Attached files
| file | filename |
|---|---|
| EX-32.2 - EXHIBIT 32.2 - FIRST CHINA PHARMACEUTICAL GROUP, INC. | v242144_ex32-2.htm |
| EX-31.1 - EXHIBIT 31.1 - FIRST CHINA PHARMACEUTICAL GROUP, INC. | v242144_ex31-1.htm |
| EX-31.2 - EXHIBIT 31.2 - FIRST CHINA PHARMACEUTICAL GROUP, INC. | v242144_ex31-2.htm |
| EX-32.1 - EXHIBIT 32.1 - FIRST CHINA PHARMACEUTICAL GROUP, INC. | v242144_ex32-1.htm |
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 10-K/A
(Amendment No. 3)
x ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended March 31, 2011
OR
¨ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE
SECURITIES EXCHANGE ACT OF 1934
For the transition period from ____________ to ____________
Commission file number: 000-54076
FIRST CHINA PHARMACEUTICAL GROUP, INC.
(Exact name of registrant as specified in its charter)
|
NEVADA
|
74-3232809
|
|
|
(State or other jurisdiction of
|
(I.R.S. Employer
|
|
|
incorporation or organization)
|
Identification No.)
|
|
|
Number 504, West Ren Min Road,
Kunming City, Yunnan Province
People’s Republic of China
|
N/A
|
|
|
(Address of principal executive offices)
|
(Zip Code)
|
Registrant’s telephone number, including area code: 852-2138-1668
Securities registered pursuant to Section 12(b) of the Act: None
Securities registered pursuant to Section 12(g) of the Act:
Common Stock, $0.001 par value
(Title of Class)
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. ¨
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
|
Large accelerated filer ¨
|
Accelerated filer ¨
|
|
|
Non-accelerated filer ¨ (Do not check if a smaller reporting company)
|
Smaller reporting company x
|
|
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the voting and non-voting common equity held by non-affiliates of the registrant as of September 30, 2010 was approximately $16,000,000 based upon the closing price of $0.80 per share reported for such date on the Over-the-Counter Bulletin Board maintained by the NASD. Shares of common stock held by each officer and director and by each person who is known to own 10% of more of the outstanding Common Stock have been excluded in that such persons may be deemed to be affiliates of the Company. This determination of affiliate status is not necessarily a conclusive determination for other purposes.
Indicate the number of shares outstanding of each of the registrant’s classes of common stock, as of the latest practicable date.
|
Common Stock
|
Outstanding at June 27, 2011
|
|
|
Common Stock, $.001 par value per share
|
59,664,480 shares
|
DOCUMENTS INCORPORATED BY REFERENCE: None
EXPLANATORY NOTE
First China Pharmaceutical Group, Inc., a Nevada corporation (the “Company”) is filing this Amendment No. 3 to its Annual Report on Form 10-K which was originally filed with the Securities and Exchange Commission (“SEC”) on July 14, 2011 and amended on September 19, 2011 and November 7, 2011 (collectively, the “Original Form 10-K”), to incorporate the Company’s revisions and responses to a comment letter from the staff of the SEC dated November 17, 2011. Except for the amended disclosures made in response to the letter of comment from the staff of the SEC, the information in this Form 10-K/A has not been updated to reflect events that occurred after July 14, 2011, the filing date of the Original Form 10-K. Accordingly, this Form 10-K/A should be read in conjunction with the Company’s filings made with the SEC subsequent to the filing of the Original Form 10-K. The following sections have been amended, without limitation:
|
|
·
|
Item 1.
|
Business
|
|
|
·
|
Item 7.
|
Management’s Discussion and Analysis of Financial Condition and Results of Operation
|
|
|
·
|
Item 8.
|
Financial Statements and Supplementary Data
|
|
|
·
|
Item 13.
|
Certain Relationships and Related Transactions, and Director Independence
|
Except as set forth above, all other information in the Company’s Original Form 10-K remains unchanged. The Company has re-filed the entire Form 10-K in order to provide more convenient access to the amended information in context.
TABLE OF CONTENTS
|
Page
|
||
|
Part I
|
||
|
Item 1
|
Business
|
1
|
|
Item 1A
|
Risk Factors
|
24
|
|
Item 1B
|
Unresolved Staff Comments
|
41
|
|
Item 2
|
Properties
|
41
|
|
Item 3
|
Legal Proceedings
|
41
|
|
Item 4
|
Removed and Reserved
|
41
|
|
Part II
|
||
|
Item 5
|
Market for Registrant’s Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities
|
42
|
|
Item 6
|
Selected Financial Data
|
43
|
|
Item 7
|
Management’s Discussion and Analysis of Financial Condition and Results of Operation
|
43
|
|
Item 7A
|
Quantitative and Qualitative Disclosures about Market Risk
|
51
|
|
Item 8
|
Financial Statements and Supplementary Data
|
51
|
|
Item 9
|
Changes in and Disagreements with Accountants on Accounting and Financial Disclosure
|
51
|
|
Item 9A
|
Controls and Procedures
|
51
|
|
Item 9B
|
Other Information
|
52
|
|
Part III
|
||
|
Item 10
|
Directors and Executive Officers and Corporate Governance
|
53
|
|
Item 11
|
Executive Compensation
|
56
|
|
Item 12
|
Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters
|
58
|
|
Item 13
|
Certain Relationships and Related Transactions, and Director Independence
|
60
|
|
Item 14
|
Principal Accounting Fees and Services
|
62
|
|
Part IV
|
||
|
Item 15
|
Exhibits, Financial Statement Schedules
|
63
|
|
Signatures
|
66
|
|
PART I
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING STATEMENTS
Some discussions in this Annual Report on Form 10-K contain forward-looking statements that have been made pursuant to the provisions of the Private Securities Litigation Reform Act of 1995. These statements involve risks and uncertainties and relate to future events or future financial performance. A number of important factors could cause our actual results to differ materially from those expressed in any forward-looking statements made by us in this Form 10-K. Forward-looking statements are often identified by words such as “believe,” “expect,” “estimate,” “anticipate,” “intend,” “project,” “plans,” “seek” and similar expressions or words which, by their nature, refer to future events. In some cases, you can also identify forward-looking statements by terminology such as “may,” “will,” “should,” “plans,” “predicts,” “potential” or “continue” or the negative of these terms or other comparable terminology.
These forward-looking statements are only predictions and involve known and unknown risks, uncertainties and other factors, including the risks in the section entitled “Risk Factors” below that may cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. In addition, you are directed to factors discussed in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section as well as those discussed elsewhere in this Form 10-K.
Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity or achievements. Except as required by applicable law, including the securities laws of the United States, we do not intend to update any of the forward-looking statements to conform these statements to actual results. However, readers should carefully review the risk factors set forth in other reports or documents the Company files from time to time with the Securities and Exchange Commission (the “SEC”), particularly the Company’s Quarterly Reports on Form 10-Q and any Current Reports on Form 8-K. All written and oral forward-looking statements made subsequent to the date of this report and attributable to us or persons acting on our behalf are expressly qualified in their entirety by this section.
As used in this Annual Report on Form 10-K, references to “dollars” and “$” are to United States dollars and, unless otherwise indicated, references to “we,” “our,” “us,” the “Company,” “FCPG,” or the “Registrant” refer to First China Pharmaceutical Group, Inc., a Nevada corporation and its wholly owned subsidiaries, First China Pharmaceutical Group Limited, a Hong Kong company, and Kun Ming Xin Yuan Tang Pharmacies Co. Ltd., a company organized under the laws of the People’s Republic of China.
ITEM 1. DESCRIPTION OF BUSINESS.
Overview
First China Pharmaceutical Group, Inc. (“FCPG” or the “Company”), formerly known as E-Dispatch Inc., was incorporated under the laws of the State of Nevada on July 31, 2007. On September 15, 2010, we closed a voluntary share exchange transaction pursuant to a Share Exchange Agreement, dated August 23, 2010 (the “Exchange Transaction”), by and among FCPG, First China Pharmaceutical Group Limited, a Hong Kong company (“FCPG HK”), and Kun Ming Xin Yuan Tang Pharmacies Co. Ltd., a company organized under the laws of the People’s Republic of China (“PRC”) and wholly owned subsidiary of FCPG HK (“XYT”). Prior to the Exchange Transaction, we were a development stage company engaged in developing a cell phone-based taxi dispatch system, and a public reporting “shell company,” as defined in Rule 12b-2 of the Securities Exchange Act of 1934, as amended. As a result of the Exchange Transaction, the FCPG HK stockholder acquired approximately 25% of our issued and outstanding common stock, FCPG HK and XYT became our wholly-owned subsidiaries, and we acquired the business and operations of FCPG HK and XYT.
1
Through our wholly-owned subsidiary, XYT, we are now engaged in drug logistics and distribution in Yunnan Province, China through drug stores, medical clinics and hospitals, as well as the wholesale distribution of medicine products, chemical agents, antibiotics, biochemistry drugs and biological preparations to hospitals and the XYT store located at the Company’s distribution facility in Kunming. XYT was founded in November 2002 and is a provincial pharmaceutical distributor that offers approximately 5,000 drugs, of which approximately 1,000 are over-the-counter drugs, approximately 1,000 are prescription drugs, approximately 2,000 are prepared Chinese medicines and approximately 1,000 are supplements. Currently, we have approximately 4,700 customers and supply approximately 10% of such customers’ inventories with a sales network that covers the entire Yunnan Province of China. XYT has its head office and warehouse at Number 504, West Ren Min Road, Kunming, City, Yunnan Province, PRC
Our continuing strategy is to build a nationwide pharmaceutical distribution network throughout China. We plan to expand our customer base through the use of the following tactics: broadening of our current product line to attract larger customers that currently do not utilize us and benefit from internet ordering and the lower prices that we offer; providing computers to customers to attract new customers as our management is unaware of any other pharmaceutical distribution company providing this benefit; and increasing our current sales force to directly target hospitals, medical clinics and pharmacies.
Background
As noted above, prior to the Exchange Transaction, the Company was known as E-Dispatch, Inc. and was a development stage company engaged in the development of a cellular phone-based taxi dispatch system. As a result of the current difficult economic environment and the Company’s lack of funding to implement its business plan, in early 2010, the Company’s Board of Directors began to analyze strategic alternatives available to the Company to continue as a going concern. Such alternatives include raising additional debt or equity financing or consummating a merger or acquisition with a partner that may involve a change in its business plan. However, the Company was unable to raise capital in order to develop or deploy its taxi dispatch system. Therefore, the Company, in consultation with its advisors, identified FCPG HK as a potential strategic acquisition that the Board of Directors believed to be in the best interest of the Company and its shareholders. FCPG HK through its operating subsidiary, XYT, was attractive to the Company because it is in a growing pharmaceutical industry, has a strong presence in Yunnan Province and has plans to grow its business throughout China. XYT and FCPG HK believed the Company to be an attractive business combination partner, due in part, to the perceived benefits of being a publicly registered company, allowing for increased access to capital raising. Accordingly, the parties entered into a letter of intent with respect to the Exchange Transaction on May 14, 2010, executed the Exchange Agreement on August 23, 2010, and closed the Exchange Transaction on September 15, 2010.
The Exchange Agreement provides for bonus payments based on two percent (2%) of the quarterly gross sales of XYT to be paid to Mr. Zhen Jiang Wang, our Chairman and Chief Executive Officer, on a quarterly basis, within fifteen (15) days after the filing of a Form 10-K or Form 10-Q with the SEC containing financial statements of the Company. The parties agreed upon Mr. Wang’s overall compensation structure of shares received and the two percent (2%) amount based on market conditions and review of other Chinese based public companies acquired by U.S. based companies with sales in a similar range as XYT, in addition to providing incentive for Mr. Wang to continue to lead and develop XYT’s business. Had this formula previously been in effect, based on the historical performance of XYT, pursuant to this bonus formula, Mr. Wang would have been due US$343,087, US$505,711 and US$553,147 for XYT’s calendar years ended December 31, 2008, 2009 and 2010, respectively. The terms of such bonus payments are set forth in the Bonus Payment Agreement by and between the Company and Mr. Wang, dated October 7, 2010. Such bonus payments shall be paid within fifteen (15) days after our 10-Q and 10-K filings are made, shall not exceed an aggregate of $1,500,000 per fiscal year, and Mr. Wang can reduce or modify, in his sole discretion, the amount due to him as long as such bonus payments do not exceed two percent (2%) of the quarterly gross sales of XYT as disclosed in our consolidated financial statements filed with our 10-Q and 10-K filings.
Mr. Wang subsequently amended the Bonus Payment Agreement in a letter to us dated October 8, 2010 (“Amendment to Bonus Agreement”), whereby Mr. Wang agreed to waive any and all right to receive any bonus payment for the Company’s fiscal year ended March 31, 2011 noting that no amounts due to Mr. Wang for the fiscal year ended March 31, 2011 would be carried forward.
2
Additionally, Mr. Wang developed and maintained relationships with certain customers whom he met prior to joining XYT. Most of these customers were small business owners, who placed high emphasis on informal relationships and individual credibility developed based on the quality of products and the conduct of business transactions. Mr. Wang devoted himself to retaining these customers, and they remained loyal to Mr. Wang as they believed in the quality of the products that he introduced and received updates on new products from Mr. Wang. There are over 35 such customers, and they generally conduct high volumes of cash sales. Therefore, due in part to the client base and social network of Mr. Wang, XYT had authorized Mr. Wang to collect receivables of XYT prior to or concurrent with product delivery for certain customers with whom he has a relationship pursuant to an Authorization Agreement between the parties and XYT’s instructions. Such receivable collections are represented as “cash sales” in the PRC (which, according to common accounting practice in China, normally arise when a company receives payment for sales by cash or by a private bank account other than a corporate bank account), and the amount of such receivables collected by Mr. Wang were US$5,945,625, US$17,270,218, and US$26,363,421 during 2007, 2008, and 2009, respectively, and US$8,071,767 for the period from April 1, 2010 through June 2010. Mr. Wang also pays certain payables (including purchased inventories from suppliers, expenses, such as commissions, notes payables, software customization, installation of the internet fulfillment system, computer equipment and even vehicles to provide expedited delivery of purchased goods) with funds from the receivables he previously collected on our behalf. We believe such an arrangement is common in the PRC and allows it and Mr. Wang to maintain favorable relationships with customers and suppliers due to prompt payments and frequent communications, reduces certain transaction costs, allows it to obtain favorable prices and discounts due to Mr. Wang’s prompt settlement of certain supplier accounts, and reduces certain liquidity risks due to daily limits and other restrictions on bank withdrawals from business accounts as the funds collected by Mr. Wang on our behalf provide an additional liquidity option for us when needed. In order to ensure that a company retains sufficient registered capital to support itself, there are limits on the funds that can be paid out each day. For a company with the registered capital of XYT, the daily limit on funds that may be liquidated (paid out) is RMB500,000 or $77,000 USD per day. By Mr. Wang providing funds for the settlement of payables, we were able to work around this liquidity restriction without having to increase its registered capital. This arrangement was informal in nature and on an as-needed basis voluntarily by Mr. Wang. When we were required to make daily payments in excess of RMB500,000 or US$77,000, Mr. Wang would pay such excess amounts personally to ensure the continuous efficient flow of payments. With a company that was approaching $25 million in annual sales, Mr. Wang was called upon a couple of times per week to top up payments that were in excess RMB500,000 or US$77,000 per day.
However, prior to the closing of the Exchange Transaction, the Company and Mr. Wang recognized that continuation of this arrangement, while appropriate and effective for XYT as a private, closely held company, should not continue as XYT became a subsidiary of a publicly traded company with multiple stockholders that would be subject to SEC and U.S. GAAP compliance. Therefore, as part of its evolution into a public company, the Company and Mr. Wang had agreed that after the Exchange Transaction, Mr. Wang should discontinue making payments and collections on behalf of XYT in order to standardize its business operations and set up a structure of effective internal controls as a public company. In fact, Mr. Wang intended to cease collecting receivables in June 2010, however, customers continued to make payments to Mr. Wang despite instructions from Mr. Wang and the Company to the contrary. While the weaning process had begun, it continued for some time for fear of losing key customers, and Mr. Wang only managed to convince all key customers and completely cease the practice of such collections on behalf of XYT as of October 31, 2011. The amounts due from Mr. Wang to us had previously been treated as a loan receivable from a related party to us and amounted to $11,799,953 as of December 31, 2009 and $2,498,924 as of December 31, 2008. To comply with U.S. GAAP, we have reclassified such amounts as a deemed distribution of a dividend to Mr. Wang, a related party, and therefore a corresponding reduction of retained earnings. Mr. Wang has not repaid any amount of the distribution.
In advance of the Exchange Transaction, XYT also applied for an increase in its registered capital and began the process of arranging for direct bank financing for many of its large customers. In the past, Mr. Wang personally provided credit to some of the Company’s largest and best customers for purchases from XYT. As XYT does not grant credit to the vast majority of its customers, the Company is introducing large credit worthy customers to banks that are interested in providing XYT customers with credit for purchases from XYT. These credit arrangements are between the bank and XYT’s customers for the purchase of XYT products. XYT is not party to the credit provided, does not guarantee or co-sign for the customer and receives no compensation from the bank or its customers for arranging this credit. By arranging credit for its customers, XYT improves its liquidity position by not having to utilize working capital to finance customer purchases.
3
Further, in advance of the Exchange Transaction, XYT applied for and received a small increase in its registered capital. XYT’s registered capital was increased from RMB2,000,000 to RMB15,080,000, or US$2,295,000. After the April 2011 financing, XYT again applied and was granted an increase in registered capital. These increases in registered capital have enabled the Company to increase the daily withdrawal limits from the bank to reduce any adverse impact on daily operations.
With the exception of these bonus payments and deemed dividend distribution to Mr. Wang, we do not contemplate any material payments or benefits to be received by the parties thereto.
Between March 18, 2011 and April 15, 2011, we entered into a form of Securities Purchase Agreement (the “SPA”) with certain accredited investors (the “Purchasers”) for the issuance and sale of one hundred and fifty four (154) Units of the Company at a purchase price of $25,000 per Unit (the “Private Offering”), for aggregate consideration of $3,850,000. Each “Unit” is comprised of (i) 27,778 shares of Company common stock, $0.001 par value per share (the “Common Stock,” and the shares of Common Stock offered referred to as the “Shares”), (ii) warrants to purchase 27,778 shares of Common Stock at an exercise price of $1.25 per share (the “Series A-1 Warrants”), and (iii) warrants to purchase 27,778 shares of Common Stock at an exercise price of $2.00 per share (the “Series A-2 Warrants”) (the Series A-1 Warrants and the Series A-2 Warrants, collectively, the “Warrants”). The Warrants expire four (4) years from the date of issuance, subject to early termination or forfeiture in accordance with certain terms and conditions of the Warrants.
The Private Offering was conducted by the Company on a “best efforts” basis wherein a minimum of 80 Units or 2,222,240 Shares, 2,222,240 Series A-1 Warrants and 2,222,240 Series A-2 Warrants, and maximum of 280 Units, or 7,777,840 Shares, 7,777,840 Series A-1 Warrants and 7,777,840 Series A-2 Warrants could be sold.
Each of the Purchasers executed an SPA and each Purchaser represented to the Company that such investor is an “accredited investor” as defined in Rule 501(a) of Regulation D of the Securities Act of 1933. The Company expects to use the net proceeds of the Private Offering, totaling $3,633,500 after deducting for certain costs and expenses of the Private Offering, for general corporate purposes, which may include funding working capital needs, marketing, acquisitions and expansion, and to further the operations of the Company. An aggregate of 4,464,480 Shares, 4,381,145 Series A-1 Warrants and 4,381,145 Series A-2 Warrants were issued in connection with the Private Offering.
Corporate Structure
As a result of the Exchange Transaction, the organizational structure of the Registrant is as follows:
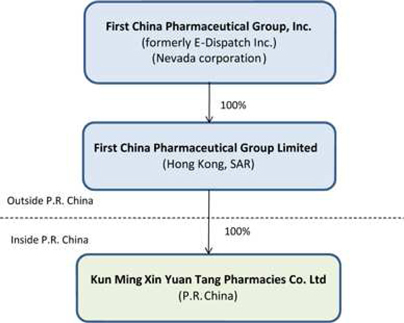
4
This corporate structure was created to establish XYT as a Wholly Foreign Owned Enterprise (“WFOE”). A WFOE is a limited liability company operating in China that is wholly owned by the foreign investors, in this case FCPG HK. FCPG HK was established on April 29, 2010 and the purpose of setting up FCPG HK is for the investment in and holding of XYT as a WFOE. On May 13, 2010, FCPG HK entered into share transfer agreements with Mr. Zhen Jiang Wang and Ms. Jing Gong, respectively, under which FCPG HK acquired all of the outstanding stock of XYT at a total consideration of RMB 2 million. On June 25, 2010, XYT received its new business license and became a WFOE under the PRC laws. The unique feature of a WFOE is that it can avoid certain problematic issues which can potentially result from dealing with a domestic joint venture partner in China. Under PRC law, as a WFOE, our PRC subsidiary XYT may pay dividends only out of its accumulated after-tax profits, if any, determined in accordance with PRC accounting standards and regulations and tax law. In addition, our PRC subsidiary is required to set aside at least 10% of its after-tax profit based on PRC accounting standards each year to its statutory surplus reserve fund until the accumulative amount of such reserve reaches 50% of its respective registered capital. These reserves are not distributable as cash dividends. The board of directors of a WFOE has the discretion to allocate a portion of its after-tax profits to its staff welfare and bonus funds. After the allocation of relevant welfare and funds, the equity owners can distribute the rest of the after-tax profits provided that all the losses of the previous fiscal year have been made up. FCPG HK may be required to repay the distributed profits if the distribution is made without the allocation of relevant welfare and funds and losses made-up. The welfare and funds refer to the statutory capital reserve as provided in Article 167 of the PRC Company Law, which requires that 10% of the company’s annual after-tax profits shall be put into the statutory capital reserve account. The losses made-up means that the after-tax profits shall be used to make up previous losses, if any, before being distributed to shareholders. In addition, according to PRC law on WFOEs, companies may be subject to a fine up to RMB5,000 as a result of non-compliance with the above rules. However, the Company believes that it has compiled with relevant rules about its statutory reserve fund.
The registered capital of XYT is US$2,295,000. The shareholders of XYT have not determined the proportion of reserve funds and bonus and welfare funds for workers and staff members, and XYT has not previously distributed any profits. If the shareholders of XYT decide to distribute profits in the future, XYT will comply with the relevant rules to withdraw statutory reserve funds to no lower than 10% of the total amount of profits after payment of tax. Therefore, there would be no penalty applicable to XYT.
Despite the extensive regulatory framework related to WFOE’s, the advantages of establishing XYT as a WFOE generally include:
|
|
·
|
Independence and freedom to implement a possible worldwide strategy of its parent company without having to consider the involvement of Chinese law;
|
|
|
·
|
Ability to formally carry out business and the ability to issue invoices to customers in RMB and receive revenues in RMB;
|
|
|
·
|
Capable of converting RMB profits to US dollars or other foreign currency for remittance to their parent company outside China; and
|
|
|
·
|
Greater protection of intellectual property rights, know-how and technology versus a joint venture structure since no partner is required and therefore the Company and XYT have more control of the IP of XYT.
|
In addition, under the PRC Enterprise Income Tax Law, effective January 1, 2008 and its implementing rules, the profits of a foreign invested enterprise which are distributed to its immediate holding company outside the PRC will be subject to a withholding tax rate of 10%. Pursuant to a special arrangement between Hong Kong and the PRC, known as the Arrangement Between the Mainland and Hong Kong Special Administrative Region on the Avoidance of Double Taxation and Prevention of Fiscal Evasion, effective August 21, 2006 (the “Arrangement”), FCPG HK may qualify for a lower rate of 5% for the profits distributed by XYT. This Arrangement remains effective unless it is terminated by a written notice from either the PRC or Hong Kong government to the other before June 30 each year following the fifth anniversary of the effective date of such Arrangement. For additional discussion, please refer to the section entitled “Risk Factors.”
5
Strategy
We believe we have a strategic advantage over certain of our competitors in Yunnan Province as we have obtained government approval to fill orders over the internet. We applied for the License of Internet Drug Information Service in May 2009 by completing an application form and providing background information on the company and its senior officers. No application fees were paid to obtain the License of Internet Drug Information Service, although a registration fee of ¥8,000, or approximately US$1,200, was paid upon the approval of this license. The application and corporate information for the License of Internet Drug Information Service was examined and approved by the provincial Yunnan Food and Drug Administration. The State Food and Drug Administration provides the provincial Yunnan Food and Drug Administration authority to examine the applicant and issue this license. We received the License of Internet Drug Information Service issued by the Yunnan Food and Drug Administration in October 2009, which remains in good standing. There are no annual fees associated with this license, which enables us to bypass municipal and county pharmaceutical distributors, market our product line, provide pricing information and provide products directly to our customers. Bypassing these layers of distribution enables us to offer products to our customers at a significantly lower price than our major competitors while maintaining our margins.
Through our own industry research and documents from the Yunnan Food and Drug Administration, we believe that there are currently 491 drug distribution companies in Yunnan Province and that 42 of these companies also possess the License of Internet Drug Information Service. However, we have completed all the forms and supporting documentation and submitted a formal application to the Yunnan Food and Drug Administration for a second internet license, the Internet Transaction Service License. There is no formal timeline to obtain approval for the Internet Transaction Service License. Company research indicates that the approval process can be in excess of one year and is subject to the changing policies of the Chinese government. While our current License of Internet Drug Information Service permits us to use the internet to market our product line, display the inventory we hold and provide pricing information, thereby, enabling customers to see our inventory and to order directly by email, fax, online “chat” or phone, the Internet Transaction Service License would allow us to provide secured access to our proprietary computer fulfillment system, advertise, list inventories, take orders, provide shipping confirmation, invoice the customer and accept payment over the internet. Through our own industry research and review by a senior Company employee of records and documents from the Yunnan Food and Drug Administration relating to licenses granted during the past eight years, our management believes that there are currently only two drug manufacturing companies in Yunnan Province that possess the Internet Transaction Service License. If we are successful in obtaining this license, management believes it will be the only drug distribution company in Yunnan Province that will possess the Internet Transaction Service License.
This application was made in September 2010 with all required documentation provided to the Yunnan Food and Drug Administration. No fees were required at the time. Typically, if there are no deficiencies in the initial application, the Yunnan Food and Drug Administration will approve the first phase of the application and send the application and supporting documentation to the State Food and Drug Administration, a department of the Yunnan provincial government, for second review and approval. Both the Yunnan Food and Drug Administration and the State Food and Drug Administration are government agencies which review the application based on the information supplied by the applicant and subjective criteria. Ultimately, approval for the Internet Transaction Service License is largely subjective, based on the application and the direction of government policy at the time. The Yunnan Food and Drug Administration has completed the first review of the application and forwarded the application to the State Food and Drug Administration for their review. There is no established time frame regarding the decline or approval of this application.
If we do not receive the Internet Transaction Service License, management does not believe our business will be materially affected, as our existing license, the Internet Drug Information License, enables us to market our products to our customers and fulfill orders. We are currently licensed to receive orders via telephone, fax and email, and payment must be made via, check, wire transfer or cash. The Internet Transaction Service License would allow us to also receive orders and payments through our website.
6
Products and Distribution
We currently distribute Chinese patent drugs (such as infusion for treating coryza of wind-cold type fructus forsythiae antidotal tablets and Liuwei Dihuang Pills), herbs (such as Leonurus and Polygonum multiflorum), pharmaceutical chemicals (such as Loratadine Tablets, Ofloxacin Eye Drops and Vitamin B2 Tablets), biological products (such as Rubella vaccine, Mumps vaccine and Hepatitis B vaccine), antibiotics (such as Amoxicillin and Acetylspiramycin), biochemical drugs (such as insulin and amino acid) and small medical instruments (such as clinical thermometers, blood pressure meters and syringes). These products are purchased by our current customers, that include licensed pharmaceutical users and retailers such as hospitals, medical clinics and pharmacies. Small licensed drug distributors also purchase these products to distribute. All customers must be verified by us as entities licensed to purchase pharmaceuticals.
The Company completed a financing of $3.6 million (net of fees) and will utilize some of these proceeds to expand its product line. As the funding fell short of the $5 to $6 million objective which would have provided sufficient capital for product line expansion and an acquisition, the Company’s short term objective is now to broaden our product line from our current 5,000 products to 20,000 to 25,000 over a 6 to 8 month period. The Company will utilize its License of Internet Drug Information Service to market and distribute these products. The utilization of the internet to distribute pharmaceuticals enables the Company to eliminate two levels in the traditional Chinese drug distribution system (see - “Business Model”), thereby enabling the Company to sell products at a lower price than competitors utilizing the traditional distribution model. As each product has a different cost structure, the Company cannot precisely state the discount each customer could receive. However, the elimination of two distribution tiers typically enables us to offer products at a 10% to 40% discount to its competitors that utilize the traditional distribution model.
Management believes that broadening its product line and offering its products at discounted prices will allow the Company to sell more products to our approximately 4,700 existing customers so as to supply our current customers with over 80% of the pharmaceutical products they require and become their primary supplier. Through ongoing discussions and relationship building the Company’s sales representatives have had with many of the Company’s customers, the Company believes that its existing customers would purchase virtually all their current inventory from the Company, if it was available. Overwhelmingly, the Company’s existing customers have indicated to sales representatives that they would prefer to buy all of their products from the Company due to our turnaround time, competitive prices and volume purchasing discounts provided. Management believes that the feedback from existing customers would lead to the Company supplying 80% of the inventory of its existing customers.
In addition to selling significantly more products to its approximately 4,700 existing customers, we also plan to aggressively attract 5,000 new primary customers that currently do not utilize us and would benefit from internet ordering and an expanded product line. The Company defines primary customers as those that it believes purchase more than 50% of their total inventory from the Company. The Company plans to implement several marketing strategies and tactics to attract 5,000 new primary customers. For instance, we believe that providing computers to customers will attract new customers as our management is unaware of any other pharmaceutical distribution company providing this benefit. The Company will also undertake an advertising campaign utilizing print advertising in industry publications. We have supplemented our existing sales force with the addition of two additional sales teams that will make calls directly to hospitals, medical clinics and pharmacies that are not customers of the Company to try and recruit them as customers; each sales team will be composed of a sales manager and 10 sales people. The sales teams also will visit existing customers to advise them of new products offered, new programs the Company has in place and to try and make sure they are ordering as much of their inventory from the Company as possible.
We maintain two websites, www.firstchinapharma.com and www.kmxyt.com. www.firstchinapharma.com is a corporate website in English and provides information regarding First China Pharmaceutical Group, Inc. www.kmxyt.com is the website for XYT, our PRC operating subsidiary. XYT’s website is in Chinese and its purpose is to enable XYT customers to obtain information on products, search inventory and to place orders.
Customers access our website at www.kmxyt.com, and our Internet Drug Information Service License enables the Company to use the internet via our website to market our product line, display the inventory we holds, provide pricing information, conduct live “chats” with customers and receive orders through email or online “chat” sessions with customers. However, customers cannot directly access our computer system and place electronic orders through www.kmxty.com, nor can they make payments through the website or over the internet. The PRC government is concerned that direct access to order pharmaceuticals could lead to drugs ending up in the hands of non licensed groups. Therefore, access to the website is only provided to customers that are screened to be authentic licensed hospitals, medical clinics, pharmacies and drug distribution companies. Customers must provide us with the appropriate government licenses prior to being issued a user ID and password to the site. Once on the site, customers can review products by drug type, manufacturer, price and other criteria. Customers can use a VOIP system built into the software to talk to our customer representatives and can place orders directly with them, by email or chat, or through the traditional telephone. In the event that we receive approval for the Internet Drug Transaction Service License, we will be able to receive customer orders and payment directly through our website. As part of our application for the Internet Drug Transaction Service License, we must prove that we have adequate security measures in place to prevent order fraud.
7
We estimate that only 10% to 15% of our current customers have access to a personal computer. As such, the vast majority of our customers cannot utilize our internet ordering system. We plan to leverage our internet ordering system by providing personal computers to our customers. We anticipate that over the next 12 months, approximately 60% of our current 4,700 customers (approximately 2,800) will be provided a personal computer and that such customers will purchase at least 60% of their drugs from us. We reserve the right to take back or to obtain full payment for the personal computers provided to our customers if they do not purchase at least 60% of their pharmaceutical orders from us or if we determine we cannot verify the total pharmaceutical orders of our customers. These high volume customers will become “members” that will be granted special recognition and benefits, including:
|
|
·
|
more favorable payments terms;
|
|
|
·
|
free personal computers for order placement;
|
|
|
·
|
access to specialty drugs; and
|
|
|
·
|
discount pricing, through volume purchases.
|
Part of what will differentiate us from other pharmaceutical distributors is the creation of a brand image for the Company. Once we have broadened our product line and commenced offering customers personal computers to facilitate online ordering, we will begin to establish ourselves as a unique brand. Customers will be offered attractive signs to be displayed in their premises to indicate that XYT/FCPG is their pharmaceutical supplier of choice and to indicate to their customers that they deal with a credible pharmaceutical supplier.
Currently, we own an over 3,000 square meter warehouse, which includes GSP certified room temperature storeroom, cool storeroom, cold storage, hazardous materials storage and gamy materials storage. Our employees do not deliver customer orders, rather, we utilize courier companies. All courier charges are paid directly by the customer. We do not have any agreements in place with any courier companies and we monitor courier costs very closely, and if any appear to be excessive, we will switch to another courier.
We have a proprietary Enterprise Resource Planning (“ERP”) system that is integrated with our internet ordering process, which enables us to directly procure pharmaceutical products from drug manufacturers and from the large national distributor, Anhui Huayuan Pharmaceutical Co. This enables us to execute a direct sales model, where some products can be shipped from the manufacturer or national distributor to our customers. We expect this strategic use of technology to significantly enhance profits of both the Company and our direct customers.
