Attached files
| file | filename |
|---|---|
| 8-K - FORM 8K - WEST PHARMACEUTICAL SERVICES INC | form8k.htm |

WEST PHARMACEUTICAL SERVICES, INC.
Solutions for Injectable Drug Delivery NYSE:WST www.westpharma.com
© 2011 by West Pharmaceutical Services, Inc., Lionville, PA.
All rights reserved. This material is protected by copyright. No part of it may be reproduced, stored in a retrieval system, or transmitted in any
form or by any means, electronic, mechanical, photocopying or otherwise, without written permission of West Pharmaceutical Services, Inc.. All
trademarks and registered trademarks are property of West Pharmaceutical Services, Inc., unless noted otherwise.
form or by any means, electronic, mechanical, photocopying or otherwise, without written permission of West Pharmaceutical Services, Inc.. All
trademarks and registered trademarks are property of West Pharmaceutical Services, Inc., unless noted otherwise.
CL King’s 9th Annual “Best Ideas” Conference
New York, NY
September 14, 2011

Safe Harbor Statement
2
Cautionary Statement Under the Private Securities Litigation Reform Act of 1995
This presentation and any accompanying management commentary contain “forward-looking statements”
as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include,
but are not limited to statements about expected financial results for 2011 and future years.
as that term is defined in the Private Securities Litigation Reform Act of 1995. Such statements include,
but are not limited to statements about expected financial results for 2011 and future years.
Each of these estimates is based on preliminary information, and actual results could differ from these
preliminary estimates. We caution investors that the risk factors listed under “Cautionary Statement” in
our press releases, as well as those set forth under the caption "Risk Factors" in our most recent Annual
Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or
supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially
from those estimated or predicted in the forward-looking statements. You should evaluate any statement
in light of these important factors. Except as required by law or regulation, we undertake no obligation to
publicly update any forward-looking statements, whether as a result of new information, future events, or
otherwise.
preliminary estimates. We caution investors that the risk factors listed under “Cautionary Statement” in
our press releases, as well as those set forth under the caption "Risk Factors" in our most recent Annual
Report on Form 10-K as filed with the Securities and Exchange Commission and as revised or
supplemented by our quarterly reports on Form 10-Q, could cause our actual results to differ materially
from those estimated or predicted in the forward-looking statements. You should evaluate any statement
in light of these important factors. Except as required by law or regulation, we undertake no obligation to
publicly update any forward-looking statements, whether as a result of new information, future events, or
otherwise.
Non-GAAP Financial Measures
Certain financial measures included in these presentation materials, and which may be referred to in
management’s discussion of the Company’s results and outlook, are Non-GAAP (Generally Accepted
Accounting Principles) financial measures. Please refer to the “Non-GAAP Financial Measures” and
“Notes to Non-GAAP Financial Measures” at the end of these materials for more information. Non-GAAP
financial measures should not be considered in isolation or as an alternative to such measures
determined in accordance with GAAP.
management’s discussion of the Company’s results and outlook, are Non-GAAP (Generally Accepted
Accounting Principles) financial measures. Please refer to the “Non-GAAP Financial Measures” and
“Notes to Non-GAAP Financial Measures” at the end of these materials for more information. Non-GAAP
financial measures should not be considered in isolation or as an alternative to such measures
determined in accordance with GAAP.

