Attached files
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
Form 10-Q
(Mark One)
x | QUARTERLY REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the quarterly period ended September 30, 2011
or
o | TRANSITION REPORT UNDER SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from __________________ to __________________
Commission file number: 000-32581
LOTUS PHARMACEUTICALS, INC.
(Exact name of registrant as specified in its charter)
NEVADA |
| 20-0507918 |
(State or other jurisdiction of incorporation or organization) |
| (I.R.S. Employer Identification No.) |
No. 16 Cheng Zhuang Road, Feng Tai District, Beijing People’s Republic of China |
|
100071 |
(Address of principal executive offices) |
| (Zip Code) |
86-10-63899868
(Registrant’s telephone number, including area code)
N/A
(Former name, former address and former fiscal year, if changed since last report)
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days.
Yes x No o
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files).
Yes x No o
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer o |
| Accelerated filer o |
Non-accelerated filer o (Do not check if smaller reporting company) |
| Smaller reporting company x |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act)
Yes o No x
Indicated the number of shares outstanding of each of the issuer’s classes of common stock, as of the latest practicable date, 27,816,455 shares of common stock are issued and outstanding as of November 10, 2011.
TABLE OF CONTENTS
|
|
|
| Page No. |
PART I - FINANCIAL INFORMATION | ||||
Item 1. |
| Financial Statements (Unaudited) |
|
|
|
| Condensed Consolidated Balance Sheets as of September 30, 2011 and December 31, 2010 |
| 4 |
|
| Condensed Consolidated Statements of Income and Comprehensive Income for the Three and |
| 5 |
|
| Condensed Consolidated Statements of Cash Flows for the Nine Months Ended September 30, 2011 and 2010 |
| 6 |
|
| Notes to Unaudited Condensed Consolidated Financial Statements |
| 7 |
Item 2. |
| Management’s Discussion and Analysis of Financial Condition and Results of Operations. |
| 30 |
Item 3. |
| Quantitative and Qualitative Disclosures About Market Risk.. |
| 42 |
Item 4T |
| Controls and Procedures. |
| 42 |
PART II - OTHER INFORMATION | ||||
Item 1. |
| Legal Proceedings. |
| 44 |
Item 1A. |
| Risk Factors. |
| 44 |
Item 2. |
| Unregistered Sales of Equity Securities and Use of Proceeds. |
| 44 |
Item 3. |
| Defaults Upon Senior Securities. |
| 44 |
Item 4. |
| (Removed and Reserved) |
| 44 |
Item 5. |
| Other Information. |
| 44 |
Item 6. |
| Exhibits. |
| 44 |
CAUTIONARY STATEMENT REGARDING FORWARD-LOOKING INFORMATION
Certain statements in this report contain or may contain forward-looking statements that are subject to known and unknown risks, uncertainties and other factors which may cause actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by such forward-looking statements. These forward-looking statements were based on various factors and were derived utilizing numerous assumptions and other factors that could cause our actual results to differ materially from those in the forward-looking statements. These factors include, but are not limited to, our ability to enforce the Contractual Arrangements, Lotus East’s strategic initiatives, economic, political and market conditions and fluctuations, U.S. and Chinese government and industry regulation, interest rate risk, U.S., Chinese and global competition, and other factors. Most of these factors are difficult to predict accurately and are generally beyond our control. Readers should consider the areas of risk described in connection with any forward-looking statements that may be made herein. Readers are cautioned not to place substantial reliance on these forward-looking statements and readers should carefully review this report in its entirety together with our Annual Report on Form 10-K for the year ended December 31, 2010 as filed with the SEC, including the risks described in Item 1A. Risk Factors. Except for our ongoing obligations to disclose material information under the Federal securities laws, we undertake no obligation to release publicly any revisions to any forward-looking statements, to report events or to report the occurrence of unanticipated events. These forward-looking statements speak only as of the date of this report, and readers should not rely on these statements without also considering the risks and uncertainties associated with these statements and our business.
2
OTHER PERTINENT INFORMATION
We maintain a web site at www.lotuspharma.com. Information on this web site is not a part of this report.
CERTAIN DEFINED TERMS USED IN THIS REPORT
Unless specifically set forth to the contrary, when used in this report the terms:
| · | “Lotus,” “we,” “us,” “our,” the “Company,” and similar terms refer to Lotus Pharmaceuticals, Inc., a Nevada corporation and its subsidiaries, |
|
|
|
| · | “Lotus International” refers to Lotus Pharmaceutical International, Inc., a Nevada corporation and a subsidiary of Lotus, |
|
|
|
| · | “Lotus Century” refers to Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd., a wholly foreign-owned enterprise (WFOE) Chinese company which is a subsidiary of Lotus, |
|
|
|
| · | “Liang Fang” refers to Beijing Liang Fang Pharmaceutical Co., Ltd., a Chinese limited liability company formed on June 21, 2000, |
|
|
|
| · | “En Ze Jia Shi” refers to Beijing En Ze Jia Shi Pharmaceutical Co., Ltd., a Chinese limited liability company formed on September 17, 1999 and an affiliate of Liang Fang, |
|
|
|
| · | “Lotus East” collectively refers to Liang Fang and En Ze Jia Shi, |
|
|
|
| · | “Consulting Services Agreements” refers to the Consulting Services Agreements dated September 20, 2006 between Lotus and Lotus East. |
|
|
|
| · | “Operating Agreements” refers to the Operating Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East, |
|
|
|
| · | “Equity Pledge Agreements” refers to the Equity Pledge Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East, |
|
|
|
| · | “Option Agreements” refers to the Option Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East, |
|
|
|
| · | “Proxy Agreements” refers to the Proxy Agreements dated September 20, 2006 between Lotus, Lotus East and the stockholders of Lotus East, |
|
|
|
| · | “Contractual Arrangements” collectively refers to the Consulting Services Agreements, Operating Agreements, Equity Pledge Agreements, Option Agreements and the Proxy Agreements, |
|
|
|
| · | “SFDA” refers to The State Food and Drug Administration, |
|
|
|
| · | “China” or the “PRC” refers to the People’s Republic of China |
|
|
|
| · | “RMB” refers to the renminbi which is the legal currency of mainland PRC of which the yuan is the principal currency, and |
|
|
|
| · | “U.S. dollars”, “dollars” and “$” refer to the legal currency of the United States. |
3
PART 1. - FINANCIAL INFORMATION
Item 1. Financial Statements.
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED BALANCE SHEETS
(UNAUDITED)
|
| September 30, 2011 |
| December 31, 2010 |
| ||
ASSETS |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT ASSETS: |
|
|
|
|
|
|
|
Cash |
| $ | 158,908 |
| $ | 1,339,972 |
|
Accounts receivable |
|
| 6,000,546 |
|
| 1,973,150 |
|
Inventories |
|
| 1,268,875 |
|
| 634,583 |
|
Prepaid expenses and other current assets |
|
| 128,805 |
|
| 593,759 |
|
|
|
|
|
|
|
|
|
Total Current Assets |
|
| 7,557,134 |
|
| 4,541,464 |
|
|
|
|
|
|
|
|
|
PROPERTY AND EQUIPMENT, net |
|
| 52,280,950 |
|
| 39,337,935 |
|
|
|
|
|
|
|
|
|
OTHER ASSETS |
|
|
|
|
|
|
|
Land use right held for development or sale |
|
| 30,195,957 |
|
| 29,236,891 |
|
Deposits and Installments on intangible assets |
|
| 9,840,982 |
|
| 9,528,419 |
|
Land use rights, net |
|
| 13,111,904 |
|
| 12,932,421 |
|
Other intangible assets, net |
|
| 7,169,988 |
|
| 7,607,485 |
|
|
|
|
|
|
|
|
|
Total Assets |
| $ | 120,156,915 |
| $ | 103,184,615 |
|
|
|
|
|
|
|
|
|
LIABILITIES AND SHAREHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
CURRENT LIABILITIES: |
|
|
|
|
|
|
|
Accounts payable |
| $ | 2,499 |
| $ | 37,829 |
|
Other payables and accrued liabilities |
|
| 723,557 |
|
| 3,441,466 |
|
Taxes payable |
|
| 10,595,533 |
|
| 2,024,565 |
|
Unearned revenue |
|
| 530,987 |
|
| 504,442 |
|
Dividend payable |
|
| 5,743 |
|
| — |
|
Due to related parties |
|
| 2,342,828 |
|
| 2,042,376 |
|
|
|
|
|
|
|
|
|
Total Current Liabilities |
|
| 14,201,147 |
|
| 8,050,678 |
|
|
|
|
|
|
|
|
|
LONG-TERM LIABILITIES: |
|
|
|
|
|
|
|
Due to related parties |
|
| 1,087,879 |
|
| 869,067 |
|
Notes payable - related parties |
|
| 5,413,778 |
|
| 5,241,829 |
|
|
|
|
|
|
|
|
|
Total Liabilities |
|
| 20,702,804 |
|
| 14,161,574 |
|
|
|
|
|
|
|
|
|
COMMITMENTS AND CONTIGENCIES |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY: |
|
|
|
|
|
|
|
Preferred stock ($.001 par value; 10,000,000 shares authorized; |
|
| 620 |
|
| 607 |
|
Common stock ($.001 par value; 100,000,000 shares authorized; |
|
| 27,801 |
|
| 26,763 |
|
Additional paid-in capital |
|
| 23,809,738 |
|
| 21,679,147 |
|
Retained earnings |
|
| 59,170,490 |
|
| 53,925,101 |
|
Statutory reserves |
|
| 6,240,202 |
|
| 6,240,202 |
|
Accumulated other comprehensive income |
|
| 10,205,260 |
|
| 7,151,221 |
|
|
|
|
|
|
|
|
|
Total stockholders’ Equity |
|
| 99,454,111 |
|
| 89,023,041 |
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Equity |
| $ | 120,156,915 |
| $ | 103,184,615 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
4
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF INCOME AND COMPREHENSIVE INCOME
(UNAUDITED)
|
| For the Three Months Ended |
| For the Nine Months Ended |
| ||||||||
|
| September 30, |
| September 30, |
| ||||||||
|
| 2011 |
| 2010 |
| 2011 |
| 2010 |
| ||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET REVENUES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Wholesale |
| $ | 15,276,053 |
| $ | 15,347,372 |
| $ | 38,585,730 |
| $ | 42,309,596 |
|
Retail |
|
| 3,768,485 |
|
| 2,957,488 |
|
| 12,070,485 |
|
| 9,652,235 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Net Revenues |
|
| 19,044,538 |
|
| 18,304,860 |
|
| 50,656,215 |
|
| 51,961,831 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COST OF REVENUES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Wholesale |
|
| 12,855,958 |
|
| 5,791,825 |
|
| 27,287,898 |
|
| 16,318,673 |
|
Retail |
|
| 2,874,778 |
|
| 2,133,906 |
|
| 9,014,537 |
|
| 6,956,438 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Cost of Revenues |
|
| 15,730,736 |
|
| 7,925,731 |
|
| 36,302,435 |
|
| 23,275,111 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
GROSS PROFIT |
|
| 3,313,802 |
|
| 10,379,129 |
|
| 14,353,780 |
|
| 28,686,720 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OPERATING EXPENSES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Selling expenses |
|
| 846,919 |
|
| 2,452,629 |
|
| 2,987,630 |
|
| 6,996,741 |
|
Research and development expenses |
|
| 470,311 |
|
| 21,517 |
|
| 1,889,535 |
|
| 21,517 |
|
General and administrative expenses |
|
| 728,164 |
|
| 1,145,412 |
|
| 4,170,350 |
|
| 3,242,617 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
| 2,045,394 |
|
| 3,619,558 |
|
| 9,047,515 |
|
| 10,260,875 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME FROM OPERATIONS |
|
| 1,268,408 |
|
| 6,759,571 |
|
| 5,306,265 |
|
| 18,425,845 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER INCOME (EXPENSE): |
|
|
|
|
|
|
|
|
|
|
|
|
|
Debt issuance costs |
|
| — |
|
| — |
|
| — |
|
| (52,226 | ) |
Other income |
|
| 47,717 |
|
| 200,074 |
|
| 141,331 |
|
| 597,016 |
|
Interest income |
|
| 439 |
|
| 525 |
|
| 2,005 |
|
| 2,711 |
|
Interest expense |
|
| (63,223 | ) |
| (59,896 | ) |
| (187,256 | ) |
| (551,726 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Other Income (Expense) |
|
| (15,067 | ) |
| 140,703 |
|
| (43,920 | ) |
| (4,225 | ) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME BEFORE INCOME TAXES |
|
| 1,253,341 |
|
| 6,900,274 |
|
| 5,262,345 |
|
| 18,421,620 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
INCOME TAXES |
|
| — |
|
| 200,348 |
|
| 149 |
|
| 470,514 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME |
|
| 1,253,341 |
|
| 6,699,926 |
|
| 5,262,196 |
|
| 17,951,106 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OTHER COMPREHENSIVE INCOME: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Foreign currency translation gain |
|
| 930,264 |
|
| 1,426,434 |
|
| 3,054,039 |
|
| 1,760,632 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
COMPREHENSIVE INCOME |
| $ | 2,183,605 |
| $ | 8,126,360 |
| $ | 8,316,235 |
| $ | 19,711,738 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET INCOME PER COMMON SHARE: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
| $ | 0.05 |
| $ | 0.25 |
| $ | 0.19 |
| $ | 0.69 |
|
Diluted |
| $ | 0.04 |
| $ | 0.25 |
| $ | 0.19 |
| $ | 0.67 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WEIGHTED AVERAGE COMMON SHARES OUTSTANDING: |
|
|
|
|
|
|
|
|
|
|
|
|
|
Basic |
|
| 27,784,732 |
|
| 26,697,892 |
|
| 27,675,063 |
|
| 26,000,584 |
|
Diluted |
|
| 28,094,644 |
|
| 27,047,556 |
|
| 27,985,245 |
|
| 26,872,434 |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
5
LOTUS PHARMACEUTICALS, INC. AND SUBSIDIARIES
CONDENSED CONSOLIDATED STATEMENTS OF CASH FLOWS
(UNAUDITED)
|
| For the Nine Months Ended |
| ||||
|
| September 30, |
| ||||
|
| 2011 |
| 2010 |
| ||
|
|
|
|
|
|
|
|
CASH FLOWS FROM OPERATING ACTIVITIES: |
|
|
|
|
|
|
|
Net income |
| $ | 5,262,196 |
| $ | 17,951,106 |
|
Adjustments to reconcile net income from operations to net cash provided by operating activities: |
|
|
|
|
|
|
|
Depreciation |
|
| 312,176 |
|
| 18,856 |
|
Amortization of intangible assets |
|
| 916,865 |
|
| 1,318,468 |
|
Amortization of deferred debt issuance costs |
|
| — |
|
| 52,226 |
|
Amortization of discount on convertible redeemable preferred stock |
|
| — |
|
| 151,553 |
|
Interest expense attributable to beneficial conversion feature of preferred shares |
|
| — |
|
| 184,660 |
|
Common shares issued for service |
|
| 721,127 |
|
| 228,525 |
|
Common shares issued for compensation |
|
| 1,283,101 |
|
| 125,250 |
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
Accounts receivable |
|
| (3,899,198 | ) |
| (110,085 | ) |
Inventories |
|
| (603,649 | ) |
| (133,004 | ) |
Prepaid expenses and other current assets |
|
| 476,647 |
|
| 553,428 |
|
Accounts payable |
|
| (35,985 | ) |
| 36,001 |
|
Other payables and accrued liabilities |
|
| (2,663,379 | ) |
| (584,286 | ) |
Taxes payable |
|
| 8,368,333 |
|
| (1,290,507 | ) |
Unearned revenue |
|
| 9,837 |
|
| 153,160 |
|
Due to related parties |
|
| 424,188 |
|
| 323,264 |
|
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY OPERATING ACTIVITIES |
|
| 10,572,259 |
|
| 18,978,615 |
|
|
|
|
|
|
|
|
|
CASH FLOWS USED IN INVESTING ACTIVITIES: |
|
|
|
|
|
|
|
Purchase of property and equipment |
|
| (11,778,133 | ) |
| (22,054,326 | ) |
|
|
|
|
|
|
|
|
NET CASH USED IN INVESTING ACTIVITIES |
|
| (11,778,133 | ) |
| (22,054,326 | ) |
|
|
|
|
|
|
|
|
CASH FLOWS FROM FINANCING ACTIVITIES: |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
NET CASH PROVIDED BY FINANCING ACTIVITIES |
|
| — |
|
| — |
|
|
|
|
|
|
|
|
|
EFFECT OF EXCHANGE RATE ON CASH |
|
| 24,810 |
|
| 27,621 |
|
|
|
|
|
|
|
|
|
NET DECREASE IN CASH |
|
| (1,181,064 | ) |
| (3,048,090 | ) |
|
|
|
|
|
|
|
|
CASH - beginning of period |
|
| 1,339,972 |
|
| 3,945,740 |
|
|
|
|
|
|
|
|
|
CASH - end of period |
| $ | 158,908 |
| $ | 897,650 |
|
|
|
|
|
|
|
|
|
SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: |
|
|
|
|
|
|
|
Cash paid for: |
|
|
|
|
|
|
|
Interest |
| $ | — |
| $ | — |
|
Income taxes |
| $ | 4,959 |
| $ | 640,897 |
|
|
|
|
|
|
|
|
|
Non-cash investing and financing activities: |
|
|
|
|
|
|
|
Common stock issued for conversion of convertible preferred stock |
| $ | — |
| $ | 4,048,200 |
|
Convertible redeemable preferred stock reclassified to permanent equity |
| $ | — |
| $ | 595,233 |
|
Common stock issued for prior service and compensation |
| $ | 116,350 |
| $ | — |
|
Convertible preferred stock issued for dividend payable |
| $ | 11,064 |
| $ | — |
|
The accompanying notes are an integral part of these unaudited condensed consolidated financial statements
6
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of presentation
The accompanying unaudited condensed consolidated financial statements of the Company have been prepared in accordance with accounting principles generally accepted in the United States of America and reflect the activities of Lotus Pharmaceuticals, Inc., its wholly owned subsidiaries, and its variable interest entities. All material intercompany transactions and balances have been eliminated in the consolidation. Certain information and footnote disclosures normally included in an annual financial statement prepared in accordance with generally accepted accounting principles in the United States have been condensed or omitted pursuant to such rules and regulations. In the opinion of management, the interim condensed consolidated financial statements reflect all adjustments (consisting of normal recurring adjustments) necessary for a fair presentation of the statement of the results for the interim periods presented. These interim condensed consolidated financial statements should be read in conjunction with the audited consolidated financial statements and notes thereto, as well as the accompanying Management’s Discussion and Analysis of Financial Condition and Results of Operations for the year ended December 31, 2010 included in the Company’s Annual Report on Form 10-K. Interim financial results are not necessarily indicative of the results that may be expected for a full year.