We do not possess any patents or trademarks. However, we possess a computer software system that has been developed exclusively for the Company that permits customers to access the Company’s inventory, marketing and ordering systems through the internet. The Company considers this computer software system, as well as the XYT brand name and Internet Drug Information Service License, to be valuable assets.
Industry
The PRC pharmaceutical distribution industry has evolved in the past 30 years from a complex, multi-tiered system that was subject to strict control at every governmental level to a competitive and increasingly market-oriented industry. From 1950 to 1979, all Chinese pharmaceutical distributors were state-owned and categorized into national, provincial and municipal-level distributors. The price markup at each level, from pharmaceutical manufacturer to end-consumer, was subject to a total markup cap of 28%. During the 1980s, the rigid three-level distribution system gave way to a more open and decentralized network. Driven by increasing demand for pharmaceutical products in the past three decades, the PRC pharmaceutical industry has experienced rapid growth. The numbers of pharmaceutical manufacturers and distributors have also increased significantly until recent years, when competition and government regulations and policies started to drive consolidation in the industry. As a result of these developments, the market volume of the PRC pharmaceutical distribution market has steadily increased.
8
Market Drivers
The significant growth of China’s population aged 60 or above is expected to drive demand for healthcare and pharmaceutical products in China. According to the PRC National Bureau of Statistics, the proportion of the population aged 60 or above in China has increased from 13.6% in 2007, or approximately 162.2 million people, to 14.5%, or approximately 193.5 million people in 2009. Rising life expectancy is also expected to contribute to the growth of China’s aging population, both as an absolute number and as a percentage of the total population. We believe that the aging population in China, which historically spends the most on healthcare, will drive the growth of the PRC healthcare and pharmaceutical industries. The prevalence of chronic health problems, such as arthritis, cardiovascular diseases and cancer, is expected to increase with the growth of China’s population aged 60 or above. In addition, as living standards continue to improve and health consciousness grows in China, many lifestyle-related diseases are also increasing and becoming more widespread. For example, sales of prescription cardiovascular medicines have been increasing, and management believes this is primarily as a result of the rising prevalence of heart disease in an aging population and increasingly unhealthy lifestyles in the population at large.
According to the China Statistical Yearbook 2009 (the “Yearbook”), from 2005 to 2009, the average per capita annual disposable income of China’s urban residents increased from approximately ¥7,943, or approximately US$1,168, to ¥12,247, or approximately US$1,800, representing a compound annual growth rate (“CAGR”) of approximately 11.4%. According to the Yearbook, China’s GDP grew at a CAGR of 16.0% from 2005 to 2009, and its per capita GDP grew from ¥14,144, or approximately US$2,080, in 2005 to approximately ¥25,125, or approximately US$3,695, in 2009, representing a CAGR of 15.4%. During this period, national income and disposable income levels increased significantly.
With rising living standards and increasing disposable income, people in China have become more health conscious. These developments have resulted in both Chinese urban and rural residents spending more on healthcare. According to the PRC National Bureau of Statistics, consumer expenditures on healthcare in China’s urban and rural areas increased from approximately ¥600.9, or approximately US$88.3, and ¥168.1, or approximately US$24.7, per person in 2005, respectively, to approximately ¥856.4, or approximately US$126, and ¥287.5, or approximately US$42.2, per person in 2009, respectively.
National Medical Insurance Program
The National Medical Insurance Program (“National Program”), which was introduced in 1999, is the largest medical insurance program in China. The National Program is funded with varying levels of contributions from the PRC Government, individual program participants and their employers.
In 1999, the National Program was originally launched as the Urban Worker Basic Medical Insurance Program (“Urban Worker Program”), a mandatory scheme covering urban workers and their minor children. In 2007, a voluntary component called the Urban Resident Basic Medical Insurance Program (“Urban Resident Program”) was further implemented as part of the National Program, to cover the rest of the urban residents that are not covered by the Urban Workers Program. The National Program provides guidance on which prescription and over-the-counter medicines are included in the National Program and to what extent the purchases of these medicines are reimbursable. See the section headed “Government Regulation — Reimbursement Under the National Medical Insurance Program” below for further information.
We believe that only a small percentage of the Chinese population can afford commercial insurance plans. Therefore, the National Program coverage is expected to expand in the future. According to the PRC National Bureau of Statistics, the percentage of PRC urban residents grew from approximately 44.9% of the total population to 46.6% from 2007 to 2009. The number of people covered by the National Program increased from approximately 94 million in 2002 to 219.4 million in 2009, representing a CAGR of 11.2%. This trend is anticipated to continue as the Twelfth Five-Year Plan government development initiative projects that the PRC urban population will increase from 47% to 52% of China’s total population between 2010 to 2015. Furthermore, the provincial and municipal authorities who are responsible for administering social medical insurance funds to cover such reimbursements have been gradually increasing funding in recent years. According to the PRC Ministry of Labor and Social Security, total funding under the national insurance program reached ¥1882.3 billion, or approximately US$276.8 billion, in 2010, representing an increase of 16.8% from 2009. The availability of funding is expected to increase significantly in the near future, primarily as a result of increased financial and policy support from various levels of the PRC government.
9
Access to Healthcare in Rural Areas
At the fifth meeting of the tenth National People’s Congress held in March 2007, the PRC Government announced its goal to accelerate the reform and development of healthcare services in the PRC and focus on building a basic healthcare system that covers both rural and urban areas. The PRC Government’s plans include providing expanded healthcare services for its rural citizens and establishing comprehensive community healthcare service centers that would provide basic medical treatment and pharmaceutical services, as well as upgrading existing class-two hospitals and state owned medical facilities. The public health service centers would be allocated based on demand and population.
In addition, the PRC Government has actively promoted the implementation of the New Rural Cooperative Medical Insurance Scheme (“New Rural Insurance Scheme”), which seeks to provide healthcare services to the vast rural areas of China. The program extends to cover approximately 2,678 counties in the PRC, which account for 93.8% of the total number of counties in the PRC. In addition, the program covers approximately 836 million rural residents, which accounts for approximately 96.0% of the total population engaged in the agricultural industry in China as of December 31, 2010. We believe that the New Rural Insurance Scheme will have a positive impact on the demand for our products in Yunnan Province, which is relatively underdeveloped with a large rural population.
PRC Healthcare Reform
In September 2008, the State Council published a draft plan to ease the difficulties and minimize the costs for PRC citizens to obtain proper healthcare treatment. On March 17, 2009, the PRC Government issued the Opinion on Deepening the Healthcare System Reform (the “Opinion”). The State Council subsequently released the Notice on Important Implementing Plans for the Healthcare System Reform 2009-2011 (the “Implementing Plan”). The goal of the healthcare reform plan is to establish a basic, universal healthcare framework to provide Chinese citizens with safe, efficient, convenient and affordable healthcare. The Opinion calls for healthcare reform to be carried out in two steps:
|
|
·
|
Step One, which will be completed by 2011, aims to increase the accessibility while reducing the cost of healthcare. During this phase, the PRC Government will build up a network of basic healthcare facilities, expand coverage of the public medical insurance system to cover 90% or more of the population, and reform the drug supply and public hospital system.
|
|
|
·
|
Step Two, which will take place between 2011 and 2020, envisions the establishment of a universal healthcare system. The entire population should be covered by public medical insurance; drugs and medical services should be accessible and affordable to citizens in all public healthcare facilities.
|
While the PRC Government has neither provided a concrete timetable nor steps to implement certain tasks, such as the public hospital reform, it has released execution guidance for other tasks. Most notably, the PRC Government has announced it will spend an additional approximately RMB 850 billion, or US$125 billion from 2009 to 2011 on the healthcare industry. A significant portion will be expended to establish a basic healthcare medical insurance regime, which aims to cover over 90% of the national population by 2011, mainly through the Urban Worker Program, Urban Resident Program and the New Rural Insurance Scheme. The PRC Government further announced that the annual subsidy for each participant will be increased from approximately RMB40, or US$5.90 to approximately RMB120, or US$17.60 for Urban Resident Program participants, and from approximately RMB 80, or US$11.76 to approximately RMB 120, or US$17.60 for New Rural Insurance Scheme participants, starting from 2010. The reform plan will also raise the cap on claim payments from four times the local average annual income to six times such income. Another significant part of the spending plan focuses on healthcare facilities. The PRC Government plans to build 29,000 rural clinics in 2009. In the next three years, the PRC government plans to build an additional 5,000 rural clinics, 2,000 county-level hospitals and 2,400 urban community clinics in under-developed areas. This substantial increase in healthcare spending is expected to expedite the growth of the healthcare industry in China.
10
Under the healthcare reform plan, the additional funding for the healthcare industry will primarily target four fundamental healthcare systems in China:
|
|
·
|
The public health services system. This system focuses on preventing disease and promoting health as a complementary alternative to medical treatment. The public health services system will provide services such as immunizations, regular physical check-ups (for senior citizens over 65 years of age and children under three years of age), pre-natal and post-natal check-ups for women, prevention of infectious or chronic diseases and other preventative and fitness activities.
|
|
|
·
|
The public medical insurance system. This system covers drugs and medical treatments for the majority of the population. The healthcare reform plan will retain the framework of the current public medical insurance schemes under the National Program, but will expand them to cover more of the population and increase the scope of treatments, raise the cap on claim payments and cover more claims at higher percentages.
|
|
|
·
|
The public healthcare delivery system. One of the primary goals of the Implementing Plan is to build more healthcare facilities and to improve the training of healthcare professionals. Beyond additional public wellness centers, the reform plan aims to place a medical clinic in every village and a hospital in every county by 2011. In addition, the PRC Government will encourage private investors to establish public non-profit hospitals.
|
|
|
·
|
The drug supply system. This system regulates pricing and how drugs will be procured prescribed and dispensed in healthcare facilities. The healthcare reform plan will focus on pricing, procurement, prescription and dispensing of essential drugs.
|
The Opinion and the Implementing Plan direct relevant governmental authorities, including the Ministry of Health, SFDA and the National Development Reform Commission, or NDRC, to adopt implementing regulations for the reforms outlined in the healthcare reform plan.
We believe the PRC healthcare reform plan will benefit our pharmaceutical distribution and other business operations, although the full impact of PRC healthcare reform on our operations is uncertain.
Industry Consolidation
We plan to rapidly expand from a provincial pharmaceutical distributor in Yunnan Province to a national distributor servicing many provinces. We believe that our ordering, fulfillment and logistics network will enable us to attract and acquire other provincial, county and municipal pharmaceutical distributors over the next 24 to 48 months.
The pharmaceutical distribution industry in China is currently highly fragmented. There were more than 13,000 Good Supply Practice (“GSP”) certified pharmaceutical distributors as of 2008 according to the South Medicine Economics Research Institute, an affiliate of the State Food and Drug Administration (“SFDA”). This fragmentation of the pharmaceutical industry has resulted in an inefficient supply chain for the distribution of most pharmaceutical products without the advanced logistics services featured in more developed markets. Given the level of fragmentation in the pharmaceutical distribution industry, we believe that only large distributors with effective nationwide distribution capabilities, value-added supply chain services and large-scale operations will thrive.
Due to competitive pressures caused by the fragmentation of the industry, the introduction of GSP requirements and other increased PRC regulatory requirements, as well as continuing price controls imposed by the PRC Government and the centralization of tender and bidding processes among public hospitals, there has been a trend towards consolidation of the pharmaceutical distribution industry in recent years. According to CAPC, the combined market share of the top three pharmaceutical distributors in China increased from 12.7% in 2003 to approximately 20.0% in 2008. The market share of the top 20 pharmaceutical distributors in the industry increased from 36.5% to 43.0% in the same period. Furthermore, the data suggest that the largest distributors benefit more from consolidation. The total market share of the ten largest distributors grew from 26.1% in 2003 to 34.5% in 2008, while that of the 11 to 20 largest companies decreased from 10.4% to 8.5% over the same period.
11
Consolidation has also occurred in the pharmaceutical distribution industries of other countries as a natural part of their evolution and development into a mature market. According to the Kaiser Foundation, a non-profit private foundation focusing on healthcare issues, between 1975 and 2000, the number of pharmaceutical distributors in the United States declined from approximately 200 to fewer than 50. Similarly, according to Booz Allen Hamilton, an international consulting firm, between 1979 and 2005, the number of pharmaceutical distributors decreased from 25 to 10 in France, from 32 to 12 in the United Kingdom, from 41 to 16 in Germany, and from 279 to 138 in Italy. The three largest pharmaceutical distributors in the U.S. held 90% of the U.S. market in terms of their share of total revenues in 2005, and the three largest European pharmaceutical distributors had 73%, 68%, 47% and 43% of the market in the United Kingdom, France, Germany, and Italy, respectively, in 2005. Overall, these three leading European pharmaceutical distributors held a market share of 64% of the pharmaceutical distribution industry in Europe in 2005.
We expect that, over time, the PRC pharmaceutical distribution industry will experience consolidation in the manner experienced in North America and Europe, as distributors seek to achieve economies of scale and optimize their resources. The trend towards consolidation in the PRC pharmaceutical industry has also been intensified by increased regulatory requirements and policies imposed by the PRC government on market participants in order to implement uniform quality control criteria for the distribution of pharmaceuticals and ensure a stable supply of safe, effective medicines throughout the country. For example, in 2003 the SFDA adopted and strictly enforced GSP certification as the relevant standard for quality control in pharmaceutical distribution. A number of smaller distributors were forced to exit the market due to the associated higher compliance costs following the adoption of GSP certification and other regulatory standards. We believe that the more rigorous regulatory standards and policies imposed by the PRC government will accelerate the trend towards consolidation in the pharmaceutical industry, and favor the continued growth of pharmaceutical distributors with large-scale, nationwide pharmaceutical distribution operations and effective quality controls that are positioned to benefit from the changes in PRC regulatory requirements and policies. In addition, the imposition of price controls imposed by the PRC government, the centralization of tender and bidding processes among public hospitals and consolidation among drug manufacturers are additional factors that will also contribute to the trend towards consolidation in the industry.
Business Model
Upon the closing of the Exchange Transaction, FCPG HK and XYT became wholly owned subsidiaries of the Registrant, and our business and operations are conducted solely through XYT.
Our business model leverages our ability to take and fulfill orders over the internet. We believe this provides us competitive advantage over other provincial distributors as we can order directly from some manufacturers as well as bypass several layers in the traditional Chinese pharmaceutical distribution model that utilizes county and municipal distributors and provide our product line directly to hospitals, clinics, pharmacies, drug stores and other health care institutions, as shown in the diagram below.
12
Traditional Distribution Model Internet Distribution Model
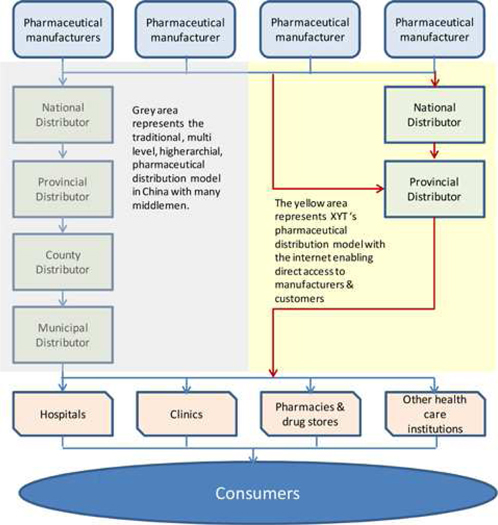
With the Company now expanding its product line to 20,000 to 25,000 products, our business model is changing to leverage the advantages of our internet ordering and fulfillment system, which will allow us to not only sell more products to our approximately 4,700 existing customers, but also attract more customers that currently do not utilize us. The bulk of these funds will be utilized to purchase inventory, which will be on a cash basis until a track record is established and net 30 day terms can be negotiated. The broader product line will include significantly more Western drugs as well as additional traditional Chinese drugs and herbs.
The foreign made drugs that we distribute are imported into China by drug importers that are licensed by the Chinese government. We are not licensed to import drugs from outside of China. In the event that trade protectionist policies are implemented by countries currently supplying Western drugs to China, such activities would adversely affect all pharmaceutical distribution companies in China, including us.
We will leverage our ability to fulfill orders over the internet by bypassing two levels of distributors and approximately 40% in total pricing mark-up. By bypassing city and county distributors, we will be able to achieve higher margins while providing pharmaceuticals to pharmacies, hospitals and clinics at costs below the traditional distribution model. We believe this will provide us with a competitive pricing advantage over our competitors as well as maintaining profit margins.
We believe that numerous provincial, county and municipal pharmaceutical distributors will be displaced by our business model. We believe that this also represents a tremendous opportunity for us as we may be able to acquire these companies and their customers and expand rapidly in Yunnan and other provinces, utilizing our corporate sales network that currently covers over 15 regions in Yunnan Province.
13
XYT Online Pharmaceutical Permit
The Interim Regulations on the Examination and Approval of Providing Drug Transaction Services on the internet became effective in China on December 1, 2005 and establishes the parameters and qualifications required for providing drug transaction services on the internet, including online drug transactions between a wholesale pharmaceutical distribution company and unrelated third parties using the website of the distribution company. These transactions are subject to inspection by, and the pharmaceutical distribution company must obtain a qualification certificate from, the provincial food and drug administration. The qualification certificate is valid for five years and may be renewed by filing for an extension at least six months prior to its expiration date and undergoing a reexamination by the relevant authority.
The Measures regarding the Administration of Drug Information Service Over the Internet which became effective on July 8, 2004 define the delivery of free publicly available drug information services over the Internet as a non-profit online drug information service. This service requires a qualification certificate from the provincial food and drug administration. The provincial food and drug administration must file its approval with the SFDA for records and make a public announcement. The qualification certificate is valid for five years and may be renewed by filing for an extension at least six months prior to its expiration date and undergoing a reexamination by the relevant authority.
We were certified to provide internet drug transaction services from the Yunnan Food and Drug Administration in October 2009 when we obtained an Internet Drug Information Service License. We can also utilize the internet fulfillment system licensed in Yunnan Province in other provinces, thereby creating an immediate advantage over competitors throughout China. Through our own industry research and documents from the Yunnan Food and Drug Administration, we believe that there are currently 491 drug distribution companies in Yunnan Province and that 42 of these companies also possess the Internet Drug Information Service License. We have also determined that in June 2009 the Yunnan Food and Drug Administration stopped accepting applications for Internet Drug Information Service Licenses due to misleading advertising by some licensees. Management has learned that this moratorium on Internet Drug Information Licenses was lifted during 2010 and new applications can now be submitted to the Yunnan Food and Drug Administration.
We have completed all the forms and supporting documentation and submitted a formal application to the Yunnan Food and Drug Administration for a second internet license, the Internet Transaction Service License. While our current License of Internet Drug Information Service permits us to use the internet to market our product line, display the inventory we hold and provide pricing information, thereby, enabling customers to see our inventory and to order directly by email, fax, online “chat” or phone, the Internet Transaction Service License would allow us to provide secured access to our proprietary computer fulfillment system, advertise, list inventories, take orders, provide shipping confirmation, invoice the customer and accept payment over the internet. Through our own industry research and documents from the Yunnan Food and Drug Administration, management believes that there are currently only two drug manufacturing companies in Yunnan Province that possess the Internet Transaction Service License. If we are successful in obtaining this license, management believes we will be the only drug distribution company in Yunnan Province that will possess the Internet Transaction Service License.
The Yunnan Food and Drug Administration has completed the first review of the application and forwarded the application to the State Food and Drug Administration for their review. There is no established time frame regarding the decline or approval of the application. Both the Yunnan Food and Drug Administration and the State Food and Drug Administration are government agencies which review the application based on the information supplied by the applicant and subjective criteria. Ultimately, approval for the Internet Transaction Service License is largely subjective, based on the application and the direction of government policy at the time. To find out if the application is approved or declined can take as short as 6 months to longer than a year.
If we do not receive the Internet Transaction Service License, we do not believe our business will be materially affected, as our existing license, the Internet Drug Information License, enables us to market our products to our customers and fulfill orders. We are currently licensed to receive orders via telephone, fax and email, and payment must be made via, check, wire transfer or cash. The Internet Transaction Service License would allow us to also receive orders and payments through our website.
14
Competition
The pharmaceutical distribution industry in China is intensely competitive, rapidly evolving and highly fragmented. In many large cities in China, we need to not only compete with other retail drugstores, but also face increasing competition pressure from discount stores, convenience stores and supermarkets. In order to maintain our competitive position in the market, we have increasingly diversified products and services by offering some non-drug products that are provided in regular convenience stores. These products include cosmetics, diapers, tissues, health drinks and small medical instruments like tweezers and scissors. Convenience stores in China are not yet licensed to carry any pharmaceutical products. In addition, we also increased our competitiveness through careful selection of store location, merchandise, and services.
With the continuous consolidation of the pharmaceutical industry and opening of new drugstore chains in large cities, we will face more competition in the industry. However, in many of our targeted second- and third- tier cities and rural areas, we are facing less competition because major drugstore chains have not entered into the market. We are in a good position to establish our standing and reputation in these targeted markets. In addition, the pharmaceutical industry has entrance barriers for new entrants due to the requirements for capital, brand name, management expertise, etc. Further, PRC laws and regulations limit a foreign investor’s ownership in retail drugstores to the maximum of 49.0% if such investor holds ownership interest in more than 30 drug stores that sell a variety of branded drugs sourced from different suppliers. This limitation, together with the complexity of the Chinese market, creates a barrier for foreign retail drugstore chain operators to enter into the PRC market. As a result, currently we do not face notable competition from foreign owned drugstore chains.
Because our network covers many cities and areas, and many of drugstores are regional, our competitors vary from region to region. Each region can have its own, among others, distinct demographics, local regulations and shopping style. We do not consider any individual regional drugstore as our major competitor, but we compete with them on an aggregate basis.
Given the fragmentation in the Chinese pharmaceutical distribution industry, distributors are under intense pressure to compete for business and maintain their profits, and must focus on a number of competitive issues, including:
|
|
·
|
Scale. Given the low margins of the distribution business, achieving economies of scale is crucial for distributors to maintain a sustainable and profitable business.
|
|
|
·
|
Quality and range of services. Customers and suppliers of pharmaceutical distributors increasingly value pharmaceutical distributors that are able to deliver one-stop shop pharmaceutical distribution services, which comprise high-quality traditional distribution services and logistics and other value-added services.
|
|
|
·
|
Geographic coverage. China’s vast territory presents significant geographical challenges that require manufacturers and distributors to develop their distribution networks and penetrate as many local markets as possible to take advantage of the growth of the Chinese market.
|
|
|
Product portfolio. The breadth of products that distributors offer is an important factor for their customers. For example, a large hospital typically needs thousands of different types of prescribed drugs. As such, distributors with an extensive portfolio are generally preferred.
|
|
|
Creditworthiness and financial stability. To minimize supply disruptions and bad debt, customers and suppliers generally select pharmaceutical distributors that are reliable commercial partners with strong financial capabilities and proven track records.
|
|
|
Price. Price competition is intense in the pharmaceutical distribution industry. However, customers and suppliers generally do not make purchasing decisions solely based on price, as they will consider the foregoing factors as well. As a result, the leading and established distributors are able to leverage their strengths to obtain better pricing terms than their weaker competitors.
|
15
|
|
·
|
Yun Nan Hong Xiang Pharmacy Co., Ltd. is a provincial bulk seller and chain store that owns over 1,000 chain stores in middle, west and south of Yun Nan.
|
|
|
·
|
Yun Nan Provincial Pharmacy Co., Ltd. is a designated supplier to many state-run hospitals.
|
|
|
·
|
Yun Nan Tong Feng Pharmacy Co., Ltd. has substantial experience in selling a wide range of products to end users.
|
Suppliers
We have significantly increased the number of suppliers that we acquire products from. We now purchase approximately 60% of our products from the following five drug manufacturers and national distributors:
|
|
·
|
Anhui Hua Yuan Pharmaceutical Co., Ltd.
|
|
|
·
|
Guangdong Guo Yi Tang Pharmaceutical Co., Ltd.
|
|
|
·
|
Yunnan Li Jie Pharmaceutical Co., Ltd.
|
|
|
·
|
Tonghua Xing Hua Pharmaceutical Co., Ltd.
|
|
|
·
|
Jilin Dong Feng Pharmaceutical Co., Ltd.
|
We believe we have excellent relationships with these suppliers.
Customers
We currently have approximately 5,000 customers, with annual sales of approximately ¥190 million. These include pharmacies (drug stores), hospitals, distribution companies and clinics. In urban areas, we typically service customers directly, utilizing internet orders and local bonded couriers. Rural areas are predominantly serviced by local sub-distributors. Our approximately 4,000 long-term downstream customers, include:
|
|
·
|
approximately 1846 independents directly serviced by internet;
|
|
|
·
|
approximately 855 sub-distributors; and
|
|
|
·
|
approximately 1246 hospital, clinics and other medical institutions.
|
Our top five customers listed below accounted for approximately 40% of the Company’s sales in 2010:
|
|
·
|
Kunming Ri Xin Shi Jia Drugstore
|
|
|
·
|
Yunnan Wan Yuan Pharmaceutical Co, Ltd.
|
|
|
·
|
Yunnan Pharmaceutical Co. Ltd
|
|
|
·
|
Yunnan Hong Xiang Yi Xin Tang Pharmaceutical Group.
|
|
|
·
|
Yunnan Bai Yao Drugstore Co., Ltd.
|
16
Mr. Wang, Chairman and Chief Executive Officer, developed and maintained relationships with certain customers whom he met prior to joining XYT. Most of these customers were small business owners, who placed high emphasis on informal relationships and individual credibility developed based on the quality of products and the conduct of business transactions. Mr. Wang devoted himself to retaining these customers, and they remained loyal to Mr. Wang as they believed in the quality of the products that he introduced and received updates on new products from Mr. Wang. There are over 35 such customers, and they generally conduct high volumes of cash sales. Therefore, due in part to the client base and social network of Mr. Wang, XYT had authorized Mr. Wang to collect receivables of XYT prior to or concurrent with product delivery for certain customers with whom he has a relationship pursuant to an Authorization Agreement between the parties and XYT’s instructions. Such receivable collections are represented as “cash sales” in the PRC (which, according to common accounting practice in China, normally arise when a company receives payment for sales by cash or by a private bank account other than a corporate bank account), and the amount of such receivables collected by Mr. Wang were US$5,945,625, US$17,270,218, and US$26,363,421 during 2007, 2008, and 2009, respectively. Mr. Wang also pays certain payables (including purchased inventories from suppliers, expenses, such as commissions, notes payables, software customization, installation of the internet fulfillment system, computer equipment and even vehicles to provide expedited delivery of purchased goods) with funds from the receivables he previously collected on behalf of the Company. The Company believes such an arrangement is common in the PRC and allows it and Mr. Wang to maintain favorable relationships with customers and suppliers due to prompt payments and frequent communications, reduces certain transaction costs, allows it to obtain favorable prices and discounts due to Mr. Wang’s prompt settlement of certain supplier accounts, and reduces certain liquidity risks due to daily limits and other restrictions on bank withdrawals from business accounts as the funds collected by Mr. Wang on behalf of the Company provide an additional liquidity option for the Company when needed. In order to ensure that a company retains sufficient registered capital to support itself, there are limits on the funds that can be paid out each day. For a company with the registered capital of XYT, the daily limit on funds that may be liquidated (paid out) is RMB500,000 or $77,000 USD per day. By Mr. Wang providing funds for the settlement of payables, the Company was able to work around this liquidity restriction without having to increase its registered capital. This arrangement was informal in nature and on an as-needed basis voluntarily by Mr. Wang. When the Company was required to make daily payments in excess of RMB500,000 or US$77,000, Mr. Wang would pay such excess amounts personally to ensure the continuous efficient flow of payments. With a company that was approaching $25 million in annual sales, Mr. Wang was called upon a couple of times per week to top up payments that were in excess RMB500,000 or US$77,000 per day.
However, prior to the closing of the Exchange Transaction, the Company and Mr. Wang recognized that continuation of this arrangement, while appropriate and effective for XYT as a private, closely held company, should not continue as XYT became a subsidiary of a publicly traded company with multiple stockholders that would be subject to SEC and U.S. GAAP compliance. Therefore, as part of its evolution into a public company, the Company and Mr. Wang had agreed that after the Exchange Transaction, Mr. Wang should discontinue making payments and collections on behalf of XYT in order to standardize its business operations and set up a structure of effective internal controls as a public company. In fact, Mr. Wang intended to cease collecting receivables in June 2010, however, customers continued to make payments to Mr. Wang despite instructions from Mr. Wang and the Company to the contrary. While the weaning process had begun, it continued for some time for fear of losing key customers, and Mr. Wang only managed to convince all key customers and completely cease the practice of such collections on behalf of XYT as of October 31, 2011. The amounts due from Mr. Wang to us had previously been treated as a loan receivable from a related party to us and amounted to $11,799,953 as of December 31, 2009 and $2,498,924 as of December 31, 2008. To comply with U.S. GAAP, we have reclassified such amounts as a deemed distribution of a dividend to Mr. Wang, a related party, and therefore a corresponding reduction of retained earnings. Mr. Wang has not repaid any amount of the distribution.
In advance of the Exchange Transaction, XYT also applied for an increase in its registered capital and began the process of arranging for direct bank financing for many of its large customers. In the past, Mr. Wang would personally provided credit to some of the Company’s largest and best customers for purchases from XYT. As XYT does not grant credit to the vast majority of its customers, the Company is introducing large credit worthy customers to banks that are interested in providing XYT customers with credit for purchases from XYT. These credit arrangements are between the bank and XYT’s customers for the purchase of XYT products. XYT is not party to the credit provided, does not guarantee or co-sign for the customer and receives no compensation from the bank or its customers for arranging this credit. By arranging credit for its customers, XYT improves its liquidity position by not having to utilize working capital to finance customer purchases.
Further, in advance of the Exchange Transaction, XYT applied for and received a small increase in its registered capital. XYT’s registered capital was increased from RMB2,000,000 to RMB15,080,000, or US$2,295,000. After the April 2011 financing, XYT again applied and was granted an increase in registered capital. These increases in registered capital have enabled the Company to increase the daily withdrawal limits from the bank to reduce any adverse impact on daily operations.
With the exception of these bonus payments and deemed dividend distribution to Mr. Wang, we do not contemplate any material payments or benefits to be received by the parties thereto.
17
Government Regulation
As a distributor and retailer of pharmaceutical products, we are subject to substantial regulation and oversight by different levels of the food and drug administration in China, in particular, the SFDA. The Law of the PRC on the Administration of Pharmaceutical Products, as amended, provides the basic legal framework for the administration of the production and sale of pharmaceutical products in China and governs the manufacturing, distributing, packaging, pricing and advertising of pharmaceutical products in China. The corresponding implementation regulations set out detailed rules with respect to the administration of pharmaceuticals in China. We are also subject to other PRC laws and regulations that are applicable to business operators, retailers and foreign-invested companies, as described below.
Distribution of Pharmaceutical Products
A distributor of pharmaceutical products must obtain a distribution permit from the relevant provincial, designated municipal, or county-level food and drug administration. The grant of such permit is subject to an inspection of the distributor’s facilities, warehouses, hygienic environment, quality control systems, personnel and equipment. The distribution permit is valid for five years, and the holder must apply for renewal of the permit within six months prior to its expiration. In addition, a pharmaceutical product distributor needs to obtain a business license from the relevant administration for industry and commerce prior to commencing its business. We have obtained necessary pharmaceutical distribution permits, and we anticipate a routine renewal of these permits and certifications at this time.
In addition, under the Supervision and Administration Rules on Pharmaceutical Product Distribution promulgated by the SFDA on January 31, 2007, and effective May 1, 2007, a pharmaceutical product distributor is responsible for its procurement and sales activities and is liable for the actions of its employees or agents in connection with their conduct of distribution on behalf of the distributor. A retail distributor of pharmaceutical products is not allowed to sell prescription pharmaceutical products, or Tier A OTC pharmaceutical products, listed in the national or provincial medical insurance catalogs without the presence of a certified in-store pharmacist. See “Reimbursement under the National Medical Insurance Program” below. We believe we are in full compliance with applicable governmental regulations.
Operations of pharmaceutical distributors shall be conducted in accordance with the Pharmaceutical Operation Quality Management Rules and shall be granted a GSP certificate under such rules by the SFDA.
Pharmaceutical distributors must keep true and complete records of any pharmaceutical products purchased, distributed or sold with the generic name of such products, specification, approval code, term, manufacturer, purchasing or selling party, price and date of purchase or sale. A pharmaceutical distributor must keep such records at least until one year after the expiration date of such products and in any case, such records must be kept for no less than three years. Penalties may be imposed for any recordkeeping violations.
Pharmaceutical distributors can only distribute pharmaceutical products obtained from those with a Pharmaceutical Manufacturing Permit and a Pharmaceutical Distribution Permit.
Good Supply Practice Standards
China has strengthened its enforcement of GSP standards since adopting it at the end of December 2004. As a result, many smaller drugstore chains or independently operated drugstores may find it difficult to meet these enhanced quality requirements for the operations of pharmacies.
GSP standards regulate wholesale and retail pharmaceutical product distributors to ensure the quality of distribution of pharmaceutical products in China. The current applicable GSP standards require pharmaceutical product distributors to implement strict controls on the distribution of medicine products, including standards regarding staff qualifications, distribution premises, warehouses, inspection equipment and facilities, management and quality control. The GSP certificate is usually valid for five years.
We possess a GSP certificate, and we anticipate a routine renewal of these certifications upon their expiration at this time.
18
Prescription Administration
Under the Rules on Administration of Prescriptions promulgated by the SFDA, effective May 1, 2007, doctors are required to include the chemical ingredients of the medicine they prescribe in their prescription and are not allowed to include brand names in their prescription. This regulation is designed to provide consumers with choices among different pharmaceutical products that contain the same chemical ingredients.
Advertisement of Pharmaceutical Products
The PRC government has adopted a series of measures regulating the advertising of pharmaceutical products. Consumers typically become familiar with a medicine through advertising and word-of-mouth recommendations by our salespeople. With increased restrictions on advertising of pharmaceutical products, pharmaceutical product manufacturers are expected to increasingly rely on retail pharmacies to build brand familiarity among the general public.
