Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Innoviva, Inc. | a11-26866_18k.htm |
| EX-99.1 - EX-99.1 - Innoviva, Inc. | a11-26866_1ex99d1.htm |
| EX-99.2 - EX-99.2 - Innoviva, Inc. | a11-26866_1ex99d2.htm |
| EX-99.3 - EX-99.3 - Innoviva, Inc. | a11-26866_1ex99d3.htm |
Exhibit 99.4
The pharmacodynamics of GSK961081 in patients with COPD
V Norris(1), C Zhu(2), C Ambery(2)
(1)GlaxoSmithKline, Stevenage, UK; (2)GlaxoSmithKline, Uxbridge, UK
Poster No. P823
INTRODUCTION
· Inhaled muscarinic antagonists (MA) and beta2-adrenoceptor agonists (BA) are the mainstay of treatment of COPD. Clinical trials in COPD patients have shown that combining agents from these two classes leads to greater efficacy and therefore long-acting bronchodilators with different mechanisms are frequently co-prescribed in patients with COPD.
· GSK961081 (formerly TD5959) is a dual pharmacophore that has both long-acting muscarinic antagonist activity and long-acting beta2-adrenoceptor agonist activity in a single molecule (MABA).(1),(2)
· This study was conducted to determine the safety, tolerability, pharmacokinetics and pharmacodynamics, both pulmonary and systemic, of inhaled GSK961081 in patients with COPD.
OBJECTIVES
Primary
· To evaluate the pulmonary pharmacodynamic profile of GSK961081 following 14 days’ dosing in patients with COPD.
Secondary
· To evaluate the safety and tolerability of GSK961081 given for 14 days in patients with COPD.
· To evaluate the systemic pharmacodynamic effects of GSK961081 given for 14 days in patients with COPD .
· To evaluate the systemic pharmacokinetics of GSK961081 given for 14 days in patients with COPD.
METHODS
· This was a randomised, double-blind, double-dummy, placebo and active comparator controlled, incomplete block crossover study.
· Male and female (of non-child bearing potential) COPD patients with FEV1 <80 % predicted and FEV1/FVC <0.7 post-bronchodilator and reversibility to salbutamol and ipratropium were enrolled.
· Long-acting inhaled bronchodilators were prohibited from 72h before screening until after completion of all treatment periods. Salbutamol was permitted as rescue medication during the study but was required to be withheld for 6h before any lung function assessment. Inhaled corticosteroids (ICS) of <1000mcg fluticasone propionate (or equivalent) were permitted provided the dose was stable for 6 weeks before screening .
· Each patient received 3 out of the following 4 treatments for 14 days, with a washout period of at least 14 days between treatments
· 400mcg GSK961081 DPI (via DISKUS®) plus placebo (matched for tiotropium) in the morning and placebo (via DISKUS) in the evening
· 1200mcg GSK961081 DPI (via DISKUS) plus placebo (matched for tiotropium) in the morning plus placebo (via DISKUS) in the evening
· placebo (via DISKUS) plus placebo (matched for tiotropium) in the morning and placebo (via DISKUS) in the evening
· 50mcg salmeterol (via DISKUS) plus 18mcg tiotropium in the morning and 50 mcg salmeterol (via DISKUS) in the evening.
· FEV1, heart rate, 12-lead ECG (QTcF), blood potassium and glucose and plasma GSK961081 were measured before and frequently after dosing. Adverse events were monitored and laboratory safety tests were done.
RESULTS
Demographics
· Fifty (16 female, 34 male) patients were randomised with 43, 29, 32 and 41 receiving placebo, 400mcg GSK961081, 1200mcg GSK961081 and tiotropium plus salmeterol, respectively.
· Patients were aged 58.3 years (range 41–74); 18 were former smokers and 32 were current smokers; 11 patients took ICS.
Safety and tolerability
· Three patients discontinued prematurely from the study
· one patient was withdrawn due to an exacerbation of COPD requiring steroid treatment whilst taking tiotropium plus salmeterol
· two withdrew for personal reasons unrelated to the study.
· There were no serious adverse events; adverse events were similar across all groups with the exception of tremor (n=2, 1200mcg dose), dysguesia (n=2, 1200mcg dose; n=2, 400mcg dose) and dry mouth (n=1, 1200mcg dose) seen after GSK961081 only.
· There were no clinically significant abnormal 12-lead ECG findings or laboratory safety test findings.
