Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Riot Blockchain, Inc. | appy_8k.htm |
| EX-99.2 - EXHIBIT 99.2 - Riot Blockchain, Inc. | ex99x2.htm |
Exhibit 99.1

November 2011
NASDAQ: APPY
AppyScore™
For Acute Appendicitis Management

2
NASDAQ: APPY
Important Cautions Regarding Forward-Looking Statements
Certain statements made in this presentation are "forward-looking statements" of AspenBio Pharma, Inc. (“AspenBio")
as defined by the Securities and Exchange Commission ("SEC"). All statements, other than statements of historical fact,
included in this presentation that address activities, events or developments that AspenBio believes or anticipates will
or may occur in the future are forward-looking statements. These statements are based on certain assumptions made
based on experience, expected future developments and other factors AspenBio believes are appropriate in the
circumstances. Such statements are subject to a number of assumptions, risks and uncertainties, many of which are
beyond the control of AspenBio. Investors are cautioned that any such statements are not guarantees of future
performance. Actual results or developments may differ materially from those projected in the forward-looking
statements as a result of many factors, including our ability to successfully complete the pilot study and clinical trial
activities for AppyScore™ required for FDA submission, obtain FDA clearance, cost effectively manufacture and generate
revenues from AppyScore and other new products, execute agreements required to successfully advance the company's
objectives, retain the management team to advance the products, overcome adverse changes in market conditions and
the regulatory environment, obtain and enforce intellectual property rights, and realize value of intangible assets.
as defined by the Securities and Exchange Commission ("SEC"). All statements, other than statements of historical fact,
included in this presentation that address activities, events or developments that AspenBio believes or anticipates will
or may occur in the future are forward-looking statements. These statements are based on certain assumptions made
based on experience, expected future developments and other factors AspenBio believes are appropriate in the
circumstances. Such statements are subject to a number of assumptions, risks and uncertainties, many of which are
beyond the control of AspenBio. Investors are cautioned that any such statements are not guarantees of future
performance. Actual results or developments may differ materially from those projected in the forward-looking
statements as a result of many factors, including our ability to successfully complete the pilot study and clinical trial
activities for AppyScore™ required for FDA submission, obtain FDA clearance, cost effectively manufacture and generate
revenues from AppyScore and other new products, execute agreements required to successfully advance the company's
objectives, retain the management team to advance the products, overcome adverse changes in market conditions and
the regulatory environment, obtain and enforce intellectual property rights, and realize value of intangible assets.
Furthermore, AspenBio does not intend (and is not obligated) to update publicly any forward-looking statements. The
contents of this presentation should be considered in conjunction with the risk factors contained in AspenBio's most
recent Annual Report on Form 10-K as filed with the SEC.
contents of this presentation should be considered in conjunction with the risk factors contained in AspenBio's most
recent Annual Report on Form 10-K as filed with the SEC.
This presentation is © 2011 AspenBio Pharma, Inc., All Rights Reserved.

