Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Orexigen Therapeutics, Inc. | d236506d8k.htm |
 1
JMP Securities Healthcare Conference
JMP Securities Healthcare Conference
September 28, 2011
September 28, 2011
Exhibit 99.1 |
 Forward-Looking Statements
Forward-Looking Statements
2
This presentation contains forward-looking statements about Orexigen Therapeutics, Inc.
Words such as "believes," "anticipates," "plans,"
"expects," "indicates," "will," "intends," "potential," "suggests," "assuming," "designed" and similar expressions are intended
to identify forward-looking statements. These statements are based on the Company's current
beliefs and expectations. These forward-looking
cardiovascular outcomes trial (CVOT); the potential for resubmission and approval of an NDA
based on interim results of the CVOT; the prospects for ultimate approval of an NDA for
Contrave; and the potential to complete a partnership or similar transaction for ex-
North American rights to Contrave and maintain the Company’s existing North American
collaboration with Takeda Pharmaeuticals. The inclusion of forward-looking
statements should not be regarded as a representation by Orexigen that any of its plans will be
achieved. Actual results may differ from those set forth in this presentation due to the risk
and uncertainties inherent in Orexigen’s business, including, without limitation:
Orexigen’s ability to maintain and raise sufficient capital to fund the CVOT and maintain its
other operations; the uncertainty of the FDA approval process, including requirements for
additional clinical and non-clinical studies or other commitments prior to the
submission and approval of an NDA for Contrave; Orexigen's ability to demonstrate that the risk
of major adverse CV events in overweight and obese subjects treated with Contrave does not
adversely affect the product candidate’s benefit-risk profile; the potential
for FDA’s planned 2012 public advisory committee meeting on obesity drug
development to result in additional NDA approval requirements for Contrave as well as
post-approval commitments; Orexigen’s dependence on Takeda Pharmaceuticals for
aspects of the development and commercialization of Contrave; reliance on third parties
to supply Contrave and assist with the development of Contrave and the regulatory submissions
related thereto; the potential for adverse safety findings relating to Contrave; intense
competition in the obesity marketplace and the potential for new products to emerge that
provide different or better therapeutic alternatives for obesity and weight loss compared to Contrave; and other risks
described in the Company's filings with the Securities and Exchange Commission (SEC). You are
cautioned not to place undue reliance on these forward-looking statements, which
speak only as of the date hereof, and Orexigen undertakes no obligation to revise or
update this presentation to reflect events or circumstances after the date hereof. Further information regarding these and
other risks is included under the heading "Risk Factors" in Orexigen’s Quarterly
Report on Form 10-Q, which was filed with the SEC on August 8, 2011 and is available
from the SEC's website (www.sec.gov) and on our website (www.orexigen.com) under the
heading "Investor Relations”. All forward-looking statements are qualified in
their entirety by this cautionary statement. This caution is made under the safe harbor
provisions of Section 21E of the Private Securities Litigation Reform Act of 1995.
