Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF ERNST & YOUNG LLP - Myrexis, Inc. | d227415dex231.htm |
| EX-31.1 - SECTION 302 CEO CERTIFICATION - Myrexis, Inc. | d227415dex311.htm |
| EX-31.2 - SECTION 302 CFO CERTIFICATION - Myrexis, Inc. | d227415dex312.htm |
| EX-32.1 - SECTION 906 CEO & CFO CERTIFICATION - Myrexis, Inc. | d227415dex321.htm |
Table of Contents
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
(Mark One)
| x | ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the fiscal year ended June 30, 2011
OR
| ¨ | TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from to
Commission file number: 001-34275
MYREXIS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 26-3996918 | |
| (State or other jurisdiction of incorporation or organization) |
(I.R.S. Employer Identification No.) | |
| 305 Chipeta Way Salt Lake City, Utah |
84108 | |
| (Address of principal executive offices) | (Zip Code) | |
Registrant’s telephone number, including area code (801) 214-7800
Securities registered pursuant to Section 12(b) of the Exchange Act:
| Title of each class | Name of each exchange on which registered | |
| Common Stock, $0.01 Par Value Per Share Preferred Share Purchase Rights |
The NASDAQ Stock Market LLC |
Securities registered pursuant to Section 12(g) of the Exchange Act: None.
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ¨ No x
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Exchange Act. Yes ¨ No x
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes x No ¨
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes ¨ No ¨
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. x
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ¨ | Accelerated filer x | Non-accelerated filer ¨ | Smaller reporting company ¨ | |||
| (Do not check if a smaller reporting company) |
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ¨ No x
The aggregate market value of the registrant’s common stock held by non-affiliates of the registrant (without admitting that any person whose shares are not included in such calculation is an affiliate) computed by reference to the price at which the common stock was last sold December 31, 2010, the last business day of the registrant’s most recently completed second fiscal quarter, was $105,522,622. As of September 6, 2011 the registrant had 26,085,690 shares of common stock outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
The following documents (or parts thereof) are incorporated by reference into the following parts of this Form 10-K: Certain information required in Part III of this Annual Report on Form 10-K is incorporated from the registrant’s Proxy Statement for the 2011 Annual Meeting of Stockholders.
Table of Contents
Forward-looking Statements
This Annual Report on Form 10-K contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934, as amended. All statements other than statements of historical facts are “forward-looking statements” for purposes of these provisions, including any statements relating to the plans and objectives of management for future operations, the progress, scope or duration of the development of any of our drug candidates, such as the size, design, population, conduct or objective of any clinical trial, the timing for initiation or completion of or availability of results from any clinical trial or for submission or approval of any regulatory filing or for meeting with regulatory authorities, or any indication for which any of our drug candidates may be developed, the benefits that may be derived from any of our drug candidates; or any statements concerning proposed new products or licensing or collaborative arrangements, any statements regarding future economic conditions or performance, and any statement of assumptions underlying any of the foregoing. In some cases, forward-looking statements can be identified by the use of terminology such as “may,” “will,” “expects,” “plans,” “anticipates,” “estimates,” “potential,” or “continue” or the negative thereof or other comparable terminology. Although we believe that the expectations reflected in the forward-looking statements contained herein are reasonable, there can be no assurance that such expectations or any of the forward-looking statements will prove to be correct, and actual results could differ materially from those projected or assumed in the forward-looking statements. Our future financial condition and results of operations, as well as any forward-looking statements, are subject to inherent risks and uncertainties, including but not limited to the risk factors set forth in Item 1A “Risk Factors” below, and for the reasons described elsewhere in this Annual Report. All forward-looking statements and reasons why results may differ included in this Annual Report are made as of the date hereof, and we assume no obligation to update these forward-looking statements or reasons why actual results might differ.
As used in this Annual Report on Form 10-K, the terms “we,” “us,” “our,” the “Company,” and “Myrexis” mean Myrexis, Inc. (unless the context indicates a different meaning). In addition, these terms refer to the former research and drug development businesses that were integrated with and operated by Myriad Genetics, Inc. prior to June 30, 2009, which are now operated by Myrexis, Inc.
2
Table of Contents
PART I
| Item 1. | BUSINESS |
Overview
We are a biotechnology company focused on the development of small-molecule compounds with novel chemical structures and distinct mechanisms of action. We have generated a strong pipeline of differentiated product candidates in oncology and autoimmune diseases. We are focused on maximizing the therapeutic and commercial value of these molecules by developing potential first-in-class and/or best-in-class treatment options for patients with unmet needs. We currently retain all rights to all of our drug candidates and programs across all geographic markets and therapeutic indications.
We recently completed an in-depth review of our drug development pipeline, incorporating extensive inputs from both internal and independent external analyses. As a result, in September 2011 we announced a strategic business decision to suspend any further development of our lead drug candidate Azixa, which was in Phase 2 development for the treatment of advanced primary and metastatic tumors with brain involvement. This decision is not based on any single factor. Our review took into consideration the accumulated data from our clinical trials to date, the evolving competitive environment in Glioblastoma multiforme, or GBM, including ongoing studies of competitive drug candidates that are in more advanced stages of development, inputs from key opinion leaders, updated cost and timing estimates, and other factors affecting the risks and opportunities relating to the development of Azixa. On the basis of these inputs, we concluded that completing the Phase 2b clinical trial would require a disproportionate investment of time and resources relative to its likelihood of technical and regulatory success, when compared to our other programs.
Our Drug Candidates
The following table summarizes our drug candidates currently in development:
| Drug Candidate |
Disease |
Development Stage |
Descriptional Status* | |||||
| Oncology |
MPC-3100 | |||||||
| Hsp90 Inhibitor | Cancer | Phase 1 | Phase 1 completed. Expect to report results in 4Q 2011. | |||||
| MPC-0767 | ||||||||
| Hsp90 Inhibitor Pro-drug |
Cancer | Preclinical | MPC-0767 is a pro-drug of MPC-3100. Expect to submit IND in 1Q 2012. | |||||
| MPC-8640 | ||||||||
| Cancer Metabolism Inhibitor Pro-drug |
Cancer | Preclinical | IND-enabling studies ongoing. MPC-8640 is an orally bioavailable pro-drug of a follow-on to our CMI drug candidate MPC-9528. | |||||
| Autoimmune Diseases |
MPI-0485520 | |||||||
| Oral Anti-interferon | Autoimmune diseases |
Preclinical | Proof of concept established in an animal model of autoimmune disease. Lead optimization is ongoing. | |||||
| * | All dates are for calendar year ending December 31. |
We currently do not have any drugs that are commercially available and none of our drug candidates have obtained approval of the U.S. Food and Drug Administration, or FDA, or any similar foreign regulatory authority.
3
Table of Contents
Our Oncology Programs
We currently have two active programs in oncology:
| • | Hsp90 Inhibitor Program. MPC-3100 is an Hsp90 inhibitor we are developing for the treatment of cancer. In the second quarter of 2009, we initiated a Phase 1 open-label, dose-finding, multiple-dose clinical trial in patients with refractory or relapsed cancers, including solid tumors, lymphomas and leukemias. This study has been completed in the clinic and we expect to report results in the fourth quarter of 2011. MPC-0767 is a novel L-alanine ester pro-drug of MPC-3100, which was designed to have improved aqueous solubility compared to MPC-3100. We expect to submit an investigational new drug application, or IND, on MPC-0767 in the first quarter of 2012. |
| • | Cancer Metabolism Inhibitor Program. MPC-8640 is our lead preclinical compound for the Cancer Metabolism Inhibitor, or CMI, program. It is currently in investigational new drug studies. |
Oncology Background and Market Opportunity
Cancers are diseases characterized by abnormal and uncontrolled cell growth and division, typically leading to tumor formation. As a tumor grows, it can directly disrupt organ function at its site of origin. In addition, cancer cells can also spread to other organs, such as the brain, bones and liver, by a process called metastasis. The growth of metastatic tumors at these new sites can disrupt the function of these other organs. There are many kinds of cancer, but all are characterized by uncontrolled growth of abnormal cells.
The World Health Organization estimates that 12.7 million people were diagnosed with cancer worldwide in 2008, and a projected 9.0 million people will die from the disease in 2015. Global cancer cases and deaths are expected to rise by around 70% in the next 20 years. The American Cancer Society estimated that approximately 1.6 million people in the United States will be diagnosed with cancer in 2011, and approximately 571,950 people will die from the disease. According to a 2009 IMS Health report, oncology products are the largest therapeutic class of pharmaceuticals in the world, with global sales of over $52.4 billion in 2009.
Our Hsp90 Inhibitor Program for the Treatment of Cancer
Background
Heat shock protein 90, or Hsp90, is a chaperone protein that plays an important role in regulating the activity and function of numerous signaling proteins, or client proteins, that trigger proliferation of cancer cells. Important client proteins in cancer include steroid hormone receptors, oncogenes, protein kinases, mutant p53, and telomerase h-TERT. Hsp90 binds and stabilizes these client proteins and inhibition of Hsp90 leads to degradation of the client proteins important for growth and, sometimes, survival of the tumor.
Early Hsp90 inhibitors were analogs of the natural product molecule geldanamycin that demonstrated promising preclinical and clinical proof of concept activity, but have been challenging to develop because of drug related toxicities, including hepatotoxicity, nephrotoxicity and pancreatitis. These toxicities do not appear to be related to inhibition of Hsp90. Additional limitations to geldanamycin derivatives include poor solubility, metabolic stability and difficulty in administration.
MPC-3100 and MPC-0767: Preclinical Development
MPC-3100 and MPC-0767, a pro-drug of MPC-3100, are fully synthetic, orally bio-available, non-geldanamycin Hsp90 inhibitors that have shown significant and broad preclinical anti-tumor activity in mouse models of human cancers. MPC-3100 has not demonstrated the same hepatic or renal toxicity in vivo as the geldanamycin analogs. MPC-3100 inhibits Hsp90 by binding to the same site as geldanamycin and has displayed potent anticancer activity in several in vitro and in vivo models. MPC-3100 significantly and dose-dependently reduced tumor growth in multiple studies conducted in mice implanted with a variety of human cancer cell lines, including colon, prostate, myeloid leukemia, small-cell lung, gastric, breast, and ovarian
4
Table of Contents
cancers. In April 2011, we reported additional preclinical data on our Hsp90 inhibitor program at the annual meeting of the American Association for Cancer Research in Orlando, Florida. The data presented included a demonstration that the combination of MPC-3100 with other targeted therapies, including erlotinib and sorafenib, showed greater in vivo anti-tumor activity compared to either agent alone, suggesting the potential of combining MPC-3100 with these targeted cancer therapies in the clinic. We also presented a preliminary assessment of the novel L-alanine ester pro-drug of MPC-3100, MPC-0767, which was designed to have improved aqueous solubility compared to MPC-3100. Animal studies showed that the pro-drug displayed pharmacokinetics comparable to MPC-3100 and equivalent efficacy, inducing significant tumor regressions. We expect to submit an IND on MPC-0767 in the first quarter of 2012.
MPC-3100: Clinical Development
We submitted an IND application for MPC-3100 in the first quarter of 2009 and initiated patient enrollment of a Phase 1 clinical trial in the second quarter of 2009 to investigate the safety and tolerability of MPC-3100, pharmacokinetics, and the potential for anti-tumor activity. This trial is an open-label, multiple-dose, dose escalation design in up to 40 subjects with refractory or relapsed cancer. Physical examination findings, electrocardiograms, pharmacokinetics, clinical laboratory parameters, and adverse events will be evaluated in subjects at each dose level to assess safety. Disease progression will be evaluated using standard clinical practice guidelines for each patient’s cancer type. In November 2009, we presented the preliminary results of this ongoing study at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics meeting in Boston. Preliminary data to date have demonstrated that MPC-3100 is orally bio-available in cancer patients with a half life of approximately 12 hours. Drug absorption has not been maximized and continues to increase with increasing dose. Plasma concentrations in patients are comparable to those found to inhibit tumor growth in non-clinical studies. Moreover, these concentrations of MPC-3100 were achieved in patients in the absence of dose-limiting toxicities. In the Phase 1 clinical study, MPC-3100 was administered orally on a daily, continuous schedule. We have completed this trial in the clinic and expect to report results from this study in the fourth quarter of 2011.
MPC-3100: Safety Summary
In the Phase 1 clinical trial, 26 subjects have been treated with MPC-3100. Five serious adverse events in four subjects have been reported as possibly related to MPC-3100: abdominal pain (single event in a single patient), respiratory failure and supraventricular tachycardia (two events in a single patient), enteritis (single event in a single patient), and renal failure (single event in a single patient).
MPC-3100 and MPC-0767: Future Clinical Development
We are conducting non-clinical studies as well as other technical, regulatory and market assessments with the objective of identifying optimal cancer indications and drug combination regimens to potentially advance one or both of our Hsp90 inhibitor compounds into Phase 2 clinical development. Our current plan is to initiate a Phase 2 study of MPC-3100 in one or more specific populations of patients with cancer in the second quarter of 2012. However, the timing of a Phase 2 trial and the compound to be tested may change depending upon the results and timing of the clinical development of MPC-0767.
Our Cancer Metabolism Inhibitor Program
MPC-8640 is our lead preclinical compound for our Cancer Metabolism Inhibitor, or CMI, program. MPC-8640 is an orally bioavailable pro-drug of a follow-on molecule to our prior CMI drug candidate, MPC-9528, that has enhanced solubility and distinct pharmacokinetic advantages and is being developed for the treatment of cancer. Both the active moiety of MPC-8640 as well as MPC-9528 inhibit Nicotinamide phosphoribosyltransferase (Nampt) in vitro and in cells at picomolar drug concentrations and are tumoricidal in every cancer line tested to date representing 17 different tumor tissue types. Both compounds display on-target activity by potently reducing Nicotinamide Adenine Dinucleotide (NAD) levels, which leads to inhibition of
5
Table of Contents
glycolysis, energy deprivation and cell death in tumor cells, while NAD in normal tissues is less affected. In preclinical efficacy studies, MPC-9528 causes dramatic tumor regressions in multiple tumor types when administered orally with either a low daily dose or higher intermittent dose regimen. Nicotinic acid is converted to NAD through an alternative pathway that is dependent upon the enzyme Nicotinic acid phosphoribosyltransferase (Naprt1) which does not involve Nampt. In tumor cell types with sufficient Naprt expression to support this NAD biosynthetic pathway, nicotinic acid (niacin, Vitamin B3) can completely block the NAD-reducing and tumoricidal activity of MPC-9528. Our studies have found that approximately 40% of tumor cell lines are deficient in Naprt1 and in these cells, nicotinic acid has little to no effect on MPC-9528 tumoricidal activity. Furthermore, in animal model studies, a combination of nicotinic acid with MPC-9528 increases the tolerated dose of MPC-9528, while still causing growth inhibition of tumors deficient in Naprt1. This demonstrates the potential to increase the therapeutic index and efficacy of a Nampt inhibitor by combining it with nicotinic acid to treat patients with tumors that are deficient in Naprt1. A diagnostic method designed to measure Naprt1 expression could be used to identify those patients with Naprt1 deficient tumors that are most likely to benefit from this combination therapy.
In April 2011, results from recent preclinical studies of MPC-9528 were presented at the annual meeting of the American Association for Cancer Research in Orlando, Florida. The studies presented included data that support the potential of Nampt inhibitors for broad spectrum tumoricidal activity as monotherapy and in a variety of combinations with other agents. Inhibition of Nampt by MPC-9528 was shown to exhibit synergistic anti-tumor activity when coupled with DNA damaging agents, such as alkylating agents and thymidylate synthase inhibitors. These common classes of chemotherapy drugs also reduce NAD cellular levels as a result of their mechanism of action, specifically by activating the NAD-consuming enzyme poly (ADP-ribose) polymerase (PARP). The mechanism of action of our Nampt inhibitors is distinct from these other agents, leading to a combined effect on cellular NAD levels and synergistic anti-tumor activity. In June 2011, additional preclinical studies on MPC-9528 and MPI-0487316, a structurally distinct Nampt inhibitor, were presented at the annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. Oral administration of either MPC-9528 or MPI-0487316 resulted in tumor regressions in animal model studies across multiple dosing schedules. MPC-8640 is a pro-drug of MPI-0487316 with enhanced solubility and distinct pharmacokinetic advantages. We are currently conducting IND enabling studies on MPC-8640.
Azixa: A Drug Candidate for the Treatment of Cancer
Background
Glioblastoma multiforme, or GBM, is a type of brain tumor and is amongst the most highly vascularized tumors, characterized by abnormal vessel structure and unique vascular cells. This vascular hyperplasia is believed to be essential to the rapid growth of the tumor and may offer an opportunity for treatment by agents that are both able to penetrate the brain and selectively disrupt tumor vasculature. The American Cancer Society estimated the incidence of primary central nervous system, or CNS, tumors in the United States in 2011 as 22,340. GBM represents approximately 15-20% of primary brain tumors and prognosis remains poor with median survival estimated to be between 12 to 18 months from the time of diagnosis.
Azixa Overview
Azixa is a novel, small-molecule drug candidate we had been developing for the treatment of advanced primary and metastatic tumors with brain involvement. Azixa acts as a microtubule destabilizing agent, causing arrest of cell division and programmed cell death, or apoptosis, in cancer cells. Azixa has also been shown to be a vascular disrupting agent, or VDA, in a mouse model of human ovarian cancer. Thus, Azixa has a dual mode of action; it induces apoptosis and acts as a VDA, resulting in tumor cell death. Importantly, in non-clinical studies, Azixa has demonstrated the unique ability to effectively cross the blood-brain barrier and accumulate in the brain. Azixa does not appear to be subject to multiple drug resistance. In September 2011, as a result of a strategic review of our programs, a decision was made to suspend further development of Azixa,
6
Table of Contents
Azixa: Clinical Development
Prior to suspending clinical development in September 2011, we had completed two Phase 1 trials and three Phase 2 trials of Azixa. The two Phase 1 trials were open-label, dose-escalating, multiple dose trials to investigate the safety, tolerability and pharmacokinetics of Azixa and to observe for any evidence of anti-tumor activity in treatment of a variety of refractory solid tumors with and without brain metastases. In these Phase 1 trials, six out of 66 subjects had stable disease ranging from five to 16 months and there was no evidence of CNS toxicities or development of peripheral neuropathies.
In 2008, we initiated a 19-patient, open-label, dose finding, multiple-dose Phase 2 clinical trial to confirm the safety profile of Azixa in combination with the chemotherapeutic agent carboplatin in subjects with recurring/relapsing GBM. All patients had failed previous standard of care treatment with temozolomide. In June of 2010, we reported results from this study at the annual meeting of the American Society of Clinical Oncology (ASCO) in Chicago. In this study, six subjects achieved stable disease and two subjects had achieved partial responses. One subject’s partial response duration was 7.8 months; the additional patient’s response was, at that time, 16 months in duration and has been classified by his physician as almost a complete response. This second patient has been off study drug for 13 months and there has been no evidence of disease recurrence. The overall response rate was 42% as defined by partial response and stable disease evaluated using Macdonald criteria.
In 2008, we also initiated an open-label, dose finding, multiple-dose Phase 2 clinical trial to confirm the safety profile of Azixa in combination with the chemotherapeutic agent temozolomide, the current standard of care for GBM and recurrent metastatic melanoma, and to look for evidence of reduced tumor burden and improved survival. This trial explored Azixa’s efficacy in 22 patients with metastatic melanoma (Stage IV) with and without confirmed CNS metastases. In November 2009, we reported initial results from this study at the AACR-NCI-EORTC Molecular Targets and Cancer Therapeutics meeting in Boston. In June of 2010, we reported final results from this study at ASCO in Chicago. The combination of Azixa at all concentrations with fixed dose temozolomide, including the previously determined single agent maximum tolerated dose of Azixa, was well-tolerated. A dose reduction of Azixa was not required when combined with temozolomide in these patients. In this study, two patients achieved partial response durations of four and 10 months. Nine patients experienced stable disease durations between three and seven months. The response rate (defined as partial response by modified RECIST criteria and stable disease) was 50% and the median PFS of patients in the metastatic melanoma study was 2.9 months. The results from this study were not statistically significant.
In the second quarter of 2009, we initiated a 56-patient, open-label Phase 2 clinical trial to evaluate Azixa as monotherapy in patients with recurrence of GBM, including a cohort of patients who have never been treated with bevacizumab and a cohort of patients who have recurrence of GBM following treatment with bevacizumab. In November of 2010, we reported results from the 25 patient bevacizumab-experienced cohort of this study at the annual meeting of the Society for NeuroOncology (SNO) in Montreal. In these patients, Azixa monotherapy was well-tolerated. One patient achieved a partial response as assessed by Macdonald criteria with two measurable tumor reductions over twelve months of Azixa treatment and four additional patients experienced stable disease. The median and mean PFS duration was 22 and 37 days, respectively. The median and mean overall survival were 94 and 105 days, respectively. In June of 2011, we reported results from the bevacizumab-naïve cohort of patients at ASCO in Chicago. There were 31 patients with recurrent GBM enrolled in this arm of the Phase 2 Azixa monotherapy study who had failed temozolomide and were naïve to treatment with bevacizumab. Two patients (6.5%) achieved partial response as assessed by Macdonald criteria. Another patient (3.2%) with two tumor lesions at baseline was observed to have no detectable disease after cycle 13 of Azixa treatment and continued treatment for 2 additional cycles of Azixa for a total of 15 cycles. A further five patients (16.1%) achieved stable disease. The median duration of stable disease was four months and the median duration of partial response was six months. The median PFS duration was 1.8 months (range 0.04-13.1) and the median overall survival was 9.9 months (range 1.1-17.2). Azixa monotherapy was well tolerated in these patients with the most common adverse events being fatigue (26%), nausea (10%), and constipation (10%).
7
Table of Contents
In December of 2010, we initiated a two arm Phase 2b trial of Azixa in patients newly diagnosed with GBM. This study was expected to enroll up to 120 newly diagnosed GBM patients at treatment centers in the United States. In September 2011, after a strategic review of the program, we made the decision to suspend further development of Azixa.
Azixa: Safety Summary
In completed and ongoing clinical trials in which a total of 168 subjects have been treated with Azixa, 14 serious adverse events in 13 subjects have been reported as possibly, probably or definitely related to Azixa: non fatal myocardial infarction (single events in three subjects), cerebral hemorrhage (single events in two subjects), cerebral ischemia (one event in one subject), troponin I increase (one event in one subject), hypersensitivity (two events in one subject), pulmonary embolism (one event in one subject), hypertension (one event in one subject), thrombocytopenia (one event in one subject), and ejection fraction decreased (single events in two subjects). To date, the overall incidence of myocardial infarction is 1.8%, the incidence of cerebral hemorrhage, hypersensitivity and ejection fraction decreased is 1.2% each, and the incidence of cerebral ischemia, pulmonary embolism, hypertension, thrombocytopenia and troponin I increase is 0.6% each.
Our Small-Molecule Autoimmune Disease Program
MPI-0485520 is our lead preclinical compound in our small-molecule anti-interferon program for autoimmune diseases. It has demonstrated proof of concept activity in an animal model of the autoimmune disease rheumatoid arthritis. A medicinal chemistry program of lead optimization is ongoing to select a candidate compound for IND-enabling studies.
Oral Anti-interferon Program for the Treatment of Autoimmune Diseases
MPI-0485520 is an orally-available small molecule that potently and selectively inhibits IKKe and TBK1and is our lead preclinical compound in our small molecule anti-interferon program for autoimmune diseases. MPI-0485520 exhibits high oral bio-availability, favorable ADME/PK properties and efficacy in an in vivo mouse model of rheumatoid arthritis. In cellular models of type I interferon production, MPI-0485520 potently inhibits induction of type I interferons (IFNa/b) following stimulation of a variety of receptors that mediate the type-I interferon to pathogens, such as TLR3, TLR4, RIG-I, and MDA-5. Inhibition of type I interferon production by IKKe/TBK1 inhibitors may benefit patients with autoimmune disorders such as rheumatoid arthritis, systemic lupus erythematosus (SLE), scleroderma, Sjögren’s syndrome, and polymyositis. In April 2011, results from our most recent preclinical studies of MPI-0485520 were presented at the European League Against Rheumatism (EULAR) Annual European Congress of Rheumatology in London. In a proof of concept study, in the well characterized collagen-induced mouse model of arthritis, mice treated with MPI-0485520 show a dose-dependent and statistically significant reduction in the severity of clinical symptoms and paw and joint histopathology, as well as lower weight loss compared to control mice. MPI-0485520 is one compound out of an extensive portfolio of potent and selective IKK epsilon/TBK1 inhibitors identified by our oral anti-interferon program. A medicinal chemistry program of lead optimization is ongoing to select a candidate compound for IND-enabling studies.
Our Strategy
Our strategy is to develop small-molecule compounds with novel chemical structures and distinct mechanisms of action that address severe medical conditions with large markets, with a focus in oncology and autoimmune diseases. Key elements of our strategy include:
| • | Advance the development of our drug candidates. We plan to advance our drug candidates based on an ongoing assessment of preclinical and clinical study results and market potential; and |
| • | Establish collaborative relationships. We may seek to establish research, development and/or commercial collaborations with other companies in order to maximize the value of our drug candidates, share risk, accelerate their path to market, and optimize value for our shareholders. |
8
Table of Contents
Intellectual Property
Our success will depend in part on our ability to obtain and maintain proprietary protection for our drug candidates, technology, and know-how, to operate without infringing on the proprietary rights of others, and to prevent others from infringing our proprietary rights. Our policy is to seek to protect our proprietary position by, among other methods, filing U.S. and foreign patent applications related to our proprietary technology, inventions, and improvements that are important to the development of our business. We also rely on trade secrets, know-how, continuing technological innovation, and in-licensing opportunities to develop and maintain our proprietary position.
We intend to seek patent protection in the United States and major foreign jurisdictions for drug compounds, pharmaceutical compositions and dosage forms, therapeutic and prophylactic methods, diagnostic methods, processes of manufacturing, and other inventions which we believe are patentable and where we believe our interests would be best served by seeking patent protection. We also rely upon trade secret rights to protect certain other technologies which may be used in discovering, characterizing, and manufacturing new therapeutic products. To further protect our trade secrets and other proprietary information, we require that our employees and consultants enter into confidentiality and invention assignment agreements. However, those confidentiality and invention assignment agreements may not provide us with adequate protection.