3
NASDAQ: APPY
The AspenBio Solution
Ÿ AspenBio is developing AppyScore, a blood-based
“Rule-Out” test for Acute Appendicitis in children and
adolescents
“Rule-Out” test for Acute Appendicitis in children and
adolescents
Ÿ Designed to help confirm that patients with abdominal
pain suspicious for appendicitis are at low to moderate
risk of the disease so they can be managed
appropriately
pain suspicious for appendicitis are at low to moderate
risk of the disease so they can be managed
appropriately
Ÿ Healthcare benefit: Fewer CTs = Reduced radiation
exposure
exposure
Ÿ Hospital benefit: Enhanced decision making, reduced
cost and increased workflow
cost and increased workflow
Ÿ Pilot study underway: Solid regulatory plan based on
regulatory feedback
regulatory feedback
Ÿ Substantial global market potential
Ÿ Significant first mover advantage
4
NASDAQ: APPY
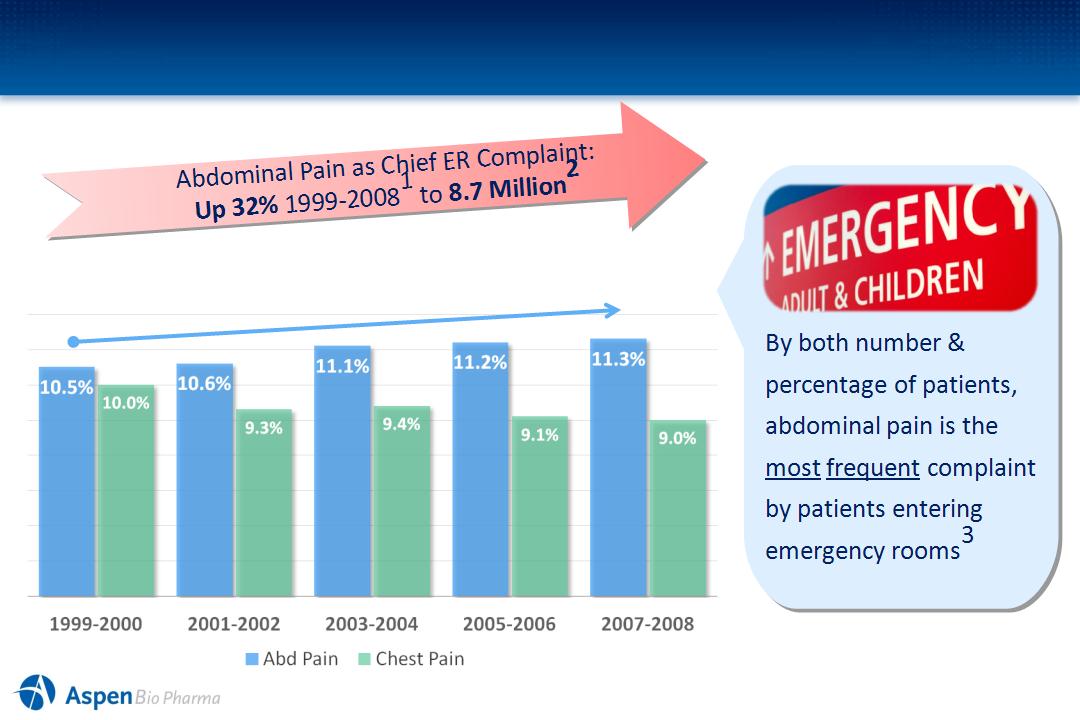
Why Focus on the Diagnosis of Abdominal Pain?
Abdominal vs. Chest Pain as % of Total ER Visits
Patients >18 years in U.S., 1999-20081
1Trend is significant (p < 0.05). Figures are based on 2-year averages. Source: CDC/NCHS, National Hospital Ambulatory Medical Care Survey 1999-2008.
²NHAMCS Data, 2008 ( All reference to abdominal pain are regarding “non- traumatic” abdominal pain)
3 Emer Med Clinics of North Amer 2001 19:123-136

5
NASDAQ: APPY
…and Why Appendicitis?
Ÿ 3.1M CT Scans annually for abdominal
pain alone2
pain alone2
Ÿ 326K appendectomies in U.S. in 20072
Ÿ Children & adolescents represent peak
age group3
age group3
Ÿ Appendicitis affects 9% men, 7%
women over lifetime3
women over lifetime3
1Ind J Radiol Imag 2006 16:4:523-532
2Source: NHDS from CDC 2007 data
3Am Journal of Epidemiology, Addis et al. Johns Hopkins Vol. 132 no. 5
Appendectomy is #1 Reason for Emergency Abdominal Surgery1

6
NASDAQ: APPY
Abdominal Pain Metrics
Patients Entering ER for Abdominal Pain & Receiving
Complete Blood Count Test (CBC) Annually (millions)
Complete Blood Count Test (CBC) Annually (millions)
Source of CBC data NHAMCS 2009
Greater than 50%
of patients
suspected of
appendicitis are
children,
adolescents and
young adults
of patients
suspected of
appendicitis are
children,
adolescents and
young adults


8
NASDAQ: APPY
Use of ER Abdominal CT Imaging is Rising Rapidly
• Use of advanced medical
imaging 1 for non-traumatic
abdominal pain is increasing
imaging 1 for non-traumatic
abdominal pain is increasing
• However, “liberal use of CT
scans in diagnosing
appendicitis in children has
not resulted in a decreased
negative appendectomy
rate”² (i.e., CT scans did not
decrease the removal of non-
diseased appendixes)
scans in diagnosing
appendicitis in children has
not resulted in a decreased
negative appendectomy
rate”² (i.e., CT scans did not
decrease the removal of non-
diseased appendixes)
Medical imaging (U.S.) in non-traumatic ER visits
Percentage per Symptom 1
Patients >18 years in U.S., 1999-2008
1 Includes CT, MRI and Ultrasound, ages 18 and older. SOURCE: CDC/NCHS, National Hospital Ambulatory Medical
Care Survey 1999-2008.
Care Survey 1999-2008.
² J Pediatr Surg. 2004 Jun;39(6):886-90; discussion 886-90.