3
Pharmaceutical Packaging Systems
Pharmaceutical Delivery Systems
• A globally diverse manufacturer of
products used primarily in containing and
administering small-volume parenteral
drugs
products used primarily in containing and
administering small-volume parenteral
drugs
• Strong competitive position
– Substantial market shares
– Proprietary technology
– Diversified customer base
– Global footprint
– Preferred products for biologics
– Long-term customer relationships
• Stability with growth potential
• Proprietary products
• Geographic expansion
• Financial strength to invest
– Reliable operating cash flow
– Well capitalized
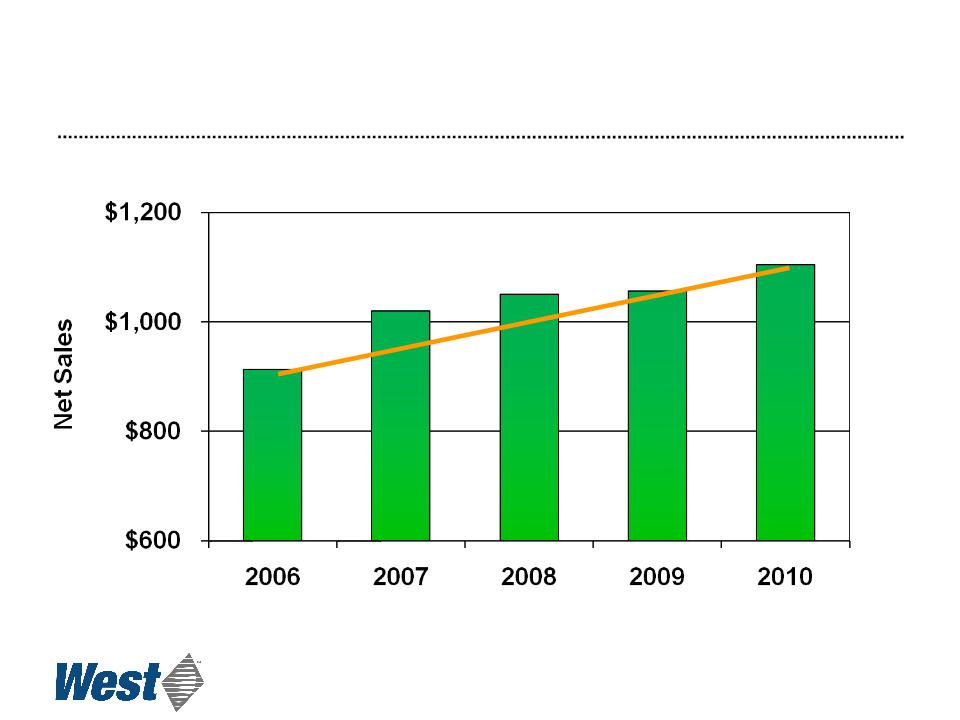
Sales
($ in millions)
($ in millions)
4
Compound annual growth rate: 4.9% (4.3% ex-currency)
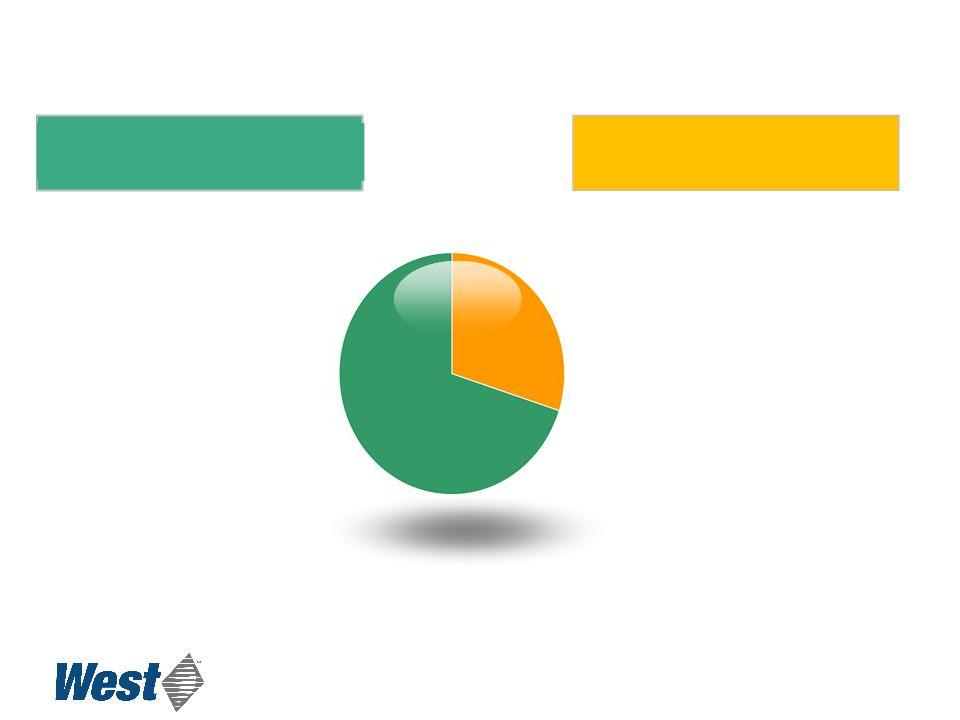
Business Segments
5
$785
$324
2010 Revenues
($ millions)
Delivery Systems
• Contract manufacturing base
• Multi-material
• Project management
• Automated assembly
• Regulated products
• Capabilities + IP = proprietary
delivery devices
delivery devices
• Proprietary devices are
expected to drive growth
expected to drive growth
Packaging Systems
• Established leadership
• Designed-in revenue base
• Diverse global capabilities
• High market shares
• Steady growth in base
• Increasing unit value of products
and geographic expansion are
expected to enhance growth
and geographic expansion are
expected to enhance growth

