Attached files
| file | filename |
|---|---|
| 8-K/A - FORM 8-K/A - ENDO HEALTH SOLUTIONS INC. | d238841d8ka.htm |
 ENDO
PHARMACEUTICALS Jefferies 2011 Global Healthcare Conference
September 27, 2011
grow. collaborate. innovate.
thrive. Exhibit
99.1 |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
2
FORWARD LOOKING STATEMENTS
This presentation contains forward-looking statements within the meaning of the Private
Securities Litigation Reform Act of 1995. Statements including words such as
“believes,” “expects,”
“anticipates,”
“intends,”
“estimates,”
“plan,”
“will,”
“may,”
“look forward,”
“intend,”
“guidance,”
“future”
or similar
expressions are forward-looking statements. Because these statements reflect our
current views, expectations and beliefs concerning future events, these
forward-looking statements involve risks and uncertainties. Investors should note
that many factors, as more fully described under the caption “Risk
Factors”
in our Form 10-K, Form 10-Q and Form 8-K filings with the Securities and Exchange
Commission and as otherwise enumerated herein or therein, could affect our future
financial results and could cause our actual results to differ materially from those
expressed in forward-looking statements contained in our Annual Report on Form
10-K. The forward-looking statements in this presentation are qualified by
these risk factors. These are factors that, individually or in the aggregate, could cause our
actual results to differ materially from expected and historical results. We assume no
obligation to publicly update any forward-looking statements, whether as a result
of new information, future developments or otherwise. |
 ENDO
PHARMACEUTICALS I.
Our Diversified Business
II.
Pharmaceuticals
III.
American Medical Systems
IV.Innovation
V.
Financial Outlook
grow. collaborate. innovate. thrive. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
4
ENDO’S INTEGRATED BUSINESS MODEL
Branded
Pharmaceuticals
Generics
Pain
Urology
Oncology
Endocrinology
Devices
& Services |
 ©2011
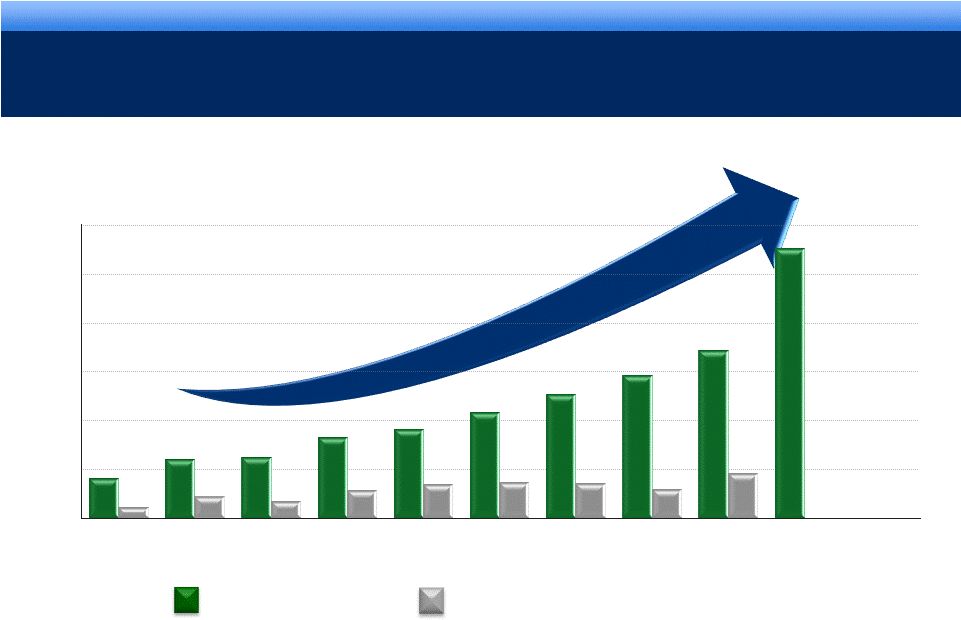
Endo Pharmaceuticals STRONG OPERATING PERFORMANCE
16% 3-YEAR CAGR FOR REVENUE*
* Revenue CAGR 2007-2010.
$mm
5
Sustaining our Growth
$3,000
$2,500
$2,000
$1,500
$1,000
$500
$0
2002
2003
2004
2005
2006
2007
2008
2009
2010
2011E
$399
$596
$615
$820
$910
$1,086
$1,261
$1,461
$1,716
$2.72B -
$2.80B
$110
$217
$171
$285
$345
$366
$356
$295
$454
grow. collaborate. innovate. thrive.