statements
include
statements
regarding:
the
study
design
for,
and
the
timing
and
feasibility
of,
the
Contrave
® |
 Orexigen
Strategic Summary Orexigen Strategic Summary
Clear path to approval defined by FDA’s OND
Clear path to approval defined by FDA’s OND
Time, cost and probability of success make approval
Time, cost and probability of success make approval
requirements feasible
requirements feasible
Regulatory Path Clarified
Regulatory Path Clarified
Obesity remains a huge market opportunity
Obesity remains a huge market opportunity
Global epidemic with increasing public health concerns
Global epidemic with increasing public health concerns
Obesity Unmet Need
Obesity Unmet Need
Contrave
Contrave
®
®
has blockbuster, global potential
has blockbuster, global potential
Target profile indicates potential first-line pharmacotherapy for
Target profile indicates potential first-line pharmacotherapy for
the majority of patients
the majority of patients
NA partner Takeda has relevant skills and experience
NA partner Takeda has relevant skills and experience
Ex-NA commercial partnership planned
Ex-NA commercial partnership planned
Orexigen Position
Orexigen Position
3 |
 Experienced
Management Team with a Strong Balance Sheet
Experienced Management Team with a Strong
Balance Sheet
Michael
Narachi,
President
and
CEO
20-Year
Veteran
of
Amgen
–
Including:
GM of Anemia Business Unit
Global Team Leader, Neupogen
Head of Licensing and Business Development
Mark
Booth,
Chief
Commercial
Officer
President, Takeda
Pharmaceuticals North America Senior Commercial Officer, Immunex and Abbott
Jay
Hagan,
Chief
Business
Officer
Managing Director,
Amgen Ventures
Head
of
Corporate
Development,
Amgen
Preston
Klassen,
M.D.,
SVP,
Head
of
Development
Nephrology Therapeutic Area Head, Amgen Suzanne
McDonald,
Consultant,
Managed
Markets & Government Affairs
VP, Managed Markets and Government Affairs, Takeda Heather
Turner,
SVP,
General
Counsel
Associate General Counsel, Conor Medsystems
Dawn
Viveash,
M.D.,
SVP,
Head
of
Regulatory Affairs
VP, Regulatory Affairs and Safety, Amylin VP,
Regulatory Affairs and Safety, Amgen & Immunex
Global Chief Safety Officer, P&U
4
End of Q2 Cash and Investments Balance of $69.7m
Currently Low Operating Expense Base and No Debt |
 5
Regulatory Update
Orexigen Position
Global Need |
 Filed
NDA
NDA Accepted
by FDA
Results
Published in
The Lancet
&
Obesity
Signed Takeda
Partnership
Positive Advisory
Committee
Meeting
The Orexigen Story –
Two Years of Successes and
Setbacks, but a Clear Path to Approval Has Emerged
The Orexigen Story –
Two Years of Successes and
Setbacks, but a Clear Path to Approval Has Emerged
2010
2011
CRL
Received
EOR –
Appeal,
Programs Halted &
New Asset Search
Initiated
FDA Feedback on
Feasible Path
Forward –
Development
Restarted
6
Q1
Q2
Q3
Q4
Q1
Q2
Q3
Q4 |
 FDA Has Provided
Guidance on Critical Elements for Feasible Trial
FDA Has Provided Guidance on Critical Elements for
Feasible Trial
Goal of trial is to demonstrate that the risk of CV events is not
unacceptably high
Primary endpoint is major CV events (MACE = CV death, MI, stroke)
Enrolled patient population should have background MACE event rate
of 1.0-1.5% per year
Success criteria at interim analysis to enable approval:
–
Upper bound of 95% CI should exclude a hazard ratio of 2.0
–
Requires ~87 endpoint events and an observed hazard ratio of <1.32
Success criteria at final (post-approval) analysis:
–
Upper bound of 95% CI should exclude a hazard ratio of 1.4
–
Requires ~371 endpoint events and an observed hazard ratio of <1.14
7 |
 Trial Focus
Enables Streamlined Approach and Drives Feasible Cost Estimates
Trial Focus Enables Streamlined Approach and Drives
Feasible Cost Estimates
Typical Phase 3 Trial
Approach for Contrave CVOT*
Comprehensive evaluation of efficacy
and general safety
Targeted clinical question allows
focused and streamlined design
Detailed data collection is required
with lengthy CRF (drives site cost)
A CRF focused on primary endpoint
(less data capture)
AE collection is broad
AE collection limited to SAE
Comprehensive labs and ancillary
assessments
Limited labs/ancillary measures
Frequent site visits
Less frequent site visits
Trial activity is focused at sites
Leverages web-based activity
Many enrollment exclusion criteria
Fewer enrollment exclusion criteria
*Trial design and cost assumptions based on current understanding of FDA correspondence. Final
cost projections require FDA agreement on trial protocol.