A Diverse, Stable Customer Base
(representative healthcare customers)
(representative healthcare customers)
PHARMACEUTICAL / BIOTECHNOLOGY
6
GENERIC
MEDICAL DEVICE

|
Category
|
Key Customers
|
Projected
Growth |
|
Diabetes
|
|
> 10 %
|
|
Oncology
|
|
> 10 %
|
|
Vaccines
|
|
> 10 %
|
|
Autoimmune
|
|
> 8%
|
|
Generics
|
|
>10%
|
IMS April 2010 Report; Business Insights 2009; GBI Research 2009
7
Therapeutic Category Growth Drivers

Business Environment
• Economic Recovery: Slowing or Double-dip
– Effects of weakness on private and public healthcare spending
– Implications of EU sovereign debt situation
– Developing economies growing (e.g., China, Brazil, India)
• Persistent Fx and commodity price volatility
• Trends in Pharmaceutical and Device markets:
– Thin new-product pipelines in the near-term
– Patent expirations for blockbuster products
– Pharma M&A reflects shift to large molecule products
– Global shift in product sourcing (e.g., India generic growth)
– More demanding regulatory environment
• Pre-approval demands on drugs and devices slow the rate of change
• Scrutiny of customers’ product quality creates opportunities
– Uncertain near and longer-term effects of US healthcare changes
8

• Company
– Demographics and increasing prevalence of chronic disease
– Increasing use of biologics to treat those
– Broader access to healthcare
• Packaging Systems Segment
– Growth in emerging markets
– Products and processes to satisfy escalating regulatory and quality
expectations
expectations
• Delivery Systems Segment
– Demand for combination products that promote safety, dosing accuracy,
ease of use, and deliver cost savings
ease of use, and deliver cost savings
– Drug product life cycle management
– Growing customer & regulatory awareness of glass quality issues
What Will Drive Growth?
9
Pharmaceutical Packaging Systems
Packaging Components for Small Volume Parenterals
Packaging Components for Small Volume Parenterals
Plungers, Tip caps,
Needle shields for Glass
Syringes
Needle shields for Glass
Syringes
Plungers, lined seals
for Glass Cartridges
for Pens
for Glass Cartridges
for Pens
10
Primary packaging components (those that touch the drug) are typically
proprietary to West and are “designed into” customers’ drug products
proprietary to West and are “designed into” customers’ drug products

Packaging Systems Segment
• Increase per-unit value
• Continued growth of prefilled syringes
• Improve operating efficiency: lean operations
• Geographic expansion - capacity investments in Asia
• Strategic acquisitions and partnerships
Growth Strategy
11