We own or have licensed rights to over 32 issued patents and over 135 patent applications in the United States and foreign countries. Issued patents expire between 2015 and 2028. Any patent applications which we have filed or will file or to which we have licensed or will license rights may not issue, and patents that do issue may not contain commercially valuable claims. In addition, any patents issued to us or our licensors may not afford meaningful protection for our products or technology, or may be subsequently circumvented, invalidated or narrowed, or found unenforceable. Our processes and potential products may also conflict with patents which have been or may be granted to competitors, academic institutions or others. As the pharmaceutical industry expands and more patents are issued, the risk increases that our processes and potential products may give rise to interferences filed by others in the U.S. Patent and Trademark Office, or to claims of patent infringement by other companies, institutions or individuals. These entities or persons could bring legal actions against us claiming damages and seeking to enjoin clinical testing, manufacturing and marketing of the related product or process. If any of these actions are successful, in addition to any potential liability for damages, we could be required to cease the infringing activity or obtain a license in order to continue to manufacture or market the relevant product or process. We may not prevail in any such action and any license required under any such patent may not be made available on acceptable terms, if at all. Our failure to obtain a license to any technology that we may require to commercialize our technologies or potential products could have a materially adverse effect on our business.
We also rely upon unpatented proprietary technology, and in the future may determine in some cases that our interests would be better served by reliance on trade secrets or confidentiality agreements rather than patents or licenses. We may not be able to protect our rights to such unpatented proprietary technology and others may independently develop substantially equivalent technologies. If we are unable to obtain strong proprietary rights to our processes or products after obtaining regulatory clearance, competitors may be able to market competing processes and products.
Others may obtain patents having claims which cover aspects of our products or processes which are necessary for, or useful to, the development, use or manufacture of our services or products. Should any other group obtain patent protection with respect to our discoveries, our commercialization of potential therapeutic products and methods could be limited or prohibited.
Material Licenses
Our rights to certain patents and technologies have been acquired through license agreements with other corporations or academic institutions. The license agreements that we consider of particular importance to our business are summarized below.
9
Table of Contents
License and Collaboration Agreement with EpiCept Corporation
In November 2003, Myriad Genetics, Inc., our former parent company, entered into a license and collaboration agreement with Maxim Pharmaceuticals, Inc. and Cytovia, Inc. All licensed rights of Maxim and Cytovia were subsequently acquired by EpiCept Corporation, and we refer to Maxim, Cytovia and EpiCept collectively herein as EpiCept. In connection with our separation from Myriad Genetics on June 30, 2009, Myriad Genetics assigned this agreement to us but will remain liable for the performance and observance of our duties and obligations under the agreement. Pursuant to this license agreement, we hold an exclusive, worldwide right to utilize certain intellectual property rights of EpiCept, including patents, patent applications and know-how that relate to Azixa, in the development and commercialization of products for the treatment or prevention of any disease or disorder. We have the right to grant sublicenses of licensed rights.
We are obligated to pay EpiCept a royalty on net sales of products subject to the license. Royalty payments range, based on sales volume, from the mid to high single digits and may be reduced by up to 50% if we are obligated to pay a royalty to a third party in order to make, use or sell such products, subject to a maximum reduction amount. The license agreement also provides for license fees, research support, and milestone payments of up to $23 million based on the occurrence of specified product development related events for each product based on a particular active ingredient, provided that milestone payments made on a product that is subsequently abandoned may be credited against milestone payment obligations for future products. We are also obligated to make payment of a percentage of certain income received from sublicensees. We are obliged to use commercially reasonable efforts to develop and commercialize licensed products in major markets, failing which, our rights in a major market could end and our licensed rights would terminate. As of June 30, 2009, Myriad Genetics had made payments totaling $4 million under the EpiCept license and collaboration agreement. As of June 30, 2011, we have made no payments under the EpiCept license and collaboration agreement.
We are responsible for filing, prosecuting and maintaining the licensed patent rights, and we bear the majority of costs related to those activities. We have the right, but not the obligation, to enforce the patent rights against infringement. We are obligated to indemnify EpiCept against any liabilities resulting from our utilization of the licensed patent rights and manufacture and commercialization of licensed products.
The license agreement ends on the later of ten years after the date of the first commercial sale of a licensed product or the expiration of EpiCept patent rights covered by the license agreement. These rights are presently not expected to expire until July 2024, based on the last patent issued to date. The license may be sooner terminated on the bankruptcy or uncured material breach of a party.
Manufacturing and Supply
We currently rely on contract manufacturers to produce drug substances and drug products required for our clinical trials under current good manufacturing practices, or cGMP, with oversight by our internal managers. We plan to continue to rely upon contract manufacturers or possibly collaboration partners to manufacture commercial quantities of our drug candidates if and when approved for marketing by the FDA. We currently rely on a single manufacturer for the preclinical or clinical supplies of each of our drug candidates and do not currently have relationships for redundant supply or a second source for any of our drug candidates. We believe that there are alternate sources of supply that can satisfy our clinical trial requirements without significant delay or material additional costs.
Sales and Marketing
We may establish our own sales and marketing capabilities if and when we obtain regulatory approval of our drug candidates. Patients in the markets for our drug candidates are largely managed by medical specialists in the areas of oncology and rheumatology. Historically, companies have experienced substantial commercial success through the deployment of specialized sales forces which can address a majority of key prescribers. Therefore, we may utilize a specialized sales force in the United States for the sales and marketing of drug
10
Table of Contents
candidates that we may successfully develop. We currently have limited marketing, sales and distribution capabilities. In order to participate in the commercialization of any of our drugs, we must develop these capabilities on our own or in collaboration with third parties. We may also choose to hire a third party to provide sales personnel instead of developing our own staff. Outside of the United States, and in situations or markets where a more favorable return may be realized through licensing commercial rights to a third party, we may license a portion or all of our commercial rights in a territory to a third party in exchange for one or more of the following: up-front payments, research funding, development funding, milestone payments and royalties on drug sales.
Competition
The development and commercialization of new drugs is highly competitive. We will face competition with respect to all drug candidates we may develop or commercialize in the future from pharmaceutical and biotechnology companies worldwide. The key factors affecting the success of any approved product will be its efficacy, safety profile, drug interactions, method of administration, pricing, reimbursement and level of promotional activity relative to those of competing drugs. If approved, we would expect our drug candidates to compete with approved drugs and potentially with drug candidates currently under development, including the following:
| • | Hsp90 Inhibitor Program. If approved, we would expect MPC-3100 and MPC-0767 to compete with other Hsp90 inhibitors currently in clinical development, including natural product derived, geldanamycin-based analogs in development (including retaspimycin from Infinity, which is in Phase 2 development) and non-geldanamycin small molecule inhibitors of Hsp90 currently in clinical development (including AUY922 from Vernalis/Novartis and ganetespib from Synta which are in Phase 2 development), as well as other cancer treatments currently approved or in clinical development. |
| • | Cancer Metabolism Inhibitor (CMI) Program. If approved, we would expect our CMI drug candidates, including MPC-8640, to compete with numerous approved products for the treatment of cancer and with other treatments currently in clinical development for the treatment of cancer. |
| • | Small-molecule Anti-interferon Program. If approved, we would expect our small-molecule anti-interferon drug candidates, including MPI-0485520, to compete with numerous approved products for the treatment of autoimmune diseases and with other treatments currently in clinical development for the treatment of autoimmune diseases. |
Many of our potential competitors have substantially greater financial, technical, and personnel resources than we do. In addition, many of these competitors have significantly greater commercial infrastructures. Our ability to compete successfully will depend largely on our ability to leverage our collective experience in drug development to:
| • | develop medicines that are differentiated from other products in the market; |
| • | obtain patent and/or proprietary protection for our medicines and technologies; |
| • | obtain required regulatory approvals; |
| • | obtain a commercial partner, especially for larger indications in oncology; and |
| • | attract and retain high-quality research, development, and commercial personnel. |
Government Regulation and Product Approval
Government authorities in the United States, at the federal, state and local level, and other countries extensively regulate, among other things, the research, development, testing, manufacture, labeling, packaging, promotion, storage, advertising, distribution, marketing and export and import of products such as those we are developing. Our drugs must be approved by the FDA through the new drug application, or NDA, process before they may be legally marketed in the United States.
11
Table of Contents
United States Government Regulation
NDA Approval Processes
In the United States, the FDA regulates drugs under the Federal Food, Drug and Cosmetic Act, or the FDCA, and implementing regulations. The process of obtaining regulatory approvals and the subsequent compliance with applicable federal, state, local and foreign statues and regulation require the expenditure of substantial time and financial resources. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may subject an applicant to administrative or judicial sanctions, any of which could have a material adverse effect on us. These sanctions could include:
| • | refusal to approve pending applications; |
| • | withdrawal of an approval; |
| • | imposition of a clinical hold; |
| • | warning letters; |
| • | product seizures; |
| • | total or partial suspension of production or distribution; or |
| • | injunctions, fines, disgorgement, or civil or criminal penalties. |
The process required by the FDA before a drug may be marketed in the United States generally involves the following:
| • | completion of preclinical laboratory tests, animal studies and formulation studies conducted according to Good Laboratory Practices, or GLPs, or other applicable regulations; |
| • | submission to the FDA of an IND, which must become effective before human clinical trials may begin; |
| • | performance of adequate and well-controlled human clinical trials according to Good Clinical Practices, or GCPs, to establish the safety and efficacy of the proposed drug for its intended use; |
| • | submission to the FDA of an NDA; |
| • | satisfactory completion of an FDA inspection of the manufacturing facility or facilities at which the product is produced to assess compliance with current Good Manufacturing Practices, or cGMPs, to assure that the facilities, methods and controls are adequate to preserve the drug’s identity, strength, quality and purity; and |
| • | FDA review and approval of the NDA. |
Once a pharmaceutical candidate is identified for development, it enters the preclinical testing stage. Preclinical tests include laboratory evaluations of product chemistry, toxicity and formulation, as well as animal studies. An IND sponsor must submit the results of the preclinical tests, together with manufacturing information and analytical data, to the FDA as part of the IND. Some preclinical or nonclinical testing may continue even after the IND is submitted. In addition to including the results of the preclinical studies, the IND will also include a protocol detailing, among other things, the objectives of the clinical trial, the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated if the first phase lends itself to an efficacy determination. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day time period, places the IND on clinical hold. In such a case, the IND sponsor and the FDA must resolve any outstanding concerns before clinical trials can begin. A clinical hold may occur at any time during the life of an IND, and may affect one or more specific studies or all studies conducted under the IND.
12
Table of Contents
All clinical trials must be conducted under the supervision of one or more qualified investigators in accordance with GCPs. They must be conducted under protocols detailing the objectives of the trial, dosing procedures, subject selection and exclusion criteria and the safety and effectiveness criteria to be evaluated. Each protocol must be submitted to the FDA as part of the IND and progress reports detailing the results of the clinical trials must be submitted at least annually to the FDA and more frequently in other situations, including the occurrence of serious adverse events. An institutional review board, or IRB, at each institution participating in the clinical trial must review and approve the protocol before a clinical trial commences at that institution and must also approve the information regarding the trial and the consent form that must be provided to each trial subject or his legal representative, monitor the study until completed and otherwise comply with IRB regulations.
Human clinical trials are typically conducted in three sequential phases that may overlap or be combined:
| • | Phase 1. The drug is initially introduced into healthy human subjects and tested for safety, dosage tolerance, absorption, metabolism, distribution and elimination. In the case of some products for severe or life-threatening diseases, such as cancer, especially when the product may be inherently too toxic to ethically administer to healthy volunteers, the initial human testing is often conducted in patients. |
| • | Phase 2. Clinical trials in a limited patient population intended to identify possible adverse effects and safety risks, to preliminarily evaluate the efficacy of the product for specific targeted diseases and to determine dosage tolerance and optimal dosage. |
| • | Phase 3. Clinical trials are undertaken to further evaluate dosage, clinical efficacy and safety in an expanded patient population at geographically dispersed clinical study sites. These studies are intended to establish the overall risk-benefit ratio of the product and provide, an adequate basis for product labeling. |
Phase 1, Phase 2, and Phase 3 testing may not be completed successfully within any specified period, if at all. The FDA or the sponsor may suspend a clinical trial at any time for a variety of reasons, including a finding that the research subjects or patients are being exposed to an unacceptable health risk or by the sponsor for business reasons. Similarly, an IRB can suspend or terminate approval of a clinical trial at its institution if the clinical trial is not being conducted in accordance with the IRB’s requirements or if the drug has been associated with unexpected serious harm to patients.
During the development of a new drug, sponsors are given an opportunity to meet with the FDA at certain points. These points may be prior to submission of an IND, at the end of Phase 2, and before an NDA is submitted. Meetings at other times may be requested. These meetings can provide an opportunity for the sponsor to share information about the data gathered to date, for the FDA to provide advice, and for the sponsor and FDA to reach agreement on the next phase of development. Sponsors typically use the end of Phase 2 meeting to discuss their Phase 2 clinical results and present their plans for the pivotal Phase 3 clinical trial that they believe will support approval of the new drug. If a Phase 2 clinical trial is the subject of discussion at an end of Phase 2 meeting with the FDA, a sponsor may be able to request a Special Protocol Assessment, the purpose of which is to reach agreement with the FDA on the design of the Phase 3 clinical trial protocol design and analysis that will form the primary basis of an efficacy claim. If such an agreement is reached, it will be documented and made part of the administrative record, and it will be binding on the FDA unless public health concerns unrecognized at the time of protocol assessment are evident, and may not be changed except under a few specific circumstances.
Concurrent with clinical trials, companies usually complete additional animal safety studies and must also develop additional information about the chemistry and physical characteristics of the drug and finalize a process for manufacturing the product in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the drug candidate and the manufacturer must develop methods for testing the quality, purity and potency of the final drugs. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the drug candidate does not undergo unacceptable deterioration over its shelf-life.
13
Table of Contents
The results of product development, preclinical studies and clinical trials, along with descriptions of the manufacturing process, analytical tests conducted on the chemistry of the drug, proposed labeling and other relevant information are submitted to the FDA as part of an NDA requesting approval to market the product. The submission of an NDA is subject to the payment of user fees, but a waiver of such fees may be obtained under specified circumstances. The FDA reviews all NDAs submitted to ensure that they are sufficiently complete for substantive review before it accepts them for filing. It may request additional information rather than accept a NDA for filing. In this event, the NDA must be resubmitted with the additional information. The resubmitted application also is subject to review before the FDA accepts it for filing.
Once the submission is accepted for filing, the FDA begins an in-depth review. NDAs receive either standard or priority review. A drug representing a significant improvement in treatment, prevention or diagnosis of disease may receive priority review. The FDA may refuse to approve an NDA if the applicable regulatory criteria are not satisfied or may require additional clinical or other data. Even if such data are submitted, the FDA may ultimately decide that the NDA does not satisfy the criteria for approval. The FDA reviews an NDA to determine, among other things, whether a product is safe and effective for its intended use and whether its manufacturing is cGMP-compliant to assure and preserve the product’s identity, strength, quality and purity. The FDA may refer the NDA to an advisory committee for review and recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendation of an advisory committee, but it generally follows such recommendations. Before approving an NDA, the FDA will inspect the facility or facilities where the product is manufactured and tested.
Satisfaction of FDA requirements or similar requirements of foreign regulatory authorities typically takes at least several years and the actual time required may vary substantially, based upon, among other things, the indication and the type, complexity and novelty of the product. Government regulation may delay or prevent marketing of potential products for a considerable period of time and impose costly requirements. Success in early stage clinical trials does not assure success in later stage clinical trials. Data obtained from clinical activities are not always conclusive and may be susceptible to varying interpretations, which could delay, limit or prevent regulatory approval. The FDA may not grant approval on a timely basis, or at all. Even if a product receives regulatory approval, the approval may be significantly limited to specific diseases and dosages or the indications for use may otherwise be limited, which could restrict the commercial application of the product. Further, even after regulatory approval is obtained, later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. Delays in obtaining, or failures to obtain, regulatory approvals for any drug candidate could substantially harm our business and cause our stock price to drop significantly. In addition, we cannot predict what adverse governmental regulations may arise from future U.S. or foreign governmental action.
Expedited Review and Approval
The FDA has various programs, including Fast Track, priority review, and accelerated approval, which are intended to expedite or simplify the process for reviewing drugs, and/or provide for approval on the basis of surrogate endpoints. Even if a drug qualifies for one or more of these programs, the FDA may later decide that the drug no longer meets the conditions for qualification or that the time period for FDA review or approval will be shortened. Generally, drugs that may be eligible for these programs are those for serious or life-threatening conditions, those with the potential to address unmet medical needs, and those that offer meaningful benefits over existing treatments. For example, Fast Track is a process designed to facilitate the development, and expedite the review of drugs to treat serious diseases and fill an unmet medical need. Priority review is designed to give drugs that offer major advances in treatment or provide a treatment where no adequate therapy exists an initial review within six months as compared to a standard review time of 10 months. Although Fast Track and priority review do not affect the standards for approval, the FDA will attempt to facilitate early and frequent meetings with a sponsor of a Fast Track designated drug and expedite review of the application for a drug designated for priority review. Accelerated approval provides an earlier approval of drugs to treat serious diseases, and that fill an unmet medical need based on a surrogate endpoint, which is a laboratory measurement or physical sign used as an
14
Table of Contents
indirect or substitute measurement representing a clinically meaningful outcome. As a condition of approval, the FDA may require that a sponsor of a drug receiving accelerated approval perform post-marketing clinical trials.
Patent Term Restoration and Marketing Exclusivity
Depending upon the timing, duration and specifics of FDA approval of the use of our drugs, some of our U.S. patents may be eligible for limited patent term extension under the Drug Price Competition and Patent Term Restoration Act of 1984, referred to as the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND, and the submission date of an NDA, plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to an approved drug is eligible for the extension and the extension must be applied for prior to expiration of the patent. The United States Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we intend to apply for restorations of patent term for some of our currently owned or licensed patents to add patent life beyond their current expiration date, depending on the expected length of clinical trials and other factors involved in the submission of the relevant NDA.
Market exclusivity provisions under the FDCA also can delay the submission or the approval of certain applications. The FDCA provides a five-year period of non-patent marketing exclusivity within the United States to the first applicant to gain approval of an NDA for a new chemical entity. A drug is a new chemical entity if the FDA has not previously approved any other new drug containing the same active moiety, which is the molecule or ion responsible for the action of the drug substance. During the exclusivity period, the FDA may not accept for review an abbreviated new drug application, or ANDA, or a 505(b)(2) NDA submitted by another company for another version of such drug where the applicant does not own or have a legal right of reference to all the data required for approval. However, an application may be submitted after four years if it contains a certification of patent invalidity or non-infringement. The FDCA also provides three years of marketing exclusivity for an NDA, 505(b)(2) NDA or supplement to an existing NDA if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant are deemed by the FDA to be essential to the approval of the application, for example, for new indications, dosages, or strengths of an existing drug. This three-year exclusivity covers only the conditions associated with the new clinical investigations and does not prohibit the FDA from approving ANDAs for drugs containing the original active agent. Five-year and three-year exclusivity will not delay the submission or approval of a full NDA; however, an applicant submitting a full NDA would be required to conduct or obtain a right of reference to all of the preclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety and effectiveness.
Orphan Drug Designation
Under the Orphan Drug Act, the FDA may grant orphan drug designation to drugs intended to treat a rare disease or condition, which is generally a disease or condition that affects fewer than 200,000 individuals in the United States, or more than 200,000 individuals in the United States and for which there is no reasonable expectation that the cost of developing and making available in the United States a drug for this type of disease or condition will be recovered from sales in the United States for that drug. Orphan drug designation must be requested before submitting an NDA. After the FDA grants orphan drug designation, the identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in or shorten the duration of the regulatory review and approval process.
If a product that has orphan drug designation subsequently receives the first FDA approval for the disease for which it has such designation, the product is entitled to orphan product exclusivity, which means that the FDA may not approve any other applications to market the same drug for the same indication, except in very
15
Table of Contents
limited circumstances, for seven years. Orphan drug exclusivity, however, also could block the approval of one of our products for seven years if a competitor obtains approval of the same drug as defined by the FDA or if our drug candidate is determined to be contained within the competitor’s product for the same indication or disease.
Pediatric Exclusivity
Section 505(a) of the FDCA, as amended by the FDA Amendments Act of 2007, permits certain drugs to obtain an additional six months of exclusivity, if the sponsor submits information requested in writing by the FDA, or a Written Request, relating to the use of the drug in children. The FDA may not issue a Written Request for studies on unapproved or approved indications or where it determines that information relating to the use of a drug in a pediatric population, or part of the pediatric population, may not produce health benefits in that population.
We have not requested or received a Written Request for such pediatric studies, although we may ask the FDA to issue a Written Request for such studies in the future. To receive the six-month pediatric market exclusivity, we would have to receive a Written Request from the FDA, conduct the requested studies in accordance with a written agreement with the FDA or, if there is no written agreement, in accordance with commonly accepted scientific principles, and submit reports of the studies. The FDA will accept the reports upon its determination that the studies were conducted in accordance with and are responsive to the original Written Request or commonly accepted scientific principles, as appropriate, and that the reports comply with the FDA’s filing requirements. The FDA may not issue a Written Request for such studies or accept the reports of the studies.
Post-approval Requirements
Once an approval is granted, the FDA may withdraw the approval if compliance with regulatory standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product may result in restrictions on the product or even complete withdrawal of the product from the market. After approval, some types of changes to the approved product, such as adding new indications, manufacturing changes and additional labeling claims, are subject to further FDA review and approval. In addition, the FDA may require testing and surveillance programs to monitor the effect of approved products that have been commercialized, and the FDA has the power to prevent or limit further marketing of a product based on the results of these post-marketing programs.
Any drug products manufactured or distributed by us pursuant to FDA approvals are subject to continuing regulation by the FDA, including, among other things:
| • | record-keeping requirements; |
| • | reporting of adverse experiences with the drug; |
| • | providing the FDA with updated safety and efficacy information; |
| • | drug sampling and distribution requirements; |
| • | notifying the FDA and gaining its approval of specified manufacturing or labeling changes; |
| • | complying with certain electronic records and signature requirements; and |
| • | complying with FDA promotion and advertising requirements. |
Drug manufacturers and other entities involved in the manufacture and distribution of approved drugs are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and some state agencies for compliance with cGMP and other laws.
We rely, and expect to continue to rely, on third parties for the production of clinical and commercial quantities of our products. Future FDA and state inspections may identify compliance issues at the facilities of our contract manufacturers that may disrupt production or distribution, or require substantial resources to correct.
16
Table of Contents
From time to time, legislation is drafted, introduced and passed in Congress that could significantly change the statutory provisions governing the approval, manufacturing and marketing of products regulated by the FDA. In addition, FDA regulations and guidance are often revised or reinterpreted by the agency in ways that may significantly affect our business and our products. It is impossible to predict whether legislative changes will be enacted, or FDA regulations, guidance or interpretations changed or what the impact of such changes, if any, may be.
Foreign Regulation
In addition to regulations in the United States, we will be subject to a variety of foreign regulations governing clinical trials and commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we must obtain approval by the comparable regulatory authorities of foreign countries before we can commence clinical trials and approval of foreign countries or economic areas, such as the European Union, before we may market product in those countries or areas. The approval process and requirements governing the conduct of clinical trials, product licensing, pricing and reimbursement vary greatly from place to place, and the time may be longer or shorter than that required for FDA approval.
Under European Union regulatory systems, a company may submit marketing authorization applications either under a centralized or decentralized procedure. The centralized procedure, which is compulsory for medicines produced by biotechnology or those medicines intended to treat AIDS, cancer, neurodegenerative disorders, or diabetes and optional for those medicines which are highly innovative, provides for the grant of a single marketing authorization that is valid for all European Union member states. The decentralized procedure provides for mutual recognition of national approval decisions. Under this procedure, the holder of a national marketing authorization may submit an application to the remaining member states. Within 90 days of receiving the applications and assessments report, each member state must decide whether to recognize approval. If a member state does not recognize the marketing authorization, the disputed points are eventually referred to the European Commission, whose decision is binding on all member states. As in the United States, we may apply for designation of a product as an orphan drug for the treatment of a specific indication in the European Union before the application for marketing authorization is made. Orphan drugs in Europe enjoy economic and marketing benefits, including up to 10 years of market exclusivity for the approved indication unless another applicant can show that its product is safer, more effective or otherwise clinically superior to the orphan-designated product.
Reimbursement
Sales of our products will depend, in part, on the extent to which the costs of our products will be covered by third-party payors, such as government health programs, commercial insurance and managed healthcare organizations. These third-party payors are increasingly challenging the prices charged for medical products and services. Additionally, the containment of healthcare costs has become a priority of federal and state governments, and the prices of drugs have been a focus in this effort. The U.S. government, state legislatures and foreign governments have shown significant interest in implementing cost-containment programs, including price controls, restrictions on reimbursement and requirements for substitution of generic products. Adoption of price controls and cost-containment measures, and adoption of more restrictive policies in jurisdictions with existing controls and measures, could further limit our net revenue and results. If these third-party payors do not consider our products to be cost-effective compared to other available therapies, they may not cover our products after approval as a benefit under their plans or, if they do, the level of payment may not be sufficient to allow us to sell our products on a profitable basis.
The Medicare Prescription Drug, Improvement, and Modernization Act of 2003, or the MMA, imposed new requirements for the distribution and pricing of prescription drugs for Medicare beneficiaries Under Part D, Medicare beneficiaries may enroll in prescription drug plans offered by private entities which will provide coverage of outpatient prescription drugs. Part D plans include both stand-alone prescription drug benefit plans
17
Table of Contents
and prescription drug coverage as a supplement to Medicare Advantage plans. Unlike Medicare Part A and B, Part D coverage is not standardized. Part D prescription drug plan sponsors are not required to pay for all covered Part D drugs, and each drug plan can develop its own drug formulary that identifies which drugs it will cover and at what tier or level. However, Part D prescription drug formularies must include drugs within each therapeutic category and class of covered Part D drugs, though not necessarily all the drugs in each category or class. Any formulary used by a Part D prescription drug plan must be developed and reviewed by a pharmacy and therapeutic committee. Government payment for some of the costs of prescription drugs may increase demand for products for which we receive marketing approval. However, any negotiated prices for our products covered by a Part D prescription drug plan will likely be lower than the prices we might otherwise obtain. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare coverage policy and payment limitations in setting their own payment rates. Any reduction in payment that results from the MMA may result in a similar reduction in payments from non-governmental payors.