8
Total cost of Contrave CVOT to interim analysis
and approval is estimated at <$100M
* |
 Probability of
Success at Interim Analysis is High Probability of Success at Interim Analysis is
High Bupropion well characterized in >50M patients over >25 years
–
Large prospective patient registries (up to 10,000 patients) and
examination of AERS have not identified evidence of increased clinical
cardiovascular events
Demonstration of non-inferior CV safety required for approval
–
Interim analysis hurdle for approval is reasonable and analogous
to the
threshold required in Type 2 DM
–
Excluding a HR of 2.0 at interim is possible with a trial result
HR as high
as 1.31
We believe Contrave CV trial is unlikely to produce SCOUT-like results
–
Bupropion is a different drug with different clinical profile
–
Risk Engine modeling of 1-year Contrave Phase 3 data predicts reduced
10-year CV risk compared to placebo
–
Use of a real-world approach to drug utilization will focus therapy on early
responders
9 |
 Estimated
Timing of Key Events That Could Deliver a 2014 Approval
Estimated Timing of Key Events That Could Deliver a
2014 Approval
Development Milestone
Estimated Time
Finalize Protocol Details and Begin CVOT
7-9 months
Enroll and Accrue 87 CV Events for Interim Analysis
18-20 months
Time to Prepare resubmission
3-4 months
Regulatory Review
6 months
Potential Approval
H2 2014
10 |
 Regulatory
Update Orexigen Position
Global Need
11 |
 Obesity
Epidemic Plus Lack of Solutions Have Created a Perfect Storm
Obesity Epidemic Plus Lack of Solutions Have
Created a Perfect Storm
12
T2DM, Driven by Obesity, Is an Epidemic
1
in
3
Adults
in
the
U.S.
May
Have
Diabetes
by
2050
(CDC)
Associated
with
Premature
Death
&
Physical/Psychosocial
Consequences
Weight Loss of 5
10% Confers Significant Benefits
Lifestyle Change is Critical, but Weight Loss Difficult to
Achieve/Maintain
Large Gaps in Current Treatment Paradigm
75M
U.S.
103M
16%
34%
43%
Serious
Disease
Attributable
to
Obesity
Treatment
Options
Needed
Increase
in
Obesity
Source: http://www.cdc.gov; http://www.fightchronicdisease.org;
Flegal KM, et al. 2010 |
 World Wide
Obesity Prevalence Projected to Increase Over the Next Decade
World Wide Obesity Prevalence Projected to
Increase Over the Next Decade
n= ~473M people
n= ~473M people
n= ~655M people
n= ~655M people
2010
2010
% Growth
% Growth
81%
81%
17%
17%
38%
38%
20%
20%
41%
41%
Region
Region
Source: EuroMonitor, 2010
Source: EuroMonitor, 2010
2020
2020
13
182
135
117
127
93
Asia Pacific
Europe
Latin America
North America
ROW
101
115
85
106
66 |
 Regulatory
Update Orexigen Position
Global Need
14 |
 Majority of
Obese are Untreated in an Environment of Limited Treatment Options Contrave
Keys to Commercial Success
Contrave
Keys to Commercial Success