12
Standard High-Value
Products Products
Revenue Opportunity ($ per unit)
Plungers and
sleeve stoppers
sleeve stoppers
Stoppers
Seals
RU seals
Westar® RU
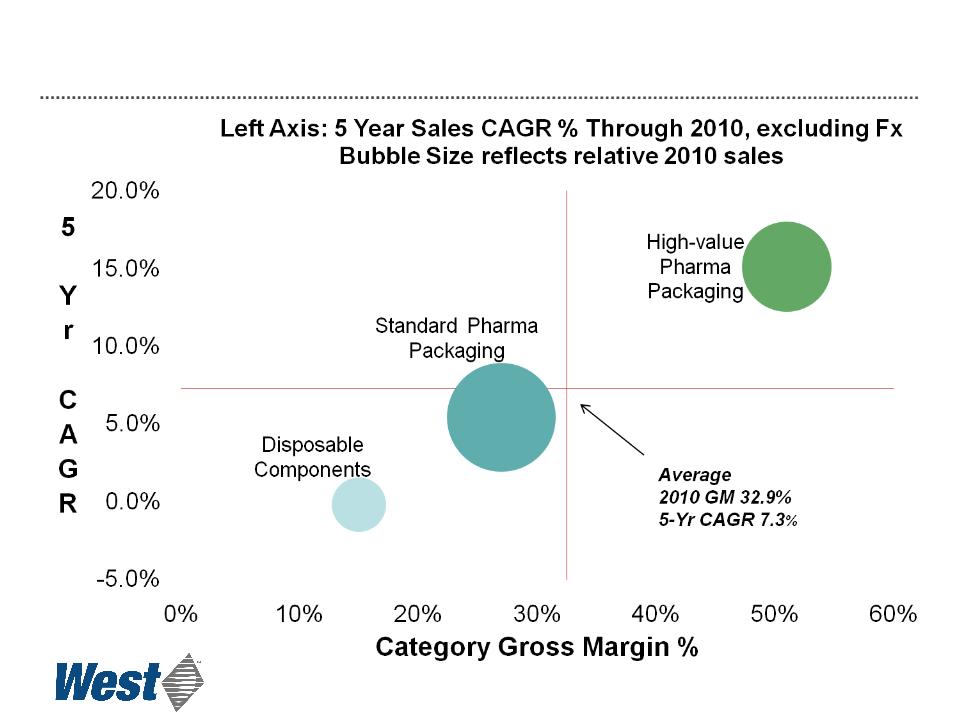
Faster Growth of High-Value Products
Pharmaceutical Packaging Systems
Pharmaceutical Packaging Systems
13

Pharmaceutical Packaging Systems
Key High-value Products
Key High-value Products
• Westar® RS (ready-to-sterilize) and Westar® RU (ready-to-use)
components
components
• Barrier film coatings (FluroTec®, Teflon® coated) enhance drug-
product stability
product stability
– Key feature for biotech products
• B-2 and LyoTec® coatings for enhanced handling and machine-
ability
ability
• Envision™ 100% vision-inspected products
– Eliminate deformation, surface and embedded defects
14
Teflon® is a registered trademark of DuPont.
FluroTec® technology is licensed from Daikyo Seiko, Ltd.
FluroTec® technology is licensed from Daikyo Seiko, Ltd.

Delivery Systems
West ConfiDose®
auto-injector system
auto-injector system
Daikyo Crystal Zenith®
Life-cycle Containment Solutions
Life-cycle Containment Solutions
SmartDose® electronic
patch injector system
patch injector system
15
MixJect® and Vial2Bag®
Custom Manufacturing of
Components and Devices
Components and Devices
Proprietary Components, Devices and Systems
MixJect®, Vial2Bag® and SmartDose® are registered trademarks of Medimop Medical Projects Ltd., a West company.
Veramyst® is a registered trademark of Glaxo Group, Inc.
Veramyst® is a registered trademark of Glaxo Group, Inc.