Reverse stock split
The Company effected a two-for-one reverse split of its common stock on December 31, 2010. Accordingly, all references to number of shares and to per share information in the condensed consolidated financial statements have been adjusted to reflect the reverse stock split on a retroactive basis.
Variable interest entities
Pursuant to Financial Accounting Standards Board Accounting Standards Codification Topic 810 “Consolidation” (“ASC 810”), the Company is required to include in its consolidated financial statements the financial statements of variable interest entity (“VIE”). ASC 810 requires a VIE to be consolidated by a company if that company is subject to a majority of the risk of loss for the VIE or is entitled to receive a majority of the VIE’s residual returns. VIE is the entity in which the Company, through contractual arrangements, bears the risk of, and enjoys the rewards normally associated with ownership of the entity, and therefore the Company is the primary beneficiary of the entity.
Lotus East is considered a VIE and the Company is the primary beneficiary. In September 2006, the Company entered into agreements with Lotus East pursuant to which the Company shall receive 100% of Lotus East’s net income. In accordance with these agreements, Lotus East shall pay consulting fees equal to 100% of its net income to the Company and the Company shall supply business consulting and other general business operation services needed to Lotus East.
The accounts of Lotus East are consolidated in the accompanying consolidated financial statements. As a VIE, Lotus East’s sales are included in the Company’s total sales, Lotus East’s income from operations is consolidated with the Company’s income from operations, and the Company’s net income includes all of Lotus East’s net income, and Lotus East’s assets and liabilities are included in the Company’s consolidated balance sheet. The VIE is entirely controlled by the Company and accordingly, none of the VIE’s net income is subtracted in calculating the net income attributable to the Company. Because of the contractual arrangements, the Company has pecuniary interest in Lotus East that requires consolidation of Lotus East’s financial statements with the Company’s consolidated financial statements. Management makes ongoing assessments of whether the Company is the primary beneficiary of Lotus East.
Use of estimates
The preparation of condensed consolidated financial statements in conformity with US GAAP requires management to make estimates and assumptions that affect certain reported amounts of assets, liabilities, revenues, expenses, and the related disclosures at the date of the financial statements and during the reporting period. Accordingly, actual results could differ from those estimates. Significant estimates include the allowance for doubtful accounts, the allowance for obsolete inventory, the useful life of property and equipment and intangible assets, assumptions used in assessing impairment of long-term assets and valuation of deferred tax assets.
7
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Fair value of financial instruments
The Company follows the provisions of ASC 820, “Fair Value Measurement”. ASC 820 clarifies the definition of fair value, prescribes methods for measuring fair value, and establishes a fair value hierarchy to classify the inputs used in measuring fair value as follows:
Level 1-Inputs are unadjusted quoted prices in active markets for identical assets or liabilities available at the measurement date.
Level 2-Inputs are unadjusted quoted prices for similar assets and liabilities in active markets, quoted prices for identical or similar assets and liabilities in markets that are not active, inputs other then quoted prices that are observable, and inputs derived from or corroborated by observable market data.
Level 3-Inputs are unobservable inputs which reflect the reporting entity’s own assumptions on what assumptions the market participants would use in pricing the asset or liability based on the best available information.
The carrying amounts reported in the balance sheets for cash, accounts receivable, inventories, prepaid expenses and other current assets, property and equipment, land use right held for development or sale, deposits and installments on intangible assets, land use rights, other intangible assets, accounts payable, other payable and accrued liabilities, taxes payable, unearned revenue, dividend payable, amounts due to related parties and notes payable approximate their fair market value. The Company did not identify any assets or liabilities that are required to be re-measured at fair value at a recurring basis in accordance with ASC 820.
Cash
The Company maintains cash accounts with various financial institutions mainly in the PRC. Balances at financial institutions of state-owned banks within the PRC are not covered by insurance. The Company has not experienced any losses for amounts in these financial institutions and believes it is not exposed to any significant risks on its cash in bank accounts.
Accounts receivable
The Company presents accounts receivable, net of an allowance for doubtful accounts, if necessary. The Company reviews the accounts receivable on a periodic basis and makes general and specific allowances when there is doubt as to the collectability of individual balances. In evaluating the collectability of individual receivable balances, the Company considers many factors, including the aging of the balance, customer’s historical payment history, its current credit-worthiness, current economic trends and changes in customer payment terms. The amount of the provision, if any, is recognized in the consolidated statement of operations within “General and Administrative Expenses”. Accounts are written off after exhaustive efforts at collection. Because of the Company’s good relationship with its customers and the efforts of the Company’s collection representative to collect outstanding receivables, the majority of the balance of the Company’s accounts receivable is aged less than six months. Management believes that the accounts receivable are fully collectable. Therefore, no allowance for doubtful accounts is deemed to be required as of September 30, 2011 and December 31, 2010.
Inventories
Inventories, consisting of raw materials, packaging materials, work-in-process and finished goods, are stated at the lower of cost or market utilizing the weighted average costing method. An allowance is established when management determines that certain inventories may not be saleable. If inventory costs exceed expected market value due to obsolescence or slow-moving or quantities in excess of expected demand, the Company will record reserves for the difference between the cost and the market value. These reserves are recorded based on estimates and reflected in cost of revenues. The Company did not record any inventory reserve at September 30, 2011 and December 31, 2010.
8
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Property and equipment
Property and equipment is stated at cost less accumulated depreciation. The cost of repairs and maintenance is expensed as incurred; major replacements and improvements are generally capitalized. When assets are retired or disposed of, the cost and accumulated depreciation are removed from the accounts, and any resulting gains or losses are included in income in the period of disposition. The Company reviews property and equipment for impairment when events or changes in circumstances indicate the recorded value may not be recoverable.
The construction-in-progress which consists of factories and office buildings under construction in China is included in property and equipment. No provision for depreciation is made on construction-in-progress until such time as the relevant assets are completed and ready for their intended use.
Depreciation is calculated on a straight-line basis over the estimated useful lives of the assets. The useful lives for property and equipment are estimated as follows:
| Useful life |
Manufacturing equipment | 10 to 15 years |
Office equipment and furniture | 3 to 10 years |
Land use rights
Land use rights are carried at cost and charged to expense on a straight-line basis over the estimated useful lives of the assets. Land use rights are amortized over the period the rights are granted, generally over 40 - 50 years.
Other intangible assets
Other intangible assets are carried at cost and charged to expense on a straight-line basis over the estimated useful lives of the assets. Other intangible assets consist of the right to receive revenues from the sale of medical supplies, intellectual right and software. The right to receive revenues from the sale of medical supplies, disclosed in Note 7, is amortized over 20 years, which is the term the Company would benefit from it. Intellectual right is being amortized over 10 years as based on the transfer agreement. Software is amortized over 3 years, its estimated useful life.
Impairment of long-lived assets
In accordance with ASC 360, “Property, Plant and Equipment”, the Company periodically reviews its long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of the assets may not be fully recoverable. The Company recognizes an impairment loss when the sum of expected undiscounted future cash flows is less than the carrying amount of the asset. The amount of impairment is measured as the difference between the asset’s estimated fair value and its book value. Fair value is an estimate using assumptions about future cash flows and may change significantly as time passes. The Company did not record any impairment charges for the three and nine months ended September 30, 2011 and 2010.
Income taxes
Deferred income taxes are recognized for the tax consequences of temporary differences by applying enacted statutory tax rates applicable to future periods to differences between the financial statement carrying amounts and the tax bases of existing assets and liabilities. The effect on deferred income taxes of a change in tax rates is recognized in income in the period that includes the enactment date. A valuation allowance is recognized if it is more likely than not that some portion, or all of, a deferred tax asset will not be realized.
The Company follows United States Generally Accepted Accounting Principles (“US GAAP”) regarding the accounting and disclosure for uncertain tax positions which prescribes a recognition threshold and measurement attribute for recognition and measurement of a tax position taken or expected to be taken in a tax return.
9
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Income taxes (continued)
The evaluation of a tax position is a two-step process. The first step is to determine whether it is more-likely-than-not that a tax position will be sustained upon examination, including the resolution of any related appeals or litigation based on the technical merits of that position. The second step is to measure a tax position that meets the more-likely-than-not threshold to determine the amount of benefit to be recognized in the financial statements. A tax position is measured at the largest amount of benefit that is greater than 50 percent likely of being realized upon ultimate settlement. Tax positions that previously failed to meet the more-likely-than-not recognition threshold should be recognized in the first subsequent period in which the threshold is met. Previously recognized tax positions that no longer meet the more-likely-than-not criteria should be de-recognized in the first subsequent financial reporting period in which the threshold is no longer met. Penalties and interest incurred related to underpayment of income tax are classified as income tax expense in the period incurred. The Company did not have any uncertain tax position as of September 30, 2011 and December 31, 2010.
US income tax returns for the years prior to 2008 are no long subject to examination by tax authorities.
Value added tax
The Company is subject to value added tax (“VAT”) for manufacturing products. The applicable VAT rate is 17% for products sold in the PRC. The amount of VAT liability is determined by applying the applicable tax rate to the invoiced amount of goods sold (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT). Sales and purchases are recorded net of VAT collected and paid as the Company acts as an agent for the government.
Revenue recognition
Product sales are recognized when title to the product has transferred to customers in accordance with the terms of the sale. The Company recognizes revenue in accordance with ASC 360. ASC 360 states that revenue should not be recognized until it is realized or realizable and earned. The Company records revenue when persuasive evidence of an arrangement exists, product delivery has occurred, the sales price to the customer is fixed or determinable, and collectability is reasonably assured.
Revenue from sales transactions where the buyer has the right to return the product is recognized at the time of sale only if the seller’s price to the buyer is substantially fixed or determinable at the date of sale, the buyer has paid the seller, or the buyer is obligated to pay the seller and the obligation is not contingent on resale of the product, the buyer’s obligation to the seller would not be changed in the event of theft or physical destruction or damage of the product, the buyer acquiring the product for resale has economic substance apart from that provided by the seller, the seller does not have significant obligations for future performance to directly bring about resale of the product by the buyer, and the amount of future returns can be reasonably estimated. At September 30, 2011 and December 31, 2010, the Company did not have any allowance for returns.
Unearned revenue
Unearned revenue consists of prepayments from customers for merchandise that has not yet been shipped. The Company recognizes the deposits as revenue at the time of shipment. At September 30, 2011 and December 31, 2010, the Company had unearned revenue of $530.987 and $504,442, respectively.
Risks and uncertainties
The Company’s operations are carried out in the PRC. Accordingly, the Company’s business, financial condition and results of operations may be influenced by the political, economic and legal environment in the PRC, and by the general state of the PRC’s economy. The Company’s operations in the PRC are subject to specific considerations and significant risks not typically associated with companies in North America. The Company’s results may be adversely affected by changes in governmental policies with respect to laws and regulations, anti-inflationary measures, currency conversion and remittance abroad, and rates and methods of taxation, among other things. Although the Company has not experienced any material losses from these risks and believes it is in compliance with existing laws and regulations, this may not necessarily be indicative of future results.
10
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Risks and uncertainties (continued)
Financial instruments which potentially subject the Company to concentrations of credit risk consist principally of cash and trade accounts receivable. Substantially all of the Company’s cash is maintained with state-owned banks within the PRC, and no deposits are covered by insurance. The Company has not experienced any losses in such accounts and believes it is not exposed to any risks on its cash in bank accounts. A significant portion of the Company’s sales are credit sales which are primarily to customers whose ability to pay is dependent upon the industry economics prevailing in these areas; however, concentrations of credit risk with respect to trade accounts receivables is limited due to generally short payment terms. The Company also performs ongoing credit evaluations of its customers to help further reduce credit risk.
Stock-based compensation
The Company accounts for stock options and other equity based compensation issued to employees in accordance with ASC 718 “Stock Compensation”. ASC 718 requires companies to recognize an expense in the statement of income at the grant date of stock options and other equity based compensation issued to employees. There were no options outstanding as of September 30, 2011 and December 31, 2010. The Company accounts for non-employee share-based awards in accordance with ASC 505-50 “Equity-based payments to non-employees”.
Shipping
Shipping costs are expensed as incurred and included in selling expenses. For the three and nine months ended September 30, 2011 and 2010, all of the shipping expenses were reimbursed by the Company’s customers.
Employee benefits
The Company’s operations and employees are all located in the PRC. The Company makes mandatory contributions to the PRC government’s health, retirement benefit and unemployment funds in accordance with the relevant Chinese social security laws. The costs of these payments are charged to the same accounts as the related salary costs in the same period as the related salary costs and are not material.
Advertising
Advertising is expensed as incurred and is included in selling expenses. Advertising expenses amounted to $0 for the three and nine months ended September 30, 2011 and 2010.
Research and development
Research and development costs are expensed as incurred. These costs primarily consist of cost of material used and salaries paid for the development of the Company’s products and depreciation related to property and equipment used and fees paid to third parties. Research and development expenses amounted to $470,311 and $21,517 for the three months ended September 30, 2011 and 2010, respectively. Research and development expenses amounted to $1,889,535 and $21,517 for the nine months ended September 30, 2011 and 2010, respectively.
Foreign currency translation
The reporting currency of the Company is the U.S. dollar. The functional currency of the parent company is the U.S. dollar, and the functional currency of the Company’s operating subsidiaries and affiliates is Chinese Renminbi (“RMB”). For the subsidiaries and affiliates whose functional currencies are the RMB, results of operations and cash flows are translated at average exchange rates during the period, assets and liabilities are translated at the unified exchange rate at the end of the period, and equity is translated at historical exchange rates. Translation adjustments resulting from the process of translating the local currency financial statements into
11
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Foreign currency translation (continued)
U.S. dollars are included in determining comprehensive income. Transactions denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing on the transaction dates. Assets and liabilities denominated in foreign currencies are translated into the functional currency at the exchange rates prevailing at the balance sheet date with any transaction gains and losses that arise from exchange rate fluctuations on transactions denominated in a currency other than the functional currency are included in the results of operations as incurred.
All of the Company’s revenue transactions are transacted in the functional currency. The Company does not enter any material transaction in foreign currencies and, accordingly, transaction gains or losses have not had, and are not expected to have, a material effect on the results of operations of the Company.
The foreign currency exchange rates were obtained from www.oanda.com. Asset and liability accounts at September 30, 2011 and December 31, 2010 were translated at 6.4018 RMB to $1.00 and at 6.6118 RMB to $1.00, respectively, which were the exchange rates on the balance sheet dates. Equity accounts were stated at their historical rate. The average translation rates applied to the statements of income and cash flows for the nine months ended September 30, 2011 and 2010 were 6.50601 RMB and 6.8164 RMB to $1.00, respectively. As a result, amounts related to assets and liabilities reported on the statement of cash flows will not necessarily agree with changes in the corresponding balances on the balance sheets.
Earnings per common share
Basic earnings per share is computed by dividing net income available to common shareholders by the weighted average number of shares of common stock outstanding during the period. Diluted income per share is computed by dividing net income by the weighted average number of shares of common stock, common stock equivalents and potentially dilutive securities outstanding during each period. Potentially dilutive common shares consist of common shares issuable upon the conversion of series A preferred stock (using the if-converted method) and common stock warrants (using the treasury stock method).
All share and per share amounts used in the Company’s condensed consolidated financial statements and notes thereto have been retroactively restated to reflect the 2-to-1 reverse split, which occurred on December 31, 2010.