Source: CDC (2009)
103M
US Obesity Rate Growth
Obesity
Rate
Blockbuster Market Size
Blockbuster Market Size
Market Growth
Market Growth
Significant Unmet Need –
Limited Competition
Contrave: Differentiated Profile
Contrave: Differentiated Profile
Takeda: Well Established and Resourced
Takeda: Well Established and Resourced
BMI
Distribution
of US Pop:
Morbidly Obese
Obese
Overweight
~ 113K People
Treated w/ Surgery
~ 2 Million People
Treated w/ Rxs
US Population
~ 1 in 3 People
in the US
are Obese
15
50%
40%
30%
20%
10%
0%
1970s
2009
2018 |
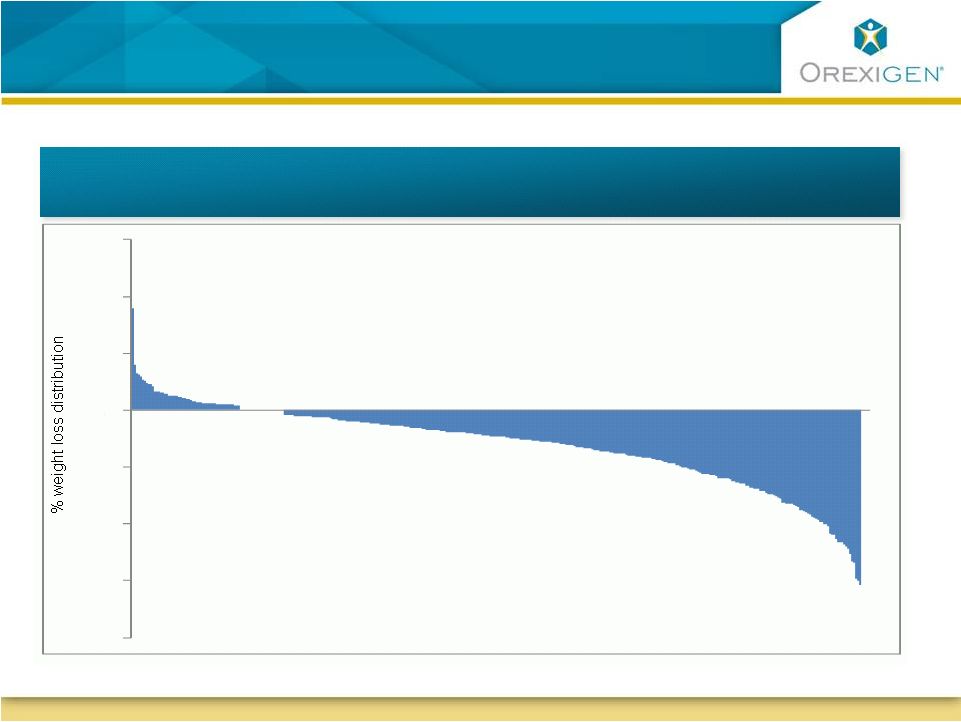 Typical
Response to Weight Loss Therapy is not Uniform
Typical Response to Weight Loss Therapy
is not Uniform
16
COR-I Weight Loss Distribution (Contrave32)
COR-I Weight Loss Distribution (Contrave32)
-
40
-
30
-
20
-
10
0
10
20
30
N=471
N=471
for
COR-I
subjects
treated
with
Contrave
32
32 |
 Our Recommended
Treatment Algorithm Focuses on Patients who Achieve a Meaningful Response
Our Recommended Treatment Algorithm Focuses on
Patients who Achieve a Meaningful Response
Our Focus: Patients
Who Lose
5%
<5% weight loss
COR-I Weight Loss Distribution (Contrave32)
N=471 for COR-I subjects treated with Contrave 32
-40
-30
-20
-10
0
10
20
30
17 |
 18
Treatment Algorithm: Identify Early Responders
for Continued Long-term Therapy
Treatment Algorithm: Identify Early Responders
for Continued Long-term Therapy
Appropriate BMI
Motivated to Adhere
to Lifestyle Change
Inadequate
Response to Diet &
Exercise
Select Responders
for Continued
Treatment
Reinforce Adherence
to Appropriate Use
Evaluate
Adherence
to
Appropriate
Use
Guidance
Assess
Response
to
Therapy
(
5%)
Screening
16-Week
Therapeutic Trial
Long-Term
Therapy |
 19
Contrave Early Responders Go on to Achieve
Substantial Long Term Weight Loss
Contrave Early Responders Go on to Achieve
Substantial Long Term Weight Loss
Weight Loss Distribution
Weight Loss Distribution
0% -
5%
Weight loss
10% -
15%
Weight Loss
Avg Wt Loss =
26.7 lbs
>15% Wt Loss
Avg Wt Loss =
43.9 lbs
Weight Gain
Represents
one
year
data
for
responders
(patients
with
5%
weight
loss
at
week
16
per
recommended
treatment
protocol)
5% -
10%
Weight Loss
Avg Wt Loss =
17.0 lbs |
 20
Contrave Responders Achieve Improvement across
Multiple Cardiometabolic Parameters
Contrave Responders Achieve Improvement across
Multiple Cardiometabolic Parameters
Improvement
(Standardized Mean Change from Baseline)
Contrave32
N=1038
Placebo
N=254
-11.3%
-8.6%
-9.5 cm
-7.2 cm
5.2 mg/dL
2.9 mg/dL
-2.5 mg/dL
1.7 mg/dL
-16.0%
-9.2%
-38.9%
-36.2%
14.7
12.3
-1.0%
-0.6%
-0.9 mm Hg
-2.8 mm Hg
-1.6 mm Hg
-2.6 mm Hg
0.2 bpm
-2.3 bpm
Proportion w/
5% Wt Loss Wk 16
51%
19%
Contrave32 Standardized Mean
Change from Baseline at 1 Year
Change from Baseline at 1 Year
Body Weight
Waist Circ.
