Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Pharmasset Inc | d251734d8k.htm |
| EX-99.1 - PRESS RELEASE - Pharmasset Inc | d251734dex991.htm |
 www.pharmasset.com
www.pharmasset.com
NASDAQ: VRUS
NASDAQ: VRUS
Exhibit 99.2
**********
**********
**********
**********
**********
**********
**********
**********
**********
**********
**********
**********
**********
**********
**********
**********
**********
*******************************************
******************************************* |
 Forward-Looking Statements
Pharmasset “Safe Harbor”
Statement under the Private Securities Litigation
Reform Act of 1995: Statements in this presentation that are not historical
facts are “forward-looking statements,”
including, without limitation,
statements that involve risks, uncertainties, and other important factors,
including, without limitation, the risk of cessation or delay of any of
the ongoing or planned clinical trials and/or our development of our
product
candidates, the risk that the results of previously conducted studies
involving our product candidates will not be repeated or observed in ongoing
or future studies involving our product candidates, the risk that our
collaboration with Roche will not continue or will not be successful, and the
risk that any one or more of our product candidates will not be successfully
developed and commercialized. For a discussion of risks, uncertainties, and
other important factors, any of which could cause our actual results to differ
from those contained in the forward-looking statements, see the section
entitled "Risk Factors" in our Annual Report on Form 10-K for the
fiscal year ended September 30, 2010 and Form 10-Q for fiscal quarter
ended June 30, 2011 filed with the Securities and Exchange Commission and
discussions of potential risks, uncertainties, and other important factors in
our subsequent filings with the Securities and Exchange Commission.
|
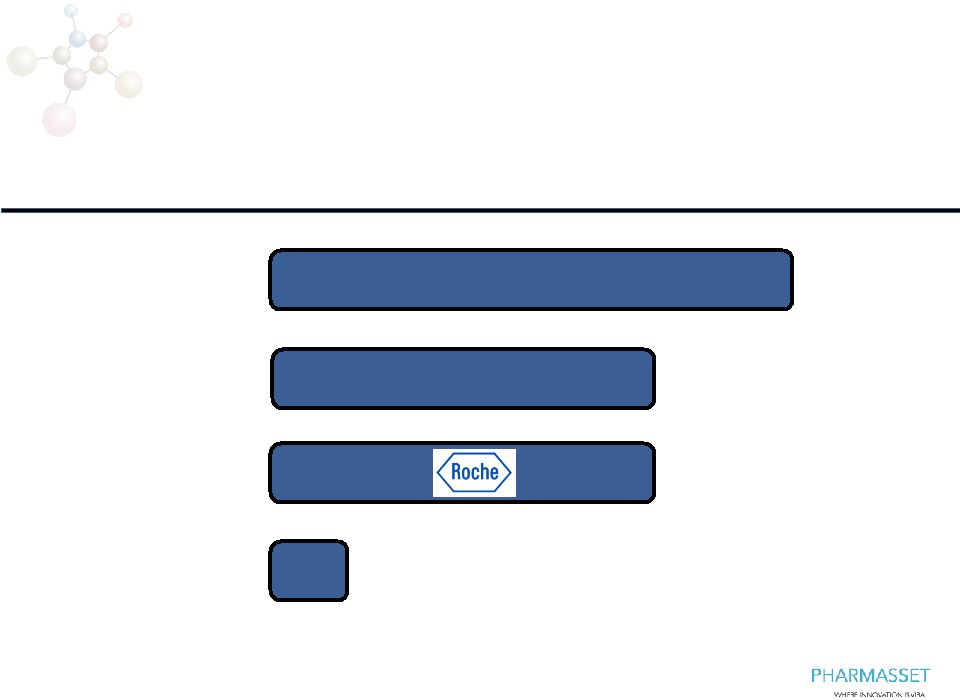 Nucleoside/tide Analog Pipeline