Pulmonary pharmacodynamics
· After 14 days’ dosing there were significant improvements in trough FEV1 for all active treatments (Figure 1)
· for 400mcg GSK961081, 1200mcg GSK961081 and tiotropium plus salmeterol adjusted mean (95% confidence interval [CI]) differences vs placebo were 0.115L (0.024, 0.205), 0.168L (0.080, 0.255) and 0.103L (0.026, 0.180), respectively (all p<0.05).
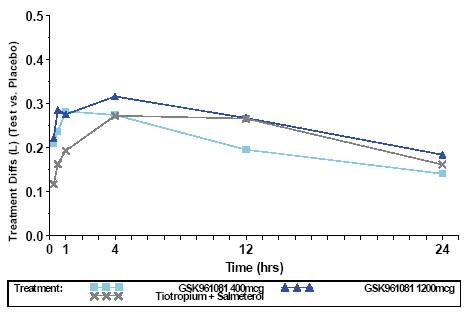
· Onset of bronchodilatation was faster for both doses of GSK961081 than for tiotropium plus salmeterol
· fifteen minutes after treatment on Day 1 adjusted mean (95% CI) difference vs placebo were 0.209L (0.156, 0.262) and 0.222L (0.170, 0.274) for 400mcg and 1200mcg GSK961081 respectively and 0.117L (0.072, 0.162) for tiotropium plus salmeterol (Figure 2).
FIGURE 1. FEV1 (L) DIFFERENCE TO PLACEBO ON DAY 14
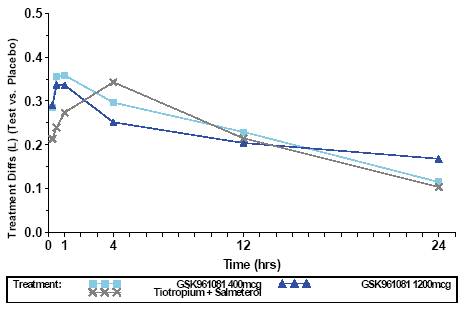
FIGURE 2. FEV1 (L) DIFFERENCE TO PLACEBO ON DAY 1
Systemic pharmacodynamics
· There was no significant difference in maximum change from baseline heart rate, glucose or QTcF 0–4h after the final dose of any active treatment vs placebo (Table 1).
· There was a small, non-clinically significant decrease in potassium 0–4h after the final dose of all active treatments (Table 1).
TABLE 1. SUMMARY OF SYSTEMIC PD FOLLOWING 14 DAYS’ DOSING
|
|
|
|
|
Diff in Adj |
|
|
|
|
Parameter (units) |
|
Comparison |
|
Mean |
|
95% CI |
|
|
Max change from baseline heart rate 0–4h (bpm) |
|
400mcg vs placebo |
|
1.58 |
|
–1.15, 4.31 |
|
|
|
1200mcg vs placebo |
|
2.60 |
|
–0.02, 5.23 |
| |
|
|
Tio+Sal vs placebo |
|
1.82 |
|
–0.69, 4.33 |
| |
|
Max change from baseline QTcF 0–4h (msec) |
|
400mcg vs placebo |
|
0.44 |
|
–3.95, 4.84 |
|
|
|
1200mcg vs placebo |
|
1.60 |
|
–2.63, 5.82 |
| |
|
|
Tio+Sal vs placebo |
|
–0.71 |
|
–4.60, 3.19 |
| |
|
Min change from baseline potassium 0–4h (mmol/L) |
|
400mcg vs placebo |
|
–0.11 |
|
–0.22, –0.01 |
|
|
|
1200mcg vs placebo |
|
–0.19 |
|
–0.29, –0.09 |
| |
|
|
Tio+Sal vs placebo |
|
–0.10 |
|
–0.19, –0.01 |
| |
|
Max change from baseline glucose 0–4h (mmol/L) |
|
400mcg vs placebo |
|
0.11 |
|
–0.11, 0.34 |
|
|
|
1200mcg vs placebo |
|
0.14 |
|
–0.07, 0.36 |
| |
|
|
Tio+Sal vs placebo |
|
0.18 |
|
–0.02, 0.38 |
|
Systemic pharmacokinetics
TABLE 2. SUMMARY OF GSK961081 PK: GEOMETRIC MEAN (95% CI)
|
Parameter |
|
|
|
400mcg |
|
1200mcg |
|
|
(units) |
|
Day |
|
N=29 |
|
N=32 |
|
|
Cmax (pg/mL) |
|
1 |
|
101 (80.4, 126) [n=29] |
|
243 (182, 323) [n=32] |
|
|
|
7 |
|
102 (87.0, 121) [n=28] |
|
256 (203, 322) [n=32] |
| |
|
|
14 |
|
119 (101, 140) [n=28] |
|
280 (220, 356) [n=32] |
| |
|
|
|
|
|
|
|
|
|
|
tmax (h)(a) |
|
1 |
|
0.48 (0.5, 1.1) [n=28] |
|
0.50 (0.5, 8.0) [n=31] |
|
|
|
7 |
|
0.50 (0.5, 0.8) [n=28] |
|
0.50 (0.5, 1.0) [n=31] |
| |
|
|
14 |
|
0.50 (0.5, 1.0) [n=28] |
|
0.50 (0.4, 1.0) [n=31] |
| |
|
|
|
|
|
|
|
|
|
|
tlast (h)(a) |
|
1 |
|
1.05 (0.5, 4.0) [n=28] |
|
4.00 (1.00, 8.00) [n=31] |
