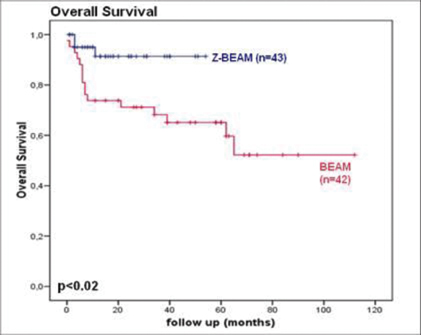
Abstract #3078 ZEVALIN-BEAM Followed by Autologous Stem Cell Transplantation Significantly Improves Overall Survival After Rituximab Containing Induction Therapy in Patients with High-Risk Aggressive B Cell Non-Hodgkin’s Lymphoma
Introduction: Adding rituximab to induction therapy has improved overall survival in patients with relapsed or refractory diffuse large B cell lymphoma (DLBCL) after high dose chemotherapy followed by autologous stem cell transplantation (AuSCT) to 50-60%. However, there is still room for improvement. Addition of ZEVALIN prior to the BEAM conditioning regimen seems feasible and results in promising data with respect to disease free and overall survival in high risk DLBCL patients even when treated with rituximab containing induction therapy. At the VU University Medical center, rituximab was added to (re-)induction therapy starting July 2001. From 2006 we started to add ZEVALIN to BEAM (Z-BEAM) in high risk DLBCL patients. In this retrospective analysis we compare outcome of Z-BEAM versus BEAM, both followed by AuSCT.
Patients and methods: All high risk DLBCL patients consolidated with high dose (radio-immuno) chemotherapy and AuSCT in CR or PR after rituximab-containing induction therapy were included. High-risk DLBCL was defined as either relapsed or refractory DLBCL or as histological transformation of indolent NHL. AuSCT was preceded by BEAM conditioning and ZEVALIN (0.4 mCi/kg, max 32 mCi, starting 2006: Z-BEAM group) or by BEAM only (BEAM group). EFS and OS were estimated using the Kaplan-Meier method and compared using the log rank test.
Results: 43 patients received Z-BEAM and 42 patients received BEAM conditioning. Median age was 56 and 52 years respectively. No significant differences in disease characteristics were seen. Median follow up (range) was 15 months (6-54) and 39 months (0-112) respectively. Overall survival was significantly better in the Z-BEAM group compared with the BEAM group (p=0.02) with an estimated 2 year overall survival of 90% vs. 65%. (fig 1.) In the first 2 years of follow up 7 patients in the Z-BEAM group relapsed compared to 11 in the BEAM group, this did not reach significance (p=0.09). Median time to recovery of neutrophils and thrombocytes was not significantly different. Moreover, there was no significant difference in TRM (no TRM in the Z-BEAM group versus 2 patients in the BEAM group). Patients who relapsed in both groups were able to receive re-induction chemotherapy and, if indicated, allogeneic SCT without being compromised by decreased bone marrow reserve or non hematological toxicities.
Conclusion: Adding 90Yttrium ibritumomab tiuxetan to the BEAM conditioning regimen preceding AuSCT leads to a significant improvement in overall survival in high risk DLBCL patients, even if they have received rituximab during (re)induction.
11500 S. Eastern Ave., Ste. 240 • Henderson, Nevada 89052 • Tel: 702-835-6300 • Fax: 702-260-7405 • www.sppirx.com • NASDAQ: SPPI
About Non-Hodgkin Lymphoma
According to the National Cancer Institute (www.cancer.gov), there are expected to be 66,360 new cases of non-Hodgkin lymphoma diagnosed and approximately 19,320 deaths in the United States in 2011. Non-Hodgkin lymphoma is defined as any of a large group of cancers of lymphocytes (white blood cells). Non-Hodgkin lymphomas can occur at any age and are often marked by lymph nodes that are larger than normal, fever, and weight loss. There are many different types of non-Hodgkin lymphoma. These types can be divided into aggressive (fast-growing) and indolent or low grade (slow-growing) types, and they can be formed from either B-cells or T-cells. Prognosis and treatment depend on the stage and type of disease.