9
NASDAQ: APPY
Ÿ Children are highly vulnerable to the oncogenic, long
term effects of exposure to high doses of ionizing
radiation (i.e., from CT)
term effects of exposure to high doses of ionizing
radiation (i.e., from CT)
Ÿ Up to 2% of all U.S. cancers will be caused by CT1
Ÿ Earlier the exposure, the more likely to die from
cancer later in life (see chart below)
cancer later in life (see chart below)
1N Engl J Med 2007; 357:2277-84
2Larson et. al., Radiology Published online April 5, 2011
Lifetime Attributable Risk of Death from Cancer
per Million Patients Exposed to 10mCy
per Million Patients Exposed to 10mCy
Age at Exposure (yr)
“Our finding of a substantial increase in the use of CT in children
who visit emergency departments in the United States
underscores the need for special attention to this vulnerable
population to ensure that imaging is appropriately ordered,
performed, and interpreted.” 2
who visit emergency departments in the United States
underscores the need for special attention to this vulnerable
population to ensure that imaging is appropriately ordered,
performed, and interpreted.” 2
CT Use in Children Increases Lifetime Cancer Risk

10
NASDAQ: APPY
Further analysis of the 2011 pilot study found that of the 50 low/mod risk subjects who had a
CT, 14 (28%) would have tested negative by our multi-marker diagnostic and hence, could
have been more conservatively managed and possibly been spared a CT scan.*
CT, 14 (28%) would have tested negative by our multi-marker diagnostic and hence, could
have been more conservatively managed and possibly been spared a CT scan.*
74/181
50/152
57/128
15/45
CT Usage Not Correlated With Appendicitis Risk
*Risk groups in the 2010 clinical study were based on a modified Alvarado scoring system (9 of the 10 Alvarado items) while the
risk groups in the 2011 pilot study were based on a new scoring systems (4 of the 10 Alvarado items).
risk groups in the 2011 pilot study were based on a new scoring systems (4 of the 10 Alvarado items).

11
NASDAQ: APPY
AppyScore Intended Use & Value Proposition
AppyScore is intended to be used as an aid to rule out appendicitis in pediatric
and adolescent patients presenting with signs, symptoms and medical history
placing them at low to moderate risk of having the disease.
and adolescent patients presenting with signs, symptoms and medical history
placing them at low to moderate risk of having the disease.
Fits emergency room & laboratory workflow
Ÿ Blood-based rule-out test
Ÿ Rapid Processing Time and
throughput
throughput
Ÿ Data accessible to hospital via
LIS system
LIS system
Ÿ Easy to deploy & use in any
hospital setting
hospital setting

12
Clinical Work Flow with AppyScore
History & Physical Exam
Conservative Management
Spares Radiation Exposure
AppyScore Testing to Help
Rule/Out Appendicitis
Rule/Out Appendicitis
CT Scan
Higher Risk
for Further
Evaluation
Evaluation
Risk
Stratification
Stratification
Signs & Symptoms
of Appendicitis
AppyScore
Test
Negative
Appendectomy
Lower Risk
AppyScore
Test Positive