On February 17, 2009, President Obama signed into law the American Recovery and Reinvestment Act of 2009. This law provides funding for the federal government to compare the effectiveness of different treatments for the same illness. A plan for the research will be developed by the Department of Health and Human Services, the Agency for Healthcare Research and Quality and the National Institutes for Health, and periodic reports on the status of the research and related expenditures will be made to Congress. Although the results of the comparative effectiveness studies are not intended to mandate coverage policies for public or private payors, it is not clear how such a result could be avoided and what if any effect the research will have on the sales of our product candidates, if any such product or the condition that it is intended to treat is the subject of a study. It is also possible that comparative effectiveness research demonstrating benefits in a competitor’s product could adversely affect the sales of our product candidates. Decreases in third-party reimbursement for our product candidates or a decision by a third-party payor to not cover our product candidates could reduce physician usage of the product candidate and have a material adverse effect on our sales, results of operations and financial condition.
The Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act of 2010 (collectively, the ACA) enacted in March 2010, is expected to have a significant impact on the health care industry. The ACA is expected to expand coverage for the uninsured while at the same time contain overall healthcare costs. With regard to pharmaceutical products, among other things, the ACA is expected to expand and increase industry rebates for drugs covered under Medicaid programs and make changes to the coverage requirements under the Medicare D program. We cannot predict the impact of the ACA on pharmaceutical companies as many of the ACA reforms require the promulgation of detailed regulations implementing the statutory provisions which has not yet occurred. In addition, the current legal challenges to the ACA, as well as congressional efforts to repeal the ACA, add to the uncertainty of the legislative changes enacted as part of the ACA.
In addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements governing drug pricing vary widely from country to country. For example, the European Union provides options for its member states to restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices of medicinal products for human use. A member state may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of our products. Historically, products launched in the European Union do not follow price structures of the United States and generally tend to be significantly lower.
18
Table of Contents
Employees
As of June 30, 2011, we had 81 full-time employees, 16 of whom hold an M.D., Ph.D, or combined M.D./Ph.D. Approximately 55 employees are engaged in development and clinical and regulatory affairs, and 26 are in general and administrative functions. Our workforce is non-unionized, and we believe our employee relations are good.
Corporate History and Available Information
We were incorporated as Myriad Pharmaceuticals, Inc. in Delaware in January 2009 as a new, wholly owned subsidiary of Myriad Genetics, Inc. in order to effect the separation and spin-off of Myriad Genetics’ research and drug development businesses as a stand-alone, independent, publicly traded company. In connection with the formation of this new subsidiary, Myriad Genetics’ existing subsidiary, Myriad Pharmaceuticals, Inc., changed its corporate name to Myriad Therapeutics, Inc. On June 30, 2009, Myriad Genetics contributed substantially all of the assets and certain liabilities of its research and drug development businesses as well as $188 million in cash and marketable securities to us and effected the spin-off of our company through the pro rata dividend distribution to its stockholders of all outstanding shares of our common stock. Effective July 1, 2010, we changed our name from Myriad Pharmaceuticals, Inc. to Myrexis, Inc. Our principal executive offices are located at 305 Chipeta Way, Salt Lake City, Utah 84108. Our telephone number is 801-214-7800 and our web site address is www.myrexis.com. We include our web site address in this Annual Report on Form 10-K only as an inactive textual reference and do not intend it to be an active link to our web site. We make available free of charge through the “Investors” section of our web site our Corporate Code of Conduct and Ethics, our Audit Committee and other committee charters and our other corporate governance policies, as well as our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, current reports on Form 8-K and all amendments to those reports as soon as reasonably practicable after such material is electronically filed with or furnished to the Securities and Exchange Commission.
19
Table of Contents
| Item 1A. | RISK FACTORS |
The risks and uncertainties described below are those that we currently believe may materially affect our company. If any of the following risks actually occur, they may materially harm our business, our financial condition and our results of operations.
Risks Relating to Our Financial Position and Our Business
We anticipate that we will incur losses for the foreseeable future and we may never achieve or sustain profitability.
We do not expect to generate the cash that is necessary to finance our operations in the short term. We incurred losses of $38.7 million, $46.9 million and $58.1 million for the years ended June 30, 2011, 2010 and 2009, respectively. We expect to continue to incur significant research and development and other significant operating expenses and capital expenditures and anticipate that we will continue to have significant expenses and losses in the foreseeable future as we:
| • | conduct clinical trials for MPC-3100 and initiate additional clinical trials, if supported by the results of such trials; and |
| • | complete preclinical development of MPC-8640, MPC-0767, and MPI-0485520 and initiate clinical trials, if supported by positive preclinical data. |
We must generate significant revenue to achieve and maintain profitability. Even if we succeed in developing and commercializing one or more of our drug candidates, we may not be able to generate sufficient revenue and we may never be able to achieve or maintain profitability.
We will require additional capital to fund our operations, and if we are unable to obtain such capital, we will be unable to successfully develop and commercialize our drug candidates.
We believe that our existing cash and investment securities will be sufficient to support our current operating plan through at least June 30, 2014. However, we will require additional capital in order to complete the clinical development of and to commercialize our drug candidates. Our future capital requirements will depend on many factors that are currently unknown to us, including:
| • | the timing of initiation, progress, results and costs of our clinical trials for MPC-3100; |
| • | the results of preclinical studies of MPC-8640, MPC-0767, and MPI-0485520 and the timing of initiation, progress, results and costs of any clinical trials that we may initiate based on the preclinical results; |
| • | the costs of establishing commercial manufacturing arrangements and of establishing sales and marketing functions, if needed; |
| • | the scope, progress, results, and cost of preclinical development, clinical trials, and regulatory review of any new drug candidates for which we may initiate development; |
| • | the costs of preparing, filing, and prosecuting patent applications and maintaining, enforcing, and defending intellectual property-related claims; |
| • | our ability to establish research collaborations and strategic collaborations and licensing or other arrangements on terms favorable to us; |
| • | the costs to satisfy our obligations under potential future collaborations; and |
| • | the timing, receipt, and amount of sales or royalties, if any, from any approved drug candidates. |
There can be no assurance that additional funds will be available when we need them on terms that are acceptable to us, or at all. If adequate funds are not available on a timely basis, we may be required to terminate or delay clinical trials or other development for one or more of our drug candidates.
20
Table of Contents
We may seek to raise any necessary funds through public or private equity offerings, debt financings or strategic alliances and licensing arrangements. We currently have an effective universal shelf registration statement pursuant to which we have $80 million in securities available for sale. We may not be able to obtain additional financing on terms favorable to us, if at all. General market conditions may make it very difficult for us to seek financing from the capital markets. We may be required to relinquish rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us, in order to raise additional funds through alliance, joint venture or licensing arrangements.
Our future success depends on our ability to retain our key executives and to attract, retain, and motivate qualified personnel.
Our President and Chief Executive Officer, Adrian N. Hobden, resigned effective July 21, 2011, and Robert Lollini, our Chief Financial Officer, was appointed our Interim President and Chief Executive Officer on July 21, 2011, and our permanent President and Chief Executive Officer effective September 6, 2011. In connection with his appointment as President and Chief Executive Officer, Mr. Lollini resigned as our Chief Financial Officer. On September 6, 2011, Andrea Kendell was appointed our Chief Financial Officer.
The competition for qualified personnel in the biotechnology field is intense and we must retain and motivate highly qualified scientific personnel. We are highly dependent on Mr. Lollini, Ms. Kendell, and Wayne Laslie, our Chief Operating Officer. There can be no assurance that we will be able to retain any of our key executives due in part to the fact that the agreements we have entered into with the principal members of our executive and scientific teams provide for employment that can be terminated by either party without cause at any time. Further, the non-compete provisions to which each employee is subject, generally expire for certain key executive officers one year from the applicable date of termination of employment, which means that these executives may be employed by a competitor of ours in the future. We have entered into retention agreements with certain of our key executive officers to reinforce and encourage continued employment and dedication without distraction from the possibility of a change in control and related events and circumstances. Although we do not have any reason to believe that we may lose the services of any of these persons in the foreseeable future, the loss of the services of any of these persons might impede the achievement of our development and commercialization objectives. Recruiting and retaining qualified scientific personnel and possibly sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our development and commercialization strategy. Our consultants and advisors may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us.
Risks Related to the Development and Regulatory Approval of Our Drug Candidates
Our success is largely dependent on the success of our drug candidates, and we cannot be certain that we will be able to obtain regulatory approval for or successfully commercialize any of these drug candidates.
We have invested significant time and financial resources in the development of our drug candidates. We anticipate that our success will depend largely on the receipt of regulatory approval and successful commercialization of our drug candidates. The future success of our clinical and pre-clinical programs will depend on several factors, including the following:
| • | our ability to provide acceptable evidence of their safety and efficacy; |
| • | receipt of marketing approval from the FDA and similar foreign regulatory authorities; |
| • | obtaining and maintaining commercial manufacturing arrangements with third-party manufacturers or establishing commercial-scale manufacturing capabilities; |
21
Table of Contents
| • | possibly establishing an internal sales force or collaborating with pharmaceutical companies or contract sales organizations to market and sell any approved drug; and |
| • | acceptance of any approved drug in the medical community and by patients and third-party payors. |
Many of these factors are beyond our control. Accordingly, we cannot assure you that we will ever be able to generate revenues through the license or sale of any of our drug candidates.
Our drug candidates are still in the early stages of development and remain subject to clinical testing and regulatory approval. If we are unable to successfully develop and test our drug candidates, we will not be successful.
To date, we have not marketed, distributed or sold any drugs. The success of our business depends substantially upon our ability to develop and commercialize our drug candidates successfully. We currently have one clinical-stage drug candidate in development, MPC-3100, which is in the early stages of development. Our drug candidates are prone to the risks of failure inherent in drug development. Before obtaining regulatory approvals for the commercial sale of MPC-3100 or any other drug candidate for a target indication, we must demonstrate with substantial evidence gathered in well-controlled clinical trials, and, with respect to approval in the United States, to the satisfaction of the FDA and, with respect to approval in other countries, similar regulatory authorities in those countries, that the drug candidate is safe and effective for use for that target indication. Satisfaction of these and other regulatory requirements is costly, time consuming, uncertain, and subject to unanticipated delays. Despite our efforts, our drug candidates may not:
| • | offer improvement over existing, comparable drugs; |
| • | be proven safe and effective in clinical trials; |
| • | meet applicable regulatory standards; or |
| • | be successfully commercialized. |
Positive results in preclinical studies of a drug candidate may not be predictive of similar results in humans during clinical trials, and promising results from early clinical trials of a drug candidate may not be replicated in later clinical trials. Interim results of a clinical trial do not necessarily predict final results. A number of companies in the pharmaceutical and biotechnology industries have suffered significant setbacks in late-stage clinical trials even after achieving promising results in early-stage development. Accordingly, the results from completed preclinical studies and clinical trials for our drug candidates may not be predictive of the results we may obtain in later stage trials or studies. Our preclinical studies or clinical trials may produce negative or inconclusive results, and we may decide, or regulators may require us, to conduct additional preclinical studies or clinical trials, or to discontinue clinical trials altogether. We may also decide to stop development of a drug candidate for other reasons. We do not expect any of our drug candidates to be commercially available for at least several years and some or all may never become commercially available.
If clinical trials for our drug candidates are prolonged or delayed, we may be unable to commercialize our drug candidates on a timely basis, which would require us to incur additional costs and delay our receipt of any revenue from potential product sales.
We cannot predict whether we will encounter problems with any of our ongoing or planned clinical trials that will cause us or any regulatory authority to delay or suspend those clinical trials or delay the analysis of data derived from them. A number of events, including any of the following, could delay the completion of our ongoing and planned clinical trials and negatively impact our ability to obtain regulatory approval for, and to market and sell, a particular drug candidate:
| • | conditions imposed on us by the FDA or any foreign regulatory authority regarding the scope or design of our clinical trials; |
22
Table of Contents
| • | delays in obtaining, or our inability to obtain, required approvals from institutional review boards, or IRBs, or other reviewing entities at clinical sites selected for participation in our clinical trials; |
| • | insufficient supply or deficient quality of our drug supply or other materials necessary to conduct our clinical trials; |
| • | delays in obtaining regulatory agreement for the conduct of our clinical trials; |
| • | lower than anticipated enrollment and retention rate of subjects in clinical trials for a variety of reasons, including size of patient population, nature of trial protocol, the availability of approved effective treatments for the relevant disease and competition from other clinical trial programs for similar indications; |
| • | serious and unexpected drug-related side effects experienced by patients in clinical trials; or |
| • | failure of our third-party contractors to meet their contractual obligations to us in a timely manner. |
Clinical trials may also be delayed or terminated as a result of ambiguous or negative interim results. In addition, a clinical trial may be suspended or terminated by us, the FDA, the IRBs at the sites where the IRBs are overseeing a trial, or other regulatory authorities due to a number of factors, including:
| • | failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols; |
| • | the imposition of a clinical hold by the FDA; |
| • | varying interpretation of data by the FDA or similar foreign regulatory authorities; |
| • | failure to achieve primary or secondary endpoints or other failure to demonstrate efficacy; |
| • | unforeseen safety issues; or |
| • | lack of adequate funding to continue the clinical trial. |
Additionally, changes in standard of care or regulatory requirements and guidance may occur and we may need to amend clinical trial protocols to reflect these changes. Such amendments may require us to resubmit our clinical trial protocols to IRBs for reexamination, which may impact the cost, timing or successful completion of a clinical trial. Such changes may also require us to reassess the viability of the program in question.
We do not know whether our clinical trials will begin as planned, will need to be restructured or will be completed on schedule, if at all. Delays in our clinical trials will result in increased development costs for our drug candidates. In addition, if we experience delays in completion of, or if we terminate, any of our clinical trials, the commercial prospects for our drug candidates may be affected and our ability to generate product revenues will be delayed. Furthermore, many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of a drug candidate.
Even if our drug candidates receive regulatory approval in the United States, we may never receive approval or commercialize our products outside of the United States.
In order to market any products outside of the United States, we must establish and comply with numerous and varying regulatory requirements of other countries regarding safety and efficacy. Approval procedures vary among countries and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries might differ from that required to obtain FDA approval. The regulatory approval process in other countries may include all of the risks detailed above regarding FDA approval in the United States as well as other risks. Regulatory approval in one country does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country may have a negative effect on the regulatory process in others. Failure to obtain regulatory approval in other countries or any delay or setback in obtaining such approval would impair our ability to develop foreign markets for our drug candidates and may have a material adverse effect on our results of operations and financial condition.
23
Table of Contents
Both before and after marketing approval, our drug candidates are subject to ongoing regulatory requirements, and if we fail to comply with these continuing requirements, we could be subject to a variety of sanctions and the sale of any approved commercial products could be suspended.
Both before and after regulatory approval to market a particular drug candidate, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion and record keeping related to the drug are subject to extensive regulatory requirements. If we fail to comply with the regulatory requirements of the FDA and other applicable U.S. and foreign regulatory authorities, we could be subject to administrative or judicially imposed sanctions, including:
| • | restrictions on the products or manufacturing processes; |
| • | warning letters; |
| • | civil or criminal penalties; |
| • | fines; |
| • | injunctions; |
| • | product seizures or detentions and related publicity requirements; |
| • | suspension or withdrawal of regulatory approvals; |
| • | total or partial suspension of production; and |
| • | refusal to approve pending applications for marketing approval of new drug candidates or supplements to approved applications. |
If side effects increase or are identified during the time our drug candidates are in development or after they are approved and on the market, we may be required to perform lengthy additional clinical trials, change the labeling of any such products, or withdraw such products from the market, any of which would hinder or preclude our ability to generate revenues.
In the second quarter of 2009, we initiated a Phase 1 open-label, dose-finding, multiple-dose clinical trial of MPC-3100, our drug candidate in development for the treatment of cancer, in patients with refractory or relapsed cancers, including solid tumors, lymphomas and leukemias. This study has been completed in the clinic and we expect to report results in the fourth quarter of 2011. In the Phase 1 clinical trial, 26 subjects have been treated with MPC-3100. Five serious adverse events in four subjects have been reported as possibly related to MPC-3100: abdominal pain (single event in a single patient), respiratory failure and supraventricular tachycardia (two events in a single patient), enteritis (single event in a single patient), and renal failure (single event in a single patient).
If the incidences of these side effects increases or other problems occur in future clinical trials, we may be required to terminate or delay clinical development of the product candidate. Furthermore, even if any of our drug candidates receives marketing approval, as greater numbers of patients use a drug following its approval, if the incidence of side effects increases or if other problems are observed after approval that were not seen or anticipated during pre-approval clinical trials, a number of potentially significant negative consequences could result, including:
| • | regulatory authorities may withdraw their approval of the product; |
| • | we may be required to reformulate such products, change the way the product is manufactured or administered, conduct additional clinical trials or change the labeling of the product; |
| • | we may become the target of lawsuits, including class action suits; and |
| • | our reputation in the market place may suffer resulting in a significant drop in the sales of the affected products. |
24
Table of Contents
Any of these events could substantially increase the costs and expenses of developing, commercializing and marketing any such drug candidates or could harm or prevent sales of any approved products.
While we choose to test our drug candidates in specific clinical indications based in part on our understanding of their mechanisms of action, our understanding may be incorrect or incomplete and, therefore, our drugs may not be effective against the diseases tested in our clinical trials.
Our rationale for selecting the particular therapeutic indications for each of our drug candidates is based in part on our understanding of the mechanism of action of these drug candidates. However, our understanding of the drug candidate’s mechanism of action may be incomplete or incorrect, or the mechanism may not be clinically relevant to the diseases treated. In such cases, our drug candidates may prove to be ineffective in the clinical trials for treating those diseases.
We deal with hazardous materials and must comply with environmental laws and regulations, which can be expensive and restrict how we do business.
Certain of our drug development activities involve the controlled storage, use, and disposal of hazardous materials. We are subject to federal, state, and local laws and regulations governing the use, manufacture, storage, handling, and disposal of these hazardous materials. Although we believe that our safety procedures for the handling and disposing of these materials comply with the standards prescribed by these laws and regulations, we cannot eliminate the risk of accidental contamination or injury from these materials. In the event of an accident, state or federal authorities may curtail our use of these materials, and we could be liable for any civil damages that result, which may exceed our financial resources and may seriously harm our business. Because we believe that our laboratory and materials handling policies and practices sufficiently mitigate the likelihood of materials liability or third-party claims, we currently carry no insurance covering such claims. An accident could damage, or force us to shut down, our operations.
Risks Related to the Commercialization of Our Drug Candidates
Even if any of our drug candidates receives regulatory approval, if the approved product does not achieve broad market acceptance, the revenues that we generate from sales of the product will be limited.
Even if drug candidates we may develop or acquire in the future obtain regulatory approval, they may not gain broad market acceptance among physicians, healthcare payors, patients, and the medical community. The degree of market acceptance for any approved drug candidate will depend on a number of factors, including:
| • | demonstration of clinical safety and efficacy compared to other products; |
| • | prevalence and severity of adverse side effects; |
| • | availability of reimbursement from government health programs and other third-party payors; |
| • | convenience and ease of administration; |
| • | cost-effectiveness; |
| • | timing of market introduction of competitive products; |
| • | other potential advantages of alternative treatment methods; and |
| • | ineffective marketing and distribution support of our products. |
If our approved drugs fail to achieve broad market acceptance, we may not be able to generate significant revenue and our business would suffer.
25
Table of Contents
If we are unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell our drug candidates, we may be unable to generate product revenue.
We do not currently have an organization for the sales, marketing and distribution of pharmaceutical products. In order to market any products that may be approved by the FDA, or similar foreign regulatory authorities, we must build our sales, marketing, managerial and other non-technical capabilities, license to a commercial partner, or make arrangements with third parties to perform these services. If we are unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, we may not be able to generate product revenue and our business would suffer.
If government and third-party payors fail to provide adequate coverage and payment rates for any of our drug candidates that receive regulatory approval, our revenue and prospects for profitability will be harmed.
In both domestic and foreign markets, the sales of any future products will depend in part upon the availability of reimbursement from third-party payors. Such third-party payors include government health programs such as Medicare, managed care providers, private health insurers, and other organizations. These third-party payors are increasingly attempting to contain healthcare costs by demanding price discounts or rebates and limiting both coverage and the amounts that they will pay for new drugs, and, as a result, they may not cover or provide adequate payment for our drug candidates. In addition, in order to show such payors that our future products are cost effective, we may have to conduct costly post-marketing clinical studies. Adequate third-party reimbursement might not be available to enable us to maintain price levels sufficient to realize an appropriate return on investment in product development.
Changes in healthcare policy could adversely affect our business.
U.S. and foreign governments continue to propose and pass legislation designed to reduce the cost of healthcare. For example, the Medicare Prescription Drug Improvement and Modernization Act of 2003, or MMA, expanded Medicare coverage for drugs purchased by Medicare beneficiaries and introduced new reimbursement methodologies. In addition, this law provided authority for limiting the number of drugs that will be covered in any therapeutic class. We do not know what impact the MMA and similar laws will have on the availability of coverage for and the price that we receive for any approved products. Moreover, while the MMA applies only to drug benefits for Medicare beneficiaries, private payors often follow Medicare policies in setting their own reimbursement policies, and any reduction in reimbursement that results from the MMA may result in similar reductions by private payors.
In March 2010, the President signed the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Affordability Reconciliation Act, together the Affordable Care Act or ACA. This law is expected to result in an increase in the number of people who are covered by both public and private insurance and is also expected to substantially change the way health care is financed by both government health program and private insurers, and significantly impact the pharmaceutical industry. The ACA contains a number of provisions that are expected to impact our business and operations in ways that may negatively affect our potential revenues in the future. For example, the ACA imposes a non-deductible excise tax on pharmaceutical manufacturers or importers that sell branded prescription drugs to U.S. government programs which we believe will increase the cost of our products. In addition, as part of the ACA’s provisions closing a funding gap that currently exists in the Medicare Part D prescription drug program (commonly known as the “donut hole”), we will be required to provide a 50% discount on branded prescription drugs sold to beneficiaries who fall within the donut hole. While it is too early to predict all the specific effects the ACA or any future healthcare reform legislation will have on our business, they could have a material adverse effect on our business and financial condition.
The availability of government reimbursement for prescription drugs is also likely to be impacted by the Budget Control Act of 2011, which was signed into law on August 2, 2011. This law is expected to result in federal spending cuts totaling between $1.2 trillion and $1.5 trillion over the next decade over half of which will include cuts in Medicare and other health related spending.
26
Table of Contents
The markets for our drug candidates are subject to intense competition. If we are unable to compete effectively, our drug candidates may be rendered noncompetitive or obsolete.
The development and commercialization of new drugs is highly competitive. We will face competition with respect to all drug candidates we may develop or commercialize in the future from pharmaceutical and biotechnology companies worldwide. The key factors affecting the success of any approved product will be its efficacy, safety profile, drug interactions, method of administration, pricing, reimbursement and level of promotional activity relative to those of competing drugs. If approved, we would expect our drug candidates to compete with approved drugs and potentially with drug candidates currently under development, including the following:
| • | Hsp90 Inhibitor Program. If approved, we would expect MPC-3100 and MPC-0767 to compete with other Hsp90 inhibitors currently in clinical development, natural product derived, geldanamycin-based analogs in development (including retaspimycin from Infinity, which is in Phase 2 development) and non-geldanamycin small molecule inhibitors of Hsp90 currently in clinical development (including AUY922 from Vernalis/Novartis and ganetespib from Synta which are in Phase 2 development), as well as other cancer treatments currently approved or in clinical development. |
| • | Cancer Metabolism Inhibitor (CMI) Program. If approved, we would expect our CMI drug candidates, including MPC-8640, to compete with numerous approved products for the treatment of cancer and with other treatments currently in clinical development for the treatment of cancer. |
| • | Small-molecule Anti-interferon Program. If approved, we would expect our small-molecule anti-interferon drug candidates, including MPI-0485520, to compete with numerous approved products for the treatment of autoimmune diseases and with other treatments currently in clinical development for the treatment of autoimmune diseases. |
Furthermore, many large pharmaceutical and biotechnology companies, academic institutions, governmental agencies, and other public and private research organizations are pursuing the development of novel drugs that target the same indications we are targeting with our drug candidates. We face, and expect to continue to face, intense and increasing competition as new products enter the market and advanced technologies become available. Many of our competitors have:
| • | significantly greater financial, technical and human resources than we have and may be better equipped to develop, manufacture and commercialize drug candidates; |
| • | more extensive experience in preclinical testing and clinical trials, obtaining regulatory approvals and manufacturing and marketing pharmaceutical products; |
| • | drug candidates that have been approved or are in late-stage clinical development; and/or |
| • | collaborative arrangements in our target markets with leading companies and research institutions. |
Competitive products may render our products obsolete or noncompetitive before we can recover the expenses of developing and commercializing our drug candidates. Furthermore, the development of new treatment methods and/or the widespread adoption or increased utilization of any vaccine or development of other products or treatments for the diseases we are targeting could render our drug candidates noncompetitive, obsolete or uneconomical. If we successfully develop and obtain approval for our drug candidates, we will face competition based on the safety and effectiveness of our drug candidates, the timing of their entry into the market in relation to competitive products in development, the availability and cost of supply, marketing and sales capabilities, reimbursement coverage, price, patent position and other factors. If we successfully develop drug candidates but those drug candidates do not achieve and maintain market acceptance, our business will not be successful.
27
Table of Contents
If a successful product liability claim or series of claims is brought against us for uninsured liabilities or in excess of insured liabilities, we could incur substantial liability.