In order to prevent misleading advertising of pharmaceutical products, the State Administration for Industry and Commerce (“SAIC”) and the SFDA jointly promulgated the Standards for Examination and Publication of Advertisements of Pharmaceutical Products and Rules for Examination of Advertisement of Pharmaceutical Products in March 2007. Under these regulations, there are prohibitions on the advertising of certain pharmaceutical products, and advertisement of prescription pharmaceutical products may only be made in authorized medical magazines. In addition, an approval must be obtained from the provincial level of food and drug administration before a pharmaceutical product may be advertised. Such approval, once obtained, is valid for one year.
Our Internet Drug Information Service License, issued by the Yunnan Food and Drug Administration enables us to use the internet to advertise and market our product line, display the inventory we hold, provide pricing information, conduct live “chats” with customers and receive orders through email. The only advertising restrictions with this license are that license holders cannot advertise drugs they do not carry in their inventory or advertise on behalf of another company.
Product Liability and Consumer Protection
Product liability claims may arise if the products sold have any harmful effect on the consumers. The injured party may claim for damages or compensation. The General Principles of the Civil Law of the PRC, which became effective in January 1987, state that manufacturers and sellers of defective products causing property damage or injury shall incur civil liabilities for such damage or injuries.
The Product Quality Law of the PRC was enacted in 1993 and amended in 2000 to strengthen the quality control of products and protect consumers’ rights and interests. Under this law, manufacturers and distributors who produce or sell defective products may be subject to confiscation of earnings from such sales, revocation of business licenses and imposition of fines, and in severe circumstances, may be subject to criminal liability.
The Law of the PRC on the Protection of the Rights and Interests of Consumers was promulgated on October 31, 1993 and became effective on January 1, 1994 to protect consumers’ rights when they purchase or use goods or services. All business operators must comply with this law when they manufacture or sell goods and/or provide services to customers. In extreme situations, pharmaceutical product manufacturers and distributors may be subject to criminal liability if their goods or services lead to the death or injuries of customers or other third parties.
The Tort Liability Law of the PRC became effective on July 1, 2010. Under this law, distributors who cannot identify the manufacturer’s fault in the defective products may be liable to consumers for torts, and after distribution of defective products, distributors and manufacturers may be subject to various remedies including product recalls.
The retail prices of some pharmaceutical products sold in China, primarily those included in the national and provincial medical insurance catalogs and those pharmaceutical products whose production or distribution are deemed to constitute monopolies, are subject to price controls in the form of fixed prices or price ceilings. Manufacturers or distributors cannot freely set or change the retail price for any price-controlled product above the applicable price ceiling or deviate from the applicable fixed price imposed by the PRC government. The prices of medicines that are not subject to price controls are determined freely at the discretion of the respective pharmaceutical companies, subject to notification to the provincial pricing authorities.
19
The retail prices of medicines that are subject to price controls are administered by the Price Control Office of the National Development and Reform Commission (“NDRC”), and provincial and regional price control authorities. The retail price, once set, also effectively determines the wholesale price of that medicine. From time to time, the NDRC publishes and updates a list of medicine that are subject to price controls. Fixed prices and price ceilings on medicine are determined based on profit margins that the relevant government authorities deem reasonable, the type and quality of the medicine, its production costs, the prices of substitute medicine and the extent of the manufacturer’s compliance with the applicable Good Manufacturing Practices (“GMP”) standards. The NDRC directly regulates the pricing of a portion of the medicine on the list, and delegates to provincial and regional price control authorities the authority to regulate the pricing of the rest of the medicine on the list. Provincial and regional price control authorities have discretion to authorize price adjustments based on the local conditions and the level of local economic development. Currently, approximately 2,600 pharmaceutical products, or approximately 22.0% of the pharmaceutical products available in China, are subject to price control. Of those, the price controls for the retail prices of approximately 1,900 pharmaceutical products are administered by the NDRC and the rest are administered by provincial and regional price control authorities.
Only the manufacturer of a medicine may apply for an increase in the retail price of the medicine, and it must either apply to the provincial price control authorities in the province where it is incorporated, if the medicine is provincially regulated, or to the NDRC, if the medicine is NDRC-regulated. For a provincially-regulated medicine, in cases where provincial price control authorities approve an application, manufacturers must file the newly approved price with the NDRC for record and thereafter the newly approved price will become binding and enforceable across China.
Since May 1998, the PRC government has ordered reductions in the retail prices of various pharmaceutical products 24 times. The latest price reduction occurred in May 2007 and affected 1,245 different pharmaceutical products, of which 524 are sold by us. As of December 31 of the following years, approximately the following percentages of the pharmaceutical products we offered were subject to price controls: 2004 - 1.5%; 2005 - 2.0%; 2006 - 7.5%; 2007-2009 - 16.0%; and 2010 - 7.0%.
Reimbursement under the National Medical Insurance Program
The PRC government has increased the availability of funding under the National Program and included more pharmaceutical products in the China’s national medical insurance scheme. Eligible participants in the National Program, mainly consisting of urban residents, are entitled to purchase medicine when presenting their medical insurance cards in an authorized Company store, provided that the medicine they purchase has been included in the national or provincial medical insurance catalogs. Depending on relevant local regulations, authorized pharmacies either sell medicine on credit and obtain reimbursement from relevant government social security bureaus on a monthly basis, or receive payments from the participants at the time of their purchases, and the participants in turn obtain reimbursement from relevant government social security bureaus.
Medicine included in the national and provincial medical insurance catalogs is divided into two tiers. Purchases of Tier A pharmaceutical products are generally fully reimbursable, except that certain Tier A pharmaceutical products are only reimbursable to the extent the medicines are used for specifically stated purposes in the medical insurance catalogs. Purchasers of Tier B pharmaceutical products, which are generally more expensive than Tier A pharmaceutical products, are required to make a certain percentage of co-payments, with the remaining amount being reimbursable. The percentage of reimbursement for Tier B OTC pharmaceutical products varies in different regions in the PRC. Factors that affect the inclusion of medicine in the medical insurance catalogs include whether the medicine is consumed in large volumes and commonly prescribed for clinical use in China and whether it is considered to be important in meeting the basic healthcare needs of the general public.
20
The PRC Ministry of Labor and Social Security, together with other government authorities, has the power to determine every two years which pharmaceutical products are included in the national medical insurance catalog, under which of the two tiers the included pharmaceutical products fall, and whether an included pharmaceutical product should be removed from the catalog. Provincial governments are required to include all Tier A pharmaceutical products listed on the national Medical Insurance Catalog in their provincial medical insurance catalogs. For Tier B pharmaceutical products listed in the national medical insurance catalog, provincial governments have the discretion to adjust the number of Tier B pharmaceutical products listed in the national medical insurance catalog that are to be included in the provincial medical insurance catalogs but the number of Tier B pharmaceutical products under adjustment shall not exceed 243. The amount in a participant’s individual account under the program varies, depending on the amount of contributions from the participant and his or her employer. Generally, participants under the National Program who are from relatively wealthier parts and metropolitan centers of China have greater amounts in their individual accounts than those from other parts of the country. Different regions in China have different requirements regarding the caps of reimbursements in excess of the amounts in the individual accounts.
Sales of Nutritional Supplements and other Food Products
According to the PRC Food Hygiene Law and Rules on Food Hygiene Certification, a distributor of nutritional supplements and other food products must obtain a food distribution certificate from relevant provincial or local health regulatory authorities. The grant of such certificate is subject to an inspection of the distributor’s facilities, warehouses, hygienic environment, quality control systems, personnel and equipment. The food distribution certificate is valid for four years, and the holder must apply for renewal of the certificate within thirty days prior to its expiration. Our certification was renewed in April 2011 for an additional four years.
Foreign Exchange Regulation
Pursuant to the Foreign Currency Administration Rules promulgated in 1996 and amended in 1997 and various regulations issued by the State Administration of Foreign Exchange (“SAFE”), and other relevant PRC government authorities, the Renminbi is freely convertible only to the extent of current account items, such as trade-related receipts and payments, interest and dividends. Capital account items, such as direct equity investments, loans and repatriation of investments, require the prior approval from the SAFE or its local counterpart for conversion of Renminbi into a foreign currency, such as U.S. dollars, and remittance of the foreign currency outside the PRC.
Payments for transactions that take place within the PRC must be made in Renminbi. Unless otherwise approved, PRC companies must repatriate foreign currency payments received from abroad. Foreign-invested enterprises may retain foreign exchange in accounts with designated foreign exchange banks subject to a cap set by the SAFE or its local counterpart. Unless otherwise approved, domestic enterprises must convert all of their foreign currency receipts into Renminbi.
Pursuant to the SAFE’s Notice on Relevant Issues Concerning Foreign Exchange Administration for PRC Residents to Engage in Financing and Inbound Investment via Overseas Special Purpose Vehicles, or the SAFE Circular No. 75, issued on October 21, 2005:
|
|
(i)
|
a PRC citizen residing in the PRC, or PRC resident, shall register with the local branch of the SAFE before it establishes or controls an overseas special purpose vehicle (“overseas SPV”), for the purpose of overseas equity financing (including convertible debts financing);
|
|
|
(ii)
|
when a PRC resident contributes the assets of or its equity interests in a domestic enterprise into an overseas SPV, or engages in overseas financing after contributing assets or equity interests into an overseas SPV, such PRC resident shall register his or her interest in the overseas SPV and the change thereof with the local branch of the SAFE; and
|
|
|
(iii)
|
when the overseas SPV undergoes a material event outside of China, such as change in share capital or merger and acquisition, the PRC resident shall, within 30 days from the occurrence of such event, register such change with the local branch of the SAFE.
|
21
On May 29, 2007, SAFE issued relevant guidance to its local branches for the implementation of the SAFE Circular No. 75. This guidance standardizes more specific and stringent supervision on the registration requirement relating to SAFE Circular No. 75 and further requires PRC residents holding any equity interests or options in SPVs, directly or indirectly, controlling or nominal, to register with SAFE.
Mr. Wang is a PRC resident who has registered with the local branch of SAFE as required under SAFE Circular No. 75.
Under the Implementing Rules of Measures for the Administration of Individual Foreign Exchange, or the Implementation Rules, issued by SAFE on January 5, 2007, PRC citizens who are granted shares or share options by an overseas listed company according to its share incentive plan are required, through a qualified PRC agent or the PRC subsidiary of such overseas listed company, to register with the SAFE and complete certain other procedures related to the share incentive plan. Foreign exchange income received from the sale of shares or dividends distributed by the overseas listed company must be remitted into a foreign currency account of such PRC citizen or be exchanged into Renminbi.
In addition, domestic wages and salaries of foreign employees outside of the PRC, as well as other rightful earnings, such as dividends, bonuses and profits, of shareholders outside of the PRC may be remitted freely out of the PRC after taxes have been paid in accordance with the provisions of the Chinese tax law with a tax certificate. Since we do not have any debt that is generated outside the PRC and do not have any employees located outside PRC, management is not aware of any material risk of paying in foreign currency in respect of those employee-related and debt-settlement amounts due to any other party located outside PRC.
Liquidation
According to the bankruptcy law of the PRC, XYT, as a WFOE, needs to have its debt to creditors settled in the priority as set forth in the relevant Bankruptcy law in China and its immediate equity holder, FCPG HK, located in Hong Kong, would be the last party to be entitled to any residual interest of the entity. Such priority of payment and distribution in the case of the liquidation of XYT does not have any different priority in respect of PRC nationals or foreigners. The priority is based on the status of being a creditor and other requirements as set forth in the Bankruptcy law in China, which does not have any discrimination or preference in respect of whether the party is a PRC national or foreigner.
Taxation
Under the Enterprise Income Tax Law (“EIT”), effective January 1, 2008, China will adopt a uniform tax rate of 25.0% for all enterprises (including foreign-invested enterprises) and revoke the current tax exemption, reduction and preferential treatments applicable to foreign-invested enterprises. However, there will be a transition period for enterprises, whether foreign-invested or domestic, that are currently receiving preferential tax treatment granted by relevant tax authorities. Enterprises that are subject to an enterprise income tax rate lower than 25.0% may continue to enjoy the lower rate and gradually transition to the new tax rate within five years after the effective date of the EIT Law. Enterprises that are currently entitled to exemptions or reductions from the standard income tax rate for a fixed term may continue to enjoy such treatment until the fixed term expires. However, the two-year exemption from enterprise income tax for foreign-invested enterprise will begin from January 1, 2008 instead of from when such enterprise first becomes profitable. Preferential tax treatments will continue to be granted to industries and projects that are strongly supported and encouraged by the state, and enterprises otherwise classified as “new and high technology enterprises strongly supported by the state” will be entitled to a 15.0% enterprise income tax rate even though the EIT Law does not currently define this term.
Provisions Regarding Mergers and Acquisitions of Domestic Enterprises by Foreign Investors
On August 8, 2006, six PRC regulatory agencies, including the Chinese Securities Regulatory Commission (“CSRC”), promulgated a rule entitled Provisions Regarding Mergers and Acquisitions of Domestic Enterprises by Foreign Investors (“the new M&A rule”) to regulate foreign investment in PRC domestic enterprises. The new M&A rule provides that the Ministry of Commerce must be notified in advance of any change-of-control transaction in which a foreign investor takes control of a PRC domestic enterprise and any of the following situations exists:
22
|
|
the PRC domestic enterprise has a well-known trademark or historical Chinese trade name in China.
|
The new M&A rule also contains a provision requiring overseas SPVs, formed for listing purposes through acquisitions of PRC domestic companies and controlled by PRC individuals, to obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange.
On September 21, 2006, the CSRC issued a clarification that sets forth the criteria and process for obtaining any required approval from the CSRC. To date, the application of this new M&A rule is unclear.
Government Support of the Pharmaceutical Industry
The PRC government has supported the growth of the drugstore industry with a series of initiatives.
Active PRC Government support
As part of its Eleventh Five-Year Plan (2006-2010), the PRC Government has actively supported the PRC healthcare industry by providing a number of incentives and enacting programs, including increased funding for building additional hospitals, research centers and other healthcare facilities, enacting healthcare reforms and standards and subsidizing healthcare services for its citizens. The PRC Government has announced it will spend an additional RMB850 billion on healthcare programs from 2009 to 2011, which will significantly bolster the PRC healthcare market.
In addition, in 2011, the PRC Government enacted its Twelfth Five-Year Plan (2011-2015). As part of the Twelfth Five-Year Plan, the PRC Government plans to integrate the distribution market of pharmaceutical products gradually to 1-3 nationwide distributors in the market whose revenues exceed RMB100 billion and approximately 20 pharmaceutical regional distributors whose revenues exceed RMB10 billion. The market share of the top 100 pharmaceutical wholesalers is projected to reach 85%, while that of the top 100 pharmaceutical retailers is projected to reach 60% as of 2015.
Anti-Corruption
The substantial majority of hospitals in China are owned and operated by the government, and revenue from hospital pharmacies constitutes a significant portion of hospitals’ revenue. Hospitals procure their supplies of pharmaceutical products in bulk from manufacturers or distributors of pharmaceutical products, and generally decide whether to include a particular medicine on their formulary based upon a number of factors, including doctors’ preference in prescribing the medicine, the cost of the medicine, the perceived efficacy of the medicine and the hospital’s budget. Decisions by hospitals regarding whether to include a particular medicine in their pharmacies could be affected by corrupt practices, including illegal kickbacks and other benefits offered by manufacturers or distributors of pharmaceutical products. These corrupt practices may also affect doctors’ decisions regarding which types of medicine to prescribe.
The PRC government has strengthened its anti-corruption measures and has organized a series of government-sponsored anti-corruption campaigns in recent years. In particular, China amended its criminal code in 2006, increasing the penalties for corrupt business practices. The amendment of the criminal code is expected to make pharmaceutical product suppliers compete for the hospitals’ business on fair and equal terms, and thus is expected to result in more growth opportunities for drugstores that are not affiliated with hospitals.
The PRC SFDA promulgated pharmaceutical product labeling regulations in March 2006, which require that pharmaceutical product labels state the generic ingredients of the pharmaceutical products and which bar the registration of any brand name for any pharmaceutical product which does not contain active ingredients. In addition, effective May 1, 2007, doctors are not permitted to include brand names in their prescriptions and required to specify the chemical ingredients of the medicines they prescribe in their prescription. These requirements are expected to have the following positive impacts on the business of non-hospital drugstores:
23
|
|
·
|
help curb corrupt practices by pharmaceutical product manufacturers and doctors;
|
|
|
·
|
ensure that patients are given better information on the medicines they purchase; and
|
|
|
·
|
weaken the hospitals’ monopoly on prescriptions and prescription pharmaceutical products.
|
Equal Opportunity for Non-Hospital Drugstores
The PRC Ministry of Health has promulgated prescription regulations requiring hospitals to allow prescriptions to be filled at non-hospital drugstores. The implementation of this regulation is expected to increase drug sales, especially prescription drug sales, in drugstore chains and independent drugstores that are not affiliated with hospitals.
Employees
We currently employ 90 individuals at our headquarters, located at Number 504, West Ren Min Road, Kunming, City, Yunnan Province, PRC.
Corporate Information
Our principal executive offices are located at FCPG HK’s and XYT’s headquarters located at Number 504, West Ren Min Road, Kunming City, Yunnan Province, People’s Republic of China, 650000, which is also our mailing address. Our telephone number is 852-2138-1668.
ITEM 1A. RISK FACTORS.
You should carefully consider the risks described below together with all of the other information included in our public filings before making an investment decision with regard to our securities. The statements contained in or incorporated into this document that are not historic facts are forward-looking statements that are subject to risks and uncertainties that could cause actual results to differ materially from those set forth in or implied by forward-looking statements. If any of the following events described in these risk factors actually occurs, our business, financial condition or results of operations could be harmed. In that case, the trading price of our common stock could decline, and you may lose all or part of your investment.
Risks Related to Our Business and Industry
Our operating results are difficult to predict, and we may experience significant fluctuations in our operating results.
Our operating results may fluctuate significantly. As a result, you may not be able to rely on period to period comparisons of our operating results as an indication of our future performance. Factors causing these fluctuations include, among others:
|
|
·
|
our ability to maintain and increase sales to existing customers, attract new customers and satisfy our customers’ demands;
|
|
|
·
|
the frequency of customer visits to pharmacies and drugstores and the quantity and mix of products our customers purchase;
|
|
|
the price we charge for our products or changes in our pricing strategies or the pricing strategies of our competitors;
|
|
|
·
|
timing and costs of marketing and promotional programs organized by us and/or our suppliers, including the extent to which we or our suppliers offer promotional discounts to our customers;
|
|
|
·
|
our ability to acquire merchandise, manage inventory and fulfill orders;
|
|
|
·
|
technical difficulties, system downtime or interruptions of our computer system, which we use for product selection, procurement, pricing, distribution and retail management processes;
|
24
|
|
·
|
the introduction by our competitors of new products;
|
|
|
·
|
the effects of strategic alliances, potential acquisitions and other business combinations, and our ability to successfully and timely integrate them into our business;
|
|
|
·
|
changes in government regulations with respect to pharmaceutical and retail industries; and
|
|
|
·
|
economic and geopolitical conditions in China and elsewhere.
|
In addition, a significant percentage of our operating expenses are fixed in the short term. As a result, a delay in generating or recognizing revenue for any reason could result in substantial operating losses.
Moreover, our business is subject to seasonal variations in demand. In particular, traditional retail seasonality affects the sales of certain pharmaceuticals and other non-pharmaceutical products. Sales of our pharmaceutical products benefit in the fourth quarter from the winter cold and flu season, and are lower in the first quarter of each year because Chinese New Year falls into the first quarter of each year and our customers generally pay fewer visits to drugstores during this period. In addition, sales of some health products are driven, to some extent, by seasonal purchasing patterns and seasonal product changes. Failure to manage the increased sales effectively in the high sale season, and increases in inventory in anticipation of sales increase could have a material adverse effect on our financial condition, results of operations and cash flow.
The commercial success of our products depends upon the degree of their market acceptance among the medical community. If our products do not attain market acceptance among the medical community, our operations and profitability would be adversely affected.
The commercial success of our products depends, in large part, upon the degree of market acceptance they achieve among the medical community, particularly among physicians, pharmacists, administrators of hospitals, clinics and other health care institutions. Physicians may not prescribe or recommend our products to patients and pharmacies, procurement departments of hospitals, clinics and other health care institutions may not purchase our products if physicians or pharmacists do not find our products attractive. The acceptance and use of our products among the medical community will depend upon a number of factors including:
|
|
·
|
perceptions by physicians, pharmacists, patients and others in the medical community about the safety and effectiveness of our products;
|
|
|
·
|
the prevalence and severity of any side effects;
|
|
|
·
|
pharmacological benefit of our products relative to competing products and products under development;
|
|
|
·
|
the efficacy and potential advantages relative to competing products and products under development;
|
|
|
·
|
relative convenience and ease of administration;
|
|
|
·
|
effectiveness of our education, marketing and distribution efforts;
|
|
|
·
|
publicity concerning our products or competing products and treatments; and
|
|
|
·
|
the price for our products and competing products.
|
If our products fail to attain market acceptance among the medical community, or if our currently marketed products cannot maintain market acceptance, our results of operations and profitability would be adversely affected.
Our success is dependent upon our ability to maintain our relationships with hospitals, clinics, pharmacies, drugstores and other health care institutions, to expand such relationships and develop new relationships.
Our business depends significantly on our relationships with hospitals, clinics, pharmacies, drugstores and other health care institutions. No assurance can be given that any such distribution channels will continue their relationships with us, and the loss of one or more of these distribution channel partners could have a material adverse effect on our business, results of operations and financial condition. Our ability to grow our business will therefore depend to a significant degree upon our ability to develop new relationships with such distribution channel partners and to expand existing relationships. No assurance can be given that new partners will be found, that any such new relationships will be successful when they are in place, or that business with current distribution channel partners will increase. Failure to develop and expand such relationships could have a material adverse effect on our business, results of operations and financial condition.
25
We may not be able to timely identify or otherwise effectively respond to changing customer preferences, and we may fail to optimize our product offering and inventory position.
The pharmaceutical industry in China is rapidly evolving and is subject to rapidly changing customer preferences that are difficult to predict. We believe that our success depends on our ability to anticipate and identify customer preferences and adapt our product selection to these preferences. In particular, we believe that we must optimize our product selection and inventory positions based on sales trends. No assurances can be given that our product selection, especially our selections of nutritional supplements and food products, will accurately reflect customer preferences at any given time. If we fail to anticipate accurately either the market for our products or customers’ purchasing habits or fail to respond to customers’ changing preferences promptly and effectively, we may not be able to adapt our product selection to customer preferences or make appropriate adjustments to our inventory positions, which could significantly reduce our revenue and have a material adverse effect on our business, financial condition and results of operations.
We face significant competition, and if we do not compete successfully against existing and new competitors, our revenue and profitability would be materially and adversely affected.
The pharmaceutical industry in China is highly competitive, and we expect competition to intensify. In addition there is a trend towards consolidation of the pharmaceutical industry in the future. Our primary competitors are other provincial pharmaceutical distributors. We compete for customers and revenue primarily on the basis of product selection, price, and timely delivery of products. Moreover, we may be subject to additional competition from new entrants to the drugstore industry in China. If the PRC government removes the barriers for foreign companies to operate majority-owned pharmaceutical distributors in China, we could face increased competition from foreign companies. Some of our larger competitors may enjoy competitive advantages, such as:
|
|
·
|
greater financial and other resources;
|
|
|
·
|
larger variety of products;
|
|
|
·
|
more extensive and advanced supply chain management systems;
|
|
|
·
|
greater pricing flexibility;
|
|
|
·
|
larger economies of scale and purchasing power;
|
|
|
·
|
more extensive advertising and marketing efforts;
|
|
|
·
|
greater knowledge of local market conditions;
|
|
|
·
|
larger sales and distribution networks.
|
As a result, we may be unable to offer products similar to, or more desirable than, those offered by our competitors, market our products as effectively as our competitors or otherwise respond successfully to competitive pressures. In addition, our competitors may be able to offer larger discounts on the same or competing products, and we may not be able to profitably match those discounts. Furthermore, our competitors may offer products that are more attractive to our customers or that render our products uncompetitive. Our failure to compete successfully could materially and adversely affect our business, financial condition, results of operation and prospects.
Our reliance on third-party manufacturers to supply our products may result in disruptions to our distribution network.
We rely on third-party manufactures to supply our products. Reliance on such third-party manufacturers involves a number of risks, including a lack of control over the manufacturing process and the potential absence or unavailability of adequate capacity. If any of our third-party manufacturers cannot or will not manufacture our products in required volumes in compliance with applicable regulations, on a cost-effective basis, in a timely manner, or at all, we will have to secure alternative manufacturers. Maintaining relationships with existing manufacturers and replacing such manufacturers may be difficult and time consuming. Any disruption of our network of manufacturers, including failure to renew existing distribution agreements with desired manufacturers, could negatively affect our product selection and our ability to effectively sell our products and could materially and adversely affect our business, financial condition and results of operations.
26
Failure to maintain optimal inventory levels could increase our inventory holding costs or cause us to lose sales, either of which could have a material adverse effect on our business, financial condition and results of operations.
We need to maintain sufficient inventory levels to operate our business successfully as well as meet our customers’ expectations. However, we must also prevent accumulating excess inventory. We are exposed to increased inventory risks due to our increased offering of private label products, rapid changes in product life cycles, changing consumer preferences, uncertainty of success of product launches, seasonality, and manufacturer backorders and other vendor-related problems. No assurances can be given that we can accurately predict these trends and events and avoid over-stocking or under-stocking products. In addition, demand for products could change significantly between the time product inventory is ordered and the time it is available for sale. When we begin selling a new product, it is particularly difficult to forecast product demand accurately. The purchase of certain types of inventory may require significant lead-time. As we carry a broad selection of products and maintain significant inventory levels for a substantial portion of our merchandise, we may be unable to sell such inventory in sufficient quantities or during the relevant selling seasons. Carrying too much inventory would increase our inventory holding costs, and failure to have inventory in stock when orders are received could cause us to lose such orders or customers, either of which could have a material adverse effect on our business, financial condition and results of operations.
The retail prices of some of our products are subject to control, including periodic downward adjustment, by PRC governmental authorities which may have an adverse effect on our profitability.
An increasing percentage of our products, primarily those included in the national and provincial Medical Insurance Catalogues, are subject to price controls in the form of fixed retail prices or retail price ceilings. In addition, the retail prices of these products are also subject to periodic downward adjustments as the PRC governmental authorities seek to make pharmaceutical products more affordable to the general public. Any future price controls or government mandated price reductions may have a material adverse affect on our financial condition and results of operations, including significantly reducing our revenue, margins and profitability.
Reimbursement may not be available for our products, which could diminish our sales or affect our ability to sell our products profitably.
Market acceptance and sales of our products also depend to a large extent on the reimbursement policies of the PRC government. The Ministry of Labor and Social Security of the PRC or provincial or local labor and social security authorities, together with other government authorities, review the inclusion or removal of drugs from the national Medical Insurance Catalog or provincial or local medical insurance catalogs for the National Medical Insurance Program every other year, and the tier under which a drug will be classified, both of which affect the amounts reimbursable to program participants for their purchases of those medicines. These determinations are made based on a number of factors, including price and efficacy. Depending on the tier under which a drug is classified in the provincial medicine catalog, a National Medical Insurance Program participant residing in that province can be reimbursed for the full cost of Tier 1 medicine and for 80% to 90% of the cost of a Tier 2 medicine. Decisions by the relevant government authorities not to include our products in the medicine catalogs may reduce the affordability of our products relative to other products included in the medicine catalogs and negatively affect the public perception regarding our products which in turn would adversely affect the sales of these products and reduce our net revenue.
Adverse publicity associated with our company or our products or similar products manufactured by our competitors could have a material adverse effect on our results of operations.
We are highly dependent upon market perceptions of the safety and quality of our products. Concerns over the safety of pharmaceutical products manufactured in China could have an adverse effect on the sale of such products, including products distributed by us. We could be adversely affected if any of our products or any similar products distributed by other companies prove to be, or are alleged to be, harmful to patients. Any negative publicity associated with severe adverse reactions or other adverse effects resulting from patients’ use or misuse of our products or any similar products manufactured by other companies could also have a material adverse impact on our results of operations. If in the future we become involved in incidents of the type described above, such problems could severely and adversely impact our product sales and reputation.
27
The continued penetration of counterfeit products into the retail market in China may damage our brand and reputation and have a material adverse effect on our business, financial condition, results of operations and prospects.
There has been continued penetration of counterfeit products into the pharmaceutical retail market in China. Counterfeit products are generally sold at lower prices than the authentic products due to their low production costs, and in some cases are very similar in appearance to the authentic products. Counterfeit pharmaceuticals may or may not have the same chemical content as their authentic counterparts, and are typically manufactured without proper licenses or approvals as well as fraudulently mislabeled with respect to their content and/or manufacturer. Although the PRC government has been increasingly active in combating counterfeit pharmaceutical and other products, there is not yet an effective counterfeit pharmaceutical product regulation control and enforcement system in China. Although we have implemented a series of quality control procedures in our procurement process, no assurances can be given that we would not be selling counterfeit pharmaceutical products inadvertently. Any unintentional sale of counterfeit products may subject us to negative publicities, fines and other administrative penalties or even result in litigation against us. Moreover, the continued proliferation of counterfeit products and other products in recent years may reinforce the negative image of retailers among consumers in China, and may severely harm the reputation and brand name of companies like us. The continued proliferation of counterfeit products in China could have a material adverse effect on our business, financial condition, results of operations and prospects.
Our geographic concentration in Yunnan Province presents certain risks that could adversely affect us.
We conduct our logistics and distribution business in Yunnan Province, China. Therefore, we are subject to risks specifically related to such region, including adverse economic effects on this region, natural disasters, local laws and regulations, including the discretion of provincial and regional price control authorities to authorize price adjustments for our products. Because of our geographic concentration, these risks could have a material adverse effect on our business and could result in significant disruptions to our business or increased operating expenses. In addition, we rely on our warehouse located in Kunming, City, Yunnan Province, to ship our products to our customers. If our warehouse was destroyed or shut down for any reason, we would incur significantly higher costs and delays associated with distribution of products during the time it takes us to reopen or replace our warehouse.
We rely on computer software and hardware systems in managing our operations, the capacity of which may restrict our growth and the failure of which could adversely affect our business, financial condition and results of operations.
We are dependent upon our integrated information management system to monitor daily operations of our drugstores and to maintain accurate and up-to-date operating and financial data for compilation of management information. In addition, we rely on our computer hardware and network for the storage, delivery and transmission of data. Any system failure which causes interruptions to the input, retrieval and transmission of data or increase in the service time could disrupt our normal operation. Although we believe that our disaster recovery plan is adequate in handling the failure of our computer software and hardware systems, no assurances can be given that we can effectively carry out this disaster recovery plan and that we will be able to restore our operation within a sufficiently short time frame to avoid our business being disrupted. Any failure in our computer software and/or hardware systems could have a material adverse effect on our business, financial condition and results of operations. In addition, if the capacity of our computer software and hardware systems fails to meet the increasing needs of our expanding operations, our ability to grow may be constrained.
28
Our dependence on the development and maintenance of the internet infrastructure could result in disruptions to our business.
We believe that our ability to fill orders over the internet provides us a competitive advantage over certain other provincial distributors as we can order directly from some manufacturers as well as bypass several layers in the traditional Chinese pharmaceutical distribution model that utilizes county and municipal distributors and provide our product line directly to hospitals, clinics, pharmacies, drug stores and other health care institutions. Therefore, we believe the success our business will depend largely on the development and maintenance of the internet infrastructure. This includes maintenance of a reliable network backbone with the necessary speed, data capacity, and security, as well as timely development of complementary products, for providing reliable internet access and services. XYT’s Kunming headquarters are supplied high speed internet capacity by China Telecom and China Netcom. Hosting of the XYT website is provided by Jian Wang Technology Co. Ltd at a cost of $90.00 per year. To protect against XYT’s website going down, the corporate servers utilize dual line server connections to both China Telecom and China Netcom. However, the internet has experienced, and is likely to continue to experience, significant growth in the numbers of users and amount of traffic. The internet infrastructure may be unable to support such demands. In addition, increasing numbers of users, increasing bandwidth requirements, or problems caused by “viruses,” “worms,” and similar programs may harm the performance of the internet. The backbone computers of the internet have been the targets of such programs. The internet has experienced a variety of outages and other delays as a result of damage to portions of its infrastructure, and it could face outages and delays in the future. These outages and delays could reduce the level of internet usage generally resulting in lower fulfillment of orders for our products over the internet, which may have a negative impact on our revenues.
Increasing government regulation of the internet could affect our business.
We are subject not only to regulations applicable to businesses generally but also to laws and regulations directly applicable to electronic commerce. The PRC government may adopt new laws and regulations applicable to electronic commerce. Currently, there are no specific internet regulations adopted by the PRC government pertaining to the pharmaceutical industry. However, in the future the PRC government may decide to regulate electronic commerce to ensure pharmaceutical drug orders do not end up in the possession of unlicensed persons. Any such legislation or regulation could dampen the growth of the internet and decrease its acceptance. If such a decline occurs, customers may decide in the future not to use the internet to fulfill their orders for our products. Any new laws or regulations in the following areas could affect our business:
|
|
·
|
user privacy;
|
|
|
·
|
the pricing and taxation of products offered over the internet;
|
|
|
·
|
the content of websites;
|
|
|
·
|
copyrights;
|
|
|
·
|
the online distribution of specific material or content over the internet; and
|
|
|
·
|
the characteristics and quality of services offered over the internet.
|
If we are unable to detect and prevent unauthorized use of confidential information, we could be subject to financial liability, our reputation could be harmed and customers may be reluctant to purchase products from us.