Delivery Systems Segment
• Realize commercial potential of CZ
• Leverage life-cycle management opportunities
• Develop new platform opportunities - combination products
• Custom solution provider
Growth Strategy
16
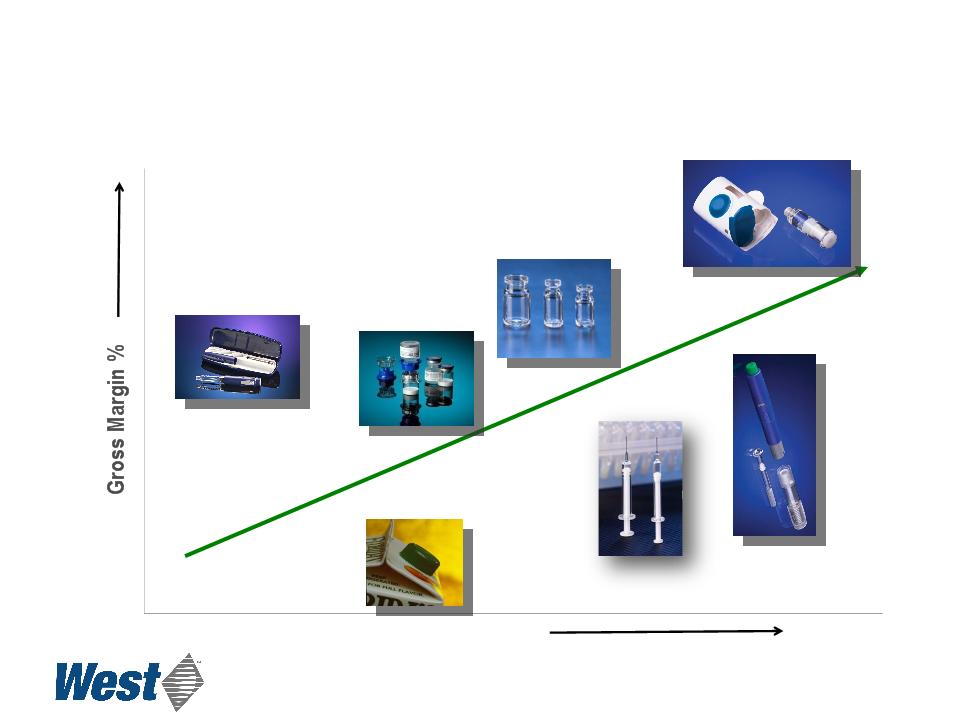
17
Revenue per-unit
Consumer product
manufacturing
manufacturing
Medical device
manufacturing
manufacturing
Mix2Vial®
CZ vials
CZ syringes
ConfiDose®
Effect of Increasing Proprietary Device Sales
Contract Manufacturing Proprietary Devices
Products
SmartDose®

Concerns With Glass Syringes
• Interaction with sensitive biologics
• Protein aggregation (silicone oil)
• Residual chemicals (tungsten, glue)
• Glass flakes
• Dimensional variation
• Variable silicone distribution
• High Cost of Quality
• Breakage
• In process/handling
• Within auto-injector systems
Siliconized Glass Syringe
Crystal Zenith Syringe
18