The use of our drug candidates in clinical trials and the sale of any products for which we obtain marketing approval expose us to the risk of product liability claims. Product liability claims might be brought against us by consumers, health care providers or others selling or otherwise coming into contact with our products. If we cannot successfully defend ourselves against product liability claims, we could incur substantial liabilities. In addition, regardless of merit or eventual outcome, product liability claims may result in:
| • | decreased demand for any approved drug candidates; |
| • | impairment of our business reputation; |
| • | withdrawal of clinical trial participants; |
| • | costs of related litigation; |
| • | distraction of management’s attention from our primary business; |
| • | substantial monetary awards to patients or other claimants; |
| • | loss of revenues; and |
| • | the inability to successfully commercialize any approved drug candidates. |
We have obtained product liability insurance coverage for our clinical trials with a $5.0 million annual aggregate coverage limit. However, our insurance coverage may not be sufficient to reimburse us for any expenses or losses we may suffer. Moreover, insurance coverage is becoming increasingly expensive, and, in the future, we may not be able to maintain insurance coverage at a reasonable cost or in sufficient amounts to protect us against losses due to liability. If and when we obtain marketing approval for any of our drug candidates, we intend to expand our insurance coverage to include the sale of commercial products; however, we may be unable to obtain this product liability insurance on commercially reasonable terms. On occasion, large judgments have been awarded in class action lawsuits based on drugs that had unanticipated side effects. A successful product liability claim or series of claims brought against us could cause our stock price to decline and, if judgments exceed our insurance coverage, could decrease our cash and adversely affect our business.
We are subject to federal and state laws prohibiting “kickbacks” and false or fraudulent claims, and state gift ban laws which, if violated, could subject us to substantial penalties. Additionally, any challenge to or investigation into our practices under these laws could cause adverse publicity and be costly to respond to, and thus could harm our business.
A federal law commonly known as the federal anti-kickback law, and several similar state laws, prohibit the payment of any remuneration that is intended to induce physicians or others either to refer patients or to acquire or arrange for or recommend the acquisition of health care products or services. Other federal and state laws generally prohibit individuals or entities from knowingly presenting, or causing to be presented, claims for payment to Medicare, Medicaid or other third-party payors that are false or fraudulent, or for items or services that were not provided as claimed.
A number of states have enacted laws that require pharmaceutical and medical device companies to monitor and report payments, gifts and other remuneration made to physicians and other health care professional and health care organizations. Some state statutes impose an outright ban on gifts to physicians. These laws are often referred to as “gift ban” or “aggregate spend” laws, and they carry substantial fines if they are violated. In addition, the ACA requires the annual reporting of certain payments and other transfers of value that are made to health care professionals in 2012 and thereafter. The federal ACA does not preempt all aspects of the similar state laws.
28
Table of Contents
In the event that we are found to have violated these laws or decide to settle a claim that we have done so, our business may be materially adversely affected as a result of any payments required to be made, restrictions on our future operations or actions required to be taken, damage to our business reputation or adverse publicity in connection with such a finding or settlement or other adverse effects relating thereto. Additionally, even an unsuccessful challenge or investigation into our practices could cause adverse publicity, and be costly to respond to, and thus could harm our business and results of operations.
Risks Related to Our Dependence on Third Parties
We rely on third parties to conduct our clinical trials, and those third parties may not perform satisfactorily, including failing to meet established deadlines for the completion of such clinical trials.
We do not have the ability to independently conduct clinical trials for our drug candidates, and we rely on third parties, such as contract research organizations, medical institutions, and clinical investigators, to perform this function. Our reliance on these third parties for clinical development activities reduces our control over these activities. Furthermore, these third parties may also have relationships with other entities, some of which may be our competitors. Although we have, in the ordinary course of business, entered into agreements with these third parties, we continue to be responsible for confirming that each of our clinical trials is conducted in accordance with its general investigational plan and protocol. Moreover, the FDA requires us to comply with regulations and standards, commonly referred to as good clinical practices, for conducting, recording and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the trial participants are adequately protected. Our reliance on third parties does not relieve us of these responsibilities and requirements. To date, we believe our contract research organizations and other similar entities with which we are working have performed well. However, if these third parties do not successfully carry out their contractual duties or meet expected deadlines, we may be required to replace them. Although we believe that there are a number of other third-party contractors we could engage to continue these activities, it may result in a delay of the affected trial. Accordingly, we may be delayed in obtaining regulatory approvals for our drug candidates and may be delayed in our efforts to successfully commercialize our drug candidates for targeted diseases.
If we do not establish strategic collaborations, we may have to alter our development plans.
Our drug development programs and potential commercialization of our drug candidates will require substantial additional cash to fund expenses. Our strategy includes collaborating with leading pharmaceutical and biotechnology companies to assist us in furthering development and potential commercialization of some of our drug candidates, in some or all geographies. It may be difficult to enter into one or more of such collaborations in the future. We face significant competition in seeking appropriate collaborators and these collaborations are complex and time-consuming to negotiate and document. We may not be able to negotiate collaborations on acceptable terms, or at all. If that were to occur, we may have to curtail the development of a particular drug candidate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of our sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms, or at all. If we do not have sufficient funds, we will not be able to bring our drug candidates to market and generate product revenue.
We have no manufacturing capacity and depend on third-party manufacturers to produce our clinical trial drug supplies.
We do not currently operate manufacturing facilities for clinical or commercial production of any of our drug candidates. We have limited experience in drug manufacturing, and we lack the resources and the capabilities to manufacture any of our drug candidates on a clinical or commercial scale. As a result, we currently
29
Table of Contents
rely on third-party manufacturers to supply, store, and distribute drug supplies for our clinical trials and anticipate future reliance on a limited number of third-party manufacturers until we increase the number of manufacturers with whom we contract. Any performance failure on the part of our existing or future manufacturers could delay clinical development or regulatory approval of our drug candidates or commercialization of any approved products, producing additional losses and depriving us of potential product revenue.
Our drug candidates require precise, high quality manufacturing. Failure by our contract manufacturers to achieve and maintain high manufacturing standards could result in patient injury or death, product recalls or withdrawals, delays or failures in testing or delivery, cost overruns, or other problems that could seriously hurt our business. Contract manufacturers may encounter difficulties involving production yields, quality control, and quality assurance. These manufacturers are subject to ongoing periodic unannounced inspection by the FDA and corresponding state and foreign agencies to ensure strict compliance with cGMP, and other applicable government regulations and corresponding foreign standards; however, we do not have control over third-party manufacturers’ compliance with these regulations and standards.
If for some reason our contract manufacturers cannot perform as agreed, we may be required to replace them. Although we believe there are a number of potential replacements as our manufacturing processes are not manufacturer specific, we may incur added costs and delays in identifying and qualifying any such replacements because the FDA must approve any replacement manufacturer prior to manufacturing our drug candidates. Such approval would require new testing and compliance inspections. In addition, a new manufacturer would have to be educated in, or develop substantially equivalent processes for, production of our drug candidates after receipt of FDA approval.
We anticipate continued reliance on third-party manufacturers if we are successful in obtaining marketing approval from the FDA and other regulatory agencies for any of our drug candidates.
To date, our drug candidates have been manufactured in small quantities for preclinical testing and clinical trials by third-party manufacturers. If the FDA or other regulatory agencies approve any of our drug candidates for commercial sale, we expect that we would continue to rely, at least initially, on third-party manufacturers to produce commercial quantities of our approved drug candidates. These manufacturers may not be able to successfully increase the manufacturing capacity for any of our approved drug candidates in a timely or economic manner, or at all. Significant scale-up of manufacturing may require additional validation studies, which the FDA must review and approve. If they are unable to successfully increase the manufacturing capacity for a drug candidate, or we are unable to establish our own manufacturing capabilities, the commercial launch of any approved products may be delayed or there may be a shortage in supply.
We depend on third-party suppliers for key raw materials used in our manufacturing processes, and the loss of these third-party suppliers or their inability to supply us with adequate raw materials could harm our business.
We rely on third-party suppliers for the raw materials required for the production of our drug candidates. Our dependence on these third-party suppliers and the challenges we may face in obtaining adequate supplies of raw materials involve several risks, including limited control over pricing, availability, quality, and delivery schedules. We cannot be certain that our current suppliers will continue to provide us with the quantities of these raw materials that we require or satisfy our anticipated specifications and quality requirements. Any supply interruption in limited or sole sourced raw materials could materially harm our ability to manufacture our products until a new source of supply, if any, could be identified and qualified. Although we believe there are several other suppliers of these raw materials, we may be unable to find a sufficient alternative supply channel in a reasonable time or on commercially reasonable terms. Any performance failure on the part of our suppliers could delay the development and commercialization of our drug candidates, including limiting supplies necessary for clinical trials and regulatory approvals, or interrupt production of the existing products that are already marketed, which would have a material adverse effect on our business.
30
Table of Contents
Risks Related to Our Intellectual Property
If we are unable to adequately protect the intellectual property relating to our drug candidates, or if we infringe the rights of others, our ability to successfully commercialize our drug candidates will be harmed.
We own or hold licenses to a number of issued patents and U.S. pending patent applications, as well as foreign patents and pending PCT applications and foreign counterparts. Our success depends in part on our ability to obtain patent protection both in the United States and in other countries for our drug candidates. Our ability to protect our drug candidates from unauthorized or infringing use by third parties depends in substantial part on our ability to obtain and maintain valid and enforceable patents. Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, our ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions. Accordingly, rights under any issued patents may not provide us with sufficient protection for our drug candidates or provide sufficient protection to afford us a commercial advantage against competitive products or processes.
In addition, we cannot guarantee that any patents will issue from any pending or future patent applications owned by or licensed to us. Even if patents have issued or will issue, we cannot guarantee that the claims of these patents are or will be valid or enforceable or will provide us with any significant protection against competitive products or otherwise be commercially valuable to us. Patent applications in the United States are maintained in confidence for up to 18 months after their filing. In some cases, however, patent applications remain confidential in the U.S. Patent and Trademark Office, or the U.S. Patent Office, for the entire time prior to issuance as a U.S. patent. Similarly, publication of discoveries in the scientific or patent literature often lag behind actual discoveries. Consequently, we cannot be certain that we or our licensors or co-owners were the first to invent, or the first to file patent applications on, our drug candidates or their use as drugs. In the event that a third party has also filed a U.S. patent application relating to our drug candidates or a similar invention, we may have to participate in interference proceedings declared by the U.S. Patent Office to determine priority of invention in the United States. The costs of these proceedings could be substantial and it is possible that our efforts would be unsuccessful, resulting in a loss of our U.S. patent position. Furthermore, we may not have identified all U.S. and foreign patents or published applications that affect our business either by blocking our ability to commercialize our drugs or by covering similar technologies.
The laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as in the United States and many companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. If we encounter such difficulties in protecting or are otherwise precluded from effectively protecting our intellectual property rights in foreign jurisdictions, our business prospects could be substantially harmed.
The patent positions of biotechnology and pharmaceutical companies, including our patent position, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated, or circumvented. We will be able to protect our proprietary rights from unauthorized use by third parties only to the extent that our proprietary technologies, drug candidates, and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets.
We license patent rights from third-party owners. Our licenses may be subject to early termination if we fail to comply with our obligations in our licenses with third parties.
We are party to a number of licenses that give us rights to third-party intellectual property that is necessary or useful for our business. We may also enter into additional licenses to third-party intellectual property in the future. Our licensors may terminate their agreements with us in the event we breach the applicable license agreement and fail to cure the breach within a specified period of time. Under our existing license agreements we
31
Table of Contents
are obligated to pay the licensor fees, which may include annual license fees, royalties, a percentage of revenues associated with the licensed technology and a percentage of sublicensing revenue. In addition, under our existing license agreements, we are required to diligently pursue the development of products using the licensed technology. If we breach any of the terms of our licenses, the licensors may terminate the agreements.
Litigation regarding patents, patent applications and other proprietary rights may be expensive and time consuming. If we are involved in such litigation, it could cause delays in bringing drug candidates to market and harm our ability to operate.
Our success will depend in part on our ability to operate without infringing the proprietary rights of third parties. The pharmaceutical industry is characterized by extensive litigation regarding patents and other intellectual property rights. Other parties may obtain patents in the future and allege that the use of our technologies infringes these patent claims or that we are employing their proprietary technology without authorization.
In addition, third parties may challenge or infringe upon our existing or future patents. Proceedings involving our patents or patent applications or those of others could result in adverse decisions regarding:
| • | the patentability of our inventions relating to our drug candidates; and/or |
| • | the enforceability, validity or scope of protection offered by our patents relating to our drug candidates. |
Even if we are successful in these proceedings, we may incur substantial costs and divert management time and attention in pursuing these proceedings, which could have a material adverse effect on us. If we are unable to avoid infringing the patent rights of others, we may be required to seek a license, defend an infringement action or challenge the validity of the patents in court. Patent litigation is costly and time consuming. We may not have sufficient resources to bring these actions to a successful conclusion. In addition, if we do not obtain a license, develop or obtain non-infringing technology, fail to defend an infringement action successfully or have infringed patents declared invalid, we may:
| • | incur substantial monetary damages; |
| • | encounter significant delays in bringing our drug candidates to market; and/or |
| • | be precluded from participating in the manufacture, use or sale of our drug candidates or methods of treatment requiring licenses. |
We may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
We rely on trade secrets to protect our proprietary technologies, especially where we do not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. We rely in part on confidentiality agreements with our employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors to protect our trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover our trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of our proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect our competitive business position.
32
Table of Contents
Risks Relating to Our Separation from Myriad Genetics
Our historical financial information for periods ending on or prior to June 30, 2009 is not necessarily representative of the results we would have achieved as a separate, publicly traded company and may not be a reliable indicator of our future results.
The historical financial information we have included in this Annual Report on Form 10-K for periods ending on or prior to June 30, 2009 may not reflect what our results of operations, financial position and cash flows would have been had we been an independent, publicly traded company during the periods presented or what our results of operations, financial position and cash flows are as an independent company. This is primarily because:
| • | such historical financial information reflects allocations for services historically provided to us by Myriad Genetics, which allocations may not reflect the costs we now incur for similar services as an independent company; and |
| • | such historical financial information does not reflect changes that resulted from our separation from Myriad Genetics, including changes in the cost structure, personnel needs, financing and operations of the contributed businesses as a result of the separation from Myriad Genetics and from reduced economies of scale. |
Following the separation, we are now responsible for the additional costs associated with being an independent, public company, including costs related to corporate governance and listed and registered securities. Therefore, our financial statements for periods prior to June 30, 2009 may not be indicative of our performance as an independent company. For additional information about our past financial performance and the basis of presentation of our financial statements, please see “Selected Historical Financial Data,” “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the notes thereto included elsewhere in this Annual Report on Form 10-K.
If the distribution of our common stock or certain internal transactions undertaken in connection with the separation are determined to be taxable for U.S. federal income tax purposes, we, our stockholders that are subject to U.S. federal income tax, and Myriad Genetics could incur significant U.S. federal income tax liabilities.
Myriad Genetics received a private letter ruling from the Internal Revenue Service regarding the U.S. federal income tax consequences of the distribution of our common stock to the Myriad Genetics stockholders substantially to the effect that the distribution, except for cash received in lieu of a fractional share of our common stock, qualified as tax-free under Sections 368(a)(1)(D) and 355 of the Internal Revenue Code of 1986, or the Code. The private letter ruling also provides that certain internal transactions undertaken in anticipation of the separation qualified for favorable treatment under the Code. The private letter ruling relied on certain facts and assumptions, and certain representations and undertakings, from us and Myriad Genetics regarding the past and future conduct of our respective businesses and other matters. Notwithstanding the private letter ruling, the Internal Revenue Service could determine on audit that the distribution or the internal transactions should be treated as taxable transactions if it determines that any of these facts, assumptions, representations or undertakings is not correct or has been violated, or that the distributions should be taxable for other reasons, including as a result of significant changes in stock or asset ownership after the distribution. If the distribution ultimately is determined to be taxable, the distribution could be treated as a taxable dividend or capital gain to stockholders who received our common stock in the separation for U.S. federal income tax purposes, and such stockholders could incur significant U.S. federal income tax liabilities. In addition, Myriad Genetics would recognize gain in an amount equal to the excess of the fair market value of our common stock distributed to Myriad Genetics stockholders on the distribution date over Myriad Genetics’ tax basis in such common shares. We and Myriad Genetics would incur significant U.S. federal income tax liabilities if it is ultimately determined that certain internal transactions undertaken in anticipation of the separation should be treated as taxable transactions.
33
Table of Contents
In addition, under the terms of the Tax Sharing Agreement we entered into with Myriad Genetics, in the event the distribution or the internal transactions were determined to be taxable and such determination was the result of actions taken after the distribution by us or Myriad Genetics, the party responsible for such failure would be responsible for all taxes imposed on us or Myriad Genetics as a result thereof. Such tax amounts could be significant.
Risks Related to Our Common Stock
Our stock price has been and is likely to continue to be volatile and the market price of our common stock may drop.
On June 12, 2009, trading of shares of our common stock began on The NASDAQ Global Market on a “when-issued” basis and has continued on a “regular” basis since July 1, 2009. However, there can be no assurance that an active trading market for our common stock will continue or be sustained in the future. There is a limited history on which to gauge the volatility of our stock price; however, since our common stock began “regular” trading on The NASDAQ Global Market on July 1, 2009 through June 30, 2011, our stock price has fluctuated from a low of $3.29 to a high of $6.81. Furthermore, the stock market has recently experienced significant volatility, particularly with respect to pharmaceutical, biotechnology, and other life sciences company stocks. The volatility of pharmaceutical, biotechnology, and other life sciences company stocks often does not relate to the operating performance of the companies represented by the stock. We cannot predict the prices at which our common stock may trade in the future. The market price of our common stock may continue to fluctuate widely, depending upon many factors, some of which may be beyond our control, including:
| • | progress in and results from our clinical trials of MPC-3100; |
| • | failure or delays in advancing our preclinical drug candidates, or other drug candidates we may develop in the future, into clinical trials; |
| • | results of clinical trials conducted by others on drugs that would compete with our drug candidates; |
| • | issues in manufacturing our drug candidates or approved products; |
| • | regulatory developments or enforcement in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | introduction of technological innovations or new commercial products by us or our competitors; |
| • | changes in estimates or recommendations by securities analysts, if any cover our common stock; |
| • | failure to secure adequate capital to fund our operations if and when needed, or the issuance of equity securities at prices below the current market price; |
| • | public concern over our drug candidates or any approved products; |
| • | litigation; |
| • | future sales of our common stock; |
| • | general market conditions; |
| • | changes in the structure of healthcare payment systems; |
| • | failure of any of our drug candidates, if approved, to achieve commercial success; |
| • | economic and other external factors or other disasters or crises; |
| • | period-to-period fluctuations in our financial results; and |
| • | overall fluctuations in U.S. equity markets. |
34
Table of Contents
These and other external factors may cause the market price and demand for our common stock to fluctuate substantially, which may limit or prevent investors from readily selling their shares of common stock and may otherwise negatively affect the liquidity of our common stock. In the past, when the market price of a stock has been volatile, holders of that stock have instituted securities class action litigation against the company that issued the stock. If any of our stockholders brought a lawsuit against us, we could incur substantial costs defending the lawsuit. Such a lawsuit could also divert the time and attention of our management.
Provisions of our charter and bylaws and Delaware law and our shareholder rights agreement, or poison pill, may make an acquisition of us or a change in our management more difficult.
Certain provisions of our restated certificate of incorporation and restated bylaws could discourage, delay, or prevent a merger, acquisition, or other change in control that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. Stockholders who wish to participate in these transactions may not have the opportunity to do so. These provisions also could limit the price that investors might be willing to pay for shares of our common stock, thereby depressing the market price of our common stock. Furthermore, these provisions could prevent or frustrate attempts by our stockholders to replace or remove our management. These provisions:
| • | allow the authorized number of directors to be changed only by resolution of our board of directors; |
| • | establish a classified board of directors, providing that not all members of our board be elected at one time; |
| • | authorize our board of directors to issue without stockholder approval blank check preferred stock; |
| • | require that stockholder actions must be effected at a duly called stockholder meeting and prohibit stockholder action by written consent; |
| • | establish advance notice requirements for stockholder nominations to our board of directors or for stockholder proposals that can be acted on at stockholder meetings; |
| • | limit who may call stockholder meetings; and |
| • | require the approval of the holders of 80% of the outstanding shares of our capital stock entitled to vote in order to amend certain provisions of our restated certificate of incorporation and restated bylaws. |
We have also implemented a shareholder rights plan (commonly known as a “poison pill”), which could make it uneconomical for a third party to acquire us on a hostile basis. In addition, because we are incorporated in Delaware, we are governed by the provisions of Section 203 of the Delaware General Corporation Law, which may, unless certain criteria are met, prohibit large stockholders, in particular those owning 15% or more of our outstanding voting stock, from merging or combining with us for a prescribed period of time.
We do not anticipate paying cash dividends, and accordingly, stockholders must rely on stock appreciation for any return on their investment.
We currently anticipate that we will retain future earnings for the development, operation and expansion of our business and do not anticipate declaring or paying any cash dividends for the foreseeable future. Therefore, the success of an investment in shares of our common stock will depend upon any future appreciation in their value. There is no guarantee that shares of our common stock will appreciate in value or even maintain the price at which our stockholders have purchased their shares.
A failure to maintain adequate internal control over financial reporting in accordance with Section 404 of the Sarbanes-Oxley Act of 2002 or prevent or detect material misstatements in our annual or interim consolidated financial statements in the future could materially harm our business and cause our stock price to decline.
As a public company, our internal control over financial reporting is required to comply with the standards adopted by the Public Company Accounting Oversight Board in compliance with the requirements of
35
Table of Contents
Section 404 of the Sarbanes-Oxley Act of 2002. Accordingly, we are currently required to document and test our internal controls and procedures to assess the effectiveness of our internal control over financial reporting. In addition, our independent registered public accounting firm is currently required to report on management’s assessment of the effectiveness of our internal control over financial reporting and the effectiveness of our internal control over financial reporting. If we are unable to maintain effective control over financial reporting, such conclusion would be disclosed in this and/or subsequent Annual Reports on Form 10-K. In the future, we may identify material weaknesses and deficiencies which we may not be able to remediate in a timely manner. If we fail to maintain effective internal control over financial reporting in accordance with Section 404, we will not be able to conclude that we have and maintain effective internal control over financial reporting or our independent registered accounting firm may not be able to issue an unqualified report on the effectiveness of our internal control over financial reporting. As a result, our ability to report our financial results on a timely and accurate basis may be adversely affected, we may be subject to sanctions or investigation by regulatory authorities, including the SEC or The NASDAQ Global Market and investors may lose confidence in our financial information, which in turn could cause the market price of our common stock to decrease. We may also be required to restate our financial statements from prior periods. In addition, testing and maintaining internal control in accordance with Section 404 requires increased management time and resources. Any failure to maintain effective internal control over financial reporting could impair the success of our business and harm our financial results.
| Item 1B. | UNRESOLVED STAFF COMMENTS |
Not applicable.
| Item 2. | PROPERTIES |
Our headquarters and facilities are located in Salt Lake City, Utah. We currently lease 87,000 square feet of office and laboratory space from Myriad Genetics, Inc., our former parent company, under a sublease with an initial term expiring January 2013 renewable at our election for a total of an additional 12 years in three-year increments.
We believe our existing facilities and equipment are well maintained and in good working condition and that our current facilities will provide adequate capacity and that additional space, if needed, will be available in the future on commercially reasonable terms.
| Item 3. | LEGAL PROCEEDINGS |
We are currently not a party to any legal proceedings that we believe will have a material impact on our financial position or results of operations.
| Item 4. | REMOVED AND RESERVED |
36
Table of Contents
PART II
| Item 5. | MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on The NASDAQ Global Market under the symbol “MYRX.” The following table sets forth the high and low sales prices for our common stock as reported by The NASDAQ Global Market for the periods indicated.
| High | Low | |||||||
| Fiscal Year Ended June 30, 2011: |
||||||||
| Fourth Quarter |
$ | 4.52 | $ | 3.29 | ||||
| Third Quarter |
$ | 4.26 | $ | 3.72 | ||||
| Second Quarter |
$ | 4.50 | $ | 3.57 | ||||
| First Quarter |
$ | 3.98 | $ | 3.56 | ||||
| High | Low | |||||||
| Fiscal Year Ended June 30, 2010: |
||||||||
| Fourth Quarter |
$ | 5.25 | $ | 3.70 | ||||
| Third Quarter |
$ | 5.43 | $ | 4.19 | ||||
| Second Quarter |
$ | 6.81 | $ | 4.75 | ||||
| First Quarter |
$ | 6.80 | $ | 3.81 | ||||
Stockholders
As of September 6, 2011, there were approximately 105 stockholders of record of our common stock and, according to our estimates, approximately 10,861 beneficial owners of our common stock.
Dividends
We have not paid cash dividends to our stockholders since our inception and we do not plan to pay cash dividends in the foreseeable future. We currently intend to retain earnings, if any, to finance our growth.
Unregistered Sales of Securities
None.
Issuer Purchases of Equity Securities
None.
37
Table of Contents
Stock Performance Graph
The graph set forth below compares the annual percentage change in our cumulative total stockholder return on our common stock, during a period commencing on June 12, 2009 (the first day of trading of our common stock on The NASDAQ Global Market) and ending on June 30, 2011 (as measured by dividing (A) the difference between our share price at the end and the beginning of the measurement period; by (B) our share price at the beginning of the measurement period) with the cumulative total return of The NASDAQ Stock Market, Inc. and the NASDAQ Biotech Index during such period. We have not paid any cash dividends on our common stock, and we do not include cash dividends in the representation of our performance. The price of a share of common stock is based upon the closing price per share as quoted on The NASDAQ Global Market on the last trading day of the year shown. The graph lines merely connect quarter-end values and do not reflect fluctuations between those dates. The comparison assumes $100 was invested on June 12, 2009 in our common stock and in each of the foregoing indices. The comparisons shown in the graph below are based upon historical data. We caution that the stock price performance shown in the graph below is not necessarily indicative of, nor is it intended to forecast, the potential future performance of our common stock.
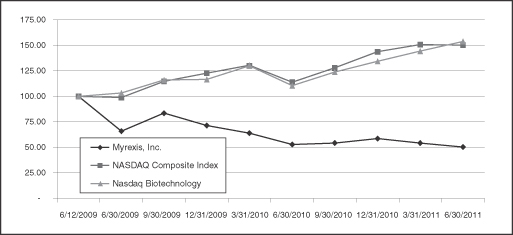
| 06/12/2009 | 06/30/2009 | 09/30/2009 | 12/31/2009 | 03/31/2010 | 06/30/2010 | 09/30/2010 | 12/31/2010 | 03/31/2011 | 06/30/2011 | |||||||||||||||||||||||||||||||
| Myrexis, Inc. |
100.00 | 66.43 | 83.71 | 71.86 | 64.57 | 53.71 | 55.14 | 59.43 | 55.00 | 51.14 | ||||||||||||||||||||||||||||||
| NASDAQ Biotechnology Index |
100.00 | 103.19 | 115.59 | 116.13 | 129.27 | 110.13 | 123.26 | 133.56 | 143.29 | 152.59 | ||||||||||||||||||||||||||||||
| NASDAQ Composite Index |
100.00 | 98.72 | 114.18 | 122.08 | 129.69 | 113.47 | 127.43 | 142.72 | 149.62 | 149.21 | ||||||||||||||||||||||||||||||
The performance graph shall not be deemed to be incorporated by reference by means of any general statement incorporating by reference this Form 10-K into any filing under the Securities Act of 1933, as amended or the Securities Exchange Act of 1934, as amended, except to the extent that we specifically incorporate such information by reference, and shall not otherwise be deemed filed or soliciting material under such acts.