The following is a reconciliation of the basic and diluted earnings per share computations for the three months ended September 30, 2011 and 2010:
| 2011 |
| 2010 | ||
Net income | $ | 1,253,341 |
| $ | 6,699,926 |
Deduct: preferred stock dividends |
| (2,435 | ) |
| — |
Net income attributable to common shareholders for basic earnings per share | $ | 1,250,906 |
| $ | 6,699,926 |
|
|
|
|
|
|
Weighted average shares used in basic computation |
| 27,784,732 |
|
| 26,697,892 |
Earnings per share: |
|
|
|
|
|
Basic | $ | 0.05 |
| $ | 0.25 |
Diluted earnings per share
| 2011 |
| 2010 | ||
Net income attributable to common shareholders for basic earnings per share | $ | 1,250,906 |
| $ | 6,699,926 |
Add: preferred stock dividends |
| 2,435 |
|
| — |
Net income attributable to common shareholders for diluted earnings per share | $ | 1,253,341 |
| $ | 6,699,926 |
12
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Earnings per common share (continued)
Diluted earnings per share (continued)
| 2011 |
| 2010 | ||
Weighted average shares used in basic computation |
| 27,784,732 |
|
| 26,697,892 |
Diluted effect of warrants |
| — |
|
| 7,576 |
Diluted effect of preferred stock |
| 309,912 |
|
| 342,088 |
Weighted average shares used in diluted computation |
| 28,094,644 |
|
| 27,047,556 |
Earnings per share: |
|
|
|
|
|
Diluted | $ | 0.04 |
| $ | 0.25 |
For the three months ended September 30, 2011 and 2010, a total of 2,001,000 and 1,938,500 warrants, respectively, have not been included in the calculation of diluted earnings per share in order to avoid any anti-dilutive effect.
The following is a reconciliation of the basic and diluted earnings per share computations for the nine months ended September 30, 2011 and 2010:
| 2011 |
| 2010 | ||
Net income | $ | 5,262,196 |
| $ | 17,951,106 |
Deduct: preferred stock dividends |
| (16,807 | ) |
| — |
Net income attributable for common shareholders for basic earnings per share | $ | 5,245,389 |
| $ | 17,951,106 |
|
|
|
|
|
|
Weighted average shares used in basic computation |
| 27,675,063 |
|
| 26,000,584 |
Earnings per share: |
|
|
|
|
|
Basic | $ | 0.19 |
| $ | 0.69 |
Diluted earnings per share
| 2011 |
| 2010 | ||
Net income attributable to common shareholders for basic earnings per share | $ | 5,245,389 |
| $ | 17,951,106 |
Add: interest expense |
| — |
|
| 24,277 |
Add: preferred stock dividends |
| 16,807 |
|
| — |
Net income attributable to common shareholders for diluted earnings per share | $ | 5,262,196 |
| $ | 17,975,383 |
|
|
|
|
|
|
Weighted average shares used in basic computation |
| 27,675,063 |
|
| 26,000,584 |
Diluted effect of warrants |
| 1,551 |
|
| 43,426 |
Diluted effect of preferred stock |
| 308,631 |
|
| 828,424 |
Weighted average shares used in diluted computation |
| 27,985,245 |
|
| 26,872,434 |
Earnings per share: |
|
|
|
|
|
Diluted | $ | 0.19 |
| $ | 0.67 |
For the nine months ended September 30, 2011 and 2010, a total of 1,938,500 and 200,000 warrants, respectively, have not been included in the calculation of diluted earnings per share in order to avoid any anti-dilutive effect.
13
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Accumulated other comprehensive income
The Company follows ASC 220 “Comprehensive Income” to recognize the elements of comprehensive income. Comprehensive income is comprised of net income and all changes to the statements of stockholders’ equity, except those due to investments by stockholders, changes in paid-in capital and distributions to stockholders. For the Company, accumulated other comprehensive income consisted of unrealized gains on foreign currency translation adjustments from the translation of financial statements from Chinese RMB to US dollars.
Segment reporting
ASC 280 requires use of the “management approach” model for segment reporting. The management approach model is based on how a company’s management organizes segments within the company for making operating decisions and assessing performance. Reportable segments are based on products and services, geography, legal structure, management structure, or any other manner in which management disaggregates a company. During the three and nine months ended September 30, 2011 and 2010, the Company operated in two business segments - (1) Wholesales segment and (2) Retail segment.
Recent accounting pronouncements
In May 2011, the FASB (“Financial Accounting Standard Board”) issued ASU (“Accounting Standards Update”) 2011-04, “Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS (International Financial Reporting Standards”)” (“ASU 2011-04”). The amendments in ASU 2011-04 result in common fair value measurement and disclosure requirements in U.S. GAAP and IFRSs. Consequently, ASU 2011-04 changes the wording used to describe many of the requirements in U.S. GAAP for measuring fair value and for disclosing information about fair value measurements. For many of the requirements, the FASB does not intend for the amendments in ASU 2011-04 to result in a change in the application of the requirements in Topic 820. ASU 2011-04 is effective prospectively for interim and annual reporting periods beginning after December 15, 2011. The Company does not expect the adoption of the provisions in ASU 2011-04 will have a significant impact on the Company’s consolidated financial statements.
In June 2011, the FASB issued ASU 2011-05, “Presentation of Comprehensive Income” (“ASU 2011-05”). In accordance with ASU 2011-05, an entity has the option to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In both choices, an entity is required to present each component of net income along with total net income, each component of other comprehensive income along with a total for other comprehensive income, and a total amount for comprehensive income. ASU 2011-05 eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. ASU 2011-05 does not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income. ASU 2011-05 is effective retrospectively for fiscal years, and interim periods within those years, beginning after December 15, 2011. The Company does not expect the adoption of this standard will have a significant impact on its consolidated financial statements.
In September 2011, the FASB issued Accounting Standards Update (“ASU”) No. 2011-08, Intangibles — Goodwill and Other (Topic 350). This Accounting Standards Update amends FASB ASC 350. This amendment specifies the change in method for determining the potential impairment of goodwill. It includes examples of circumstances and events that the entity should consider in evaluating whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. The amendments are effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. The Company does not expect the adoption of the provisions in ASU 2011-08 will have a significant impact on the Company’s consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on the Company’s consolidated financial statements upon adoption.
14
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BASIS OF PRESENTATION AND SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES (Continued)
Reclassifications
Certain prior period amounts have been reclassified to conform to the current period presentation. Leasing income was reclassified from retail revenue into other income. A portion of retail revenue was reclassified into wholesale revenue and a portion of cost of revenues – retail was reclassified into cost of revenues – wholesale. These reclassifications have no material impact on the previously reported financial position, results of operations and cash flows.
NOTE 2 – INVENTORIES
At September 30, 2011 and December 31, 2010, inventories consisted of the following:
|
| 2011 |
| 2010 | ||
Raw materials |
| $ | 280,974 |
| $ | 157,165 |
Packaging materials |
|
| 30,344 |
|
| 4,830 |
Finished goods |
|
| 957,557 |
|
| 472,588 |
Total |
| $ | 1,268,875 |
| $ | 634,583 |
NOTE 3 – PREPAID EXPENSES AND OTHER CURRENT ASSETS
At September 30, 2011 and December 31, 2010, prepaid expenses and other current assets consist of the following:
|
| 2011 |
| 2010 | ||
Advanced payment for research and development expenses |
| $ | — |
| $ | 453,734 |
Prepaid rent expenses |
|
| 126,871 |
|
| 123,343 |
Prepaid service fees |
|
| 1,575 |
|
| — |
Security deposit |
|
| 359 |
|
| 16,682 |
Total |
| $ | 128,805 |
| $ | 593,759 |
NOTE 4 – PROPERTY AND EQUIPMENT
At September 30, 2011 and December 31, 2010, property and equipment consists of the following:
| Useful life |
|
| 2011 |
|
| 2010 |
|
Office equipment and furniture | 3-10 Years |
| $ | 264,867 |
| $ | 254,564 |
|
Manufacturing equipment | 10-15 Years |
|
| 5,969,644 |
|
| 5,780,040 |
|
Construction-in-progress |
|
|
| 56,752,183 |
|
| 43,361,865 |
|
Total |
|
|
| 62,986,694 |
|
| 49,396,469 |
|
Less: accumulated depreciation |
|
|
| (10,705,744 | ) |
| (10,058,534 | ) |
Total |
|
| $ | 52,280,950 |
| $ | 39,337,935 |
|
At September 30, 2011, construction-in-progress amounted to $56,752,183, representing (i) costs incurred on the 1000 MU (approximately 667,000 square meters) of land for construction of a new manufacturing plant of approximately $7.5 million located in Cha Ha Er Industrial Park in Inner Mongolia, China, and (ii) costs incurred for construction of a new complex building of approximately $49.3 million in Beijing, China.
15
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 4 – PROPERTY AND EQUIPMENT (Continued)
Upon completion of the construction-in-progress and ready for their intended uses, such assets will be classified to their respective property and equipment categories.
For the three months ended September 30, 2011 and 2010, depreciation expense amounted to $100,879 and $5,936, respectively, of which $0 was included in cost of revenues since the Company did not perform any production during the three months ended September 30, 2011 and 2010. For the nine months ended September 30, 2011 and 2010, depreciation expense amounted to $312,176 and $18,856, respectively, of which $0 was included in cost of revenues since the Company did not perform any production during the nine months ended September 30, 2011 and 2010.
NOTE 5 – LAND USE RIGHT HELD FOR DEVELOPMENT OR SALE
The Company purchased 1,000 MU (approximately 667,000 square meters) of land in Inner Mongolia in 2008 (See Inner Mongolia New Facility under Note 13). The area for Inner Mongolia land which is held for development or sale is 900 MU (approximately 600,000 square meters). The area for the rest of Inner Mongolia land on which the Company expects to build a facility is 100 MU (approximately 67,000 square meters, See note 7).
Land use right held for development or sale is accounted for at the lower of cost or market. It is considered as long-term asset and free of amortization as it is not used in operations. Management intends to co-develop the land with another entity or sell it to a third party, but such partner or buyer has not been found yet. The Company’s management believe that the fair value of the land use right as of September 30, 2011 and concluded that the fair value of the right exceeded the carrying value and no impairment loss is recorded.
As of September 30, 2011 and December 31, 2010, land use right held for development or sale consists of the following:
|
| Lower of cost or market | ||||
|
| 2011 |
| 2010 | ||
Inner Mongolia land use right – 900 MU |
| $ | 30,195,957 |
| $ | 29,236,891 |
Total |
| $ | 30,195,957 |
| $ | 29,236,891 |
NOTE 6 – DEPOSITS AND INSTALLMENTS ON INTANGIBLE ASSETS
Deposit and Installments on a Chinese Class I drug patent-Laevo-Bambuterol
Pursuant to the technology transfer agreement the Company entered into in April 2008 (See Technology Transfer Agreement under Note 13), the Company previously made a deposit for future milestone requirements in order to acquire a Chinese Class I drug patent. Accordingly, $3,124,121 (RMB 20 million) was classified as a deposit on a patent as of September 30, 2011. Such deposit will be returned to the Company after all milestone payments in connection with the technology transfer agreement has been made.
Also, the Company has arranged an installment payment plan on the Chinese Class I drug patent to obtain the patent based on clinic milestones, as stipulated in the signed contract. The Company made $5,779,625 (RMB 37 million) as installment payments on the intangible assets as of September 30, 2011. The Company will need to make additional installment payments of approximately $1.7 million (RMB 11 million) to obtain the patent. The various milestone payments have been recorded as an asset and not expensed because all such payments are fully refundable if the studies are not ratified by the SFDA.
In addition, the Company expects to incur approximately $7.8 million (RMB 50 million) in research and development expense related to the Laevo-Bambuterol drug that has entered in Clinical Trial I in the next two years. The Company recorded $470,311 and $21,517 in research and development expenses for this drug for the three months ended September 30, 2011 and 2010, respectively. The Company recorded $1,889,535 and $21,517 in research and development expenses for this drug for the nine months ended September 30, 2011 and 2010, respectively.
16
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 6 – DEPOSITS AND INSTALLMENTS ON INTANGIBLE ASSETS (Continued)
Installments on Gliclazide-Controlled Release Tablets
Pursuant to the new drug patent transfer agreement the Company entered into in February 2009 (See New Drug Patent Transfer Agreement under Note 13), the Company has made the first installment to the transferor to obtain the patent. Hence, the Company recorded $937,236 (RMB 6 million) as installment payment on intangible assets as of September 30, 2011. In order to acquire the patent, the Company needs to make additional installments of approximately $469,000 (RMB 3 million). These payments have been recorded as an asset and not expensed as they are fully refundable if the studies are not ratified by the SFDA.
In addition, the Company expects to incur approximately $1.25 million (RMB 8 million) in research and development expense related to the Gliclazide-Controlled Release Tablets that was accepted by the China SFDA for medicine registration application in the next two years. The Company did not record any research and development expense for the drug for the three and nine months ended September 30, 2011 and 2010.
NOTE 7 – LAND USE RIGHTS AND OTHER INTANGIBLE ASSETS
Land Use Rights
All land in the PRC is owned by the PRC government and cannot be sold to any individual or company. The Company has recorded the amounts paid to acquire long-term interests to utilize land underlying the Company’s facilities as land use rights. This type of arrangement is common for the use of land in the PRC. Land use rights are amortized on the straight-line method over the terms of the land use rights, which range from 40 to 50 years. The Company acquired one parcel of land use right in Beijing and the other parcel of land use right in Inner Mongolia, China. The Company has received land use right certificate on the parcel of land in Beijing. However, as of the filing date of this report, the Company has not received land use right certificate on the parcel of land in Inner Mongolia. The delay is common in China. As described elsewhere in this report, the area for the Inner Mongolia land on which the Company expects to build a facility is 100 MU (approximately 67,000 square meters).The Company acquired the parcel of Beijing land use right in the amount of approximately $10.4 million (RMB 66,769,504) and the 100 MU (approximately 67,000 square meters) Inner Mongolia land use right in the amount of approximately $3.5 million (RMB 22,366,000), which are included in land use rights.
At September 30, 2011 and December 31, 2010, land use rights consist of the following:
|
| 2011 |
| 2010 |
| ||
Beijing land use right |
| $ | 10,429,802 |
| $ | 10,098,537 |
|
Inner Mongolia land use right |
|
| 3,493,705 |
|
| 3,382,740 |
|
Total |
|
| 13,923,507 |
|
| 13,481,277 |
|
Less: accumulated amortization |
|
| (811,603 | ) |
| (548,856 | ) |
Total |
| $ | 13,111,904 |
| $ | 12,932,421 |
|
The projected amortization expense for land use rights attributed to future periods is as follows:
Twelve-month periods ending September 30: |
| Expense | |
2012 |
| $ | 326,324 |
2013 |
|
| 326,324 |
2014 |
|
| 326,324 |
2015 |
|
| 326,324 |
2016 |
|
| 326,324 |
2017 and thereafter |
|
| 11,480,284 |
Total |
| $ | 13,111,904 |
17
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 7 – LAND USE RIGHTS AND OTHER INTANGIBLE ASSETS (Continued)
Other Intangible Assets
On October 9, 2006, the Company entered into a five-year loan agreement (the “Loan Agreement”) and a contract with Wu Lan Cha Bu Emergency Hospital (“Wu Lan”), whereby the Company agreed to lend Wu Lan approximately $4.7 million (RMB 30 million) for the construction of a hospital ward in Inner Mongolia, China. In exchange for the loan, Wu Lan agreed to grant the Company an exclusive right to supply all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty years. In October 2006, the Company’s chief executive officer, Dr. Zhongyi Liu (hereafter, “Dr. Liu”), made this loan to Wu Lan on behalf of the Company. On October 21, 2006, the Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Dr. Liu, except the rights to receive revenues from the sale of medical and disposable medical treatment apparatus (“Revenue Rights”). Since Dr. Liu accepted the assignment with all the risks and obligations but no right to revenues from the sale of medical and disposable medical treatment apparatus, the Company agreed to pay Dr. Liu compensation of approximately $1.4 million (RMB 9 million) in five (5) equal annual installments of approximately $281,000 (RMB 1.8 million) commencing October 21, 2006. Accordingly, the Company recorded an intangible asset of approximately $1.4 million (RMB 9 million) related to the exclusive rights to provide all medicines and disposable medical treatment apparatus to Wu Lan for a period of twenty (20) years. The Company amortizes this exclusive right over a term of 20 years.
The Company entered into an intellectual rights transfer contract with Beijing Yipuan Bio-Medical Technology Co., Ltd. in December 2008 to acquire the drug property right of Yipubishan. The intellectual property right is valued at a fixed amount of RMB 54 million (approximately $8 million). The Company paid the transfer fee in full for the intellectual property right to Yipuan. The intellectual property right has a term of 10 years and will not expire until December 31, 2018. The Company amortizes the intellectual property right over the term of the intellectual property right.
At September 30, 2011 and December 31, 2010, other intangible assets consist of the following:
|
| 2011 |
| 2010 |
| ||
Revenue rights |
| $ | 1,405,855 |
| $ | 1,361,203 |
|
Intellectual rights |
|
| 8,435,128 |
|
| 8,167,216 |
|
Software |
|
| 11,558 |
|
| 11,192 |
|
Total |
|
| 9,852,541 |
|
| 9,539,611 |
|
Less: accumulated amortization |
|
| (2,682,553 | ) |
| (1,932,126 | ) |
Total |
| $ | 7,169,988 |
| $ | 7,607,485 |
|
The projected amortization expense for other intangible assets attributed to future periods is as follows:
Twelve-month periods ending September 30: |
| Expense | |
2012 |
| $ | 913,936 |
2013 |
|
| 913,805 |
2014 |
|
| 913,805 |
2015 |
|
| 913,805 |
2016 |
|
| 913,805 |
2017 and thereafter |
|
| 2,600,832 |
Total |
| $ | 7,169,988 |
Amortization expense amounted to approximately $309,389 and $441,849 for the three months ended September 30, 2011 and 2010, respectively. Amortization expense amounted to approximately $916,865 and $1,318,468 for the nine months ended September 30, 2011 and 2010, respectively.