Revenue
Cash Flow from Operations |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
6
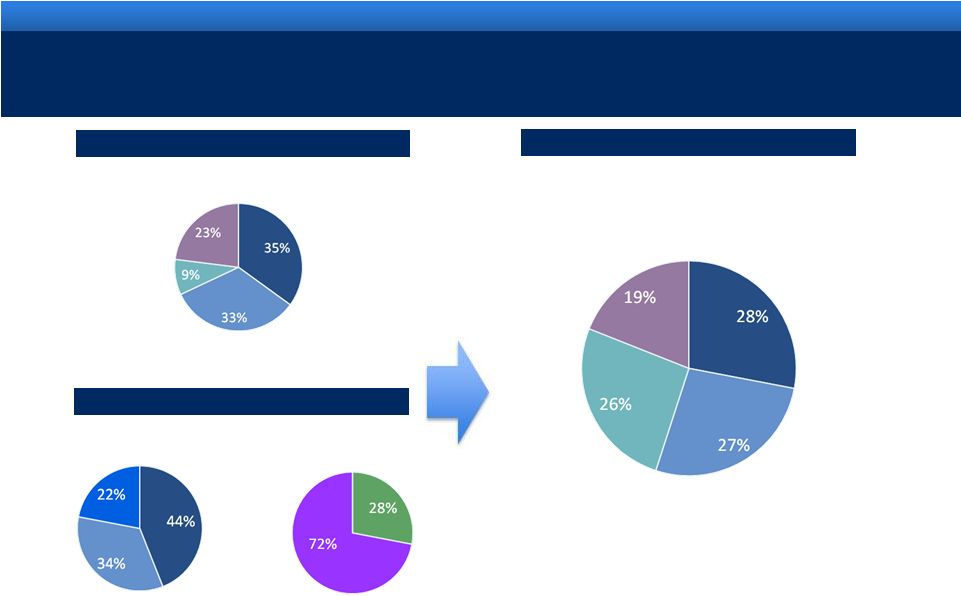
DIVERSIFIED HEALTHCARE SOLUTIONS COMPANY
Lidoderm®
Generic
Men’s
Health
TTM 6/30/11: $2.25B
(1)
TTM 6/30/11: $0.54B
(2)
TTM 6/30/11: $2.79B
(3)
Revenue mix
Endo
AMS
Pro Forma
1.
Represents TTM of pro forma 2010 acquisitions (Qualitest, HealthTronics and Penwest,
excluding AMS) 2.
Includes $1.1MM of revenue from the uterine health business, which was divested during the
first quarter 2010. 3.
Includes full year of AMS acquisition
Other Branded
Devices &
Services
Women’s
Health
BPH
Lidoderm®
Generic
Other Branded
Devices &
Services
Revenue mix
Revenue mix
International
US |
 ©2011
Endo Pharmaceuticals STRONG CORE BUSINESS SUPPORTING GROWTH
7
grow. collaborate. innovate. thrive. |
 8
AMERICAN MEDICAL SYSTEMS ACQUISITION
©2011 Endo Pharmaceuticals
grow. collaborate. innovate. thrive. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
9
Leading Provider of Devices to Pelvic Health Space
•
Men’s Health: Solutions for male incontinence and erectile
dysfunction prostheses
•
Women’s Health: Solutions for female incontinence and pelvic
floor repair
•
Benign Prostatic Hyperplasia (BPH) Therapy: Laser treatments
Strong Product Portfolio Supported by Robust Future
Product Cadence
•
Solid market share in several categories that have historically
grown in the mid-to-high single digit range over the long-term
•
Near-term growth driven by new product launches and
initiatives such as the GreenLight XPS console and MoXy fiber
Seasoned Management Team and High Quality Assets
•
Team has significant experience running a growing devices
business
•
Four global facilities –
two in the US (Minnetonka, MN and San
Jose, CA) and two in Europe (Breukelen, Netherlands and
Athlone, Ireland)
9
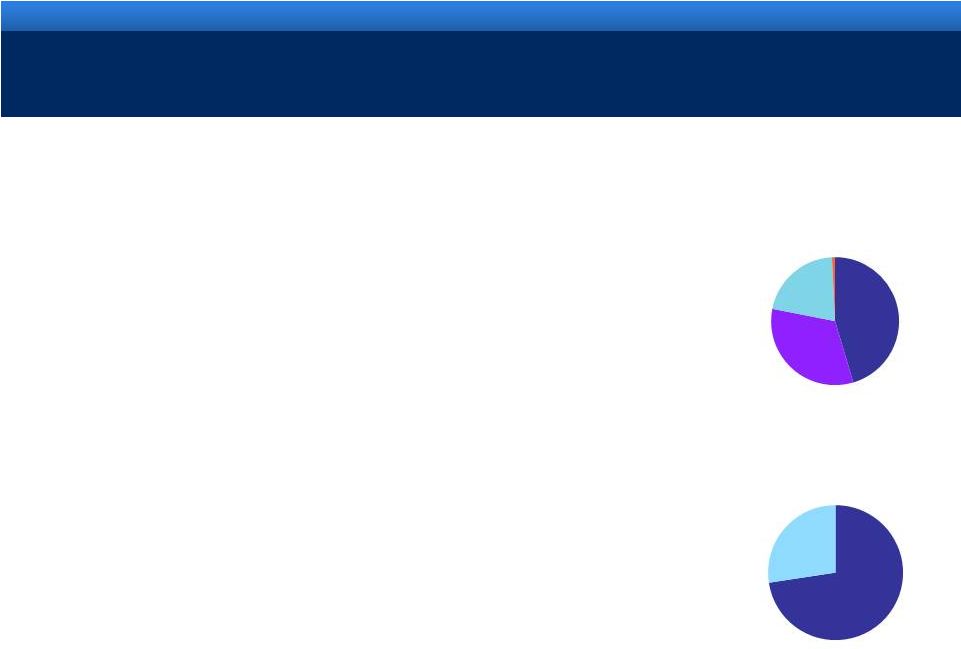
AMS COMPANY PROFILE
Revenue by Segment
FY 2010
Revenue by Geography
FY 2010
United States 73%
International
27%
Men's Health
45%
Women's
Health
33%
BPH
21%
Other
1% |
 10
AMS –
PRODUCT PORTFOLIO
Product
Description
AMS 800®
Artificial Urinary
Sphincter
InVance®
Less invasive treatment
for moderate
incontinence
AdVance®
Less invasive treatment
for mild to moderate
incontinence
UroLume®
Endoprosthesis for non-