Daikyo CZ Solution
with Daikyo FluroTec®
with Daikyo FluroTec®
• Reduces:
– Drug exposure to extractables
– Risk of protein aggregation caused by silicone oil in the drug product
– Returns and in-process clean-ups caused by broken glass
– Risk of delamination and glass-particulate contamination
• Consistent piston release and travel forces without using silicone oil
19
Pharma Industry Drug Life-Cycle
Management
Management
Phase I
Phase II
Phase III
Post-Market Life Cycle Management
8 - 10 years
2 - 3 years
2 - 3 years
Regulatory
Approval
Discovery
20
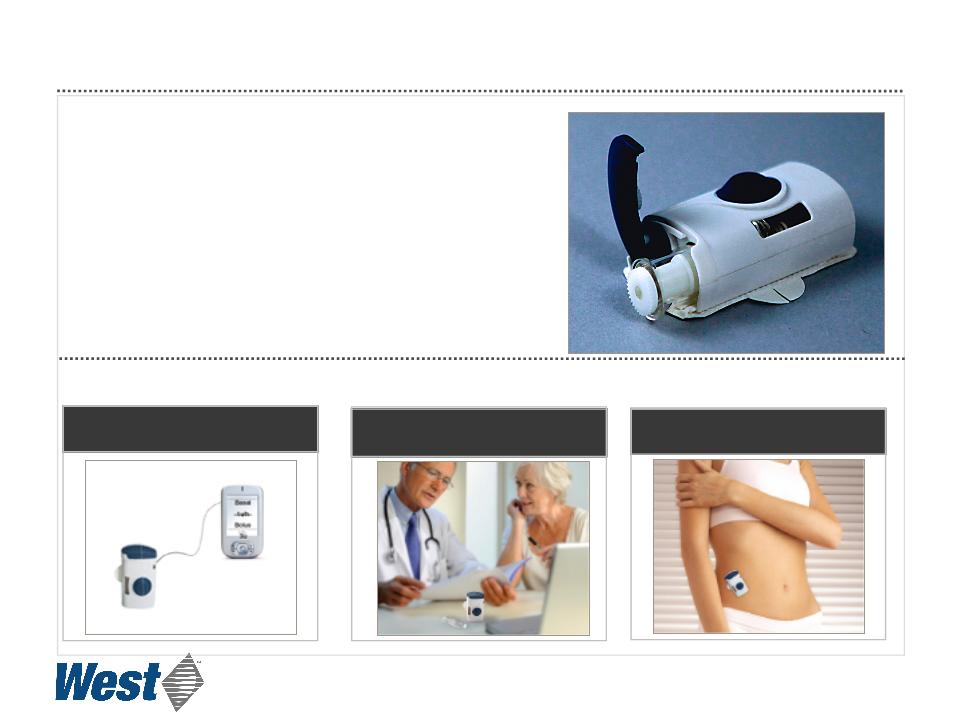
SmartDose®
Electronic Patch Injector
Programmed by PDA or PC
Dose may be customized
Applied and activated by patient
21
• Controlled, subcutaneous, micro-infusion delivery
of high volumes and high viscosity drugs
of high volumes and high viscosity drugs
• Prefilled cartridge, no need for user filling
• Based on Daikyo CZ cartridge
• Compact
• Hidden needle for safety
• Single push-button operation
Prototype Operation
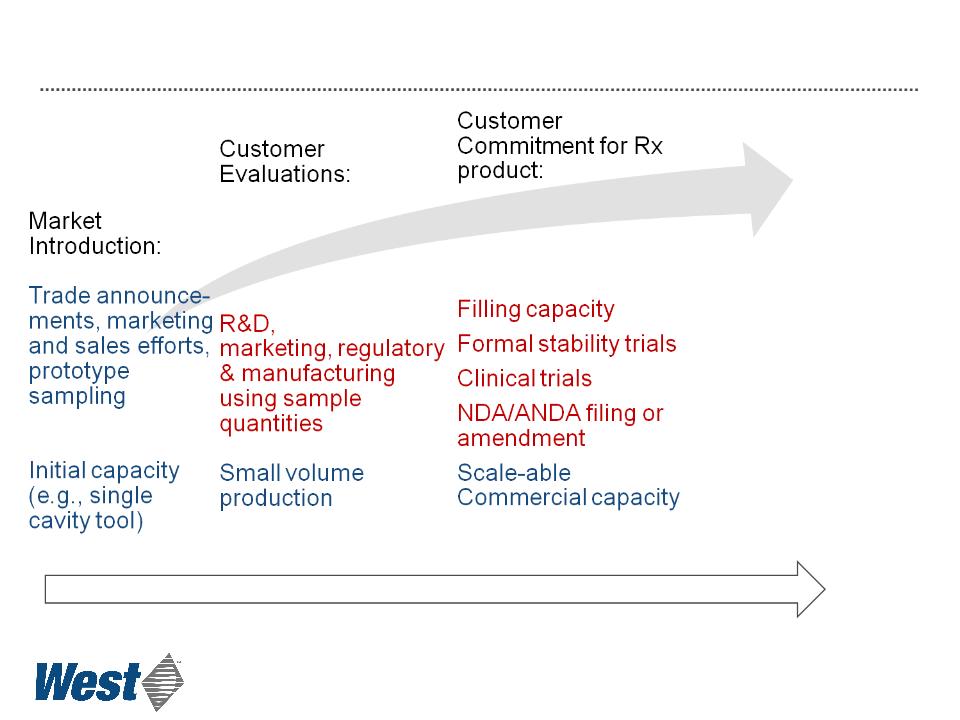
New Product Commercialization
Schematic representation of order and timing of West & Customer activities
Schematic representation of order and timing of West & Customer activities
22
Commercial Sales:
Rx Product approval
Commercial
Production
Production
1 2
4 5 6
Approximate timing (years)