13
NASDAQ: APPY
Competencies Match Requirements for Appendicitis Test Development

14
NASDAQ: APPY
Multi-Marker Approach Enhances Product Performance
Examples of Recent FDA-Cleared Single Marker vs. Multi-marker Tests
Examples of Recent FDA-Cleared Single Marker vs. Multi-marker Tests
|
FDA-Cleared
Test |
# Bio-
Markers |
Application
|
Sensitivity
|
Specificity
|
NPV
|
|
CA 125 1
|
1
|
Tumor
Marker (primarily Ovarian) |
77
|
68
|
87
|
|
Fujirebio -
ROMA 2 |
2
|
Ovarian
Cancer |
88
|
67
|
96
|
|
Vermillion -
Ova 11 |
5
|
Ovarian
Cancer |
94
|
35
|
93
|
1 Miller et al. Performance of the American College of Obstetricians and Gynecologists’ Ovarian Tumor Referral
Guidelines With a Multivariate Index Assay. Obstet Gynecol. 2011; 117(6): 1298-1306
Guidelines With a Multivariate Index Assay. Obstet Gynecol. 2011; 117(6): 1298-1306
2 Fujirebio press release. 6 Sept 2011

15
NASDAQ: APPY
2011 AspenBio Interim Pilot Study Summary
* Data as of October 2011.
|
Pilot Study
Metrics & Milestones*
|
|
|
Number of Sites
|
11
|
|
Patients Enrolled
|
406
|
|
Prevalence of Appendicitis
|
28%
|
|
% of Patients who Underwent CT
|
37%
|
|
Samples Tested for Multi-Markers
|
197
|
|
Enrollment Completion (~500 Patients)
|
Nov 11
|
|
Follow-up FDA Meeting Request
|
Q4/11
|

16
NASDAQ: APPY
Ÿ Ongoing pilot study designed to
evaluate the performance of
patented MRP 8/14 biomarker
and AspenBio’s Candidate
Multi-Marker Panel
evaluate the performance of
patented MRP 8/14 biomarker
and AspenBio’s Candidate
Multi-Marker Panel
Ÿ Interim analysis pilot study
results indicate use of multiple
biomarkers as diagnostic test
format provided improved
outcomes
results indicate use of multiple
biomarkers as diagnostic test
format provided improved
outcomes
Ÿ MRP 8/14 biomarker provided
outcomes generally consistent
with results noted in prior trials
outcomes generally consistent
with results noted in prior trials
Interim Data from 2011 Pilot Study (197 Patients)
MRP 8/14 vs. Candidate Multi-Marker Panel
Ÿ Preliminary results are encouraging, but are not conclusive
Ÿ Additional development work, analysis and completion of the
full 500-patient study is necessary prior to commencement of a
pivotal trial
full 500-patient study is necessary prior to commencement of a
pivotal trial
Ÿ *All three improvements using the multi-marker test are
collectively important
collectively important
|
|
MRP 8/14
|
Multi-Marker
|
||
|
|
Results
|
95% CI
|
Results*
|
95% CI
|
|
Sensitivity
|
90%
|
74 - 96
|
95%
|
87 - 99
|
|
Specificity
|
33%
|
25 - 42
|
40%
|
35 - 52
|
|
NPV
|
93%
|
81 - 98
|
97%
|
93 - 99
|

17
NASDAQ: APPY
1 Thachil et al. Amer J of Med 123:1 pp 17-19 (2009)
FDA Guidance Suggests Following D-Dimer Assay Model
D-Dimer Aids in
“Ruling Out”
Blood Clots1
“Ruling Out”
Blood Clots1
AppyScore
designed to Aid
in “Ruling Out”
Appendicitis
designed to Aid
in “Ruling Out”
Appendicitis
|
D-Dimer and AppyScore
|
||
|
Share Similar Regulatory Pathways
|
||
|
|
|
|
|
|
D-Dimer
|
AppyScore
|
|
Condition Being Evaluated
|
Pulmonary
Embolism |
Appendicitis
|
|
Rule-Out Test to Aid in Patient
Management (Low Disease Risk) |
Yes
|
Yes
|
|
Probability of the Disease
|
Low
|
Low
|
|
Use of Pretest Probability
|
Yes
|
Yes
|
|
Negative Predictive Value
|
High
|
High
|
|
Specificity
|
Moderate
|
Moderate
|
|
Competition
|
Significant
|
None
|
|
Number of Test Manufacturers
|
14
|
One
|