38
Table of Contents
| Item 6. | SELECTED FINANCIAL DATA |
The following table sets forth selected financial information as of and for each of the years in the five-year period ended June 30, 2011, which has been derived from our (1) audited financial statements as of June 30, 2011and 2010 and for the years ended June 30, 2011, 2010 and 2009, which are included elsewhere in this Form 10-K; and (2) audited financial statements as of June 30, 2009, 2008 and 2007 and for the years ended June 30, 2008 and 2007, which are not included elsewhere in this Form 10-K. Because our historical financial information for periods ending on or prior to June 30, 2009 reflects allocations for services historically provided to us by Myriad Genetics, the selected financial information presented below for such periods may not be indicative of our results of operations and financial position as an independent company. The selected financial information presented for the years ended June 30, 2011 and 2010, reflects our performance as an independent company. See “Risk Factors—Risks Relating to Our Separation from Myriad Genetics.”
The selected financial data below should be read in conjunction with our audited financial statements (and notes thereon) and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” included elsewhere in this Form 10-K.
| Years ended June 30, | ||||||||||||||||||||
| In thousands (except per share data) | 2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||||||
| Statement of Operations Data: |
||||||||||||||||||||
| Research revenue |
$ | 185 | $ | 90 | $ | 5,456 | $ | 6,774 | $ | 11,841 | ||||||||||
| Pharmaceutical revenue |
— | — | — | 100,000 | (1) | — | ||||||||||||||
| Other revenue |
— | — | — | 4,000 | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total revenues |
185 | 90 | 5,456 | 110,774 | 11,841 | |||||||||||||||
| Costs and expenses: |
||||||||||||||||||||
| Research and development expense |
22,296 | 28,222 | 54,611 | 121,526 | (2) | 94,929 | ||||||||||||||
| Selling, general and administrative expense |
17,239 | 19,984 | 8,981 | (7) | 20,600 | 10,250 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total costs and expenses |
39,535 | 48,206 | 63,592 | 142,126 | 105,179 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Operating loss |
(39,350 | ) | (48,116 | ) | (58,136 | ) | (31,352 | ) | (93,338 | ) | ||||||||||
| Other income (expense), net |
642 | 1,165 | — | (3,017 | )(3) | 653 | ||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss |
$ | (38,708 | ) | $ | (46,951 | ) | $ | (58,136 | ) | $ | (34,369 | ) | $ | (92,685 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Net loss per basic and diluted share (4) |
$ | (1.52 | ) | $ | (1.91 | ) | $ | (2.43 | ) | $ | (1.43 | ) | $ | (3.87 | ) | |||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| As of June 30, | ||||||||||||||||||||
| In thousands | 2011 | 2010 | 2009 | 2008 | 2007 | |||||||||||||||
| Balance Sheet Data: |
||||||||||||||||||||
| Cash, cash equivalents and marketable securities (5) |
$ | 115,878 | $ | 147,453 | $ | 188,005 | $ | — | $ | — | ||||||||||
| Current liabilities |
3,310 | 4,250 | 4,576 | 46,568 | 10,875 | |||||||||||||||
| Total assets |
121,260 | 154,108 | 193,677 | 15,746 | 16,244 | |||||||||||||||
| Total shareholders’ / parent equity (6) |
117,950 | 149,858 | 189,101 | (30,822 | ) | 5,369 | ||||||||||||||
| (1) | Represents a nonrefundable upfront payment from A/S Lundbeck for the former drug candidate Flurizan. |
| (2) | Amount includes an accrued $20 million sublicense fee payable related to Flurizan. |
| (3) | Amount includes the write-off of the cost basis investment in Encore Pharmaceuticals. |
| (4) | For years ended June 30, 2009, 2008 and 2007, pro forma net loss per share calculated based on the 23,974,211 shares issued in connection with the spin-off. |
| (5) | Prior to June 30, 2009, all cash and investments were held and managed by Myriad Genetics. |
| (6) | Balances prior to June 30, 2009 represent Myriad Genetics’ net investment (or capital deficiency) in Myrexis. |
| (7) | Amount includes a $9.0 million credit recorded in fiscal 2009, resulting from the difference in an estimated sub-license fee accrual recorded in fiscal 2008 and amounts actually paid in 2009. |
39
Table of Contents
Quarterly Financial Data (Unaudited)
| Quarter Ended | ||||||||||||||||
| In thousands | June 30, 2011 |
March 31, 2011 |
December 31, 2010 |
September 30, 2010 |
||||||||||||
| Research revenue |
$ | — | $ | 55 | $ | 23 | $ | 107 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
— | 55 | 23 | 107 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and expenses: |
||||||||||||||||
| Research and development expense |
3,651 | 7,935 | 4,995 | 5,715 | ||||||||||||
| Selling, general, and administrative expense |
3,349 | 5,088 | 4,240 | 4,562 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
7,000 | 13,023 | 9,235 | 10,277 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(7,000 | ) | (12,968 | ) | (9,212 | ) | (10,170 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income (expense), net |
(992 | )(1) | 125 | 1,349 | (2) | 160 | ||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (7,992 | ) | $ | (12,843 | ) | $ | (7,863 | ) | $ | (10,010 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| Quarter Ended | ||||||||||||||||
| June 30, 2010 |
March 31, 2010 |
December 31, 2009 |
September 30, 2009 |
|||||||||||||
| Research revenue |
$ | — | $ | 30 | $ | — | $ | 60 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total revenue |
— | 30 | — | 60 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Costs and expenses: |
||||||||||||||||
| Research and development expense |
6,935 | 7,190 | 8,217 | 5,880 | ||||||||||||
| Selling, general, and administrative expense |
894 | (3) | 6,926 | 6,928 | 5,236 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total costs and expenses |
7,829 | 14,116 | 15,145 | 11,116 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Operating loss |
(7,829 | ) | (14,086 | ) | (15,145 | ) | (11,056 | ) | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Other income, net |
22 | 363 | 355 | 425 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Net loss |
$ | (7,807 | ) | $ | (13,723 | ) | $ | (14,790 | ) | $ | (10,631 | ) | ||||
|
|
|
|
|
|
|
|
|
|||||||||
| (1) | Reflects a $1.1 million impairment loss of certain fixed assets. |
| (2) | Reflects a one-time $1.2 million grant received in November 2010 as a part of the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code. |
| (3) | Reflects reimbursement of $1.5 million in stipulated expenses and a $2.9 million termination fee in connection with the proposed merger with Javelin Pharmaceuticals, Inc. that was terminated in April 2010. |
40
Table of Contents
| Item 7. | MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion and analysis should be read together with “Selected Financial Data,” and the financial statements and the related notes appearing elsewhere in this Form 10-K. This discussion and analysis contains forward-looking statements that involve risks, uncertainties and assumptions. Information regarding these forward-looking statements can be found in the preface to Part I, Item 1 “Business” of this Form 10-K. Our actual results may differ materially from those anticipated in these forward-looking statements as a result of certain factors, including, but not limited to, those set forth under “Risk Factors” and elsewhere in this Form 10-K.
Overview
We are a biotechnology company focused on the development of small-molecule compounds with novel chemical structures and distinct mechanisms of action. We have generated a strong pipeline of differentiated product candidates in oncology and autoimmune diseases. We are focused on maximizing the therapeutic and commercial value of these molecules by developing potential first-in-class and/or best-in-class treatment options for patients with unmet needs.
We were incorporated as Myriad Pharmaceuticals, Inc. in Delaware in January 2009 as a new, wholly owned subsidiary of Myriad Genetics, Inc. in order to effect the separation and spin-off of Myriad Genetics’ research and drug development businesses as a stand-alone, independent, publicly traded company. In connection with the formation of this new subsidiary, Myriad Genetics’ existing subsidiary, Myriad Pharmaceuticals, Inc., changed its corporate name to Myriad Therapeutics, Inc., and we adopted the name of Myriad Pharmaceuticals, Inc. which was subsequently changed to Myrexis, Inc. effective July 1, 2010. On June 30, 2009, Myriad Genetics contributed substantially all of the assets and certain liabilities of its research and drug development businesses as well as $188 million in cash and marketable securities to us and effected the spin-off of our company through a pro rata dividend distribution to its stockholders of all outstanding shares of our common stock.
We operate in one reportable operating segment, drug development.
During the years ended June 30, 2011 and 2010, we reported $185,000 and $90,000, respectively in revenues associated with research services related to short-term research agreements and a net loss of $38.7 million and $47.0 million, respectively.
In June 2010, we announced several strategic initiatives to focus our efforts on our oncology pipeline and to conserve our financial resources, including a reduction in workforce affecting 21 employees.
On March 29, 2011, we announced a corporate reorganization to focus resources on our current portfolio of clinical and preclinical drug candidates. The reorganization included an immediate reduction in our workforce by 57 employees or approximately 41%. The reduction was primarily in our internal drug discovery group and related support functions. In addition, we have stopped all contract research services activity. In connection with the reorganization, we recorded severance costs of approximately $3.0 million in the three months ended March 31, 2011, which were fully paid before June 30, 2011. These expenses reflected in the statement of operations include $0.5 million in general and administrative and $2.5 million in research and development for year ended June 30, 2011.
In September 2011, we announced we had completed an in-depth review of our drug development pipeline, incorporating extensive inputs from both internal and independent external analyses. As a result, we made a strategic business decision to suspend any further development of our lead drug candidate Azixa, which is in Phase 2 development for the treatment of advanced primary and metastatic tumors with brain involvement. This decision was not based on any single factor. Our review took into consideration the accumulated data from our clinical trials to date, the evolving competitive environment in GBM, including ongoing studies of competitive
41
Table of Contents
drug candidates that are in more advanced stages of development, inputs from key opinion leaders, updated cost and timing estimates, and other factors affecting the risks and opportunities relating to the development of Azixa. On the basis of these inputs, we concluded that completing the Phase 2b clinical trial would require a disproportionate investment of time and resources relative to its likelihood of technical and regulatory success, when compared to our other programs.
We expect to incur significant net losses for the foreseeable future and that such losses will fluctuate from quarter to quarter and that such fluctuations may be substantial.
Our drug research and development expenses include costs incurred for our drug candidates Currently, the only costs we track by each drug candidate are external costs such as services provided to us by clinical research organizations, manufacturing of drug supply, and other outsourced research. We do not assign or allocate internal costs such as salaries and benefits, facilities costs, lab supplies and the costs of preclinical research and studies to individual development programs. We also incurred costs related to external research collaborations from our research services business. We track all underlying principal costs associated with our research collaborations. All development costs for our drug candidates and external research collaborations are expensed as incurred. Our research and development expense for Azixa (for which development was recently suspended), our clinical-stage drug candidate, MPC-3100, our preclinical-stage drug candidates, MPC-9528, MPC-8640and MPC-0767, and our discontinued drug candidates MPC-4326, MPC-9055, and Flurizan during the fiscal years ended June 30, 2011, 2010 and 2009 are as follows:
| Years Ended June 30, | ||||||||||||
| (In thousands) | 2011 | 2010 | 2009 | |||||||||
| External costs, drug candidates: |
||||||||||||
| Azixa |
$ | 1,388 | $ | 2,998 | $ | 4,279 | ||||||
| MPC-4326 |
(144 | )(1) | 1,720 | 9,175 | ||||||||
| MPC-3100 |
1,202 | 2,568 | 2,952 | |||||||||
| MPC-0767 |
278 | — | — | |||||||||
| MPC-9055 |
— | — | 2,867 | |||||||||
| MPC-9528 |
264 | 14 | — | |||||||||
| MPC-8640 |
121 | — | — | |||||||||
| Flurizan |
— | — | (9,861 | )(2) | ||||||||
|
|
|
|
|
|
|
|||||||
| Sub-total direct costs |
3,109 | 7,300 | 9,412 | |||||||||
| Internal costs, drug candidates |
5,318 | 5,965 | 7,508 | |||||||||
| Preclinical development costs |
13,157 | 13,812 | 35,677 | |||||||||
| External research collaborations |
712 | 1,145 | 2,014 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total research and development |
$ | 22,296 | $ | 28,222 | $ | 54,611 | ||||||
|
|
|
|
|
|
|
|||||||
| (1) | Amount includes a $0.2 million credit recorded in fiscal 2011 resulting from a favorable change in estimate for outside clinical services which were later terminated due to the discontinuation of the program. |
| (2) | Amount includes a $9.0 million credit recorded in fiscal 2009, resulting from the difference in an estimated sub-license fee accrual recorded in fiscal 2008 and amounts actually paid in 2009. |
The timing and amount of any future expenses, completion dates, and revenues for our drug candidates is not readily determinable due to the early stage of these development programs.
We do not know if we will be successful in developing any of our drug candidates. While expenses associated with the completion of our current clinical programs are expected to be substantial and increase, we believe that accurately projecting total program-specific expenses through commercialization is not possible at this time. The timing and amount of these expenses will depend upon the costs associated with potential future clinical trials of our drug candidates, and the related expansion of our development organization, regulatory
42
Table of Contents
requirements, advancement of our preclinical programs and product manufacturing costs, many of which cannot be determined with accuracy at this time. We are also unable to predict when, if ever, material net cash inflows will commence from our drug candidates. This is due to the numerous risks and uncertainties associated with the duration and cost of clinical trials, which vary significantly over the life of a project as a result of unanticipated events arising during clinical development, including:
| • | the scope, rate of progress, and expense of our clinical trials and other development activities; |
| • | the length of time required to enroll suitable subjects; |
| • | the number of subjects that ultimately participate in the trials; |
| • | the efficacy and safety results of our clinical trials and the number of additional required clinical trials; |
| • | the terms and timing of regulatory approvals; |
| • | our ability to market, commercialize, manufacture and supply, and achieve market acceptance for our drug candidates that we are developing or may develop in the future; and |
| • | the filing, prosecuting, defending or enforcement of any patent claims or other intellectual property rights. |
A change in the outcome of any of the foregoing variables in the development of a drug candidate could mean a significant change in the costs and timing associated with the development of that drug candidate. For example, if the FDA or another regulatory authority were to require us to conduct clinical trials beyond those which we currently anticipate to complete clinical development of a drug candidate, or if we experience significant delays in the enrollment of patients in any of our clinical trials, we would be required to expend significant additional financial resources and time on the completion of clinical development.
Critical Accounting Policies and Use of Estimates
Critical accounting policies are those policies which are both important to the portrayal of a company’s financial condition and results and require management’s most difficult, subjective or complex judgments, often as a result of the need to make estimates about the effect of matters that are inherently uncertain. Our critical accounting policies are as follows:
| • | income taxes; |
| • | clinical trial expenses; |
| • | share-based payment expense; and |
| • | impairment of long-lived assets. |
Income Taxes
Our income tax provision is based on income before taxes and is computed using the liability method in accordance with Accounting Standards Codification, or ASC, 740—Income Taxes. Deferred tax assets and liabilities are determined based on the difference between the financial statement and tax basis of assets and liabilities using tax rates projected to be in effect for the year in which the differences are expected to reverse. Significant estimates are required in determining our provision for income taxes. Some of these estimates are based on interpretations of existing tax laws or regulations, or the expected results from any future tax examinations. Various internal and external factors may have favorable or unfavorable effects on our future provision for income taxes. Those factors include, but are not limited to, changes in tax laws, regulations and/or rates, the results of any future tax examinations, changing interpretations of existing tax laws or regulations, changes in estimates of prior years’ items, past levels of R&D spending, acquisitions, changes in our corporate structure, and changes in overall levels of income before taxes all of which may result in periodic revisions to our provision for income taxes.
43
Table of Contents
Developing our provision for income taxes, including our effective tax rate and analysis of potential uncertain tax positions, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowance we deem necessary to offset deferred tax assets. We have established a valuation allowance to fully offset our deferred tax assets. Our judgment and tax strategies are subject to audit by various taxing authorities. While we believe we have provided adequately for our uncertain income tax positions in our consolidated financial statements, an adverse determination by these taxing authorities could have a material adverse effect on our consolidated financial condition, results of operations or cash flows. Interest and penalties on income tax items are included as a component of overall income tax expense.
Clinical Trial Expenses
The cost of our clinical trials is based, in part, on estimates of the services received and efforts expended pursuant to contracts with numerous clinical trial centers and clinical research organizations, or the CROs. In the normal course of business, we contract with third parties to perform various clinical trial activities in the ongoing development of our drug candidates. The financial terms of these agreements vary from contract to contract, are subject to negotiation and may result in uneven payment flows. Payments under the contracts depend on factors such as the achievement of certain events, the successful enrollment of patients or the completion of portions of the clinical trial or similar conditions. The objective of our accrual policy is to match the recording of expenses in our financial statements to the actual services received and efforts expended. As such, we recognize direct expenses related to each patient enrolled in a clinical trial on an estimated cost-per-patient basis as services are performed. In addition to considering information from our clinical operations group regarding the status of our clinical trials, we rely on information from CROs, such as estimated costs per patient, to calculate our accrual for direct clinical expenses at the end of each reporting period. For indirect expenses, which relate to site and other administrative costs to manage our clinical trials, we rely on information provided by the CRO, including costs incurred by the CRO as of a particular reporting date, to calculate our indirect clinical expenses. In the event of early termination of a clinical trial, we would recognize expenses in an amount based on our estimate of the remaining non-cancelable obligations associated with the winding down of the clinical trial, which we would confirm directly with the CRO.
If our CROs were to either under or over report the costs that they have incurred or if there is a change in the estimated per patient costs, it could have an impact on our clinical trial expenses during the period in which they report a change in estimated costs to us. Adjustments to our clinical trial accruals primarily relate to indirect costs, for which we place significant reliance on our CROs for accurate information at the end of each reporting period.
Share-Based Payment Expense
Share-based compensation expense standards set accounting requirements for “share-based” compensation to employees, including employee stock purchase plans, and require us to recognize, as expense, in our statements of operations, the grant date fair value of our stock options and other equity-based compensation. The determination of grant date fair value is estimated using an option-pricing model, which includes variables such as the terms of each grant, the expected volatility of our share price, the exercise behavior of our employees, interest rates, and dividend yields. These variables are projected based on our historical data, experience, and other factors. Changes in any of these variables could result in material adjustments to the expense recognized for share-based payments.
In connection with the separation and spin-off from Myriad Genetics and related transactions, each outstanding Myriad Genetics stock option was converted into an adjusted Myriad Genetics common stock option, exercisable for the same number of shares of common stock as the original Myriad Genetics option, and a new Myrexis common stock option, exercisable for one-fourth of the number of shares of common stock as the original Myriad Genetics option. An adjusted exercise price of each converted option was determined in
44
Table of Contents
accordance with Section 409A and Section 422 of the Internal Revenue Code of 1986, as amended. All other terms of the converted options remain the same however; the vesting and expiration of the converted options will be based on the optionholder’s continuing employment with Myriad Genetics or Myrexis, as applicable, following the separation.
As a result of the option modifications that occurred in connection with the separation from Myriad Genetics, Myriad Genetics measured the potential accounting impact of these option modifications. Based upon the analysis, which included a comparison of the fair value of the modified options granted to our employees and directors immediately after the modification with the fair value of the original option immediately prior to the modification, it was determined that there was no incremental compensation expense. All unrecognized compensation expense at June 30, 2009 that is related to Myriad Genetics options and Myrexis options that are held by current Myrexis employees and directors will be recognized by us over the remaining vesting term of the option. All such expense relating to Myrexis options held by current and former Myriad Genetics employees, directors or consultants will not be recognized by us.
Impairment of Long-Lived Assets
We assess the impairment of long-lived assets when events or changes in circumstances indicate that the carrying value of the assets or the asset grouping may not be recoverable. Factors that we consider in deciding when to perform an impairment review include significant negative industry or economic trends, and significant changes or planned changes in our use of the assets. We measure the recoverability of assets that will continue to be used in our operations by comparing the carrying value of the asset grouping to our estimate of the related total future undiscounted net cash flows. If an asset grouping’s carrying value is not recoverable through the related undiscounted cash flows, the asset grouping is considered to be impaired. The impairment is measured by comparing the difference between the asset grouping’s carrying value and its fair value. Fair value is the price that would be received from selling an asset in an orderly transaction between market participants at the measurement date. Long-lived assets such as intangible assets and property, plant and equipment are considered non-financial assets, and are recorded at fair value only when an impairment charge is recognized.
Results of Operations
The balance sheets as of June 30, 2011 and 2010, and notes related thereto reflect the balances of Myrexis as an independent company. For all periods prior to June 30, 2010, amounts reflected in the results of operations are components of Myriad Genetics that constituted the research and drug development businesses that were separated. Those financial statements have been prepared using Myriad Genetics’ historical costs basis of the assets and liabilities of the various activities that reflect the combined results of operations, financial condition and cash flows of us as a component of Myriad Genetics. Specific costs attributable to our operations have been included in the financial statements. The financial statements also include some proportional cost allocations of certain common costs of Myriad Genetics because these expenses were not specifically identified at the subsidiary level. The basis of these allocations includes full-time equivalent employees for the respective periods presented, square footage, and other appropriate allocation drivers.
The financial information for the years ended June 30, 2011 and 2010, reflects the financial position, results of operations and cash flows for Myrexis as a stand-alone entity. The financial information for the year ended June 30, 2009 in the financial statements reflects allocations for services historically provided to us by Myriad Genetics and does not include all of the expenses that would have been incurred had we been a separate, stand-alone publicly traded entity, including, but not limited to, costs to implement accounting, human resource, payroll, purchasing, information technology, legal and other business functions and systems.
45
Table of Contents
Years ended June 30, 2011 and 2010
Research revenue is comprised of research services related to short-term research agreements. Research revenue for the fiscal year ended June 30, 2011 was $185,000 compared to $90,000 in the prior year. The 105% increase in research revenue was primarily attributable to increased research services related to short-term research agreements that were completed during the 2011 fiscal year. As a result of the March 2011 corporate reorganization, we have stopped all contract research services activity going forward.
Research and development expenses are comprised primarily of salaries and related personnel costs, laboratory supplies, equipments costs, and costs associated with our clinical trials. Research and development expenses for the fiscal year ended June 30, 2011 were $22.3 million compared to $28.2 million in 2010. This 21% decrease was primarily due to:
| • | decreased internal preclinical developments costs of approximately $0.7 million resulting from a reduction in headcount; |
| • | decreased external drug candidate costs associated with our HIV drug candidate of $1.9 million, decreased costs of $1.6 million associated with the development of Azixa and the timing of the Phase 2b trial initiation, and decreased costs of $1.4 million associated with MPC-3100 due to the completion of current studies; and |
| • | decreased external research collaboration costs of $0.4 million associated with a reduction in headcount. |
We expect our research and development expenses will fluctuate over the next several years as we conduct additional clinical trials to support the clinical development of our Hsp90 inhibitor program, and advance other drug candidates into clinical development. We expect to see reduced external drug development costs as a result of the decision to suspend further development of Azixa which will be partially offset by increased costs associated with our preclinical-stage drug candidates. We expect to see a reduction in internal development expenses related to salaries and benefits and lab supplies as a result of the March 2011 corporate reorganization.
General and administrative expenses consist primarily of salaries and related personnel costs for business development, executive, legal, finance and accounting, information technology, human resources, and allocated facilities expenses. General and administrative expenses for the fiscal year ended June 30, 2011 were $17.2 million, compared to $20.0 million in 2010. The decrease in general and administrative expenses of 14% was due primarily to a decrease in expenses as a result of a reduction in headcount in June 2010. We incurred $3.1 million in external acquisition expenses in connection with the proposed merger with Javelin Pharmaceuticals, Inc. that was terminated in April 2010. These expenses were offset by $1.5 million in stipulated expenses reimbursed by Javelin plus a termination fee of $2.9 million. These reimbursed expenses are presented in the financials for the year ended June 30, 2010, as an offset to total general and administrative costs. We expect our general and administrative expenses to decrease as a result of the March 2011 corporate reorganization.
Other income (expense) for the fiscal year ended June 30, 2011 was $0.6 million compared to $1.2 for the fiscal year ended June 30, 2010. Other income reflects interest income and realized gains on our marketable securities offset by a loss on impairment of assets of $1.1 million and $0.2 million for the years ended June 30, 2011 and 2010, respectively. Other income for the year ended June 30, 2011, includes a $1.2 million one-time grant received in November 2010 as a part of the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code.
Years ended June 30, 2010 and 2009
Research revenue for the fiscal year ended June 30, 2010 was $90,000 compared to $5.5 million in the prior year. The 98% decrease in research revenue was primarily attributable to the completion of genomic sequencing research collaboration and a long-term contract that were completed during the 2009 fiscal year. Research revenue from our research collaboration agreements is recognized using a proportional performance methodology.
46
Table of Contents
Research and development expenses for the fiscal year ended June 30, 2010 were $28.2 million compared to $54.6 million in 2009. This 48% decrease was primarily due to:
| • | decreased external drug development costs of approximately $7.5 million resulting from the discontinuance of our HIV drug candidate MPC-4326, |
| • | decreased external drug development costs of approximately $1.3 million resulting from the timing of studies related to Azixa; |
| • | a decrease of $2.9 million in expenditures relating to MPC-9055, a compound that did not transfer from Myriad Genetics to Myrexis in connection with the spin-off; |
| • | a $9.0 million credit from the former drug candidate Flurizan recorded in fiscal 2009 resulting from the difference in an estimated sublicense fee accrual recorded in fiscal 2008 and amounts actually paid in 2009; and |
| • | a decrease of approximately $21.9 million in preclinical development costs as a result of a reduction in headcount. |
General and administrative expenses for the fiscal year ended June 30, 2010 were $20.0 million, compared to $9.0 million in 2009. The increase in general and administrative expenses of 122% was due primarily to an increase in expenses associated with being a separate, stand-alone publicly traded entity, including, but not limited to, costs to implement accounting, human resource, payroll, purchasing, information technology, legal and other business functions and systems. In addition to these certain costs, we incurred $3.1 million in external acquisition expenses in connection with the proposed merger with Javelin Pharmaceuticals, Inc. that was terminated in April 2010. These expenses were offset by $1.5 million in stipulated expenses reimbursed by Javelin plus a termination fee of $2.9 million. These reimbursed expenses are presented in the financials for the year ended June 30, 2010, as an offset to total general and administrative costs.