Our Long-Term Focus
• Pharmaceutical Packaging Systems
– Organic growth of 3-5% per year
– Margin expansion from efficiency, product mix
– Capital investments target enhanced quality and value
• Pharmaceutical Delivery Systems
– Deliver the potential of Daikyo CZ products
– Stronger mix of healthcare-consumable contract manufacturing
– Grow proprietary safety and delivery systems
• Financial discipline
– Operating cash flow supports R&D and capital spending
– Deliver returns (ROIC) that regularly exceed cost of capital (WACC)
– Maintain quarterly dividend
– Align incentives with financial performance and value creation
23

|
($ millions)
|
June 30,
2011
|
December 31,
2010 |
|
Cash and cash equivalents
|
$110.4
|
$110.2
|
|
Debt
|
$373.5
|
$358.4
|
|
Equity
|
$696.0
|
$625.7
|
|
Net debt to total invested capital
|
27.4%
|
28.4%
|
|
Working capital
|
$317.7
|
$266.9
|
Selected Financial Information
• Balance Sheet
• Dividend
– Most recent quarter: $0.17 ($0.68 annually at current rate)
– Annual increases for 18 consecutive years
24

Summary Second Quarter Results
$ millions, except per-share data
$ millions, except per-share data
(1) These are Non-GAAP Financial Measures and exclude the effects of:
• In the 2011 period, restructuring and related pre-tax charges of $1.3, income of $0.7 from the reduction of
acquisition-related contingencies; and a pre-tax charge of $2.1 for special separation benefits.
acquisition-related contingencies; and a pre-tax charge of $2.1 for special separation benefits.
• In the 2010 period, restructuring and related pre-tax charges of $0.4, and $0.5 of tax expense from discrete items.
Further explanations of these items were included in the press release announcing the second-quarter 2011 results, which is
available through the Company’s website, www.westpharma.com, and in Form 10-Q for the second quarter of 2011.
available through the Company’s website, www.westpharma.com, and in Form 10-Q for the second quarter of 2011.
|
|
Three Months Ended
June 30,
|
|
|
2011
|
2010
|
|
|
Net Sales
|
$ 307.9
|
$ 281.8
|
|
Gross Profit
|
84.6
|
83.2
|
|
Reported Operating Profit
|
27.8
|
30.5
|
|
Adjusted Operating Profit (1)
|
30.5
|
30.9
|
|
Reported Diluted EPS
|
$ 0.57
|
$ 0.62
|
|
Adjusted Diluted EPS(1)
|
$ 0.62
|
$ 0.64
|
25

26
Pharmaceutical Packaging Systems
Pharmaceutical Delivery Systems
• Strong competitive position
– Substantial market shares
– Proprietary technology
– Diversified customer base
– Global footprint
– Preferred products for biologics
– Long-term customer
relationships
relationships
• Stability with growth potential
• Proprietary products
• Geographic expansion
• The financial strength to invest
– Reliable operating cash flow
– Well capitalized
Summary

On average, approximately 100 million components manufactured by West and
our Global Partners are used to enhance the quality of healthcare worldwide
every day.
our Global Partners are used to enhance the quality of healthcare worldwide
every day.
Global Partners with Daikyo Seiko, Ltd. and West Pharmaceutical services Mexico, S.A. de
C.V.
C.V.