19
NASDAQ: APPY
AspenBio Positioned to Build Product Portfolio for
Abdominal Pain
Abdominal Pain
Ÿ AspenBio continues to build a comprehensive understanding of the
biology of appendicitis and abdominal pain
biology of appendicitis and abdominal pain
Ÿ As the result of numerous studies, we have amassed a large & unique
store of blood samples with diagnostic information
store of blood samples with diagnostic information
Ÿ 2,000+ samples, growing to 3,000+
following Pivotal Trial
Ÿ Further study of these samples could provide rare insight into markers
related to other causes of abdominal pain
related to other causes of abdominal pain
Ÿ This resource provides AspenBio a head start in the development of
diagnostic products focused on appendicitis and abdominal pain
diagnostic products focused on appendicitis and abdominal pain
Present Next Future

20
NASDAQ: APPY
Highly-Experienced Team w/ Strong Track Records of
Success
Success
§ Steve Lundy - President & CEO
MicroPhage, GeneOhm Sciences (Acquired by BD),
Dianon Systems (Acquired by LabCorp), Bayer
MicroPhage, GeneOhm Sciences (Acquired by BD),
Dianon Systems (Acquired by LabCorp), Bayer
Commercial leadership in several high growth
diagnostic companies (Dianon, AVL, GeneOhm)
resulting in combined exit value of >$1 billion
diagnostic companies (Dianon, AVL, GeneOhm)
resulting in combined exit value of >$1 billion
§ Steve Tyrrell- Head of Research & Development
Founder & CTO of Eveia Medical, VP R&D Biosafe
Laboratories, R&D Osborn Laboratories
Founder & CTO of Eveia Medical, VP R&D Biosafe
Laboratories, R&D Osborn Laboratories
Led the Research & Development of 10 commercial
products with primary emphasis in In Vitro
diagnostics
products with primary emphasis in In Vitro
diagnostics
§ Michael Wandell - Clinical & Regulatory Affairs
Epigenomics, Benaroya Research Institute, Home
Access Health Corp., Genetic Systems
Access Health Corp., Genetic Systems
Seven major diagnostics cleared or approved by FDA;
extensive world-wide regulatory approvals
extensive world-wide regulatory approvals
§ Erik Miller, VP Marketing & Bus. Development
Biosite (Acquired by Alere), Safety Syringes, & Luminex
Biosite (Acquired by Alere), Safety Syringes, & Luminex
Launched & managed Triage® line, UltraSafe® Safety
Devices, Luminex 200™ platform & consumable
(MagPlex®) driving key sustainable high-margin
businesses
Devices, Luminex 200™ platform & consumable
(MagPlex®) driving key sustainable high-margin
businesses
Directors with diagnostic experience
§ John Landon - Prior director of Digene, chair of Cholestech,
and Vice President and General Manager, DuPont Medical
and Vice President and General Manager, DuPont Medical
Products

21
NASDAQ: APPY
|
Summary Capitalization
|
|
|
Market Cap. (10/20/11)
|
$18.7M
|
|
Shares Outstanding*
|
8.0M
|
|
Public Float, est.
|
7.8M
|
|
|
|
|
Ownership Summary
|
|
|
Institutional Holdings, est.
|
26.4%
|
|
Insider Holdings, est.
|
3.0%
|
(mrq) - most recent quarter as of June 2011
Key Stats: APPY (NASDAQ CM)
|
Summary Balance Sheet
|
|
|
Cash & Equiv. (9/30/11)
|
$4.8M
|
|
Total Assets (mrq)
|
$12.0M
|
|
Total Liabilities (mrq)
|
$5.7M
|
*Post split as of 8/1/11

22
NASDAQ: APPY
AspenBio Key Takeaways
Ÿ AppyScore: Designed to Help Confirm that
Patients with Abdominal Pain Suspicious for
Appendicitis are at low to Moderate Risk of the
Disease so they can be Managed Appropriately
Patients with Abdominal Pain Suspicious for
Appendicitis are at low to Moderate Risk of the
Disease so they can be Managed Appropriately
Ÿ Patient Benefit: Fewer CTs = Reduced radiation
exposure
exposure
Ÿ Hospital Benefit: Enhanced Decision Making,
Reduced Cost and Increased Workflow
Reduced Cost and Increased Workflow
Ÿ Pilot Study Underway: Solid Regulatory Plan
Based on Regulatory Feedback
Based on Regulatory Feedback
Ÿ Recent ISO 13485 Certification Achieved
Ÿ Substantial Global Market Potential
Ÿ Significant First Mover Advantage