Focused on HCV
Product
Product
& Territories
& Territories
Pre-clinical
Pre-clinical
Phase 1
Phase 1
Phase 2
Phase 2
RG7128
RG7128
US HIV co-promotion,
Latin America, Korea
PSI-7977
PSI-7977
Worldwide
Phase 3
Phase 3
PSI-938
PSI-938
a
a
Worldwide
a
PSI-352938
Preclinical/
Preclinical/
Research
Research
NDA
NDA
2H13
2014
2014
3 |
 PSI-7977 Phase 2 Data Support
IFN-free, Pan-GT Registrational Program
High SVR across studies, across HCV GTs, No Resistance
PROTON –
91% SVR HCV GT1 (43/47)
–
No non-responders; no virologic failure or resistance; 1
relapse
–
No discontinuations related to PSI-7977
–
Results independent of predictors of interferon response
PROTON –
96% SVR HCV GT2/GT3 (24/25)
–
No non-responders; no virologic failure or resistance; no
relapse
–
Results independent of predictors of interferon response
ATOMIC
–
“12+0”
HCV GT1 (n=50)
–
Rapid enrollment; establish safety database for Phase 3 start
ELECTRON –
Proof of Concept for IFN-free
–
Data
to
be
presented
at
AASLD
Nov
6
th
4 |
 PSI-7977 Safety Database Supports
IFN-free, Pan-GT Registrational Program
No identified safety signal for PSI-7977 to date
>300 patients PSI-7977 400 mg QD x 12 wks
No discontinuations due to PSI-7977
No Maximum Tolerated Dose
–
Single doses up to 1200 mg
All Required Clinical Pharmacology Complete
–
Support enrollment of relevant HCV pt populations
•
Methadone & QT data at AASLD
5 |
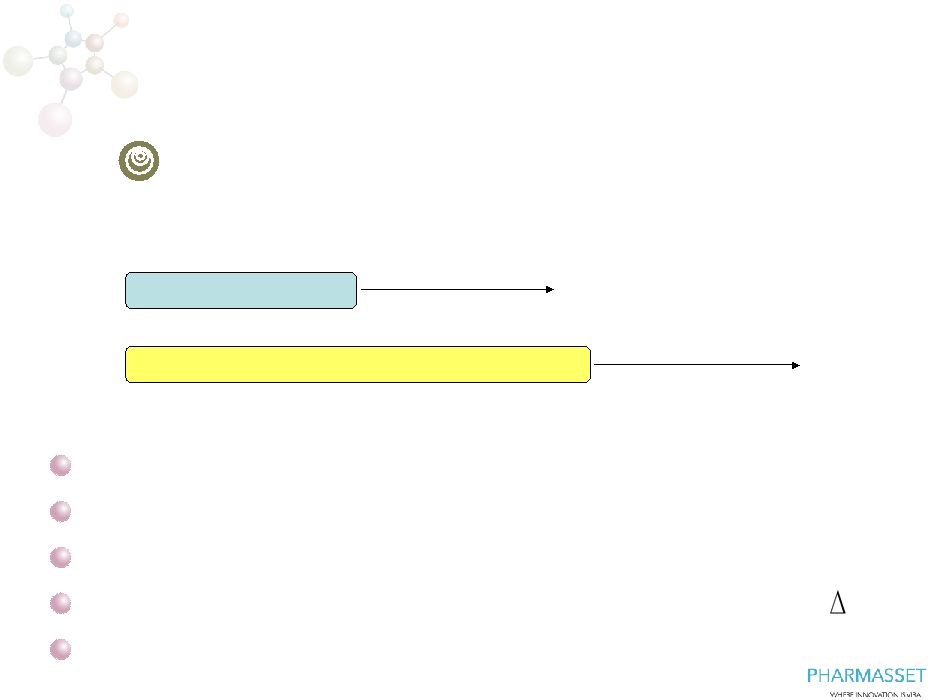 Phase
3 FISSION: PSI-7977/RBV in
Treatment-Naïve Patients with HCV GT2 or GT3
0
36
12
24
N=~250
N=~250
PSI-7977 400mg + RBV
Peg-IFN + RBV
SVR12
SVR12
~500 treatment naïve patients with HCV GT2 or GT3
HCV GT3 enrolled 3:1 over HCV GT2
International enrollment
SVR
12
endpoint,
superiority
design
:
assumes
15%
Initiating trial –
first patient, first dose by end of 2011
6
FISSION |
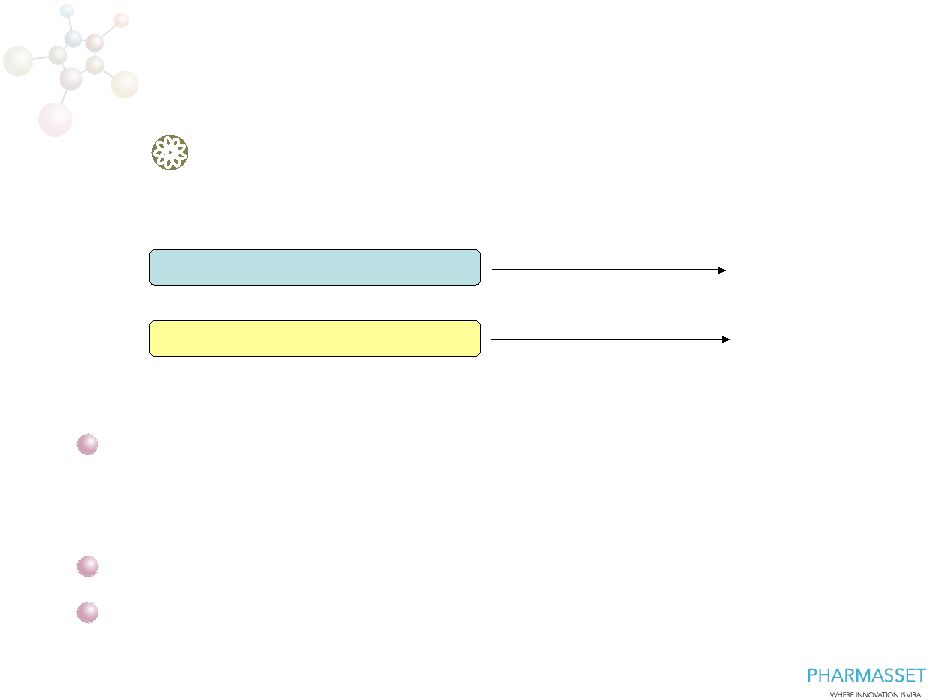 Phase
3 POSITRON: PSI-7977/RBV in Patients with HCV
GT2 or GT3 Who Cannot Take Interferon
0
12
24
N=~150
N=~75
PSI-7977 400mg QD + RBV
PSI-7977 placebo + RBV placebo