We rely to a large extent upon our information technology systems and infrastructure which are potentially vulnerable to breakdown, malicious intrusion and random attack. Likewise, data privacy breaches by employees and others with permitted access to our systems may pose a risk that sensitive data may be exposed to unauthorized persons or to the public. While we have taken measures for the protection of data and information technology, no assurances can be given that our efforts will prevent breakdowns or breaches in our systems that could adversely affect our business.
We expect to rely on encryption and authentication technology to provide secure transmission of confidential information over the internet, including confidential customer information. Advances in computer capabilities, new discoveries in the field of cryptography or other events or developments could result in a compromise or breach of the technologies used to protect sensitive transaction data. If any such compromise of our security, or the security of our customers, were to occur, it could result in misappropriation of proprietary information or interruptions in operations and have an adverse impact on our reputation and our business could suffer. Additionally, we might be required to expend significant capital and other resources to protect against security breaches or to rectify problems caused by any security breach.
29
Uninsured claims and litigation could adversely impact our operating results.
The distribution of drugs, including medicine products, chemical agents, antibiotics, biochemistry drugs and biological preparations entails an inherent risk of harm to the patient and, therefore, product liability. In extreme situations, pharmaceutical product manufacturers and distributors may be subject to criminal liability if their goods or services lead to the death or injuries of customers or other third parties. If a product liability claim is brought against us, it may, regardless of merit or eventual outcome, result in damage to our reputation, breach of contract with our customers, decreased demand for our products, costly litigation, product recalls, loss of revenue, and the inability to commercialize some products. Although we have general business insurance coverage, to the extent deemed prudent by our management and to the extent insurance is available, no assurances can be given that the nature and amount of the insurance coverage will be sufficient to fully indemnify us against liabilities arising out of pending and future claims and litigation. Our insurance coverage is subject to deductibles or self-insured retentions and contain certain coverage exclusions. The insurance does not cover damages from breach of contract by us, alleged fraud or deceptive trade practices. Insurance and customer agreements do not provide complete protection against losses and risks, and our results of operations could be adversely affected by unexpected claims not covered by insurance.
The decision of the Yunnan Food and Drug Administration to issue licenses to fill orders over the internet to our competitors would have an adverse effect on our business and diminish our competitive advantage over such competitors.
We have obtained government approval to fill orders over the internet in Yunnan Province, thereby bypassing municipal and county pharmaceutical distributors and provide products directly to retailers and in some cases, customers. We believe that our ability to fill orders over the internet provides us a competitive advantage over certain of our competitors. Bypassing the additional layers of distribution enables us to offer products to our customers at a significantly lower price than our major competitors while maintaining our margins. In 2010, the Yunnan Food and Drug Administration lifted a moratorium and began accepting new applications for these licenses. If the Yunnan Food and Drug Administration issues licenses to fill orders over the internet to our competitors, we would lose our competitive advantage resulting from our license to fill orders over the internet.
Our certificates, permits, and licenses related to our business are subject to governmental control and renewal and failure to obtain renewal will cause all or part of our operations to be terminated.
We are subject to various PRC laws and regulations pertaining to the pharmaceutical industry. We have attained certificates, permits, and licenses required for the operation of our business. Our distribution permit is valid for five years and renewal of such permit is subject to an inspection of our facilities, warehouse, hygienic environment, quality control systems, personnel and equipment. In addition, we possess an Internet Drug Information Service License in Yunnan Province issued by the Yunnan Food which enables us to provide internet drug transaction services. Such license is valid for five years and will expire in November 2014 and in order to renew the license, we must undergo reexamination. Additionally, as a distributor of nutritional supplements and other food products we must also have a food hygiene certificate from relevant provincial or local health regulatory authorities which is valid for four years. We have Good Supply Practice Standards certificates which currently have expiration dates of nearly five years. We intend to apply for renewal of our licenses, permits and certificates, but no assurances can be given that we will be successful in obtaining such renewals. In the event that trade protectionist policies are implemented by countries currently supplying Western drugs to China, such activities would adversely affect all pharmaceutical distribution companies in China, including XYT. In the event that we are not able to import Western drugs due to trade protection measures or to meet any new requirements imposed on our business by the appropriate regulatory authorities or are unable to renew our certificates, permits and licenses, all or part of our operations may be terminated. Furthermore, if escalating compliance costs associated with governmental standards and regulations restrict or prohibit any part of our operations, it may adversely affect our operation and profitability.
Our failure to retain and attract qualified personnel could harm our business.
We believe that our success depends in part on our ability to attract, train and retain qualified personnel, including sales personnel. Competition for qualified personnel is intense and we may not be able to hire sufficient personnel to achieve our goals or support the anticipated growth in our business. If we fail to attract and retain qualified personnel, our business will suffer.
30
If we are unable to protect our intellectual property from infringement, our business and prospects may be harmed.
As sales of our private label products increasingly account for a substantial portion of our revenue, we consider our brand name and trade names to be valuable assets. Although we currently have no trademarks under PRC law, we would have the exclusive right to use a trademark for products for which such trademark has been registered with the PRC Trademark Office of State Administration for Industry and Commerce (“SAIC”). In addition, no assurances can be given that we will be able to obtain any trademarks for which we may apply in the future.
Moreover, we may be unable to prevent third parties from using our brand name without authorization and we may not have adequate remedies for such violations. Unauthorized use of our brand name by third parties may adversely affect our business and reputation, including the perceived quality and reliability of our products.
We also rely on trade secrets to protect our know-how and other proprietary information, including pricing, purchasing, promotional strategies, customer lists and/or suppliers lists. However, trade secrets are difficult to protect. While we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors or advisors may unintentionally or willfully disclose our information to competitors. In addition, confidentiality agreements, if any, executed by the foregoing persons may not be enforceable or provide meaningful protection for our trade secrets or other proprietary information in the event of unauthorized use or disclosure. If we were to enforce a claim that a third party had illegally obtained and was using our trade secrets, our enforcement efforts could be expensive and time-consuming, and the outcome is unpredictable. In addition, if our competitors independently develop information that is equivalent to our trade secrets or other proprietary information, it will be even more difficult for us to enforce our rights and our business and prospects could be harmed.
Litigation may be necessary in the future to enforce our intellectual property rights or to determine the validity and scope of the intellectual property rights of others. However, because the validity, enforceability and scope of protection of intellectual property rights in the PRC are uncertain and still evolving, we may not be successful in prosecuting these cases. In addition, any litigation or proceeding or other efforts to protect our intellectual property rights could result in substantial costs and diversion of our resources and could seriously harm our business and operating results. Furthermore, the degree of future protection of our proprietary rights is uncertain and may not adequately protect our rights or permit us to gain or keep our competitive advantage. If we are unable to protect our trade names, trade secrets and other propriety information from infringement, our business, financial condition and results of operations may be materially and adversely affected.
We may be exposed to intellectual property infringement and other claims by third parties which, if successful, could disrupt our business and have a material adverse effect on our financial condition and results of operations.
Our success depends, in large part, on our ability to use our proprietary information and know-how without infringing third party intellectual property rights. As we increase our sales of private label products, and as litigation becomes more common in China, we face a higher risk of being the subject of claims for intellectual property infringement, invalidity or indemnification relating to other parties’ proprietary rights. Our current or potential competitors, many of which have substantial resources, may have or may obtain intellectual property protection that may prevent, limit or interfere with our ability to make, use or sell our products in China. Moreover, the defense of intellectual property suits, including trademark infringement suits, and related legal and administrative proceedings can be both costly and time consuming and may significantly divert the efforts and resources of our management personnel. Resolving intellectual property infringement claims may also require us to enter into license agreements. No assurances can be given that we would be able to obtain license agreements on commercially reasonable terms. A successful claim of intellectual property infringement could subject us to significant damages and may prevent us from manufacturing or selling the affected product. Any of these events could have a material adverse effect on our profitability and financial condition.
31
We depend substantially on the continuing efforts of our executive officers, and our business and prospects may be severely disrupted if we lose their services.
Our future success is dependent on the continued services of the key members of our management team, including Mr. Zhen Jiang Wang, our Chairman and Chief Executive Officer, Ms. Jing Gong, our President, Mr. Yong Kang Chen, our Senior Vice President, Quality Control and Ms. Yi Jia Li, our Chief Financial Officer. We do not maintain key man life insurance on any of our executive officers and directors. If one or more of our executive officers are unable or unwilling to continue in their present positions, we may not be able to replace them readily, if at all. Therefore, our business may be severely disrupted, and we may incur additional expenses to recruit and retain new management. The process of hiring suitably qualified personnel is also often lengthy. If our recruitment and retention efforts are unsuccessful in the future, it may be more difficult for us to execute our business strategy.
Changes in economic conditions and consumer confidence in China may influence the industry in which we operate, consumer preferences and spending patterns.
Our business and revenue growth primarily depend on the size of the retail market of pharmaceutical products in China. As a result, our revenue and profitability may be negatively affected by changes in national, regional or local economic conditions and consumer confidence in China. We are susceptible to changes in economic conditions, consumer confidence and customer preferences of the Chinese population. External factors beyond our control that affect consumer confidence include unemployment rates, levels of personal disposable income, national, regional or local economic conditions, natural disasters, extreme weather conditions, disease outbreaks and acts of war or terrorism. Changes in economic conditions and consumer confidence could adversely affect consumer preferences, purchasing power and spending patterns. In addition, natural disasters, extreme weather conditions, disease outbreaks and acts of war or terrorism may cause damage to our facilities, disrupt the supply of the products we offer or adversely impact consumer demand. Any of these factors could have a material adverse effect on our business, financial condition and results of operations.
We are subject to critical accounting policies and actual results may vary from our estimates.
We follow generally accepted accounting principles in the United States in preparing our financial statements. As part of the preparation of such financial reports, we must make many estimates and judgments concerning future events, which affect the value of the assets and liabilities, contingent assets and liabilities, and revenue and expenses reported in our financial statements. We believe that these estimates and judgments are reasonable, and we make them in accordance with our accounting policies based on information available at the time. However, actual results could differ from our estimates, and this could require us to record adjustments to expenses or revenues that could be material to our financial position and results of operations in the future.
We may not be able to manage our expansion of operations effectively and failure to do so could strain our management, operational and other resources, which could materially and adversely affect our business and growth potential.
We have grown rapidly since our inception and we anticipate continued expansion of our business to address growth in demand for our products, as well as to capture new market opportunities. The continued growth of our business has resulted in, and will continue to result in, substantial demands on our management, operational and other resources. In particular, we believe that the management of our growth will require, among other things:
|
|
·
|
our ability to expand our market reach in China beyond the Yunnan Province;
|
|
|
·
|
our ability to continue to identify new customers;
|
|
|
·
|
our ability to optimize product offerings and increase sales of private label products;
|
|
|
·
|
our ability to control procurement cost and optimize product pricing;
|
|
|
·
|
our ability to control operating expenses;
|
|
|
·
|
our ability to improve reporting systems and procedures;
|
|
|
·
|
information technology system enhancement;
|
|
|
·
|
strengthening of financial and management controls;
|
|
|
·
|
increased marketing, sales and sales support activities; and
|
32
|
|
·
|
hiring, training and managing of new personnel, including sales personnel.
|
If we are not able to manage our growth successfully, our business and prospects would be materially and adversely affected.
We may acquire other businesses, license rights to products or form alliances with third-parties, which could cause us to incur significant expenses and could negatively affect our profitability.
We may pursue acquisitions, licensing arrangements, and strategic alliances, as part of our business strategy. We may not complete these transactions in a timely manner, on a cost-effective basis, or at all, and may not realize the expected benefits. If we are successful in making an acquisition, the products that are acquired may not be successful or may require significantly greater resources and investments than originally anticipated. We may not be able to integrate acquisitions successfully into our existing business and could incur or assume significant debt and unknown or contingent liabilities. This may result in increased borrowing costs and interest expense.
We may need additional capital and may not be able to obtain it on acceptable terms or at all, which could adversely affect our liquidity and financial position; the issuance of additional equity would result in dilution to our shareholders.
We may need to raise additional capital if our expenditures exceed our current expectations due to changed business conditions or other future developments. Our future liquidity needs and other business reasons may require us to sell additional equity or debt securities or obtain a credit facility. The sale of additional equity securities or securities convertible or exchangeable to our equity securities would result in additional dilution to our stockholders. The incurrence of additional indebtedness would result in increased debt service obligations and could result in operating and financing covenants that restrict our operational flexibility. Our ability to raise additional funds in the future is subject to a variety of uncertainties, including:
|
|
·
|
our future financial condition, results of operations and cash flows;
|
|
|
·
|
general market conditions for capital-raising activities by pharmaceutical companies; and
|
|
|
·
|
economic, political and other conditions in China and elsewhere.
|
No assurances can be given that we will be able to obtain additional capital in a timely manner or on commercially acceptable terms or at all.
Future acquisitions are expected to be a part of our growth strategy, and could expose us to significant business risks.
One of our strategies is to grow our business through acquisition of other pharmaceutical distributor companies. However, no assurances can be given that we will be able to identify and secure suitable acquisition opportunities. Our ability to consummate and integrate effectively any future acquisitions on terms that are favorable to us may be limited by the number of attractive acquisition targets, internal demands on our resources and, to the extent necessary, our ability to obtain financing on satisfactory terms for larger acquisitions, if at all.
Moreover, if an acquisition candidate is identified, the third parties with whom we seek to cooperate may not select us as a potential partner or we may not be able to enter into arrangements on commercially reasonable terms or at all. The negotiation and completion of potential acquisitions, whether or not ultimately consummated, could also require significant diversion of management’s time and resources and potential disruption of our existing business. Furthermore, no assurances can be given that the expected synergies from future acquisitions will actually materialize. In addition, future acquisitions could result in the incurrence of additional indebtedness, costs, and contingent liabilities. Future acquisitions may also expose us to potential risks, including risks associated with:
|
|
·
|
the integration of new operations, services and personnel;
|
|
|
·
|
unforeseen or hidden liabilities;
|
|
|
·
|
the diversion of financial or other resources from our existing businesses;
|
|
|
·
|
our inability to generate sufficient revenue to recover costs and expenses of the acquisitions; and
|
33
|
|
·
|
the potential loss of, or harm to, relationships with employees or customers.
|
Any of the above could significantly disrupt our ability to manage our business and materially and adversely affect our business, financial condition and results of operations.
Risks Related to Our Corporate Structure
Transactions among our affiliates are subject to scrutiny by the PRC tax authorities and a finding that we or any of our consolidated entities owe additional taxes could have a material adverse impact on our net income and the value of an investment in our common stock.
Under PRC law, arrangements and transactions among related parties may be subject to audit or challenge by the PRC tax authorities. If any of the transactions we have entered into among our consolidated entities are challenged by the PRC tax authorities to be not on an arm’s-length basis, or to result in an unreasonable reduction in our PRC tax obligations, the PRC tax authorities have the authority to disallow our tax deduction claims, adjust the profits and losses of our respective PRC consolidated entities and assess late payment fees and other penalties. Our net income may be materially reduced if our tax liabilities increase or if we are otherwise assessed late payment fees or other penalties.
The approval of the China Securities Regulatory Commission, or the CSRC, may be required in connection with the Exchange Transaction, which set up our WFOE structure, under PRC regulations.
On August 8, 2006, six PRC regulatory agencies: the PRC Ministry of Commerce, or MOFCOM, the State Assets Supervision and Administration Commission, or SASAC, the State Administration for Taxation, the State Administration for Industry and Commerce, the China Securities Regulatory Commission, or CSRC, and the SAFE, jointly adopted the Regulations on Mergers and Acquisitions of Domestic Enterprises by Foreign Investors, or M&A Rule, which became effective on September 8, 2006. The M&A Rule purports, among other things, to require offshore special purpose vehicles, or SPVs, formed for overseas listing purposes through acquisitions of PRC domestic companies and controlled by PRC companies or individuals, to obtain the approval of the CSRC prior to publicly listing their securities on an overseas stock exchange.
There are substantial uncertainties regarding the interpretation and application of current or future PRC laws and regulations, including the M&A Rule. Although we do not believe that our Exchange Transaction, where we indirectly acquired XYT and converted it to a WFOE, required prior CSRC approval, the interpretation and application of the M&A Rule remains unclear. Accordingly, we cannot assure you that the Exchange Transaction, wherein we indirectly acquired the WFOE, did not require the prior approval of the CSRC. If CSRC approval was required for us to consummate the indirectly acquisition of the WFOE, our failure to obtain or delay in obtaining the CSRC approval would subject us to sanctions imposed by the CSRC and other PRC regulatory agencies. These sanctions could include fines and penalties on our operations in China, restriction or limitation on our ability to pay dividends outside of China, and other forms of sanctions that may cause a material and adverse effect on our business, results of operations and financial conditions. However, the M&A Rule does not stipulate the specific penalties, so we are not able to predict what penalties we may face, and how such penalties will affect our business operations or future strategy.
Risks Related to Doing Business in China
Adverse changes in political and economic policies of the PRC government could have a material adverse effect on the overall economic growth of China, which could reduce the demand for our products and materially and adversely affect our competitive position.
All of our business operations are conducted in China and all of our sales are made in China. Accordingly, our business, financial condition, results of operations and prospects are affected significantly by economic, political and legal developments in China. The Chinese economy differs from the economies of most developed countries in many respects, including:
34
|
|
·
|
the degree of government involvement;
|
|
|
·
|
the level of development;
|
|
|
·
|
the growth rate;
|
|
|
·
|
the control of foreign exchange;
|
|
|
·
|
access to financing; and
|
|
|
·
|
the allocation of resources.
|
While the Chinese economy has grown significantly in the past 25 years, the growth has been uneven, both geographically and among various sectors of the economy. The PRC government has implemented various measures to encourage economic growth and guide the allocation of resources. Some of these measures benefit the overall Chinese economy, but may also have a negative effect on us. The Chinese government may not continue to pursue these policies or may significantly alter them to our detriment from time to time with little, if any, prior notice. Changes in policies, laws and regulations or in their interpretation or the imposition of confiscatory taxation, restrictions on currency conversion, restrictions or prohibitions on dividend payments to stockholders, governmental control over capital investments or changes in tax regulations applicable to us, devaluations of currency or the nationalization or other expropriation of private enterprises could have a material adverse effect on our business.
The Chinese economy has been transitioning from a planned economy to a more market-oriented economy. Although the PRC government has in recent years implemented measures emphasizing the utilization of market forces for economic reform, the reduction of state ownership of productive assets and the establishment of sound corporate governance in business enterprises, a substantial portion of the productive assets in China is still owned by the PRC government. The continued control of these assets and other aspects of the national economy by the PRC government could materially and adversely affect our business. The PRC government also exercises significant control over China’s economic growth through the allocation of resources, controlling payment of foreign currency-denominated obligations, setting monetary policy and providing preferential treatment to particular industries or companies. Since late 2003, the PRC government implemented a number of measures, such as raising bank reserves against deposit rates to place additional limitations on the ability of commercial banks to make loans and raise interest rates, in order to decrease the growth rate of specific segments of China’s economy which it believed to be overheating. These actions, as well as future actions and policies of the PRC government, could materially affect our liquidity and access to capital and our ability to operate our business. Nationalization or expropriation could even result in the total loss of our investment in China and in the total loss of our stockholders’ investment.
New labor laws in the PRC may adversely affect our results of operations.
On January 1, 2008, the PRC government promulgated the Labor Contract Law of the PRC, or the New Labor Contract Law. The New Labor Contract Law imposes greater liabilities on employers and significantly impacts the cost of an employer’s decision to reduce its workforce. Further, it requires certain terminations to be based upon seniority and not merit. In the event we decide to significantly change or decrease our workforce, the New Labor Contract Law could adversely affect our ability to enact such changes in a manner that is most advantageous to our business or in a timely and cost effective manner, thus materially and adversely affecting our financial condition and results of operations.
If political relations between the United States and China worsen, our stock price may decrease and we may have difficulty accessing U.S. capital markets.
At various times during recent years, the United States and China have had significant disagreements over political and economic issues. Controversies may arise in the future between these two countries. Any political or trade controversies between the United States and China, whether or not directly related to our business, could adversely affect the market price of our common stock and our ability to access U.S. capital markets.
35
Uncertainties with respect to the PRC legal system could limit the protections available to you and us.
The PRC legal system is a civil law system based on written statutes. Unlike the common law system in the United States, prior court decisions may be cited for reference but have limited precedential value. Since 1979, PRC legislation and regulations have significantly enhanced the protections afforded to various forms of foreign investments in China. We conduct all of our business through our subsidiary established in China. Thus we are generally subject to laws and regulations applicable to foreign investment in China and, in particular, laws applicable to wholly foreign-owned enterprises. However, since many laws, rules and regulations are relatively new and the PRC legal system continues to rapidly evolve, the interpretations of many laws, regulations and rules are not always uniform and enforcement of these laws, regulations and rules involve uncertainties, which may limit legal protections available to us. For example, we may have to resort to administrative and court proceedings to enforce the legal protection that we enjoy either by law or contract. However, since PRC administrative and court authorities have significant discretion in interpreting and implementing statutory and contractual terms, it may be more difficult to evaluate the outcome of Chinese administrative and court proceedings and the level of legal protection we enjoy in China than in more developed legal systems. These uncertainties may impede our ability to enforce the contracts we have entered into with our business partners, customers and suppliers. In addition, such uncertainties, including the inability to enforce our contracts, could materially and adversely affect our business and operations. Furthermore, intellectual property rights and confidentiality protections in China may not be as effective as in the United States or other countries. Accordingly, we cannot predict the effect of future developments in the PRC legal system, particularly with regard to the Chinese pharmaceutical industry and retail industry, including the promulgation of new laws, changes to existing laws or the interpretation or enforcement thereof, or the preemption of local regulations by national laws. These uncertainties could limit the legal protections available to us and our investors. In addition, any litigation in China may be protracted and result in substantial costs and diversion of our resources and management attention.
The fluctuation of foreign currency exchange rates could materially impact our financial results.
Since we conduct our operations in China, our business is subject to foreign currency risks, including currency exchange rates fluctuations and difficulties in converting Renminbis into U.S. dollars. The exchange rates between the Renminbi and the U.S. dollar, Euro and other foreign currencies is affected by, among other things, changes in China’s political and economic conditions. On July 21, 2005, the PRC government changed its decade-old policy of pegging the value of the Renminbi to the U.S. dollar. Under the new policy, the Renminbi is permitted to fluctuate within a narrow and managed band against a basket of foreign currencies. There remains significant international pressure on the PRC government to adopt a more flexible currency policy, which could result in a further and more significant appreciation of the Renminbi against the U.S. dollar. In addition, appreciation or depreciation in the value of the Renminbi relative to the U.S. dollar would affect our financial results reported in U.S. dollar terms without giving effect to any underlying change in our business, financial condition and results of operations.
Because our assets are located outside of the United States and most of our directors and officers reside outside of the United States, it may be difficult for investors to enforce their rights based on United States federal securities laws or any United States court judgments against us and our officers and directors.
Our operating company and all of our assets are located in the PRC. In addition, most of our directors and officers reside outside of the United States. It may therefore be difficult for investors in the United States to enforce their legal rights based on the civil liability provisions of the United States federal securities laws against us in the courts of either the United States or PRC and, even if civil judgments are obtained in United States courts, to enforce such judgments in PRC courts. Further, it is unclear if extradition treaties now in effect between the United States and the PRC would permit effective enforcement against us or our officers and directors of criminal penalties, under the United States federal securities laws or other United States laws.
Restrictions under PRC law on our PRC operating subsidiary’s ability to make dividends and other distributions could materially and adversely affect our ability to grow, make investments or complete acquisitions that could benefit our business, pay dividends to, and otherwise fund and conduct our businesses.
Substantially all of our revenues are earned by our PRC operating subsidiary, XYT. However, PRC regulations restrict the ability of our PRC subsidiary to make dividends and other payments to its offshore parent company. PRC legal restrictions permit payments of dividend by our PRC subsidiary only out of its accumulated after-tax profits, if any, determined in accordance with PRC accounting standards and regulations. Our PRC subsidiary is also required under PRC laws and regulations to allocate at least 10% of our annual after-tax profits determined in accordance with PRC GAAP to a statutory general reserve fund until the amounts in said fund reaches 50% of our registered capital. Allocations to these statutory reserve funds can only be used for specific purposes and are not transferable to us in the form of loans, advances or cash dividends. Any limitations on the ability of our PRC subsidiary to transfer funds to us could materially and adversely limit our ability to grow, make investments or acquisitions that could be beneficial to our business, pay dividends and otherwise fund and conduct our business.
36
Restrictions on currency exchange may limit our ability to receive and use our sales revenue effectively.
All of XYT’s sales revenue and expenses are denominated in RMB. Under PRC law, the RMB is currently convertible under the “current account,” which includes dividends and trade and service-related foreign exchange transactions, but not under the “capital account,” which includes foreign direct investment and loans. Currently, our PRC operating subsidiary may purchase foreign currencies for settlement of current account transactions, including payments of dividends to the Company, without the approval of the State Administration of Foreign Exchange, or SAFE, by complying with certain procedural requirements. However, the relevant PRC government authorities may limit or eliminate our ability to purchase foreign currencies in the future. Since a significant amount of our future revenue will be denominated in RMB, any existing and future restrictions on currency exchange may limit our ability to utilize revenue generated in RMB to fund our business activities outside China that are denominated in foreign currencies.
Foreign exchange transactions by our PRC operating subsidiary under the capital account continue to be subject to significant foreign exchange controls and require the approval of or need to register with PRC government authorities, including SAFE. In particular, if our PRC operating subsidiary borrows foreign currency through loans from us or other foreign lenders, these loans must be registered with SAFE, and if we finance the subsidiary by means of additional capital contributions, these capital contributions must be approved by certain government authorities, including the Ministry of Commerce, or MOFCOM, or their respective local counterparts. These limitations could affect their ability to obtain foreign exchange through debt or equity financing.
There are significant uncertainties under the EIT relating to the withholding tax liabilities of XYT, and dividends payable by XYT to FCPG HK may not qualify to enjoy the treaty benefits.
Under the EIT and its implementing rules, the profits of a foreign invested enterprise which are distributed to its immediate holding company outside the PRC will be subject to a withholding tax rate of 10%. Pursuant to a tax arrangement between Hong Kong and the PRC, such rate may be lowered to 5% if a Hong Kong resident enterprise owns over 25% of a PRC company. XYT is currently wholly-owned by FCPG HK. However, the 5% withholding tax rate does not automatically apply and approvals from competent local tax authorities are required before an enterprise can enjoy any benefits under the relevant taxation treaties. Moreover, according to the Notice of the State Administration of Taxation on Issues regarding the Administration of the Dividend Provision in Tax Treaties promulgated on February 20, 2009, for a tax treaty to be applicable, certain requirements must be satisfied, including: (1) the taxpayer must be the beneficial owner of the relevant dividends; (2) for corporate recipients to enjoy the favorable tax treatment under the tax treaty as direct owners of a PRC enterprise, such corporate recipients must satisfy the direct ownership thresholds at all times during the 12 consecutive months preceding the receipt of the dividends. On August 24, 2009, the State Administration of Taxation issued the Administrative Measures for Non-resident Enterprises to Enjoy Treatments under Tax Treaties (For Trial Implementation), which became effective on October 1, 2009, requiring non-resident enterprises to obtain an approval from the competent tax authority in order to enjoy the treatments under tax treaties. Further, the State Administration of Taxation promulgated the Notice on How to Understand and Recognize the “Beneficial Owner” in Tax Treaties on October 27, 2009, which limits the “beneficial owner” to individuals, enterprises or other organizations normally engaged in substantive operations, and set forth certain adverse factors on the recognition of such “Beneficial Owner.” XYT has not yet applied for such approvals because it has not declared or paid dividends, and does not intend to declare or pay dividends. XYT will apply for such approvals when it intends to declare and pay dividends. There is no assurance that the PRC tax authorities will approve the 5% withholding tax rate on dividends received by FCPG HK from XYT.
37
We are subject to uncertainty related to the tax systems in China and any uncertainty in taxation could negatively affect our business, results of operations and financial condition.
China currently has a number of laws related to various taxes imposed by both national and regional governmental authorities. Applicable taxes include value-added tax (VAT), corporate income tax (profits tax), and payroll (social) taxes, together with others. In contrast to more developed market economies, laws related to these taxes have not been in force for a significant period, and interpretive regulations are often unclear or nonexistent. Often, there are differing opinions regarding legal interpretation, both among and within government ministries and organizations, resulting in uncertainties and areas of conflict. Matters of taxation, customs and currency control, as well as other areas, are subject to review and investigation by a number of governmental authorities, who are enabled by law to impose extremely severe fines and penalties. Any regulatory uncertainty in taxation or other areas could negatively affect us through increased operating costs, which could have a material adverse effect on our business, results of operations and financial condition.
In particular with respect to VAT, in China, receipts for payment are different from VAT invoices as VAT invoices are issued only when customers require the VAT invoice after payment is made. As such, if a customer does not require a VAT invoice, no VAT invoice would be issued. Input VAT invoices are used to net off the output tax but not allowed to be used alone to reclaim money. The input VAT invoices are only valid for three months from the issue date. Therefore, if output VAT tax is less than input VAT tax, then the surplus input VAT invoices would be wasted. Therefore, businesses are very careful when they require VAT invoices. In this case, as most customers of XYT did not require VAT invoices, XYT postponed the requirement for input VAT invoices from suppliers to such time that customers require output VAT invoices. Therefore, the Company was not obligated to remit such VAT amount to the government taxing authority. Even though the Company has not yet received any notice about any additional VAT taxes owed from the tax office, for the year ended March 31, 2011, XYT had accrued a VAT tax payable balance of US$11,238,596, and if asked to pay such balance immediately by the government taxing authority, it could have adverse effects on our business and financial condition, and we may be subject to potential penalties and fines for not making payments earlier.
Risks Relating to our Securities and our Status as a Public Company
The relative lack of public company experience of our management team may put us at a competitive disadvantage.
Our management team lacks public company experience and is generally unfamiliar with the requirements of the United States securities laws and U.S. Generally Accepted Accounting Principles, which could impair our ability to comply with legal and regulatory requirements such as those imposed by Sarbanes-Oxley Act of 2002. The individuals who now constitute our senior management team have never had responsibility for managing a publicly traded company. Such responsibilities include complying with federal securities laws and making required disclosures on a timely basis. Specifically, we lack personnel with formal training to properly analyze and record complex transactions in accordance with U.S. GAAP. Our current Chief Financial Officer, Yi Jia Li, has over 10 years of accounting and financial management experience and graduated from Yun Nan University in 1999, majoring in Accounting and Auditing. Ms. Li is a certified Accounting Professional in the PRC, receiving this designation in 2002; however, she does not have a Certified Public Accountant (U.S.) or Certified Management Accountant designation. We will seek to add individual(s) to our Board of Directors and to management positions with additional U.S. GAAP expertise and will continue to provide the current members of the Board of Directors and senior management with educational opportunities to further study U.S. GAAP. To that end, subsequent to our fiscal year ended March 31, 2011, on May 6, 2011, we appointed Mr. Jack Zwick as a member of the Board of Directors. Mr. Zwick was subsequently appointed to our Audit Committee in June 2011. Mr. Zwick is a certified public accountant and founding member of Zwick Maddox & Banyai PLLC, certified public accountants, in Michigan. Mr. Zwick holds a Bachelor of Arts degree in Accountancy and a Masters of Science in Taxation from Wayne State University. He is a member of the American Institute of Certified Public Accountants and the Michigan Association of Certified Public Accountants, and previously taught accounting and audit related classes for the Michigan Association of CPAs and Ohio Society of CPAs. As such, Mr. Zwick is very knowledgeable on U.S. GAAP.
Notwithstanding these efforts, our senior management may not be able to implement programs and policies in an effective and timely manner that adequately responds to increased legal, regulatory compliance and reporting requirements. Our failure to comply with all applicable requirements could lead to the imposition of fines and penalties and distract our management from attending to the growth of our business.
38
We will continue to incur significant costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance requirements.
As a public company we incur significant legal, accounting and other expenses under the Sarbanes-Oxley Act of 2002, together with rules implemented by the Securities and Exchange Commission and applicable market regulators. These rules impose various requirements on public companies, including requiring certain corporate governance practices. Our management and other personnel will need to devote a substantial amount of time to these new compliance requirements. Moreover, these rules and regulations will increase our legal and financial compliance costs and will make some activities more time-consuming and costly.
In addition, the Sarbanes-Oxley Act requires, among other things, that we maintain effective internal controls for financial reporting and disclosure controls and procedures. In particular, we must perform system and process evaluations and testing of our internal controls over financial reporting to allow management and our independent registered public accounting firm to report on the effectiveness of our internal controls over financial reporting, as required by Section 404 of the Sarbanes-Oxley Act. Our testing, or the subsequent testing by our independent registered public accounting firm, may reveal deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses. Compliance with Section 404 may require that we incur substantial accounting expenses and expend significant management efforts. If we are not able to comply with the requirements of Section 404 in a timely manner, or if our accountants later identify deficiencies in our internal controls over financial reporting that are deemed to be material weaknesses, the market price of our stock could decline and we could be subject to sanctions or investigations by the SEC or other applicable regulatory authorities.
A limited public trading market exists for our common stock, which makes it more difficult for our stockholders to sell their common stock in the public markets.
Our common stock is currently traded under the symbol “FCPG.OB,” but currently with low or no volume, based on quotations on the “Over-the-Counter Bulletin Board,” meaning that the number of persons interested in purchasing our common stock at or near bid prices at any given time may be relatively small or non-existent. This situation is attributable to a number of factors, including the fact that we are a small company which is still relatively unknown to stock analysts, stock brokers, institutional investors, and others in the investment community that generate or influence sales volume, and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company such as ours or purchase or recommend the purchase of our stock until such time as we became more viable. Additionally, many brokerage firms may not be willing to effect transactions in the securities. As a consequence, there may be periods of several days or more when trading activity in our stock is minimal or non-existent, as compared to a seasoned issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse effect on share price. We cannot give you any assurance that a broader or more active public trading market for our common stock will develop or be sustained, or that trading levels will be sustained.