18
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 8 – OTHER PAYABLES AND ACCRUED LIABILITIES
At September 30, 2011 and December 31, 2010, other payables and accrued liabilities consist of the following:
|
| 2011 |
| 2010 | ||
Construction payable |
| $ | — |
| $ | 120,996 |
Refundable deposit |
|
| 158,549 |
|
| 153,513 |
Accrued sales commission |
|
| — |
|
| 1,512,447 |
Accrued payroll and employees benefit |
|
| 392,159 |
|
| 1,280,394 |
Other |
|
| 172,849 |
|
| 374,116 |
Total |
| $ | 723,557 |
| $ | 3,441,466 |
NOTE 9 – TAXES
Income Tax
The Company is subject to income taxes on an entity basis on income arising in or derived from the tax jurisdiction in which each entity is domiciled.
Lotus Pharmaceuticals Inc. was incorporated in the United States and has an aggregate U.S. net operating loss carryforward of approximately $8,241,000 as of September 30, 2011, subject to the Internal Revenue Code Section 382, which places a limitation on the amount of taxable income that can be offset by net operating losses after a change in ownership. The net operating loss carries forward for United States income taxes, which may be available for offsetting against future taxable U.S. income. These carryforwards will expire, if not utilized, through 2031.
Management believes that the realization of the benefits from these loss carryforwards appears uncertain due to the Company’s limited operating history and continuing losses for United States income tax purposes. Accordingly, the Company has provided a 100% valuation allowance on the deferred tax asset to reduce the asset to zero. Management will review this valuation allowance periodically and make adjustments as needed. The valuation allowance at September 30, 2011 and December 31, 2010 was approximately $2,802,000 and $2,036,000, respectively. The net change in the valuation allowance was an increase of approximately $766,000 and $434,000 during the nine months ended September 30, 2011 and 2010, respectively. The consolidated income is earned overseas and will continue to be indefinitely reinvested in overseas operations. Accordingly, no provision has been made for U.S. deferred taxes related to future repatriation of these earnings, nor is it practicable to estimate the amount of income taxes that would have to be provided if we concluded that such earnings will be remitted in the future.
Deferred tax assets and liabilities are provided for significant income and expense items recognized in different years for income tax and financial reporting purposes. Temporary differences, which give rise to a net deferred tax asset for the Company as of September 30, 2011 and December 31, 2010 is as follows:
|
| 2011 |
| 2010 |
| ||
Tax benefit of net operating loss carryforward |
| $ | 2,802,000 |
| $ | 2,036,000 |
|
Valuation allowance |
|
| (2,802,000 | ) |
| (2,036,000 | ) |
Net deferred tax asset |
| $ | — |
| $ | — |
|
Lotus Pharmaceutical International, Inc. was incorporated in the United States and it was an affiliated group of Lotus Pharmaceuticals Inc. for United States income tax purpose and it did not have any business activity. Therefore, no income tax provision was made for Lotus Pharmaceutical International, Inc.
Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. and Lotus East were incorporated in the PRC and are subject to PRC income tax which is computed according to the relevant laws and regulations in the PRC.
19
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 9 – TAXES (Continued)
Income Tax (continued)
Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd. did not have any business activity for PRC income tax purpose. Accordingly, no income tax provision was made for Lotus Century Pharmaceutical (Beijing) Technology Co., Ltd pursuant to the related PRC income tax law.
Beijing Liang Fang was subject to 25% income tax rate since January 1, 2009. Located in Inner Mongolia, Liang Fang’s branch received income tax exemption for its fiscal 2011, 2010 and 2009 taxable income from the Cha You Qian Qi government situated in Inner Mongolia, P.R.C. on June 3, 2008.
The table below summarizes the differences between the U.S. statutory federal rate and the Company’s effective tax rate for the nine months ended September 30, 2011 and 2010:
| 2011 |
| 2010 |
|
US statutory rate | 34.00% |
| 34.00% |
|
Foreign income not recognized in the US | (34.00% | ) | (34.00% | ) |
China statutory rate | 25.00% |
| 25.00% |
|
China income tax exemption | (25.00% | ) | (22.45% | ) |
Effective income tax rate | 0.00% |
| 2.55% |
|
The estimated tax savings as a result of the income tax exemption from Liang Fang’s branch for the three months ended September 30, 2011 and 2010 amounted to approximately $351,000 and $1,615,000, respectively. The net effect on earnings per share had the income tax been applied would decrease basic earnings per share for the three months ended September 30, 2011 and 2010 from $0.05 to $0.03 and from $0.25 to $0.19, respectively. The estimated tax savings as a result of the income tax exemption from Liang Fang’s branch for the nine months ended September 30, 2011 and 2010 amounted to approximately $1,934,000 and $4,497,000, respectively. The net effect on earnings per share had the income tax been applied would decrease basic earnings per share for the nine months ended September 30, 2011 and 2010 from $0.19 to $0.12 and from $0.69 to $0.52, respectively.
Value Added Tax
Enterprises or individuals who sell commodities, engage in repair and maintenance or import and export goods in the PRC are subject to a value added tax, or VAT, in accordance with Chinese laws. The applicable VAT tax rate is 17% for products sold in the PRC. The amount of VAT liability is determined by applying the applicable tax rate to the invoiced amount of goods sold (output VAT) less VAT paid on purchases made with the relevant supporting invoices (input VAT).
VAT on sales and VAT on purchases amounted to approximately $3,238,000 and $280,000 for the three months ended September 30, 2011 and $3,246,000, and $343,000 for the three months ended September 30, 2010, respectively. VAT on sales and VAT on purchases amounted to approximately $8,612,000 and $944,000 for the nine months ended September 30, 2011 and $8,990,000, and $867,000 for the nine months ended September 30, 2010, respectively. Sales and purchases are recorded net of VAT collected and paid as the Company acts as an agent. As of September 30, 2011 and December 31, 2010, the VAT payable amounted to $9,663,161 and $1,874,078, respectively.
Taxes Payable
At September 30, 2011 and December 31, 2010, taxes payable (prepaid) are as follows:
|
| 2011 |
| 2010 |
| ||
Value added tax |
| $ | 9,663,161 |
| $ | 1,874,078 |
|
Corporation income tax |
|
| (62,954 | ) |
| (56,223 | ) |
Other taxes |
|
| 995,326 |
|
| 206,710 |
|
Total |
| $ | 10,595,533 |
| $ | 2,024,565 |
|
20
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – RELATED PARTY TRANSACTIONS
Notes Payable – Related Parties
Notes payable - related parties consisted of the following at September 30, 2011 and December 31, 2010:
|
| 2011 |
| 2010 | ||
Note to Guoan Song, father of Zhenghong Song who is the spouse of the company’s CEO, Zhongyi Liu, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% at September 30, 2011 and December 31, 2010), and unsecured. |
| $ | 815,735 |
| $ | 789,826 |
Note to Guixin Zheng, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% at September 30, 2011 and December 31, 2010), and unsecured. |
|
| 1,765,910 |
|
| 1,709,822 |
Note to Zhaozhao Ma, employee, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% at September 30, 2011 and December 31, 2010), and unsecured. |
|
| 707,242 |
|
| 684,779 |
Note to Zhongyi Liu, CEO and director, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% at September 30, 2011 and December 31, 2010), and unsecured. |
|
| 1,505,459 |
|
| 1,457,643 |
Note to Zhenghong Song, spouse of the Company’s Chief Executive Officer, Zhongyi Liu, due on December 30, 2015 with variable annual interest at 80% of current bank rate (4.6869% at September 30, 2011 and December 31, 2010), and unsecured. |
|
| 619,432 |
|
| 599,759 |
|
|
|
|
|
|
|
Total notes payable – related parties, long term |
| $ | 5,413,778 |
| $ | 5,241,829 |
For the three months ended September 30, 2011 and 2010, the Company recorded a total interest expense of $63,223 and $59,896 related to those loans, respectively. For the nine months ended September 30, 2011 and 2010, the Company recorded a total interest expense of $187,256 and $178,729 related to those loans, respectively.
Due to Related Parties
The Chief Executive Officer of the Company Dr. Zhongyi Liu and his spouse and several key employees of the Company, from time to time, provided advances to the Company for working capital purposes. During the nine months ended September 30, 2011 and 2010, the Company did not repay any of these advances. At September 30, 2011 and December 31, 2010, the Company owed to its Chief Executive Officer and his spouse and other employees an aggregate of $769,314 and $744,879, respectively. These advances are short-term in nature and non-interest bearing and unsecured.
As discussed in Note 7, the Company entered into a five-year loan agreement and a contract with Wu Lan, whereby the Company agreed to lend Wu Lan approximately $4.7 million (RMB 30 million) for the construction of a hospital ward in Inner Mongolia, China. The Company’s CEO, Dr. Liu, made this loan to Wu Lan on behalf of the Company. In return, the Company entered into an assignment agreement whereby the Company assigned all of its rights, obligations, and receipts under the Loan Agreement to Dr. Liu, except the rights to receive revenues from the sale of medical and disposable medical treatment apparatus. Since Dr. Liu accepted the assignment with all the risks and obligations but had no right to revenues from the sale of medical and disposable medical treatment apparatus, the Company agreed to pay Dr. Liu compensation of an aggregate of approximately $1.4 million (RMB 9 million) in 5 equal annual installments of approximately $281,000 (RMB 1.8 million) started from October 21, 2006. For the nine months ended September 30, 2011 and 2010, the Company did not pay anything to Dr. Liu for the liability incurred by the assignment in the agreement mentioned above. At September 30, 2011 and December 31, 2010, amounts due under this assignment agreement were $1,103,888 and $1,068,827, respectively, and have been included in current portion of due to related parties on the accompanying condensed consolidated balance sheets, respectively.
21
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 10 – RELATED PARTY TRANSACTIONS (Continued)
Due to Related Parties (continued)
At September 30, 2011 and December 31, 2010, the Company has recorded accrued interest relating to notes payable - related parties of $1,087,879 and $869,067, respectively, which have been included in due to related parties – long-term on the accompanying condensed consolidated balance sheets. The accrued and unpaid interest relating to notes payable will be paid in full on the due date of the notes in accordance with the loan agreement. Therefore, the accrued interest is long-term in nature.
During the nine months ended September 30, 2011 and 2010, the Chief Executive Officer of the Company, Dr. Zhongyi Liu, from time to time, made payments to various unrelated third parties on behalf of the Company. At September 30, 2011 and December 31, 2010, the Company owed its Chief Executive Officer $469,626 and $228,670, respectively. These advances are short-term in nature, non-interest bearing and unsecured.
For the nine months ended September 30, 2011, a summary of activities in due to related parties is as follows:
|
| Assignment |
| Working |
| Accrued |
| Payments made |
| Total |
| |||||
Balance - December 31, 2010 |
| $ | 1,068,827 |
| $ | 744,879 |
| $ | 869,067 |
| $ | 228,670 |
| $ | 2,911,443 |
|
Additions |
|
| — |
|
| — |
|
| 187,256 |
|
| 313,791 |
|
| 501,047 |
|
Repayment |
|
| — |
|
| — |
|
| — |
|
| (76,859 | ) |
| (76,859 | ) |
Foreign currency fluctuations |
|
| 35,061 |
|
| 24,435 |
|
| 31,556 |
|
| 4,024 |
|
| 95,076 |
|
Balance – September 30, 2011 |
| $ | 1,103,888 |
| $ | 769,314 |
| $ | 1,087,879 |
| $ | 469,626 |
| $ | 3,430,707 |
|
NOTE 11 – STOCKHOLDERS’ EQUITY
February 2008 Preferred Stock and Warrant Purchase Agreement
On February 25, 2008 (“Closing Date”), pursuant to a Convertible Redeemable Preferred Share and Warrant Purchase Agreement (the “Purchase Agreement”) by and among the Company, Dr. Zhongyi Liu and Mrs. Zhenghong Song (the “Founders”), and accredited investors (each a “Purchaser” and collectively, the “Purchasers”), the Company issued to the Purchasers an aggregate of 5,747,118 shares of the Company’s Preferred Stock, par value $0.001 per share, at a price equal to $0.87 per share (the “Preferred Shares”). In February 2008, the convertible redeemable preferred stock was deemed debt due to the mandatory redeemable feature of the Preferred Stock according to ASC 480 “Accounting for Certain Financial Instruments with Characteristics of both Liabilities and Equity”. In addition, the Company issued to the Purchasers warrants to purchase up to 1,436,777 shares of the Company’s common stock in the aggregate. The Warrants have an exercise price of $2.41. The warrants are exercisable for a period of five years from the closing date. Holders of the warrants may not exercise the warrants if the exercise would result in the holder beneficially owning more than 4.99% of the Company’s outstanding common shares. That limitation may be waived by a holder of the warrants upon not less than 61 days written notice to the Company.
Each of these Preferred Shares was convertible into 0.5 share of the Company’s common stock (as adjusted for stock splits, stock dividends, reclassification and the like), pays an 8% dividend annually, payable in additional Convertible Preferred Shares and also pays any dividend to be paid on the common shares on an as-converted basis. Until May 25, 2010, the Preferred Shares could be redeemed at the option of the Purchasers at the redemption price of $0.87 per share (as adjusted for stock splits, stock dividends, reclassification and the like), and no other capital stock of the Company may be redeemable prior to the Preferred Shares. Holders of Preferred Shares may not convert Preferred Shares to common shares if the conversion would result in the holder beneficially owning more than 4.99% of the Company’s outstanding common shares. That limitation may be waived by a holder of Preferred Shares upon not less than 61 days written notice to the Company. The Preferred Shares vote with the common stock on an as converted basis, except that the Preferred Shares are not entitled to vote for directors. The Company is prohibited from taking certain corporate actions, including, without limitation, issuing securities senior to the Preferred Shares, selling substantially all assets, repurchasing securities and declaring or paying dividends, without the approval of the holders of a majority of the Preferred Shares then outstanding.
22
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 – STOCKHOLDERS’ EQUITY (Continued)
February 2008 Preferred Stock and Warrant Purchase Agreement (continued)
Under the designations of related agreements, rights and preferences of the Preferred Stock, the Company could be required to redeem the Preferred Stock at the option of the holder for a period of 90 days beginning on February 25, 2010. The redemption price which is equal to $0.87 per share plus any accrued but unpaid dividends must be paid in cash, in one lump sum within one month from the end of the 90 days period. Following the end of said 90 days period, the Series A Preferred Stock is not redeemable. None of the preferred stockholders required the redemption of the Preferred Stock by the end of the applicable redemption period. Since the redemption feature of the Preferred Stock lapsed, the Company reclassified the existing carrying amount of the convertible Preferred Stock from debt to equity on May 26, 2010 in accordance with the provisions of ASC 480. Accordingly, the 684,176 shares of outstanding convertible Preferred Stock were recorded as equity on May 26, 2010.
In February 2011, the Company issued 12,717 shares of Preferred Stock to the holders of Series A Preferred Stock for their dividends. These Preferred Shares have the same terms as the Preferred Shares issued in February 2008 per the February 2008 Preferred Stock and Warrant Purchase Agreement.
During the period from May 26, 2010 through September 30, 2011, 77,069 shares of preferred stock were converted into 38,535 shares of common stock.
Common Stock
In January 2011, the Company issued 5,000 shares of its common stock to a lawyer in connection with legal service rendered. The shares were valued at the fair value of $2.27 per share on the grant date. In connection with the issuance of these shares, the Company reduced accrued expense by $11,350.
In January 2011, the Company issued 80,000 shares of its common stock to its four newly appointed directors in connection with service rendered and to be rendered. The shares were valued at the fair value of $2.05 per share on the grant date. In connection with the issuance of these shares, the Company recorded director fees of $164,000.
In January 2011, the Company issued 340,000 shares of its common stock to four consultants in connection with services rendered and to be rendered. The shares were valued at the fair value of $2.05 per share on the grant date. In connection with the issuance of these shares, the Company recorded consulting expense of $697,000.
In January 2011, the Company issued 30,000 shares of its common stock to its vice president of corporate development in connection with services rendered and to be rendered. The shares were valued at the fair value of $2.05 per share on the grant date. In connection with the issuance of these shares, the Company recorded stock-based compensation of $61,500.
In January 2011, the Company issued 47,383 shares of its common stock to its chief executive officer in connection with services rendered. The shares were valued at the fair value of $2.216 per share on the grant date. In connection with the issuance of these shares, the Company reduced accrued expense by $105,000.
In January 2011, the Company issued 451,263 shares of its common stock to its chief executive officer in connection with services rendered and to be rendered. The shares were valued at the fair value of $2.216 per share on the grant date. In connection with the issuance of these shares, the Company recorded stock-based compensation of $1,000,001.