Other income (expense) for the fiscal year ended June 30, 2010 was $1.2 million compared to $0.0 for the fiscal year ended June 30, 2009. Other income reflects interest income and realized gains on our marketable securities offset by a loss on disposal of assets. Prior to June 30, 2009, all cash and investments were held and managed by Myriad Genetics and, as a result, we recognized no interest income in our financial statements.
Liquidity and Capital Resources
Net cash used in operating activities was $33.5 million during the fiscal year ended June 30, 2011 compared to $40.4 million used by operating activities during the prior fiscal year. The change in cash flow from operating activity can be attributed primarily to the higher net loss in fiscal 2010 offset, in part, by higher non-cash charges associated with share-based compensation recorded in fiscal 2010.
Our investing activities provided $14.8 million in cash during the fiscal year ended June 30, 2011 compared to $54.4 million used during the prior fiscal year. The change is primarily due to the maturities and selling of our marketable investment securities. In addition, we invested $2.1 million in capital expenditures during the fiscal year ended June 30, 2010 in leasehold improvements, furniture and equipment for the new facility that we took occupancy of in January 2010 compared to $0.1 million during the fiscal year ended June 30, 2011. We anticipate our investment in additional equipment and leasehold improvements will be minimal in the future.
Approximately $1.9 million in cash was provided by financing activities during fiscal 2011, compared to $2.3 million during the prior fiscal year. Financing activities in fiscal 2011 and 2010 were comprised primarily of cash proceeds from the exercise of stock awards.
As of June 30, 2011, we had $115.9 million in cash, cash equivalents and marketable securities, a decrease of $31.6 million from $147.5 million as of June 30, 2010. We believe that with our existing capital resources, we
47
Table of Contents
will have adequate funds to maintain our current and planned operations through at least June 30, 2014, although no assurance can be given that changes will not occur that would consume available capital resources before such time and we may need or want to raise additional financing within this period of time. Our future capital requirements, cash flows, and results of operations could be affected by and will depend on many factors that are currently unknown to us, including:
| • | progress in and results from our clinical trial of MPC-3100; |
| • | failure or delays in advancing our preclinical drug candidates, or other drug candidates we may develop in the future, into clinical trials; |
| • | results of clinical trials conducted by others on drugs that would compete with our drug candidates; |
| • | issues in manufacturing our drug candidates or approved products; |
| • | regulatory developments or enforcement in the United States and foreign countries; |
| • | developments or disputes concerning patents or other proprietary rights; |
| • | introduction of technological innovations or new commercial products by us or our competitors; |
| • | changes in estimates or recommendations by securities analysts, if any cover our common stock; |
| • | failure to secure adequate capital to fund our operations if and when needed, or the issuance of equity securities at prices below the current market price; |
| • | public concern over our drug candidates or any approved products; |
| • | litigation; |
| • | future sales of our common stock; |
| • | general market conditions; |
| • | changes in the structure of healthcare payment systems; |
| • | failure of any of our drug candidates, if approved, to achieve commercial success; |
| • | economic and other external factors or other disasters or crises; |
| • | period-to-period fluctuations in our financial results; and |
| • | overall fluctuations in U.S. equity markets. |
We may require significant additional funds earlier than we currently expect in order to conduct additional clinical trials and conduct additional preclinical activities. Because of the numerous risks and uncertainties associated with the development and commercialization of our drug candidates, we are unable to estimate the amounts of increased capital outlays and operating expenditures associated with our current and anticipated clinical trials.
To the extent our capital resources are insufficient to meet our future capital requirements, we will need to finance our future cash needs through public or private equity offerings, collaboration agreements, debt financings or licensing arrangements. However, the credit markets and the financial services industry have recently been experiencing a period of unprecedented turmoil and upheaval that have made equity and debt financing more difficult to obtain. Additional funding may not be available to us on acceptable terms or at all. In addition, the terms of any financing may adversely affect the holdings or the rights of our stockholders. For example, if we raise additional funds by issuing equity securities or by selling convertible debt securities, further dilution to our existing stockholders may result. If we raise funds through collaboration agreements or licensing arrangements, we may be required to relinquish rights to our technologies or drug candidates, or grant licenses on terms that are not favorable to us.
48
Table of Contents
If adequate funds are not available, we may be required to terminate, significantly modify or delay our research and development programs, reduce planned commercialization efforts, or obtain funds through collaborators that may require us to relinquish rights to our technologies or drug candidates that we might otherwise seek to develop or commercialize independently. We may elect to raise additional funds even before we need them if the conditions for raising capital are favorable. We have an effective universal shelf registration statement on Form S-3 pursuant to which we have up to $80 million of securities available for issuance.
Off-Balance Sheet Arrangements
None.
Contractual Obligations
The following table represents our contractual obligations as of June 30, 2011 (in thousands):
| Total | Less than one year |
1-3 Years | 4-5 Years | More than 5 years |
||||||||||||||||
| Contractual services |
$ | 3,019 | $ | 460 | $ | 2,559 | $ | — | $ | — | ||||||||||
| Lease obligations |
5,861 | 3,802 | 2,059 | — | — | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
| Total |
$ | 8,880 | $ | 4,262 | $ | 4,618 | $ | — | $ | — | ||||||||||
|
|
|
|
|
|
|
|
|
|
|
|||||||||||
Contractual services represent financial commitments for drug development and clinical trial activities. The expected timing of payment for the obligations listed above is estimated based on currently available information. The actual timing and amount of such payments may differ depending on the timing of goods or services received and other factors. The table above only includes payment obligations that are fixed or determinable. The table excludes potential milestone payments we may be required to pay under license agreements in the aggregate of up to $23 million based on the progress of our drug candidates, as the likelihood and timing of such payments is not yet determinable. The table also excludes royalties payable to third parties based on future sales of any of our drug candidates that may be approved for sale in the future, as the amount, timing, and likelihood of any such payments are unknown.
Effects of Inflation
We do not believe that inflation has had a material impact on our business, revenues, or operating results during the periods presented.
| Item 7A. | QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We maintain a portfolio of cash, cash equivalents and short term and long term marketable securities which are subject to interest rate risk. Our investments consist primarily of highly liquid securities of various types and maturities of two years or less, with a maximum average maturity of 12 months. Changes in interest rates affect the fair market value of these marketable investment securities. After a review of our marketable securities as of June 30, 2011 and 2010, we have determined, hypothetically, that in the event of an increase of 100 basis points in a key market interest rate, the resulting decrease in fair market value of our marketable investment securities would not have a material effect on our financial condition or on our financial statements as a whole.
49
Table of Contents
| Item 8. | FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
The information required by this Item 8 is included at the end of this Annual Report on Form 10-K beginning on page F-1.
| Index to Financial Statements |
Number | |||
| F-2 | ||||
| F-3 | ||||
| Statements of Operations for the Years Ended June 30, 2011, 2010 and 2009 |
F-4 | |||
| F-5 | ||||
| Statements of Cash Flows for the Years Ended June 30, 2011, 2010 and 2009 |
F-6 | |||
| F-7 | ||||
50
Table of Contents
| Item 9. | CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
Not applicable.
| Item 9A. | CONTROLS AND PROCEDURES |
1. Disclosure Controls and Procedures
We maintain disclosure controls and procedures (Disclosure Controls) within the meaning of Rules 13a-15(e) and 15d-15(e) of the Securities Exchange Act of 1934, as amended, or the Exchange Act. Our Disclosure Controls are designed to ensure that information required to be disclosed by us in the reports we file or submit under the Exchange Act, such as this Annual Report on Form 10-K, is recorded, processed, summarized and reported within the time periods specified in the Securities and Exchange Commission’s rules and forms. Our Disclosure Controls are also designed to ensure that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Our Chief Executive Officer and Chief Financial Officer, after evaluating the effectiveness of our Disclosure Controls as of the end of the period covered by this Annual Report on Form 10-K, have concluded that, based on such evaluation, our Disclosure Controls were effective to ensure that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms, and is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
2. Internal Control Over Financial Reporting
(a) Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) promulgated under the Exchange Act as a process designed by, or under the supervision of, a company’s principal executive and principal financial officers and effected by the company’s board of directors, management and other personnel to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with U.S. generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that:
| • | pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; |
| • | provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and |
| • | provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of the company’s assets that could have a material effect on the financial statements. |
Because of those inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Our management assessed the effectiveness of our internal control over financial reporting as of June 30, 2011. In making their assessment, management used the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission (COSO) in Internal Control—Integrated Framework. Based on our assessment, management believes that, as of June 30, 2011, our internal control over financial reporting is effective based on those criteria.
51
Table of Contents
Our independent registered public accounting firm has issued its report on the effectiveness of our internal control over financial reporting. This report appears below.
(b) Attestation Report of the Registered Public Accounting Firm
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Myrexis, Inc.
We have audited Myrexis, Inc.’s internal control over financial reporting as of June 30, 2011, based on criteria established in Internal Control—Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission (the COSO criteria). Myrexis, Inc.’s management is responsible for maintaining effective internal control over financial reporting, and for its assessment of the effectiveness of internal control over financial reporting included in the accompanying Management’s Annual Report on Internal Control over Financial Reporting. Our responsibility is to express an opinion on the company’s internal control over financial reporting based on our audit.
We conducted our audit in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether effective internal control over financial reporting was maintained in all material respects. Our audit included obtaining an understanding of internal control over financial reporting, assessing the risk that a material weakness exists, testing and evaluating the design and operating effectiveness of internal control based on the assessed risk, and performing such other procedures as we considered necessary in the circumstances. We believe that our audit provides a reasonable basis for our opinion.
A company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles. A company’s internal control over financial reporting includes those policies and procedures that (1) pertain to the maintenance of records that, in reasonable detail, accurately and fairly reflect the transactions and dispositions of the assets of the company; (2) provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that receipts and expenditures of the company are being made only in accordance with authorizations of management and directors of the company; and (3) provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use, or disposition of the company’s assets that could have a material effect on the financial statements.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
In our opinion, Myrexis, Inc. maintained, in all material respects, effective internal control over financial reporting as of June 30, 2011, based on the COSO criteria.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), the balance sheets of Myrexis, Inc. as of June 30, 2011 and 2010, and the related statements of operations, stockholders’ equity and comprehensive income (loss), and cash flows for each of the three years in the period ended June 30, 2011, and our report dated September 13, 2011 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Salt Lake City, Utah
September 13, 2011
52
Table of Contents
(c) Change in Internal Control over Financial Reporting
There were no changes in our internal control over financial reporting, identified in connection with the evaluation of such internal control that occurred during the fourth quarter of our last fiscal year that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
| Item 9B. | OTHER INFORMATION |
Not applicable.
53
Table of Contents
PART III
| Item 10. | DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Management and Corporate Governance,” “Section 16(a) Beneficial Ownership Reporting Compliance” and “Code of Conduct and Ethics” in our Proxy Statement for the 2011 Annual Meeting of Stockholders.
| Item 11. | EXECUTIVE COMPENSATION |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Compensation Discussion and Analysis,” “Executive Officer and Director Compensation,” “Management and Corporate Governance-Committees of the Board of Directors and Meetings,” “Management and Corporate Governance-Compensation Committee Interlocks and Insider Participation,” “Compensation Committee Report” and “Risks Related to Compensation Practices and Policies” in our Proxy Statement for the 2011 Annual Meeting of Stockholders.
| Item 12. | SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Security Ownership of Certain Beneficial Owners and Management” and “Equity Compensation Plan Information” in our Proxy Statement for the 2011 Annual Meeting of Stockholders.
| Item 13. | CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
The response to this item is incorporated by reference from the discussion responsive thereto under the captions “Certain Relationships and Related Person Transactions” and “Management and Corporate Governance-Director Independence” in our Proxy Statement for the 2011 Annual Meeting of Stockholders.
| Item 14. | PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The response to this item is incorporated by reference to the discussion responsive thereto in the proposal entitled “Independent Registered Public Accounting Firm” in our Proxy Statement for the 2011 Annual Meeting of Stockholders.
54
Table of Contents
PART IV
| Item 15. | EXHIBITS AND FINANCIAL STATEMENT SCHEDULES |
(a) The following documents are filed as part of this Annual Report on Form 10-K:
1. Financial Statements
See “Index to Financial Statements” at Item 8 of this Annual Report on Form 10-K.
2. Financial Statement Schedules
The financial statements beginning on page F-1 are filed as part of this Annual Report on Form 10-K. Other financial statement schedules have not been included because they are not applicable or the information is included in the financial statements or notes thereto.
3. Exhibits
The exhibits which are filed with or incorporated by reference into this Annual Report on Form 10-K are set forth in the Exhibit Index to this Annual Report on Form 10-K, which is incorporated herein by reference.
55
Table of Contents
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the Registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly authorized, on September 13, 2011.
| MYREXIS, INC. | ||
| By: | /s/ ROBERT J. LOLLINI | |
| Robert J. Lollini | ||
| President and Chief Executive Officer | ||
Pursuant to the requirements of the Securities Exchange Act of 1934, this report has been signed below by the following persons on behalf of the Registrant and in the capacities indicated below and on the dates indicated.
| Signatures |
Title |
Date | ||||
| By: | /s/ ROBERT J. LOLLINI |
President, Chief Executive Officer and | September 13, 2011 | |||
| Robert J. Lollini | Director (principal executive officer) |
|||||
| By: | /s/ ANDREA KENDELL |
Chief Financial Officer | September 13, 2011 | |||
| Andrea Kendell | (principal financial and accounting officer) | |||||
| By: | /s/ GERALD P. BELLE |
Chairman of the Board | September 13, 2011 | |||
| Gerald P. Belle | ||||||
| By: | /s/ JOHN T. HENDERSON |
Director | September 13, 2011 | |||
| John T. Henderson, M.D. | ||||||
| By: | /s/ DENNIS H. LANGER |
Director | September 13, 2011 | |||
| Dennis H. Langer, M.D., J.D. | ||||||
| By: | /s/ ROBERT FORRESTER |
Director | September 13, 2011 | |||
| Robert Forrester | ||||||
| By: | /s/ TIMOTHY R. FRANSON |
Director | September 13, 2011 | |||
| Timothy R. Franson, M.D. | ||||||
56
Table of Contents
Myrexis, Inc.
Years Ended June 30, 2011, 2010 and 2009
| Page | ||||
| F-2 | ||||
| Financial Statements: |
||||
| F-3 | ||||
| Statements of Operations for the Years Ended June 30, 2011, 2010 and 2009 |
F-4 | |||
| F-5 | ||||
| Statements of Cash Flows for the Years Ended June 30, 2011, 2010 and 2009 |
F-6 | |||
| F-7 | ||||
F-1
Table of Contents
Report of Independent Registered Public Accounting Firm
The Board of Directors and Stockholders
Myrexis, Inc.
We have audited the accompanying balance sheets of Myrexis, Inc. as of June 30, 2011 and 2010, and the related statements of operations, stockholders’ equity and comprehensive income (loss), and cash flows for each of the three years in the period ended June 30, 2011. These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on these financial statements based on our audits.
We conducted our audits in accordance with the standards of the Public Company Accounting Oversight Board (United States). Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement. An audit includes examining, on a test basis, evidence supporting the amounts and disclosures in the financial statements. An audit also includes assessing the accounting principles used and significant estimates made by management, as well as evaluating the overall financial statement presentation. We believe that our audits provide a reasonable basis for our opinion.
In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of Myrexis, Inc. at June 30, 2011 and 2010, and the results of its operations and its cash flows for each of the three years in the period ended June 30, 2011, in conformity with U.S. generally accepted accounting principles.
We also have audited, in accordance with the standards of the Public Company Accounting Oversight Board (United States), Myrexis, Inc.’s internal control over financial reporting as of June 30, 2011, based on criteria established in Internal Control-Integrated Framework issued by the Committee of Sponsoring Organizations of the Treadway Commission and our report dated September 13, 2011 expressed an unqualified opinion thereon.
/s/ Ernst & Young LLP
Salt Lake City, Utah
September 13, 2011
F-2
Table of Contents
June 30, 2011 and 2010
(In thousands, except per share amounts)
| 2011 | 2010 | |||||||
| Assets | ||||||||
| Current assets: |
||||||||
| Cash and cash equivalents |
$ | 19,189 | $ | 35,911 | ||||
| Marketable investment securities |
86,446 | 102,965 | ||||||
| Prepaid expenses and other assets |
1,861 | 453 | ||||||
|
|
|
|
|
|||||
| Total current assets |
107,496 | 139,329 | ||||||
|
|
|
|
|
|||||
| Equipment and leasehold improvements: |
||||||||
| Equipment |
4,320 | 6,035 | ||||||
| Leasehold improvements |
1,192 | 1,160 | ||||||
|
|
|
|
|
|||||
| 5,512 | 7,195 | |||||||
| Less accumulated depreciation |
2,197 | 1,199 | ||||||
|
|
|
|
|
|||||
| Net equipment and leasehold improvements |
3,315 | 5,996 | ||||||
|
|
|
|
|
|||||
| Long-term marketable investment securities |
10,243 | 8,577 | ||||||
| Other assets |
206 | 206 | ||||||
|
|
|
|
|
|||||
| Total assets |
$ | 121,260 | $ | 154,108 | ||||
|
|
|
|
|
|||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current liabilities: |
||||||||
| Accounts payable |
$ | 1,210 | $ | 1,927 | ||||
| Accrued liabilities |
2,100 | 2,323 | ||||||
|
|
|
|
|
|||||
| Total current liabilities |
3,310 | 4,250 | ||||||
|
|
|
|
|
|||||
| Commitments and contingencies |
||||||||
| Stockholders’ equity: |
||||||||
| Preferred stock, $0.01 par value, authorized 5,000 shares; no shares issued and outstanding |
— | — | ||||||
| Common stock, $0.01 par value, authorized 60,000 shares; issued and outstanding 26,053 shares at June 30, 2011; issued and outstanding 25,214 shares at June 30, 2010 |
261 | 252 | ||||||
| Additional paid-in capital |
203,301 | 196,532 | ||||||
| Accumulated other comprehensive income |
47 | 25 | ||||||
| Accumulated deficit |
(85,659 | ) | (46,951 | ) | ||||
|
|
|
|
|
|||||
| Total stockholders’ equity |
117,950 | 149,858 | ||||||
|
|
|
|
|
|||||
| Total liabilities and stockholders’ equity |
$ | 121,260 | $ | 154,108 | ||||
|
|
|
|
|
|||||
See accompanying notes to financial statements.
F-3
Table of Contents
Years ended June 30, 2011, 2010 and 2009
(In thousands, except per share amounts)
| 2011 | 2010 | 2009 | ||||||||||
| Research revenue |
$ | 185 | $ | 90 | $ | 5,456 | ||||||
|
|
|
|
|
|
|
|||||||
| Total revenue |
185 | 90 | 5,456 | |||||||||
|
|
|
|
|
|
|
|||||||
| Costs and expenses: |
||||||||||||
| Research and development expense |
22,296 | 28,222 | 54,611 | |||||||||
| General and administrative expense |
17,239 | 19,984 | 8,981 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total costs and expenses |
39,535 | 48,206 | 63,592 | |||||||||
|
|
|
|
|
|
|
|||||||
| Operating loss |
(39,350 | ) | (48,116 | ) | (58,136 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Other income (expense), net |
642 | 1,165 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net loss |
$ | (38,708 | ) | $ | (46,951 | ) | $ | (58,136 | ) | |||
|
|
|
|
|
|
|
|||||||
| Loss per basic and diluted share |
$ | (1.52 | ) | $ | (1.91 | ) | $ | (2.43 | )(1) | |||
| Weighted-average shares used to compute net loss per basic and diluted share |
25,513 | 24,545 | 23,974 | (2) | ||||||||
| (1) | Pro forma loss per basic and diluted share for fiscal year ended June 30, 2009. |
| (2) | Pro forma shares used to compute net loss per basic and diluted share for fiscal year ended June 30, 2009. |
See accompanying notes to financial statements.
F-4
Table of Contents
Statements of Stockholders’ Equity and Comprehensive Income (Loss)
Years ended June 30, 2011, 2010 and 2009
(In thousands)
| Common Stock | Additional Paid-In Capital |
Accumulated Deficit |
Unrealized (Loss) Gain on Available-for- sale securities |
Parent Company Investment |
Total Stockholders’ Equity |
|||||||||||||||||||||||
| Shares | Amount | |||||||||||||||||||||||||||
| Balance at June 30, 2008 |
— | $ | — | $ | — | $ | — | $ | — | $ | (30,822 | ) | $ | (30,822 | ) | |||||||||||||
| Net transfers from parent |
— | — | — | — | — | 90,054 | 90,054 | |||||||||||||||||||||
| Contribution of cash and investments from parent |
— | — | — | — | 461 | 187,544 | 188,005 | |||||||||||||||||||||
| Contribution of net operating assets and liabilities to Myrexis, Inc. and issuance of common shares to Myriad Genetics stockholders |
23,974 | 240 | 188,400 | — | — | (188,640 | ) | — | ||||||||||||||||||||
| Net loss and comprehensive loss |
— | — | — | — | — | (58,136 | ) | (58,136 | ) | |||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at June 30, 2009 |
23,974 | 240 | 188,400 | 461 | — | 189,101 | ||||||||||||||||||||||
| Comprehensive Income: |
||||||||||||||||||||||||||||
| Net loss |
— | — | — | (46,951 | ) | — | — | (46,951 | ) | |||||||||||||||||||
| Change in unrealized gains on marketable investment securities |
— | — | — | — | (436 | ) | — | (436 | ) | |||||||||||||||||||
| Total comprehensive loss |
(47,387 | ) | ||||||||||||||||||||||||||
| Issuance of common stock for cash upon exercise of options and employee stock purchase plan |
1,240 | 12 | 2,332 | — | — | — | 2,344 | |||||||||||||||||||||
| Share-based payment expense |
— | — | 5,800 | — | — | — | 5,800 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at June 30, 2010 |
25,214 | 252 | 196,532 | (46,951 | ) | 25 | — | 149,858 | ||||||||||||||||||||
| Comprehensive Income: |
||||||||||||||||||||||||||||
| Net loss |
— | — | — | (38,708 | ) | — | — | (38,708 | ) | |||||||||||||||||||
| Change in unrealized gains on marketable investment securities |
— | — | — | — | 22 | — | 22 | |||||||||||||||||||||
|
|
|
|||||||||||||||||||||||||||
| Total comprehensive loss |
(38,686 | ) | ||||||||||||||||||||||||||
| Issuance of common stock for cash upon exercise of options and employee stock purchase plan |
839 | 9 | 1,937 | — | — | — | 1,946 | |||||||||||||||||||||
| Share-based payment expense |
— | — | 4,832 | — | — | — | 4,832 | |||||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
| Balance at June 30, 2011 |
26,053 | $ | 261 | $ | 203,301 | $ | (85,659 | ) | $ | 47 | — | $ | 117,950 | |||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||||
See accompanying notes to financial statements.
F-5
Table of Contents
Years ended June 30, 2011, 2010 and 2009
(In thousands)
| 2011 | 2010 | 2009 | ||||||||||
| Cash flows from operating activities: |
||||||||||||
| Net loss |
$ | (38,708 | ) | $ | (46,951 | ) | $ | (58,136 | ) | |||
| Adjustments to reconcile net loss to net cash used in operating activities: |
||||||||||||
| Depreciation and amortization |
1,661 | 1,347 | 2,854 | |||||||||
| Loss on impairment of assets |
1,112 | 224 | — | |||||||||
| Share-based compensation expense |
4,832 | 5,800 | 10,445 | |||||||||
| Gain on sale of marketable investment securities |
(5 | ) | (43 | ) | — | |||||||
| Write-off of in process research and development |
— | — | 7,000 | |||||||||
| Changes in operating assets and liabilities: |
||||||||||||
| Prepaid expenses |
(1,003 | ) | (213 | ) | 390 | |||||||
| Accounts receivable |
— | — | 4,547 | |||||||||
| Other assets, long-term |
(411 | ) | (206 | ) | — | |||||||
| Accounts payable |
(711 | ) | 1,927 | — | ||||||||
| Accrued liabilities |
(222 | ) | (2,253 | ) | (25,782 | ) | ||||||
| Deferred revenue |
— | — | (2,000 | ) | ||||||||
|
|
|
|
|
|
|
|||||||
| Net cash used in operating activities |
(33,455 | ) | (40,368 | ) | (60,682 | ) | ||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from investing activities: |
||||||||||||
| Capital expenditures for equipment and leasehold improvements |
(93 | ) | (2,135 | ) | (375 | ) | ||||||
| Acquisition of in-process research and development |
— | — | (7,000 | ) | ||||||||
| Purchase of marketable investment securities |
(142,428 | ) | (183,875 | ) | — | |||||||
| Proceeds from sale of marketable investment securities |
29,099 | 32,794 | — | |||||||||
| Proceeds from maturity of marketable investment securities |
128,209 | 98,779 | — | |||||||||
| Change in other assets |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by (used in) investing activities |
14,787 | (54,437 | ) | (7,375 | ) | |||||||
|
|
|
|
|
|
|
|||||||
| Cash flows from financing activities: |
||||||||||||
| Net proceeds from common stock issued under share-based compensation plans |
1,946 | 2,344 | — | |||||||||
| Net change in investment from parent |
— | — | 196,429 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net cash provided by financing activities |
1,946 | 2,344 | 196,429 | |||||||||
|
|
|
|
|
|
|
|||||||
| Net increase (decrease) in cash and cash equivalents |
(16,722 | ) | (92,461 | ) | 128,372 | |||||||
| Cash and cash equivalents at beginning of year |
35,911 | 128,372 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Cash and cash equivalents at end of year |
$ | 19,189 | $ | 35,911 | $ | 128,372 | ||||||
|
|
|
|
|
|
|
|||||||
| Supplemental cash flow information: |
||||||||||||
| Fair value adjustment on marketable investment securities recorded to stockholders’ equity |
22 | (436 | ) | 461 | ||||||||
See accompanying notes to financial statements.