Shareholders should be aware that, according to SEC Release No. 34-29093, the market for “penny stocks” has suffered in recent years from patterns of fraud and abuse. Such patterns include (1) control of the market for the security by one or a few broker-dealers that are often related to the promoter or issuer; (2) manipulation of prices through prearranged matching of purchases and sales and false and misleading press releases; (3) boiler room practices involving high-pressure sales tactics and unrealistic price projections by inexperienced sales persons; (4) excessive and undisclosed bid-ask differential and markups by selling broker-dealers; and (5) the wholesale dumping of the same securities by promoters and broker-dealers after prices have been manipulated to a desired level, along with the resulting inevitable collapse of those prices and with consequent investor losses. Our management is aware of the abuses that have occurred historically in the penny stock market. Although we do not expect to be in a position to dictate the behavior of the market or of broker-dealers who participate in the market, management will strive within the confines of practical limitations to prevent the described patterns from being established with respect to our securities. The occurrence of these patterns or practices could increase the future volatility of our share price.
39
We do not anticipate paying any dividends in the foreseeable future. If and when we decide to pay dividends, any dividends or proceeds from liquidation will be subject to the approval of the relevant Chinese government agencies.
We currently intend to retain any future earnings for funding growth. We do not anticipate paying any dividends in the foreseeable future. As a result, stockholders should not rely on an investment in our securities if they require dividend income. Our assets are located inside China. Under the laws governing foreign-invested enterprises in China, dividend distribution and liquidation are allowed but subject to special procedures under the relevant laws and rules. If and when made, any dividend payment will be subject to the decision of the board of directors of our Chinese operating company, XYT, and subject to foreign exchange rules governing such repatriation. Any liquidation is subject to both the relevant government agency’s approval and supervision as well the foreign exchange control. This may generate additional risk for our investors in case of dividend payment and liquidation.
Our stock is categorized as a penny stock. Trading of our stock may be restricted by the SEC’s penny stock regulations which may limit a shareholder’s ability to buy and sell our stock.
Our stock is categorized as a penny stock. The SEC has adopted Rule 15g-9 which generally defines “penny stock” to be any equity security that has a market price (as defined) less than US$ 5.00 per share or an exercise price of less than US$ 5.00 per share, subject to certain exceptions. Our securities are covered by the penny stock rules, which impose additional sales practice requirements on broker-dealers who sell to persons other than established customers and accredited investors. The penny stock rules require a broker-dealer, prior to a transaction in a penny stock not otherwise exempt from the rules, to deliver a standardized risk disclosure document in a form prepared by the SEC which provides information about penny stocks and the nature and level of risks in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock, the compensation of the broker-dealer and its salesperson in the transaction and monthly account statements showing the market value of each penny stock held in the customer’s account. The bid and offer quotations, and the broker-dealer and salesperson compensation information, must be given to the customer orally or in writing prior to effecting the transaction and must be given to the customer in writing before or with the customer’s confirmation. In addition, the penny stock rules require that prior to a transaction in a penny stock not otherwise exempt from these rules, the broker-dealer must make a special written determination that the penny stock is a suitable investment for the purchaser and receive the purchaser’s written agreement to the transaction. These disclosure requirements may have the effect of reducing the level of trading activity in the secondary market for the stock that is subject to these penny stock rules. Consequently, these penny stock rules may affect the ability of broker-dealers to trade our securities. We believe that the penny stock rules discourage investor interest in and limit the marketability of our common stock.
FINRA sales practice requirements may also limit a stockholder’s ability to buy and sell our stock.
In addition to the “penny stock” rules described above, the Financial Industry Regulatory Authority (“FINRA”) has adopted rules that require that in recommending an investment to a customer, a broker-dealer must have reasonable grounds for believing that the investment is suitable for that customer. Prior to recommending speculative low priced securities to their non-institutional customers, broker-dealers must make reasonable efforts to obtain information about the customer’s financial status, tax status, investment objectives and other information. Under interpretations of these rules, FINRA believes that there is a high probability that speculative low priced securities will not be suitable for at least some customers. The FINRA requirements make it more difficult for broker-dealers to recommend that their customers buy our common stock, which may limit your ability to buy and sell our stock and have an adverse effect on the market for our shares.
A large number of shares may be eligible for future sale and may depress our stock price.
We may be required, under terms of future financing arrangements, to offer a large number of common shares to the public, or to register for sale by future private investors a large number of shares sold in private sales to them. Sales of substantial amounts of common stock, or a perception that such sales could occur, and the existence of options or warrants to purchase shares of common stock at prices that may be below the then-current market price of our common stock, could adversely affect the market price of our common stock and could impair our ability to raise capital through the sale of our equity securities, either of which would decrease the value of any earlier investment in our common stock.
40
Shares of our common stock that have not been registered under the Securities Act of 1933, as amended, regardless of whether such shares are restricted or unrestricted, are subject to resale restrictions imposed by Rule 144, including those set forth in Rule 144(i) which apply to a “shell company.” In addition, any shares of our common stock that are held by affiliates, including any received in a registered offering, will be subject to the resale restrictions of Rule 144(i).
Pursuant to Rule 144 of the Securities Act of 1933, as amended (“Rule 144”), a “shell company” is defined as a company that has no or nominal operations; and, either no or nominal assets; assets consisting solely of cash and cash equivalents; or assets consisting of any amount of cash and cash equivalents and nominal other assets. As such, we were a “shell company” pursuant to Rule 144 prior to the consummation of the Exchange Transaction, and as such, sales of our securities pursuant to Rule 144 are not able to be made until a period of at least twelve months has elapsed from the date on which the Company’s Current Report on Form 8-K has been filed with the Commission reflecting the Company’s status as a non- “shell company.” Therefore, any restricted securities we sell in the future or issue to consultants or employees, in consideration for services rendered or for any other purpose will have no liquidity until and unless such securities are registered with the Commission and/or until a year after the date of the filing of the Company’s Current Report on Form 8-K and we have otherwise complied with the other requirements of Rule 144. As a result, it may be harder for us to fund our operations and pay our consultants with our securities instead of cash. Furthermore, it will be harder for us to raise funding through the sale of debt or equity securities unless we agree to register such securities with the Commission, which could cause us to expend additional resources in the future. Our previous status as a “shell company” could prevent us from raising additional funds, engaging consultants, and using our securities to pay for any acquisitions (although none are currently planned), which could cause the value of our securities, if any, to decline in value or become worthless. Lastly, any shares held by affiliates, including shares received in any registered offering, will be subject to the resale restrictions of Rule 144(i).
ITEM 1B. UNRESOLVED STAFF COMMENTS.
As a smaller reporting company, as defined in Rule 12b-2 of the Securities Exchange Act of 1934, we are not required to provide the information required by this item.
ITEM 2. PROPERTIES.
FCPG HK’s and XYT’s headquarters are located at Number 504, West Ren Min Road, Kunming City, Yunnan Province, People’s Republic of China, 650000. In addition to the head office located at this address, XYT’s 3,000 square meter warehouse is also located here. This property is leased for a ten year term, commencing April 1, 2005 and ending March 31, 2015, with a total rental and property management fee of RMB580,000 (approximately US$85,000).
The Company also maintains an executive office at Room 1301, 13th Floor, CRE Building, 303 Hennessy Road Wanchai, Hong Kong.
ITEM 3. LEGAL PROCEEDINGS.
There are no material pending legal proceedings to which we are a party or to which any of our property is subject, nor are there any such proceedings known to be contemplated by governmental authorities. None of our directors, officers or affiliates is involved in a proceeding adverse to our business or has a material interest adverse to our business.
ITEM 4. REMOVED AND RESERVED.
41
PART II
|
ITEM 5.
|
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES.
|
Market for Registrant’s Common Equity
Our common stock is currently listed for trading on the Over-the-Counter Bulletin Board maintained by the Financial Industry Regulatory Authority (“FINRA”) under the Symbol: “FCPG.” The table below lists the high and low closing prices per share of our common stock since our stock was first traded on May 27, 2010, as quoted on the Over-the-Counter Bulletin Board. Prior to that time, there was no public market for our common stock.
|
Fiscal Year Ending March 31, 2011
|
High
|
Low
|
||||||
|
First Quarter - June 30, 2010
|
$ | 1.00 | $ | 1.00 | ||||
|
Second Quarter - September 30, 2010
|
$ | 0.80 | $ | 0.80 | ||||
|
Third Quarter - December 31, 2010
|
$ | 1.98 | $ | 1.72 | ||||
|
Fourth Quarter - March 31, 2011
|
$ | 1.29 | $ | 1.18 | ||||
Trading in our common stock has been sporadic and the quotations set forth above are not necessarily indicative of actual market conditions. All prices reflect inter-dealer prices without retail mark-up, mark-down, or commission and may not necessarily reflect actual transactions.
Approximate Number of Holders of Common Stock
As of June 27, 2011, there were 62 shareholders of record of our common stock. Such number does not include any shareholders holding shares in nominee or “street name.”
Dividends
We have not declared any cash dividends in the two most recent fiscal years. The declaration of future cash dividends, if any, will be at the discretion of the Board of Directors and will depend on our earnings, if any, capital requirements and financial position, general economic conditions and other pertinent conditions. It is our present intention not to pay any cash dividends in the near future.
Securities Authorized for Issuance Under Equity Compensation Plans
There are no options, warrants or convertible securities outstanding pursuant to an equity compensation plan.
Recent Sales of Unregistered Securities
The information set forth below describes our issuance of securities without registration under the Securities Act of 1933, as amended, during the year ended March 31, 2011, that were not previously disclosed in a Quarterly Report on Form 10-Q or in a Current Report on Form 8-K:
On April 15, 2011, the Company entered into a form of Securities Purchase Agreement (the “SPA”) for the third and final closing of a private placement offering (the “Private Offering”) with certain accredited investors (the “Purchasers”) for the issuance and sale of seven (7) Units of the Company at a purchase price of $25,000 per Unit, for aggregate consideration of $175,000. Each “Unit” is comprised of (i) 27,778 shares of Company common stock, $0.001 par value per share (the “Common Stock,” and the shares of Common Stock offered referred to as the “Shares”), (ii) warrants to purchase 27,778 shares of Common Stock at an exercise price of $1.25 per share (the “Series A-1 Warrants”), and (iii) warrants to purchase 27,778 shares of Common Stock at an exercise price of $2.00 per share (the “Series A-2 Warrants”) (the Series A-1 Warrants and the Series A-2 Warrants, collectively, the “Warrants”). The Warrants expire four (4) years from the date of issuance, subject to early termination or forfeiture in accordance with certain terms and conditions of the Warrants. This third and final closing is in addition to the initial closing of one hundred and fourteen (114) Units for $2,850,000 in proceeds and the second closing of thirty three (33) Units for $825,000 in proceeds, both as previously disclosed in Current Reports on Form 8-K filed with the SEC.
42
An aggregate of 210,001 Shares, 210,001 Series A-1 Warrants and 210,001 Series A-2 Warrants were issued in connection with the third and final closing. The Shares and Warrants were issued in reliance upon Rule 506 of Regulation D of the Securities Act, and comparable exemptions for sales to “accredited” investors under state securities laws.
As disclosed in the Company’s Current Report on Form 8-K filed May 10, 2011, on May 6, 2011, the Company entered into a Board Advisory Agreement (the “Agreement”) with Jack Zwick, whereby Mr. Zwick consented to serve as a director of the Company. In accordance with the Agreement, on May 23, 2011, the Company issued to Mr. Zwick 200,000 shares of Company Common Stock, vesting monthly over a period of two years in connection with his service as a director (“Zwick Shares”). The offer and sale of the Zwick Shares was effected without registration in reliance on the exemption afforded by Regulation D and/or Section 4(2) of the Securities Act.
ITEM 6. SELECTED FINANCIAL DATA.
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
|
ITEM 7.
|
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS.
|
This discussion summarizes the significant factors affecting the operating results, financial condition, liquidity and cash flows of the Company and its subsidiaries for the fiscal years ended March 31, 2011 and 2010. The discussion and analysis that follows should be read together with our consolidated financial statements and the notes to the consolidated financial statements included elsewhere in this Annual Report on Form 10-K. Except for historical information, the matters discussed in this section are forward looking statements that involve risks and uncertainties and are based upon judgments concerning various factors that are beyond the Company’s control. Consequently, and because forward-looking statements are inherently subject to risks and uncertainties, the actual results and outcomes may differ materially from the results and outcomes discussed in the forward-looking statements. You are urged to carefully review and consider the various disclosures made by us in this report.
OVERVIEW
As a result of the Exchange Transaction, through our wholly-owned subsidiary, XYT, we are now engaged in drug logistics and distribution in Yunnan Province, China through drug stores, medical clinics and hospitals, as well as the wholesale distribution of medicine products, chemical agents, antibiotics, biochemistry drugs and biological preparations to hospitals and the XYT store located at the Company’s distribution facility in Kunming. XYT was founded in November 2002 and is a provincial pharmaceutical distributor that offers approximately 5,000 drugs, of which approximately 1,000 are over-the-counter drugs, approximately 1,000 are prescription drugs, approximately 2,000 are prepared Chinese medicines and approximately 1,000 are supplements. Currently, XYT has approximately 4,700 customers and supplies approximately 10% of such customers’ inventories with a sales network that covers the entire Yunnan Province of China. XYT believes it has a strategic advantage over certain of its competitors in Yunnan Province as it has obtained government approval to fill orders over the internet. XYT received the License of Internet Drug Information Service issued by the Yunnan Food and Drug Administration in October 2009. This license enables XYT to bypass municipal and county pharmaceutical distributors, market XYT’s product line, provide pricing information and provide products directly to XYT customers. Bypassing these layers of distribution enables XYT to offer products to its customers at a significantly lower price than its major competitors while maintaining its margins.
Our continuing strategy is to build a nationwide pharmaceutical distribution network throughout China. We plan to expand our customer base through the use of the following tactics: broadening of our current product line to attract larger customers that currently do not utilize us and benefit from internet ordering and the lower prices that we offer; providing computers to customers to attract new customers as our management is unaware of any other pharmaceutical distribution company providing this benefit; and increasing our current sales force to directly target hospitals, medical clinics and pharmacies.
43
Our management’s discussion and analysis of our financial condition and results of operations are only based on XYT’s current drug logistics and distribution business in China. Key factors affecting our results of operations include revenues, cost of revenues, operating expenses and income and taxation.
Critical Accounting Policies and Estimates
Our consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“US GAAP”), and this requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and the related disclosure of contingent assets and liabilities at the dates of the consolidated financial statements as well as the reported amounts of revenues and expenses during the reporting periods. We base our estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances. Accordingly, actual results may differ significantly from these estimates under different assumptions or conditions. Significant estimates include:
Valuation of accounts receivable
An allowance for impairment of trade and other receivables is established when there is objective evidence that we will not be able to collect all amounts due according to the original terms of receivables. The amount of the allowance is the difference between the receivables’ carrying amount and the present value of estimated future cash flows, discounted at the effective interest rate computed at initial recognition. The amount of the allowance is recognized in the income statement.
Inventories
Net realizable value of inventories is the estimated selling price in the ordinary course of business, less the estimated costs of completion and the estimated costs necessary to make the sale. For the years ended March 31, 2011 and 2010, the Company recorded no allowance for slow-moving and obsolete inventories.
Deferred income taxes
Income taxes are accounted for under the asset and liability method. Deferred tax assets and liabilities are recognized for the future tax consequences attributable to differences between the financial statement carrying amounts of existing assets and liabilities and their respective tax bases and operating loss and tax credit carry-forwards. Deferred tax assets are reduced by a valuation allowance to the extent management concludes it is more likely than not that the assets will not be realized. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the statements of income and comprehensive income in the periods that includes the enactment date.
Useful lives of plant and machinery
Depreciation of property, plant and equipment is calculated to write off the cost, less their estimated residual value, if any, using the straight-line method over their estimated useful lives. The principal depreciation periods are as follows:
|
Machinery
|
5 years
|
|
Motor Vehicles
|
4 years
|
|
Office equipment
|
3 years
|
44
Recently Issued Accounting Pronouncements
In April 2009, the FASB issued FSP 157-4, Determining Fair Value When The Volume And Level Of Activity For The Asset Or Liability Have Significantly Decreased And Identifying Transactions That Are Not Orderly (“FSP 157-4”). FSP 157-4 provides additional guidance for estimating fair value in accordance with SFAS 157 when the volume and level of activity for the asset or liability have significantly decreased. FSP 157-4 also includes guidance on identifying circumstances that indicate a transaction is not orderly. FSP 157-4 is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009. FSP 157-4 does not require disclosures for earlier periods presented for comparative purposes at initial adoption. In periods after initial adoption, FSP 157-4 requires comparative disclosures only for periods ending after initial adoption. The adoption of the provisions of FSP 157-4 is not anticipated to materially impact on the Company’s results of operations or the fair values of its assets and liabilities.
In May 2009, the FASB issued FSP SFAS 165 “Subsequent Events.” The objective of this Statement is to establish general standards of accounting for and disclosure of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. SFAS 165 is effective for the interim and annual periods ending after June 15, 2009, which is now codified as FASB ASC 855 “Subsequent Events.” The adoption of FASB ASC 855 did not have a material impact on the Company’s financial position, results of operations and cash flows. Effective February 24, 2010, the Company adopted Accounting Standards Update (“ASU”) No. 2010-09, “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements,” which removes the requirement to disclose the date through which subsequent events have been evaluated. The adoption of the ASU did not have a material impact on the Company’s financial position, results of operations and cash flows.
In June 2009, the FASB issued SFAS No. 166 Accounting For Transfers Of Financial Assets (“SFAS 166”). This statement is intended to improve the relevance, representational faithfulness, and comparability of the information that a reporting entity provides in its financial reports about a transfer of financial assets; the effects of a transfer on its financial position, financial performance, and cash flows; and a transferor’s continuing involvement in transferred financial assets. This Statement must be applied as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009, and is required to be adopted by the Company in the first quarter of fiscal year 2011. Earlier application is prohibited. This Statement must be applied to transfers occurring on or after the effective date. The Company does not expect the adoption of SFAS 166 to have a material impact on its financial position, results of operations and cash flows.
In June 2009, the FASB issued SFAS No. 167, “Amendments to FASB Interpretation No. 46(R),” which is codified as ASC 810. ASC 810 amends FASB Interpretation No. 46(R), “Variable Interest Entities” for determining whether an entity is a variable interest entity (“VIE”) and requires an enterprise to perform an analysis to determine whether the enterprise’s variable interest or interests give it a controlling financial interest in a VIE. Under ASC 810, an enterprise has a controlling financial interest when it has a) the power to direct the activities of a VIE that most significantly impact the entity’s economic performance and b) the obligation to absorb losses of the entity or the right to receive benefits from the entity that could potentially be significant to the VIE. ASC 810 also requires an enterprise to assess whether it has an implicit financial responsibility to ensure that a VIE operates as designed when determining whether it has power to direct the activities of the VIE that most significantly impact the entity’s economic performance.
ASC 810 also requires ongoing assessments of whether an enterprise is the primary beneficiary of a VIE, requires enhanced disclosures and eliminates the scope exclusion for qualifying special-purpose entities. ASC 810 shall be effective as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009, for interim periods within that first annual reporting period, and for interim and annual reporting periods thereafter. Earlier application is prohibited. ASC 810 is effective for the Company in the first quarter of fiscal 2011. The Company is currently evaluating the effect of ASC 810 on its financial statements and results of operation and is currently not yet in a position to determine such effects.
In June 2009, the FASB issued SPAS 168, “The FASB Accounting Standards Codification™ and the Hierarchy of Generally Accepted Accounting Principles - a replacement of FASB Statement No 162,” which supersedes all existing non-SEC accounting and reporting standards. The codification does not change GAAP but rather organizes it into a new hierarchy with two levels; authoritative and non-authoritative. All authoritative GAAP carries equal weight and is organized in a topical structure. The adoption of SPAS 168 did not have a material impact on the Company’s financial position, results of operations and cash flows.
45
In August 2009, the FASB issued Accounting Standards Update (“ASU”) No. 2009-05, “Measuring Liabilities at Fair Value,” which is codified as ASC 820, “Fair Value Measurements and Disclosures.” This Update provides amendments to ASC 820-10, Fair Value Measurements and Disclosures –Overall, for the fair value measurement of liabilities. This Update provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using a valuation technique that uses the quoted price of the identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities when traded as assets, or that is consistent with the principles of ASC 820. The amendments in this Update also clarify that when estimating the fair value of a liability, a reporting entity is not required to include a separate input or adjustment to other inputs relating to the existence of a restriction that prevents transfer of the liability.
The amendments in this Update also clarify that both a quoted price in an active market for the identical liability at the measurement date and the quoted price for the identical liability when traded as an asset in an active market when no adjustments to the quoted price of the assets are required are Level 1 fair value measurements. ASC 820 is effective for the first reporting period (including interim periods) beginning after August 28, 2009. The adoption of this Update did not have a significant impact to the Company’s financial statements.
In September 2009, the FASB issued ASU No. 2009-06, “Income Taxes (Topic 740)—Implementation Guidance on Accounting for Uncertainty in Income Taxes and Disclosure Amendments for Nonpublic Entities,” and it provides implementation guidance on accounting for uncertainty in income taxes effective for interim and annual reporting period ending on or after September 15, 2009. The adoption of ASU No. 2009-06 did not have any impact on the Company’s financial position, results of operations and cash flows.
In December 2009, the FASB issued ASU No. 2009-17, “Improvements to Financial Reporting by Enterprises Involved with Variable Interest Entities (“ASU 2009-17”).” ASU 2009-17 amends the variable-interest entity guidance in FASB ASC 810-10-05-8 to clarify the accounting treatment for legal entities in which equity investors do not have sufficient equity at risk for the entity to finance its activities without financial support. ASU 2009-17 shall be effective as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009. ASU 2009-17 is effective for the Company in the first quarter of fiscal 2011. The Company is currently evaluating the effect of ASU 2009-17 on its consolidated financial statements and results of operation and is currently not yet in a position to determine such effects.
In January 2010, the FASB issued ASU No. 2010-06 “Fair Value Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements.” This update provides amendments to Subtopic 820-10 that required new disclosures for 2 situations: (i) a reporting entity should disclose separately the amount of significant transfer in and out of Level 1 and 2 fair value measurements and describe the reasons for the transfers; (ii) in the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuance, and settlements. None of the new pronouncements has current application to the Company.
In February 2010, the FASB issued ASU No. 2010-09 “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements.” This Update provides amendments to: (i) an entity that either (a) is an SEC filer or (b) is a conduit bond obligor for conduit debt securities that are traded in a public market (a domestic or foreign stock exchange or an over-the-counter market, including local or regional markets), is required to evaluate subsequent events through the date that the financial statements are issued. If an entity meets neither of those criteria, then it should evaluate subsequent events through the date the financial statements are available to be issued; (ii) the glossary of Topic 855 is amended to include the definition of SEC filer; (iii) an entity that is an SEC filer is not required to disclose the date through which subsequent events have been evaluated; (iv) the glossary of Topic 855 is amended to remove the definition of public entity; and (v) the scope of the reissuance disclosure requirements is refined to include revised financial statements only.
In May 2010, the FASB issued ASU No. 2010-19 “Foreign Currency (Topic 830): Foreign Currency Issues: Multiple Foreign Currency Exchange Rates (SEC Update).” The purpose of this Update is to codify the SEC Staff Announcement made at the March 18, 2010 meeting of the FASB Emerging Issues Task Force (EITF) by the SEC Observer to the EITF. The Staff Announcement provides the SEC Staff’s view on certain foreign currency issues related to investments in Venezuela. None of the new pronouncements has current application to the Company.
46
In December 2010, the FASB issued Update No. 2010-28 “Intangibles—Goodwill and Other (Topic 350): When to Perform Step 2 of the Goodwill Impairment Test for Reporting Units with Zero or Negative Carrying Amounts.” The amendments in this Update modify Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For those reporting units, an entity is required to perform Step 2 of the goodwill impairment test if it is more likely than not that a goodwill impairment exists. In determining whether it is more likely than not that a goodwill impairment exists, an entity should consider whether there are any adverse qualitative factors indicating that an impairment may exist. The qualitative factors are consistent with the existing guidance and examples in paragraph 350-20-35-30, which requires that goodwill of a reporting unit be tested for impairment between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. For nonpublic entities, the amendments are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. Nonpublic entities may early adopt the amendments using the effective date for public entities.
None of the above new pronouncements has current application to us, but may be applicable to our future financial reporting.
Comparison of the Years Ended March 31, 2011 and 2010
The following table sets forth certain information regarding our results of operation.
|
Years Ended March 31
|
||||||||
|
2011
|
2010
|
|||||||
|
Statements of Operations Data
|
||||||||
|
Sales
|
$
|
28,999,198
|
$
|
25,723,902
|
||||
|
Cost of Sales
|
(23,749,000)
|
(21,045,598)
|
||||||
|
Gross Profit
|
5,250,198
|
4,678,304
|
||||||
|
Selling, general and administrative expenses, and other expenses
|
(1,831,141)
|
(981,382)
|
||||||
|
Income tax expense
|
(1,085,378)
|
(934,463)
|
||||||
|
Net Income
|
2,333,679
|
2,762,459
|
||||||
|
Effects of foreign currency translation conversion
|
202,391
|
21,037
|
||||||
|
Comprehensive income
|
2,536,070
|
2,783,496
|
||||||
Sales
Our sales of Chinese patent drugs, antibiotics, bio-chemicals, chemical preparations, and biologicals totaled US$28,999,198 for the year ended March 31, 2011, an increase of 13% from US$25,723,902 for the year ended March 31, 2010. This increase in sales was primarily due to increased selling efforts by XYT and FCPG HK’s staff and the addition of several products with high profit margins. In addition, several new hospitals were added as customers which contributed to increased sales volume. Further, we had the ability to market our product line over the internet in 2011 due to our receipt, in November 2010, of Chinese government approval to market our product line in such manner. We began to realize the benefits of internet marketing from November 2010 onward, resulting in increased sales for the year ended March 31, 2011. We were not able to undertake such internet marketing efforts during the year ended March 31, 2010.
Cost of sales
Cost of sales consists primarily of material cost, labor cost, and related expenses which are directly attributable to the selling of pharmaceutical products. Cost of sales for the year ended March 31, 2011 increased by 13% to US$23,749,000 from US$21,045,598 for the year ended March 31, 2010. The largest component of our cost of sales is the purchase price for our goods. This increase in cost of sales percentage from the prior year is relatively consistent with XYT and FCPG HK’s percentage increase in sales described above, reflecting its increased selling efforts. Specifically, our increased selling efforts are a result of our ability to market our product line over the internet in 2011 due to our receipt, in November 2010, of Chinese government approval to market our product line in such manner. We were not able to undertake such internet marketing efforts (and incur such related costs) during the year ended March 31, 2010.
47
Although we anticipate that the cost of sales will increase due to inflationary price increases, we do not believe that such increases will be material for fiscal year 2012. We anticipate that beyond 2011, our price for materials and other production costs will continue to increase due to inflation. If our costs of sales increase, this may have a negative effect on our net income because due to market conditions and competitive conditions, we may not be able to increase the price for our products in proportion to the increase in costs of goods sold.
Gross profit
Gross profit for the year ended March 31, 2011 was US$5,250,198, an increase of 12%, from US$4,678,304 for the year ended March 31, 2010. The increase in gross profit reflects increased overall sales as described above, as well as the sale of certain higher margin products. Specifically, our freeze-dry powder injector and antibiotics products have higher margins than our Chinese medicine products. Our increased costs of sales for the year ended March 31, 2011 was partially offset by the increased sales of our higher margin products (freeze-dry powder injector and antibiotics) for the year ended March 31, 2011.
Selling, general and administrative expenses, and others
Selling, general and administrative expenses increased by 87% to US$1,831,141 for the year ended March 31, 2011 from US$981,382 for the year ended March 31, 2010. The increase is largely due to an increase in legal and accounting expenses and administrative expenses from $162,471 for the year ended March 31, 2010 to $1,356,288 for the year ended March 31, 2011. The increase in administrative expenses was mainly due to the securities issuance cost paid to placement agents.
Income tax expense
Income tax expense for the year ended March 31, 2011 was US$1,085,378 compared to income tax expense of US$934,463 for the year ended March 31, 2010. The 16% increase was due to the increase in profits in FCPG HK of $0 for the year ended March 31, 2010 to $2,883,208 for the year ended March 31, 2011, where we were subject to a 16.5% income tax rate.
Net income
Our net income for the year ended March 31, 2011 was US$2,333,679, a decrease of 16%, from US$2,762,459 for the year ended March 31, 2010. This decrease was primarily due to the increase in selling, general and administrative expenses, specifically, our increased administrative expenses of $1,356,288 attributable to the securities issuance cost paid to placement agents.
Effects of foreign currency translation conversion
We recognized a gain of US$202,391 on the effects of foreign currency conversion for the year ended March 31, 2011, as compared to a gain of US$21,037 for the year ended March 31, 2010. This change is due to differences in exchange rates used during the years for converting from XYT’s functional currency of Renminbi to U.S. dollars for financial reporting purposes.
Comprehensive income
Our comprehensive income decreased by 9% from US$2,783,496 for the year ended March 31, 2010 to US$2,536,070 for the year ended March 31, 2011. The decrease is attributable to the above-mentioned increases in sales and gross profits, offset in part by selling and administrative expenses and income tax expenses.
48
Liquidity and Capital Resources
Overview
As of March 31, 2011, we had cash and equivalents on hand of US$740,368, and a working capital deficit of US$5,718,593. We believe that our cash on hand and working capital will be sufficient to meet our operational cash requirements through December 31, 2011. If XYT does not meet its revenue objectives over that period, the Company may need to sell additional equity securities, which could result in dilution to current stockholders, or seek additional loans. The incurrence of indebtedness would result in increased debt service obligations and could require the Company to agree to operating and financial covenants that would restrict its operations. Financing may not be available in amounts or on terms acceptable to the Company, if at all. Any failure by us to raise additional funds on terms favorable to us, or at all, could limit our ability to expand our business operations and could harm our overall business prospects.
Substantially all of our revenues are earned by XYT, our PRC subsidiary. However, PRC regulations restrict the ability of our PRC subsidiary to make dividends and other payments to their offshore parent company. Pursuant to the law of PRC on foreign-capital enterprises, when XYT decides to distribute profits, reserve funds and bonus and welfare funds for workers and staff members shall be withdrawn from the profits after a foreign-capital enterprise has paid income tax in accordance with the provisions of the Chinese tax law. The proportion of reserve funds to be withdrawn shall not be lower than 10% of the total amount of profits after payment of tax; the withdrawal of reserve funds may be stopped when the total cumulative reserve has reached 50% of the registered capital. The proportion of bonus and welfare funds for workers and staff members to be withdrawn shall be determined by the foreign-capital enterprise of its own accord. Companies may be subject to a fine up to 5,000 RMB as a result of non-compliance of such rules. The registered capital of XYT is US$2,295,000. Shareholders of XYT had not determined the proportion of reserve funds and bonus and welfare funds for workers and staff members, and XYT had not distributed any profits previously. If we decide to distribute profits in the future, XYT will comply with the relevant rules to withdraw statutory reserve funds which will be no lower than 10% of the total amount of profits after payment of tax.
XYT had previously recorded as a receivable an amount due from a related party to the Company of $11,799,953 as of December 31, 2009 and $2,498,924 as of December 31, 2008. To comply with U.S. GAAP, the Company has reclassified such amounts as a deemed distribution of a dividend to Mr. Wang, a related party, and therefore a corresponding reduction of retained earnings. Prior to such reclassification, we contemplated access to the cash collected by Mr. Wang to be used to finance a portion of our operations in the interim between bonus compensation payments to Mr. Wang. As a result of the foregoing reclassification, we may experience potential shortfalls from not collecting the receivables from Mr. Wang and our liquidity position could be affected such that we will not have the opportunity to use these earnings to fund our operations for any period of time, and may need to pursue direct bank financing or sell additional equity securities to meet our operational cash requirements. We are in the process of trying to obtain a line of credit from a bank to be used for the purchase of inventories to cover any potential shortfalls in capital resulting from not collecting the receivables from Mr. Wang. There are no assurances that we will be successful or that we will be presented with terms that will be satisfactory to us.
Our cash needs are primarily for working capital to support our operations, the purchase of inventory and future strategic acquisitions. We presently finance our operations through revenue from the sale of our products and services, the private placement of equity and debt securities and short-term bank borrowings. As of March 31, 2011, we had outstanding bank loans from Kunming Wuhua Branch of Fudian Bank in the amounts of US$13,114 and US$758,897, with annual interests rates of 7.97% and 5.56%, respectively, and maturity dates of April 26, 2011 and December 8, 2011, respectively. We believe that our existing capital resources are sufficient to meet our current obligations and operating requirements, but will not be sufficient to meet our more aggressive growth and acquisition plans and that we will need to raise additional capital in the next 12 months. In order to meet our planned two to four strategic acquisitions, we estimate requiring US$6 million in capital. We will consider debt or equity offerings or institutional borrowing as potential means of financing, however, there are no assurances that we will be successful or that we will obtain terms that are favorable to us.
49
In addition to the US$6 million for acquisitions, XYT will also require financing to expand its product line. The Company completed a financing of $3.6 million (net of fees) and will utilize some of these proceeds to expand its product line. As the funding fell short of the $5 to $6 million objective which would have provided sufficient capital for product line expansion and an acquisition, the Company’s short term objective is now to broaden our product line from our current 5,000 products to 20,000 to 25,000 over a 6 to 8 month period. This will allow XYT to not only sell more products to its existing customers, but also attract more customers that currently do not utilize XYT. The bulk of these funds will be utilized to purchase inventory, which will be on a cash basis until a track record is established and net 30 day terms can be negotiated. The broader product line will include significantly more Western drugs as well as traditional Chinese drugs and herbs. In addition, the Company may be required to raise capital in order to make acquisitions in furtherance of its growth plan. Although funds from operations are adequate to conduct the Company’s current operations, it will need to raise capital in order to grow through acquisitions.