HDL
LDL
Triglycerides
hsCRP
IWQOL
HbA1c
SBP
DBP
Heart Rate
-0.4
0
0.4
0.8
1.2
1.6
2
2.4 |
 21
Contrave: Well Characterized Safety Profile
Contrave: Well Characterized Safety Profile
Delivers Safety Experience Consistent with
Delivers Safety Experience Consistent with
20+ Year History of Underlying Agents
20+ Year History of Underlying Agents
Known Areas of Focus for Development Program –
Known Areas of Focus for Development Program –
CV Effects, Seizure, Psychiatric Effects, Creatinine
CV Effects, Seizure, Psychiatric Effects, Creatinine
General Safety Profile Established in >4500 Patients with
General Safety Profile Established in >4500 Patients with
Single Approval Deficiency to Evaluate CV Safety
Single Approval Deficiency to Evaluate CV Safety
Theoretical CV Risk Remains an FDA Concern
Theoretical CV Risk Remains an FDA Concern
OND Outlined a Feasible Plan to Evaluate CV Outcomes
OND Outlined a Feasible Plan to Evaluate CV Outcomes |
 22
SUCCESSFUL
BRAND BUILDER
Actos ~$3B in U.S.
Sales in 2009
Prevacid ~$3B in U.S.
Sales in 2009
SCALE
Top 15 Pharma
Significant U.S. Presence
Primary Care Focus
COMMITMENT
TO OBESITY
Partnership
with Amylin
Stated
Strategy
DOMAIN
EXPERIENCE
Leader in
Cardiometabolic
Care
Excellence in
Life Cycle
Management
Takeda is the Ideal Partner for Contrave
Takeda is the Ideal Partner for Contrave |
 23
Competitive Product Profile Attractive to Patients
Competitive Product Profile Attractive to Patients
Women Dominate the Market
No Addictive Concerns
Responders Maintained Meaningful
Weight Loss
No Abuse Liability
No DEA Limitations on
Distribution Expected
73%
73%
Sources: Orexigen Quantitative Market Research 2009; IMS NDTI Audit Data, 6
Months Ending 3/09
Female, Many of
Childbearing Age
Represents
one
year
data
for
responders
(patients
with
5%
weight
loss
at
week 16 per recommended treatment protocol) |
 24
Orexigen Strategic Summary
Orexigen Strategic Summary
Clear path to approval defined by FDA’s OND
Clear path to approval defined by FDA’s OND
Time, cost and probability of success make approval
Time, cost and probability of success make approval
requirements feasible
requirements feasible
Obesity remains a huge market opportunity
Obesity remains a huge market opportunity
Global epidemic with increasing public health concerns
Global epidemic with increasing public health concerns
Contrave has blockbuster, global potential
Contrave has blockbuster, global potential
Target profile indicates potential broad use for the majority of
Target profile indicates potential broad use for the majority of
patients
patients
NA partner Takeda has relevant skills and experience
NA partner Takeda has relevant skills and experience
Ex-NA commercial partnership planned
Ex-NA commercial partnership planned
Regulatory Path Clarified
Regulatory Path Clarified
Obesity Unmet Need
Obesity Unmet Need
Orexigen Position
Orexigen Position |
 25 |