F-6
Table of Contents
June 30, 2011, 2010, and 2009
| (1) | Organization and Summary of Significant Accounting Policies |
| (a) | Organization and Business Description |
Myrexis, Inc. (“Myrexis” or the “Company”) is a biotechnology company focused on the development of small-molecule compounds with novel chemical structures and distinct mechanisms of action. We have generated a strong pipeline of differentiated product candidates in oncology and autoimmune diseases. We are focused on maximizing the therapeutic and commercial value of these molecules by developing potential first-in-class and/or best-in-class treatment options for patients with unmet needs.
On June 2, 2009 the Myriad Genetics, Inc. (“MGI”) Board of Directors approved a plan to separate its molecular diagnostic business from its research and drug development businesses. In order to carry out the proposed separation of the research and drug development businesses MGI created a new wholly owned subsidiary, a Delaware corporation into which the research operations along with substantially all of the assets (and employees) of the pharmaceuticals business and associated intellectual property rights (including patents) and cash were contributed. In connection with the formation of this new subsidiary, MGIs’ existing subsidiary, Myriad Pharmaceuticals, Inc., changed its corporate name to Myriad Therapeutics, Inc., and the newly formed subsidiary adopted the name of Myriad Pharmaceuticals, Inc., and, effective July 1, 2010, changed its name to Myrexis, Inc.
The shares of Myrexis were distributed on June 30, 2009 to MGI stockholders of record as of June 17, 2009 as a pro-rata, tax-free dividend. MGI has received a private letter ruling from the Internal Revenue Service affirming the tax-free nature of the spin-off. The separation resulted in Myrexis operating as an independent entity with its own publicly traded common stock. MGI no longer has any ownership or other form of interest in Myrexis subsequent to the separation. Following the separation, the Company’s operations consist solely of the operations described herein.
In connection with the separation, Myrexis and MGI entered into a series of agreements, including a separation agreement, a sublease agreement, an employee matters agreement, and a tax sharing agreement. See note 2 for further discussion regarding these agreements.
The Company’s focus is to develop therapeutic products to treat patients with unmet medical needs. Myrexis researchers have made important discoveries that point to novel disease pathways that may pave the way for the development of new classes of drugs. The Company’s operations are located in Salt Lake City, Utah.
| (b) | Basis of Accounting |
The balance sheets as of June 30, 2011 and 2010 and notes related thereto, reflect the balances of Myrexis as an independent company. For purposes of preparing the statements of operations for the year ended June 30, 2009, Myrexis has been allocated certain expenses from MGI but has not been allocated the underlying productive assets, such as, certain information systems equipment that were not assigned to Myrexis but for which Myrexis has benefited from the assets. Such expenses have been reflected in the statements of operations as expense allocations from MGI for this period.
Management believes that the assumptions underlying the financial statements for years ended prior to June 30, 2010, are reasonable. The financial information in those financial statements does not include all expenses that would have been incurred had Myrexis been an independent publicly traded entity. As such, the financial information herein does not reflect the financial position, results of operations or cash flows of Myrexis in the future or what they would have been, had Myrexis been a
F-7
Table of Contents
separate, stand-alone entity during the periods presented. Specific costs attributable to Myrexis operations have been included in Myrexis’s financial statements. The financial statements also include some proportional cost allocations of certain common costs of MGI and Myrexis because these expenses were not specifically identified at the subsidiary level. The basis of these allocations includes full-time equivalent employees for the respective periods presented, square footage, and other appropriate allocation drivers.
| (c) | Use of Estimates |
The preparation of the financial statements in accordance with U.S. generally accepted accounting principles requires Myrexis management to make estimates and assumptions relating to the reported amounts of assets and liabilities and the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the period. Significant items subject to such estimates and assumptions include the carrying amount of certain accrued liabilities and share-based compensation. Actual results could differ from those estimates presented herein.
| (d) | Cash and Cash Equivalents |
The Company considers all cash on deposit, money market accounts, and highly liquid debt instruments purchased with original maturities of three months or less to be cash and cash equivalents. The Company maintains cash and cash equivalents in bank deposit and other investment accounts which, at times, may exceed federally insured limits.
| (e) | Myriad Genetics, Inc. Net Investment (Capital Deficiency) |
The balance sheet as of June 30, 2011 and 2010 reflects the balances of Myrexis as an independent company. Amounts reflected in the statements of operations of Myrexis for the year ended June 30, 2009, represent a combination of various components of MGI. The statement of operations for the years ended June 30, 2011 and 2010 is representative of Myrexis as a stand-alone company. Because a direct ownership relationship did not exist among all the components comprising Myrexis, MGI’s investment in Myrexis is shown in lieu of stockholder’s equity in the financial statements for the year ended June 30, 2009. The net investment account represents the cumulative investments in, distributions from and earnings (loss) of Myrexis.
Prior to the spin-off, all cash and investments were held and managed by MGI. Accordingly, cash used to pay Myrexis expenses or cash collected from collaboration agreements by MGI on behalf of Myrexis prior to June 30, 2009 are recorded as an increase or decrease in the MGI net investment (capital deficiency).
| (f) | Loss Per Share |
The loss per basic and diluted share is calculated by dividing net loss by the weighted-average number of shares outstanding during the reported period. For the year ended June 30, 2009, the computation of pro forma net loss per basic and diluted share and the weighted-average shares outstanding are calculated based on the 23,974,211 shares issued in connection with the spin-off on June 30, 2009.
For the year ended June 30, 2011, there were outstanding potential common equivalent shares of 2,613,945 compared to 2,004,904, in the same period in 2010 which were excluded from the computation of diluted earnings per share because the effect would have been anti-dilutive. These potential dilutive common equivalent shares may be dilutive to basic earnings per share in future periods.
The calculation of diluted loss per share is the same as the basic loss per share since the inclusion of any potentially dilutive securities would be anti-dilutive.
F-8
Table of Contents
| (g) | Fair Value Disclosure |
At June 30, 2011and 2010, the carrying value of the Company’s other receivables, accounts payable and accrued expenses approximates fair value, principally because of the short term nature of the assets and liabilities.
| (h) | Revenue Recognition |
Revenue from non-refundable upfront license fees where the Company has continuing involvement is recognized ratably over the development or agreement period or upon termination of a development or license agreement when the Company has no ongoing obligation.
Research revenue includes revenue from research services agreements, milestone payments, and technology licensing agreements. In applying the principles of revenue recognition to research and technology license agreements the Company considers the terms and conditions of each agreement separately to arrive at a proportional performance methodology of recognizing revenue. Such methodologies involve recognizing revenue on a straight-line basis over the term of the agreement, as underlying research costs are incurred, or on the basis of contractually defined output measures such as units delivered. The Company makes adjustments, if necessary, to the estimates used in its calculations as work progresses and it gains experience. The principal costs under these agreements are for personnel expenses to conduct research and development but also include costs for materials and other direct and indirect items necessary to complete the research under these agreements. Actual results may vary from estimates. Payments received on uncompleted long-term contracts may be greater than or less than incurred costs and estimated earnings are recorded as other receivables or deferred revenues.
| (i) | Research and Development Expenses |
Research and development expenses consist primarily of costs associated with the clinical trials of Myrexis product candidates, development materials, compensation and related benefits for research and development personnel, costs for consultants, and various overhead costs. Research and development costs are expensed as incurred.
| (j) | General and Administrative Expenses |
General and administrative expenses for the year ended June 30, 2010, include $1.5 million in reimbursed stipulated expenses and a $2.9 million termination fee in connection with the proposed merger with Javelin Pharmaceuticals, Inc. that was terminated in April 2010. For the year ended June 30, 2010, the Company incurred expenses of $3.1 million in external acquisition expenses which are offset by the fees described above. General and administrative expenses for the year ended June 30, 2009, reflect a decrease in commercialization efforts resulting from the discontinuance of the drug candidate Flurizan.
| (k) | Equipment and Leasehold Improvements |
Equipment and leasehold improvements are stated at cost. Depreciation and amortization are computed using the straight-line method based on the lesser of estimated useful lives of the related assets or lease terms. Equipment items have depreciable lives of five years. Leasehold improvements are depreciated over the shorter of the estimated useful lives or the associated lease terms, which range from three to fifteen years. For the years ended June 30, 2011, 2010, and 2009, the Company recorded depreciation expense of $1.7 million, $1.2 million, and $2.7 million, respectively.
| (l) | Impairment of Long-Lived Assets |
Long-lived assets are reviewed for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability of assets to be
F-9
Table of Contents
held and used is measured by a comparison of the carrying amount of an asset to future net cash flows expected to be generated by the asset. If the carrying amount of an asset exceeds its estimated future cash flows, an impairment charge is recognized in the amount by which the carrying amount of the asset exceeds the fair value of the asset. Assets to be disposed of are reported at the lower of the carrying amount or fair value less costs to sell. For the years ended June 30, 2011 and 2010, $1.1 million and $0.2 million was recorded for impairment of assets. No impairments of long-lived assets were recorded for the year ended June 30, 2009.
| (m) | Other Assets |
Other assets are comprised of purchased intellectual property, a purchased library of chemical compounds and a security deposit for the sublease agreement entered into with MGI to provide for the lease of office and laboratory space in a new facility. Management reviews the valuation of these investments for possible impairment as changes in facts and circumstances indicate that potential impairment should be assessed.
The library of chemical compounds and related purchased intellectual property were fully amortized during the year ended June 30, 2010. Myrexis has also reassessed the useful lives of its other assets and has determined that the estimated useful lives are appropriate.
For the years ended June 30, 2011, 2010, and 2009, the Company recorded amortization expense of $0, $95,000, and $125,000, respectively, related to these assets.
| (n) | Income Taxes |
The Company recognizes income taxes under the asset and liability method. This approach requires the recognition of deferred tax assets and liabilities for the expected future tax consequences of temporary differences between the carrying amounts and the tax bases of assets and liabilities.
The provision for income taxes, including the effective tax rate and analysis of potential tax exposure items, if any, requires significant judgment and expertise in federal and state income tax laws, regulations and strategies, including the determination of deferred tax assets and liabilities and any estimated valuation allowances deemed necessary to recognize deferred tax assets at an amount that is more likely than not to be realized. The Company’s filings, including the positions taken therein, are subject to audit by various taxing authorities. While the Company believes it has provided adequately for its income tax liabilities in the consolidated financial statements, adverse determinations by these taxing authorities could have a material adverse effect on the consolidated financial condition, results of operations or cash flows.
For periods prior to fiscal 2010, the Company’s operations have been included in MGI’s consolidated U.S. federal and state income tax returns. The provision for income taxes has been determined as if the Company had filed separate income tax returns under its existing structure for the periods presented. Accordingly, the effective tax rate of the Company in future years could vary from its historical effective tax rates depending on the Company’s future legal structure and related tax elections. The historical net operating loss and research credit carryforwards generated by Myrexis prior to the separation remained with MGI.
| (o) | Share-based compensation |
Certain Myrexis employees who were MGI’s employees prior to the separation hold stock options and participated in the MGI employee stock option and stock purchase plans. Share-based compensation expense in the accompanying financial statements for the year ended June 30, 2009 is recognized based on MGI’s share-based payment expense for such Myrexis employees and certain allocated share-based compensation expense relating to general and administrative employees of MGI.
F-10
Table of Contents
The Company recognizes compensation expense using a fair-value based method for costs related to stock options and other equity-based compensation. The expense is measured based on the grant date fair value of the awards that are expected to vest, and the expense is recorded over the applicable requisite service period. For time-based stock options and restricted stock, compensation expense is recognized over the vesting period from the vesting commencement date using the straight-line method. In the absence of an observable market price for a share-based award, the fair value is based upon a valuation methodology that takes into consideration various factors, including the exercise price of the award, the expected term of the award, the current price of the underlying shares, the expected volatility of the underlying share price based on peer companies, the expected dividends on the underlying shares and the risk-free interest rate.
| (p) | Marketable Investment Securities |
The Company has classified its marketable investment securities as available-for-sale. These securities are carried at estimated fair value with unrealized holding gains and losses, net of the related tax effect, included in accumulated other comprehensive income (loss) in stockholders’ equity until realized. Gains and losses on investment security transactions are reported on the specific-identification method. Dividend and interest income are recognized when earned.
A decline in the market value of any available-for-sale security below cost that is deemed other than temporary results in a charge to earnings and establishes a new cost basis for the security. Losses are charged against “Other income (expense)” when a decline in fair value is determined to be other than temporary. The Company reviews several factors to determine whether a loss is other than temporary. These factors include but are not limited to: (i) the extent to which the fair value is less than cost and the cause for the fair value decline, (ii) the financial condition and near term prospects of the issuer or declines in credit risk, (iii) the length of time a security is in an unrealized loss position and (iv) the Company more likely than not, holding securities for a period of time sufficient to allow for any anticipated recovery in fair value. The Company recognized no impairments on available-for-sale securities for the years ended June 30, 2011, 2010 and 2009.
| (q) | Segment and Related Information |
ASC 280, Segment Reporting, redefines how operating segments are determined and requires disclosure of certain financial and descriptive information about a company’s operating segments. Myrexis’ business consists primarily of pharmaceutical development and related research activities. Accordingly, the Company operates in one reportable business segment.
The Company’s revenues were derived from research performed in the United States. Additionally, all of the Company’s long-lived assets are located in the United States.
| (2) | Spin-Off of Myrexis, Inc. |
On June 30, 2009, MGI separated its molecular diagnostic business from its research and drug development businesses through the spin-off of Myrexis. MGI contributed substantially all of the assets and certain liabilities from its research and drug development businesses and $188 million of cash and marketable securities to Myrexis. All outstanding shares of the Company were then distributed to MGI’s stockholders of record on June 17, 2009 as a pro-rata, tax-free dividend of one share of Myrexis common stock for every four shares of MGI’s common stock.
On June 30, 2009, the Company entered into a Separation and Distribution Agreement with MGI that set forth the terms and conditions of the separation of the Company from MGI. The Separation and Distribution Agreement sets forth a framework for the relationship between the Company and MGI following the separation regarding principal transactions necessary to separate the companies, including: (i) the contribution of substantially all of the assets and certain liabilities of MGI’S research and drug development businesses and cash and marketable securities of approximately $188 million to the Company;
F-11
Table of Contents
and (ii) the distribution by MGI, as of 11:59 p.m. (EDT) on June 30, 2009, of all outstanding shares of Myrexis common stock to MGI’s stockholders in the form of a pro rata dividend of one share of Myrexis common stock for every four shares of MGI’s common stock outstanding to stockholders of record on June 17, 2009. This agreement also sets forth other provisions that govern certain aspects of the Company’s relationship with MGI after completion of the separation and also provides for the allocation of assets, liabilities and obligations between the Company and MGI in connection with the separation.
In addition, on June 30, 2009 the Company entered into other definitive agreements in connection with the spin-off, including (1) a Tax Sharing Agreement that generally governs the parties’ respective rights, responsibilities and obligations after the separation with respect to taxes (2) a Sublease Agreement that provides for the sublease from MGI to the Company of certain office and laboratory space to be utilized by Myrexis in its operations and (3) an Employee Matters Agreement that allocates liabilities and responsibilities relating to employee compensation, benefit plans, programs and other related matters in connection with the separation, including the treatment of outstanding incentive awards and certain retirement and welfare benefit obligations.
| (3) | Marketable Investment Securities |
The amortized cost, gross unrealized holding gains, gross unrealized holding losses, and fair value for available-for-sale securities by major security type and class of security at June 30, 2011 and 2010 were as follows (in thousands):
| Amortized cost |
Gross unrealized holding gains |
Gross unrealized holding losses |
Estimated fair value |
|||||||||||||
| June 30, 2011: |
||||||||||||||||
| Available-for-sale: |
||||||||||||||||
| Money market funds |
$ | 18,071 | $ | — | $ | — | $ | 18,071 | ||||||||
| Corporate bonds and notes |
13,963 | 12 | — | 13,975 | ||||||||||||
| Federal agency issues |
82,431 | 40 | (6 | ) | 82,465 | |||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 114,465 | $ | 52 | $ | (6 | ) | $ | 114,511 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Amortized cost |
Gross unrealized holding gains |
Gross unrealized holding losses |
Estimated fair value |
|||||||||||||
| June 30, 2010: |
||||||||||||||||
| Available-for-sale: |
||||||||||||||||
| Money market funds |
$ | 34,593 | $ | 1 | $ | — | $ | 34,594 | ||||||||
| Corporate bonds and notes |
31,556 | — | (21 | ) | 31,535 | |||||||||||
| Federal agency issues |
79,415 | 45 | — | 79,460 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 145,564 | $ | 46 | $ | (21 | ) | $ | 145,589 | |||||||
|
|
|
|
|
|
|
|
|
|||||||||
Cash and cash equivalents of $19.2 million at June 30, 2011 consist of cash and money market funds. In addition, the Company holds $200,000 restricted cash in an 18-month certificate of deposit as collateral for a corporate purchasing card program and $48,000 in a restricted cash account as collateral for office equipment. These amounts are included in long-term marketable securities on the balance sheet as of June 30, 2011.
F-12
Table of Contents
Maturities of debt securities classified as available-for-sale are as follows at June 30, 2011 (in thousands):
| Amortized cost |
Estimated fair value |
|||||||
| Available-for-sale: |
||||||||
| Due within one year |
$ | 86,396 | $ | 86,446 | ||||
| Due after one year through three years |
9,998 | 9,994 | ||||||
|
|
|
|
|
|||||
| $ | 96,394 | $ | 96,440 | |||||
|
|
|
|
|
|||||
| (4) | Fair Value Measurements |
The fair value of the Company’s financial instruments reflects the amounts that the Company estimates to receive in connection with the sale of an asset or paid in connection with the transfer of a liability in an orderly transaction between market participants at the measurement date (exit price). The fair value hierarchy prioritizes the use of inputs used in valuation techniques into the following three levels:
Level 1—quoted prices in active markets for identical assets and liabilities.
Level 2—observable inputs other than quoted prices in active markets for identical assets and liabilities; quoted prices in markets that are not active; or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the assets or liabilities. Some of the Company’s marketable securities primarily utilize broker quotes in a non-active market for valuation of these securities.
Level 3—unobservable inputs.
The majority of the Company’s financial instruments are valued using quoted prices in active markets or based on other observable inputs. The following table sets forth the fair value of the Company’s financial assets that the Company re-measured:
| (In thousands) | Level 1 | Level 2 | Level 3 | Total | ||||||||||||
| June 30, 2011 |
||||||||||||||||
| Money market funds |
$ | 18,071 | $ | — | $ | — | $ | 18,071 | ||||||||
| Corporate bonds and notes |
— | 13,975 | — | 13,975 | ||||||||||||
| Federal agency issues |
— | 82,465 | — | 82,465 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 18,071 | $ | 96,440 | $ | — | $ | 114,511 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Level 1 | Level 2 | Level 3 | Total | |||||||||||||
| June 30, 2010 |
||||||||||||||||
| Money market funds |
$ | 34,594 | $ | — | $ | — | $ | 34,594 | ||||||||
| Corporate bonds and notes |
— | 31,535 | — | 31,535 | ||||||||||||
| Federal agency issues |
— | 79,460 | — | 79,460 | ||||||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| Total |
$ | 34,594 | $ | 110,995 | $ | — | $ | 145,589 | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
As of June 30, 2011, the Company has no investments which were measured using unobservable (Level 3) inputs.
| (5) | Leases |
The Company entered into a sublease agreement with MGI effective July 1, 2009, as amended on November 11, 2009, and February 19, 2010, that provides for the sublease of certain office and laboratory space. The sublease for the Company’s facility took effect January 4, 2010 for a period of three years from
F-13
Table of Contents
the commencement date with the option to extend for an additional four three-year periods. For the period ended June 30, 2009, an allocation of these lease costs is reflected in the financial statements using square footage of space occupied and other methodologies described in note 11.
Rental expense for the years ended June 30, 2011 and 2010 was $3.8 million and $3.6 million, respectively. Rental expense allocated to Myrexis was $2.7 million in 2009.
The table is reflective of the new facility sublease. As of June 30, 2011 the future minimum lease payments under the sublease agreement are as follows (in thousands):
| Fiscal year ending: |
||||
| 2012 |
$ | 3,802 | ||
| 2013 |
2,059 | |||
|
|
|
|||
| $ | 5,861 | |||
|
|
|
| (6) | Share-Based Compensation |
Myrexis Share Based Compensation Plans
The Company adopted two equity incentive plans, the Myrexis, Inc. 2009 Employee, Director and Consultant Equity Incentive Plan (the “Equity Incentive Plan”) and the Myrexis, Inc. 2009 Employee Stock Purchase Plan (the “ESPP”). At June 30, 2011, the Company was authorized to issue a total of 7.3 million shares under the plans. On July 1, 2011, (a) the number of shares of common stock reserved for issuance under the Equity Incentive Plan was increased from 7,260,690 to 8,563,259 pursuant to an “evergreen” provision, which provides for an annual increase equal to the lesser of 2,400,000 shares, 5% of the Company’s then outstanding shares of common stock, or such other amount as the board of directors may determine, and (b) the number of shares of common stock reserved for issuance under the ESPP was increased from 1,000,000 to 1,500,000 pursuant to an “evergreen” provision, which provides for an annual increase equal to the lesser of 500,000 shares, 2% of the Company’s then outstanding shares of common stock, or such other amount as the board of directors may determine.
The Equity Incentive Plan provides for the issuance of common stock based awards, including restricted stock, restricted stock units, stock options, stock appreciation rights and other equity based awards the Company directors, officers, employees and consultants. In addition, pursuant to the separation agreements the plan authorizes the issuance of stock options to certain current and former directors, officers, employees and consultants of MGI who were option holders of MGI at June 30, 2009.
The ESPP is intended to qualify as an “employee stock purchase plan” under Section 423 of the Internal Revenue Code of 1986, as amended. Full-time employees of Myrexis who will own less than five percent of Myrexis, Inc’s outstanding shares of common stock will be eligible to contribute a percentage of their base salary, subject to certain limitations, over the course of six-month offering periods for the purchase of shares of common stock. The purchase price for shares of common stock purchased under the ESPP will equal 85 percent of the fair market value of a share of common stock at the beginning or end of the relevant six-month offering period, whichever is lower.
In connection with the separation from MGI and related transactions, each outstanding MGI stock option was converted into an adjusted MGI common stock option, exercisable for the same number of shares of common stock as the original MGI option, and a new Myrexis common stock option, exercisable for one-fourth of the number of shares of common stock as the original MGI option. All other terms of the converted options remained the same. However, the vesting and expiration of the converted options is based on the optionholder’s continuing employment with either MGI or Myrexis, as applicable, following the separation. The adjusted exercise price of each converted option was determined in accordance with Section 409A and Section 422 of the Code, as follows:
| • | The per share exercise price of each such MGI converted option is equal to the product of (i) the per share exercise price of the original MGI option multiplied by (ii) a fraction, the numerator of which is |
F-14
Table of Contents
| the closing MGI’s stock price on the day after the distribution, and the denominator of which is the ex-dividend closing stock price of MGI on the day of the distribution plus one-quarter of the “when-issued” Myrexis stock price on the day of the distribution. |
| • | The per share exercise price of each such Myrexis converted option is equal to the product of (i) the per share exercise price of the original MGI option multiplied by (ii) a fraction, the numerator of which is the closing Myrexis stock price on the day after the distribution, and the denominator of which is the ex-dividend closing stock price of MGI on the day of the distribution plus one-quarter of the “when-issued” Myrexis stock price on the day of the distribution. |
Accordingly, in connection with the separation and related transactions, the Company issued stock options to current and former directors, officers, employees and consultants of MGI and Myrexis.
The exercise price of options granted during the period ended June 30, 2011 was equivalent to the fair value of the stock on the date of grant. The number of shares, terms, and vesting periods are determined by the Company’s board of directors or a committee thereof on an option-by-option basis. Options generally vest ratably over service periods of four years and expire ten years from the date of grant. As of June 30, 2011, 2,175,995 shares were available for future grant under the Equity Incentive Plan.
The fair value of each option grant is estimated on the date of the grant using the Black-Scholes option-pricing model with the following weighted-average assumptions used for grants for the fiscal years ended June 30:
| 2011 | 2010 | 2009 | ||||
| Risk-free interest rate |
1.4% | 2.1% | 2.4% | |||
| Expected dividend yield |
0% | 0% | 0% | |||
| Expected lives (in years) |
6.0 - 7.0 | 3.6 - 4.0 | 4.7 - 5.7 | |||
| Expected volatility |
75.4% | 65.7% | 42% |
Expected option lives and volatilities are based on historical data of MGI and other factors. For the year ended June 30, 2009, the assumptions used were based on inputs related to grants issued prior to the spin-off. The differences in the assumptions are a result of Myrexis operating as an independent company.
A summary of option activity is as follows:
| 2011 | 2010 | 2009 | ||||||||||||||||||||||
| Number of shares |
Weighted average exercise price |
Number of shares |
Weighted average exercise price |
Number of shares |
Weighted average exercise price |
|||||||||||||||||||
| Options outstanding at beginning of year |
3,592,227 | $ | 3.28 | 3,592,372 | $ | 2.42 | — | $ | — | |||||||||||||||
| Options granted |
844,060 | 3.84 | 1,326,064 | 4.61 | 3,592,372 | 2.42 | ||||||||||||||||||
| Less: |
||||||||||||||||||||||||
| Options exercised |
(562,562 | ) | 2.22 | (954,522 | ) | 1.73 | — | — | ||||||||||||||||
| Options canceled or expired |
(624,741 | ) | 4.26 | (371,687 | ) | 3.80 | — | — | ||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Options outstanding at end of year |
3,248,984 | 3.42 | 3,592,227 | 3.28 | 3,592,372 | 2.42 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Options exercisable at end of year |
1,542,307 | 2.76 | 1,525,053 | 2.36 | 1,781,044 | 1.87 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Options vested and expected to vest |
3,086,509 | 3.38 | 3,316,003 | 3.19 | 3,294,764 | 2.34 | ||||||||||||||||||
|
|
|
|
|
|
|
|||||||||||||||||||
| Weighted average fair value of options granted during the year |
2.53 | 2.33 | — | |||||||||||||||||||||
F-15
Table of Contents
The following table summarizes information about the stock options outstanding under our Equity Incentive Plan for both Myrexis and MGI employees at June 30, 2011:
| Range
of |
Options outstanding | Options exercisable | ||||||||||||||||||
| Number outstanding at June 30, 2011 |
Weighted average remaining contractual life (years) |
Weighted average exercise price |
Number exercisable at June 30, 2011 |
Weighted average exercise price |
||||||||||||||||
| $ 0.59 - 2.06 |
817,731 | 4.52 | $ | 1.53 | 734,145 | $ | 1.46 | |||||||||||||
| 2.07 - 3.86 |
1,238,329 | 7.06 | 3.55 | 335,462 | 3.15 | |||||||||||||||
| 4.03 - 4.67 |
869,211 | 7.11 | 4.49 | 390,025 | 4.44 | |||||||||||||||
| 4.73 - 4.83 |
323,713 | 6.40 | 4.83 | 82,675 | 4.83 | |||||||||||||||
|
|
|
|
|
|||||||||||||||||
| 3,248,984 | 6.37 | 3.42 | 1,542,307 | 2.76 | ||||||||||||||||
|
|
|
|
|
|||||||||||||||||
The fair-value of each Myrexis stock option issued pursuant to the separation was based on an allocation of the unamortized fair-value of the original MGI stock option from which it was derived. Myrexis recognizes share based compensation expense relating to both Myrexis and MGI options held by current directors, officers, employees and consultants of Myrexis. Share based compensation expense relating to Myrexis options held by current and former directors, officers, employees and consultants of MGI will be recognized by MGI.