About ZEVALIN® and the ZEVALIN Therapeutic Regimen
ZEVALIN (ibritumomab tiuxetan), Injection for intravenous use is indicated for the treatment of patients with previously untreated follicular non-Hodgkin lymphoma (NHL), who achieve a partial or complete response to first-line chemotherapy. ZEVALIN is also indicated for the treatment of patients with relapsed or refractory, low-grade or follicular B-cell non-Hodgkin lymphoma.
ZEVALIN is a CD20-directed radiotherapeutic antibody. The ZEVALIN therapeutic regimen consists of two components: rituximab, and Yttrium-90 (Y-90) radiolabeled ZEVALIN for therapy. ZEVALIN builds on the combined effect of a targeted biologic monoclonal antibody augmented with the therapeutic effects of a beta-emitting radioisotope.
Important ZEVALIN® Safety Information
Deaths have occurred within 24 hours of rituximab infusion, an essential component of the ZEVALIN therapeutic regimen. These fatalities were associated with hypoxia, pulmonary infiltrates, acute respiratory distress syndrome, myocardial infarction, ventricular fibrillation, or cardiogenic shock. Most (80%) fatalities occurred with the first rituximab infusion. ZEVALIN administration can result in severe and prolonged cytopenias in most patients. Severe cutaneous and mucocutaneous reactions, some fatal, can occur with the ZEVALIN therapeutic regimen.
Please see full Prescribing Information, including BOXED WARNINGS, for ZEVALIN and rituximab. Full prescribing information can be found at www.ZEVALIN.com.
11500 S. Eastern Ave., Ste. 240 • Henderson, Nevada 89052 • Tel: 702-835-6300 • Fax: 702-260-7405 • www.sppirx.com • NASDAQ: SPPI
About Spectrum Pharmaceuticals, Inc.
Spectrum Pharmaceuticals is a biotechnology company with fully integrated commercial and drug development operations with a primary focus in hematology and oncology. The Company’s strategy is to acquire, develop and commercialize a broad and diverse pipeline of late-stage clinical and commercial products. The Company markets two oncology drugs, FUSILEV and ZEVALIN and has two drugs, apaziquone and belinostat, in late stage development along with a diversified pipeline of novel drug candidates. The Company has assembled an integrated in-house scientific team, including clinical development, medical research, regulatory affairs, biostatistics and data management, formulation development, and has established a commercial infrastructure for the marketing of its products. The Company also leverages the expertise of its worldwide partners to assist in the execution of its strategy. For more information, please visit the Company’s website at www.sppirx.com.
Forward-looking statement – This press release may contain forward-looking statements regarding future events and the future performance of Spectrum Pharmaceuticals that involve risks and uncertainties that could cause actual results to differ materially. These statements are based on management’s current beliefs and expectations. These statements include but are not limited to statements that relate to our business and its future, including certain company milestones, Spectrum’s ability to identify, acquire, develop and commercialize a broad and diverse pipeline of late-stage clinical and commercial products, leveraging the expertise of partners and employees, around the world to assist us in the execution of our strategy, and any statements that relate to the intent, belief, plans or expectations of Spectrum or its management, or that are not a statement of historical fact. Risks that could cause actual results to differ include the possibility that our existing and new drug candidates, may not prove safe or effective, the possibility that our existing and new applications to the FDA may not receive approval, and other regulatory agencies in a timely manner or at all, the possibility that our existing and new drug candidates, if approved, may not be more effective, safer or more cost efficient than competing drugs, the possibility that our efforts to acquire or in-license and develop additional drug candidates may fail, our lack of sustained revenue history, our limited marketing experience, our dependence on third parties for clinical trials, manufacturing, distribution and quality control and other risks that are described in further detail in the Company’s reports filed with the Securities and Exchange Commission. We do not plan to update any such forward-looking statements and expressly disclaim any duty to update the information contained in this press release except as required by law.
SPECTRUM PHARMACEUTICALS, INC.®, ZEVALIN®, and FUSILEV® are registered trademarks of Spectrum Pharmaceuticals, Inc. REDEFINING CANCER CARE™ and the Spectrum Pharmaceutical logos are trademarks owned by Spectrum Pharmaceuticals, Inc.
© 2011 Spectrum Pharmaceuticals, Inc. All Rights Reserved.
11500 S. Eastern Ave., Ste. 240 • Henderson, Nevada 89052 • Tel: 702-835-6300 • Fax: 702-260-7405 • www.sppirx.com • NASDAQ: SPPI