surgical candidates
AMS 700®
Semi-rigid malleable
prostheses/inflatable
prostheses for treatment
of Erectile Dysfunction
Product
Description
Monarc®
Self-fixating, subfascial
hammock for treatment of
stress incontinence
MiniArc®
Single-Incision Sling for
stress incontinence
Elevate®
Transvaginal pelvic floor
repair system (requires no
external incisions)
Product
Description
GreenLight™
Photo Vaporization / laser
therapy designed to
remove prostatic tissue
StoneLight®
Laser treatment of urinary
stones
SureFlex™
Fiber optic line designed
for holmium laser
lithotripsy
TherMatrx®
Less invasive tissue
ablation technique for
men not yet to the point
of urethral obstruction
Men’s Health
Women’s Health
BPH
©2011 Endo Pharmaceuticals
grow. collaborate. innovate. thrive. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
A COMMITMENT TO INNOVATION
11
11
Branded Pharmaceuticals
Medical Devices
Generic Pharmaceuticals
•
Key Therapeutic Areas
•
Pain
•
Oncology
•
Endocrinology
•
Semi-Virtual Model
•
Global Partnerships
•
Discovery
•
Early Development
•
Development Pipeline
•
Anticipate filing 14 ANDAs in
2011
•
50 ANDAs currently under
review by FDA
•
Expect 14 ANDA approvals in
2011
5 ANDA approvals YTD
•
Supplements strong
commercial base growth
•
Continually enhancing existing
products
•
Recent advances:
GreenLight™
XPS Laser
Console
MoXy™
Laser Fiber
AdVance™
XP (OUS)
•
Developing treatments in new
areas
•
Exploring new emerging
technologies
OPANA®
ER
Formulation designed to be crush-resistant
AVEED
TM
Long Acting Injectable Testosterone
Urocidin
TM
Bladder Cancer
Octreotide Implant
Acromegaly**
Androgen Receptor Agonist***
Castration Resistant Prostate Cancer
* There can be no assurance that any of these development programs will be
successful or if successful, the products will ultimately be approved by FDA. **
Granted orphan drug designation *** Licensed from Orion Corporation for joint
development and commercialization |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
12
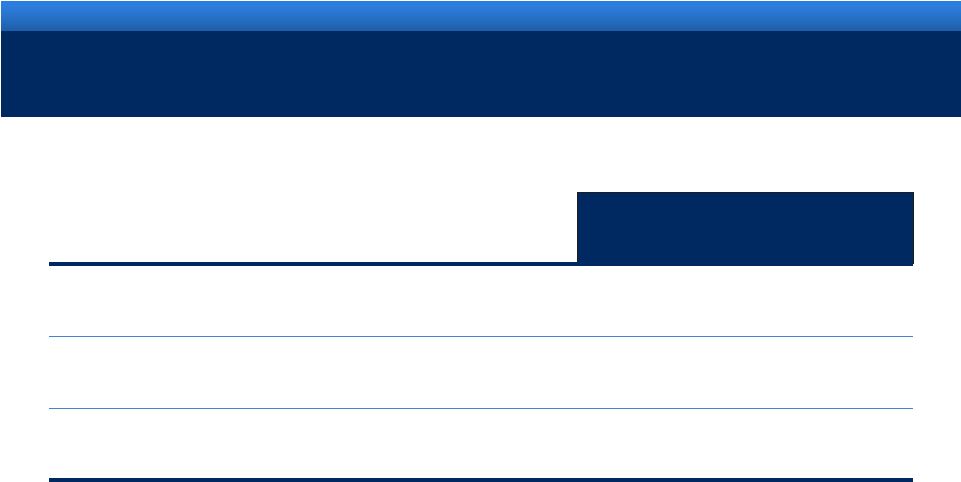
2011 ENDO GUIDANCE
Guidance
Revenue range
$2.72B -
$2.80B
Adjusted diluted EPS range
$4.55 -
$4.65
Reported (GAAP) diluted EPS range
$2.22 -
$2.32 |
 ENDO
PHARMACEUTICALS grow. collaborate. innovate. thrive. |
 grow.