In February 2011, the Company issued 30,000 shares of its common stock to a key person in connection with services rendered. The shares were valued at the fair value of $1.92 per share on the grant date. In connection with the issuance of these shares, the Company recorded compensation expense of $57,600.
23
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 11 – STOCKHOLDERS’ EQUITY (Continued)
Common Stock (continued)
In July 2011, the Company issued 26,847 shares of its common stock to an attorney in connection with legal service rendered. The shares were valued at $0.45 per share on the grant date based on market price. In connection with the issuance of these shares, the Company recorded professional fees of $102,081.
In August 2011, the Company issued 9,724 shares of its common stock to an attorney in connection with legal service rendered. The shares were valued at $0.617 per share on the grant date based on market price. In connection with the issuance of these shares, the Company recorded professional fees of $6,000.
In September 2011, the Company issued 17,225 shares of its common stock to an attorney in connection with legal service rendered. The shares were valued at $0.351 per share on the grant date based on market price. In connection with the issuance of these shares, the Company recorded professional fees of $6,046.
Statutory Reserve
The Company is required to make appropriations to a statutory reserve, based on after-tax net income determined in accordance with generally accepted accounting principles of the PRC (“PRC GAAP”). Appropriations to the statutory reserve is required to be at least 10% of the after tax net income determined in accordance with PRC GAAP until the reserve is equal to 50% of the entities’ registered capital.
The statutory reserve fund is non-distributable other than during liquidation and can be used to fund previous years’ losses, if any, and may be utilized for business expansion or converted into share capital by issuing new shares to existing shareholders in proportion to their shareholding or by increasing the par value of shares currently held by them, provided that the remaining statutory reserve balance after such issue is not less than 25% of the registered capital.
The Company’s statutory reserve has reached 50% of its registered capital as of March 31, 2010. As such, no additional reserve was recorded during the nine months ended September 30, 2011. The Company’s statutory reserve was $6,240,202 at September 30, 2011 and December 31, 2010.
Stock Warrants
There were no stock warrants issued, terminated/forfeited and exercised during the nine months ended September 30, 2011.
The following table summarizes the shares of the Company’s common stock issuable upon exercise of warrants outstanding and exercisable at September 30, 2011:
Warrants outstanding and exercisable | ||||||||
Exercise |
| Number |
| Weighted average |
| Weighted average | ||
$ | 1.74 |
| 62,500 |
| 0.37 |
| $ | 1.74 |
| 2.40 |
| 1,436,777 |
| 1.41 |
|
| 2.40 |
| 2.41 |
| 301,723 |
| 1.41 |
|
| 2.41 |
| 3.00 |
| 125,000 |
| 0.32 |
|
| 3.00 |
$ | 3.82 |
| 75,000 |
| 3.28 |
|
| 3.82 |
|
|
| 2,001,000 |
| 1.38 |
| $ | 2.47 |
24
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 – SEGMENT INFORMATION
The following information is presented in accordance with ASC 280, “Segment Reporting”. In the nine months ended September 30, 2011 and 2010, the Company operated in two reportable business segments: (1) Wholesale segment: the manufacture and distribution of pharmaceutical products; and (2) Retail segment: the retailing of western and traditional Chinese medications and medical treatment equipment through its own ten drug stores and direct sales to other drug stores located in Beijing China. The Company’s reportable segments are strategic business units that offer different products. They are managed separately based on the fundamental differences in their operations.
Selected financial information with respect to these reportable business segments for the three months ended September 30, 2011 and 2010 was as follows:
For the three months ended September 30, 2011 |
| Wholesale |
| Retail |
| Unallocated |
| Total |
| ||||
Net revenues |
| $ | 15,276,053 |
| $ | 3,768,485 |
| $ | — |
| $ | 19,044,538 |
|
Cost of revenues |
|
| (12,855,958 | ) |
| (2,874,778 | ) |
| — |
|
| (15,730,736 | ) |
Operating expenses |
|
| — |
|
| — |
|
| (2,045,394 | ) |
| (2,045,394 | ) |
Other income (expense) |
|
| — |
|
| — |
|
| (15,067 | ) |
| (15,067 | ) |
Income tax |
|
| — |
|
| — |
|
| — |
|
| — |
|
Net income |
| $ | 2,420,095 |
| $ | 893,707 |
| $ | (2,060,461 | ) | $ | 1,253,341 |
|
For the three months ended September 30, 2010 |
| Wholesale |
| Retail |
| Unallocated |
| Total |
| ||||
Net revenues |
| $ | 15,347,372 |
| $ | 2,957,488 |
| $ | — |
| $ | 18,304,860 |
|
Cost of revenues |
|
| (5,791,825 | ) |
| (2,133,906 | ) |
| — |
|
| (7,925,731 | ) |
Operating expenses |
|
| — |
|
| — |
|
| (3,619,558 | ) |
| (3,619,558 | ) |
Other income (expense) |
|
| — |
|
| — |
|
| 140,703 |
|
| 140,703 |
|
Income tax |
|
| — |
|
| — |
|
| (200,348 | ) |
| (200,348 | ) |
Net income |
| $ | 9,555,547 |
| $ | 823,582 |
| $ | (3,679,203 | ) | $ | 6,699,926 |
|
Selected financial information with respect to these reportable business segments for the nine months ended September 30, 2011 and 2010 was as follows:
For the nine months ended September 30, 2011 |
| Wholesale |
| Retail |
| Unallocated |
| Total |
| ||||
Net revenues |
| $ | 38,585,730 |
| $ | 12,070,485 |
| $ | — |
| $ | 50,656,215 |
|
Cost of revenues |
|
| (27,287,898 | ) |
| (9,014,537 | ) |
| — |
|
| (36,302,435 | ) |
Operating expenses |
|
| — |
|
| — |
|
| (9,047,515 | ) |
| (9,047,515 | ) |
Other income (expense) |
|
| — |
|
| — |
|
| (43,920 | ) |
| (43,920 | ) |
Income tax |
|
| — |
|
| — |
|
| (149 | ) |
| (149 | ) |
Net income |
| $ | 11,297,832 |
| $ | 3,055,948 |
| $ | (9,091,584 | ) | $ | 5,262,196 |
|
For the nine months ended September 30, 2010 |
| Wholesale |
| Retail |
| Unallocated |
| Total |
| ||||
Net revenues |
| $ | 42,309,596 |
| $ | 9,652,235 |
| $ | — |
| $ | 51,961,831 |
|
Cost of revenues |
|
| (16,318,673 | ) |
| (6,956,438 | ) |
| — |
|
| (23,275,111 | ) |
Operating expenses |
|
| — |
|
| — |
|
| (10,260,875 | ) |
| (10,260,875 | ) |
Other income (expense) |
|
| — |
|
| — |
|
| (4,225 | ) |
| (4,225 | ) |
Income tax |
|
| — |
|
| — |
|
| (470,514 | ) |
| (470,514 | ) |
Net income |
| $ | 25,990,923 |
| $ | 2,695,797 |
| $ | (10,735,614 | ) | $ | 17,951,106 |
|
25
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 12 – SEGMENT INFORMATION(Continued)
The Company does not allocate operating expenses, other income/expense and income tax expense to its reportable segments, because these activities are managed at a corporate level. In addition, the specified amounts for depreciation and amortization, interest expense and income tax expense are not included in the measure of segment profit or loss reviewed by the chief operating decision maker and these specified amounts are not regularly provided to the chief operating decision maker. Therefore, the Company has not disclosed depreciation and amortization, interest expense and income tax expense for each reportable segment.
Asset information by reportable segment is not reported to or reviewed by the chief operating decision maker and, therefore, the Company has not disclosed asset information for each reportable segment. Substantially all of the Company’s assets are located in China.
NOTE 13 – COMMITMENTS AND CONTINGENCIES
Technology Transfer Agreement
In April 2008, one of the Company’s affiliates, En Ze Jia Shi, entered into a Technology Transfer Agreement with Dong Guan Kai Fa Biological Medicine LTD (“Dong Guan”) pursuant to which Dong Guan agreed to transfer the technology material, new medicine research and rights to the Chinese patent of the anti-asthma new medicine R-BM to En Ze Jia Shi on an exclusive basis in exchange for a transfer technology fee of approximately $7.5 million (RMB 48 million) to be paid at various intervals. Under the terms of the agreement, En Ze Jia Shi is obligated to:
| · | complete the filing with the SFDA of the medicine’s clinical research ratification document, |
|
|
|
| · | complete the clinical research, |
|
|
|
| · | complete the medicine’s trial production, and |
|
|
|
| · | provide raw materials and formulation related documentation and apply for the new medicine certification and production approval. |
In addition to the payment of the technology transfer fee, En Ze Jia Shi is responsible for paying all costs associated with its responsibility under the agreement which are presently estimated at $7.8 million (RMB 50 million). RMB of 12,880,000 (approximately $2 million) was incurred as of September 30, 2011. Lotus East intends to use its working capital to fund the related costs.
Dong Guan is responsible for preparing and transferring the clinical research and application documents as well as assisting En Ze Jia Shi in the completing the clinical research and applying for the new medicine certification and production approval documents.
Under the terms of the agreement, the technology transfer fee is to be paid upon the following schedule:
| · | Approximately $1.56 million (RMB 10 million) is due by the 15th business day following the receipt of the processing notice of the receipt of the clinical application and all related material from SFDA is received, |
|
|
|
| · | Approximately $1.25 million (RMB 8 million) is due by the 10th business day after the receipt of the medicine’s clinical ratification document, |
|
|
|
| · | Approximately $1.56 million (RMB 10 million) is due by the 15th business day after the medicine’s Phase I clinical study is completed and ratification from the SFDA is obtained, and |
|
|
|
| · | Approximately $3.12 million (RMB 20 million) is due by the 10th business day after the medicine’s Phase II clinical study is completed and ratification from the SFDA is obtained. |
26
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 13 – COMMITMENTS AND CONTINGENCIES (Continued)
Technology Transfer Agreement (continued)
As of September 30, 2011, the Company has made payments of $5.8 million (RMB 37 million) in accordance to the above schedule and the drug is on the Phase I clinical study now.
En Ze Jia Shi paid Dong Guan a deposit of approximately $3.12 million (RMB 20 million) in April 2008 which is to be returned to En Ze Jia Shi within 10 days after the transfer technology fee is fully paid. In the event Dong Guan should be unable to timely return the deposit, it will pay En Ze Jia Shi a late fee and En Ze Jia Shi is entitled to damages for Dong Guan’s failure to timely return the deposit.
The intellectual property arising from the agreement will be jointly shared by the parties. In addition, En Ze Jia Shi has guaranteed that both parties must jointly apply for related government grants prior to when the new medicine is marketed. Upon receipt of the government grants En Ze Jia Shi guaranteed that the grant monies will be shared equally by both parties. As of September 30, 2011, the Company has not received any government grant. The agreement can be terminated by Dong Guan if En Ze Jia Shi should fail to make any of the aforedescribed payments in which event the patent rights would revert to Dong Guan and it is entitled to transfer the project rights to a third party.
Once the drug is marketed, the Company is obligated to pay 3% of revenue derived from the drug to Dong Guan.
Inner Mongolia New Facility
In June 2008, one of the Company’s affiliates, Liang Fang, entered into an agreement with Cha You Qian Qi Economy Commission, a governmental agency (“Cha You”) related to the construction of a pharmaceutical plant in Cha You’s Cha Ha Er Industrial Garden District in Inner Mongolia. The new facility, which will be comprised of approximately 40,000 square meters situated on 600 MU of land (approximately 400,200 square meters), will be used to expand Liang Fang’s business. The Company was subsequently granted the right to expand the land use right area to 1,000 MU (approximately 667,000 square meters) (See note 5 and note 7). The project will require a total investment of RMB 320 million, or approximately $49,986,000 besides the payment for the land use right. The construction of the project began in August 2008 and the Company anticipates that it will take a few years to complete the construction of the project.
Liang Fang intends to use its present working capital together with bank loans and/or government grants and/or third party financing to fund the project. The funds are required to be invested over the estimated construction period of the project. As of September 30, 2011, Liang Fang has paid approximately $3.5 million (approximately RMB 22.37 million) of the total investment that was recorded as land use rights on the accompanying balance sheet. Liang Fang, however, has not secured either the bank loans or government grants and does not have sufficient working capital to complete this project without securing substantial funds from third party sources.
Under the terms of the agreement, Cha You agreed to abate fees associated with water resources, waste and other relative supplies for a period of 30 years and agreed to ensure that the land use tax to be paid by Liang Fang after it begins normal production will be at the lowest tax rate imposed for five years. Once the project is completed, for a period of eight years the local reserved portion of the imposed corporation income tax will be returned to Liang Fang.
New Drug Patent Transfer Agreement
In February 2009, one of the Company’s affiliates, En Ze Jia Shi, entered into a New Drug Patent Transfer Agreement with Beijing Huicheng Ruixiang Pharmaceutical Technology Co. LTD (“Huicheng”) pursuant to which Huicheng agreed to transfer the patent technology and related research materials about the Chinese drug of Gliclazide-Controlled Released Tablets to En Ze Jia Shi on an exclusive basis in exchange for a transfer patent fee of approximately $1.4 million (RMB 9 million) to be paid at various intervals. Under the terms of the agreement, En Ze Jia Shi is obligated to:
27
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 13 – COMMITMENTS AND CONTINGENCIES (Continued)
New Drug Patent Transfer Agreement (continued)
| · | Finishing other needed related technical materials of this new medicine and providing the legal invoice for raw materials and purchase agreement, |
|
|
|
| · | Providing enough raw materials, to enable Huicheng to prepare new medicine of 100,000 dosage units, and standard samples for experiments and research, |
|
|
|
| · | Providing the choice basis and quality standards of the package materials, |
|
|
|
| · | Paying for organization, seal, signature, field-exam, registration and related fees including registration and examination fees, registration evaluation fees etc. for the new medicine registration materials, |
|
|
|
| · | Completing clinical research and paying related fees if the new medicine is required for clinical research, and |
|
|
|
| · | Making payment to Huicheng according to specific schedule mentioned in the agreement. |
In addition to the payment of the patent transfer fee, En Ze Jia Shi is responsible for paying all costs associated with its responsibility under the agreement which are presently estimated at approximately $1.25 million (RMB 8 million). Lotus East intends to use its working capital to fund the project costs.
Huicheng Ruixiang Pharmaceutical Technology Co. Ltd. is obligated to
| · | Provide the patent of the new medicine, |
|
|
|
| · | Provide the related technical materials and the original records of the experiments which satisfy the requirement of the fifth classified chemical drug registration by Drug Registration Administration Method (2005) issued by Chinese SFDA. The Huicheng has to provide above mentioned materials to En Ze Jia Shi in 30 days after it received first installment payment and qualified documents from En Ze Jia Shi, |
|
|
|
| · | Provide the new medicine registration samples with 100,000 dosage units, and |
|
|
|
| · | Supplement and improve the technical materials according to the requirement of the Evaluation Center of Chinese State Drug Administration. |
Under the terms of the agreement, the technology transfer fee is to be paid upon the following schedule:
| · | Approximately $0.94 million (RMB 6 million) is due by the 90th business day following the receipt of the Notice of China Accepted Patent and Notice of Medicine Registration Application, |
|
|
|
| · | Approximately $0.23 million (RMB 1.5 million) is due by the 30th business day after the receipt of the medicine’s clinical ratification document, and |
|
|
|
| · | Approximately $0.23 million (RMB 1.5 million) is due by the 30th business day after the production ratification from the SFDA is obtained. |
En Ze Jia Shi made the first installment of approximately $0.94 million (RMB 6 million) to Huicheng in May 2009 which is to be returned to En Ze Jia Shi if it cannot obtain the production ratification from the SFDA due to any fault caused by Huicheng. The Company is waiting for China government’s approval for Clinical Trial for this drug now.
28
LOTUS PHARMACEUTICALS INC. AND SUBSIDIARIES
NOTES TO UNAUDITED CONDENSED CONSOLIDATED FINANCIAL STATEMENTS
NOTE 14 – CONCENTRATIONS
Customer
No customer accounted for more than 10% of the Company’s total sales for the three and nine months ended September 30, 2011 and 2010, respectively.
Suppliers
Two major suppliers provided approximately 50% of the Company’s purchases of raw materials and third party manufactured finished goods for the nine months ended September 30, 2011 and the Company did not have any amount of advance to these suppliers as of September 30, 2011.
One major supplier provided approximately 18% of the Company’s purchases of raw materials and third party manufactured finished goods for the nine months ended September 30, 2010 and the Company did not have any amount of advance to the supplier as of September 30, 2010.
NOTE 15 – SUBSEQUENT EVENTS
In October 2011, the Company issued 15,528 shares of its common stock to an attorney in connection with legal service rendered. The shares were valued at $0.389 per share on the grant date based on market price. In connection with the issuance of these shares, the Company recorded professional fees of $6,040.
29
Item 2. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
The following discussion of our financial condition and results of operation should be read in conjunction with the financial statements and the notes to those statements that are included elsewhere in this report. Our discussion includes forward-looking statements based upon current expectations that involve risks and uncertainties, such as our plans, objectives, expectations and intentions. Actual results and the timing of events could differ materially from those anticipated in these forward-looking statements as a result of a number of factors. We use words such as “anticipate,” “estimate,” “plan,” “project,” “continuing,” “ongoing,” “expect,” “believe,” “intend,” “may,” “will,” “should,” “could,” and similar expressions to identify forward-looking statements.