Our liquidity could be further impacted by external factors such as any significant decrease in the market price of pharmaceutical products as a result of downward periodic price adjustments enforced by the PRC government seeking to make pharmaceutical products more affordable to the general public, and reimbursement policies of the PRC Ministry of Labor and Social Security. Although we have yet to be materially impacted by such factors, each or a combination of such factors may result in lower sales, increased expenses and costs and result in lower profitability and cash flow, thus reducing our liquidity. We may also in the future apply for and receive government subsidies. Although we have not applied for any such subsidies to date, any such subsidies which we may receive in the future may increase our liquidity.
Net cash provided by (used in) operating activities
Net cash used in operating activities for the year ended March 31, 2011 was US$2,949,086, compared to net cash used in operating activities of US$641,622 for the year ended March 31, 2010. This increase in cash used in operating activities was primarily due to net income of US$2,333,679, and offset by accounts receivable of US$8,868,241. The nature of our accounts receivable constitutes outstanding orders from our customers. Our accounts receivable has increased in the year ended March 31, 2011 because of the aggressive increase of sales and marketing efforts of FCPG HK, whose customers are located in mainland China.
Net cash provided by (used in) investing activities
Net cash used in investing activities was US$289,828 for the year ended March 31, 2011, compared to US$2,885 for the year ended March 31, 2010, related to the purchase of property, plant and equipment and an investment in a subsidiary. Specifically, we used $266,101 for the purchase of XYT.
Net cash provided by financing activities
Net cash provided by financing activities was US$3,815,759 for the year ended March 31, 2011 and was comprised of a convertible promissory note and a private offering of units as part of our capital raising efforts to fund our market expansion and expand our product line, offset in part by repayment of borrowings from Kunming Wuhua Branch of Fudian Bank and increase in restricted cash. Restricted cash consists of US$338,970. Net cash provided by financing activities during the year ended March 31, 2010 was US$49,829.
Off-Balance Sheet Arrangements
We have not entered into any other financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
Contractual Obligations
The following table outlines payments due under our significant contractual obligations over the periods shown, exclusive of interest:
50
|
Payments due by Period
|
||||||||||||||||||||
|
Contractual Obligations
|
||||||||||||||||||||
|
At March 31, 2011
|
Less than
|
One to
|
Three to
|
More Than
|
||||||||||||||||
|
|
One Year
|
Three Years
|
Five Years
|
Five Years
|
Total
|
|||||||||||||||
|
Operating Lease Obligations
|
$ | - | $ | 113,835 | $ | 75,890 | $ | 75,890 | $ | 265,615 | ||||||||||
|
Long-Term Debt Obligations
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
Capital Expenditure Obligations
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
Purchase Obligations
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
|
Other Long-Term Liabilities
|
$ | - | $ | - | $ | - | $ | - | $ | - | ||||||||||
The above table outlines our obligations as of March 31, 2011 and does not reflect any changes in our obligations that have occurred after that date.
|
ITEM 7A.
|
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK.
|
We are a smaller reporting company as defined by Rule 12b-2 of the Securities Exchange Act of 1934 and are not required to provide the information under this item.
|
ITEM 8.
|
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA.
|
The financial statements annexed to this Form 10-K for the year ended March 31, 2011 begin on page F-1 and have been examined by our independent accountants, Parker Randall CF (H.K.) CPA Limited.
|
ITEM 9.
|
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE.
|
As previously disclosed on a Current Report on Form 8-K filed with the SEC on August 7, 2009, Moore and Associates, Chartered was dismissed as our independent accountant on August 7, 2009. On August 7, 2009, we engaged Seale and Beers, CPAs as our new independent registered public accounting firm. As previously disclosed on a Current Report on Form 8-K filed with the SEC on October 23, 2009, on October 18, 2009, we dismissed Seale and Beers, CPAs, which had not provided any services to our company from the period between its engagement and dismissal. On October 8, 2009, our Board of Directors approved the appointment of Li & Company, PC as our independent registered public accounting firm. As previously disclosed on a Current Report on Form 8-K filed with the SEC on August 26, 2010, on August 24, 2010, Li & Company, PC resigned as our independent registered public accounting firm, and our Board of Directors approved the appointment of Parker Randall CF (H.K.) CPA Limited as our independent registered public accounting firm, effective August 25, 2010. In connection with each of these changes in accountants, there were no disagreements (as that term is used in Item 304(a)(1)(iv) of Regulation S-K) or reportable events (as described in Item 304(a)(1)(v) of Regulation S-K).
|
ITEM 9A.
|
CONTROLS AND PROCEDURES.
|
Evaluation of Disclosure Controls and Procedures
We carried out an evaluation, under the supervision and with the participation of our management, including our Principal Executive Officer along with our Principal Financial Officer, of the effectiveness of the design of our disclosure controls and procedures (as defined by Exchange Act Rules 13a-15(e) or 15d-15(e)) as of March 31, 2011 pursuant to Exchange Act Rule 13a-15. Based upon that evaluation, our Principal Executive Officer along with our Principal Financial Officer concluded that our disclosure controls and procedures are not effective as of March 31, 2011 in ensuring that information required to be disclosed by us in reports that we file or submit under the Exchange Act is recorded, processed, summarized, and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. This conclusion is based on findings that constituted material weaknesses. A material weakness is a deficiency, or a combination of control deficiencies, in internal control over financial reporting such that there is a reasonable possibility that a material misstatement of the Company’s interim financial statements will not be prevented or detected on a timely basis.
51
Management’s Report on Internal Control Over Financial Reporting
In performing the above-referenced assessment, our management identified the following material weaknesses:
|
|
i)
|
We lack personnel with the experience to properly analyze and record complex transactions in accordance with U.S. GAAP.
|
|
|
ii)
|
We have insufficient quantity of dedicated resources and experienced personnel involved in reviewing and designing internal controls. As a result, a material misstatement of the interim and annual financial statements could occur and not be prevented or detected on a timely basis.
|
|
|
iii)
|
We have not achieved the optimal level of segregation of duties relative to key financial reporting functions.
|
|
|
iv)
|
We did not perform an entity level risk assessment to evaluate the implication of relevant risks on financial reporting, including the impact of potential fraud related risks and the risks related to non-routine transactions, if any, on our internal control over financial reporting. Lack of an entity-level risk assessment constituted an internal control design deficiency which resulted in more than a remote likelihood that a material error would not have been prevented or detected, and constituted a material weakness.
|
|
|
v)
|
We did not have an audit committee or an independent audit committee financial expert. While not being legally obligated to have an audit committee or independent audit committee financial expert, it is the management’s view that to have an audit committee, comprised of independent board members, and an independent audit committee financial expert is an important entity-level control over our financial statements.
|
We are currently reviewing our disclosure controls and procedures related to these material weaknesses and expect to implement changes in the near term, including identifying specific areas within our governance, accounting and financial reporting processes to add adequate resources and personnel to potentially mitigate these material weaknesses. To that end, on June 2, 2011, our Board of Directors formed an audit committee and adopted an audit committee charter. Our Board of Directors appointed Mr. Gregory D. Tse and Mr. Jack Zwick, current independent members of the Board of Directors, to serve on the audit committee. Our Board of Directors has determined that Jack Zwick is an “audit committee financial expert,” as defined in Item 407(d)(5) of Regulation S-K.
Our present management will continue to monitor and evaluate the effectiveness of our internal controls and procedures and our internal controls over financial reporting on an ongoing basis and are committed to taking further action and implementing additional enhancements or improvements, as necessary and as funds allow.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate. All internal control systems, no matter how well designed, have inherent limitations. Therefore, even those systems determined to be effective can provide only reasonable assurance with respect to financial statement preparation and presentation.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal controls over financial reporting that occurred during the quarterly period ended March 31, 2011 that have materially affected, or are reasonably likely to materially affect, our internal controls over financial reporting. We believe that a control system, no matter how well designed and operated, cannot provide absolute assurance that the objectives of the control system are met, and no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, within any company have been detected.
ITEM 9B. OTHER INFORMATION.
None.
52
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE.
Set forth below are the present directors and executive officers of the Company. Note that there are no other persons who have been nominated or chosen to become directors nor are there any other persons who have been chosen to become executive officers. Other than as set forth below, there are no arrangements or understandings between any of the directors, officers and other persons pursuant to which such person was selected as a director or an officer
|
Name
|
Age
|
Position
|
||
|
Zhen Jiang Wang
|
53
|
Chairman and Chief Executive Officer
|
||
|
Gregory D. Tse
|
51
|
Director
|
||
|
Jack Zwick
|
75
|
Director
|
||
|
Jing Gong
|
37
|
President
|
||
|
Yong Kang Chen
|
73
|
Senior Vice President, Quality Control
|
||
|
Yi Jia Li
|
41
|
Chief Financial Officer
|
The Board of Directors is comprised of only one class. All of the directors serve for a term of one year and until their successors are elected at the Company’s Annual Shareholders’ Meeting and are qualified, subject to removal by the Company’s shareholders. Each executive officer serves, at the pleasure of the Board of Directors, for a term of one year and until his successor is elected at a meeting of the Board of Directors and is qualified.
Our Board of Directors believes that all members of the Board and all executive officers encompass a range of talent, skill, and experience sufficient to provide sound and prudent guidance with respect to our operations and interests. The information below with respect to our directors and executive officers includes each individual’s experience, qualifications, attributes, and skills that led our Board of Directors to the conclusion that he or she should serve as a director and/or executive officer.
Biographies
Set forth below are brief accounts of the business experience during the past five years of each director, executive officer and significant employees of the Company.
Zhen Jiang Wang
Mr. Wang founded XYT in 2002, served as its general manager until 2009, and is currently serving as its Executive Director. Prior to establishing XYT, Mr. Wang served as Vice President of the Sales Department of Yun Nan Provincial Pharmacies Co., Ltd from 1998 to 2001, general manager of Yun Nan Tuo Xin Equipments and Electronics Trading Co. Ltd from 1994 to 1997, and prior to that, as Executive Director of Kun Ming Feng Ning Department Store. Mr. Wang is a 1994 graduate from He Bei Architecture Institute in the People’s Republic of China, majoring in Architecture Engineering. Mr. Wang was appointed to the Company’s Board of Directors due to his tremendous knowledge of the pharmaceutical industry in China, as well as his corporate leadership experience in China. The Company believes that Mr. Wang’s knowledge of the pharmaceutical business environment of the PRC will be an invaluable resource as the Company seeks to expand its business in the PRC.
Gregory D. Tse
Mr. Tse has over 25 years of international finance, marketing, media, PR and advertising experience with an extensive brand management track record in North America, Hong Kong and China. In China, Mr. Tse has helped many international brands to enter into the marketplace before moving on to become one of China’s first communications/media M&A specialists. Mr. Tse most recently served as Head of China Advisory for Calneva Financial Group from July 2004 to the present date, providing investment banking services for merger and acquisitions in the information technology, media, energy, infrastructure and natural resources areas. Previously, Mr. Tse’s media and marketing communications career included heading up several multinational advertising and PR agencies, including from May 1997 to June 2004, when Mr. Tse served as Managing Director at Publicis China, a communications group, were he managed the China national offices. Mr. Tse has also served as a member of the Board of Directors of i-Level Media Group Incorporated (PK: ILVL) from July 2008 to January 2009. Mr. Tse has also traveled extensively in China as the Chief Communications Officer for CORA (China’s Old Revolution Area), a NGO with a mandate to develop China’s rural areas, and started many humanitarian projects to fund education there. Mr. Tse graduated from the School of Architecture at University of Waterloo, Canada. Mr. Tse was appointed to the Company’s Board of Directors due to his over 25 years of experience, primarily in China, as well as his public company board and management experience. The Company believes that Mr. Tse’s knowledge of investment banking services and mergers and acquisitions will be an invaluable resource as the Company may seek additional capital to expand its business in the PRC. The Company also believes that Mr. Tse’s knowledge of brand management and marketing will aid the Company in expanding the brand awareness of its pharmaceutical distribution operations.
53
Jack Zwick
Mr. Zwick is a certified public accountant and a founding and current member of Zwick Maddox & Banyai PLLC, certified public accountants, in Michigan, since 1994. Mr. Zwick is also currently the Senior Vice President of Finance of Sunrise Sports & Entertainment, LLC and the Chief Financial Officer of American BioCare, Inc., a public company, positions he has held since 2008 and September 2010, respectively. Mr. Zwick has extensive experience as an audit partner for both public and private companies in several industries, including real estate, manufacturing, pharmaceutical, theatre, concert, recreation, sports franchises, depleting assets, hospitality and service organizations. He has worked with clients in a wide variety of areas, ranging from accounting and auditing services, and SEC work which included initial public offerings of corporations and public limited partnerships, to tax consulting and litigation support. Mr. Zwick is currently the audit partner for all Nederlander operations, an international company involved in theatre and concert facilities throughout the United States and in the United Kingdom. He was previously the audit partner for the Detroit Red Wings, a National Hockey League team, a previous member of the audit committee of Health Chem Corporation, a public company, and a member of the board and chairman of the audit committee of Solar Energy Initiatives Corporation, a public company (OTCBB: SNRY). Further, Mr. Zwick is a former managing partner of the Detroit, Michigan office of Laventhol & Horwath, an international CPA firm, and a former executive director with Grant Thornton, an international CPA firm.
Mr. Zwick holds a Bachelor of Arts degree in Accountancy and a Masters of Science in Taxation from Wayne State University. He is a member of the American Institute of Certified Public Accountants and the Michigan Association of Certified Public Accountants, and previously taught accounting and audit related classes for the Michigan Association of CPAs and Ohio Society of CPAs.
Jing Gong
Ms. Jing Gong is the General Manager of FCPG HK and XYT. She is one of the founding shareholders of XYT and has broad experience in retailing and the pharmaceutical industry. She was the General Manager of Kun Ming Feng Ning Department Store and the sales manager of Yun Nan Tuo Xin Equipments and Electronics Trading Co. Ltd prior to helping establish XYT. Ms. Gong is a graduate from He Bei University, PRC in 1999, majoring in Economic Management.
Yong Kang Chen
Mr. Yong Kang Chen is the Senior Vice President, Quality Control of FCPG HK and XYT. Mr. Chen has been involved in the pharmaceutical industry for over 47 years. He has been the General Manager of XYT since 2002. He has also held the following positions within the pharmaceutical industry in China:
|
•
|
Director of pharmaceutical section of No. 1 People’s Hospital of Dong Chuan City.
|
|
•
|
Director of Pharmaceutical Inspection Department of Dong Chuan City.
|
|
•
|
Vice Director of Dong Guan Bureau of Hygiene.
|
|
•
|
Director of Dong Guan Science and Technology Committee.
|
|
•
|
Director of Kun Ming Pharmaceutical Inspection Department.
|
|
•
|
Chief Supervisor of Kun Ming Municipal Pharmacies Co., Ltd.
|
Mr. Chen Graduated from Nan Jing Pharmaceutical College in 1963, majoring in Pharmacy.
54
Yi Jia Li
Ms. Yi Jia Li is the Chief Financial Officer of XYT and FCPG HK. Ms. Li joined XYT in 2009 after working as the financial manager for Yun Nan Rui Ming Audio and Video Equipments Co., Ltd. from 2005 to 2009 and the accounting manager for Yun Nan Bai Feng Real Estate Co., Ltd. from 2000 to 2005. Ms. Li Graduated from Yun Nan University in 1999, majoring in Accounting and Auditing. Ms. Li is a certified Accounting Professional in the PRC, receiving this designation in 2002.
Family Relationships
There are no family relationships between or among any of our directors, executive officers and incoming directors or executive officers.
Involvement in Certain Legal Proceedings
No director, executive officer, significant employee or control person of the Company has been involved in any legal proceeding listed in Item 401(f) of Regulation S-K in the past 10 years.
Audit Committee
During the fiscal year ended March 31, 2011, we did not have a standing audit committee of the Board of Directors, or any committee performing a similar function. Our Board of Directors performed the function of an audit committee.
On June 2, 2011, our Board of Directors established an audit committee in accordance with section 3(a)(58)(A) of the Exchange Act, which currently consists of Jack Zwick, as Chairman of the audit committee, and Gregory D. Tse. Both Jack Zwick and Gregory D. Tse satisfy the independence requirements established by The New York Stock Exchange, Inc., The NASDAQ National Market, and the Securities and Exchange Commission. The Charter of the audit committee of the Board of Directors sets forth the responsibilities of the audit committee. The primary function of the audit committee is to oversee and monitor the Company’s accounting and reporting processes and the audits of the Company’s financial statements. The audit committee did not hold any meetings during the fiscal year ended March 31, 2011 as it was not yet established by the Company’s Board of Directors.
Audit Committee Financial Expert
Our Board of Directors has determined that Jack Zwick is an “audit committee financial expert,” as defined in Item 407(d)(5) of Regulation S-K. As disclosed above, Jack Zwick is “independent,” as defined by requirements set forth by The New York Stock Exchange, Inc., The NASDAQ National Market, and the Securities and Exchange Commission.
Other Board Committees
We do not currently have a standing nominating or compensation committee of the Board of Directors, or any committee performing similar functions. Our Board of Directors performs the functions of nominating and compensation committees.
Director Nominations
As of March 31, 2011, we did not effect any material changes to the procedures by which our shareholders may recommend nominees to our Board of Directors.
55
Compliance with Section 16(a) of the Securities Exchange Act of 1934
Section 16(a) of the Securities Exchange Act requires our executive officers and directors, and persons who own more than 10% of our common stock, to file reports regarding ownership of, and transactions in, our securities with the Securities and Exchange Commission and to provide us with copies of those filings.
Based solely on our review of the copies of such forms received by us, or written representations from certain reporting persons, we believe that, except as set forth below, during fiscal year ended March 31, 2011, our officers, directors and greater than 10% percent beneficial owners complied with all applicable filing requirements, except that Battalion Capital Partners, LLC did not file a Form 3 or a Form 4 as a beneficial owner of greater than 10% of our common stock in connection with either the acquisition and disposition thereof.
Code of Ethics
The Company has adopted a Code of Ethics applicable to all Company directors, officers and employees which is available on our website at: http://www.firstchinapharma.com/
ITEM 11. EXECUTIVE COMPENSATION.
General Philosophy
Our Board of Directors is responsible for establishing and administering the Company’s executive and director compensation.
Executive Compensation
The following summary compensation table indicates the cash and non-cash compensation earned from the Company during the fiscal years ended March 31, 2011 and 2010 by the current and former executive officers of the Company and each of the other two highest paid executives or directors, if any, whose total compensation exceeded $100,000 during those periods.
Summary Compensation Table
|
Name and Principal
Position
|
Year
|
Salary
|
Bonus
|
Stock
Awards
|
Option
Awards
|
Non-Equity
Incentive Plan
Compensation
|
Nonqualified
deferred
compensation
earnings
|
All Other
Compensation
|
Total
|
|||||||||||||||||||||||||
|
Zhen Jiang Wang (1)
|
2011
|
$ | 8,825 | - | - | - | - | - | $ | 18,033,038 | $ | 18,041,863 | ||||||||||||||||||||||
|
Chairman, Chief Executive Officer
|
2010
|
$ | 8,825 | - | - | - | - | - | $ | 12,009,958 | $ | 12,018,783 | ||||||||||||||||||||||
|
Jing Gong
|
2011
|
$ | 8,825 | - | - | - | - | - | - | $ | 8,825 | |||||||||||||||||||||||
|
President
|
2010
|
$ | 8,825 | - | - | - | - | - | - | $ | 8,825 | |||||||||||||||||||||||
|
Yi Jia Li
|
2011
|
$ | 6,000 | - | - | - | - | - | - | $ | 6,000 | |||||||||||||||||||||||
|
Chief Financial Officer
|
2010
|
$ | 6,000 | - | - | - | - | - | - | $ | 6,000 | |||||||||||||||||||||||
|
Yong Kang Chen
|
2011
|
$ | 4,600 | - | - | - | - | - | - | $ | 4,600 | |||||||||||||||||||||||
|
Senior Vice President, Quality Control
|
2010
|
$ | 4,600 | - | - | - | - | - | - | $ | 4,600 | |||||||||||||||||||||||
|
Former Executive Officers
|
||||||||||||||||||||||||||||||||||
|
Roderick Macutay(2)
|
2011
|
$ | - | - | - | - | - | - | - | $ | - | |||||||||||||||||||||||
|
Former Director, President and Treasurer
|
2010
|
$ | - | - | - | - | - | - | - | $ | - | |||||||||||||||||||||||
|
Tina Suava (3)
|
2011
|
$ | - | - | - | - | - | - | - | $ | - | |||||||||||||||||||||||
|
Former Director and Secretary
|
2010
|
$ | - | - | - | - | - | - | - | $ | - | |||||||||||||||||||||||
|
Aidan Hwuang(4)
|
2011
|
$ | - | - | - | - | - | - | - | $ | - | |||||||||||||||||||||||
|
Former Director, President, Chief Financial Officer and Secretary
|
2010
|
$ | - | - | - | - | - | - | - | $ | - | |||||||||||||||||||||||
56
(2) On June 8, 2010, Mr. Macutay resigned as President and Treasurer of the Company. As a condition to closing the Exchange Agreement, effective September 15, 2010, Mr. Macutay resigned from the Company’s Board of Directors.
(3) Ms. Suava resigned as our Secretary and Director on June 8, 2010.
(4) As a condition to closing the Exchange Agreement, effective September 15, 2010, Mr. Hwuang resigned as the Company’s President, Chief Financial Officer and Secretary. Effective February 11, 2011, Mr. Hwuang resigned as a member of the Company’s Board of Directors. The Company agreed to pay Mr. Hwuang US$150,000 pursuant to the terms of that certain Mutual Release and Settlement Agreement, dated February 11, 2011, by and between the Company and Mr. Hwuang.
Other than as noted above, none of our executive officers or directors received, nor do we have any arrangements to pay out, any bonus, stock awards, option awards, non-equity incentive plan compensation, or non-qualified deferred compensation.
Employment Agreements
The Company is party to employment agreements with two of its executive officers, Yong Kang Chen and Yi Jia Li, providing for monthly salaries of RMB2,600 (approximately US$385) and RMB3,400 (approximately US$500), respectively. Each employment agreement commenced on June 1, 2009 and will terminate on May 31, 2012. The employment agreements each provide for the Company to arrange social insurance for the executive officers and the termination by the Company or executive officer upon 30 days notice upon the occurrence of a limited number of circumstances.
Compensation of Directors
Other than the Board Advisory Agreement we have entered into with Jack Zwick, as discussed below, we have no standard arrangement to compensate directors for their services in their capacity as directors. Directors are not paid for meetings attended. However, we intend to review and consider future proposals regarding board compensation. All travel and lodging expenses associated with corporate matters are reimbursed by us, if and when incurred.
The following table sets forth compensation paid to our non-executive directors for the fiscal year ended March 31, 2011.
57
|
Name
|
Fees Earned
or Paid
in Cash
($)
|
Stock
Awards
($)
|
Option
Awards
($)
|
Non-Equity
Incentive Plan
Compensation
($)
|
Nonqualified
Deferred
Compensation
Earnings
($)
|
All
Other
Compensation
($)
|
Total
($)
|
|||||||||||||||||||||
|
Jack Zwick(1)
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||||||
|
Gregory D. Tse
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
$
|
-
|
||||||||||||||
|
(1)
|
Mr. Zwick was appointed May 6, 2011.
|
On May 6, 2011, we entered into a Board Advisory Agreement with Jack Zwick, whereby Mr. Zwick consented to serve as a director of the Company. Pursuant to the Board Advisory Agreement, Mr. Zwick will receive two hundred thousand (200,000) shares of Company common stock, vesting monthly over a period of two years, in connection with his service as a director and a consulting fee of $2,000 per month.
Stock Option Plans - Outstanding Equity Awards at Fiscal Year End
None.
Pension Table
None.
Retirement Plans
We do not offer any annuity, pension, or retirement benefits to be paid to any of our officers, directors, or employees in the event of retirement. There are also no compensatory plans or arrangements with respect to any individual named above which results or will result from the resignation, retirement, or any other termination of employment with our company, or from a change in the control of our Company.
Compensation Committee
The Company does not have a separate Compensation Committee. Instead, the Company’s Board of Directors reviews and approves executive compensation policies and practices, reviews salaries and bonuses for other officers, administers the Company’s stock option plans and other benefit plans, if any, and considers other matters.
Risk Management Considerations
We believe that our compensation policies and practices for our employees, including our executive officers, do not create risks that are reasonably likely to have a material adverse effect on our Company.
|
ITEM 12.
|
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS.
|
Security Ownership
The following table sets forth certain information as of June 27, 2011, with respect to the beneficial ownership of our common stock for (i) each director and officer, (ii) all of our directors and officers as a group, and (iii) each person known to us to own beneficially five percent (5%) or more of the outstanding shares of our common stock. As of June 27, there were 59,664,480 shares of common stock outstanding.
58
To our knowledge, except as indicated in the footnotes to this table or pursuant to applicable community property laws, the persons named in the table have sole voting and investment power with respect to the shares of common stock indicated.
|
Name and Address of
Beneficial Owner(1)
|
Shares Beneficially Owned
|
Percentage
Beneficially Owned
|
||||||
|
Directors and Executive Officers
|
||||||||
|
Zhen Jiang Wang
Chairman and Chief Executive Officer
People West Road 504, Room 819
West Ren Min Road
Kunming
Yunnan Province
PRC
|
15,000,000 | 25.1 | % | |||||
|
Jing Gong
President
People West Road 504, Room 819
West Ren Min Road
Kunming
Yunnan Province
PRC
|
― | ― | ||||||
|
Yong Kang Chen
Senior Vice President, Quality Control
West Circle Road 291
Block B, 2403
Wuhua District
Kunming
Yunnan Province
PRC
|
― | ― | ||||||
|
Yi Jai Li
Chief Financial Officer
News Road No. 447
Kunming
Yunnan Province
PRC
|
― | ― | ||||||
|
Gregory D. Tse
Director
1155 Yu Yuan Road
Building 4, Suite 103
Shanghai, PRC 200050
|
― | ― | ||||||
|
Jack Zwick(2)
Director
Zwick & Banyai, PLLC
20750 Civic Center Drive
Suite 418
Southfield, MI 48075
|
100,001 | * | ||||||
|
All Officers and Directors as a Group
|
15,100,001 | 25.3 | % | |||||
|
5% Stockholders
|
||||||||
|
None.
|
||||||||
59
* Less than 1%.
|
(1)
|
Beneficial ownership has been determined in accordance with Rule 13d-3 under the Exchange Act. Pursuant to the rules of the SEC, shares of common stock which an individual or group has a right to acquire within 60 days pursuant to the exercise of options or warrants are deemed to be outstanding for the purpose of computing the percentage ownership of such individual or group, but are not deemed to be beneficially owned and outstanding for the purpose of computing the percentage ownership of any other person shown in the table.
|
|
(2)
|
Mr. Zwick has entered into that certain Board Advisory Agreement dated May 6, 2011 pursuant to which he has been granted 200,000 shares of the Company’s common stock subject to certain vesting and forfeiture restrictions. As of June 27, 2011, 16,667 shares had vested. Mr. Zwick is also the beneficial owner of 27,778 shares of our common stock and a right to 27,778 shares underlying Series A-1 warrants to purchase our common stock, and 27,778 shares underlying Series A-2 warrants to purchase our common stock.
|
Securities Authorized for Issuance Under Equity Compensation Plans
None.
Non-Cumulative Voting
The holders of our shares of common stock do not have cumulative voting rights, which means that the holders of more than 50% of such outstanding shares, voting for the election of Directors, can elect all of the Directors to be elected, if they so choose. In such event, the holders of the remaining shares will not be able to elect any of our Directors.
Transfer Agent
Our transfer agent is Routh Stock Transfer Inc., and is located at 6860 N. Dallas Parkway, Suite 200, Plano, Texas, 75024. Their telephone number is (972) 381-2782.
|
ITEM 13.
|
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE.
|
Related Party Transactions
As of September 30, 2010, FCPG HK purchased from its former shareholder, an amount of $5,995,513 of the inventory which had all been sold out by FCPG HK before March 31, 2011.
We are party to employment agreements with two of our executive officers, Yong Kang Chen and Yi Jia Li, providing for monthly salaries of RMB2,600 (approximately US$385) and RMB3,400 (approximately US$500), respectively. Each employment agreement commenced on June 1, 2009 and will terminate on May 31, 2012. The employment agreements each provide for the Company to arrange social insurance for the executive officers and the termination by the Company or executive officer upon 30 days notice upon the occurrence of a limited number of circumstances.
60
As noted above, XYT, our subsidiary, entered into an Authorization Agreement, dated July 12, 2006, with Mr. Wang, our Chairman and Chief Executive Officer. In accordance with the Authorization Agreement, XYT had authorized Mr. Wang to collect receivables of XYT prior to or concurrent with product delivery for certain customers with whom he has existing relationships. The amount of such receivables collected by Mr. Wang totaled US$5,945,625, US$17,270,218, and US$26,363,421 during 2007, 2008, and 2009, respectively. Mr. Wang also paid certain payables (including purchased inventories from suppliers, expenses, such as commissions, notes payables, software customization, installation of the internet fulfillment system, computer equipment and even vehicles to provide expedited delivery of purchased goods) with funds from the receivables he previously collected on behalf of the Company. We believe such an arrangement is common in the PRC, and it allowed both us and Mr. Wang to maintain favorable relationships with customers and suppliers due to prompt payments and frequent communication, to reduce certain transaction costs, to obtain favorable prices and discounts due to Mr. Wang’s prompt settlement of certain supplier accounts, and to reduce certain liquidity risks due to daily limits and other restrictions on bank withdrawals from business accounts as the funds collected by Mr. Wang on behalf of the Company provide an additional liquidity option for the Company when needed. In order to ensure that a company retains sufficient registered capital to support itself, there are limits on the funds that can be paid out each day. For a company with the registered capital of XYT, the daily limit on funds that may be liquidated (paid out) is RMB500,000 or $77,000 USD per day. By Mr. Wang providing funds for the settlement of payables, the Company was able to work around this liquidity restriction without having to increase its registered capital. This arrangement was informal in nature and on an as-needed basis voluntarily by Mr. Wang. When the Company was required to make daily payments in excess of RMB500,000 or US$77,000, Mr. Wang would pay such excess amounts personally to ensure the continuous efficient flow of payments. With a company that was approaching $25 million in annual sales, Mr. Wang was called upon a couple of times per week to top up payments that were in excess RMB500,000 or US$77,000 per day.
However, prior to the closing of the Exchange Transaction, the Company and Mr. Wang recognized that continuation of this arrangement, while appropriate and effective for XYT as a private, closely held company, should not continue as XYT became a subsidiary of a publicly traded company with multiple stockholders that would be subject to SEC and U.S. GAAP compliance. Therefore, as part of its evolution into a public company, the Company and Mr. Wang had agreed that after the Exchange Transaction, Mr. Wang should discontinue making payments and collections on behalf of XYT in order to standardize its business operations and set up a structure of effective internal controls as a public company. In fact, Mr. Wang intended to cease collecting receivables in June 2010, however, customers continued to make payments to Mr. Wang despite instructions from Mr. Wang and the Company to the contrary. While the weaning process had begun, it continued for some time for fear of losing key customers, and Mr. Wang only managed to convince all key customers and completely cease the practice of such collections on behalf of XYT as of October 31, 2011. The amounts due from Mr. Wang to us had previously been treated as a loan receivable from a related party to us and amounted to $11,799,953 as of December 31, 2009 and $2,498,924 as of December 31, 2008. To comply with U.S. GAAP, we have reclassified such amounts as a deemed distribution of a dividend to Mr. Wang, a related party, and therefore a corresponding reduction of retained earnings. Mr. Wang has not repaid any amount of the distribution.
Further, the Company has entered into a Bonus Payment Agreement with Mr. Wang, our Chairman and Chief Executive Officer, dated October 7, 2010 (“Bonus Agreement”), whereby we have agreed to pay Mr. Wang a bonus payment of two percent (2%) of the quarterly gross sales of XYT, as calculated and disclosed in our consolidated financial statements filed with our Form 10-K and Form 10-Q filings. Such bonus payments shall be paid within fifteen (15) days after such respective filings are made, shall not exceed an aggregate of $1,500,000 per fiscal year, and Mr. Wang can reduce or modify, in his sole discretion, the amount due to him as long as such bonus payments do not exceed two percent (2%) of the quarterly gross sales of XYT. Mr. Wang subsequently amended the Bonus Agreement in a letter to us dated October 8, 2010 (“Amendment to Bonus Agreement”), whereby Mr. Wang agreed to waive any and all right to receive any bonus payment for the Company’s fiscal year ended March 31, 2011 noting that no amounts due to Mr. Wang for the fiscal year ended March 31, 2011 would be carried forward.
As discussed above, on September 15, 2010, we closed the Exchange Transaction, by and among FCPG, FCPG HK, and XYT, the wholly owned subsidiary of FCPG HK. Our Chairman and Chief Executive Officer, Mr. Wang, was the sole stockholder of FCPG HK. As a result of the Exchange Transaction, we issued a total of 15,000,000 shares of our common stock to Mr. Wang in exchange for 100% of the capital stock of FCPG HK and Mr. Wang was appointed our Chairman and Chief Executive Officer.
On May 6, 2011, we entered into a Board Advisory Agreement with Jack Zwick, whereby Mr. Zwick consented to serve as a director of the Company. Pursuant to the Board Advisory Agreement, Mr. Zwick will receive two hundred thousand (200,000) shares of Company common stock, vesting monthly over a period of two years, in connection with his service as a director and a consulting fee of $2,000 per month.
61
Review, Approval or Ratification of Transactions with Related Persons
Although we have adopted a Code of Ethics, we still rely on our Board to review related party transactions on an ongoing basis to prevent conflicts of interest. Our Board reviews a transaction in light of the affiliations of the director, officer or employee and the affiliations of such person’s immediate family. Transactions are presented to our Board for approval before they are entered into or, if this is not possible, for ratification after the transaction has occurred. If our Board finds that a conflict of interest exists, then it will determine the appropriate remedial action, if any. Our Board approves or ratifies a transaction if it determines that the transaction is consistent with the best interests of the Company.