collaborate. innovate. thrive. ©2011 Endo Pharmaceuticals
14
14
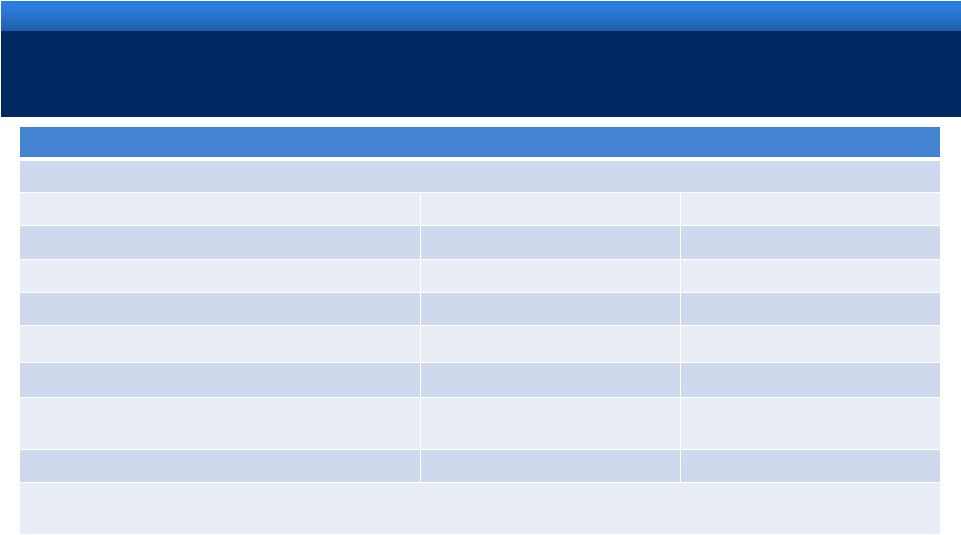
RECONCILIATION OF NON-GAAP MEASURES
14
For an explanation of Endo’s reasons for using non-GAAP measures, see Endo’s
Current Report on Form 8-K filed today with the Securities and Exchange Commission
Reconciliation of Projected GAAP Diluted Earnings Per Share to Adjusted Diluted Earnings Per
Share Guidance for the Year Ending December 31, 2011 Lower End of Range
Upper End of Range
Projected GAAP diluted income per common share
$2.22
$2.32
Upfront and milestone-related payments to partners
$0.27
$0.27
Amortization of commercial intangible assets and inventory step-up
$1.94
$1.94
Acquisition and integration costs related to recent acquisitions.
$0.40
$0.40
Interest expense adjustment for ASC 470-20 and the amortization of
the premium on debt acquired from Indevus
$0.16
$0.16
Tax effect of pre-tax adjustments at the applicable tax rates and
certain other expected cash tax savings as a result of recent
acquisitions
($0.44)
($0.44)
Diluted adjusted income per common share guidance
$4.55
$4.65
The company's guidance is being issued based on certain assumptions including:
•Certain of the above amounts are based on estimates and there
can be no assurance that Endo will achieve these results
•Includes all completed business development transactions as of
September 27, 2011 |
 ENDO
PHARMACEUTICALS Jefferies 2011 Global Healthcare Conference
September 27, 2011
grow. collaborate. innovate.
thrive. |