OVERVIEW
We develop, manufacture, and sell pharmaceuticals in the PRC. We produce medicine and drugs in the forms of tablets, capsules, granules, eye-drops, and freeze-dried powder injection. We have established markets for several drugs that are self-branded or self-patented, including (i) Maixin - valsartan capsules for the treatment of hypertension, (ii) Muxin – eye drops for the treatment of glaucoma and (iii) Yipubishan - octreotide Acetate Injection solution for the treatment of gastric ulcers. Our drug development is focused on the treatment of cerebro-cardiovascular disease, asthma, and diabetes. We have a nationwide sales network to directly and indirectly sell to hospitals, clinics and drug stores in approximately 30 provinces in China. Additionally, through our 10 retail pharmacy locations and direct sales to other drug stores in Beijing, China, we sell western and traditional Chinese medications and medical treatment equipment, and generate ancillary revenues from the leasing of retail space to third party vendors at our retail stores.
To be in compliance with China’s regulations on foreign ownership in the pharmaceutical industry and to consolidate the financial information of our operating entities, Lotus East, we operate our business in China through the Contractual Arrangements with Lotus East. The contractual relationship among the above companies as follows:
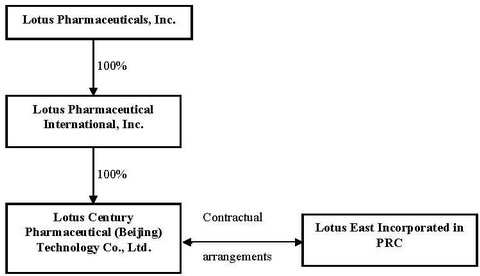
In April 2010, the term of Contractual Arrangements was approved and extended by the board of directors from 10 years to 30 years, thereby establishing the termination date of such agreements as 2036.
When used in this section, and except as may be set forth otherwise, the terms “we,” “us,” “ours,” and similar terms includes Lotus Pharmaceuticals Inc. and its subsidiaries, Lotus International and Lotus Century, as well as Lotus East.
30
CRITICAL ACCOUNTING POLICIES AND ESTIMATES
Our discussion and analysis of our financial condition and results of operations are based upon our consolidated financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States. The preparation of these consolidated financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, revenues and expenses, and related disclosure of contingent assets and liabilities. On an ongoing basis, we evaluate our estimates based on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions. These critical accounting policies were discussed in more detail in the Management’s Discussion and Analysis of Financial Condition and Results of Operations contained in our Annual Report on Form 10-K for the year ended December 31, 2010 and in Note 1 to the unaudited condensed consolidated financial statements contained herein.
RECENT ACCOUNTING PRONOUNCEMENTS
In May 2011, the FASB issued ASU 2011-04, “Amendments to Achieve Common Fair Value Measurement and Disclosure Requirements in U.S. GAAP and IFRS” (“ASU 2011-04”). The amendments in ASU 2011-04 result in common fair value measurement and disclosure requirements in U.S. GAAP and IFRSs. Consequently, ASU 2011-04 changes the wording used to describe many of the requirements in U.S. GAAP for measuring fair value and for disclosing information about fair value measurements. For many of the requirements, the FASB does not intend for the amendments in ASU 2011-04 to result in a change in the application of the requirements in Topic 820. ASU 2011-04 is effective prospectively for interim and annual reporting periods beginning after December 15, 2011. We do not expect the adoption of the provisions in ASU 2011-04 will have a significant impact on our consolidated financial statements.
In June 2011, the FASB issued ASU 2011-05, “Presentation of Comprehensive Income” (“ASU 2011-05”). In accordance with ASU 2011-05, an entity has the option to present the total of comprehensive income, the components of net income, and the components of other comprehensive income either in a single continuous statement of comprehensive income or in two separate but consecutive statements. In both choices, an entity is required to present each component of net income along with total net income, each component of other comprehensive income along with a total for other comprehensive income, and a total amount for comprehensive income. ASU 2011-05 eliminates the option to present the components of other comprehensive income as part of the statement of changes in stockholders’ equity. ASU 2011-05 does not change the items that must be reported in other comprehensive income or when an item of other comprehensive income must be reclassified to net income. ASU 2011-05 is effective retrospectively for fiscal years, and interim periods within those years, beginning after December 15, 2011. We do not expect this standard will have a significant impact on our consolidated financial statements.
In September 2011, the FASB issued Accounting Standards Update (“ASU”) No. 2011-08, Intangibles — Goodwill and Other (Topic 350). This Accounting Standards Update amends FASB ASC 350. This amendment specifies the change in method for determining the potential impairment of goodwill. It includes examples of circumstances and events that the entity should consider in evaluating whether it is more likely than not that the fair value of a reporting unit is less than its carrying amount. The amendments are effective for annual and interim goodwill impairment tests performed for fiscal years beginning after December 15, 2011. We do not expect the adoption of the provisions in ASU 2011-08 will have a significant impact on our consolidated financial statements.
Other accounting standards that have been issued or proposed by the FASB or other standards-setting bodies that do not require adoption until a future date are not expected to have a material impact on our consolidated financial statements upon adoption.
RESULTS OF OPERATIONS
Nine Months Ended September 30, 2011 Compared to Nine Months Ended September 30, 2010
Net Revenues
Total net revenues for the nine months ended September 30, 2011 were $50,656,215 as compared to total net revenues of $51,961,831 for the nine months ended September 30, 2010, a decrease of $1,305,616 or 2.5%. For the nine months ended September 30, 2011 and 2010, total net revenues consisted of the following:
31
|
| 2011 |
| 2010 | ||
Wholesale |
| $ | 38,585,730 |
| $ | 42,309,596 |
Retail |
|
| 12,070,485 |
|
| 9,652,235 |
Total net revenues |
| $ | 50,656,215 |
| $ | 51,961,831 |
Wholesale revenues by product categories were as follows ($ in thousands):
|
| Nine Months Ended September 30, |
| Increase |
| Percentage | ||
|
| 2011 |
| 2010 |
| (Decrease) |
| Change |
Mai Xin | $ | 3,713 | $ | 7,141 | $ | (3,428 | ) | (48)% |
Mu Xin |
| 8 |
| 7,746 |
| (7,738 | ) | (100)% |
Yipubishan |
| 3,910 |
| 4,964 |
| (1,054 | ) | (21)% |
Three drugs with exclusive distribution rights |
| 9,509 |
| 5,460 |
| 4,049 |
| 74 % |
Twelve drugs with non-exclusive distribution rights |
| 1,235 |
| 12,186 |
| (10,951 | ) | (90)% |
Other miscellaneous drugs |
| 20,211 |
| 4,813 |
| 15,398 |
| 320 % |
Total | $ | 38,586 | $ | 42,310 | $ | (3,724 | ) | (9)% |
For the nine months ended September 30, 2011, wholesale revenues decreased $3,723,866 or 8.8%, compared to wholesale revenues for the nine months ended September 30, 2010. The decrease was primarily attributable to a decrease in sales of approximately $7,738,000 from Mu Xin (an eye drop) which is one of our self-branded products since our sales quantity for Mu Xin decreased by approximately 100% due to the termination of our out-sourcing manufacture agreement, a decrease in sales of approximately $3,428,000 from Mai Xin (Valsartan) which is one of our self-branded products, though we lowered our wholesale distribution price for Mai Xin by 11.0% due to competitive conditions, our sales quantity for this drug decreased by 44.2%, a decrease in sales of approximately $1,054,000 from Yipubishan which is one of our self-branded products, though we decreased our wholesale distribution price for Yipubishan by 9.0% in order to deal with competition, our sales quantity for this drug decreased by 17.4%, and a decrease in sales of approximately $10,951,000 from twelve third party manufactured products distributed through our wholesale distribution channel for which we act as a non-exclusive wholesale distributor. For these twelve drugs, although we decreased our average wholesale distribution price by 11.7%, our total sales quantities decreased by 89.0%. This was partially offset by an increase in revenues of approximately $4,049,000 from three third party manufactured products (the names of these three drugs are Recombinant Human Erythropoietin Injection, Recombinant Human Interleukin-2 Injection and Recombinant Human Granulocyte Stimulating Factor Injection) for which we hold exclusive distribution rights, and an increase in revenues of approximately $15,398,000 from other miscellaneous drugs distributed through our wholesale distribution channel. For the three drugs for which we hold exclusive distribution rights, although we increased our average wholesale distribution price by 45.6% due to the increase in our purchase price, our total sales quantities from the three drugs increased by 14.1%. We believe that, in the nine months ended September 30, 2011, numerous drug sales agents sold drugs for which they hold non-exclusive rights in some regions in which they did not have any distribution rights. In order to compete with our competitors, we lowered our wholesales distribution price of these drugs for which we hold non-exclusive distribution rights and concentrated our sales efforts on these drugs for which we hold exclusive distribution rights and other miscellaneous drugs. As a result, our sales from the twelve drugs for which we hold non-exclusive distribution rights decreased and our sales from the three drugs for which we hold exclusive distribution rights and other miscellaneous drugs increased.
For the nine months ended September 30, 2011, retail revenues increased by $2,418,250 or 25.1%, compared to retail revenues for the nine months ended September 30, 2010. The increase was mainly attributable to an increase in revenue from our own ten drug stores of approximately $177,000 and an increase in revenue from our direct sales to other Over-the-Counter drug stores in Beijing of approximately $2,241,000. Due to the growth and success of our OTC Drug Division’s sales force, our retail revenues for the nine months ended September 30, 2011 substantially increased. We expect our retail revenue from our own ten drug stores will remain at its current level with small growth and our retail revenue from our direct sales to other drug stores in Beijing will continue to increase in the future.
Cost of Revenues
Overall, cost of revenues for the nine months ended September 30, 2011 increased $13,027,324 or 56.0% as compared to the total cost of revenues for the nine months ended September 30, 2010. Our total cost of revenues as a percentage of total net revenues for the nine months ended September 30, 2011 increased to 71.7% from 44.8% for the nine months ended September 30, 2010.
32
Cost of revenues for our wholesale operations by product categories were as follows ($ in thousands):
|
| Nine Months Ended September 30, |
| Increase |
| Percentage | ||
|
| 2011 |
| 2010 |
| (Decrease) |
| Change |
Mai Xin | $ | 1,464 | $ | 1,894 | $ | (430 | ) | (23)% |
Mu Xin |
| 1 |
| 2,391 |
| (2,390 | ) | (100)% |
Yipubishan |
| 638 |
| 544 |
| 94 |
| 17 % |
Three drugs with exclusive distribution rights |
| 4,326 |
| 2,766 |
| 1,560 |
| 56 % |
Twelve drugs with non-exclusive distribution rights |
| 533 |
| 4,559 |
| (4,026 | ) | (88)% |
Other miscellaneous drugs |
| 20,326 |
| 4,165 |
| 16,161 |
| 388 % |
Total | $ | 27,288 | $ | 16,319 | $ | 10,969 |
| 67 % |
Cost of revenues as a percentage of net revenues from our wholesale operations increased to 70.7% for the nine months ended September 30, 2011 from 38.6% for the nine months ended September 30, 2010. The increase in cost of revenues as a percentage of net revenues from our wholesale operations was primarily attributable to: (i) In the nine months ended September 30, 2011, we lowered our wholesales price of drugs for which we hold non-exclusive distribution rights as discussed above and decreased our wholesales price for the drugs Mai Xin (Valsartan) and Yipubishan; (ii) There was a slight increase in our unit cost; (iii) We lowered our wholesales price for other miscellaneous drugs distributed through our wholesale channel and caused a negative gross profit margin of approximately 0.6% from the wholesales of other miscellaneous drugs. We want to incentivize our wholesale distributors and earn their loyalties by lowering our wholesales price and letting our distributors obtain greater profits from our lowered distribution price since competition is so keen. We expect the situation to continue in the rest of fiscal 2011 until our Beijing new facility comes into use in fiscal 2012.
Cost of revenues as a percentage of net revenues from our retail operations for the nine months ended September 30, 2011 increased to 74.7% from 72.1% for the nine months ended September 30, 2010. The increase was primarily attributable to the increase in our purchase price for retail drugs and our inability to pass the increase to our customers due to the China government’s restrictive controls on drugs sales prices. We expect our total cost of revenues as a percentage of total net revenues will remain in its current level in the near future.
Gross Profit
Overall, our gross profit for the nine months ended September 30, 2011 was $14,353,780 or 28.3% of total net revenues, as compared to $28,686,720 or 55.2% of total net revenues for the nine months ended September 30, 2010.
Gross profit margins for our wholesale operations by product categories were as follows:
|
| Nine Months Ended September 30, |
| Increase | ||
|
| 2011 |
| 2010 |
| (Decrease) |
Mai Xin |
| 60.6 % |
| 73.5 % |
| (12.9)% |
Mu Xin |
| 84.9 % |
| 69.1 % |
| 15.8 % |
Yipubishan |
| 83.7 % |
| 89.0 % |
| (5.3)% |
Three drugs with exclusive distribution rights |
| 54.5 % |
| 49.3 % |
| 5.2 % |
Twelve drugs with non-exclusive distribution rights |
| 56.9 % |
| 62.6 % |
| (5.7)% |
Other miscellaneous drugs |
| (0.6)% |
| 13.5 % |
| (14.1)% |
Total |
| 29.3 % |
| 61.4 % |
| (32.1)% |
Our gross profit from our wholesale operations for the nine months ended September 30, 2011 was $11,297,832 or 29.3% of net wholesale revenues, as compared to $25,990,923 or 61.4% of net wholesale revenues for the nine months ended September 30, 2010.
Our gross profit from our retail operations for the nine months ended September 30, 2011 was $3,055,948 or 25.3% of net retail revenues, as compared to $2,695,797 or 27.9% of net retail revenues for the nine months ended September 30, 2010.
Operating Expenses
Total operating expenses for the nine months ended September 30, 2011 were $9,047,515, as compared to total operating expenses of $10,260,875 for the nine months ended September 30, 2010, a decrease of $1,213,360 or 11.8%. This decrease included the following:
33
For the nine months ended September 30, 2011, selling expenses amounted to $2,987,630 as compared to $6,996,741 for the nine months ended September 30, 2010, a decrease of $4,009,111 or 57.3%. For the nine months ended September 30, 2011, selling expenses as a percentage of total net revenues was 5.9% while for the nine months ended September 30, 2010, selling expenses as a percentage of total net revenues was 13.5%. In the nine months ended September 30, 2011, we decreased the unit price of those drugs for which we hold non-exclusive distribution rights as described in elsewhere of this report and we lowered the unit sale price of two self-branded products which are Mai Xin (Valsartan) and Yipubishan and we lowered our wholesales price for other miscellaneous drugs distributed through our wholesale channel. Therefore, our distributors can obtain greater profits from our lowered distribution price for these wholesale drugs and our distributors got less commission from us. In this way, we transferred a portion of our profits to our distributors instead of paying them commission. Namely, as a result of these lowered distribution prices, our sales representatives were able to profitably sell these drugs with our less payment of commission for their sales of these drugs. Therefore, our selling expenses as a percentage of total net revenues decreased significantly. We expect our selling expenses will maintain at its current level in the remaining part of fiscal 2011.
For the nine months ended September 30, 2011, research and development expenses amounted to $1,889,535 as compared to $21,517 for the nine months ended September 30, 2010, an increase of $1,868,018 or 8,681.6% The increase was attributable to the research and development expenses for clinical trial of the Laevo-Bambuterol drug. We expect our research and development expense to continue to increase in the near future since the Laevo-Bambuterol drug is in clinical trials and Gliclazide-Controlled Release Tablets and Isosorbide Mononitrate Tablets are waiting for approval to initiate clinical trials.