As of June 30, 2011, unrecognized compensation expense related to the unvested portion of MGI’s stock options granted to Myrexis employees and the unvested portion of Myrexis stock options granted was approximately $3.6 million and will be recognized over a weighted-average period of 2.18 years.
On September 24, 2010, the Company issued 141,094 restricted stock units under the Equity Incentive Plan at a fair value of $3.86. As of June 30, 2011, the unrecognized compensation expense related to unvested restricted stock units was approximately $0.5 million and will be recognized over a weighted-average period of 2.53 years. The total intrinsic value of options exercised during fiscal year ended June 30, 2011 was $1.0 million. The aggregate intrinsic value of options outstanding was approximately $1.9 million and the aggregate intrinsic value for options fully vested was approximately $1.7 million as of June 30, 2011.
Activity with respect to outstanding restricted stock units for the year ended June 30, 2011 is as follows:
| Number of shares |
Weighted average grant date fair value |
|||||||
| Balance at June 30, 2010 |
144,466 | $ | 4.03 | |||||
| Granted |
141,094 | 3.86 | ||||||
| Cancelled |
(54,657 | ) | 3.97 | |||||
| Vested |
(55,302 | ) | 4.03 | |||||
|
|
|
|||||||
| Balance at June 30, 2011 |
175,601 | 3.91 | ||||||
|
|
|
|||||||
Share-based compensation expense recognized for Myrexis employees included in the statements of operations for the fiscal years ended June 30, 2011, 2010 and 2009 is as follows (in thousands):
| 2011 | 2010 | 2009 | ||||||||||
| Research and development |
$ | 2,086 | $ | 2,455 | $ | 8,991 | ||||||
| General and administrative |
2,746 | 3,345 | 1,454 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total employee stock-based compensation expense |
$ | 4,832 | $ | 5,800 | $ | 10,445 | ||||||
|
|
|
|
|
|
|
|||||||
F-16
Table of Contents
For the years ended June 30, 2011 and 2010, Myrexis employees purchased 221,191 and 197,342 shares, respectively, under the Myrexis ESPP. For the year ended June 30, 2009, Myrexis employees participated in the employee stock purchase plan offered by MGI (the “MGI ESPP”), and purchased 44,979 shares. Compensation expenses associated with Myrexis employees participating in the Myrexis ESPP for the years ended June 30, 2011 and 2010 and the MGI ESPP for the year ended June 30, 2009 were approximately $360,000, $318,000, and $340,000, respectively. The fair value of shares issued under the Myrexis ESPP and the MGI ESPP, as applicable, was calculated using the Black-Scholes option-pricing model with the following weighted-average assumptions for the fiscal years ended June 30:
| 2011 | 2010 | 2009 | ||||||||||
| Risk-free interest rate |
0.2 | % | 0.2 | % | 0.5 | % | ||||||
| Expected dividend yield |
0 | % | 0 | % | 0 | % | ||||||
| Expected lives (in years) |
0.5 | 0.5 | 0.5 | |||||||||
| Expected volatility |
75 | % | 75 | % | 54 | % | ||||||
| (7) | Income Taxes |
Income tax expense (benefit) consists of the following:
| (In thousands) | Year ended June 30, | |||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Current: |
||||||||||||
| Federal |
$ | — | $ | — | $ | — | ||||||
| State |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Current |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Deferred: |
||||||||||||
| Federal |
(13,032 | ) | (18,007 | ) | (22,650 | ) | ||||||
| State |
(2,380 | ) | (2,900 | ) | (4,620 | ) | ||||||
| Change in valuation allowance |
15,412 | 20,907 | 27,270 | |||||||||
|
|
|
|
|
|
|
|||||||
| Total Deferred |
— | — | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Total income tax expense (benefit) |
$ | — | $ | — | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
The differences between income taxes at the statutory federal income tax rate and income taxes reported in the consolidated statements of operations were as follows:
| Year ended June 30, | ||||||||||||
| 2011 | 2010 | 2009 | ||||||||||
| Federal income tax expense at the statutory rate |
(34.0 | )% | (34.0 | )% | (34.0 | )% | ||||||
| State income taxes, net of federal benefit |
(3.3 | ) | (3.3 | ) | (3.3 | ) | ||||||
| Research and development credits, net of the federal tax on state credits |
(5.9 | ) | (1.7 | ) | (9.6 | ) | ||||||
| Tax basis differences from spin off transaction |
— | (5.7 | ) | — | ||||||||
| Incentive stock option and employee stock purchase plan expense |
2.3 | — | — | |||||||||
| Uncertain tax positions, net of federal benefit on state positions |
1.1 | 0.2 | — | |||||||||
| Change in valuation allowance |
39.8 | 44.5 | 46.9 | |||||||||
|
|
|
|
|
|
|
|||||||
| — | % | — | % | — | % | |||||||
|
|
|
|
|
|
|
|||||||
F-17
Table of Contents
The significant components of the Company’s deferred tax assets and liabilities were comprised of the following at June 30, 2011 and 2010:
| (In thousands) | Year ended June 30, | |||||||
| 2011 | 2010 | |||||||
| Net operating loss carryforwards |
$ | 28,699 | $ | 17,267 | ||||
| Intangible assets |
1,319 | 1,401 | ||||||
| Accrued vacation |
236 | 348 | ||||||
| Stock compensation expense |
2,914 | 1,607 | ||||||
| Research and development credits |
3,069 | 775 | ||||||
| Property, plant and equipment |
589 | (401 | ) | |||||
| Other, net |
35 | — | ||||||
| Liability for unrecognized tax benefits |
(541 | ) | (90 | ) | ||||
|
|
|
|
|
|||||
| Total net deferred tax assets before valuation allowance |
36,320 | 20,907 | ||||||
| Less valuation allowance |
(36,320 | ) | (20,907 | ) | ||||
|
|
|
|
|
|||||
| Net deferred tax assets |
$ | — | $ | — | ||||
|
|
|
|
|
|||||
For the year ended June 30, 2009, the Company’s operations were included in MGI’s consolidated U.S. federal and state income tax returns. The income tax provision for that year has been determined as if the Company had filed a separate income tax return under its existing structure for that period. All net operating loss carryforwards and research tax credits generated prior to June 30, 2009 by the Company were retained by MGI upon the separation of the companies. Accordingly, the Company had no material deferred tax assets and liabilities at June 30, 2009.
Due to losses incurred by the Company, it has determined that it is more likely than not that the Company’s deferred tax assets will not be realized. Accordingly, a valuation allowance has been established for the full amount of the Company’s deferred tax assets. The valuation allowance increased $15.4 million and $20.9 million for the years ended June 30, 2011 and 2010, respectively. The net change in the valuation allowance for the year ended June 30, 2009 was recorded at MGI and did not carry over to the Company.
At June 30, 2011, the Company had total federal and state tax net operating loss carryforwards of approximately $76.9 million. If not utilized, these operating loss carryforwards expire beginning in 2030 through 2031. None of the net operating loss carryforwards are subject to the limitations imposed by Section 382 of the Internal Revenue Code. The Company had approximately $2.5 million of federal research tax credits, which can be carried forward to reduce federal income taxes. Additionally, the Company had approximately $0.9 million of Utah research tax credits, which can be carried forward to reduce Utah income taxes. If not utilized, the federal and Utah research tax credit carryforwards expire in 2031 and 2025, respectively.
Approximately $10.7 million of net operating losses result from ‘excess tax benefits’ as defined by ASC guidance. As such, they are not included in deferred tax assets. They will be recognized as additional paid-in-capital only upon realization of the tax benefit.
The Company has adopted the provisions of ASC Topic 740 Subtopic 10 Section 05, which addresses the accounting for uncertainty in tax positions. ASC guidance requires that the impact of a tax position be recognized in the financial statements if that position is more likely than not of being sustained on audit, based on the technical merits of the position.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows:
| Year ended June 30, | ||||||||||||
| (In thousands) | 2011 | 2010 | 2009 | |||||||||
| Unrecognized tax benefits at beginning of year |
$ | 90 | $ | — | $ | — | ||||||
| Gross increases—current year tax positions |
451 | 90 | — | |||||||||
|
|
|
|
|
|
|
|||||||
| Unrecognized tax benefits at end of year |
$ | 541 | $ | 90 | $ | — | ||||||
|
|
|
|
|
|
|
|||||||
F-18
Table of Contents
Approximately $541,000 of the total unrecognized tax benefits as of June 30, 2011, if recognized, would affect the effective tax rate. The Company does not anticipate that unrecognized tax benefits will significantly increase or decrease within 12 months of the reporting date. Interest and penalties related to uncertain tax positions are included as a component of income tax expense.
The Company files U.S. and various state income tax returns. The 2009 and 2010 tax years (the Company’s only tax years as a separate entity) will remain subject to examination for three years after their filing. The Company’s federal tax return and state tax returns are not currently under examination. Annual tax provisions include amounts considered necessary to pay assessments that may result from examination of the Company’s tax returns. However, the amount ultimately paid upon resolution of issues may differ materially from the amount accrued.
| (8) | Stockholders’ Equity |
Comprehensive income
The components of the Company’s comprehensive income are as follows:
| June 30, | ||||||||
| (In thousands) | 2011 | 2010 | ||||||
| Net loss |
$ | (38,708 | ) | $ | (46,951 | ) | ||
| Other comprehensive loss: |
||||||||
| Change in unrealized gain (loss) on marketable securities |
22 | (436 | ) | |||||
|
|
|
|
|
|||||
| Total comprehensive loss |
$ | (38,686 | ) | $ | (47,387 | ) | ||
|
|
|
|
|
|||||
| (9) | Employee Deferred Savings Plan |
During fiscal years 2011, 2010 and 2009, the Myrexis employees participated in a deferred savings plan which qualifies under Section 401(k) of the Internal Revenue Code. Substantially all of the Myrexis employees were covered by the plan. Myrexis made matching contributions of 50% of each employee’s contribution with the employer’s contribution not to exceed 4% of the employee’s compensation. Myrexis contributions to the plan were $470,000, $552,000, and $594,000, for the years ended June 30, 2011, 2010, and 2009, respectively. Prior to the spin-off on June 30, 2009, matching contributions were made by MGI under the MGI deferred savings plan.
| (10) | Collaborative Agreements |
During the year ended June 30, 2011, Myrexis did not enter into any new collaborative agreements.
In May 2008, Myrexis entered into a collaboration agreement with H. Lundbeck A/S (“Lundbeck”) granting certain marketing rights for Myrexis’s therapeutic candidate Flurizan. Under the terms of the agreement Lundbeck paid Myrexis a $100 million non-refundable fee, and agreed to pay future royalties, sales-based milestones, and share certain development costs.
On June 30, 2008, based on results from the U.S. Phase III clinical trial, Myrexis announced its intention to discontinue all Flurizan development activities. Both Myrexis and Lundbeck concluded that Flurizan had no future economic value and that Myrexis had no continuing substantive obligations to Lundbeck. Based on this conclusion, Myrexis recognized the $100 million as pharmaceutical revenue in the accompanying statements of operations for the year ended June 30, 2008.
Upon receipt of the up-front payment from Lundbeck in June 2008, Myrexis also recorded a one-time sublicense expense of $20 million which represented the potential amount payable to a third party for the license of the Flurizan compound. The amount was recorded as research and development expense and a related accrued liability at June 30, 2008. In March 2009, this liability was settled for $11 million. Accordingly, the Company recognized a credit to its research and development expense of $9 million during the fiscal year ended June 30, 2009.
F-19
Table of Contents
In June 2006, the Company entered into a research collaboration to apply its high-speed genomic sequencing capability and bioinformatics expertise to deliver molecular genetic information to the collaborator. Revenue related to this collaboration is recognized when completed information is delivered to the collaborator. Under this agreement the Company recognized research revenue of $0 and $3.1 million for the fiscal years ended June 30, 2010 and 2009, respectively.
In June 2004, the Company entered into a five-year, research agreement to utilize its expertise to characterize pathogen-host protein interactions. Revenue related to this collaboration was recognized on a cost-to-cost basis. Under this agreement the Company recognized research revenue of $0 and $2.2 million for the fiscal years ended June 30, 2010 and 2009, respectively.
| (11) | Related Party Transactions |
For the period ended June 30, 2009, the Company’s operations were fully integrated with MGI, including executive services, finance, treasury, corporate income tax, human resources, legal services and investor relations. The accompanying financial statements reflect the application of certain estimates and allocations of operating expenses. Management believes the methods used to allocate these operating expenses are reasonable. The allocation methods include relative time devoted by executive management on Myrexis business and related benefit received by Myrexis for other services such as costs associated with being a public company and other services. Allocation of expenses for these services of $6,105,000 for the year ended June 30, 2009, are reflected in total operating expenses in the statements of operations. Operating expenses for the year ended June 30, 2011 and 2010, reflect the costs associated with being an independent publicly traded company.
| (12) | Asset Acquisitions |
On January 20, 2009, Myrexis purchased certain in-process research and development assets related to the HIV candidate MPC-4326 from Panacos Pharmaceuticals, Inc. The assets were determined to be in-process research and development assets and were charged to expense on the acquisition date. The aggregate purchase price was $7 million, which represented cash consideration. Myrexis has assumed control of all clinical and commercial development of MPC-4326.
| (13) | Stockholder Rights Plan |
In June 2009, the Company declared a dividend of one preferred stock purchase right (a “Right”) for each outstanding share of the Company’s common stock to be paid on June 30, 2009 (the “Record Date”) to holders of record of the Common Stock immediately following the distribution of shares of Common Stock to the stockholders of MGI. Each Right entitles the registered holder to purchase from the Company one one-thousandth of a share of Series A Junior Participating Preferred Stock. The rights have certain anti-takeover effects since, under certain circumstances, the rights allow the Company’s stockholders (other than an acquiror) to acquire common stock in the Company or equivalent consideration at a substantial discount if a person or persons acting as a group, directly or indirectly, obtain control of 15% of more of the Company’s Common Stock. The Rights are not currently exercisable and will expire at the close of business on June 30, 2019, unless earlier redeemed or exchanged by the Company.
| (14) | Commitments and Contingencies |
MGI had entered into a license agreement for exclusive rights to utilize certain intellectual property rights related to the drug candidate Azixa. Pursuant to the Separation Agreement, Myrexis has assumed all rights and obligations under this the license agreement. Under this agreement Myrexis may pay milestone payments totaling up to $23 million. Payment of milestones is based on the occurrence of potential future events, including the initiation of certain human clinical trials, filing of a New Drug Application with the Food and Drug Administration, receipt of regulatory approval, and specific revenue targets.
F-20
Table of Contents
Various legal claims have been filed against Myrexis that relate to the ordinary course of business and are currently pending resolution. In the opinion of management and upon consultation with legal counsel, the ultimate resolution of these matters is not expected to have a material adverse effect on the financial position or future results of operations of Myrexis.
| (15) | Other Income |
Other income was $0.6 million, $1.2 million and $0 for the years ended June 30, 2011, 2010 and 2009. Other income in the year ended June 30, 2011 includes a one-time $1.2 million grant received in November 2010 as a part of the qualifying therapeutic discovery project under section 48D of the Internal Revenue Code, interest income and realized gains on Myrexis’s marketable securities, offset by an impairment loss on assets of $1.1 million as a result of Myrexis’s corporate reorganization in March 2011. Other income for the same period in 2010 reflects interest income and realized gains on Myrexis’s marketable securities, offset by a loss on disposal of assets of $0.2 million.
| (16) | Reorganization |
On March 29, 2011, Myrexis announced a corporate reorganization to focus resources on the Company’s current portfolio of clinical and preclinical drug candidates. The reorganization included an immediate reduction in workforce by 57 employees or approximately 41%. The reduction was primarily in the Company’s internal drug discovery group and related support functions. In addition, Myrexis has stopped providing other contract research services. In connection with the restructuring, Myrexis recorded severance costs of approximately $3.0 million which were fully paid before June 30, 2011. These expenses reflected in the statement of operations include $0.5 million in general and administrative and $2.5 million in research and development for year ended June 30, 2011.
Also, in conjunction with the reorganization, the Company assessed whether there were indicators of impairment of certain fixed assets and has evaluated whether the carrying value of assets with impairment indicators is recoverable. During the fourth quarter, management reassessed its ability to use or sell certain assets. For instance, certain assets that management had planned to sell will now be abandoned due to the difficulty of selling, or negligible economic benefit. As a result of management’s assessment, $1.1 million in impairment loss was recognized during the year ended June 30, 2011.
F-21
Table of Contents
EXHIBIT INDEX
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
|||||||||
| 2.1 | Separation and Distribution Agreement, dated June 30, 2009, by and between the Registrant and Myriad Genetics, Inc. | 8-K (Exhibit 2.1) |
7/7/09 | 001-34275 | ||||||||||
| 3.1 | Amended and Restated Certificate of Incorporation of the Registrant | 10-K (Exhibit 3.1) |
9/13/10 | 001-34275 | ||||||||||
| 3.1.1 | Certificate of Designation, Preferences and Rights of Series A Junior Participating Preferred Stock | 10-K (Exhibit 3.1.1) |
9/13/10 | 001-34275 | ||||||||||
| 3.1.2 | Certificate of Amendment to Restated Certificate of Incorporation of the Registrant | 10-K (Exhibit 3.1.2) |
9/13/10 | 001-34275 | ||||||||||
| 3.2 | Amended and Restated Bylaws of the Registrant | 10-K (Exhibit 3.2) |
9/13/10 | 001-34275 | ||||||||||
| 4.1 | Form of Common Stock Certificate of the Registrant | 10/A (Exhibit 4.1) |
6/8/09 | 001-34275 | ||||||||||
| 4.2 | Shareholder Rights Agreement between the Registrant and American Stock Transfer & Trust Company, LLC, dated June 30, 2009, which includes as Exhibit B the form of Right Certificate | 8-A (Exhibit 4.1) |
6/30/09 | 001-34275 | ||||||||||
| Agreements with Myriad Genetics, Inc. |
||||||||||||||
| 10.1 | Tax Sharing Agreement, dated June 30, 2009, by and between the Registrant and Myriad Genetics, Inc. | 8-K (Exhibit 10.1) |
7/7/09 | 001-34275 | ||||||||||
| 10.2 | Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 8-K (Exhibit 10.2) |
7/7/09 | 001-34275 | ||||||||||
| 10.2.1 | Amendment No. 1, effective November 11, 2009, to Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 10-Q (Exhibit 10.1) |
11/12/09 | 001-34275 | ||||||||||
| 10.2.2 | Amendment No. 2, dated February 19, 2010, to Sublease Agreement, effective July 1, 2009, by and between the Registrant and Myriad Genetics, Inc. | 10-Q (Exhibit 10.2) |
5/17/10 | 001-34275 | ||||||||||
| 10.3 | Employee Matters Agreement, dated June 30, 2009, by and between the Registrant and Myriad Genetics, Inc. | 8-K (Exhibit 10.3) |
7/7/09 | 001-34275 | ||||||||||
Table of Contents
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
|||||||||
| Agreements with Respect to Collaborations, Licenses, Research and Development |
||||||||||||||
| †10.4 | License and Collaboration Agreement, dated November 19, 2003, by and among Myriad Genetics, Inc., Maxim Pharmaceuticals, Inc., and Cytovia, Inc. (now known as Epicept Corporation), as assigned to the Registrant on June 30, 2009 |
10/A (Exhibit 10.4) |
5/11/09 | 001-34275 | ||||||||||
| Equity Compensation Plans |
||||||||||||||
| *10.5 | 2009 Employee, Director and Consultant Stock Plan (the “2009 Plan”) | 10/A (Exhibit 10.6) |
6/8/09 | 001-34275 | ||||||||||
| *10.5.1 | Form of Stock Option Agreement under the 2009 Plan | 10/A (Exhibit 10.6.1) |
6/8/09 | 001-34275 | ||||||||||
| *10.5.2 | Form of Restricted Stock Unit Agreement under the 2009 Plan | 10/A (Exhibit 10.6.2) |
6/8/09 | 001-34275 | ||||||||||
| *10.5.3 | Form of Incentive Stock Option Agreement under the 2009 Plan for Rollover Options issued under the Myriad Genetics, Inc. 2003 Employee, Director and Consultant Stock Option Plan, as amended (the “MGI 2003 Plan”) | 10/A (Exhibit 10.6.3) |
6/8/09 | 001-34275 | ||||||||||
| *10.5.4 | Form of Non-Qualified Stock Option Agreement under the 2009 Plan for Rollover Options issued under the MGI 2003 Plan | 10/A (Exhibit 10.6.4) |
6/8/09 | 001-34275 | ||||||||||
| *10.5.5 | Form of Incentive Stock Option Agreement under the 2009 Plan for Rollover Options issued under the Myriad Genetics, Inc. 2002 Employee, Director and Consultant Stock Option Plan, as amended (the “MGI 2002 Plan”) | 10/A (Exhibit 10.6.5) |
6/8/09 | 001-34275 | ||||||||||
| *10.5.6 | Form of Non-Qualified Stock Option Agreement under the 2009 Plan for Rollover Options issued under the MGI 2002 Plan | 10/A (Exhibit 10.6.6) |
6/8/09 | 001-34275 | ||||||||||
| *10.6 | 2009 Employee Stock Purchase Plan | 10/A (Exhibit 10.7) |
6/8/09 | 001-34275 | ||||||||||
| Agreements with Executive Officers and Directors |
||||||||||||||
| 10.7 | Form of Indemnification Agreement between the Registrant and its directors and officers | 10/A (Exhibit 10.8) |
5/29/09 | 001-34275 | ||||||||||
| *10.8 | Non-Employee Director Compensation Policy, as amended November 11, 2010 | 10-Q (Exhibit 10.1) |
2/9/11 | 001-34275 | ||||||||||
| *10.9 | Form of Employment Agreement between the Registrant and its officers | 10-K (Exhibit 10.10) |
9/28/09 | 001-34275 | ||||||||||
| *10.10 | Offer Letter between the Registrant and Robert J. Lollini, dated January 30, 2009 |
10-K (Exhibit 10.11) |
9/28/09 | 001-34275 | ||||||||||
| *10.11 | Management Performance—Incentive Bonus Program Fiscal Year 2010 | 10-K (Exhibit 10.12) |
9/28/09 | 001-34275 | ||||||||||
Table of Contents
| Exhibit Number |
Exhibit Description |
Filed with this Report |
Incorporated by Reference herein from Form or Schedule |
Filing Date |
SEC File / Registration Number |
|||||||||
| *10.12 | Executive Severance and Change in Control Agreement by and between the Registrant and Adrian N. Hobden, dated February 1, 2010 | 8-K (Exhibit |
2/4/10 | 001-34275 | ||||||||||
| *10.13 | Form of Executive Severance and Change in Control Agreement entered into between the Registrant and the Executive Officers of the Registrant listed on Schedule I attached thereto, on February 1, 2010 | 8-K (Exhibit 10.2) |
2/4/10 | 001-34275 | ||||||||||
| *10.14 | First Amendment, dated June 3, 2010, to Executive Severance and Change in Control Agreement by and between the Registrant and Barbara Berry, dated February 1, 2010 | 8-K (Exhibit 10.1) |
6/9/10 | 001-34275 | ||||||||||
| *10.15 | Consulting Agreement by and between the Registrant and Barbara Berry, dated June 8, 2010 | 10-K (Exhibit 10.16) |
9/13/10 | 001-34275 | ||||||||||
| *10.16 | Consulting Agreement by and between the Registrant and Edward Swabb, dated June 18, 2010 | 10-K (Exhibit 10.17) |
9/13/10 | 001-34275 | ||||||||||
| *10.17 | Notice of Termination and Release by and between the Registrant and Barbara Berry, dated June 3, 2010 | 10-K (Exhibit 10.18) |
9/13/10 | 001-34275 | ||||||||||
| *10.18 | Notice of Termination and Release by and between the Registrant and Edward Swabb, dated June 18, 2010 | 10-K (Exhibit 10.19) |
9/13/10 | 001-34275 | ||||||||||
| *10.19 | Separation Agreement by and between the Registrant and Adrian N. Hobden, dated July 21, 2011 | 8-K (Exhibit 10.1) |
7/22/11 | 001-34275 | ||||||||||
| 21.1 | List of Subsidiaries of the Registrant | S-4 (Exhibit 21.1) |
2/12/10 | 333-164890 | ||||||||||
| 23.1 | Consent of Ernst & Young LLP, Independent Registered Public Accounting Firm | X | ||||||||||||
| 31.1 | Certification of the Chief Executive Officer under Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||||
| 31.2 | Certification of the Chief Financial Officer under Section 302 of the Sarbanes-Oxley Act of 2002 | X | ||||||||||||
| 32.1 | Certification of the Chief Executive Officer and the Chief Financial Officer under Section 906 of the Sarbanes- Oxley Act of 2002 | X | ||||||||||||
| * | Management contract, compensatory plan or arrangement. |
| † | Confidential portions of these documents have been filed separately with the Securities and Exchange Commission pursuant to a request for confidential treatment. |