SVR12
SVR12
~225 Patients with HCV GT2 or GT3
–
Cannot take interferon
–
International trial
Primary endpoint SVR12, superiority design
Initiate in early 2012
7
POSITRON |
 Phase
3 NEUTRINO: PSI-7977/RBV in Patients with HCV
Who Cannot Take Interferon
0
12
24
N=~210
N=~70
PSI-7977 400mg QD + RBV
PSI-7977 placebo + RBV placebo
SVR12
SVR12
Final study design will be based on emerging data:
–
QUANTUM:
Planned
Interim
analysis
of
first
225
patients
–
ELECTRON:
•
PSI-7977/RBV in prior null and treatment-naïve patients with
HCV GT1
•
Additional arms in prior non-responders with GT2/GT3
Primary endpoint SVR12, superiority design
Initiate trial in mid-2012
8
NEUTRINO |
 Phase
3 Expense with shareholder support
Overall Phase 3 Costs Manageable
–
3 core studies: 1,005 patients @ $100K/pt = $101Million
Strong Balance Sheet
–
$188M as of 6/30/2011
–
Cash use in 3Q11: $22.6M
Key Catalysts Ahead
–
PSI-7977 + RBV in GT1 pts
(ELECTRON) : 1H2012 –
QUANTUM
interim data (all GTs) : 2Q2012
–
ATOMIC
interim data (non GT2/3): 2Q2012
No Near Term Financing Planned
9 |
 First IFN-Free Regimen in Phase 3
Phase 3 IFN-free program with PSI-7977/RBV
–
All 12 week regimens
–
FISSION
trial in HCV GT2/3 treatment naïve patients
–
POSITRON
trial in HCV GT2/3 patients who cannot take IFN
–
NEUTRINO
trial in HCV patients who cannot take IFN
Anticipate NDA filing in Second half of 2013
–
Submit all 3 Phase 3 studies
–
Safety database of >1,250 patients
–
Broad label covering multiple genotypes
We believe the future of HCV treatment is a short duration
of interferon free therapy
10 |
 www.pharmasset.com
www.pharmasset.com
NASDAQ: VRUS
NASDAQ: VRUS
************
************
************
************
************
************
************
************
************
************
************
************
************
************
************
************
************
***************************************************
***************************************************
*************************************************** |