Director Independence
During the fiscal year ended March 31, 2011, we had one independent director on our board, Gregory D. Tse. As of the date hereof, we believe that Jack Zwick can also be classified as an independent director. We evaluate independence by the standards for director independence established by applicable laws, rules, and listing standards including, without limitation, the standards for independent directors established by The New York Stock Exchange, Inc., the NASDAQ National Market, and the Securities and Exchange Commission.
Subject to some exceptions, these standards generally provide that a director will not be independent if (a) the director is, or in the past three years has been, an employee of ours; (b) a member of the director’s immediate family is, or in the past three years has been, an executive officer of ours; (c) the director or a member of the director’s immediate family has received more than $120,000 per year in direct compensation from us other than for service as a director (or for a family member, as a non-executive employee); (d) the director or a member of the director’s immediate family is, or in the past three years has been, employed in a professional capacity by our independent public accountants, or has worked for such firm in any capacity on our audit; (e) the director or a member of the director’s immediate family is, or in the past three years has been, employed as an executive officer of a company where one of our executive officers serves on the compensation committee; or (f) the director or a member of the director’s immediate family is an executive officer of a company that makes payments to, or receives payments from, us in an amount which, in any twelve-month period during the past three years, exceeds the greater of $1,000,000 or two percent of that other company’s consolidated gross revenues.
|
ITEM 14.
|
PRINCIPAL ACCOUNTING FEES AND SERVICES.
|
The following table shows the fees paid or accrued by us for the audit and other services provided by Parker Randall CF (H.K.) CPA Limited for the fiscal periods shown.
|
March 31,
2011
|
March 31,
2010
|
|||||||
|
Audit Fees
|
$
|
82,000
|
$
|
6,500
|
||||
|
Audit Related Fees
|
—
|
1,500
|
||||||
|
Tax Fees
|
—
|
—
|
||||||
|
All Other Fees
|
—
|
—
|
||||||
|
Total
|
$
|
82,000
|
$
|
8,000
|
||||
Audit fees consist of fees billed for professional services rendered for the audit of our financial statements and review of the interim financial statements included in quarterly reports and services that are normally provided by the above auditors in connection with statutory and regulatory fillings or engagements
In the absence of a formal audit committee, the full Board of Directors pre-approves all audit and non-audit services to be performed by the independent registered public accounting firm in accordance with the rules and regulations promulgated under the Securities Exchange Act of 1934, as amended. The Board of Directors pre-approved 100% of the audit, audit-related and tax services performed by the independent registered public accounting firm for the fiscal year ended March 31, 2011. The percentage of hours expended on the principal accountant’s engagement to audit the Company’s financial statements for the most recent fiscal year that were attributed to work performed by persons other than the principal accountant’s full-time, permanent employees was 0%.
62
PART IV
ITEM 15. EXHIBITS AND FINANCIAL STATEMENT SCHEDULES.
(a) Financial Statements:
|
Page
|
||
|
Report of Independent Registered Accounting Firm
|
F-2
|
|
|
Consolidated Balance Sheets as of March 31, 2011 and 2010
|
F-3
|
|
|
Consolidated Statements of Income and Comprehensive Income for the Years Ended March 31, 2011 and 2010
|
F-5
|
|
|
Consolidated Statements of Shareholders’ Equity for the Years Ended March 31, 2011 and 2010
|
F-7
|
|
|
Consolidated Statements of Cash Flow for the Years Ended March 31, 2011 and 2010
|
F-9
|
|
|
Notes to Consolidated Financial Statements
|
|
F-11-F-35
|
(b) Exhibits:
|
Number
|
Description
|
|
|
2.1
|
Share Exchange Agreement, dated August 23, 2010 (incorporated by reference to Exhibit 2.1 of the Registrant’s Current Report on Form 8-K filed on August 24, 2010)
|
|
|
3.1
|
Articles of Incorporation of the Registrant, dated July 31, 2007, including all amendments to date (incorporated by reference to Exhibit 3.1 of the Registrant’s Current Report on Form 8-K filed September 21, 2010)
|
|
|
3.2
|
Amended and Restated Bylaws of the Registrant, as amended, dated June 1, 2010 (incorporated by reference to Exhibit 3.2 of the Registrant’s Current Report on Form 8-K filed on June 30, 2010)
|
|
|
4.1
|
Form of Stock Specimen Certificate (incorporated by reference to Exhibit 4.1 of the Registrant’s Registration Statement on Form S-1 filed on May 28, 2008)
|
|
|
10.1
|
Labour Contract, commencing June 1, 2009, by and between Yun Nan Xin Yuan Tang Pharmacies Co. Ltd. and Yong Kang Chen (incorporated by reference to Exhibit 10.2 of the Registrant’s Current Report on Form 8-K filed September 21, 2010)
|
|
|
10.2
|
Labour Contract, commencing June 1, 2009, by and between Yun Nan Xin Yuan Tang Pharmacies Co. Ltd. and Yi Jia Li (incorporated by reference to Exhibit 10.3 of the Registrant’s Current Report on Form 8-K filed September 21, 2010)
|
|
|
10.3
|
Lease Agreement, commencing April 1, 2005, by and between Kun Ming Xin Yuan Tang Pharmaceuticals Co., Ltd. and No. 1 Residents Group of Liang Yuan Community (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K filed September 21, 2010)
|
|
|
10.4
|
Convertible Promissory Note of the Company, dated October 3, 2010, to Sierra Growth Inc., as amended and assigned to Caledonia Partners LLC (incorporated by reference to Exhibit 10.1 of the Registrant’s Quarterly Report on Form 10-Q filed February 22, 2011)
|
|
|
10.4(a)
|
Amendment to the Convertible Promissory Note Dated October 3, 2010, dated October 4, 2010 (incorporated by reference to Exhibit 10.1(a) of the Registrant’s Quarterly Report on Form 10-Q filed February 22, 2011)
|
|
|
10.4(b)
|
Amendment to the Convertible Promissory Note Dated October 3, 2010, dated January 21, 2011 (incorporated by reference to Exhibit 10.1(b) of the Registrant’s Quarterly Report on Form 10-Q filed February 22, 2011)
|
63
|
10.5
|
Bonus Payment Agreement, dated October 7, 2010, by and between the Company and Mr. Zhen Jiang Wang (incorporated by reference to Exhibit 10.3 of the Registrant’s Quarterly Report on Form 10-Q filed February 22, 2011)
|
|
|
10.5(a)
|
Amendment to Bonus Payment Agreement, dated October 8, 2010 (incorporated by reference to Exhibit 10.3(a) of the Registrant’s Quarterly Report on Form 10-Q filed February 22, 2011)
|
|
|
10.6
|
Authorization Agreement, dated as of July 12, 2006, between XYT and Mr. Zhen Jiang Wang (incorporated by reference to Exhibit 10.4 of the Registrant’s Current Report on Form 8-K filed on January 19, 2011)
|
|
|
10.7
|
Mutual Release and Settlement Agreement, dated February 11, 2011, by and between the Company and Aidan Hwuang (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K filed on January 19, 2011)
|
|
|
10.8
|
Convertible Promissory Note of the Company, dated December 22, 2010, to Caledonia Partners LLC (incorporated by reference to Exhibit 10.2 of the Registrant’s Quarterly Report on Form 10-Q filed February 17, 2011)
|
|
|
10.9
|
Form of Securities Purchase Agreement (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K filed March 23, 2011)
|
|
|
10.10
|
Form of Series A-1 Warrant (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K filed April 6, 2011)
|
|
|
10.11
|
Form of Series A-2 Warrant (incorporated by reference to Exhibit 10.2 of the Registrant’s Current Report on Form 8-K filed April 6, 2011)
|
|
|
10.12
|
Authorization Agreement, dated as of July 12, 2006, between XYT and Mr. Zhen Jiang Wang (incorporated by reference to Exhibit 10.4 of the Registrant’s Current Report on Form 8-K filed on January 19, 2011)
|
|
|
10.13
|
Board Advisory Agreement with Jack Zwick, dated May 6, 2011 (incorporated by reference to Exhibit 10.1 of the Registrant’s Current Report on Form 8-K filed on May 10, 2011)
|
|
|
16.1
|
Letter from Moore and Associates, Chartered, dated August 7, 2009 (incorporated by reference to Exhibit 16.1 of the Registrant’s Current Report on Form 8-K filed on August 7, 2009)
|
|
|
16.2
|
Letter from Moore and Associates, Chartered, dated September 1, 2009 (incorporated by reference to Exhibit 16.1 of the Registrant’s Current Report on Form 8-K/A filed on September 2, 2009)
|
|
|
16.3
|
Letter from Seale and Beers, CPAs, dated October 19, 2009 (incorporated by reference to Exhibit 16.1 of the Registrant’s Current Report on Form 8-K filed on October 23, 2009)
|
|
|
16.4
|
Letter from Li & Company, PC, dated August 25, 2010 (incorporated by reference to Exhibit 16.1 of the Registrant’s Current Report on Form 8-K filed on August 26, 2010)
|
|
|
21
|
First China Pharmaceutical Group Limited, a Hong Kong company; Kun Ming Xin Yuan Tang Pharmacies Co. Ltd., a company organized under the laws of the People’s Republic of China
|
|
|
31.1
|
Certification of Chief Executive Officer pursuant to Section 302 of the Sarbanes Oxley Act of 2002*
|
|
|
31.2
|
Certification of Chief Financial Officer pursuant to Section 302 of the Sarbanes Oxley Act of 2002*
|
64
|
32.1
|
Certification of Chief Executive Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
|
|
32.2
|
Certification of Chief Financial Officer pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002*
|
|
|
99.1
|
License of Internet Drug Information Service (incorporated by reference to Exhibit 99.3 of the Registrant’s Current Report on Form 8-K/A filed on December 6, 2010)
|
|
|
99.2
|
|
Enterprise Legal Person Business License of XYT (incorporated by reference to Exhibit 99.3 of the Registrant’s Current Report on Form 8-K/A filed on January 19, 2011)
|
*Filed Herewith
65
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
FIRST CHINA PHARMACEUTICAL GROUP, INC.
|
||
|
(Registrant)
|
||
|
Date: December 2, 2011
|
By:
|
/s/ Zhen Jiang Wang
|
|
Zhen Jiang Wang
|
||
|
Chief Executive Officer (Principal
|
||
|
Executive Officer)
|
||
|
Date: December 2, 2011
|
By:
|
/s/ Yi Jia Li
|
|
Yi Jia Li
|
||
|
Chief Financial Officer (Principal
|
||
|
Financial Officer and Principal
|
||
|
Accounting Officer)
|
||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the registrant and in the capacities and on the dates indicated.
|
Signatures
|
Title(s)
|
Date
|
||
|
/s/ Zhen Jiang Wang
|
Chief Executive
|
|||
|
Zhen Jiang Wang
|
Officer and Director
|
December 2, 2011
|
||
|
(Principal Executive Officer)
|
||||
|
/s/ Yi Jia Li
|
Chief Financial Officer
|
December 2, 2011
|
||
|
Yi Jia Li
|
(Principal Financial Officer and
|
|||
|
Principal Accounting Officer)
|
||||
|
/s/ Jack Zwick
|
Director
|
December 2, 2011
|
||
|
Jack Zwick
|
||||
|
/s/ Gregory D. Tse
|
Director
|
December 2, 2011
|
||
|
Gregory D. Tse
|
66
FIRST CHINA PHARMACEUTICAL GROUP, INC.
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
|
Page
|
||
|
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
|
F-2
|
|
|
CONSOLIDATED BALANCE SHEET
|
F-3
|
|
|
CONSOLIDATED STATEMENT OF INCOME
|
F-5
|
|
|
CONSOLIDATED STATEMENT OF STOCKHOLDERS’ EQUITY
|
F-7
|
|
|
CONSOLIDATED STATEMENT OF CASH FLOWS
|
F-9
|
|
|
NOTES TO THE CONSOLIDATED FINANCIAL STATEMENTS
|
|
F-11- F-35
|
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Equity Holders of
First China Pharmaceutical Group, Inc.
We have audited the accompanying consolidated balance sheets of First China Pharmaceutical Group, Inc. (“the Company”) as of March 31, 2011 and 2010, and the related consolidated statements of income and comprehensive income, stockholders’ equity and cash flows for the years ended March 31, 2011 and 2010. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of March 31, 2011 and 2010, and the results of its operations and its cash flows for the years ended March 31, 2011 and 2010 in conformity with accounting principles generally accepted in the United States of America.
As discussed in Note 3 to the financial statements, the Company has restated the consolidated financial statements for the year ended March 31, 2011.
/s/ Parker Randall CF
Parker Randall CF (H.K.) CPA Limited
Certified Public Accountant
Hong Kong
June 27, 2011 except for Note (15) to the Consolidated Financial Statements as at July 12, 2011 and except for Note 3 on September 15, 2011.
F-2
FIRST CHINA PHARMACEUTICAL GROUP, INC.
CONSOLIDATED BALANCE SHEETS AS OF THE END OF MARCH 31, 2011 AND 2010
(Stated in US Dollars)
|
As of
|
As of
|
||||||||||
|
March 31,
|
March 31,
|
||||||||||
|
Notes
|
2011
|
2010
|
|||||||||
|
Restated
|
Restated
|
||||||||||
| $ | $ | ||||||||||
|
ASSETS
|
|||||||||||
|
Current Assets
|
|||||||||||
|
Cash and cash equivalents
|
740,368 | 158,766 | |||||||||
|
Restricted cash
|
7 | 1,052,135 | 682,514 | ||||||||
|
Other receivables
|
2,143,982 | - | |||||||||
|
Inventories
|
890,185 | 4,671,322 | |||||||||
|
Account receivables
|
8,881,014 | - | |||||||||
|
Total Current Assets
|
13,707,684 | 5,512,602 | |||||||||
|
Non-current Assets
|
|||||||||||
|
Due from a related party
|
3 | - | - | ||||||||
|
Plant and equipment, net
|
4 | 5,139 | 848 | ||||||||
|
Intangible assets, net
|
5 | 2,052 | 2,798 | ||||||||
|
Total Non-current Assets
|
7,191 | 3,646 | |||||||||
|
Total Assets
|
13,714,875 | 5,516,248 | |||||||||
F-3
FIRST CHINA PHARMACEUTICAL GROUP, INC.
CONSOLIDATED BALANCE SHEETS AS OF THE END OF MARCH 31, 2011 AND 2010
(Stated in US Dollars)
|
As of
|
As of
|
|||||||||
|
March 31,
|
March 31,
|
|||||||||
|
Notes
|
2011
|
2010
|
||||||||
|
Restated
|
Restated
|
|||||||||
| $ | $ | |||||||||
|
LIABILITIES AND STOCKHOLDERS’ EQUITY
|
||||||||||
|
Current Liabilities
|
||||||||||
|
Short-term borrowings
|
772,010 | 732,461 | ||||||||
|
Other payable and accrued liabilities
|
6 | 12,819,702 | 8,919,168 | |||||||
|
Due to a related party
|
3 | 12,752 | - | |||||||
|
Notes payable
|
7 | 2,630,336 | - | |||||||
|
Income tax payable
|
3,191,477 | 2,021,627 | ||||||||
|
Total Current Liabilities
|
19,426,277 | 11,673,256 | ||||||||
|
Non-current Liabilities
|
||||||||||
|
Convertible Promissory Notes
|
8 | 886,386 | - | |||||||
|
Total Non-current Liabilities
|
886,386 | - | ||||||||
|
Total Liabilities
|
20,312,663 | 11,673,256 | ||||||||
|
STOCKHOLDERS’ EQUITY
|
||||||||||
|
Common stock
|
||||||||||
|
Common stock, $0.001 par value; 200,000,000 shares authorized; 58,264,471 shares issued and outstanding as of March 31, 2011; and 45,000,000 shares issued and outstanding as of March 31, 2010 respectively.
|
9 | 58,264 | 45,000 | |||||||
|
Treasury stock
|
10 | 5,000 | - | |||||||
|
Additional paid-in capital
|
9 | 3,249,067 | 221,101 | |||||||
|
Retained earnings
|
3 | (10,229,389 | ) | (6,539,988 | ) | |||||
|
Accumulated other comprehensive income - foreign currency translation adjustments
|
319,270 | 116,879 | ||||||||
|
Total Stockholders’ Equity
|
(6,597,788 | ) | (6,157,008 | ) | ||||||
|
Total Liabilities and Stockholders’ Equity
|
13,714,875 | 5,516,248 | ||||||||
See accompanying notes to the financial statements
F-4
FIRST CHINA PHARMACEUTICAL GROUP INC.
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
FOR THE YEAR ENDED MARCH 31, 2011 AND 2010
(Stated in US Dollars)
|
Year ended
|
Year ended
|
||||||||||
|
March 31,
|
March 31,
|
||||||||||
|
Notes
|
2011
|
2010
|
|||||||||
| $ | $ | ||||||||||
|
Net sales
|
28,999,198 | 25,723,902 | |||||||||
|
Costs of sales
|
(23,749,000 | ) | (21,045,598 | ) | |||||||
|
Gross Profit
|
5,250,198 | 4,678,304 | |||||||||
|
Selling expenses
|
(645,396 | ) | (790,612 | ) | |||||||
|
Administrative expenses
|
(1,382,276 | ) | (168,053 | ) | |||||||
|
Income from Operation
|
3,222,526 | 3,719,639 | |||||||||
|
Other income/(expenses)
|
251,959 | (23,870 | ) | ||||||||
|
Interest income
|
8,922 | 12,362 | |||||||||
|
Interest expense
|
(64,350 | ) | (11,209 | ) | |||||||
|
Income before Tax
|
3,419,057 | 3,696,922 | |||||||||
|
Income tax
|
11 | (1,085,378 | ) | (934,463 | ) | ||||||
|
Net Income
|
2,333,679 | 2,762,459 | |||||||||
|
Other Comprehensive Income
|
|||||||||||
|
Foreign currency translation adjustments
|
202,391 | 21,037 | |||||||||
|
Total Comprehensive Income
|
2,536,070 | 2,783,496 | |||||||||
F-5
FIRST CHINA PHARMACEUTICAL GROUP INC.
CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
FOR THE YEAR ENDED MARCH 31, 2011 AND 2010
(Stated in US Dollars)
|
Year ended
|
Year ended
|
||||||||||
|
March 31,
|
March 31,
|
||||||||||
|
Notes
|
2011
|
2010
|
|||||||||
| $ | $ | ||||||||||
|
Basic Earnings per Common Share
|
14 | ||||||||||
|
Weight average number of common shares outstanding
|
51,249,632 | 45,000,000 | |||||||||
|
Earnings per share - Basic
|
0.0495 | 0.0619 | |||||||||
|
Diluted earnings per common share
|
14 | ||||||||||
|
Adjusted weighted average number of shares
|
51,392,732 | 45,000,000 | |||||||||
|
Earnings per share - Diluted
|
0.0493 | 0.0619 | |||||||||
See accompanying notes to the financial statements
F-6
FIRST CHINA PHARMACEUTICAL GROUP INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
FOR THE YEAR ENDED MARCH 31, 2011 AND 2010
(Stated in US Dollars) (Restated)
|
Common stock
|
Additional
|
Other comprehensive
income
|
|||||||||||||||||||||||||||||
|
Note
|
Shares
|
Amount
|
Treasury
Shares
|
paid-in
capital
|
Retained
Earnings
|
Foreign currency
transaction adjustment
|
Total
|
||||||||||||||||||||||||
|
As restated
|
|||||||||||||||||||||||||||||||
|
(Note 3)
|
|||||||||||||||||||||||||||||||
| $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||
|
Balance as of March 31, 2009
|
45,000,000 | 45,000 | - | 221,101 | 2,707,511 | 95,842 | 3,069,454 | ||||||||||||||||||||||||
|
Net income
|
- | - | - | - | 2,762,459 | - | 2,762,459 | ||||||||||||||||||||||||
|
Foreign currency translation Adjustments
|
- | - | - | - | - | 21,037 | 21,037 | ||||||||||||||||||||||||
|
Deemed distribution of dividend
|
3 | - | - | - | - | (12,009,958 | ) | - | (12,009,958 | ) | |||||||||||||||||||||
|
Balance as of March 31, 2010
|
45,000,000 | 45,000 | - | 221,101 | (6,539,988 | ) | 116,879 | (6,157,008 | ) | ||||||||||||||||||||||
F-7
FIRST CHINA PHARMACEUTICAL GROUP INC.
CONSOLIDATED STATEMENTS OF SHAREHOLDERS’ EQUITY
FOR THE YEAR ENDED MARCH 31, 2011 AND 2010
(Stated in US Dollars)
|
Other comprehensive income
|
|||||||||||||||||||||||||||||||
|
Common stock
|
Additional
|
Foreign
Currency
|
|||||||||||||||||||||||||||||
|
Note
|
Shares
|
Amount
|
Treasury
Shares
|
paid-in
capital
|
Retained
Earnings
|
transaction adjustment
|
Total
|
||||||||||||||||||||||||
|
As restated
|
|||||||||||||||||||||||||||||||
|
(Note 3)
|
|||||||||||||||||||||||||||||||
| $ | $ | $ | $ | $ | $ | ||||||||||||||||||||||||||
|
Balance as of March 31, 2010
|
45,000,000 | 45,000 | - | 221,101 | (6,539,988 | ) | 116,879 | (6,157,008 | ) | ||||||||||||||||||||||
|
Reorganization of FCPG HK and XYT on reverse acquisition
|
9 | 15,000,000 | 15,000 | - | (298,431 | ) | - | - | (283,431 | ) | |||||||||||||||||||||
|
Cancellation of common stock of the Company
|
10 | (5,000,000 | ) | (5,000 | ) | 5,000 | - | - | - | - | |||||||||||||||||||||
|
Equity component of issued convertible notes
|
8 | - | - | - | 113,747 | - | - | 113,747 | |||||||||||||||||||||||
|
Issuance of private offering units
|
9 | 3,166,692 | 3,167 | - | 2,846,833 | - | - | 2,850,000 | |||||||||||||||||||||||
|
Share-based payment for service on private offering units
|
9 | 97,779 | 97 | 365,817 | - | - | 365,914 | ||||||||||||||||||||||||
|
Net Income
|
- | - | - | - | 2,333,679 | - | (2,333,679 | ) | |||||||||||||||||||||||
|
Deemed distribution of dividend
|
3 | - | - | - | - | (6,023,080 | ) | - | (6,023,080 | ) | |||||||||||||||||||||
|
Foreign currency translation Adjustments
|
- | - | - | - | - | 202,391 | 202,391 | ||||||||||||||||||||||||
|
Balance as of March 31, 2011
|
58,264,471 | 58,264 | 5,000 | 3,249,067 | (10,229,389 | ) | 319,270 | (6,597,788 | ) | ||||||||||||||||||||||
See accompanying notes to the financial statements
F-8
FIRST CHINA PHARMACEUTICAL GROUP INCORPORATION
CONSOLIDATED STATEMENT OF CASH FLOWS
FOR THE YEAR ENDED MARCH 31, 2011 AND 2010
(Stated in US Dollars)
|
Year Ended March 31
|
||||||||
|
2011
|
2010
|
|||||||
| $ | $ | |||||||
|
Cash Flows from Operating Activities
|
||||||||
|
Net income
|
2,333,679 | 2,762,459 | ||||||
|
Adjustments to reconcile net income to net cash provided by operating activities:
|
||||||||
|
Depreciation and amortization
|
4,010 | 7,687 | ||||||
|
Interest expense on convertible notes
|
5,132 | - | ||||||
|
Shared based payment for placement agent
|
365,914 | - | ||||||
|
Changes in operating assets and liabilities:
|
||||||||
|
Due from a related party
|
(5,399,802 | ) | (6,408,267 | ) | ||||
|
Other receivable
|
(39,157 | ) | - | |||||
|
Account Receivables
|
(8,868,241 | ) | - | |||||
|
Inventories
|
3,880,840 | 867,297 | ||||||
|
Other receivables
|
(2,100,872 | ) | - | |||||
|
Other payable and accrued liabilities
|
3,199,575 | 112,342 | ||||||
|
Notes payable
|
2,584,457 | - | ||||||
|
Income tax payable
|
1,085,379 | 2,016,860 | ||||||
|
Net cash used in operating activities
|
(2,949,086 | ) | (641,622 | ) | ||||
|
Cash Flows from Investing Activities
|
||||||||
|
Additions in plant and equipment
|
(3,731 | ) | (2,885 | ) | ||||
|
Acquisition of a subsidiary
|
(293,428 | ) | - | |||||
|
Cash contributed by the legal acquirer
|
7,331 | - | ||||||
|
Net cash used in investing activities
|
(289,828 | ) | (2,885 | ) | ||||
|
Cash flows from financing activities
|
||||||||
|
Capital contributions from stockholders
|
1,282 | - | ||||||
|
Due to a related party
|
1,240 | - | ||||||
|
New borrowings raised
|
1,189,114 | 730,734 | ||||||
|
Repayment of borrowings
|
(881,907 | ) | - | |||||
|
Net proceed from convertible notes
|
995,000 | - | ||||||
|
Net proceed from private offering units
|
2,850,000 | - | ||||||
|
Decrease (increase) in restricted cash
|
(338,970 | ) | (680,905 | ) | ||||
|
Net cash provided by financing activities
|
3,815,759 | 49,829 | ||||||
F-9
FIRST CHINA PHARMACEUTICAL GROUP INCORPORATION
CONSOLIDATED STATEMENT OF CASH FLOWS
FOR THE YEAR ENDED MARCH 31, 2011 AND 2010
(Stated in US Dollars)
|
Year Ended March 31
|
||||||||
|
2011
|
2010
|
|||||||
| $ | $ | |||||||
|
Effect of foreign currency translation on cash and cash Equivalents
|
4,757 | 2,147 | ||||||
|
Net increase/(decrease) in cash and cash equivalents
|
581,602 | (592,531 | ) | |||||
|
Cash and cash equivalents - beginning of period
|
158,766 | 751,297 | ||||||
|
Cash and cash equivalents at the end of period
|
740,368 | 158,766 | ||||||
|
Supplemental disclosures for cash flow information:
|
||||||||
|
Cash paid for:
|
||||||||
|
Interest
|
45,559 | 11,209 | ||||||
See accompanying notes to the financial statements
F-10
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
1.
|
ORGANIZATION AND PRINCIPAL ACTIVITIES
|
First China Pharmaceutical Group, Inc., (the “Company”), incorporated in Nevada as of July 31, 2007, is a public reporting “shell company”, as defined in Rule 12b-2 of the securities Exchange Act of 1934, as amended. As of May 14, 2010, the Company amended its Articles of Incorporation to change its name from “E-Dispatch Inc.” to “First China Pharmaceutical Group, Inc.”.
First China Pharmaceutical Group limited (the “FCPG HK”) was incorporated in Hong Kong as of April 29, 2010 under the Companies Ordinance of Hong Kong. As of September 15, 2010, the Company issued 15,000,000 shares in exchange for 100% of the issued and outstanding common stock of FCPG HK. The newly issued shares represented 25% of the Company’s issued and outstanding common stock.
Kun Ming Xin Yuan Tang Pharmacies Co. Ltd. (“XYT”) was established under the laws of the People’s Republic of China (“PRC”) as of November 12, 2002 with a paid-in capital of RMB 2,000,000 as of December 31, 2010. As of June 25, 2010, FCPG HK acquired XYT, of which became FCPG HK’s wholly owned subsidiary.
The Company and the Subsidiaries (collectively the “Group”) are principally engaged in drug logistics and distribution in Yunnan Province, China through drug stores, medical clinics and hospitals.
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
|
|
(a)
|
Basis of Preparation and Presentation
|
As of September 15, 2010, the Company acquired FCPG HK and XYT. The Company was a shell company and its accounting acquirer is FCPG HK and XYT on the date before September 15, 2010. According to the division of Corporation Finance Financial Reporting Manual 1170.1, financial information of a registrant’s predecessor is required for all periods prior to the succession, with no lapse in audited periods or omission of other information required about the registrant. Any interim period of the predecessor prior to its acquisition by the registrant should be audited when audited financial statements for the period after the acquisition are presented. Schedules required by S-X Article 12 are required for predecessor entities.
The Share Exchange is being accounted for as a reverse acquisition presumed to be effected on March 31, 2010 instead of September 15, 2010. The consolidated statements of balance sheet as of March 31, 2011 and the statement of income and comprehensive income and conceded statements of cash flows for the year ended March 31, 2011 have been prepared for the Group. The statements of balance sheet as of March 31, 2010 and the statement of income and comprehensive income and conceded statements of cash flows for the year ended March 31, 2010 have been prepared for the predecessor, XYT on a comparative basis. Information presented for comparative purposes in the consolidated financial statements is also to be retroactively adjusted to reflect the legal parent’s (the Company’s) legal capital.
F-11
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(CONT’D)
|
|
(a)
|
Basis of Preparation and Presentation (Cont’d)
|
The Group’s consolidated financial statements have been prepared in accordance with generally accepted accounting principles in the United States of America for illustrative of predecessor and successor financial information.
This basis of accounting differs in certain material respects from that used for the preparation of the books of account of the Group, which are prepared in accordance with the accounting principles and the relevant financial regulations applicable to enterprises with limited liabilities established in the PRC (“PRC GAAP”), the accounting standards used in the places of their domicile. The accompanying financial statements reflect necessary adjustments recorded in the books of account of the Group to present them in conformity with US GAAP.
In the opinion of the management of the Group, all adjustments, which are of a normal recurring nature, necessary for a fair statement of the results for the years have been made.
|
(b)
|
Use of Estimates
|
In preparing of the financial statements in conformity with accounting principles generally accepted in the United States of America, management makes estimates and assumptions that affect the reported amounts of assets and liabilities and disclosures of contingent assets and liabilities at the dates of the financial statements, as well as the reported amounts of revenues and expenses during the reporting periods. These accounts and estimates include, but are not limited to, the valuation of due from related parties, inventories and the estimation on useful lives of plant and machinery and intangible assets. Actual results could differ from those estimates.
|
(c)
|
Concentration of Credit Risk
|
Financial instruments that potentially expose the Group to concentrations of credit risk consist primarily of cash and cash equivalents, other receivable, restricted cash and due from related parties. The Group places its cash with financial institutions with high-credit ratings and quality. The Group conducts periodic reviews of the related party financial conditions and payment practices.
No single external customer exceeded 10% of the Group’s total revenue for the periods presented.
The Company relies on supplies from numerous vendors. The Company had only one vendor that exceeded 10% of the Group’s total purchase, that accounted for approximately 20% of total purchases for the year ended March 31, 2011.
F-12
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(CONT’D)
|
|
(d)
|
Cash and Cash Equivalents
|
The Group considers all highly liquid investments with initial maturities of six months or less to be cash equivalents.
|
(e)
|
Restricted Cash
|
Deposits in banks for securities of notes payable that are restricted in use are classified as restricted cash under current assets.
|
(f)
|
Trade and Other Receivables
|
Trade and other receivables are recognized initially at fair value and subsequently measured at amortized cost using the effective interest method, less allowance for impairment. An allowance for impairment of trade and other receivables is established when there is objective evidence that the Group will not be able to collect all amounts due according to the original terms of receivables. The amount of the allowance is the difference between the receivable’s carrying amount and the present value of estimated future cash flows, discounted at the effective interest rate computed at initial recognition. The amount of the allowance is recognized in the statement of income and comprehensive income.
|
(g)
|
Inventories
|
Inventories are finished goods purchased from outsiders of the Company and stated at the lower of cost and net realizable value. Cost is determined using the weighted average basis. The cost of inventories, principally comprising purchase cost and other costs incurred in bringing the inventories to their present location and condition. Net realizable value is the estimated selling price in the ordinary course of business, less the estimated costs of completion and the estimated costs necessary to make the sale.
F-13
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(CONT’D)
|
|
(h)
|
Plant and Equipment
|
Plant and equipment are stated at cost less depreciation and accumulated impairment loss. Cost represents the purchase price of the asset and other costs incurred to bring the asset into its existing use. Maintenance, repairs and betterments, including replacement of minor items, are charged to expense; major additions to physical properties are capitalized.
Depreciation of plant and equipment is calculated to written off the cost, less their estimated residual value, if any, using the straight-line method over their estimated useful lives. The principal depreciation periods are as follows:
|
Office equipment
|
3 years
|
|
Other equipment
|
5 years
|
|
Motor vehicles
|
4 years
|
|
(i)
|
Intangible Assets
|
Intangible assets are stated at cost less amortization and accumulated impairment loss. The intangible assets of the Group represent software in used. The intangible assets are amortized over their estimated useful lives of 10 years using the straight-line method.
|
(j)
|
Revenue Recognition
|
Revenue from sales of the Group’s products is recognized when the significant risks and rewards of ownership have been transferred to the buyer at the time of delivery and the sales price is fixed or determinable and collection is reasonably assured.
When the customers checked and accepted the products, the collection is reasonably assured. Since the nature of the products, the type of their customers and their distribution methods are substantially similar, the revenue recognition policy on variable products is the same.
F-14
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(CONT’D)
|
|
(k)
|
Income taxes
|
The Group uses the asset and liability method of accounting for income taxes pursuant to SFAS No. 109 “Accounting for Income Taxes”. Under the asset and liability method of SFAS 109, deferred tax assets and liabilities are recognized for the future tax consequences attributable to temporary differences between the financial statements carrying amounts of existing assets and liabilities and loss carry forwards and their respective tax bases. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled.
|
(l)
|
Comprehensive Income
|
Comprehensive income is defined to include all changes in equity except those resulting from investments by owners and distributions to owners. Among other disclosures, all items that are required to be recognized under current accounting standards as components of comprehensive income are required to be reported in a financial statement that is presented with the same prominence as other financial statements. The Group’s current component of other comprehensive income is the foreign currency translation adjustments.