For the nine months ended September 30, 2011, general and administrative expenses were $4,170,350, as compared to general and administrative expenses of $3,242,617 for the nine months ended September 30, 2010, an increase of $927,733 or 28.6%. These changes are summarized below:
|
| 2011 |
| 2010 | ||
Salaries and related benefits |
| $ | 1,639,442 |
| $ | 496,841 |
Amortization and depreciation expenses |
|
| 1,229,041 |
|
| 1,337,324 |
Rent |
|
| 233,896 |
|
| 232,047 |
Travel and entertainment |
|
| 28,713 |
|
| 5,538 |
Professional fees |
|
| 932,432 |
|
| 1,037,575 |
Other |
|
| 106,826 |
|
| 133,292 |
Total |
| $ | 4,170,350 |
| $ | 3,242,617 |
The primary changes in these expenses for the nine months ended September 30, 2011 as compared to the nine months ended September 30, 2010 included the following:
| · | Salaries and related benefits increased $1,142,601 or 230.0%, which was mainly attributable to an increase in salaries and employees’ benefits of approximately $309,000 and an increase in bonuses of approximately $834,000 which included a bonus paid to Dr. Liu, our Chief Executive Officer, of approximately $776,000. We increased our salaries and related benefits in order to motivate our staff and to ensure a stable and loyal work team for our long term benefit and success. We anticipate that our salaries and related benefits will remain at its current level in the near future. |
|
|
|
| · | Amortization of our intangible assets and depreciation on our property and equipment decreased by $108,283 or 8.1%, which was primarily attributable to the decrease in amortization from intangible assets of approximately $401,000 offset by an increase in depreciation on property and equipment of approximately $293,000. During the nine months ended September 30, 2011, we did not amortize the land use right which was held for development since it was not used in operations while we charged amortization of approximately $443,000 for the same land use right to general and administrative expenses in the nine months ended September 30, 2010 as it was put in use in the period. For the nine months ended September 30, 2011, we did not generate any production activity and we charged depreciation of approximately $299,000 from our manufacturing machinery and equipment to general and administrative expenses, whereas, for the nine months ended September 30, 2010, we did not charge any depreciation from our manufacturing machinery and equipment to general and administrative expenses. In the fourth quarter of fiscal 2010, we charged depreciation for the whole fiscal year of 2010 from our manufacturing machinery and equipment to general and administrative expenses. |
|
|
|
| · | Travel and entertainment increased $23,175 or 418.5% which was mainly attributable to the increased travel expenses of approximately $23,000 for the nine months ended September 30, 2011 due to the increased travel activities as compared to the travel and entertainment expenses for the nine months ended September 30, 2010. |
34
| · | Professional fees decreased $105,143 or 10.1%, which was primarily attributable to a decrease in consulting fees related to our consultants’ service of approximately $83,000 for corporate affairs and development, and a decrease in fees paid for other miscellaneous service of approximately $22,000. |
|
|
|
| · | Other general and administrative expenses, which included office supplies, general management fees, car insurance, postage and other office expenses, decreased by $26,466 or 19.9% reflecting efforts at reducing non-sales related corporate activities as well as stricter controls on corporate spending. |
Income from Operations
As a result of the forgoing, we reported income from operations of $5,306,265 for the nine months ended September 30, 2011, as compared to income from operations of $18,425,845 for the nine months ended September 30, 2010, a decrease of $13,119,580 or 71.2%.
Other Income (Expense)
For the nine months ended September 30, 2011, we had a total net other expense of $43,920, as compared to a total net other expense of $4,225 for the nine months ended September 30, 2010, an increase of $39,695 or 939.5%. The change in total net other income (expense) was primarily attributable to:
| · | For the nine months ended September 30, 2011, we did not have any debt issuance costs, as compared to $52,226 for the nine months ended September 30, 2010. The decrease was attributable to the decrease in amortization amount on debt issuance costs associated with the issuance of February 2008 Series A Convertible Preferred Stock for which we amortized on a 24-month period and began our amortization on the February 2008 debt issuance costs in March 2008. Therefore, the debt issuance costs were fully amortized in February 2010. |
|
|
|
| · | For the nine months ended September 30, 2011, other income was $141,331 as compared to $597,016 for the nine months ended September 30, 2010, a decrease of $455,685 or 76.3%. The other income was leasing income. We sublease certain portions of our retail stores and counter spaces to various other vendors which generate leasing revenue. The significant decrease in leasing income was primarily attributable to less spaces being rented out. In late 2010, the State Food and Drug Administration began to require that companies that produce and sell food and drug decrease the ratio of the area they rent out in these companies’ operation facility in order to prevent any defective food and drug from getting into Beijing. As a result, our leasing revenue decreased substantially. |
|
|
|
| · | For the nine months ended September 30, 2011, interest expense was $187,256 as compared to $551,726 for the nine months ended September 30, 2010, a decrease of $364,470 or 66.1%. The decrease in interest expense was primarily attributable to a decrease in amortization of debt discount associated with the February 2008 Series A Convertible Preferred Stock of approximately $151,000, a decrease in accrued dividend for the February 2008 Series A Convertible Preferred Stock of approximately $37,000, and a decrease in interest of approximately $185,000 from the beneficial conversion feature of the second year interest shares for the February 2008 Series A Convertible Preferred Stock, offset by an increase in interest of approximately $9,000 in connection with the related parties notes payable. |
Income Taxes
For the nine months ended September 30, 2011, our income tax expense was $149, as compared to $470,514 for the nine months ended September 30, 2010, a decrease of $470,365 or 99.97%. The decrease in income tax expense was mainly attributable to the decrease in taxable income generated by our operating entities.
Net Income
As a result of these factors, we reported net income of $5,262,196 for the nine months ended September 30, 2011 as compared to net income of $17,951,106 for the nine months ended September 30, 2010. This translated to basic earnings per common share of $0.19 and $0.69, and diluted earnings per common share of $0.19 and $0.67, for the nine months ended September 30, 2011 and 2010, respectively.
35
Other Comprehensive Income
The functional currency of our operating subsidiaries and affiliates is the Chinese Renminbi (“RMB”). The financial statements of our operating subsidiaries and affiliates are translated to U.S. dollars using period end rates of exchange for assets and liabilities, and average rates of exchange for the period for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations. As a result of these translations, which are a non-cash adjustment, we reported a foreign currency translation gain of $3,054,039 for the nine months ended September 30, 2011 as compared to $1,760,632 for the nine months ended September 30, 2010. This non-cash gain had the effect of increasing our reported comprehensive income.
Comprehensive Income
As a result of our foreign currency translation gains, we had comprehensive income for the nine months ended September 30, 2011 of $8,316,235, compared with $19,711,738 for the nine months ended September 30, 2010.
Three Months Ended September 30, 2011 Compared to Three Months Ended September 30, 2010
Net Revenues
Total net revenues for the three months ended September 30, 2011 were $19,044,538 as compared to total net revenues of $18,304,860 for the three months ended September 30, 2010, an increase of $739,678 or 4.0%. For the three months ended September 30, 2011 and 2010, total net revenues consisted of the following:
|
| 2011 |
| 2010 | ||
Wholesale |
| $ | 15,276,053 |
| $ | 15,347,372 |
Retail |
|
| 3,768,485 |
|
| 2,957,488 |
Total net revenues |
| $ | 19,044,538 |
| $ | 18,304,860 |
Wholesale revenues by product categories were as follows ($ in thousands):
|
| Three Months Ended September 30, |
| Increase |
| Percentage | ||
|
| 2011 |
| 2010 |
| (Decrease) |
| Change |
Mai Xin | $ | 603 | $ | 2,589 | $ | (1,986 | ) | (77)% |
Mu Xin |
| — |
| 2,836 |
| (2,836 | ) | (100)% |
Yipubishan |
| 838 |
| 1,921 |
| (1,083 | ) | (56)% |
Three drugs with exclusive distribution rights |
| 2,227 |
| 1,819 |
| 408 |
| 22 % |
Twelve drugs with non-exclusive distribution rights |
| — |
| 4,100 |
| (4,100 | ) | (100)% |
Other miscellaneous drugs |
| 11,608 |
| 2,082 |
| 9,526 |
| 458 % |
Total | $ | 15,276 | $ | 15,347 | $ | (71 | ) | (0.5)% |
For the three months ended September 30, 2011, wholesale revenues decreased $71,319 or 0.5%, compared to wholesale revenues for the three months ended September 30, 2010. The decrease was primarily attributable to a decrease in sales of approximately $2,836,000 from Mu Xin (an eye drop) which is one of our self-branded products since our sales quantity for Mu Xin decreased by 100% due to the termination of our out-sourcing manufacture agreement, a decrease in sales of approximately $1,986,000 from Mai Xin (Valsartan) which is one of our self-branded products, although we increased our wholesale distribution price for Mai Xin by 5.3% due to the increase in our purchase price for the drug, our sales quantity for the drug decreased by 79.5%, a decrease in sales of approximately $1,083,000 from Yipubishan which is one of our self-branded products, though we increased our wholesale distribution price for Yipubishan by 7.0% in order to deal with the increase in our purchase price, our sales quantity for this drug decreased by 61.9%, and a decrease in sales of approximately $4,100,000 from twelve third party manufactured products distributed through our wholesale distribution channel for which we act as a non-exclusive wholesale distributor since our sales quantity for these twelve drugs decreased by 100%. This was partially offset by an increase in revenues of approximately $408,000 from three third party manufactured products (the names of these three drugs are Recombinant Human Erythropoietin Injection, Recombinant Human Interleukin-2 Injection and Recombinant Human Granulocyte Stimulating Factor Injection) for which we hold exclusive distribution rights, and an increase in revenues of approximately $9,526,000 from other miscellaneous drugs distributed through our wholesale distribution channel. For the three drugs for which we hold exclusive distribution rights, while we increased our average wholesale distribution price by 72.3% due to the increase in our purchase price for these three drugs, our total sales quantities from these three drugs decreased by 33.3% In the third quarter of fiscal 2011, we concentrated our sales efforts on these drugs for which we hold exclusive distribution rights and other miscellaneous drugs. Therefore, our sales from the three drugs for which we hold exclusive distribution rights and other miscellaneous drugs increased. We expect our total wholesale revenue will maintain at its current quarter level in the remaining part of fiscal 2011.
36
For the three months ended September 30, 2011, retail revenues increased by $810,997 or 27.4%, compared to retail revenues for the three months ended September 30, 2010. The increase was mainly attributable to an increase in revenue from our own ten drug stores of approximately $35,000 and an increase in revenue from our direct sales to other Over-the-Counter drug stores in Beijing of approximately $776,000. Due to the growth and success of our OTC Drug Division’s sales force, our retail revenues for the third quarter of fiscal 2011 substantially increased. We expect our retail revenue from our own ten drug stores will remain in its current level with small growth and our retail revenue from our direct sales to other drug stores in Beijing will continue to increase in the future.
Cost of Revenues
Overall, cost of revenues for the three months ended September 30, 2011 increased $7,805,005 or 98.5% as compared to the total cost of revenues for the three months ended September 30, 2010. Our total cost of revenues as a percentage of total net revenues for the three months ended September 30, 2011 increased to 82.6% from 43.3% for the three months ended September 30, 2010.
Cost of revenues for our wholesale operations by product categories were as follows ($ in thousands):
|
| Three Months Ended September 30, |
| Increase |
| Percentage | ||
|
| 2011 |
| 2010 |
| (Decrease) |
| Change |
Mai Xin | $ | 311 | $ | 666 | $ | (355 | ) | (53)% |
Mu Xin |
| — |
| 873 |
| (873 | ) | (100)% |
Yipubishan |
| 133 |
| 211 |
| (78 | ) | (37)% |
Three drugs with exclusive distribution rights |
| 967 |
| 926 |
| 41 |
| 4 % |
Twelve drugs with non-exclusive distribution rights |
| — |
| 1,540 |
| (1,540 | ) | (100)% |
Other miscellaneous drugs |
| 11,445 |
| 1,575 |
| 9,870 |
| 627 % |
Total | $ | 12,856 | $ | 5,791 | $ | 7,065 | ) | 122 % |
Cost of revenues as a percentage of net revenues from our wholesale operations increased to 84.2% for the three months ended September 30, 2011 from 37.7% for the three months ended September 30, 2010. The increase in cost of revenues as a percentage of net revenues from our wholesale operations was mainly attributable to: (i) the change of product mix; (ii) There was an increase in our unit cost and we only passed a portion of the increase to our customers; (iii) We lowered our wholesales price for other miscellaneous drugs distributed through our wholesale channel and generated a slight gross profit margin of approximately 1.4% from the wholesales of other miscellaneous drugs. We want to incentivize our wholesale distributors and earn their loyalties by lowering our wholesales price and letting our distributors obtain greater profits from our lowered distribution price since competition is so keen. We expect the situation would be continued in the rest of fiscal 2011 until our Beijing new facility comes into use in fiscal 2012.
Cost of revenues as a percentage of net revenues from our retail operations for the three months ended September 30, 2011 increased to 76.3% from 72.2% for the three months ended September 30, 2010. The increase was primarily attributable to the increase in our purchase price for retail drugs and our inability to pass the increase to our customers due to the China government’s restrictive controls on drugs sales prices. We expect our total cost of revenues as a percentage of total net revenues will remain in its current quarter level in the near future.
Gross Profit
Overall, our gross profit for the three months ended September 30, 2011 was $3,313,802 or 17.4% of total net revenues, as compared to $10,379,129 or 56.7% of total net revenues for the three months ended September 30, 2010.
Gross profit margins for our wholesale operations by product categories were as follows:
|
| Three Months Ended September 30, |
| Increase | ||
|
| 2011 |
| 2010 |
| (Decrease) |
Mai Xin |
| 48.4 % |
| 74.3 % |
| (25.9)% |
Mu Xin |
| — |
| 69.2 % |
| (69.2)% |
Yipubishan |
| 84.2 % |
| 89.0 % |
| (4.8)% |
Three drugs with exclusive distribution rights |
| 56.6 % |
| 49.1 % |
| 7.5 % |
Twelve drugs with non-exclusive distribution rights |
| — |
| 62.4 % |
| (62.4)% |
Other miscellaneous drugs |
| 1.4 % |
| 24.3 % |
| (22.9)% |
Total |
| 15.8 % |
| 62.3 % |
| (46.5)% |
37
Our gross profit from our wholesale operations for the three months ended September 30, 2011 was $2,420,095 or 15.8% of net wholesale revenues, as compared to $9,555,547 or 62.3% of net wholesale revenues for the three months ended September 30, 2010. We expect that our gross profit margin from our wholesale operations will maintain in its current quarter level in the near future.
Our gross profit from our retail operations for the three months ended September 30, 2011 was $893,707 or 23.7% of net retail revenues, as compared to $823,582 or 27.8% of net retail revenues for the three months ended September 30, 2010. We expect that our gross profit margin from our retail operations will maintain in its current quarter level in the near future.
Operating Expenses
Total operating expenses for the three months ended September 30, 2011 were $2,045,394, as compared to total operating expenses of $3,619,558 for the three months ended September 30, 2010, a decrease of $1,574,164 or 43.5%. This decrease included the following:
For the three months ended September 30, 2011, selling expenses amounted to $846,919 as compared to $2,452,629 for the three months ended September 30, 2010, a decrease of $1,605,710 or 65.5%. For the three months ended September 30, 2011, selling expenses as a percentage of total net revenues was 4.4% while for the three months ended September 30, 2010, selling expenses as a percentage of total net revenues was 13.4%. In the three months ended September 30, 2011, we lowered our wholesales price for other miscellaneous drugs distributed through our wholesale channel. Therefore, our distributors can obtain greater profits from our lowered distribution price for these wholesale drugs and our distributors receive lower commissions from us. In this way, we transferred a portion of our profits to our distributors instead of paying them commissions. Namely, as a result of these lowered distribution prices, our sales representatives were able to profitably sell these drugs with lower commissions for their sales of these drugs. Therefore, our selling expenses as a percentage of total net revenues decreased. We expect our selling expenses will maintain at its current level in the remaining part of fiscal 2011.
For the three months ended September 30, 2011, research and development expenses amounted to $470,311 as compared to $21,517 for the three months ended September 30, 2010, an increase of $448,794 or 2,085.8%. The increase was attributable to the research and development expenses for clinical trial of the Laevo-Bambuterol drug. We expect our research and development expense to continue to increase in the near future since the Laevo-Bambuterol drug is in clinical trials and Gliclazide-Controlled Release Tablets and Isosorbide Mononitrate Tablets are waiting for approval to initiate clinical trials.
For the three months ended September 30, 2011, general and administrative expenses were $728,164, as compared to general and administrative expenses of $1,145,412 for the three months ended September 30, 2010, a decrease of $417,248 or 36.4%. These changes are summarized below:
|
| 2011 |
| 2010 | ||
Salaries and related benefits |
| $ | 122,823 |
| $ | 173,511 |
Amortization and depreciation expenses |
|
| 410,268 |
|
| 447,785 |
Rent |
|
| 78,970 |
|
| 80,861 |
Travel and entertainment |
|
| 10.080 |
|
| 5,372 |
Professional fees |
|
| 73,152 |
|
| 389,158 |
Other |
|
| 32,871 |
|
| 48,725 |
Total |
| $ | 728,164 |
| $ | 1,145,412 |
The primary changes in these expenses for the three months ended September 30, 2011 as compared to the three months ended September 30, 2010 included the following:
| · | Salaries and related benefits decreased $50,688 or 29.2%, which was mainly attributable to the decrease in salaries of approximately $51,000 reflecting normal business fluctuations. We anticipate that our salaries and related benefits will remain at its current level in the near future. |
38
| · | Amortization of our intangible assets and depreciation on our property and equipment decreased by $37,517 or 8.4%, which was primarily attributable to the decrease in amortization from intangible assets of approximately $132,000 offset by an increase in depreciation on fixed assets of approximately $95,000. During the three months ended September 30, 2011, we did not amortize the land use right which was held for development since it was not used in operations while we charged amortization of approximately $132,000 for the same land use right to general and administrative expenses in the three months ended September 30, 2010 as it was put in use in the period. For the three months ended September 30, 2011, we did not generate any production activity and we charged depreciation of approximately $95,000 from our manufacturing machinery and equipment to general and administrative expenses, whereas, for the three months ended September 30, 2010, we did not charge any depreciation from our manufacturing machinery and equipment to general and administrative expenses. In the fourth quarter of fiscal 2010, we charged depreciation for the whole fiscal year of 2010 from our manufacturing machinery and equipment to general and administrative expenses. |
|
|
|
| · | Professional fees decreased $316,006 or 81.2%, which was primarily attributable to a decrease in fees related to our consultants’ service of approximately $278,000 for corporate affairs and development, and a decrease in fees paid for other miscellaneous service of approximately $38,000. |
|
|
|
| · | Other general and administrative expenses, which included office supplies, general management fees, car insurance, postage and other office expenses, decreased by $15,854 or 32.5% reflecting efforts at reducing non-sales related corporate activities as well as stricter controls on corporate spending. |
Income from Operations
As a result of the forgoing, we reported income from operations of $1,268,408 for the three months ended September 30, 2011, as compared to income from operations of $6,759,571 for the three months ended September 30, 2010, a decrease of $5,491,163 or 81.2%.