|
|
|
7 |
|
2.00 (0.5, 6.0) [n=28] |
|
6.00 (2.0, 6.1) [n=31] |
| |
|
|
14 |
|
2.00 (1.0, 24.1) [n=28] |
|
11.80 (2.0, 24.4) [n=31] |
| |
|
|
|
|
|
|
|
|
|
|
AUC(0–t) (pg.h/mL) |
|
1 |
|
75.1 (53.6, 105) [n=29] |
|
264 (170, 409) [n=32] |
|
|
|
7 |
|
99.8 (69.9, 143) [n=28] |
|
459 (324, 652) [n=32] |
| |
|
|
14 |
|
134 (98.1, 183) [n=28] |
|
705 (506, 983) [n=32] |
| |
|
|
|
|
|
|
|
|
|
|
AUC(0–2) (pg.h/mL) |
|
1 |
|
160 (125, 204) [n=10] |
|
311 (266, 364) [n=28] |
|
|
|
7 |
|
144 (127, 164) [n=18] |
|
341 (296, 393) [n=31] |
| |
|
|
14 |
|
157 (130, 189) [n=19] |
|
374 (328, 427) [n=31] |
|
(a)Median (range)
N = number of patients
n = number of patients with non-missing observation
AUC = area under the curve. CI = Confidence interval
· Systemic exposure to GSK961081 increased from Day 1 to Day 14 (Table 2). Maximum plasma concentration (Cmax) and AUC from 0–2h post-dose (AUC[0–2]) had an accumulation ratio of approximately 1.2-fold (Day 14/Day 1).
· AUC to the last quantifiable concentration (AUC[0–t]) had an accumulation ratio of approximately 2.2-fold (Day 14/Day 1), which is likely a reflection of the increase in tlast over the dosing period.
CONCLUSIONS
· After 14 days’ dosing with GSK961081 there were significant improvements in trough FEV1.
· There was no significant difference in maximum change from baseline, heart rate, glucose or QTcF 0–4h after the final dose of any active treatment vs placebo. There was a small decrease in potassium 0–4h after the final dose of all active treatments.
· Adverse events were similar across all groups with the exception of tremor (n=2, 1200mcg dose), dysguesia (n=2, 1200mcg dose; n=2, 400mcg dose) and dry mouth (n=1, 1200mcg dose) seen after GSK961081 only.
· GSK961081 was well tolerated and showed significant bronchodilatation meriting further evaluation as a potential therapy for COPD.
References
(1) Aiyar J, et al. In vitro characterization of TD-5959: a novel bifunctional molecule with muscarinic antagonist and beta2-adrenergic agonist activity. Am J Respir Crit Care Med 179;2009:A4552.
(2) Pulido-Rios MT, et al. TD-5959: a novel bifunctional muscarinic antagonist beta2-adrenergic agonist with potent and sustained in vivo bronchodilator activity in guinea pigs. Am J Respir Crit Care Med 179;2009:A6195.
Acknowledgements
· We thank the following Investigators and their staff and patients
· Professor E Bateman, University of Cape Town, Lung Institute, South Africa
· Dr T Conje, Farmos Parexel, South Africa
· Dr O Kornmann, University of Mainz, Germany
· Dr J DuToit, Qdot Pharma, South Africa.
· Study funded by GlaxoSmithKline (MAB104958; NCT00478738).
· Editorial support (in the form of copyediting) was provided by David Cutler at Gardiner-Caldwell Communications and was funded by GlaxoSmithKline.
|
|
|
|
| |
|
Presented at the European Respiratory Society Annual Congress, Amsterdam, The Netherlands, September 24–28, 2011 | |