F-15
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(CONT’D)
|
|
(m)
|
Foreign Currency Translation
|
The Group maintains its financial statements in the functional currency. The functional currency of the Company is US dollar (“USD”, “$”), the functional currency of FCPG HK is Hong Kong dollar (“HKD”), and the functional currency of XYT is Renminbi (“RMB”). Monetary assets and liabilities denominated in currencies other than the functional currency are translated into the functional currency at rates of exchange prevailing at the balance sheet dates. Transactions denominated in currencies other than the functional currency are translated into the functional currency at the exchanges rates prevailing at the dates of the transaction. Exchange gains or losses arising from foreign currency transactions are included in the determination of net income for the respective periods.
For financial reporting purposes, the financial statements of the Company, FCPG HK and XYT which are prepared using the functional currency have been translated into USD. Assets and liabilities are translated at the exchange rates at the balance sheet dates and revenue and expenses are translated at the average exchange rates and stockholders’ equity is translated at historical exchange rates. Any translation adjustments resulting are not included in determining net income but are included in foreign exchange adjustment to other comprehensive income, a component of stockholders’ equity.
HKD is pegged to USD and hence there is no significant translation adjustment impact on these financial statements.
RMB is not freely convertible into foreign currency and all foreign exchange transactions must take place through authorized institutions. No representation is made that the RMB amounts could have been, or could be, converted into USD at the rates used in translation.
|
(n)
|
Financial instruments
|
The carrying amounts of all financial instruments approximate fair value. The carrying amounts of cash and cash equivalents, restricted cash, due from (to) a related party, notes payable, other payable and accrued liabilities and income tax payable approximate their fair values due to the short-term nature of these items. The carrying amounts of short-term borrowings approximate the fair value based on the Company’s expected borrowing rate for debt with similar remaining maturities and comparable risk.
It is management’s opinion that the Company is not exposed to significant interest, price or credit risks arising from these financial instruments.
F-16
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(CONT’D)
|
|
(o)
|
Recent Accounting Pronouncements
|
In April 2009, the FASB issued FSP 157-4 “Determining Fair Value When the Volume and Level of Activity for the Asset or Liability Have Significantly Decreased and Identifying Transactions That Are Not Orderly”. FSP 157-4 provides additional guidance for estimating fair value in accordance with SFAS 157 when the volume and level of activity for the asset or liability have significantly decreased. FSP 157-4 also includes guidance on identifying circumstances that indicate a transaction is not orderly. FSP 157-4 is effective for interim and annual reporting periods ending after June 15, 2009, with early adoption permitted for periods ending after March 15, 2009. FSP 157-4 does not require disclosures for earlier periods presented for comparative purposes at initial adoption. In periods after initial adoption, FSP 157-4 requires comparative disclosures only for periods ending after initial adoption.
In May 2009, the FASB issued FSP SFAS 165 “Subsequent Events”. The objective of this Statement is to establish general standards of accounting for and disclosures of events that occur after the balance sheet date but before financial statements are issued or are available to be issued. SFAS 165 is effective for the interim and annual periods ending after June 15, 2009, which is now codified as FASB ASC 855 “Subsequent Events”. The adoption of FASB ASC 855 did not have a material impact on the Company’s financial position, results of operations and cash flows. Effective February 24, 2010, the Company adopted Accounting Standards Update (“ASU”) No. 2010-09, “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements”, which removes the requirement to disclose the date through which subsequent events have been evaluated.
In June 2009, the FASB issued SFAS No. 166 “Accounting for Transfers of Financial Assets – an amendment of FASB Statement No. 140”. This statement is intended to improve the relevance, representational faithfulness, and comparability of the information that a reporting entity provides in its financial reports about a transfer of financial assets; the effects of a transfer on its financial position, financial performance, and cash flows; and a transferor’s continuing involvement in transferred financial assets. This Statement must be applied as of the beginning of each reporting entity’s first annual reporting period that begins after November 15, 2009, and is required to be adopted by the Company in the first quarter of fiscal year 2011. Earlier application is prohibited. This Statement must be applied to transfers occurring on or after the effective date.
F-17
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(CONT’D)
|
|
(o)
|
Recent Accounting Pronouncements (Cont’d)
|
In June 2009, the FASB issued SFAS 168, “The FASB Accounting Standards CodificationTM and the Hierarchy of Generally Accepted Accounting Principles - a replacement of FASB Statement No 162”, which supersedes all existing non-SEC accounting and reporting standards. The codification does not change GAAP but rather organizes it into a new hierarchy with two levels: authoritative and non-authoritative. All authoritative GAAP carries equal weight and is organized in a topical structure.
In August 2009, the FASB issued Accounting Standards Update (“ASU”) No. 2009-05, “Measuring Liabilities at Fair Value”, which is codified as ASC 820, “Fair Value Measurements and Disclosures”. This Update provides amendments to ASC 820-10, Fair Value Measurements and Disclosures –Overall, for the fair value measurement of liabilities. This Update provides clarification that in circumstances in which a quoted price in an active market for the identical liability is not available, a reporting entity is required to measure fair value using a valuation technique that uses the quoted price of the identical liability when traded as an asset, quoted prices for similar liabilities or similar liabilities when traded as assets, or that is consistent with the principles of ASC 820. The amendments in this Update also clarify that when estimating the fair value of a liability, a reporting entity is not required to include a separate input or adjustment to other inputs relating to the existence of a restriction that prevents transfer of the liability.
The amendments in this Update also clarify that both a quoted price in an active market for the identical liability at the measurement date and the quoted price for the identical liability when traded as an asset in an active market when no adjustments to the quoted price of the assets are required are Level 1 fair value measurements. ASC 820 is effective for the first reporting period (including interim periods) beginning after August 28, 2009.
In September 2009, the FASB issued ASU No. 2009-06, “Income Taxes (Topic 740)—Implementation Guidance on Accounting for Uncertainty in Income Taxes and Disclosure Amendments for Nonpublic Entities”, and it provides implementation guidance on accounting for uncertainty in income taxes effective for interim and annual reporting period ending on or after September 15, 2009.
F-18
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
2.
|
SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES(CONT’D)
|
|
(o)
|
Recent Accounting Pronouncements (Cont’d)
|
The amendments in this Update also clarify that both a quoted price in an active market for the identical liability at the measurement date and the quoted price for the identical liability when traded as an asset in an active market when no adjustments to the quoted price of the assets are required are Level 1 fair value measurements. ASC 820 is effective for the first reporting period (including interim periods) beginning after August 28, 2009.
In September 2009, the FASB issued ASU No. 2009-06, “Income Taxes (Topic 740)—Implementation Guidance on Accounting for Uncertainty in Income Taxes and Disclosure Amendments for Nonpublic Entities”, and it provides implementation guidance on accounting for uncertainty in income taxes effective for interim and annual reporting period ending on or after September 15, 2009.
In January 2010, the FASB issued ASU No. 2010-06 “Fair Value Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements”. This update provides amendments to Subtopic 820-10 that required new disclosures for 2 situations - i) A reporting entity should disclose separately the amount of significant transfer in and out of Level 1 and 2 fair value measurements and describe the reasons for the transfers; ii) In the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuance, and settlements. None of the new pronouncements has current application to the Company.
In February 2010, the FASB issued ASU No. 2010-09 “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements”. This Update provides amendments to i) An entity that either (a) is an SEC filer or (b) is a conduit bond obligor for conduit debt securities that are traded in a public market (a domestic or foreign stock exchange or an over-the-counter market, including local or regional markets) is required to evaluate subsequent events through the date that the financial statements are issued. If an entity meets neither of those criteria, then it should evaluate subsequent events through the date the financial statements are available to be issued; ii) The glossary of Topic 855 is amended to include the definition of SEC filer; iii) An entity that is an SEC filer is not required to disclose the date through which subsequent events have been evaluated; iv) The glossary of Topic 855 is amended to remove the definition of public entity; v) The scope of the reissuance disclosure requirements is refined to include revised financial statements only.
F-19
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
|
(o)
|
Recent Accounting Pronouncements (Cont’d)
|
In January 2010, the FASB issued ASU No. 2010-06 “Fair Value Measurements and Disclosures (Topic 820): Improving Disclosures about Fair Value Measurements”. This update provides amendments to Subtopic 820-10 that required new disclosures for 2 situations - i) A reporting entity should disclose separately the amount of significant transfer in and out of Level 1 and 2 fair value measurements and describe the reasons for the transfers; ii) In the reconciliation for fair value measurements using significant unobservable inputs (Level 3), a reporting entity should present separately information about purchases, sales, issuance, and settlements. None of the new pronouncements has current application to the Company.
In February 2010, the FASB issued ASU No. 2010-09 “Subsequent Events (Topic 855): Amendments to Certain Recognition and Disclosure Requirements”. This Update provides amendments to i) An entity that either (a) is an SEC filer or (b) is a conduit bond obligor for conduit debt securities that are traded in a public market (a domestic or foreign stock exchange or an over-the-counter market, including local or regional markets) is required to evaluate subsequent events through the date that the financial statements are issued. If an entity meets neither of those criteria, then it should evaluate subsequent events through the date the financial statements are available to be issued; ii) The glossary of Topic 855 is amended to include the definition of SEC filer; iii) An entity that is an SEC filer is not required to disclose the date through which subsequent events have been evaluated; iv) The glossary of Topic 855 is amended to remove the definition of public entity; v) The scope of the reissuance disclosure requirements is refined to include revised financial statements only.
In May 2010, the FASB issued ASU No. 2010-19 “Foreign Currency (Topic 830): Foreign Currency Issues: Multiple Foreign Currency Exchange Rates (SEC Update)”. The purpose of this Update is to codify the SEC Staff Announcement made at the March 18, 2010 meeting of the FASB Emerging Issues Task Force (EITF) by the SEC Observer to the EITF. The Staff Announcement provides the SEC staff’s view on certain foreign currency issues related to investments in Venezuela. None of the new pronouncements has current application to the Company.
In December 2010, the FASB issued Update No. 2010-28 “Intangibles—Goodwill and Other (Topic 350): When to Perform Step 2 of the Goodwill Impairment Test for Reporting Units with Zero or Negative Carrying Amounts”. The amendments in this Update modify Step 1 of the goodwill impairment test for reporting units with zero or negative carrying amounts. For those reporting units, an entity is required to perform Step 2 of the goodwill impairment test if it is more likely than not that a goodwill impairment exists. In determining whether it is more likely than not that a goodwill impairment exists, an entity should consider whether there are any adverse qualitative factors indicating that an impairment may exist. The qualitative factors are consistent with the existing guidance and examples in paragraph 350-20-35-30, which requires that goodwill of a reporting unit be tested for impairment between annual tests if an event occurs or circumstances change that would more likely than not reduce the fair value of a reporting unit below its carrying amount. For nonpublic entities, the amendments are effective for fiscal years, and interim periods within those years, beginning after December 15, 2011. Nonpublic entities may early adopt the amendments using the effective date for public entities.
The Company does not anticipate that the adoption of the above recent accounting pronouncements will have a material impact on these financial statements.
F-20
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
2. SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (CONT’D)
|
(p)
|
Correction of an Error in Previously Issued Financial Statements
|
Pursuant to ASC 250-10-50-7, when financial statements are restated to correct an error, the entity shall disclose that its previously issued financial statements have been restated, along with a description of the nature of the error. The entity also shall disclose both of the following:
|
|
a.
|
The effect of the correction on each financial statement line item and any per-share amounts affected for each prior period presented;
|
|
|
b.
|
The cumulative effect of the change on retained earnings or other appropriate components of equity or net assets in the statement of financial position, as of the beginning of the earliest period presented.
|
F-21
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
3.
|
DEEMED DISTRIBUTION OF DIVIDEND
|
The Company had a loan receivable from a related party in the amount of $18,033,038 as of March 31, 2011 and $12,009,958 as of March 31, 2010, which the SEC had deemed to be treated as a distribution to the related party and therefore a reduction of retained earnings. As a result, the Company has restated the financial statements in accordance with US Generally Accepted Accounting Principles (GAAP) as requested by the US Securities and Exchange Commission (SEC).
The following table presents the financial statements accounts which are restated:
|
Note
|
Pre restated
|
Adjustments
|
Post restated
|
|||||||||||
|
For the year ended March 31, 2010
|
||||||||||||||
|
Due from related party
|
(i)
|
12,009,958 | (12,009,958 | ) | - | |||||||||
|
Retained earning
|
(i)
|
5,469,970 | (12,009,958 | ) | (6,539,988 | ) | ||||||||
|
Deemed distribution of dividend
|
(i)
|
- | 12,009,958 | 12,009,958 | ||||||||||
|
Note
|
Pre restated
|
Adjustments
|
Post restated
|
|||||||||||
|
For the year ended March 31, 2011
|
||||||||||||||
|
Due from related party
|
(i)
|
18,033,038 | (18,033,038 | ) | - | |||||||||
|
Retained earning
|
(i)
|
7,803,649 | (18,033,038 | ) | (10,229,389 | ) | ||||||||
|
Deemed distribution of dividend
|
(i)
|
- | 18,033,038 | 18,033,038 | ||||||||||
|
|
(i)
|
Restated amount due from a related party (shareholder) and treated as a distribution to the related party and therefore a reduction of retained earnings. The nature of the error was a matter of disclosure of the presentation of consolidated financial statements in conformity with the United States Generally Accepted Accounting Principles.
|
F-22
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
4.
|
PLANT AND EQUIPMENT, NET
|
|
As of
|
As of
|
|||||||
|
March 31,
|
March 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
$
|
$
|
|||||||
|
Cost
|
||||||||
|
Office equipment
|
14,042 | 9,888 | ||||||
|
Other equipment
|
30,791 | 29,719 | ||||||
|
Motor vehicles
|
- | 21,710 | ||||||
|
Total cost
|
44,833 | 61,317 | ||||||
|
Accumulated depreciation
|
(39,694 | ) | (60,469 | ) | ||||
|
Property, plant and equipment, net
|
5,139 | 848 | ||||||
|
5.
|
INTANGIBLE ASSET, NET
|
|
As of
|
As of
|
|||||||
|
March 31,
|
March 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
$
|
$
|
|||||||
|
Cost
|
8,469 | 8,174 | ||||||
|
Accumulated amortization
|
(6,417 | ) | (5,376 | ) | ||||
|
Intangible asset, net
|
2,052 | 2,798 | ||||||
|
6.
|
OTHER PAYABLE AND ACCRUED LIABILITIES
|
|
As of
|
As of
|
|||||||
|
March 31,
|
March 31,
|
|||||||
|
2011
|
2010
|
|||||||
|
$
|
$
|
|||||||
|
Value added tax payable
|
11,238,596 | 8,477,337 | ||||||
|
Other payables
|
1,593,858 | 441,831 | ||||||
| 12,832,454 | 8,919,168 | |||||||
F-23
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
7.
|
NOTES PAYABLE
|
The notes payable which were issued by the Company with bank guarantees are secured by the restricted cash.
|
8.
|
CONVERTIBLE PROMISSORY NOTES
|
On October 3, 2010, we issued a convertible promissory note to Sierra Growth Inc. (the “Sierra Note”) in the principal amount of up to $400,000, with interest on the unpaid principal at a rate of 5.0% simple interest per annum. The Sierra Note matures on October 3, 2015. The outstanding principal and accrued but unpaid interest thereon may be converted (1) upon a qualified debt or equity financing, in which case the holder of the Sierra Note would receive for the same promissory note or class and series of stock, respectively, issued in such qualified financing; (2) upon mutual agreement by the holder and the Company, in which case the holder would receive a number of shares of common stock based on a conversion price equal to the trailing volume weighted average price; or (3) upon a reorganization, consolidation or merger of the Company, in which case the holder would receive a number of shares of common stock based on a conversion price equal to the trailing volume weighted average price. On October 4, 2010, Sierra Growth Inc. assigned the Sierra Note to Caledonia Partners LLC (the “Caledonia Note I”), and on January 21, 2011, Caledonia Partners LLC agreed to extend the total funds available to the Company under the Sierra Note to $500,000. As of March 31, 2011, the Company has drawn $495,000 under the Caledonia Note I and no share conversion occurred.
On December 22, 2010, we issued a convertible promissory note to Caledonia Partners LLC (the “Caledonia Note II”) in the principal amount to $500,000, with interest on the unpaid principal at a rate of 5.0% simple interest per annum. The Caledonia Note matures on December 22, 2015. The outstanding principal and accrued but unpaid interest thereon may be converted (1) upon a qualified debt or equity financing, in which case the holder of the Caledonia Note would receive for the same promissory note or class and series of stock, respectively, issued in such qualified financing; (2) upon mutual agreement by the holder and the Company, in which case the holder would receive a number of shares of common stock based on a conversion price equal to the trailing volume weighted average price; or (3) upon a reorganization, consolidation or merger of the Company, in which case the holder would receive a number of shares of common stock based on a conversion price equal to the trailing volume weighted average price. As of March 31, 2011 the Company has drawn $500,000 under the Caledonia Note and no share conversion occurred.
F-24
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
8.
|
CONVERTIBLE PROMISSORY NOTES (Cont’d)
|
The discount rate of the convertible promissory notes were calculated based on the 5-year bank loan interest yield rate and the treasury yield rate by 6.87%~8.67% per annum under the Caledonia Note I due to different issuance date and 7.98% per annum under the Caledonia Note II. The discount amounts on the convertible promissory notes were allocated to the additional paid-in capital.
The details of convertible promissory notes as of March 31, 2011 are as follows
|
As of March 31, 2011
|
||||||||||||
|
Caledonia Note I
|
Caledonia Note II
|
Total
|
||||||||||
|
Convertible notes payable:
|
||||||||||||
|
-par value
|
495,000 | 500,000 | 995,000 | |||||||||
|
-discount on liabilities
|
(54,224 | ) | (59,523 | ) | (113,747 | ) | ||||||
|
-interest payable adjustment
|
2,380 | 2,753 | 5,133 | |||||||||
| 443,156 | 443,230 | 886,386 | ||||||||||
F-25
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
9.
|
COMMON STOCK
|
As of March 31, 2010, the Company had 100,000,000 common shares authorized. As of June 25, 2010, the Company’s stockholders approved a proposal to amend the Company’s articles of incorporation to increase the total number of authorized shares of common stock from 100,000,000 to 200,000,000. The effective date of the amendment was June 29, 2010. As of March 31, 2011, the Company had 200,000,000 common shares authorized.
Reorganization of FCPG HK and XYT on reverse acquisition
On August 23, 2010, the Company entered into a voluntary share exchange agreement with FCPG HK Group (Exchange Agreement). Accordingly, an additional 15,000,000 shares of the Company would be issued to the sole selling shareholder of FCPG HK Group, in exchange for 100% of the issued and outstanding common stock of FCPG HK Group. The newly issued shares would represent 25% of the Company’s issued and outstanding common stock. The issuance date was September 15, 2010.
Issuance of private offering units
|
As of March 31, 2011
|
||||||||||||||||||||||||
|
Investors (i)
|
Placement agent(ii)
|
Total
|
||||||||||||||||||||||
|
Shares/
quantity
|
Fair
value
|
Shares/
quantity
|
Fair
value
|
Shares/
quantity
|
Fair
value
|
|||||||||||||||||||
|
$
|
$
|
$
|
||||||||||||||||||||||
|
Common stock
|
3,166,692 | 3,167 | 97,779 | 97 | 3,264,471 | 3,264 | ||||||||||||||||||
|
Additional paid-in capital
|
||||||||||||||||||||||||
|
-Common stock component
|
964,025 | 124,081 | 1,088,106 | |||||||||||||||||||||
|
-Series A-1 Warrants
|
3,166,692 | 944,385 | 97,779 | 121,251 | 3,264,471 | 1,065,636 | ||||||||||||||||||
|
-Series A-2 Warrants
|
3,166,692 | 938,423 | 97,779 | 120,485 | 3,264,471 | 1,058,908 | ||||||||||||||||||
| 6,333,384 | 2,846,833 | 195,558 | 365,817 | 6,528,942 | 3,212,650 | |||||||||||||||||||
|
Total
|
N/A | 2,850,000 | N/A | 365,914 | N/A | 3,215,914 | ||||||||||||||||||
(i) On March 18, 2011, the Company entered into a form of Securities Purchase Agreement (the “SPA”) and consummated an initial closing of a private placement offering (the “Private Offering”) with certain accredited investors (the “Purchasers”) for the issuance and sale of one hundred and fourteen (114) Units of the Company at a purchase price of $25,000 per Unit, for aggregate consideration of $2,850,000.
F-26
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
9.
|
COMMON STOCK (Cont’d)
|
Issuance of private offering units (Cont’d)
Each “Unit” is comprised of (i) 27,778 shares of Company common stock, $0.001 par value per share (the “Common Stock,” and the shares of Common Stock offered referred to as the “Shares”), (ii) warrants to purchase 27,778 shares of Common Stock at an exercise price of $1.25 per share (the “Series A-1 Warrants”), and (iii) warrants to purchase 27,778 shares of Common Stock at an exercise price of $2.00 per share (the “Series A-2 Warrants”) (the Series A-1 Warrants and the Series A-2 Warrants, collectively, the “Warrants”). The Warrants expire four (4) years from the date of issuance, subject to early termination or forfeiture in accordance with certain terms and conditions of the Warrants.
(ii) Net proceeds to the Company was approximately $2,723,500, after deducting for certain costs and expenses paid to the placement agent set forth in the SPA. 97,779 shares, 97,779 Series A-1 Warrants and 97,779 Series A-2 Warrants were issued for share-based payment to the placement agent.
An aggregate of 3,264,471 Shares, 3,264,471 Series A-1 Warrants and 3,264,471 Series A-2 Warrants were issued in connection with the initial closing as of March 23, 2011. The Shares and Warrants were issued in reliance upon Rule 506 of Regulation D and/or Regulation S of the Securities Act, and comparable exemptions for sales to “accredited” investors under state securities laws.
The quoted market price on common stock for the Company as of March 23, 2011 was $1.27 per share from OTCBB Market. The fair value on Series A-1 and A-2 Warrants were $1.24 and $1.23 per share, respectively under Black-Scholes Model.
|
10.
|
TREASURY SHARES
|
|
|
As of November 17, 2010, 5,000,000 shares had been cancelled and returned to the Company as Treasury Shares.
|
F-27
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
11.
|
INCOME TAX
|
XYT, organized under the laws of the People’s Republic of China (“PRC”) is subject to PRC Enterprises Income Tax ("EIT") with the tax rate of 25%.
FCPG HK was incorporated in Hong Kong under the Companies Ordinance of Hong Kong is subject to HK Profit tax with the tax rate of 16.5%.
A reconciliation of the tax expense applicable to income before tax using the statutory rate to the tax expense at the effective tax rate is as follows:
|
Year ended
March 31, 2011
|
Year ended
March 31, 2010
|
|||||||
|
$
|
$
|
|||||||
|
Income before tax
|
3,419,057 | 3,696,922 | ||||||
|
Tax effect of tax losses not recognized
|
246,073 | - | ||||||
|
Tax at the statutory rate
|
839,305 | 934,463 | ||||||
|
Effective income tax expenses
|
1,085,378 | 934,463 | ||||||
F-28
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
12.
|
COMMITMENTS AND CONTINGENCIES
|
There was no amount due to commitments and contingencies except that XYT leases the office and warehouse under non-cancelable operating lease agreement that expires in 2018.
|
Payments due by Period
|
||||||||||||||||||||
|
Contractual Obligations
|
Less than
|
One to
|
Three to
|
More Than
|
||||||||||||||||
|
As of March 31, 2011
|
One Year
|
Three Years
|
Five Years
|
Five Years
|
Total
|
|||||||||||||||
|
Operating Lease Obligations
|
$ | - | $ | 113,835 | $ | 75,890 | $ | 75,890 | $ | 265,615 | ||||||||||
|
13.
|
SEGMENT INFORMATION
|
No segment information is disclosed as the Company is engaged in the sales of Chinese patent drug, antibiotics, bio-chemicals, chemical preparations and biological. The nature of the products, the type of their customers and their distribution methods are substantially similar. The Company operates in a single segment in the PRC.
|
14.
|
EARNINGS PER SHARE
|
The Company reports basic and diluted earnings per share in accordance with ASC Topic 260, “Earnings Per Share”. Basic earnings/ (loss) per share is computed by dividing net comprehensive income/ (loss) by weighted average number of shares of common stock outstanding during the period. Diluted earnings per share is computed by dividing net comprehensive income by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during the period. Common equivalent shares are excluded from the computation in periods for which they have an anti-dilutive effect. Stock options for which the exercise price exceeds the average market price over the period are anti-dilutive and, accordingly, are excluded from the calculation.
As of March 31, 2011, the comprehensive income was $2,536,070 and the weighted average number of common shares outstanding for the fiscal year ended March 31, 2011 was 51,249,632. The adjusted weighted average number of common shares outstanding and potentially diluted securities for the fiscal year ended March 31, 2011 was 51,392,732, respectively.
F-29
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
15.
|
SUBSEQUENT EVENT
|
Second private offering units
On March 31, 2011, the Company entered into a form of Securities Purchase Agreement (the “SPA”) for the second closing of a previously announced private placement offering (the “Private Offering”) with certain accredited investors (the “Purchasers”) for the issuance and sale of thirty three (33) Units of the Company at a purchase price of $25,000 per Unit, for aggregate consideration of $825,000. Each “Unit” is comprised of (i) 27,778 shares of Company common stock, $0.001 par value per share (the “Common Stock,” and the shares of Common Stock offered referred to as the “Shares”), (ii) warrants to purchase 27,778 shares of Common Stock at an exercise price of $1.25 per share (the “Series A-1 Warrants”), and (iii) warrants to purchase 27,778 shares of Common Stock at an exercise price of $2.00 per share (the “Series A-2 Warrants”) (the Series A-1 Warrants and the Series A-2 Warrants, collectively, the “Warrants”). The Warrants expire four (4) years from the date of issuance, subject to early termination or forfeiture in accordance with certain terms and conditions of the Warrants. This second closing is in addition to the initial closing of one hundred and fourteen (114) Units for $2,850,000 in proceeds as disclosed in Note 9 Common Stock. Aggregate consideration was $825,000 and net proceeds to the Company was approximately $750,750, after deducting for certain costs and expenses set forth in the SPA. An aggregate of 990,008 Shares, 990,008 Series A-1 Warrants and 990,008 Series A-2 Warrants were issued in connection with the second closing.
Increase in share capital on XYT
As of April 2, 2011, the amount injected by FCPG HK of $2 million (RMB13.08 million) was released by PRC State Administration of Foreign Exchange, into the share capital of XYT. The capital verification report was issued as of April 12, 2011 by a local accounting firm in PRC.
Year end date change
On May 6, 2011, the Company's Board of Directors changed the Company’s fiscal year end from March 31 to December 31, effective immediately.
Corporate re-organization & Intended disposal of XYT
The Company has always had a vision to eventually expand its operations throughout China, Hong Kong, other Asian countries and perhaps the Middle East. It has also been an objective of the Company to list on the Hong Kong Stock Exchange as soon as it qualifies.
In order to better facilitate these objectives, reduce the tax structure of the company, and operate in a common law jurisdiction, the Company has initiated a re-organization that will move its corporate operations to Hong Kong. These include its executive management, financial accounting, information technology and investor relations. The benefits of this move are significant - as a Chinese domiciled company, the Company currently pays VAT of 17% and corporate income tax of 25%. By moving the corporate functions to Hong Kong, the Company will not have to pay VAT and the profit tax is only 16.5%.
F-30
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
15.
|
SUBSEQUENT EVENT
|
Corporate re-organization & Intended disposal of XYT
The Company projects that this re-organization will save the Company tens of millions of dollars in taxes over the next 5 years alone. As part of this re-organization FCPG HK will dispose its interests in XYT through an arm's length transaction. FCPG HK will contract with XYT to act as the supplier of its logistics in filling orders in Yunnan province. XYT will be contractually bound to only provide services to FCPG HK. FCPG HK will retain ownership of the customers generated by XYT and the internet license. The internet software utilized to conduct the business will also continue to be used exclusively by FCPG HK.
Future acquisitions will be made by a similar re-structuring and will utilize the acquired company's logistics in each province to fulfill orders that are processed by FCPG HK.
The Company is now negotiating with an independent third party on an arm's length basis to acquire XYT. As Management has decided to dispose of its interest in XYT in line with its corporate strategic development of having FCPG HK undertake sales, product ordering and accounting, therefore all the operating results, assets and liabilities of XYT are shown below separately as 'Segment for discontinued operation'.
Management is in the process of negotiating contractual agreements with an independent third party to acquire XYT and enter agreements that will have XYT continue to provide logistical support to FCPG HK and not undertake in any business activities that will be in direct competition with FCPG HK now or in the future.
F-31
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
15.
|
SUBSEQUENT EVENT
|
Corporate re-organization & Intended disposal of XYT
The financial results attributable to the Company’s interest in XYT will be presented as discontinued operations. The following table summarizes the results of XYT included in the consolidated balance sheet as of March 31, 2011 and 2010:
|
As of
March 31, 2011
|
As of
March 31, 2010
|
|||||||
|
$
|
$
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
57,177 | 158,766 | ||||||
|
Restricted cash
|
1,052,135 | 682,514 | ||||||
|
Other receivables
|
39,852 | - | ||||||
|
Inventories
|
890,185 | 4,671,322 | ||||||
|
Non-current Assets
|
||||||||
|
Due from a related party
|
24,028,551 | 12,009,958 | ||||||
|
Plant and equipment, net
|
5,139 | 848 | ||||||
|
Intangible assets, net
|
2,052 | 2,798 | ||||||
|
Total assets
|
26,075,091 | 17,526,206 | ||||||
|
Current Liabilities
|
||||||||
|
Short-term borrowings
|
772,010 | 732,461 | ||||||
|
Other payables and accrued liabilities
|
12,247,186 | 8,919,168 | ||||||
|
Notes payable
|
2,630,336 | - | ||||||
|
Due to FCPG HK
|
495,347 | - | ||||||
|
Income tax payable
|
2,715,062 | 2,021,627 | ||||||
|
Total liabilities
|
18,859,941 | 11,673,256 | ||||||
|
Paid-in capital
|
266,101 | 266,101 | ||||||
|
Reserve
|
8,169,832 | - | ||||||
|
Retained earnings
|
(1,568,979 | ) | 5,469,970 | |||||
|
Accumulated other comprehensive income - foreign currency translation adjustments
|
348,196 | 116,879 | ||||||
|
Total Stockholders’ Equity
|
7,215,150 | 5,852,950 | ||||||
|
Total liabilities and stockholders' equity
|
26,075,091 | 17,526,206 | ||||||
F-32
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
15.
|
SUBSEQUENT EVENT
|
Corporate re-organization & Intended disposal of XYT
The following table summarizes the results of XYT included in the consolidated statement of income for the year ended March 31, 2011 and 2010:
|
Year ended
March 31, 2011
|
Year ended
March 31, 2010
|
|||||||
|
$
|
$
|
|||||||
|
Sales
|
14,601,942 | 25,723,902 | ||||||
|
Cost of sales
|
(11,929,814 | ) | (21,045,598 | ) | ||||
|
Selling expenses
|
(644,924 | ) | (790,612 | ) | ||||
|
Administrative expenses
|
(185,346 | ) | (168,053 | ) | ||||
|
Other expenses
|
(64,689 | ) | (23,870 | ) | ||||
|
Interest income
|
8,922 | 12,362 | ||||||
|
Interest expenses
|
(45,559 | ) | (11,209 | ) | ||||
|
Income(loss) before taxes
|
1,740,532 | 3,696,922 | ||||||
|
Income tax
|
(609,649 | ) | (934,463 | ) | ||||
|
Net income
|
1,130,883 | 2,762,459 | ||||||
F-33
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
15.
|
SUBSEQUENT EVENT
|
Corporate re-organization & Intended disposal of XYT
For the purpose of clarity in presentation, Management presents below the balance sheet and income statements of FCPG HK alone for year ended March 31, 2011 and 2010:
|
As of
March 31, 2011
|
As of
March 31, 2010
|
|||||||
|
$
|
$
|
|||||||
|
ASSETS
|
||||||||
|
Current Assets
|
||||||||
|
Cash and cash equivalents
|
624,049 | - | ||||||
|
Due from FCPG US
|
500,000 | - | ||||||
|
Account receivables
|
8,881,014 | - | ||||||
|
Other receivables
|
2,103,488 | - | ||||||
|
Non-current Assets
|
||||||||
|
Investment in XYT
|
293,851 | - | ||||||
|
Total assets
|
12,402,402 | - | ||||||
|
Current Liabilities
|
||||||||
|
Other payables and accrued liabilities
|
294,746 | - | ||||||
|
Due to a related party
|
5,995,513 | - | ||||||
|
Due to FCPG US
|
3,223,490 | - | ||||||
|
Income tax payable
|
476,415 | - | ||||||
|
Total liabilities
|
9,990,164 | - | ||||||
|
Paid-in capital
|
1,282 | - | ||||||
|
Retained earnings
|
2,407,479 | - | ||||||
|
Accumulated other comprehensive income - foreign currency translation adjustments
|
3,477 | - | ||||||
|
Total Stockholders’ Equity
|
2,412,238 | - | ||||||
|
Total liabilities and stockholders’ equity
|
12,402,402 | - | ||||||
F-34
FIRST CHINA PHARMACEUTICAL GROUP INC.
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
FOR THE YEAR ENDED March 31, 2011 AND 2010
(Stated in US Dollars)
|
15.
|
SUBSEQUENT EVENT
|
Corporate re-organization & Intended disposal of XYT
The following table summarizes the results of FCPG HK included in the consolidated statement of income for the year ended March 31, 2011 and 2010:
|
Year ended
March 31, 2011
|
Year ended
March 31, 2010
|
|||||||
|
$
|
$
|
|||||||
|
Sales
|
14,397,256 | - | ||||||
|
Cost of sales
|
(11,819,186 | ) | - | |||||
|
Administrative expenses
|
(11,510 | ) | - | |||||
|
Other income
|
316,648 | - | ||||||
|
Income(loss) before taxes
|
2,883,208 | - | ||||||
|
Income tax
|
(475,729 | ) | - | |||||
|
Net income
|
2,407,479 | - | ||||||
F-35