Other Income (Expense)
For the three months ended September 30, 2011, we had a total net other expense of $15,067, as compared to a total net other income of $140,703 for the three months ended September 30, 2010. The change in total net other income (expense) was primarily attributable to:
| · | For the three months ended September 30, 2011, other income was $47,717 as compared to $200,074 for the three months ended September 30, 2010, a decrease of $152,357 or 76.2%. The other income was leasing income. We sublease certain portions of our retail stores and counter space to various other vendors which generate leasing revenue. The significant decrease in leasing income was primarily attributable to less spaces being rented out. In late 2010, the State Food and Drug Administration began to require that companies that produce and sell food and drug decrease the ratio of the area they rent out in these companies’ operation facility in order to prevent any defective food and drug from getting into Beijing. As a result, our leasing revenue decreased substantially. |
Income Taxes
For the three months ended September 30, 2011, our income tax expense was $0, as compared to $200,348 for the three months ended September 30, 2010, a decrease of $200,348 or 100.0%. Liang Fang did not generate any taxable income during the third quarter of fiscal 2011 while Liang Fang generated some taxable income during the third quarter of fiscal 2010. Therefore the income tax expense was zero for the third quarter of fiscal 2011.
Net Income
As a result of these factors, we reported net income of $1,253,341 for the three months ended September 30, 2011 as compared to net income of $6,699,926 for the three months ended September 30, 2010. This translated to basic earnings per common share of $0.05 and $0.25, and diluted earnings per common share of $0.04 and $0.25, for the three months ended September 30, 2011 and 2010, respectively.
39
Other Comprehensive Income
The functional currency of our operating subsidiaries and affiliates is the Chinese Renminbi (“RMB”). The financial statements of our operating subsidiaries and affiliates are translated to U.S. dollars using period end rates of exchange for assets and liabilities, and average rates of exchange for the period for revenues, costs, and expenses. Net gains and losses resulting from foreign exchange transactions are included in the consolidated statements of operations. As a result of these translations, which are a non-cash adjustment, we reported a foreign currency translation gain of $930,264 for the three months ended September 30, 2011 as compared to $1,426,434 for the three months ended September 30, 2010. This non-cash gain had the effect of increasing our reported comprehensive income.
Comprehensive Income
As a result of our foreign currency translation gains, we had comprehensive income for the three months ended September 30, 2011 of $2,183,605, compared with $8,126,360 for the three months ended September 30, 2010.
Liquidity and Capital Resources
Liquidity is the ability of a company to generate funds to support its current and future operations, satisfy its obligations and otherwise operate on an ongoing basis.
At September 30, 2011 and December 31, 2010, we had a cash balance of $158,908 and $1,339,972, respectively. These funds are distributed in financial institutions located in China.
Our working capital decreased $3,134,799 to working capital deficit of $6,644,013 at September 30, 2011 from working capital deficit of $3,509,214 at December 31, 2010. This decrease in working capital is primarily attributed to:
| · | a decrease in cash of approximately $1,181,000, |
|
|
|
| · | a decrease in prepaid expenses and other current assets of approximately $465,000, |
|
|
|
| · | an increase in taxes payable of approximately $8,571,000 which was mainly attributable to the increase in value-added taxes payable of approximately $7,789,000 and the increase in other taxes payable of approximately $789,000 offset by an increase in prepaid corporation income taxes of approximately $7,000, |
|
|
|
| · | an increase in due to related parties of approximately $300,000, |
offset by
| · | an increase in accounts receivable of approximately $4,027,000 due to the credit provided to more customers in order to encourage customers to purchase our product, |
|
|
|
| · | an increase in inventories of approximately $634,000, |
|
|
|
| · | a decrease in other payables and accrued liabilities of approximately $2,718,000 due to the payments made in fiscal 2011 to pay off the fiscal 2010 accrued sales commission of approximately $1,562,000 and the fiscal 2010 accrued employees bonus of approximately $937,000 and the fiscal 2010 accrued expenses for services of approximately $116,000 and the fiscal 2010 accrued payables for construction of approximately $125,000, offset by an increase in payables from other miscellaneous items of approximately $22,000. |
The changes in asset and liabilities discussed above is based on a comparison of amounts on our balance sheets as of September 30, 2011 and December 31, 2010 and does not necessarily reflect changes in assets and liabilities reflected on our cash flow statement, for which we use the average foreign exchange rate during the period to calculate these changes.
Our balance sheet as of September 30, 2011 also reflects notes payable to related parties of $5,413,778 due on December 30, 2015 which was a series of working capital loans made to us since December 31, 2005 by the Company’s Chief Executive Officer, his wife, Chief Executive Officer’s father-in-law and two employees of the Company. These loans bear interest based on a floating annual interest rate, which is 80% of China bank interest rate and are unsecured. During the nine months ended September 30, 2011, we did not repay any portion of the principal of these loan balances.
40
Net cash provided by operating activities for the nine months ended September 30, 2011 was $10,572,259 as compared to net cash provided by operating activities of $18,978,615 for the nine months ended September 30, 2010. For the nine months ended September 30, 2011, net cash provided by operating activities was primarily attributable to net income of approximately $5,262,000 and the add back of non-cash charges such as: depreciation of approximately $312,000, amortization of intangible assets of approximately $917,000, common shares issued for service of approximately $ 721,000 and common shares issued for compensation of approximately $ 1,283,000. In addition, the receipt of cash from changes in operating assets and liabilities, such as: a decrease in prepaid expenses and other current assets of approximately $477,000, an increase in taxes payable of approximately $8,368,000 which was mainly attributable to the increase in value-added taxes payable, and an increase in due to related parties of approximately $424,000, offset by the use of cash from changes in operating assets and liabilities, such as: an increase in accounts receivable of approximately $3,899,000 due to the credit provided to more customers in order to encourage customers to purchase our product, an increase in inventories of approximately $604,000, and a decrease in other payables and accrued liabilities of approximately $2,663,000 mainly due to the payments made in fiscal 2011 for fiscal 2010 accrued sales commission, employees bonus and accrued expenses for service and accrued payables for construction.
For the nine months ended September 30, 2010, net cash provided by operating activities was primarily attributable to net income of approximately $17,951,000 and the add back depreciation of approximately $19,000, amortization of intangible assets of approximately $1,318,000, amortization of deferred debt issuance costs of approximately $52,000, amortization of discount on convertible redeemable preferred stock of approximately $152,000, interest expense attributable to beneficial conversion feature of preferred shares of approximately $185,000, common shares issued for service of approximately $229,000 and common shares issued for compensation of approximately $125,000 and the changes in operating assets and liabilities, such as: a decrease in prepaid expenses and other current assets of approximately $553,000, an increase in unearned revenue of approximately $153,000 and an increase in due to related parties of approximately $323,000, offset by an increase in accounts receivable of approximately $110,000, an increase in inventories of approximately $133,000, a decrease in other payables and accrued liabilities of approximately $584,000 and a decrease in taxes payable of approximately $1,291,000.
Net cash used in investing activities for the purchase of property and equipment for the nine months ended September 30, 2011 and 2010 amounted to approximately $11,778,000 and $22,054,326, respectively.
Net cash provided by financing activities for the nine months ended September 30, 2011 and 2010 was $0.
We reported a net decrease in cash for the nine months ended September 30, 2011 of $1,181,064 as compared to a net decrease in cash of $3,048,090 for the nine months ended September 30, 2010.
We noted the negative working capital and believe this is a temporary trend giving the fact that we still have positive cash flow from operations. We believe our working capital situation will be turned around in the near future. Lotus East has historically funded its capital expenditures from its working capital. As of September 30, 2011, Lotus East has contractual commitments of approximately $52 million related to a Technology Transfer Agreement and the construction of the new facility in Inner Mongolia and a New Drug Patent Transfer Agreement. While it intends to fund the costs with its existing working capital associated with the Technology Transfer Agreement and the New Drug Patent Transfer Agreement and a portion of the construction of the new facility in Inner Mongolia, it is dependent upon the continued growth of its operations and prompt payment of outstanding accounts receivables by its customers to ensure that it has sufficient cash for these commitments. In case of emergency for cash, we would like to dispose the 900 MU (approximately 600,000 square meters) Inner Mongolia land use right which was purchased in 2008 and held for development or sale now. Management estimate that we can receive cash of approximately $35 million from the disposal of the 900 MU Inner Mongolia land use right. Our ability to fully fund the costs associated with the new facility in Inner Mongolia is materially dependent upon our ability to obtain secured bank financing and/or government grants and/or other third party financing.
There is no guarantee that Lotus East can obtain these financings on favorable terms at the right time. Although the Chinese government has announced an economic stimulation plan, there is no guarantee that we will be awarded the government grants successfully. While Lotus East’s management believes the Company will be successful in securing the necessary funding through its increasing revenue, faster collections on receivables, and continuing discussions with various commercial banks, there are no assurances that the funding will be available in the amounts or at the time required to meet Lotus East’s commitments. In the event that Lotus East is not successful in obtaining the funds it needs for the Technology Transfer Agreement and the New Drug Patent Transfer Agreement, it is possible that it could default under the terms of the two agreements and forfeit any funds paid to date. If Lotus East fails to obtain all of the funding necessary to complete the construction of the new facility in Inner Mongolia, which is estimated to be approximately $50 million in the next few years, approximately $34.9 million for the payments on the land use right which is refundable if the Chinese local government does not grant it land use right certificate, could be returned to Lotus East.
41
Contractual Obligations and Off-Balance Sheet Arrangements
Contractual Obligations
We have certain fixed contractual obligations and commitments that include future estimated payments. Changes in our business needs, cancellation provisions, changing interest rates, and other factors may result in actual payments differing from the estimates. We cannot provide certainty regarding the timing and amounts of payments. We have presented below a summary of the most significant assumptions used in our determination of amounts presented in the tables, in order to assist in the review of this information within the context of our consolidated financial position, results of operations, and cash flows. The total of contractual obligations and commitments does not include any payments made by us.
The following table summarizes our contractual obligations as of September 30, 2011, and the effect these obligations are expected to have on our liquidity and cash flows in future periods.
|
| Payments due by period | |||||||||||||
|
| Total |
| Less than |
| 1-3 |
| 3-5 |
| 5+ | |||||
Related parties indebtedness |
| $ | 7,286,980 |
| $ | 1,873,202 |
| $ | — |
| $ | 5,361,684 |
| $ | — |
Interest payment on notes payable – related parties |
| $ | 1,087,879 |
| $ | — |
| $ | — |
| $ | 1,014,586 |
| $ | — |
Technology purchase obligations |
| $ | 1,718,266 |
| $ | 859,133 |
| $ | 859,133 |
| $ | — |
| $ | — |
New drug patent purchase obligations |
| $ | 468,618 |
| $ | 468,618 |
| $ | — |
| $ | — |
| $ | — |
Construction obligations in Inner Mongolia |
| $ | 49,985,941 |
| $ | — |
| $ | 18,744,728 |
| $ | 31,241,213 |
| $ | — |
Total contractual obligations |
| $ | 60,547,684 |
| $ | 3,200,953 |
| $ | 19,603,861 |
| $ | 37,742,870 |
| $ | — |
Off-balance Sheet Arrangements
We have not entered into any other financial guarantees or other commitments to guarantee the payment obligations of any third parties. We have not entered into any derivative contracts that are indexed to our shares and classified as shareholder’s equity or that are not reflected in our consolidated financial statements. Furthermore, we do not have any retained or contingent interest in assets transferred to an unconsolidated entity that serves as credit, liquidity or market risk support to such entity. We do not have any variable interest in any unconsolidated entity that provides financing, liquidity, market risk or credit support to us or engages in leasing, hedging or research and development services with us.
Item 3. Quantitative and Qualitative Disclosures About Market Risk.
Not applicable for a smaller reporting company.
Item 4T. Controls and Procedures.
Our internal control over financial reporting is a process designed by or under the supervision of our principal executive and principal financial officers to provide reasonable assurance regarding the reliability of financial reporting and the preparation of consolidated financial statements for external purposes in accordance with generally accepted accounting principles. Our internal control over financial reporting includes policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect transactions and dispositions of assets; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of consolidated financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures are being made only in accordance with authorizations of management and the directors of the Company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the Company’s assets that could have a material effect on our consolidated financial statements.
Our management does not expect that our disclosure controls or our internal controls over financial reporting will prevent all errors and frauds. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control systems are met. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues, if any, within a company have been detected. Such limitations include the fact that human judgment in decision-making can be faulty and that breakdowns in internal control can occur because of human failures, such as simple errors or mistakes or intentional circumvention of the established process.
42
Disclosure Controls and Procedures
Disclosure controls and procedures refer to controls and other procedures designed to ensure that information required to be disclosed in the reports we file or submit under the Securities Exchange Act is recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC and that such information is accumulated and communicated to our management, including our chief executive officer and chief financial officer, as appropriate, to allow timely decisions regarding required disclosure. In designing and evaluating our disclosure controls and procedures, management recognizes that any controls and procedures, no matter how well designed and operated, can provide only reasonable assurance of achieving the desired control objectives, and management is required to apply its judgment in evaluating and implementing possible controls and procedures.
As required by Rule 13a-15 under the Exchange Act, our management has carried out an evaluation, with the participation and under the supervision of our Chief Executive Officer and our Chief Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures as of September 30, 2011. As discussed in more detail below, our Chief Executive Officer and our Chief Financial Officer concluded that our disclosure controls and procedures were not effective as of September 30, 2011, due to the material weaknesses that we identified in internal control over financial reporting in our annual report on Form 10-K for the fiscal year ended December 31, 2010 (the “Form 10-K”).
Remediation Measures of Material Weakness
We have implemented, or plan to implement, the measures described below under the supervision and guidance of our management to remediate the material weaknesses identified in the Form 10-K and to strengthen our internal controls over financial reporting. Key elements of the remediation effort include, but are not limited to, the following initiatives, which have been implemented, or are in the process of implementation, as of the date of filing of this interim report:
| · | We plan to engage a consultant or consulting firm in fiscal 2011, to review, evaluate and identify inadequacies of our existing internal control procedures, and to make recommendation and implement changes as necessary for the improvement of our internal controls. |
|
|
|
| · | We will continue to bring in additional qualified financial personnel for the accounting department to further strengthen our financial reporting function. We have started training our internal accounting staff in US GAAP and financial reporting requirements. |
|
|
|
| · | Due to our size and nature, segregation of all conflicting duties may not always be possible and may not be economically feasible. However, to the extent possible, we will implement procedures to assure that the initiation of transactions, the custody of assets and the recording of transactions will be performed by separate individuals. |
We believe that the foregoing steps will remediate the material weakness identified in the Form 10-K, and we will continue to monitor the effectiveness of these steps and make any changes that our management deems appropriate.
A material weakness (within the meaning of PCAOB Auditing Standard No. 5) is a deficiency, or a combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of our annual or interim financial statements will not be prevented or detected on a timely basis. A significant deficiency is a deficiency, or a combination of deficiencies, in internal control over financial reporting that is less severe than a material weakness, yet important enough to merit attention by those responsible for oversight of the company’s financial reporting.
Changes in Internal Control over Financial Reporting
There have been no changes in our internal control over financial reporting during the period covered by this report that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
43
PART II - OTHER INFORMATION
Item 1. Legal Proceedings.
None.
Item 1A. Risk Factors.
There are no material changes from the risk factors previously disclosed in the Annual Report on Form 10-K for the year ended December 31, 2010, filed with the SEC on March 28, 2011.
Item 2. Unregistered Sales of Equity Securities and Use of Proceeds.
None.
Item 3. Defaults Upon Senior Securities.
None.
Item 4. (Removed and Reserved)
Item 5. Other Information.
None.
Item 6. Exhibits.
No. |
| Description |
| Rule 13a-14(a)/ 15d-14(a) Certification of Chief Executive Officer | |
|
|
|
| Rule 13a-14(a)/ 15d-14(a) Certification of Chief Financial Officer | |
|
|
|
| Section 1350 Certification of Chief Executive Officer | |
|
|
|
| Section 1350 Certification of Chief Financial Officer | |
|
|
|
101* |
| XBRL data files of Financial Statements and Notes contained in this Quarterly Report on Form 10-Q. |
* In accordance with Regulation S-T, the Interactive Data Files in Exhibit 101 to the Quarterly Report on Form 10-Q shall be deemed “furnished” and not “filed.”
44
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned thereunto duly authorized.
| Lotus Pharmaceuticals, Inc. | |
|
|
|
|
|
|
Date: November 10, 2011 | By: | /s/ Zhongyi Liu |
| Zhongyi Liu | |
| Chief Executive Officer and President, principal executive officer | |
|
|
|
Date: November 10, 2011 | By: | /s/ Yan Zeng |
| Yan Zeng | |
| Chief Financial Officer, principal financial officer | |
45
